Chapter 7 Wound healing
Introduction
Wound healing is a complex issue with several interrelated and simultaneous phases. Knowledge of the anatomy of the skin and the physiology of wound healing is essential in order to care competently for patients with wounds created during a surgical procedure and those that occur as a result of pathology or trauma. An understanding of normal coagulation (i.e. blood clotting or haemostasis) and the methods used in surgery to enhance it are critical as this informs many of the activities of the surgeon and instrument nurse. Scientific evidence has led to changes in wound management, with a preponderance of new and varied wound care products available. Wound management requires not only knowledge of the properties of dressings but also an understanding of the healing process. The perioperative nurse requires knowledge of the various dressings and drains available and their rationale for use. Knowledge and understanding of the basic concepts of wound healing and wound care provide the new practitioner with confidence in caring for patients in the perioperative setting.
Skin anatomy
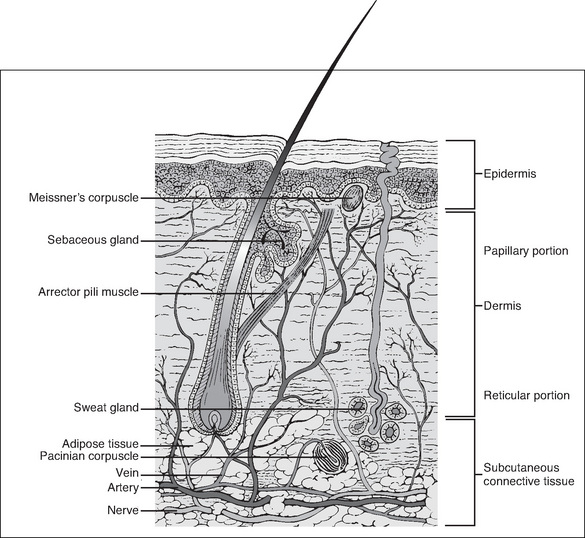
Knowledge of the anatomy of the skin and its associated structures is important in order to understand the physiology of wound healing. The skin consists of the dermis (a type I collagen) and the epidermis, which together compose the outermost layer (Wysocki, 2007). The epidermis is made up of many overlapping layers of epidermal cells and has no blood vessels, so this layer is avascular, receiving its nutrients from blood vessels in the underlying dermis (Wysocki, 2007). The structures associated with the skin, including the capillaries, lymph channels, hair follicles, sebaceous glands, sweat glands and nerve endings, are located in the dermis. Other individual cells, such as mast cells, melanocytes and fibroblasts, are also found in the dermis (Fig 7-1).
The next layer of tissue is the subcutaneous layer. This layer contains adipose or fatty tissue, which is yellow, and greasy or slippery to touch, with globules of fat that frequently dislodge. Blood vessels, lymphatics and nerves are found within the subcutaneous layer. The fascia, the next layer, is a thin membrane that fully encapsulates muscle. It is often glossy in appearance, transparent and separates the subcutaneous layer from muscles, tendons and bones (Wysocki, 2007).
Wound healing
A wound is an injury that disrupts the continuity of body tissue, with or without tissue loss, and which may be intentional or unintentional. Wounds may be surgical, traumatic or chronic (McEwen, 2007; Phillips, 2007).
Intentional wounds
Surgical site incisions and/or excision constitute intentional wounds; an incision is a cut or an opening into intact tissue, whereas an excision is the removal of tissue. Other types of intentional wounds include occlusion, such as occlusion banding, which is used to treat haemorrhoids, or occlusion using clips to block the passage of the fallopian tubes. Wounds can also be created using chemicals applied to the skin or other tissue intentionally to cause inflammation and re-epithelialisation (e.g. performing a facial peel during plastic surgery).
Unintentional wounds (or traumatic injuries)
Unintentional wounds can be classified by cause—mechanical, thermal or chemical destruction. For example, wounds can occur following trauma (mechanical), as a result of being burnt (thermal) or from contact with chemicals such as acid (chemical).
Chronic wounds
A chronic wound has not completed the usual wound healing process in the expected time frame (Ramundo, 2007). These wounds are caused by an underlying pathophysiological process. For example, a decubitus ulcer, which may be caused by compromised circulation over bony prominences or venous ulcers, develops due to venous stasis or arterial insufficiency. On assessment, a wound that does not appear to be healing by approximately 14–21 days is at risk of becoming a chronic wound.
The phases of wound healing
Wound healing depends on many local and systemic factors and is a complex process that involves a series of cellular processes and biochemical events. There is disagreement among researchers about the exact number of phases of wound healing but all agree that it is complex and that there is some overlapping of these phases because they occur almost simultaneously (Benbow, 2007; Doughty & Sparks-Defries, 2007; Karukonda et al., 2000; Schultz, 2007; Schultz et al., 2003). Following haemostasis, healing progresses through three phases:
The same basic biochemical and cellular processes are involved in the healing of all soft tissue injuries, whether they are acute or chronic.
Haemostasis and the inflammatory phase
The process of inflammation produces classic symptoms: redness, heat, swelling, pain and decreased function. Early and late inflammatory responses differ and each phase involves different biochemical mediators and cells that respond by:
The inflammatory response is immediate, non-specific, self-limiting and lasts for 3–4 days. It is also referred to as the defensive phase of healing because it is essential to enable healing to occur (Trask et al., 2006). It is induced by:
Haemostasis
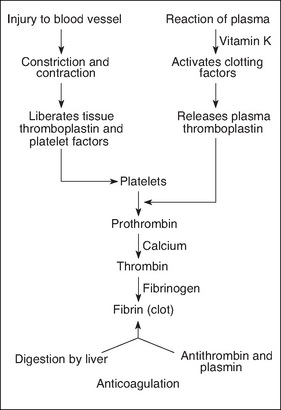
Following injury, bleeding in large vessels must be artificially stopped or the patient will suffer from hypovolaemia and, without treatment, death will occur. Following injury, blood vessels briefly constrict. Platelets accumulate at the damaged site, adhere to one another and form a platelet plug (Benbow, 2007). When damage occurs to endothelial cells, which line the blood vessel walls, collagen fibres are exposed. When collagen fibres contact the platelets, an important release of adenosine phosphate (ADP), histamine and serotonin occurs. The coagulation cascade is also triggered (Karukonda et al., 2000; Trask et al., 2006). The mechanism of haemostasis is shown in Figure 7-2.
Biochemical mediator release
Mast cells are found in the extracellular spaces close to blood vessels. These are the most important activators of the inflammatory response. Activation occurs in two ways. Firstly, as a result of degranulation, preformed granular contents are released into the extracellular matrix (e.g. histamine release). Secondly, certain pain producing mediators (e.g. prostaglandins) are synthesised in response to the injury stimulus; leukotrines, which cause increased vascular permeability and exudation, are also produced. Mast cell degranulation also attracts leucocytes to the site of injury; these cells phagocytose damaged tissue and fight bacteria (Trask et al., 2006).
Vasodilation
Some mediators, such as serotonin and histamine, are termed vasoactive amines because they cause vasodilation and increased vessel permeability. Increased blood flow to the injured tissue causes redness. This arteriolar dilation increases pressure in the microcirculation, which causes development of an exudate made up of plasma and cells; the exudate pushes into the surrounding tissue, which causes localised oedema and swelling.
Increased capillary permeability
As blood becomes more viscous and sticky due to plasma leakage, the microcirculation slows down. Consequently, leucocytes in circulation migrate to the cell walls and adhere there. Released biochemical mediators stimulate the endothelial cells lining the capillaries and venules, causing them to retract, and a space is created at the cell junction. Consequently, leucocytes are then able to squeeze out of circulation into the surrounding tissue.
Phagocytosis
Phagocytosis is the process whereby neutrophils, monocytes and macrophages remove debris and bacteria by engulfing them. In the process, neutrophils die and pus may form. Importantly, macrophages produce growth factors by releasing angiogenesis factor, which is needed for production of capillary and lymphatic buds, and fibroblast-activating factor, which attracts fibroblasts. This process initiates wound repair.
Plasma protein systems
The inflammatory response activates three key plasma protein systems. The complement system activates and assists inflammation and the immune process, and directly destroys cells. The clotting system traps bacteria in injured tissue and interacts with platelets to prevent haemorrhage. The kinin system helps control vascular permeability.
Generally, most surgical wounds are sealed within hours of closure. This seal unites the wound and is a barrier, to a degree, to bacterial invasion.
Reconstructive phase (proliferative)
The reconstructive phase occurs 3–4 days after injury and lasts for about 2 weeks. During this phase the wound is filled in, sealed and then shrinks. The wound is initially sealed by a blood clot containing fibrin, which traps erythrocytes, leucocytes and platelets. Fibrin is created by the activation of the coagulation cascade, and the fibrin in the clot provides a framework for collagen molecules.
Fibroblasts
Fibroblasts synthesise collagen and other connective tissue proteins. They multiply rapidly and enter the wound, forming fibres that bridge the wound edges and restore tissue continuity (Trask et al., 2006). Collagen is the most abundant protein in the body and is the material of tissue repair. It cannot be produced without iron, vitamin C or oxygen. Collagen is produced within 6 days of fibroblasts entering a wound.
Granulation
Repair continues as granulation tissue grows inwards from the surrounding healthy tissue. Granulation tissue is filled with new capillaries, giving it a red, granular appearance. It is surrounded by fibroblasts and macrophages. Capillary buds sprout out of vascular endothelial cells and extend into the debrided areas, eventually forming capillaries. Loops form when these capillaries anastomose and leak neutrophils and erythrocytes, causing further debridement of the wound. Capillaries differentiate into arterioles and venules as repair continues; lymphatics are formed in the same way.
Epithelialisation
As a clot is being dissolved and granulation tissue formed, the healing wound must be protected. This occurs during a process by which epithelial cells grow into the wound from surrounding healthy tissue. Macrophages secrete a factor that attracts epithelial cells, which migrate under a clot or seal. Eventually these epithelial cells contact other migrating cells and seal the wound; migration/proliferation then ceases. However, the epithelial cells remain active, undergoing differentiation and giving rise to various epidermal layers (Trask et al., 2006). This process is hastened when the wound is moist.
Wound contraction
Wound contraction occurs over 6–12 days and is the final reconstructive phase necessary to close all wounds, especially those that heal by secondary intention. Granulation tissue contains specialised cells called myofibroblasts, which cause wound contraction (Trask et al., 2006). The scar will form at this point and may appear red to pink in colour (Myers, 2004). (Note: Wounds heal side to side and not end to end.)
Maturation phase
The maturation phase is the final phase, commencing 2–3 weeks after injury and continuing for several years. Scar tissue is remodelled as collagen type III is replaced by collagen type I, which is much stronger. Capillaries regress, leaving the wound avascular and thus pale, and localized itching subsides. Within 2–3 weeks after maturation begins, the scar has gained two thirds of its maximal strength. However, at best, the repaired tissue will regain only 80% of its original tensile strength. Only epithelial, hepatic and bone marrow cells are capable of mitotic regeneration (i.e. they can regenerate and function as before). Other cells are replaced by fibrous tissue. The healing process is the same for all wounds, although the composition of healed tissue may differ.
Types of wound closure
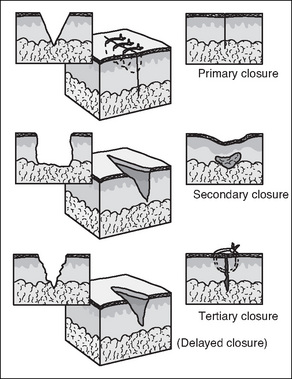
The three mechanisms by which surgical wounds may be closed and subsequently heal are: primary intention, secondary intention and delayed primary closure or tertiary intention (Fig 7-3).
Primary intention
Surgical (and other) clean wounds heal by a process of collagen synthesis, which seals the wound. This is facilitated by minimal tissue loss and approximation of wound edges, with sutures, clips or tapes. There is no dead space on closure and contamination is held to a minimum by adherence to aseptic technique. Very little epithelialisation is required for healing and most wounds are sealed with fibrin several hours after closure (McEwen, 2007; Phillips, 2007).
Secondary intention
Secondary intention (granulation) healing occurs when there is loss of tissue and the wound cannot be closed; consequently, the wound edges are not approximated. Healing occurs by granulation, eventual re-epithelialisation and wound contraction (Harvey, 2005). The wound will heal spontaneously as long as the dermal base is preserved. Secondary intention healing takes longer than primary intention healing and produces extensive scarring. However, it is often the best option for large open wounds (e.g. decubitus ulcers), traumatic wounds or in wounds where infection is present (McEwen, 2007;Myers, 2004).
Delayed primary closure
When approximation and suturing is delayed intentionally by 3 or more days, or is secondary for the purpose of walling off an area of gross infection or where extensive tissue has been removed, then healing by tertiary intention/delayed primary closure occurs. This method may also be used for haemodynamically unstable trauma patients (McEwen, 2007). The wound edges are closed 4–6 days postoperatively/post-trauma after meticulous debridement (Benbow, 2007).
Box 7-1 Subcutaneous management of vertical incisions with 3 cm or more of subcutaneous fat
Surgical wound infections have been shown to be multifactorial. Obesity or an increased depth of subcutaneous fat have been regarded as a significant risk factor for developing a surgical site infection. The multifactorial nature of wound infection was explored in a study by Cardosi et al. (2006), with no definitive conclusion as to the causative factors in any given patient. The researchers found no significant correlation between the depth of subcutaneous fat and the incidence of wound complications or wound disruption.
Wound classification
Wounds are classified into the following four types (Box 7-2):
Box 7-2 Classification of surgical wounds from the Centers for Disease Control
Class I Clean wounds
Clean wounds are defined as uninfected operative wounds in which no inflammation is encountered and the respiratory, alimentary, genital or uninfected urinary tract are not entered. Additionally, clean wounds are a result of elective surgery with primary closure and, if necessary, drained with a closed drainage device. Examples include eye surgery, hernia repair, breast surgery, neurosurgery (non-traumatic), cardiac or peripheral vascular surgery (Phillips, 2007).
Class II Clean–contaminated wounds
Clean–contaminated wounds are operative wounds in which the respiratory, alimentary, genital or uninfected urinary tract is entered under controlled conditions and without unusual contamination. Specifically, operations involving the biliary tract, appendix, vagina and oropharynx are included, providing there is no evidence of infection or no major break in aseptic technique. Examples include gastrectomy, cholecystectomy (without spillage), elective appendicectomy, cystoscopy and/or cystoscopy/transurethral resection (negative urine cultures), total abdominal hysterectomy, dilation and curettage of the uterus, Caesarean section and tonsillectomy (not infected at time of surgery) (Phillips, 2007).
Class III Contaminated wounds
Contaminated wounds are classified as those contaminated in surgery that involves open, fresh, traumatic wounds, or with major breaks in sterile technique, or with gross spillage from the gastrointestinal tract, and incisions in which acute non-purulent inflammation is encountered. Examples include rectal surgery, laparotomy (with significant spillage), traumatic wounds (e.g. gunshot, stab wounds—non-perforation of viscera) or acute inflammation of any organ without frank pus present (e.g. acute appendicitis or cholecystitis) (Phillips, 2007).
Class IV Dirty (infected) wounds
Old traumatic wounds with retained devitalised tissue and wounds that involve existing clinical infection or perforated viscera and/or delayed primary closure wounds are classified as dirty wounds. This classification suggests an infectious process was present prior to surgery. Examples include debridement, incision and drainage of, for example, an abscess, total evisceration, perforated viscera, amputation or patients with positive preoperative blood cultures.
Debridement
Debridement involves the removal of dead or necrotic tissue and the manual removal of microorganisms, often by irrigation. The aim of debridement is to provide a clean surface with a minimum of microorganisms and dead tissue that provides a focus for infection and physically obstructs contraction of the wound and closure of the wound edges. Different types of debridement materials are available (Ramundo, 2007). Sharp debridement has been described as what usually happens in the operating room (Ramundo, 2007).
Surgical haemostasis
Surgical haemostasis is the deliberate halting of blood flow. It is essential to wound management and is a necessary and ongoing process during surgery. It is necessary to prevent the patient experiencing the physiological effects of excessive blood loss, which would lead to an increased length of stay in hospital. Additionally, bleeding from the operative site reduces visibility for the surgeon, and is a risk factor for developing a surgical site infection (Rubin, 2006). The mechanisms used encourage the formation of a blood clot, thereby stopping the flow of blood into the surgical site. Surgical haemostasis is achieved by several means, which are classed as mechanical, chemical or electrosurgical; several other methods are unclassified.
Methods of surgical haemostasis
Mechanical haemostasis
Mechanical haemostasis is achieved by compressing the ends of severed vessels until the normal clotting mechanisms have sealed the vessel. Various methods are used to achieve mechanical haemostasis:
Instruments
Instrument clamps are used to hold a small amount of tissue or the end of a blood vessel. The haemostat or artery forceps is the most commonly used instrument for achieving haemostasis. Often, the pressure of clamping a blood vessel is sufficient to achieve haemostasis.
Ligature/ties
Ligatures are made from suture material and are available in pre-packaged standard lengths, pre-cut lengths or as ligature reels, in which the material is wound around a spool. Ties may be passed to the surgeon in several ways depending on the surgery being performed and the individual preference of the surgeon. Standard length ties may be divided and cut into halves, thirds or quarters, depending on the depth of the tissue being ligated. Ties can be grasped within the jaws of a haemostat/artery or tissue forceps, threaded through an ‘eyed’ needle or simply handed to the surgeon. Ties handed to the surgeon should be held taut like a guitar string.
Ligating clips
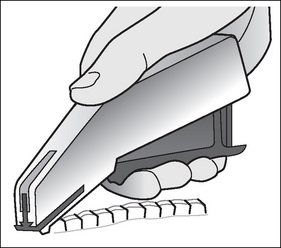
Ligating clips are small, V-shaped, staple-like devices that are designed to be pinched shut over the ends of tissue. They are made from surgical stainless steel, titanium or absorbable polymer. When placed on a blood vessel and closed shut, ligating clips occlude the lumen and stop the bleeding from the vessel (Fig 7-4). Ligating clips are available in small plastic carriers, preloaded with multiple clips, which require individual loading onto a sterile instrument. Ligating clips are also available in a purpose-designed, disposable, preloaded instrument that delivers and closes the clips.
Bone wax
Bone wax is small balls of processed bees wax, which is smeared along the open edge of the bone, acting as a barrier to stop oozing from the cut bone surface.
Packing
Surgical sponges or packs are used for packing a wound site, and effectively place pressure on the wound edges or in a body cavity to reduce bleeding. The surgeon may require the packs to be moistened with a warm sterile irrigating solution. Warm packs promote haemostasis by accelerating the coagulation mechanism (Phillips, 2007).
Pledgets
Pledgets are small pieces of Teflon material that are used to reinforce a suture line where bleeding may occur through the needle holes, effectively placing pressure on the site to reduce bleeding. The pledgets remain in place as part of the suture.
Patties
Compressed, absorbent, radiopaque, cottonoid patties are available in various sizes and are used to absorb blood and compress delicate areas to stop blood flow. They are commonly used in surgical procedures on the brain, spine and spinal cord. The instrument nurse counts the patties, moistens them with normal saline, presses out excess moisture and keeps them flat before use.
Tourniquets
A tourniquet compresses the underlying vessels, thus restricting the blood flow to a limb or digit, creating a bloodless field for the procedure. A pneumatic tourniquet is used when the compression of blood flow to a limb will reduce blood flow (e.g. this might be required during open reduction and internal fixation of a fractured ankle). Applying an elastic band, Penrose drain or the finger of a glove can reduce blood flow to the toes and fingers (Phillips, 2007).
Chemical haemostasis
A number of products are available to provide chemical haemostasis. The effect of these products is the formation of a blood clot. Some examples of the most commonly used products are outlined below.
Absorbable gelatin
Absorbable gelatin (Gelfoam) is an absorbable haemostatic agent made from porcine gelatin, which is compressed into a pad or powder form. As a pad, it is available in an assortment of sizes that can be cut to the desired size without crumbling. The gelatin sponge is not soluble and absorbs up to 45 times its own weight in blood. It is frequently soaked in thrombin or adrenaline solution. It is handed moist to the surgeon for use. When placed in an area of capillary bleeding, fibrin is deposited in the interstices and the sponge swells, forming a clot. Gelatin powder is mixed with sterile saline to make a paste for absorbing blood (Phillips, 2007).
Absorbable collagen sponge
Haemostatic sponges (Colostat) of bovine collagen origin are applied dry to oozing or bleeding sites. The collagen activates the coagulation mechanism, especially the aggregation of platelets, to accelerate clot formation.The material dissolves as haemostasis occurs and any residual will absorb in the wound. The sponge must be kept dry and should be applied with dry gloves or instruments. It is applied directly to the bleeding surface as supplied from the sterile package. Absorbable collagen is contraindicated in the presence of infection or where blood or other fluids have pooled (Phillips, 2007).
Oxidised cellulose
Absorbable oxidised cellulose comes in the form of a knitted fabric (Surgicel) and is applied dry to bleeding areas. It may be sutured to, wrapped around or held firmly against a bleeding site, or laid dry on an oozing surface until haemostasis is obtained. Oxidation of the cellulose acts rapidly to form a clot when it comes in contact with whole blood. As it reacts with blood, it increases in size to form a gel, and stops bleeding in areas in which bleeding is difficult to control by other means of haemostasis. If left on oozing surfaces, it will absorb 10 times its own weight with minimal tissue reaction. Oxidised cellulose is inactivated in the presence of thrombin (Phillips, 2007).
Thrombin
Thrombin (Thrombostat) is an enzyme that is extracted from dried beef blood; it is used topically. Thrombin accelerates coagulation of blood and controls capillary bleeding. It unites rapidly with fibrinogen to form a clot. It is available as a powder that is reconstituted immediately prior to use. It can be used alone or soaked into a gelatin sponge. Thrombin is used as a topical application only and is never injected (Phillips, 2007).
Oxytocin
Oxytocin is a hormone produced in the pituitary gland, which can also be prepared synthetically for therapeutic injection. It is commonly used in obstetric and gynaecological surgery. Oxytocin causes the uterine muscle to contract, thus putting pressure on the blood vessels, reducing bleeding (Phillips, 2007).
Adrenaline
Adrenaline is a naturally occurring hormone produced by the adrenal gland. It is also prepared commercially. It acts as a vasoconstrictor, reducing the flow of blood to the surgical site. Topical adrenaline can be applied to bleeding surfaces. Local anaesthesia often contains adrenaline to reduce the blood flow to the skin, promoting surgical haemostasis (Phillips, 2007).
Fibrin glue
Fibrin glue acts as a biological adhesive and haemostatic agent. It is composed of fibrinogen, cryoprecipitate from human plasma, calcium chloride and reconstituted thrombin of bovine origin (Phillips, 2007). Upon application directly to tissues, thrombin converts fibrinogen to fibrin to produce a clot. Fibrin glue may be used in deeper tissues to control bleeding and approximate tissues. A liquid gel or aerosol spray can deliver the fibrin glue.
Electrosurgical haemostasis
Electric current can be used to cut or coagulate most tissues: fat, fascia, muscle, internal organs and vessels. Electrosurgery is used to a greater or lesser extent in all surgical specialities. Electrosurgical unit (ESU), lasers and ultrasonic machines are the types most frequently seen in the operating room. These machines come with a range of disposable ‘tools’ that surgeons can use to achieve ongoing haemostasis.
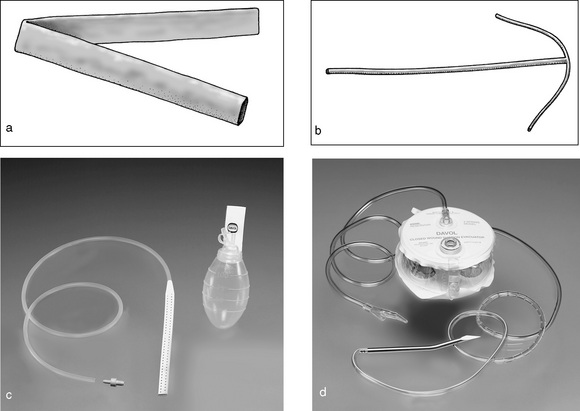
Electrosurgical unit
The ESU provides a high-frequency electric current to cut tissue and to coagulate bleeding points. The size and flow of the current generate heat as it meets resistance in passage through tissue. The current can be passed through the tissues without causing stimulation of muscle or nerves. A grounding pad is required. Two different types of electrosurgical machines (Fig 7-5), which act to seal blood vessels using different types of energy, are available.
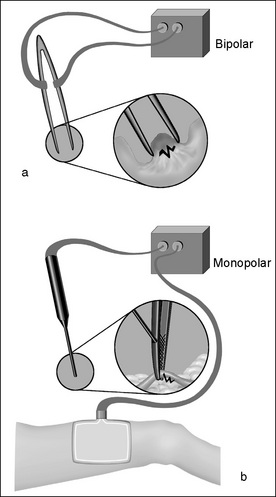
Figure 7-5 Electrosurgical haemostasis: (a) bipolar and (b) monopolar diathermy (Henry & Thompson, 2005, p 110).
Laser
Laser light is used for controlling bleeding or for the ablation and excision of tissues. The laser concentrates and intensifies a light beam of a single wavelength. The thermal energy of this beam may simultaneously cut, coagulate and/or vaporise tissue. The laser wound is characterised by minimal bleeding and no postoperative oedema. Different lasers have selected uses, depending on the form of the wavelength (Phillips, 2007).
Ultrasonic scalpel
The titanium blades of this scalpel move by a rapid ultrasonic motion that cuts and coagulates tissue simultaneously. It generates less heat than the ESU and, therefore, does not damage adjacent tissues. Vibrations from the tool denature protein molecules, producing a coagulum that seals bleeding vessels. The continuous vibration of the denatured protein generates heat within the tissue to cause deeper coagulation. Because electricity is not required to produce coagulative effects on tissue, a grounding pad is not required (Phillips, 2007).
Haemostatic scalpel
The sharp steel blade of the haemostatic scalpel seals blood vessels as it cuts through the tissue. When the surgeon activates the handle, the blade transfers thermal energy to tissues as the sharp edge cuts through them. The temperature can be adjusted between 110°C and 270°C. The resultant rapid haemostasis with minimal tissue damage promotes wound healing and may eliminate the need for blood replacement. Because electric current from the microcircuitry does not pass through the tissues, a grounding pad is not required (Phillips, 2007).
Additional methods of haemostasis
Embolisation
In embolisation, a haemostatic agent is placed inside a blood vessel, deliberately blocking it, in order to prevent the risk of a more serious haemorrhage. Several substances are used as embolic agents, as well as devices such as coils. This technique is often used in neurosurgery to embolise a cerebral aneurysm or the blood supply to an arteriovenous malformation, thereby negating the need for surgery, or to help the surgeon control bleeding during the surgical procedure (Phillips, 2007).
Sclerotherapy
Sclerotherapy is the injection of a coagulant to stop or reduce venous bleeding. Phenol plus alcohol is a sclerotherapy agent used to thrombose external haemorrhoids (Phillips, 2007).
Wound closure
The goal of wound closure is to approximate the wound edges, eliminate dead space, distribute tension evenly along the suture line, and maintain the tensile strength across the suture line until sufficient tissue tensile strength is achieved. The closure of a surgical wound is performed after adequate haemostasis has occurred. The strength of the wound is related to the condition of the tissue and the number of stitches in the edges. Care is taken not to place more sutures than necessary to approximate the edges. The amount of tissue incorporated into each stitch directly influences the rate of healing. Wound closures often include deep and superficial sutures but may also include staples, clips, tapes and glues. There are indications for each method of closure, along with advantages, disadvantages and special considerations (Lai, 2004).
Skilful wound closure requires knowledge of good surgical technique, as well as knowledge of the properties of the suture material and needle. While the actual suturing technique is largely left to the surgeon, the perioperative nurse needs a broad knowledge of suture materials, their properties and how to handle the sutures safely in order to assist the surgeon. Chapter 8 has a detailed section on suture materials.
Wound closure methods
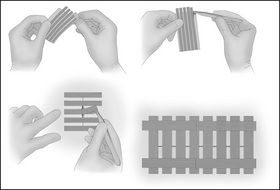
The traditional methods of wound closure include (Fig 7-6):
Figure 7-7 demonstrates suturing techniques for wound closure.
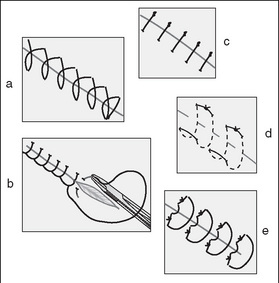
Figure 7-7 Examples of suturing techniques (Phillips, 2007, p 557): (a) simple continuous; (b) continuous locking; (c) simple interrupted; (d) horizontal mattress; (e) vertical mattress.
Single layer closure
A single layer continuous suture line is commonly used in abdominal closure. It is subject to great pulling forces, and the ‘bites’ of the needle are deliberately large so that the force is more evenly distributed over the area.
Multiple layer closure
As the name suggests, the wound is closed in multiple layers, effectively eliminating the dead space, thus promoting wound healing.
Simple continuous sutures
In this method, the suture is anchored at one end of the wound and proceeds towards the opposite end, taking even bites of tissue. The suture is then anchored at the other end of the wound. This method is quicker than tying several knots, as for interrupted sutures (see below).
Simple interrupted sutures
With simple interrupted sutures, each suture is placed and tied individually. This method is generally considered to be the most secure method of suturing. Variations in the configuration of the stitch itself are designed to exert forces in different ways.
Knot placement
Interrupted stitches require individual knots and, therefore, placement of the knot can influence how well the wound heals and the cosmetic result. Principles concerning knots and knot tying include the following:
Continuous running/locking (blanket stitch)
In this method, a single suture is passed in and out of the tissue layers and looped through the free end before the needle is passed through the tissue for another stitch. Each new stitch locks the previous stitch.
Subcutaneous sutures
Subcutaneous sutures are placed under the epidermis of the skin. This method of closure provides good cosmetic results as there is no skin perforation with the suture needle. It is suitable in the absence of oedema or infection.
Retention sutures
Interrupted, non-absorbable retention or stay sutures are placed alongside the primary suture line to relieve lateral tension on the suture line, with the aim of reducing the risk of wound dehiscence. The tissue through which retention sutures are passed includes skin, subcutaneous tissue and fascia, and may include the rectus abdominis muscle and peritoneum in an abdominal incision. After abdominal surgical procedures, retention sutures are used frequently in patients in whom slow wound healing is expected because of:
Other methods of wound closure
Staples
Most operating suites have a large collection of mechanical stapling devices that are used for internal ligation, division, resection, anastomosis and closure of the skin and fascia layers. These disposable devices provide the important benefit of reducing tissue handling, thereby reducing the length of the procedure. When fired, the staples take on a ‘B’ shape, permitting nutrients to permeate the tissue and promote healing. Stapling devices fire a single staple, one or two straight rows or circular rows of staples. Refills are also available for some stapling devices. Some stapling devices incorporate blades; this permits the simultaneous division of tissue that has been ligated between rows of staples (Phillips, 2007).
Skin staples/clips are used to close the skin layer of an incision (Fig 7-8). The clips are made from non-corroding metal and delivered by a preloaded disposable device. Skin staples can be used in the presence of infection as the strength of the material is not affected by the increased inflammatory response, which can weaken some suture materials (Phillips, 2007).
Tapes
Skin tapes are adhesive-backed nylon or polypropylene tapes, which can be used separately or in conjunction with subcuticular skin closure, providing extra strength (Fig 7-9). Other examples of surgical tapes include an adherent device that is laced up the centre to reduce the tension in a large wound (Phillips, 2007).
Tissue adhesives
Tissue adhesives have been available for some 20 years and have been used most commonly in the emergency room, particularly for children with minor traumatic wounds. Tissue adhesives provide good closure without causing any pain, plus there is no need to remove sutures at a later time. Patients are able to shower because the adhesive also provides a waterproof barrier. Synthetic adhesives are glue-like adhesives that polymerise to bind tissue edges together. Biological and synthetic adhesives, such as fibrin glue (Box 7-3), have great potential application in the area of haemostasis as previously presented (Phillips, 2007). Tissue adhesives may be used for microsurgical anastomoses of blood vessels, nerves and fallopian tubes. They may also be used for reconstruction of the middle ear, to fix ocular implants, to close superficial lacerations and fistula tracts, and to secure skin grafts.
Box 7-3 Sample fibrin glue compound and supplies
Instructions
Mix CaCl and thrombin in the specimen cup. Draw mixture into a 20-mL syringe and attach a 14-gauge IV catheter. Draw cryoprecipitate into the second syringe with a 14-gauge catheter on the end. Both syringes are discharged over the wound at the same time. The fibrin glue will form a clot over the wound.
Implantable tissue repair and replacement material
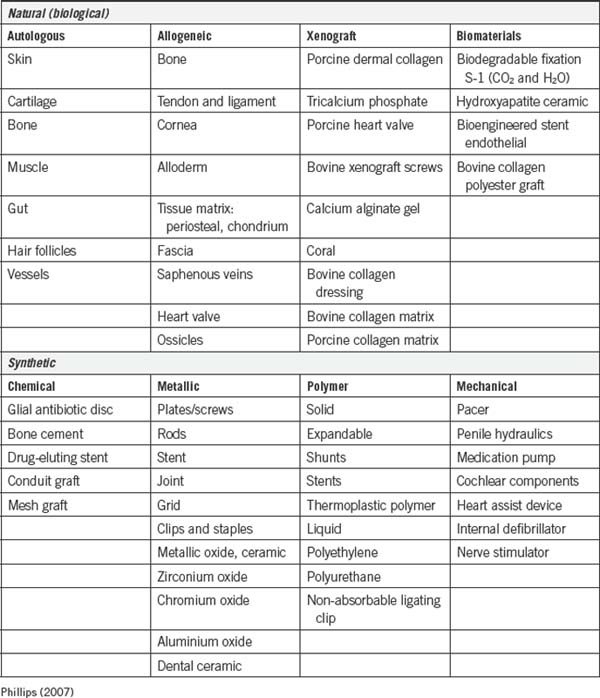
Tissue deficiencies may require additional reinforcement or bridging material (Table 7-1) to obtain adequate wound healing. Sometimes, the edges of fascia, for example, cannot be brought together without excessive tension. In obese patients or older patients the fascia cannot withstand this tension because of weakness caused by the infiltration of fat. Biological or synthetic mesh materials are used to fill congenital, traumatic or acquired defects in fascia or a body wall and to reinforce fascia, as in hernia repair (Phillips, 2007). Implants must be sterile and compatible with the recipient. They should not be handled excessively to prevent damage to the surface or contamination from the field. Implants can be permanent or temporary and composed of many materials. Some implants used are mechanical.
Wound care
Dressings
Dressings are applied to a surgical incision or wound site for the first 24–48 hours to provide the best environment for wound healing to occur. A dressing serves several purposes:
When considering the most suitable dressing, each wound is assessed in terms of the type of wound, its location, depth and the patient’s comorbidities. The type of dressing selected is also based on the surgeon’s or specialty preference.
Types of dressings
Dressings are classified according to their main function: primary or secondary dressings. Primary dressings are placed directly over the wound. The function of these dressings is to absorb drainage away from the wound edge. This layer of dressing should be non-stick unless debridement is necessary. Secondary dressings are placed directly over the primary dressing. The function of secondary dressings can include haemostasis by compression, absorption of excess drainage and protection of the wound from trauma (McEwen, 2007).
One layer dressings
One layer dressings are sterile, transparent, occlusive dressings that are suitable for clean, incised wounds. Multiple studies have shown that the use of occlusive dressings promotes wound healing two to six times faster than in a wound exposed to air; surgical site infection rates are significantly lower under an occlusive dressing compared to a non-occlusive dressing (Fletcher et al., 2007).
Skin closure dressing (island dressing)
Skin closure or island dressings consist of a non-stick pad in the centre (to absorb drainage) and either an occlusive-type material or adherent woven gauze, which anchors the dressing to the skin. Fletcher et al. (2007) recommend the use of surgical island dressings on clean, incised surgical wounds.
Dry sterile dressing
Dry sterile dressings are applied to dry incised wounds where there is no drainage. Dry dressings are not used on denuded wounds or those with large amount of drainage as they adhere to the wound, causing trauma on removal.
Three layer dressings
Three layer dressings are used when moderate to heavy drainage is expected. The contact layer acts as a passageway for the secretion and exudates that emanate from a draining wound. It must conform to body contours regardless of the site and extent of the wound, and must stay in intimate contact with the wound surface for at least 48 hours, yet be non-adherent for painless removal. The intermediate layer absorbs secretions that pass through the contact layer. It should be layered but not excessively bulky, nor apply pressure that could compromise circulation. The outer layer holds the contact and intermediate layers in proper position. It should be conforming and stretchable to avoid constriction if oedema develops.
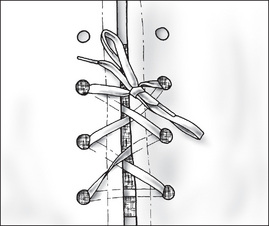
Non-allergenic tape is the most frequently used material to hold the dressing in place. Depending on the site of the wound, an elastic bandage may be used because it provides gentle even pressure and gives firm support. Montgomery straps (Fig 7-10) are used to hold bulky dressings in place that require frequent changes or wound inspections (Henry & Thompson, 2005).
Pressure dressing
Bulky dressings are added to the immediate layer of a three layer dressing. This dressing acts to eliminate dead space and prevent haematoma or oedema. They also distribute pressure evenly; absorb extensive drainage; encourage wound healing and minimise scarring by influencing wound tension; and immobilise a body area or support soft tissues when muscles are being moved. A pressure dressing helps to provide comfort to the patient postoperatively. These dressings are often used in plastic, knee and breast surgery (Henry & Thompson, 2005).
Stent dressing
A stent dressing is a method of applying pressure and stabilising tissues when it is impossible to dress an area, such as on the face or neck.
Bolster/tie-over dressing
Dressing materials may be sutured in place to exert an even pressure over autografted wounds to prevent haematoma or seroma formation.
Wet-to-dry dressings
Wet-to-dry dressings are used when debridement of the wound is required; the saline-soaked gauze dries and debrides the wound on removal. This process is used to facilitate new tissue growth. These dressings are painful and their removal and/or replacement may be conducted in the operating room under anaesthetic.
Wet-to-wet dressings
Normal saline or other medicinal solutions are applied to dressings to maintain an optimal environment for wound healing. Wet-to-wet dressings are less painful than wet-to-dry dressings but may also be applied or changed in the operating room under sterile conditions.
Vacuum-assisted dressings
These dressings are a closed-system dressing used for difficult to heal wounds with large amounts of drainage. The dressing comprises an absorbent sponge to draw away fluid, an occlusive adherent dressing to seal the system, tubing to facilitate the drainage and a vacuum pump, which gently aspirates the fluid and holds it in a storage unit (Fig 7-11). The use of vacuum-assisted dressings has increased in recent years for the treatment of wounds in patients with compromised healing (Heller et al., 2006). However, some evidence suggests that the drains may contribute to the formation of fistulas (Rao et al., 2007).
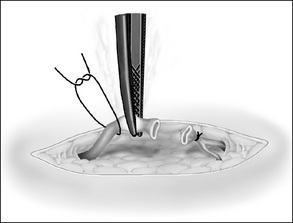
Drains
Drains are often inserted during surgery to provide a pathway allowing blood, lymph, intestinal secretions, bile, pus, air or urine to be transported away from the surgical site (Box 7-4). Drains can be used prophylactically or therapeutically. A drain is inserted prophylactically to remove unwanted fluid, air or secretions from around the surgical site, to promote wound healing, to provide a mechanism to observe for haemorrhage (Gurusamy & Samraj, 2007) and to reduce postoperative pain (Shen et al., 2003). The presence of a collection of fluid, air or secretions is thought to act as a medium in which microbes grow, leading to a surgical site infection.
Box 7-4 Routine abdominal drainage for uncomplicated laparoscopic cholecystectomy
In this study, the benefits and harm caused by the insertion of a closed suction drain after an uncomplicated laparoscopic cholecystectomy were assessed. The study analysed a group of six trials involving 741 patients: 361 with drain versus 380 with no drain. The study explored the reason why drains are often used, which was prevention of intra-abdominal collections, postoperative shoulder pain, nausea and vomiting. The authors concluded that the use of a drain after a laparoscopic cholecystectomy increased wound infection and delayed discharge from hospital.
The use of prophylactic drains in surgery continues to be controversial. Several recent studies have found insufficient evidence to support their use (McCarthy et al., 2005; Parker et al., 2007; Tjeenk et al., 2005;). The presence of a wound drainage system does not necessarily reduce the incidence of surgical site infection or haematoma (Gurusamy & Samraj, 2007; Gurusamy et al., 2007). Indeed, in some cases the presence of a drain is thought to increase the risk of surgical site infections by providing a route for the migration of bacteria around the tube into the wound (Fletcher et al., 2007).
A drain that is inserted therapeutically is used to reduce the amount of an already present collection, which may be necrotic or purulent material. Drains are usually inserted at the time of surgery primarily through a small stab incision near the operative site. Drains may or may not be sutured to the skin.
Some drains act by directing the fluid away through the lumen of the tube itself, other drains have a small fenestration at the tip, which drains into a closed system, and other drains act by ‘wicking’ the fluid away by capillary action into an absorbent dressing or drainage bag (Phillips, 2007).
The perioperative nurse must document clearly in the patient’s medical record the type of drain, its location and whether the drain is sutured in place, and ensure that the drain is working properly before the patient leaves the operating room.
Drain types
Passive drains
Passive drains use gravity and capillary action to move unwanted fluids away from the operative site. A Penrose drain (Fig 7-12) is a soft latex tube, of varying sizes, often used for the superficial drainage of abscesses. A Yeates drain is a soft, silicone corrugated drain, which acts by capillary action and gravity into a dressing or drainage bag; it is often used to drain post-appendicectomy wounds (Phillips, 2007).
Active drains
Active drains are attached to an external source of vacuum to create a negative pressure in the wound. Active drains include closed wound suction systems, sump drains and chest drains.
Closed wound suction
Closed drainage systems, such as the Jackson-Pratt or haemovac drains (Fig 7-12), are sterile, self-contained drainage units. The closed unit minimises the pathway of pathogens to the wound site. They may be used with or without suction; that is, they can be active or passive drains. The negative pressure in the reservoir acts to draw fluid gently away from the wound site into the reservoir.
Specialised drains
The T-tube drainage system is a soft latex drain that is inserted into the common bile duct, allowing bile to be drained away.
Urinary drainage
A urinary or ureteral catheter provides continuous drainage of the bladder or kidneys during and after the surgical procedure. The balloon of a urinary catheter maintains pressure on the bladder neck, which helps control bleeding after a transurethral prostatectomy and can be used to facilitate bladder irrigation. The urinary catheter is also used to monitor the patient’s haemodynamic status.
Conclusion
The perioperative nurse must show sound assessment, planning, implementation and evaluation of all aspects of wound management during the perioperative period. The results of a surgical site infection may be devastating to patients and their family and continue to be a costly problem for hospitals. Wound healing, assessment, haemostasis, wound closure and wound management have been explored in this chapter in more detail to empower the perioperative nurse to care for patient wounds skilfully.
Critical thinking exercises
1 Potential wound problems
Mr Jones is scheduled for a liver resection on tomorrow’s list. He weighs 60 kg, is 175 cm tall and has a previous history of alcohol abuse.
Identify three potential problems that Mr Jones may encounter. Address the following:
Benbow M. Healing and wound classification. Journal of Community Nursing. 2007;21:26-32.
Cardosi R., Drake J., Holmes S., et al. Subcutaneous management of vertical incisions with 3 or more centimetres of subcutaneous fat. American Journal of Obstetrics & Gynecology. 2006;195:607-616.
Corbett L.Q., Milne C.T. Wound care in the age of PPS: tools for survival. Home Health Care Management Practice. 2001;13:93.
Curtis K., Ramsden C., Friendship J. Emergency and trauma nursing. Sydney: Elsevier; 2007.
Doughty D., Sparks-Defries B. Wound healing physiology. In Bryant R., Nix D., editors: Acute and chronic wounds: current management concepts, (3rd ed.), St Louis: Mosby, 2007. (pp. 56–81)
Fletcher N., Sofianos D., Brantling Berkes M., Obremskey W.T. Prevention of perioperative infection. Journal of Bone & Joint Surgery. 2007;89:1605-1618.
Gurusamy, K. S., & Samraj, K. (2007). Wound drains after incisional hernia repair. Cochrane Database of Systematic Reviews, 1, CD005570.
Gurusamy, K. S., Samraj, K., Mullerat, P., Davidson, B. R. (2007). Routine abdominal drainage for uncomplicated laparoscopic cholecystectomy. Cochrane Database of Systematic Reviews, 4, CD006004.
Harvey C. Wound healing. Orthopaedic Nursing. 2005;24(2):143-159.
Heller L., Levin S.L., Butler C.E. Management of abdominal wound dehiscence using vacuum assisted closure in patients with compromised healing. American Journal of Surgery. 2006;191:165-172.
Henry M., Thompson J. Clinical surgery, (2nd ed.). London: Saunders; 2005.
Karukonda S., Flynn T., Boh E., et al. The effects of drugs on wound healing: part 1. International Journal of Dermatology. 2000;39(4):250-257.
Lai, S. Y. (2004). Sutures and needles. eMedicine. Retrieved April 29, 2007, from http://www.emedicine.com/ent/topic38.htm.
McCarthy C.M., Disa J.J., Pusic A.L., et al. The effect of closed-suction drains on the incidence of local wound complications following tissue expander/implant reconstruction: a cohort study. Plastic and Reconstructive Surgery. 2005;119(7):2018-2022.
McEwen D. Wound healing, dressings and drains. In Rothrock J., editor: Alexander’s care of the patient in surgery, (13th ed.), St Louis: Mosby, 2007. (pp. 263–273)
Myers B. Wound management: principles and practice. New Jersey: Prentice Hall; 2004.
Parker, M. J., Livingstone, V., Clifton, R., McKee, A. (2007). Closed suction surgical wound drainage after orthopaedic surgery. Cochrane Database of Systematic Reviews, 3, CD001825
Phillips N., editor. Berry & Kohn’s operating room technique, (11th ed.), St Louis: Mosby, 2007.
Ramundo J. Wound debridement. In Bryant R., Nix D., editors: Acute and chronic wounds: current management concepts, (3rd ed.), St Louis: Mosby, 2007. (pp. 176–192)
Rao M., Burke D., Finan P.J., Sagar P.M. The use of vacuum-assisted closure of abdominal wounds: a word of caution. Colorectal Discourse. 2007;9:266-268.
Rothrock J. Alexander’s care of the patient in surgery, (13th ed). St Louis: Mosby; 2007.
Rubin R. Surgical wound infection: epidemiology, pathogenesis, diagnosis and management. BMC Infectious Diseases. 2006;6:171.
Shen C.C., Wu M.P., Lu C.H., et al. Effects of closed suction drainage in reducing pain after laparoscopic-assisted vaginal hysterectomy. American Association of Gynaecologic Laparoscopists. 2003;10(2):210-214.
Schultz G. Molecular regulation of wound healing. In Bryant R., Nix D., editors: Acute and chronic wounds: current management concepts, (3rd ed.), St Louis: Mosby, 2007. (pp. 82–99)
Schultz G., Sibbald G., Falanga V., et al. Wound bed preparation: a systematic approach to wound management. Wound Repair and Regeneration. 2003;11:1-28.
Tjeenk R.M., Peeters M.P., van den Ende E., et al. Wound drainage versus non-drainage for proximal femoral fractures: a prospective randomised study. International Journal of Trauma Surgery. 2004;36:100-104.
Trask B.C., Rote N., Huether S. Innate immunity: inflammation. In McCance K., Huether S., editors: Pathophysiology the biologic basis for disease in adults and children, (5th ed.), St Louis: Mosby, 2006. (pp. 175–209)
Wysocki A. Anatomy and physiology of skin and soft tissue. In Bryant R., Nix D., editors: Acute and chronic wounds: current management concepts, (3rd ed.), St Louis: Mosby, 2007. (pp. 39–55)
Carless P.A., Henry D.A., Anthony D.M. Fibrin sealant use for minimising peri-operative allogenic blood transfusion. Cochrane Database of Systematic Reviews. 2, 2003. CD004171
Choy P.Y.G., Bissett I.P., Docherty J.G., et al. Stapled versus handsewn methods for ileocolic anastomoses. Cochrane Database of Systematic Reviews. 3, 2007. CD004320
Collis N., McGuiness C.M., Batchelor A.G. Drainage in breast reduction surgery: a prospective randomised intra-patient trail. British Journal of Plastic Surgery. 2005;58:286-289.
Evans J. Massive tissue loss: burns. In Bryant R., Nix D., editors: Acute and chronic wounds: current management concepts, (3rd ed.), St Louis: Mosby, 2007. (pp. 361–390)
Hall J.C., Hall J.L. The measurement of wound infection after breast surgery. The Breas Journal. 2004;10(5):412-415.
Hangstrom A. Perceived barriers to implementation of a successful sharps safety program. AORN Journal. 2006;83(2):391-397.
Hess C., Kirsner R. Orchestrating wound healing: assessing and preparing the wound bed. Advances in Skin and Wound Care. 2003;16(5):246-257.
Lusby P.E., Coombes A., Wilkinson J.M. Honey: a potent agent for wound healing? Journal of Wound, Ostomy and Continence Nurses. 2002;29(6):295-300.
McCallum I., King P.M., Bruce J. Healing by primary versus secondary intention after surgical treatment for pilonidal sinus. Cochrane Database of Systematic Reviews. 4, 2007. CD006213
Nadkarni M.S., Rangole A.K., Sharma R.K., et al. Influence of surgical technique on axillary seroma formation: a randomized trial. Australian and New Zealand Journal of Surgery. 2007;77(5):385-389.
Parker M.J., Livingstone V., Clifton R., McKee A. Closed suction surgical wound drainage after orthopaedic surgery. Cochrane Database of Systematic Reviews. 3, 2007. CD001825
Perry J., Jagger J. Slash sharps risk for surgical personnel. AORN Journal. 2005;36(11):28-29.
Sessler D.I. Non-pharmacological prevention of surgical wound infection. Anesthesiology Clinics of North America. 2006;24:279-297.
Vilar-Compte D., Roldan-Martin R., Robles-Vidal C., Volkow P. Surgical site infection (SSI) rates among patients who underwent mastectomy after introduction of SSI prevention policies. Infection Control and Hospital Epidemiology. 2006;27(8):829-834.
Wang N.D., Doty D.B., Doty J.R., et al. BioGlue: a protective barrier after pericardiotomy. Journal of Cardiac Surgery. 2007;22:295-299.
Yarboro S.R., Baum E.J., Dahners L.E. Locally administered antibiotics for prophylaxis against surgical wound infection. Journal of Bone and Joint Surgery. 2007;89:929-933.