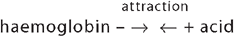29 FLUIDS AND ELECTROLYTES, ACIDS AND BASES
INTRODUCTION
The cells of the body live in a fluid environment with an electrolyte and acid–base concentration maintained within a narrow range. Changes in electrolyte concentration affect the electrical activity of nerve and muscle cells and cause movement of fluid in or out of cells. Alterations in acid–base balance disrupt cellular functions. Fluid fluctuations also affect blood volume and cellular function. Disturbances in these functions are common, particularly in those who are hospitalised, and can progress to become life-threatening. Understanding how alterations occur and how the body compensates or corrects the disturbance is important for understanding many pathophysiological conditions.
Balance of fluids and electrolytes is controlled mainly by the renal system, so that a healthy kidney is essential to maintaining homeostasis. Important hormones that act on the kidneys such as antidiuretic hormone (ADH) and the renin-angiotensin-aldosterone system are essential to normal fluid and electrolyte balance. Hormone disturbances are likely to interfere with fluid and electrolyte balance (refer to Chapter 11). Acid–base balance is extremely well controlled in the body. Normally, blood pH is maintained within a very narrow range and corrections are implemented if pH starts to fall outside the normal range. Both the lungs and the kidneys are critical organs in maintaining acid–base balance.
FLUID BALANCE
The distribution of body fluids
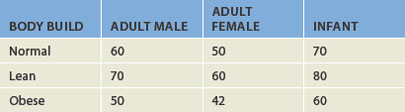
All fluids within the body compartments constitute the total body water, which is about 60% of adult body weight (see Table 29-1) but is higher in infants. The volume of total body water is usually expressed as a percentage of body weight in kilograms — 1 L of water weighs 1 kg and hence the total volume of body water for a 70 kg male is about 42 L, but it is important to remember that this is an approximate value only. The rest of the body weight is fat and solids such as bone, which consists mainly of the molecules fats, proteins and carbohydrates. It is important also to consider that data on the ‘average’ body size are based on a 70 kg male, which is not a particularly large person. Fluid levels are actually lower in those who are obese (refer to Table 29-1), due to the lower proportion of fluid stored in fat cells; hence, those who are obese are more susceptible to dehydration.
Body fluids are distributed among functional compartments or spaces. Intracellular fluid (ICF) comprises all the fluid within cells, which is about two-thirds of total body water. Extracellular fluid (ECF) is all the fluid outside the cells, which constitutes the remaining fluid volume and is further divided into smaller compartments: the interstitial fluid between cells (outside the blood vessels) and the plasma or intravascular fluid (see Figure 29-1). Note that the plasma volume of 3.5 L shown in Figure 29-1 is substantially lower than the average total blood volume of 5 L, as the blood volume consists of plasma (the liquid component of blood) plus the cellular components. Additional smaller compartments of extracellular fluid include lymph, cerebrospinal fluid, synovial fluid, sweat, urine, and pleural, peritoneal, pericardial and intraocular fluids.
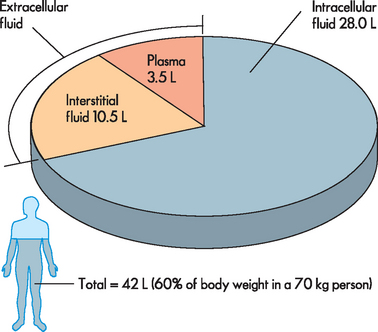
FIGURE 29-1 The distribution of body fluids.
The majority of the total body water is located within the cells in the intracellular fluid. The extracellular fluid is located mainly in the interstitial fluid compartment and plasma.
Although the amount of fluid within the various compartments is relatively constant, water and electrolytes are exchanged between the compartments to maintain their unique compositions. The constant balancing between body compartments allows the body to maintain homeostasis.
Water intake and output
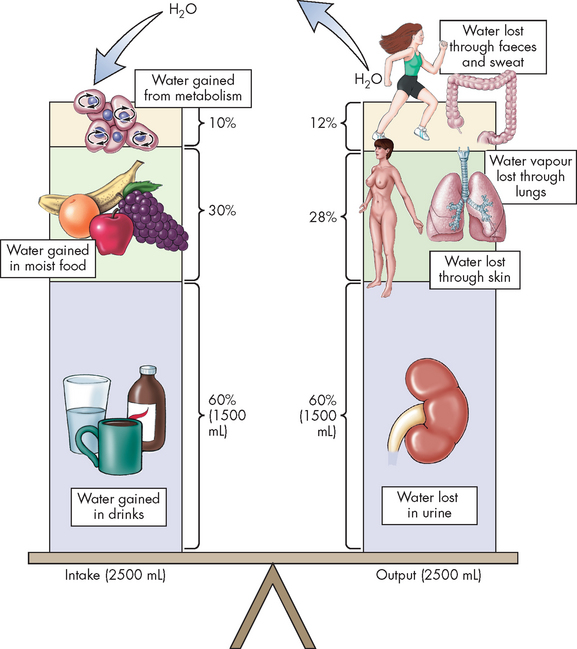
Daily fluid intake may fluctuate widely, although the body regulates water volume within a relatively narrow range. The primary sources of body water are drinking, ingestion of water from food and water obtained from the metabolism of food. Normally, most water is lost through urine, with lesser amounts lost through faeces, sweat and lungs (insensible water loss) (see Figure 29-2). Overall, approximately 2.5 L of fluid enters and exits the circulation per day; this balance of input and output maintains homeostasis.
Water movement between the plasma and interstitial fluid
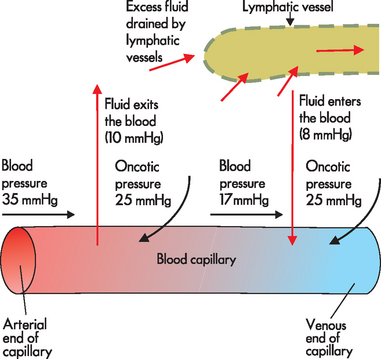
Water crosses cell membranes easily to be exchanged between fluid compartments. As blood flows from the arterial to the venous end of the capillary, two main forces determine whether fluid moves (1) out of the capillary and into the interstitial space or (2) back into the capillary from the interstitium:
 Blood pressure, also known as capillary hydrostatic pressure, is present within all blood vessels. In addition to pushing the blood along, this pressure tends to force water outwards from the capillary to the interstitial space.
Blood pressure, also known as capillary hydrostatic pressure, is present within all blood vessels. In addition to pushing the blood along, this pressure tends to force water outwards from the capillary to the interstitial space. Blood or capillary oncotic pressure occurs as proteins tend to attract fluid; there is a high level of protein in the blood and hence there is a reasonable amount of oncotic pressure, which draws water from the interstitial space into the capillary.
Blood or capillary oncotic pressure occurs as proteins tend to attract fluid; there is a high level of protein in the blood and hence there is a reasonable amount of oncotic pressure, which draws water from the interstitial space into the capillary.There are also additional forces in the interstitium, which tend to be quite low:
 Interstitial hydrostatic pressure, due to the presence of fluid within the interstitium, facilitates the inward movement of water from the interstitial space into the capillary, as well as into the lymphatic vessels (as explained below).
Interstitial hydrostatic pressure, due to the presence of fluid within the interstitium, facilitates the inward movement of water from the interstitial space into the capillary, as well as into the lymphatic vessels (as explained below). Interstitial oncotic pressure, due to proteins in the interstitium, osmotically attracts water from the capillary into the interstitial space.
Interstitial oncotic pressure, due to proteins in the interstitium, osmotically attracts water from the capillary into the interstitial space.We now consider how these forces interact in the movement of fluids, starting with an examination of the pressures within the capillary. At the arterial end of the capillary, capillary hydrostatic pressure exceeds capillary oncotic pressure and so fluid exits the capillary and moves into the interstitial space (see Figure 29-3). At the venous end of the capillary, capillary hydrostatic pressure is actually less than at the arterial end, mainly due to the rapid exit of water from the arterial end. Capillary oncotic pressure is the same at the venous and arterial ends, as the amount of protein in the vessel does not change. As a result, capillary oncotic pressure exceeds capillary hydrostatic pressure at the venous end and so fluid is attracted back into the capillary (see Figure 29-3). The pressure of fluid within the interstitial space is extremely low, as the fluid that is not drawn back into the bloodstream is constantly drained by the lymphatic vessels. A reasonably large volume of 3 L is drained from the interstitium by the lymphatic system per day. Similarly, oncotic pressure within the interstitial space is also very low, as there is very little protein in this area. Therefore, it is the pressures within the capillary that determine whether fluid exits or enters the bloodstream.
Water movement between the interstitial fluid and intracellular fluid
Water moves from the interstitial space into the cells primarily as a function of osmotic forces. The oncotic force of proteins, which is relatively constant within the cell, draws water into the intracellular fluid. Water crosses cell membranes freely, so the osmolality of the total body water is normally at equilibrium. This means that if the cell is low in fluid, it is easily corrected by fluid crossing the cell membrane and entering the cell, so homeostasis of fluid balance at the cell is maintained (see Figure 29-4). On the other hand, if the total body fluid volume is low, water will exit the cells. Unfortunately, even the sensitive neurons of the brain cannot be protected from this water loss, which may explain why some people experience headache when they have not consumed enough water. This can progress to more severe symptoms if the lack of fluid is not addressed.
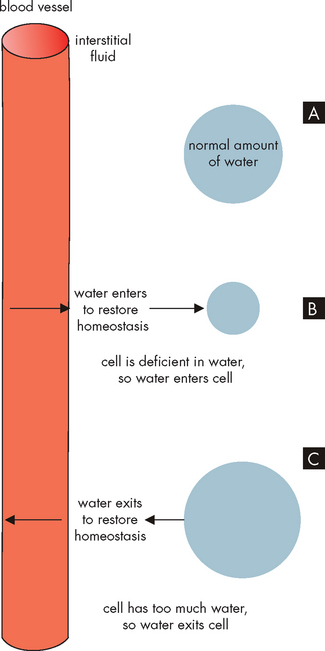
FIGURE 29-4 Water movement between the extracellular fluid (plasma and interstitial space surrounding the cells) and the intracellular fluid.
In A, a normal amount of water is inside the cell. In B, a low intracellular fluid volume draws water from the plasma and interstitium, while in C a high intracellular fluid volume requires water to be moved to the plasma.
Alterations in water movement
Oedema
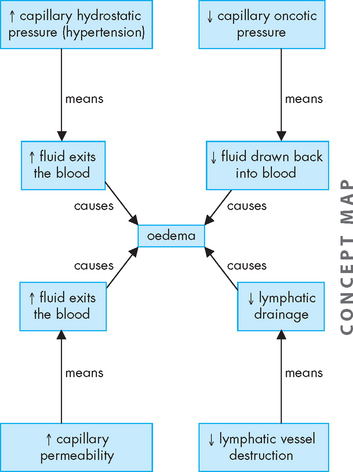
Oedema, from the Greek word ‘to swell’, is the excessive accumulation of fluid within the interstitial spaces. The forces that promote fluid movement from the capillaries into the tissues are increased capillary hydrostatic pressure, lowered plasma oncotic pressure, increased capillary membrane permeability and lymphatic channel obstruction (see Figure 29-5).1
PATHOPHYSIOLOGY
Conditions that cause increased sodium and water retention lead to increased blood volume, which increases blood hydrostatic pressure, thereby contributing to oedema. Hypertension (which is common in Australia and New Zealand; see Chapter 23), congestive heart failure and renal failure are common causes of oedema. Venous obstruction, particularly in the lower limbs, may result from decreased venous return due to lack of use of the skeletal muscle pump during standing or while remaining seated on long-haul flights, thereby pushing fluid from the capillaries into the interstitial spaces and causing oedema. This actually occurs in healthy people. For example, when you have been standing upright for a lengthy period of time your feet will swell as fluid moves into the interstitial spaces but is not reabsorbed at the same rate. Upon moving or lying down the fluid will quickly reabsorb and the swelling in your feet will subside. It is when oedema is in greater amounts and is associated with underlying disease that it is of most concern.
Decreases in the amount of protein in the blood will lower plasma oncotic pressure. The decreased oncotic attraction of fluid within the capillaries causes filtered capillary fluid to remain in the interstitial spaces, resulting in oedema. This may occur with decreased plasma albumin (a main plasma protein) production, due to liver disease or protein malnutrition. Plasma proteins are also lost in some kidney diseases (see Chapter 30), serous drainage from open wounds, haemorrhage and burns.
Increased capillary permeability is an important part of the inflammatory process, as it allows leucocytes to exit the blood and reach the tissues. This increased permeability also occurs with trauma such as burns or crushing injuries, neoplastic disease and allergic reactions. Proteins escape from the vascular space, which draws fluid into the interstitial spaces, leading to oedema.
The lymphatic system normally collects and drains interstitial fluid. When lymphatic channels are blocked or surgically removed, proteins and fluid accumulate in the interstitial spaces causing lymphoedema.2 For example, lymphoedema of the arm or leg occurs after surgical removal of the axillary or femoral lymph nodes, respectively, for treatment of carcinoma.
CLINICAL MANIFESTATIONS
Oedema may be localised or generalised. Localised oedema is usually limited to a site of trauma, as in a sprained ankle or the swelling of an area after surgery. Another kind of localised oedema occurs within organ systems and is usually a serious pathophysiological condition. These types of localised oedema include cerebral oedema, pulmonary oedema, pleural effusion (fluid accumulation in the pleural space around the lungs), pericardial effusion (within the membrane around the heart) and ascites (accumulation of fluid in the peritoneal space). Generalised oedema is manifested by a more uniform distribution of fluid in the interstitial spaces. It occurs in gravity-dependent areas of the body, in the feet and legs when standing and in the sacral area and buttocks when supine (lying face up). It can be identified by pressing on tissues overlying bony prominences — a pit left in the skin indicates oedema, known as pitting oedema (see Figure 29-6).
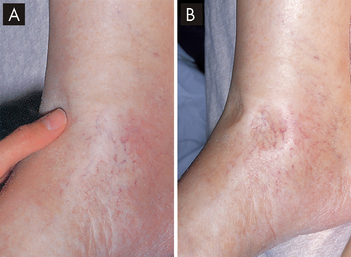
FIGURE 29-6 Pitting oedema in a patient with cardiac failure.
A The limb is oedamatous and when fingertip pressure is applied a depression appears in the tissue. B A depression (‘pit’) remains in the oedema for some minutes after firm fingertip pressure is applied.
Source: Forbes CD, Jackson WD. Color atlas and text of clinical medicine. 3rd edn. London: Mosby; 2003.
Oedema is usually apparent as weight gain, swelling and puffiness, tight-fitting clothes and shoes, limited movement of affected joints and symptoms associated with the underlying pathological condition. Fluid accumulations make it more difficult for nutrients and waste products to diffuse between the capillaries and tissues. Blood flow may also be impaired. Therefore, wounds heal more slowly and, with prolonged oedema, the risks of infection and pressure sores over bony prominences increase.
EVALUATION AND TREATMENT
The underlying cause of the oedema should be determined and treated. Elevating the feet assists in lessening lower limb oedema. Restricting fluid intake and using diuretics to promote fluid excretion by the kidneys are often helpful.
Fluid spacing
When the distribution of fluid is normal between the intracellular and extracellular fluids, this is known as first spacing. In the development of oedema, second spacing occurs, as there is an excess of fluid located in the interstitial spaces. This excess fluid in the interstitium can be treated by changing pressures (such as elevating the limb to enhance venous return and lymphatic drainage) or using diuretic agents that cause water loss from the plasma, hence drawing the excess fluid from the interstitium back to the plasma.
Alternatively, fluid may accumulate in an area that is not able to be drained easily; for example, fluid will pool around the damaged tissue of a burns site. Because this is not a normal fluid compartment that is exchanged with other body fluids, this is known as third spacing. It is difficult to return third space fluid to the body circulation. Dehydration may result, as fluid in the third space is not available for the circulation. Importantly, increasing fluid intake to correct the dehydration can worsen the third space fluid. Large amounts of water in the third space is difficult to treat. A main concern when large amounts of fluid accumulate in the third space is the risk of hypovolaemia and circulatory shock (see Chapter 23).
Water balance
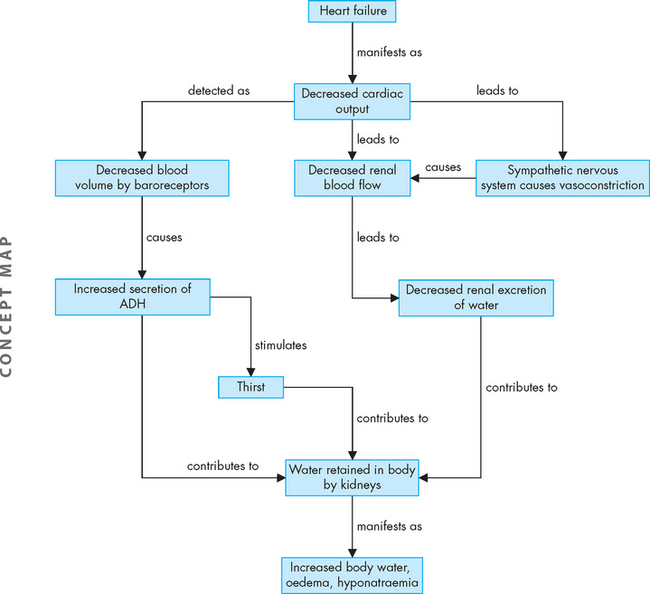
Water balance is important in maintaining appropriate blood volume; blood volume is linked with blood pressure, such that a low blood volume (hypovolaemia) causes hypotension; this is compensated by tachycardia in the otherwise healthy person. Fluid loss (dehydration) may occur from vomiting, diarrhoea or excessive sweating. For example, fluid is lost in someone with diarrhoea, as it passes through the intestines too quickly to be absorbed to replenish the blood volume. Fluid can also move from the plasma into the cells lining the intestines to replace the fluid loss, which may result in hypovolaemia (see Figure 29-7). Tachycardia is one way for the body to maintain homeostasis of blood flow to tissues until the overall water balance can be increased. Alternatively, hypervolaemia (increased blood volume), seen after an excessive intake of water, can cause hypertension and bradycardia.
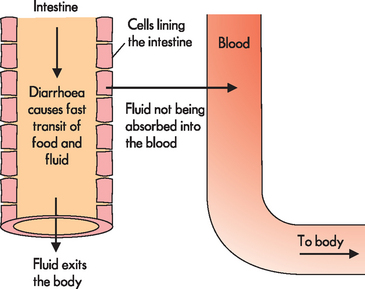
FIGURE 29-7 Diarrhoea causes fluid to exit the body rapidly, so that it cannot be absorbed into the bloodstream.
Water balance is regulated by antidiuretic hormone (ADH), which is secreted when the total plasma concentration (osmolality) increases or the circulating blood volume or blood pressure decreases. Increased plasma concentration occurs with either water deficit or sodium excess. The increased concentration stimulates hypothalamic osmoreceptors, which stimulate the sensation of thirst and result in drinking (see Figure 29-8). In addition to causing thirst, these osmoreceptors signal the posterior pituitary to release ADH, which increases the reabsorption of water by the kidneys, so that water is retained rather than being lost in the urine (see Chapter 28). Urine concentration increases and the reabsorbed water increases the plasma volume, which in turn decreases plasma concentration, returning it towards normal.
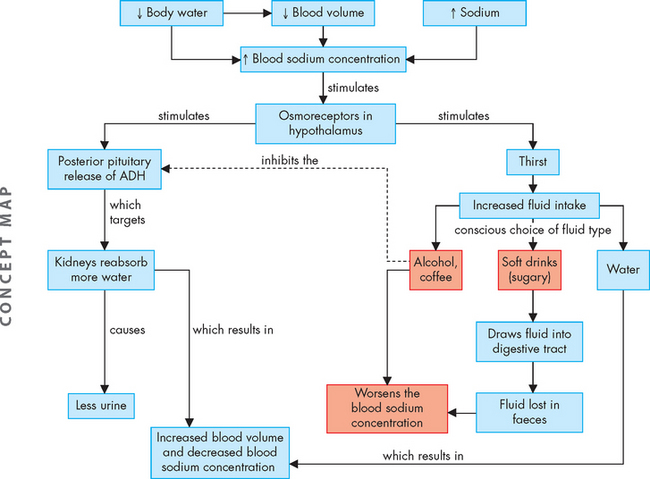
FIGURE 29-8 Mechanisms of fluid replacement to increase blood volume and decrease blood concentration.
Note that the fluid that is consumed may not always be particularly useful in causing rehydration.
Water is the most effective fluid for rehydrating, as other fluid choices such as caffeinated drinks, alcohol and sugary soft drinks may actually contribute to dehydration (see Figure 29-8).
With further fluid loss, other receptors are activated in addition to the hypothalamic osmoreceptors. Volume-sensitive receptors in the right and left atria of the heart and thoracic vessels, as well as the baroreceptors (nerve endings that are sensitive to changes in stretch and pressure) found in the aorta and carotid sinus become activated. These receptors stimulate the release of ADH from the pituitary gland, which then promotes the restoration of plasma volume and blood pressure.
ELECTROLYTE BALANCE
Sodium, chloride and potassium balance
The kidneys and hormones have a central role in maintaining sodium and water balance. Because water follows the osmotic gradients established by changes in salt concentration, sodium and water balance are intimately related. Sodium and potassium are regulated by the renal effects of aldosterone.
Sodium
Sodium (Na+) is the main extracellular fluid ion and is a cation (positively charged ion). Along with the anions (negatively charged ions) chloride and bicarbonate, sodium regulates extracellular osmotic forces and therefore is a key regulator of water balance. Sodium is important in other functions, including working with potassium and calcium in neuron signalling, and regulating the acid–base balance (see ‘Acid–base balance’ below). The distribution of electrolytes in body compartments is summarised in Table 29-2.
Table 29-2 THE DISTRIBUTION OF ELECTROLYTES IN BODY COMPARTMENTS
| ELECTROLYTES | EXTRACELLULAR FLUID (mmol/L) | INTRACELLULAR FLUID (mmol/L) |
|---|---|---|
| Cations | ||
| Sodium | 135–145 | 10 |
| Potassium | 3.5–5.0 | 150–160 |
| Calcium | 2.15–2.60 | 0 |
| Magnesium | 0.8–1.0 | 24 |
| Anions | ||
| Bicarbonate | 22–32 | 12 |
| Chloride | 95–110 | 4 |
| Phosphate | 0.8–1.5 | 100 |
| Proteins | 16 | 65 |
Source: The Royal College of Pathologists of Australasia. RCPA Manual. 6th edn. Available at www.rcpamanual.edu.au/sections/pathologytest, accessed June 2010.
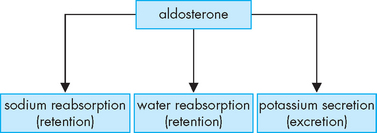
The kidneys maintain normal plasma sodium concentration within a narrow range (135–145 mmol/L). After being filtered at the kidneys, the reabsorption of sodium through the kidney tubules is tightly controlled. Hormonal regulation of sodium balance is mediated by aldosterone secreted from the adrenal cortex. Aldosterone secretion is influenced by both circulating blood volume and plasma concentrations of sodium and potassium (aldosterone is secreted when sodium levels are decreased or potassium levels are increased). Aldosterone increases the reabsorption of sodium and the secretion of potassium by the kidneys. As a result, sodium concentration of the extracellular fluid is enhanced and potassium is excreted with the urine (see Figure 29-9; a full discussion of the important renin-angiotensin-aldosterone system can be found in Chapter 28).
Chloride
Chloride (Cl−) is in a high concentration in the extracellular fluid and its negative charge allows it to interact with the positively charged sodium to balance the electrical charge in the form of sodium chloride (NaCl, which is table salt). The transport of chloride is generally passive and follows the active transport of sodium, so that increases or decreases in sodium are proportional to changes in chloride. The concentration of chloride tends to be the ‘opposite’ to the concentration of bicarbonate (HCO3−), the other major extracellular anion, so that when there are high amounts of chloride, there are low concentrations of bicarbonate, and vice versa.
Potassium
Potassium (K+) is the major intracellular electrolyte; it is positively charged and is essential for normal cellular function. The intracellular fluid concentration of potassium is 150–160 mmol/L, while the extracellular fluid concentration is 3.5–5.0 mmol/L. The difference in concentration is maintained by the sodium–potassium pump (which requires ATP to pump the electrolytes against their concentration gradients), which returns 3 molecules of sodium to the extracellular fluid and 2 molecules of potassium to the intracellular fluid with each cycle.
As the predominant intracellular ion, potassium exerts a major influence in regulating intracellular fluid concentration. In addition, potassium has a vital role in neuronal function, as it is essential for maintaining the resting membrane potential and mediating the action potential for the transmission of nerve impulses. It is also essential for neural control and muscular function of cardiac, skeletal and smooth muscle contractions. For this reason, disturbances in the potassium balance require urgent medical attention, as disruptions to neurons and cardiac muscle are life-threatening.
The kidneys are the most efficient regulator of the potassium balance. Potassium is freely filtered by the nephrons of the kidney, with most potassium subsequently being reabsorbed into the blood. However, potassium is also secreted by the kidneys as well. The fact that potassium is filtered, reabsorbed and secreted by the kidneys indicates that it is tightly regulated in the blood. Changes in the rate of filtrate (urine) flow through the kidneys also influence potassium secretion. When the flow rate is high, as with the use of diuretics, potassium is secreted into the urine.3
Box 29-1 THERE’S NO K IN POTASSIUM
Have you ever wondered why the symbol for potassium is K, yet there is no ‘K’ in potassium? This is because the symbols for the chemical elements are based on Latin words. Fortunately, the names of some chemical elements in English are similar to Latin, so learning their symbols is somewhat easier.
| ENGLISH | LATIN | SYMBOL |
|---|---|---|
| Sodium | Natrium | Na+ |
| Potassium | Kalium | K+ |
| Calcium | Calcium | Ca2+ |
| Chloride | Chlorum | Cl− |
Besides conserving sodium, aldosterone also regulates potassium. When plasma potassium concentration increases, aldosterone is released, stimulating the excretion of potassium into the urine by the kidneys. Aldosterone also increases the secretion of potassium from the sweat glands. Changes in the acid–base balance (or pH) also affect the potassium balance, as hydrogen ions (H+) inside the cell cause potassium to shift out of the cell to the extracellular fluid to maintain the balance of cations across the cell membrane (see Figure 29-17).
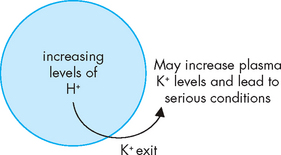
FIGURE 29-17 Acidosis can cause potassium to exit from the cell.
This allows the level of positive charge to remain normal within the cell. However, it may result in hyperkalaemia.
Potassium tolerance is the ability of the body to adapt to increased levels of potassium intake over time. A sudden increase in potassium may be fatal, but if the intake of potassium is slowly increased, the kidneys can increase urinary excretion and maintain the potassium balance.
Alterations in sodium, chloride and water balance
Alterations in sodium and water balance are closely related.4 Water imbalance may develop with gains or losses of salt. Likewise, sodium imbalance occurs with alterations in body water volume. Generally, these alterations can be classified as changes in tonicity — the change in the concentration of solutes with relation to water (see Chapter 3). Alterations can therefore be classified as isotonic, hypertonic or hypotonic (see Table 29-3).
Table 29-3 WATER AND SOLUTE IMBALANCES
| TONICITY | MECHANISM |
|---|---|
| Isotonic imbalance | Gain or loss of extracellular fluid resulting in a concentration equivalent to a 0.9% sodium chloride (salt) solution (normal saline); no shrinking or swelling of cells |
| Hypertonic imbalance | Imbalance that results in an extracellular fluid concentration more than 0.9% salt solution (i.e. water loss or solute gain); cells shrink in a hypertonic fluid |
| Hypotonic imbalance | Imbalance that results in an extracellular fluid concentration less than 0.9% salt solution (i.e. water gain or solute loss); cells swell in a hypotonic fluid |
Isotonic alterations
Total body water changes may be accompanied by proportional changes in electrolytes, so that osmolality remains within the normal range of 280–300 mOsm/kg.5 For example, if an individual loses pure plasma or extracellular fluid, the fluid volume is depleted but the concentration and type of electrolytes remain in the normal range. The term isotonic refers to a solution that has the same concentration of solutes as the normal plasma concentration.
Hypovolaemia
Hypovolaemia is a low extracellular fluid volume (and hence low blood volume), which is caused by insufficient fluid intake, haemorrhage, vomiting or diarrhoea, or high doses of diuretic medications. This results in a decrease in the extracellular fluid volume, with weight loss, dryness of skin and mucous membranes, and decreased urine output. In hypovolaemia, both the fluid and extracellular sodium are lost together, as compared with dehydration, which is just the loss of water. Indicators of hypovolaemia include a rapid heart rate, flattened neck veins and normal or decreased blood pressure. In severe states, hypovolaemic shock can occur (see Chapter 23). Controlled rehydration using fluid and sodium is implemented.
Hypervolaemia
Hypervolaemia is a high extracellular fluid volume (and increased blood volume), which is most commonly the result of excessive administration of intravenous fluids (saline), hypersecretion of aldosterone (causing retention of sodium and water) or the effects of corticosteroid drugs (which cause renal reabsorption of sodium and water). As plasma volume expands, weight gain occurs. The diluting effect of excess plasma volume leads to a decreased haematocrit and decreased plasma protein concentration, which can be ascertained using blood tests. The neck veins may distend and the blood pressure increases. Increased capillary hydrostatic pressure leads to oedema. Ultimately, pulmonary oedema and heart failure may develop. Fluid intake should be restricted to correct the hypervolaemia.
Hypertonic alterations
Hypertonic fluid refers to fluid where the solute concentration is higher than in the blood (and thus the solution also has less water than the blood). Within the body, such alterations develop when the concentration of the extracellular fluid is elevated above normal osmolality (greater than 300 mOsm/kg).5 The most common causes are increased concentration of sodium (hypernatraemia) or deficit of extracellular fluid water. In both instances, the extracellular fluid hypertonicity seen in the plasma attracts water from the intracellular spaces, causing intracellular fluid dehydration.
PATHOPHYSIOLOGY
Hypernatraemia occurs when sodium levels in the extracellular fluid or plasma are elevated and may be caused by either an acute gain in sodium or a net loss of water (see Figure 29-10).6 Although sodium is mainly in the extracellular fluid, increased levels of sodium cause intracellular dehydration, as fluid will move out of the cells and into the extracellular fluid. This movement of water may cause hypervolaemia; however, if there is also an accompanying water loss with the hypernatraemia, both intracellular and extracellular dehydration may occur. Because chloride follows sodium, hypernatraemia is accompanied by hyperchloraemia (elevated chloride levels); however, no specific symptoms are associated with chloride excess.
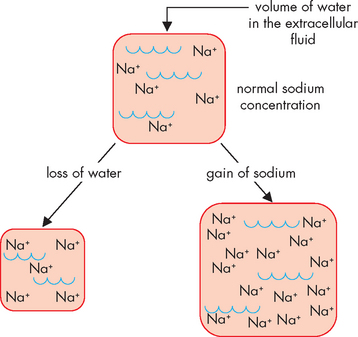
FIGURE 29-10 Hypernatraemia may result from either loss of water or a gain in sodium in the extracellular fluid.
As a result, in either case the concentration of sodium increases.
Dehydration, particularly in the elderly, is a common finding. Increased sodium concentration due to water deprivation or water loss is associated with fever or respiratory infections, which increase the respiratory rate and enhance water loss from the lungs. Similarly, profuse sweating can increase fluid loss. Elderly patients have a decreased thirst mechanism, making them more vulnerable to the effects of fluid loss, as insufficient water intake means that fluid is not being replaced. Decreased secretion of ADH (due to diabetes insipidus) or its decreased effectiveness (due to renal disease) can lead to insufficient retention of fluid by the kidneys, resulting in water loss. On the other hand, diabetes mellitus leads to glucose passing into the urinary filtrate — this glucose attracts water and hence water is lost from the body in urine.
High amounts of dietary sodium rarely cause hypernatraemia in a healthy individual, as excess sodium is eliminated from the body by the kidneys. However, a high-sodium diet is not advised for those with illness or the majority of the population as this may contribute to hypertension. High sodium levels can occur with oversecretion of aldosterone, leading to increased sodium retention by the kidneys; this is associated with hypervolaemia as water is reabsorbed with the sodium.
CLINICAL MANIFESTATIONS
The higher concentration of sodium in the extracellular fluid draws water out of the cells and intracellular dehydration ensues. Thirst, fever, dry mucous membranes and restlessness are associated with hypernatraemia as a result of water loss. Central nervous system symptoms include muscle twitching and hyperreflexia (hyperactive reflexes). Convulsions are the most serious symptoms.
EVALUATION AND TREATMENT
Investigations should be directed at assessing the presence of diabetes insipidus and appropriate treatment. Water loss should be replaced, although this must be administered slowly to avoid serious complications such as cerebral oedema or death.7
PATHOPHYSIOLOGY
Dehydration refers to water deficit, but the term dehydration is commonly also used to indicate both sodium and water loss.7 Pure water deficit is rare because most people have access to water. Individuals who are comatose or paralysed continue to have insensible water losses through the skin and lungs with a minimal obligatory formation of urine. Hyperventilation caused by fever can contribute to water deficit. The most common cause of water loss is increased renal excretion of water due to impaired function or diabetes insipidus (decreased ADH; see Chapter 11).
CLINICAL MANIFESTATIONS
Marked water deficit is manifested by symptoms of dehydration: thirst, dry skin and mucous membranes, elevated temperature, weight loss and concentrated urine (with the exception of diabetes insipidus, when the urine is dilute). Skin turgor (ability to move back into normal place when pinched) may be normal or decreased. Symptoms of hypovolaemia include tachycardia, weak pulses and postural hypotension (a decrease in blood pressure with movement from lying or sitting to standing).
EVALUATION AND TREATMENT
Comparing plasma and urinary osmolarity assists in determining the magnitude of dehydration. In addition, physical examination and patient history are important in ascertaining dehydration causation. Determining the cause, such as assessing the patient for diabetes insipidus, directs treatment. Oral or intravenous rehydration therapy may be sufficient, although some patients may require administration of an antidiuretic hormone agent.
Hypotonic alterations
Hypotonic fluids are those that are more dilute than the blood. Hypotonic imbalances occur when the concentration of the extracellular fluid is less than normal (less than 280 mOsm/kg).5 The most common cause is sodium deficit (hyponatraemia) or water excess. Either leads to intracellular overhydration and cell swelling (oedema). When there is a sodium deficit, less water is required in the extracellular fluid and hence water moves into the cells. The plasma volume then decreases, leading to symptoms of hypovolaemia. With water excess, increases in both the intracellular and the extracellular fluid volume occur, causing symptoms of hypervolaemia and water intoxication with cerebral and pulmonary oedema.
PATHOPHYSIOLOGY
Hyponatraemia develops when the serum sodium concentration falls below 135 mmol/L. This may result from insufficient sodium due to sodium loss or inadequate sodium intake, or dilution of the body’s sodium level by water excess (see Figure 29-11).8
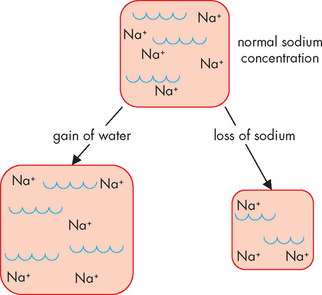
FIGURE 29-11 Hyponatraemia may result from either gain of water or loss of sodium from the extracellular fluid, thereby decreasing the sodium concentration.
Pure sodium deficits are usually caused by vomiting, diarrhoea, gastrointestinal suctioning or burns, or due to renal losses of sodium from the use of diuretics. Inadequate intake of dietary sodium is rare but possible in individuals on low-sodium diets or if fasting. Insufficient aldosterone secretion allows both sodium and water to be lost from the body by the kidneys. Hyponatraemia is also a common adverse effect of diuretics, particularly the loop diuretics (such as frusemide), which cause excretion of water and sodium. Hypochloraemia (low blood chloride) may also occur with hyponatraemia, as chloride is associated with sodium.
Dilutional hyponatraemia occurs when the amount of sodium is diluted by the amount of water. This is one reason why patients are not rehydrated intravenously using water only — saline is infused, as it replaces both sodium and water simultaneously. Nevertheless, hyponatraemia is the most common electrolyte abnormality observed in patients in hospital and this can be attributable to intravenous fluid administration without sufficient sodium.9 For instance, replacement of fluid loss with intravenous 5% dextrose in water can be detrimental, as the glucose is metabolised to carbon dioxide and water, leaving a water solution that has a diluting effect. For this reason, most hospital patients receive a mixture of intravenous fluids if they cannot take fluids orally, and these will replace both water and sodium deficits.
Excessive sweating may stimulate thirst and the intake of large amounts of water, which dilute sodium. Hyponatraemia can also result from the syndrome of inappropriate antidiuretic hormone secretion (see Chapter 11), such that increased amounts of water that are retained dilute the sodium. During renal failure or heart failure, renal excretion of water is impaired, resulting in hyponatraemia (see Figure 29-12). Also, hyperglycaemia in diabetes mellitus increases extracellular fluid concentration and attracts water out from the intracellular fluid; this in turn dilutes the concentration of sodium and other electrolytes.
CLINICAL MANIFESTATIONS
Deficits of sodium alter the cells’ ability to depolarise and repolarise normally and hence can have severe implications for the neuron action potential (see Chapter 6). Behavioural and neurological changes characteristic of hyponatraemia include lethargy, confusion, apprehension, depressed reflexes, seizures and coma. Hyponatraemia often indicates that the fluid volume is increased (hypervolaemia), which can lead to increased intracranial volume and life-threatening brain swelling. Pure sodium losses that cause hypovolaemia have symptoms of hypotension, tachycardia and decreased urine output. Weight gain, oedema and ascites are characteristic of hyponatraemia with normal or increased blood volume.
EVALUATION AND TREATMENT
Simply restricting the amount of water ingested in these patients usually alleviates the sodium imbalance and is a common treatment. Fluid should not be administered if the patient has accompanying oedema.10 If the hyponatraemia is accompanied by a decrease in fluid volume, then fluid volume replacement with normal saline may be used.
PATHOPHYSIOLOGY
When the body is functioning normally, it is almost impossible to retain an excess of total body water, because any increase in water consumption is soon balanced by an increase in the excretion of water by the kidneys. Some individuals with psychogenic disorders develop water intoxication from compulsive water drinking. Acute renal failure, severe congestive heart failure and liver cirrhosis can contribute to water excess, as can intravenous infusion of 5% dextrose in water. Decreased urine formation from renal disease or decreased renal blood flow contributes to water excess. The overall effect is dilution of the extracellular fluid, with water also moving into the intracellular spaces by osmosis. Water excess is usually accompanied by hyponatraemia. Excess retention of water also occurs with the syndrome of inappropriate secretion of antidiuretic hormone (see Chapter 11), whereby the kidneys are prevented from excreting excess water and hence water is retained in the body.
CLINICAL MANIFESTATIONS
The symptoms of water excess are related to the rate at which water is increased, known as water loading. Acute excesses cause confusion and convulsions. Weakness, nausea, muscle twitching, headache and weight gain are common symptoms of long-term water accumulation.
EVALUATION AND TREATMENT
Correct diagnosis of the cause of the water excess is essential to guiding treatment. Fluid restrictions are necessary until the underlying cause is adequately treated. As the extracellular solutes are diluted in the excess water, electrolytes including sodium may need correction.
Alterations in potassium balance
PATHOPHYSIOLOGY
Potassium deficiency, or hypokalaemia, develops when the serum potassium concentration falls below 3.5 mmol/L. In clinical practice, the intracellular level of potassium cannot be measured accurately, but generally, lowered serum potassium indicates loss of total body potassium. Therefore, with potassium loss from the extracellular fluid, the concentration gradient change favours movement of potassium from the cells to the extracellular fluid. The intracellular/extracellular fluid concentration ratio is maintained, but total body potassium is depleted (see Figure 29-13).
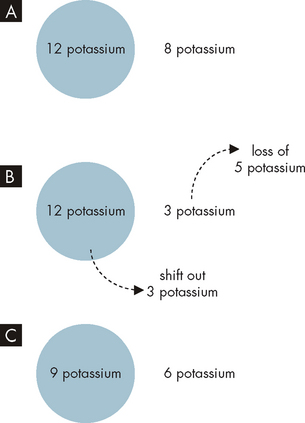
A Normal intracellular and extracellular levels of potassium, with a concentration gradient established. B Loss of 5 potassium from the extracellular fluid causes 3 potassium from inside the cell to shift out. C Although the overall numbers of potassium are now lessened, the concentration gradient from inside to outside of the cell remains normal, due to the potassium shift from the intracellular fluid to the extracellular fluid. Note: the numbers used are arbitrary.
Potassium shifts from the intracellular fluid to the extracellular fluid in conditions such as diabetic ketoacidosis: the increased hydrogen ion (H+) or acid concentration in the extracellular fluid causes acid to shift into the cell and, as a consequence, potassium exits the cell. A normal level of potassium is maintained in the plasma, but potassium continues to be lost in the urine, causing a deficit in total body potassium. Severe, even fatal, hypokalaemia may occur if insulin is used to treat diabetic emergency of hyperglycaemia without also providing potassium supplements. Thus, total body potassium depletion becomes evident when insulin treatment and rehydration therapy are initiated.
Losses of potassium from body stores are usually caused by gastrointestinal and renal disorders. Diarrhoea, intestinal drainage tubes or fistulae and laxative abuse also result in hypokalaemia. Normally, only small amounts of potassium are excreted in the faeces each day, but with diarrhoea, the loss of fluid and electrolytes, including potassium, can be substantial. Vomiting or continuous nasogastric suctioning is often associated with potassium depletion due to alkalosis (see ‘Acid–base balance’ below). Conditions that cause high aldosterone secretion (described above) also result in renal losses of potassium.
Other factors that contribute to the development of hypokalaemia include reduced intake of potassium and increased losses of body potassium. Dietary deficiency of potassium is rare but may occur in elderly individuals who have both a low protein intake and an inadequate intake of fruits and vegetables, and in individuals with alcoholism. Generally, reduced potassium intake becomes a problem when combined with other causes of potassium depletion.
Many diuretics, such as frusemide, are potassium-wasting as they cause potassium excretion by the kidneys and hence contribute to hypokalaemia. For example, diuretics that inhibit the reabsorption of sodium chloride cause sodium and water to be excreted — this increased fluid volume passing through the kidney tubules prevents adequate reabsorption of potassium (resulting in renal potassium excretion). Similarly, renal diseases also cause increased fluid excretion, which limits the ability to retain potassium in the body. Several antibiotics are known to cause hypokalaemia by increasing the rate of potassium excretion.
CLINICAL MANIFESTATIONS
Neuromuscular and cardiac effects of hypokalaemia are evident with severe loss of potassium. Neuron and muscular excitability decreases, causing skeletal muscle weakness, smooth muscle atony and cardiac arrhythmias.11 A wide range of other dysfunctions may result from potassium deficiency (see Table 29-4), as potassium is the main intracellular ion.
Table 29-4 CLINICAL MANIFESTATIONS OF POTASSIUM ALTERATIONS
| ORGAN SYSTEM | HYPOKALAEMIA | HYPERKALAEMIA |
|---|---|---|
| Cardiovascular | ||
| Nervous | ||
| Gastrointestinal | ||
| Kidneys | ||
| Skeletal and smooth muscle |
Symptoms occur in relation to the rate of potassium depletion. Slow losses of potassium may allow potassium to gradually shift from the intracellular fluid to the extracellular fluid, restoring the potassium concentration gradient towards normal, with less severe neuromuscular changes. With acute and severe losses of potassium, changes in neuromuscular excitability are more profound. Skeletal muscle weakness occurs initially in the larger muscles of the legs and arms and ultimately affects the diaphragm and depresses ventilation. Paralysis and respiratory arrest can occur. Loss of smooth muscle tone is manifested by constipation, intestinal distension, anorexia, nausea and vomiting.
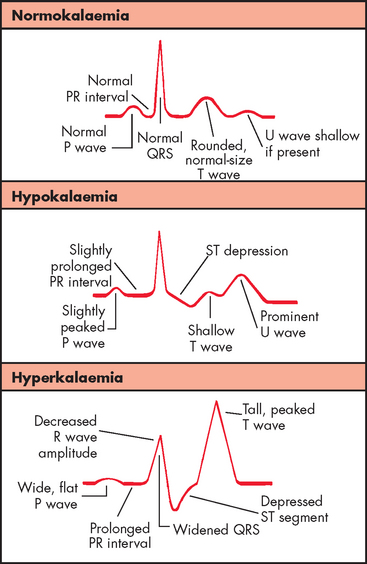
The cardiac effects of hypokalaemia are related also to changes in membrane excitability. Because potassium contributes to the repolarisation phase of the action potential, hypokalaemia delays ventricular repolarisation. Various dysrhythmias may occur, including sinus bradycardia, atrioventricular block and paroxysmal atrial tachycardia. The characteristic changes in the ECG reflect delayed repolarisation — for instance, the amplitude of the T wave decreases, the amplitude of the U wave increases and the ST segment is depressed (see Figure 29-14). In severe states of hypokalaemia, P waves peak and the QRS complex is prolonged. Hypokalaemia also increases the risk of toxicity caused by digoxin (see Chapter 23).
EVALUATION AND TREATMENT
ECG allows for monitoring of cardiac function during hypokalaemia. Oral or intravenous potassium chloride replacement is instituted cautiously to prevent hyperkalaemia. Including foods higher in potassium can be used for those at risk of continual hypokalaemia.
PATHOPHYSIOLOGY
Elevation of extracellular fluid potassium above 5.5 mmol/L constitutes hyperkalaemia.12 Because of efficient renal excretion, increases in total body potassium are relatively rare: acute increases are handled quickly through increased cellular uptake and renal excretion of body potassium excesses (by the effects of aldosterone). Potassium excesses may be caused by increased intake, a shift of potassium from cells to the extracellular fluid or decreased renal excretion.
If renal function is normal, slow increases in potassium intake are usually well tolerated by the kidneys, although short-term potassium increases can be too much for the kidneys to excrete. Dietary excesses of potassium are uncommon, but accidental ingestion of potassium salt substitutes can cause toxicity.
Cell damage results in the release of potassium from inside the cell to the extracellular fluid. This may occur during trauma, burns, massive crushing injuries, hypoxia or extensive surgery. For this reason, someone who has an injury that restricts the circulation is at risk of hyperkalaemia, as the cells that are damaged release their potassium; then, when the restriction is removed, this released potassium can enter the circulation.
In acidosis, hydrogen ions shift into the cells in exchange for intracellular potassium; hyperkalaemia and acidosis therefore often occur together (see ‘Acid–base balance’ below). Because insulin promotes cellular entry of potassium, insulin deficits that occur with diabetic ketoacidosis are accompanied by hyperkalaemia.
Decreased renal excretion of potassium is associated with hyperkalaemia. Renal failure that results in oliguria (low urine output) is accompanied by elevations of serum potassium. Decreases in the secretion or renal effects of aldosterone can also cause decreases in the urinary excretion of potassium. Potassium-sparing diuretics (e.g. spironolactone), which inhibit sodium reabsorption and potassium secretion by the kidneys, may also contribute to hyperkalaemia.
CLINICAL MANIFESTATIONS
During mild hyperkalaemia, increased neuromuscular irritability may be manifested as restlessness, intestinal cramping and diarrhoea. Severe hyperkalaemia causes muscle weakness, loss of muscle tone and paralysis. Hyperkalaemia causes decreased cardiac conduction and more rapid repolarisation of heart muscle. In mild states of hyperkalaemia, the more rapid repolarisation is reflected in the ECG as narrow and taller T waves with a shortened QT interval. Severe hyperkalaemia depresses the ST segment, prolongs the PR interval and widens the QRS complex due to decreased conduction velocity (see Figure 29-14). Alterations in cardiac conduction can cause ventricular fibrillation and cardiac arrest.
Changes in the ratio of intracellular to extracellular potassium concentration contribute to the symptoms of hyperkalaemia (see Table 29-4). The neuromuscular effects of hyperkalaemia are related to the increase in the rate of repolarisation and the presence of other contributing factors such as acidosis. Long-term increases in extracellular potassium concentration result in shifts of potassium into the cells, because the tendency is to maintain a normal ratio of intracellular to extracellular potassium concentrations. Acute elevations of extracellular potassium affect neuromuscular irritability as this ratio is disrupted too quickly to be maintained.
Insulin helps regulate plasma potassium levels by stimulating the sodium–potassium pump, thus promoting the movement of potassium into liver and muscle cells after eating. Insulin can also be used to treat hyperkalaemia. Dangerously low levels of plasma potassium can result when insulin is given while potassium levels are depressed. Potassium balance is especially significant in the treatment of conditions requiring insulin administration, such as type 1 diabetes mellitus.
EVALUATION AND TREATMENT
Elevated potassium levels may be fatal, so urgent correction is necessary. If potassium levels are dangerously high, dialysis may be necessary to remove the potassium from the blood. Immediate correction of any associated acid–base disturbance (see ‘Acid–base balance’ below) is also vital. Assessing and treating the underlying cause is critical — for example, adequate control of blood glucose levels in the diabetic is important in preventing future imbalance.
Calcium, phosphate and magnesium
Interestingly, these electrolytes were once considered to be of lower importance, but increasing evidence of patient prognosis has led to a greater understanding that an appropriate balance of these ions is essential. In fact, it is now common to test these electrolytes in blood tests, along with sodium and potassium, so that alterations can be identified and treated prior to becoming life-threatening.
Calcium
The most critical function of calcium (Ca2+) is in neuronal signalling, as calcium influx is necessary to allow neuronal communication; therefore, calcium homeostasis is usually well-regulated (see Chapter 10). Appropriate calcium concentrations also allow muscle contraction (remember that the heart and diaphragm are critical muscles that require calcium to contract). Stores of calcium in bone are broken down to release the calcium when plasma calcium levels are low. Calcium is found in a higher concentration in the extracellular fluid than within the cells. The main control of plasma calcium levels is by parathyroid hormone; consequently, treatments for the parathyroid gland or the thyroid gland may limit parathyroid hormone secretion, resulting in hypocalcaemia.
Hypercalcaemia
Hypercalcaemia (greater than normal amounts of calcium in the blood) is largely a result of hyperparathyroidism (see Chapter 11). Elevated parathyroid hormone causes increased calcium levels to be released from bone back into the blood, as well as increasing blood calcium at the kidneys and intestines. Symptoms include alteration of neural and muscular function, such as muscle weakness and arrhythmias (linked to abnormally strong contractions). Impaired renal function can lead to loss of water reabsorption and kidney stones.
Hypocalcaemia
Hypocalcaemia (calcium deficiency in the blood) can occur due to hypoparathyroidism or renal failure. Inability of the parathyroid gland to produce adequate parathyroid hormone will not allow calcium levels to be adequate; this may occur with surgery of the thyroid or parathyroid glands. When kidney function is impaired, phosphate ion is retained and calcium cannot be reabsorbed (and hence exits the body in the urine). Muscle spasm and tetany are common, and intestinal cramping and convulsions also occur. There is also a risk of cardiac arrest.
Phosphate
Phosphate (P+) is mainly an intracellular ion; that is, it is found in higher concentrations inside the cell compared with extracellular concentrations. In addition, stores of phosphate are found within bone, where it contributes to bone structure, and it has an important role in acid–base balance. It is also part of the molecule of energy, ATP (adenosine triphosphate), necessary for powering the processes of all body cells; thus, it is needed in all cells and the effects of imbalance are apparent throughout the body. The most significant effects of imbalance are those found in the vital organs, namely the brain and heart — if there is insufficient energy for the neurons or the heart to function, a life-threatening emergency will quickly occur.
Hypophosphataemia
Hypophosphataemia (low serum phosphate) may be caused by intestinal malabsorption due to vitamin D deficiency, long-term alcohol abuse, excessive antacid use or increased excretion of phosphates by the kidneys in response to hyperparathyroidism. The patient who is only mildly hypophosphataemic will not exhibit symptoms until the condition worsens. Symptoms include altered neuron and muscle function, such as altered function of the heart and lungs — cardiac failure or cardiac arrest can result in severe cases.13,14 The patient may also progress to convulsions and coma. Phosphate is measured routinely with other electrolytes in blood tests; a patient with very low levels of phosphate will require aggressive replacement therapy (intravenous phosphate).
Magnesium
Magnesium (Mg2+) is in higher concentrations within the intracellular fluid than outside of cells, with large stores found in bones. In a similar manner to phosphates, magnesium is essential for intracellular processes such as energy metabolism, membrane transport of ions and contraction of muscles (including those of the heart and lungs). It is also important for the normal function of the sodium–potassium pump, which assists in restoring normal concentrations of sodium and potassium across the cell membrane. Due to its diverse roles in cell metabolism, deficiency of magnesium can cause disturbances throughout body cells.
Hypomagnesaemia
Hypomagnesaemia (deficiency of magnesium) is linked to cardiovascular disease and is common in approximately 10% of hospitalised patients, even being apparent in 40% of hospitalised patients who have other disturbances in electrolyte balance.15 Renal losses of magnesium are the most common cause and relate to kidney dysfunction and diuretic use. Hypomagnesaemia is also common following surgery,16 as well as in alcoholism. Symptoms include irritability and muscle cramps, leading to convulsions and hypotension. Intravenous replacement of magnesium is usually necessary and can rectify cardiac arrhythmias and assist with ATP production. However, the recommended dosage for administration is variable.15
ACID–BASE BALANCE
Acid–base balance must be regulated within a narrow range for the body to function normally. Slight changes in amounts of hydrogen ions can significantly alter biological processes in cells and tissues.17 Neurons are particularly susceptible to changes in acid levels and their function is altered if the acid–base balance is not maintained. Most pathological conditions disturb acid–base balance, producing conditions possibly more harmful than the disease process itself.
Acid and pH
Acidic substances are produced by normal body processes. For example, cells that operate using aerobic metabolism (with oxygen) produce carbon dioxide as a by-product, which is then converted into acidic molecules (see below). In addition, cells that use anaerobic metabolic processes (without oxygen) also produce acid — in this case, lactic acid. Body acids are formed as end products of the metabolism of protein, carbohydrate and fat. This must be balanced by the amount of basic or alkaline substances in the body to maintain normal pH, as changes in acid levels alter body processes, as well as being particularly harmful to neurons. For this reason, multiple systems control acid–base balance in the body.
The concentration of acid or hydrogen ion, H+, in the blood is maintained in a narrow pH range of 7.35 to 7.45. A pH of less than 7.35 is defined as acidosis and a pH greater than 7.45 is defined as alkalosis (alkaline meaning the same as a base, the opposite to an acid) (see Table 29-5). Changes in pH are life-threatening; for instance, a blood pH of 6.8 is too acidic to sustain the body and a pH of 7.8 is too alkaline (see Figure 29-15). Certain conditions can greatly displace blood pH. One example is when exercising and approaching exhaustion, such as during a maximal exercise test. The severe metabolic effects can drive the blood pH below 7. However, in comparison to disease states, this effect is only temporary and the body will rapidly correct the body’s pH back to homeostatic balance. Furthermore, the individual will not be able to continue with the exercise due to the metabolic imbalance.
| BODY FLUID | pH | FACTORS AFFECTING pH |
|---|---|---|
| Gastric juices | 1.0–3.0 | Hydrochloric acid production |
| Urine | 5.0–6.0 | H+ ion excretion from waste products |
| Arterial blood | 7.35–7.45 | pH is slightly higher because there is less carbonic acid |
| Venous blood | 7.37 | pH is slightly lower because there is more carbonic acid |
| Cerebrospinal fluid | 7.32 | Decreased bicarbonate and higher carbon dioxide content decrease pH |
| Pancreatic fluid | 7.8–8.0 | Contains bicarbonate produced by exocrine cells |
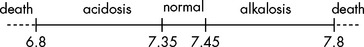
FIGURE 29-15 Normal pH range is 7.35 to 7.45.
Below this is acidosis, and above this alkalosis. Changes in pH that cannot be corrected are fatal.
The lungs and kidneys are the major organs involved in regulating acid–base balance. These systems work together to regulate short- and long-term changes in acid–base status. We now examine how these systems work to achieve pH homeostasis.
Buffer systems
Buffering occurs in response to changes in acid–base status. Buffers are substances that can absorb excessive acid (hydrogen ion, H+) or base (hydroxyl ion, OH−) and prevent significant fluctuations in pH. The buffer systems are located in both the intracellular and the extracellular fluid compartments and they function at different rates (see Table 29-6). The most important plasma buffer systems are carbonic acid-bicarbonate and the protein haemoglobin.
| BUFFER SYSTEM | MECHANISM | RATE |
|---|---|---|
| Protein buffer and ionic shifts | Protein buffers bind acid; exchange of intracellular potassium and sodium for hydrogen | 2–4 hours |
| Lungs | Regulates retention or elimination of carbon dioxide and therefore carbonic acid concentration | Minutes–hours |
| Kidneys | Bicarbonate reabsorption and regeneration, ammonia formation, phosphate buffering | Hours–days |
Acid–base fluctuations are essentially controlled on three levels: (1) by protein buffers; (2) by the lungs; and (3) by the kidneys. The protein buffering system consists of substances that are present in the blood that are able to provide short-term, immediate balancing of acid–base changes. The lungs then contribute and are efficient in removing acid, which is related to respiratory processes — namely, due to carbon dioxide levels. Finally, the kidneys are responsible for the main, longer-term control of acid–base balance and are able to remove excess acid (or base) from a variety of sources. These three systems contribute to acid–base homeostasis.
Renal and respiratory adjustments to changes in pH are known as compensation. The respiratory system compensates for changes in pH by increasing or decreasing carbon dioxide by changing ventilation. The renal system compensates by producing more acidic or more alkaline urine. Correction occurs when the values for both components of the buffer pair (carbonic acid and bicarbonate) return to normal levels.
Protein buffering
Proteins have negative charges that allow them to combine with hydrogen ions, which are positively charged, to buffer excess acid. This occurs mainly inside cells where the level of protein is high:
This means that the acid inside the cells can be buffered by the high level of intracellular protein. Haemoglobin, apart from its vital roles in oxygen and carbon dioxide transport, is also an excellent intracellular buffer. It is a protein and can therefore bind with acid hydrogen:
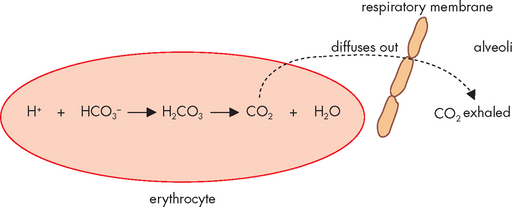
Haemoglobin is also critical for being able to buffer carbon dioxide — when carbon dioxide is combined with water, it forms carbonic acid, which then splits into hydrogen ion (acid) and bicarbonate ion (see Figure 29-16). This occurs in a number of processes as part of acid–base balance and it is the most important relationship to remember, as it allows you to see that carbon dioxide actually leads to acid production. This process occurs inside red blood cells, where the hydrogen ion can bind with haemoglobin, thereby buffering the blood. Haemoglobin not saturated with oxygen (found in venous blood) is actually a better buffer than haemoglobin saturated with oxygen (arterial blood) — this is particularly convenient, as it is the venous blood that collects the acid produced from cells. In this manner, the acid can be buffered in the blood, until it can be transported to the lungs for elimination.
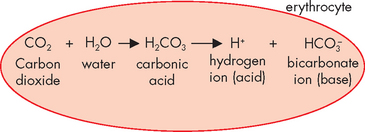
FIGURE 29-16 Carbon dioxide combines with water to form carbonic acid, which then splits into hydrogen ion (acid) and bicarbonate ion (base).
Finally, cells can exchange acid and potassium. When acid levels rise, which causes acidosis, increased levels of acid inside the cells causes potassium to exit the cells (Figure 29-17) — in this way, the overall level of positive charge inside the cells remains more stable. However, hyperkalaemia may result, which can lead to serious pathophysiological conditions (see previous section).
Respiratory buffering
The bicarbonate buffer system is a major extracellular buffer, which works with the respiratory system (discussed in Chapter 24). Carbon dioxide, produced from cellular processes, combines with water (present as body fluid, inside and outside of cells) in the manner described above. Increases in carbon dioxide contribute directly to increased amounts of acid and hence lowered pH (see Figure 29-18).
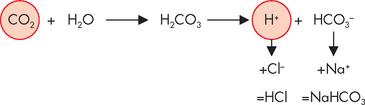
FIGURE 29-18 Increased levels of carbon dioxide result in increased levels of acid being produced (and a lowered pH).
In this figure, note that the bicarbonate ion combines with sodium to form sodium bicarbonate. It is also possible for the bicarbonate ion to combine with potassium and magnesium, which have lesser roles in acid–base balance. HCl = hydrochloric acid; NaHCO3 = sodium bicarbonate.
Once the haemoglobin carrying its hydrogen ion returns to the lungs, the acid can be eliminated from the body by the steps occurring in reverse — this results in the formation of carbon dioxide, which is removed from the body simply by exhaling (see Figure 29-19). The lungs are able to remove excess carbon dioxide in this way relatively quickly, by simply increasing the respiratory rate.
Renal buffering
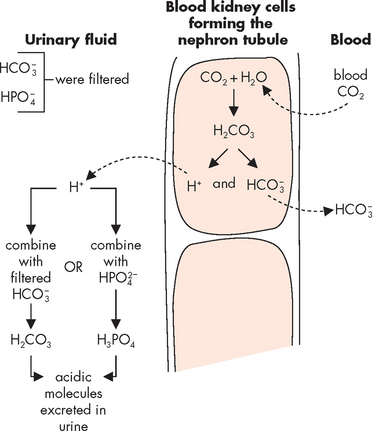
The kidneys have the main role in acid–base balance, especially in long-term regulation. They have the ability to make relatively large changes as necessary to restore the balance. The nephrons are able to remove acid from the blood, as well as produce new bicarbonate ions for the blood, to enable more buffering ability in the blood. Figure 29-20 shows how carbon dioxide diffuses from the blood into the kidney tubule cells and is then secreted into the urinary filtrate. Here, the acid is combined with other molecules to form carbonic acid, which exits the body in the urine. At the same time, the kidneys regenerate new bicarbonate from carbon dioxide and water. The nephrons can also reabsorb some of the bicarbonate ion that was filtered, as an additional means of supplying more bicarbonate to the blood when needed to buffer more acid.
Acid–base imbalances
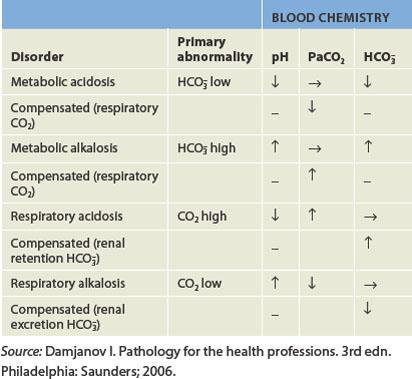
Pathophysiological changes in the concentration of hydrogen ion in the blood lead to acid–base imbalances.18 When the pH of arterial blood is less than 7.35, this is termed acidaemia. A systemic increase in hydrogen ion concentration is termed acidosis, which is the more commonly used term in clinical practice. In alkalaemia, the pH of arterial blood is greater than 7.45. Accordingly, a systemic decrease in hydrogen ion concentration is termed alkalosis, a term that is also commonly used in clinical practice. These pH disturbances are often associated with serious pathophysiological deterioration and may be caused by metabolic or respiratory processes (summarised in Table 29-7).
Respiratory acidosis
Respiratory acidosis occurs when the pH is less than 7.35 due to hypoventilation (see Figure 29-21A). This occurs when alveolar ventilation (gas exchange at the alveoli) decreases and there is a build-up of carbon dioxide in the body (hypercapnia). The arterial carbon dioxide pressure (PaCO2) becomes greater than 45 mmHg, which lowers the pH. Respiratory acidosis can be acute or chronic. Common causes include depression of the respiratory centre (i.e. from drugs or head injury), respiratory muscle paralysis, disorders of the chest wall (e.g. fractured ribs) and disorders of the lung (pneumonia, pulmonary oedema, emphysema, asthma, bronchitis). Compensation (to correct the pH abnormality) occurs in the kidneys by increasing the elimination of hydrogen ions in the urine and retaining bicarbonate, which causes an increase in pH back to the normal range.
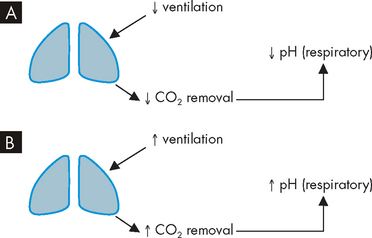
FIGURE 29-21 Respiratory causes of acid–base disturbances.
A Decreased ventilation leads to low pH, or respiratory acidosis. B Increased ventilation leads to high pH, or respiratory alkalosis.
The signs and symptoms seen often include headache, blurred vision, breathlessness, restlessness and apprehension followed by lethargy, disorientation, muscle twitching, tremors, convulsions and coma. The respiratory rate increases to correct the acidosis. The skin may be warm and flushed as the elevated carbon dioxide causes vasodilation.
Respiratory alkalosis
Respiratory alkalosis occurs when alveolar hyperventilation causes an excessive reduction in plasma carbon dioxide levels (hypocapnia),19 resulting in a carbon dioxide level of less than 35 mmHg (see Figure 29-21B). Respiratory alkalosis can also be chronic or acute. The most common cause of respiratory alkalosis is anxiety leading to hyperventilation, triggered by a stressful event like a pathophysiology exam! Hypoxaemia (caused by pulmonary disease, congestive heart failure or high altitudes), hypermetabolic states (fever, anaemia), hysteria and gram-negative sepsis stimulate hyperventilation. The kidneys compensate by decreasing hydrogen excretion and bicarbonate reabsorption in the nephrons.
The central and peripheral nervous systems are stimulated by respiratory alkalosis, causing dizziness, confusion, tingling of extremities (paraesthaesias), convulsions and coma. Cerebral vasoconstriction reduces cerebral blood flow, which is actually useful in the short term if the patient has raised intracranial pressure. Deep and rapid breathing is the primary symptom that causes respiratory alkalosis.
Metabolic acidosis
In metabolic acidosis, acids due to sources other than carbonic acid increase. Alternatively, bicarbonate is lost from the extracellular fluid, such as during severe diarrhoea (see Figure 29-22A). This can occur either quickly or over an extended period of time. The buffering systems normally compensate for excess acid and maintain arterial pH within the normal range. When acidosis is severe, buffers become depleted and cannot compensate adequately to correct the decrease in pH.
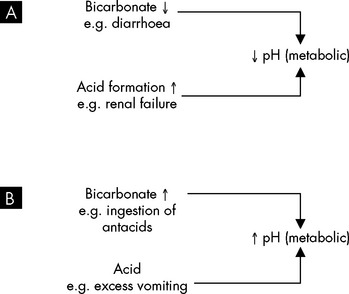
FIGURE 29-22 Metabolic causes of acid–base disturbances.
A Metabolic acidosis occurs due to decreased pH, caused by increased levels of acid or decreased bicarbonates. B Metabolic alkalosis occurs when pH is raised by increased bicarbonate or loss of acid.
Metabolic acidosis is manifested by changes in the function of the neurological, respiratory, gastrointestinal and cardiovascular systems. Early symptoms include headache and lethargy, which progress to coma in severe acidosis. The respiratory system’s efforts to compensate for the increase in metabolic acids result in what is termed Kussmaul respiration — deep, rapid and sustained breaths. This represents the body’s attempt to increase pH by exhaling excess carbon dioxide, which decreases carbonic acid. Other symptoms include anorexia, nausea, vomiting, diarrhoea and abdominal discomfort. Death can result in the most severe and prolonged cases, preceded by arrhythmias and hypotension.
Metabolic alkalosis
Metabolic alkalosis occurs when there is an excessive loss of metabolic acids or bicarbonate levels increase (Figure 29-22B).20 When acid loss is caused by vomiting, the kidneys’ compensation is not very effective because the loss of chloride (an anion) in the vomitus in the form of hydrochloric acid (HCl) actually stimulates renal retention of bicarbonate (an anion), which can contribute to the alkalosis. Hyperaldosteronism can also lead to alkalosis as a result of sodium bicarbonate retention and loss of hydrogen and potassium. Some diuretics (such as frusemide and thiazide) may produce a mild alkalosis because they promote greater excretion of sodium, potassium and chloride than bicarbonate.
Some common signs and symptoms of metabolic alkalosis are weakness, muscle cramps, hyperactive reflexes, tetany, shallow slow respirations, confusion and convulsions. The manifestations vary with the cause and severity of the alkalosis. The symptoms of hyperactive reflexes and tetany occur because alkalosis increases binding of calcium to plasma proteins, thus decreasing blood calcium levels. The decreased calcium levels cause excitable cells to become hypopolarised (membrane potential is towards threshold), initiating an action potential more easily and causing muscle contraction.
PAEDIATRICS AND THE DISTRIBUTION OF BODY FLUIDS
At birth, total body water represents about 75–80% of body weight; this decreases to about 67% during the first year of life. Physiological loss of body water amounting to 5% of body weight occurs as the infant adjusts to a new environment. Infants are particularly susceptible to significant changes in body fluids because of their high metabolic rate and greater body surface area. Consequently, they have a greater fluid intake and output in relation to their body size. In addition, the immature kidneys are less able to reabsorb water, so the amount of water excreted is relatively higher than in adults. This can contribute to the infant’s susceptibility for developing dehydration. Renal mechanisms of fluid and electrolyte conservation may not be mature enough to counter abnormal losses related to vomiting or diarrhoea, thereby allowing dehydration to occur. Symptoms of dehydration include thirst, decreased urine output, decreased body weight, decreased skin elasticity, sunken fontanels, absent tears, dry mucous membranes, increased heart rate and irritability. Dehydration due to fluid loss is a leading cause of death in newborns and children, and therefore restoration of fluids is imperative.
Total body water slowly decreases to 60–65% of body weight in adulthood. At adolescence, the percentage approaches adult levels and differences in the sexes appear. Males have a greater percentage of body water because of increased muscle mass, and females have more body fat because of the influence of oestrogen and thus have less body water.
AGEING AND THE DISTRIBUTION OF BODY FLUIDS
The further decline in the percentage of total body water in the elderly is in part the result of a decreased free fat mass and decreased muscle, as well as reduced ability to regulate sodium and water balance. Kidneys are less efficient in producing concentrated urine, and sodium-conserving responses are sluggish. Thirst perception also may decline and loss of cognitive function can influence access to beverages. Healthy older adults can adequately maintain their hydration status. When disease is present, a decrease in body fluid content and dehydration can become life-threatening.21,22
Fluid balance
 Body fluids are distributed among functional compartments and are classified as intracellular fluid (ICF) and extracellular fluid (ECF). The main divisions of extracellular fluid are the plasma (within blood vessels) and interstitial fluid between cells (outside of the blood).
Body fluids are distributed among functional compartments and are classified as intracellular fluid (ICF) and extracellular fluid (ECF). The main divisions of extracellular fluid are the plasma (within blood vessels) and interstitial fluid between cells (outside of the blood). Water moves between the intracellular fluid and extracellular fluid compartments principally by osmosis.
Water moves between the intracellular fluid and extracellular fluid compartments principally by osmosis. Water moves between the plasma and interstitial fluid by osmosis and hydrostatic pressure, which occur across the capillary membrane. Movement across the capillary wall occurs due to this balance between hydrostatic and osmotic forces. Fluid in the interstitium is drained by the lymphatic vessels.
Water moves between the plasma and interstitial fluid by osmosis and hydrostatic pressure, which occur across the capillary membrane. Movement across the capillary wall occurs due to this balance between hydrostatic and osmotic forces. Fluid in the interstitium is drained by the lymphatic vessels. Oedema is a problem of fluid distribution that results in the accumulation of fluid within the interstitial spaces.
Oedema is a problem of fluid distribution that results in the accumulation of fluid within the interstitial spaces. The pathophysiological process that leads to oedema is related to an increase in forces favouring fluid movement out of capillaries into tissues such as increased capillary hydrostatic pressure, lowered plasma oncotic pressure, increased capillary permeability or blockages of lymphatic channels.
The pathophysiological process that leads to oedema is related to an increase in forces favouring fluid movement out of capillaries into tissues such as increased capillary hydrostatic pressure, lowered plasma oncotic pressure, increased capillary permeability or blockages of lymphatic channels.Electrolyte balance
 The movement of electrolytes and water is related: sodium is usually followed by water and chloride, while potassium moves in the opposite direction to sodium across cell membranes.
The movement of electrolytes and water is related: sodium is usually followed by water and chloride, while potassium moves in the opposite direction to sodium across cell membranes. Water balance is regulated by the sensation of thirst and by antidiuretic hormone, which is secreted in response to an increase in plasma osmolality or a decrease in circulating blood volume.
Water balance is regulated by the sensation of thirst and by antidiuretic hormone, which is secreted in response to an increase in plasma osmolality or a decrease in circulating blood volume. Sodium is the main electrolyte in the extracellular fluid and hence it is a main contributor to fluid volume in this compartment. Sodium is critical for a number of functions, including the normal function of neurons and muscle.
Sodium is the main electrolyte in the extracellular fluid and hence it is a main contributor to fluid volume in this compartment. Sodium is critical for a number of functions, including the normal function of neurons and muscle. Sodium balance is regulated by aldosterone, which increases reabsorption of sodium from the urine into the blood by the distal convoluted tubule of the kidneys.
Sodium balance is regulated by aldosterone, which increases reabsorption of sodium from the urine into the blood by the distal convoluted tubule of the kidneys. Hypovolaemia is a low blood volume, while hypervolaemia is a high blood volume. Corresponding changes in blood pressure occur with these conditions.
Hypovolaemia is a low blood volume, while hypervolaemia is a high blood volume. Corresponding changes in blood pressure occur with these conditions. Hypernatraemia (elevated plasma sodium levels) may be caused by an acute increase in sodium or a loss of water.
Hypernatraemia (elevated plasma sodium levels) may be caused by an acute increase in sodium or a loss of water. Water deficit, or hypertonic dehydration, is rare but can be caused by hyperventilation, lack of access to water, pure water losses, arid climates and increased renal elimination of water.
Water deficit, or hypertonic dehydration, is rare but can be caused by hyperventilation, lack of access to water, pure water losses, arid climates and increased renal elimination of water. Hyponatraemia (lowered plasma sodium concentration) usually causes movement of water into the cells.
Hyponatraemia (lowered plasma sodium concentration) usually causes movement of water into the cells. Hyponatraemia may be caused by sodium loss, inadequate sodium intake or dilution of the body’s sodium level with excess water.
Hyponatraemia may be caused by sodium loss, inadequate sodium intake or dilution of the body’s sodium level with excess water. Water excess is rare but can be caused by compulsive water drinking, decreased urine formation or the syndrome of inappropriate secretion of antidiuretic hormone.
Water excess is rare but can be caused by compulsive water drinking, decreased urine formation or the syndrome of inappropriate secretion of antidiuretic hormone. Potassium is the predominant intracellular fluid ion; it regulates intracellular fluid osmolality, maintains the resting membrane potential and mediates action potentials in neurons and muscle.
Potassium is the predominant intracellular fluid ion; it regulates intracellular fluid osmolality, maintains the resting membrane potential and mediates action potentials in neurons and muscle. The mechanism of potassium tolerance or adaptation allows the body to accommodate slowly to increased levels of potassium intake.
The mechanism of potassium tolerance or adaptation allows the body to accommodate slowly to increased levels of potassium intake. Hypokalaemia (low plasma potassium concentration) indicates loss of total body potassium and may be caused by reduced potassium intake, a shift from extracellular fluid to intracellular fluid potassium, increased aldosterone or increased renal excretion.
Hypokalaemia (low plasma potassium concentration) indicates loss of total body potassium and may be caused by reduced potassium intake, a shift from extracellular fluid to intracellular fluid potassium, increased aldosterone or increased renal excretion. Hyperkalaemia (elevated plasma potassium levels) may be caused by increased potassium intake, a shift from intracellular fluid to extracellular fluid potassium or decreased renal excretion.
Hyperkalaemia (elevated plasma potassium levels) may be caused by increased potassium intake, a shift from intracellular fluid to extracellular fluid potassium or decreased renal excretion. Calcium is a necessary ion in neuronal signalling and blood clotting and is stored in the bone. Calcium is mainly controlled by parathyroid hormone levels.
Calcium is a necessary ion in neuronal signalling and blood clotting and is stored in the bone. Calcium is mainly controlled by parathyroid hormone levels. Hypercalcaemia (high plasma calcium concentration) is usually caused by elevated parathyroid hormone and may cause kidney stones and cardiac arrhythmia.
Hypercalcaemia (high plasma calcium concentration) is usually caused by elevated parathyroid hormone and may cause kidney stones and cardiac arrhythmia. Hypocalcaemia (low plasma calcium concentration) is usually related to inadequate levels of parathyroid hormone; a serious complication is cardiac arrest.
Hypocalcaemia (low plasma calcium concentration) is usually related to inadequate levels of parathyroid hormone; a serious complication is cardiac arrest.Acid–base balance
 Hydrogen ions (acid) must be concentrated within a narrow range if the body is to function normally. Hydrogen ion concentration is expressed as pH and indicates the balance in acid or base.
Hydrogen ions (acid) must be concentrated within a narrow range if the body is to function normally. Hydrogen ion concentration is expressed as pH and indicates the balance in acid or base. The normal pH of the blood is 7.35 to 7.45. Acidosis is defined as a pH less than 7.35 and alkalosis as a pH above 7.45. Significant changes in pH are life-threatening.
The normal pH of the blood is 7.35 to 7.45. Acidosis is defined as a pH less than 7.35 and alkalosis as a pH above 7.45. Significant changes in pH are life-threatening. The renal and respiratory systems, together with the body’s protein buffer systems, are the principal regulators of acid–base balance.
The renal and respiratory systems, together with the body’s protein buffer systems, are the principal regulators of acid–base balance. The lungs and kidneys act to compensate for changes in pH by increasing or decreasing ventilation and by producing more acidic or more alkaline urine.
The lungs and kidneys act to compensate for changes in pH by increasing or decreasing ventilation and by producing more acidic or more alkaline urine. Respiratory acidosis occurs with a decrease in alveolar ventilation, which in turn causes hypercapnia (an increase in carbon dioxide) and increases in carbonic acid concentration.
Respiratory acidosis occurs with a decrease in alveolar ventilation, which in turn causes hypercapnia (an increase in carbon dioxide) and increases in carbonic acid concentration. Respiratory alkalosis occurs with alveolar hyperventilation and excessive reduction of carbon dioxide or hypocapnia with decreases in carbonic acid.
Respiratory alkalosis occurs with alveolar hyperventilation and excessive reduction of carbon dioxide or hypocapnia with decreases in carbonic acid.Karolina, a female patient in her mid-70s, has been admitted to hospital from the nursing home where she has been living for the last 3 years. Over the course of this time, her renal function has deteriorated despite being prescribed the diuretic drug frusemide, to assist in renal excretion of sodium and water. Karolina presents to hospital in a dehydrated state with a decreased serum potassium level (2.6 mmol/L). Aggressive intravenous fluid and potassium replacement therapy over the next 48 hours restore the potassium back to the normal range but cause hyponatraemia (130 mmol/L).