For spinal anesthesia, the value of ropivacaine and levobupivacaine compared with bupivacaine is doubtful. Given the small doses administered, a reduction in risk for systemic local anesthetic toxicity is not a consideration. Further, it is not clear that ropivacaine produces spinal anesthesia of similar quality to that provided by bupivacaine. The FDA has not approved ropivacaine or levobupivacaine for intrathecal administration. Thus, in the United States, bupivacaine remains the predominant agent for spinal anesthesia for cesarean delivery.
Hyperbaric spinal lidocaine or mepivacaine (60 to 80 mg) may be used when the obstetrician can reliably perform cesarean delivery in less than 45 minutes. The use of hyperbaric lidocaine for spinal anesthesia remains controversial because of concerns about transient neurologic symptoms (see Chapter 32).
Adjuvant Agents.
Adjuvant medications contribute to spinal anesthesia by different mechanisms from those of local anesthetics. For cesarean delivery, adjuvant agents improve the quality of intraoperative anesthesia, prolong postoperative analgesia, and reduce the dose, and therefore the side effects, of local anesthetics. Opioids, dextrose, and epinephrine are commonly used adjuvants; neostigmine and clonidine are two agents undergoing clinical investigation.
Opioids have been observed to improve intraoperative and postoperative comfort for patients undergoing spinal anesthesia for cesarean delivery. Intraoperatively, this effect can be observed through a reduction in local anesthetic drug doses and the need for analgesic supplementation.178,179 In a systematic review of intraoperative and postoperative analgesic efficacy and adverse effects of intrathecal opioids, Dahl et al.180 indicated that 24% of patients undergoing cesarean delivery with spinal hyperbaric bupivacaine alone required supplemental intraoperative analgesia. Opioids augment the quality and prolong the duration of local anesthetic-induced blockade, an effect most likely modulated by A-delta (pinprick) and C (cold) nerve fibers; muscle function (A-alpha nerve fibers) does not appear to be affected.181 The mechanism for the opioid-induced prolongation of sensory block remains unclear but may include modulation of sensory input at the spinal and supraspinal level as well as an alteration of consciousness of peripheral sensations.182
An additional advantage of intrathecal opioid administration is its salutary effect on the incidence of intraoperative nausea and vomiting. During periods of visceral stimulation (i.e., exteriorization of the uterus and fascial stimulation during closure), patients often complain of nausea. The addition of spinal fentanyl in doses of 10 to 25 µg to lidocaine or bupivacaine decreases the incidence of nausea and/or vomiting during cesarean delivery.183,184 Clinicians commonly add both a lipid- and water-soluble opioid to the local anesthetic for spinal anesthesia for cesarean delivery. This practice takes advantage of the fast onset of the lipid-soluble agent and the prolonged duration of the water-soluble agent (Figure 26-5) (see Chapter 13). Controversy exists as to whether the co-administration of a lipid-soluble agent (e.g., fentanyl, sufentanil) with a water-soluble agent (e.g., morphine) leads to diminished response to the water-soluble agent (i.e., acute opioid tolerance). Cooper et al.185 observed that spinal fentanyl 25 µg or saline added to plain bupivacaine 10 mg resulted in no difference in intravenous patient-controlled analgesia morphine consumption within the first 6 hours after cesarean delivery; however, between 6 and 23 hours, there was a 63% increase in morphine use in the group that received fentanyl. In a study of 40 women undergoing cesarean delivery using intrathecal fentanyl (0, 5, 10, or 25 µg) combined with hyperbaric bupivacaine 12 mg and morphine 0.2 mg, Carvalho et al.186 observed higher postoperative pain scores in the patients who received fentanyl but no differences among groups in postoperative intravenous patient-controlled analgesia morphine consumption. Women who received no fentanyl had a higher incidence of intraoperative nausea and vomiting, suggesting that fentanyl is an important adjunct for intraoperative anesthesia.186 We recommend the administration of both a lipid- and a water-soluble opioid when spinal anesthesia is administered for cesarean delivery.
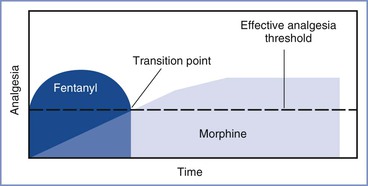
FIGURE 26-5 Schematic illustration of the pharmacokinetic and pharmacodynamic activities resulting from the neuraxial administration of a lipid-soluble opioid (e.g., fentanyl) and a water-soluble opioid (e.g., morphine) for analgesia. The transition point varies according to the opioid drugs and doses administered. For most commonly used opioids, this transition point occurs in the postoperative period.
The optimal dose of spinal opioids is influenced by the type, dose, and baricity of the accompanying local anesthetic and the presence of other adjuvants. Dahl et al.180 performed a systematic review of spinal fentanyl administered in doses ranging from 2.5 to 60 µg to augment spinal anesthesia for cesarean delivery. Studies included in the analysis were pooled into two groups based on spinal fentanyl dose (15 to 35 µg and 40 to 60 µg); there was no difference between groups in the need for supplemental intraoperative analgesia. Postoperative pruritus, nausea, and vomiting were significantly reduced with administration of doses less than 35 µg, although no meaningful postoperative analgesia was produced at these doses. Manullang et al.187 found that spinal fentanyl 20 µg was superior to intravenous ondansetron 4 mg for the prevention of perioperative nausea (but not vomiting) during spinal anesthesia for cesarean delivery. Spinal doses of fentanyl 10 to 25 µg are commonly used for cesarean delivery anesthesia (Table 26-5).180,188
Spinal sufentanil 2.5 to 20 µg has been used with bupivacaine for cesarean delivery. In a study of 37 parturients undergoing elective cesarean delivery with sufentanil (0, 10, 15, or 20 µg) added to hyperbaric bupivacaine 10.5 mg, Courtney et al.189 found better quality and longer duration of analgesia in all sufentanil groups than in the control group, with similar Apgar scores, umbilical cord blood gas measurements, and Early Neonatal Neurobehavioral Scale (ENNS) scores. No cases of respiratory depression occurred. Braga Ade et al.190 randomly assigned parturients to receive hyperbaric bupivacaine 12.5 mg with sufentanil (0, 2.5, 5, or 7.5 µg). Analgesia lasted longer with sufentanil 5 and 7.5 µg, and pruritus and somnolence were more pronounced with 7.5 µg. Thus, there appears to be little justification for giving a dose of sufentanil greater than 5 µg in this setting.
Preservative-free morphine and diamorphine can improve intraoperative comfort of parturients during cesarean delivery; however, both drugs are primarily used for providing prolonged (12 to 24 hours) postcesarean analgesia (see Chapter 28). Spinal morphine has a latency of 30 to 60 minutes for onset of analgesia,191 and it produces significant analgesia with acceptable side-effect profiles when given in doses ranging from 0.1 to 0.25 mg. Palmer et al.192 conducted a dose-response study of morphine added to hyperbaric bupivacaine 12.75 mg; morphine 0.1 mg provided analgesia comparable to that provided by doses as high as 0.5 mg. The occurrence of pruritus, but not nausea and vomiting, appeared to be dose related.
Intrathecal diamorphine is used in the United Kingdom for postoperative analgesia. It is metabolized to the two active compounds 6-acetyl morphine and morphine. Diamorphine is more lipophilic than morphine, enabling a rapid onset (6 to 9 minutes, similar to fentanyl) but a potentially shorter duration of action.193 Saravanan et al.178 observed that intrathecal diamorphine 0.4 mg combined with bupivacaine 12.5 mg resulted in an intraoperative supplementation rate of less than 5%; however, the incidence of nausea and vomiting was 56% and the incidence of pruritus was 80%.
Neuraxial administration of water-soluble opioids such as morphine is associated with delayed respiratory depression (6 to 18 hours after administration) (see Chapter 28). Postoperative monitoring protocols should observe for respiratory depression, which although infrequent, can lead to mortality, particularly in high-risk patients (e.g., those with sleep apnea or obesity).180,194 Many opioids, including morphine and diamorphine, may outlast the antagonism provided by naloxone (approximately 90 minutes).195
Most spinal local anesthetics are prepared in dextrose to make the agents hyperbaric. For example, commercially available hyperbaric bupivacaine contains 8.25% dextrose (82.5 mg/mL), and hyperbaric lidocaine contains 7.5% dextrose (75 mg/mL). The amount of dextrose required to make a meaningful clinical difference in a spinal technique with local anesthetic agents has not been well characterized. Baricity is defined as the ratio of the density of the local anesthetic solution to the density of CSF measured at the same temperature. The density of CSF is lower in women than men, particularly during pregnancy and the immediate postpartum period196; even so, CSF density is significantly greater than that of local anesthetics and opioids in the absence of dextrose.196
The intrathecal administration of an alpha-adrenergic agonist (e.g., epinephrine, clonidine) increases the density of sensory and motor blockade and may prolong the duration of blockade as well as contribute to postcesarean analgesia. Abouleish197 observed that intrathecal epinephrine 0.1 to 0.2 mg, when combined with hyperbaric bupivacaine, improved the quality of intraoperative analgesia and prolonged both sensory and motor blockade by approximately 15% in comparison with bupivacaine alone. However, Randalls et al.198 observed that the addition of epinephrine 0.3 mg to hyperbaric bupivacaine 12.5 mg for elective cesarean delivery increased the incidence of nausea.
Spinal clonidine, in doses of 60 to 150 µg, improves intraoperative analgesia, decreases shivering, and reduces peri-incisional hyperalgesia in women undergoing cesarean delivery; however, it has been associated with hypotension and sedation.199 This agent is not used commonly in the United States, although it may be considered in specific circumstances (e.g., when neuraxial opioid analgesia is contraindicated). The FDA has issued a “black box” warning against its use in obstetric patients because of concerns about hemodynamic instability.
In women undergoing cesarean delivery, spinal neostigmine in doses up to 100 µg significantly reduced postoperative pain with no effect on FHR or Apgar scores; however, 100% of patients who received 100 µg complained of nausea.200 Chung et al.201 observed that 74% of parturients undergoing cesarean delivery with spinal neostigmine 25 µg with hyperbaric bupivacaine 12 mg had significant nausea and vomiting, which persisted for greater than 1 hour despite multiple antiemetic agents. In a dose-response investigation in nonpregnant healthy volunteers, spinal doses of neostigmine as low as 6.25 µg were associated with a high incidence of side effects, including prolonged motor blockade, nausea, and vomiting.202 Collectively, these studies suggest that the high incidence of nausea associated with intrathecal neostigmine limits its clinical use.
At many institutions, the spinal agents and doses are standardized so that consistent results are obtained during the provision of spinal anesthesia for cesarean delivery. Such standardization enables the anesthesia, obstetric, and nursing staff to anticipate predictable onset and recovery characteristics and respond to physiologic responses that are outside the norm. Standardization of drugs and doses may also result in fewer errors. At our institution, spinal anesthesia for cesarean delivery is provided with 0.75% hyperbaric bupivacaine 12 mg, fentanyl 10 µg, and morphine 0.2 mg. Administration of these doses of fentanyl and morphine allows administration of the same volume (0.2 mL) of fentanyl 50 µg/mL and morphine 1 mg/mL, thereby helping to prevent dosing errors. The use of a tuberculin or insulin syringe to prepare the opioids further improves measurement accuracy.
Epidural Anesthesia
The use of epidural anesthesia for nonelective cesarean delivery has increased, primarily as a result of the greater use of epidural analgesia during labor. However, the overall use of epidural anesthesia is becoming less common for elective cesarean delivery when an epidural catheter is not already in situ (e.g., for labor analgesia or as a potential mode of anesthesia in a laboring individual at high risk for urgent cesarean delivery) (see Figure 26-3), in part because the resulting block is less reliable than that with spinal anesthesia. Initiation of CSE anesthesia offers both rapid onset and reliable spinal anesthesia coupled with the ability to augment or prolong the blockade through the epidural catheter. The dural puncture performed as part of CSE anesthesia may enhance movement of drugs injected into the epidural space across the dura-arachnoid into the subarachnoid space.203 Epidural local anesthetic and opioid doses are generally 5 to 10 times greater than doses given intrathecally; this difference results from the requirement for penetration of nerve roots as they traverse the epidural space, the greater capacity of the epidural space, and the presence of the epidural venous plexus, which becomes progressively more engorged during pregnancy. Greater systemic absorption of anesthetic agents occurs with epidural anesthesia than with spinal anesthesia, and the risk for local anesthetic toxicity is a real possibility with local anesthetic injection for epidural anesthesia, but not for spinal anesthesia.
Possible advantages of the epidural technique include a slower onset of sympathetic blockade; this may allow compensatory mechanisms to attenuate the severity of hypotension. A catheter-based technique also allows titration of the level, density, and duration of anesthesia. Continuous postcesarean analgesia can be provided through an epidural catheter.
Local Anesthetic Agents
The most common local anesthetic used for the initiation and maintenance of epidural anesthesia for cesarean delivery is 2% lidocaine with epinephrine (see Table 26-6). The epidural administration of lidocaine in concentrations less than 2%, or without the addition of epinephrine (which augments the analgesia through alpha-adrenergic receptor blockade204), may result in anesthesia that is inadequate for surgery.205 Hillyard et al.206 observed a significantly lower incidence of intraoperative block supplementation with 2% lidocaine with epinephrine 1 : 200,000 or 0.75% ropivacaine compared with 0.5% bupivacaine or 0.5% levobupivacaine.
TABLE 26-6
Drugs Used for Epidural Anesthesia for Cesarean Delivery
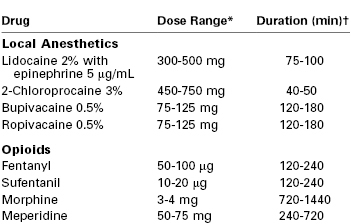
* Both the mass and volume of local anesthetic affect the extent and quality of anesthesia. The usual volume of local anesthetic solution administered into the epidural space at the indicated concentrations is 15 to 25 mL. More mass/volume is required for initiating epidural anesthesia de novo; conversely, less is required if epidural labor analgesia is being extended to surgical anesthesia.
† For the local anesthetics, the duration is defined as the time to two-segment regression. For the opioids, the duration is defined as the period of analgesia (or time to first request for a supplemental analgesic drug).
A 3% solution of 2-chloroprocaine has the most rapid onset and the shortest duration of action of available local anesthetics given epidurally. These characteristics make it an excellent choice for emergency cesarean delivery (see later discussion) because the dose is administered rapidly, and even if unintentional intravenous administration of drug were to occur, the sequelae would likely be less severe than the similar administration of an amide local anesthetic agent. Administration of 2-chloroprocaine has been associated with neurologic sequelae, possibly associated with the antioxidant sodium bisulfite, and paralumbar muscle spasms and pain, believed to be a result of calcium chelation by the preservative EDTA. Current preparations of 2-chloroprocaine do not contain either an antioxidant or a preservative (see Chapter 13). Epidural administration of 2-chloroprocaine may be associated with a rapid onset of hypotension and an apparent reduction in the clinical efficacy of subsequently administered epidural opioids or local anesthetics. Toledo et al.207 observed that the analgesic effect of epidural morphine administered 30 minutes before 2-chloroprocaine did not appear to be mitigated; however, administration of morphine before 2-chloroprocaine in the setting of emergency cesarean delivery is not possible. These considerations limit the use of 2-chloroprocaine to those situations in which the rapid onset of anesthesia is paramount.
Surgical anesthesia can be produced with epidural administration of 0.5% bupivacaine; however, the slow onset of neuroblockade and the risk for cardiovascular sequelae from unintentional intravascular injection (or systemic absorption) limit the contemporary use of this agent. (The risk for cardiovascular sequelae resulted in a proscription against the epidural administration of 0.75% bupivacaine in obstetric patients by the FDA.208) The single-isomer, levorotatory local anesthetics 0.5% to 0.75% ropivacaine and 0.5% levobupivacaine may be preferable to racemic bupivacaine because of their better safety profiles and earlier recovery, although a significant portion of the improved safety profile is due to the lower potency of these agents (e.g., 0.5% bupivacaine is more potent than 0.5% levobupivacaine or 0.5% ropivacaine).209 Bader et al.210 compared 30 mL of epidural 0.5% levobupivacaine with racemic 0.5% bupivacaine in women undergoing elective cesarean delivery; they observed no differences in the block onset or resolution, signal-averaged ECG results, complications, or maternal and fetal plasma pharmacokinetic profiles between the treatment groups. After administration of 25 mL of 0.5% levobupivacaine or 0.5% racemic bupivacaine for epidural anesthesia in women undergoing cesarean delivery, Faccenda et al.211 observed no difference in onset, spread, or duration of sensory block between the agents, although levobupivacaine produced lower limb motor blockade of longer duration but less intensity. Datta et al.212 demonstrated that the onset, duration, and regression of sensory blockade with 0.5% ropivacaine was similar to that provided by 0.5% bupivacaine, although a faster onset and longer duration of motor blockade was observed with bupivacaine. The free concentrations of ropivacaine were approximately twice those of bupivacaine in both maternal and neonatal blood at delivery; however, these measurements were less than the concentrations shown to be toxic in animals.
Adjuvant Agents
As with spinal anesthesia, adjuvant medications are used for their intrinsic properties and to reduce the dose and side effects of local anesthetic agents. The use of epidural adjuvants can improve the quality of intraoperative anesthesia and result in less motor blockade as well as enhance postoperative analgesia (see Chapter 28).
Whereas some anesthesia providers administer an epidural opioid with the initial therapeutic dose of local anesthetic, others delay opioid administration until after the umbilical cord is clamped to prevent transfer of opioid to the fetus (see Chapter 28). The onset of analgesia is dictated by complex pharmacokinetics; however, the lipid-soluble opioids (e.g., fentanyl, sufentanil) have greater availability, more rapid onset, and more rapid clearance than the water-soluble opioids (e.g. morphine).213
The administration of epidural fentanyl (50 to 100 µg) results in activity at both spinal and supraspinal sites of action,214 improves the intraoperative quality of anesthesia during cesarean delivery,215,216 and does not appear to adversely affect the neonate.217 The optimal dose of epidural fentanyl has not been determined for patients undergoing cesarean delivery; however, Eichenberger et al.218 observed a segmental effect of epidural fentanyl 100 µg, but not 50 µg, on experimental pain in nonpregnant patients.
Epidural sufentanil (10 to 20 µg), when added to 0.5% bupivacaine with epinephrine 5 µg/mL, provides significantly better intraoperative anesthesia and longer postoperative analgesia than bupivacaine and epinephrine alone, with minimal maternal side effects and no adverse neonatal effects.219 Epidural sufentanil is approximately five times as potent as epidural fentanyl, but when equipotent doses are administered, no differences between the agents in onset, quality, or duration of analgesia have been observed.220,221
Epidural administration of the hydrophilic drug morphine provides prolonged postcesarean analgesia. In a dose-response study of epidural morphine 1.25, 2.5, 3.75, and 5 mg, Palmer et al.222 found 3.75 mg to be an optimal dose beyond which postcesarean analgesia (as measured by patient-controlled analgesia morphine demands) was no better. Extended-release epidural morphine 10 mg provides better postoperative analgesia than epidural morphine 4 mg, with no differences in nausea, pruritus, or sedation scores.223
Epidural diamorphine (2.5 to 3 mg) is commonly used in the United Kingdom for providing prolonged postcesarean analgesia.224 Optimal dose-finding studies of epidural diamorphine have not been performed; however, Bloor et al.225 observed that duration and quality of analgesia from epidural diamorphine 3 mg was similar to that provided by spinal diamorphine 0.3 mg, with significantly less pruritus.
Epidural clonidine (75 to 200 µg) combined with morphine or fentanyl reduces the requirement for postcesarean morphine analgesia.226 Eisenach et al.226 demonstrated an additive rather than synergistic effect of epidural clonidine and fentanyl in producing postcesarean analgesia. Common side effects include hypotension and sedation. Currently, epidural clonidine has only one specific neuraxial indication in the United States (intractable cancer pain), and the package insert has a “black box” FDA warning stating that “epidural clonidine is not recommended for obstetrical, postpartum and perioperative pain management.”
Epidural neostigmine produces a modest amount of postcesarean analgesia when given after umbilical cord clamping. Kaya et al.227 investigated the administration of 75, 150, or 300 µg of epidural neostigmine in women undergoing elective cesarean delivery. An increase in intraoperative shivering and sedation was observed in the 300-µg group only; a dose-independent reduction in postoperative pain and sedation was observed in all groups.
Epinephrine is frequently added to the local anesthetic agent to minimize systemic absorption and peak blood level of local anesthetic, increase the density of sensory and motor blockade, and prolong the duration of anesthesia.204,228,229 Bernards et al.230 observed that the pharmacokinetic effects of epinephrine co-administered with an opioid vary with the opioid and the sampling site. In the lumbar epidural space, epinephrine lengthened the mean residence time of morphine but shortened that of fentanyl and sufentanil.
The epidural administration of epinephrine in preeclamptic women is controversial (see Chapter 36).231 Animal and clinical studies suggest that epidural epinephrine 0.1 mg does not decrease uterine blood flow.232,233 Alahuhta et al.234 used maternal Doppler ultrasonography and fetal M-mode echocardiography to evaluate the hemodynamic effects of the addition of epidural epinephrine 5 µg/mL (1 : 200,000) to 0.5% bupivacaine for cesarean delivery; maternal diastolic pressure, but not systolic pressure or uterine blood flow, was decreased with the addition of epinephrine. By contrast, in a similar study in hypertensive women, Alahuhta et al.235 observed that the addition of epidural epinephrine 5 µg/mL (1 : 200,000) to 0.5% bupivacaine significantly reduced uteroplacental blood flow but did not affect umbilical arterial blood flow or pH measurements at delivery.
When combined with local anesthetic for epidural anesthesia, the usual epinephrine concentration is 2.5 or 5 µg/mL (i.e., 1 : 400,000 or 1 : 200,000). The addition of epinephrine to a solution of plain local anesthetic just prior to administration results in a solution that has a higher pH than commercially prepared epinephrine-containing products, which use (low-pH) antioxidants to preserve the efficacy of the epinephrine (see Chapter 13). Thus, use of freshly prepared solutions hastens the onset of anesthesia.
The addition of sodium bicarbonate results in a solution with more local anesthetic molecules in a non-ionized state, which hastens the onset and augments the quality of the local anesthetic blockade, particularly if sodium bicarbonate is added to a low-pH solution (see later discussion).
Combined Spinal-Epidural Anesthesia
The CSE technique incorporates the rapid and predictable onset of spinal blockade with the ability to augment anesthesia by injection of additional drug through the epidural catheter.236,237 In 1981, Brownridge236 reported the first use of the CSE technique for cesarean delivery through separate spinal and epidural needles introduced at different interspaces. Carrie and O'Sullivan237 subsequently described the needle-through-needle technique via a single interspace for cesarean delivery; this has become the more popular technique. Compared with a conventional epidural anesthetic technique for cesarean delivery, Davies et al.238 reported that the CSE technique resulted in a faster onset, greater motor blockade, and lower pain scores at delivery; moreover, no differences were observed in the incidence of maternal hypotension, nausea, or headache, the use of supplemental analgesics, or overall patient satisfaction.
Additional advantages of the CSE technique include (1) use of the epidural needle as an introducer for a longer spinal needle when attempts with a traditional introducer and spinal needle have failed and (2) use of a spinal needle (and return of CSF through the needle) to “confirm” the correct positioning of the epidural needle in the epidural space. The CSE technique also allows use of a low dose of local anesthetic to initiate spinal anesthesia (associated with a lower incidence of hypotension), followed by use of the epidural catheter to extend intraoperative anesthesia or provide postoperative analgesia.
Conventional spinal doses (e.g., 12 mg) of hyperbaric bupivacaine are most often used to provide CSE anesthesia for cesarean delivery; however, a satisfactory block has been reported with plain bupivacaine drug doses as low as 4.5 mg.170 Although the use of lower amounts of local anesthetic is enabled by the presence of the epidural catheter (because additional agents can be administered if discomfort occurs), the block achieved with the CSE technique may be inherently different from the block achieved with a single-shot spinal technique with the same dose(s) of medication. Goy et al.239 positioned men undergoing surgery in the right lateral position for initiation of neuraxial anesthesia and demonstrated that the median effective doses of intrathecal hyperbaric bupivacaine (to achieve a T6 sensory level of anesthesia for 60 minutes) for the CSE and spinal techniques were 9.2 mg and 11.4 mg, respectively. The investigators speculated that the use of the loss-of-resistance to air (during introduction of the epidural needle) resulted in a reduction in lumbar CSF volume and a subsequently higher sensory blockade. Similarly, after initiating neuraxial analgesia with intrathecal bupivacaine 10 mg in parturients undergoing elective cesarean delivery, Ithnin et al.240 observed median sensory levels of C6 and T3 with the CSE and spinal techniques, respectively. The CSE technique was performed with loss-of-resistance to air (2 mL); however, after administration of the spinal medications, the epidural catheter was not inserted. The investigators speculated that the loss of negative pressure in the epidural space created by the introduction of the epidural needle was responsible for the observed differences. However, when investigators from the same institution performed the same anesthetic techniques for cesarean delivery in laboring women, no differences in the block characteristics were observed.241 The reasons for these different results are unclear.
The sequential CSE technique uses a lower dose of spinal bupivacaine (7.5 to 10 mg) followed by incremental injection of local anesthetic through the epidural catheter to achieve a T4 level of anesthesia.242,243 The purported advantage of this approach is a lower incidence of hypotension. Thoren et al.243 observed a more gradual onset of hypotension and a lower initial sensory level with the CSE compared with the single-shot spinal technique (T7 and T4, respectively); however, all parturients in the CSE group required additional doses of local anesthetic through the epidural catheter. The sequential CSE technique may be of particular advantage in high-risk parturients (e.g., significant cardiac disease) in whom avoidance of severe hypotension can be vitally important.
Another CSE technique is the extradural volume extension (EVE) technique.242,244 Intrathecal administration of a small dose of local anesthetic is followed by the administration of saline through the epidural catheter. In a review of this technique, McNaught and Stocks242 observed a higher cephalad spread of one to four dermatomal segments. However, the effect of EVE may depend on the initial dose and baricity of local anesthetic, the time interval between spinal and epidural injection, the volume of epidural saline, and the outcomes measured. Kucukguclu et al.244 found no clinical differences when 10 mL of epidural saline was administered within 5 minutes of intrathecal injection of either hyperbaric or plain 0.5% bupivacaine 8 to 9 mg with fentanyl 20 µg. Similarly, Loubert et al.245 found no difference in sensory or motor blockade when spinal hyperbaric bupivacaine 7.5 mg was administered with or without 5 mL of epidural saline injected immediately after the spinal dose.
Potential drawbacks of CSE techniques include an untested epidural catheter and hypotension. Yun et al.128 reported greater severity and duration of hypotension when the CSE technique was administered in the sitting position than in the lateral decubitus position. The hypotension may be related to the delay in moving the patient from the sitting to the supine (with leftward tilt) position. Alternatively, greater hypotension may result from a higher level of sympathetic blockade with the CSE technique.
Extension of Epidural Labor Analgesia
The extension of epidural labor analgesia to surgical anesthesia sufficient for cesarean delivery can be accomplished with one of several local anesthetic agents. The selection of agent often depends on the urgency of the case. Extension of epidural analgesia can be initiated as preparations are being made to move the patient from the labor room to the operating room. Whether an in situ epidural catheter should be used for an extension attempt depends on a number of factors, including the quality of the existing labor analgesia. If obtaining satisfactory epidural labor analgesia has been problematic (e.g., one-sided or “patchy” analgesia), replacing the catheter and using a spinal or CSE technique may be a better method to attain rapid and effective anesthesia. In a systematic review and meta-analysis, Bauer et al.246 observed that the incidence of failed conversion of labor analgesia to cesarean delivery anesthesia is greater when an increasing number of epidural boluses have been required to produce sufficient labor analgesia, a greater urgency for cesarean delivery exists, and a nonobstetric anesthesiologist is managing the case.
Specific local anesthetic and adjuvant solutions may influence whether the quality and level of epidural anesthesia is adequate for cesarean delivery. Lucas et al.247 compared the extension of existing labor epidural analgesia among three solutions: 0.5% bupivacaine, 2% lidocaine with epinephrine 5 µg/mL (1 : 200,000), and a 50 : 50 combination of the two solutions. They observed no difference among groups in the time required to obtain a bilateral loss to cold sensation at T4. Similarly, although the study was likely underpowered, Bjornestad et al.248 observed no significant difference in onset of anesthesia between epidural administration of 3% 2-chloroprocaine and that of 2% lidocaine with freshly added epinephrine 5 µg/mL; median onset was 8 minutes (range of 4 to 13 minutes) in the 2-chloroprocaine group and 5 minutes (range of 2 to 22 minutes) in the lidocaine group. However, given the time taken to prepare the lidocaine with epinephrine solution, the investigators concluded that use of a pre-prepared solution, such as 2-chloroprocaine, may be preferred. Of interest, 30% and 20% of patients in the 2-chloroprocaine and lidocaine groups, respectively, required intravenous alfentanil for supplementation of anesthesia.
Alkalinization of the local anesthetic solution not only increases the speed of onset but also improves the quality and prolongs the duration of neuroblockade.249 Alkalinization shifts more of the local anesthetic molecules to the non-ionized, lipid-soluble form, which allows the local anesthetic to pass more easily through the lipid neuronal membrane surrounding the sodium channel. Although this phenomenon can be demonstrated for all local anesthetics, alkalinization is most often performed with local anesthetic agents of short and medium duration (e.g., 2-chloroprocaine, lidocaine). Typically, 1 mL of 8.4% sodium bicarbonate (1 mEq/mL) is added to 10 mL of lidocaine or 2-chloroprocaine. Longer-acting agents (e.g., bupivacaine, ropivacaine, levobupivacaine) easily precipitate with the addition of sodium bicarbonate. Precipitation occurs with the addition of less than 0.2 mEq of bicarbonate to 20 mL of 0.5% bupivacaine.250 Alkalinization exerts the greatest effect when it is freshly mixed with the local anesthetic solution; however, the mixture is relatively stable. Tuleu et al.251 evaluated the stability of pH-adjusted lidocaine with epinephrine, prepared with 2 mEq of sodium bicarbonate added to 20 mL of 2% lidocaine and epinephrine 0.1 mg. Although the local anesthetic activity was unchanged, the epinephrine showed evidence of partial degradation at 6 hours.
Lam et al.249 evaluated the extension of a T10 level of epidural labor analgesia with the addition of 1.2 mL of 8.4% sodium bicarbonate (or saline) to 12 mL of premixed 2% lidocaine with epinephrine 5 µg/mL (1 : 200,000) and fentanyl 75 µg (Figure 26-6). The mean times to attain a T6 anesthesia level with and without bicarbonate were 5.2 minutes and 9.7 minutes, respectively. Gaiser et al.252 evaluated the addition of 2 mL of 8.4% sodium bicarbonate to 23 mL of either 1.5% lidocaine with epinephrine 5 µg/mL (1 : 200,000) or 3% 2-chloroprocaine; the mean onset time to extend the T10 analgesia level to a surgical level of anesthesia was 4.4 minutes for lidocaine and 3.1 minutes for 2-chloroprocaine. Malhotra and Yentis253 evaluated the use of 20 mL of 0.5% levobupivacaine, with and without fentanyl 75 µg, to extend a T9 labor analgesia level to a T4 sensory level. The onset time did not differ between groups, averaging 10 to 11 minutes.
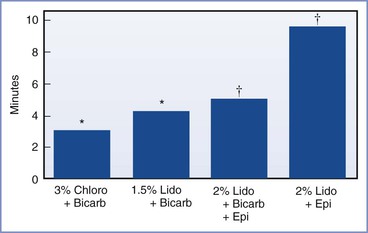
FIGURE 26-6 Onset time for extension of existing labor analgesia blockade (T10 sensory level) with different local anesthetic preparations. Results between the two studies cannot be directly compared owing to differences in labor analgesia regimens, different sensory testing methods and target levels, and presence of epidural opioids. The 2% lidocaine with epinephrine solution was premixed. The epinephrine concentration was 5 µg/mL. Chloro, 2-chloroprocaine; Bicarb, bicarbonate; Lido, lidocaine; Epi, epinephrine 5 µg/mL. *To T4 sensory level. (Data modified from Gaiser RR, Cheek TG, Gutsche BB. Epidural lidocaine versus 2-chloroprocaine for fetal distress requiring urgent cesarean section. Int J Obstet Anesth 1994; 3:208-10.) †To T6 sensory level. (Data modified from Lam DT, Ngan Kee WD, Khaw KS. Extension of epidural blockade in labour for emergency Caesarean section using 2% lidocaine with epinephrine and fentanyl, with or without alkalinisation. Anaesthesia 2001; 56:790-4.)
Extension of a T10 level of analgesia to a T4 level of anesthesia typically requires a volume of 15 to 20 mL of local anesthetic with one or more adjuvants. At our institution the extension of epidural labor analgesia begins with assessment of the quality of analgesia. For emergency cesarean delivery, we often initiate the extension of epidural anesthesia in the labor room by giving 5 to 10 mL of alkalinized 2% lidocaine (with epinephrine) or 3% 2-chloroprocaine. The sensory blockade is assessed after transfer of the patient to the operating room; if the blockade is bilateral and moving in a cephalad direction, an additional 5 to 10 mL is administered to bring the sensory level to T4. The use of this fractionated dosing schedule offers several advantages, including (1) greater hemodynamic stability during patient transfer; (2) assessment of the evolving sensory level before administration of the full dose of local anesthetic; (3) minimization of dural sac compression (by a large volume epidural injection),254 which enables a less difficult and safer conversion to spinal anesthesia if extension of epidural anesthesia is not successful (see later discussion); and (4) early sensory blockade at the incision site, so that surgery can be initiated in emergency cases before establishment of a full T4 sensory level. The extension of epidural analgesia to epidural anesthesia in the labor room is controversial.255 Some anesthesia providers delay epidural administration of additional local anesthetic until the patient has arrived in the operating room. However, this practice may increase the risk for failed epidural anesthesia, necessitating the induction of general anesthesia with its attendant risks (see later discussion).
General Anesthesia
Although neuraxial techniques are typically preferred when anesthesia is provided for cesarean delivery, there are some clinical situations in which the administration of general anesthesia is considered the most appropriate option (see Table 26-3). In addition, general anesthesia offers an advantage in cases in which uterine relaxation would be beneficial (e.g., cesarean delivery as part of an ex utero intrapartum treatment [EXIT] procedure).
The basic elements for preparation and care of the obstetric patient undergoing cesarean delivery also apply to the patient undergoing general anesthesia (Box 26-8; see Table 26-2). The preanesthetic evaluation should focus on assessment of physical characteristics (e.g., airway) and comorbidities. The consent process should feature the risks associated with airway management, aspiration, and awareness. The importance of a careful airway evaluation cannot be overemphasized (see Chapter 30); pregnancy-induced changes in the upper airway may be exacerbated during labor. Kodali et al.256 used acoustic reflectometry to show that soft tissue mucosal edema in both the oral (incisor teeth to oropharyngeal junction) and pharyngeal (oropharyngeal junction to the glottis) tissue increases during labor; these changes occurred over an average labor duration of 11 hours, of which 75 minutes was in the second stage, and resulted in worsening of the airway classification compared with the prelabor evaluation. Failed intubation, failed ventilation and oxygenation, and pulmonary aspiration of gastric contents remain leading anesthesia-related causes of maternal death.150 If the airway evaluation suggests the possibility of a difficult intubation, consideration should be given to the placement of a neuraxial catheter during early labor, even if it is not used to provide labor analgesia.34
Preparation
All pregnant patients requiring surgical anesthesia should be considered at risk for pulmonary aspiration of gastric contents (see Chapter 29). Attempts should be made to minimize both the risk for maternal aspiration and the risk for pulmonary injury if aspiration occurs. Fasting policies should be shared with all members of the obstetric care team. We administer metoclopramide 10 mg and ranitidine 50 mg intravenously between 30 and 60 minutes before induction of general anesthesia, to diminish gastric volume and gastric acid secretion, respectively.73 A clear, nonparticulate antacid (sodium citrate 30 mL) should also be administered within 30 minutes of surgery to neutralize gastric acid71; the antacid may be particularly relevant in the emergent situation when metoclopramide and ranitidine have not had the necessary time to exert their pharmacologic effects.
If the patient has airway characteristics that herald difficult mask ventilation or intubation, preparations should be made to perform an awake tracheal intubation (see Chapter 30). Preparations include administering an antisialagogue (e.g., glycopyrrolate), judicious sedation (e.g., midazolam), and topical airway anesthesia (e.g., aerosolized lidocaine). Glossopharyngeal and laryngeal nerve blocks may also be considered.
The patient should be placed supine with left uterine displacement. The head, neck, and shoulders should be optimally positioned for airway management (i.e., the sniffing position) (see Figure 30-8). Routine monitoring should be established, including ECG, pulse oximetry, blood pressure, and capnography. Preoxygenation (denitrogenation) with 100% oxygen should be performed to delay the onset of hypoxemia during apnea; this hypoxemia occurs more rapidly due to the pregnancy-induced decrease in functional residual capacity and increase in oxygen consumption. In a computer simulation of the respiratory and cardiovascular systems during pregnancy, McClelland et al.257 noted that the presence of labor, high BMI, and sepsis further accelerated oxyhemoglobin desaturation during apnea; by contrast, multiple gestation and hemorrhage appeared to have minimal effects. Ideally, preoxygenation is accomplished by 3 minutes of tidal-volume breathing with a tight-fitting face mask.258 Although four maximal deep breaths over 30 seconds with an FIO2 of 1.0 can achieve a similar PaO2, the same protection against rapid oxyhemoglobin desaturation is not afforded, owing to differences in tissue and venous compartment oxygen reserves.257,258 The method of eight deep breaths over 1 minute appears to provide better protection from oxyhemoglobin desaturation during apnea than the four deep breath over 30-second method.259
In contrast to most surgical procedures, the patient's abdomen is prepared and draped before induction of general anesthesia to minimize fetal exposure to general anesthesia. After the surgical drapes have been applied and the operating personnel are ready at the tableside, the surgeon should be instructed to delay the incision until the anesthesia provider confirms correct placement of the endotracheal tube and gives verbal instructions to proceed with surgery.
Induction
A rapid-sequence induction is initiated with denitrogenation/preoxygenation followed by administration of an induction agent, paralysis, and cricoid pressure; mask ventilation is not performed, to prevent unintentional insufflation of the stomach. Whether rapid-sequence induction should be employed, particularly in an appropriately fasted, nonlaboring patient presenting for elective cesarean delivery, has been questioned.260 Further, the value of cricoid pressure has been challenged owing to (1) physiologic evidence demonstrating that cricoid pressure reduces lower esophageal sphincter pressure, (2) anatomic investigations showing an inability to completely occlude the esophagus, (3) a lack of clinical outcome data that confirm that cricoid pressure reduces the incidence of aspiration, and (4) the frequent misapplication of the technique itself.261 The technique for cricoid pressure begins with an assistant applying 10 newtons (N) of force, with one N being the unit of force required to accelerate a mass of 1 kilogram 1 meter per second squared. (Force cannot be represented by mass alone, but as a practical guide to the amount of force to apply, 10 N is approximately equivalent to the downward force exerted by a weight of 1 kg.) Following loss of consciousness, the amount of force is increased to 30 N. Application of the full amount of force while the patient is still awake can provoke active retching and regurgitation. In some cases, cricoid pressure may be briefly released to enable a successful intubation; not infrequently the benefit of release outweighs the risk for regurgitation. Cricoid pressure should then be reapplied until the correct endotracheal tube position is confirmed.
Historically, thiopental (4 to 5 mg/kg) has been the most frequently used induction agent. Because of recent lack of availability, and also lack of familiarity, it is now rarely used in the United States, although it is still used in other countries. Propofol (2 to 2.8 mg/kg) is now commonly used to induce general anesthesia for cesarean delivery. Propofol, in a dose sufficient for induction and to prevent maternal awareness (2.5 mg/kg), depresses the infant more than thiopental. In the presence of hemodynamic instability, ketamine (1 to 1.5 mg/kg) or etomidate (0.3 mg/kg) should be substituted for propofol. Paralysis is achieved by succinylcholine (1 to 1.5 mg/kg) in 30 to 40 seconds; a peripheral nerve stimulator can be used to confirm neuromuscular blockade, because the presence of fasciculations is an unreliable sign. Administration of a defasciculating dose of a nondepolarizing muscle relaxant is not recommended, because it may delay the onset of neuromuscular blockade with succinylcholine. Pregnancy appears to be associated with less severe succinylcholine-induced fasciculations and muscle pain.262
Rocuronium (1 mg/kg) may provide intubating conditions similar to those provided with succinylcholine (1 mg/kg) for cesarean delivery263 and is a viable alternative in situations in which succinylcholine should be avoided (e.g., malignant hyperthermia, myotonic dystrophy, spastic paraparesis). The use of a priming preinduction dose of a nondepolarizing muscle relaxant is not recommended during pregnancy, because it may result in complete paralysis and increase the risk for aspiration.264 Enhanced activity of nondepolarizing agents may also be observed in patients receiving magnesium sulfate (e.g., for seizure prophylaxis in preeclamptic women or for fetal neuroprotection).265 Sugammadex, a modified gamma-cyclodextrin, has been demonstrated to be effective in providing rapid recovery (a train-of-four ratio of greater than 0.9) without recurarization from moderate and profound rocuronium-induced neuromuscular blockade in parturients undergoing cesarean delivery.266 Sugammadex has not been approved for use in all countries owing to concerns regarding hypersensitivity and allergic reactions.
A small-diameter cuffed endotracheal tube (i.e., 6.5 or 7.0 mm) should be used during pregnancy; the use of a flexible stylet within the endotracheal tube optimizes the first attempt at intubation. Tissue trauma and airway edema may occur with repeated attempts at intubation. Correct endotracheal tube placement should be confirmed by auscultation in both axillae and over the stomach to detect inadvertent endobronchial and esophageal intubation, respectively. Expired end-tidal carbon dioxide may be detected transiently with an esophageal intubation; thus, the anesthesia provider should observe ongoing evidence of a normal capnographic tracing and adequate maternal oxyhemoglobin saturation as well as bilateral thoracic movement and breath sounds. If doubts persist, the anesthesia provider can perform direct laryngoscopy or fiberoptic bronchoscopy to confirm the correct placement of the endotracheal tube in the trachea. If incorrect endotracheal tube placement is promptly recognized, extubation (with continued cricoid pressure) will often allow another attempt without the need for additional muscle relaxant.
Anticipation of a difficult endotracheal intubation, or a failed intubation attempt, should invoke the difficult airway algorithm and a call for assistance (see Chapter 30). Options include (1) allowing the patient to awaken, (2) using alternative techniques to place an endotracheal tube, and (3) using alternative airway devices. The laryngeal mask airway (LMA) does not prevent pulmonary soiling with gastric contents as efficiently as an endotracheal tube, but it can be a lifesaving device in situations of failed intubation. Han et al.267 reported clinically effective airway management with a classic LMA, which was placed successfully on the first attempt in 98% of 1067 healthy parturients undergoing elective cesarean delivery with general anesthesia. Cricoid pressure was maintained throughout the cesarean delivery, and no adverse sequelae occurred. Utilizing an LMA with a higher seal pressure than a simple LMA, and a built-in gastric draining tube, Halaseh et al.268 reported successful placement of the LMA in 98% of 3000 healthy parturients undergoing elective cesarean delivery; they observed only one case of regurgitation without aspiration. Cricoid pressure was not maintained after confirmation of successful LMA placement. In both of these studies, patients were excluded if they had symptoms of gastropharyngeal reflux, known/predicted difficult airway, and a prepregnancy BMI greater than 30 kg/m2. A number of variations to the classic LMA have been developed that may facilitate airway management in specific situations (see Chapter 30). Emergency airway equipment should be immediately available in all obstetric operating rooms.34
Maintenance
The goals for anesthetic maintenance include (1) adequate maternal and fetal oxygenation, with maintenance of normocapnia for pregnancy; (2) appropriate depth of anesthesia to promote maternal comfort and a quiescent surgical field and to prevent awareness and recall; (3) minimal effects on uterine tone after delivery; and (4) minimal adverse effects on the neonate.
Fetal oxygenation appears maximal when a maternal FIO2 of 1.0 is used269; however, in the absence of fetal compromise, an FIO2 of 0.3 appears to provide sufficient oxygenation while minimizing the production of oxygen free radicals (see earlier discussion). Although the use of a higher FIO2 can increase maternal arterial and umbilical venous blood oxygen content, this action has not been observed to result in differences in 1- or 5-minute Apgar or neurobehavioral scores.270,271 As a consequence, in the absence of fetal compromise, inspired oxygen concentrations should be guided by pulse oximetry rather than provision of an arbitrarily set level of FIO2.
Maternal ventilation should maintain normocapnia, which at term gestation is a PaCO2 of 30 to 32 mm Hg. Excessive ventilation can cause uteroplacental vasoconstriction and a leftward shift of the oxyhemoglobin dissociation curve, which may result in compromised fetal oxygenation.272 On the other hand, hypercapnia can lead to maternal tachycardia and is also undesirable.
Initially, high fresh-gas flows should be used to ensure an adequate end-tidal concentration of the volatile halogenated agent. No specific volatile halogenated agent has been demonstrated to be superior to another. The anesthetic requirements for volatile halogenated agents are diminished 25% to 40% during pregnancy.273 End-tidal levels of halogenated agent greater than 1 to 1.5 times the minimum alveolar concentration (MAC) may reduce the effect of oxytocin on uterine tone and lead to greater blood loss after delivery.274 A bispectral index measurement less than 60 typically requires more than 0.75 MAC of a volatile halogenated agent combined with 50% nitrous oxide and has been suggested to prevent intraoperative awareness and recall in parturients undergoing general anesthesia275; however, this target bispectral index value requires further study in pregnant women. Yoo et al.276 observed lower bispectral index values with a standardized sevoflurane–50% nitrous oxide anesthetic in women with prior labor compared with women without prior labor; in addition, plasma norepinephrine concentrations were higher at both baseline and delivery in the laboring group. Similarly, Erden et al.277 observed lower volatile agent requirements to maintain a target bispectral index value between 40 and 55 in parturients who were in labor compared with nonlaboring women; this finding could not be explained by differences in plasma concentrations of progesterone, prolactin, or cortisol.277 Postoperative analgesia requirements for the first 24 hours were also less in the group who had labored.276
In clinical practice, approximately 1.0 MAC of a volatile halogenated agent is typically administered between tracheal intubation and delivery, and the concentration of the volatile agent is then reduced to 0.5 to 0.75 MAC after delivery. Nitrous oxide 50% in oxygen is often added to reduce the required concentration of volatile agent, thereby mitigating adverse effects on uterine tone. The administration of a benzodiazepine (e.g., midazolam) after delivery may reduce the risk for maternal awareness.
Intravenous opioids are often withheld until after delivery to minimize the potential for neonatal respiratory depression; however, there may be circumstances in which maternal hemodynamic stability or blunting of responses to airway manipulation and surgical stimulation favor the administration of opioids during the induction of general anesthesia. The rapid onset and efficacy of intravenous lipid-soluble agents (e.g., remifentanil, fentanyl, alfentanil) make them ideal for mitigating the responses to laryngoscopy and intubation.278 Intraoperatively, the prolonged activity of water-soluble agents (e.g., morphine, hydromorphone) can be useful to minimize volatile anesthetic use and for the provision of intraoperative and postoperative analgesia (see Chapter 27).
Given the pregnancy-induced stretching of the abdominal wall, additional neuromuscular blockade is seldom necessary in the parturient who has an adequate depth of anesthesia (with administration of both a volatile agent and an opioid). A small dose of a short-acting nondepolarizing agent (or an infusion of succinylcholine) may be administered, with maternal response monitored with a peripheral nerve stimulator, if additional muscle relaxation is indicated.
Although differences in maternal and umbilical artery acid-base status have been observed in women who underwent elective general anesthesia compared with epidural anesthesia for cesarean delivery, similar neonatal outcomes were demonstrated.279 With any anesthetic technique, consideration should be given to the duration of the U-D interval; in a study evaluating spinal anesthesia, when the U-D interval was longer than 180 seconds, lower Apgar scores and greater fetal acidosis were observed.137 (This result most likely reflects difficulty in delivering the baby rather than a direct effect of the anesthetic agents.) General anesthetic agents can redistribute from the neonatal fat to the central circulation and lead to secondary depression of neonatal ventilatory effort; thus, the presence of a pediatrician (or another neonatal provider) is advisable until a normal ventilatory pattern is observed.
Emergence and Extubation
When the patient awakens, extubation should be undertaken with the patient in a semirecumbent position. The patient should demonstrate purposeful response to verbal commands and return of protective airway reflexes before tracheal extubation. In a review of anesthesia-related maternal deaths between 1985 and 2003 in the state of Michigan, Mhyre et al.280 observed that deaths associated with hypoventilation or airway obstruction did not occur at induction and tracheal intubation but rather during emergence, extubation, or recovery from anesthesia. Risk factors associated with mortality were obesity and African-American race, which may have delayed the visual recognition of cyanosis; medical management and medication issues were also identified. The American Society of Anesthesiologists Practice Guidelines for Postanesthetic Care suggest that pulse oximetry is associated with early detection of hypoxemia; the guidelines recommend periodic assessment of airway patency, respiratory rate, and oxygen saturation during emergence and recovery.281 If repeated airway manipulation, massive hemorrhage, or emergency hysterectomy has occurred, delayed extubation and/or transfer to an intensive care unit (ICU) should be considered.
Pharmacology
Thiopental.
Historically, the barbiturates (e.g., thiopental [thiopentone], methohexital, thiamylal) have been the induction agents most commonly used for cesarean delivery. Extensive published data have confirmed the safety and efficacy of thiopental for induction of anesthesia in patients undergoing cesarean delivery at various gestational ages. Thiopental 4 mg/kg provides a rapid and reliable induction of anesthesia. As a negative inotrope and vasodilator, thiopental can cause decreased cardiac output and blood pressure,282 which may result in significant hypotension in hypovolemic patients. Some investigators have attempted to minimize this effect by using a lower dose of thiopental in combination with ketamine or propofol, with varying success.
Thiopental rapidly crosses the placenta. In 11 healthy subjects who underwent induction of general anesthesia with thiopental, the mean umbilical artery–to–umbilical vein ratio was 0.87 with an induction-to-delivery (I-D) interval that ranged from 8 to 22 minutes.283 Fetal-to-maternal concentration ratios after a single thiopental dose exposure in other studies in term infants exhibited a range of 0.43 to 0.96.284 The equilibration of thiopental occurs relatively rapidly in the fetus; however, fetal brain concentrations rarely exceed the threshold required for neonatal depression. With a maternal induction dose of 4 mg/kg, umbilical vein concentrations of thiopental are well below the arterial plasma concentrations necessary to produce anesthesia in adults.285 However, with large induction doses (8 mg/kg), thiopental can produce significant neonatal depression.286
The following theories have been proposed to explain the clinical occurrence of an unconscious mother but an awake neonate: (1) preferential uptake of thiopental by the fetal liver, which is the first organ perfused by blood coming from the umbilical vein286; (2) the higher relative water content of the fetal brain287; (3) rapid redistribution of the drug into the maternal tissues, which causes a rapid reduction in the maternal-to-fetal concentration gradient; (4) nonhomogeneity of blood flow in the intervillous space; and (5) progressive dilution by admixture with the various components of the fetal circulation. Because of this rapid equilibration of thiopental and the low fetal brain concentration of thiopental, there is no advantage in delaying delivery until thiopental concentrations decline. There is no evidence that thiopental causes adverse fetal effects when the I-D time is prolonged.
Propofol.
Propofol is an intravenous induction agent with a rapid onset, rapid recovery, and favorable side-effect profile, which includes a low incidence of nausea and vomiting. Induction with propofol can result in pain on injection and a reduction in maternal blood pressure and cardiac output. The pharmacokinetics of propofol are similar in pregnant and nonpregnant women, except for a more rapid clearance observed during pregnancy, which may partially reflect drug removal through blood loss and the delivery of the infant and placenta.
When given as an intravenous bolus, by continuous infusion, or both, propofol rapidly crosses the placenta and results in an umbilical vein–to–maternal vein (UV/MV) ratio of approximately 0.7.288 In an in vitro human placenta study, Soares de Moura et al.289 observed that propofol produced vasodilation of fetal placental blood vessels and decreased the effect of various vasoconstrictors, most likely through the inhibition of calcium influx through the smooth muscle sarcolemma; intralipid, the propofol carrier solution, was not responsible for these effects. Celleno et al.290 randomly assigned 40 mothers undergoing cesarean delivery with general anesthesia to receive either propofol 2.8 mg/kg or thiopental 5 mg/kg for induction of anesthesia; they observed significantly lower Apgar and neurobehavioral scores in the propofol group. The I-D and U-D intervals in the two groups were nearly identical. Five infants exposed to propofol had profound muscular hypotonus at birth and at 5 minutes, and one newborn was somnolent. Other studies have found no effect of propofol on neurobehavioral scores or the time to sustained spontaneous respiration with induction bolus doses of propofol 2.5 mg/kg or with infusion doses less than 6 mg/kg/h.291,292 However, higher doses of propofol (9 mg/kg/h) have been correlated with a low Neurologic and Adaptive Capacity Score (NACS).293
Compared with thiopental, propofol results in a greater incidence of maternal hypotension,294 which may more effectively attenuate the response to laryngoscopy and intubation at the risk of reduced uteroplacental blood flow. Moreover, in women undergoing cesarean delivery, Celleno et al.295 demonstrated that propofol 2.4 mg/kg, in comparison with thiopental 5 mg/kg, resulted in electroencephalographic patterns consistent with a lighter depth of anesthesia, which was confirmed by the presence of clinical signs of light anesthesia in 50% of the patients. Other studies have not observed significant hypotension after the administration of propofol (2 to 2.8 mg/kg) or lower umbilical cord blood gas and pH measurements than after administration of thiopental (4 to 5 mg/kg)291,296 or thiamylal (3 to 4 mg/kg).297 However, one report noted a transient but severe episode of maternal bradycardia after administration of propofol followed by succinylcholine for rapid-sequence induction.298 This effect has also been demonstrated in pregnant ewes; one animal experienced severe bradycardia that led to a sinus arrest.299
In a study of nonpregnant women, the interaction of propofol and ketamine was found to be additive at hypnotic and anesthetic endpoints; the cardiostimulant effects of ketamine appear to offset the cardiodepressant effects of propofol.300
Propofol provides a vehicle for bacterial growth, has undergone less investigation in pregnant women (especially preterm parturients), is painful on administration, and may lead to a higher incidence of adverse maternal and neonatal effects. Thus, propofol does not offer a significant benefit over thiopental for the induction of general anesthesia for cesarean delivery.
Ketamine.
The sympathomimetic properties of ketamine make it an ideal induction agent in the setting of an urgent cesarean delivery in a patient with hypotension or an acute exacerbation of asthma.301 Ketamine is an analgesic, hypnotic, and amnestic agent associated with minimal respiratory depression; it is often used to supplement a neuraxial technique that may not be providing optimal anesthesia. Ketamine's effect is likely related to antagonism of the N-methyl-D-aspartate (NMDA) receptor.
An induction dose of ketamine 1 mg/kg is associated with an increase in blood pressure immediately after induction, and a further increase is observed after laryngoscopy and intubation.302 Such an increase can be desirable in the bleeding hypotensive patient but should be avoided in the parturient with hypertension (e.g., preeclampsia). However, in experimental animal models, ketamine was sometimes associated with direct myocardial depression, decreased cardiac output, and hypotension.282
Studies in pregnant ewes suggest that the use of ketamine is not associated with a reduction in uterine blood flow.303 Ketamine is associated with dose-dependent increases in uterine tone, but a single induction dose does not increase uterine tone at term gestation.304 Using an induction dose of ketamine 0.7 mg/kg, Craft et al.303 observed a 39% increase in resting uterine tone with no effect on uterine blood flow in gravid ewes.
Ketamine rapidly crosses the placenta. No neonatal depression is observed with doses less than 1 mg/kg.305 At higher doses, low Apgar scores, neonatal respiratory depression, and need for resuscitation have been reported.305 Apgar scores and umbilical cord blood gas and pH measurements at delivery with ketamine are similar to those with thiopental.306,307 A formulation of the purified S+ isomer of ketamine is available for clinical use in some countries outside the United States. In chronically instrumented pregnant sheep, Strumper et al.308 found that the effects of the isomer were similar to those of the racemic mixture in terms of maternal and fetal hemodynamics and uterine perfusion; however, the S+ isomer was associated with a smaller increase in maternal and fetal PCO2 than that seen with racemic ketamine in spontaneously breathing animals.
The emergence delirium and hallucinations experienced with ketamine, particularly in the unpremedicated patient, have limited the adoption of this drug as a routine induction agent for cesarean delivery. If ketamine is used, a benzodiazepine should be administered to decrease the incidence of these psychomimetic effects.309 Maternal awareness may still occur after an induction dose of ketamine 1 to 1.5 mg/kg,310 but the incidence is lower than with thiopental 4 mg/kg or a mixture of ketamine 0.5 mg/kg and thiopental 2 mg/kg.311 The incidence of maternal awareness can also be diminished with the co-administration of a benzodiazepine.
When used to maintain general anesthesia with 50% nitrous oxide in oxygen for cesarean delivery, a continuous infusion of ketamine (70 µg/kg/minute) was followed by a higher incidence of factual recall and postoperative pain than seen with a volatile anesthetic technique.312 Ngan Kee et al.306 found that patients who received ketamine 1 mg/kg for induction had lower postoperative consumption of morphine than patients who received thiopental 4 mg/kg for induction (anesthesia was maintained with nitrous oxide and isoflurane). Some investigators have suggested that lower doses of ketamine (0.5 to 0.7 mg/kg) combined with thiopental or propofol may be preferable to the administration of any one individual agent. There appears to be limited advantage to this approach with thiopental in terms of maternal recall, maternal hemodynamic status, and neonatal neurobehavioral scores.313,314 Whether ketamine, given as a bolus or infusion initiated after infant delivery, can provide postcesarean analgesia and modulate pain remains controversial (see later discussion).
Etomidate.
Etomidate is an intravenous induction agent that produces rapid onset of anesthesia with minimal effects on cardiorespiratory function. This property makes it ideal for parturients who are hemodynamically unstable or who would not tolerate hemodynamic aberrations well (e.g., patients with severe cardiac disease).315 With an induction dose of 0.2 to 0.3 mg/kg, etomidate undergoes rapid hydrolysis, thereby allowing rapid recovery.316 Intravenous administration of etomidate may cause pain and involuntary muscle movements in unpremedicated patients; etomidate is also associated with nausea and vomiting, potential activation of seizures in patients with an epileptogenic foci, and an impaired glucocorticoid response to stress.317
Etomidate crosses the placenta rapidly; however, large variations in the UV/MV ratio (0.04 to 0.5) have been reported.316 Downing et al.318 observed that an induction dose of etomidate 0.3 mg/kg was associated with better neonatal acid-base measurements and overall clinical condition than with thiopental 3.5 mg/kg. A transient (< 6 hours) reduction in neonatal cortisol production has been observed when an induction dose of etomidate is used for cesarean delivery319; however, the clinical relevance of this finding is unclear.
Midazolam.
Midazolam is a short-acting, water-soluble benzodiazepine that has few adverse hemodynamic effects and provides hypnosis and amnesia. Although most commonly used as a premedicant prior to anesthesia, midazolam can be used as an induction agent for cesarean delivery. Crawford et al.320 observed that induction with either midazolam 0.3 mg/kg or thiopental 4 mg/kg resulted in similar maternal hemodynamic responses and Apgar scores. By contrast, Bland et al.321 reported that midazolam 0.2 mg/kg for induction of anesthesia resulted in a higher incidence of low Apgar scores and longer time to spontaneous respiration in the neonates than thiopental 3.5 mg/kg. Umbilical cord blood gas measurements did not differ between the two groups; however, the infants exposed to midazolam had lower neurobehavioral scores, body temperature, general body tone, and arm recoil. These differences did not persist at 4 hours after delivery. There are few indications for the use of midazolam for the induction of general anesthesia for cesarean delivery; it should be used only when there are relative or absolute contraindications to the use of other agents.
Muscle Relaxants.
Muscle relaxants are commonly used before delivery to provide optimal intubation and operating conditions. Most muscle relaxants are highly ionized with low lipid solubility; thus, they do not undergo significant placental transfer.
The depolarizing agent succinylcholine (1 to 1.5 mg/kg) is the muscle relaxant of choice for most parturients undergoing rapid-sequence induction of general anesthesia. Maternal administration provides adequate intubating conditions within approximately 45 seconds of intravenous administration. Succinylcholine is a highly ionized and water-soluble molecule, and only small amounts cross the placenta. Although high doses of succinylcholine (2 to 3 mg/kg) can result in detectable levels in umbilical cord blood, very large doses (10 mg/kg) are required to lead to placental transfer sufficient to cause neonatal muscle weakness.322
Succinylcholine is rapidly metabolized by plasma pseudocholinesterase, the concentration of which is decreased during pregnancy; however, in most patients this effect is offset by the pregnancy-induced increase in volume of distribution. Thus, recovery from succinylcholine is not prolonged, unless the patient has extremely low levels of pseudocholinesterase or atypical pseudocholinesterase.323 The administration of metoclopramide may also prolong succinylcholine-induced neuromuscular blockade, perhaps by inhibiting plasma pseudocholinesterase324; this effect is rarely (if ever) clinically significant. The return of neuromuscular function should be confirmed before additional doses of muscle relaxant are given.
Rocuronium is a suitable alternative to succinylcholine when a nondepolarizing agent is preferred for rapid-sequence induction (e.g., history of malignant hyperthermia). Abouleish et al.325 observed that rocuronium (0.6 mg/kg) administered with an induction dose of thiopental (4 to 6 mg/kg) provided good to excellent intubating conditions in pregnant women after a mean interval of 79 seconds and maximal intubating conditions in 98 seconds. Rocuronium did not adversely affect neonatal Apgar scores, acid-base measurements, time to sustained respiration, or neurobehavioral scores. Neuromuscular blockade was reversed satisfactorily at the end of cesarean delivery. Magorian et al.326 demonstrated that rocuronium 1.2 mg/kg resulted in an onset of paralysis similar to that provided by succinylcholine (55 seconds), but it had a significantly longer clinical duration of action.
Vecuronium 0.1 mg/kg may be administered when the use of succinylcholine is contraindicated; however, its onset of action is significantly slower than that of rocuronium (144 seconds).326 Hawkins et al.327 evaluated the use of two methods of vecuronium administration for rapid-sequence induction of anesthesia for elective cesarean delivery. One group of women received vecuronium 0.01 mg/kg as a priming dose before administration of 0.1 mg/kg 4 to 6 minutes later; the other group received 0.2 mg/kg as a single bolus. The mean onset time for both groups (177 seconds and 175 seconds, respectively) was much longer than that for succinylcholine; moreover, the duration of blockade was prolonged (73 minutes in the priming group and 115 minutes in the bolus group). The same group performed a separate study in women undergoing postpartum tubal ligation; the mean duration of action of vecuronium was significantly longer in these women (57 minutes) than in nonpregnant controls (35 minutes).328 Vecuronium crosses the placenta in small amounts; however, neonatal outcome, as assessed by Apgar scores and NACS, does not appear to be adversely affected.329
Atracurium is a less desirable agent for rapid-sequence induction because the high dose required for a rapid onset of action may result in significant histamine release, which may cause hypotension. The isomer cisatracurium does not have these undesirable side effects, but its relatively slow onset makes it less optimal than other alternatives.330
Regardless of the choice of agent, laryngoscopy and intubation should not be attempted until adequate muscle relaxation has occurred. The use of a nerve stimulator allows an objective assessment of the onset and duration of the neuromuscular blockade. Residual neuromuscular blockade can be reversed with neostigmine and glycopyrrolate. To diminish the risk for aspiration, the anesthesia provider should confirm that the patient responds appropriately to verbal commands before tracheal extubation.
Nitrous Oxide.
Nitrous oxide is an inhalational agent commonly used in the setting of a cesarean delivery because of its minimal effects on maternal blood pressure and uterine tone. The use of nitrous oxide allows for a reduction in the concentration of the volatile halogenated agent. (High concentrations of a volatile halogenated agent decrease uterine tone.) Administration of 50% to 67% nitrous oxide in oxygen without another anesthetic agent does not provide complete anesthesia and can result in maternal awareness in 12% to 26% of cases.331,332
Nitrous oxide is transferred rapidly across the placenta, where fetal tissue uptake reduces the fetal arterial concentration for the first 20 minutes. Karasawa et al.333 evaluated the relationship between duration of exposure to nitrous oxide 67% and the resulting UV/MA nitrous oxide concentration ratios; they observed different ratios according to duration of exposure: 2 to 9 minutes (0.37), 9 to 14 minutes (0.61), and 14 to 50 minutes (0.70). Apgar scores at 1 minute inversely correlated with duration of anesthesia, an effect observed in other studies.334 The use of a lower concentration (e.g., 50%) of nitrous oxide may reduce but not eliminate these neonatal effects. Piggott et al.269 randomly assigned parturients undergoing general anesthesia to receive either 100% oxygen or 50% nitrous oxide in oxygen, both supplemented by isoflurane (1.5 MAC for the first 5 minutes and 1.0 MAC thereafter). Neonates exposed to nitrous oxide required more resuscitation, although no significant differences were observed in Apgar scores.
Volatile Halogenated Agents.
Volatile halogenated agents are perhaps the most commonly used agents for maintaining general anesthesia for cesarean delivery. Volatile halogenated agents produce central nervous system and cardiovascular effects in a dose-dependent manner; of particular concern for the obstetric patient are the resulting decreases in blood pressure (which may result in reduced uterine blood flow) and uterine tone. The uptake and delivery of a volatile halogenated agent is determined by inspired partial pressure, blood flow, and the blood/gas/tissue partition coefficient. The alveolar partial pressure of volatile agents during pregnancy follows known patterns of equilibration; the following commonly used agents are listed in order of more rapid to slower equilibration: nitrous oxide, desflurane, sevoflurane, and isoflurane. Volatile halogenated agents cross the placenta rapidly and equilibrate quickly with fetal tissues.335 Neonatal depression may occur. This is typically not a clinical issue when volatile anesthetic agents are used for emergency cesarean delivery, because the delivery usually occurs before much of the volatile agent crosses the placenta (particularly if uteroplacental insufficiency is the reason for emergency delivery). Also, the maternal hemodynamic response to laryngoscopy and intubation typically offsets any hypotension that might result from administration of a volatile halogenated agent before delivery.
Munson and Embro336 evaluated three concentrations (0.5, 1.0, and 1.5 MAC) of isoflurane, enflurane, and halothane in an in vitro study of gravid and nongravid uterine myometrial strips; the amount of depression of uterine contractile activity was dose related for each agent and was similar for the three agents. In a similar study, Dogru et al.337 evaluated the effect of 0.5, 1.0, and 2.0 MAC of desflurane and sevoflurane on gravid uterine myometrial strips; a dose-dependent decrease in uterine contractions was observed. Importantly, the duration, amplitude, and frequency of oxytocin-induced uterine contractions were affected in a dose-dependent manner and were completely inhibited at 2 MAC.337 The same group of investigators also reported that 1 MAC of desflurane inhibits the amplitude of oxytocin-induced myometrial contractions to a lesser extent than sevoflurane338 and that pregnant myometrium is more sensitive than nonpregnant myometrium to the inhibitory effects of volatile halogenated agents.339 These effects may influence maternal blood loss after delivery.
Lower amounts of volatile halogenated agents are required during pregnancy. Gin et al.340 demonstrated a 28% lower MAC for isoflurane in pregnant women at 8 to 12 weeks' gestation than in nonpregnant women. The same group of investigators found a 27% and 30% decrease in MAC of halothane and enflurane, respectively.341 These findings were correlated with an increase in progesterone level. In an animal model, Datta et al.342 demonstrated that long-term administration of progesterone was associated with a reduction in the MAC of halothane in rabbits. Chan and Gin343 observed that the reduction in MAC persists for 24 to 36 hours postpartum, with a gradual return to normal values by 72 hours.
Opioids.
All opioids, particularly those with high lipid solubility (e.g., remifentanil, fentanyl, sufentanil), readily pass through the placenta to the fetus. As a consequence, the administration of opioids is usually avoided until after delivery to reduce the risk for neonatal depression. However, the hemodynamic stability provided by opioids during airway manipulation and surgery may be valuable in select settings, particularly in the presence of maternal cardiac disease, neurologic conditions, and preeclampsia or hypertension.
The administration of remifentanil has been observed to mitigate the hemodynamic responses to intubation and surgery but result in significant neonatal depression. Even with the administration of relatively low doses of remifentanil (0.5 µg/kg bolus followed by 0.15 µg/kg/min continuous infusion until peritoneal incision), Draisci et al.344 observed that Apgar scores and umbilical cord blood pH measurements were lower in the infants exposed to remifentanil than in the infants whose mothers received fentanyl 5 µg/kg after delivery; some neonates exposed to remifentanil required tracheal intubation. There were no significant differences between groups in maternal hemodynamics or maternal plasma concentrations of catecholamines and growth hormone.
The highly lipid-soluble agent fentanyl rapidly crosses the placenta. Fentanyl is 60% to 80% protein bound; thus, approximately one third is available for transfer across the placenta.345 Despite its low lipid solubility, morphine has a fetal-to-maternal blood concentration ratio of 0.96 at 5 minutes346; this rate of equilibration and the production of active metabolites are relevant considerations in the use and timing of intravenous morphine administration.
Meperidine is highly lipid soluble and is 50% to 70% protein bound; maternal administration results in a mean fetal-maternal blood concentration ratio of 0.75.347 The production of the active metabolite normeperidine, which can accumulate in both the mother and neonate and result in respiratory and neurobehavioral alterations, limits the use of meperidine as a principal analgesic agent during cesarean delivery; however, after delivery of the neonate, an intravenous dose of 12.5 to 25 mg is useful for treatment of shivering in the mother.
Maternal respiratory depression, as well as nausea and emesis during the intraoperative and postoperative periods, represent significant concerns in parturients given intravenous opioids.
Local Anesthesia
As a method used primarily for supplementation of neuraxial anesthesia, the infiltration of local anesthesia can also be used to facilitate an emergency cesarean delivery. This latter technique has been well described and is used predominantly in developing countries, where contemporary anesthesia techniques may not be readily available. Few contemporary obstetricians are familiar or proficient with this technique in developed countries.
The success of local infiltration depends on the obstetrician's making a midline abdominal incision, avoiding use of retractors, and not exteriorizing the uterus. In settings in which an anesthesia provider might not be readily available, the obstetrician might begin surgery with the aid of local infiltration; after delivery of the infant, the achievement of temporary hemostasis, and the arrival of the anesthesia provider, surgery may be completed once general anesthesia has been induced.
Local infiltration is performed in sequential steps as the operation progresses (Box 26-9).348 The use of 0.5% lidocaine with epinephrine is recommended; the use of a more concentrated solution is likely to result in systemic toxicity. A 25-gauge spinal needle is used to make the intracutaneous injection; the needle is inserted just below the umbilicus and is directed in the midline toward the symphysis pubis. Approximately 10 mL of local anesthetic is required to create a skin wheal that extends from the symphysis pubis to the umbilicus. The subcutaneous injection is also performed for the full length of the planned incision with 10 to 20 mL of local anesthetic. Ideally, the obstetrician should then wait for 3 to 4 minutes to allow the local anesthetic agent to exert its effect before making the skin incision.
A vertical skin incision is made between the umbilicus and the symphysis pubis and is extended down to the rectus fascia. The obstetrician then infiltrates local anesthetic into the rectus fascia and rectus muscles by making three to five laterally directed injections on each side. The needle should be passed between the rectus sheath and the transversus muscle at an angle of 10 to 15 degrees and a depth of 3 to 5 cm; aspiration is performed, and 2 to 3 mL of local anesthetic is injected at each site with an additional 1 mL injected with needle withdrawal. The obstetrician should also make oblique injections at the upper and lower poles of the incision. The local anesthetic will spread freely in the rectus sheath, but it takes 4 to 5 minutes for anesthesia to be complete. The suprapubic area must also be generously infiltrated to ensure blockade of the branches of the iliohypogastric nerve. The disadvantage of the rectus sheath block is the large volume (40 to 50 mL) of local anesthetic required; a less effective alternative that requires less volume and time is to raise a longitudinal paramedian wheal in the rectus fascia on each side of the midline and to infiltrate the suprapubic region.
The obstetrician then extends the incision through the rectus sheath, and the peritoneum is grasped with forceps clamps. If the patient has pain, the parietal peritoneum may be infiltrated with 5 to 10 mL of local anesthetic and then incised. The visceral peritoneum overlying the area of the uterine incision is injected with 10 mL of local anesthetic and is then incised and reflected appropriately. Paracervical infiltration with 5 to 10 mL of local anesthetic may block pain impulses from the uterus and cervix.
A uterine incision is made, and the infant is delivered. The surgeon must avoid forceful retraction and blunt dissection of tissue planes, and uterine manipulation should be kept to a minimum. A support person at the head of the table who can provide coaching and reassurance to the mother is invaluable.
The major disadvantages of local infiltration anesthesia are patient discomfort and the potential for systemic local anesthetic toxicity, given that as much as 100 mL of local anesthetic solution is required. The latter disadvantage may be especially problematic in the absence of a skilled anesthesia provider to assist with maternal resuscitation. Another disadvantage is the amount of time required for maximal anesthesia to develop; maternal discomfort often accompanies an urgent delivery performed with this form of anesthesia. Finally, local infiltration does not provide satisfactory operating conditions in the event of a surgical complication (e.g., uterine laceration, broad ligament hematoma).
Cesarean delivery with use of local infiltration, if successful, has the advantages of preserving maternal cardiovascular stability and a patent airway while allowing the initiation of surgery in emergency cases. However, the technique is frequently associated with incomplete maternal anesthesia, which subsequently presents significant management issues, because the surgical procedure has commenced, positioning options are limited, and the consequences of the operative procedure (e.g., hemorrhage) may require immediate attention.
Additional peripheral nerve blockade techniques (e.g., transversus abdominis plane blockade349) may successfully augment local anesthesia infiltration; however, the degree to which the maternal experience is enhanced and the timing required to place these blocks deserve further investigation.
Recovery From Anesthesia
Cesarean delivery represents a major abdominal surgical procedure with significant anatomic, physiologic, and hormonal sequelae, even when it is performed electively without complications in a healthy parturient. The risk for adverse outcomes is greater in the presence of significant maternal comorbidity or in the setting of surgical complications (e.g., massive blood loss, cesarean hysterectomy).350
Cohen et al.351 evaluated revised PACU discharge criteria in their tertiary care center for patients who received neuraxial anesthesia for cesarean delivery. They suggested that the majority of patients undergoing cesarean delivery could meet revised discharge criteria (i.e., presence of a normal level of consciousness, stable vital signs, adequate analgesia, and ability to flex the knees) within 60 minutes, a period that would shorten the average duration of PACU stay and result in cost savings. However, 26% to 36% of patients remained in the PACU for up to 180 minutes because of pain, sedation, nausea and vomiting, pruritus, prolonged neuroblockade, and/or drug treatment. In addition, 16% to 22% remained in the PACU for up to 210 minutes for cardiovascular (e.g., bleeding, hypertension, hypotension, tachycardia) or respiratory events. Moreover, the study did not include the most seriously ill or highest-risk patients, who were transferred directly to an ICU.
The 2001 National Sentinel Caesarean Section Audit in the United Kingdom reported that 10% of women undergoing cesarean delivery required admission to a high-dependency unit.352 Moreover, 3.5% of these women required subsequent transfer to an ICU. Preexisting comorbid conditions accounted for the majority (80%) of these ICU admissions; a smaller fraction were due to the medical emergency (e.g., uterine rupture, placental abruption) that prompted the cesarean delivery.352 Although ICU admission is uncommon in obstetric patients, it occurs more frequently (approximately 9 per 1000 patients) after cesarean delivery.352
Of concern, inadequate postoperative care has been cited as a recurring factor in maternal deaths (see Chapter 40). The ASA Practice Guidelines for Obstetric Anesthesia state that “appropriate equipment and personnel should be available to care for obstetric patients recovering from major neuraxial or general anesthesia.”34 Similarly, the National Obstetric Anaesthetic Service Guidelines from the United Kingdom state that postoperative care of the patient undergoing cesarean delivery should meet the same standard of care as required for any postoperative patient.353
Oral Intake
Mangesi and Hofmeyr354 performed a systematic review of six randomized clinical trials comparing early with delayed oral intake of fluids and foods after cesarean delivery; they found that the early consumption (within 4 to 8 hours) was associated with a shorter time to return of bowel sounds and a shorter hospital stay. No differences were reported in nausea and vomiting, abdominal distention, time to bowel activity, paralytic ileus, or need for analgesia. The NICE guidelines state that “women who are recovering well and who do not have complications … can eat and drink when they feel hungry or thirsty.”355
Removal of Urinary Catheter
There are no differences in the incidence of urinary retention after general anesthesia and epidural anesthesia following cesarean delivery.356 Risk factors for postpartum urinary retention after cesarean delivery include the use of postoperative opioid analgesia (particularly when given via an epidural catheter), multiple gestation, and a low BMI.357 Most urinary catheters are removed either immediately after cesarean delivery or within 24 hours; there are no differences between these two options in regard to postoperative urinary retention, dysuria, urgency, fever, positive microscopy, or length of hospital stay.358 In the pregnant population, the return of bladder sensation of fullness after neuraxial techniques appears to be a function of time, rather than urinary volume.359 The return of bladder sensation after spinal anesthesia for cesarean delivery (with hyperbaric bupivacaine and fentanyl) takes longer (mean of 374 minutes [IQR 172 to 692]) than return of sensation after patient-controlled epidural analgesia for vaginal delivery (mean of 234 minutes [IQR 95 to 382]).359
Postoperative Assessment and Discharge
The anesthesia provider should assess for recovery of motor and sensory function if a neuraxial technique was administered. Women should be reassured that breast-feeding is safe, even after general anesthesia, and that postoperative analgesics have a favorable safety profile. Early mobility and ambulation should be encouraged.