Chapter 30 Drugs Affecting the Lower Gastrointestional Tract
This chapter reviews the various medications that are used to treat illnesses or disorders affecting the lower gastrointestinal (GI) tract. These include laxatives, antidiarrhoeal drugs and drugs used in the treatment of inflammatory bowel disease. Many GI disorders—such as constipation, diarrhoea, inflammatory bowel disease and irritable bowel syndrome—negatively impact on affected individuals and knowledge of the drugs used can improve the quality of a person’s life.
Key abbreviations
IBD inflammatory bowel disease
Key background
THE small intestine, comprising the duodenum, jejunum and ileum, begins at the pyloric sphincter, coils through the abdominal cavity and connects with the large intestine at the ileocaecal sphincter. The small intestine is actually about 6–7m long but under normal conditions muscle tone keeps it to about 3 m in length. Chyme remains in the small intestine for 3–5 hours, during which time more than 90% of the nutrients and water are absorbed. Any undigested material passes to the large intestine.
The caecum, colon and rectum make up the large intestine, which is about 1.5 m long. The distal 2.5 cm of the rectum is known as the anal canal. The final stages of digestion in the large intestine occur through bacterial action, including fermentation of carbohydrate, with the release of carbon dioxide, hydrogen and methane gas. These bacteria also synthesise vitamin K, which is absorbed by simple diffusion in the colon. Around 400–800 mL water are also reabsorbed in the large intestine. Decomposition of bilirubin contributes to the brown colour of faeces. Secretion of mucus by the lining of the large intestine protects the bowel from the rough undigested faecal matter. Peristaltic movements push the faeces to the rectum, where they are expelled through the reflex action known as defecation.
Distension of the rectum by faeces stimulates stretch receptors. This sends sensory information to the central nervous system via the sacral segment of the spinal cord. Parasympathetic motor impulses to the colon, rectum and anus result in contraction of rectal muscles. This increases pressure in the rectum which, coupled with contraction of the diaphragm and abdominal muscles, relaxation of the internal sphincter and voluntary relaxation of the external sphincter, results in expulsion of faeces through the anus.
Diarrhoea is characterised by defecation of liquid faeces occurring as a result of decreased absorption by the small and large intestine, accumulation of non-reabsorbable solutes (osmotic diarrhoea) or excessive secretion in the small intestine and colon (secretory diarrhoea). In contrast, constipation results from either infrequent or difficult defecation of hard, dry faeces. Causes of constipation include disordered bowel habits, various disease states, lack of dietary fibre, inadequate fluid intake or certain drugs, such as codeine and morphine (see Chapter 15). Overall ∼10% of adults report symptoms of constipation and the incidence is higher in women and the elderly (Wald et al 2008).
Two disorders affecting the entire lower GIT are diarrhoea and constipation. Others affecting the large intestine include diverticular disease, which has no specific therapy; inflammatory bowel disease (IBD), which is the collective term used to describe ulcerative colitis and Crohn’s disease (see Clinical Interest Box 30-1)); irrit able bowel syndrome; and carcinoma. Haemorrhoids (varicosities of the external or internal haemorrhoidal veins) are also common.
Clinical interest box 30-1 Crohn’s disease
Manifestations of Crohn’s disease, described by Dr Burrill D Crohn in 1932, occur as a result of a complex process that involves infiltration of the full thickness of the bowel wall with macrophages and lymphocytes. As the disease progresses, the lining of predominantly the terminal ileum and colon develop deep linear ulcers interspersed with normal tissue, which gives rise to the characteristic cobblestone appearance. Collagen deposition is common and this often leads to stricture formation.
Crohn’s disease is a chronic recurring inflammatory disease characterised by episodes of active flare-up and periods of remission when people are relatively free of symptoms such as abdominal pain, diarrhoea, nausea and tiredness. Treatment of the disease involves the use of various drugs with the main aim of inducing and maintaining remission. In about 60% of cases surgery may be required to remove affected portions of the bowel. The cause of Crohn’s disease is unknown but associations have been made with smoking, the increased intake of simple sugars, increased intestinal permeability, genetic factors and infection with Mycobacterium paratuberculosis. Recent studies of the genetic aetiology of Crohn’s disease have identified several susceptibility regions on different chromosomes. Mutations in one particular region on chromosome 16q12 are strongly associated with Crohn’s disease in people of European descent. The region of interest has been implicated in proinflammatory cytokine induction and mutations in this region lead to an abnormal protein that could alter the activation of NFκB, a transcription factor that is involved in regulating cellular activity in response to stress and injury and also in pathways of the immune response (Schreiber et al 2004).
Bowel function is often a major concern, particularly constipation in the elderly and diarrhoea in children and immunosuppressed patients. Constipation is defined as difficult faecal evacuation as a result of hardness and perhaps infrequent movements. Everyone has regular bowel movements that range from three per day to three per week. Changes to bowel habits should be investigated thoroughly and causative factors eliminated before instituting drug therapy.
Chronic constipation is sometimes simply caused by a lack of dietary fibre and reduced fluid intake. Often, however, the underlying cause is organic disease such as tumours; bowel obstruction; metabolic abnormalities, such as diabetes mellitus or hypercalcaemia; rectal disorders; diseases of the liver, gallbladder or muscles; neurological abnormalities, such as multiple sclerosis and Parkinson’s disease; or pregnancy. Other factors that contribute to constipation include a failure to respond to defecation impulses, a sedentary lifestyle characterised by insufficient exercise and impaired physical mobility.
Constipation is often an adverse reaction of many commonly used drugs, and simply changing or stopping drug therapy may be all that is required to restore normal bowel habit. Drugs causing constipation include aluminium antacids, anticholinergics, tricyclic antidepressants, opioids and the calcium channel blockers verapamil and amlodipine. Additionally the atypical antipsychotic drug clozapine causes constipation that may be fatal especially if combined with other drugs that also have anticholinergic effects. In patients prescribed clozapine constipation should be treated in order to prevent development of serious complications.
The elderly appear to have a higher incidence of constipation, often because of multiple illnesses that require a variety of medications. The ageing process itself is associated with a decline in both physiological function and physical activity, and people who suffer from disorders of the GIT frequently complain of constipation (see Clinical Interest Box 30-2). On the other hand, a person may complain of constipation when no organic disease or lesion can be found.
Clinical interest box 30-2 Alternatives to laxative therapy
The elderly often use or misuse laxatives because of the view that regularity implies a daily bowel movement. As lifestyle factors may predispose to constipation, health-care professionals should obtain from the person a dietary and laxative history, including use of herbal preparations. Encouragement to simply increase fluid intake and undertake a regular exercise routine, such as a daily walk or active and passive exercise for bedridden clients, may prove beneficial.
Individuals who regularly consume a low-fibre diet or foods that tend to harden stools (such as cheese, hard-boiled eggs, liver, cottage cheese, foods high in sugar content and rice) should be encouraged to increase their intake of dietary fibre. A high-fibre diet with adequate fluid intake helps reduce constipation by stimulating bowel activity. High-fibre foods include orange juice with pulp or fresh citrus fruits, bran or whole-grain cereals and breads and leafy vegetables. Other fruits high in dietary fibre are prunes, bananas, figs and dates. Prunes (Prunus domestica) contain a laxative substance in addition to being high in dietary fibre. Daily consumption of prunes or a small glass of prune juice is therefore often suggested for constipation. Avoid fibre supplements in nonambulatory patients or those who are on restricted or limited fluid intake.
Drugs that affect the lower gastrointestinal tract
Laxatives
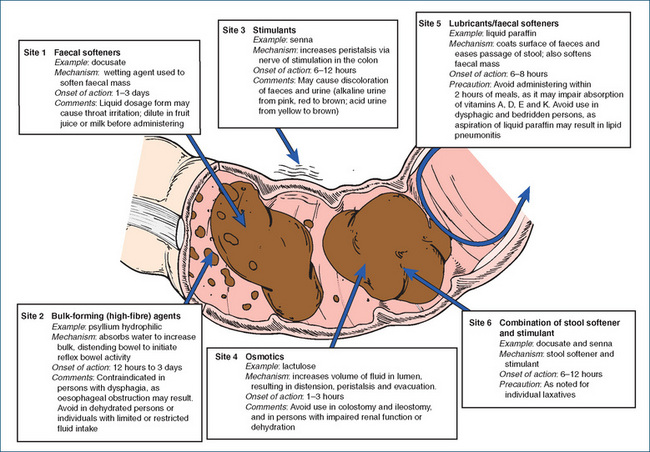
Laxatives are drugs given to enhance transit of food through the intestine. The duration of treatment with laxatives should be as short as possible, and the desirability of limiting reliance on laxatives should be discussed with the person. Figure 30-1 summarise the types of laxatives and Clinical Interest Box 30-3 describes some traditional medicines from Australian plants.
Clinical interest box 30-3 Australian medicinal plants
Australian Aborigines have long used plant-derived astringents such as mucilage or tannins for symptomatic treatment of GI tract disorders.
Astringents obtained from exudates, barks and roots of several plants, most commonly eucalypt and wattle species, can be used to treat diarrhoea and dysentery. Exudates, commonly known as kinos, provide a rich source of the astringent tannins. Kinotannic acid is thought to be the active ingredient. Exudates of the river red gum (Eucalyptus camaldulensis) are dissolved in water and drunk, as are those of the coastal sheoak (Casuarina equisetifolia) and sassafras (Cinnamonium oliveri). Queensland Aborigines use the inner roots, leaves and boiled fruits (named jelly boys) of the dysentery tree (Grewia retusifolia) for relief of gastric upsets. The fruits form a jelly-like substance, which is ingested. Similarly, the roots and stems of some species of orchid (Cymbidium albuciflorum) provide a rich source of mucilage or pseudostarch.
Stomach upsets of unspecified cause are treated with infusions of either the bark of black wattle (Acacia mearnsii) or the leaves of wild or native raspberry (Rubus hillii). Small balls of white clay, perhaps similar to kaolin, may be chewed, sometimes with pieces of termite mounds. The termite mound pieces might also provide nutritional benefit, as they are a rich source of iron, with levels as high as 2%.
Natural purgatives or laxatives include the mildly active ingredient mannitol, found in the sugary exudate of manna gum (Eucalyptus viminalis). The leaves and pods of the native senna (Cassia pleurocarpa or Cassia australis) also provide a laxative effect. Although the active constituents are not known, the leaves do contain triterpenes.
Bulk-forming laxatives
Bulk laxatives absorb water and increase the volume, bulk and moisture of non-absorbable intestinal contents, thereby distending the bowel and initiating reflex bowel activity. The laxatives constituting this group are natural plant gums such as psyllium (ispaghula) husk (see Clinical Interest Box 30-4), bran and Sterculia and semisynthetic cellulose derivatives such as methylcellulose. These agents are polysaccharide polymers that are not broken down by normal digestive processes. They stimulate peristalsis by increasing the bulk of the stool through absorption of water in the colon. This mechanism of laxative action is a normal stimulus and is one of the least harmful. These drugs do not interfere with absorption of food, but need to be administered with sufficient fluids to ensure an adequate effect.
Clinical interest box 30-4 Psyllium
Ispaghula consists of the dried ripe seeds of Plantago ovata, commercially known as Spanish or French psyllium seed. Psyllium hydrophilic mucilloid (e.g. Metamucil) is a powder that contains around 50% powdered husk (outer epidermis) of psyllium seeds and 50% dextrose or sucrose. The husk swells rapidly in water and this mixture is used to treat constipation because it promotes the formation of a soft, water-retaining, gelatinous residue in the lower bowel within 12–72 hours. In addition, it has a demulcent effect on inflamed mucosa. Large quantities can cause abdominal distension and there is a risk of intestinal obstruction. In general, the dosage is in the order of 4–7 g, administered 1–3 times daily with 250 mL liquid (refer to the product information for full details).
The effect of these laxatives may not be apparent for 12–24 hours, and their full effect may not be achieved until the second or third day after administration. Some health-care practitioners maintain that bran and dried fruits (e.g. prunes, prune juice and figs) exert the same effect, and they prefer to suggest these foods rather than the bulk-forming laxatives. Although bulk-forming laxatives are indicated for constipation, they have also been found to improve stool consistency in diarrhoea and for colostomy and ileostomy patients. Adverse reactions are minimal, the most commonly reported being flatulence and bulky stools.
Faecal softening agents
Faecal softening agents act as dispersing wetting agents, facilitating mixture of water and fatty substances within the faecal mass, producing soft faeces. The faecal softening agents include docusate, liquid paraffin and poloxamer. They are commonly used to treat acute constipation and to prevent straining, e.g. after bowel surgery.
Docusate acts like a detergent, permitting water and fatty substances to penetrate and to become well mixed with the faecal material. It may also inhibit water absorption from the bowel and stimulate water secretion into the GI tract. Softened stools are usually excreted in 1–3 days after oral administration and about 15 minutes after rectal administration. These agents may be used for patients with rectal impaction, haemorrhoids, postpartum constipation and painful conditions of the rectum and anus, and for people who should avoid straining during defecation (e.g. after rectal surgery). Docusate may be useful for immobile patients, especially children. Adverse reactions are infrequent, and oral formulations should be given with plenty of fluid.
Liquid paraffin, a mixture of liquid hydrocarbons obtained from petroleum, is not digested and absorption is minimal. Liquid paraffin penetrates and coats the faecal mass and prevents excessive absorption of water. Liquid paraffin is especially useful when it is desirable to keep faeces soft and when straining must be avoided, as after abdominal surgery, rectal operations, repair of hernias, eye surgery, aneurysm or myocardial infarction, or for prevention of haemorrhoidal tearing.
Liquid paraffin can impair the absorption of fat-soluble vitamins A, D, E and K. If liquid paraffin is taken with meals, gastric emptying time may be delayed. An objection to its use is that in large doses it tends to leak or seep from the rectum, which can cause anal pruritus and interfere with healing of postoperative wounds in the region of the anus and perineum. This leakage is often an embarrassment to the patient. Although absorption of liquid paraffin is limited, after prolonged use it may cause a chronic inflammatory reaction in tissues where it is found.
Poloxamer is a surfactant that increases penetration of fluid into faeces thereby softening the faecal mass. It is primarily used in infants and children. Care should be exercised as continued or high use may lead to abdominal discomfort and excessive fluid and electrolyte loss, particularly potassium.
Stimulant laxatives
Stimulant laxatives promote accumulation of water and increase peristalsis in the colon by irritating intramural sensory nerve plexi endings in the mucosa. The principal stimulant laxatives are bisacodyl, sodium picosulfate and preparations of senna. These agents promote accumulation of water and electrolytes in the lumen and stimulate nerve endings to increase intestinal motility. The stimulant laxatives usually act in 6–12 hours. Their primary effect is on the small and large intestines, which explains their tendency to produce cramping. Stimulant laxatives are used in preparation for diagnostic and surgical bowel procedures. Adverse effects of stimulant laxatives include abdominal cramping and fluid and electrolyte imbalance. With the exception of spinal patients, these agents are not recommended for regular use. Table 30-1 compares the stimulant laxatives in use today.
Table 30-1 Stimulant laxatives
| NAME | ONSET OF ACTION (h) | REMARKS |
| Bisacodyl | 6–12 (oral) | To prevent premature dissolving of enteric coating and GI irritation, bisacodyl should not be taken with, or within 1 hour of ingestion of, milk or antacids |
| Sodium picosulfate | 10–14 | Often used in preparation for surgery |
| Senna | 6–12 | Crude senna may cause urine discoloration |
Bisacodyl is a relatively non-toxic laxative agent that stimulates peristalsis on contact with the mucosa of the colon. The enteric coating is formulated to dissolve in intestinal fluids and, when released, produces its stimulating effects on the colon. It should not be chewed, crushed or taken with milk or antacids because it can irritate the stomach, manifesting as severe abdominal cramps. If antacids are to be taken, they should be taken at least several hours apart from the bisacodyl. The tablets produce evacuation of the bowel in 6–12 hours, and suppositories and enemas act within 15–60 minutes. The suppositories may cause a burning sensation and proctitis.
Senna is obtained from the dried leaves of the Cassia plant. It produces a thorough bowel evacuation in 6–12 hours, and this may be accompanied by abdominal pain or gripping. It is found in proprietary remedies such as Laxettes, Sennetabs and Senokot.
Osmotic and saline laxatives
The osmotic laxatives glycerol, lactulose (Drug Monograph 30-1) and sorbitol are not absorbed. By exerting an osmotic effect, they increase the volume of fluid in the lumen. This increased volume accelerates the transfer of the gut contents and leads to increased defecation. Glycerol suppositories are available in adult, child and infant sizes. These suppositories act as osmotic agents by absorbing water, but they also lubricate and increase stool bulk. Local irritation of the mucous membrane of the rectum may promote peristalsis, and evacuation occurs 5–30 minutes after insertion.
Mechanism of action
Lactulose is a semisynthetic disaccharide of galactose and fructose. In the GI tract, the normal colonic bacteria (Lactobacillus and Bacteroides, Escherichia coli and Streptococcus faecalis) metabolise lactulose to organic acids, primarily lactic, acetic and formic acids. These acids produce an osmotic effect, an increase in fluid accumulation, distension, peristalsis and bowel movement within 24–72 hours. Lactulose is indicated for constipation and is used to decrease blood ammonia levels in people with hepatic encephalopathy secondary to chronic liver disease. The latter effect is thought to result from the trapping by the lactulose of intestinal ammonia (as NH4+) and hence the excretion of excess ammonia in the faeces.
Pharmacokinetics
Absorption is minimal (<1%) after oral administration; this exceedingly small dose is excreted via the kidneys.
Drug interactions
The effectiveness of lactulose can be reduced if it is used concomitantly with an antibiotic that destroys the normal colonic bacteria. Conflicting reports exist regarding the concomitant use of neomycin and lactulose. Closer monitoring of patients should occur with concomitant oral antibiotic therapy.
Adverse reactions
These include flatulence, intestinal cramps, increased thirst and belching. Excessive doses might produce some diarrhoea and nausea (caused by the sweet taste).
Saline laxatives retain and increase the water content of faeces by virtue of an osmotic effect and stimulate peristalsis. Saline laxatives are soluble salts (e.g. magnesium salts, sodium salts, polyethylene glycol [PEG] electrolyte solutions) that are only slightly absorbed from the alimentary canal. Because of their osmotic effect, they retain and increase the water content of faeces. The water in the intestinal lumen produces fluid accumulation and distension, leading to peristalsis and eventual evacuation of bowel contents. The result is a faecal mass of liquid or semi-liquid stools. The laxative dose promotes laxation in 6–8 hours, whereas a cathartic dose works in less than 3 hours.
The intestinal membrane is not entirely impermeable to the passage of saline laxatives, and as much as 20% of the salt may be absorbed. Electrolyte disturbances have been reported with their long-term daily use, and sodium salts should be avoided in patients with congestive cardiac failure. Renal impairment can lead to the accumulation of magnesium and sodium ions, and hence significant electrolyte disturbances. A common formulation of magnesium sulfate is Epsom Salts.
Isosmotic solutions containing PEG and electrolytes are marketed as GI solutions specifically for bowel evacuation before surgery or GI diagnostic procedures. These powders (ColonLYTELY, Movicol and Glycoprep-C) consist of a mixture of PEG (a non-absorbable osmotic substance) with sodium salts (sulfate, bicarbonate and chloride) and potassium chloride that is isosmotic with body fluids. The large volume of non-absorbable fluid, commonly 2–4 litres, leads to copious watery diarrhoea. Because it is isosmotic, dehydration does not occur. These products can cause failure of regular medication, e.g. the oral contraceptive pill. All the saline laxatives can cause nausea, vomiting, bloating and electrolyte disturbances, and are used with caution in people with intestinal obstruction or suspected perforation.
Antidiarrhoeal drugs
The term diarrhoea generally describes the increased passage of semi-liquid or liquid stools. Causes are numerous. Diarrhoea may be acute, with a sudden onset in a previously healthy individual, lasting about 3 days to 1–2 weeks; this is usually self-limiting and resolves without sequelae. Chronic diarrhoea can last for 3–4 weeks or more, with recurring passage of liquid stools, and may be accompanied by fever, anorexia, nausea, vomiting, weight reduction and chronic weakness. In many instances, chronic diarrhoea in adults signifies an underlying disease that necessitates definitive treatment directed to the organic cause. Persistent diarrhoea in any age group, but particularly in infants, can lead to significant fluid and electrolyte disturbances and circulatory collapse.
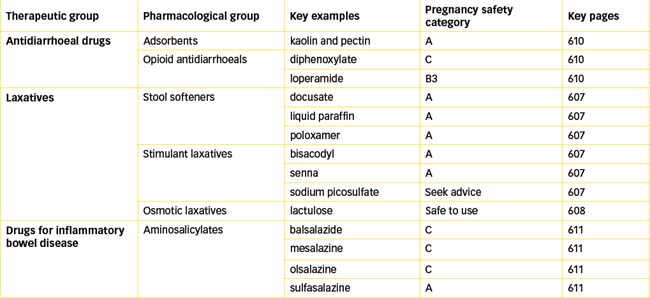
In some circumstances, specific anti-infective drug treatment is indicated but, in the main, the rationale for the use of antidiarrhoeal drugs relates to relief of symptoms and the prevention of fluid and electrolyte loss. These drugs should not be used in infants and children with acute diarrhoea, as their use may delay expulsion of organisms and does not reduce fluid and electrolyte loss (AMH 2010). Many OTC antidiarrhoeal drugs contain limited amounts of opioids (codeine, loperamide, diphenoxylate), aluminium hydroxide, attapulgite, kaolin, pectin and belladonna alkaloids (hyoscyamine, hyoscine and atropine).
In addition to the causes listed in Table 30-2, many drugs can cause diarrhoea; these include NSAIDs, antibiotics, cytotoxic agents, magnesium-containing antacids and laxatives.
Table 30-2 Causes of acute and chronic diarrhoea
| CAUSES OF ACUTE DIARRHOEA |
| CAUSES OF CHRONIC DIARRHOEA |
Adsorbents
Adsorbents are said to act by coating the intestinal mucosa, adsorbing the bacteria or toxins causing the diarrhoea and passing them out with the stools. Definitive studies confirming their proposed mechanism of action and their usefulness in treating diarrhoea have not been conducted. Examples of OTC drugs in this class are activated aluminium hydroxide (Kaomagma) and aluminium hydroxide with kaolin and pectin kaolin and pectin (Kaomagma with pectin).
Each of the formulations available has specific dosing information; in general, an initial higher dose is taken, followed by a further lower dose after each loose bowel movement until the diarrhoea has been controlled. Caution should be exercised if other medications are given concurrently with adsorbents, as they can bind to other drugs (e.g. antibiotics, anticoagulants, digoxin, salicylates, H2-receptor antagonists and phenothiazines) and interfere with their absorption.
Opioid antidiarrhoeals
Loperamide, diphenoxylate (see Drug Monograph 30-2) and codeine are OTC opioids that activate opioid receptors in the gut wall, resulting in a reduction in secretions and inhibition of propulsive movements in the gut. This slows the passage of intestinal contents and allows reabsorption of water and electrolytes, reducing stool frequency.
Drug monograph 30-2 Diphenoxylate
Mechanism of action
Diphenoxylate is chemically related to pethidine and inhibits intestinal propulsive motility by acting directly on opioid (μ) receptors on intestinal smooth muscles. The addition of atropine has no therapeutic benefit but it is included in the formulation to discourage abuse.
Pharmacokinetics
Peak plasma concentrations occur after 2 hours, and a half-life of 2.5 hours indicates rapid metabolism. Diphenoxylate undergoes hydrolysis and conjugation in the liver; the metabolites are excreted principally in bile and eliminated via the faeces. A small amount of unchanged drug is excreted via the urine.
Adverse reactions
Common adverse reactions include abdominal pain, nausea, vomiting and constipation. Rash, dizziness and paralytic ileus occur rarely. Accidental or deliberate overdosage can produce additional symptoms of flushing, hyperthermia, tachycardia, dry mouth, agitation, pinpoint pupils, lethargy, respiratory depression and coma.
Drug interactions
The following effects can occur when diphenoxylate and atropine are given with the drugs listed. Alcohol. Concurrent use can result in increased CNS-depressant effects of alcohol. Anticholinergics or other drugs with anticholinergic effects (e.g. tricyclic antidepressants). An increase in anticholinergic effects may result. A dosage adjustment might be required. Monoamine oxidase inhibitors (MAO inhibitors). Concurrent use with diphenoxylate can result in a hypertensive crisis. Avoid or a potentially serious drug interaction could occur.
These agents are indicated for short-term treatment of diarrhoea and for reducing the frequency and fluidity of motions in people with an intestinal stoma. Adverse reactions are usually minimal. Diphenoxylate is available only in combination with atropine to discourage misuse and the combination (Lomotil) can produce dizziness, dry mouth and blurred vision as a result of the muscarinic antagonist properties of atropine.
Inflammatory bowel disease
Inflammatory bowel disease (IBD) includes ulcerative colitis and Crohn’s disease (seeClinical Interest Box 30-1). Although initially recognised in developed countries IBD is a global problem without geographical boundaries. Smoking is a well-recognised risk factor for Crohn’s disease with smokers having twice the risk of a person who has never smoked (Yamamoto et al 2009). Genetic and environmental factors are thought to play a role in both of these conditions, and management includes not only drug therapy but also consideration of dietary and lifestyle factors. Excellent information can be obtained from the Australian Crohn’s and Colitis Association (ACCA). Management is primarily aimed at inducing and maintaining remission of the disease state and preventing complications such as fistulae and abscesses. This in turn improves quality of life and ensures adequate nutrition. The latter is particularly important in children with IBD, as adequate nutrition is essential to growth and sexual development.
Ulcerative colitis is also a chronic inflammatory bowel disorder but, unlike Crohn’s disease, it affects only the colon. It is characterised by diffuse mucosal inflammation and clinical symptoms include bloody diarrhoea, abdominal pain and rectal urgency. Similar to Crohn’s disease individuals experience acute flare-ups of the disease and long periods of remission. In a proportion of patients the disease is so severe a colectomy will be required. Aggressive pharmacotherapy limits progression and maintains remission in patients who adhere to their drug regimen (Howell 2008).
Drug therapy for inflammatory bowel disease
Current therapy (summarised in Table 30-3) for these conditions includes corticosteroids (e.g. prednisolone and budesonide), which are discussed in detail in Chapter 35; the 5-aminosalicylates (5-ASA), which include balsalazide, sulfasalazine, mesalazine and olsalazine; and the immunosuppressants, such as azathioprine, mercaptopurine and methotrexate (discussed in Chapter 47). Newer agents such as immunoglobulins and interferons are also being investigated for their use in IBD, and currently Crohn’s disease is an indication for the use of the TNF-α antagonists adalimumab and infliximab (discussed in Chapter 47).
Table 30-3 Drugs used for the treatment of ulcerative colitis and crohn’s disease
| ULCERATIVE COLITIS | CROHN’S DISEASE | |
| Mild–moderate disease | 5-ASA +/− prednisolone | Prednisolone |
| Budesonide (controlled ileal release formulation) | ||
| Severe disease | Methylprednisolone sodium succinate or hydrocortisone | Methylprednisolone sodium succinate or hydrocortisone |
| Infliximab | ||
| Chronic active disease | Azathioprine or mercaptopurine or infliximab | Azathioprine or mercaptopurine or methotrexate plus folic acid |
| Maintenance therapy | 5-ASA +/− azathioprine or mercaptopurine | Azathioprine or mercaptopurine or methotrexate plus folic acid (if azathioprine or mercaptopurine are not tolerated) |
| Infliximab |
Source: Adapted from Therapeutic Guidelines, Gastrointestinal, Version 4, 2006.
5-Aminosalicylates (5-ASA)
Sulfasalazine consists of the sulfonamide antibiotic sulfapyridine, linked to the anti-inflammatory drug 5-aminosalicyclic acid also called mesalazine. Sulfasalazine is poorly absorbed and in the colon it is split by bacteria into sulfapyridine and mesalazine, which is the active component effective in the treatment of IBD (see Drug Monograph 30-3). Olsalazine (a dimer of two molecules of 5-ASA) and balsalazide (5-ASA linked to 4-aminobenzoyl-β-alanine, an inert carrier) are azo-bonded prodrugs that following azo reduction by anaerobic bacteria in the colon release the active drug mesalazine directly to the colon. Variability in efficacy and tolerability may be related to the different formulations.
Drug monograph 30-3 Mesalazine
Mechanism of action
The exact mechanism of action of mesalazine (5-ASA) is unknown but it is thought to exert an anti-inflammatory effect by inhibiting the production of (1) inflammatory mediators of the cyclooxygenase and lipoxygenase pathways, (2) platelet activating factor and (3) interleukin-1. It also inhibits activation of B cells and the production of oxygen radicals (Hanauer 2004).
Pharmacokinetics
Disintegration of the enteric-coated formulation (Pentasa) takes place in the small bowel about 5 hours after administration, and about 80% of the drug is available to exert its action on the intestinal mucosa. In addition to oral formulations mesalazine can be delivered directly into the rectum or left colon by using enemas (Salofalk, Pentasa), foam preparations (Salofalk) and suppositories (Pentasa). Mesalazine is metabolised by acetylation forming N-acetyl-5-ASA, and 20%–40% of the dose is excreted in faeces and 30%–50% in urine. The plasma half-life is 0.5–1 hour.
Adverse reactions
These are more common with higher doses, and include headache, nausea, rash, abdominal discomfort and diarrhoea.
Irritable bowel syndrome
The cause of irritable bowel syndrome (IBS) is unknown. The condition is common and affects up to 20% of adults in the industrialised world. Although IBS is often thought of as a predominantly female condition, symptoms are found equally in men and women. The female tag to the syndrome probably arose because women more frequently seek medical advice. Symptoms of IBS include long-term recurrent abdominal pain, change in bowel habits, anorexia, nausea, bloating and flatulence. The condition is often precipitated by stress and anxiety and may occur after severe intestinal infection.
Management of IBS varies enormously and no real consensus on treatment exists. Dietary manipulation (e.g. exclusion-type diets) and supplements (e.g. wheat bran, fibre) are popular approaches, as is psychotherapy. Although beneficial in multiple settings increase in dietary fibre may worsen symptoms especially in those most affected by constipation and may lead to bloating and flatulence.
The use of drug therapy is still debated and includes antispasmodic agents (e.g. hyoscine, hyoscamine), loperamide if diarrhoea predominates and short-term use of laxatives if constipation predominates. It has been shown that IBS sufferers improve significantly when treated with Chinese herbal medicines. Among the many herbal medicines that have calming properties, peppermint oil has found its way into mainstream medicine (see Clinical Interest Box 30-5). Although conflicting studies have been published, the current balance of data tends to support a role for peppermint oil in the treatment of IBS.
Clinical interest box 30-5 Peppermint oil
Peppermint (Mentha x piperita) belongs to the mint family (Labiatae). Peppermint oil is obtained by distillation from the fresh flowering tops and consists mainly of menthol (50%–60%), ketones (as menthone, 5%–30%) and 5%–10% esters.
Menthol is thought to act as the antispasmodic, relaxing intestinal muscle most probably through antagonism of calcium. Unlike peppermint tea, the oil can cause heartburn, bradycardia, skin rash, allergic reactions, headache, muscle tremor and ataxia. Release of peppermint oil in the mouth can cause local irritation of the mouth and oesophagus, and capsules should not be broken or chewed. Mintec capsules contain 0.2 mL pepper mint oil; the initial dosage is one capsule three times daily 30 minutes before food, increasing to 1–2 capsules three times daily (AMH 2010; Goh & Roufogalis 2001).
Key points
 Drugs affecting the lower GI tract include laxatives and antidiarrhoeal medications, and specific drugs used for the treatment of inflammatory bowel disease (e.g. mesalazine) and irritable bowel syndrome (e.g. peppermint oil).
Drugs affecting the lower GI tract include laxatives and antidiarrhoeal medications, and specific drugs used for the treatment of inflammatory bowel disease (e.g. mesalazine) and irritable bowel syndrome (e.g. peppermint oil). Diarrhoea is characterised by defecation of liquid faeces occurring as a result of decreased absorption by the small and large intestine, accumulation of non-reabsorbable solutes (osmotic diarrhoea) or excessive secretion in the small intestine and colon (secretory diarrhoea).
Diarrhoea is characterised by defecation of liquid faeces occurring as a result of decreased absorption by the small and large intestine, accumulation of non-reabsorbable solutes (osmotic diarrhoea) or excessive secretion in the small intestine and colon (secretory diarrhoea). Constipation results from either infrequent or difficult defecation of hard, dry faeces and causes include disordered bowel habits, various disease states, lack of
Constipation results from either infrequent or difficult defecation of hard, dry faeces and causes include disordered bowel habits, various disease states, lack of
dietary fibre, inadequate fluid intake or certain drugs, such as codeine and morphine.
 Bulk laxatives (e.g. psyllium, bran, methylcellulose) absorb water and increase the volume, bulk and moisture of nonabsorbable intestinal contents, thereby distending the bowel and initiating reflex bowel activity.
Bulk laxatives (e.g. psyllium, bran, methylcellulose) absorb water and increase the volume, bulk and moisture of nonabsorbable intestinal contents, thereby distending the bowel and initiating reflex bowel activity. Faecal softening agents act as dispersing wetting agents, facilitating mixture of water and fatty substances within the faecal mass, producing soft faeces. The faecal softening agents include docusate, liquid paraffin and poloxamer.
Faecal softening agents act as dispersing wetting agents, facilitating mixture of water and fatty substances within the faecal mass, producing soft faeces. The faecal softening agents include docusate, liquid paraffin and poloxamer. Stimulant laxatives promote accumulation of water and increase peristalsis in the colon by irritating intramural sensory nerve plexi endings in the mucosa. The principal stimulant laxatives are bisacodyl, sodium picosulfate and preparations of senna.
Stimulant laxatives promote accumulation of water and increase peristalsis in the colon by irritating intramural sensory nerve plexi endings in the mucosa. The principal stimulant laxatives are bisacodyl, sodium picosulfate and preparations of senna. Osmotic laxatives are not absorbed and, because they exert an osmotic effect, they increase the volume of fluid in the lumen. The osmotic laxatives glycerol, lactulose and sorbitol are not absorbed.
Osmotic laxatives are not absorbed and, because they exert an osmotic effect, they increase the volume of fluid in the lumen. The osmotic laxatives glycerol, lactulose and sorbitol are not absorbed. The term diarrhoea generally describes the increased passage of semi-liquid or liquid stools. Persistent diarrhoea in any age group, but particularly in infants, can lead to significant fluid and electrolyte disturbances and circulatory collapse.
The term diarrhoea generally describes the increased passage of semi-liquid or liquid stools. Persistent diarrhoea in any age group, but particularly in infants, can lead to significant fluid and electrolyte disturbances and circulatory collapse. Loperamide, diphenoxylate and codeine are OTC opioid antidiarrhoeal drugs that activate opioid receptors in the gut wall, resulting in a reduction in secretions and inhibition of propulsive movements in the gut.
Loperamide, diphenoxylate and codeine are OTC opioid antidiarrhoeal drugs that activate opioid receptors in the gut wall, resulting in a reduction in secretions and inhibition of propulsive movements in the gut. Inflammatory bowel disease (IBD) includes ulcerative colitis and Crohn’s disease. Genetic and environmental factors are thought to play a role in both of these conditions, and management includes not only drug therapy but also consideration of dietary and lifestyle factors.
Inflammatory bowel disease (IBD) includes ulcerative colitis and Crohn’s disease. Genetic and environmental factors are thought to play a role in both of these conditions, and management includes not only drug therapy but also consideration of dietary and lifestyle factors. Current therapy for ulcerative colitis and Crohn’s disease includes corticosteroids (e.g. prednisolone and budesonide); the 5-aminosalicylates (5-ASA), which include balsalazide, sulfasalazine, mesalazine and olsalazine; and the immunosuppressants, such as azathioprine, mercaptopurine and methotrexate.
Current therapy for ulcerative colitis and Crohn’s disease includes corticosteroids (e.g. prednisolone and budesonide); the 5-aminosalicylates (5-ASA), which include balsalazide, sulfasalazine, mesalazine and olsalazine; and the immunosuppressants, such as azathioprine, mercaptopurine and methotrexate.Review exercises
Australian Medicines Handbook 2010. Adelaide: AMH, 2010.
Bensoussan A., Talley N.J., Hing M., Menzies R., Guo A., Ngu M. Treatment of irritable bowel syndrome with Chinese herbal medicine: a randomised controlled trial. Journal of the American Medical Association. 1998;280:1585-1589.
Brookes M.J., Green J.R.B. Maintenance of remission in Crohn’s disease. Current and emerging therapeutic options. Drugs. 2004;64(10):1069-1089.
Carter M.J., Lobo A.J., Travis S.P.L. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2004;53(Suppl V):V1-V6.
Casburn-Jones A.C., Farthing M.J.G. Management of infectious diarrhoea. Gut. 2004;53:296-305.
Cribb A.B., Cribb J.W. Wild Medicine in Australia. Sydney: Collins; 1988.
Goh P.P., Roufogalis B.D. Peppermint: a herb for calming irritable bowel? Australian Pharmacy Trade. 2001;8:22-23. February
Hanauer S.B. Review article: aminosalicylates in inflammatory bowel disease. Alimentary Pharmacology. 2004;20(Suppl 4):60-65.
Howell H.R. Ulcerative colitis: achieving and maintaining remission. US Pharmacist. 2008;33:30-38.
Lassak E.V., McCarthy T. Australian Medicinal Plants. Sydney: New Holland Publishers; 2001.
Lichtenstein G.R., Bengtsson B., Hapten-White L., Rutgeerts P. Oral budesonide for maintenance of remission of Crohn’s disease: A pooled safety analysis. Alimentary Pharmacology and Therapeutics. 2009;29:643-653.
Low T. Bush Medicine: A Pharmacopoeia of Natural Remedies. Sydney: Angus & Robertson; 1990.
Schreiber S., Hanpe J., Nikolaus S., et al. Exploration of the genetic aetiology of inflammatory bowel disease—implications for diagnosis and therapy. Alimentary Pharmacology and Therapeutics. 2004;20(Suppl 4):1-8.
Selby W.S. Current issues in Crohn’s disease. Medical Journal of Australia. 2003;178(11):532-533.
Therapeutic Guidelines, Gastrointestinal, Version 4. Therapeutic Guidelines Limited, 2006.
Wahed M., Louis-Auguste J.R., Baxter L.M., et al. Efficacy of methotrexate in Crohn’s disease and ulcerative colitis patients unresponsive or intolerant to azathioprine/mercaptopurine. Alimentary Pharmacology and Therapeutics. 2009;30:614-620.
Wald A., Scarpignato C., Mueller-Lissner S., et al. A multinational survey of prevalence and patterns of laxative use among adults with defined constipation. Alimentary Pharmacology and Therapeutics. 2008;28:917-930.
Yamamoto T., Nakahigashi M., Saniabadi A.R. Diet and inflammatory bowel disease—epidemiology and treatment. Alimentary Pharmacology and Therapeutics. 2009;30:99-112.
Zola N., Gott B. Koorie Plants, Koorie People: Traditional Aboriginal Food, Fibre and Healing Plants of Victoria. Melbourne: Koorie Heritage Trust, Globe Press; 1992.