Oral unit dosage forms
Introduction
Tablets and capsules (oral unit dosage forms) are the most popular way of delivering a drug for oral use. They are convenient for the patient and are usually easy to handle and identify. They are produced by the pharmaceutical industry, where quality assurance ensures a high accuracy of dose within each individual dosage form. They are free from the problems of stability found in aqueous mixtures and suspensions. Packaging in blister packs can also enhance the stability of these dosage forms. The main disadvantages are that there is a slower onset of action relative to liquids and some people have difficulty swallowing solid oral dosage forms, e.g. the very young or very old.
Tablets
Tablets are solid preparations each containing a single dose of one or more active ingredient(s). They are normally prepared by compressing uniform volumes of particles, although some tablets are prepared by moulding.
Many different types of tablet are available, which may be in a variety of shapes and sizes. The types include dispersible, effervescent, chewable, sublingual and buccal tablets, lozenges, tablets for rectal or vaginal administration and solution tablets. Some tablets are designed to release the drug after a time lag, or slowly for a prolonged drug release or sustained drug action (see Ch. 29). The design of these modified-release tablets uses formulation techniques to control the biopharmaceutical behaviour of the drug. In addition to the drug(s), several excipients must be added. These will aid the process of tableting and ensure that the active ingredient will be released as intended. Excipients include:
 Diluents. These add bulk to make the tablet easier to handle. Examples include lactose, mannitol, sorbitol and calcium carbonate
Diluents. These add bulk to make the tablet easier to handle. Examples include lactose, mannitol, sorbitol and calcium carbonate
 Binders. These enable granules to be prepared which improves the flow properties of the mixture during manufacture. Examples include polyvinylpyrrolidone and microcrystalline cellulose
Binders. These enable granules to be prepared which improves the flow properties of the mixture during manufacture. Examples include polyvinylpyrrolidone and microcrystalline cellulose
 Disintegrants. These encourage the tablet to break into smaller particles after ingestion. Examples include modified cellulose and modified starch
Disintegrants. These encourage the tablet to break into smaller particles after ingestion. Examples include modified cellulose and modified starch
 Lubricants, glidants, antiadherents. These are essential for the flow of the tablet material into the tablet dies and preventing sticking of the compressed tablet in the punch and die. Examples of lubricants are magnesium and calcium stearate, sodium lauryl sulphate and sodium stearyl fumarate. Colloidal silica is usually the glidant of choice. Talc and magnesium stearate are effective antiadherents
Lubricants, glidants, antiadherents. These are essential for the flow of the tablet material into the tablet dies and preventing sticking of the compressed tablet in the punch and die. Examples of lubricants are magnesium and calcium stearate, sodium lauryl sulphate and sodium stearyl fumarate. Colloidal silica is usually the glidant of choice. Talc and magnesium stearate are effective antiadherents
 Miscellaneous agents may be added, such as colours and flavours in chewable tablets.
Miscellaneous agents may be added, such as colours and flavours in chewable tablets.
Some tablets have coatings, such as sugar coating or film coating. Coatings can protect the tablet from environmental damage, mask an unpleasant taste, aid identification of the tablet and enhance its appearance. Enteric (gastro-resistant) coatings on tablets resist dissolution or disruption of the tablet in the stomach, but not in the intestine. This is useful when a drug is destroyed by gastric acid, is irritating to the gastric mucosa, or when bypassing the stomach, aids drug absorption.
Dispensing of tablets
Many tablets in the UK and other countries are packaged by the manufacturer into patient packs suitable for issue to the patient without repacking by the pharmacist. Patient information leaflets are also contained in these patient packs. When dispensing these packs to patients, the pharmacist must ensure that they are labelled correctly, according to the prescriber’s instructions (see Ch. 31), and that the patient is counselled on the use of the medication (see Ch. 25).
For some controlled-release tablets, variations in bioavailability may occur with different brands. It is important that patients are given the brand that they are stabilized on in order to maintain therapeutic outcome. Examples where this is important include theophylline, lithium and phenytoin.
Tablets may also be supplied in a bulk container. The required number of tablets needs to be counted out (see Ch. 30) and placed in a suitable container for dispensing to the patient (see Ch. 32). It is important to minimize errors by ensuring that the correct bulk container has been selected and the correct drug dispensed. The pharmacist should verify this by checking the label of the bulk container and by examining the shape, size and markings on the dispensed tablets where appropriate, with the prescription. A copy of the patient information leaflet should be included.
Some tablets are supplied in a strip-packed form where each tablet has its own blister. A development of this is the calendar pack where the day or date on which the tablet is to be taken is indicated on the pack.
Shelf-life and storage of tablets
Most tablets should be stored in airtight packaging, protected from light and extremes of temperature. When stored properly, they generally have a long shelf-life. The expiry date will be printed on the package or the individual strip packs. Some tablets need to be stored in a cool place, e.g. Ketovite® and Leukeran® (chlorambucil) (both stored between 2 and 8°C). Some tablets contain volatile drugs, e.g. glyceryl trinitrate, and must be packed in glass containers with tightly fitting metal screw caps (see Ch. 32).
Containers for tablets
Strip or blister packs are dispensed in a paperboard box and tablets counted from bulk containers are placed in amber glass or plastic containers with airtight, child-resistant closures.
Special labels and advice on tablets
Most tablets should be swallowed with a glass, or ‘draught’, of water. A draught of water refers to a volume of water of about 50 mL. This prevents the dosage from becoming lodged in the oesophagus, which can cause problems such as ulceration. Tablets may be coated and shaped to aid swallowing.
Some tablets should be dissolved or dispersed in water before taking, e.g. effervescent tablets. Other tablets, particularly those with coatings or modified-release properties, should be swallowed whole. There are also some tablets which should be chewed or sucked before swallowing. Appropriate labels should be placed on the container (see Ch. 31).
Coated tablets, e.g. enteric (gastro-resistant) coatings, require specific advice on avoiding indigestion remedies at the same time of day, as these will affect the pH of the stomach, and therefore cause premature breakdown of the enteric coating on the tablet.
Buccal and sublingual tablets are not swallowed whole because they will not have their intended therapeutic effect. Figure 39.1 illustrates the positioning for buccal tablets. Sublingual tablets are placed under the tongue.
Capsules
Capsules are solid preparations intended for oral administration made with a hard or soft gelatin shell. One (or more) medicament is enclosed within this gelatin container. Most capsules are swallowed whole, but some contain granules which provide a useful premeasured dose for administering in a similar way to a powder, e.g. formulations of pancreatin. Some capsules enclose enteric-coated pellets, e.g. Erymax® (erythromycin). Capsules are elegant, easy to swallow and can be useful in masking unpleasant tastes. Capsules may also be used to hold oils for inhalation, e.g. Karvol® or for rectal and vaginal administration, e.g. Gyno-Daktarin® (miconazole nitrate) (see Ch. 37).
Soft shell capsules
A soft gelatin capsule consists of a flexible solid shell containing powders, non-aqueous liquids, solutions, emulsions, suspensions or pastes. Such capsules allow liquids to be given as solid dosage forms, e.g. cod-liver oil. They also offer accurate dosage, improved stability and overcome some of the problems of dealing with powders. They are formed, filled and sealed in one manufacturing process.
Hard shell capsules
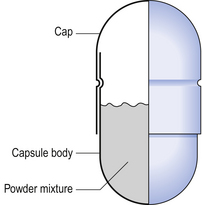
Empty capsule shells are made from gelatin and are clear, colourless and essentially tasteless. Colourings and markings can be easily added for light protection and to ease identification. The shells are used in the preparation of most manufactured capsules and for the extemporaneous compounding of capsules. The shell comprises two sections, the body and the cap, both being cylindrical and sealed at one end. Powder or particulate solid, such as granules and pellets, can be placed in the body and the capsule closed by bringing the body and cap together (Fig. 39.2). Some capsules have small indentations on the body and cap which ‘lock’ together.
Compounding of capsules
Occasionally hand filling of capsules may be required, particularly in a hospital pharmacy or when preparing materials for clinical trials. A suitable size of capsule shell should be selected so that the finished capsule looks reasonably full. Hard shell capsules are available in eight sizes. These are listed in Table 39.1, with the corresponding approximate capacity (based on lactose). The bulk density of a powder mixture will also affect the choice of capsule size.
Calculations for compounding capsules
The recommended minimum weight for filling a capsule is 100 mg. If the required weight of the drug is smaller than this, a diluent should be added by trituration (see Ch. 38). If the quantity of the drug for a batch of capsules is smaller than the minimum weighable amount, 100 mg on a Class B balance, then trituration will also be required. Lactose and starch are commonly used diluents. To allow for small losses of powder, an excess should be calculated, e.g. two extra capsules (see Ch. 38).
Filling capsules
The number of capsules to be filled should be counted and set to one side. This avoids the danger of contaminating empty capsules. The powder to be encapsulated should be finely sifted (180 μm sieve) and prepared. Magnesium stearate (up to 1% weight in weight (w/w)) and silica may be added as a lubricant and glidant respectively, to aid filling of the capsule. Various methods of filling capsules on a small scale are possible.
Filling from a powder mass
The prepared powder can be placed on a clean tile or piece of demy paper and powder pushed into the capsule body with the aid of a spatula until the required weight has been enclosed. The empty capsule body could also be ‘punched’ into a heap of powder until filled. Alternatively, create a small funnel from demy paper and fill the capsule body with the required weight. Gloves should be worn to protect the capsules from handling.
Filling with weighed aliquots
Weighed aliquots of powder may be placed on paper and channelled into the empty capsule shell. A sharp fold in the paper helps direct the powder. Alternatively, simple apparatus is available for small-scale manufacture of larger numbers of capsules. A plastic plate with rows of cavities to hold the empty capsule bodies is used, different rows holding different sizes of capsules. A plastic bridge containing a row of holes corresponding to the position of the capsule cavities can then be used to support a long-stemmed funnel. The end of the funnel passes into the mouth of the capsule below. The stem of the funnel should be as wide as possible for the size of the capsule to assist with powder flow. A weighed aliquot of powder can then be poured into the capsule via the funnel. A thin plastic rod or wire may be used to ‘tamp’ the powder to break blockages or to lightly compress the material inside the capsule. After filling the capsule, the cap can be fitted loosely and the weight checked before sealing.
Capsules are subject to tests for uniformity of weight and content of active ingredient, and uniformity of content where the content of active ingredient is<2 mg or<2% by weight of the total capsule fill.
Shelf-life and storage of capsules
If stability data are not available for extemporaneously filled capsules, then a short expiry date (up to 4 weeks) should be given. Manufactured capsules will be assigned expiry dates on the container and the packed strips or blister packs. Most capsules need to be stored in a cool, dry place.
Containers for capsules
The containers used for capsules are similar to those for tablets. Some capsules are susceptible to moisture absorption, and desiccants may be included in the packaging.
Special labels and advice on capsules
Capsules should be swallowed whole with a glass of water or other liquid. Advice may be sought from the pharmacist about whether it is acceptable to empty the contents of a capsule onto food or into water for ease of swallowing. In giving this advice, the release characteristics of the dosage form should be considered; for instance, whether it is an enteric-coated or prolonged-release formulation.
Other oral unit dosage forms
Pastilles
These contain a glycerol and gelatin base. They are sweetened, flavoured and medicated and are popular over-the-counter remedies for soothing coughs and sore throats.
Formulation notes. Magnesium stearate is added to act as a lubricant to aid flow of the powder into the capsule; 10 mg is not weighable, so a trituration must be carried out. Lactose acts as a diluent to bring the weight of each capsule fill to 100 mg.
Trituration for magnesium stearate:
| Magnesium stearate | 100 mg |
| Lactose | 900 mg |
Take a 100 mg portion of this mixture, which will contain 10 mg of magnesium stearate and 90 mg of lactose.
Method of preparation. Sieve the powders using a 180 μm sieve. Prepare the magnesium stearate triturate. Weigh 100 mg of haloperidol, and mix this with the magnesium stearate triturate in a mortar and pestle. Gradually add 800 mg of lactose to this mixture, by doubling-up. This gives a total powder quantity of 1000 mg (equivalent to 10 × 100 mg capsules). Fill the capsule shells (size 4 or 5) with 100 mg aliquots, checking the weight of each capsule before sealing. Pack eight capsules in an amber glass or plastic tablet container with a child-resistant closure.
Storage and shelf-life. Store in a cool, dry place and protect from light. Expiry date of 2 weeks, since stability in capsule form is unknown.
Key Points
 Tablets and capsules are the most common dosage forms
Tablets and capsules are the most common dosage forms
 Excipients are added to improve manufacture, handling and release of the drug
Excipients are added to improve manufacture, handling and release of the drug
 Tablets and capsules should be swallowed normally with about 50 mL of water
Tablets and capsules should be swallowed normally with about 50 mL of water
 Some tablets are designed to be chewed, dissolved, swallowed whole or delivered by the buccal or sublingual route
Some tablets are designed to be chewed, dissolved, swallowed whole or delivered by the buccal or sublingual route
 Tablets cannot be made extemporaneously, but capsules are filled when preparing for clinical trials
Tablets cannot be made extemporaneously, but capsules are filled when preparing for clinical trials
 Capsule size is selected so that they look reasonably full
Capsule size is selected so that they look reasonably full
 The minimum weight of contents in an extemporaneous capsule is 100 mg
The minimum weight of contents in an extemporaneous capsule is 100 mg
 Medicated glycerol-gelatin-based pastilles are popular for coughs and sore throats
Medicated glycerol-gelatin-based pastilles are popular for coughs and sore throats