Neurobehavioral tests have been developed to detect subtle changes in organized behavior in the newborn. These tests include the Brazelton Neonatal Behavioral Assessment Scale (NBAS), the Early Neonatal Neurobehavioral Scale (ENNS), and the Neurologic and Adaptive Capacity Score (NACS). All the tests are subjective and complex and lack specificity.
Other perinatal factors appear to have a more important effect on neonatal test performance than the choice of local anesthetic.208 Indeed, neurobehavioral tests have been shown not to be a reliable measure of drug effect in the newborn.209
Preterm Fetus and Newborn
It has become axiomatic that the preterm infant is more vulnerable than the term infant to the effects of analgesic and anesthetic drugs. Causes of enhanced drug sensitivity in the preterm newborn that have been postulated are as follows: (1) less protein is available for drug binding, (2) higher levels of bilirubin are present and may compete with the drug for protein binding, (3) greater access of the drug to the CNS occurs because of a poorly developed blood-brain barrier, (4) the preterm infant has greater total body water and less fat content, and (5) the preterm infant has a diminished ability to metabolize and excrete drugs. Unfortunately, few systematic studies have determined the maternal and fetal pharmacokinetics and pharmacodynamics of drugs throughout gestation; nevertheless, these deficiencies of the preterm infant may not be as serious as we have been led to believe. Although the plasma albumin and AAG concentrations are lower in the preterm fetus, these factors primarily affect drugs that are highly bound to these proteins. Most local anesthetics, however, exhibit only low to moderate degrees of binding in fetal plasma.90,91
The placenta efficiently eliminates fetal bilirubin. Thus, the hyperbilirubinemia of prematurity normally occurs in the postpartum period. Bupivacaine has been implicated as a possible cause of neonatal jaundice.210,211 High affinity of the drug for fetal erythrocyte membranes may lead to a decrease in filterability and deformability, which may render red blood cells more prone to hemolysis.211 However, increased bilirubin production has not been demonstrated in newborns whose mothers received bupivacaine for epidural anesthesia during labor and cesarean delivery.212,213
Greater total body water in the preterm fetus results in a larger volume of distribution for drugs. Thus, to achieve equal blood concentrations, the immature fetus must receive a greater amount of drug transplacentally than the mature fetus.
The diminished ability to metabolize or excrete drugs associated with prematurity is certainly not a universal phenomenon. One study of the pharmacokinetics of lidocaine in preterm newborns noted that plasma clearance was similar to that in adults.196
During anesthesia for preterm labor, concerns about drug effects on the newborn are far less important than the prevention of asphyxia and trauma to the fetus. Indeed, healthy preterm fetal lambs tolerated clinically relevant plasma concentrations of lidocaine (e.g., approximately 1.5 µg/mL) as well as mature ones.19,214
Asphyxia
Circulatory adaptations important for fetal survival during asphyxia result in increased blood flow and oxygen delivery to vital organs (e.g., heart, brain, adrenal glands).194 Little information exists about the effects of local anesthetics on these fetal responses. Adaptation to asphyxia was unaffected in mature fetal lambs exposed to lidocaine.194 In contrast, lidocaine adversely affected asphyxiated preterm fetal lambs, which experienced a further deterioration of acid-base status and a reduction in cardiac output and blood flow to the brain and heart (Figure 13-6).215 Also in asphyxiated preterm fetal lambs, exposure to bupivacaine reduced blood flow to vital organs; however, FHR, blood pressure, and acid-base measurements did not change.216
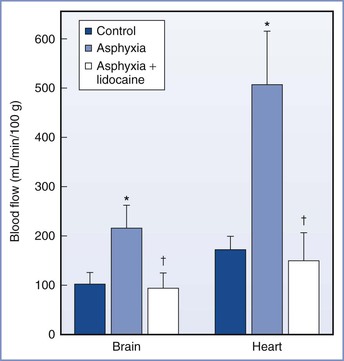
FIGURE 13-6 Blood flow to the brain and heart in the preterm fetal lamb before and during asphyxia and during exposure to lidocaine while asphyxiated (mean ± SEM). *Significantly different from control. †Significantly different from asphyxia. (Modified from Morishima HO, Pedersen H, Santos AC, et al. Adverse effects of maternally administered lidocaine on the asphyxiated preterm fetal lamb. Anesthesiology 1989; 71:110-5.)
After performing an in vitro study using perfused human placentas, Johnson et al.217 suggested that bupivacaine might be preferable to lidocaine in the presence of fetal acidosis because the greater maternal protein binding of bupivacaine may limit its placental transfer. However, this methodology does not consider the potential for greater fetal tissue uptake of bupivacaine (than of lidocaine) because bupivacaine is more lipid soluble and more protein bound than lidocaine.
In 1997, Santos et al.216 reported that the effects of bupivacaine appeared less severe than those of lidocaine in asphyxiated preterm fetal lambs. However, the lidocaine data were generated in a separate experiment reported in 1989.215 There are inherent limitations in a historical comparison of two studies performed 8 years apart. Further, it is unclear whether these findings are applicable to humans because both lidocaine and bupivacaine have enjoyed a long history of safe use in obstetric anesthesia practice; prospective clinical studies are required before one drug can be recommended over the other in the setting of fetal asphyxia.
Opioids
Neuraxial opioid administration is unique in that it produces analgesia without loss of sensation or proprioception. Opioids are often co-administered with local anesthetic agents during intrapartum administration of neuraxial analgesia and anesthesia.
The term opioid refers to a series of compounds that are related to opium. These compounds may be classified as follows: (1) naturally occurring (e.g., morphine), (2) semisynthetic compounds (e.g., dihydromorphone), and (3) synthetic compounds (e.g., fentanyl) (Box 13-4). The only three naturally occurring opioids of clinical significance are morphine, codeine, and papaverine. These substances can be obtained from the poppy plant known botanically as Papaver somniferum. Development of synthetic drugs with morphine-like properties has led to development of the broad term opioid. These substances bind to several subpopulations of opioid receptors with resulting morphine-like effects. More than 30 years ago, identification of a dense concentration of opioid receptors in the dorsal horn of the spinal cord led to the use of neuraxial opioids as important adjuncts in obstetric anesthesia.
Molecular Structure
Naturally occurring opioids of significance can be divided into two distinct chemical classes, phenanthrenes (e.g., morphine) and benzylisoquinolines (e.g., papaverine) (Figure 13-7). The phenanthrenes are five-ring structures, and the benzylisoquinolines are three-ring structures. The semisynthetic opioids are morphine derivatives that have undergone relatively simple modification of the morphine molecule. For example, substitution of an ester for the hydroxyl group on carbon 6 of morphine results in hydromorphone (Figure 13-8). Synthetic opioids can be classified into the following four groups: (1) morphinan derivatives (e.g., levorphanol), (2) diphenyl or methadone derivatives (e.g., methadone, D-propoxyphene), (3) benzomorphan derivatives (phenazocine, pentazocine), and (4) phenylpiperidines (e.g., meperidine, fentanyl, sufentanil).
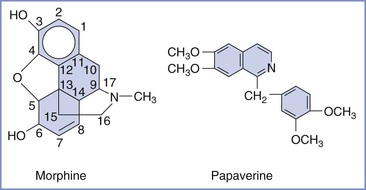
FIGURE 13-7 Naturally occurring opioids: phenanthrenes (e.g., morphine) and benzylisoquinolines (e.g., papaverine).
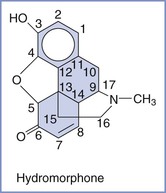
FIGURE 13-8 Semisynthetic opioids are morphine derivatives. For example, substitution of an ester for the hydroxyl group on carbon 6 of morphine results in hydromorphone.
Structurally, opioids are complex three-dimensional compounds that often exist as two optical isomers (e.g., morphine).218 Usually the levorotary isomer is the only isomer capable of producing analgesia. Analgesic activity of the opioid compound depends on its stereochemical structure.219 Even relatively minor molecular alterations (e.g., extent of ionization) can produce significant alterations in the pharmacologic activity of the opioid.
Morphine is the prototypical opioid. It is a five-ring structure that conforms to a T shape.220 Three of the rings lie in one plane, and the other two rings are perpendicular to the plane. This forms the basis for the T (Figure 13-9). Morphine demonstrates several other characteristics that are common to other opioids: (1) a tertiary, positively charged basic nitrogen; (2) a quaternary carbon that is separated from the basic nitrogen by an ethane chain and attached to a phenyl group; (3) a phenolic hydroxyl group (morphine derivatives) or a ketone group (meperidine); and (4) the presence of an aromatic ring.220
A phenylpiperidine structure (i.e., an aromatic ring attached to a six-member ring containing five carbons and one nitrogen) is also part of the morphine molecule and is present in some other opioids (e.g., fentanyl) (Figure 13-10).220 Phenylalanine and tyrosine moieties are structural elements that are important to all opioids, including endogenous neurotransmitters and modulators.221,222 The poppy plant synthesizes morphine from two tyrosine molecules; many opioids contain a structure that is similar to alanine.220
Mechanism of Action
Since first described in 1979,223 neuraxial opioid administration has become a mainstay in obstetric anesthesia practice. Clinical and laboratory research has focused on the mechanisms of synaptic transmission as well as the study of opioids and neurotransmitters that modulate this transmission.
Pain perception involves a complex series of nociceptive transmissions that begin with stimulation of sensory nerves in the periphery, resulting in generation of action potentials within the spinal cord and synaptic transmission to other supraspinal sites. Intraspinal administration of an opioid exploits the pharmacology of pain-modulating and pain-relieving systems that exist within the spinal cord (see Figure 20-9). In early studies, Yaksh224 demonstrated that morphine could produce selective suppression of nociceptive processing without affecting motor function, sympathetic tone, or proprioception when it was administered to the superficial layers of the dorsal horn of the spinal cord. However, when small amounts of opioid were administered to the cortex, the effects on nociceptive processing were negligible. Collectively, this work demonstrated that small doses of opioid can be selectively administered to a receptor site (i.e., spinal cord) and produce profound analgesia. In contrast, systemic administration of a much larger dose of opioid results in activation of multiple central and peripheral receptors to produce analgesia, but with unwanted side effects.
All opioids produce analgesia by binding to G protein–coupled opioid receptors. Activation of opioid receptors subsequently inhibits both adenylate cyclase– and voltage-gated calcium channels. Inhibition of these calcium channels inhibits the release of excitatory afferent neurotransmitters, including glutamate, substance P, and other tachykinins.225,226 The result is inhibition of ascending nociceptive stimuli from the dorsal horn of the spinal cord.
Opioid receptors are nonuniformly distributed throughout the CNS. Although parenterally administered opioids most likely have both direct spinal and supraspinal effects, neuraxially administered opioids block the transmission of pain-related information by binding at presynaptic and postsynaptic receptor sites in the dorsal horn of the spinal cord (i.e., Rexed laminae I, II, V) (Figure 13-11). However, the rate and extent of neuraxial analgesia depends largely on the specific drug's physicochemical properties and ability to reach the opioid receptors in the spinal cord.
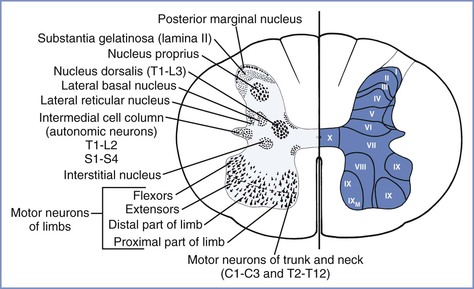
FIGURE 13-11 Architecture of the spinal cord, showing the gray matter nuclei (left) and Rexed laminae (right). (From Ross BK, Hughes SC. Epidural and spinal narcotic analgesia. Clin Obstet Gynecol 1987; 30:552-65.)
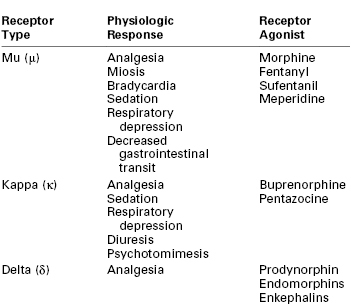
The following three broad classes of opioid receptors have been identified: (1) mu (μ) receptor for morphine type, (2) kappa (κ) receptor for ketocyclazocine type, and (3) delta (δ) receptor.225 A fourth receptor, the opioid receptor–like-1 (ORL-1) receptor, has structural homology to the classic opioid receptors, but its endogenous ligand, orphanin (OFQ) (also called nociceptin [N]), binds poorly to the classic opioid receptors.227 Its role in pain modulation is not well characterized but appears distinctive from that of the classic opioid system.225 Each opioid receptor is encoded by a different gene and mediates different physiologic effects (Table 13-3). Although all of these receptors may be involved with pain processing, the μ or κ receptors have the most important clinical pharmacologic effects.
The distinct receptor subtypes have significance in neuraxial opioid administration and drug development. Common pharmacologic effects (e.g., analgesia, respiratory depression) of morphine are mediated by μ-opioid receptors. Functional subclasses of μ-opioid receptors have been characterized; however, only one gene has been identified for the μ-opioid receptor. Some specific functions have been ascribed to μ-opioid receptor subtypes, including mediation of respiratory depression and spinal opioid analgesia by μ2 receptors and production of supraspinal analgesia by μ1 receptors.228 Although subtype-specific μ agonists may have greater efficacy and less toxicity, no receptor-specific agents have been developed for clinical use.
Morphine also has effects at κ- and δ-opioid receptors when higher doses are administered. Responsible for analgesic, sedative, dysphoric, and diuretic effects,225 κ receptors are located both within the CNS and peripherally.229 Peripheral κ-opioid receptor agonists have been shown to modulate visceral pain, particularly in conditions that involve inflammation.229
The δ receptor is responsible for mediating some of the analgesic effects of the endogenous opioids (e.g., enkephalins, prodynorphan, pro-opiomelanocortin, pro-orphanin, endomorphins) in the spinal cord.230 Few of the opioids have effects at the δ receptor in clinically relevant doses, but if a μ agonist is administered in a high enough dose to treat an opioid-tolerant patient, the drug may be less selective and produce δ effects.
Pharmacokinetics and Pharmacodynamics
Many of the pharmacologic differences observed among neuraxially administered opioids depend on an opioid's ability to reach opioid receptors. An opioid's physicochemical properties, especially lipophilicity or hydrophilicity, largely determine the bioavailability of neuraxially administered opioids as well as the drug's ability to produce spinally mediated analgesia.
Before G protein–receptor activation can occur, the opioid must undergo a series of complex processes. Although several mechanisms have been proposed to explain the movement of opioids from the epidural space to the spinal cord, studies demonstrate that the only relevant mechanism is diffusion through the spinal meninges.231-233 The opioid must traverse the dura and arachnoid membranes, diffuse through the CSF, and cross the pia membrane to reach the spinal cord (Figure 13-12). Once the drug reaches the surface of the spinal cord, it must diffuse through the white matter and then the gray matter to reach the site of action, the dorsal horn.234 The rate and extent of opioid transfer to receptors largely depend on a drug's physicochemical properties, particularly lipid solubility, because competing processes (e.g., uptake into the epidural fat or systemic circulation) limit the agent's diffusion to opioid receptors. Greater lipid solubility of a drug results in more rapid onset of analgesia. For example, fentanyl is a highly lipid-soluble opioid (i.e., 600 times more lipid soluble than morphine); therefore, it has a more rapid onset of action than morphine (Table 13-4).
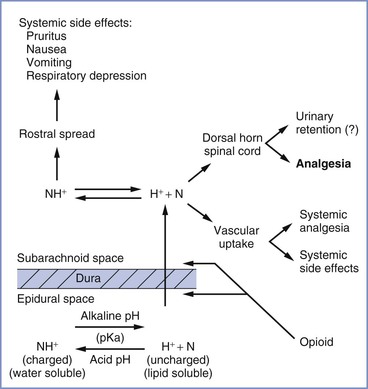
FIGURE 13-12 Epidural opioids traverse the dura and arachnoid membranes, diffuse through cerebrospinal fluid, and cross the pia membrane before reaching the spinal cord. Several factors, including physicochemical properties (e.g., pKa), affect the distribution of opioids within the neuraxis. (From Ross BK, Hughes SC. Epidural and spinal narcotic analgesia. Clin Obstet Gynecol 1987; 30:552-65.)
TABLE 13-4
Physicochemical Properties of Opioids Used for Neuraxial Analgesia
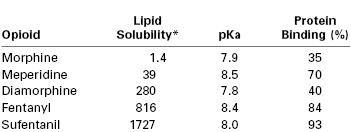
* Octanol-water partition coefficient.
Data from Camu F, Vanlersberghe C. Pharmacology of systemic analgesics. Best Pract Res Clin Anaesthesiol 2002; 16:475-88; and McLeod GA, Munishankar B, Columb MO. Is the clinical efficacy of epidural diamorphine concentration-dependent when used as analgesia for labour? Br J Anaesth 2005; 94:229-33.
Latency, potency, and duration are also affected by other physicochemical properties, including molecular weight, pKa, and protein binding. For example, the lower the pKa, the greater the percentage of opioid existing in uncharged form (i.e., the anionic base) at a pH of 7.4. In the uncharged form, opioids penetrate the dura mater and dorsal horn more easily, resulting in a more rapid onset of analgesia.
The boundaries of the epidural space are the vertebral bodies, ligaments, and spinal meninges. Fat and the epidural venous plexus account for a large volume of the epidural space. The spinal meninges consist of the dura, arachnoid, and pia mater. Of these membranes, the arachnoid is the primary barrier for drug transfer from the epidural space to the spinal cord.235 The arachnoid mater has multiple layers of overlapping cells that represent both a hydrophilic domain (consisting of extracellular and intracellular fluid) and a hydrophobic domain (the cell membranes).232 For an opioid to navigate the arachnoid, it must diffuse through both domains before entering the CSF. Therefore, drugs of intermediate hydrophobicity move most readily across the arachnoid. Other physical characteristics of drugs (e.g., molecular weight) do not appear to play an important role in determining redistribution from the epidural space to the subarachnoid space.232
The efficacy of a drug also depends on its physicochemical properties, particularly lipid solubility. For example, the amount of drug that is sequestered in the epidural fat is entirely dependent on the drug's octanol-to-buffer distribution coefficient.236 Consequently, lipophilic drugs (e.g., fentanyl) with a high octanol-to-buffer coefficient may never reach the arachnoid membrane and may partition in epidural fat. This lack of drug transfer across the meninges results in poor CSF bioavailability. To evaluate movement of opioids from the epidural to the subarachnoid space, Bernards et al.236 used a porcine model to continuously sample opioid concentrations in the epidural and intrathecal spaces. Using microdialysis techniques, the investigators measured the redistribution of morphine, alfentanil, fentanyl, and sufentanil out of the epidural space. (These opioids were administered by epidural bolus injection.) Opioid concentrations were measured over time in the epidural space, subarachnoid space, systemic venous plasma, and epidural venous plasma. Results suggested that there was a strong linear relationship between lipid solubility and mean residence time, indicating that more lipid-soluble opioids spent a longer time in the epidural space. Consequently, these drugs partition themselves into the epidural fat with ongoing slow release back into the epidural space. Because of their long residence time in the epidural space, more lipid-soluble drugs are found in lower concentrations in the CSF (i.e., decreased bioavailability to opioid receptors in the dorsal horn).
Several human studies have evaluated whether epidurally administered fentanyl produces analgesia by a selective spinal mechanism or by systemic absorption and redistribution. Results of studies of lipophilic opioids (administered by epidural infusion) have suggested that low concentrations of lipophilic opioids are subject to rapid vascular uptake from the epidural space or sequestration in epidural fat, thereby limiting access to the spinal cord.237-239 However, other studies have suggested the occurrence of a spinal effect when lipophilic opioids are administered by epidural bolus injection240 or by epidural infusion of short duration.241 Ginosar et al.242 compared the analgesic effects of epidural bolus injection and epidural infusion of fentanyl in human volunteers. Study results suggested that epidural fentanyl infusion produced analgesia by uptake into the systemic circulation with redistribution to brain and peripheral opioid receptors. However, epidural bolus administration of fentanyl produced analgesia by selective spinal mechanisms. These results were consistent with previous reports that an epidural fentanyl bolus results in a larger amount of fentanyl in the epidural space than occurs at any time during an epidural infusion, leading to the greater availability of drug to activate opioid receptors in the dorsal horn of the spinal cord.
Although hydrophilic drugs (e.g., morphine) are subject to less systemic and epidural fat uptake than lipophilic drugs, the transfer of the former into the CSF is an inefficient process because they have difficulty in crossing the lipid bilayer of the arachnoid. However, despite these inefficiencies, morphine content in the spinal cord is significantly greater than lipophilic drug (e.g., fentanyl) content,243 and morphine has much greater bioavailability in the spinal cord than do fentanyl and sufentanil.236,243 In summary, although morphine clearly produces analgesia via a spinal mechanism, the extent of spinal analgesia produced by the neuraxial administration of fentanyl is less clear.
After a drug reaches the subarachnoid space, either by diffusion across the meninges or by direct injection into the CSF, its effects depend on its lipid solubility. All opioids produce at least some analgesia by spinal-specific mechanisms. Movement of these drugs within the CSF depends on their physicochemical properties. Drugs can diffuse within the CSF in either a cephalad or a caudad direction. Both morphine and fentanyl have been shown to move rapidly within the CSF.244 Lipophilic drugs can also return to the epidural space by traversing previously mentioned structures.
Ummenhofer et al.243 used a porcine model to investigate intrathecal administration of opioids. These investigators found that lipophilic opioids have a very large volume of distribution compared with hydrophilic drugs; the volume of distribution of sufentanil was 40 times greater than that of morphine. The reason is sufentanil's extreme lipid solubility, with the drug rapidly leaving the CSF and entering the epidural fat, from which it is absorbed systemically.245
The ultimate goal of neuraxial opioid administration is for the drug to penetrate the dorsal horn of the spinal cord and activate μ-opioid receptors. A drug's ability to move from the CSF to the dorsal horn depends on its physicochemical properties. Of the clinically relevant opioids, morphine has the most favorable physicochemical properties to allow penetration of the dorsal horn of the spinal cord (i.e., gray matter). Because of its extreme lipid solubility, sufentanil redistributes itself or partitions itself on the superficial layer (i.e., white matter) of the spinal cord.243 Data suggest that the spinal bioavailability of the hydrophilic drugs (e.g., morphine, hydromorphone) is greater than that of hydrophobic opioids (e.g., fentanyl, sufentanil).
An extended-release formulation of morphine has been developed to prolong the duration of a single epidural injection of morphine or obviate the need for a continuous catheter. Multivesicular liposomal preparations gradually release morphine so that a larger epidural dose can be administered, providing analgesia for up to 48 hours (see Chapter 28). Studies that have compared extended-release epidural morphine (EREM) 10 to 15 mg with conventional epidural morphine for provision of analgesia after cesarean delivery have determined that EREM provided superior and prolonged analgesia.246,247 The most recent American Society of Anesthesiologists Practice Guidelines for the Prevention, Detection, and Management of Respiratory Depression Associated with Neuraxial Opioid Administration state that “the literature reports no significant difference in the frequency of respiratory depression when [EREM] is compared with conventional (i.e., immediate release) epidural morphine.”248 However, in a study of patients undergoing cesarean delivery, Atkinson Ralls et al.149 observed that epidural administration of 20 to 35 mL of epidural lidocaine 2% with fentanyl 1 hour before administration of EREM (8 mg) increased the mean (± SD) peak plasma concentration (Cmax) in the EREM group (11.1 ± 4.9 ng/mL) compared with a group that received a CSE anesthetic technique without epidural medication (8.3 ± 7.1 ng/mL) (P = .038). Further, the EREM group had an increased incidence of vomiting, hypotension, and use of supplemental oxygen. Patients who receive EREM should be monitored at least once every hour during the first 12 hours after administration and at least once every 2 hours for the next 12 hours (i.e., from 12 to 24 hours).248 After 24 hours, monitoring should be performed at least once every 4 hours for a minimum of 48 hours. Increased intensity and duration of monitoring should be considered in patients at increased risk for respiratory depression (e.g., obesity) or in the setting of concomitant administration of opioid analgesics or sedative-hypnotics by other routes. Extra precautions should be taken when patients receive EREM within 1 hour of large doses of local anesthetic149 or when unintentional spinal administration occurs.249
In summary, the onset and duration of analgesia as well as side effects produced by neuraxial opioid administration depend on the specific type of opioid receptor that is activated as well as the dose, lipid solubility, and rate of movement and clearance of the opioid in the CSF.
Pharmacogenetics
Pain associated with labor and delivery is influenced by a multitude of physiologic, psychosocial, and environmental factors. However, genetic variations have also been suggested to alter a patient's sensation, experience, and perception of pain. Recent advances in genomic research have led to identification of more than 100 variants (polymorphisms) in the μ-opioid receptor gene (OPRM1).250 Although inconsistent, results of some studies suggest that a single-nucleotide polymorphism (SNP) in OPRM1 at position 118 (initially known as 118A>G, now annotated 304A>G; rs1799971) may influence responses to opioid analgesics.251 The allelic frequency of the 304A>G polymorphism is population dependent; it is more common in Asians and less frequent in whites and blacks.
The role of the 304A>G polymorphism has been investigated in obstetric anesthesia. Using both up-down sequential allocation and random allocation methods, Landau et al.252 estimated the ED50 of intrathecal fentanyl, administered as part of a CSE technique for labor analgesia in nulliparous women with and without the 304A>G variant. The ED50 of intrathecal fentanyl in women with the variant allele was lower than in women without the allele (1.5- to 2-fold difference). However, when Wong et al.253 investigated the effect of the 304A>G allele on the duration of intrathecal fentanyl labor analgesia (25 µg), they found no significant difference in the duration of analgesia or in the treatment of breakthrough pain in women with the variant allele. Camorcia et al.254 examined the effect of the 304A>G variant on the ED50 of epidural sufentanil in nulliparous women. Similar to the findings of the Landau et al. study,252 the estimated ED50 was significantly lower in women with the variant allele (20.2 µg; 95% CI, 14.2 to 23.6) compared with women without the variant (25.2 µg; 95% CI, 23.2 to 26.4) (P = .03).
The potential role of the 304A>G polymorphism in influencing opioid analgesic requirements after cesarean delivery has been investigated in several studies. Wong et al.,253 in a mixed race/ethnicity population, found no difference in duration of intrathecal morphine analgesia or need for supplemental analgesia in women carrying the variant allele. In contrast, Sia et al.255 reported that Asian women with the variant allele had increased breakthrough pain (as assessed by patient-controlled intravenous morphine requirements) after intrathecal administration of morphine compared with women without the variant allele. In a second study, the variant allele was found to independently predict increased postoperative morphine use in women undergoing cesarean delivery.256
The results of these studies are difficult to reconcile.257 Although genetic components may influence patients' responses to nociceptive stimuli, current evidence suggests that genetic polymorphism in OPRM1 plays a minor role, if any, in opioid pain management.251,257 A recent meta-analysis examining the 304A>G variant of OPRM1 failed to identify a strong association between this variant allele and the response to opioids in different clinical settings.251
Toxicity
Any agent that is injected into the epidural or subarachnoid space should be administered with caution owing to the potential for neurotoxicity and permanent neurologic damage. Although there is concern about injecting any type of medication into the neuraxis, the epidural space is more forgiving than the subarachnoid space (see Chapter 32). In many cases, clinicians have injected medications that were not well tested in animal models. Yaksh and Collins258 have urged careful administration of neuraxial drugs, stating that “studies in animals should precede human use of spinally administered drugs.”
The most commonly administered neuraxial opioids in obstetric patients are preservative-free morphine, fentanyl, and sufentanil. Preservative-free morphine is commercially available for both epidural and intrathecal administration. To evaluate preservative-free morphine for potential neurotoxicity, Abouleish et al.259 examined the short- and long-term effects of intrathecal morphine injection in monkeys. The meninges, nerve roots, and dorsal root ganglia were examined macroscopically and microscopically in both the study and the control groups. The researchers found no evidence of demyelination, arachnoiditis, or necrosis in either group.
Fentanyl is also available in a preservative-free formulation. Despite its widespread clinical use, few studies have assessed the histologic, physiologic, or clinical evidence of neurotoxicity with spinally administered fentanyl. One in vitro study evaluated the effects of fentanyl administration on nerve conduction.260 Histopathologic studies of isolated rabbit vagus nerve axons did not show localized nerve damage after nerves were bathed in an isotonic solution of fentanyl. When axons were bathed in a hypotonic solution of fentanyl, permanent conduction deficits were noted. However, in vivo, relatively large doses of fentanyl would be required to create a hypotonic intrathecal environment.
Although no formal neurotoxicology studies have evaluated sufentanil administration in humans, there are no clinical reports of neurotoxicity despite its widespread use. In one study, sufentanil was administered to cats through an indwelling intrathecal catheter over 5 days.261 Sabbe et al.262 administered clinically relevant doses of intrathecal sufentanil to dogs over several weeks and reported no histopathologic changes. In a sheep model, Rawal et al.263 demonstrated dose-dependent spinal cord histopathologic changes after intrathecal administration of sufentanil (50 to 100 µg) every 6 hours for 72 hours. These doses are much larger than those used in clinical practice. It is possible that these findings reflect an artifact of experimental design (e.g., the frequent administration of a large-volume, hypotonic preparation).
Despite the paucity of data about possible neurotoxicity, both fentanyl and sufentanil are widely used in clinical practice. These drugs are not approved by the FDA for neuraxial use. However, there are no published reports of neurologic deficits after epidural or intrathecal administration of either agent in humans, and these drugs appear to be safe for neuraxial administration. In general, anesthesia providers should exercise extreme caution before injecting any untested agent into the spinal or epidural space, in order to prevent irritation or damage to neural structures.
Side Effects
Neuraxial opioid administration is associated with beneficial effects as well as potential complications and side effects. Intrathecal administration of relatively large doses of morphine is associated with a high incidence of side effects, including somnolence, nausea and vomiting, pruritus, and respiratory depression (Table 13-5). However, epidural and intrathecal injection of more lipid-soluble opioids have fewer side effects.
TABLE 13-5
Incidence of Adverse Side Effects after Intrathecal Injection of 0.5 or 1.0 mg of Morphine
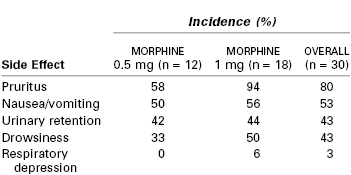
Modified from Abboud TK, Shnider SM, Dailey PA, et al. Intrathecal administration of hyperbaric morphine for the relief of pain in labour. Br J Anaesth 1984; 56:1351-60.
Sensory Changes
An early study evaluating intrathecal sufentanil in laboring women reported sensory changes and hypotension, although no local anesthetics were administered.264 Other investigators have reported high cervical sensory blockade associated with mental status changes, dysphagia, dyspnea, and automatisms after intrathecal sufentanil injection.265-268 These symptoms are likely to be related to a dose-dependent opioid effect rather than neuraxial blockade–induced sympathectomy.269 Further, these changes do not predict the quality or duration of analgesia or degree of hemodynamic change.269 These sensory changes can be clinically significant, especially when they extend to the cervical dermatomes. Patients may feel that they cannot breathe or swallow, an effect that can be distressing. Fortunately, neither intrathecal sufentanil nor fentanyl affects the efferent limb of the nervous system, and motor function is not impaired. Patients should be reassured that their respiratory efforts are not impaired and that these symptoms will subside in 30 to 60 minutes. One report described the use of naloxone to treat the sensory changes associated with intrathecal sufentanil.265
Nausea and Vomiting
Nausea and vomiting are common during labor and delivery. Intrapartum nausea and vomiting can occur from a variety of causes, including pregnancy, physiology of labor itself, pain associated with labor, and parenteral administration of an opioid that may have preceded the neuraxial opioid administration. Therefore, it is difficult to determine the incidence of nausea and vomiting as direct side effects of neuraxial analgesia. Although the mechanism of neuraxial opioid–mediated nausea is unclear, there are suggestions that it may be caused by modulation of afferent input to the area postrema (i.e., the chemoreceptor trigger zone) or at the nucleus of the tractus solitarius, a key relay station in the visceral sensory network.270 Interestingly, nausea is more common after intrathecal administration of opioids to patients who have undergone cesarean delivery than in patients who received the same intrathecal regimen during labor and delivery. Norris et al.271 reported that women receiving epidural or intrathecal opioid analgesia during labor had an incidence of nausea and vomiting of only 1.0% and 2.4%, respectively.
A number of treatments are available with minimal side effects. A meta-analysis suggested that metoclopramide administration (10 mg) before initiation of spinal anesthesia or after delivery resulted in a significant reduction in intraoperative nausea and vomiting as well as early postoperative nausea and vomiting.272 One explanation for metoclopramide's efficacy is that it promotes gastric emptying. Ondansetron is also used in many centers for prophylaxis and treatment of opioid-induced nausea. In a study comparing transdermal scopolamine 1.5 mg, intravenous ondansetron 4 mg, and placebo, scopolamine was an effective prophylactic medication against nausea in parturients who received intrathecal morphine for analgesia after cesarean delivery.273 However, the use of scopolamine may be limited by bothersome side effects, including dry mouth, drowsiness, and blurred vision. George et al.274 performed a systematic review of randomized, controlled trials comparing prophylaxis or treatment of nausea and vomiting using one of the 5-hydroxytryptamine-3 (5-HT3) receptor antagonists or placebo in women receiving spinal anesthesia with intrathecal morphine for cesarean delivery. The authors determined that 5-HT3 receptor antagonists reduced the incidence of postoperative nausea and vomiting as well as the need for postoperative rescue antiemetic therapy when compared with placebo.
Although droperidol is effective for the treatment of nausea, the FDA has issued a “black box” warning against its use because of concern for QT-interval prolongation in association with droperidol administration. Intravenous cyclizine 50 mg was shown to be superior to dexamethasone 8 mg in reducing nausea after intrathecal morphine administration for cesarean delivery.275 Another systematic review of randomized controlled trials compared dexamethasone with placebo for the prevention of postoperative nausea and vomiting in patients receiving neuraxial morphine as part of a neuraxial technique.276 Results suggested that dexamethasone is an effective antiemetic agent, and doses used for antiemetic prophylaxis enhanced postoperative analgesia compared with placebo.
Pruritus
Pruritus is the most common side effect of neuraxial opioid administration.264,277 Presentation is highly variable, but the incidence and severity seem to be dose dependent, especially with epidural opioid administration.278 Onset of the pruritus occurs shortly after analgesia develops, and even small doses of intrathecal sufentanil may produce significant pruritus.279 Some observers have noted a segmental pruritus, especially with lipophilic opioids. For example, patients often complain of perineal and truncal pruritus after intrathecal sufentanil injection.264 Pruritus occurs more commonly with intrathecal opioid administration than with epidural administration (in one study,271 41.4% versus 1.3%, respectively). The incidence and severity of pruritus may be reduced by administration of a lower dose of opioid279,280 or co-administration of the opioid with a local anesthetic.281 Many patients do not complain about the pruritus and appear asymptomatic; however, when questioned, they acknowledge the symptom.
Although the cause of opioid-induced pruritus is unknown, it appears to be unrelated to histamine release.282 Some investigators have suggested that pruritus results from a perturbation of sensory input resulting from rostral spread of the opioid within the CSF to the trigeminal nucleus or subnucleus caudalis.282 Itch-specific neuronal pathways may interact with pain pathways so that continuing activity of the pain-processing system suppresses activity in the spinal itch-processing neurons. Consequently, if pain is inhibited, pruritus can be unmasked (e.g., intrathecal morphine–induced pruritus). Pruritus can also be inhibited by pain (e.g., antipruritic effect of scratching).283
The serotoninergic system may contribute to modulation of pain by providing a balance between nociception and anti-nociception in the network of pain-processing neurons.284,285 The dorsal horn of the spinal cord and the spinal tract of the trigeminal nerve are abundant in 5-HT3 receptors. Because morphine is known to activate 5-HT3 receptors by a mechanism independent of opioid receptors,286 it is postulated that morphine may directly stimulate 5-HT3 receptors and may cause intrathecal morphine–induced pruritus. Consequently, occupation of 5-HT3 receptors by a 5-HT3–receptor antagonist potentially prevents the pruritus.
Iatrou et al.287 performed a randomized, double-blind, placebo-controlled study to evaluate the prophylactic effects of ondansetron and dolasetron in the treatment of intrathecal morphine–induced pruritus. Study results demonstrated that patients who received preemptive 5-HT3–receptor antagonists reported significantly less pruritus and pruritus of less severity during the first 8 postoperative hours than patients who received placebo. The frequency of pruritus was reduced by 48% and 70% for ondansetron and dolasetron, respectively, compared with placebo. A quantitative systematic review evaluated the efficacy of prophylactic 5-HT3 receptor antagonists for the prophylaxis and treatment of neuraxial opioid-induced pruritus. The investigators determined that prophylactic 5-HT3 receptor antagonists did not alter the incidence of pruritus compared with placebo but did reduce the incidence of severe pruritus and the need for therapy.274 Additionally, 5-HT3 receptor antagonists were efficacious for the treatment of established pruritus.
Other treatments of intrathecal opioid–induced pruritus include administration of intravenous naloxone (40 to 80 µg) or diphenhydramine (25 mg). Despite the probability that the pruritus is unrelated to histamine release, there may be some benefit from the modest sedation that follows diphenhydramine administration. Administration of nalbuphine (2.5 to 5 mg intravenously288,289 or 10 mg subcutaneously288,290) may also be helpful in reducing symptoms. The advantage of nalbuphine compared with naloxone is that it is less likely to reverse neuraxial opioid analgesia.289 Although propofol 10 to 20 mg was found effective for the treatment of pruritus in several studies in nonobstetric patients, its efficacy was no better than placebo in an obstetric study.291 Regardless of the chosen treatment, pruritus can contribute significantly to patient dissatisfaction and should be treated promptly upon request.
Hypotension
Decreased blood pressure was reported in early studies that evaluated intrathecal opioid administration.264,269 Although hypotension occurs in 5% to 10% of parturients who receive intrathecal opioids,264,269 the incidence is higher when a local anesthetic or clonidine is added to the opioid. Early reports suggested that hypotension was due to a sympathectomy, but later work suggests that hypotension results from pain relief292 and decreased maternal levels of catecholamines, especially epinephrine.293 Wang et al.294 demonstrated that intrathecal opioids block the afferent information from A-delta and C-fibers to the spinal cord but that efferent nerve impulses (e.g., sympathetic efferents) are not directly blocked.
Respiratory Depression
All opioids can cause respiratory depression regardless of their route of administration. When opioids are administered either epidurally or intrathecally, the following factors affect the risk for respiratory depression: (1) choice of drug and its pharmacokinetics, (2) drug dose, and (3) concomitantly administered CNS depressants. The most important factor affecting the onset time of respiratory depression induced by intrathecal opioids is lipid solubility.270 Respiratory depression may occur within minutes after the administration of a lipophilic opioid (e.g., fentanyl, sufentanil) because of rapid absorption of the opioid from the CSF to lipophilic tissues.234 Its subsequent clearance and elimination are similar to those of the drug when injected intravenously; thus the “time frame” for respiratory depression is short. In contrast, hydrophilic drugs (e.g., morphine, hydromorphone) are associated with a delayed onset of respiratory depression. This potentially serious side effect occurs because these hydrophilic opioids remain in the CSF for several hours. Although this characteristic improves the bioavailability of these opioids, rostral migration and absorption of the drug into the respiratory centers in the brainstem can produce respiratory depression 6 to 12 hours after injection (Figure 13-13).
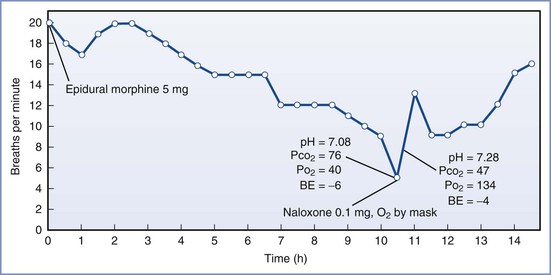
FIGURE 13-13 Respiratory rate of an obstetric patient who received 5 mg of morphine after cesarean delivery and who experienced delayed respiratory depression. (From Leicht CH, Hughes SC, Dailey PA, et al. Epidural morphine sulfate for analgesia after cesarean section: a prospective report of 1000 patients [abstract]. Anesthesiology 1986; 65:A366.)
The dose of opioid has also been shown to be an important factor in the occurrence of respiratory depression. The usual dose of intrathecal morphine for analgesia after cesarean delivery is 0.1 to 0.2 mg. Not surprisingly, an early report of respiratory depression occurred after administration of intrathecal morphine 1 mg.295 In a dose-response study, Palmer et al.296 concluded that there was little justification for giving more than 0.1 mg of intrathecal morphine for analgesia after cesarean delivery. In a dose-response study of epidural morphine administration after cesarean delivery, investigators concluded that the quality of analgesia increases as the dose of epidural morphine increases to 3.75 mg but that increasing the dose to 5 mg does not improve analgesia.297
Studies in nonobstetric surgical patients suggest that the risk for respiratory depression after the epidural administration of EREM is also dose related.298,299 Carvalho et al.247 compared EREM (10 mg) administration with standard epidural morphine (4 mg) administration in healthy women undergoing cesarean delivery and found that EREM reduced opioid consumption for 48 hours without significant risk for respiratory depression. However, the authors cautioned that the study's small sample size may not accurately reflect the true incidence of respiratory depression in obstetric patients who have received the extended-release preparation.
Although most cases of respiratory depression associated with sufentanil administration occur with larger doses, respiratory depression has also been reported with as little as 10 µg of intrathecal sufentanil administered for labor analgesia.300,301 Larger doses (e.g., 15 µg) have not been found to produce better or more prolonged analgesia but do result in increased plasma opioid concentrations and a higher risk for respiratory depression. In a female volunteer study, Lu et al.245 reported that doses of intrathecal sufentanil larger than 12.5 µg did not produce a proportionate increase in intensity or duration of analgesia. Similarly, there is little benefit to increasing the dose of intrathecal fentanyl beyond 25 µg when it is used as the sole agent for labor analgesia. These higher doses (i.e., more than 10 µg of sufentanil or more than 25 µg of fentanyl) should not be used in routine clinical practice. Respiratory depression has been reported with as little as 100 µg of epidural fentanyl.302
Several case reports have implicated previous parenteral administration of opioid as a contributing factor in respiratory arrest associated with intrathecal sufentanil administration in laboring women.303,304 For example, Jaffee et al.305 reported a case of apnea and unresponsiveness in a parturient who had received several doses of intravenous fentanyl in the 4 hours before intrathecal sufentanil administration. Although the pregnancy-induced increase in respiratory drive continues throughout labor and into the postpartum period and may provide some protection against respiratory depression, respiratory depression is the most serious side effect of neuraxial opioid administration.
Practice guidelines from the American Society of Anesthesiologists recommend that all patients who receive neuraxial opioids should be monitored for adequacy of ventilation (e.g., respiratory rate, depth of respiration), oxygenation (e.g., pulse oximetry when appropriate), and level of consciousness.248 In patients who receive a single neuraxial injection of a lipophilic opioid (e.g., fentanyl), monitoring should be continual for the first 20 minutes after administration, followed by monitoring at least once per hour until 2 hours have passed. In patients who receive a single neuraxial injection of a hydrophilic opioid (e.g., morphine), monitoring should be performed at least hourly for the first 12 hours and then at least every 2 hours for the next 12 hours after opioid administration (and then every 4 hours for another 24 hours in patients who receive epidural EREM). For patients who receive a continuous infusion of a neuraxial opioid, monitoring should be performed hourly during the first 12 hours, every 2 hours for the next 12 hours, and then every 4 hours for the duration of the opioid infusion. In addition, the guidelines state that greater duration and intensity of monitoring and/or additional methods of monitoring may be indicated in patients at increased risk for respiratory depression (e.g., obesity, obstructive sleep apnea, concomitant administration of opioid analgesics by other routes).
Urinary Retention
Urinary retention is a bothersome side effect of intraspinal opioid administration. The incidence varies widely. It is more common with neuraxial opioid administration than with intramuscular or intravenous administration of equivalent doses. Urinary retention is unrelated to systemic absorption and is dose independent. The onset of urinary retention appears to parallel the onset of analgesia. Evidence suggests that the rapid onset of this side effect is produced by relaxation of the detrusor muscle (Figure 13-14),306 which most likely results from the sacral spinal action of opioids. Urinary retention can be treated with naloxone; however, because many parturients require catheterization for other reasons, urinary retention is often treated with bladder catheterization.
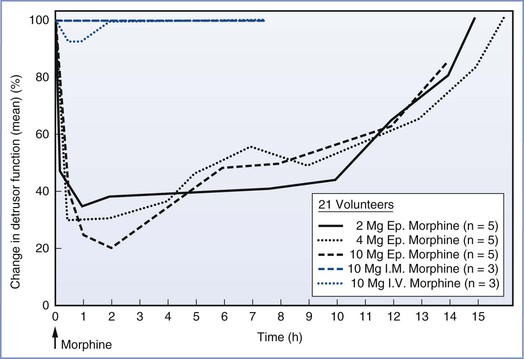
FIGURE 13-14 Urodynamic effects of epidural (Ep.), intramuscular (I.M.), and intravenous (I.V.) morphine administration in male volunteers. Depression of detrusor muscle function persisted for many hours after epidural morphine administration. This did not occur with parenteral opioids and may represent a local spinal cause (i.e., opioid receptors). (From Rawal N, Mollefors K, Axelsson K, et al. An experimental study of urodynamic effects of epidural morphine and of naloxone reversal. Anesth Analg 1983; 62:641-7.)
Delayed Gastric Emptying
Labor may delay gastric emptying, and opioids may further exacerbate this delay (see Chapter 29). Parenterally administered opioids are known to delay gastric emptying in laboring women.307 However, clinically useful doses of epidural fentanyl have minimal effects on gastric emptying. Intrathecal administration of fentanyl produces greater delays in gastric emptying than epidural administration.308 Delays in gastric emptying may increase the risk for nausea and vomiting and also increase the risk for aspiration if general anesthesia is necessary for emergency cesarean delivery.
Recrudescence of Herpes Simplex Virus Infections
Genital herpes infection (herpes simplex virus [HSV]) is the most common type of herpes-virus infection during pregnancy309; however, oral HSV infections (common cold sore or fever blister) resulting from reactivation of latent HSV infection also occur during pregnancy. Reports have suggested a relationship between neuraxial opioid administration and reactivation of oral herpes infection.310 Crone et al.311 reported a 10% incidence of reactivation after cesarean delivery in patients who had received epidural morphine, compared with a 1% incidence in similar patients who did not receive epidural morphine. These observations have been confirmed in two prospective studies.312,313 Davies et al.314 reported an increased incidence of postpartum herpes infection in patients with a history of HSV-1 who had received intrathecal morphine. Two case reports have reported an association between intraspinal administration of fentanyl and meperidine and reactivation of oral herpes infection.315,316
The mechanism of herpes reaction is unknown.310 Viral reactivation is known to occur with exposure to ultraviolet light, immunosuppression, trauma, and fever. Proposed causes include (1) a skin trigger mechanism, whereby pruritus and scratching trigger reactivation; (2) an altered immunologic response317; and (3) a ganglion trigger mechanism, whereby the intraspinal opioid spreads rostrally and binds to the trigeminal nerve.318 The ganglion trigger mechanism involves an alteration of sensory modulation that results in reactivation. We are unaware of any serious maternal or neonatal complications that have resulted from neuraxial administration of an opioid and reactivation of oral herpes infection.
Placental Transfer and Fetal and Neonatal Effects
Neuraxial opioid administration may have a direct effect on the infant (i.e., respiratory depression at delivery) that results from systemic absorption of the opioid followed by transplacental transfer. The fetus may also be affected indirectly by opioid-related maternal side effects (i.e., hypoxemia, respiratory depression).
Neonatal Depression
Systemic opioid absorption can result in neonatal respiratory depression, which is sometimes observed after systemic opioid administration during labor.319,320 Neuraxial opioid analgesia techniques may result in better Apgar scores and umbilical cord blood gas and pH measurements at delivery. Despite the rapid systemic uptake of intrathecally administered opioids, the neuraxial analgesia requires the administration of smaller doses of opioid.
Several studies have evaluated neonatal outcome after continuous maternal epidural infusion of opioids and local anesthetics.321-323 Collectively, these studies have demonstrated that maternal epidural opioid administration by continuous infusion rarely results in drug accumulation and subsequent neonatal depression. Reynolds et al.324 performed a systematic review of randomized and nonrandomized studies comparing epidural with systemic opioid analgesia. They reviewed 12 trials with a total study population of 2102 parturients. Epidural analgesia was associated with better umbilical cord blood acid-base measurements than systemic opioids, suggesting that placental perfusion and gas exchange was well preserved despite maternal sympathetic blockade and effective analgesia. Although not all of the studies used neuraxial opioid infusions, the researchers suggested that replacement of systemic opioids with modest doses of neuraxial opioids not only produces superior analgesia but also may have a favorable effect on neonatal outcome.324
Fetal Heart Rate Abnormalities
Although epidural or intrathecal opioid administration has little direct effect on FHR,325 worrisome abnormalities such as late decelerations and fetal bradycardia have been observed after intrathecal lipophilic opioid administration. Several reports have described the abrupt onset of fetal bradycardia after intrathecal administration of fentanyl or sufentanil.326-328 Clarke et al.327 suggested that the bradycardia is an indirect effect of decreased circulating maternal epinephrine associated with the rapid onset of analgesia. Epinephrine has a tocolytic effect and causes uterine relaxation by stimulating β2-adrenergic receptors. Consequently, reduced epinephrine levels may lead to increased uterine tone. Because uteroplacental perfusion occurs during periods of uterine diastole (i.e., uterine relaxation), uterine tachysystole may result in diminished uteroplacental perfusion and fetal hypoxia. Norepinephrine is known to have a uterine-stimulating effect329; thus the decrease in epinephrine concentration alongside an unchanged norepinephrine concentration may produce uterine hyperactivity and fetal compromise.
Other mechanisms may also be relevant. Van de Velde et al.330 questioned the catecholamine imbalance theory, because intrathecal bupivacaine combined with low-dose sufentanil (1.5 µg) produced analgesia similar to that provided by intrathecal sufentanil (7.5 µg), but the incidence of fetal bradycardia was higher with sufentanil 7.5 µg. Russell et al.331 demonstrated that intravenous opioids have central effects, altering the release of oxytocin and vasopressin and inducing uterine hyperactivity. Lipid-soluble opioids undergo rapid systemic redistribution after neuraxial injection; therefore, even neuraxial opioids may have central effects.
Initial reports indicated that the incidence of FHR abnormalities with intrathecal opioid analgesia was 15% to 20%.264,332 One published report suggests that uterine tachysystole and fetal bradycardia may follow administration of either intrathecal or epidural analgesia during labor.333 FHR tracings were assessed after administration of either intrathecal sufentanil or epidural bupivacaine. There were no observed differences in the incidence of FHR abnormalities (i.e., recurrent late decelerations and/or bradycardia) between groups (22% in the intrathecal sufentanil group versus 23% in the epidural bupivacaine group).333 In contrast, Mardirosoff et al.334 performed a systematic review of all randomized trials comparing intrathecal with non-intrathecal administration of opioids in laboring women. Twenty-four trials met criteria; the study population included 3513 women. The relative risk for FHR abnormalities in patients receiving spinal opioids was 1.81 (95% CI, 1.04 to 3.14). The risks of cesarean delivery for FHR abnormalities were similar in the two groups (6.0% for intrathecal administration versus 7.8% for other methods).
In a prospective study, Van de Velde et al.330 investigated whether intrathecal sufentanil 7.5 µg produced more FHR abnormalities than either conventional epidural analgesia or intrathecal bupivacaine combined with sufentanil 1.5 µg. The high-dose sufentanil group had more FHR abnormalities (i.e., late decelerations, fetal bradycardia) but less hypotension than the low-dose sufentanil/bupivacaine group. The incidence of FHR abnormalities was similar in the low-dose sufentanil/bupivacaine and conventional epidural analgesia groups. The rates of cesarean delivery for FHR abnormalities were similar in the three groups. Although FHR abnormalities are worrisome, most published trials have not reported a higher risk for emergency cesarean delivery with intrathecal opioid analgesia.330,334,335 However, Gambling et al.336 reported more operative deliveries for nonreassuring fetal status after administration of intrathecal sufentanil 10 µg than with parenteral meperidine analgesia. The study's conclusions, however, were limited in that the two study groups differed in frequency of FHR assessment.
In a randomized trial, Abrao et al.337 evaluated the effects of CSE versus traditional epidural analgesia on basal uterine tone and the occurrence of FHR abnormalities. Use of the CSE technique was the only independent predictor of an increase in basal intrauterine pressure of 10 mm Hg or more (OR, 3.53; 95% CI, 1.21 to 10.36; P = .022). The authors also demonstrated that the only predictor of FHR abnormalities was an increase in intrauterine pressure after initiation of analgesia (OR, 18.62; 95% CI, 4.46 to 77.72). A decrease in visual analog scale pain scores immediately after administration of analgesia was also correlated with an increased probability of increased intrauterine pressure and FHR abnormalities. Although there were no emergency cesarean deliveries that resulted from either neuraxial technique, the authors concluded that more studies are needed to better understand the effects of the CSE technique on labor progress and fetal physiology.337 However, in a letter-to-the-editor, Landau et al.338 suggested that the analgesic techniques were not equipotent. Additionally, monitoring for FHR abnormalities was only performed for 15 minutes. Given that the onset of analgesia is slower with epidural compared with CSE techniques, FHR abnormalities may occur earlier after intrathecal analgesia.
Given the potential risk for fetal bradycardia after neuraxial analgesia in laboring women, the FHR should be monitored before and after the initiation of epidural and intrathecal analgesia. FHR changes are usually transient and may be managed successfully with conservative measures, including (1) supplemental oxygen administration, (2) position changes to relieve aortocaval compression, (3) vasopressor therapy to treat hypotension, (4) discontinuation of oxytocin infusion, (5) intravenous fluid bolus administration, and (6) administration of a tocolytic agent for persistent uterine tachysystole.
Historically, intravenous or subcutaneous terbutaline was used to treat persistent uterine tachysystole. More recently, nitroglycerin has been used with some success. Nitroglycerin has several advantages compared with terbutaline. First, nitroglycerin has a short duration of action and labor resumes shortly after the period of tachysystole. In addition, nitroglycerin rarely produces significant hypotension, and if hypotension occurs, it is easily treated. Several studies have evaluated nitroglycerin for the treatment of uterine hypertonus. Mercier et al.339 described consistent success in treating FHR abnormalities resulting from uterine tachysystole after the administration of one or two doses of nitroglycerin (60 to 90 µg), and Bell340 described the successful use of sublingual nitroglycerin (400 µg) in the treatment of uterine tachysystole. In a randomized trial comparing intravenous terbutaline 250 µg to nitroglycerin 400 µg for the treatment of intrapartum tachysystole and nonreassuring FHR tracings, acute intrauterine resuscitation success rates were similar between the two groups (72% versus 64% for terbutaline and nitroglycerin, respectively; P = .38), but the incidence of tachysystole 10 minutes after drug administration was lower in the terbutaline group.341 Therefore, if there is no response within 2 to 3 minutes of nitroglycerin administration, terbutaline 0.25 mg (250 µg) should be administered and preparations should be made for emergency cesarean delivery if the fetal bradycardia does not resolve.
Adjuvants
Epinephrine
Epinephrine is often added to epidural and spinal local anesthetic solutions to increase the duration of anesthesia, reduce peak plasma drug concentrations, improve block reliability, and intensify analgesia/anesthesia.342-344 Uptake of epinephrine varies with the choice and concentration of local anesthetic as well as the concentration of epinephrine. The effect of epinephrine is greater when it is combined with lidocaine than when it is combined with bupivacaine.159,198 Even concentrations of epinephrine as low as 3.3 µg/mL (1 : 300,000) have been shown to be effective in reducing the plasma concentrations of lidocaine.344
The efficacy of epinephrine depends on the specific local anesthetic as well as the site of injection. Epinephrine prolongs the duration of epidural lidocaine anesthesia by reducing uptake of local anesthetic into the systemic circulation through constriction of the epidural venous plexus. This effect helps maintain the concentration of local anesthetic at the site of injection. During epidural administration, epinephrine provides optimal results when added to lidocaine in a concentration of 5 µg/mL (1 : 200,000); this concentration of epinephrine nearly doubles the duration of epidural lidocaine anesthesia.345 In contrast to lidocaine, the addition of epinephrine 3.3 µg/mL to epidural bupivacaine 0.5% had no effect on maternal venous plasma concentrations of drug in laboring women.346 Similarly, Reynolds et al.347 observed no effect when epinephrine 5 µg/mL was added to bupivacaine during administration of epidural anesthesia for cesarean delivery. Epinephrine did not prolong the epidural anesthesia produced by ropivacaine,348 nor did it alter absorption of lidocaine after subarachnoid injection.349 In contrast, one group reported that the addition of epinephrine to bupivacaine resulted in a 50% decrease in maternal plasma concentrations of bupivacaine after paracervical block.350
Greater reliability and intensity of the block are sometimes observed when epinephrine is added to epidurally administered local anesthetics. Epinephrine has intrinsic analgesic effects that are produced by stimulation of α2-adrenergic receptors. These presynaptic adrenergic receptors are found at the terminals of primary afferent neurons. They can also be found centrally on neurons in superficial laminae of the spinal cord and in several brainstem nuclei.
In addition to the intrinsic analgesic effects of epinephrine, the inherent lipid solubility of each local anesthetic affects the degree of sensory blockade. Each local anesthetic has a lipid-to-water partition coefficient that determines the drug uptake between the aqueous and lipid phases within the spinal canal. The outcome of competition between these lipid and aqueous phases depends on the lipid solubility of the local anesthetic. If a local anesthetic is more lipid soluble, the advantage of adding epinephrine to the local anesthetic is less significant. For example, the lipid-to-water partition coefficient of lidocaine is 2.7. When epinephrine is added to lidocaine, there is marked improvement in the intensity of the block. However, because bupivacaine has a lipid-to-water partition coefficient 10 times greater than that of lidocaine, the effect of epinephrine on a bupivacaine block is less pronounced. Because ropivacaine has a lipid-to-water partition coefficient similar to that of lidocaine, epinephrine will intensify a ropivacaine block. However, the duration of the block remains unchanged.
Despite the advantages of epinephrine, concern remains about the effects of epinephrine on uterine blood flow and the maternal cardiovascular system. In healthy fetuses, epidural administration of epinephrine does not affect umbilical cord blood flow. However, in fetuses with increased vascular resistance, epidural epinephrine administration can increase the umbilical artery S/D ratio.351 Studies of the effects of epinephrine on the placental transfer of local anesthetics have yielded contradictory results. In rabbits, epinephrine did not affect the F/M ratio of bupivacaine.352 As a result of the addition of epinephrine, the F/M ratio for bupivacaine has been found to be increased353 or unchanged.346,350,354 For lidocaine, the F/M ratio has variously been reported to be increased,198,355 decreased,344 or unchanged.356
Bicarbonate
The addition of sodium bicarbonate to a local anesthetic solution increases the pH closer to the pKa of the local anesthetic. This change increases the proportion of drug in un-ionized form that is available to penetrate the nerve sheath and membrane, thereby accelerating the onset of the block and decreasing the minimum concentration required for conduction blockade.357 Most studies have demonstrated that the addition of sodium bicarbonate to lidocaine, bupivacaine, or 2-chloroprocaine hastens the onset of epidural blockade by as much as 10 minutes.148,358-360 The speed of onset of a ropivacaine block does not seem to be affected by alkalinization, but as with the other local anesthetics, evidence suggests that alkalinization intensifies epidural ropivacaine anesthesia and improves spread to sacral dermatomes.361 The effects of alkalinization are most pronounced in epinephrine-containing solutions, particularly commercially prepared epinephrine-containing formulations. These solutions are prepared at a lower pH, ranging from 3.2 to 4.2.362 The lower pH of these solutions helps preserve the epinephrine but increases the latency of onset.
Sodium bicarbonate 1 mEq/mL (8.4%) may be freshly added to local anesthetic solutions shortly before use (Table 13-6). Alkalinization of bupivacaine must be performed carefully because the margin between satisfactory alkalinization and complete precipitation is very narrow. All local anesthetics have a tendency to precipitate, so solutions containing bicarbonate should be inspected for precipitation before being administered.
TABLE 13-6
Alkalinization of Local Anesthetic Solutions
| Local Anesthetic | Sodium Bicarbonate (mL)* |
| Lidocaine | 1.0 |
| Bupivacaine | 0.1 |
| 2-Chloroprocaine | 0.3 |
* Sodium bicarbonate 8.4% (1 mEq/mL) added to 10 mL local anesthetic solution. Suggested doses are from Warren DT, Neal JM, Bernards CM. Neuraxial anesthesia. In Longnecker DE, Newman MF, Brown DL, Zapol WM, editors. Anesthesiology. 2nd edition. New York, McGraw-Hill, 2012. Available at http://www.accessanesthesiology.com/content/56638559. Accessed August 2013..
Hypotension occurs more frequently with epidural administration of an alkalinized local anesthetic than with administration of an unbuffered solution.363 This likely results from an accelerated onset of sympathetic blockade. Carbonated salts of local anesthetics can also be administered for a rapid onset of epidural blockade. However, these drugs have limited availability. Like alkalinized local anesthetics, these preparations are more likely to produce hypotension.
Clonidine
α2-Adrenergic agonists (e.g., clonidine) have been investigated as adjuvants to local anesthetics and opioids to improve analgesic efficacy without increasing side effects. The advantage of clonidine is its ability to provide analgesia without affecting sensation or producing motor blockade.364 However, epidural and intrathecal administration of α2-adrenergic agonists are known to produce hypotension, probably by acting on α2-adrenergic receptors on preganglionic cholinergic neurons.365 In addition, α2-adrenergic agonists produce dose-dependent sedation, which results from α2-adrenergic stimulation in the locus ceruleus.366
Neuraxial clonidine has been administered for labor as well as analgesia after cesarean delivery. It exerts its effects by binding to α2-adrenergic receptors located on primary afferent terminals of the spinal cord, substantia gelatinosa, and brainstem nuclei367 as well as via a cholinergic mechanism.368 Conduction blockade is produced by increases in potassium conductance and in acetylcholine and norepinephrine in the CSF, leading to decreased release of substance P and subsequent analgesia.369
Approximately 70% of alpha-adrenergic receptors on human myometrium are α2-adrenergic receptors370; therefore, the potential effects of clonidine on labor and delivery have been evaluated. In an in vitro study, clonidine directly enhanced the frequency and amplitude of human myometrial contraction.371 α2-Adrenergic receptor stimulation in the uterus could theoretically enhance uterine contractions and decrease uterine blood flow.365 Indeed, in animal studies, large doses of clonidine produced a decrease in FHR.372 This effect probably resulted from direct fetal transfer of drug and from direct and indirect effects on baroreflexes. However, this effect is unlikely to occur with clinical doses of neuraxial clonidine.
Multiple studies have evaluated neuraxial clonidine administration in humans as an analgesic adjunct during labor and delivery (see Chapter 23). When combined with local anesthetics and opioids, lower doses may be used, resulting in less hypotension and sedation. The FDA has issued a “black box” warning against its use in obstetric patients because of concerns about hemodynamic instability after its use. Therefore, clonidine is rarely used for labor analgesia in North America; however, it is more widely used in some European countries. Clonidine may be particularly useful in women in whom other epidural analgesics are contraindicated or in those who have breakthrough pain with standard local anesthetic/opioid solutions despite a functioning epidural catheter. In this setting, the bolus administration of clonidine 75 µg without a local anesthetic is not usually associated with hypotension. It appears safe to add small doses of intrathecal clonidine (15 to 30 µg) to opioids or local anesthetics, but side effects must be treated promptly to avoid fetal compromise.
Epidural clonidine has been administered for analgesia after cesarean delivery. One study suggested that epidural clonidine (400 to 800 µg) provided postoperative analgesia, but a continuous infusion was required after 6 hours.373 Others have demonstrated that epidural clonidine (75 to 150 µg) lengthens the duration of postoperative analgesia without increasing the incidence of side effects.374,375
Neostigmine
Both nicotinic and muscarinic cholinergic receptors are present in the dorsal horn of the spinal cord. Neostigmine prevents breakdown of acetylcholine in the spinal cord. The acetylcholine then binds to muscarinic and nicotinic receptors of the spinal cord.376-378 Stimulation of muscarinic receptors facilitates release of gamma-aminobutyric acid (GABA) in the dorsal horn of the spinal cord, resulting in analgesia.368,379 Neostigmine and clonidine use a common pathway to produce analgesia mediated through acetylcholine release.
Several studies have evaluated the addition of neostigmine to intrathecal labor analgesics. Although results of these studies were inconsistent in terms of prolonging the duration of labor analgesia, all studies found that intrathecal administration of neostigmine produced severe nausea unresponsive to standard antiemetics.380,381 These important gastrointestinal side effects limit its clinical use despite its ability to potentiate the analgesic effects of intrathecal opioids and clonidine.
Epidural neostigmine alone has limited efficacy. Neostigmine appears to be more effective at alleviating somatic pain than visceral pain.382,383 Visceral afferents are located deep within the spinal cord. Because neostigmine has low lipid solubility, it has a limited ability to traverse biologic membranes. When it is administered without other agents, it is unable to reach these visceral afferents responsible for much of labor pain.384 This accounts for the limited efficacy of neostigmine epidurally administered as the sole agent. However, when combined with epidural sufentanil or clonidine for initiation of analgesia, neostigmine produces selective analgesia without side effects.385,386 Large doses of neostigmine can potentially reduce uteroplacental blood flow by CNS activation and direct stimulation of uterine contractions.387 When administered for analgesia after cesarean delivery, epidural neostigmine (75 to 300 µg) produced modest analgesia without nausea or vomiting, but the incidence of sedation was increased.388 Neostigmine is not routinely used in clinical practice, and its neuraxial administration is not approved by the FDA.
References
1. Aberg G. Toxicological and local anaesthetic effects of optically active isomers of two local anaesthetic compounds. Acta Pharmacol Toxicol (Copenh). 1972;31:273–286.
2. McClure JH. Ropivacaine. Br J Anaesth. 1996;76:300–307.
3. Graf BM, Abraham I, Eberbach N, et al. Differences in cardiotoxicity of bupivacaine and ropivacaine are the result of physicochemical and stereoselective properties. Anesthesiology. 2002;96:1427–1434.
4. Strichartz GR. The inhibition of sodium currents in myelinated nerve by quaternary derivatives of lidocaine. J Gen Physiol. 1973;62:37–57.
5. Bromage PR. Continuous lumbar epidural analgesia for obstetrics. Can Med Assoc J. 1961;85:1136–1140.
6. Lee GY, Kim CH, Chung RK, et al. Spread of subarachnoid sensory block with hyperbaric bupivacaine in second trimester of pregnancy. J Clin Anesth. 2009;21:482–485.
7. Fagraeus L, Urban BJ, Bromage PR. Spread of epidural analgesia in early pregnancy. Anesthesiology. 1983;58:184–187.
8. Butterworth JF, Walker FO, Lysak SZ. Pregnancy increases median nerve susceptibility to lidocaine. Anesthesiology. 1990;72:962–965.
9. Datta S, Lambert DH, Gregus J, et al. Differential sensitivities of mammalian nerve fibers during pregnancy. Anesth Analg. 1983;62:1070–1072.
10. Bader AM, Datta S, Moller RA, Covino BG. Acute progesterone treatment has no effect on bupivacaine-induced conduction blockade in the isolated rabbit vagus nerve. Anesth Analg. 1990;71:545–548.
11. O'Brien JE, Abbey V, Hinsvark O, et al. Metabolism and measurement of chloroprocaine, an ester-type local anesthetic. J Pharm Sci. 1979;68:75–78.
12. Kuhnert BR, Kuhnert PM, Philipson EH, et al. The half-life of 2-chloroprocaine. Anesth Analg. 1986;65:273–278.
13. Monedero P, Hess P. High epidural block with chloroprocaine in a parturient with low pseudocholinesterase activity. Can J Anaesth. 2001;48:318–319.
14. Santos AC, Pedersen H, Morishima HO, et al. Pharmacokinetics of lidocaine in nonpregnant and pregnant ewes. Anesth Analg. 1988;67:1154–1158.
15. Bloedow DC, Ralston DH, Hargrove JC. Lidocaine pharmacokinetics in pregnant and nonpregnant sheep. J Pharm Sci. 1980;69:32–37.
16. Downing JW, Johnson HV, Gonzalez HF, et al. The pharmacokinetics of epidural lidocaine and bupivacaine during cesarean section. Anesth Analg. 1997;84:527–532.
17. Kuhnert BR, Philipson EH, Pimental R, et al. Lidocaine disposition in mother, fetus, and neonate after spinal anesthesia. Anesth Analg. 1986;65:139–144.
18. Kuhnert BR, Knapp DR, Kuhnert PM, Prochaska AL. Maternal, fetal, and neonatal metabolism of lidocaine. Clin Pharmacol Ther. 1979;26:213–220.
19. Pedersen H, Santos AC, Morishima HO, et al. Does gestational age affect the pharmacokinetics and pharmacodynamics of lidocaine in mother and fetus? Anesthesiology. 1988;68:367–372.
20. Wood M, Wood AJ. Changes in plasma drug binding and alpha 1-acid glycoprotein in mother and newborn infant. Clin Pharmacol Ther. 1981;29:522–526.
21. Fragneto RY, Bader AM, Rosinia F, et al. Measurements of protein binding of lidocaine throughout pregnancy. Anesth Analg. 1994;79:295–297.
22. Pihlajamaki K, Kanto J, Lindberg R, et al. Extradural administration of bupivacaine: pharmacokinetics and metabolism in pregnant and non-pregnant women. Br J Anaesth. 1990;64:556–562.
23. Tucker GT, Mather LE. Clinical pharmacokinetics of local anaesthetics. Clin Pharmacokinet. 1979;4:241–278.
24. Santos AC, Arthur GR, Lehning EJ, Finster M. Comparative pharmacokinetics of ropivacaine and bupivacaine in nonpregnant and pregnant ewes. Anesth Analg. 1997;85:87–93.
25. Kuhnert PM, Kuhnert BR, Stitts JM, Gross TL. The use of a selected ion monitoring technique to study the disposition of bupivacaine in mother, fetus, and neonate following epidural anesthesia for cesarean section. Anesthesiology. 1981;55:611–617.
26. Reynolds F, Taylor G. Maternal and neonatal blood concentrations of bupivacaine: a comparison with lignocaine during continuous extradural analgesia. Anaesthesia. 1970;25:14–23.
27. Mather LE, Thomas J. Bupivacaine binding to plasma protein fractions. J Pharm Pharmacol. 1978;30:653–654.
28. Wulf H, Munstedt P, Maier C. Plasma protein binding of bupivacaine in pregnant women at term. Acta Anaesthesiol Scand. 1991;35:129–133.
29. Zhan Q, Huang S, Geng G, Xie Y. Comparison of relative potency of intrathecal bupivacaine for motor block in pregnant versus non-pregnant women. Int J Obstet Anesth. 2011;20:219–223.
30. Arthur GR, Feldman HS, Covino BG. Comparative pharmacokinetics of bupivacaine and ropivacaine, a new amide local anesthetic. Anesth Analg. 1988;67:1053–1058.
31. Lee A, Fagan D, Lamont M, et al. Disposition kinetics of ropivacaine in humans. Anesth Analg. 1989;69:736–738.
32. Datta S, Camann W, Bader A, VanderBurgh L. Clinical effects and maternal and fetal plasma concentrations of epidural ropivacaine versus bupivacaine for cesarean section. Anesthesiology. 1995;82:1346–1352.
33. Oda Y, Furuichi K, Tanaka K, et al. Metabolism of a new local anesthetic, ropivacaine, by human hepatic cytochrome P450. Anesthesiology. 1995;82:214–220.
34. Moller R, Covino BG. Cardiac electrophysiologic properties of bupivacaine and lidocaine compared with those of ropivacaine, a new amide local anesthetic. Anesthesiology. 1990;72:322–329.
35. Porter JM, Kelleher N, Flynn R, Shorten GD. Epidural ropivacaine hydrochloride during labour: protein binding, placental transfer and neonatal outcome. Anaesthesia. 2001;56:418–423.
36. Dailey PA, Hughes SC, Rosen MA, et al. Effect of cimetidine and ranitidine on lidocaine concentrations during epidural anesthesia for cesarean section. Anesthesiology. 1988;69:1013–1017.
37. Brashear WT, Zuspan KJ, Lazebnik N, et al. Effect of ranitidine on bupivacaine disposition. Anesth Analg. 1991;72:369–376.
38. Ramanathan J, Bottorff M, Jeter JN, et al. The pharmacokinetics and maternal and neonatal effects of epidural lidocaine in preeclampsia. Anesth Analg. 1986;65:120–126.
39. Debon R, Chassard D, Duflo F, et al. Chronobiology of epidural ropivacaine: variations in the duration of action related to the hour of administration. Anesthesiology. 2002;96:542–545.
40. Shafer SL, Lemmer B, Boselli E, et al. Pitfalls in chronobiology: a suggested analysis using intrathecal bupivacaine analgesia as an example. Anesth Analg. 2010;111:980–985.
41. De Jong RH, Robles R, Corbin RW. Central actions of lidocaine—synaptic transmission. Anesthesiology. 1969;30:19–23.
42. Liu PL, Feldman HS, Giasi R, et al. Comparative CNS toxicity of lidocaine, etidocaine, bupivacaine, and tetracaine in awake dogs following rapid intravenous administration. Anesth Analg. 1983;62:375–379.
43. Scott DB. Evaluation of the toxicity of local anaesthetic agents in man. Br J Anaesth. 1975;47:56–61.
44. Covino BG, Vassallo HG. Local Anesthetics: Mechanisms of Action and Clinical Use. Grune & Stratton: New York; 1976.
45. Englesson S, Grevsten S. The influence of acid-base changes on central nervous system toxicity of local anaesthetic agents. II. Acta Anaesthesiol Scand. 1974;18:88–103.
46. Englesson S. The influence of acid-base changes on central nervous system toxicity of local anaesthetic agents. I. An experimental study in cats. Acta Anaesthesiol Scand. 1974;18:79–87.
47. Burney RG, DiFazio CA, Foster JA. Effects of pH on protein binding of lidocaine. Anesth Analg. 1978;57:478–480.
48. de Jong RH, Ronfeld RA, DeRosa RA. Cardiovascular effects of convulsant and supraconvulsant doses of amide local anesthetics. Anesth Analg. 1982;61:3–9.
49. Morishima HO, Finster M, Arthur GR, Covino BG. Pregnancy does not alter lidocaine toxicity. Am J Obstet Gynecol. 1990;162:1320–1324.
50. Santos AC, Pedersen H, Harmon TW, et al. Does pregnancy alter the systemic toxicity of local anesthetics? Anesthesiology. 1989;70:991–995.
51. Morishima HO, Pedersen H, Finster M, et al. Bupivacaine toxicity in pregnant and nonpregnant ewes. Anesthesiology. 1985;63:134–139.
52. Clarkson CW, Hondeghem LM. Mechanism for bupivacaine depression of cardiac conduction: fast block of sodium channels during the action potential with slow recovery from block during diastole. Anesthesiology. 1985;62:396–405.
53. Thomas RD, Behbehani MM, Coyle DE, Denson DD. Cardiovascular toxicity of local anesthetics: an alternative hypothesis. Anesth Analg. 1986;65:444–450.
54. Coyle DE, Porembka DT, Sehlhorst CS, et al. Echocardiographic evaluation of bupivacaine cardiotoxicity. Anesth Analg. 1994;79:335–339.
55. Feldman HS, Arthur GR, Covino BG. Comparative systemic toxicity of convulsant and supraconvulsant doses of intravenous ropivacaine, bupivacaine, and lidocaine in the conscious dog. Anesth Analg. 1989;69:794–801.
56. Reiz S, Haggmark S, Johansson G, Nath S. Cardiotoxicity of ropivacaine—a new amide local anaesthetic agent. Acta Anaesthesiol Scand. 1989;33:93–98.
57. Nancarrow C, Rutten AJ, Runciman WB, et al. Myocardial and cerebral drug concentrations and the mechanisms of death after fatal intravenous doses of lidocaine, bupivacaine, and ropivacaine in the sheep. Anesth Analg. 1989;69:276–283.
58. Scott DB, Lee A, Fagan D, et al. Acute toxicity of ropivacaine compared with that of bupivacaine. Anesth Analg. 1989;69:563–569.
59. Polley LS, Columb MO, Naughton NN, et al. Relative analgesic potencies of ropivacaine and bupivacaine for epidural analgesia in labor: implications for therapeutic indexes. Anesthesiology. 1999;90:944–950.
60. Capogna G, Celleno D, Fusco P, et al. Relative potencies of bupivacaine and ropivacaine for analgesia in labour. Br J Anaesth. 1999;82:371–373.
61. Ngan Kee WD, Ng FF, Khaw KS, et al. Determination and comparison of graded dose-response curves for epidural bupivacaine and ropivacaine for analgesia in laboring nulliparous women. Anesthesiology. 2010;113:445–453.
62. Dony P, Dewinde V, Vanderick B, et al. The comparative toxicity of ropivacaine and bupivacaine at equipotent doses in rats. Anesth Analg. 2000;91:1489–1492.
63. Chazalon P, Tourtier JP, Villevielle T, et al. Ropivacaine-induced cardiac arrest after peripheral nerve block: successful resuscitation. Anesthesiology. 2003;99:1449–1451.
64. Huet O, Eyrolle LJ, Mazoit JX, Ozier YM. Cardiac arrest after injection of ropivacaine for posterior lumbar plexus blockade. Anesthesiology. 2003;99:1451–1453.
65. Yoshida M, Matsuda H, Fukuda I, Furuya K. Sudden cardiac arrest during cesarean section due to epidural anaesthesia using ropivacaine: a case report. Arch Gynecol Obstet. 2008;277:91–94.
66. Albright GA. Cardiac arrest following regional anesthesia with etidocaine or bupivacaine. Anesthesiology. 1979;51:285–287.
67. Valenzuela C, Snyders DJ, Bennett PB, et al. Stereoselective block of cardiac sodium channels by bupivacaine in guinea pig ventricular myocytes. Circulation. 1995;92:3014–3024.
68. Mazoit JX, Boico O, Samii K. Myocardial uptake of bupivacaine. II. Pharmacokinetics and pharmacodynamics of bupivacaine enantiomers in the isolated perfused rabbit heart. Anesth Analg. 1993;77:477–482.
69. Santos AC, DeArmas PI. Systemic toxicity of levobupivacaine, bupivacaine, and ropivacaine during continuous intravenous infusion to nonpregnant and pregnant ewes. Anesthesiology. 2001;95:1256–1264.
70. Lyons G, Columb M, Wilson RC, Johnson RV. Epidural pain relief in labour: potencies of levobupivacaine and racemic bupivacaine. Br J Anaesth. 1998;81:899–901.
71. Lacassie HJ, Habib AS, Lacassie HP, Columb MO. Motor blocking minimum local anesthetic concentrations of bupivacaine, levobupivacaine, and ropivacaine in labor. Reg Anesth Pain Med. 2007;32:323–329.
72. Foxall G, McCahon R, Lamb J, et al. Levobupivacaine-induced seizures and cardiovascular collapse treated with Intralipid. Anaesthesia. 2007;62:516–518.
73. Bucklin BA, Warner DS, Choi WW, et al. Pregnancy does not alter the threshold for lidocaine-induced seizures in the rat. Anesth Analg. 1992;74:57–61.
74. Kim YJ, McFarlane C, Warner DS, et al. The effects of plasma and brain magnesium concentrations on lidocaine-induced seizures in the rat. Anesth Analg. 1996;83:1223–1228.
75. Kasten GW, Martin ST. Resuscitation from bupivacaine-induced cardiovascular toxicity during partial inferior vena cava occlusion. Anesth Analg. 1986;65:341–344.
76. Marx GF. Cardiotoxicity of local anesthetics—the plot thickens. Anesthesiology. 1984;60:3–5.
77. Moller RA, Datta S, Fox J, et al. Effects of progesterone on the cardiac electrophysiologic action of bupivacaine and lidocaine. Anesthesiology. 1992;76:604–608.
78. Moller RA, Datta S, Strichartz GR. Beta-estradiol acutely potentiates the depression of cardiac excitability by lidocaine and bupivacaine. J Cardiovasc Pharmacol. 1999;34:718–727.
79. Moller RA, Covino BG. Effect of progesterone on the cardiac electrophysiologic alterations produced by ropivacaine and bupivacaine. Anesthesiology. 1992;77:735–741.
80. Hawkins JL, Chang J, Palmer SK, et al. Anesthesia-related maternal mortality in the United States: 1979-2002. Obstet Gynecol. 2011;117:69–74.
81. Bern S, Weinberg G. Local anesthetic toxicity and lipid resuscitation in pregnancy. Curr Opin Anaesthesiol. 2011;24:262–267.
82. Neal JM, Mulroy MF, Weinberg GL, et al. American Society of Regional Anesthesia and Pain Medicine checklist for managing local anesthetic systemic toxicity: 2012 version. Reg Anesth Pain Med. 2012;37:16–18.
83. de Jong RH, Bonin JD. Benzodiazepines protect mice from local anesthetic convulsions and deaths. Anesth Analg. 1981;60:385–389.
84. Rosen MA, Thigpen JW, Shnider SM, et al. Bupivacaine-induced cardiotoxicity in hypoxic and acidotic sheep. Anesth Analg. 1985;64:1089–1096.
85. Saitoh K, Hirabayashi Y, Shimizu R, Fukuda H. Amrinone is superior to epinephrine in reversing bupivacaine-induced cardiovascular depression in sevoflurane-anesthetized dogs. Anesthesiology. 1995;83:127–133.
86. Vanden Hoek TL, Morrison LJ, Shuster M, et al. Part 12: cardiac arrest in special situations: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122:S829–S861.
87. Weinberg GL, Palmer JW, VadeBoncouer TR, et al. Bupivacaine inhibits acylcarnitine exchange in cardiac mitochondria. Anesthesiology. 2000;92:523–528.
88. Spence AG. Lipid reversal of central nervous system symptoms of bupivacaine toxicity. Anesthesiology. 2007;107:516–517.
89. Rosenblatt MA, Abel M, Fischer GW, et al. Successful use of a 20% lipid emulsion to resuscitate a patient after a presumed bupivacaine-related cardiac arrest. Anesthesiology. 2006;105:217–218.
90. Kennedy RL, Miller RP, Bell JU, et al. Uptake and distribution of bupivacaine in fetal lambs. Anesthesiology. 1986;65:247–253.
91. Kennedy RL, Bell JU, Miller RP, et al. Uptake and distribution of lidocaine in fetal lambs. Anesthesiology. 1990;72:483–489.
92. Covino BG, Marx GF, Finster M, Zsigmond EK. Prolonged sensory/motor deficits following inadvertent spinal anesthesia. Anesth Analg. 1980;59:399–400.
93. Ready LB, Plumer MH, Haschke RH, et al. Neurotoxicity of intrathecal local anesthetics in rabbits. Anesthesiology. 1985;63:364–370.
94. Barsa J, Batra M, Fink BR, Sumi SM. A comparative in vivo study of local neurotoxicity of lidocaine, bupivacaine, 2-chloroprocaine, and a mixture of 2-chloroprocaine and bupivacaine. Anesth Analg. 1982;61:961–967.
95. Gissen A. J. DS, Lambert. The chloroprocaine controversy. II Is chloroprocaine neurotoxic? Reg Anesth. 1984;9:135–145.
96. Fibuch EE, Opper SE. Back pain following epidurally administered Nesacaine-MPF. Anesth Analg. 1989;69:113–115.
97. Taniguchi M, Bollen AW, Drasner K. Sodium bisulfite: scapegoat for chloroprocaine neurotoxicity? Anesthesiology. 2004;100:85–91.
98. Yoos JR, Kopacz DJ. Spinal 2-chloroprocaine for surgery: an initial 10-month experience. Anesth Analg. 2005;100:553–558.
99. Rigler ML, Drasner K, Krejcie TC, et al. Cauda equina syndrome after continuous spinal anesthesia. Anesth Analg. 1991;72:275–281.
100. Schneider M, Ettlin T, Kaufmann M, et al. Transient neurologic toxicity after hyperbaric subarachnoid anesthesia with 5% lidocaine. Anesth Analg. 1993;76:1154–1157.
101. Bainton CR, Strichartz GR. Concentration dependence of lidocaine-induced irreversible conduction loss in frog nerve. Anesthesiology. 1994;81:657–667.
102. Lambert LA, Lambert DH, Strichartz GR. Irreversible conduction block in isolated nerve by high concentrations of local anesthetics. Anesthesiology. 1994;80:1082–1093.
103. Li DF, Bahar M, Cole G, Rosen M. Neurological toxicity of the subarachnoid infusion of bupivacaine, lignocaine or 2-chloroprocaine in the rat. Br J Anaesth. 1985;57:424–429.
104. Dripps RD, Vandam LD. Long-term follow-up of patients who received 10,098 spinal anesthetics: failure to discover major neurological sequelae. J Am Med Assoc. 1954;156:1486–1491.
105. Pollock JE, Liu SS, Neal JM, Stephenson CA. Dilution of spinal lidocaine does not alter the incidence of transient neurologic symptoms. Anesthesiology. 1999;90:445–450.
106. Freedman JM, Li DK, Drasner K, et al. Transient neurologic symptoms after spinal anesthesia: an epidemiologic study of 1,863 patients. Anesthesiology. 1998;89:633–641.
107. Zaric D, Christiansen C, Pace NL, Punjasawadwong Y. Transient neurologic symptoms after spinal anesthesia with lidocaine versus other local anesthetics: a systematic review of randomized, controlled trials. Anesth Analg. 2005;100:1811–1816.
108. Aouad MT, Siddik SS, Jalbout MI, Baraka AS. Does pregnancy protect against intrathecal lidocaine-induced transient neurologic symptoms? Anesth Analg. 2001;92:401–404.
109. Philip J, Sharma SK, Gottumukkala VN, et al. Transient neurologic symptoms after spinal anesthesia with lidocaine in obstetric patients. Anesth Analg. 2001;92:405–409.
110. Bhole MV, Manson AL, Seneviratne SL, Misbah SA. IgE-mediated allergy to local anaesthetics: separating fact from perception: a UK perspective. Br J Anaesth. 2012;108:903–911.
111. Phillips JF, Yates AB, Deshazo RD. Approach to patients with suspected hypersensitivity to local anesthetics. Am J Med Sci. 2007;334:190–196.
112. Incaudo G, Schatz M, Patterson R, et al. Administration of local anesthetics to patients with a history of prior adverse reaction. J Allergy Clin Immunol. 1978;61:339–345.
113. Fisher M. Intradermal testing after anaphylactoid reaction to anaesthetic drugs: practical aspects of performance and interpretation. Anaesth Intensive Care. 1984;12:115–120.
114. Chandler MJ, Grammer LC, Patterson R. Provocative challenge with local anesthetics in patients with a prior history of reaction. J Allergy Clin Immunol. 1987;79:883–886.
115. Palmer CM, Voulgaropoulos D. Management of the parturient with a history of local anesthetic allergy. Anesth Analg. 1993;77:625–628.
116. Levy JH, Yegin A. Anaphylaxis. What is monitored to make a diagnosis? How is therapy monitored? Anesthesiol Clin North America. 2001;19:705–715.
117. Magness RR, Rosenfeld CR. Mechanisms for attenuated pressor responses to alpha-agonists in ovine pregnancy. Am J Obstet Gynecol. 1988;159:252–261.
118. Zucker-Pinchoff B, Ramanathan S. Anaphylactic reaction to epidural fentanyl. Anesthesiology. 1989;71:599–601.
119. Cibils LA. Response of human uterine arteries to local anesthetics. Am J Obstet Gynecol. 1976;126:202–210.
120. Noren H, Lindblom B, Kallfelt B. Effects of bupivacaine and calcium antagonists on human uterine arteries in pregnant and non-pregnant women. Acta Anaesthesiol Scand. 1991;35:488–491.
121. Fishburne JI Jr, Greiss FC Jr, Hopkinson R, Rhyne AL. Responses of the gravid uterine vasculature to arterial levels of local anesthetic agents. Am J Obstet Gynecol. 1979;133:753–761.
122. Santos AC, Karpel B, Noble G. The placental transfer and fetal effects of levobupivacaine, racemic bupivacaine, and ropivacaine. Anesthesiology. 1999;90:1698–1703.
123. Chestnut DH, Weiner CP, Herrig JE. The effect of intravenously administered 2-chloroprocaine upon uterine artery blood flow velocity in gravid guinea pigs. Anesthesiology. 1989;70:305–308.
124. Biehl D, Shnider SM, Levinson G, Callender K. The direct effects of circulating lidocaine on uterine blood flow and foetal well-being in the pregnant ewe. Can Anaesth Soc J. 1977;24:445–451.
125. Gintautas J, Kraynack B, Havasi G, et al. Responses of isolated uterine arteries to local anesthetic agents. Proc West Pharmacol Soc. 1981;24:191–192.
126. Gintautas J, Kraynack BJ, Warren PR, et al. Effects of adenine nucleotides on lidocaine induced contractions in isolated uterine artery. Proc West Pharmacol Soc. 1980;23:299–300.
127. Alahuhta S, Rasanen J, Jouppila P, et al. The effects of epidural ropivacaine and bupivacaine for cesarean section on uteroplacental and fetal circulation. Anesthesiology. 1995;83:23–32.
128. Giles WB, Lah FX, Trudinger BJ. The effect of epidural anaesthesia for caesarean section on maternal uterine and fetal umbilical artery blood flow velocity waveforms. Br J Obstet Gynaecol. 1987;94:55–59.
129. Kopacz DJ, Carpenter RL, Mackey DC. Effect of ropivacaine on cutaneous capillary blood flow in pigs. Anesthesiology. 1989;71:69–74.
130. Tuvemo T, Willdeck-Lund G. Smooth muscle effects of lidocaine, prilocaine, bupivacaine and etiodocaine on the human umbilical artery. Acta Anaesthesiol Scand. 1982;26:104–107.
131. Noren H, Kallfelt B, Lindblom B. Influence of bupivacaine and morphine on human umbilical arteries and veins in vitro. Acta Obstet Gynecol Scand. 1990;69:87–91.
132. Halevy S, Rossner KL, Liu-Barnett M, et al. The response of umbilical vessels, with and without vascular endothelium, to local anesthesia in low pO2 and hypercarbia. Reg Anesth. 1995;20:316–322.
133. Morishima HO, Heymann MA, Rudolph AM, et al. Transfer of lidocaine across the sheep placenta to the fetus: hemodynamic and acid-base responses of the fetal lamb. Am J Obstet Gynecol. 1975;122:581–588.
134. Marx GF, Elstein ID, Schuss M, et al. Effects of epidural block with lignocaine and lignocaine-adrenaline on umbilical artery velocity wave ratios. Br J Obstet Gynaecol. 1990;97:517–520.
135. Marx GF, Patel S, Berman JA, et al. Umbilical blood flow velocity waveforms in different maternal positions and with epidural analgesia. Obstet Gynecol. 1986;68:61–64.
136. Lindblad A, Marsal K, Vernersson E, Renck H. Fetal circulation during epidural analgesia for caesarean section. Br Med J (Clin Res Ed). 1984;288:1329–1330.
137. McGaughey HS Jr, Corey EL, Eastwood D, Thornton WN. Effect of synthetic anesthetics on the spontaneous motility of human uterine muscle in vitro. Obstet Gynecol. 1962;19:233–240.
138. Morishima HO, Covino BG, Yeh MN, et al. Bradycardia in the fetal baboon following paracervical block anesthesia. Am J Obstet Gynecol. 1981;140:775–780.
139. Belitzky R, Delard LG, Novick LM. Oxytocic effect of intramyometrial injection of procaine in a pregnant woman. Am J Obstet Gynecol. 1970;107:973–975.
140. Kaynak G, Iskender A, Albayrak M, et al. In vivo comparison of the effects of bupivacaine and levobupivacaine on the pregnant rat myometrium using electrohysterogram. Gynecol Obstet Invest. 2012;73:43–47.
141. Camann WR, Hartigan PM, Gilbertson LI, et al. Chloroprocaine antagonism of epidural opioid analgesia: a receptor-specific phenomenon? Anesthesiology. 1990;73:860–863.
142. Grice SC, Eisenach JC, Dewan DM. Labor analgesia with epidural bupivacaine plus fentanyl: enhancement with epinephrine and inhibition with 2-chloroprocaine. Anesthesiology. 1990;72:623–628.
143. Coda B, Bausch S, Haas M, Chavkin C. The hypothesis that antagonism of fentanyl analgesia by 2-chloroprocaine is mediated by direct action on opioid receptors. Reg Anesth. 1997;22:43–52.
144. Toledo P, McCarthy RJ, Ebarvia MJ, et al. The interaction between epidural 2-chloroprocaine and morphine: a randomized controlled trial of the effect of drug administration timing on the efficacy of morphine analgesia. Anesth Analg. 2009;109:168–173.
145. Hess PE, Snowman CE, Hahn CJ, et al. Chloroprocaine may not affect epidural morphine for postcesarean delivery analgesia. J Clin Anesth. 2006;18:29–33.
146. Cohen SE, Thurlow A. Comparison of a chloroprocaine–bupivacaine mixture with chloroprocaine and bupivacaine used individually for obstetric epidural analgesia. Anesthesiology. 1979;51:288–292.
147. Corke BC, Carlson CG, Dettbarn WD. The influence of 2-chloroprocaine on the subsequent analgesic potency of bupivacaine. Anesthesiology. 1984;60:25–27.
148. Chestnut DH, Geiger M, Bates JN, Choi WW. The influence of pH-adjusted 2-chloroprocaine on the quality and duration of subsequent epidural bupivacaine analgesia during labor: a randomized, double-blind study. Anesthesiology. 1989;70:437–441.
149. Atkinson Ralls L, Drover DR, Clavijo CF, Carvalho B. Prior epidural lidocaine alters the pharmacokinetics and drug effects of extended-release epidural morphine (DepoDur®) after cesarean delivery. Anesth Analg. 2011;113:251–258.
150. van Kleef JW, Veering BT, Burm AG. Spinal anesthesia with ropivacaine: a double-blind study on the efficacy and safety of 0.5% and 0.75% solutions in patients undergoing minor lower limb surgery. Anesth Analg. 1994;78:1125–1130.
151. Coppejans HC, Vercauteren MP. Low-dose combined spinal-epidural anesthesia for cesarean delivery: a comparison of three plain local anesthetics. Acta Anaesthesiol Belg. 2006;57:39–43.
152. D'Angelo R, James RL. Is ropivacaine less potent than bupivacaine? Anesthesiology. 1999;90:941–943.
153. Polley LS, Columb MO, Naughton NN, et al. Relative analgesic potencies of levobupivacaine and ropivacaine for epidural analgesia in labor. Anesthesiology. 2003;99:1354–1358.
154. Benhamou D, Ghosh C, Mercier FJ. A randomized sequential allocation study to determine the minimum effective analgesic concentration of levobupivacaine and ropivacaine in patients receiving epidural analgesia for labor. Anesthesiology. 2003;99:1383–1386.
155. Vercauteren MP, Hans G, De Decker K, Adriaensen HA. Levobupivacaine combined with sufentanil and epinephrine for intrathecal labor analgesia: a comparison with racemic bupivacaine. Anesth Analg. 2001;93:996–1000.
156. Beilin Y, Guinn NR, Bernstein HH, et al. Local anesthetics and mode of delivery: bupivacaine versus ropivacaine versus levobupivacaine. Anesth Analg. 2007;105:756–763.
157. Bader AM, Tsen LC, Camann WR, et al. Clinical effects and maternal and fetal plasma concentrations of 0.5% epidural levobupivacaine versus bupivacaine for cesarean delivery. Anesthesiology. 1999;90:1596–1601.
158. Syme MR, Paxton JW, Keelan JA. Drug transfer and metabolism by the human placenta. Clin Pharmacokinet. 2004;43:487–514.
159. Tucker GT, Mather LE. Properties, absorption, and disposition of local anesthetic agents. Cousins MJ, Bridenbaugh PO. Neural Blockade in Clinical Anesthesia and Management of Pain. Lippincott-Raven: Philadelphia; 1998:55–96.
160. Ueki R, Tatara T, Kariya N, et al. Comparison of placental transfer of local anesthetics in perfusates with different pH values in a human cotyledon model. J Anesth. 2009;23:526–529.
161. Biehl D, Shnider SM, Levinson G, Callender K. Placental transfer of lidocaine: effects of fetal acidosis. Anesthesiology. 1978;48:409–412.
162. Brown WU Jr, Bell GC, Alper MH. Acidosis, local anesthetics, and the newborn. Obstet Gynecol. 1976;48:27–30.
163. Morishima HO, Covino BG. Toxicity and distribution of lidocaine in nonasphyxiated and asphyxiated baboon fetuses. Anesthesiology. 1981;54:182–186.
164. Hamshaw-Thomas A, Reynolds F. Placental transfer of bupivacaine, pethidine and lignocaine in the rabbit: effect of umbilical flow rate and protein content. Br J Obstet Gynaecol. 1985;92:706–713.
165. Hamshaw-Thomas A, Rogerson N, Reynolds F. Transfer of bupivacaine, lignocaine and pethidine across the rabbit placenta: influence of maternal protein binding and fetal flow. Placenta. 1984;5:61–70.
166. Vella LM, Knott C, Reynolds F. Transfer of fentanyl across the rabbit placenta. Effect of umbilical flow and concurrent drug administration. Br J Anaesth. 1986;58:49–54.
167. Petersen MC, Moore RG, Nation RL, McMeniman W. Relationship between the transplacental gradients of bupivacaine and alpha 1-acid glycoprotein. Br J Clin Pharmacol. 1981;12:859–862.
168. Thomas J, Long G, Moore G, Morgan D. Plasma protein binding and placental transfer of bupivacaine. Clin Pharmacol Ther. 1976;19:426–434.
169. Johnson RF, Cahana A, Olenick M, et al. A comparison of the placental transfer of ropivacaine versus bupivacaine. Anesth Analg. 1999;89:703–708.
170. Petrie RH, Paul WL, Miller FC, et al. Placental transfer of lidocaine following paracervical block. Am J Obstet Gynecol. 1974;120:791–801.
171. Mazze RI, Dunbar RW. Plasma lidocaine concentrations after caudal, lumbar epidural, axillary block, and intravenous regional anesthesia. Anesthesiology. 1966;27:574–579.
172. Giasi RM, D'Agostino E, Covino BG. Absorption of lidocaine following subarachnoid and epidural administration. Anesth Analg. 1979;58:360–363.
173. Idanpaan-Heikkila JE, Taska RJ, Allen HA, Schoolar JC. Placental transfer of diazepam-14 C in mice, hamsters and monkeys. J Pharmacol Exp Ther. 1971;176:752–757.
174. Shnider SM, Way EL. The kinetics of transfer of lidocaine (Xylocaine) across the human placenta. Anesthesiology. 1968;29:944–950.
175. Roe DA, Little BB, Bawdon RE, Gilstrap LC 3rd. Metabolism of cocaine by human placentas: implications for fetal exposure. Am J Obstet Gynecol. 1990;163:715–718.
176. Van Petten GR, Hirsch GH, Cherrington AD. Drug-metabolizing activity of the human placenta. Can J Biochem. 1968;46:1057–1061.
177. Sturrock JE, Nunn JF. Cytotoxic effects of procaine, lignocaine and bupivacaine. Br J Anaesth. 1979;51:273–281.
178. Lee H, Bush KT, Nagele RG. Time-lapse photographic study of neural tube closure defects caused by xylocaine in the chick. Teratology. 1988;37:263–269.
179. Lee H, Nagele RG. Neural tube defects caused by local anesthetics in early chick embryos. Teratology. 1985;31:119–127.
180. Anderson PL, Bamburg JR. Effects of local anesthetics on nerve growth in culture. Dev Neurosci. 1981;4:273–290.
181. O'Shea KS, Kaufman MH. Neural tube closure defects following in vitro exposure of mouse embryos to Xylocaine. J Exp Zool. 1980;214:235–238.
182. Stygall K, Mirsky R, Mowbray J. The effect of local anaesthetics and barbiturates on myogenesis and myotube integrity in rat skeletal muscle cultures. J Cell Sci. 1979;37:231–241.
183. Ramazzotto LJ, Curro FA, Paterson JA, et al. Toxicological assessment of lidocaine in the pregnant rat. J Dent Res. 1985;64:1214–1217.
184. Fujinaga M, Mazze RI. Reproductive and teratogenic effects of lidocaine in Sprague-Dawley rats. Anesthesiology. 1986;65:626–632.
185. Martin LV, Jurand A. The absence of teratogenic effects of some analgesics used in anaesthesia: additional evidence from a mouse model. Anaesthesia. 1992;47:473–476.
186. Smith RF, Wharton GG, Kurtz SL, et al. Behavioral effects of mid-pregnancy administration of lidocaine and mepivacaine in the rat. Neurobehav Toxicol Teratol. 1986;8:61–68.
187. Smith RF, Kurkjian MF, Mattran KM, Kurtz SL. Behavioral effects of prenatal exposure to lidocaine in the rat: effects of dosage and of gestational age at administration. Neurotoxicol Teratol. 1989;11:395–403.
188. Heinonen OP, Slone D, Shapiro S. Birth Defects and Drugs in Pregnancy. Publishing Sciences Group: Littleton, MA; 1977.
189. Friedman JM. Teratogen update: anesthetic agents. Teratology. 1988;37:69–77.
190. Fletcher S, Carson R, Reynolds F, et al. Plasma total and free concentrations of bupivacaine and lignocaine in mother and fetus following epidural administration, singly or together. Int J Obstet Anesth. 1992;1:135–140.
191. Tucker GT, Boyes RN, Bridenbaugh PO, Moore DC. Binding of anilide-type local anesthetics in human plasma. II. Implications in vivo, with special reference to transplacental distribution. Anesthesiology. 1970;33:304–314.
192. Ehrnebo M, Agurell S, Jalling B, Boreus LO. Age differences in drug binding by plasma proteins: studies on human foetuses, neonates and adults. Eur J Clin Pharmacol. 1971;3:189–193.
193. Finster M, Morishima HO, Boyes RN, Covino BG. The placental transfer of lidocaine and its uptake by fetal tissues. Anesthesiology. 1972;36:159–163.
194. Morishima HO, Santos AC, Pedersen H, et al. Effect of lidocaine on the asphyxial responses in the mature fetal lamb. Anesthesiology. 1987;66:502–507.
195. Blankenbaker WL, DiFazio CA, Berry FA Jr. Lidocaine and its metabolites in the newborn. Anesthesiology. 1975;42:325–330.
196. Mihaly GW, Moore RG, Thomas J, et al. The pharmacokinetics and metabolism of the anilide local anaesthetics in neonates. I. Lignocaine. Eur J Clin Pharmacol. 1978;13:143–152.
197. Morishima HO, Finster M, Pedersen H, et al. Pharmacokinetics of lidocaine in fetal and neonatal lambs and adult sheep. Anesthesiology. 1979;50:431–436.
198. Brown WU, Bell GC, Lurie AO, et al. Newborn blood levels of lidocaine and mepivacaine in the first postnatal day following maternal epidural anesthesia. Anesthesiology. 1975;42:698–707.
199. Lieberman BA, Rosenblatt DB, Belsey E, et al. The effects of maternally administered pethidine or epidural bupivacaine on the fetus and newborn. Br J Obstet Gynaecol. 1979;86:598–606.
200. Finster M, Poppers PJ, Sinclair JC, et al. Accidental intoxication of the fetus with local anesthetic drug during caudal anesthesia. Am J Obstet Gynecol. 1965;92:922–924.
201. Morishima HO, Pedersen H, Finster M, et al. Toxicity of lidocaine in adult, newborn, and fetal sheep. Anesthesiology. 1981;55:57–61.
202. Bosnjak ZJ, Stowe DF, Kampine JP. Comparison of lidocaine and bupivacaine depression of sinoatrial nodal activity during hypoxia and acidosis in adult and neonatal guinea pigs. Anesth Analg. 1986;65:911–917.
203. Badgwell JM, Heavner JE, Kytta J. Bupivacaine toxicity in young pigs is age-dependent and is affected by volatile anesthetics. Anesthesiology. 1990;73:297–303.
204. Loftus JR, Holbrook RH, Cohen SE. Fetal heart rate after epidural lidocaine and bupivacaine for elective cesarean section. Anesthesiology. 1991;75:406–412.
205. Hehre FW, Hook R, Hon EH. Continuous lumbar peridural anesthesia in obstetrics. VI. The fetal effects of transplacental passage of local anesthetic agents. Anesth Analg. 1969;48:909–913.
206. Abboud TK, Afrasiabi A, Sarkis F, et al. Continuous infusion epidural analgesia in parturients receiving bupivacaine, chloroprocaine, or lidocaine—maternal, fetal, and neonatal effects. Anesth Analg. 1984;63:421–428.
207. Becker JH, Schaap TP, Westerhuis ME, et al. Intrapartum epidural analgesia and ST analysis of the fetal electrocardiogram. Acta Obstet Gynecol Scand. 2011;90:1364–1370.
208. Kuhnert BR, Harrison MJ, Linn PL, Kuhnert PM. Effects of maternal epidural anesthesia on neonatal behavior. Anesth Analg. 1984;63:301–308.
209. Halpern SH, Littleford JA, Brockhurst NJ, et al. The neurologic and adaptive capacity score is not a reliable method of newborn evaluation. Anesthesiology. 2001;94:958–962.
210. Campbell N, Harvey D, Norman AP. Increased frequency of neonatal jaundice in a maternity hospital. Br Med J. 1975;2:548–552.
211. Clark DA, Landaw SA. Bupivacaine alters red blood cell properties: a possible explanation for neonatal jaundice associated with maternal anesthesia. Pediatr Res. 1985;19:341–343.
212. Gale R, Ferguson JE 2nd, Stevenson DK. Effect of epidural analgesia with bupivacaine hydrochloride on neonatal bilirubin production. Obstet Gynecol. 1987;70:692–695.
213. Alkan S, Tiras U, Dallar Y, Sunay D. Effect of anaesthetic agents administered to the mothers on transcutaneous bilirubin levels in the neonates. Acta Paediatr. 2010;99:993–996.
214. Smedstad KG, Morison DH, Harris WH, Pascoe P. Placental transfer of local anaesthetics in the premature sheep fetus. Int J Obstet Anesth. 1993;2:34–38.
215. Morishima HO, Pedersen H, Santos AC, et al. Adverse effects of maternally administered lidocaine on the asphyxiated preterm fetal lamb. Anesthesiology. 1989;71:110–115.
216. Santos AC, Yun EM, Bobby PD, et al. The effects of bupivacaine, L-nitro-L-arginine-methyl ester, and phenylephrine on cardiovascular adaptations to asphyxia in the preterm fetal lamb. Anesth Analg. 1997;85:1299–1306.
217. Johnson RF, Herman NL, Johnson HV, et al. Effects of fetal pH on local anesthetic transfer across the human placenta. Anesthesiology. 1996;85:608–615.
218. Snyder SH. Opiate receptors and internal opiates. Sci Am. 1977;236:44–56.
219. Beckett AH. Analgesics and their antagonists: some steric and chemical considerations. I. The dissociation constants of some tertiary amines and synthetic analgesics, the conformations of methadone-type compounds. J Pharm Pharmacol. 1956;8:848–859.
220. Thorpe DH. Opiate structure and activity—a guide to understanding the receptor. Anesth Analg. 1984;63:143–151.
221. Portoghese PS, Alreja BD, Larson DL. Allylprodine analogues as receptor probes: evidence that phenolic and nonphenolic ligands interact with different subsites on identical opioid receptors. J Med Chem. 1981;24:782–787.
222. Gorin FA, Balasubramanian TM, Cicero TJ, et al. Novel analogues of enkephalin: identification of functional groups required for biological activity. J Med Chem. 1980;23:1113–1122.
223. Wang JK, Nauss LA, Thomas JE. Pain relief by intrathecally applied morphine in man. Anesthesiology. 1979;50:149–151.
224. Yaksh TL. Spinal opiate analgesia: characteristics and principles of action. Pain. 1981;11:293–346.
225. Yaksh TL, Wallace MS. Opioids, analgesia, and pain management. Brunton LL. Goodman and Gilman's The Pharmacologic Basis of Therapeutics. 12th edition. McGraw-Hill Professional: New York; 2011:481–526.
226. Al-Hasani R, Bruchas MR. Molecular mechanisms of opioid receptor-dependent signaling and behavior. Anesthesiology. 2011;115:1363–1381.
227. Fioravanti B, Vanderah TW. The ORL-1 receptor system: are there opportunities for antagonists in pain therapy? Curr Top Med Chem. 2008;8:1442–1451.
228. Pasternak GW. Multiple opiate receptors: deja vu all over again. Neuropharmacology. 2004;47(Suppl 1):312–323.
229. Riviere PJ. Peripheral kappa-opioid agonists for visceral pain. Br J Pharmacol. 2004;141:1331–1334.
230. Porreca F, Heyman JS, Mosberg HI, et al. Role of mu and delta receptors in the supraspinal and spinal analgesic effects of [D-Pen2, D-Pen5]enkephalin in the mouse. J Pharmacol Exp Ther. 1987;241:393–400.
231. Bernards CM, Hill HF. The spinal nerve root sleeve is not a preferred route for redistribution of drugs from the epidural space to the spinal cord. Anesthesiology. 1991;75:827–832.
232. Bernards CM, Hill HF. Physical and chemical properties of drug molecules governing their diffusion through the spinal meninges. Anesthesiology. 1992;77:750–756.
233. Bernards CM, Sorkin LS. Radicular artery blood flow does not redistribute fentanyl from the epidural space to the spinal cord. Anesthesiology. 1994;80:872–878.
234. Bernards CM. Understanding the physiology and pharmacology of epidural and intrathecal opioids. Best Pract Res Clin Anaesthesiol. 2002;16:489–505.
235. Bernards CM, Hill HF. Morphine and alfentanil permeability through the spinal dura, arachnoid, and pia mater of dogs and monkeys. Anesthesiology. 1990;73:1214–1219.
236. Bernards CM, Shen DD, Sterling ES, et al. Epidural, cerebrospinal fluid, and plasma pharmacokinetics of epidural opioids. I. Differences among opioids. Anesthesiology. 2003;99:455–465.
237. Coda BA, Brown MC, Schaffer RL, et al. A pharmacokinetic approach to resolving spinal and systemic contributions to epidural alfentanil analgesia and side-effects. Pain. 1995;62:329–337.
238. Coda BA, Brown MC, Risler L, et al. Equivalent analgesia and side effects during epidural and pharmacokinetically tailored intravenous infusion with matching plasma alfentanil concentration. Anesthesiology. 1999;90:98–108.
239. Miguel R, Barlow I, Morrell M, et al. A prospective, randomized, double-blind comparison of epidural and intravenous sufentanil infusions. Anesthesiology. 1994;81:346–352.
240. Liu SS, Gerancher JC, Bainton BG, et al. The effects of electrical stimulation at different frequencies on perception and pain in human volunteers: epidural versus intravenous administration of fentanyl. Anesth Analg. 1996;82:98–102.
241. D'Angelo R, Gerancher JC, Eisenach JC, Raphael BL. Epidural fentanyl produces labor analgesia by a spinal mechanism. Anesthesiology. 1998;88:1519–1523.
242. Ginosar Y, Riley ET, Angst MS. The site of action of epidural fentanyl in humans: the difference between infusion and bolus administration. Anesth Analg. 2003;97:1428–1438.
243. Ummenhofer WC, Arends RH, Shen DD, Bernards CM. Comparative spinal distribution and clearance kinetics of intrathecally administered morphine, fentanyl, alfentanil, and sufentanil. Anesthesiology. 2000;92:739–753.
244. Eisenach JC, Hood DD, Curry R, Shafer SL. Cephalad movement of morphine and fentanyl in humans after intrathecal injection. Anesthesiology. 2003;99:166–173.
245. Lu JK, Schafer PG, Gardner TL, et al. The dose-response pharmacology of intrathecal sufentanil in female volunteers. Anesth Analg. 1997;85:372–379.
246. Carvalho B, Riley E, Cohen SE, et al. Single-dose, sustained-release epidural morphine in the management of postoperative pain after elective cesarean delivery: results of a multicenter randomized controlled study. Anesth Analg. 2005;100:1150–1158.
247. Carvalho B, Roland LM, Chu LF, et al. Single-dose, extended-release epidural morphine (DepoDur) compared to conventional epidural morphine for post-cesarean pain. Anesth Analg. 2007;105:176–183.
248. Horlocker TT, Burton AW, Connis RT, et al. American Society of Anesthesiologists: Practice guidelines for the prevention, detection, and management of respiratory depression associated with neuraxial opioid administration. Anesthesiology. 2009;110:218–230.
249. Gerancher JC, Nagle PC. Management of accidental spinal administration of extended-release epidural morphine. Anesthesiology. 2008;108:1147–1149.
250. Ikeda K, Ide S, Han W, et al. How individual sensitivity to opiates can be predicted by gene analyses. Trends Pharmacol Sci. 2005;26:311–317.
251. Walter C, Lötsch J. Meta-analysis of the relevance of the OPRM1 118A>G genetic variant for pain treatment. Pain. 2009;146:270–275.
252. Landau R, Kern C, Columb MO, et al. Genetic variability of the mu-opioid receptor influences intrathecal fentanyl analgesia requirements in laboring women. Pain. 2008;139:5–14.
253. Wong CA, McCarthy RJ, Blouin J, Landau R. Observational study of the effect of mu-opioid receptor genetic polymorphism on intrathecal opioid labor analgesia and post-cesarean delivery analgesia. Int J Obstet Anesth. 2010;19:246–253.
254. Camorcia M, Capogna G, Stirparo S, et al. Effect of mu-opioid receptor A118G polymorphism on the ED50 of epidural sufentanil for labor analgesia. Int J Obstet Anesth. 2012;21:40–44.
255. Sia AT, Lim Y, Lim EC, et al. A118G single nucleotide polymorphism of human mu-opioid receptor gene influences pain perception and patient-controlled intravenous morphine consumption after intrathecal morphine for postcesarean analgesia. Anesthesiology. 2008;109:520–526.
256. Tan EC, Lim EC, Teo YY, et al. Ethnicity and OPRM variant independently predict pain perception and patient-controlled analgesia usage for post-operative pain. Mol Pain. 2009;5:32.
257. Wong CA. The promise of pharmacogenetics in labor analgesia … tantalizing, but not there yet. Int J Obstet Anesth. 2012;21:105–108.
258. Yaksh TL, Collins JG. Studies in animals should precede human use of spinally administered drugs. Anesthesiology. 1989;70:4–6.
259. Abouleish E, Barmada MA, Nemoto EM, et al. Acute and chronic effects of intrathecal morphine in monkeys. Br J Anaesth. 1981;53:1027–1032.
260. Power I, Brown DT, Wildsmith JA. The effect of fentanyl, meperidine and diamorphine on nerve conduction in vitro. Reg Anesth. 1991;16:204–208.
261. Yaksh TL, Noueihed RY, Durant PA. Studies of the pharmacology and pathology of intrathecally administered 4-anilinopiperidine analogues and morphine in the rat and cat. Anesthesiology. 1986;64:54–66.
262. Sabbe MB, Grafe MR, Mjanger E, et al. Spinal delivery of sufentanil, alfentanil, and morphine in dogs: physiologic and toxicologic investigations. Anesthesiology. 1994;81:899–920.
263. Rawal N, Nuutinen L, Raj PP, et al. Behavioral and histopathologic effects following intrathecal administration of butorphanol, sufentanil, and nalbuphine in sheep. Anesthesiology. 1991;75:1025–1034.
264. Cohen SE, Cherry CM, Holbrook RH Jr, et al. Intrathecal sufentanil for labor analgesia—sensory changes, side effects, and fetal heart rate changes. Anesth Analg. 1993;77:1155–1160.
265. Scavone BM. Altered level of consciousness after combined spinal-epidural labor analgesia with intrathecal fentanyl and bupivacaine. Anesthesiology. 2002;96:1021–1022.
266. Fragneto RY, Fisher A. Mental status change and aphasia after labor analgesia with intrathecal sufentanil/bupivacaine. Anesth Analg. 2000;90:1175–1176.
267. Hamilton CL, Cohen SE. High sensory block after intrathecal sufentanil for labor analgesia. Anesthesiology. 1995;83:1118–1121.
268. Currier DS, Levin KR, Campbell C. Dysphagia with intrathecal fentanyl. Anesthesiology. 1997;87:1570–1571.
269. Riley ET, Ratner EF, Cohen SE. Intrathecal sufentanil for labor analgesia: do sensory changes predict better analgesia and greater hypotension? Anesth Analg. 1997;84:346–351.
270. Chaney MA. Side effects of intrathecal and epidural opioids. Can J Anaesth. 1995;42:891–903.
271. Norris MC, Grieco WM, Borkowski M, et al. Complications of labor analgesia: epidural versus combined spinal epidural techniques. Anesth Analg. 1994;79:529–537.
272. Mishriky BM, Habib AS. Metoclopramide for nausea and vomiting prophylaxis during and after Caesarean delivery: a systematic review and meta-analysis. Br J Anaesth. 2012;108:374–383.
273. Harnett MJ, O'Rourke N, Walsh M, et al. Transdermal scopolamine for prevention of intrathecal morphine-induced nausea and vomiting after cesarean delivery. Anesth Analg. 2007;105:764–769.
274. George RB, Allen TK, Habib AS. Serotonin receptor antagonists for the prevention and treatment of pruritus, nausea, and vomiting in women undergoing cesarean delivery with intrathecal morphine: a systematic review and meta-analysis. Anesth Analg. 2009;109:174–182.
275. Nortcliffe SA, Shah J, Buggy DJ. Prevention of postoperative nausea and vomiting after spinal morphine for Caesarean section: comparison of cyclizine, dexamethasone and placebo. Br J Anaesth. 2003;90:665–670.
276. Allen TK, Jones CA, Habib AS. Dexamethasone for the prophylaxis of postoperative nausea and vomiting associated with neuraxial morphine administration: a systematic review and meta-analysis. Anesth Analg. 2012;114:813–822.
277. Abboud TK, Shnider SM, Dailey PA, et al. Intrathecal administration of hyperbaric morphine for the relief of pain in labour. Br J Anaesth. 1984;56:1351–1360.
278. Lyons G, Columb M, Hawthorne L, Dresner M. Extradural pain relief in labour: bupivacaine sparing by extradural fentanyl is dose dependent. Br J Anaesth. 1997;78:493–497.
279. Wong CA, Scavone BM, Loffredi M, et al. The dose-response of intrathecal sufentanil added to bupivacaine for labor analgesia. Anesthesiology. 2000;92:1553–1558.
280. Bernard JM, Le Roux D, Barthe A, et al. The dose-range effects of sufentanil added to 0.125% bupivacaine on the quality of patient-controlled epidural analgesia during labor. Anesth Analg. 2001;92:184–188.
281. Asokumar B, Newman LM, McCarthy RJ, et al. Intrathecal bupivacaine reduces pruritus and prolongs duration of fentanyl analgesia during labor: a prospective, randomized controlled trial. Anesth Analg. 1998;87:1309–1315.
282. Ganesh A, Maxwell LG. Pathophysiology and management of opioid-induced pruritus. Drugs. 2007;67:2323–2333.
283. Ikoma A, Rukwied R, Stander S, et al. Neurophysiology of pruritus: interaction of itch and pain. Arch Dermatol. 2003;139:1475–1478.
284. Zeitz KP, Guy N, Malmberg AB, et al. The 5-HT3 subtype of serotonin receptor contributes to nociceptive processing via a novel subset of myelinated and unmyelinated nociceptors. J Neurosci. 2002;22:1010–1019.
285. Arcioni R, della Rocca M, Romano S, et al. Ondansetron inhibits the analgesic effects of tramadol: a possible 5-HT3 spinal receptor involvement in acute pain in humans. Anesth Analg. 2002;94:1553–1557.
286. Fan P. Nonopioid mechanism of morphine modulation of the activation of 5-hydroxytryptamine type 3 receptors. Mol Pharmacol. 1995;47:491–495.
287. Iatrou CA, Dragoumanis CK, Vogiatzaki TD, et al. Prophylactic intravenous ondansetron and dolasetron in intrathecal morphine-induced pruritus: a randomized, double-blinded, placebo-controlled study. Anesth Analg. 2005;101:1516–1520.
288. Morgan PJ, Mehta S, Kapala DM. Nalbuphine pretreatment in cesarean section patients receiving epidural morphine. Reg Anesth. 1991;16:84–88.
289. Cohen SE, Ratner EF, Kreitzman TR, et al. Nalbuphine is better than naloxone for treatment of side effects after epidural morphine. Anesth Analg. 1992;75:747–752.
290. Davies GG, From R. A blinded study using nalbuphine for prevention of pruritus induced by epidural fentanyl. Anesthesiology. 1988;69:763–765.
291. Beilin Y, Bernstein HH, Zucker-Pinchoff B, et al. Subhypnotic doses of propofol do not relieve pruritus induced by intrathecal morphine after cesarean section. Anesth Analg. 1998;86:310–313.
292. Riley ET, Walker D, Hamilton CL, Cohen SE. Intrathecal sufentanil for labor analgesia does not cause a sympathectomy. Anesthesiology. 1997;87:874–878.
293. Cascio M, Pygon B, Bernett C, Ramanathan S. Labour analgesia with intrathecal fentanyl decreases maternal stress. Can J Anaesth. 1997;44:605–609.
294. Wang C, Chakrabarti MK, Whitwam JG. Specific enhancement by fentanyl of the effects of intrathecal bupivacaine on nociceptive afferent but not on sympathetic efferent pathways in dogs. Anesthesiology. 1993;79:766–773.
295. Glynn CJ, Mather LE, Cousins MJ, et al. Spinal narcotics and respiratory depression. Lancet. 1979;2:356–357.
296. Palmer CM, Emerson S, Volgoropolous D, Alves D. Dose-response relationship of intrathecal morphine for postcesarean analgesia. Anesthesiology. 1999;90:437–444.
297. Palmer CM, Nogami WM, Van Maren G, Alves DM. Postcesarean epidural morphine: a dose-response study. Anesth Analg. 2000;90:887–891.
298. Gambling D, Hughes T, Martin G, et al. A comparison of Depodur, a novel, single-dose extended-release epidural morphine, with standard epidural morphine for pain relief after lower abdominal surgery. Anesth Analg. 2005;100:1065–1074.
299. Viscusi ER, Martin G, Hartrick CT, et al. Forty-eight hours of postoperative pain relief after total hip arthroplasty with a novel, extended-release epidural morphine formulation. Anesthesiology. 2005;102:1014–1022.
300. Greenhalgh CA. Respiratory arrest in a parturient following intrathecal injection of sufentanil and bupivacaine. Anaesthesia. 1996;51:173–175.
301. Katsiris S, Williams S, Leighton BL, Halpern S. Respiratory arrest following intrathecal injection of sufentanil and bupivacaine in a parturient. Can J Anaesth. 1998;45:880–883.
302. Brockway MS, Noble DW, Sharwood-Smith GH, McClure JH. Profound respiratory depression after extradural fentanyl. Br J Anaesth. 1990;64:243–245.
303. Lu JK, Manullang TR, Staples MH, et al. Maternal respiratory arrests, severe hypotension, and fetal distress after administration of intrathecal, sufentanil, and bupivacaine after intravenous fentanyl. Anesthesiology. 1997;87:170–172.
304. Ferouz F, Norris MC, Leighton BL. Risk of respiratory arrest after intrathecal sufentanil. Anesth Analg. 1997;85:1088–1090.
305. Jaffee JB, Drease GE, Kelly T, Newman LM. Severe respiratory depression in the obstetric patient after intrathecal meperidine or sufentanil. Int J Obstet Anesth. 1997;6:182–184.
306. Rawal N, Mollefors K, Axelsson K, et al. An experimental study of urodynamic effects of epidural morphine and of naloxone reversal. Anesth Analg. 1983;62:641–647.
307. O'Sullivan GM, Sutton AJ, Thompson SA, et al. Noninvasive measurement of gastric emptying in obstetric patients. Anesth Analg. 1987;66:505–511.
308. Kelly MC, Carabine UA, Hill DA, Mirakhur RK. A comparison of the effect of intrathecal and extradural fentanyl on gastric emptying in laboring women. Anesth Analg. 1997;85:834–838.
309. Stagno S, Whitley RJ. Herpesvirus infections of pregnancy. II. Herpes simplex virus and varicella-zoster virus infections. N Engl J Med. 1985;313:1327–1330.
310. Bauchat JR. Focused review: neuraxial morphine and oral herpes reactivation in the obstetric population. Anesth Analg. 2010;111:1238–1241.
311. Crone LA, Conly JM, Clark KM, et al. Recurrent herpes simplex virus labialis and the use of epidural morphine in obstetric patients. Anesth Analg. 1988;67:318–323.
312. Crone LA, Conly JM, Storgard C, et al. Herpes labialis in parturients receiving epidural morphine following cesarean section. Anesthesiology. 1990;73:208–213.
313. Boyle RK. Herpes simplex labialis after epidural or parenteral morphine: a randomized prospective trial in an Australian obstetric population. Anaesth Intensive Care. 1995;23:433–437.
314. Davies PW, Vallejo MC, Shannon KT, et al. Oral herpes simplex reactivation after intrathecal morphine: a prospective randomized trial in an obstetric population. Anesth Analg. 2005;100:1472–1476.
315. Valley MA, Bourke DL, McKenzie AM. Recurrence of thoracic and labial herpes simplex virus infection in a patient receiving epidural fentanyl. Anesthesiology. 1992;76:1056–1057.
316. Acalovschi I. Herpes simplex after spinal pethidine. Anaesthesia. 1986;41:1271–1272.
317. Gieraerts R, Navalgund A, Vaes L, et al. Increased incidence of itching and herpes simplex in patients given epidural morphine after cesarean section. Anesth Analg. 1987;66:1321–1324.
318. Scott PV, Fischer HB. Spinal opiate analgesia and facial pruritus: a neural theory. Postgrad Med J. 1982;58:531–535.
319. Smith CV, Rayburn WF, Allen KV, et al. Influence of intravenous fentanyl on fetal biophysical parameters during labor. J Matern Fetal Med. 1996;5:89–92.
320. Rayburn WF, Smith CV, Parriott JE, Woods RE. Randomized comparison of meperidine and fentanyl during labor. Obstet Gynecol. 1989;74:604–606.
321. Bader AM, Fragneto R, Terui K, et al. Maternal and neonatal fentanyl and bupivacaine concentrations after epidural infusion during labor. Anesth Analg. 1995;81:829–832.
322. Porter J, Bonello E, Reynolds F. Effect of epidural fentanyl on neonatal respiration. Anesthesiology. 1998;89:79–85.
323. Elliott RD. Continuous infusion epidural analgesia for obstetrics: bupivacaine versus bupivacaine-fentanyl mixture. Can J Anaesth. 1991;38:303–310.
324. Reynolds F, Sharma SK, Seed PT. Analgesia in labour and fetal acid-base balance: a meta-analysis comparing epidural with systemic opioid analgesia. BJOG. 2002;109:1344–1353.
325. Viscomi CM, Hood DD, Melone PJ, Eisenach JC. Fetal heart rate variability after epidural fentanyl during labor. Anesth Analg. 1990;71:679–683.
326. Friedlander JD, Fox HE, Cain CF, et al. Fetal bradycardia and uterine hyperactivity following subarachnoid administration of fentanyl during labor. Reg Anesth. 1997;22:378–381.
327. Clarke VT, Smiley RM, Finster M. Uterine hyperactivity after intrathecal injection of fentanyl for analgesia during labor: a cause of fetal bradycardia? Anesthesiology. 1994;81:1083.
328. Kahn L, Hubert E. Combined spinal epidural (CSE) analgesia, fetal bradycardia, and uterine hypertonus. Reg Anesth Pain Med. 1998;23:111–112.
329. Segal S, Csavoy AN, Datta S. The tocolytic effect of catecholamines in the gravid rat uterus. Anesth Analg. 1998;87:864–869.
330. Van de Velde M, Teunkens A, Hanssens M, et al. Intrathecal sufentanil and fetal heart rate abnormalities: a double-blind, double placebo-controlled trial comparing two forms of combined spinal epidural analgesia with epidural analgesia in labor. Anesth Analg. 2004;98:1153–1159.
331. Russell JA, Gosden RG, Humphreys EM, et al. Interruption of parturition in rats by morphine: a result of inhibition of oxytocin secretion. J Endocrinol. 1989;121:521–536.
332. Honet JE, Arkoosh VA, Norris MC, et al. Comparison among intrathecal fentanyl, meperidine, and sufentanil for labor analgesia. Anesth Analg. 1992;75:734–739.
333. Nielsen PE, Erickson JR, Abouleish EI, et al. Fetal heart rate changes after intrathecal sufentanil or epidural bupivacaine for labor analgesia: incidence and clinical significance. Anesth Analg. 1996;83:742–746.
334. Mardirosoff C, Dumont L, Boulvain M, Tramer MR. Fetal bradycardia due to intrathecal opioids for labour analgesia: a systematic review. BJOG. 2002;109:274–281.
335. Albright GA, Forster RM. Does combined spinal-epidural analgesia with subarachnoid sufentanil increase the incidence of emergency cesarean delivery? Reg Anesth. 1997;22:400–405.
336. Gambling DR, Sharma SK, Ramin SM, et al. A randomized study of combined spinal-epidural analgesia versus intravenous meperidine during labor: impact on cesarean delivery rate. Anesthesiology. 1998;89:1336–1344.
337. Abrao KC, Francisco RP, Miyadahira S, et al. Elevation of uterine basal tone and fetal heart rate abnormalities after labor analgesia: a randomized controlled trial. Obstet Gynecol. 2009;113:41–47.
338. Landau R, Carvalho B, Wong C, et al. Elevation of uterine basal tone and fetal heart rate abnormalities after labor analgesia: a randomized controlled trial. Obstet Gynecol. 2009;113:1374.
339. Mercier FJ, Dounas M, Bouaziz H, et al. Intravenous nitroglycerin to relieve intrapartum fetal distress related to uterine hyperactivity: a prospective observational study. Anesth Analg. 1997;84:1117–1120.
340. Bell E. Nitroglycerin and uterine relaxation. Anesthesiology. 1996;85:683.
341. Pullen KM, Riley ET, Waller SA, et al. Randomized comparison of intravenous terbutaline vs nitroglycerin for acute intrapartum fetal resuscitation. Am J Obstet Gynecol. 2007;197:414.e1–414.e6.
342. Lee BB, Ngan Kee WD, Plummer JL, et al. The effect of the addition of epinephrine on early systemic absorption of epidural ropivacaine in humans. Anesth Analg. 2002;95:1402–1407.
343. Sakura S, Sumi M, Morimoto N, Saito Y. The addition of epinephrine increases intensity of sensory block during epidural anesthesia with lidocaine. Reg Anesth Pain Med. 1999;24:541–546.
344. Abboud TK, David S, Nagappala S, et al. Maternal, fetal, and neonatal effects of lidocaine with and without epinephrine for epidural anesthesia in obstetrics. Anesth Analg. 1984;63:973–979.
345. Liu SS, Lin Y. Local anesthetics. Barash PG, Cullen BF, Stoelting RK, et al. Clin Anesthesia. 6th edition. Lippincott Williams & Wilkins: Philadelphia; 2009:531–548.
346. Abboud TK, Sheik-ol-Eslam A, Yanagi T, et al. Safety and efficacy of epinephrine added to bupivacaine for lumbar epidural analgesia in obstetrics. Anesth Analg. 1985;64:585–591.
347. Reynolds F, Laishley R, Morgan B, Lee A. Effect of time and adrenaline on the feto-maternal distribution of bupivacaine. Br J Anaesth. 1989;62:509–514.
348. Cederholm I, Anskar S, Bengtsson M. Sensory, motor, and sympathetic block during epidural analgesia with 0.5% and 0.75% ropivacaine with and without epinephrine. Reg Anesth. 1994;19:18–33.
349. Denson DD, Bridenbaugh PO, Turner PA, et al. Neural blockade and pharmacokinetics following subarachnoid lidocaine in the rhesus monkey. I. Effects of epinephrine. Anesth Analg. 1982;61:746–750.
350. Beazley JM, Taylor G, Reynolds F. Placental transfer of bupivacaine after paracervical block. Obstet Gynecol. 1972;39:2–6.
351. Alahuhta S, Rasanen J, Jouppila P, et al. Uteroplacental and fetal circulation during extradural bupivacaine-adrenaline and bupivacaine for caesarean section in hypertensive pregnancies with chronic fetal asphyxia. Br J Anaesth. 1993;71:348–353.
352. Laishley RS, Carson RJ, Reynolds F. Effect of adrenaline on placental transfer of bupivacaine in the perfused in situ rabbit placenta. Br J Anaesth. 1989;63:439–443.
353. Reynolds F, Taylor G. Plasma concentrations of bupivacaine during continuous epidural analgesia in labour: the effect of adrenaline. Br J Anaesth. 1971;43:436–440.
354. Abboud TK, Afrasiabi A, Zhu J, et al. Bupivacaine/butorphanol/epinephrine for epidural anesthesia in obstetrics: maternal and neonatal effects. Reg Anesth. 1989;14:219–224.
355. Abboud TK, Kim KC, Noueihed R, et al. Epidural bupivacaine, chloroprocaine, or lidocaine for cesarean section—maternal and neonatal effects. Anesth Analg. 1983;62:914–919.
356. Thomas J, Climie CR, Long G, Nighjoy LE. The influence of adrenaline on the maternal plasma levels and placental transfer of lignocaine following lumbar epidural administration. Br J Anaesth. 1969;41:1029–1034.
357. Wong K, Strichartz GR, Raymond SA. On the mechanisms of potentiation of local anesthetics by bicarbonate buffer: drug structure-activity studies on isolated peripheral nerve. Anesth Analg. 1993;76:131–143.
358. DiFazio CA, Carron H, Grosslight KR, et al. Comparison of pH-adjusted lidocaine solutions for epidural anesthesia. Anesth Analg. 1986;65:760–764.
359. Ackerman WE, Denson DD, Juneja MM, et al. Alkalinization of chloroprocaine for epidural anesthesia: effects of pCO2 at constant pH. Reg Anesth. 1990;15:89–93.
360. Ackerman WE, Juneja MM, Denson DD, et al. The effect of pH and pCO2 on epidural analgesia with 2% 2-chloroprocaine. Anesth Analg. 1989;68:593–598.
361. Ramos G, Pereira E, Simonetti MP. Does alkalinization of 0.75% ropivacaine promote a lumbar peridural block of higher quality? Reg Anesth Pain Med. 2001;26:357–362.
362. Berrada R, Chassard D, Bryssine S, et al. In vitro effects of the alkalinization of 0.25% bupivacaine and 2% lidocaine. Ann Fr Anesth Reanim. 1994;13:165–168.
363. Parnass SM, Curran MJ, Becker GL. Incidence of hypotension associated with epidural anesthesia using alkalinized and nonalkalinized lidocaine for cesarean section. Anesth Analg. 1987;66:1148–1150.
364. Yaksh TL. Pharmacology of spinal adrenergic systems which modulate spinal nociceptive processing. Pharmacol Biochem Behav. 1985;22:845–858.
365. Eisenach JC, Tong CY. Site of hemodynamic effects of intrathecal alpha 2-adrenergic agonists. Anesthesiology. 1991;74:766–771.
366. Correa-Sales C, Rabin BC, Maze M. A hypnotic response to dexmedetomidine, an alpha-2 agonist, is mediated in the locus coeruleus in rats. Anesthesiology. 1992;76:948–952.
367. Gaumann DM, Brunet PC, Jirounek P. Hyperpolarizing afterpotentials in C fibers and local anesthetic effects of clonidine and lidocaine. Pharmacology. 1994;48:21–29.
368. Chiari A, Eisenach JC. Spinal anesthesia: mechanisms, agents, methods, and safety. Reg Anesth Pain Med. 1998;23:357–362.
369. De Kock M, Eisenach J, Tong C, et al. Analgesic doses of intrathecal but not intravenous clonidine increase acetylcholine in cerebrospinal fluid in humans. Anesth Analg. 1997;84:800–803.
370. Jacobs MM, Hayashida D, Roberts JM. Human myometrial adrenergic receptors during pregnancy: identification of the alpha-adrenergic receptor by [3H] dihydroergocryptine binding. Am J Obstet Gynecol. 1985;152:680–684.
371. Sia AT, Kwek K, Yeo GS. The in vitro effects of clonidine and dexmedetomidine on human myometrium. Int J Obstet Anesth. 2005;14:104–107.
372. Eisenach JC, Castro MI, Dewan DM, Rose JC. Epidural clonidine analgesia in obstetrics: sheep studies. Anesthesiology. 1989;70:51–56.
373. Mendez R, Eisenach JC, Kashtan K. Epidural clonidine analgesia after cesarean section. Anesthesiology. 1990;73:848–852.
374. Capogna G, Celleno D, Zangrillo A, et al. Addition of clonidine to epidural morphine enhances postoperative analgesia after cesarean delivery. Reg Anesth. 1995;20:57–61.
375. Massone ML, Lampugnani E, Calevo MG, et al. The effects of a dose of epidural clonidine combined with intrathecal morphine for postoperative analgesia. Minerva Anestesiol. 1998;64:289–296.
376. Seybold VS. Distribution of histaminergic, muscarinic and serotonergic binding sites in cat spinal cord with emphasis on the region surrounding the central canal. Brain Res. 1985;342:291–296.
377. Yaksh TL, Dirksen R, Harty GJ. Antinociceptive effects of intrathecally injected cholinomimetic drugs in the rat and cat. Eur J Pharmacol. 1985;117:81–88.
378. Bartolini A, Ghelardini C, Fantetti L, et al. Role of muscarinic receptor subtypes in central antinociception. Br J Pharmacol. 1992;105:77–82.
379. Baba H, Kohno T, Okamoto M, et al. Muscarinic facilitation of GABA release in substantia gelatinosa of the rat spinal dorsal horn. J Physiol. 1998;508(Pt 1):83–93.
380. Owen MD, Ozsarac O, Sahin S, et al. Low-dose clonidine and neostigmine prolong the duration of intrathecal bupivacaine-fentanyl for labor analgesia. Anesthesiology. 2000;92:361–366.
381. D'Angelo R, Dean LS, Meister GC, Nelson KE. Neostigmine combined with bupivacaine, clonidine, and sufentanil for spinal labor analgesia. Anesth Analg. 2001;93:1560–1564.
382. Lauretti GR, Lima IC. The effects of intrathecal neostigmine on somatic and visceral pain: improvement by association with a peripheral anticholinergic. Anesth Analg. 1996;82:617–620.
383. Lauretti GR, Mattos AL, Reis MP, Pereira NL. Combined intrathecal fentanyl and neostigmine: therapy for postoperative abdominal hysterectomy pain relief. J Clin Anesth. 1998;10:291–296.
384. Lauretti GR, de Oliveira R, Reis MP, et al. Study of three different doses of epidural neostigmine coadministered with lidocaine for postoperative analgesia. Anesthesiology. 1999;90:1534–1538.
385. Roelants F, Lavand'homme PM. Epidural neostigmine combined with sufentanil provides balanced and selective analgesia in early labor. Anesthesiology. 2004;101:439–444.
386. Roelants F, Lavand'homme PM, Mercier-Fuzier V. Epidural administration of neostigmine and clonidine to induce labor analgesia: evaluation of efficacy and local anesthetic-sparing effect. Anesthesiology. 2005;102:1205–1210.
387. Nelson KE, D'Angelo R, Foss ML, et al. Intrathecal neostigmine and sufentanil for early labor analgesia. Anesthesiology. 1999;91:1293–1298.
388. Kaya FN, Sahin S, Owen MD, Eisenach JC. Epidural neostigmine produces analgesia but also sedation in women after cesarean delivery. Anesthesiology. 2004;100:381–385.