CHAPTER 27 Skin integrity and wound care
At the completion of this chapter and with some further reading, students should be able to:
• Describe the structure of the skin
• Describe the functions of the skin
• Identify the risk factors for loss of skin integrity
• Discuss the wound-healing process
• Describe the different wound-healing intentions
• Describe the classifications of wound types
• Identify the principles of wound management
• Describe the differences between acute and chronic wounds
• Describe the procedures related to care of surgical wounds
• Describe the care required to prevent and manage pressure injuries
• Discuss the differences between arterial and venous leg ulcers
• Describe the care required to prevent and manage skin tears
• Describe the management of loss of skin integrity related to burns
• Describe the major manifestations of skin disorders
• Complete an assessment for an individual with impaired skin integrity
• Assist in planning and implementing nursing care for the individual with a loss of skin integrity
• Identify the purpose of commonly used wound dressings
• List appropriate nursing interventions for the individual with impaired skin integrity
The skin has several functions, which are largely concerned with protection of the body against infection, physical trauma and ultraviolet radiation. Any disorder that disrupts normal skin function will affect the efficiency with which it carries out its functions, and may place the physiological integrity of the individual at risk. The effects that disorders of the skin have on the individual range from minor and temporary to major and life threatening. Some serious skin disorders such as burns affect the individual to the extent that self-concept and body image are severely impaired. Wound management is a major role of the nurse. It is important that all nurses understand normal wound healing and the variances that can occur with ageing and disease processes. There is an abundance of literature on wound management and a vast array of wound-care products. The aim of this chapter is to assist the nurse to increase their knowledge in this rapidly changing area.
After I had open heart surgery, I was worried about the scars on my chest and what other people would think when they saw them. I thought I would never be able to wear low-cut tops or dresses again—which I love wearing. But the nurses explained to me that with the new dressings available today the wound will heal very well and the scar will become less noticeable over time. I hope so!!
THE INTEGUMENTARY SYSTEM
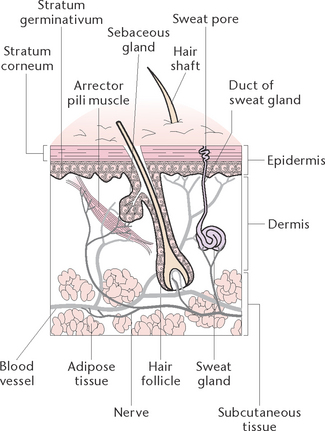
The integumentary system consists of the skin and its appendages; the hair, nails, sweat and sebaceous glands. The skin (or integument) is the largest organ of the body, covering about 7500 cm2 of surface area in an average adult (Fig 27.1). It is a protective barrier to the outside world, plays a vital role in homeostasis and also provides a major means of communication through touch and sensation. The appendages of the skin, hair, nails and glands, arise from the epidermis but are present in the dermis.
Structure of the skin
The skin is comprised of two basic layers: the epidermis and dermis. Under the dermis is a layer of adipose tissue called subcutaneous tissue. While this layer is not considered to be part of the skin, subcutaneous tissue does protect and insulate the deeper tissues.
The epidermis
The epidermis is the thin outermost layer and is composed of epithelial cells arranged in layers of stratified epithelium. The number of layers varies according to the amount of wear and tear experienced; for example, there are many more layers on the soles of the feet and the palms of the hands than there are between the toes and the fingers.
The epidermis is divided into two layers. The horny layer (stratum corneum) is the uppermost layer, and consists of about 30 layers of dead, flattened keratinised cells. These keratinocytes contain a waterproof hard protein substance called keratin. Keratin’s waterproofing properties protect the body and prevent escape of fluid from the deeper tissues. Keratin is also responsible for the formation of hair and nails. The germinative layer (stratum germinativum) is the deeper layer of the epidermis. It is here that new cells are constantly being formed and pushed upwards to replace cells that die and are rubbed off. Millions of new cells are produced daily and are pushed up away from the source of nutrition, to become part of the outermost layer.
Melanocytes are present in the germinative layer. Their function is to produce a brown pigment called melanin. Melanin gives colour to the skin and protects the body against the damaging effects of ultraviolet rays in sunlight. Brown-toned skin results when large amounts of melanin are produced, whereas light-toned skin results when the body produces less melanin.
The epidermis does not contain any blood vessels, but receives its essential substances from fluid that comes from the blood supply to the dermis. As cells are pushed towards the surface, away from the source of nutrition, they die and are eventually rubbed off. Thousands of dead epithelial cells are flaked off every day, which means that they are deposited on clothing and on every surface touched. They become part of the dust in a room, serve as food for mites and harbour microorganisms. A person sheds about 0.5 kg of dead cells per year, much of which goes down the bathroom drain.
The patterns of lines and ridges in the epidermis are due to projections in the dermis called papillae. On the fingertips these patterns are the fingerprints, which are different in every individual. For this reason, fingerprints are useful for purposes of identification. Nails are formed from the stratum corneum and are composed of modified epithelium.
The dermis
The dermis consists of white fibrous tissue containing many elastic fibres. Elasticity of the skin is essential to allow for changes in the size of a part of the body without tearing, such as the abdominal area during pregnancy. In old age the fibres become less elastic, causing wrinkles and folds to appear in the skin. The following structures are contained in the dermis.
Network of blood vessels
The blood vessels transport blood containing oxygen and nutrients to the dermis and transport blood containing wastes such as carbon dioxide away from the dermis. The blood vessels also play a role in regulating body temperature. If the body temperature is elevated the dermal capillaries become engorged with blood, which allows loss of body heat from the skin surface through radiation. If the environmental temperature is low, blood vessels in the skin constrict, conserving body heat by reducing radiation from the body.
Nerve endings
The dermis has a rich nerve supply consisting of several types of nerve endings. Each type of nerve ending reacts to a different stimulus, such as pain, touch, pressure and temperature. Impulses are transmitted from the nerve endings to the brain for interpretation.
Hair follicles and hairs
Hairs grow from hair follicles, which are deep pouch-like cavities in the skin. Although hair follicles are present in most areas of the skin, they are not found on the palms of the hands or the soles of the feet. Hair is composed of modified epithelium and grows from roots deep in the follicles. The part of a hair projecting above the epidermis is called the shaft. Hair colour reflects the amount of pigment, generally melanin, in the epidermis. Hair is a protection from the elements and from trauma; for example, the scalp hair and eyebrows are barriers against sunlight, and the nasal hairs filter inhaled air.
Hair growth is influenced by the sex hormones oestrogen and testosterone. An excessive growth of hair is called hirsutism. Like other cells that compose the skin, the hair cells also become keratinised. The hair that we brush, blow dry and curl is a collection of dead keratinised cells. Hair colour is genetically controlled and is determined by the type and amount of melanin. The absence of melanin produces white hair. Grey hair is due to a mixture of pigmented and non-pigmented hairs. Red hair is due to a modified type of melanin that contains iron. Hair is important cosmetically. Hair loss can be very distressing for some people. The most common type of hair loss is male-pattern baldness. It is a hereditary condition characterised by a gradual loss of hair with ageing.
Arrector pili muscles
The arrector pili muscles are minute involuntary muscles, with one end attached to a hair follicle and the other end to the dermis. When these muscles contract, for example, during fear or exposure to cold, the follicles and hairs become erect. Contraction of the muscles also causes some elevation of the skin around the hairs, giving rise to the ‘goose pimple’ appearance. The contraction of the arrector pili muscles increases heat production. This response is called shivering.
Sebaceous glands
Sebaceous glands are small glands, most of which open into hair follicles. The glands produce sebum, which is an oily substance and a lubricant that keeps the skin soft and moist and prevents the hair from becoming brittle. Combined with sweat, sebum forms a moist, oily acidic film that is mildly antibacterial. During periods of increased hormonal activity, such as adolescence, sebaceous glands become very active and the skin becomes oilier.
Sweat glands
Sweat glands, which are widely distributed, are either eccrine or apocrine. Eccrine glands are present all over the body and produce a clear perspiration. Apocrine glands are found mainly in the axillary and genital areas, and secrete sweat that has a strong characteristic odour. Sweat glands are coiled in appearance, with a straight duct that releases sweat onto the surface of the skin through an opening called a pore.
Sweat glands play a part in regulating body temperature. They excrete large amounts of sweat when the external, or body, temperature is high. When sweat evaporates off the skin’s surface it carries large amounts of body heat with it. Sweat consists of water that contains sodium chloride, phosphates, urea, ammonia and other waste products. Under normal circumstances, the amount of sweat secreted by an individual is about 700 mL/day. Under some conditions, such as strenuous physical exertion or pyrexia, the amount can be increased to as much as 1500 mL/day. Much of the water lost through the skin evaporates immediately, so it is not noticeable and is called insensible perspiration. Sweat that makes the skin damp and is noticeable is called sensible perspiration.
Functions of the skin
The major functions in which the skin and its appendages play a role are protection, thermoregulation, metabolism and sensory perception.
Protection
The skin is the first line of defence against the external environment. It provides a barrier to a variety of harmful agents, such as microorganisms, radiant energy and chemical substances. The skin acts as a barrier to harmful agents only as long as it remains intact. The waterproof quality of the outer layer prevents excess water absorption and abnormal loss of body fluids. The skin contains nerve endings that are sensitive to painful stimuli. The nerve endings transmit impulses to the brain that alert the individual that damage is occurring.
Thermoregulation
The skin plays a major role in the maintenance of constant body temperature. Blood conducts heat from internal structures to the skin for dissipation. The skin dissipates excess body heat by radiation, conduction, convection and evaporative cooling. Body temperature is controlled by the hypothalamus, which is the heat-regulating centre in the brain. This centre is sensitive to the temperature of the blood passing through it and also receives sensory stimuli from nerve endings in the skin that react to heat and cold (thermoreceptors). The hypothalamus in turn relays impulses requiring vasodilation and activation of the sweat glands (for cooling), or vasoconstriction and inhibition of sweat glands (for heat retention). Thus, the hypothalamus acts like a thermostat that initiates heat-losing activities when the body temperature begins to rise and heat-retaining activities when the body temperature starts to fall.
Metabolism
The skin assists in the regulation of fluid and electrolyte balance by eliminating water and small amounts of sodium chloride through the sweat glands. Sweat consists of 99.4% water, 0.2% salts, and 0.4% urea and other wastes. In the presence of sunlight or ultraviolet radiation, the skin begins the process of forming vitamin D (calciferol), a substance required for absorbing calcium and phosphates from food.
Sensory perception
Through perception of a painful stimulus, the skin causes an avoidance reaction, while other receptors perceive sensations of pressure and touch. The skin is therefore an agent of communication between the outside environment and the body, as the activity of sensory nerve endings informs the individual of what is happening outside the body.
Factors that affect skin integrity
Many risk factors can affect the integrity of the skin, including:
• Neurological factors, such as paraplegia, quadriplegia and multiple sclerosis
• Poor nutrition and hydration status
• Systemic and local circulation and oxygenation
• Presence or absence of excessive moisture
• Vascular conditions such as peripheral vascular disease
• Exposure to pressure, friction, shearing forces or burns
• Diseases such as diabetes mellitus
• Immunological suppression as a result of systemic conditions or medications
• Trauma from falls, accidents or burns
• Inappropriate or ill-fitting prostheses and footwear
• Skin disorders, e.g. genetic factors, idiopathic causes, hypersensitivity rashes.
WOUND HEALING
Wound healing is a dynamic and complex process and consists of four stages: haemostasis, the inflammation stage, the reconstruction phase and the maturation phase. The process of wound healing begins at the moment of injury and can continue for some years.
Haemostasis
The first stage of wound healing is haemostasis, which has three components: vasoconstriction, platelet response and the biochemical response. Vasoconstriction occurs when the bleeding in the wound is arrested by spasm in the arteries, arterioles and capillaries.
The platelet response is commonly described as the formation of the platelet plug. When platelets come into contact with parts of a damaged blood vessel, such as collagen or endothelium, their characteristics change. They become larger and irregular in shape and stick to the collagen fibres in the wall of the vessels and to each other. The platelets release various chemicals: serotonin, prostaglandins, phospholipids and adenosine diphosphate (ADP) that attract more platelets, which stick to the original platelets and form the plug. This platelet plug is very effective in preventing blood loss in a small vessel.
The biochemical component is the formation of a blood clot through the processes of the intrinsic and extrinsic clotting pathways, clot retraction and fibrinolysis. This is a complex process involving different clotting factors that are released from the damaged tissue. A clot is developed and retraction of the wound takes place.
The next stage of the healing process is termed tissue repair. This stage also has three phases: inflammation, reconstruction and maturation, which overlap each other and have varying time intervals.
The inflammation phase
This phase begins the moment that injury is incurred. The capillaries contract and thrombose to facilitate haemostasis. Vasodilation of the surrounding tissues occurs in response to the release of histamine and other vasoactive chemicals. This process causes increased blood flow to the surrounding tissue, which produces erythema, swelling, heat and discomfort, such as throbbing. A variety of white blood cells called polymorphonuclear leucocytes arrives at the site of the wound as a defence response and is involved in the immune response to fight infection. Polymorphs, macrophages and their associated growth factors produce various local and systemic effects. This phase continues for about 3 days.
The reconstruction phase
This is a time of cleaning and temporary replacement of tissue. The polymorphs kill bacteria, and the phagocytic macrophages digest the dead bacteria and debris to clean up the wound. Dermal repair is necessary if the wound is one of full thickness. New blood capillaries are developed (angiogenesis) and granulation tissue, which consists largely of collagen, is laid down. Epithelial cells migrate over the granulation tissue from the surrounding wound edges, hair follicles, sweat or sebaceous glands in the wound. These cells are very fragile. When the wound is covered the epithelium begins to thicken to 4–5 layers, forming the epidermis. Wound contraction then occurs, reducing the overall size of the wound. This phase can continue for 2–24 days.
The maturation phase
This is commonly known as the remodelling phase. The matrix of collagen cells is reorganised and strengthened. This phase can continue for about 24 days to 1 year. The wound is still at risk during this phase and should be protected.
Healing intentions
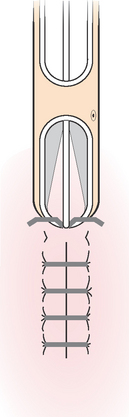
When the wound has minimal tissue loss the edges can be brought together by sutures or clips; as in a surgical wound, the wound is said to heal by primary intention, or first intention. Granulation tissue is not obvious. Healing by secondary intention occurs when wound edges cannot be brought together, as with a gaping wound. Granulation tissue fills in the wound until re-epithelialisation takes place and a large scar results. Third intention, or delayed primary intention, healing occurs when wound closure is delayed for a few days so that an infected or contaminated wound can be debrided (dirt, foreign objects, damaged tissue and cellular debris are removed from a wound or burn to prevent infection and promote wound healing). Closure of contaminated wounds is usually delayed until all layers of wound tissue show no signs of infection, usually within 4–10 days. At other times some wounds need surgical intervention such as the application of skin grafts or flaps to speed the healing process and reduce the risk of infection. Clinical Interest Box 27.1 provides details on skin grafts.
CLINICAL INTEREST BOX 27.1 Skin grafts
Skin grafts speed up the healing process and reduce the risk of infection. Grafts may be partial or full thickness. Skin grafts are classified as:
• Autograft: a surgical relocation of skin to the wound from another site of the body. A second wound results and is known as the donor site
• Allograft: a donor graft of skin between allogenic individuals, such as from one person to another
• Xenograft: a donor graft of tissue transplanted between different species, such as tissue of porcine origin transplanted to a human being
• Cultured: the cultivation of epidermis from a small amount of epithelial cells taken from the donor or recipient’s body and cultivated under laboratory conditions to form epidermis, before being transplanted back to the individual.
Factors that affect wound healing
TYPES OF WOUNDS
Septic and aseptic wounds
Clean wound
These wounds are made under aseptic conditions such as surgery, and heal by primary intention. These wounds generally do not require drainage.
Clean-contaminated wound
This is a wound made under aseptic conditions, but involving a body cavity that normally harbours microorganisms, such as the gastrointestinal, respiratory or urinary tract.
Contaminated wound
This term applies to a wound in which microorganisms are likely to be present, and includes open, traumatic and accidental wounds and surgical wounds in which a break in asepsis occurred.
Acute and chronic wounds
Acute wounds
Acute wounds in early stages are frequently not colonised with bacteria, but infection can become a complication. Although infection cannot always be prevented, care should be taken to minimise transmission by thorough aseptic technique when attending to the wound. Examples of acute wounds are those made by surgical incision or traumatic injury. An example of a surgical wound is a skin flap (see Clinical Interest Box 27.2).
CLINICAL INTEREST BOX 27.2 Skin flaps
A flap is a surgical relocation of tissue from one part of the body to another part to reconstruct a primary defect. This creates a secondary defect that will require skin grafting or primary closure.
Types of flaps
Skin, or cutaneous, flaps are grafts of tissue consisting of skin and superficial fascia. Composite tissue flaps are described according to the type of tissue they are composed of, for example, fasciocutaneous flap.
Flaps can be classified as free and pedicle. A free flap is the relocation of skin and subcutaneous tissue as a complete segment, with an anastomosis of the segment’s blood supply to vessels at the affected site. A pedicle flap is the surgical transfer of skin and subcutaneous tissue to another body site. Blood supply to the flap is maintained via a vascular pedicle attached to the body donor site.
Chronic wounds
Chronic wounds are highly contaminated predisposing the wound to infection. Clinical infection depends on the virulence of the bacteria and the resistance of the host. Clinical Interest Box 27.3 provides the differences between inflammatory response and infection. An example of a chronic wound is a venous leg ulcer.
WOUND MANAGEMENT
Wound management employs the principles of moist wound healing, that is, keeping the wound bed moist enough to facilitate the movement of new epithelial across the granulation tissue.
The benefits of moist wound healing are:
• Less injury and damage to cells on removal of the dressing
• More efficient autolytic debridement of necrotic tissue
• Less risk of transmission of microorganisms
• Fewer dressing changes and therefore less disturbance of the wound, resulting in reduced costs in dressing consumables and reduced workload for care staff
• The client’s lifestyle is interrupted less and, with most dressings, the client can continue to shower as usual.
Wound assessment
An initial holistic assessment of the client includes a comprehensive health history, demographics, nutritional status, psychosocial status, medication, mobility and activity, vascularity and current health status. Assessment of the wound identifies the wound characteristics, cause of the wound (e.g. traumatic, surgical), appearance of the wound bed, exudate (amount, type), peri-wound skin condition, type of wound (skin tear, pressure injury), location, size of the wound and its chronicity. A wound management history should be obtained to determine previously effective or non-effective treatments. Presence of infection is assessed. Pain management is a vital aspect of care and impacts on the quality of life for the individual with a wound. Clinical Interest Box 27.4 outlines the principles of wound management.
Assessment tools capture essential components of the client and wound and include medical/surgical history of the client, clinical history of the wound, relevant investigations to assist diagnosis of aetiology and local wound characteristics. Consistency in the information collected can be obtained in tools such as the mnemonic tool HEIDI: History (H), Examination (E), Investigation (I), Diagnosis (D) and Indicators (I) (Harding et al 2007). Specialised assessment tools are available for pressure injuries: National Pressure Ulcer Advisory Panel (NPUAP)/Agency for Health Care Policy and Research (AHCPR) staging system (see Pressure Injuries) (NPUAP & EPUAP 2009); PSST: Pressure Sore Status Tool, automated version known as the Wound Intelligence System (Bates-Jensen 1997); and PUSH: the NPUAP Pressure Ulcer Scale for Healing (NPUAP 2011). Other general tools are the WHS: Wound Healing Scale (Krasner 1997) and the SWHT: Sussman Wound Healing Tool (Sussman & Bates-Jensen 1998).
Description of wounds
Wound location
Anatomical description, for example, lower left leg, upper right arm. This allows consistency in assessment.
Exudate
Wound bed preparation
Schultz and colleagues (2003) define wound bed preparation as ‘… the management of the wound to accelerate endogenous healing or to facilitate the effectiveness of other therapeutic measures’. Wound bed preparation uses an algorithm called the TIME principle. These principles identify four key areas of the chronic non-healing wound, though the principles can also be applied to acute wounds (Schultz 2007). All chronic wounds start as acute wounds but fail to progress and get trapped in the inflammatory phase of healing with bacterial and biochemical imbalance. TIME provides a systematic approach to wound care. The four elements are T (tissue non-viable or deficient), I (infection or inflammation), M (moisture imbalance) and E (edge of wound non-advancing or undermining epidermal margin) (Schultz et al 2003). The wound bed preparation concept has gained international recognition as a framework that provides a structured approach to wound care.
Tissue non-viable
Non-viable tissue is black (necrotic) dead tissue that impairs the growth of new tissue, obscures the true depth of a wound and is a haven for bacterial growth preventing wound healing. Non-viable tissue also increases the risk of wound infection. Serial debridement is required to remove the tissue to, for example, produce a healthy wound bed.
Infection/inflammation
All wounds contain bacteria at some level often without harmful effects. Wound infection results from an imbalance of the host’s (the client) immune system and its ability to combat bacteria (host resistance), and the virulence of the microorganism: some bacteria have greater disease producing ability than others.
Failure to control bioburden can lead to infection especially in the immune-compromised client. Chronic wounds are likely to be colonised with bacterial or fungal microorganisms due to the nature of the open wound and tend to be polymicrobial. The presence of slough and necrotic tissue provides an environment for bacterial growth. It is when these bacteria interfere with wound healing that intervention is required.
Infection in acute or surgical wounds is relatively easy to recognise—spreading redness, local pain and swelling, peri-wound area warm to the touch. Infection in the chronic wound might be less obvious. Delayed healing can be an indicator of infection common to most wounds (Schultz et al 2003). Other signs can be an increase or change in wound exudate, or discolouration of the granulation tissue depending on the cause of the wound (Cutting et al 2005) (see Clinical Interest Box 27.5). It is recognised with increasing evidence that different wound types exhibit specific characteristics of infection (WUWHS 2008).
Bacteria can also cause prolonged inflammation by stimulating a continuing influx of neutrophils that release cytotoxic enzymes, free oxygen radicals and inflammatory mediators inducing a cycle of continuing tissue damage (Han et al 2011). Biofilms are also increasingly thought to play a role in prolonging inflammation and delaying healing (Wolcott et al 2010).
The risk of infection increases when ‘any factor that debilitates the client, impairs immune resistance or reduces tissue perfusion’ (WUWHS 2008). Comorbidities such as diabetes mellitus, arterial, cardiovascular or respiratory disease, renal impairment, malignancy, malnutrition, obesity, rheumatoid arthritis or an immune-compromised state contribute to an increased risk of infection. Certain medications also affect wound healing and increase the risk of infection, such as corticosteroids, cytotoxic drugs and immunosuppressants (WUWHS 2008). In elective surgery the amount of blood loss, the type and length of the procedure and a lack of pre-operative warming can influence the development of postoperative wound infection.
Biofilms in wounds
It is theorised that biofilms play a role in the delayed healing of chronic wounds. A biofilm is a community of multiple bacteria that attach to surfaces or each other to form highly complex communities embedded in a matrix that can be difficult to remove as the matrix offers protection from antimicrobials and the host immune system. There is an increasing awareness that microorganisms colonising a wound may be present as a biofilm (Percival & Bowler 2004). Acute bacterial infections are considered to result from growth of planktonic cells (single free floating bacteria) while many chronic bacterial infections involve biofilms. James et al (2008) found 60% of chronic wounds had characteristics of a biofilm compared to only 6% in acute wounds and evidence that biofilms exist in chronic wounds such as pressure injuries, diabetic foot ulcers and venous leg ulcers. Wound swabbing for microscopy and culturing will fail to detect biofilms in wounds and special molecular techniques are necessary.
Difficulties in treating biofilm-related conditions suggest that prevention is a more feasible option and the speed at which biofilms form necessitates rapid therapeutic action. Wolcott et al (2010) demonstrated that sharp debridement followed by the application of topical antimicrobials provides a time-dependent window of antibiotic sensitivity and is effective at targeting bacteria and supressing their regrowth.
Moisture imbalance
Exudate production is a normal part of wound healing. Excessive wound exudate can lead to maceration of the peri-wound skin. Absorbent dressings assist with removal to re-establish moisture balance. Too little moisture in a wound desiccates the cells and in this situation additional moisture, for example, a hydrogel dressing, can help maintain a moist wound bed. Chronic wound fluid differs from acute wound fluid. Chronic wound fluid contains high levels of tissue enzymes known as matrix metalloproteinases (MMPs) that are destructive to new tissue growth (Trengove et al 1999).
Pain
Pain is a significant factor affecting the quality of life in clients with wounds and in one study of people living with a chronic leg ulcer was found to be the most common feature strongly expressed (Douglas 2001).
Causes of pain vary and include procedural, operative, incident and background pain (Briggs et al 2004). Assessment of pain occurs with an initial history taking and is performed before, during and after wound care and documented in the person’s medical records. The goal is to minimise pain. Pain scoring can indicate how the wound is progressing, for example, worsening pain can be a sign of infection. Visual or numerical scales can be used to assist in the assessment of pain. Providing adequate and effective analgesia is vital in controlling pain especially at dressing changes and can include oral and topical analgesia.
Dressing selection can affect wound pain. Dressings that provide a moist wound environment and can stay in place for several days, and therefore require less frequent dressing changes, reduce the risk of trauma to the wound and surrounding skin. Soft silicone dressings facilitate non-traumatic removal and can be considered to reduce pain at dressing changes. Avoiding unnecessary handling of the wound at dressing changes can also lessen the pain experienced by the person. Asking and documenting the types of interventions that worsen or lessen wound pain contribute to the continuing assessment of a client’s pain history.
Wound debridement
Wound debridement is intended to remove necrotic tissue or fibrous slough from a wound to clean and prepare the wound bed for healing. Several methods are used and the method of debridement will depend on the amount of non-viable tissue to be removed, the vascularity of the area and how quickly the tissue needs to be removed. For example, in the case of a burn injury eschar must be surgically removed to allow skin grafting.
Autolytic debridement
This method occurs spontaneously in most wounds as macrophages and proteolytic enzymes liquefy and separate non-viable tissue and slough, promoting granulation tissue. Dressings that create or maintain a moist wound environment will assist autolysis. Hydrogels will donate water to necrotic tissue or dry fibrin slough. Hydrocolloids gel on contact with wound exudate and create a moist environment. Film dressings are permeable to water vapour but impermeable to water, bacteria and microorganisms and help to create and maintain a moist wound environment.
Enzymatic debridement
Enzymatic debridement is a less common method of debridement. Enzymatic agents are applied topically to the wound and work with endogenous enzymes to remove non-viable tissue. They need to be replaced frequently to maintain effectiveness. Several enzyme agents are available but not in all countries.
Mechanical debridement
Wet-to-dry
Wet-to-dry dressings are one of the oldest methods of mechanical wound debridement. Wet gauze dressings are applied to the wound and when dry, stripped from the wound, indiscriminately removing necrotic tissue or fibrin slough. They can also remove healthy tissue, causing bleeding. They are not generally recommended as they can cause pain to the client (see Clinical Interest Box 27.6).
CLINICAL INTEREST BOX 27.6 Clinical note
It is important to note that in cases where peripheral vasculature is poor, or has not been assessed and investigated, aggressive debridement and moist wound dressings are not indicated as new tissue growth cannot be supported.
(Adapted from WUWHS 2008, Best Practice Statement 2011)
Hydro-surgical debridement
Hydro-surgical debridement is a form of mechanical debridement though under more controlled conditions. Hydro-surgical debridement uses a high-pressure water jet pushed through a suitable hose to the tip of a procedure-specific handpiece. This method is fast and precise in the hands of a skilled operator such as a surgeon for excising unwanted tissue. Its use in practice is mostly in burn injuries and vascular leg ulcers.
Biological (larval therapy) debridement
In the biological debridement method, sterile maggots are applied to a wound, covered with a sterile semi-permeable dressing and allowed to sit for 1 to 3 days before being removed. The maggots will only ‘eat’ non-viable tissue and fibrin slough. The procedure is painless though not socially agreeable to all clients and nurses.
Surgical sharp debridement
Surgical sharp debridement is the fastest and most effective method of debridement. With this method all non-viable tissue and slough is removed until a clean, healthy wound bed is exposed. Sharp scissors, scalpels, forceps and curettes can be used to remove dead tissue. Pain can be experienced with this method and a topical anaesthetic cream may need to be applied prior to debridement. When pain is experienced, smaller, more frequent debridements can be undertaken. Bleeding can be controlled with light pressure.
Managing wound infection
The deleterious effect of infection in wounds is well recognised. Effectively managing wound infection requires optimising the host response, minimising risk factors where possible, reducing bacterial load and managing signs and symptoms such as fever and pain. Regular re-evaluation of the client and the wound and adjustment in treatment is necessary to assess and optimise the effectiveness of treatment. A systemic antibiotic should be considered for locally infected wounds and spreading infection in a wound.
Managing bioburden (removal of non-viable tissue, preventing and controlling infection and maintaining moisture balance) is essential for wounds to progress to healing. This should be directed by the clinical response of the tissue and client to the treatment and not dictated by strict times; for example, 2 weeks of antibiotic therapy or the length of time an antimicrobial dressing is used. This means adjusting therapy to the clinical signs and symptoms of infection with the use of topical antimicrobial agents. For example, if the wound is progressing after a week of topical antimicrobial therapy then the therapy may be stopped. If the wound is unchanged after the introduction of therapy, then reassessment and alternative topical therapies need to be used (WUWHS 2008). Gaining early control requires detection of subtle changes especially in the chronic wound such as a reduction in wound odour and reduction in pain. Wound infection requires prompt management to prevent the spread of infection systemically.
Diagnosing wound infection is a combination of clinical signs and symptoms (see Clinical Interest Box 27.5), full client evaluation, assessment of medications and comorbidities. Microbiological analysis can be used to assist diagnosis. This can be achieved by wound swabbing, wound biopsy or needle aspiration. Wound swabbing might help to detect the causative microorganisms but can also be misleading as it will detect only surface microorganisms not those in the deeper tissue and there is little supporting evidence of the role of wound swabs in identifying infection (WUWHS 2008). Results from a wound swab should not be used in isolation to diagnose a wound infection. When a wound swab is to be taken to assist in diagnosing the causative microorganism, clean the wound first and where appropriate debride non-viable tissue. There is no agreed best technique (e.g. Z-technique, Levine, cleansing or no cleansing before swabbing) for wound swabbing. It has been reported that the Levine technique is perhaps the more accurate method for identifying infection (Angel et al 2011; Gardner et al 2006). This technique uses a swab that is rotated over a 1 cm2 area of the wound surface with enough pressure to squeeze fluid from within the wound tissue.
To prevent further wound contamination or cross contamination, infection control procedures should be used to protect the client and the wound. This includes thorough hand hygiene and the use of suitable protective clothing and gloves at dressing changes. The wound should be cleansed at each dressing change with an appropriate wound cleanser; this can be sterile normal saline or water. Absorbent dressings are used to manage excess exudate and pus. Topical negative pressure therapy can be used for wounds with high volumes of exudate. Serial debridement of non-viable tissue will reduce the medium for bacterial growth and remove biofilms.
Topical antimicrobial dressings
Topical antimicrobial dressings contain agents that are capable of killing bacteria (bacteriocidal) commonly found in wounds. These agents are impregnated into various wound dressing materials and include silver, iodine, honey, chlorhexidine, polyhexamethylene biguanide (PHMB) and glucose oxidase enzyme systems. Topical antimicrobial dressings can assist in treating local infection in wounds and reduce the risk of infection in immune-compromised people at risk of infection, such as those with diabetes. Systemic antibiotic use should be considered in spreading infection in a wound. Topical antibiotics should be avoided for use on infected wounds to minimise the risk of allergy and the emergence of bacterial resistance (WUWHS 2008).
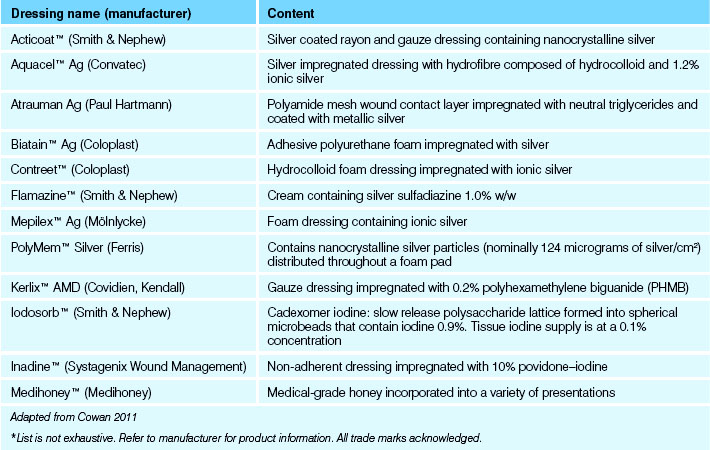
Examples of these dressings are listed in Table 27.1 and Clinical Interest Box 27.7 outlines the difference between disinfectants, antibiotics and antiseptics.
Table 27.1 Examples of topical antimicrobial dressings*
| Dressing name (manufacturer) | Content |
|---|---|
| Acticoat™ (Smith & Nephew) | Silver coated rayon and gauze dressing containing nanocrystalline silver |
| Aquacel™ Ag (Convatec) | Silver impregnated dressing with hydrofibre composed of hydrocolloid and 1.2% ionic silver |
| Atrauman Ag (Paul Hartmann) | Polyamide mesh wound contact layer impregnated with neutral triglycerides and coated with metallic silver |
| Biatain™ Ag (Coloplast) | Adhesive polyurethane foam impregnated with silver |
| Contreet™ (Coloplast) | Hydrocolloid foam dressing impregnated with ionic silver |
| Flamazine™ (Smith & Nephew) | Cream containing silver sulfadiazine 1.0% w/w |
| Mepilex™ Ag (Mölnlycke) | Foam dressing containing ionic silver |
| PolyMem™ Silver (Ferris) | Contains nanocrystalline silver particles (nominally 124 micrograms of silver/cm2) distributed throughout a foam pad |
| Kerlix™ AMD (Covidien, Kendall) | Gauze dressing impregnated with 0.2% polyhexamethylene biguanide (PHMB) |
| Iodosorb™ (Smith & Nephew) | Cadexomer iodine: slow release polysaccharide lattice formed into spherical microbeads that contain iodine 0.9%. Tissue iodine supply is at a 0.1% concentration |
| Inadine™ (Systagenix Wound Management) | Non-adherent dressing impregnated with 10% povidone–iodine |
| Medihoney™ (Medihoney) | Medical-grade honey incorporated into a variety of presentations |
* List is not exhaustive. Refer to manufacturer for product information. All trade marks acknowledged.
Adapted from Cowan 2011
CLINICAL INTEREST BOX 27.7 Disinfectants, antibiotics and antiseptics
Disinfectants
Non-selective agents that kill or remove microorganisms from inert surfaces such as work surfaces, medical instruments. They are not intended for use on the body or human tissue.
Antibiotics
Act selectively to inhibit or kill microorganisms. They can be administered either topically (not usually recommended) or systemically (orally or intravenously). Increasing resistance is an international concern amongst healthcare practitioners.
Antiseptics
Non-selective substances that inhibit multiplication of organisms (bacteriostatic) or kill (bactieriocidal). They can be used on intact skin and some open wounds and do not rely on the bloodstream for access to the wound so are useful for ischaemic wounds. They may have toxic effects on human cells. Low levels of bacterial resistance due to action at multiple sites within the microbial cell.
(Adapted from WUWHS 2008)
Wound dressings
There are many different types of wound dressings available from numerous manufacturers; therefore, the clinician involved in wound care requires a sound clinical knowledge of how the main groups of dressings perform. Dressings do not heal wounds, but appropriately selected dressings can enhance the body’s ability to heal. Maintaining a moist wound bed provides the optimum environment to promote healing (Winter 1963) and forms the foundation for contemporary wound care practice.
The ideal dressing will have many of the attributes listed below:
• Maintains a moist wound environment
• Provides an effective bacterial barrier
• Protects wound and surrounding tissues
• Promotes comfort and reduces pain
• Maintains an optimal wound temperature and pH
• Assists to prevent or manage clinical infection
• Covers wound from view of client and/or significant others
Dressings are generally categorised by mode of action or material. Holistic assessment and individual focused outcomes identify the characteristics that determine the specific ideal dressing. The criteria listed above will be available in a dressing product which can be selected to meet the needs of the wound—to promote healing and achieve the desired client-focused outcomes. Clinical Interest Box 27.8 gives some examples of dressings and their actions.
CLINICAL INTEREST BOX 27.8 Examples and action of wound dressings*
(Adapted from Cowan 2011)
* List is not exhaustive. Please refer to manufacturer for product information. All trade marks acknowledged.
Wound dressings can be classified into three main types:
Wound-hydration products
Wound-hydration products provide additional moisture to dry wounds or wounds with dead (necrotic) tissue and are usually in the form of a gel. These products are composed of mostly water, bound together by cross-linked polymers. They can be used as cavity fillers in smaller wounds with minimal exudate. These dressings require a secondary dressing to hold the gel in the wound and require daily to third-daily changing depending on how quickly the gel is utilised by the wound. These dressings are not suitable for wounds with high amounts of exudate.
Moisture-retentive dressings
Moisture-retentive dressings keep the wound bed moist, assisting keratinocytes to migrate across the surface of the wound. These dressings are used on light-to-moderately exuding wounds. They generally have a waterproof outer layer and are composed of hydrophilic particles (cellulose) and come in various thicknesses, shapes and sizes. These dressings aid the process of natural autolysis and stimulate granulation and re-epithelialisation. They retain moisture and are the ideal secondary dressing to support wound hydration. They can be left in position for several days, do not adhere to the wound, allow the passage of oxygen to the wound and are impermeable to microorganisms and water. The dressings are usually of such a nature that visual inspection can be made of the wound. These semi-permeable film dressings are comfortable for the client and alleviate pain because they protect exposed cutaneous nerve endings.
Exudate-management dressings
Exudate-management products absorb exudate from the wound surface and hold it within the dressing as well as transpiring fluid. These dressings are calcium alginates, which are composed of polysaccharides derived from seaweed; polyurethane foam dressings; hydrofibre dressings, which are made from pure cellulose (hydrocolloids) pectin and gelatine; and the combination dressings, which marry the technologies of hydrocolloids and the absorption capacity of baby nappy technology.
Negative pressure wound therapy
Negative pressure wound therapy (NPWT) is the application of sub-atmospheric pressure to a wound. This type of therapy has increased in use over the past 10 years and is now a regular aspect of wound care, with several versions available (Henderson et al 2010). It is used to absorb and control large volumes of exudate that are difficult to manage with absorbent dressings. It can be used on a variety of wound types: abdominal dehiscence, diabetic foot ulcers, burns, venous leg ulcers, surgical infections, skin grafts, orthopaedic trauma and soft tissue trauma. It is considered when the wound is not healing in the expected time frame, the wound is in an awkward location or difficult size to dress or a reduction in wound size is required before surgical closure (Henderson et al 2010).
NPWT is a closed suction and drainage system and consists of a wound filler, an adhesive wound sealer with a drainage tube connected to a vacuum pump that has an attached fluid collection canister. These devices are portable, allowing the client to mobilise and to be managed in the community. Pressure settings vary from −80 mmHg to −125 mmHg and depend on the wound type. For example, a thin skin graft will require less pressure than an abdominal wound dehiscence. Negative pressure can be applied continuously or intermittently, though continuous suction is more common, especially for wounds with large volumes of exudate.
Two types of wound interface are used: gauze and foam. Both have been shown to be effective (Campbell et al 2008). Foam tends to produce a thicker granulation tissue with gauze producing a less thick but denser granulation tissue (Borgquist et al 2010). A wound contact layer is often placed under the foam interface when there is concern there may be complications, or there is exposure of vulnerable structures (Dunbar et al 2005). Tissue ingrowth in the foam interface has been reported in the published literature (Borgquist et al 2009), which is why a wound contact layer is placed under the foam. The function of the wound filler is to deliver negative pressure to the wound. Use of either depends on various factors. For example, foam may be used in areas of large tissue loss where contraction of tissue is required before closure. Gauze may be more beneficial when the wound surface is irregular and cosmetic outcome is important.
A newer smaller device is also available that does not rely on a fluid canister for exudate collection, making it extremely portable. This device (PICO™, Smith & Nephew Australia Pty Ltd) has a specialised dressing that absorbs and transpires exudate. The dressing has a tube connection to a small pump that delivers a fixed negative pressure (−80 mmHg). NPWT offers several benefits:
• Stimulates new tissue growth (granulation)
• Reduces tissue oedema and manages wound exudate
• Increases the rate of wound healing reducing the wound area
• Helps control bacterial burden (removes exudate which contains bacteria)
Certain factors need to be considered before applying NPWT:
• Effective management of client symptoms
• Dimensions of the wound and ease of application
• Care setting, e.g. home, hospital and who will care for therapy
• Effective debridement of wound bed
• Ability of client to give consent to treatment (adapted from Henderson et al 2010).
There are also several contraindications for NPWT:
• Necrotic tissue in the wound
• Exposed organs or blood vessels
• Malignancy in the wound bed (except in palliative care cases)
Therapy should be stopped when there is uniform granulation tissue in the wound and the wound’s depth has decreased.
Clinical Interest Box 27.9 discusses the selection of dressings while Clinical Interest Box 27.10 outlines the basic factors to consider when selecting a dressing, and Clinical Interest Box 27.11 addresses a myth about wound cleansing.
CLINICAL INTEREST BOX 27.9 The right dressing for the right wound
Modern wound dressings improve healing time, reduce pain, reduce the time required to dress the wound and require less frequent changes. It is important to use the most appropriate dressing for the wound at the appropriate stage of healing. One dressing will not suit the entire wound healing process. Match the action of the dressing to the aim of the treatment, as follows:
| Condition of wound bed | Aim of treatment | Examples of dressings |
|---|---|---|
| Non-viable tissue (black, hard, dehydrated, necrotic) | Rehydrate, debride, promote granulation tissue | Hydrogels |
| Slough (soft, yellow, creamy or fibrous) | Debride slough, promote granulation, absorb excess exudate | Hydrogels, alginates, hydroactive dressings, cadexomer iodine, hydrocolloids, foams |
| Granulating (red, moist) | Retain moisture, promote and protect granulation tissue and epithelialisation, absorb excess exudate | Foams, alginates, hydroactive dressings, hydrocolloids, hydrogels |
| Epithelialising (pink wound, evidence of epithelial growth on surface) | Retain moisture, promote and protect epithelialisation | Hydrocolloids (thin), non-adherent dressings, polyurethane films |
| Infected (local or spreading) | Treat infection with systemic antibiotics (spreading infection), topical antimicrobial dressings, absorb excess exudate, debride slough | Silver-impregnated dressings, silver foams, cadexomer iodine |
CLINICAL INTEREST BOX 27.10 Factors to consider when selecting a dressing
• Wound type: superficial, full thickness, cavity
• Wound description: granulating, epithelialising, necrotic, sloughy
• Wound characteristics: dry, moist, heavily exuding, malodorous, excessively painful, difficult to dress, bleeds easily
• Bacterial profile: sterile, colonised, infected, infected and potential source of cross-infection
CLINICAL INTEREST BOX 27.11 A myth in wound cleansing
Myth: Clients with wounds should not let the wound come into contact with shower water.
Fact: Showering a client with a postoperative wound does not increase the risk of infection or slow the healing process. It does promote a sense of wellbeing and health associated with cleanliness. Showering of chronic wounds and ulcers may be undertaken with caution. Tap water should not be used if declared unsuitable for drinking. Tap should be run for 15 seconds prior to use.
PATHOPHYSIOLOGICAL EFFECTS AND MAJOR MANIFESTATIONS OF SKIN DISORDERS
Pathophysiological influences and effects
The major factors that affect normal structure and functions of the skin can generally be classified into six categories:
Genetic factors
Genetic factors determine skin colour and the amount and distribution of hair. Congenital skin disorders include birthmarks, hypopigmentation (albinism) and a condition called ichthyosis, which involves excessive scaling or thickening of the outermost skin layer. Heredity also plays a role in predisposition to the development of acne and atopic dermatitis.
Idiopathic causes
Many skin disorders have no one known cause, for example, vitiligo and psoriasis. Other skin disorders may be associated with emotional or physical stress but there does not seem to be any one identifiable cause.
Hypersensitivity
Some individuals have a tendency to react adversely to contact with various substances, for example, when a substance is inhaled, ingested or comes in contact with the skin. Some allergic reactions are manifested in alterations in the skin; for example, reddening and itching of the skin may be side effects of certain medications.
Trauma
Damage to the skin can result from exposure to extremes of temperature, from prolonged pressure on the skin or from physical injuries resulting in lacerations, punctures or abrasions.
Neoplasia
Any abnormal growth of new tissue, whether benign or malignant, is called a neoplasm. Examples include calluses, which can develop on the toes from friction and chronic pressure, or keloid scarring, which can result after injury to the skin. Benign or malignant neoplasms may develop from any type of cell in the skin, but the melanocytes and keratinocytes are the cells most frequently involved. A mole (naevus) is a common type of benign skin tumour. Some benign epithelial cell lesions may develop into malignant neoplasms.
Infections and infestations
If the skin is broken and pathogenic microorganisms gain entry, infection may result.
Primary skin infections are commonly caused by bacteria, fungi and viruses. Secondary skin infections may occur in conditions such as stasis dermatitis, in which impaired circulation damages skin cells of the lower limbs.
Systemic infections, such as measles, chickenpox and some sexually transmitted infections, also result in manifestations on the skin. Skin infestations occur when parasites such as lice or mites invade and subsist on the skin.
Major manifestations of skin disorders
Various structural and functional changes accompany skin disorders.
Pruritus
Pruritus (itching) is one of the more common and distressing symptoms of a skin disorder. Pruritus is thought to result from a disruption in the skin nerve endings. Scratching to relieve pruritus can result in tissue damage and infection, thereby causing further discomfort.
Lesions
Depending on the type of skin disorder, one or a variety of lesions may be present. Observation of the client includes assessing any lesions to determine their shape, size and distribution. Table 27.2 lists and describes the various types of skin lesions. Some types of lesions may discharge fluid, which is referred to as exudate.
| Term | Description | Examples |
|---|---|---|
| Bulla | Elevated, filled with clear fluid. Similar to a vesicle, but larger | Pemphigus vulgaris, drug eruptions, partial thickness burns |
| Comedo | A plug of secretion contained in a follicle | Acne |
| Crust | A superficial mass caused by dried exudate | Impetigo, eczema |
| Cyst | Encapsulated mass in the dermis or subcutaneous layer. May be raised or fat, and contain fluid or solid material | Sebaceous cyst |
| Erosion | Moist, red, depressed break in the epidermis. Follows rupture of a vesicle or bulla | Chickenpox |
| Excoriation | Superficial break in the skin | Scratches, abrasions |
| Fissure | Deep, linear, red crack or break exposing the dermis | Tinea pedis |
| Macule | Small circumscribed discolouration, e.g. red, white, tan or brown | Freckle, rubella, scarlet fever |
| Nodule | Circumscribed, elevated area—usually 1–2 cm in diameter | Ganglion, acne |
| Papule | Circumscribed, elevated, firm palpable area | Mole, wart, pimple |
| Plaque | Elevated, rough fat-topped areas | Psoriasis, seborrhoeic warts |
| Pustule | A vesicle or bulla containing pus | Acne, furuncle, folliculitis, impetigo |
| Scale | Mass of exfoliated epidermis | Dandruff, psoriasis |
| Scar (cicatrix) | Ranges from a thin line to thick, irregular fibrous tissue. May be white, pink or red | Healed surgical incision or wound |
| Tumour | Elevated, solid formation | Lipoma, melanoma, fibroma |
| Ulcer | Depressed circumscribed area involving loss of the epidermis, exposing the dermis, and may involve subcutaneous tissue | Decubitus ulcer, stasis ulcer |
| Vesicle | Circumscribed, elevated superficial area filled with clear fluid | Blister, herpes simplex infection, contact dermatitis |
| Weal | Transitory, elevated irregularly-shaped swelling of the epidermis | Urticaria, insect bites |
Alterations in sensation
In addition to pruritus, the individual may experience other abnormal skin sensations such as numbness, tingling, burning or pain.
Alterations in skin colour
Disorders of the skin may be accompanied by darkened areas of skin (hyperpigmentation), patches of pale skin (hypopigmentation) or inflammation. Burned skin may be reddened, blanched or charred, depending on the extent of the burn. Cold injuries can result in red areas, as in chilblains, or in extreme pallor, as in frostbite.
Alterations in skin temperature
In certain skin disorders such as bacterial infection the skin may feel hot to touch, whereas in other conditions such as frostbite the skin is cool to touch.
Alterations in texture
Abnormalities of texture, for example, roughness or hardness, may result from the presence of certain types of lesions such as scabs or papules. Scaling may occur, or the skin may be thick, wrinkled or atrophied. Some skin disorders may result in areas of oedema; for example, injuries from heat or cold.
SPECIFIC DISORDERS OF THE SKIN
Genetic disorders
Genetic disorders are those that are present at birth, become evident soon after birth or those that may be passed on to the next generation.
Acne vulgaris is a chronic inflammatory condition involving the sebaceous glands and the pilosebaceous follicles, particularly of the face. A blackhead forms and blocks the opening of a sebaceous gland, which becomes infected. Later, a pustule forms. This condition is most often present in adolescents and young adults. Familial tendencies are thought to contribute to the cause or exacerbation of acne. Other causative factors include endocrine imbalances, use of oral contraceptives, hormone therapy, emotional stress and lack of personal hygiene.
Ichthyosis is any one of several inherited conditions in which the skin is dry, hyperkeratotic and fissured, resembling fish scales. It usually appears at, or shortly after, birth. Ichthyosis vulgaris is the most common type and the least severe.
Idiopathic disorders
Idiopathic disorders are those in which no definite cause can be identified.
Psoriasis is a chronic skin disorder characterised by red patches covered by thick, dry, silvery scales. The lesions may be present on any part of the body but are more common on the extensor surfaces of the elbows and knees and on the scalp. Psoriasis can be exacerbated by trauma, infection, stress and the use of specific systemic medications.
Pityriasis rosea is thought to be caused by a virus, and is characterised by a scaling, pink macular rash that spreads over the trunk and other parts of the body. The condition is self-limiting and usually disappears within 4–6 weeks.
Vitiligo is a benign disorder consisting of irregular patches of skin totally lacking in pigment.
Seborrhoeic dermatitis is a chronic inflammatory condition characterised by dry or moist, red scaly eruptions. Common sites are the scalp, eyelids, face and trunk. The scales have a greasy feel and yellow crusts. Cradle cap is one form of seborrhoeic dermatitis.
Hypersensitivity disorders
These disorders result from an immediate or delayed reaction after exposure to a certain substance.
Contact dermatitis is caused by an irritant substance that comes into direct contact with the skin, such as detergents, hair dye, metals, preservatives, perfumes or specific fabrics. The resultant inflammation and skin rash may be mild or severe, depending on the individual’s response. Chronic exposure to an irritant may result in the skin becoming reddened, scaly or cracked.
Atopic dermatitis usually occurs when there is a history of asthma and/or hay fever. The condition is characterised by pruritus, redness of the skin, papules and thickening of the skin. Common sites are the face and neck, behind the knees and in the cubital fossae and on the back of the hands.
Urticaria is a pruritic skin eruption characterised by transient weals with well-defined red margins and pale centres. Urticaria (hives) is most frequently caused by foods, insect bites and inhalants. Specific types of urticaria are associated with systemic diseases. Pruritus associated with urticaria is frequently intense and is commonly accompanied by stinging, numbness or prickling sensations. Urticaria may also be a manifestation of an adverse reaction to a drug, and the skin lesions may appear almost immediately or several days after the drug has been absorbed. Drugs responsible for such adverse reaction include acetylsalicylic acid, penicillin and codeine.
Pemphigus vulgaris is an uncommon disorder of the skin and mucous membranes, characterised by the formation of large bullae containing clear fluid. The disorder is thought to result from an autoimmune response, and may be fatal if untreated. The bullae erupt, ooze and bleed readily, and death is often due to a secondary bacterial infection or loss of blood protein.
Trauma
A traumatic injury, which involves damage to the skin, may be due to direct force, penetration or extremes of temperature.
Erythrocyanosis (chilblains) is redness and swelling of the skin as a result of excessive exposure to cold. Burning, itching, blistering and ulceration may occur; the areas most commonly affected are the toes, fingers, nose and ears.
Frostbite is the traumatic effect of extreme cold on the skin and subcutaneous tissues, characterised by pallor of the exposed areas, such as the nose, ears, fingers and toes. Vasoconstriction and damage to blood vessels impair local circulation, resulting in oedema, anoxia and necrosis.
Immersion (trench) foot is a condition of the skin on the feet that develops from continued exposure to wetness and coldness, such as prolonged immersion in cold water. The feet appear pale, cold and swollen, and the individual experiences tingling followed by loss of sensation.
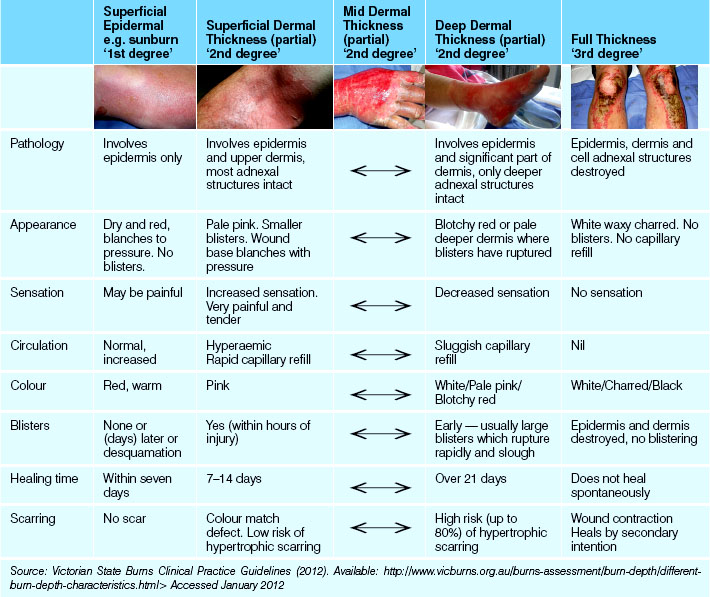
Burns are injuries to the body tissues caused by heat, electricity or chemicals. Thermal burns include injuries caused by flame, steam or hot liquids. Electrical burns result from contact with an electrical current, and chemical burns most often result from contact with caustic substances. A burn may be minor or major, and the degree of local effects and systemic consequences depend on many factors, including the severity of the burn and the age of the individual. (More information on burns and the care of clients with burns is provided later in this chapter.)
Neoplasia
A keloid is a benign overgrowth of fibrous tissue at the site of a wound to the skin. The new tissue is elevated, thickened and reddened. Most keloids flatten and become less noticeable over a period of years. Keloids are more likely to develop if a wound has been infected or if the edges of a wound have been poorly aligned during healing.
Sebaceous cysts are one type of epithelial cyst and consist of a capsule containing a soft yellow–white material. These benign cysts are elevated and firm and range in size from about 0.2–5.0 cm.
A lipoma is a common benign tumour composed of adipose tissue, which is generally encapsulated in the subcutaneous layer of the skin. Lipomas vary in size and most frequently occur on the neck, back, thighs or forearms.
Neurofibromatosis is a congenital condition characterised by numerous neurofibromas of the skin and nerves, by café-au-lait spots on the skin and in some cases by abnormalities of the muscles, bones and internal organs. Many large, pedunculated soft-tissue tumours may develop.
Basal cell carcinoma is a malignant lesion characterised by a shallow ulcer surrounded by a raised well-defined edge. Basal cell carcinomas may also be referred to as rodent ulcers. The most common site is the face, particularly the nose, eyelids and cheeks. Basal cell carcinomas usually occur in people aged over 40 and, as metastasis is rare, the prognosis is favourable.
Squamous cell carcinoma is a malignant lesion characterised by a firm, elevated painless nodule. The most common sites are areas of the body most often exposed to ultraviolet rays. Squamous cell carcinoma is most frequently seen in men over age 55 and, as metastasis is probable, this neoplasm has a higher mortality rate than does basal cell carcinoma.
A melanoma is a malignant tumour that arises from melanocytes. The incidence of melanoma seems to be related to prolonged exposure to the sun, particularly by fair-skinned people. Because metastatic dissemination is relatively common, the mortality rate is high. In its pre-malignant stage, a melanoma appears as a flat, irregularly pigmented macule. Colour changes appear as the melanoma becomes malignant and invasive, with the colour ranging from red, brown and blue to black. Melanoma can occur on any part of the body but most frequently occurs in areas of the skin exposed to sunlight. There are many types of melanoma and, because of its invasive nature, the nodular type is the most serious. Australians have the highest rate of malignant melanoma in the world, and the incidence is particularly high in Queensland and other tropical regions.
Infections and infestations
Because the surface of the body is constantly exposed to large numbers of pathogenic microorganisms, the skin is a potential area for infection. In addition, dermatological problems are often the result of infestation by parasites. Many factors increase a person’s vulnerability to a skin infection, including ill-health, poor standard of hygiene or a break in the continuity of the skin. Bacterial skin infections include carbuncles, erysipelas, folliculitis, furuncles (boils), impetigo and paronychia.
A carbuncle is a cluster of staphylococcal abscesses or boils containing purulent matter. Eventually pus discharges to the skin surface through numerous openings.
Erysipelas is an acute streptococcal inflammatory infection involving subcutaneous tissue. The skin of the affected area is bright red and oedematous, with a sharply defined border. The area may develop vesicles and the individual commonly experiences pain and an elevated body temperature.
Folliculitis is a common infection of the hair follicles, caused by staphylococci. Superficial or deep pustules are evident, and the most common site is the face.
A furuncle (boil) is an infection caused by either staphylococci or streptococci. A furuncle starts as a painful, hard, deep follicular abscess, and the overlying skin is hot to touch. The area becomes soft and opens to discharge a core of tissue and pus.
Impetigo is an acute contagious disorder of the superficial layers of the skin, caused by either staphylococci or streptococci. The condition begins as local erythema and progresses to pruritic vesicles which ooze, with the exudate from the lesion forming a yellow-coloured crust. Lesions usually form on the face and spread locally.
Paronychia is a painful inflammatory infection of the tissue around the nails.
Viral skin infections include herpes simplex and herpes zoster infections, and verrucae.
Herpes simplex virus (HSV) has an affinity for the skin and usually produces small irritating or painful fluid-filled blisters on the skin and mucous membranes. HSV-1 infections tend to occur in the facial area, particularly around the mouth and nose, whereas HSV-2 infections are usually limited to the genital region. The blisters erupt and thin yellow crusts form as the lesions begin to heal.
Acute infection with herpes zoster, or varicella-zoster virus (V-ZV), is characterised by the development of very painful vesicular skin eruptions that follow the underlying route of cranial or spinal nerves inflamed by the virus. After about 1 week the vesicles develop crusts, and the condition may last several weeks. The pain may last for much longer.
A verruca (wart) is caused by the human papilloma virus, and presents as a firm skin lesion with a rough surface. Different types of verrucae include those that commonly affect the hands, fingers or knees; and those that affect the genito-anal region.
Fungal skin infections include candidiasis and tinea. Candidiasis is any infection caused by a species of Candida, usually Candida albicans, characterised by pruritus, a white exudate, peeling and easy bleeding. Oral or vaginal thrush are common topical manifestations of candidiasis, as are red eroded patches in the genito-anal region.
Tinea (ringworm) is a group of fungal skin diseases caused by dermatophytes of several kinds. It is characterised by itching, scaling and painful lesions. Types of tinea include tinea capitis, affecting the scalp, tinea pedis, affecting the feet, and tinea corporis, affecting non-hairy smooth skin on the body.
Infestations of the skin by parasites include scabies and pediculosis. Scabies is a condition caused by a mite, Sarcoptes scabiei, and characterised by a papular rash, intense pruritus and excoriation of the skin from scratching. The sites most commonly affected are the thin-skinned areas between the fingers, flexor surfaces of the wrists and the inner aspect of the thigh. The mite burrows into the outer layers of the skin, where the female lays eggs. Small, thread-like red streaks appear where the mite has burrowed into the skin.
Pediculosis is infestation by blood-sucking lice, which causes intense pruritus, often resulting in excoriation from scratching. Different varieties of pediculi affect the hair on the scalp, the body or the pubic area. The lice can be seen with the naked eye, and their eggs (nits) can be seen as small pear-shaped bodies attached to the hairs.
Diagnostic tests
To diagnose specific disorders of the skin a variety of tests may be performed.
Direct examination
A lesion may be examined using a magnifying lens, or a Wood’s lamp may be used to determine the presence of fungal infections. Fungal infections such as ringworm show a characteristic fluorescence under black light.
Skin biopsy
The medical officer obtains a sample of skin or part of a lesion for pathological examination. Certain lesions may be surgically excised to provide sufficient tissue for histological diagnosis.
Microscopic examination
Specimens for microscopic examination may be obtained by gently scraping the scales or crusts of lesions. Exudate from oozing lesions may also be obtained for microscopic examination.
Skin testing
Skin testing may be performed to determine which substance or substances cause a hypersensitive reaction. Patch testing provides a means for assessing contact sensitivity. One or more suspected allergens are placed on a hairless part of the body. The test site is later examined for a visible reaction.
CARE OF THE INDIVIDUAL WITH A SKIN DISORDER
Although nursing care is planned to meet the individual’s needs, according to their specific skin disorder, the nursing care of a person with any dermatosis generally involves the following aspects.
Relief of pruritus
Itching, which can be a source of considerable distress, is a feature of many skin disorders. The natural response to pruritus is to scratch, and scratching can cause further discomfort and may lead to tissue damage and infection. While medical therapy is aimed at resolving the problem responsible for the pruritus, certain nursing measures can be employed to provide some relief:
• As heat tends to aggravate itching, the room should be maintained at a moderate and comfortable temperature. The bedclothes and personal clothing should be light, loose and cool
• Soothing tepid baths may be helpful in alleviating the itching
• Diversions that are of interest to the individual, such as reading or watching television, may be helpful.
Topical applications
The application of local soothing preparations or topical medications may be prescribed. The most common mediums used to apply medications to the skin are creams, lotions, ointments and pastes, or powders. Medications that are mixed with the appropriate medium for topical application include: anti-inflammatory drugs such as corticosteroids, antipruritic agents such as tar or corticosteroids, antiseptics such as phenol and antibiotics such as neomycin. Specific substances to be added to the bath water may also be prescribed. For example, oatmeal, bath oils or coal-tar preparations may be prescribed when large areas of the body surface are affected. The nurse must ensure that the bath water is at a comfortable temperature and should be aware that many skin disorders result in changes in sensory perception, so it is essential that the water is not too hot.
Whenever topical applications have been prescribed, the nurse must know the level of responsibilities and the regulations regarding administration of medications in the healthcare facility and geographical area in which they work. Each type of topical medication requires proper application, and the nurse must know the amount to use, whether gloves are required during application and the signs of any adverse effects of the medication. The five rights of administration of medication (right person, right medication, right time, right dose, right route) are just as relevant for topical applications as they are for systemic medications.
In addition to topical applications, systemic medications may be prescribed, such as analgesics to relieve pain, antibiotics to combat infection or mild sedatives to promote adequate rest.
Dressings may be prescribed as part of the local treatment of skin disorders. Moist dressings may be used in the management of acute inflammatory skin disorders. Any solution that is used to soak the dressing should be warmed to body temperature. Occlusive dressings may be applied over a topical medication, to promote penetration of the drug into the epidermis.
Maintenance of fluid and nutritional balance
Fluid and electrolyte balance may be disrupted because of loss of fluid in exudate from skin lesions. It is important to ensure that adequate fluid replacement is provided to compensate for any abnormal fluid loss.
A diet that is rich in protein may be prescribed to replenish losses and to promote healing. It is important to ascertain whether the individual is allergic to any specific foods, as skin disorders can be caused or aggravated by food allergies. Any known allergens must be eliminated from the diet.
Preventing infection
Any disruption to the integrity of the skin increases the risk of infection, so measures such as the following should be implemented to protect the skin:
• After bathing or showering, the skin should be gently patted dry. Brisk rubbing could cause damage to already tender skin
• The nails should be kept short and clean to prevent damage from scratching, and the person should be encouraged to resist scratching. It is important to explain that scratching increases the risk of skin trauma and infection. For some people, such as a young child or a disoriented person, mittens may be placed over the hands to reduce skin damage by scratching
• All dressings and applications of topical substances must be performed aseptically
• If the skin disorder is contagious, isolation precautions may be implemented to prevent the spread of infection to others.
Providing psychological support
A severe skin disorder may cause distress and embarrassment to the person who has it. They may be self-conscious about their appearance, and their body image may be severely impaired. If the person feels that other people will avoid contact because of their unsightly appearance, they may experience anxiety or depression, both of which may be exacerbated if the disorder is likely to result in permanent disfigurement. To assist the client with a skin disorder, the nurse should be careful not to demonstrate any distaste or repugnance. It is important that the client and their significant others are kept well informed about the disorder and its likely outcome. The client should be given the opportunity to express their emotions and fears, such as the fear of disfigurement or alienation from their loved ones.
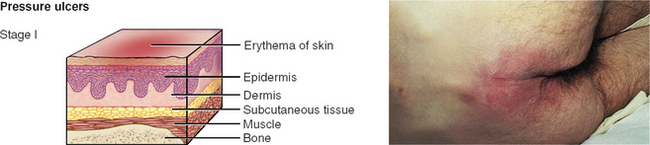
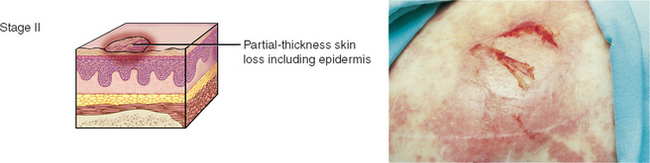
PRESSURE INJURIES
Pressure injuries are defined as ‘A localised injury to the skin and/or underlying tissue usually over a bony prominence, as a result of pressure, shear and/or friction, or a combination of these factors’ (Australian Wound Management Association (AWMA) 2010). Pressure injuries have previously been referred to as pressure ulcers/areas, decubitus ulcers, trophic ulcers and bedsores. The AWMA has now adopted the term ‘pressure injuries’ to fall in line with other international pressure injury guidelines (NPUAP & EPUAP 2009). The prevalence of pressure injuries remains high even with the implementation of guidelines and interventions to reduce their occurrence. Australian prevalence rates have been reported at between 9% and 29.4% with rates declining by 9–16% over a 3-year period (Asimus 2011; Mulligan et al 2009; Quality and Safety Branch Department of Human Services Victoria 2006). In Australia, hospital-acquired pressure injuries are reported at rates between 6% and 68% (Mulligan et al 2009; Quality and Safety Branch Department of Human Services Victoria 2006) and have been estimated to independently increase hospital length of stay by over 4 days (Graves et al 2005), deflecting the use of these bed-days from other conditions and placing a significant economic and clinical burden on the Australian healthcare system. In the US Healthcare System pressure injuries are considered a ‘never event’ being largely preventable and have been reported as the most expensive preventable adverse event/medical error, consuming over US$3.8 million annually (Shreve et al 2010). This cost exceeds postoperative infection (US$3.7 million) and mechanical complication of device, implant or graft (US$1.1 million) (Shreve et al 2010). From 2008 the US medical system ceased reimbursement to facilities for hospital-acquired grade three and four pressure injuries, an indication of the seriousness the system is taking to control the occurrence of pressure injuries (CMS 2012).
Pressure injuries result from ischaemic hypoxia of the tissues owing to prolonged pressure on the area when it is over a hard surface such as a mattress or chair. Impaired mobility or activity and impaired sensory perception of the individual affects the ability of the individual to recognise when there is pain and to change position (see Clinical Interest Box 27.12). Pressure impairs blood circulation and the area is deprived of essential nutrients and oxygen, causing cellular death. The major factors contributing to loss of local capillary blood supply are pressure and shearing forces; however, these can be exacerbated by predisposing factors classed as either intrinsic or extrinsic. A combination, of these factors increases the risk of pressure injury.
CLINICAL INTEREST BOX 27.12 Individuals with increased exposure to pressure
(Adapted from AWMA & NZWCS 2011b)
The greater the pressure on the skin, the more the tissue is compressed. Pressure on the skin over bony prominences can distort the blood vessels to such an extent that the blood flow is interrupted. Pressure can also occlude lymphatic vessels, and the consequent accumulation of toxic substances can contribute to cell damage. Recent research in the laboratory on rats suggests pressure injuries may result from sustained deformation of the tissue, causing an ischaemia-reperfusion injury where there is a cycle of ischaemia and reperfusion of the tissue during prolonged pressure, which contributes to tissue damage (Loerakker et al 2011).
Pressure injuries form in a few hours but may take many months to heal; preventing them is therefore of prime importance. Ensuring adequate blood supply to pressure areas is critical as altered blood supply is the primary cause of pressure injuries. Blood supply to promote tissue healing is dependent on adequate circulation that brings antibodies and leucocytes to combat infection, as well as nutrients and oxygen for regeneration of tissues.
Pressure injuries can develop on body areas when they are subjected to prolonged or excessive pressure (Fig 27.2). The two most frequently reported locations are the sacrum and heels (Quality and Safety Branch Department of Human Services Victoria 2006).
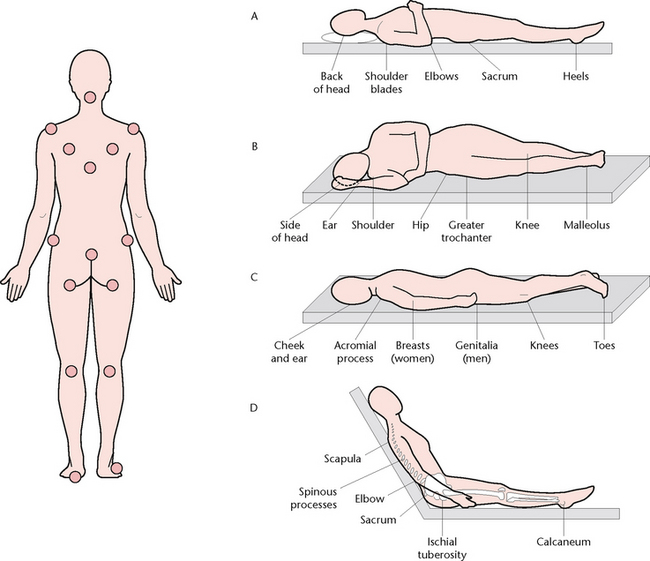
Figure 27.2 Pressure injury sites. A: In the supine position B: In the lateral position C: In the prone position D: In the sitting position
Classification of pressure injuries
Pressure injuries are categorised into four main stages with two sub-categories (NPUAP & EPUAP 2009; AWMA & NZWCS 2011b). Stages I and II are generally considered partial thickness wounds (though the skin remains intact in a stage one pressure injury) and stage III and IV and considered full thickness injuries. Tissue destruction can continue through the layers of tissue unless measures are taken early to relieve pressure and increase local tissue perfusion (Box 27.1).
Box 27.1 NPUAP/EPUAP pressure injury classification system
(AWMA & NZWCS 2011b. Images from deWit (2005))
STAGE I PRESSURE INJURY: NON-BLANCHABLE ERYTHEMA
• Intact skin with non-blanchable redness of a localised area usually over a bony prominence.
• Darkly pigmented skin may not have visible blanching; its colour may differ from the surrounding area.
• The area may be painful, firm, soft, warmer or cooler compared to adjacent tissue.
• May be difficult to detect in individuals with dark skin tones.
• May indicate ‘at risk’ persons (a heralding sign of risk).
STAGE II PRESSURE INJURY: PARTIAL THICKNESS SKIN LOSS
• Partial thickness loss of dermis presenting as a shallow, open wound with a red-pink wound bed, without slough.
• May also present as an intact or open/ruptured serum-filled blister.
• Presents as a shiny or dry, shallow ulcer without slough or bruising (Note: bruising indicates suspected deep tissue injury).
• Stage II PI should not be used to describe skin tears, tape burns, perineal dermatitis, maceration or excoriation.
STAGE III PRESSURE INJURY: FULL THICKNESS SKIN LOSS
• Full thickness tissue loss. Subcutaneous fat may be visible but bone, tendon or muscle are not exposed. Slough may be present but does not obscure the depth of tissue loss. May include undermining and tunnelling.
• The depth of a stage III PI varies by anatomical location. The bridge of the nose, ear, occiput and malleolus do not have subcutaneous tissue and stage III PIs can be shallow. In contrast, areas of significant adiposity can develop extremely deep stage III PIs. Bone or tendon is not visible or directly palpable
STAGE IV PRESSURE INJURY: FULL THICKNESS TISSUE LOSS
• Full thickness tissue loss with exposed bone, tendon or muscle. Slough or eschar may be present on some parts of the wound bed.
• The depth of a stage IV pressure injury varies by anatomical location. The bridge of the nose, ear, occiput and malleolus do not have subcutaneous tissue and these PIs can be shallow. Stage IV PIs can extend into muscle and/or supporting structures (e.g. fascia, tendon or joint capsule) making osteomyelitis possible. Exposed bone or tendon is visible or directly palpable.
UNSTAGEABLE PRESSURE INJURY: DEPTH UNKNOWN
• Full thickness tissue loss in which the base of the PI is covered by slough (yellow, tan, grey, green or brown) and/or eschar (tan, brown or black) in the PI bed.
• Until enough slough/eschar is removed to expose the base of the PI, the true depth, and therefore the stage, cannot be determined. Stable (dry, adherent, intact without erythema or fluctuance) eschar on the heels serves as the body’s natural biological cover and should not be removed.
SUSPECTED DEEP TISSUE INJURY: DEPTH UNKNOWN
• Purple or maroon localised area or discoloured, intact skin or blood-filled blister due to damage of underlying soft tissue from pressure and/or shear. The area may be preceded by tissue that is painful, firm, mushy, boggy, warmer or cooler as compared to adjacent tissue.
• Deep tissue injury may be difficult to detect in individuals with dark skin tone.
• Evolution may include a thin blister over a dark wound bed. The PI may further evolve and become covered by thin eschar. Evolution may be rapid, exposing additional layers of tissue even with optimal treatment.
Stage I pressure injury: pressure injury presenting as intact skin with non-blanchable redness of a localised area usually over a bony prominence.
Stage II pressure injury: partial thickness loss of dermis presenting as a shallow, open wound with a red-pink wound bed, without slough.
Stage III pressure injury: pressure injury presenting as full thickness tissue loss in which subcutaneous fat may be visible but bone, tendon or muscle are not exposed.
Stage IV pressure injury: pressure injury presenting as full thickness tissue loss with exposed bone, tendon or muscle.
Unstageable pressure injury: depth unknown: Full thickness tissue loss in which the base of the pressure injury is covered by slough (yellow, tan, grey, green or brown) and/or eschar (tan, brown or black) in the pressure injury bed.
Suspected deep tissue injury: depth unknown: Purple or maroon localised area or discoloured, intact skin or blood-filled blister due to damage of underlying soft tissue from pressure and/or shear. The area may be preceded by tissue that is painful, firm, mushy, boggy, warmer or cooler as compared to adjacent tissue.
Intrinsic factors
Intrinsic factors are characteristics specific to an individual. These factors reduce the skin’s tolerance to pressure by affecting the vasculature, lymphatic system and supporting structures. Factors include age, mobility, circulation, nutrition, comorbidities and mobility. Chronic illnesses, particularly those affecting tissue perfusion and oxygen delivery (e.g. peripheral vascular disease) and sensation, also contribute to an increased risk of pressure injury development.
Age
People aged over 65 years are at greatest risk of pressure injuries with the risk increasing over 75 years of age (AWMA & NZWCS 2011b).
Nutritional status
Inadequate nutrition prevents efficient cellular growth and repair, and renders the tissues more vulnerable to damage. A poorly nourished person will usually have less protection over the bony prominences and be more vulnerable to the effects of prolonged pressure.
Body type
As the sites of maximum skin compression are located over bony prominences, a thin person is more at risk. Conversely, an obese person may be at risk due to shearing forces and added pressure caused by body weight pressing on the skin.
Mobility
Altered mobility and activity restricts an individual’s ability to redistribute pressure on weight-bearing areas.
Neurological factors
Reduced sensitivity to pressure or pain may occur in certain disease states, such as spinal cord injury, paralysis, multiple sclerosis or diabetes mellitus. Transmission of impulses from receptors in the skin or to the muscles may be impaired, so that the person does not receive the ‘message’ to change position regularly, a normal body defence mechanism. Other factors can also impact the nervous system such as the use of epidural analgesia, which reduces this protection mechanism (Angel et al 2004).
Vascular factors
Reduced peripheral tissue perfusion may result from certain disease states, such as atherosclerosis, cardiac failure or diabetes mellitus. As reduced local tissue oxygenation and nutrition are the primary cause of pressure injuries, people with vascular perfusion abnormalities are at increased risk.
Extrinsic factors
Extrinsic factors are those derived from the individual’s environment. The main identified factors are pressure (discussed above), shear, friction and moisture; all of these impact on the ability of the skin to tolerate pressure.
Shearing forces and friction
When pressure is applied to the skin at an angle, movement of the layers of the skin over one another may cause distortion of the tissue. The mechanical shearing forces involved distort and occlude the dermal capillaries, resulting in thrombosis and capillary occlusion, and tissue necrosis. Friction is also a mechanical force between the skin and another surface, such as bed linen, which creates resistance between the two surfaces. Friction immobilises the epidermis while the deeper layers move, and is an important factor in a client whose skin is fragile and at risk.
Moisture
Moisture on the skin can be from incontinence (urinary and faecal), wound exudate, perspiration, mucus or saliva. Prolonged exposure to moisture causes maceration, which weakens the connective fibres of the skin and alters the resilience of the epidermis to external forces (AWMA & NZWCS 2011b). Abrasions are more likely to form from friction between the skin and the surface under the individual, making shearing forces more severe. Actions such as excessive washing with soap, unsafe manual handling or rubbing can also damage the skin’s integrity. Incontinence of urine or liquid faeces can result in incontinence-associated dermatitis, which can be inaccurately described as a stage I or stage II pressure injury (Black 2011) and it is important to be able to clinically distinguish between pressure damage and incontinence-associated skin damage. Incontinence-associated skin damage creates an added risk of exposure to bacterial and faecal enzymes that alter the normal acidic pH of the skin and may lead to an increased risk of skin infection.
Pressure injury risk assessment
Assessing the potential of an individual for pressure injury development is a combination of the use of a recognised risk assessment tool, skin and nutritional assessment and clinical judgment. Although the use of a risk assessment tool can assist management and provide a structured approach to an individual’s care plan it should not be used in isolation. A head-to-toe assessment is made on admission to a facility, including those entering an emergency department, and at regular intervals throughout their stay. Any change in the individual’s medical and mental condition also requires reassessment.
Risk assessment
It is important to identify clients at risk of pressure injuries and various assessment tools have been designed to assist healthcare professionals. Implementation of a valid and reliable risk assessment tool is considered best practice and is used as a guide to identify an individual at risk of pressure injury development. The tools are intended as a preventive measure to facilitate the implementation of a rational preventive regimen. Several risk assessment tools have been developed with the common ones cited in the published literature being the Braden Scale for Predicting Pressure Sore Risk© (Braden Scale) (Table 27.3), the Norton Scale (Table 13.3) and the Waterlow Score. Most encompass a scale or scoring system and include clinical risk factors such as mobility, nutrition, mental condition, activity, incontinence, sensory perception, friction and shear. A cumulative score is reached which is used to determine the type of intervention measures to be utilised for ongoing care.
Nutritional assessment
Nutritional screening identifies malnutrition, dehydration and comorbidities and allows for appropriate interventions. In the elderly who may already have a poor diet, comorbidities (e.g. diabetes), malabsorption and infection can affect wound healing and nutritional screening can help to identify dietary deficits to guide management. The Mini Nutritional Assessment Short Form (MNA-SF) is an example of a validated nutritional assessment tool that can be used in the elderly population with pressure injuries (AWMA 2001c). Assessment includes: weight, height and BMI, food intake, dental and oral health, drugs that can interfere with appetite, swallowing difficulties and biochemical investigations to detect anaemia, haemoglobin and serum albumin levels. Individuals found to be at risk of nutritional deficit must be referred to a dietitian for assessment and other specialist healthcare practitioners (e.g. speech and occupational therapists) where appropriate (EPUAP & NPUAP 2009).
Skin assessment
Skin is assessed for erythema, blanching response, moisture damage, infection (e.g. fungal infection), oedema, induration (hardness), localised heat and any sign of loss of integrity (e.g. dry skin). It is important to include observation for pressure damage from medical devices such as oxygen tubing and drain tubes.
Psychosocial assessment
As part of a risk assessment tool mental or cognitive status is assessed as an impaired status can affect the individual’s ability to comply with treatment (e.g. identify when to change position, unplanned removal of devices due to confusion). Also assessed is their social history, previous disease experience, social support, individual preferences for the type of care to be received, culture and ethnicity, financial support, quality of life and goals of care (AWMA & NZWCS 2011b).
Wound assessment
Pressure injuries should be assessed by their size, depth, location and degree of tissue (and bony) necrosis. They are classified on the degree of tissue loss (see Box 27.1). The classifications are useful for documentation of the degree of injury and when considering treatment options, especially for debridement. Undermining and tunnelling are included as part of the assessment for stages III and IV pressure injuries when there is extension of the wound under the skin such that the wound cavity is larger than the skin opening. Documentation of the assessment findings and stage of pressure injury in the individual’s medical records is necessary to provide a comparative review with future assessments and to guide treatment interventions to assess progress of wound healing.
Prevention and management of pressure injuries
Consideration of psychosocial issues, pain, altered body image, and effects on activities of daily living are necessary to provide individual care.
Skin protection
Protection from pressure, friction and shear forces is identified as the most important measure to reduce the risk of pressure injuries. Appropriate positioning, use of pressure redistribution surfaces, skin hygiene and adequate nutrition all play a role. Local measures include appropriate manual handling techniques when transferring and changing the position of an individual (e.g. using transfer devices) that can lift the client off the bed to avoid friction and shearing. It is not advisable and cannot be recommended to massage or vigorously rub the individual’s skin as part of a pressure injury protocol and is contraindicated where there is ‘acute inflammation and where there is the possibility of damaged blood vessels or fragile skin’ (EPUAP & NPUAP 2009). Water-based and pH neutral skin cleansers and creams are suggested to maintain skin hydration. Barrier creams help to maintain hydration and prevent maceration.
Nutrition
Wound healing increases the energy demands on the body. The nutritional requirements of a stage I pressure injury will differ to those of a client with a stage IV injury and will be affected by the volume of exudate leaking from the wound. As the wound heals nutritional requirements will also change. Recently available Australian and New Zealand guidelines suggest increased protein intake (or the inclusion of protein building blocks such as arginine supplements in the diet) and inclusion of vitamin and mineral supplements to the individual’s usual diet (TTDWCG 2011). These guidelines also offer recommendations based on the probable risk of healing due to nutritional issues. If a person is unable to tolerate oral food or fluids, an alternative method of meeting nutritional needs, such as gastric tube or total parenteral nutrition (IV) feeding, may be necessary.
Support surfaces
Specialised support surfaces aim to reduce the interface pressure between the skin and the surface on which the individual is placed, such as the mattress on a bed, trolley, operating table or chair. Support surfaces work by increasing the amount of the body’s surface area in contact with the surface or alternating the contact with the surface. Using support surfaces does not remove the need for regular skin inspection and care and regular repositioning of the individual. Selection is based on mobility of the individual in the bed, comfort, the microclimate and the care setting. Various types of pressure redistribution devices are available and they fall into two main categories: constant low pressure (reactive) and active devices. Reactive devices can be powered (e.g. low air loss mattress/beds) or non-powered (e.g. foam/gel/air/combination mattresses). Mattresses should be checked for ‘bottoming out’. This means there must be at least 5 cm between the individual’s lowest bony prominence and the mattress when the individual is lying supine. Heel protection should place the heel completely off the bed and distribute weight along the calf avoiding additional pressure on the Achilles tendon. Synthetic sheepskins, fluid filled gloves, donut devices or cut-outs should be avoided as they shift the pressure to another body surface area.
Positioning
Positioning is an essential component of pressure injury prevention and care. Repositioning of the individual is designed to relieve or redistribute pressure points. It provides an opportunity to observe the skin condition and offer basic nursing care such as food or fluids and interaction with the individual. Repositioning regimens such as frequency, position and evaluation of the regimen must be documented in the individual’s medical records. When lying in bed, individuals should be placed in a 30 degree tilted side-lying position (right side, back, left side) or 30 degree inclined recumbent position. When head-of-bed elevation is required avoid a slouched position, which places extra pressure on the sacrum and coccyx. Use aids such as pillows to support the upper body and prevent the individual slipping towards the foot of the bed. An overhead handgrip may be used to lift and relieve pressure from the buttocks and sacral area. More frequent, smaller shifts in position may be necessary for some individuals intolerant of major changes (e.g. post surgery pain). The individual sitting in a chair is encouraged to lift up from the seat frequently while sitting, to relieve pressure and facilitate tissue perfusion in the buttocks and sacral area. The person is assisted in this action if unable to lift independently. Avoid positioning the individual on medical devices such as drainage tubes; these can create areas of localised pressure. Splints, plaster casts, braces and bandages should be checked regularly and adjusted to relieve pressure. Individual factors need to be considered to be effective in preventing prolonged pressure on any area. Using correct manual handling techniques can avoid shearing when repositioning an individual. The person must be moved safely avoiding dragging the skin along any surface, and surfaces such as bed linen must be kept smooth and dry. In healthcare, annual mandatory competency in use of aids and positioning is suggested to maintain best practice.
Wound care including infection management
Wound bed preparation is used as the tool to guide pressure injury wound care. Debridement of non-viable tissue after vascular assessment maximises the healing ability of the wound. In large pressure injuries therapies such as negative pressure wound therapy (NPWT) may be beneficial to reduce the size of the wound and manage large volumes of exudate. Local wound infection is managed aggressively to avoid spreading infection and speed the healing process. More information on this issue can be found in the wound management section of this chapter.
Specific pressure injury assessment tools such as the Pressure Ulcer Scale for Healing (PUSH Tool) (NPUAP 1998) monitor changes in a pressure injury. The PUSH Tool measures three parameters: size of the wound (length × width), exudate amount and tissue type (e.g. necrotic, slough, granulating, epithelialising). Similarly to a risk assessment tool, it gives a score for each parameter with the sum giving a total score. It is designed to provide a quick reference to whether the wound is healing.
Skin tears
Skin tears are injuries caused by shearing, friction or blunt trauma. The skin injury can present as a laceration or skin flap, with separation of epidermis and/or dermis. At highest risk are the elderly due to skin fragility as ageing takes place. Neonates are also at risk because of the immaturity of their skin. These events cause pain and distress to individuals and their families, and in the elderly can often be difficult to heal.
There appears to be no national and uniform language to describe and classify skin tears. The most commonly cited definition of a skin tear is that offered by Payne and Martin (Payne & Martin 1993), which states that a skin tear is:
A traumatic wound occurring principally on the extremities of older adults, as a result of friction alone or shearing and friction forces which separate the epidermis from the dermis (partial thickness wound) or which separate both the epidermis and the dermis from underlying structures (full thickness wound).
Prevalence
National figures on incidence and prevalence rates of skin tears are difficult to obtain, as there is no national reporting mechanism. These wounds often go unreported, especially in the community (Carville et al 2007; White 2001). In one Australian state survey skin tears were found to be the most prevalent wound in the aged care setting (16%), followed by pressure injuries (10%) (Mulligan et al 2009). Tears were the third most common wound type in the hospital setting, with over half of these being hospital acquired (Mulligan et al 2009). This correlates with a 16% incidence rate in a 120-bed aged care facility (White et al 1994). Another survey of forty-four aged care facilities found a prevalence rate of 54% (Edwards 1998). White found 64% of RNs working in high care residential aged care facilities reported more than one skin tear a week, with 29% indicating they became aware of between three and five skin tears a week (White 2001).
Skin elasticity and tensile strength decrease as ageing skin loses subcutaneous and dermal tissue and serum composition, which diminishes skin surface moisture.
Risk factors
Risk factors for skin tears in elderly are complicated by dehydration, poor nutrition and hydration, cognitive impairment, impaired vision or altered mobility and decreased sensation. Further identified risks include age >85; female; long-term steroid use, which thins the skin; a history of skin tears; stiffness and spasticity; dry, fragile skin; polypharmacy; presence of ecchymoses (bleeding into the subcutaneous tissue); dependence for activities of daily living such as use of assistive devices; applying and removing stockings; removal of tape; having blood drawn; transfers and falls; and cardiac, respiratory or vascular comorbidities. There are currently no validated risk assessment tools for skin tears.
Clinical Scenario Box 27.1
Mr Darcy, an 88-year-old man, was discharged from hospital following admission for a urinary tract infection. As he has no family, Mr Darcy was taken home by hospital transport and was escorted into his home where he lives alone, and placed in a lounge chair. Two days later, a nurse from hospital in the home did a follow-up visit on Mr Darcy. On arrival the nurse found Mr Darcy still sitting in the lounge chair; he had not moved from the chair since his arrival at home, 2 days prior. He had been incontinent of both urine and faeces. Mr Darcy made minimal eye contact and was not able to give coherent answers to questions.
While assisting Mr Darcy, the nurse noted a large lesion on his sacrum. Mr Darcy was transferred to an acute care facility where surgical debridement took place, identifying a stage IV pressure injury on his sacrum.
• What would be the recommendation for care for Mr Darcy?
• What other allied health professionals would you include in your care of Mr Darcy?
• What specialised wound dressing regimen will Mr Darcy require and what are the expected outcomes?
• Before Mr Darcy is discharged, what additional assessments will he require? Will you recommend that he is discharged back to his own home?
Classification for skin tears
Until recently there was only one recognised skin tear classification system, the Payne Martin Classification for Skin Tears (Payne & Martin 1990). This system was later revised to include sub-classifications (see Clinical Interest Box 27.13) of three main categories and five types of skin tears (Payne & Martin 1993).
CLINICAL INTEREST BOX 27.13 Payne & Martin skin tear classification system
Category I: Skin tears without tissue loss
Category II: Skin tears with partial tissue loss
Category III: Skin tears with complete tissue loss
(Payne RL, Martin ML. Defining and classifying skin tears: Need for a common language. Ostomy/Wound Management. 1993 Jun; 39(5):16–20, 22–4, 26. Used with permission from HMP Communications)
Recent Australian research has developed a new skin tear classification the system, the STAR skin tear classification system, based on consensus from Australian wound nurse experts (Carville et al 2007). This system uses five categories depending on the degree of skin loss (Fig 27.3).
Prevention
• Practise skin hygiene and responsible bathing: avoid the use of soap; use pH neutral cleansers
• Apply barrier creams or emollients to hydrate the skin and increase suppleness
• Where adhesive products are used consider the use of silicone-based adhesives or dressings that have a silicone wound contact layer
• Provide appropriate nutrition and hydration
• Use safe clothing that is easy to apply and remove and is protective
• Address environmental risk factors by padding surfaces, removing falls risks and providing uncluttered areas and adequate lighting
• Use correct manual handling techniques for turning, transferring and positioning
• Trim individual’s finger and toe nails and carer’s finger nails.
Assessment
Assessment of a skin tear is the same as for other wound types. The cause of the skin tear must be established if possible to implement preventive measures. Full client assessment, which includes comorbidities, client age, medical and surgical history, medications and general health status, will determine the potential for wound healing.
Other factors to consider are:
• Wound dimensions (length, width and depth)
• Size and depth of skin and skin flap
• Condition of the peri-wound skin, e.g. fragility
• Signs and symptoms of infection where the skin tear is more than a few days old.
The arms and lower leg are reported to be the most common location for skin tears (Payne & Martin 1993).
Management
There is no national consensus or guidelines in the management of skin tears to guide clinicians in their treatment of skin tears (Ratliff 2007; Stephen-Haynes & Carville 2011).
As for any other type of wound implement best practice wound management principles to promote healing. Essential are assessment (as discussed above), documentation, cleansing, moist wound healing principles, dressing selection and consideration of pain and potential infection.
Treatment regimens are best aimed at using low or non-adherent dressings which, when removed, will not strip the skin. Soft silicone or silicone-impregnated dressings are gentle to the skin and provide security to the skin flap. Foam dressings are also gentler to fragile skin and absorb exudate leaking from the wound. Skin sealants or protectants can be used to create a thin film between the skin and the dressing. Adhesive dressings such as film dressings and hydrocolloid dressings are generally discouraged because of the risk of further damage to the skin on removal.
Skin tears in the elderly do not respond well to suturing or staples, as the skin is too thin and fragile.
Steps to manage a skin tear
Where infection is a concern systemic antibiotics or a topical antimicrobial dressing, such as a silver-containing dressing, may be used to help manage infection.
Helpful tip: Applying a crepe bandage to secure a non-adherent dressing will reduce the risk of further injury from the use of tape and dressing removal and use of a silicone dressing will also reduce the risk of additional trauma to the skin tear on dressing removal.
LEG ULCERS
Chronic leg ulceration is a common and often recurrent condition, most commonly affecting the over-60 age group. Baker & Stacey (1994) found that 58% of leg ulcers had been present for more than 3 months and 24% for more than 1 year, indicating the chronicity of the problem. Leg ulceration is not a disease but rather a symptom of an underlying disease process. Leg ulcers decrease an individual’s quality of life, often causing severe discomfort, inconvenience and social isolation, as well as incurring loss of productivity in the workforce (Douglas 2001; Rich & McLachlan 2003). Non-compliance with treatment of venous leg ulcers is a common problem, which leads to a failure to heal and recurrence. However, there are many different types of leg ulcers and it is important to differentiate between them so that the correct management strategies can be followed. Leg ulcers caused by underlying venous disease are the most common type.
The cost of managing and treatment for leg ulcers on the Australian healthcare system is substantial, with the average cost in 2002 of $27 493 per wound (Santamaria et al 2002). This cost rises with the extended length of time taken to heal these wounds. Leg ulcers incur cost in hospital admissions and in the community nursing setting because of the need for frequent home visits (Johnson 2007).
Primary causes
Leg ulcers are classified according to the vasculature involved:
• Arterial, involving arteries and arterioles
• Venous, involving veins and venules
• Mixed arterial–venous, involving arteries, arterioles, veins and venules
Arterial leg ulcers
The underlying cause of pure arterial ulcers is a lack of arterial blood supply caused by peripheral arterial disease, though this usually presents a more complex problem with coexisting disease such as heart disease or diabetes. A history of cardiovascular disease, stroke or intermittent claudication may indicate arterial disease. Predisposing factors are atherosclerosis, advanced age, diabetes mellitus, hypertension, obesity and smoking. These ulcers are frequently located between toes or at the tip of the toes, over phalangeal heads, metatarsal heads or on the side of the sole of the foot and above the lateral malleolus. Arterial ulcers can present with a well-demarcated edge and a deep pale wound base. The ulcer may be painful unless diabetic neuropathy is present and the leg takes on a hairless, thin, shiny, appearance with dry skin. The toenails may be thickened and often brittle. The leg is pale when elevated and cool to touch. Pedal pulses are often diminished or absent.
Venous leg ulcers
Ulcers caused by underlying venous disease are referred to as venous ulcers. They can be defined as a ‘full thickness defect of the skin that persists due to venous disease of the lower leg’ (AWMA Guidelines 2011). Venous disease can occur for various reasons, a common cause being a deep vein thrombosis (DVT) that damages the valves or walls of the deep veins on the leg (post-thrombotic damage). Where the valves in the large veins (superficial and deep) are damaged they fail to close properly allowing venous blood to move up and down the vein. Blood is allowed to flow back down the leg (retrograde flow) and this is known as venous reflux. Additional pressure is created in the veins, and fails to fall when the combined actions of the calf muscle pump and foot pumps contract through walking. This is referred to as ambulatory venous hypertension. Over time the raised pressure in the veins causes fluid to leak into the tissues resulting in oedema (accumulation of fluid in extravascular tissue) and eventual skin ulceration.
These ulcers can take weeks or months to heal and accurate diagnosis of the underlying aetiology is essential to ensure correct management. Compression therapy using bandages or stockings is the accepted management option and has been shown to heal venous leg ulcers more rapidly compared to no compression (O’Meara et al 2009). Compression bandages or stockings aid venous return of blood to the heart (orthograde flow) and a reduction in venous reflux (see ‘Compression therapy for venous leg ulcers’ below).
In obtaining a diagnosis of venous disease the person may have a history of:
• Venous disease, confirmed by duplex ultrasound
• Varicose veins and/or surgery
• Family history of leg ulceration
Predisposing risk factors are:
Leg ulcers with underlying venous disease usually occur on the lower leg. They can be small in the pretibial area and anterior to the medial malleolus or larger and circumferential. These ulcers generally have uneven edges and a ruddy granulation tissue and appear to be superficial compared with the arterial ulcer. Discomfort is relieved by elevation of the leg. Venous ulcers are often accompanied by oedema, with fluid seepage and maceration of the surrounding skin. Pruritus and scale formation occurs. The leg can take on a reddish–brown pigmentation caused by haemosiderin, oedema is present and the leg can be quite firm to the touch. There is usually evidence of old healed ulcers, tortuous superficial veins and the limb may be warm to touch. Foot and leg pulses are present. Clinical Interest Box 27.14 outlines the difference between venous and arterial ulcers.
CLINICAL INTEREST BOX 27.14 Differentiating between venous and arterial ulcers
(Carville K., 2012. Wound Care Manual, 6E, Perth Silver Chain Nursing Association, Perth WA, p. 127)
| Indicator | Arterial | Venous |
|---|---|---|
| Predisposing factors | ||
| Associated changes in the leg and skin | ||
| Ulcer location | ||
| Ulcer characteristics | ||
| Pain | ||
| Surrounding area | May have neuropathy | Leaking exudate can cause maceration and excoriation |
| Foot/leg pulses | Diminished or absent foot | Usually normal |
Diagnostic tools for the assessment of leg ulcers
• Comprehensive medical and surgical history
• Physical examination including palpation of specific relative pulses
• Doppler ultrasound measurements—ankle/brachial pressure index (ABPI)*
• Photoplethysmography (PPG) measurement of venous refill
Arterial leg ulcer management strategies
The management of arterial leg ulcers is very different from the management of venous leg ulcers:
Compression therapy for venous leg ulcers
Compression therapy is indicated in the management of pure venous leg ulcers. The aim is to reduce the venous hypertension in the leg through compression of the veins using strong bandages or stockings. This will return extracellular fluid to the venous system, reducing limb oedema. Compression of the lower leg narrows the major veins reducing the local blood volume and redirecting blood to the heart. It also narrows the diameter of the vessels increasing the velocity of flow through the vein. Compression can be applied as bandages, stockings or pneumatic compression (dynamic compression). These systems compress the entire lower leg and are contraindicated for clients with poor arterial circulation, as this circulation can be further compromised. Pressure is determined by the tension (stiffness) of the bandage, the circumference of the limb and the width and number of layers applied (Thomas & Fram 2003). A pressure of 30–40 mmHg measured at the ankle aims to achieve a reduction in venous hypertension though it does not account for different limb sizes, bandage application techniques, posture and movement of the limb, condition of the bandage or stocking and skill of the bandager. The pressure may decline over a period of time and re-bandaging is required. Pressure beneath the bandage is measured in three main ways: working pressure (when the client is walking), resting pressure (at rest) and a third factor called the static stiffness index (SSI), which is the increase in pressure per centimetre increase in the circumference of the leg, or the difference in pressure between the supine and standing position (Partsch 2005a). A high SSI (above 10 mmHg) is considered a more inelastic compression system that produces a higher pressure when standing than when lying (Partsch 2005b).
Venous and mixed vessel disease ulcers are assessed for suitability to have compression therapy; the type and degree of pressure is determined upon completion of investigations and test. A medical practitioner or advanced practice nurse recommends the appropriate compression bandaging system to apply. An initial trial of bandaging is monitored for increased pain, discolouration of the toes, change of sensation (tingling or loss of sensation), increased oedema in the toes and client compliance. If a client is unable to tolerate compression bandages safely, lower compression levels can be applied to build tolerance. The type of bandage or stocking must also try to suit the lifestyle and ability of the client in self-management.
Compression bandages
Compression bandages are generally divided into elastic and inelastic bandages. This classification is suitable for a more powerful single layer elastic bandage but does not account for multilayer systems that use two or three layers of weak elastic bandages to achieve compression. Elastic bandages are highly extensible and accommodate changes in the shape of the limb with the contraction of the calf muscle when walking. This gives minimal changes in pressure beneath the bandage and sustained compression at rest. Inelastic bandages have minimal extensibility and greater fluctuations in pressure between walking and resting. Pressure is highest with walking as the expanded calf muscle pushes against the rigid bandage. Multilayer bandage systems of three or four layers combine inelastic and elastic bandages with a padding layer and/or crepe bandage where the components have different functions of protection, padding and retention. A Cochrane Review found multilayer compression systems (compression bandages) to be more effective than single layer systems (O’Meara et al 2009).
The application of compression bandages requires advanced skills and knowledge to ensure correct application. A normal shaped leg has a narrower ankle and wider calf circumference. Compression levels are graduated when a bandage is applied with constant tension and overlap, with a higher pressure achieved at the ankle due to its narrower circumference, reducing by approximately 50% when it reaches below the knee (tibial tuberosity).
Graduated compression stockings (GCS)
Graduated compression stockings have been shown to be as effective as multilayer compression systems (AWMA 2010). They are generally used for clients with healed ulcers to prevent recurrence. Regular use and replacement is necessary to maintain compression.
Pneumatic compression
Pneumatic compression is the application of an inflatable boot to the leg continuously, intermittently or in sequential cycles. It is used in the prevention of deep vein thrombosis, though there is some evidence to suggest it may play a role in the healing of venous leg ulcers.
Preventing recurrence
Venous leg ulcer recurrence may be prevented with the use of long-term compression at the strongest compression tolerated by the client (Nelson et al 2000) to increase compliance. In clients with superficial venous insufficiency surgery and compression may be more effective than compression on its own (Barwell et al 2004).
Client education and support
Leg ulcer education is centred on prevention of recurrence, chronic disease management and general health and lifestyle issues such as nutrition. In some areas leg ulcer clubs have been created to promote these aspects of care and to provide support and education about leg ulcers. These clubs also provide and allow ownership of the person’s wound and disease (Kapp et al 2010).
BURN INJURIES
Burns are considered one of the most traumatic injuries for a person to sustain. The physical trauma of a burn goes beyond the damage to the skin. A major burn can cause systemic complications in the short to medium term and physical and emotional complications in the medium to long term.
Dry heat, moist heat, chemicals, electricity or radiation can cause burn injuries. When a heat source comes in contact with the skin, the transfer of heat causes structural damage. Heat causes coagulation of the protein in tissue cells (cell damage starts at 41°C; protein coagulation at 50°C), and the depth of the burn is related to the amount of thermal energy dissipated in the skin, which is dependent on the temperature and duration of exposure.
After a burn injury, intravascular fluid is lost as the capillaries become more permeable. As the intravascular fluid volume decreases, the venous return and cardiac output fall. The clinical signs of ‘shock’ become apparent and tissue hypoxia threatens internal organs such as the kidneys. Other changes occur in organ function, in electrolyte levels and in metabolic function. Subsequently, complications of major burns can involve any body system because all systems are stressed during the injury and during healing.
Classification of burns
Burn injuries are classified according to the depth of tissue injury. Where burn injuries were classified by first, second and third degree the classifications now reflect the anatomical thickness of the skin layers: epidermal, superficial dermal, mid dermal, deep dermal and full thickness (Table 27.4).
Epidermal burns
In superficial burns only the epidermis is involved, with the basal layer of the epithelium remaining intact. The skin is dry, red (erythematous) and oedematous and the skin is hypersensitive. Superficial burns generally heal within 7 days without scarring and treatment is usually conservative to manage pain, maintain adequate hydration and moisturise the burned area.
Superficial dermal burns
Partial-thickness burns have damage to the epidermis and to the superficial dermis (papillary dermis). They may blister as serum is released from injured blood vessels and have a moist surface. They can be painful, blanch with pressure and have brisk capillary return indicating blood vessels are intact. If the blisters burst, the area beneath is pink, swollen and wet. When the superficial dermis has been injured, the raw area is resurfaced with epithelium growing from the undamaged walls of the sweat glands and hair follicles. Healing is usually complete within 14 days if there are no complications with minimal scarring, though there may be reduced pigmentation of the skin.
Deep partial thickness burns
Deep partial thickness burns (mid dermal burns) extend into the reticular dermis. These burns may blanch with pressure but capillary return is sluggish or absent as blood vessels are destroyed; there is also less leakage of serum. Blisters are not usually present and the burn surface is moist or dry. Deeper partial thickness burns result in diminished sensation, as the dermal nerve endings are destroyed and the burn can be painless. Healing is slower as more skin structures, such as hair follicles, are destroyed. These burn injuries may require early surgical excision of dead tissue and skin grafting to speed recovery to reduce the risk of infection and minimise scarring. These burns will take longer to heal (>21 days).
Deep dermal burns
Deep dermal burns have damage to all layers of the skin and the injury may extend to underlying fat, muscle and bone. Epidermal appendages are destroyed and the burn area is painless. The appearance of the burned area can vary and may be white, waxy, tan coloured, red or charred. The area is dry and feels hard. Because all layers of the skin are destroyed early surgical excision of dead tissue and skin grafting or covering with artificial skin is performed, to assist closure of the burn area. Scarring will occur and, depending on the anatomical region involved, there may be functional impairment. These burns also take longer to heal (>21 days).
Full-thickness burns
Burns of this depth have damage to all layers of the skin, the subcutaneous fat layer, muscle and often bone. The damaged tissue appears charred, firm and dry. There is no pain and limited or no movement of involved extremities. These burns may result from an electrical current or prolonged contact with flame; for example, an unconscious client unable to move from the source of the burn. They require surgical intervention for dead tissue removal and skin grafting. Scarring will occur to the damaged areas.
Assessing burn severity
An experienced burn clinician should assess burn injuries, especially burns that require admission to hospital. The severity of a burn is largely dependent on its size and depth. Assessing burn depth and size correctly is important as treatment pathways differ depending on the severity of the burn. Furthermore, the greater the severity, the greater the amount of fluid lost from the body, and the greater the degree of shock that ensues. To assess the size of a burn accurately, a chart is used on which the size is expressed as a percentage of the total body surface.
Two methods exist to calculate burn size: the ‘rule of nines’ (Fig 27.4) and the Lund–Browder method (Fig 27.5). The rule of nines divides the body into areas that equal 9% which, when totalled, equal 99% with the genitalia accounting for the remaining 1%. For example, the front and back of each leg is 9%, each arm is 4.5%; the upper and lower torso is 9%. This method is quick and easy though less accurate than the Lund–Browder method, which is more accurate as it allows for changes in body proportion with age. The numbers on each body portion on the chart indicate the percentage of body surface for that part. Both types of chart provide a means of determining the extent of the burn or the total body surface area (%TBSA). Areas of erythema are not included in the calculations.
Causes of burn injury
While there are many ways a burn injury can be sustained, for example, thermal, chemical, electrical, friction or abrasion, all burn injuries cause cell death and tissue ischaemia. The Jackson’s Burn Wound Model (Singh et al 2007) describes three areas of tissue injury: zone of coagulation (necrosis) nearest the source of injury, where there is irreversible tissue damage; zone of stasis (ischaemia), where there is damaged tissue and decreased circulation, but potentially viable tissue; and the zone of hyperaemia, in which there is oedema and reversible increased blood flow. Interventions that preserve tissue perfusion in the zones of stasis and hyperaemia potentially decrease burn wound progression or deepening tissue damage (necrosis). Where interventions are suboptimal there is the potential for a partial thickness burn to progress to a full thickness burn in the first 3 to 5 days, thus causing burn wound conversion and making burn depth assessment difficult.
Thermal burns
Flame and scald burns are the most common types of thermal injury (ANZBA nd) and are caused by three main mechanisms: conduction (coming into contact with hot objects e.g. hot fluids (scald burns)), radiation (e.g. heat lamp) and convection (e.g. explosion). Scald injuries account for over 55% of burns in children especially those aged 10 years or less (ANZBA nd).
Chemical burns
Chemical burns result from direct contact with the substance or through ingestion or inhalation of acids, alkalis or organic materials. The severity of the burn depends on the type and concentration (pH) of the agent and the duration of contact. Clients with chemical burns should have treatment commenced at the scene of the injury. Contaminated clothing should be removed and the area irrigated with clean water, especially burns to the eyes, which require a minimum of two litres of water, before transfer to hospital (Cinat & Smith 2006).
Electrical and lightning burns
Faulty electrical wiring, contact with overhead power lines and high voltage power cables can all be sources of electrical burns. The tissue damage from these burns can be underestimated due to the internal damage that results, as it is not always visible on admission and the skin can appear normal yet have muscular tissue destruction beneath it. There will be an exit wound as the electrical current has travelled through the body. Cardiopulmonary function must be assessed in these clients. Lightning burns can cause cardiac arrest and serious nerve damage.
Friction or abrasion burns
Friction burns are becoming more common, especially in children who injure themselves on exercise machines such as a treadmill. In adults friction burns result from car and motorbike accidents (road rash) (ANZBA nd).
Inhalation
There is a risk of inhalation injury in clients who have been in fires as there is exposure to smoke, especially in an enclosed area such as a house fire. Inhalation injury should also be suspected and assessed in clients with facial burns, singed nasal hairs and ash in the oropharynx. These burn injuries should always be referred to hospital for full assessment and monitoring.
Management of the client with burn injuries
Management is determined by the depth and size of the burn. Effective treatment must be in place in the first 24 hours to reduce the damaging effects of inflammation and to avoid burn wound conversion and deeper tissue injury and it begins with effective first aid. Smaller superficial burns may be managed in the outclient setting. Full thickness and sub-dermal burns should be hospitalised and managed in a specialist burn centre. Nursing Care Plan 27.1 provides an overview of nursing care for a client with a burn injury.
Nursing care plan 27.1 A client with a burn injury
Identification of issue
One of the many nursing diagnoses that needs to be considered by the nurse is disturbance in self-concept related to changes in appearance associated with tissue loss and scarring, alterations in motor and sensory function, dependence on others to meet basic needs, changes in usual lifestyle and roles and inability to participate in usual sexual activities
Short-term and long-term goals
The client will demonstrate beginning adaptation to changes in appearance, body functioning, lifestyle and roles, evidenced by the verbalisation of feelings of self-worth and sexual adequacy
The client will maintain relationships with significant others and have an active participation in activities of daily living
The client will take an active interest in personal appearance, have a willingness to participate in social activities and begin to plan a lifestyle to meet the restrictions imposed by the burn injury
Actions and rationale
The nurse will determine the meaning of the effects of the burn injury to the client by encouraging them to verbalise feelings and noting the non-verbal responses to the changes experienced
The nurse will assist the client to identify strengths and qualities that have a positive effect on self-concept
The nurse will implement measures to assist the client to cope with the effects of the burn injury by:
— Reinforcing the reason for wound-care procedures
— Not encouraging the use of a mirror until the client is ready
— Arranging for a visit by another burns client who has successfully adjusted to a similar burn injury
— Reducing fear and anxiety, reassuring the client regarding nightmares if they occur and reinforcing that these will gradually stop
— Including the client in all aspects of planning care and allowing choices when possible to increase the client’s control
— Providing diversional activities according to the client’s interests
— Clarifying misconceptions about future limitations on activity and sexual function
— Encouraging family and significant others to allow the client to do self-care as much as possible, as this assists in building self-esteem
— Assisting the client to be well informed, and communicating with all members of the multidisciplinary team
— Monitoring the client for signs and symptoms of depression and referring them to the appropriate discipline for assistance
First aid for burn injuries
Adequate and appropriate first aid is vital to the successful management of a burn injury. Applying cool running water for the first 20 minutes within the first 3 hours of a burn injury is considered to be best practice for a burn injury (Cuttle et al 2010). It can limit the extent of burn depth, the amount of pain and reduce the area of skin affected by the burn. Clients with larger burns need to be monitored for hypothermia if cool running water is applied for longer periods. Lotions, creams, butter, aloe vera, toothpaste and ice are all to be avoided as they either mask the full extent of the burn or can interfere with the ongoing management. There is no agreement on the type of burn management a burn injured client should receive prior to transfer and consultation should be with the treating burn surgeon.
When to refer to a specialist burn centre
The Australian and New Zealand Burn Association (ANZBA 2004) provides criteria for when a client needs to be referred for specialist treatment:
• Burns greater than 10% of total body surface area (TBSA)
• Burns of special areas—face, hands, feet, genitalia, perineum and major joints
• Full-thickness burns greater than 5% of TBSA
• Burns with an associated inhalation injury
• Circumferential burns of the limbs or chest
• Burns in the very young or very old, or pregnant women
• Burns in people with preexisting medical disorders that could complicate management, prolong recovery or increase mortality
Emergency department management
The aims of initial management are to minimise further tissue damage, stabilise the client, identify and assess further injuries and initiate fluid resuscitation. Assessment is made regarding the cause of the burn (flame, chemical, electrical), the time of injury, type of first aid given, tetanus history and loss of consciousness. Assessment of burn depth, size and location is then made. Burn management should not preclude resuscitation and emergency management of the client.
Hospital management
Once the client is stabilised management will depend on a range of factors with the main aspects of care being:
Fluid resuscitation
If the burned area is not too great (e.g. less than 15% in an adult, less than 10% in a child), the body generally compensates adequately for the fluid loss, and only the provision of extra oral fluids is indicated. In more extensive or severe burns, fluid loss is a major cause of shock, so fluid replacement is of prime importance. Fluid resuscitation rates are calculated from the time of injury and are based on the size and depth of the burn, age of the client and preexisting illnesses and other factors such as dehydration. The medical officer calculates the volume of fluid replacement required and prescribes the type of fluid to be administered. There are various resuscitation formulas; however, these should be used only as a guide to fluid replacement. Intravenous fluid administration is through peripheral and central access in large burns. Extensive full-thickness burns may also require a blood transfusion to compensate for a reduction in erythrocyte numbers. Intravenous fluid replacement with electrolytes is continually calculated and adjusted according to need. Clients with electrical and inhalation injuries have higher fluid requirements.
Monitoring techniques that may be performed to assess fluid requirements include:
• Insertion of a urinary catheter to enable hourly monitoring of urine output. Hydration aims at maintaining a urine output of 0.5–1.0 mL/kg/hour in an adult and 0.5–2.01 mL/kg/hour in a child (Cinat & Smith 2006; NSW Health 2008)
• Advanced monitoring methods including central venous pressure measurements, oxygen consumption and delivery, serum lactate, pulmonary artery catheter measurements and monitoring of base deficit (Cinat & Smith 2006).
Wound management
Burn wound management is aimed at preventing infection and providing early skin coverage with dressings or skin grafts. The wound can change in depth and appearance over the first few days and treatment plans should reflect these changes. Wound care consists of regular observation, assessment, cleansing and debridement. Showers and bedside cleansing are used to remove old dressings and loose necrotic skin. Occlusive dressings maintain a moist wound environment, enhance the healing process and reduce the risk of scarring as well as reducing exposure to contamination. These are suitable for superficial dermal burns. Deeper burns require an antimicrobial dressing to decrease microbial load and reduce the risk of infection. Moist wound healing produces better cosmetic outcomes with less scarring. Negative pressure wound therapy (NPWT), though used less frequently in burn wounds, is placed over skin grafts to improve graft take (Runkel et al 2010). Permanent and temporary skin covers are discussed below.
Infection prevention is a priority and the most serious threat to further tissue necrosis and wound sepsis. Methods of preventing infection include:
• Aseptic dressing techniques when applying wound dressings (disposable gowns, gloves, masks, handwashing)
• Topical antimicrobial dressings (silver dressings)
• Use of sterile linen on the bed
• Enhancing natural defences, e.g. by providing a high protein/kilojoule diet and treating any anaemia.
Escharotomies
Eschar is dead tissue which may present in circumferential full thickness burns. This development causes increased tissue pressure, which may impair circulation and lead to ischaemic necrosis. An escharotomy (release of skin and subcutaneous tissue) is a surgical procedure performed to release constriction of the eschar to prevent secondary ischaemic necrosis. A longitudinal incision is made through the eschar and is extended across joints. As the eschar does not contain nerve endings the procedure is painless, but is still performed under an anaesthetic. It results in open wounds, which provide a portal for infection.
Prevention of infection
The burn wound provides an ideal culture medium for a range of microorganisms. Preventing infection is achieved by preventing contamination of the wound and preventing invasion of microorganisms into the tissues and bloodstream. A specialised burns unit may be available where the air is filtered, the client is received into a bed prepared with sterile linen and isolation techniques are used to protect against infection. When a specialised unit is not available, the client is generally nursed in a single room using protective isolation techniques.
Debridement of dead tissue
In Australia it is now the accepted standard of care for partial and full thickness burn injuries to be treated by early surgical excision of dead tissue (within 72 hours of injury) to prepare the burn area for grafting and primary closure. Blood flow is markedly increased by day 4. The client must be stabilised and fully resuscitated. Early excision (in the first 24 hours) has been shown to: reduce blood loss in a paediatric population (Desai 1990); reduce mortality in clients without inhalation injury (reduces bacteraemia limiting sepsis and multi-organ failure); and reduce length of hospital stay (Ong et al 2006). It also removes the major route of infection. Serial operations may be required within the first several days.
Primary closure
Specialised dressings, skin closure with skin grafts or skin substitutes can all be used to achieve primary closure of a burn wound. All methods aim to achieve the same outcome of promoting healing, preventing infection and preventing scar development and contraction of scar tissue. Common methods of closure include skin grafting (split and full thickness), application of biological dressings (allografts, xenografts) or biosynthetic dressings.
Skin grafting
The ideal burn wound closure is with autograft; that is, using the client’s own skin. It is performed as the treatment of some deeper partial thickness burns and in the treatment of all full thickness burns. Skin grafting promotes healing, prevents infection and prevents the contraction of scars. It is performed as early as possible in the treatment of some partial thickness burns and in the treatment of all full thickness burns. Split skin grafting involves the removal of thin sheets of the epidermis and some dermis (split thickness skin graft) or all of the dermis (full thickness skin graft) from a donor site, often the thigh, buttocks or back, and placing the skin over the debrided burned area.
In extensive burns the sheets of skin can be ‘meshed’ to give a net-like appearance and increase the body surface area to be covered. A full-thickness graft includes the epidermis and the entire dermal layer, and is generally used when full-thickness skin loss has occurred.
Skin from a source other than the client’s own body may be used as a temporary cover for large burned areas, to decrease fluid loss. The donor site is generally covered with a non-adherent dressing, a topical antimicrobial to reduce the risk of infection and graft failure and layers of dressing material to absorb blood and serum that ooze from the wound.
To promote successful transplantation, the graft must be immobilised to prevent displacement. Immobilisation is achieved by one of several methods, such as suturing, and the graft area is covered for protection. Successful ‘take’ of the graft depends on many factors, including absence of infection in the area, prevention or elimination of haematomas or seromas and adequate blood circulation.
Skin substitutes
Common methods of temporary or permanent skin coverage are biological (e.g. allografts, xenograft) or biosynthetic skin substitutes (e.g. Biobrane, PELNAC, Integra, Apligraf). Skin substitutes provide early coverage, reduce the risk of wound contamination and infection and reduce pain and fluid loss. They increase the rate of healing and decrease scarring. They are typically used in full thickness burns and generally consist of a bi-layered structure of an outer epidermal structure and an inner more biologically active dermal layer. They may also deliver growth factors. Some require a secondary procedure with split thickness skin grafting. The outer layer peels off as the wound heals underneath them. Early separation of the outer layer from the regenerated skin indicates an infection or a deeper wound requiring surgical treatment. Some surgeons will place a layer of nanocrystalline silver dressing over the skin substitute to reduce the risk of infection and loss of the dressing.
Skin from a source other than the client’s own body (allograft (cadaver skin) or xenograft (porcine skin)) may be used as a temporary cover for large burned areas, to decrease fluid loss (see Clinical Interest Box 27.1).
Bioengineered skin substitutes (e.g. cultured epithelial autografts) involve new techniques in which sections of the individual’s unburned skin are grown in the laboratory, and later used to graft onto their burned areas.
Non-biological dressings
Superficial partial thickness burns may be managed with suitable dressings. This method of treatment has expanded over the last several years. The application of silver-containing dressings is used to prevent infection. Silver sulfadiazine (SSD) cream has been the most commonly used burn dressing; however, this treatment in the most part has been replaced by silver dressings (e.g. Acticoat™) that require less frequent changing, decreasing manipulation of the burn injury and graft site. These dressings are held in position with bandages and can remain in place for 3 to 7 days before being changed. Dressing changes are performed using strict aseptic precautions. The client is given an analgesic before the dressing change or may be given a general anaesthetic before the procedure is performed. After the dressings have been removed, the wounds are cleansed and further debridement is performed where necessary before the burns are redressed.
Providing adequate nutrition
In addition to the loss of erythrocytes and protein-rich serum, the hypermetabolic response of the body to a burn rapidly depletes stores of energy. The individual must be provided with adequate carbohydrates, protein, fats, minerals, vitamins and fluid. Energy and protein requirements are generally assessed using a formula, and oral dietary intake is often augmented by nasogastric feeding or parenteral therapy to meet these requirements. Accurate monitoring of tolerance to the diet enables continual assessment of nutritional requirements. Nursing responsibilities include adherence to feeding protocols, daily calorie counts, understanding the enteral formulas and regular communication with the dietitian.
Pain management
Superficial burn injury pain can be severe, as nerve endings are exposed. In deeper burn injuries the nerve endings are destroyed, and the person may say the burn does not hurt. Pain management is complex and challenging because the perception of pain has physical and psychological origins. Pain should be recognised from the client’s perspective and is the responsibility of the entire burn team. Inadequate pain management is distressing to the client, the client’s family and the burn team. Analgesic medications are commonly administered together with anxiolytic medications. Nursing measures also include explanation and assurance to the client to reduce fear, and careful positioning may be beneficial. The nurse has a responsibility to observe the client continually and to be directed by the client on the level of analgesia required. (More information on pain management is provided in Ch 31.)
Promoting mobility
Because of the nature of the injury, and its management, a person with burns may experience prolonged periods of immobility. Thus, the individual is subject to the complications of immobility, such as venous thrombosis, pneumonia, pressure injuries and contractures. (See Ch 26 for measures to manage problems associated with immobility.) Regular and careful position changes are essential. Clients must be positioned for comfort and repositioned frequently to prevent pressure injuries and contractures. Splints and other devices may be used to improve positioning and to prevent contractures. The client’s limbs should be correctly positioned to decrease joint flexion.
Physiotherapy is essential for a person who is immobile, and involves chest physiotherapy and range of movement (ROM) exercises. ROM exercises may be performed actively or passively, and the degree of motion should be recorded to document progress. Ambulation is started as soon as possible and the nurse may be required to assist. If skin grafting has been performed, the client will be required to increase ambulation slowly, as too rapid ambulation may damage new grafts.
Scar and contracture management
Current management of burn scars comprises a combination of therapies from non-operative interventions to surgery for contracture release. Pressure placed directly over the healed burn tissue is commenced as soon as the new epithelium can tolerate the pressure without causing friction. Clients with severe and extensive burns will have scarring and will normally have a tailored elastic compression garment applied. These garments are custom made to fit exactly over the burned area. Pressure garments are required to be worn between 6 months and 2 years after healing. Silicone gel sheets are also used to manage scar tissue. These can be worn under a pressure garment or on their own. Other treatments include prolonged stretching and splinting and mobilisation and massage of the scar. It is usual practice for non-operative methods to be applied before surgery is used. Surgery is required for contractures and where vital functional areas are exposed such as the eyelids.
Providing psychological support
As a burn injury can have a devastating emotional impact on the individual and their significant others, psychological support is an extremely important aspect of care. After the injury, the person will experience various emotions such as fear of death, fear of disability and fear of disfigurement. Feelings of despair, anger, frustration, depression and guilt may all be experienced at any time after the burn.
Communication between team members caring for the client is important to achieve adequate support for both them and their significant others. The nurse can help to provide support by:
• Developing a trusting relationship and being available to listen. The nurse should encourage the client to talk through their feelings and fears
• Providing information about all the procedures and equipment in a way that can be understood. Fear will be reduced if information is provided to the client and significant others in a way that facilitates understanding of the injury, the treatment and the prognosis. People who are experiencing stress often have difficulty in remembering, so information should be repeated whenever necessary
• Assisting the client to adapt gradually to body changes, and by being supportive when the client views their injury, particularly for the first time
• Supporting the client’s coping mechanisms for as long as necessary. The nurse should understand that any expressions of anger, aggression or intolerance are not being directed at the nurse personally. Rather, it is the person’s way of reacting to the injury and its consequences
• Involving the client in all aspects of care as much as possible, to promote independence and self-esteem.
Preparation for discharge
Before discharge from hospital, it is important that the client understands that the healed burned and grafted skin is very fragile and will break down more easily than normal skin. The client must be informed that these areas are very vulnerable to extremes of temperature, irritant substances and physical trauma. As part of discharge preparation the nurse should advise the client, or the parents of the young child, to:
• Avoid sunlight on the burned areas for at least 12 months. Burned and grafted skin tans unevenly and unpredictably and burns easily. Continuous use of sunscreen (SPF 30 or higher) is recommended.
• Use non-irritant soaps and cosmetics on the burn areas
• Exercise care if engaging in activities such as sports in which there is the possibility that the burned areas may be bumped or damaged
• Wear clothing made from soft fabrics that will not irritate the healed burned or grafted areas
• Continue with prescribed management of the areas. Management may include the continual wearing of a tailored compression garment, or application of a cream to the areas. The person may also be required to follow a planned program of exercises designed to improve mobility
• Seek appropriate help, such as psychological counselling, when there is difficulty in adapting to a changed physical appearance.
Complications of burns
In a severe burn all body systems become involved as the body attempts to maintain homeostasis and complications can involve any body system.
Shock
Shock is due to loss of fluid from the circulation. The greatest loss of fluid occurs at the site of the burn, but loss also occurs throughout the body. Substances such as kinins, released by the body in response to the injury, increase capillary permeability, allowing serum to escape into the tissues. This loss of serum causes reduced blood volume (hypovolaemia), which may lead to severe shock. As a result of hypovolaemia, cardiac output decreases, blood pressure falls and acute renal failure and death may follow.
Infection and graft failure
Infection may occur if microorganisms gain entry through areas where the skin has been damaged or destroyed. Although a burn wound is usually sterile initially, the area provides an ideal culture medium for a wide range of microorganisms. Necrotic tissue, oedema and transudate all provide a nutrient medium for bacteria. The individual’s resistance to infection is reduced by factors such as shock, anaemia and electrolyte imbalance.
Septicaemia
Septicaemia occurs when there is bacterial invasion of the tissues and bloodstream, and is a major cause of death in severely burned individuals. Septic shock is a form of shock that occurs in septicaemia, when endotoxins are released from bacteria in the bloodstream. The endotoxins cause decreased vascular resistance, resulting in a drastic fall in blood pressure. Pyrexia, tachycardia, rapid ventilations, confusion and coma may also occur.
Pulmonary problems
Pulmonary damage can occur from smoke, chemical inhalation or carbon monoxide intoxication. In the acute period, pulmonary oedema may develop, and later pulmonary complications include pneumonia. The cause of death in many burn injury clients is usually a pulmonary complication/s such as pneumonia (Cinat & Smith 2006).
Psychological effects
A person who has been severely burned is likely to experience depression, anxiety and difficulty in adjusting to the consequences of injury. The person’s significant others are likely to experience similar emotions. If a child has been burned, the parents are also likely to experience severe guilt feelings about the incident.
Contractures, hypertrophic and keloid scars
Scar formation is a normal part of wound healing and in linear wounds is minimal. In large surface area wounds scars can create problems. Contractures, hypertrophic scarring and keloid formation frequently result after healing of a burn injury. Contractures occur over joints and functional areas. These are characterised by their interference with normal function of any joint that they cross and deformity of mobile structures. True keloid scars tend to extend beyond the margin of the original scar and continue to grow, failing to undergo scar maturation and size reduction. They can also recur after excision. Hypertrophic scars mature and reduce in size. They tend to stay within the boundaries of the original wound and after excision will tend to be a better scar than the original.
SURGICAL WOUNDS
Care of a surgical wound is directed towards promoting healing and preventing infection. In the immediate postoperative period, assessing for haemorrhage is a major responsibility of the nurse. Both the dressing and the bed linen under the client should be checked for signs of haemorrhage. Any increase in blood staining on the dressing or an increase of blood in a drainage tube or bottle must be reported immediately to the nurse in charge. Strict asepsis is necessary when caring for surgical wounds and the nurse must follow the individual healthcare facility’s protocol.
Adequate oxygenation of the tissues is promoted by deep breathing and coughing exercises, and early ambulation to promote full lung expansion will enhance oxygenation of the blood. Adequate circulation of blood to the area should be promoted to transport all the substances required for healing and combating infection. Adequate blood volume can be maintained by ensuring sufficient fluid intake, and circulation can be stimulated by exercises and mobility.
Movement of the area should be restricted in the early stages of healing. If strain is placed on a wound, the newly formed granulation tissue may tear. The wound area should be stabilised, rested and supported.
Surgical wound management
To provide the conditions necessary for healing, the wound must be free of dead or infected tissue, protected against external agents which may delay healing and provided with a moist environment, which is conducive to healing.
Controversy exists about many aspects of wound care, such as the type of dressing applied, frequency of dressing changes, cleansing procedures and solutions used. Most healthcare facilities develop their own protocols and procedures for wound management and it is the nurse’s responsibility to know these procedures and be aware of the protocols and regulations relating to wound care.
Changing a wound dressing
If a dressing is to be changed, sterile equipment and aseptic techniques are used to prevent cross-infection. To prevent the spread of airborne microorganisms, activities in the room should be reduced to a minimum, windows and doors should be closed and conversation should be limited while the equipment and wound are exposed to the air. A suggested technique for performing a wound dressing is outlined in Procedural Guideline 27.1. The equipment required includes:
• A sterile dressing pack that consists of:
• Prescribed skin-cleansing solution
Procedural Guideline 27.1 Dressing a wound
| Review and carry out the standard steps for all nursing procedures/interventions |
| Action | Rationale |
|---|---|
| Explain the procedure | Reduces anxiety |
| Inspect the dressing | To determine dressing requirements |
| Ensure privacy and assist the client to assume an appropriate position, ensuring that only the wound area is exposed | Promotes comfort and facilitates performance of the procedure |
| Ensure adequate lighting | Facilitates observation of the wound |
| Wash and dry hands thoroughly | Prevents cross-infection |
| Assemble the required equipment, using aseptic technique | Prevents contamination of sterile items |
| Place the equipment in a convenient location near the bedside | Facilitates performance of the procedure |
| Perform hand hygiene, don gloves if required | Prevents cross-infection |
| Use forceps to remove any dressing from the wound and place it in the waste receptacle | Prevents cross-infection |
| Position dressing towels around the wound | Creates a sterile field |
| Observe the wound for union, signs of infection, exudate, infammation and healing | Evaluates condition of the wound, and stage of healing |
| Cleanse the wound in accordance with the practices of the healthcare facility, medical officer’s orders or as directed by the nurse in charge. Clean from a clean area towards a less clean area | Avoids transferring wound exudate and normal fora from the surrounding skin into the wound, and thus prevents wound contamination |
| Using forceps, apply a clean dressing over the wound and secure it in position. Or, if ordered, the wound may be left exposed to the air | Protects the wound from infection and irritation, e.g. from clothing |
| Remove gloves and towels and place them in an appropriate receptacle | Prevents cross-infection |
| Assist the individual to reassume a comfortable position | Promotes comfort |
| Remove and attend to the equipment appropriately. Perform hand hygiene | Prevents cross-infection |
| Report and document the procedure | Appropriate care can be planned and implemented |
Drainage tubes
At the time of operation, the surgeon may place a drain tube through a separate ‘stab’ incision near the principal wound. A drain promotes healing by providing an exit for blood, serum and debris that may otherwise accumulate and result in postoperative swelling, pain or infection. The type of drain inserted depends on the site and extent of the wound; a drain may be freestanding or attached to a drainage bag, intermittent suction or to a self-contained disposable drainage system that supplies its own suction.
When a free drainage is inserted, a sterile safety pin is usually passed through the tubing just above skin level to prevent the drain from slipping into the incision. A dressing is placed between the pin and the skin. Alternatively, the drain may be secured in position by one or two sutures. An absorbent dressing may be placed around the drain to collect exudate, or a pouch or bag may be applied to the surrounding skin for drainage collection. A closed-wound drain consists of tubing connected to a vacuum unit. Using aseptic technique, the container is emptied as necessary. Vacuum in the container is re-established each time the container is emptied. The unit is always positioned below wound level to promote drainage by gravity. The exit drain site can be protected by an absorbent or occlusive dressing.
Drain tube removal
The length of time a drain remains in position depends on several factors, such as the amount of drainage required. Before its removal, a drain may be rotated and shortened daily to prevent the body tissues adhering to it and also to promote healing. A suggested technique for rotating and shortening a drain tube is outlined in Procedural Guideline 27.2.
Procedural Guideline 27.2 Shortening a drain tube
| Review and carry out the standard steps for all nursing procedures/interventions |
| Action | Rationale |
|---|---|
| Follow the steps described in the guideline for dressing a wound, up to and including cleaning the wound | The stab wound is cleansed to remove exudate, thus preventing contamination |
| Using the stitch cutter, remove any suture securing the tube in the wound | Enables the tube to be rotated, if necessary, and shortened |
| If the tube is round, gently rotate it | Rotation of the tube frees any adherent granulation tissue |
| Withdraw the tube the prescribed length, e.g. 1.25 cm | Tube must only be shortened the prescribed length, to allow the wound to heal from within |
| Secure tube with sterile safety pin below level of planned cut | Prevents tube from slipping into the wound |
| Cut off excess tube | Prevents it pressing on the wound |
| Place and secure a clean dressing or pouch over the tube | Protects the skin from irritation from wound drainage |
| A gauze dressing is generally placed between the pin and the skin, and another dressing or pouch placed over the tube | Protects the skin from irritation |
| Remove and discard gloves and towels | Prevents cross-infection |
| Assist the client to reassume a comfortable position | Promotes comfort |
| Remove and attend to the equipment appropriately. Perform hand hygiene | Prevents cross-infection |
| Report and document the procedure | Appropriate care can be planned and implemented |
The nurse is required to perform this procedure only if it is within the scope of practice and if institutional policy permits. As this action may be quite painful, administration of a prescribed analgesic 30 minutes before the procedure is usually necessary. The equipment required includes:
• A sterile tray containing sterile:
• Artery forceps (if required)
Removal of a drain tube requires a similar technique to that for dressing a wound and shortening a drain tube. Any retaining suture is removed and the tube is withdrawn steadily and gently to minimise discomfort. If the tube is attached to a suction device, the nurse must ascertain whether suction is to be discontinued before removal of the tube. Generally, suction is discontinued to avoid damaging the tissues as the tube is being withdrawn. Check the healthcare facility’s protocol.
Sutures and clips
When surgery has been completed, the wound edges are approximated and held together by sutures, clips or staples. Generally a single line of sutures or clips is sufficient for closure. In some instances, tension sutures are also required to ensure closure and to provide additional support for the wound. Suturing methods include intermittent and continuous. With intermittent (interrupted) suturing the surgeon ties each individual suture. Continuous suturing is a series of sutures with only two knots: one at the beginning and one at the end of the suture line. The manner in which the suture crosses and penetrates the skin determines the method of removal. Figure 27.6 illustrates two methods of achieving skin closure.
When healing has progressed well, sutures, clips and staples are removed, usually within 7–10 days after insertion. The wound is checked for union of the edges before sutures or clips are removed. Sometimes alternate sutures or clips are removed one day, and the remainder are removed the following day. The most important principle in suture removal is never to pull the visible portion of a suture through underlying tissue, as pulling the exposed portion of the suture through tissues may lead to infection. The nurse may be required to perform this procedure if it is within the specified role and function, and if institutional policy permits. Sutures are generally removed using a disposable sterile stitch cutter, and staples or clips are removed using a sterile staple extractor. Figure 27.7 illustrates the technique of staple removal. A suggested technique for removing sutures or staples is outlined in Procedural Guideline 27.3. The equipment required includes:
Procedural Guideline 27.3 Removal of sutures and staples
| Review and carry out the standard steps for all nursing procedures/interventions |
Summary
The integumentary system consists of the skin and its appendages: the hair, nails, sweat and sebaceous glands. The skin is the largest organ of the body and is composed of two layers. The epidermis is the thin outermost layer composed of epithelial cells, many of which are keratinocytes. Keratin has waterproofing qualities and is also responsible for the formation of hair and nails. Melanin, which is produced by cells in the deeper layer of the epidermis, gives colour to the skin and protects the body against the damaging effects of ultraviolet rays in sunlight. The dermis, or deeper layer of the skin, contains blood vessels, nerve endings, hair follicles and hairs, arrector pili muscles, sebaceous glands and sweat glands. Nails and hair protect certain areas of the body, for example, nails protect the tips of toes and fingers, while hair protects areas such as the scalp. The major functions of the skin are protection, thermoregulation, sensory perception, excretion, immunity, synthesis of vitamin D and to act as a blood reservoir.
Surgical wounds are the result of intervention by the surgeon and can range from an uncomplicated suture line to a more complex wound requiring drainage tubes or skin grafts. A surgical wound must be treated with strict aseptic technique and the nurse must be cognisant of the individual health organisation’s protocols.
Pressure ulcers result from ischaemic hypoxia of the tissues due to prolonged pressure in an area of the body, usually over a bony prominence. Both intrinsic and extrinsic predisposing factors contribute to the development of a pressure ulcer. Pressure ulcers can be classified as stages I–IV, depending upon the amount of tissue damage. Risk assessment and preventive strategies that are employed by the nurse play an important role in ensuring that the incidence of pressure ulcers, which are largely predictable and preventable, is kept to a minimum. The nurse engages the multidisciplinary team to assist in the prevention of pressure ulcers within the vulnerable client population such as the elderly and frail.
Skin tears are a traumatic injury that can occur especially to the frail elderly population. Preventive strategies that pay particular attention to the environment and equipment used for caring for this vulnerable age group are important.
Leg ulcers are a costly and debilitating disorder and the nurse must be able to differentiate between the different types to employ the correct wound management method. The use of compression bandaging, which can be quite effective in the management of venous leg ulcers, is contraindicated and dangerous for the management of arterial leg ulcers.
Dry heat, moist heat, chemicals, electricity or radiation can cause a burn injury. The depth of tissue injury describes a burn and the size of the burn is estimated by using either the Lund–Browder method or the rule of nines. Complications include: shock, infection, scarring and contracture and adverse psychological effects. Medical and nursing management of a client with burn injury will vary according to the severity of the injury. The care of the individual involves fluid and electrolyte replacement, prevention of infection, provision of adequate nutrition, relief of pain, promotion of mobility, providing psychological support and promoting healing of the injury.
The effects that disorders of the skin have on the individual range from minor and temporary, to major and life threatening. Some serious skin disorders affect the individual to the extent that self-concept and body image are severely impaired. Disorders of the skin may have a single cause or may result from several interrelated factors, while for some disorders the cause is unknown. Tests used to diagnose skin disorders include direct examination, biopsy, microscopic examination and skin testing.
In all skin integrity deficits the psychological support for the individual is of major importance. Pain management and nutritional support are also vital. The correct method of wound care is essential and must be given with a clear understanding of the normal healing processes and the effect that certain pathologies have on those processes.
The website of the Australian Wound Management Association (www.awma.com.au) is a recommended resource for nurses wanting to enhance their knowledge, as it provides up-to-date information, links to other wound associations throughout the world and future wound management conferences. Membership of the Australian Wound Management Association entitles the member to receive a copy of the Australian wound care journal Wound Practice and Research.
1. Bruce, 77, is admitted to the acute-care hospital for a right total knee replacement. He has been fairly immobile for some time because of deterioration in his right knee. Bruce also has diabetes mellitus and is obese. Bruce’s postoperative recovery is marked by pain and subsequent wound infection. He experiences a pressure injury to his right heel. What measures could have been taken to prevent Bruce from developing a pressure injury?
2. What discharge instructions would you give a client about assessing for signs of wound infection?
3. What factors do you take into account in terms of selecting an appropriate dressing product for a given wound type?
4. After changing a client’s position, you observe redness over the bony prominences. What type of assessment must you perform to obtain correct information regarding pressure injury risk?
1. What are the major functions of skin?
2. What are the major factors that affect skin integrity?
3. Describe the four (4) stages of the wound-healing process.
4. What factors can affect wound healing?
5. State the benefits of moist wound healing.
6. What are the major manifestations of skin disorders?
7. What are the four (4) stages of a pressure injury?
8. What care is required to prevent and manage pressure injuries?
9. What care is required to prevent skin tears?
10. What is the difference between an arterial and a venous ulcer?
References and Recommended Reading
Angel D, Lloyd P, Carville K, et al. The clinical efficacy of two semi-quantitative wound-swabbing techniques in identifying the causative organism(s) in infected cutaneous wounds. International Wound Journal. 2011;8:176–185.
Angel D, Sieunarine K, Hunduma N, et al. Postoperative pressure ulcers in vascular clients after epidural analgesia: case reports. Primary Intention. 2004;12:34–38.
Asimus M, MacLellan, Li PI. Pressure ulcer prevention in Australia: the role of the nurse practitioner in changing practice and saving lives. International Wound Journal. 2011;8(5):508–513.
Australian and New Zealand Burn Association (ANZBA). Criteria for specialist burn treatment. Online. Available: www.anzba.org.au/index.php?option=com_content&view=article&id=51&Itemid=58, 2004.
Australian and New Zealand Burn Association (ANZBA). (nd) Bi-national Burns Registry Annual Report 2009–2010. Online. Available: www.med.monash.edu.au/assets/docs/sphpm/bi-nbr-annual-report.pdf.
Australian Wound Management Association. Clinical Practice Guidelines. West Leederville, WA: Cambridge Publishing, 2001.
Australian Wound Management Association. Clinical Practice Guidelines for the Prediction and Prevention of Pressure Ulcers. West Leederville WA: Cambridge Publishing, 2001.
Australian Wound Management Association. Standards for Wound Management. West Leederville, WA: Cambridge Publishing, 2001.
Australian Wound Management Association (AWMA) & New Zealand Wound Care Society (NZWCS) (2011a) Australian and New Zealand Clinical Practice Guideline for Prevention and Management of Venous Leg Ulcers
Australian Wound Management Association (AWMA), New Zealand Wound Care Society (NZWCS). Pan Pacific Clinical Practice Guideline for the Prevention and Management of Pressure Injury Draft for Consultation. Online. Available: www.awma.com.au/publications/2011_awma_pan_pacific_pig_draft.pdf, 2011.
Baker S, Stacey M. Epidemiology of chronic leg ulcers in Australia. Australian and New Zealand Journal of Surgery. 1994;64:258–261.
Bale S, Jones V. Wound Care Nursing: a Client-Centred Approach. Edinburgh: Mosby Elsevier, 2006.
Barwell J, Davies C, Deacon J, et al. Comparison of surgery and compression and compression alone in chronic venous ulceration (ESCHAR study): a randomised controlled trial. The Lancet. 2004;363:1854–1859.
Bates-Jensen BM. Pressure ulcer assessment and documentation: the pressure sore status tool. In: Krasner D, Kane D. Chronic Wound Care. 2nd edn. Wayne, NJ: Health Management Publications Inc; 1997:38.
Black JM, Gray M, Bliss DZ, et al. MASD part 2: incontinence-associated dermatitis and intertriginous dermatitis: a consensus. Journal of Wound Ostomy Continence Nursing. 2011;38(4):359–370.
Borgquist O, Gustafsson L, Ingemansson R, et al. Tissue ingrowth into foam but not into gauze during negative pressure wound therapy. Wounds. 2009;21:302–309.
Borgquist O, Ingemansson R, Malmsjö M. Micro- and macromechanical effects on the wound bed by negative pressure wound therapy using gauze and foam. Ann Plastic Surgery. 2010;64:789–793.
Brandeis GH, Berlowitz DR, Katz P. Are pressure ulcers preventable? A survey of experts. Advances in Skin & Wound Care: The Journal for Prevention and Healing. 2001;14(5):244–248.
Briggs M, Ferris FD, Glynn C, et al. Principles of best practice: Minimising pain at wound dressing-related procedures. A consensus document. London: MEP Ltd, 2004.
Campbell PE, Smith GS, Smith JM. Retrospective clinical evaluation of gauze-based negative pressure wound therapy. International Wound Journal. 2008;5:280–286.
Carville K. Wound Care Manual. Perth WA: 6E, Perth Silver Chain Nursing Association, 2012;127.
Carville K, Lewin G, Newall N, et al. STAR: a consensus for skin tear classification. Primary Intention. 2007;15(1):18–28.
Centers for Medicare and Medicaid Services. Hospital-acquired conditions (present on admission indicators). Online. Available: http://cms.gov/HospitalAcqCond/01_Overview.asp#TopofPage, 2012.
Cinat ME, Smith MM. Acute burn management. In: Sood R, Achauer BM. Sood’s Burn Surgery: Reconstruction and Rehabilitation. Philadelphia: Saunders; 2006:50–76.
Cowan T, ed. Wound Care Handbook 2011–2012, 4th edn., UK: Mark Allen Healthcare, 2011.
Crisp J, Taylor C. Potter & Perry’s Fundamentals of Nursing, 3rd edn., Sydney: Elsevier, 2009.
Cuddigan J, Ayello EA, Sussman C. National Pressure Ulcer Advisory Panel. Pressure Ulcers in America: Prevalence, Incidence, and Implications for the future. Reston, VA: NPUAP, 2002.
Cutting K, Harding KG. Criteria for identifying wound infection. Journal of Wound Care. 1994;3:198–201.
Cuttle L, Kempf M, Liu P-Y, et al. The optimal duration and delay of first aid treatment for deep partial thickness burn injuries. Burns. 2010;36:673–679.
deWit SC. Fundamental Concepts and Skills for Nursing, 2nd edn., Philadelphia: WB Saunders; 2005:278.
Desai MH, Herndon DN, Broemeling L, et al. Early burn wound excision significantly reduces blood loss. Annals of Surgery. 1990;211:753–762.
Douglas V. Living with a chronic leg ulcer: an insight into clients’ experiences and feelings. Journal of Wound Care. 2001;10:355–360.
Dunbar A, Bowers DM, Holderness H, Jr. Silicone net dressing as an adjunct with negative pressure wound therapy. Ostomy Wound Management. 2005;51(suppl 11A):21–22.
Edwards H, Gaskill D, Nash R. Treating skin tears in nursing home residents: A pilot study comparing four different types of dressings. International Journal of Nursing Practice. 1998;4:25–32.
European Pressure Ulcer Advisory Panel (EPUAP) & National Pressure Ulcer Advisory Panel (NPUAP). Prevention and treatment of pressure ulcers: a quick reference guide. Washington DC: National Pressure Ulcer Advisory Panel, 2009.
European Wound Management Association (EWMA). Position Document: Identifying criteria for wound infection. London: MEP Ltd, 2005.
Gardner SE, Frantz RA, Saltzman CL, et al. Diagnostic validity of three swab techniques for identifying chronic wound infection. Wound Repair and Regeneration. 2006;14:548–557.
Graves N, Birrell F, Whitby M. Effect of pressure ulcers on hospital length of stay. Infection Control and Hospital Epidemiology. 2005;26:293–297.
Han A, Zenilman JM, Melendez JH, et al. The importance of a multifaceted approach to characterising the microbial flora of chronic wounds. Wound Repair and Regeneration. 2011;19:532–541.
Harding K, Gray D, Timmons J, et al. Evolution or revolution? Adapting to complexity in wound management. International Wound Journal. 2007;4(Suppl. 2):1–12.
Henderson V, Timmons J, Hurd T, et al. NPWT in everyday practice made easy. Wounds International. 1(5), 2010. Online. Available: www.woundsinternational.com
Herlihy B, Maebus NK. The Human Body in Health and Illness, 2nd edn. St Louis: Elsevier Science, 2003.
Hess CT. Skin & Wound Care. Philadelphia: Lippincott Williams & Wilkins, 2008.
James GA, Swogger E, Wolcott R, et al. Biofilms in chronic wounds. Wound Repair and Regeneration. 2008;16:27–44.
Joanna Briggs Institute. Solutions, techniques and pressure for wound cleansing. Best Practice. 2006;10:1–4.
Johnson M. The prevalence of leg ulcers in older people: implications for community nursing. Public Health Nursing. 2007;12:269–275.
Kapp S, Miller C, Sayers V, et al. The LUPP: effectiveness of a multimedia client education package. Wound Practice and Research. 2010;18:80–90.
Kimble R. Tangential debridement. In: Granick MS, Gamelli RL. Surgical Wound Healing and Management. New York: Informa Healthcare; 2007:1–16.
Krasner D. Pressure ulcers: assessment, classification and management. In: Krasner D, Kane D. Chronic Wound Care. 2nd edn. Wayne, NJ: Health Management Publications Inc; 1997:152–157.
Loerakker S, Manders E, Strijkers GJ, et al. The effects of deformation, ischaemia, and reperfusion on the development of muscle damage during prolonged loading. Journal of Applied Physiology. 2011. Online. Available: http://jap.physiology.org/content/111/4/1168.abstract (doi:10.1152/japplphysiol.00389.2011)
Marieb EN. Essentials of Human Anatomy & Physiology, 8th edn. San Francisco: Pearson/Benjamin Cummings, 2006.
Mulligan S, Scott L, Prentice J, et al. WoundsWest Wound Prevalence Survey 2009 State-wide Report. Western Australia: Ambulatory Care Services, Department of Health, 2009. Online. Available: www.health.wa.gov.au/woundswest/docs/WWWPS_09_state_report.pdf
National Pressure Ulcer Advisory Panel. PUSH Tool 3.0. Online. Available. http://www.npuap.org, 1998.
National Pressure Ulcer Advisory Panel (NPUAP) & European Pressure Ulcer Advisory Panel (EPUAP). Prevention and Treatment of Pressure Ulcers: Clinical Practice Guideline. Washington, DC: NPUAP, 2009. Online. Available: http://www.npuap.org/pr2.htm
Nelson A, Bell-Syer SEM, Cullum NA, et al, Compression for preventing recurrence of venous ulcers. Cochrane Database of Systematic Reviews, 2000. doi:10.1002/14651858.CD002303 Issue 4. Art. No.: CD002303.
O’Meara S, Cullum NA, Nelson EA, Compression for venous leg ulcers. Cochrane Database of Systematic Reviews, 2009. doi:10.1002/14651858.CD000265.pub2. Issue 1. Art. No.: CD000265.
Ong YS, Samuel M, Song C. Meta-analysis of early excision of burns. Burns. 2006;32:145–150.
Partsch H. The static stiffness index: a simple method to assess the elastic property of compression material in vivo. Dermatological Surgery. 2005;31:625–630.
Partsch H. The use of pressure change on standing as a surrogate measure of the stiffness of a compression bandage. European Journal of Vascular Endovascular Surgery. 2005;30:415–421.
Payne R, Martin M. Defining and classifying skin tears: Need for a common language. A critique and revision of the Payne-Martin classification system for skin tears. Ostomy Wound Management. 1993;39:16–20.
Percival S, Bowler PG. Biofilms and their potential role in wound healing. Wounds. 2004;16:234–240.
Quality and Safety Branch, Department of Human Services Victoria. PUPPS 3––Pressure ulcer point prevalence survey. Statewide report 2006. Online. Available: www.health.vic.gov.au/pressureulcers/downloads/pupps3.pdf, 2006.
Ratliff CR, Fletcher KR. Skin tears: A review of the evidence to support prevention and treatment. Ostomy Wound Management. 2007;53:32–34. 36, 38–40
Rich A, McLachlan L. How living with a leg ulcer affects people’s daily life: a nurse-led study. Journal of Wound Care. 2003;12:51–54.
Runkel N, Krug E, Berg L, et al. International Expert Panel on Negative Pressure Wound Therapy (NPWT-EP). Evidence-based recommendations for the use of Negative Pressure Wound Therapy in traumatic wounds and reconstructive surgery: steps towards an international consensus. Injury. 2010;42(Suppl 1):S1–12.
Santamaria N, Austin D, Clayton L. A multisite clinical evaluation of Alfred/Medseed Wound Imaging System prototype. Wound Practice and Research. 2002;10:120–125.
Schultz GS. The physiology of wound bed preparation. In: Granick MS, Gamelli RL. Surgical Wound Healing and Management. New York: Informa Healthcare; 2007:1–16.
Schultz GS, Sibbald G, Falang V, et al. Wound bed preparation: a systematic approach to wound management. Wound Repair and Regeneration. 2003;11:1–28.
Shreve J, van Den Bos J, Gray T, et al. The economic measurement of medical errors. Milliman Inc., 2010. Online. Available: www.soa.org/files/pdf/research-econ-measurement.pdf
Singh V, Devgan L, Bhat S, et al. The pathogenesis of burn wound conversion. Annals of Plastic Surgery. 2007;59:109–115.
Stephen-Haynes J, Carville K. Skin tears made easy. Wounds International. 2(4), 2011. Online. Available: www.woundsinternational.com
Sussman C, Bates-Jensen B. Tools to measure wound healing. In: Sussman C, Bates-Jensen B. Wound care. A collaborative practice manual for physical therapists and nurses. Gaithersburg: Aspen Publishers; 1998:103.
Thomas S, Fram P. Laboratory-based evaluation of a compression-bandaging system. Nursing Times. 2003;99(40):24–28.
Trans Tasman Dietetic Wound Care Group (TTDWCG). Evidence based practice guidelines for the dietetic management of adults with pressure injuries. Online. Available: http://daa.asn.au/wp-content/uploads/2011/09/Trans-Tasman-Dietetic-Wound-Care-Group-Pressure-Injury-Guidelines-2011.pdf, 2011.
Trengove NJ, Stacey MC, MacAuley S, et al. Analysis of the acute and chronic wound environments: the role of proteases and their inhibitors. Wound Repair and Regeneration. 1999;7:442–452.
White MW, Karam S, Cowell B. Skin tears in frail elders: a practical approach to prevention. Geriatric Nursing. 1994;15:95–99.
White W. Skin tears: a descriptive study of the opinions, clinical practice and knowledge base of RNs caring for the aged in high care facilities. Primary Intention. 2001;9:138–149.
Wolcott RD, Rumbaugh DJ, James G, et al. Biofilm maturity studies indicates sharp debridement opens a time-dependent therapeutic window. Journal of Wound Care. 2010;19:320–328.
World Union of Wound Healing Societies (WUWHS). Principles of best practice: Wound infection in clinical practice. An international consensus. Online. Available: www.woundsinternational.com/clinical-guidelines/wound-infection-in-clinical-practice-an-international-consensus/page-1, 2008.
Wounds UK. Best Practice Statement: The use of topical antiseptic/antimicrobial agents in wound management, 2nd edn. Online. Available: www.wounds-uk.com, 2011.
Australian and New Zealand Burn Association (ANZBA), www.anzba.org.au/.
Department of Health, Victoria. Pressure Ulcers. Online. Available: www.health.vic.gov.au/pressureulcers/education.htm, 2012.
NSW Health. Burn Transfer Guidelines — NSW Severe Burn Injury Service, 2nd edn. Online. Available: www.health.nsw.gov.au/policies/gl/2008/pdf/GL2008_012.pdf, 2008.
The Alfred Hospital & the Royal Children’s Hospital (Victoria). (nd) Burns Management Guidelines. Online. Available: http://www.vicburns.org.au/about.html.