Management of Opioid-Induced Adverse Effects
Nausea and Vomiting in Patients Receiving Long-Term Opioid Therapy
Postoperative Nausea and Vomiting (PONV)
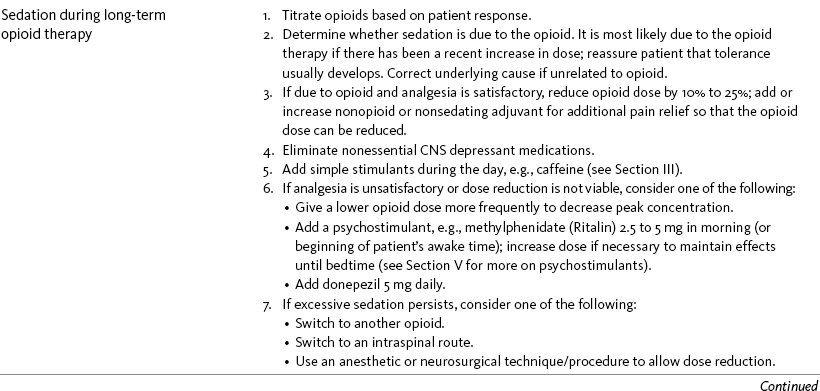
Sedation or Cognitive Impairment During Long-Term Opioid Therapy
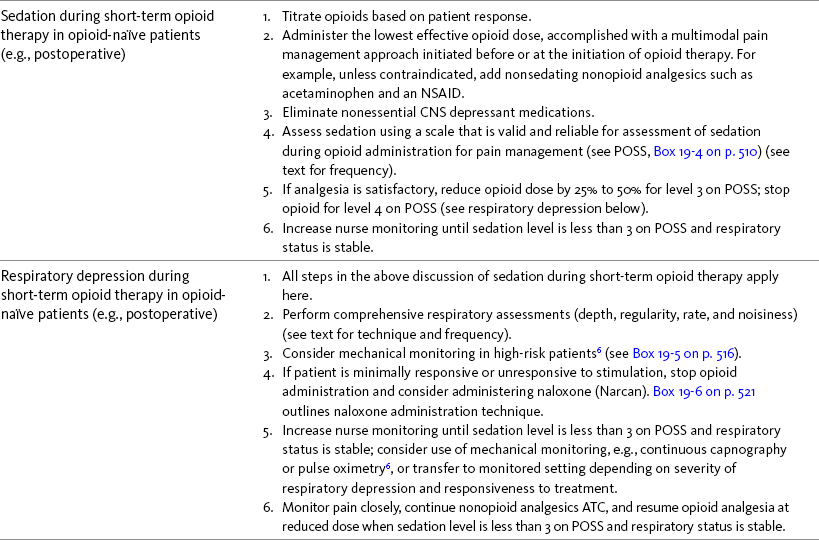
Sedation During Short-Term Opioid Therapy in Opioid-Naïve Patients
IN opioid-naïve patients, common opioid adverse effects include constipation, nausea and vomiting, sedation, pruritus, and mental confusion and clouding. Respiratory depression is less common but is the most feared adverse effect. As the patient becomes opioid tolerant, these adverse effects, except for constipation, tend to subside. Other less common opioid adverse effects include urinary retention, dry mouth, sweating, orthostatic hypotension, delirium, myoclonic jerks, and seizures. The underlying mechanisms of opioid adverse effects, even the most common, are not completely understood (Hanks, Cherny, Fallon, 2004). A number of factors influence the development of opioid adverse effects including patient age, co-morbidities, prior opioid exposure, concurrent administration of other drugs, and route of administration (Hanks, Cherny, Fallon, 2004). This explains why there is great individual variation in their development, and why most must be managed by using an individualized approach.
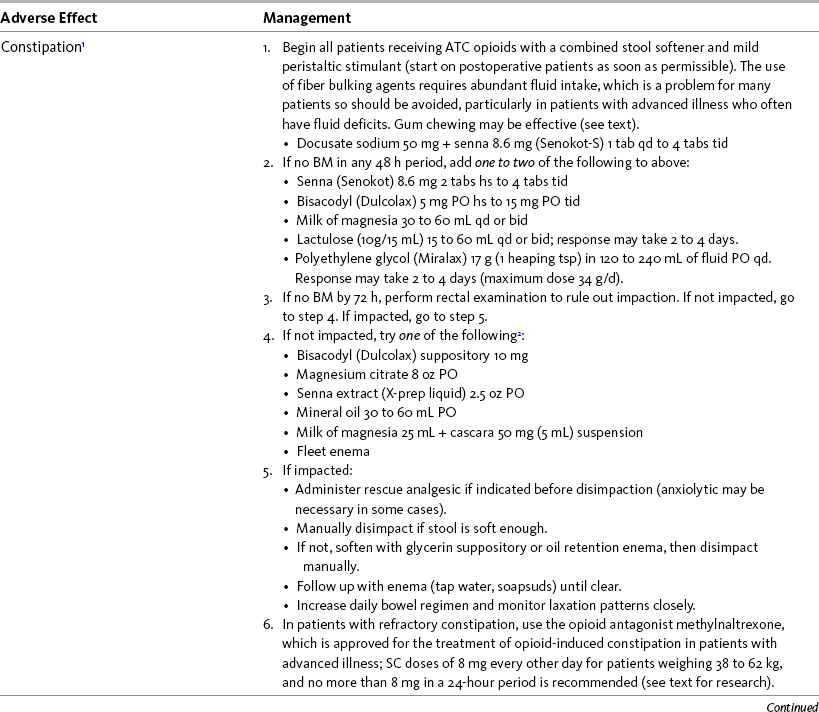
Prevention rather than treatment of opioid adverse effects is an important principle of pain management. Most adverse effects are dose dependent (Sinatra, 2009; Zhao, Chung, Hanna, et al., 2004). Therefore a practical approach includes the use of nonsedating analgesics that have an opioid dose-sparing effect, such as nonopioids and local anesthetics, so that the lowest effective opioid dose can be given. For some patients, simply decreasing the opioid dose is sufficient to eliminate or make an adverse effect tolerable (Pasero, McCaffery, 2003). Based on clinical experience, a decrease of 25% usually is sufficient to initiate a meaningful reduction in an adverse effect; if this dose change can be tolerated without severe pain, it is reasonable to attempt it. Again, based on clinical observation, a trial of a lower dose is least effective as a strategy for addressing constipation, presumably because the dose that produces constipation is approximately 4-fold less than the analgesic dose (Yuan, 2005) and because the symptom is so commonly multidetermined (see the discussion that follows).
The following is a discussion of many of the opioid adverse effects. Table 19-1 is a guide to preventing and managing the common ones. See Table 11-1 on p. 285 for information on the specific opioid receptor binding sites of each adverse effect.
Constipation
Opioids work in both the peripheral and central nervous systems to suppress neuronal excitability and inhibit neurotransmitter release from enteric neurons that innervate the secretory glands. This can result in delayed gastric emptying, slowed bowel motility, and decreased peristalsis (Murphy, 2006; Thomas, 2008; Wood, 2005). The result is slow-moving, hard stool that is difficult to pass. At its worst, GI dysfunction can result in unresolved ileus, fecal impaction, and obstruction (Kehlet, 2005; Wood, 2005). GI dysfunction is worsened by the presence of other conditions of advanced disease, such as ascites or tumors (Thomas, 2008).
Constipation is the most common opioid adverse effect and the one that is most often persistent (Gutstein, Akil, 2006; Hanks, Cherny, Fallon, 2004). It requires a preventive approach, regular assessment, and aggressive management if symptoms are detected. Factors contributing to the problem of constipation in patients taking opioids include advanced age, immobility, abdominal disease, and concurrent medications (Hanks, Cherny, Fallon, 2004; Hinrichs, Huseboe, 2001). Most patients placed on ATC opioid analgesics should be directed to take laxatives regularly. Although some clinicians do not endorse prophylactic treatment of a subpopulation that has no other risk factors and is younger, active, and well-nourished, there is general acceptance of the value of prophylaxis in others. A coadministered laxative usually must be continued as long as the patient takes opioids.
The goals of prophylactic treatment of constipation are to maximize stool volume, keep stool soft, and enhance peristalsis (Thomas, 2008). Although it has been suggested that a complete bowel movement at least every 3 days without difficulty is ideal (Goodheart, Leavitt, 2006), the frequency of defecation is less important than comfortable evacuation (Yuan, 2005). Attention to diet and exercise in addition to providing for privacy and convenience for patients are important aspects of bowel management but are insufficient alone to prevent opioid-induced constipation. Natural fiber and large amounts of fluid are a preferred strategy unless intrinsic bowel disease (usually partial obstruction) increases the risk associated with more intraluminal volume, the approach increases adverse effects such as bloating, or the patient finds this strategy unpalatable. Bulk laxatives, such as psyllium (Metamucil), are relatively contraindicated unless fluid intake is adequate, because of an increased risk of fecal impaction and obstruction (Thomas, 2008).
Stool softeners alone appear inadequate, and the usual initial therapy is a combination of stool softener and mild peristaltic stimulant, such as senna (e.g., Senokot-S) (Hanks, Cherny, Fallon, 2004; Thomas, 2008). Stool softeners are detergents and allow better water penetration into stool; stimulant laxatives induce peristalsis (Thomas, 2008).
The simple activity of chewing gum also seems to stimulate bowel motility (Schuster, Grewal, Greaney, et al., 2006). In a small prospective study, 34 patients undergoing elective open sigmoid resections were randomized into two groups: gum chewing or a control group. The patients chewing sugarless gum three times a day for one hour passed flatus, had their first bowel movement, and were discharged significantly sooner than the control group. More studies are needed, but gum chewing appears harmless and may help with constipation. Other tips on managing constipation are listed in Table 19-1. Tables containing the various classifications and properties of laxatives and the amount of fiber in common foods can be found in Curry, C. E., & Butler, D. M. (2006). Constipation. In R. R. Berardi, A. L. Kroon, J. H. McDermott, et al. (Eds.), Handbook of nonprescription drugs. An interactive approach to self care, ed 15, pp. 299-326, Washington DC, American Pharmacists Association. Tools with established reliability and validity for assessment of bowel function and constipation (Downing, Kuziemsky, Lesperance, et al., 2007; Goodman, Low, Wilkinson, 2005; Hinrichs, Huseboe, 2001; McMillan, Williams, 1989) are available. Figure 19-1 provides the Victoria Bowel Performance Scale (BPS), a simple-to-use 9-point tool. See also Form IV-1 on p. 546 at the end of Section IV. It contains valuable information that should be given to all patients receiving opioid therapy.
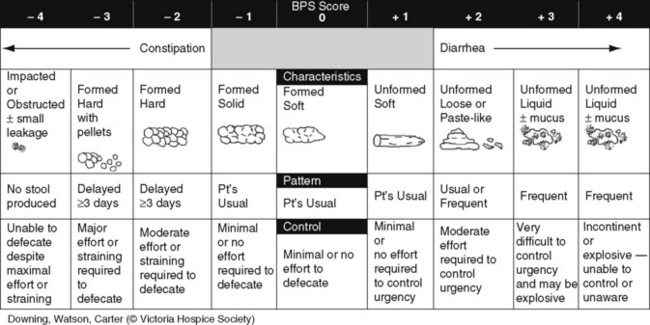
Figure 19-1 Victoria Bowel Performance Scale (BPS). From Downing, G. M., Kuziemsky, C., Lesperance, M., et al. (2007). Development and reliability testing of the Victoria Bowel Performance Scale (BPS). J Pain Symptom Manage, 34(5), 513-522.
Opioid Antagonists for Bowel Dysfunction
Although receptors in the central nervous system (CNS) are involved in the pathophysiology of opioid-induced constipation, the effect appears to be mediated predominantly by GI mu opioid receptors (Thomas, Karver, Cooney, et al., 2008). This observation led to a search for a peripherally acting mu opioid antagonist that could specifically reverse opioid-induced bowel dysfunction without reversing analgesia (Thomas, 2008).
When taken orally, the opioid antagonists naloxone, naltrexone, and nalmefene are absorbed systemically, and although they can effectively reverse bowel dysfunction, they can cross the blood-brain barrier, reverse central opioid receptors and analgesia, and produce withdrawal symptoms (Becker, Galandi, Blum, 2007; Goodheart, Leavitt, 2006; Liu, Wittbrodt, 2002; Thomas, 2008). This outcome is least likely to occur with naloxone, which has a very limited oral bioavailability (around 3%). Although there is a risk of systemic absorption with associated return of pain and withdrawal, the risk with this drug is low, and oral naloxone (20 to 40 mg) has been used clinically for refractory constipation (Meissner, Leyndecker, Mueller-Lissner, et al., 2009).
In contrast to these other antagonists, methylnaltrexone (Relistor) and alvimopan (Entereg) have the potential to block opioid actions mediated by peripheral opioid receptors while sparing actions mediated by opioid receptors in the CNS (Portenoy, Thomas, Moehl Boatwright, et al., 2008). Methylnaltrexone has approval in the United States for the treatment of opioid-induced constipation in patients with advanced illness (Wyeth Pharmaceuticals, 2009), and alvimopan is approved for acceleration of the time to upper and lower GI recovery following partial large or small bowel resection with primary anastomosis (GlaxoSmithKline, 2009). Methylnaltrexone is given subcutaneously (8 mg for patients weighing 38 to 62 kg every other day and no more than one dose in a 24-hour period), and alvimopan is taken orally (12 mg 30 minutes to 5 hours prior to surgery, then 12 mg twice daily for up to 7 days for a maximum of 15 doses). Methylnaltrexone is not approved for IV use but has been used by this route for treatment of nausea, pruritus, and urinary retention (Gold Standard Clinical Pharmacology, 2009).
A systematic review of 20 studies that were conducted on the use of methylnaltrexone and alvimopan for treatment of constipation concluded that further research with larger numbers of patients and varying types of pain are required (Becker, Galandi, Blum, 2007). Another analysis of 22 studies concluded that there is insufficient evidence for the safety and efficacy of naloxone or nalbuphine for the treatment of opioid-induced bowel dysfunction and that further research is needed to fully assess the role of alvimopan and methylnaltrexone in therapy (McNicol, Boyce, Schumann, et al., 2008). Following is a discussion of some of the research on methylnaltrexone. See pp. 491-493, Postoperative Ileus, for more on alvimopan.
One of the first studies conducted on methylnaltrexone was a double-blind study of 22 adults who were enrolled in a methadone maintenance program and had methadone-induced constipation (Yuan, Foss, O’Connor, et al., 2000). The subjects were randomized to receive either IV methylnaltrexone (up to 0.365 ) or placebo infusion over 9 minutes. All of the subjects who received methylnaltrexone had abdominal cramping followed by laxation on day 1 or day 2; none of those who received placebo had these effects. The effects of methadone maintenance were not reversed in this study.
A 2-week double-blind trial randomized 133 patients with incurable cancer or other end-stage disease who had been taking stable doses of opioid analgesics for 2 or more weeks to receive either SC methylnaltrexone (0.15 ) or placebo (Thomas, Karver, Cooney, et al., 2008). The median dose of morphine equivalent the patients were taking was 100 mg and 150 mg in the methylnaltrexone and placebo groups, respectively, and the patients were constipated at baseline. Laxation occurred within 4 hours of the first study dose in 48% of those who received methylnaltrexone compared with 15% of those who received placebo, and 52% had laxation without a rescue laxative within 4 hours after two or more of the first four study doses compared with 8% in the placebo group. Adverse effects were mild or moderate (e.g., abdominal pain, flatulence, nausea, increased body temperature, and dizziness) in 8% and 13% of those in the methylnaltrexone and placebo groups, respectively. Life-threatening adverse events were assessed as related to the primary illness. Eighty-nine of the patients in this study entered a 3-month, open-label extension study in which methylnaltrexone was administered to all of them. The response rate in those who had received methylnaltrexone and placebo in the double-blind phase was 45% to 58% and 48% to 52%, respectively. Similar adverse effects occurred in the open-label phase as in the double-blind phase. Analgesia was maintained throughout both phases of this study.
Methylnaltrexone was studied in a randomized, parallel-group, repeated dose, dose-ranging trial that included a one-week double-blind phase followed by an open-label phase for up to 3 weeks (Portenoy, Thomas, Moehl Boatwright, et al., 2008). The patients (N = 33) in this study had terminal or end-stage diseases and were receiving palliative care and long-term opioid therapy with stable doses for at least 2 weeks (mean and median opioid morphine equivalent dose = 289.9 mg and 180 mg, respectively) and reported ongoing constipation. They were randomized to receive 1, 5, 12.5, or 20 mg of SC methylnaltrexone. Doses between 5 mg and 20 mg (0.05 to 0.5 ) induced a bowel movement within 4 hours of drug administration significantly more often than a dose of 1 mg (less than 0.05 ), and there was no dose-response relationship above 5 mg/day. Approximately 50% of the patients responded with doses 5 mg or more within 4 hours and maintained favorable effects with repeated doses. These doses produced effective and rapid relief of constipation without producing pain flare or opioid withdrawal symptoms. All of the patients in this study experienced at least one adverse effect related to treatment; however, most were mild and not related to the dose of methylnaltrexone; abdominal pain was the most common. More recent research (N = 52) showed methylnaltrexone (0.15 ) given SC every other day for 2 weeks to patients with advanced illness resulted in a higher percentage of patient-rated improvements in bowel status, prompt and predictable laxation, and less use of other laxation techniques (e.g., laxatives and enemas) compared with placebo (Chamberlain, Cross, Winston, et al., 2009) (see Table 19-1).
Postoperative Ileus
Postoperative ileus is the temporary impairment of GI motility following surgery (Moore, Kalff, Bauer, 2005). Kehlet (2005) defines it as the time from surgery until passage of flatus or stool and tolerance of diet and describes it as part of the normal pathophysiologic response to surgical injury with multiple underlying mechanisms. As such, it requires a multimodal approach to preventing and treating it (Gannon, 2007; Kehlet, 2005). It is characterized by delayed gastric emptying, dilation of the small bowel and colon, loss of normal propulsive contractile patterns, and inability to pass gas or stool (Moore, Kalff, Bauer, 2005). Unresolved ileus is a postoperative complication that can cause significant discomfort, pulmonary morbidity, delayed rehabilitation, prolonged hospitalization, and increased cost of care (Kehlet, 2005; Mythen, 2005).
The effectiveness of traditional measures to reduce the incidence of postoperative ileus, such as nasogastric (NG) tube drainage and avoidance of early fluid and food intake, have been questioned (Mythen, 2005; Viscusi, Gan, Leslie, et al., 2009; Wilmore, Kehlet, 2008). A systematic review of the literature concluded that routine NG decompression after abdominal surgery did not accomplish any of the intended goals, patients with ileus recovered earlier without an NG tube, and the practice of routine NG decompression should be abandoned in favor of selective use (Nelson, Tse, Edwards, 2005). When used, NG tubes should be removed as soon as possible to avoid adverse effects including fever, atelectasis, and pneumonia (Holte, Kehlet, 2002; Kehlet, 2005; Saclarides, 2006).
Oral intake is traditionally restricted in the early postoperative period, and although a Cochrane Collaboration Review found early feeding to be safe, no significant difference in postoperative ileus could be found between early and delayed oral fluids and food after major abdominal surgery (Charoenkwan, Phillipson, Vutyavanich, 2007). Nevertheless, research is ongoing regarding its impact on ileus (Saclarides, 2006), and postoperative rehabilitation protocols that include early feeding have produced impressive results (Wilmore, Kehlet, 2001). Oral intake has been successfully initiated as early as 6 hours after colonic surgeries that use an anastomosis (Basse, Hjort Jakobsen, Billesbolle, et al., 2000; Basse, Raskov, Hjort Jakobsen, et al., 2002; Muller, Zalunardo, Hubner, et al., 2009).
Gum chewing has been suggested as a novel and inexpensive approach to reducing ileus. More research with larger numbers of patients are needed to clarify a role (Gannon, 2007), but one randomized controlled trial (N = 34) found patients who chewed gum after open sigmoid resection experienced a significantly faster return of GI function and shorter length of stay than those who did not chew gum (Schuster, Grewal, Greaney, et al., 2006). An earlier smaller study (N =19) of patients undergoing laparoscopic colectomy found similar results (Asao, Kuwano, Nakamura, et al., 2002).
Fluid excess can be detrimental to GI motility and should be avoided during and after colorectal surgery (Kehlet, 2005). Further research is needed to clarify the optimal amount and composition of fluid that should be administered for the various surgical procedures (Wilmore, Kehlet, 2008).
Randomized controlled trials demonstrate a reduction in duration of ileus from approximately 5 days to 3 days with the use of laparoscopic surgery (Kehlet, 2005). More randomized controlled research is needed to confirm the impact of laparoscopic techniques, but the additional benefits of reduced pain and opioid requirements can be expected to improve bowel function (Kehlet, 2005; Schwenk, Haase, Neudecker, et al., 2005).
Although early ambulation has not been shown to reduce the duration of ileus, immobility may lead to other complications, so aggressive mobilization is recommended as part of the overall strategy to reduce postoperative ileus (Kehlet, Holte, 2001). To this end, effective pain management is critical. Opioids slow bowel motility and contribute to ileus (Wood, 2005); therefore, administration of the lowest effective opioid dose (or, in some cases, avoiding opioids entirely) during the perioperative period is an important strategy in patients at high risk for ileus, such as those having open colorectal surgery. Continuous epidural analgesia with local anesthetics provides effective pain management for these types of surgeries, produces a positive effect on the stress response, and reduces postoperative ileus (Jorgensen, Wetterslev, Moiniche, et al., 2001). Because inhibitory neural reflexes are mediated through the sympathetic enteric nervous system and contribute to postoperative ileus, mid-to-low thoracic epidural catheter placement is essential (Kehlet, 2005). Very low thoracic and lumbar epidural analgesia and epidural opioids have no positive effects on ileus (Holte, Kehlet, 2002; Kehlet, 2005); however, low-dose opioid (e.g., morphine less than 1 mg/h) added to a sufficient amount of local anesthetic (e.g., bupivacaine 0.25%) epidurally will improve analgesia and preserve the ileus-reducing effect of epidural analgesia (Kehlet, 2005). Epidural analgesia should be provided for 2 to 3 days following major surgery.
For many years, the only effective pharmacologic agent for treatment of ileus was the prokinetic agent cisapride, but it was removed from the market because of potential cardiac adverse effects (Kehlet, 2005; Holte, Kehlet, 2002). Metoclopramide has been used as well but has not been shown to reduce postoperative ileus (Kehlet, Holte, 2001). There are no randomized controlled studies evaluating the use of laxatives to reduce ileus.
Opioid Antagonists for Management of Ileus
A major advance in the management of postoperative ileus is the approval of alvimopan (Entereg) for the acceleration of the time to upper and lower GI recovery following partial large or small bowel resection with primary anastomosis (GlaxoSmithKline, 2009). Alvimopan is a synthetic peripherally acting mu opioid receptor antagonist taken orally (12 mg 30 minutes to 5 hours prior to surgery, then 12 mg twice daily for up to 7 days for a maximum of 15 doses) (Kraft, 2007). Randomized controlled trials have shown that the drug can reduce ileus, shorten hospital length of stay, and is well tolerated with adverse effects similar to placebo after major abdominal surgery (Becker, Blum, 2009; Leslie, 2005; Neary, Delaney, 2005; Sinatra, 2006; Viscusi, Gan, Leslie, et al., 2009; Viscusi, Goldstein, Witkowski, et al., 2006). Methylnaltrexone, another peripherally acting mu opioid antagonist, has also been shown to reduce ileus (Viscusi, Gan, Leslie, et al., 2009) but is approved and used most often for the treatment of opioid-induced constipation in patients with advanced illness (see previous discussion earlier in the chapter). The opioid antagonists naloxone and nalmefene are not selective for the mu opioid receptors in the GI tract and should not be used for the prevention or treatment of ileus (Kraft, 2007).
In summary, postoperative ileus has multiple underlying mechanisms and is influenced by a number of factors. Management requires the implementation of a multimodal approach that focuses on prevention. Strategies include continuous thoracic epidural analgesia, opioid-sparing analgesic techniques, peripheral mu opioid antagonists, laparoscopic surgical techniques, and avoidance of routine NG tube, fluid excess, and immobility (see Table 19-1).
Nausea and Vomiting in Patients Receiving Long-Term Opioid Therapy
Initiating or increasing opioid therapy may cause nausea through both peripheral and central mechanisms that stimulate the chemoreceptor trigger zone in the brain, slowing GI mobility, and sensitizing the labyrinth vestibular system (needed for balance and equilibrium) (Gibbison, Spencer, 2009; Hanks, Cherny, Fallon, 2004; Pleuvry, 2009). Nausea is most common with the initial opioid dose and usually subsides within weeks of opioid therapy (Fine, Portenoy, 2007). In some patients, nausea is persistent and severe. Intractable nausea and vomiting in terminally ill patients have a significant negative impact on quality of life and function (Wood, Shega, Lynch, et al., 2007). Further investigation is warranted if it is suspected that factors other than opioid therapy are the cause.
The incidence and severity of nausea does not justify prophylactic treatment, with the possible exception of those patients who have a history of severe opioid-induced nausea and vomiting (Fine, Portenoy, 2007; Hanks, Cherny, Fallon, 2004). However, nausea should be treated aggressively once it occurs. Once controlled, the antiemetic can be tapered after a week to determine if the patient has developed tolerance to the emetogenic effects of the opioid. If not, treatment should be resumed and another tapering trial attempted again in one week (Fine, Portenoy, 2007).
A variety of antiemetics is available to treat nausea and can be selected based on assumptions concerning the underlying mechanism (see Table 19-1). A dopamine antagonist, such as prochlorperazine, is most often selected, but the occurrence of nausea immediately after eating, or nausea associated with early satiety and bloating, suggests the occurrence of delayed gastric emptying, which in turn, supports a trial of a prokinetic drug, such as metoclopramide (Reglan) (Fine, Portenoy, 2007). A retrospective chart review led researchers to recommend risperidone 1 mg daily for refractory nausea and vomiting in advanced cancer patients (Okamoto, Tsuneto, Matsuda, et al., 2007). When nausea occurs in patients with medical illness and is both severe and presumably determined by several causes, combination therapy should be considered. For example, a prospective, multicenter, phase II clinical trial administered daily IV granisetron (Kytril) (3 mg) and dexamethasone (Decadron) (8 mg) to 24 patients with intestinal obstruction who were refractory to previous antiemetic treatment (Tuca, Roca, Sala, et al., 2009). This regimen controlled nausea and vomiting in 86.9% of the patients.
Slow and steady opioid titration helps to reduce nausea. Eliminating nonessential drugs that may be contributing to nausea may be helpful as well (Fine, Portenoy, 2007). Adjustments in diet and activity plus the use of relaxation techniques can also be effective remedies (Coyle, Cherny, Portenoy, 1995). Table 19-1 outlines approaches commonly used to treat opioid-induced nausea and vomiting.
Postoperative Nausea and Vomiting (PONV)
Nausea and vomiting are among the most unpleasant of the adverse effects associated with surgery and are the cause of low patient satisfaction and higher cost of care in patients who have them compared with those who do not (Habib, Gan, 2004; Watcha, 2000). Many patients consider postoperative nausea and vomiting (PONV) to be as debilitating as the pain associated with the surgery. A questionnaire administered to postoperative patients revealed that they place high value on not having PONV and are willing to pay a significant amount for an effective antiemetic (Gan, Sloan, de L Dear, et al., 2001). PONV also is associated with detrimental effects, including aspiration of vomitus, tension on sutures, increased intracranial and intraocular pressure, and fluid and electrolyte imbalance. Not only does PONV have a negative impact on patient outcomes, but it can increase the burden on nursing staff (Miaskowski, 2009). It was once described as the “big little problem” by clinicians who manage it (Watcha, White, 1995).
Opioids are among a number of factors that increase the incidence of PONV. Box 19-1 lists the primary risk factors. A review of the literature described other factors in addition to this established list, including history of migraine, presence of preoperative anxiety, and the use of longer-acting versus shorter-acting opioids (Gan, 2006). It is important to note that postoperative pain, particularly incident pain, is associated with a higher incidence of postoperative vomiting (Chia, Kuo, Liu, et al., 2002; Ho, Gan, 2009).
Despite being listed as a risk factor in accepted guidelines (see Box 19-1), there is controversy about whether or not the type of surgical procedure influences the incidence of PONV (Habib, Gan, 2010; Scuderi, 2010). Researchers conducted a retrospective review of oncology surgeries from their electronic database to evaluate the impact of type of surgery on antiemetic administration within the first 2 hours of PACU admission and found that patients who underwent neurologic, head or neck, and abdominal surgeries received significantly more antiemetic in the PACU than patients who underwent integumetary-musculoskeletal (e.g., puncture procedures of the skin or muscles) and superficial (e.g., breast or axillary, endoscopic) surgeries (Ruiz, Kee, Frenzel, et al., 2010). In a commentary about this research, Habib and Gan (2010) discuss several limitations of this study and problems with methodology, and they conclude that large well-designed studies are needed to firmly establish the type of surgery as a risk factor (Habib, Gan, 2010).
Consensus guidelines present a number of recommendations for the management of PONV (Gan, Meyer, Apfel, et al., 2003, 2007). Algorithms that incorporate guideline recommendations are available (American Society of PeriAnesthesia Nurses, 2006; Gan, Meyer, Apfel, et al., 2007), and the December 2006 focus issue of the Journal of PeriAnesthesia Nursing is devoted entirely to content on PONV. Below is a summary of the major guideline recommendations in adults followed by a more detailed discussion of various antiemetics and strategies for treatment of PONV (see Table 19-1).
• Identify patients at high risk for PONV (see Box 19-1). There is no consensus on how many risk factors a patient must have to warrant the designation of high risk (Apfel, Kranke, Eberhart, et al., 2002; Gan, Meyer, Apfel, et al., 2003; van den Bosch, Kalkman, Vergouwe, et al., 2005). A simple scoring system (the Apfel risk score) developed by Apfel and colleagues (1999) is widely used and has been shown to be reliable and valid for this purpose. It is based on four predictors: female gender, prior history of motion sickness or PONV, nonsmoking status, and the use of postoperative opioids. If no or only one factor is present, the incidence of PONV varies from 10% to 21%. If two or more are present, the risk rises to 39% to 78% (Apfel, Laara, Koivuranta, et al., 1999).
• Reduce baseline risk factors, e.g., implement multimodal analgesic strategies to treat postoperative pain so that no opioid or the lowest effective dose of opioid can be given. This may also include the use of effective nonpharmacologic strategies such as relaxation techniques, acupuncture, and acupressure (Lee, Done, 1999; Nunley, Wakim, Guinn, 2008; Roscoe, Bushunow, Jean-Pierre, et al., 2009). Although nondrug interventions may be helpful, the mainstay of PONV treatment is pharmacologic (Wilhelm, Dehoorne-Smith, Kale-Pradhan, 2007). The use of regional rather than general anesthesia is widely recommended to reduce PONV, although some surgical procedures (e.g., cesarean, some major orthopedic surgeries) are associated with a high incidence of PONV despite using regional anesthesia (Borgeat, Ekatodramis, Schenker, 2003).
• Administer PONV prophylaxis using one to two interventions to patients at moderate risk for PONV. For example, administer dexamethasone (Decadron) before anesthesia induction and a serotonin receptor antagonist (e.g., ondansetron [Zofran]) at the end of surgery. It is important to consider that research has shown that single-drug prophylaxis has a high failure rate and is associated with a resultant increased cost of care (Gan, Meyer, Apfel, et al., 2007; Watcha, 2000), so combinations of two antiemetics rather than a single antiemetic prophylactically are preferred.
• Administer PONV prophylaxis using two or more interventions (multimodal approach) to patients at high risk for PONV. For example, administer dexamethasone before anesthesia induction, IV total anesthesia (IVTA) propofol (Diprivan), a serotonin receptor antagonist at the end of surgery, and IV propofol rescue doses in the PACU. A prospective study of 376 patients at high risk for PONV revealed that the administration of three or more prophylactic antiemetics produced the largest reduction in PONV, but despite this aggressive treatment, 30% still experienced symptoms severe enough to interfere with function (White, O’Hara, Roberson, et al., 2008). A prospective observational study concluded similarly that compared with lower Apfel risk scores, a high Apfel risk score (see above) was associated with a higher incidence of emetic sequelae in the first 24 hours after surgery despite the prophylactic administration of multiple antiemetics (White, Sacan, Nuangchamnong, et al., 2008).
• Do not administer prophylactic antiemetic treatment to low-risk patients as this is not supported by current practice (Apfel, Korttila, Abdalla, et al., 2004; Gan, Meyer, Apfel, et al., 2003, 2007); however, provide antiemetic treatment to patients with PONV who did not receive prophylaxis or in whom prophylaxis failed.
Antiemetics
There are numerous antiemetic drug options available (Carlisle, Stevenson, 2006), and selection should be based on evidence of efficacy and safety as well as consideration of cost (see Table 19-1). A systematic review concluded that no one antiemetic agent is superior to another (Wilhelm, Dehoorne-Smith, Kale-Pradhan, 2007). A multicenter study of over 4000 patients at high risk for PONV (greater than 40% risk per Apfel risk scoring system [Apfel, Laara, Koivuranta, et al., 1999]) and undergoing a variety of types of surgeries were randomized to receive one of the following interventions: ondansetron (4 mg IV) or no ondansetron; dexamethasone (4 mg IV) or no dexamethasone; droperidol (Inapsine) (1.25 mg IV) or no droperidol; propofol or a volatile anesthetic (i.e., isoflurane, desflurane, or sevoflurane); nitrogen or nitrous oxide; and remifentanil or fentanyl. Because propofol has been shown to reduce PONV (see the paragraphs that follow), twice as many patients were assigned to the propofol group to ensure adequate power to compare treatments. All of the antiemetics were similarly effective; ondansetron, dexamethasone, and droperidol reduced risk by approximately 26%, propofol by 19%, and nitrogen by 12%. Droperidol, which has a “Black Box” warning for potential QTc prolongation and torsades de pointes, was safe (see later in the chapter for more on droperidol). The researchers pointed out that the clinical implication of their findings is that, because the interventions were similarly effective, the safest and least expensive treatment should be used first.
The glucocorticoid dexamethasone is an excellent choice antiemetic because numerous studies have shown it to be effective, safe, and inexpensive (Gan, Meyer, Apfel, et al., 2007) (see Table 19-1). It may be given prophylactically before induction of anesthesia as well as for established PONV (Golembiewski, Chernin, Chopra, 2005) and has been shown to have similar efficacy to the serotonin antagonist tropisetron, which is not available in the United States (Wang, Ho, Uen, et al., 2002). Dexamethasone is administered as a single 4 mg to 8 mg IV bolus dose most often and has been combined in multimodal treatment plans with a number of other agents including ondansetron (Zofran) (Paech, Rucklidge, Lain, et al., 2007; Pan, Lee, Harris, 2008), granisetron (Kytril) (Fujii, Saitoh, Tanaka, et al., 1999; Gan, Coop, Philip, et al., 2005), dolasetron (Anzemet) (Coloma, White, Markowitz, et al., 2002; Rusch, Arndt, Martin, et al., 2007), droperidol (Sanchez-Ledesma, Lopez-Olaondo, Pueyo, et al., 2002), metoclopramide (Reglan) (Wallenborn, Gelbrich, Bulst, et al., 2006), and haloperidol (Haldol) (Chu, Shieh, Tzeng, et al., 2008; Rusch, Arndt, Martin, et al., 2007). Adverse effects are rare with short-term use (Gan, Meyer, Apfel, et al., 2007).
The serotonin receptor antagonists dolasetron, granisetron, and ondansetron are most effective when administered at the end of surgery (Gan, Meyer, Apfel, et al., 2007) (see Table 19-1). The newest serotonin antagonist palonosetron (Aloxi) is approved for prevention of chemotherapy-induced nausea and has been shown to significantly decrease PONV in a dose-related manner (0.075 mg IV significantly better than 0.025 mg IV) when administered immediately prior to anesthesia induction (Candiotti, Kovac, Melson, et al., 2008). The serotonin receptor antagonists are often combined with other antiemetics in multimodal PONV prophylaxis regimens. This practice is supported by a meta- analysis of 33 randomized controlled trials (3447 patients), which concluded that various combinations of a serotonin antagonist with dexamethasone or droperidol were equally effective with similar adverse effects, and the combinations were more effective than a serotonin antagonist alone (Habib, El-Moalem, Gan, 2004). A 2001 systematic review of the literature found that the serotonin antagonists available at the time were similarly effective for treatment of established PONV and that lower doses were as effective as higher doses, so the lowest dose in the dosing range was recommended (Kazemi-Kjellberg, Henzi, Tramer, 2001).
The serotonin antagonists are reported to prevent postoperative vomiting better than nausea (Gan, Meyer, Apfel, et al., 2007; Kazemi-Kjellberg, Henzi, Tramer, 2001); however, an analysis of data from 5161 patients concluded that ondansetron prevents both symptoms equally well (Jokela, Cakmakkaya, Danzeisen, et al., 2009). Ondansetron 8 mg twice daily is effective in an orally disintegrating tablet formulation (Zofran ODT) (Gan, Franiak, Reeves, 2002; Grover, Mathew, Hegde, 2009; Hartsell, Long, Kirsch, 2005). The most common adverse effect of the serotonin antagonists is headache (Kazemi-Kjellberg, Henzi, Tramer, 2001).
The anticholinergic scopolamine delivered via a transdermal patch (Transderm-Scop, Transderm-V) has been shown to prevent PONV with minimal adverse effects when applied preoperatively (White, Tang, Song, et al., 2007) (see Table 19-1). One patch (1.5 mg) should be applied behind the ear preoperatively, taking into account its 2- to 4-hour onset of action, and it can provide relief for up to 72 hours. A second patch may be applied after the first is removed at 72 hours. Transdermal scopolamine may be combined with other antiemetics in a multimodal treatment plan (Kranke, Morin, Roewer, et al., 2002). A randomized study of 126 patients undergoing cosmetic surgery found that the transdermal scopolamine patch combined with IV ondansetron (4 mg) was more effective in reducing PONV than ondansetron plus a placebo patch (Sah, Ramesh, Kaul, et al., 2009). Another study also found the combination to be more effective than ondansetron alone (Gan, Sinha, Kovac, et al., 2009). Transdermal scopolamine has also been used to reduce nausea and vomiting associated with intrathecal morphine post–cesarean section (Harnett, O’Rourke, Walsh, et al., 2007). Adverse effects include dry mouth, sedation, and visual disturbances; older patients may be more sensitive to CNS adverse effects, such as dizziness and agitation (Golembiewski, Chernin, Chopra, 2005).
Research in the 1990s demonstrated that the IV sedative hypnotic propofol at subhypnotic doses (5 to 10 mg IV push q 4 to 6 h or 0.5 to 1 per hour continuous infusion) reduced the overall incidence of PONV in patients at high risk for PONV without untoward sedative or cardiovascular (CV) effects compared with placebo (Ewalenko, Janny, Dejonckheere, et al., 1996). Since then, the drug has gained in popularity as a component of multimodal approaches designed to reduce PONV (Eberhart, Mauch, Morin, et al., 2002; Scudieri, James, Harris, et al., 2000) (see Table 19-1). A randomized controlled study administered 90 patients undergoing laparoscopic cholecystectomy one of the following regimens: (1) a multimodal management strategy that involved the use of TIVA propofol plus droperidol and ondansetron, (2) IV propofol at induction followed by inspired isoflurane/nitrous oxide-based anesthesia, droperidol, and ondansetron, or (3) TIVA with no other antiemetic prophylaxis (Habib, White, Eubanks, et al., 2004). The droperidol (0.625 mg) was administered at induction and ondansetron (4 mg) was administered at the end of surgery in groups 1 and 2. Complete response rate (no PONV and no rescue antiemetic) at 2 hours and 24 hours after surgery was 90% and 80% in group 1, 63% and 63% in group 2, and 66% and 43% in group 3. Patient satisfaction was also higher in group 1. The researchers noted, however, that the higher cost of propofol compared with volatile anesthetics and its short-lived antiemetic effect (limited to early postoperative period) makes it suitable for use only in patients at very high risk for PONV.
Droperidol and the “Black Box” Warning
Since its approval for use in general anesthesia in the 1970s, the butyrophenone droperidol (Inapsine) has been used to effectively and cost-efficiently treat PONV (White, 2002). In 2001, the United States Food and Drug Administration (U.S. FDA) issued a “Black Box” warning that described the drug’s potential to cause prolonged QTc interval and torsade de pointes, a life-threatening cardiac dysrhythmia (Martinez, Moos, Dahlen, 2006). However, citing a lack of documentation of cardiac adverse events, the FDA warning has been widely criticized by anesthesia experts (Gan, White, Scuderi, et al., 2002; White, 2002). A review of the 273 reported adverse events involving the use of droperidol at doses of 1.25 mg or less (customary for treatment of PONV) revealed extensive use of the drug (over 11 million ampules sold in 2001) (Habib, Gan, 2003). Of the 273 adverse events, 74 and 17 were cases of possible cardiac events and torsades de pointes or prolonged QTc interval, respectively. The researchers concluded that there was no evidence of a cause-and-effect relationship between the occurrence of arrhythmias and small-dose droperidol (1.25 mg or less). A later randomized controlled trial of 120 patients undergoing outpatient surgery found no statistically significant increase in QTc interval compared with placebo during general anesthesia and no evidence of any droperidol-induced QTc prolongation after surgery (White, Song, Abrao, et al., 2005). The drug is recommended in doses less than 1.25 mg as a first-line option in evidence-based PONV management guidelines (Gan, Meyer, Apfel, et al., 2003, 2007) (see Table 19-1).
Novel Approaches to the Management of PONV
Some novel and relatively inexpensive approaches have been tried for the management of PONV. Researchers applied nicotine patches to patients undergoing laparoscopic cholecystectomy under general anesthesia based on research that shows cigarette smoking reduces risk of PONV (Ionescu, Badescu, Acalovschi, 2007). Patients in this study (N = 75) were randomized according to their cigarette smoking status: (1) nonsmokers, (2) patients who had stopped smoking at least 5 years prior and received one 16.6 mg nicotine patch, and (3) patients who currently smoked. There was a 20% reduction in PONV in patients in group 2 compared with group 1 and no difference between group 2 and group 3.
Intraoperative supplemental oxygen has been suggested as a strategy to reduce PONV, but research is conflicting. One early study showed that the incidence of PONV was significantly reduced in patients following laparoscopic gynecologic surgery who received 80% supplemental intraoperative oxygen (22%) and those who received 8 mg of ondansetron at induction (30%) compared with patients who received 30% supplemental oxygen intraoperatively (44%) (Goll, Akca, Grief, et al., 2001). However, a later study showed that neither 30% nor 80% intraoperative oxygen administration reduced PONV in 100 patients undergoing ambulatory gynecologic laparoscopy (Purhonen, Turunen, Ruohoaho, et al., 2003). Intraoperative supplemental oxygen did not decrease the incidence of PONV post–cesarean section delivery with neuraxial anesthesia either (Phillips, Broussard, Sumrall, et al., 2007).
Studies have shown that wrist acustimulation/acupressure (acupuncture point P6 [pericardium 6]) (ReliefBand®) reduces PONV (Gan, Jiao, Zenn, et al., 2004; Lee, Fan, 2009; Nunley, Wakim, Guinn, 2008; Roscoe, Bushunow, Jean-Pierre, et al., 2009) and that when combined with ondansetron (4 mg), the response rate to acustimulation is increased and quality of recovery and patient satisfaction are improved (Coloma, White, Ogunnaike, et al., 2002; White, Issioui, Hu, et al., 2002). The optimal time to administer acustimulation for antiemetic prophylaxis is after surgery (White, Hamza, Recart, et al., 2005). Figure 19-2 shows the location of acupuncture point P6.
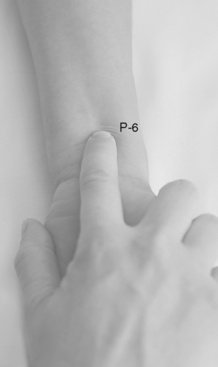
Figure 19-2 Wrist acustimulation for PONV: Location of acupuncture point P-6. From Focks, C. (2008). Atlas of acupuncture, Philadelphia, Churchill Livingstone.
A meta-analysis of research on gum chewing during the early postoperative period following colectomy concluded that the practice may enhance GI recovery and reduce length of stay (Purkayastha, Tilney, Darzi, et al., 2008). Improved GI recovery may contribute to a lower incidence of PONV.
Effective, Safe, and Inexpensive Treatment
By far, the most effective, safest, and least expensive way to treat PONV is to reduce the opioid dose whenever possible. Postoperative opioid orders should include the expectation that nurses will consider decreasing the opioid dose by 25% prior to or in conjunction with pharmacologic treatment of moderate-to-severe PONV (see following patient example and Form 17-1 on p. 464 for an example of how decreases in opioid dose can be included in opioid order sets). Decreasing the opioid dose is facilitated by adding or increasing a nonopioid, such as an NSAID or acetaminophen, or adding a local anesthetic to the epidural opioid solution to provide additional pain relief. If patients are too nauseated to take oral nonopioids, they may be given rectally (see Section III and Chapter 14 in this section for more on rectal administration).
Approaches with Little or No Effectiveness
In their 2007 guidelines, the American Society for Ambulatory Anesthesia concluded that there is a lack or limited evidence of a prophylactic effect for metoclopramide (10 mg IV), ginger root, and cannabinoids for PONV (Gan, Meyer, Apfel, et al., 2007). Metoclopramide is reported to be no more effective than placebo for its treatment (Gan, 2002); however, one randomized controlled study (N = 3140) found doses of 25 to 50 mg of metoclopramide in combination with dexamethasone administered intraoperatively reduced the frequency of PONV without a high incidence of adverse effects (Wallenborn, Gelbrich, Bulst, et al., 2006). Doses this high are not recommended; the drug has been reported to produce extrapyramidal symptoms, even in low doses (i.e., 10 mg) (Moss, Hansen, 2008).
Although promethazine (Phenergan) has been used for many years and has efficacy for the treatment of established PONV (Habib, Breen, Gan, 2005; Gan, Meyer, Apfel, et al., 2007; Habib, Reuveni, Taguchi, et al., 2007; Moser, Caldwell, Rhule, 2006), it is associated with significant adverse effects, including excessive sedation, respiratory depression, dysphoria, dystonia, and extrapyramidal symptoms (McGee, Alexander, 1979; Sheth, Verrico, Skledar, et al., 2005). Further, significant tissue damage can occur when promethazine is given intravenously (Institute for Safe Medication Practices, 2006a, 2006b). A “Black Box” warning added to promethazine prescribing information describes these injuries and the risk of unintentional intra-arterial injection and recommends deep IM injection of the drug; SC injection is contraindicated (U.S. FDA, 2009c). It is common practice to administer promethazine based on the belief that it will enhance opioid analgesia; however, early research dispelled this misconception (McGee, Alexander, 1979), and the practice is discouraged (Pasero, Portenoy, McCaffery, 1999). If promethazine is used, low doses are recommended, particularly in older adults (Habib, Breen, Gan, 2005; Moser, Caldwell, Rhule, 2006). Doses of 6.25 mg were found to be as effective as higher doses (e.g., 12 mg) (Habib, Reuveni, Taguchi, et al., 2007).
Prochlorperazine (Compazine) provided better relief of nausea and vomiting than promethazine in patients in the emergency department (ED) (Ernst, Weiss, Park, et al., 2000). Prochlorperazine has a faster onset and causes less sedation than promethazine as well (Golembiewski, Chernin, Chopra, 2005).
Another commonly used drug is hydroxyzine (Vistaril), but the doses that would be required to produce analgesia create significant risk of respiratory depression that is not reversible by naloxone (Gordon, 1995). IM hydroxyzine is especially irritating to the muscle and soft tissue and can produce sterile abscesses, so this practice is discouraged as well. Other older drugs—dimenhydrinate (Dramamine) (Kothari, Boyd, Bottcher, et al., 2000) and diphenhydramine (Benadryl)—are occasionally used for PONV but can cause significant sedation and dizziness. Antiemetics with better efficacy and safety should be considered before these drugs are used for treatment of PONV (Gan, Meyer, Apfel, et al., 2007) (see Table 19-1).
The routine use of NG tubes during surgery as a means of reducing PONV is not recommended. A large case control study (N = 4055) evaluated the association between NG tube use and the incidence of nausea, emesis, and overall PONV and showed no reduction in the incidence of these three outcome measures; the incidence of PONV was 44.4% with intraoperative NG tube versus 41.5% in controls (Kerger, Mascha, Steinbrecher, et al., 2009).
Biliary Spasm
Opioids increase smooth muscle tone in the biliary tract, especially in the sphincter of Oddi, which regulates the flow of bile and pancreatic fluids. This can result in a decrease in biliary and pancreatic secretions and a rise in bile duct pressure (Fukuda, 2005). Patients may experience epigastric distress and occasionally biliary spasm from this effect.
All opioids are capable of causing constricture of the sphincter of Oddi and the biliary tract (“biliary spasm”) and do so in a drug- and dose-dependent manner (Fukuda, 2005). This effect is complex with multiple underlying mechanisms (Helm, Venu, Geenen, et al., 1988). Research on this response is somewhat conflicting with regard to differences between the various opioids. The research that has been done has never shown much clinical relevance in humans (Lee, Cundiff, 1998; Spiegel, 2001). Meperidine produces a dual effect on the biliary tract (Fukuda, 2005); at low concentrations it inhibited the response of the common bile duct to electrical stimulation, and in higher concentrations it produced an excitatory effect and increased spontaneous contractions in guinea pigs (Goldberg, Vatashsky, Haskel, et al., 1987). A common misconception is that meperidine causes less constricture of the sphincter of Oddi than other opioids, but research does not support this (see the paragraphs that follow).
Early research showed morphine increased bile duct pressure in animals (Coelho, Runkel, Herfarth, et al., 1986) and humans (Zsigmond, Vieira, Duarte, et al., 1993). A study of 36 patients without common bile duct stones or anatomic abnormalities who were undergoing cholecystectomy demonstrated that morphine increased the frequency of sphincter of Oddi motility more than meperidine (Thune, Baker, Saccone, et al., 1990); however, an earlier study showed fentanyl, meperidine, morphine, and pentazocine caused a rise in bile duct pressure of 99.5%, 61.3%, 52.7%, and 15.1%, respectively in humans (Radnay, Brodman, Mankikar, et al., 1980). In other words, morphine produced less of a rise than both fentanyl and meperidine. Although pentazocine produced the smallest rise in this study, it causes dysphoria, anxiety, nightmares, depersonalization, and hallucinations, has an analgesic ceiling, and is not recommended for the management of any type of pain (see Chapter 13).
Later randomized controlled research showed fentanyl and sufentanil had no effect on common bile duct diameter in 17 patients during cholecystectomy, and the researchers recommended fentanyl and sufentanil in patients in whom spasm of the common bile duct should be avoided (Vieira, Zsigmond, Duarte, et al., 1994). Remifentanil, another lipophilic, short-acting mu opioid, was shown to cause a shorter delay in biliary tract drainage into the duodenum in 6 healthy volunteers than had been previously reported in studies of morphine and meperidine (Fragen, Vilich, Spies, et al., 1999).
The agonist-antagonist opioids (in addition to pentazocine above) have also been researched for their effect on the sphincter of Oddi. One early study showed that fentanyl, morphine, and meperidine increased common bile duct pressure more than butorphanol or placebo in 50 patients undergoing cholecystectomy (Radnay, Duncalf, Novakovic, et al., 1984). Later research found no differences between butorphanol, nalbuphine, and placebo in patients undergoing cholecystectomy (Vieira, Zsigmond, Duarte, et al., 1993); however, a more recent study found that nalbuphine caused a significant stimulatory effect on the sphincter of Oddi in 17 patients with suspected sphincter of Oddi dysfunction when used as a premedication for endoscopy; the researchers recommended against its use for endoscopic diagnosis of this condition (Madacsy, Bertalan, Szepes, et al., 2003). As discussed in Chapter 13, the agonist-antagonists are not recommended as first-line opioid analgesics for the treatment of pain.
The Meperidine Misconception
Although institutional quality improvement initiatives have resulted in a significant decline in the use of meperidine over the years (Gordon, Jones, Goshman, et al., 2000), the drug continues to be a first-choice analgesic of many prescribers (Seifert, Kennedy, 2004). This is particularly true for the management of pain during GI procedures and in patients with pancreatitis or cholecystitis; however, there are many disadvantages to the use of meperidine for pain management, including accumulation of its toxic metabolite with repeated dosing and its inappropriateness in older adults (Latta, Ginsberg, Barkin, 2002) (see Chapter 13).
A review of the literature concluded that morphine may be of more benefit than meperidine by offering analgesia without the risks associated with meperidine and that there are no studies or evidence to indicate morphine is contraindicated for use in acute pancreatitis (2001). Low-dose transdermal fentanyl (12.5 to 25 mcg) caused no significant changes in sphincter of Oddi pressure in patients with pancreatitis in one study and was described as the ideal analgesic for treatment of pancreatitis pain (Koo, Moon, Choi, et al., 2009). An interesting letter to the editor suggested that the choice of meperidine over other opioids is not based on evidence and persists because of the perpetuation of the misconception that meperidine has no effect on the sphincter of Oddi (Lee, Cundiff, 1998). The authors equated this to the “medical equivalent of an urban legend.” The best course of action is to avoid meperidine for all types of pain, including procedural pain and pancreatitis pain, and rely instead on other mu agonist opioids, such as morphine, hydromorphone, or fentanyl.
Pruritus
Pruritus (itching) is an adverse effect, not an allergic reaction to opioids (Ho, Gan, 2009). Its incidence ranges from 18% to 40% in the postoperative setting depending on the route of opioid administration and opioid administered (Wheeler, Oderda, Ashburn, et al., 2002). It is one of the most common adverse effects when opioids are delivered by the intraspinal routes (Ganesh, Maxwell, 2007; Wheeler, Oderda, Ashburn, et al., 2002) and is more common with intraspinal morphine than hydromorphone and fentanyl (Dabu-Bondoc, Franco, Sinatra, 2009).
Pruritus is sometimes generalized all over the body but usually is localized to the face, neck, or upper thorax (Ho, Gan, 2009). It rarely is accompanied by a rash. It can range from being annoying to so severe that it interferes with sleep, accomplishment of daily activities, and quality of life (Pittelkow, Loprinzi, 2004).
Pruritus, like pain, is transmitted via unmyelinated C-fiber nociceptors from the periphery (skin) to the CNS (dorsal horn of the spinal cord) where they synapse with itch-specific secondary neurons (Waxler, Dadabhoy, Stojiljkovic, et al., 2005) (see Section I). Secondary neurons then transmit the signal to the thalamus and sematosensory cortex. Opioids reduce tonic inhibition of this itch-specific pathway and allow spontaneous activity of central itch neurons (Ho, Gan, 2009). This pathway is the only one identified so far, but others are likely to exist, and further research is needed to more clearly understand all of the underlying mechanisms of pruritus (Waxler, Dadabhoy, Stojiljkovic, et al., 2005).
Although itch is transmitted by a subset of C-fibers that are different from those that transmit pain, the two sensations seem to be interrelated; painful stimuli can inhibit itching, and inhibition of pain processing may enhance itching. Several substances have been identified as mediators of itch, including histamine, prostaglandins, and serotonin (Waxler, Dadabhoy, Stojiljkovic, et al., 2005). When applied into the epidermis, histamine stimulates histamine receptors on the itch-specific C-fibers, which can cause the itching sensation. There are two types of histamine receptors in the skin (H1 and H2), and clinicians have taken advantage of this in treating some types of pruritus. This may explain why the combination of the H1 antagonist cetirizine (Zyrtec) and the H2 antagonist cimetidine (Tagamet) was significantly more effective than diphenhydramine (Benadryl) and placebo for burn-related pruritus (Baker, Zeller, Klein, et al., 2001).
Pruritus associated with advanced illness may be caused by multiple factors related to the disease process and is particularly challenging to treat. The reader is referred to an excellent overview of the anatomy and physiology and treatment options for pruritus in patients with advanced disease in Pittelkow, M. R., & Loprinzi, C. L. (2004). Pruritus and sweating in palliative medicine. In D. Doyle, G. Hanks, N. I. Cherny, et al. (Eds.), Oxford textbook of palliative medicine, ed 3, pp. 573-587, New York, Oxford Press. The treatment options presented in this chapter are used primarily for postoperative pruritus.
Assessment of Pruritus
The use of a self-report tool to assess pruritus may be helpful, particularly in cases of intractable pruritus. A 4-point verbal rating scale (VRS-4) and an 11-point verbal numeric rating scale (VNRS-11) are used most often (Jenkins, Spencer, Weissgerber, 2009). The VRS-4 matches a score to the patient’s description of itch intensity: 0 = no itching; 1 = mild itching; 2 = moderate itching; 3 = severe itching; the VNRS-11 is used similarly to the numerical rating scale (NRS) for pain intensity assessment: 0 = no itch and 10 = the worst imaginable level of itching. A study of 50 parturients demonstrated a strong correlation between the two scales, leading the researchers to conclude that each verbal descriptor on the VRS-4 could be substituted with a quantifiable range on the VNRS-11, i.e., 1 to 3 = mild itching; 4 to 7 = moderate itching; and 8 to 10 = severe itching (Jenkins, Spencer, Weissgerber, 2009).
Pharmacologic Management of Pruritus
Antihistamines such as diphenhydramine relieve only itch caused by histamine release, such as insect bites, urticaria, and allergic skin reactions. Although they are widely used, there is no strong evidence that antihistamines relieve opioid-induced pruritus (Grape, Shug, 2008). Some suggest that they may be more effective for pruritus caused by systemic opioids than neuraxial opioids, but research is lacking (Waxler, Dadabhoy, Stojiljkovic, et al., 2005). Patients may report being less bothered by itching after taking an antihistamine, but this is likely the result of sedating effects (Ho, Gan, 2009). Sedation can be problematic in those already at risk for excessive sedation, such as postoperative patients, as this can lead to life-threatening respiratory depression (Anwari, Iqbal, 2003) (see later in this chapter). Thus careful monitoring of sedation levels is recommended when antihistamines are combined with opioid administration, and they should not be administered if patients are excessively sedated.
Prostaglandins do not elicit pruritus when applied to the skin, but they do act synergistically with histamine to potentiate the histamine-elicited itch (Waxler, Dadabhoy, Stojiljkovic, et al., 2005). Topical ketorolac tromethamine has been shown to relieve ocular itching, most likely through its inhibition of prostaglandin synthesis (Donshik, Pearlman, Pinnas, et al., 2000) (see Section III).
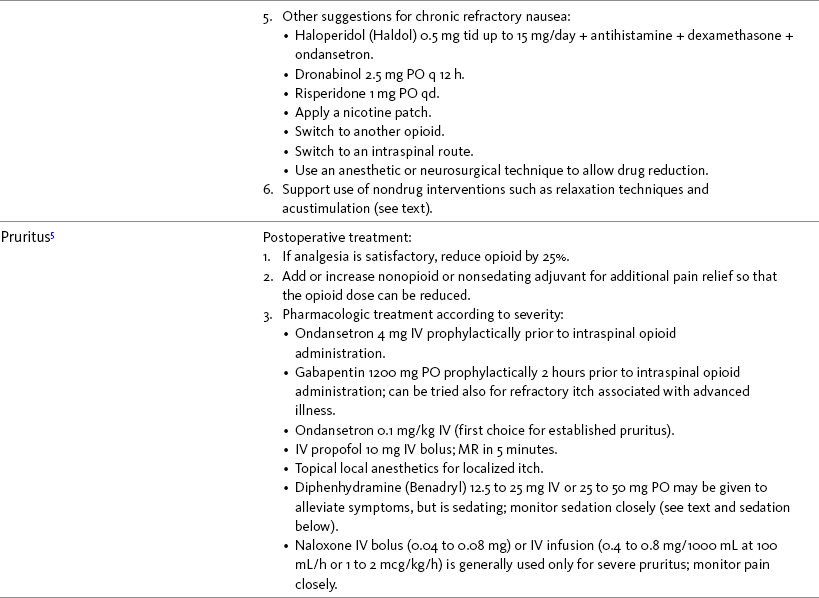
It is thought that serotonin (5-HT) acts on 5-HT3 receptors to generate the sensation of pruritus (Waxler, Dadabhoy, Stojiljkovic, et al., 2005). This helps to explain why the serotonin receptor antagonists have been used to successfully treat pruritus caused by intraspinal opioids. A prospective study randomized 105 patients to receive ondansetron 4 mg, dolasetron 12.5 mg, or placebo 30 minutes before spinal morphine and bupivacaine anesthesia (Iatrou, Dragoumanis, Vogiatzaki, et al., 2005). Patients in both treatment groups experienced significantly less pruritus during the first 8 postoperative hours than the placebo group, and severe pruritus was noted only in those who received placebo. Others have reported similar results with ondansetron for prevention of pruritus from intrathecal morphine (Yeh, Chen, Lin, et al., 2000) and fentanyl (Gurkan, Toker, 2002). However, no benefit was found with the combination of dexamethasone (8 mg) and ondansetron (4 mg) for prevention of intrathecal morphine-induced pruritus (Szarvas, Chellapuri, Harmon, et al., 2003). Ondansetron orally disintegrating tablets (ODT) 8 mg, ondansetron IV 4 mg, or placebo was administered prior to intrathecal morphine in 150 men undergoing surgery, and although there were no significant differences in PONV, the incidences of pruritus were 56%, 66%, and 86% in the ODT, ondansetron IV, and placebo groups, respectively (Pirat, Tuncay, Torgay, et al., 2005).
Established pruritus is responsive to treatment with ondansetron as well. A randomized controlled study of 80 women with moderate to severe pruritus following intrathecal morphine for cesarean section were given ondansetron (4 mg) or placebo (Charuluxananan, Somboonviboon, Kyokong, et al., 2000). Relief was achieved in 80% of those who received ondansetron compared with 36% of those who received placebo. The recurrence rates within 4 hours after administration were 12% and 70% for ondansetron and placebo, respectively. Nausea and vomiting were also significantly less in the ondansetron group. A case report described the treatment of intractable pruritus with 4 mg of IV ondansetron following spinal fentanyl and bupivacaine anesthesia (Henry, Tetzlaff, Steckner, 2002).
An interesting study found that gabapentin (Neurontin) was effective in reducing the incidence of intrathecal morphine-induced pruritus (Sheen, Ho, Lee, et al., 2008). Patients (N = 86) were randomized prior to limb surgery to receive 1200 mg of gabapentin or placebo orally 2 hours prior to surgery. The incidence of pruritus was 47.5% in the gabapentin group and 77% in the placebo group. The severity of pruritus was greater, its onset shorter, and duration longer in the placebo group. The researchers suggested that the effectiveness of gabapentin may be related to its action on central neurons and the fact that intrathecal morphine-induced pruritus is a “neurogenic itch.” Anticonvulsants such as gabapentin are used to treat pruritus associated with advanced illness as well (Pittelkow, Loprinzi, 2004).
Subanesthetic doses of IV propofol (e.g., 10 mg) have been shown to relieve pruritus associated with intrathecal morphine and are suggested as a second-line option after administration of a serotonin receptor antagonist (Grape, Schug, 2008). One study randomized 50 postoperative patients who had intrathecal morphine-induced pruritus to receive 10 mg of IV propofol or placebo; a dose was repeated 5 minutes later in patients who had a pruritus score of more than 2 on scale of 0 to 5 (Borgeat, Wilder-Smith, Saiah, et al., 1992). Treatment failure was defined as the persistence of a pruritus score of greater than 2 at 5 minutes after treatment. The success rate was 86% and 16% in the propofol and placebo groups, respectively. In contrast, a later randomized controlled study of 29 women with intrathecal morphine-induced pruritus following cesarean section found no difference between propofol 10 mg IV and placebo (Beilin, Bernstein, Zucker-Pinchoff, et al., 1998).
Topical local anesthetics such as EMLA or lidocaine patch 5% may provide relief of pruritus in some patients. These preparations are not convenient for generalized pruritus, but may be helpful for localized areas that are particularly bothersome (Pittelkow, Loprinzi, 2004).
Opioid Antagonists for Pruritus
Opioid antagonists, such as naloxone and naltrexone, are sometimes used to treat pruritus; however, this practice risks reversal of analgesia if the doses administered are too high. Ultra low-dose IV naloxone boluses (0.04 to 0.08 mg) or infusions (0.4 to 0.8 mg/liter IV fluid at 100 mL/h or 0.1 to 0.2 mcg/kg/h) or naltrexone (6 to 9 mg orally) may be effective in some patients with severe pruritus (Dabu-Bondoc, Franco, Sinatra, 2009; Grape, Schug, 2008) (see Table 19-1). The agonist antagonist opioid nalbuphine 4 mg IV given prior to intrathecal morphine for cesarean section for prevention of pruritus had a better success rate (20%) than ondansetron 4 mg IV (13%), ondansetron 8 mg (12%), or placebo (6%) (Charuluxananan, Kyokong, Somboonviboon, et al., 2003). Pain must be monitored closely when opioid antagonists are used.
The peripherally-acting opioid antagonist, methylnaltrexone, indicated for treatment of opioid-induced constipation, was shown to reduce the sensation of skin itch in healthy volunteers who were given IV morphine (Yuan, Foss, O’Connor, et al., 1998). Clinical research and more options from this group of drugs are needed, but these agents may have a role in the management of a variety of opioid-induced adverse effects in the future (Bates, Foss, Murphy, 2004).
Switching to Another Opioid
Switching to another opioid may relieve pruritus but is usually reserved for patients with pruritus that has been unresponsive to other treatments. A case report described intractable itching that began when morphine was initiated in a patient with small round cell tumor of the pelvis and was not improved when the patient was switched to fentanyl and then to hydromorphone (Tarcatu, Tamasdan, Moryl, et al., 2007). Diphenhydramine and hydroxyzine also had no effect. When oxycodone and a low-dose naloxone infusion (0.25 mcg/kg/h) were started, the patient’s pruritus dramatically improved. Pain control was achieved with titrated doses of oxycodone (60 mg every 3 hours) and IV hydromorphone PRN. Naloxone was discontinued seven days later with no recurrence of itching. The authors suggested that the resolution of pruritus was because the oxycodone created a balance between mu and kappa opioid receptors through a predominantly kappa agonist effect. (Morphine, hydromorphone, and fentanyl bind primarily to mu opioid receptors.) Further, the authors discounted a singular role for naloxone since itching did not recur after it was discontinued.
Effective, Safe, and Inexpensive Treatment
A common clinical observation is that patients with postoperative opioid-induced pruritus have well-controlled pain. This may be because, as mentioned, the two sensations of pain and itch seem to be interrelated; painful stimuli can inhibit itching, and inhibition of pain processing may enhance itching. This helps to explain why a small decrease in the opioid dose is such an efficient solution to opioid-induced pruritus. Opioid dose reduction is by far the single most effective, safest, and least expensive treatment for pruritus. Postoperative opioid orders should include the expectation that nurses will decrease the opioid dose by 25% prior to or in conjunction with pharmacologic treatment of moderate-to-severe pruritus (see Form 17-1 on p. 464 for an example of how decreases in opioid dose can be included in opioid order sets). Patients usually tolerate this small reduction in opioid dose without any loss of analgesia and experience a significant reduction or complete resolution of their pruritus. Decreasing the opioid dose is facilitated by adding or increasing a nonopioid, such as an NSAID or acetaminophen, or adding a local anesthetic to the epidural opioid solution to provide additional pain relief.
In summary, pruritus should be treated according to its severity or prevented based on its expected severity (see Table 19-1). The use of a self-report pruritus assessment scale is recommended to help determine severity and effectiveness of treatment in patients with intractable pruritus. Intraspinal opioid-induced pruritus should be prevented with administration of a serotonin antagonist, particularly when a single-bolus intraspinal technique is used. Serotonin antagonists may be helpful for established pruritus as well. Mild facial and chest pruritus may be relieved by cold compresses. The easiest and most effective treatment for pruritus is to decrease the opioid dose if possible. Opioid agonist-antagonists and opioid antagonists should be reserved for severe pruritus, and the patient should be watched closely for any increase in pain if these are used.
Hypotension
Research studies vary widely in their definitions of hypotension, so the reported incidences vary widely. Some studies do not provide any definition, but most describe hypotension as a systolic arterial pressue of less than 80 mm Hg to less than 100 mm Hg and/or a greater than 20% decrease in arterial pressure. The overall rate of hypotension related to postoperative pain management is thought to be 4.7%, with the lowest incidence associated with IV PCA and the highest with epidural analgesia (Cashman, Dolin, 2004). An audit of over 2500 patients cared for by an acute pain service who had received a variety of analgesic techniques reported hypotension in just 4 patients due to bupivacaine and fentanyl, all with a sensory block higher than T5 (Tsui, Irwin, Wong, et al., 1997) (see Chapter 15 for discussion of thoracic epidural analgesia).
Opioids have no effect on myocardial contractility or output and, therefore, do not produce severe hemodynamic instability (i.e., severe hypotension); however, they can produce dose-related, asymptomatic bradycardia (Harris, Kotob, 2006; Ho, Gan, 2009). This is thought to be related to stimulation of the vagal nuclei in the medulla (Ho, Gan, 2009). The exception to this is meperidine, which has intrinsic antimuscarinic properties and can increase resting heart rate. Morphine can indirectly cause hypotension through the release of histamine, which causes vasodilation (Harris, Kotob, 2006), and this effect varies among individuals (Ho, Gan, 2009).
The opioid doses commonly used for pain management rarely cause hypotension (Ho, Gan, 2009). Cashman and Dolin (2004) appropriately point out that research shows that many factors other than analgesic technique (e.g., surgical factors) influence hypotension. When it does occur, it is more likely to be in individuals with high sympathetic tone, such as those with pain or poor cardiac function, or in patients who are hypovolemic. In fact, addressing pain is important because it may be contributing to hemodynamic instability. In other words, opioids should not be withheld for fear of causing hypotension.
When hypotension is a concern, it can be minimized by administering the opioid slowly, keeping the patient supine, and optimizing intravascular volume (Harris, Kotob, 2006; Ho, Gan, 2009). Therapy can begin with a small dose while closely observing patient response. Administration of opioids via slow IV infusion may be appropriate in some patients (Harris, Kotob, 2006).
Caution is recommended when administering morphine and any other histamine-releasing opioid to patients with cor pulmonale as deaths have been reported with their use in this population (Harris, Kotob, 2006). Opioids that do not release histamine are fentanyl and sufentanil (Ho, Gan, 2009). See Chapter 15 and Table 15-7 on pp. 428-429 for discussion and treatment of hypotension as an unwanted effect of intraspinal local anesthetics.
Urinary Retention
Opioids increase smooth muscle tone in the bladder and ureters and can cause bladder spasm and urgency (Hanks, Cherny, Fallon, 2004). An opioid-induced increase in sphincter tone can make urination difficult. The central effects of opioids may reduce a patient’s attention to bladder stimuli, which can result in urinary retention. Urinary retention is not a common adverse effect of opioids but is observed most often in older-aged men (Hanks, Cherny, Fallon, 2004). A review of the literature revealed an incidence of urinary retention requiring catheterization to be 23% in the postoperative setting (Dolin, Cashman, 2005).
Neuraxial opioids are associated with a higher incidence of urinary retention than systemic opioids, and intrathecal opioid administration has the highest reported incidence of urinary retention (35.6%) (Wheeler, Oderda, Ashburn, et al., 2002). Although the mechanism is not fully understood, urinary retention from neuraxial opioids is thought to be the result of spinally-mediated inhibition of parasympathetic outflow (Bates, Foss, Murphy, 2004; Dabu-Bondoc, Franco, Sinatra, 2009). The addition of local anesthetic to the opioid intraspinally can compound urinary retention. It is seen less often in patients receiving thoracic than lumbar epidural analgesia, so dermatomal level of the neuraxial blockade has been cited as a possible contributing factor (Dabu-Bondoc, Franco, Sinatra, 2009; Wu, 2005). It is likely that there are multiple causes and risk factors including age, type of surgery, lack of ambulation, and abnormal voiding history. The incidence of urinary retention in the PACU (16%) was influenced only by amount of intraoperative fluids and bladder volume on entry to the PACU in one study (Keita, Diouf, Tubach, et al., 2005).
Low-dose naloxone has been used to treat urinary retention but can reverse analgesia and is not recommended (Dabu-Bondoc, Franco, Sinatra, 2009; Wang, Pennefather, Russell, 1998). A double-blind study found that both naloxone (0.01 IV) and the peripheral opioid antagonist methylnaltrexone (0.3 IV) reversed remifentanil-induced urinary retention in 13 male volunteers; however, whereas methylnaltrexone appeared to work via peripheral mechanisms, naloxone reversed central opioid effects (Rosow, Gomery, Chen, et al., 2007). Clinical research is needed to determine the role of the peripheral opioid antagonists in the treatment of opioid-induced urinary retention.
In and out bladder catheterization may be necessary and sufficient to relieve urinary retention in postoperative patients. For refractory urinary retention and for urinary retention in patients with persistent pain, indwelling catheterization is recommended rather than repeated catheterizations if the opioid dose cannot be reduced.
The common practice of using indwelling urinary catheters in patients receiving thoracic epidural analgesia has been questioned. A prospective study of 100 consecutive patients receiving continuous thoracic epidural analgesia for colon resection demonstrated that removal of indwelling urinary catheters 24 hours after surgery was well tolerated with just 8 patients requiring a single in and out catheterization and 1 requiring indwelling recatheterization (Basse, Werner, Kehlet, 2000). A later study of 49 patients receiving thoracic PCEA removed indwelling urinary catheters within 12 to 48 hours after surgery and found just 5 patients (10%) required recatheterization (Ladak, Katznelson, Muscat, et al., 2009). Others have found similar results (Chia, Wei, Chang, et al., 2009). If urinary catheters are used, removing them as soon as possible after surgery to reduce pain, improve mobility, and prevent infection is recommended (Pasero, Belden, 2006; Wilmore, Kehlet, 2008).
Tolerance to opioid-induced urinary retention does develop. As with the other opioid-induced adverse effects, decreasing the opioid dose, if possible, is the most effective treatment.
Myoclonus
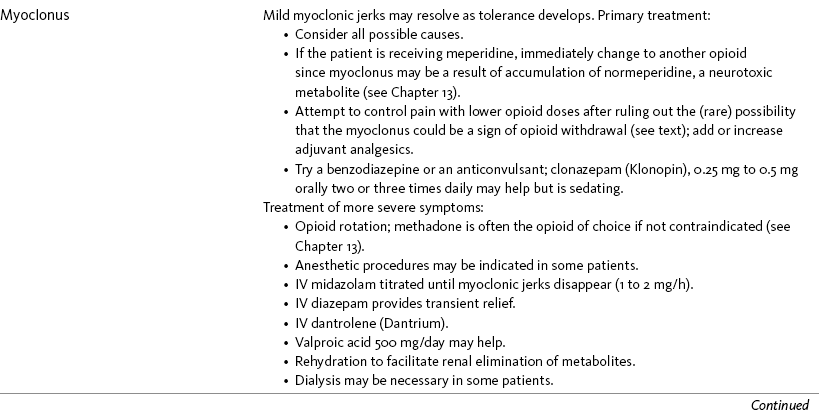
Myoclonus is sudden, brief, involuntary muscle contractions arising from the CNS (Harris, Kotob, 2006). They can be multifocal, occurring in different places in the body. Mild and infrequent myoclonus is common in patients taking opioids with a prevalence of 83% (Glare, Walsh, Sheehan, 2006) and may resolve as tolerance develops (Coyle, Cherny, Portenoy, 1995); however, occasionally they are severe and can cause increased breakthrough pain during myoclonic episodes (Hanks, Cherny, Fallon, 2004). Myoclonic jerks usually are experienced only by patients receiving high doses of opioids (Hagen, Swanson, 1997; Han, Arnold, Bond, et al., 2002; Okon, George, 2008). Although all opioids can produce myoclonus, the effect is most prominent with meperidine, presumably from normeperidine accumulation (Hagen, Swanson, 1997) (see Chapter 13). One case report suggested that the patient’s myoclonus might have been related to opioid withdrawal, underscoring the need to consider all possible causes for symptoms to determine the appropriate treatment (Han, Arnold, Bond, et al., 2002).
If the patient is taking meperidine, switching to another opioid should be done immediately to prevent further possible accumulation of normeperidine and subsequent seizures. For other opioids, primary treatment includes attempts to control pain with lower opioid doses and by adding or increasing adjuvant analgesics (Coyle, Cherny, Portenoy, 1995; Hanks, Cherny, Fallon, 2004). A benzodiazepine or anticonvulsant may be tried (Hanks, Cherny, Fallon, 2004). For example, clonazepam (Klonopin) 0.25 mg to 0.5 mg orally two or three times daily may help to control jerking, but patients may dislike the sedation it produces.
Rotation to a new opioid is instituted if symptoms worsen despite primary treatment (Mercadante, Ferrera, Villari, et al., 2009) (see Chapter 18). The muscle relaxant dantrolene (Dantrium) has been used to reduce symptoms (Mercadante, 1995), and anesthetic procedures may be indicated in some patients (Coyle, Cherny, Portenoy, 1995). A series of five case reports described treatment of severe toxicity and myoclonus. Carbamazepine (Tegretol) and phenytoin (Dilantin) were ineffective, valproic acid 500 mg/day was of uncertain benefit, and large doses of diazepam (Valium) provided only transient relief; however, cessation of the offending opioid, rotation to a new opioid, and midazolam infusion, titrated upward until myoclonic jerks disappeared (1 to 2 mg/h), successfully controlled severe myoclonus in 2 patients (Hagen, Swanson, 1997). Rehydration to facilitate renal elimination of metabolites is recommended (Morita, Tei, Tsunoda, et al., 2002). Dialysis may be necessary (Hagen, Swanson, 1997) (see Table 19-1).
Mental Status Changes
Confusion, disorientation, and cognitive impairment are among the most feared of opioid-induced adverse effects for patients and families. It occurs in 28% to 83% of patients near the end of life, depending on the population studied and criteria used to define it (Casarett, Inouye, 2001). Mild cognitive impairment and occasional hallucinations may occur when opioid therapy is initiated and with significant dose increases (Hanks, Cherny, Fallon, 2004). Patients can be reassured that these are transient and will resolve within days to a couple of weeks. Unresolved delirium may necessitate switching to another opioid. An open-label trial described switching patients experiencing delirium related to morphine toxicity to transdermal or parenteral fentanyl (Morita, Takigawa, Onishi, et al., 2005). Others have advocated the use of methadone in this situation (Mercadante, Ferrera, Villari, et al., 2009) (see Chapter 13). Table 19-1 outlines treatment options. See also Chapter 20 for more on cognitive effects during long-term opioid therapy.
Delirium in the terminally ill has a variety of manifestations, including hallucinations, disorientation, clouding consciousness, fear, and paranoia. Opioids usually are not a cause but may be a contributing factor. Terminally ill patients with delirium tend to be taking higher opioid doses than those who do not have delirium (Gagnon, Allard, Masse, et al., 2000), but a higher dose requirement could be related to underlying pathologic processes that contribute to delirium as well. Other potential causes of delirium are major organ failure, neoplastic involvement of the CNS, sepsis, hypoxia, and electrolyte disorders, such as hypercalcemia (Hanks, Cherny, Fallon, 2004).
Up to half of delirium episodes are not noted by clinicians, underscoring the importance of a careful history and thorough evaluation that includes an accurate assessment of the patient’s baseline status (Casarett, Inouye, 2001). A variety of tools are available for the assessment of delirium (Schuurmans, Deschamps, Markham, et al., 2003; Timmers, Kalisvaart, Schuurmans, et al., 2004). The Confusion Rating Scale allows evaluation of observable behaviors, and the Neecham Confusion Scale includes vital signs and oxygen saturation levels, which may provide early signs of delirium (Williams, 1991). The Delirium Observation Screening (DOS) Scale (Schuurmans, Shortridge-Baggett, Duursma, 2003) (Form 19-1) is a simple screening tool that can be easily integrated into routine care (Gagnon, Allard, Masse, et al., 2000). The Confusion Assessment Measure (CAM) (Inouye, van Dyck, Alessi, et al., 1990) is often used to confirm the diagnosis of delirium (Gagnon, Allard, Masse, et al., 2000; Schuurmans, Shortridge-Baggett, Duursma, 2003; Vaurio, Sands, Wang, et al., 2006). (See templates for some of the tools at http://www.clintemplate.org/groups/15/) (see Table 19-1).
Form 19-1 Delirium Observation Screening (DOS) Scale. Maximum DOS score is 13. A score of less than 3 = not delirious; a score of 3 or more = probably delirious (contact primary provider). From Schuurmans, M. J., Shortridge-Baggett, L. M., & Duursma, S. A. (2003). The delirium observation screening scale: A screening instrument for delirium. Res Theory Nurs Pract, 17(1), 31-50.
Postoperative Delirium
The reported incidence of postoperative delirium is 10% to 60%, and it is most frequent in older adults (Vaurio, Sands, Wang, et al., 2006). This number is likely to increase as more old than young adults have surgery (Amador, Goodwin, 2005). Delirium is associated with prolonged hospital stay, greater use of hospital resources, and increased cost of care (Demeure, Fain, 2006). Despite this, there is little well-controlled research on the incidence and prevention of delirium in the hospitalized patient (Siddiqi, Holt, Britton, et al., 2007).
Similar to the terminally ill, delirium tends to be underdiagnosed in the postoperative setting (Amador, Goodwin, 2005). It is characterized by alterations in orientation, consciousness, memory, thought processes, and behavior (Demeure, Fain, 2006). Patients may demonstrate either hyperactivity or hypoactivity and may be lucid at times, but typically the symptoms are worse at night (“sundowning”). A variety of delirium screening and diagnostic tools are available (see previous discussion and Form 19-1). Table 19-1 provides treatment suggestions.
Opioids are often blamed for the occurrence of postoperative confusion; however, little research supports the assertion that they are a major cause. In fact, pain and other factors, such as sleep disturbance, type of surgery, choice of opioid, and method of administering opioids, have been directly linked to postoperative cognitive impairment. As described in the terminally ill, the importance of an accurate assessment with consideration of the patient’s baseline cognitive status is critical in the confused postoperative patient; many patients undergoing surgery have pre-existing confusion and cognitive impairment and should be assessed as “at risk” for postoperative confusion. Box 19-2 lists the risks associated with postoperative delirium.
Poorly managed pain appears to be an important risk factor for postoperative delirium. Early research showed that a decline in mental status was linked to poor pain control in patients aged 50 to 80 in one study (Duggleby, Lander, 1994). Pain, not analgesic intake, predicted mental decline. More recently, a study was conducted in 330 consecutively admitted patients (age 65 years or older) scheduled for cardiac surgery (Vaurio, Sands, Wang, et al., 2006). Interviews and baseline assessments of neurologic, functional, and cognitive status and pain were completed within 48 hours before surgery and repeated at 24 and 48 hours after surgery. Both the presence of postoperative pain and increased pain postoperatively were found to be independent predictors of postoperative delirium in these patients and to have a greater impact on the development of delirium than any of the other factors evaluated (e.g., age, type of anesthesia, postoperative medications, and preoperative cognitive status). There was also an ordered relationship between levels of postoperative pain and risk of delirium; those with severe pain were at a greater risk of developing delirium than those with moderate pain. An important finding was that neuraxial analgesia and IV PCA carried the same risk despite the lower opioid doses given neuraxially. Similarly, an earlier study reported no difference in the incidence of delirium between IV fentanyl and epidural fentanyl analgesia for pain following total knee replacement (Williams-Russo, Urquhart, Sharrock, et al., 1992). In contrast, fewer complications, including mental confusion, were found in older adult men receiving IV PCA morphine compared with those receiving IM morphine (Egbert, Parks, Short, et al., 1990).
The choice of opioid used to control pain also may influence the incidence of mental confusion, but research demonstrating differences among the various opioids is lacking. One exception is meperidine, which is more likely than other opioid drugs to cause delirium in postoperative patients of all ages (Fong, Sands, Leung, 2006). In a case control study (N = 91 with 1 to 2 controls), meperidine more than doubled the risk of delirium when given either epidurally or IV (Marcantonio, Juarez, Goldman, et al., 1994). It has also been shown to be the only opioid to have a negative impact on mood (Latta, Ginsberg, Barkin, 2002), sometimes the first sign of neurotoxicity. Early research found that IV PCA fentanyl produced less depression of postoperative cognitive function than IV PCA morphine (Herrick, Ganapathy, Komar, et al., 1996). IV PCA morphine and IV PCA hydromorphone provided similar analgesic efficacy, adverse effects, mood, and cognitive function in another study (Rapp, Egan, Ross, et al., 1996). Although the patients receiving hydromorphone experienced improved mood, they also demonstrated poorer cognitive performance than those receiving morphine.
In summary, these research findings reinforce that factors other than dose of opioid, such as quality of pain control, play a more important role in the development of delirium. Studies and clinical observations suggest that improvement in pain management practices, including the administration of appropriate doses of opioids, is one way to reduce confusion after surgery. The common reaction of many clinicians to abruptly discontinue the opioid when a patient develops confusion may worsen the confusion and is not recommended. After careful evaluation of all potential causes, a better approach is to add or increase a nonopioid or nonsedating adjuvant analgesic and decrease the opioid dose if a decrease is thought to be necessary (see Table 19-1).
Sedation or Cognitive Impairment During Long-Term Opioid Therapy
Most patients experience sedation at the beginning of opioid therapy and whenever the opioid dose is increased significantly (Hanks, Cherny, Fallon, 2004). The incidence of sedation in patients receiving oral morphine for cancer pain is 20% to 60% (Cherny, Ripamonti, Pereira, et al., 2001), and it is often cited as a reason for inability to titrate an opioid to pain relief (Harris, Kotob, 2006). The underlying mechanisms of opioid-induced sedation or cognitive impairment are unclear, but are thought to be related to their effects on cholinergic pathways that modulate cortical arousal and information processing (Harris, Kotob, 2006). Opioids inhibit REM sleep and other aspects of normal sleep due to anticholinergic activity (Slatkin, Rhiner, Bolton, 2001; Young-McCaughan, Miaskowski, 2001). They also depress sensitivity of central and peripheral chemoreceptors to carbon dioxide, which has the potential to diminish wakefulness (Slatkin, Rhiner, Bolton, 2001). Sometimes patients experience persistent sedation during long-term opioid treatment if other sedating drugs are taken or there are underlying conditions that cause sedation, such as metabolic disturbances (Coyle, Cherny, Portenoy, 1995). Care must be taken not to confuse sedation with exhaustion and the need to “catch up” on sleep when poorly controlled pain is finally controlled (Coyle, Cherny, Portenoy, 1995).
Although a disturbing adverse effect, especially for patients on long-term opioid therapy, they can be reassured that tolerance to sedation and cognitive impairment usually develops over a period of days to weeks (Hanks, Cherny, Fallon, 2004). The clinical presentation ranges from drowsiness to somnolence, and the degree of sedation is used to guide its treatment (Pasero, 2009b; Young-McCaughan, Miaskowski, 2001). If possible, the opioid dose is decreased to treat sedation. If opioid dose reduction cannot be done or is unsuccessful, adding a psychostimulant is an option. Modafinil (Provigil) 200 mg in the morning, or methylphenidate (Ritalin) at 2.5 mg to 5 mg in the morning and repeated midday are the most commonly selected psychostimulants (see Chapter 31). Doses can be titrated upward. A report of 6 cases also described the use of the acetylcholinesterase inhibitor donepezil (Aricept) 5 mg daily in patients taking the equivalent of 200 mg/day of oral morphine and experiencing excessive sedation and unstable pain control (Slatkin, Rhiner, Bolton, 2001). Patients demonstrated improved alertness after donepezil was started, which allowed the clinicians to better optimize the pain management regimen.
If significantly impaired, patients should be discouraged from performing dangerous activities, such as driving or operating complicated mechanical equipment, until sedation subsides (see the section that follows for more on driving during long-term opioid therapy). Severe sedation precedes respiratory depression, and if sedation is severe or is worsening, it should be addressed promptly, typically either by dose reduction or a switch to a different opioid (Harris, Kotob, 2006) (see Chapter 18). Alternative methods of pain control, such as intraspinal opioids and/or local anesthetics, must be considered if the effect cannot be mitigated with systemic therapy (Coyle, Cherny, Portenoy, 1995). Table 19-1 provides treatment suggestions for patients receiving long-term opioid therapy.
Opioids and Driving Ability
For many people, driving an automobile is a daily activity that is a key element to maintaining their independence, engaging in employment, and remaining socially active. They are essentially disabled if they are deprived of the freedom to drive. As the use of long-term opioid therapy gains greater favor, especially in the care of patients with persistent noncancer pain, it is of great importance to the patient, physician, and public to determine their ability to drive safely.
This problem has been studied since the early 1960s when methadone maintenance therapy was introduced (Dole, Nyswander, 1965). One of the first studies compared the driving records of 401 patients before and after methadone maintenance therapy with 182 drivers selected at random (Gordon, 1973). During methadone treatment, patients did not differ significantly from the comparison group in rates of accidents or in convictions for driving offenses. Interestingly, while using heroin the patients had had fewer accidents, a finding that had no satisfactory explanation. (Of interest, another similar study found that heroin users had a slightly better driving record on heroin than they did on methadone [Maddux, Williams, Ziegler, 1977]). Gordon’s conclusion was the same as many studies to follow: that taking an opioid (methadone in this case) on a long-term basis does not have a significant detrimental effect on the ability to drive.
Before embarking on an exploration of the effects on driving ability of long-term opioid therapy for persistent pain, it is important to point out that those patients who take opioids short-term, those in whom long-term opioid therapy is being initiated, and those on long-term therapy in whom opioid doses are being increased are different circumstances. Studies of the psychomotor and cognitive effects of opioids in these groups are generally different from those discussed in relation to long-term stable opioid therapy.
Extensive reviews of the literature have examined studies on the effects of a single opioid dose or an increased dose of opioid in patients on long-term therapy (Chapman, Byas-Smith, Reed, 2002; Zacny, 1996). These reviews reveal that this is a complex topic and that results of various studies are inconclusive. Results depend upon a multitude of factors such as type of opioid administered, dose of opioid, use of other medications, and whether or not the patient is in pain. For example, it appears that in opioid-naïve, healthy volunteers without pain, psychomotor and cognitive functioning are impaired more by some opioids (e.g., mixed-agonist-antagonists such as buprenorphine and pentazocine) than others (e.g., codeine and morphine). However, this may have little relevance to patients with pain, since pain itself impairs cognition (Lorenz, Beck, Bromm, 1997), and pain relief may improve cognitive functioning (Jamison, Schein, Vallow, et al., 2003). For example, treatment of neuropathic pain with both low and high doses of levorphanol resulted in improvement in some measures of cognitive performance (Rowbotham, Twilling, Davies, et al., 2003).
In a landmark study of patients with cancer, the cognitive effects of stable doses of opioids compared with changes in opioid dose in patients on long-term opioid therapy was conducted by Bruera and others (Bruera, Macmillan, Hanson, et al., 1989). Twenty inpatients with cancer who had increased their opioid dose by at least 30% in the 3 days before testing were compared with 20 other inpatients who had been on a stable opioid dose for at least 7 days. A variety of opioids were used. Compared with the group on a stable opioid dose, the group taking the recently increased dose reported more drowsiness after the dose, and significantly more cognitive impairment occurred 45 minutes after opioid administration compared with several hours afterwards.
The immediate cognitive effects following a dose of opioid are often observed by clinicians and are supported by other studies. The immediate effects of 130 mg of dextropropoxyphene showed impairment was greater when measured 2 hours after than 4 hours after a dose (Saarialho-Kere, Julkunen, Mattila, et al., 1988). Thinking that the immediate effects of an opioid will persist seems to cause some clinicians to fear long-term use of opioids. In correcting this misconception, an explanation of the effects of tolerance may be helpful.
Cognitive performance was examined in a study comparing 6 female patients with persistent noncancer pain before and after being treated with modified-release morphine (Lorenz, Beck, Bromm, 1997). The first testing was before initiation of morphine, and the second examination was after the patients reported sufficient pain relief and had been on a stable dose for at least 3 days. Doses ranged from 30 to 150 mg of modified-release morphine per day. During the second testing session, behavioral and physiologic indicators of vigilance and cognitive function revealed lack of sedation and improved alertness. The authors suggested that these effects might be due in part to the possibility that pain relief made it unnecessary to constantly shift attention between pain and other activities.
The above studies and others have resulted in the recommendation that after a person’s stable opioid dose is increased, caution should be exercised during the first few hours after taking the dose and until the patient has been on a stable dose for 7 days. Cognitive performance during the first few days of opioid use and during the first few hours after a given dose seems likely to be worse. However, after about 7 days of stable opioid dosing, cognitive effects such as sedation seem to subside.
Effects of long-term opioid therapy on driving ability were studied by comparing 16 patients with persistent noncancer pain on opioids for 6 months or longer with 327 cerebrally compromised patients from another study who had off-road and on-road testing for driving ability (Galski, Williams, Ehle, 2000). In the study of the cerebrally impaired patients, certain off-road tests were found to predict success or failure in the on-road evaluation. The patients on opioids were given the off-road tests, and the results were compared with those of the cerebrally impaired patients who had undergone the same evaluation and then passed or failed the on-road evaluation. Generally the patients on opioid therapy performed better than the cerebrally compromised patients who passed the on-road test. Patients on long-term opioid therapy had faster reaction times and did not manifest any major problems with coordination, but they had greater difficulty following instructions and a tendency toward impulsivity, that is, hurrying and sacrificing accuracy for speed. Nevertheless, the results give general support for the ability of patients on long-term opioid therapy to pass actual on-road driving tests.
Twenty-nine patients with persistent noncancer pain (primarily low back pain) on long-term modified-release oxycodone were compared with 90 healthy volunteers to assess their driving ability (Gaertner, Radbruch, Giesecke, et al., 2006). Attention reaction, visual orientation, motor coordination, and vigilance were evaluated. This included a test battery that followed the German national recommendations on tests to determine driving ability. Permission to drive is usually denied if the score is below the sixteenth percentile. Using this definition of driving ability, there was no significant difference between the patients receiving modified-release oxycodone and the control group. The authors concluded that long-term treatment with modified-release oxycodone did not prohibit driving. However, they recommended individual assessment.
Another study using the German national recommendations on tests to determine driving ability compared 90 healthy controls with 21 patients with noncancer pain who had been treated with transdermal fentanyl for at least 4 weeks without a dose change in the last 12 days (Sabatowski, Schwalen, Rettig, et al., 2003). The median dose of transdermal fentanyl was 50 mcg/h with a range of 25 to 400 mcg/h. Of the 21 patients on transdermal fentanyl, all but 1 considered themselves fit to drive while the one “didn’t know.” Based on test results, the authors concluded that patients with noncancer pain on a stable dose of transdermal fentanyl do not have any clinically significant impairment of psychomotor or cognitive function that would prevent them from driving a car.
Of note is the fact that in the above study all patients except 1 correctly assumed they were able to drive. This suggests the wisdom of asking patients about their ability to drive and also of comparing these answers in future studies to actual ability to drive.
Driving performance, cognition, and balance were also studied before and after adding transdermal fentanyl to treatment regimens for 23 patients with persistent noncancer pain (Menefee, Frank, Crerand, et al., 2004). After patients were stabilized on a dose for 1 month, the tests were repeated. The median dose was 50 mcg/h. Once the dose of transdermal fentanyl had stabilized, it did not have a negative effect on driving performance, reaction time, or cognition. In fact, there was improvement on some measures of cognitive performance.
A poster presentation (Furlan, Lakha, Yegneswaran, et al., 2007) reported on the results of a literature search for controlled studies of long-term opioid use and the effect on driving. Although 1720 studies were located, only 31 were usable. They found that only one study tested driving on the road and two on a driving simulator and that, in these studies, stable opioid doses did not impair driving ability. In all 31 studies, there were 79 evaluations, and 27 found impairment, but no details about this impairment were given. The authors concluded that the quality of the studies was very low, but stated, “stable doses of opioids do not affect driving performance in a chronic pain population” (p. 127).
A structured evidence-based review, which is different from a meta-analysis, was conducted to examine whether long-term stable opioid dosing caused impairment in driving ability (Fishbain, Cutler, Rosomoff, et al., 2003). A literature search revealed 209 references, but only 48 were relevant to addressing driving abilities of patients on stable doses of opioids. These articles were reviewed in detail and then sorted and tabulated in several topic areas such as psychomotor abilities, cognitive functioning, and motor vehicle violations. Based on guidelines developed by the Agency for Health Care Policy and Research (AHCPR), the studies were categorized according to various levels of evidence, from well-designed controlled studies to case reports, and then categorized as to their strength and consistency of evidence. Following are the results of this evidenced-based review of studies of patients on long-term stable doses of opioids:
1. Psychomotor abilities—moderate, generally consistent evidence for no impairment
2. Psychomotor abilities immediately after being given new, additional doses of opioids—strong consistent evidence on multiple studies for there being no impairment of psychomotor abilities
3. Cognitive abilities—inconclusive evidence to support there being no cognitive impairment
4. Incidence of motor vehicle violations/motor vehicle accidents in the general population versus comparable controls of opioid-maintained patients—strong consistent evidence for no greater incidence in motor vehicle violations/motor vehicle accidents
5. Impairment as measured in driving simulators or off/on road driving as compared to controls—consistent evidence of no impairment in opioid-maintained patients
The authors of the above study concluded that overall the majority of studies appeared to indicate that patients on stable doses of long-term opioid therapy are not impaired in regard to driving skills. The authors also pointed out that the most relevant evidence were those studies that showed that opioid-tolerant patients appear to perform driving skills as well as controls, as stated in number 5 above. Nevertheless, they recommended that the decision to drive be made on an individual basis and specified the recommendations in Box 19-3.
A structured evidence-based literature review using methodology similar to the above study was conducted to determine if there was evidence of an association between opioid use and intoxicated driving, motor vehicle accidents (MVA), and MVA fatalities (Fishbain, Cutler, Rosomoff, et al., 2002). Except for those subjects identified as being on methadone maintenance therapy, no information was provided on how long the subjects had been taking opioids. The evidence indicated that opioids probably are not related to intoxicated driving, MVA, or MVA fatalities, supporting the recommendation that patients on opioids be allowed to drive. However, again, this decision should be made on an individual basis.
Compared with patients with noncancer pain on long-term opioid therapy, determining if patients with cancer on long-term opioid therapy are able to drive is a more complex matter (Brandman, 2005). Patients with cancer often have co-morbidities, neurologic changes, other medications, and advancing disease, causing their driving ability to change over time. The reader is referred to the specific guidelines given in the Brandman (2005) article for helping patients and physicians determine safe driving ability in patients with cancer on long-term opioid therapy. Some of these suggestions include asking occupational therapists to perform a driving assessment of the patient and considering the use of a CNS stimulant such as methylphenidate or modafinil for patients who report drowsiness.
In a medicolegal case report that presented the details of the case of a patient with persistent low back pain on long-term opioid therapy, the authors submitted some recommendations on how pain physicians should approach the problem of whether patients should be allowed to drive while on long-term opioid therapy (Fishbain, Lewis, Cole, et al., 2007). Although their literature review indicated that such patients could drive safely once they had developed a tolerance to the sedating effects of opioids, they offered specific guidelines such as suggesting that some form of informed consent concerning the risks of driving while on long-term opioid therapy should be obtained. Specific points they felt should be discussed with the patient on long-term opioid therapy included advising the patient of the current literature on driving while on this therapy, that whether or not to drive was their own personal decision, and that if they decided to drive they should obey certain rules. Some of these rules were not to drive for 4 to 5 days after beginning opioid treatment, not to drive if they felt sedated, and not to use alcohol or other illicit drugs before driving. The reader is referred to this article for information on other recommendations. Similar recommendations are available in Fishbain, Cutler, Rosomoff, et al., 2003 (see Box 19-3).
Not only patients and their families but also policymakers should be informed that the majority of the current literature shows that driving ability is not necessarily impaired in patients on stable doses of opioids. Without this knowledge, those responsible for enacting laws related to driving may restrict patients taking opioids. For example, in 1995 a guide was distributed to the British medical community recommending that people taking morphine-like opioids should not drive (as reported in Zacny, 1996). Zacny (1996) pointed out that the science of opioids and functioning should be used when making policy decisions, rather than hearsay and speculation.
In summary, whether or not a patient taking opioid analgesics can drive must be decided on an individual basis. Some patients will be able to drive safely, but others may be impaired by opioids. Impairment may be brief such as at the beginning of opioid therapy or following significant increases in dose. Some opioids cause more impairment for an individual patient than others, and a change in opioid may remedy the situation. It seems prudent to warn all patients that driving ability may be compromised and that they should not drive if they feel drowsy, dizzy, or impaired in any way.
It should be noted that the concern over effects of medication on driving ability should not be limited to opioids. Currently there seems to be a bias against the use of opioids that has helped fuel the concern about how these analgesics affect driving ability. However, other medication deserves attention too. Studies of older adults indicate that medications such as antidepressants or benzodiazepines (Ray, Fought, Decker, 1992), especially long–half-life benzodiazepines (Hemmelgarn, Suissa, Huang, et al., 1997) are at greater risk for motor vehicle crashes than those on opioids. Cognitive impairment was studied by comparing 13 patients taking benzodiazepines only with 13 patients taking opioids only (Hendler, Cimini, Long, 1980). The results showed that benzodiazepines were far more likely to produce cognitive impairment. In a study of 40 licensed drivers, age 25 to 44, the effects of fexofenadine (60 mg, Allegra), diphenhydramine (50 mg, Benadryl), alcohol (approximately 0.1% blood alcohol concentration), and placebo on driving performance were studied (Weiler, Bloomfield, Woodworth, et al., 2000). Driving performance was the poorest after taking diphenhydramine, impairing driving even more than alcohol.
Sedation During Short-Term Opioid Therapy in Opioid-Naïve Patients
Unfortunately, for patients receiving short-term opioid therapy, time does not allow for development of tolerance to sedation. In addition to affecting the patient’s ability to participate in the recovery process, if left untreated, excessive sedation can progress to clinically significant opioid-induced respiratory depression in opioid-naïve patients (Mcintyre, Upton, 2008; Vila, Smith, Augustyniak, et al., 2005).
Sedation Assessment
The observation that excessive sedation precedes opioid-induced respiratory depression (Abou hammoud, Simon, Urien, et al., 2009; Taylor, Voytovich, Kozol, 2003) indicates that systematic sedation assessment is an essential aspect of the care of opioid-naïve patients receiving opioid therapy (Nisbet, Mooney-Cotter, 2009; Pasero, 2009b). The importance of monitoring sedation to prevent clinically significant respiratory depression cannot be overemphasized. As the American Pain Society (APS) (2003) succinctly states, “No patient has succumbed to (opioid-induced) respiratory depression while awake” (p. 35).
Nursing assessment of opioid-induced sedation is convenient, inexpensive, and takes minimal time to perform (Pasero, 2009b). To assess sedation, the nurse should ask the patient a simple question, such as “What did you have for breakfast today?” then observe the patient’s ability to stay awake and answer the question. Patients who are excessively sedated will typically have difficulty keeping their eyes open and will fall asleep while responding, usually in the middle of a sentence. Nursing technicians (and other health care team members, including ancillary staff such as pastoral care counselors and dieticians) should be trained to watch for and report these signs of excessive sedation if evident and to routinely observe how alert patients are every time they obtain vital signs. Touching the patient to provide care and even simply entering the room can arouse a patient, giving the false impression of an acceptable level of sedation. Therefore, it is essential that the nurse or nursing technician observe the patient without stimulation for long enough to ensure an accurate evaluation.
Sedation Scales
A simple, easy-to-understand sedation scale that includes what should be done at each level of sedation should be used to enhance accuracy and consistency of assessment and treatment, monitor trends, and communicate effectively between members of the health care team. The use of a scale to assess sedation during opioid pain management is common in hospitals in the United States; a 2006 survey of members of the APS and the American Society for Pain Management Nursing (ASPMN) email list serve revealed that all but one respondent (N = 64) used some type of sedation scale during opioid therapies, such as IV PCA and epidural analgesia (Pasero, unpublished data, 2006). The most commonly used scale among those surveyed was the Pasero Opioid-Induced Sedation Scale (POSS) (Box 19-4). A later survey (N = 92) conducted by the ASPMN of its members found a high use of sedation scales; however, the survey did not ask the respondents the purpose for using the selected scales, so it was not possible to evaluate whether the scales were being used appropriately (Pasero, unpublished data, 2009). For example, the Aldrete scale, which is used to determine readiness for discharge from the postanesthesia care unit (PACU) by scoring several criteria (including level of consciousness), was listed as the most commonly used scale (53.3%); the POSS, which is used for assessment of opioid-induced sedation during pain management, was the second most commonly used scale (45.5%).
Note in Box 19-4 that the POSS links nursing interventions to the various levels of sedation. Research has shown that nurses find this approach helpful in making the appropriate decisions on how to proceed with opioid treatment. A descriptive survey-based study compared three sedation scales, the Inova Sedation Scale (ISS) (the researchers’ scale), Richmond Agitation and Sedation Scale (RASS) (Sessler, Gosnell, Grap, et al., 2002), and the POSS (Nisbet, Mooney-Cotter, 2008, 2009). This study invited nurses (N = 96) to participate in an online survey that presented a scenario describing a patient with excessive sedation during IV PCA therapy. The participants were asked to determine the patient’s level of sedation using each of the sedation scales and select the course of action they would take. The percentage of correct sedation score responses with each scale was 46.9% (ISS), 76% (RASS), and 79.2% (POSS); however, the most important finding of this study was that when using the POSS, nurses selected the correct nursing action significantly more often (80.2%) than when using the RASS (68.8%) or the ISS (67.7%). Validity for the POSS and RASS for sedation assessment during pain management was established by a panel of experts for this study, but a lack of discrimination between scale items on the ISS prevented establishment of its validity. Reliability for the scales using Cronbach’s alpha was 0.803 (ISS), 0.770 (RASS), and 0.903 (POSS). Another group of researchers found similar results when they compared the POSS and RASS in patients receiving opioids for pain management (Dempsey, Davidson, Cahill, et al., 2008). The POSS demonstrated reliability of 0.909 (Cronbach’s alpha) in this study.
Sedation scales such as the RASS (Ely, Truman, Shintani, et al., 2003) and the Ramsay Scale (Ramsay, Savege, Simpson, et al., 1974; Olson, Lynn, Thoyre, et al., 2007) have been tested for assessment during goal-directed (purposeful) sedation during procedures and in ventilated critically ill patients but are not recommended for use during opioid administration for pain management because they incorporate assessment of other conditions in addition to sedation (Pasero, McCaffery, 2002; Pasero, 2009b). For example, most goal-directed sedation scales link anxiety or agitation to the scale (Peck, Down, 2009), which complicates assessment because agitation and anxiety are not indicators of increasing opioid-induced sedation. Patients may be either calm or anxious and sedated. Further, the scales used for purposeful sedation were not developed for assessment of unwanted sedation during opioid administration for pain management. Similarly, simple sedation scales such as the POSS were not developed for assessment of purposeful, goal-directed sedation. It is recommended that institutions use two different sedation scales depending on the goal of treatment—one scale for assessment during purposeful sedation (the goal is to produce sedation) and a simpler scale for sedation assessment during opioid administration for pain management (the goal is to prevent sedation) (Pasero, 2009b). As mentioned, PACU nurses often use scoring systems (e.g., Aldrete) that include level of consciousness to determine readiness for discharge, which is acceptable for that area; however, a simple scale such as the POSS is more appropriate for ongoing assessment of opioid-induced sedation after transfer to the clinical unit. A review of sedation scales for assessment of goal-directed sedation can be found in: Odom-Forren, J. & Watson, D. (2005). Practical guide to moderate sedation/analgesia. St. Louis, Mosby. See Table 19-1 and later discussion of the frequency of monitoring sedation and respiratory depression.
Coadministration of Other Sedating Drugs
The administration of IM opioids to opioid-naïve patients receiving IV or epidural analgesia is unnecessary, can result in excessive sedation and clinically significant respiratory depression due to unpredictable absorption (see Chapter 14), and should be avoided. Instead, patients who are receiving IV or epidural opioid analgesia and experiencing uncontrolled pain should be carefully reevaluated. Clinician-administered IV or epidural supplemental doses and increases in the dose of the mainstay opioid usually are indicated (see Chapters 15 and 17).
Although not necessarily contraindicated with parenteral and intraspinal analgesia, muscle relaxants and anxiolytics can produce sedation. Therefore, prior to administering them, it is best to obtain approval for their administration from the prescriber responsible for the pain management plan (e.g., anesthesia provider with postoperative epidural analgesia). Antihistamines are often used to treat pruritus but also must be used with caution as they can be extremely sedating (see earlier discussion and Section V). Sedation can be problematic in those already at risk for excessive sedation, such as postoperative patients, as this can lead to life-threatening respiratory depression (Anwari, Iqbal, 2003). When these drugs are administered, increased monitoring of sedation is recommended, and they should not be administered if patients are excessively sedated.
Patients with Severe Pain and Excessive Sedation
It is important to note that the presence of sedation does not necessarily mean that patients are comfortable. Research shows that caring for patients with both severe pain and excessive sedation is common and presents major challenges to providing safe opioid therapy. In a time-course study of sedation and analgesia in 73 patients in the PACU after major surgery, 52 (73%) patients became sedated during titration necessitating discontinuation of titration; 21 remained awake, and titration was continued (Paqueron, Lumbroso, Mergoni, et al., 2002). Among those in whom titration was stopped because of sedation, 25% had a pain level higher than 50 on the VAS and only 50% had satisfactory pain relief (VAS less than 30). The findings of this study are important to consider when titrating opioids, i.e., that sedation can occur before pain is completely relieved and that sleep during opioid titration is not normal sleep but primarily the result of the sedative effects of the opioid.
Another time course study was conducted in 228 patients in the PACU following major orthopedic surgery who were titrated to either pain relief (VAS of 3 or less) or excessive sedation (Ramsay score higher than 2—a score of 3 indicates response to verbal commands only) (Abou Hammoud, Simon, Urien, et al., 2009). Titration was stopped when analgesia was achieved or an adverse effect occurred such as respiratory rate less than 12 breaths/min, oxygen saturation less than 95%, or sleep (sedation). Titration was stopped in nearly one half (45%) of the patients because of excessive sedation, but no serious adverse events (e.g., respiratory depression) occurred. The effectiveness of morphine was found to be increased in patients who had a decreased delay between extubation and titration, low initial VAS, and intraoperative multimodal analgesia (NSAID and/or acetaminophen administration). Analgesia was attained with a mean of 4 bolus doses and a mean morphine dose of 11 mg. The researchers emphasized that a multimodal approach is essential and that patients may benefit from earlier initiation of nonopioids (e.g., preoperatively). They proposed that higher amounts of bolus doses (e.g., 4 to 5 mg) rather than smaller bolus doses given at shorter intervals may be more effective in controlling severe pain, but appropriately pointed out that this could also increase adverse effects and compromise safety. An important finding was that 20 mg of titrated morphine was associated with a high incidence of excessive sedation, underscoring the need to use other approaches to manage pain that will allow lower opioid doses (i.e., multimodal analgesia). The effectiveness of stopping titration or reducing opioid dose when sedation is excessive is also demonstrated in this study by the lack of clinically significant respiratory depression.
A case control study demonstrated similar challenges in managing patients who have severe pain and also are excessively sedated. Patients in the PACU (N = 285) were titrated with IV morphine (2 to 3 mg) boluses every 5 minutes and then evaluated at 24 hours postoperatively (Lentschener, Tostivint, White, et al., 2007). PACU titration was discontinued when pain relief (VAS less than 3) was achieved or if adverse effects occurred such as excessive sedation (Ramsay score more than 3, sluggish or no response to loud voice or pain). Of the 285 patients, 26 were discharged from the PACU excessively sedated (mean Ramsay score 4) with uncontrolled pain (mean VAS 6.5). These patients were matched with 52 patients who were discharged not excessively sedated with adequate pain relief. The sedated patients received significantly more morphine in the PACU (mean 12 mg vs. 5 mg), had a two-times longer stay in the PACU (mean 240 min vs. 120 min), more often recalled having moderate-to-severe pain in the PACU, and had poorer quality sleep and higher pain scores at 24 hours after surgery.
An observational study compared pain, oxygen saturation levels, and sedation levels in three groups of patients: those receiving conscious sedation for colonoscopy (N = 18), those receiving IV PCA for postoperative pain management (N = 15), and those receiving nurse-controlled analgesia (PRN schedule) (N = 20) (Taylor, Voytovich, Kozol, 2003). The researchers reported that the analgesics and sedatives given were chosen by surgeons or anesthesiologists and adjusted to optimize analgesia, sedation, and safety, but exact drugs and doses were not provided in the publication of this study. Patients in group 2 (PCA) had trends in sedation levels greater than or equal to patients in group 1 (conscious sedation) during the first 10 hours postoperatively, and 72.7% of those receiving PCA were excessively sedated within the first 4 hours after discharge from the PACU. The lowest level of sedation was in group 3 (nurse controlled). Of interest, oxygen saturation was well maintained in all groups throughout the study. Pain scores declined steadily during the study in patients in groups 1 and 2 (average mean maximal pain score = 6.5/10) and undulated in group 3 (average mean maximal pain score = 5.5).
These studies add to a growing body of research showing that sedation precedes analgesia and despite being sedated, some patients will report pain. Nevertheless, opioid doses should not be increased (titration should be stopped) in patients who are excessively sedated, and they should be watched closely until they are no longer sedated. The APS (2003) recommends close monitoring of sedation and respiratory status for at least 3 hours after the peak of the last dose administered. Other options to achieve comfort such as adding or increasing the dose of nonopioids should be implemented. See the following discussion and Table 19-1 for the recommended approach for prevention and treatment of sedation and respiratory depression in these complex patients.
Treatment of Excessive Sedation
Opioid-induced sedation is dose-related (Abou Hammoud, Simon, Urien, et al., 2009; Aubrun, Monsel, Langeron, et al., 2001); therefore, opioid orders and hospital protocols should include the expectation that nurses will stop titration or promptly decrease the opioid dose whenever excessive sedation is detected. For example, the opioid dose should be decreased by 25% to 50% in patients receiving PCA or epidural analgesia who have a sedation level of 3 on the POSS (see Box 19-4 and see Form 17-1 [p. 464] for an example of how decreases in dose can be included in opioid order sets). Monitoring of sedation level and respiratory status is then increased in frequency (e.g., every 15 to 30 minutes) until the sedation level is less than 3 and the respiratory status is stable (see p. 517 for respiratory assessment). Nonsedating nonopioid analgesics can be added or increased to facilitate opioid dose reduction. Routine administration of these agents as part of a multimodal approach initiated preoperatively or as soon as the patient is started on opioid therapy (e.g., admission to the PACU) is essential to help prevent excessive sedation from occurring later in the course of care. Giving less opioid more frequently to decrease peak concentration may be effective in reducing sedation in some cases. For example, large PCA doses and long lockout (delay) intervals can be reduced in patients who report excessive sedation following PCA injection. Catheter migration to either the intravascular or intrathecal space should be ruled out in patients receiving epidural analgesia who have a sudden change in sedation level (see Box 15-4 on p. 433). See the following patient example and Table 19-1 for treatment of opioid-induced sedation during short-term opioid therapy and the following section on prevention and treatment of clinically significant respiratory depression.
Respiratory Depression
Respiratory depression is assessed on the basis of what is normal for a particular individual. Respiratory depression associated with opioid use usually is described as clinically significant when there is a decrease in the rate and depth of respirations from baseline, rather than just by a specific number of respirations per minute. This means that in some cases even patients breathing less than 10 breaths/minute may not have respiratory depression if they are breathing deeply (Pasero, Portenoy, McCaffery, 1999). Respiratory rates of less than 8 breaths/min have been described as severe respiratory depression (Dahan, Aarts, Smith, 2010). Oxygen (O2) saturation and exhaled carbon dioxide (end-tidal [ET] CO2) are increasingly used to define respiratory depression, particularly in research. Postoperative hypoxemia has been characterized as an oxygen saturation level of less than 90% and severe hypoxemia as less than 85% for more than 6 minutes per hour (Wheatley, Shepherd, Jackson, et al., 1992). It is important, however, for clinicians to understand that respiratory rate and oxygen saturation are surrogate indicators of ventilatory drive and provide little information about the effects of a drug on the ventilatory system (Dahan, Aarts, Smith, 2010). Arterial carbon dioxide and inspired minute ventilation are direct measures, but continuous monitoring of these is difficult in the clinical setting (Dahan, Aarts, Smith, 2010) (see later in this chapter for a discussion of mechanical monitoring).
Respiratory Depression During Long-Term Opioid Therapy
Clinically significant respiratory depression is the most feared of the opioid-induced adverse effects, but like sedation, tolerance to respiratory depression develops over a period of days to weeks and rarely occurs if opioids are titrated according to patient response (Hanks, Cherny, Fallon, 2004). The longer the patient receives opioids, the wider the margin of safety. Therefore, fear of respiratory depression in patients who have been receiving opioids for more than 1 week should not pose a barrier to administering adequate opioid doses (see later discussion of the postoperative opioid tolerant patient). Similar to opioid-naïve patients, when respiratory depression occurs in an opioid-tolerant individual, it is preceded by increased sedation; however, if appropriate steps are taken to address persistent sedation, respiratory depression is unusual in patients receiving long-term opioid therapy (Hanks, Cherny, Fallon, 2004).
Respiratory Depression in Opioid-Naïve Patients
The incidence of opioid-induced respiratory depression in opioid-naïve patients is unclear, mainly because of variations in the definition of respiratory depression used in research (Cashman, Dolin, 2004; Dahan, Aarts, Smith, 2010). This has resulted in reported incidences based on a variety of indicators, including respiratory rate, number of hypoxemic or hypercarbic episodes, and the need for treatment with naloxone (rescue) (Cashman, Dolin, 2004). The incidence of respiratory depression may be much higher than reported here because of the lack of consensus on definitions and uniform reporting as well as the reliance on retrospective data, the source of most published reports, which may not record the reasons for interventions (Etches, 1994).
Incidence of respiratory depression varies according to the method of opioid administration. A small study (N = 38) reported that no episodes of respiratory depression occurred in patients who were randomized to receive 10 mg of morphine by the IV or the IM route of administration (Tveita, Thoner, Klepstad, et al., 2008). A systematic review of IV PCA reported a range of respiratory depression of 0.6% to 15.2% when hypoventilation and oxygen desaturation were used as indicators (Walder, Schafer, Henzi, et al., 2001). Another systematic review of the incidences of respiratory depression associated with IM, IV PCA, and epidural opioids found wide variations in indicators resulting in wide ranges of incidences (Cashman, Dolin, 2004). Using hypoventilation and oxygen desaturation as indicators, the researchers reported mean incidences of between 0.8% to 37.0% (IM), 1.2% to 11.5% (IV PCA), and 1.1% to 15.1% (epidural). They concluded that an incidence of 1% or less (defined by a low respiratory rate) can be expected in patients receiving IV PCA or epidural analgesia and cared for by an acute pain service. They noted that if oxygen desaturation is used as a definer, a much higher percentage should be expected (see the discussion of respiratory assessment later in the chapter). In a review of the literature, Dahan and colleagues state that the many studies that have been conducted on patients receiving IV PCA yield an incidence of respiratory depression between 0.2% and 0.5% (Dahan, Aarts, Smith, 2010); however, it has been pointed out that many of the studies of IV PCA were published in the early 1990s before emphasis was placed on reducing patients’ pain intensity ratings, and the incidence of oversedation and respiratory depression may actually be much higher today (Taylor, Voytovich, Kozol, 2003).
A prospective study of nearly 3000 patients receiving either PCEA morphine with basal rate (N = 1670) or IV PCA morphine with basal rate (N = 1026) after major surgery found a higher incidence of respiratory depression (defined as less than 8 breaths/min) in the IV PCA group (1.2%) than in the epidural group (0.04%) (Flisberg, Rudin, Linner, et al., 2003) (see Chapter 17 for safe use of a basal rate with IV PCA). In their neuraxial analgesia practice guidelines, the American Society of Anesthesiologists (ASA) reported an incidence of 0.01% to 7% with single-bolus intrathecal morphine and 0.08% to 3% with single-bolus epidural morphine (ASA Task Force on Neuraxial Opioids, 2009). They suggested that the range of incidence for respiratory depression with single-bolus neuraxial opioids (0.01% to 7%) is similar to that of IM, IV, and IV PCA opioids and recommended continuous epidural opioids over parenteral opioids as a way of reducing the risk of respiratory depression.
A significantly higher incidence of respiratory depression than had been previously reported was found when continuous pulse oximetry (O2 saturation) and capnography (ETCO2) were utilized to evaluate respiratory depression in a study of 178 postoperative patients receiving morphine or meperidine by IV PCA (Overdyk, Carter, Maddox, et al., 2007). The incidence of oxygen desaturation (less than 90%) lasting 2 or more minutes was 21.4% and lasting 3 or more minutes was 11.8%; the incidence of bradypnea (rate less than 10 breaths/min) lasting 2 or more minutes was 58.4% and lasting 3 or more minutes was 41%. Only 1 patient (0.6%) required naloxone treatment. The researchers attributed the low need for naloxone rescue to the monitoring equipment’s audible alarm that arouses a patient and summons a nurse, but they suggested that more research is needed to verify this (see p. 518 for a discussion of mechanical monitoring). An interesting finding in this study was that despite consuming approximately two times more opioid, patients receiving a basal rate with IV PCA had a significantly lower incidence of bradypnea (32% vs. 53%) and desaturation (8% vs. 17%) than those who did not have a basal rate. The reason for this was not clear.
Risk Factors for Opioid-Induced Respiratory Depression
There is no set dose of an opioid that is safe for all patients. All opioid-naïve patients are at risk for clinically significant respiratory depression when they receive their first dose of an opioid. Even opioid-tolerant patients have an elevated risk if they are given a significant amount of opioid in addition to their usual amount, for example, the patient who takes an opioid analgesic for persistent pain and receives several IV opioid bolus doses in the PACU followed by IV PCA with a basal rate for ongoing acute postoperative pain (Pasero, 2009b). Any patient who required a large amount of opioid in a relatively short period of time to achieve comfort (e.g., more than 10 mg morphine equivalent in an opioid-naïve patient in the PACU) is at risk for subsequent respiratory depression (Dahan, Aarts, Smith, 2010; Lotsch, Dudziak, Freynhagen, et al., 2006) and should be watched closely for at least 3 hours past the time of the expected peak analgesic blood concentration of the last opioid dose (APS, 2003) (see Table 16-1 on pp. 444-446). A study of morphine pharmacokinetics and pharmacodynamics in the immediate postoperative period found that 20 mg of IV morphine during titration was associated with a high likelihood of sedation (Abou Hammoud, Simon, Urien, et al., 2009). A disturbing case report supports this conclusion (Lotsch, Dudziak, Freynhagen, et al., 2006).
The first 24 hours after surgery is a particularly high-risk period for postoperative patients. A retrospective case control study examined 62 patients who experienced a respiratory event. It found that 77.4% had the respiratory event in the first 24 hours postoperatively, and 56.5% of these experienced the event within the first 12 hours postoperatively (mean time to event was 20.48 hours after the end of surgery) (Taylor, Kirton, Staff, et al., 2005). Risk factors for the event were identified as age greater than 65 years, coexisting chronic obstructive pulmonary disease, having one or more co-morbidities, and receiving hydromorphone. Morphine was described as a “protective factor.” The researchers suggested that the elevated risk of respiratory depression with hydromorphone was due to its higher potency but did not provide dosing information, so it is unclear if equianalgesic opioid doses were administered (see Chapter 11 for a discussion of potency and equianalgesia). Randomized controlled trials have not demonstrated differences between morphine and hydromorphone in terms of the incidences of sedation and respiratory depression (Chang, Bijur, Meyer, et al., 2006; Hong, Flood, Diaz, 2008). A retrospective analysis of medical records compared adverse effects associated with IV PCA morphine, hydromorphone, and fentanyl and found no difference in sedation among the three opioids; an incidence of respiratory depression (respiratory rate less than 12 breaths/min or oxygen saturation less than 90%) of 8%, 7%, and 4%, respectively, was reported, but this was not statistically significant (Hutchison, Chon, Tucker, et al., 2006). One randomized controlled trial (N = 56) compared remifentanil and fentanyl administered by IV infusion for postoperative pain and reported three episodes of serious respiratory depression in patients who received remifentanil and none in those who received fentanyl; however, other causes for the episodes could not be ruled out (Choi, Koo, Nam, et al., 2008). More well-controlled research with large numbers of patients is needed to conclude differences in risk of respiratory depression among the various opioids; however, the time it takes an opioid to reach its analgesic site of action must be considered during titration (Lotsch, Dudziak, Freynhagen, et al., 2006).
Because pain stimulates respiration (Borgbjerg, Nielsen, Franks, 1996), patients in whom pain is controlled after a period of poor control are at risk for respiratory depression and should be watched closely until steady state is reached. As mentioned, others at risk for opioid-induced respiratory depression are older opioid-naïve patients (age 65 or older) and those who have coexisting conditions such as chronic pulmonary disease or major organ failure (Dahan, Aarts, Smith, 2010; Lucas, Vlahos, Ledgerwood, 2007; Taylor, Kirton, Staff, et al., 2005).
Individuals with obstructive sleep apnea (OSA) are at particularly high risk for opioid-induced respiratory depression (ASA, 2006; Dahan, Aarts, Smith, 2010) as are obese patients (body mass index [BMI] 35 kg/m2 or more) with or without OSA (Overdyk, Ahmed, 2009). One group of researchers recommends routine mechanical monitoring postoperatively in all patients undergoing bariatric surgery for obesity because of their elevated risk for severe and prolonged epiosodes of hypoxemia (Gallagher, Haines, Osterlund, et al., 2010). A high incidence of sleep-disordered breathing has been reported in patients receiving long-term opioid therapy, particularly in those taking methadone for pain management (Webster, Choi, Desai, et al., 2008). Clinicians are encouraged to establish processes in their insitutions that will help to identify patients with possible OSA before opioid therapy is initiated (e.g., preoperatively). The ASA provides a list of clinical signs that are suggestive of OSA, such as frequent snoring; neck circumference of 17 inches in men or 16 inches in women; and BMI 35 kg/m2 or more, as well as recommendations for the management of OSA in the perioperative setting (ASA, 2006). All patients and family members should be asked on admission or as soon as possible thereafter about whether the patient snores, awakens spontaneously to take a deep breath, or has apneic episodes (even brief) during sleep (Pasero, 2009b). The family is especially important to query as many individuals who have these characteristics are often not aware that they do (see the discussion later in the chapter). If any of the signs of OSA are reported, further evaluation, such as a sleep study to diagnose OSA, may be warranted before surgery or administration of sedating agents. At the very least, the primary physician and anesthesia provider should be made aware of the findings.
As mentioned, some therapies are associated with a higher incidence of respiratory depression than others, such as neuraxial single-bolus dosing (ASA, 2006) and IV PCA with a basal rate (continuous infusion) (APS, 2003; Dahan, Aarts, Smith, 2010; Pasero, McCaffery, 2004) (see Chapter 17). It is important for nurses to increase sedation and respiratory status assessments in patients receiving opioid therapy and concomitant administration of sedating agents, such as benzodiazepines and anxiolytics. Patients who are given antihistamines for treatment of adverse effects, such as pruritus, should also be watched closely as these increase sedation and can lead to respiratory depression (Anwari, Iqbal, 2003). Box 19-5 lists these and other risk factors for opioid-induced respiratory depression.
Prevention of Opioid-Induced Respiratory Depression
Clinically significant opioid-induced respiratory depression in opioid-naïve patients can be prevented by careful opioid titration and close nurse monitoring of sedation (see Box 19-4) and respiratory status (Pasero, 2009b; Pasero, McCaffery, 2002) (see later in this chapter for a discussion of mechanical monitoring). A principle of safe pain management is to administer the lowest effective opioid dose necessary to achieve satisfactory pain control (Pasero, Portenoy, McCaffery, 1999). This is best accomplished with the use of a multimodal analgesic approach that combines drugs with different underlying mechanisms, such as acetaminophen, NSAIDs, and local anesthetics (see Chapter 12). Lower opioid doses have the potential to reduce the incidence and severity of adverse effects like sedation and respiratory depression. Unless contraindicated, the administration of acetaminophen, an NSAID, or both preoperatively or before opioid therapy is initiated (e.g., on admission to the PACU) is recommended in all patients receiving short-term opioid therapy for nociceptive pain (e.g., surgery).
In all patients with elevated risk, starting opioid doses should be decreased 25% to 50%. Mechanical monitoring may also be indicated in some patients with high risk factors or who develop excessive sedation during opioid therapy (see following discussion). The expectation that nurses will reduce opioid doses promptly whenever advancing sedation is detected and increase monitoring frequency is essential for safe opioid administration (see previous discussion of sedation assessment). These actions should be included in all opioid orders and hospital protocols and taught in nursing orientation and ongoing educational courses (see Form 17-1 on p. 464 for an example of how decreases in opioid dose can be included in opioid order sets).
Respiratory Assessment
Ventilation is driven by a balance between arterial partial pressure of oxygen (PaO2) and arterial partial pressure of carbon dioxide (PaCO2) (Cretikos, Bellomo, Hillman, et al., 2008). Respiratory rate reflects the body’s attempts to maintain that balance (e.g., tidal volume is increased to correct hypoxemia and hypercarbia). This helps to explain why abnormal respiratory rate is often a predictor of serious adverse events and critical illness (Cretikos, Bellomo, Hillman, et al., 2008; McBride, Knight, Piper, et al., 2005; Ridley, 2005).
Efforts to improve the quality and documentation of respiratory assessment, such as rigorous education of staff and evaluation of performance, are needed in all health care settings (Cretikos, Bellomo, Hillman, et al., 2008; Hogan, 2006). Research shows that assessment of respiratory rate alone is inadequate for determining ventilatory adequacy and propensity for respiratory depression (Dahan, Aarts, Smith, 2010; Overdyk, Carter, Maddox, 2006). Further, clinical observation suggests that staff often do not actually count the number of times a minute a patient breathes, and if it is counted, it is not counted long enough to obtain an accurate respiratory rate. This is supported by research that showed that respiratory rate was assessed and recorded in just 30% (McBride, Knight, Piper, et al., 2005), 50% (Hogan, 2006), and 58% (Edwards, Murdin, 2001) of patients on general care nursing units. One study found that almost 77% of patients who suffered an adverse event, such as unexpected cardiac arrest, unplanned ICU admission, and unexpected death, had a high proportion of missing documentation of vital signs, with respiratory rate being documented the least (Chen, Hillman, Bellomo, et al., 2009). A retrospective analysis showed that even when the diagnosis was of a respiratory nature (e.g., asthma, chronic obstructive pulmonary disease), respiratory rate was not documented in 27% of admissions and in only 65% of patients with chest symptoms in the ED (Edwards, Murdin, 2001). The introduction of a new vital signs chart and an early modified warning score in one hospital improved documentation of respiratory rate from approximately 30% to nearly 70% (McBride, Knight, Piper, et al., 2005). Similar results were found in another study with this approach (Hogan, 2006).
Although respiratory rate is an important parameter to obtain (Cretikos, Bellomo, Hillman, et al., 2008), it is emphasized again that clinically significant respiratory depression is not defined by a specific number of breaths/minute. Rather, it is defined by several characteristics of a patient’s respiratory status. A comprehensive assessment of respiratory status goes hand-in-hand with sedation assessment and constitutes more than counting a patient’s respiratory rate over a 30- or 60-second period (Pasero, 2009b) (see the discussion of sedation earlier in the chapter). A proper respiratory assessment requires the nurse or nurse technician to watch the rise and fall of the patient’s chest to determine rate, depth, and regularity of respirations (Stemp, Ramsay, 2005). Current rate and quality is compared with baseline, and trends are noted (Pasero, 2009b).
Snoring Is a Warning Sign.: Listening to the sound of the patient’s respiration is critical. Snoring indicates airway obstruction and must be attended to promptly with repositioning (Isono, Tanaka, Nishino, 2002) and, depending on the severity, a request for respiratory therapy consultation and further evaluation for the presence of obstructive sleep apnea (OSA) (ASA, 2006). Even subtle snoring can evolve into complete obstruction in a sedated patient, and so must be promptly addressed and monitoring increased (Pasero, 2009b).
Patients and family members often report that snoring is “normal” because the patient snores at home. This thinking can lead to fatal consequences. In the home setting, patients are usually awakened by their own snoring and ineffectual respiration, or a family member awakens them; however, in the context of opioid administration and perhaps other sedating medications, patients may be too sedated to self-arouse. Under these circumstances, snoring is an ominous sign and requires the nurse to arouse and further evaluate the patient to avert disaster (Pasero, 2009b) (see the discussion that follows).
Frequency of Sedation and Respiratory Assessment
Assessment of sedation level and respiratory status should be done together at the same frequency. The risk factors previously discussed in this chapter and in Box 19-5 are considered when determining the frequency of assessment. Although there is no set frequency for sedation and respiratory assessment that fits all patients, the most dangerous time for a patient in terms of developing respiratory depression is during the first 24 hours of opioid therapy for moderate-to-severe pain in opioid-naïve patients and others at risk for respiratory depression (Pasero, 2009b; Taylor, Voytovich, Kozol, 2003; Taylor, Kirton, Staff et al., 2005). Therefore, sedation and respiratory assessment should be performed every hour for the first 12 hours after opioid therapy is initiated, and, if stable, this may be followed by a frequency of every 2 hours for the next 12 hours of opioid therapy. Other vital signs may be obtained as required by the primary provider’s routine orders (e.g., every 4 hours). The ASA recommends this same protocol for patients receiving neuraxial analgesic therapies except that frequency may be every hour for just 2 hours after a single bolus neuraxial dose of a lipophilic opioid, such as fentanyl (ASA Task Force on Neuraxial Opioids, 2009). After 24 hours of opioid therapy, most patients are able to go to every-4-hour sedation and respiratory assessments, but this must be individualized and based on the patient’s condition.
Nurses are expected to evaluate each patient initially and on an ongoing basis during opioid therapy for the need for more or less frequent monitoring based on the patient’s risk factors and current status. If a patient’s condition warrants closer monitoring than can be provided on the current clinical unit, the patient should be promptly moved to a more closely monitored setting (Pasero, 2009b). Institutions are encouraged to establish protocols that support the nurse’s role in ensuring patient safety during opioid administration (see Table 19-1).
Assessment of the Sleeping Patient.: Nurses often ask if they should awaken sleeping patients to determine level of sedation. It is acceptable to allow a patient to sleep who has been receiving stable opioid doses and demonstrates optimal respiratory status determined by a comprehensive respiratory assessment as described above (ASA, 2009; Pasero, 2009b). However, patients must be aroused if there is any question about whether they are sleeping normally or are sedated. Research discussed earlier showed that sleep during morphine titration is mainly the result of the sedative effects of the opioid (Lovovschi, Aubrun, Bonnet, et al., 2008). It is important to realize that arousal will stimulate respiration; to obtain a more accurate picture of the patient’s respiratory status, the comprehensive respiratory assessment should be performed before arousing the sleeping patient (ASA, 2009; Pasero, 2009b).
It is reassuring to know that patients who are sleeping normally and have well-controlled pain will fall back to sleep after they are aroused for a sedation assessment if one is necessary. Those who do not fall back to sleep require further evaluation because they may be experiencing pain and the need for additional analgesia. Note that patients who achieve pain control and fall asleep after a period of poor pain control should be carefully assessed to be sure that what seems to be normal sleep is not actually excessive sedation.
Mechanical Monitoring During Short-Term Opioid Therapy
The risk factors listed in Box 19-5 are considered when determining who might benefit from mechanical monitoring (e.g., pulse oximetry [O2 saturation] or capnography [ETCO2]) during opioid therapy. Having a risk factor for respiratory depression does not automatically mean the patient must receive mechanical monitoring; however, careful consideration of the severity of the factor and the patient’s general health and current condition is essential to decision making. Mechanical monitoring should also be considered in patients who require naloxone administration for clinically significant respiratory depression until their condition is stable (see the discussion of reversing opioid-induced respiratory depression later in the chapter).
The most common technologies used for mechanically monitoring patients during opioid administration are pulse oximetry and capnography; however, research is lacking on their effectiveness in reducing episodes of clinically significant opioid-induced respiratory depression. As mentioned earlier, a low incidence of rescue naloxone (one patient, 0.6%) in a study of 178 patients who were monitored with capnography and pulse oximetry during IV PCA was attributed to the monitoring equipment’s audible alarm that arouses a patient and summons a nurse, but the researchers suggested that more research is needed to verify this (Overdyk, Carter, Maddox, et al., 2007). A systematic review of randomized controlled trials that compared postoperative patients with and without pulse oximetry found that the incidence of hypoxemia in the PACU was 1.5 to 3 times less with pulse oximetry but could find no significant difference in postoperative complications between those who received pulse oximetry (10%) and those who did not (9.4%) (Pedersen, Moller, Pedersen, 2003).
Pulse Oximetry.: Oxygen saturation monitoring via finger-sensor pulse oximetry is commonly used but has several drawbacks that can have significant consequences, particularly when used on clinical units (i.e., outside of PACU, ICU, and ED). Research shows that finger-sensor pulse oximetry is associated with a high incidence of technical false alarms from the loss of signal and poor signal quality when blood flow is low to the sensor area or when patients move (Eisenkraft, 2006; Reich, Timcenko, Bodian, et al., 1996). Forehead-sensor technology may provide a solution to the problem of technical false alarms but has not proven to be superior to finger-sensor technology (Agashe, Coakley, Mannheimer, 2006; Blaylock, Brinkman, Carver, et al., 2008). Nurses often do not respond to alarms, particularly if the alarm self-resolves within a short period of time (Overdyk, Carter, Maddox, et al., 2007). It is important for nursing staff to appreciate that alarm resolution does not necessarily mean the patient is safe—all alarms should be evaluated. Sometimes nurses disable alarms when repeated false alarms occur in some patients. Instead, when repeated false alarms occur, the patient’s primary health care provider should be consulted to determine the need for mechanical monitoring. Patients often become annoyed and remove the oximetry sensor as well, so initial and ongoing patient teaching on the purpose of monitoring is required but not always provided.
Some institutions implement periodic pulse oximetry readings (spot checks) that are taken usually when the patient’s vital signs are obtained; however, this practice may lead to inaccurate assumptions about the patient’s respiratory status. The process of applying the pulse oximeter sensor is likely to stimulate the patient to take a deep breath, which can yield a higher oxygen saturation than the patient has when not stimulated. If mechanical monitoring is warranted, it should be done continuously (Pasero, 2009b).
Finally, as mentioned earlier, oxygen saturation is a measure of gas exchange rather than a direct indicator of ventilatory efficacy; it provides limited information on the effects of an opioid on ventilatory control (Dahan, Aarts, Smith, 2010). In addition, a primary disadvantage of pulse oximetry is that it will yield high oxygen saturation readings despite the presence of respiratory depression in patients who are receiving supplemental oxygen (Overdyk, Ahmed, 2009; Overdyk, Carter, Maddox, 2006). If a patient requires both supplemental oxygen and mechanical monitoring and pulse oximetry is the only mechanical monitoring available, the patient should be moved to a more closely monitored setting (Overdyk, Ahmed, 2009; Pasero, 2009b).
End-Tidal CO2 Monitoring (Capnography).: Expired CO2 is considered a highly reliable measure of the quality of ventilation and, unlike pulse oximetry, is an early indicator of impending respiratory depression (Kopka, Wallace, Reilly, et al., 2007). The technology to monitor ETCO2 is rapidly advancing and is likely to rival or replace pulse oximetry as the primary method for monitoring patients in the perioperative, procedural, critical care, and emergency settings. Current technology utilizes a nasal catheter to detect expired CO2, but transcutaneous sensors have been researched (Kopka, Wallace, Reilly, et al., 2007). The latter may suffer the same drawbacks of pulse oximetry in terms of sensitivity to blood flow. Further research is needed.
Modular technology that links continuous capnography and pulse oximetry monitoring to analgesic devices, such as PCA pumps, is available. Such a device can be programmed to alarm and discontinue opioid administration whenever the device detects a reading outside of the set threshold (e.g., oxygen saturation less than 90% or end-tidal CO2 more than 50 mm Hg or less than 30 mm Hg). Research is lacking regarding the safe minimal settings for these parameters and the effectiveness of this technology in reducing the incidence of clinically significant respiratory depression. Anecdotally, nurses report that this feature is frequently triggered for reasons unrelated to the patient’s physiologic status, such as signal failure (discussed earlier in the chapter), and they cite the subsequent loss of pain control and difficulty regaining control as major drawbacks to the technology.
The use of capnography during opioid administration is evolving. The reader is referred to an excellent website (http://www.capnography.com) that provides extensive information and access to research about capnography including the physiology of capnography, clinical uses, and advances in technology.
Summary Points on Mechanical Monitoring.: In summary, further research is needed to determine who will benefit from mechanical monitoring during opioid therapy. More data are needed to assess the clinical significance of the oxygen desaturation and hypercarbia that occur during opioid therapy. As technology improves, it is likely to become more commonplace, but it is extremely important to remind staff members that the use of mechanical monitoring does not absolve them of sedation and respiratory assessments (Pasero, 2009b). Mechanical monitoring can be an extremely valuable technology in some patients, but excessive use can lull staff into a false sense of security and a tendency to ignore alarms. Institutions are encouraged to form a multidisciplinary task force to establish guidelines for the rational use of mechanical monitoring during short-term opioid therapies. Clinician decision making about whether to use mechanical monitoring or not is enhanced when it is based on the patient’s risk factors and current condition.
Transfer of Care: Hand-off Communication
Pain control should be included in the criteria for discharge from one area of care to another. Some EDs, short-stay units, outpatient surgery units, and PACUs establish a comfort-function goal of at least 4/10 before discharge (see Section II); however, the expectation that all patients must be discharged from these areas with pain ratings below an arbitrary number can lead to the unsafe administration of further opioid doses to patients who are excessively sedated (Blumstein, Moore, 2003; Lucas, Vlahos, Ledgerwood, 2007). Instead, achieving optimal pain relief is best viewed on a continuum, with the primary objective being to provide both effective and safe analgesia. Although it is not always possible to achieve a patient’s comfort-function goal within the short time the patient is in these areas, the comfort-function goal provides direction for ongoing care. Important information to give to the nurse assuming care of the patient is the patient’s comfort-function goal, how close the patient is to achieving it, what has been done thus far to achieve it (analgesics, doses, and times of administration), and how well the patient has tolerated the administration of analgesics (adverse effects).
The transferring nurse should also alert staff on the receiving clinical unit of a patient’s risk factors for respiratory depression so that appropriate monitoring can be initiated (see Box 19-5). For example, opioid-naïve patients who require high opioid doses (e.g., more than 10 mg of IV morphine or its equivalent) during titration for acute pain are at higher risk for respiratory depression (Dahan, Aarts, Smith, 2010; Lotsch, Dudziak, Freynhagen, et al., 2006) and must be watched closely for at least 3 hours after the peak concentration of the last dose has passed (APS, 2003); the highest risk for respiratory depression is during the entire first 24 postoperative hours. Mechanical monitoring is warranted in patients with diagnosed or suspected obstructive sleep apnea or pulmonary disease. Providing the postoperative patient’s ASA Patient Status Classification is recommended as a simple way to further communicate the patient’s risk for respiratory depression.
Reversing Respiratory Depression: Naloxone Administration
The opioid antagonist naloxone has been used since the early 1970s to reverse respiratory depression related to opioid overdose (Buck, 2002). It is administered IV and has a rapid onset of action (2 minutes) with a peak concentration in 10 minutes, duration of 1 to 4 hours, and a half-life of 30 to 81 minutes (Leavitt, 2009). The extent and duration of naloxone reversal of opioid effects varies and is dependent on many factors, such as specific opioid used, opioid dose, method of administration, and concurrent medications (Dahan, Aarts, Smith, 2010).
If it is necessary to use naloxone to reverse clinically significant respiratory depression, it should be titrated very carefully (APS, 2003). Sometimes more than one dose of naloxone is necessary because naloxone has a shorter duration (1 hour in most patients) than most opioids (Dahan, Aarts, Smith, 2010); however, giving too much naloxone or giving it too fast can precipitate severe pain that is extremely difficult to control and increase sympathetic activity leading to hypertension, tachycardia, ventricular dysrhythmias, pulmonary edema, and cardiac arrest (Brimacombe, Archdeacon, Newell, et al., 1991; O’Malley-Dafner, Davies, 2000). Box 19-6 outlines the procedure for correctly administering naloxone to an adult. Hospital protocols and opioid orders should include the expectation that nurses will administer naloxone in accordance with the procedure outlined in Box 19-6 whenever a patient is found to have clinically significant opioid-induced respiratory depression.
In physically dependent patients, withdrawal syndrome can be precipitated by naloxone administration; patients who have been receiving opioids for more than 1 week may be exquisitely sensitive to antagonists (APS, 2003). If sedation and respiratory depression occurs during administration of transdermal fentanyl, the patch should be removed immediately, and if naloxone is necessary, treatment will be needed for a prolonged period and the typical approach involves a naloxone infusion over a 12- to 24-hour period (e.g., mix 0.8 mg of naloxone in 250 mL of IV solution and run at 9 to 20 mL/h to administer approximately 0.2 to 0.6 mg/h naloxone). The patient must be closely monitored for at least 24 hours after discontinuation of the transdermal fentanyl.
IV access for naloxone administration usually is maintained for 24 hours after a stable epidural opioid dose has been achieved. After that time, naloxone rarely is needed because most patients are alert and, in many cases, are ready for a decrease in the epidural opioid dose.
Although no formulation is commercially available in the United States, naloxone has been administered intranasally. One randomized controlled trial found an equally safe and favorable response within 10 minutes of administering 2 mg intranasally or intramuscularly in the prehospital setting for suspected heroin overdose (Kerr, Kelly, Dietze, et al., 2009). A retrospective study compared intranasal naloxone with IV naloxone for opioid overdose in the prehospital setting and found the mean time to clinical response was longer in those who received intranasal naloxone, but the researchers suggested that the response time was equivalent when the time required to prepare an IV injection of naloxone is considered (Robertson, Hendey, Stroh, et al., 2009). In both of these studies, patients who received intranasal naloxone were more likely to require an additional rescue dose of naloxone.
Inappropriate Use of Opioid Antagonists
More often than clinically significant respiratory depression, cancer patients receiving long-term opioid treatment may develop confusion and be somewhat sedated but remain easily aroused with an acceptable respiratory rate and depth. Great care must be taken to prevent the administration of an opioid antagonist drug to patients exhibiting such symptoms because the symptoms may be caused by disease progression rather than opioid overdose. This is not an emergency situation, and observation for a few hours is the best diagnostic and therapeutic approach (Manfredi, Ribeiro, Chandler, et al., 1996). If a terminally ill patient cannot be aroused and an opioid overdose is suspected, an opioid antagonist drug is warranted only if it is clear that this is not a natural progression of the disease. Only small doses are given very slowly because opioid-tolerant patients are extremely sensitive to an opioid antagonist and easily experience withdrawal symptoms and loss of pain control.
Conclusion
The most common opioid adverse effects include constipation, nausea and vomiting, sedation, pruritus, and mental confusion and clouding. Respiratory depression is less common but is the most feared effect. As the patient becomes opioid tolerant, these adverse effects, except for constipation, tend to subside. A number of factors influence the development of opioid adverse effects, including patient age, co-morbidities, prior opioid exposure, concurrent administration of other drugs, and route of administration. This underscores the need for an individualized approach to preventing and managing the effects. Prevention rather than treatment of opioid adverse effects is an important principle of pain management. Most adverse effects are dose dependent, which is why an effective and practical approach to treatment of these is to administer the lowest effective opioid dose.