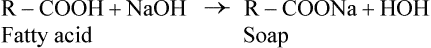Chemistry of Lipids
Lipids, carbohydrates and proteins form bulk of the organic matter in living systems. However, unlike the other two, lipids are not well defined chemically. In general, lipids are a heterogeneous group of water-insoluble, oily or greasy substances that can be extracted with non-polar solvents (e.g. benzene or ether), but not with the polar solvents. Because of their insolubility in aqueous solutions, body lipids are mostly found in isolated compartments. For example, droplets of triacylglycerols are present in adipocytes and some lipids are membrane bound. When present in blood, they form complexes with proteins so that they can be transported in the aqueous plasma. In contrast to other major biomolecules, lipids do not form polymers.
In this chapter, various types of lipids have been described, with a special emphasis on structure-function relationships. After going through this chapter, the student should be able to understand:
Lipids serve as fuel molecules, highly concentrated energy stores, signal molecules, components of cell membranes, and carriers of fat soluble vitamins, e.g. A, D, E and K. A layer of subcutaneous fat acts as thermal insulator and acts as a cushion, which protects several delicate organs by absorbing mechanical shocks. Both lipids and lipid derivatives serve as vitamins and hormones (Table 3.1 ).
Table 3.1
Lipids and their major biological functions in the human body
| Lipid | Biological function |
| Triacylglycerols | Energy storage, thermal insulation |
| Waxes | Keeps skin lubricated and waterproof |
| Phospholipids | Membrane components, detergents, surfactant, second messengers |
| Sphingolipids | Components of membranes, especially of nervous tissue and the myelin sheath |
| Sterols | Precursors of biologically useful compounds, such as bile acids, steroids, sex hormones, vitamin D, component of membranes |
The building blocks of most lipids are fatty acids. Some lipids such as cholesterol and terpenes lack fatty acids. However, these lipids are potentially related to fatty acids because they are synthesized from the cata-bolic end product of fatty acid degradation (i.e. acetyl CoA).
I Fatty Acids
A General Characteristics
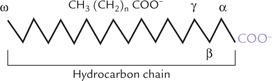
The fatty acid molecule consists of a long hydrocarbon chain with a polar carboxyl group at its end (Fig. 3.1). Since the pK value of the carboxylate group is around 4.85 (between 4.7 and 5.0), it rapidly ionizes at the physiological pH to form the carboxylate ion (COO–). The carboxylate ion has polar characteristics, with high affinity for water (hydrophilic). However, the predominant portion of the fatty acid molecule is the long hydrocarbon chain in which carbon atoms are in their lowest oxidation state. Being non-polar in nature, hydrocarbon chain accounts for predominantly non-polar character of the fatty acid molecule. This in turn accounts for the oily or greasy nature of lipids.
Thus, a fatty acid molecule contains both polar (hydro-philic) and non-polar (hydrophobic) regions; such molecules are called amphipathic molecules. Fatty acids are simplest of all amphipathic substances in the body.
Naturally occurring fatty acids contain even number of carbon atoms (most contain 14 to 24). Of these, most abundant are the fatty acids containing 16 or 18 carbon atoms. The fatty acids with hydrocarbon chains containing one or more double bonds are called unsaturated fatty acids, whereas those lacking any double bonds are referred to as saturated fatty acids.
Functions
Fatty acids perform following functions in the body:
1. Membrane lipids: They are the components of the more complex membrane lipids.
2. Fuel molecules: They are components of stored fat in the form of triacylglycerols.
3. Hormones: Derivatives of fatty acids serve as hormones (such as prostaglandins) and intracellular second messengers (such as IP3 and DAG).
4. Numerous proteins are covalently modified by fatty acids. Palmitic acid and myristic acid, for example, are directly attached to some proteins.
B Nomenclature
The nomenclature of fatty acids is based on the following characteristics of the hydrocarbon chain:
As shown in Table 3.2, the abbreviation 18;1 indicates an 18-carbon fatty acid having a single double bond. Likewise, 18;2 indicates an 18-C fatty acid with two double bonds. Δn indicates position of these bonds; for example, Δ9,12 indicates double bonds at C-9 and C-12, starting from the carboxyl end.
Table 3.2
Some naturally occurring fatty acids
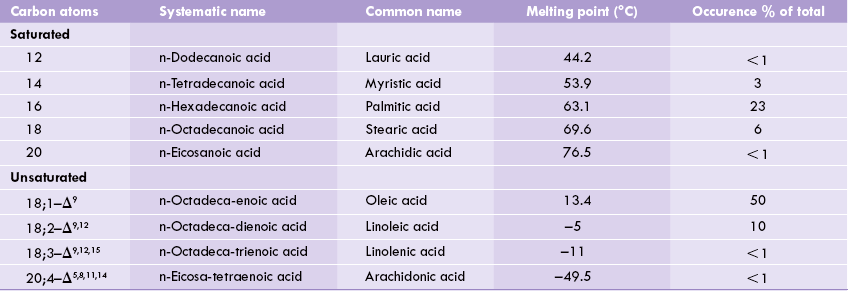
1 8;2—Δ9,12 implies 18-C fatty acid with 2 double bonds at C-9 and C-12, 20;4-Δ5,8,11,14 implies 20-C fatty acid with 4 double bonds at C-5, C-8, C-l 1, and C-14.
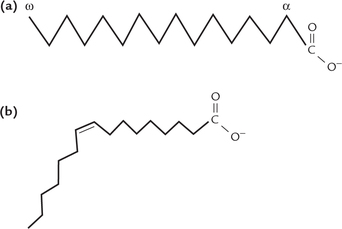
Thus, the 18-C saturated fatty acid (Fig. 3.2a ) is called octadecanoic acid: octa (8) and deca (ten) imply chain length (18 carbons). Its common name is stearic acid and it is the most abundant fatty acid in nature. Introduction of a double bond at the C-9 position results in the formation of octadeca (mono) enoic acid (common name, oleic acid). Similarly, the 18-C fatty acid having two double bonds, at C-9, and C-12, is called octadeca-dienoic acid (common name linoleic acid), and that having three double bonds, at C-9, C-12 and C-15 positions, is called octadeca-trienoic acid (common name linolenic acid). The latter two, and all others that contain more than one double bond, are known as polyun-saturated fatty acids (PUFA).
The double bonds are in cis geometrical configuration (Fig. 3.2b); this configuration produces a rigid bend in the aliphatic chain. They are non-conjugated, i.e. they are spaced at three-carbon intervals.
The carbon atom at the non-polar end of the aliphatic chain is called the omega (to) carbon. Fatty acids can also be named by counting the carbon atoms from the ω carbon. Thus, the octadecatrienoic acid (linolenic acid) having double bonds at C-9, C-12 and C-15, can also be designated as a fatty acid of ω-3 series. This is because it contains a double bond attached to C-3 when counted from the non-polar (ω) end. Similarly octadeca-dienoic acid belongs to the ω-6 series.
C Properties
Solubility
Fatty acids are predominantly non-polar in nature because of the long hydrocarbon chain. This accounts for the insolubility of lipids in water and other polar solvents, and solubility in the non-polar solvents.
Melting Point
Melting point of fatty acids is dependent on two factors:
1. Level of unsaturation of hydrocarbon chain: The melting point falls as the number of double bonds increases.
2. Chain length of fatty acid: Increase in chain length results in elevating the melting point. Saturated fatty acids of less than eight carbon atoms are liquid at physiological temperature, whereas those containing more than 10 are solids at this temperature.
In saturated fatty acids, hydrocarbons chains are in an extended conformation (Fig. 3.2a) and so can be packed together into a compact structure.
In unsaturated fatty acids, compact packing is prevented due to presence of rigid bends in their hydrocarbon chains; these bends are produced by the double bonds. Consequently, the unsaturated chains are loosely packed and are, therefore, more easily disturbed by thermal energy. This accounts for the lower melting point of the latter. For example, melting point of stearic acid (69.6 °C) is about fivefolds higher than that of oleic acid (13.4 °C).
Soap Formation
Fatty acids react with alkalies, such as NaOH or KOH, to produce the corresponding salts, called soaps.
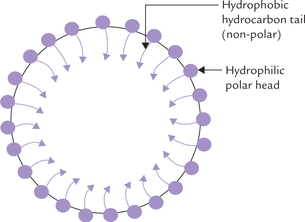
Soaps are amphipathic molecules where the ionized carboxyl group constitutes the polar head, and the hydrocarbon tail forms the non-polar portion. Because of their amphipathic nature, soaps can disperse oily or greasy substances into finer droplets; the process is called emulsification. The hydrophilic polar portion of the soap molecule remains at the surface, forming a stable cover, whereas the hydrocarbon tail extends into the interior to cause the dispersion (Fig. 3.3 ).
Calcium or magnesium soaps of fatty acids are highly insoluble, and hence do not emulsify oily substances. They precipitate as white, insoluble curds.
Hydrogenation
The double bonds of unsaturated fatty acids can be hydrogenated in the presence of catalysts, e.g. nickel to yield the corresponding saturated fatty acids.
Halogenation
Double bonds of unsaturated fatty acids are capable of adding halogens, the result of which are dihalogen fatty acids. This property is often used to assess the extent of unsaturation in fats and oils via reaction with iodine to obtain the so-called iodine number (number of gm of iodine required to saturate 100 gm of the lipid). The higher the iodine number, the more unsaturated the oil.
Oxidation
Unsaturated fatty acids can spontaneously react with atmospheric oxygen to form fatty acid peroxides, fatty acids epoxides and fatty acid aldehydes. Fat which undergoes this type of oxidation becomes rancid. Rancid fat is unpleasant in taste and smell and can be toxic. Rancidity is prevented by anti-oxidants, e.g. vitamin E. Degree of rancidity is measured by acid number, defined as the number of milligrams of KOH required to neutralize fatty acids in a gram of fat.
D Essential Fatty Acids
The lipid biosynthetic capacity of the body can generate various fatty acids needed by the body. Key exceptions to these are the highly unsaturated fatty acids, e.g. linoleic acid and linolenic acid, containing unsaturated sites beyond carbon 9 (Table 3.2). Lack of endogenous synthesis makes their consumption in diet-essential, and hence they are called essential fatty acids.
It is important to note that arachidonic acid, a 20-C unsaturated fatty acid (Table 3.2) can be synthesized only from linoleic acid (Chapter 11). Therefore, in deficiency of linoleic acid, arachidonic acid also becomes an essential fatty acid.
Biomedical Importance
Essential fatty acids (PUFAs) are important for following reasons:
1. Membrane components: They are components of phospholipids and form biomembranes. Because the double bonds are in cis configuration, the PUFA molecules are sickle-shaped and so they increase fluidity of biomembranes. They are essential components of mito-chondrial membranes, and therefore, their deficiency decreases efficiency of biological oxidation.
2. Synthesis of eicosanoids: The eicosanoids are biologically active lipids that are derived from polyunsatu-rated 20-carbon fatty acids, mainly arachidonic acid. They are short lived (half-life < 5 minutes), and act as paracrine or autocrine messengers within tissue of origin. Arachidonic acid can be processed to eicosanoids by two alternative pathways: the cyclooxygenase pathway which produces the prostaglandins, prostacyclin and thromboxane; and the lipoxygenase pathway, which produces, among other products, the leukotrienes (see Chapter 12 for further details).
3. Effect on serum cholesterol: Ingestion of PUFAs increases esterification and excretion of cholesterol, thereby lowering serum cholesterol level. Hence, EFAs have anti-atherogenic effect, which is augmented by their fibrinolytic activity (Chapter 12).
4. Role of vision: Dietary linolenic acid produces docosa-hexanoic acid in retinal photoreceptor membranes. It enhances the electrical response of the photorecep-tors to illumination.
5. Structural elements: PUFAs are present in high concentrations in the lipids associated with structural elements of tissues.
Deficiency of EFAs
Approximately 2–3% of daily calorie intake should be accounted by EFAs. Dietary deficiency is characterized by scaly dermatitis, poor wound healing and hair loss. However, the deficiency symptoms are rare due to wide distribution of EFAs. They are most commonly seen in patients suffering from severe fat malabsorption, in patients kept on parenteral nutrition for long, and in infants fed on low-fat milk formulas. The symptoms are readily cured by dietary linoleic acids.
A diet that supplies as little as 1% of the calories in from of EFAs is considered adequate. Interestingly, the attempts to induce a deficiency syndrome by selective omission of linolenic acid from diet have been unsuccessful, not only in humans but also in animals. Details regarding metabolism of EFAs, are given in Chapter 11.
E Branched Chain Fatty Acids
Most naturally occurring fatty acids are of the straight chain variety, though lesser amounts of branched chain fatty acids (for example, phytanic acid) also occur in nature.
Significant amount of this fatty acid is present in dairy products. Defective catabolism of phytanic acid causes Refsum’s disease Refsum’s disease, which is characterized by accumulation of phytanic acid in plasma and tissues (Chapter 11).
II Classification of Lipids
Lipids are classified into three groups—simple lipids, compound lipids and derived lipids (Table 3.3 ). Simple lipids are esters of fatty acids with alcohol. Compound lipids contain, besides fatty acids and alcohols, non-lipid polar groups like phosphate, carbohydrates, etc. Substances derived from the above groups of lipids by hydrolysis are termed derived lipids. These include fatty acids, mono-glycerides, diacylglycerides, quinones and steroids.
Table 3.3
Classification of lipids according to their chemical structure

1. Sphingomyelins and glycolipids may be grouped together as sphingolipids.
2. Cholesterol, cholesteryl esters and triacylglycerols are termed neutral lipids because they are uncharged.
A Simple Lipids
The major simple lipids are the triacylglycerols and the waxes. They are neutral molecules, devoid of an electric charge. Moreover, they lack any polar group. This accounts for their hydrophobicity and water repellent properties. They serve as major fuel reserves in the animal and the plant cells.
Triacylglycerols
Triacylglycerols are the simplest and the most abundant lipids, also referred to as fats, neutral fats or triglycerides. They are fatty acid esters of glycerol and are the major storage fats in animal cells. Triacylglycerols stored in the adipose tissue in humans can meet the energy requirements of body for several weeks.
Structure
A triacylglycerol molecule is an ester of three fatty acids and a trihydroxy-alcohol, called glycerol (Fig. 3.4 ). The three carbon atoms of glycerol are designated as α, β and α’, or more commonly as 1, 2, 3; the β carbon is chiral. Because about two-third of all fatty acids in the human organism are unsaturated, triacylglycerols reflect this usually by containing two molecules of unsaturated and one molecule of a saturated fatty acid. Such triacylg-lycerols are called mixed triacylglycerols. By contrast, the simple triacylglycerol have all three hydroxyl groups of the glycerol esterified to the same fatty acid. For example, three stearic acids bound to glycerol makes a simple tri-acylglycerol, called tristearin.
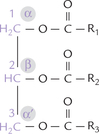
Fig. 3.4 Basic composition of a triacylglycerol. The glycerol backbone is coloured. The fatty acids esterified with the second carbon (R2-COOH) and the third carbon (R3-COOH) are usually unsaturated fatty acids, whereas (R1-COOH) is a saturated fatty acid.
Oils, butter, and other food fats are complex mixtures of simple and mixed triacylglycerols containing fatty acids of varying chain lengths and unsaturation.
A triacylglycerol molecule lacks any free polar group because the polar hydroxyl groups of glycerol and the carboxyl groups of fatty acids are involved in ester linkage. This imparts the triacylglycerols a complete non-polar character.
The monoacylglycerols (monoglycerides) and diacyl-glycerols (diglycerides) have only one and two fatty acids, respectively, bound to glycerol.
Properties
Properties of a triacylglycerol depend on the nature of the constituent fatty acids.
1. Melting point: Triacylglycerols containing only saturated fatty acids, such as tristearin and tripalmitin, are solids at physiologic temperature (37°C) since melting point of the saturated fatty acids is relatively higher (Table 3.2). Conversely, the triacylglycerol that contain only unsaturated fatty acids, such as tri-olein, are liquids at room temperature. The latter can be solidified chemically by partial hydrogenation; the process causes conversion of the unsaturated fatty acids to the saturated ones. Since shorter chain fatty acids also have lower melting point, butter, which is rich in such fatty acids, has a soft consistency.
2. Solubility: Lack of any polar group makes the triacylg-lycerols insoluble in water. However, they are soluble in the non-polar solvents, such as ether, benzene and chloroform.
3. Specific gravity: Triacylglycerols have a lower specific gravity than water. Therefore, when mixed with water, they float above to form the upper layer.
4. Hydrolysis: Triacylglycerols undergo hydrolysis when boiled with strong acids. Lipases, the gastrointestinal esterases, catalyze the hydrolysis at milder physiological conditions prevailing in the human organism. These enzymes are quite specific, and they do not necessarily remove all three fatty acid molecules from a triglyceride molecule. Thus, pancreatic lipase, the main lipid digestive enzyme, catalyzes the removal of fatty acids from positions 1 and 3 only.
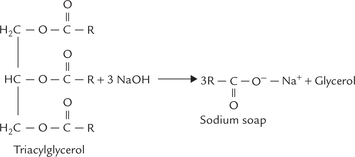
Hydrolysis of triacylglycerols by alkalis, such as NaOH and KOH, is called saponification (i.e. “soap formation”). It yields a mixture of sodium or potassium salts of the fatty acids (called soaps) and glycerol (Fig. 3.5 ).
Saponification number: The number of milligrams of KOH required to saponify the free and combined fatty acids in one gram of a given fat is called saponification number. Fats having short chains with more carboxyl groups will have higher saponification number when compared to those having long chain fatty acids.
5. Autoxidation: When exposed to air, the triacylglycerols containing polyunsaturated fatty acids, are attacked by molecular oxygen at the double bonds of the hydrocarbon chains to yield complex products. This process is responsible for the spoiled taste of the rancid fats.
Functions
Triacylglycerols store chemical energy, and act as thermal insulators and mechanical shock absorbers.
1. Storage of chemical energy: Large triacylglycerol stores are present in the fat cells (adipocytes) of the adipose tissue. Subcutaneous tissues, viscera, and the abdominal cavity contain adipose tissue. Within cytoplasm of these cells, droplets of triacylglycerols coalesce to form a large globule which may fill most of the cell volume. The cells not only store triacylglycerols, but also mobilize them as fuel molecules that are transported to other tissues by blood.
Triacylglycerols store huge amounts of energy: Body can store unlimited amounts of triacylglycerols, which can sustain biological functions for several weeks. In a typical 70 kg man, the triacylglycerols constitute about 11 kg of his total body weight, which may provide about 100,000 kcal. If this amount of energy were stored in glycogen, at least 66 kg glycogen would have been required, and his total body weight would have been at least 55 kg greater. Evidently, triacylglycerols are more concentrated source of energy. This is accounted by their highly reduced and anhydrous nature.
• The reduced nature of the hydrocarbon chains means that carbons are present in lowest oxidation state. This accounts for a higher energy yield (9 kcal/g) upon complete oxidation of fatty acids, in contrast with about 4 kcal/g for carbohydrates and proteins.
• The anhydrous nature means that water molecules do not associate with triacylglycerols because of their hydrophobic character, and so space requirement is minimal. In contrast, the polar carbohydrates and proteins are highly hydrated: 1 g of dry glycogen binds about 2 g of water. Consequently, 1 g of nearly anhydrous fat stores more than six times as much energy as 1 g of hydrated glycogen.
These facts clearly explain why 11 kg of stored anhydrous triacylglycerols are equivalent to about 66 kg hydrated glycogen.
Waxes
Waxes are esters of long chain unsaturated fatty acids, having 14–36 carbon atoms, with long chain alcohols (consisting of 16–22 carbons). In vertebrates, the waxes are secreted by skin glands. They keep the skin lubricated, water proof and pliable. Birds secrete large amount of waxes, which prevents any unwanted accumulation of water on their feathers because of the water repellent property of the waxes. This is especially important in flying birds whose body weight has to be kept light. Leaves of many plants also have a protective cover of waxes.
B Compound Lipids
Compound lipids differ from simple lipids in having an additional non-lipid portion. Nearly 60% of lipids in the brain and most of the membrane lipids belong to this class. In contrast to the non-polar nature of the simple lipids, the compound lipids are amphipathic molecules. They can be subclassified into the following categories:
• Phospholipids which contain an additional phosphate group.
• Glycolipids which contain carbohydrate groups (Table 3.2).
Phospholipids
These are amphipathic molecules with non-polar aliphatic (hydrocarbon) "tails" and polar (phosphoryl-X) "heads". They are major components of the biological membranes, accounting for more than half of the total lipids in most membranes. The polar heads of the phospholipid tend to extend to exterior, whereas the non-polar tails move towards the interior, where they associate with other non-polar constituents of the membrane including glycolipids, cholesterol and some proteins.
Phospholipids can be further subdivided into two groups: phosphoglycerides and sphingomyelins. Glycerol phosphate forms the backbone structure of the phos-phoglycerides while an amino alcohol, sphingosine is present in the sphingomyelins.
Phosphoglycerides
Their basic structure is very similar to that of triacylgly-cerol (one fatty acid each is esterified to two of the carbon atoms of glycerol) except the third carbon that is esterified to phosphoric acid. The structure so formed is phosphatidic acid (diacylglycerol 3-phosphate) or phos-phatidate molecule, as shown in Figure 3.6 . Phosphatidate occurs only sparingly in the free form, and is not a major membrane lipid itself. However, it forms the baseline structure from which various phosphoglycerides or glyc-erophospholipids are derived.
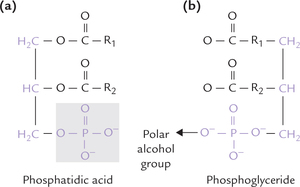
Fig. 3.6 (a) The structure of phosphatidic acid, the parent compound and biosynthetic precursor of phosphoglycerides, (b) A polar alcohol group is attached to the phosphate group to form a phosphoglyceride.
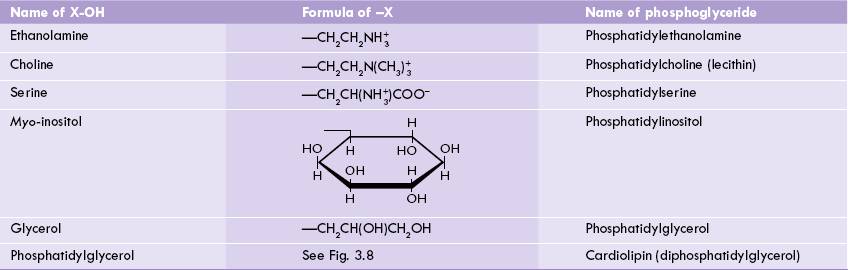
The phosphoglycerides are derived from phosphatidic acid by attachment of a polar alcohol group to the phosphate group (of phosphatidic acid) by a phosphodiester bond. The common alcohol groups of phosphoglycerides are the ethanolamine, choline, the amino acid serine, myoinositol and glycerol (Table 3.4 ).
Plasmalogens and cardiolipins are less common types of phosphoglycerides. The phosphate group in these phosphoglycerides is negatively charged at physiologic pH values, and also, the polar alcohol (X-OH) bound to the phosphate is either charged or has a high hydrogen-bonding potential. Together with phosphate, it forms the hydrophilic head group of the molecule, and the two fatty acids form two hydrophobic tails.
Thus, phosphoglycerides are amphipathic, the hydro-philic and hydrophobic parts being combined in the same molecule. In common phosphoglycerides, the fatty acid in position 1 is usually saturated (either palmitic or stearic acid), whereas that in position 2 is unsaturated.
Two less common phosphoglycerides are plasmalo-gens (mainly in myocardium) and cardiolipins.
Plasmalogens
The widespread plasmalogens are distinguished from phosphoglycerides by the presence of an α-β-unsaturated fatty alcohol, rather than a fatty acid residue, in position 1. It is linked with carbon 1 of glycerol by an ether bond (Fig. 3.7 ).
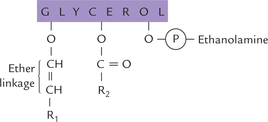
Fig. 3.7 Structure of plasmalogen. R1 is hydrocarbon chains of fatty alcohol esterified to C-1 of glycerol, and R2 is hydrocarbon chain of fatty acid esterified to C-2 of glycerol.
Usually ethanolamine is present in the hydrophilic head groups of plasmalogens (ethanolamine plasmalogens).
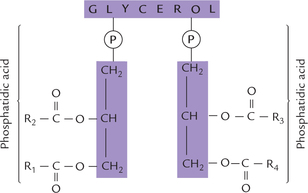
Cardiolipins
Two molecules of phosphatidic acid esterified through their phosphate groups to a glycerol molecule yield a cardiolipin molecule (Fig. 3.8 ). It is a complex molecule of fatty acids, three glycerol molecules and two phosphate ions. Cardiolipin is an important component of the inner mitochondrial membrane and some bacterial membranes. Cardiolipin is the only glycerophospholipid that is antigenic.
Functions of phosphoglycerides
1. Membrane lipids: Phosphoglycerides are easily the most abundant membrane lipids, accounting for more than half of the total lipids (55–70%) in most membranes. They form the well known lipid bilayer structures because of their amphipathic nature. The bilayer structure forms a permeability barrier in which their hydrophilic head groups try to surround themselves with water, whereas hydrophobic tails avoid water, and so move towards the interior (Chapter 7). Cardiolipins and plasmalo-gens are relatively less common; the former is present in abundance only in the inner mitochondrial membrane, whereas plasmalogens are present in most tissues, especially in muscle and nervous tissue, where they account for up to 10% of the total phospholipids.
2. Surfactants: Most phosphoglycerides function only as structural components of biological membranes, but one phosphoglyceride serves a special function as the major constituent of lung surfactant. It is dipalmitoyl phosphatidylcholine (dipalmitoyl lecithin), secreted by cells in the alveolar wall. It reduces surface tension by forming a thin bilayer film that lines the alveolar wall.
Thus, dipalmitoyl-phosphatidylcholine helps to maintain shape of the alveoli and to prevent their collapse due to the high surface tension of the surrounding aqueous medium. Within the uterus, fetus is bathed in amniotic fluid, therefore, surfactant is not required. However, after the birth, surfactants are needed to prevent lung collapse. Normally, the fetus starts producing surfactants after the 30th week of gestation, and adequate levels are reached at term. However, in premature infants, the adequate levels are not reached at the time of birth, which results in respiratory distress (Case 3.1). This condition accounts for 15–20% of neonatal deaths in western countries.
3. Generation of second messengers: Two intracellular signals (or second messengers), inositol triphosphate and diacylglycerol are generated from a membrane phospholipid, phosphatidyl inositol bisphosphate (Chapter 29).
4. Anchoring certain proteins to cell membrane: The phos-phoglycerides, being amphipathic, can interact with non-polar as well as polar substances. Thus, they readily interact with the polar group of proteins as also with the cell membranes (which are predominantly non-polar). This makes possible anchoring of the proteins to biological membranes.
5. Biologic detergents: Phosphoglycerides are excellent biologic detergents; this action is especially important in the small intestine, where lipids must be emulsified for digestion purposes. The detergent property is also accounted by amphipathic nature of phosphoglycerides.
6. Lipoprotein structure: Lipids are transported as lipo-proteins and phosphoglycerides are essential structural components of lipoproteins (Chapter 12).
Lysophospholipids: These are the compounds formed from phosphoglycerides by the removal of one of their fatty acid moiety. They are produced by action of the enzymes, phospholipases. They account for 1-2% of total phospholipids in the living system. Some lysophospho-lipids possess potent haemolytic action. The haemolytic action of snake venom is because of its ability to generate lysophospholipids (since it is rich in phospholipases). However, toxic action of venoms cannot be solely attributed to this mechanism.
Sphingomyelins
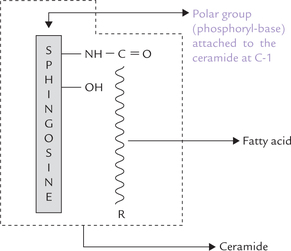
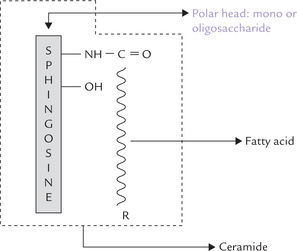
Nervous tissue, especially myelins of nerves, and the erythrocyte membranes are rich in sphingomyelins, which are characterized by having an 18-C amino-alcohol, known as sphingosine (Greek word, "sphingein" means to bind tightly). The hydroxyl groups in sphingosine are present at carbons 1 and 3, and amino group is present at carbon 2 (Fig. 3.9 ). A fatty acid gets attached at the amino group at C-2 to form a sphingosine derivative called ceramide (Fig. 3.10 ). Free forms of sphingosine and ceramide occur in nature in negligible amounts.
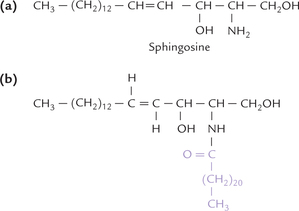
Fig. 3.9 Structure of sphingosinea. (a) Sphingosine, an 18-carbon amino alcohol, with an amino group at C-2, and hydroxyl groups at C-1 and C-3, (b) Ceramide, formed by covalent attachment of fatty acid to the amino group at C-2.
Of the two hydroxyl groups in ceramide, the one at C-S remains unsubstituted, and the one at C-1 carries a variable substituent (a phosphorylbase, e.g. phosphocho-line, phosphoethanolamine). The phosphorylbase forms the polar head group of the sphingomyelin.
Sphingosine + Fatty acid = Ceramide
Ceramide + Phosphoryl base = Sphingomyelin
Like phosphoglycerides (and other sphingolipids, discussed below) the sphingomyelin molecule has two hydro-phobic tails. Only one of these comes from a fatty acid; the other one is the hydrocarbon tail of sphingosine itself.
Recall that sphingomyelins can be grouped together with glycolipids as sphingolipids (both contain sphin-gosine; Table 3.3). They serve as essential membrane components, as discussed in the next section.
Glycolipids
The glycolipids are formed by attachment of a carbohydrate component (mono- or oligosaccharide) to ceramide (Fig. 3.11 ). They are commonly referred to as glyco-sphingolipids (Greek word, glykos means “sweet”) because of presence of sphingosine backbone. Depending on the nature of the carbohydrate component attached, four types of glycolipids are recognized: cerebrosides, sul-phatides, globosides and gangliosides (Table 3.3).
Cerebrosides
These are ceramide monosaccharides: a monosaccharide is attached to ceramide. The monosac-charide is commonly either galactose (galactocerebro-side) or glucose (glucocerebroside). Galactocerebroside is the most common cerebroside found in membranes, whereas glucocerebroside serves primarily as an intermediate in the synthesis and degradation of the more complex glycosphingolipids.
Members of a group of glucocerebrosides (or galacto-cerebrosides) may differ from each other in the type of fatty acid attached to the sphingosine. The fatty acids commonly present are the C-24 fatty acids, such as ligno-ceric acid, nervonic acid, cerebronic acid and oxyner-vonic acids. The cerebrosides formed by these four fatty acids are kerasin, nervon, cerebron and oxynervon respectively.
Sulphatides
These are sulphuric acid esters of galactocer-ebroside, found predominantly in nervous tissue.
Gangliosides
They are similar to globosides except that they also contain sialic acid. They are found primarily in the ganglion cells of the central nervous system, particularly at the nerve endings. The oligosaccharide chains of gangliosides contain hexoses, hexosamines, and characteristically one or more molecules of a sialic acid, a C-9 sugar, also called N-acetylneuraminic acid (NANA). It is an acid sugar derivative that characteristically occupies terminal positions in the oligosaccharide chain.
Gangliosides can be classified on the basis of the number of component NANA molecules and by the length (and sequence) of their oligosaccharide units. GM means a ganglioside with a single (mono) NANA residue, whereas GD, GT and GQ would indicate two, three and four NANA residues in the molecule, respectively. The number after GM—for example, GM1, GM2 or GM3—indicates the sequence of sugars:
1. represents the sequence Gal-GalNAc-Gal-Glc-ceramide,
2. means GalNAc-Gal-Glc-ceramide, and
3. is Gal-Glc-ceramide. The structures of GM3 and GM2 ganglioside, for example, are shown below:
The numbers (GM1, GM2, GM3) are derived from the relative mobility of the glycolipids on thin layer chroma-tography—GM1 are the largest and therefore, migrate most slowly.
The complex structure of gangliosides is built up step-wise, one sugar residue at a time, in the Golgi apparatus. These complex sugars are degraded by a series of exo-glycosidases in lysosomes. They are of medical interest because several lipid storage disorders involve the accumulation of NANA-containing glycosphingolipids in cells. These disorders lead to serious impairment of development of nervous system (Chapter 11).
Functions of Glycolipids
1. The glycolipids are essential components of biological membranes (though less abundant than phos-phoglycerides), with highest concentration in plasma membrane. Maximum amount is found in nervous tissue: galactocerebrosides in sulphated form are important constituents of myelin, and gangliosides and galactocerebrosides are abundant in grey matter of the brain. They are typically located in the outer layer of the plasma membrane, where they interact with the extracellular environment.
2. Sphingolipids are known to play a role in cell-cell interactions, growth, and development. They are highly antigenic, and have been identified in several tumour antigens, blood group antigens, and the embryonic antigens specific for particular stages of fetal development.
3. The gangliosides act as receptors for toxic agents and some pathogens such as Vibrio cholerae, influenza virus, and tetanus toxin. In various malignant disorders, when the cells lose control of cell division and growth, there is a dramatic change in the glycolipid composition of the membrane.
C Derived Lipids
Unsaponifiable compounds obtained by hydrolysis of simple or compound lipids are called derived lipids, e.g. steroids, ketone bodies, and quinones (Table 3.3).
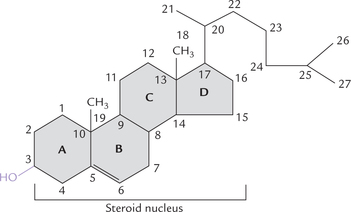
Steroids
Steroids are complex molecules consisting of four fused carbon rings. The designation of rings and numbering of carbon atoms is shown in Figure 3.12 . There is a phenanthrene nucleus made of three six-membered rings (A, B and C rings) and a cyclopentane which forms D ring. The steroid nucleus in a fully saturated form is known as cyclo-pentanopherhydrophenanthrene. The alcohol derivatives of steroids, in which one or more OH groups are present in the steroid nucleus, are termed sterols. Cholesterol, ergosterol, coprosterol and sitosterol are some important sterols.
In animal tissues, cholesterol is the major sterol. It has a single polar head group, i.e. the hydroxyl group at the C-3 position and the rest of the molecule is non-polar. It is present in two forms in serum: free and esterified. The latter is esterified with a fatty acid molecule at the third carbon hydroxyl group. The esterified cholesterol is an important component of plasma lipoproteins and the outer cell membrane.
Functionally, cholesterol is a very important molecule being precursor for three useful compounds: the bile acids, the steroid hormones, and vitamin D. Cholesterol is an essential component of all biological membranes. With its ring system and a solitary hydroxyl group, it is least water-soluble of all membrane lipids. Disorders of cholesterol metabolism play a role in aetiology of cardiovascular diseases and cholesterol is a major component of gall stones.
In plants and yeasts a different membrane steroid, phy-tosterol, is present, and most bacteria have no steroid at all.
Colour reactions of sterols: The chloroform solution of a sterol is dehydrated by strong dehydrating agents (e.g. acetic anhydride and strong H2SO4) to form coloured products. This forms the basis for detection of cholesterol by Salkowski and Liebermann Burchard reactions.
Other Lipid Derivatives
A detailed account of the other lipid derivatives such as lipoproteins, ketone bodies (Chapter 11) quinones poly-isoprenoid compounds including tocopherol and carot-enoids (Chapter 18) are discussed in appropriate chapters.
Exercises
Essay type questions
1. Name the phospholipids and state their biological importance. What products are released upon complete hydrolysis of a phospholipid molecule?
2. Name essential fatty acids. Which one of these is most important and why? Discuss biomedical importance of polyunsaturated fatty acids.
3. Give an account of lipid-classification giving examples from each class/subclass. Describe structure and functions of cholesterol in human body.
 Lipids are a diverse group of molecules that are soluble in organic solvents, and in contrast to other major types of biomolecules, do not form polymers.
Lipids are a diverse group of molecules that are soluble in organic solvents, and in contrast to other major types of biomolecules, do not form polymers.