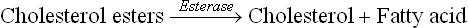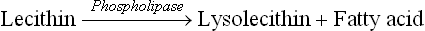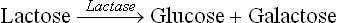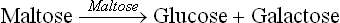Gastrointestinal pathobiochemistry
Digestion, absorption and subsequent assimilation of dietary components are responsible for the transformation of food to flesh. This process is complex and depends on integrated activities of various organs, mainly those of the alimentary tract.
A battery of enzymes and cofactors present in various gastrointestinal secretions are essential for digestion. Coordinated action of these enzymes causes disintegration of the large polymers that constitute bulk of the ingested nutrients. The process occurs in a stepwise and controlled fashion which ensures degradation of macronutrients like proteins, lipids and carbohydrates to their building block precursors. This is essential because only small molecules can be absorbed from the gastrointestinal wall.
In this chapter, mode of action of various proteolytic, lipolytic and other enzymes of digestion and mechanism of absorption of various products of digestion are described. This is followed by description of biochemical tests for assessing gastrointestinal and hepatic functions.
After going through this chapter, the student should be able to understand:
• Digestion and absorption of major nutrients: carbohydrates, proteins and lipids.
• Organ function tests: pancreatic function tests, intestinal function tests and liver function tests.
I Digestion and Absorption
Most nutrients are large polymers. Their disintegration requires coordinated action of a number of enzymes and associated cofactors. Approximately 30 g ofdigestive enzymes are secreted per day. The whole process of digestion consists of hydrolytic cleavage reactions catalyzed by these enzymes, in which macromolecular nutrients are hydrolyzed to their monomeric building blocks.
The extent to which various nutrients are hydrolyzed, and then utilized varies. Utilization of starch and glycogen is nearly complete: these molecules are completely degraded to their monomeric unit, glucose, which is then readily absorbed. On the other extreme are the indigestible compounds like dietary fibres, which remain unutilized and are excreted as such. The major processes involved in digestion and absorption are:
1. Mechanical homogenization of food and mixing of the ingested solids with gastrointestinal secretions.
• digestive enzymes that hydrolyze dietary polymers to oligomers and dimers.
• electrolytes, acids or bases to provide an environment for optimum digestion.
3. Hydrolysis of the oligomers and dimers by intestinal surface enzymes.
A Carbohydrates
Carbohydrates provide a major share of the daily caloric requirement. Dietary carbohydrates consist of digestible compounds such as starch, glycogen, lactose and sucrose. In addition, certain indigestible fibres of plant origin, such as cellulose, hemi-cellulose, pentosans, and inulin are present in normal diet, which cannot be degraded by digestive enzymes of non-ruminants.
Since starch and glycogen provide bulk of the dietary carbohydrates, they will be considered first in some detail. Starch is a plant polysaccharide, consisting of linear chains of glucose molecules linked by α(1→4) glycosidic linkages, and branch points linked by α(1→6) glycosidic linkages (Chapter 2). Glycogen is a polysaccharide of animal origin, having a similar structure as starch, but is more extensively branched. Digestion of these polysaccharides begins in mouth in humans by action of the salivary enzyme, α-amylase. This enzyme hydrolyzes the α(1→4) bonds to release smaller oligosaccharide fragments. However, it gets little time to act because as soon as the food reaches the stomach, it is acidified and the acidic pH stops the action of this enzyme (optimum pH for salivary amylase is 6.9). No further digestion of carbohydrates occurs in the stomach.
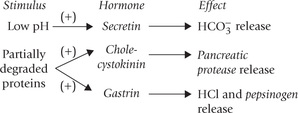
When the acidified gastric contents reach duodenum, the low pH stimulates release of secretin, an intestinal hormone, which helps elevation of pH to neutral range. It does so by stimulating bicarbonate release from the exocrine pancreas. Neutral pH is optimum for the action of the pancreatic amylase, the principal enzyme for digestion of starch (and glycogen). Like salivary amylase, it is also a dextrinogenic endosaccharidase, specific for hydrolyzing the α(1 →4) linkages. The term endo implies that it is capable of hydrolyzing those bonds that lie towards the core of the starch molecule. This distinguishes amylases (salivary and pancreatic) from the exosaccharidases of plant origin, which can act only on the terminal α(1 →4) linkages. Amylases cannot hydrolyze the branch linkages, i.e. α(1→6) linkages, being specific only for the α (1→4) linkages.
Extensive action of α-amylase in intestine cleaves the starch molecule into smaller fragments such as maltose, maltriose, and short oligosaccharides. The oligosaccharides may be linear or α-limit dextrins (5–9 glucose units with a branch point). Further hydrolysis of these products is carried out by surface enzymes of the small intestinal epithelium cells. These enzymes are also referred to as brush border enzymes. They are firmly attached to the cell surfaces with their catalytic domains protruding into the intestinal lumen.
Some brush border enzymes and their actions are as below:
1. Maltase: This enzyme possesses α(1→4) glucosidase activity which enables it to cleave maltose into two glucose residues. It can also hydrolyze short linear oligosaccharides of up to 9-carbon unit length.
2. Lactase: It degrades lactose into glucose and galactose. Its action is slower than the other brush-broder enzymes which have excess capacity to hydrolyze their substrates. Moreover, quantity of the lactase is just about sufficient to degrade the lactose that is presented to it. This is in contrast to the other brush border enzymes, synthesis of which can be induced, if required.
3. Isomaltase or sucrase: This enzyme is initially synthesized as a single polypeptide chain, which is later cleaved into two subunits, each having a distinct enzymatic activity. Both these subunits get embedded in the brush border, where:
In addition, some other disaccharidases and oligosaccharidases are present in the intestinal brush border, as summarized in Table 26.1 .
Table 26.1
Disaccharidases and oligosaccharidases of the intestinal brush border
| Enzyme | Cleavage specificity |
| Maltase | Maltose, maltotriose; also acts as exoglycosidase on α(1→4) bonds at the non-reducing end of starch and starch-derived oligosaccharides |
| Lactase* | Lactose; also cellobiose# |
| Cerebrosidase* | Gluco- and galactocerebroside |
| Sucrase | Sucrose; also maltose and maltotriose |
| Isomaltase | α(1→6) bonds in isomaltose and α-limit dextrins |
| Trehalase | Trehalose>• |
*The lactase and cerebrosidase activities reside in two different globular domains of the same polypeptide.
#Cellobiose is a disaccharide of two glucose residues in β(1→4) glycosidic linkage.
•Trehalose is a disaccharide with a structure of α-D-glucopyranosyl-α-D-glucopyranoside (in 1,1 glycosidic linkage); common only in mushrooms.
Thus, concerted action of various enzymes results in breakdown of dietary carbohydrates to produce monosaccharides such as D-glucose, D-galactose, and D-fructose. These compounds are absorbed by facilitated diffusion, i.e. through mediation of the carrier proteins present in the plasma membrane (Chapter 7). Based on studies, carried out in rat-intestine, it has been estimated that the relative rates of absorption of the monosaccharides are as follows: glucose, 100%; galactose, 11%; fructose, 43%; mannose, 10% and xylose, 15%.
Transport of glucose and galactose into the cell can be coupled with passive diffusion of sodium ions. As the sodium diffuses across the cell membrane of enterocyte along the concentration gradient, glucose and galactose are also transported along with it. This mode of transport is referred to as the secondary active transport (Chapter 7). After absorption, the monosaccharides pass into portal circulation.
B Proteins
Digestion of Dietary Proteins Begins in the Stomach
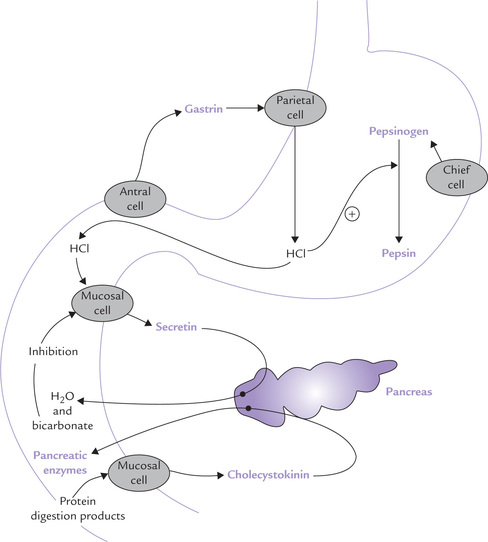
The gastric secretion contains hydrochloric acid and pepsinogen, a zymogen. Both play important role in protein digestion. The most potent stimuli for gastric secretion are dietary proteins: partially digested proteins and amino acids are more effective stimuli than the intact food proteins. These compounds first stimulate release of a hormone, gastrin, from the gastric antrum (Fig. 26.1 ). Gastrin in turn stimulates the gastric parietal cells (to release hydrochloric acid) and the chief cells (to release pepsinogen). Pepsinogen, an inactive precursor form, is subsequently converted to pepsin which is primarily responsible for the digestive activity of gastric juice.
Hydrochloric acid performs several important functions.
(a) It acts as an antiseptic and lowers the pH of the food mixture to about 1.5–2.5. This pH range is optimum for the action of pepsin.
(b) Hydrochloric acid brings about denaturation (unfolding) of the polypeptide chains, which exposes the peptide bonds for enzymatic action.
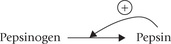
(c) Low pH (below 5) induces the conversion of pepsinogen to its activated form, pepsin, by removal of 42 amino acids from the N-terminal of the polypeptide chain.
Further conversion proceeds autocatalytically, i.e. pepsin itself activates more of pepsinogen molecules (Fig. 26.1).
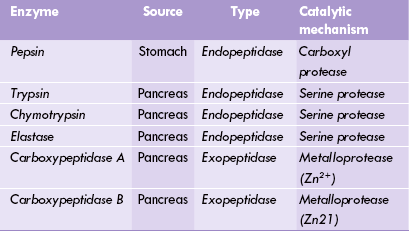
Pepsin is an endopeptidase, i.e. it cleaves the peptide linkages lying towards the core of the polypeptide chain. Moreover, it is site-specific in action, cleaving only those peptide bonds whose carboxy terminal amino acid is an aromatic amino acid (phenylalanine, tyrosine, or tryptophan). Its action yields partially disintegrated proteins.
Bulk of Protein Digestion Takes Place in Duodenum
As the acidic gastric contents reach duodenum, their low pH acts as stimulus for duodenal mucosa, inducing it to release secretin (Fig. 26.1) (Secretin was the first hormone ever to be identified). Its predominant action is to stimulate release of water and bicarbonate ions from the exocrine pancreas. The bicarbonate ions elevate pH of the duodenal contents towards the neutral range, which is optimal for the action of pancreatic enzymes, including those of protein digestion (Table 26.2 ). These enzymes are secreted in response to another hormone released from the duodenal mucosa, i.e. cholecystokinin, earlier known as pancreozymin. The release of this hormone is stimulated by the products of protein digestion.
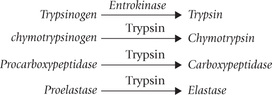
The pancreatic proteases are released in the form of inactive precursors, the form in which they are stored in the exocrine cells. Subsequently, the inactive precursors (zymogens) are activated in the intestinal lumen. This arrangement acts as safeguard against autodigestion of pancreatic cells since the activated pancreatic enzymes that are capable of digesting the cellular components are generated away from the cell.
Trypsinogen, chymotrypsinogen, procarboxypeptidase and proelastase are some examples of inactive precursors of the pancreatic proteases. Trypsinogen is initially activated by a duodenal enzyme, enterokinase which is located in the brush border. Once trypsin has been generated in this way, it acts as a major stimulus for the activation of other zymogens: chymotrypsinogen, procarboxypeptidase and proelastase.
Like pepsin, the pancreatic proteases—trypsin, chymotrypsin and elastase—are also site-specific endopeptidases.
• Trypsin cleaves those peptide bonds the carboxy terminal of which is contributed by the basic amino acid (lysine and arginine).
• Chymotrypsin hydrolyzes the ones in which an aromatic amino acid forms the carboxy terminal.
• Elastase acts on the peptide linkages at carboxy terminal of small amino acids such as valine, leucine and alanine.
In contrast with these enzymes, the carboxypeptidase is referred to as an exopeptidase (Table 26.2). This is because carboxypeptidases (both A and B type) act only on the terminal peptide bond of the polypeptide chain: their action catalyzes successive removal of a single amino acid from the carboxy terminal of the polypeptide chain. Cleavage specificity of type A is hydrophobic amino acid at carboxy terminal, and that of type B is basic amino acid at carboxy terminal.
Final Stages of Digestion Occur in Small Intestine
Various peptidases are released in small intestine which act at multiple sites. This greatly enhances the overall efficiency and speed of proteolysis. Their concerted action cleaves the polypeptide chain into multiple oligopeptide fragments.
In final stages of protein digestion, hydrolysis of these oligopeptides into constituent amino acids occurs. Enzymes operating at this stage are called peptidases (dipeptidase and aminopeptidase). They are located in the brush border membrane. Aminopeptidase catalyzes removal of a single amino acid from the N-terminal end of the oligopeptide fragment. The dipeptidase, as is evident from its name, cleaves dipeptides to yield two amino acids.
Absorption
Amino acids produced by the action of these enzymes are actively absorbed, being coupled with passive diffusion of sodium (i.e. secondary active transport). There appears to be at least six different carrier proteins for amino acid transport across the luminal mucosa. The dipeptides and tripeptides can also be absorbed, although at a much slower rate. About 5% of the ingested proteins remain unabsorbed and are excreted in faeces.
C Lipids
An average diet provides 90–100 g lipids, 90% of which are triacylglycerols. The dietary triacylglycerols are termed saturated or unsaturated fats depending on whether the glycerol backbone is esterified with the saturated fatty acids or the unsaturated fatty acids. The other dietary lip-ids are phospholipids and cholesterol esters. In addition to dietary lipids, the gastrointestinal system has to handle about 1 g of cholesterol and 5 g of lecithin that enter intestine through bile each day.
Digestion of lipids is more complex than that of proteins or carbohydrates because the hydrophobic nature of lipids limits the digestive process to the lipid-water interphase. This is in contrast with the proteins and the carbohydrates, which being hydrophilic are thoroughly exposed to action of hydrolytic enzymes. For instance, the enzymes catalyzing digestion of carbohydrates and proteins, such as endosaccharidase and endopeptidases are capable of hydrolyzing the internal bonds of these molecules, including those lying near the core.
Digestion of lipids starts in the mouth by action of the enzyme lingual lipase, which is secreted by the sublingual glands. As the food mixes with saliva, this enzyme begins hydrolytic removal of the fatty acids esterified to the C-1 and the C-3 of the triacylglycerol molecule. As a result, the dietary triacylglycerol is converted to the corresponding diacylglycerol and then to monoacylglycerol.
The hydrolytic process continues in the stomach also due to action of another lipolytic enzyme, the gastric lipase.
The partially hydrolyzed food then enters the duodenum in the form of an unstable emulsion. Further digestion occurs in the duodenum, where the following processes occur:
Emulsification
The emulsion is stabilized and subsequently dispersed by the action of bile-salts, namely sodium taurocholate and sodium glycocholate (Chapter 12). These compounds are synthesized from cholesterol in hepatocytes, and secreted into the duodenum through the hepatobiliary route. The bile-salts are major constituents of bile.
Bile salts are amphipathic in nature, i.e. they have a polar portion (termed head) and a non-polar portion (called tail). The polar heads form a hydrophilic coating on surface of the lipid particles which face the aqueous exterior. The non-polar tails, on the other hand, extend into the interior of these particles (see Fig. 3.3). The above-stated action of the bile salts is supplemented by phospholipids and other emulsifiers already present ir food. This results in dispersion of the lipid particles into the finer emulsion droplets. As a result, the surface area of the lipid: water interphase, on which the digestive enzymes can act, is increased several folds.
Enzymatic Hydrolysis
The pancreatic lipase is the principal enzyme of fat digestion, carrying out bulk of the digestion in the duodenum. This enzyme is secreted in an inactive precursor form, prolipase, along with its cofactor, called colipase. The latter is also initially secreted in a precursor form called procolipase.
The colipase positions itself on the surface of a fat particle and helps in anchoring the pancreatic lipase to the fat particle (Fig. 26.2 ). The enzyme then carries out fat degradation: the triacylglycerol particles are hydrolyzed to fatty acids, diacylglycerol and monoacylglycerol.
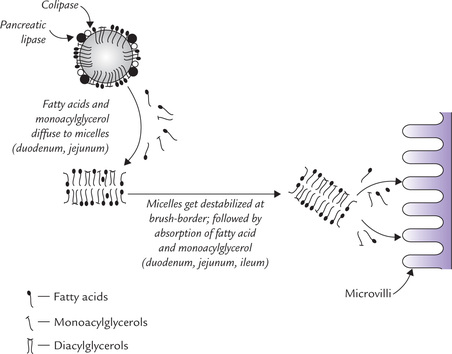
Fig. 26.2 Enzymatic hydrolysis of fat droplet, micelle formation and absorption of products of digestion in brush border.
As in case of other pancreatic enzymes, the neutral pH required for the optimum activity of lipase is attained by action of bicarbonate ions.
Hydrolysis of other dietary lipids, cholesterol esters and phospholipids also occurs in the duodenum as below:
Micelle Formation
The fatty acids and other simple molecules formed by enzymatic hydrolysis of the dietary lipids are aggregated with bile salts to form mixed micelles. In this type of micelle, aggregation of different types of molecules occurs. The polar heads of the bile salts from the hydrophilic exterior that faces the aqueous medium; non-polar tails of the bile salts form the hydrophobic core, in which fatty acids and monoacylglycerols are held. A single emulsion particle can from 106 micelles, each of about 20 nm diameter. Micelles can accommodate small quantities of cholesterol, carotene and other such non-polar substances as well.
Absorption
Absorption of the products of lipid digestion occurs by passive diffusion, mainly in the proximal jejunum. The micellar complex gets destabilized when it faces the relatively acidic pH prevailing at the brush border of enterocytes. Liberation of the monoacylglycerols, fatty acids, cholesterol and other non-polar substances, held in the hydrophobic interior, occurs as a result of the destabilization. This is followed by passive diffusion of these substances across the luminal cell membrane. Some fatty acids such as oleic acid and linoleic acid cross the luminal cell membrane by facilitated diffusion to enter the enterocyte.
Within the enterocyte, resynthesis of triacylglycerols and cholesterol esters occurs in endoplasmic reticulum. These lipids, together with small quantities of phospholipids and apolipoprotein B-48 are incorporated into chylomicrons (Chapter 12). The chylomicrons cross the cell membrane (serosal aspect) of enterocytes and pass into the lymphatic vessels. The triacylglycerols having short chain fatty acids directly enter the portal circulation. Some short chain fatty acids of chain length of 6–10 carbon atoms do not even need esterification in the cells and can directly enter the portal circulation.
Digestion and absorption of nucleic acids are discussed in Chapter 20.
II Organ Function Tests
A Gastric Function Tests
These tests evaluate acid secretion by the stomach. A sample of gastric juice is obtained by aspiration via a nasogastric tube. The parameters generally measured in the sample are titrable acidity and acid output. However, titrable acidity is affected spuriously by two factors, dilution and neutralization. The aspirated juice may be diluted by food, saliva, pancreatic secretion or bile. Further, these secretions may be alkaline in nature and hence tend to neutralize gastric acid.
However, in spite of these limitations, useful information has been obtained by these simple biochemical tests. Acid output may be measured under basal conditions, or after administration of some gastric acid-stimulating factor, as discussed below:
The sample of gastric juice is collected over a defined time period (usually one hour) by nasogastric aspiration. Following an overnight fast, the patient is directed to swallow a nasogastric tube, passed through one of his nostrils. The tube is directed radiologically and positioned with its tip in the gastric antrum. Samples of the gastric juice are collected by suction every 15 minutes, for a total period of one hour, i.e. basal secretion. Volume of the secretion collected in one hour is recorded and its acid content, i.e. basal acid output or BAO is determined by titration with alkali (Normal BAO: 0–17 mmol/h). The patient is kept fasting till end, which ensures that the gastric cells remain unstimulated. The acid output may also be measured following stimulation of the gastric secretion, i.e. stimulated secretion. The stimulus is provided by test meal or by exogenous agents such as histamine and pentagastrin. The test results are tabulated as below:

The physiological stimulation by test meal may lead to inaccurate results because the food components may dilute the gastric secretion and neutralize its acid content. Measurement of acid secretion following histamine secretion is, therefore, the more widely used test. Histamine interacts with specific receptors on gastric cells called H2 receptors, which in turn leads to stimulation of acid secretion. However, use of histamine suffers from a serious drawback. Histamine also interacts with another type of receptor called H1 receptor. The latter interaction elicits a widespread vasodilatation which evokes several undesirable side effects, including headache and flushing. Fortunately, prior administration of benadryl, an H1 blocker, prevents these effects without affecting the acid secretion.
Pentagastrin, a synthetic analogue of gastrin, is a highly potent and predictable stimulus for acid secretion. Subcutaneous administration of pentagastrin stimulates the parietal cells to secrete maximum acids, i.e. maximum acid output (MAO).
The aforementioned tests are principally used to investigate the conditions in which gastric acid secretion is either excessive or inadequate. Excessive gastric acid secretion (hyperchlorhydria) is an important factor in pathogenesis of duodenal ulcers. Decreased acid secretion (hypochlorhydria) is seen in conditions where gastric mucosa is damaged, such as chronic gastritis and gastric carcinoma. When the damage is so extensive that no acid output can be detected by the aforementioned tests the condition is called achlorhydria. This condition is most frequently seen in patients with atrophic gastritis, but also occurs in pernicious anaemia and gastric carcinoma.
Newer tests like endoscopy and contrast radiography make direct visualization of gastric mucosa possible, and therefore the mentioned tests (involving measurement of acid output) are becoming obsolete. However, they are still of much use in the following conditions:
1. Zollinger Ellison syndrome: The underlying defect in this condition is carcinoma of the gastrin-producing cells. Excess gastrin causes a persistent stimulation of the parietal cells, resulting in marked increase in the acid output even in the unstimulated state. Consequently, the ratio of the basal acid output and the maximum acid output rises up to 0.6% (Normal: BAO, 0–17 mmol/h; MAO, 4.7–58.4 mmol/h).
2. Pernicious anaemia: Extensive damage of the gastric mucosa occurs in this disorder, resulting in achlorhydria.
3. Following gastrectomy: Gastrectomy entails removal of a portion of the acid-secreting stomach wall. It is performed for treating acid hypersecretion.
Insulin secretion test is performed to check whether vagus nerve has been properly sectioned in the patients treated by vagotomy. Vagotomy is performed for treating gastric hypersecretion. The test is based on the principle that insulin-induced hypoglycaemia stimulates vagal center, which in turn serves as a potent stimulus for acid secretion. Therefore, in normal subjects, insulin injection is followed by increased acid secretion. However, following vagotomy, this is not expected, provided the vagus nerve has been properly sectioned.
In a typical test (Hollander test), 0.1–0.2 units/kg body weight of regular insulin is injected, which is followed by sampling of the gastric juice. The test, however, is not commonly used due to associated risk of hypoglycaemia.
Measurement of plasma gastrin concentration is now the first-line test for the investigation of atypical peptic ulceration such as duodenal ulcer resistant to medical treatment, recurrent ulcer after surgery, multiple duodenal ulcers and jejunal ulcers.
B Pancreatic Function Tests
Changes in both internal and external secretions of the pancreas may be observed in pancreatic diseases. As regards the internal secretions (insulin, glucagon, etc.) conditions in which the α- and the (β-cells are affected, have already been considered (Chapter 15). The tests described below are mostly concerned with exocrine secretions of the organ, i.e. sodium bicarbonate, and the enzymes, amylase, lipase, and the group of proteinases, still referred to as trypsin. The tests can be divided into two categories: direct tests and indirect tests.
Direct Tests
These involve quantitative estimation of the output of fluid, bicarbonate, and enzymes in the sample obtained by duodenal intubation, following pancreatic stimulation.
Collection of duodenal contents is always preceded by an overnight fast. On the morning of the test, the pancreatic secretions are stimulated by exogenous hormones or by test meal. This is followed by collection and analysis of the duodenal contents.
Secretin-cholecystokinin Test
The stimulus for the pancreatic secretion is provided by administration of hormones, secretin and cholecystokinin. During collection of the duodenal contents, special care is taken to prevent contamination by gastric secretion. A double lumen tube is used for this purpose, which consists of a longer and a shorter tube placed together. The length of longer tube is about 25 cm more than the shorter one. The tube is positioned, under radiological control, in such a way that the shorter tube ends at pyloric antrum and the longer one in the duodenum near the opening of the pancreatic duct. Continuous aspiration in both these tubes permits recovery of uncontaminated duodenal contents from the longer tube. The samples are collected in containers chilled in ice.
At the end of the collection period, the total volume of the aspirate is measured and the total bicarbonate output and the enzyme activities are determined. Different investigators favour different enzymes, but amylase and a proteolytic enzyme (trypsin or chymotrypsin) are probably best. The range for normal response is very wide; Table 26.3 gives typical lower limits of the normal response.
Table 26.3
| Test | Lower limit of normal response |
| Volume of aspirate | 150 mL |
| Peak bicarbonate concentration | 90 mmol/L |
| Peak tryptic activity | 30 lU/mL |
| Peak amylase activity | 270 lU/mL |
The sensitivity of this test for the diagnosis of exo-crine pancreatic-insufficiency is approximately 85% and the specificity, approximately 90%. The test has high diagnostic significance in a number of pancreatic disorders, discussed below.
Diagnostic significance
Following an episode of acute pancreatitis, the bicarbonate and the enzyme outputs are depressed, but return to normal as the pancreas recovers (Case 26.1). In chronic pancreatic insufficiency, values of the bicarbonate and enzyme outputs are persistently decreased, indicating extensive acinar damage. The fall in bicarbonate secretion is more marked in the early stages than the (reduction in) output of enzymes, which is observed late in the disease process. In fact, such a dissociation is highly suggestive of chronic pancreatitis. In carcinoma pancreas, particularly when the growth is located in the head region, secretion of enzymes tends to be affected to an extent greater than that of the bicarbonate ions. However, progression of any pancreatic disease eventually results in depression of both. Such changes may be observed in extra-pancreatic disorders, such as duodenal ulcer, gall bladder disease and haemochromatosis.
Other Direct Tests
Several simpler tests have been developed in which a physiological stimulation is employed for stimulating the exocrine pancreatic secretion. Most common is the Lundh test in which the stimulus is provided by a test meal containing corn oil, skimmed milk powder and dextrose. The samples are collected by a single lumen tube for a period of 2 hours and analyzed for tryptic activity. Reduced activity (positive test) is seen in about 90% of the cases of chronic pancreatitis but in only 79% of cases of pancreatic carcinoma. The test is most definite for tumours of head of the pancreas.
Indirect Tests
Determination of Enzymes in Serum and Urine
In pancreatic disorders involving exocrine cell damage, the pancreatic enzymes may be absorbed into the portal blood stream and be found in increased quantities in the peripheral blood (Case 26.1). Increased urinary excretion may also follow.
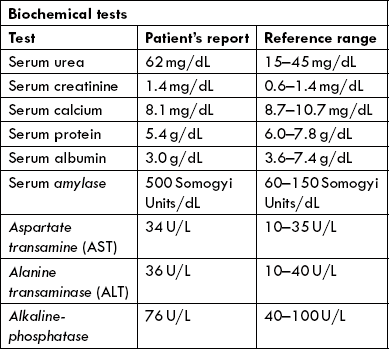
Serum amylase
It is a digestive enzyme secreted by pancreas and salivary glands. Most of the amylase present in blood circulation is of pancreatic origin, therefore a pancreatic pathology is commonest cause of increased serum amylase activity. In acute pancreatitis, greatest elevation of the enzyme activity is observed (often exceeds 1000 Somogyi units per 100 mL), whereas in chronic pancreatitis and carcinoma of the pancreas the serum enzymes are raised to a lower extent. Less significant increase occurs in acute parotitis, mumps or calculous disease of the salivary glands, and in some abdominal conditions such as perforated peptic ulcer, acute peritonitis and intestinal obstruction. Urinary amylase in most conditions is increased when the serum amylase is increased.
Serum lipase
It is an enzyme for lipid digestion secreted by exocrine pancreas. Its activity is likewise increased in acute pancreatitis.
Study of faeces
Since pancreatic enzymes are primarily involved in the digestive process, in severe pancreatic deficiency evidence of impaired digestion of foodstuff may be obtained from the stools. Analysis of fecal matter shows increased fat (steatorrhoea) and excess nitrogen. Quantitative estimation of the average daily excretion of fat and nitrogen is made when the patient is given a standard diet containing known amounts of fat and protein.
Determination of sweat chloride
This test is important in the diagnosis of cystic fibrosis (fibrocystic disease of the pancreas or mucoviscidosis). There may be an abnormal increase in the chloride content of sweat: a concentration above 60 mEq/L is diagnostic of this condition. The genes which undergo mutation in this autosomal recessive disease have been recently identified and sequenced (Case 7.1).
Tubeless Pancreatic Function Tests
The patient is administered certain labelled compound that may serve as substrate for a pancreatic enzyme. The digestion product of this compound is then measured, usually in urine sample. Decreased excretion indicates impaired digestion and hence pancreatic insufficiency. The following two tests are commonly employed.
Fluorescein dilaurate test
The substrate used in this test is fluorescein dilaurate. When administered orally, it is hydrolyzed by pancreatic esterase to release fluorescein. The latter is absorbed in the small intestine, conjugated in liver to form fluorescein glucuronide and excreted in urine. Since bile salts are also needed for the activity of esterase, the test effectively assesses combined pancreaticobiliary function.
False positive results are obtained in case of any defect in hepatic conjugation, intestinal absorption or renal excretion. To avoid any such error of interpretation, an equivalent amount of free fluorescein is given on the following day and urinary excretion of this compound is measured. The results are then compared.
14C PABA test
A synthetic peptide, BT-PABA is the labelled compound administered in this test. It is hydrolyzed to PABA by chymotrypsin and excreted in urine. The PABA excretion in the urine is measured and the results are expressed as ratio of the amount excreted to the dose administered. This test is also affected by the extra-pancreatic factors mentioned earlier, but the sensitivity and specificity are high (up to 90%).
Demonstration of an Associated Abnormality
Certain pancreatic disorders are associated with hyperlipidaemia or hypercalcaemia. Therefore, detection of these biochemical aberrations indirectly indicates a pancreatic disorder.
Some familial disorders involving pancreas are associated with aminoacidurias. Measurement of these parameters serves as a useful pointer towards the above stated pancreatic disorders.
C Intestinal Function Test
Test for Carbohydrate Digestion and Absorption
A number of tests are available to study disorders affecting the carbohydrate digestion and absorption. Some of the most commonly used tests are given here.
Tests of Disaccharidase Deficiency
In these tests, the blood glucose response is observed following oral administration of a disaccharide. The principle of these tests is that the administered disaccharide is hydrolyzed to the constituent monosaccharide units by the intestinal brush border disaccharidases; for example, lactose and maltose, the commonly used disaccharides, are hydrolyzed as below:
Glucose, produced by the action of disaccharidases, is then absorbed to cause elevation of blood glucose level. Thus, the blood glucose response serves as an indicator of the disaccharidase activity. In case of impairment of the activity of lactase (or maltase), the blood glucose elevation following administration of lactose (or maltose) is impaired.
The commonest of the disaccharidase deficiency disorders is lactose intolerance, in which lactase is deficient. In the affected children, the blood glucose response, following oral administration of lactose (50 g), is considerably depressed as compared to the normal subjects.
Tests for Monosaccharide Absorption
These tests involve oral administration of a monosaccharide followed by measurement of its plasma concentration and urinary excretion. In some disorders affecting intestinal absorption, the blood concentration as well as the urinary output of the ingested monosaccharide are depressed. The test results are, however, not affected by deficiency of the digestive enzymes.
A number of monosaccharides were initially tried, but xylose has been found most suitable for this test. It is a plant monosaccharide, that is rapidly absorbed (without undergoing any change in intestine) and excreted as such. Xylose is rapidly eliminated in urine, there being no renal threshold for it.
Different investigators have recommended different doses of xylose, although 5 g is the most commonly used dose. The compound is administered orally. Blood xylose concentration is determined in a sample drawn one hour after the administration. Urinary excretion of this compound is measured in samples collected at regular intervals, for a total period of 5 hours. In a normal subject, the blood xylose concentration reaches a maximum of 250 mg/dL, and the urinary excretion exceeds 4 g in the 5-hour period.
Apart from the rare congenital abnormalities of the glucose transport system, decreased absorption mostly occurs as a consequence of an impaired absorptive surface area. Abnormal results are always found in severe celiac disease and disorders of the proximal small intestine (Case 26.2). However, in milder diseases, the test results may not be affected. Therefore, the xylose excretion test cannot be used as a screening test for malabsorption, but may be useful in differential diagnosis of steatorhea as an alternative method.
Other monosaccharides (especially glucose) have been tried instead of xylose. But the tests involving glucose lack specificity because the results are affected in several extraintestinal disorders as well (for example, in diabetes).
Hydrogen Breath Test
This test provides convenient alternative for more cumbersome tests than just described. It is especially useful for the diagnosis of lactose intolerance. As mentioned earlier, in this disorder the lactose cannot be hydrolyzed and therefore, starts accumulating in the intestine. Bacterial fermentation of the accumulated lactose produces a number of by products including hydrogen, which is exhaled. Since hydrogen is not normally present in the expired air, its detection in breath serves as an indicator of the disaccharidase deficiency.
Others
Less common disaccharidase deficiencies include maltase deficiency and sucrase-isomaltase deficiency. The definitive tests for the specific diagnosis of these conditions are histopathological examinations of the biopsy sample. Measurement of the appropriate enzymes in biopsy sample also has high diagnostic value (Case 26.2).
III Liver Function Tests
A number of biochemical tests, referred to as liver function tests, are available to make a quantitative assessment of the hepatic cell activity. These tests involve measurement of certain parameters in blood, urine, and stool samples, and in some cases, in exhaled air. Since these parameters are directly related to function of the hepatocytes, alternation of their levels may reflect hepatic disorders. Thus, they serve as the first line investigations for the diagnosis of these disorders.
These tests are also useful for follow up studies in chronic hepatic disorders; periodic estimations of these parameters provide important information regarding response to therapy.
Liver function tests are based on various functions of this organ and can be accordingly divided into five categories:
A. Tests based on excretory function of liver.
B. Tests based on role of liver in intermediary metabolism.
C. Tests based on synthetic function of liver.
D. Tests based on detoxification function of liver.
E. Tests based on diagnostic enzymes.
A Tests Based on Excretory Function of Liver
Serum Bilirubin Estimation
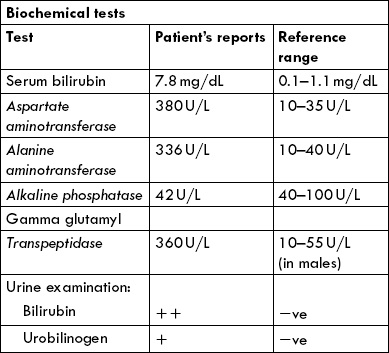
Bilirubin, a catabolic end product of haem, is generated in reticulo-endothelia system, metabolized in liver and excreted by biliary system (Chapter 16). The normal plasma concentration of bilirubin is less than 1.0 mg/dL; however, increased concentration (> 3.0 mg/dL) is readily recognized clinically because of the yellow colour that the bilirubin imparts to the skin (jaundice).
Estimation of serum bilirubin is based on its reaction with diazo reagent (van den Bergh reaction) to form purple coloured azobilirubin. Because of its solubility in the aqueous medium, the conjugated bilirubin (bilirubin diglucuronide) is capable of directly reacting with diazo reagent. It is, therefore, referred to as the direct bilirubin. The unconjugated bilirubin, on the other hand, being non-polar, reacts only after it has been solubilized by methanol. It is referred to as the indirect bilirubin.
Estimation of direct and indirect bilirubin is useful for delineating the predominant hepatic pathology. In obstructive pathology, predominant rise occurs in indirect bilirubin. On the other hand, in case of hepatocellular damage, rise in serum concentrations of both direct and indirect bilirubin occurs.
Further details of bilirubin metabolism and the biochemical tests based thereupon are discussed in Chapter 16.
BSP Elimination Test
BSP is a drug that is taken up from the blood circulation by the liver where it is metabolized. The metabolized drug is subsequently excreted through bile. When a measured dose of BSP is administered, rate of its disappearance from the blood circulation depends on the functioning liver cell mass. Normally, only about 5% of the administered dose remains in blood circulation 45 minutes after the administration. In cases of hepatic impairment, the ability of the damaged cells to take up the circulating drug falls. Consequently, a larger percentage of the administered drug is detectable in blood after 45 minutes.
B Tests Based on Role of Liver in Intermediary Metabolism
Liver is the major organ for metabolism of carbohydrates and proteins; in lipid metabolism its importance stands next only to adipose tissue. The under-mentioned tests are based on the role of liver in intermediary metabolism.
Galactose Tolerance Test
Liver is the major organ for metabolism of galactose. In case of hepatic impairment, metabolism of galactose proceeds at a rate much slower than normal. Half-life of the circulating galactose, which is 12 ± 2.6 minutes, increases in this instance.
The test is carried out by giving a measured dose of galactose intravenously and estimating its blood level at specific intervals. In case of hepatic impairment, the blood galactose levels remain elevated for a longer time and to a greater extent. Galactose tolerance test is one of the most sensitive of all liver function tests.
Amino Acid Profile
Serum amino acids are taken up from blood circulation by hepatocytes, where amino acid oxidases metabolize them. For example, aromatic amino acid oxidase brings about catabolism of the aromatic amino acids: tyrosine, tryptophan and phenylalanine. The enzyme activity is depressed in hepatic disorders, resulting in elevated blood level of these (aromatic) amino acids. Thus, circulating levels of the aromatic amino acids relate inversely with liver cell function.
Conversely, levels of the branched chain amino acids, namely leucine, isoleucine and valine fall in hepatic disorders. Therefore, amino acid profile is a useful indictor for the diagnosis of hepatic disorders.
C Tests Based on Synthetic Functions of Liver
Serum Protein Estimation
Liver is an important site for the synthesis of a variety of biomolecules, including plasma proteins. In fact, all subfractions of the plasma proteins, with sole exception of γ-globulins, are synthesized in liver. In most hepatic disorders, impaired synthesis of these molecules occurs, resulting in hypo-proteinaemia and hypoalbuminaemia. Albumin: globulin (A:G) ratio is a more reliable indicator of hepatic dysfunction than total protein concentration.
Prothrombin Time
Hepatocytes synthesize most coagulation factors, including factor II (prothrombin). Prothrombin levels (assessed by measuring prothrombin time) may be reduced in hepatocellular damage because of impaired synthesis. Vitamin K is necessary for the synthesis of prothrombin, and therefore, deficiency of vitamin K is accompanied by a bleeding tendency and a prolonged prothrombin time. This can be corrected by parenteral administration of vitamin K (provided hepatocellular function is normal).
D Tests Based on Detoxification Function of Liver
Several endogenously produced metabolites and exogenously administered chemicals (xenobiotics) that are potentially hazardous, are converted to relatively harmless substances in hepatocytes (Chapter 15). This process, known as detoxification, involves oxidation and attachment of polar groups, which render these substances hydrophilic so that they can be excreted through the renal or the hepatobiliary routes.
Arterial Ammonia Levels
Ammonia is a highly toxic substance that is produced in the peripheral tissues from amino-group catabolism. It is also produced in colon by bacterial fermentation, and in muscles by adenine breakdown. It is carried to liver (as amino-group of glutamine) where it is channeled into urea cycle. Since urea cycle operates only in liver, in hepatic impairment, decreased ammonia detoxification occurs, which leads to elevated blood ammonia levels.
The arterial ammonia level serves as a more reliable indicator of hepatic impairment than the venous-blood ammonia level. This is because the latter keeps fluctuating due to continuous addition of ammonia in veins from various sources. These levels are further increased due to formation of venous shunts that bypass the liver.
14C Amino-Antipyrine Excretion Test
The labelled 14C amino-antipyrine is catabolized in liver; the end product is labelled (14C) carbon dioxide, which is subsequently exhaled. In hepatic disorders, decreased catabolism of this compound occurs, resulting in decreased 14C carbon dioxide in breath.
Thus, measurement of the labelled 14CO2 in the exhaled air is a useful indicator of hepatocellular damage.
E Tests Based on Diagnostic Enzymes
Diagnostic utility of enzymes like transaminases, alkaline phosphatase, γ-glutamyl transpeptidase and 5’-nucleotidase in hepatic disorders was described earlier in Chapter 5. Some of these enzymes are finding increasing use in the diagnosis, being early indicators and sensitive markers of the hepatic disorders. Estimation of some enzymes which were earlier used as research procedures have been successfully introduced in clinical laboratories as routine tests; for example, serum arginase activity is a highly specific and sensitive indicator of hepatocellular damage.
The battery of tests described finds use not only for diagnosis of liver disorders, but also for their treatment and follow up (Case 26.3). These tests reflect a pathological process and not any disease specifically. Nowadays, highly sophisticated diagnostic tools based on imaging techniques are available. But their routine use is not possible due to the high costs involved. Therefore, the biochemical tests retain their utility and still remain the first-line investigations. A decision to go for the imaging techniques is usually taken based on the reports of these tests.
 Along the length of the gut, various fluids, electrolytes, acids or bases, and enzymes are added to aid in mixing, hydration and digestion of food.
Along the length of the gut, various fluids, electrolytes, acids or bases, and enzymes are added to aid in mixing, hydration and digestion of food.