Assessment Tools
Tools for Initial Pain Assessment
Criteria for Selecting of Pain Rating Scales for Use in Daily Clinical Practice
Principles of Using Pain Rating Scales
Teaching Patients and Families How to Use a Pain Rating Scale
Patients Who Deny Pain or Refuse Pain Relief
Selection of Pain Rating Scales for Patients Who Have Difficulty with the Commonly Used Self-Report Scales
Assessment of Neuropathic Pain
Assessment of Breakthrough Pain
Flow Sheets for Analgesic Infusions in Acute Care
Pain Diaries for Outpatient Care
Clinic Record for Reassessment of Patients with Persistent Pain Who Are Taking Opioids
TRADITIONALLY, asking patients about pain has been avoided. The fear of causing pain by suggesting the possibility of pain and the fear of increasing pain by focusing the patient’s attention on it are but a few of the reasons given for not discussing pain. However, no studies have shown that asking the patient about pain increases pain. A literature review concluded that when patients attend to and rate their pain levels, it usually lowers, not raises, their pain levels (Cruise, Broderick, Porter, et al., 1996). To explore this result, these researchers asked patients with chronic rheumatoid arthritis (RA) to complete pain diaries for 1 week, rating their pain and mood seven times a day. The findings did not indicate that this intense self-assessment of pain increased pain, but pain was not lessened either.
Perhaps questioning the patient about pain also has been avoided because it might identify a pain problem that the clinician did not know how to handle or was fearful of relieving. The clinician might fear that opioids would be necessary and that use of them would cause addiction or death by respiratory depression. “How are you feeling today?” is still about as close as many clinicians get to assessing pain. As one nurse explained, “I find it hard to talk about pain. I think it’s the fear of stirring something up. . . . If you’re really going to talk about pain, you have to be able to handle things properly” (de Schepper, Francke, Abu-Saad, 1997, p. 425).
Thus, routine assessment of pain is a relatively new idea and is recognized as being essential in the prevention of inadequate pain relief. The following material presents practical tools for initial and ongoing assessments that may be used to facilitate regular pain assessment in a variety of clinical settings.
Tools for Initial Pain Assessment
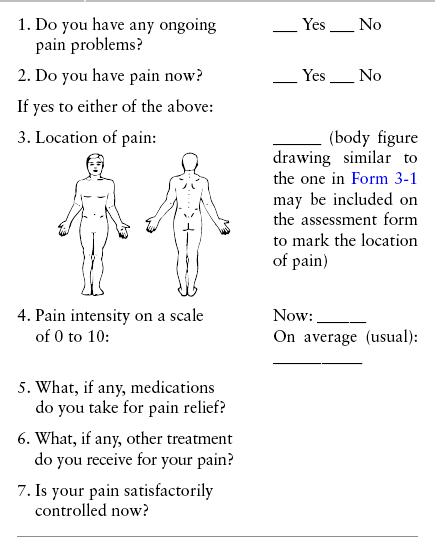
When patients are admitted to a health care facility, such as a hospital or an outpatient clinic, nurses perform general admission assessments. Along with such information as the patient’s self-care abilities and nutritional needs, a section should be included to identify pain problems. Patients may have chronic pain conditions for which they are already receiving treatment, or pain may be the primary reason for admission. Examples of questions that are appropriate for routine admission assessments are shown in Box 3-1.
The purpose of these questions is to identify new or ongoing pain problems. If a pain problem is ongoing and the patient already has an effective pain treatment plan, steps should be taken to ensure that the plan is continued. If a treatment plan must be developed, further assessments using the Initial Pain Assessment Tool (Form 3-1, p. 51) or the Brief Pain Inventory (BPI; Form 3-2, p. 53) may be indicated.
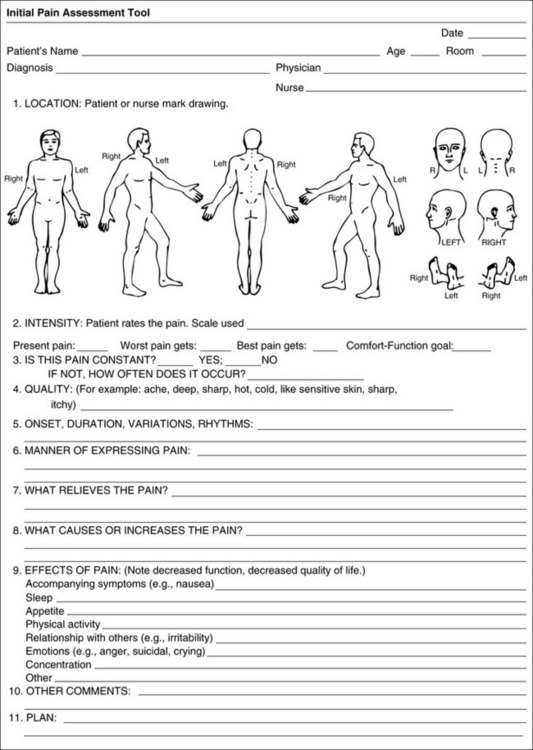
Form 3-1 May be completed by patients or used by clinicians to interview patients. From Pasero, C., & McCaffery, M. Pain assessment and pharmacologic management, p. 51, St. Louis, Mosby. Pasero C, McCaffery M. May be duplicated for use in clinical practice.
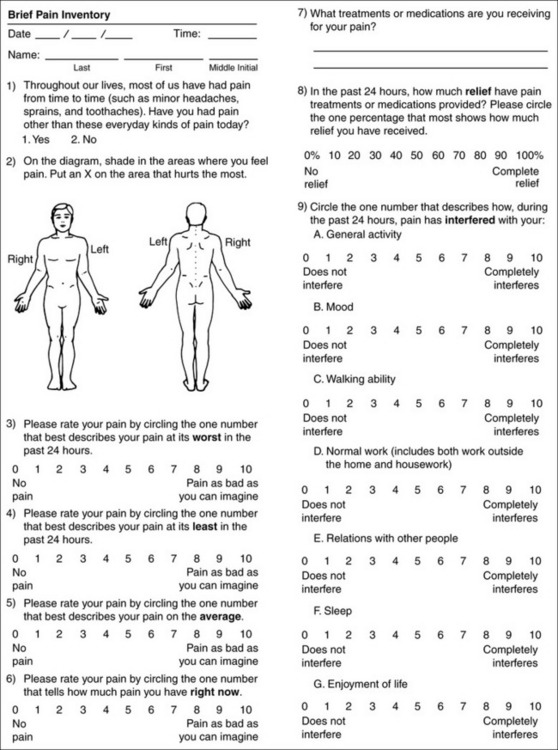
Form 3-2 From Pasero, C., & McCaffery, M. Pain assessment and pharmacologic management, p. 53, St. Louis, Mosby. Pasero C, McCaffery M. May be duplicated for use in clinical practice.
Tools for Initial Overall Pain Assessment
Two tools widely used for overall initial pain assessments are referred to as Initial Pain Assessment Tool (see Form 3-1) and the BPI (see Form 3-2). Both of these were included in the cancer pain clinical guidelines published by the Agency for Health Policy and Research (Jacox, Carr, Payne, et al., 1994a), and the BPI is included in the revised cancer pain guidelines published by APS (Miaskowski, Cleary, Burney, et al., 2005). The patient or the clinician may complete these tools. Another overall pain assessment tool often used in research and clinical practice is the short-form McGill Pain Questionnaire (Melzack, 1987).
These pain assessment tools need not be used for all patients with pain. The policies and procedures of a health care agency must identify the criteria necessary for these tools to be considered. Certainly if the patient has chronic pain problems that are not satisfactorily controlled, an overall pain assessment tool should be completed. Hospices and pain treatment centers may need far more extensive initial overall assessment tools than those offered here. For patients with acute pain that is not easily controlled by the usual pain treatments (e.g., intravenous patient-controlled analgesia [IV PCA] following surgery), an overall assessment also is indicated.
Both the Initial Pain Assessment Tool and the BPI, described later, attempt to assess some aspects of suffering. Suffering, like pain, is subjective, but it goes beyond simply feeling pain. Pain may exist without suffering. Suffering eludes definition but has been characterized as an individual’s experience of threat to self, a meaning given to events such as pain or loss (Kahn, Steeves, 1996). Suffering involves the person’s evaluations of the significance or meaning of pain (Spross, 1993). To some extent, suffering is similar to an impairment in quality of life. Items on the assessment tools directed at the effects of pain on various aspects of living are an attempt to assess some aspects of the patient’s suffering.
Initial Pain Assessment Tool
The Initial Pain Assessment Tool (Form 3-1) may be completed by the patient or used to guide the clinician in collecting information about the patient’s pain. A discussion of each assessment point follows.
1. Location of pain. This is most easily and quickly accomplished by asking the patient to mark the location on the figure drawings. Alternately, the clinician may ask the patient to point to the locations of pain on his or her own bodies, and the clinician can mark figure drawings. If there is more than one site of pain, letters (A, B, C, etc.) may be used to distinguish the various sites. These letters may be used in answering the remainder of the questions.
2. Intensity. The pain rating scale used by the patient is identified. The patient is asked to rate pain intensity for present pain, worst pain, the least pain felt, and comfort-function goals (pain rating that will not interfere with necessary or desired functioning, such as ambulation and decreased anxiety). If the patient has more than one site of pain, the letter designations mentioned simplify recording. For example, for present pain intensity, the recording might be A = 4, B = 6. A time period may be specified for answering the next questions about pain intensity. For example, worst pain intensity may be asked in relation to the past 24 hours or the past week.
3. Is this pain constant? If not, how often does it occur? These questions help screen for the presence of breakthrough pain (BTP), defined as a transitory increase in pain that occurs on a background of otherwise controlled chronic pain. If the patient says the pain is constant, this rules out BTP. But if the patient say the pain is not constant, further questioning is indicated, specifically asking the patient whether there are temporary flares of pain that are more intense than the constant pain. The Screening Tool for Pain Flares—Breakthrough Pain, Form 3-6 (p. 103), and the Assessment of Pain Flares—Breakthrough Pain, Form 3-7 (p. 104), may be helpful. Roughly 50% or more of patients with chronic, constant pain also have BTP that might be overlooked.

Form 3-6 From Pasero, C., & McCaffery, M. Pain assessment and pharmacologic management, p. 103, St. Louis, Mosby. Pasero C, McCaffery M. May be duplicated for use in clinical practice.
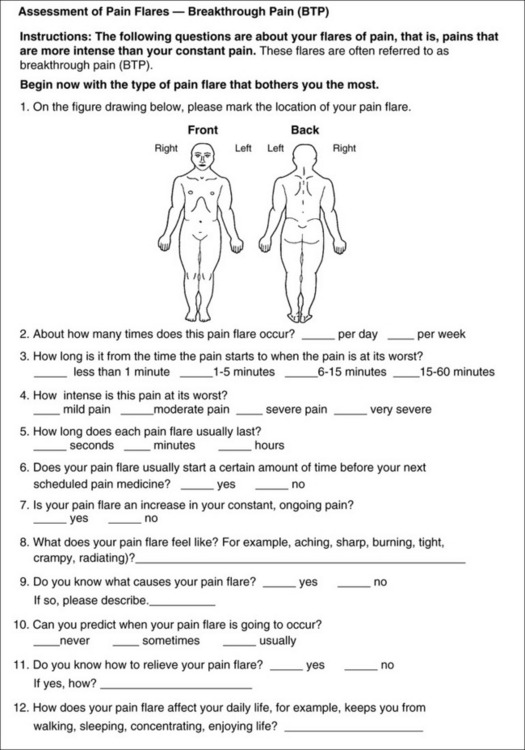
Form 3-7 This form may be used for clinicians to interview patients or may be completed by the patients themselves. It is appropriate for patients with chronic pain who have controlled baseline pain and breakthrough pain. This form should not be used unless baseline pain is usually controlled. These questions should be answered in relation to the type of pain flare that bothers the patient the most. Determine whether the patient has more than one type of pain flare, and consider using this assessment tool for each one. From Pasero, C., & McCaffery, M. Pain assessment and pharmacologic management, pp. 104-105, St. Louis, Mosby. Data from Bennett, D., Burton, A. W., Fishman, S, et al. (2005). Consensus panel recommendations for the assessment and management of breakthrough pain. Pharmacol Ther, 30(5), 296-301; Hagen, N. A., Stiles, C., Nekolaichuk, C., et al. (2008). The Alberta breakthrough pain assessment tool for cancer patients: A validation study using a Delphi process and patient think-aloud interviews. J Pain Symptom Manage, 35(2), 136-152; Portenoy, R. K., Bennett, D. S., Rauck, R., et al. (2006). Prevalence and characteristics of breakthrough pain in opioid-treated patients with chronic noncancer pain. J Pain, 7(8), 583-591. Pasero C, McCaffery M. May be duplicated for use in clinical practice.
4. Quality of pain. This information is helpful in diagnosing the underlying pain mechanism. Soreness is commonly more likely to be indicative of somatic pain, whereas burning or knifelike pain is more likely to be indicative of neuropathic pain. This information may have direct implications for the type of pain treatment chosen. For example, an anticonvulsant (an adjuvant analgesic; see Section V) may be indicated for knifelike pain. One study found that the quality of pain seemed to cluster in three groups: (1) paroxysmal pain sensations, such as shooting, sharp, and radiating pains; (2) superficial pain, such as itchy, cold, sensitive, and tingling pains; and (3) deep pain, such as aching, dull, cramping, and throbbing pain (Victor, Jensen, Gammaitoni, et al., 2008).
If the patient has difficulty describing pain, the clinician should ask the patient about the appropriateness of possible descriptors, such as throbbing, shooting, sharp, cramping, aching, tender, pricking, burning, or pulling. For the patient who continues to have difficulty, try asking him or her, “What could you do to me to make me feel the pain you have?”
5. Onset, duration, variations, rhythms. To detect variations and rhythms, ask the patient, “When did this pain begin?” “Is the pain better or worse at certain times, certain hours of the day or night, or certain times of the month?”
6. Manner of expressing pain. Ask the patient if he or she is hesitant or embarrassed to discuss the pain or whether the patient tries to hide it from others. Ask the patient if using the pain rating scale is acceptable.
7. What relieves the pain? If the patient has had pain for a while, he or she may know which medications and doses are helpful and may have found some nondrug methods, such as cold packs, helpful. If appropriate, these methods should be continued.
8. What causes or increases the pain? A variety of activities, body positions, and other events may increase pain, and efforts can be made to avoid them or to provide additional analgesia at those times.
9. Effects of pain. These items help to identify how pain affects the patient’s quality of life and how pain interferes with recovery from illness. Information obtained in this section may be useful in developing pain management goals. If pain interferes with sleep, a major goal may be to identify a pain rating that will allow the patient to sleep through the night without being awakened by pain.
10. Other comments. No tool is comprehensive. This space simply allows for information the patient may wish to add.
11. Plan. Immediate and long-range plans can be mentioned here and developed in greater detail as time passes.
Brief Pain Inventory
The BPI (Form 3-2, p. 53) is a 9-item tool that gathers information about pain severity and rates level of pain interference with seven key areas of function. Regular use of this tool helps track progress in treating pain intensity and degree of pain interference with general activity, mood, walking ability, normal work (both work outside the home and house work), relations with other people, sleep, and enjoyment of life. The BPI is relatively short and easy for patients to complete, taking about 10 to 15 minutes. It has been used successfully with older adults (Ersek, Turner, Cain, et al., 2004; Kemp, Ersek, Turner, 2005).
The BPI assessment tool has been used extensively in research too. It has reasonable validity and reliability (Daut, Cleeland, 1982; Daut, Cleeland, Flanery, 1983) and has proven useful in a variety of clinical settings (Cleeland, Gonin, Hatfield, et al., 1994). The BPI has been translated into other languages, including Japanese (Uki, Mendoza, Cleeland, et al., 1998); Vietnamese (Cleeland, Ladinsky, Serlin, et al., 1988); Chinese (Wang, Mendoza, Gao, et al., 1996); the Philippine language (Cleeland, Nakamura, Mendoza, et al., 1996); Russian (Kalyadina, Ionova, Ivanova, et al., 2008); and French (Serlin, Mendoza, Nakamura, et al., 1995). The BPI has been shown to be reliable and valid in several patient populations, such as patients with cancer pain (Cleeland, Gonin, Hatfield, 1994) and with chronic noncancer pain (Tan, Jensen, Thornby, et al., 2004); in patients 6 months after cardiac surgery (Gjeilo, Stenseth, Wahba, et al., 2007); and in Canadian veterans with pain who are suffering from traumatic stress (Poundja, Fikretoglu, Guay, et al., 2007). A modified version of the BPI has been validated in patients with painful diabetic peripheral neuropathy (Zelman, Gore, Dukes, et al., 2005).
Questions on the BPI focus on pain during the past 24 hours.
• Question 1 asks if the patient has experienced pain other than common everyday kinds of pain and if the patient has experienced that pain today.
• Question 2 asks the patient to identify the location of that pain in the figure drawing.
• Questions 3 through 6 ask the patient to use a pain rating scale of 0 to 10 to rate pain at its worst and least in the past 24 hours and its intensity on average and right now.
• Question 7 asks about treatment or medication the patient is receiving for pain.
• Question 8 asks the percentage of pain relief provided by these treatments.
• Question 9 has seven parts that attempt to identify how much pain has interfered with the patient’s life, including general activity, mood, work, sleep, and enjoyment of life.
Pain Intensity Rating Scales
Pain intensity rating scales used in daily clinical practice deal with how much pain the patient is feeling. When a patient uses the scale to report pain, it is called a self-report scale. Numerous self-report scales for measuring pain intensity exist. They have been referred to by many different names, but often each scale has no standardized title or definition. No “best” scale exists, but some are more practical and more widely used in clinical practice than others. Following is a discussion of criteria for selecting pain rating scales for daily clinical practice and a discussion of the visual analogue scale (VAS) and specific pain rating scales appropriate for clinical use in assessing pain intensity in cognitively intact adolescents, adults, and older persons. Later in this chapter, tools for assessing patients who have difficulty with the commonly used self-report scales or who are unable to self-report are discussed.
Criteria for Selecting Pain Rating Scales for Use in Daily Clinical Practice
Criteria for selecting self-report pain rating scales for use in daily clinical practice are summarized in Box 3-2. Most important, the pain rating scale selected must have been tested and found to be reliable and valid. Reliability means that the scale consistently measures pain intensity from one time to the next, and validity means that the scale accurately measures pain intensity. Probably the most fundamental aspect of validity for pain rating scales is that they demonstrate sensitivity to changes in the magnitude of pain (Herr, Spratt, Garand, et al., 2007). For example, they must be sensitive enough to measure effects of analgesic medication. (For more detailed information about the reliability and validity of a variety of pain measures, refer to Jensen [2003] and Herr, Spratt, Mobily, et al. [2004]).
Usually the main purpose of using pain rating scales in clinical practice is to identify the intensity of pain over time for the purpose of evaluating the effectiveness of interventions to relieve pain. Scales used to obtain self-reports of pain intensity fail to provide other information that is important to assessing pain and determining pain treatment. This is one of the reasons for remembering to use the Initial Pain Assessment Tool (see Form 3-1) or the Brief Pain Inventory (see Form 3-2).
Certain open-ended questions are also used in addition to the pain rating scale. Research with older adults (N = 312) has shown that an open-ended pain question such as “Tell me about your pain, aches, soreness, or discomfort” when initiating pain assessment discussions provided significantly more pain information than asking the patient to rate his or her pain or simply asking “How are you feeling?” (McDonald, Shea, Rose, et al., 2009). The latter question resulted in the least amount of information about pain. Thus, clinicians should be aware that simply asking a patient to rate the intensity of pain provides useful but not complete information about important aspects of the pain and the implications for treatment.
Unfortunately, pain rating scales seem to invite creativity. Sometimes colors are added to a preexisting scale such that different colors are used to indicate different intensities of pain, or colors are added to a faces scale. Some of the problems with this practice are that some health care facilities do not have copiers that duplicate in color and that research into colors as indicators of pain intensity is limited. Colors are subject to personal preferences and cultural differences and are best avoided. Another change to a preexisting valid and reliable scale is drawing hats on a faces rating scale. When a validated and reliable scale is changed in any of these ways, additional research is required to establish the reliability and validity of the new scale. Sometimes clinicians develop and use an entirely new scale without testing it for reliability and validity. Such energy is better spent on promoting the routine use of a single, simple pain rating scale that has already been established as being valid and reliable.
As stated in Box 3-2, the tool also should be sufficiently graded so it can capture changes in pain intensity and should be easily understood, easy to score, and liked by patients and staff. The tool should place a low burden on staff; that is, it should be quickly explainable and easily scored. It should also be inexpensive and easy to duplicate. The tool should also be appropriate for patients of various cultures and available in several languages.
Visual Analogue Scale
The tool that is probably used most frequently in research but is inappropriate for clinical use is the VAS. It is difficult to say how long pain rating scales have been used, but the VAS has been used for assessment of subjective phenomena such as loudness of a sound for more than 80 years (Freyd, 1923; Hayes, Patterson, 1921). At some point the VAS began to be used in pain research, and it is a reliable and valid instrument (Huskisson, 1974; Jenson, Karoly, 2001).
Currently, the term VAS is used loosely and inaccurately to refer to several different pain intensity scales such as the Numerical Rating Scale (NRS). However, the VAS has a fairly precise definition as a horizontal (sometimes vertical) 10-cm line (100 mm) with word anchors at the extremes, such as “no pain” on one end and “pain as bad as it could be” on the other end (Figure 3-1). The patient is asked to make a mark on the line to represent pain intensity. In this manual, the term VAS refers only to a horizontal or vertical straight line with word anchors at the ends.
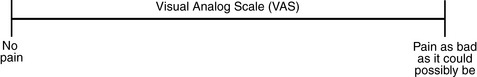
Figure 3-1 This horizontal visual analog scale (VAS) for rating pain intensity is a 10-cm line with word anchors. Patients are asked to mark the line. Although the VAS is frequently used in research, it is not recommended for clinical practice because scoring is time-consuming. From Pasero, C., & McCaffery, M. Pain assessment and pharmacologic management, p. 55, St. Louis, Mosby. Pasero C, McCaffery M. The scale is in the public domain. May be duplicated for use in clinical practice.
Probably because the VAS is reliable and valid and is commonly used in research, it is sometimes used in clinical practice. However, it has several disadvantages in that setting. Although the VAS is usually easy to administer (unless the patient has an injury to the arm or hand or is lying down) and easy to reproduce, scoring is time consuming. As mentioned, the patient is asked to mark the line to indicate the level of pain. A number is then obtained by measuring in millimeters up to the point the patient has indicated. Also, without specific numbers being arranged along the VAS line, it is difficult to use the scale to discuss pain rating goals with the patient, especially over the telephone. Further, patients tend to have more difficulty understanding and using a VAS than the other scales such as the NRS (Herr, Spratt, Mobily, et al., 2004; Jensen, Karoly, 1992; Peters, Patijn, Lame, 2007). In research comparing the VAS with the verbal descriptor scale (VDS) and with 11-point and 21-point numeric box scales, patients made more mistakes using the VAS (Peters, Patijn, Lame, 2007). (A box scale shows connected boxes along a vertical or horizontal line with numbers in the boxes, such as 0 to 10 or 0 to 20. Figure 3-2 is an example.)
Recommended Pain Intensity Rating Scales
After we (the authors) considered a wide array of pain intensity rating scales, the self-report scales we now recommend for use in clinical practice with cognitively intact adolescents, adults, and older adults consist of the NRS plus a faces pain rating scale (Box 3-3). This gives the patient a choice of using either the NRS or a faces scale. Some patients are unable to understand the NRS but are able to use one of the faces scales. Other patients simply do not like the NRS. For example, in a study of 267 hospitalized patients ranging in age from 16 to 91 years, almost half preferred the Wong-Baker FACES Pain Rating Scale (FACES) to the 0 to 10 NRS or the VAS (Carey, Turpin, Smith, et al., 1997).
Specifically, we suggest a horizontal NRS using 0 to 10 (0 = no pain; 10 = worst possible pain) presented visually. The patient is asked to report a number or mark the scale. Or the patient may choose a faces scale. We recommend that the NRS be combined with either (a) the Wong-Baker FACES Pain Rating Scale (FACES), using 0 to 10 to represent the 6 faces; or (b) the Faces Pain Scale-Revised (FPS-R), again using 0 to 10 to represent the 6 faces (Figures 3-3 and 3-4). No single-word anchor has been identified as being the best one for the number 10 on the 0 to 10 scale. Examples of word anchors that have been used fairly frequently are “worst imaginabale pain,” “worst possible pain,” “most intense pain imaginable,” “terrible pain,” and “pain as bad as it can be.”
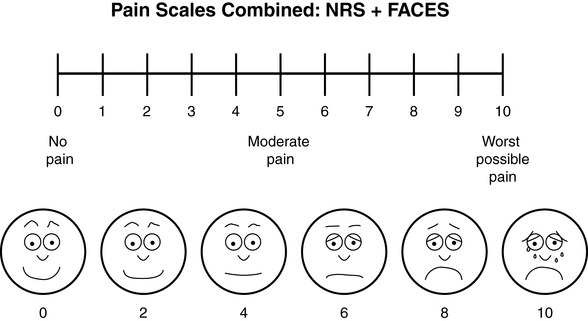
Figure 3-3 Example of how the Numerical Rating Scale (NRS) can be combined with the Wong-Baker FACES Pain Rating (FACES) in a horizontal format with a 0-to-10 metric and placed on the same paper or card to present to patients. They have a choice of pain rating scales. If the NRS is not easily understood, the FACES scale is an alternative. From Hockenberry, M. J., Wilson, D., & Winkelstein, M. L. (2005). Wong’s essentials of pediatric nursing, ed 7, St Louis, Mosby, p. 1259. Used with permission. From Pasero, C., & McCaffery, M. (2011). Pain assessment and pharmacologic management, p. 56, St. Louis, Mosby. Permission to use the FACES scale for purposes other than clinical practice can be obtained at http://www.us.elsevierhealth.com/FACES/. The NRS is in the public domain. May be duplicated for use in clinical practice.
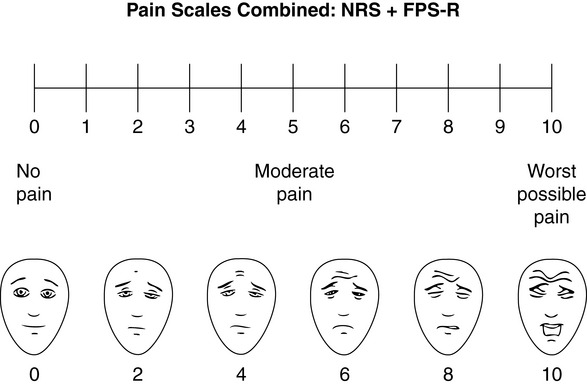
Figure 3-4 Example of how the Numerical Rating Scale (NRS) can be combined with the FPS-R in a horizontal format with a 0-to-10 metric and placed on the same paper or card to present to patients. Patients have a choice of pain rating scales. If the NRS is not easily understood, the FPS-R scale is an alternative. As appears in Pasero, C., & McCaffery, M. (2011). Pain assessment and pharmacologic management, p. 57, St. Louis, Mosby. This figure has been reproduced with permission of the International Association for the Study of Pain. May be duplicated for use in clinical practice. Permission to use the FPS-R for purposes other than clinical practice or research can be obtained by e-mailing IASPdesk@iasp-pain.org. The NRS is in the public domain.
Numerical Rating Scale: The NRS is sometimes presented as a 0 to 5 scale or as a vertical line, but in this manual, unless otherwise specified, NRS refers to a 0 to 10 horizontal scale with numbers between 0 and 10. Although occasionally the 0 to 5 scale is appropriate for patients who are unable to comprehend more items on a scale, the 0 to 10 is now widely accepted. In a survey of health care professionals that asked them which scale they preferred for use in clinical practice, the majority (70%) favored 0 to 10 (von Baeyer, Hicks, 2000). Regarding use of the horizontal line, it is worth noting that research indicates that the vertical VAS may be more sensitive and easier for patients to use, especially patients who are under the stress of having a narrowed visual field (Gift, 1989; Gift, Plaut, Jacox, 1986). Further, some research has found that the vertical format may be more easily understood by older persons (Herr, Garand, 2001; Herr, Mobily, 1993). Therefore, the vertical NRS (Figure 3-5, A) may be advisable for some patient populations or may be presented as an alternative to patients who have difficulty with the horizontal scale (Figure 3-5, B).
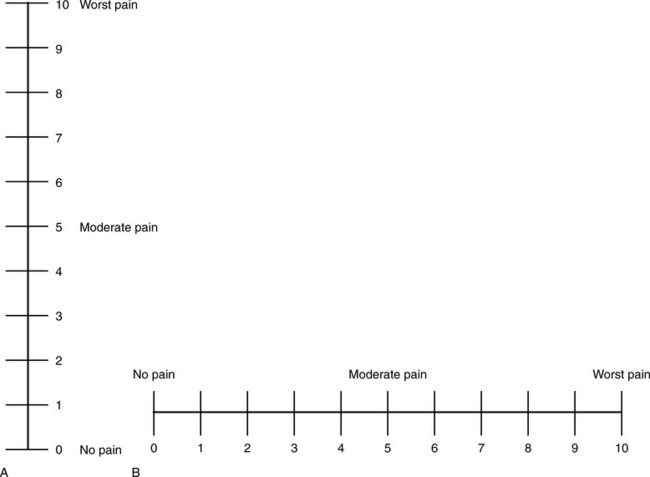
Figure 3-5 The vertical version (A) of the numeric rating scale may be more easily understood than the horizontal version (B). From Pasero, C., & McCaffery, M. (2011). Pain assessment and pharmacologic management, p. 58, St. Louis, Mosby. The scale is in the public domain. May be duplicated for use in clinical practice.
The reliability and validity of the 0 to 10 NRS are well established (Jensen, Karoly, 1992). Other major strengths of the 0 to 10 NRS are that most patients and clinicians are familiar with it, and it is easily administered. The universal adoption of a 0 to 10 NRS has been promoted for some time (Dalton, McNaull, 1998; Paice, Cohen, 1997; von Baeyer, Hicks, 2000). Also, numeric scales are well liked. In a group of younger adults and a group of older adults, one study compared the VAS, a 21-point numeric rating scale, a verbal descriptor scale, the 11-point (0 to 10) verbally presented numeric rating scale, and a faces pain scale (Herr, Spratt, Mobily, et al., 2004). Both the younger and the older groups preferred the 21-point numeric rating scale. Research conducted with younger and older surgical patients suggests that the 0-10 NRS has good psychometric properties and is the preferred scale when compared to a verbal descriptor scale, a horizontal VAS, and a vertical VAS (Gagliese, Weizblit, Ellis, et al., 2005).
Probably the most frequent way the NRS is presented is verbally, without a visual aid. This method is appealing because, unless the patient’s ability to speak is compromised, such as in the presence of an endotracheal tube, the patient can provide verbal responses at any time and in any position and regardless of difficulty in using the arms or hands. However, presentation of the NRS without a visual aid is not recommended because of the high error rate. Research has shown that when the NRS is presented verbally, without a written copy of the scale, patients make more mistakes, such as giving a score higher than 10 or a number between whole numbers (Herr, Spratt, Garand, 2007). However, when a 21-point box scale (numbers in boxes from 0 to 100 in increments of 5) and a verbally administered 0 to 10 box scale were compared, the results supported the validity of a verbally administered 0 to 10 point scale while showing a visual of the box scale, strongly suggesting that the NRS should be shown visually along with asking the patient to verbally rate pain on a 0 to 10 scale (Jensen, Miller, Fisher, 1998).
Therefore, when the NRS is used, every effort should be made to show the patient a copy of the scale. Patient reporting-error rates when using the 0 to 10 NRS in the absence of a visual presentation are sufficiently great for some to suggest that hospitals rethink their choice of using 0 to 10 without a visual aid (Feldt, 2007). Rather than abandoning the highly popular verbally presented 0 to 10 scale, a more practical solution is to make multiple copies of the 0 to 10 NRS along with whichever faces scale is chosen and post them in patients’ rooms, clinic areas, treatment rooms and other places where patients and clinicians might discuss pain.
As discussed earlier, pain rating scales seem to invite change and creativity without testing for reliability or validity. One problem with the 0 to 10 self-report scale is that some clinicians try to attach behaviors to each of the numbers. In one study of sedated patients, researchers tried to substitute behaviors for the sedated patients’ self-report of pain, overriding the patients’ stated pain levels (Salmore, 2002). However, there is no evidence that pain ratings by a sedated patient are less reliable than behaviors. The study in question requires further investigation.
When patients, such as very cognitively impaired patients and unconscious patients, are unable to self-report pain one must begin to rely on known pathology and behavioral indicators (see Box 3-9, p. 123). Otherwise, the gold standard for measuring pain intensity is a self-report, such as a number or word.
Another problem we (the authors) have seen across the United States is clinicians’ attaching behaviors to each number on the NRS and asking patients to state a number to indicate their pain levels. These scales are seldom published but are nevertheless used in clinical practice in some institutions. In one example of a 0 to 10 NRS with behaviors attached to each number, the number 5 was accompanied by the statement that pain “can’t be ignored for more than 30 minutes.” This has not been researched and is negated by the statements of many patients who have practiced learning to ignore pain. This also requires timing how long patients can ignore pain, which is cumbersome, time consuming, and a little on the humorous side. The same scale stated with the number 7 that pain “interferes with sleep,” again contradicted by patients who have learned to use sleep as a coping mechanism. (Readers may want to review the section on the acute pain model, presented earlier in this manual, on pp. 28-29). Again, on the humorous side, that same scale stated with the number 10 that “pain makes you pass out.” Again, no research substantiates this, and it makes it almost impossible for patients to rate pain as a 10 because they would be unconscious. Patients’ behavioral responses vary enormously and cannot be pigeonholed at one particular level of pain. Patients cannot be told how they have to behave in order to rate their pain at a particular level.
A similar problem has arisen with a published functional pain scale (Gloth, Scheve, Stober, et al., 2001). To associate numbers with levels of activity seems like a reasonable way to assess function but not pain. On this scale the number 5 is the highest level of pain, and the behavior listed for 5 is that the pain is so severe that the patient is unable to communicate verbally. Therefore, on this scale, if patients say their pain level is 5, they must reconsider because they are able to communicate that score verbally. Further, it seems somewhat ridiculous to think that patients writhing in pain and rating pain at 5 would not be allowed to do so.
These attempts to assign behaviors to pain levels seem to reflect the desire to make pain levels uniform within and among patients. However, what is a 7 for one patient on a scale of 0 to 10 is not going to be a 7 for all other patients. And a given level of pain that is a 7 for a patient on one day may not be a 7 for that patient the next day. Some patients with chronic pain have commented that as their pain increases over time, what they had considered severe pain is now what they would rate as moderate pain.
One error, mentioned earlier, that is fairly common in research and clinical practice is that some patients select a point between numbers on a 0 to10 scale, such as 6.5. This deserves some explanation. Although difficult to handle when a computer is programmed to accept only 11 points of scale, a review of research shows that people can actually distinguish among 21 levels of pain (Jensen, Miller, Fisher, 1998). Further, research with older adults that compared a faces scale, a 0 to 5 verbal rating scale, and a vertical and a horizontal 21-point box scale, the horizontal 21-point box scale emerged as the best scale with respect to psychometrics and regardless of mental status (Chibnall, Tait, 2001). But the 0 to 10 scale is well entrenched, and using a 0 to 20 scale in clinical practice does not seem practical at this time. However, even when compared with the VAS, a 21-point box scale may be the instrument of choice for research in mixed populations, such as various levels of cognitive ability (Herr, Spratt, Mobily, et al., 2004; Peters, Patijn, Lame, 2007).
The 0 to 10 NRS has been translated into many languages. A few translations are seen in Figure 3-6 (p. 60). Further, there is preliminary evidence that a 0 to 10 NRS (presented with a visual) has higher reliability in illiterate patients when compared to the VAS or a verbal rating scale (such as no pain, mild, moderate, severe, unbearable) (Ferraz, Quaresma, Aquino, et al., 1990). Results are difficult to generalize because the study was conducted only with Portuguese patients.


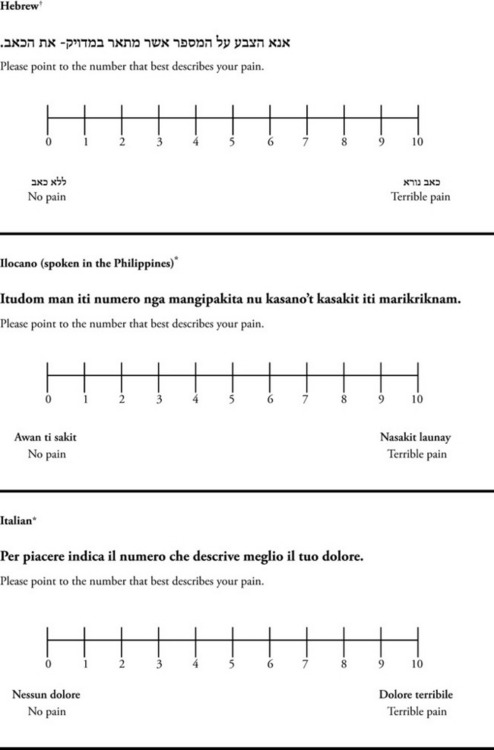
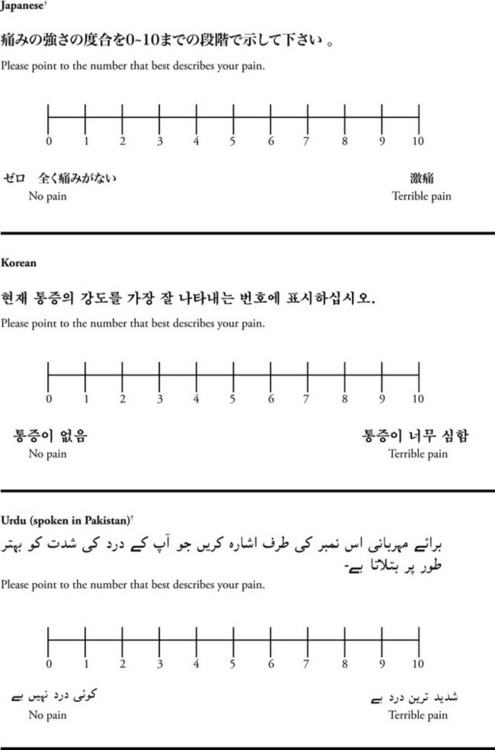
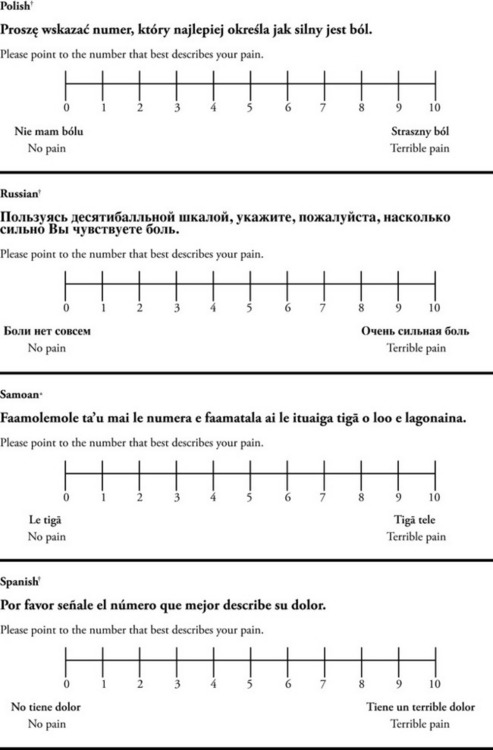

Figure 3-6 Translations of 0-10 numerical rating scale.
*Courtesy of Pain-Management Committee, St. Francis Medical Center, Honolulu, NY.
†Compiled by Josephine Musto, St. Vincent’s Hospital and Medical Center, New York, NY. From Pasero, C., & McCaffery, M. (2011). Pain assessment and pharmacologic management, pp. 60–65. St. Louis, Mosby. These scales are in the public domain. May be duplicated for use in clinical practice.
Wong-Baker FACES Pain Rating Scale: Work on the Wong-Baker FACES Pain Rating Scale (henceforth referred to as the FACES scale) began in 1981 by Donna Wong, a nurse consultant, and Connie Morain Baker, a child life specialist, who were working in a burn unit. In 1988, Wong and Baker published a study of 150 children aged 3 to 18 years. The FACES scale was presented in a circular format and was compared to five other scales: (1) a simple descriptive scale with numbers assigned to five adjectives on a horizontal line; (2) a numeric scale with the numbers 0 to 10 on a horizontal line; (3) a five-glasses scale in which the glasses contained varying amounts of water, ranging from no water to a full glass; (4) a chips scale using five white plastic chips; and (5) a color scale that included six colors. This study demonstrated the initial reliability and validity of the FACES scale, and no single scale demonstrated superior validity or reliability. The most preferred scale was the FACES scale. Because the FACES scale was presented in a circular format, the results of this study are difficult to compare with those of the numerous other studies in which these faces and other faces scales are presented to children and adults in a horizontal format. Other information concerning the development of this scale and its use in children is available at http://www.mosbysdrugconsult.com/WOW/faces.html and http://www.mosbysdrugconsult.com/WOW/facesStatisticalAnalysis.html. Unfortunately, several studies of reliability and validity listed at this website are unpublished.
The FACES scale was developed for use with children, but is it appropriate for some adults? Indeed, some studies of adults have revealed that they often prefer the faces scales to other scales. For example, in one study of 267 adults, the FACES, VAS, and NRS were compared, and the FACES was preferred (Carey, Turpin, Smith, et al., 1997). In another study of children and their parents, a comparison of five faces scales revealed that the parents as well as the children preferred the FACES scale (Chambers, Giesbrecht, Craig, et al., 1999). The cartoon-like features of this scale seem to contribute to this preference. The high preference for the FACES scale is one reason for combining the FACES scale with the NRS to give adults a choice in pain rating scales (see Figure 3-3, p. 56). Of the various faces scales, the FACES scale is currently probably the most widely used in both children and adults in the United States.
In a study of 37 older adults in a long-term care facility, some of whom were cognitively impaired and others cognitively intact, pain was assessed using the VAS, VRS, McGill Word Scale (Melzack, 1975), and FACES scale (Wynne, Ling, Remsburg, 2000). The FACES scale was completed by 61% of the participants, the McGill Word Scale by 51%, and the VAS by 57%. Patients with cognitive impairment had more difficulty completing the instruments. Nevertheless, more participants were able to complete the FACES scale than the other scales.
The FACES scale meets many of the criteria for selecting a pain rating scale for use in clinical practice (see Box 3-2, p. 54). The faces do not depict age, gender, or culture. The FACES scale has been translated into several languages (Figure 3-7, pp. 66-67; translations are available at http://www.mosbysdrugconsult.com/WOW/faces Translations.html). It is therefore appropriate for patients of various cultures. It is often preferred by adults when they compare it to other scales. It is quickly explained and easily scored, easily and quickly understood, well-liked by clinicians and patients, and inexpensive to duplicate.
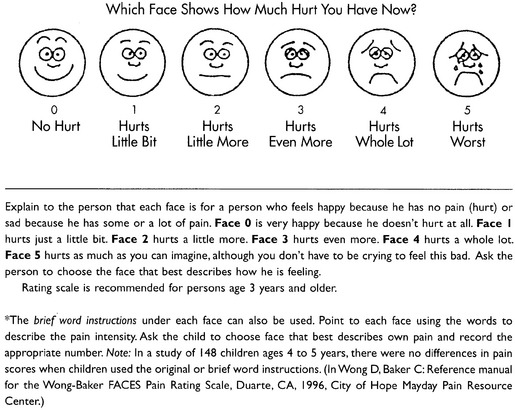

Figure 3-7 Translations of Wong-Baker FACES Pain Rating Scale. From Hockenberry, M. J., Wilson, D., & Winkelstein, M. L. (2005). Wong’s essentials of pediatric nursing, ed 7, St. Louis, Mosby, p. 1259. Used with permission. From Pasero, C., & McCaffery, M. (2011). Pain assessment and pharmacologic management, p. 66-67, St. Louis, Mosby. Permission to use the FACES scale for purposes other than clinical practice can be obtained at http://www1.us.elsevierhealth.com/FACES/. The NRS is in the public domain. May be duplicated for use in clinical practice.
No pain rating scale is perfect, and several objections have been made regarding the use of the FACES scale. One frequently mentioned concern has been that the presence of tears in the last face, depicting the greatest pain, might cause both children and adults to be reluctant to select that face if they were not crying or if they wanted to appear more stoic. Similarly, tearful patients may avoid selecting a face that shows no tears. The instructions for the scale attempt to avert this problem by telling a patient that a person does not have to cry to select the tearful face or may be crying and select a face without tears. Still, patients have been concerned. In a pilot study of 267 adults conducted in preparation for comparing scales that included the FACES scale, clinicians found that patients who selected the faces with tears said something about the tears, highlighting the need to include the instruction that one does not have to be crying to select the face with tears (Carey, Turpin, Smith, et al., 1997).
Some clinicians have asked about simply eliminating the tears on the FACES scale when they copy it for clinical use. However, this could affect the validity of the scale.
In one study of parents and their children, a similar problem occurred in adults’ understanding of the instructions for using the FACES scale (Chambers, Giesbrecht, Craig, et al., 1999). The researchers reported that some parents experienced difficulty understanding how to apply the scale to judge pain in their children when they were given the same verbal instructions as their children received, which included the statement that a person does not have to cry to select the last face with tears. Nevertheless, as an example, one parent commented that the child had a lot of pain but that his face never looked like the face with tears, so the adult selected a face at the lower end of the scale. This requires further investigation but suggests that when the FACES scale is used with adults, directions that are given for children may need to be clarified for the adults.
Another concern arises over the fact that a smiling face rather than a neutral face is placed at the beginning of the scale to denote no pain. In the FACES scale, the second face has a slight smile, and the third face is neutral. Some other faces scales, such as the FPS-R described next, begin with a neutral face to represent no pain. A person with no pain who is not smiling might select the third neutral face on the FACES scale, resulting in falsely high pain ratings. One study comparing five faces scales, two beginning with a smiling face and three beginning with a neutral face, used by children 5 to 12 years old and their parents, found that both the children and their parents gave higher pain scores when the smiling faces scales were used (Chambers, Giesbrecht, Craig, et al., 1999). The implications of these findings have been disputed by Wong and Baker (2001). However, another study of children and their parents comparing five faces scales, including the FACES scale and a color scale, found that scales beginning with a smiling face produced significantly higher pain ratings (Chambers, Hardial, Craig, et al., 2005). Children’s pain ratings were the highest when the FACES scale was used.
Thus, when adults as well as children use the smiling faces scales, higher pain ratings may result than when faces scales begin with a neutral face. This problem can be circumvented by using a faces scale that begins with a neutral face, and we suggest the FPS-R, presented next.
Another objection to the FACES scale is the difficulty in obtaining studies of reliability and validity. As mentioned earlier, on the website that lists studies of the reliability and validity of the FACES scale, several are unpublished and difficult to obtain.
Faces Pain Scale-Revised: The FPS-R is a modification of a scale with seven faces developed by Bieri, Reeves, Champion, and others (1990). A scale with seven faces does not adapt well to the 0 to 10 metric used by the majority of pain scales. The revised scale has six faces, so it is easily presented with the 0 to 10 metric (see Figure 3-4, p. 57) (Hicks, von Baeyer, Spafford, et al., 2001). Although numbers beneath the faces are not used when the scale is shown to young children, they are developmentally appropriate to use with adults. In fact by the age of eight years most children are able to use a 0 to 10 scale (Spagrud, Piira, von Baeyer, 2003).
The FPS-R was developed for use in preschool and school-age children. Initially, two studies were designed to establish the psychometric properties of the scale in children experiencing nonclinical pain and clinical pain (Hicks, von Baeyer, Spafford, et al., 2001). Comparing the FPS-R and the VAS, the validity of the FPS-R was established in a study of 76 children aged 5 to 12 years old having ear piercing. The next study established validity of the FPS-R in a group of 90 children 4 to 12 years of age experiencing clinical pain associated with hospitalization. The FPS-R was compared with a color analog scale and the VAS.
Studies have also shown that the FPS-R is appropriate for adults. One study compared the FPS-R with a verbal descriptor scale, the NRS and the Iowa Pain Thermometer in 40 cognitively intact and 28 cognitively impaired older minority adults, including African-Americans (74%), Hispanics (16%), and Asians (10%) (Ware, Epps, Herr, et al., 2006). The study was conducted in an acute care facility in the southern United States. The reliability and validity of the FPS-R were supported in this group, and the FPS-R was preferred by African-Americans and Hispanics and those who were cognitively impaired.
Another study of adults in Spain compared the Spanish version of the FPS-R with a pain thermometer (Miro, Huguet, Nieto, et al., 2005). Using five hypothetic painful situations, 177 cognitively intact subjects aged 65 years and older were asked to rate pain. The study provided preliminary evidence of the scale’s reliability and validity in this group. Also, subjects preferred the FPS-R. The study established the usefulness of the FPS-R in cognitively intact elderly patients.
In a study conducted in China of 173 Chinese surgical patients, aged 18 to 78 years, reliability, validity, and scale preference were studied by comparing the FPS-R, VAS, NRS, and a verbal descriptor scale (Li, Liu, Herr, 2007). Patients were interviewed preoperatively and asked to rate any vividly recalled pain and anticipated postoperative pain intensity. Then they were asked for pain intensity ratings on the day of surgery through the sixth postoperative day. On the last day they were asked their scale preference. All four scales had good reliability and validity. The FPS-R had a low error rate, and almost half of the subjects preferred the FPS-R. The researchers concluded that the FPS-R was the best one for Chinese adults.
In summary, the reliability and validity of the FPS-R has been established in the following groups of adults:
• Cognitively impaired and intact older adults who were Asian, African-American, or Hispanic. The last two groups preferred the FPS-R.
• Cognitively intact elderly Spanish patients; most preferred the FPS-R.
• Cognitively intact Chinese adults ranging in age from 18 to 78 years old; almost half preferred the FPS-R; the FPS-R had a low error rate.
These studies ensure that the FPS-R meets some of the criteria for selecting a pain rating scale for use with adults in daily clinical practice (see Box 3-2, p. 54). Research establishes it as being reasonably valid and reliable in children and adults. It is developmentally appropriate not only for children but also for adults as young as 18 years and for cognitively intact and cognitively impaired elderly. It is easily understood and well liked by patients. It has demonstrated appropriateness for patients of various cultures, such as minorities, Chinese, and Spanish. Further, the tool is available in approximately 30 languages (Figure 3-8). It is inexpensive and easily explained, scored, and recorded.
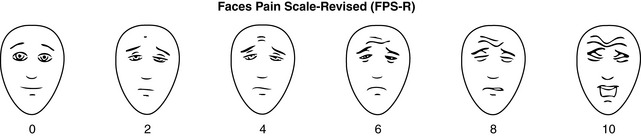

















Figure 3-8 Translations of Faces Pain Scale-Revised. From Hicks, C. L, von Baeyer, C. L., Spafford, P. A., et al. (2001). The Faces Pain Scale-Revised: Toward a common metric in pediatric pain measurement. Pain, 93, 173-183. As appears in Pasero, C., & McCaffery, M. (2011). Pain assessment and pharmacologic management, pp. 69-85, St. Louis, Mosby. Permission to use the FPS-R for purposes other than clinical practice or research can be obtained by e-mailing IASPdesk@iasp-pain.org. This figure has been reproduced with permission of the International Association for the Study of Pain. May be duplicated for use in clinical practice.
The FPS-R minimizes some concerns associated with the FACES scale. The first face is neutral, not smiling, so it lowers the possibility of artificially high scores. Tears, which might be objectionable to some patients, are not shown on any of the faces of the FPS-R scale, including the one that denotes the most severe pain.
Principles of Using Pain Rating Scales
Whichever pain rating scale is chosen for a patient should be used consistently with that patient. Using the same pain rating scale makes it possible to compare the pain ratings. Also, using the same scale is obviously easier for the patient than switching from one scale to another. Further, using the same pain rating scales and the same metric (that is, 0 to 10) throughout the institution facilitates communication about patients’ pain. Standardization is helpful within a given health care system, so that the same scale is used in the emergency department, the hospital, and the outpatient care services. A standard pain rating scale minimizes confusion for both patients and staff.
The primary reason for using pain rating scales is to evaluate the effectiveness of pain treatment plans. Reassessment is essential. The frequency with which pain ratings are obtained depends on the situation. The Guideline for the Management of Cancer Pain in Adults and Children (Miaskowski, Cleary, Burney, et al., 2005) recommends that at “a minimum pain should be reassessed and documented:
• at regular intervals after a management plan is initiated,
• with each new report of pain; and
• at an appropriate interval after each pharmacologic or nonpharmacologic intervention, such as 15 to 30 minutes after parenteral drug therapy or 1 hour after oral administration of an analgesic” (p. 28).
When pain is out of control, such as a 10 on a scale of 0 to 10, and a rapidly acting analgesic is used, pain ratings every 5 minutes may be appropriate. However, at the beginning of treatment, asking the patient for a pain rating may be entirely inappropriate. When a patient is obviously in pain or is not focused enough to learn to use a pain rating scale, pain treatment should proceed without pain ratings. Once a patient can use a pain rating scale, pain assessment may be necessary as often as every 15 minutes until pain is brought under control, then every 1 to 2 hours for 24 hours, followed by every 4 hours. Once pain has been well controlled, however, in hospitalized patients, obtaining pain ratings every 8 to 12 hours may be sufficient; in nursing homes a daily or even weekly pain assessment may be appropriate. In home care, once pain has been controlled, patients are not ordinarily asked to keep records of pain ratings.
An intricate part of using pain rating scales to evaluate pain management is to set goals for treatment. These may be referred to as comfort-function goals and are discussed later. Basically, it is essential to know what pain rating is necessary for individual patients.
Teaching Patients and Families How to Use a Pain Rating Scale
It is not unusual for clinicians to claim that patients cannot use pain rating scales. However, even in cognitively impaired elderly patients in nursing homes, 83% who report pain are able to use at least one type of pain rating scale (Ferrell, Ferrell, Rivera, 1995). Another study that included older cognitively impaired minority patients used a verbal descriptor scale (no pain, mild pain, moderate pain, severe pain, extreme pain, and most intense pain imaginable), a 0 to 10 NRS, a FPS-R, and an Iowa Pain Thermometer (Figure 3-9, p. 87) and found that all these scales were relatively easy for these patients to use (Ware, Epps, Herr, et al., 2006). Therefore, most adolescents and adults who are not cognitively impaired should be able to learn to use a pain rating scale. It is simply a matter of having a plan that ensures that when patients are admitted to clinical settings, someone is responsible for teaching all patients about the pain rating scale, having patients demonstrate their understanding of it, and documenting this in the records.
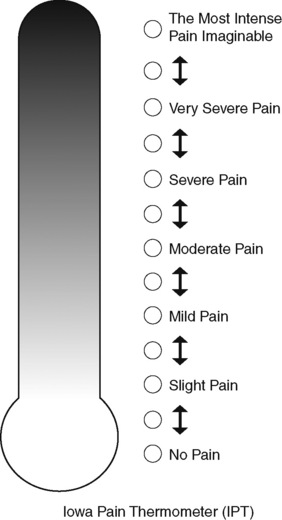
Figure 3-9 Iowa Pain Thermometer (IPT). From Herr, K., Spratt, K., Spratt, K. F., et al. (2006). Evaluation of the Iowa Pain Thermometer and other selected pain intensity scales in younger and older adult cohorts using controlled clinical pain: A preliminary study. Pain Manag Nurs, 7(2), 44-52. From Pasero, C., & McCaffery, M. (2011). Pain assessment and pharmacologic management, p. 87, St. Louis, Mosby. May be duplicated for use in clinical practice.
The steps for teaching the 0 to 10 NRS are in Box 3-4 on p. 86. Steps 1, 2, and 3 provide information; steps 4 and 5 ask the patient to demonstrate understanding of the information; and Step 6 focuses on the goals for comfort and function/recovery. Along with each step are specific examples of what may be said to the patient and family. A teaching brochure titled “Understanding Your Pain: Using a Pain Rating Scale” is patterned after the steps in Box 3-4 and is available at www.endo.com.
Patients tend to have a narrow concept of the word pain, often restricting its use to excruciating and intolerable sensations. The patient’s concepts of pain may be expanded by explaining that pain includes several different uncomfortable sensations, such as tightness and pressure, and by using the words aching and hurting (Step 3). In a study of four different ethnic groups—Hispanics, American Indians, blacks, and whites—participants were asked to describe and rate painful experiences. Findings revealed that in all groups, the word ache was used for mild pain, hurt for moderate pain, and pain for the most intense discomfort (Gaston-Johansson, Albert, Fagan, et al., 1990).
Setting the Comfort-Function Goal
How much pain is too much pain? When clinical practice guidelines first began to be published, the need for a goal was recognized. One of the first guidelines stated: “Determine the level of pain above which adjustment of analgesia or other interventions will be considered” (Acute Pain Management Guideline Panel, 1992, p. 7). The primary reason for using pain rating scales is to evaluate the effectiveness of the pain treatment plan. To do this, it is essential to set goals, as described in Box 3-4, Step 6. In a review of studies of primary care physicians’ goals for pain treatment, goals for pain relief were the single best predictor of the quality of pain management (Green, Anderson, Baker, et al., 2003).
The purpose of establishing a comfort-function goal is to identify how much pain can exist without interfering with the function and quality of life; that is, activities that the patient needs or wishes to perform. Goals should be as concrete as possible. For example, identifying a pain rating of 3 for accomplishing recovery is too general, whereas identifying a pain rating of 3 for ambulation is much more concrete. The goal is always established by working with the patient and identifying goals that are consistent with what the patient wants. An examination of longitudinal data from quality assurance studies revealed that charted goals for pain relief were often higher than the goals patients reported wanting (Ward, Gordon, 1996). However, clinicians should not establish comfort-function goals in the absence of input from patients.
Comfort-function goals are appropriate for both acute pain and persistent pain, and they encompass physical as well as emotional status and cognitive-behavioral activities. The patient should be assured that reported pain ratings above the goal will result in consideration of additional interventions.
When a clinician works with a patient to set comfort-function goals, the patient must understand that the intent is not to identify the highest pain level the patient can tolerate, but rather to identify how much pain can exist without interfering with function. In other words, what level of pain may be noticeable but not bothersome?
With some patients, such as those with persistent pain, multiple goals may be appropriate, but for most patients, setting more than one goal can be avoided by identifying the pain levels that would allow the patients to perform the function that is most painful but also of great importance. Following are examples of how this is done in various settings.
A study of patients with moderate to severe persistent pain related to osteoarthritis (OA), metastatic cancer, and low back pain were found to prefer the terms manageable and tolerable when describing a day of pain control to the term acceptable (Zelman, Smith, Hoffman, et al., 2004). Many of the participants objected to the phrase “an acceptable day of pain control” because it suggested that it would be possible to have days when their painful situations were acceptable or when they could experience full pain relief, a condition they felt was not possible. On the other hand, interviews with some patients indicated that it may be that patients whose pain is well controlled do not object to the term acceptable. Thus, objection to the term acceptable may indicate that pain is not well controlled.
The patients in the study identified that a manageable or tolerable day of pain control included (1) taking the edge off pain (note that this phrase may signal that pain should be reduced); (2) performing valued activities; (3) relief from dysphoria and irritability; (4) reduced adverse effects caused by medications; and (5) feeling well enough to socialize. These may be common themes in setting goals with patients who have persistent pain.
When patients are asked what level of pain they desire, some will report zero. The process of setting comfort-function goals helps set realistic goals because zero pain is not always possible. In fact, research involving patients with rheumatic diseases showed that on a 100-mm VAS, patients may interpret scores of less than 10 mm as no pain and that scores less than 25 mm may be considered to be relatively normal (Sokka, 2005). However, once the goal is achieved, such as a pain rating of 2 out of 10, the possibility of even better pain relief can always be considered. Over time or with experience, goals may change. For example, if a patient find that the pain rating goal is too high to facilitate frequent use of the incentive spirometer postoperatively, it should be changed to a lower number.
For some patients, especially those with persistent uncontrolled pain, setting goals may take place in stages. For example a patient, in sickle cell crisis may have had uncontrolled severe pain that has lasted for days and deprived him or her of sleep. The first objective may be to provide enough relief for the patient to get uninterrupted sleep. Once this is achieved, another goal related to activities may be established.
It may be difficult to engage some patients with chronic persistent pain of noncancer origin in pain management plans. They may be depressed and reluctant to participant for fear of failure (Filoramo, 2007). Many have tried multiple methods of pain control that have failed, or they have failed to accomplish their goals in the past. It is especially important in these cases to set goals that are concrete, that incorporate the patient’s personal goals, and that occur in stages that are achievable. For example, beginning with a goal that establishes a pain rating of 3 out of 10 when walking for 5 minutes may be relatively easy for a patient to accomplish. After this goal is achieved, the next goal might be to walk for 10 minutes. This approach incorporates the concept of pacing; that is, stopping an activity before it becomes painful. In this way, a patient begins to believe that improvement in function is attainable and becomes more strongly committed to the plan of care.
Not only does setting a comfort-function goal help the entire team, including the patient and family, to know what the pain-treatment plan should achieve, it also helps the patient to see how pain relief contributes to recovery or improves quality of life. Not all patients understand the importance of pain relief or the possible harmful effects of pain. Some patients do not expect to have their pain relieved, are frightened of taking opioid analgesics, or value a stoic response to pain. These patients may be reluctant to set a low pain rating as the goal. By setting pain rating goals that correspond to function, patients learn that pain relief helps them to recover faster from circumstances such as surgery. In cases of persistent pain, patients learn that pain control puts them back in control of their daily lives rather than allowing pain to control their lives.
The patient’s comfort-function goal should be visible on all records where pain ratings are recorded, such as bedside flow sheets. Whether the goal has been achieved or not should also be routinely included at change-of-shift hand-off reports. If the pain rating is below the goal, documentation should show which efforts were made to achieve the desired goal. For outpatient pain management, a diary or flow sheet kept by the patient (discussed later; see Form 3-11, p. 116) is one way to document and make visible progress toward goals. Establishing comfort-function goals for individual patients and holding clinicians accountable for attempting to achieve those goals may help clinicians to avoid basing pain-management decisions on their own biases.

Form 3-11 From Pasero, C., & McCaffery M. Pain assessment and pharmacologic management, p. 116, St. Louis, Mosby. Pasero C, McCaffery M. May be duplicated for use in clinical practice.
Research Related to Setting Comfort-Function Goals
Research helps to guide the process of setting a comfort-function goal by suggesting that certain pain levels are more than a patient should attempt to tolerate. The findings of several studies of different cultures have found that on a 0 to 10 NRS, pain ratings of 5 or more interfere significantly with daily function (Cleeland, 1984; Cleeland, Gonin, Hatfield, et al. 1994; Serlin, Mendoza, Nakamura, et al., 1995). The data from several countries (Philippines, France, China, and the United States) confirmed that pain ratings of 5 or greater were clearly more significant in their functional impact than pain ratings of 4 or less (Cleeland, Serlin, Nakamura, et al., 1997). In a study of 271 patients with AIDS-related pain, ratings on the BPI interference items (see Form 3-2, p. 53) found that patients with pain ratings up to 4 out of 10 reported that pain interfered most with mood and enjoyment of life (Breitbart, McDonald, Rosenfeld, et al., 1996). Patients with pain ratings of 5 or 6 out of 10 reported somewhat higher levels of interference on the BPI items, with pain again interfering most with mood and enjoyment of life. Patients with pain of 7 or more out of 10 reported even greater pain interference on the BPI items, with pain interfering most with walking ability, general activity, and sleep. Another study of 274 patients with AIDS-related pain revealed that higher levels of pain intensity resulted in increased levels of depressive symptoms and psychological distress (Rosenfeld, Brietbart, McDonald, et al., 1996). Thus, as pain intensity increases, it is associated with greater impairment in functional ability and psychologic distress.
Of note, one study using the BPI scales showed that a change in pain intensity from 4 (out of 10) to 5 was associated with a greater increase in interference with activity and enjoyment of life than any other change in pain intensity (Daut, Cleeland, 1982). Another study across four cultures, using the 0 to 10 NRS, also found that both a change in pain intensity from 4 to 5 and also a change from 6 to 7 were more significant than other increases in terms of interference with function (Serlin, Mendoza, Nakamura, et al., 1995). This suggests that preventing pain from increasing from 4 to 5 out of 10 is of great importance.
Other research suggests that 4, rather than 5, is the point at which pain significantly interferes with function. The results using the BPI to assess 111 patients with pain and advanced cancer showed that on a 0 to 10 scale, pain ratings of 4 or greater interfered markedly with activity, and interference with enjoyment increased markedly between scores of 6 and 7 (Twycross, Harcourt, Bergl, 1996). Another study of women during childbirth found that almost all patients (93%) wanted more analgesia when the NRS score was greater than 3 (Berlin, Hossain, Bodian, 2003). In a study of titrating drug doses for patients with neuropathic pain, patients were satisfied with their pain relief and stopped increases of drug doses at an average level of 3 out of 10 (Gilron, Bailey, Tu, et al., 2005). These findings and others, combined with clinical experience, have led many clinicians to the conclusion that pain ratings greater than 3 signal the need to revise the pain treatment plan by higher doses of analgesics or different medications and other interventions (Cleeland, Syrjala, 1992; Syrjala, 1993). In addition, even temporary pain at a level of 6 or more should mandate immediate intervention.
This information helps in guiding patients who select goals of 4, 5, or 6 out of 10. Such patients need to be cautioned that pain ratings this high may significantly interfere with function. Further, clinicians should ask these patients why they do not want better pain relief. In research in patients with persistent pain, mentioned earlier, almost all patients said that they did not expect pain to diminish to below 4 or 5 out of 10 without excessive interference from medications’ adverse effects (Zelman, Smith, Hoffman, et al., 2004). Patients felt they were trading pain relief for lower function. Clinicians should pursue this problem with patients and determine whether the adverse effects can be managed. If the problem is related to opioids, the addition of other medications such as nonopioids or adjuvants for pain relief can be explored. Or the adverse effects may respond to treatment. If the adverse effect of concern is sedation, patients can be informed that increases in opioid dosages may initially cause significant cognitive impairment but that with stable dosages of opioid this effect usually subsides in about a week (Bruera, Macmillan, Hanson, et al., 1989). If the adverse effect is constipation, a more aggressive bowel regime can be put in place to prevent this effect. (See Section IV for additional information about management of opioid-induced adverse effects.)
Minimum Clinically Important Changes: These changes are sometimes referred to as minimal important changes. In the process of adjusting pain treatment plans, such as titrating opioids, to accomplish the comfort-function goal, it is helpful to know how much reduction in pain intensity will be important to patients. A statistically significant decrease in pain intensity may not be meaningful to a patient. Therefore, it is important to look at the degree of reduction in pain that is clinically meaningful from the patient’s perspective. Several studies have found that approximately a 30% reduction in pain intensity in cases of acute or persistent pain is minimally clinically meaningful but that it depends on the baseline intensity of pain (Farrar, Young Jr., LaMoreaux, et al., 2001; Jensen, Chen, Brugger, 2003; Ostelo, Devo, Stratford, 2008; Sloman, Wruble, Rosen, et al., 2006). For example, a clinical reduction of 2 points on the 0 to 10 NRS may have clinical relevance for someone with mild pain but is of little or no relevance to someone with severe pain (Cepeda, Aficano, Polo, 2003). Specifically, a 2-point reduction in pain intensity from 4 to 2 on a 0 to 10 NRS (a 50% reduction) is likely to be meaningful to a patient with mild pain, but a 2-point reduction from 10 to 8 (a 20% reduction) may not be meaningful and may provide “only some relief” to a patient with severe pain.
Patients Who Deny Pain or Refuse Pain Relief
When a patient denies pain, clinicians must accept this rating because a patient’s self-report is the single most reliable indicator of pain. However, when a patient’s behavior, known pathology, or other findings suggest the existence of pain, clinicians are responsible for exploring this seeming contradiction with the patient and family. Sometimes a patient acknowledges the pain but refuses analgesics. Clinicians must respect this decision but, again, the reasons should be discussed with the patient and family. Giving a patient information or considering other approaches to pain management may result in the admission of pain or the acceptance of measures to relieve it.
Reluctance to report pain or to use analgesics obviously results in poor pain relief. The reasons patients may act this way have been shown to be associated with a variety of specific concerns (Ward, Goldberg, Miller-McCauley, et al., 1993); many further reasons are supported by a survey of laymen’s attitudes (Mayday Fund, 1993). The relationships between patients’ attitudes about pain and its treatment and their willingness to report pain and take analgesics were investigated using the Barriers Questionnaire (BQ), an 8-item tool (Ward, Goldberg, Miller-McCauley, et al., 1993). The patients in the study had chronic cancer pain. The patients’ responses to the BQ revealed that poor pain control was related to concerns about addiction (the most problematic misconception), adverse effects, and tolerance (the need to “save” the pain medicine); the belief that increased pain meant increased disease; concern that complaining about pain would distract physicians from curing illness; and the belief that “good” patients do not complain about pain.
The BQ was later revised to reflect changes in pain management, such as patients’ believing that pain medicine could weaken the immune system, and the result was the 27-item Barriers Questionnaire-II (BQII), a reliable and valid measure of patient-related barriers to the management of cancer pain (Gunnarsdottir, Donovan, Serlin, et al., 2002). The BQ-II confirmed that inadequate analgesia was related to higher scores than those found in the group that used adequate pain medication. Further study of the BQ-II has resulted in the Barriers Questionnaire Short Form, a 9-item survey shown in Form 3-3 (p. 91)(Ward, personal communication, 2008).

Form 3-3 As appears in Pasero, C., & McCaffery, M. (2011). Pain assessment and pharmacologic management, p. 91, St. Louis, Mosby. From Gunnarsdottir, S., Donovan, H., Serlin, R. C., et al. (2002). Patient-related barriers to pain management: The Barriers Questionnaire II (BQ-II). Pain 99(3), 385-396; Heidrich, S., Ward, S., Julesberg, K., et al. (2003). Conducting intervention research through the Cancer Information Service: A feasibility study. Oncology Nurs Forum, 30(1), 131-134. © Ward S. May be duplicated for use in clinical practice.
The BQ Short Form is recommended for use with patients and their families to help identify what may be causing a patient with cancer to deny pain or refuse analgesics. For patients newly diagnosed with a disease that may become painful eventually, the BQ Short Form may be administered before pain occurs to identify barriers to pain control before problems arise. Depending on the patient population, clinicians may wish to adapt the BQ Short Form by omitting some items or adding others, such as concerns about finances, hiding pain from the family and others, distrust of caregivers, not being a “sissy,” or wanting to avoid highly technologic methods of pain control.
Immediately after the BQ Short Form is administered, clinicians should discuss the results with the patient. The patient and family should be assured that many others have similar concerns, but that the concerns need not be problems. Appropriate patient teaching should follow. However, one session of patient teaching probably will not be sufficient to reassure the patient and family.
Correct information about addiction is almost always needed. One study found that the impact of concern about addiction may be somewhat alleviated by the way the prescriber presents information about the medication (Dawson, Sellers, Spross, 2005). More than half of the patients who received prescriptions for opioids said they would be very likely to take them if the prescribers advised them that it would not be addictive if used according to instructions. However, this statement is not entirely true. Correct use of opioids does not protect patients from developing addictive disease. It is more accurate, as discussed in Chapter 2, to say that that far less than 1% of patients exposed to chronic opioid use will become addicted. Unfortunately, this may not be as reassuring to patients as being told that they simply will not become addicted if they follow instructions. For this reason, another strategy clinicians use is to tell patients to ask themselves a question: If you were not in pain would you want to take this medication? A “no” answer means they have not become addicted. (See Section IV for more information about talking with patients and families about addiction.)
Most literature that teaches patients about pain control covers many of the concerns listed in the BQ Short Form. A teaching brochure concerning taking oral opioids that addresses many of the concerns patients have, including addiction, adverse effects, and tolerance, is titled “Understanding Your Pain: Taking Oral Opioid Analgesics” and is available at www.endo.com. One use of the BQ Short Form is to evaluate patient-education interventions by having the patient or family complete the BQ Short Form before and after information has been provided.
A consistent finding has been that patients who used inadequate analgesics had higher barrier scores on the BQ than did those who used adequate analgesics (Lin, Ward, 1995; Ward, Goldberg, Miller-McCauley, et al., 1993; Ward, Hernandez, 1994). Although misconceptions about pain management exist in patients who do not have pain as well as in those who have cancer-related pain (Yeager, Miaskowski, Dibble, et al., 1997), some research has suggested that these misconceptions may exist because current pain is undertreated (Dawson, Sellers, Spross, 2005). Patients’ beliefs about pain management seem to be shaped in part by the fact that their pain management provides less than adequate pain relief. Thus, not only do patients’ education about misconceptions require attention, so do patients’ pain management regimens.
One of the most frustrating occurrences in pain management is knowing that a patient with pain does not have to suffer, yet the patient refuses help. The BQ Short Form may help clinicians begin the process of exploring why a particular patient has decided to endure pain. Despite receiving accurate information about misconceptions and being given choices regarding methods of pain management, some patients, for cultural or religious reasons, choose to experience pain. These decisions must be respected, and at some point clinicians must refrain from further efforts to change these patients’ minds.
It is interesting that denial of pain is not always deliberate or conscious. Sometimes patients have narrow concepts of pain and deny its presence when asked. Following-up with other terms, such as aching or hurting, may uncover the existence of pain.
Other situations in which pain is denied are related to the energizing effect of visits from physicians or health team members. Before and after such visits, the patient may report pain. But encounters during hospital rounds or clinic visits sometimes have powerful positive placebo effects, and a patient honestly reports feeling fine at that time. Clinicians must remember to question the patient further and to check the patient’s record to determine what occurred before the visit.
Some patients who have experienced pain for a long time seem to lose their frame of reference. They may forget what it is like not to have pain. As one patient reported after reluctantly taking an analgesic, “I didn’t know I had pain. I guess I had been in pain too long to know the difference.”
Sometimes pain is not reported not because of any wish to deny pain, but because the patient believes that the health care provider knows about the pain. The patient may reason that the clinicians knows about his or her disease or surgery and knows that pain is present, so it is unnecessary to tell them. For example, a study comparing adolescents’ pain ratings with their perceptions of nurses’ evaluations of their pain revealed that adolescents perceived that nurses know how much pain they are experiencing (Favaloro, Touzel, 1990).
Although self-report is considered the gold standard for determining the presence and severity of pain (AGS, 2002; APS, 2003; Hadjistavropoulos, Herr, Turk, et al., 2007), older adults often deny or underreport the presence of pain. A number of misbeliefs or concerns underlie lack of pain reporting by older adults, including the belief that pain is to be expected with aging and should be endured; the desire to avoid bothering clinicians or distracting them from focusing on the primary disease; expecting the health care providers to anticipate pain based on condition and diagnoses; fear of the meaning of pain; and fear that acknowledging pain will lead to unpleasant diagnostic tests, hospitalization, and loss of independence (Gagliese, 2001; Herr, Garand, 2001; McAuliffe, Nay, O’Donnell, et al, 2008). Given the high prevalence of common conditions that can be painful in older adults, clinicians must be proactive in asking about pain and exploring possible reasons for denial. (See the previous section on denial of pain, pp. 89-92).
Older adults may deny the presence of pain but when asked about other sensations, such as aching, hurting, or discomfort, respond positively (Closs, Briggs, 2002; McDonald, Shea, Rose, et al., 2009). In the continuum of pain severity, some older adults consider pain to be the most extreme sensation, so clinicians must ask about pain using additional descriptors. Use of open-ended questions without social desirability (such as “Tell me about your pain, aches, soreness, or discomfort”) and use of follow-up questions significantly increased the amount of important pain information obtained from older adults with OA pain (McDonald, Shea, Rose, et al., 2009). If older patients cannot self-report verbally, it may be possible to learn about the presence of pain by establishing communication by using a head nod or eye blink. A variety of approaches can help solicit self-reports of pain in older persons with cognitive impairment.
Many pain conditions may not cause discomfort at rest, but movement or other activity may precipitate a pain response (Husebo, Strand, Moe-Nilssen, et al., 2008; Shega, Rudy, Keefe, et al., 2008). So, a denial of pain at rest should be followed by assessment during activity that is likely to cause pain, such as transferring, range of motion, or ambulation, especially in those who may have difficulty reporting.
Selection of Pain Rating Scales for Patients Who Have Difficulty with the Commonly Used Self-Report Scales
As cognitive impairment advances in the older patient, obtaining a reliable pain report becomes more difficult (Hadjistavropoulos, 2005; Kelley, Siegler, Reid, 2008). Many (Pesonen, Kauppila, Tarkkila, et al, 2009) will be unable to use the 0 to 10 pain rating scale previously described but will be able to self-report using other scales. No clear method has been determined to establish a patient’s ability to use a self-report scale reliably, but an attempt should be made. When reliability is an issue, it does not negate the importance of attempting to obtain a self-report in those with cognitive impairment. The MMSE is often used in research but is not readily reproducible for clinical use (Folstein, Folstein, McHugh, 1975). However, another simple tool may be used.
One approach is the Pain Screening Tool (Buffum, Miaskowski, Sands, 2001) (Box 3-5, #5). Focusing on their current pain, patients are asked to rate the intensity on a 0 to 3 scale and to say a word that describes their pain. Following 1 minute of distracting conversation, a patient is asked to recall the number and word. He or she receives 1 point for being able to provide the number and 1 point for providing the word. Half a point is given for recalling the word and half a point for recalling the number. A score of 3 suggests that the patient can use a self-report scale reliably. Most important, a score of less than 3 provides evidence that the patient cannot report pain reliably and that another pain assessment tool is necessary, such as a behavioral observation tool (see p. 126 for a discussion of behavioral tools). Another approach to evaluate whether the patient understands the use of the pain scale is to ask him or her to show on a pain scale where a severe pain would be located and to compare that to where the patient shows a mild pain would be located. This approach is discussed earlier in this chapter in the section about teaching patients how to use pain rating scales (see Box 3-4, p. 86).
Asking about pain in the present, that is “right now,” decreases issues related to memory losses that increase with advancing cognitive impairment. Studies have shown that asking older patients to identify pain severity that is dependent on memory, such as worst pain, least pain, or pain in the past week, may provide unreliable responses. (Bergh, Sjostrom, Oden, et al., 2000; Closs, Barr, Briggs, et al., 2004; Feldt, Ryden, Miles, 1998; Kelly, Siegler, Reid, 2008; Taylor, Herr, 2003). Soliciting reports of current pain and reassessing for change and for response to intervention are recommended. Box 3-5 illustrates strategies that can be used to increase success in soliciting self-report and pain scale use in older adults with cognitive impairment.
For older adults with mild to moderate cognitive impairment, a number of pain intensity scales have adequate reliability and validity (Hadjistavropoulos, Herr, Turk, et al., 2007; Weiner, Herr, 2002). There is wide individual variability in preference for and understanding of format, so no single tool is recommended for all patients. Organizations should identify several options that can be used when assessing pain severity in older patients with cognitive impairment. Tool formats that are acceptable and clinically useful include NRSs (Chibnall, Tait, 2001; Gagliese, Weizblit, Ellis, et al., 2005; Herr, Spratt, Mobily, et al., 2004); verbal descriptor scales (Closs, Barr, Briggs, et al., 2004; Herr, Spratt, Garand, et al., 2007; Herr, Spratt, Mobily, et al., 2004); and faces pain scales, such as FPS-R (Chibnall, Tait, 2001; Kaasalainen, Crook, 2003; Scherder, Sergeant, Swaab, 2003; Taylor, Herr, 2003). These scales are discussed earlier in the chapter (pp. 55-85) and are addressed here in relation to their use in older patients who have difficulty with commonly used scales such as the 0 to 10. Many older adults who are verbal simply have difficulty with scales that provide too many options, such as a 0 to 10 scale, but may be able to use a simpler scale such as a verbal descriptor scale that includes only four options (none, mild, moderate, severe) (Caraceni, Cherny, Fainsinger, et al., 2002; Closs, Barr, Briggs, et al., 2004; Dworkin, Turk, Farrar, et al., 2005). Although a 4-point scale is less sensitive to change, a scale with fewer options is preferable to no self-report at all. A substantial number of older adults (with and without cognitive impairment) have difficulty responding to an NRS, particularly if it is administered verbally and without a visual representation (Kaasalainen, Crook, 2003; Wynne, Ling, Remsburg, 2000). Vertical presentation (see Figure 3-5, A, p. 58) may be easier for persons with alterations in abstract thinking and often is preferred by older adults (Herr, Spratt, Mobily, et al., 2004).
Verbal descriptor scales (e.g., none, slight, mild, moderate, severe, extreme, most intense pain possible) have good reliability and validity, increased sensitivity to change, and a very low failure rate. Further, the verbal descriptor scale has been identified as the instrument preferred by many older adults, and it has strong clinical utility (Chibnall, Tait, 2001; Gagliese, Katz, 2003; Herr, Spratt, Mobily, et al., 2004; Kaasalainen, Crook, 2003; Pautex, Herrmann, Le Lous, et al., 2005; Pesonen, Kauppila, Tarkkila, et al., 2009; Taylor, Herr, 2003).
A recent quasiexperimental study compared the use of five pain intensity scales, including a novel pain thermometer called the Iowa Pain Thermometer (IPT; Figure 3-9, p. 87), with verbal descriptors in a sample of 61 younger and 36 older adults with osteoarthritic pain (Herr, Spratt, Garand, et al., 2007). The IPT provides a range of adjectives that have been supported in earlier research, including no pain, slight pain, mild pain, moderate pain, severe pain, very severe pain, the most intense pain imaginable (Herr, Spratt, Mobily, et al., 2004), along with a thermometer to assist understanding of the tool by older persons who have difficulties with abstract thinking. The IPT had the lowest failure rate of all pain intensity scales evaluated, was sensitive in detecting changes in pain following intervention, and was most preferred by both younger and older patients, including those with cognitive impairment. This and other studies provide further support for the IPT, as well as for the NRS (a 0 to 20 scale with 5-point intervals); the VDS (same adjectives as the IPT but no thermometer); and the FPS (7 line-drawn faces) in cognitively impaired elders (Taylor, Harris, Epps, et al., 2005; Ware, Epps, Herr, et al., 2006).
A simplified version of the verbal descriptor scale that was evaluated in long-term care includes only the adjectives none, mild, moderate, and severe (Closs, Barr, Briggs, et al., 2004). Treatment decisions are based on level of pain severity, and although this simple tool may be most useful for those with advanced dementia, it may be less sensitive than a verbal descriptor scale to detect changes in pain that move from severe to moderate or moderate to severe.
The FPS (7 faces) (Bieri, Reeves, Champion, et al., 1990) was revised in studies with children and presented in a 6-face version that is scored 0 to 10 (FPS-R), presented earlier in this chapter, p. 69) (Hicks, von Baeyer, Spafford, et al., 2001; Spagrud, Piira, von Baeyer, 2003). Subsequent studies in older populations have shown the FPS-R to be reliable and valid and to be the pain intensity scale preferred by cognitively impaired older African-Americans, Hispanics, and Chinese elders (Li, Liu, Herr, et al., 2007; Ware, Epps, Herr, et al., 2006). Although testing of the FPS-R in older Caucasian adults has not been published, the comparability of the FPS and FPS-R in children and the validity established in minority elders suggest that it would be appropriate in the general older-adult population. It should be noted that the FPS-R tends to correlate less strongly with other pain intensity scales, suggesting it may be capturing other characteristics besides intensity (e.g., pain affect). This does not negate its usefulness, but clinicians should be aware that it may be capturing a broader view of pain.
A consideration in tool selection that has not received much attention in the literature is the impact of a patient’s education and literacy on tool use and understanding. Low education level might suggest the need to focus tool selection on the simplest and most concrete options; however, education has not been a significant factor in failure to use pain intensity scales correctly in older adults (Gagliese, Weizblit, Ellis, et al., 2005; Herr, Spratt, Garand, et al., 2007). A study presenting a new scale for patients with low education, the Full Cup Test (FCT), demonstrated a statistically significant correlation between the FCT and the VAS (a 100-mm horizontal line with word anchors at the extremes; see p. 55 for discussion of and an image of VAS) and the ability to complete this alternative tool reliably in a sample of 114 patients with 9.23 years of education (Ergun, Say, Ozer, et al., 2007). Of 14 patients with low education, 3 could not understand the VAS, but all were able to complete the FCT. However, the literature shows that many older persons are unable to complete the VAS regardless of education level (Herr, Spratt, Mobily, et al., 2004). Calculation of a pain score using the FCT is cumbersome, so a copy of the tool is not included here, but it is mentioned and referenced, should the reader need such a tool.
Language challenges impact our ability to evaluate pain and are a consideration in tool selection. These challenges may relate to inability to read or write but also to those whose primary language is not English. A study of Portuguese patients with RA compared those who were literate (N = 66) with those who could not read or write in Portuguese (N = 25) using a VAS, VRS (separate boxes for no pain, mild, moderate, severe, unbearable), and NRS (boxes with 0 to 10) (Ferraz, Quaresma, Aquino, et al., 1990). The NRS that uses numbers rather than the VRS with word descriptors had higher reliability in both groups. Tools that avoid words, such as the FPS-R, may be useful options in older persons who are illiterate, dyslexic, or non-English speaking, although testing in these samples is needed.
In addition to pain intensity scales, the use of a pain map or figure drawing such as an enlargement of the figure in Form 3-1 (p. 51) can be a useful tool in identifying the location of pain. Even those with dementia have been able to use this approach to communicate the location of their pain (Weiner, Peterson, Logue, et al., 1998; Wynne, Ling, Remsburg, 2000). In those with cognitive impairment, information about pain location can guide approaches to care that minimize the activities and movements that precipitate discomfort. Knowledge of pain location can also help clinicians decide when anticipatory pain intervention would be useful (e.g., prior to range of motion exercises or transfer of a patient who located pain in the shoulders and hip joints).
In summary, the goals are to identify a pain assessment tool that older patients with cognitive impairment can use easily, to use the same tool consistently in each assessment, to establish a comfort-function goal, and to document the reports of pain in an accessible location in the medical records. The organization should identify valid and reliable tools from which to select for use with patients having varying levels of cognitive impairment (see Patient Example, below). A reasonable approach would be to have a vertical NRS such as 0 to 5, a VDS, and the FPS-R as options for this population. Unfortunately, no research has yet tested the 0-to-5 NRS in the cognitively impaired population. Development of documentation strategies that keep pain assessment information in a visible place is needed to monitor changes in pain and response to treatment. Table 3-1 (p. 96) provides a summary of research information on pain-intensity tools recommended for use in older adults with cognitive impairment.
Table 3-1
Pain Intensity Tools Recommended for Use with Older Adults Who Have Difficulty with Commonly Used Self-Report Scales
1Closs, S. J., Barr, B., Briggs, M., et al. (2004). A comparison of five pain assessment scales for nursing home residents with varying degrees of cognitive impairment. J Pain Symptom Manage 27, 196-205.
2Herr, K., Spratt, K., Garand, L., et al. (2007). Evaluation of the Iowa pain thermometer and other selected pain intensity scales in younger and older adult cohorts using controlled clinical pain: A preliminary study. Pain Medicine, 8(7), 585-600.
3Taylor, L. J., Harris, J., Epps, C. D., et al. (2005). Psychometric evaluation of selected pain intensity scales for use with cognitively impaired and cognitively intact older adults. Rehabilitation Nursing, 30, 55-61.
4Ware, L. J., Epps, C., Herr, K., et al. (2006). Evaluation of the Revised Faces Pain Scale, verbal descriptor scale, numeric rating scale, and Iowa Pain Thermometer in older minority adults. Pain Management Nursing, 7(3), 117-125.
5Li, L., Liu, X, & Herr, K. (2007). Postoperative pain intensity assessment: A comparison of four scales in Chinese adults. Pain Medicine, 8(3), 223-234.
6Miro, J., Huguet, A., Nieto, R., et al. (2005). Evaluation of reliability, validity, and preference for a pain intensity scale for use with the elderly. The Journal of Pain, 6(11), 727-735.
As appears in Pasero, C., & McCaffery, M. Pain assessment and pharmacologic management, p. 96, St. Louis, Mosby. Pasero C, McCaffery M. May be duplicated for use in clinical practice.
Assessment of Neuropathic Pain
Characteristics of Neuropathic Pain
The International Association for the Study of Pain defines neuropathic pain as “pain initiated or caused by a primary lesion or dysfunction of the nervous system” (Merskey, Bogduk, 1994, p. 212). This remains the current definition and includes neuropathies, postherpetic neuralgia, and radiculopathy. There are, however, a variety of clinical conditions that have neuropathic features but do not exactly fit this definition because they do not seem to involve an injury or dysfunction of the nervous system (Audette, Emenike, Meleger, 2005; Fishbain, Lewis, Cutler, et al., 2008). These conditions include complex regional pain syndrome, fibromyalgia, and others in which whether they represent neuropathic pain is controversial.
Neuropathic (pathologic) pain is distinctly different from physiologic (nociceptive) pain (see Section I). It is pain sustained by abnormal processing of sensory input by the peripheral or central nervous system, most often as a result of injury. There are numerous causes of nervous system damage that can result in neuropathic pain, including toxins, infection, viruses, metabolic disturbances, nutritional deficiencies, and trauma (Pasero, 2004).
The types of neuropathic pain are commonly divided into categories on the basis of the mechanism thought to be primarily responsible for causing the pain (i.e., peripheral or central nervous system activity) (see Figure I-1, p. 2). Note that in some cases the original injury occurs in the peripheral nerves (e.g., amputation), but the mechanisms that underlie the pain (e.g., phantom pain) seem to be generated primarily in the central nervous system.
Screening Tools and Measurement Tools for Neuropathic Pain
In relation to neuropathic pain, two types of assessment scales may be used: screening tools and measurement tools. Tools have been developed for screening populations of patients with chronic pain to distinguish neuropathic pain from other types of chronic pain (Bennett, Attal, Backonia, et al., 2007). Those that are self-report tools and do not require examination by a clinician are reliable and valid and are easy to score; they include the ID Pain tool (Portenoy, 2006); the Self-Administered Leeds Assessment of Neuropathic Symptoms and Signs (S-LANSS) tool (Bennett, Smith, Torrance, et al., 2005); and the painDETECT tool (Freynhagen, Baron, Gockel, et al., 2006). This type of tool may be especially useful for non-pain specialists in primary care settings. Once neuropathic pain has been identified, the need to refer a patient to a pain specialist can be evaluated, and measurement tools such as the Neuropathic Pain Scale (NPS) (Galer, Jensen, 1997) may be used to measure the qualities of neuropathic pain over time and evaluate the effects of various treatments (see discussion of the NPS below).
Because of its brevity, simplicity, reliability, and validity, the complete ID Pain tool has been selected for inclusion here (Form 3-4). This tool was validated using data from two independent multicenter study samples (Portenoy, 2006). It is composed of a drawing, five sensory descriptor items requiring a yes or no answer, and one item relating to whether pain is located in the joints, which is used to identify nociceptive pain. The scale is self-administered, is easily scored, and can be used to quickly identify patients who have neuropathic components to their pain. Scores range from –1 to 5, with 3 being identified as the cut-off point for neuropathic pain. Scores of 3 or higher indicate the likely presence of a neuropathic component of the pain and justify a more detailed evaluation.
Form 3-4 As appears in Pasero, C., McCaffery, M. (2011). Pain assessment and pharmacologic management, p. 97, St. Louis, Mosby. Used with permission. From Portenoy, R. (2006). Development and testing of a neuropathic pain screening questionnaire: ID Pain. Curr Med Res Opin, 22(1), 1555-1565. May be duplicated for use in clinical practice.
In the late 1990s a brief yet comprehensive assessment tool, the NPS, was designed specifically to measure the qualities of neuropathic pain (Galer, Jensen, 1997). The complete tool is included here (Form 3-5). Studying 288 patients with peripheral neuropathic pain, the researchers identified eight common qualities: sharp, hot, dull, cold, sensitive (like raw skin or a sunburn), itchy, and deep versus surface pain. These are included on the NPS as items 2 through 7 and item 10. Each item is rated on a 0 to 10 scale (0 = none; 10 = most imaginable). The tool is easy for most patients to learn, takes about 5 minutes to complete, and is sensitive to the effects of treatment. It also has been translated into Italian (Negri, Bettaglio, Demartini, et al., 2002). One limitation of the scale is that it does not include some pain qualities typical of neuropathic pain, such as numbness, tingling (pins and needles), and electric shock (Jensen, Dworkin, Gammaitoni, et al., 2005).
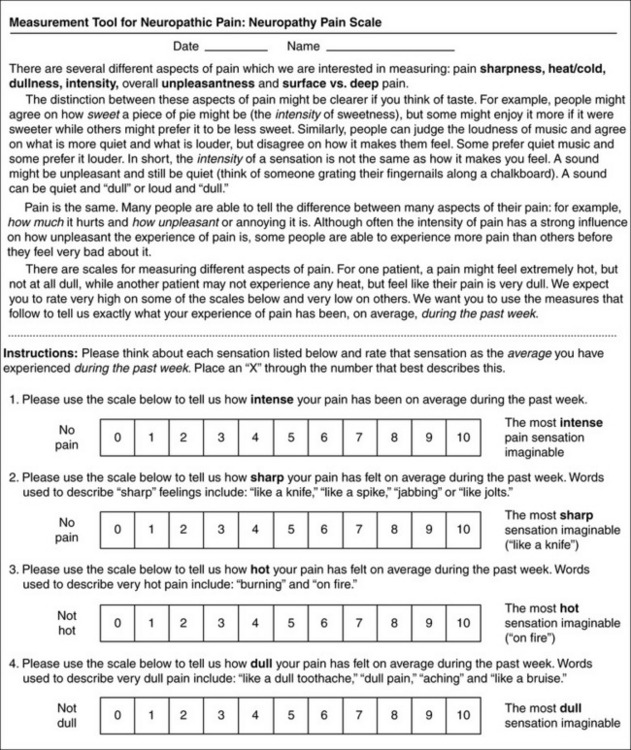

Form 3-5 From Pasero, C., McCaffery, M. (2011). Pain assessment and pharmacologic management, pp. 99-100, St. Louis, Mosby. From Galer, B. S., & Jensen, M. P. (1997). Development and preliminary validation of a pain measure specific to neuropathic pain: The neuropathic pain scale. Neurology, 48, 337-338. © Jensen MP, Galer BS. Permission for all use in research activities is required and must be obtained by contacting the MAPI Research Trust (http://www.mapiresearch.fr).
The NPS also appears to be a useful tool in the assessment of patients with central pain caused by multiple sclerosis. In a study of 141 patients with chronic pain related to multiple sclerosis, the NPS was found to be a valid and reliable assessment tool and possibly useful in measuring treatment outcomes (Rog, Nurmikko, Friede, et al., 2007). It was also found to be versatile in that it was reliable and valid when administered face-to-face as well as by postal survey.
In a study of 159 patients with diabetic-related foot pain, the NPS was administered before, during, and after treatment with placebo versus opioid (modified-release oxycodone) (Jensen, Friedman, Bonzo, et al., 2006). More participants receiving opioids responded to the six NPS items than those receiving placebos. The three NPS items that were most responsive to opioid treatment as compared to placebo were pain intensity, unpleasantness, and deep pain. The results support the validity of the NPS for detecting change in neuropathic pain with treatment, and this is probably the first study to show a significant effect of opioid treatment on specific pain sensations.
A study of 470 patients with peripheral neuropathic pain, low back pain, or OA, demonstrated the utility of the NPS for assessing changes in pain after treatment with a lidocaine patch (Jensen, Dworkin, Gammaitoni, et al., 2005). With the exception of cold, each of the qualities of pain on the NPS showed significant change after treatment in each of the three diagnostic groups, supporting the validity of the NPS for detecting change in pain. Eventually, this type of research might help to identify which qualities of pain respond to which analgesics and to better understand the mechanisms of analgesic effects.
The words used by patients to describe pain may indicate the underlying pain mechanism. For example, postherpetic neuralgia is more likely to be described as sharp, less cold, and more sensitive and itchy than other types of neuropathic pain (Galer, Jensen, 1997). Patients with diabetic neuropathy who described sharp and shooting pain were found in one study to be more likely to respond to clonidine than those who did not use these descriptors (Byas-Smith, Max, Muir, et al., 1995). Although the NPS was not designed to discriminate between neuropathic and nonneuropathic pain, further study of patients with persistent pain has shown that the NPS appears to be able to do this (Fishbain, Lewis, Cutler, et al., 2008).
In summary, the NPS may be a practical tool in the initial assessment of patients suspected of having neuropathic components in their pain, such as low back pain. It may also be useful in evaluating the effects of treatment of patients with neuropathic pain. As the tool is developed further it may become a practical method of distinguishing one type of neuropathic pain from another. As mentioned, the NPS or the ID Pain tool can be provided to patients presenting to the emergency department with conditions such as low back pain so they can complete it while waiting to be evaluated. This will help clinicians to better determine the type of pain patients are having and the appropriate treatment to prescribe.
For patients who do not have neuropathic pain initially, routine pain assessment tools completed at each visit to the clinician might include a list of the eight descriptors on the NPS. The patient could be asked to indicate whether any of these sensations had been noted since the last visit. In that way, a patient who did not originally have neuropathic pain might be identified, and changes in the progression of pain and in underlying pathologic conditions may be assessed and treated more quickly. This method of identifying neuropathic pain has not been tested for reliability and validity as has the ID Pain form, but it may be a practical method to use in some situations.
Along with the NPS, there is another self-report scale that is a reliable and valid instrument for measuring the qualities of neuropathic pain: the Neuropathic Pain Symptom Inventory (NPSI). The reader may wish to compare this scale with the NPS to determine which is preferred. The NPSI tool is available in both English and French (Bouhassira, Attal, Fermanian, et al., 2004).
Assessment of Breakthrough Pain
No universally accepted definition of BTP exists. It is defined differently in different countries and in different specialties, and definitions have varied over the years (Payne, 2007). Probably the first comprehensive definition of BTP was developed in relation to research involving patients with persistent cancer-related pain and was defined as “a transitory increase in pain to greater than moderate intensity (that is, to an intensity of ‘severe’ or ‘excruciating’) which occurred on a baseline pain of moderate intensity or less (that is, no pain or pain of ‘mild’ or ‘moderate’ intensity)” (Portenoy, Hagen, 1990, p. 274). It is pain that breaks through other pain that is well controlled. BTP has also been studied in relation to persistent noncancer pain. A definition that is commonly used now in clinical practice is that BTP is a transitory increase in pain that occurs on a background of otherwise controlled persistent pain (Portenoy, Bennett, Rauck, et al., 2006).
Characteristics of Breakthrough Pain
BTP is divided into three subtypes: incident, idiopathic, and end-of-dose. Incident BTP is further divided into two types: predictable and unpredictable (Box 3-6). Idiopathic pain is not associated with any known cause. End-of-dose pain refers to pain returning before the interval between doses elapses. Defining these types of BTP may assist with selecting a form of treatment. Use of a short-acting opioid is commonly the treatment for incident pain and idiopathic pain, whereas an increase in the dosage of around-the-clock analgesic or a decrease in the interval between doses is usually the treatment for end-of-dose BTP.
In a review of 5 studies of cancer populations, BTP was found to occur in 50% to 90% of patients with persistent pain, confirming that BTP is a significant problem for patients with cancer-related pain (Portenoy, Bennett, Rauck, et al., 2006). In one study of BTP in 164 patients with persistent cancer pain, the median number of episodes was 6 per day, and the median interval from onset to peak was 3 minutes (Portenoy, Payne, Jacobsen, 1999). Almost two thirds (61.7%) could identify precipitants such as movement, but almost half (48.2%) stated that BTP was never predictable. Patients with BTP experienced more functional impairment and psychologic distress than did patients with controlled background pain and no BTP. BTP has not been as well studied in patients with persistent noncancer pain, but in a survey of 228 patients with noncancer pain, 74% had BTP (Portenoy, Bennett, Rauck, et al., 2006). The most common cause for their persistent pain was low back pain (52%). The median number of episodes of BTP per day was 2, median time to maximum intensity was 10 minutes, and median duration was 60 minutes. Patients identified a precipitant for 69% of the occurrences of BTP, and it was almost always activity related. Onset could not be predicted in 45% of bouts of BTP and could be predicted only sometimes in 31%.
A study of 43 patients with persistent noncancer pain assessed for impact of BTP on quality of life (Taylor, Webster, Chun, et al., 2007). In response to questions about how BTP affected patients’ lives, the two questions that 93% of the patients found affected their lives the most (“quite a bit or very much”) were related to general activity levels and the ability to work both outside the home and in the home doing housework. The next category most commonly rated (86%) as affecting their lives the most was enjoyment of life.
Tools for Assessment of Breakthrough Pain
Only a few tools have been developed for use in clinical practice to screen for or to assess BTP. Some have been used only for research purposes, and others are meant only for clinician assessment; that is, they guide a clinician’s interview of a patient. A list of questions that clinicians can use to interview patients about BTP has been published by a multidisciplinary panel (Bennett, Burton, Fishman, et al., 2005). A pain assessment algorithm has been published; it begins with the clinician asking a patient questions that screen for BTP and then follows up with specific questions about the BTP (Portenoy, Bennett, Rauck, et al., 2006). This was used in a research study of BTP in opioid-treated patients with noncancer pain and was modified from one originally developed for patients with cancer-related pain, neither of which has been validated (Portenoy, Payne, Jacobsen, 1999). Another published tool for assessing BTP, the Alberta Breakthrough Pain Assessment Tool, was developed for research with cancer patients (Hagen, Stiles, Nekolaichuk, et al., 2008). Some of the questions must be completed by the clinician as they interview the patient, and one section of the tool is suitable for completion by the patient. Evidence suggests that this tool is understandable by patients and clinicians, so further validation of the tool is warranted.
A screening tool for BTP is difficult to develop because BTP is not easily characterized when baseline pain has not been controlled. Baseline pain can be defined as almost always being present, continuous, steady, or constant or would be if medication did not control the pain. Thus, a screening tool for BTP in clinical practice is best designed to be used after baseline pain has been determined to exist and has been well managed.
Most patients with persistent pain who have controlled baseline pain experience only one type of BTP. In a study of 228 patients with persistent noncancer pain and controlled baseline pain, 74% had one or more types of BTP (Portenoy, Bennett, Rauck, et al., 2006). Of these patients, 88.7% had one type of BTP, and the remainder of the patients had up to three types of BTP. Precipitants were identified for 69% of the BTP episodes, and 92% of them were activity related. In a study of 164 patients with chronic cancer pain and controlled baseline pain, 51.2% experienced one or more types of BTP (Portenoy, Payne, Jacobsen, 1999). Of these patients, 83.1% experienced only one type of BTP, and the remainder experienced as many as three types of BTP. Precipitants of BTP could be identified by 61.7% of patients, with movement being the most common, 20.4%. However, almost half of the patients said that precipitants were never predictable.
Forms 3-6 (p. 103) and 3-7 (pp. 104-105) have been developed to provide simple, short forms to screen for and assess BTP. These forms have not been tested for reliability and validity, but they have been created in the absence of such forms in the hope that they will encourage assessment of BTP until simple, reliable, and valid forms are available. The short screening tool may be included in an already existing initial pain assessment tool, as is done with Form 3-1 (p. 51), or may be included in the clinician’s interview with the patient. The purpose of this form is to identify patients with persistent, constant, controlled baseline pain who also have pain that breaks through the controlled pain; that is, BTP. When such a patient is identified, he or she may complete the assessment form, or the clinician may use the form to ask the patient about BTP.
A detailed pain diary is commonly recommended as being the best account of the occurrence, predictability, severity, and duration of BTP (Payne, 2007). The Pain Control Diary, Form 3-11 (p. 116), may be used for this purpose and perhaps modified to include columns for noting physical activity at the time pain started and the time pain ended. A daily pain diary that may be more suitable for assessing BTP can be found at http://www.healthinaging.org/public_education/pain/my_pain_diary.pdf.
Reassessment
Once the initial assessments of the patient is complete and treatment for pain relief has begun, reassessment of the patient is essential to determine the effects of treatment. This is facilitated by using a variety of types of flow sheets. Flow sheets for pain management are work sheets that document progress toward achieving and maintaining pain-management goals. They are used for ongoing evaluation of pain treatments.
Following is a discussion of paper-based flow sheets for analgesic infusions in acute care, pain control diaries for outpatient care, and a standardized record for reassessing patients with chronic pain who are taking opioids. The current focus on patient safety as well as efficient administration of care has brought about the introduction of the electronic health record to patient care. Also called the electronic medical record, the electronic health record is a comprehensive patient-centered clinical information system that contains data from multiple care settings as well as from the patient. (See Appendix C [pp. 837-857] for an overview of the electronic medical record, including its implementation in the clinical setting and examples of screens, such as pain management order sets and flow sheets, medication administration records, and assessment alerts.)
Flow Sheets for Analgesic Infusions in Acute Care
Obtaining pain ratings before and after the administration of analgesics is especially important because clinicians tend to assume that analgesics are safe and effective. Research has verified that the use of pain flow sheets, compared with the routine narrative charting in nursing notes, is effective in improving pain management (e.g., decreasing pain intensity and sedation) (Brown, 1992; Faries, Mills, Goldsmith, et al., 1991; McMillan, Williams, Chatfield, et al., 1988).
Flow sheets must be tailored to the patient population, clinical setting, and type of pain being managed. For example, some are designed for staff to complete for hospitalized patients using IV PCA for severe pain. Typically, flow sheets include, at a minimum, columns for time, pain ratings, facts about the analgesic administered (e.g., dose, route) or other pain treatment, and adverse effects pertinent to the situation. Adverse effects pertinent to the use of opioids for severe pain in opioid-naive patients (those not taking regular daily doses of opioids) include sedation level and respiratory status. Information about pain ratings should be obtained and recorded near the time of the event, whenever possible. For example, in a study of 60 patients being discharged from the postanesthesia care unit, many were unable to recall the pain ratings they gave when they were admitted to the unit (DeLoach, Higgins, Caplan, et al., 1998).
Frustration about the failure of staff nurses to reassess and document the effects of analgesics in acute care inpatient settings is frequently reported by nurses attending programs on pain management and is often a topic discussed on the nursing issues LISTSERV provided by the American Pain Society (APS) and the American Society for Pain Management Nursing. Research reflects this problem as well. In an Australian study of 52 nurses caring for 364 postoperative patients, 316 pain-related activities occurred in 74 observation periods (2 hours each), but only 4.4% were reassessments after analgesic administration (Bucknall, Manias, Botti, 2007). Before or during the observation period, 147 patients received analgesics but in only 13.6% was the pain reassessed. Most of these reassessments were opportunistic in that they were performed when the nurse was in the patient’s room carrying out other nursing activities such as administering other medications. Some have argued that reassessment following analgesic administration occurs but is not documented. However, this study was of actual nursing activity at the patient’s bedside, not documentation, and still reassessment of administered analgesics was substantially lacking.
In the United States, abstracts of the medical records of 709 older patients hospitalized for hip fracture were made for the purpose of determining nurses’ documentation of their pain assessments, including reassessments following administration of non-PCA analgesics (Herr, Titler, Schilling, et al., 2004). For the first 24-hour period following admission, 2965 analgesics were administered, and only 21.8% (646) were followed by reassessment of pain within 60 minutes. For the entire 72-hour period, only 15.3% (1497) of 9782 analgesic administrations were following by reassessments of pain within 60 minutes. In a study of frail elderly patients postoperatively who received analgesics, only 4% were reassessed following the analgesic (MacDonald, Hilton, 2001).
The medical records were reviewed for reassessment of 52 critically ill intubated patients who had had 183 pain episodes (Gelinas, Fortier, Viens, 2004). Pharmacologic interventions were used 89% of the time, and nonpharmacologic interventions were used less than 25% of the time. However, almost 40% of the time, pain was not reassessed after an intervention. Self-report was recorded for only 1 patient. Reassessment of patients consisted primarily of recorded behavioral and physiologic manifestations. Some of the physiologic indicators are questionable signs of pain, and although they were recorded within 1 hour of an intervention, it is not clear that they were done to reassess pain.
Another study of 117 charts of 80 inpatients and 37 outpatients with cancer whose pain had been documented in their charts revealed that reassessment after analgesic treatment was reported in 34% of the outpatient charts and 44% of the inpatient charts (Cohen, Easley, Ellis, et al., 2003). The more favorable results shown in this study may have occurred because charts that did not document pain at all were excluded from the sample, meaning that pain may have existed, and treatment may have been provided but not documented. For example, the researchers found one outpatient setting with no charts that documented pain at all, and the staff said they simply did not document pain.
Reassessment of pain after administration of an opioid is not just a documentation issue, it is also an issue of patient safety following administration of a drug that could lead to death. Reassessment should be the basis of planning for further intervention, in particular knowing when change in the treatment plan is needed. For example, measures such as decreasing the opioid dosage in a patient who is excessively sedated can be implemented to prevent life-threatening respiratory depression. Range orders for opioid analgesics simply cannot be effectively implemented unless the previous dose of analgesic is followed by reassessment of pain intensity and adverse effects. (See Section IV for additional information about opioid-range orders and treatment of opioid-induced adverse effects.)
Most health care facilities have incorporated guidelines for pain documentation into their policies and procedures and have provided space for documentation of basic pain assessments in standard nursing care forms; flow sheets are often used for analgesic infusions and IV bolus administration. Unit-specific quality improvement activities and ongoing education initiatives routinely include documentation of pain assessment following interventions for pain relief. In cases in which compliance is exceptionally low, management may decide to implement disciplinary measures such as linking documentation compliance to the nurses’ annual performance reviews.
In the initial treatment of moderate to severe pain with opioids, the patient’s pain rating is usually recorded every 1 to 2 hours during the first 24 hours, then every 4 hours if pain is controlled and other observations, such as sedation level, show the opioid is safe. When the patient appears to be sleeping at the time a pain rating should be obtained, it is often questioned whether to awaken the patient. Several factors should be considered. Not all patients who appear to be sleeping are actually sleeping. Further, sleep does not mean pain has been relieved. When a patient appears to be sleeping, his or her name may be called in a normal tone of voice. If the patient does not rouse and has acceptable respiratory status, pain assessment could be delayed until the patient awakens, and “appears to be sleeping” could be charted. However, even this approach has its limits, because sleep does not mean absence of pain. Therefore, if the medication has been given previously and the patient has rated relief as satisfactory, when that same medication is given again it is not necessary to awaken the patient after administration. However, if the patient has pain that is still out of control, discuss the situation with the patient and tell him or her that he or she may be awakened to determine whether the medication is providing effective pain relief.
Creative ways of reminding nurses to obtain and document pain ratings are often implemented as well. For example, hospital-wide competitions for prizes to be awarded to the unit demonstrating the highest compliance record can be good incentives for improving documentation. Some hospitals have installed, outside of patients’ rooms, colored flags (similar to those seen in clinics or office-based practices) that can be moved to a position perpendicular to the wall after an analgesic has been given so as to provide visual reminders to nurses of the need for reassessments. Enlisting the help of other staff members (e.g., nursing technicians or even patients can remind nurses of the need for reassessment at a specific time). (See the discussion of pain diaries on pp. 110-119 and oral PCA in Chapter 12.)
As health care facilities move toward the use of electronic health records, computer software has the potential to become an excellent tool for improving the documentation of pain. Carefully placed prompts and alerts can remind and even force clinicians to document pain assessment before moving on to the next computer screen. (See Appendix C for additional information about electronic health records.)
Following is a discussion of a flow sheet for use in acute care settings in which patients with severe pain are being treated with opioids. Examples are provided.
Characteristics of the Flow Sheet for Analgesic Infusions in Acute Care
The flow sheet for analgesic infusions, such as IV PCA, epidural analgesia, and continuous perineural blockade, provides a quick and easy assessment and evaluation tool (Form 3-8). Because the flow sheet provides a comprehensive snapshot of how well a patient is tolerating therapy, it is often used for documentation of single or intermittent clinician-administered bolus doses as well. Flow sheets are used primarily in hospital settings where nurses are responsible for recording the information.
Form 3-8 The back of the 24-hour pain management flow sheet provides space for comments when patients or infusion systems are outside of the norm. Comments can be made at any time by any member of the health care team and do not necessarily coincide with the flow sheet. From Pasero, C., & McCaffery, M. Pain assessment and pharmacologic management, pp. 107-108, St. Louis, Mosby. Pasero C, McCaffery M. May be duplicated for use in clinical practice.
Most flow sheets are designed to prevent duplicate documentation. Space can be provided on the back of the flow sheet for documenting detailed information (e.g., description of how severe pain or an adverse effect was brought under control; see Forms 3-9 and 3-10 as examples). A mechanism such as an asterisk can be incorporated into the flow sheet for referring other members of the health care team to this detailed information. For ease in using the flow sheet, the type of information and the order in which it is recorded in the columns of the flow sheet should correspond with the type and order of the information displayed on the screens of the institution’s infusion pump. This explains why there is such wide variability from one institution to another in terms of the information found on flow sheets and the way it is presented.

Form 3-9 Back of 24-hour pain management flow sheet provides space for comments when patient or infusion system is outside of the norm. Comments can be made at any time by any member of the health care team and do not necessarily coincide with the flow sheet. Patient assessment columns on the front of the flow sheet show that the patient had satisfactory pain control while awake, but after sleeping and 5 hours of no sef-administered PCA doses, the patient was awakened by pain. Pain was brought under control with two clinician-administered bolus doses and the addition of a low-dose basal rate. The patient’s pain, respiratory status, LOS, and adverse effects are recorded every 2 hours during the first 24 hours after surgery. Site assessment and pump parameters are recorded only every 8 hours. From Pasero, C., & McCaffery, M. Pain assessment and pharmacologic management, pp. 111-112, St. Louis, Mosby. Pasero C, McCaffery M. May be duplicated for use in clinical practice.
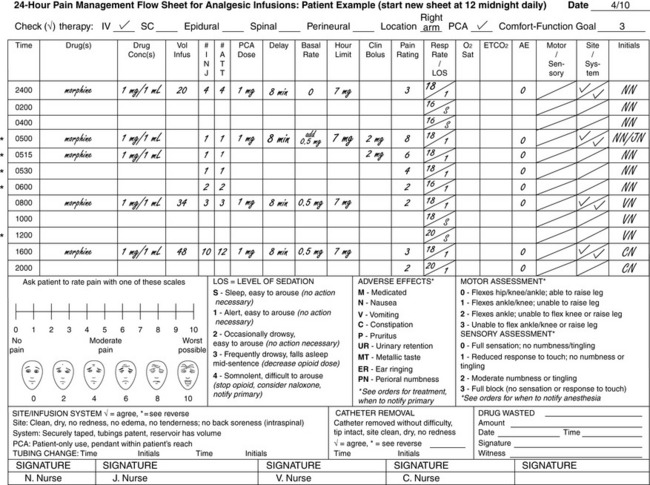
Form 3-10 Back of 24-hour pain management flow sheet provides space for comments when patient or infusion system is outside of the norm. Comments can be made at any time by any member of the health care team and do not necessarily coincide with the flow sheet. Patient assessment columns on the front of the flow sheet show that the patient had adequate pain control with ratings of 0-1, which are less than the patient’s comfort-function goal of 3, and that the analgesic dose was safe with LOS of 2 or 1-2. Itching (pruritus) is the only adverse effect and was handled by decreasing the basal rate. Note that the patient’s pain, respiratory status, LOS, and adverse effects are recorded every 2 hours during the first 24 hours after surgery. Site assessment and pump parameters are recorded only every 8 hours. From Pasero, C., & McCaffery, M. Pain assessment and pharmacologic management, pp. 113-114, St. Louis, Mosby. Pasero C, McCaffery M. May be duplicated for use in clinical practice.
The example of the flow sheet for analgesic infusions shown in Form 3-8 has numbered spaces and columns that match the numbered explanations that follow.
1. Date. A new flow sheet can be started at 12 midnight (2400) every 24 hours or when space for documentation runs out. The day, month, and year are entered.
2. Route of administration. Analgesic therapies documented on flow sheets are usually given by the IV, subcutaneous (SC), epidural, spinal (or intrathecal), intrapleural, or perineural route of administration.
3. Location. Examples of locations include the extremity in which an IV is inserted for IV PCA, the targeted nerve or nerve plexus for continuous perineural blockade, and lumbar or thoracic epidural catheter placement.
4. PCA. A checkmark is made if the therapy is patient controlled (PCA).
5. Comfort-function goal. The comfort-function goal is a pain level that enables the patient to participate in specific recovery activities with relative ease (e.g., 2/10 to 3/10, discussed on pp. 85-88). The patients is encouraged to set a comfort-function goal, which is recorded in the space provided at the top right-hand corner of the flow sheet. Pain ratings greater than the patient’s comfort-function goals should trigger further assessment and possible intervention. This information can also be used for quality-improvement purposes to help ensure unrelieved pain is being addressed appropriately.
6. Time. At various times during therapy, patient assessment and infusion data are recorded in the column spaces provided (see following numbers 7 through 23 for detailed descriptions of these entries). The analgesic prescription (also called pump parameter), which include, a the drug(s) and concentration(s), PCA doses, delay (or lockout) intervals, basal rate, and hour limit, are recorded typically only every 4 to 8 hours. Double-checks of pump programming are performed as safeguards against errors and require two clinicians or pharmacists to confirm that the drug(s) and concentration(s) listed on the reservoir label and the pump programming match the prescription. Practices vary among institutions as to when double-checks must be performed, but they are usually performed when therapy is initiated, when a prescription is changed, and when a patient’s care is transferred from one care area to another (e.g., from postanesthetia care unit to clinical unit). Other data found in most pumps are the number of successful injections (INJ) and attempts (ATT) the patient makes to obtain a PCA dose during therapy and the administration of clinician boluses. Pain ratings, nurse-monitored respiratory status and sedation level (LOS), oxygen saturation, and carbon dioxide concentration if mechanical monitoring is prescribed, and presence of adverse effects (AE) usually are recorded every 1 to 2 hours for the first 24 hours of analgesic infusion therapies in opioid-naive patients. After that time, these parameters usually can be monitored every 4 hours, and mechanical monitoring, if prescribed, often is discontinued or performed PRN in stable patients. Motor and sensory assessments in patients receiving local anesthetic are usually recorded every 4 to 8 hours. Site and system assessments are documented at least every 8 hours.
7. and 8. Drug(s) and concentration(s). After checking the drug reservoir labels against the prescriptions, the name of the drug(s) and the concentration(s) in the drug reservoir are recorded in this column every 4 to 8 hours and when prescriptions are changed, new reservoirs are added, or clinicians’ boluses are administered. The drug concentrations, which are the amounts of drug per 1 milliliter of solution (X mg/mL, X mcg/mL) programmed into the pump are also checked for accuracy against the drug concentrations recorded on the drug reservoir labels.
9. Volume infused (sometimes called “given” volume). The amount of solution delivered since the start of therapy or since the last time the amount was cleared from the pump is recorded every 4 to 8 hours. (This feature is not available on all pumps; some pumps have volume remaining rather than volume infused, and some have both.) Some institutions record a running total of volume infused; others require pumps to be cleared at the end of every shift.
10. and 11. Injections and attempts. The term injections means the number of times patients self-administer a dose of PCA. Attempts are the number of times a patient presses the PCA button (pendant), whether a dose is delivered or not. If this information is available (some disposable pumps do not offer this information), these amounts can be recorded for patients receiving PCA. Most PCA pumps store in memory the number of injections and attempts every hour. This information is used to help determine the type of interventions that would most benefit the patient (e.g., patient teaching, increase dosage) (see Section IV).
12. PCA dose. The amount patients receive each time a PCA bolus dose is self-administered is recorded every 4 to 8 hours.
13. Delay (or lockout) interval. The number of minutes that must elapse between doses of PCA is recorded every 4 to 8 hours.
14. Basal rate. The amount of the continuous infusion is recorded every 4 to 8 hours. Many pumps bypass this display screen if a basal rate has not been programmed.
15. Hour limit. The total amount the patient can receive in 1 or 4 hours by PCA dose, and basal rate is recorded every 4 to 8 hours. Some pumps are programmed to bypass this display screen (see Section IV for more about hour limits).
16. Clinician bolus. Clinician-administered bolus doses (supplemental, breakthrough doses) are recorded when administered.
17. Pain rating. The patient’s pain rating is usually recorded every 1 to 2 hours during the first 24 hours, then every 4 hours if pain is controlled. The pain rating scales are provided in a box in the lower left side of the flow sheet. A pain rating higher than patient’s comfort-function goal (upper right-hand corner of flow sheet) should trigger further assessment and action (see item 5).
18. Respiratory rate and level of sedation (LOS). Two of the most important assessments in the opioid-naive patient are that of respiratory status (nurse monitored), which includes respiratory rate, depth, regularity, and noisiness, and level of sedation. These two parameters are recorded every 1 to 2 hours during the first 24 hours, then every 4 hours if the they are stable; respiratory rate and LOS are recorded on the face of the flow sheet, and any excessive sedation or abnormalities or trends and changes from baseline in respiratory rate, depth, regularity, or noisiness (e.g., snoring) require an asterisk notation (*) on the front of the flow sheet and detailed information on the back. After the first 24 hours, significant increases in opioid dose warrant the return to respiratory and sedation monitoring every 1 to 2 hours until stable patterns are observed. Depending on the respiratory status and sedation levels, further action may be necessary. The Pasero Opioid-Induced Sedation Scale (POSS) is used to assess LOS and is shown on the bottom half of the flow sheet (see item 26). On this scale, sedation levels 3 and 4 require action; a sedation level of 3 should trigger further assessment and a decrease in the opioid dose; a sedation level of 4 requires a clinician to stop opioid administration, consider naloxone administration, and notify the prescriber. (See Section IV for additional information about opioid-induced sedation and respiratory depression.)
19. Oxygen (O2) saturation. When continuous or intermittent oxygen saturation monitoring (pulse oximetry) is prescribed, readings should be recorded with the same frequency as those of respiratory status and level of sedation.
20. End tidal carbon dioxide (ETCO2) concentration. When continuous or intermittent ETCO2 monitoring (capnography) is prescribed, readings should be recorded with the same frequency as respiratory status and level of sedation.
21. Adverse effects (AE). Using the code for adverse effects (bottom of flow sheet; see item 27), the presence of adverse effects is recorded every 1 to 2 hours during the first 24 hours, then every 4 hours if the patient has no adverse effects. The letter M recorded above or next to an adverse effect signifies that the patient was given medication to treat the adverse effects. Depending on the adverse effects, further action may be warranted and noted in the comment section on the back of the flow sheet (see also Section IV).
22. Motor/sensory. Assessment for detection of motor or sensory deficits in patients receiving local anesthetic is usually performed every 4 to 8 hours. Modified versions of the Bromage Scale and the Inova Sensory Assessment Scale (Nisbet, personal communication, 2008), both shown in the bottom half of the flow sheet (see item 28), are used for motor and sensory assessment, respectively. The level at which the anesthesia provider should be notified depends on the therapy and individual provider practice; orders or protocol should stipulate this information (see Section IV for more about regional analgesia techniques).
23. Site/system. This column is used to document that the infusion sites and systems, from a patient’s access to the infusion pumps, have been assessed and are functioning properly. Usually, this information is documented as often as IV site assessment is documented in the institution (e.g., every 8 hours or every shift) and whenever patients are experiencing inadequate pain control. A check mark (√) in the space provided signifies acceptable assessment findings (agreement with the statement at the bottom of the flow sheet; see item 29). If a patient is receiving PCA, the check mark also signifies patient-only use and that the PCA pendant is within the patient’s reach. An asterisk in this space means that detailed information is provided on the back of the flow sheet.
24. Initials. The initials of the clinician who performs the assessment, pump programming, clinician-administered boluses, or double checks (see item 26) are recorded in the box in the last column. All initials on the flow sheet should have a matching signature at the bottom of the flow sheet (see item 32).
25. Pain rating scale. The first section, at the bottom of the flow sheet, provides an example of the 0-to-10 NRS and the 0-to-10 FPS-R for clinicians’ reference.
26. Level of sedation. The second section, at the bottom half of the flow sheet, contains the POSS for clinicians’ reference to guide documentation of LOS. (See item 18 and Section IV for more about this scale.)
27. Adverse effects. The third section at the bottom half of the flow sheet, contains the codes for recording various adverse effects (see item 21).
28. Motor and sensory assessment. The third section, at the bottom half of the flow sheet, contains the modified version of the Bromage Scale for motor assessment and the Inova Sensory Assessment Scale (Nisbet, personal communication, 2008) for sensory assessment to help clinicians in documentation (see item 22 and Section IV for more about regional analgesia techniques).
29. Site/infusion system: A description of acceptable infusion sites, systems, and PCA setups for clinicians’ reference, as well as space to record infusion tubing changes, are provided at the bottom of the flow sheet (see item 23).
30. Catheter removal. Space is provided for documentation of catheter removal. A check mark (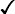 ) signifies agreement with the description; an asterisk (*) means that detailed information is recorded on the back of the flow sheet.
) signifies agreement with the description; an asterisk (*) means that detailed information is recorded on the back of the flow sheet.
31. Drug waste. Space is provided for documentation of drug waste, including the signature of the clinician wasting the drug and the clinician witnessing the waste.
32. Signature. The signatures of the clinicians who perform the assessments, tubing changes, double checks, and catheter removal are recorded at the bottom of the flow sheet. All initials on the flow sheet should have a matching signature.
The medication administration record (MAR) is used to document analgesic use when patients are switched from analgesic infusions to oral pain medication. Then, wherever vital signs are recorded is the most logical place to document pain ratings. Space should be provided for recording the institution’s minimum standards for pain assessment (e.g., pain rating at least every 8 hours) and to record pain ratings at the time analgesics are administered and at the time of reassessment after administration. When pain is difficult to control, the Pain Control Record (Form 3-8) can be kept at the bedside for closer monitoring.
Examples of the use of the analgesic infusion flow sheet for patients receiving IV PCA (Form 3-9) and patients receiving epidural analgesia (Form 3-10) are shown on pp. 111-114.
Pain Diaries for Outpatient Care
A pain control diary is a type of flow sheet for a patient to use in the outpatient setting, although there may be instances when it is appropriate to use it in inpatient settings too. Increasing numbers of patients with pain are being cared for on an outpatient basis. Patients with severe and sometimes escalating pain related to cancer are often cared for at home by family caregivers. Patients with moderate to severe pain related to surgical procedures are discharged from the hospital quickly, sometimes on the same day of the surgery. Both types of patients might benefit from the use of a pain diary.
Because the flow sheets or diaries of patients at home are kept by patients or their families, they must be particularly easy to use. Form 3-11 (p. 116) presents a paper pain diary designed for these circumstances. A patient teaching brochure titled “Understanding Your Pain: Using a Pain Rating Scale” contains an almost identical diary and is available at www.endo.com. It is similar to the diary presented in our previous book (McCaffery, Pasero, 1999). A nurse with chronic pain reported that she modified the diary for the purpose of clarifying for her physicians her pain intensity and the effectiveness of treatments (Aguirre, Nevidjon, Clemens, 2008). It helped her to identify trends in her pain and what worsened her pain. This adaptation of the diary is available at http://download.lww.com/wolterskluwer_vitalstream_com/PermaLink/NAJ_108_6_Aguirre_1059_SDC1.pdf.
As illustrated in Form 3-11, a paper diary for outpatients usually includes, at the minimum, columns or spaces for time, pain rating, medication taken or pain treatment, effect of the pain treatment on the pain rating, and comments, such as related activities or adverse effects. To avoid burdening patients and their families, certain criteria should be established regarding when to keep the record and for how long. The instructions for the pain diary in Form 3-11 stipulate that the record is to be kept only until an effective pain management plan has been developed. The example shown in Form 3-12 concerns a patient with chronic, progressive, cancer-related pain whose pain has escalated.

Form 3-12 Patient example. This patient has increasing pain resulting from metastasizing cancer. He has been receiving the following analgesics around the clock every day: ibuprofen 400 mg tid (8 am, 2 pm, 8 pm); duloxetine 30 mg bid (8 am and 8 pm); modified-release morphine (MS Contin) 100 mg q12h (8 am and 8 pm). His breakthrough dose (BTD) is short-acting morphine (MS IR) 30 mg PO q2h as needed. Until 2 days ago he was taking BTDs twice a day. That relieved his pain to the level of 3 or less and met his goals of walking around his home and sleeping through the night uninterrupted by pain. This record reveals that his pain is no longer controlled. As indicated at the top of the diary, he needs to complete this diary when he has problems related to his pain medicines. The diary reveals that his pain ratings are now greater than 3, that pain awakens him at night, and that he stays in bed. The patient talks with the nurse at 10 am, reads the diary to the nurse, and says that this is approximately what had happened during the past 2 days. The nurse contacts the physician and the decision is made to increase his morphine doses by approximately 30% to 50% to 45 mg of short-acting MS q2h and to 130 mg of modified-release morphine q12h. (When a dose of opioid is safe but ineffective, an increase of 25% usually produces a small increase in pain relief.) From previous prescription, the patient has modified-release morphine tablets 30 mg on hand, and the nurse instructs the patient to take one 30 mg tablet now along with short-acting MS 45 mg (½ 30 mg MS IR), which he does at 10:30 am. The entry at 12:30 pm shows that pain is reduced to the goal of 3, and the patient can sleep. Because of the increase in pain over 3 days, the patient is instructed to make an appointment to see the physician the next day. From Pasero, C., McCaffery, M. Pain assessment and pharmacologic management, p. 117, St. Louis, Mosby. Pasero C, McCaffery M. May be duplicated for use in clinical practice.
A paper diary may also be helpful for patients having ambulatory surgery. In a study of 20,817 patients who underwent same-day surgery, pain was the most common reason for unanticipated return to the hospital, occurring in 38% of the patients whose return was not anticipated (Coley, Williams, DaPos, et al., 2002). In ambulatory surgery, patients are usually discharged before there is time to establish the effectiveness of the oral analgesics they will be taking at home. Patients known to be at risk for moderate to severe pain, such as those having certain orthopedic procedures, may be asked to complete this diary about every 2 hours for the remainder of the day. The patients then have records that can be referred to when the clinicians contact them or the patients call with unrelieved pain or unmanageable adverse effects. Information from the pain control diary will be helpful in correcting the problem. For example, unrelieved pain may be due to failure to take the analgesic, or the analgesic prescription may be inadequate. Ideally, clinicians contact high risk patients the evening of discharge rather than the next day. Attempts to manage problems can be initiated before patients spend an uncomfortable night and develop complications.
In a hospitalized patient for whom the switch from parenteral or spinal analgesia to oral analgesia is not going smoothly, this paper pain diary may be modified and kept by the patients at the bedside. The information may be summarized in the nursing notes. Pain ratings every 2 hours may expedite identification of effective oral analgesic plans.
For certain periods of time, weeks or months, a daily pain diary may be indicated for a patient with chronic noncancer pain, such as headaches or low back pain. This type of record is especially important when medications or other treatments are being evaluated for continued use or are being adjusted to improve their effectiveness.
Poor recall of pain underscores the importance of asking some patients to keep records of pain management in the outpatient setting. The patient’s recall of pain often influences both the diagnosis and the treatment of pain. In one study of patients with chronic headache, pain ratings recorded in daily pain diaries kept over approximately 1 week were compared with patients’ memories of pain felt during that time (Eich, Reeves, Jaeger et al., 1985). Recall of pain intensity for maximum, usual, and minimum levels was influenced by the intensity of the pain felt at the time of the interview. The patients recalled their previous levels of pain as being more severe than they actually had been when the intensity of their present pain was high but recalled them as being less severe when their present pain intensity was low. In another study of patients with persistent pain, results with regard to recall of pain were similar (Smith, Safer, 1993). In addition, findings revealed that patients with lower present pain ratings also recalled their use of medication as having been less than it actually was.
In a study of patients receiving nerve blocks for pain, pain ratings were obtained before and after the blocks as well as 2 days and 2 weeks after the procedure (Porzelius, 1995). Findings revealed that memory distortions were common. At 2 days and 2 weeks after the nerve block, many patients recalled that the pain they had felt immediately after receiving the block was higher than they had reported at the time.
In outpatient care of patients with chronic pain, it is common to ask them, at each clinic visit, about their average or usual pain over the past week. Clinicians must realize that when precise information about pain ratings is needed so as to determine further treatment, daily records are more accurate than recall. Under these circumstances, a daily pain control diary is indicated.
Some patients will be asked to complete paper pain diaries only when problems are encountered, as in the example in Form 3-12. That patient was awakened by moderate to severe pain, and several supplemental doses were taken. Had the patient and family member not kept a diary, variations in pain ratings and the times breakthrough doses were taken, especially during the night, may not have been recalled accurately.
One of the limitations of paper diaries, when patients are asked to keep them over weeks or longer in the outpatient setting, is that some patients complete diaries immediately before they are to be turned in, or a week’s diary may be completed in advance (Gendreau, Hufford, Strone, 2003). Others have found that patients completing paper diaries are frequently noncompliant, tending to fabricate data if they have not completed the requested information at the times requested (Litt, Cooney, Morse, 1998). The use of electronic diaries that are programmed to prohibit invalid entries are helpful with respect to compliance (Hadjistravropoulos, Herr, Turk, et al., 2007). Momentary data are less likely to be falsified (Schwartz, Stone, 1998). The advantages of using electronic diaries are portability, low cost, ease of sharing data, and the availability of numerous software applications (Smith, Sheplock, 1999). Nevertheless their use is still limited by the much lower cost of paper dairies.
To study compliance with keeping paper diaries versus electronic diaries, 80 patients with chronic pain were asked to complete diaries; 40 patients kept paper diaries, and 40 patients kept electronic diaries (on palmtop computers) for 21 days, making entries three times a day (Stone, Shiffman, Schwartz, et al., 2002). Unknown to the patients, the paper diary was equipped with photosensors that identified the times at which entries were made so as to compare actual compliance with reported compliance. Among those who kept the paper diaries, reported compliance was 90%, but actual compliance was 11% to 20%. Actual compliance with the electronic diaries was 94%. The low compliance by those keeping the paper diaries is all the more remarkable considering the subjects were given $150 to participate in the study.
No other study has yet reported a comparison between compliance with electronic diary entries versus compliance with paper diary entries. The relatively higher compliance with paper diaries reported in the following studies may not be as high as actual compliance; that is, entries may not actually have been made at the times indicated in the paper diaries. In one study, 36 patients with chronic low back pain were asked to monitor their pain for 1 year (Jamison, Raymond, Levine, et al., 2001). Of the 36, 20 used both electronic diaries and paper diaries, and 16 used paper diaries alone. Two-way messaging available with the electronic diaries seemed to encourage the patients to use them, and they preferred them to the paper diaries. Further, patients were much more compliant with the monitoring of pain when using the electronic diaries (89.9%) than when using the paper diaries (55.9%). However, noncompliance resulted primarily from failure to return the paper diaries. Analysis of the data gathered by telephone calls and paper diaries suggested that the data in the electronic diaries were reliable and valid.
In another study, 155 cancer patients with metastasized bone pain were asked to complete daily paper pain diaries to determine the usefulness for themselves of doing so (Schumacher, Koresawa, West, et al., 2002). These were simple diaries that the patients were asked to complete once a day. (A copy of the diary is published in the Schumacher citation.) The pain diary was reported by 74% of the patients to be useful, and 10% felt it clearly was not. Very few patients and caregivers found the diary to be burdensome. No compliance data were reported. A group of 159 patients with cancer-related pain were asked to record their pain twice daily in a paper pain diary for 2 months at home (de Wit, van Dam, Hanneman, et al., 1999). Even in seriously ill patients, compliance was high (85.9%). High compliance may have been due in part to the guidance given by specially trained nurses and to the practice in completing the diaries before discharge. Although 37% of the patients reported that the diaries were of no benefit, 60% found that they helped them to cope with pain, giving them a sense of control over their pain. However, for patients with stable pain, the use of pain diaries may not be beneficial.
In a study of 91 patients with persistent noncancer pain, the patients used electronic diaries to monitor their pain during 2 weeks (Stone, Broderick, Schwartz, et al., 2003). Patients were divided into three groups and asked to monitor their pain 3, 6, or 12 times per day. Compliance was 94% or higher. Patients did not feel this was a burden and were willing to participate again in a similar study. One purpose of this study was to determine whether completing momentary diaries of pain would cause a shift in levels of pain over time (referred to as reactivity), and little support was found for such an occurrence.
In a crossover trial, 36 patients with persistent pain were asked to monitor their pain, mood, activity interference, medical use, and pain location in either paper or electronic diaries for 2 weeks (Marceau, Link, Jamison, et al., 2007). Patients reported that the electronic diary was easier to use than the paper diary, and 61% said that they would continue using it if given the choice. No compliance data were reported in this study. A serendipitous finding was that the patients using the electronic diary more frequently reported that their providers suggested medication changes based on information in the electronic diaries.
An emerging type of pain diary is an ordinary paper diary together with a digital pen and wireless mobile Internet technology. The digital pen looks, feels, and functions like an ordinary ballpoint pen. The strokes made by the pen are recorded and transferred by the Internet. In a small study of 12 palliative care patients using this technology, the patients reported that they found it easy to use despite severe illness and difficulties in comprehending the technology (Lind, Karlsson, Fridlund, 2008). The patients were asked to record immediate pain ratings three times a day and to complete a question about consumed doses. The patients could transmit their assessments at any time of any day. Caregivers gave the patients feedback on received assessments. It was this aspect of the methodology that the patients seemed to like the most. In postinterviews it was discovered that the patients believed that the transferred assessments were monitored by the caregivers around the clock, although the patients were not told that. Although the patients always recorded their pain at the requested times, initially they had difficulty using the VAS, and none of the patients understood how to mark the boxes to indicate the times at which they had taken their extra doses. Clearly, better directions should have been given. Nonetheless, the medical records indicated that the information from the assessments was useful and resulted in quick medical responses to changes in the patients’ statuses.
Research thus far suggests that electronic diaries and possibly digital pens are preferable to paper diaries when data are needed for 1 week or longer. Patients prefer electronic diaries to paper diaries, and compliance is better with electronic diaries. Based on the one study involving digital pens, it seems that this technology may be even better. Perhaps most important, it appears likely that data from electronic diaries or digital pens are most useful to physicians in making medication adjustments for patients.
When paper diaries, digital pens, or electronic diaries are used, the patient and family should receive explanations of the purpose of using them, along with information about how and when to complete the diaries and practice sessions with the diaries. Electronic diaries and digital pens are not always available in clinical practice, so paper diaries will serve as an alternative.
The low actual compliance with the use of paper diaries reported in one study (Stone, Shiffman, Schwartz, et al., 2002) suggests that when patients are asked to use paper diaries, it may be wise to caution them about the tendency to record information much later than it actually occurred, about the fact that recall is often inaccurate, and about the need to be honest about the times at which they actually make their entries. The most effective way to encourage compliance may be frequent feedback between clinician and patient. Calling the patient, perhaps every day or two, to remind him or her to complete the diary may help the patient to feel that the entries are of value. Perhaps the most important encouragement to complete the diaries lies in clinicians’ use of diary information to adjust care. Doing so gives the patient a reason to use the diary. In the studies mentioned, the patients responded very positively to having regular contact with clinicians and to feeling that their data were being used. Compliance may also be increased by decreasing the number of entries expected per day, although some patients did not feel overburdened by making as many as 12 entries per day (Stone, Broderick, Schwartz, et al., 2003).
Clinic Record for Reassessment of Patients with Persistent Pain Who Are Taking Opioids
A standardized tool for assessing patients with persistent pain who are receiving opioid therapy is almost essential to determine the effects of opioid management and to identify problems, such as undertreatment of pain, adverse effects, or problematic patient behaviors. Relevant domains for monitoring are often referred to as the Four As: Analgesia, Activities of daily living, Adverse effects, and Aberrant drug-related behaviors (Nicholson, Passik, 2007). The Pain Assessment and Documentation Tool (PADT), which assesses these four domains, has been published (Passik, Kirsh, Whitcomb, et al., 2004). The original, longer tool was field-tested by 27 clinicians who applied it to the assessments of 388 patients receiving long-term opioid therapy for persistent noncancer pain. The result is the PADT, a brief, two-sided chart note that can be included in a patient’s medical records (Form 3-13). It should require only a few minutes of a clinician’s time to complete.
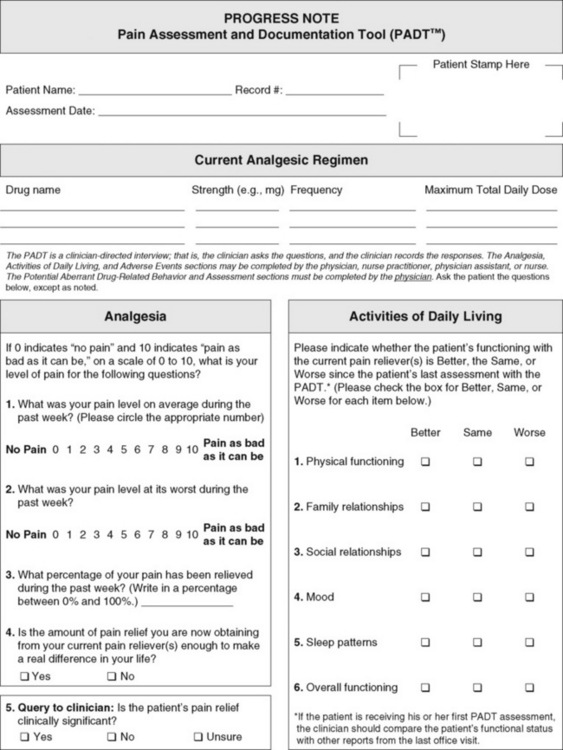
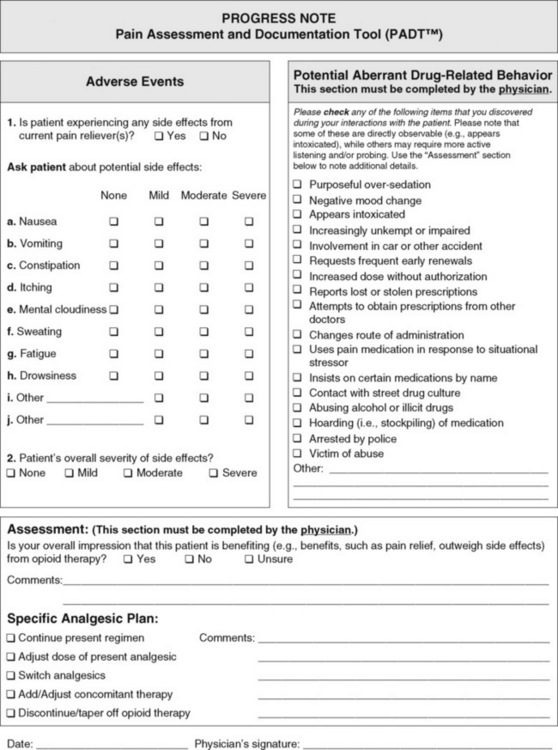
Form 3-13 The revised pain assessment and documentation tool shown as a two-sided chart note. As appears in Pasero, C., & McCaffery, M. (2011). Pain assessment and pharmacologic management, pp. 120-121, St, Louis, Mosby. Janssen Pharmaceutica Products, L.P. May be duplicated for use in clinical practice.
The PADT assesses the four As in four sections of the tool. In the first section, Analgesia is assessed using the 0 to 10 scale. The second section, Activities of daily living, includes physical functioning, social relationships, and sleep patterns. The third section, Adverse effects, includes the effects that sometimes accompany chronic opioid use, such as nausea, constipation, and mental cloudiness. The fourth section assesses Aberrant drug-related behaviors, including those that may indicate undertreatment of pain, or abuse, or addictive disease, and they require differential diagnoses.
Aberrant drug-related behavior is too often interpreted as being indicative of abuse or addictive disease, and little thought may be given to the fact that the behavior may be caused by undertreatment of pain. The term aberrant drug-related behavior refers to behavior that is inconsistent with the expressed intentions of the prescriber, such as taking the drug for sleep when it was intended for pain relief (Fine, Portenoy, 2007). Another example of aberrant behavior is hoarding opioids during periods of reduced symptoms for later use when pain is worse or for occasions when the opioid is unexpectedly unavailable, such as a mail-order pharmacy’s being late in filling the prescription or the local pharmacy’s being temporarily out of the medication. Clearly, the presence of aberrant drug taking behavior should not automatically result in denying a patient opioid therapy.
The ultimate goals of using the PADT are to optimize analgesia, to improve activities of daily living, to minimize adverse analgesic effects, and to minimize aberrant drug behaviors. Further validation of the PADT is under way. The published tool is presented along with a survey of 19 clinicians who participated in the development of the PADT, revealing that 63.2% felt the tool would also be helpful in documenting behaviors or concerns for legal purposes (Passik, Kirsh, Whitcomb, et al., 2004).
Communication Strategies
Pain is best managed by using a team approach. Seldom does any one clinician possess the time, knowledge, and skill to provide all the care required by a patient with pain. Usually the care of a single patient requires at least the involvement of a prescriber, nurse, and pharmacist, and sometimes many other health care professionals representing various disciplines.
Nurses have emerged as pivotal members of the pain management team. As has been known in the palliative care setting for decades, the nurse is the cornerstone of the team approach to caring for patients with pain. The nurse uses, as necessary, the expertise of all team members, applies their recommendations to the care of individual patients, and reports to team members regarding the success of the plan or the problems that emerge and require revision of the plan.
The assessment tools previously discussed are important methods of fostering a team approach. Standardization of these tools and other communication formats facilitates communication and minimizes delays in making appropriate changes in pain treatment plans.
A team approach requires collaboration among the clinicians and the patients and their families. In simple terms, collaborative practice means working together, not against each other. Following are some basic requirements for a team/collaborative approach to pain management, many of which have already been incorporated in the assessment tools presented in this chapter. The numbers correspond to those in Box 3-7.
1. Common goals. In each specific situation, the prescriber, nurse, patient, and others involved in the care must agree on a common goal, referred to as the comfort-function goals (discussed on pp. 85-88). This is often a simple matter of identifying activities, emotional states, or cognitive abilities that a patient values or needs to perform and determining the pain ratings that would be necessary for achieving the goal of performing. Simply put, if the team does not know where it is going, it will not know when it gets there or whether it has not yet arrived.
2. Common language. Obviously, communication is facilitated when all people involved are using the same words to refer to important elements of the situation. Pain rating scales are an example of using a common language. One of the most essential components of pain management is assessment of pain intensity. Pain rating scales that are valid, reliable, and easy to understand and use have been discussed in this chapter. For each individual patient, a single pain rating scale appropriate for that patient should be chosen and should be used by all team members when they discuss pain management among themselves and with the patient and family.
Among clinicians, another component of a common language is using standardized assessment tools and formats for written communication and documentation. Examples of these, already discussed in this chapter, are the flow sheets.
3. Common knowledge. Because pain is a relatively new scientific discipline, lack of education about pain management is to be expected. Knowledge of pain management varies widely among clinicians and in various clinical situations. For this reason, clinical practice guidelines have been developed by professional organizations such as the APS. They may be used to provide clinicians with a quick summary of current recommendations for pain management. One or more of them can be distributed to clinicians and referred to for guidance each time a question arises about the assessment and treatment of pain. An example of a brief guideline that applies to many clinical areas is published by the APS and is titled “Principles of Analgesic Use in the Treatment of Acute Pain and Cancer Pain” (2003). This guideline might be given to prescribers, nurses, and pharmacists during orientation. Home care agencies might mail this guideline to referring physicians with a cover letter explaining that the agency is aiming to implement these recommendations.
Other guidelines published by the APS include those that focus on pain management in sickle cell disease (Benjamin, Dampier, Jacox, et al., 1999), cancer in adults and children (Miaskowski, Cleary, Burney, et al., 2005), and various types of arthritis (2002). Although a considerable amount of essential information is contained in the clinical practice guidelines, the single most important directive is that clinicians must accept a patient’s report of pain. Repeated reference to this recommendation may well be necessary.
4. Regular communication. Common goals, language and knowledge must be shared by team members on a regular and predictable basis. In some instances, such as the care of patients with persistent pain, regular communication takes place at a weekly team meeting. In other situations, such as the care of surgical patients, communication make take the form of pain ratings recorded every 4 hours on a flow sheet and included at the change-of-shift or hand-off report. Home health care agencies may use a standardized format to fax or e-mail updates to referring prescribers and may follow up with telephone calls when there are problems. Care should be taken, however, to report the positive too, not just the problems.
Box 3-8 lists some very simple and basic reminders to prepare clinicians for communicating unrelieved pain to colleagues. It is not a comprehensive list, but it includes the essential information needed in a conversation about improving the patients’ pain management.
Pain Assessment in Patients Who Cannot Self-Report
Before discussing strategies for assessing pain in patients who cannot self-report, it is important to correct a common misconception—that patients who are cognitively impaired do not experience as much pain as those who are cognitively intact. Recent studies examining mechanisms and differences in pain transmission and perception in older adults with dementia document that the pain transmission process is unaltered, although cognitive processing and interpretation of the pain stimulus may be impaired, resulting in different presentations or responses to painful stimuli (Gibson, Voukelatos, Amers, et al., 2001; Karp, Shega, Morone, et al., 2008; Kunz, Mykius, Scharmann, et al, 2009; Scherder, Oosterman, Swaab, et al., 2005; Scherder, Sergeant, Swaab, 2003; Schuler, Njoo, Hestermann, et al., 2004). Cognitive impairment often results in less pain reported, even though there is no evidence that cognitive impairment reduces the ability to feel painful stimuli (Benedetti, Arduino, Vighetti, et al., 2004; Reynolds, Hanson, DeVellis, et al., 2008; Scherder, Herr, Pickering, 2009). Lower reporting and recognition of pain in cognitively impaired patients add to the vulnerability of these older adults and to the smaller likelihood of their receiving treatment that can mediate the profound consequences of untreated pain. Proactive recognition of pain is important and requires understanding the likelihood of underreporting of pain and using methods recommended to recognize pain in those who cannot self-report.
As cognitive impairment advances, obtaining self-reports may not be possible so other approaches are necessary to recognize presence of pain. A hierarchical approach to identifying the presence of pain in nonverbal patients has been recommended and provides a comprehensive strategy for assessing pain in the vulnerable older adults unable to self-report (Buffum, Hutt, Chang, et al., 2007; Herr, Bjoro, Decker, 2006; McCaffery, Pasero, 1999; Pasero, McCaffery, 2005) (Box 3-9). This approach includes the following steps: (1) determine ability to self-report, and if unable to obtain self-report, document why and continue with the following steps; (2) investigate for possible pathologies and procedures that might produce pain; (3) observe for possible behaviors that may signal pain; (4) incorporate surrogate reporting; and (5) attempt a trial of analgesic even if only suspicious of pain; and evaluate whether this causes a reduction in the behavioral indicators thought to be related to pain. If a patient is unable to self-report, evidence indicative of pain in any of items 2 through 5 is sufficient to assume pain present (APP) (Pasero, McCaffery, 2002).
Strategies for obtaining self-reports in the cognitively impaired were presented earlier, and efforts should be made to secure self-reports. For those unable to report, searching for potential causes of pain or discomfort is an important approach to identifying pain. Becoming familiar with common diagnoses, conditions, and procedures known to be painful can increase awareness of the possibility of pain in those who cannot report. Box 3-10 provides a list of potential causes of pain that guide review of a patient’s medical history and physical examinations and the search for potentially painful conditions.
Behavioral observation is a key strategy for recognizing the presence of pain and can include direct observation of behaviors or obtaining reports from proxies or surrogates, including certified nursing assistants (CNAs), family members, or other caregivers. For patients with dementia, the scope of potential pain indicators is much broader than common pain behaviors, such as grimacing, moaning, groaning, bracing, rubbing, and guarding. Clinicians should be familiar with typical and atypical behavioral presentations (e.g., agitation, restlessness, irritability, combativeness, resisting care, changes in appetite or sleep, or usual activities) that are potential pain indicators and warrant evaluation.
The American Geriatrics Society (AGS) provided a list of pain indicators that may represent pain in cognitively impaired older adults (AGS, 2002) (Table 3-2). The framework is a list of potential pain indicators that can guide observation. Behaviors that are most frequently observed during direct observation are being identified through ongoing study. Shega, Rudy, Keefe, and others (2008) recently found that grimacing was the most common behavior observed, during a guided movement protocol, in both cognitively impaired and cognitively intact older persons with persistent low back pain. They also noted that the frequency of other behaviors differed based on cognitive status, with more rubbing and guarding and less bracing in the cognitively impaired. Facial expression is emerging as a reliable and prominent behavior that can detect and judge severity of pain in both those with and without dementia (Chapman, 2008; Kunz, Scaharmann, Hemmeter, et al., 2007). Studies are needed to examine the frequency of less typical pain behaviors occurring in natural circumstances to guide behavioral tool refinement and pain recognition.
Table 3-2
Common Pain Behaviors in Cognitively Impaired Older Persons
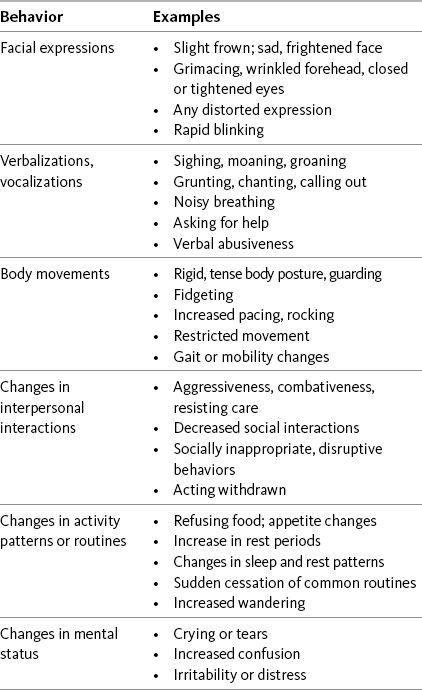
Note: Some patients demonstrate little or no specific behaviors in association with severe pain.
As appears in Pasero, C., & McCaffery, M. (2011). Pain assessment and pharmacologic management, p. 125, St. Louis, Mosby. Used with permission from American Geriatrics Society Panel on Persistent Pain in Older Adults. (2002). The management of persistent pain in older persons. J Am Geriatr Soc, 50, S211. May be duplicated for use in clinical practice.
Clinicians should solicit information about pain presence, typical patterns of behavior, and judgment of current pain severity from surrogates or proxies who have knowledge about the patients (e.g., family, CNA). In the long-term care setting, the CNA plays an important role and has been shown to be effective in recognizing presence of pain (Mentes, Teer, Cadogan, 2004; Nygaard, Jarland, 2006), whereas in the acute care setting, family members may be most familiar with typical pain behaviors or changes in usual activities that might suggest presence of pain (Nygaard, Jarland, 2006; Shega, Hougham, Stocking, et al., 2004).
Although observation of behavior occurs commonly in clinical practice, a standardized approach to regular observation and documentation that facilitates consistency and more effective communication across provider and setting is needed. Use of a behavioral pain assessment tool is one part of this template (see Step 3, Box 3-9, p. 123) and requires careful selection and use to ensure that vulnerable older patients with pain are recognized and treated. A discussion of behavioral pain assessment tools follows.
The final step in the hierarchical approach to pain assessment in those unable to self-report involves the use of a trial of analgesic to validate whether the suspicion of pain based on information obtained from the previously mentioned steps, such as observation of behavior, is valid. This is especially important when considering atypical presentations of pain by patients when the causes are uncertain. Blinded trials comparing analgesic and placebo interventions on pain-related outcomes are few, and the findings are mixed. Two randomized controlled trials focused on the use of acetaminophen and its effect on agitation and function. The first trial found no effect on agitation among nursing home residents, although the acetaminophen dosing of 1500 mg per day may not have provided a therapeutic level of analgesia (Buffum, Sands, Miaskowski, et al., 2004), whereas the second trial demonstrated increased levels of social activity and interaction after a therapeutic dose of acetaminophen (3000 mg/day), although no significant change in agitation was noted (Chibnall, Tait, Harman, et al., 2005). A study of low-dose opioid treatment (20 milliequivalent morphine/24 h) compared with placebo showed significant effect on agitation in the over-85 age group only (Manfredi, Breuer, Wallenstein, et al., 2003).
Kovach and colleagues evaluated a systematic method of assessing and treating behaviors that may be pain-related in patients with severe dementia in a clinical protocol called the Serial Trial Intervention, which incorporates an analgesic trial (Kovach, Noonan, Schlidt, et al., 2006). In a randomized controlled trial involving 114 subjects residing in 14 nursing homes, those who received the intervention had significantly less discomfort and more frequent return of behavioral symptoms to baseline than those who did not.
An analgesic trial can be used to confirm suspicion that a nonverbal older person is experiencing pain. It also serves as the foundation for developing a pain treatment plan when the trial confirms the presence of pain. Box 3-11 provides a guide for use of an analgesic trial and development of a treatment plan for suspected pain in cognitively impaired older adults. Because many of the potential pain indicators could also be caused by other conditions related to the dementia diagnosis, it is useful to understand the rationale for attempting an analgesic trial before psychiatric management. Compared with psychotropic intervention, response to an analgesic intervention is more rapid, the adverse reactions are usually less severe, and pain will not be obscured by the sedative properties of psychotropic agents (Herr, Bjoro, Decker, 2006). Psychiatric intervention can be considered if behaviors do not improve with an analgesic trial. The limited studies suggest that analgesic trials to validate pain in persons with behavioral manifestations of potential pain hold promise, although algorithms for this approach need further development and testing.
At any step in the process described, pain may be suspected but difficult to confirm. However, when any information indicating pain is obtained in any of items 2 through 5 in Box 3-9 (p. 123), the clinician may assume pain is present and document it with the abbreviation APP if that acronym is approved by the institution (Pasero, McCaffery, 2002). For example, if a patient shows no behavioral signs of pain, the presence of pathology or procedures that are normally painful is sufficient to assume pain is present (Pasero, McCaffery, 2002).
Physiologic measures are not included in the hierarchy of pain assessment techniques because of their limited sensitivity to pain. It is a misconception that elevated vital signs can be used to verify severe pain, and autonomic responses to pain, including vital signs, are often decreased in persons with dementia and thus not accurate indicators of pain presence (Kunz, Mykius, Scharmann, et al., 2009). Details related to this are discussed earlier (p. 26). For example, in the critical care unit, vital signs are readily observable but they are influenced by many factors other than pain (Gelinas, Johnston, 2007). Elevated vital signs in patients with moderate to severe chronic pain are usually not evident. Thus, absence of elevated vital signs does not mean absence of pain.
Types of Behavioral Pain Assessment Tools
Tools for the observation of pain behaviors have been developed to assist in the recognition of pain in those unable to self-report. A number of reviews have critiqued existing behavioral tools and similarly concluded that no tool is currently recommended for general use across all populations and settings (Hadjistavropoulos, 2005; Herr, Bjoro, Decker, 2006; Stolee, Hillier, Esbaugh, et al., 2005; van Herk, van Dijk, Baar, et al., 2007; Zwakhalen, Hamers, Abu-Saad, et al., 2006). However, additional research into some existing tools and the development of new tools continue to broaden the selection of instruments for consideration. When selecting a standard observation tool for implementation, it is important for clinicians to consider the psychometric properties of a tool and its clinical utility and appropriateness for the setting and population. The next section provides information about existing tools that can guide clinicians in determining which tool to incorporate into a comprehensive approach to pain assessment in persons unable to self-report. Because of rapid changes in the field and ongoing research, monitoring for new developments is recommended.
The Mayday Fund provided support for the development of resources to assist clinicians in behavioral pain assessment tool selection, available in the resource section at the Pain Resource Center at the City of Hope (http://.prc.coh.org/PAIN-NOA.htm). A detailed critique of existing pain tools, a brief summary of tool strengths and challenges, information regarding tool access and permission, and a comparison across tools are available at the website.
There are a variety of ways to categorize the pain behavior assessment tools available for use with nonverbal older patients. One approach is to organize them as pain behavior checklists, pain behavior intensity scales, and a combination of both. Alternatively, tools can be organized by whether they are direct-observation tools or informant-based tools. Direct-observation tools can usually be completed by those without prior knowledge of the patients as they gather information about specific behaviors observed during a specified period or activity. They identify the presence or absence of the behavior and may include a rating of the behavior’s intensity or frequency (Feldt, 2000; Snow, Weber, O’Malley, et al., 2004; Warden, Hurley, Volicer, 2003). Informant-based tools (also called surrogate/proxy tools) rely on information from family members, caregivers, and health care providers familiar with the individuals’ baseline behaviors and they can detect changes in activities and behaviors from usual presentation. There also may be overlap in direct observation and informant report. Regardless of the categorization of tools, several considerations are necessary in determining the best approach for given populations and settings.
A key consideration is tool specificity versus sensitivity. Because of individual variability in behavioral presentation related to pain, short, direct observation tools that focus on key common pain behaviors (e.g., grimacing, moaning, bracing) may recognize pain in those who present with these common pain behaviors (specificity) but may fail to detect pain in those who present with more atypical changes in activity or behavior (sensitivity) (e.g., decreased appetite, increased sleeping, greater agitation, acting withdrawn, or increased irritability). Longer comprehensive tools that include more potential pain indicators may be more sensitive but less specific. This means that more patients who present with less common pain behaviors will be identified, but in some of those, pain may not be the cause of their behaviors. Follow-up evaluation is necessary to determine the causes of the behaviors observed.
One factor influencing tool use is whether behavior is observed at rest, during or following some activity, or during an activity intended to evoke pain. Studies have illustrated that observation at rest is misleading and can lead to incorrect judgment about the presence of pain (Feldt, 2000; Hadjistavropoulos, LaChapelle, MacLeod, et al., 2000; Husebo, Strand, Moe-Nilssen, et al., 2008). Thus, observation during movement or activity is recommended. The timing of the observation should be consistent and should be part of the assessment procedure in persons requiring behavioral observation (e.g., during morning care, transfers, or ambulation).
In patients with severe dementia, studies support the ability of surrogates to recognize pain’s presence, but not accurately rate its severity (Manfredi, Breuer, Wallenstein, et al., 2003; Pautex, Herrmann, Michon, et al., 2007; Shega, Hougham, Stocking, et a., 2004). This may be related to individual variability in patients’ behavioral presentations. For example, one patient with severe dementia may present with agitation and aggressive behavior, whereas another may become withdrawn and quiet. Their behavioral presentations are quite different, but they both could be experiencing moderate pain; this indicates that it is unreliable to determine the severity of pain on the basis of the severity of restlessness and agitation. Some authors have attempted to establish a direct relationship between behavior scores and pain intensity, such as adding up the score on a behavioral tool and equating it with pain intensity. Given current knowledge about this topic, number or intensity of behaviors cannot be added up to equal a pain score. We emphasize that no self-report means no pain-intensity rating (Pasero, McCaffery, 2005). The low correlations shown in most studies between surrogate judgment of pain severity and patient reports of severity contribute to concern about using behavioral scores as pain-intensity ratings (vanHerk, vanDijk, Biemold, 2009). Further, if clinicians could reliably determine the intensity of pain in patients who cannot self-report, there would be no need for behavioral assessment tools (Zwakhalen, Hamers, Abu-Saad, et al., 2006).
Certain studies that have focused on direct observation of typical pain behaviors (such as grimacing, vocalizations, and guarding) show stronger correlations between pain behaviors and pain severity (Horgas, Nichols, Schapson, et al., 2007; Husebo, Strand, Moe-Nilssen, et al., 2007). However, the behaviors observed were typical pain behaviors, and the circumstances were controlled, so the results might not apply to assessments involving broader circumstances and less typical behaviors. Future research may reveal approaches that guide judgments of pain’s severity on the basis of behaviors. However, until there is stronger evidence to support this relationship, the score on a pain behavior assessment tool is not the same as a pain-intensity rating scale, and the two should not be directly compared (Herr, Coyne, Key, et al., 2006). However, for an individual patient, an increasing score on a behavioral pain assessment tool usually indicates increasing pain, and a decreasing score probably represents a decrease in pain.
Selection of a tool is determined by the tool’s reliability and validity, the setting of the care and the population to be assessed, the availability of knowledgeable caregivers, and the clinical utility. Foremost, a pain assessment tool must have at least moderate reliability and validity. A tool that is not reliable and valid is not useful for supporting clinical judgments. It is very tempting to develop a new tool or adapt an existing tool to meet a clinical need. For example, a tool such as the Face, Legs, Activity, Cry, and Consolability (FLACC) may seem like a good tool to use with older persons. However, the FLACC was based on children’s development and has not yet been demonstrated to be reliable and valid for older persons (Baiardi, Parzuchowski, Kosik, et al., 2002). A recent study of the FLACC in citically ill patients who could not self-report included older adults, but it was not clear how many of the population were 65 to 70 years and older (Voepel-Lewis, Zanotti, Dammeyer, 2010). Therefore, further study of the FLACC in older patients is warranted. Considerable time and effort are required to establish a tool’s validity and reliability, which are essential before integrating a tool into clinical use.
One key aspect of a tool’s validity is its sensitivity to change—a critical aspect of a tool’s utility that has received limited attention in the research to date. Several of the existing tools have been evaluated for their abilities to detect responses to intervention (e.g., analgesia) (Cohen-Mansfield, Lipson, 2008; Morello, Jean, Alix, et al., 2007). Ensuring that tools can detect response to treatment is part of the development of a tool’s validity.
The setting of care is important in evaluating the appropriateness of a tool. For example, a number of tools that have been developed require observation over time and knowledge of patients’ usual behaviors, so they would not be useful in acute care settings to look for procedural pain or postoperative pain. In acute care settings, a short, direct-observation tool may be well suited for use with patients who have acute pain problems and are unknown to the nursing staff. In the long-term care setting, a short, direct-observation tool that can be used on a regular basis by CNAs during usual care combined with a more comprehensive screening tool that is completed at regular intervals may work well. Finally, a pain assessment tool that requires excessive training, is perceived as being too complex, or requires too much time to administer will not be helpful in promoting regular clinical use of the tool. Careful consideration of these characteristics can guide the selection of an appropriate tool. Detailed critiques and summaries of tools are available at http://pcr.coh.org/PAIN-NOA.htm.
Tools for Assessing Pain in Cognitively Impaired Patients and Others Who Cannot Self-Report
For those caring for older adults in long-term care facilities, the minimum data set (MDS) may be considered a source of assessment data. The MDS is a federally mandated system for the assessment of residents of long-term care nursing facilities. It systematizes the assessment of each resident’s functional, mental, psychosocial, and medical status at admission and at regular intervals thereafter (Kovach, Noonan, Griffie, et al., 2002). However, concerns have been raised regarding the underreporting of pain in cognitively impaired residents when reports are based on MDSs (Chu, Schnelle, Cadogan, et al., 2004; Cohen-Mansfield, 2004). A revised MDS 3.0 has been under development and evaluation and includes additional pain assessment content, including documentation of behaviors related to pain. Once implemented, the value of the revised MDS may be improved although evaluation of its ability to detect pain in nonverbal patients and to demonstrate response to intervention will be needed.
A number of standardized tools for pain assessment have been developed and are at various stages of testing and refinement. Following is a description of selected tools that merit consideration, depending on the setting and population. We have chosen not to include discussions of tools that do not have support for use with older adults with cognitive impairment or that focus primarily on the detection of discomfort rather than pain. Information about other tools can be obtained at http://prc.coh.org/PAIN-NOA.htm.
Recommended Tools for Patients Who Cannot Self-Report
No perfect behavioral assessment tool exists, but based on current research and clinical utility, the three tools we recommend for use in clinical practice with patients who cannot self-report are (1) Checklist of Nonverbal Pain Indicators (CNPI); (2) The Pain Assessment in Advanced Dementia (PAINAD); and (3) Pain Assessment Checklist for Seniors with Severe Dementia (PACSLAC). These tools are described, and an overview of them is provided in Table 3-3. Other tools are also described.
Table 3-3
Overview of Selected Behavioral Pain Assessment Scales for Older Adults Unable to Self-Report
Note: The tools selected for inclusion were those with the highest scores based on overall evaluation of psychometric properties and utility.
1Feldt, 2000; Jones et al., 2005; Nygaard, Jarland, 2006.
2Warden, Hurley, Volicer, 2003; DeWaters et al., 2008; Leong, Chong, Gibson, 2006; Zwakhalen, Hamers, Bergen, 2006; Cohen-Mansfield, Lipson, 2008.
3Fuchs-Lacelle, Hadjistavropoulos, 2004: Fuchs-Lacelle, Hadjistavropoulos, 2005; Zwakhalen, Hamers, Bergen, 2006.
From Pasero, C., & McCaffery, M. (2011). Pain assessment and pharmacologic management, p. 129, St. Louis, Mosby. Adapted and used with permission from Hadjistavropolous, T., Herr, K., Turk, D., et al. (2007). An interdisciplinary expert consensus statement on assessment of pain in older persons. Clin J Pain 23(1), S1-S43. May be duplicated for use in clinical practice.
The tools presented here are in varying stages of development, and further testing may establish greater reliability and validity; however, considerable detail is presented regarding the current stages of the research that establishes their psychometric properties. Research terminology unfamiliar to some readers has been used. To facilitate understanding of the research findings, Table 3-4 provides definitions of selected research-related terms.
Table 3-4
Definitions of Selected Research-Related Terms
| Term | Definition |
| Content or face validity | The examination of tool items to determine whether the items look like they are measuring the concept of interest (i.e., pain) |
| Construct validity | The ability of a tool to measure what it is intended to measure. Concurrent, convergent, and discriminant validity are forms of construct validity. |
| Concurrent validity | The ability of a tool to differentiate among individuals in performance or behavior by comparing performance against a similar tool. For example, a numeric rating scale should be strongly related to scores reported on a verbal descriptor scale. |
| Convergent validity | The relationship of a tool to other tools measuring the same concept. For example, in comparing a new tool measuring ability to perform ADLs to a standardized ADL tool, a strong relationship supports convergent validity. |
| Discriminant validity | The relationship of a tool to other tools measuring different concepts. For example, a tool measuring pain intensity and a tool measuring hostility would not be expected to be strongly related to each other and support divergent validity. |
| Internal consistency | The extent to which the items in a tool measure the same concept. For example, in the Brief Pain Inventory the pain interference items all examine the impact of pain on function and thus has high internal consistency. |
| Inter-rater reliability | The degree of agreement among different raters independently scoring the same data in the same way. For example, two raters observe 10 patients and record their observations on the PAINAD. The more their scorings agree, the higher the inter-rater reliability. |
| Normative data and cutoffs | The normal or average scores to be expected on a given measure for a representative sample. Cutoffs are the scores on a scale that determine whether the subject meets or does not meet the criteria. For example, normative data from a number of studies were used to establish cutoffs for mild, moderate, and severe pain on the numeric rating scale. |
ADL, Activities of daily living.
As appears in Pasero, C., & McCaffery, M. (2011). Pain assessment and pharmacologic management, p. 130, St. Louis, Herr K. May be duplicated for use in clinical practice.
The Checklist of Nonverbal Pain Indicators (CNPI) (Feldt, 2000) is a brief, clinically useful observation list of six behavior items that are scored as being present or absent at rest and during movement in cognitively impaired older adults. (Form 3-14 on p. 131 shows the tool as it is used in clinical practice.) The tool includes only the common behaviors that reveal pain, thus limiting the measure’s ability to capture the full range of possible pain behaviors. Preliminary testing provided initial support for use of the tool with older adults in acute care settings, although internal consistency was low. Recent evaluation in long-term care (Jones, Fink, Hutt, et al., 2005) demonstrated support of the CNPI as a measure of pain’s severity; increases in CNPI scores were associated with increases in self-reports of pain (Jones, Fink, Hutt, et al., 2005). However, 50% of residents reporting pain had no visible indicators of pain, which raises concerns about the tool’s sensitivity and its ability to detect persistent pain in those unable to report. Cutoff scores for determining the presence and severity of pain are not available. Another study, conducted in a group of Norwegian nursing homes, provides evidence of acceptable test-retest and inter-rater reliabilities and concurrent validity, as well as the tool’s practicality when administered by various categories of nursing personnel (Nygaard, Jarland, 2006). Further testing in acute care and long-term care settings in the United States is recommended.
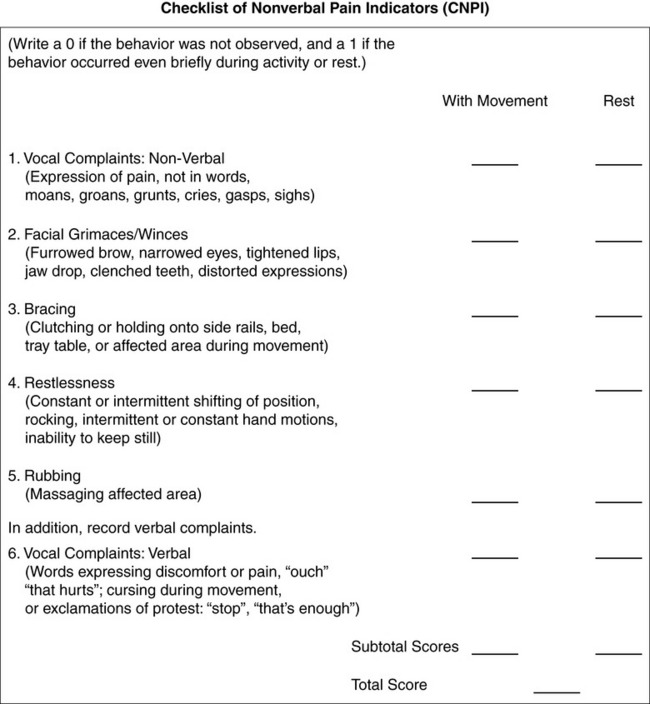
Form 3-14 As appears in Pasero, C., McCaffery, M. (2011). Pain assessment and pharmacologic management, p. 131, St. Louis, Mosby. From Feldt, K. S. (1996). Treatment of pain in cognitively impaired versus cognitively intact post-hip-fractured elders. Doctoral dissertation, University of Minnesota Dissertation Abstracts International, 57-09B, 5574; Feldt, K. S. (2000). Checklist of nonverbal pain indicators. Pain Manag Nurs, 1(1). 13-21. May be duplicated for use in clinical practice.
The Pain Assessment in Advanced Dementia (PAINAD) Scale (Warden, Hurley, Volicer, 2003) was developed as a short, easy-to-use observation tool for assessing pain in individuals with advanced dementia. (Form 3-15 on pp. 132-134 shows the tool as it is used in clinical practice.) The PAINAD is an adaptation of the Discomfort Scale for Dementia of the Alzheimer Type (DS-DAT) (Hurley, Volicer, Hanrahan, et al., 1992) and the FLACC (Merkel, Voepel-Lewis, Shayevitz, et al., 1997) and includes five items: breathing, negative vocalization, facial expression, body language, and consolability. These items are not comprehensive, and the ability to detect pain in those with less obvious changes in behavior may be compromised. No data attach level of pain severity (mild, moderate, or severe) to the number obtained in the tool, although higher scores do represent greater reports of pain.
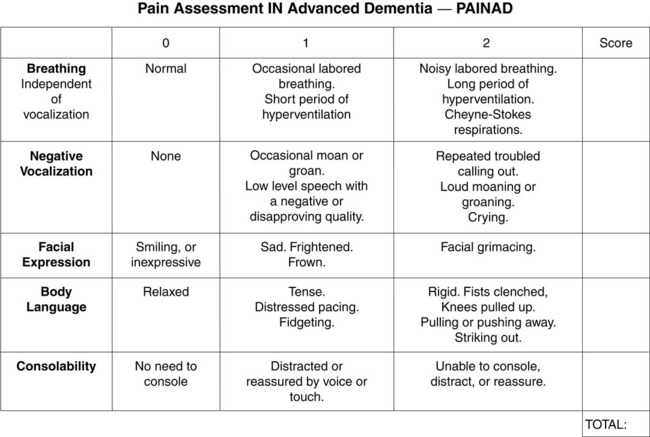


Form 3-15 As appears in Pasero, C., & McCaffery, M. (2011). Pain assessment and Pharmacologic management, pp. 132-134, St. Louis, Mosby. From Warden, V., Hurley, A. C., & Volicer, L. (2003). Development and psychometric evaluation of the pain assessment in advanced dementia (PAINAD) scale. JAMA, 4(1), 9-15. May be duplicated for use in clinical practice.
The PAINAD has been translated into four languages and tested, and three additional English studies have provided additional information about the tool’s psychometrics and utility. Subsequent studies have provided support for its construct and its concurrent validity and ability to detect pain and to differentiate between groups with and without pain in both long-term and acute care settings (Costardi, Rozzini, Costanzi, et al., 2007; DeWaters, Faut-Callahan, McCann, et al., 2008; Leong, Chong, Gibson, 2006; Schuler, Becker, Kaspar, et al., 2007; van Iersel, Timmerman, Mullie, 2006; Zwakhalen, Hamers, Abu-Saad, et al., 2006).
One study that compared the PAINAD to other tools in terms of their abilities to detect change resulting from treatment raises questions regarding the tool’s sensitivity (Cohen-Mansfield, Lipson, 2008). Follow-up studies demonstrate good internal consistency, inter-rater reliability, and test-retest reliability. Several studies question the utility of including the items breathing and consolability, but removal of these items did not demonstrate improved internal consistency. Further study is needed to investigate the tool’s sensitivity to change in behavior in response to treatment.
The Pain Assessment Checklist for Seniors with Limited Ability to Communicate (PACSLAC) (Fuchs-Lacelle, Hadjistavropoulos, 2004), developed by a Canadian team, is an observational tool that uses direct observation and familiar caregiver information to assess both common and uncommon pain behaviors. (Form 3-16, pp. 135-136, shows the tool as it is used in clinical practice.) The PACSLAC is potentially a clinically useful behavior checklist that appears to be simple to use for assessing and monitoring changes in persons with dementia and diverse presentations of pain-related behavior. The tool is comprehensive; 60 indicators address all six pain behavior categories included in the AGS guidelines. Prospective evaluation has supported the tool’s reliability and validity (Fuchs-Lacelle, Hadjistavropoulos, 2005; Zwakhalen, Hamers, Berger, 2006; Zwakhalen, Hamers, Berger, 2007) and has done a factor analysis to determine the most efficient and useful indicator set for clinical use. Because of the length of the tool, it may not be suitable for acute care settings. However, clinicians report that it actually takes very little time to complete the form once they are familiar with it. Even if the tool is not formally used in the acute care setting, it can be posted in the clinical units as a resource for identifying behaviors.

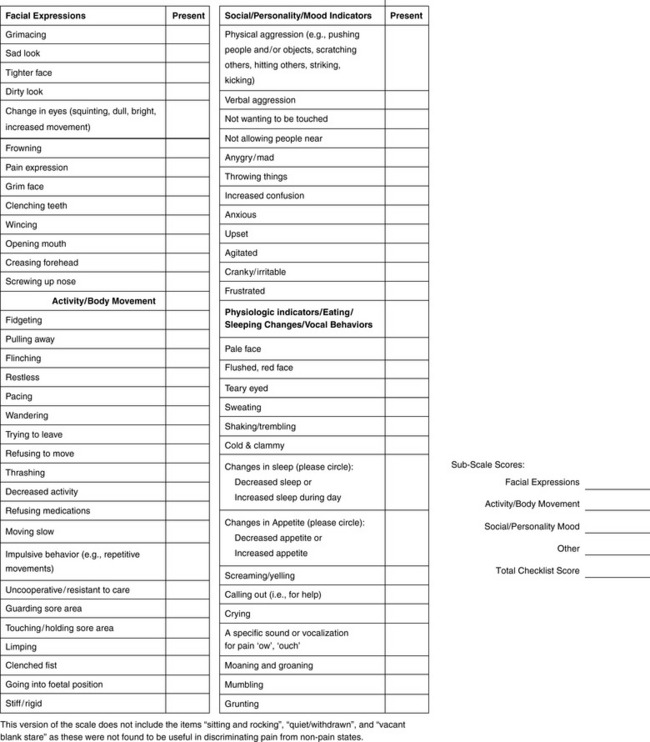
Form 3-16 Shannon Fuchs-Lacelle and Thomas Hadijistavropoulos are the worldwide copyright holders of this version of the PACSLAC. The PACSLAC may not be reproduced without permission. For permission to reproduce the PACLSAC, contact Thomas.hadjistavropoulos@uregina.ca.
A study was conducted to determine whether systematic pain assessment of older adults with severe dementia in a nursing home, using the PACSLAC, would improve pain management and decrease nursing stress in comparison with a control group (Fuchs-Lacelle, Hadjistavropoulos, Lix, 2008). The most common pain-related diagnosis was arthritis. The experimental group of nurses was asked to complete the PACSLAC regularly, that is, at least three times a week. The use of PRN medications by the experimental group increased more over time than it did in the control group. Further, the nurses in the experimental group reported a decrease in work-related emotional exhaustion over time; the control group did not. Rather than increasing stress in the experimental group, the addition of a 60-item assessment tool to their workload led them to find that regular use of the assessment tool reduced their stress. One explanation for this result is that pain caused disruptive and aggressive behavior, and assessment identified this pain and led to increased analgesic medication. This reduced patients’ pain and hence decreased disruptive behaviors, which in turn decreased the nurses’ stress.
The revised PACSLAC-D (Zwakhalen, Hamers, Berger, 2006) is a 24-item tool but does not include items based on changes in behavior or activity. Because of this revision, the PACLSAC and PACSLAC-D psychometric properties should be considered independently. The PACSLAC has good internal consistency, inter-rater reliability, and intra-rater reliability. The PACSLAC-D also shows good reliability estimates in preliminary testing. Preliminary normative data and cutoffs are provided but require validation in larger, more diverse samples. Further refinement of the PACSLAC has been reported, with a shorter tool including 18 valid and reliable indicators confirmed (van Nispen tot Pannerdan, Candel, Zwakhalen, et al., 2009). Additional factor analysis in English-speaking samples and evaluation of sensitivity in detecting treatment effects are recommended.
Pain Screens and Patient Examples
The development and use of pain screens and flow sheets or other methods of documentation, including electronic medical records, are important to ensure consistency in the practice and communication of relevant assessment information. Forms 3-17 through 3-20, pp. 137-140, provide examples of a pain screen for use with older patients unable to self-report and a flow sheet for monitoring pain behaviors.

Form 3-17 This form coordinates information that may be used in the initial assessment of pain in patients who are unable to provide self-report. Both the PACSLAC and the PAINAD are included in the flow sheet because they are both good options for monitoring pain behavior changes depending on patients’ circumstances. The PACSLAC may be used on a less frequent basis as a comprehensive screen for potential pain indicators, whereas the PAINAD may be used on a regular basis for patients with observable pain behaviors and the need for frequent reassessment. From Pasero, C., & McCaffery, M. (2011). Pain assessment and pharmacologic management, p. 137, St. Louis, Mosby. May be duplicated for use in clinical practice.

Form 3-18 The patient described above is in a long-term care facility. He is 78 years old and has been nonverbal since a cerebrovascular accident 6 months ago. He does not appear to be oriented to his environment, but he seems to recognize his wife, who visits several times a week. The PAINAD was used for this patient because he presents with observable behaviors indicative of pain and requires regular reassessment. The score of 6 (out of 10) on the PAINAD and the specific behaviors noted by the nurse and the family’s reports of pain severity strongly support the conclusion that pain is present in this patient unable to self-report. From Pasero, C., & McCaffery, M. (2011). Pain assessment and pharmacologic management, p. 138, St. Louis, Mosby. May be duplicated for use in clinical practice.

Form 3-19 Ongoing pain assessment in the absence of a self-report. This form is used after completion of the Pain Screen, Forms 3-17 and 3-18. From Pasero, C., & McCaffery, M. Pain assessment and pharmacologic management, p. 139, St. Louis, Mosby. Pasero C, McCaffery M. May be duplicated for use in clinical practice.
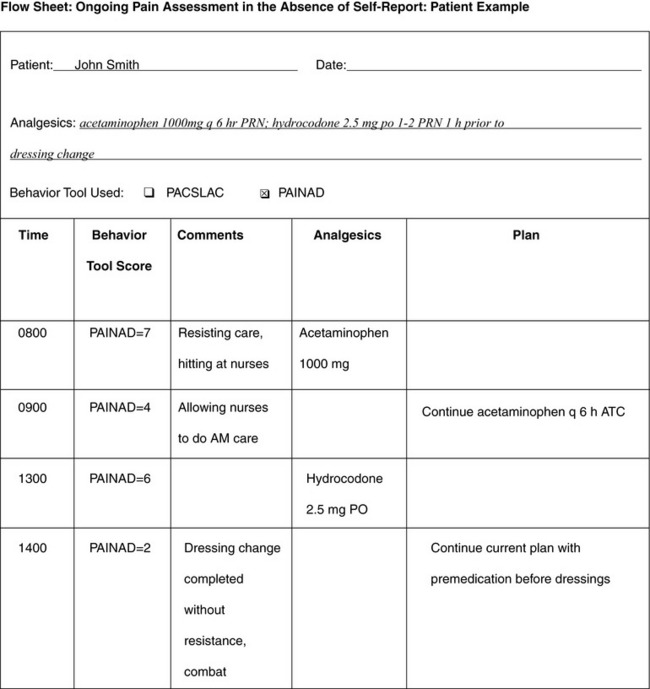
Form 3-20 This form shows observation of Mr. Smith using the PAINAD. His scores suggest a high level of pain along with aggressive behavior. Treatment is initiated, and reassessment shows improvement in his pain behavior score. A low-dose opioid is added to his regimen for use prior to dressing changes, and his pain behavior score, based on the PAINAD, suggests effective pain management with current treatment plan. ATC, Around the clock. From Pasero, C., & McCaffery, M. Pain assessment and pharmacologic management, p. 140, St. Louis, Mosby. Pasero C, McCaffery M. May be duplicated for use in clinical practice.
Other Tools in Development
Several other tools are in development for use in clinical practice. Some of them are discussed briefly; however, new tools and further evaluation of existing tools occur regularly in the literature. Readers are encouraged to study the literature so as to stay abreast of new research findings.
The Abbey Pain Scale (ABBEY) (Abbey, Piller, DeBellis, et al., 2004) is an Australian informant-based tool that measures pain intensity in people with late-stage dementia. The tool attempts to measure acute pain, chronic pain, and acute-on-chronic pain and produces ranges of scores to determine pain intensity. The rationale for mixing and differentiating pain types, pain behaviors, and pain causes is not clear nor is the justification for the pain intensity ranges. Although a recent evaluation of the use of the ABBEY with palliative care patients found that the staff perceived the tool to be helpful in judging pain (van Iersel, Timmerman, Mullie, 2006), facial expressions, vocalizations, and body language were identified as being the best and easiest pain indicators to observe. Information about the tool’s reliability and validity is limited, and tool revision and additional testing in well-designed studies are recommended.
The Doloplus 2 (Lefebvre-Chapiro, 2001; http://www.doloplus.com) is a French tool developed for the multidimensional assessment of pain in nonverbal older adults. It has been translated into several languages, including English, although none of the studies available were conducted using the English-translated tool. The Doloplus 2 is a comprehensive tool based on behavior change that addresses many key indicators noted in the literature and in the AGS persistent pain guidelines and that includes three subscales with 10 items focusing on somatic reactions, psychomotor reactions, and psychosocial reactions. The items are rated according to increasing severity of pain, and the result is a total score that ranges from 0 to 30, with 5 being identified as the threshold for pain. Justification of the cutoff score is not available in the English literature. Recent studies conducted in French, Norwegian, and Dutch samples have been published in English and provide strong support of the tool’s reliability, validity, and clinical utility (Holen, Saltvedt, Fayers, et al., 2005; Pautex, Herrmann, Michon, et al., 2007; Pautex, Michon, Guedira, et al., 2006; Zwakhalen, Hamers, Berger, 2006). Translation issues are evident, and further study or description regarding the use of Doloplus 2 in English-speaking populations is needed.
Elderly Pain Caring Assessment 2 (EPCA-2) (Morello, Jean, Alix, et al., 2007) was developed by a team of French physicians in long-term care facilities to evaluate both persistent and acute pain in non-verbal older adults. The tool relies on caregiver familiarity with a patient to report changes in behavior. The tool has eight items that comprise five of the six categories of nonverbal pain behaviors noted in the AGS persistent pain guidelines. The items are observed prior to and during caregiving; the time needed for observation and completion of the tool is 15 minutes. The hierarchy of pain behaviors according to pain intensity appears logical, but no conceptual basis for the ordering is offered. In other words, in the fifth subscale item (“Observations during caregiver intervention”), “restless” is rated lower than is “aggressive,” and this seems a logical order. However, validation of the ordering of items in each subscale of the tool has not been provided.
Preliminary internal consistency and inter-rater reliabilities are moderate to strong. There was high convergent and discriminant validity and strong responsiveness to treatment. The tool requires training and time for proper administration and has not been validated in English-speaking samples, limiting its clinical utility in the United States.
The CNA Pain Assessment Tool (CPAT) (Cervo, Raggi, Bright-Long, et al., 2007) is an informant-based assessment tool for use by certified nursing assistants (CNAs) for the assessment of pain in patients who have been diagnosed with severe dementia. The tool uses 12 items and collapses them into 5 categories, including 3 of the 6 categories of pain indicators consistent with AGS pain guidelines: facial expressions, body movements, and verbalizations. The tool requires approximately 1 minute to administer, with an uncomplicated forced choice numeric scoring of 0 to 5 and a score of 1 or greater requiring further action by the CNAs. Initial validity was established comparing ratings between patients with and without pain.
Subsequent evaluation of the CPAT (Cervo, Bruckenthal, Chen, et al., 2009; Cervo, Raggi, Bright-Long, et al., 2007) noted acceptable levels of both interrater reliability and test-retest reliability. Construct validity was established comparing ratings before and after a known painful or uncomfortable event and criterion validity established by comparing to another established pain scale, the DS-DAT. Based on a practicality survey, the CPAT was shown to be clinically useful and feasible. The CPAT requires further evaluation of ability to measure severity of pain and response to treatment.
The Mobilization-Observation-Behavior-Intensity-Dementia Pain Scale (MOBID) (Cervo, Raggi, Bright-Long, et al., 2007; Husebo, Strand, Moe-Nilssen, et al., 2007) is a nurse-administered instrument used to observe pain behaviors and infer pain intensity at rest and with standardized guided activity. It is intended for use with patients who have severe cognitive impairment and chronic musculoskeletal pain and are living in long-term care settings. A patient is guided through five structured activities (mobilization of both hands, both arms, and both legs; turning in bed; sitting at bedside) and the presence and intensity of pain are rated by nurses on an 11-point NRS for sounds, facial expressions, and defense, with increased behaviors indicating increased pain (e.g., combative behaviors when moved). The tool does not include subtle cues or changes in behavior and may limit recognition of pain in those presenting atypically. The MOBID has good internal consistency. There was a wide range of inter-rater reliability for the presence of pain behaviors, but reliability was better for pain intensity. The MOBID was able to detect increased pain with movement, and there was a linear trend among external raters toward more pain behaviors being associated with higher pain intensity. Recent follow-up evaluation supports reliability of MOBID scoring during video evaluation of behaviors in older persons with severe dementia (Huesebo, Strand, Moe-Nilssen, et al., 2009). More evidence is needed to support the validity of the tool for both the presence of pain behaviors and inferred pain intensity.
The Non-Communicative Patient’s Pain Assessment Instrument (NOPPAIN) (Snow, Weber, O’Malley, et al., 2004) is an instrument that focuses on nursing assistants’ (nursing technicians’) observations and assessments of pain in patients with dementia at rest and with movement. Nursing assistants observe for pain-related behaviors as they perform common caregiving tasks. The NOPPAIN contains common pain behaviors, not subtle cues or changes in behavior. Because the NOPPAIN is based on proxy reports of pain intensity, evidence is needed to support the validity of the judgments of intensity of pain behaviors as indicators of pain severity. Preliminary support for the tool’s reliability and validity was reported based on responses to video simulations, rather than on actual observation of patients. One follow-up study provides support for the convergent validity of proxy reports by means of detailed behavioral coding in patient videos (Horgas, Nichols, Schapson, et al., 2007). The tool’s reliability and validity have been established by means of video coding by nursing assistants and patient videos scored by nursing students in a laboratory setting. Although ease of administration by nursing assistants is a strength of the tool, evaluation of nursing assistants’ abilities to use the scale in actual patient care situations is needed.
Pain Assessment in Noncommunicative Elderly Persons (PAINE) (Cohen-Mansfield, 2006) is an informant-based assessment tool developed to assess pain in noncommunicative elders. The tool includes a comprehensive list of 22 behaviors representing 4 of the 6 pain behavior categories consistent with the AGS persistent pain guidelines. The tool has been administered only by research assistants and has not been studied for feasibility in the clinical setting. No information about the time or skill level needed to complete the PAINE is reported. Preliminary evaluation suggests good internal consistency and good inter-rater reliability, even among staff members who have varying familiarity with patients. Although procedures are unclear and difficult to follow, it appears that the PAINE has construct validity and good correlation with other tools testing responsiveness to intervention (Cohen-Mansfield, Lipson, 2008). However, PAINE is weakly correlated with other observational and self-report assessment tools. Further evaluation of reliability and validity when used by nursing assistants directly is needed.
Two newer instruments that warrant consideration are the Rotterdam Elderly Pain Observation Scale (REPOS) (van Herk, van Dijk, Tibboel, et al., 2009) and the Mahoney Pain Scale (Mahoney, Peters, 2008). These tools were not available to include in the critical review completed in 2008 on the City of Hope Pain Resource Center website and discussed in this chapter. However, the reader is encouraged to access the most recent information when reviewing possible tools for consideration.
Conclusion
Clinicians should carefully review the tools in light of the particular population and setting so as to determine which tools are good matches. As noted earlier, considerable ongoing research has the goals of revising existing tools so as to maximize their reliability, validity, and clinical usefulness; providing additional information about tools’ psychometrics; and developing new approaches to pain assessment in this vulnerable population. Readers should monitor the literature for updates on progress in this area.
A pain policy that addresses comprehensive and appropriate assessment of pain in all patients must include procedures for identifying and monitoring pain in older persons who are cognitively impaired or nonverbal. The process should be comprehensive and should incorporate procedures that address the issues discussed here.