IONTOPHORESIS AND PHONOPHORESIS
LOW-LEVEL LASER THERAPY (LLLT)
▪ Physical agents should be incorporated into the plan of care with other therapy interventions.
▪ When using cryotherapy, monitor for signs of cold sensitivity.
▪ The use of thermal agents should be avoided if the sympathetic autonomic efferents are not functioning due to nerve injury or vessel repair.
▪ Neuromuscular electrical nerve stimulator treatments should be monitored by the therapist to ensure quality contractions.
▪ More evidence is needed to support the use of physical agents in hand therapy practice.
Physical agents are included in the plan of care for hand and upper extremity patients to decrease pain, increase range of motion, increase muscle strength, and facilitate tissue healing. To use physical agents in a safe and judicious manner, therapists should have a strong foundation of the biophysical properties, clinical indications, precautions, and contraindications. Therapists need to perform a comprehensive clinical examination to determine whether the use of a physical agent is warranted and to determine treatment effectiveness. It is beyond the scope of this chapter to cover all aspects of the use of physical agents to the depth that they would be covered in an entry-level therapy course. Key texts are available for the reader who desires additional information.1,2 In recent years, several states have adjusted practice act requirements; therefore, readers are advised to investigate state licensure laws to determine individual professional limitations or requirements before applying physical agents in the clinic.
In the first part of this chapter, the theory and principles of each group of physical agents commonly used in hand therapy are discussed. In the second part of this chapter, the clinical application of physical agents to address impairments common to patients with hand and upper extremity injuries is discussed.
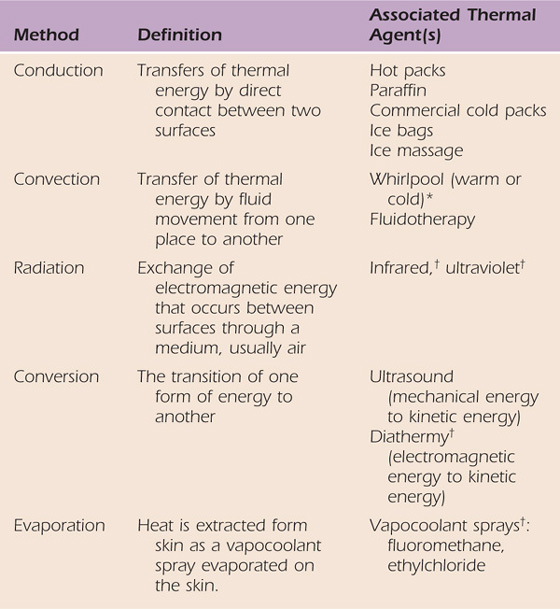
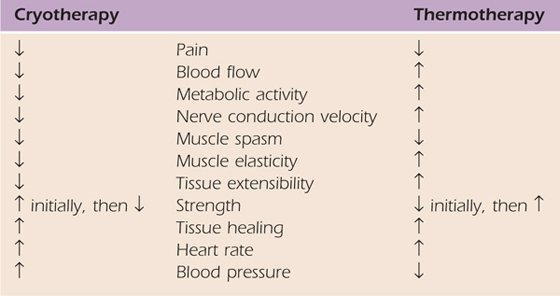
Thermal agents rely on several methods of heat transfer to alter the temperature of the target tissue. Table 117-1 reviews the different methods of heat transfer and the associated thermal agents. Thermotherapy consists of superficial and deep heating agents that increase tissue temperature at different levels of penetration. Cryotherapy techniques reduce tissue temperature by removing heat from the tissues in direct contact with the cold agent. Thermal agents should always be used as adjuncts to other therapeutic interventions that will help achieve the established therapy goals. Therapists should understand the biophysical principles, clinical indications, precautions, and contraindications for safe and effective use of thermal agents. Box 117-1 outlines the precautions and contraindications for the use of thermal agents; in particular, impaired sensation and/or circulation will limit the use of thermal agents due to diminished temperature regulation capabilities. The vasa nervosum and nervi vasorum control vasomotor response in arteries. If the nerve is injured and/or impaired, these sympathetic autonomic efferents may not be able to communicate with the nearby artery to signal the appropriate vasomotor response to an environmental temperature change and tissue damage may occur3 (Fig. 117-1). Information on treatment parameters and application techniques for ultrasound, superficial heating agents, and cryotherapy are outlined in Table 117-2, online.
Table 117-1 Methods of Heat Transfer

*Whirlpools not commonly used in practice today as thermal agents.
†Not commonly used in hand therapy practice.
Box 117-1 Precautions and Contraindications for Physical Agents
1. Decreased sensation to thermal or painful stimuli
2. Vascular compromise or disease
3. Recent or potential hemorrhage
2. Area of untreated infection; okay 24 hours after antibiotic therapy started
3. Acute inflammation or injury (wait at least 48 hours)
4. Corn allergy (Fluidotherapy)
5. Not advised to use in combination with liniments, heat rubs, and herbal packs
1. Identified cold-sensitive conditions
c. Paroxysmal cold hemoglobinuria
d. Raynaud’s phenomenon or disease
2. Decreased sensation to thermal or painful stimuli
3. Vascular compromise or disease
1. Include heat contraindications listed
2. Should not be performed over the eyes, heart, or reproductive organs
3. Should not be performed over a pregnant uterus
4. Should not be used within a known malignant area
5. Should not be used with patients with cardiac pacemakers
6. Caution and selective use over epiphyseal plates of children
Lower intensity may be needed due to refractory effects with
1. Metal plates, screws, pins, external fixators
2. Joint replacement components
ELECTROTHERAPY CONTRAINDICATIONS
1. Unshielded cardiac pacemakers or other implanted electrical devices (may interfere with normal operation)
2. Should not be performed over a pregnant uterus
3. Should not be performed over carotid sinus
4. Should not be performed over thoracic region of body
5. Should not be used within area of deep venous thrombosis; risk of thrombus or embolism
1. Pain serving a protective function
2. Area of infection, malignancy, or peripheral vascular disease; U.S. Food and Drug Administration does not directly state, but increases in circulation may exacerbate these conditions
3. Excessive adipose tissue may require higher current amplitudes
4. Decreased sensation to thermal or painful stimuli (direct current)
5. Damaged or fragile skin or open skin surface
6. Contraindications, allergies, or previous adverse reactions associated with medications or substances used in iontophoresis
7. Adverse skin reactions at electrode interface
b. Allergic reaction to adhesive polymer electrodes
c. Direct current, especially from cathode
d. Unbalanced biphasic pulsed-current waveforms
2. Altered cardiorespiratory status, e.g., acute myocardial infarction, congestive heart failure, high blood pressure, low blood pressure, chronic obstructive pulmonary disease
Figure 117-1 Schematic of the sympathetic autonomic efferents that regulate the vasomotor response with tissue temperature changes.
Table 117-2 General Application Procedures of Thermal Agents
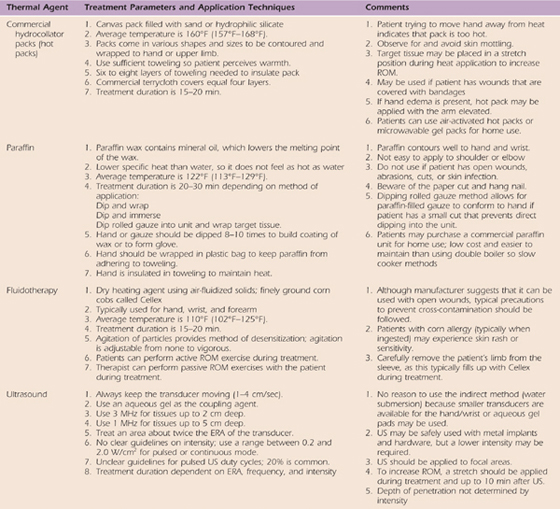

Superficial heating agents include hydrocollator packs, air-activated heat wraps, paraffin, heating pads, and Fluidotherapy (Chattanooga Medical, Chattanooga, Tennessee). These agents can increase tissue temperature as much as 2 cm in depth; therefore, they work well in the hand and wrist. The depth of penetration will depend on (1) the physical agent used, (2) the composition of the target tissue, (3) the duration of treatment, (4) the initial temperature difference, and (5) how long the agent stays hot.
Tissue temperatures must be elevated between 40°C and 45°C (104°F–113°F) to achieve therapeutic benefit.4 Above this range, there is the potential for tissue injury and below this range the therapeutic benefits may not be achieved because the heating is considered to be mild. The proposed benefits of therapeutic heating include vasodilation or increased blood flow, decreased pain, increased tissue extensibility, and decreased muscle tension.
The increase in blood flow is limited primarily to the skin located beneath the heat source. Vasodilation occurs in response to the heat to regulate tissue temperature to prevent a burn. There is a secondary vasodilation response in coordination with a spinal cord reflex that is stimulated by the heating of cutaneous afferents. No change in skeletal muscle blood flow is expected. Along with the increased blood flow, metabolic rate (cellular activity) and oxygen saturation within the local tissue increase.5 Although these effects are only observed while the tissue temperature is elevated, these hemodynamic and metabolic effects may facilitate tissue healing.
Elevation of tissue temperature creates some temporary neuromuscular effects that may reduce pain and muscle tension. These changes only occur when the tissue temperature is elevated and maintained. As tissue temperature decreases, the neuromuscular effects will reverse. Sensory nerve conduction velocity will increase with elevated temperature.6,7 Because the increase in temperature is temporary, the therapeutic benefit is unclear, but it may contribute to a reduction in pain. Elevation of muscle temperature decreases the firing rate of the muscle spindle afferents (type II afferents) and increases the firing rate of the type Ib afferents from the Golgi tendon organs.4,8 These changes in firing rates will lead to a decrease in alpha motor neuron activity and thus a reduction in muscle (extrafusal fiber) activity. Superficial heat may not sufficiently increase muscle temperature to achieve these neuromuscular effects, but the heating of skin does decrease gamma efferent firing, which will elicit a similar response.9 Basically, skeletal muscles heated to at least 42°C (107.6°F) will relax and be more flexible. The therapeutic benefits may be decreased pain and increased range of motion, especially if muscle guarding or spasm is present.
There are several proposed mechanisms for pain modulation with heat modalities. The counterirritant theory suggests that thermal receptors in the target tissue are activated and conduct impulses about temperature increases in the tissue environment more quickly than impulses from pain fibers.10 This physiologic response is related to the gating mechanism of pain.11 Increases in tissue temperature also increase the activation threshold of pain fibers (nociceptors), which may reduce the number of pain signals sent to the spinal cord. The elevated threshold of nociceptors may also be related to a decrease in chemical mediators within the target tissue that either activate or sensitize nociceptors. The increased blood flow associated with temperature elevation may remove these chemical mediators and decrease nociceptor activity in the local tissue.
Tissue extensibility also increases with temperature increases.12,13 The viscosity of ground substance in collagen connective tissues such as joint capsule, ligaments, and tendons decreases. Due to the neuromuscular effects, there is increased muscle flexibility. Although these effects are seen only while the tissue temperature is elevated, the decreased perception of joint stiffness allows the patient to participate in range of motion exercises.
Ultrasound is considered a deep heating agent. It is probably the most widely used physical agent in hand therapy practice. Treatments are likely to be ineffective if the (1) ultrasound treatment is not focused on the correct structure, (2) dosing parameters are incorrect, and (3) transducer is moved too quickly. Electrical energy is converted into mechanical energy (sound propagation) via the piezoelectric effect. A ceramic or quartz piezoelectric crystal located within the transducer expands and contracts from the applied electrical current (Fig. 117-2). The crystal creates an electric voltage potential that replicates the sound wave pattern determined by the selected frequency. The sound waves are initially propagated longitudinally into the target tissue and then transversely at bone or metal interfaces. These sound waves leave the transducer as a collimated focused beam similar to a flashlight. The larger the sound head, the more collimated the beam. Molecules and cells within the target tissue expand and contract to produce vibration (Fig. 117-3). The vibration increases kinetic energy of the molecules, which increases tissue temperature, provided the ultrasound was delivered in the continuous mode with sufficient intensity.14
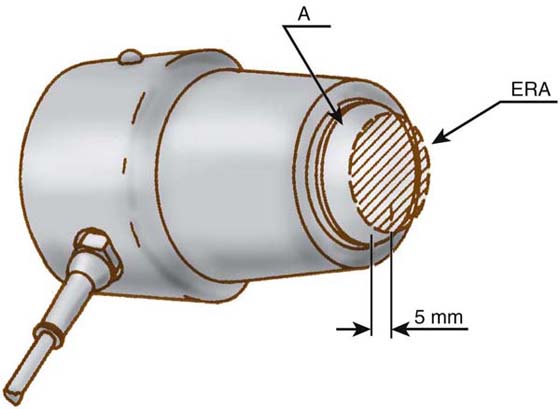
Figure 117-2 The shaded area is the piezoelectric crystal (transducer) mounted in the ultrasound applicator. The effective radiating area (ERA) of the transducer is noted. (Diagram with permission of Henley International, Sugar Land, Texas.)
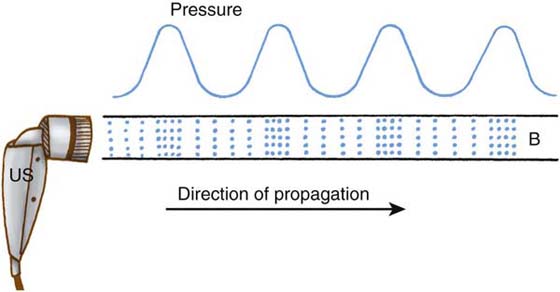
Figure 117-3 Diagram of a collimated ultrasound beam (B) coming from an ultrasound (US) applicator. The associated pressure wave is diagrammed above. Areas of increased molecular concentration (:::) are condensations. Areas of decreased molecular concentration are rarefactions (:.:). (From Ziskin MC, McDiarmid T, Michlovitz SL. Therapeutic ultrasound. In: Michlovitz SL, ed. Thermal Agents in Rehabilitation, 2nd ed. Philadelphia: FA Davis, 1990.)
Frequency determines the depth of penetration within the target tissue. The collimated beam is more divergent with the 1-MHz transducer, which will transmit sound waves through the superficial tissue layers so that the ultrasound energy will be absorbed in deeper tissues at 2 to 5 cm. Energy is absorbed in the superficial tissue layers up to 2 cm with the 3-Hz sound head. Ultrasound is absorbed within tissues with a high protein content (e.g., collagen); thus, collagen-rich connective tissues such as tendon, ligament, and joint capsule absorb sound waves well.14
Acoustic impedance occurs at tissue interfaces such as bone. Sound waves will be reflected away from the bone either in the opposite direction or transversely. Standing waves may occur if the longitudinal incident waves are superimposed on reflected sound waves. Standing waves are known as “hot spots” and have the potential to cause tissue damage. Excessive heating of the periosteum at the bone interface may also cause pain due to standing waves. If this occurs during treatment, the patient usually pulls away from the transducer and reports pain. This is a clinical sign of standing wave formation, and treatment should be modified or discontinued for that session. Standing waves can be avoided if the transducer is kept moving, if the 3-MHz frequency is selected, and if the intensity is not too high.14
The effective radiating area (ERA) is the portion of the transducer that actually produces and emits sound waves. The beam nonuniform ratio (BNR) compares the maximum point intensity on the transducer (spatial peak intensity) with the average spatial intensity across the sound head (spatial average intensity). The lower the BNR is, the more evenly distributed the energy from the transducer. Optimally, the BNR should be 1 : 1, but manufacturing guidelines require the BNR to be 6 : 1 or lower. Most units on the market today have an ERA equal to the size of the transducer face plate with the perimeter emitting less energy than the center of the face plate and a BNR of 6 : 1. The manufacturer specifications or equipment manuals supply this information. A variety of sizes of applicators are available for use, ranging from 0.5 to 5.0 cm2.
Ultrasound waves will markedly attenuate in air. Therefore, air between the applicator face and body surface must be eliminated or minimized. A coupling medium such as a commercially available water-soluble gel is spread in a layer between the applicator and skin surface. If a small irregular area is to be treated with ultrasound, a small applicator with a gel pad interface can be used.15 Coupling through a water bath16 or water-filled balloon15 is less efficient.
Therapists have the option to select continuous or pulsed-wave ultrasound treatments. With pulsed-wave ultrasound, the intensity is periodically interrupted so that no ultrasound energy is being produced during the off time in the duty cycle. Continuous-wave ultrasound produces thermal and nonthermal effects, whereas pulsed-wave ultrasound emphasizes the nonthermal effects of ultrasound because heat does not really accumulate. This is a result of the dissipation of heat by conduction during the off time of the pulse period. Duty cycles may be calculated by dividing the duration of the pulse (on time) by the pulse period (on time + off time). Therefore, if the pulse is 2 seconds of a 10-second pulse period, the duty cycle would be 20%.14 Figure 117-4 shows a typical pulsed-wave ultrasound pattern. The physiologic effects of a particular duty cycle are unclear. Low-duty cycles of 10% or 20% have been studied in chronic wound healing, but no other work has been demonstrated at high-duty cycles.
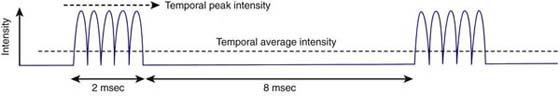
Figure 117-4 A typical pulsing pattern. The total pulse period is 10 msec. The pulse duration is 2 msec. The duty cycle is 0.20 (20%). (From Ziskin MC, McDiarmid T, Michlovitz SL. Therapeutic ultrasound. In: Michlovitz SL, ed. Thermal Agents in Rehabilitation, 2nd ed. Philadelphia: FA Davis, 1990.)
Spatial average intensity is the rate at which ultrasound energy (in watts) is delivered and averaged over the area of the transducer (in square centimeters).14 Intensity is the strength of the ultrasound treatment. All factors held constant, the greater the intensity is, the greater the tissue temperature elevation with continuous ultrasound. Most units display spatial average intensity. When using pulsed-wave ultrasound, the temporal average intensity can be calculated by multiplying the duty cycle by the spatial average intensity. For example, if the spatial average intensity selected was 2.0 W/cm2 and the duty cycle is 20%, the temporal average intensity is 0.4 W/cm2. Although the temporal average intensity over the pulse period is low, it is still 2.0 W/cm2 during the pulse period, which may be too high for some target tissues in the upper extremity. There are no clear-cut guidelines on how to select a treatment intensity, but the World Health Organization does set an upper limit of 3.0 W/cm2 ultrasound units. The correct intensity will achieve the desired goal and do no harm.
Selecting continuous- or pulsed-wave mode determines whether the thermal or nonthermal effects will predominate during the ultrasound treatment. Thermal effects are related to temperature elevation and are the same as those for the superficial heating agents discussed previously. The two primary nonthermal effects are cavitation and acoustic streaming.14 Cavitation is the formation of gas bubbles within the cells in response to the vibration within the target tissue. Stable cavitation may enhance cellular diffusion, but unstable cavitation may results in cell implosion and tissue damage.4 Unstable cavitation can be avoided by selecting the pulsed-wave mode and using lower treatment intensities in the continuous-wave mode. Acoustic streaming is the movement of fluids and gas bubbles within cells in response to vibration. Acoustic streaming is theorized to facilitate cell membrane permeability and ion exchange, which have potential tissue healing benefits.17
Ultrasound has all the same clinical indications as the superficial heating agents discussed previously, but it focuses on smaller treatment areas and may target structures greater than 2 cm in depth. Most structures in the wrist and hand can be sufficiently heated to elevate tissue temperature to a therapeutic level, but ultrasound as a deep heating agent is needed for similar indications at the forearm, elbow, and shoulder. Ultrasound may promote tissue healing first by increasing blood flow and oxygenation due to thermal effects and second as a result of the nonthermal effects. Studies of delayed-healing wounds have demonstrated that low-intensity pulsed-wave ultrasound, which emphasizes nonthermal effects, releases growth factors from macrophages, increases angiogenesis, and facilitates collagen production.17 These benefits promote the fibroplasia phase of tissue healing. During remodeling, tensile strength may increase when thermal ultrasound is combined with controlled stress exercises. Increased tissue extensibility will facilitate collagen reorganization and gains in tensile strength.
The physiologic effects of cold are for the most part the exact opposite of those associated with thermotherapy. Table 117-3 reviews these comparisons. Cold is primarily used after acute injury or surgery to control pain and edema. The normal patient response to cold in terms of sensory changes is the feeling of cold followed by burning, pain/discomfort, tingling, and numbness.18 Although the timing is not standardized among patients, the order of these sensory changes is consistent. It is this author’s experience that patients do not tolerate ice bags or ice baths applied directly to the hand, but do find ice applied directly to the shoulder or elbow tolerable. Commercial cold packs are more commonly used at the hand and wrist.
Table 117-3 Comparisons of Physiologic Effects of Heat Versus Cold
The application of cold results in decreased blood flow and vasoconstriction in response to decreases in tissue temperature.19,20 Vasoconstriction is an immediate response to attempt to regulate temperature. There is a decrease in blood viscosity, which further reduces blood flow. Prolonged cold exposure such as in an ice bath below 10°C (50°F) may result in a reflex vasodilation called the hunting response.20 This response is usually observed in the skin, but it may occur in deeper tissues as well.
There may also be a decrease in the inflammatory response if cold is applied immediately after injury.19-22 Due to the decrease in blood flow, the chemical mediators associated with inflammation such as histamine and prostaglandins may not be able to circulate and promote vasodilation. Decreased blood flow also reduces metabolic activity, including the synthesis of prostaglandins that mediate inflammation. There is also a decrease in oxygen uptake within cells due to reduced metabolic demand. With prolonged cold exposure, this may result in hypoxia and tissue damage, as is observed in frostbite and peripheral nerve palsy.20,21
The mechanisms of pain modulation are similar to those with heat based on the gating mechanism and counterirritant theory.10,11 In addition, decreased blood flow may lessen the inflammatory response and further modulate pain. Reduced edema means that there will be less tissue distention and therefore less pain. A decrease in the circulating chemical mediators that promote inflammation and activate or sensitize nociceptors will also modulate pain.
The patient may report an increase in joint stiffness or at least the perception of increased stiffness after the application of cold. The viscosity of the ground substance will increase with decreases in tissue temperature. Although muscle tension will decrease in response to cold, which may reduce muscle spasm, guarding, or spasticity, the muscles will be less flexible to movement.23-25 With cold exposure, decreased nerve conduction velocity26 and muscle spindle and Golgi tendon organ activity may also contribute to decreases in pain.
There are several cold sensitivity symptoms and conditions that need to be addressed before applying cold to hand and upper extremity patients. It is normal for the skin underlying the cold source to become red during cryotherapy because of hyperemia or vasodilation of skin blood vessels. The entire area should be red. However, if wheals or hives develop as a result of a histamine reaction, this is an allergic reaction to cold. It is called local cold urticaria, and the wheals are raised with red borders and blanching in the center.20,21 They are usually warm to touch (Fig. 117-5). This is the most common cold sensitivity condition observed, and the cold treatment should cease if a wheal develops.
Systemic urticaria is a systemic allergic reaction that results in flushing of the face, increased heart rate, and a sharp decrease in blood pressure that can lead to syncope or lightheadness.20 Raynaud’s disease or phenomenon is a vasospastic disorder that occurs in response to cold. It usually affects the fingers, and in response to cold, the fingers will blanch (pallor), become blue/purple (cyanosis), turn bright red (rubor), and then return to normal color. This cyclic response is associated with autoimmune disorders such as lupus erythematosus and rheumatoid arthritis. It has also been associated with nerve compression syndromes and trauma.20 Patients likely know whether they have ever had any of these responses to cold, so be sure to ask about them when obtaining your patient’s history during the clinical examination.
Cryoglobulinemia is associated with autoimmune conditions, chronic liver disease, multiple melanoma, and infections. In response to cold exposure, an abnormal blood protein forms a gel, decreasing blood flow and resulting in ischemia and eventually gangrene.20 Paroxysmal cold hemoglobinuria20 is a condition in which hemoglobin is released and lysed red blood cells can be identified in urine, although the urine is not red. Anemia develops in the patient, which may be mild or severe. Although neither of these cold sensitivity problems are common, therapists should ask patients about these conditions when obtaining their history and monitor the patients’ response to cryotherapy carefully because the sequelae can be severe.
Electrotherapy may be used for pain modulation, for muscle re-education, and to facilitate tissue healing. Electrotherapeutic currents are delivered across the skin using surface electrodes to disperse the current within the target tissues. There are a variety of electromedical devices on the market that offer one or more therapeutic currents, current modulators, and channels. The clinical line-powered units typically offer more choices, so that one device could be used for a variety of clinical indications. Battery-powered portable units also offer a range of therapeutic currents and current modulators, but they are limited to two channels and have fewer options in current selection. Portable units may be used in the clinic, but also by patients at home if they have appropriate durable medical equipment (DME) coverage with their insurance plan. Portable units may be used during functional activities, whereas line-powered units are limited to the area near the wall plug. Portable units may be used for pain modulation while the patient performs “controlled” therapeutic exercises or activities or to augment muscle strength during functional activities such as reaching.
Electrodes are available in a variety of sizes, shapes, and materials. The success of electrotherapy begins with appropriate electrode selection, preparation, and placement. Electrodes for the hand and upper limb need to be able to conform to the target area and serve as an efficient conductor of the electrotherapeutic current. Some electrode materials are better conductors of electricity and have less resistance to current flow at the skin–electrode interface.
Metal electrodes, usually tin, insulated with a rubber or plastic backing on the side that does not contact the skin are good conductors of current with a wet sponge interface. However, this type of electrode does not conform well to the body and is often not available in a small size needed for the wrist and hand. The sponges are another concern in that the same sponges should not be used among different patients because they are a source of bacteria if not properly cleaned and stored. For this reason alone, most clinics do not use this type of electrode.
Flexible carbon and rubber electrodes use an aqueous coupling gel similar to ultrasound. These electrodes need to be secured with tape or Nylatex wraps (Chattanooga Group, Chattanooga, Tennessee). They are fine to use in the clinic, if properly cleaned and maintained, but are cumbersome for patients to use at home. This is especially true for patients with hand injuries who may have only one hand that can prepare the electrodes.
A variety of commercially available electrodes for single or multiple use have been developed. These electrodes are coated with an adhesive polymer and come in a variety of shapes and sizes. Most have a pigtail coming from the electrode so that the lead wire from the electrotherapy unit can easily be secured to the electrode. The reusable electrodes may be used for multiple applications provided that adequate skin preparation and electrode cleansing are performed. The single-use electrodes have an adhesive quality similar to that of an adhesive bandage, which is a more appropriate choice for patients with sensitive skin and those who experienced an adverse reaction to another electrode. They are meant to be used once and thrown away. The adhesive polymers typically have more resistance to current flow than the wet sponge or aqueous gel, but their ease of application makes them a favorite among therapists and patients. The adhesive polymer electrodes enhance compliance. They are also suited to clinical use (provided they are not shared among different patients to maintain infection control standards and prevent cross-contamination among patients). Patients may have adverse reactions (allergic reaction) to the adhesive polymers. If this occurs the electrodes should be replaced with another type, and the reaction should be reported to the electrode vendor (Fig. 117-6, online).
Figure 117-6 There are a variety of electrode options available for clinical application. A, Reusuable electrodes come in a variety of shapes and sizes. They are secured to the patient with an adhesive polymer backing. The pigtail allows the leadwire from the electrotherapy unit to be easily secured. This type of electrode can easily be used on the hand and upper extremity due to the smaller sizes. B, Metal and sponge electrode that attaches to the target tissue using the Nylatex wrap. These electrodes usually do not confirm well to the hand and upper extremity even in a smaller size. C, Flexible carbon electrodes that can conduct electricity via a wet sponge or ultrasound transmission gel. They may be secured with paper tape or Nylatex wrap. These work best on the proximal upper extremity. D, The bow-tie shaped resusable electrode conforms well to the hand and may be placed circumferentially around the digits.
Skin preparation in the area where the electrodes will be placed is essential to promote decreased skin resistance to current flow. It should make the treatment more comfortable because less current should be needed to achieve the desired clinical response. The skin should be cleansed with soap and water or rubbing alcohol to remove dirt, dry skin cells, oils, and lotions. This is important for all types of electrotherapy, but it is considered essential when performing iontophoresis. The alcohol swab is even packaged with the iontophoresis electrodes. If the skin is hairy and the electrodes cannot make uniform contact, the hair should be clipped or shaved. This author prefers trimming the hair with scissors rather than shaving. Shaving creates skin irritability, which may be exacerbated by the electrotherapeutic current.
There are three types of therapeutic currents: direct, alternating, and pulsed. The therapeutic current is not determined by the power source of the electromedical device. Oscillators and potentiometers within the electromedical device create the type of therapeutic current to be used for the treatment.
Direct current (DC) is defined as the unidirectional flow of charged particles that continues when the circuit is closed and stops when the circuit is opened.27 The direction of flow is determined by the polarity selected. This type of current is used primarily for iontophoresis in hand therapy, but it may also be used for wound healing and to stimulate denervated muscle. The recognition of polarity is important when a treatment method requires a specific polarity such as iontophoresis. The negative electrode, termed the cathode, attracts positive ions from the tissue, and the anode, or positive electrode, attracts negative ions from the tissue.
An alkaline reaction will build up under the cathode, and an acidic reaction will build up under the anode; both may cause a skin reaction under the electrodes. The typical skin reaction is redness termed hyperemia, but a full-thickness burn is always a potential. The most common adverse reaction is blister formation under the electrode, especially the cathode, because the alkaline reaction is more caustic to human skin. The commercial electrodes used for iontophoresis contain buffering agents to minimize the pH changes under the electrodes and therefore minimize risk of skin irritation or burn. Therapists should consult their vendors to determine whether the buffering agents work for both the acidic and alkaline reactions.
The size of the electrode will also reduce the risk of skin irritation or burn. The electrode should be large enough to cover the target tissue and disperse the current evenly. If the electrode is too small (≤1 cm in diameter), the current cannot be dispersed and irritation or a burn is more likely. The manufacturers of iontophoresis electrodes have already accounted for this issue in the sizing of their electrodes. Therapists should avoid making “homemade” electrodes when using any type of electrotherapy, but especially with DC. Your electrode made out of aluminum foil and gauze does not contain a buffering system and is not approved by the U.S. Food and Drug Administration (FDA). The foil may not adhere well, and “hot spots” where the current density is too high may develop. The FDA approves all electrodes, so if there is a problem with a patient, you will be glad that you used an FDA-approved electrode.
Alternating current (AC) is the bidirectional flow of charged particles.27 It is typically represented as a sinusoidal waveform, but it can be symmetrical or asymmetrical. A common example of a stimulator using AC is interferential current (IFC). IFC is typically used for pain modulation. IFC uses a high carrier frequency to overcome skin resistance to current flow, so it is usually considered a comfortable form of electrotherapy.
AC may be modulated and acts in a fashion similar to a biphasic pulsed current. Burst-modulated AC current or “Russian” stimulation is used for muscle strengthening. Bursting of current results in phases of current delivery that act similarly to individual pulses, but the current density is higher than with most pulsed (see next section) currents. Although commonly used for larger muscle groups such as the quadriceps and hamstrings, this type of current may be comfortably used for neuromuscular re-education in the hand and upper extremity.
Most electrotherapy units that have IFC will also have the burst-modulated AC for muscle strengthening. This may be confusing to therapists, but is an easy way to remember that IFC is used for pain modulation, so there is no way to establish on/off time ratios for muscle contractions with the IFC controls.
Pulsed current is the flow of charged particles, unidirectional or bidirectional, that is delivered in finite periods of time, usually microseconds, before the next electrical event. The time between electrical events or pulses does not equate to on or off time cycles. Pulsed currents are primarily used for pain modulation or neuromuscular re-education. On an oscilloscope, the visual representation of the pulse is called a waveform.27
Waveforms can be monophasic or biphasic, depending on the direction of current flow. With monophasic waveforms, polarity does not change; current flow is unidirectional. In biphasic waveforms, each pulse has two phases that cross the isoelectric line of polarity; current flow is bidirectional. Biphasic pulses can be symmetrical or asymmetrical in shape (Fig. 117-7). Symmetrical biphasic waveforms are balanced; the area within each phase is equal but opposite polarity. The phases look like mirror images. Asymmetrical biphasic waveforms can be balanced or unbalanced.27 Therapists want to use only balanced asymmetrical biphasic waveforms in clinical practice. The manufacturer’s specifications located on either a product information sheet or in the user’s manual will indicate that an asymmetrical biphasic waveform is balanced with the phrase “zero net DC effect.” This just means that even though the phases may not look equal, the total electrical charge in each direction is the same. In an unbalanced waveform, this is not the case, so the sensation under one stimulation electrode will be more intense than under the counterpart electrode. Reputable electromedical device vendors provide only balanced asymmetrical biphasic waveforms.
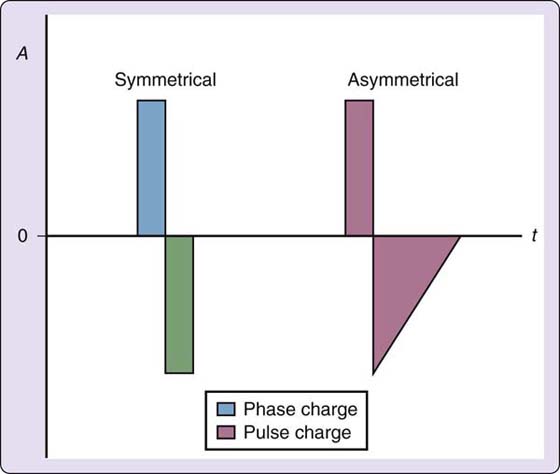
Figure 117-7 Biphasic pulses can be symmetrical or asymmetrical in shape. Pulse charge combines both phases of the pulse and phase charge keeps the phases separate. In a symmetrical biphasic pulse, the pulse charge is the same in each phase. A, current amplitude in milliamps; t, time in microseconds; 0, the zero point, or isoelectric line.
Amplitude is another term for intensity. Both terms are used interchangeably in the peer-reviewed literature, on devices, and among clinicians. The therapist and/or the patient has full adjustability of the amplitude controls on any device. When the intensity of one phase is measured, the highest point of the phase is called the peak amplitude. When intensity measurements are taken for biphasic pulses, the distance from the isoelectric line of one phase to its peak and the distance between the isoelectric line of the other phase to its peak are combined for a peak-to-peak amplitude. The device manual will provide information on whether you are setting peak or peak-to-peak amplitude (Fig. 117-8).
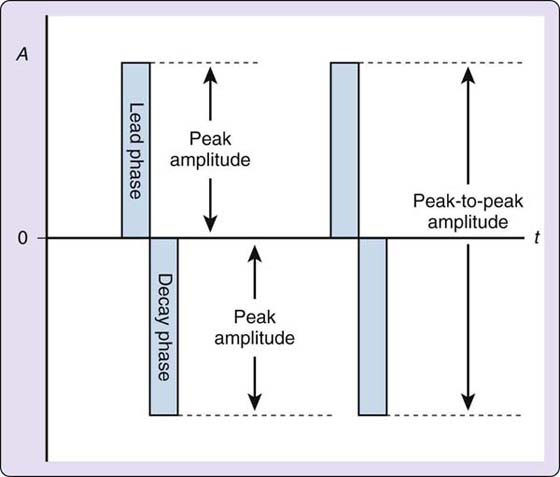
Figure 117-8 Peak amplitude versus peak-to-peak amplitude. A, current amplitude in milliamps; t, time in microseconds; 0, the zero point, or isoelectric line.
Phase duration is the time that elapses from the beginning of the phase to the end of the phase. If the waveform is biphasic, then the two phases, the lead and decay phases, would constitute the pulse. Pulse duration is the time that elapses from the beginning of the pulse to the end of the pulse. Pulse duration is also known as pulse width, but because the unit of measure is in microseconds (time), duration is the more appropriate term. Phase or pulse duration exists only for pulsed current units and it is either preset or fully adjustable within the unit (Fig. 117-9). For clinical applications, a device with a fully adjustable pulse duration is preferred. Check the device manual to determine whether you are setting the phase or pulse duration because this varies among units. Many muscle stimulators have the therapist set the phase duration.
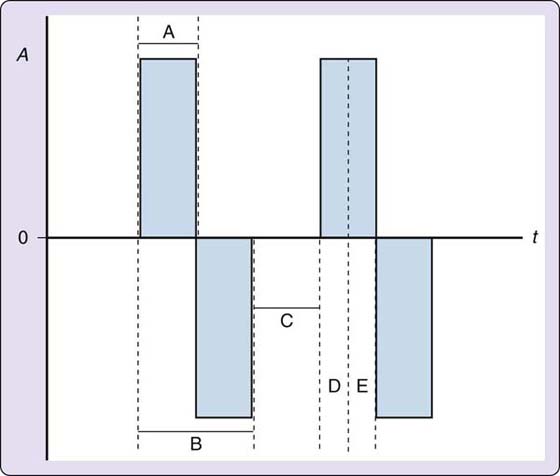
Figure 117-9 Time-dependent characteristics of a pulse or waveform. A, current amplitude in milliamps; t, time in microseconds; 0, the zero point, or isoelectric line. A, phase duration; B, pulse duration; C, interpulse interval; D, rise time; E, fall time.
There may be intervals of time between phases called interphase intervals. An interpulse interval is the time between pulses. As the frequency of pulses increases, the time between pulses will need to decrease to accommodate the increase in pulses. The time that it takes the leading edge of a phase to go from the isoelectric line to peak amplitude is known as the rise time, and the time that it takes the phase to return to the isoelectric line is called the decay of fall time (see Fig. 117-9).
Pulse charge is both amplitude and time dependent. It is considered the “power of the waveform.” In other words, there is a certain level of amplitude and pulse duration that must be achieved to produce the desired clinical response in addition to frequency. Some electromedical devices allow the therapist to determine the pulse charge in microcoulombs (see Fig. 117-7).
Frequency is also known as pulse rate on pulsed-current generators and as beat frequency on IFC units. Essentially, frequency is the number of pulses (pulsed current), beats (IFC), and cycles (AC) that are delivered in 1 second. If the frequency is set at five pulses per second (pps), then the interpulse interval times are longer than if the frequency was set at 100 pps.
Pulse charge is determined by pulse duration and amplitude. Thus, frequency, pulse duration, and amplitude are the three primary parameters that need to be manipulated to produce one of three clinical responses: (1) perceptible tingling, (2) muscle twitching, and (3) tetanizing muscle contractions. Devices that to not deliver pulsed current like IFC will rely on frequency and amplitude to achieve the clinical response to treatment.
Many electrotherapy devices have a built-in ramp-up to gradually increase to peak amplitude or clinical response. This prevents a startle response in the patient and typically makes the treatment more comfortable. Some muscle stimulators have controls to allow the therapist to establish the appropriate ramp-up and ramp-down to ease in and out of tetanized muscle contractions.
Other current modulators will modify amplitude, pulse or phase duration, and frequency during continuous sensory-level stimulation (perceptible tingling) to prevent sensory receptor accommodation. If the tingling response is held constant, eventually the sensory receptors in the target tissue will perceive the unchanged stimulus and will no longer fire in response to the electrical stimulus. Current modulators minimize the risk of this occurring during a treatment session for pain modulation. Portable pulsed-current generators, commonly called transcutaneous electrical nerve stimulation (TENS) units, have several current modulators that may work alone or in conjunction with another. IFC units also have current modulators called scan, which is amplitude modulation, and sweep, which is frequency modulation. Current modulators never increase the parameters established by the therapist; they only modify the parameters by decreasing from the selected levels (Figs. 117-10 and 117-11).
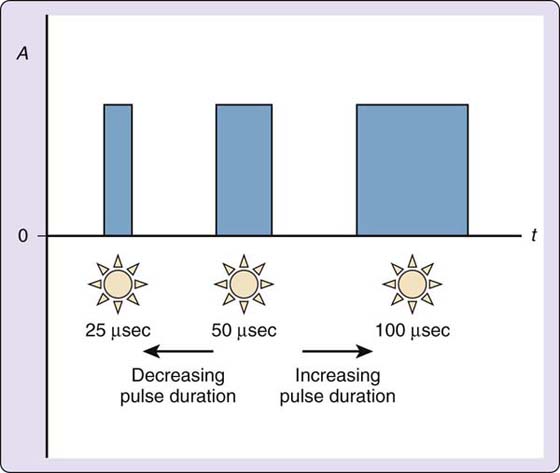
Figure 117-10 Pulse duration modulation. A, current amplitude in milliamps; t, time in microseconds; 0, the zero point, or isoelectric line.
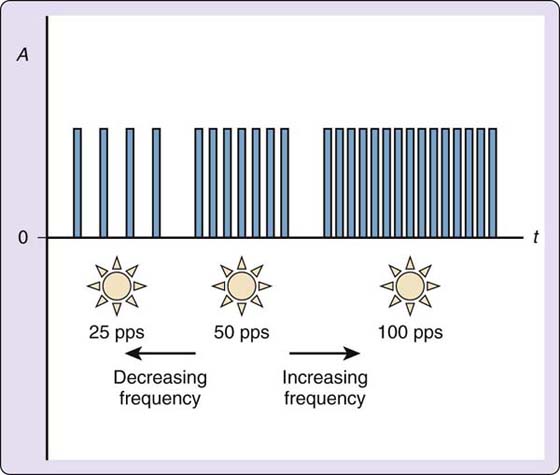
Figure 117-11 Frequency modulation. A, current amplitude in milliamps; t, time in microseconds; 0, the zero point, or isoelectric line.
In many applications of therapeutic current, it is desirable to alternate periods of electrical stimulation with periods of no stimulation. Many electrotherapy units allow the therapist to establish on and off time cycles. This is a requirement for neuromuscular electrical stimulation. A typical cycling for muscle stimulation for strengthening is 10 seconds of stimulation followed by 50 seconds of rest. This rest period is to minimize muscle fatigue.
Box 117-1 outlines the precautions and contraindications for the use of electrotherapy. Therapists should carefully review the patient’s history to determine that it is safe to use electrotherapy. Adverse reactions to electrodes are the most common problem, so the patient’s skin should be carefully inspected before and after treatment.
Electroanalgesia is a general term to describe the outcome of using electrical stimulation for pain modulation. TENS has been commonly used to describe pain suppression with electrotherapy since Melzack and Wall11 proposed the gate-control theory in 1965. Three modes of stimulation have been described to promote electroanalgesia.27 Table 114-3 reviews the common parameters used for each of the levels and the theoretical level of pain modulation.
Sensory- and motor-level stimulation are commonly used to modulate peripheral nociceptive and/or neurogenic mediated pain.28 Electrode placements are on or near the site of the painful tissue. Sensory-level stimulation, also known as high rate or conventional TENS, creates a clinical response of perceptible tingling. Motor-level stimulation, also known as low rate or “acupuncture-like” TENS, creates a clinical response of muscle twitching. At low current amplitudes, motor-level and sensory-level stimulation are very similar. The underlying mechanism for reducing pain is that the electrical current will stimulate large primary sensory afferents that may block or “gate” impulses from primary nociceptive (Ad and C) fibers. Patients typically have an immediate response to the stimulation and maintain the analgesic effect during the treatment and for a short time thereafter.28,29
Motor-level stimulation may be used at higher current amplitudes to cause vigorous muscle twitching. In this situation, electrodes are typically placed over motor points, peripheral nerves, or segmental nerve roots that correlate with the site of pain. A motor point is where the motor nerve branch pierces the muscle belly for innervation. Vigorous muscle twitching may be uncomfortable, so it is not recommended for acutely painful, irritable conditions. This author finds it most beneficial for pain of muscular origin. Higher amplitude motor-level stimulation may activate central mechanisms of pain modulation, including the release of endogenous opiates.28,29
Treatment duration in the clinic for both motor- and sensory-level electroanalgesia is usually 20 minutes; however, if used at home, electroanalgesia may be used as much as is needed to control pain. Patients can use it more or less depending on the carryover between breaks from stimulation and the pain intensity. There is little residual analgesic effect, but this may be condition and patient dependent. Sensory-level stimulation may be incorporated into a controlled exercise program as demonstrated by Rizk30 and Cannon31 with patients with shoulder adhesive capsulitis and flexor tenolysis, respectively. The twitching muscle contractions used in motor-level stimulation are not conducive to being combined with controlled exercise.
Pulsed-current generators referred to as TENS units and IFC units are commonly used stimulators for pain modulation. Both have current modulators as discussed previously to prevent accommodation to prolonged sensory-level stimulation. TENS units are portable and easily obtained for home use. Portable and line-powered IFC units are also available, but not all vendors carry the portable ones. The IFC unit is coded as a muscle stimulator and not a pain device, so this can be a problem with DME coverage and rental agreements. The high volt pulse current stimulator (HVPC) may also be used for acute or postoperative pain modulation. It does not have the current modulators found on the IFC or the pulsed-current generators, so it is not recommended for long-term pain control.
Noxious-level stimulation, also known as electroacupuncture, and brief intense TENS are used when other methods of pain modulation have failed and in the management of complex regional pain syndrome or centrally mediated pain conditions. The underlying mechanism for using this form of electroanalgesia is to apply a painful stimulus to target the release of endogenous opiates. This treatment is uncomfortable at first, but patients report pain relief 1 to 3 days after the treatment.28,29 The painful stimulation is applied to motor or acupuncture points until a burning sensation is perceived. There may be a small red burn left by the probe. To use this technique, a special unit is required (Fig. 117-12).
The specialized unit uses a long pulse duration in the milliseconds compared with the microseconds used on the other units. The frequency may be set very low (1–5 pps) or very high (100 pps), based on therapist’s preference. The amplitude is increased to the highest level that the patient can tolerate. These parameters more readily stimulate pain fibers rather than sensory or motor fibers, as indicated on the strength duration curves for these nerve fibers28,29 (Fig. 117-13, online).
Figure 117-13 Strength duration curves for sensory, motor, and pain fibers (C).
The primary clinical indication for NMES is to increase muscle strength by stimulating the intact peripheral nerve to achieve a muscle contraction.27 As a result of increasing strength, active range of motion increases, disuse atrophy decreases, and, if present, edema may decrease. The term functional electrical stimulation may be used if NMES is being combined with functional training, such as orthosis substitution at the shoulder, where NMES reduces a shoulder subluxation instead of a sling or brace. NMES selectively stimulates large muscle fibers, or the fast-twitch fibers, first. This is in contrast to volitional or active exercise, which first recruits the small muscle fibers, or slow-twitch type II fibers. With postsurgical immobilization, the large fibers quickly atrophy, and NMES can selectively recruit these fibers for stimulation.32 This makes NMES a valuable tool as an adjunct to active exercise for atrophy reduction and to increase strength.
Electrically elicited contractions are more forceful than volitional contractions due to the fiber recruitment order and the synchronous firing of motor units (motor neuron + muscle fibers that it innervates). As a result, they also produce more fatigue.32 Patients will not be able to complete as many electrically elicited contractions during an NMES session compared with volitional exercise. Higher frequencies also increase fatigue due to the increased firing rate of the motor units.
Pulsed or burst-modulated AC may be used for NMES.27,32,33 There are no studies that indicate that one type of electrotherapeutic current is better than another for hand and upper extremity muscles. Therapist preference and equipment availability are the likely decision-making factors. The waveforms used with pulsed-current generators may be monophasic or biphasic (asymmetrical or symmetrical, respectively). Some vendors indicate that monophasic and asymmetrical biphasic pulsed-current waveforms are more effective for small muscles in the hand and forearm. In this author’s experience, the type of current has not had much of an impact on treatment outcome.
Frequency needs to be set within the range of 30 to 80 pps to achieve a tetanic contraction.27,32,33 Typical frequencies used for the upper extremity muscles are 35 to 55 pps. It is important to establish ramp up and ramp down times to create a smooth tetanic muscle contraction. Typically, a 2-second ramp up is sufficient followed by a 1-second ramp down. In patients with spasticity or high tone, longer ramp times, up and down, are recommended.32 On most units, this ramp time is included in the on time or contraction time of the muscle, so therapists may need to increase the on time to account for the ramp times to allow sufficient contraction time at peak amplitude.
The current amplitude needs to be set high enough to allow a strong tetanic contraction.27,32,33 The limiting factors are the patient’s tolerance and fatigue. The patient needs to be informed that the strongest contractions produce the greatest strength gains. Ideally, the patient should perform 10 strong quality contractions; therefore, depending on muscle fatigue, the on/off time cycle ratios need to be between 1:3 and 1:12.33 Therapists should look for units with the greatest range of customization to allow appropriate rest between contractions.
On a pulsed-current unit, ideally there should be a fully adjustable pulse duration setting. The variable pulse duration allows for some fine tuning of motor unit recruitment, so as pulse duration is increased, more motor units may be recruited, and fewer are recruited as pulse duration is decreased. The latter is important to prevent current overflow into adjacent muscles. The variable pulse duration feature is particularly useful when stimulating reinnervated muscles after peripheral nerve injuries. The units with fixed pulse durations of 250 to 300 µsec may not have sufficient pulse duration for the reinnervated muscles.
NMES devices should have two channels to allow reciprocal motion on each channel.27,33 There should also be an interrupt switch if the patient needs to stop the treatment immediately. For functional training, it is nice to have external triggers such as a heel or hand switch. These external triggers override on/off time cycles. The heel switch is particularly useful during gait training, even for the upper extremity if trying to restore arm swing.
Electrode preparation and placement are essential to achieving the desired muscle contraction. Because NMES can be uncomfortable, it is recommended that high quality, low impedance electrodes be used. The clinic should also be stocked with a variety of sizes to accommodate the size range of the upper extremity muscles from the large biceps to the small opponens pollicis. The electrodes and configuration should “fit” the muscle, and the electrodes should be on or near the motor point of the desired muscle (Fig. 117-14). A bipolar electrode configuration, two electrodes over the target muscle, is suitable for larger muscles of the proximal upper extremity and superficial muscles in the forearm such as the wrist extensors (Fig. 117-15). With biphasic pulsed currents, it may be necessary to apply electrodes of different sizes to the area stimulated, thus increasing current density under the smaller electrode and thereby obtaining a stronger tissue response. A monopolar configuration is recommended when several muscles are located close together as in the forearm and hand. A probe electrode works best for a monopolar electrode setup, especially during the initial application, because the probe makes it easier to locate small muscles. The electrode to complete the circuit is placed away from the target tissue, proximally on the upper extremity33 (Fig. 117-16).
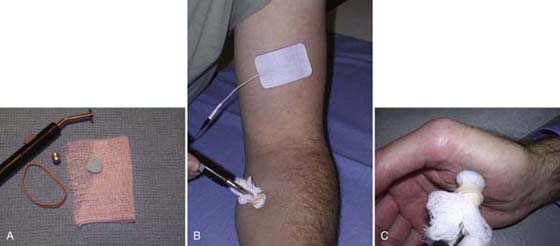
Figure 117-16 Monopolar electrode configuration. A, Probe setup. B, Probe electrode placed over motor point, and electrode to complete the circuit placed proximal to the target tissue. C, Probe electrode used to stimulate atrophied thenar muscles.
This author believes that NMES should be an “attended” treatment by the therapist to establish appropriate parameters, modify parameters as needed, and achieve a good treatment outcome. Poor outcomes result from sloppy, hastily prepared, unattended treatments. Once the parameters and electrode placement have been established, the therapist should monitor for 10 strong quality muscle contractions. Fibrillation or poor quality contractions are a sign of fatigue. This author recommends that if fatigue occurs after seven or eight contractions, the treatment for that session should be discontinued and the parameters for the next session adjusted. However, if it occurs in the first five contractions, stop the treatment and adjust the on/off time cycle to increase off time. You may be able to decrease the on time, but it will depend on the initial setting. If the on time is set for 10 seconds and you have 3 seconds allotted for ramp, you really cannot reduce the on time. If the on time is greater than 7 seconds at peak amplitude, decrease it to 7 seconds. If you used an on/off ratio of 1 : 5, increase the off time to a 1 : 8 or 1 : 10 ratio. Frequency may also be lowered, but it must stay high enough to achieve tetany, at least 35 pps.
Iontophoresis and phonophoresis are used to deliver analgesics and anti-inflammatory agents parenterally. Iontophoresis delivers ionizable substances through the skin using DC. The polar effects of DC create electrostatic repulsion of like charges (likes repel, opposites attract).34 Phonophoresis is the use of ultrasound to enhance the delivery of topically applied medications.35 The potential benefits to transdermal delivery include direct application to the target tissue, resulting in higher concentrations of medication in the target tissue, and gastrointestinal absorption or liver metabolism factors are eliminated. Injections produce higher concentrations of medication to the target tissue, but this is an invasive procedure with a potential risk of injury, and the injections are frequently painful to the recipient.35
In the late 1980s, treatment-specific DC generators with a maximum current output of 4 mA were developed with complementary electrode systems (Fig. 117-17). One electrode is called the delivery or receptacle electrode and contains the ionizable substance. The other is the return or dispersive electrode made out of adhesive polymer that completes the circuit. Today, most delivery electrodes contain a buffering agent to minimize pH changes beneath the electrode. There is no buffering agent in the return electrode. Review the electrode considerations discussed in the direct current section.
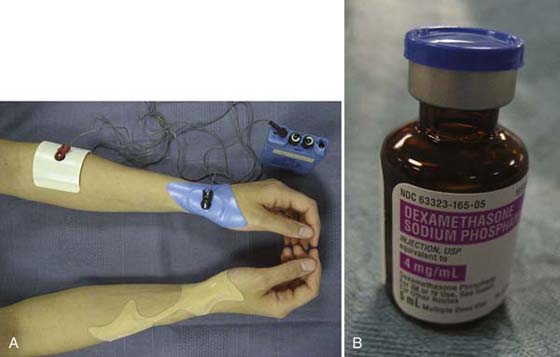
Figure 117-17 A, Iontophoresis setup for de Quervain’s tenosynovitis on the left wrist and transdermal patch without direct current on the right wrist. B, Bottle of injectable dexamethasone sodium phosphate. Note: Polarity is not indicated on the bottle. (Photographs courtesy of Marilyn Lee, OTR/L, CHT.)
Iontophoresis is commonly used to reduce pain associated with tendinopathies such as tennis elbow and de Quervain’s tenosynovitis, calcific tendinitis, and painful scar. Treatment outcome may be influenced by current amplitude, treatment duration, pH changes under the electrodes, correct polarity, drug concentration, and using more than one substance within the delivery electrode.
The typical treatment is to deliver a current dose of 40 mA/min.34 Assuming the patient can tolerate a current amplitude of 4 mA, the treatment time to deliver the current dose of 40 mA/min would be 10 minutes. Not all patients can tolerate this current dose, so longer treatment times may be necessary. It is unclear whether 4 mA is the current amplitude necessary to drive electrostatic repulsion, and there is no clinical measure to determine how much substance is delivered in the 40 mA/min. In most cases, the electrodes remain saturated after treatment.
Dexamethasone sodium phosphate (4 mg/mL), a common anti-inflammatory medication in injectable form, is commonly used for the treatment of tendinopathies. It is not clear whether this is an appropriate concentration for iontophoresis. The cathode is used to deliver the dexamethasone sodium phosphate because an animal study revealed larger deposits of drug by-product within the target tissue.28 Before 1992, therapists were using the anode or combining the dexamethasone sodium phosphate with lidocaine to mimic an injection and switching the polarities midway through the treatment. Very few clinical data exist regarding the appropriate parameters of iontophoresis, especially related to the medications.
Less is known about the effective parameters for phonophoresis. Transmission quality of the ultrasound is affected by the coupling medium used with the drug.36 Therapists should follow application guidelines used for ultrasound treatments. Byl35 completed an extensive review of phonophoresis that may be useful for additional reading. Recent reviews indicate that phonophoresis is not a superior treatment to iontophoresis37 or ultrasound alone.38
LASER is an acronym for light amplification by stimulated emission of radiation. LLLT received FDA approval in the United States in 2002 for the treatment of pain associated with carpal tunnel syndrome and in 2004 for iliotibial band syndrome. The infrared lamp on LLLT units was approved for the management of minor muscle and joint pain in 2003.39 It is currently billed as an unlisted modality. LLLT generates light of a single wavelength that is coherent (travels in a straight line), monochromatic, and polarized (concentrated beam). It produces the proposed physiologic changes by creating photochemical reactions within cells. It does not produce a thermal or sensory effect.39,40
It is theorized that the light applied to the target tissue is absorbed by chromophores within cellular organs, especially mitochondria. A chromophore is part of a molecule responsible for color. Hemoglobin, lycopene, and beta-carotene are chromophores. The proposed physiologic effects of LLLT include (1) increased adenosine triphosphate production, (2) increased oxygen consumption, (3) decreased prostaglandin synthesis, (4) decreased edema, (5) decreased cell membrane permeability of neurons, (6) increased levels of serotonin and endorphins, (7) increased lymphatic flow, and (8) increased skin circulation. Therefore, the clinical indications are to reduce pain and inflammation and promote tissue healing associated with musculoskeletal conditions such as carpal tunnel syndrome and tennis elbow.39-43
Because this is a relatively new physical agent in the United States, the application and treatment parameters are not well standardized. The gallium aluminum arsenide infrared semiconductor (GaA1As) (830-nm wavelength) is most commonly used. Gallium arsenide (GaAs) (910-nm wavelength) and helium neon (HeNe) (632.8-nm wavelength) are also used in therapy. Some protocols call for the use of more than one laser for treatment. All these lasers fall in the near-infrared region of the electromagnetic spectrum. The longer wavelength penetrates deeper, but is more divergent.39,40
LASER should not be used over an area of malignancy or in pregnant women, and patients should avoid using products containing photosensitizers. Therapists should avoid irradiation of the retina, anterior neck, endocrine glands, hemorrhagic lesions, epiphyseal plates, sympathetic ganglia, vagus nerve, and mediastinum. It is not recommended for patients with epilepsy, decreased sensation, fever, or infection. It is recommended that both the patient and the therapist wear goggles during treatment.40
Therapists should consider the following parameters during treatment and for documentation: treatment location and surface area treated, dose (expressed in J/cm2), power (in watts), treatment duration (seconds), type of laser, frequency, pulsed- or continuous-wave mode, and, of course, patient response. The literature for this agent is quite limited for treatment applications and outcomes, and this author has limited experience in using laser. Proponents of LLLT will need to educate their colleagues and conduct clinical research to determine the most effective treatment parameters.
This section focuses on the application of physical agents in clinical practice. The decision to use a physical agent to treat impairments identified during the examination process is influenced by patient factors (other than the impairments), clinic factors, and administrative considerations. The components of clinical reasoning to reach a determination about the use of a physical agent include the following.
What are the results of the patient examination? The results of the examination will indicate the list of patient impairments that may be treated with physical agents. The tests and measures performed will serve as baseline values to determine treatment effectiveness and outcome. Most importantly, examination results should reveal any comorbidities and other patient conditions that may make the use of a physical agent unsafe or suggest that the physical agent should be used with caution. Finally, the therapist will develop a hypothesis as to the source(s) of the present impairment(s). Using sound clinical reasoning and experience, the therapist can determine the physiologic appropriateness of physical agent use.
What is the expected duration of this episode of care (visits/week, number of weeks)? Insurance copayments are on the rise for therapy visits, so many patients want to reduce their number of clinic visits to save money. If the patient is not going to be seen more than once weekly and if only followed for a short duration, the use of a physical agent in the clinic may not be suitable to achieve goals. In this case, emphasis should be placed on physical agents that may be used at home.
What physical agent equipment is available to me? Most therapy clinics are well stocked with traditional physical agents such as cryotherapy, superficial heat agents, ultrasound, and electromedical devices. Insurance reimbursement for these agents is low or none. There is a lack of evidence regarding the use of physical agents in clinical practice for all impairments, so it would be difficult to support the purchase of new or additional equipment to supply all types of agents. If considering a unit for home use, the therapist needs to determine whether it is appropriate to use the agent at home, and if so, he or she needs to find out whether the patient has DME coverage.
What other treatments do I plan to incorporate into my plan of care? Some physical agents may be used to treat more than one impairment, so agents that have multiple indications may be best suited for patients with more than one impairment. Other therapeutic interventions may be combined with the use of a physical agent such as “heat and stretch.” Clinical reasoning and experience will enhance physical agent selection.
What is the evidence for best practice in the current literature? Although it is presented last, it is certainly not the least important. There is a growing body of systematic reviews and meta-analyses in the literature that may support or refute the use of a physical agent for a particular impairment. Therapists need to continue their professional development to learn how to seek evidence of best practice and then need to carry it out on a regular basis (see Chapter 143).
The use of physical agents for pain modulation is the most common indication in hand therapy practice. Every physical agent presented in this chapter has the ability to reduce pain, including NMES to reduce shoulder subluxations.44,45 Figure 117-18, online, is a clinical decision-making tree that puts the information presented in the previous section into practical use. After a comprehensive assessment of pain, the therapist can determine the source of pain mediation28,46 (see Chapter 114). Physical agents appear to work best on peripheral nociceptive or neurogenic pain of short duration and appear to be less effective on complex pain syndromes.46-51
As previously mentioned, the selection of the agent will depend on a review of the precautions and contraindications. In the case of peripheral nerve injury, thermal agents may need to be avoided or used with caution (see Box 117-1 and Fig. 117-1).
Associated impairments may determine the selection. This is particularly true for pain associated with edema and inflammation. Cryotherapy techniques or low-intensity, pulsed-wave ultrasound may be the most beneficial22,28 (Fig. 117-19). Interstitial edema can contribute to pain caused by increased pressure on mechanoreceptors or through chemical mediators located within the inflammatory exudate that sensitize or activate nociceptors.52 Cold, compression, elevation, active exercise, and retrograde massage have been recommended for edema management.52-54 Whirlpools, cold or warm, should not be used on the injured hand because the dependent position may increase edema.
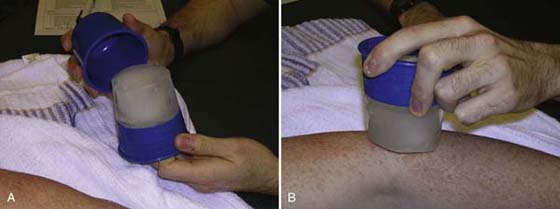
Figure 117-19 A, Commercial ice massage cup. Protects therapist’s hands from cold and is reusable B, Ice massage to the lateral epicondyle.
Mendel and Fish55 studied the use of electrical stimulation for edema control. They concluded from all their experimental investigations that sensory-level cathodal high-voltage, pulsed-wave current, applied immediately after injury, retarded edema formation in the frog model. Griffin and colleagues56 were unable to demonstrate these findings in the edematous hand, but concluded that pneumatic intermittent compression may be beneficial. This author believes that the use of electrical stimulation to manage edema is not practical, and newer edema management techniques such as manual lymph drainage and the use of short-stretch bandages show much promise (see Chapter 63 through 65).
Low-intensity, pulsed-wave ultrasound has been shown to facilitate wound healing in chronic dermal wounds.17 An associated benefit may be a reduction of interstitial edema and pain. Low-intensity, pulsed-wave ultrasound is thought to accelerate the early stages of inflammation, enhancing wound-healing rates by promoting the release of histamine from degranulating mast cells. Increased fibroblastic activity during inflammation and early proliferation has also been documented.
The application of heat and controlled stress results in increased tissue extensibility during and shortly after the treatment is applied. This is due to the viscoelastic properties of connective tissue and the response to preconditioning.12,13 Controlled stress should be applied simultaneously with heating (Fig. 117-20). This is an ideal preconditioning technique that will allow the therapist to determine how compliant the tissues are to controlled stress.57 Depending on the target tissue, superficial or deep heat may be used. Both active and passive range of motion will benefit from preconditioning.
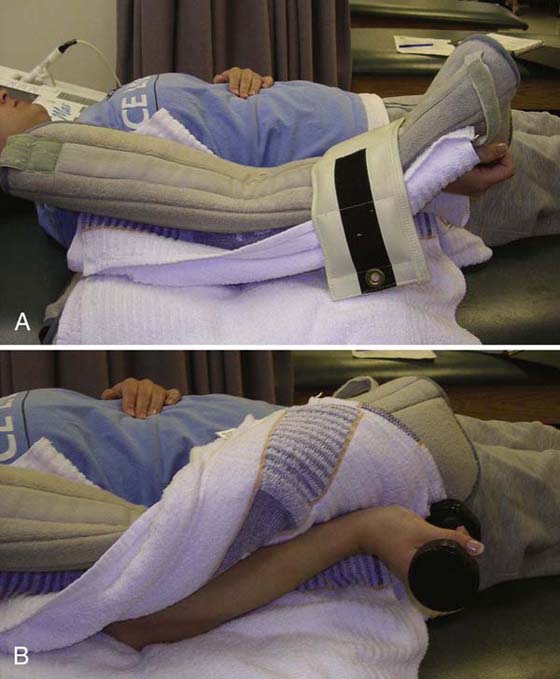
Figure 117-20 Application of heat and controlled stress with a hot pack to the elbow as elbow is being stretched into extension. A, The use of a cuff weight on the forearm allows for stress to be placed on elbow and the patient can relax. B, The use of a dumbbell is not recommended because the patient cannot relax, and stress is likely to be put on the wrist, not on the elbow.
The application of heat may also enhance motion by decreasing pain, altering contractile properties of muscle, and increasing blood flow. Heat will also decrease the perception of joint stiffness. The application of cryotherapy may enhance motion by decreasing pain and edema. Although joint stiffness may be perceived, the benefits of pain and edema reduction may improve range of motion gains. Both active and passive range of motion will benefit from decreased pain and edema.
NMES may be used to augment muscle strength to improve active range of motion. As previously mentioned, the stronger the contractions are, the greater the strength gains. NMES is particular useful in the presence of disuse atrophy and to promote learning after immobilization. NMES does not take the place of volitional exercise, but it can enhance the active range of motion gains. NMES should be discontinued when the patient is able to perform a manual muscle test against gravity with minimal resistance (F+ contraction).32,33 The emphasis on volitional exercise will promote the normal muscle physiology in terms of recruitment order and rate coding of the motor units. This may assist coordination and proprioception during functional activities.
Other than the application of heat and controlled stress for preconditioning, physical agents are not used to resolve joint contracture. Low-load prolonged stress in the form of orthotic intervention is this author’s first choice to resolve joint stiffness or tendon tightness (see Chapter 122 through 125). NMES has been used in the neurologic population to contract the antagonist to a muscle with spasticity in the hopes of reducing spasticity and increasing motion.58
Wound, scar, and tendon management are covered elsewhere (see Chapters 18, 26, and 36). The use of low-intensity, pulsed-wave ultrasound and electrical stimulation for delayed-healing wounds is well established.17,59 However, the incidence of delayed-healing wounds is quite low in hand therapy. Additional information on the use of physical agents, especially ultrasound, with tissue healing may be found in recent literature reviews.60,61
The two primary interests for rehabilitation after tendon repair are to prevent rupture or gapping and to maximize tendon gliding. Physical agents can have a role in both by preconditioning the tissue before active exercise is performed. This will decrease the drag or work of flexion requirements on the healing tendon during gliding exercise, which may enhance gliding without increasing the risk of rupture or gapping.
There has been uncertainty about the use of ultrasound over tendon repair sites, with the notion that use during the first few weeks would attenuate or damage the repair.62,63 Basic science studies using animal models (chickens and rabbits) have demonstrated that there are no adverse effects from the use of ultrasound.64-66 However, it is not practical to apply ultrasound over the area of tendon repair while sutures are still in place. Since this debate began, postoperative tendon management has progressed to more vigorous controlled stress, specifically early active mobilization. The benefits of ultrasound may not be superior to the early controlled stress alone. The use of ultrasound with recently repaired tendons is likely to remain controversial, but it does warrant further research by the hand surgeon and therapist.
In postsurgical tendon repairs, NMES can be applied to the affected muscle group to aid in strong muscle contraction to assist in tendon gliding. Proper timing is extremely important. After tendon repair, application of NMES at high intensity is the equivalent of performing resistive activities. It is necessary to wait at least 6 weeks after surgical repair before applying NMES at a level to elicit strong muscle contraction. After tenolysis, NMES may be applied earlier, at 7 to 10 days after surgery, if tendon integrity and nutrition are considered good, but care should be taken not to overexercise the involved tendon, thereby causing an inflammatory response. If the tendons are frayed, electrical stimulation must be delayed. It is good practice to discuss the introduction of NMES for tendon gliding with the surgeon who performed the tendon repair or tenolysis.
A compelling issue in practice today is the need to support our therapeutic interventions with the best available evidence. Unfortunately, there is a lack of strong evidence to support the use of physical agents with any of the impairments covered in this chapter. However, there has been an abundance of meta-analyses and systematic reviews to remind us that the evidence in the current literature is limited and weak.38,42-44,47-51,68-75
There are many reasons for the lack of evidence in the therapy literature, but even more compelling is the lack of studies on patients with hand and upper extremity conditions. Currently, the practice of hand therapy has a lack of trained researchers, limited grant funding opportunities, and a lack of support to conduct well-designed clinical trials. These factors affect our ability to report new research. Control groups are needed, but it is difficult to deny care to patients. Therapists are overburdened with the administrative paperwork that goes along with patient care, so there is little time to establish good research procedures. Therapists also do not have sufficient administrative support or consistent support from referring physicians to refer patients to studies. Patients also do not consistently participate in long-term follow-up.
Comparison of existing studies is also difficult, which adds to the problems of poor-quality studies omitted from systematic reviews. Studies that offer one-time-only treatments cannot be compared with studies that offer multiple sessions. It is difficult to compare studies when terminology used for physical agent parameters is not consistent in the field of rehabilitation or they are omitted from published papers. Comparison of impairments is difficult without establishing operational definitions for the types of pain or other measurable characteristics of impairments. Studies that address pain management for acute pain cannot be compared with studies of chronic pain management. The underlying sources of pain mediation are completely different.
Despite all these factors, further clinical research is needed to determine the value of physical agents as adjuncts to the other interventions used in the care of patients with hand and upper extremity injuries.
Several factors need to be considered in the clinical decision-making process to use a physical agent as part of the plan of care for a hand and upper extremity patient. Reviewing the results of the examination to determine how a physical agent may contribute to treatment goals and the potential contraindications or precautions will promote safe and judicious use. Although evidence is limited to support the use of physical agents, therapists can provide documentation to support their use with individual patients by collecting and re-evaluating baseline tests and measures to assess treatment outcome.
1. Michlovitiz SL, Nolan TP. Modalities for Therapeutic Intervention. 4th ed Philadelphia: F. A. Davis Company; 2005.
2. Cameron MH. Physical Agents in Rehabilitation: From Research to Practice. 3rd ed St. Louis: W.B. Saunders Co.; 2008.
3. Sunderland S. Nerves and Nerve Injuries. 2nd ed New York: Churchill Livingstone; 1978.
4. Lehmann JF, deLateur BJ. Therapeutic heat. In: Lehman JF, ed. Therapeutic Heat and Cold. 4th ed Baltimore: Williams & Wilkins; 1990.
5. Abramson DI, Mitchell RE, Tuck S, et al. Changes in blood flow, oxygen uptake, and tissue temperatures produced by the topical application of wet heat. Arch Phys Med Rehabil. 1961;42:305–318.
6. Currier DP, Kramer JF. Sensory nerve conduction: heating effects of ultrasound and infrared. Physiother Can. 1982;34:241.
7. Halle JS, Scoville CR, Greathouse DG. Ultrasound’s effect on the conduction latency of the superficial radial nerve in man. Phys Ther. 1981;61:345–350.
8. Mense S. Effects of temperature on the discharges of muscle spindles and tendon organs. Pfleugers Archives. 1978;374:159–166.
9. Michlovitz SL, Rennie S. Heat therapy modalities: beyond the fake and bake. In: Michlovitz SL, Nolan TP, eds. Modalities for Therapeutic Intervention. 4th ed Philadelphia: FA Davis; 2005:63–65.
10. Gammon SD, Starr I. Studies on the relief of pain by counterirritation. J Clin Invest. 1941;20:13–20.
11. Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150:971–979.
12. Lehmann JF. Effect of therapeutic temperatures on tendon extensibility. Arch Phys Med Rehabil. 1970;51:481–487.
13. Warren GC, Lehmann JF, Koblanksi JN. Heat and stretch procedures: an elevation using rat tail tendon. Arch Phys Med Rehabil. 1976;57:122.
14. Sparrow KJ. Therapeutic ultrasound. In: Michlovitz SL, Nolan TP, eds. Modalities for Therapeutic Intervention. 4th ed Philadelphia: FA Davis; 2005:79–86.
15. Klucinec B, Scheidler M, Denegar C, et al. Transmissivity of coupling agents used to deliver ultrasound through indirect methods. J Orthop Sports Phys Ther. 2000;30:263–269.
16. Draper DO, Sunderland S, Kirkendall DT, Ricard M. A comparison of temperature rise in human calf muscles following applications of underwater and topical gel ultrasound. J Orthop Sports Phys Ther. 1993;17:247–251.
17. Dyson M. Role of ultrasound in wound healing. In: McCulloch JM, Kloth LC, Feedar JA, eds. Wound Healing Alternatives in Management. 2nd ed Philadelphia: FA Davis; 1995:318–345.
18. Bugaj R. The cooling, analgesic, and rewarming effects of ice massage on localized skin. Phys Ther. 1975;55:11–19.
19. Enwemeka CS, Allen C, Avila P, et al. Soft tissue thermodynamics before, during, and after cold pack therapy. Med Sci Sports Exerc. 2002 Jan;34(1):45–50.
20. Michlovitz SL. Heat therapy modalities: frozen peas and more. In: Michlovitz SL, Nolan TP, eds. Modalities for Therapeutic Intervention. 4th ed Philadelphia: FA Davis; 2005:43–60.
21. Swenson C, Swärd L, Karlsson J. Cryotherapy in sports medicine. Scand J Med Sci Sports. 1996 Aug;6(4):193–200.
22. Daniel DM, Stone ML, Arendt DL. The effect of cold therapy on pain, swelling, and range of motion after anterior cruciate ligament reconstructive surgery. Arthroscopy. 1994;10:530–533.
23. Knuttsson E, Mattsson E. Effects of local cooling on monosynaptic reflexes in man. Scand J Rehabil Med. 1969;1:126–132.
24. Eldred E, Linsley DF, Buchwald JS. Effects of cooling on mammalian muscle spindles. Exp Neurol. 1960;2:144.
25. Newton M, Lehmkuhl D. Muscle spindle response to body heating and localized muscle cooling: implications for relief of spasticity. J Am Phys Ther Assoc. 1965;45:91–105.
26. Herrera E, Sandoval MC, Camargo DM, Salvini TF. Motor and sensory nerve conduction are affected differently by ice pack, ice massage, and cold water immersion. Phys Ther. 2010 Apr;90(4):581–591.
27. Section on Clinical Electrophysiology, American Physical Therapy Association. Electrotherapeutic Terminology in Physical Therapy. Alexandria, VA: APTA Publications; 1990.
28. Fedorczyk J. The role of physical agents in modulating pain. J Hand Ther. 1997;10:110–121.
29. Snyder-Mackler L. Electrical stimulation for pain control. In: Robinson AJ, Snyder-Mackler L, eds. Clinical Electrophysiology: Electrotherapy and Electrophysiologic Testing. 2nd ed Baltimore: Williams & Wilkins; 1995:285–292.
30. Rizk TE, Christopher RP, Pinals RS, et al. Adhesive capsulitis (frozen shoulder): a new approach to its management. Arch Phys Med. 1983;64:29–33.
31. Cannon NM. Enhancing flexor tendon glide through tenolysis and hand therapy. J Hand Ther. 1989;3:122.
32. Robinson AJ. Neuromuscular electrical stimulation for control of posture and movement. In: Robinson AJ, Snyder-Mackler L, eds. Clinical Electrophysiology: Electrotherapy and Electrophysiologic Testing. 2nd ed Baltimore: Williams & Wilkins; 1995:157–210.
33. Delitto A, Snyder-Mackler L, Robinson AJ. Electrical stimulation of muscle: techniques and applications. In: Robinson AJ, Snyder-Mackler L, eds. Clinical Electrophysiology: Electrotherapy and Electrophysiologic Testing. Baltimore: Williams & Wilkins; 1995:123–153.
34. Ciccone CD. Iontophoresis. In: Robinson AJ, Snyder-Mackler L, eds. Clinical Electrophysiology: Electrotherapy and Electrophysiologic Testing. Baltimore: Williams & Wilkins; 1995:335–358.
35. Byl NN. The use of ultrasound as an enhancer for transcutaneous drug delivery: phonophoresis. Phys Ther. 1995;75:539.
36. Cameron MH, Monroe LG. Relative transmission of ultrasound by media customarily used for phonophoresis. Phys Ther. 1992;72:142–148.
37. Hoppenrath T, Ciccone CD. Is there evidence that phonophoresis is more effective than ultrasound in treating pain associated with lateral epicondylitis? Phys Ther. 2006 Jan;86(1):136–140.
38. Klaiman MD, Shrader JA, Danoff JV, et al. Phonophoresis versus ultrasound in the treatment of common musculoskeletal conditions. Med Sci Sports Exerc. 1998;30:1349–1355.
39. Enwemeka CS. Therapeutic light. Rehab Manag. 2004 Jan-Feb;17(1):20–25. 56-57
40. Bukowski EL, Dellagatta EM. Electromagnetic radiation: laser, ultraviolet, and diathermy. In: Michlovitz SL, Nolan TP, eds. Modalities for Therapeutic Intervention. 4th ed Philadelphia: FA Davis; 2005:141–148.
41. Enwemeka CS, Parker JC, Dowdy DS, et al. The efficacy of low-power lasers in tissue repair and pain control: a meta-analysis study. Photomed Laser Surg. 2004 Aug;22(4):323–329.
42. Woodruff LD, Bounkeo JM, Brannon WM, et al. The efficacy of laser therapy in wound repair: a meta-analysis of the literature. Photomed Laser Surg. 2004 Jun;22(3):241–247.
43. Bjordal JM, Lopes-Martins RA, Joensen J, et al. A systematic review with procedural assessments and meta-analysis of low level laser therapy in lateral elbow tendinopathy (tennis elbow). BMC Musculoskelet Disord. 2008 May 29;9:75.
44. Chantraine A, Baribeault A, Uebelhart D, Gremion G. Shoulder pain and dysfunction in hemiplegia: effects of functional electrical stimulation. Arch Phys Med Rehabil. 1999;80:328–331.
45. Yu DT, Chae J, Walker ME, et al. Intramuscular neuromuscular electric stimulation for poststroke shoulder pain: a multicenter randomized clinical trial. Arch Phys Med Rehabil. 2004;85:695–704.
46. Fedorczyk JM, Michlovitz SL. Pain and limited motion. In: Michlovitz SL, Nolan TP, eds. Modalities for Therapeutic Intervention. 4th ed Philadelphia: FA Davis; 2005:185–206.
47. Chapman CE. Can the use of physical modalities for pain control be rationalized by the research evidence? Can J Physiol Pharmacol. 1991;69:704–712.
48. van der Windt D, van der Heijden G, van den Berg S, et al. Ultrasound therapy for musculoskeletal disorders: a systematic review. Pain. 1999;81:257–271.
49. Meyler WJ, deJongste MJ, Rolf CA. Clinical evaluation of pain treatment with electrostimulation: a study of TENS in patients with different pain syndromes. Clin J Pain. 1994;10:22–27.
50. Carroll D, Moore RA, McQuay HJ, et al. Transcutaneous electrical nerve stimulation (TENS) for chronic pain. [Systematic Review]. Cochrane Database Syst Rev. 2004;2.
51. Gam AN, Johannsen F. Ultrasound therapy in musculoskeletal disorders: a meta-analysis. Pain. 1995;63:85–91.
52. Flowers KR. Edema: differential management based on the stages of wound healing. In: Hunter JM, Mackin EJ, Callahan AD, eds. Rehabilitation of the Hand: Surgery and Therapy. St. Louis: CV Mosby; 1995:87–91.
53. Sorenson MK. The edematous hand. Phys Ther. 1989;69:1059–1064.
54. Walsh MT, Muntzer E. Wound management. In: Stanley BG, Tribuzi SM, eds. Concepts in Hand Rehabilitation. Philadelphia: FA Davis; 1992:167–177.
55. Mendel FC, Fish DR. New perspectives in edema control via electrical stimulation. J Athl Train. 1993;28:63–74.
56. Griffin JW, Newsome LS, Stralka SW, et al. Reduction of chronic posttraumatic hand edema: a comparison of high voltage pulsed current, intermittent pneumatic compression, and placebo treatments. Phys Ther. 1990;70:279–286.
57. Flowers KR. A proposed decision hierarchy for splinting the stiff joint, with an emphasis on force application parameters. J Hand Ther. 2002 Apr-Jun;15(2):158–162.
58. Glinsky J, Harvey L. Efficacy of electrical stimulation to increase muscle strength in people with neurological conditions: a systematic review. Physiother Res Int. 2007;12(3):175–194.
59. Kloth LC. Electrical stimulation in tissue repair. In: McCulloch JM, Kloth LC, Feedar JA, eds. Wound Healing Alternatives in Management. 2nd ed Philadelphia: FA Davis; 1995:275–314.
60. Michlovitz SL. Is there a role for ultrasound and electrical stimulation following injury to tendon and nerve? J Hand Ther. 2005 April-June;18(2):292–296.
61. Nussbaum E. The influence of ultrasound on healing tissues. J Hand Ther. 1998;11:140–147.
62. Stevenson JH, Pang CY, Lindsay WK. Functional, mechanical, and biomechanical assessment of ultrasound therapy on tendon healing in the chicken toe. Plast Reconstr Surg. 1986;77:965–972.
63. Enwemeka CS, Rodriquez O, Mendosa S. The biomechanical effects of low intensity ultrasound on healing tendons. Ultrasound Med Biol. 1990;16:801–807.
64. Enwemeka CS. The effects of therapeutic ultrasound on tendon healing: a biomechanical study. Am J Phys Med Rehabil. 1989;68:283–287.
65. Roberts M, Rutherford JH, Harris D. The effect of ultrasound on flexor tendon repairs in the rabbit. J Hand Surg. 1982;14B:17–20.
66. Turner SM, Powell ES, Ng CS. Effect of ultrasound on the healing of cockerel tendon: is collagen cross-linkage a factor? J Hand Surg. 1989;14B:428–433.
67. Magness J, Garrett T, Erickson D. Swelling of the upper extremity during whirlpool baths. Arch Phys Med Rehabil. 1970;51:297–299.
68. Enwemeka CS, Parker JC, Dowdy DS, et al. The efficacy of low-power lasers in tissue repair and pain control: a meta-analysis study. Photomed Laser Surg. 2004;4:323–329.
69. Vanderthommen M, Duchateau J. Electrical stimulation as a modality to improve performance of the neuromuscular system. Exerc Sport Sci Rev. 2007;35:180–185.
70. Dehail P, Duclos C, Barat M. Electrical stimulation and muscle strengthening. Ann Readapt Med Phys. 2008;51:441–451.
71. Speed CA. Therapeutic ultrasound in soft tissue lesions. Rheumatology. 2001;40:1331–1336.
72. MacAuley D. Ice therapy: how good is the evidence? Int J Sports Med. 2001;22:379–384.
73. Bleakley C, McDonough S, MacAuley D. The use of ice in the treatment of soft-tissue injury: A systematic review of randomized controlled trials. Am J Sports Med. 2004;32:251–261.
74. Andres BM, Murrell GA. Treatment of tendinopathy: what works, what does not, and what is on the horizon. Clin Orthop Relat Res. 2008;466:1539–1554.
75. Robertson VJ, Baker KG. A review of therapeutic ultrasound: effectiveness studies. Phys Ther. 2001;81:1339–1350.