Misconceptions that Hamper Assessment and Treatment of Patients Who Report Pain
Patient versus Caregivers/Family
Health Care Conditions that Influence Clinicians’ Judgments of Pain
Pain Threshold: Uniform versus Variable
Pain Tolerance: High versus Low
Behavioral and Physiologic Responses to Pain
Acute Pain Model versus Adaptation
Responses Clinicians Expect of Patients with Pain
Patients’ Knowledge of Clinicians’ Expectations of Pain Behaviors
Correction of the Misconceptions Concerning the Acute Pain Model
Seeking Drugs versus Seeking Pain Relief
Definitions Related to Addiction
Likelihood of Occurrence of Addictive Disease
Addiction as a Stigmatizing Label: Societal versus Medical Views
Misconceptions about the Meaning of Positive Placebo Responses
Misconceptions about Using Placebos for Pain Relief
Recommendations against the Use of Placebos
REGARDLESS of what patients say about their pain, the subjectivity of pain seems to invite speculation from everyone—clinicians, families, and acquaintances—about the “true” nature of patients’ pain. Numerous reasons are given by clinicians to explain why they find it difficult to accept some patients’ reports of pain and why they fail to respond with appropriate treatment. These reasons include lack of a known cause for the pain or lack of behavioral indicators such as grimacing. Still other reasons remain less obvious, often below the level of awareness, but still cause clinicians to have doubts. For example, the patients’ gender or ethnic origins may unknowingly influence clinicians’ decisions about pain management.
Subjectivity of Pain
Patient versus Caregivers/Family
Who is the authority on patients’ pain? Whose pain is it? Clinicians sometimes believe they know more about the patient’s pain than the patient does. No matter how appealing that belief may be, it is false. Nevertheless, privately or among themselves, clinicians may comment about a patient, “He doesn’t have as much pain as he thinks he does” or “The pain is not that bad,” implying that the clinicians are the true authorities on patients’ pain. In research cited earlier, clinicians’ tendencies to underestimate patients’ pain is noted.
No objective measures of pain exist. The sensation of pain is completely subjective. Pain cannot be proved or disproved. One definition of pain used in clinical practice says “Pain is whatever the experiencing person says it is, existing whenever he says it does” (McCaffery, 1968, p. 95). Statements by the American Pain Society (APS) have echoed the same approach to patients’ reports of pain by statements such as:
• “The clinician must accept the patient’s report of pain.” (APS, 2003, p. 1)
• “Self-report should be the primary source of pain assessment when possible.” (APS, 2003, p. 33).
• “…the patient’s self-report should be used as the foundation for the pain assessment.” (Miaskowski, Cleary, Burney, et al., 2005, p. 19)
The gold standard for assessing the existence and intensity of pain is patients’ self-reports. No other source of information has ever been shown to be more accurate or reliable than what a patient says. Patients’ behaviors, the opinions of nurses and physicians delivering care, patients’ vital signs—none of these is as reliable as patients’ reports of pain and should never be used instead of what a patient says.
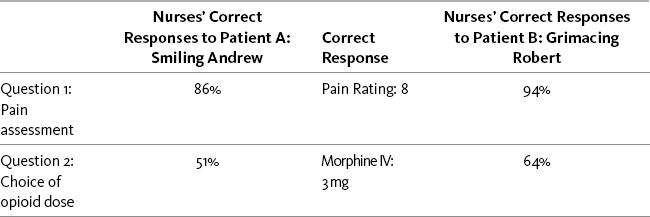
The Andrew-Robert survey presented in Box 1-1 (p. 18) illustrates what happens when clinicians do not adopt the patient’s self-report as the standard for assessment of pain intensity. This survey has been used by many nurse educators in hundreds of educational programs to explain the necessity of accepting the patient’s report of pain as the standard for assessment. The survey has become so familiar to staff nurse educators that it is often referred to simply as the Andrew-Robert survey. Several publications have reported the results of studies using this survey and modifications of it (McCaffery, Ferrell, 1991a, 1991b, 1992a, 1992b, 1994a, 1997a; McCaffery, Ferrell, O’Neil-Page, 1992). A summary of survey findings from 1990 to 2006 has been published by the originators of the survey (McCaffery, Pasero, Ferrell, 2007). The results of the Andrew-Robert survey as presented in Table 2-1 are based on the responses of 615 registered nurses who attended pain programs throughout the United States. The survey was administered to registered nurses before the pain conference began. In viewing the nurses’ responses to pain assessment (question 1), it is apparent that not all nurses understood that the patient’s self-report of pain is the single most reliable indicator of pain. Both of these patients reported their postoperative pain as 8, but 14% of the nurses did not record 8 for the smiling patient and 5% did not record 8 for the grimacing patient. Those nurses who did not record 8 falsified the record and made it impossible for the next nurse to evaluate previous treatment for pain.
Table 2-1
Nurses’ Responses to the Survey: Assessment and Use of Analgesics*
*Data collected in 2006; N = 615.
As appears in Pasero, C., & McCaffery, M. (2011). Pain assessment and pharmacologic management, p. 21, St. Louis, Mosby. Data from McCaffery, M., Pasero, C., & Ferrell, B. (2007). Nurses’ decisions about opioid dose. Am J Nurs, 107, 35-39. McCaffery M, Pasero C, Ferrell B. May be duplicated for use in clinical practice.
The percentages of nurses who recorded what the patients said are high but there is no room for false entries in the chart. If these results were from a single institution, then one would have to say that up to 14% of the nurses were not recording what the patients said, and this is made more complex because we do not know who they are. Thus, all recordings of pain intensity would have to be viewed with some suspicion.
The Andrew-Robert survey is a quick and easy survey to use in small and large populations of nurses. The results of the survey help to generate discussion of common problems that arise in patient care. Another brief and more comprehensive survey of staff knowledge is titled Pain Knowledge and Attitude Survey and is available in Appendix A.
The survey findings illustrate what is sometimes seen in clinical practice—each person caring for a patient may have a different opinion about the intensity of a patient’s pain. Without a standard for assessing pain, chaos quickly ensues. For example, four different clinicians caring for the same patient may arrive at four different pain ratings, all of which are different from the patient’s own pain rating (and usually are underestimations of a patient’s pain). How do you resolve five different pain ratings so that intervention can be planned?
The need to establish the patient’s self-report as the standard becomes apparent. There appears to be no alternative. It is reassuring to realize that the validity and reliability of patients’ self-reports of pain are testified to in the numerous double-blind studies of analgesics, in which the patients’ pain ratings always determine the analgesic effect of the drugs being tested. Initial recommended doses of analgesics and equianalgesic charts have relied on the patient’s self-report for decades.
When a patient reports pain, the health care professional’s responsibility is to accept and respect the report and to proceed with appropriate assessment and treatment based upon the self-report. All reports of pain are taken seriously. When difficulties arise in accepting the patient’s report of pain, some of the strategies listed in Box 2-1 may be helpful.
Health Care Conditions that Influence Clinicians’ Judgments of Pain
Numerous factors influence clinicians’ tendencies to doubt patients’ reports of pain. This section discusses the effects of length of time caring for patients with pain, environmental cues that promote suspicion of patients’ pain reports, and the effects of the nature of one’s clinical practice.
Studies report conflicting results about the effects of length of time caring for patients with pain. As reported earlier, clinicians frequently underestimate patients’ pain ratings. This has been explained as being a process of habituation. Repeated exposure to patients’ pain seems to promote the development of insensitivity to pain (Kappesser, Williams, Prkachin, 2006). Yet other studies have not shown this (Everett, Patterson, Marvin, 1994; Hamers, van den Hout, Halfens, 1997). In a study of 50 registered nurses randomly selected from 2 general surgical units, two general medical units, and a combined intensive care unit/coronary care unit, participants completed an instrument that contained 60 vignettes describing a patient’s illness or injury (Dudley, Holm, 1984). Nurses were instructed to rate each patient on the degree of pain and psychologic distress. Nurses inferred significantly less pain than psychologic distress. Correlation analysis revealed a markedly weak and statistically insignificant correlation between years of practice and pain scores.
Other factors that may influence clinicians’ estimates of patients’ pain may be access to the patients’ self-reports and the atmosphere of suspicion surrounding particular patients. In one study, 60 physicians and nurses from the emergency department (ED) and 60 from the oncology setting watched videotapes of facial expressions of patients with shoulder pain undergoing range-of-motion exercises (Kappesser, Williams, Prkachin, 2006). Participants were divided into three groups and given different information: Group 1, videotape only; Group 2, the same videotape plus the patients’ reported pain intensity; and Group 3, the same videotape plus being told the patient pain reports and that some of the people were faking pain to obtain opioid drugs. All participants were asked to rate the patients’ pain, and participants in groups 2 and 3 were asked to state whether they had the impression that any of the patients had faked pain or hidden pain. Clinicians in all three groups underestimated patient pain intensity. The least discrepancy between clinicians’ and patients’ pain ratings occurred when the clinicians were given the patients’ self-reports but were not told that some patients were faking pain to obtain opioids.
The greatest discrepancy occurred when clinicians were given the patients’ pain reports but were told that patients might be faking pain to obtain opioids. This suggests that a clinical atmosphere of suspicion, such as sometimes occurs in trauma units or EDs, may cause an increase in clinicians’ doubting patients’ reports of pain. In all clinical areas clinicians need to be cognizant of the attitudes they convey when they discuss patient care with their colleagues. Managers and supervisors can be alert to stigmatizing labels and help staff to avoid them. For example, the term drug-seeking, which has no universally accepted definition, commonly conveys that a patient is addicted to opioids, abusing pain medicine, or manipulative (McCaffery, Grimm, Pasero, et al., 2005). In fact, the American Society for Pain Management Nurses (ASPMN; 2002) recommends that this term not be used because it creates bias and prejudice that subsequently have negative effects on pain management.
The nature of a particular clinical practice may influence clinician estimates of patient pain intensity. The effect of repeatedly inflicting pain on patients was studied by using brain scans to compare the responses of physicians who practice acupuncture with the responses of naïve participants while watching animated visual stimuli depicting body parts in both a nonpainful situation (being touched by a Q-tip) and a potentially painful situation (acupuncture [being pricked by needles]) (Cheng, Lin, Liu, et al., 2007). The acupuncturists rated these situations as being significantly less painful and unpleasant than did the naïve control participants.
In addition, the brain scans supported the notion that mechanisms are triggered in the areas of the brain that regulate emotions and cognitive control. The authors commented that without some regulatory mechanism, it is likely that clinicians would experience personal distress and anxiety that would interfere with their ability to perform. Acupuncturists know that they may be inflicting pain and seem to have learned throughout their training to inhibit the empathy-pain response.
Many other clinical practices, such as burn care and surgery, involve repeated infliction of pain. A study of burn care found that staff members who had spent more time working with burned patients believed débridement was less painful than did the staff members who had spent less time working with burned patients (Perry, Heidrich, 1982). To continue to manage pain effectively, such clinicians need to be aware of their tendencies to underestimate the painful impacts of what they do and to recognize that doing so may be an essential mechanism that allows them to continue to give care. Rather than be embarrassed or ashamed of this reaction, they can acknowledge it to themselves (and perhaps others) and try to compensate by obtaining and listening carefully to information from their patients.
Other examples are clinicians who perform venipunctures or bone marrow aspirations daily; they may become less sensitive to the amount of pain they are inflicting. Clinicians who work in a pain clinic several days a week may grow weary of and less sensitive to patients who continue to report pain despite their best efforts to relieve the pain. Again, to continue to practice effectively in such conditions it may be necessary to make a conscious personal inventory that honestly admits to having less empathy for patients as time goes by. Once this is acknowledged, the clinician can plan a systematic approach to obtaining information from the patient and ensure that it is acted upon.
Considerations When Doubts Arise
On occasion, accepting and acting on the patient’s report of pain are difficult. Because pain cannot be proved, health care professionals are vulnerable to being fooled by the patient who wishes to lie about pain. However, although accepting and responding to the report of pain will undoubtedly result in giving analgesics to some patients who do not have pain, doing so ensures that everyone who does have pain receives attentive responses. Health care professionals do not have the right to deprive a patient of appropriate assessment and treatment simply because they believe a patient is lying.
An important distinction exists between believing the patient’s report of pain and accepting the report. Following the recommendations of the clinical practice guidelines does not require that the clinician agree 100% with what the patient says. Clinicians are not required to believe a patient but are required to accept what a patient says, convey acceptance to the patient, and take the appropriate action. Clinicians are entitled to their personal doubts and opinions, but they cannot be allowed to interfere with appropriate patient care. Box 2-1 summarizes some strategies that can be used when the patient’s report of pain is not accepted.
Although accepting the patient’s report of pain occasionally results in being fooled, no stigma or blame should be attached to being duped. In any relationship, each party has certain responsibilities. Fault is assigned to the parties who fail to meet their responsibilities (Wesson, Smith, 1990). If the clinicians fulfills his or her responsibility to respond to all reports of pain with appropriate assessment and treatment, the clinician will be able to say, “Although I was probably fooled by some patients, I never failed to help those who did have pain. No one can find fault with my behavior or professional conduct.”
Furthermore, accepting the patient’s report of pain avoids an adversarial relationship. When a clinician conveys to a patient that a report of pain has not been accepted, it amounts to accusing the patient of lying. Understandably, this is upsetting and frightening to a patient who has asked the health care provider for help with pain. Much has been written in the literature about the distinction between suffering and pain. It is worth noting that in his analysis of suffering, Eric Cassell (1982) mentions that one source of suffering is physicians who do not validate a patient’s pain but rather ascribe it to psychologic causes or accuse the patient of faking. Clinicians ask patients to trust them. At some point clinicians must return the favor.
The term malingering may be encountered when a patient’s report of pain is doubted. Malingering can be defined as a conscious effort to produce symptoms such as pain for the purpose of deceiving or misrepresenting the facts, usually for monetary or other gains. Malingering seems to be suspected most commonly in patients with persistent pain, especially low back pain. A review of the literature prior to 1999 reveals 68 references to research of persistent pain malingering or disease simulation (Fishbain, Cutler, Rosomoff, et al., 1999). These studies conclude that malingering does occur, perhaps in 1.25% to 10.4% of patients, but these figures are not reliable because of the quality of the studies. This review concludes that there are no reliable methods of identifying malingering. The International Association for the Study of Pain notes that the process of identifying malingering is, in the final analysis, a legal, not a medical, process (Merskey, Bogduk, 1994).
On reflection, one might ask whether it is justifiable to be suspicious of all patients in an attempt to avoid being fooled by the few who lie. It is a burden to the clinician and an insult to the patient to wrestle with potential dishonesty at each encounter.
Pain Threshold: Uniform versus Variable
Pain threshold may be defined as the point at which an increasing intensity of stimuli is felt as painful. Several decades ago, preliminary research erroneously suggested that everyone perceives the same intensity of pain from the same stimuli (Hardy, Wolff, Goodell, 1943). This has been called the uniform pain threshold. However, further research failed to support the uniform pain threshold theory (Beecher, 1956). For half a century it has been known that comparable stimuli in different people do not produce the same intensities of pain. A uniform relationship does not exist between tissue damage and pain. A given type of tissue damage may produce more or less pain than one might expect. Not only does pain intensity vary among patients, but duration and other characteristics also vary.
In a small study, healthy volunteer subjects were asked to rate experimental pain consisting of nerve shocks. Given the same intensity of pain stimulus, various persons did not always give the same pain ratings, and the same intensity of pain stimulus given to the same person repeatedly did not always result in the same pain rating (Mader, Blank, Smithline, et al., 2003).
The idea that a particular patient “shouldn’t hurt that much” probably is based on the misconception that comparable stimuli produce comparable pain in different people. A more appropriate appraisal might be that a painful event hurts one patient more than it hurts another. Concluding that one patient is exaggerating the pain is judgmental and may result in a number of potentially harmful effects, such as failing to detect a complication or providing inadequate analgesia. When a patient reports pain that is considerably more than expected, it is always wise to reassess that patient.
Pain Tolerance: High versus Low
Pain tolerance is not the same as pain threshold; it may be defined as the duration or intensity of pain that a person is willing to endure. An example of high pain tolerance is the willingness to endure prolonged and severe pain without desiring relief, whereas low pain tolerance might be the desire for relief of brief, mild pain.
Pain tolerance varies from person to person and within the same person depending on numerous factors, such as past experiences with pain, coping skills, motivation to endure pain, and energy level. For example, a patient may be willing to endure intense pain during childbirth to minimize the infant’s exposure to medications but may be unwilling to endure mild episiotomy pain later if she is not breast-feeding.
Society places a high value on a high pain tolerance. The findings of one study suggest that nurses do not like patients who have severe pain or who are perceived as coping poorly with their pain (Salmon, Manyande, 1996). When patients were perceived as being unable to cope with pain, they were evaluated by the nurses as demanding and were unpopular with the staff.
Low pain tolerance seems to be regarded as a weakness, a character flaw, a lack of will power, or perhaps even self-indulgence. The implication appears to be that the patient should be stronger and muster the energy to cope with pain more successfully.
The routine use of the phrase complains of pain suggests a negative attitude toward patients with pain and may reflect a desire for patients to cope better and talk less about pain. Saying that patients “report pain” is much less evaluative. It is probably worthwhile making a conscious effort to avoid using the word complain. The reader may note that throughout this book, the authors never use the word complain in reference to patients’ reports of pain.
One cannot escape the fact that this society and many others value a stoic response to pain, which is probably very closely aligned with valuing a high pain tolerance. No doubt most readers share this value. However, health care providers must guard against requiring this of patients and certainly must avoid criticizing patients who are unable to meet this expectation. Patients have a right to determine their own pain tolerance.
Expecting a high pain tolerance may translate into deciding that patients ought to be able to tolerate particular painful experiences. There may be procedures; persistent pain conditions, such as arthritis or low back pain; surgeries; wound care; or a variety of other circumstances. In cases of procedural pain, it is not unusual for clinicians to voice their belief that providing sedation/analgesia is unnecessary for very short, but very painful procedures, believing that patients should be able to “tolerate” brief pain.
A common misconception is that increased experience with pain should teach a person to be more tolerant of it and better able to cope with it. However, repeated experience with pain often teaches a person how severe pain can become and how difficult it is to get pain relief. Thus, a person who has repeated experiences with pain may have higher levels of anxiety and lower pain tolerance. In one study of adults, previous surgeries appeared to result in greater pain intensity and emotion during later surgical experiences (Wells, 1989).
Identifying the patient’s pain tolerance is a critical part of providing pain relief. Setting pain rating and activity goals (discussed later in this section) is an effort to identify the level of pain a patient can endure without distress and still perform necessary activities easily.
Patients, as well as clinicians, value stoicism. When a patient is unable to meet his or her own expectations of being able to tolerate unavoidable pain or minimize behavioral expressions of pain, the clinician can at least minimize the psychologic trauma to the patient by conveying that the patient’s response to pain is fully acceptable. Simply saying, “This is tough. You’re doing well,” may help reduce the patient’s distress.
Behavioral and Physiologic Responses to Pain
Acute Pain Model versus Adaptation
The acute pain model says that if the patient has pain, visible signs of discomfort, behavioral or physiologic, will be present. Examples of behavior usually expected of patients with pain include grimacing, rigid body posture, limping, frowning, or crying. Physiologically, elevated vital signs are commonly expected. Clinicians and laymen alike usually fail to appreciate that both physiologic and behavioral adaptation occurs, leading to periods of minimal or no signs of pain. Absence of behavioral or physiologic signs of pain does not necessarily mean absence of pain.
The acute pain model is of limited value for assessing pain. When pain is sudden or severe, behavioral and physiologic indicators may be present for a brief time. However, very quickly the patient may make an effort to cease behaviors such as crying or moaning. Behavioral adaptation or suppression of pain behaviors may occur because the patient values the stoic response or simply becomes exhausted. Physiologic indicators such as increased blood pressure (BP) or heart rate may also disappear. In a healthy individual, the body seeks homeostasis or equilibrium, returning to the former physiologic state despite severe pain. Or a patient may have a medical condition that causes low BP, such as hypothyroidism or dehydration, which has a much greater impact on vital signs than pain has. In such patients, sudden, severe pain may elevate the vital signs only briefly and minimally.
The APS addresses the misconceptions about the acute pain model by stating the following:
• With regard to acute pain, the APS (2003) says, “Often but not always [italics ours], it is associated with objective physical signs of sympathetic branch autonomic nervous system activity, including tachycardia, hypertension. . . .” (p. 2).
• With regard to persistent cancer pain, the APS (2003) says, “The lack of objective signs may prompt the inexperienced clinician to wrongly conclude the patient does not appear to be in pain” (p. 3).
Responses Clinicians Expect of Patients with Pain
Nurses’ responses to the Andrew-Robert survey (see Table 2-1, p. 21) reveal that patients’ behavioral responses have significant effect on nurses’ pain assessments and treatment decisions (McCaffery, Pasero, Ferrell, 2007). The only difference between Andrew and Robert is their behavior—Andrew smiles and laughs with visitors, whereas Robert lies in bed and grimaces. This simple difference has a startling effect on nurses’ decisions about opioid doses.
Although the patients were exactly alike except for their behaviors, the nurses were more likely to increase the morphine dose for the grimacing patient. Both patients had received morphine, 2 mg IV, 2 hours before; half-hourly pain ratings had ranged from 6 to 8 out of 10 and were currently 8; and no clinically significant adverse effects such as sedation had occurred. The pain rating goal was 2. Nurses were given a choice of administering no morphine or 1 mg, 2 mg, or 3 mg IV. Morphine, 3 mg IV, was the correct choice for both patients because the previous dose of 2 mg was safe but ineffective. However, only 50.6% of the nurses would increase the dose for the smiling patient, whereas 64.3% would increase the dose for the grimacing patient. Both patients were undertreated, but at least 14% of the nurses knew that it was safe to increase the dose for the smiling patient, but they did not. Over the years since 1990, other similar vignette surveys conducted by these authors have shown similar findings, which show little improvement in nurses’ choices of opioid doses (McCaffery, Pasero, Ferrell, 2007).
The same discouraging results were found when the Andrew-Robert survey was adapted to reflect the needs of elderly patients in a long-term care facility (Katsma, Souza, 2000). The participants were 89 licensed nurses working in long-term care facilities. As in the original vignette, one patient was described as smiling and the other as grimacing. After receiving medication, hourly pain ratings were 6 to 8, and the patients showed no serious adverse effects. In response to the question about which analgesic dose they would now give, only 30% correctly selected a higher dose for the smiling patient, but 43% did so for the grimacing patient.
These same biases about behavior also exist in laypersons. The Andrew-Robert survey was revised to be appropriate for a nonnurse audience and was administered to 85 college students who were not enrolled as medical or nursing majors (McCaffery, Ferrell, 1996a). College students’ responses to assessment and relief of pain showed trends similar to those of practicing nurses. The smiling patient’s pain rating was accepted by 38% of the college students, whereas 55% accepted the grimacing patient’s report of pain.
Vital signs also influence nurses’ willingness to record a patient’s report of pain. A survey using the same format as the original Andrew-Robert survey was constructed; the only difference between the patients was their vital signs (McCaffery, Ferrell, 1992a, 1992b). One patient had low to normal vital signs, and the other patient had elevated vital signs. The responses of 166 nurses revealed that more nurses were willing to accept the report of severe pain from the patient with elevated vital signs than from the patient with low to normal vital signs.
As part of the Missoula Demonstration Project, a 15-year process to study end-of-life issues, surveys about pain knowledge and attitudes were sent to 942 nurses and produced 311 responses (Mayer, Torma, Byock, et al., 2001). The survey revealed that 93% of the nurses knew that vital signs were not reliable indicators of pain, but 75% said that vital signs moderately or greatly influenced their decisions to treat or not to treat pain.
In a small study designed to identify the criteria nurses use to assess postoperative pain, 10 nurses were interviewed about their pain assessments of 30 postoperative patients (Kim, Schwartz-Barcott, Tracy, et al., 2005). The strategy most frequently reported by the nurses relied on the patient’s appearance and drew on past experience of which physical signs to look for, such as facial expression, body movement, and heart rate. For example, nurses mentioned frowning or wincing as being indicative of pain and a patient’s being able to fall asleep as a sign of little or no pain. The researchers commented on the fact that the predominant strategy of looking for objective signs of pain was in marked contrast to current guidelines that emphasize the patient’s self-report of pain.
Expectations that certain behaviors indicate pain also influence the prescribing of analgesics. To identify factors that affect physicians’ decisions to prescribe opioids for persistent noncancer patients, the records of 191 patients referred to a pain center were examined to determine pain severity, physical findings, pain duration, age, gender, observed pain behaviors, reported functional limitations, and affective distress (Turk, Okifuji, 1997). Of all these variables, only observed pain behaviors were significantly related to receiving opioid prescriptions. In other words, patients who exhibited pain behaviors were most likely to receive opioids for pain relief. The extent of physical findings and the severity of the pain did not appear to influence the decision to prescribe opioids. Because opioids are prescribed for the purpose of pain relief, it would seem more logical to find that severity of pain determined prescription of opioids, but this was not the case.
Similar findings also were reported regarding patients with low back pain. Decisions about lumbar surgery were not made on the basis of physical pathologic conditions but rather on behaviors demonstrated by patients during their evaluations (Waddell, Main, Morris, et al., 1984). As summarized by Turk and Okifuji (1997), “physicians appear to believe that behavioral demonstrations of pain, such as limping and grimacing, indicate something important about the nature of the patient’s pain and the need for prescribing specific treatments such as surgery and opioids” (p. 334).
Patients’ Knowledge of Clinicians’ Expectations of Pain Behaviors
Interviews with patients who had used intravenous patient-controlled analgesia (IV PCA) for opioid administration after surgery revealed that a major reason for patients’ valuing PCA was related to the fact that PCA decreased the need to interact with the nursing staff regarding pain (Hall, Salmon, 1997; Taylor, Hall, Salmon, 1996a, 1996b). Not only did patients see PCA as being better than waiting for a nurse to administer opioids intramuscularly, they also believed it protected them from having to show distress to the nurses.
Apparently many patients with pain are aware of the behaviors expected of them, or they learn them quickly. Patients may learn from early childhood experiences with pain, television, the responses of their clinicians, and a variety of other sources how to behave to signal others that pain exists and that help is needed. It is a common observation that patients with pain change their behavior in the presence of clinicians and in other selected circumstances. Patients may appear to be calm and to read or have lively telephone conversations, but as soon as clinicians enter, patients may replace these activities with a solemn facial expression and may even grimace, moan, and restrict movement—just the behaviors clinicians want to see when patients report pain, but not necessarily the behavior patients prefer.
Consider what might happen if the patients in the Andrew-Robert survey were roommates. It would not take long for smiling Andrew to realize that grimacing Robert was receiving better pain relief, and the reason probably would be quite apparent to smiling Andrew. Andrew might take pride in looking energetic and happy in front of his visitors or may find that such distraction is very helpful as a coping mechanism. But if he wants better pain relief, he may decide to change that behavior, at least during the time clinicians are present. When clinicians see this change occur, they often regard patients as being manipulative, not realizing that it is the expectations that clinicians convey to patients that cause this behavior change.
Taking this hypothetical situation further, by succumbing to expectations of pain behavior to obtain relief, Andrew may begin to jeopardize his recovery. For example, both patients might be told to ambulate. Smiling Andrew may walk the hall until he begins to hurt and then go to the nurses’ station and ask for something for relief. The staff may feel that if Andrew can be this active, he could not hurt enough to require an analgesic. Grimacing Robert, on the other hand, may remain in bed grimacing rather than ambulating and may also ask for an analgesic. Robert may very well be more likely to receive the analgesic than Andrew. Andrew may then learn to stay in bed, prolonging his recovery time and increasing the risk of complications, but increasing his chances of receiving pain relief.
Comparable circumstances were observed in a study of cancer patients (Cleeland, Gonin, Hatfield, et al., 1994). The more active patients were more likely to receive inadequate pain management.
Because clinicians differ in how they expect patients to behave in response to pain, patients may have difficulty learning which behaviors will effectively convince which clinicians. An analysis of staff and patient behavior in an orthopedic unit revealed that the staff assessed pain by observing patients’ behaviors and that their expectations about how patients should express their pain varied within and between shifts (Wiener, 1975). Some, but not all, patients were adept at reading the explicit and implicit cues given by staff and changed their behaviors accordingly. Patients sometimes felt forced to use tactics that they believed were unacceptable but were expected by the staff and were necessary to obtain pain relief.
Expecting patients to behave in certain ways to verify their pain becomes more confusing when clinicians are especially particular about the intensity or type of behavior that a patient should display. Sometimes clinicians say that a patient does not appear to be in pain, but when a patient exhibits pain behavior, clinicians may say that a patient is making too much fuss about the pain.
Correction of the Misconceptions Concerning the Acute Pain Model
One of the most interesting aspects of the misconception that the patient’s behavioral response is more reliable than the patient’s self-report of pain is that it is totally illogical. Virtually every human being has had the personal experience of trying to hide pain and to function in spite of it, smiling when appropriate and even deliberately using humor as distraction. One of the oldest maxims in health care is that laughter is the best medicine. Why couldn’t the nurses and college students responding to the Andrew-Robert survey use this folk wisdom and their own personal experiences with pain to realize that patients with severe pain most certainly may smile and laugh? Nurses are probably the largest group of clinician advocates for nondrug pain relief measures, of which distraction and laughter are highly ranked. And no research has ever even suggested that smiling and joking are incompatible with feeling pain. Why clinicians seem to have such difficulty accepting and acting on reports of pain from smiling and active patients remains a mystery, at least to these authors.
A substantial amount of research refutes the value of the acute pain model, showing that neither behavioral indicators nor physiologic responses are dependably related to the intensity of a patient’s pain. Physiologic indicators appear to be even less valuable than a patient’s behavioral responses to pain.
Most nurses seem to have been taught to use elevated vital signs to assess or verify the presence of pain, especially severe pain. However, in literature reviews, investigators found very little research that supported using physiologic manifestations as specific indicators of pain (Herr, Coyne, Key, et al., 2006; van Cleve, Johnson, Pothier, 1996). In a study of 1063 patients admitted to the ED with conditions that could be verified to be painful, such as fractures and nephrolithiasis, no clinically significant associations were identified between pain scores self-reported on a scale of 0 to 10 and heart rate, BP, or respiratory rate (Marco, Plewa, Buderer, et al., 2006). In a study published in French, investigators found that absence of increased vital signs does not mean absence of pain (reported in Gelinas, Johnston, 2007).
Critical care nurses usually consider vital signs to be relevant indicators of pain, possibly because the signs are readily available, and it is often difficult to obtain pain reports from critically ill patients, whose behavioral responses may be compromised and who may be unconscious, intubated, or otherwise unable to communicate. In a study involving 30 conscious patients in an intensive care unit, no significant relationship was found between physiologic indicators and patients’ self-reports of pain, reinforcing the fact that physiologic indicators should not be considered primary indicators for pain assessment (Gelinas, Johnston, 2007). In other words, physiologic indicators were not related to patients’ reports of pain.
In another study of 755 patients, primarily in intensive care units, physiologic responses were monitored while the patients were undergoing tracheal suctioning (Arroyo-Novoa, Figueroa-Ramos, Puntillo, et al., 2007). Statistically significantly higher increases were noted in heart rate and systolic and diastolic BP, but these changes were not clinically significant. The authors suggest that methods of measuring these physiologic parameters may not be sensitive enough to capture the response to acute pain. For further discussion of the limited usefulness of physiologic measures in assessing pain, see the discussion of patients who are critically ill in Chapter 4, pp. 143-147.
The usefulness of physiologic measures of pain is further compromised by the presence of dementia in older adults. In a small study of adults 65 years old and older, 50 of whom were cognitively intact and 44 of whom had varying degrees of dementia, heart rate and other physiologic responses were monitored before and during venipuncture (Porter, Malhotra, Wolf, et al., 1996). Increasing severity of dementia was associated with the blunting of physiologic responses as measured by diminished heart rate increase in the preparatory phase and during venipuncture.
Thus, little research supports vital signs as being relevant indicators of pain, although they can be used as indicators of the need for further assessment of pain. A position statement by the ASPMN regarding assessment of pain in nonverbal patients (available at http://www.aspmn.org/Organization/documents/NonverbalJournalFINAL.pdf) suggests that physiologic indicators should not be used alone to assess pain but should be considered cues for further assessment of the possibility of pain (Herr, Coyne, Key, et al., 2006).
Considerable research demonstrates that the behavioral expressions of pain that clinicians expect to see in patients are often absent. In the late 1970s, investigators interviewed 102 adult patients with various types of pain, acute and persistent (Jacox, 1979). Many patients did not report pain and made strong efforts to conceal it. When the patients were asked whether they discussed pain with others, 70% said no or were ambivalent. When they were asked how they responded to pain, 66% said they tried to remain calm and not show pain. More than a decade later a similar finding was reported in a study of 45 patients with pain related to lung cancer (Wilkie, Keefe, 1991). Of the patients, 42% revealed that they coped with pain by trying not to let others know about it (Wilkie, Keefe, 1991).
Some researchers have attempted to use facial movements as a method of studying malingering. A review of the literature found that the results of these studies were inconsistent and concluded that it is unclear whether facial expressions of pain can be used as a reliable method for identifying malingering (Fishbain, Cutler, Rosomoff, et al., 1999). A few years after this literature review, facial expressions were studied in 40 patients with low back pain who were videotaped during rest and painful straight-leg raises (Hill, Craig, 2002). During painful movement they were asked to express their pain genuinely or to pretend that it did not hurt. Without moving, they were asked to fake pain. Although distinctions could be made between faked and genuine painful facial expressions, this research confirmed the difficulty of discriminating between them.
Patients with pain may deliberately engage in certain behaviors that are incompatible with those of the acute pain model but are helpful in coping with pain. In a study of 13 patients with pain related to advanced cancer, the patients reported that behaviors they used to control their pain included watching television (9 patients) or chatting with family and friends (Wilkie, Lovejoy, Dodd, et al., 1988). A questionnaire survey of 53 patients with persistent cancer pain asked them to identify and rate the effectiveness of the self-initiated, noninvasive pain control measures they used to cope with their pain (Fritz, 1988). Patients rated laughing as being the most effective.
Recently, researchers have identified that patients actually smile during painful situations, yet they did not find one tool for behavioral observation to assess pain that included smiling (Kunz, Prkachin, Lautenbacher, 2009). The facial expression of pain has been studied in both experimental and clinical research, and facial expression is often a part of behavioral observation tools to assess pain in both children and adults who cannot self-report. When these researchers viewed several videotapes of patients undergoing painful stimulation, one of the most unexpected findings in response to pain was the oblique raising of the lip that results in a smile. Their research revealed that the percentage of patients who smile at least once during painful stimulation ranges from 22% to 57%. A satisfactory explanation for this requires more research, but clinicians should not discount pain just because the patient smiles. Among other things, the smile could represent embarrassment over other behavioral responses to pain, an attempt to mask feelings of pain, or a willingness to endure the pain.
Sleep may be mistakenly equated with lack of pain, but even patients with severe pain may sleep. Some patients use sleep to help control their pain (Wilkie, Lovejoy, Dodd, et al., 1988). In one study, 100 patients were interviewed about the experiences of pain and sleep following abdominal surgery (Closs, 1992). Pain was the most common cause of sleep disturbance at night, demonstrating that pain occurs during sleep. Analgesics helped more patients get back to sleep than any other intervention. Also, about half of the patients felt that pain was worse at night.
Further, an appreciation of the fact that sleeping patients may have pain is demonstrated in analgesic research. When the effectiveness of analgesics is studied, trained observers ask patients to rate their pain at specific intervals, such as every hour, after the administration of the analgesic. When the observer finds the patient asleep at the time a pain rating is required, the observer awakens the patient to obtain a pain rating (Forbes, 1991).
This information does not necessarily indicate that in clinical practice sleeping patients should always be awakened to assess pain. If a patient has been given an analgesic and assessment of pain ratings afterward has shown that the analgesic has been effective, when the patient is given further doses, there usually is no need to awaken the patient to assess pain rating following a dose. Pain assessments can be made when the patient awakens. However, it may be wise to awaken the patient for the next analgesic dose if the analgesic lasts only 4 hours and the patient has continuous pain. This option can be discussed with the patient, explaining that if he or she is allowed to sleep beyond 4 hours, the pain may return to awaken him or her. If this happens, pain control is jeopardized. The patient must notify the nurse or obtain the analgesic on his or her own, take the analgesic, and then wait for it to be effective. In this scenario, a patient’s sleep is interrupted for longer than it would have been had the patient been awakened at 4 hours and given an analgesic before pain returned. Once this is explained to a patient, he or she may opt to be awakened or to wait to be awakened by pain. If the latter is chosen, a patient should be cautioned to notify the nurse immediately so the analgesic can be given. (If a patient’s sedation levels needs to be assessed, then it may be wise to awaken the patient. See the discussion of sedation levels on pp. 510-511.)
Very little research has been done to evaluate pain control using around-the-clock (ATC) dosing of analgesics versus administering them as needed, and the results are often inconclusive. However, one small study compared these two types of dosing schedules in 35 patients following abdominal surgery (De Conno, Ripamonti, Gamba, et al., 1989). Patients who received ATC analgesia had significantly better pain control, slept longer, and spent more time out of bed rather than lying down over the first postoperative week. Another study of medical inpatients compared ATC dosing of opioids to a control group and found that patients with ATC dosing reported lower pain intensity ratings than those reported by the control group yet did not take higher doses than the control group (Paice, Noskin, Vanagunas, et al., 2005).
Sedation is erroneously equated with analgesia. However, in a study of sedation and pain relief in the postanesthesia care unit, researchers found that opioid-induced sedation did not ensure adequate self-reported pain relief (Lentschener, Tostivint, White, et al., 2007). About half of the 26 patients who experienced opioid-induced sedation had persistently high pain scores in the postanesthesia care unit and during the initial 24-hour postoperative period. Further, morphine-induced sedation did not suppress the patients’ memories of early postoperative pain. Still another study confirmed that opioid-induced sedation could not arbitrarily be equated with analgesia (Paqueron, Lumbroso, Mergoni, et al., 2002). Of patients receiving intravenous morphine in whom morphine was discontinued because of sedation, 25% still had pain levels on the Visual Analog Scale (VAS) above 50. (In patients receiving morphine whose pain relief is unacceptable, a multimodal approach to analgesia should be considered; see pp. 9-10.)
Many sedating drugs, such as benzodiazepines and phenothiazines, are given to patients who are experiencing pain, but most of them provide no analgesia, and the resulting sedation may limit the amount of opioid that can be given safely to a patient in pain. Except for pain related to muscle spasm, benzodiazapines do not relieve pain. Further, available phenothiazines neither relieve pain nor potentiate opioid analgesia (APS, 2003).
When a patient’s report of pain is not accepted and acted on, an effective strategy is to ask, “Why is it so difficult to believe this patient has pain?” When the problem revolves around expecting the acute-pain model to exist, the answer is likely to be “He doesn’t look like he’s in pain.” When you find yourself thinking this or hearing someone say it, try asking, “How would this person have to act for us to believe he has pain?” A clinician might answer that a patient in that much pain would grimace or be less active. Unfortunately, expecting a patient to “act like he is in pain” may lead to those behaviors and contribute to manipulative behavior and physical harm such as disability or complications resulting from decreased function.
Causes of Pain
When a patient reports pain and the cause clearly is established, clinicians are almost always more willing to treat the pain than when the cause of pain is in doubt. Surveys of nurses’ responses to hypothetical patients who report pain show that nurses tend to assume less intense pain when no physical pathologic condition is present (Halfens, Evers, Abu-Saad, 1990; Taylor, Skelton, Butcher, 1984) and when pain is persistent rather than acute (Burgess, 1980; Taylor, Skelton, Butcher, 1984). Also, nurses take fewer actions to relieve pain in patients with persistent pain (Burgess, 1980).
Lack of Physical Evidence of Pain
A previously suggested strategy for addressing clinicians’ reluctance to accept a patient’s report of pain is also useful here. Once again, try asking, “Why is it so difficult to believe this patient has pain?” If lack of a known physical cause is the reason, the answer is likely to be “There’s no reason for this patient to hurt.” A more accurate and appropriately humble response would be that we are as yet unable to establish the cause of the pain (Teasell, Merskey, 1997).
Statements that may help us reconsider our misconceptions include a reminder that the study of pain is a new and inexact science and that it would be foolish of us to think that we will be able to determine the causes of all the pains that patients report. It also may be helpful to articulate the underlying thought process, which is “We seem to be thinking that if there is pain, there is a cause. If there is a cause, we can find it. If we cannot find the cause, there is no pain.” Once it has been stated, we begin to recognize the absurdity of this idea.
Available assessment tools are not infallible and do not exhaust all possible means for determining the causes of pain. This has been especially true of chronic noncancer pain. In a study of 60 patients with persistent pain who had been referred to a diagnostic center, the overall rate of inaccurate or incomplete diagnosis at referral was 66.7% (Hendler, Kozikowski, 1993). In particular, neuropathic pain, which is often severe burning or shooting pain, tends to be underdiagnosed. It is not detectable by ordinary diagnostic tests because nerves, not muscles or other somatic structures, are involved.
Sometimes the physical cause of pain is known, but the pain is more intense or lasts longer than expected. In the 1980s (when hospital stays were longer than they are now), a study examined the belief that postoperative pain subsides rapidly over the first 3 days and is negligible by the fourth day. Research did not support this. Of 88 patients on a general surgical unit, 31% had pain that persisted beyond day 4, often related to being older or to complications such as infection (Melzack, Abbott, Zackon, et al., 1987). These patients typically received inadequate pain control because less effective analgesics were prescribed. Probably the staff believed there was no reason for these patients to hurt that much or for that long.
Clearly, the causes of pain cannot always be determined. This does not mean that the pain is absent or that clinicians are entitled to ignore a patient’s report of pain. Pain is subjective, and it seems rather easy to engage in faulty reasoning about it. An analogy that may clarify our responsibility to such patients is our response to patients with objective symptoms that have unknown causes. For example, if a patient vomits, we may not know why it has occurred, but because it is objective (an undeniable symptom), we treat it anyway. The cause of the vomiting is sought, but meanwhile treatment, such as antiemetics, is provided. Pain deserves the same respect as objective symptoms.
Belief that Noncancer Pain Is not as Painful as Cancer Pain
When persistent pain is not associated with a terminal illness, especially when the cause of the pain is unclear, that pain seems to be regarded as being more suspicious, less painful, or less in need of relief. Doubting the trustworthiness of the patient with persistent noncancer pain is thought by some to be at the heart of most treatment problems that arise with these patients (Richeimer, Case, 2004). For example, the patient’s motive for seeking care may be under suspicion. The cause of pain may not be identifiable or obvious, and the clinician may be suspicious that a patients is seeking opioids or disability compensation (Victor, Richeimer, 2005). Negative attitudes toward patients with noncancer low back pain have been recognized for many years (Burgess, 1980; Wiener, 1975).
Even when the causes of pain are known, non-life-threatening pain is less likely to be treated than is pain associated with terminal illnesses. In one study of physicians treating patients with cancer, inadequate pain management was more likely when pain was not attributed to cancer (Cleeland, Gonin, Hatfield, et al., 1994). A mail survey responded to by 368 physicians in Michigan asked them to identify their treatment goals for acute pain, cancer pain, pain due to terminal illness, and persistent noncancer pain (Green, Wheeler, Marchant, et al., 2001). Although their goals for pain relief for terminally ill patients and patients with cancer pain were similar, the goals were significantly lower for patients with persistent noncancer pain.
Surveys of laypeople also reveal differing attitudes toward various types of pain. A telephone survey of 1000 Americans asked whether high doses of analgesics should be prescribed for any of these three conditions: severe persistent pain, cancer pain, and rheumatoid arthritis (RA) (The Mayday Fund, 1998). Approximately 80% supported high doses for cancer pain, approximately 70% supported high doses for severe persistent pain, but only about 50% supported high doses for RA. Perhaps both the public and clinicians tend to believe that some types of pain should be tolerated or are not very painful. In any case, the study reflects the tendency to provide less aggressive analgesia for one type of noncancer pain.
However, non-life-threatening conditions can result in very intense and prolonged pain. In a survey of 204 people with persistent noncancer pain, respondents revealed that their average length of time in pain was 9.5 years, with a range of 6 months to 74 years (Hitchcock, Ferrell, McCaffery, 1994). They were in pain an average of 80% of the time. For 30% the usual intensity of pain was severe, with a rating of 4 to 5 out of 5.
A common temporal classification of pain types is acute, cancer, and nonmalignant. Turk (2002) proposes that the mechanisms underlying cancer pain and nonmalignant pain are no different and that it makes no sense to discriminate between cancer pain and nonmalignant pain. He says that to do so results in paying insufficient attention to knowledge gained about cancer pain, and he suggests that greater effort be made to classify and treat pain according to the underlying mechanisms causing that pain as opposed to basing all treatment of persistent pain on whether the pain is caused by cancer. As a consequence, both cancer pain and persistent nonmalignant pain are now more often grouped together and referred to as persistent or prolonged pain.
Treating noncancer pain in the same way as cancer pain does raise legitimate concerns about the prolonged administration of medications. Certainly the chronic use of opioids remains controversial to some extent because of questions about the occurrence of addictive disease, hyperalgesia, hormonal changes, and other outcomes. (See Addiction on pp. 32-42 and Opioid Analgesics in Section IV for discussions of these conditions.) Prolonged use of nonsteroidal antiinflammatory drugs is of even greater concern than the use of opioids because of the possibility of life-threatening gastrointestinal bleeding or adverse cardiovascular events. (See Nonopioid Analgesics in Section III for a discussion of this subject.) Thus, long-term treatment by pharmacologic measures poses many challenges but cannot be ignored as a possible source of relief for persistent pain.
Implication that Anxiety or Depression Is the Cause
In a previously described study, 50 registered nurses completed an instrument that contained 60 vignettes describing patients’ illnesses or injuries (Dudley, Holm, 1984). Nurses were instructed to rate the patients on the degree of their pain and psychologic distress. Nurses inferred significantly less pain than psychologic distress, whereas in actuality, patients were experiencing both. Unfortunately, patients are then likely to receive psychologic support but not the analgesic needed to decrease pain.
When the physical cause of pain is unknown or seems insufficient to account for the severity of pain a patient reports, clinicians may attribute the pain to the patient’s emotional state and cease treating the pain. A comment that suggests this is so is, “The patient is just upset.” It is interesting that in a survey of the public’s attitude toward stress and pain, 95% of respondents agreed with the statement that stress increases pain (The Mayday Fund, 1998). Actually, evidence that stress increases pain is limited.
An erroneous and simplistic view promoted around the middle of the 20th century was that physical and psychologic causes of pain were mutually exclusive; that is, that pain is caused by either organic or psychologic factors (IASP ad hoc Subcommittee for Psychology Curriculum, 1997). Gagliese and Katz (2000) state that Melzack’s gate control theory argues against the simplistic thinking that leads to categorizing pain as being either organic or psychogenic. The authors believe that medically unexplained pain is not caused by psychopathology and that thinking that separates mind and body should be abandoned. Trying to differentiate between psychogenic and physical pain is usually fruitless. Pain caused solely by psychologic factors is rare, as is pain caused solely by physical causes. Most pain is a combination of physical and psychologic factors and is best treated as such. The subsequent discussion concerns the as yet unclear relationships between pain and anxiety or depression.
The International Association for the Study of Pain defines pain as “an unpleasant sensory and emotional experience which we primarily associate with tissue damage or describe in terms of such damage, or both” (Mersky, Bogduk, 1994, p. 210). By definition, pain is always unpleasant and always subjective, so pain is always an emotional experience. But what are the relationships between pain and various emotions? A common assumption is that anxiety or depression makes pain worse, but this is not always true.
One review of the literature found that research findings are conflicting and inconsistent regarding the relationship between depression and pain and between anxiety and pain (Zimmerman, Story, Gaston-Johansson, et al., 1996). Another literature review pointed out that a high proportion of patients with persistent pain have some kind of depressive syndrome; however, the depression may precede, follow, or develop concomitantly with the persistent pain (Dellemijn, Fields, 1994).
For the purposes of discussion, it is helpful to consider how anxiety and depression affect coping with pain as well as how they affect the intensity of pain. Although certain levels of anxiety are actually helpful in mobilizing appropriate coping mechanisms, high levels of anxiety and possibly any level of depression may adversely affect a patient’s ability to cope with pain.
Roughly 50% of patients with persistent pain also have depression or an anxiety disorder (Weisberg, Boatwright, 2007). It has been relatively well established that persistent pain is often the precipitating factor for mood disorders and for anxiety disorders, yet the interaction between persistent pain and such disorders is less well understood (Weisberg, Boatwright, 2007). Depression may adversely affect coping by causing a patient to be less motivated to engage in activities and try new ideas for rehabilitation or treatment.
Pain-related anxiety or pain-related fear may also immobilize patients. Such fear or anxiety may cause hypervigilence and avoidance of activities that a patient fears may cause pain. This concern with avoiding painful activities may result in a patient’s having difficulty freeing attention to focus on nonpainful activities (Vlaeyen, Crombez, 2007). One approach to helping these patients to improve their functioning has been exercise and graded activity programs in which patients gradually increase their physical activities. Another approach is verbal reassurance, but this can have an opposite effect unless done carefully. Simply telling patients not to worry, that they do not have a severe disease, or that their tests have shown negative results may increase their distress. Fearful patients may become more puzzled because they still hurt and no explanations have been offered. Such patients need credible explanations of why they hurt (Vlaeyen, Crombez, 2007).
How anxiety and depression influence pain intensity is an even more complicated issue. Anxiety certainly is associated with many types of pain, and depression is common in patients with persistent pain. However, the cause-and-effect relationship is unclear. Does anxiety cause pain or is it the result of pain? Is depression the cause or the result of pain? Some studies show a relationship between depression and pain, and some do not. Likewise, anxiety has been correlated with increased pain in some studies but not in others. Anxiety unrelated to pain may actually decrease pain, possibly because the anxiety increases the production of endorphins (Janssen, Arntz, 1996).
In a study of 120 patients with cancer pain, no difference was found in pain intensity measures or functional status between the depressed patients and those who were not depressed (Grossman, Sheidler, Sweeden, et al., 1991). In other words, the depression associated with pain did not alter a patient’s report of the intensity of the pain. In another study of patients who had cancer, the presence of depression, hostility, and anxiety did not correlate with the effectiveness of attempts at pain relief (Cleeland, 1984). Cleeland warns us that when patients do not respond to analgesics, clinicians should be wary of blaming this result on the patients’ depression rather than on inappropriate analgesic therapy.
The relationship between preoperative-state and postoperative-state anxiety and pain magnitude has been addressed by a few studies. (State anxiety is situational, whereas trait anxiety is a general level of anxiety.) A study of 96 patients after coronary artery bypass graft surgery examined the relationship between postoperative-state anxiety and the perception of postoperative pain. The results showed that even in the range of low to moderate state anxiety, higher anxiety postoperatively was associated with higher pain intensities (Nelson, Zimmerman, Barnason, et al., 1998). The correlations between postoperative-state anxiety and postoperative pain ranged from low to moderate but were nevertheless statistically significant. However, again, the direction of causality has not been established. In other words, although increased pain and increased anxiety may occur together, it is not always clear whether the anxiety causes the pain or the pain causes the anxiety.
An exploratory study of 24 patients undergoing varicose vein surgery examined the relationship between pain ratings and preoperative state anxiety (Terry, Niven, Brodie, et al., 2007). Pre- and postoperative-state anxiety and pain ratings in the postoperative period were significantly positively correlated. However, the actual postoperative pain ratings were relatively low, with a mean VAS rating of 28.1 mm on a 100-mm linear scale. Nevertheless, the authors point out that the findings show the need to decrease anxiety in the clinical setting whenever possible. Suggestions about how to reduce anxiety were not included.
Another small study of women undergoing termination of pregnancy investigated the links between preoperative-state and trait anxiety and pain magnitude (Pud, Amit, 2005). About 1 hour before the procedure the women completed Spielberger’s State-Trait Anxiety Inventory. Postoperatively, patients were asked to mark a 100-mm VAS at 50, 30, and 60 minutes. State anxiety was able to predict pain magnitude at 15 minutes, and trait anxiety predicted pain magnitude at 30 minutes. The authors explained this finding on the basis that state anxiety was expected to disappear when patients felt the immediate threat had been removed, whereas patients with trait anxiety needed more time to relax. The authors suggest that patients with high anxiety may require additional information relevant to the impending pain along with effective pain-relief protocols.
The belief that anxiety causes pain is reflected in the common practice of combining anxiolytics and opioids. Based on a literature review, Pud and Amit (2005) found that two methods, information giving and relaxation techniques, were beneficial in relieving anxiety and pain but that the use of pharmacologic approaches to anxiety such as benzodiazepines was more controversial. In one study, lorazepam (Ativan) preoperatively did not significantly relieve pain (Wiebe, Podhradsky, Dijak, 2003). The findings in a study of postoperative patients who had access to IV PCA morphine and midazolam (Versed) separately revealed that use of midazolam did not influence pain scores or amount of PCA morphine used (Egan, Ready, Nessly, et al., 1992). In another study of postoperative pain, the administration of diazepam (Valium) preoperatively was shown to have an ongoing antianalgesic effect on morphine analgesia (Gear, Miaskowski, Heller, et al., 1997). In a double-blind study, combinations of various doses of midazolam and meperidine were administered to 150 patients with postoperative pain and, once again, midazolam did not significantly enhance the analgesic effect of the opioid (Miller, Eisenkraft, Cohen, et al., 1986).
No doubt exists that pain results in considerable distress for many patients, causing anxiety, depression, and hostility and interfering with all domains of quality of life (e.g., Ferrell, Grant, Funk, et al., 1997; Zimmerman, Story, Gaston-Johansson, et al., 1996). Until the relationships among pain and anxiety, depression, and other emotional states are clarified, the most practical initial approach to patients who are both in pain and anxious or depressed probably is to assume that pain causes these emotional responses rather than to assume that the emotional responses cause or intensify pain. Anxiety and depression appear to be normal responses to pain. When a patient is both in pain and anxious, initial intervention probably should be aimed at reducing the pain. The APS (2003) states, “In anxious patients with pain, opioid titration should precede treatment with benzodiazepines” (p. 46). Pain relief may well reduce the anxiety and minimize the need for a benzodiazepine. Likewise, for patients who are in pain and depressed, the most logical initial approach probably is to relieve the pain, which may then reduce the depression. If anxiety or depression persists following pain relief, other interventions, such as behavioral and pharmacologic approaches, are indicated.
Addiction
Seeking Drugs versus Seeking Pain Relief
Perhaps the most common reason for not accepting and acting on a patient’s report of pain is the belief that the patient is or will become addicted to an opioid. A previously suggested strategy for addressing clinicians’ reluctance to accept a patient’s report of pain is to ask, “Why is it so difficult to believe this patient has pain?” Suspicion of addiction is often the answer. Clinicians may say, “The patient is drug seeking,” “He just wants drugs,” or “He’s getting addicted.” At present, in many clinical settings, the term drug seeking, though poorly defined, is used to mean addiction to opioids, abuse of opioids, or manipulative behavior (McCaffery, Grimm, Pasero, et al., 2005). Although patients seek drugs for many legitimate purposes, such as treating an infection, diabetes, or heart disease, somehow the term drug seeking has become associated with seeking opioids for reasons clinicians believe are inappropriate.
Questions, discussed later, that may be helpful in clarifying the confusion that surrounds beliefs about addiction are:
Definitions Related to Addiction
Tolerance to opioids and physical dependence on opioids are not the same as addiction to opioids, but these three terms are often confused. Following are the definitions proposed by the American Academy of Pain Medicine (AAPM), the APS, and the American Society of Addiction Medicine (ASAM) (AAPM, APS, ASAM, 2001; APS, 2003):
• Physical dependence is a normal response that occurs with repeated administration of an opioid for more than 2 weeks and cannot be equated with addictive disease. It is a state of adaptation that is manifested by the occurrence of withdrawal symptoms when the opioid is suddenly stopped or rapidly reduced or an antagonist such as naloxone (Narcan) is given. When the opioid is no longer needed for pain relief, withdrawal symptoms usually are easily suppressed by the natural, gradual reduction of the opioid as pain decreases or by gradual, systematic reduction, referred to as tapering (not detoxification, which is a term used when opioids are decreased in a person with addictive disease).
• Tolerance is also a normal response that occurs with regular administration of an opioid and consists of a decrease in one or more effects of the opioid (e.g., decreased analgesia, decreased sedation or decreased respiratory depression). It cannot be equated with addictive disease. Tolerance to analgesia usually occurs in the first few days to 2 weeks of opioid therapy but is uncommon after that. It may be treated with increases in dose. However, disease progression, not tolerance to analgesia, appears to be the reason for most dose escalations. Stable pain usually results in stable doses. Fortunately, tolerance to opioid-induced respiratory depression, sedation, and other adverse effects (except for constipation) occur to some degree within a few days of starting regular doses of opioids. So tolerance poses very few clinical problems.
• Opioid addiction or addictive disease is a chronic neurologic and biologic disease. Its development and manifestations are influenced by genetic, psychosocial, and environmental factors. No single cause of addiction, such as taking an opioid for pain relief, has been found. It is characterized by behaviors that include one or more of the following: impaired control of drug use, compulsive use, continued use despite harm, and craving. The diagnosis of addictive disease is not based on a single event but is based on a pattern of behavior that is observed over time. Most pain specialists and addiction specialists agree that in patients treated with prolonged opioid therapy, addictive disease is not a predictable drug effect and does not usually occur. The actual risk is not known. The disease of addiction is complex and multicausal and occurs over time, certainly not as a result of one hospital experience.
Each of these conditions—physical dependence, tolerance, and addictive disease—is a separate entity requiring different treatment. One may occur alone, any two together, or all three together. Physical dependence and tolerance are a result of repeated administration of the opioid and should be expected if regular daily doses of opioids are taken for 2 to 4 weeks or longer. Addiction, however, is a rare consequence of using opioids for pain relief.
Pseudoaddiction, as the name implies, is a mistaken diagnosis of addictive disease. The term was first used and the behaviors described by Weissman and Haddox (1989). Patients with undertreated pain may manifest behaviors very similar to those typical of addictive disease, such as escalating demands for more or different medications, repeated requests for opioids before the prescribed interval between doses has elapsed, illicit drug use (such as obtaining opioids from others), and deception (APS, 2003). Pseudoaddiction can be distinguished from true addictive disease in that the behaviors resolve when pain is effectively treated.
Likelihood of Occurrence of Addictive Disease
To address concerns about the likelihood of the occurrence of addictive disease in patients who receive opioids for pain relief, two separate questions must be addressed:
• What is the likelihood that a patient will develop addictive disease as the result of taking opioids for pain relief?
• What is the likelihood that a patient taking opioids for pain relief already has addictive disease?
Inconsistency in Defining Terms Related to Problematic Opioid-Taking Behavior
To attempt to answer either question, one must admit that it is extremely difficult to design studies about addiction and that the findings of studies done to date are certainly open to question. One of the major difficulties in interpreting and comparing these studies is inconsistency in the definitions and criteria used to determine addictive disease, opioid abuse, and other problematic drug-taking behaviors (a term usually used to cover all uses of opioids that are of concern). Many of the studies used the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV; APA, 1994) criteria for substance dependence, a term usually equated with addictive disease. This definition of addiction, however, is not the same as that proposed by AAPM, the APS, and the ASAM (2001), cited above. The term substance abuse, also used in these studies and defined by DSM-IV, is not the same as substance dependence or addictive disease. Substance abuse as it applies to opioids is generally reserved for behaviors somewhat similar to those of addictive disease but not as severe. The behaviors are destructive to the life of the individual and persist over time. They may include use of opioids in situations in which drug use is physically hazardous such as driving an automobile, or legal problems may occur such as forging prescriptions. The term substance misuse as it applies to opioids is generally reserved for behaviors less severe than substance abuse, such as calling for early refills, not taking the opioid as prescribed, giving the medication to someone else, or unauthorized dose escalation.
Since the DSM-IV (1994) does not use the term addictive disease but rather the term substance dependence, which incorporates symptoms of addiction, this creates confusion. DSM-IV (1994) incorporates symptoms of addiction. To minimize confusion, an effort has been made to resolve this (Heit, Gourlay, 2009). Pain specialists argue that dependence is too misleading because it gets confused with physical dependence, which is expected, and physical dependence differs from DSM-IV’s use of the term substance dependence. As the DSM-IV manual is being updated, pain specialists are encouraging the American Psychiatric Association DSM-5 committee to restore the term addiction (Heit, Gourlay, 2009). One of the committee’s objections to this is that the word addiction is too stigmatizing. However, in recognition of the need to clarify terminology and establish addiction as a disease (Heit, Gourlay, 2009), the National Institute on Drug Abuse (NIDA) may be changed to the National Institute on Diseases of Addiction. Another example of the term addiction being more commonly used by professionals is the creation of the ASAM. Use of the word addiction promotes understanding that it is a disease and should be treated as one. As this book goes to print, the decision of the DSM-5 committee is not known.
Despite this confusion about the meaning of terms used in research when referring to addictive disease, we review some of the studies to identify trends in the currently available information.
Development of Addictive Disease in Patients Who Are Taking Opioids
In response to the first question, the findings of several studies have shown that addiction as a result of using opioids for pain relief occurs in less than 1% of patients. Possibly the most interesting research in this area was conducted in the 1970s during a time when heroin was widely used as an analgesic in England. Heroin was used for a variety of types of pain, such as postoperative pain and pain associated with terminal illness. In fact, the popular “magic” mixture called Brompton’s Cocktail, which originated in England, contained heroin, cocaine, gin, and honey. It was modified and used in the United States in the 1970s for pain relief in terminal illness. Many clinicians are unaware that the Brompton’s Cocktail initially was used in England as an analgesic for postthoracotomy pain (Kerrane, 1975). Thus the use of heroin as an analgesic was widespread in England in the 1970s. In two studies of more than 500 patients taking regular doses of heroin orally or parenterally for pain relief for weeks or months, no patients could be documented as having become addicted (Twycross, 1974; Twycross, Wald, 1976).
In a frequently quoted study by the Boston Collaborative Drug Surveillance Program (Porter, Jick, 1980), a review of their files to determine the incidence of opioid addiction in hospitalized medical patients identified 11,882 patients of 39,946 patients who received at least one opioid preparation and had no history of addictive disease. Only 4 cases (less than 1%) of reasonably well-documented addictive disease could be identified. The findings of this study can be questioned for several reasons, such as failure to state a working definition of addiction and the difficulty of carefully evaluating such a large number of patients, but it is one attempt to answer the question of how likely it is that persons without a history of addictive disease will develop it as a result of taking opioids for pain relief.
Another large study shows the same trend. Questionnaires about pain management were sent to 151 burns units in the United States, and 181 staff members responded from 93 burn units (Perry, Heidrich, 1982). These respondents had an average experience of more than 6 years on a burn unit and represented the accumulated knowledge of at least 10,000 hospitalized burn patients. Not one case of addictive disease resulting from the use of opioids could be documented in patients with no history of addictive disease.
In a literature review of addiction as a consequence of taking opioid analgesics, findings revealed that more than 24,000 patients had been studied (Friedman, 1990). Yet only 7 (less than 1%) patients could be documented as having become addicted as a result of receiving opioids for pain relief.
In a large prospective study of 15,160 veterans who were chronic users of opioids for persistent noncancer pain, the rate of newly physician-diagnosed opioid abuse or opioid dependence was 2% (Edlund, Steffick, Hudson, et al., 2007). These patients had no diagnosed opioid abuse or opioid dependence in the previous 2 years. The investigators felt that the rate of 2% of opioid abuse or opioid dependence might be lower than the actual occurrence, but it is important to note that this figure includes both opioid abuse (such as unauthorized escalation of dose or obtaining opioids from multiple sources) and opioid dependence (a term more commonly equated with addiction) and therefore may reflect less than a 2% occurrence of addictive disease.
In a metaanalysis of studies of the safety and effectiveness of opioids in patients receiving opioids for 6 months or longer for persistent noncancer pain, 115 studies were identified but only 17 met inclusion criteria (Noble, Tregear, Treadwell, et al., 2008). Of these, only 7 studies specifically mentioned opioid addiction, but the criteria for addiction and methods of monitoring were unclear. Nevertheless, in the 2042 patients in these 7 studies, only 1 patient was reported as having possibly developed opioid addiction (Anderson, Burchiel, 1999), for an overall addiction rate of 0.042%.
In an evidence-based review of all available studies of the development of abuse, addiction, and aberrant drug-related behaviors in patients who had persistent pain and were exposed to chronic opioid analgesic therapy, only four studies preselected patients for no previous or current history of abuse/addiction (Fishbain, Cole, Lewis, et al., 2008). In these studies the percentage of abuse/addiction was calculated to be 0.19%. In other words, in patients with no previous or current history of abuse/addiction, chronic use of opioids resulted in only 0.19% of abuse/addiction.
A more recent and much better designed study examined the likelihood of addiction occurring in patients with no history of addictive disease who received controlled-release oxycodone for as long as 3 years (Portenoy, Farrar, Backonja, et al., 2007). This study was exceptional in that a systematic effort was made to identify problems in each study patient with taking drugs both before and during the study. Patients who self-reported past or present substance or alcohol abuse were excluded. In addition to self-report, some of the patients were also screened by the Drug Abuse Screening Test-20 questionnaire to establish eligibility. At each 3-month visit, physician investigators also completed brief questionnaires indicating whether patients showed any signs of problematic drug-taking behavior. If the response was positive, the physicians answered further questions and, if they were concerned about drug use, their assessments were further evaluated by a panel of experts who rated physicians’ reports as positive, possible, alleged, or negative for abuse, misuse, or withdrawal. This study revealed that of 227 patients only 6 cases (2.6%) of possible drug misuse could be identified but none (0) were actual addictive disease. In other words, there were no cases of new addiction after taking controlled-release oxycodone for as along as 3 years.
That study is not meant to reflect the likelihood of drug abuse or addiction already existing in patients with pain who are receiving chronic opioid therapy. Rather, it is, so far, the best controlled study to shed light on the likelihood that addictive disease develops in patients with pain who are treated by chronic opioid therapy and do not have a history of addictive disease or abuse. The finding was that 0 patients without histories of addictive disease developed addictive disease in the course of chronic opioid therapy. This investigation is reassuring and substantiates the findings of previous studies that suggest that the likelihood that addictive disease will develop as a result of the administration of opioids to treat pain in a population of patients with no history of addiction or abuse is likely to be below 1%.
Preexisting Addictive Disease in Patients Who Are Taking Opioids
To address the second question, how many patients being treated with opioids already had addictive disease prior to treatment with opioids, requires examining other types of studies. This question is most often raised in relation to patients with persistent pain. Again, the findings of these studies are open to question because of the recurring problem of the absence of a universally accepted definition of addictive disease.
Based on their review of the literature, Nicholson and Passik (2007) found that some studies indicate that the rate of addiction to illicit drugs, prescribed opioids, and alcohol in the population that has persistent pain is approximately the same as it is in the general population; it ranges from 6% to 10% (Savage, 2002). Other studies report a wide range of prevalence of current substance abuse or dependence in patients receiving opioids for persistent pain. As a reminder, prevalence data cannot be used to establish how many patients became addicted to opioids as a result of receiving them for pain relief.
In a review of the literature in 1992 (Fishbain, Rosomoff, Rosomoff), 24 articles were found that addressed the prevalence of substance dependence, but this information was limited in part by the lack of agreement on how addictive disease was defined. Only seven studies used acceptable diagnostic criteria for drug misuse. Six studies found that the prevalence of addictive disease ranged from 3.2 % to 16%, and one study reported 18.9% prevalence of opioid dependence. A study in Denmark revealed similar findings of a 3% to 19% prevalence of addictive disorders in patients with persistent noncancer pain (Breivik, 2005).
In a metaanalysis of five studies examining the prevalence of current substance use disorders in patients receiving opioid therapy for persistent back pain (Martell, O’Connor, Kerns, et al., 2007), only one study was judged to be of good quality (Brown, Patterson, Rounds, et al., 1996). In this study the current prevalence estimate of substance-use disorders was 23% for those receiving opioids for persistent back pain and for those with back pain who were not receiving opioids. Again, adequate definitions were lacking, but this prevalence may be high because it covers both abuse and addictive disease.
In a study of 801 patients receiving daily opioid therapy for persistent noncancer pain, the current prevalence of substance abuse or dependence (based on criteria in the DMS-IV) was 9.7% (Fleming, Balousek, Klessig, et al., 2007). The current prevalence of opioid-use disorder was 3.8%, four times higher than the 0.9% in the general population. Once more, it is important to note that this covers abuse as well as addictive disease.
The prevalence of addictive disease in acute care varies markedly between one population and another. It is estimated that addictive disorders occur in approximately 19% to 25% of hospitalized patients and in 40% to 60% of patients who sustain major trauma (Savage, 2002). Another estimate is that 25% to 40% of hospitalized patients have prior problems related to drug or alcohol addiction (Kissen, 1997).
In summary, it is difficult to determine the likelihood that patients with persistent pain already have addictive disease when they are placed on chronic opioid therapy. Studies to date are helpful but do not use the same definitions of addictive disease, so their findings are in question. Perhaps the best guess is the suggestion by Nicholson and Passik (2007), cited earlier, that the rate of addiction to alcohol and other drugs in the population with persistent pain is approximately the same as it is in the general population, and that ranges from 6% to 10%. In the acute care setting the prevalence is higher because many medical illnesses such as cancer are complications of addictive disease, and alcohol and other drugs are often involved in trauma, burns, and orthopedic injuries.
Addiction as a Stigmatizing Label: Societal versus Medical Views
An understanding of some of the stigma clinicians associate with addiction arises from confusion related to society’s general views of addiction (the moral and criminal model), which are at odds with the validated view of addiction as a disease (the disease model; Table 2-2) (Compton, 1999). Taken to its extreme, the moral model of addiction views substance abuse as a shameful behavior in which good and moral persons do not participate. It is seen as a behavior that is the individual’s fault and that an individual can freely choose that behavior or not. Because addicts in this model appear unwilling to adhere to the so-called virtuous path, they are viewed as being at fault for any negative consequences arising from their drug use and commonly are considered less worthy of care and concern than patients suffering a “faultless” disease (Malone, 1996).
Table 2-2
Models of Addiction: Society’s Views of Addiction

*This is the validated medical model to be used in clinical practice.
From Pasero, C., & McCaffery, M. Pain assessment and pharmacologic management, p. 36, St. Louis, Mosby. McCaffery M, Pasero C, Compton P. May be duplicated for use in clinical practice.
Somewhat different, but resulting in the same negative, judgmental view of addiction, is the criminal or criminal justice model. It is illegal in this country and many others around the world to use certain drugs for the purpose of experiencing their psychoactive effects, so persons possessing or ingesting drugs are criminals and are subject to punishment and imprisonment (Compton, 1999). The predominance of the criminal justice view of addiction is reflected in the high percentage of Americans incarcerated in federal (59.5%) and state prisons (22.3%) for drug offenses (Office of National Drug Control Policy [ONDCP], 1997). Although the use of alcohol is legal, laws against drunk driving and public intoxication criminalize excessive alcohol use too. It is interesting that addiction is the only recognized disease for which its sufferers risk arrest rather than medical care when impaired by its symptoms. Thus, in the criminal justice model, like the moral model, persons suffering addictive disease are characterized as bad, unethical, and deserving of criminal justice or social sanctions.
Unlike the moral and criminal justice models, the medical model views addiction as a disease, specifically as a disease acquired by the brain. Like any disease, addiction has identifiable risk factors and a demonstrated pathophysiologic basis, can be diagnosed according to a well-described cluster of signs and symptoms, follows a predictable pattern of progression, and can be managed and treated. Unlike the moral and criminal justice models, the medical model of addiction places no blame or negative social value on the sufferer. The focus is on understanding the factors that result in the disease’s development and progression and on evaluating methods of prevention and treatment. Punishing and stigmatizing addicted individuals are not viewed as being effective methods of intervention for addiction in the disease model, whereas treatment approaches, including pharmacotherapy, cognitive-behavioral skill training, group support, and lifestyle change are (Compton, 1999).
Unfortunately, drug abuse and addiction often are treated as crimes rather than as public health problems (Cotton, 1994). Consequently, clinicians judgmentally regard addicts as bad people rather than focusing on addiction as a bad disease that requires treatment. So the label of addiction is stigmatizing and should be avoided. The term addiction should be used only to refer to a brain disease that requires appropriate treatment.
When a patient is referred to as being addicted or drug seeking, it is helpful to ask, “Has the patient been diagnosed as being an addict? If so, how does this affect our plan for pain relief? How do we plan to treat the addictive disease?” If a patient is addicted, he or she still deserves the best possible pain relief that can be provided safely. The following position is unethical: I can provide safe and effective pain relief for this patient, but I will not because I do not believe the patient deserves it.
Behaviors Mistaken as Indicators of Addiction and What They Could Mean
The following material discusses patients who are labeled, not diagnosed, as being addicted or are referred to as drug seeking. These patients have not been assessed appropriately for the presence of addiction, and a diagnosis of addiction probably is not written in these patients’ charts. Yet clinicians may continue to say that these patients are addicted or are drug seeking. These stigmatizing words can adversely influence patients’ pain treatment plans and other aspects of care. Clinicians may be reluctant to accept patients’ reports of pain and may be opposed to providing pain relief.
To detect and correct this situation, several questions may be explored with the staff:
• Has the patient been diagnosed by qualified clinicians as having addictive disease?
• If this is a label, not a diagnosis, what has the patient done to cause us to believe he or she is drug seeking or addicted?
• Is there another way to explain behaviors that seem to indicate drug seeking or addiction? Acknowledge that the behaviors may mean addiction but could be something else.
• Could the patient who is seeking opioids actually be seeking pain relief?
Some clinicians seem to believe that addicted patients are easily identified by the presence of certain behaviors. The list of “aberrant drug-related behaviors that raise concern about the potential for addiction,” originated by Portenoy (1994) and subsequently revised (Fine, Portenoy, 2007), is one such list that is often misinterpreted. The list is divided into behaviors more suggestive of addiction (such as prescription forgery or multiple episodes of prescription loss) and those less suggestive of addiction (such as drug hoarding or requesting specific drugs). As Portenoy (1994; 1996; Fine, Portenoy, 2007) states, the behaviors require differential diagnosis because they may also indicate unrelieved pain or confusion about drug intake; they exist on a continuum, and no single behavior is diagnostic of addictive disease. Table 2-3 includes some of these behaviors.
Table 2-3
Misconceptions: Behaviors Indicating Addiction
| Behaviors that Are Commonly Mistaken as Indicators of Addiction or Drug Seeking | Corrections and Comments (What Else Could it Be?) |
| Patient is a “frequent flier,” often visiting one or more emergency departments (EDs) to obtain opioid analgesics. | This is not desirable behavior, but it may be caused by inadequate pain treatment. If treatment in the ED results in poor pain relief or if staff convey that the patient comes in too often, the patient may go to another ED for additional pain relief or to decrease the frequency of visits to a single ED. The patient may have persistent pain problems that are not being well managed by private prescribers, so the patient is forced to seek help in the ED. If the patient returns often to the ED, a plan should be developed and placed on file to document previous assessments, the effectiveness of treatments, and recommendations for initiating pain relief on subsequent ED visits. |
| Patient obtains opioids from another prescriber despite being told not to do so. | This is not desirable behavior, but as in the above situation, it may reflect poor pain management. For example, a patient’s prescriber may prescribe long-acting opioids plus short-acting opioids at doses that do not relieve the pain. Out of desperation, the patient may try taking additional doses (an unauthorized escalation of dose) and find that doing so successfully controls the pain. When the prescriber refuses to increase the dose, the patient may seek help from other prescribers. Obviously, a solution to this situation is for the original prescriber to increase the dose or try alternative methods of controlling the patient’s pain. |
| Patient tells inconsistent stories about pain or medical history. | It is well recognized that a patient’s memories of illnesses and treatments are often inconsistent from one time to another. This includes recall of pain and of its treatment. The patient may forget, combine, or separate incidents or may remember events as having occurred more recently than they did. Recall is influenced by intensity of pain at the time of interview and by emotional state. Cognitive impairment, adverse effects of medications, and psychiatric illness can also influence recall. To minimize the chance of inconsistent stories, a clinician may take several actions: interview the patient about pain in the same manner each time (e.g., using pain rating scales or pain assessment forms) or begin with the most recent episode and work backward in time. |
| Patient asks for refills because a prescription was lost or stolen. | Patients with cognitive impairment may misplace medications. Other patients may be unaware of the abuse potential of opioids and fail to protect supplies from friends and family members. They need to be cautioned about how to secure their prescriptions carefully. Patients should be told to make a police report when theft is suspected. However, some patients suspect theft because they have taken more than they realize and have run out of medication. The adequacy of the treatment plan then needs to be reassessed. |
| Patient is manipulative (e.g., changing behavior when clinician arrives). | Undertreatment of pain may lead to manipulative behavior. When patients report pain and clinicians do not respond by providing adequate relief, patients may become less than honest with the clinicians. This may result in behavior changes, such as more grimacing or reporting the pain at a higher intensity. |
| Patient tells nurse where to give the opioid or how fast (e.g., “Give it fast and close to the port”). | This is most likely to occur in a patient who has become familiar with this route of administration and has discovered that giving it faster and closer to the port results in faster pain relief. It is also possible that a patient enjoys some nonanalgesic effects by this method, such as sudden feelings of relaxation and reduced anxiety. |
| Patient requests analgesics by name, dose, interval between doses, and/or route of administration (e.g., “I’ll need two Vicodin every 4 hours,” or “Morphine, 10 mg IV, works best for my headaches”). | This is likely to be a well-educated patient who probably has had pain previously or has persistent pain. Patients need to be educated about all their medications, including analgesics. If the patient was diabetic and talking about insulin requirements, it would be welcomed information. The patient is providing helpful information for the pain treatment plan. |
| Patient may be a clock watcher and may ask for analgesics in advance of a specified time. The patient may say, “I’ll need my next dose in about 30 minutes.” | Sometimes analgesics are prescribed at intervals longer than their durations. When the patient asks for a dose before the interval elapses, the clinician often tell the patient how much time he or she must wait. For example, “You can’t have your next pill for 2 hours.” Because the patient must wait in pain for 2 hours, he or she is likely to note the time and ask for the medication as soon as the 2 hours pass. The patient may then find that it takes the nurse another 30 minutes to deliver the dose. A patient may work with this reality by calculating when the next dose can be given and asking for it 30 minutes in advance. This situation strongly suggests that the patient’s opioid prescription should be changed to a longer acting opioid or the interval between doses should be shortened. |
| Patient enjoys the opioid (e.g., is happy, active, or wants to leave the unit to smoke). | Once pain is relieved, it is natural for a patient to feel happier and engage in more activities, such as talking and ambulating. It may appear that a patient is “high” or euphoric, but it is simply a return to normal mood, perhaps with some elation at being in less pain. Wanting to go to a smoking area for a cigarette is certainly understandable if the patient regularly smokes. Withdrawal symptoms from nicotine can occur rather quickly and may result in a patient feeling irritable and having an intense desire to smoke. Further, nicotine has analgesic properties and may provide additional mild to moderate pain relief. Nicotine also decreases the incidence of PONV. |
| Patient says he or she is allergic to everything except one particular opioid. | Allergy to opioids is rare, but patients often mistake adverse effects, such as nausea, vomiting, and itching, for allergic reactions. These may have been poorly managed adverse effects, or patients may indeed have more severe adverse effects with some opioids than others, and these should be avoided. If a patient is convinced that he or she experiences greater effectiveness with one opioid than with others, it is possible that he or she will try to avoid the others by saying he or she is allergic to them. Even when an analgesic is not terribly effective, a patient may be afraid to try another analgesic for fear the results will be even worse. If it is not necessary for a patient to change to another opioid, he or she should receive what is preferred. If a change is necessary, perhaps because the preferred opioid has an active metabolite that is accumulating (a potential problem with meperidine), then selection of another opioid should depend on careful assessment to determine whether a patient is allergic or has experienced unmanaged or unmanageable adverse effects resulting from other opioids. |
From Pasero, C., & McCaffery, M. Pain assessment and pharmacologic management, pp. 38-39, St. Louis, Mosby. McCaffery M, Pasero C. May be duplicated for use in clinical practice.
However, Table 2-3 is based primarily on the findings of a study that attempted to identify behaviors that may cause nurses to refer to a patient as being drug seeking and to identify what nurses think the term drug seeking means (McCaffery, Grimm, Pasero, et al., 2005). The nurses believed that when the term drug seeking is used, it is very likely to mean that a patient is addicted to opioids, is abusing pain medicine, or is manipulative. The behaviors that would cause the majority of the nurses in this study (N = 369) to refer to patients as being drug seeking were: they went to various EDs to get opioids; they told inconsistent stories about pain or medical history; or they asked for a refill because the prescription had been lost or stolen. The majority of nurses (82% to 85%) agreed that drug seeking has a negative meaning, and the majority of nurses (91% to 93%) denied using the term in charting. Thus, the term drug seeking should be avoided in clinical practice. A position paper, Pain Management in Patients with Addictive Disease, issued by ASPMN (2002) recommends that the term drug seeking not be used because it creates prejudice and barriers to care.
Again, none of the behaviors in the previously mentioned study of drug seeking is diagnostic of addiction. Like the list of aberrant drug-taking behaviors, the drug-seeking behaviors may indicate pseudoaddiction, and improved pain control is recommended as one way to identify this (Passik, Kirsh, 2004; Portenoy, 1994).
The first column in Table 2-3 lists behaviors that seem to cause clinicians, especially nurses, to think that patients have addictive disease. The second column lists comments made about what these behaviors may indicate other than addiction, and suggestions are made for remedying some of the problems.
Some of the behaviors in Table 2-3 can be explained easily by simply looking at other logical options mentioned in the table. In some cases, knowledge about opioid analgesia (discussed in detail in Section IV) is helpful. Other comments in Table 2-3 revolve around some complex issues that are discussed here.
Patient Seeks Pain Relief Persistently, Repeatedly, or from Several Sources: Several of the behaviors listed in Table 2-3 probably reflect inadequate pain management, which causes a patient to make multiple or persistent attempts to obtain analgesia. Research has established that many clinicians lack knowledge of the principles of analgesic use (e.g., McCaffery, Robinson, 2002). No doubt this is a major reason for clinicians’ failing to see that a poor pain treatment plan is responsible for certain patient behaviors. Clinicians may believe that the pain relief provided for a patient should be effective. When it is not, clinicians may label the patient or blame him or her rather than the treatment plan. For example, a patient who is a clock watcher probably should receive a longer acting opioid, or the interval between doses should be changed. The patient should not be labeled as an addict for trying to make the best of inadequate analgesia by asking for it in a timely manner.
Patients who receive inadequate analgesia in the ED or from a physician should not be labeled as being addicted or drug seeking if they seek help elsewhere. One study found that one third of patients presenting to EDs with pain did not have their pain resolved (Johnston, Gangon, Pepler, 2005). At follow-up 1 week later, approximately one third of patients still could not return to normal activities. Understandably, these patients might return to the ED or seek relief elsewhere. For many patients, improving their pain-treatment plans would negate the need to return to the ED or to see other physicians.
Recognizing the limits of what can be done to help certain patients may enable clinicians to be less impatient and frustrated. In a study of 46 heavy users of EDs in two West Coast inner-city trauma-center hospitals, findings revealed that many of them were homeless, poor, or disabled (Malone, 1996). Many had no family and most had one or more chronic medical problems. It is not too surprising that these vulnerable populations of patients might not follow up on prescribed treatment or suggestions to obtain additional help or might seek pain relief from clinics or places other than the ED. Further, some patients may not be able to take time off from work to follow up on referrals to pain clinics or may not have the transportation to get there.
Some individuals who come repeatedly to EDs are seeking treatment for pain, and because they visit the ED frequently, it is likely that they have a persistent pain condition. Migraine headache, sickle cell crisis, and flares of low back pain are common. Planning for the next visit is far more practical than feeling angry with the patient or labeling him or her as being addicted when he or she returns. A carefully developed treatment plan greatly facilitates a patient’s care and discourages the tendency to label the patient because he or she has a persistent problem.
An example of an innovative approach to the care of “frequent flyers” is the use of a passport document (a small booklet or plasticized card) for patients with frequent sickle cell crises. The booklet summarizes the initial comprehensive clinical assessment and contains a written pain-treatment plan that has been developed and agreed on by patients, families, and caregivers (Beyer, Platt, Kinney, et al., 1994). The ED also may develop and have on file protocols or individualized treatment plans for other patients who are seen regularly (Nichols, 1996).
Such treatment plans help clinicians to realize the vast differences in opioid requirements and to appreciate that the same dose is not effective for everyone. For identical painful problems, some patients require eight or more times the amount required by other patients (APS, 2003). Patients with moderate to severe low back pain of noncancer origin are also frequent users of many EDs. Again, individualized treatment plans or protocols are helpful. In this group of patient, an additional aid is to assess for neuropathic pain, which seems to be commonly overlooked. While patients are waiting to be seen, an assessment tool like the ID Pain tool or the Neuropathic Pain Scale, discussed later in this section (see Forms 3-4 and 3-5, pp. 97 and 99, for copies of these tools), can be completed by the patients to help discriminate between nonneuropathic pain and neuropathic pain (Fishbain, Lewis, Cutler, et al., 2008). Differing treatment approaches must be used for pain of neuropathic origin as opposed to pain that is nociceptive. Opioids are helpful for both types of pain but often are inadequate for neuropathic pain. Adjuvant analgesics, such as certain anticonvulsants and antidepressants (see Section V, Adjuvant Analgesics), have been identified as being effective for many types of neuropathic pain.
An aggressive protocol for rapid titration of fentanyl, a short-acting opioid, is useful for many patients who present with moderate to severe pain. When these patients are discharged from the ED, careful choice of an oral opioid is essential to keep moderate to severe pain under control. The automatic use of a prescription for one to two tablets of oral hydrocodone or oxycodone with acetaminophen (e.g., Vicodin or Percocet) is often inadequate, and the patient then returns to the ED or seeks pain relief elsewhere.
Patient Gives Inconsistent Stories about Pain or Medical Histories: It is well recognized that patients’ memories of past symptoms, treatments, illnesses, and episodes of care are inconsistent from one time to another (Barsky, 2002). Yet when patients with pain report inconsistently about their pain, they may be suspected of drug seeking. Unreliability of recall is influenced by many factors, such as psychiatric condition, cognitive impairment, medication adverse effects, current pain intensity, or simple difficulty in recalling details that occurred some time ago or even recently.
Barsky (2002) states that one of the major sources of contradictory information is variance, situations in which patients answer the same question differently on differing occasions. Patient variance takes several forms:
• Patients forget and therefore underreport the incidence of previous symptoms. Memory of dates is particularly problematic, and patients may knowingly resort to guesswork. Forgetting may occur when the information is embarrassing or stigmatizing such as alcohol abuse.
• Patients have a tendency to combine multiple, separate incidents into a single one. This is called recomposition.
• Patients may engage in forward telescoping, which means that remote events tend to be displaced forward in time and are remembered as having occurred more recently.
One study looked at a health maintenance organization patients’ recall of all outpatient visits made within a 12-month period. This information was compared with their medical records. Patients recalled only 41% of the visits recorded in their medical records, and there was a 28% incidence of false recall in which patients recalled a visit of which there was no record (Means, Nigam, Zarrow, et al., 1989).
In a study of 125 patients with a long history of facial pain, self-reported dates of onset of pain were compared with onset dates that patients had reported 7 years earlier (Raphael, Marbach, 1997). The dates reported differed by more than a year in 74% of the patients. As time went by, patients underestimated how chronic their pain had been. They recalled pain as having begun more recently than had actually been recorded in their records.
A review of the literature revealed that persistent pain that varies in intensity over weeks and months may be especially difficult to remember accurately (Smith, Safer, 1993). In patients experiencing persistent pain, the intensity of present pain affects the recall of their pain and medication use for 1 day to several weeks previously. Pain is recalled as having been less severe when present pain is at relatively low intensity and as having been more severe when present pain is at relatively high intensity. Likewise, medication use is recalled as having been less frequent when a patient’s present level of pain is low. In one study, patients with persistent headache noted past pain as having been more intense when they were currently having pain than when asked about pain during a period of less intense pain (Eich, Reeves, Jaeger, et al., 1985). When pain was less intense, they remembered past pain as having been milder than they had rated it when they were actually experiencing it. Therefore, a patient’s memory of symptoms since the previous inquiry may actually depend on how severe the symptoms are at the time of the present inquiry.
The patient’s emotional state also influences memory. Researchers concluded in one study that patients who had anxiety or depression at the time of the interview were more likely to recall somatic symptoms that they had not remembered to report 1 year earlier (Simon, Gureje, 1999).
Therefore, clinicians should not be surprised that sometimes patients tell inconsistent stories about their pain. Barsky (2002) suggests several ways to minimize this effect.
• Try to interview the patient about pain in the same manner each time. Having a standardized form similar to the Brief Pain Inventory (see Form 3-2, p. 53) may be helpful.
• Interpret the patient’s responses consistently, such as clarifying what the patient means by “a lot of pain,” by using a pain rating scale such as 0 (no pain) to 10 (worst pain imaginable).
• Note the patient’s current state, such as current level of pain, anxiety, or depression, at the time of the interview. As mentioned earlier, this state can influence a patient’s report of pain. If a patient is feeling well at the time of the interview, he or she is less likely to remember details.
• Try to establish an anchor point, such as an important event (e.g., new job, graduation), for the information being recalled. Patients remember health events more easily when they have occurred close to a landmark event.
• Begin with the most recent episode and work back-ward in time.
The fact that patients may tell inconsistent stories about their pain or medical histories should come as no surprise to clinicians. Some techniques can be used to minimize this effect, but when it occurs, patients should not automatically be labeled drug seekers or addicted persons.
Patient is Manipulative: Misconceptions about the acute pain model and expectations clinicians have about how patients with pain should act were discussed on p. 27. That material is relevant here as we discuss why patients may be manipulative. In brief, research shows that clinicians expect patients to act like they are in pain by displaying certain behaviors, such as limping or frowning, and by not displaying other behaviors, such as smiling or being active. The Andrew-Robert vignette research illustrated this by comparing nurses’ responses to smiling patients and to grimacing patients. If patients’ behaviors do not meet the clinicians’ expectations, for example, if they smile while reporting severe pain, the reports of pain might not be accepted and that pain might be undertreated. In an example reported earlier in this chapter, researchers looked at the prescribing practices of physicians in a pain center; they were treating patients with persistent noncancer pain and found that the prescribing of opioids was more likely to be based on whether the patients exhibited pain behaviors than on pain severity or the extent of physical findings (Turk, Okifuji, 1997).
When patients are undertreated for pain, it is understandable that they may begin to change their behaviors in an attempt to find out how to convince clinicians that they need better pain relief. Fisher (2004) has pointed out that undertreatment of pain can easily cause a patient to be less than honest with a prescriber. When a patient is referred to as being manipulative, clinicians may mean that they suspect the patient of lying by rating pain level higher than it actually is or by claiming to be allergic to all opioids except one, so as to obtain the opioid of choice. Clinicians might also observe that a patient sometimes acts differently in the presence of clinicians than when alone or with friends. In fact, these suspicions and observations may be accurate if a patient’s pain has been undertreated when the patient acted differently. When manipulation is suspected, the question that should be asked is “Have I ignored the patient’s other attempts to get pain relief? Is the patient now changing behavior to convince me to provide better pain relief?”
Patient Enjoys Pain Relief: When some patients receive pain relief, especially relief from moderate to severe pain, their behaviors tend to change. Whereas they were quiet, immobile, or perhaps crying prior to receiving analgesia, afterward they may become active, happy, and talkative. This is normal behavior—it’s the way people act when they are not in pain. However, especially when patients have received opioids, some clinicians think this behavior means the patients are getting high on the medication.
One of the behaviors often mentioned by nurses at full-day conferences we (MM, CP) presented and noted in the commentary section of our drug-seeking research (McCaffery, Grimm, Pasero, et al., 2005) is the patient leaving the clinical unit to smoke a cigarette. Why this disturbs some nurses and is viewed as such a negative behavior is unclear. Based on our survey about drug seeking, a few nurses seem to think that going out to smoke indicates opioid addiction or that it is somehow the reason for a patient requesting opioid analgesia.
However, the desire to smoke has other more likely explanations. Regular smokers are probably physically dependent on nicotine. After abrupt cessation, a patient usually begins to crave cigarettes after a few hours. Within 24 hours of nicotine cessation, the patient may experience withdrawal symptoms, such as depression, irritability, anxiety, frustration, anger, or restlessness (Schmitz, Schneider, Jarvik, 1997). These uncomfortable feelings easily explain why the patient wants to obtain enough pain relief to be able to ambulate to an area where smoking is allowed.
Another, less well-known reason for a patient in pain to want to smoke is that some research has shown that nicotine has mild to moderate analgesic properties. In a study of 20 female nonsmoking patients who received one dose of intranasal nicotine at the conclusion of uterine surgery found that pain scores during the first day were significantly lower than those of the control group, and morphine use was less (Flood, Daniel, 2004). No hypertension or tachycardia was noted. The authors speculated that this might have occurred because the patients receiving nicotine were experiencing less pain.
That study included only nonsmokers, who could not have been in withdrawal from nicotine postoperatively. So patients with pain who smoke and want to smoke a cigarette may not simply be relieving their withdrawal symptoms. In a series of three experimental studies using a heat stimulus and nicotine nasal spray, both smokers and nonsmokers were included (Perkins, Grobe, Stiller, et al., 1994). Nicotine decreased pain sensitivity and, overall, no significant differences were noted in relation to whether the subjects were smokers or nonsmokers.
Some studies have found that the analgesic effects of nicotine are almost absent or are much lower in females than in males (e.g., Shapiro, Jarvik, 1998), but the previously mentioned study that included only females does not support this finding. However, Shapiro and Jarvik (1998) found that among men (but not women), smokers had significantly higher pain thresholds and tolerance levels than did nonsmokers. Hence, it appears that whether or not patients are smokers, nicotine is likely to have mild to moderate analgesic effects, especially in males.
One last note about the possible benefits of smoking is related to postoperative nausea and vomiting (PONV). Several studies have found that PONV is likely to be less severe in patients who have a history of smoking (Apfel, Laara, Koivuranta, et al., 1999; Gan, Meyer, Apfel, 2007). This benefit of smoking seems to be conferred on nonsmokers when a nicotine patch is used after surgical procedures. PONV was studied in three groups of patients: (1) nonsmokers who had never smoked; (2) nonsmokers who had previously smoked but had given up smoking for at least 5 years and who had received a nicotine patch (16.6 mg) perioperatively; and (3) current smokers (Ionescu, Badescu, Acalovschi, 2007). The researchers found a significant reduction in the incidence of PONV in groups two (nicotine patch) and three (smokers), and there was no significant difference between these two groups. They concluded that use of a nicotine patch might reduce the incidence of PONV.
Pain Relief from Placebos
A placebo may be defined as any medication or procedure, including surgery, that produces an effect in a patient because of its implicit or explicit intent, not because of its specific physical or chemical properties. An example is a saline injection for analgesia. When a patient responds to a placebo in accordance with its intent, it is called a positive placebo response. Administration of a medication at a known subtherapeutic dose (e.g., 0.05 mg of morphine in an adult) is also considered a placebo (Sullivan, Terman, Peck, et al., 2005).
Placebos are often appropriately used as controls in preliminary research into the effects of new medications. New drugs are compared with placebos and must demonstrate more favorable effects than placebos to warrant further investigation of or marketing of the drugs. In such studies, patients or volunteers are informed of the nature of the project and are told that placebos may be given, along with other medications. To be included in the study, persons must give informed consent. Unfortunately, placebos also may be used clinically in a deceitful manner and without informed consent.
Misconceptions about the Meaning of Positive Placebo Responses
Occasionally, when the question is asked, “Why is it so difficult to believe this patient has pain?” the answer is that the patient reported satisfactory pain relief after receiving a placebo. Pain relief resulting from a placebo is mistakenly believed to invalidate patients’ reports of pain. This may result in the patient being deprived of pain-relief measures.
Common misconceptions about pain relief resulting from a placebo are that patients are malingering (lying about pain) or the pain is psychogenic rather than being physical in origin. The fallacy of this is apparent in the results of research using placebos as controls in the evaluation of potential analgesic drugs. Beginning in the 1950s, numerous placebo-controlled analgesic studies were conducted with postoperative patients. Thus we know the answer to this question: What percentage of patients will report adequate relief of pain after having received a placebo injection the day after abdominal surgery? The answer commonly quoted is 36% (Beecher, 1955; Evans, 1974; Goodwin, Goodwin, Vogel, 1979); however, no fixed percentage of the population responds to placebos (Wall, 1992).
In fact, researchers find that the belief that overall, the positive placebo effects occur approximately one third of the time in any individual circumstance is incorrect (Moerman, Harrington, 2005). Beecher’s (1955) study (as reported in Turner, Deyo, Loeser, et al., 1994), which proposed that on average, one third of the time a placebo would relieve pain, was based on a review of 15 studies of patients suffering a variety of painful conditions; and the placebo response rate ranged from 15% to 58%. In trials of antisecretory medications for peptic ulcer disease, one study examined the 4-week endoscopically verified healing rates in 117 control groups and showed that the placebo response ranged from 0% to 100% (Moerman, 2000).
If, on average, one third of patients who have obvious physical stimuli for pain (abdominal surgery) report pain relief after a placebo injection, clearly placebos cannot be used to diagnose malingering, psychogenic pain, or any psychologic problem and cannot be used to justify denying the patient pain relief. Quite simply, all we can conclude is that sometimes placebos are effective in relieving moderate to severe pain of known physical origin.
Although it is well documented that positive placebo responses occur, why placebos relieve pain remains poorly understood. Many theories have been proposed, including operant conditioning, faith, anxiety reduction, expectation, and endorphin release (Finniss, Benedetti, 2007; Grevert, Albert, Goldstein, 1983; Levine, Gordon, Fields, 1978; Lidstone, de la Fuente-Fernandez, Stoessl, 2005; Price, Chung, Robinson, 2005; Richardson, 1994). Yet none has been proved, and the mystery remains. It is interesting that in studying the role of endogenous opioids in producing a positive placebo response, researchers have demonstrated that placebo-induced respiratory depression may occur and can be reversed by naloxone (Finniss, Benedetti, 2005).
Misconceptions about Using Placebos for Pain Relief
Because placebos can provide pain relief in a rather large portion of patients, why wouldn’t they be a legitimate option for pain relief? One reason placebos are not appropriate as pain treatment is that a positive placebo response cannot be predicted in an individual, and a placebo may be effective for one person at one time and not another (Sullivan, Terman, Peck, 2005). Although deceitful use of placebos is not recommended, efforts to enhance legitimate therapies with positive placebo effects is encouraged (Brody, 1997). This may be as simple as saying to the patient when an injection of morphine is administered, “This medication usually is a very effective way to relieve pain, and we can adjust it as necessary.” Placebo effects occur with both inert substances such as saline injections and with proven analgesics such as opioids.
Another reason for not using placebos for pain relief is that they may produce harm as well as benefit. Research shows that placebos may mimic active drugs, may produce adverse effects (such as respiratory depression, as mentioned earlier) and toxic reactions, may worsen symptoms (negative placebo effects), and may directly affect many body organs (Benedetti, Amanzio, Baldi, et al., 1998; Lavin, 1991; Wolf, Pinsky, 1954). Therefore, substituting a placebo for an opioid cannot be justified on the basis of avoiding adverse effects, such as sedation or preventing addiction.
Unfortunately, placebos are most likely to be used in patients the staff regard as being difficult. The most extensive study of placebo use was undertaken in the 1970s (Goodwin, Goodwin, Vogel, 1979). Researchers surveyed placebo knowledge of and use by 60 physicians and 39 nurses in a university teaching hospital. Most of the physicians (78%) had ordered placebos, and most of the nurses (82%) had administered them. The reasons given for administering placebos revolved around proving the patients “wrong” and administering to patients who were disliked, who did not obtain pain relief by standard treatment, or who had frustrated or angered the staff. Not only does this deceitful use of placebos in place of appropriate therapy violate patients’ rights to the highest quality of care possible, it clearly poses a moral, ethical, and professional danger to the clinicians.
Perhaps the most important reason for not using placebos in the assessment and treatment of pain is that deception is involved. The clinician lies to the patient. Deceit is harmful to both patients and health care professionals. When discovered (and it usually is), it may permanently damage a patient’s trust in health care professionals.
Legal and ethical considerations involved in the deceitful use of placebos in pain management include liability for fraud, malpractice, breach of contract, and medical negligence (Fox, 1994). Nurses should examine their nurse practice acts and the policies of their state boards of nursing for guidance. For example, in 1997, the Board of Registered Nursing in California specifically stated that the use “of placebos for management of pain would not fulfill informed consent parameters” (p. 12).
Placebo use violates the rights of all patients who receive them outside of the context of approved clinical trials in which patients have given informed consent to participate. Even under these circumstances, placebo use may not be justified when the drug being studied could be evaluated by comparing it with an effective medication instead of a placebo (Hampton, 2006; Rothman, Michels, 1994; Sullivan, Terman, Peck, 2005).
There are no individuals for whom and no conditions for which placebos are the recommended treatment. Literature review shows no basis for the assumption that placebo pain medication is useful to patients (Kleinman, Brown, Librach, 1994). Therefore, the use of placebos inevitably deprives patients of more appropriate methods of assessment or treatment. In other words, there is always a better way to assess and treat patients with pain than to administer a placebo.
Recommendations against the Use of Placebos
Avoidance of deceitful use of placebos is recommended in the APS clinical guideline “Principles of Analgesic Use in the Treatment of Acute Pain and Cancer Pain”: “Do not use placebos to assess the nature of pain. . . . The deceptive use of placebos and the misinterpretation of the placebo response to discredit patients’ pain reports are unethical and should be avoided” (APS, 2003, p. 37).
Nursing organizations have issued placebo policy statements that strongly recommend against the use of placebos in pain management. The Oncology Nursing Society (ONS) (McCaffery, Ferrell, Turner, 1996) developed the first position statement. Soon afterward, 27 or more professional organizations, such as the National Association of Orthopaedic Nurses (NAON), endorsed the statement. The ONS position statement focuses on patients with diagnoses of cancer, but the article accompanying its publication verifies that the facts and ethical issues presented are equally applicable to other patients with pain, regardless of their diagnoses.
The ASPMN (2004) adopted a position statement on placebo use similar to that of ONS, the primary difference being that the opposition to placebo use in pain management applies to all patients, regardless of age or diagnosis. The ASPMN’s position statement says, “placebos should not be used by any route of administration in the assessment and/or management of pain in any individual regardless of age or diagnosis” (p. 1). Box 2-2 summarizes the key elements to consider concerning the use of placebos in clinical practice.
Debate about Use of Placebos in n-of-1 Trials
In 2005, the APS issued a position paper about placebo use that was in agreement with the statement by the ASPMN except for one thing—to find the least invasive or damaging treatment for patients. The APS states that “trials involving alternation between active and placebo treatments in a single patient (known as “n-of-1 trials”) might provide clinically useful and scientifically valid information” (Sullivan, Terman, Peck, et al., 2005, p. 216). n of 1 literally means the number of subjects in the trial is 1. In such cases, treatment decisions might be made on the basis of a positive placebo response, and a patient might be denied active treatment. The APS states that ethically, such treatment requires a patient’s consent, so a patient should be informed prior to the treatments that a placebo will be used as one of the treatments.
As an example, a patient who is being evaluated for an anesthetic block or injection would be told that a placebo would be used, and then the patient might receive a placebo injection of saline and later an injection of local anesthetic. If the patient responded positively to the placebo, active treatment using a local anesthetic might be denied. This does not seem consistent with other parts of the APS position that include the statement that “placebo response does not prove that pain is not ‘real’ or unworthy of any real treatment” (Sullivan, Terman, Peck, 2005, p. 216). The APS also states that placebo use might “delay specific treatments, thereby leading to disease or symptom escalation” (Sullivan Terman, Peck, 2005, p. 216). Thus, the APS seems to contradict its own statement about making a decision to withhold treatment based on a positive placebo response.
The ASPMN does not agree with the APS’s support of the n-of-1 trial for some of the same reasons stated earlier by the APS as well as for other reasons (McCaffery, Arnstein, 2006). The ASPMN’s position statement on use of placebos in pain management (2004) says that the ASPMN “supports the use of placebos only in Institutional Review Board (IRB)-approved clinical trials” (p. 1). Further, this policy states that “placebo use for the assessment and/or treatment of pain, including the evaluation of response to pain treatments, constitutes fraud and deception” (p. 4). The ASPMN agrees with the need to identify patients who will not benefit from treatments that are irreversible or invasive, but it does not agree that the n-of-1 trial accomplishes this. There is no research that provides strong support for this method of determining whether to provide a patient with active treatment for pain relief.
N-of-1 trials pose several problems. The sequence of injections (placebo versus local anesthetic) is not random; clinicians are not blinded as to which is being injected; the trials are not done with the oversight of an IRB; and the effects when a placebo is administered might be caused simply by the puncture or the instillation of fluid (McCaffery, Arnstein, 2006). Also, patients may respond positively to placebos once and never do so again. Further, patients who do not get pain relief from the local anesthetic the first time it is administered might respond later after proper dosing and titration. And, finally, the placebo response tends to be more common and more pronounced with highly invasive procedures such as spinal nerve blocks. In one study of seven patients with complex regional pain syndrome who were receiving a nerve block with saline followed by a local anesthetic, significant pain relief occurred after saline in six of the seven patients (Price, Long, Wilsey, et al., 1998). Thus, in n-of-1 trials involving invasive procedures, the positive placebo effect is very likely to occur and would not seem to be an adequate reason for denying patients active treatment.
An even greater danger exists when n-of-1 trials are supported. It opens the door to uses of placebos in situations other than invasive procedures. For example, hospitalized patients with cancer pain caused by widespread metastases might be taking large doses of opioids and, under the conditions of an n-of-1 trial, could be given placebos. If patients reported pain relief, the opioids might be discontinued (McCaffery, Arnstein, 2006).
Institutional Policies Concerning Placebos
For the protection of patients and staff, written institutional policies about placebo use appear to be essential. The current frequency of deceitful or inappropriate placebo use in the assessment and treatment of pain is unknown, but it undoubtedly exists. Articles on this topic have appeared regularly through the years in the professional literature, and misunderstandings persist (Arnstein, 2006; Fox, 1994; Fox, McCaffery, Ferrell, et al., 1995; Grace, 2006; Haddad, 1993; Kleinman, Brown, Librach, 1994; McCaffery, Arnstein, 2006; McCaffery, Ferrell, 1994a; McCaffery, Ferrell, 1997b; McCaffery, Ferrell, Pasero, 1998; McCaffery, Pasero, 1995; Oh, 1994; Wall, 1993).
Surveys of nurses reveal that they are susceptible to becoming participants in deceitful use of placebos. Surveys completed by 601 nurses during 1995 revealed that a significant percentage of these nurses exhibited a high level of readiness to participate in deceitful placebo use (McCaffery, Ferrell, 1996b). About one of five nurses (19%) thought pain was a clinical problem for which placebos could be used. Even more respondents (27%) endorsed placebo use for anxiety; 25% believed placebos were appropriate in patients whose symptoms (pain) were questionable. Clearly, if placebos were suggested or prescribed, many nurses would participate in the action.
All hospitals and other health care agencies should have written policies that address unethical use of placebos. When policies are in place, communication among health care providers is less difficult. If placebos are prescribed, the nurse may use the existing placebo policy as a point of departure for communicating concerns about the patient’s quality of care and for explaining why a nurse will not administer placebos, even when a prescription exits.
The ASPMN’s (2004) position paper concerning placebos states that a policy is necessary to ensure that no patient receives a placebo unless it is in the context of an IRB-approved clinical trial. This should include mechanisms for reporting a clinician who violates the policy and the actions to be taken to censure such an individual. The policy should also provide a means of protecting any person who reports inappropriate use of placebos or refuses to participate in the use of placebos outside the context of an IRB-approved clinical trial. Other guidelines for developing a policy may be found in the ASPMN (2004) position paper and in Box 2-2.
Other Hidden Biases and Misconceptions
Biases and misconceptions that lead to inadequate pain management are not new. They are evident in recent history. In the late 1800s, many physicians and scientists believed that cultural and intellectual development were accompanied by increased sensitivity to pain (de Moulin, 1979). In 1892, Mitchell said, “The savage does not feel pain as we do.” Mitchell suggested that increased capacity for feeling pain began at the end of the 18th century. Van den Berg (1963) was more exact, stating that pain sensitivity increased between 1780 and 1845. Racial and class prejudices were also apparent. In the Old South of the 1700s and 1800s, black people were considered insensitive to pain and underwent limb amputation without anesthesia. However, upper-class white women were believed to have “delicate nerves,” and the same surgeon would use ether or chloroform to deliver a baby (Pernick, 1985). No doubt many of these physicians believed that they were practicing medicine in professionally and ethically acceptable ways. Clinicians today probably believe the same thing about their clinical practices, but what will be said of our pain management in the year 2099?
Do we know when our biases are showing? Following is a brief overview of research findings related to some biases that are likely to escape conscious awareness but influence clinical practice:
• Physical appearance. Physicians infer more pain in unattractive patients and in those who express pain (e.g., by facial expression); they infer less pain in attractive patients and in those who do not express pain (Hadjistavropoulos, Ross, von Baeyer, 1990).
• Gender. Most studies seem to show that women are more likely to be undertreated for pain than are men. Workups by physicians in response to five common types of pain, including back pain, headache, and chest pain, were found to be significantly more extensive in men than in women (Armitage, Schneiderman, Bass, 1979). In a study of cancer patients, inadequate pain management was more likely in females than in males (Cleeland, Gonin, Hatfield, et al., 1994). In a study of differences between patients and physicians in their assessment of pain intensity in an ED, patients and physicians rated pain on arrival and on discharge from the ED (Marquie, Raufaste, Lauque, et al., 2003). Gender played a complex role, but one finding was that male physicians rated the pain of female patients as being less than that of male patients when the cause of pain was obvious. Using vignettes of patients with surgical pain, nurses’ medication choices for men and women were compared, revealing that nurses were significantly more likely to undertreat pain in women than in men (Cohen, 1980). In a survey of 368 physicians in Michigan, using vignettes of patients with pain, significantly more physicians chose optimal pain treatment for men than they did for women (Green, Wheeler, 2003). Physicians more frequently chose the optimal pain management response for men undergoing prostatectomy (56.2%) than for women following myomectomy (42%) and following cesarean section (44.5%). They also chose the optimal pain management response more frequently for metastatic prostate cancer (16.3%) than for metastatic breast cancer (10.7%) However, gender biases are not always predictable. In one study of patients with persistent nonmalignant pain, men were no more likely than women to receive opioids (Turk, Okifuji, 1997).
The gender of the clinicians may further complicate assessment. In one laboratory study, men reported significantly less pain to female experimenters than to male experimenters (Levine, De Simone, 1991). In another experimental study, volunteer subjects tolerated pain longer when they were tested by an experimenter of the opposite sex (Kallai, Barke, Voss, 2004).
• Age. In a study of 56 men who were 65 or older and undergoing major elective surgery, 16 received no parenteral postoperative analgesics (Short, Burnett, Egbert, et al., 1990). On the basis of vignette research, reports of severe pain are more likely to be accepted from elderly than from young-adult patients, but the elderly are less likely to receive adequate doses of opioid analgesics (McCaffery, Ferrell, 1991b). In a study of 1492 nursing homes, data from 13,625 patients with cancer who were 65 or older revealed that 4003 reported daily pain and more than 25% of them received no analgesic. One of the risk factors for failing to receive any analgesic included being 85 years old or older (Bernabei, Gambassi, Lapane, et al., 1998). In a study of 1360 patients admitted to the ED with documented pain, patients older than 50 years were half as likely to receive analgesia as were younger patients (Heins, Grammas, Heins, et al., 2006). The undertreatment of older patients continued with regard to discharge analgesics.
• Race/culture. Racial and ethnic minorities are at high risk for receiving inadequate pain relief. A longitudinal study that took place between 1993 and 2005 analyzed data from the National Hospital Ambulatory Medicine Care Survey and showed that the number of patients who received opioids to manage pain increased steadily and significantly (Pletcher, Kertesz, Kohn, et al., 2008). However, the use of opioids in nonwhites and Hispanics lagged behind that in whites. For the 13-year period, the overall rate of opioid use was 31% for whites, 23% for blacks, 24% for Hispanics, and 28% for Asians and others. In 2005, the rate for whites was 40% but was 32% for all others. In a survey of an ED in a large teaching hospital, Hispanic patients were twice as likely as non-Hispanic whites to receive no analgesia (Todd, Samaroo, Hoffman, 1993). In a later study of ED practices, 90 white and 127 black patients with extremity fractures were compared regarding the likelihood of their receiving analgesics, and the findings were similar, with 74% of whites and 57% of blacks receiving analgesics, despite similar reports of pain in their medical records (Todd, Deaton, D’Adamo, et al., 2000). In other words, disparities in the prescription of analgesics could not be attributed to differences in pain assessments. In a study to determine whether differences in waiting times for pain treatment in EDs existed in adults of ethnic and minority groups suffering from long-bone fractures, 234 European-American, African-American, and Hispanic patients were included (Epps, Ware, Packard, 2008). All reported substantial pain caused by long-bone fractures, but a significant difference in waiting times was found between Hispanic and European-American patients; Hispanic patients waited an average of 102 minutes for the first dose of analgesic, and European-Americans waited an average of 67 minutes, a 35-minute difference. The African-American group was too small for any conclusions to be drawn about that group. These last two studies clearly indicate that assessment is not enough and, indeed, that patients’ reports of pain might not be accepted by clinicians. Hence, pain relief is not forthcoming or is delayed. In a study of outpatients with metastatic cancer who were treated at 54 locations, patients seen at centers treating predominantly minorities were three times more likely than those treated elsewhere to have received inadequate pain management (Cleeland, Gonin, Hatfield, et al., 1994). In other words, in centers treating predominantly minorities, cancer patients were found to be three times more likely to have inadequate pain management (Cleeland, Gonin, Hatfield, et al., 1994). In a study of outpatients with cancer pain, 65% of minority patients did not receive appropriate analgesic prescriptions for their pain, compared with 50% of nonminority patients (Cleeland, Gonin, Baez, et al., 1997). Hispanic patients, in particular, were less likely to experience adequate analgesia. In a study of 454 patients with postoperative pain who were receiving IV PCA opioids, the amount of opioid prescribed for Asians, blacks, Hispanics, and whites differed, with blacks and whites being prescribed more analgesic than Hispanics. No differences were found among these groups as to the amount of opioid self-administered (Ng, Dimsdale, Rollnik, et al., 1996).
A review of the literature concerning studies of racial disparities in pain management acknowledged that disparities do exist but that the magnitude of the disparities was, for the most part, small (Ezenwa, Ameringer, Ward, et al., 2006).
• Personal experience with pain. In a study of vignette descriptions of patients, nurses who had experienced intense pain inferred higher levels of pain in patients than did nurses who had not experienced intense pain (Holm, Cohen, Dudas, et al., 1989). By contrast, a study of 29 nurses who had not undergone any surgery and 33 who had undergone surgery found that nurses who had not experienced wound pain resulting from surgery estimated the intensity of wound pain to be higher than did those who had experienced surgical wound pain (Ketovuori, 1987). A study of nurses, physicians, and medical and nursing students in medical centers in Israel were given questionnaires that elicited demographic information along with whether or not they had consumed opioids (Pud, 2004). Those who had consumed opioids considered pain management a lower priority than did the group that had not consumed opioids.
• “Irresponsible” lifestyle. In a vignette survey, 452 nurses’ answers about assessments and analgesic choices for patients described as risk takers, consumers of alcohol, or unemployed were compared with their responses to patients described as typically middle class (McCaffery, Ferrell, O’Neil-Page, 1992). The nurses said they themselves would provide the same care for both kinds of patients, but they believed their colleagues would treat the patients differently, tending to disbelieve and undertreat the “irresponsible” patients. Nurses revealed that they did not want personal values to interfere with their quality of care, but that they needed assistance (e.g., permission to say they do not like a certain patient and discussion about how to prevent this from interfering with care).
In a study of 167 nurses working in the medical-surgical and critical care units and in EDs, two vignettes were used to determine whether the level of social acceptability of a patient’s behavior at the time of injury would affect pain management (Hazelett, Powell, Androulakakis, 2002). In one vignette, the patient injured himself while playing football with his son. In the other vignette, the patient injured himself while driving intoxicated, a less socially acceptable behavior. The vignettes were randomly distributed, and each nurse received one vignette. Significantly more nurses would have correctly increased the dose of morphine for the patient who had injured himself playing football with his son compared with the patient who had injured himself while driving intoxicated.
Conclusion
The preceding discussion of biases, misconceptions, and misunderstandings that cause clinicians to doubt a patient’s report of pain and refuse to take action to relieve pain covers only a few of the many misunderstandings that may lead to inadequate pain management. Some of them are well hidden from consciousness and very difficult to correct. It is, therefore, foolish to believe that we provide an equally high quality of care for all of our patients who are experiencing pain, regardless of our personal values, preferences, or painful experiences. No doubt all of us have conscious and unconscious biases and misconceptions that may adversely affect the care we provide for patients with pain.
This sounds like a harsh judgment of humankind, but it may be true. A veritable mountain of literature published during the past three decades attests to the undertreatment of pain. Much of this literature is consistent with the hypothesis that human beings, including health care providers in all societies, have strong tendencies or motivations to deny or discount pain, especially severe pain, and to avoid relieving the pain. Certainly we should struggle to identify and correct personal tendencies that lead to inadequate pain management, but this may not be a battle that can be won. Perhaps it is best to assume that there are far too many biases to overcome and that the best strategy is to establish policies and procedures that protect patients and ourselves from being victims of these influences. The next chapter includes some simple tools that can be made mandatory in clinical practice as some ways to hold clinicians accountable for assessing and treating pain.