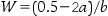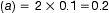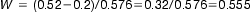Parenteral products
 Reasons for parenteral administration
Reasons for parenteral administration
 Routes available for parenteral administration
Routes available for parenteral administration
 Forms and types of parenteral product
Forms and types of parenteral product
 Design of containers for the administration of parenteral products
Design of containers for the administration of parenteral products
Introduction
In practice, parenteral products are often regarded as dosage forms that are implanted, injected or infused directly into vessels, tissues, tissue spaces or body compartments. Parenteral products are often used for drugs that cannot be given orally. This may be because of patient intolerance, the instability of the drug, or poor absorption of the drug if given by the oral route. From the site of administration the drug is transported to the site of action. With developing technology, parenteral therapy is being used outside the hospital or clinic environment: at a patient’s home or their workplace, allowing self-administration.
Parenteral therapy is used to:
 Administer drugs if the oral route cannot be used
Administer drugs if the oral route cannot be used
 Deliver drugs to the unconscious patient
Deliver drugs to the unconscious patient
Parenteral injections are either administered directly into blood for a fast and controlled effect or into tissues outside the blood vessels for a local or systemic effect. An intravenously administered (IV) injection will rapidly increase the concentration of drug in the blood plasma, but this concentration falls due to the reversible transfer of the drug from blood plasma into body tissues, a process known as distribution. An IV infusion administers a large volume of fluid at a slow rate and ensures that the drug enters the general circulation at a constant rate. A steady state is reached when the rate of drug addition equals the rate of drug loss in the blood plasma. When infusion is stopped, elimination of the drug from the body generally follows first-order kinetics.
Following subcutaneous (SC) and intramuscular (IM) injection, there is a delay in the systemic effects of the drug due to the time taken for the drug to first pass through the walls of the capillaries before entering into the blood. This occurs by passive diffusion that is promoted by the concentration gradient across the capillary wall. The drug concentration in the blood plasma rises to a peak level and then falls due to distribution to the tissues followed by metabolism and excretion.
Administration procedures
IV injections and infusions
The vein that is selected for administering the formulation depends on the size of the delivery needle or catheter, the type and volume of fluid to be administered and the rate at which the fluid is to be administered. The fluids are administered into a superficial vein, commonly on the back of the hand or in the internal flexure of the elbow. The intravenous route is widely used to administer parenteral products, but it must not be used to administer water-in-oil emulsions or suspensions.
SC injections
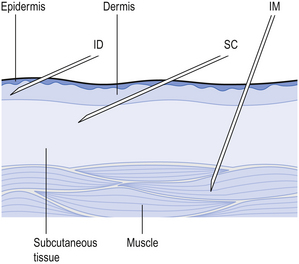
These are injected into the loose connective and adipose tissue immediately beneath the skin in the abdomen, the upper back, the upper arms and the lateral upper hips (Fig. 41.1). Typically, the volume injected does not exceed 1 mL. Following administration, the site of the injection, the body temperature, age of the patient and the degree of massaging of the injection site will all affect the drug distribution.
IM injections
Small-volume aqueous solutions, solutions in oil and suspensions are administered directly into the body of a relaxed muscle, such as the gluteal muscle in the buttock, the deltoid muscle in the shoulder and the vastus lateralis of the thigh (Fig. 41.1).
Other routes of parenteral administration include intradermal, intra-arterial, intracardiac, intraspinal and intra-articular.
Products for parenteral use
Parenteral products include injection, infusion and implantation.
Injections
These are subdivided into small- and large-volume parenteral fluids. Small-volume parenteral fluids are sterile, pyrogen-free injectable products. They are packaged in volumes up to 100 mL. Small-volume parenteral fluids are packed as:
Single-dose ampoules
Most small-volume parenteral fluids are currently packaged as either ampoules or vials. Glass ampoules are thin-walled containers made of Type I borosilicate glass (see Fig. 32.2). Injections packaged in glass ampoules are manufactured by filling the product into the ampoules, which are then heat sealed. To achieve the quality required of these products, the packaged solution must be sterile and practically free of particles. These products are prepared in clean room conditions (see Ch. 40). Opening glass ampoules may contaminate the product with glass particles; this is a hazard to the patient. Modern glass ampoules have weakened necks to reduce the number of particles.
Plastic ampoules are prepared, filled and sealed by a procedure known as blow-fill-seal in which the semi solid plastic is blow moulded and formed into ampoules. These containers are filled with the product and immediately sealed. This system is only used to package simple solutions due to absorption of the drug by the plastic. When the ampoule is opened only a few particles are released into the solution.
Ampoules should have a reliable seal that can be readily leak tested and will not deteriorate during the lifetime of the product. Ampoules do not contain added antimicrobial preservatives. The ampoule must contain a slight excess volume of product. This is necessary to allow the nominal injection volume to be drawn into a syringe.
Multiple-dose vials
These are composed of a thick-walled glass container that is sealed with a rubber closure. The closure is kept in position by an aluminium seal (see Fig. 32.4), then covered with a plastic cap. The cap is removed before a needle, attached to a syringe, is inserted through the rubber closure to withdraw a dose of product. The contents of the vial may be removed in several portions.
There are disadvantages with the use of glass vials. Fragments of the closure may be released into the product when the needle is inserted through the closure. There is also the risk of interaction between the product and the closure. Repeated withdrawal of injection solution from these containers increases the risk of microbial contamination of the product. These products must, therefore, contain an antimicrobial preservative unless the medicine itself has antimicrobial activity. An example of such a multidose product is insulin.
Prefilled syringes
With these devices, the injection solution is aseptically filled into sterile syringes. The packed solution has a high level of sterility assurance and does not contain an antimicrobial preservative. The final product is available for immediate use. Prefilled syringes are becoming increasingly common.
Administration of small-volume parenteral products
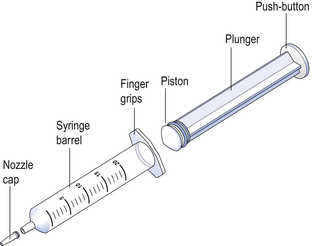
Hypodermic syringes and needles are extensively used for administering small volumes of parenteral formulations to the patient. These syringes have been sterilized by ethylene oxide gas or by gamma irradiation following packaging. Various sizes of hypodermic syringes are available. They are composed of a barrel, having a graduated scale, together with a plunger and a headpiece, known as a piston (Fig. 41.2). These components are often made of polypropylene.
Formulation of parenteral products
Vehicles for injections
The vehicle provides the highest proportion of the formulation and should not be toxic nor have any therapeutic activity.
Water for Injections
Water for Injections is the most extensively used vehicle in parenteral formulations, Water for Injections must be free of pyrogens and have a high level of chemical purity. The BP considers that Water for Injections can only be prepared by distillation.
Pyrogens
Pyrogens are fever-producing substances. Water is the greatest source of pyrogens in parenteral products. Pyrogens can be removed in the preparation of water for injections by distillation. Water that is free from pyrogens is termed apyrogenic.
Microbial pyrogens arise from components of Gram-negative and Gram-positive bacteria, fungi and viruses. Non-microbial pyrogens are for example some steroids and plasma components.
Parenteral products must be prepared in conditions that reduce microbial contamination because bacteria contaminating aqueous solutions can release pyrogens. Contaminated solutions will become more pyrogenic with the passage of time. Therefore, these products must be sterilized shortly after preparation.
Dry heat at 250°C for 30 min is the most common method of inactivating pyrogens.
Non-aqueous solvents
Water-miscible co-solvents, such as glycerin and propylene glycol, are used as vehicles in small-volume parenteral fluids. They are used to increase the solubility of drugs and to stabilize drugs degraded by hydrolysis.
Metabolizable oils are used to dissolve drugs that are insoluble in water. For example, steroids, hormones and vitamins are dissolved in vegetable oils. These formulations are administered by intramuscular injection.
Additives
Various additives, such as antimicrobial agents, antioxidants, buffers, chelating agents and tonicity-adjusting agents, are included in injection formulations. Their purpose is to produce a safe and elegant product. Both the types and amounts of additives to be included in formulations are given in the appropriate monograph in the BP.
Antimicrobial agents
Antimicrobial agents are added to inhibit the growth of microbial organisms that may accidentally contaminate the product during use, for example, multiple-dose vials. The antimicrobial agents must be stable and effective in the parenteral formulation. Rubber closures have been shown to take up antimicrobial preservatives from the injection solution. Preservative uptake is more significant with natural and neoprene rubber and much less significant with butyl rubber closures.
Antioxidants
Many drugs in aqueous solutions are easily degraded by oxidation. Small-volume parenteral products of these drugs often contain an antioxidant. Bisulfites and metabisulfites are commonly used antioxidants in aqueous injections. Antioxidants must be carefully selected for use in injections to avoid interaction with the drug. Injections may, in addition to antioxidants, also contain chelating agents such as EDTA or citric acid, which remove trace elements.
Buffers
The ideal pH of parenteral products is pH7.4. If the pH is above pH9, tissue necrosis may result, while below pH3, pain and phlebitis can occur.
Buffers are included in injections to maintain the pH of the packaged product. Changes in pH can arise through interaction between the product and the container. Acetate, citrate and phosphate buffers are commonly used in parenteral products.
Tonicity-adjusting agents
Isotonic solutions have the same osmotic pressure as blood plasma and do not damage the membrane of red blood cells. Hypotonic solutions have a lower osmotic pressure than blood plasma and cause blood cells to swell and burst because of fluids passing into the cells by osmosis. Hypertonic solutions have a higher osmotic pressure than plasma and as a result the red blood cells lose fluids and shrink. Thus, the BP states that aqueous solutions for large-volume infusion fluids, together with aqueous fluids for subcutaneous, intradermal and intramuscular administration, should be made isotonic. Intrathecal injections must also be isotonic to avoid serious changes in the osmotic pressure of the cerebrospinal fluid. Aqueous hypotonic solutions are made isotonic by adding either sodium chloride, glucose or, occasionally, mannitol. The latter two agents are incompatible with some drugs. If the solution is hypertonic, it is made isotonic by dilution.
Injection solutions are often made isotonic with 0.9% sodium chloride solution. The amount of solute, or the required dilution necessary to make a solution isotonic, can be determined from the freezing point depression. The freezing point depression of blood plasma and tears is−0.52°C. Thus, solutions that freeze at−0.52°C have the same osmotic pressure as body fluids. Hypotonic solutions have a smaller freezing point depression and require the addition of a solute to depress the freezing point to−0.52°C.
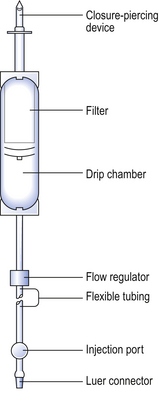
The amount of adjusting substance added to these solutions may be calculated from the equation:
where W=percentage concentration of adjusting substance in the final solution, a=freezing point depression of the unadjusted hypotonic solution, b=freezing point depression of a 1% weight in volume (w/v) concentration of the adjusting substance.
An extensive list of freezing point depression values is detailed in the Pharmaceutical Codex (1994: 53–64) (Example 41.1).
Other methods that are used to estimate the amount of adjusting substances required to make a solution isotonic include:
Details of these methods are given in the Pharmaceutical Codex (1994: 64–67).
Units of concentration
The concentration of the components in parenteral products may be expressed in various ways (see also Ch. 19):
 Percentage weight/volume. Examples include: magnesium sulfate injection 50%, sodium chloride intravenous infusion 0.9%
Percentage weight/volume. Examples include: magnesium sulfate injection 50%, sodium chloride intravenous infusion 0.9%
 Weight per unit volume. Examples include: atropine sulfate 600 μg/mL or ephedrine hydrochloride injection 30 mg/mL
Weight per unit volume. Examples include: atropine sulfate 600 μg/mL or ephedrine hydrochloride injection 30 mg/mL
 Millimoles per unit volume. Examples include: potassium chloride solution, strong (sterile) contains 2 mmol each of K+ and Cl− per mL; Calcium Chloride Injection BP contains 2.5 mmol of Ca2+ and 5 mmol of Cl− in 5 mL.
Millimoles per unit volume. Examples include: potassium chloride solution, strong (sterile) contains 2 mmol each of K+ and Cl− per mL; Calcium Chloride Injection BP contains 2.5 mmol of Ca2+ and 5 mmol of Cl− in 5 mL.
During the formulation of injections and infusions, the units of interest are the ions of electrolytes and the molecules of non-electrolytes. For molecules, 1 millimole (mmol) is the weight in milligrams corresponding to its relative molecular mass. A mole of an ion is its relative atomic mass weighed in grams. The number of moles of each of the ions of a salt in solution depends on the number of each ion in the molecule of the salt (Example 41.2).
Special injections
These are more complex formulations than solutions for injection.
Suspensions
Suspensions for injection contain<5% of drug solids with a mean particle diameter within the range 5–10 μm. Owing to the presence of particles in these formulations, these injections are more difficult to process and sterilize than solutions for injection. During the manufacture of suspensions for injection, the components are prepared and sterilized separately. They are then aseptically combined (see Ch. 29). The final product cannot be filter sterilized, owing to the presence of particles in the formulation. Powders for use in sterile suspensions can be sterilized by gas, but gas residues must be avoided.
Dried injections
With these products the dry sterile powder is aseptically added to a sterile vial. Alternatively, a sterile filtered solution can be freeze dried in a vial. The dry drug powder is reconstituted with a sterile vehicle before use.
Non-aqueous injections
Drugs that are insoluble in an aqueous vehicle can be formulated in solution using an oil as the vehicle. Several oils are used in these formulations, including arachis oil and sesame oil, which are easily metabolized. These viscous injections give a depot effect with slow release of the drug and are administered by intramuscular injection.
Large-volume parenteral products
These are formulated as single-dose injections that are administered by intravenous infusion. They are sterile aqueous solutions or emulsions with water for injections as the main component. It is important that they are free of particles. During the administration of these fluids, additional drugs are often added to the fluids (see Ch. 40). This may be carried out by the injection of small-volume parenteral products to the administration set of the fluid, or by the ‘piggyback’ method. In this procedure, a second, but smaller, volume infusion of an additional drug is added to the intravenous delivery system.
Large-volume parenteral products include:
 Infusion fluids to deliver drugs or restore fluid or electrolyte imbalance
Infusion fluids to deliver drugs or restore fluid or electrolyte imbalance
All of these products have direct contact with blood or are introduced into a body cavity.
Large-volume parenteral fluids must be terminally heat sterilized. While water for injections is the main component of these products, they also incorporate other ingredients including:
 Carbohydrates, e.g. dextrose, sucrose and dextran
Carbohydrates, e.g. dextrose, sucrose and dextran
 Lipid emulsions which contain vegetable or semisynthetic oil
Lipid emulsions which contain vegetable or semisynthetic oil
Most large-volume parenteral fluids are clear aqueous solutions, except for the oil-in-water emulsions. The production of emulsions for infusion is highly specialized as they are destabilized by heat.
Production of large-volume parenteral products
The fluids are produced and filled into containers in a high-standard clean room environment (see Ch. 46). The use of stringent quality assurance procedures is essential to ensure the quality of the products.
In commercial manufacturing facilities, the fluids are packaged from a bulk container into the product container using high-speed filling machines. Just before the fluid enters the container, particulate matter is removed from the fluid by passing it through an in-line membrane filter. Immediately after filling, the neck of each glass bottle is sealed with a tight-fitting rubber closure that is kept in place with a crimped aluminium cap. The outer cap is also aluminium and an outer tamper-evident closure is used.
When using plastic bags, the preformed plastic bag is aseptically filled and immediately heat sealed. As an alternative, a blow-fill-seal system can be used. Blow-fill-seal production decreases the problems with product handling, cleaning and particulate contamination. Following filling of the product into containers, the fluids are examined for particulate matter and the integrity of container closures established.
Moist heat is used to sterilize parenteral products, irrigation solutions and dialysis fluids as soon as possible after the containers have been filled. Plastic containers must be sterilized with an over-pressure during the sterilization cycle to avoid the containers bursting.
Containers and closures
Large-volume parenteral fluids are packaged into:
The containers and closures that are used for packaging parenteral products must:
Glass bottles are rarely used these days, but may be used for products that are incompatible with plastic containers. If used they require the use of an air inlet filter device for pressure equilibration within the container. Particles of glass can be released into the injection fluids. Damage to the neck of the bottles may result in contamination of the container contents from the external environment. Owing to these difficulties with glass containers, plastic containers have become widely used.
PVC collapsible bags are used to package most infusion fluids. They are designed with a port for the attachment of the administration set and an additive port for the addition of small-volume parenteral fluids.
The disadvantages of plastic bags are that:
 They permit a high moisture penetration
They permit a high moisture penetration
 They require an extended sterilization time due to the heat resistance of the PVC
They require an extended sterilization time due to the heat resistance of the PVC
 Moist heat sterilization requires air ballasting to avoid pouch explosion.
Moist heat sterilization requires air ballasting to avoid pouch explosion.
Semi-rigid plastic containers are used for volumes of 100 mL for electrolyte solutions, 3 L for TPN solutions and up to 5 L for dialysis solutions.
Semi-rigid bags are designed with two ports. One port allows the attachment of the administration set. The other port permits the addition of small-volume parenteral products or small-volume infusion fluids. They have a graduated scale that can be read either in an inverted or upright position (Fig. 41.3). To enable containers of large-volume parenteral fluids to be suspended from a drip stand for administration, bags are made with an eyelet opening that suspends the bag.
Administration of large-volume parenteral fluids
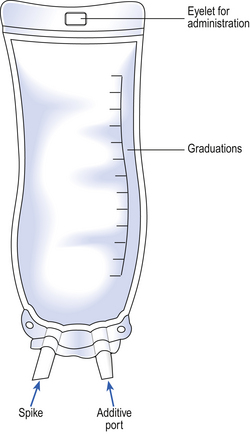
Most large-volume parenteral fluids are administered to the patient by a parenteral route using the standard infusion set specified in British Standard 2463 (Part 2, 1989). These sets are packaged as sterile units intended for single use (Fig. 41.4). Fluid moves through them by gravity.
Labelling
Batch-produced products have identical labels attached to both the product and the outer packaging carton that is used for transport. With flexible plastic containers, the labelling requirements are commonly printed directly on to the container prior to filling. With bags containing TPN fluids, a label is placed on the bag itself and an identical label is attached to the outer plastic cover on the bag. Labels are attached to infusion fluid containers. The labels on parenteral fluids should include the following details:
 Product identity and details of the contained volume
Product identity and details of the contained volume
 Solution strength in terms of the amount of active ingredient in a suitable dose-volume
Solution strength in terms of the amount of active ingredient in a suitable dose-volume
 Batch number and product expiry date
Batch number and product expiry date
 For TPN solutions, the name of the patient, the unit number, ward and infusion rate.
For TPN solutions, the name of the patient, the unit number, ward and infusion rate.
Containers often carry a warning label to discard the remaining product when treatment is completed.
Aseptic dispensing
Most parenteral fluids are terminally moist heat sterilized. However, some products are aseptically compounded from sterile ingredients in the hospital pharmacy. These products are prepared and dispensed for individual patients. Examples of aseptically prepared products are TPN fluids (see Ch. 44) and the aseptic reconstitution of freeze-dried formulations. These freeze-dried products are often reconstituted using either water for injections or 0.9% sodium chloride injection. Aseptic dispensing is performed in a Grade A clean room environment or a Grade A isolator chamber (see Ch. 40). The dispensing of these products relies on good aseptic procedures to ensure the sterility of the product. Owing to the absence of terminal sterilization, it is important that manufacture is performed using rigorous quality assurance procedures. Aseptically dispensed products are given a very limited expiry time.
Admixtures
These are prepared by adding at least one sterile injection to an intravenous infusion fluid for administration. The injections to be added are packed in an ampoule or vial, or may be reconstituted from a solid. These additions should be carried out using aseptic procedures in a Grade A environment within an isolator cabinet or clean room facility. This environment is required to maintain the sterility of the product and avoid contamination of the product with particulate matter, microorganisms and pyrogens. Following the additions, a sealing cap may be placed over the additive port of the infusion bag to prevent further, potentially incompatible, additions at ward level. Hospital pharmacies often have a centralized intravenous additive service (CIVAS), as detailed in Chapter 46. These facilities ensure that additions to infusion fluids are carried out in a suitable environment.
Infusion devices
There are situations that require strict control of the volume of fluids that are infused into a patient. Accurate flow control with infusion devices is vital for patient safety and for optimum efficacy of the infusion. A range of delivery systems are available that regulate the volume of fluid administered and are used both in the hospital and for the self-administration of fluids by patients at home.
The selection of an infusion device for the self-administration of medicines by patients requires careful consideration of several factors including:
Irrigation solutions
These solutions are applied topically to bathe open wounds and body cavities. They are sterile solutions for single use only. Examples of irrigation fluids are 0.9% w/v sodium chloride solution or sterile water for irrigation. Most irrigation fluids are now available in rigid plastic bottles. Urological irrigation solutions are used for surgical procedures. They are usually sterile water or sterile glycine solutions and are used to remove blood and maintain tissue integrity during an operation.
Water for irrigation is sterilized distilled water that is free of pyrogens. The water is packed in containers and is intended for use on one occasion only. The containers are sealed and sterilized by moist heat.
Peritoneal dialysis fluids
Peritoneal dialysis involves the administration of dialysis solutions directly into the peritoneum by way of an indwelling catheter. The fluid is then drained after a ‘dwell-time’ to remove toxic waste products from the body. Peritoneal dialysis solutions are sterile solutions manufactured to the same standards as parenteral fluids. The composition of peritoneal dialysis fluid simulates potassium-free extracellular fluid. These fluids are packaged in volumes of 3–5 L in plastic containers that are similar to the bags used for TPN (see Ch. 44).
Haemodialysis
In this dialysis procedure, blood is removed and returned to the patient by way of a catheter, or a double needle arrangement, using a fistula where an artery and vein are joined together. The dialysis procedure involves the use of an artificial disposable membrane within a ‘dialyser’ machine that acts as an artificial kidney. An electrolyte fluid, simulating body fluid, bathes one side of the membrane, with blood from the patient on the other side. There is no direct contact between the blood and the dialyser fluid. Thus fluids for haemodialysis are not required to be sterile or free of pyrogens or particulate matter.
Blood products
These products are not usually identified as sterile products although they are commonly packaged as sterile large-volume parenteral fluids. These biological products include albumin, human plasma and blood protein fractions. All these products must be treated to inactivate virus contamination prior to packaging. This is usually achieved by specialized heat treatment or filtration. These products are unstable to heat sterilization. Therefore, they are filter sterilized and then aseptically filled into containers in large-scale production facilities. Most of these products are packed as liquids, although a few blood protein fractions such as factor VIII and factor IX are freeze dried. The collection, management and distribution of these products are carried out by the blood transfusion service.
Key Points
 Convention uses the term ‘parenteral’ for dosage forms which are placed directly into the body
Convention uses the term ‘parenteral’ for dosage forms which are placed directly into the body
 The three main routes are IV, IM and SC
The three main routes are IV, IM and SC
 Parenteral products are sterile forms used for injection, infusion or implantation
Parenteral products are sterile forms used for injection, infusion or implantation
 Small volumes are packed in glass or plastic ampoules
Small volumes are packed in glass or plastic ampoules
 Multiple-dose injections must have an antimicrobial preservative
Multiple-dose injections must have an antimicrobial preservative
 Water for injections must be used as the aqueous ingredient in all injections
Water for injections must be used as the aqueous ingredient in all injections
 Water for irrigations is used in large volumes to irrigate body cavities and other areas
Water for irrigations is used in large volumes to irrigate body cavities and other areas
 Pyrogens cause fever and must be eliminated from water for injections and water for irrigations
Pyrogens cause fever and must be eliminated from water for injections and water for irrigations
 Additives to injections include antimicrobial preservatives, antioxidants, buffers, tonicity adjusters and co-solvents
Additives to injections include antimicrobial preservatives, antioxidants, buffers, tonicity adjusters and co-solvents
 Injection solutions for SC, intradermal, IM, intrathecal and large-volume IV use should be made isotonic
Injection solutions for SC, intradermal, IM, intrathecal and large-volume IV use should be made isotonic
 Large-volume parenteral products, include infusion fluids, TPN, dialysis fluids and irrigation solutions
Large-volume parenteral products, include infusion fluids, TPN, dialysis fluids and irrigation solutions
 All large-volume parenteral products must be sterilized after filling into their final containers
All large-volume parenteral products must be sterilized after filling into their final containers
 Large-volume parenteral products may be packaged in glass bottles, semi-rigid or collapsible plastic containers
Large-volume parenteral products may be packaged in glass bottles, semi-rigid or collapsible plastic containers
 When aseptic dispensing is required, rigorous quality assurance is essential and a short expiry date is given to the product
When aseptic dispensing is required, rigorous quality assurance is essential and a short expiry date is given to the product