Chapter 35 Pharmacology of the adrenal cortex
This chapter describes the endocrine functions of the adrenal glands, including the synthesis, secretion and functions of the glucocorticoids and mineralocorticoids, and reviews the rhythms and controls that influence adrenal gland functions. The glucocorticoids affect numerous normal and pathological processes in the body and are often used for their anti-inflammatory and immunosuppressant effects. The routes of administration and clinical uses of glucocorticoids are discussed, as well as the recommended method for discontinuing corticosteroid treatment, major adverse effects of the glucocorticoids and potentially serious drug interactions. The clinical use of synthetic analogues of the mineralocorticoids is also described.
Key abbreviations
ACE angiotensin-converting enzyme
ACTH adrenocorticotrophic hormone (corticotrophin)
CBG corticosteroid-binding globulin
Key background: the adrenal glands
IT has been recognised for thousands of years that women suffering from chronic inflammatory conditions such as rheumatoid arthritis experience relief from their symptoms during pregnancy. The first suggestion that this effect might be due to a hormone was made in 1930. The so-called ‘compound E’ was shown in the 1940s to be the steroid hormone cortisone, produced in the adrenal glands from cholesterol; the levels of cortisone and its metabolites are indeed markedly elevated during pregnancy. The clinical benefits of the hormones were immediately recognised but they remained very rare and expensive until the 1950s, when chemical methods for the synthesis of the steroid structures were developed. Since then, hundreds of steroids have been synthesised and tested for specific anti-inflammatory and immunosuppressant effects, and for actions in various endocrine and reproductive glands.
Anatomy
The adrenal glands1 are located just above the kidneys in the retroperitoneal space, in capsules of connective tissue (see Figure 33-1). Each adrenal gland consists of two separate endocrine organs: the inner medulla surrounded by the outer cortex. They differ in their embryological development, functions and control but share a common blood supply. The adrenal medulla can be considered best in relation to the sympathetic nervous system: the medulla is innervated by preganglionic sympathetic fibres and secretes the catecholamine hormones/neurotransmitters adrenaline and noradrenaline (see Unit 3). In a situation of stress, both the adrenal medulla and adrenal cortex are ‘fired up’ to help the body respond and adapt, in different ways (see Clinical Interest Box 33-2 for the mechanisms involved). Normally, a reaction to serious stress causes a prompt and measurable increase in release of adrenaline, noradrenaline, hydrocortisone and aldosterone, which operate together to maintain the cardiovascular tone essential to survival.
Pathology
In adrenal insufficiency, or in the absence of the adrenal cortex, there is both gluco- and mineralocorticoid deficit. Sodium reabsorption is inhibited and potassium excretion decreases; hyperkalaemia and mild acidosis occur, and a powerful and uncontrolled loss of extracellular fluid can lead to a state of hypovolaemic shock. The animal cannot respond to stress; survival is possible only under rigidly controlled non-stressful conditions with available food and water and a high sodium intake.
Because the corticosteroids have so many physiological actions, pathological conditions affecting the adrenal cortex in which there are glucocorticoid deficiencies (Addison’s disease) or excesses (Cushing’s syndrome), or mineralocorticoid excess (Conn’s syndrome), have widespread and potentially severe manifestations throughout the body (see Clinical Interest Box 35-1).
Clinical interest Box 35-1 Addison’s, cushing’s and conn’s
ADDISON’S DISEASE: DEFICIENCIES OF CORTICOSTEROIDS
CUSHING’S SYNDROME: EXCESS OF GLUCOCORTICOSTEROIDS
CONN’S SYNDROME: EXCESS OF MINERALOCORTICOSTEROIDS
Note: Liquorice has significant indirect mineralo corticoid activity: persons ‘addicted’ to liquorice as a candy can suffer sodium retention and raised blood pressure.
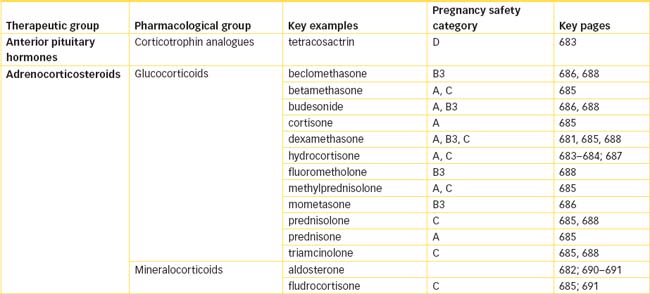
Adrenocorticosteroids
The adrenal cortex synthesises three important classes of hormones based on the steroid structure—the corticosteroids (or adrenocorticoids):
This chapter discusses the glucocorticoids and mineralocorticoids; androgens are discussed in Unit 12.
Synthesis of adrenal cortex hormones
Cholesterol, which the body uses for the biosynthesis of corticosteroids, is synthesised and stored in the adrenal cortex. The general pathways for synthesis of the adrenal cortex hormones are shown in Figure 35-1 (and the chemical structures of typical steroids in Figure 33-3). Note that there is no large store of corticosteroids in the body, so the rate of synthesis from plasma cholesterol determines the rate of release. The rate-limiting step, the synthesis of pregnenolone from cholesterol, is regulated by ACTH.
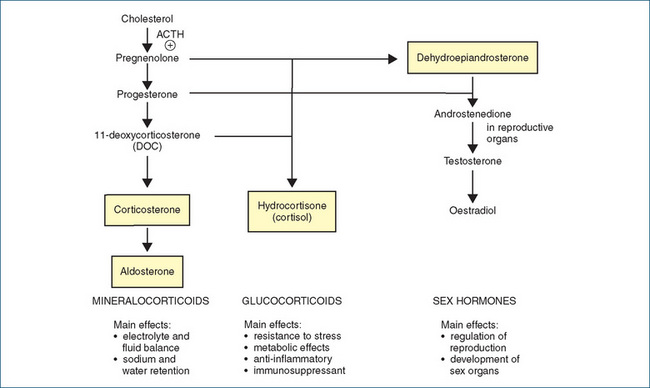
Figure 35-1 Biosynthesis of adrenal cortex hormones. Hormones shown in boxes are produced in the adrenal cortex in physiologically active amounts. The chemical structures of some naturally-occurring steroids are shown in Figure 33-3.
Levels of hydrocortisone ( = cortisol) are measured in many tests of hypothalamic–pituitary–adrenal (HPA) axis function, and in recovery of function after cessation of treatment (see Figure 35-3, later). For example, in Cushing’s syndrome the increased secretion of hydrocortisone is not reduced following dexamethasone administration, whereas in Addison’s disease levels of hydrocortisone do not rise after ACTH stimulation (see review by Ho and Torpy [2007]).
Corticosteroid synthesis inhibitors
The synthetic pathways can be blocked if there are deficiencies of the enzymes required and by enzyme inhibitors such as the drugs aminoglutethimide, etomidate, ketoconazole, mitotane and metyrapone (the latter is mainly of research and diagnostic interest); these drugs thus inhibit or suppress adrenal cortex function. Mitotane, a DDT (insecticide) analogue, also has adrenolytic properties useful in treating adrenocortical cancer. (Aminoglutethimide, a drug that inhibits the enzymatic conversion of cholesterol to pregnenolone, thereby blocking the synthesis of all adrenal steroids, was previously indicated for the treatment of Cushing’s syndrome associated with adrenal carcinoma, ectopic ACTH-dependent tumours and adrenal gland hyperplasia, and advanced breast cancer in men and in postmenopausal women. There were many adverse effects and it was frequently abused by athletes; it has recently been withdrawn in Australia.)
Secretion of adrenal cortex hormones
Two rhythms appear to influence glucocorticoid release: circadian (diurnal, daily) rhythm and ultradian (less than daily) rhythm. A circadian rhythm, a pattern based on a 24-hour cycle with the repetition of certain physiological processes, is controlled by the dark–light and sleep– wakefulness cycles via the limbic system. People living a normal day–night cycle (sleeping in the dark at night) will have raised plasma hydrocortisone levels in the early morning hours that reach a peak after they are awake. These levels then slowly fall to very low levels in the evening and during the early phase of sleep. The import ance of this rhythm is emphasised by the finding that corticosteroid therapy is more potent when given at midnight than when given at noon. To simulate the natural diurnal rhythm when corticosteroids are administered as drugs, daily doses are usually divided, with two-thirds given in the morning and one-third at night.
In humans, there are also 4–8 bursts of adrenal glucocorticoid release that occur over each 24 hours, which may follow peaks in the release of corticotrophin-releasing factor (CRF) and ACTH. Although the basal production rate averages 30 mg every 24 hours, under stressful conditions (trauma, major surgery or infection) there is a reserve capacity production of up to 300 mg daily. Increases in glucocorticoid production may be proportional to increases in the release of ACTH by the anterior pituitary gland.
Steroid hormones are not stored in the body; they are synthesised when needed so the rate of synthesis determines the rate of release and plasma levels. The corticosteroids are transported in the plasma highly protein-bound to albumin and to corticosteroid-binding globulin (CBG). They are metabolised to hydroxy- derivatives and then undergo conjugation and glucuronidation in the liver before excretion by the kidneys.
Control of adrenal cortex hormones
Hypothalamic and pituitary control
Corticosteroid synthesis depends on stimulation of the adrenal cortex by pituitary corticotrophin (ACTH), which is governed by CRF from the hypothalamus (see Figure 35-2). Corticotrophin secretion fluctuates with a circadian rhythm, with high levels in the early morning and trough levels in the evening. The rhythms are disrupted by long transmeridian airline flights and take several days to be restored. This rhythm in turn determines the circadian rhythm in secretion of corticosteroids.
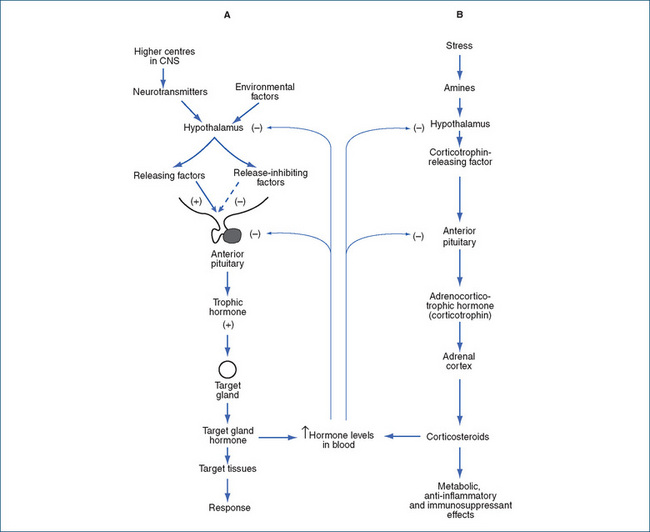
Figure 35-2 Levels of endocrine control. Various internal and external factors may inhibit or stimulate the hypothalamus to secrete inhibitory or releasing factors, which increase (+) or decrease (–) output of hormones from the anterior pituitary gland, and ultimately hormone release from target glands. Short and long negative feedback loops ‘damp down’ further release. A Typical pattern of levels of controls. B Example in the adrenal cortex, showing negative feedback control of release of hypothalamic corticotrophin-releasing factor and of pituitary corticotrophin (ACTH) by high levels of adrenocorticosteroids.
Corticotrophin is a 39-amino-acid polypeptide. When administered clinically, it tends to be antigenic; hence a synthetic analogue tetracosactrin (24 amino acids) has been developed. It has similar actions to the natural hormone, i.e. trophic actions on adrenal cortex cells, increasing the synthesis and release of corticosteroids (mainly glucocorticoids), and regulating enzymes for steroidogenesis. It is administered parenterally in diagnostic tests of adrenal cortex function: administration should result in a rapid rise in cholesterol synthesis and release of hydrocortisone into the bloodstream. A sustained-release depot preparation for IM injection is also available, for use in treatment of exacerbations of multiple sclerosis and of infantile convulsions.
ACTH secretion is suppressed by somatostatin (GH release-inhibiting hormone—see Drug Monograph 33-1); a new analogue pasireotide that is more selective for suppression of ACTH secretion has potential efficacy in treatment of Cushing’s disease.
Negative feedback control
Increased levels of corticosteroids, in the usual negative feedback fashion, inhibit the adrenal glucocorticoid system by inhibiting the release of CRF from the hypothalamus and also inhibiting the release of ACTH from the anterior pituitary. This is referred to as suppression of the hypothalamic–pituitary–adrenal (HPA) axis.
Structure–activity relationships
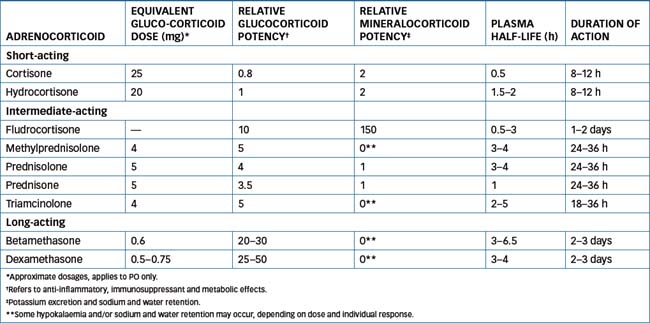
Many thousands of steroid compounds have been synthesised and their pharmacological actions tested in attempts to enhance particular actions or pharmacokinetic properties. The synthesis of steroids with little or no mineralocorticoid activity was a great advance, as the most useful clinical effects are those of the glucocorticoids, i.e. anti-inflammatory and immunosuppressant actions. Mineralocorticoid effects such as hypokalaemia, hypertension and oedema are then adverse effects. Several commonly used corticosteroids are compared for potencies in Table 35-1.
Glucocorticoids
Physiological actions of glucocorticoids
Hydrocortisone (cortisol) is considered the prototype glucocorticoid hormone; synthetic analogues have similar effects in the body. These include general metabolic effects, anti-inflammatory and immunosuppressant actions, and negative feedback effects on the HPA axis. Some mineralocorticoid effects may also occur, as the specificity between the two types of steroids is not absolute.
Metabolic effects
Carbohydrate metabolism
Glucocorticoids decrease glucose uptake into cells and glucose utilisation, while increasing gluconeogenesis; thus they help to maintain the blood sugar level and liver and muscle glycogen content. This can produce hyperglycaemia and glycosuria, i.e. glucocorticoids are diabetogenic: they can aggravate diabetes, unmask latent diabetes and cause insulin resistance.
Protein metabolism
Glucocorticoids facilitate the breakdown of protein in muscle and extrahepatic tissues, which leads to increased plasma amino acid levels. Glucocorticoids increase the trapping of amino acids by the liver and stimulate the deamination of amino acids. Subsequent inhibition of protein synthesis can delay wound healing and cause muscle wasting and osteoporosis. In young people, these effects can inhibit growth.
Fat metabolism
Glucocorticoids promote mobilisation of fatty acids from adipose tissue, increasing their concentration in the plasma and their use for energy. Despite this effect, individuals taking glucocorticoids for long periods may accumulate fat stores (‘moon face’, ‘buffalo hump’) because of redistribution of fat. The effects of glucocorticoids on fat metabolism are complex and are thought to occur through metabolic actions of catecholamines.
Calcium balance
Glucocorticoids tend to decrease calcium absorption from the gut and increase its excretion via the kidneys, causing an overall negative calcium balance. In response, bone is resorbed by osteoclastic activity, raising blood calcium levels. Chronically, this can lead to osteoporosis.
Blood pressure and stress responses
Glucocorticoids potentiate the vasoconstrictor action of noradrenaline, partly by inhibiting extraneuronal uptake of catecholamines. When glucocorticoids are absent, the vasoconstricting action of the catecholamines is diminished and blood pressure falls.
Both CRH and arginine vasopressin are released in response to acute and chronic stress and, via activation of pro-opiomelanocortin in anterior pituitary cells, cause release of ACTH and hence of glucocorticosteroids which help maintain homeostasis. This sudden release is believed to be a protective mechanism: without steroid release (or administration), hypotension and shock may occur. Simultaneous release of adrenaline and noradrenaline from the adrenal medulla has a synergistic action with the corticosteroids.
Central nervous system effects
Corticosteroids affect mood and behaviour, and possibly cause neuronal or brain excitability. Glucocorticoid receptor function is impaired in major depression, resulting in reduced negative feedback on the HPA axis and increased secretion of CRH; it is thought that hyperactivity of the HPA is involved in causing depression. Some people on exogenous corticosteroids report euphoria or depression, insomnia, anxiety and increased motor activity; chronic high doses can lead to psychoses. It has been suggested that prolonged stress during childhood and adolescence can lead to depression and psychotic disorders in adulthood, via increased activity of the HPA, leading to impaired NMDA and glutamate functioning in cortical neural networks.
Suppression of the hypothalamic–pituitary–adrenal axis
High levels of circulating corticosteroids have negative feedback effects on secretion of CRF and ACTH, thus suppressing the HPA axis, leading to decreased secretion of glucocorticoids and, in the long term, atrophy of the adrenal cortex. This leaves the body unable to cope immediately with stress, infection or immune challenge.
‘Pharmacological’ actions
When hormones are administered in doses that lead to higher than normal (physiological) levels in the body, the doses are said to be ‘pharmacological’. Hydrocortisone (cortisol) is taken as the ‘gold standard’ corticosteroid, so relative affinities of other steroids at glucocorticoid receptors can be compared and relative potencies calculated; for example, three drugs of choice for glucocorticoid (anti-inflammatory) activity are prednisolone (4 times the potency of hydrocortisone), dexamethasone (30 times) and betamethasone (30 times)—all with minimal sodiumretaining activity. For mineralocorticoid activity, the drugs of choice are aldosterone (500 times the potency of hydrocortisone) and fludrocortisone (150 times), both having much lower anti-inflammatory activity.
Relative potencies and doses
Table 35-1 lists relative potencies, typical doses and some pharmacokinetic data for corticosteroids. Note that the corticosteroids that are always administered topically (mometasone, clobetasone, beclomethasone, ciclesonide and fluticasone, on the skin and in the nasal passages and airways) cannot readily be compared with the older oral corticosteroids for systemic potencies and pharmacokinetic parameters. Budesonide was originally introduced as an anti-asthma preventive, given by inhalation. It is now available in controlled-release capsules for treatment of Crohn’s disease, with the main action in the GIT; the intrinsic potency of budesonide at the glucocorticoid receptor is approximately 15 times higher than that of prednisolone. There is a high first-pass effect (85%–90%), and duration of action is extended by the prolonged-release formulation.
Anti-inflammatory action
Glucocorticoids, especially hydrocortisone, in larger than physiological doses can stabilise lysosomal membranes and prevent movement of neutrophils and release of proteolytic enzymes during inflammation. They can also suppress virtually all the vascular and cellular events in the inflammatory response, both immediate events and late processes, including wound healing and repair. By stimulating the production of the mediator protein lipocortin (also called annexin-1), they inhibit phospholipase-A2, inhibiting the production from damaged cell membranes of many mediators, including prostaglandins, thromboxanes, prostacyclin and leukotrienes. Because phospholipase-A2 is involved much earlier in the pathways for synthesis of inflammatory mediators than is cyclo-oxygenase, the corticosteroids inhibit production of many more mediators than do the non-steroidal anti-inflammatory drugs (see Chapter 47).
Immunosuppressant actions
Glucocorticoids can cause atrophy of the thymus and decrease the number of lymphocytes, plasma cells and eosinophils in blood. By blocking the production and release of cytokines and other mediators, corticosteroids interfere with the integrated roles of T and B lymphocytes, macrophages and monocytes in immune and allergic responses.
Mechanism of action
The general mechanism of action of the glucocorticoids is as for most steroids:
In the case of the glucocorticoids, there are many molecular forms of the glucocorticoid receptor (GR) expressed in tissues, many genes are targeted and there is increased synthesis of various kinase enzymes and antiinflammatory mediators, including lipocortin (annexin-1). At the same time, there is decreased synthesis of other enzymes, including cyclo-oxygenase-2 and collagenase, and hence suppression of pro-inflammatory mediators, including histamine, some cytokines, prostaglandins and leukotrienes (see reviews by van der Laan and Meijer [2008] and De Bosscher and Haegeman [2009]). Thus steroid effects can be mediated via genes and transcription and via signalling pathways and mediators.
Agents that selectively antagonise glucocorticoid receptors are currently being researched actively; such drugs could potentially be useful in therapy of Cushing’s disease, diabetes, obesity, neuropathic pain and glaucoma. Early research led to the non-selective GR antagonist RU-486 (mifepristone), used in medical termination of pregnancy.
Clinical aspects
Doses in clinical use
In low doses (physiological levels), glucocorticoids are used in replacement therapy, e.g. in Addison’s disease, adrenal insufficiency or hypopituitarism. A typical daily adult dose of hydrocortisone is 10–30 mg, with two-thirds in the morning and one-third in the evening. Doses are increased (doubled or trebled) in times of stress, e.g. during intercurrent illness, before surgery and after trauma. In acute adrenal insufficiency, higher doses (100 mg every 4–8 hours) may be required IV or IM (see Drug Monograph 35-1). Higher doses (pharmacological levels) such as 100 mg hydrocortisone are also used for the anti-inflammatory and immunosuppressant effects. Critically ill patients, e.g. those in intensive care units and with respiratory distress syndrome, may require high-dose corticosteroids due to suppression of the HPA and to tissue resistance to glucocorticoids.
Drug monograph 35-1 Hydrocortisone
Hydrocortisone is the prototype glucocorticoid, used clinically as replacement therapy for adrenocortical insufficiency and in many inflammatory and immune disorders (see ‘Clinical Aspects: Indications’). It has some mineralocorticoid (salt-retaining) effects.
Pharmacokinetics
After oral administration, hydrocortisone is readily absorbed and circulates bound to plasma proteins (>90%). It is metabolised in the liver and most body tissues by hydroxylation and glucuronidation, and metabolites are excreted in the urine. Peak plasma concentrations are reached in about 1 hour, the elimination half-life is about 1.5–2 hours, and the duration of action is 8–12 hours.
Adverse reactions
Adverse effects can occur in most systems and tissues, including musculoskeletal, cardiovascular, gastrointestinal, dermatological, neurological, endocrine, immunological, haematological, ophthalmic and metabolic effects. Chronic administration leads to suppression of the hypothalamic–pituitary–adrenal axis, and excessive doses to Cushingoid effects.
Warnings
Use with caution in patients with hyper tension, colitis, diverticulitis, open-angle glaucoma, liver or kidney disease, oral herpes lesions, hyperlipidaemia, hypothyroidism, hypoalbuminaemia, psychotic tendencies, osteoporosis, systemic lupus erythematosus or uncontrolled infections (and many other conditions). Patients should carry an alerting card or wear a bracelet, giving details of dosage and emergency instructions.
Contraindications
Avoid use in persons with corticosteroid hypersensitivity, HIV infection or AIDS, heart disease, heart failure, severe kidney disease, chickenpox, measles, peptic ulcer, oesophagitis, systemic fungal infection, diabetes mellitus, herpes simplex infection (eye), myasthenia gravis or tuberculosis. Corticosteroids transfer into breast milk and may cause adverse effects in the infant.
Drug interactions
(see also Drug Interactions 35-1) Important interactions occur with hepatic-enzyme inducers, which shorten the half-life of hydrocortisone, and with oral contraceptives, which may prolong the half-life. Hydrocortisone (as with other mineralocorticoids) can increase potassium excretion, hence there are potential interactions with diuretics and digoxin (increased sensitivity).
Dosage and administration
Dosage is individualised depending on the disease and the patient’s response; a typical adult dosage is 20 mg PO in the morning and 10 mg at night. Dosage is increased to cover other illness or surgery; a mineralocorticoid may also be required. In acute adrenal insufficiency, the adult dose IV or IM is 100 mg repeated every 6–8 hours for 24 hours.
Indications
Corticosteroids have been tried in virtually every condition that may have an inflammatory or immune pathology, including:
They are also used as replacement therapy in patients with suppressed HPA axis (e.g. after several months of glucocorticoid therapy), before surgery or in times of stress.
Specialised uses
Topical glucocorticoids
Dozens of topical steroid preparations are available, in many dosage forms (creams, gels, ointments, eye-drops, ear-drops, eye ointments, lotions, shampoos, suppositories) and in many combinations (e.g. with antibacterials or keratolytic). Potencies of topically administered corticosteroids vary: fluorinated compounds are particularly potent (e.g. fluorometholone eye-drops).
For skin disorders, topical glucocorticosteroids are used for their anti-inflammatory and antimitotic actions, in inflammatory and pruritic eruptions, hyperplastic conditions, infiltrative disorders such as eczema, and psoriasis. There are many advantages of topical preparations, including broad applicability, rapid action, stable formulations, compatibility and ease of use, with no pain or odour and few systemic adverse effects. Local adverse effects include vasoconstriction, skin striae, atrophy and infections. Typical drugs include betamethasone, desonide, triamcinolone and methylprednisolone; these are discussed in greater detail in Chapter 48.
Ocular (eye) and otic (ear) formulations of corticosteroids commonly include dexamethasone, fluorometholone, prednisolone, triamcinolone or hydrocortisone, in drops or ointments. An antibacterial antibiotic is sometimes included in the formulation to treat or prevent infections (see Chapters 31 and 32 and Drug Monograph 32–1).
Prednisolone is formulated for rectal administration (as suppositories or a retention enema) for use in inflammatory bowel disease (ulcerative colitis, Crohn’s disease) and other painful inflammatory conditions of the rectum and anus. Budesonide capsules are used for similar indications.
Inhaled glucocorticoids
The glucocorticoids are potent anti-inflammatory agents because of their actions in decreasing the degranulation of mast cells and in the synthesis of inflammatory mediators and new antibodies. They are also effective immunosuppressants, so they are extremely useful as preventers in asthma, in which corticosteroids are administered by inhalation to decrease bronchial hyper-reactivity and minimise the pathophysiological changes (oedema, excess mucus) (see Chapter 28). Administration directly to the airways via a metered-dose inhaler or nebuliser decreases the incidence of systemic adverse reactions.
The available inhaled corticosteroids are: beclomethasone (Drug Monograph 28-4), budesonide, fluticasone and ciclesonide. Note that corticosteroids do not bronchodilate, so they may be used after an inhaled bronchodilator, which increases penetration of the anti-inflammatory agent into the smaller airways.
Similar corticosteroids are formulated as nasal sprays, for nasopharyngeal administration, in conditions such as allergic rhinitis and nasal polyps.
Intralesional administration
Glucocorticoids are used for musculoskeletal and joint pain (e.g. tennis elbow), usually by intralesional or intraarticular (joint) injection given by specialised practitioners. Long-acting corticosteroids are administered, but not more than 3–4 times per year or joint damage can occur.
Pharmacokinetic aspects of glucocorticoids
Routes of administration
These very frequently used drugs have been administered by virtually every imaginable route and formulation, including PO, IM, IV, by inhalation, topically and locally (by dermal, inhalational, intra-articular, ocular, otic, nasal, intralesional and per rectum). Local administration to the site of action is preferred if possible, as this allows lower doses to be used, fewer systemic adverse effects are likely and a more rapid and direct action occurs.
Alternate-day dosage
If glucocorticoids must be given orally, alternate-day therapy is preferred, as this minimises the risk of systemic adverse effects, especially suppression of the HPA axis, growth suppression in children, raised blood sugar levels, protein catabolism, bone loss, infections and mineralocorticoid effects. A drug is selected from the shortor intermediate-acting corticosteroids and, when the patient’s condition is stabilised on a particular dosage, the schedule is tapered down on one day and increased on the next until the patient is taking about 2–3 times the previous daily dose on every alternate day.
Absorption and distribution
Glucocorticoids are well absorbed after oral, topical or local administration. Parenterally (IM) and topically, the soluble esters (phosphate and succinate) are rapidly absorbed, while the poorly soluble agents (acetate, acetonide, diacetate, hexacetonide and valerate) are slowly but completely absorbed and act as depots in the tissues for slow release of hormone. Rectally, about 20% of the drug is absorbed normally but, if the rectum is inflamed, absorption may increase by up to 50%.
Steroids, being lipophilic, diffuse well into cells; they are transported around the body in the bloodstream bound to albumin and to CBG.
Metabolism and excretion
The natural hormone cortisone must be hydroxylated to hydrocortisone before it is active; the same is true for the synthetic analogue prednisone, activated to prednisolone (thus cortisone and prednisone are prodrugs). As with most drugs, steroids are metabolised by sulfation and glucuronidation to inactive metabolites, which are eventually excreted in the urine. The fluorinated adrenocorticoids are more slowly metabolised than the other compounds.
Half-lives
The elimination half-lives of the drugs may be relatively short, as the administered drugs are rapidly metabolised (e.g. the half-life of hydrocortisone is about 90 min). The biological half-life and duration of action, however, may last for several hours or days, as the actions initiated by the hormone—enzyme activation and protein synthesis—continue in tissues long after the drug has diffused away from the receptor and been eliminated (see Table 35-1).
Adverse drug reactions and drug interactions
There are many adverse reactions from the use of glucocorticoids, especially after prolonged administration. Like the drugs’ actions, they can be summarised as cushing oid effects, mainly on metabolism; pituitary– adrenal suppression effects (adrenal atrophy, decreased growth, decreased response to stress or infection); and mineralocorticoid effects (hypertension, oedema).
Withdrawal from corticosteroids
Suppression of the HPA axis is unpredictable: it is unlikely with daily doses lower than 7.5 mg prednisolone or equivalent, or during treatment periods shorter than 3 weeks. While a corticosteroid is being given exogenously, a suppressed HPA is mainly a problem during periods of stress. As shown in Figure 35-3, it can take many months, even up to a year, for HPA functions to recover after cessation of therapy, during which time the body is at risk as the adrenal gland cannot rapidly respond to demand for synthesis of steroids. This leads to hazards of sudden withdrawal of therapy, so doses should be reduced gradually.
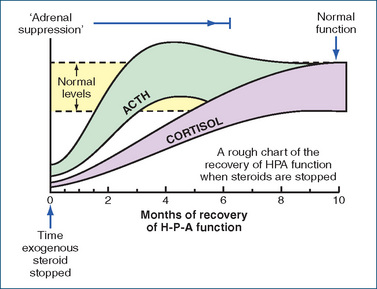
Figure 35-3 Recovery of hypothalamic–pituitary–adrenal (HPA) functions after cessation of administration of exogenous steroid; levels of ACTH (corticotrophin) rise to normal over about 2 months, and thereafter levels of cortisol ( = hydrocortisone) gradually return to normal. Figure reproduced from Sweeney 1990, with permission.
One recommended protocol for tapering down doses is as follows: the dose should be reduced by the equivalent of 2.5–5 mg prednisone every 3–7 days until a physiological dose of about 5 mg is reached. If the patient’s condition worsens, the dose must be increased until stabilised, then resume tapering the dose more gradually (Speight & Holford 1997).
Steroid sparing
Another approach to reduce suppression of the HPA is to use as adjunctive therapy the immunosuppressant drugs that act by different mechanisms—e.g. the calcineurin inhibitors (cyclosporin, tacrolimus) or cytotoxic immunosuppressants (azathioprine, methotrexate).
Suppression of the HPA is also less likely with alternate-day corticosteroid therapy, if the dose is given in the morning rather than the evening, and after topical or inhaled dose compared to oral or systemic doses.
Adverse drug reactions
Adverse reactions include euphoria, headache, insomnia, restlessness, anxiety, psychiatric changes, an increase in appetite (anorexia with triamcinolone), hyperpigmentation, increased hair growth, lowered resistance to infections, visual disturbances (cataracts, glaucoma), increased urination or thirst and decreased growth in children. Parenterally at an injection site, redness, swelling, rash, pain, tingling or numbness may occur.
Chronic use may result in abdominal pain, gastrointestinal bleeding, peptic ulcers, round face, ‘buffalo hump’, acne, weight gain, muscle cramps, weakness, osteoporosis, irregular heart rate, nausea, vomiting, bone pain, increased bruising and difficulty in wound healing. Diabetes mellitus and hyperglycaemia can occur or be unmasked; masking of signs and symptoms of other pathological conditions can occur and confuse the diagnosis.
Drug interactions
Potentially, adverse interactions can occur with many drugs; these are summarised in Drug Interactions 35-1.
Drug interactions 35-1 Glucocorticoids
The following effects may potentially occur when a corticosteroid is given with the drugs listed below.
| Drug | Possible effects and management |
| Aminoglutethimide (recently withdrawn in Australia) | Suppresses adrenal function, therefore corticotrophin is not adminstered concurrently. Aminoglutethimide can increase the metabolism of many drugs (especially warfarin and dexamethasone), reducing their half-lives; doses may need to be increased. When aminoglutethimide is given, glucocorticoid supplements are often prescribed; hydrocortisone is recommended. |
| Amphotericin B (parenteral) | May result in severe hypokalaemia. If given concurrently, monitor serum potassium levels closely |
| Antacids, e.g. magnesium trisilicate; bile-acid-binding resins | When given concurrently, a decrease in steroid absorption may result. Doses should be separated by at least 2 hours, and steroid dosage increase may be necessary |
| Antidiabetic drugs (oral) or insulin; other drugs affecting blood glucose concentration (see Table 36-2) | Glucocorticoids may elevate serum glucose levels, hence dosage adjustment of one or both drugs may be necessary |
| Aprepitant (antiemetic) | Increases concentration of dexamethasone and methylprednisolone; oral doses of steroid may need to be halved |
| -conazole antifungal agents (itraconazole, ketoconazole) | Reduce the metabolism and enhance the clinical effects of some glucocorticoids, including inhaled budesonide; chronic administration should be monitored and dosage of glucocorticoid may need to be reduced |
| Digitalis glycosides | May result in increased potential for toxicity (arrhythmias) associated with hypokalaemia |
| Diuretics | The sodium- and fluid-retaining effects of the adrenocorticoids may reduce the effectiveness of diuretic agents. Monitor closely for oedema and fluid retention. Potassium-depleting diuretics given with adrenocorticoids may result in severe hypokalaemia, whereas the effects of potassium-sparing diuretics may be decreased. Monitor serum potassium levels and patient response closely |
| Hepatic-enzyme-inducing agents | Barbiturates, carbamazepine, phenytoin and others may decrease the adrenocorticoid effect because of increased metabolism. Monitor serum hydrocortisone levels closely; dosage increase may be necessary. A benzodiazepine is safer |
| Potassium supplements | These reduce the effect of either one or both medications on serum potassium levels. Monitor serum levels if given concurrently |
| Vaccines, live virus and other immunisations | Generally, immunisations are not recommended for patients receiving immunosuppressant doses of glucocorticoids, as the immunisation effect will be reduced and the patient may develop neurological complications or develop the viral disease |
Steroid resistance
In many conditions in which steroids are used chronically (asthma, chronic obstructive pulmonary disease, inflammatory bowel disease), some patients develop reduced responsiveness to the steroid therapy. This is known as ‘steroid resistance’, and means that the conditions become difficult to treat. Some mechanisms of development of steroid resistance include:
Strategies for dealing with steroid resistance include administration of other types of immunosuppressants and/or of drugs targeting other processes in the disease (see review by Creed and Probert [2007]).
Mineralocorticoids
Aldosterone
The other main group of steroid hormones secreted by the adrenal cortex are the mineralocorticoids, of which a natural hormone is aldosterone.
Secretion and control
Aldosterone is synthesised in the adrenal zona glomerulosa, the outer edge of the adrenocortical tissue. Aldosterone production is regulated primarily by the renin–angiotensin system and the concentration of circulating serum potassium (see Unit 5), rather than by stimulation of the adrenal cortex by ACTH. A drop in the circulating arterial volume or pressure, or low sodium levels in the kidney tubules (e.g. loss of blood, excessive diuresis or low salt intake), stimulates receptors in the juxtaglomerular apparatus within the renal afferent arterioles (see Figure 23-4). As a result, renin (a proteolytic enzyme) is released and acts on angiotensinogen (an α2-globulin synthesised by the liver) to form angiotensin I. When the angiotensin I passes through the pulmonary circulation, and also in the kidneys, two amino acids are cleaved from it by angiotensinconverting enzyme (ACE) to form angiotensin II, an octapeptide that stimulates the adrenal cortex zona glomerulosa to produce aldosterone. A rise in plasma potassium concentration also directly stimulates the adrenal cortex output of aldosterone; whereas aldosterone secretion is suppressed by an elevation of sodium levels in the blood, e.g. by excessive dietary salt intake.
Physiological actions
Aldosterone’s primary function is to regulate sodium and potassium balance in the blood. It increases sodium reabsorption by increasing the functions of sodium channels and sodium–potassium pumps in the membranes of kidney tubule luminal cells. It thus restricts the loss of sodium and its accompanying anions, chloride and bicarbonate, and thereby helps to maintain extracellular fluid volume, resulting in raised blood pressure. It stimulates potassium secretion by the renal tubular cells in the distal and collecting tubules, while simultaneously enhancing the cells’ reabsorption of sodium. Hence renin release leads to actions that counteract the initiating salt depletion or decrease in blood pressure or volume—an important homeostatic mechanism. (Note that liquorice, a candy made from a natural root, also has mineralocorticoid actions and can cause high blood pressure.)
Clinical uses
Aldosterone is several thousand times more potent as a mineralocorticoid than is hydrocortisone. In adrenal cortex insufficiency, replacement of a glucocorticoid and sometimes a mineralocorticoid also is necessary. The clinical use of aldosterone has been limited because of its cost, short half-life and relative unavailability, and because it is best administered parenterally; hence synthetic analogues such as fludrocortisone are administered (see Drug Monograph 35-2). In high doses, aldosterone analogues have a negative-feedback effect on the pituitary secretion of ACTH and on adrenal cortex secretion of endogenous steroids (see Figure 35-2).
Drug monograph 35-2 Fludrocortisone
Fludrocortisone has very potent mineralocorticoid activity, for which it is mainly used, with strong glucocorticoid effects as well. It acts primarily on the renal distal convoluted tubule to reabsorb sodium, enhance excretion of potassium and hydrogen and raise blood pressure. It is indicated for the treatment of Addison’s disease (adrenocortical insufficiency) and salt-losing adrenogenital syndrome, and is also used in orthostatic hypotension.
Pharmacokinetics
Fludrocortisone has good oral absorption and a half-life of about 3.5 hours in the plasma, with a half-life of biological activity in the body of 18–36 hours and duration of action of 24–48 hours. It is highly protein-bound, and metabolites produced in the liver and kidneys are excreted by the kidneys.
Drug interactions
The main interactions with fludrocortisone are due to its causing potassium loss and hence hypokalaemia; interactions with amphotericin B, digitalis glycosides, diuretics and potassium supplements are as for glucocorticoids (see Drug Interactions 35-1). Potassium levels should be monitored and supplements given as necessary.
Adverse reactions
These include salt and water retention, severe or persistent headaches, hypertension, dizziness, oedema of the lower extremities, joint pain, hypokalaemia and increased weakness. Such adverse reactions should be reported immediately to the prescriber. Heart failure may be exacerbated by fluid and electrolyte disturbances. At the low doses of mineralocorticoids usually used, serious glucocorticoid adverse effects are unlikely.
Warnings and contraindications
Use with caution in patients with peripheral oedema, acute glomerulonephritis, liver impairment, hypothyroidism, hyperthyroidism, chronic nephritis, infections or osteoporosis. Avoid use in persons with fludrocortisone hypersensitivity, heart disease, hypertension or kidney function impairment. During chronic administration, periodic monitoring of serum electrolytes, and dietary sodium restriction and potassium supplementation, are advisable.
Antagonists of aldosterone have salt- and water-losing actions and potassium-retaining effects, hence are useful as potassium-sparing diuretics—see spironolactone and eplerenone, Chapter 25.
Key points
 The corticosteroids (glucocorticoids and mineralocorticoids) are steroidal hormones that are synthesised in and released from the adrenal cortex.
The corticosteroids (glucocorticoids and mineralocorticoids) are steroidal hormones that are synthesised in and released from the adrenal cortex. Release of glucocorticosteroids is controlled by hypothalamic corticotrophin-releasing hormone and pituitary gland corticotrophin (ACTH), and is subject to circadian rhythms.
Release of glucocorticosteroids is controlled by hypothalamic corticotrophin-releasing hormone and pituitary gland corticotrophin (ACTH), and is subject to circadian rhythms. The many important pharmacological actions of the glucocorticoids (metabolic, anti-inflammatory and immunosuppressant) have led to their extensive use in medicine.
The many important pharmacological actions of the glucocorticoids (metabolic, anti-inflammatory and immunosuppressant) have led to their extensive use in medicine. The mineralocorticoids act in the kidneys to reabsorb sodium and water, and enhance the excretion of potassium and hydrogen. They are controlled by the renin–angiotensin system and by potassium and sodium levels.
The mineralocorticoids act in the kidneys to reabsorb sodium and water, and enhance the excretion of potassium and hydrogen. They are controlled by the renin–angiotensin system and by potassium and sodium levels. The actions of these hormones are vitally important in helping the body to maintain homeostasis, particularly in times of stress.
The actions of these hormones are vitally important in helping the body to maintain homeostasis, particularly in times of stress. Higher than normal doses are required for critically ill patients, and for those undergoing acute stress.
Higher than normal doses are required for critically ill patients, and for those undergoing acute stress.Review exercises
dosing schedule? Name three additional points to be discussed with a patient concerning the clinical use of an oral corticosteroid.
References and further reading
Australian Medicines Handbook 2010. Adelaide: AMH, 2010.
Bennett A.O., Maxwell R. Stress and anxiety in schizophrenia and depression: glucocorticoids, corticotrophin-releasing hormone and synapse regression. Australian and New Zealand Journal of Psychiatry. 2008;42(12):995-1002.
Clark R.D. Glucocorticoid receptor antagonists. Current Topics in Medicinal Chemistry. 2008;8(9):813-838.
Creed T.J., Probert C.S.J. Steroid resistance in inflammatory bowel disease: mechanisms and therapeutic strategies. Alimentary Pharmacology and Therapeutics. 2007;25(2):111-122.
De Bosscher K., Haegeman G. Minireview: latest perspectives on anti-inflammatory actions of glucocorticoids. Molecular Endocrinology. 2009;23(3):281-291.
De Martin M., Pecori Giraldi F., Cavagnini F. Cushing’s disease. Pituitary. 2006;9(4):279-287.
Endocrinology Expert Group. Therapeutic Guidelines: Endocrinology, version 4. Melbourne: Therapeutic Guidelines Limited; 2009.
Ho J., Torpy D.J. Evaluation of adrenocortical function in adults. Australian Prescriber. 2007;30(6):147-149.
Igaz P., Tombol Z., Szabo P.M., Racz K. Steroid biosynthesis inhibitors in the therapy of hypercortisolism: theory and practice. Current Medicinal Chemistry. 2008;15(26):2734-2747.
Pelaia G., Vatrella A., Cuda G., Maselli R., Marsico S.A. Molecular mechanisms of corticosteroid actions in chronic inflammatory airway diseases. Life Sciences. 2003;72(14):1549-1561.
Rogerson F.M., Brennan F.E., Fuller P.J. Mineralocorticoid receptor binding, structure and function. Molecular and Cellular Endocrinology. 2004;217(1-2):203-212.
Speight T.M., Holford N.H.G. Avery’s Drug Treatment, 4th edn. Auckland: Adis; 1997.
Sweeney G. Clinical Pharmacology: A Conceptual Approach. New York: Churchill Livingstone; 1990.
Van der Laan S., Meijer O.C. Pharmacology of glucocorticoids: beyond receptors. European Journal of Pharmacology. 2008;585(2-3):483-491.
MIMS Annual OnLine More weblinks at: http://evolve.elsevier.com/AU/Bryant/pharmacology