Special Topics
Although orthopedic injuries including fractures and dislocations constitute some of the most common reasons for extremity imaging in emergency medicine, other conditions of the extremities can be dangerous to the patient and diagnostically challenging to the physician, creating medical–legal risk. In this section, we consider these conditions, discuss the limits of diagnostic imaging, and point out diagnostic pitfalls that may arise during evaluation.
Arterial Injuries and Other Arterial Pathology
We discussed in other sections of this chapter the evaluation of arterial vascular injuries in the context of knee dislocation. Vascular injury can result from a variety of other blunt and penetrating trauma mechanisms. In addition, the emergency physician may encounter nontraumatic cases of suspected vascular insufficiency, requiring confirmation with diagnostic imaging. Although x-ray may reveal extensive vascular calcifications related to peripheral arterial disease, it does not provide information about the patency of the vessel (Figure 14-129). Diagnostic modalities for assessing vascular structures include ultrasound, conventional angiography, CTA, and MRA.
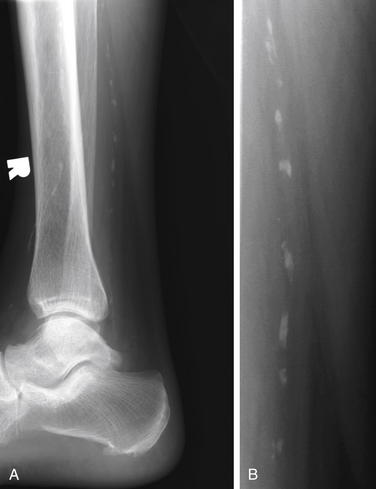
Figure 14-129 Vascular calcifications.
Calcifications within blood vessels betray the presence of peripheral arterial disease in this patient. However, plain x-ray does not reveal essential information about the patency of the vascular lumen. Ultrasound, conventional angiography, CT angiography, and magnetic resonance angiography provide this information. A, Lateral ankle. B, Close-up.
Advantages of ultrasound include portability, lack of ionizing radiation exposure, and absence of the need for vascular contrast agents, with their attendant risks for allergy, renal dysfunction, and other systemic toxicity (see Chapter 7 for a discussion of iodinated contrast and Chapter 15 for a discussion of gadolinium). In the extremities, ultrasound can assess flow and identify aneurysms and intimal flaps. Some structures can be difficult to visualize, depending on the patient’s body habitus. In addition, ultrasound does not allow ready imaging of the complete course of vessels from their origins at the aorta, which may be important in cases such as vascular insufficiency from aortic dissection or atherosclerotic disease.
Conventional angiography (Figure 14-130), although the historical criterion standard, has the disadvantages of being an invasive procedure requiring a team of specialist interventional radiologists, high radiation exposure, and use of iodinated contrast. However, it allows imaging of the aorta and extremity branch vessels. In addition, some lesions such as intimal flaps or vascular stenoses can be treated with endovascular stenting. Some vascular thromboses can be treated with local administration of thrombolytic agents during angiography.
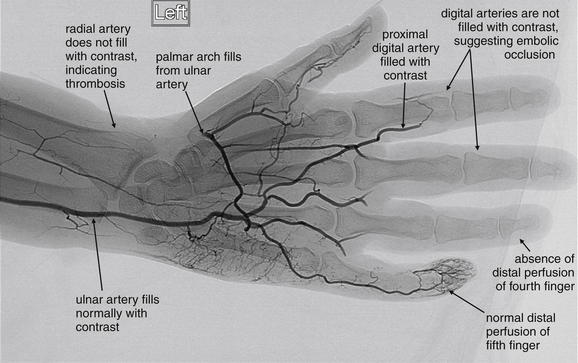
Figure 14-130 Radial artery thrombosis, angiogram.
This patient presented with acute left arm pain, as well as pallor of the palm and fingers. The patient had no palpable radial pulse. An angiogram was performed to determine the site of vascular occlusion. The radial artery does not fill with contrast, indicating proximal occlusion. The digital arteries of the second through fourth fingers do not fill with contrast, suggesting embolic occlusion. The digital artery on the lateral aspect of the fifth finger fills with contrast. The palmar arch fills from the patient’s ulnar artery.
CTA has multiple advantages, including multiplanar and three-dimensional imaging of vessels from the aorta to the distal extremities (Figure 14-131; see also Figure 14-105). CTA of the extremities in the setting of injury is comparable in sensitivity to conventional angiography (around 95%), with moderate specificity (87%).67 Chapter 7 describes principles of CTA in the setting of pulmonary embolism and aortic dissection, which also apply to peripheral CTA. In CTA of the extremities, the contrast bolus is followed as it passes through the aorta to the vessels of the upper or lower extremities. Unlike conventional angiography, a single contrast bolus can be used to construct a detailed map of an entire vascular system. Other advantages of CTA include wide availability and the ability to assess other injuries, including thoracoabdominal and bony injuries with the same modality.
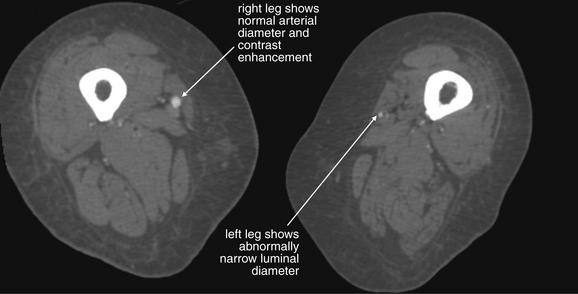
Figure 14-131 Peripheral arterial disease, CT angiography (CTA).
This 60-year-old female presented with severe lower extremity pain, left greater than right. Although this had been going on intermittently for some months, the pain was now present at rest. She had developed bilateral purpura of her toes extending to her midfoot in the past few hours. The patient underwent CTA, which demonstrated a short segment of nearly complete occlusion of the left common femoral artery and proximal superficial femoral artery. The patient underwent surgical thrombectomy. CTA can provide detailed three-dimensional or multiplanar images of the lower extremities for assessment of arterial injury or peripheral vascular diseases including dissections, aneurysms, and occlusions. Advantages of CT include its speed and accessibility. It compares favorably with digital angiography in sensitivity and specificity, but it offers no therapeutic options, unlike formal angiography that offers the possibility of clot lysis, extraction, or stenting. CTA of the extremities requires approximately 150 mL of injected contrast material. Consider the full diagnostic and therapeutic plan for patients with renal disease before ordering this test. If the treatment plan will not be influenced by CTA, avoid unnecessary risk for contrast nephropathy.
MRA is less readily available in many emergency departments, especially outside of normal business hours. However, it offers three-dimensional imaging capability, accuracy similar to CT and conventional angiography, an absence of radiation exposure, and outstanding soft-tissue evaluation for concurrent injuries to muscle and joint structures.
Deep Venous Thrombosis
Diagnostic imaging for DVT is discussed extensively in Chapter 7, in association with a discussion of pulmonary embolism diagnosis. Ultrasound remains the primary modality for assessment (Figure 14-132). Serial compression of the deep venous system is performed from the inguinal ligament to the popliteal fossa. Normal veins are readily compressible, whereas thrombosed veins may be deformed but do not compress with pressure applied with the ultrasound probe. In a metaanalysis of studies of ultrasound, the sensitivity of compression ultrasound for proximal DVT was 93.8% (95% CI = 92.0%-95.3%) with specificity of 97.8% (95% CI = 97.0%-98.4%).100 Addition of color Doppler techniques improves sensitivity slightly, to around 96% for proximal DVT, but reduces specificity to around 94%.100 Repeated ultrasound 1 to 2 weeks after an initial negative study has been recommended in the past, but detects DVT in only about 1% of patients with initial negative studies. Withholding anticoagulation in patients with negative ultrasound appears safe, with only 0.6% of patients with normal ultrasound having interval development of thromboembolism at 3-month follow-up.101 CT venography has comparable sensitivity and specificity to ultrasound (Figure 14-133). CT allows assessment for pelvic thrombus that cannot be detected with ultrasound but also exposes the patient to substantial radiation and to iodinated contrast, neither of which occurs with ultrasound. Magnetic resonance venography is an alternative in patients with suspected pelvic thrombus and contraindications to radiation or iodinated contrast exposure. See Chapter 7 for additional details.
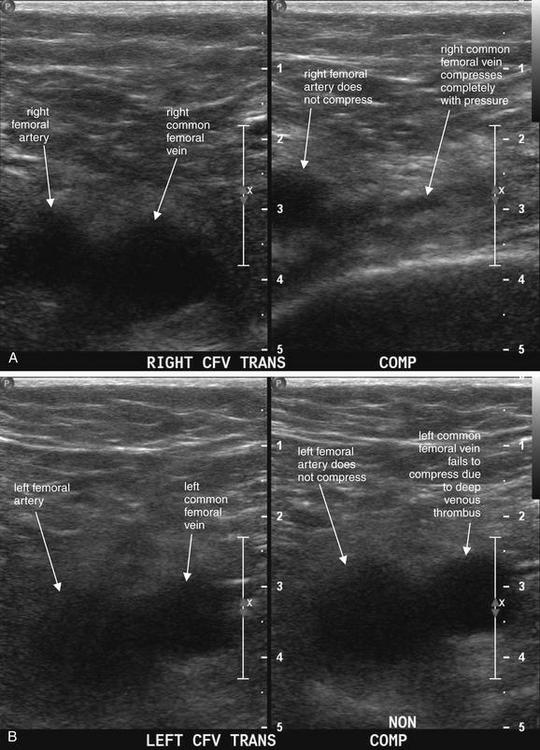
Figure 14-132 Deep venous thrombosis (DVT).
This 61-year-old male presented with abdominal pain and a history of adenocarcinoma of the colon. He underwent abdominal CT, which incidentally suggested DVT of the left common femoral vein. Ultrasound of his lower extremities is shown. A, Normal ultrasound of the patient’s right common femoral vein. The same location is shown without compression (left) and with application of compression (right). The artery remains visible in both images, because the degree of compression is insufficient to compress the high-pressure artery. In contrast, the normal common femoral vein is easily compressed. B, Abnormal ultrasound of the patient’s left leg. This documents left common femoral vein DVT. Again, the artery and vein are seen without compression (left), and compression is applied yet the vein does not compress (right). This is because of the presence of an acute deep venous thrombus. New thrombus is generally hypoechoic (black) like liquid blood. As thrombus ages, it may become hyperechoic (white) and recognizable even without compression. In this case, a DVT is diagnosed without use of Doppler ultrasound. Although it is common for emergency physicians to refer to “Dopplering the legs” to detect DVT, in reality serial compression ultrasound along the length of a vein is usually sufficient to evaluate for thrombus. Doppler technology can be useful in equivocal cases to document augmentation of venous flow with squeezing of the calf muscles. The patient’s CT scan is shown in Figure 14-133 for comparison.
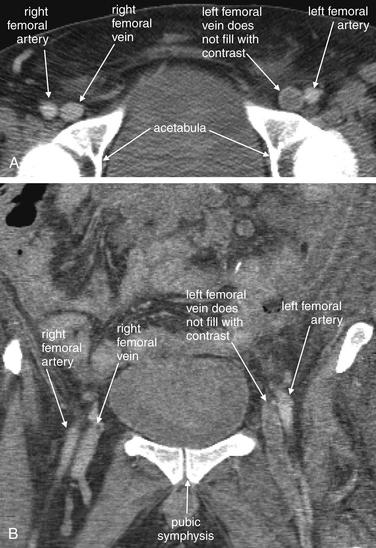
Figure 14-133 Deep venous thrombosis (DVT).
This 61-year-old male presented with abdominal pain and a history of adenocarcinoma of the colon. He underwent abdominal CT, which incidentally suggested DVT of the left common femoral vein. Ultrasound of his lower extremities is shown in Figure 14-132. Although DVT was an unexpected finding in this patient, who was undergoing CT for abdominal pain, computed tomography venography (CTV) is sometimes used intentionally to diagnosis DVT. Proponents of CTV have encouraged its use in conjunction with pulmonary computed tomography angiography (CTA) for the diagnosis of pulmonary embolism. However, others have argued that CTV is unnecessary because it adds cost and radiation exposure to a patient’s evaluation. Ultrasound remains the standard modality for diagnosis of DVT. In selected circumstances, CTV may be the best test available. For example, if a patient’s legs are inaccessible to ultrasound because of cast material or overlying soft-tissue wounds, CTV can provide diagnostic information. External orthopedic fixators that interfere with ultrasound diagnosis of DVT may also prevent CTV from making the diagnosis because of metallic streak artifact. CTV does potentially provide information not available from ultrasound, including evaluation of the pelvic veins. Diagnostic CTV requires the same conditions as pulmonary CTA: a rapid injection of a large volume of iodinated contrast material. Patients with poor renal function or contrast allergies may be unable to undergo the procedure. DVT is diagnosed when a filling defect is visualized, resulting from an obstructing DVT preventing contrast from entering a segment of vessel. A, Axial section showing a filling defect in the left femoral vein. This slice has been cropped to show the region of interest. B, Coronal reconstruction. This image shows the extent of the thrombus.
Sutter et al.102 reported that important incidental findings were found in about 3% of patients undergoing lower extremity ultrasound for suspected DVT, including pseudoaneurysms, arterial occlusive disease, vascular graft complications, compartment syndrome, and tumor. The authors noted that low clinical probability of DVT, in conjunction with a negative D-dimer, is often used to avoid the use of ultrasound but question whether other important causes of lower extremity signs and symptoms might be missed with this approach.
Compartment Syndrome
Compartment syndrome, an increase in the pressure of a fascial compartment, leading to decreased perfusion and potential ischemic infarction of compartment contents, is not a radiologic diagnosis. Compartment syndrome can occur in the absence of fractures, although the most common injury resulting in compartment syndrome is fracture of the tibial diaphysis, which should prompt consideration of compartment syndrome (Figures 14-134 and 14-135). Research has examined the role of MRI, ultrasound, nuclear scintigraphy, and infrared imaging, but these techniques are not well validated.103
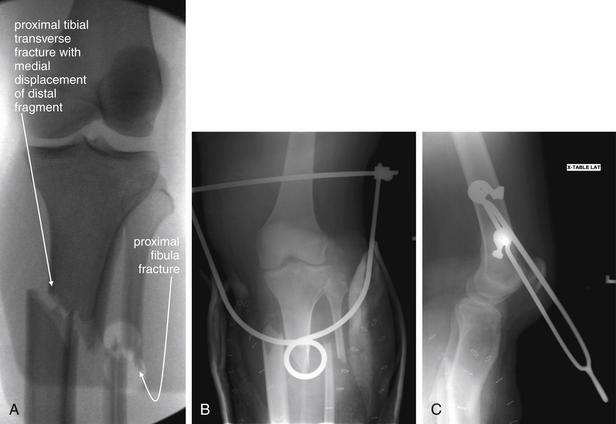
Figure 14-134 Compartment syndrome with tibial fracture.
This 25-year-old pregnant female presented with life-threatening injuries, including an open book pelvic fracture. After an emergency Cesarean section in the trauma bay, she was taken to the operating room, where additional stabilization was performed. A, Fluoroscopy of her deformed left lower leg showed proximal tibial and fibular transverse fractures with significant medial displacement. Her calf compartments were noted to be taut. Compartment pressures were 55 mm Hg for the deep posterior compartment, 39 mm Hg for the superficial posterior compartment, 42 mm Hg for the anterior compartment, and 46 mm Hg for the lateral compartment. Emergency fasciotomy was performed. B, C, Postoperative x-rays show persistently displaced tibial and fibular fractures. The radiopaque device is a traction pin through the distal femur. Although compartment syndrome is not a radiographic diagnosis, remember that proximal tibial fractures are associated with the development of compartment syndrome.
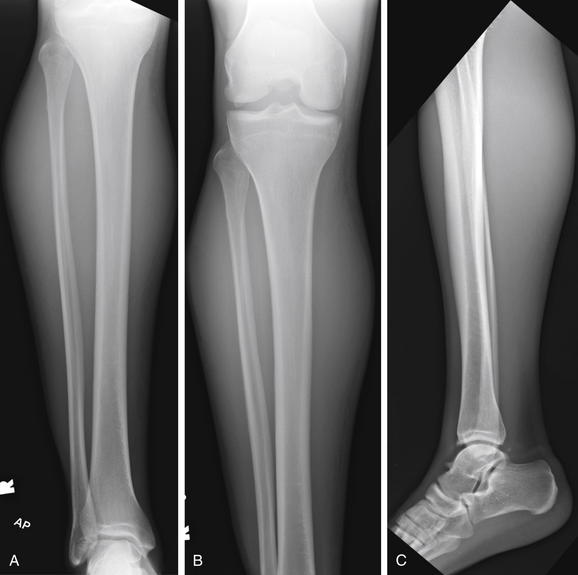
Figure 14-135 Compartment syndrome.
This 22-year-old male presented with calf pain and swelling 2 days after his right calf was stepped on during a rugby match. He complained of numbness in the lateral aspect of his foot. In addition, his dorsalis pedis pulse was diminished to palpation and his right calf was extremely tense. A, B, Anterior–posterior views. C, Lateral view. X-rays were obtained, showing no fractures. The patient’s posterior compartment pressures were elevated to 70 mm Hg, and he underwent four-compartment fasciotomy for compartment syndrome. This revealed necrosis of the medial third of the gastrocnemius, which was surgically debrided. Compartment syndrome can occur in the absence of fractures. Normal radiographs do not rule out the diagnosis.
Magnetic Resonance Imaging in Compartment Syndrome
Rominger et al.104 performed a case-control study with 15 patients with compartment syndrome (10 “described as “manifest,” and 5 described as “imminent”) and 5 normal volunteers. In the 10 advanced cases of compartment syndrome, MRI demonstrated abnormalities of muscle architecture on T1-weighted images and increased signal on spin–echo T2-weighted sequences. Affected compartments showed increased enhancement with gadolinium. However, in 4 of the 5 patients with earlier manifestations of compartment syndrome, MRI was normal. The ability of MRI to identify early changes of compartment syndrome and to distinguish these changes from local muscle edema caused by trauma is not established, and MRI should not be used to exclude or confirm compartment syndrome at this time.
Ultrasound in Compartment Syndrome
Standard ultrasound is likely not useful in excluding or confirming compartment syndrome. In a study of healthy athletes, measurements with ultrasound were not reproducible.105 Experimental techniques show promise but are not ready for clinical application.103
Nuclear Scintigraphy in Compartment Syndrome
In the setting of chronic exertional compartment syndrome, nuclear scintigraphy with 99- technetium-99m–methoxyisobutylisonitrile was 80% sensitive and 97% specific in 46 patients.106 However, the sensitivity and specificity in acute compartment syndrome, in which traumatic injuries to limbs may cause additional imaging abnormalities, is unknown. The technique should not be used to exclude acute compartment syndrome until validated through further research.
Infrared and Near-Infrared Noninvasive Monitoring for Compartment Syndrome
Infrared imaging of lower extremities shows promise in detection of compartment syndrome. In a study of 164 trauma patients, 11 with compartment syndrome, anterior surface temperatures of affected extremities showed greater differences between proximal and distal measurements than did unaffected extremities.107 However, the technique requires further validation. Near-infrared spectroscopy provides a continuous transcutaneous measure of tissue oxygenation and may offer a future method of early detection of compartment syndrome.108
Necrotizing Fasciitis and Other Soft-Tissue Infections
Imaging of necrotizing soft-tissue infections, including necrotizing fasciitis, has not been examined in large methodologically rigorous studies because of the relative rarity of the condition. X-ray, ultrasound, and MRI all have been described as having utility, but the sensitivity and specificity of the modalities is not known.
X-ray findings of necrotizing fasciitis include soft-tissue air tracking along muscle fascial boundaries (Figure 14-136).
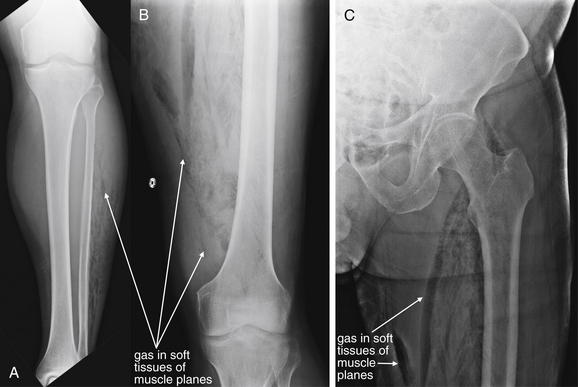
Figure 14-136 Necrotizing fasciitis.
This 71-year-old male with aplastic anemia presented with fevers to 38.9°C, leg weakness, and extreme leg pain. Initially, the patient was felt to have neuropathic pain and weakness, possibly indicating spinal pathology such as epidural abscess. He rapidly developed crepitus of his legs. X-rays of the patient’s legs were obtained, followed by noncontrast CT (Figure 14-137) A, Anterior–posterior (AP) tibia and fibula. B, AP femur. C, AP hip. Air is seen dissecting in muscle planes of the legs. On x-ray, air appears black. Given the wide distribution of air, a focal abscess is unlikely, and necrotizing fasciitis with gas-producing organisms should be suspected.
Ultrasound can demonstrate adipose tissue, fascial, and muscle changes in necrotizing fasciitis. Parenti et al.109 reported results in 32 patients with confirmed necrotizing fasciitis but found sensitivity of only 47% for muscle changes and 56% for fascial changes when ultrasound was performed by operators noted to be particularly skilled at soft-tissue examination. Because no normal group was examined in this study, the specificity could not be calculated.
Contrast-enhanced CT can demonstrate the extent of disease, although no contrast is needed to demonstrate soft-tissue air (Figure 14-137).109 Wysoki et al.110 reviewed CT scans in 20 patients with pathologically confirmed necrotizing fasciitis. Soft-tissue air tracking along fascial planes (sensitivity = 55%), asymmetrical fascial thickening and fat stranding (sensitivity = 80%), and associated deep abscesses (sensitivity = 35%) were seen. Again, the specificity cannot be calculated, because no disease-free patients were included in this study. Other case reports suggest utility of CT in diagnosis.111-112
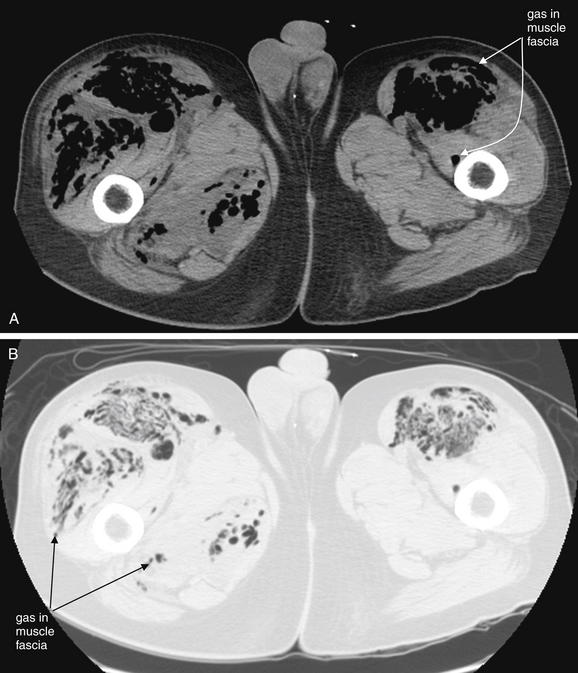
Figure 14-137 Necrotizing fasciitis.
Same patient as in Figure 14-136, where x-rays of the patient’s legs are shown. Noncontrast CT is shown here. A, Soft-tissue window. B, Lung window, same slice. If the diagnosis is highly suspected and x-rays are nondiagnostic, noncontrast CT is very sensitive for air. Air appears black on all CT window settings and is particularly evident on lung window, which makes all other tissues white. However, do not delay surgical consultation, antibiotic therapy, and surgical debridement to obtain diagnostic imaging once the diagnosis is suspected. This patient was taken to the operating room, and disarticulation of the hips was performed. He died of septic shock hours later. Blood cultures grew Clostridium perfringens—the feared gas gangrene organism of trench warfare in World War I.
Small studies have explored the role of MRI for the diagnosis of necrotizing fasciitis. Seok et al.113 compared MRI findings in 11 patients with surgically confirmed necrotizing fasciitis and 8 patients with pathologically confirmed pyomyositis. MRI findings in necrotizing fasciitis included a peripheral, bandlike, hyperintense signal in muscles on fat-suppressed, T2-weighted images (73%) and peripheral, bandlike, contrast enhancement of muscles, neither of which were seen in pyomyositis patients. In addition, necrotizing fasciitis patients showed thin, smooth enhancement of deep fascia in 82% of cases, compared with 13% of pyomyositis patients. In all necrotizing fasciitis patients, the superficial and deep fascia and muscle showed hyperintense signals on T2-weighted images and contrast enhancement on fat-suppressed, contrast-enhanced, T1-weighted images. However, the small size of this study and the absence of a normal control group limit the findings.
Brothers et al.114 reported results of MRI in nine patients with suspected necrotizing fasciitis of the lower extremity. Absence of gadolinium enhancement on T1-weighted images was seen in six patients with surgically proven fascial necrosis. MRI showed fascial inflammation (defined as low signal intensity on T1-weighted images and high signal intensity on T2-weighted images) in all nine patients, including three who ultimately did not have a clinical diagnosis of necrotizing fasciitis.
Schmid et al.115 compared MRI findings with surgical findings, autopsy, and clinical outcomes in 17 patients with suspected necrotizing fasciitis. MRI detected all 11 surgically proven cases of necrotizing fasciitis, based on imaging findings of deep fascial involvement with fluid collections, thickening, and enhancement after contrast administration. MRI was false positive in one case of cellulitis. Other case reports suggest utility of MRI in identifying early necrotizing fasciitis.116-117
Because necrotizing fasciitis is a time-sensitive and life-threatening infection, operative therapy should never be delayed for imaging when the diagnosis is strongly suspected.118 Studies of MRI are too limited at this time to determine whether MRI can exclude early necrotizing fasciitis. Presumably, imaging findings early in the course of disease might be subtler and less sensitive. MRI may not be perfectly specific and might prompt unnecessary operation in some patients.
Septic Arthritis
Septic arthritis is typically proven by culture of joint aspirates, in conjunction with clinical examination findings, synovial fluid Gram stain, and cell counts. The role of diagnostic imaging includes guidance of joint aspiration (e.g., using ultrasound in aspiration of the hip) and assessment of joints that are not successfully aspirated.
Can Ultrasound Rule Out Septic Arthritis of the Hip?
Zawin et al.119 reviewed results in 96 children with suspected septic arthritis of the hip. Among 40 with normal ultrasound of the hip, none had septic arthritis (95% CI = 0%-9%). Ultrasound detected hip effusions in 56 children; 31 had attempted ultrasound-guided joint aspiration, 29 successfully. Of these patients, 15 had joint aspirates consistent with septic arthritis. The authors concluded that a normal ultrasound of the hip rules out septic arthritis. Ultrasound-guided joint aspiration was highly successful in this series.
Can Magnetic Resonance Imaging Distinguish Septic Arthritis From Other Causes of Inflammation?
Karchevsky et al.120 reviewed 50 consecutive cases of MRI of septic arthritis and described the frequency of various findings (Table 14-14). Graif et al.121 compared MRI findings in 19 patients with septic joint and 11 with uninfected inflamed joints. Although combinations of findings made the diagnosis of septic joint more likely, no single MRI abnormality could confirm or exclude joint infection, because the findings in both conditions were similar and none showed statistically significant differences between the two groups (Table 14-15).
TABLE 14-14 Magnetic Resonance Imaging Findings of Septic Arthritis and Associated Osteomyelitis
| Finding | Frequency |
|---|---|
| Synovial enhancement | 98% |
| Perisynovial edema | 84% |
| Joint effusions | 70% |
| Fluid outpouching | 53% |
| Fluid enhancement | 30% |
| Synovial thickening | 22% |
| Marrow bare area changes | 86% |
| Marrow abnormal T2 signal | 84% |
| Marrow abnormal gadolinium enhancement | 81% |
| Marrow abnormal T1 signal | 66% |
Adapted from Karchevsky M, Schweitzer ME, Morrison WB, Parellada JA. MRI findings of septic arthritis and associated osteomyelitis in adults. AJR Am J Roentgenol 182:119-122, 2004.
TABLE 14-15 Magnetic Resonance Imaging Findings in Septic and Nonseptic Arthritis
| Finding | Septic Arthritis Frequency | Nonseptic Arthritis Frequency |
|---|---|---|
| Effusion | 79% | 82% |
| Fluid outpouching | 79% | 73% |
| Fluid heterogeneity | 21% | 27% |
| Synovial thickening | 68% | 55% |
| Synovial periedema | 63% | 55% |
| Synovial enhancement | 94% | 88% |
| Cartilage loss | 53% | 30% |
| Bone erosions | 79% | 38% |
| Bone erosions enhancement | 77% | 43% |
| Bone marrow edema | 74% | 38% |
| Bone marrow enhancement | 67% | 50% |
| Soft-tissue edema | 63% | 78% |
| Soft-tissue enhancement | 67% | 71% |
| Periosteal edema | 11% | 10% |
Adapted from Graif M, Schweitzer ME, Deely D, Matteucci T: The septic versus nonseptic inflamed joint: MRI characteristics. Skeletal Radiol 28:616-620, 1999.
Lee et al.122 retrospectively compared imaging findings in 9 pediatric patients with septic arthritis and 14 with transient synovitis of the hip. The authors documented signal intensity changes in bone marrow of the femoral head and neck on fat-suppressed, T1- and T2-weighted image sequences in patients with septic joint but not in those with transient synovitis. Similarly, Kwack et al.123 reviewed MRI findings in 9 patients with septic hip arthritis and 11 with transient synovitis. The authors found statistically significant differences in the frequency of some findings between the two groups. These two small studies suggest but do not prove that MRI can distinguish between the two conditions.
Osteomyelitis
Osteomyelitis can occur in the absence of any plain radiographic changes in its early phases. Later changes of osteomyelitis include osteolysis and soft-tissue swelling (Figures 14-138, 14-139, and 14-141). Soft-tissue air may also be seen. Unfortunately, by the time that these late findings develop, significant clinical deterioration and irreversible bone damage may be present. A prospective study of 110 consecutive patients with suspected diabetes-related osteomyelitis of the foot found the sensitivity of x-ray to be 63%, with specificity of 87%.124
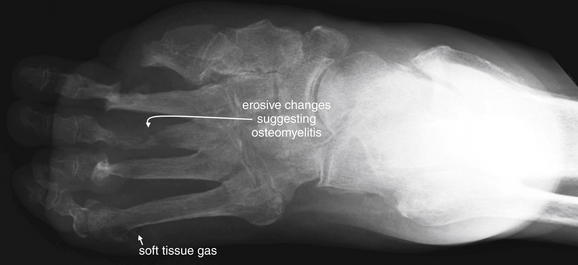
Figure 14-138 Charcot foot with osteomyelitis.
This 81-year-old male with diabetes was noted to have a foul-smelling foot wound by his daughter. She noted maggots in the wound, prompting an emergency department visit. The patient had wounds on the distal third, fourth, and fifth toes. He denied having any foot pain. There are extensive erosive changes of the metatarsals with fractures through the second and third distal metatarsal diaphyses—findings concerning for osteomyelitis. Gas is present in the soft tissues around the lateral aspect of the distal fifth metatarsal diaphysis, which could indicate gas-forming organisms or air entering through a wound. A number of other findings are present. The fourth proximal interphalangeal joint is subluxed. There has been great toe and first metatarsal amputation. There is diffuse degenerative joint disease, osteopenia, and soft-tissue swelling.
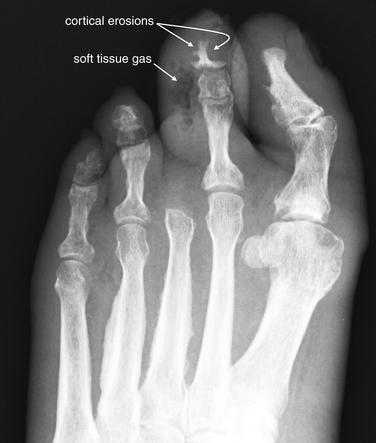
Figure 14-139 Diabetic foot infections.
This 61-year-old female with diabetes presented with fever, foot pain, and a foul-smelling foot wound with erythema and purulent discharge. This anterior–posterior view shows cortical irregularities of the second toe’s distal and middle phalanges, with adjacent soft-tissue swelling. A collection of rounded lucencies in the soft tissues are gas bubbles. The patient underwent amputation of her left second toe.
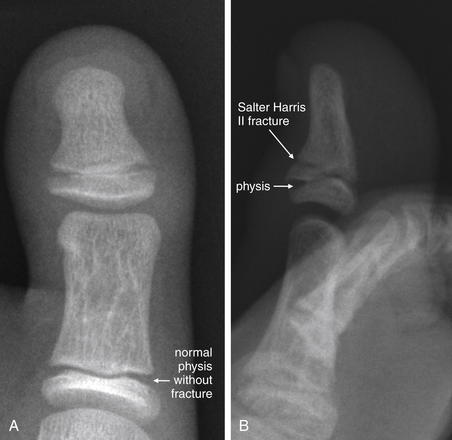
Figure 14-140 Stubbed toe with Salter-Harris II fracture.
This 8-year-old male presented with left great toe pain after stubbing his toe. Examination showed an abrasion just proximal to the cuticle, which the patient reported was bleeding before emergency department arrival. A, Anterior–posterior (AP) view of the great toe. B, Lateral view. Lateral x-ray shows a fracture line extending through the metaphysis to the physis—a Salter-Harris II fracture. The AP view does not clearly reveal this injury. Two perpendicular views should be assessed when evaluating for fracture. Abrasions or lacerations overlying fractures constitute open fractures, with risk for osteomyelitis. See Figure 14-141.
Earlier detection of osteomyelitis can be performed with MRI or bone scan. Nawaz et al.124 reported MRI to be 91% sensitive and 78% specific for diabetic osteomyelitis of the foot. In a study of 78 children, unenhanced MRI was equal to enhanced MRI in sensitivity and specificity for osteomyelitis, although use of gadolinium-enhancement increased reader confidence.125 Johnson et al.126 retrospectively reviewed 74 consecutive cases of MRI of the foot for suspected osteomyelitis and found that confluent decreased T1 marrow signal in a medullary distribution was 95% sensitive and 91% specific for osteomyelitis. Kan et al.127 retrospectively reviewed MRI following surgical interventions in 34 pediatric cases and 96 controls and concluded that iatrogenic soft-tissue and bone edema resulting from recent surgical intervention did not affect the diagnostic accuracy of MRI.
In a study of 51 patients with suspected osteomyelitis, Morrison et al.128 reported that fat-suppressed, contrast-enhanced MRI was more sensitive (88%) and specific (93%) for osteomyelitis than was nonenhanced magnetic resonance (sensitivity = 79%, specificity = 53%) or three-phase bone scan (sensitivity = 61%, specificity = 33%).
Earlier studies suggest that combining multiple nuclear scintigraphy tests (e.g., gallium and technetium-99m) can increase the accuracy for osteomyelitis in children. However, nuclear scintigraphy scans can be falsely positive in conditions such as fracture and juvenile rheumatoid arthritis.129
FDG-PET has been investigated in prospective comparison with MRI. Its sensitivity and specificity were 81% and 93%, respectively. In patients with contraindications to MRI, FDG-PET is an alternative. Its specificity is higher than that of MRI and can complement MRI when the diagnosis is uncertain.124
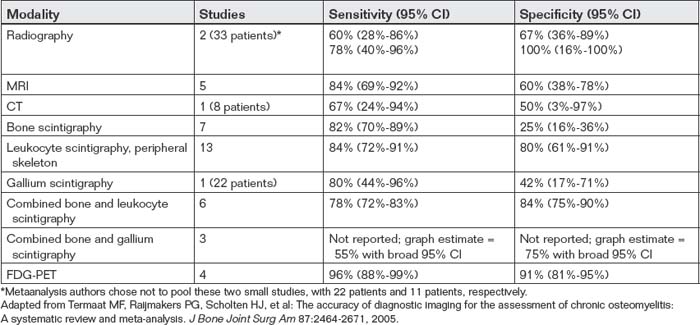
Which Imaging Modality Is Best for Diagnosis of Chronic Osteomyelitis?
The many studies of imaging of osteomyelitis makes selection of the best imaging test difficult. From 3384 studies identified in Medline, Embase, and Current Contents databases, a systematic review and metaanalysis identified only 23 clinical studies meeting strong methodologic standards and addressing diagnostic imaging of chronic osteomyelitis, defined as osteomyelitis requiring more than one episode of treatment or lasting more than 6 weeks.130 The results are summarized in Table 14-16. According to this metaanalysis, FDG-PET appears most sensitive and specific, but is not widely used in the emergency department setting. However, controversy remains about the best diagnostic modality, with some authors advocating MRI.131
Foreign Bodies
Dense foreign bodies, such as those composed of metal, stone, or glass, are usually visible on plain x-ray, because they are considerably denser than body soft tissues and similar to bone density (Figure 14-142). Multiple orthogonal views may be required to identify such foreign bodies, because they may not be visible if projected directly over bone of similar density. Less dense foreign bodies, such as those composed of wood, rubber, plastic, or other organic material (e.g., the defensive spines of marine organisms) pose a greater dilemma (Figure 14-143). These materials are often invisible on x-ray but can sometimes be detected using ultrasound and MRI. No large studies comparing these modalities exist.
In a case series of eight patients, rubber foreign bodies from puncture wounds through rubber-soled shoes were not detected by x-ray in 50% of cases, with serious wound complications including osteomyelitis arising in some patients.132
In a controlled study using simulated wounds in chicken thighs, x-ray using standard extremity exposures had no sensitivity for wood and was 10% sensitive for rubber foreign bodies, with 90% specificity in both cases. Soft-tissue exposure x-ray was 10% sensitive for detection of wood and rubber foreign bodies, with 90% specificity. In comparison, high-frequency ultrasound was 90% sensitive and 80% specific.133 Imaging was interpreted by blinded radiologists in this study.
Case reports suggest value of ultrasound in detection of radiolucent soft-tissue foreign bodies, including small fragments not detected with CT.134-138 In a prospective human study of 131 wounds in pediatric patients, sensitivity and specificity for foreign body detection were 67% and 97%, respectively, for bedside ultrasound performed by an attending pediatric emergency physician and 58% and 90%, respectively, for x-ray interpreted by a radiologist.139 However, in a prospective cadaver study involving 900 ultrasound examinations, ultrasound performed by trained emergency physicians was only 52.6% sensitive and 47.2% specific for detection of a variety of small foreign bodies composed of wood, metal, plastic, or glass.140 Why this low sensitivity and specificity? Several factors may account for the discrepancy between this study and studies in living humans. First, the gold standard in the preceding cadaver study was extremely strong, because foreign bodies were placed in the cadavers by the investigators. In contrast, in the preceding pediatric human study, the gold standard is less robust, because the diagnostic standard was removal of a foreign body. It is possible that x-ray and ultrasound both missed some foreign bodies, which were then not detected and removed from the wound.139 Thus the pediatric study may overestimate the sensitivity of x-ray and ultrasound. In addition, the cadaver study examined the sensitivity and specificity of ultrasound for detection of very small foreign bodies—2.5 mm3 or less in total volume and 5 mm or less in longest dimension, with insertion depths of up to 3 cm.140
In case reports, MRI has been reported to demonstrate soft-tissue foreign bodies, though no large studies validate its sensitivity and specificity.141-142 CT also has been reported to be useful in detection of radiolucent foreign bodies, though large studies are lacking.134,143
Do All Glass-Inflicted Wounds Require X-ray for Evaluation of Foreign Body?
A common principle in emergency medicine practice is to x-ray all wounds resulting from glass because of concern for an unrecognized retained foreign body. In a prospective study of 264 wounds, glass foreign bodies were found in 8.7%. In 2 (1.5%) of 134 superficial wounds (no deeper than subcutaneous fat), a foreign body was detected on x-ray but not on examination. In 10 (7.7%) of 130 deeper wounds, glass was detected with x-ray but not on examination. Consequently, in superficial wounds amenable to adequate exploration, x-ray may be unnecessary to evaluate for a glass foreign body.144
Gout
Gout is not typically a radiographic diagnosis, but x-rays are sometimes obtained to assess for competing diagnoses, including septic joint, osteomyelitis, and retained foreign body. In advanced cases (Figure 14-144), gout can create a characteristic appearance of periarticular and articular bony erosions, which must be recognized to avoid mistaken diagnosis of lytic lesions of osteomyelitis. Other x-ray findings associated with gout include soft-tissue opacities (less dense than bone but denser than surrounding soft tissue), representing tophi, and osteophyte formation at the margins of bony erosions and soft-tissue opacities. X-ray sensitivity for gout is reported to be 31%, with specificity of 93%, although small study sizes result in wide CIs. In addition, the diagnostic standard in some studies is poor, casting uncertainty on the reported diagnostic characteristics of x-ray. Ultrasound has a reported sensitivity of 96% but a lower specificity (73%). Ultrasound findings of gout include bright stippling and hyperechoic soft tissues, consistent with tophi.145
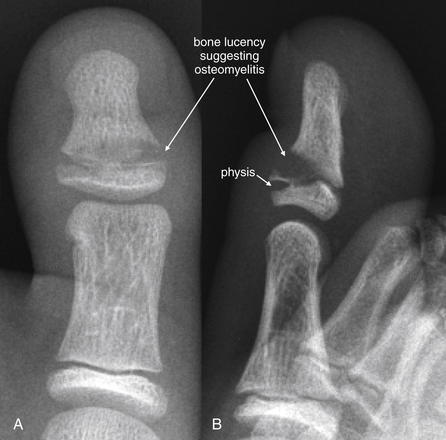
Figure 14-141 Stubbed toe osteomyelitis.
Same patient as in Figure 14-140. X-rays obtained 2 weeks after initial injury. A, Anterior–posterior view of the great toe. B, Lateral view. A lucency is visible in the metaphysis at the site of the prior Salter-Harris II fracture, suggesting osteomyelitis. Subtle soft-tissue injuries such as abrasions and subungual hematoma can indicate the presence of an open fracture. Given that Salter-Harris injuries can be radiographically occult, consider antibiotics when soft-tissue defects overlie physes in pediatric patients.
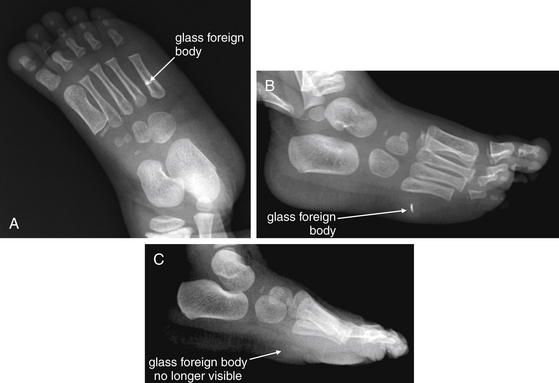
Figure 14-142 Foreign body x-ray, glass.
This 20-month-old female presented with a suspected glass foreign body. One week prior, she had stepped on a broken drinking glass and had been favoring her left foot since that time. Her parents had noted pus draining from the wound on the day of presentation. A, Anterior–posterior view of the foot. B, Oblique view of the foot. C, Lateral x-ray obtained after foreign body removal. Ordinary glass foreign bodies are typically visible on x-ray. Glass need not be of an unusual type, such as leaded glass, to be seen on x-ray. Two orthogonal views should be obtained to localize the foreign body. A, The foreign body appears to overlie the fifth metatarsal. B, The foreign body is visible in the plantar soft tissues. Exploration of the wound in the left foot showed one glass fragment, which was removed. C, No foreign body is visible.
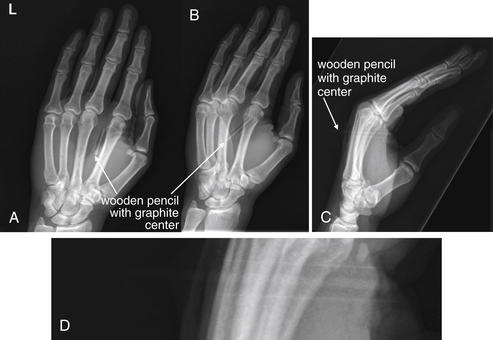
Figure 14-143 Foreign body x-ray, wood.
This 49-year-old right-hand-dominant teacher fell on a pencil while wrestling with a student. The pencil pierced the left hand from a volar to dorsal direction. A, Anterior–posterior x-ray. B, Oblique x-ray. C, Lateral x-ray. D, Expanded view from C. A pencil fragment pierces the soft tissues between the second and the third metacarpals without underlying bone fracture. The pencil appears intact. The graphite rod in the center of the pencil is visible as an opacity because of its high density, whereas the wood is seen as a lucency against the soft tissues, resulting from its low density. Wood is often not visible on x-ray. The paint on the pencil is faintly visible as lines parallel to the graphite center.
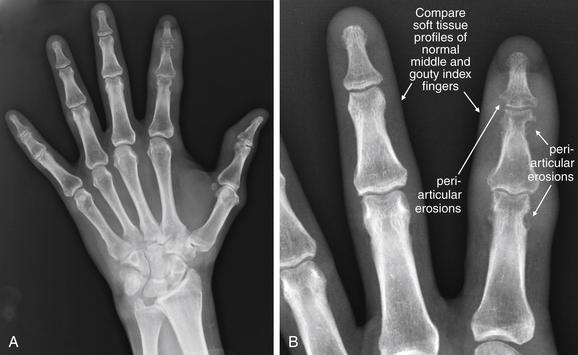
This 58-year-old male with a history of gout complained of left index finger swelling, erythema, and pain. His x-rays show well-defined periarticular erosions with overhanging edges at the index finger proximal interphalangeal (PIP) and distal interphalangeal (DIP) joints. Soft-tissue swelling is also seen at DIP and PIP joints of the index finger. These are characteristic findings of crystalline deposition disorders such as gout. A, Anterior–posterior view of the hand. B, Close-up.
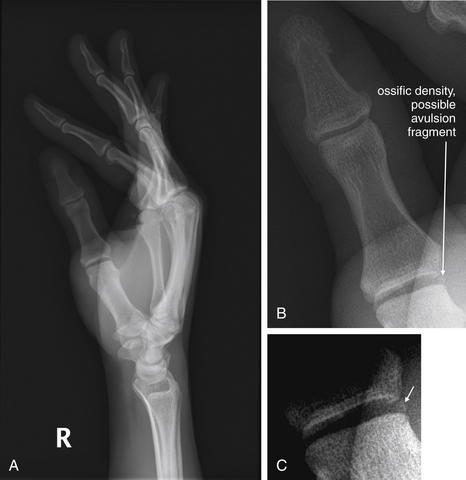
Figure 14-145 Gamekeeper’s thumb.
A 36-year-old male, with pain at the metacarpophalangeal (MCP) joint of the thumb after a bicycling accident. A, Lateral view of the hand. B, C, Close-ups. Gamekeeper’s thumb refers to avulsion of the ulnar collateral ligament (UCL) from the base of the thumb, usually occurring from forced thumb abduction. An avulsion fracture of proximal thumb at the MCP joint may accompany this injury. The avulsion fragment may be seen at the ulnar aspect of the proximal portion of the proximal phalanx of the thumb. When no fracture is seen, stress views of the thumb are sometimes used to assess the integrity of the UCL. Normal neutral position x-rays do not rule out this ligamentous injury. In this patient, a tiny ossific density is seen at the ulnar thumb base, possibly representing a tiny avulsion fragment.
Rickets
Rickets, the demineralization of bone at growth plates in children most commonly because of vitamin D deficiency, can be an incidental radiographic diagnosis in the emergency department.146 Risk factors include dark skin color and prolonged breast feeding, which predispose a child to vitamin D deficiency. X-ray findings can include decreased bone density, bowing of weight-bearing long bones, greenstick fractures from low bone density, metaphyseal lucencies, a coarsened trabecular pattern, and cortical tunneling from secondary hyperparathyroidism. The metaphyses of long bones may demonstrate splaying. Anterior rib ends may appear expanded.147 The findings can be subtle; an emergency physician may recognize that an abnormality is present, but the specific diagnosis may require the interpretation of a pediatric radiologist. Emergency physicians should be attuned to the possibility of this diagnosis, which is still seen in developed countries including the United States, particularly in immigrant populations who may lack vitamin D supplementation.148 Moreover, in pediatric fractures occurring with minimal force, the possibility of rickets and rare conditions such as osteogenesis imperfecta should be considered, along with nonaccidental trauma (described earlier).146
Lead
Chronic lead toxicity may result in a radiodensity in the distal metaphyseal plate in children. So-called lead lines in the long bones of children represent growth arrest lines. They are not pathognomonic of lead toxicity but may be seen with long-standing lead levels greater than 40 μg/dL.149 X-ray should not be used to rule out lead toxicity because of poor sensitivity with acute ingestions. Instead, a whole-blood lead level is the criterion standard.150
Summary
Extremity imaging is an essential part of emergency medicine practice. In some common clinical scenarios such as ankle injury, clinical decision rules can assist in imaging decisions and reduce the need for radiography. Although x-rays suffice for evaluation of many extremity injuries and nontraumatic pathology, in selected cases such as scaphoid fracture and injuries to the Lisfranc joint, the emergency physician must be aware of the poor sensitivity of x-ray and the potential necessity of advanced imaging such as MRI. Emergency physicians must recognize patterns of high-risk injuries and understand when imaging is required for associated conditions including vascular trauma. MRI, CT, ultrasound, angiography, and nuclear scintigraphy play specialty roles for evaluation of subtle but important conditions such as osteomyelitis. Some important extremity conditions such as compartment syndrome remain clinical diagnoses at this time, with little added diagnostic value of imaging.
1. Kim E.E., Pjura G.A., Lowry P.A., et al. Osteomyelitis complicating fracture: Pitfalls of 111In leukocyte scintigraphy. AJR Am J Roentgenol. 1987;148:927-930.
1a. Ludwig H., Kumpan W., Sinzinger H. Radiography and bone scintigraphy in multiple myeloma: a comparative analysis. Br J Radiol. 1982;Br J Radiol(651):173-181.
2. Seabold J.E., Palestro C.J., Brown M.L., et al. Procedure guideline for gallium scintigraphy in inflammation: Society of Nuclear Medicine. J Nucl Med. 1997;38:994-997.
2a. Shortt C.P., Gleeson T.G., Breen K.A., McHugh .J., et al. Whole-Body MRI versus PET in assessment of multiple myeloma disease activity. AJR Am J Roentgenol. 2009;192(4):980-986.
3. Brehaut J.C., Stiell I.G., Visentin L., Graham I.D. Clinical decision rules “in the real world”: How a widely disseminated rule is used in everyday practice. Acad Emerg Med. 2005;12:948-956.
4. Stiell I.G., Wells G.A., McDowell I., et al. Use of radiography in acute knee injuries: Need for clinical decision rules. Acad Emerg Med. 1995;2:966-973.
5. Stiell I.G., Greenberg G.H., Wells G.A., et al. Derivation of a decision rule for the use of radiography in acute knee injuries. Ann Emerg Med. 1995;26:405-413.
6. Stiell I.G., Greenberg G.H., Wells G.A., et al. Prospective validation of a decision rule for the use of radiography in acute knee injuries. JAMA. 1996;275:611-615.
7. Emparanza J.I., Aginaga J.R. Validation of the Ottawa knee rules. Ann Emerg Med. 2001;38:364-368.
8. Bachmann L.M., Haberzeth S., Steurer J., ter Riet G. The accuracy of the Ottawa knee rule to rule out knee fractures: A systematic review. Ann Intern Med. 2004;140:121-124.
9. Khine H., Dorfman D.H., Avner J.R. Applicability of Ottawa knee rule for knee injury in children. Pediatr Emerg Care. 2001;17:401-404.
9a. Bulloch B., Neto G., Plint .A., et al. Validation of the Ottawa Knee Rule in children: a multicenter study. Ann Emerg Med. 2003;42(1):48-55.
9b. Vijayasankar D., Boyle A.A., Atkinson P. Can the Ottawa knee rule be applied to children? A systematic review and meta-analysis of observational studies. Emerg Med J Apr. 2009;26(4):250-253.
9c. Seaberg D.C., Yealy D.M., Lukens T., et al. Multicenter comparison of two clinical decision rules for the use of radiography in acute, high-risk knee injuries. Ann Emerg Med. 1998;32(1):8-13.
10. Seaberg D.C., Jackson R. Clinical decision rule for knee radiographs. Am J Emerg Med. 1994;12:541-543.
11. Lee J.H., Weissman B.N., Nikpoor N., et al. Lipohemarthrosis of the knee: A review of recent experiences. Radiology. 1989;173:189-191.
12. Henckel J., Richards R., Lozhkin K., et al. Very low-dose computed tomography for planning and outcome measurement in knee replacement: The imperial knee protocol. J Bone Joint Surg Br. 2006;88:1513-1518.
13. Lemburg S.P., Lilienthal E., Heyer C.M. Growth plate fractures of the distal tibia: Is CT imaging necessary? Arch Orthop Trauma Surg, 2010.
14. Boutis K., Narayanan U.G., Dong F.F., et al. Magnetic resonance imaging of clinically suspected Salter-Harris I fracture of the distal fibula. Injury. 2010.
15. Kocher M.S., Kasser J.R. Orthopaedic aspects of child abuse. J Am Acad Orthop Surg. 2000;8:10-20.
16. Loder R.T., Feinberg J.R. Orthopaedic injuries in children with nonaccidental trauma: Demographics and incidence from the 2000 kids’ inpatient database. J Pediatr Orthop. 2007;27:421-426.
17. Lane W.G., Rubin D.M., Monteith R., Christian C.W. Racial differences in the evaluation of pediatric fractures for physical abuse. JAMA. 2002;288:1603-1609.
18. Clements R.H., Reisser J.R. Scapulothoracic dissociation: A devastating injury. J Trauma. 1996;40:146-149.
19. Ebraheim N.A., An H.S., Jackson W.T., et al. Scapulothoracic dissociation. J Bone Joint Surg Am. 1988;70:428-432.
20. Rubenstein J.D., Ebraheim N.A., Kellam J.F. Traumatic scapulothoracic dissociation. Radiology. 1985;157:297-298.
21. Ridpath C.A., Nork S., Linnau K., Mann F.A. Scapulothoracic dissociation: Are there reliable chest radiographic findings? Emergency Radiology. 2001;8:304-307.
22. Throckmorton T., Kuhn J.E. Fractures of the medial end of the clavicle. J Shoulder Elbow Surg. 2007;16:49-54.
23. Alyas F., Curtis M., Speed C., et al. MR imaging appearances of acromioclavicular joint dislocation. Radiographics. 2008;28:463-479. quiz 619
24. Vaatainen U., Pirinen A., Makela A. Radiological evaluation of the acromioclavicular joint. Skeletal Radiol. 1991;20:115-116.
25. Cook D.A., Heiner J.P. Acromioclavicular joint injuries. Orthop Rev. 1990;19:510-516.
26. Bossart P.J., Joyce S.M., Manaster B.J., Packer S.M. Lack of efficacy of “weighted” radiographs in diagnosing acute acromioclavicular separation. Ann Emerg Med. 1988;17:20-24.
27. Wojtys E.M., Nelson G. Conservative treatment of grade III acromioclavicular dislocations. Clin Orthop Relat Res. 1991:112-119.
28. Zacchilli M.A., Owens B.D. Epidemiology of shoulder dislocations presenting to emergency departments in the United States. J Bone Joint Surg Am. 2010;92:542-549.
29. Widjaja A.B., Tran A., Bailey M., Proper S. Correlation between Bankart and Hill-Sachs lesions in anterior shoulder dislocation. ANZ J Surg. 2006;76:436-438.
30. Workman T.L., Burkhard T.K., Resnick D., et al. Hill-Sachs lesion: Comparison of detection with MR imaging, radiography, and arthroscopy. Radiology. 1992;185:847-852.
31. Pancione L., Gatti G., Mecozzi B. Diagnosis of Hill-Sachs lesion of the shoulder: Comparison between ultrasonography and arthro-CT. Acta Radiol. 1997;38:523-526.
32. Tadros A.M., Lunsjo K., Czechowski J., et al. Usefulness of different imaging modalities in the assessment of scapular fractures caused by blunt trauma. Acta Radiol. 2007;48:71-75.
33. Haapamaki V.V., Kiuru M.J., Koskinen S.K. Multidetector CT in shoulder fractures. Emerg Radiol. 2004;11:89-94.
34. Landin L.A., Danielsson L.G. Elbow fractures in children: An epidemiological analysis of 589 cases. Acta Orthop Scand. 1986;57:309-312.
35. Louahem D.M., Bourelle S., Buscayret F., et al. Displaced medial epicondyle fractures of the humerus: Surgical treatment and results. A report of 139 cases. Arch Orthop Trauma Surg. 2010;130:649-655.
36. Wilson N.I., Ingram R., Rymaszewski L., Miller J.H. Treatment of fractures of the medial epicondyle of the humerus. Injury. 1988;19:342-344.
37. Fowles J.V., Slimane N., Kassab M.T. Elbow dislocation with avulsion of the medial humeral epicondyle. J Bone Joint Surg Br. 1990;72:102-104.
38. Yates C., Sullivan J.A. Arthrographic diagnosis of elbow injuries in children. J Pediatr Orthop. 1987;7:54-60.
39. Walsh JJ IV, Patterson LA, Rectenwald J: Medial humeral condyle fracture. (Accessed at http://emedicine.medscape.com/article/1231290-overview.)
40. Ertl JP: Lateral humeral condyle fracture. (Accessed at http://emedicine.medscape.com/article/1231199-diagnosis.)
41. Milch H. Fractures and fracture dislocations of the humeral condyles. J Trauma. 1964;4:592-607.
42. Jakob R., Fowles J.V., Rang M., Kassab M.T. Observations concerning fractures of the lateral humeral condyle in children. J Bone Joint Surg Br. 1975;57:430-436.
43. O’Dwyer H., O’Sullivan P., Fitzgerald D., et al. The fat pad sign following elbow trauma in adults: Its usefulness and reliability in suspecting occult fracture. J Comput Assist Tomogr. 2004;28:562-565.
44. Amis A.A., Miller J.H. The mechanisms of elbow fractures: An investigation using impact tests in vitro. Injury. 1995;26:163-168.
45. Goldfarb C.A., Yin Y., Gilula L.A., et al. Wrist fractures: What the clinician wants to know. Radiology. 2001;219:11-28.
46. Essex-Lopresti P. Fractures of the radial head with distal radio-ulnar dislocation: Report of two cases. J Bone Joint Surg Br. 1951;33B:244-247.
47. Edwards G.S.Jr., Jupiter J.B. Radial head fractures with acute distal radioulnar dislocation: Essex-Lopresti revisited. Clin Orthop Relat Res. 1988:61-69.
48. Pliefke J., Stengel D., Rademacher G., et al. Diagnostic accuracy of plain radiographs and cineradiography in diagnosing traumatic scapholunate dissociation. Skeletal Radiol. 2008;37:139-145.
49. Webster A.P., Goodacre S., Walker D., Burke D. How do clinical features help identify paediatric patients with fractures following blunt wrist trauma? Emerg Med J. 2006;23:354-357.
50. Ali A., Hamman J., Mass D.P. The biomechanical effects of angulated boxer’s fractures. J Hand Surg Am. 1999;24:835-844.
51. Soyer A.D. Fractures of the base of the first metacarpal: Current treatment options. J Am Acad Orthop Surg. 1999;7:403-412.
52. Foster R.J., Hastings H.II. Treatment of Bennett, Rolando, and vertical intraarticular trapezial fractures. Clin Orthop Relat Res. 1987:121-129.
53. Howard F.M. Fractures of the basal joint of the thumb. Clin Orthop Relat Res. 1987:46-51.
54. Coons M.S., Green S.M. Boutonniere deformity. Hand Clin. 1995;11:387-402.
55. Husain S.N., Dietz J.F., Kalainov D.M., Lautenschlager E.P. A biomechanical study of distal interphalangeal joint subluxation after mallet fracture injury. J Hand Surg Am. 2008;33:26-30.
56. Chang Y.H., Tu Y.K., Yeh W.L., Hsu R.W. Tibial plateau fracture with compartment syndrome: A complication of higher incidence in Taiwan. Chang Gung Med J. 2000;23:149-155.
57. American College of Radiology. ACR appropriateness criteria: Acute trauma to the knee, 2008. (Accessed at http://www.acr.org/SecondaryMainMenuCategories/quality_safety/app_criteria/pdf/ExpertPanelonMusculoskeletalImaging/AcuteTraumatotheKneeDoc2.aspx.)
58. Wicky S., Blaser P.F., Blanc C.H., et al. Comparison between standard radiography and spiral CT with 3D reconstruction in the evaluation, classification and management of tibial plateau fractures. Eur Radiol. 2000;10:1227-1232.
59. Aderinto J., Walmsley P., Keating J.F. Fractures of the tibial spine: Epidemiology and outcome. Knee. 2008;15:164-167.
60. Vidyadhara S, Rao SK, Shetty MS. Tibial plateau fractures. In: Emedicine Orthopedics, 2009. (Accessed at http://emedicine.medscape.com/article/1249872-diagnosis.)
61. Yu J.S., Goodwin D., Salonen D., et al. Complete dislocation of the knee: Spectrum of associated soft-tissue injuries depicted by MR imaging. AJR Am J Roentgenol. 1995;164:135-139.
62. Dennis J.W., Jagger C., Butcher J.L., et al. Reassessing the role of arteriograms in the management of posterior knee dislocations. J Trauma. 1993;35:692-695. discussion 695-7
63. Barnes C.J., Pietrobon R., Higgins L.D. Does the pulse examination in patients with traumatic knee dislocation predict a surgical arterial injury? A meta-analysis. J Trauma. 2002;53:1109-1114.
64. Abou-Sayed H., Berger D.L. Blunt lower-extremity trauma and popliteal artery injuries: Revisiting the case for selective arteriography. Arch Surg. 2002;137:585-589.
65. Hutto J.D., Reed A.B. Endovascular repair of an acute blunt popliteal artery injury. J Vasc Surg. 2007;45:188-190.
66. Papavassiliou V.G., Dervisis C.I., Argitis V.P., et al. Endovascular repair of a traumatic popliteal arteriovenous fistula. Vasa. 2006;35:53-55.
67. Rieger M., Mallouhi A., Tauscher T., et al. Traumatic arterial injuries of the extremities: Initial evaluation with MDCT angiography. AJR Am J Roentgenol. 2006;186:656-664.
67a. Busquets A.R., Acosta J.A., Colon E., et al. Helical computed tomographic angiography for the diagnosis of traumatic arterial injuries of the extremities. J Trauma. 2004;56:625-628.
67b. Inaba K., Potzman J., Munera F., et al. Multi-slice CT angiography for arterial evaluation in the injured lower extremity. J Trauma. 2006;60:502-506. discussion 506-507
67c. Willmann J.K., Baumert B., Schertler T., et al. Aortoiliac and lower extremity arteries assessed with 16-detector row CT angiography: prospective comparison with digital subtraction angiography. Radiology. 2005;236:1083-1093.
68. Fleiter T.R., Mervis S. The role of 3D-CTA in the assessment of peripheral vascular lesions in trauma patients. Eur J Radiol. 2007;64:92-102.
69. Potter H.G., Weinstein M., Allen A.A., et al. Magnetic resonance imaging of the multiple-ligament injured knee. J Orthop Trauma. 2002;16:330-339.
70. Matheson G.O., Clement D.B., McKenzie D.C., et al. Stress fractures in athletes. A study of 320 cases. Am J Sports Med. 1987;15:46-58.
71. Coady C.M., Micheli L.J. Stress fractures in the pediatric athlete. Clin Sports Med. 1997;16:225-238.
72. Heyworth B.E., Green D.W. Lower extremity stress fractures in pediatric and adolescent athletes. Curr Opin Pediatr. 2008;20:58-61.
73. Tytherleigh-Strong G.M., Keating J.F., Court-Brown C.M. Extra-articular fractures of the proximal tibial diaphysis: Their epidemiology, management and outcome. J R Coll Surg Edinb. 1997;42:334-338.
74. Panchbhavi VK. Pilon fractures. In: Emedicine Orthopedics, 2010. (Accessed at http://emedicine.medscape.com/article/1233429-overview update May 13, 2010.)
75. Duchesneau S., Fallat L.M. The Tillaux fracture. J Foot Ankle Surg. 1996;35:127-133. discussion 89
76. Spiegel P.G., Mast J.W., Cooperman D.R., Laros G.S. Triplane fractures of the distal tibial epiphysis. Clin Orthop Relat Res. 1984:74-89.
77. El-Karef E., Sadek H.I., Nairn D.S., et al. Triplane fracture of the distal tibia. Injury. 2000;31:729-736.
78. Chan G.M., Yoshida D. Fracture of the lateral process of the talus associated with snowboarding. Ann Emerg Med. 2003;41:854-858.
79. McCrory P., Bladin C. Fractures of the lateral process of the talus: A clinical review. “Snowboarder’s ankle. Clin J Sport Med. 1996;6:124-128.
80. Moehring H.D., Tan R.T., Marder R.A., Lian G. Ankle dislocation. J Orthop Trauma. 1994;8:167-172.
81. Chaminade B., Zographos S., Utheza G. [Double measurement of the Bohler angle: Prognostic value of radiological angles in posterior facet fractures of the calcaneus]. Rev Chir Orthop Reparatrice Appar Mot. 2001;87:712-717.
82. Isaacs J., Baba M., Szomor Z. The diagnostic accuracy of Bohler’s angle in fractures of the calcaneus. J Bone Joint Surg Br. 2008;92B:178.
83. Knight J.R., Gross E.A., Bradley G.H., et al. Boehler’s angle and the critical angle of Gissane are of limited use in diagnosing calcaneus fractures in the ED. Am J Emerg Med. 2006;24:423-427.
84. Rammelt S., Zwipp H. Calcaneus fractures: Facts, controversies and recent developments. Injury. 2004;35:443-461.
85. ACR appropriateness criteria: Suspected Ankle Fracture. 2008. American College of Radiology. Available at: http://www.acr.org/SecondaryMainMenuCategories/quality_safety/app_criteria/pdf/ExpertPanelonMusculoskeletalImaging/SuspectedAnkleFracturesDoc21.aspx.
86. Preidler K.W., Peicha G., Lajtai G., et al. Conventional radiography and MR imaging in patients with hyperflexion injuries of the foot: Diagnostic accuracy in the detection of bony and ligamentous changes. AJR Am J Roentgenol. 1999;173:1673-1677.
87. Peicha G., Preidler K.W., Lajtai G., et al. [Diagnostic value of conventional roentgen image, computerized and magnetic resonance tomography in acute sprains of the foot: A prospective clinical study]. Unfallchirurg. 2001;104:1134-1139.
88. Preidler K.W., Brossmann J., Daenen B., et al. MR imaging of the tarsometatarsal joint: Analysis of injuries in 11 patients. AJR Am J Roentgenol. 1996;167:1217-1222.
89. Crim J. MR imaging evaluation of subtle Lisfranc injuries: The midfoot sprain. Magn Reson Imaging Clin N Am. 2008;16:19-27. v
90. Rammelt S., Heineck J., Zwipp H. Metatarsal fractures, Injury. 2004;35(Suppl 2):SB77-SB86.
91. Lawrence S.J., Botte M.J. Jones’ fractures and related fractures of the proximal fifth metatarsal. Foot Ankle. 1993;14:358-365.
92. Nunley J.A. Fractures of the base of the fifth metatarsal: The Jones fracture. Orthop Clin North Am. 2001;32:171-180.
93. Chuckpaiwong B., Queen R.M., Easley M.E., Nunley J.A. Distinguishing Jones and proximal diaphyseal fractures of the fifth metatarsal. Clin Orthop Relat Res. 2008;466:1966-1970.
94. Clapper M.F., O’Brien T.J., Lyons P.M. Fractures of the fifth metatarsal: Analysis of a fracture registry. Clin Orthop Relat Res. 1995:238-241.
95. Heineck J., Wolz M., Haupt C., et al. Fifth metatarsal avulsion fracture: A rational basis for postoperative treatment. Arch Orthop Trauma Surg. 2009;129:1089-1092.
96. O’Malley M.J., Hamilton W.G., Munyak J. Fractures of the distal shaft of the fifth metatarsal “Dancer’s fracture. Am J Sports Med. 1996;24:240-243.
97. Pinckney L.E., Currarino G., Kennedy L.A. The stubbed great toe: A cause of occult compound fracture and infection. Radiology. 1981;138:375-377.
98. Kensinger D.R., Guille J.T., Horn B.D., Herman M.J. The stubbed great toe: Importance of early recognition and treatment of open fractures of the distal phalanx. J Pediatr Orthop. 2001;21:31-34.
99. American College of Radiology. ACR appropriateness criteria: Soft-tissue masses, 2009. (Accessed at http://www.acr.org/SecondaryMainMenuCategories/quality_safety/app_criteria/pdf/ExpertPanelonMusculoskeletalImaging/SoftTissueMassesDoc19.aspx.)
99a. American College of Radiology. ACR Appropriateness Criteria. Primary Bone Tumors. 2009. Available at: http://www.acr.org/SecondaryMainMenuCategories/quality_safety/app_criteria/pdf/ExpertPanelonMusculoskeletalImaging/BoneTumorsDoc4.aspx.)
100. Goodacre S., Sampson F., Thomas S., et al. Systematic review and meta-analysis of the diagnostic accuracy of ultrasonography for deep vein thrombosis. BMC Med Imaging. 2005;5:6.
101. Birdwell B.G., Raskob G.E., Whitsett T.L., et al. The clinical validity of normal compression ultrasonography in outpatients suspected of having deep venous thrombosis. Ann Intern Med. 1998;128:1-7.
102. Sutter M.E., Turnipseed S.D., Diercks D.B., et al. Venous ultrasound testing for suspected thrombosis: Incidence of significant non-thrombotic findings. J Emerg Med. 2009;36:55-59.
103. Shadgan B., Menon M., O’Brien P.J., Reid W.D. Diagnostic techniques in acute compartment syndrome of the leg. J Orthop Trauma. 2008;22:581-587.
104. Rominger M.B., Lukosch C.J., Bachmann G.F. MR imaging of compartment syndrome of the lower leg: A case control study. Eur Radiol. 2004;14:1432-1439.
105. Jerosch J., Geske B., Sons H.U., Winkelmann W. [The value of sonography in assessing intracompartmental pressure in the anterior tibial compartment]. Ultraschall Med. 1989;10:206-210.
106. Edwards P.D., Miles K.A., Owens S.J., et al. A new non-invasive test for the detection of compartment syndromes. Nucl Med Commun. 1999;20:215-218.
107. Katz L.M., Nauriyal V., Nagaraj S., et al. Infrared imaging of trauma patients for detection of acute compartment syndrome of the leg. Crit Care Med. 2008;36:1756-1761.
108. Gentilello L.M., Sanzone A., Wang L., et al. Near-infrared spectroscopy versus compartment pressure for the diagnosis of lower extremity compartmental syndrome using electromyography-determined measurements of neuromuscular function. J Trauma. 2001;51:1-8. discussion 9
109. Parenti G.C., Marri C., Calandra G., et al. [Necrotizing fasciitis of soft tissues: Role of diagnostic imaging and review of the literature]. Radiol Med. 2000;99:334-339.
110. Wysoki M.G., Santora T.A., Shah R.M., Friedman A.C. Necrotizing fasciitis: CT characteristics. Radiology. 1997;203:859-863.
111. Schulze M., Overkamp D., Joanoviciu S., Horger M. [Necrotizing fasciitis: CT-imaging findings]. Rofo. 2008;180:587-590.
112. Jean-Charles N., Sadler M.A. Necrotizing perineal fasciitis in two paraplegic nursing-home residents: CT imaging findings. Abdom Imaging. 2001;26:443-446.
113. Seok J.H., Jee W.H., Chun K.A., et al. Necrotizing fasciitis versus pyomyositis: Discrimination with using MR imaging. Korean J Radiol. 2009;10:121-128.
114. Brothers T.E., Tagge D.U., Stutley J.E., et al. Magnetic resonance imaging differentiates between necrotizing and non-necrotizing fasciitis of the lower extremity. J Am Coll Surg. 1998;187:416-421.
115. Schmid M.R., Kossmann T., Duewell S. Differentiation of necrotizing fasciitis and cellulitis using MR imaging. AJR Am J Roentgenol. 1998;170:615-620.
116. Sato T., Hagiwara K., Matsuno H., et al. A case of necrotizing fasciitis caused by coagulase-negative staphylococcus: Utility of magnetic resonance imaging for the preoperative diagnosis of necrotizing fasciitis. J Infect Chemother. 2005;11:160-163.
117. Zittergruen M., Grose C. Magnetic resonance imaging for early diagnosis of necrotizing fasciitis. Pediatr Emerg Care. 1993;9:26-28.
118. Drake D.B., Woods J.A., Bill T.J., et al. Magnetic resonance imaging in the early diagnosis of group A beta streptococcal necrotizing fasciitis: A case report. J Emerg Med. 1998;16:403-407.
119. Zawin J.K., Hoffer F.A., Rand F.F., Teele R.L. Joint effusion in children with an irritable hip: US diagnosis and aspiration. Radiology. 1993;187:459-463.
120. Karchevsky M., Schweitzer M.E., Morrison W.B., Parellada J.A. MRI findings of septic arthritis and associated osteomyelitis in adults. AJR Am J Roentgenol. 2004;182:119-122.
121. Graif M., Schweitzer M.E., Deely D., Matteucci T. The septic versus nonseptic inflamed joint: MRI characteristics. Skeletal Radiol. 1999;28:616-620.
122. Lee S.K., Suh K.J., Kim Y.W., et al. Septic arthritis versus transient synovitis at MR imaging: Preliminary assessment with signal intensity alterations in bone marrow. Radiology. 1999;211:459-465.
123. Kwack K.S., Cho J.H., Lee J.H., et al. Septic arthritis versus transient synovitis of the hip: Gadolinium-enhanced MRI finding of decreased perfusion at the femoral epiphysis. AJR Am J Roentgenol. 2007;189:437-445.
124. Nawaz A., Torigian D.A., Siegelman E.S., et al. Diagnostic performance of FDG-PET, MRI, and plain film radiography (PFR) for the diagnosis of osteomyelitis in the diabetic foot. Mol Imaging Biol. 2010;12:335-342.
125. Averill L.W., Hernandez A., Gonzalez L., et al. Diagnosis of osteomyelitis in children: Utility of fat-suppressed contrast-enhanced MRI. AJR Am J Roentgenol. 2009;192:1232-1238.
126. Johnson P.W., Collins M.S., Wenger D.E. Diagnostic utility of T1-weighted MRI characteristics in evaluation of osteomyelitis of the foot. AJR Am J Roentgenol. 2009;192:96-100.
127. Kan J.H., Hilmes M.A., Martus J.E., et al. Value of MRI after recent diagnostic or surgical intervention in children with suspected osteomyelitis. AJR Am J Roentgenol. 2008;191:1595-1600.
128. Morrison W.B., Schweitzer M.E., Bock G.W., et al. Diagnosis of osteomyelitis: Utility of fat-suppressed contrast-enhanced MR imaging. Radiology. 1993;189:251-257.
129. Lewin J.S., Rosenfield N.S., Hoffer P.B., Downing D. Acute osteomyelitis in children: Combined Tc-99m and Ga-67 imaging. Radiology. 1986;158:795-804.
130. Termaat M.F., Raijmakers P.G., Scholten H.J., et al. The accuracy of diagnostic imaging for the assessment of chronic osteomyelitis: A systematic review and meta-analysis. J Bone Joint Surg Am. 2005;87:2464-2471.
131. Schwegler B., Stumpe K.D., Weishaupt D., et al. Unsuspected osteomyelitis is frequent in persistent diabetic foot ulcer and better diagnosed by MRI than by 18F-FDG PET or 99mTc-MOAB. J Intern Med. 2008;263:99-106.
132. Chang H.C., Verhoeven W., Chay W.M. Rubber foreign bodies in puncture wounds of the foot in patients wearing rubber-soled shoes. Foot Ankle Int. 2001;22:409-414.
133. Turkcuer I., Atilla R., Topacoglu H., et al. Do we really need plain and soft-tissue radiographies to detect radiolucent foreign bodies in the ED? Am J Emerg Med. 2006;24:763-768.
134. Bray H., Stringer D.A., Poskitt K., et al. Maple tree knee: A unique foreign body—value of ultrasound and CT examination. Pediatr Radiol. 1991;21:457-458.
135. Dean A.J., Gronczewski C.A., Costantino T.G. Technique for emergency medicine bedside ultrasound identification of a radiolucent foreign body. J Emerg Med. 2003;24:303-308.
136. Dumarey A., De Maeseneer M., Ernst C. Large wooden foreign body in the hand: Recognition of occult fragments with ultrasound. Emerg Radiol. 2004;10:337-339.
137. Hung Y.T., Hung L.K., Griffith J.F., et al. Ultrasound for the detection of vegetative foreign body in hand: A case report. Hand Surg. 2004;9:83-87.
138. Yanay O., Vaughan D.J., Diab M., et al. Retained wooden foreign body in a child’s thigh complicated by severe necrotizing fasciitis: A case report and discussion of imaging modalities for early diagnosis. Pediatr Emerg Care. 2001;17:354-355.
139. Friedman D.I., Forti R.J., Wall S.P., Crain E.F. The utility of bedside ultrasound and patient perception in detecting soft tissue foreign bodies in children. Pediatr Emerg Care. 2005;21:487-492.
140. Crystal C.S., Masneri D.A., Hellums J.S., et al. Bedside ultrasound for the detection of soft tissue foreign bodies: A cadaveric study. J Emerg Med. 2009;36:377-380.
141. Nelson E.W., DeHart M.M., Christensen A.W., Smith D.K. Magnetic resonance imaging characteristics of a lead pencil foreign body in the hand. J Hand Surg Am. 1996;21:100-103.
142. Varma D.G., Ro J.Y., Guo S.Q., Moulopoulos L.A. Magnetic resonance imaging appearance of foreign body granulomas of the upper arms. Clin Imaging. 1994;18:39-42.
143. Kantarci M., Ogul H., Karasen R.M. Detection of a giant wooden foreign body with multidetector computed tomography and multiplanar reconstruction imaging. Am J Emerg Med. 2007;25:211-213.
144. Orlinsky M., Bright A.A. The utility of routine x-rays in all glass-caused wounds. Am J Emerg Med. 2006;24:233-236.
145. Rettenbacher T., Ennemoser S., Weirich H., et al. Diagnostic imaging of gout: Comparison of high-resolution US versus conventional X-ray. Eur Radiol. 2008;18:621-630.
146. Rennie L.M., Beattie T.F., Wilkinson A.G., et al. Incidental radiological diagnosis of rickets. Emerg Med J. 2005;22:534-537.
147. Renton P. Radiology of rickets, osteomalacia and hyperparathyroidism. Hosp Med. 1998;59:399-403.
148. Rowe P.M. Why is rickets resurgent in the USA? Lancet. 2001;357:1100.
149. Marcus S: Toxicity, lead. (Accessed at http://emedicine.medscape.com/article/815399-overview.)
150. Committee on Environmental Health. Lead exposure in children: Prevention, detection, and management. Pediatrics. 2005;116:1036-1046.
151. Bachmann L.M., Kolb E., Koller M.T., et al. Accuracy of Ottawa ankle rules to exclude fractures of the ankle and mid-foot: Systematic review. BMJ. 2003;326:417.
152. Stiell I.G., Wells G.A., Hoag R.H., et al. Implementation of the Ottawa knee rule for the use of radiography in acute knee injuries. JAMA. 1997;278:2075-2079.
153. Seaberg D.C., Yealy D.M., Lukens T., et al. Multicenter comparison of two clinical decision rules for the use of radiography in acute, high-risk knee injuries. Ann Emerg Med. 1998;32:8-13.
154. Tandberg D., Sherbring M. A mnemonic for the Salter-Harris classification. Am J Emerg Med. 1999;17:321.
155. Post M. Current concepts in the treatment of fractures of the clavicle. Clin Orthop Relat Res. 1989:89-101.