Nonprescription Nonopioids
JUDGING from the rows of nonprescription analgesics found in a well-stocked pharmacy, millions of Americans are self-medicating with nonopioids to treat their aches and pains. As a group, these analgesics are marketed to the public as “pain relievers.”
Many nonprescription pain relievers are merely different doses of either acetaminophen or aspirin. Quite a few contain both aspirin and acetaminophen. The combination of acetaminophen and aspirin has raised concern about enhanced renal toxicity, and some find this combination questionable. Other common ingredients in nonprescription pain relievers are buffering agents, caffeine, and antihistamines. (See Patient Medication Information Forms III-4 and III-5 on pp. 256-259).
Buffered Aspirin
Buffered aspirin has been compared with nonbuffered aspirin, and endoscopic evaluation shows no difference in the amount of gastric damage produced by either one. Enteric-coated aspirin does not decrease the incidence of local irritation either (Bhatt, Scheiman, Abraham, et al., 2008; Laine, 2001). Nevertheless, some individuals report less dyspepsia and prefer these formulations.
Caffeine
Caffeine has been used to augment pain relief since research in the early 1980s showed that it produced a 40% acetaminophen dose-sparing effect (Zhang, 2001). Other research found that caffeine in combination with acetaminophen (but not alone) produced a significant enhanced and prolonged analgesic effect with onset of analgesia within 30 minutes and lasting for up to 3 hours (Renner, Clarke, Grattan, et al., 2007). Caffeine produces a synergistic effect with a variety of other analgesics as well, including opioids and NSAIDs (Diamond, Freitag, 2001; Mitchell, van Zanten, Inglis, et al., 2008). Consumption of caffeinated coffee has even been found to enhance the analgesic effect of nicotine (Nastase, Ioan, Braga, et al., 2007) (see Section V for more on nicotine analgesia). The underlying mechanisms of caffeine’s action are unclear, but theories include that it blocks adenosine receptors, inhibits COX-2, or simply changes emotional state (Zhang, 2001).
Caffeine is often combined with nonopioids for the treatment of migraine and tension-type headaches. Combinations produce better pain relief than any of the analgesics alone for this type of pain (Diamond, Balm, Freitag, 2000; Diamond, Freitag, 2001). Research has not confirmed the optimal dose of caffeine for increasing the analgesia of nonopioids, but the minimal effective dose appears to be 65 mg (APS, 2003; Zhang, 2001). While nonopioid analgesia may be enhanced with this amount of caffeine, patients often also experience dizziness and nervousness (Zhang, 2001).
A randomized controlled study found that a single dose (2 tablets) of the combination of aspirin (250 mg), acetaminophen (250 mg), and caffeine (65 mg) produced significantly better and faster pain relief than a single dose of ibuprofen (200 mg) or placebo for acute migraine (Goldstein, Silberstein, Saper, et al., 2006). In contrast, a study of patients following outpatient general surgery found that acetaminophen plus ibuprofen produced fewer adverse effects and higher patient satisfaction than the combination of acetaminophen, codeine, and caffeine (Mitchell, van Zanten, Inglis, et al., 2008). However, the difference in findings in this study may have been due to codeine, which is associated with a relatively high incidence of adverse effects and wide variability in efficacy due to dependence on CYP2D6 metabolism; 10% of Caucasians are poor metabolizers and derive little or no analgesia from codeine (Stamer, Stuber, 2006) (see Chapter 13 for more on codeine). Other research supports the use of caffeine in the postoperative setting; randomized-controlled trials and an extensive meta-analysis of 30 trials concluded that caffeine significantly enhanced postoperative pain relief when combined with other analgesics (Fitzgerald, Buggy, 2006). Despite this evidence, caffeine is rarely prescribed as a component of a postoperative multimodal analgesic regimen.
IV caffeine has been researched in advanced cancer patients experiencing adverse psychomotor effects associated with high doses of opioids and found to have little effect (Mercandante, Serretta, Casuccio, 2001). Pain significantly decreased, but this was not statistically superior to placebo. Caffeine produced improvements in speed of finger tapping, but there was no improvement in cognitive outcomes as measured by testing of visual memory and number and digit recall. (See Section V for use of caffeine to counteract opioid-induced sedation.)
Because caffeine is a CNS stimulant, its effect on the cardiovascular (CV) system has been a concern. Some adults are very sensitive to caffeine and respond to even small doses with tremors, increased heart rate, and insomnia. Individuals with CV risks must exercise caution in the amount of caffeine they consume or avoid it altogether. For most people, moderate intake of caffeine (e.g., 200 to 300 mg, which is more than the amount required to augment nonopioid analgesia) is considered safe (Mayo Clinic.com, 2008). Heavy use (500 to 600 mg/day; 5 to 7 cups of coffee/day) is associated with a significant increase in adverse effects. Chronic repeated exposure to caffeine can increase the risks for development of analgesic-overuse headache, chronic daily headache, and physical dependency (Shapiro, 2008). Discontinuing caffeine after long-term use can lead to a withdrawal syndrome with headache as a dominant symptom.
An inexpensive way to reap the benefits of a caffeine/nonopioid combination is to avoid commercially compounded preparations and simply buy generic forms of the ingredients separately (e.g., aspirin and acetaminophen) and ordinary caffeine-containing beverages as a source of the dose of caffeine required for enhanced analgesia. Individuals often report resolution of pain, particularly headache pain, after drinking caffeinated beverages. Stimulant products also often contain a sufficient amount of caffeine (e.g., No-Doz has 200 mg caffeine).
A variety of food products contain caffeine. Chocolate lovers may be dismayed to learn that most chocolate contains very little caffeine (e.g., 9 mg). However, 1.45 ounces of dark, semisweet chocolate contains 31 mg, requiring at least 3 ounces to obtain the desired analgesic effect. The popularity of energy drinks, which contain large amounts of caffeine (e.g., 300 mg), has prompted calls for changes in labeling to include stronger warnings of the dangers of excessive caffeine intake (Johns Hopkins Medicine, 2008). See Table 9-1 for the caffeine content of common beverages, food, and over-the-counter (OTC) drugs.
Table 9-1
Content of Caffeine in Selected Beverages, Food, and OTC Drugs
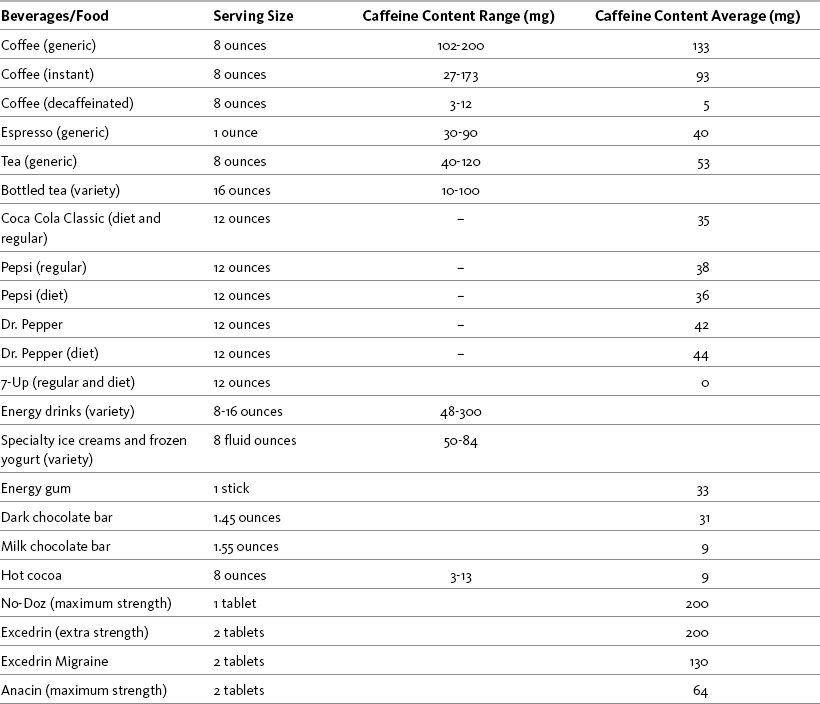
Note: The analgesia of a dose of nonopioid may be augmented by 65 to 200 mg of caffeine. The ideal caffeine dose has not been established.
From Pasero, C., & McCaffery, M. Pain assessment and pharmacologic management, p. 241, St. Louis, Mosby. Data from Center for Science in the Public Interest. Available at http://www.cspinet.org/new/cafchart.htm. Accessed December 24, 2008; Energy fiend. Available at http://www.energyfiend.com/the-caffeine-database. Accessed December 24, 2008. Pasero C, McCaffery M. May be duplicated for use in clinical practice.
Analgesic Nephropathy
For several years, concerns have been raised regarding the potential for caffeine, co-formulated with nonopioids, to initiate or sustain analgesic overuse causing kidney disease (Feinstein, Heinemann, Dalessio, et al., 2000; Fox, Seibers, 2003; van der Woude, Heinemann, Graf, et al., 2007). This is widely referred to as “analgesic nephropathy” (van der Woude, Heinemann, Graf, et al., 2007). Extensive reviews of the literature, case control studies, and expert panel opinions have concluded that there is insufficient evidence to support the claim that caffeine (in the absence of phenacetin) causes analgesic nephropathy or that such a phenomenon even exists since clear criteria for it have not been established (Feinstein, Heinemann, Dalessio, et al., 2000; Fox, Seibers, 2003; Michielsen, Heinemann, Mihatsch, et al., 2009; van der Woude, Heinemann, Graf, et al., 2007; Zhang, 2001).
The true culprit of the controversy appears to be a chemical substance called phenacetin, which was introduced in the late 1800s as an analgesic and antipyretic. In the 1970s and 1980s it was available in combination with other agents, such as aspirin and caffeine, for OTC analgesia. Phenacetin has psychotropic properties, which have been blamed for reports of abuse and overdosing of phenacetin-containing analgesics (Fox, Siebers, 2003). The chemical was withdrawn from the U.S. market in 1983 after research and clinical observations showed an association between phenacetin and renal pelvis and bladder cancer (DHHS, 2008). There have been no approved commercially-available products containing phenacetin since its withdrawal.
Antihistamines
As discussed early in Section I, histamines promote pain transmission (Raffa, 2001). Further research is needed to clarify the underlying mechanisms, but three histamine receptors appear to be primary sites of action. The antinociceptive properties of morphine were enhanced in H1 and H2 receptor knockout mice (H1 and H2 receptors are genetically “turned off”) indicating a role (Mobarakeh, Takahashi, Sakurada, et al., 2006). The analgesic action of the centrally-acting nonopioid nefopam was modulated by H3 receptors but not H1 and H2 receptors in other animal research (Girard, Pansart, Coppe, et al., 2004).
Despite histamine’s role in nociception, antihistamines are poor pain relievers, and there is little evidence to support common claims of enhanced pain relief and dose-sparing effects when they are combined with other analgesics (APS, 2003; Fitzgerald, Buggy, 2006). For example, very little research could be found to support the use of antihistamines for analgesia in the postoperative setting; however, the phenothiazine-derived antihistamine promethazine (Phenergan) is commonly used in the postoperative setting based on a misconception that it potentiates opioid analgesia (APS, 2003). One study of patients following abdominal hysterectomy showed that those who received preoperative promethazine (0.1 mg/kg) consumed less postoperative PCA morphine than those who received the same dose of the drug postoperatively (Chia, Lo, Liu, et al., 2004). However, another study found no differences in opioid-sparing effects among patients following abdominal hysterectomy who were randomized to receive different amounts of diphenhydramine (Benadryl) or placebo at induction plus morphine PCA postoperatively (Lin, Yeh, Yen, et al., 2005).
The use of antihistamines in the postoperative setting, where patients are given anesthesia and other sedating medications, may be dangerous because it can produce an additive sedative effect and increase the risk of respiratory depression (Anwari, Iqbal, 2003). Patients must be watched very closely and antihistamine administration discontinued if increasing sedation is detected (see Chapter 19).
Antihistamines, such as phenyltoloxamine and diphenhydramine, are commonly combined with nonopioids, such as acetaminophen, aspirin, and ibuprofen, and promoted as night-time pain relievers (Table 9-2). Occasionally, antihistamines are administered for bone pain associated with chemotherapy-induced neutropenia when other strategies are ineffective; however, research is lacking on this approach (Pangilinan, 2008).
Table 9-2
Selected Nonprescription Analgesics: Acetaminophen, Aspirin, and Combination Analgesics
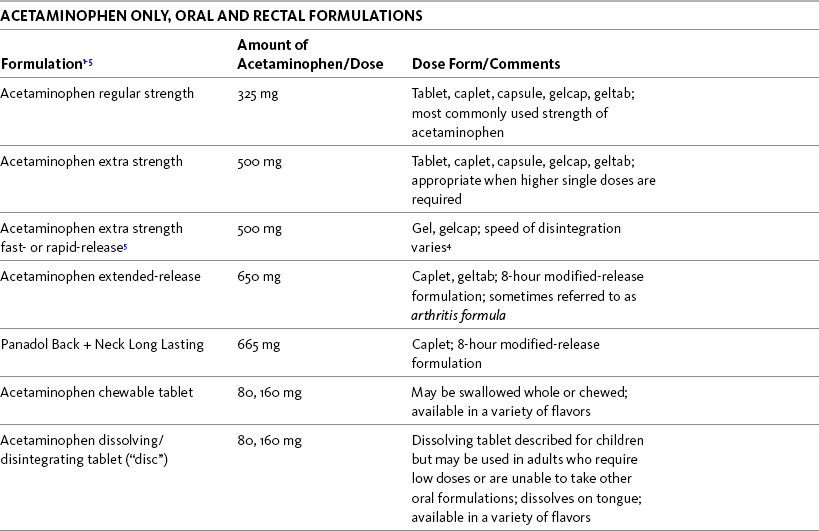


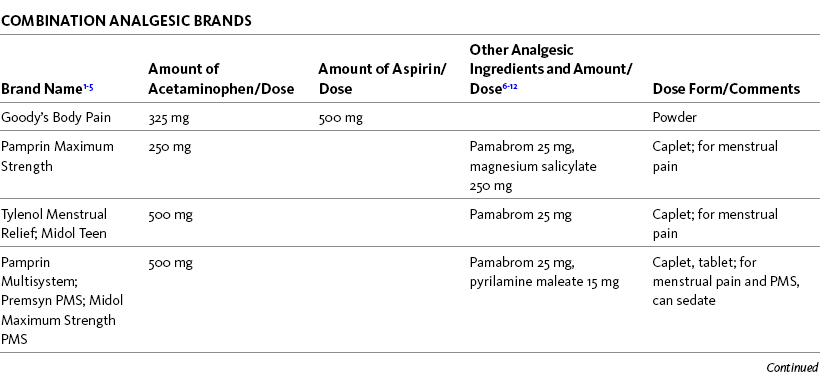
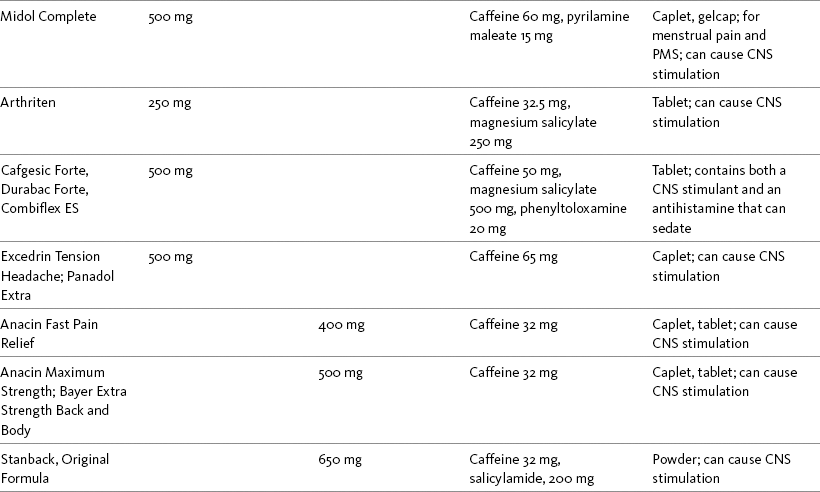


1The nonopioids listed in this table are available under a variety of brand names and manufactured by a variety of pharmaceutical companies. Active and inactive ingredients vary, and some oral solutions contain alcohol and dyes. Product availability changes frequently; this is not intended to be a comprehensive list. Reading specific product labeling is recommended.
2Tablets are round or oblong/elliptical-shaped; caplets are oblong/elliptical-shaped; capsules, geltabs, and gelcaps are usually oblong/elliptical-shaped and usually contain gelatin in coating.
3Many manufacturers offer caplets and tablets with delayed-release or enteric coating promoted as gastro-protective (see text for more about enteric coating and gastro-protection).
4Products vary in the time it takes to dissolve and release the active drug. This can range from less than 1 minute for non-enteric coated aspirin to more than 35 minutes for liqui-gels (see http://cruftbox.com/cruft/docs/dissolve.html).
5Some formulations are described as fast- or rapid-release. Formulations vary; some contain the active ingredient within liquid and some have holes in the coating or an exposed gelcap center which facilitates faster disintegration and release of the drug.
6Pamabrom is a diuretic.
7Pyrilamine maleate, diphenhydramine, and phenyltoloxamine are antihistamines.
8Caffeine is a central nervous system stimulant.
9Magnesium salicylate is an NSAID.
10Salicylamide has analgesic and antipyretic properties.
11Sodium is a buffering agent in this formulation.
12Phenylalanine is an amino acid.
From Pasero, C., & McCaffery, M. Pain assessment and pharmacologic management, pp. 242-245, St. Louis, Mosby. Data from Berardi, R. R., Kroon, L. A., McDermott, J. H., et al. (2006). Handbook of non-prescription drugs: An interactive approach to self-care, ed 15, Washington, DC, American Pharmacists Association; Brunton, L. L., Lazo, J. S., & Parker, K. L. (Eds.). (2006). Goodman & Gilman’s the pharmacological basis of therapeutics, ed 11, New York, McGraw-Hill; Clinical Pharmacology Online. Gold Standard, Inc. Available at http://clinicalpharmacology.com. Accessed January 4, 2009; Lacy, C. F., Armstrong, L. L., & Goldman, M. P., et al. (Eds.). (2010). Drug information handbook 2009-2010, ed 8, Hudson, OH, American Pharmacists Association and Lexi-Comp; United States Food and Drug Administration (FDA) orange book. Available at www.fda.gov/cder/orange/. Accessed January 8, 2009. Pasero C, McCaffery M. May be duplicated for use in clinical practice.
Conclusion
Analgesics available without a prescription (OTC) include many formulations and brands of aspirin and acetaminophen as well as ibuprofen, naproxen, and ketoprofen (see Tables 9-2 and 9-3) (see also Patient Medication Information Forms III-4 and III-5 on pp. 256-259). More NSAIDs are likely to become available as OTC analgesics. Clinicians need to be familiar with doses and ingredients of those used by the individual patient. Clinicians also should know that sometimes a nonprescription NSAID (e.g., ibuprofen) will be less expensive than the same dose by prescription.
Table 9-3
Selected Nonprescription NSAIDs Other Than Acetaminophen and Aspirin
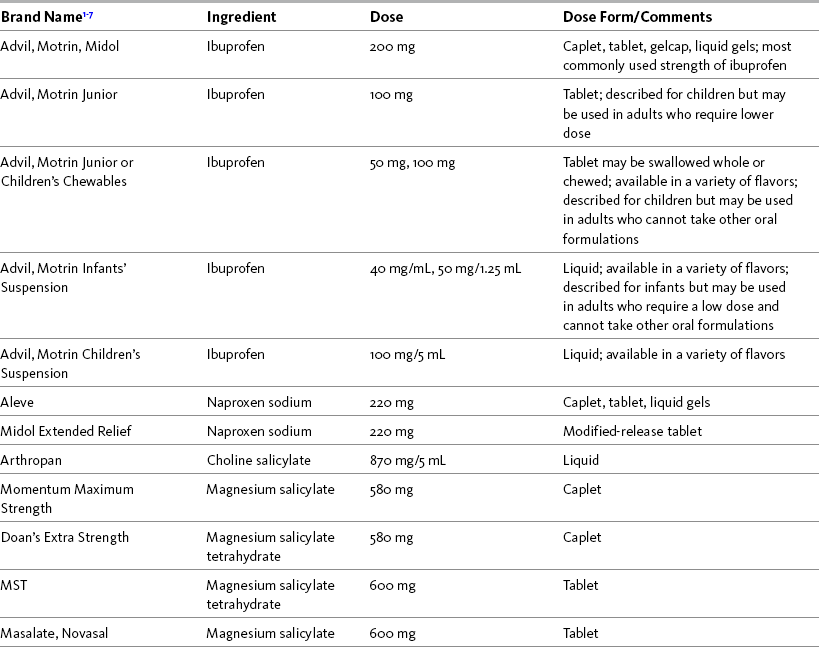
1Product availability changes frequently; this is not intended to be a comprehensive list.
2Many of the active ingredients in this table are available by prescription in other strengths and formulations (e.g., modified-release, suspensions).
3Active and inactive ingredients vary, and some oral solutions contain alcohol and dyes. Reading specific product labeling is recommended.
4Tablets are round or oblong/elliptical-shaped; caplets are oblong/elliptical-shaped; capsules, geltabs, and gelcaps are usually oblong/elliptical-shaped and usually contain gelatin in coating
5Many manufacturers offer caplets and tablets with delayed-release or enteric coating promoted as gastro-protective (see text for more about enteric coating and gastro-protection).
6Products vary in the time it takes to dissolve and release the active drug. This can range from less than 1 minute for non-enteric coated aspirin to more than 35 minutes for liqui-gels (see http://cruftbox.com/cruft/docs/dissolve.html).
7Some formulations are described as fast or rapid-release. Formulations vary; some contain the active ingredient within liquid and some have holes in the coating or an exposed gelcap center, which facilitates faster disintegration and release of the drug.
From Pasero, C., & McCaffery, M. Pain assessment and pharmacologic management, p. 246, St. Louis, Mosby. Data from Berardi, R. R., Kroon, L. A., McDermott, J. H., et al. (2006). Handbook of non-prescription drugs: An interactive approach to self-care, ed 15, Washington, DC, American Pharmacists Association; Brunton, L. L., Lazo, J. S., & Parker, K. L. (Eds.). (2006). Goodman & Gilman’s the pharmacological basis of therapeutics, ed 11, New York, McGraw-Hill; Clinical Pharmacology Online. Gold Standard, Inc. Available at http://clinicalpharmacology.com. Accessed January 4, 2009; Lacy, C. F., Armstrong, L. L., & Goldman, M. P., et al. (Eds.). (2010). Drug information handbook 2009-2010, ed 8, Hudson, OH, American Pharmacists Association and Lexi-Comp; United States Food and Drug Administration (FDA) orange book. Available at www.fda.gov/cder/orange/. Accessed January 8, 2009. Pasero C, McCaffery M. May be duplicated for use in clinical practice.