Edema: Therapist’s Management
NET CAPILLARY FILTRATION AND EFFECT ON EDEMA
EDEMA AND STAGES OF WOUND HEALING
Evaluation techniques should be reproducible and standardized.
▪ Use minimal pressure needed to traction the skin.
▪ Pressure will vary slightly depending on skin texture.
▪ Strokes should be directed and open in direction of desired flow.
This chapter will cover the definition of edema, physiology, stages of wound healing, assessment of edema, and treatment techniques available to address local edema with intact (although overwhelmed) venous, arterial, and lymphatic systems.
The physiology and treatment of lymphedema is discussed separately in Chapter 64. Many of the same principles used in management of lymphedema can be applied to decrease local edema through facilitating more efficient lymphatic drainage. “Manual Edema Mobilization: Treatment for Edema in the Subacute Hand” is reviewed in Chapter 65. The reader is encouraged to refer to these additional chapters for more information on these specialized techniques.
Persistent edema presents a constant challenge to hand surgeons and hand therapists. If unresolved, it will delay healing and can result in pain and stiffness, thereby compromising functional results.
Edema is the accumulation of excessive fluid in the intercellular spaces.1,2 The process of controlling fluid accumulation involves a variety of factors that influence capillary filtration and lymph drainage. Both vascular and nonvascular processes affect fluid accumulation.
All cells are bathed in extracellular fluid. This fluid can be divided into two main components: the interstitial fluid and the blood plasma.1,2 The interstitial fluid is outside of the closed vascular system.3 Blood plasma is the fluid noncellular component of blood in which red blood cells, white blood cells, and platelets are suspended to collectively form total blood volume.1,2 This circulating blood tissue permeates the vascular system and flows through the heart, arteries, capillaries, and veins.
The arterial system brings oxygen and nutrients to the cells, whereas the venous system is responsible for waste and carbon dioxide removal. The exchange of nutrients and cellular waste between the tissues and the circulating blood takes place at the level of the capillaries, primarily through diffusion and filtration. The capillary wall consists of a single layer of highly permeable endothelial cells and is surrounded by a basement membrane. The diameter of the capillary is just large enough for red blood cells and other blood cells to pass through.1-3 Blood enters the capillaries through arterioles and metarterioles and exits through venules. It is rare that any single functional cell is more than 20 to 30 µm away from a capillary.1,2 Oxygen and glucose are in higher concentration in the bloodstream than in the interstitial fluid and diffuse into the interstitial fluid, whereas carbon dioxide diffuses in the opposite direction.2,3 Proteins are too large to diffuse easily through the capillary membrane and primarily flow linearly along the capillary.1 However, small amounts of proteins leak out of the blood capillaries into the interstitium, where they accumulate in the interstitial fluid.1,2 The lymphatic system is responsible for returning proteins that have accumulated in the interstitial spaces back into the venous system until the system is back in balance. Lymph is fluid collected from tissues; it flows via lymphatic vessels through the lymph nodes and drains into the venous system.1-3 Figure 63-1 illustrates the exchange process between substances in the interstitial fluid spaces with blood and lymphatic capillaries.
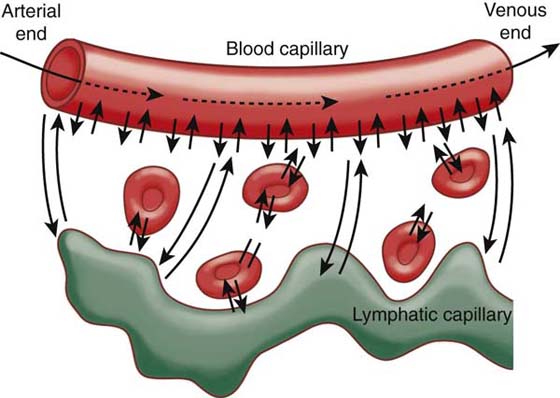
Figure 63-1 Diffusion of fluid molecules and dissolved substances between the capillary and interstitial fluid spaces. (From Guyton AC, Hall JE. Textbook of Medical Physiology. 9th ed. Philadelphia: WB Saunders, 1996.)
The spaces between cells are collectively called the interstitium. The fluid between cells (or interstitial fluid) is derived by filtration from the capillaries. There is a constant exchange of fluid between the intercellular tissue spaces and the blood plasma across the capillary membrane. Fluid in the interstitium is trapped by proteoglycan filaments, which cause the fluid to have the characteristics of a gel.1,2 This “tissue gel” contains almost the same constituents as plasma, except for proteins, because proteins do not filter out of the capillaries easily. Water molecules, electrolytes, nutrients, and cellular waste diffuse through the interstitium rapidly, although fluid flows very poorly in the tissue gel.1,2 Generally, there is only a very slight amount of fluid that is free from these proteoglycan filaments and not trapped in the tissue gel. In normal tissues, the “free” fluid is usually much less than 1%. When tissues start to develop edema, the gel can swell from 30% to 50% to accommodate the increased volume of interstitial free fluid.1,2 After this point, the gel cannot accommodate additional fluid, and the amount of free fluid increases. The amount of the free fluid may expand to equal more than half of the interstitial fluid,1,2 and the interstitial fluid volume can increase to several hundred percent above normal in seriously edematous tissues.2 Pitting edema is made up of large amounts of free fluid in the tissues that can be displaced briefly by pressure, leaving a pit that slowly fills with fluid flowing back from the surrounding tissues2 (Fig. 63-2 online). Brawny edema results when fluid in the interstitium becomes clotted with fibrinogen, preventing it from moving freely, or when tissue cells rather than the interstitium swells.2 Brawny edema is firm to the touch.
Figure 63-2 Patient with pitting edema of the hand. Note the “pit” left by the therapist’s thumb (arrow).
Lymphedema refers to the specific type of edema caused by accumulation of protein-rich fluid in the extracellular space of skin and subcutaneous tissue, which results from obstruction of superficial extremity lymphatics.4 Most edemas are caused by increased capillary filtration, which overwhelms lymph drainage (a condition called “dynamic insufficiency”). Lymphedema represents failure of the lymphatic system to drain lymph from a defined region, usually a limb, a condition called “mechanical insufficiency”.5 The lymphatic vessels are responsible for resorbing excess fluid as well as cells, proteins, lipids, microorganisms, and debris from tissues. The lymphatic system influences the volume of interstitial fluid and the interstitial fluid pressure as it compensates to balance the rate of protein and fluid leakage from the blood capillaries.
Net filtration of fluid across the capillary membrane is determined by the balance between the forces that tend to force fluid outward into the interstitial spaces (filtration) and the forces that move fluid inward (resorption). Normally, the filtration pressures slightly exceed the resorption pressures, and the lymphatics balance the system by pulling excess fluid and proteins out of the interstitial spaces and returning them to the blood.1,2
Substances are transferred between the plasma and interstitial fluid primarily by diffusion through the capillary membrane. This diffusion provides continual mixing between the interstitial fluid and the plasma.1,2 Lipid-soluble substances such as oxygen and carbon dioxide can diffuse directly through the cell membranes. Lipid-insoluble substances, such as chloride ions, sodium ions, and glucose, as well as water, diffuse through capillary pores or periodic intercellular clefts that connect the interior of the capillary with the exterior. These clefts or slit pores are about 20 times the diameter of a water molecule but slightly less than the diameters of plasma proteins such as an albumin molecule. The net rate of diffusion depends on the concentration difference between the two sides of the capillary membrane.
Minute vesicles are also located on the surface of the endothelial cells.1-3 These vesicles transport plasma and extracellular fluid through the capillary wall by imbibing small amounts and then moving slowly through the endothelial cells, releasing the contents to the outer surface.1-3 Fluid exchange depends on the properties of the capillary walls and the pressures acting across the capillary membrane. Capillary permeability is selective and is affected by pressures and relative concentrations on either side of the membrane and on the integrity of the membrane itself.6 Capillary permeability is increased following external injury, operative trauma, and burns.7
The primary four pressures that influence net capillary filtration are the capillary (hydrostatic) pressure, the interstitial fluid (hydrostatic) pressure, the plasma colloid osmotic pressure, and the interstitial fluid colloid osmotic pressure. The capillary pressure is the blood pressure in the capillaries that tends to move fluid from the capillary outward through the membrane at any given point.1,2,6,7 This pressure is greater at the arterial end than at the venous end of the capillary, resulting in fluid filtering out at the arterial end and being reabsorbed at the venous end of the blood capillaries.
The interstitial fluid pressure tends to force fluid inward through the capillary membrane when it is positive but outward when it is negative. This can be subdivided into the pressure of the fluid within the gel (integral pressure) and the free-fluid pressure. There is a slight difference between these pressures caused by the osmotic pressure of the gel. When acute edema develops, the free-fluid portion swells. Normally, in loose tissue, interstitial free-fluid pressure is slightly less than atmospheric pressure and exerts a suction force, drawing fluid out of the capillaries.1,2 The direct cause of edema is positive pressure in the interstitial fluid spaces.2
Colloid osmotic or oncotic pressures are the pressures created by dissolved proteins, causing osmosis of fluid. The plasma colloid osmotic pressure draws fluid inward through the membrane, whereas the interstitial fluid colloid osmotic pressure draws fluid outward through the membrane. The concentration of protein in the plasma is generally two to three times that of the interstitial fluid. Approximately 75% of the plasma colloid osmotic pressure results from albumin. The plasma colloid osmotic pressure is important in preventing loss of fluid volume from the blood into the interstitial spaces.
The capillary pressure, negative interstitial free-fluid pressure, and interstitial fluid colloid osmotic pressure all tend to force fluid outward, whereas the plasma colloid osmotic pressure causes osmosis of fluid inward. The balance of pressure between these four forces is called the net capillary filtration pressure. There is usually a higher outward force (filtration pressure) at the arterial end of the capillary because of the higher capillary pressure at this end, and a higher inward force, or resorption pressure, is seen at the venous end. About nine-tenths of what is filtered out at the arterial end of the capillary is reabsorbed back in at the venous end, with the remaining fluid going through the lymph vessels.1,2 When the net filtration pressure rises excessively, too much fluid is moved outward into the interstitial spaces for the lymphatics to manage, and extracellular edema results.
Abnormal leakage of fluid from the capillaries into the extracellular (interstitial) spaces can be caused by increased capillary pressure (as with arteriolar dilation, venular constriction, increased venous pressure, failure of venous pumps, or lack of active muscular activity), decreased plasma proteins (as with loss of proteins from denuded skin areas in burns and wounds), increased capillary permeability (as results from release of histamine and related substances, from kinins such as bradykinin, or from bacterial infections), or blockage of lymph return.1-3 All of these will result in an increased volume of interstitial fluid with subsequent expansion of the extracellular fluid volume.1,2
Widespread edema may be caused by heart failure, loss of proteins in the urine or decreased ability to produce proteins, and excessive kidney retention of salt and water.1-3 Trauma can cause venous obstruction or arteriolar dilation and thus result in increased capillary pressure and a higher net filtration pressure.6 The capillary filtration pressure will also be elevated by local or general heating that causes arterial dilation. Burns not only decrease plasma proteins (as discussed earlier) but also lead to increased capillary permeability and may allow fluid to spill into the tissues as a result of damage to the integrity of the capillary endothelium or enlarged capillary pores.6 Release of histamine, bradykinin, and substance P is part of an initial inflammatory response and will increase capillary permeability and blood flow, allowing large quantities of fluid and protein, including fibrinogen, to leak into the tissue. This in turn will cause increased interstitial fluid to accumulate.2
Intracellular edema can occur in conditions in which the metabolic systems of tissues are depressed or lack adequate nutrition to the cells, and it may occur in inflamed tissue areas as cell membranes increase their permeability to sodium and other ions, with subsequent osmosis of water into the cells.1,2
The nature and treatment of edema differ for the three stages of wound healing. The stages are described here and consist of the inflammatory phase, which usually lasts the first 3 to 5 days; the fibroplastic or proliferative phase, which may last 2 to 6 weeks depending on the nature and degree of injury; and the maturation or remodeling phase, which may last from 6 months to 2 years.8,9
Edema is the first and most obvious reaction of the hand to injury. Most wounds have an excess of fluid content early in the healing process. Release of histamine and bradykinin increases capillary permeability and is part of a normal acute inflammatory reaction that occurs in response to tissue injury from a variety of causes, including trauma, heat, chemicals, and bacteria. Edema in the early phase of wound healing is liquid, soft, and easy to mobilize and reduce. At this stage, the excess fluid or transudate consists mainly of water and dissolved electrolytes.10 This type of edema should not alarm the therapist as long as the principles of compression, elevation, use of cold, and active motion are observed to minimize pooling of blood in injured areas.11,12 However, excessive edema can inhibit wound healing by decreasing arterial, venous, and lymphatic flow.13
The primary function of the inflammatory phase is to wall off the injured area, dispose of injury by-products through phagocytosis, and prepare for the fibroplastic repair phase. Chemical mediators initially cause vasoconstriction, followed by vasodilation. Increased cell permeability allows passage of fluid and white blood cells through cell walls to form plasma.12 Leukocytes and other phagocytic cells accumulate at the site of injury to clean up the debris, and fibrinogen is converted to fibrin. Key to treatment in the inflammatory phase of wound healing is pain control and a balance between gentle active range of motion in an elevated position and rest of the involved structures. Excessive exercise in this phase can delay clot formation and increase inflammation.12 Heat is also contraindicated in the inflammatory stage, because it will cause further vasodilation and increase membrane permeability, capillary infiltration, and arterial blood flow, which will result in additional edema.
Fibroplasia begins as early as 3 to 5 days after injury and lasts from 2 to 6 weeks, depending on the extent of the wound.8,9 During the fibroplastic or repair stage of wound healing, repair of the injured tissue is initiated. Fibroplasia is characterized by increased capillary growth, increased fibroblasts, and new collagen synthesis. Clinically, scar production is heightened, and the wound begins to gain tensile strength.
Edema that persists into the fibroplasia phase is of particular concern to the surgeon and therapist. This edema is likely to become an ongoing problem unless early intervention is applied. The edema fluid becomes more viscous from the elevated protein content, and the excess fluid is called exudate.10 The protein-rich fluid or exudate associated with edema causes fibrosis and thickening of the tissues, with subsequent shortening of structures such as ligaments and tendons. As fibrin is deposited between tissue layers, organized adhesions result between structures such as tendons and their sheaths, joint capsules, synovial membranes, and fascial layers.6,14 Structures will continue to swell, thicken, and shorten, and eventually will be replaced by dense fibrous tissue.11,15 The greater the edema and the longer it persists, the more extensive the scarring and the resultant pain, adhesions, disfigurement, and disability. All tissues—vessels, nerves, joints, and intrinsic muscles—become involved in a state of reduced nutrition and inelasticity. The combination of persistent edema with immobilization and poor positioning ultimately results in a stiff hand and must be circumvented.
If the lymphatic system becomes blocked or metabolites are allowed to accumulate in the interstitial spaces, colloid osmotic pressure is increased by the relative increase in concentration of proteins, again resulting in a higher capillary net filtration pressure. The coagulating effect of tissue exudates causes the interstitial and lymphatic fluid to clot, preventing the fluid from being expelled by pressure and resulting in brawny edema in the spaces surrounding the injured cells.1 Brawny or nonpitting edema can also be caused by swelling of the tissues cells from trauma, disease, or inadequate nutrition.1
The final phase of healing is called the maturation or remodeling phase; it is initiated as fibroplasia subsides. In this stage, tissue remodeling and realignment are achieved by placing tensile stresses on collagen fibers. At this point, persistent, stagnant edema may have led to fibrosis with elevated protein content. Chronic edema will result in stretching of the tissue spaces, necessitating long-term use of continuous-compression garments to maintain gains made in edema reduction. If allowed to progress to the maturation phase, edema will become hard, thick, and brawny as the result of connective tissue infiltration and fibrosis. In the worst scenario, edema compromises arterial flow, causing anoxia and impaired metabolic circulation and cellular nutrition, and necrosis of tissues may ensue.
The prevention and treatment of edema are of paramount importance during all phases of management of the injured hand.16 Measures must be taken before edema is visible, because interstitial fluid volume will increase 30% above normal before detection.2 According to Brand and Thompson,17 as much as 50 ml of edematous fluid can accumulate in the hand without being noticed “by a busy therapist dealing with a succession of patients.” More recently, Kelly also comments that “a 30% fluid overload may occur before swelling is visible.”18
After surgery or trauma, the extremity should be positioned above the heart as much of the time as possible except following arterial injury/repair. In this case the hand should be slightly below the level of the heart to prevent undue pressure on the repaired artery. If the hand must be immobilized, when possible it should be positioned in the intrinsic-plus position of flexed metacarpophalangeal (MCP) joints (to 70 degrees) and extended interphalangeal (IP) joints to prevent shortening of the MCP joint collateral ligaments and IP volar plates. The wrist should be positioned in neutral or slight extension. The first webspace also must be maintained via thumb abduction and extension. This is particularly true with burn patients, whose wounds will contract as they heal. If the intrinsic-plus position is not feasible, the patient’s hand should be positioned in the best approximation and the orthosis adjusted when feasible.19 In some cases injured and repaired structures of the hand may require alternative positioning.
Active ROM and tendon gliding exercises are especially important in the fibroplasia stage of healing to prevent the development of adhesions. Orthotic positioning will help to maintain and increase ROM. All joints that are not required to be immobilized should be able to move freely in the cast or orthosis and taken through their full ROM.
Edema in its early stages is reversible. If edema can be controlled early, subsequent scar formation is minimized in comparison with the scar that forms if edema is prolonged and brawny. Postoperative efforts are directed toward minimizing edema and promoting uncomplicated wound healing. Patient education is vital. Beginning with the initial treatment in the hospital or clinic, the patient must be made aware of the factors that can exacerbate or alleviate edema.
As discussed previously, a significant amount of edema may accumulate in a hand without visible detection.17 For this reason, the ability to establish and measure changes in edema with standardized and reliable procedures is critical for the effective management of edema. Edema will fluctuate daily with changes in diet, activity level, water retention, temperature, and time of day. Therefore, it is important to measure both the involved and uninvolved extremities to obtain a relative comparison between the two, ideally at the same time of day and in the same position. Measures should be taken at the initial evaluation and routinely thereafter with one of the three techniques described below.
The volumeter, figure-of-eight method, and truncated circumferential method of measuring edema are all reliable techniques to measure edema. A high correlation between water displacement and geometric (truncated) formulas has been well documented.20-26 Studies are also available that assess volumetry and truncated measures as a tool to look at change in edematous arms over a period of time. Both measures have been found to be accurate, although the methods are not interchangeable.22,24-26 Total volumes for both of these measures are provided in units of cubic centimeters, which can be converted directly to milliliters. The figure-of-eight method provides a final measure in centimeters. A high correlation between the figure-of-eight method and volumetry has been established in three separate studies,27-29 as well as in a study by Maihafer et al. that used slightly different landmarks.30
The volumeter is a standardized tool that allows the therapist to measure hand edema by measuring the amount of water the limb displaces.31,32 Waylett-Rendall and Seibly32 have shown that the volumeter is reliable within ± 10 mL (1%) if successive measures are performed by the same examiner. A subsequent study done by King33 assessed the effect of water temperature on hand volume during volumetric measurement. Although there was a significant difference in volumes using extreme temperatures (41°F versus 113°F; 5°C versus 45°C), it was found that the use of “cool” (68°F; 20°C) versus “tepid” (95°F; 35°C) water does not appear to alter hand volume readings sufficiently to be of concern.33
The difference in volume of dominant versus nondominant hands was studied by Van Velze et al.34 for 263 male laborers. They concluded that, on average, the left nondominant hand was 3.43% smaller (16.9 mL) than the right dominant hand for male laborers.34
The effect of exercise of the asymptomatic hand on volumetric and sensory status has been studied by McGough and Surwasky.35 Their results on 20 subjects suggest that exercise influences volumetric measurements. Specifically, females in the study demonstrated a 3.6% increase in volumetrics immediately after exercise, with a decline in volume at 10 minutes after exercise to 2.4%.35 The males in the study demonstrated a 5.2% volume increase immediately after exercise, with a decline in volume at 10 minutes after exercise to 5%. McGough and Surwasky encourage further statistical investigation on a larger scale of the role of hand dominance, gender, and age on hand volume.
It is important to follow the standardized procedure for the volumeter and to standardize alternative techniques as much as possible. The volumetric kit includes the volumeter, the 800-mL collection beaker, and a 500-mL graduated cylinder (Fig. 63-3A online). Instructions for the standardized procedure to use with the commercially available volumeters accompany each volumeter purchased from equipment companies. The volumeter should be placed on exactly the same spot on the same level surface for each use. Readings should also be taken on exactly the same spot, since most floors and tables are not completely level. The volumeter should be filled with room temperature water (68°F to 95°F) until it overflows, the overflow discarded, and the beaker placed under the volumeter’s spout. Once all jewelry has been removed from the patient’s hand, the hand should be lowered into the volumeter with the thumb facing the spout and the forearm in pronation until the webspace between the middle and ring fingers firmly straddles the rod (Fig. 63-3B online). The sides of the hand should not come into contact with the sides of the volumeter. Any variations in this position should be documented. Water will overflow into the beaker. This position must be maintained until water stops spilling from the spout. The water from the beaker can than be poured into the graduated cylinder and measured. This same process is then completed with the other hand. Both hands should always be measured.6,31,36,37
Figure 63-3 A, The volumeter, beaker, and graduated cylinder used to collect and measure the amount of water displaced by an edematous hand. B, The hand placed in pronation inside the volumeter, with the ring and middle fingers straddling the rod and the webspace in contact with the bar.
Truncated surface measures are derived by dividing the arm into cones, determining the volume of each cone, and summing these to determine total arm volume. There are two basic formulas for this, depending on whether measures are taken every 4 cm (or less) or every 10 cm (or less) (Box 63-1). Commercial computer programs are available that automate the computations once the therapist has determined the landmarks and circumferences, or the formulas can be entered into a spreadsheet program, such as Microsoft Excel. Landmarks are recorded as the distance in centimeters measured from the third fingertip or nail bed (Fig. 63-4A) with the first landmark being at the MCP, all joints neutral as able, and the arm pronated. Additional landmarks should be taken every 4 to 10 cm. as well as anywhere that the shape of the arm changes significantly, such as the wrist and elbow. The same landmarks should be used on the uninvolved arm and in all subsequent reassessments. It is important to use the same measuring tape and to apply consistent tension. One way to accomplish this is to have the tape straddle the landmark with the larger number always proximal, and with the ball of the tape always hanging just inside and below the arm where it is being measured (Fig. 63-4B). The tape should be taut and lay flat against the skin, without being so tight as to wrinkle the skin.
Box 63-1 Formulas for Calculating Limb Volume from Truncated Surface Measures
Formula for every 4 cm: Volume = circumference2/π for each segment
Formula for every 10 cm: Volume = h[(Ct × Ct) + (Ct × Cb) + (Cb × Cb)]12π
Where h is the height of the cone, Ct is the circumference at the top of the cone, and Cb is the circumference at the bottom of the cone, for each segment.
For example, the first cone could be the area between the MCP joints and wrist. Assuming these are 8 cm apart (height), the MCP joints (top of cone) measure 16 cm, and the wrist (bottom of cone) measures 18 cm, the formula for this segment would be:
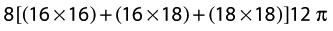
For the next segment/cone, the wrist will now be the top of the cone, and the distal forearm at the bottom of the cone. Assuming that these landmarks are 10 cm apart and the measure at the distal forearm is 20 cm; the formula for this segment would be:
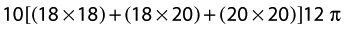
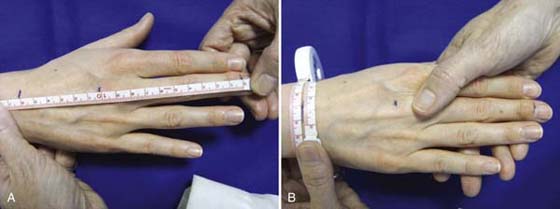
Figure 63-4 A, Landmarks are recorded in centimeters from the proximal cuticle or fingertip of the long finger, with all joints in neutral extension and the arm pronated, if able, for truncated circumferential measures. Variations in joint positioning must be noted. B, To enhance repeatability, circumferential measurements must be taken the same way and in the same position each time. Measurements should straddle the landmark, the tape measure should be taut without providing tension to the skin, and the portion of the extremity being measured should be parallel to the ground or table and positioned the same way as when the landmarks were determined. The tape should be placed with the higher numbers proximal to the landmark, the lower numbers distal. This therapist measures with the case of the tape measure resting just inside and below the extremity (to provide a constant and reproducible force).
Figure 63-5A to E illustrates the figure-of-eight technique as described by Pellecchia.29 It uses four landmarks to provide one cumulative number in centimeters as a measure of edema. Measures are taken with the wrist in neutral and the fingers adducted. The tape is placed at (Fig. 63-5A) the medial wrist, distal to the ulnar styloid; crosses the ventral wrist to (Fig. 63-5B) the distal radial styloid; crosses the dorsal wrist diagonally to the (Fig. 63-5C) fifth MCP joint; and finally crosses the wrist dorsally to return to the (Fig. 63-5D) distal ulnar styloid. It is important to provide consistent tension on the tape measure.
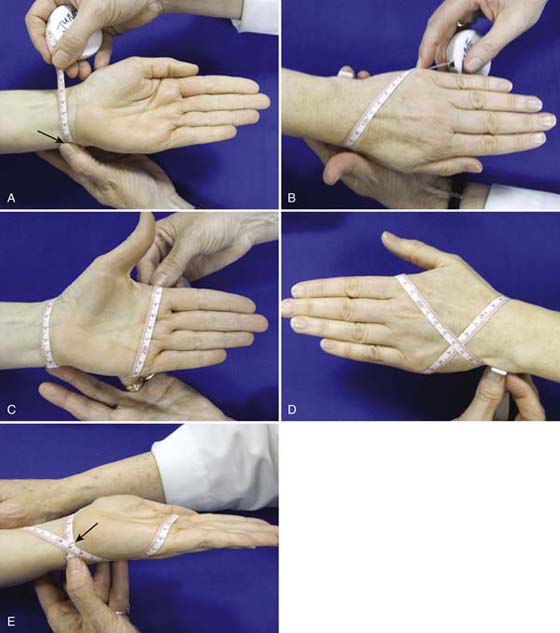
Figure 63-5 The figure-of-eight method of evaluation. A, Starting with the tape at the distal medial edge of the ulnar styloid;B, the tape is brought volarly across to the (distal) radial styloid and diagonally across the dorsum of the hand to the fifth MCP joint;C, the tape is brought across the volar distal palmar crease to the second MCP;D, finally, the tape is brought diagonally across the dorsum of the hand back to the starting point at the distal medial ulna. E, The recorded measure is taken where the two numbers intersect.
At least one of these three measures should be appropriate for any patient with edema. All three can be used with hand edema; volumes or truncated measures can be used with edema that extends to the forearm. Truncated measures are especially useful for patients with large arms or diffuse edema that makes using the volumeter difficult, and for patients with pins, fixators, or open wounds that preclude use of the volumeter. For early-stage edema that is not observable clinically, the patient may describe heaviness or fullness in the extremity, pain, tingling, or numbness.18 Measurements may not reflect this early-stage edema; however, the clinician should institute edema management techniques.
The specific techniques used to promote resolution of edema vary depending on whether the edema is in the inflammatory, fibroplastic, or maturation phase of wound healing.
The following modalities can be used in combination and are discussed in more detail below: cold, elevation, active motion, lymphatic massage, intermittent compression, continuous passive motion (CPM), compressive bandages, electrical stimulation, other electrical modalities, and hyperbaric oxygen.
Cold application may be helpful in limiting edema formation by producing vasoconstriction and reducing metabolic rate and arteriolar blood flow, reducing membrane permeability and capillary infiltration.14,38,39 However, careful attention to vascular status is required when cold packs are used, because excessive cooling may result in tissue ischemia and damage. Cold is contraindicated in the presence of arterial compromise or repair. Cold is especially helpful during the initial inflammatory phase; it performs better than heat or contrast baths in existing studies35,39,40 and should be used in combination with other principles of edema management, such as elevation and compression.
McMaster38 found improved edema control with 20-minute applications of cold over 10 therapy sessions. Cote found that cold application resulted in better control of edema than heat or contrast baths.40 Stockle39 studied the reduction of foot or ankle edema using an intermittent impulse compression device, continuous cryotherapy, or cool packs and found reductions of 74%, 70%, and 45%, respectively. Bleakely41 conducted a systematic review and reported that many more high-quality trials are needed to provide evidence-based guidelines in the treatment of acute soft tissue injuries. Bleakely concluded that ice was more effective than no ice after knee surgery, and more effective with exercise or electrical stimulation; moreover, Bleakely found little evidence to suggest that ice with compression was more effective than compression alone. Ice or cold packs are used with an interface lining such as a towel between the skin and the pack. The duration of use is usually for 10 to 15 minutes.
Elevation uses gravity to enhance venous and lymphatic flow out of traumatized areas. Elevation is especially indicated in early-stage edema such as immediately after surgery. For elevation to be effective, a gentle decline from distal to proximal with the entire limb slightly above the level of the heart should be achieved. In other words, the hand should be slightly higher than the wrist, the wrist should be slightly higher than the elbow, and the elbow should be positioned slightly higher than the shoulder. Pillows or wedge supports can be used to position the extremity appropriately at a table or in bed. If arterial systems are compromised or if elevation causes ischemia of the extremity, the level of elevation will have to be altered. When arterial occlusion is diagnosed, the arm should be lowered below the heart. When venous occlusion is diagnosed, the extremity should be elevated.42 While increased edema is empirically evident with dependent positioning, there are no actual studies that demonstrate decreased edema formation with elevation above the heart.43 However, the complications of edema are much more difficult to treat than the prevention of edema, and it is prudent to utilize simple procedures such as elevation at least to the level of the heart to minimize the possibility of this development.
Precautions must be observed for the replanted arm, hand, or digit. In this case excessive elevation is to be avoided, because it can stress the arterial system. Elevation for the transplanted limb should be at the level of the heart and modified according to the patient’s arterial and venous status.44 Healthy replanted digits are warm and pink. Arterial occlusion may be indicated by a cool, pale digit, whereas venous insufficiency is associated with a dusky hue in the replanted part. The therapist should instruct the patient to make sure that the elbow is not kept in a flexed position, because this may create an obstruction to venous drainage. A sudden increase in edema or an alteration in color or temperature should be reported immediately to the surgeon.11
In addition to facilitating venous and lymphatic outflow from the limbs, elevation decreases the hydrostatic pressure in the blood vessels, which in turn decreases the capillary filtration pressure at the arterial end.7 As discussed previously, hydrostatic pressure occurs in the vascular system because of the weight of blood in the vessels.3 Peripheral venous and arterial pressures are influenced by gravity. The pressure in any vessel held below heart level is increased by gravity, and the pressure in any vessel held above the level of the heart is decreased by gravity.3 When the hand is lower than the heart, the intravascular pressure is increased, in turn increasing the capillary filtration pressure.7 Consequently, interstitial fluid will accumulate in the dependent hand. In contrast, keeping the limb above the level of the heart decreases the intravascular pressure and decreases the capillary filtration pressure.3
Active exercises create muscle pumping, soft tissue movement, and compression of veins and lymphatic vessels, all of which are helpful in edema control.44-47 Strong muscular contractions assist in venous and lymphatic drainage.47 Active motion prevents stagnation of tissue fluids that can result from lack of use.
Schuind and Burny48 state that the postcapillary blood pressure facilitating venous return is less than 15 mm Hg, whereas the hydrostatic pressure opposing venous return from the dependent hand is approximately 35 mm Hg. Active movement is required to produce venous return.48 There is also data supporting the benefits of exercise in enhancing lymph flow and improving resorption.5,49 It is important to include the more proximal joints in a ROM program. These joints will become stiff if not taken through their ROM, and active pumping of the proximal muscles will help clear any proximal edema, thus allowing more efficient drainage of distal edema. An effective exercise that incorporates elevation and active motion of the digits and shoulders is to have the patient elevate both arms over the head and make firm fists at least 25 repetitions each hour.11,50
Light cardiopulmonary exercise will stimulate the lymphatic system and assist with fluid uptake via the thoracic duct, which penetrates the diaphragm.
Lymphatic massage can mobilize tissue fluid and increase lymphatic flow.7 Lymphatic massage can increase the frequency of lymphatic vessel contraction, thereby increasing the transport capacity of the lymphatic system.19,51 Stimulation of the lymphatic system through massage has been shown to be helpful in both the lympedema population53 and the hand therapy population.53 Simple lymphatic drainage concepts can be applied to local edema and are discussed below.
Superficial lymphatic capillaries have anchoring filaments that attach them to the surrounding tissue. Stretch to the overlying skin causes “clefts” in the capillary walls to open and accept fluid from the surrounding tissue. Only minimal pressure is needed to stimulate this process. In fact, Eliska54 has shown that pressure above 60 mm Hg will cause mechanical occlusion of the capillaries and be counterproductive to fluid reabsorption. The exact force required for an optimal response will vary slightly depending on skin texture but should be the minimum force required to traction the skin without sliding on the skin. In Dr. Vodder’s words, “The drainage must be performed softly, harmoniously, rhythmically, and with supple hands. … The hands should be so supple and alive that the dry skin can be massaged.”55 While the exact pressure must be adapted to the findings, it should never elicit pain.55 A variety of types of strokes can be used, but the simplest is the stationary circle, variably referred to as a U or J or L stroke. The bottom of the stroke at the base of the J or L catches the skin distally, and then longitudinal traction is provided to the skin in a proximal direction to the limit of the skin’s elasticity. Massage should never be painful, and the individual strokes should always go in the direction toward the regional lymph nodes. The palmar surface of the hand should be used when possible, or several fingers together for smaller areas so as to avoid point pressure.
Before providing simple lymphatic massage to the local area of edema, it is important to create a path for the edema. A useful analogy is a traffic jam caused by a wreck up the street. Before backed-up traffic can begin to move, the wreck has to be pulled off the highway. Once it is cleared away, the traffic farthest ahead (the most “proximal” traffic) will move first, eventually allowing the traffic at the very end (most distal) of the jam to flow again. Likewise, regional lymph nodes have to be stimulated to increase uptake proximally and provide a path for distal edema to follow. It is important to start (clear) proximally, work distally to the local edema, and then move this edema proximally by providing light, proximally directed, skin tractioning massage strokes to the skin between the edema and the regional lymph nodes. Simple lymphatic drainage should start and end proximally. Each stroke should be performed slowly and take 2 to 3 seconds, to allow for refilling and emptying of the lymphatic vessels (see video online).51 With successive strokes, the therapist will be able to feel the underlying edematous tissue soften and flatten. It is also important to remember that edema will have to be rerouted around scars/incisions, since it cannot go through scars. Each skin area should be massaged a minimum of 5 to 10 times, with more attention to specific areas as needed.
Simple lymphatic massage is contraindicated any time stimulation of the circulatory system must be minimized or avoided. This includes, but is not limited to, co-morbidities such as deep vein thrombosis, pulmonary embolism, untreated infection, untreated cancer, chronic heart failure, or renal failure. Simple lymphatic massage is specific to local edema for the hand therapy population with an intact lymphatic system and is not appropriate for generalized edema or lymphedema. The reader is encouraged to review Chapters 64 and 65 for further information on these highly specialized techniques.
Massage techniques such as retrograde massage, tube wrapping, and string wrapping are to be avoided.
Intermittent pneumatic compression may be helpful in decreasing edema during the inflammatory phase by facilitating the lymphatic system’s ability to resorb proteins, cellular components, and fatty acids and by assisting in the removal of these during the fibroplasia phase.12 Intermittent compression has been shown to be an effective adjunct to lymphatic massage to reduce lymphedema in conjunction with lymphatic massage,52,56,57 and as an adjunct to edema with high-voltage electrical stimulation,59 although there is variability as to the treatment protocols with regard to pressure, duration, and frequency.13,47,49,58-60 Its efficacy with the hand therapy population has been mixed.58,59,61 Intermittent compression increases tissue hydrostatic pressure and acceleration of the lymphatic and venous flow.7 This in turn drives lymphatic fluid back into the venous system. However, while intermittent compression is able to displace fluid, it does not stimulate the lymphatic system directly and does not increase uptake of proteins.60 As a result, gains made with intermittent compression pumping with protein-rich edemas may not be stable and may reverse quickly as proteins continue to pull fluid back into the interstitial spaces. To be effective, the intermittent pump pressure must be greater than the 25–mm Hg29 mean capillary pressure and should not exceed 60 mm Hg in patients with an intact lymphatic system. One of the problems with intermittent compression pumps is that it is difficult to measure exactly how much pressure is transferred to the lymphatic vessels through the skin, which will be different for brawny edema than for pitting types of edema. Segers et al.62 found wide discrepancies between the target pressure as set by the controls and the actual pressures delivered inside the cuff chambers, with the actual pressures being as much as 80% higher. As mentioned previously, excessive pressures may damage the lymphatic vessels and result in further increases in edema. Intermittent compression should be used cautiously and primarily with patients who are not able to master self lymphatic massage.
Treatment duration guidelines range from 30 minutes to 2 hours for the patient with acute edema. Leduc et al.60 advise that pneumatic compression not exceed 40 mm Hg for patients with compromised lymphatic systems. The limb should be elevated during treatment with intermittent compression. The compression-to-release ratio is usually 3 : 1 or 4 : 1.13 The amount of pressure must be adjusted according to each patient’s diagnosis and condition. Intermittent pneumatic compression should be used with caution with acute injuries, since premature use can result in increased bleeding.10 Acute fractures are a relative contraindication, since the pressure could result in unwanted movement/displacement of the fracture. Stable fractures can begin with a low amount of pressure once enough healing has taken place to tolerate the pressure, but the treatment should be carefully supervised by the therapist. The presence of open wounds does not prohibit intermittent pressure as long as sterile dressings are used. Felt pads can be positioned around pins to prevent pressure on them. More chronic edema will require higher pressures and longer treatment times.
Several different models of pneumatic pumps provide compression, including single-chamber and multichamber pumps. A multichamber sequential-pressure sleeve inflates from distal to proximal and is theoretically more effective at returning fluid to the lymphatic system by preventing backflow. One concern with the single-chamber unit is that it may merely redistribute fluid into areas of low hydrostatic pressure and spread the edema over a larger area.63
Intermittent compression is contraindicated in patients with infection or with any patient who has a medical condition who may not be able to safely handle the additional pressure or additional fluid load into their circulatory system. This includes patients with congestive heart failure, cardiac or renal insufficiency, pulmonary edema, deep vein thrombosis, or vascular compromise. It should be used with caution in patients with impaired sensation.
CPM is another method available for the therapist to consider to reduce hand edema in postoperative patients, especially patients who have undergone surgical release procedures such as tenolysis or capsulectomy. This group of patients may be predisposed to the development of adhesions and hand stiffness. Hand CPM units include parameters that can be set by the therapist to control the specific ROM desired and velocity of movement. Many units include an MCP joint block to isolate IP joint motion. CPM is most effective during the early phases of wound healing and early scar formation. CPM in the pain-free ROM allows for improved gliding, nutrition of tissues, and scar lengthening.64 CPM may also have a role with patients who have an impaired ability to actively use their hand, such as patients who have hemiplegia secondary to a cerebrovascular accident.46 Passive ROM will maintain joint mobility in the specified range and can improve lymphatic flow.
In the acute stage of wound healing, compression limits the amount of space available for swelling to accumulate.12 Compression may decrease fibroblast synthesis of collagen by decreasing the blood flow and causing local hypoxia,13 which is especially important in the fibroplasia phase. Pressure may mechanically force fluid out of the tissue.13 In the later stages of wound healing, compression maintains gains made in edema reduction by reducing capillary filtration. Compressive garments help reinforce tissue hydrostatic pressure and facilitate venous and lymphatic flow. The use of compressive garments or wrapping is especially important with brawny (fibrotic) edema and should always be used following pneumatic pumping or lymphatic massage to maintain reductions in edema.
Various compressive dressings are available commercially. These include compressive stockinets, tubular elastic bandages, finger sleeves, pressure garments and gloves, short-stretch bandages, finger sleeves and wraps, self-adherent bandages, and Isotoner gloves (Isotoner, Cincinnati, Ohio), among others.47 Short stretch bandages provide more support and controlled compression than elastic wrapping for the arm. Any type of circular elastic bandages, such as tubigrip tubular bandages, have the potential to create a tourniquet effect and should be used cautiously and monitored carefully; these types of compressive garments should be limited to alert patients during waking hours so as to allow for appropriate monitoring of circulation.
For stubborn or brawny edema, foam or chip bags made from small pieces of varied-density foam can be used under a wrap to soften these areas. Light foam over a larger area will improve tolerance to the wrap by distributing the forces, while dense foam over a problem area such as the back of the hand provides more compression. Chip bags provide significant local pressure and are especially helpful for brawny areas but may be less tolerated. As with any bandaging, the extremity should be wrapped from distal to proximal to assist with lymphatic and venous drainage, and a spiral or figure-of-eight technique (not circular) should be used.
Specialized soft finger wraps such as Elastomull (BSN Medical, Inc., Charlotte, North Carolina) can be used to facilitate lymphatic function and provide gentle compression to the hand without restricting ROM. These wraps anchor at the wrist and cross the dorsum of the hand to start at the small finger or thumb, depending on the direction of the wrap (Fig. 63-6). This type of wrap provides superior ability to provide even pressure while accommodating to changes in joint position. While originally intended as a technique to include all the digits, this same technique can be applied to a single edematous digit. These finger wraps can be worn safely in a reliable patient with normal sensation for 24 hours.
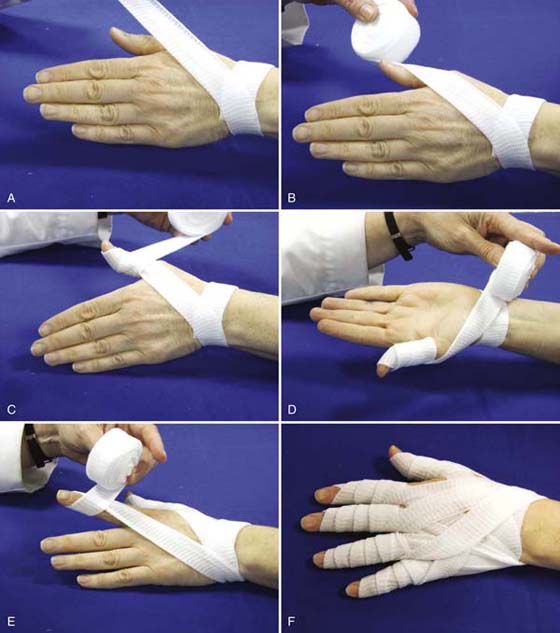
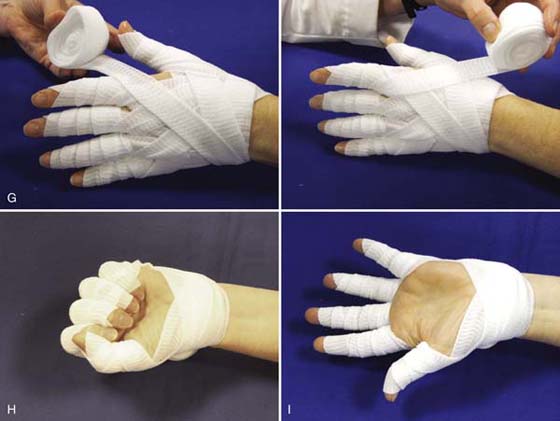
Figure 63-6 A, Elastomull is anchored at the wrist and wrapped diagonally across the back of the hand to (B) the outermost aspect of the distal thumb. C, The wrap is then spiraled around the thumb from distal to proximal with each layer overlapping the previous layer by one-half width. D, From the base of the thumb the wrap continues around the base of the wrist, leaving the palm free. E, This technique is continued for each of the digits, each time anchoring around the wrist and leaving the palm free. F, Dorsal view of completed wrap. G (1 and 2), For additional support at the webspaces, additional wrap can be anchored at the wrist and wrapped once around each finger. H, The finger wrap provides gentle compression without limiting motion, and is well tolerated. I, Volar view of completed wrap with palm free.
Self-adherent wrap is another alternative for small areas, such as edematous digits. Care must be taken not to apply tension while wrapping, because this can restrict circulation. Patients must be instructed to carefully monitor the finger for circulation. There are two techniques for using self-adherent wrap; 1-inch wrap can be applied in an overlapping spiral (see Fig. 63-7 online), or, for patients who have difficulty applying this technique, a larger wrap can be wrapped once around the finger and cinched (distal to proximal) to provide more gentle compression (Fig. 63-8 online). Lowell65 reports improved results in edema control using Coban (3M, St. Paul, Minnesota) over standard dressings for a patient with bilateral hand burns.
Figure 63-7 A, A 1-inch self-adherent wrap can be spiraled around an edematous finger and wrapped proximally, overlapping by one-half width. B, Completed wrap.
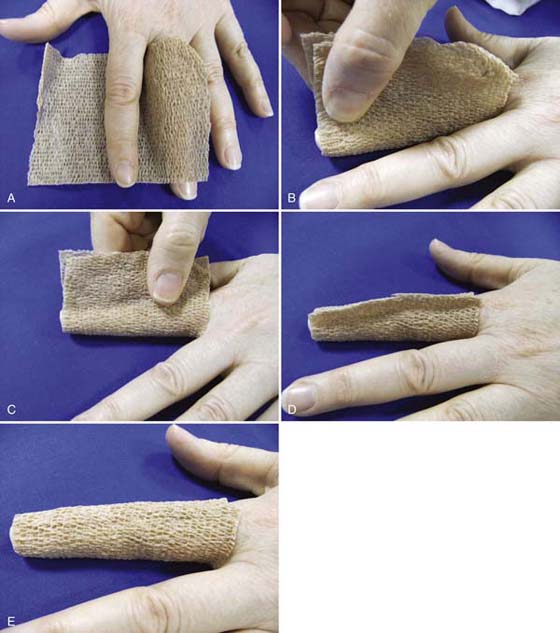
Figure 63-8 A, A 3- or 4-inch self-adherent wrap is wrapped around the digit and cinched distal to proximal. B, Wrap is cinched starting distally. C, Self-adherent wrap is cinched to form a seam. D, Excess wrap is trimmed, leaving a small seam. E, The seam can then be flattened.
Compressive garments are contraindicated for patients with arterial compromise, new skin grafts, or unhealed burn wounds.
All forms of external compression must be monitored by the patient to make sure that capillary flow is not restricted. The patient who is using compression wrapping at home must be instructed to look for any signs of compromised circulation, such as changes in color, coldness, or numbness in the fingertip(s).
High-voltage pulsed direct current (HVPC) has been hypothesized to decrease edema by reducing microvascular permeability to plasma proteins.45,66-69 It is hypothesized that the current reduces edema by repelling negatively charged proteins in the edematous interstitial spaces.67,69 It is suggested that when the negative polarity of HVPC repels negatively charged cells and protein, a fluid shift occurs.67 High-voltage current includes intensities greater than 100 V. Research results are mixed.
The effect of HVPC in reducing edema has been assessed in animal experiments with frogs, rats, and hamsters.45,60,66,68,70 Other rat studies have yielded disappointing results. Mohr,71 Cosgrove,72 and Cook73 did not find significant differences using subcontraction high voltage with Sprague-Dawley rats. A recent study by Hahm74 found that using low-frequency electrical stimulation of acupoint sites for Sprague-Dawley rats resulted in decreased edema at 6 and 12 hours postinjury, but that high-frequency (5× muscle twitch) electrical stimulation did not have an effect on edema. Thorton75 found that different strains of rats responded differently to electrical stimulation and that Sprague-Dawley rats did not respond whereas other strains did. Chu et al.76 studied the effect of direct current on wound edema after full-thickness burn injury in rats and found that direct electric current had a beneficial effect in reducing wound edema after burn injury but that at least 8 hours of treatment were required to achieve a sustained maximum effect.
Human studies include work by Stralka, Griffen, Faghri Cheing, and Man.58,69,77-79 Stralka et al.69 conducted a study on the use of HVPC in reducing chronic hand edema in conjunction with a wrist orthosis that demonstrated significant decreases for hand edema and pain following treatment. Griffin et al.58 compared the effectiveness of a single-treatment HVPC, intermittent pneumatic compression, and placebo-HVPC. Differences between the HVPC and placebo-HVPC group approached but did not reach statistical significance (P = 0.04). Reduction in hand edema was significant for the intermittent pneumatic compression group compared with the placebo-HVPC group, at the P = 0.01 level. Faghri77 studied the effects of neuromuscular stimulation (NMS)-induced muscle contraction and elevation and concluded that NMS was more effective for reduction of edema than elevation alone for hand edema in flaccid cerebrovascular accident patients. However, Geurts80 found Fagri’s statistics “insufficient and at specific points erroneous.” Man79 found no significant difference with NMS used with ankle sprains.
Transcutaneous electrical nerve stimulation has not been found helpful with treatment of edema.81
All patients who are candidates for electrical modalities must be cleared medically before initiating this type of therapy. The use of electrical modalities is contraindicated in patients who have a pacemaker, cardiac arrhythmias, seizures, myocardial disease, or any other condition that may be adversely affected by current. Although electrical stimulation may be helpful in decreasing edema in any of the three stages of wound healing, it has been suggested that intensities that produce muscle contractions should be avoided in the inflammatory phase because they may increase clotting time.12
Previous human studies on the use of low-level laser therapy for edema control primarily following ankle sprains has yielded mixed results and has not provided any conclusive support for using low-level laser therapy with musculoskeletal conditions.10,82 More recently, positive results were obtained by Sergioulas83 with ankle edema and Ozkan84 with edema following injury to digital flexor tendons; these studies have used different treatment parameters and instrumentation.
The nonthermal effects of ultrasound include acoustic streaming and cavitation.85 Nonthermal ultrasound modulates membrane properties, alters cellular proliferation, and produces increases in proteins associated with inflammation and injury repair including vasodilation of arterioles and activation of adhesion molecules.85 Fyfe and Chahl86 found significant but short-term reductions in the edema response using .79 Hz applied for 2 or 4 minutes with male (Wistar) albino rats following injections of silver nitrate. Human research studies using ultrasound to reduce ankle edema have utilized a variety of parameters and have yielded conflicting results.10 Hashish87 applied very low level ultrasound at .1 W/cm2 following bilateral surgical extraction of molars and achieved greater edema reduction in the placebo group than the test group. Additional research is needed to determine the best parameters and efficacy of ultrasound to decrease edema in the hand therapy population.
In summary, there are a variety of modalities available to assist in edema reduction, but more research is needed to determine the appropriate treatment parameters and overall efficacy with specific approaches to upper extremity edema. The reader is referred to Chapter 117 in this text for further information on the use of physical agents in hand rehabilitation.
Hyperbaric oxygen (HBO) therapy is a treatment wherein the entire body is treated with 100% oxygen at greater than normal atmospheric pressures. This treatment increases the concentration of oxygen in the blood plasma and cells. HBO has a potential role with any condition wherein blood flow and oxygen delivery are compromised. In a double-blind randomized, placebo-controlled study, Kiralp et al. found HBO therapy to be an effective and well-tolerated method for decreasing pain and edema in patients with chronic regional pain syndrome.88
The best treatment of edema is to prevent and minimize its occurrence through the use of atraumatic surgical techniques, appropriate postoperative dressings and positioning, judicious and monitored use of cold, comfortable elevation, and early motion when possible. If edema persists, the patient should be instructed in lymphatic massage and an appropriate level of continuous compression. A variety of other clinical treatment options can be considered to further enhance arterial systems, venous return, and lymphatic flow. Therapists must be judicious in selecting the most appropriate approaches for each patient given the level of supportive evidence in the literature, other treatment considerations, and clinical time constraints. Control of edema is essential to optimize the benefits of therapy and, ultimately, hand function.
1. Guyton AC. Capillary fluid exchange, interstitial fluid dynamics, and lymph flow. In: Guyton AC, Hall JE, eds. Human Physiology and Mechanism of Disease. 6th ed Philadelphia: WB Saunders; 1997:130–142.
2. Guyton AC, Hall JE. Textbook of Medical Physiology. 10th ed Philadelphia: WB Saunders; 2000.
3. Ganong WF. The general and cellular basis of medical physiology. In: Ganong WF, ed. Review of Medical Physiology. 20th ed Stamford: Conn.: Appleton & Lange; 2001:1–49.
4. Daane S, Poltoratszy P, Rockwell WB. Post mastectomy lymphedema management: evaluation of the complex decongestive therapy technique. Ann Plast Surg. 1998;40:128.
5. Mortimer PS. Therapy approaches for lymphedema. Angiology. 1997;48:87.
6. Schultz-Johnson K. Volumetrics: A Literature Review. Santa Monica. Calif.: Upper Extremity Technology; 1988.
7. Vasudevan SV, Melvin JL. Upper extremity edema control: rationale of the techniques. Am J Occup Ther. 1979;33:520.
8. Flowers KR. Edema: differential management based on the stages of wound healing. In: Hunter JM, Schneider LH, Mackin EJ, Callahan AD, eds. Rehabilitation of the Hand: Surgery and Therapy. 4th ed St Louis: Mosby; 1995.
9. Smith KL. Wound healing. In: Hunter JM, Schneider LH, Mackin EJ, Callahan AD, eds. Rehabilitation of the Hand: Surgery and Therapy. 4th ed St Louis: Mosby; 1995.
10. Micholvitch SL, Nolan TP Jr. Modalities for Therapeutic Intervention. 4th ed Philadelphia: FA Davis; 2005.
11. Mackin EJ. Prevention of complications in hand therapy. In: Complications of Hand Surgery, Hand Clinics. Philadelphia: WB Saunders; 1986:429. 2
12. Prentice WE. Guidelines for using therapeutic modalities in rehabilitation. In: Prentice WE, ed. Therapeutic Modalities in Sports Medicine. 3rd ed St Louis: Mosby; 1994.
13. Walsh M, Muntzer E. Wound management. In: Stanley BG, Tribuzi SM, eds. Concepts in Hand Rehabilitation. Philadelphia: FA Davis; 1992.
14. Bunnell S. Bunnell’s Surgery of the Hand. Philadelphia: JB Lippincott; 1970.
15. Weeks P, Wray RC. Management of the stiff hand. In: Weeks PM, ed. Management of Acute Hand Injuries. St Louis: Mosby; 1978.
16. Hunter JM. Salvage of the burned hand. Surg Clin North Am. 1967;47:1060.
17. Brand PW, Thompson DE. Mechanical resistance. In: Brand PW, Hollister A, eds. Clinical Mechanics of the Hand. 2nd ed St Louis: Mosby; 1993.
18. Kelly DG. A Primer on Lymphedema. Upper Saddle River, New Jersey Prentice Hall; 2002.
19. Villeco JP, Mackin EJ, Hunter JM. Edema: therapist’s management. In: Mackin EJ, Callahan AD, Skirven TM, eds., et al. Rehabilitation of the Hand and Upper Extremity. 5th ed St Louis: CV Mosby; 2002.
20. Briele HA, Schneebaum S, Barnicle M, Briele CS. Method of measurement for volume of an extremity. Surg Gynecol Obstet. 1989;169:349–351.
21. Casley-Smith JR. Measuring and representing peripheral oedema and its alterations. Lymphology. 1994;27:56–70.
22. Casley-Smith JR. Measuring peripheral odema and bioimpedance. Lymphology. 1995;28:43–47.
23. Karges JR, Mark BE, Stikeleather SJ, Worrell T. Concurrent validity of upper-extremity volume estimates: comparison of calculated volume derived from girth measurements and water displacement volume. Phys Ther. 2003;83(2):134–145.
24. Latchford S, Casley-Smith JR. Estimating limb volumes and alterations in peripheral edema from circumferences measured at different intervals. Lymphology. 1997;39:161–164.
25. Megans AM, Harris SR, Kim-Sing C, McKenzie DC. Measurement of upper extremity volume in women after axillary dissection for breast cancer. Arch Phys Med Rehabil. 2001;82:1639–1644.
26. Sander AP, Hajer NM, Hemenway K, Miller AC. Upper-extremity volume measurements in women with lymphedema: a comparison of measurements obtained via water displacement with geometrically determined volume. Phys Ther. 2002;82(12):1201–1212.
27. Dewey WS, Hedman TL, Chapman TT, et al. The reliability and concurrent validity of the figure-of-eight method of measuring hand edema in patients with burns. J Burn Care Res. 2007;28(1):157–162.
28. Leard JS, Breglio L, Fraga L, et al. Reliability and concurrent validity of the figure-of-eight method of measuring hand size in patients with hand pathology. J Orthop Sports Phys Ther. 2004;34(6):335–340.
29. Pellecchia G. Figure-of eight method of measuring hand size: reliability and concurrent validity. J Hand Ther. 2003;16(4):300–305.
30. Maihafer GC, Llewellyn MA, Pillar WJ, et al. A comparison of the figure-of eight method and water volumetry in measurement of hand and wrist size. J Hand Ther. 2003;16(4):305–310.
31. Brand PW. Methods of clinical measurement of the hand. In: Brand PW, Hollister A, eds. Clinical Mechanics of the Hand. 2nd ed St Louis: CV Mosby; 1993.
32. Waylet-Rendall J, Seibly D. A study of the accuracy of a commercially available volumeter. J Hand Ther. 1991;4:10.
33. King TI II. The effect of water temperature on hand volume during volumetric measurement using the water displacement method. J Hand Ther. 1993;6:202.
34. Van Velze CA, Kluever J, van der Merwd CA, Mannen U. The difference in volume of dominant and non-dominant hand. J Hand Ther. 1991;4:6.
35. McGough CE, Surwasky ML. Effect of exercise on volumetric and sensory status of the asymptomatic hand. J Hand Ther. 1991;4:1771.
36. Jaffe R, Farney-Mokris S. Edema. In: Casanova JS, ed. Clinical Assessment Recommendations. 2nd ed Chicago: American Society of Hand Therapists; 1992.
37. NC Medical: Hand volumeter for measuring edema by fluid displacement: directions for use (instruction sheet, not dated).
38. Hecht PG, Bachmann S, Booth RE Jr, Rothman RH. Effects of thermal therapy on rehabilitation after total knee arthroplasty: a prospective randomized study. Clin Orthop Relat Res. 1983;Sep(178):198–201.
39. Stockle U, Hoffmann R, Schutz M, et al. Fastest reduction of posttraumatic edema: continuous cryotherapy or intermittent impulse compression? Foot Ankle Int. 1997;18(7):432–438.
40. Cote DJ, Prentice WE Jr, Hooker DN, Shields EW. Comparison of three treatment procedures for minimizing ankle sprain swelling. Phys Ther. 1988;68(7):1072–1076.
41. Bleakley C, McDonough S, MacAuley D. The use of ice in the treatment of acute soft-tissue injury: a systematic review of randomized controlled trials. Am J Sports Med. 2004;32(1):251–261.
42. Steichen JB, Idler RS. Surgical aspects of replantation and revascularization. In: Hunter JM, Schneider LH, Mackin EJ, Callahan AD, eds. Rehabilitation of the Hand: Surgery and Therapy. 3rd ed St Louis: Mosby; 1990.
43. Fagen DJ. A controlled clinical trial of postoperative hand elevation at home following day-case surgery. J Hand Surg. 2004;29(5):4548–4560.
44. Buncke HJ, Jackson RL, Buncke GM, Chan SWL. Surgical and rehabilitative aspects of replantation and revascularization of the hand. In: Hunter JM, Schneider LH, Mackin EJ, Callahan AD, eds. Rehabilitation of the Hand: Surgery and Therapy. 4th ed St Louis: Mosby; 1995.
45. Bettany JA, Fish DR, Mendel FC. Influence of high voltage pulsed direct current on edema formation following impact injury. Phys Ther. 1990;70:219.
46. Giudice ML. Effects of continuous passive motion and elevation on hand edema. Am J Occup Ther. 1990;44:914.
47. Sorenson MK. The edematous hand. Phys Ther. 1989;69:1059.
48. Schuind F, Burny F. Can algodystrophy be prevented after hand surgery? Hand Clin. 1997;13:455.
49. Brennan MJ, Miller LT. Overview of treatment options and review of the current role and use of compression garments, intermittent pumps, and exercise management of lymphedema. Cancer. 1998;83(12 suppl Am):2812. 15
50. Beasley RW. Vascular injuries. In: Beasley RW, ed. Hand Injuries. Philadelphia: WB Saunders; 1981.
51. Weissleder H, Schuchhardt. Physiology. In: Weissleder H, Schuchhardt C, eds. Lymphedema Diagnosis and Therapy. 3rd ed Köln, Germany: Viavital Verlag GmbH; 2001.
52. Mosely AL, Carati CJ, Piller NB. A systematic review of common conservative therapies for arm lymphoedema secondary to breast cancer treatment. Ann Oncol. 2007;18:639–646.
53. Haren K, Backman C, Wiberg M. Effect of manual lymph drainage as described by Vodder on oedema of the hand after fracture of the distal radius: a prospective clinical study. Scand J Plast Reconstr Surg Hand Surg. 2000;34(4):367–372.
54. Eliska O, Eliskova M. Are peripheral lymphatics damaged by high pressure manual massage? Lymphology. 1995;28:21–30.
55. Wittlinger G, Wittlinger H. Textbook of Dr. Vodder’s Manual Lymph Drainage. 6th ed Heidelberg: Karl F. Haug Verlag; 1998.
56. Bunce I, Mirolo R, Hennessy JM, et al. Post mastectomy lymphoedema treatment and measurement. Med J Aust. 1994;161:125–128.
57. Szuba A, Achalu R, Rockson SG. Decongestive lymphatic therapy for patients with breast carcinoma-associated lymphedema: a randomized, prospective study of a role for adjunctive intermittent pneumatic compression. Cancer. 2002.2260–2267.
58. Griffin JW, Newsome LS, Stralka SW, Wright PE. Reduction of chronic posttraumatic hand edema: a comparison of high voltage pulsed current, intermittent pneumatic compression and placebo treatments. Phys Ther. 1990;70:279.
59. Ramesh M, Morrissey B, Healy JB, et al. Effectiveness of the A-V impulse hand pump. J Bone Joint Surg. 1999;81B:229–233.
60. Leduc O, Leduc A, Bourgeois P, Belgrado JP. The physical treatment of upper limb edema. Cancer. 1998;15(12 suppl Am):2835.
61. Roper TA, Redford S, Tallis RC. Oedematous hand in hemiplegic stroke: a randomized controlled study. Age Aging. 1999;28:9–13.
62. Segers P, Belgrado JP, Leduc A, et al. Excessive pressure in multichambered cuffs used for sequential compression therapy. Phys Ther. 2002;82(10):1000–1008.
63. Ause-Ellias KL. The effect of mechanical compression on chronic hand edema after burn injury: a preliminary report. J Burn Care Rehabil. 1994;15:29.
64. King JW. Traumatic injuries of the hand. In: Stanley BG, Tribuzi SM, eds. Concepts in Hand Rehabilitation. Philadelphia: FA Davis; 1992.
65. Lowell M, Pirc P, Ward RS, et al. Effect of 3M Coban self-adherent wraps on edema and function of the burned hand: a case study. J Burn Care Rehabil. 2003;24(4):253–258.
66. Bettany JA, Fish DR, Mendel FC. The effect of high volt pulsed direct current on edema formation following hyperflexion injury. Arch Phys Med Rehabil. 1990;71:677.
67. Newton R. High-voltage pulsed current: theoretical bases and clinical application. In: Nelson RM, Currier DP, eds. Clinical Electrotherapy. 2nd ed Norwalk, Conn.: Appleton & Lange; 1991.
68. Reed BV. Effect of high voltage pulsed electronical stimulation on microvascular permeability to plasma proteins, a possible mechanism in minimizing edema. Phys Ther. 1988;68:491.
69. Stralka SW, Jackson JA, Lewis AR. A randomized clinical trial of high voltage pulsed, direct current built into a wrist splint. AAOHN J. 1998;46:233–236.
70. Taylor K, Fish DR, Mendel FC, Burton HW. Effect of a single 30-minute treatment of high voltage pulsed current on edema formation in frog hind limbs. Phys Ther. 1992;72:63.
71. Mohr T, Akers TK, Wessman HC. Effect of high voltage stimulation on blood flow in the rat hind limbs. Phys Ther. 1987;67(4):526–533.
72. Cosgrove KA, Alon G, Bell SF, et al. The electrical effect of two commonly used clinical stimulators on traumatic edema in rats. Phys Ther. 1992;72(3):227–233.
73. Cook HA, Morales M, La Rosa EM, et al. Effects of electrical stimulation on lymphatic flow and limb volume in the rat. Phys Ther. 1994;74(11):1040–1046.
74. Hahm TS. The effect of 2 Hz and 100 Hz electrical stimulation of acupoint on ankle sprain in rats. J Korean Med Sci. 2007;22(2):347–351.
75. Thorton RM, Mendel FC, Fish DR. Effects of electrical stimulation on edema formation in different strains of rats. Phys Ther. 1998;78(4):386–394.
76. Chu CS, Matylevich NP, McManus AT, et al. Direct current reduces wound edema after full-thickness burn injury in rats. J Trauma. 1996;40:738–743.
77. Faghri PD. The effects of neuromuscular stimulation-induced muscle contraction versus elevation on hand edema in CVA patients. J Hand Ther. 1997;10:29.
78. Cheing GL, Wan JW, Kai Lo S. Ice and pulsed electromagnetic field to reduce pain and swelling after distal radius fractures. J Rehabil Med. 2005;37(6):372–377.
79. Man IOW, Morrissey MC, Cywinski JK. Effect of neuromuscular electrical stimulation on ankle swelling in the early period after ankle sprain. Phys Ther. 2007;87:53–65.
80. Guerts AC, Viaaxhwea BA, van Limbeek J, Ribbers GM. Systematic review of aetiology of post-stroke hand oedema and shoulder-hand syndrome. Scand J Rehabil Med. 2000;32(1):4–10.
81. Resende MA, Sabino GG, Candido CR, et al. Local transcutaneous electrical stimulation (TENS) effects in experimental inflammatory edema and pain. Eur J Pharmacol. 2004;504(3):217–222.
82. De Bie RA, de Vet HCW, Lenssen TF, et al. Low level laser therapy in ankle sprains: a randomized clinical trial. Arch Phys Med Rehabil. 1998;79(11):1415–1420.
83. Stergioulas A. Low-level laser treatment can reduce edema in second degree ankle sprains. J Clin Laser Med Surg. 2004;22(2):125–128.
84. Ozkan N, Altan L, Bingol U, et al. Investigation of the supplementary effect of GaAs laser therapy on the rehabilitation of human digital flexor tendons. J Clin Laser Med Surg. 2004;22(2):105–110.
85. Johns LD. Nonthermal effects of therapeutic ultrasound: the frequency resonance hypothesis. J Athl Train. 2002;37(3):293–299.
86. Fyfe MC, Chahl LA. The effect of ultrasound on experimental oedema in rats. Ultrasound Med Biol. 1980;6:107–111.
87. Hashish I, Hai HK, Harvey W, et al. Reduction of postoperative pain and swelling by ultrasound treatment: a placebo effect. Pain. 1988;33(3):303–311.
88. Kiralp MZ, Yildiz S, Vural D, et al. Effectiveness of hyperbaric oxygen therapy in the treatment of complex regional pain syndrome. J Int Med Res. 2004;32(3):258–262.