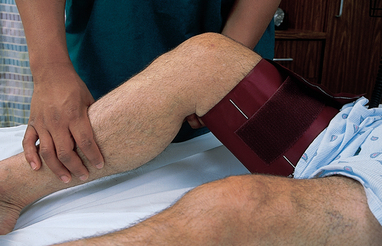
Body temperature
Physiology and regulation
The body temperature is the difference between the amount of heat produced by body processes and the amount of heat lost to the external environment:
Heat produced – Heat lost = Body temperature
Despite extremes in environmental conditions and physical activity, temperature-control mechanisms keep the body’s core temperature (temperature of the deep tissues) relatively constant (Figure 28-1). Surface temperature fluctuates depending on blood flow to the skin and the amount of heat lost to the external environment. While there is no single temperature that is ‘normal’ for all people, the body’s tissues and cells function best within a relatively narrow temperature range, the normal range being approximately 36.2–37.5°C (Gordon and Craft, 2011).
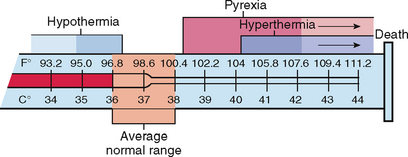
FIGURE 28-1 Ranges of normal temperature values and abnormal body temperature alterations.
From Potter PA, Perry AG 2013 Fundamentals of nursing, ed 8. St Louis, Mosby.
Nurses can use knowledge of temperature-control mechanisms to understand and promote temperature regulation. The balance between heat lost and heat produced, or thermoregulation, is precisely regulated by physiological and behavioural mechanisms.
Neural and vascular control
Body temperature is controlled in the anterior portion of the hypothalamus (preoptic area) at a set-point that is normal for each individual. This thermoregulatory centre is monitored by peripheral thermoreceptors in the skin and mucous membranes and central thermoreceptors in internal organs including the hypothalamus. A fall in environmental temperature activates heat-production mechanisms such as vasoconstriction of blood vessels to reduce blood flow to the skin and extremities. When vasoconstriction is ineffective in preventing additional heat loss, compensatory heat production is stimulated through voluntary muscle contraction and muscle shivering. Disease or trauma to the hypothalamus or to the spinal cord, which carries hypothalamic messages, can cause serious alterations in temperature control.
Heat production
Heat production within the body is the by-product of metabolism, skeletal muscle tone and contraction, and chemical thermogenesis. Metabolism refers to the chemical reactions in all body cells. Chemical reactions require energy in the form of adenosine triphosphate (ATP). Any ATP metabolic process results in the production of heat. Food is the main fuel source for metabolism; its energy is transferred to ATP which in turn transfers energy to body cells. The amount of energy used for metabolism is the metabolic rate. When metabolism increases, additional heat is produced and vice versa.
Heat loss
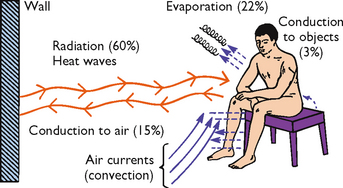
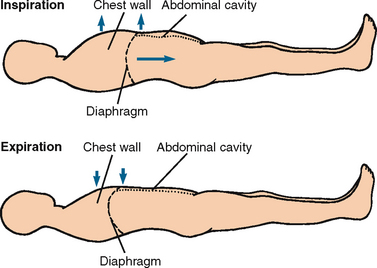
Heat loss and heat production occur simultaneously. The skin’s structure and exposure to the environment result in constant normal heat loss through radiation, conduction, convection and evaporation (see Figure 28-2). Diaphoresis is profuse sweating associated with an elevated body temperature, physical exertion, exposure to heat, and mental or emotional stress.
Behavioural control
Individuals voluntarily act to maintain a comfortable body temperature when exposed to temperature extremes. The ability of a person to control body temperature depends on the degree of temperature extreme, the person’s ability to sense feeling comfortable or uncomfortable, thought processes or emotions and the person’s mobility or ability to remove or add clothes. Body temperature control is difficult if any of these abilities are absent or lost. Infants can sense uncomfortable warm conditions, but need help to change their environment. Older adults may need help in detecting cold environments and minimising heat loss. Illness, a decreased level of consciousness or impaired thought processes all result in an inability to recognise the need to change behaviour for temperature control. When temperatures become extremely high or low, health-promoting behaviours such as removing or adding clothing have a limited effect on controlling temperature.
An essential aspect of the nurse’s role is the assessment and modification of variables that place patients at high risk of ineffective thermoregulation.
Factors affecting body temperature
Age
At birth the newborn leaves a warm, relatively constant environment and enters one in which temperatures fluctuate widely. Extra care is needed to protect the newborn because their temperature-control mechanisms are immature. A newborn loses up to 30% of body heat through the head and therefore needs to wear a cap to prevent heat loss. When protected from environmental extremes, the newborn’s body temperature is maintained within the range of 35.5–37.5°C.
The older adult has a narrower range of body temperatures than the younger adult. The average body temperature of older adults is approximately 36°C. Older adults are particularly susceptible to temperature extremes because of deterioration in control mechanisms, particularly poor vasomotor control (control of vasoconstriction and vasodilation), reduced amounts of subcutaneous tissue, reduced sweat gland activity and reduced metabolism.
Exercise
Muscle activity requires an increased blood supply and increases carbohydrate and fat breakdown. This increased metabolism increases heat production and thus body temperature. Prolonged strenuous exercise, such as long-distance running, can temporarily raise body temperatures up to 41°C.
Hormone level
Women generally experience greater fluctuations in body temperature than men. Menstrual cycle hormonal variations cause body temperature fluctuations. When progesterone levels are low, the body temperature is a few tenths of a degree below the baseline level; the lower temperature persists until ovulation occurs. During ovulation, greater amounts of progesterone raise the body temperature to previous baseline levels or higher. These variations can predict the most fertile time to achieve pregnancy.
Body temperature changes also occur in women during menopause (cessation of menstruation). Postmenopausal women may experience periods of intense body heat and sweating lasting from 30 seconds to 5 minutes with an intermittent increase in skin temperature of up to 4°C (hot flushes). This is due to the instability of the vasomotor controls for vasodilation and vasoconstriction (Brashers, 2006).
Circadian rhythm
Body temperature follows a circadian pattern, peaking late afternoon (1800–2200 hours) and falling early morning (0200–0400 hours). Interestingly, temperature patterns are not automatically reversed in people who work at night and sleep during the day; it takes 1–3 weeks for the cycle to reverse. In general, the circadian temperature rhythm does not change with age.
Stress
Physical and emotional stress increase body temperature through hormonal and neural stimulation. These physiological changes increase metabolism, which increases heat production.
Environment
Environment influences body temperature. If temperature is assessed in a very warm room, a person may be unable to regulate body temperature by heat-loss mechanisms, and the body temperature will be elevated. If the person has just been outside in the cold without warm clothing, body temperature may be low because of extensive radiant and conductive heat loss. Infants and older adults are most likely to be affected by environmental temperatures, because their thermoregulatory mechanisms are less efficient.
Fever
Fever (or pyrexia) occurs as a part of the inflammatory response caused by trauma, surgery, infection, immune responses and tissue damage. For example, in the immediate postoperative period it is common for a patient to develop a fever as part of the normal inflammatory response to surgery. During fever, white-blood-cell production is stimulated and there is a decrease in the concentration of iron in the blood plasma, suppressing bacterial growth. Fever also stimulates the production of antiviral interferon.
Fever is a host defence response, a febrile response, to invasion from exogenous pyrogens, including microbial pathogens such as bacteria, viruses, mycobacteria and fungi as well as non-microbial antigens such as inflammatory agents and drugs. During the febrile response, the set-point in the thermoregulatory centre is reset to maintain a higher level of body temperature. This higher temperature is maintained through increased heat production, especially through peripheral vasoconstriction and behavioural measures such as covering oneself with blankets in response to chills even though the body temperature is elevated.
The febrile response is a coordinated series of events to defend the body against these invading organisms through the intentional elevation of the body’s core temperature—a mean 1°C increase in body temperature (for that specific measurement site). For example, normal axillary temperature ranges from 34.7–37.4°C with a mean of 36.5°C; 1°C above the mean is 37.5°C. However, body temperature can be raised by excessive clothing, physical activity, hot weather and the digestion of food. Hyperthermia—passive heat gain greater than the body’s capability to dissipate heat—is distinct from fever and the two should not be confused. Temperatures accepted as representative of fever are (El-Radhi and others, 2009):
| ≥ 38.0°C | |
| ≥ 37.6°C | |
| ≥ 36.5°C | |
| ≥ 37.6°C. |
The widespread and continued prevalence of the febrile response in mammals, reptiles, amphibians and fish suggests that fever is an adaptive response, even though it places substantial demands on the body through increased metabolic demands. In humans and most mammals, fever has an upper limit ranging from 41°C to 42°C. When humans are in a thermoneutral environment, febrile rises in body temperature tend to range from 0.5°C to 3°C, with most infections producing fevers between 38.5°C and 40.5°C, and an average fever of 39.5°C.
Three phases of fever
The cold phase begins as the hypothalamic set-point is reset to a higher level (see Figure 28-3). This phase lasts approximately 10–40 minutes, during which all heat-producing mechanisms are activated and there is a rapid, steady rise in temperature. Heat production increases oxygen demands by 3–5 times normal resting levels, contributing to a hypermetabolic state. In this state there are associated increases in heart and respiratory rates and thirst. Vasoconstriction causes the skin to look pale with cyanotic nail beds, and to feel cool and dry. During this period, the person experiences chills and rigors, and feels cold even though the body temperature is rising.
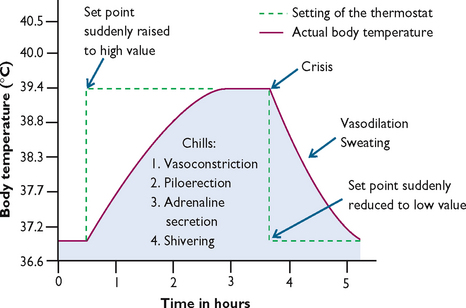
FIGURE 28-3 Effect of changing the set-point of the hypothalamic temperature control during a fever.
Modified from Guyton AC, Hall JE 2011 Textbook of medical physiology, ed 12. Philadelphia, Saunders.
During the hot phase the body has reached a new set-point and maintains the body temperature at this new higher temperature. The length of this phase depends on the time it takes to eradicate the pyrogenic cytokines responsible for the raised set-point. Higher temperatures in this phase are maintained through a balance in heat production and heat loss. Skin is flushed and warm and the individual feels hot. Basal metabolic rate remains high, so tachycardia and thirst continue. Other symptoms associated with this phase include drowsiness, headache, photophobia, reduced activity and appetite, feelings of weakness and/or restlessness and sometimes convulsions. This phase ends when the underlying cause of fever has been treated and/or eliminated by the body, resulting in a decrease in set-point to normal.
The defervescence phase or the ‘breaking’ of the fever occurs when there is a sudden decline in circulating pyrogenic cytokines and resetting of the hypothalamic set-point back to normal. Heat loss mechanisms take over and heat production is inhibited. The skin feels warm and is flushed due to vasodilation and sweating, which can exacerbate existing dehydration. Finally, the temperature returns to normal and the patient becomes afebrile.
Patterns of fever
In the past, patterns of fever were used to assist in diagnosis, although there is much less focus given to these patterns during febrile illnesses today. There are only a few diseases that show a specific pattern of fever, yet the same disease may also present as a different pattern. Today, diagnosis of febrile illnesses is based on laboratory investigations, enabling an accurate diagnosis even before a specific fever pattern emerges.
Benefits and costs of fever
Fever is beneficial in a normal healthy person in the home setting, but seriously ill children and adults can become severely compromised by the additional physiological strain of fever. For every 1°C above normal temperature, there are associated physiological changes. Metabolic, heart and respiratory rates increase by 13%, 20 beats per minute and 4–5 respirations per minute, respectively. There is an associated increase in oxygen consumption of 10–12% and an insensible fluid loss of 20%. Increased fluid loss associated with reduced intake leads to dehydration, the most common and dangerous side effect of fever. During the cold stage, blood pressure increases and glomerular filtration rates decrease; this reverses during the hot phase. Increased urine output assists in the removal of the additional metabolic wastes from the catabolic febrile state.
When fever is prolonged, the risk of dehydration increases and anorexia, secondary to generalised weakness and malaise, is common. Psychological effects include apathy, confusion, delirium and withdrawal from people and activities. These physiological and psychological effects of fever are important considerations for nurses and those caring for febrile children and adults. Fever should be reduced in those who are placed at risk due to the additional physiological burden from the febrile response. This includes children and adults who are seriously ill, and those who have cardio-respiratory, neurological or metabolic disorders or are malnourished, dehydrated or have epileptic lesions and may not tolerate the additional physiological demands during fever. In children, fever may trigger convulsions in those with a seizure disorder or a predisposition to febrile convulsions.
Febrile convulsions
Febrile convulsions were defined as:
a seizure in association with a febrile illness in the absence of a central nervous system (CNS) infection or acute electrolyte imbalance in children older than 1 month of age without prior afebrile seizures
by the International League Against Epilepsy (1993). Approximately 30–40% of children who have one febrile convulsion will have another.
Most febrile convulsions (75–85%) are simple, lasting less than 10 minutes. Complex febrile convulsions lasting longer than 15 minutes occur in 9% of cases. Simple febrile convulsions are brief (<15 minutes), bilateral, tonic–clonic seizures of short duration followed by a brief postictal period after which the child readily returns to their pre-morbid baseline state. Complex febrile convulsions are focal, unilateral or prolonged seizures lasting longer than 15 minutes or multiple convulsions within the same illness. Febrile convulsions longer than 30 minutes indicate febrile status epilepticus and occur in 5% of febrile convulsions. Large epidemiological studies have concluded that simple febrile convulsions are benign, common events in children without a history of afebrile convulsions or intracranial involvement associated with rectal temperatures above 38°C.
Febrile convulsions are precipitated by any febrile illness (e.g. otitis media, pneumonia, tonsillitis or influenza) or environmental factors that raise the body temperature. Immunisations such as diphtheria–pertussis–tetanus and measles are environmental precipitants of febrile convulsions. In children who have an environmentally precipitated febrile convulsion, 50% have a genetic predisposition to febrile convulsions.
Less than 2–5% of all children under the age of 5 years will have a febrile convulsion. Predictors of an initial febrile convulsion are a febrile convulsion in a first- or second-degree relative, neonatal discharge at 30 days or later, very pre-term birth, parental report of slow development, more febrile episodes per year, or attending day care. In children with a febrile illness, additional factors include the peak temperature during the illness and the underlying illness. For example, gastroenteritis has a lower risk for febrile convulsions than otitis media or other causes of fever.
No long-term effects have been identified in children aged 6–12 years following febrile convulsions. Large epidemiological studies report that measures to prevent additional febrile convulsions are unlikely to alter the long-term outcome of most children who have a febrile convulsion.
Management of febrile children
Fever does not always need to be treated, although it should be treated in those it places at risk of further complications. Fever has a role to play in supporting the body’s defence against invading pyrogens, although there is consensus that temperatures above 40°C should be avoided, as there are reduced immunological benefits at these temperatures (Walsh, 2008).
Appropriate management of febrile children includes careful observation of the child’s response to fever, preventing dehydration, supporting the febrile response and reducing distressing symptoms such as pain and discomfort with recommended doses of analgesics. Carers can determine the degree of illness from the child’s interactions with the environment. This is achieved through observing the child’s alertness, playfulness or irritability and consolability, in addition to physical observations such as petechiae, bulging fontanels, nasal flaring and response to stimuli. If a child is shivering and vasoconstricted in a warm environment, it is safe to assume their thermostatic set-point has been raised. If they become flushed and perspire, cooling mechanisms are functioning.
It is important for carers to be watchful when administering antipyretics to dehydrated or severely malnourished children and those with hepatic or renal impairment. In a febrile, irritable, uncomfortable child, analgesia is warranted. Antipyresis is warranted in children with underlying neurological or cardiopulmonary disease (Walsh, 2008). Parents learn to manage fever from healthcare professionals, drawing on their knowledge. This makes it imperative that healthcare professionals have current fever management knowledge based on the latest scientific evidence (see Box 28-2).
BOX 28-2 ADVICE FOR PARENTS WHEN CARING FOR A FEBRILE CHILD
• Mild to moderate fever is beneficial and supports the immune system.
• Observe the child: focus on the child’s wellbeing rather than temperature.
• Dress the child in light clothing.
• Encourage fluids: small, frequent drinks of clear liquid (e.g. water or diluted juice).
• Light blanket for children who are cold or shivering.
• Selectively reduce fevers with medications when fever is:
• Medication dosages for children up to 6 years:
• Do not continue giving regular medication for >48 hours without having the child assessed by a medical or nurse practitioner.
• Seek advice from your healthcare provider if there is no improvement in 48 hours, or if the child:
Adapted from Walsh A 2008 Fever management in children. Aust J Pharm 89(4):66–9.
Hyperthermia
Hyperthermia is an elevated body temperature related to the body’s inability to promote heat loss or reduce heat production. Whereas fever is an upward shift in the set-point, hyperthermia results from an overload of the body’s thermoregulatory mechanisms (Guyton and Hall, 2011). Any disease or trauma to the hypothalamus can impair heat-loss mechanisms. Malignant hyperthermia is a hereditary condition of uncontrolled heat production, occurring when susceptible people receive certain anaesthetic drugs.
Prolonged exposure to the sun or high environmental temperatures can overwhelm the body’s heat-loss mechanisms and depress hypothalamic function. These conditions cause heatstroke, a dangerous heat emergency with a high mortality rate. People at risk include the very young and very old; a diagnosis of cardiovascular disease, hypothyroidism, diabetes or alcoholism; people taking medications that decrease the body’s ability to lose heat (e.g. phenothiazines, anticholinergics, diuretics, amphetamines and beta-adrenergic receptor antagonists); and those who exercise or work strenuously (e.g. athletes, construction workers and farmers). Signs and symptoms of heatstroke include hot, dry skin, body temperature sometimes as high as 45°C, tachycardia, hypotension, giddiness, confusion, delirium, excessive thirst, nausea, muscle cramps, visual disturbances and even incontinence. Victims of heatstroke do not sweat because of severe electrolyte loss and hypothalamic malfunction. Heatstroke with a temperature greater than 40.5°C produces tissue damage to the cells of all body organs. The brain may be the first organ affected, because of its sensitivity to electrolyte imbalances. As the condition progresses, a person becomes unconscious with fixed, non-reactive pupils. Permanent neurological damage occurs unless cooling measures are rapidly started.
Hypothermia
Heat loss during prolonged exposure to cold overwhelms the body’s ability to produce heat, causing hypothermia. In hypothermia the body at first increases metabolic rate (to increase heat production), increases vasoconstriction (to decrease heat loss), shunts blood from the peripheral vascular bed to the core (to reduce heat loss) and increases shivering (to increase heat production). Hypothermia is classified by core temperature measurements (Box 28-3). Some people are more prone to hypothermia than others (Box 28-4). It can be caused unintentionally, such as by falling through the ice of a frozen lake. Hypothermia may also be intentionally induced during surgical procedures, to reduce metabolic demand and the body’s need for oxygen.
BOX 28-3 CLASSIFICATION OF HYPOTHERMIA
| Mild | 33.1-36°C |
| Moderate | 30.1-33°C |
| Severe | 27-30°C |
| Profound | < 27°C |
BOX 28-4 PATIENTS AT RISK OF HYPOTHERMIA
NEONATES
Can lose as much as 4.5°C immediately after delivery, as a result of heat evaporation. They also have a larger surface-to-mass ratio and a small amount of subcutaneous tissue.
OLDER PATIENTS
Often have a decrease in level of thyroxine and therefore a decreased ability to increase metabolic rate and heat production. Have a decreased ability in vasomotor response, including decreased ability to produce heat through shivering.
Hypothermia usually develops gradually and may go unnoticed for several hours. When skin temperature drops to 35°C, the person suffers uncontrolled shivering, loss of memory, depression and poor judgment. As the body temperature falls below 34.4°C, cyanosis occurs, and heart and respiratory rates and blood pressure fall. If hypothermia progresses, cardiac dysrhythmias, loss of consciousness and unresponsiveness to painful stimuli occurs. In cases of severe hypothermia, a person may demonstrate clinical signs similar to death (e.g. lack of response to stimuli and extremely slow respirations and pulse). The assessment of core temperature is critical when hypothermia is suspected. A special low-reading thermometer may be required, because standard devices often do not register below 35°C.
Frostbite occurs when the body is exposed to subnormal temperatures. Ice crystals forming inside the cell can result in permanent circulatory and tissue damage. Areas particularly susceptible to frostbite are the earlobes, tip of the nose, and fingers and toes. The injured area is white, waxy and firm to the touch, and sensation is lost in the affected area. Intervention includes gradual warming measures, analgesia, and protection of the injured tissue.
Sites for measuring body temperature
There are several sites for measuring core and surface body temperature. The non-invasive sites used most commonly for temperature measurement include the tympanic membrane, mouth and axilla (Box 28-5). The rectum is an invasive site, which is occasionally used.
Oral, axillary and skin temperature sites rely on effective blood circulation at the measurement site. The heat of the blood is conducted to the thermometer probe. Non-invasive chemically prepared thermometer patches can also be applied to the skin.
Tympanic temperature relies on the radiation of body heat to an infra-red sensor. Tympanic temperature is considered a core temperature because the tympanic membrane shares the same arterial blood supply as the hypothalamus.
The temperature obtained varies, depending on the site used, but should be between 36°C and 38°C. Research findings from numerous studies are contradictory; however, it is generally accepted that rectal temperatures are usually 0.5°C higher than oral temperatures, and axillary temperatures are usually 0.5°C lower than oral temperatures (Guyton and Hall, 2011). Each of the common temperature measurement sites has advantages and disadvantages (Box 28-6). The nurse chooses the safest and most accurate site for the patient (Skill 28-1). The same site should be used when repeated measurements are necessary.
BOX 28-6 ADVANTAGES AND DISADVANTAGES OF SELECT TEMPERATURE MEASUREMENT SITES
TYMPANIC MEMBRANE SENSOR
• Minimal patient repositioning required
• Very rapid measurement (2–5 seconds)
• Can be obtained without disturbing or waking patient
• Unaffected by oral intake of food, fluids, smoking
• Can be used for tachypnoeic patients
• More accurately reflects core temperature than any other method (Nimah and others, 2006).
ORAL
• Affected by ingestion of fluids or foods, smoke and oxygen delivery (Guyton and Hall, 2011)
• Should not be used with patients who have had oral surgery, trauma, history of epilepsy or shaking chills
• Should not be used with infants, small children or confused, unconscious or uncooperative patients
Guyton AC, Hall J 2011 Textbook of medical physiology, ed 12. Philadelphia, Saunders.; Lu S-H, Leasure A-R, Dai Y-T 2010 A systematic review of body temperature variations in older people. J Clin Nurs 19(1–2):4–16.; Nimah MM, Bshesh K, Callahan JD and others 2006 Infrared tympanic thermometry in comparison with other temperature measurement techniques in febrile children. Pediatr Crit Care Med 7(1):48–55.
SKILL 28-1 Measuring body temperature
EQUIPMENT
| STEPS | RATIONAL | |||
|---|---|---|---|---|
| 1. Assess for signs and symptoms of temperature alterations and for factors that influence body temperature. | Physical signs and symptoms may indicate abnormal temperature. Nurse can accurately assess nature of variations. | |||
| 2. Determine any previous activity that would interfere with accuracy of temperature measurement. When taking oral temperature, wait 15-20 min before measuring temperature orally if patient has smoked or ingested hot or cold liquids or foods. | Smoking or oral intake of food or fluids can cause false temperature readings in oral cavity. | |||
| 3. Determine appropriate temperature site and device for patient. | Chosen based on patient requirements, advantages and disadvantages of each site (see Box 28-6). | |||
| 4. Explain how temperature will be taken and importance of maintaining proper position until reading is completed. | ||||
| 5. Perform hand hygiene. | Reduces transmission of microorganisms. | |||
| 6. Help patient assume comfortable position that provides easy access to temperature site. | Ensures comfort and accuracy of temperature reading. | |||
| 7. Obtain temperature reading. | ||||
| A. Oral temperature measurement with electronic thermometer | ||||
| Use of oral probe cover, which can be removed without physical contact, minimises need to wear gloves. | ||||
| Charging provides battery power. Ejection button releases plastic probe cover from tip. | ||||
| Soft plastic cover will not break in patient’s mouth and prevents transmission of microorganisms between patients. | ||||
| Heat from superficial blood vessels in sublingual pocket produces temperature reading. With electronic thermometer, temperatures in right and left posterior sublingual pocket are significantly higher than in area under front of tongue. | ||||
| Maintains proper position of thermometer during recording. | ||||
| Probe must stay in place until signal occurs to ensure accurate reading. | ||||
| Reduces transmission of microorganisms. | ||||
| Protects probe from damage. Returning probe automatically causes digital reading to disappear. | ||||
| Reduces transmission of microorganisms. | ||||
| Maintains battery charge. | ||||
| B. Axillary temperature measurement with electronic thermometer | ||||
| Provides privacy. | ||||
| Provides easy access to axilla. | ||||
| Exposes axilla for correct thermometer probe placement. | ||||
| Charging provides battery power. Ejection button releases plastic cover from probe. | ||||
| Soft plastic cover prevents transmission of microorganisms between patients. | ||||
| Maintains proper position of probe against blood vessels in axilla. | ||||
| Probe must stay in place until signal occurs to ensure accurate reading. | ||||
| Reduces transmission of microorganisms. | ||||
| Protects probe from damage. Returning probe automatically causes digital reading to disappear. | ||||
| Restores comfort and promotes privacy. | ||||
| Reduces transmission of microorganisms. | ||||
| Maintains battery charge. | ||||
| C. Tympanic membrane temperature with electronic thermometer | ||||
| Ensures comfort and exposes auditory canal for accurate temperature measurement. | ||||
| Base provides battery power. Removal of handheld unit from base prepares it to measure temperature. Ejection button releases plastic probe cover from tip. | ||||
| Lens cover must be unimpeded by dust, fingerprints or earwax to ensure clear optical pathway. | ||||
| Correct positioning of the probe with respect to ear canal ensures accurate readings. | ||||
| The ear tug straightens the external auditory canal, allowing maximum exposure of the tympanic membrane. | ||||
| Depression of scan button causes infra-red energy to be detected. Otoscope tip must stay in place until signal occurs to ensure accurate reading. | ||||
| Reduces transmission of microorganisms. Automatically causes digital reading to disappear. | ||||
| Lens cover must be free of cerumen to maintain optical path. | ||||
| Protects sensory tip from damage. | ||||
| Restores comfort and sense of wellbeing. | ||||
| Reduces transmission of microorganisms. | ||||
| 8. Discuss findings with patient as needed. | Promotes participation in care and understanding of health status. | |||
| 9. If temperature is assessed for the first time, establish temperature as baseline if it is within normal range. | Used to compare future temperature measurements. | |||
| 10. Compare temperature reading with patient’s previous baseline and acceptable temperature range for patient’s age group. | Normal body temperature fluctuates within narrow range; comparison reveals presence of abnormality. Improper placement or movement of thermometer can cause inaccuracies. Second measurement confirms initial findings of abnormal body temperature. | |||
| RECORDING AND REPORTING | HOME CARE CONSIDERATIONS |
|---|---|
Thermometers
The types of thermometers used for determining body temperature are electronic and disposable. The nurse must be skilled in the use of the selected measurement device.
ELECTRONIC THERMOMETERS
The electronic thermometer consists of a rechargeable battery-powered display unit, a thin wire cord and a temperature-processing probe covered by a disposable plastic sheath (Figure 28-4). One form of electronic thermometer uses a pencil-like probe. Separate non-breakable probes are available for oral and rectal use. The oral probe can also be used for axillary temperature measurement. Within 20–50 seconds of insertion, a reading appears on the display unit. A sound signals when the peak temperature reading has been measured.
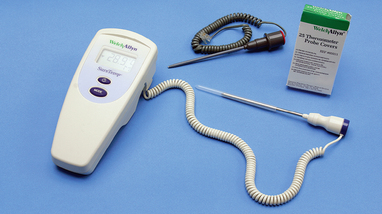
FIGURE 28-4 Electronic thermometer. Blue probe is for oral or axillary use. Red probe is for rectal use.
From Potter PA, Perry AG 2013 Fundamentals of nursing, ed 8. St Louis, Mosby.
Another form of electronic thermometer is used exclusively for tympanic temperature (Figure 28-5). An otoscope-like speculum with an infra-red sensor tip detects heat radiated from the tympanic membrane. The head is stabilised, the ear pulled straight back (children) or up and back (adults), and within 2–5 seconds of placement of the speculum in the auditory canal a reading appears on the display unit. A sound signals when the peak temperature reading has been measured.

FIGURE 28-5 Tympanic thermometer with probe cover being inserted into auditory canal.
Image: iStockphoto/tbradford.
The greatest advantages of electronic thermometers are ease of insertion, readings within seconds, being easy to read and the plastic sheath being unbreakable and ideal for children. Their expense is a major disadvantage.
DISPOSABLE THERMOMETERS
Disposable, single-use thermometers are thin strips of plastic with a temperature sensor at one end. The sensor consists of a matrix of dot-like indentations that contain chemicals which melt and change colour at different temperatures. They are used for oral or axillary temperatures, particularly with children (Figure 28-6). Single-use thermometers are inserted the same way as any oral or axillary thermometer. The thermometer is removed after 3 minutes and read after waiting about 10 seconds for the colour change to stabilise.
FIGURE 28-6 Disposable, single-use thermometer strip.
From Potter PA, Perry AG 2013 Fundamentals of nursing, ed 8. St Louis, Mosby.
Another form of disposable thermometer is a temperature-sensitive patch or tape. Applied to the forehead or abdomen, the patch changes colour at different temperatures. Both forms of disposable thermometers are useful for screening patients, especially infants, for altered temperature, and are not appropriate for monitoring temperature therapies.
Interpretation of results
Interpretation of temperature readings is based on norms, patient baseline temperature and clinical situation as well as the nurse’s knowledge of pathophysiology. An important part of the process of making a nursing diagnosis is identifying the cause or aetiology of the problem, as well as the defining characteristics relevant to the patient.
Nursing interventions
Prevention is the key for patients at risk of altered body temperature. The very old and the very young are most at risk of developing altered body temperature, because of a lack of ability to modify the environment in conjunction with ineffective thermoregulation mechanisms. Other people at risk include those who are debilitated by trauma, stroke, diabetes, drug or alcohol intoxication, sepsis, Raynaud’s disease and alterations in mental health status. People without adequate home heating, shelter, diet or clothing are also at risk.
Prevention involves educating patients, family members and friends. Nursing interventions for diagnoses of hyperthermia and hypothermia are directed towards promoting balance between heat production and heat loss. Level of activity, temperature of the environment and clothing are all considered. For example, avoiding strenuous exercise in hot, humid weather; drinking fluids such as water or clear fruit juices before, during and after exercise; wearing light, loose-fitting, light-coloured clothes; avoiding exercising in areas with poor ventilation; wearing a hat when outdoors; and gradual exposure to hot climates. The ability of an elderly patient or the mother of an infant to explain appropriate actions to take during hot weather is important in monitoring the elimination of a risk factor and in demonstrating the success of patient and family education.
Managing fever
The goal of fever management is to reduce heat production, increase heat loss and prevent complications (see Box 28-7). The procedures used to intervene and treat an elevated temperature depend on the fever’s cause, its adverse effects, its intensity and duration. Following consultation with the medical practitioner, the nurse may be required to obtain samples of urine, blood or sputum or wound-site specimens for culture and sensitivities to determine the cause of fever and direct treatment.
BOX 28-7 NURSING MEASURES FOR PATIENTS WITH A FEVER
ASSESSMENT
INTERVENTION (UNLESS CONTRAINDICATED)
• Obtain blood cultures if indicated. Blood specimens are obtained to coincide with temperature spikes when the antigen-producing organism is most prevalent.
• Initiate therapies to minimise heat production.
— Reduce the frequency of activities that increase oxygen demand such as excessive turning and ambulation.
• Initiate therapies to maximise heat loss;
• Initiate therapies to meet requirements of increased metabolic rate:
• Initiate therapies to promote patient comfort:
• Identify onset and duration of febrile episode phases.
Antipyretics such as paracetamol or non-steroidal anti-inflammatory drugs (NSAIDs, e.g. indomethacin) may be prescribed to reduce fever. Paracetamol increases heat loss by reducing the production of prostaglandins in the brain and spinal cord. Other medications that effect body temperature include corticosteroids. This group of medications acts within cells to prevent the release of chemicals that are normally involved in producing immune and allergic responses, resulting in inflammation. Consequently, they affect the body’s ability to fight infection and are not used to treat fever. It is important, however, to be aware of their effect in suppressing the immune system and masking the typical signs of infection.
Non-pharmacological therapy for fever includes methods that increase heat loss by evaporation, conduction, convection or radiation. Nursing measures are aimed at enhancing comfort, reducing metabolic demands and providing nutrients to meet increased energy needs (Box 28-7). Nursing measures to enhance body cooling must avoid the stimulation of shivering. Traditionally, nurses have used tepid sponge baths and cooling fans; however, use of these methods over antipyretic medications may lead to shivering. Shivering is counterproductive because of the heat produced by muscle activity. Vigorous shivering can increase energy expenditure up to 400% (Guyton and Hall, 2011). Shivering intensity ranges from palpable but not visible to violent extremity contractions.
Children and older adults are at risk of dehydration because they can quickly lose large amounts of fluids in proportion to their bodyweight. Prevention of dehydration is managed by accurate documentation of intake and output records, daily weighs and adequate fluid intake. Identifying preferred fluids and encouraging oral fluid intake is an important nursing intervention. It is recommended that antipyretics such as paracetamol in combination with tepid sponge baths is the most effective approach to reducing fever rather than using paracetamol alone (Joanna Briggs Institute, 2010). Care must be taken, however, not to induce shivering. Tepid sponging is not recommended for fever reduction in children (Joanna Briggs Institute, 2009).
Managing hypothermia
The priority treatment for hypothermia is to prevent a further decrease in body temperature. Wet clothes are removed and replaced with dry clothing, and the person is wrapped in blankets. When the patient reaches an emergency department, treatment depends on the severity of the condition. Warmed intravenous fluids, heating blankets, and warm fluids may be used. Patients are monitored closely for cardiac irregularities and electrolyte imbalances. In the acute care setting, patients undergoing surgery may become hypothermic as a consequence of the cooler operating room environment and the effects of the anaesthetic (Kurz, 2008). To avoid the complications associated with hypothermia, use of a warming system such as circulating water garments or forced-air warming are more effective than using extra blankets (Galvão and others, 2010).
Clinical decision making: body temperature
Knowledge of the physiology of body temperature regulation positions the nurse to assess the patient’s response to temperature alterations and to intervene safely and effectively. Independent measures can be implemented to increase or minimise heat loss, to promote heat conservation and to increase comfort. Many measures can also be taught to family members, parents of children or other caregivers.
If treatment is effective, body temperature will return to a normal range, other vital signs will stabilise and the patient will report a sense of comfort. The hypothermic patient will also demonstrate a temperature and other vital signs within the normal ranges and report feeling comfortable.
ELEVATED BODY TEMPERATURE
ASSESSMENT
Mr Coburn is a 45-year-old man being treated for pneumonia. His skin is warm and dry to touch. His face is flushed, and he appears to have laboured breathing. He reports that he smokes one packet of cigarettes per day and recently began expectorating yellow-green sputum. Vital signs obtained are: blood pressure 116/62 mmHg, radial pulse 98 beats per minute, regular and bounding; respiratory rate 26 breaths per minute; SpO2 98% on room air; tympanic temperature 39.2°C.
NURSING DIAGNOSIS: Altered body temperature (febrile) related to infectious process.
PLANNING
| GOALS | EXPECTED OUTCOMES |
|---|---|
| Patient will regain normal range of body temperature within next 24 hours. | Body temperature will decline at least 1°C within next 8 hours. |
| Patient will attain sense of comfort and rest within next 48 hours. | Patient will report increased satisfaction with rest and sleep pattern. |
| Patient will report increase in energy level within next 3 days. | |
| Fluid and electrolyte balance will be maintained during next 3 days. |
| INTERVENTIONS† | RATIONALE |
|---|---|
| Fever treatment | |
| Promotes heat loss through conduction and convection. | |
| Antipyretics reduce set point. | |
| Activity and stress increase metabolic rate, contributing to heat production. | |
| Fluids lost through insensible water loss require replacement. |
†Intervention classification labels from Bulechek GM, Butcher HK, McCloskey Dochterman J 2008 Nursing interventions classification (NIC), ed 5. St Louis, Mosby.
• CRITICAL THINKING
Marcia, aged 40 years, had a hysterectomy this morning. As you collect her vital signs this evening you note her body temperature is 37.8°C. Marcia asks you if this is normal. How would you respond? Explain the relevance of Marcia’s temperature. What additional assessment will you conduct?