Anesthetic Complications
Awareness and Recall
Cesarean delivery is considered to be a high-risk procedure for the occurrence of intraoperative awareness, defined as the spontaneous postoperative recall of an event that occurred during general anesthesia.62 The following factors contribute to the risk for maternal awareness during cesarean delivery: (1) the avoidance of sedative premedications, (2) the deliberate use of a low concentration of a volatile halogenated agent, (3) the use of muscle relaxants, (4) the reduction in dose of anesthetic agents during hypotension or hemorrhage, (5) the presence of a partial neuraxial blockade in parturients requiring conversion to general anesthesia after failed neuraxial anesthesia, and (6) the (mistaken) assumption that high baseline sympathetic tone is responsible for intraoperative tachycardia in parturients.
Concern about neonatal depression and uterine atony associated with volatile halogenated agents has led to administration of relatively low doses of these agents. Administration of a barbiturate induction agent followed by nitrous oxide 50% in oxygen resulted in maternal awareness in 12% to 26% of cases.331,332 Using an isolated forearm technique, King et al.360 assessed 30 women undergoing cesarean delivery with thiopental 250 mg, succinylcholine infusion, and 0.5% halothane in 50% nitrous oxide; the majority of patients signaled pain in the first minute. The incidence of recall with this anesthetic regimen was approximately 1%.361 The use of higher concentrations of a volatile halogenated agent has subsequently become a more common practice, leading to an incidence of maternal awareness of approximately 0.26%.62 However, the result of increasing the depth of maternal anesthesia is that neonates born to women who receive general anesthesia tend to have lower Apgar and neurobehavioral scores, particularly when the I-D interval exceeds 8 minutes.362
The optimal doses and concentrations of anesthetic agents to prevent awareness remain unclear, in part because of the difficulty in assessing awareness. Studies have evaluated several tools for assessment of depth of maternal anesthesia, including the electroencephalogram, brainstem auditory evoked potentials, and the bispectral index.62,276,311 The bispectral index is an empirically derived electroencephalographic parameter in which values less than 60 are suggested to predict a low probability of intraoperative recall and awareness.363 With each of these monitoring devices, the threshold for awareness will need further validation, particularly during pregnancy364; moreover, many of these devices are not suitable for the emergency conditions under which most general anesthetics for cesarean delivery are administered.
Yeo et al.365 evaluated 20 women undergoing cesarean delivery and noted that an end-tidal concentration of 1% sevoflurane (approximately 0.5 MAC) with 50% nitrous oxide resulted in a range of bispectral index values between 52 and 70; no patient experienced intraoperative dreams, recall, or awareness. Using a similar regimen, Yoo et al.276 demonstrated that bispectral index values were lower during general anesthesia in women who were in labor prior to cesarean delivery than in nonlaboring women; the nonlaboring group did not reliably have values less than 60, and the mean value at tracheal intubation was 64 ± 10. Ittichaikulthol et al.366 found that mean bispectral index values in women undergoing cesarean delivery with 3% and 4.5% desflurane in 50% nitrous oxide were 62 ± 8 and 49 ± 12, respectively. Chin et al.275 observed that an 80% probability of maintaining bispectral index values less than 60 required a sevoflurane concentration of at least 1.2% to 1.3%.
Although pregnancy diminishes anesthetic requirements by 25% to 40%,273 administration of 0.5 MAC of a volatile halogenated agent may not reliably provide adequate depth of anesthesia to consistently prevent maternal awareness. Robins and Lyons62 have recommended a larger induction dose of barbiturate (e.g., thiopental 5 to 7 mg/kg instead of 3 to 4 mg/kg), an end-tidal volatile anesthetic concentration greater than 0.8 MAC, the highest concentration of nitrous oxide compatible with appropriate oxygenation, and the administration of an opioid and a benzodiazepine after delivery. Intravenous induction or infusion techniques that may reduce the risk for maternal awareness include the administration of repeat doses of thiopental,365 the use of ketamine,310 or a combination of thiopental and ketamine.313 Midazolam 0.075 mg/kg provides 30 to 60 minutes of anterograde amnesia when given to women undergoing elective cesarean delivery under epidural anesthesia.367 Induction of general anesthesia with propofol 2.4 mg/kg compared with thiopental 5 mg/kg has been associated with a significantly greater incidence (50% versus 10%) of rapid low-voltage (8-9 Hz) waves, suggestive of a light plane of anesthesia.295 Infusion of propofol 8 mg/kg/h with 67% nitrous oxide has also been used with satisfactory amnestic effect.368 Propofol exhibits an amnestic effect that is not dependent on the degree of sedation; however, the effect is significantly less than that with midazolam.369
The psychological morbidity associated with awareness should not be underestimated.62 Further investigations into the anesthetic regimens and monitoring necessary to prevent awareness and recall in pregnant women undergoing operative procedures are needed. These studies should incorporate the growing data on gender- and pregnancy-related differences in pharmacokinetics and pharmacodynamics of drugs used for anesthesia.370,371
Paradoxically, the issue of recall is not limited to the administration of general anesthesia. In women undergoing cesarean delivery with a neuraxial technique who desire treatment for anxiety, the administration of anxiolytic or hypnotic agents may result in a lack of recall of delivery, which is typically undesirable.
Dyspnea
After the initiation of neuraxial anesthesia, the patient may complain of dyspnea. The most common cause of this complaint is hypotension (causing hypoperfusion of the brainstem); therefore, the complaint of difficulty in breathing should prompt immediate assessment of blood pressure and treatment, if appropriate. Other causes of dyspnea are the blunting of thoracic proprioception, the partial blockade of abdominal and intercostal muscles, and the recumbent position, which increases the pressure of the abdominal contents against the diaphragm. The sensation of dyspnea appears related to the cephalad extent of the sensory blockade and may be mitigated by using a low-dose hyperbaric spinal bupivacaine technique in women undergoing cesarean delivery.372
Despite these changes, significant respiratory compromise is unlikely, primarily because the neuraxial blockade rarely affects the cervical nerves that control the diaphragm. Lirk et al.373 evaluated the effects of spinal bupivacaine 10 mg, ropivacaine 20 mg, and levobupivacaine 10 mg, all with fentanyl 15 µg, on women undergoing cesarean delivery. Reductions in the functional vital capacity (3 to 6%) and peak expiratory flow rate (6 to 13%) were observed; however, the findings had no apparent clinical significance, were similar for all local anesthetics, and did not differ for sensory blockade that extended higher, versus no higher, than the T4 dermatome.
If the patient loses the ability to vocalize, demonstrate a strong hand grip, and/or maintain normal oxyhemoglobin saturation (e.g., symptoms suggestive of high spinal anesthesia), a rapid-sequence induction with cricoid pressure and placement of an endotracheal tube should be performed to maintain ventilation and prevent pulmonary soiling with gastrointestinal contents.
Hypotension
Hypotension is a common sequela of neuraxial anesthesia and, if severe and sustained, may lead to impairment of uteroplacental perfusion and result in fetal hypoxia, acidosis, and neonatal depression or injury.374 Severe maternal hypotension can also have adverse maternal outcomes, including altered consciousness, pulmonary aspiration, apnea, and cardiac arrest.
Although not universally accepted, most investigators accept the following definitions for maternal hypotension: (1) a decrease in systolic blood pressure of more than 20% to 30% from baseline measurements or (2) a systolic blood pressure lower than 100 mm Hg.375 Neuraxial anesthetic techniques produce hypotension through blockade of sympathetic nerve fibers, which control vascular smooth muscle tone. Several studies using noninvasive measures of cardiac output have demonstrated that cardiac output commonly increases after spinal anesthesia, even in the presence of a phenylephrine infusion and fluid administration.376-378 These studies emphasize that spinal anesthesia–induced hypotension is principally related to a marked decrease in systemic vascular resistance, rather than decreased cardiac output. The rate and extent of the sympathetic involvement, and subsequently the severity of hypotension, are determined by the onset and spread of the neuraxial blockade379; hypotension may be less common with epidural anesthesia than with spinal anesthesia because of the slower onset of neuroblockade and the earlier recognition and treatment.380
Risk Factors for Hypotension
A number of studies have attempted to identify pregnant women at increased risk for development of hypotension. Of interest, women with severe preeclampsia381 or in established labor appear less likely to experience hypotension during administration of spinal anesthesia for cesarean delivery.
Using a modified orthostatic challenge (i.e., “tilt test”), Kinsella and Norris382 were unable to establish a correlation in the observed change in blood pressure or heart rate with hypotension after spinal anesthesia. Similarly, Frölich and Caton383 could not establish a correlation between orthostatic blood pressure and heart rate changes and the hypotension that developed after spinal anesthesia; however, the investigators found that patients with a baseline heart rate higher than 90 bpm had a 83% chance (positive predictive value) of experiencing marked hypotension (decrease in blood pressure > 30%), whereas patients with a baseline heart rate lower than 90 bpm had a 75% chance (negative predictive value) of not experiencing marked hypotension.
Dahlgren et al.384 hypothesized that the response of pregnant women to a preoperative supine stress test would predict the occurrence of maternal symptoms, a need for ephedrine, or a decrease in blood pressure below 80 mm Hg during administration of spinal anesthesia for cesarean delivery. The supine stress test was considered positive if it was associated with (1) an increase in maternal heart rate greater than 10 bpm, (2) a decrease in systolic blood pressure of more than 15 mm Hg, or (3) signs and symptoms related to the supine position (e.g., nausea, dizziness). These investigators found that the preoperative stress test had a sensitivity of 69% and a specificity of 92% in identifying those who would have hypotension.
Investigators have used other methods, including assessment of heart rate variability385,386 and noninvasive measurements of systemic vascular resistance (e.g., thoracic impedance387) in an attempt to identify parturients at risk for neuraxial anesthesia–induced hypotension for cesarean delivery. Hanss et al.385,388 suggested that the low- to high-frequency ratio of heart rate variability could identify pregnant patients at risk for severe spinal anesthesia–induced hypotension and could guide prophylactic treatment with fluid and vasopressors. To date, predicting which parturients will have hypotension after neuraxial anesthesia for cesarean delivery has not proven feasible clinically and will likely require more sophisticated studies that employ a number of different methodologies; the reason may be the myriad of factors that control the autonomic, physiologic, and hormonal changes and hemodynamic responses that occur during pregnancy.
Prevention of Hypotension
A number of strategies can mitigate hypotension after spinal anesthesia for cesarean delivery, including fluid administration, vasopressor administration, lower local anesthetic doses, leg elevation or wrapping, and left uterine displacement.
The use of intravenous fluid to prevent hypotension can be manipulated by (1) timing of administration, either prior to (preload) or coincident with (co-load) the intrathecal injection, and/or (2) type of fluid, either crystalloid or colloid. Rate of fluid administration may also play a role. A crystalloid preload is minimally effective, even when volumes as great as 30 mL/kg are infused.93 By contrast, a colloid preload consistently reduces the incidence and severity of hypotension. Ueyama et al.389 compared the administration of 1500 mL of lactated Ringer's solution and 500 mL and 1000 mL of hydroxyethyl starch solution (HES) 6% prior to spinal anesthesia for cesarean delivery; the incidence of hypotension (systolic blood pressure < 100 mm Hg and < 80% of baseline) was 75%, 58%, and 17%, respectively. Significant increases in intravascular volume and cardiac output, as measured by indocyanine green spectrophotometry, were observed in the HES groups (Figure 26-7). At 30 minutes, 100% of the HES volume, versus 28% of the lactated Ringer's volume, remained within the intravascular space.
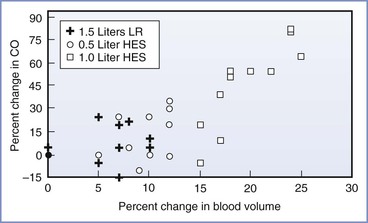
FIGURE 26-7 The relationship between the changes (%) in blood volume and cardiac output after volume preload in parturients undergoing spinal anesthesia. Cardiac output (CO) was estimated with indocyanine green pulse spectrophotometry methodology. HES, hydroxyethyl starch solution; LR, lactated Ringer's solution. (Modified from Ueyama H, He Y, Tanigami H, et al. Effects of crystalloid and colloid preload on blood volume in the parturient undergoing spinal anesthesia for elective cesarean section. Anesthesiology 1999; 91:1571-6.)
In one trial,94 the rapid administration of a crystalloid co-load (20 mL/kg in 10 minutes, initiated immediately on induction of spinal anesthesia) was more effective than crystalloid preload in preventing hypotension; however, a meta-analysis of randomized trials comparing crystalloid preload to co-load did not find a difference in the incidence of hypotension.95 Similarly, no difference in the incidence of hypotension is observed with a colloid preload versus co-load, likely reflecting the intravascular dwell time of colloid.95 Additionally, co-load administration of colloid is as effective, and likely more effective, than co-load administration of crystalloid.376
The cost and associated pruritus, mild coagulation abnormalities, and potential for allergic reaction to colloid starch solutions, particularly with first-generation agents, have tempered their widespread use. Possible fetal and neonatal effects related to the type and timing of maternal fluid administration deserve further investigation; for example, the rapid administration of 1500 to 2000 mL of fluid can release atrial natriuretic peptide, which may result in vasodilation and reduced sensitivity to vasoconstrictors.390 Our current practice is to administer a rapid crystalloid co-load (approximately 15 mL/kg) to healthy parturients undergoing elective cesarean delivery with spinal anesthesia. Parturients at high risk for hypotension (e.g., history of supine hypotension syndrome), or at high risk for the adverse consequences of hypotension (e.g., hypertrophic cardiomyopathy), receive a colloid preload (500 mL).
Vasopressor agents, including ephedrine and phenylephrine, can be titrated to maintain maternal blood pressure and have been observed to be more effective than crystalloid solution or placebo in preventing spinal anesthesia–induced hypotension. In a meta-analysis, Lee et al.391 concluded that ephedrine was superior to placebo in the prevention of spinal anesthesia–induced hypotension for women undergoing cesarean delivery. Although greater doses of ephedrine provided more effective prophylaxis, hypotension was still observed and reactive hypertension and umbilical artery metabolic acidosis were more common.
Traditionally, ephedrine was used to prevent and treat the hypotension associated with neuraxial anesthesia because of fear that pure alpha-adrenergic agonists would decrease uterine blood flow. However, phenylephrine is equally efficacious to ephedrine for the prevention and treatment of hypotension, and it is less likely to depress umbilical arterial blood pH and base excess.392 Phenylephrine crosses the placenta at a lower rate than ephedrine and undergoes greater fetal metabolism than ephedrine.393 Presumably, fetal ephedrine contributes to stimulation of fetal metabolism, resulting in lower pH and base excess than phenylephrine.394
There is some controversy as to whether vasopressors should be administered as a continuous infusion or bolus, particularly for the prevention of hypotension. When administered as an infusion, phenylephrine infusion rates of 25 to 100 µg/min are usually used to prevent hypotension.395,396 However, the number of interventions to maintain systolic blood pressure within 20% of baseline are lower for phenylephrine infusions of 25 and 50 µg/min than for infusions of 75 and 100 µg/min because of the number of interventions required to treat hypertension with the higher doses. Using an up-down sequential allocation study design, the effective phenylephrine bolus dose for preventing hypotension in 95% of patients (ED95) was estimated at 159 µg (95% CI, 122 to 371 µg).397 Reflex decreases in heart rate and reductions in cardiac output may occur with phenylephrine; however, this does not appear to affect umbilical cord blood gas measurements or neonatal Apgar scores in uncompromised infants delivered via elective cesarean delivery.396 The effect on infants that have been subjected to intrauterine compromise remains unclear.
One study demonstrated a salutary effect of a prophylactic infusion of ephedrine combined with phenylephrine compared with infusion of ephedrine alone.398 However, another study comparing infusions of different ephedrine-to-phenylephrine ratios found that as the proportion of ephedrine increased, the incidence of hypotension and nausea/vomiting also increased, whereas umbilical cord blood pH and base excess were decreased.399
The titration of vasopressor infusions often requires frequent infusion rate adjustments, and this mode of administration may be more cumbersome than bolus administration of the same agents. Thus, some clinicians prefer to administer a bolus dose of vasopressor to prevent or treat hypotension. Loughrey et al.400 tested various ratios of ephedrine combined with phenylephrine, administered as a bolus, but were unable to identify a combination that reliably prevented hypotension yet avoided hypertension. Methods under investigation include a closed-loop feedback computer-controlled infusion of phenylephrine for maintaining blood pressure during spinal anesthesia.401
The combination of fluid and vasopressor administration may be the most effective regimen to prevent hypotension. Ngan Kee et al.402 reduced the incidence of spinal anesthesia–associated hypotension to almost zero (1.9%) by combining a rapid crystalloid co-load with a prophylactic phenylephrine infusion (beginning at 100 µg/min); the incidence of hypotension was 28% in the women who received phenylephrine without the co-load. No difference in neonatal outcome was observed between groups.
The use of lower doses of spinal local anesthetic is associated with a lower incidence of hypotension, particularly when high and low doses are compared (e.g., hyperbaric bupivacaine 6.5 versus 9.5 mg, or 3.75 versus 9 mg; and plain bupivacaine 5 versus 10 mg combined with fentanyl 25 µg).403 However, the desire to use a low dose of spinal local anesthetic (e.g., bupivacaine ≤ 8 mg) should be tempered by the potential for an increased requirement for intraoperative supplemental analgesia or conversion to general anesthesia.404 The optimal local anesthetic dose is likely influenced by a number of factors, including technical factors (e.g., precision of dose, spinal level of injection, concomitant opioid use, positioning of patient during and after the block), and other factors (e.g., genetic sensitivity, patient expectations, differences in operative technique); these factors are often not controlled in studies of local anesthetic dose.403 Anesthesia providers who administer an intermediate or low dose of local anesthetic should consider the use of a catheter-based technique (continuous spinal or CSE anesthesia), given the frequent need (up to 40%) for supplemental administration of additional local anesthetic through the catheter.403
Physical methods to prevent hypotension include the use of lower limb compression bandages or pneumatic compression devices, which have demonstrated some success375 and may assist in preventing thromboembolic complications. Compared with the supine position, left uterine displacement does not consistently reduce the occurrence of maternal hypotension during cesarean delivery,375 most likely reflecting the variable presence and significance of supine hypotension syndrome. However, in some studies, adequate left uterine displacement was associated with higher umbilical arterial blood pH measurements and better neonatal outcomes than the supine position, indicating that maternal blood pressure measured at the level of the brachial artery may not always predict uteroplacental perfusion. It is not known a priori who will experience hypotension; more important, diminished uterine blood flow may occur in the presence or absence of maternal hypotension. Thus, we consider the use of left uterine displacement mandatory during anesthesia for cesarean delivery.
Treatment of Hypotension
The ideal treatment of hypotension would be reliable, titratable, easy to use, and devoid of maternal and fetal side effects. Almost 40 years ago, ephedrine, a mixed alpha- and beta-adrenergic receptor agonist, emerged as the leading choice for the treatment of hypotension on the basis of studies demonstrating its efficacy and apparent superiority (over other agents) in protecting and/or restoring uterine blood flow in gravid ewes and other pregnant animal models.405 By contrast, other agents, including metaraminol and phenylephrine, while restoring maternal blood pressure, were associated with a decrease in uterine artery blood flow and fetal pH.405
Contemporary animal studies have provided a mechanistic understanding of these effects. During pregnancy vasopressors appear to constrict the femoral artery more than the uterine artery, which increases blood pressure and protects uterine blood flow. This differential pressor effect is greater for ephedrine than metaraminol.406 A second mechanism appears to be the up-regulation of nitric oxide synthase (NOS) in the uterine artery during pregnancy.407 The presence of NOS potentially makes this artery less sensitive to vasopressors; this effect may be further augmented by ephedrine, a drug observed to independently cause the release of NOS.
In a quantitative systematic review, Lee et al.392 noted that the use of ephedrine for treatment of maternal hypotension during administration of spinal anesthesia for cesarean delivery was associated with lower umbilical cord blood pH measurements than the use of phenylephrine. This surprising clinical result (which differs from results in animal studies) may reflect interspecies differences in vascular smooth muscle physiology, control of blood flow, and drug metabolism. Also, this result may reflect the fetal effects of ephedrine given to the mother. Cooper et al.394 developed a measurement index by subtracting the umbilical vein PCO2 from the umbilical artery PCO2 to determine the amount of CO2 generated by the fetus; the index indicated a higher metabolic production of CO2 in fetuses whose mothers received ephedrine. Ngan Kee et al.393 observed that the greater depression of fetal pH and base excess with ephedrine compared with phenylephrine appears related to its ability to cross the placenta to a greater extent, undergo less early metabolism or redistribution in the fetus, and consequently produce greater fetal concentrations of lactate, glucose, and catecholamines.
The NICE guidelines state that phenylephrine and ephedrine are equally effective as vasopressors.408 Given the efficacy of phenylephrine in the treatment of hypotension and the better umbilical cord blood acid-base measurements associated with its use in clinical studies, many anesthesia providers now use phenylephrine as a first-line agent for the prevention and treatment of maternal hypotension.409 Regardless of the vasopressor agent selected to treat hypotension, therapy should be administered as soon as the blood pressure begins to decrease, rather than after the occurrence of clinically significant hypotension.410 In addition, vasopressor administration strategies can optimize maternal, and potentially fetal, hemodynamics and well-being by maintaining blood pressure near baseline, instead of lower target goals such as 80% or 90% of baseline measurements.411 Ephedrine is usually administered intravenously in bolus doses of 5 to 10 mg. Phenylephrine may be administered intravenously in bolus doses of 50 to 100 µg or by continuous infusion beginning at 25 to 50 µg/min,395 with titration to maintain maternal arterial blood pressure at or near baseline and avoidance of maternal bradycardia.378 Administration of ephedrine may lead to tachycardia, as well as tachyphylaxis. By contrast, phenylephrine may result in reflex maternal bradycardia, which, if treated with an anticholinergic agent in the absence of hypotension, may result in significant hypertension.
Failure of Neuraxial Blockade
“Failed” neuraxial anesthesia can be defined as neuroblockade insufficient in extent, density, or duration to provide anesthesia for cesarean delivery. Four to 13 percent of epidural anesthetics and 0.5% to 4% of spinal anesthetics fail to provide sufficient anesthesia for the initiation or completion of cesarean delivery.412,413 Epidural techniques are more often associated with failure, given that the catheter is often placed during early labor, and over time the catheter may migrate out of the epidural space. Factors that may correlate with failed extension of labor epidural anesthesia for cesarean delivery include a higher number of bolus doses for the provision of labor analgesia (i.e., treatment of breakthrough pain), patient characteristics (e.g., obesity, distance from the skin to the epidural space), and the time elapsed between placement of the epidural catheter and cesarean delivery.413
The causes of failure of neuraxial techniques include anatomic, technical, and obstetric factors. Steps to reduce the likelihood of epidural block failure include meticulous attention to technical detail, the administration of a solution that contains both a local anesthetic and an opioid, and a better understanding of the characteristics of epidural versus spinal blockade. Moreover, the patient should be prepared to expect the sensation of deep pressure and movement yet be reassured that reports of discomfort or pain will be addressed promptly. Initiation of surgery should be delayed until adequate thoracic and sacral sensory blockade has been achieved; on rare occasions, in the setting of an urgent procedure for which a developing epidural block is present at T10 but has yet to achieve a T4 level, surgery can commence with the understanding that adjuvant treatments or alternative forms of anesthesia may be required.
Evaluation of intraoperative pain requires (1) determination of the location and extent of discomfort, (2) evaluation of the sensory level of anesthesia, (3) assessment of the current status of the surgery (e.g., incision, delivery, uterine repair, skin closure), and (4) assessment of the presence of confounding factors (e.g., hemorrhage, anxiety). Shoulder pain can originate from irritation of the diaphragm (usually by amniotic fluid or blood) and is mediated by the phrenic nerve (C3 to C5); prolonged abduction and extension of the arms can also cause discomfort. Additional discomfort can occur from visceral stimulation such as uterine manipulation, which often involves the greater splanchnic nerve (T5 to T10). Alternatively, the extent of the block may be adequate but the density of neuroblockade of the large nerve fibers in the lumbosacral plexus may be inadequate. Inadequate anesthesia can result from regression of the block from a cephalad or caudad direction.
Management of breakthrough pain should begin with acknowledgement of the patient's discomfort and a consideration of the fetal (e.g., presence of nonreassuring fetal status), surgical (e.g., ongoing or anticipation of prolonged surgery), and anesthetic (e.g., maternal airway examination, BMI) implications, as well as the anesthesia provider's experience. Emergent or ongoing surgery may require administration of general anesthesia. If no block exists, the surgery has not begun, and time allows, neuraxial anesthesia can be repeated; whether an epidural or spinal technique is attempted depends on the previously mentioned factors. If an inadequate, partial block exists in an elective situation, either the surgery can be postponed (to allow resolution of the partial block) or a second neuraxial technique may be performed with caution.
The disadvantages of replacing a failed neuraxial block with epidural anesthesia include (1) the potential for local anesthetic toxicity (particularly after epidural administration of a large dose of local anesthetic for the initial attempt), (2) the time required to establish an adequate block, and (3) the unpredictable reliability and quality of the resulting block. As a consequence, many practitioners intent on replacing a failed epidural technique suggest the cautious use of a technique with an intrathecal component (i.e., spinal, CSE, or continuous spinal technique).414
The performance of a spinal technique in the setting of a partial but failed epidural or spinal anesthetic technique is controversial. In this setting, intrathecal administration of a standard intrathecal dose of bupivacaine may result in a high spinal block.415 Radiographic evidence suggests that the dural sac is compressed by prior epidural drug administration.254 Thus, when performing a spinal anesthetic technique after failed epidural or spinal anesthesia, the anesthesia provider should consider (1) using a different interspace to avoid the anatomic distortions (e.g., from the loss of resistance to saline or previous needle passes) or difficulties; (2) reducing the dose of bupivacaine (with the chosen dose depending on the extent of existing neuroblockade); (3) placing the patient in a semi-sitting (Fowler's) position to limit cephalad spread of the local anesthetic; (4) using a CSE technique with a small intrathecal dose of local anesthetic, and, if necessary, titrating the sensory level with additional drugs administered through the epidural catheter; and (5) intentionally placing an epidural catheter into the intrathecal space for administration of continuous spinal anesthesia. This last strategy may be especially useful in obese patients in whom the technical difficulty of the neuraxial approach may otherwise limit success.
If discomfort is reported after the start of surgery, it is often helpful to ask the surgeons to halt the operation while an assessment is made. If an epidural catheter is in place, an alkalinized local anesthetic with an opioid (e.g., 3% 2-chloroprocaine with fentanyl) should be administered. The density of epidural anesthesia may be improved by “repainting the fence.” An additional dose of local anesthetic (20% to 30% of the initial dose [e.g., 4 to 7 mL]) is administered approximately 20 minutes after the initial dose. This second dose serves to improve the density of neuroblockade without extending the sensory level. Some anesthesia providers routinely administer this supplemental dose, without waiting for a patient's complaint of breakthrough pain.
Intravenous administration of an opioid (fentanyl), inhalation of nitrous oxide (40% to 50% in oxygen), or intravenous anxiolysis (midazolam) may be helpful for the treatment of breakthrough pain. Severe pain may require intravenous ketamine in 5- to 10-mg increments. However, care should be taken, because the administration of multiple agents can result in significant sedation, loss of consciousness, and the presence of psychomimetic and amnestic effects. The obstetrician can infiltrate the wound or instill the peritoneal cavity with local anesthetic; however, at this point, the induction of general anesthesia with tracheal intubation is often necessary.
If the anesthesia provider anticipates that the duration of the surgical procedure will be longer than the predicted duration of epidural or CSE anesthesia, additional local anesthetic (with or without an opioid) should be administered before anticipated regression of neuroblockade (see Table 26-6). The usual dose to maintain neuroblockade is half of the initial dose.
High Neuraxial Blockade
It is not uncommon for the parturient to report mild dyspnea or reduced ability to cough, especially if the neuraxial blockade has achieved a T2 sensory level. If impaired phonation, unconsciousness, respiratory depression, or significant impairment of ventilation occurs, administration of general anesthesia should be performed. High neuraxial blockade may also result in cardiovascular sequelae, including bradycardia and hypotension. An easy method to diagnose a clinically significant high neuraxial blockade is to ask the patient to make a fist (“squeeze your fingers”). A weak hand grasp indicates high thoracic and cervical motor blockade.
High neuraxial block can be caused by several mechanisms, including an exaggerated spread of spinal or epidural drugs and unintentional intrathecal or subdural administration of an “epidural dose” of local anesthetic. The rapid epidural administration of a large volume of local anesthetic solution in the presence of a large-bore dural puncture (e.g., after a “wet tap”) may also result in high neuroblockade.
Nausea and Vomiting
Nausea and vomiting are regulated by the chemoreceptor trigger zone and the vomiting center, which are located in the area postrema and the medullary lateral reticular formation, respectively. The vomiting center receives impulses from the vagal sensory fibers in the gastrointestinal tract, the semicircular canals and ampullae (labyrinth) of the inner ear, higher cortical centers, the chemoreceptor trigger zone, and intracranial pressure receptors. Impulses from these structures are influenced by dopaminergic, muscarinic, tryptaminergic, histaminic, and opioid receptors, which are subsequently the targets for antiemetic agents. Efferent impulses from the vomiting center are transmitted through the vagus, phrenic, and spinal nerves to the abdominal muscles, which causes the physical act of vomiting.
Preoperative Nausea and Vomiting
Nausea and vomiting may occur separately or in combination and are not uncommon during pregnancy. A number of metabolic, endocrine, and anatomic changes have been implicated in the genesis of gestational nausea and vomiting, including human chorionic gonadotropin, estrogen, progesterone, prostaglandins, and immune system dysregulation.416 When vomiting is sufficiently severe to produce weight loss, dehydration, acidosis from starvation, alkalosis from loss of hydrochloric acid in vomitus, and hypokalemia, it is referred to as hyperemesis gravidarum (see Chapter 16). This disorder most commonly occurs in early pregnancy, but as many as 10% of pregnant women have nausea and vomiting that persist beyond 22 weeks' gestation.416 Severe and persistent hyperemesis may result in maternal and fetal morbidity.
The presence of delayed gastric emptying during labor and administration of opioids are risk factors for nausea and vomiting before cesarean delivery.
Intraoperative Nausea and Vomiting
Intraoperative nausea and vomiting associated with cesarean delivery can be variable in incidence and presentation, depending on preexisting symptoms, anesthetic and obstetric techniques, and preventive and therapeutic measures. The incidence of nausea may be as high as 80%, particularly when the anesthesia provider specifically assesses for the presence of intraoperative symptoms; symptoms occur frequently with exteriorization of the uterus.7 Anesthetic causes of intraoperative nausea and vomiting include hypotension and increased vagal activity; nonanesthetic causes include surgical stimuli, bleeding, medications (e.g., uterotonic agents, antibiotics), and motion at the end of surgery.417 Many of these elements occur simultaneously.
Hypotension is among the most common sequelae associated with the administration of neuraxial anesthesia. Centrally, hypotension may lead to cerebral and brainstem hypoperfusion, which results in stimulation of the medullary vomiting center. Peripherally, hypotension may cause gut ischemia with release of emetogenic substances (e.g., serotonin) from the intestine.418 Strict maintenance of intraoperative blood pressure can reduce the occurrence of emesis; Datta et al.410 observed that the incidence of intraoperative nausea and vomiting was 66% when the blood pressure decreased more than 30% from baseline, but was less than 10% when blood pressure was maintained at baseline with ephedrine. Similarly, Ngan Kee et al.411 demonstrated progressive increases in intraoperative nausea and vomiting when blood pressure control with an infusion of phenylephrine was less aggressive; the incidence of nausea and vomiting was 4% when blood pressure was maintained at 100% of baseline, 16% when maintained at 90% of baseline, and 40% when maintained at 80% of baseline during spinal anesthesia for cesarean delivery.
Uterotonic agents may also contribute to intraoperative nausea and vomiting. Ergot alkaloids may cause nausea and vomiting by interacting with dopaminergic and serotoninergic receptors. Oxytocin causes nausea and vomiting primarily as a result of the hypotension produced through release of nitric oxide and atrial natriuretic peptide.419 A 29% incidence of nausea and a 9% incidence of vomiting have been reported with an intravenous bolus of oxytocin 5 units during elective cesarean delivery with neuraxial anesthesia.420 Administration of 15-methyl prostaglandin F2α causes nausea through the stimulation of smooth muscles of the gastrointestinal tract; a 10% incidence of nausea and vomiting has been observed after administration of 250 µg intramuscularly.417
Surgical stimuli, including exteriorization of the uterus, intra-abdominal manipulation, and peritoneal traction, can cause visceral pain and subsequent nausea through the stimulation of vagal fibers and activation of the vomiting center; despite high levels of thoracic sensory block obtained for cesarean delivery anesthesia, visceral pain may still occur, particularly after the neuraxial administration of a local anesthetic without opioid.421 The administration of neuraxial opioids reduces visceral pain–induced nausea and vomiting. Neuraxial fentanyl both improves the quality of neuraxial anesthesia and decreases intraoperative nausea; the minimal effective doses are 6.25 µg given intrathecally and 50 µg given epidurally, respectively.180
Postoperative Nausea and Vomiting
Risk factors for postoperative nausea and vomiting have not been specifically studied in obstetric patients; however, studies have identified risk factors in nonobstetric patients receiving general or neuraxial anesthesia (Box 26-10).422,423 Apfel et al.423 identified the following four highly predictive factors for postoperative nausea and vomiting in nonobstetric patients after general anesthesia, which may have relevance in the pregnant population: (1) female gender, (2) history of motion sickness or postoperative nausea and vomiting, (3) nonsmoking status, and (4) the use of perioperative opioids. The incidence of postoperative nausea and vomiting was 10% if the patient had no risk factors, 21% for one, 39% for two, 61% for three, and 79% for four. A subset of pregnant women may have a lower threshold for nausea and vomiting associated with motion.416 Changes in position and transfer to and on the stretcher may stimulate afferent neural pathways that trigger emesis. Because the histamine-1 (H1) and muscarinic cholinergic pathways play primary roles in this response, antihistamine and anticholinergic agents should be considered first-line treatments.424 Postoperative nausea and vomiting may be related to postoperative ileus, which in turn is influenced by the effect of opioids on the gastrointestinal tract, the activation of the sympathetic nervous system, the occurrence of intestinal wall inflammation, and the presence of volume overload or edema.416
Prophylaxis and Treatment of Nausea and Vomiting
Preventing maternal hypotension may be the best means of preventing nausea and vomiting (see earlier). Several options exist for the pharmacologic prophylaxis of nausea and vomiting, and several different classes of drugs are available (Table 26-7). Although various algorithms have been developed to prevent postoperative nausea and vomiting, primarily targeting the nonpregnant patient population, none has been universally successful.425 However, the prophylactic use of these agents either before or after umbilical cord clamping during cesarean delivery with neuraxial anesthesia has been demonstrated to be highly effective. Balki and Carvalho417 suggested an algorithm consisting of metoclopramide as a first-line agent, dimenhydrinate as a second-line agent, and ondansetron or granisetron as a third-line agent. Multimodal therapies may eventually prove the most effective.
TABLE 26-7
Agents for Prevention of Nausea and Vomiting in Women Undergoing Cesarean Delivery with Neuraxial Anesthesia*
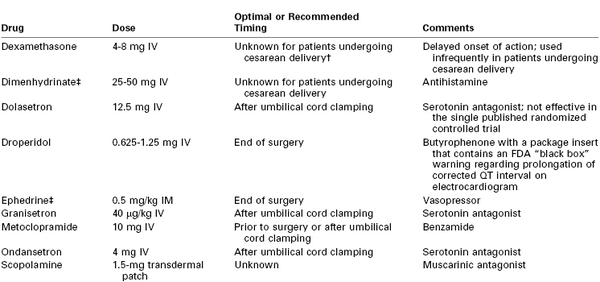
* If nausea and vomiting occur despite prophylaxis, the anesthesia provider should consider administration of a drug from a different pharmacologic class. There is no evidence that a second administration of the same drug within 6 hours provides additional benefit.
† Studies in nonobstetric patients suggest that administration of dexamethasone at induction of anesthesia results in better efficacy, but the optimal timing in obstetric patients is unclear.
‡ Has not been studied in patients undergoing cesarean delivery with neuraxial anesthesia; however, this drug has proved effective for prophylaxis of postoperative nausea and vomiting in studies of nonobstetric patients after administration of general anesthesia.
IM, intramuscularly; IV, intravenously.
Metoclopramide is the agent most frequently given, owing to its favorable prokinetic effects. Common side effects include dizziness, drowsiness, and fatigue; more rare side effects include extrapyramidal reactions and acute dystonias. In a meta-analysis of 11 studies of 702 patients undergoing cesarean delivery, Mishriky and Habib75 found that intravenous metoclopramide 10 mg, administered before neuraxial blockade, resulted in a significant reduction in intraoperative nausea (RR, 0.27; 95% CI, 0.16 to 0.45) and vomiting (RR, 0.14; 95% CI, 0.03 to 0.56) when metoclopramide, rather than placebo, was administrated before neuraxial blockade. This approach was more effective than giving metoclopramide after delivery, an approach that also resulted in a significant reduction in perioperative nausea and vomiting. Early postoperative nausea (RR, 0.47; 95% CI, 0.26 to 0.87) and vomiting (RR, 0.45; 95% CI, 0.21 to 0.93) were also reduced with metoclopramide.
Abouleish et al.426 found that ondansetron was more effective than placebo for the prevention of intraoperative nausea and vomiting during cesarean delivery with spinal anesthesia. Pan and Moore427 observed that when administered after umbilical cord clamping, ondansetron 4 mg was more effective than metoclopramide 10 mg in preventing nausea (26% versus 51%) but not vomiting (15% versus 18%). In a systematic review, Griffiths et al.428 concluded that the use of a serotonin antagonist (e.g., ondansetron, granisetron) in women undergoing neuraxial anesthesia for cesarean delivery was associated with a reduction in intraoperative nausea (but not vomiting) and postoperative nausea and vomiting, compared with placebo. Furthermore, serotonin antagonists were found to be more effective in reducing postoperative nausea than dopamine antagonists (e.g., metoclopramide, droperidol).428
In an animal toxicology study that was followed by a human clinical trial, Han et al.429 demonstrated no histologic changes in spinal cord tissues after epidural administration of ondansetron in rats. Moreover, in 80 women undergoing elective cesarean delivery with CSE anesthesia, the incidence of postoperative nausea was lower in women randomized to receive an epidural infusion of ondansetron than in women who received an intravenous infusion (8 mg over 48 hours) at both 24 and 48 hours.429 However, future investigations are needed to validate the safety of the neuraxial administration of ondansetron before this route of administration can be recommended.
In a meta-analysis, Allen et al.430 found that a single intravenous dose of dexamethasone 5 to 10 mg (but not 2.5 mg) compared with placebo reduced the incidence of postoperative nausea (23% versus 41%, respectively) and vomiting (20% versus 36%, respectively) in women who received neuraxial morphine for cesarean delivery. In a subgroup analysis, the authors identified a lower incidence of postoperative nausea and vomiting in women who received epidural morphine; however, they were unable to draw definitive conclusions regarding the effect of dexamethasone on postoperative nausea and vomiting in women who received spinal morphine because of the small sample size.
Harnett et al.431 observed that the administration of a transdermal scopolamine 1.5-mg patch after umbilical cord clamping was as effective as ondansetron 4 mg in the prevention of nausea and vomiting after cesarean delivery with spinal anesthesia (bupivacaine 12 mg with fentanyl 10 µg and morphine 0.2 mg); compared with placebo, both drugs resulted in a reduction in the incidence of postoperative emesis from approximately 60% to approximately 40%. The use of scopolamine may be limited by side effects, particularly dry mouth and blurry vision. The results of a systematic review suggest that the use of an anticholinergic agent (e.g., scopolamine, glycopyrrolate) in women undergoing neuraxial anesthesia for cesarean delivery results in a significant reduction in intraoperative nausea but not vomiting.428
Alternative therapies may play a role in preventing or treating perioperative nausea and vomiting. Several studies have found a favorable effect of acupressure on the P6 acupoint (on the inner aspect of the wrist). After spinal anesthesia for cesarean delivery, acupressure has been observed to result in a lower incidence of nausea (36% and 15%, respectively) and vomiting (17% and 9%, respectively), when compared with placebo.432 Two systematic reviews of randomized controlled trials involving a total of 649 women undergoing neuraxial anesthesia for cesarean delivery concluded that despite heterogeneity in the data, P6 stimulation (compared with placebo) appears to reduce intraoperative nausea but not intraoperative vomiting or postoperative nausea or vomiting.428,433
One study found that the administration of supplemental oxygen (FIO2 of 0.7) between umbilical cord clamping and the end of cesarean delivery with neuraxial anesthesia was not associated with a lower incidence of nausea and vomiting than the administration of room air.434 This finding is consistent with a meta-analysis435 of randomized controlled trials in the nonobstetric population; there was no difference in the incidence of nausea and vomiting with the use of supplemental oxygen (FIO2 of 0.8) compared with the use of lower oxygen concentrations (FIO2 of 0.3 to 0.4).435
A systematic review428 suggests that the administration of subhypnotic doses of propofol (0.5 to 1.5 mg/kg/h) for the reduction of intraoperative and postoperative nausea and vomiting in women undergoing neuraxial anesthesia for cesarean delivery is more effective than placebo; however, there are insufficient data to compare this method with other therapies.428
Perioperative Pain
In contrast to surveys performed in the general surgical patient population, in which patients have revealed a primary concern for postoperative nausea and vomiting, a survey performed in obstetric patients during their expectant parent class indicated that pain during and after cesarean delivery was their greatest concern.436 Inadequate neuraxial anesthesia leading to pain during labor or cesarean delivery was the most frequent “damaging event” in obstetric claims (31%) against the National Health Service in the United Kingdom from 1995 to 2007.437 Although pain during cesarean delivery was generally classified as “mild” or “moderate” (when accompanied by post-traumatic stress disorder), it represented an especially frequent cause of low-severity obstetric claims.437 Pain due to inadequate neuraxial anesthesia is also a common cause of obstetric complaints in the American Society of Anesthesiologists Closed-Claims Project database (see Chapter 33).
A preoperative discussion about pain and discomfort can help allay patient concerns. The anesthesia provider should (1) explain that there may be some deep pressure, pain, or discomfort during cesarean delivery performed with a neuraxial technique; (2) reassure the patient that the anesthesia provider will be present throughout the operation to administer additional analgesics or general anesthesia if necessary; (3) ensure and document adequacy of neuraxial blockade before the start of surgery; (4) communicate with the patient frequently during the procedure, specifically about pain or discomfort; and (5) treat pain when it arises, in agreement with the patient's wishes. During the postoperative visit, the anesthesia provider should address any concerns that may have arisen during or after surgery.
The anesthetic technique for the cesarean delivery may be altered because of postoperative pain management considerations. For example, an epidural catheter–based technique may be optimal for the patient with a significant pain history (e.g., sickle cell vaso-occlusive crises, chronic pain syndromes, drug-seeking behavior) so that the epidural catheter may be used for postoperative pain management.
By directly activating spinal and supraspinal opioid receptors, epidurally and spinally administered opioids blunt nociceptive input and produce analgesia of greater intensity than parenterally or intramuscularly administered doses.214 A number of opioids have been used in the epidural and spinal spaces; however, morphine has emerged as the leading agent for postcesarean analgesia, owing to its long duration of action and low cost (see Chapter 28). When morphine is administered intrathecally or epidurally, doses of 0.1 mg and 3.75 mg, respectively, appear to provide optimal analgesia after cesarean delivery.192,222 Neuraxial morphine has a peak analgesic effect at 60 to 90 minutes but continues to provide effective analgesia for up to 24 hours.222 Thus, intrathecal morphine is often co-administered with the local anesthetic and lipid-soluble opioid administered for spinal anesthesia, and epidural morphine is administered intraoperatively, after the umbilical cord is clamped, to allow sufficient time for the onset of epidural morphine analgesia before the regression of epidural anesthesia.
The local anesthetic selected for epidural anesthesia may influence postoperative analgesia. The epidural administration of 2-chloroprocaine has been observed to adversely affect the subsequent efficacy of epidural morphine analgesia,438 although this remains a matter of some dispute.439 The mechanism for this potential interaction remains unknown, however, the use of 2-chloroprocaine should be limited to emergency situations in which rapid augmentation of epidural anesthesia is desired (see earlier discussion).
Adverse effects of neuraxial morphine include pruritus, nausea and vomiting, urinary retention, and delayed respiratory depression. Frequent evaluations (hourly for the first 12 hours, and then every 2 hours for another 12 hours) should be conducted.440 Postpartum women who are morbidly obese or have preexisting respiratory issues (sleep apnea) are at greater risk for respiratory depression.
Postoperative pain has at least two components, somatic and visceral. A multimodal approach with different agents (e.g., ketamine) and techniques (e.g., infiltration, peritoneal spraying, transversus abdominis plane [TAP] block) provides the most effective postcesarean analgesia (see Chapters 27 and 28). The administration of nonsteroidal anti-inflammatory drugs has been associated with potential adverse effects (platelet dysfunction, uterine atony), and some investigators have expressed concerns related to neonatal exposure through breast milk. However, the American Academy of Pediatrics441 has stated that ibuprofen and ketorolac are compatible with breast-feeding.
Pruritus
The incidence of pruritus with the administration of opioids can be as high as 30% to 100%, and pruritus is more commonly observed when opioids are administered intrathecally than epidurally. Pruritus may be generalized or localized to regions of the nose, face, and chest and is typically self-limited in duration. The particular combinations and doses of opioid and local anesthetic may influence the incidence and severity of pruritus, and the addition of epinephrine to an opioid–local anesthetic solution has been observed to worsen pruritus.442 Pruritus does not represent an allergic reaction to the neuraxial opioid. If flushing, urticaria, rhinitis, bronchoconstriction, or cardiac symptoms also occur, an allergic reaction to another substance should be considered.
The cause of neuraxial opioid–induced pruritus is not known, although multiple theories have been proposed. They include µ-opioid receptor stimulation at the medullary dorsal horn, antagonism of inhibitory transmitters, and activation of an “itch center” in the central nervous system.443 Pharmacologic prophylaxis or treatment of pruritus may include an opioid antagonist, an opioid agonist/antagonist, droperidol, a serotonin antagonist (e.g., ondansetron), and/or a subhypnotic dose of propofol (Table 26-8).443 Intravenous administration of granisetron 3 mg may reduce the severity but not the incidence of intrathecal morphine–induced pruritus when compared with administration of ondansetron 8 mg.444 Dexamethasone in doses of 2.5 to 10 mg has not been found to reduce the incidence of pruritus associated with neuraxial morphine in women undergoing cesarean delivery.430 Although opioid antagonists, such as naltrexone and naloxone, and partial agonist/antagonists, such as nalbuphine, are currently the most effective treatments for pruritus, a single dose or continuous intravenous infusion of any of these agents may reverse analgesia. Because the primary mechanisms of opioid-induced pruritus appears unrelated to histamine release, antihistamines seldom represent a viable treatment option, although some benefit may be derived from the accompanying sedative qualities of these agents.
TABLE 26-8
Agents for Prevention or Treatment of Pruritus in Women Undergoing Cesarean Delivery
| Drug Class | Drug and Dose | Comments |
| Opioid antagonists | Naloxone infusion 1-2 µg/kg/h IV Naltrexone 6-9 mg PO | May reverse analgesia |
| Opioid agonist/antagonist | Nalbuphine 2.5-5 mg IV | |
| Sedative/hypnotic agent | Propofol 10-20 mg IV | Subhypnotic dose with conflicting evidence regarding efficacy in treating pruritus |
| Serotonin antagonist | Ondansetron 0.1 mg/kg IV | Conflicting evidence regarding efficacy in treating pruritus |
| Butyrophenone | Droperidol 1.25 mg IV | Package insert contains an FDA “black box” warning regarding prolongation of corrected QT interval on electrocardiogram |
IV, intravenously; PO, orally.
Hypothermia and Shivering
Perioperative hypothermia and shivering are commonly observed in women undergoing cesarean delivery, with a reported incidence of 66% and 85%, respectively.445,446 Hypothermia has been associated with a number of adverse outcomes in nonpregnant surgical patients, including wound infection, coagulopathy, increased blood and transfusion requirements, increased oxygen consumption, decreased metabolism, and prolonged recovery.447 In a systematic evaluation of randomized trials of normothermic and mildly hypothermic (34° C to 36° C) nonpregnant surgical patients, Rajagopalan et al.448 observed that even hypothermia less than 1° C below normal body temperature was associated with a 16% increase in blood loss (95% CI, 4% to 26%) and a 22% increase in risk for transfusion (95% CI, 3% to 37%).
Normally, core body temperature is tightly regulated within a narrow range of 36° C to 37° C. During pregnancy, despite an increase in maternal basal metabolic rate and energy released by the developing fetal and uteroplacental unit, maternal core temperature decreases, reaching a nadir at 12 weeks postpartum (36.4° C).449 Major causes of hypothermia during cesarean delivery are most likely related to core-to-periphery heat redistribution due to diminished vasoconstriction and shivering, particularly after neuraxial blockade, and impairment of centrally mediated thermoregulatory control.447
The onset and severity of hypothermia and shivering are associated with the patient's baseline thermal status, the perioperative environment, and the anesthetic technique and agents selected. Saito et al.450 observed that spinal anesthesia reduced the initial core temperature of patients undergoing cesarean delivery more rapidly than epidural anesthesia, but the overall incidence of shivering was similar. However, the severity of shivering was significantly less in the spinal group, possibly through the induction of a lower shivering threshold or the inhibition of thermoregulatory control as a function of the number of blocked dermatomes.451
The effect of neuraxial opioids on thermoregulation and shivering in patients undergoing cesarean delivery is not fully understood. Intravenously administered meperidine 12.5 to 25 mg is one of the most effective antishivering drugs known and is unique among opioids in producing this effect at doses not typically associated with respiratory depression. The mechanism of this effect does not appear to be related to κ-opioid receptor activity or inhibition of cholinergic receptors; instead, central α2B-adrenergic receptor stimulation may be involved.452 The α2-adrenergic receptor agonists clonidine (150 µg) and dexmedetomidine are effective antishivering agents that can lower the shivering threshold,452 although both agents may have limited use during pregnancy because of the potential to cause sedation, bradycardia, and hypotension. Other modalities have been used to prevent and treat hypothermia and shivering. Preoperative patient warming using forced air has been shown to reduce the incidence of perioperative and postoperative core hypothermia and shivering in patients undergoing cesarean delivery with epidural anesthesia.453 In contrast, a subsequent study found that perioperative forced-air warming did not prevent maternal hypothermia after cesarean delivery with spinal anesthesia.445 Lower limb wrapping has also been observed to have no effect on the incidence of hypothermia or shivering.454
Obstetric Complications
Postpartum Hemorrhage
A leading cause of maternal and fetal morbidity and mortality worldwide, mild to moderate obstetric hemorrhage can be masked by pregnancy-related physiologic changes. Underestimation of blood loss and inadequacy of resuscitation remain common problems (see Chapter 38).
Failure of the uterus to contract (uterine atony) after delivery accounts for most cases of postpartum hemorrhage and remains a leading cause of postpartum hysterectomy and blood transfusion. Each minute, 600 to 700 mL of blood flows through the placental intervillous spaces; thus, obstetric hemorrhage can rapidly result in maternal shock. Uterine atony occurs more commonly after cesarean delivery than after vaginal delivery, perhaps as a reflection of the condition(s) that prompted the cesarean delivery or possibly because surgery disrupts the normal postpartum response to uterotonic hormones and pharmacologic agents. Risk factors for uterine atony include (1) high parity, (2) an overdistended uterus (multiple gestation, macrosomia, polyhydramnios), (3) prolonged labor (augmented by oxytocin), (4) chorioamnionitis, (5) abnormalities in placentation (placenta accreta, increta, or percreta), (6) retained placental tissue, and (7) poor perfusion of the uterine myometrium (e.g., with hypotension).
Initial efforts to control uterine atony include uterine massage and exogenous oxytocin administration. Postpartum oxytocin is administered in a wide range of doses, methods, and timing patterns (e.g., before or after delivery of the placenta),455 although small doses of oxytocin are sufficient to produce adequate uterine contraction after cesarean delivery in most women.455 The effective bolus dose necessary for adequate uterine tone in 90% (ED90) of nonlaboring women undergoing cesarean delivery oxytocin is 0.35 unit456; in laboring women who have received approximately 10 hours of oxytocin augmentation, the ED90 is 2.99 units.457 George et al.458 estimated the ED90 of an oxytocin infusion to be 0.29 unit/min (95% CI, 0.15 to 0.43).
Women receiving oxytocin augmentation for labor have greater blood loss despite higher oxytocin doses; this appears to originate from signal attenuation and desensitization of the oxytocin receptors in a time- and concentration-dependent manner.459-462 Continued high-dose oxytocin exposure in the postpartum period can lead to acute receptor desensitization and render the myometrium less responsive to additional oxytocin but not to other uterotonic agents.462 Pregnancy causes a 180-fold increase in the concentration of oxytocin receptors with a significant proportion of this increase occurring just before the onset of labor463; this change in receptor number may have relevance to parturients who are delivering preterm infants.
Tsen and Balki455 have suggested a “rule of 3's” (oxytocin 3 units, 3-minute evaluation intervals, 3 total doses, and oxytocin 3 units/h for maintenance) protocol for the administration of oxytocin after delivery. The oxytocin 3 units is given as a slow bolus or as an infusion (30 units oxytocin in 500 mL of normal saline [50 mL]), at a rate no faster than over a period of 15 seconds. Uterine tone is reassessed again at 3 and 6 minutes; if inadequate, an additional dose of oxytocin 3 units is given. If uterine atony persists after three total doses of oxytocin, other uterotonic agents should be employed (see later discussion). The specific timing of the initial dose of oxytocin varies by individual practitioner; insufficient data are available to determine whether oxytocin administration immediately on emergence of the infant's shoulder or body or after placental delivery makes a difference in overall blood loss during cesarean delivery. After the establishment of adequate uterine tone, an infusion of 3 units/h for up to 5 hours is recommended.455
The administration of oxytocin as a rapid intravenous bolus causes hypotension and may result in cardiovascular collapse464,465; patients with preeclampsia may have an unpredictable hemodynamic response to oxytocin administration (i.e., decrease in cardiac output).466 Oxytocin has a direct relaxing effect on the vascular smooth muscle, which leads to decreased systemic vascular resistance, hypotension, and tachycardia.467 Tachycardia also may result from a direct effect on specific oxytocic receptors in the myocardium and subsequently result in alterations in atrioventricular conduction and myocardial repolarization.467 Chest pain and signs suggestive of myocardial ischemia and anaphylaxis may occur. Owing to the structural similarity of oxytocin to vasopressin, water intoxication may occur and, when severe, can lead to hyponatremia, confusion, convulsions, and coma.
If oxytocin proves ineffective, the ergot alkaloid derivative methylergonovine (0.2 mg) may be given intramuscularly to enhance uterine tone; onset time is within 10 minutes and the effect persists for 3 to 6 hours. Intravenous administration (in small divided doses) should be performed only with great caution, because intense vasoconstriction may lead to acute hypertension, seizures, cerebrovascular accident, retinal detachment, and myocardial arrest468; this possibility is of special concern in patients with preeclampsia or cardiac disease. Methylergonovine also has additive hemodynamic effects when given with sympathomimetic agents, such as ephedrine and phenylephrine. Nausea and vomiting are common side effects, which most likely reflect a direct central nervous system effect. The co-administration of oxytocin and ergometrine has been demonstrated to improve uterine contractions (as measured by the requirement for additional uterotonic agents) compared with the administration of oxytocin alone; however, the estimated blood loss was not different between groups, and nausea and vomiting were more prevalent with the oxytocin-ergometrine combination.469
Uterine sensitivity to prostaglandins increases with advancing gestation. 15-Methyl prostaglandin F2α causes a dose-dependent increase in the force and the frequency of uterine contractions. The initial recommended dose is 250 µg given intramuscularly; this dose can be repeated if necessary at 15- to 90-minute intervals up to a maximum of eight doses.470 Whether 15-methylprostaglandin F2α is more effective than oxytocin is controversial471; however, it clearly has a role in the treatment of refractory uterine atony.472 In approximately 20% of women, the following side effects occur (listed in descending order of frequency): diarrhea, hypertension, vomiting, fever, flushing, and tachycardia.472 Bronchospasm, pulmonary vasoconstriction, and oxyhemoglobin desaturation may also occur.
Rectal or sublingual administration of prostaglandin E1 (misoprostol) is a uterotonic agent with a rapid onset of action. A systematic review of prophylactic misoprostol (given orally or sublingually in doses ranging from 400 to 800 µg) administered for the active management of the third stage of labor concluded that it is more effective than placebo in preventing severe postpartum hemorrhage but less effective than conventional injectable uterotonic agents.473 Several large studies have assessed misoprostol's role in the treatment of postpartum hemorrhage. An editorial concluded that the drug was no more effective than oxytocin and was associated with more side effects.474 These side effects include hyperthermia and severe shivering. There may be a prophylactic role for this drug in low-resource settings where oxytocin is not available.
Preparation for Blood Loss
When risk factors for hemorrhage are identified, several preparatory steps can be considered. Iron supplementation and use of recombinant human erythropoietin are effective therapies for producing red blood cells, particularly in patients with preexisting anemia, renal failure, and/or reasons for preoperative donation of autologous blood.475 Antepartum erythropoietin administration may be of value in pregnant women at high risk for hemorrhage; however, additional investigation is needed to determine the optimal dosing, goals of therapy, and side-effect profiles. Hypertension, a problem associated with the use of erythropoietin in patients with renal failure, is a relevant concern during pregnancy. Although normal pregnancy is associated with a twofold to fourfold increase in maternal erythropoietin levels, isolated studies of the effect of erythropoietin on placental vessels suggest that dose-dependent vasoconstriction occurs.476 Observation of high erythropoietin levels in hypertensive and preeclamptic parturients has fueled speculation that erythropoietin participates in the humoral mechanisms responsible for preeclampsia and fetal growth restriction (also known as intrauterine growth restriction).477 A hyperglycosylated analogue of recombinant human erythropoietin (darbepoetin) has a threefold longer terminal half-life and results in a more rapid and greater erythropoietic response than recombinant human erythropoietin. This novel protein may be useful in the setting of anticipated or actual obstetric hemorrhage if concerns about its adverse effects are alleviated.475
The efficacy of preoperative autologous blood donation is limited by the maximum life span of stored blood; collection can start no sooner than 6 weeks before a planned delivery, with an average unit collection interval of 3 to 7 days. This method may be of some use in a woman with maternal antibodies to red blood cell antigens. The technique seems safe but has limited applicability and efficacy in obstetric patients (see Chapter 38).
Acute normovolemic hemodilution has the advantage of reducing the risk for administrative errors and bacterial contamination and allowing the infusion of whole blood replete with functional coagulation factors and platelets. This technique may reduce the need for transfusion in selected patients, and it may be acceptable to Jehovah's Witness patients at increased risk for blood loss during cesarean delivery.
The use of intraoperative red blood cell salvage in obstetric patients is gaining greater acceptance.478 In the past, obstetric anesthesia providers have expressed concern that intraoperative cell salvage might precipitate amniotic fluid embolism. Allam et al.478 noted that “existing cell salvage systems differ in their ability to clear contaminants and all require the addition of a leucocyte depletion filter.” The cell salvage process does not remove all fetal red blood cells and hemoglobin, and maternal isoimmunization may occur.479 Intraoperative cell salvage may be used to prevent morbidity and mortality in parturients who refuse homologous blood or in cases of intractable hemorrhage that may overwhelm blood bank supplies (see Chapter 38).34
Response to Blood Loss
Underestimation of blood loss and inadequate resuscitation are common problems in the management of obstetric hemorrhage. Rapid volume replacement is more important in maintaining tissue perfusion and oxygenation than the type of administered fluid. Colloids and blood products should be considered early, along with a request for assistance, establishment of a second large-bore intravenous catheter, and use of pressurized transfusion equipment. Many institutions require performance of a blood type and screen for parturients at high risk for hemorrhage undergoing a trial of labor for a planned vaginal delivery and in all parturients undergoing cesarean delivery. The immediate availability of two to four units of crossmatched packed red blood cells should be considered when the potential for significant blood loss appears imminent, such as in women suspected placenta accreta. In situations in which the need for emergency blood transfusion precedes the availability of crossmatched blood, type-specific (or type O, Rh-negative blood) should be administered. All institutions should consider the development of a massive transfusion protocol.480 Continued blood loss and hemodynamic instability despite transfusion of packed red blood cells is often an indication for placement of an arterial line and invasive central venous pressure monitoring; however, restoration of circulating volume takes precedence. Urine output, heart rate, blood pressure, and transthoracic or transesophageal echocardiography (TTE or TEE)481 assessments can assist in the rapid evaluation of the adequacy of volume resuscitation.
Fortunately, most pregnant women are healthy and tolerate modest blood loss well. Also, concerns about uteroplacental perfusion and fetal oxygenation are no longer present after delivery of the infant. The ASA Task Force on Blood Component Therapy482 has concluded that transfusion of packed red blood cells is rarely indicated when the hemoglobin concentration is greater than 10 g/dL and is almost always indicated when the hemoglobin concentration is less than 6 g/dL. Transfusion of platelets is rarely indicated unless the platelet count is less than 100,000/mm3 (unless platelet dysfunction and microvascular bleeding are present), and replacement of fibrinogen is rarely indicated unless the fibrinogen concentration is less than 150 mg/dL in the presence of microvascular bleeding. However, decreases in fibrinogen concentrations at levels higher than this threshold may serve as an early predictor of the severity of obstetric hemorrhage.483
The prophylactic placement of intravascular balloon occlusion catheters can facilitate the timely control of obstetric bleeding in some parturients at high risk for hemorrhage.484 Harnett et al.50 have recommended the placement of an epidural catheter prior to an intravascular balloon catheter for the following reasons: (1) once the balloon catheter is placed, flexion of the hips (during positioning for a neuraxial anesthetic technique) is discouraged, because it may result in balloon dislodgement or occlusion and subsequent thrombosis; (2) epidural anesthesia seems preferable to the use of local anesthesia with sedation for balloon catheter placement; (3) during balloon catheter placement, small amounts of heparin are sometimes used, and it seems preferable to have the epidural catheter in place prior to anticoagulation; and (4) should untoward events (e.g., fetal compromise, vessel rupture) occur during the procedure, the epidural catheter allows for rapid extension of anesthesia for cesarean delivery. With prior planning, operative procedures, including cesarean delivery, can be performed successfully under neuraxial anesthesia in the interventional radiology suite.50
When uterine bleeding occurs postpartum, the use of uterine tamponade balloon catheters has been demonstrated to tamponade and potentially treat intrauterine sources of bleeding and allow time to correct coagulopathy.485 A number of balloon catheters have been used by practitioners for uterine tamponade (e.g., Sengstaken-Blakemore esophageal balloon, Foley urinary catheters). Commercial balloon catheters designed specifically for uterine tamponade are now available. Some of the catheter designs allow for uterine cavity drainage, with variable success in function and ability to identify ongoing, significant bleeding.485 The balloons are often left in place for 24 to 48 hours, along with a vaginal pack and continued antibiotic administration. Distention of the uterus causes discomfort, and pain relief should be provided, either with systemic agents or epidural analgesia if a catheter is in situ. We typically provide continuous epidural analgesia, or patient-controlled epidural analgesia, with a dilute solution of local anesthetic and a lipid-soluble opioid, similar to that used for labor analgesia. The epidural catheter, regardless if used for analgesia, is typically kept in place until the uterine balloon is deflated to allow for rapid transition to surgical anesthesia if necessary.
The treatment of postpartum hemorrhage with human recombinant factor VIIa (rFVIIa), a vitamin K–dependent protein licensed for the treatment of bleeding episodes in patients who have hemophilia A or B with inhibitors to factor VIII or factor IX, remains controversial (see Chapter 38). Off-label administration of rFVIIa has resulted in cessation or reduction of severe bleeding refractory to standard hematologic or hemostatic support in a variety of animal and human trials and case reports or series.486 Administration of rFVIIa promotes clotting primarily through the extrinsic (tissue factor) pathway with some activation of the intrinsic pathway and should be combined with best practice use of blood products (i.e., blood product component therapy). Administered as an intravenous dose of 60 to 100 µg/kg, rFVIIa has been observed to have a clinical effect within 10 minutes in some cases. The half-life of the drug is 2 to 6 hours, and side effects consist primarily of hypertension, hypotension, bradycardia, renal dysfunction, and thromboembolism.486 Observational trials in the setting of traumatic injury are being performed, but to date there have been no large randomized studies of rFVIIa in obstetric patients.
Obstetric Hysterectomy
The incidence of cesarean hysterectomy and emergency postpartum hysterectomy ranges from 0.03% to 0.33% in different hospital settings and countries.487 Over a 14-year period in the United States, the rate of peripartum hysterectomy per 100,000 deliveries increased from 71.6 (1994 to 1995) to 82.6 (2006 to 2007); this increased rate was associated with an increased rate of abnormal placentation (e.g., placenta previa, placenta accreta) from 32.9 to 40.5 and an increased rate of uterine atony from 11.2 to 25.9 per 100,000 deliveries.488 Increasing rates of cesarean delivery, failed trial of labor, infection, intrauterine fetal death, and disseminated intravascular coagulation are other factors associated with cesarean hysterectomy. Improvements in ultrasonography, color flow Doppler ultrasonography, and magnetic resonance imaging have allowed earlier identification of some women with placenta accreta; however, limitations in diagnostic sensitivity and specificity exist.489
Cesarean hysterectomy is considered a high-risk procedure owing to the vascularity and size of the uterus and the distorted anatomic relationships. Bladder and ureteral injuries are common. Prior to performance of hysterectomy, various conservative medical and surgical treatment modalities may be attempted (see Chapter 38). The ligation or embolization of major and collateral uterine vessels with the assistance of an interventional radiologist is becoming more common, particularly in tertiary care settings.484
The amount of blood loss depends in part on whether the hysterectomy is elective or an emergency. In a prospective review of all obstetric hysterectomies performed at five university hospitals (1984 to 1987), Chestnut et al.490 found an average blood loss of 1319 mL and 2526 mL in 25 elective and 21 emergency cases, respectively, with an average replacement of 1.6 units and 6.6 units of blood, respectively. In a more recent study, Briery et al.491 performed a multicenter retrospective review of operative and postoperative outcomes in 30 elective and 35 emergency cesarean hysterectomies; the mean (± SD) blood loss for elective and emergency cases was 1963 ± 1180 mL and 2597 ± 1369 mL, with 33% and 66% of the patients requiring blood transfusion, respectively.
The anesthesia provider should consult with the obstetric team to discuss risk factors and the planned course of management. Preparations for the management of potentially massive blood loss should be made, which may include the placement of an epidural catheter and prophylactic intravascular balloon catheters (see earlier discussion). An elective cesarean hysterectomy, or a cesarean delivery in which the patient is at high risk for emergency hysterectomy, is not a contraindication to a neuraxial anesthetic technique, although a catheter-based technique is recommended. Moreover, the occurrence of an emergency cesarean hysterectomy does not necessitate immediate conversion to general anesthesia. Consideration should be given to (1) maternal history (e.g., number of prior abdominal operative procedures, which may lead to more scarring and adhesions); (2) the presence of risk factors (e.g., extent of placentation abnormality, coagulation status); (3) the potential difficulty of conversion to general anesthesia if required (e.g., presence of difficult airway, level of assistance available); (4) the desires and experience of the obstetric team; and (5) the desires of the patient. Chestnut et al.490 observed in their review that none of the 12 patients who received continuous epidural anesthesia for cesarean delivery (8 patients from the elective group and 4 from the emergency group) required intraoperative induction of general anesthesia for hysterectomy. In addition, there was no evidence that epidural anesthesia significantly affected blood loss, crystalloid replacement, or requirement for transfusion.
Given the morbidity and mortality associated with a peripartum hysterectomy, particularly if it is accompanied by significant blood loss, postoperative observation of the patient in a critical care setting should be considered. Wright et al.492 used the Nationwide Inpatient Sample database to compare women who underwent a peripartum hysterectomy (n = 4,967) with women who had a nonobstetric hysterectomy (n = 578,179) from 1998 to 2007; women in the peripartum hysterectomy group were nine and five times more likely to have bladder and ureteral injuries, respectively, and they had greater rates of reoperation, postoperative hemorrhage, wound complications, and venous thromboembolism. Perioperative cardiovascular, pulmonary, gastrointestinal, renal, and infectious morbidities were also higher for women who had peripartum hysterectomy (P < .001 for all). In addition, women undergoing peripartum hysterectomy had a higher rate of blood transfusion (46% versus 4%), a longer hospital stay, and a higher rate of perioperative mortality.492
Thromboembolic Events
Thromboembolic events constitute a major reason for peripartum maternal mortality, and risks for the development of pregnancy-associated thrombosis include an operative delivery, physiologic changes of pregnancy, and medical history and comorbidities (e.g. obesity, hemoglobinopathies, hypertension, and smoking).493 The risk for venous thromboembolism appears highest in the first postpartum week.493 A number of prophylactic interventions have been evaluated; however, the trials have been small and unable to provide robust results.494 Recommended thromboprophylaxis measures include hydration, early mobilization, pneumatic compression devices, and, in high-risk patients, pharmacologic prophylaxis (see Chapter 39).
References
1. Todman D. A history of caesarean section: from ancient world to the modern era. Aust N Z J Obstet Gynaecol. 2007;47:357–361.
2. Kochanek KD, Kirmeyer SE, Martin JA, et al. Annual summary of vital statistics: 2009. Pediatrics. 2012;129:338–348.
3. Gibbons L, Belizan JM, Lauer JA, et al. Inequities in the use of cesarean section deliveries in the world. Am J Obstet Gynecol. 2012;206:331 e1–19.
4. Clark SL, Belfort MA, Hankins GD, et al. Variation in the rates of operative delivery in the United States. Am J Obstet Gynecol. 2007;196:526 e1–5.
5. National Institutes of Health. State-of-the-science conference statement: Cesarean delivery on maternal request. March 27-29, 2006. Obstet Gynecol. 2006;107:1386–1397.
6. Jacobs-Jokhan D, Hofmeyr G. Extra-abdominal versus intra-abdominal repair of the uterine incision at caesarean section. Cochrane Database Syst Rev. 2004;(4).
7. Siddiqui M, Goldszmidt E, Fallah S, et al. Complications of exteriorized compared with in situ uterine repair at cesarean delivery under spinal anesthesia: a randomized controlled trial. Obstet Gynecol. 2007;110:570–575.
8. Nafisi S. Influence of uterine exteriorization versus in situ repair on post-Cesarean maternal pain: a randomized trial. Int J Obstet Anesth. 2007;16:135–138.
9. Belizan JM, Althabe F, Cafferata ML. Health consequences of the increasing caesarean section rates. Epidemiology. 2007;18:485–486.
10. Pallasmaa N, Ekblad U, Gissler M. Severe maternal morbidity and the mode of delivery. Acta Obstet Gynecol Scand. 2008;87:662–668.
11. Ecker JL, Frigoletto FD Jr. Cesarean delivery and the risk-benefit calculus. N Engl J Med. 2007;356:885–888.
12. Clark SL, Belfort MA, Dildy GA, et al. Maternal death in the 21st century: causes, prevention, and relationship to cesarean delivery. Am J Obstet Gynecol. 2008;199:36.e1–36.e5.
13. Visco AG, Viswanathan M, Lohr KN, et al. Cesarean delivery on maternal request: maternal and neonatal outcomes. Obstet Gynecol. 2006;108:1517–1529.
14. Hansen AK, Wisborg K, Uldbjerg N, Henriksen TB. Risk of respiratory morbidity in term infants delivered by elective caesarean section: cohort study. BMJ. 2008;336:85–87.
15. MacDorman MF, Declercq E, Menacker F, Malloy MH. Infant and neonatal mortality for primary cesarean and vaginal births to women with “no indicated risk,” United States, 1998-2001 birth cohorts. Birth. 2006;33:175–182.
16. Segal S, Su M, Gilbert P. The effect of a rapid change in availability of epidural analgesia on the cesarean delivery rate: a meta-analysis. Am J Obstet Gynecol. 2000;183:974–978.
17. Anim-Somuah M, Smyth RM, Jones L. Epidural versus non-epidural or no analgesia in labour. Cochrane Database Syst Rev. 2011;(12).
18. Van de Velde M, Teunkens A, Hanssens M, et al. Intrathecal sufentanil and fetal heart rate abnormalities: a double-blind, double placebo-controlled trial comparing two forms of combined spinal epidural analgesia with epidural analgesia in labor. Anesth Analg. 2004;98:1153–1159.
19. Mardirosoff C, Dumont L, Boulvain M, Tramer MR. Fetal bradycardia due to intrathecal opioids for labour analgesia: a systematic review. BJOG. 2002;109:274–281.
20. Bucklin BA, Hawkins JL, Anderson JR, Ullrich FA. Obstetric anesthesia workforce survey: twenty-year update. Anesthesiology. 2005;103:645–653.
21. The Royal College of Obstetricians and Gynaecologists. The Management of Breech Presentation. Guideline No. 20. Royal College of Obstetricians and Gynaecologists: London; 1999.
22. American College of Obstetricians and Gynecologists. External cephalic version. ACOG Practice Bulletin No. 13. (Reaffirmed 2012). Obstet Gynecol. 2000;95:1–7.
23. Sultan P, Carvalho B. Neuraxial blockade for external cephalic version: a systematic review. Int J Obstet Anesth. 2011;20:299–306.
24. Goetzinger KR, Harper LM, Tuuli MG, et al. Effect of regional anesthesia on the success rate of external cephalic version: a systematic review and meta-analysis. Obstet Gynecol. 2011;118:1137–1144.
25. Dufour P, Vinatier D, Puech F. The use of intravenous nitroglycerin for cervico-uterine relaxation: a review of the literature. Arch Gynecol Obstet. 1997;261:1–7.
26. Dufour P, Vinatier D, Vanderstichele S, et al. Intravenous nitroglycerin for intrapartum internal podalic version of the second non-vertex twin. Eur J Obstet Gynecol Reprod Biol. 1996;70:29–32.
27. Carvalho B. Nitroglycerin to facilitate insertion of a labor epidural. Anesthesiology. 2005;102:872.
28. Ekerhovd E, Brannstrom M, Weijdegard B, Norstrom A. Nitric oxide synthases in the human cervix at term pregnancy and effects of nitric oxide on cervical smooth muscle contractility. Am J Obstet Gynecol. 2000;183:610–616.
29. Morgan PJ, Kung R, Tarshis J. Nitroglycerin as a uterine relaxant: a systematic review. J Obstet Gynaecol Can. 2002;24:403–409.
30. Chandraharan E, Arulkumaran S. Acute tocolysis. Curr Opin Obstet Gynecol. 2005;17:151–156.
31. Mercier FJ, Benhamou D. Nitroglycerin for fetal head entrapment during vaginal breech delivery? Anesth Analg. 1995;81:654–655.
32. Rosaeg OP, Yarnell RW, Lindsay MP. The obstetrical anaesthesia assessment clinic: a review of six years experience. Can J Anaesth. 1993;40:346–356.
33. Rai MR, Lua SH, Popat M, Russell R. Antenatal anaesthetic assessment of high-risk pregnancy: a survey of UK practice. Int J Obstet Anesth. 2005;14:219–222.
34. American Society of Anesthesiologists Task Force on Obstetric Anesthesia. Practice guidelines for obstetric anesthesia. Anesthesiology. 2007;106:843–863.
35. American Society of Anesthesiologists Task Force on Preanesthesia Evaluation. Practice advisory for preanesthesia evaluation. Anesthesiology. 2012;116:522–538.
36. Hoehner PJ. Ethical aspects of informed consent in obstetric anesthesia—new challenges and solutions. J Clin Anesth. 2003;15:587–600.
37. Barkham M, Peters G, Pace N. Ethical and medico-legal aspects of obstetric anaesthesia. Anaesth Intensive Care Med. 2005;127–129.
38. Worthington R. Ethical dichotomies and methods of seeking consent. Anaesthesia. 2004;59:525–527.
39. Jenkins K, Baker AB. Consent and anaesthetic risk. Anaesthesia. 2003;58:962–984.
40. White SM, Baldwin TJ. Consent for anaesthesia. Anaesthesia. 2003;58:760–774.
41. Lanigan C, Reynolds F. Risk information supplied by obstetric anaesthetists in Britain and Ireland to mothers awaiting elective caesarean section. Int J Obstet Anesth. 1995;4:7–13.
42. Bethune L, Harper N, Lucas DN, et al. Complications of obstetric regional analgesia: how much information is enough? Int J Obstet Anesth. 2004;13:30–34.
43. Jackson GN, Robinson PN, Lucas DN, et al. What mothers know, and want to know, about the complications of general anaesthesia. Acta Anaesthesiol Scand. 2012;56:585–588.
44. Plaat F, McGlennan A. Women in the 21st century deserve more information: disclosure of material risk in obstetric anaesthesia. Int J Obstet Anesth. 2004;13:69–70.
45. National Collaborating Centre for Women's and Children's Health (Commissioned by the National Institute for Clinical Excellence). Clinical guideline: Caesarean section. [Available at] http://www.nice.org.uk/nicemedia/live/13620/57162/57162.pdf [Accessed January 2013] .
46. Paris JJ, Crone RK, Reardon FE. Ethical context for physician refusal of requested treatment. J Perinatol. 1991;11:273–275.
47. Khan KS, Wojdyla D, Say L, et al. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006;367:1066–1074.
48. Hannah ME, Hannah WJ, Hewson SA, et al. Planned caesarean section versus planned vaginal birth for breech presentation at term: a randomised multicentre trial. Term Breech Trial Collaborative Group. Lancet. 2000;356:1375–1383.
49. Stones RW, Paterson CM, Saunders NJ. Risk factors for major obstetric haemorrhage. Eur J Obstet Gynecol Reprod Biol. 1993;48:15–18.
50. Harnett MJ, Carabuena JM, Tsen LC, Kodali BS. Anesthesia for interventional radiology in parturients at risk of major hemorrhage at cesarean section delivery. Anesth Analg. 2006;103:1329–1330.
51. American Society of Anesthesiologists. Standards for Basic Anesthetic Monitoring. [Available at] http://www.asahq.org/For-Members/Standards-Guidelines-and-Statements.aspx [Accessed January 2013] .
52. Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005;111:697–716.
53. Pomini F, Scavo M, Ferrazzani S, et al. There is poor agreement between manual auscultatory and automated oscillometric methods for the measurement of blood pressure in normotensive pregnant women. J Matern Fetal Med. 2001;10:398–403.
54. Zakowski MI, Ramanathan S, Baratta JB, et al. Electrocardiographic changes during cesarean section: a cause for concern? Anesth Analg. 1993;76:162–167.
55. Palmer CM, Norris MC, Giudici MC, et al. Incidence of electrocardiographic changes during cesarean delivery under regional anesthesia. Anesth Analg. 1990;70:36–43.
56. Charbit B, Albaladejo P, Funck-Brentano C, et al. Prolongation of QTc interval after postoperative nausea and vomiting treatment by droperidol or ondansetron. Anesthesiology. 2005;102:1094–1100.
57. Charbit B, Mercier FJ, Benhamou D. Modification of Tp-e and QTc intervals during caesarean section under spinal anaesthesia. Anaesthesia. 2010;65:956–957.
58. Jonsson M, Hanson U, Lidell C, Norden-Lindeberg S. ST depression at caesarean section and the relation to oxytocin dose: a randomised controlled trial. BJOG. 2010;117:76–83.
59. Moran C, Ni Bhuinneain M, Geary M, et al. Myocardial ischaemia in normal patients undergoing elective Caesarean section: a peripartum assessment. Anaesthesia. 2001;56:1051–1058.
60. London MJ, Hollenberg M, Wong MG, et al. Intraoperative myocardial ischemia: localization by continuous 12-lead electrocardiography. Anesthesiology. 1988;69:232–241.
61. Shen CL, Ho YY, Hung YC, Chen PL. Arrhythmias during spinal anesthesia for Cesarean section. Can J Anaesth. 2000;47:393–397.
62. Robins K, Lyons G. Intraoperative awareness during general anesthesia for cesarean delivery. Anesth Analg. 2009;109:886–890.
63. Tully L, Gates S, Brocklehurst P, et al. Surgical techniques used during caesarean section operations: results of a national survey of practice in the UK. Eur J Obstet Gynecol Reprod Biol. 2002;102:120–126.
64. American College of Obstetricians and Gynecologists. Fetal monitoring prior to scheduled cesarean delivery. ACOG Committee Opinion No. 382. Obstet Gynecol. 2007;110:961–962.
65. Kirschling TE. The Handbook on Storing and Securing Medications. 2nd edition. Joint Commission Resources, Inc.: Oakbrook Terrace, IL; 2009.
66. U.S. Department of Justice Drug Enforcement Administration. Controlled substance schedules. [Available at] http://www.deadiversion.usdoj.gov/schedules/index.html [Accessed January 2013] .
67. U.S. Department of Health and Human Services Center for Medicare and Medicaid Service. Hospital Conditions of Participation. CMS-3122F, 72 FR 68672. [Available at] http://www.cms.gov/Regulations-and-Guidance/Regulations-and-Policies/QuarterlyProviderUpdates/downloads/cms3122f.pdf [Accessed January 2013] .
68. Wong CA, Loffredi M, Ganchiff JN, et al. Gastric emptying of water in term pregnancy. Anesthesiology. 2002;96:1395–1400.
69. Wong CA, McCarthy RJ, Fitzgerald PC, et al. Gastric emptying of water in obese pregnant women at term. Anesth Analg. 2007;105:751–755.
70. James CF, Gibbs CP. An evaluation of sodium citrate solutions. Anesth Analg. 1983;62:241.
71. Hauptfleisch JJ, Payne KA. An oral sodium citrate-citric acid non-particulate buffer in humans. Br J Anaesth. 1996;77:642–644.
72. Babaei A, Bhargava V, Aalam S, et al. Effect of proton pump inhibition on the gastric volume: assessed by magnetic resonance imaging. Aliment Pharmacol Ther. 2009;29:863–870.
73. Paranjothy S, Griffiths JD, Broughton HK, et al. Interventions at caesarean section for reducing the risk of aspiration pneumonitis. Cochrane Database Syst Rev. 2010;(1).
74. Kjaer K, Comerford M, Kondilis L, et al. Oral sodium citrate increases nausea amongst elective Cesarean delivery patients. Can J Anaesth. 2006;53:776–780.
75. Mishriky BM, Habib AS. Metoclopramide for nausea and vomiting prophylaxis during and after Caesarean delivery: a systematic review and meta-analysis. Br J Anaesth. 2012;108:374–383.
76. Murphy DF, Nally B, Gardiner J, Unwin A. Effect of metoclopramide on gastric emptying before elective and emergency caesarean section. Br J Anaesth. 1984;56:1113–1116.
77. Smaill F, Hofmeyr GJ. Antibiotic prophylaxis for cesarean section. Cochrane Database Syst Rev. 2002.
78. American College of Obstetricians and Gynecologists. Use of prophylactic antibiotics in labor and delivery. ACOG Practice Bulletin No. 120. Obstet Gynecol. 2011;117:1472–1483.
79. Alfirevic Z, Gyte GM, Dou L. Different classes of antibiotics given to women routinely for preventing infection at caesarean section. Cochrane Database Syst Rev. 2010;(1).
80. Pevzner L, Swank M, Krepel C, et al. Effects of maternal obesity on tissue concentrations of prophylactic cefazolin during cesarean delivery. Obstet Gynecol. 2011;117:877–882.
81. Costantine MM, Rahman M, Ghulmiyah L, et al. Timing of perioperative antibiotics for cesarean delivery: a metaanalysis. Am J Obstet Gynecol. 2008;199:301.e1–301.e6.
82. Tita AT, Hauth JC, Grimes A, et al. Decreasing incidence of postcesarean endometritis with extended-spectrum antibiotic prophylaxis. Obstet Gynecol. 2008;111:51–56.
83. Birnbach DJ, Meadows W, Stein DJ, et al. Comparison of povidone iodine and DuraPrep, an iodophor-in-isopropyl alcohol solution, for skin disinfection prior to epidural catheter insertion in parturients. Anesthesiology. 2003;98:164–169.
84. Reynolds F. Neurological infections after neuraxial anesthesia. Anesthesiol Clin. 2008;26:23–52.
85. Kinsella SM, Girgirah K, Scrutton MJ. Rapid sequence spinal anaesthesia for category-1 urgency caesarean section: a case series. Anaesthesia. 2010;65:664–669.
86. Ward KA, Spokes PJ, McAnulty JM. Case-control study of risk factors for hospitalization caused by pandemic (H1N1) 2009. Emerg Infect Dis. 2011;17:1409–1416.
87. American Society of Anesthesiologists Task Force on Infectious Complications Associated with Neuraxial Techniques. Practice advisory for the prevention, diagnosis, and management of infectious complications associated with neuraxial techniques. Anesthesiology. 2010;112:530–545.
88. Sakuragi T, Ishino H, Dan K. Bactericidal activity of clinically used local anesthetics on Staphylococcus aureus. Reg Anesth. 1996;21:239–242.
89. Seeberger MD, Staender S, Oertli D, et al. Efficacy of specific aseptic precautions for preventing propofol-related infections: analysis by a quality-assurance programme using the explicit outcome method. J Hosp Infect. 1998;39:67–70.
90. Scott DA, Fox JA, Cnaan A, et al. Resistance to fluid flow in veins. J Clin Monit. 1996;12:331–337.
91. de la Roche MR, Gauthier L. Rapid transfusion of packed red blood cells: effects of dilution, pressure, and catheter size. Ann Emerg Med. 1993;22:1551–1555.
92. Hepner DL, Tsen LC. Fluid management in obstetrics. Hahn R, Prough DS, Svensen CH. Perioperative Fluid Therapy. Informa Healthcare: New York; 2007:405–422.
93. Mercier FJ. Fluid loading for cesarean delivery under spinal anesthesia: have we studied all the options? Anesth Analg. 2011;113:677–680.
94. Dyer RA, Farina Z, Joubert IA, et al. Crystalloid preload versus rapid crystalloid administration after induction of spinal anaesthesia (coload) for elective caesarean section. Anaesth Intensive Care. 2004;32:351–357.
95. Banerjee A, Stocche RM, Angle P, Halpern SH. Preload or coload for spinal anesthesia for elective Cesarean delivery: a meta-analysis. Can J Anaesth. 2010;57:24–31.
96. Morgan PJ, Halpern SH, Tarshis J. The effects of an increase of central blood volume before spinal anesthesia for cesarean delivery: a qualitative systematic review. Anesth Analg. 2001;92:997–1005.
97. Bulach R, Myles PS, Russnak M. Double-blind randomized controlled trial to determine extent of amnesia with midazolam given immediately before general anaesthesia. Br J Anaesth. 2005;94:300–305.
98. Nishiyama T. Dose-finding study of intravenous midazolam for sedation and amnesia during spinal anesthesia in patients premedicated with intramuscular midazolam. J Anesth. 2004;18:257–261.
99. Olde E, van der Hart O, Kleber R, van Son M. Posttraumatic stress following childbirth: a review. Clin Psychol Rev. 2006;26:1–16.
100. Frölich MA, Burchfield DJ, Euliano TY, Caton D. A single dose of fentanyl and midazolam prior to Cesarean section have no adverse neonatal effects. Can J Anaesth. 2006;53:79–85.
101. Kinsella SM, Lohmann G. Supine hypotensive syndrome. Obstet Gynecol. 1994;83:774–788.
102. Marx GF, Bassell GM. Hazards of the supine position in pregnancy. Clin Obstet Gynaecol. 1982;9:255–271.
103. Pirhonen JP, Erkkola RU. Uterine and umbilical flow velocity waveforms in the supine hypotensive syndrome. Obstet Gynecol. 1990;76:176–179.
104. Kinsella SM. Lateral tilt for pregnant women: why 15 degrees? Anaesthesia. 2003;58:835–836.
105. Cluver C, Novikova N, Hofmeyr GJ, Hall DR. Maternal position during caesarean section for preventing maternal and neonatal complications. Cochrane Database Syst Rev. 2010;(6).
106. Morgan DJ, Paull JD, Toh CT, Blackman GL. Aortocaval compression and plasma concentrations of thiopentone at caesarean section. Br J Anaesth. 1984;56:349–354.
107. Jones SJ, Kinsella SM, Donald FA. Comparison of measured and estimated angles of table tilt at Caesarean section. Br J Anaesth. 2003;90:86–87.
108. Paech MJ. Should we take a different angle in managing pregnant women at delivery? Attempting to avoid the ‘supine hypotensive syndrome. Anaesth Intensive Care. 2008;36:775–777.
109. Loke GP, Chan EH, Sia AT. The effect of 10 degrees head-up tilt in the right lateral position on the systemic blood pressure after subarachnoid block for Caesarean section. Anaesthesia. 2002;57:169–172.
110. Hignett R, Fernando R, McGlennan A, et al. A randomized crossover study to determine the effect of a 30 degrees head-up versus a supine position on the functional residual capacity of term parturients. Anesth Analg. 2011;113:1098–1102.
111. Dixon BJ, Dixon JB, Carden JR, et al. Preoxygenation is more effective in the 25 degrees head-up position than in the supine position in severely obese patients: a randomized controlled study. Anesthesiology. 2005;102:1110–1115.
112. Mhyre JM. Anesthetic management for the morbidly obese pregnant woman. Int Anesthesiol Clin. 2007;45:51–70.
113. Miyabe M, Namiki A. The effect of head-down tilt on arterial blood pressure after spinal anesthesia. Anesth Analg. 1993;76:549–552.
114. Miyabe M, Sato S. The effect of head-down tilt position on arterial blood pressure after spinal anesthesia for cesarean delivery. Reg Anesth. 1997;22:239–242.
115. Sinclair CJ, Scott DB, Edstrom HH. Effect of the Trendelenberg position on spinal anaesthesia with hyperbaric bupivacaine. Br J Anaesth. 1982;54:497–500.
116. Tsen LC. Neuraxial techniques for labor analgesia should be placed in the lateral position. Int J Obstet Anesth. 2008;17:146–149.
117. Polley LS. Neuraxial techniques for labor analgesia should be placed in the lateral position. Int J Obstet Anesth. 2008;17:149–152.
118. Jones AY, Dean E. Body position change and its effect on hemodynamic and metabolic status. Heart Lung. 2004;33:281–290.
119. Suonio S, Simpanen AL, Olkkonen H, Haring P. Effect of the left lateral recumbent position compared with the supine and upright positions on placental blood flow in normal late pregnancy. Ann Clin Res. 1976;8:22–26.
120. Andrews PJ, Ackerman WE 3rd, Juneja MM. Aortocaval compression in the sitting and lateral decubitus positions during extradural catheter placement in the parturient. Can J Anaesth. 1993;40:320–324.
121. Armstrong S, Fernando R, Columb M, Jones T. Cardiac index in term pregnant women in the sitting, lateral, and supine positions: an observational, crossover study. Anesth Analg. 2011;113:318–322.
122. Vincent RD, Chestnut DH. Which position is more comfortable for the parturient during identification of the epidural space? Int J Obstet Anesth. 1991;1:9–11.
123. Igarashi T, Hirabayashi Y, Shimizu R, et al. The fiberscopic findings of the epidural space in pregnant women. Anesthesiology. 2000;92:1631–1636.
124. Hirabayashi Y, Shimizu R, Fukuda H, et al. Effects of the pregnant uterus on the extradural venous plexus in the supine and lateral positions, as determined by magnetic resonance imaging. Br J Anaesth. 1997;78:317–319.
125. Bahar M, Chanimov M, Cohen ML, et al. Lateral recumbent head-down posture for epidural catheter insertion reduces intravascular injection. Can J Anaesth. 2001;48:48–53.
126. Bahar M, Chanimov M, Cohen ML, et al. The lateral recumbent head-down position decreases the incidence of epidural venous puncture during catheter insertion in obese parturients. Can J Anaesth. 2004;51:577–580.
127. Hirasawa Y, Bashir WA, Smith FW, et al. Postural changes of the dural sac in the lumbar spines of asymptomatic individuals using positional stand-up magnetic resonance imaging. Spine (Phila Pa 1976). 2007;32:E136–E140.
128. Yun EM, Marx GF, Santos AC. The effects of maternal position during induction of combined spinal-epidural anesthesia for cesarean delivery. Anesth Analg. 1998;87:614–618.
129. Fox GS, Houle GL. Acid-base studies in elective caesarean sections during epidural and general anaesthesia. Can Anaesth Soc J. 1971;18:60–71.
130. Cogliano MS, Graham AC, Clark VA. Supplementary oxygen administration for elective Caesarean section under spinal anaesthesia. Anaesthesia. 2002;57:66–69.
131. Khaw KS, Wang CC, Ngan Kee WD, et al. Effects of high inspired oxygen fraction during elective caesarean section under spinal anaesthesia on maternal and fetal oxygenation and lipid peroxidation. Br J Anaesth. 2002;88:18–23.
132. Ryerson EG, Block AJ. Oxygen as a drug. Burton GG, Hodgkin JE, Ward JJ. Respiratory Care: A Guide to Clinical Practice. 3rd edition. JB Lippincott: Philadelphia; 1991:319–339.
133. Harrop-Griffiths AW, Ravalia A, Browne DA, Robinson PN. Regional anaesthesia and cough effectiveness: a study in patients undergoing caesarean section. Anaesthesia. 1991;46:11–13.
134. Kelly MC, Fitzpatrick KT, Hill DA. Respiratory effects of spinal anaesthesia for caesarean section. Anaesthesia. 1996;51:1120–1122.
135. Khaw KS, Ngan Kee WD, Lee A, et al. Supplementary oxygen for elective Caesarean section under spinal anaesthesia: useful in prolonged uterine incision-to-delivery interval? Br J Anaesth. 2004;92:518–522.
136. McCord JM. The evolution of free radicals and oxidative stress. Am J Med. 2000;108:652–659.
137. Rogers MS, Murray HG, Wang CC, et al. Oxidative stress in the fetal lamb brain following intermittent umbilical cord occlusion: a path analysis. BJOG. 2001;108:1283–1290.
138. Rogers MS, Wang W, Mongelli M, et al. Lipid peroxidation in cord blood at birth: a marker of fetal hypoxia during labour. Gynecol Obstet Invest. 1997;44:229–233.
139. Yamada T, Yoneyama Y, Sawa R, Araki T. Effects of maternal oxygen supplementation on fetal oxygenation and lipid peroxidation following a single umbilical cord occlusion in fetal goats. J Nihon Med Sch. 2003;70:165–171.
140. Buhimschi IA, Buhimschi CS, Pupkin M, Weiner CP. Beneficial impact of term labor: nonenzymatic antioxidant reserve in the human fetus. Am J Obstet Gynecol. 2003;189:181–188.
141. Thorp JA, Trobough T, Evans R, et al. The effect of maternal oxygen administration during the second stage of labor on umbilical cord blood gas values: a randomized controlled prospective trial. Am J Obstet Gynecol. 1995;172:465–474.
142. Khazin AF, Hon EH, Hehre FW. Effects of maternal hyperoxia on the fetus. I. Oxygen tension. Am J Obstet Gynecol. 1971;109:628–637.
143. Vento M, Asensi M, Sastre J, et al. Resuscitation with room air instead of 100% oxygen prevents oxidative stress in moderately asphyxiated term neonates. Pediatrics. 2001;107:642–647.
144. Saugstad OD, Ramji S, Soll RF, Vento M. Resuscitation of newborn infants with 21% or 100% oxygen: an updated systematic review and meta-analysis. Neonatology. 2008;94:176–182.
145. Shibli KU, Russell IF. A survey of anaesthetic techniques used for caesarean section in the UK in 1997. Int J Obstet Anesth. 2000;9:160–167.
146. Weiniger CF, Ivri S, Ioscovich A, et al. Obstetric anesthesia units in Israel: a national questionnaire-based survey. Int J Obstet Anesth. 2010;19:410–416.
147. Palanisamy A, Mitani AA, Tsen LC. General anesthesia for cesarean delivery at a tertiary care hospital from 2000 to 2005: a retrospective analysis and 10-year update. Int J Obstet Anesth. 2011;20:10–16.
148. Ismail S, Huda A. An observational study of anaesthesia and surgical time in elective caesarean section: spinal compared with general anaesthesia. Int J Obstet Anesth. 2009;18:352–355.
149. Hawkins JL, Koonin LM, Palmer SK, Gibbs CP. Anesthesia-related deaths during obstetric delivery in the United States, 1979-1990. Anesthesiology. 1997;86:277–284.
150. Hawkins JL, Chang J, Palmer SK, et al. Anesthesia-related maternal mortality in the United States: 1979-2002. Obstet Gynecol. 2011;117:69–74.
151. Chestnut DH. Anesthesia and maternal mortality. Anesthesiology. 1997;86:273–276.
152. Afolabi BB, Lesi FE. Regional versus general anaesthesia for caesarean section. Cochrane Database Syst Rev. 2012;(10).
153. Morgan BM, Aulakh JM, Barker JP, et al. Anaesthetic morbidity following caesarean section under epidural or general anaesthesia. Lancet. 1984;1:328–330.
154. Reynolds F, Seed PT. Anaesthesia for Caesarean section and neonatal acid-base status: a meta-analysis. Anaesthesia. 2005;60:636–653.
155. Russell IF. Levels of anaesthesia and intraoperative pain at caesarean section under regional block. Int J Obstet Anesth. 1995;4:71–77.
156. Russell IF. A comparison of cold, pinprick and touch for assessing the level of spinal block at caesarean section. Int J Obstet Anesth. 2004;13:146–152.
157. Bourne TM, deMelo AE, Bastianpillai BA, May AE. A survey of how British obstetric anaesthetists test regional anaesthesia before caesarean section. Anaesthesia. 1997;52:901–903.
158. Burns SM, Barclay PM. Regional anaesthesia for caesarean section. Curr Anaesth Crit Care. 2000;11:73–79.
159. Riley ET, Cohen SE, Macario A, et al. Spinal versus epidural anesthesia for cesarean section: a comparison of time efficiency, costs, charges, and complications. Anesth Analg. 1995;80:709–712.
160. Garry M, Davies S. Failure of regional blockade for caesarean section. Int J Obstet Anesth. 2002;11:9–12.
161. Kuhnert BR, Philipson EH, Pimental R, et al. Lidocaine disposition in mother, fetus, and neonate after spinal anesthesia. Anesth Analg. 1986;65:139–144.
162. Kuhnert BR, Zuspan KJ, Kuhnert PM, et al. Bupivacaine disposition in mother, fetus, and neonate after spinal anesthesia for cesarean section. Anesth Analg. 1987;66:407–412.
163. Tsen LC, Hepner DL. Needles used for spinal anesthesia. Expert Rev Med Devices. 2006;3:499–508.
164. Kestin IG. Spinal anaesthesia in obstetrics. Br J Anaesth. 1991;66:596–607.
165. Greene NM. Distribution of local anesthetic solutions within the subarachnoid space. Anesth Analg. 1985;64:715–730.
166. Carvalho B, Durbin M, Drover DR, et al. The ED50 and ED95 of intrathecal isobaric bupivacaine with opioids for cesarean delivery. Anesthesiology. 2005;103:606–612.
167. Sarvela PJ, Halonen PM, Korttila KT. Comparison of 9 mg of intrathecal plain and hyperbaric bupivacaine both with fentanyl for cesarean delivery. Anesth Analg. 1999;89:1257–1262.
168. Vercauteren MP, Coppejans HC, Hoffmann VL, et al. Small-dose hyperbaric versus plain bupivacaine during spinal anesthesia for cesarean section. Anesth Analg. 1998;86:989–993.
169. Ben-David B, Miller G, Gavriel R, Gurevitch A. Low-dose bupivacaine-fentanyl spinal anesthesia for cesarean delivery. Reg Anesth Pain Med. 2000;25:235–239.
170. Bryson GL, Macneil R, Jeyaraj LM, Rosaeg OP. Small dose spinal bupivacaine for Cesarean delivery does not reduce hypotension but accelerates motor recovery. Can J Anaesth. 2007;54:531–537.
171. Norris MC. Height, weight, and the spread of subarachnoid hyperbaric bupivacaine in the term parturient. Anesth Analg. 1988;67:555–558.
172. Hartwell BL, Aglio LS, Hauch MA, Datta S. Vertebral column length and spread of hyperbaric subarachnoid bupivacaine in the term parturient. Reg Anesth. 1991;16:17–19.
173. McDonald SB, Liu SS, Kopacz DJ, Stephenson CA. Hyperbaric spinal ropivacaine: a comparison to bupivacaine in volunteers. Anesthesiology. 1999;90:971–977.
174. Ogun CO, Kirgiz EN, Duman A, et al. Comparison of intrathecal isobaric bupivacaine-morphine and ropivacaine-morphine for Caesarean delivery. Br J Anaesth. 2003;90:659–664.
175. Khaw KS, Ngan Kee WD, Wong EL, et al. Spinal ropivacaine for cesarean section: a dose-finding study. Anesthesiology. 2001;95:1346–1350.
176. Khaw KS, Ngan Kee WD, Wong M, et al. Spinal ropivacaine for cesarean delivery: a comparison of hyperbaric and plain solutions. Anesth Analg. 2002;94:680–685.
177. Gautier P, De Kock M, Huberty L, et al. Comparison of the effects of intrathecal ropivacaine, levobupivacaine, and bupivacaine for Caesarean section. Br J Anaesth. 2003;91:684–689.
178. Saravanan S, Robinson AP, Qayoum Dar A, et al. Minimum dose of intrathecal diamorphine required to prevent intraoperative supplementation of spinal anaesthesia for Caesarean section. Br J Anaesth. 2003;91:368–372.
179. Choi DH, Ahn HJ, Kim MH. Bupivacaine-sparing effect of fentanyl in spinal anesthesia for cesarean delivery. Reg Anesth Pain Med. 2000;25:240–245.
180. Dahl JB, Jeppesen IS, Jorgensen H, et al. Intraoperative and postoperative analgesic efficacy and adverse effects of intrathecal opioids in patients undergoing cesarean section with spinal anesthesia: a qualitative and quantitative systematic review of randomized controlled trials. Anesthesiology. 1999;91:1919–1927.
181. Dickenson AH. Spinal cord pharmacology of pain. Br J Anaesth. 1995;75:193–200.
182. Ginosar Y, Columb MO, Cohen SE, et al. The site of action of epidural fentanyl infusions in the presence of local anesthetics: a minimum local analgesic concentration infusion study in nulliparous labor. Anesth Analg. 2003;97:1439–1445.
183. Palmer CM, Voulgaropoulos D, Alves D. Subarachnoid fentanyl augments lidocaine spinal anesthesia for cesarean delivery. Reg Anesth. 1995;20:389–394.
184. Dahlgren G, Hultstrand C, Jakobsson J, et al. Intrathecal sufentanil, fentanyl, or placebo added to bupivacaine for cesarean section. Anesth Analg. 1997;85:1288–1293.
185. Cooper DW, Lindsay SL, Ryall DM, et al. Does intrathecal fentanyl produce acute cross-tolerance to i.v. morphine? Br J Anaesth. 1997;78:311–313.
186. Carvalho B, Drover DR, Ginosar Y, et al. Intrathecal fentanyl added to bupivacaine and morphine for cesarean delivery may induce a subtle acute opioid tolerance. Int J Obstet Anesth. 2012;21:29–34.
187. Manullang TR, Viscomi CM, Pace NL. Intrathecal fentanyl is superior to intravenous ondansetron for the prevention of perioperative nausea during cesarean delivery with spinal anesthesia. Anesth Analg. 2000;90:1162–1166.
188. Shende D, Cooper GM, Bowden MI. The influence of intrathecal fentanyl on the characteristics of subarachnoid block for caesarean section. Anaesthesia. 1998;53:706–710.
189. Courtney MA, Bader AM, Hartwell B, et al. Perioperative analgesia with subarachnoid sufentanil administration. Reg Anesth. 1992;17:274–278.
190. Braga Ade F, Braga FS, Poterio GM, et al. Sufentanil added to hyperbaric bupivacaine for subarachnoid block in Caesarean section. Eur J Anaesthesiol. 2003;20:631–635.
191. Fournier R, Van Gessel E, Macksay M, Gamulin Z. Onset and offset of intrathecal morphine versus nalbuphine for postoperative pain relief after total hip replacement. Acta Anaesthesiol Scand. 2000;44:940–945.
192. Palmer CM, Emerson S, Volgoropolous D, Alves D. Dose-response relationship of intrathecal morphine for postcesarean analgesia. Anesthesiology. 1999;90:437–444.
193. Husaini SW, Russell IF. Intrathecal diamorphine compared with morphine for postoperative analgesia after caesarean section under spinal anaesthesia. Br J Anaesth. 1998;81:135–139.
194. Carvalho B. Respiratory depression after neuraxial opioids in the obstetric setting. Anesth Analg. 2008;107:956–961.
195. Kaufman RD, Gabathuler ML, Bellville JW. Potency, duration of action and pA2 in man of intravenous naloxone measured by reversal of morphine-depressed respiration. J Pharmacol Exp Ther. 1981;219:156–162.
196. Lui AC, Polis TZ, Cicutti NJ. Densities of cerebrospinal fluid and spinal anaesthetic solutions in surgical patients at body temperature. Can J Anaesth. 1998;45:297–303.
197. Abouleish EI. Epinephrine improves the quality of spinal hyperbaric bupivacaine for cesarean section. Anesth Analg. 1987;66:395–400.
198. Randalls B, Broadway JW, Browne DA, Morgan BM. Comparison of four subarachnoid solutions in a needle-through-needle technique for elective caesarean section. Br J Anaesth. 1991;66:314–318.
199. Lavand’homme PM, Roelants F, Waterloos H, et al. An evaluation of the postoperative antihyperalgesic and analgesic effects of intrathecal clonidine administered during elective cesarean delivery. Anesth Analg. 2008;107:948–955.
200. Krukowski JA, Hood DD, Eisenach JC, et al. Intrathecal neostigmine for post-cesarean section analgesia: dose response. Anesth Analg. 1997;84:1269–1275.
201. Chung CJ, Kim JS, Park HS, Chin YJ. The efficacy of intrathecal neostigmine, intrathecal morphine, and their combination for post-cesarean section analgesia. Anesth Analg. 1998;87:341–346.
202. Liu SS, Hodgson PS, Moore JM, et al. Dose-response effects of spinal neostigmine added to bupivacaine spinal anesthesia in volunteers. Anesthesiology. 1999;90:710–717.
203. Cappiello E, O’Rourke N, Segal S, Tsen LC. A randomized trial of dural puncture epidural technique compared with the standard epidural technique for labor analgesia. Anesth Analg. 2008;107:1646–1651.
204. Sakura S, Sumi M, Morimoto N, Saito Y. The addition of epinephrine increases intensity of sensory block during epidural anesthesia with lidocaine. Reg Anesth Pain Med. 1999;24:541–546.
205. Sakura S, Sumi M, Kushizaki H, et al. Concentration of lidocaine affects intensity of sensory block during lumbar epidural anesthesia. Anesth Analg. 1999;88:123–127.
206. Hillyard SG, Bate TE, Corcoran TB, et al. Extending epidural analgesia for emergency Caesarean section: a meta-analysis. Br J Anaesth. 2011;107:668–678.
207. Toledo P, McCarthy RJ, Ebarvia MJ, et al. The interaction between epidural 2-chloroprocaine and morphine: a randomized controlled trial of the effect of drug administration timing on the efficacy of morphine analgesia. Anesth Analg. 2009;109:168–173.
208. Albright GA. Cardiac arrest following regional anesthesia with etidocaine or bupivacaine. Anesthesiology. 1979;51:285–287.
209. Santos AC, DeArmas PI. Systemic toxicity of levobupivacaine, bupivacaine, and ropivacaine during continuous intravenous infusion to nonpregnant and pregnant ewes. Anesthesiology. 2001;95:1256–1264.
210. Bader AM, Tsen LC, Camann WR, et al. Clinical effects and maternal and fetal plasma concentrations of 0.5% epidural levobupivacaine versus bupivacaine for cesarean delivery. Anesthesiology. 1999;90:1596–1601.
211. Faccenda KA, Simpson AM, Henderson DJ, et al. A comparison of levobupivacaine 0.5% and racemic bupivacaine 0.5% for extradural anesthesia for caesarean section. Reg Anesth Pain Med. 2003;28:394–400.
212. Datta S, Camann W, Bader A, VanderBurgh L. Clinical effects and maternal and fetal plasma concentrations of epidural ropivacaine versus bupivacaine for cesarean section. Anesthesiology. 1995;82:1346–1352.
213. Bernards CM, Shen DD, Sterling ES, et al. Epidural, cerebrospinal fluid, and plasma pharmacokinetics of epidural opioids (part 1): differences among opioids. Anesthesiology. 2003;99:455–465.
214. Ginosar Y, Riley ET, Angst MS. The site of action of epidural fentanyl in humans: the difference between infusion and bolus administration. Anesth Analg. 2003;97:1428–1438.
215. Helbo-Hansen HS, Bang U, Lindholm P, Klitgaard NA. Maternal effects of adding epidural fentanyl to 0.5% bupivacaine for caesarean section. Int J Obstet Anesth. 1993;2:21–26.
216. Gaffud MP, Bansal P, Lawton C, et al. Surgical analgesia for cesarean delivery with epidural bupivacaine and fentanyl. Anesthesiology. 1986;65:331–334.
217. Helbo-Hansen HS, Bang U, Lindholm P, Klitgaard NA. Neonatal effects of adding epidural fentanyl to 0.5% bupivacaine for caesarean section. Int J Obstet Anesth. 1993;2:27–33.
218. Eichenberger U, Giani C, Petersen-Felix S, et al. Lumbar epidural fentanyl: segmental spread and effect on temporal summation and muscle pain. Br J Anaesth. 2003;90:467–473.
219. Vertommen JD, Van Aken H, Vandermeulen E, et al. Maternal and neonatal effects of adding epidural sufentanil to 0.5% bupivacaine for cesarean delivery. J Clin Anesth. 1991;3:371–376.
220. Madej TH, Strunin L. Comparison of epidural fentanyl with sufentanil: analgesia and side effects after a single bolus dose during elective caesarean section. Anaesthesia. 1987;42:1156–1161.
221. Grass JA, Sakima NT, Schmidt R, et al. A randomized, double-blind, dose-response comparison of epidural fentanyl versus sufentanil analgesia after cesarean section. Anesth Analg. 1997;85:365–371.
222. Palmer CM, Nogami WM, Van Maren G, Alves DM. Postcesarean epidural morphine: a dose-response study. Anesth Analg. 2000;90:887–891.
223. Carvalho B, Roland LM, Chu LF, et al. Single-dose, extended-release epidural morphine (DepoDur) compared to conventional epidural morphine for post-cesarean pain. Anesth Analg. 2007;105:176–183.
224. Hallworth SP, Fernando R, Bell R, et al. Comparison of intrathecal and epidural diamorphine for elective caesarean section using a combined spinal-epidural technique. Br J Anaesth. 1999;82:228–232.
225. Bloor GK, Thompson M, Chung N. A randomised, double-blind comparison of subarachnoid and epidural diamorphine for elective caesarean section using a combined spinal-epidural technique. Int J Obstet Anesth. 2000;9:233–237.
226. Eisenach JC, D’Angelo R, Taylor C, Hood DD. An isobolographic study of epidural clonidine and fentanyl after cesarean section. Anesth Analg. 1994;79:285–290.
227. Kaya FN, Sahin S, Owen MD, Eisenach JC. Epidural neostigmine produces analgesia but also sedation in women after cesarean delivery. Anesthesiology. 2004;100:381–385.
228. Mather LE, Tucker GT, Murphy TM, et al. The effects of adding adrenaline to etidocaine and lignocaine in extradural anaesthesia II: Pharmacokinetics. Br J Anaesth. 1976;48:989–994.
229. Murphy TM, Mather LE, Stanton-Hicks M, et al. The effects of adding adrenaline to etidocaine and lignocaine in extradural anaesthesia. I. Block characteristics and cardiovascular effects. Br J Anaesth. 1976;48:893–898.
230. Bernards CM, Shen DD, Sterling ES, et al. Epidural, cerebrospinal fluid, and plasma pharmacokinetics of epidural opioids (part 2): effect of epinephrine. Anesthesiology. 2003;99:466–475.
231. Hadzic A, Vloka J, Patel N, Birnbach D. Hypertensive crisis after a successful placement of an epidural anesthetic in a hypertensive parturient: case report. Reg Anesth. 1995;20:156–158.
232. Albright GA, Jouppila R, Hollmen AI, et al. Epinephrine does not alter human intervillous blood flow during epidural anesthesia. Anesthesiology. 1981;54:131–135.
233. Marx GF, Elstein ID, Schuss M, et al. Effects of epidural block with lignocaine and lignocaine-adrenaline on umbilical artery velocity wave ratios. Br J Obstet Gynaecol. 1990;97:517–520.
234. Alahuhta S, Rasanen J, Jouppila R, et al. Effects of extradural bupivacaine with adrenaline for caesarean section on uteroplacental and fetal circulation. Br J Anaesth. 1991;67:678–682.
235. Alahuhta S, Rasanen J, Jouppila P, et al. Uteroplacental and fetal circulation during extradural bupivacaine-adrenaline and bupivacaine for caesarean section in hypertensive pregnancies with chronic fetal asphyxia. Br J Anaesth. 1993;71:348–353.
236. Brownridge P. Epidural and subarachnoid analgesia for elective caesarean section. Anaesthesia. 1981;36:70.
237. Carrie LE, O'Sullivan G. Subarachnoid bupivacaine 0.5% for caesarean section. Eur J Anaesthesiol. 1984;1:275–283.
238. Davies SJ, Paech MJ, Welch H, et al. Maternal experience during epidural or combined spinal-epidural anesthesia for cesarean section: a prospective, randomized trial. Anesth Analg. 1997;85:607–613.
239. Goy RW, Chee-Seng Y, Sia AT, et al. The median effective dose of intrathecal hyperbaric bupivacaine is larger in the single-shot spinal as compared with the combined spinal-epidural technique. Anesth Analg. 2005;100:1499–1502.
240. Ithnin F, Lim Y, Sia AT, Ocampo CE. Combined spinal epidural causes higher level of block than equivalent single-shot spinal anesthesia in elective cesarean patients. Anesth Analg. 2006;102:577–580.
241. Lim Y, Teoh W, Sia AT. Combined spinal epidural does not cause a higher sensory block than single shot spinal technique for cesarean delivery in laboring women. Anesth Analg. 2006;103:1540–1542.
242. McNaught AF, Stocks GM. Epidural volume extension and low-dose sequential combined spinal-epidural blockade: two ways to reduce spinal dose requirement for caesarean section. Int J Obstet Anesth. 2007;16:346–353.
243. Thoren T, Holmstrom B, Rawal N, et al. Sequential combined spinal epidural block versus spinal block for cesarean section: effects on maternal hypotension and neurobehavioral function of the newborn. Anesth Analg. 1994;78:1087–1092.
244. Kucukguclu S, Unlugenc H, Gunenc F, et al. The influence of epidural volume extension on spinal block with hyperbaric or plain bupivacaine for Caesarean delivery. Eur J Anaesthesiol. 2008;25:307–313.
245. Loubert C, O’Brien PJ, Fernando R, et al. Epidural volume extension in combined spinal epidural anaesthesia for elective caesarean section: a randomised controlled trial. Anaesthesia. 2011;66:341–347.
246. Bauer ME, Kountanis JA, Tsen LC, et al. Risk factors for failed conversion of labor epidural analgesia to cesarean delivery anesthesia: a systematic review and meta-analysis of observational trials. Int J Obstet Anesth. 2012;21:294–309.
247. Lucas DN, Ciccone GK, Yentis SM. Extending low-dose epidural analgesia for emergency Caesarean section: a comparison of three solutions. Anaesthesia. 1999;54:1173–1177.
248. Bjornestad E, Iversen OL, Raeder J. Similar onset time of 2-chloroprocaine and lidocaine + epinephrine for epidural anesthesia for elective Cesarean section. Acta Anaesthesiol Scand. 2006;50:358–363.
249. Lam DT, Ngan Kee WD, Khaw KS. Extension of epidural blockade in labour for emergency Caesarean section using 2% lidocaine with epinephrine and fentanyl, with or without alkalinisation. Anaesthesia. 2001;56:790–794.
250. Peterfreund RA, Datta S, Ostheimer GW. pH adjustment of local anesthetic solutions with sodium bicarbonate: laboratory evaluation of alkalinization and precipitation. Reg Anesth. 1989;14:265–270.
251. Tuleu C, Allam J, Gill H, Yentis SM. Short term stability of pH-adjusted lidocaine-adrenaline epidural solution used for emergency caesarean section. Int J Obstet Anesth. 2008;17:118–122.
252. Gaiser RR, Cheek TG, Adams HK, Gutsche BB. Epidural lidocaine for cesarean delivery of the distressed fetus. Int J Obstet Anesth. 1998;7:27–31.
253. Malhotra S, Yentis SM. Extending low-dose epidural analgesia in labour for emergency Caesarean section—a comparison of levobupivacaine with or without fentanyl. Anaesthesia. 2007;62:667–671.
254. Higuchi H, Adachi Y, Kazama T. Effects of epidural saline injection on cerebrospinal fluid volume and velocity waveform: a magnetic resonance imaging study. Anesthesiology. 2005;102:285–292.
255. Regan KJ, O'Sullivan G. The extension of epidural blockade for emergency Caesarean section: a survey of current UK practice. Anaesthesia. 2008;63:136–142.
256. Kodali BS, Chandrasekhar S, Bulich LN, et al. Airway changes during labor and delivery. Anesthesiology. 2008;108:357–362.
257. McClelland SH, Bogod DG, Hardman JG. Pre-oxygenation and apnoea in pregnancy: changes during labour and with obstetric morbidity in a computational simulation. Anaesthesia. 2009;64:371–377.
258. Gambee AM, Hertzka RE, Fisher DM. Preoxygenation techniques: comparison of three minutes and four breaths. Anesth Analg. 1987;66:468–470.
259. Soro Domingo M, Belda Nacher FJ, Aguilar Aguilar G, et al. Preoxygenation for anesthesia. Rev Esp Anestesiol Reanim. 2004;51:322–327.
260. de Souza DG, Doar LH, Mehta SH, Tiouririne M. Aspiration prophylaxis and rapid sequence induction for elective cesarean delivery: time to reassess old dogma? Anesth Analg. 2010;110:1503–1505.
261. Paech MJ. “Pregnant women having caesarean delivery under general anaesthesia should have a rapid sequence induction with cricoid pressure and be intubated”. Can this ‘holy cow’ be sent packing? Anaesth Intensive Care. 2010;38:989–991.
262. Thind GS, Bryson TH. Single dose suxamethonium and muscle pain in pregnancy. Br J Anaesth. 1983;55:743–745.
263. Perry JJ, Lee JS, Sillberg VA, Wells GA. Rocuronium versus succinylcholine for rapid sequence induction intubation. Cochrane Database Syst Rev. 2008;(2).
264. Cherala S, Eddie D, Halpern M, Shevde K. Priming with vecuronium in obstetrics. Anaesthesia. 1987;42:1021.
265. Guay J, Grenier Y, Varin F. Clinical pharmacokinetics of neuromuscular relaxants in pregnancy. Clin Pharmacokinet. 1998;34:483.
266. Puhringer FK, Kristen P, Rex C. Sugammadex reversal of rocuronium-induced neuromuscular block in Caesarean section patients: a series of seven cases. Br J Anaesth. 2010;105:657–660.
267. Han TH, Brimacombe J, Lee EJ, Yang HS. The laryngeal mask airway is effective (and probably safe) in selected healthy parturients for elective Cesarean section: a prospective study of 1067 cases. Can J Anaesth. 2001;48:1117–1121.
268. Halaseh BK, Sukkar ZF, Hassan LH, et al. The use of ProSeal laryngeal mask airway in caesarean section—experience in 3000 cases. Anaesth Intensive Care. 2010;38:1023–1028.
269. Piggott SE, Bogod DG, Rosen M, et al. Isoflurane with either 100% oxygen or 50% nitrous oxide in oxygen for caesarean section. Br J Anaesth. 1990;65:325–329.
270. Matthews P, Dann WL, Cartwright DP, Taylor E. Inspired oxygen concentration during general anaesthesia for caesarean section. Eur J Anaesthesiol. 1989;6:295–301.
271. Parpaglioni R, Capogna G, Celleno D, Fusco P. Intraoperative fetal oxygen saturation during Caesarean section: general anaesthesia using sevoflurane with either 100% oxygen or 50% nitrous oxide in oxygen. Eur J Anaesthesiol. 2002;19:115–118.
272. Levinson G, Shnider SM, DeLorimier AA, Steffenson JL. Effects of maternal hyperventilation on uterine blood flow and fetal oxygenation and acid-base status. Anesthesiology. 1974;40:340–347.
273. Palahniuk RJ, Shnider SM, Eger EI 2nd. Pregnancy decreases the requirement for inhaled anesthetic agents. Anesthesiology. 1974;41:82–83.
274. Lertakyamanee J, Chinachoti T, Tritrakarn T, et al. Comparison of general and regional anesthesia for cesarean section: success rate, blood loss and satisfaction from a randomized trial. J Med Assoc Thai. 1999;82:672–680.
275. Chin KJ, Yeo SW. A BIS-guided study of sevoflurane requirements for adequate depth of anaesthesia in Caesarean section. Anaesthesia. 2004;59:1064–1068.
276. Yoo KY, Jeong CW, Kang MW, et al. Bispectral index values during sevoflurane-nitrous oxide general anesthesia in women undergoing cesarean delivery: a comparison between women with and without prior labor. Anesth Analg. 2008;106:1827–1832.
277. Erden V, Erkalp K, Yangin Z, et al. The effect of labor on sevoflurane requirements during cesarean delivery. Int J Obstet Anesth. 2011;20:17–21.
278. Ngan Kee WD, Khaw KS, Ma KC, et al. Maternal and neonatal effects of remifentanil at induction of general anesthesia for cesarean delivery: a randomized, double-blind, controlled trial. Anesthesiology. 2006;104:14–20.
279. Petropoulos G, Siristatidis C, Salamalekis E, Creatsas G. Spinal and epidural versus general anesthesia for elective cesarean section at term: effect on the acid-base status of the mother and newborn. J Matern Fetal Neonatal Med. 2003;13:260–266.
280. Mhyre JM, Riesner MN, Polley LS, Naughton NN. A series of anesthesia-related maternal deaths in Michigan, 1985-2003. Anesthesiology. 2007;106:1096–1104.
281. American Society of Anesthesiologists Task Force on Postanesthetic Care. Practice guidelines for postanesthetic care. Anesthesiology. 2013;118:291–307.
282. Horwitz LD. Effects of intravenous anesthetic agents on left ventricular function in dogs. Am J Physiol. 1977;232:H44–H48.
283. Morgan DJ, Blackman GL, Paull JD, Wolf LJ. Pharmacokinetics and plasma binding of thiopental. II: Studies at cesarean section. Anesthesiology. 1981;54:474–480.
284. Norman E, Westrin P, Fellman V. Placental transfer and pharmacokinetics of thiopentone in newborn infants. Arch Dis Child Fetal Neonatal Ed. 2010;95:F277–F282.
285. Kosaka Y, Takahashi T, Mark LC. Intravenous thiobarbiturate anesthesia for cesarean section. Anesthesiology. 1969;31:489–506.
286. Finster M, Morishima HO, Mark LC, et al. Tissue thiopental concentrations in the fetus and newborn. Anesthesiology. 1972;36:155–158.
287. Flowers CE Jr. The placental transmission of barbiturates and thiobarbiturates and their pharmacological action on the mother and the infant. Am J Obstet Gynecol. 1959;78:730–742.
288. Gin T, Gregory MA, Chan K, Oh TE. Maternal and fetal levels of propofol at caesarean section. Anaesth Intensive Care. 1990;18:180–184.
289. Soares de Moura R, Silva GA, Tano T, Resende AC. Effect of propofol on human fetal placental circulation. Int J Obstet Anesth. 2010;19:71–76.
290. Celleno D, Capogna G, Tomassetti M, et al. Neurobehavioural effects of propofol on the neonate following elective caesarean section. Br J Anaesth. 1989;62:649–654.
291. Yau G, Gin T, Ewart MC, et al. Propofol for induction and maintenance of anaesthesia at caesarean section: a comparison with thiopentone/enflurane. Anaesthesia. 1991;46:20–23.
292. Gin T, Gregory MA, Chan K, et al. Pharmacokinetics of propofol in women undergoing elective caesarean section. Br J Anaesth. 1990;64:148–153.
293. Gregory MA, Gin T, Yau G, et al. Propofol infusion anaesthesia for caesarean section. Can J Anaesth. 1990;37:514–520.
294. Grounds RM, Twigley AJ, Carli F, et al. The haemodynamic effects of intravenous induction: comparison of the effects of thiopentone and propofol. Anaesthesia. 1985;40:735–740.
295. Celleno D, Capogna G, Emanuelli M, et al. Which induction drug for cesarean section? A comparison of thiopental sodium, propofol, and midazolam. J Clin Anesth. 1993;5:284–288.
296. Moore J, Bill KM, Flynn RJ, et al. A comparison between propofol and thiopentone as induction agents in obstetric anaesthesia. Anaesthesia. 1989;44:753–757.
297. Abboud TK, Zhu J, Richardson M, et al. Intravenous propofol vs thiamylal-isoflurane for caesarean section, comparative maternal and neonatal effects. Acta Anaesthesiol Scand. 1995;39:205–209.
298. Baraka A. Severe bradycardia following propofol-suxamethonium sequence. Br J Anaesth. 1988;61:482–483.
299. Alon E, Ball RH, Gillie MH, et al. Effects of propofol and thiopental on maternal and fetal cardiovascular and acid-base variables in the pregnant ewe. Anesthesiology. 1993;78:562–576.
300. Hui TW, Short TG, Hong W, et al. Additive interactions between propofol and ketamine when used for anesthesia induction in female patients. Anesthesiology. 1995;82:641–648.
301. Corssen G, Gutierrez J, Reves JG, Huber FC Jr. Ketamine in the anesthetic management of asthmatic patients. Anesth Analg. 1972;51:588–596.
302. McDonald JS, Mateo CV, Reed EC. Modified nitrous oxide or ketamine hydrochloride for cesarean section. Anesth Analg. 1972;51:975–985.
303. Craft JB Jr, Coaldrake LA, Yonekura ML, et al. Ketamine, catecholamines, and uterine tone in pregnant ewes. Am J Obstet Gynecol. 1983;146:429–434.
304. Oats JN, Vasey DP, Waldron BA. Effects of ketamine on the pregnant uterus. Br J Anaesth. 1979;51:1163–1166.
305. Little B, Chang T, Chucot L, et al. Study of ketamine as an obstetric anesthetic agent. Am J Obstet Gynecol. 1972;113:247–260.
306. Ngan Kee WD, Khaw KS, Ma ML, et al. Postoperative analgesic requirement after cesarean section: a comparison of anesthetic induction with ketamine or thiopental. Anesth Analg. 1997;85:1294–1298.
307. Peltz B, Sinclair DM. Induction agents for Caesarean section: a comparison of thiopentone and ketamine. Anaesthesia. 1973;28:37–42.
308. Strumper D, Gogarten W, Durieux ME, et al. The effects of S+-ketamine and racemic ketamine on uterine blood flow in chronically instrumented pregnant sheep. Anesth Analg. 2004;98:497–502.
309. Dich-Nielsen J, Holasek J. Ketamine as induction agent for caesarean section. Acta Anaesthesiol Scand. 1982;26:139–142.
310. Baraka A, Louis F, Dalleh R. Maternal awareness and neonatal outcome after ketamine induction of anaesthesia for Caesarean section. Can J Anaesth. 1990;37:641–644.
311. Gaitini L, Vaida S, Collins G, et al. Awareness detection during caesarean section under general anaesthesia using EEG spectrum analysis. Can J Anaesth. 1995;42:377–381.
312. Mankowitz E, Downing JW, Brock-Utne JG, et al. Total intravenous anaesthesia using low-dose ketamine infusion for caesarean section: a comparison with a standard inhalation anaesthetic technique. S Afr Med J. 1984;65:246–250.
313. Krissel J, Dick WF, Leyser KH, et al. Thiopentone, thiopentone/ketamine, and ketamine for induction of anaesthesia in caesarean section. Eur J Anaesthesiol. 1994;11:115–122.
314. Schultetus RR, Hill CR, Dharamraj CM, et al. Wakefulness during cesarean section after anesthetic induction with ketamine, thiopental, or ketamine and thiopental combined. Anesth Analg. 1986;65:723–728.
315. Orme RM, Grange CS, Ainsworth QP, Grebenik CR. General anaesthesia using remifentanil for caesarean section in parturients with critical aortic stenosis: a series of four cases. Int J Obstet Anesth. 2004;13:183–187.
316. Esener Z, Sarihasan B, Guven H, Ustun E. Thiopentone and etomidate concentrations in maternal and umbilical plasma, and in colostrum. Br J Anaesth. 1992;69:586–588.
317. Bergen JM, Smith DC. A review of etomidate for rapid sequence intubation in the emergency department. J Emerg Med. 1997;15:221–230.
318. Downing JW, Buley RJ, Brock-Utne JG, Houlton PC. Etomidate for induction of anaesthesia at caesarean section: comparison with thiopentone. Br J Anaesth. 1979;51:135–140.
319. Reddy BK, Pizer B, Bull PT. Neonatal serum cortisol suppression by etomidate compared with thiopentone, for elective caesarean section. Eur J Anaesthesiol. 1988;5:171–176.
320. Crawford ME, Carl P, Bach V, et al. A randomized comparison between midazolam and thiopental for elective cesarean section anesthesia. I. Mothers. Anesth Analg. 1989;68:229–233.
321. Bland BA, Lawes EG, Duncan PW, et al. Comparison of midazolam and thiopental for rapid sequence anesthetic induction for elective cesarean section. Anesth Analg. 1987;66:1165–1168.
322. Kvisselgaard N, Moya F. Investigation of placental thresholds to succinylcholine. Anesthesiology. 1961;22:7–10.
323. Shnider SM. Serum cholinesterase activity during pregnancy, labor and the puerperium. Anesthesiology. 1965;26:335–339.
324. Kao YJ, Turner DR. Prolongation of succinylcholine block by metoclopramide. Anesthesiology. 1989;70:905–908.
325. Abouleish E, Abboud T, Lechevalier T, et al. Rocuronium (Org 9426) for caesarean section. Br J Anaesth. 1994;73:336–341.
326. Magorian T, Flannery KB, Miller RD. Comparison of rocuronium, succinylcholine, and vecuronium for rapid-sequence induction of anesthesia in adult patients. Anesthesiology. 1993;79:913–918.
327. Hawkins JL, Johnson TD, Kubicek MA, et al. Vecuronium for rapid-sequence intubation for cesarean section. Anesth Analg. 1990;71:185–190.
328. Hawkins JL, Adenwala J, Camp C, Joyce TH 3rd. The effect of H2-receptor antagonist premedication on the duration of vecuronium-induced neuromuscular blockade in postpartum patients. Anesthesiology. 1989;71:175–177.
329. Dailey PA, Fisher DM, Shnider SM, et al. Pharmacokinetics, placental transfer, and neonatal effects of vecuronium and pancuronium administered during cesarean section. Anesthesiology. 1984;60:569–574.
330. Eikermann M, Peters J. Nerve stimulation at 0.15 Hz when compared to 0.1 Hz speeds the onset of action of cisatracurium and rocuronium. Acta Anaesthesiol Scand. 2000;44:170–174.
331. Warren TM, Datta S, Ostheimer GW, et al. Comparison of the maternal and neonatal effects of halothane, enflurane, and isoflurane for cesarean delivery. Anesth Analg. 1983;62:516–520.
332. Crawford JS. Awareness during operative obstetrics under general anaesthesia. Br J Anaesth. 1971;43:179–182.
333. Karasawa F, Takita A, Fukuda I, Kawatani Y. Nitrous oxide concentrations in maternal and fetal blood during caesarean section. Eur J Anaesthesiol. 2003;20:555–559.
334. Palahniuk RJ, Cumming M. Foetal deterioration following thiopentone-nitrous oxide anaesthesia in the pregnant ewe. Can Anaesth Soc J. 1977;24:361–370.
335. Dwyer R, Fee JP, Moore J. Uptake of halothane and isoflurane by mother and baby during caesarean section. Br J Anaesth. 1995;74:379–383.
336. Munson ES, Embro WJ. Enflurane, isoflurane, and halothane and isolated human uterine muscle. Anesthesiology. 1977;46:11–14.
337. Dogru K, Yildiz K, Dalgic H, et al. Inhibitory effects of desflurane and sevoflurane on contractions of isolated gravid rat myometrium under oxytocin stimulation. Acta Anaesthesiol Scand. 2003;47:472–474.
338. Yildiz K, Dogru K, Dalgic H, et al. Inhibitory effects of desflurane and sevoflurane on oxytocin-induced contractions of isolated pregnant human myometrium. Acta Anaesthesiol Scand. 2005;49:1355–1359.
339. Gultekin H, Yildiz K, Sezer Z, Dogru K. Comparing the relaxing effects of desflurane and sevoflurane on oxytocin-induced contractions of isolated myometrium in both pregnant and nonpregnant rats. Adv Ther. 2006;23:39–46.
340. Gin T, Chan MT. Decreased minimum alveolar concentration of isoflurane in pregnant humans. Anesthesiology. 1994;81:829–832.
341. Chan MT, Mainland P, Gin T. Minimum alveolar concentration of halothane and enflurane are decreased in early pregnancy. Anesthesiology. 1996;85:782–786.
342. Datta S, Migliozzi RP, Flanagan HL, Krieger NR. Chronically administered progesterone decreases halothane requirements in rabbits. Anesth Analg. 1989;68:46–50.
343. Chan MT, Gin T. Postpartum changes in the minimum alveolar concentration of isoflurane. Anesthesiology. 1995;82:1360–1363.
344. Draisci G, Valente A, Suppa E, et al. Remifentanil for cesarean section under general anesthesia: effects on maternal stress hormone secretion and neonatal well-being: a randomized trial. Int J Obstet Anesth. 2008;17:130–136.
345. Craft JB Jr, Coaldrake LA, Bolan JC, et al. Placental passage and uterine effects of fentanyl. Anesth Analg. 1983;62:894–898.
346. Gerdin E, Rane A, Lindberg B. Transplacental transfer of morphine in man. J Perinat Med. 1990;18:305–312.
347. Nation RL. Meperidine binding in maternal and fetal plasma. Clin Pharmacol Ther. 1981;29:472–479.
348. Bonica JJ. Local-regional analgesia for abdominal delivery. Bonica JJ. Obstetric Analgesia and Anesthesia. FA Davis: Philadelphia; 1967:527–538.
349. Mei W, Jin C, Feng L, et al. Bilateral ultrasound-guided transversus abdominis plane block combined with ilioinguinal-iliohypogastric nerve block for cesarean delivery anesthesia. Anesth Analg. 2011;113:134–137.
350. Waterstone M, Bewley S, Wolfe C. Incidence and predictors of severe obstetric morbidity: case-control study. BMJ. 2001;322:1089–1093.
351. Cohen SE, Hamilton CL, Riley ET, et al. Obstetric postanesthesia care unit stays: reevaluation of discharge criteria after regional anesthesia. Anesthesiology. 1998;89:1559–1565.
352. Thomas J, Paranjothy S. The National Sentinel Caesarean Section Audit Report: Clinical Effectiveness Support Unit of the Royal College of Obstetricians and Gynaecologists. Royal College of Obstetricians and Gynaecologists: London; 2001.
353. Guidelines for Obstetric Anaesthesia Services. Association of Anaesthetists of Great Britain and Ireland and Obstetric Anaesthetists’ Association: London; 1998.
354. Mangesi L, Hofmeyr GJ. Early compared with delayed oral fluids and food after caesarean section. Cochrane Database Syst Rev. 2002;(3).
355. National Institute for Health and Clinical Excellence. Caesarean section. NICE clinical guideline 132. [Available at] http://www.nice.org.uk/nicemedia/live/13620/57163/57163.pdf [Accessed January 2013] .
356. Sharma KK, Mahmood TA, Smith NC. The short term effect of obstetric anaesthesia on bladder function. J Obstet Gynaecol. 1994;14:254–264.
357. Liang CC, Chang SD, Chang YL, et al. Postpartum urinary retention after cesarean delivery. Int J Gynaecol Obstet. 2007;99:229–232.
358. Onile TG, Kuti O, Orji EO, Ogunniyi SO. A prospective randomized clinical trial of urethral catheter removal following elective cesarean delivery. Int J Gynaecol Obstet. 2008;102:267–270.
359. Foon R, Toozs-Hobson P, Millns P, Kilby M. The impact of anesthesia and mode of delivery on the urinary bladder in the postdelivery period. Int J Gynaecol Obstet. 2010;110:114–117.
360. King H, Ashley S, Brathwaite D, et al. Adequacy of general anesthesia for cesarean section. Anesth Analg. 1993;77:84–88.
361. Moir DD. Anaesthesia for Caesarean section: an evaluation of a method using low concentrations of halothane and 50 per cent of oxygen. Br J Anaesth. 1970;42:136–142.
362. Datta S, Ostheimer GW, Weiss JB, et al. Neonatal effect of prolonged anesthetic induction for cesarean section. Obstet Gynecol. 1981;58:331–335.
363. Glass PS, Bloom M, Kearse L, et al. Bispectral analysis measures sedation and memory effects of propofol, midazolam, isoflurane, and alfentanil in healthy volunteers. Anesthesiology. 1997;86:836–847.
364. Sanders RD, Avidan MS. Evidence is lacking for interventions proposed to prevent unintended awareness during general anesthesia for cesarean delivery. Anesth Analg. 2010;110:972–973.
365. Yeo SN, Lo WK. Bispectral index in assessment of adequacy of general anaesthesia for lower segment caesarean section. Anaesth Intensive Care. 2002;30:36–40.
366. Ittichaikulthol W, Sriswasdi S, Prachanpanich N, et al. Bispectral index in assessment of 3% and 4.5% desflurane in 50% N2O for caesarean section. J Med Assoc Thai. 2007;90:1546–1550.
367. Kanto J, Aaltonen L, Erkkola R, Aarimaa L. Pharmacokinetics and sedative effect of midazolam in connection with caesarean section performed under epidural analgesia. Acta Anaesthesiol Scand. 1984;28:116–118.
368. Tsai PS, Huang CJ, Hung YC, Cheng CR. Effects on the bispectral index during elective caesarean section: a comparison of propofol and isoflurane. Acta Anaesthesiol Sin. 2001;39:17–22.
369. Polster MR, Gray PA, O'Sullivan G, et al. Comparison of the sedative and amnesic effects of midazolam and propofol. Br J Anaesth. 1993;70:612–616.
370. Dahan A, Kest B, Waxman AR, Sarton E. Sex-specific responses to opiates: animal and human studies. Anesth Analg. 2008;107:83–95.
371. Hoymork SC, Raeder J. Why do women wake up faster than men from propofol anaesthesia? Br J Anaesth. 2005;95:627–633.
372. Fan SZ, Susetio L, Wang YP, et al. Low dose of intrathecal hyperbaric bupivacaine combined with epidural lidocaine for cesarean section—a balance block technique. Anesth Analg. 1994;78:474–477.
373. Lirk P, Kleber N, Mitterschiffthaler G, et al. Pulmonary effects of bupivacaine, ropivacaine, and levobupivacaine in parturients undergoing spinal anaesthesia for elective caesarean delivery: a randomised controlled study. Int J Obstet Anesth. 2010;19:287–292.
374. Corke BC, Datta S, Ostheimer GW, et al. Spinal anaesthesia for Caesarean section: the influence of hypotension on neonatal outcome. Anaesthesia. 1982;37:658–662.
375. Cyna AM, Andrew M, Emmett RS, et al. Techniques for preventing hypotension during spinal anaesthesia for caesarean section. Cochrane Database Syst Rev. 2006;(4).
376. McDonald S, Fernando R, Ashpole K, Columb M. Maternal cardiac output changes after crystalloid or colloid coload following spinal anesthesia for elective cesarean delivery: a randomized controlled trial. Anesth Analg. 2011;113:803–810.
377. Dyer RA, Piercy JL, Reed AR, et al. Hemodynamic changes associated with spinal anesthesia for cesarean delivery in severe preeclampsia. Anesthesiology. 2008;108:802–811.
378. Langesaeter E, Dyer RA. Maternal haemodynamic changes during spinal anaesthesia for caesarean section. Curr Opin Anaesthesiol. 2011;24:242–248.
379. Mark JB, Steele SM. Cardiovascular effects of spinal anesthesia. Int Anesthesiol Clin. 1989;27:31–39.
380. Ng K, Parsons J, Cyna AM, Middleton P. Spinal versus epidural anaesthesia for caesarean section. Cochrane Database Syst Rev. 2004;(2).
381. Aya AG, Vialles N, Tanoubi I, et al. Spinal anesthesia-induced hypotension: a risk comparison between patients with severe preeclampsia and healthy women undergoing preterm cesarean delivery. Anesth Analg. 2005;101:869–875.
382. Kinsella SM, Norris MC. Advance prediction of hypotension at cesarean delivery under spinal anesthesia. Int J Obstet Anesth. 1996;5:3–7.
383. Frölich MA, Caton D. Baseline heart rate may predict hypotension after spinal anesthesia in prehydrated obstetrical patients. Can J Anaesth. 2002;49:185–189.
384. Dahlgren G, Granath F, Wessel H, Irestedt L. Prediction of hypotension during spinal anesthesia for Cesarean section and its relation to the effect of crystalloid or colloid preload. Int J Obstet Anesth. 2007;16:128–134.
385. Hanss R, Bein B, Francksen H, et al. Heart rate variability-guided prophylactic treatment of severe hypotension after subarachnoid block for elective cesarean delivery. Anesthesiology. 2006;104:635–643.
386. Chamchad D, Arkoosh VA, Horrow JC, et al. Using heart rate variability to stratify risk of obstetric patients undergoing spinal anesthesia. Anesth Analg. 2004;99:1818–1821.
387. Ouzounian JG, Masaki DI, Abboud TK, Greenspoon JS. Systemic vascular resistance index determined by thoracic electrical bioimpedance predicts the risk for maternal hypotension during regional anesthesia for cesarean delivery. Am J Obstet Gynecol. 1996;174:1019–1025.
388. Hanss R, Bein B, Ledowski T, et al. Heart rate variability predicts severe hypotension after spinal anesthesia for elective cesarean delivery. Anesthesiology. 2005;102:1086–1093.
389. Ueyama H, He YL, Tanigami H, et al. Effects of crystalloid and colloid preload on blood volume in the parturient undergoing spinal anesthesia for elective Cesarean section. Anesthesiology. 1999;91:1571–1576.
390. Pouta AM, Karinen J, Vuolteenaho OJ, Laatikainen TJ. Effect of intravenous fluid preload on vasoactive peptide secretion during Caesarean section under spinal anaesthesia. Anaesthesia. 1996;51:128–132.
391. Lee A, Ngan Kee WD, Gin T. A dose-response meta-analysis of prophylactic intravenous ephedrine for the prevention of hypotension during spinal anesthesia for elective cesarean delivery. Anesth Analg. 2004;98:483–490.
392. Lee A, Ngan Kee WD, Gin T. A quantitative, systematic review of randomized controlled trials of ephedrine versus phenylephrine for the management of hypotension during spinal anesthesia for cesarean delivery. Anesth Analg. 2002;94:920–926.
393. Ngan Kee WD, Khaw KS, Tan PE, et al. Placental transfer and fetal metabolic effects of phenylephrine and ephedrine during spinal anesthesia for cesarean delivery. Anesthesiology. 2009;111:506–512.
394. Cooper DW, Carpenter M, Mowbray P, et al. Fetal and maternal effects of phenylephrine and ephedrine during spinal anesthesia for cesarean delivery. Anesthesiology. 2002;97:1582–1590.
395. Allen TK, George RB, White WD, et al. A double-blind, placebo-controlled trial of four fixed rate infusion regimens of phenylephrine for hemodynamic support during spinal anesthesia for cesarean delivery. Anesth Analg. 2010;111:1221–1229.
396. Stewart A, Fernando R, McDonald S, et al. The dose-dependent effects of phenylephrine for elective cesarean delivery under spinal anesthesia. Anesth Analg. 2010;111:1230–1237.
397. Tanaka M, Balki M, Parkes RK, Carvalho JC. ED95 of phenylephrine to prevent spinal-induced hypotension and/or nausea at elective cesarean delivery. Int J Obstet Anesth. 2009;18:125–130.
398. Mercier FJ, Riley ET, Frederickson WL, et al. Phenylephrine added to prophylactic ephedrine infusion during spinal anesthesia for elective cesarean section. Anesthesiology. 2001;95:668–674.
399. Ngan Kee WD, Lee A, Khaw KS, et al. A randomized double-blinded comparison of phenylephrine and ephedrine infusion combinations to maintain blood pressure during spinal anesthesia for cesarean delivery: the effects on fetal acid-base status and hemodynamic control. Anesth Analg. 2008;107:1295–1302.
400. Loughrey JP, Yao N, Datta S, et al. Hemodynamic effects of spinal anesthesia and simultaneous intravenous bolus of combined phenylephrine and ephedrine versus ephedrine for cesarean delivery. Int J Obstet Anesth. 2005;14:43–47.
401. Ngan Kee WD, Tam YH, Khaw KS, et al. Closed-loop feedback computer-controlled infusion of phenylephrine for maintaining blood pressure during spinal anaesthesia for caesarean section: a preliminary descriptive study. Anaesthesia. 2007;62:1251–1256.
402. Ngan Kee WD, Khaw KS, Ng FF. Prevention of hypotension during spinal anesthesia for cesarean delivery: an effective technique using combination phenylephrine infusion and crystalloid cohydration. Anesthesiology. 2005;103:744–750.
403. Benhamou D, Wong C. Neuraxial anesthesia for cesarean delivery: what criteria define the “optimal” technique? Anesth Analg. 2009;109:1370–1373.
404. Rucklidge MW, Paech MJ. Limiting the dose of local anaesthetic for caesarean section under spinal anaesthesia—has the limbo bar been set too low? Anaesthesia. 2012;67:347–351.
405. Ralston DH, Shnider SM, DeLorimier AA. Effects of equipotent ephedrine, metaraminol, mephentermine, and methoxamine on uterine blood flow in the pregnant ewe. Anesthesiology. 1974;40:354–370.
406. Tong C, Eisenach JC. The vascular mechanism of ephedrine's beneficial effect on uterine perfusion during pregnancy. Anesthesiology. 1992;76:792–798.
407. Li P, Tong C, Eisenach JC. Pregnancy and ephedrine increase the release of nitric oxide in ovine uterine arteries. Anesth Analg. 1996;82:288–293.
408. Soltanifar S, Russell R. The National Institute for Health and Clinical Excellence (NICE) guidelines for caesarean section, 2011 update: implications for the anaesthetist. Int J Obstet Anesth. 2012;21:264–272.
409. Riley ET. Editorial I: Spinal anaesthesia for Caesarean delivery: keep the pressure up and don't spare the vasoconstrictors. Br J Anaesth. 2004;92:459–461.
410. Datta S, Alper MH, Ostheimer GW, Weiss JB. Method of ephedrine administration and nausea and hypotension during spinal anesthesia for cesarean section. Anesthesiology. 1982;56:68–70.
411. Ngan Kee WD, Khaw KS, Ng FF. Comparison of phenylephrine infusion regimens for maintaining maternal blood pressure during spinal anaesthesia for Caesarean section. Br J Anaesth. 2004;92:469–474.
412. Pan PH, Bogard TD, Owen MD. Incidence and characteristics of failures in obstetric neuraxial analgesia and anesthesia: a retrospective analysis of 19,259 deliveries. Int J Obstet Anesth. 2004;13:227–233.
413. Bauer ME, Kountanis JA, Tsen LC, et al. Risk factors for failed conversion of labor epidural analgesia to cesarean delivery anesthesia: a systematic review and meta-analysis of observational trials. Int J Obstet Anesth. 2012;21:294–309.
414. Carvalho B. Failed epidural top-up for cesarean delivery for failure to progress in labor: the case against single-shot spinal anesthesia. Int J Obstet Anesth. 2012;21:357–359.
415. Furst SR, Reisner LS. Risk of high spinal anesthesia following failed epidural block for cesarean delivery. J Clin Anesth. 1995;7:71–74.
416. Lee NM, Saha S. Nausea and vomiting of pregnancy. Gastroenterol Clin North Am. 2011;40:309–334.
417. Balki M, Carvalho JC. Intraoperative nausea and vomiting during cesarean section under regional anesthesia. Int J Obstet Anesth. 2005;14:230–241.
418. Borgeat A, Ekatodramis G, Schenker CA. Postoperative nausea and vomiting in regional anesthesia: a review. Anesthesiology. 2003;98:530–547.
419. Pinder AJ, Dresner M, Calow C, et al. Haemodynamic changes caused by oxytocin during caesarean section under spinal anaesthesia. Int J Obstet Anesth. 2002;11:156–159.
420. Dansereau J, Joshi AK, Helewa ME, et al. Double-blind comparison of carbetocin versus oxytocin in prevention of uterine atony after cesarean section. Am J Obstet Gynecol. 1999;180:670–676.
421. Alahuhta S, Kangas-Saarela T, Hollmen AI, Edstrom HH. Visceral pain during caesarean section under spinal and epidural anaesthesia with bupivacaine. Acta Anaesthesiol Scand. 1990;34:95–98.
422. Gan TJ. Risk factors for postoperative nausea and vomiting. Anesth Analg. 2006;102:1884–1898.
423. Apfel CC, Laara E, Koivuranta M, et al. A simplified risk score for predicting postoperative nausea and vomiting: conclusions from cross-validations between two centers. Anesthesiology. 1999;91:693–700.
424. Kreis ME. Postoperative nausea and vomiting. Auton Neurosci. 2006;129:86–91.
425. Kranke P, Eberhart LH, Gan TJ, et al. Algorithms for the prevention of postoperative nausea and vomiting: an efficacy and efficiency simulation. Eur J Anaesthesiol. 2007;24:856–867.
426. Abouleish EI, Rashid S, Haque S, et al. Ondansetron versus placebo for the control of nausea and vomiting during Caesarean section under spinal anaesthesia. Anaesthesia. 1999;54:479–482.
427. Pan PH, Moore CH. Comparing the efficacy of prophylactic metoclopramide, ondansetron, and placebo in cesarean section patients given epidural anesthesia. J Clin Anesth. 2001;13:430–435.
428. Griffiths JD, Gyte GM, Paranjothy S, et al. Interventions for preventing nausea and vomiting in women undergoing regional anaesthesia for caesarean section. Cochrane Database Syst Rev. 2012;(9).
429. Han DW, Hong SW, Kwon JY, et al. Epidural ondansetron is more effective to prevent postoperative pruritus and nausea than intravenous ondansetron in elective cesarean delivery. Acta Obstet Gynecol Scand. 2007;86:683–687.
430. Allen TK, Jones CA, Habib AS. Dexamethasone for the prophylaxis of postoperative nausea and vomiting associated with neuraxial morphine administration: a systematic review and meta-analysis. Anesth Analg. 2012;114:813–822.
431. Harnett MJ, O’Rourke N, Walsh M, et al. Transdermal scopolamine for prevention of intrathecal morphine-induced nausea and vomiting after cesarean delivery. Anesth Analg. 2007;105:764–769.
432. Harmon D, Ryan M, Kelly A, Bowen M. Acupressure and prevention of nausea and vomiting during and after spinal anaesthesia for caesarean section. Br J Anaesth. 2000;84:463–467.
433. Allen TK, Habib AS. P6 stimulation for the prevention of nausea and vomiting associated with cesarean delivery under neuraxial anesthesia: a systematic review of randomized controlled trials. Anesth Analg. 2008;107:1308–1312.
434. Phillips TW Jr, Broussard DM, Sumrall WD 3rd, Hart SR. Intraoperative oxygen administration does not reduce the incidence or severity of nausea or vomiting associated with neuraxial anesthesia for cesarean delivery. Anesth Analg. 2007;105:1113–1117.
435. Orhan-Sungur M, Kranke P, Sessler D, Apfel CC. Does supplemental oxygen reduce postoperative nausea and vomiting? A meta-analysis of randomized controlled trials. Anesth Analg. 2008;106:1733–1738.
436. Carvalho B, Cohen SE, Lipman SS, et al. Patient preferences for anesthesia outcomes associated with cesarean delivery. Anesth Analg. 2005;101:1182–1187.
437. Szypula K, Ashpole KJ, Bogod D, et al. Litigation related to regional anaesthesia: an analysis of claims against the NHS in England 1995-2007. Anaesthesia. 2010;65:443–452.
438. Karambelkar DJ, Ramanathan S. 2-Chloroprocaine antagonism of epidural morphine analgesia. Acta Anaesthesiol Scand. 1997;41:774–778.
439. Hess PE, Snowman CE, Hahn CJ, et al. Chloroprocaine may not affect epidural morphine for postcesarean delivery analgesia. J Clin Anesth. 2006;18:29–33.
440. American Society of Anesthesiologists Task Force on Neuraxial Opioids. Practice guidelines for the prevention, detection, and management of respiratory depression associated with neuraxial opioid administration. Anesthesiology. 2009;110:218–230.
441. American Academy of Pediatrics Committee on Drugs. Transfer of drugs and other chemicals into human milk. Pediatrics. 2001;108:776–789.
442. Douglas MJ, Kim JH, Ross PL, McMorland GH. The effect of epinephrine in local anaesthetic on epidural morphine-induced pruritus. Can Anaesth Soc J. 1986;33:737–740.
443. Szarvas S, Harmon D, Murphy D. Neuraxial opioid-induced pruritus: a review. J Clin Anesth. 2003;15:234–239.
444. Tan T, Ojo R, Immani S, et al. Reduction of severity of pruritus after elective caesarean section under spinal anaesthesia with subarachnoid morphine: a randomised comparison of prophylactic granisetron and ondansetron. Int J Obstet Anesth. 2010;19:56–60.
445. Butwick AJ, Lipman SS, Carvalho B. Intraoperative forced air-warming during cesarean delivery under spinal anesthesia does not prevent maternal hypothermia. Anesth Analg. 2007;105:1413–1419.
446. Roy JD, Girard M, Drolet P. Intrathecal meperidine decreases shivering during cesarean delivery under spinal anesthesia. Anesth Analg. 2004;98:230–234.
447. Sessler DI. Temperature monitoring and perioperative thermoregulation. Anesthesiology. 2008;109:318–338.
448. Rajagopalan S, Mascha E, Na J, Sessler DI. The effects of mild perioperative hypothermia on blood loss and transfusion requirement. Anesthesiology. 2008;108:71–77.
449. Hartgill TW, Bergersen TK, Pirhonen J. Core body temperature and the thermoneutral zone: a longitudinal study of normal human pregnancy. Acta Physiol (Oxf). 2011;201:467–474.
450. Saito T, Sessler DI, Fujita K, et al. Thermoregulatory effects of spinal and epidural anesthesia during cesarean delivery. Reg Anesth Pain Med. 1998;23:418–423.
451. Leslie K, Sessler DI. Reduction in the shivering threshold is proportional to spinal block height. Anesthesiology. 1996;84:1327–1331.
452. Sessler DI. Thermoregulatory defense mechanisms. Crit Care Med. 2009;37:S203–S210.
453. Horn EP, Schroeder F, Gottschalk A, et al. Active warming during cesarean delivery. Anesth Analg. 2002;94:409–414.
454. Sun HL, Ling QD, Sun WZ, et al. Lower limb wrapping prevents hypotension, but not hypothermia or shivering, after the introduction of epidural anesthesia for cesarean delivery. Anesth Analg. 2004;99:241–244.
455. Tsen LC, Balki M. Oxytocin protocols during cesarean delivery: time to acknowledge the risk/benefit ratio? Int J Obstet Anesth. 2010;19:243–245.
456. Carvalho JC, Balki M, Kingdom J, Windrim R. Oxytocin requirements at elective cesarean delivery: a dose-finding study. Obstet Gynecol. 2004;104:1005–1010.
457. Balki M, Ronayne M, Davies S, et al. Minimum oxytocin dose requirement after cesarean delivery for labor arrest. Obstet Gynecol. 2006;107:45–50.
458. George RB, McKeen D, Chaplin AC, McLeod L. Up-down determination of the ED90 of oxytocin infusions for the prevention of postpartum uterine atony in parturients undergoing Cesarean delivery. Can J Anaesth. 2010;57:578–582.
459. Robinson C, Schumann R, Zhang P, Young RC. Oxytocin-induced desensitization of the oxytocin receptor. Am J Obstet Gynecol. 2003;188:497–502.
460. Phaneuf S, Asboth G, Carrasco MP, et al. Desensitization of oxytocin receptors in human myometrium. Hum Reprod Update. 1998;4:625–633.
461. Magalhaes JK, Carvalho JC, Parkes RK, et al. Oxytocin pretreatment decreases oxytocin-induced myometrial contractions in pregnant rats in a concentration-dependent but not time-dependent manner. Reprod Sci. 2009;16:501–508.
462. Balki M, Cristian AL, Kingdom J, Carvalho JC. Oxytocin pretreatment of pregnant rat myometrium reduces the efficacy of oxytocin but not of ergonovine maleate or prostaglandin F2-alpha. Reprod Sci. 2010;17:269–277.
463. Zingg HH. Vasopressin and oxytocin receptors. Baillieres Clin Endocrinol Metab. 1996;10:75–96.
464. Bolton TJ, Randall K, Yentis SM. Effect of the Confidential Enquiries into Maternal Deaths on the use of Syntocinon at caesarean section in the UK. Anaesthesia. 2003;58:277–279.
465. Langesaeter E, Rosseland LA, Stubhaug A. Haemodynamic effects of repeated doses of oxytocin during Caesarean delivery in healthy parturients. Br J Anaesth. 2009;103:260–262.
466. Langesaeter E, Rosseland LA, Stubhaug A. Haemodynamic effects of oxytocin in women with severe preeclampsia. Int J Obstet Anesth. 2011;20:26–29.
467. Svanstrom MC, Biber B, Hanes M, et al. Signs of myocardial ischaemia after injection of oxytocin: a randomized double-blind comparison of oxytocin and methylergometrine during Caesarean section. Br J Anaesth. 2008;100:683–689.
468. Abouleish E. Postpartum hypertension and convulsion after oxytocic drugs. Anesth Analg. 1976;55:813–815.
469. Balki M, Dhumne S, Kasodekar S, et al. Oxytocin-ergometrine co-administration does not reduce blood loss at caesarean delivery for labour arrest. BJOG. 2008;115:579–584.
470. Dildy GA 3rd. Postpartum hemorrhage: new management options. Clin Obstet Gynecol. 2002;45:330–344.
471. Chou MM, MacKenzie IZ. A prospective, double-blind, randomized comparison of prophylactic intramyometrial 15-methyl prostaglandin F2-alpha, 125 micrograms, and intravenous oxytocin, 20 units, for the control of blood loss at elective cesarean section. Am J Obstet Gynecol. 1994;171:1356–1360.
472. Oleen MA, Mariano JP. Controlling refractory atonic postpartum hemorrhage with Hemabate sterile solution. Am J Obstet Gynecol. 1990;162:205–208.
473. Tuncalp Ö, Hofmeyr GJ, Gülmezoglu AM. Prostaglandins for preventing postpartum haemorrhage. Cochrane Database Syst Rev. 2012;(8).
474. Elati A, Weeks A. Misoprostol for the management of postpartum haemorrhage. BMJ. 2011;342:d2877.
475. de Souza A, Permezel M, Anderson M, et al. Antenatal erythropoietin and intra-operative cell salvage in a Jehovah's Witness with placenta praevia. BJOG. 2003;110:524–526.
476. Resch BE, Gaspar R, Sonkodi S, Falkay G. Vasoactive effects of erythropoietin on human placental blood vessels in vitro. Am J Obstet Gynecol. 2003;188:993–996.
477. Teramo KA, Hiilesmaa VK, Schwartz R, et al. Amniotic fluid and cord plasma erythropoietin levels in pregnancies complicated by preeclampsia, pregnancy-induced hypertension and chronic hypertension. J Perinat Med. 2004;32:240–247.
478. Allam J, Cox M, Yentis SM. Cell salvage in obstetrics. Int J Obstet Anesth. 2008;17:37–45.
479. Ralph CJ, Sullivan I, Faulds J. Intraoperative cell salvaged blood as part of a blood conservation strategy in Caesarean section: is fetal red cell contamination important? Br J Anaesth. 2011;107:404–408.
480. Gutierrez MC, Goodnough LT, Druzin M, Butwick AJ. Postpartum hemorrhage treated with a massive transfusion protocol at a tertiary obstetric center: a retrospective study. Int J Obstet Anesth. 2012;21:230–235.
481. Dennis AT. Transthoracic echocardiography in obstetric anaesthesia and obstetric critical illness. Int J Obstet Anesth. 2011;20:160–168.
482. American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Practice guidelines for perioperative blood transfusion and adjuvant therapies. Anesthesiology. 2006;105:198–208.
483. Charbit B, Mandelbrot L, Samain E, et al. The decrease of fibrinogen is an early predictor of the severity of postpartum hemorrhage. J Thromb Haemost. 2007;5:266–273.
484. Gonsalves M, Belli A. The role of interventional radiology in obstetric hemorrhage. Cardiovasc Intervent Radiol. 2010;33:887–895.
485. Georgiou C. Balloon tamponade in the management of postpartum haemorrhage: a review. BJOG. 2009;116:748–757.
486. Pacheco LD, Saade GR, Gei AF, Hankins GD. Cutting-edge advances in the medical management of obstetrical hemorrhage. Am J Obstet Gynecol. 2011;205:526–532.
487. Daskalakis G, Anastasakis E, Papantoniou N, et al. Emergency obstetric hysterectomy. Acta Obstet Gynecol Scand. 2007;86:223–227.
488. Bateman BT, Mhyre JM, Callaghan WM, Kuklina EV. Peripartum hysterectomy in the United States: nationwide 14-year experience. Am J Obstet Gynecol. 2012;206:63 e1–8.
489. Nguyen D, Nguyen C, Yacobozzi M, et al. Imaging of the placenta with pathologic correlation. Semin Ultrasound CT MR. 2012;33:65–77.
490. Chestnut DH, Dewan DM, Redick LF, et al. Anesthetic management for obstetric hysterectomy: a multi-institutional study. Anesthesiology. 1989;70:607–610.
491. Briery CM, Rose CH, Hudson WT, et al. Planned vs emergent cesarean hysterectomy. Am J Obstet Gynecol. 2007;197:154 e1–5.
492. Wright JD, Devine P, Shah M, et al. Morbidity and mortality of peripartum hysterectomy. Obstet Gynecol. 2010;115:1187–1193.
493. American College of Obstetricians and Gynecologists. Thromboembolism in pregnancy. ACOG Practice Bulletin No. 123. Obstet Gynecol. 2011;118:718–729.
494. Tooher R, Gates S, Dowswell T, Davis LJ. Prophylaxis for venous thromboembolic disease in pregnancy and the early postnatal period. Cochrane Database Syst Rev. 2010;(5).
* Outside the operating room, and before the onset of labor, maternal blood pressure is ideally measured (using an appropriately sized cuff with a bladder length that is 80% and a width that is at least 46% of the arm circumference) after a rest period of 10 minutes or more, with the pregnant woman sitting or lying on her left side with her arm at the level of the right atrium. The onset (phase 1) and disappearance (phase 5) of Korotkoff sounds correspond to systolic and diastolic pressures, respectively.52
* The Institute of Safe Medicine Practices (ISMP) has recommended that health care providers never use µg as an abbreviation for micrograms, but rather they should use mcg (http://www.ismp.org/tools/errorproneabbreviations.pdf, Accessed February 2013). The use of the symbol µg is frequently misinterpreted and involved in harmful medication errors. The abbreviation may be mistaken for mg (milligrams), which would result in a 1000-fold overdose. The symbol µg should never be used when communicating medical information, including pharmacy and prescriber computer order entry screens, computer-generated labels, labels for drug storage bins, and medication administration records. However, most scholarly publications have continued to use the abbreviation µg. The editors have chosen to retain the use of the abbreviation µg throughout this text. However, the editors recommend the use of the abbreviation mcg in clinical practice.