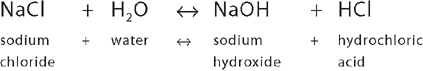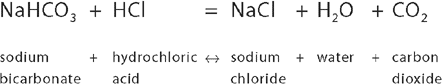1 INTRODUCTION TO CLINICAL SCIENCE
INTRODUCTION
Pathophysiology is the study of changes in body function that result from a disease or disorder. This altered condition may arise from an abnormal process within the body, or it may be the body’s response to a foreign substance that has entered the body. Pathophysiological changes can be short term and hence corrected relatively quickly, or they may form the basis of an underlying long-term condition that remains with the patient for decades. In addition, the functional changes may be minor and not noticeable, or they may be more extensive, producing distinct clinical symptoms.
Pathophysiology focuses on alterations in function, rather than alterations in structure (pathology is the study of changes in structure). However, because structure and function are related, a fundamental understanding of structural changes is necessary. When exploring pathophysiology, knowledge of the altered body process at the microscopic level constitutes an important link to understanding the effects and symptoms experienced by the patient. In addition, knowledge of the workings of the body at this microscopic level allows you to understand why the appropriate drugs and other treatment options are useful. In order to understand diseases, you first need grounding in the normal structure and functions of the human body. Therefore, studying pathophysiology also means understanding the disciplines of anatomy and physiology.
Anatomy is the study of normal body structure — what the body and individual components look like, and how the body is organised. It includes the normal anatomical relationships between structures, as well as the appearance of the individual components. Physiology is the study of normal body functions — the activities of the body and how its individual components work. Anatomy and physiology are often studied together, because the structure and functions of organs are interrelated.
In addition, having a basic knowledge of some concepts from the physical sciences, particularly chemistry and physics, contributes to understanding how the body works. The extremely small components of the body are actually molecules and electrolytes from the field of chemistry, while pressure changes during breathing can be explained using physics. Within this textbook, the focus on the physical sciences concentrates on using the sciences as a means to understand body processes.
After learning some basic principles from the physical sciences, as well as normal anatomy and physiology, you will have the essential knowledge to commence studies in pathophysiology — the biological and physical manifestations of a disease or disorder that represent a disruption to normal physiological processes.
ESSENTIAL PATHOPHYSIOLOGY
Pathophysiology and clinical manifestations
Many terms are associated with pathophysiology and these can be confusing, primarily because there is sometimes an overlap in meaning. Nonetheless, it is important that you understand these terms, as they are used throughout this text and you will hear them in clinical practice. We will introduce the terms working through an example of a patient, Mr Jones, to allow you to see how theory relates to clinical practice. Mr Jones has presented to hospital with chest pain, and in the following section we explore how we might learn about his condition (see Figure 1-1).
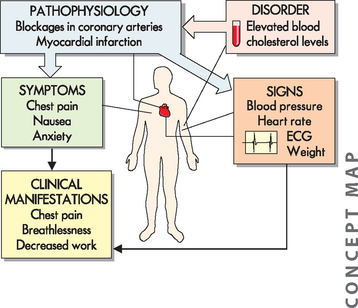
FIGURE 1-1 The relationship between pathophysiology, a disorder, signs and symptoms, and clinical manifestations.
Mr Jones presents to hospital with chest pain, breathlessness and a decreased ability to perform work. Upon examination, he also states that he feels nauseous and anxious. His blood pressure, heart rate, electrocardiogram (ECG) and weight are measured and considered in the diagnosis. A blood test reveals an elevated blood cholesterol level, a condition that contributes to the pathophysiological process of atherosclerosis (blockage of the coronary arteries).
Pathophysiology is the study of alterations to normal function that result from a disease or disorder. In clinical applications, the pathophysiology of the patient’s condition allows us to relate causes of disease processes to corresponding changes in the body. In our example, we need to consider the range of data that inform us about Mr Jones. This data comes from various sources, including a physical examination, medical history, family history, laboratory tests, medical imaging and other diagnostic tools.
Mr Jones’ chest pain may be due to blocked coronary arteries, and diagnostic tools to confirm this would include blood tests to measure his level of cholesterol and other associated substances, as well as an electrocardiogram (ECG), which looks at electrical conduction through the heart. This would provide the clinician with information about the extent of the coronary artery blockage, as well as any history of any heart disease. For example, Mr Jones’ blood tests show an increased level of cholesterol in the blood, which can result in the pathophysiological condition of blockage of the coronary arteries (known as atherosclerosis) and lead to myocardial infarction, commonly referred to as heart attack.
A sign is a measurement or recording of something that is objective, such as a patient’s heart rate, temperature, weight or blood glucose level. You would expect that different healthcare professionals measuring the signs of one patient at one point in time would observe and record the same results. For Mr Jones, these signs include his heart rate, blood pressure and ECG findings. The healthcare professional observes these signs and combines them with the symptoms to provide evidence about the extent and type of the pathophysiological process. A symptom is a more subjective indication of the patient’s experience as reported by the patient — such as the amount of pain, or feeling bloated. Symptoms can vary from person to person, even if their clinical signs are similar. Mr Jones may describe the pain being more intense in the left of his chest and his left arm and less painful in his right shoulder. Another patient with the same medical condition may report differing degrees of pain. The patient’s perception of symptoms is individual but must be recognised. For example, if a patient reports discomfort due to nausea, this experience needs to be recognised and addressed, regardless of whether another person would consider the nausea to be of concern.
Signs and symptoms are often considered together in practice, because both sources of information are used to assist with diagnosis. Healthcare professionals become quite expert in recognising particular signs and symptoms in diagnosing and treating diseases, and therefore they are particularly relevant to you as a student of health science. In this textbook, we usually consider signs and symptoms together as clinical manifestations and so use this term to encompass the range of consequences that would usually be anticipated for a person with a particular disorder. Clinical manifestations may be quite localised to a particular region of the body, or they may be systemic to the whole body, such as fatigue. Clinical manifestations for Mr Jones include chest pain and breathlessness, which in turn impair his ability to perform physical tasks (see Figure 1-1).
Disorders and diseases
A disorder (or condition) refers to disturbances or abnormality of function and indicates incomplete health. (Other general terms that are used include illness and sickness.) An example of a disorder is hypertension (or high blood pressure), where the cardiovascular system is altered, which can lead to other body systems being affected. The disorder is unable to be corrected by the body.
Syndrome refers to a set of signs and symptoms that occur together and are often specific to a particular syndrome, but usually the actual cause of the syndrome is unknown. For example, metabolic syndrome is associated with a range of criteria and provides a link between obesity and the development of diabetes mellitus. There are several risk factors for this syndrome; however, the exact pathophysiological processes are not fully understood.
Conversely, disease is a specific term reserved for the characteristic or distinguishing features that correspond to a particular pathophysiological condition. A disease usually has a well-defined series of events (including the cause, signs and symptoms) and a corresponding set of diagnostic and treatment strategies. For example, coronary heart disease refers to blockage of the coronary arteries of the heart, which decreases the flow of oxygen and nutrients to heart muscle. This causes chest pain and may lead to complete blockage and death of the tissue.
The aetiology of a disorder or disease refers to the underlying cause. In some cases, the aetiology may be well known, while in other cases the underlying cause of the illness is poorly understood.
The onset of disease
The onset of disease refers to how quickly a disease develops; this information usually assists in determining the correct diagnosis.
An acute disease or condition usually develops quickly and resolves or heals quickly, but can be mild, severe or even fatal. However, because acute diseases usually last for only a relatively short period, often there is no permanent associated damage. Sometimes, acute diseases do not require complicated treatment procedures: it may be suitable to ensure that the individual is well-hydrated and resting to allow the process to ‘run its course’. An example of an acute disease is pharyngitis (commonly referred to as a sore throat), resulting from inflammation of the upper airways and usually caused by a virus. In this case, treatment consists of support and monitoring for any worsening of symptoms.
Conversely, a chronic disease or condition develops more gradually and lasts for a longer time, even a lifetime. Chronic diseases may also recur frequently. Similar to acute diseases, chronic diseases can be mild, severe or fatal. The most typical chronic diseases are often mild early in the disease process, when the patient is often unaware of the early stages of the disease, but by the time the disease has progressed substantially, there is usually permanent damage. Many chronic diseases are also insidious, meaning that onset is gradual such that the disease is well-established before it is detected. The first symptoms are often vague and non-specific and do not alert the patient or healthcare staff. Such symptoms may include tiredness, weakness or loss of weight. The most prevalent chronic diseases in Australia and New Zealand are heart disease and cancer. Despite the best efforts of the patient and the medical treatment available, it is quite difficult to say that a patient has been completely cured of a chronic disease.
Population-level indicators of disease
Epidemiology is the study of factors that affect the health of populations and it includes information about each type of disease, how often it occurs and the type of people who acquire the disease, such as children, females, the elderly or a particular racial group. This information is vitally important for health professionals in determining who is likely to acquire the disease and the success of treatments. In this section we explain the main terms used to describe disease in a population.
The incidence of a disease refers to the number of new cases that have been diagnosed and confirmed; it is usually calculated as the number of cases that are diagnosed within one year. It illustrates what is happening currently in the population, and is particularly useful for considering those diseases that appear to be increasing within a community. In addition, this data is an indication of the effectiveness of various national health strategies that are currently being promoted in Australia and New Zealand in an attempt to decrease the incidence of particular diseases, such as asthma and diabetes mellitus.
In contrast, the prevalence of a disease includes the number of new cases, as well as those who have been diagnosed previously. It is used as an overall indication of the total number of people who are affected by a disease at a particular time, regardless of whether they have been diagnosed for a short or long time. We often refer to the prevalence of a disease in this textbook as it provides a measure of how common a disease is in the population.
The prevalence of a disease can also be considered by looking at the morbidity, which is the proportion of the population with the disease (relating the number with the disease to the number without the disease). The term ‘morbidity’ is often restricted to those disorders that result in a substantial loss of function and therefore present a significant impact on individuals concerned as well as the healthcare system. Co-morbidity refers to the presence of another disease or condition in the same patient or group of patients. The presence of co-morbidities increases the risk of significant health problems. Co-morbidities are particularly relevant when studying heart disease. For example, hypertension (high blood pressure) and hyperlipidaemia (high levels of lipids in the blood) are co-morbidities that contribute to worsening of heart disease.
Another way of examining the severity of a disease at the population level is to examine the mortality rate (or death rate). Diseases with a high mortality rate are of great concern regarding the health of the community. Individuals with an increased number of co-morbidities often have a greater risk of death, and this contributes significantly to the mortality rate of particular diseases.
In this textbook, we have used the latest available population-level data as a guide to which conditions you are most likely to encounter clinically. Accordingly, we focus on the most prevalent diseases in Australia and New Zealand, which are remarkably similar. You will thus learn about the current health status of these countries, as well as which conditions have the greatest impact on our healthcare system and are therefore particularly common within the hospital environment.
Age groups within the population
Diseases and disorders may be more pertinent in certain age groups. The population is generally split into the following age groups:
 Ageing: adults over the age of 65. As in many other countries, this part of the population is increasing in Australia and New Zealand.
Ageing: adults over the age of 65. As in many other countries, this part of the population is increasing in Australia and New Zealand.Such age groups are relevant for health professionals because the anatomical and physiological differences between the groups may impact on the clinical presentation and pathophysiological processes of the diseases that individuals acquire. Furthermore, certain populations are more prone to particular diseases; for example, cancer is usually associated with older individuals. Finally, when determining treatment options, social and psychological differences between the groups usually need to be considered, as these may impact on the implementation and even the success of the treatment.
Evaluation and treatment
Combining information from a patient’s history, signs and symptoms, laboratory tests and medical imaging allows medical staff to make a diagnosis. Establishing the correct diagnosis is important, because identification of the disease forms the basis for determining the appropriate treatment. Treatment options are often surgical or medical. Surgery involves an operation or a procedure to correct an abnormality or to remove an area of tissue; it ranges from minor skin procedures through to major surgery requiring opening of a body region and carrying a life-threatening risk for the patient. Treatment using medicine relies on the use of drugs or pharmacological agents. In this textbook we focus on why particular drugs are useful, whereas studies of pharmacology include more-detailed information on dosing regimens and the safe administration of drugs.
Other treatment and management options may include dietary changes or vitamin supplements, exercise, rest or avoiding triggers that worsen the condition (such as allergens). In many cases, these options may be as important as surgical and medical treatments.
ESSENTIAL ANATOMY
Anatomy refers to the structure of the body, and is an essential discipline for all students of the health professions. Not only do you need to know the names of the main body parts, such as the organs, blood vessels and bones, but you also need a basic understanding of the language of anatomy. This language includes some directional terms that are more specific than in everyday language. For example, consider the word ‘upward’. If you wish to move a part of the body upward, the movement may vary depending on whether you are lying, standing or sitting. For clarity, anatomical language provides more precise descriptive terms.
Anatomical position
One of the first considerations in studying anatomy is the actual positioning of the patient’s body. Some patients will be sitting, some will be lying down and others will be curled up asleep on the bed — not to mention children who cannot keep still! How then can you describe the location of particular structures if patients’ bodies are not always in the same position? The answer is to describe the features of the body based on the anatomical position. This is a reference position, and it is useful to take a moment to put your own body into the anatomical position as you read this — it is a great way of helping you to remember the position. Stand upright, with your arms by your side and palms forward, your thumbs facing out (away from your legs; see Figure 1-2). You will notice that in the anatomical position, the inside of the elbow is always visible from the front; otherwise, it would be difficult to distinguish between the ‘front’ and ‘back’ of the arm. The patient does not move into the anatomical position to make things easier for you; rather, you need to consider the patient’s body as if it were in anatomical position, and to use that reference position whenever you think of or write about body parts.
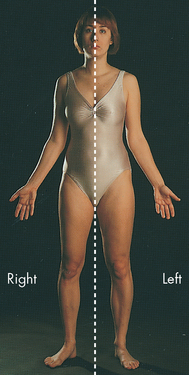
FIGURE 1-2 The anatomical position and bilateral symmetry.
In the anatomical position, the body is in an erect, or standing, posture with the arms at the sides and palms forward. The head and feet are also pointing forward. The dotted line shows the axis of the body’s bilateral symmetry: the right and left sides of the body are mirror images of each other.
Source: Based on Thibodeau GA, Patton KT. Anatomy & physiology. 6th edn. St Louis: Mosby; 2007.
Another aspect of learning anatomical terminology is not actually learning new words, but learning how to appropriately use the words ‘left’ and ‘right’ — these must be used to describe the patient’s left and right sides (see Figure 1-2). This can take a bit of getting used to. It is easier if you imagine the organ or limb of interest as part of your own body — then identifying left from right is not so confusing. Diagrams of the body or parts of the body are often included in patients’ medical records, so it is particularly important that you can identify the correct orientation. Internal organs also need to be recognised and positioned correctly, separately from the rest of the body, for specific uses such as transplantation, and some surgeries and emergency medicine.
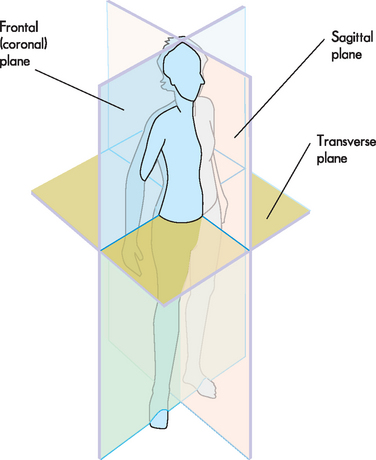
Body sections and planes
Correct orientation of organs is necessary not only when the organs are actually exposed or removed from the body, but also for regular viewing of images of internal structures, such as MRI (magnetic resonance imaging) and X-ray images. Look at the three images in Figure 1-3. Although these images all show the brain, each one appears quite different, and at first it may seem that they cannot be from the same structure. However, it is important to remember that each image is a different two-dimensional view through a three-dimensional structure. Two-dimensional views can be taken from different angles, and therefore medical terminology requires us to use descriptions that inform the orientation of the image.
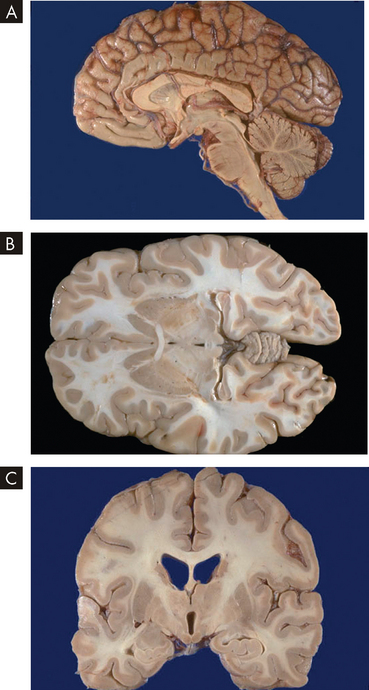
FIGURE 1-3 Anatomical sections through the brain.
A Midsagittal section; B transverse section; and C frontal section.
Source: Klatt EC. Robbins & Cotran atlas of pathology. 2nd edn. Philadelphia: Saunders; 2010.
The following terminology is used, based on the concept of passing a plane through the body to produce a two-dimensional anatomical section (see Figure 1-4):
 The sagittal plane divides the body into left and right. The term midsagittal means the exact middle of the body (through the nose and navel).
The sagittal plane divides the body into left and right. The term midsagittal means the exact middle of the body (through the nose and navel). The transverse plane divides the body into upper and lower — imagine a horizontal slice being inserted into the body (in the anatomical position of course, so the body would be upright).
The transverse plane divides the body into upper and lower — imagine a horizontal slice being inserted into the body (in the anatomical position of course, so the body would be upright).Anatomical directional terminology
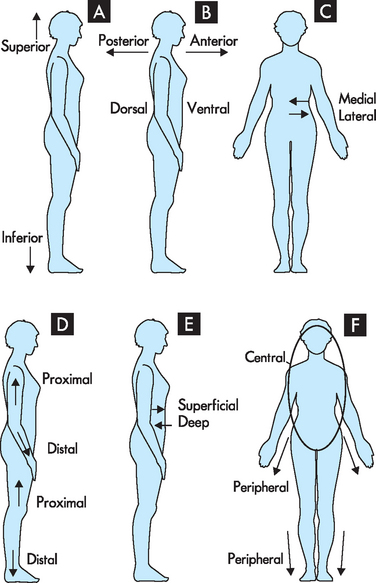
Anatomical directional terminology is used to give specific directions about body structures. For example, how would you describe the location of a suspicious mole on a patient’s back? The back has a reasonably large area, and if the patient has many moles, finding a particular mole again may be difficult. It would be useful to be able to describe the location of the mole so that it can easily be found again. This is where directional terms are useful. The terms comprise opposite pairs. It is worth committing these terms to memory; you may like to stand up and point them out on yourself. Most terms have a similar meaning to their use in everyday language, which should assist you to remember the definitions (see Figure 1-5).
 Superior means towards the head; inferior means towards the feet. For example, the nose is superior to the navel (remember: the monarch is superior as the head of the kingdom; the inferior servants bow down towards the monarch’s feet). The transverse section divides the body into superior and inferior (see Figure 1-5A).
Superior means towards the head; inferior means towards the feet. For example, the nose is superior to the navel (remember: the monarch is superior as the head of the kingdom; the inferior servants bow down towards the monarch’s feet). The transverse section divides the body into superior and inferior (see Figure 1-5A). Anterior means towards the front; posterior means towards the back. For example, the navel is anterior to the spine (remember: insect antennae are at the front of their bodies, while you sit down on your posterior). A coronal section divides the body into anterior and posterior. In humans, additional terms are used to describe anterior and posterior: ventral (anterior) and dorsal (posterior — towards the vertebral surface or back; dorsal actually means the same as superior in a four-legged animal) (see Figure 1-5B).
Anterior means towards the front; posterior means towards the back. For example, the navel is anterior to the spine (remember: insect antennae are at the front of their bodies, while you sit down on your posterior). A coronal section divides the body into anterior and posterior. In humans, additional terms are used to describe anterior and posterior: ventral (anterior) and dorsal (posterior — towards the vertebral surface or back; dorsal actually means the same as superior in a four-legged animal) (see Figure 1-5B). Medial means towards the middle or midsagittal section (the vertical line that extends through the nose and navel); lateral means away from the midline, or towards the side. For example, the nose is medial to the ear (remember: medial sounds like middle, while being a lateral thinker means that you can think ‘sideways’ about an issue — also, ipsilateral means on the same side, while contralateral means on the other side) (see Figure 1-5C).
Medial means towards the middle or midsagittal section (the vertical line that extends through the nose and navel); lateral means away from the midline, or towards the side. For example, the nose is medial to the ear (remember: medial sounds like middle, while being a lateral thinker means that you can think ‘sideways’ about an issue — also, ipsilateral means on the same side, while contralateral means on the other side) (see Figure 1-5C). Proximal means towards the point of attachment to the body trunk; distal means further from the point of attachment. For example, the foot is distal to the knee (remember: something in close proximity is nearby, while distant is further away) (see Figure 1-5D).
Proximal means towards the point of attachment to the body trunk; distal means further from the point of attachment. For example, the foot is distal to the knee (remember: something in close proximity is nearby, while distant is further away) (see Figure 1-5D). Superficial means towards the body skin or surface; deep means towards the body centre or core. For example, the stomach is deep to the skin (remember: if you have a superficial relationship with someone you know only the outer things about them; if you have a deep relationship with someone you know some deeper secrets about them) (see Figure 1-5E).
Superficial means towards the body skin or surface; deep means towards the body centre or core. For example, the stomach is deep to the skin (remember: if you have a superficial relationship with someone you know only the outer things about them; if you have a deep relationship with someone you know some deeper secrets about them) (see Figure 1-5E). Central means towards the body core, usually the head and trunk; peripheral means towards the body periphery or extremities (towards the hands and feet). For example, the brain is central, while the nerves that can sense pain in the foot are located peripherally (remember: centre means towards the middle or main part of something, while peripheral refers to something spread out or further away) (see Figure 1-5F).
Central means towards the body core, usually the head and trunk; peripheral means towards the body periphery or extremities (towards the hands and feet). For example, the brain is central, while the nerves that can sense pain in the foot are located peripherally (remember: centre means towards the middle or main part of something, while peripheral refers to something spread out or further away) (see Figure 1-5F).Body cavities and quadrants
In broad terms, the central part of the body, namely the head and trunk, can be divided into cavities (see Figure 1-6). The dorsal cavity is in the dorsal (back) part of the body and contains the central nervous system. It is subdivided into the cranial cavity above and the spinal cavity below: the cranial cavity within the skull encloses the brain, while the spinal cavity within the bony vertebrae contains the spinal cord. The ventral cavity is on the ventral (front) side of the trunk and contains most of the internal organs of the trunk. It is subdivided into the thoracic cavity and the abdominopelvic cavity. The superior part of the ventral cavity is the thoracic cavity within the chest (containing the heart, lungs, trachea and oesophagus). The thoracic cavity is subdivided into the mediastinum in the centre and the pleural cavities to the sides. The inferior part of the ventral cavity is known as the abdominopelvic cavity, which is subdivided into the abdominal cavity and the pelvic cavity. The abdominal cavity contains the stomach and intestines, pancreas, gallbladder, spleen, kidneys and adrenal glands; and the pelvic cavity contains the internal organs of reproduction and the urinary bladder.
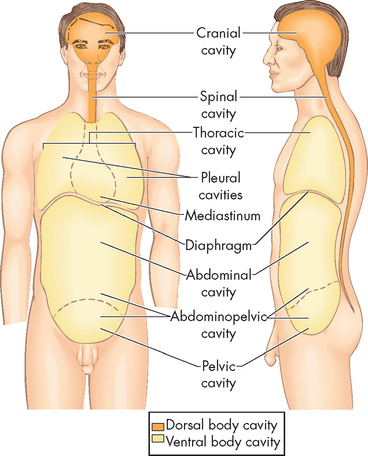
FIGURE 1-6 Major body cavities.
The dorsal cavity is in the dorsal (back) part of the body and contains the central nervous system. The ventral cavity is on the ventral (front) side of the trunk and contains most of the internal organs of the trunk.
Source: Patton KT, Thibodeau GA. Anatomy & physiology. 7th edn. St Louis: Mosby; 2010.
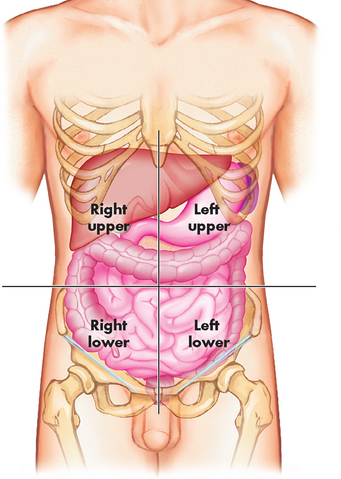
The abdominal quadrants are formed by passing a line through the umbilicus (navel) to form the left and right upper quadrants, and left and right lower quadrants (see Figure 1-7). These terms are particularly useful for locating an area of interest, such as when a patient is describing where he or she is experiencing pain. The right upper quadrant contains the liver, gallbladder and part of the large intestine; the left upper quadrant contains the stomach, pancreas, spleen and some large intestine; the right lower quadrant contains the right kidney, appendix and part of the small and large intestines; and the left lower quadrant contains the left kidney and part of the small and large intestines. We introduce the functions of these organs in the section ‘Essential physiology’.
Health science terminology
Many of the words that you will encounter in your studies are derived from Greek and Latin and will be easier to understand if you can split the words into their component parts. Some of the most commonly used prefixes (start of words) are shown in Table 1-1, while suffixes (end of words) are shown in Table 1-2. You may already be familiar with several of these. A full listing of prefixes and suffixes is provided in Appendix B for easy reference.
| PREFIX | MEANING |
|---|---|
| a-, an- | lack of |
| auto- | self |
| adip- | fat |
| cardio- | heart |
| cerebro- | brain |
| dys- | difficulty |
| endo- | inner, within |
| epi- | over |
| erythro- | red |
| exo-, extra- | outside |
| haem- | blood |
| hepat- | liver |
| hyper- | excess |
| hypo- | deficient |
| inter- | between |
| intra- | within |
| iso- | equal |
| leuco- | white |
| myo- | muscle |
| nephro- | kidney |
| osteo- | bone |
| peri- | around |
| pneum- | lungs, respiration |
| pulm- | lung |
| SUFFIX | MEANING |
|---|---|
| -aemia | in the blood |
| -cyte | cell |
| -ectomy | cutting out |
| -ia | state or condition of |
| -itis | inflammation |
| -ology | study of |
| -oma | tumour |
| -stemy | surgical opening |
For example, one term that you will notice repeatedly throughout this text, as well as in your career in the health industry, is hypoxia: ‘hypo’ meaning deficient, ‘ox’ for oxygen and ‘ia’ for a condition. So hypoxia is the condition of having low oxygen in the tissues. A similar term is hypoxaemia: ‘hypo’ meaning deficient, ‘ox’ for oxygen and ‘aemia’ for in the blood. Both hypoxia and hypoxaemia refer to insufficient levels of oxygen, but one refers to tissues and the other to blood.
It is worth mentioning several spelling conventions that differ between Australia/New Zealand and the United States. For example, in Australia/New Zealand we use the prefix ‘haem-’ and suffix ‘-aemia’, whereas Americans use ‘hem-’ and ‘-emia’. There are also some name differences between the United States and Australia/New Zealand. One very important example is the substance adrenaline, which is made by the body and is also used as a drug in cardiac arrest situations. In the United States, adrenaline is known as epinephrine (similarly, noradrenaline is known as norepinephrine).
ESSENTIAL PHYSIOLOGY
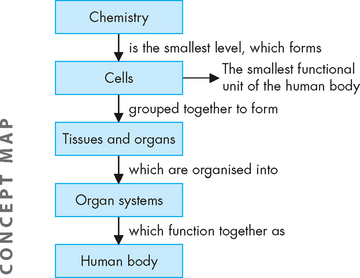
Physiology is the study of normal body functions and forms a basis for understanding subsequent changes to the body during disorder and disease. Each structure in the body is specialised to perform a unique and important function. These functions are undertaken at the smallest level, the cell. Cells work together in functional units called organs, which are grouped into organ systems. Physiology and pathophysiology are usually approached at the level of the organ systems, with knowledge of the cellular level giving more detail of the mechanisms involved. The organ systems are interrelated and dependent on each other to achieve a fully functional body.
The hierarchy from microscopic to whole body level
The smallest functional unit of the human body is the cell. Cells can be viewed only by using a microscope: an average cell diameter is between 7 and 100 micrometres (1 micrometre is 1/1000 of a millimetre). Cells are often squashed closely together with little space between them. In schematic diagrams they may be depicted as simplified squares or circles, but cells are three-dimensional, so you should visualise them more like cubes or spheres. The individual cells function by constantly exchanging material with the surrounding fluid. For example, nutrients and oxygen enter the cell; and wastes and carbon dioxide exit the cell. Cell size and function depend on cell type.
The components of the cell may be examined on a substantially smaller scale to reveal the underlying chemistry. This chemistry is often the key to understanding cellular changes that result from disease, as well as how drugs are able to provide effective treatment. On a larger scale, cells are arranged together as tissues, and tissues are organised to form organs (see Figure 1-8). Organ systems consist of organs working together to achieve a common goal. The organ systems are interrelated and depend on each other to achieve a fully functional and healthy body. As the components of a system work closely together, functions are usually studied according to systems.
Organ systems
An overview of the function of different parts of the body is useful, as it provides an introduction to all areas of the body. Each organ system works with the other organ systems to achieve a healthy human body.
 The nervous system consists of the brain, spinal cord and neurons, which sense variables throughout the body and stimulate the muscles to contract. The brain provides critical regulation over all body processes, as well as having the extremely sophisticated tasks of personality, emotion and memory.
The nervous system consists of the brain, spinal cord and neurons, which sense variables throughout the body and stimulate the muscles to contract. The brain provides critical regulation over all body processes, as well as having the extremely sophisticated tasks of personality, emotion and memory. The endocrine system consists of the organs, glands and cells that secrete hormones to regulate the anatomy and physiology of particular targets; the endocrine system assists the nervous system in this function.
The endocrine system consists of the organs, glands and cells that secrete hormones to regulate the anatomy and physiology of particular targets; the endocrine system assists the nervous system in this function. The immune system consists of a range of cell types and tissues that protect and defend the body from destruction by foreign particles including bacteria, viruses and cancer cells.
The immune system consists of a range of cell types and tissues that protect and defend the body from destruction by foreign particles including bacteria, viruses and cancer cells. The integumentary system comprises the skin and mucous membranes (in areas without skin) that form the external covering of the body. It provides a barrier that assists with the protection of the body from foreign substances.
The integumentary system comprises the skin and mucous membranes (in areas without skin) that form the external covering of the body. It provides a barrier that assists with the protection of the body from foreign substances. The haematological system comprises the blood and bone marrow that form the blood components. The general functions of the blood are the transport of nutrients and wastes, and protection (working closely with the immune system).
The haematological system comprises the blood and bone marrow that form the blood components. The general functions of the blood are the transport of nutrients and wastes, and protection (working closely with the immune system). The musculoskeletal system (muscles and bones) allows the body to move, as well as providing physical support for the internal organs. The functions of this system are far more critical than just allowing us to move — for example, breathing requires the function of the diaphragm (a large muscle), while the bones of the skull protect the brain.
The musculoskeletal system (muscles and bones) allows the body to move, as well as providing physical support for the internal organs. The functions of this system are far more critical than just allowing us to move — for example, breathing requires the function of the diaphragm (a large muscle), while the bones of the skull protect the brain. The cardiovascular system consists of the heart and blood vessels, which provide the means for the blood to travel to every body cell.
The cardiovascular system consists of the heart and blood vessels, which provide the means for the blood to travel to every body cell. The respiratory system (respiratory tract and lungs) exchanges oxygen and carbon dioxide with the environment, as well enabling the production of speech.
The respiratory system (respiratory tract and lungs) exchanges oxygen and carbon dioxide with the environment, as well enabling the production of speech. The digestive system includes the mouth, oesophagus, stomach, intestines, liver, gallbladder and pancreas. The overall purpose of this system is to break down food into small products that can be absorbed into the bloodstream and become available for all body cells. Undigested food leaves the body as the faeces.
The digestive system includes the mouth, oesophagus, stomach, intestines, liver, gallbladder and pancreas. The overall purpose of this system is to break down food into small products that can be absorbed into the bloodstream and become available for all body cells. Undigested food leaves the body as the faeces. In the urinary system, fluid balance is performed by the kidneys, with excess fluid and a range of wastes exiting the body in the urine. Other components of the system include the urinary bladder for temporary storage of urine, the ureters and the urethra, which drains urine from the body.
In the urinary system, fluid balance is performed by the kidneys, with excess fluid and a range of wastes exiting the body in the urine. Other components of the system include the urinary bladder for temporary storage of urine, the ureters and the urethra, which drains urine from the body. The reproductive system consists of glands and organs that allow for the production of offspring. Although the anatomy of this system is distinctly different between males and females, it is the functioning of the two sexes together that achieves reproduction.
The reproductive system consists of glands and organs that allow for the production of offspring. Although the anatomy of this system is distinctly different between males and females, it is the functioning of the two sexes together that achieves reproduction.Each system needs to function adequately to maintain a healthy body. In a scientific approach, the essential nature of the reproductive system may be questioned; however, this system is vital for the production of offspring, thereby providing new life and continuation of the species.
An overriding theme for all body processes is homeostasis, whereby the body maintains stable conditions despite changes in the environment. This vital concept is explored in Chapter 2.
ESSENTIAL CHEMISTRY
Before turning to the details of pathophysiology, it is necessary for you to understand some basic principles of chemistry, because the components of the body are made of chemical structures. This section provides an important background for your understanding of the human body. Rest assured that only chemistry that is essential and relevant for your study of pathophysiology has been included.
Elements
The body is composed of a number of elements (an element is a substance that cannot be broken down any further). Fortunately, you will have already heard of most of the relevant elements: the four listed in Table 1-3 are the main elements that make up most of the human body.
Table 1-3 MAJOR ELEMENTS OF THE HUMAN BODY
| NAME | SYMBOL |
|---|---|
| Carbon | C |
| Hydrogen | H |
| Oxygen | O |
| Nitrogen | N |
Ions and electrolytes
Elements in the body that have an electrical charge are called ions. A positively charged ion is called a cation and a negatively charged ion is an anion. The main ions of the human body and their charges are listed in Table 1-4. If an ion has two or more positive charges, the number of charges is written just before the ‘+’ sign in superscript, such as Ca2+. If an ion has only one positive charge, the number ‘1’ does not need to be written beside the ‘+’ sign; for example, Na+ has one positive charge.
Table 1-4 COMMON ELECTROLYTES (IONS) OF THE HUMAN BODY
| NAME | SYMBOL |
|---|---|
| Cation | |
| Sodium | Na+ |
| Potassium | K+ |
| Hydrogen | H+ |
| Calcium | Ca2+ |
| Magnesium | Mg2+ |
| Anion | |
| Chloride | Cl− |
| Hydroxide | OH− |
| Bicarbonate | HCO3− |
| Sulfate | SO42– |
| Phosphate | PO43– |
Ions with opposite charges attract each other. This allows the chemical substances to exist as a unit. An example that you will no doubt be familiar with is common table salt, NaCl, which occurs because the positive sodium and negative chloride interact, as follows:
This equation is written with a double-headed arrow, indicating that the process can move from left to right or from right to left, depending on the conditions at the time. This means that the sodium and chloride ions can exist independently of each other or combine to form sodium chloride. This process is vital to the body: by reversing chemical reactions, the body can maintain chemical equilibrium (or balance).
Ions are also electrolytes, meaning they have the ability to conduct electricity. In water, NaCl will separate back out (dissociate) into the individual ions Na+ and Cl−. Electrolytes are of particular interest to healthcare staff who need to identify and replace lost electrolytes in patients who are suffering from vomiting, diarrhoea or blood loss. In fact, electrolyte replacements consist largely of the electrolytes covered in this section. Electrolytes are measured in millimoles per litre (mmol/L).
Molecules and compounds
Molecules contain two or more of the same element together and compounds contain two or more different elements together. Some of the main molecules and compounds found in the human body are listed in Table 1-5. You will no doubt have heard of many of them, and you can now become familiar with their symbols as well. The symbols contain a code for understanding how a compound is formulated; for example, carbon dioxide is written as CO2 — that is, one carbon atom (shown by the C) and two oxygen atoms (shown by the subscript ‘2’ after the O). Another clue is the use of ‘di’ in ‘dioxide’ — ‘di’ means two, indicating the presence of two oxygen atoms in the molecule.
Table 1-5 COMMON MOLECULES AND COMPOUNDS OF THE HUMAN BODY
| NAME | SYMBOL | DESCRIPTION |
|---|---|---|
| Water | H2O | 2 hydrogen, 1 oxygen |
| Oxygen | O2 | 2 oxygen |
| Carbon dioxide | CO2 | 1 carbon, 2 oxygen |
| Glucose | C6H12O6 | 6 carbon, 12 hydrogen, 6 oxygen |
| Sodium bicarbonate | NaHCO3 | 1 sodium, 1 hydrogen, 1 carbon, 3 oxygen |
Water
Two-thirds of your body weight is water; all body processes depend on adequate amounts of water. Body fluids such as blood, brain fluid (cerebrospinal fluid), mucus and saliva are water-based, and most substances dissolve in water.
The following general properties of water have significance for the body:
 It absorbs heat. Liquid water increases and decreases temperature relatively slowly. This is particularly useful in that it can prevent sudden and severe changes in the temperature of body fluids.
It absorbs heat. Liquid water increases and decreases temperature relatively slowly. This is particularly useful in that it can prevent sudden and severe changes in the temperature of body fluids. It is a lubricant. Water is an important component of lubricating fluids such as saliva, and it allows organs to slide against other tissues without causing friction.
It is a lubricant. Water is an important component of lubricating fluids such as saliva, and it allows organs to slide against other tissues without causing friction.Substances that mix well with water are described as being hydrophilic (‘hydro’ = water, ‘philic’ = loving); while substances that do not mix well with water are hydrophobic (‘phobic’ = hating). Think about oily or fatty deposits that accumulate on greasy pans in the kitchen — these molecules are definitely hydrophobic and cannot be easily removed using water alone.
Water molecules comprise two hydrogen atoms and one oxygen atom, thus forming H2O. These elements can separate out (dissociate), thereby producing a hydrogen ion (H+) and a hydroxide ion (OH−):
Again, this is a reversible reaction. If we consider what might happen to salt when it is mixed with water, we see that the salt and water particles separate out and form new complexes:
The new complexes are NaOH, sodium hydroxide, and HCl, hydrochloric acid. It is useful to understand how these ions and compounds behave within the watery environment of the body, as electrolytes can dissolve in water and form different substances.
Acids and bases
An acid is a substance that contains a hydrogen ion, H+. Hydrochloric acid, HC1, is an acid, and can release the H+ when mixed in water:
Foods such as lemons and vinegar are acidic and have a characteristic sour flavour. The contents of the stomach are highly acidic, as this assists in the digestion of food, as well as destroying bacteria in the food.
A base or alkaline substance is the opposite of an acid. While the term ‘base’ is used to mean the opposite of acidic in the discipline of chemistry, in biology we usually prefer the term ‘alkaline’. Household products such as bleach and oven cleaner are quite alkaline and have a slippery feel (although it is not recommended that you allow these products to contact your skin).
An alkaline substance will take up the H+ that has been released from an acid. For example, if hydrochloric acid, HCl, is mixed with the compound sodium hydroxide, NaOH, the hydroxide will take up the H+ as follows:
If your stomach contents are excessively acidic and cause severe pain, you may use antacids, which are alkaline substances that bind with some of the excess acid. The bicarbonate ion HCO3− is used on many occasions by the body to assist in neutralising acid, as it is able to take up the H+ released by an acid:
For example, the stomach secretes large amounts of hydrochloric acid. This extremely acidic environment is important to facilitate the functioning of the stomach in digesting food. Because this highly acidic environment is harmful to body tissues, the stomach lining has a range of protective properties to prevent acidic destruction. However, the intestines do not have the protective properties that are found within the stomach, so as the acidic content moves into the intestines, the acid must be neutralised to prevent damage to the intestinal tissues. When hydrochloric acid from the stomach mixes with sodium bicarbonate (NaHCO3) present within the intestines, the following chemical reaction occurs:
In this way, the acid is neutralised by the sodium bicarbonate, leaving behind a solution of salt and water. In fact, the hydrogen ions are used to make molecules of water. This neutralisation of acid is essential to protect the lining of the intestines.
The pH scale is used to indicate the relative proportion of acids and bases. The scale starts at pH 0 for the strongest acids, progresses through pH 7, which is neutral, and continues to pH 14, which is the strongest base. You will notice in Figure 1-9 that the pH of blood is 7.35 to 7.45, which is quite a narrow range. Any deviation outside that range must be quickly corrected to keep the body functioning properly (this is discussed in Chapter 29).
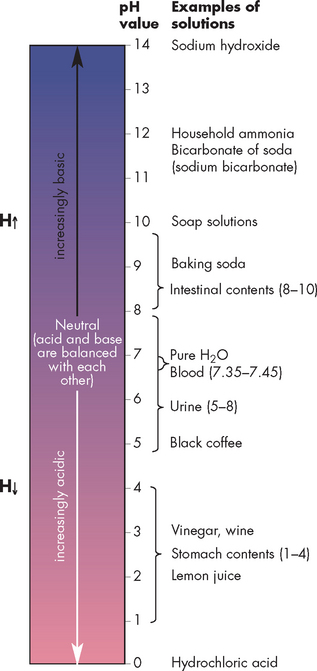
FIGURE 1-9 The pH value of various solutions.
The scale indicates the H+ concentration. A pH of 0 is most acidic, whereas a pH of 14 is most alkaline. The pink colouring indicates the acidic range and the blue colouring the alkaline range.
Source: Based on Herlihy B. The human body in health and illness. 3rd edn. St Louis: Saunders; 2007.
Maintaining an adequate balance between acidic and alkaline substances is tightly controlled within the human body. Normal body processes produce acids, such as lactic acid from working muscles, which requires buffering. If acids are allowed to accumulate, the delicate balance between acid and alkaline would be altered. The brain is particularly sensitive to fluctuations in acid–base balance, so the respiratory system and urinary system work together in removing excess acid. In the long term, the acids produced by the body are excreted in the urine, which is normally an acidic fluid for this reason. While excessive alkaline fluctuations are also detrimental for the body, this is less likely to occur than acid accumulation, because acid is a by-product of many processes.
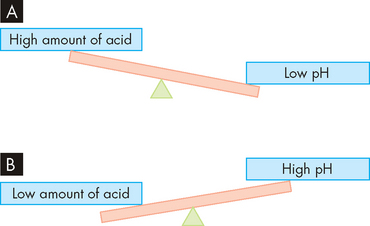
Acidosis and alkalosis
The normal pH of the blood is maintained in the range 7.35 to 7.45, making it slightly alkaline. A pH below 7.35 indicates that the blood has too much acid (acidosis), while a pH above 7.45 indicates that the blood is too alkaline (alkalosis). Remembering the relationship between acid and pH is easier if you think about a see-saw (see Figure 1-10). Substantial changes in pH leading to acidosis and alkalosis are significant problems for the human body, as most body processes depend on a normal acid–base balance.
Chemical reactions
In addition to chemical reactions where molecules are added together to form new molecules, such as when the body manufactures hormones (called synthesis or anabolism), another type of chemical reaction occurs when one molecule is broken down into smaller molecules (called catabolism), an example being digestion of food. Metabolism refers to all the chemical reactions that take place within the body.
Energy
We derive the energy to fuel the many and varied processes of the body from food. However, we cannot use this energy directly — it must first be transferred to a molecule called adenosine triphosphate, usually abbreviated to ATP. Adenosine triphosphate is the storage form for energy, so any time you consider energy usage, ATP will be involved.
Molecules of life
There are three main categories of molecules that accommodate most of the compounds of the body: proteins, lipids and carbohydrates. These are also the three main nutrient groups from the food we eat.
Proteins
A protein is a large molecule consisting of many amino acids linked together. An amino acid is the smallest type of protein: a couple of amino acids joined together make a peptide, several more linked together become a polypeptide and even more become a protein. The ability of proteins to function within the body is dependent on the three-dimensional structure (folding) of this large molecule. A protein molecule consists largely of carbon, hydrogen, oxygen and nitrogen.
The functions of proteins are widespread, as they contribute to the structure of body tissues such as muscle and bone. In fact, it would be difficult to list a body function that does not depend on proteins. Proteins are also responsible for muscle contraction, and special proteins called enzymes have an important role in speeding up some body functions. As an everyday example, enzymes in laundry detergents break down food stains quickly so that clothes are cleaned; laundry detergents without enzymes do not have this advantage, so that food stains may be only partly removed by the end of the wash cycle. In the body, not only do enzymes have the important function of breaking down food, but a wide variety of different enzymes enable thousands of cellular processes to operate quickly and efficiently. You will encounter an extensive array of protein functions as you learn about the body systems.
Proteins from food are found in large amounts in animal products such as red and white meat, dairy products, legumes (peas and beans), cereals and nuts. After you consume these large molecules, the proteins are digested and broken down into the small amino acids, which the body then uses to produce new proteins.
Lipids
Lipids consist of fats (solid at room temperature) and oils (liquid at room temperature) comprised of the molecule glycerol, with some fatty acids linked to it. An example that you may know is triglyceride — one glycerol with three fatty acids attached. The level of triglycerides in the body can be monitored using blood tests to indicate risk of heart disease. A lipid molecule consists largely of carbon, hydrogen and some oxygen.
Some lipids are used to store energy for the body; stored fat is also valuable in helping to protect some of the body’s internal organs. In addition, lipids provide the main structural component of the cell membrane, the boundary around body cells. One lipid of particular interest is cholesterol, and although excessive cholesterol contributes to the development of heart disease, appropriate levels of cholesterol are necessary for body functions such as the production of hormones. A range of other functions are attributed to different types of lipid molecules.
Lipids from food are found in solids (usually of animal origin) such as butter and liquids (usually plant-derived) such as vegetable oils.
Carbohydrates
Similar to proteins, there are specific names for smaller and larger molecules of carbohydrates: the smallest are monosaccharides (mono = one; saccharide = sugar) or simple sugars, with the main one of interest being glucose; disaccharides (di = two) consist of two sugars, such as sucrose; and polysaccharides (poly = many) consist of many sugars linked together, such as starch found in potatoes. A carbohydrate molecule consists largely of carbon, hydrogen and oxygen.
One of the key functions of carbohydrates is the use of glucose as a main fuel source for a number of processes. In later chapters, you will learn the processes involved in keeping glucose levels adequate in the body and how abnormal levels of glucose can lead to severe illness (such as diabetes mellitus). Dietary carbohydrates also contribute to the overall health of the intestines.
Carbohydrates from food are found in breads, cereals, fruits and vegetables. Some carbohydrates cannot be digested by the intestines and these constitute dietary fibre.
Nucleic acids
The nucleic acids are another category of molecules in the body, and these form our genetic information. Although you may not have heard of nucleic acids, you most certainly will have heard of the type DNA (deoxyribonucleic acid) — the other type being ribonucleic acid (RNA). These important molecules store genetic information: DNA contains the code, while RNA carries a copy of the code for when it needs to be replicated. The main elements of these nucleic acids are carbon, hydrogen, oxygen, nitrogen and phosphorus, and these are organised into molecules that link together and form a double strand. We consider the role of DNA and RNA in Chapter 5 when we consider how cells replicate.
ESSENTIAL PHYSICS
Pressure is a measure of force and it has some important applications when studying the human body. The main pressure that you already know about in the body is blood pressure: this pressure occurs within the body due to the presence of blood in an enclosed space (the blood vessels) — the force generated by the heart is the main generator of this pressure. Atmospheric pressure in the environment is another example of pressure, and it is critical in allowing air to move in and out of the lungs during respiration.
Pressure within an enclosed area of the body
Pressure occurs within the body due to the presence of gases, solids or fluids within confined spaces. These substances exert a pressure on the walls that enclose them. In the blood vessel system, for example, the enclosed blood vessels maintain the pressure provided by the contraction of the heart (see Figure 1-11A). The substances within the bloodstream exert this pressure by pushing against the walls of the blood vessels. As a result, any small spaces in the blood vessel lining will allow small substances to exit the vessels — this is how valuable substances such as oxygen and water move from within the blood vessels to nearby cells that need them (see Figure 1-11B).
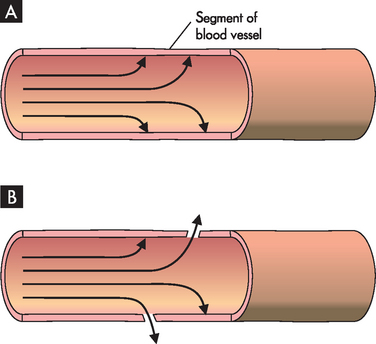
FIGURE 1-11 Pressure within enclosed blood vessels.
A The presence of blood within the enclosed system forces pressure against the blood vessel walls. B Small spaces in the blood vessel lining allow substances to exit the area of higher pressure.
This concept may be explored by looking at the relationship between pressure and the number of particles. Consider two containers of the same size, one with more particles of oxygen than the other. The container with more particles of oxygen is under a higher pressure (see Figure 1-12). If there was an opportunity to do so, the particles of oxygen would move out of the container in A where it is crowded with oxygen to the container in B where more space is available. Thus in the blood vessels, substances move from an area where they are in high abundance (and hence higher pressure) to an area where they are in a lower abundance (and lower pressure). So oxygen and water move from an area of higher pressure in the blood vessels to an area of lower pressure outside the vessels. Within the cardiovascular system, the pressure inside the arteries is particularly high, while pressure in the veins is considerably lower — this facilitates the blood moving from the arteries towards the veins.
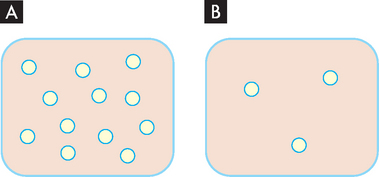
FIGURE 1-12 Different pressures with different numbers of particles.
A The pressure of oxygen within an enclosed space. B Lower pressure of oxygen in region when there is less oxygen present.
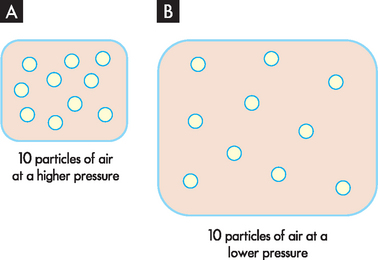
Changing the volume of the container changes the pressure within. For example, although there are 10 particles of air in both containers in Figure 1-13, the pressure is lower in B, as the particles are more spread out. So, one way of lowering pressure within an area is to increase the size of the container.
Pressure from the atmosphere
Atmospheric pressure is essentially the weight of the air and it occurs due to the weight of particles within the air pushing down on everything within the environment, including you. Atmospheric pressure is measured by a barometer, hence the term ‘barometric pressure’.
In applications relating to the human body, pressure is usually measured in millimetres of mercury, abbreviated to mmHg. At sea level, atmospheric pressure is 760 mmHg (or 1 atmosphere). Atmospheric pressure decreases at higher altitudes — as a result, there are fewer particles of substances such as oxygen within the air. So if you could be rapidly transported from sea level to the top of Mount Everest, the highest mountain in the world at 8848 metres above sea level, you would not have sufficient oxygen to survive and would collapse and die within a very short space of time. This is why aircraft cabins are pressurised when flying to increase the pressure to normal sea-level conditions.
During breathing, air enters and exits the body through the respiratory tract, and exchanges with atmospheric air. It is the changes in pressure within the respiratory system that allow the atmospheric air to be drawn in and out of the lungs. Pressure measurements in the lungs are stated as variations from barometric pressure, rather than percentages of it, and barometric pressure is actually considered to be zero, with pressure variations given as up or down from zero. Hence a respiratory pressure of −1 mmHg is 1 mmHg lower than atmospheric pressure — in other words, 759 mmHg.
During inspiration (breathing in), the relative size of the lungs increases, causing a lowering of pressure inside the lungs compared with the outside pressure (see Figure 1-14). Since particles move away from an area of higher pressure towards an area of lower pressure, air from the atmosphere is drawn into the lungs. It is the same principle that causes air to rush out of a balloon — as the balloon is inflated, more and more air is forced into the enclosed area; when the air in the balloon is released, the air moves from the high-pressure area within the balloon to the atmosphere outside.
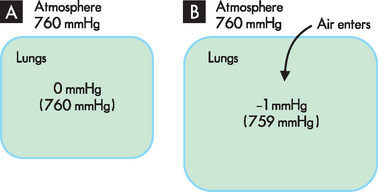
FIGURE 1-14 Movement of air into the lungs during breathing.
A Before breathing in, the pressure within the lungs matches that in the atmosphere. B During breathing in, the pressure within the lungs decreases because the lungs get bigger: as a result, air from the atmosphere is drawn into the lungs.
Essential pathophysiology
 Pathophysiology is the study of altered body function. Related disciplines include pathology (altered structure), anatomy (normal body structure) and physiology (normal body function).
Pathophysiology is the study of altered body function. Related disciplines include pathology (altered structure), anatomy (normal body structure) and physiology (normal body function). A sign is the measurement of a variable that is objective (such as heart rate). A symptom is a variable that is subjective (such as chest pain) and it may be interpreted differently from patient to patient. Signs and symptoms are considered together as clinical manifestations.
A sign is the measurement of a variable that is objective (such as heart rate). A symptom is a variable that is subjective (such as chest pain) and it may be interpreted differently from patient to patient. Signs and symptoms are considered together as clinical manifestations. A disorder (or condition) is a disturbance in function. A syndrome is a group of signs and symptoms, often with an unknown cause. A disease is a well-defined group of signs and symptoms, usually with a set of treatment options.
A disorder (or condition) is a disturbance in function. A syndrome is a group of signs and symptoms, often with an unknown cause. A disease is a well-defined group of signs and symptoms, usually with a set of treatment options. Acute disease usually develops and resolves quickly, whereas chronic disease lasts a longer time. Both types may be mild or severe. Insidious disease develops slowly without being apparent in the early stages.
Acute disease usually develops and resolves quickly, whereas chronic disease lasts a longer time. Both types may be mild or severe. Insidious disease develops slowly without being apparent in the early stages. Incidence refers to the number of new cases diagnosed; prevalence refers to all those people with the disease, whether diagnosed recently or previously.
Incidence refers to the number of new cases diagnosed; prevalence refers to all those people with the disease, whether diagnosed recently or previously. Morbidity refers to the proportion of people with a disease and is usually used in relation to diseases that have a significant impact on health. Co-morbidity refers to the presence of another disease. Mortality is an indication of the death rate associated with a disease.
Morbidity refers to the proportion of people with a disease and is usually used in relation to diseases that have a significant impact on health. Co-morbidity refers to the presence of another disease. Mortality is an indication of the death rate associated with a disease.Essential anatomy
 The anatomical position is a reference position. It is adopted by standing, facing forward, with the palms of the hands facing forward too.
The anatomical position is a reference position. It is adopted by standing, facing forward, with the palms of the hands facing forward too. A sagittal section is a view taken by dividing the body vertically; a transverse section divides the body into upper and lower; and a coronal section divides the body into front and back.
A sagittal section is a view taken by dividing the body vertically; a transverse section divides the body into upper and lower; and a coronal section divides the body into front and back. Superior means towards the head; inferior means towards the feet. Anterior (ventral) means towards the front; posterior (dorsal) means towards the back. Medial means towards the mid-line; lateral means away from the mid-line. Proximal means closer to the point of attachment; distal means further away. Superficial means towards the body surface; deep means towards the body core. Central means towards the body core; peripheral means towards the hands and feet.
Superior means towards the head; inferior means towards the feet. Anterior (ventral) means towards the front; posterior (dorsal) means towards the back. Medial means towards the mid-line; lateral means away from the mid-line. Proximal means closer to the point of attachment; distal means further away. Superficial means towards the body surface; deep means towards the body core. Central means towards the body core; peripheral means towards the hands and feet.Essential physiology
Essential chemistry
 An element is a substance that cannot be broken down any further. The main elements of the body are carbon, hydrogen, nitrogen and oxygen.
An element is a substance that cannot be broken down any further. The main elements of the body are carbon, hydrogen, nitrogen and oxygen. An element with an electrical charge is known as an ion. A positive ion is a cation; a negative ion is an anion. Ions are also electrolytes, as they have the ability to conduct water. Electrolyte balance within the body is of particular interest in the healthcare setting.
An element with an electrical charge is known as an ion. A positive ion is a cation; a negative ion is an anion. Ions are also electrolytes, as they have the ability to conduct water. Electrolyte balance within the body is of particular interest in the healthcare setting. Water is a large component of the body, and is able to absorb heat as well as provide lubrication. A hydrophilic substance mixes well with water; a hydrophobic substance does not mix well with water.
Water is a large component of the body, and is able to absorb heat as well as provide lubrication. A hydrophilic substance mixes well with water; a hydrophobic substance does not mix well with water. An acid contains hydrogen ions; an alkaline substance (or base) is able to bind with (or accept) the hydrogen ion. Acid and alkaline substances are usually balanced or buffered in the body. A normal blood pH is 7.35–7.45; levels below 7.35 indicate acidosis and above 7.45 indicate alkalosis.
An acid contains hydrogen ions; an alkaline substance (or base) is able to bind with (or accept) the hydrogen ion. Acid and alkaline substances are usually balanced or buffered in the body. A normal blood pH is 7.35–7.45; levels below 7.35 indicate acidosis and above 7.45 indicate alkalosis. Proteins consist of amino acids and are used widely in the structure and function of a diverse array of processes throughout the body. Enzymes are specific proteins that allow chemical reactions in the body to occur quickly.
Proteins consist of amino acids and are used widely in the structure and function of a diverse array of processes throughout the body. Enzymes are specific proteins that allow chemical reactions in the body to occur quickly. Lipids are made of glycerol and fatty acids. They are used as a storage form for excess energy, as well as providing support and insulation for organs.
Lipids are made of glycerol and fatty acids. They are used as a storage form for excess energy, as well as providing support and insulation for organs.Essential physics
 Pressure in an enclosed area of the body causes substances to move from an area of higher pressure to an area of lower pressure. This assists the blood to travel from arteries to veins.
Pressure in an enclosed area of the body causes substances to move from an area of higher pressure to an area of lower pressure. This assists the blood to travel from arteries to veins.Mr Jones is in hospital as he has been experiencing chest pain. Investigations reveal that he has high levels of cholesterol in his blood. Mr Jones takes the opportunity to talk with you to learn more about what is happening in his body. He explains that his chest pain has actually been occurring over the past few weeks. He asks you the following questions: