Other Challenges in Pain Assessment
SOME patients with pain are simply incapable of providing self-reports of pain. They may be cognitively impaired or unconscious. Such circumstances necessitate using other, less reliable indicators of pain, always being mindful that in the absence of self-reports all else is an educated guess.
This group includes patients who have a wide range of communication difficulties, from those who are conscious but unable to speak to those who are unconscious and, of course, unable to speak. Examples are adults who have cognitive impairment, as discussed in the previous chapter; who have severe emotional disturbances; who are intubated; who are developmentally delayed; who speak a language other than English; or whose educational or cultural backgrounds are significantly different from those of the health care team (APS, 2003). Such patients pose assessment challenges and are at risk for undertreatment.
Patients who are critically ill, intellectually disabled, mentally ill, or unconscious are discussed in this chapter. Cultural considerations are also included.
Patients Who Are Critically Ill
Some critically ill patients are mistakenly believed to be unconscious or pain-free, but pain in critically ill patients has been well documented for some time. For example, patients with endotracheal tubes and those who have received neuromuscular blocking agents such as pancuronium (which does not alter sensitivity to pain) may be fairly alert, and some are fully capable of feeling pain and are able to self-report pain if given the appropriate opportunity. In a sample of 24 patients in an intensive care unit (ICU), interviews after transfer from the ICU revealed that all but 1 patient recalled their ICU stays, and 63% recalled moderate to severe pain (Puntillo, 1990). The patients described pain caused by surgical incisions, movement, coughing, endotracheal suctioning, and chest tube removal. Those who could not talk (80% had endotracheal tubes) described numerous behaviors they used in attempts to tell staff they were in pain, such as signaling with their eyes and moving their legs up and down. These patients are actually capable of giving self-reports of pain. Some can use writing materials and can be taught to use a pain rating scale. Some can use the call button, which should be placed within easy reach. Others can be asked questions and be taught to signal with their eyes that pain is present. For example, the clinician can establish with the patient that when asked about pain, squeezing the eyes once means yes and twice means no.
Clinicians are prone to overlook pain at rest as well as pain resulting from common procedures such as turning and suctioning. In a study of 30 critically ill, traumatically injured patients, pain was measured at rest in a supine position and after being turned onto their sides (Stanik-Hutt, Soeken, Belcher, 2001). On a visual analog scale (VAS) (0 to 100 mm), mean at-rest scores were 34.5, which indicates mild pain bordering on moderate pain. Immediately after the turns, the mean scores were 48.1 on the VAS, indicating moderate pain. Terms commonly used to describe pain at rest included throbbing, sharp, sore, hurting, and annoying. After turning, the pain was often described with the same words used for pain at rest (except for hurting) plus pressing, pulling, tender, tiring, and miserable. Of these patients, 13 were not included in the study because they refused to be turned, giving as the reason severe pain at rest or anticipated pain with turning. Their pain at rest was 47.1 on the VAS. Based on their review of the literature, the researchers concluded that pain intensity at rest in this study was in the same range as that in other studies of critically ill patients.
Endotracheal suctioning also causes considerable pain. In a study of 45 adults having cardiovascular (CV) surgery, patients rated their pain during endotracheal suctioning on a 0-to-10 scale (Puntillo, 1994). The mean pain intensity score was 4.5, and more than one third of the patients reported a pain intensity of 7 or greater, indicating severe pain. Patients described the pain as tiring-exhausting, stabbing, tender, sharp, and heavy.
The results of the Thunder Project II, a multisite study, provide extensive information about procedural pain in acute and critically ill patients (Puntillo, White, Morris, et al., 2001). In a total of 153 clinical sites, information was collected from 6201 patients, 5957 of whom were 18 years or older. Patients were alert and able to answer questions. Data were obtained about pain related to six procedures: turning, wound drain removal, tracheal suctioning, femoral catheter removal, placement of a central venous catheter, and nonburn-wound dressing change. Adults rated their procedural pain intensity as 2.65 to 4.93 on a 0-to-10 numerical rating scale (NRS). The most painful and distressing procedure for adults was turning; the mean pain intensity was 4.93 and the mean distress score was 3.47 on a scale of 0 to 10. After turning, the most painful procedures in adults, in descending order, with mean pain intensity in parentheses, were wound drain removal (4.67); wound care (4.42); tracheal suctioning (3.94); central line placement (2.72); and femoral sheath removal (2.65). Pain at rest was usually described as aching, and procedural pain was often described as sharp.
Thus, critically ill patients experience mild to moderate pain throughout the day. At rest, the pain is commonly mild, but turning, which usually occurs at least every 2 hours, is associated with moderate pain and, compared to other painful procedures, is the most distressing.
In a follow-up to the study just described, Puntillo, Wild, Morris, and others (2002) examined analgesic and local anesthetic administration in the 5957 adults they assessed prior to and during the six procedures. Undertreatment of procedural pain was apparent. More than 63% of the patients received no analgesics before or during the procedures, and less than 20% received opioids. The patients most likely to receive opioids were those undergoing femoral sheath removal (28.6%), and those least likely to were undergoing tracheal suctioning (3.7%). A total of 18.4% of patients received local anesthetics, and 89.5% of them were undergoing placement of a central venous catheter. Part of this undertreatment may be the result of failure to assess pain or anticipate pain. All of these patients were alert, and pain could have been assessed at least during the procedure or prior to the procedure for those who had already experienced the procedure, such as turning or tracheal suctioning.
Further analysis of data available from the previously mentioned study of 5957 critically ill adults revealed information about behavioral responses associated with the six procedures being studied (Puntillo, Morris, Thompson, et al., 2004). All the patients in this study were alert and able to self-report, but many critical care patients are unable to provide self-reports of pain because of intubation, motor impairments, cultural or language barriers, or altered levels of consciousness. Behavioral responses to pain may then become one of the most valuable contributions to pain assessment. A 30-item behavioral tool was used to observe patients’ behaviors before and during the procedures. Significantly more behaviors were exhibited by patients with procedural pain than by those without that pain. Behaviors specific to procedural pain were grimacing, rigidity, wincing, shutting of eyes, verbalizations, moaning, and clenching of fists.
The FLACC (Face, Legs, Activity, Cry, Consolability) Behavioral Scale has recently been studied in 29 critically ill adults and children who could not self-report pain (Voepel-Lewis, Zanotti, Dammeyer, 2010). This is the first study to provide support of the FLACC in this population, but further study is warranted.
Several behavioral scales for use with adult patients in critical care and unable to self-report are being developed, and the psychometric properties of six of these scales have been compared (Li, Puntillo, Miaskowski, 2008). They are listed here with references that include the actual tool:
• The Behavioral Pain Rating Scale (BPRS) (Mateo, Krenzischek, 1992)
• The Behavioral Pain Scale (BPS) (Payen, Bru, Bosson, et al., 2001)
• Pain Behavior Assessment Tool (PBAT) (Puntillo, Morris, Thompson, et al., 2004)
• Critical-Care Pain Observation Tool (CPOT) (Gelinas, Fillion, Puntillo, et al., 2006)
• Pain Assessment and Intervention Notation (PAIN Algorithm) (Puntillo, Miaskowski, Kehrie, et al., 1997)
• Nonverbal Pain Scale (NVPS) (Odhner, Wegman, Freeland, et al., 2003).
Review of these assessment tools revealed that none had undergone vigorous validation or had been accepted as standardized measures, but two showed good evidence of validity and reliability—the BPS and the CPOT (Li, Puntillo, Miaskowski, 2008). Both are described here, and both are easy to use. However, we feel that the CPOT is a slightly better choice for clinical practice because it includes four behavioral domains instead of the three that are in the BPS, and because the CPOT divides the domain of patients on ventilators into two groups, patients who are intubated and patients who are not; whereas the BPS addresses only ventilated patients. Therefore, the actual tool for the CPOT is included (Form 4-1).
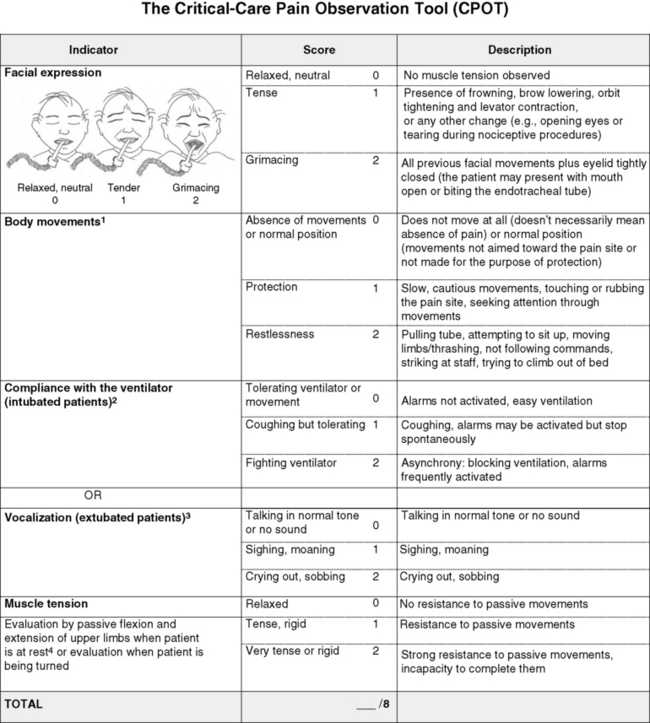
Form 4-1 As appears in Pasero, C., & McCaffery, M. (2011). Pain assessment and pharmacologic management, p. 145, St. Louis, Mosby. Instructions Form modified from Gélinas, C., Fillion, L., Puntillo, K. A., et al. (2006). Validation of the Critical-Care Pain Observation Tool (CPOT) in adult patients. Am J Crit Care, 15(4), 420-427. 1Puntillo, K. A., Miaskowski, C., Kehrle, K., et al. (1997). Relationship between behavioral and physiological indicators of pain, critical care self-reports of pain, and opioid administration. Crit Care Med, 25(7), 1159-1166; 2Devlin, J. W., Boleski, G., Mlynarek, M., et al. (1999). Motor activity assessment scale: A valid and reliable sedation scale for use with mechanically ventilated patients in an adult surgical intensive care unit. Crit Care Med, 27(7), 1271-1275; 3Harris, C. E., O’Donnell, C. MacMillan, R. R., et al. (1991). Use of propofol by infusion for sedation of patients undergoing haemofiltration: Assessment of the effect of haemofiltration on the level of sedation and on blood propofol concentration. J. Drug Dev, 4(Suppl 3), 37-39; 4Payen, J. F., Bru, O., Bosson, J. L., et al. (2001), Assessing pain in the critically ill sedated patients by using a behavioral pain scale. Crit Care Med, 29(12), 2258-2263; 5Mateo, O. M., Krenzischek, D. A. (1992). A pilot study to assess the relationship between behavioral manifestations and self-report of pain in postanesthesia care unit patients. J Post Anes Nurs, 7(1), 15-21; 6Ambuel, B., Hamlett, K. W., Marx, C. M., et al. (1992). Assessing distress in pediatric intensive care environments: The COMFORT scale. J Pediat Pscych, 17(1), 95-109. Gélinas C.
The CPOT evaluates four behavioral domains: facial expressions, movements, muscle tension, and ventilator compliance (Gelinas, Fillion, Puntillo, et al., 2006) . Each domain has three behaviors, which are scored from 0 to 2. The critical review of assessment tools used with seriously ill patients points out that a weakness of the CPOT is that generally low scores were reported in all patients during painful procedures (Li, Puntillo, Miaskowski, 2008). This should be kept in mind when using the tool, and clinicians should remember that pain behaviors are merely indicators of pain and that the number of behaviors do not equate with pain intensity (Pasero, McCaffery, 2005).
The sensitivity and specificity of the CPOT was studied in a sample of 105 adults in an ICU following cardiac surgery (Gelinas, Harel, Fillion, et al., 2009). Pain was evaluated during the painful procedure of turning. Sensitivity was high during turning, meaning that the tool could detect when pain was not present. The specificity of the CPOT was also high; that is, it correctly identified the patients who did have pain. This reduces the likelihood of administering an analgesic to a patient who does not have pain.
The BPS consists of three behavioral domains: facial expression, movements of upper limbs, and compliance with ventilation. Each domain contains four behaviors, each rated from 1 to 4 (Payen, Bru, Bosson, et al., 2001). In the critical review of assessment tools used with critically ill patients, the point is made that the observation of no body movement is equated with a pain-free state in the BPS (Li, Puntillo, Miaskowski, 2008). However, no body movement may simply reflect sedation, restraints, or other restrictions of movement. Another criticism concerns the numbering system, which starts with 1, meaning no pain behavior. It is suggested that starting with 0 more readily reflects no pain behaviors.
The American Society for Pain Management Nursing (ASPMN) published a position statement on pain assessment in the nonverbal patient (available at http://www.aspmn.org/Organization/documents/NonverbalJournalFINAL.pdf) that includes a section about intubated and unconscious patients (Herr, Coyne, Key, et al., 2006). Recommendations for assessment utilize the template for the hierarchy of pain measures, as presented previously (see Box 3-9, p. 123):
• The hierarchy begins with an attempt to obtain a self-report. If that fails, the following indicators should be assessed, and one can assume pain is present (APP) if any of the measures indicates pain. Again, the abbreviation APP may be used for documentation if approved by institutional policy and procedure.
• Potential causes of pain, such as procedures or existing painful conditions, are next on the hierarchy. These should include noting that pain at rest can cause aching and that the following procedures have been found to be painful: turning (the most painful of this group), wound drain removal, tracheal suctioning, femoral catheter removal, placement of a central venous catheter, and nonburn wound dressing change.
• Following assessment for causes of pain, clinicians look for patient behaviors that may indicate pain. This is the point at which a behavioral pain assessment tool is used, and we recommend using the CPOT for critically ill patients.
• Next, reports of pain or observations of behaviors that might indicate pain are elicited, when possible, from people who know the patient well, such as caregivers and family members. They may know of previously existing painful conditions such as arthritis that have been causing pain.
• Finally, when in doubt about the presence of pain or when wanting to confirm the suspicion of pain, an analgesic trial may be helpful. The analgesic trial is used as the basis for developing the pain treatment plan once pain has been confirmed. (See Boxes 3-9 and 3-11, pp. 123 and 126, for more ideas about using the analgesic trial.)
Physiologic indicators of pain such as changes in blood pressure are omitted from the hierarchy of pain assessment techniques. Their limited usefulness in assessing pain is discussed in Chapter 2, pp. 27-28. They are occasionally helpful but, especially in critically ill patients, these measures are often influenced by factors other than pain, such as medications, mechanical ventilation, change in level of consciousness, and patients’ illnesses or surgeries. In critically ill patients, behavioral indicators have been found to provide more valid information for pain assessment than physiologic indicators (Arbour, Gelinas, 2009; Gelinas, Arbour, 2009; Gelinas, Johnston, 2007).
For example, a study of 254 critically ill patients in intensive care units who were mechanically ventilated (144 conscious and 114 unconscious) examined potential behavioral and physiologic indicators of pain and their association with self-reports of pain (Gelinas, Arbour, 2009). These patients were observed at rest, during a nociceptive procedure (such as endotracheal suctioning), and 20 minutes postprocedure. Behaviors were measured with the CPOT, and physiologic indicators were obtained from the available monitoring. Patients able to self-report pain were asked if they had pain or not immediately after being scored with the CPOT. This prevented bias in the raters because the CPOT was used before requesting a self-report. Based on the patient’s self-report, only the CPOT score, not changes in vital signs, could predict the presence or absence of pain; consequently, only behavioral observation was recommended for pain assessment in unconscious patients. The researchers noted that changes in vital signs could be used as a cue to begin further assessment of pain.
Probably because the physiologic changes are so readily able to be observed in the ICU, critical care nurses are tempted to use them as indicators of pain, especially in patients who are unable to self-report. However, as mentioned above, many patients actually are able to self-report pain if the right approaches are used, such as eye blinking and the CPOT. Research strongly recommends use of behaviors to assess pain in unconscious patients and warns that vital signs should be used with caution (Gelinas, Arbour, 2009).
Patients Who Are Unconscious
Some critically ill patients appear to be unconscious due to injury such as brain trauma or disease, sedating medications, or neuromuscular blocking agents. But unconscious patients also exist outside the critical care setting. For example, patients in vegetative states may be found in long-term care facilities. Terminally ill patients may become unconscious as death approaches.
If unconsciousness is defined as no awareness of self or environment, how is it possible that an unconscious patient could feel pain and suffer from it? The problem is that unconsciousness is not easily determined, and there are varying levels of unconsciousness. A minimally conscious state is described as one in which there is some evidence of awareness of self and environment, and a persistent vegetative state is defined as wakefulness without awareness of self or environment (Boly, Faymonville, Schnakers, et al., 2008). Anecdotal evidence and some research strongly suggest that quite a few patients who appear to be unconscious and unresponsive to painful stimuli actually feel and recall pain. Thus, clinicians should assume that the unconscious patient may feel pain and should provide analgesia if anything known to be painful is present.
In a study of 100 critically ill patients whose records indicated that they were unconscious, interviews with the patients revealed that only about one quarter of them were actually unaware of themselves and their surroundings (Lawrence, 1995). The remaining three quarters had some awareness of themselves and their environments, and about 25% were able to hear and to feel pain.
During general anesthesia, some patients experience periods of awareness that they recall postoperatively. In a study of 26 patients who had experienced awareness during general anesthesia, hearing sounds and feeling paralyzed were reported by 89% and 85%, respectively (Moerman, Bonke, Oosting, 1993). Of those patients, 10% reported feeling pain. Aftereffects such as sleep disturbances, nightmares, and flashbacks occurred in 70%, and pain was recalled by one half of these patients. In another study of 45 patients who experienced awareness during general anesthesia, 8 patients reported feeling severe pain (Schwender, Kunze-Kronawitter, Dietrich, et al., 1998). A large study of 3921 patients undergoing general anesthesia identified an incidence of 1% (39 patients) who experienced awareness with recall (Errando, Sigl, Robles, et al., 2008). Pain was felt by 11 patients.
An anecdotal report illustrates the tragedy of assuming an unconscious patient does not feel pain. A terminally ill patient was receiving intravenous morphine for cancer-related pain; as her disease progressed, she gradually became unresponsive to voices and no longer grimaced during position changes, which previously had been painful for her. Because she appeared to be unconscious and unresponsive to painful stimuli, morphine was no longer administered. After 3 days the patient regained consciousness and immediately asked for morphine. The patient reported that during the apparent “coma,” she had heard voices and felt intense pain (Stephens, 1994).
Regarding pain in terminally ill patients who appear to be unconscious, a recommendation made almost 3 decades ago is worth repeating: “When patients are no longer able to verbally communicate whether they are in pain or not, the best approach is to assume that their cancer is still painful and to continue them on their regular medications…. a therapeutic narcotic level should be maintained…. continued narcotics simply ensure that the death will be as peaceful and painless as possible” (Levy, 1985, pp. 397-398).
Whether patients in persistent vegetative states feel pain remains controversial. This state is defined as complete unawareness of self and environment, including lack of the cortical capacity to feel pain. However, some have voiced reservations about assuming that patients in vegetative states do not feel pain and have suggested that, until it can be proved otherwise, clinicians should assume that such patients feel pain and should treat that pain (Bushnell, 1997; Klein, 1997).
This position is also advocated by researchers who studied brain activation during noxious stimulation consisting of electrical stimulation of the median nerve, comparing 5 patients in minimally conscious states, 15 normal controls, and 15 patients in persistent vegetative states (Boly, Faymonville, Schnakers, et al., 2008). No brain area was less activated in patients in minimally conscious states than in the controls. All areas of the cortical pain matrix showed greater activation in these patients than in patients in persistent vegetative states. The researchers concluded that this evidence confirmed the need for analgesic treatment for patients in minimally conscious states. They pointed out, however, that misdiagnosis of patients in persistent vegetative states and minimally conscious states is common and concluded that analgesic treatment is indicated in both groups. Analgesics for patients in persistent vegetative states are indicated also to prevent potentially damaging defensive hormonal reactions such as the production of adrenal stress hormones, despite the possible absence of feelings of pain.
For some unconscious patients the CPOT, discussed earlier (see Form 4-1, pp. 145-146), may be appropriate if they are capable of responding in the four behavioral domains: facial expressions, movements, muscle tension, and ventilator compliance (Gelinas, Fillion, Puntillo, et al., 2006). If these behaviors are absent, documenting procedures or pathologies that probably cause pain may be relied upon to assume pain is present (APP), as is suggested in Box 3-9 (p. 123). A behavioral tool is appropriate only with patients who are able to respond with the tool’s requisite behaviors.
Clinicians often wonder which dose of opioid to administer on an ongoing basis to a patient who cannot respond with any behaviors to indicate the effectiveness of a dose one way or another. In such cases, opioid therapy should be initiated and maintained at the recommended starting dose (e.g., 2.5 mg/hr morphine IV for adults in possibly severe pain). However, clinicians should not hesitate to increase doses if there are any behaviors whatsoever that might indicate pain (see Chapter 27 for more about analgesic administration in the ICU).
Patients Who Are Intellectually Disabled
The term developmental disabilities encompasses mental or physical disabilities that are evident in childhood and continue throughout life (Craig, 2006). Intellectual disability (ID), as defined for this chapter, is a type of developmental disability in which some form of intellectual disability or cognitive impairment is noted in childhood. The ID may or may not be accompanied by physical disability. Conversely, physical disabilities such as cerebral palsy are not always accompanied by mental disabilities. IDs are commonly defined by IQ scores. An IQ score of 50 to 70 indicates mild cognitive impairment, a score of 35 to 49 indicates moderate impairment, a score of 20 to 34 indicates severe impairment, and profound impairment is an IQ score below 20 (Bottos, Chambers, 2006; Grossman, 1983). Individuals with mild cognitive impairment make up 85% of those with IDs and usually acquire about a sixth grade level of academic skills (American Psychiatric Association [APA], 1994). Moderate cognitive impairment occurs in about 10% of cases of ID, and severe and profound impairment constitute 1% to 4% of cases of ID.
This chapter focuses on adults with IDs, that is, adults who have been cognitively impaired since birth or early childhood. This state is not to be confused with that of adults who have been cognitively intact for most of their lives but become cognitively impaired in their later years. Dementia is an example of a cognitive impairment that occurs late in life in individuals who were cognitively intact previously. The literature can be confusing because the publications that use the term cognitive impairment in their titles may be focusing on adults who have become impaired as they have grown older or on adults who have been cognitively impaired since childhood. Pain assessment strategies previously discussed in the section on cognitively impaired older patients probably are not useful in assessing pain in adults with mental retardation. Adults who have been cognitively impaired since childhood require assessment tools that have been specially designed for them because these individuals do not have the same understandings and experiences as adults who have been cognitively intact for many years and become cognitively impaired in later life.
Most of the research on pain and ID has been conducted in infants and children, but many of the issues raised in those studies, such as assessment, are regarded as being relevant to the care of adults with IDs (Symons, Shinde, Gilles, 2008). Consequently, some studies of children are reviewed here even though this book focuses on adults. Some pain assessment tools used with children with IDs have been adapted and studied in adults with IDs.
A long-standing misconception is that persons with IDs, or mental retardation, are insensitive or indifferent to pain (Symons, Shinde, Gilles, 2008). After reviewing several studies of children with IDs, Symons and others (2008) concluded that caregivers of children with IDs frequently underestimated pain intensity. Consequently, these individuals are undertreated. This conclusion appears to be based on observations of behavior, not measurements of sensitivity to pain. It is hypothesized that what may be confusing about behavioral observations of these patients is that behavioral indicators of pain may be delayed or unconventional, contributing to the belief that this population has a lowered sensitivity to pain or a greater tolerance. However, testing of patients with mild intellectual disabilities by measuring heat-pain thresholds showed that their pain threshold was significantly lower than that of normal controls (Defrin, Pick, Peretz, et al., 2004). Thus, these individuals not only are sensitive to pain but also may be more sensitive to some types of pain than persons without IDs.
Along the same lines, children with autism have also been thought to be insensitive to pain, but this is not supported by research. In a study comparing 21 children with autism with 22 without autism, all of them undergoing venipuncture, the children with autism manifested significant facial reactions quite similar to those in the unimpaired group but characterized by more facial activity (Nader, Oberlander, Chambers, et al., 2004). A perplexing finding was that the children who were the most reactive during the venipuncture had been identified by the parents as being less sensitive and less reactive to pain.
Understanding the misconceptions of caregivers of children with disabilities may be helpful in recognizing the possibility that the same misconceptions may occur in reference to adults with IDs. The degree of agreement between parent and child regarding pain intensity was examined in 68 children with spina bifida who were able to communicate (Clancy, McGrath, Oddson, 2005). Children rated their pain, and their parents completed a proxy report of the pain. Parents tended to underestimate the intensity of the pain their children felt. In another study, 65 caregivers of children with IDs completed questionnaires about their beliefs regarding pain in this population and revealed complex beliefs (Breau, MacLaren, McGrath, et al., 2003). Caregivers believed that children’s sensations of pain increased as the severity of the ID increased. They also believed that children with mild cognitive impairment might overreact to pain. Perhaps most alarming was the finding that the more caregivers had learned about ID, the more they believed that those with IDs experienced less pain than those without IDs. The authors suggested several reasons for this finding, including the possibility that the information that was being taught was inaccurate or misunderstood.
Educational materials for caregivers of individuals with IDs should be carefully examined. Caregivers may enter the learning situation already harboring misconceptions. If these are not addressed, they may persist. Underestimation of pain by caregivers of individuals with IDs may result in underreporting of pain to clinicians, leading to undertreatment.
In a literature review, Bottos and Chambers (2006) identified types of pain likely to occur in individuals with IDs. Pain in children with IDs, particularly cerebral palsy, includes gastrointestinal discomfort, such as gastroesophageal reflux and constipation, and musculoskeletal pain. Children with autism also tend to experience gastrointestinal disorders. Individuals with Down syndrome are prone to developing leukemia, hence experiencing all the usual pain associated with the disease, diagnostic tests, and treatment. They also tend to have hip abnormalities and oral health problems such as periodontal disease. In adults with severe to profound mental retardation, pain is more often chronic than acute. An extensive review of the literature about this population revealed that a significant percentage of these people experience significant intensities of pain on a daily basis (Bodfish, Harper, Deacon, et al., 2006).
A review of the literature found that in the adult population of individuals with cerebral palsy, as much as 84% reported one or more types of chronic pain; 56% experienced pain daily and 53% reported moderate to severe pain (Bottos, Chambers, 2006). In one study of 63 women with cerebral palsy, of the 53 who had pain, the most common sites were the head (28%), back (26%), and arms (23%) (Turk, Geremski, Rosenbaum, et al., 1997). Activities of daily living, such as assisted sitting, walking, stretching, toileting, and standing, are especially painful and common, being reported by 35% to 93% of individuals with cerebral palsy (Bottos, Chambers, 2006). Some of the musculoskeletal pain may worsen with aging. It is important to note that studies of the prevalence of pain in adults with cerebral palsy have usually been restricted to those with mild or no cognitive impairment, although those with greater cognitive impairment are not known to be less sensitive to pain or to have fewer types of pain. In fact, the prevalence of pain in children with IDs is higher than in healthy children, and children with greater cognitive impairment tend to experience the most intense and most frequent pain but are least able to communicate their pain (Bottos, Chambers, 2006). Thus, studies suggest that the prevalence of pain in adults with IDs is higher than in adults without IDs.
Assessment of individuals with IDs is extremely challenging and, although considerable research has gone into creating assessment tools for children with IDs, few studies have included adults with IDs. A large majority of adults with IDs achieve a sixth grade level of functioning, so many may be able to provide self-reports of pain, and others with greater cognitive impairment may also be able to self-report. The greatest challenge is the assessment of those without verbal ability. The following studies suggest what to look for as indicators of pain in adults with IDs who cannot self-report.
To identify differences in the pain indicators used by nurses to assess pain in children and adults with severe versus profound IDs, a questionnaire consisting of 158 possible indicators of pain was completed by 109 nurses (Zwakhalen, van Dongen, Hamers, et al., 2004). More than 50% of the nurses found the following seven indicators to be very important: moaning during manipulation, crying during manipulation, painful facial expression during manipulation, swelling, screaming during manipulation, not using the affected body part, and moving the body in “a specific way of behaving.” Pain appeared to be assessed differently in those with severe versus profound IDs. Physiologic indicators, such as gasping for breath, vomiting, and turning red in the face, were scored somewhat higher by the group of nurses specializing in individuals with profound IDs (N = 47). Nurses specializing in the care of persons with severe IDs (N = 42) gave the highest score to “unusual way of crying.” Other indicators used by nurses specializing in the care of those with severe IDs included seeking comfort and being grouchy. (The remaining 53 nurses were taking care of both groups of patients.)
Interviews with eight nurses caring for patients with learning disabilities, who might be in pain but who could not communicate their feelings verbally, identified numerous behaviors they used as indicators of pain (Donovan, 2002). The ages of the patients were not given but some were probably adults because mention was made of nurses’ developing close relationships with patients over a long period of time. Change in facial expression was the most frequently cited nonverbal behavior indicative of pain or distress. Crying was also mentioned frequently. Other behaviors the nurses thought were possible indicators of pain were pacing, out-stretched arms, self-harm such as picking at the skin, aggressive behaviors such as biting or kicking others, pallor, altered rates of breathing, wincing, clenching teeth, and changes in activities such as avoiding weight bearing. One nurse described a patient with limited verbal ability that used the word arm for pain in any part of the body. These nurses emphasized the importance of caring for patients over time so that changes in behavior could be noted. Because changes in usual daily activities are often mentioned as clues to the presence of pain, it is noteworthy that these nurses mentioned that the patients were often determined to carry out normal activities even when seriously injured.
In one study, 40 adults with IDs who were undergoing intramuscular injections were studied to identify the usefulness of facial expressions in detecting pain (LaChapelle, Hadjistavropoulos, Craig, 1999). Only 65% of the subjects were able to provide self-reports. Observing the intensity of facial expressions, specifically brow lowering and chin raising, proved to be useful for assessing pain in these adults. Further, the frequency and intensity of facial expressions did not vary with level of cognitive functioning. In other words, persons with levels of ID that prevented self-report of pain showed facial expressions similar to those shown by persons with less intellectual impairment, providing further evidence against pain insensitivity in persons with IDs.
Somewhat contrary to that study, which found that facial expression did not vary with level of cognitive functioning, another study of adults with IDs found that facial expressions differed in individuals with mild to moderate cognitive impairment compared to those with severe to profound ID (Defrin, Lotan, Pick, 2006). In a study of 159 adults with varying levels of mental retardation and a control group of 38 with normal cognition, behavior was observed before and during the painful stimulus of a vaccination. During the vaccination, facial expression increased in those with mild to moderate IDs and in those without impairment. However, in those with severe to profound impairment, 47% to 50% exhibited stillness, or a “freezing reaction” (face and body not moving for several seconds), suggesting that pain assessment tools based only on facial expressions might contribute to the misconception that these patients are insensitive to pain. An increase in cognitive impairment was associated with a significant increase in freezing. In a few patients, freezing was accompanied by a full-blown smile, and some laughed, reactions that could easily be misinterpreted as insensitivity to pain. Freezing was exhibited by only 8% to 13% of individuals with mild to moderate IDs.
One reason for the differing conclusions in the studies described may be related to the sizes of the samples. It is possible that the study of 40 adults (LaChapelle, Hadjistavropoulos, Craig, 1999) did not include a large enough sample to allow for the detection of differences in behavior among the levels of ID, whereas the study of 159 adults with IDs (Defrin, Lotan, Pick, 2006) did allow for such detection.
Two behavioral pain scales were used in the study of 159 adults with ID: the Non-Communicating Children’s Pain Checklist-Revised (NCCPC-R) and the Facial Action Coding System (FACS) (Defrin, Lotan, Pick, 2006). The FACS is impractical to use in daily clinical practice because it involves coding video tapes. The NCCPC-R involves observing and rating behaviors (over a 2-hour period) and was found to be more sensitive to changes occurring in individuals at all levels of cognitive functioning, whereas the FACS was more sensitive to those with mild to moderate IDs. The NCCPC-R consists of 30 behaviors divided into seven categories: vocal, eating/sleeping, social, facial, activity, body/limb, and physiologic signs (Breau, McGrath, Camfield, et al., 2002). An observer scores the frequency of occurrence of the behaviors on a scale of 0 to 3: not at all, just a little, fairly often, and very often. The NCCPC-R was found to be reliable for measuring acute pain in adults with IDs.
One problem with the NCCPC-R is that it was validated by using a 2-hour observation period, which is impractical in many clinical settings. For that reason Breau, McGrath, and Zabalia (2006) recommend using the Non-Communicating Children’s Pain Checklist-Postoperative Version (NCCPC-PV) over a 10-minute period when a 2-hour period is not feasible. Research using the NCCPC-PV in adults with IDs and acute pain is needed. A variety of versions of the NCCPC are currently being researched in various populations with IDs. So far, the NCCPC-R and the NCCPC-PV have the greatest support for use with children because of their psychometric properties.
Assessment of location of pain may be difficult, especially in those with Down syndrome. In a study involving cold stimuli applied to the wrists and the temples of 26 individuals with Down syndrome, all were able to express themselves verbally but had some difficulty locating the site of the cold stimuli (Hennequin, Morin, Feine, 2000). In a later study of parents’ perceptions of pain in their children with Down syndrome (ages 1 to 39 years), the parents reported having greater difficulty in identifying locations of pain in their children with Down syndrome than in their children without Down syndrome (Hennequin, Faulks, Allison, 2003). Their abilities improved as the children grew older.
Two of the pain assessment tools that have been researched in adults with severe to profound mental retardation are the Pain and Discomfort Scale (PADS) (developed from the NCCPC-R) and the Pain Examination Procedure (PEP), and it is recommended that they be used together (Bodfish, Harper, Deacon, et al., 2006). These tools have acceptable levels of reliability and validity. The research into the development of these tools is extensive and is summarized in the chapter by Bodfish and others (2006) cited earlier. Studies indicate that the PADS is sensitive to nonverbal signs of pain in adults with severe IDs and is sensitive to everyday pain, acute pain responses, chronic pain, and effects of treatments to relieve pain. The authors found that administering the PADS plus the PEP takes trained personnel approximately 10 minutes. However, training for administering the PADS and PEP is extensive. A CD-ROM that includes a manual and workbook for the PADS and a DVD training video are available by calling 919-966-4896.
Other researchers disagree with the soundness of using the PADS in adults with IDs (Burkitt, Breau, Salsman, et al., 2009). They state that research to date is insufficient to support the general use of the PADS with adults with IDs. Indeed, further research is needed to identify pain assessment tools for this population. Meanwhile, the PADS plus the PEP may be a good alternative to using no tool at all.
Another potential tool, the Chronic Pain Scale for Nonverbal Adults with Intellectual Disabilities (CPS-NAID) (Form 4-2), is being researched (Burkitt, Breau, Salsman, et al., in press; reported in Breau, Burkett, 2009). This scale was arrived at by having two observers use the NCCPC-R to observe 16 nonverbal adults with IDs and chronic or recurrent pain conditions for a period of 5 minutes under conditions of pain and no pain. The observers also rated the pain using a VAS. Analyses indicated that six items should be removed from the NCCPC-R, resulting in a 24-item scale. This yielded 94% sensitivity to pain and 87% specificity for the presence of pain. These researchers concluded that the CPS-NAID has sound psychometric properties and can be used to assess chronic pain in adults with IDs. They think that this tool is superior to the original NCCPC-R, but further studies are needed to compare the two.
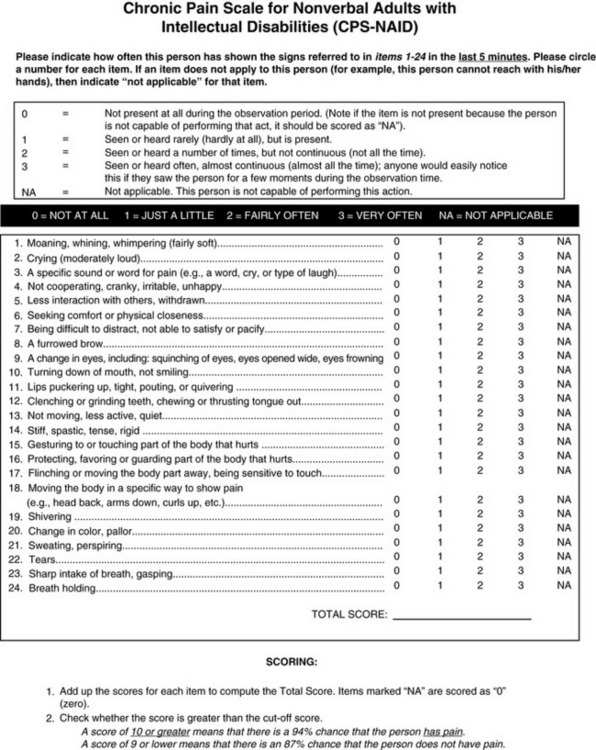
Form 4-2 As appears in Pasero, C., & McCaffery M. (2011). Pain assessment and pharmacologic management, p. 152, St. Louis, Mosby. From Burkitt, J. C., Breau, L. M., Salsman, S., et al. (2009). Pilot study of the feasibility of the non-communicating children’s pain checklist revised for pain assessment for adults with intellectual disabilities. J Pain Manage, 2(1).
An attempt was made to establish cutoff points for pain on the basis of the total score of the CPS-NAID. However, it was based on proxy pain reports. As mentioned previously, in the section on behavioral scales for cognitively impaired adults, given current knowledge, scores on behavioral pain scales do not equate with pain-intensity scores (Pasero, McCaffery, 2005). In most studies of pain assessment tools, there are low correlations between proxy judgments of pain severity and patients reports of severity. This raises concern about comparing pain-behavior scores with pain-intensity scores. If clinicians could reliably determine the intensity of pain in patients who cannot self-report, there would be no need for a behavioral assessment tool (Zwakhalen, Hamers, Abu-Saad, et al., 2006).
The hierarchy of pain assessment techniques (see Box 3-9, p. 123) is helpful in the assessment of patients with IDs. Following are the steps in that hierarchy, with comments related to assessing adults with IDs, starting with attempts to obtain self-reports.
• Try to obtain a self-report. A large majority of adults with IDs achieve a sixth grade level of academic skill and may be able to use some of the self-report scales mentioned in the section on self-report tools for adults who have difficulty with the commonly used tools such as the 0 to 10 NRS (pp. 92-95).
• Identify pathologic conditions and procedures that commonly cause pain, paying particular attention to those sources of pain characteristic of the type of ID. For example, individuals with cerebral palsy or autism often experience gastrointestinal discomfort such as gastroesophageal reflux. Individuals with Down syndrome are prone to developing leukemia, with all the usual pain associated with the disease and its treatment. Chronic pain is very common, one source being musculoskeletal pain in cerebral palsy.
• Observe behaviors indicative of pain. Changes in facial expression, especially brow lowering and chin raising, are probably the most frequently cited indicators of pain in persons with IDs, and they do not seem to vary with the level of cognitive functioning. Numerous other behaviors are cited in research into pain in adults with IDs. Some behaviors indicative of pain are unconventional, such as kicking others, picking at own skin, or freezing. Consider using the CPS-NAID (see Form 4-2) to assess chronic pain in adults with IDs.
• Physiologic indicators such as vital signs are not usually very helpful in assessing pain, but in the ID literature physiologic indicators often refer to the following behaviors, which may be helpful in assessing individuals with severe or profound IDs: gasping for breath, vomiting, and becoming red in the face.
• Obtaining information from caregivers who know the patient well, sometimes over a period of years, is a common way of assessing pain in individuals with IDs and is high on the hierarchy of pain assessment.
• Finally, when in doubt about the presence of pain or when wanting to confirm the suspicion of pain, an analgesic trial may be helpful. Assess for changes in behavior following use of an analgesic. (See Box 3-11, p. 126, for suggestions concerning analgesic trials.)
Patients Who Are Mentally Ill
Most of this part of the chapter focuses on assessment of pain in patients who have one of two mental disorders: (1) schizophrenia, in which decreased sensitivity to pain (hyppoalgesia) or impaired pain responsiveness has been documented in some patients (Singh, Giles, Nasrallah, 2006); and (2) posttraumatic stress disorder (PTSD), in which both decreased and increased sensitivity to pain have been reported(Geuze, Westenberg, Jochims, et al., 2007). The occurrence and the perception of pain in persons who have these two mental disorders are examined. Depression and anxiety in relation to pain are discussed briefly in Chapter 2 (pp. 31-32).
Schizophrenia
The extent of apparent insensitivity to pain in patients with schizophrenia can be extreme, as revealed in some clinical case reports. One such example is a patient with diagnosed schizophrenia for 20 years who burned his arm so severely it had to be amputated (Virit, Savas, Altindag, 2008). The patient explained, “I put my hand on the burning flames of the LPG (liquefied petroleum gas) cylinder in order to get warm…. I did not feel any pain” (p. 384).
Insensitivity to pain by schizophrenic patients has been suggested for more than 100 years, and failure to respond to pain is clearly associated with increased morbidity and mortality rates. A literature review of physical diseases in patients with schizophrenia documented the long-held observation that many types of physical illness, such as CV diseases, HIV infections, and dental problems, are more common in these patients than in the general population (Leucht, Burkard, Henderson, et al., 2007). This is thought to be caused in part by the stigma attached to patients with schizophrenia; it leads to undertreatment. Further, the tendency to ascribe patients’ expressions of physical illness to the mental illness sometimes leads to missing a medical diagnosis. In addition, patients themselves simply fail to report symptoms of physical illness. It appears that patients with schizophrenia who fail to respond to pain also seek medical care at later stages of disease. For example, in a small study of 55 patients with schizophrenia undergoing appendectomy, 34 appendixes were perforated and 9 were gangrenous (Cooke, Magas, Virgo, et al., 2007).
In a literature search for studies of postoperative complications in seriously mentally ill patients, 10 studies relating to patients with schizophrenia were identified (Copeland, Zeber, Pugh, et al., 2008). Of the studies, 9 had fewer than 100 patients, but one large retrospective study included 466 patients with schizophrenia and 338,257 patients without schizophrenia (Daumit, Pronovost, Anthony, et al., 2006). Those with schizophrenia had higher rates of postoperative complications, such as respiratory failure and deep-vein thrombosis, than did patients without schizophrenia. Again, it appears that patients with schizophrenia are insensitive to pain and seek medical care at later stages of disease.
One small study of 50 schizophrenic patients and 25 controls found that those with schizophrenia reported significantly lower pain intensity postoperatively and consumed 60% less analgesic medication (Kudoh, Ishihara, Matsuki, 2000). This shows that schizophrenic patients seem to be less responsive to postoperative pain. Nevertheless, postoperative pain in schizophrenic patients is a risk factor for postoperative confusion (Kudoh, Takahira, Katagal, et al., 2002), which occurs more commonly in schizophrenic patients than in those without schizophrenia. Although pain scores do not reflect very much conscious awareness of pain, adequate postoperative pain relief is necessary to prevent postoperative confusion (Kudoh, 2005).
The lack of responsiveness to pain of schizophrenic patients is poorly understood. One way of comprehending the denial of pain and the low pain ratings in the presence of noxious stimuli that ordinarily cause severe pain is that the mental illness overwhelms the patient’s thought processes (e.g., hallucinations, intrusive thoughts), and the patient simply does not focus on pain. Patients with schizophrenia also may be preoccupied with their psychotic symptoms and may suffer from fatigue and a lack of drive, consequently failing to seek help for physical symptoms (Leucht, Burkard, Henderson, et al., 2007). Some studies find that patients with schizophrenia, when compared to healthy controls, have higher sensory perception thresholds, such as the perception of warmth and the onset of thermal pain. This trait seems to be caused by abnormalities in information processing (Jochum, Letzsch, Greiner, et al., 2006). The tests for these sensations required sustained attention, and the researchers noted that the patients had difficulty focusing. A review of 12 studies of individuals with schizophrenia revealed that their responses to experimental pain were diminished (Potvin, Marchand, 2008).
Antipsychotic medications have sometimes been thought to have analgesic effects, and that could help explain seeming decreased sensitivity to pain in individuals with schizophrenia. However, in the previously mentioned study, antipsychotic medications did not alter the finding of high sensory thresholds. Drug-free patients also had hypoalgesic responses. Therefore, hypoalgesia could not be explained solely by the effects of antipsychotic drugs (Potvin, Marchand, 2008).
Another explanation for hypoalgesia is derived from a review of the literature that suggests that seeming pain insensitivity may be a familial trait rather than a function of the psychotic state (Singh, Giles, Nasrallah, 2006). One study compared the responses to finger pressure by college students who did not have diagnoses of schizophrenia, but who had relatives who did, with the responses of students with no family history of schizophrenia (Hooley, Delgado, 2001). Pain threshold and tolerance were higher in the healthy adults who had relatives diagnosed with schizophrenia, suggesting that pain insensitivity may be a familial trait.
To summarize, apparent insensitivity to pain in patients with schizophrenia may reflect a true lack of pain sensitivity or may be caused by other factors such as failure to focus on pain or respond to pain in the presence of a psychotic state. Unfortunately, a review of the literature did not produce sufficient evidence of whether hypoalgesia is present in schizophrenic patients when they are stable as well as during acute phases of psychosis (Potvin, Marchand, 2008).
More recently, researchers have questioned whether some patients with schizophrenia are insensitive to pain or just seem insensitive to pain because of communication impairments that accompany schizophrenia (Bonnot, Anderson, Cohen, et al., 2009). A review of the scientific literature on pain in patients with schizophrenia yielded 431 articles, of which 57 were considered relevant to examining sensitivity to pain. These included 9 case reports, 23 clinical studies, 20 experimental research, and 5 review articles. Only 1 experimental study used a neurophysiologic measure of pain reactivity, and it showed a normal pain threshold in schizophrenia. Misconceptions about patients with schizophrenia being insensitive to pain are probably derived from observations that many of these patients show reduced pain reactivity in the form of decreased behavioral responses and self-reported pain.
How can pain be assessed in patients who do not report pain or report low intensities of pain? A survey was conducted of 74 staff members of a geriatric psychiatry service to explore pain assessment and management issues in that population (Stolee, Hillier, Esbaugh, et al., 2007). Part of the survey consisted of a list of 37 potential indicators of pain, and staff were asked to rate the extent to which they agreed or disagreed that these could be indicators of pain. Those pain indicators that were endorsed most often were facial grimacing or wincing, verbal pain complaints, diagnoses of painful conditions (such as arthritis), body positioning, and groaning or moaning. The accuracy of these indicators of pain was not assessed. Also, in this population of patients both mental illness and cognitive impairment existed, so the extent to which each condition influenced the perception of pain is unknown. However, the majority of respondents (more than 80%) were not so much interested in specific behaviors but rather in identifying changes in usual behaviors as indicators of pain in patients with mental illness. This preliminary research provides a clue to the assessment of pain in patients with schizophrenia, namely, notation of changes in the patients’ usual behaviors.
Many behaviors, such as personality changes, sleep disturbances, and fatigue, may occur as the result of either pain or exacerbations in mental illness (McCaffery, Pasero, 2001; Rutledge, Donaldson, 1998). Because apparent insensitivity to pain and impaired pain responsiveness are common in mental illness, it is recommended that such behaviors be interpreted as potential indicators of pain and that the pain be treated first, before attempting to determine whether the behaviors are a result of a mental disorder.
The finding of the appearance of insensitivity to pain in many, but not all, patients with schizophrenia presents clinicians with assessment challenges. In some ways assessment is comparable to that in previous recommendations that pain in nonverbal patients and those unable to respond with behavioral indicators should be assumed and treated when usually painful underlying pathologies or procedures are present. Because pain is rarely experienced as a hallucination (Watson, Chandarana, Merskey, 1981), any report of pain should always be taken seriously.
The assessment of pain in patients with schizophrenia should not differ from the assessment of pain in mentally healthy individuals. For example, if an institution’s policy recommends assessment of pain during every shift, it should also be done on units caring for patients with mental illness, bearing in mind that a patient with schizophrenia may deny pain, even in the presence of a disease known to be painful such as an infected appendix.
For the assessment of pain in adult patients with schizophrenia, information from the previous discussion can be applied to the hierarchy-of-pain assessment techniques, Box 3-9 (p. 123), as follows:
• As in all patients, give priority to obtaining self-reports of pain. Beware of ascribing reports of pain to the mental illness. If reports of pain are ignored, diagnosis of physical illness may be delayed, subjecting a patient to complications such as a ruptured appendix. Pain is rarely a hallucination. In fact, clinicians should maintain a high index of suspicion of pain in these patients because they are known for late reporting of the symptoms of physical illnesses.
In schizophrenic patients with the intellectual capacity for self-reporting of pain, reports of no pain or mild pain may indicate lack of responsiveness to pain, not lack of pain. However, each patient should be assessed for a history of lack of responsiveness to pain.
• Identify pathologic conditions and procedures that commonly cause pain such as surgery. Consider obtaining a consultation for assistance in diagnosing a physical illness. Clinicians skilled in the care of patients with mental illness may not be as skilled in the detection of physical illness.
• Observe behaviors indicative of pain. Consider the possibility that pain is present when changes in patients’ usual behaviors occur, such as increased fatigue, sleep disturbances, postoperative confusion, or personality changes. In one study, clinicians identified facial grimacing or wincing and groaning or moaning as common indicators of pain. Studies have shown that clinicians value changes in usual behavior as the most important indicators of pain. “Absence of pain reactivity does not mean absence of pain sensitivity” (Bonnot, Anderson, Cohen, 2009, p. 246).
• Obtain information from caregivers and medical records regarding past or current pain, potential causes of pain, and any indications of lack of responsiveness to pain.
• Finally, when in doubt about the presence of pain or when wanting to confirm the suspicion of pain, an analgesic trial may be helpful. Behavior changes that could be caused by either pain or exacerbation of mental illness should first be treated as pain before initiating treatment for the mental illness. Assessment of behavior after a trial use of analgesic may help to determine whether changes in behavior are due to a physical illness or to the mental illness.
In conclusion, when addressing the problem of pain in patients with serious mental illness, two important considerations are (1) patients with chronic pain who also have mental disorders have a poorer quality of life than those without mental disorders; and (2) better treatment outcomes are likely when both pain and the mental illness are treated than when only one or the other is treated (Nicholas, 2007). Thus, whether the mental illness causes pain or is a consequence of pain, both pain and the mental illness should be treated.
Posttraumatic Stress Disorder
Posttraumatic stress disorder (PTSD) is an anxiety disorder that can occur following an extremely traumatic event that involves being threatened by or witness to a situation that involves death or injury (APA, 1994). Traumatic events may include military combat, physical attacks, torture, or severe automobile accidents. The prevalence rates of individuals’ at risk for developing PTSD, such as combat veterans and victims of criminal violence, range from 3% to 58%.
Soldiers returning from the Iraqi war report very high levels of combat exposure, with 90% describing being shot at and a high percentage report handling dead bodies, knowing someone who was injured or killed, or killing an enemy combatant (Hoge, Castro, Messer, et al., 2004) Research has shown that soldiers with extensive combat exposure are four times more likely to develop PTSD than those with low combat exposure (Kulka, Schlenger, Fairbank, et al., 1990). In a convenience sample of Iraqi veterans, 71% had pain, 57% had PTSD, and 64% had mild traumatic brain injury; 36% had all three (Tan, Fink, Dao, et al., 2009). This population also displayed compromised autonomic nervous system function, suggesting that autonomic abnormality may contribute to, result from, or be a useful marker of symptoms of pain in traumatic brain injury and PTSD.
Soon after a traumatic event, “psychic numbing” or “emotional anesthesia” occurs; it is a symptom of decreased responsiveness to the external world (APA, 1994). Symptoms of PTSD usually begin within the first 3 months of the precipitating event, and approximately half of patients experiencing such symptoms completely recover within 3 months, whereas many others have persistent symptoms for longer than 12 months. PTSD is characterized by distress or impairment in functioning, socially or occupationally; persistent anxiety; sleep disturbances; and recurrent nightmares about the traumatic event. The patient may develop hypervigilence, irritability, exaggerated startle responses; and difficulty concentrating. When persons are exposed to events that are reminders of the original traumatic event, extreme psychologic distress or physiologic reactions commonly occur. Usually, they make deliberate efforts to avoid all reminders.
The prevalence of PTSD has been estimated to be between 6% and 9% or more in the general population of the United States (Breslau, 2001). Unfortunately, PTSD is much more common and is often underdiagnosed in primary care and mental health settings. For example, in a study of 295 psychiatric outpatients, 46% had PTSD but only 7% were diagnosed as such by physicians (Villano, Rosenblum, Magura, et al., 2007).
The presence of high levels of pain during the peritrauma period has been associated with increased risk for the development of PTSD (Norman, Stein, Dimsdale, et al., 2008). Related to this finding is a study of 24 children admitted to the hospital for acute burns (Saxe, Stoddard, Courtney, et al., 2001). The children were assessed for PTSD while in the hospital and 6 months after discharge. All children received morphine, but those who received higher doses of morphine showed greater reduction in PTSD symptoms over 6 months. It is interesting that in another study, of 147 soldiers in Iraq who were treated in a military treatment center for burns and had had at least one surgery, 119 who had received ketamine during surgery were compared with 28 who had not (McGhee, Maani, Garza, et al., 2008). The prevalence of PTSD was 27% in those who had received ketamine and 46% in those who had not received ketamine despite the fact that their burns were larger and their injury severity scores higher. A pilot study of placebo versus 40 mg of propranolol (Inderal) four times a day following acute, psychologically traumatic events found that propranolol had a preventive effect on subsequent PTSD (Pitman, Sanders, Zusman, et al., 2002). This offers the hope that ketamine, propranolol, morphine, or perhaps some other medications can be used soon after trauma or injury to prevent or reduce the symptoms of PTSD.
When PTSD involves a physically painful injury, it has been suggested that pain may be a flashback to the original traumatic injury (Asmundson, Coons, Taylor, et al., 2002). Therefore, it may be possible to prevent or minimize the development of pain-related PTSD flashbacks by assessing pain and treating it with analgesics at an early stage. Another small study (N = 6) of veterans with both chronic pain and PTSD examined the effects of using components of cognitive processing therapy for PTSD and cognitive behavioral therapy for chronic pain (Otis, Keane, Kerns, et al., 2009). A 12-session integrated treatment for veterans with both chronic pain and PTSD was developed and used by the participants. Although half the participants withdrew from the study, participants appeared to benefit from the treatment, and further research is being conducted to evaluate the efficacy of this approach.
PTSD and chronic pain often occur together and are frequently observed within the Department of Veterans Affairs health care system (Otis, Keane, Kerns, 2003). When patients with PTSD are being assessed, chronic pain is commonly identified, regardless of the nature of the traumatic experience, and vice versa; when patients with chronic pain are being assessed, PTSD is commonly found too (Asmundson, Coons, Taylor, et al., 2002; Schwartz, Bradley, Penza, et al., 2006). In a study of 295 psychiatric outpatients, 24% had both PTSD and chronic severe pain (Villano, Rosenblum, Magura, et al., 2007). In another study of 85 veterans seeking treatment for PTSD, 66% had chronic pain diagnoses (Shipherd, Keyes, Jovanovic, et al., 2007).
Unfortunately, because of the stigma placed on seeking psychiatric help, most veterans do not seek help from this source. If they develop painful conditions, they are more likely to seek help from pain clinics (DeCarvalho, Whealin, 2006). Because PTSD may hamper treatment for chronic pain, pain specialists should know about the possibility that PTSD can coexist with chronic pain and should watch for symptoms, such as being easily startled, hypervigilence, isolation, and use of alcohol or other substances to deal with PTSD or pain. An appropriate screening tool for PTSD should be used.
Studies have reported both increased and decreased sensitivity to pain in individuals with PTSD (Geuze, Westenberg, Jochims, et al., 2007). Decreased sensitivity to pain in patients with PTSD has been documented in only a few experimental studies. In a small study of 12 veterans with PTSD and 12 without PTSD, neuroimaging was done while each group was exposed to heat stimuli (Geuze, Westenberg, Jochims, et al., 2007). Subjects rated pain using an NRS of 0 to 100. Compared to controls, individuals with PTSD rated the heat stimuli as being significantly less painful, and neuroimaging revealed an analgesic response and altered pain processing in certain brain areas.
In another small, double-blind, crossover study, eight Vietnam veterans with PTSD and a control group of eight veterans without PTSD, matched for combat severity, watched a videotape of dramatized combat (Pitman, van der Kolk, Orr, et al., 1990). None were receiving opioid analgesics. The tape was viewed by each group while under the effects of naloxone hydrochloride or placebo. In the placebo condition, those with PTSD experienced a 30% decrease in pain ratings, but no decreases in pain ratings occurred in the naloxone condition, which suggests that naloxone blocked endogenous opioid analgesia. The veterans without PTSD showed no decrease in pain ratings under either condition. It appeared that veterans with PTSD had opioid-mediated analgesic responses (endogenous opioids). Thus, decreased sensitivity to pain in patients with PTSD appears to occur briefly when a patient is exposed to acute pain or something that triggers memories of or reminds the patient of the original trauma.
Patients with PSTD and chronic pain tend to have increased sensitivity to pain. The mechanisms most often proposed to understand the cooccurrence of chronic pain and PTSD are shared vulnerability and mutual maintenance (Asmundson, Coons, Taylor, et al., 2002). For example, in terms of the concept of shared vulnerability, it seems possible that anxiety sensitivity is shared by patients with both PTSD and chronic pain. With respect to the concept of mutual maintenance, it seems possible that certain cognitive or affective components of chronic pain exacerbate symptoms associated with PTSD and that certain affective and behavioral components of PTSD exacerbate symptoms associated with chronic pain.
Analysis of pain intensity was compared in three groups, all members of which had chronic pain; but one group had accident-related pain and high PTSD symptoms, one group had accident-related pain and few or no symptoms of PTSD, and the third group had pain that was not accident related and had no symptoms of PTSD (Geisser, Roth, Bachman, et al., 1996). Patients with accident-related pain and strong PTSD symptoms reported higher levels of pain than did the other two groups.
To examine sensitivity to pain, three groups of subjects—32 outpatients with combat- and terror-related PTSD, 29 outpatients with anxiety disorder, and 20 healthy individuals—were compared (Defrin, Ginzburg, Solomon, et al., 2008). The first two groups were randomly selected from an outpatient psychiatric clinic and contacted by telephone to request participation in the study. Higher rates of chronic pain, more intense chronic pain, and more painful body regions were found in the subjects with PTSD than in the other two groups. Further, the greater the severity of PTSD, the greater the severity of chronic pain.
A review of the literature also confirms this finding (Smith, Egert, Winkel, et al., 2002). In addition to being at high risk for PTSD, individuals with persistent pain who do develop PTSD are also likely to experience more intense and pervasive pain than those who do not develop PTSD.
In the study by Defrin and others (2008), differences emerged when the groups were tested for measurement of warmth, cold, light touch, and heat-pain thresholds and for responses to acute suprathreshold heat and mechanical stimuli. Although the patients with PTSD were less sensitive to thresholds for warmth, cold, touch, and heat-pain than the other two groups, they found the suprathreshold heat and mechanical stimuli much more intense than did the other groups. The researchers offered several possible explanations for this paradoxical finding, such as altered sensory processing in subjects with PTSD, and recommended further research. Other researchers suggest that the increased sensitivity to suprathreshold pain may reflect in part that the subjects were exposed to this type of pain for a longer time (repeated painful stimuli) in the experimental situation than when tested for the heat-pain threshold in which they were simply asked to identify the point at which the heat stimulus became painful (Asmundson, Katz, 2008).
A study of 145 individuals with diagnoses of HIV/AIDS who also had persistent pain was conducted to examine the relationship between PTSD and pain (Smith, Egert, Winkel, et al., 2002). In this sample, approximately half (51.8%) had diagnoses of PTSD. Individuals with PTSD reported higher levels of pain at its worst, worse physical and mental health, and higher levels of pain that interfered with daily activities and mood than did those without PTSD. One obvious implication of the study is that individuals with HIV should be assessed for symptoms of PTSD.
A study of 93 patients diagnosed with the chronic pain condition of fibromyalgia syndrome (FMS) was undertaken to determine the prevalence of PTSD-like symptoms (not the diagnostic category of PTSD) and to evaluate the relationship between PTSD symptoms, FMS symptoms, and disability (Sherman, Turk, Okifuji, 2000). More than half (56%) of the sample reported significant levels of PTSD-like symptoms, and this group reported significantly greater levels of pain, interference with life, emotional distress, difficulty adapting to FMS, depressive symptoms, and disability than did individuals without clinically significant levels of PTSD symptoms. Another study of PTSD and FMS yielded similar results. The frequency of occurrence of PTSD was examined in 77 patients with FMS, and 57% were found to have significant levels of PTSD symptoms (Cohen, Neumann, Haiman, et al., 2002). The most frequently reported traumatic event associated with PTSD was the unexpected death of a loved one (as is true for the other patients with PTSD). These patients did not regard their FMS as a traumatic event.
Clearly PTSD symptomatology is prevalent in individuals with FMS and probably has a negative impact on coping with FMS, suggesting that interventions for the treatment of PTSD, such as stress management, may be helpful for patients with FMS. These interventions overlap with interventions for persistent pain and probably can be incorporated easily in a pain management program for patients with FMS.
The relationship between analgesic use and PTSD was studied in 173 African-Americans who were outpatients at a mental health center (Schwartz, Bradley, Penza, et al., 2006). Those with PTSD, 43.5% of the sample, used significantly more opioid and nonopioid analgesic medications than did those who did not have PTSD. Of the patients with PTSD, 68% were prescribed analgesics, and 59% received opioids, whereas only 51% of patients without PTSD received analgesics, 42% of them receiving opioids. (Pain ratings were not reported in this article.) Patients receiving opioids had the highest severity of PTSD symptoms. PTSD was notably underdiagnosed in this group of patients; only 6.5% of the total sample had documented diagnoses of PTSD.
Chronic pain and PTSD often coexist, and PTSD exacerbates chronic pain but is frequently underdiagnosed. It is therefore critical to assess patients with chronic pain for the symptoms of PTSD and to assess patients with PTSD for chronic pain. This makes it possible to recognize and arrange for both the pain and the PTSD to be addressed. A convenient screening tool for PTSD in the primary care setting is the Primary Care PTSD (PC-PTSD) screen shown in Form 4-3 (Prins, Quimette, Kimerling, et al., 2003). It is psychometrically sound, identifying PTSD in 78% of 188 men and women in a primary care setting at the Veterans Administration. It is brief, written at an eighth grade reading level, and easy to score. It consists of four yes/no questions, and a positive answer to two or more items suggests the need for further assessment of PTSD.
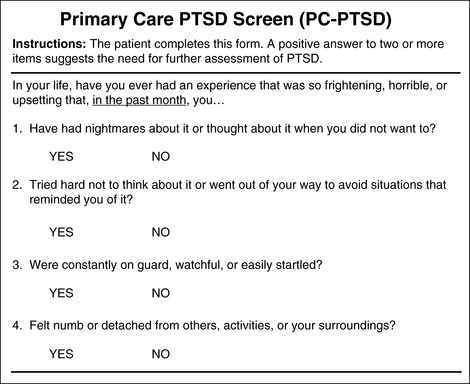
Form 4-3 As appears in Pasero, C., & McCaffery, M. (2011). Pain assessment and pharmacologic management, p. 158, St. Louis, Mosby. Used with permission. From Prins, A., Ouimette, P., Kimerling, R., et al. (2003). The primary care PTSD screen (PC-PTSD): Development and operating characteristics. Prim Care Psychiat, 9(1), 9-14. May be duplicated for use in clinical practice.
Based on the previous discussion of PTSD, suggestions for assessment of pain and PTSD, along with possible prevention of or decrease in symptoms of PTSD are as follows:
• Obtain self-reports of pain as indicated in the hierarchy of pain assessment techniques (see Box 3-9, p. 123). PTSD itself does not seem to hinder the ability to provide a self-report of pain, but chronic pain is often underdiagnosed, although it is common in patients with PTSD. Other steps in the hierarchy of pain assessment should be followed if self-report is hampered by some other condition such as brain injury.
• At times of extremely traumatic events, assess for pain and, if present, treat it aggressively in the hope of preventing or decreasing the symptoms of PTSD. If individuals are unable to respond at the time of the traumatic event and physical stimuli suggest that pain is present, assume that pain is present and treat it aggressively as a prophylactic measure
• Clinicians treating patients with PTSD should assess for the cooccurrence of chronic pain.
• Clinicians treating patients with chronic pain, especially soldiers with long exposure to combat, should assess for the cooccurrence of PTSD or PTSD symptomatology. Watch for symptoms such as hypervigilence, isolation, being easily startled, and use of alcohol and other drugs to cope with pain or PTSD. A useful screening tool is PC-PTSD (Form 4-3).
• If a patient with PTSD also has pain, recognize the possibility of increased sensitivity to pain, higher pain ratings, and the need for more analgesia than required by other patients with comparable injuries but no PTSD.
• Be alert to diagnoses such as the HIV and advanced cancer that expose individuals to a number of psychologically traumatic events, such as receiving the diagnosis, disclosing the diagnosis to others and, in some cases such as infection by HIV, infecting significant others. Assess for symptoms of PTSD and chronic pain.
To summarize the implications of these assessment suggestions, when PTSD or PTSD symptomatology and pain cooccur, a cognitive-behavioral therapy program is appropriate but will have to be modified to address both problems (Asmundson, Coons, Taylor, et al., 2002). No data yet exist to suggest that either PTSD or chronic pain should be treated first when they cooccur. Thus, treating the conditions simultaneously seems indicated.
Specific treatment of coexisting pain and PTSD is beyond the scope of this chapter, but DeCarvalho and Whealin (2006) suggest additional practical ideas that can be quickly initiated after PTSD is suspected. Educate the patient about the symptoms of PTSD, and provide written materials when possible. One short patient education sheet about chronic pain and PTSD can be obtained from http://www.ncptsd.va.gov/ncmain/ncdocs/fact_shts/fs_chronic_pain_and_patients.html?opm=1&rr=rr102&srt=d&echorr=true. When possible, give returning service members with pain and possible PTSD the option of attending a military or a civilian pain clinic. This may reduce their fear of stigmatization. Relaxation therapy may be helpful, although some patients find that it allows for disturbing intrusive thoughts.
Cultural Considerations
As racial and cultural diversity increases in the United States, clinicians are increasingly likely to care for patients from backgrounds quite different from their own. There is little doubt that various ethnic groups express pain and suffering differently. The same can be said of people in general. Fortunately, many of the pain assessment tools used in English in the United States can be used successfully in other cultures if they are translated into the appropriate languages. Culture affects behavioral responses to pain and treatment preferences. However, patients of many different cultures may be assessed using similar pain assessment tools, and the findings will have similar meanings across cultures. Following is a reminder of some of the assessment tools discussed in this section that have been used in other cultures.
This section provides translations of the horizontal 0 to 10 NRS (see Figure 3-6, pp. 60-65); the Wong-Baker FACES Pain Rating Scale (see Figure 3-7, pp. 66-67); and the Faces Pain Scale-Revised (FPS-R) (see Figure 3-8, pp. 69-85) into numerous languages. However, some minor changes may be made when necessary. For example, a study of Chinese patients’ responses to a VAS revealed that it was a suitable method for assessing pain intensity but that the vertical presentation was more quickly understood than the horizontal line, probably because traditionally, the Chinese read vertically downward and from right to left, not left to right (Aun, Lam Collett, 1986). If difficulties are encountered with the horizontal Chinese translation in Figure 3-6 (pp. 60-65), readers are encouraged to make a vertical presentation with 10 at the top.
The reliability and validity of the FPS-R has been established in several different cultural groups. In one study, the reliability and validity of the FPS-R was established in cognitively impaired and cognitively intact older adults who were Asian, African-American, and Hispanic (Ware, Epps, Herr, et al., 2006). The last two groups preferred the FPS-R. This pain rating scale has also been found to be reliable and valid in cognitively intact elderly Spanish patients (Miro, Huguet, Nieto, et al., 2005) and in cognitively intact Chinese adults ranging in age from 18 to 78 years (Li, Liu, Herr, 2007). In the latter group almost half preferred the FPS-R, and the FPS-R had a low error rate. The FPS-R is available in approximately 30 languages (see Figure 3-8, pp. 69-85).
The Brief Pain Inventory (BPI) (see Form 3-2, p. 53) has also consistently demonstrated a high degree of reliability and validity when administered to cancer patients from countries other than the United States. The BPI has been translated into a number of languages, including Japanese (Uki, Mendoza, Cleeland, et al., 1998); Vietnamese (Cleeland, Ladinsky, Serlin et al., 1988); Chinese (Wang, Mendoza, Gao, et al., 1996); the Philippine language (Cleeland, Nakamura, Mendoza, et al., 1996); Russian (Kalyadina, Ionova, Ivanova, et al., 2008); and French (Serlin, Mendoza, Nakamura, et al., 1995). Using the BPI, several studies of various cultures (e.g., Kalyadina, Ionova, Ivanova, et al., 2008) have found that on a 0 to 10 pain rating scale, pain ratings of 5 or more interfere significantly with daily function (Cleeland, 1984; Cleeland, Gonin, Hatfield, 1994; Serlin, Mendoza, Nakamura, et al., 1995). Although behavioral expression of pain may differ considerably among cultures, pain ratings and the impact of pain on quality of life appear to be very similar.
Some of the behavioral tools developed for assessment of pain in nonverbal patients with dementia have been translated into other languages and used in other countries, suggesting that many behaviors indicative of pain are similar in different cultures. The Pain Assessment in Advanced Dementia (PAINAD) (see Form 3-15, pp. 132-134) has been translated and tested in five languages: English (DeWaters, Faut-Callahan, McCann, et al., 2008); Italian (Costardi, Rozzini, Costanzi, et al., 2007); Belgian (Dutch translation) (van Iersel, Timmerman, Mullie, 2006); the Netherlands (Dutch translation) (Zwakhalen, Hamers, Abu-Saad, et al., 2006); and German (Schuler, Becker, Kaspar, et al., 2007). The Doloplus 2 was developed in the French language and later translated into English for further testing (Lefebvre-Chapiro, The Doloplus Group, 2001). The Elderly Pain Caring Assessment 2 (EPCA-2) was developed by a French team and is being validated in English-translated samples (Morello, Jean, Alix, et al., 2007).
The usefulness of basic pain assessment tools across cultures is not sufficient to ensure a high quality of care for patients of various cultural backgrounds. Clinicians must be particularly mindful of the fact that the more difference there is between a patient and a clinician, the more difficult it is for the clinician to assess and treat the patient. As an example, in a study of 50 hospitalized patients who spoke Arabic, a comparison of the patients’ pain ratings with those of nurses who shared the mother tongue and those who did not revealed that nurses sharing the mother tongue with patients were much more likely to provide pain ratings similar to those of their patients than were the other nurses (Harrison, Busabir, Al-Kaabi, et al., 1996).
Another study of 32 nurses’ responses to 60 patients with cholecystectomy pain compared the nurses’ estimates of pain to those of each of two ethnic groups, Anglo-American women and Mexican-American women (Calvillo, Flaskerud, 1993). The majority of nurses (45.8%) were Anglo-American and the remainder were a mix of ethnicities. Although there were no significant differences between the two ethnic groups’ self-reports of pain, nurses assigned more pain to Anglo-Americans than to Mexican-Americans. Clearly, clinicians must recognize the possibility of this problem and guard against it by always obtaining and acting on a patient’s self-report of pain, not their own beliefs about a patient’s pain.
In discussing pain it is helpful to know that three terms—pain, hurt, and ache—seem to be used similarly across cultures to describe pain intensity. The term pain is usually used for the most intense pain, followed by hurt; ache means the least pain. This was shown to be true in a study of Hispanics, American Indians, blacks, and whites (Gaston-Johansson, Albert, Fagan, et al., 1990). These terms are also basic words used in the Swedish language to describe pain intensity (Gaston-Johansson, 1984), as well as in the Dutch language (Francke, Theeuwen, 1994). So it is clear that people of diverse ethnic, cultural, and educational backgrounds use similar words to describe the intensity of pain.
The Chinese population has been studied with regard to concepts of pain, and some information is applicable to pain assessment in that group (Chen, Miaskowski, Dodd, et al., 2008). The Chinese culture includes Confucianism, which maintains that pain is an essential element of life and is a trial or a sacrifice that must be endured. A Confucian would rather endure pain and not report it to a clinician. Confucians tend to report pain only when it is unbearable. However, sometimes a patient will report pain to a close family member and ask him or her to report the pain to the clinician. Note that on the hierarchy of pain assessment, one of the steps is to ask people familiar with a patient whether they think the patient has pain. Their reports of pain may be more accurate than a patient’s denial of pain. A clinician should also explore the beliefs of a Chinese patient and, when a patient wishes to bear pain, explain the harmful effects of pain. Ultimately, a clinician may have to support a patient’s wish to endure pain. A clinician should also assess how a patient wishes to handle pain. A patient may desire Chinese medicines or acupuncture instead of analgesics. However, care must be taken to realize that not all Chinese patients share the beliefs described. Believing that individuals within the same cultural group are alike is a misconception.
The Chinese culture is not the only one in which enduring pain may be regarded as valuable (Mazanee, Tyler, 2003). In fact, many clinicians reading this probably have also noted in some white Americans the same desire to bear pain and not report it until it is severe. Overall, patients of various cultures may share more similarities than differences. To enhance cultural sensitivity, clinicians must work closely with patients and their families to identify mutual goals of care that take into account different preferences, such as the relative values placed on being free of pain versus being fully alert. Whenever possible, health care practices that are desired by a patient or are specific to the patient’s cultural group should be included in patient care if he or she wishes. Teaching materials in patients’ own languages may be secured or developed, and cultural resources such as interpreters may be useful (McGuire, Sheidler, 1993).
Some have urged nurses to become “culturally competent,” but we share the belief of others who maintain that it is really a matter of nursing competence—that is, encouraging nurses to be sensitive to the differences among all individuals (Dreher, MacNaughton, 2002). Understanding the characteristics of a culture may be helpful in understanding some of the behaviors seen in patients, but that knowledge does not tell us what to expect of individual patients.
Far too many cultures are represented in the patient population to allow consideration of all of them in this section. Further, pain assessment has not been studied in all cultures. Nevertheless, clinicians are encouraged to seek information about the specific cultural backgrounds of their patients through exploration with the patients and through publications. Information about the beliefs concerning health and illness of 17 different cultures is available in Giger and Davidhizar (2008). Care of oncology patients of Hispanic and Japanese-American backgrounds is discussed by Kagawa-Singer (1987). Other articles address pain in Arab-Americans (Reizien, Meleis, 1986); Chinese-Americans (Louie, 1985); and African-Americans (Capers, 1985).
As discussed previously in this chapter, clinicians must realize that despite their assessments of pain, those assessments might not be acted upon appropriately because of hidden biases such as patients’ ethnicities. A few salient points are worth emphasizing. One example that represents several similar studies showed that in 217 patients, black patients, as compared with white patients, were undertreated for pain in the emergency department, despite similar notations of pain reports in the medical records (Todd, Deaton, D’Adamo, et al., 2000). A few years later, in a much larger study of patients with pain (N = 374,891) admitted to the emergency department, white patients were more likely to receive opioids than were black, Hispanic, Asians, and other patients (Pletcher, Kertesz, Kohn, et al., 2008). These are probably “hidden” biases; that is, ones that we are not aware of consciously. Those are the ones that are most difficult to recognize and stop before we undertreat patients.
Section II Conclusion
SECTION II is the most important section in this book. The essential message about pain assessment is easy to summarize: Ask patients about their pain; accept and respect what they say; intervene to relieve their pain according to their pain management goals; and, once intervention has been implemented, ask them again about their pain to evaluate the intervention. It is a circle of assessment, intervention, and reassessment.
It would be unfair to lead the reader to believe that pain assessment will automatically result in improved pain care. Studies described in previous chapters have given examples of how assessment results in better pain management, but this is not always true. Unfortunately, even when seemingly appropriate assessment is performed and appropriate steps are initiated, the outcome for pain care may not be improved. For example, to determine whether systematic pain assessment information would alter a physician’s clinical practice, a group of case coordinators used a pain assessment battery to assess a group of elderly patients and reported the results to physicians in the experimental group (Hadjistavropoulos, MacNab, Lints-Martindale, et al., 2009). Physician action in the experimental group was compared to a control group of 56. There were no significant differences between experimental and control groups with respect to medications prescribed or overall pain intensity.
On the other hand, sometimes assessment seems to improve patient care. In a study of mechanically ventilated patients who received analgesia on day 2 of their stay in the ICU, investigators compared those who were assessed for pain (513 patients) with those who were not assessed for pain (631 patients) (Payen, Bosson, Chanques, et al., 2009). Those who were assessed for pain were more likely to receive sedation level assessment, nonopioids, dedicated analgesia during pain procedures, fewer hypnotics, and lower daily doses of midazolam. Patients who were assessed for pain also had a shorter duration of mechanical ventilation and a reduced duration of stay in the ICU. This study suggests a possible link between pain assessment and clinical practices, but it does not establish a cause and effect relationship between pain assessment and improved outcomes in this group. Further study is needed to determine if pain assessment is truly linked to improved length of stay in the ICU, and if not, why not.
Although some improvement has been shown in pain assessment, there may be little progress in alleviating pain. From 1992 to 2001, data from 20 studies at 8 hospitals analyzed pain intensity and pain documentation. There was an increased frequency of pain assessments but no significant decrease in pain intensity or in pain interference with activities (Gordon, Pellino, Miaskowski, et al., 2002). Even with appropriate assessment, many patients continue to suffer pain needlessly.
Nevertheless, without assessment and reassessment of the patient with pain, none of the pain relief measures presented in the following chapters will be useful. If assessment is not used to formulate and implement appropriate pain relief measures, assessment will be largely useless. Even if pain assessment results in appropriate pain relief measures, reassessment is essential. Seemingly appropriate pain relief measures may fail to relieve pain or be harmful to the patient. As essential as assessment is, it is wasted without follow up.
Research tells us that we have a long way to go in improving the care of patients with pain. We can assess patients with pain fairly well, but we need to learn how to teach all members of the health care team to use them. No doubt many of our biases stop us from taking the next step—formulating appropriate intervention for pain relief. It took us 15 to 20 years to develop pain assessment tools, and even today we have not yet established their routine use in many clinical areas, including ICUs and long-term care areas. It is not a hopeless process, but it is a long and difficult one. Numerous pain assessment tools are now available for many patient populations, but assessment alone is not enough. Efforts must be made to act upon that assessment. One approach is to identify comfort-function goals and hold ourselves accountable for intervening until these goals are achieved.
References
Abbey, J., Piller, N., De Bellis, A., et al. The Abbey pain scale: A 1-minute numerical indicator for people with end-stage dementia. International Journal of Palliative Nursing. 2004;10(1):6–13.
Acute Pain Management Guideline Panel. Acute pain management in adults: Operative procedures. Quick reference guide for clinicians, 1992. [AHCPR Pub. No. 92-0019, Rockville, MD, Agency for Health Care and Research, Public Health Service, U.S. Department of Health and Human Services].
Aguirre, L.L., Nevidjon, B.M., Clemens, A.E. Pain diaries. The American Journal of Nursing. 2008;108(6):36–39.
American Academy of Pain Medicine (AAPM), American Pain Society (APS), American Society of Addiction Medicine (ASAM) Definitions related to the use of opioids for the treatment of pain, 2001. Available at http://www.ampainsoc.org/advocacy/opioids2.htm
American Geriatrics Society (AGS) Panel on Persistent Pain in Older Persons. Clinical practice guidelines: The management of persistent pain in older persons. Journal of the American Geriatrics Society. 2002;50(6 Suppl):S205–S224.
American Pain Society (APS). Guideline for the management of acute and chronic pain in sickle cell disease. Glenview, IL: APS, 1999.
American Pain Society (APS). Principles of analgesic use in the treatment of acute pain and cancer pain, 5th ed. Glenview, IL: APS, 2003.
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV), 4th ed. Washington, DC: American Psychiatric Association, 1994.
American Society for Pain Management Nurses (ASPMN) ASPMN position statement: pain management in patients with addictive disease. Pensacola, FL: ASPMN; 2002 Available at http://www.aspmn.org/Organization/documents/addictions_9pt.pdf [Accessed February 3, 2008].
American Society for Pain Management Nurses (ASPMN) Position statement on use of placebos in pain management, 2004. Available at http://www.aspmn.org/pdfs/Use%20of%20Placebos.pdf [Accessed April 3, 2008].
American Society for Pain Management Nurses (ASPMN) Authorized and unauthorized (“PCA by proxy”) dosing of analgesic infusion pumps, 2006. Available at http://www.aspmn.org/Organization/documents/PCAbyProxy-final-EW_004.pdf [Accessed February 3, 2008].
Anderson, J.V.C., Burchiel, K.J. A prospective study of long-term intrathecal morphine in the management of chronic nonmalignant pain. Neurosurgery. 1999;44(2):289–300. [discussion 300–301].
Apfel, C.C., Laara, E., Koivuranta, M., et al. A simplified risk score for predicting postoperative nausea and vomiting: conclusions from cross-validations between two centers. Anesthesiology. 1999;91(3):693–700.
Arbour, C., Gelinas, C. Are vital signs valid indicators of pain in postoperative cardiac surgery ICU adults? Intens Crit Care Nurs. 2009. [doi: 10.1016/j.iccn.2009.11.003.].
Armitage, K.J., Schneiderman, L.J., Bass, R.A. Response of physicians to medical complaints in men and women. JAMA: The Journal of the American Medical Association. 1979;241(20):2186–2187.
Arnstein, P. Placebo: no relief for Ms. Mahoney’s pain. The American Journal of Nursing. 2006;106(2):54–57.
Arroyo-Novoa, C.M., Figueroa-Ramos, M.I., Puntillo, K.A., et al. Pain related to tracheal suctioning in awake acutely and critically ill adults: a descriptive study. Intensive & Critical Care Nursing. 2007;24(1):20–27.
Asmundson, G.J.G., Coons, M.J., Taylor, S., et al. PTSD and the experience of pain: research and clinical implications of shared vulnerability and mutual maintenance models. Canadian Journal of Psychiatry. Revue Canadienne de Psychiatrie. 2002;47(10):930–937.
Asmundson, G.J.G., Katz, J. Understanding pain and posttraumatic stress disorder comorbidity: Do pathological responses to trauma alter the perception of pain? Pain. 2008;138:247–249.
ASP ad hoc Subcommittee for Psychology Curriculum. Curriculum on pain for students in psychology. Seattle: International Association for the Study of Pain, 1997.
Au, E., Loprinzi, C., Chodapkar, M., et al. Regular use of a verbal pain scale improves the understanding of oncology inpatient pain intensity. Journal of Clinical Oncology. 1994;12:2751–2755.
Audette, J.F., Emenike, E., Meleger, A.L. Neuropathic low back pain. Current Pain and Headache Reports. 2005;9:168–177.
Aun, C., Lam, Y.M., Collett, B. Evaluation of the use of visual analogue scale in Chinese patients. Pain. 1986;25:215–221.
Baiardi, J., Parzuchowski, J., Kosik, C., et al. Examination of the reliability of the FLACC Pain Assessment Tool with cognitively impaired elderly. Chicago: Paper presented at the Annual National Conference of Gerontological Nurse Practitioners, 2002.
Barsky, A.J. Forgetting, fabricating, and telescoping. A.M.A. Archives of Internal Medicine. 2002;162:981–984.
Beecher, H.K. Limiting factors in experimental pain. Journal of Chronic Diseases. 1956;4:11–21.
Beecher, H.K. The powerful placebo. JAMA: The Journal of the American Medical Association. 1955;159:1602–1606.
Benedetti, F., Amanzio, M., Baldi, S., et al. The specific effects of prior opioid exposure on placebo analgesia and placebo respiratory depression. Pain. 1998;75:313–319.
Benedetti, F., Arduino, C., Vighetti, S., et al. Pain reactivity in Alzheimer patients with different degrees of cognitive impairment and brain electrical activity deterioration. Pain. 2004;111(1–2):22–29.
Benjamin, L.J., Dampier, C.D., Jacox, A.K., et al. Guideline for the management of acute and chronic pain in sickle cell disease. Glenview, IL: American Pain Society, 1999. [APS Clinical Practice Guidelines, Series No. 1].
Bennett, D., Burton, A.W., Fishman, S., et al. Consensus panel recommendations for the assessment and management of breakthrough pain. Pharmacology & Therapeutics. 2005;30(5):296–301.
Bennett, M.I., Attal, N., Backonia, M.M., et al. Using screening tools to identify neuropathic pain. Pain. 2007;127:199–203.
Bennett, M.I., Smith, B.H., Torrance, N., et al. The S-LANSS score for identifying pain of predominantly neuropathic origin: validation for use in clinical and postal research. The Journal of Pain. 2005;6(3):149–158.
Bergh, I., Sjostrom, B., Oden, A., et al. An application of pain rating scales in geriatric patients. Aging (Milano). 2000;12:380–387.
Berlin, Y., Hossain, S.J., Bodian, C.A. The numeric rating scale and labor epidural analgesia. Anesthesia and Analgesia. 2003;96:1794–1798.
Bernabei, R., Gambassi, G., Lapane, K., et al. Management of pain in elderly patients with cancer. SAGE study group. Systematic assessment of geriatric drug use via epidemiology. JAMA: The Journal of the American Medical Association. 1998;279:1877–1882.
Beydoun, A., Backonja, M.M. Mechanistic stratification of antineuralgic agents. Journal of Pain and Symptom Management. 2003;25:S18–S35.
Beyer, J.E., Denyes, M.J., Villarruel, A.M. The creation, validation, and continuing development of the oucher: A measure of pain intensity in children. Journal of Pediatric Nursing. 1992;7(5):335–346.
Beyer, J.E., Platt, A., Kinney, T., et al. Assessment of pain in adults and children with sickle cell disease. In: Shapiro B.S., Schechter N.L., Ohene-Frempong K., eds. Sickle cell disease related pain assessment and management: Conference proceedings. Mt. Desert, ME: New England Regional Genetics, 1994.
Bieri, D., Reeves, R., Champion, G., et al. The Faces Pain Scale for the self-assessment of the severity of pain experienced by children: development, initial validation, and preliminary investigation for ratio scale properties. Pain. 1990;41(2):139–150.
Bijur, P.E., Berard, A., Esses, D.J., et al. Lack of influence of patient self-report of pain intensity on administration of opioids for suspected long-bone fractures. The Journal of Pain. 2006;7(6):438–444.
Bodfish, J.W., Harper, V.N., Deacon, J.M., et al. Issues in pain assessment for adults with severe to profound mental retardation. In: Oberlander T.F., Symons F.J., eds. Pain in children and adults with developmental disabilities. Baltimore: Paul H. Brookes, 2006.
Boly, M., Faymonville, M.E., Schnakers, C., et al. Perception of pain in the minimally conscious state with PET activation: An observational study. Lancet Neurology. 2008;7(11):1013–1020.
Bonnot, O., Anderson, G.M., Cohen, D., et al. Are patients with schizophrenia insensitive to pain? A reconsidertion of the question. The Clinical Journal of Pain. 2009;25(3):244–252.
Bottos, S., Chambers, C.T. The epidemiology of pain in developmental disabilities. In: Oberlander T.F., Symons F.J., eds. Pain in children and adults with developmental disabilities. Baltimore: Paul H. Brookes, 2006.
Bouhassira, D., Attal, N., Fermanian, J., et al. Development and validation of the Neuropathic Pain Symptom Inventory. Pain. 2004;108(3):248–257.
Breau, L.M., MacLaren, J., McGrath, P.J., et al. Caregivers’ beliefs regarding pain in children with cognitive impairment: relation between pain sensation and reaction increases with severity of impairment. The Clinical Journal of Pain. 2003;19(6):335–344.
Breau, L.M., McGrath, P.J., Camfield, C.S., et al. Psychometric properties of the non-communicating children’s pain checklist-revised. Pain. 2002;99(1–2):349–357.
Breau, L.M., McGrath, P.J., Zabalia, M. Assessing pediatric pain and developmental disabilities. In: Oberlander T.F., Symons F.J., eds. Pain in children and adults with developmental disabilities. Baltimore: Paul H. Brookes, 2006.
Breitbart, W., McDonald, M.V., Rosenfeld, B., et al. Pain in ambulatory AIDS patients. I: pain characteristics and medical correlates. Pain. 1996;68(2–3):315–321.
Breivik, H. Opioids in chronic non-cancer pain, indications and controversies. European Journal of Pain (London, England). 2005;9(2):127–130.
Breslau, N. The epidemiology of posttraumatic stress disorder: what is the extent of the problem? The Journal of Clinical Psychiatry. 2001;62(Suppl. 17):16–22.
Bridge, D., Thompson, S.W.N., Rice, A.S.C. Mechanisms of neuropathic pain. British Journal of Anaesthesia. 2001;87(1):12–26.
Brody, H. The doctor as therapeutic agent: a placebo effect research agenda. In: Harrington A., ed. The placebo effect: An interdisciplinary exploration. Cambridge, MA: Harvard University Press, 1997.
Brown, J. Nurses’ analgesic choices and postoperative patients’ perceived pain: the effect of a pain flow sheet. Am J Pain Manage. 1992;2:192–197.
Brown, R.L., Patterson, J.J., Rounds, L.A., et al. Substance abuse among patients with chronic low back pain. The Journal of Family Practice. 1996;43(2):152–160.
Bruera, E., Macmillan, K., Hanson, J., et al. The cognitive effects of the administration of narcotic analgesics in patients with cancer pain. Pain. 1989;39(1):13–16.
Bucknall, T., Manias, E., Botti, M. Nurses’ reassessment of postoperative pain after analgesic administration. The Clinical Journal of Pain. 2007;23(1):1–7.
Buffum, M.D., Hutt, E., Chang, V.T., et al. Cognitive impairment and pain management: Review of issues and challenges. Journal of Rehabilitation Research and Development. 2007;44(2):315–330.
Buffum, M.D., Miaskowski, C., Sands, L. A pilot study of the relationship between discomfort and agitation in patients with dementia. Geriatric Nursing. 2001;22(2):80–85.
Buffum, M.D., Sands, L., Miaskowski, C., et al. A clinical trial of the effectiveness of regularly scheduled versus as-need administration of acetaminophen in the management of discomfort in older adults with dementia. Journal of the American Geriatrics Society. 2004;52(7):1093–1097.
Burgess, M.M. Nurses’ pain ratings of patients with acute and chronic low back pain. Charlottesville, VA: University of Virginia School of Nursing, 1980. [Unpublished master’s thesis].
Burkitt, J.C., Breau, L.M., Salsman, S., et al. Pilot study of the feasibility of the non-communicating children’s pain checklist revised for pain assessment for adults with intellectual disabilities. J Pain Manage. 2(1), 2009.
Bushnell, M.C. Commentaries. European Journal of Pain (London, England). 1997;1:167.
Byas-Smith, M.G., Max, M.B., Muir, J., et al. Transdermal clonidine compared to placebo in painful diabetic neuropathy using a two-stage “enriched enrollment design.”. Pain. 1995;60(3):267–274.
California Board of Registered Nursing (BRN). BRN focuses on pain management. BRN Report. 1997;10(1):12.
Calvillo, E.R., Flaskerud, J.H. Evaluation of the pain response by Mexican American and Anglo American women and their nurses. Journal of Advanced Nursing. 1993;18(3):451–459.
Capers, C.F. Nursing and the Afro-American client. Topics in Clinical Nursing. 1985;7(3):11–17.
Caraceni, A., Cherny, N., Fainsinger, R., et al. Pain measurement tools and methods in clinical research in palliative care: Recommendations of an Expert Working Group of the European Association of Palliative Care. Journal of Pain and Symptom Management. 2002;23(3):239–255.
Carey, S.J., Turpin, C., Smith, J., et al. Improving pain management in an acute care setting. Orthopaedic Nursing/National Association of Orthopaedic Nurses. 1997;16(4):29–36.
Carpenter, J.S., Brockopp, D. Comparison of patients’ ratings and examination of nurses’ responses to pain intensity rating scales. Cancer Nursing. 1995;18(4):292–298.
Cassell, E.J. The nature of suffering and the goals of medicine. The New England Journal of Medicine. 1982;306:639–645.
Cepeda, M.S., Aficano, J.M., Polo, R., What decline in pain intensity is meaningful to patients with acute pain?. Dostrovsky, J.O., Carr, D.B., Kolzenburg, M., eds. Proceedings of the 10th World Congress on Pain. Seattle: IASP Press; 2003;24:601–609. [Prog Pain Res Management].
Cervo, F., Bruckenthal, P., Bright-Long, L., et al. Pain assessment in nursing home residents with dementia: Psychometric properties and clinical utility of the CAN Pain Assessment Tool (CPAT). JAMDA. 2009;10(7):505–510.
Cervo, F.A., Raggi, R.P., Bright-Long, L.E., et al. Use of the certified nursing assistant pain assessment tool (CPAT) in nursing home residents with dementia. American Journal of Alzheimer’s Disease and Other Dementias. 2007;22(2):112–119.
Chambers, C.T., Craig, K.D. An intrusive impact of anchors in children’s faces pain scales. Pain. 1998;78:27–37.
Chambers, C.T., Giesbrecht, K., Craig, et al. A comparison of faces scales for the measurement of pediatric pain: children’s and parents’ ratings. Pain. 1999;83:25–35.
Chambers, C.T., Hardial, J., Craig, K.D., et al. Faces scales for the measurement of postoperative pain intensity in children following minor surgery. The Clinical Journal of Pain. 2005;21(3):277–285.
Chapman, C.R. Progress in pain assessment: The cognitively compromised patient. Current Opinion in Anesthesiology. 2008;21:610–616.
Chen, L.M., Miaskowski, C., Dodd, M., et al. Concepts within the Chinese culture that influence the cancer pain experience. Cancer Nursing. 2008;31(2):103–108.
Cheng, Y., Lin, C.P., Liu, H.L., et al. Expertise modulates the perception of pain in others. Current Biology: CB. 2007;17:1706–1713.
Chibnall, J.T., Tait, R.C. Pain assessment in cognitively impaired and unimpaired older adults: a comparison of four scales. Pain. 2001;92:173–186.
Chibnall, J.T., Tait, R.C., Harman, B., et al. Effect of acetaminophen on behavior, well-being, and psychotropic medication use in nursing home residents with moderate-to-severe dementia. Journal of the American Geriatrics Society. 2005;53:1921–1929.
Chu, L., Schnelle, J., Cadogan, M., et al. Using the minimum data set to select nursing home residents for interview about pain. Journal of the American Geriatrics Society. 2004;52(12):2057–2061.
Clancy, A.C., McGrath, P.F., Oddson, B.E. Pain in children and adolescents with spina bifida. Developmental Medicine and Child Neurology. 2005;47:27–34.
Cleeland, C.S. The impact of pain on the patient with cancer. Cancer. 1984;54:2635–2641.
Cleeland, C.S., Gonin, R., Baez, L., et al. Pain and treatment of pain in minority patients with cancer: The Eastern Cooperative Oncology Group Minority Outpatient Pain Study. Annals of Internal Medicine. 1997;127:813–816.
Cleeland, C.S., Gonin, R., Hatfield, A.K., et al. Pain and its treatment in out-patients with metastatic cancer: The Eastern cooperative oncology group’s outpatient study. The New England Journal of Medicine. 1994;330:592–596.
Cleeland, C.S., Ladinsky, J.L., Serlin, J.R.D., et al. Multidimensional measurement of cancer pain: comparisons of US and Vietnamese patients. Journal of Pain and Symptom Management. 1988;3:23–27.
Cleeland, C.S., Nakamura, Y., Mendoza, T.R., et al. Dimensions of the impact of cancer in a four country sample: New information from multidimensional scaling. Pain. 1996;67:267–273.
Cleeland, C.S., Ryan, K.M. Pain assessment: global use of the brief pain inventory. Annals of the Academy of Medicine. 1994;23:129–138.
Cleeland, C.S., Serlin, R., Nakamura, Y., et al, Effects of culture and language on ratings of cancer pain and patterns of functional interference. Jensen, T.S., Turner, J.A., Wiesenfeld-Hallin, Z., eds. Proceedings of the 8th World Congress on Pain, 1997;8:35–51. [Pain Research & Management].
Cleeland, C.S., Syrjala, K.L. How to assess cancer pain. In: Turk D.C., Melzack R., eds. Handbook of pain assessment. New York: Guilford Press, 1992.
Closs, S.J. Patients’ night-time pain, analgesic provision and sleep after surgery. International Journal of Nursing Studies. 1992;29(4):381–392.
Closs, S.J., Barr, B., Briggs, M., et al. A comparison of five pain assessment scales for nursing home residents with varying degrees of cognitive impairment. Journal of Pain and Symptom Management. 2004;27(3):196–205.
Closs, S.J., Briggs, M. Patients’ verbal descriptions of pain and discomfort following orthopedic surgery. International Journal of Nursing Studies. 2002;39(5):563–572.
Cohen, F.L. Postsurgical pain relief: Patients’ status and nurses’ medication choices. Pain. 1980;9:265–274.
Cohen, H., Neumann, L., Haiman, Y., et al. Prevalence of post-traumatic stress disorder in fibromyalgia patients: Overlapping symptoms or post-traumatic fibromyalgia syndrome? Seminars in Arthritis and Rheumatism. 2002;32(1):38–50.
Cohen, M.Z., Easley, M.K., Ellis, C., et al. Cancer pain management and the Joint Commission on Accreditation of Healthcare Organizations’s (JACHO’s) pain standards: an institutional challenge. Journal of Pain and Symptom Management. 2003;25(6):519–527.
Cohen-Mansfield, J., Lipson, S. The utility of pain assessment for analgesic use in persons with dementia. Pain. 2008;134(1–2):16–23.
Cohen-Mansfield, J. Pain assessment in noncommunicative elderly persons: PAINE. The Clinical Journal of Pain. 2006;22(6):569–575.
Cohen-Mansfield, J. The adequacy of the minimum data set assessment of pain in cognitively impaired nursing home residents. Journal of Pain and Symptom Management. 2004;27(4):343–351.
Coley, K.C., Williams, B.A., DaPos, S.V., et al. Retrospective evaluation of unanticipated admissions and readmissions after same-day surgery and associated costs. Journal of Clinical Anesthesia. 2002;14:349–353.
Compton, P. Substance abuse. In McCaffery M., Pasero C., eds.: Pain: Clinical manual, 2nd ed., St. Louis, MO: Mosby, 1999.
Cooke, B.K., Magas, L.T., Virgo, K.S., et al. Appendectomy for appendicitis in patients with schizophrenia. American Journal of Surgery. 2007;193:41–48.
Copeland, L.A., Zeber, J.E., Pugh, M.J., et al. Postoperative complications in the seriously mentally ill: A systematic review of the literature. Annals of Surgery. 2008;248(1):31–38.
Costardi, D., Rozzini, L., Costanzi, C., et al. The Italian version of the pain assessment in advanced dementia (PAINAD) scale. Archives of Gerontology and Geriatrics. 2007;44(2):175–180.
Cotton, P. “Harm reduction” approach may be middle ground. JAMA: The Journal of the American Medical Association. 1994;271(21):1641–1645.
Craig, K. The construct and definition of pain in developmental disability. In: Oberlander T.F., Symons F.J., eds. Pain in children and adults with developmental disabilities. Baltimore: Paul H. Brookes, 2006.
Cruise, C.E., Broderick, J., Porter, L., et al. Reactive effects of diary self-assessment in chronic pain patients. Pain. 1996;67:253–258.
Dalton, J.A., McNaull, F. A call for standardizing the clinical rating of pain intensity using a 0 to 10 rating scale. Cancer Nursing. 1998;21:46–69.
Daumit, G.L., Pronovost, P.J., Anthony, C.B., et al. Adverse events during medical and surgical hospitalizations for persons with schizophrenia. Archives of General Psychiatry. 2006;63:267–272.
Daut, R.L., Cleeland, C.S. The prevalence and severity of pain in cancer. Cancer. 1982;50:1913–1918.
Daut, R.L., Cleeland, C.S., Flanery, R.C. Development of the Wisconsin Brief Pain Questionnaire to assess pain in cancer and other diseases. Pain. 1983;17:197–210.
Dawson, R., Sellers, D.E., Spross, J.A. Do patients’ beliefs act as barriers to effective pain management behaviors and outcomes in patients with cancer-related or noncancer-related pain? Oncology Nursing Forum. 2005;32(2):363–374.
De Conno, F., Ripamonti, C., Gamba, A., et al. Treatment of post-operative pain: comparison between administration at fixed hours and “on demand” with intra-muscular analgesics. European Journal of Surgical Oncology: The Journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 1989;15:242–246.
de Moulin, D. A historical-phenomenological study of bodily pain in western man. Bulletin of the History of Medicine. 1979;48(4):540–570.
de Schepper, A.M.E., Francke, A.L., Abu-Saad, H.H. Feelings of powerlessness in relation to pain: Ascribed causes and reported strategies. Cancer Nursing. 1997;20:422–429.
de Wit, R., van Dam, F., Hanneman, M., et al. Evaluation of the use of a pain diary in chronic cancer pain patients at home. Pain. 1999;79:89–99.
DeCarvalho, L.T., Whealin, J.M. What pain specialists need to know about posttraumatic stress disorder in operation Iraqi freedom and operation enduring freedom returnees. J Musculoskel Pain. 2006;14(3):37–45.
Defrin, R., Ginzburg, K., Solomon, Z., et al. Quantitative testing of pain perception in subjects with PTSD: Implications for the mechanism of the coexistence between PTSD and chronic pain. Pain. 2008;138:450–459.
Defrin, R., Lotan, M., Pick, C.G. The evaluation of acute pain in individuals with cognitive impairment: A differential effect of level of impairment. Pain. 2006;124:312–320.
Defrin, R., Pick, C.G., Peretz, C., et al. A quantitative somatosensory testing of pain threshold in individuals with mental retardation. Pain. 2004;108:58–66.
Dellemijn, P.L.I., Fields, H.L. Do benzodiazepines have a role in chronic pain management? Pain. 1994;57:137–152.
DeLoach, L.J., Higgins, M.S., Caplan, A.B., et al. The visual analog scale in the immediate postoperative period: Intrasubject variability and correlation with a numeric scale. Anesthesia and Analgesia. 1998;56:102–106.
DeWaters, T., Faut-Callahan, M., McCann, J.J., et al. Comparison of self-reported pain and the PAINAD scale in hospitalized cognitively impaired and intact older adults after hip fracture surgery. Orthopedic Nursing. 2008;27(1):21–28.
Dix, P., Sandhar, B., Murdoch, J., et al. Pain on medical wards in a district general hospital. British Journal of Anaesthesia. 2004;92(2):235–237.
Donovan, J. Learning disability nurses’ experiences of being with clients who may be in pain. Journal of Advanced Nursing. 2002;38(5):458–466.
Dreher, M., MacNaughton, N. Cultural competence in nursing: Foundation or fallacy? Nursing Outlook. 2002;50(5):181–186.
Dudley, S.R., Holm, K. Assessment of the pain experience in relation to selected nurse characteristics. Pain. 1984;18(2):179–186.
Dworkin, R.H., Turk, D.C., Farrar, J.T., et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113(1–2):9–19.
Edlund, M.J., Steffick, D., Hudson, T., et al. Rick factors for clinically recognized opioid abuse and dependence among veterans using opioids for chronic non-cancer pain. Pain. 2007;129(3):355–362.
Egan, K.J., Ready, L.B., Nessly, M., et al. Self-administration of midazolam for postoperative anxiety: A double-blinded study. Pain. 1992;49:3–8.
Eich, E., Reeves, J.L., Jaeger, B., et al. Memory for pain: Relation between past and present pain intensity. Pain. 1985;23:375–379.
Epps, C.D., Ware, L.J., Packard, A. Ethnic wait time differences in analgesic administration in the emergency department. Pain Management Nursing. 2008;9(1):26–32.
Ergun, U., Say, B., Ozer, G., et al. Trial of a new pain assessment tool in patients with low education: The full cup test. International Journal of Clinical Practice. 2007;61(10):1692–1696.
Errando, C.L., Sigl, J.C., Robles, M., et al. Awareness with recall during general anaesthesia: A prospective observational evaluation of 4001 patients. British Journal of Anaesthesia. 2008;101(2):178–185.
Ersek, M., Turner, J.A., Cain, K.C., et al, Chronic pain self-management for older adults: A randomized controlled trial. BMC Geriatrics 2004;4:7. Available at http://www.biomedcentral.com/1471-2318/4/7 [Accessed August 18, 2009].
Evans, F.J., The placebo response in pain reduction. Advances in neurology. New York: Raven Press; 1974;Vol. 4.
Everett, J.J., Patterson, D.R., Marvin, J.A. Pain assessment from patients with burns and their nurses. The Journal of Burn Care & Rehabilitation. 1994;15:194–198.
Ezenwa, M.O., Ameringer, S., Ward, S.E., et al. Racial and ethnic disparities in pain management in the United States. Journal of Nursing Scholarship. 2006;38(3):225–233.
Faries, J.E., Mills, D.S., Goldsmith, K.W., et al. Systematic pain records and their impact on pain control: A pilot study. Cancer Nursing. 1991;14:306–313.
Farrar, J.I., Young, J.P., Jr., LaMoreaux, L., et al. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94:149–158.
Favaloro, R., Touzel, B. A comparison of adolescents’ and nurses’ postoperative pain ratings and perceptions. Pediatric Nursing. 1990;16:414–417.
Feldt, K. Pain measurement: present concerns and future directions. Pain Medicine (Malden, Mass.). 2007;8(7):541–542.
Feldt, K.S. The checklist of nonverbal pain indicators (CNPI). Pain Management Nursing. 2000;1(1):13–21.
Feldt, K.S., Ryden, M.B., Miles, S. Treatment of pain in cognitively impaired compared with cognitively intact older patients with hip-fracture. Journal of the American Geriatrics Society. 1998;46(9):1079–1085.
Ferraz, M.B., Quaresma, M.R., Aquino, L.R., et al. Reliability of pain scales in the assessment of literate and illiterate patients with rheumatoid arthritis. The Journal of Rheumatology. 1990;17(8):1022–1024.
Ferrell, B.A., Ferrell, B.R., Rivera, L. Pain in cognitively impaired nursing home patients. Journal of Pain and Symptom Management. 1995;10:591–598.
Ferrell, B.R., Grant, M., Funk, B., et al. Quality of life in breast cancer. Part I: Physical and social well-being. Cancer Nursing. 1997;20:398–408.
Filoramo, M.A. Improving goal setting and goal attainment in patients with chronic noncancer pain. Pain Management Nursing. 2007;8(2):96–101.
Fine, B.G, Portenoy, R.K. A clinical guide to opioid analgesia. Vendome Group, 2007.
Finniss, D.G., Benedetti, F. Mechanisms of the placebo response and their impact on clinical trials and clinical practice. Pain. 2005;114(1–2):3–6.
Finniss, D.G., Benedetti, F. Placebo analgesia, nocebo hyperalgesia. Pain Clin Updates. 2007;15(1):4.
Fishbain, D.A., Cole, B., Lewis, J., et al. What percentage of chronic nonmalignant pain patients exposed to chronic opioid analgesic therapy develop abuse/addiction and/or aberrant drug-related behaviors? A structured evidence-based review. Pain Medicine (Malden, Mass.). 2008;9(4):444–459.
Fishbain, D.A., Cutler, R., Rosomoff, H.L., et al. Chronic pain disability exaggeration/malingering and subamaximal effort research. The Clinical Journal of Pain. 1999;15:244–274.
Fishbain, D.A., Lewis, J.E., Cutler, R., et al. Can the neuropathic pain scale discriminate between non-neuropathic and neuropathic pain? Pain Medicine (Malden, Mass.). 2008;9(2):149–160.
Fishbain, D.A., Rosomoff, H.L., Rosomoff, R.S. Drug abuse, dependence, and addiction in chronic pain patients. The Clinical Journal of Pain. 1992;8:77–85.
Fisher, F.B. Interpretation of “aberrant” drug behaviors. J Am Phys Surg. 2004;9(1):25–28.
Fleming, M.F., Balousek, S.L., Klessig, C.L., et al. Substance use disorders in a primary care sample receiving daily opioid therapy. The Journal of Pain. 2007;8(7):573–582.
Flood, P., Daniel, D. Intranasal nicotine for postoperative pain treatment. Anesthesiology. 2004;101:1417–1421.
Folstein, M.L., Folstein, S.E.M., McHugh, P.R. Mini-mental state: A practical method for grading the cognitive status of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198.
Fong, C.M. Ethnicity and nursing practice. Topics in Clinical Nursing. 1985;7:1–10.
Forbes, J.A., The nurse-observer: observation methods and training. Max, M.B., Portenoy, R.K., Laska, E.M., eds. Adv Pain Res Therapy, 1991;18:607–620.
Fox, A.E. Confronting the use of placebos for pain. The American Journal of Nursing. 1994;94(9):42–46. [(pseudonym)].
Fox, A.E., McCaffery, M., Ferrell, B., et al. A place for placebos? Reply. The American Journal of Nursing. 1995;95(2):18. [(pseudonym)].
Francke, A.L., Theeuwen, I. Inhibition in expressing pain: A qualitative study among Dutch surgical breast cancer patients. Cancer Nursing. 1994;17:193–199.
Freyd, M.J. The graphic rating scale. Journal of Educational Psychology. 1923;14:83.
Freynhagen, R., Baron, R., Gockel, J., et al. painDETECT: A new screening questionnaire to detect neuropathic components in patients with back pain. Current Medical Research and Opinion. 2006;22(10):1911–1920.
Friedman, D.P. Perspectives on the medical use of drugs of abuse. Journal of Pain and Symptom Management. 1990;5:S2–S5.
Fritz, D.J., Noninvasive pain control methods used by cancer outpatients. Oncology Nursing Forum, 1988;Suppl.:108.
Fuchs-Lacelle, S., Hadjistavropoulos, T. Development and preliminary validation of the pain assessment checklist for seniors with limited ability to communicate (PACSLAC). Pain Management Nursing. 2004;5(1):37–49.
Fuchs-Lacelle, S., Hadjistavropoulos, T. A checklist for pain assessment in LTC-PACSLAC: Pain assessment checklist for seniors with limited ability to communicate. Can Nurs Home. 2005;16(4):4–7.
Fuchs-Lacelle, S., Hadjistavropoulos, T., Lix, L. Pain assessment as intervention: A study of older adults with severe dementia. The Clinical Journal of Pain. 2008;24(8):697–707.
Gagliese, L. Assessment of pain in the elderly. In: Turk D.C., Melzack R., eds. Handbook of pain assessment. New York: Guilford Press, 2001.
Gagliese, L., Katz, J. Medically unexplained pain is not caused by psychopathology. Pain Research & Management. 2000;5(4):251–257.
Gagliese, L., Katz, J. Age differences in postoperative pain are scale dependent: A comparison of measures of pain intensity and quality in younger and older surgical patients. Pain. 2003;103:11–20.
Gagliese, L., Weizblit, N., Ellis, W., et al. The measurement of postoperative pain: A comparison of intensity scales in younger and older surgical patients. Pain. 2005;117:412–420.
Galer, B.S., Jensen, M.P. Development and preliminary validation of a pain measure specific to neuropathic pain: The neuropathic pain scale. Neurology. 1997;48:332–338.
Gan, T.J., Meyer, T.A., Apfel, C.C. Society for ambulatory anesthesia guidelines for the management of postoperative nausea and vomiting. Anesthesia and Analgesia. 2007;105:1615–1628.
Gaston-Johansson, F. Pain assessment: differences in quality and intensity of the words pain, ache, and hurt. Pain. 1984;20:69–76.
Gaston-Johansson, F., Albert, M., Fagan, E., et al. Similarities in pain descriptions of four different ethnic-culture groups. Journal of Pain and Symptom Management. 1990;5:94–100.
Gear, R.W., Miaskowski, C., Heller, P.H., et al. Benzodiazepine mediated antagonism of opioid analgesia. Pain. 1997;71:25–29.
Geisser, M.E., Roth, R.S., Bachman, J.E., et al. The relationship between symptoms of post-traumatic stress disorder and pain, affective disturbance and disability among patients with accident and non-accident related pain. Pain. 1996;66(2–3):207–214.
Gelinas, C., Arbour, C. Behavioral and physiologic indicators during a nociceptive procedure in conscious and unconscious mechanically ventilated adults: Similar or different? Journal of Critical Care. 2009;24:628e7. [628e17].
Gelinas, C., Fillion, L., Puntillo, K.A., et al. Validation of the critical-care pain observation tool in adult patients. American Journal of Critical Care. 2006;15(4):420–427.
Gelinas, C., Fortier, M., Viens, C., et al. Pain assessment and management in critically ill intubated patients: A retrospective study. American Journal of Critical Care. 2004;13(2):126–135.
Gelinas, C., Harel, F., Fillion, L., et al. Sensitivity and specificity of the critical-care pain observation tool for the detection of pain in intubated adults after cardiac surgery. Journal of Pain and Symptom Management. 2009;37(1):58–67.
Gelinas, C., Johnston, C. Pain assessment in the critically ill ventilated adult: Validation of the critical-care pain observation tool and physiologic indicators. The Clinical Journal of Pain. 2007;23(6):497–505.
Gendreau, M., Hufford, M.R., Stone, A.A. Measuring clinical pain in chronic widespread pain: Selected methodological issues. Best Practice & Research. Clinical Rheumatology. 2003;17:575–592.
Geuze, E., Westenberg, G.M., Jochims, A., et al. Altered pain perception in veterans with posttraumatic stress disorder. Archives of General Psychiatry. 2007;64:76–85.
Gibson, S.J., Voukelatos, X., Amers, D., et al. An examination of pain perception and cerebral event-related potentials following carbon dioxide laser stimulation in patients with Alzheimer’s disease and age-matched control volunteers. Pain Research & Management. 2001;6(3):126–132.
Gift, A. Visual analogue scales: Measurement of subjective phenomena. Nursing Research. 1989;38:286–288.
Gift, A.G., Plaut, D.M., Jacox, A.K. Psychologic and physiologic factors related to dyspnea in subjects with chronic obstructive pulmonary disease. Heart and Lung. 1986;15:595–601.
Giger, J.N., Davidhizar, R.E. Transcultural nursing: Assessment and intervention, 5th ed. St. Louis, MO: Mosby, 2008.
Gilron, J., Bailey, J.M., Tu, D., et al. Morphine, gabapentin, or their combination for neuropathic pain. The New England Journal of Medicine. 2005;352:1324–1334.
Gjeilo, K.H., Stenseth, R., Wahba, A., et al. Validation of the brief pain inventory in patients six months after cardiac surgery. Journal of Pain and Symptom Management. 2007;34(6):648–656.
Gloth, F.M., Scheve, A.A., Stober, C.V., et al. The Functional Pain Scale: Reliability, validity, and responsiveness in an elderly population. Journal of the American Medical Directors Association. 2001;2:110–114.
Goodwin, J.S., Goodwin, J.M., Vogel, A.A. Knowledge and use of placebos by house officers and nurses. Annals of Internal Medicine. 1979;91:106–110.
Gordon, D.B., Pellino, T.A., Miaskowski, C., et al. A 10-year review of quality improvement monitoring in pain management: Recommendations for standardized outcome measures. Pain Management Nursing. 2002;3(4):116–130.
Gordon, D.B., Ward, S.E. Correcting patient misconceptions about pain. The American Journal of Nursing. 1995;95(7):43–45.
Grace, P.J. The clinical use of placebos. The American Journal of Nursing. 2006;106(2):58–61.
Graffam, S. Congruence of nurse-patient expectations regarding nursing intervention in pain. Nursing Leadership. 1981;4(2):12–15.
Green, C.R., Anderson, K.O., Baker, T.A., et al. The unequal burden of pain: Confronting racial and ethnic disparities in pain. Pain Medicine (Malden, Mass.). 2003;4(3):277–294.
Green, C.R., Wheeler, J.R.C. Physician variability in the management of acute postoperative and cancer pain: A quantitative analysis of the Michigan experience. Pain Medicine (Malden, Mass.). 2003;4(1):8–20.
Green, C.R., Wheeler, J.R.C., Marchant, B., et al. Analysis of the physician variable in pain management. Pain Medicine (Malden, Mass.). 2001;2(4):317–327.
Grevert, P., Albert, L.H., Goldstein, A. Partial antagonism of placebo analgesia by naloxone. Pain. 1983;16:129–143.
Grossman, H.J. Classification in mental retardation. Washington, DC: American Association on Mental Deficiency, 1983.
Grossman, S.A., Sheidler, V.R., Swedeen, K., et al. Correlation of patient and caregiver ratings of cancer pain. Journal of Pain and Symptom Management. 1991;6:53–57.
Gunnarsdottir, S., Donovan, H.S., Serlin, R.C., et al. Patient-related barriers to pain management: The barriers questionnaire II (BQ-II). Pain. 2002;99:385–396.
Haddad, A. Ethics in action: What would you do? RN. 1993;56(3):21–24.
Hadjistavropoulos, H.D., Ross, M.A., von Baeyer, C.L. Are physicians’ ratings of pain affected by patients’ physical attractiveness? Social Science & Medicine. 1990;31(1):69–72.
Hadjistavropoulos, T. Assessing pain in older persons with severe limitations in ability to communicate. In: Gibson S., Weiner D., eds. Pain in the elderly. Seattle:: IASP Press, 2005.
Hadjistavropoulos, T., Herr, K., Turk, D.C., et al. An interdisciplinary expert consensus statement on assessment of pain in older persons. The Clinical Journal of Pain. 2007;23(1):S1–S43.
Hadjistavropoulos, T., LaChapelle, D., MacLeod, F., et al. Measuring movement-exacerbated pain in cognitively impaired frail elders. The Clinical Journal of Pain. 2000;16(1):54–63.
Hadjistavropoulos, T., MacNab, Y.C., Lints-Martindale, A., et al. Does routine pain assessment result in better care? Pain Research & Management. 2009;14(3):211–216.
Hagen, N.A., Stiles, C., Nekolaichuk, C., et al. The Alberta breakthrough pain assessment tool for cancer patients: A validation study using a Delphi process and patient think-aloud interviews. Journal of Pain and Symptom Management. 2008;35(2):136–152.
Halfens, R., Evers, G., Abu-Saad, H. Determinants of pain assessment by nurses. International Journal of Nursing Studies. 1990;27(1):43–49.
Hall, G.M., Salmon, P. Patient-controlled analgesia: Who benefits? Anaesthesia. 1997;52:401–402.
Hamers, J.P.H., van den Hout, M.A., Halfens, R.J.G. Differences in pain assessment and decisions regarding the administration of analgesics between novices, intermediates and experts in pediatric nursing. International Journal of Nursing Studies. 1997;34:325–334.
Hampton, T. Are placebos in advanced cancer trials ethically justified? Journal of American Medical Association. 2006;296(3):265–266.
Hardy, J.D., Wolff, H.G., Goodell, H. Pain threshold in man. Proc Assoc Res Nerv Ment Dis. 1943;23:1.
Harrison, A., Busabir, A.A., Al-Kaabi, A.O., et al. Does sharing a mother tongue affect how closely patients and nurses agree when rating the patient’s pain, worry and knowledge? Journal of Advanced Nursing. 1996;24:229–235.
Hayes, M.H.S., Patterson, D.G. Experiment development of the graphic rating scale. Psychological Bulletin. 1921;18:98–99.
Hazelett, S., Powell, C., Androulakakis, V. Patients’ behavior at the time of injury: Effect on nurses’ perception of pain level and subsequent treatment. Pain Management Nursing. 2002;3(1):28–35.
Heins, A., Grammas, M., Heins, J.K., et al. Determinants of variation in analgesic and opioid prescribing practice in an emergency department. Journal of Opioid Management. 2006;2(6):335–340.
Heit, H.A., Gourlay, D.L. DSM-V and the definitions: Time to get it right. Pain Medicine (Malden, Mass. ). 2009;10(5):784–786.
Hendler, N.H., Kozikowski, J.G. Overlooked diagnoses in chronic pain patients involved in litigation. Psychosomatics. 1993;34(6):494–504.
Hennequin, M., Faulks, D., Allison, P.J. Parents’ ability to perceive pain experienced by their child with Down syndrome. Journal of Orofacial Pain. 2003;17:347–353.
Hennequin, M., Morin, C., Feine, J.S. Pain expression and stimulus localisation in individuals with Down syndrome. Lancet. 2000;356:1882–1887.
Herr, K., Bjoro, K., Decker, S. Tools for assessment of pain in nonverbal older adults with dementia: A state-of-the-science review. Journal of Pain and Symptom Management. 2006;31(2):170–192.
Herr, K., Coyne, P.F., Key, T., et al, Pain assessment in the nonverbal patient: Position statement with clinical practice recommendation. Pain Management Nursing, 2006;7(2):44–52. Available at http://www.aspmn.org/Organization/documents/NonverbalJournalFINAL.pdf [Accessed August 18, 2009].
Herr, K., Spratt, K.F., Garand, L., et al. Evaluation of the Iowa Pain Thermometer and other selected pain intensity scales in younger and older adult cohorts using controlled clinical pain: A preliminary study. Pain Medicine (Malden, Mass.). 2007;8(7):585–600.
Herr, K., Titler, M.G., Schilling, M.L., et al. Evidence-based assessment of acute pain in older adults: Current nursing practices and perceived barriers. The Clinical Journal of Pain. 2004;20:331–340.
Herr, K.A., Garand, L. Assessment and measurement of pain in older adults. Clinics in Geriatric Medicine. 2001;17:457–478.
Herr, K.A., Mobily, P.R. Comparison of selected pain assessment tools for use with the elderly. Applied Nursing Research: ANR. 1993;6:39–46.
Herr, K.A., Spratt, K.S., Mobily, P.R., et al. Pain intensity assessment in older adults: Use of experimental pain to compare psychometric properties and usability of selected pain scales with younger adults. The Clinical Journal of Pain. 2004;20(4):207–219.
Hester, N.O. Integrating pain assessment and management into the care of children with cancer. In: McGuire D.B., Yarbro C.H., Ferrell B.R., eds. Cancer pain management. Boston: Jones and Bartlett, 1995.
Hicks, C.L., von Baeyer, C.L., Spafford, P.A., et al. The Faces Pain Scale-Revised: Toward a common metric in pediatric pain measurement. Pain. 2001;93:173–183.
Hill, M.L., Craig, K.D. Detecting deception in pain expressions: The structure of genuine and deceptive facial displays. Pain. 2002;98:135–144.
Hitchcock, L.S., Ferrell, B.R., McCaffery, M. The experience of “chronic nonmalignant pain.”. Journal of Pain and Symptom Management. 1994;9(5):312–318.
Hoge, C.W., Castro, C.A., Messer, S.C., et al. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. The New England Journal of Medicine. 2004;351(Suppl. 1):13–22.
Holen, J.C., Saltvedt, I., Fayers, P.M., et al. The Norwegian Doloplus-2, a tool for behavioral pain assessment: Translation and pilot-validation in nursing home patients with cognitive impairment. Palliative Medicine. 2005;19(5):411–417.
Holm, K., Cohen, F., Dudas, S., et al. Effect of personal pain experience on pain assessment. Image–Journal of Nursing Scholarship. 1989;21:72–75.
Hooley, J., Delgado, M. Pain insensitivity in the relatives of schizophrenia patients. Schizophrenia Research. 2001;47:265–273.
Horgas, A.L., Nichols, A.L., Schapson, C.A., et al. Assessing pain in persons with dementia: Relationships among the non-communicative patient’s pain assessment instrument, self-report, and behavioral observations. Pain Management Nursing. 2007;8(2):77–85.
Hurley, A.C., Volicer, B.J., Hanrahan, P.A., et al. Assessment of discomfort in advanced Alzheimer patients. Research in Nursing & Health. 1992;15(5):369–377.
Husebo, B., Strand, L., Moe-Nilssen, R., et al. Pain behavior and pain intensity in older persons with severe dementia: Reliability of the MOBID pain scale by video uptake. Scandinavian Journal of Caring Sciences. 2009;23(1):180–189.
Husebo, B.S., Strand, L.I., Moe-Nilssen, R., et al. Mobilization-observation-behavior-intensity-dementia pain scale (MOBID): Development and validation of a nurse-administered pain assessment tool for use in dementia. Journal of Pain and Symptom Management. 2007;34(1):67–80.
Husebo, B.S., Strand, L.I., Moe-Nilssen, R., et al. Who suffers most? Dementia and pain in nursing home patients: A cross-sectional study. Journal of the American Medical Directors Association. 2008;9(6):427–433.
Husskisson, E.C. Measurement of pain. Lancet. 1974;1:1127–1131.
IASP ad hoc Committee for Psychology Curriculum. Curriculum on pain for students in psychology. Seattle: International Association for the Study of Pain, 1997.
Ionescu, D., Badescu, C., Acalovschi, I. Nicotine patch for the prevention of postoperative nausea and vomiting. Clinical Drug Investigation. 2007;27(8):559–564.
Jacox, A. Assessing pain. The American Journal of Nursing. 1979;79:895–900.
Jacox, A., Carr, D.B., Payne, R., et al. Management of cancer pain: Clinical practice guideline No. 9. Rockville, MD: Agency for Healthcare Policy and Research, U.S. Department of Health and Human Services, Public Health Service, 1994. [AHCPR Pub. No. 94-0592].
Jacox, A., Carr, D.B., Payne, R., et al. Management of cancer pain: Adults. Quick reference for clinicians. Clinical practice guideline No. 9. Rockville, MD: Agency for Healthcare Policy and Research, U.S. Department of Health and Human Services, Public Health Service, 1994. [AHCPR Pub. No. 94-0593].
Jamison, R.N., Raymond, S.A., Levine, J.G., et al. Electronic diaries for monitoring chronic pain: 1-year validation study. Pain. 2001;91:277–285.
Janssen, S.A., Arntz, A. Anxiety and pain: Attentional and endorphinergic influences. Pain. 1996;56:145–150.
Jensen, M.P. The validity and reliability of pain measures in adults with cancer. The Journal of Pain. 2003;4(7):2–21.
Jensen, M.P., Chen, C., Brugger, A.M. Interpretation of visual analog scale ratings and change scores: An .analysis of two clinical trials of postoperative pain. The Journal of Pain. 2003;4(7):407–414.
Jensen, M.P., Dworkin, R.H., Gammaitoni, A.R., et al. Assessment of pain quality in chronic neuropathic and nociceptive pain clinical trials with Neuropathic Pain Scale. The Journal of Pain. 2005;6(2):98–106.
Jensen, M.P., Friedman, M., Bonzo, D., et al. The validity of the Neuropathic Pain Scale for assessing diabetic neuropathic pain in a clinical trial. The Clinical Journal of Pain. 2006;22(1):97–103.
Jensen, M.P., Karoly, P. Self-report scales and procedures for assessing pain in adults. In: Turk D.C., Melzack R., eds. Handbook of pain assessment. New York: Guilford Press, 1992.
Jensen, M.P., Miller, L., Fisher, L.D. Assessment of pain during medical procedures: A comparison of three scales. The Clinical Journal of Pain. 1998;14:343–349.
Jensen, M.P., Turner, J.A., Romano, J.M. What is the maximum number of levels needed in pain intensity measurement? Pain. 1994;58:387–392.
Jenson, M.P., Karoly, P. Self-report scales and procedures for assessing pain in adults. In Melzack R., Turk D.C., eds.: Handbook of pain assessment, 2nd ed., New York: Guilford Press, 2001.
Jochum, T., Letzsch, A., Greiner, W., et al. Influence of antipsychotic medication on pain perception in schizophrenia. Psychiatry Research. 2006;142:151–156.
Johnston, C.C., Gangon, A.J., Pepler, C.J., et al. Pain in the emergency department with one-week follow-up of pain resolution. Pain Research & Management. 2005;10:67–70.
Joint Commission on Accreditation of Healthcare Organizations. Accreditation manual for hospitals. Oakbrook Terrace, IL: The Commission, 1994.
Jones, K.R., Fink, R., Hutt, E., Vojir, C., et al. Measuring pain intensity in nursing home residents. Journal of Pain and Symptom Management. 2005;30(6):519–527.
Kaasalainen, S., Crook, J. A comparison of pain-assessment tools for use with elderly long-term-care residents. The Canadian Journal of Nursing Research. 2003;35(4):58–71.
Kagawa-Singer, M. Ethnic perspectives of cancer nursing: Hispanics and Japanese-Americans. Oncology Nursing Forum. 1987;14:59–65.
Kahn, D.L., Steeves, R.H. The experience of suffering. In: Ferrell B.R., ed. Suffering. Boston: Jones and Bartlett, 1996.
Kallai, I., Barke, A., Voss, U. The effects of experimenter characteristics on pain reports in women and men. Pain. 2004;112:142–147.
Kalyadina, S.A., Ionova, T.I., Ivanova, M.O., et al. Russian brief pain inventory: Validation and application in cancer pain. Journal of Pain and Symptom Management. 2008;35(1):95–102.
Kappesser, J., Williams, A.C., Prkachin, K.M. Testing two accounts of pain underestimation. Pain. 2006;124:109–116.
Karp, J., Shega, J., Morone, N., et al. Advances in understanding the mechanisms and management of persistent pain in older adults. British Journal of Anaesthesia. 2008;101(1):111–120.
Katsma, D.L., Souza, C.H. Elderly pain assessment and pain management knowledge of long-term care nurses. Pain Management Nursing. 2000;1(3):88–95.
Katz, E.R., Sharp, B., Kellerman, J., et al. Beta-endorphin immunoreactivity and acute behavioral distress in children with leukemia. The Journal of Nervous and Mental Disease. 1982;170:72–77.
Kelley, A., Siegler, E., Reid, C. Pitfalls and recommendations regarding the management of acute pain among hospitalized patients with dementia. Pain Medicine. 2008;9(5):581–586.
Kemp, C.A., Ersek, M., Turner, J.A., A descriptive study of older adults with persistent pain: Use and perceived effectiveness of pain management strategies. BMC Geriatrics 2005;5:12. Available at http://www.biomedcentral.com/1471-2318/5/12 [Accessed August 18, 2009].
Kerrane, T.A. The Brompton Cocktail. Nursing Mirror. 1975;140:59.
Ketovuori, H. Nurses’ and patients’ conceptions of wound pain and the administration of analgesics. Journal of Pain and Symptom Management. 1987;2(4):213–218.
Kim, H.S., Schwartz-Barcott, D., Tracy, S.M., et al. Strategies of pain assessment used by nurses on surgical units. Pain Management Nursing. 2005;6(1):3–9.
Kissen, B. Medical management of alcoholic patients. In: Kissen B., Begleiter H., eds. The biology of alcoholism, vol. 5, Treatment and rehabilitation of the chronic alcoholic. New York: Plenum, 1997.
Klein, M. Perception of pain in the persistent vegetative state? European Journal of Pain (London, England). 1997;1:165–167.
Kleinman, I., Brown, P., Librach, L. Placebo pain medication. Archives of Family Medicine. 1994;3:453–457.
Kovach, C.R., Noonan, P.E., Griffie, J., et al. The assessment of discomfort in dementia protocol. Pain Management Nursing. 2002;3(1):16–27.
Kovach, C.R., Noonan, P.E., Schlidt, A.M., et al. The Serial Trial Intervention: An innovative approach to meeting needs of individuals with dementia. Journal of Gerontological Nursing. 2006;32(4):18–25.
Krivo, S., Reidenberg, M.M. Assessment of patient’s pain. The New England Journal of Medicine. 1995;334:59.
Kudoh, A. Perioperative management for chronic schizophrenic patients. Anesthesia and Analgesia. 2005;101:1867–1872.
Kudoh, A., Ishihara, H., Matsuki, A. Current perception thresholds and postoperative pain in schizophrenic patients. Regional Anesthesia and Pain Medicine. 2000;25:475–479.
Kudoh, A., Takahira, Y., Katagal, H., et al. Schizophrenic patients who developed postoperative confusion have increased nor-epinephrine and cortisol secretion. Neuropsychobiol. 2002;46:7–12.
Kulka, R.A., Schlenger, W., Fairbank, J., et al. Trauma and the Vietnam War generation. New York: Bruner/Mazel, 1990.
Kunz, M., Mykius, V., Scharmann, S., et al. Influence of dementia on multiple components of pain. European Journal of Pain. 2009;13:317–325.
Kunz, M., Prkachin, K., Lautenbacher, S. The smile of pain. Pain. 2009;145:273–275.
Kunz, M., Scharmann, S., Hemmeter, U., et al. The facial expression of pain in patients with dementia. Pain. 2007;133(1-3):221–228.
LaChapelle, D.L., Hadjistavropoulos, T., Craig, K.D. Pain measurement in persons with intellectual disabilities. The Clinical Journal of Pain. 1999;15(1):13–23.
Larue, F., Fontaine, A., Colleau, S.M. Underestimation and undertreatment of pain in HIV disease: Multicentre study. BMJ (Clinical Research Ed.). 1997;3144:23–28.
Lavin, M.R. Placebo effects on mind and body. JAMA: The Journal of the American Medical Association. 1991;265:1753–1754.
Lawrence, M. The unconscious experience. American Journal of Critical Care. 1995;4:227.
Leavitt, F., Sweet, J.J. Characteristics and frequency of malingering among patients with low back pain. Pain. 1986;25:357–364.
Lefebvre-Chapiro, S. The Doloplus 2 scale: Evaluating pain in the elderly. Eur J Palliat Care. 2001;8(5):191–194.
Lentschener, C., Tostivint, P., White, P.F., et al. Opioid-induced sedation in the postanesthesia care unit does not ensure adequate pain relief: A case-control study. Anesthesia and Analgesia. 2007;105(4):1143–1147.
Leong, I.Y., Chong, M.S., Gibson, S.J. The use of a self-reported pain measure, a nurse-reported pain measure and the PAINAD in nursing home residents with moderate and severe dementia: A validation study. Age and Ageing. 2006;35(3):252–256.
Leucht, S., Burkard, T., Henderson, J., et al. Physical illness and schizophrenia: A review of the literature. Acta Psychiatrica Scandinavica. 2007;116:317–333.
Levine, F.M., De Simone, L.L. The effects of experimenter gender on pain report in male and female subjects. Pain. 1991;44:69–72.
Levine, J.D., Gordon, N.C., Fields, H. The mechanism of placebo analgesia. Lancet. 1978;2:654–657.
Levy, M. Pain management in advanced cancer. Seminars in Oncology. 1985;12:394–410.
Li, L., Liu, X., Herr, K. Postoperative pain intensity assessment: A comparison of four scales in Chinese adults. Pain Medicine (Malden, Mass.). 2007;8(3):223–234.
Li, D., Puntillo, K., Miaskowski, C. A review of objective pain measures for use with critical care adult patients unable to self-report. The Journal of Pain. 2008;9(1):2–10.
Lidstone, S.C., de la Fuente-Fernandez, R., Stoessl, J.A. The placebo response as a reward mechanism. Semin Pain Med. 2005;3:37–42.
Lin, C., Ward, S. Patient-related barriers to cancer pain management in Taiwan. Cancer Nursing. 1995;18(1):16–22.
Lind, L., Karlsson, D., Fridlund, B. Patients’ use of digital pens for pain assessment in advanced palliative home healthcare. International Jorunal of Medical Informatics. 2008;77:129–136.
Litt, M.D., Cooney, N.L., Morse, P. Ecological momentary assessment (EMA) with treated alcoholics: Methodological problems and potential solutions. Health Psychology. 1998;17:48–52.
Louie, K.B. Providing health care to Chinese clients. Topics in Clinical Nursing. 1985;7:18–25.
MacDonald, V., Hilton, B. Postoperative pain management in frail elderly adults. Orthopaedic Nursing/National Association of Orthopaedic Nurses. 2001;20:63–76.
Mader, T.F., Blank, F.S.J., Smithline, H.A., et al. How reliable are the pain scores? A pilot study of 20 healthy volunteers. Journal of Emergency Nursing. 2003;29(4):322–325.
Mahoney, A, Peters, L. The Mahoney Pain Scale: Examining pain and agitation in advanced dementia. American Journal of Alzheimer’s Disease & Other Dementias. 2008;23(3):250–261.
Malone, R.E. Almost like family: Emergency nurses and frequent flyers. Journal of Emergency Nursing. 1996;22(3):176–183.
Manfredi, P.L., Breuer, B., Wallenstein, S., et al. Opioid treatment for agitation in patients with advanced dementia. International Journal of Geriatric Psychiatry. 2003;18(8):700–705.
Marceau, L.D., Link, C., Jamison, R.N., et al. Electronic diaries as a tool to improve pain management: Is there any evidence? Pain Medicine (Malden, Mass.). 2007;6(S3):S101–S109.
Marco, C.A., Plewa, M.C., Buderer, N., et al. Self-reported pain scores in the emergency department: Lack of association with vital signs. Academic Emergency Medicine. 2006;13:974–979.
Marquie, L., Raufaste, E., Lauque, D., et al. Pain rating by patients and physicians: evidence of systematic pain miscalibration. Pain. 2003;102:289–296.
Martell, B.A., O’Connor, P.G., Kerns, R.D., et al. Systematic review: Opioid treatment for chronic back pain: Prevalence, efficacy, and association with addiction. Annals of Internal Medicine. 2007;146:116–127.
Mateo, O., Krenzischek, D. A pilot study to assess the relationship between behavioral manifestations and self-report of pain in postanesthesia care unit patients. Journal of Post Anesthesia Nursing. 1992;7:15–21.
Maxwell, C. Sensitivity and accuracy of the visual analogue scale: A psycho-physical classroom experiment. British Journal of Clinical Pharmacology. 1978;6:17–24.
Mayday Fund. 1993 Pain Survey. New York: The Mayday Fund, 1993.
Mayday Fund. Pain in America: A survey of American attitudes toward pain. New York: The Mayday Fund, 1998.
Mayer, D.D.M., Torma, L., Byock, I., et al. Speaking the language of pain. The American Journal of Nursing. 2001;101(2):44–49.
Mazanee, P., Tyler, M.K. Cultural considerations in end-of-life care. The American Journal of Nursing. 2003;103(3):50–58.
McAuliffe, L., Nay, R., O’Donnell, M., et al. Pain assessment in older people with dementia: Literature review. Journal of Advanced Nursing. 2008;65(1):2–10.
McCaffery, M. Nursing practice theories related to cognition, bodily pain, and man-environment interactions. Los Angeles: University of California at Los Angeles Students’ Store, 1968.
McCaffery, M., Arnstein, P. The debate over placebos in pain management. The American Journal of Nursing. 2006;106(2):62–65.
McCaffery, M., Ferrell, B. How would you respond to these patients in pain? Nursing. 1991;21(6):34–37.
McCaffery, M., Ferrell, B. Opioid analgesics: Addiction. Age Conc. 1992;1(5):1,4.
McCaffery, M., Ferrell, B. Are nurses’ analgesic choices influenced by the pain rating scale used? 0-5 versus 0-10. ASPMN Pathways. 1995;4(1):3, 6.
McCaffery, M., Ferrell, B.R., O’Neil-Page, E. Does life-style affect your pain-control decisions? Nursing. 1992;22(4):58–61.
McCaffery, M., Ferrell, B.R., Pasero, C.L. When the physician prescribes a placebo. The American Journal of Nursing. 1998;98(1):52–53.
McCaffery, M., Ferrell, B.R., Turner, M. Ethical issues in the use of placebos in cancer pain management. Oncology Nursing Forum. 1996;23:1587–1593.
McCaffery, M., Ferrell, B.R. Patient age: Does it affect your pain-control decisions? Nurs. 1991;21(9):44–48.
McCaffery, M., Ferrell, B.R. Does the gender gap affect your pain-control decisions? Nurs. 1992;22(8):48–51.
McCaffery, M., Ferrell, B.R. How vital are vital signs? Nurs. 1992;22(1):42–46.
McCaffery, M., Ferrell, B.R. Opioid analgesics: Nurses’ knowledge of doses and psychological dependence. Journal of Nursing Staff Development. 1992;8(2):77–84.
McCaffery, M., Ferrell, B.R. Say no to placebos. The American Journal of Nursing. 1994;94(11):20.
McCaffery, M., Ferrell, B.R. Nurses’ knowledge about cancer pain: A survey of five countries. Journal of Pain and Symptom Management. 1995;10(5):356–369.
McCaffery, M., Ferrell, B.R. Correcting misconceptions about pain assessment and use of opioid analgesics: Educational strategies aimed at public concerns. Nursing Outlook. 1996;44(4):184–190.
McCaffery, M., Ferrell, B.R. Current placebo practice and policy. ASPMN Pathways. 1996;5(4):12–14. [1].
McCaffery, M., Ferrell, B.R. Nurses’ knowledge of pain assessment and management: How much progress have we made? Journal of Pain and Symptom Management. 1997;14(3):175–188.
McCaffery, M., Ferrell, B.R. Pain and placebos: Ethical and professional issues. Orthopaedic Nursing/National Association of Orthopaedic Nurses. 1997;16(5):8–11.
McCaffery, M., Grimm, M.A., Pasero, C., et al. On the meaning of “drug seeking.”. Pain Management Nursing. 2005;6(4):122–136.
McCaffery, M., Pasero, C., Ferrell, B.R. Nurses’ decisions about opioid dose. The American Journal of Nursing. 2007;107(12):35–39.
McCaffery, M., Pasero, C. Assessment and treatment of patients with mental illness. The American Journal of Nursing. 2001;101(7):69–70.
McCaffery, M., Pasero, C. Pain: Clinical manual, 2nd ed. St. Louis, MO: Mosby, 1999.
McCaffery, M., Pasero, C. Are there circumstances that justify deceitful placebo use? Pediatric Nursing. 1995;21(6):588.
McCaffery, M., Robinson, E.S. Your patient is in pain: Here’s how you respond. Nursing. 2002;32(10):36–45.
McCaffery, M., Vourakis, C. Assessment and relief of pain in chemically dependent patients. Orthopaedic Nursing/National Association of Orthopaedic Nurses. 1992;11(2):13–27.
McDonald, D.D., Shea, M., Rose, L., et al. The effect of pain question phrasing on older adult pain information. Journal of Pain and Symptom Management. 2009;37(6):1050–1060.
McGhee, L.L., Maani, C.V., Garza, T.H., et al. The correlation between ketamine and posttraumatic stress disorder in burned severe members. The Journal of Trauma. 2008;64(2 Suppl.):S195–S198.
McGrath, P.J., Beyer, J., Cleeland, C., et al. Report of the subcommittee on assessment and methodological issues in the management of pain in childhood cancer. Pediatrics. 1990;86(Suppl. 5):814–817.
McGuire, D.B, Sheidler, V.R. Pain. In Groenwald S.L., Frogge M.H., Goodman M., et al, eds.: Cancer nursing: principles and practice, (8th ed.)., Chicago: Jones and Bartlett, 1993.
McIlveen, K.H., Morse, J.M. The role of comfort in nursing care: 1900-1980. Clinical Nursing Research. 1995;4:127–148.
McMillan, S.C., Williams, F.A., Chatfield, R., et al. A validity and reliability study of two tools for assessing and managing pain. Oncology Nursing Forum. 1988;15:735–741.
Means, B., Nigam, A., Zarrow, M., et al. Autobiographical memory for health-related events. Vital and Health Statistics. 1989;6:1–37.
Melzack, R. The McGill Pain Questionnaire: Major properties and scoring methods. Pain. 1975;1(3):277–299.
Melzack, R. The short-form McGill Pain Questionnaire. Pain. 1987;30:191–197.
Melzack, R., Abbott, F.V., Zackon, W., et al. Pain on a surgical ward: A survey of the duration and intensity of pain and the effectiveness of medication. Pain. 1987;29:67–72.
Mentes, J.C., Teer, J., Cadogan, M.P. The pain experience of cognitively impaired nursing home residents: Perceptions of family members and certified nursing assistants. Pain Management Nursing. 2004;5(3):118–125.
Merkel, S.I., Voepel-Lewis, T., Shayevitz, J.R., et al. The FLACC: A behavioral scale for scoring postoperative pain in young children. Pediatric Nursing. 1997;23(3):293–297.
Merskey H., Bogduk N., eds. Classification of chronic pain, 2nd ed., Seattle: IASP Press, 1994.
Miaskowski, C., Cleary, J., Burney, R., et al. Guideline for the management of cancer pain in adults and children. Glenview, IL: American Pain Society, 2005. [APS Clinical practice Guidelines Series, No. 3].
Miller, R., Eisenkraft, J.B., Cohen, M., et al. Midazolam as an adjunct to meperidine analgesia for postoperative pain. The Clinical Journal of Pain. 1986;2:37–43.
Miro, J., Huguet, A., Nieto, R., et al. Evaluation of reliability, validity, and preference for a pain intensity scale for use with the elderly. The Journal of Pain. 2005;6(11):727–735.
Mitchel, S.W. Civilization and pain. JAMA: The Journal of the American Medical Association. 1982;18:108.
Moerman, D.E. Cultural variations in the placebo effect: Ulcers, anxiety, and blood pressure. Medical Anthropology Quarterly. 2000;14:1–22.
Moerman, D.E., Harrington, A. Making space for the placebo effect in pain medicine. Semin Pain Med. 2005;3:2–6.
Moerman, H., Bonke, B., Oosting, J. Awareness and recall during general anesthesia. Anesthesiology. 1993;76(3):454–464.
Morello, R., Jean, A., Alix, M., et al. A scale to measure pain in non-verbally communicating older patients: The EPCA-2 study of its psychometric properties. Pain. 2007;133(1–3):87–98.
Mularski, R.A., White-Chu, F., Overbay, D., et al. Measuring pain as the fifth vital sign does not improve quality of pain management. J Gen Int Med. 2006;2(6):607–612.
Nader, R., Oberlander, T.F., Chambers, C.T., et al. Expression of pain in children with autism. The Clinical Journal of Pain. 2004;20(2):88–97.
Negri, E., Bettaglio, R., Demartini, L., et al. Validation of the Italian version of the Neuropathic Pain Scale and its clinical applications. Minerva Anestesiologica. 2002;68:95–104.
Nelson, F.V., Zimmerman, L., Barnason, S., et al. The relationship and influence of anxiety on postoperative pain in the coronary artery bypass graft patent. Journal of Pain and Symptom Management. 1998;15:102–109.
Ng, B., Dimsdale, J.E., Rollnik, J.D., et al. The effect of ethnicity on prescriptions for patient-controlled analgesia for post-operative pain. Pain. 1996;66:9–12.
Nicholas, M.K. Mental disorders in people with chronic pain: An international perspective. Pain. 2007;129:231–232.
Nichols, R. Pain during sickle-cell crises. The American Journal of Nursing. 1996;96:59–60.
Nicholson, B., Passik, S.D. Management of chronic noncancer pain in the primary care setting. Southern Medical Journal. 2007;100(10):1028–1036.
Nisbet, A. Inova Sensory Assessment Scale (Personal e-mail communication). Falls Church, VA: Inova Fairfax Hospital, 2008.
Noble, M., Tregear, S.J., Treadwell, J.R., et al. Long-term opioid therapy for chronic noncancer pain: A systematic review and meta-analysis of efficacy and safety. Journal of Pain and Symptom Management. 2008;35(2):214–228.
Norman, S.B., Stein, M.B., Dimsdale, J.E., et al. Pain in the aftermath of trauma is a risk factor for post-traumatic stress disorder. Psychological Medicine. 2008;38:533–542.
Nygaard, H.A., Jarland, M. The checklist of nonverbal pain indicators (CNPI): Testing of reliability and validity in Norwegian nursing homes. Age and Ageing. 2006;35(1):79–81.
Odhner, M., Wegman, D., Freeland, N., et al. Assessing pain control in nonverbal critically ill adults. Dimensions of Critical Care Nursing: DCCN. 2003;22(6):260–267.
Office of National Drug Control Policy. The national drug control strategy. Washington, DC: GPO, 1997.
Oh, V.M.S. The placebo effect: Can we use it better? BMJ (Clinical Research Ed.). 1994;309:69–70.
Ostelo, R.W.J.G., Devo, R.A., Stratford, P. Interpreting change scores for pain and functional status in low back pain. Spine. 2008;33(1):90–94.
Otis, J.D., Keane, T.M., Kerns, R.D. An examination of the relationship between chronic pain and post-traumatic stress disorder. Journal of Rehabilitation Research and Development. 2003;40(5):397–406.
Otis, J.D., Keane, T.M., Kerns, R., et al. The development of an integrated treatment for veterans with comorbid chronic pain and posttraumatic stress disorder. Pain Medicine (Malden, Mass.). 2009;10(7):1300–3011.
Paice, J.A., Cohen, F.L. Validity of a verbally administered numeric rating scale to measure cancer pain intensity. Cancer Nursing. 1997;20:88–93.
Paice, J.A., Noskin, G.A., Vanagunas, A., et al. Efficacy and safety of scheduled dosing of opioid analgesics: A quality improvement study. The Journal of Pain. 2005;6(10):639–643.
Paqueron, X., Lumbroso, A., Mergoni, P., et al. Is morphine-induced sedation synonymous with analgesia during intravenous morphine titration? British Journal of Anaesthesia. 2002;89:697–701.
Parmelee, P.A., Smith, B.D., Katz, I.R. Pain complaints and cognitive status among elderly institution residents. Journal of the American Geriatrics Society. 1993;41:517–522.
Pasero, C. Pathophysiology of neuropathic pain. Pain Management Nursing. 2004;5(4 Suppl. 1):3–8.
Pasero, C., McCaffery, M. Pain in the critically ill: New information reveals that one of the simplest procedures—turning—can be the most painful one. The American Journal of Nursing. 2002;102(1):59–60.
Pasero, C., McCaffery, M. No self-report means no pain-intensity rating. The American Journal of Nursing. 2005;105(10):50–53.
Passik, S.D., Kirsh, K.L., Whitcomb, L., et al. A new tool to assess and document pain outcomes in chronic pain patients receiving opioid therapy. Clinical Therapeutics. 2004;26(4):552–561.
Passik, S.D., Kirsh, K.L. Opioid therapy in patients with a history of substance abuse. CNS Drugs. 2004;18(1):13–25.
Pautex, S., Herrmann, F., Le Lous, P., et al. Feasibility and reliability of four pain self-assessment scales and correlation with an observational rating scale in hospitalized elderly demented patients. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 2005;60(4):524–529.
Pautex, S., Herrmann, F.R., Michon, A., et al. Psychometric properties of the Doloplus-2 observational pain assessment scale and comparison to self-assessment in hospitalized elderly. The Clinical Journal of Pain. 2007;23(9):774–779.
Pautex, S., Michon, A., Guedira, M., et al. Pain in severe dementia: Self-assessment or observational scales? Journal of the American Geriatrics Society. 2006;54(7):1040–1045.
Payen, J.F., Bosson, J.L., Chanques, G., et al. Pain assessment is associated with decreased duration of mechanical ventilation in the intensive care. Anesthesiology. 2009;111(6):1308–1316.
Payen, J.F., Bru, O., Bosson, J.L., et al. Assessing pain in critically ill sedated patients by using a behavioral pain scale. Critical Care Medicine. 2001;29(12):2258–2263.
Payne, R. Recognition and diagnosis of breakthrough pain. Pain Medicine (Malden, Mass.). 2007;8(Suppl. 1):S3–S7.
Perkins, K.A., Grobe, J.E., Stiller, R.L., et al. Effects of nicotine on thermal pain detection in humans. Experimental and Clinical Psychopharmacology. 1994;2(1):95–106.
Pernick, M.S. A calculus of suffering. New York: Columbia University Press, 1985.
Perry, S., Heidrich, G. Management of pain during débridement: A survey of U.S. burn units. Pain. 1982;13:267–280.
Pesonen, A., Kauppila, T., Tarkkila, P., et al. Evaluation of easily applicable pain measurement tools for the assessment of pain in demented patients. Acta Anaesthesiologica Scandinaviac. 2009;53:657–664.
Peters, M.L., Patijn, J., Lame, I. Pain assessment in younger and older pain patients: psychometric properties and patient preference of five commonly used measures of pain intensity. Pain Medicine (Malden, Mass.). 2007;8(7):601–610.
Pitman, R.K., Sanders, K.M., Zusman, R.M., et al. Pilot study of secondary prevention of posttraumatic stress disorder with propranolol. Biological Psychiatry. 2002;51(2):189–192.
Pitman, R.K., van der Kolk, S.P., et al. Naloxone-reversible analgesic response to combat-related stimuli in posttraumatic stress disorder: A pilot study. Archives of General Psychiatry. 1990;47(6):541–544.
Pletcher, M.J., Kertesz, S.G., Kohn, M.A., et al. Trends in opioid prescribing by race/ethnicity for patients seeking care in US emergency departments. JAMA: The Journal of the American Medical Association. 2008;299:70–78.
Portenoy, R., for the ID Pain Steering Committee. Development and testing of a neuropathic pain screening questionnaire. ID Pain. Current Medical Research and Opinion. 2006;22(1):1555–1565.
Portenoy, R.K. Opioid therapy for chronic nonmalignant pain: Current status. In: Fields H.L., Liebeskind J.C., eds. Progress in pain research and management, Vol. 1: Pharmacological approaches to the treatment of chronic pain: New concepts and critical issues. Seattle: IASP Press, 1994.
Portenoy, R.K. Opioid analgesics. In: Portenoy R.K., Kanner R.M., eds. Pain management theory and practice. Philadelphia: FA Davis, 1996.
Portenoy, R.K., Bennett, D.S., Rauck, R., et al. Prevalence and characteristics of breakthrough pain in opioid-treated patients with chronic noncancer pain. The Journal of Pain. 2006;7(8):583–591.
Portenoy, R.K., Farrar, J.T., Backonja, M.M., et al. Long-term use of controlled-release oxycodone for noncancer pain: Results of a 3-year registry study. The Clinical Journal of Pain. 2007;23(4):287–299.
Portenoy, R.K., Hagen, N.A. Breakthrough pain: Definition, prevalence and characteristics. Pain. 1990;41:273–281.
Portenoy, R.K, Payne, D., Jacobsen, P. Breakthrough pain: Characteristics and impact in patients with cancer pain. Pain. 1999;81:128–134.
Porter, F.L., Malhotra, K.M., Wolf, C.M., et al. Dementia and response to pain in the elderly. Pain. 1996;68:413–421.
Porter, J., Jick, H. Addiction rare in patients treated with narcotics. The New England Journal of Medicine. 1980;302(2):123.
Porzelius, J. Memory for pain after nerve-block injections. The Clinical Journal of Pain. 1995;11:112–120.
Potvin, S., Marchand, S. Hypoalgesia in schizophrenia is independent of antipsychotic drugs: A systematic quantitative review of experimental studies. Pain. 2008;138:70–78.
Poundja, J., Fikretoglu, D., Guay, S., et al. Validation of the French version of the brief pain inventory in Canadian veterans suffering from traumatic stress. Journal of Pain and Symptom Management. 2007;33(6):720–726.
Price, D.D., Chung, K.C., Robinson, M.E. Conditioning, expectation, and desire for relief in placebo analgesia. Semin Pain Med. 2005;3:15–21.
Price, D.D., Long, S., Wilsey, B., et al. Analysis of peak magnitude and duration of analgesia produced by local anesthetics injected into sympathetic ganglia of complex regional pain syndrome patients. The Clinical Journal of Pain. 1998;14:216–226.
Prins, A., Quimette, P., Kimerling, R., et al. The primary care PTSD screen (PC-PDST): development and operating characteristics. Prim Care Psychiat. 2003;9(1):9–14.
Pud, D. Personal past experience with opioid consumption affects attitudes and knowledge related to pain management. Pain Management Nursing. 2004;5(4):153–159.
Pud, D., Amit, A. Anxiety as a predictor of pain magnitude following termination of first-trimester pregnancy. Pain Medicine (Malden, Mass.). 2005;6(2):143–148.
Puntillo, K.A. Pain experiences of intensive care unit patients. Heart and Lung. 1990;19(5):526–533.
Puntillo, K.A. Dimensions of procedural pain and its analgesic management in critically ill surgical patients. American Journal of Critical Care. 1994;3:116–122.
Puntillo, K.A., Miaskowski, C., Kehrie, K., et al. Relationship between behavioral and physiological indicators of pain, critical care patients’ self-reports of pain, and opioid administration. Critical Care Medicine. 1997;25:1159–1166.
Puntillo, K.A., Morris, A.B., Thompson, C.L., et al. Pain behaviors observed during six common procedures: Results from Thunder Project II. Critical Care Medicine. 2004;32(2):421–427.
Puntillo, K.A., White, C., Morris, A.B., et al. Patients’ perceptions and responses to procedural pain: Results from Thunder Project II. American Journal of Critical Care. 2001;10(4):238–251.
Puntillo, K.A., Wild, L.R., Morris, A.B., et al. Practices and predictors of analgesic interventions for adults undergoing painful procedures. American Journal of Critical Care. 2002;11(5):315–429.
Raphael, K.G., Marbach, J.J. When did your pain start? Reliability of self-reported age of onset of facial pain. Pain. 1997;13:352–359.
Reizien, A., Meleis, A. Arab-Americans’ perceptions of and responses to pain. Critcial Care Nurse. 1986;6:30–37.
Reynolds, K., Hanson, L., DeVellis, R., et al. Disparities in pain management between cognitively intact and cognitively impaired nursing home residents. Journal of Pain and Symptom Management. 2008;35(4):388–396.
Richardson, P.H. Placebo effects in pain management. Pain Reviews. 1994;1:15–32.
Richeimer, S.H., Case, G.A. Ethical issues and problems of trust in the management of chronic pain. In Warfield C.A., Bajwa Z.H., eds.: Principles and practice of pain management, 2nd ed., New York: McGraw-Hill, 2004.
Rog, D.J., Nurmikko, T.J., Friede, T., et al. Validation and reliability of the neuropathic pain scale (NPS) in multiple sclerosis. The Clinical Journal of Pain. 2007;23(6):473–481.
Rosenfeld, B., Brietbart, W., McDonald, M.V., et al. Pain in ambulatory AIDS patients. II: Impact of pain on psychological functioning and quality of life. Pain. 1996;68:323–328.
Ross, R.S., Bush, J.P., Crummette, B.D. Factors affecting nurses’ decisions to administer prn analgesic medication to children after surgery: An analog investigation. Journal of Pediatric Psychology. 1991;16:151–167.
Rothman, K.J., Michels, K.B. The continuing unethical use of placebo controls. The New England Journal of Medicine. 1994;331:394–397.
Rutledge, D.R., Donaldson, N.E. Pain assessment and documentation. Part II: Special populations of adults. Online J Clin Innov. 1998;1(6):1–29.
Ryan, P., Vortherms, R., Ward, S. Cancer pain: knowledge, attitudes of pharmacologic management. Journal of Gerontological Nursing. 1994;20(1):7–16.
Salmon, P., Manyande, A. Good patients cope with their pain: Postoperative analgesia and nurses’ perceptions of their patients’ pain. Pain. 1996;68:63–68.
Salmore, R. Development of a new pain scale: Colorado behavioral numerical pain scale for sedated adult patients undergoing gastrointestinal procedures. Gastroenterology Nursing. 2002;25(6):257–264.
Savage, S.R. Assessment for addiction in pain-treatment settings. The Clinical Journal of Pain. 2002;18:S28–S38.
Saxe, G., Stoddard, F., Courtney, D., et al. Relationship between acute morphine and the course of PTSD in children with burns. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40(8):915–921.
Scherder, E., Herr, K., Pickering, G., et al. Pain in dementia. Pain. 2009;145:276–278.
Scherder, E., Oosterman, J., Swaab, D., et al. Recent developments in pain in dementia. BMJ (Clinical Research Ed.). 2005;330(7489):461–464.
Scherder, E.J., Sergeant, J.A., Swaab, D.F. Pain processing in dementia and its relation to neuropathology. Lancet Neurology. 2003;2(11):677–686.
Schmitz Schneider, M.E., Jarvik, N. G. Nicotine. In Lowinson J.H., Ruiz P., Millman R.B., et al, eds.: Substance abuse: A comprehensive textbook, 3rd ed., Baltimore: Lippincott Williams & Wilkins, 1997.
Schuler, M., Njoo, N., Hestermann, M., et al. Acute and chronic pain in geriatrics: Clinical characteristics of pain and the influence of cognition. Pain Medicine (Malden, Mass.). 2004;5(3):253–262.
Schuler, M.S., Becker, S., Kaspar, R., et al. Psychometric properties of the German Pain Assessment in Advanced Dementia Scale (PAINAD-G) in nursing home residents. Journal of the American Medical Directors Association. 2007;8(6):388–395.
Schumacher, K.L., Koresawa, S., West, C., et al. The usefulness of a daily pain management diary for outpatients with cancer-related pain. Oncology Nursing Forum. 2002;29(9):1304–1313.
Schwartz, A.C., Bradley, R., Penza, K.M., et al. Pain medication use among patients with posttraumatic stress disorder. Psychosomatics. 2006;47(2):136–142.
Schwartz, J.E., Stone, A.A. Strategies for analyzing ecological momentary assessment data. Health Psychology. 1998;17:6–16.
Schwender, D., Kunze-Kronawitter, H., Dietrich, P., et al. Conscious awareness during general anaesthesia: Patients’ perceptions, emotions, cognition and reactions. British Journal of Anaesthesia. 1998;80:133–139.
Serlin, R.C., Mendoza, T.R., Nakamura, Y., et al. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain. 1995;61:277–284.
Shapiro, D., Jarvik, M.E. Pain inhibition, nicotine, and gender. Experimental and Clinical Psychopharmacology. 1998;6(1):96–106.
Shega, J.W., Hougham, G.W., Stocking, C.B., et al. Pain in community-dwelling persons with dementia: Frequency, intensity, and congruence between patient and caregiver report. Journal of Pain and Symptom Management. 2004;28(6):585–592.
Shega, J.W., Rudy, T., Keefe, F.J., et al. Validity of pain behaviors in persons with mild to moderate cognitive impairment. J Am Geriatri Soc. 2008;56(9):1631–1637.
Sherman, J.J., Turk, D.C., Okifuji, A. Prevalence and impact of posttraumatic stress disorder-like symptoms on patients with fibromyalgia syndrome. The Clinical Journal of Pain. 2000;16:127–134.
Shipherd, J.C., Keyes, M., Jovanovic, T., et al. Veterans seeking treatment for posttraumatic stress disorder: What about comorbid chronic pain? Journal of Rehabilitation Research and Development. 2007;44(2):153–166.
Short, L.M., Burnett, M.L., Egbert, A.M., et al. Medicating the postoperative elderly: How do nurses make their decisions? Journal of Gerontological Nursing. 1990;16(7):12–17.
Simon, G.E., Gureje, O. Stability of somatization disorder and somatization symptoms among primary care patients. Archives of General Psychiatry. 1999;56:90–95.
Singh, M.K., Giles, L.L., Nasrallah, H.A. Pain insensitivity in schizophrenia: Trait or state marker? Journal of Psychiatric Practice. 2006;12:90–102.
Sloan, P.A., Donelly, M.B., Schwartz, R.W., et al. Cancer pain assessment and management by house staff. Pain. 1996;67:475–481.
Sloman, R., Wruble, A.W., Rosen, G., et al. Determination of clinically meaningful levels of pain reduction in patients experiencing acute postoperative pain. Pain Management Nursing. 2006;7(4):153–158.
Smith, M.P., Sheplock, G.J. The anesthesiologist’s guide to pain computing. Regional Anesthesia and Pain Medicine. 1999;24:458–462.
Smith, M.V., Egert, J., Winkel, G., et al. The impact of PTSD on pain experience in persons with HIV/AIDS. Pain. 2002;98:9–17.
Smith, W.B., Safer, M.A. Effects of present pain level on recall of chronic pain and medication use. Pain. 1993;55:355–361.
Snow, A.L., Weber, J.B., O’Malley, K.J., et al. NOPPAIN: A nursing assistant-administered pain assessment instrument for use in dementia. Dementia and Geriatric Cognitive Disorders. 2004;17(3):240–246.
Sokka, T. Assessment of pain in rheumatic diseases. Clinical and Experimental Rheumatology. 2005;23:S77–S84.
Spagrud, L.J., Piirao, T., von Baeyer, C.L. Children’s self-report of pain intensity. The American Journal of Nursing. 2003;103(12):62–64.
Spross, J.A. Pain, suffering, spiritual well-being: assessment and interventions. Qual Life. 1993;2:71–79.
Stanik-Hutt, J.A., Soeken, K.L., Belcher, A.E., et al. Pain experiences of traumatically injured patients in a critical care setting. American Journal of Critical Care. 2001;10(4):252–259.
Stephens, S.T. A promise to Billie. Nursing. 1994;24(4):96.
Stolee, P., Hillier, L.M., Esbaugh, J., et al. Instruments for the assessment of pain in older persons with cognitive impairment. Journal of the American Geriatrics Society. 2005;53(2):319–326.
Stolee, P., Hillier, L.M., Esbaugh, J., et al. Pain assessment in a geriatric psychiatry program. Pain Research & Management. 2007;12(4):273–280.
Stone, A.A., Broderick, J.E., Schwartz, J.E., et al. Intensive momentary reporting of pain with an electronic diary: Reactivity, compliance, and patient satisfaction. Pain. 2003;104:343–351.
Stone, A.A., Shiffman, S., Schwartz, J.E., et al. Patient non-compliance with paper diaries. British Medical Journal. 2002;324:1193–1194.
Sullivan, M., Terman, G.W., Peck, B., et al. APS position statement on the use of placebos in pain management. The Journal of Pain. 2005;6(4):215–217.
Symons, F.J., Shinde, S.K., Gilles, E. Perspectives on pain and intelleactual disability. Journal of Intellectual Disability Research. 2008;52(4):275–286.
Syrjala, K.L. The measurement of pain. In: Yarbo C.H., McGuire D.B., eds. Cancer pain: Nursing management. Orlando, FL: Grune & Stratton, 1987.
Syrjala, K.L. Integrating medical and psychological treatments for cancer pain. In: Chapman C.R., Foley K.M., eds. Current and emerging issues in cancer pain: Research and practice. New York: Raven Press, 1993.
Tan, T., Fink, B., Dao, T.K., et al. Associations among pain, PTSD, and heart rate. variability in veterans of operation enduring and Iraqi freedom: A pilot study. Pain Medicine (Malden, Mass.). 2009;10(7):1237–1245.
Tan, G., Jensen, M.P., Thornby, J.I., et al. Validation of the brief pain inventory for chronic nonmalignant pain. The Journal of Pain. 2004;5(2):133–137.
Taylor, A.G., Skelton, J.A., Butcher, J. Duration of pain condition and physical pathology as determinants of nurses’ assessments of patients in pain. Nursing Research. 1984;33:4–8.
Taylor, D.R., Webster, L.R., Chun, S.Y., et al. Impact of breakthrough pain on quality of life in patients with chronic, noncancer pain: Patient perceptions and effect of treatment with oral transmucosal fentanyl citrate (OTFC, ACTIQ). Pain Medicine (Malden, Mass.). 2007;8(3):281–288.
Taylor, L.J., Harris, J., Epps, C.D., et al. Psychometric evaluation of selected pain intensity scales for use with cognitively impaired and cognitively intact older adults. Rehabilitation Nursing. 2005;30(2):55–61.
Taylor, L.J., Herr, K. Pain intensity assessment: A comparison of selected pain intensity scales for use in cognitively intact and cognitively impaired African American older adults. Pain Management Nursing. 2003;4(2):87–95.
Taylor, N.M., Hall, G.M., Salmon, P. Is patient-controlled analgesia controlled by the patient? Social Science & Medicine. 1996;43:1137–1143.
Taylor, N.M., Hall, G.M., Salmon, P. Patients’ experiences of patient-controlled analgesia. Anaesthesia. 1996;51:525–528.
Teasell, R.W., Merskey, H. Chronic pain disability in the workplace. Pain Research & Management. 1997;2(4):197–204.
Terry, R., Niven, C., Brodie, E., et al. An exploration of the relationship between anxiety; expectations and memory for postoperative pain. Acute Pain. 2007;9:135–143.
Todd, K.H., Deaton, C., d’Adamo, A.P., et al. Ethnicity and analgesic practice. Annals of Emergency Medicine. 2000;35(1):11–16.
Todd, K.H., Samaroo, N., Hoffman, J.R. Ethnicity as a risk factor for inadequate emergency department analgesia. JAMA: The Journal of the American Medical Association. 1993;269(12):1537–1539.
Turk, D.C. Remember the distinction between malignant and benign pain? Well, forget it. The Clinical Journal of Pain. 2002;18(2):75–76.
Turk, D.C., Okifuji, A. What factors affect physicians’ decisions to prescribe opioids for chronic noncancer pain patients? The Clinical Journal of Pain. 1997;13:330–336.
Turk, M.A., Geremski, C.A., Rosenbaum, P.F., et al. The health status of women with cerebral palsy. Archives of Physical Medicine and Rehabilitation. 1997;78:S10–S17.
Turner, J.A., Deyo, R.A., Loeser, J.D., et al. The importance of placebo effects in pain treatment and research. Journal of American Medical Association. 1994;271(20):1609–1614.
Twycross, R.G. Clinical experience with diamorphine in advanced malignant disease. International Journal of Clinical Pharmacology, Therapy and Toxicology. 1974;9:184–198.
Twycross, R., Harcourt, J., Bergl, S. A survey of pain in patients with advanced cancer. Journal of Pain and Symptom Management. 1996;12:273–282.
Twycross, R.G., Wald, S.J., Long-term use of diamorphine in advanced cancer. Bonica, J.J., Albe-Fessard, D., eds. Advances in pain research and therapy, Vol. 1. New York: Raven Press, 1976.
Uki, J., Mendoza, T., Cleeland, C., et al. A brief cancer pain assessment tool in Japanese: The utility of the Japanese brief pain inventory—BPI-J. Journal of Pain and Symptom Management. 1998;16(6):364–373.
van Cleve, L., Johnson, L., Pothier, P. Pain responses of hospitalized infants and children to venipuncture and intravenous cannulation. Journal of Pediatric Nursing. 1996;11:161–168.
van den Berg, J.H. Leven in meervoud. Nijkerk, GF: Callenback, 1963.
van Herk, R., van Dijk, M., Baar, F.P., et al. Observation scales for pain assessment in older adults with cognitive impairments or communication difficulties. Nursing Research. 2007;56(1):34–43.
van Herk, R., van Dijk, M., Biemold, N., et al. Assessment of pain: Can caregivers or relatives rate pain in nursing home residents? Journal of Clinical Nursing. 2009;18:2478–2485.
van Herk, R., van Dijk, M., Tibboel, D., et al. The Rotterdam Elderly Pain Observation Scale (REPOS): A New Behavioral Pain Scale for Non-Communicative Adults and Cognitively Impaired Elderly Persons. J Pain Manage. 2009;1(4):367–378.
van Iersel, T., Timmerman, D., Mullie, A. Introduction of a pain scale for palliative care patients with cognitive impairment. International Journal of Palliative Nursing. 2006;12(2):54–59.
van Nispen tot Pannerden, S., Candel, M., Zwakhalen, S., et al. An item response theory-based assessment of the Pain Assessment Checklist for Seniors with Limited Ability to Communicate (PACSLAC). The Journal of Pain. 2009;10(8):844–853.
Victor, L., Richeimer, S.H. Trustworthiness as a clinical variable: The problem of trust in the management of chronic, nonmalignant pain. Pain Medicine (Malden, Mass.). 2005;6(5):385–391.
Victor, T.W., Jensen, M.P., Gammaitoni, A.R., et al. The dimensions of pain quality: Factor analysis of the pain quality assessment scale. The Clinical Journal of Pain. 2008;24(6):550–555.
Villano, C.L., Rosenblum, A., Magura, S., et al. Prevalence and correlates of posttraumatic stress disorder and chronic severe pain in psychiatric outpatients. Journal of Rehabilitation Research and Development. 2007;44(2):167–178.
Virit, O., Savas, H.A., Altindag, A. Lack of pain in schizophrenia: A patient whose arm was burned and amputated. General Hospital Psychiatry. 2008;30:384–385.
Vlaeyen, J.W.S., Crombez, G. Fear and pain. Pain Clin Updates. 2007;15(6):104.
Voepel-Lewis, T., Zanotti, J., Dammeyer, J.A. Reliability and validity of the face, legs, activity, cry, consolability behavioral tool in assessing acute pain in critically ill patients. American Journal of Critical Care. 2010;19(1):55–62.
von Baeyer, C.L., Hicks, C.L. Support for a common metric for pediatric pain intensity scales. Pain Research & Management. 2000;5(2):157–160.
Von Roenn, J.H., Cleeland, C.S., Gonin, R., et al. Physician attitudes and practice in cancer pain management: A survey from the Eastern Cooperative Oncology Group. Annals of Internal Medicine. 1993;119:121–126.
Waddell, G., Main, C.J., Morris, E.W., et al. Chronic low-back pain, psychological distress, and illness behavior. Spine. 1984;9:209–213.
Wall, P.D. The placebo effect: An unpopular topic. Pain. 1992;51(1):1–3.
Wall, P.D. Reply to E. Leskowitz. Pain. 1993;53(1):115.
Wang, X.S., Mendoza, T.R., Gao, S.Z., et al. The Chinese version of the Brief Pain Inventory (BPI-C): Its development and use in a study of cancer pain. Pain. 1996;67:407–416.
Ward, S. (Personal communication.) Madison, WI. University of Wisconsin School of Medicine and Public Health, 2008.
Ward, S.E., Goldberg, N., Miller-McCauley, V., et al. Patient-related barriers to management of cancer pain. Pain. 1993;52:319–324.
Ward, S.E., Gordon, D.B. Patient satisfaction and pain severity as outcomes in pain management: A longitudinal view of one setting’s experience. Journal of Pain and Symptom Management. 1996;11(4):242–251.
Ward, S.E., Hernandez, L. Patient related barriers to management of cancer pain in Puerto Rico. Pain. 1994;58:233–238.
Warden, V., Hurley, A.C., Volicer, L. Development and psychometric evaluation of the pain assessment in advanced dementia (PAINAD) scale. Journal of the American Medical Directors Association. 2003;4(1):9–15.
Ware, L., Epps, D.F., Herr, K., et al. Evaluation of the revised faces pain scale, verbal descriptor scale, numeric rating scale, and Iowa pain thermometer in older minority adults. Pain Management Nursing. 2006;7(3):117–125.
Watson, G.D., Chandarana, P.C., Merskey, H. Relationships between pain and schizophrenia. The British Journal of Psychiatry. 1981;138:33–36.
Weibe, E., Pudhradsky, L., Dijak, V. The effect of lorazepam on pain and anxiety in abortion. Contraception. 2003;67:219–221.
Weiner, D.K., Herr, K. Comprehensive interdisciplinary assessment and treatment planning: An integrative overview. In: Weiner D., Herr K., Rudy T., eds. Persistent pain in older adults: An interdisciplinary guide for treatment. New York: Springer, 2002.
Weiner, D.K., Peterson, B.L., Logue, P., et al. Predictors of pain self-report in nursing home residents. Aging. 1998;10:411–420.
Weisberg, J.N., Boatwright, B.A. Mood, anxiety and personality traits and states in chronic pain. Pain. 2007;133(1–3):1–2.
Weissman, D.E., Haddox, J.D. Opioid pseudoaddiction—an iatrogenic syndrome. Pain. 1989;36(3):363–366.
Wells, N. Management of pain during abortion. Journal of Advanced Nursing. 1989;14:56–62.
Wesson, D.R., Smith, D.E. Prescription drug abuse: patients, physician, and cultural responsibilities. The Western Journal of Medicine. 1990;152(5):613–616.
Whipple, B., Komisaruk, B.R. Elevation of pain threshold by vaginal stimulation in women. Pain. 1985;21:357–367.
Wiener, C.L. Pain assessment on an orthopedic ward. Nursing Outlook. 1975;23:508–516.
Wilkie, D., Lovejoy, N., Dodd, M., et al. Cancer pain control behaviors: Description and correlation with pain intensity. Oncology Nursing Forum. 1988;15(6):723–731.
Wilkie, D.F., Keefe, F.J. Coping strategies of patients with lung cancer-related pain. The Clinical Journal of Pain. 1991;7:292–299.
Wolf, S., Pinsky, R.H. Effects of placebo administration and occurrence of toxic reactions. JAMA: The Journal of the American Medical Association. 1954;155:339–341.
Wong, D., Baker, C. Pain in children: Comparison of assessment scales. Pediatric Nursing. 1988;14:9–17.
Wong, D., Baker, C. Reference manual for the Wong-Baker FACES pain rating scale. Tulsa, OK: Wong & Baker, 1995.
Wong, D., Baker, C.M. Smiling face as anchor for pain intensity scales. Pain. 2001;89:295–297.
Wynne, C.F., Ling, S.M., Remsburg, R. Comparison of pain assessment instruments in cognitively intact and cognitively impaired nursing home residents. Geriatric Nursing (New York, N. Y.). 2000;21(1):20–23.
Yeager, K.A., Miaskowski, C., Dibble, S., et al. Differences in pain knowledge in cancer patients with and without pain. Cancer Practice. 1997;5(1):39–45.
Zalon, M.L. Nurses’ assessment of postoperative patients’ pain. Pain. 1993;54:329–334.
Zelman, D.C., Gore, M., Dukes, E., et al. Validation of a modified version of the brief pain inventory for painful diabetic peripheral neuropathy. Journal of Pain and Symptom Management. 2005;29(4):401–410.
Zelman, D.C., Smith, M.Y., Hoffman, D., et al. Acceptable, manageable, and tolerable days: Patient daily goals for medication management of persistent pain. Journal of Pain and Symptom Management. 2004;28(5):474–487.
Zimmerman, L., Story, K.T., Gaston-Johansson, F., et al. Psychological variables and cancer pain. Cancer Nursing. 1996;19:44–53.
Zwakhalen, S.M., Hamers, J.P., Abu-Saad, H.H., et al. Pain in elderly people with severe dementia: A systematic review of behavioural pain assessment tools. BMC Geriatrics. 2006;6:3.
Zwakhalen, S.M., Hamers, J.P., Berger, M.P. Improving the clinical usefulness of a behavioural pain scale for older people with dementia. Journal of Advanced Nursing. 2007;58(5):493–502.
Zwakhalen, S.M.G., Hamers, J.P.H., Berger, M.P.F. The psychometric quality and clinical usefulness of three pain assessment tools for elderly people with dementia. Pain. 2006;126(1–3):210–220.
Zwakhalen, S.M.G., van Dongen, K.A.J., Hamers, J.P.H., et al. Pain assessment in intellectually disabled people: Non-verbal indicators. Journal of Advanced Nursing. 2004;45(3):236–245.