Chapter 40 Oxygenation
Mastery of content will enable you to:
• Define the key terms listed.
• Describe the structure and function of the cardiovascular and pulmonary systems.
• Identify physiological processes of cardiac output, myocardial blood flow and coronary artery circulation.
• Describe the relationship of cardiac output, stroke volume (preload, afterload, contractility) and heart rate.
• Identify physiological processes involved in ventilation, perfusion and exchange of oxygen and carbon dioxide.
• Describe neural and chemical regulation of ventilation.
• Describe the impact of a patient’s level of health, age, lifestyle and environment on tissue oxygenation.
• Identify and describe clinical outcomes as a result of disturbances in conduction, altered cardiac output, impaired valvular function, myocardial ischaemia and impaired tissue perfusion.
• Identify and describe clinical outcomes of ventilation/perfusion mismatch, diffusion impairment, alveolar hypoventilation and alveolar hyperventilation.
• Identify nursing care interventions in the primary care, acute care and restorative and extended-care settings that promote oxygenation.
Scientific knowledge base
Oxygen is required to sustain life. The function of the cardiac and respiratory systems is to supply the body’s oxygen demands. Cardiopulmonary physiology involves delivery of deoxygenated blood to the right side of the heart and then to the pulmonary circulation where oxygen uptake occurs. Oxygenated blood then moves from the lungs to the left side of the heart and is subsequently delivered to the tissues. Blood is oxygenated and carbon dioxide eliminated through the mechanisms of ventilation, the mechanical movement of gas into and out of the lungs, and respiration, the exchange of oxygen and carbon dioxide at tissue level. Neural and chemical regulators control respiratory rate and depth in response to changing tissue oxygen demands (Brashers 2010a, 2010b).
Cardiovascular physiology
The function of the cardiovascular system is to deliver oxygen, nutrients and other substances to the tissues and to remove the waste products of cellular metabolism through the cardiac pump, the circulatory vascular system and the integration of other systems, for example respiratory, gastrointestinal and renal systems (Brashers, 2010b).
Structure and function
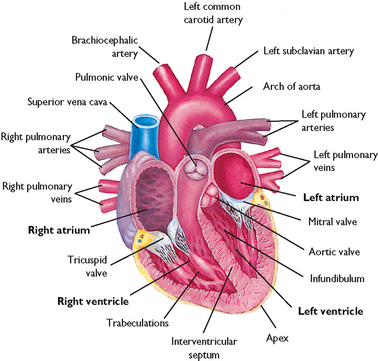
The right ventricle pumps blood through the pulmonary circulation while the left ventricle pumps blood to the systemic circulation, supplying oxygen and nutrients to the tissues and removing carbon dioxide and wastes (Figures 40-1 and 40-2). The circulatory system exchanges oxygen, carbon dioxide, nutrients and waste products between the blood and the tissues.
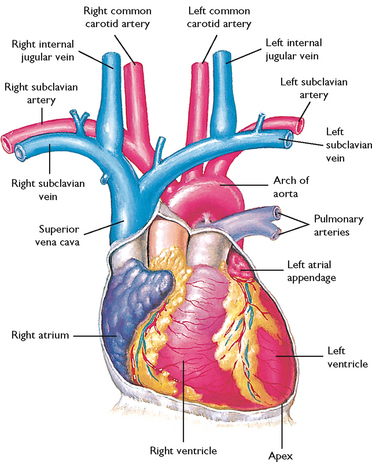
FIGURE 40-1 Diagram showing serially connected pulmonary and systemic circulation. Right heart chambers propel unoxygenated blood through the pulmonary circulation; left heart chambers propel oxygenated blood through the systemic circulation.
From Canobbio MM 1990 Cardiovascular disorders. St Louis, Mosby.
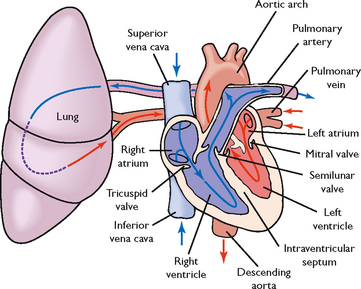
FIGURE 40-2 Schematic representation of blood flow through the heart. Arrows indicate direction of flow.
From Lewis SM and others 2004 Medical–surgical nursing: assessment and management of clinical problems, ed 6. St Louis, Mosby.
MYOCARDIAL PUMP
The pumping action of the heart is essential for the maintenance of oxygen delivery. Decreased pump effectiveness, as occurs in heart failure, results in a diminished stroke volume—the volume of blood ejected from the ventricles during each contraction. Shock, haemorrhage and dehydration decrease pump effectiveness by reducing the volume of circulating blood, thereby decreasing stroke volume.
The chambers of the heart, the right and left atria and ventricles, fill during diastole and partially empty during systole. The effectiveness of the diastolic and systolic events of the cardiac cycle can be assessed by monitoring the patient’s blood pressure (see Chapter 27). The myocardial fibres have contractile properties that enable them to stretch during filling. In a healthy heart this stretch is proportionally related to the strength of contraction. As the myocardium stretches, the strength of the subsequent contraction increases; this is known as Starling’s law of the heart (or Frank–Starling relationship). In the diseased heart, Starling’s law does not apply because the stretch of the myocardium is beyond the heart’s physiological limits. The subsequent contractile response results in insufficient ventricular ejection (volume), and blood begins to ‘back up’ in the pulmonary (left heart failure) or systemic (right heart failure) circulation.
CARDIAC BLOOD FLOW
Blood flow through the heart is unidirectional. There are four heart valves that ensure this forward blood flow (Figure 40-3). During ventricular diastole, the atrioventricular (mitral and tricuspid) valves open and blood flows from the higher-pressure atria into the relaxed ventricles. The closure of these valves represents S1, or the first heart sound of auscultation. After ventricular filling, the systolic phase begins. As the systolic intraventricular pressure rises, the atrioventricular valves close, preventing the backflow of blood into the atria, and ventricular contraction begins (isovolumetric contraction). During the systolic phase, ventricular pressure rises, causing the semilunar (aortic and pulmonic) valves to open. As the ventricles eject blood, the intraventricular pressure falls and the semilunar valves close, thus preventing the backflow of blood from the aorta and pulmonary artery into the ventricles. Closure of the aortic and pulmonic valves represents S2, or the second heart sound. Patients with valvular disease may have backflow or regurgitation of blood through an incompetent valve, causing a murmur or click that is heard on auscultation (see Chapter 27).
CORONARY ARTERY CIRCULATION
To maintain adequate blood flow to the pulmonary and systemic circulation, myocardial blood flow must supply sufficient oxygen and nutrients to the myocardium itself. Blood in the atria and ventricles does not supply oxygen and nutrients to the myocardium. The coronary circulation is the branch of the systemic circulation that supplies the myocardium with oxygen and nutrients and removes waste. The coronary arteries fill during ventricular diastole (Brashers, 2010b). The right and left coronary arteries arise from the aorta just above and behind the aortic valve through openings called the coronary ostia (coronary openings). The left coronary artery generally feeds the left atrium and ventricle, while the right coronary artery feeds the right atrium and ventricle. However, there is variation in branching of coronary arteries among individuals (Figure 40-4) (Box 40-1).
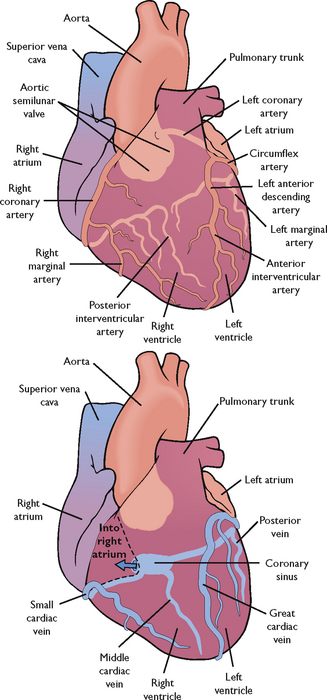
FIGURE 40-4 Coronary arteries and veins.
From Lewis SM and others 2004 Medical–surgical nursing: assessment and management of clinical problems, ed 6. St Louis, Mosby.
RIGHT CORONARY ARTERY
Part of posterior and inferior surface of left ventricle
Adapted from Bond EF 2010 Cardiac anatomy and physiology. In Woods SL, Froelicher ESS, Motzer SU and others, editors, Cardiac Nursing, ed 6. Philadelphia, Lippincott Williams & Wilkins.
SYSTEMIC CIRCULATION
The arteries and veins of the systemic circulation deliver nutrients and oxygen to the tissues and remove waste from them. Oxygenated blood flows from the left ventricle by way of the aorta and into large systemic arteries. These arteries branch into smaller arteries, then into arterioles and finally into the smallest vessels, the capillaries. At capillary level the exchange of oxygen and carbon dioxide, nutrients and wastes occurs, resulting in tissue oxygenation. The waste products exit the capillary network by way of the venules that join to form veins. These veins form larger veins, which carry deoxygenated blood to the right side of the heart, where it is returned to the pulmonary circulation.
BLOOD FLOW REGULATION
The amount of blood ejected from the left ventricle each minute is the cardiac output. The normal cardiac output is 4–6 L/min in the healthy 70 kg adult at rest. The circulating volume of blood changes according to the oxygen and metabolic needs of the body. For example, during exercise, pregnancy and fever the cardiac output increases, but during sleep it decreases. Cardiac output is represented by the following formula:
Cardiac output (CO) = Stroke volume (SV) × Heart rate (HR)
Cardiac output in the older adult may be affected by increased arterial wall tension and moderate myocardial hypertrophy due to an increased systolic blood pressure.
Stroke volume is the amount of blood ejected from the left ventricle with each contraction. It can be affected by the amount of blood in the left ventricle at the end of diastole (preload), the resistance to left ventricular ejection (afterload) and myocardial contractility (see section below).
Preload is essentially the end-diastolic volume. As the ventricles fill, they stretch. The greater the stretch on the ventricle, the greater the contraction and the greater the stroke volume (Starling’s law). In clinical situations, the preload and subsequent stroke volume can be manipulated by changing the amount of circulating blood volume. For example, in the patient with haemorrhagic shock, fluid therapy and replacement of blood increases volume, thus increasing the preload and cardiac output. If volume is not replaced, preload decreases, the cardiac output decreases and ultimately the venous return to the right atrium decreases, further decreasing preload and cardiac output.
Afterload is the resistance to left ventricular ejection—the work the heart must overcome to fully eject blood from the left ventricle. The diastolic aortic pressure is a good clinical measure of afterload. In a patient with an acute hypertensive crisis, the afterload is increased, increasing the cardiac workload. Afterload in this situation can be manipulated by decreasing systemic blood pressure.
The continuous monitoring of cardiovascular haemodynamics is usually performed in critical-care units. Some step-down or special-care units may also have the capability to continuously monitor haemodynamics.
MYOCARDIAL CONTRACTILITY
Myocardial contractility also affects stroke volume and cardiac output. Poor contraction of the myocardium decreases the amount of blood ejected by the ventricles during each contraction. Myocardial contractility can be increased by drugs that increase the force of contraction, such as digitalis preparations and sympathomimetic drugs (drugs that mimic the effects of the sympathetic nervous system), e.g. adrenaline. Injury to the myocardial muscle, such as an acute myocardial infarction, can cause a decrease in myocardial contractility. The myocardium of the older adult is more rigid and slower in recovering its contractility (Steven, 2011).
Heart rate affects blood flow because of the interaction between rate and diastolic filling time. With a sustained heart rate greater than 160 beats per minute, diastolic filling time decreases, decreasing stroke volume and cardiac output. The heart rate of the older adult is slow to increase under stress. With the normal ageing process there are significant changes to cardiac structure and function, and the stroke volume may increase to increase the cardiac output and blood pressure (Jet, 2008).
CONDUCTION SYSTEM
The rhythmic relaxation and contraction of the atria and ventricles depend on continuous, organised transmission of electrical impulses. These impulses are generated and transmitted by way of the cardiac conduction system (Figure 40-5).
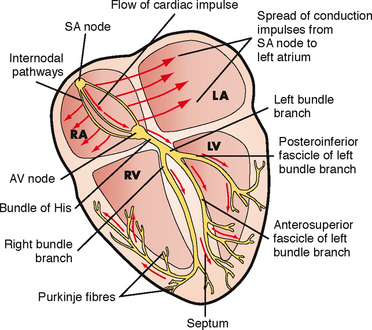
FIGURE 40-5 Conduction system of the heart. LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle; SA, sinoatrial; AV, atrioventricular.
From Lewis SM and others 2007 Medical–surgical nursing: assessment and management of clinical problems, ed 7. St Louis, Mosby.
The heart’s conduction system generates the necessary action potentials that conduct the impulses required to initiate the electrical chain of events resulting in the heartbeat. The autonomic nervous system, consisting of sympathetic and parasympathetic branches, influences the rate of impulse generation as well as the speed of transmission through the conductive pathway and the strength of atrial and ventricular contractions. Sympathetic nerve fibres, which increase the rate of impulse generation and the speed of impulse transmission, innervate all parts of the atria and ventricles. Parasympathetic fibres from the vagus nerve, which decrease this rate, also innervate these parts, as well as the sinoatrial and atrioventricular nodes (Brashers, 2010b).
The conduction system originates with the sinoatrial (SA) node, the ‘pacemaker’ of the heart. The SA node is in the right atrium next to the entrance of the superior vena cava (Brashers, 2010b). Impulses are initiated at the SA node at an intrinsic rate of 60–100 beats per minute. The resting adult rate is approximately 75 beats per minute. Resting heart rate for infants and children varies with age: 80–150 beats per minute for age 3 months to 2 years, 70–110 beats per minute for 2–10 years and 55–90 beats per minute for over 10 years (Hockenberry and others, 2009).
The electrical impulses are then transmitted through the atria along intra-atrial pathways to the atrioventricular (AV) node. The AV node mediates impulses between the atria and the ventricles. The intrinsic rate of the normal AV node is 40–60 beats per minute. The AV node assists atrial emptying by delaying the impulse before transmitting it through the bundle of His and the ventricular Purkinje network. The intrinsic rate of the bundle of His and the ventricular Purkinje network is 20–40 beats per minute.
An electrocardiogram (ECG) reflects the electrical activity of the conduction system. It monitors the regularity and path of the electrical impulse through the conduction system; however, it does not reflect muscular work of the heart. This electrical impulse is required to stimulate the myocardium to then generate the mechanical event of ventricular contraction. The normal sequence on the ECG is called normal sinus rhythm (NSR) (Figure 40-6).
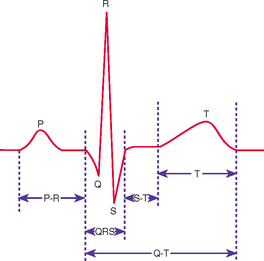
FIGURE 40-6 Normal ECG waveform.
From Potter PA, Perry AG 2013 Fundamentals of Nursing, ed 8. St Louis, Mosby.
NSR implies that the impulse originates at the SA node and follows the normal sequence through the conduction system. The P wave represents the electrical conduction through both atria and is normally small and rounded. Atrial contraction follows the P wave. The P–R interval represents the impulse travel time through the AV node and the bundle of His to the Purkinje fibres. The normal length of the P–R interval is 3–5 small squares on the ECG paper (0.12–0.20 second). An increase in the P–R of > 5 small squares (0.20 second) indicates that there is a block in the impulse transmission through the AV node, whereas a P-R interval of < 2 small squares (0.08 second) indicates the initiation of the electrical impulse from a source other than the SA node. A P–R interval of > 5 small squares (0.20 second) should be reported.
The QRS complex indicates that the electrical impulse has travelled through the ventricles. Normal QRS duration is 1.5–3 small squares on the ECG paper (0.06–0.12 second). An increase in QRS duration indicates a delay in conduction time through the ventricles. Ventricular contraction usually follows the QRS complex. A QRS complex wider than 3 small squares (0.12 second) should be reported.
The T wave represents ventricular repolarisation. The T wave is normally rounded, rising more slowly from the baseline than it returns. T waves can be flattened or inverted in hypokalaemia and peaked in hyperkalaemia.
The S–T segment extends from the end of the QRS (J point) to the start of the T wave and should be at the baseline. Significant displacement from the baseline may be indicative of disease processes such as acute coronary syndrome (Jacobson, 2010a).
Respiratory physiology
Most cells in the body obtain their energy from chemical reactions involving oxygen and the elimination of carbon dioxide. The exchange of oxygen and carbon dioxide occurs between environmental air and the blood (Figure 40-7). There are three steps in the process of oxygenation: ventilation, diffusion and perfusion of lungs (Brashers, 2010a). For the exchange of oxygen and carbon dioxide to occur, the organs, nerves and muscles of respiration must be intact and the central nervous system able to regulate the respiratory cycle. The respiratory centre lies in the brainstem, which contains a group of neurons (dorsal respiratory group) that regulates the automatic rhythm of respiration. The pneumotaxic and apneustic centres of the respiratory centre adjust inspiratory rate and depth. Central chemoreceptors detect slight changes in pH in spinal fluid, reflecting arterial carbon dioxide (see Chapter 39). Changes in carbon dioxide and therefore pH are detected and inspiratory rate and depth adjusted accordingly. Peripheral chemoreceptors detect arterial carbon dioxide and oxygen, but predominantly oxygen, and are therefore principally responsible for adjusting respiration in response to hypoxaemia. Central chemoreceptors are more sensitive than peripheral chemoreceptors (Brashers, 2010a).
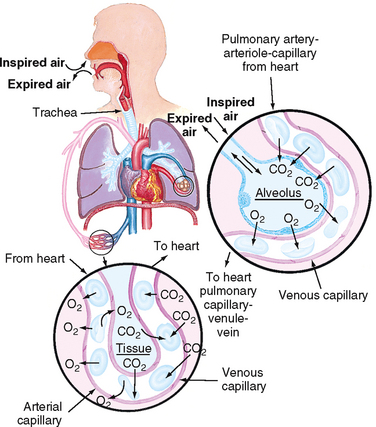
FIGURE 40-7 Structures of the pulmonary system.
From Thompson J and others 1993 Mosby’s manual of clinical nursing, ed 3. St Louis, Mosby.
Structure and function
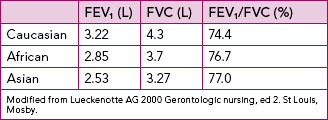
Normal adult respiratory rate ranges between 12 and 20 breaths per minute. Respiratory rate in infants and children varies: 25–30 breaths per minute for age 3 months to 2 years, 19–25 breaths per minute for 2–10 years, and 16–19 breaths per minute for over 10 years (Hockenberry and others, 2009). Respiration can be altered by conditions or diseases that change the structure and function of the lung. The respiratory muscles, pleural space, lungs and alveoli (Figure 40-8) are essential for ventilation, perfusion and exchange of oxygen and carbon dioxide (Box 40-2). The pleura is a membrane which adheres to the lungs and then folds back to connect to the chest wall. The visceral pleura adheres to the lungs, while the parietal pleura adheres to the chest wall. The area between these two is the pleural space, normally a potential space containing a small amount of fluid and negative in pressure relative to atmosphere (Brashers, 2010a). Problems arise if the negative pressure of this space is not maintained, as for example when air (pneumothorax), blood (haemothorax) or fluid (pleural effusion) enters the space (Brashers, 2010a).
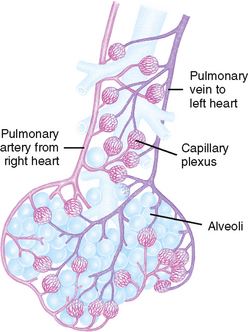
FIGURE 40-8 Alveoli at the terminal end of the lower airway.
From Thompson J and others 1993 Mosby’s manual of clinical nursing, ed 3. St Louis, Mosby.
BOX 40-2 MAJOR ANATOMICAL STRUCTURES OF THE THORAX AND THEIR FUNCTIONS
INSPIRATORY MUSCLES
DIAPHRAGM
Contraction causes the diaphragm to descend, creating a negative pleural pressure and increasing the vertical dimension of the lungs, which contributes to inflation of the lungs. The increase in vertical dimension and the decrease in intrapulmonary pressure (negative with respect to atmospheric pressure) cause air to enter the lungs.
EXPIRATORY MUSCLES
PLEURAL SPACE
The pleural space is a potential space that is only a thin film of liquid lying between the outer layer of the lung (visceral pleura) and the inner layer of the chest cavity (parietal pleura). It permits a smooth, gliding movement of the lungs along the chest wall. Normally, air is not present in the pleural space.
LUNGS
LEFT (TWO LOBES) AND RIGHT (THREE LOBES)
The lungs transfer oxygen from the atmosphere into the alveoli and carbon dioxide from the alveoli to the lungs to be excreted as a waste product. They also filter toxic material from circulation and metabolise compounds such as angiotensin I, bradykinin and prostaglandins.
Ventilation is the process of moving gases into and out of the lungs. Normal ventilation occurs at a rate of approximately 4 L/min. Ventilation requires coordination of the muscular and elastic properties of the lung and thorax, as well as intact innervation. The major inspiratory muscle of respiration is the diaphragm. It is innervated by the phrenic nerve derived from spinal nerves C3, C4 and C5. A mnemonic to aid memory of the innervation of the diaphragm is ‘C3, 4, 5 keeps the diaphragm alive’.
WORK OF BREATHING
Breathing is the effort required to expand and contract the lungs. The work of breathing is determined by the degree of compliance of the lungs, airway resistance, presence of active expiration, and use of accessory muscles of ventilation.
Inspiration is an active process, stimulated by chemical receptors in the aorta. Expiration is a passive process that depends on the elastic recoil properties of the lungs, requiring little or no muscle work. Elastic recoil is produced by elastic fibres in lung tissue and by surface tension in the fluid film lining the alveoli. Surfactant is the chemical produced in the lungs by alveolar type 2 cells that reduces the surface tension of the alveoli and keeps them from ‘sticking shut’ when collapsed (Brashers, 2010a). Patients with advanced chronic obstructive pulmonary disease (COPD) lose the elastic recoil of the lungs and thorax. As a result, the patient’s work of breathing is increased.
Accessory muscles of respiration can increase lung volume during inspiration, though not as effectively as the diaphragm. Accessory muscles include the sternocleidomastoid and scalene muscles on the side of the neck and the intercostal muscles (Brashers, 2010a). Patients with COPD, especially emphysema, frequently use these muscles to increase lung volume. During assessment the nurse may see obvious movement of these neck muscles and retraction of the muscles between the ribs. Prolonged use of the accessory muscles of respiration does not promote effective ventilation and causes fatigue.
Compliance is the ability of the lungs to distend or to expand in response to increased intra-alveolar pressure. Compliance is decreased in diseases such as pulmonary oedema, interstitial and pleural fibrosis, and congenital or traumatic structural abnormalities such as kyphosis (curving of the spine causing a roundness of the back) or fractured ribs.
Airway resistance is the pressure difference between the mouth and the alveoli in relation to the rate of flow of inspired gas. Airway resistance can be increased by an airway obstruction, small-airway disease (such as asthma) and tracheal oedema. When resistance is increased, the amount of air travelling through the anatomical airways is decreased.
Decreased lung compliance, increased airway resistance, active expiration or the use of accessory muscles increases the work of breathing, resulting in increased energy expenditure. To meet this expenditure, the body increases its metabolic rate, and the need for oxygen, as well as for the elimination of carbon dioxide, increases. This sequence is a vicious cycle for a patient with impaired ventilation, causing further deterioration of respiratory status and the ability to oxygenate adequately.
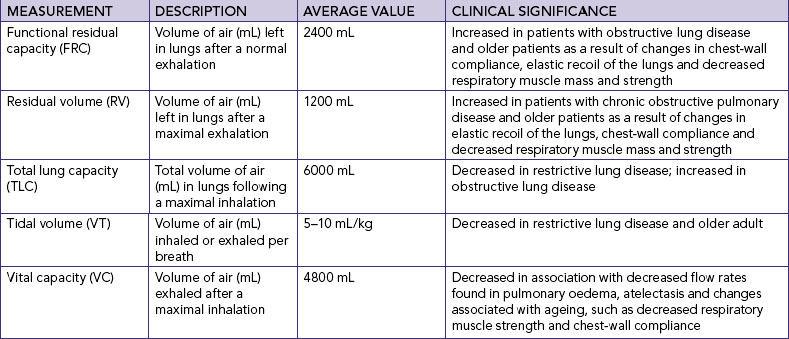
LUNG VOLUMES AND CAPACITIES
Spirometry is used to measure the volume of air entering or leaving the lungs. Variations in lung volumes may be associated with health states such as pregnancy, exercise, obesity, or obstructive and restrictive conditions of the lungs. The amount of surfactant, degree of compliance and strength of respiratory muscles can affect pressures and volumes within the lungs.
The volume of air in the lungs is measured in various ways and combined to describe lung capacities (see pulmonary function tests later in the chapter).
Gases are moved into and out of the lungs through pressure changes (Figure 40-9). Intrapleural pressure is negative relative to atmospheric pressure, which is 760 mmHg at sea level. For air to flow into the lungs, intrapleural pressure must become more negative, setting up a pressure gradient between the atmosphere and the alveoli.
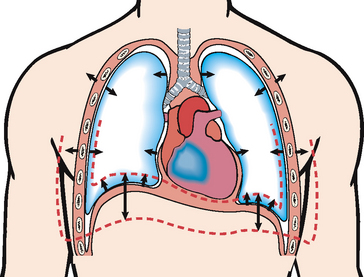
FIGURE 40-9 Frontal section of chest showing movement of the lung and chest wall during inspiration and expiration. During inspiration the inspiratory muscles contract and the chest expands. Alveolar pressure becomes subatmospheric with respect to pressure at the airway opening, and air flows into the lungs. During expiration the inspiratory muscles relax. Recoil of the lung causes alveolar pressure to exceed pressure at the airway opening and air to flow out of the lungs. Single arrows show excursion of the lungs and chest wall. Double arrows show movement of the lung bases.
From Lewis SM and others 2004 Medical–surgical nursing: assessment and management of clinical problems, ed 6. St Louis, Mosby.
PULMONARY CIRCULATION
The main function of the pulmonary circulation is to move blood to and from the alveolocapillary membrane for gas exchange to occur. The pulmonary circulation is a reservoir for blood so that the lung can increase its blood volume without large increases in pulmonary artery or venous pressures. The pulmonary circulation also acts as a filter, removing small thrombi before they can reach vital organs.
The pulmonary circulation begins at the pulmonary artery, which receives poorly oxygenated mixed venous blood from the right ventricle. Blood flow through this system depends on the pumping ability of the right ventricle, which has an output of approximately 5 L/min. The flow continues from the pulmonary artery through the pulmonary arterioles to the pulmonary capillaries, where blood comes in contact with the alveolar–capillary membrane and the exchange of oxygen and carbon dioxide occurs. The now oxygen-rich blood then circulates through the pulmonary venules and pulmonary veins, returning to the left atrium.
Pressure and resistance within the pulmonary circulatory system is lower than that within the systemic circulatory system. The walls of the pulmonary vessels are thinner and contain less smooth muscle. The lung accepts the total cardiac output from the right ventricle and, except in some specific pathophysiological circumstances, does not direct blood flow from one region to another.
EXCHANGE OF OXYGEN AND CARBON DIOXIDE
Oxygen and carbon dioxide are exchanged in the alveoli and the capillaries of the body tissues. Oxygen is transferred from the alveoli of the lungs to the pulmonary circulation, and carbon dioxide is transferred from the pulmonary circulation to the alveoli to be exhaled as a waste product. At the tissue level, oxygen is transferred from the arterial blood to tissues, and carbon dioxide is transferred from tissues to the venous blood to return to the pulmonary circulation and alveoli and be exhaled. This transfer depends on the process of diffusion.
Diffusion is the movement of molecules from an area of higher concentration to an area of lower concentration (see Chapter 39). Diffusion of oxygen and carbon dioxide occurs at the alveolar–capillary membrane. Oxygen diffuses into the pulmonary circulation from the alveoli and carbon dioxide diffuses from the pulmonary circulation into the alveoli to be expired.
OXYGEN TRANSPORT
The oxygen transport system consists of the respiratory and cardiovascular systems. Delivery depends on the amount of oxygen entering the lungs (ventilation), blood flow to the lungs and tissues (perfusion), rate of diffusion, and oxygen-carrying capacity. The capacity of the blood to carry oxygen is influenced by the amount of dissolved oxygen in the plasma, amount of haemoglobin and tendency of haemoglobin to bind with oxygen. Only a relatively small amount of required oxygen, less than 1%, is dissolved in the plasma (Brashers, 2010a). Most oxygen is transported by haemoglobin, which serves as a carrier for oxygen and carbon dioxide. The haemoglobin molecule combines with oxygen to form oxyhaemoglobin. The formation of oxyhaemoglobin is easily reversible, allowing haemoglobin and oxygen to dissociate, which frees oxygen to enter tissues.
CARBON DIOXIDE TRANSPORT
Carbon dioxide diffuses into red blood cells and is rapidly hydrated into carbonic acid (H2CO3) because of the presence of carbonic anhydrase. The carbonic acid then dissociates into hydrogen (H+) ions and bicarbonate (HCO3−) ions. The hydrogen ion is buffered by haemoglobin, and the HCO3−diffuses into the plasma (see Chapter 39). In addition, some of the carbon dioxide in red blood cells reacts with amino acid groups, forming carbamino compounds. This reaction can occur rapidly without the presence of an enzyme. Reduced haemoglobin (deoxyhaemoglobin) can combine with carbon dioxide more easily than oxyhaemoglobin, and therefore venous blood transports the majority of carbon dioxide.
REGULATION OF RESPIRATION
Regulation of respiration is necessary to ensure sufficient oxygen intake and carbon dioxide elimination to meet the body’s demands (e.g. during exercise, infection or pregnancy). Neural and chemical regulators control the process of respiration. Neural regulation includes the central nervous system control of respiratory rate, depth and rhythm. Chemical regulation involves the influence of chemicals such as carbon dioxide and hydrogen ions on the rate and depth of respiration (Box 40-3).
BOX 40-3 NEURAL AND CHEMICAL REGULATION OF RESPIRATION
NEURAL REGULATION
Maintains rhythm and depth of respiration and balance between inspiration and expiration.
Factors affecting oxygenation
Adequacy of ventilation, perfusion and transport of oxygen to the tissues is influenced by four types of factors: (1) physiological, (2) developmental, (3) behavioural and (4) environmental. Physiological factors are outlined below; developmental, behavioural (lifestyle) and environmental factors are discussed in the following section (nursing knowledge base).
Physiological factors
Any condition that affects cardiopulmonary functioning directly affects the body’s ability to meet oxygen demands. The general classifications of cardiac disorders include disturbances in conduction, impaired valvular function, myocardial hypoxia, cardiomyopathic conditions and peripheral tissue hypoxia. Respiratory disorders are caused by one or more of the following factors: ventilation/perfusion (V/Q) mismatch, diffusion impairment, alveolar hypoventilation and alveolar hyperventilation.
Other physiological processes affecting oxygenation are presented in Table 40-1.
TABLE 40-1 PHYSIOLOGICAL PROCESSES AFFECTING OXYGENATION
| PROCESS | EFFECT ON OXYGENATION |
|---|---|
| Airway obstruction | Limits delivery of inspired oxygen to alveoli |
| Anaemia | Decreases oxygen-carrying capacity of blood |
| Decreased chest wall motion (e.g. from musculoskeletal impairments) | Prevents lowering of diaphragm and reduces anteroposterior diameter of thorax on inspiration, reducing volume of air inspired |
| Fever, pregnancy, infection | Increases metabolic rate and tissue oxygen demand |
| High altitude | Atmospheric oxygen concentration is lower and inspiratory oxygen concentration decreases |
| Toxic inhalant | Decreases oxygen-carrying capacity of blood |
DECREASED OXYGEN-CARRYING CAPACITY
Haemoglobin carries 99% of the diffused oxygen to tissues (Brashers, 2010a). Anaemia and inhalation of toxic substances decrease the oxygen-carrying capacity of blood by reducing the amount of available haemoglobin to transport oxygen. Anaemia, a lower than normal haemoglobin level, is a result of decreased haemoglobin production, increased red blood cell destruction and/or blood loss. Patients complain of fatigue, decreased activity tolerance and increased breathlessness, as well as pallor (especially seen in the conjunctiva of the eye) and an increased heart rate.
Carbon monoxide is the most common toxic inhalant that decreases the oxygen-carrying capacity of blood. The affinity for haemoglobin to bind with carbon monoxide is greater than 200 times its affinity to bind with oxygen, creating a functional anaemia. Because of the bond’s strength, carbon monoxide is not easily dissociated from haemoglobin, making the haemoglobin unavailable for oxygen transport.
DECREASED INSPIRED OXYGEN CONCENTRATION
When the concentration of inspired oxygen declines, the oxygen carried by the blood is decreased. Decreases in the fraction of inspired oxygen concentration (FiO2) can be caused by an upper-airway obstruction (e.g. croup in the child) or lower-airway obstruction (e.g. COPD in the adult) limiting delivery of inspired oxygen to alveoli; decreased environmental oxygen, such as at high altitudes; or decreased inspiration as a result of an incorrect oxygen concentration setting on respiratory therapy equipment.
HYPOVOLAEMIA
Conditions such as shock and severe dehydration resulting from extracellular fluid loss and reduced circulating blood volume cause hypovolaemia. With a significant fluid loss, the body tries to adapt by increasing the heart rate and peripheral vasoconstriction through sympathetic nervous system stimulation to increase the volume of blood returned to the heart and, in turn, increase the cardiac output. Consider the child with vomiting and diarrhoea from gastroenteritis. The nursing assessment will likely reveal a child who speaks minimally and is tachypnoeic (Airway and Breathing); pale, tachycardic with capillary refill greater than 3 seconds (Circulation); and is listless (Disability). The loss in circulating volume caused by the extreme fluid loss from the vomiting and diarrhoea means that preload and therefore stroke volume (SV) fall, dropping cardiac output (CO). Remember that CO = SV × HR. The sympathetic nervous system responds by increasing HR (tachycardic) and causing vasoconstriction which increases afterload (pale and capillary refill greater than 3 seconds), all to try to improve CO. At the same time, the respiratory centre is stimulated to increase respiratory rate (tachypnoeic) in an effort to deliver more oxygen. As CO has fallen, perfusion to the brain is diminished and so the child is listless.
INCREASED METABOLIC RATE
Increases in metabolic activity result in an increased oxygen demand. When body systems are unable to meet this increased demand, the level of oxygenation declines. An increased metabolic rate is a normal physiological response to pregnancy, wound healing and exercise because the body is building tissue. Most people can meet the increased oxygen demand and do not display signs of oxygen deprivation. Fever increases the tissues’ need for oxygen, and as a result carbon dioxide production also increases. If the febrile state persists, the metabolic rate remains high and the body begins to break down protein stores, resulting in muscle wasting and decreased muscle mass. Respiratory muscles such as the diaphragm and intercostal muscles are also wasted. The body attempts to adapt to the increased carbon dioxide levels by increasing the rate and depth of respiration. The patient’s work of breathing increases and the patient eventually displays signs and symptoms of hypoxaemia. Those patients with pulmonary diseases are at greater risk of hypoxaemia and hypercapnoea due to V/Q mismatch and alveolar hypoventilation (see later in the chapter). Assessment findings include an increased rate and depth of respiration, use of the accessory muscles of respiration, pursed-lip breathing and decreased activity tolerance.
CONDITIONS AFFECTING CHEST WALL MOVEMENT
Any condition that reduces chest wall movement can result in decreased ventilation. If the diaphragm cannot fully descend with breathing, the volume of inspired air decreases and less oxygen is delivered to the alveoli and subsequently to tissues. For example, thoracic or abdominal injury or surgery will cause considerable pain, which inhibits diaphragmatic and chest wall movement, reducing inspiration and therefore delivery of oxygen.
PREGNANCY
As the fetus grows during pregnancy, the greater size of the uterus pushes abdominal contents upwards against the diaphragm. In the last trimester of pregnancy, the inspiratory capacity declines, resulting in dyspnoea on exertion and increased fatigue.
OBESITY
Obese patients have reduced lung volumes from the heavy lower thorax and abdomen, particularly when in the recumbent and supine positions. Obese patients have a reduction in compliance as a result of encroachment of the abdomen into the chest, increased work of breathing and decreased lung volumes, and they may have fatigue and carbon dioxide retention. In some patients an obesity–hypoventilation syndrome develops in which oxygenation is decreased and carbon dioxide is retained, resulting in daytime sleepiness. The obese patient is also susceptible to pneumonia after an upper respiratory tract infection because the lungs cannot fully expand and pulmonary secretions are not mobilised in the lower lobes.
MUSCULOSKELETAL ABNORMALITIES
Musculoskeletal impairments in the thoracic region reduce oxygenation. Such impairments may result from abnormal structural configurations, trauma, muscular diseases and diseases of the central nervous system. Abnormal structural configurations impairing oxygenation include those that affect the rib cage, such as pectus excavatum (abnormal development of ribs causing caved in appearance), and those that affect the vertebral column, such as kyphosis.
TRAUMA
Two or more ribs fractured in two or more places results in a flail chest, a condition in which fractures cause instability in part of the chest wall. The unstable chest wall allows the lung underlying the injured area to contract on inspiration and bulge on expiration, resulting in hypoxia. Chest wall or upper abdominal incisions may also decrease chest wall movement as the patient uses shallow respirations to minimise chest wall movement to avoid pain. Narcotic analgesia, e.g. morphine, is often prescribed to relieve the pain, thereby facilitating improved chest wall expansion. Should excessive or high doses of narcotic analgesics be administered, this may depress the respiratory centre in the brain, further decreasing respiratory rate and chest-wall expansion and therefore decreasing oxygenation.
NEUROMUSCULAR DISEASES
Diseases such as muscular dystrophy affect oxygenation of tissues by decreasing the patient’s ability to expand and contract the chest wall. Ventilation is impaired, and atelectasis, hypercapnoea and hypoxaemia can occur. Myasthenia gravis and Guillain-Barré syndrome affect respiratory functioning and result in alveolar hypoventilation (see later in the chapter). Myasthenia gravis interferes with the normal transmission of impulses from nerves to muscles, involving the whole body, including muscles of respiration. Guillain-Barré syndrome causes inflammation and paralysis of muscle groups, which usually results in an ascending pattern of paralysis. Respiratory muscles become paralysed as paralysis ascends to the thoracic region.
CENTRAL NERVOUS SYSTEM ALTERATIONS
Diseases or trauma involving the medulla oblongata and spinal cord may result in impaired respiration. When the medulla oblongata (contains the respiratory centre) is affected, neural regulation of respiration is damaged and abnormal breathing patterns may develop. If the phrenic nerve is damaged, the diaphragm may not descend, thus reducing inspiratory lung volumes and causing hypoxaemia. Cervical trauma at vertebrae C3 to C5 can result in paralysis of the phrenic nerve. Spinal cord trauma below the fifth cervical vertebra usually leaves the phrenic nerve intact but damages nerves that innervate the intercostal muscles, preventing anteroposterior chest expansion.
INFLUENCES OF CHRONIC DISEASE
Oxygenation can be decreased as a direct consequence of chronic disease. It can also be decreased as a secondary effect, as with anaemia. The physiological response to chronic hypoxaemia is the development of a secondary polycythaemia. This adaptive response is the body’s attempt to increase the amount of circulating haemoglobin to increase the available oxygen-binding sites.
Alterations in cardiac functioning
Illnesses and conditions that affect cardiac rhythm, strength of contraction, blood flow through the atria and ventricles, myocardial blood flow and peripheral circulation cause alterations in cardiac functioning.
Disturbances in conduction
Some disturbances in conduction are a result of electrical impulses that do not originate from the SA node. These rhythm disturbances are called arrhythmias, meaning a deviation from the normal sinus rhythm (Table 40-2). Arrhythmias may occur as a primary conduction disturbance as a response to ischaemia, valvular abnormality, anxiety or drug toxicity; caffeine, alcohol or tobacco use; or as a complication of acid–base or electrolyte imbalance (see Chapter 39).
TABLE 40-2 COMMON CARDIAC ARRHYTHMIAS
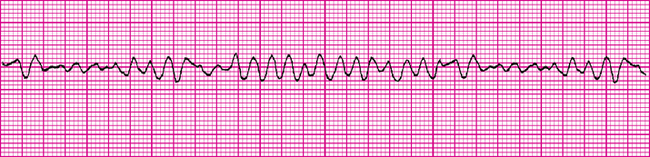
Aehlert B 2011 ECGs made easy, ed 4. St Louis, Mosby; Australian Resuscitation Council (ARC) 2010 Guideline 8, cardiopulmonary resuscitation. Melbourne, ARC. Online. Available at www.resus.org.au 30 May 2011; Moser DB, Riegel B 2008 Cardiac nursing: a companion to Braunwald’s Heart disease. Philadelphia, Saunders.
Images from Aehlert (2011).
Arrhythmias are classified by site of impulse origin and mechanism of the cardiac response. Cardiac response can be one of tachycardia (> 100 beats per minute), bradycardia (< 60 beats per minute), a premature (early) beat or a blocked (delayed or absent) beat. Tachyarrhythmia can lower cardiac output and blood pressure. Tachyarrhythmia reduces cardiac output by decreasing diastolic filling time. Bradyarrhythmia lowers cardiac output because of the decreased heart rate.
Abnormal impulses originating above the ventricles are referred to as supraventricular arrhythmias. The abnormality of the wave form is the configuration and placement of the P wave. Ventricular conduction usually remains normal and a normal QRS complex is observed. Junctional arrhythmias represent an abnormal site of impulse conduction above or below the AV node. The P wave can occur before, during or after the QRS complexes and is often inverted if visible. Because the beat originates above the ventricle, ventricular conduction and the QRS complex are usually normal. One of the most common supraventricular arrhythmias is atrial fibrillation and occurs most frequently with ageing and in heart failure. There is disorganised chaotic depolarisation of the atria at a very rapid rate (400–600 beats/min) resulting in fibrillatory waves. Conduction of many of these beats is blocked at the AV node and conduction through the ventricle is usually normal. The ventricular rate varies but is usually quite rapid. Due to the lack of synchrony between atria and ventricles, thrombi can form in the atria, potentially causing a stroke when sinus rhythm is restored (Jacobson, 2010b). Patients often require anticoagulation, usually with warfarin. A new anticoagulant, dabigatran, appears superior with fewer side effects (Talati and White, 2011). Management focuses on either rate control or rhythm control, with neither approach holding an outcome advantage over the other (Van Gelder and others, 2002; Wyse and others, 2002).
Ventricular arrhythmias represent an ectopic site of impulse formation within the ventricles. The configuration of the QRS complex is usually widened and bizarre. P waves may or may not be present; often they are buried in the QRS complex. Ventricular tachycardia and ventricular fibrillation are life-threatening arrhythmias that require immediate intervention. Ventricular tachycardia is considered a life-threatening arrhythmia because of the decreased cardiac output and the potential to deteriorate into ventricular fibrillation (Jacobson, 2010b).
Altered cardiac output
Failure of the myocardium to eject sufficient volume to the systemic and pulmonary circulations can result in heart failure. Failure of the myocardial pump results from primary coronary artery disease, cardiomyopathic conditions, valvular disorders and pulmonary disease.
LEFT-SIDED HEART FAILURE
Left-sided heart failure is an abnormal condition characterised by impaired functioning of the left ventricle due to elevated pressures. If left ventricular failure is significant, the amount of blood ejected from the left ventricle drops greatly, resulting in decreased cardiac output. Consider the patient experiencing an acute episode of heart failure. The nursing assessment will likely reveal a person who speaks in phrases, is tachypnoeic, is using accessory muscles of respiration, has crackles on auscultation of lung fields, has low oxygen saturations (Airway and Breathing) and is hypotensive and tachycardic with capillary refill greater than 3 seconds (Circulation). As the left ventricle is unable to pump effectively and stroke volume and therefore CO is reduced (hypotensive), sympathetic nervous system stimulation increases HR (tachycardic) and causes vasoconstriction (capillary refill greater than 3 seconds) to compensate. The increased volume and therefore pressure in the left ventricle due to its ineffective pumping action causes the pressure to be reflected backwards into the pulmonary circulation. The increased pressure forces fluid out of the pulmonary circulation into the interstitium and alveoli (crackles on auscultation), impairing oxygenation (low oxygen saturations). The respiratory centre is stimulated, resulting in an increased respiratory rate (tachypnoeic) to compensate and try to deliver more oxygen. Other assessment findings may include decreased activity tolerance, dizziness and confusion as a result of tissue hypoxia from the diminished cardiac output. Also, cough and paroxysmal nocturnal dyspnoea may be evident due to the pulmonary congestion.
RIGHT-SIDED HEART FAILURE
Right-sided heart failure results from impaired functioning of the right ventricle characterised by venous congestion in the systemic circulation. Right-sided heart failure more commonly results from pulmonary disease or as a result of long-term left-sided failure. The primary pathological factor in right-sided failure is elevated pulmonary vascular resistance (PVR). As the PVR continues to rise, the right ventricle must generate more work, and the oxygen demand of the heart increases. As the failure continues, the amount of blood ejected from the right ventricle declines, and blood begins to ‘back up’ in the systemic circulation. Clinically, the patient has weight gain due to fluid retention, distended neck veins, hepatomegaly and splenomegaly and dependent peripheral oedema.
Impaired valvular function
Valvular heart disease is an acquired or congenital disorder of a cardiac valve characterised by stenosis and obstructed blood flow or valvular degeneration and regurgitation of blood. When stenosis occurs in the semilunar valves (aortic and pulmonic valves), the adjacent ventricles must work harder to move the ventricular volume beyond the stenotic valve. Over time, the stenosis can cause the ventricle to hypertrophy (enlarge), and if the condition is untreated, left- or right-sided heart failure can occur. If stenosis occurs in the atrioventricular valves (mitral and tricuspid valves), the atrial pressure rises, causing the atria to hypertrophy. When regurgitation occurs, there is a backflow of blood into an adjacent chamber. For example, in mitral regurgitation the mitral leaflets do not close completely. When the ventricle contracts, blood escapes back into the atria, causing a murmur or ‘whooshing’ sound (see Chapter 27).
Myocardial ischaemia
Myocardial ischaemia results when the supply of blood to the myocardium from the coronary arteries is insufficient to meet the oxygen demands of the organ. Atherosclerotic plaque develops, narrowing the lumen of the coronary artery and leading to thrombus formation and eventually occlusion. Two common manifestations of this ischaemia are angina pectoris and myocardial infarction. These collectively are termed acute coronary syndrome. When a patient first presents with ischaemic type chest pain, the diagnosis of acute coronary syndrome is made until it is determined whether the presentation is angina pectoris or a myocardial infarction. This prevents delay in management (Aroney and others, 2006).
ANGINA
Angina pectoris is usually a transient imbalance between myocardial oxygen supply and demand due to reversible myocardial ischaemia. The condition results in chest pain that is aching, sharp, tingling or burning, or that feels like pressure. The chest pain may be left-sided or substernal and may radiate to the left or both arms, and to the jaw, neck and back. In some patients, anginal pain may not radiate. The pain can last from 1 to 15 minutes. Patients report that pain is often precipitated by activities that increase myocardial oxygen demand (e.g. exercise, anxiety or stress). The pain is usually relieved with rest and coronary vasodilators, the most common being a glyceryl trinitrate preparation, e.g. Anginine (Brashers, 2010c).
MYOCARDIAL INFARCTION
Myocardial infarction results from sudden decreases in coronary artery blood flow or an increase in myocardial oxygen demand without adequate coronary artery perfusion. Infarction occurs when ischaemia (which is reversible) remains untreated or inadequately treated and becomes necrosis (which is not reversible) of myocardial tissue (Brashers, 2010c).
Chest pain associated with myocardial infarction is usually described as crushing, squeezing or stabbing. The pain may be retrosternal and left praecordial, and it may radiate down the left arm to the neck, jaws, teeth, epigastric area and back. The pain occurs at rest or exertion, lasts more than 30 minutes and is unrelieved by rest, position change or sublingual glyceryl trinitrate administration.
Research indicates that there is a significant difference between men and women in relation to coronary artery disease. It is known that women do not always present the same type of symptoms as men (Miracle, 2010). Symptoms are likely to be more subtle in women, with atypical presentation such as a sensation of indigestion, heaviness or general discomfort. Dyspnoea, fainting, nausea, fatigue and palpitations are also common symptoms in women. A first presentation of coronary artery disease in women is likely to be angina, while in men a first presentation is more likely to be a myocardial infarction (Miracle, 2010). Oestrogen replacement in healthy postmenopausal women may reduce and prevent coronary artery disease. Additional risk factors for coronary artery disease in women include menopause and hormonal contraceptives, such as birth-control pills.
Alterations in respiratory functioning
Illnesses and conditions that affect ventilation or oxygen transport cause alterations in respiratory functioning. The main alterations are ventilation/perfusion (V/Q) mismatch, diffusion impairment, alveolar hypoventilation, alveolar hyperventilation and hypoxia.
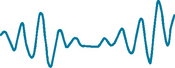
Alveolar hyperventilation
The goal of ventilation is to produce a normal arterial carbon dioxide tension (PaCO2) between 35 and 45 mmHg and maintain a normal arterial oxygen tension (PaO2) between 95 and 100 mmHg. Arterial oxygen levels can be monitored using a non-invasive oxygen saturation monitor. The normal range is 95–100%.
Alveolar hyperventilation is a state of ventilation in excess of that required to eliminate the normal venous carbon dioxide produced by cellular metabolism. Anxiety, infections, drugs or an acid–base imbalance can induce alveolar hyperventilation, as well as hypoxia associated with pulmonary embolus or shock. Acute anxiety can lead to alveolar hyperventilation and may cause loss of consciousness from excess carbon dioxide exhalation. Fever can cause alveolar hyperventilation. For each increase of 1°C there is a 12% increase in metabolic rate, thereby increasing carbon dioxide production. The clinical response is an increased rate and depth of respiration.
Alveolar hyperventilation may also be chemically induced. Salicylate (aspirin) poisoning causes excessive stimulation of the respiratory centre as the body attempts to compensate for excess carbon dioxide. Amphetamines also increase ventilation by raising carbon dioxide production. Alveolar hyperventilation can also occur as the body tries to compensate for metabolic acidosis by producing a respiratory alkalosis. For example, the patient with diabetes mellitus who has gone into diabetic ketoacidosis is producing large amounts of metabolic acids. The respiratory system tries to correct the acid–base imbalance by increasing the respiratory rate. Ventilation increases to reduce the amount of carbon dioxide available to form carbonic acid (see Chapter 39).
Alveolar hyperventilation produces many signs and symptoms that can be assessed (Table 40-3). Haemoglobin does not release oxygen to tissues as readily, and tissue hypoxia results. As symptoms worsen, the patient may become more agitated, which further increases the respiratory rate and can result in respiratory alkalosis.
TABLE 40-3 DIFFERENTIATING ALVEOLAR HYPERVENTILATION FROM HYPOVENTILATION
| HYPERVENTILATION | HYPOVENTILATION |
|---|---|
| FEATURES | |
| CARDIOVASCULAR SIGNS AND SYMPTOMS | |
| NEUROLOGICAL SIGNS AND SYMPTOMS | |
| OTHER | |
| Acid–base and electrolyte imbalances | Acid–base and electrolyte imbalances |
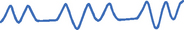
Alveolar hypoventilation
Alveolar hypoventilation occurs when alveolar ventilation is inadequate to meet the body’s oxygen demand or to eliminate sufficient carbon dioxide. As alveolar ventilation decreases, PaCO2 is elevated (hypercapnoea). Severe atelectasis can produce alveolar hypoventilation. Atelectasis is a collapse of the alveoli that prevents normal diffusion of oxygen and carbon dioxide. As alveoli collapse, less of the lung can be ventilated and alveolar hypoventilation occurs.
Patients with COPD have a progressive deterioration of the airways, leading to a loss of lung function resulting in alveolar hypoventilation. Some have a chronically elevated PaCO2 and low PaO2. In this instance, the central chemoreceptors primarily responsible for detecting changes in pH (and PaCO2) are less sensitive, and peripheral chemoreceptors are now the primary stimulus to ventilation. Remember that peripheral chemoreceptors are more sensitive to oxygen than carbon dioxide (Brashers, 2010a). A small percentage of these patients in response to supplemental oxygen will experience a significant elevation in PaCO2, which causes drowsiness and lethargy. This is known as hypercapnoeic encephalopathy or CO2 narcosis (Simmons & Simmons, 2004). To avoid this complication, supplemental oxygen therapy should be titrated to achieve oxygen saturations of 88–92% (Beasley and others, 2011).
Signs and symptoms of alveolar hypoventilation are presented in Table 40-3. If untreated, the patient’s status can rapidly decline. Convulsions, unconsciousness and death can result. Treatment for alveolar hyperventilation and alveolar hypoventilation requires improving tissue oxygenation, restoring ventilatory function, treating the underlying cause and achieving acid–base balance.
Ventilation/perfusion mismatch
For oxygenation to occur, both adequate ventilation and adequate perfusion is needed. This is termed the ventilation/perfusion (V/Q) ratio and is normally approximately 4 : 5 (ventilation 4 L/min : perfusion 5 L/min). If there is impairment with either mechanism, a V/Q mismatch occurs. The patient with pneumonia will have V/Q mismatch due to impaired ventilation, while a patient with a pulmonary embolism will have a V/Q mismatch due to impaired perfusion. The overall result is a lowering of oxygen (Pierce, 2007).
Diffusion impairment
The rate of diffusion of oxygen can be affected by the thickness of the alveolar–capillary membrane and the available surface area for diffusion. Increased thickness of the membrane impedes diffusion because oxygen takes longer to diffuse across. Patients with pulmonary oedema or pneumonia have an increased thickness of the alveolar–capillary membrane, resulting in slowed diffusion, slowed exchange of oxygen and impaired delivery of oxygen to tissues. The surface area of the membrane can be altered as a result of a chronic disease (e.g. emphysema), an acute disease (e.g. pneumothorax) or a surgical process (e.g. lobectomy). The alveolar–capillary membrane can be destroyed or may thicken, changing the rate of diffusion. When fewer alveoli are functioning, the surface area is decreased. This alteration in membrane thickness and/or surface area which results in impaired gas exchange is termed diffusion impairment (Pierce, 2007).
Hypoxia
Hypoxia is inadequate tissue oxygenation at the cellular level. This can result from a deficiency in oxygen delivery or oxygen utilisation at the cellular level. Hypoxia can be caused by:
1. a decreased haemoglobin level and lowered oxygen-carrying capacity of the blood
2. a diminished concentration of inspired oxygen, which may occur at high altitudes
3. the inability of the tissues to extract oxygen from the blood, as with cyanide poisoning
4. diffusion impairment as in pneumonia
5. poor tissue perfusion with oxygenated blood, as with shock
6. V/Q mismatch as with multiple rib fractures or chest trauma.
The clinical signs and symptoms of hypoxia include apprehension, restlessness, inability to concentrate, a declining level of consciousness, dizziness and behavioural changes (Box 40-4). The patient with a narcotic overdose, such as a heroin overdose, which depresses the respiratory centre leading to a decreased ventilatory drive, may display signs of alveolar hypoventilation. During early stages of hypoxia, the blood pressure is elevated unless the condition is caused by shock. As the hypoxia worsens, the respiratory rate may decline as a result of respiratory muscle fatigue.
BOX 40-4 HYPOXIA—SIGNS AND SYMPTOMS
Decreased ability to concentrate
Decreased level of consciousness
Cyanosis, blue discolouration of the skin and mucous membranes caused by the presence of desaturated haemoglobin in capillaries, is a late sign of hypoxia. The presence or absence of cyanosis is not a reliable measure of oxygenation status. Central cyanosis, observed in the tongue, soft palate and conjunctiva of the eye, where blood flow is high, indicates hypoxaemia. Peripheral cyanosis, seen in the extremities, nail beds and earlobes, is often a result of vasoconstriction and stagnant blood flow.
Hypoxia is a life-threatening condition. Untreated, it can produce cardiac arrhythmias that result in death. Hypoxia is managed by administration of oxygen and treatment of the underlying cause, such as airway obstruction.