Chapter 9 Individual and Lifespan Aspects of Drug Therapy
The administration of drugs to pregnant women, children and the elderly varies because of numerous factors specific to each of these groups. Such variations may be individual and are altered by both physiological and pathophysiological factors present at different ages. Drug therapy in pregnant women may increase the risk of fetal abnormalities; in children, miscalculated doses of common drugs may cause adverse reactions; and in the elderly, polypharmacy may contribute to drug toxicity. Optimising drug therapy across the lifespan of an individual is a challenging process and requires a thorough understanding by health-care professionals of the effects of ageing on pharmacodynamic and pharmacokinetic processes.
Key abbreviations
DRUG therapy in the pregnant woman, infant, child and the elderly patient may differ from that in the rest of the adult population; for example, drugs taken by the pregnant woman may reach the fetus via the maternal circulation and cause birth defects. Drugs consumed by a breastfeeding woman may be excreted in milk; if the drug concentrations are high enough this can cause adverse effects in the breastfed infant. Children and the elderly are often more sensitive to medications; in infants, this is because of immaturity of organs and some enzymes whereas, in the elderly, organ function may be compromised or impaired. Management of drug therapy throughout the lifespan of an individual must vary in line with the changes that occur to normal physiological and biochemical systems through ageing.
Drug use during pregnancy
During pregnancy, any chemical or drug substance consumed and absorbed may reach the fetus by way of the maternal circulation or be transferred to the neonate via breast milk. Drug use during pregnancy should be avoided or limited to only those women who absolutely require treatment and where the benefit to the mother is considered greater than the risk to the fetus. All women should be counselled to avoid exposure to all unnecessary drugs (including complementary medicines) and chemicals throughout their pregnancies. With the recent trends towards higher birth rates in the older age groups of 35–39 and 40–44 years, it is likely that many more women will already be on medications for existing chronic medical conditions (e.g. asthma or diabetes) when they become pregnant. This will increase the risk of exposure of the fetus to maternal drugs. Similarly, many women may require drug therapy as a result of the pregnancy, e.g. to treat nausea and vomiting.
If it is necessary to administer drug therapy, the most important variables to be considered include:
These should be adjusted carefully to avoid harmful effects.
A major problem with drug use is that the effects on the embryo may occur before a woman is aware she is pregnant. Women of childbearing age who are not using contraceptives and who are sexually active should be prescribed drugs carefully and should be instructed to use over-the-counter medications cautiously if they are contemplating pregnancy. Education and prevention are considered the best options.
Drug pregnancy categories
Even though many drugs cross the placenta, the potential for inducing adverse fetal effects depends on the drug type, drug concentration and fetal age. Drugs taken during the first trimester may cause congenital malformations; those taken during the second and third trimesters may result in perturbation of functional and growth development, while those administered close to labour may affect the birth process and the neonate.
The Australian Drug Evaluation Committee (ADEC) has established seven pregnancy categories (A, B1, B2, B3, C, D and X) to indicate the level of risk to the fetus of drugs used at the recommended therapeutic doses (Table 9-1). Although this schedule is useful clinically, a range of drugs in use today have not been rated and many of the studies have been performed only in animals. In this scheme, which is used throughout Australasia, category A drugs are considered the least problematic, while drugs in category X are considered the most dangerous and should not be used in pregnancy or when contemplating pregnancy.
Table 9-1 Adec drugs-in-pregnancy risk categories
| Category | Definition |
| A | Drugs which have been taken by a large number of pregnant women and women of childbearing age, without any proven increase in the frequency of malformations or other direct or indirect harmful effects on the fetus having been observed |
| B1 | Drugs which have been taken by only a limited number of pregnant women and women of childbearing age without an increase in the frequency of malformation or other direct or indirect harmful effects on the human fetus having been observed. Studies in animals have not shown evidence of an increased occurrence of fetal damage |
| B2 | Drugs which have been taken by only a limited number of pregnant women and women of childbearing age without an increase in the frequency of malformations or other direct or indirect harmful effects on the human fetus having been observed. Studies in animals are inadequate or may be lacking, but available data show no evidence of an increased occurrence of fetal damage |
| B3 | Drugs which have been taken by only a limited number of pregnant women and women of childbearing age without an increase in the frequency of malformations or other direct or indirect harmful effects on the human fetus having been observed. Studies in animals have shown evidence of an increased occurrence of fetal damage, the significance of which is considered uncertain in humans |
| C | Drugs which, owing to their pharmacological effects, have caused, or may be suspected of causing, harmful effects on the human fetus or neonate without causing malformations. These effects may be reversible. Accompanying texts should be consulted for further details |
| D | Drugs which have caused, are suspected to have caused or may be expected to cause, an increased incidence of human fetal malformations or irreversible damage. These drugs may also have adverse pharmacological effects. Accompanying texts should be consulted for further details |
| X | Drugs which have such a high risk of causing permanent damage to the fetus that they should not be used in pregnancy or when there is a possibility of pregnancy |
Note: For drugs in the B1, B2 and B3 categories, human data are lacking or inadequate and sub categorisation is therefore based on available animal data. The allocation of a B category does NOT imply greater safety than the C category. Drugs in Category D (e.g. anticonvulsants) are not absolutely contraindicated in pregnancy; moreover, in some cases the D category has been assigned on the basis of ‘suspicion’.
Due to legal considerations in this country, sponsor companies have, in some cases, applied a more restrictive category than can be justified on the basis of the available data.
In some cases there may be discrepancies between the published Product Information and the information in this booklet due to the process of ongoing document revision.
These categories are subject to ongoing revision and amendment. Readers are asked to consult the Therapeutic Goods Administration website: www.tga.gov.au/docs/html/medpreg.htm.
Reproduced from: Australian Drug Evaluation Committee. Prescribing Medicines in Pregnancy, 4th edn. Canberra: Government Publishing Service, 1999. Copyright Commonwealth of Australia, reproduced by permission.
Any contemplation of the use of drug therapy in a pregnant woman should include consideration of the risk–benefit ratio. This ratio is evaluated based on the mother’s condition and the potential beneficial effect of the drug(s) to the mother and the risk to the developing fetus. It is important to appreciate that no drug can really be labelled as totally safe. Typically, information on drugs not included in the ADEC categories provides non-specific warnings such as ‘safe use in pregnancy has not been established’ or ‘it is not known whether … can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity’. Statements of this type led Koren et al to conclude that ‘these typical disclaimers, although understandable from the medico legal standpoint, put large numbers of women and their physicians in difficult situations’ (Koren et al 1998).
Many historical examples highlight the problems of drug use in pregnancy and the issues faced by pregnant women and health-care providers. The use of the oestrogen diethylstilboestrol (DES) during pregnancy initially did not cause any problems; however, during the 1970s, it was linked to an increased risk of vaginal and cervical cancer in female offspring and to genital abnormalities in both male and female offspring. It has been postulated that DES taken by the mother during the first trimester of pregnancy accumulated in the fetus, which was unable to metabolise it, resulting in problems later in life. The incidence was low (0.01–0.1%) (Food and Drug Administration 1985) but the origin of the problem—a drug taken during developmental stages in pregnancy that has the potential to cause problems in offspring in later life—is still a concern.
The first trimester is when the developing embryo is most vulnerable to the teratogenic effects of various drugs and chemicals. Health-care professionals should keep in mind that drugs in this context include prescription and over-the-counter drugs, complementary medicines, alcohol (see Clinical Interest Box 9-1), drugs of abuse and any other chemical substance that the mother is exposed to during this time.
Clinical interest box 9-1 Fetal alcohol syndrome
Alcohol consumption during pregnancy increases substantially the risk of fetal abnormalities. While the exact amount of alcohol that causes harm is unknown, recent evidence suggests that one drink a week is associated with the possibility of mental health problems (Sayal et al 2007). Alcohol easily crosses the placenta entering the fetal bloodstream and causing fetal alcohol syndrome (FAS), a series of congenital abnormalities. The symptoms of FAS include small head (microcephaly), low birth weight, mental and growth retardation, impaired coordination, irritability in infancy, hyperactivity in childhood, cardiac murmurs, cleft lip or palate, hernias and many other neurological and structural abnormalities (see picture).
Australian and New Zealand Guidelines published in 2006* are clearly at odds with the United States Surgeon General, the American College of Obstetricians and Gynecologists and the American Academy of Pediatrics that urge abstinence from alcohol during pregnancy.
The National guidelines state that women ‘may consider not drinking at all’, ‘should never become intoxicated’ and ‘if they choose to drink, over a week, should have less than 7 standard drinks, and, on any one day, no more than 2 standard drinks (spread over at least two hours)’. In addition ‘an abstinencebased approach is not recommended, in part because it could result in disproportionate anxiety among women with an unplanned pregnancy, many of whom consume some alcohol before they know they are pregnant, but usually without harmful 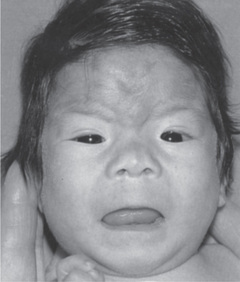 consequences for the infant’. These National Guidelines have met with opposition (Whitehall 2007) and sustained public education aimed at preventing FAS is clearly important as the perceptions of women as to a safe level of alcohol consumption vary enormously. Abstinence clearly reduces the risk of FAS.
consequences for the infant’. These National Guidelines have met with opposition (Whitehall 2007) and sustained public education aimed at preventing FAS is clearly important as the perceptions of women as to a safe level of alcohol consumption vary enormously. Abstinence clearly reduces the risk of FAS.
* National clinical guidelines for the management of drug use during pregnancy, birth and the early development years of the newborn. Ministerial Council on Drug Strategy. Sydney: NSW Health, 2006. http://www.health.nsw.gov.au/pubs/2006/ncg_druguse.html [Aug 2009].
Source: Streissguth et al 1991.
Embryo development
Drugs can exert a beneficial therapeutic effect in the fetus, cause predictable adverse effects in the fetus and act as teratogens (any substance that interferes with normal fetal development causing one or more developmental abnormalities), mutagens (any physical or chemical agent that causes a genetic mutation or increases the mutation rate) or carcinogens (any agent that causes the development of cancer or increases its incidence). Each embryo undergoes precisely programmed steps from cell proliferation to differentiation to organogenesis. The critical periods for drug effects on the fetus are the first 2 weeks of rapid cell proliferation, when exposure to drugs can be lethal to the embryo, and weeks 3–12 of pregnancy, the period of organogenesis. This is when the extremities (arms, fingers, legs and toes), central nervous system, muscles and organs are developing most rapidly (Figure 9-1).
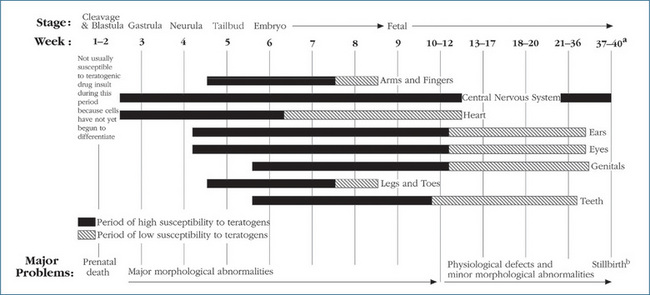
Figure 9-1 Variation in teratogenic susceptibility of organ systems during stages of human intrauterine development.
aAverage time from fertilisation to parturition is 39 weeks.
bDrugs administered during this period may cause neonatal depression at birth (or other effects directly related to the pharmacological effect of the administered drug).
Reprinted, by permission from Problems in Pediatric Drug Therapy, 4th edn, 2002, by the American Pharmaceutical Association.
Thalidomide is a well-known example of a drug with teratogenic effects during organogenesis. It was used widely, mostly in Europe, as a sedative–hypnotic drug from the 1950s to the early 1960s. Children whose mothers received this drug during pregnancy displayed abnormal limb development (phocomelia), a rare birth defect characterised by the absence or malformation of arms or legs. When administered after the 10th–12th week of pregnancy, physiological or behavioural alterations and growth delays were more likely. Thalidomide is currently available in Australia for the treatment of erythema nodosum leprosum and multiple myeloma after conventional therapy has failed. The history of this product is well recognised and every precaution is instituted if it is prescribed currently for a woman of childbearing age.
The abuse of cocaine during pregnancy has resulted in spontaneous abortions, fetal hypoxia, premature delivery and congenital abnormalities (skull defects, cardiac abnormality), and cerebral infarction or stroke. At birth, the newborn may exhibit symptoms of cocaine drug withdrawal (irritability, increased respiratory and heart rates, diarrhoea, irregular sleeping patterns and poor appetite). It has been reported that long-term behavioural patterns, such as poor attention spans and a decrease in organisational skills, may also occur in infants born to cocaine-abusing women (Hall et al 1990).
During pregnancy a woman with epilepsy will still require an antiepileptic agent, as uncontrolled epilepsy is potentially a life-threatening situation for both the mother and the child. Use of antiepileptic drugs is not without risk as they increase (2–3-fold) the risk of fetal abnormalities. The risk increases with increased number of antiepileptic drugs in comparison to treatment with a single antiepileptic drug. Fetal malformations include congenital heart disease, cleft lip or palate, neural tube defects and urogenital effects. Estimation of the teratogenic effects of antiepileptic drugs is ongoing and national registers have been established around the world. These registers include the North American Antiepilepsy Drug (AED) registry for pregnant women who are taking antiepileptic drugs (http://www.epilepsyfoundation.org/research/aedpregreg.cfm) and the Australian Pregnancy Register of Antiepileptic Drugs for Women in Pregnancy (http://www.epilepsy-society.org.au/pages/documents/APR_info.pdf).
Table 9.2 summarises information on some antiepileptic drugs and teratogenic effects (Lander 2008). Other chronic medical disorders in the mother that also need to be treated during pregnancy include diabetes, hypertension and asthma. All chronic maternal conditions require close monitoring and careful selection of therapies to minimise or reduce the risk of fetal abnormalities and maternal disease complications.
Table 9-2 Antiepileptic drugs in pregnancy
| Drug | Potential birth defect(S) | Specific considerations |
| Carbamazepine (ADEC Category D) | Spina bifida | Current data from the Australian register suggests malformation rate does not appear to differ from untreated women with epilepsy |
| Clonazepam (ADEC Category B3) | No risks identified to date | May cause drowsiness in breastfed neonate |
| Lamotrigine (ADEC Category D) | Oral clefts and significant dose-related teratogenesis | Maternal clearance of lamotrigine changes during the course of pregnancy necessitating dose adjustment. Extensively excreted in breast milk |
| Levetiracetam (ADEC Category B3) | Teratogenic risk is unknown at present | Secreted in breast milk but neonatal concentrations appear low |
| Valproate (ADEC Category D) | Substantial risk of major malformations including spina bifida. Risk increases with doses >1000 mg/day | Where possible drug should be avoided in reproductive women |
Maternal pharmacokinetics
Many physiological changes that occur during pregnancy affect the pharmacokinetics of drugs. Although pregnancy does not directly affect drug absorption from the gastrointestinal tract, it does delay gastric emptying and decreases motility, which can increase or decrease drug absorption; for example, drugs that require an acidic environment for absorption may have a delayed absorption pattern because of the typical decrease in production of hydrochloric acid in the stomach during pregnancy.
Changes in the woman’s body mass and fluid distribution may also change the volume of distribution of a drug. During pregnancy, there is an increase in maternal plasma volume (30%–50%) and a 25% increase in body fat. The latter may affect the distribution of drugs that are deposited in fatty tissues and can result in a fall in their plasma concentrations. Maternal renal blood flow and glomerular filtration rate also increase during the first 8 months of pregnancy, while hepatic drug-metabolising enzyme activity can either increase or decrease during pregnancy.
The effects of these changes on drug therapies are difficult to predict, as the alterations can vary enormously between individual pregnant women. Although induction or inhibition of drug metabolism has been reported this really depends on the specific CYP enzymes involved. For example, in pregnant women metabolism of caffeine is decreased, but the metabolism of the antiepileptics phenytoin and levetiracetam is increased, often necessitating an adjustment in dosage during pregnancy. The changes in renal function, which in general cause an increase in the elimination rate of drugs excreted by the kidney, may also lead to the need for dosage adjustment. If drugs are absolutely necessary during pregnancy, they should be carefully selected and titrated to the desired clinical response.
Fetal pharmacokinetics
Although the placenta functions to protect the fetus, many drugs ingested during pregnancy cross it. The fetus is then at risk of the pharmacological and teratogenic effects of the drug. The transfer of a drug across the placenta depends on the physicochemical properties of the drug, protein binding, lipid solubility and duration of exposure to the drug. Transfer of drugs primarily involves simple diffusion, although other transport processes may also be involved. In pregnancy, low-molecular-weight drugs (250–500) freely cross the placenta, while drugs of molecular weight over 1000 (e.g. heparin) cross very poorly. In late gestation, the enhanced uteroplacental blood flow and the thinner membranes that separate maternal blood flow and placental capillaries result in an increased placental transfer of un-ionised, lipophilic (lipid-soluble) non-protein-bound drugs. Disease states such as pregnancy-induced hypertension or diabetes mellitus can also affect drug transfer.
Most drugs cross the placenta by simple diffusion; therefore fetal plasma drug concentrations may equal maternal concentrations. Certain drugs are contraindicated during pregnancy or are used only when the risk–benefit situation has been carefully reviewed and discussed with the woman. Examples of drugs considered to be teratogenic in humans, along with the critical time periods and potential defects, are given in Table 9-3.
Table 9-3 drugs and teratogenic effects in the human fetus
| Drugs | Critical time period | Potential deffect |
| Alcohol (chronic use) | <12 weeks | Heart defects, CNS abnormalities |
| >24 weeks | Delay in development, low birth weight | |
| Androgens | >10 weeks | External female genitalia masculinisation |
| Angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor antagonists | 1st–3rd trimester | Renal dysgenesis, defects in skull ossification, prolonged renal failure and hypotension in neonates |
| Carbamazepine | <30 days after conception | Neural tube defects, craniofacial defects |
| Clomipramine | 3rd trimester | Neonatal lethargy, hypotonia, cyanosis |
| Cocaine | 2nd–3rd trimester | Abruptio placentae |
| 3rd trimester | Premature labour and delivery, intracranial bleeding | |
| Cyclophosphamide | 1st trimester | CNS malformations, secondary cancer |
| Isotretinoin | >15 days after conception | Hydrocephalus, CNS abnormalities, fetal death |
| Lithium | <2 months | Ebstein’s anomaly and other heart defects |
| Methotrexate | 6–9 weeks after conception | Skull ossification defect, limb and craniofacial defects |
| Phenytoin | 1st trimester | Craniofacial defects, underdevelopment of phalanges or nails, impaired neurological development |
| Streptomycin | All trimesters | Eight nerve toxicity |
| Tetracycline | >20 weeks | Stained teeth, bone growth defect |
| Valproic acid | <1 month after conception | Neural tube defects |
| 1st trimester | Craniofacial defects | |
| Vitamin A (high doses and parenteral) | 1st trimester | Fetal abnormalities including urinary tract malformations, growth retardation |
| Warfarin | 1st–3rd trimester | CNS and skeletal defects, low birth weight (<10th percentile), hearing loss |
Modified from: Jacqz-Aigrain Koren 2005; Katzung 2007.
Drug metabolism in the fetus
Drugs that cross the placenta enter the fetal circulation via the umbilical vein, and 40%–60% of umbilical venous blood enters the liver with the remainder entering directly into the fetal circulation. Drug effects in the fetus can be more significant and prolonged than in the mother because the fetus in general has immature liver drug-metabolising enzymes and thus metabolises drugs differently from adults. The expression of these enzymes varies depending on the developmental stage: activity can be detected at 5–8 weeks, and by 12–14 weeks the fetus has about 30% of the capacity of adults, while full maturation of drugmetabolising capacity is not evident until 1 year after birth (Gow et al 2001). The limited ability of the fetus to metabolise drugs may in some circumstances be detrimental. Increased exposure of the fetus to a drug or its metabolites can occur if the metabolite produced by the fetus is toxic and binds to fetal proteins, or if metabolism by the fetal liver results in the formation of a water-soluble metabolite that does not readily cross the placenta. Under these circumstances, drugs and drug metabolites accumulate in amniotic fluid, resulting in increased fetal exposure.
Enhanced exposure to drugs can also occur because the fetus has a slower overall rate of drug excretion, as the only connection to the environment is via the placenta. Drugs that are ‘excreted’ by the fetal kidneys may pass into the amniotic fluid and be reabsorbed by the fetus, thus increasing its exposure. Not every drug causes detrimental effects on the fetus but unfortunately some do. At this stage, our knowledge of the role of fetal drug-metabolising enzymes and a possible link with teratogenesis is incomplete.
Drugs given before delivery, such as barbiturates or opioid analgesics, may result in withdrawal symptoms in the neonate. These symptoms include hyperactivity, increased irritability, persistent crying, convulsions and sudden death. Multiple drugs are associated with neonatal withdrawal (abstinence) symptoms, including alcohol, cocaine, benzodiazepines, some antidepressants and opiates (including heroin, methadone and buprenorphine).
Drug use during lactation
Data on infant drug absorption, distribution, metabolism and excretion are scant and conflicting. Almost all forms of drugs in maternal circulation can be readily transferred to the colostrum and breast milk. Because drugs or their metabolites are handled by different pathways in the infant and the fetus, the impact of maternal medications on the infant is probably less, as the drug is diluted in the maternal circulation and the amount of milk swallowed is often small. However, immaturity of the neonate’s hepatic and renal systems may limit the infant’s capacity for further metabolism and excretion.
In general, the proven benefits of continuing breastfeeding must be weighed on an individual basis against the risks of exposure of the infant to maternal medications. Although the mammary glands are a relatively insignificant route for maternal drug excretion and the drug concentration in breast milk is usually less than the actual maternal dose, the infant’s actual dose depends largely on the volume of milk consumed. Thus a single measurement of a drug in human milk will not accurately reflect the total dose the infant receives.
The drug concentration in the maternal circulation depends on the relationship of several factors: dosing and route of administration, the drug’s distribution, its protein binding and maternal metabolism and excretion. The mammary alveolar epithelium consists of a lipid barrier with water-filled pores; thus it is more permeable to drugs during the colostrum stage of milk production (first week of life). Drug factors that enhance drug excretion into milk are higher degree of ionisation, low molecular weight, greater fat solubility and higher concentration. Transfer of a drug or its metabolites into milk occurs by both passive diffusion and carrier-mediated transport.
It is believed that absorptive processes in the infant’s gastrointestinal tract and drug distribution are similar to those in the adult and that lipid-soluble drugs are well absorbed. The infant’s age (and therefore the amount of drug-containing milk consumed) and the relative immaturity of the infant’s important organs bear greatly on the outcome. The following factors are also relevant:
The extreme variability among drug effects and the infant’s capacity to handle exposure to a drug often makes it difficult to decide whether the mother should take a drug and whether or not she should breastfeed.
If human milk contains small fixed amounts of substances absorbed by the mother, it is usually recommended that breastfeeding be temporarily interrupted (usually for 24–72 hours) and the breasts pumped to remove drug-containing milk. Less often, it is advisable to stop breastfeeding altogether. Taking medications after a feed may also lead to reduced traces of drug in breast milk before the next feed and hence to reduced effects on the infant. If any diagnostic radioisotope testing is scheduled, breastfeeding is interrupted until all the radioactive substance is absent from milk samples. Breastfeeding is also contraindicated:
Also, it is recommended that certain drugs be avoided while breastfeeding because they may either cause adverse effects in the infant or suppress lactation (see Box 9-1).
Box 9-1 Examples of drugs that should be avoided during breastfeeding
Source: Ito 2000; Katzung 2007; AMH 2010.
Drug metabolism in children
Neonates
Drug use in children requires advanced knowledge and skills. Newborns require special consideration because they lack many of the protective mechanisms of older children and adults. Their skin is thin and permeable, their stomachs lack acid and their lungs lack much of the mucus barrier. Neonates regulate body temperature poorly and become dehydrated easily. After the transition from in utero to life, neonates are solely dependent on their own drug-metabolising enzymes to metabolise drugs and chemicals. Delayed maturation of hepatic drug functionalisation and conjugation enzymes may account for the toxicity of some drugs in the newborn (see Clinical Interest Box 9-2). Expression of CYPs changes markedly during development; CYP2E1 activity surges within hours of birth as does that of CYP2D6. During the first week of life activity of CYP2C9 and CYP2C19 becomes evident while activity of CYP1A2 appears at 1–3 months of age (Kearns et al 2003). This immaturity of metabolic capacity results in slower drug clearance and prolonged elimination. For example, phenobarbitone plasma half-life is 70–500 hours in neonates (younger than 7 days), 20–70 hours in infants (younger than 1 month), 20–80 hours in children 1–15 years of age, and 60–180 hours in young adults (Walson 1997); for theophylline the half-life is 13–26 hours in neonates and 3–4 hours in children (Katzung 2007).
Clinical interest box 9-2 Grey baby syndrome
The grey baby syndrome was first reported in 1959 after treatment with the antibiotic chloramphenicol, which at that time was administered either orally or intravenously. The illness began 2–9 days after treatment and the early symptoms included vomiting and refusal to suckle, progressing to rapid and irregular respiration, periods of cyanosis, abdominal distension and loose, green faeces. Within 24 hours the baby became flaccid, turned an ash-grey colour and became hypothermic. Toxicity was found to be due to high plasma concentrations of chloramphenicol. The mechanisms responsible for producing the grey baby syndrome were later identified as failure of the drug to be glucuronidated by the immature glucuronosyltransferase enzymes in the neonatal liver, and the inadequate excretion by the immature kidneys of the unconjugated drug (Chambers 2006).
Similar to the CYPs the UGTs also have unique maturation profiles. For example, glucuronidation of paracetamol is decreased in newborns and young children in comparison to adolescents and adults, which reflects the delayed development of UGT1A6 and UGT1A9. Specific pharmacokinetic factors affecting drug use in neonates are reviewed in Table 9-4.
Table 9-4 Factors influencing drug dosing in neonates
| Physiological process | Changes in neonates | Type of drugs affected |
| Absorption | ||
| Gastric PH | Increased to 6–8 for first 24 hours; then usually a 10–15-day achlorhydria | Acid-labile drugs such as oral penicillin are better absorbed. Oral forms of phenobarbitone or phenytoin have reduced bioavailability |
| Gastric emptying time | Prolonged, usually 6–8 hours | Oral absorption of penicillin increased; that of phenytoin and phenobarbitone decreased |
| Distribution | ||
| Total body water (TBW) content Adipose (fat) content |
75%–79% 5%–12% (∼1% in preterm infants) |
Average adults have about 60% TBW and 25%–45% fat. There are vast differences in drug distribution across the age span. Water-soluble drugs have a larger volume of distribution in newborns; fat-soluble drugs have considerably less. Drug dosage adjustments are largely based on this factor |
| Protein binding | Decreased | Highly protein-bound drugs may require dose adjustment to avoid toxicity |
| Metabolism | ||
| Hepatic metabolism | Functional immaturity of drugmetabolising enzyme systems | Potent or potentially toxic drugs requiring liver metabolism are slowly metabolised; lower doses are necessary for such drugs (especially chloramphenicol and theophylline, among others) |
| Renal excretion | ||
| Glomerular filtration Tubular secretion |
Decreased Decreased |
Drugs excreted by filtration or secretion will accumulate in the neonate; dose adjustments are necessary (especially for aminoglycosides and digoxin) |
Infants/children
When the infant is 1 year old, drug absorption, distribution and excretion are in general similar to that of an adult. The exception is hepatic metabolism where there is evidence of an age-dependent increase in hepatic clearance in comparison to adults. This increased drug clearance in children under the age of 10 years often necessitates a higher weightbased drug dosage. This concept is illustrated with the example of phenobarbitone. The child reaches adult parameters at puberty, so drugs primarily eliminated by hepatic metabolism may require dosage adjustment and this must be individually determined and carefully monitored.
Renal excretion is decreased in neonates. Glomerular filtration rate reaches adult capacity between 8 and 12 months, while tubular function does not mature until the infant is around 12 months old. For drugs excreted primarily by the kidneys, the plasma half-life will be prolonged during the first week of life (the lower the renal drug clearance, the longer the drug half-life in the body). These developmental changes can alter dramatically the clearance of drugs that are renally excreted. Tobramycin is eliminated principally by glomerular filtration and in preterm newborns the dosing interval is 36–48 hours while in term newborns it is ∼24 hours.
Many drugs that are safe and effective for adults may not have been tested for use with children, nor have doses been established because of the complex medico-legal issues associated with the conduct of clinical trials involving children. Often a standard paediatric medication dosage is non-existent and doses are usually calculated according to the weight or body surface area of the child. Calculation formulae based on the child’s weight and age related to the adult and on adult dose are inaccurate and should not be used. Children are not small adults and their pharmacodynamic and pharmacokinetic differences will definitely affect the dose of drug needed to produce a therapeutic effect. An infant’s body composition is about 75% water (adults have 50%–60%) and an infant has less fat content than the adult; therefore, water-soluble drugs are generally administered in larger doses to infants and children in proportion to body weight than to adults. A good example of this is the water-soluble drug gentamicin, an intravenous antibiotic. Recommended dosages (normal renal function) (IM/IV) from the Australian Medicines Handbook 2010 are:
Although pharmaceutical companies are increasing their marketing of paediatric drug products, many medications are still available only in the standard adult dosages; therefore, health-care professionals must be able to calculate such products to the correct paediatric dosage. If the calculated dose in mg/kg exceeds the usual adult dose, the recommended adult dose should be used instead (AMH 2010).
Body surface area as a basis for drug dosage
It was suggested years ago that drug dosages be calculated on size or the proportion of body surface area (BSA) to weight. Although BSA has been proposed to be a more accurate indicator of drug clearance and thus dosage, large inter-individual variations and difficulty in calculations challenge this premise. For some drugs, body weight is sufficient whereas, for others, BSA may be more accurate. Nomograms for the estimation of BSA from weight and height can be found in common drug guides. When specific dosage information is not available, specialist information should be sought from the drug information services in major hospitals.
Although rules have been devised for converting adult dosage schedules to those for infants and children, it must be emphasised that no rules or charts are adequate to guarantee safety of dosage at any age, particularly in the neonate. Always take care to check whether the drug doses are expressed as either a mg/kg/dose or on a mg/kg/24 hours basis. No method takes into account all variables, particularly individual tolerance differences. The calculated dose is a guide for initiating therapy but the severity of the primary disorder, the presence of coexisting conditions, clinical response and therapeutic drug monitoring all contribute to ascertaining the optimal dose.
Topical medication use in children
Children have a large skin surface area in proportion to body mass. The skin of neonates has a thinner stratum corneum and is particularly permeable. Although adults absorb more medication through intact skin than was previously believed, children are even more at risk than adults from systemic absorption of topical medications due to enhanced cutaneous perfusion and hydration of the epidermis throughout childhood. The discoveries that hexachlorophene could cause encephalopathy in newborns and that topically applied boric acid could cause systemic poisoning testify to the hazard of applying drugs to children’s skin, especially in prolonged contact or over areas of broken skin.
A dearth of pharmacokinetic and pharmacodynamic information on drug use in children remains a problem for health-care professionals. There are numerous reasons why pharmaceutical companies and medical researchers are unwilling to study the effects of drugs in children but ‘many paediatricians argue that it is unethical not to undertake drug trials in children. It is not acceptable that children require medicines that have not been properly tested’ (Stephenson 2001). A source of comprehensive information is the UK paediatric formulary ‘Medicines for Children’ produced jointly by the Royal College of Paediatrics and Child Health and the Neonatal and Paediatric Pharmacists Group (http://www.bnf.org/bnf/).
Drug use in the elderly
Australians and New Zealanders can, on average, expect to live long and relatively healthy lives. Life expectancy has in general increased in all developed countries (76–80 years) but it is not uniform across all population groups (64 years in less developed countries). The elderly represent a significant proportion of the population (expected to increase to 973 million over the period 2000–2030) and in general they:
Because the elderly are the most rapidly increasing segment of the population, an understanding of age-related alterations in pharmacokinetics and pharmacodynamics is necessary. Furthermore, the higher incidence of chronic diseases in the elderly often results in an increase in the number of prescriptions, over-the-counter medications and home remedies prescribed or self-selected. The age of medical specialisation has in some ways added to this problem because multiple physicians, assessing the patient independently of each other, may prescribe a variety of medications, often without rationalising the total number of drugs the patient is currently taking. This practice is often referred to as polypharmacy, the use of numerous medications concurrently.
Polypharmacy can be a dangerous practice that may increase the risk of drug interactions and adverse reactions and the need for, or prolongation of, hospitalisation. To minimise the risks associated with use of multiple medications and adverse drug reactions in this population, an understanding of the ageing process and the associated changes in pharmacokinetics and pharmacodynamics is essential.
Physiological changes of ageing
Ageing persons undergo a variety of physiological changes that may increase their sensitivity to drugs and drug-induced adverse reactions (Figure 9-2). The loss in total body water and lean body mass (decreased volume of drug distribution) in many elderly patients may require initiation of therapy at a lower adult dose or re-evaluation of dosages of polar drugs already in use. The criterion for dosage should be shifted from age to weight. Some older patients weigh no more than the average large child and some weigh a lot less, yet they are often prescribed the larger adult doses.
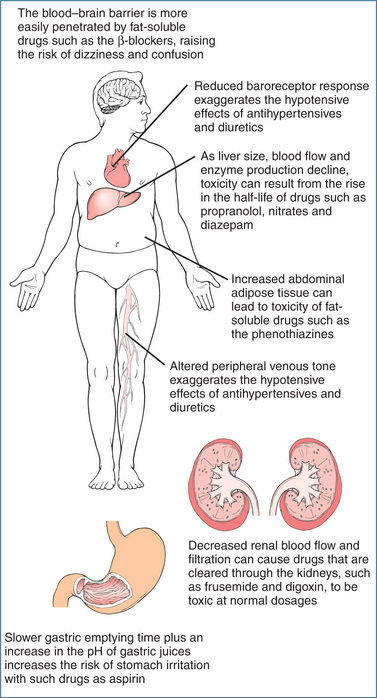
Figure 9-2 How pharmacokinetics change with age.
Source: Goodman & Gorlin 1977, courtesy Dr Charles Linder, Medical College of Georgia.
Pharmacokinetics is altered in the ageing patient because of reduced gastric acid and slowed gastric motility, resulting in unpredictable rates of dissolution and absorption of drugs. Changes in absorption may occur when acid production decreases, altering the absorption of weakly acidic drugs such as barbiturates; however, few studies of drug absorption have shown clinically significant changes occurring with advanced age.
Changes in body composition, such as an increased proportion of body fat and decreased total body water, plasma volume and extracellular fluid, have been noted in the elderly. The increased proportion of body fat increases the volume of distribution of some lipid-soluble drugs (e.g. benzodiazepines), prolonging half-life. The risk of toxicity with hydrophilic or water-soluble drugs increases as total body water decreases. Digoxin, theophylline, lithium and gentamicin are examples of hydrophilic drugs that may accumulate, causing adverse effects.
Although plasma albumin is reduced in malnutrition or acute illness, alpha-1 acid glycoprotein is increased during acute illness. These changes in plasma protein binding in the elderly may theoretically contribute to drug interactions or an enhanced pharmacological effect but the clinical relevance is limited.
Hepatic drug metabolism is also affected by ageing. For drugs with a high extraction ratio (refer to Chapter 8) the clearance is rate-limited by blood flow and hence with an age-related decrease in liver blood flow the hepatic extraction of high clearance drugs will be affected. Drugs that are metabolised by functionalisation reactions (reduction, oxidation, hydroxylation or demethylation) may have a decreased metabolism, while conjugative metabolism (glucuronidation, acetylation, sulfonation etc) appears not to be affected by ageing. Disorders common to the ageing person, such as congestive heart failure, may impair liver function and decrease the metabolism of drugs, increasing the risk of drug accumulation and toxicity.
Renal function may be impaired because of loss of nephrons, decreased blood flow and decreased glomerular filtration rate. A reduction in renal function is also secondary to heart failure. Decreased renal clearance may cause increased plasma drug concentrations and longer half-lives of drugs and active metabolites excreted by the kidney. Drugs that are highly dependent on the kidneys for excretion include the aminoglycosides, ciprofloxacin, digoxin, lithium and numerous other drugs.
Alterations in pharmacokinetics
It has been estimated that 70%–80% of all adverse drug reactions in the elderly are dose-related. The physiological changes previously discussed may result in decreases in drug metabolism, distribution and renal excretion; therefore higher blood and tissue levels of drugs and/or their metabolites may result in an increased incidence of adverse drug reactions. The half-life of diazepam increases from 20 hours in a 20-year-old to 90 hours in individuals in their 80s because of the increase in volume of distribution in the elderly person. For drugs primarily excreted by the kidneys, reduced or impaired renal function may result in drug accumulation and perhaps toxicity, as a significant proportion of the elderly population have some degree of renal dysfunction. When appropriate, determination of creatinine clearance and therapeutic drug monitoring will help optimise dosing in the elderly with compromised renal function.
Alterations in pharmacodynamics
Changes in target-organ or receptor sensitivity in the elderly may result in either a greater or lesser than normal drug effect. The reason for this alteration is unknown but it may be due to a decrease in the number of receptors at the site or an altered receptor response (second-messenger effect) subsequent to drug binding. The elderly often exhibit a decreased response to β-agonists and -antagonists, but they have a greater response (CNS depression) with diazepam. It has also been reported that the muscarinic receptor density in the cortex tends to decrease with ageing, so the elderly are often very sensitive to anticholinergic medications. Often noted adverse effects of anticholinergic drugs in the elderly include confusion, dry mouth, blurred vision, constipation and urinary retention. Refer to Chapter 11 for additional information on anticholinergic drugs. The elderly are also more sensitive to the effects of psychotropic drugs (e.g. extrapyramidal symptoms, postural hypotension) and to the central nervous system effects of benzodiazepines (Jackson et al 2009).
Medication use in the elderly
Effective use of medicines in the elderly is problematic with numerous reports of adverse drug reactions resulting in hospitalisations, less than optimum treatment when guidelines are available and discrepancies with drugs prescribed occurring after discharge from hospital. Longacting benzodiazepines, for example, have been associated with daytime sedation and an increased risk of falls, while the antidepressant amitriptyline has been reported to have a higher incidence of anticholinergic adverse effects and orthostatic hypotension than other drugs of that category. The prescribing of such medications in the aged person increases the risk of adverse drug reactions, and perhaps injury. Table 9-5 lists medications commonly prescribed for the elderly, with the most common adverse reactions reported.
Table 9-5 Commonly prescribed medications in the elderly
| Drugs | Adverse reactions |
| Aminoglycoside antibiotics (gentamicin etc) | Ototoxicity (hearing impairment or loss), renal impairment or failure |
| Analgesics, opioids | Confusion, constipation, urinary retention (morphine and others), nausea, vomiting, respiratory depression |
| Anticholinergics, antispasmodics, especially antihistamines, antiparkinsonian drugs, atropine etc | Blurred vision, dry mouth, constipation, confusion, urinary retention, nausea, delirium |
| Anticoagulants (heparin, warfarin) | Bleeding episodes, haemorrhage, increase in drug-interaction potential |
| Antihypertensive medications | Sedation, orthostatic hypotension, sexual dysfunction, CNS alterations, nausea |
| Aspirin, aspirin-containing products | Tinnitus, gastric distress, ulcers, gastrointestinal bleeding |
| Digoxin, especially at higher dosages | Nausea, vomiting, cardiac arrhythmias, visual disorders, mental status changes, hallucinations |
| Diuretics (thiazides, frusemide etc) | Electrolyte disorders, rash, fatigue, leg cramps, dehydration |
| Hypnotics/sedatives (diazepam etc) | Confusion, daytime sedation, gait disturbances, lethargy, increased forgetfulness, depression, delirium |
| H2-receptor antagonists (ranitidine etc) | Confusion, depression, mental status alterations |
| Non-steroidal anti-inflammatory drugs (NSAIDs) | Gastric distress, gastrointestinal bleeding, ulceration |
| Psychotropics (neuroleptic agents) | Sedation, confusion, hypotension, drug-induced parkinsonian effects, tardive dyskinesia |
| Tricyclic antidepressants (amitriptyline, doxepin and others) | Confusion, cardiac arrhythmias, seizures, agitation, anticholinergic effects, tachycardia etc |
Overprescribing and inappropriate prescribing in the elderly are common problems often exacerbated by unrecognised changes in pharmacokinetics and pharmacodynamics occurring as a consequence of the ageing process. Practical points in prescribing medications for the elderly (adapted from Pillans and Roberts 2001) include:
In summary, the elderly are perceived to have a greater sensitivity to drugs, especially to CNS-acting medications. If medication review is not undertaken for possible overtreatment or undertreatment, the quality use of medicines in the elderly will be suboptimal. Optimising drug therapy across a person’s lifespan is a challenging process and requires a thorough understanding by healthcare professionals of the effects of ageing on pharma codynamic and pharmacokinetic processes.
Key points
 Drug use during pregnancy should be avoided or limited to only those women who absolutely require treatment and when the benefit to the mother is considered greater than the risk to the fetus.
Drug use during pregnancy should be avoided or limited to only those women who absolutely require treatment and when the benefit to the mother is considered greater than the risk to the fetus. Although many drugs cross the placenta, the potential for inducing adverse fetal effects depends on the drug type, drug concentration and fetal age.
Although many drugs cross the placenta, the potential for inducing adverse fetal effects depends on the drug type, drug concentration and fetal age. The Australian Drug Evaluation Committee (ADEC) has established seven pregnancy categories (A, B1, B2, B3, C, D and X) to indicate the level of risk to the fetus of drugs used at the recommended therapeutic doses.
The Australian Drug Evaluation Committee (ADEC) has established seven pregnancy categories (A, B1, B2, B3, C, D and X) to indicate the level of risk to the fetus of drugs used at the recommended therapeutic doses. Drugs can exert a beneficial therapeutic effect in the fetus or cause predictable adverse effects in the fetus or act as a teratogen, mutagen or carcinogen.
Drugs can exert a beneficial therapeutic effect in the fetus or cause predictable adverse effects in the fetus or act as a teratogen, mutagen or carcinogen. The transfer of drugs across the placenta depends on the physicochemical properties of the drug, protein binding and lipid solubility.
The transfer of drugs across the placenta depends on the physicochemical properties of the drug, protein binding and lipid solubility. Drug effects in the fetus can be more significant and prolonged than in the mother because the fetus in general has immature liver drug-metabolising enzymes in comparison with adults.
Drug effects in the fetus can be more significant and prolonged than in the mother because the fetus in general has immature liver drug-metabolising enzymes in comparison with adults. Drug factors that enhance drug excretion into breast milk are high degree of ionisation, low molecular weight, fat solubility and concentration.
Drug factors that enhance drug excretion into breast milk are high degree of ionisation, low molecular weight, fat solubility and concentration. The disposition of drugs in children differs from that in adults because of factors such as growth, maturation of drug-metabolising enzymes, plasma and tissue binding and physiological maturation of organ systems.
The disposition of drugs in children differs from that in adults because of factors such as growth, maturation of drug-metabolising enzymes, plasma and tissue binding and physiological maturation of organ systems.Review exercises
References and further reading
Australian Drug Evaluation Committee. Prescribing Medicines in Pregnancy, 4th edn. Canberra: Government Publishing Service; 1999.
Australian Medicines Handbook 2010. Adelaide: AMH, 2010.
Chambers H.F. Protein synthesis inhibitors and miscellaneous antibacterial agents. In Brunton L.L., Lazo J.S., Parker K.L., editors: Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 11th edn, New York: McGraw-Hill, 2006.
Food Drug Administration. Recommendations of DES Task Force. FDA Drug Bulletin. 1985;15:40-42.
Gow P.J., Ghabrial H., Smallwood R.A., et al. Neonatal hepatic drug elimination. Pharmacology and Toxicology. 2001;88:3-15.
Hall W.C., Talbert R.L., Ereshefsky L. Cocaine abuse and its treatment. Pharmacotherapy. 1990;10:47-65.
Ito S. Drug therapy for breast-feeding women. New England Journal of Medicine. 2000;343:118-126.
Jackson S., Jansen P., Mangoni A., editors. Prescribing for Elderly Patients. Chichester, UK: Wiley-Blackwell, 2009.
Jacqz-Aigrain E., Koren G. Effects of drugs on the fetus. Seminars in Fetal & Neonatal Medicine. 2005;10:139-147.
Jennings J.C. Guide to medication use in pregnant and breastfeeding women. Pharmacy Practice News. 1996;23:10.
Katzung B.G. Basic and Clinical Pharmacology, 10th edn. New York: McGraw-Hill; 2007.
Kearns G.L., Abdel-Rahman S.M., Alander S.W., et al. Developmental pharmacology–drug disposition, action and therapy in infants and children. New England Journal of Medicine. 2003;349:1157-1167.
Koren G., Pastuszak A., Ito S. Drugs in pregnancy. New England Journal of Medicine. 1998;338:1128-1137.
Lander C.M. Antiepileptic drugs in pregnancy and lactation. Australian Prescriber. 2008;31:70-72.
Pagliaro L.A., Pagliaro A.M., editors. Problems in Pediatric Drug Therapy, 4th edn, Washington: American Pharmaceutical Association, 2002.
Pillans P.I., Roberts M.S. How to optimise use of medications in the elderly. Medicine Today. 2001;2:60-64.
Sayal K., Heron J., Golding J., et al. Prenatal alcohol exposure and gender differences in childhood mental health problems. Paediatrics. 2007;119:426-434.
Stephenson T. Medicines for children—the last century and the next. Archives of Disease in Childhood. 2001;85:177-179.
Streissguth A.P., Aase J.M., Clarren S.K., et al. Fetal alcohol syndrome in adolescents and adults. Journal of the American Medical Association. 1991;265:1961-1967.
Walson P.D. Paediatric clinical pharmacology and therapeutics. Ch. 3. In Speight T.M., Holford N.H.G., editors: Avery’s Drug Treatment, 4th edn, Auckland: Adis International, 1997.
Whitehall J.S. National guidelines on alcohol use during pregnancy: a dissenting opinion. Medical Journal of Australia. 2007;186:35-37.
Willcox S.M., Himmelstein D.U., Woolhandler S. Inappropriate drug prescribing for the community-dwelling elderly. Journal of the American Medical Association. 1994;272:292-296.