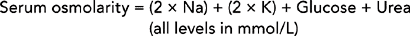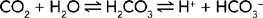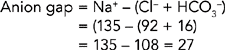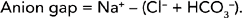Chapter 39 Fluid, electrolyte and acid–base balance
Mastery of content will enable you to:
• Use knowledge of the distribution, composition, movement and regulation of body fluids and electrolytes to discuss physiological mechanisms that maintain fluid balance in all body compartments.
• Develop a beginning understanding of the meaning of changes in laboratory results associated with common fluid, electrolyte and acid–base imbalances.
• Demonstrate an ability to apply theoretical concepts to clinical practice in the determination of appropriate screening, prescription and monitoring of intravenous (IV) fluids and electrolyte administration in patients in ambulatory and acute care settings.
• Discuss reasons for nursing interventions for patients with fluid, electrolyte and acid–base imbalances.
• Construct a plan of care (assessment, plan, implementation and evaluation) for maintaining fluid, electrolyte and acid–base balance:
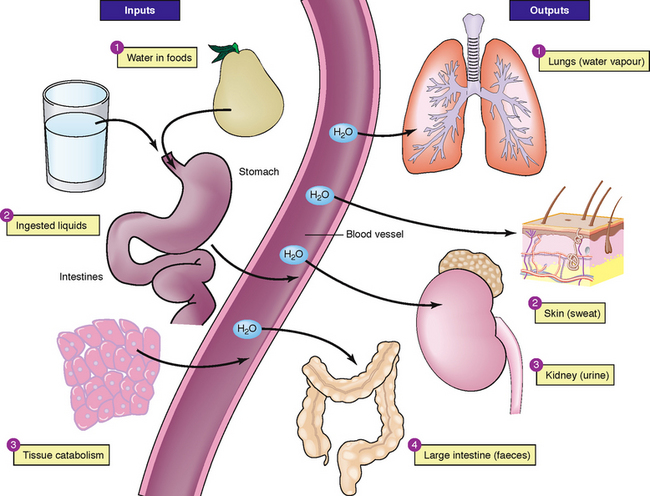
Fluid and electrolyte balance is where all compartments of the body contain the appropriate concentration of water and electrolytes. Nurses need to understand the distribution of body fluids, constituents of body fluids and processes used by the body to maintain fluid and electrolyte balance (Figure 39-1). Knowledge of these processes is essential in the early identification and understanding of factors contributing to fluid and electrolyte imbalances and in monitoring the effectiveness of appropriate management. Nurses must assume that each and every patient has a fluid and electrolyte imbalance until proven otherwise. Thus, the nurse must comprehensively assess fluid and electrolyte status for each and every patient and be able to articulate, at any time throughout the shift, the clinical indicators which prove the patient’s degree of fluid and electrolyte balance or imbalance.
Scientific knowledge base
Water is the largest single component of the body and a healthy, mobile, well-oriented adult can usually maintain normal fluid, electrolyte and acid–base balances because of the body’s adaptive physiological mechanisms.
Application of knowledge of fluid and electrolyte balance to practice
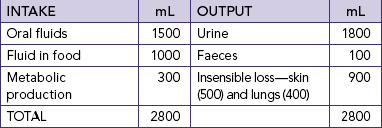
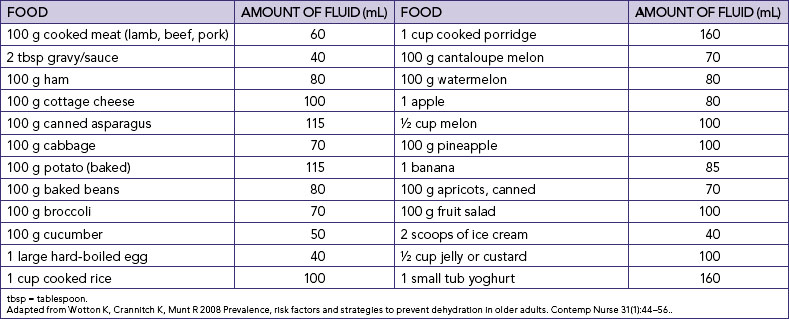
Nurses who are aware of their own fluid and electrolyte requirements and factors contributing to an imbalance in a range of different contexts will be better prepared to assess, identify and manage early indicators of fluid and electrolyte imbalance. For example, knowledge of average daily intake and output in healthy adults (Table 39-1) will assist in determining the amount of maintenance fluids required. The information in Table 39-1 can be used to demonstrate the risks to fluid and electrolyte balance for a person who decreases dietary intake and only consumes usual fluid intake. Students should also think about the impact on electrolyte balance if this person was only drinking water.
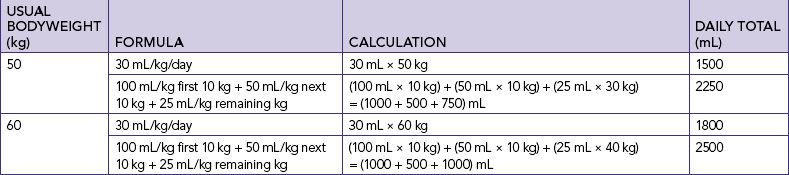
Nurses’ knowledge underpinning fluid and electrolyte balance needs to be more specific than described above. Many formulas exist for calculating the amount of fluid required per day. A general guide for the amount of fluid required in a healthy adult is 30 mL/kg/day (Eaton and others, 1999). If, for example, a student weighed 50 kg they would require 30 mL × 50 kg = 1500 mL fluid per day. There are also more-specific formulas for calculating the amount of fluid required per day. For example, fluid required per day for a healthy adult can be calculated as 100 mL/kg for the first 10 kg + 50 mL/kg for next 10 kg + 25 mL/kg for the remaining weight in kg (Austin, 1996). Table 39-2 demonstrates the difference between fluid requirements using the general formula and this more-specific formula for individuals weighing 50 kg and 60 kg.
Naturally, the daily fluid and electrolyte requirements depend on the person’s wellness, activity level and environmental conditions. Consider, for example, fluid and electrolyte loss and appropriate type and amount of fluid and electrolyte replacement for a person playing tennis in a temperature of 36°C, a bricklayer building an outside wall in summer in an environmental temperature of 39°C or a child with diarrhoea and vomiting with a temperature of 38.6°C.
As determining the appropriateness of urine output is important in the assessment of each of the situations described above, nurses should also be aware of the expected urine output based on gender and weight. The minimum urine output is the minimum amount of urine production required to ensure adequate renal function and to eliminate the body of waste products. A general rule is that urine output indicative of adequate renal function should be equal to, or more than, 30 mL/h. This is only a general guide and may lead to over- or under-estimation of the adequacy of urine output. A more specific formula for adequate urine output is 0.5 mL/kg for an adult male and 0.4 mL/kg for an adult female; infants and children weighing < 30 kg require 1 ml/kg/h. Thus, the expected urine output for a male weighing 65 kg is 34.5 mL/h and for a female weighing 65 kg is 26 mL/h.
Students should aim to develop the ability to use concepts covered in this chapter in developing knowledge of what is normal and why patients are at risk for fluid and electrolyte and acid–base imbalances, skills in comprehensive assessment, and knowledge of the appropriate fluid and electrolyte replacement therapy for different imbalances.
Distribution of body fluids
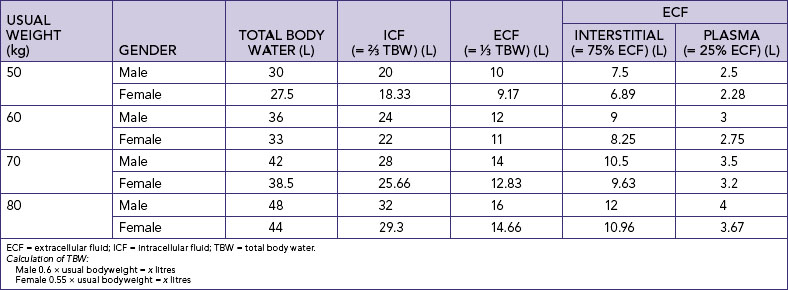
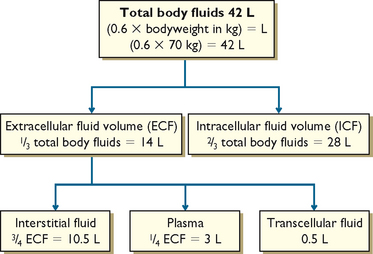
Total body water (TBW) is generally about 60% of bodyweight, with the following variations: 80% in newborns; 60% in adult males; 50% in adult females; and around 50% in obese people and 70% in lean people. Nurses need to understand these differences in usual TBW based on age, usual weight and gender. TBW calculations for an adult male are 0.6 × usual bodyweight = TBW (litres) and for an adult female 0.55 × usual bodyweight = TBW (litres) (Table 39-3).
Body fluids are distributed in two distinct compartments, one containing intracellular fluids and the other extracellular fluids (Figure 39-2). Almost two-thirds of TBW is in the intracellular compartment (intracellular fluid, ICF); the other third is extracellular (extracellular fluid, ECF). Various semipermeable cell membranes separate TBW into these and further compartments, allowing each compartment to function relatively independently (Patton and Thibodeau, 2010).
Intracellular fluid comprises all fluid within body cells. This fluid contains dissolved solutes essential to fluid and electrolyte balance and metabolism. In adult males, approximately 40% of bodyweight is ICF; in females it is 35% (Patton and Thibodeau, 2010). Extracellular fluid is all the fluid outside the cells. ECF is divided into two main smaller compartments: interstitial fluid and intravascular fluid. Interstitial fluid is the fluid between the cells and outside the blood vessels; intravascular fluid is blood plasma and lymph. Normally, about 25% of the ECF is in the intravascular compartment and the other 75% is interstitial fluid. Other extracellular fluids are transcellular fluid, cerebrospinal fluid (found in the ventricles of brain and surrounding the brain and spinal cord); synovial fluid (found inside synovial joint capsules); gastrointestinal tract fluids (saliva and gastric, pancreatic and intestinal juices); eye and ear fluids (vitreous and aqueous humour); pleural, pericardial and peritoneal fluids; and glomerular filtrate (Patton and Thibodeau, 2010).
Composition of body fluids
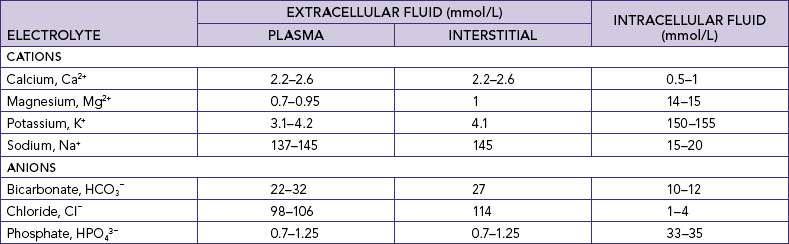
Body water contains substances that are sometimes called minerals or salts but are technically known as electrolytes. An electrolyte is an element or compound that, when dissolved in water, separates into ions and is able to carry an electrical current. An ion is an atom or molecule which possesses a positive or a negative charge. Positively charged ions are cations (sodium Na+, potassium K+, calcium Ca2+, magnesium Mg2+, iron Fe2+ and hydrogen H+). Negatively charged electrolytes are anions (chloride Cl−, bicarbonate HCO3−, sulfate SO42–, phosphate HPO43– and protein anions) (Table 39-4).
Electrolytes are measured in millimoles per litre (mmol/L). One mole (mol) is defined as the molecular (or atomic) weight of a substance, in grams. One millimole (mmol) is thus equal to one thousandth of a mole, in milligrams. In clinical practice, measurement of an amount of a substance is reported as the concentration of that substance in a particular volume; thus mass per unit of volume. The unit of measurement frequently used in the clinical setting is millimoles per litre (mmol/L). Millimoles per litre is a measure of the weight of a particular ion (the solute) dissolved in 1 L of a liquid (the solvent). The liquid formed by dissolving the solute in the solvent is called a solution.
Students should possess knowledge of the function of electrolyte anions and cations and their role in maintaining electrolyte balance in the human body in order to understand the patient’s maintenance and restorative dietary and fluid needs. If, for example, the patient has elevated levels of potassium (cation) then they are at risk for cardiac arrhythmias, whereas low potassium (cation) may result in paralysis. Decreased levels of calcium (cation) and magnesium (cation) may result in muscle spasms. Nurses must understand the reason why the consumption of just glucose or sodium solutions in individuals experiencing diarrhoea and vomiting will be insufficient to replace the electrolyte lost.
Electrolytes differ between ICF and ECF (Table 39-4). The body fluids and electrolytes in the ICF and ECF compartments are also in a constant state of mobility. Electrolyte distribution is a primary determinant of water concentration in a particular compartment.
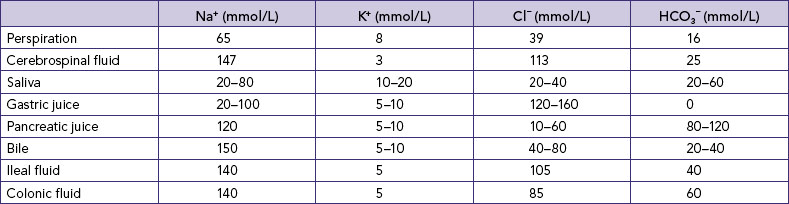
Knowledge of the electrolytes in different body fluids (Table 39-5) can assist nurses in identifying potential fluid and electrolyte imbalances when fluids are lost from particular sources, and therefore in understanding the appropriateness of the composition of fluids used to replace specific losses. Clearly there will be a difference in the electrolytes lost for a patient with a temperature of 39°C who is diaphoretic (major loss by perspiration), a patient who has an ileostomy (loss of ileal fluid) and a patient who is vomiting (loss of gastric juice)—and therefore a difference in the replacement fluids required.
• CRITICAL THINKING
Your next-door neighbour Mary calls you over to talk about what fluid she needs to give her 4-year-old child who has had acute diarrhoea with some vomiting for the past 24 hours. Respond to Mary’s question with a well-developed rationale based on your knowledge of fluid and electrolyte balance as to whether she should give either, both or none of the lemonade and Gatorade she has purchased.
Minerals (e.g. iron, zinc and magnesium; see Table 39-6) are constituents of all body tissues and fluids and are important in maintaining physiological processes. Minerals serve as cofactors in the thousands of enzyme-controlled body reactions; act as catalysts in nerve response, muscle contraction and metabolism of nutrients in foods; regulate electrolyte balance and hormone production; and strengthen skeletal structures (Patton and Thibodeau, 2010). Minerals do not work alone, but in balance with one another and with the metabolism of proteins, carbohydrates, fats and vitamins. An imbalance in one mineral can cause a chain reaction of deficiencies.
TABLE 39-6 MAJOR MINERALS AND THEIR FUNCTION
Movement of body fluids
Fluids and electrolytes constantly shift from compartment to compartment to facilitate body processes such as tissue oxygenation, acid–base balance and urine formation. Cell membranes separating the body fluid compartments are selectively permeable, allowing water to pass through them easily. Most ions and molecules pass through them more slowly. Fluids and solutes move across these membranes by four processes: osmosis, diffusion, filtration and active transport.
Osmosis
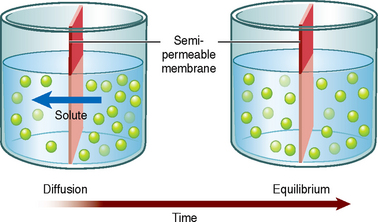
Osmosis is the net movement of water molecules through a selectively permeable membrane down a water potential gradient; the water molecules move from an area of high water potential (low solute concentration, called hypotonic) to an area of low water potential (high solute concentration, called hypertonic) (Figure 39-3) (Haynie, 2001). The membrane is permeable to the solvent (water) but is impermeable to the solute. The rate of osmosis depends on the concentration of the solutes in the solution, the temperature of the solution, the electrical charges of the solutes and the differences between the osmotic pressures exerted by the solutions. The concentration of a solution is measured in osmols, which reflect the amount of a substance in solution in the form of molecules, ions or both.
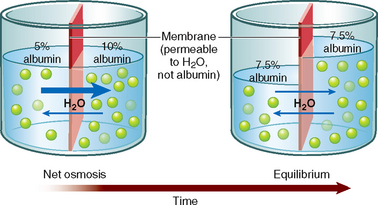
FIGURE 39-3 Osmosis through a semi-permeable membrane.
From Patton KT, Thibodeau GA 2010 Anatomy and physiology, ed 7. St Louis, Mosby.
Osmotic pressure is the drawing power for water in osmosis, and depends on the number of molecules in solution. If the concentration of the solute is greater on one side of the selectively permeable membrane, the rate of osmosis is quicker with a more rapid transfer of solvent across the membrane. This continues until equilibrium is reached. The osmotic pressure of a solution is called its osmolality, which is expressed in osmols per kilogram (Osm/kg) or milliosmols per kilogram (mOsm/kg) of the solution. The normal serum osmolality is 280–295 mOsm/kg.
Review abstract
Zinc is an essential trace element. It acts as a cofactor in numerous transcription factors and enzymes systems that augment auto-debridement and keratinocyte migration during wound repair. Zinc also assists the body to resist bacterial toxins. Zinc deficiency, whether caused by inadequate dietary intake or hereditary factors, can delay wound healing.
Oral zinc supplements may be beneficial in the treatment of patients with leg ulcers or slowly healing wounds. The therapeutic value of oral zinc supplements for faster healing of surgical wounds has not, however, been proven and requires further research. Topical application of zinc has been shown to be superior to oral zinc supplements in reduction of super-infections and necrotic material.
The action of topical zinc is thought to be from enhanced local defence mechanisms and increased collagenolytic activity. Sustained release of the zinc ions from topical applications is thought to stimulate the epithelialisation of wounds.
This review article suggests that topical zinc therapy is underappreciated even though clinical evidence emphasises its importance in autodebridement, anti-infective action and promotion of epithelialisation.
Osmolarity is the number of osmoles of solute in a litre of solution (Osm/L). Although the terms osmolality and osmolarity are often used interchangeably, the osmolality of a substance in serum is about 6% higher than its osmolarity because of proteins and lipids present in plasma. Debate exists on the accuracy of different calculations of serum osmolarity, but one which can be used at the bedside using the patient’s haematology results is (Erstad, 2003):
Table 39-7 shows the use of this formula for patients with normal, high and low osmolarity. Osmolarity is the measure used to evaluate serum and urine in clinical practice; if the ECF is too hypotonic, water will readily fill cells, increasing their volume and potentially lysing them (breaking down the cell wall, called cytolysis). As the osmolarity of ECF is approximately equal to that of ICF, serum osmolarity is a guide to intracellular osmolarity. Changes in extracellular osmolarity may result in changes in both ECF and ICF volume.
Solutions are classified as hypertonic, isotonic or hypotonic. A solution with the same osmolarity as blood plasma is called isotonic. A hypertonic solution (a solution of higher osmotic pressure) pulls fluid from cells; an isotonic solution (a solution of same osmotic pressure) expands the body’s fluid volume without causing a fluid shift from one compartment to another; and a hypotonic solution (a solution of lower osmotic pressure) moves fluid into the cells, causing them to enlarge (Figure 39-4). Each of these actions occurs through osmosis.
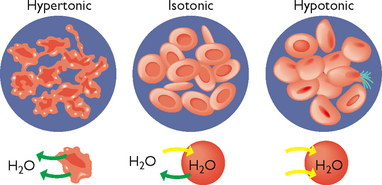
FIGURE 39-4 Hypertonic, isotonic and hypotonic solutions and their effect on cells.
From LadyofHats, Wikimedia Commons http://commons.wikimedia.org/wiki/File:Osmotic_pressure_on_blood_cells_diagram.svg
The process of osmosis within the body may be better understood by examining what would happen if a human cell was placed in a hypotonic, isotonic or hypertonic salt solution (Table 39-8).
TABLE 39-8 OSMOTIC RESPONSE TO HYPOTONIC, ISOTONIC AND HYPERTONIC SALINE SOLUTIONS
| SALT SOLUTION IN WHICH THE CELL IS PLACED | DEFINITION | OSMOTIC RESPONSE |
|---|---|---|
| Hypotonic saline solution | Dilute solution, with a higher water concentration than the cell | Cell will gain water through osmosis |
| Isotonic saline solution | Solution with exactly the same water concentration as the cell | No net movement of water across the cell membrane |
| Hypertonic saline solution | Concentrated solution, with a lower water concentration than the cell | Cell will lose water by osmosis |
The osmotic pressure of the blood is affected by plasma proteins, especially albumin. Albumin exerts colloid osmotic or oncotic pressure, which tends to keep fluid in the intravascular compartment. Knowledge of the actions of colloid osmotic pressure is essential if nurses are to understand situations causing decreases in osmotic pressure and reasons for the use of intravenous (IV) colloids and crystalloids. Where patients lose plasma (plasma proteins), for example in burns or ascites, there will be insufficient osmotic pressure to maintain fluid in the intravascular compartment. The resultant increase in plasma proteins in the interstitial compartment will continue to draw fluid away from the intravascular compartment. Thus, although there are sufficient plasma proteins in the body they are in the incorrect compartment; ultimately, failure to correct insufficient circulating plasma proteins will result in circulatory collapse.
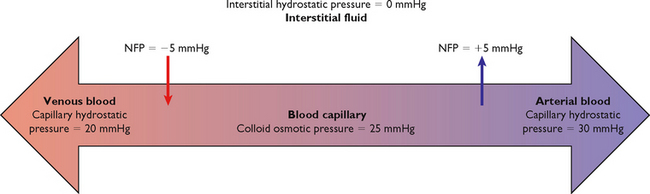
The net filtration pressure (NFP) is the difference between the hydrostatic pressure (pressure exerted by the fluid) and the oncotic pressure. Fluid leaves the capillary and enters the interstitial space when hydrostatic pressure is greater than oncotic. Fluid enters the capillary from the interstitial space when hydrostatic pressure is less than oncotic.
Fluid moves out of the arterial end of the capillary into the interstitial space because of a positive NFP of +5 mmHg, the difference between the hydrostatic pressure (30 mmHg) and the oncotic pressure (25 mmHg). Thus with this dominant hydrostatic pressure, fluid and diffusible solutes move out of the capillary into the interstitial space, and thus flow out of the circulation (Figure 39-5).
Fluid moves out of the interstitial space into the venous end of the capillary because of a negative NFP of –5 mmHg, the difference between the hydrostatic pressure (20 mmHg) and the oncotic pressure (25 mmHg). Oncotic pressure therefore dominates at the venous end of the capillary, and fluid and diffusible solutes move into the capillary from the interstitial space (Figure 39-5). The excess fluid and solutes remaining in the interstitial space are returned to the intravascular compartment by the lymph channels (Patton and Thibodeau, 2010).
A patient who has had a breast and lymph nodes removed because of breast cancer is at risk for the development of lymphoedema because of decreased return of proteins to the circulation (via the lymph) from the interstitial space. This results in increased fluid and proteins (increased oncotic pressure) in the interstitial space and the consequent development of localised oedema.
Diffusion
Diffusion is the movement of a solute (which can be a dissolved gas) in a solution across a semipermeable membrane from an area of higher solute concentration to an area of lower solute concentration (Figure 39-6) to ensure the maintenance of equilibrium and the even distribution of the solute in the solution. For example, if a small amount of red food colouring was added to a glass of water without stirring, over time the red food colouring molecules would evenly diffuse through the whole glass, giving an overall pink rather than a red appearance. A physiological example is the movement of oxygen and carbon dioxide between the alveoli and blood vessels in the lungs. The difference between the two concentrations is known as a concentration gradient.
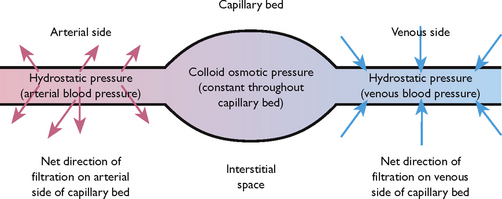
Filtration
Filtration is the process by which water and diffusible substances move together in response to fluid (hydrostatic) pressure. This process is active in capillary beds, where hydrostatic pressure differences determine the movement of water (Figure 39.7). When there is increased hydrostatic pressure on the venous side of the capillary bed, as occurs in congestive heart failure (CHF), the normal movement of water from the interstitial space into the intravascular space by filtration is reversed, resulting in an accumulation of excess fluid in the interstitial space, known as oedema.
Active transport
Unlike diffusion, osmosis and filtration, active transport requires metabolic activity and expenditure of energy to move materials across cell membranes. This allows cells to admit larger molecules than they would otherwise be able to admit, or to move molecules ‘uphill’ from areas of lesser concentration to areas of greater concentration. An example of active transport is the sodium and potassium pump (Figure 39-8). Sodium is pumped out of the cell and potassium is pumped in, against the concentration gradient. This process makes it possible to keep a higher concentration of potassium in the ICF and a higher concentration of sodium in the ECF.
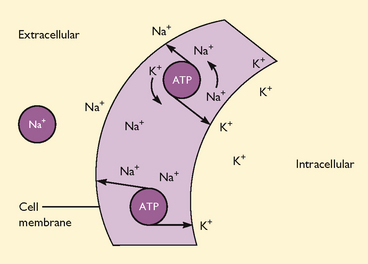
FIGURE 39-8 Sodium–potassium pump.
From Lewis SM and others 2008 Medical–surgical nursing: assessment and management of clinical problems, ed 6. St Louis, Mosby.
Active transport is enhanced by carrier molecules in a cell, which bind themselves to incoming molecules. For example, glucose is able to enter cells after it binds with the transport vehicle insulin. Active transport is the mechanism by which cells absorb glucose and other substances to carry out metabolic activities.
Regulation of body fluids
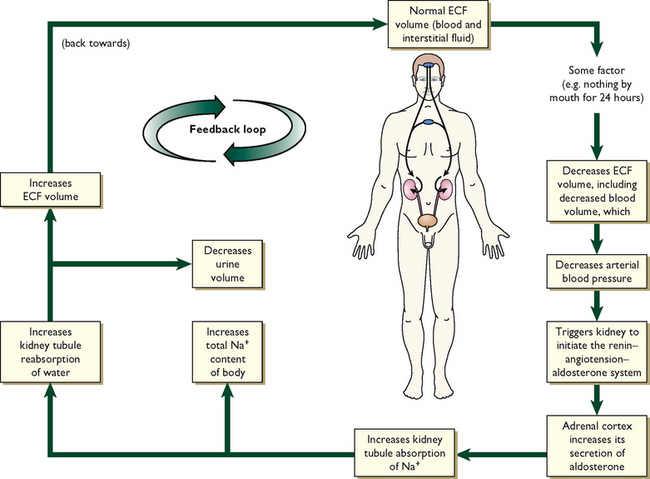
Body fluids are regulated by fluid intake, hormonal controls and fluid output. This physiological balance is termed homeostasis (Lewis and others, 2008). In health, a number of feedback mechanisms work together to maintain homeostasis (Figure 39-9).
Fluid intake
Fluid intake is regulated mainly through the thirst mechanism. Thirst is the conscious desire for water and is one of the major factors that determine fluid intake (Patton and Thibodeau, 2010). The thirst-control centre is located within the hypothalamus in the brain. The thirst reflex is stimulated by three processes:
• a decrease in saliva results in dry mucosa in the mouth and pharynx
• an increase in blood osmotic pressure stimulates osmoreceptors in the hypothalamus
• a decrease in blood volume leads to the renin–angiotensin–aldosterone system (RAAS) stimulating the thirst centre in the hypothalamus (Figure 39-10). A decrease in blood volume can be due to hypovolaemia, a reduction in extracellular fluid volume that results in decreased tissue perfusion. Hypovolaemia can be caused by loss of either salt or water (e.g. with vomiting, diarrhoea or third space fluid shift). The term dehydration is more specific, and is the loss of only water; it results in imbalance between water and salts in the body and therefore leads to hypernatraemia.
The osmoreceptors continually monitor the solute concentration of the plasma. Osmoreceptors indirectly monitor ‘water balance’ and control the distribution of water between intracellular and extracellular fluid. Osmoreceptors respond to changes in osmotic pressure and tonicity caused by an excess or a deficit of water. When osmolality increases, the hypothalamus is stimulated. Increased plasma osmolality can occur with any condition that interferes with the oral ingestion of fluids, or it can occur with the intake of hypertonic fluids. The hypothalamus will also be stimulated when excess fluid is lost and hypovolaemia occurs, as in excessive vomiting and haemorrhage. If the thirst centre is activated, fluid deficit has already occurred to some extent. The response of the thirst reflex to fluid deficit is, however, diminished by older age, hypothalamic dysfunction, diminished cognitive function and in infants. Infants are at a higher risk than adults for dehydration because of a physiological inability of their renal tubules to concentrate urine, a higher metabolic rate, a larger body surface area and a poorly developed thirst mechanism.
HORMONAL REGULATION
Hormones regulate fluid intake through various mechanisms.
Antidiuretic hormone (ADH) is stored in the posterior pituitary gland. The osmoreceptors in the hypothalamus are stimulated to release ADH if there is an increase in blood osmolality. ADH works directly on the renal tubules and collecting ducts to increase their permeability to water. This in turn causes water to return to the intravascular space circulation, which dilutes the blood and decreases its osmolality. As the body attempts to compensate, the patient will experience a temporary decrease in urinary output. When the blood has been sufficiently diluted, the osmoreceptors stop the release of ADH and urinary output is restored.
Aldosterone is released by the adrenal cortex in response to increased plasma potassium levels or as a part of the RAAS to counteract hypovolaemia. It acts on the distal portion of the renal tubule to increase the reabsorption (saving) of sodium and the secretion and excretion of potassium and hydrogen. Because sodium retention leads to water retention, the release of aldosterone acts as a volume regulator (Patton and Thibodeau, 2010).
Renin, a proteolytic enzyme secreted by the kidneys, responds to decreased renal perfusion secondary to a decrease in extracellular volume. Renin acts to produce angiotensin I, which causes some vasoconstriction. However, angiotensin I almost immediately becomes reduced by an enzyme’s action to angiotensin II. Angiotensin II then causes massive selective vasoconstriction of many blood vessels and relocates and increases the blood flow to the kidney, improving renal perfusion. Angiotensin II also stimulates the release of aldosterone when the sodium concentration is low (Patton and Thibodeau, 2010).
Fluid output
Fluid output occurs through four organs: the kidneys, the skin, the lungs and the gastrointestinal (GI) tract. The kidneys are the major regulatory organs of fluid balance. They receive approximately 180 L of plasma to filter each day and produce 1200–1500 mL of urine daily (see Table 39-1).
Water loss from the skin is regulated by the sympathetic nervous system, which activates sweat glands. Water loss from the skin can be a sensible or an insensible loss. An average of 400–500 mL of sensible and insensible fluid is lost via the skin each day (Patton and Thibodeau, 2010). With fever, another 50 to 75 mL/day may be lost for each 1°C of temperature elevation above normal. Insensible water loss from the skin is continuous, not usually perceived by the individual, at around 0.4–0.5 mL/kg/h, and can increase significantly with pyrexia. Sensible water loss from the skin occurs through excess perspiration and can be perceived by the patient or by the nurse through inspection. The amount of sensible perspiration is directly related to the stimulation of the sweat glands.
The lungs expire about 400 mL of water daily. This insensible water loss may increase in response to changes in respiratory rate and depth. In addition, devices for administering oxygen can increase insensible water loss from the lungs.
The GI tract plays a vital role in fluid regulation. Approximately 3–6 L each day of isotonic fluid is moved into the GI tract and then returns to the extracellular fluid. Under normal conditions, the average adult loses only 100–200 mL of the 3–6 L each day through faeces. However, in the presence of a disease process, for example diarrhoea, the GI tract may become the site of a large amount of fluid loss. This loss may have a significant impact on maintaining normal fluid regulation.
Regulation of electrolytes
Cations
Major cations within the body fluids include sodium (Na+), potassium (K+), calcium (Ca2+) and magnesium (Mg2+). Cations interchange when one cation leaves the cell and is replaced by another. This occurs because cells tend to maintain electrical neutrality. Regulation of individual cations is included in Table 39-9.
Anions
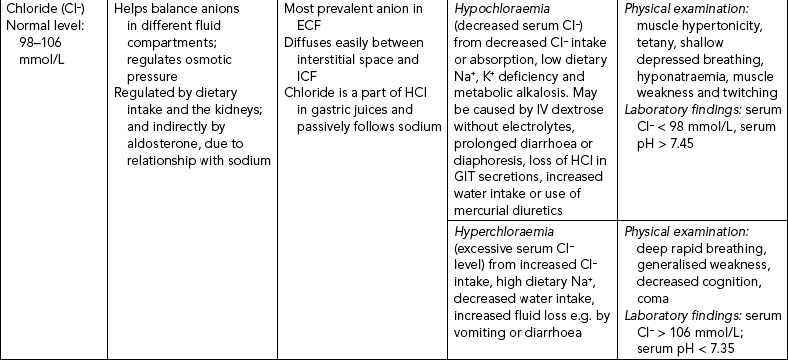
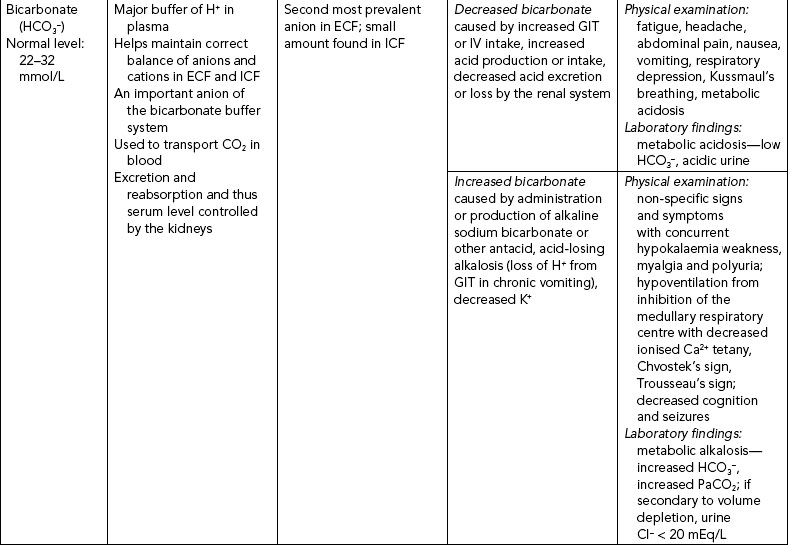
The three major anions of body fluids are chloride (Cl−), bicarbonate (HCO3−) and phosphate (PO43–) ions. Their regulation is included in Table 39-10.
Regulation of acid–base balance
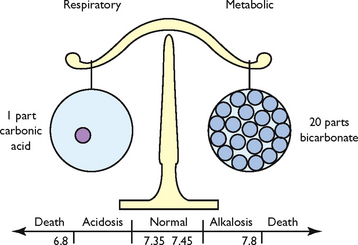
Metabolic processes maintain a steady balance between acids and bases to maintain optimal functioning of the cells. Arterial pH is an indirect measurement of hydrogen ion (H+) concentration: the higher the pH, the more acidic the solution (contains more H+ ions); a lower pH means a more alkaline solution (fewer H+ ions). Thus the pH is a scale for measuring the acidity or alkalinity of a fluid. A pH value of 7 is neutral, below 7 is acid, and above 7 is alkaline. Normal pH values in arterial blood range from 7.35 to 7.45.
The pH is regulated by two broad control systems, the chemical buffer systems (bicarbonate, phosphate and protein buffer systems) and the physiological buffer systems (the respiratory and renal response systems) (Patton and Thibodeau, 2010). A hydrogen ion buffer is a substance or a group of substances that can absorb or release H+ to correct an acid–base imbalance. Acid–base balance exists when the net rate at which the body produces acids or bases equals the rate at which acids or bases are excreted. This balance results in a stable concentration of hydrogen ions in body fluids, expressed as the pH value. Normal hydrogen ion levels are necessary to maintain cell membrane integrity and the speed of cellular enzymatic actions. Although buffers can raise the pH of body fluids, they do not remove the H+ from the body; this is left to the physiological buffers (Patton and Thibodeau, 2010).
Chemical regulation
Several buffering agents bind hydrogen ions to prevent or reduce changes in pH. Extracellular buffers include bicarbonate and ammonium ions; intracellular buffers include proteins and phosphate ions.
The most important chemical buffer in ECF is the carbonic acid and bicarbonate buffer system which reacts to imbalances within seconds (Figure 39-11). The equation below demonstrates how hydrogen ions (H+) and carbon dioxide (CO2) concentrations are directly related to each other, in that an increase in one causes an increase in the other. An increase in carbon dioxide concentration produces an increase in hydrogen ions; an increase in hydrogen ions results in increased carbon dioxide production (McCance and Huether, 2006). In the bicarbonate buffering system, carbon dioxide (CO2) moves reversibly through carbonic acid (H2CO3) to form hydrogen ions and bicarbonate ions (HCO3−):
A second type of chemical buffering occurs with the absorption or release of hydrogen ions by cells. This buffering occurs after the carbonic acid and bicarbonate buffering, and takes 2–4 hours. The positively charged hydrogen ion is exchanged with another positively charged ion, frequently potassium (K+). In conditions with excess acid, a hydrogen ion enters the cell and a potassium ion leaves the cell and enters the ECF, thus causing elevated serum potassium.
Another buffer is the haemoglobin–oxyhaemoglobin system. Carbon dioxide diffuses into the red blood cells (RBCs) and forms carbonic acid. The carbonic acid dissociates into hydrogen ions and bicarbonate ions. The hydrogen ions attach to haemoglobin, and the bicarbonate ion becomes available for buffering by exchanging with extracellular chloride (McCance and Huether, 2006).
The last buffer is the chloride shift within RBCs. When blood is oxygenated in the lungs, bicarbonate diffuses into the cells and chloride travels from the haemoglobin to the plasma to maintain electrical neutrality. The reverse occurs when carbon dioxide moves into RBCs in tissue capillary beds.
Physiological regulation
The two physiological buffers in the body are the lungs and the kidneys. The excretion of carbon dioxide resulting from metabolism is controlled mainly by the lungs, and the excretion of hydrogen and bicarbonate ions is controlled by the kidneys.
The lungs respond rapidly to acid–base imbalances to return the pH towards normal before the biological buffers act. Increased levels of hydrogen ions and carbon dioxide provide the stimulus for respiration. When the concentration of hydrogen ions is altered, the lungs react to correct the imbalance by altering the rate and depth of respiration. For example, when metabolic acidosis is present, respirations are increased, resulting in a greater amount of carbon dioxide being exhaled; this results in a decrease in the acidic level. Conversely, when metabolic alkalosis is present, the lungs retain carbon dioxide by decreasing the respirations, thereby increasing the acidic level (Madias and Adrogue, 2003).
Although the kidneys take from a few hours to several days to regulate acid–base imbalance, they are ‘the most powerful of the acid–base regulatory systems’ (Guyton and Hall 2006). In acidosis (excess acid):
• cells in the renal tubules reabsorb more bicarbonate ions from the tubule fluid
• cells in the renal collecting duct secrete more hydrogen ions and generate more bicarbonate ions
• there is increased formation of ammonia through the ammonia mechanism (see below).
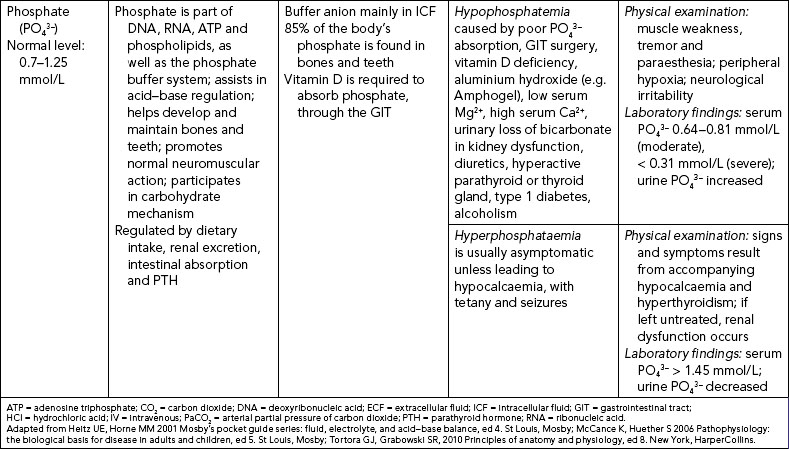
In addition, the kidneys use a phosphate ion (PO43–) to excrete hydrogen ions by forming phosphoric acid (H3PO4); sulfuric acid (H2SO4) may also be excreted. The ammonia mechanism regulates acid–base balance by certain amino acids being chemically changed within the renal tubules into ammonia; this, in the presence of hydrogen ions, forms ammonium and is excreted in the urine, hence releasing hydrogen ions from the body (Roth and Chan, 2001).
In alkalosis (acid deficit), the kidneys excrete more bicarbonate ions by decreasing hydrogen ion secretion from the epithelial cells of the renal tubules, and lowering rates of glutamine metabolism and ammonium ion excretion.
Disturbances in electrolyte, fluid and acid–base balances
Disturbances in electrolyte, fluid or acid–base balances seldom occur alone, and disrupt many other normal body processes. When there is a loss of body fluids because of dehydration, burns, illnesses or trauma or an increase of body fluids because of hypervolaemia, acute renal failure or CHF, the patient is also at risk of electrolyte imbalances. In addition, some untreated electrolyte imbalances (e.g. potassium loss) result in acid–base disturbances.
Electrolyte imbalances
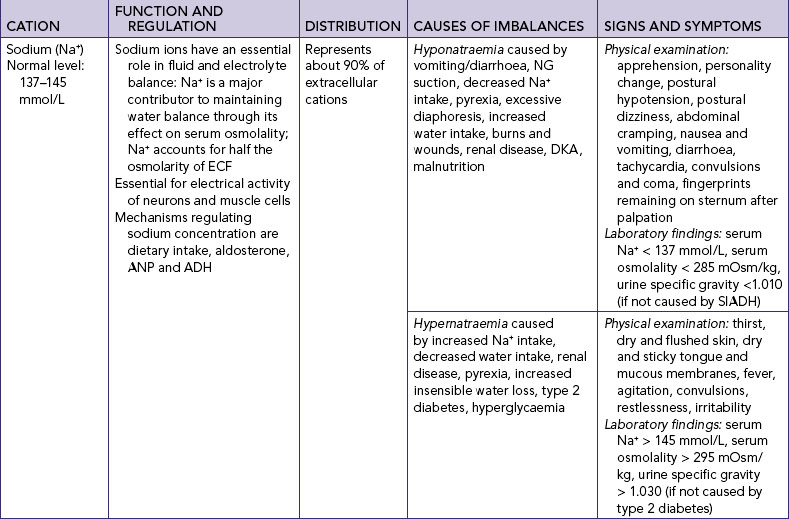
SODIUM IMBALANCES
Hyponatraemia is a lower-than-normal concentration of sodium in the blood (serum), which can occur with a net sodium loss or net water excess (Table 39-9). It occurs frequently in seriously ill patients but can also occur in water intoxication, when someone drinks too much water too quickly, leading to haemodilution. There are multiple examples of this both in hospitals and in the community. Almond and others (2005) studied 488 runners in the 2002 Boston marathon; 13% of runners were found to have hyponatraemia (a serum sodium concentration of ≤135 mmol/L) and 0.6% critical hyponatraemia (≤120 mmol/L). In this situation hyponatraemia was associated with substantial weight gain, consumption of more than 3 litres of fluids during the race, female gender and low body mass index (BMI).
Nurses’ awareness of the effect of medications on serum sodium levels should increase early identification of hyponatraemia through regular appraisal of serum electrolyte results. An association has been found between hyponatraemia and prescription of antidepressant medication (Mannesse and others, 2010; Movig and others, 2002). Selective serotonin re-uptake inhibitors (SSRIs) were associated with an increased risk of hyponatraemia compared with other classes of antidepressant drugs. The risk of hyponatraemia increased in elderly patients also prescribed diuretics (Movig and others, 2002).
Clinical indicators and treatment depend on the cause of the hyponatraemia and association with normal, decreased or increased ECF volume (McCance and Huether, 2006). The usual situation is a loss of sodium without a loss of fluid, resulting in decreased ECF osmolality. The body initially adapts by reducing water excretion and thus sodium excretion to maintain serum osmolality at near-normal levels. As the sodium loss continues, the body continues to preserve the blood and interstitial (tissue) volume. As a result, the sodium in ECF becomes diluted.
Hypernatraemia is a greater-than-normal concentration of ECF sodium, caused by excess water loss or an overall sodium excess (see Table 39-9). When the cause of hypernatraemia is increased aldosterone secretion, sodium is retained and potassium is excreted. When hypernatraemia occurs, the body attempts to conserve as much water as possible through renal reabsorption.
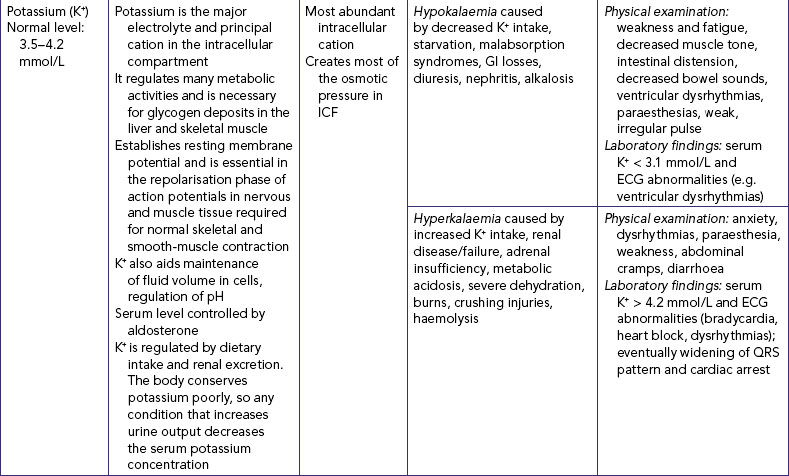
POTASSIUM IMBALANCES
Hypokalaemia is an inadequate amount of potassium in ECF (see Table 39-9). When severe, hypokalaemia can affect cardiac conduction and function. Because the normal levels of serum potassium are low, there is little tolerance for fluctuations. The most common cause of hypokalaemia is the use of potassium-wasting diuretics such as thiazide and loop diuretics.
Hyperkalaemia is a greater-than-normal amount of serum potassium. Severe hyperkalaemia produces marked cardiac conduction abnormalities (see Table 39-9). The main cause of hyperkalaemia is renal failure and thus decreased excretion of potassium.
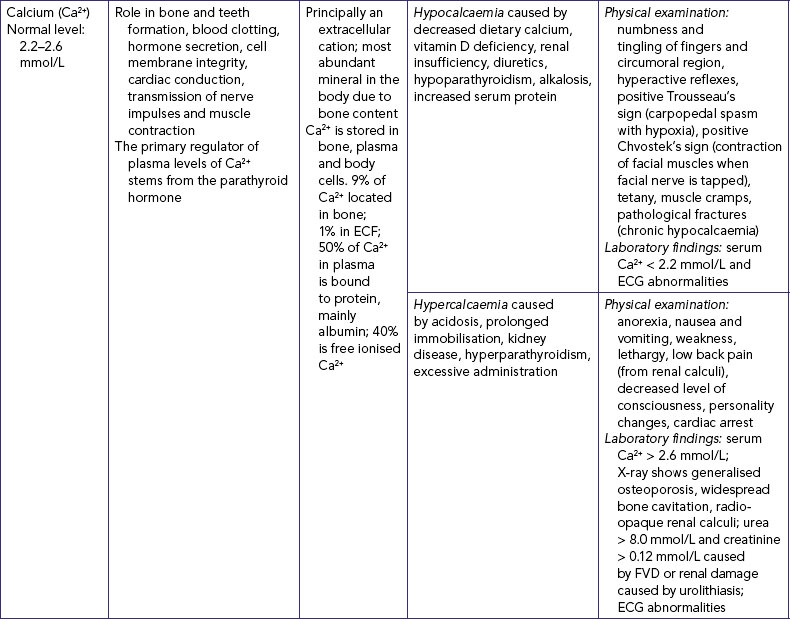
CALCIUM IMBALANCES
Hypocalcaemia represents a drop in serum and/or ionised calcium levels. It can result from illnesses which directly affect the thyroid and parathyroid glands (see Table 39-9) and from renal insufficiency (in which the kidneys’ inability to excrete phosphorus causes the phosphorus level to rise and the calcium level to decline). Signs and symptoms can be related to a diminished function of the neuromuscular, cardiac and renal systems.
Hypercalcaemia is an increase in the total serum concentration of calcium and/or ionised calcium. Hypercalcaemia is frequently a symptom of an underlying disease resulting in excess bone reabsorption with release of calcium.
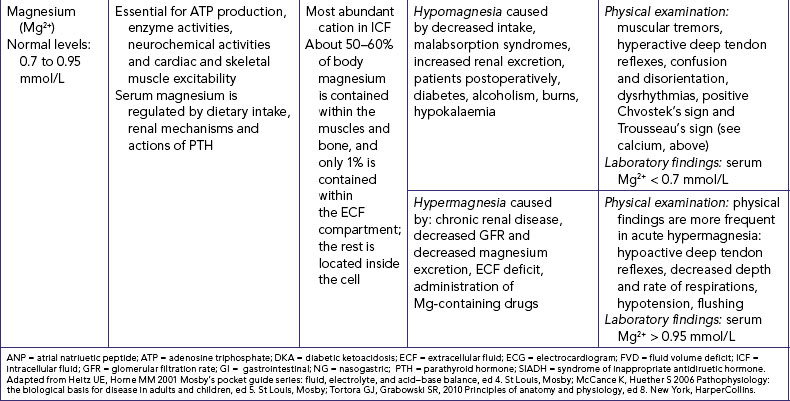
MAGNESIUM IMBALANCES
Disturbances in magnesium levels result in changes in neuromuscular excitability (see Table 39-9). Hypomagnesaemia, a decrease in serum magnesium, occurs with malnutrition and with malabsorption disorders. Signs and symptoms are directly related to the neuromuscular system. Hypermagnesaemia, an increase in serum magnesium levels, depresses skeletal muscles and nerve function. Hypermagnesaemia occurs during renal failure, aldosterone deficiency and hypothyroidism and by excess intake of antacids containing Mg2+. Hypermagnesaemia causes hypotension, muscle weakness, nausea, vomiting and altered mental functioning (Lewis and others, 2008).
CHLORIDE IMBALANCES
Hypochloraemia occurs when serum chloride levels fall below normal (see Table 39-10). Vomiting or prolonged and excessive nasogastric or fistula drainage can result in hypochloraemia because of the loss of hydrochloric acid. The use of loop and thiazide diuretics also results in increased chloride loss, as sodium is excreted. When serum chloride levels fall, metabolic alkalosis results as the body adapts by increasing reabsorption of the bicarbonate ion to maintain electrical neutrality. Hyperchloraemia occurs when the serum chloride levels rise above normal (see Table 39-10), which usually occurs when serum bicarbonate levels fall or sodium levels rise.
Hypochloraemia and hyperchloraemia rarely occur as single disease processes, but are commonly associated with acid–base imbalance. There is no single set of symptoms associated with these two alterations.
Fluid disturbances
The basic types of fluid imbalance are isotonic and osmolar. Isotonic deficit and excess exist when water and electrolytes are gained or lost in equal proportions. In contrast, osmolar imbalances are losses or excesses of only water, so that the concentration (osmolality) of the serum is affected. Table 39-11 lists the causes and symptoms of common fluid disturbances.
TABLE 39-11 FLUID DISTURBANCES
| CAUSES | SIGNS AND SYMPTOMS |
|---|---|
| ISOTONIC IMBALANCES | |
| Fluid volume deficit (FVD)—water and electrolytes lost in equal or isotonic proportions | |
|
Physical examination: postural hypotension, decreased pulse pressure, decreased MAP, tachycardia, dry mucous membranes, poor skin turgor, thirst, confusion, rapid weight loss*, slow vein filling, lethargy, oliguria, weak pulse Laboratory findings: urine specific gravity: 1.025; increased haematocrit level, 50%; and increased BUN level, > 8 mmol/L (haemoconcentration) |
|
| Fluid volume excess (FVE)—water and sodium retained in isotonic proportions | |
|
Physical examination: rapid weight gain, peripheral oedema, hypertension, increased pulse pressure, increased MAP, polyuria (if renal mechanisms are normal), increased jugular venous pressure, increased venous pressure, bi-basal lung crepitations Laboratory findings: decreased haematocrit level, 35%; and decreased BUN level, > 3 mmol/L (haemodilution) |
|
| OSMOLAR IMBALANCES | |
| Hyperosmolar imbalance—dehydration | |
| Hypo-osmolar imbalance—water excess | |
BUN = blood urea nitrogen; MAP = mean arterial pressure (of blood); SIADH = syndrome of inappropriate antidiuretic hormone.
*With third space fluid shift, there is either no change or an increase in weight
Acid–base balance disturbances
Arterial blood gas (ABG) analysis is the best way to evaluate acid–base balance. Measurement of ABGs involves analysis of six components: pH, PaCO2, PaO2, oxygen saturation, base excess and HCO3−. Deviation from a normal value will indicate that the patient is experiencing an acid–base imbalance.
pH
pH measures hydrogen ion (H+) concentration in the body fluids. Even a slight change can be potentially life-threatening. An increase in H+ concentration makes a solution more acidic; a decrease makes the solution more alkaline. Normal pH values are 7.35–7.45 (acidic is < 7.35, and alkaline is > 7.45).
PaCO2
PaCO2 is the partial pressure of carbon dioxide in arterial blood, and reflects the depth of pulmonary ventilation. The normal range is 35–45 mmHg. A PaCO2 of < 35 mmHg is an indication of hyperventilation. As rate and depth of respiration increase, more carbon dioxide is exhaled and the carbon dioxide concentration decreases. Hypoventilation causes increased PaCO2, above 45 mmHg. As rate and depth of respiration decrease, less carbon dioxide is exhaled and more is retained, increasing the concentration of carbon dioxide.
PaO2
PaO2 is the partial pressure of oxygen in arterial blood. It has no primary role in acid–base regulation if it is within normal limits. The normal range is 80–100 mmHg. A PaO2 of less than 60 mmHg (hypoxaemia) can lead to anaerobic metabolism, resulting in lactic acid production and metabolic acidosis. There is a normal decline in PaO2 in older adults. Hypoxaemia also may cause hyperventilation, resulting in respiratory alkalosis (McCance and Huether, 2006).
OXYGEN SATURATION
Oxygen saturation (SaO2) is the percentage of available haemoglobin bound to oxygen. Oxygen saturation can be affected by changes in temperature, pH and PaCO2. Normal range is 95–99%.
BASE EXCESS
Base excess is the amount of blood buffer (haemoglobin and bicarbonate) that exists. The normal range is –2 to +2. A high value indicates alkalosis and can result from the ingestion of large amounts of sodium bicarbonate solutions (some antacids), citrate excess with rapid blood transfusions or intravenous infusion of sodium bicarbonate to correct ketoacidosis. A low value indicates acidosis and is usually the result of the elimination of too many bicarbonate ions. An example is diarrhoea and prolonged loss (vomiting or nasogastric suction) of deep GI contents that force the bicarbonate-containing fluid to be lost instead of being absorbed (Berry and Pinard, 2002).
BICARBONATE
Serum bicarbonate (HCO3−) is the major renal component of acid–base balance, and is excreted and produced by the kidneys to maintain a normal acid–base environment. It is the principal buffer of the ECF of the body, and once bicarbonate is in the ECF it is maintained at a concentration of 20 times that of the fluid concentration of carbonic acid (Berry and Pinard, 2002). Normal range is 22–26 mmol/L. Less than 22 mmol/L usually indicates metabolic acidosis; more than 26 mmol/L indicates metabolic alkalosis.
Types of acid–base imbalances
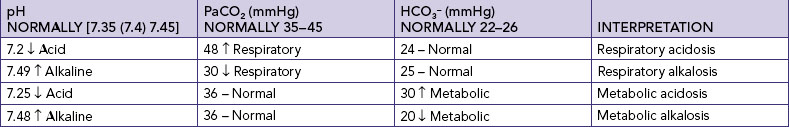
The four main types of acid–base imbalance are respiratory acidosis, respiratory alkalosis, metabolic acidosis and metabolic alkalosis (Table 39-12).
TABLE 39-12 ACID–BASE IMBALANCES
PaCO2 = partial pressure of carbon dioxide in arterial blood; PaO2 = partial pressure of oxygen in arterial blood;
RESPIRATORY ACIDOSIS
Respiratory acidosis is marked by an increased arterial carbon dioxide concentration (PaCO2), excess carbonic acid (H2CO3) and an increased hydrogen ion concentration (decreased pH). With respiratory acidosis, the cerebrospinal fluid and brain cells become acidic, causing neurological changes. Hypoxaemia occurs because of respiratory depression, resulting in further neurological impairment. Electrolyte changes such as hyperkalaemia and hypercalcaemia may accompany the acidosis.
RESPIRATORY ALKALOSIS
Respiratory alkalosis is marked by decreased PaCO2 levels and increased pH. Like respiratory acidosis, respiratory alkalosis can begin outside the respiratory system (e.g. anxiety with hyperventilation) or within the respiratory system (e.g. initial phase of an asthma attack).
METABOLIC ACIDOSIS
Metabolic acidosis results from abnormal loss of bicarbonate or accumulation of excess metabolic acids causing high acid content of the blood (Guyton and Hall, 2006). Identification of the cause of metabolic acidosis requires an analysis of serum electrolytes to detect an anion gap. An anion gap reflects unmeasurable anions present in plasma, and is calculated by subtracting the sum of chloride and bicarbonate levels from the plasma sodium level (Table 39-13). The anion gap is thus an artefact of measurement, and not a physiological reality. Anion gap is an ‘artificial’ and calculated measure that is representative of the serum ions not routinely measured when testing serum electrolytes. For example, the anions (e.g. lactate, acetoacetate, sulfate) produced during metabolic acidosis are not measured as part of the usual laboratory profile.
TABLE 39-13 DIFFERENTIATION OF CAUSES OF METABOLIC ACIDOSIS FROM EXAMINATION OF NORMAL VS ELEVATED ANION GAP
Adapted from Heitz UE, Horne MM 2001 Mosby’s pocket guide series: fluid, electrolyte, and acid–base balance, ed 4. St Louis, Mosby; McCance K, Huether S 2006 Pathophysiology: the biological basis for disease in adults and children, ed 5. St Louis, Mosby; Tortora GJ, Grabowski SR, 2010 Principles of anatomy and physiology, ed 8. New York, HarperCollins; Wotton K, Crannitch K, Munt R 2008 Prevalence, risk factors and strategies to prevent dehydration in older adults. Contemp Nurse 31(1):44–56.
Mrs Sinclair was admitted with blood pressure 90/70 mmHg, pulse 126 beats per minute, respirations 32 breaths per minutes and a 2-day history of nausea and vomiting, excessive thirst and polyuria. She was recently diagnosed with type 2 diabetes. Her arterial blood gas results reveal metabolic acidosis; calculation of the anion gap using laboratory information reveals a high anion gap of 27.
Calculating the anion gap is useful in distinguishing between anion-gap and non-anion-gap metabolic acidosis, differentiating between causes of metabolic acidosis, assessing biochemical severity of acidosis, and monitoring response to treatment. If the laboratory report does not include an anion gap it can be calculated from the formula
Table 39-13 indicates reasons for normal and increased anion gap.
METABOLIC ALKALOSIS
Metabolic alkalosis is marked by the heavy loss of acid from the body or by increased levels of bicarbonate. The most common causes of acid loss are vomiting and gastric suction. Other causes include the overcorrection of metabolic acidosis, loss of chloride, use of diuretics causing resorption of bicarbonate, hyperaldosteronism and excessive ingestion of alkali (Berry and Pinard, 2002).
• CRITICAL THINKING
Your friend meets with you for coffee after playing cricket (bowling and fielding) on a day when the air temperature was 38.9°C. He states he is not feeling very well, has a severe headache and would prefer just water. On asking what he has consumed during the day, he states he had around 4 litres of plain water.
Discuss the appropriateness of his intake throughout the day, using what you know about the fluid he would have lost and required replacement therapy.