Postoperative Pain and Its Management
Introduction
Postoperative pain is perceived by patients as one of the more obnoxious aspects of surgical procedures. Acute postoperative pain is a complex physiological reaction to tissue injury, visceral distention, or disease. It is a manifestation of autonomic, psychological, and behavioral responses that result in patient-specific unpleasant, unwanted sensory and emotional experiences. Until recently, surgeons and anesthesiologists did not recognize the importance of postoperative pain. This situation has changed and today pain control is considered a mandated part of the comprehensive surgical postoperative experience. In fact, various hospitals in the United States are now undertaking post-discharge surveys to determine the degree of postoperative pain control while in the hospital.
Developments in understanding the epidemiology and pathophysiology of pain have focused greater attention on a multimodal approach to the management of postoperative pain in an effort to improve quality of life, increase functionality, enhance activities of daily living, and reduce physiological and emotional morbidity. These approaches have progressed and have led to the establishment of the postoperative analgesia service or acute pain service (Nasir et al 2011). This service consists of a multidisciplinary group of clinicians specialized in pain management who apply an ever-increasing array of modalities to attenuate postoperative pain. Guidelines for the treatment of postoperative pain have been developed to provide safe and effective therapies with evidence-based recommendations (Rosenquist and Rosenberg 2003). Innovative changes in acute pain service guidelines continue to be made around the world (American Society of Anesthesiologists 2004, Australian and New Zealand College of Anaesthetists 2007, Royal College of Anaesthetists 2010).
This chapter reviews the pathophysiology of pain, examines some pharmacologic considerations, and compares the use of oral and parenteral analgesics, central neuraxial analgesics, and adjuvant pharmacotherapies. Peripheral nerve blocks (PNBs) that have application for postoperative pain relief are described, as well as some non-pharmacologic interventions. Globally, the use of PNBs for postoperative analgesia has increased dramatically with the recent utilization of ultrasound. Incorporation of this knowledge into clinical practice is the basis and rationale for effective management of acute postoperative pain. Although a large proportion of patients presently experience moderate to severe pain after surgery (Apfelbaum et al 2003, Phillips et al 2010), better understanding of the mechanisms and therapeutic options should result in improved pain control in the future.
Physiology of Postoperative Pain
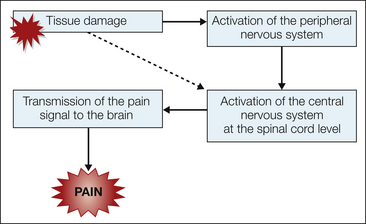
Nociception refers to the detection, transduction, and transmission of noxious stimuli. Stimuli generated from thermal, mechanical, or chemical tissue damage may activate nociceptors (Basbaum et al 2009). Nociceptors can be divided into exteroceptors, which receive stimuli from skin surfaces, and interoceptors, which are located in the walls of viscera or deeper body structures. In addition to nociceptors, the skin is richly innervated with specialized somatosensory receptors that are sensitive to other forms of stimulation. Each sensory unit includes an end-organ receptor, accompanying axon, dorsal root ganglion, and axon terminals in the spinal cord. In contrast to other special somatosensory receptors, nociceptors exhibit high response thresholds. Surgical incisions and wounds stimulate these nerve endings and produce a painful sensation in the brain (Fig. 46-1). Surgery can damage nerves and promote tissue inflammation, which leads to peripheral and central sensitization (Latremoliere and Woolf 2009, discussed fully in Chapters 1, 3, and 6).
Preclinical Models of Postoperative Pain
Understanding of postoperative pain pathways in humans has been greatly enhanced by studies of incisional pain in animal models (Brennan 2011). The rat plantar foot incision model produces both thermal and mechanical (von Frey filaments) hypersensitivity to applied stimuli for about a 10-day period (Brennan et al 1996). Moreover, the model exhibits both primary hyperalgesia (stimuli in the immediate vicinity of the incision) and secondary hyperalgesia (stimuli at some distance from the wound) (Zahn and Brennan 1999). Decreased spontaneous activity is another means of assessing pain in animal surgical models because patients with postoperative pain are less willing to move around. This has been demonstrated in rats after laparotomy (Martin et al 2004), thoracic muscle incision (Kroin et al 2006), and knee surgery (Buvanendran et al 2008).
Clinical Research Studies of Surgical Pain
Experimental incision-induced primary hyperalgesia has been demonstrated in humans (Kawamata et al 2002). After a forearm incision, mechanical hypersensitivity 3 mm from the incision site lasted for 2 days. There was also a short-lived secondary hyperalgesia.
Patients receiving flank incisions for nephrectomy had a large area of mechanical hypersensitivity to von Frey filament stimulation that lasted at least 7 days after surgery, thus indicating persistent secondary hyperalgesia (Stubhaug et al 1997). Similarly, after an abdominal incision for colonic resection, patients had a large area of mechanical hypersensitivity for at least 3 days after surgery (Lavand’homme et al 2005).
Pharmacological Treatment of Postoperative Pain
Surgical incisions evoke a series of biochemical changes in the spinal cord. The specificity of these biochemicals can be studied via their modulation by intrathecal analgesic agents. Some biochemical compounds (e.g., the neurotransmitter glutamate) are activated early in the pain response to surgery, whereas others may appear much later (Fig. 46-2). Although it was speculated that such biochemical knowledge, mostly obtained from animal studies, should facilitate the development of new analgesic or anti-hyperalgesic drugs, this optimism has been shown to be unfounded by the many recent unsuccessful clinical trials, which suggests that either our preclinical models do not closely match human pain or the drug targets are inappropriate (Woolf 2010).
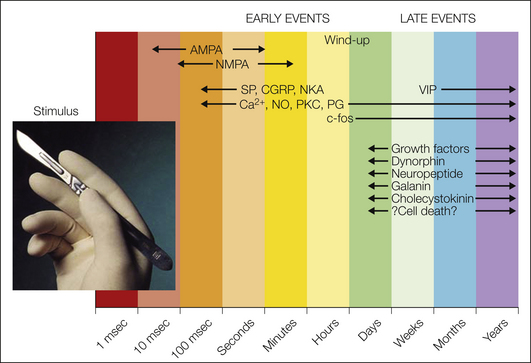
Figure 46-2 Time course of biochemical changes following surgical incision.
AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; CGRP, calcitonin gene–related peptide; NKA, neurokinin A; NMDA, N-methyl-D-aspartate; PG, prostaglandin; PKC, protein kinase C; SP, substance P; VIP, vasoactive intestinal polypeptide.
Opioids
μ-Opioid agonists (e.g., morphine) have been the mainstay in the management of moderate to severe postoperative pain for the past hundred years. They directly interact with the most powerful endogenous pain-reducing system of the body. Commercially available analgesic opioids cross the blood–brain barrier and act in both the brain and spinal cord. Opioids can also be administered epidurally or occasionally intrathecally to control postoperative pain while reducing systemic exposure.
A wide choice of opioids are available for management of postoperative pain: morphine, hydromorphone, fentanyl, sufentanil, buprenorphine, meperidine, hydrocodone, and oxycodone. Long-acting opioids have the advantage of providing a steady plasma level of the narcotic, thereby preventing the wide fluctuations in plasma levels that can lead to inconsistent analgesia. However, proper timing and dosing of the long-acting opioids are critical to obtain the optimal patient outcome.
Side Effects
The main side effects of opioids in the postoperative setting are gastrointestinal, including postoperative nausea and vomiting (PONV), ileus, and constipation; urinary retention; pruritus; central nervous system effects, including sedation and somnolence; and respiratory depression (Wheeler et al 2002). In a comparison of three opioids administered intravenously via patient-controlled anesthesia (PCA), fentanyl produced fewer adverse reactions and lower pain scores than did morphine or hydromorphone (Hutchison et al 2006). No difference was found in the quantity of opioid used when converted to equivalence. In determining the dose and drug for PCA, it is critical to examine the pharmacokinetics of the therapeutic agent. Intravenous (IV) opioids that have a short half-life may require a continuous infusion to maintain a steady plasma level in addition to the PCA mode. However, extreme caution needs to be exercised before continuous modes of opioids are administered via PCA to patients at high risk, such as those prone to sleep apnea or patients with a compromised respiratory system.
Opioid-Induced Hyperalgesia
A problem with opioid use that has recently caused some concern is opioid-induced hyperalgesia (OIH; Koppert and Schmelz 2007, Chu et al 2008). In addition to studies of this phenomenon in animal (Woolf 1981) and human experimental models, some clinical trials have also shown that perioperative opioids can increase postoperative pain or postoperative opioid consumption (Fishbain et al 2009). Therefore, even though opioids are deemed to be the “gold standard” for management of postoperative pain, paradoxically they may also facilitate postoperative pain in humans following abdominal and orthopedic surgery. Furthermore, there appears to be a positive relationship between the intraoperative opioid dose and the postoperative opioid requirement (Chia et al 1999). Consequently, what appears to be short-term tolerance to an opioid may not in fact be due to a decrease in its efficacy (pharmacological tolerance) but rather be a result of enhanced pain sensitivity (OIH) as manifested by an apparent decrease in the effectiveness of morphine. Distinguishing between these two phenomena has significant implications for the management of postoperative pain. If rapid escalation of the opioid dose in the immediate postoperative period fails to provide beneficial effects, one must consider the possibility of OIH. If the latter cause is suspected, a reduction in the opioid dose or switching to an alternative opioid (opioid rotation) may be beneficial. Moreover, the use of adjuvant drugs should also be considered because they not only contribute to an opioid-sparing effect but may also potentially result in a reduction in OIH. Knowledge in this area is just beginning to emerge, and the current information is not sufficient to propose new guidelines for the use of opioids in the postoperative period.
Opioids and Sleep Apnea and Monitoring
First recognized 35 years ago, an estimated 18 million Americans have obstructive sleep apnea (OSA). Of the people suffering from OSA, it is estimated that it is undiagnosed in 80–90%. Obesity (body mass index >29 kg/m²) has been found to be strongly associated with OSA (Benumof 2002). A known or presumptive diagnosis of OSA in a patient scheduled for surgery can influence postoperative analgesic management. Hence it is recommended that a history of nocturnal snoring and/or apnea and a history of daytime sleepiness be sought routinely in every obese adult patient preoperatively (Harrison et al 2003), in addition to evaluating a scoring system to determine whether OSA is present per recommendations of the American Society of Anesthesiologists (2006). Patients with OSA are particularly sensitive to the depressant effects of opioids, sedatives, and tranquilizers. Opioids have been shown to increase sleep and decrease arousal mechanisms. In a patient without OSA, the hypoxemia and hypercapnia that ensue following the use of opioids and other sedatives trigger the carotid chemoreceptors and the respiratory receptors of the brain stem to increase respiratory drive. However, in individuals with OSA, this protective physiological response is particularly vulnerable to the effects of opioids and other sedatives. Therefore, in these individuals, it is recommended that opioid analgesia be avoided and a multimodal analgesic regimen that includes regional analgesia be used during the postoperative period. In addition, it is important that patients continue their continuous positive airway pressure settings during the perioperative period and that oxygen saturation be monitored more frequently.
Respiratory Depression and Opioids
The Anesthesia Patient Safety Foundation (APSF) believes that clinically significant drug-induced respiratory depression (oxygenation and/or ventilation) in the postoperative period remains a serious patient safety risk factor that continues to be associated with significant morbidity and mortality. The APSF came to the following conclusions and recommendations reflecting the majority opinions (consensus): “Future technology developments may improve the ability to more effectively utilize continuous electronic monitoring of oxygenation and ventilation in the postoperative period. However, maintaining the status quo while awaiting newer technology is not acceptable.” Intermittent “spot checks” of oxygenation (pulse oximetry) and ventilation (nursing assessment) are not adequate to reliably recognize clinically significant evolving drug-induced respiratory depression in the postoperative period. Continuous electronic monitoring of oxygenation and ventilation should be available and considered for all patients and would reduce the likelihood of unrecognized clinically significant opioid-induced depression of ventilation in the postoperative period. Continuous electronic monitoring should complement and not replace traditional intermittent nursing assessment and vigilance. All patients should have their oxygenation monitored by continuous pulse oximetry. Capnography or other monitoring modalities that measure the adequacy of ventilation and airflow are indicated when supplemental oxygen is needed to maintain acceptable oxygen saturation. Although careful preoperative screening for conditions that may be associated with increased risk for postoperative respiratory insufficiency (OSA, obesity, chronic opioid therapy) is recommended and may be part of a graduated continuous monitoring adoption plan, applying electronic monitoring selectively based on perceived increased risk is likely to miss respiratory depression in patients without risk factors. Continuous monitoring of oxygenation and ventilation from a central location (telemetry or comparable technology) is desirable. This information needs to be reliably transmitted to the health care professional caring for the patient at the bedside. Structured assessment of the level of sedation or consciousness is a critical component of the nurse’s routine postoperative patient assessment for detecting respiratory depression. Nurse and physician education is critical to ensure an understanding of the physiology and pharmacology of drug-induced respiratory depression, the potential obscuring impact of patient arousal on respiratory depression during clinical assessment, and the interference of supplemental oxygen administration on detection of progressive hypoventilation when pulse oximetry is the only continuous electronic monitor. Continuous electronic monitoring systems should integrate multiple physiologic parameters to identify clinically significant changes earlier and more reliably. The APSF is aware of hospital systems that have adopted continuous capnography in combination with pulse oximetry—or in lieu of pulse oximetry. The APSF acknowledges that because of limited health care resources, implementation of these conclusions and recommendations may be part of a graduated continuous electronic monitoring adoption plan. However, institution of these conclusions and recommendations must not be delayed while awaiting newer technology. The APSF advocates increased public and private investment in research to develop monitors with high reliability and ease of use. The APSF believes that multimodal analgesia techniques need to be used more often to decrease the use of opioids alone for management of postoperative pain.
Dual-Acting Agents (Tapentadol, Tramadol)
Recently, a drug with a dual mode of action, tapentadol, was approved for moderate to severe pain but has not yet gained widespread use in clinical practice (Afilalo et al 2010). It exerts its analgesic action via the μ-opioid receptor and norepinephrine reuptake inhibition. Combining both effects in a single molecule eliminates the potential for the drug–drug interactions inherent in multiple-drug therapy. The analgesic effects of tapentadol are independent of metabolic activation, and it has minimal metabolites. The dual mode of analgesia is synergistic, as demonstrated by preclinical work. An immediate-release formulation of tapentadol was approved by the Food and Drug Administration (FDA) and has been used in the United States since 2008, with 50, 75, and 100 mg and the long-acting drug being approved in 2011. The drug is schedule II, and therefore all precautions that must be followed for other drugs in this category need to be strictly adhered to. The equipotent analgesic dose of 100 mg of tapentadol to oxycodone is 15 mg, and it needs to be administered every 4–6 hours.
This compound also has activity at the descending spinal pathway and hence may prove to be a very useful analgesic as more clinical experience is obtained in the postoperative setting. With equipotent doses of the narcotics, the incidence of PONV is lower with tapentadol than with oxycodone (Etropolski et al 2011). The concept of obtaining equipotent analgesia with decreased PONV can be of great benefit in treating postoperative pain and can lead to earlier discharge with significant cost savings (Kwong et al 2010). However, further clinical trials need to be carried out to demonstrate this benefit.
Tramadol may be appropriate for mild to moderate postoperative pain or in conjunction with a cyclooxygenase-2 (Cox-2) inhibitor for pain after anterior cruciate ligament (ACL) surgery (Bourne 2004). It is a centrally acting analgesic that binds to μ-opioid receptors and inhibits the reuptake of norepinephrine and serotonin. Tramadol is effective as a low-dose opioid for postoperative pain and has very low risk for respiratory depression. The combination of tramadol and acetaminophen can provide analgesia for moderate to severe postoperative pain. Tramadol has low risk for abuse and is not a scheduled drug.
Non-opioid Anti-hyperalgesics
In this section we discuss the use of adjuvant drugs (Buvanendran and Kroin 2007) during the postoperative period following major surgery. They are used as adjuvants to opioids or local anesthetics and are an integral part of the multimodal analgesia protocols (Buvanendran and Kroin 2009) to be discussed later.
NSAIDs and Cox-2–Selective Inhibitors
Non-steroidal anti-inflammatory drugs (NSAIDs) are a diverse group of compounds with analgesic, antipyretic, and anti-inflammatory activity. Today, NSAIDs are the most widely prescribed drugs in the world, with sales in excess of $2 billion in the United States and $6–8 billion worldwide. Prostaglandins, including prostaglandin E2 (PGE2), are responsible for reducing the pain threshold at the site of injury (peripheral sensitization). The primary site of action of NSAIDs is believed to be in the periphery, although recent research indicates that central inhibition of Cox-2 may also play an important role in modulating nociception (Buvanendran et al 2006). NSAIDs inhibit the synthesis of prostaglandins, thus diminishing the hyperalgesic state after surgical trauma. NSAIDs are useful as the sole analgesic after minor surgical procedures and may have a significant opioid-sparing effect after major surgery. Recent practice guidelines for management of acute pain in the perioperative setting specifically state that “unless contraindicated, all patients should receive around-the-clock regimen of NSAIDs, COX-2 inhibitors, or acetaminophen” (American Society of Anesthesiologists 2004).
A parenteral formulation of ketorolac tromethamine has been available for many years for the treatment of postoperative pain. As with any mixed Cox-1/Cox-2 inhibitor, the primary concern is the increased postoperative bleeding that has been documented for NSAIDs as result of their Cox-1 component (Marret et al 2003). Other injectable NSAIDs such as ibuprofen (just approved in the United States) are also becoming available. Postoperative patients who have ileus and when bleeding is not a concern may benefit from the injectable NSAID formulations. Newer NSAID injectables are currently in the final phases of clinical trials.
Unlike other NSAIDs, Cox-2–selective inhibitors, when used in the perioperative setting, have the advantages of not increasing the risk for bleeding and fewer gastrointestinal side effects. However, concern for adverse cardiovascular events with chronic use has resulted in the elimination of Cox-2–selective inhibitors, except celecoxib, from the United States. Despite the favorable reports on celecoxib versus placebo for management of postoperative pain (Derry et al 2008, White et al 2011), most patients were still dependent on rescue opioids. Celecoxib should therefore be considered as part of a multimodal anesthesia protocol.
Rofecoxib is a Cox-2–selective inhibitor that is no longer used because of adverse cardiovascular events. However, biochemical data obtained during clinical trials in which rofecoxib was given orally before joint replacement surgery revealed the mechanisms by which Cox-2 inhibition reduces postoperative pain (Buvanendran et al 2006). Following total hip arthroplasty in the placebo group, PGE2 increased at the peripheral site (hip drain), but in the rofecoxib groups, hip drain PGE2 was reduced. In addition, hip drain PGE2 was positively correlated with poorer functional recovery. Cerebrospinal fluid (CSF) PGE2 also increased in the placebo group after surgery, whereas it was decreased in the rofecoxib group. As in the case of hip drain fluid, CSF PGE2 was also positively correlated with the intensity of postoperative pain.
Acetaminophen (Paracetamol): Oral and Intravenous
Acetaminophen (paracetamol) does not have peripheral anti-inflammatory activity but acts centrally to reduce PGE2 and fever. Moreover, it has analgesic properties and fewer side effects than NSAIDs do. Oral acetaminophen has been available for postoperative pain management for more than a century. Greater use is now being made of IV acetaminophen as an analgesic for many surgical procedures (Macario and Royal 2011). IV acetaminophen provides more predictable bioavailability and has a predictable onset when compared with enteral routes of administration. However, hepatotoxicity associated with aniline derivatives is a concern. IV acetaminophen is the first in the class of IV non-opioid, non-NSAID analgesics currently in use in the United States and became available in 2010. IV acetaminophen has been demonstrated to be a safe and efficacious parenteral analgesic agent across a wide array of postoperative settings, from minor outpatient to complicated or major inpatient surgery. It has the potential to provide significant therapeutic improvement in the treatment of fever and acute postoperative pain. There appears to be much benefit to incorporating acetaminophen as part of a multimodal analgesia regimen.
Gabapentoids (Gabapentin, Pregabalin)
Use of anticonvulsants that bind to the α2δ subunit of voltage-gated calcium channels, the gabapentoids, has increased in the past decade for many types of chronic pain, and it is starting to be used in postoperative pain settings (Gilron 2007). Pregabalin has been shown to have a more favorable pharmacokinetic profile than gabapentin, including increased bioavailability, longer half-life, and increased potency (Randinitis et al 2003).
NMDA Antagonists (Ketamine, Memantine, Dextromethorphan, Magnesium)
Because L-glutamate is the most important excitatory neurotransmitter in the central nervous system, blocking glutamate receptors offers an attractive method of reducing afferent stimulation of the spinal cord and therefore blocking pain transmission (Salter 2005). In particular, many drugs or compounds that reduce central glutamate excitation are antagonists of the N-methyl-D-aspartate (NMDA) subtype of glutamate receptor. Although there are two other ion-gated glutamate receptor subtypes, the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and kainite receptors, as well as G protein–coupled glutamate receptors, none of these pharmacological subtypes are represented by drugs in clinical use for pain.
Ketamine, a non-competitive NMDA antagonist, has been used as a general anesthetic and analgesic for the past three decades. It has demonstrated some analgesic or anti-hyperalgesic potential in a large number of clinical trials of postoperative pain, although it has generally been used as an adjuvant medication to opioids, local anesthetics, or other analgesic agents (Subramaniam et al 2004, Bell et al 2005, Remérand et al 2009). High doses of ketamine have been implicated in causing psychomimetic effects (excessive sedation, cognitive dysfunction, hallucinations, nightmares), but subanesthetic or low doses of ketamine have demonstrated significant analgesic efficacy without these side effects. Low-dose ketamine has not been associated with adverse pharmacological effects on respiration, cardiovascular function, nausea, vomiting, urinary retention, and constipation/prolonged adynamic postoperative ileus. An IV bolus at the beginning of surgery followed by a 24-hour infusion decreased morphine consumption in patients undergoing total hip arthroplasty (Remérand et al 2009). More interestingly, patients receiving ketamine had a decreased incidence of chronic pain. At 6 months, 21% of placebo- and 8% of ketamine-receiving patients had persistent pain. Similar results have been found by others, albeit in opiate-dependent patients undergoing lumbar spine surgery (Loftus et al 2010). A ketamine infusion of 10 μg/kg/min was started at the beginning of surgery after a bolus of 0.5 mg/kg was administered and terminated at skin closure. Significant results included decreased postoperative morphine requirements and lower pain scores 6 weeks postoperatively.
Memantine was first synthesized in the 1960s and found to antagonize the NMDA receptor in the 1980s. It is completely absorbed from the gastrointestinal tract with maximal plasma concentrations occurring between 3 and 8 hours after oral administration. Approximately 80% of the dose administered remains as the parent drug. Its mean terminal elimination half-life is 60–100 hours. Although memantine does not appear to be beneficial as an analgesic therapy for long-term established chronic neuropathic pain, it may be a useful adjunct when used early in specific settings such as the initial phases of phantom limb pain or soon after surgery on opioid-tolerant subjects (Buvanendran and Kroin 2008). Ketamine causes memory deficits, reproduces with impressive accuracy the symptoms of schizophrenia, is widely abused, and induces vacuoles in neurons at moderate concentrations and cell death at higher concentrations. Memantine, in contrast, is well tolerated; although instances of psychotic side effects have been reported, in placebo-controlled clinical studies the incidence of side effects is remarkably low.
Dextromethorphan and its metabolite dextrorphan have been found to antagonize NMDA receptors in brain slices (Wong et al 1988). Although dextromethorphan is an open-channel blocker similar to ketamine, it produces fewer psychotomimetic effects, probably because of its lower affinity for the NMDA receptor (LePage et al 2005).
α2-Adrenergic Agonists (Clonidine, Dexmedetomidine)
In addition to the opiate system, α2-adrenergic activation represents an inherent pain control network of the central nervous system. α2-Adrenergic receptors are abundant in the substantia gelatinosa of the dorsal horn in both rats and humans and appear to be the primary site of action where α2-adrenergic agonists can inhibit somatic pain (Unnerstall et al 1984, Yaksh 1985). This receptor system also exists in the brain, where its activation can produce sedation. Cardiovascular depression from α2-adrenergic agonists can occur at both brain and spinal cord sites (Eisenach and Tong 1991). These side effects of sedation and sympathetic inhibition limit the use of α2-adrenergic agonists to just an adjuvant role as analgesics.
Clonidine was originally used to control blood pressure and heart rate. However, it is now known that it also has antinociceptive properties in both rodents and humans. Clonidine binds to α2-adrenergic receptors in the central nervous system, as well as to imidazoline receptors in the brain (Kahn et al 1999). It has been hypothesized that clonidine acts at α2-adrenergic receptors in the spinal cord to stimulate the release of acetylcholine, which acts at both the muscarinic and nicotinic subtypes for postoperative pain relief (Duflo et al 2005). Clonidine has been administered by various systemic routes as an adjuvant to reduce postoperative pain: orally, intravenously, and as a transdermal patch. The results of such studies have been mixed. Better results were observed when clonidine was added as an adjuvant to epidural analgesics or to local anesthetics for PNB.
Since its approval for clinical use, dexmedetomidine has been used for sedation during surgery and in the postoperative period. Dexmedetomidine is an α2-adrenergic agonist with even better selectivity for that receptor than clonidine has (Coursin et al 2007). For postoperative pain control, it is primarily used as an IV adjuvant to opioids.
Glucocorticoids
There is a long history of using glucocorticoids to reduce inflammation and postoperative pain in many surgical procedures (Salerno and Herman 2006). Glucocorticoids (corticosteroids) are steroids that bind with high affinity to the glucocorticoid receptor in the cytosol of cells. There are multiple sites of action at which glucocorticoid-activated receptors produce anti-inflammatory and immunosuppressive effects (Fleischli and Adams 1999). However, the powerful anti-inflammatory nature of corticosteroids, through inhibition of prostaglandin synthesis, may also have detrimental side effects with high or repeated dosing.
Dexamethasone is a synthetic glucocorticoid with high potency and a long duration of action (half-life of 2 days), but it has no mineralocorticoid activity. Prostaglandins are one of the main inducers of inflammation after tissue injury, and one of the mechanisms by which glucocorticoids reduce prostaglandin synthesis is by inhibiting the expression of Cox-2 (Hay and de Belleroche 1998). Studies using dexamethasone for postoperative pain relief have produced mostly positive results, especially with surgical procedures involving a large amount of tissue trauma, such as orthopedic and neurological surgery (Masferrer et al 1994). A recent review concluded that a single preoperative IV dose of dexamethasone (4–8 mg) reduces postoperative pain after ambulatory surgery (Jakobssen 2010). In a meta-analysis of perioperative dexamethasone, preoperative administration of dexamethasone produced a more consistent analgesic effect than intraoperative administration did (De Oliveira et al 2011). In addition to reducing inflammation, dexamethasone can also reduce PONV.
Acetylcholine Esterase Inhibitors (e.g., Neostigmine) and Cholinergic Drugs (e.g., Nicotine)
Acetylcholine esterase inhibitors and muscarinic receptor agonists increase pain thresholds (Eisenach 1999). Muscarinic receptors occur at high density in the superficial dorsal horn, and it is hypothesized that nearby cholinergic neurons stimulate these receptors to reduce postoperative pain (Decker et al 2004). Acetylcholine may cause analgesia through direct action on the M1 and M3 spinal cholinergic muscarinic receptor and nicotinic receptor subtypes and indirectly through stimulation of release of the second messenger nitric oxide in the spinal cord.
The acetylcholinesterase inhibitor neostigmine, when administered systemically, cannot access spinal cord cholinergic receptors because the compound does not cross the blood–brain barrier. However, intrathecal and epidural administration of neostigmine provides effective postoperative analgesia. Its clinical use, however, is limited by significant side effects, in particular, nausea, vomiting, and sedation (Kaya et al 2004, Ho et al 2005).
Neuronal nicotinic acetylcholine receptors are ligand-gated ion channels. Agonist activation allows cations to enter the cell. Nicotine is a classic agonist at these receptors, and newer nicotinic compounds such as epibatidine have been studied in pain models. However, even with intrathecal administration to limit systemic side effects (e.g., adverse effects on autonomic function), these agonists do not produce consistent analgesia (Decker et al 2004).
Antidepressants
Tricyclic antidepressants (TCAs) have an analgesic effect that has been demonstrated to be independent of their antidepressant effect (Dworkin et al 2003). The pharmacological actions of TCAs can be linked to their effect as a calcium channel antagonist, sodium channel antagonist, presynaptic reuptake inhibitor of monoamines such as serotonin and norepinephrine, and NMDA receptor antagonist (Colombo et al 2006). More specifically, the analgesic effect is believed to occur primarily via inhibition of reuptake of norepinephrine rather than serotonin at spinal dorsal horn synapses, with secondary activity at sodium channels (Sawynok et al 2001). Within the class of TCAs, variation exists between inhibition of norepinephrine and serotonin. The tertiary amine agents (e.g., amitriptyline and imipramine) demonstrate a balance in their ability to inhibit norepinephrine and serotonin, whereas the secondary amines (e.g., nortriptyline and desipramine) favor the inhibition of norepinephrine.
Serotonin–norepinephrine reuptake inhibitors (SNRIs) (e.g., duloxetine, venlafaxine, and desvenlafaxine) inhibit the reuptake of both serotonin and norepinephrine and are referred to as dual inhibitors or “selective serotonin–norepinephrine inhibitors.” SNRIs’ lack of anticholinergic side effects results in a distinct advantage over traditional TCAs (Sindrup et al 2003). For example, duloxetine is a potent, balanced inhibitor of serotonin and norepinephrine reuptake. Venlafaxine inhibits serotonin reuptake at lower dosages and inhibits both serotonin and norepinephrine reuptake at higher dosages (Dworkin et al 2007).
Cannabinoids
These compounds are potent analgesics in animal models. Several clinical trials have been conducted, but most of them have demonstrated no significant analgesic effect superior to placebo (Beaulieu 2006). In fact, some of the trials demonstrated an increase in scores on the visual analog scale (VAS) with nabilone (oral synthetic cannabinoid) when used in the acute postoperative setting. However, these classes of drugs seem to be promising in patients with chronic pain.
Local Anesthetics
One of the first uses of local anesthetics for anesthesia occurred in the late 19th century and involved cocaine. The chemical structure of local anesthetics in clinical use consists of an aromatic (lipophilic) benzene ring linked to an amino group (hydrophilic) via either an ester or amide intermediate chain. The intermediate link classifies the local anesthetic as either an ester (procaine, chloroprocaine, tetracaine, and cocaine) or an amide (lidocaine, prilocaine, mepivacaine, bupivacaine, etidocaine, and ropivacaine).
Local anesthetics are sodium channel blockers. They do not affect resting membrane potential but rather formation and propagation of the action potential. At peripheral nerves the reduction in sodium influx leads to a decrement in action potential formation and propagation.
Systemic Local Anesthetics
In patients undergoing abdominal surgery, perioperative IV infusion of lidocaine reduced postoperative pain intensity and opioid consumption (with earlier return of bowel function), but patients undergoing tonsillectomy, total hip arthroplasty, and coronary artery bypass surgery derived no benefit from lidocaine (McCarthy et al 2010).
Multimodal Analgesia
The theory behind multimodal anesthesia is that agents with different mechanisms of analgesia may have synergistic effects—or at least additive effects—in preventing or treating acute pain when used in combination. Thus, multimodal analgesia captures the effectiveness of individual agents at optimal dosages that maximize efficacy while attempting to minimize side effects from any one analgesic. These regimens must be tailored to individual patients while keeping in mind the procedure being performed, side effects of the individual medications, and patients’ pre-existing medical conditions (Buvanendran and Kroin 2009). The concept and theory of multimodal analgesia are not new; however, several novel pharmacological agents have emerged and can be added to the drug regimen to be used in this fashion. It is vital to realize that blocking the neuronal pathway with local anesthetics during surgery does not decrease the humoral biochemical responses that occur during surgery; the latter have to be inhibited by administering systemic pharmacological therapy (Buvanendran et al 2006). This is especially important in ambulatory or fast-track surgery protocols to hasten postoperative recovery and decrease hospitalization time (Kehlet and Wilmore 2008, Buvanendran and Thillainathan 2010).
Opioids Plus NSAIDs or Cox-2 Inhibitors
In a double-blind placebo-controlled study, patients undergoing major surgery (abdominal or orthopedic) received 30 mg ketorolac, 10 mg ketorolac, or placebo spray on recovering from general anesthesia (Moodie et al 2008). All patients then used a PCA morphine pump for the next 40 hours. Mean morphine consumption over the initial 24 hours was lower in the 30 mg ketorolac group (37.8 mg) than in the placebo group (56.5 mg) and the 10 mg ketorolac group (54.3 mg). Pain reduction over the first 6 hours postoperatively was higher in the 30 mg ketorolac group than in the placebo group. The incidence of opioid-related adverse events, such as nausea or pruritus, did not differ between groups.
In a recent review, celecoxib was as effective as the NSAID ibuprofen for acute postoperative pain (Derry et al 2008). One group of patients undergoing total knee arthroplasty under spinal anesthesia received the Cox-2–selective inhibitor celecoxib, 200 mg, 1 hour before surgery and every 12 hours for 5 days (Huang et al 2008). The other group received placebo at the same time points. Over the first 24 hours, PCA morphine use was less in the celecoxib group (15.1 mg) than in the placebo group (19.7 mg). Over the 48-hour period, VAS scores for pain at rest were lower in the celecoxib group than in the placebo group, but there was no difference in pain scores with ambulation. Celecoxib also increased knee range of motion over the first 3 postoperative days. The incidence of PONV did not differ by group. As expected with a Cox-2–selective inhibitor, there were no differences in intraoperative or postoperative blood loss between groups. Etoricoxib is a new Cox-2–selective inhibitor with a long half-life (not currently approved in the United States). With administration of etoricoxib versus placebo 1 hour before thyroid surgery under general anesthesia, postoperative IV oxycodone use during the initial 6 hours was not reduced in the etoricoxib group (Smirnov et al 2008). However, paracetamol–codeine tablet use over the 7–24-hour postoperative period was less in the etoricoxib group (2.1 g) than in the placebo group (4.1 g). Pain scores and the incidence of adverse events did not differ among groups. In summary, although most studies on the use of NSAIDs in multimodal analgesia show opioid sparing, few show a statistical reduction in opioid-related adverse effects.
Opioids Plus Acetaminophen
Oral acetaminophen is well documented in postoperative pain treatment. However, in a systematic review of patients who received PCA morphine for at least 24 hours after major surgery, NSAIDs and Cox-2 inhibitors were more effective than acetaminophen in reducing morphine consumption (Maund et al 2011). In addition, unlike NSAIDs, acetaminophen did not reduce nausea or vomiting, although like NSAIDs, it reduced pruritus. In a systematic review of the morphine-sparing effect of acetaminophen combined with PCA, its efficacy and effects on opioid-related adverse effects were evaluated (Remy et al 2005). Seven prospective randomized controlled trials involving 265 patients in the group with PCA morphine plus acetaminophen and 226 patients in the group with PCA morphine alone were included. Outcome measures included morphine consumption over the first 24 hours after surgery, patient satisfaction, and the incidence of opioid-related adverse effects, including PONV, sedation, urinary retention, pruritus, and respiratory depression. Acetaminophen combined with PCA morphine induced a significant morphine-sparing effect but did not change the incidence of morphine-related adverse effects in the postoperative period.
Opioids Plus Gabapentoids
Clinical trials have demonstrated pain-relieving and opioid-sparing effects of pregabalin and gabapentin in patients with acute postoperative pain (Dauri et al 2009, Zhang et al 2011). Some studies have indicated that gabapentin decreases the incidence of opioid-related side effects (nausea, vomiting, and pruritus). A meta-analysis concluded that a single preoperative dose of gabapentin, 1200 mg or less, reduces pain scores and opioid consumption in the first 24 hours postoperatively (Ho et al 2006). Continuing administration of gabapentin, in addition to a single preoperative dose, appears to have benefit; when given for 4 days postoperatively, opioid consumption and some opioid-related side effects were reduced following total knee arthroplasty (Clarke et al 2009).
Buvanendran and colleagues (2010) demonstrated that pregabalin (300-mg one-time dose followed by 150 mg twice a day) administered in the perioperative period for total knee arthroplasty not only reduced opioid consumption in the acute postoperative period and improved surgical outcome with greater range of motion but also decreased the development of chronic pain 6 months after surgery. The optimal dosage of pregabalin has as yet not been determined; higher doses of pregabalin (600 mg), though effective in decreasing postoperative opioid consumption, are associated with an increased incidence of dizziness, blurred vision, and headache. Incorporating anticonvulsants into a multimodal regimen appears to offer not only short-term benefits but also long-term benefits, such as decreased chronic pain and improved functional outcomes, when continued throughout the immediate postoperative period for certain procedures such as orthopedic surgery.
Opioids Plus NMDA Antagonists
In patients undergoing total knee replacement surgery and general anesthesia, ketamine or placebo was given during surgery (0.2 mg/kg followed by 2 μg/kg/min) and through the second postoperative day (10 μg/kg/min) (Aveline et al 2009). PCA morphine use was less over the 48-hour postoperative period in the ketamine group (50.5 mg) than in the placebo group (72.1 mg). Pain scores were lower at rest and with movement in the ketamine group at all times. Time to achieve 90-degree knee flexion was shorter in the ketamine group, and the incidence of PONV was less. Patients undergoing major abdominal surgery and general anesthesia were randomized into three groups: perioperative ketamine (intraoperatively, 0.5 mg/kg and then 2 μg/kg/min; postoperatively, 2 μg/kg/min for next 48 hours), intraoperative ketamine only, or placebo (Zakine et al 2008). PCA morphine use was less in the perioperative ketamine group (27 mg) than in the intraoperative ketamine (48 mg) or placebo (50 mg) groups. Interestingly, pain scores at 24 and 48 hours were lower in both the perioperative and intraoperative ketamine groups than in the placebo group. The incidence of PONV was greater in the placebo than in the perioperative ketamine group.
However, other studies have failed to show any opioid-sparing effect of ketamine. After major gynecological surgery under general anesthesia, ketamine (0.15 mg/kg before incision and then combined PCA ketamine, 0.5 mg/mL, and morphine, 1 mg/mL, for 48 hours) or placebo (PCA morphine alone) did not reduce PCA morphine requirements (Aubrun et al 2008). The total postoperative ketamine dose was 44 mg. Pain scores were also no different between groups. After pediatric (12–18 years) scoliosis surgery, intraoperative ketamine (0.5 mg/kg and then 4 μg/kg/min) did not reduce postoperative PCA morphine use over the next 24, 48, or 72 hours in comparison to placebo (Engelhardt et al 2008). Pain scores and the incidence of PONV were not different between groups. The lack of a clinical effect in these two studies may be due to a low ketamine dose (about 0.2 μg/kg/min) (Aubrun et al 2008) or not continuing the dose into the postoperative period (Engelhardt et al 2008).
Dextromethorphan has been reported to produce a modest reduction in postoperative opioid (meperidine) consumption when given intramuscularly before laparoscopic cholecystectomy (Yeh et al 2004). Given orally before incision and continued for 2 days following surgery for bone malignancy, dextromethorphan reduced patient-controlled epidural analgesia (PCEA) (ropivacaine/fentanyl) requirements postoperatively by 30–50% (Weinbroum et al 2003). However, a large oral dextromethorphan (200 mg every 8 hours) dose given postoperatively after knee surgery produced only a moderate reduction in morphine requirements (29%) and no reduction in postoperative pain levels (Wadhwa et al 2001). A systematic review (28 double-blind studies) of perioperative dextromethorphan for postoperative pain concluded that the drug has the potential to be a safe adjunctive agent to opioid analgesia but the results were inconsistent (Duedahl et al 2006). The route of administration may be important for beneficial effects. Because of the controversial data, dextromethorphan is not currently recommended for routine management of postoperative pain.
Opioids Plus α2-Adrenergic Agonists
Patients undergoing abdominal total hysterectomy under general anesthesia were randomized to receive morphine, 1 mg/mL, alone or dexmedetomidine, 5 μg/mL, plus morphine, 1 mg/mL, via PCA for postoperative analgesia over a 24-hour period (Lin et al 2009). Patients receiving dexmedetomidine plus morphine required less morphine (23 mg) than the morphine-alone group did (33 mg) over the 0–24-hour postoperative period. Postoperative pain scores at rest or with movement and the incidence of nausea during the 4–24-hour period were lower in the group receiving dexmedetomidine plus morphine. Blood pressure and the heart rate were lower in the group receiving dexmedetomidine plus morphine, but the decrease was small. In another study, patients undergoing laparoscopic bariatric surgery with general anesthesia were randomized to four intraoperative IV infusion groups: dexmedetomidine, 0.2, 0.4, or 0.8 μg/kg/hr, or placebo (Tufanogullari et al 2008). More patients in the placebo group required antiemetic therapy than in dexmedetomidine groups. However, PCA morphine use 0–48 hours after surgery and pain scores through day 7 did not differ among groups. It may be that dexmedetomidine must be given during the postoperative period to reduce PCA morphine use. Because of the many side effects of systemic clonidine administration, such as hypotension, bradycardia, and sedation, the spinal route is preferred.
Epidural or Intrathecal Local Anesthetics
Epidural analgesia and spinal/epidural analgesia have been used increasingly in recent years to control pain during and after labor (Loubert et al 2011; also see Chapter 55). When continuous epidural regimens are applied in a well-functioning acute pain service setting (Viscusi 2008), side effects are few and the benefits outweigh the risks. A meta-analysis of epidural PCA of opioids showed that it produced superior pain control in comparison to IV PCA for all types of surgeries (Wu et al 2005). Epidural analgesia also provided better pain relief than systemic opioids did for abdominal aortic surgery, and the overall incidence of cardiovascular complications, myocardial infarction, acute respiratory failure, gastrointestinal complications, and renal insufficiency was significantly reduced (Nishimori et al 2006).
Local Anesthetics and Opioids
Epidural administration of opioids and local anesthetics has evolved over the past decade. The advantages of epidural administration of these drugs include a reduced incidence of side effects and a diminished propensity for opioid-induced ventilatory depression when compared with the intrathecal route. When a drug is placed in the epidural space, it must first cross the dura before it can reach the spinal cord. Besides the physical barrier presented by the dura, the epidural space is highly vascularized, and significant redistribution of drug to the systemic circulation occurs. The epidural space also contains fat and the dorsal and ventral roots of the spinal nerves, all of which can serve as a repository for lipophilic agents.
The influence of these factors can be demonstrated by examination of the pharmacokinetics of epidurally administered hydrophilic (morphine) and lipophilic (fentanyl) opioids. Because diffusion of drugs across the dura is both concentration and time dependent, it is necessary to administer significantly larger amounts of drugs than those that effectively saturate spinal opiate receptors. When these factors are considered, the margin of therapeutic safety and the decrease in side effects with epidural administration make this route preferred for postoperative analgesia.
Effective analgesia with epidural infusions administered at a continuous rate may take as long as 3–4 hours to achieve. Delay in the onset of effective analgesia can be reduced by adjusting the infusion rate to provide the equivalent of a small (5–10 mL) bolus of the epidural solution over a period of 5–15 minutes before beginning the maintenance infusion. This allows an adequate concentration of the analgesic drugs to be present at their sites of action in a shorter time. In addition to a reduction in adverse effects, another advantage of continuous epidural infusion over an epidural bolus injection is the ability to titrate the amount of analgesia. Although morphine usually provides 12 hours of pain relief after a single epidural injection, wide variability has been reported in the duration of effective analgesia (4–24 hours), depending on the site and extent of surgical trauma and the age of the patient. Because of this variability, it becomes difficult to titrate uniform levels of analgesia. A continuous infusion provides easier analgesic titration, particularly when shorter-acting opioids such as fentanyl are used. Fentanyl has an onset of action within 4–5 minutes and a peak effect within 20 minutes. Because of its rapid onset, it becomes much easier to adjust the dosage, observe the desired effect, and titrate to an optimal intensity of analgesia. Morphine, in contrast, has an onset time of 30 minutes with the time to peak effect ranging from 60 to 90 minutes.
Mirroring the evolution of PCA, a refinement in the delivery of analgesics by the epidural route is the use of superimposed patient-controlled bolus doses with a continuous basal infusion. Early application of this technique for delivery of epidural analgesia used relatively large intermittent demand doses alone or combined with a low-rate continuous infusion, and the intermittent demand doses provided the preponderance of analgesia. This dosing paradigm has reduced efficacy because of the fluctuations in analgesia occurring as a consequence of the large intermittent bolus dosing. Using higher basal infusion rates and smaller patient-activated bolus doses, continuous infusion maintains a more constant intensity of analgesia, whereas the bolus doses provide supplemental analgesia for transient increases in analgesic requirements. PCEA is particularly useful for managing dynamic changes in pain related to patient activity (e.g., coughing, chest physiotherapy). The development of new infusion devices has allowed such combined modes of administration of epidural analgesia to be readily delivered.
Caudal Anesthesia
Caudal nerve blocks play a minor role in the management of acute postoperative pain in adults. Because they are technically more difficult to perform in adults than other efficacious forms of lumbar epidural blocks, they are used less frequently in adults than in the pediatric population. Continuous caudal analgesia for postoperative pain has limited utility because of the difficulty of securing a catheter, but it may have a role in selected patients, such as those who have undergone extensive lumbar or thoracic spine surgery. Pediatric (“kiddie”) caudals have become popular for intraoperative supplementation and postoperative pain relief.
Epidural Adjuvants
Adrenergic receptors of the α2 class modulate nociceptive impulses in the dorsal horn of the spinal cord, as well as throughout the central nervous system. Agonists of these receptors produce antinociception with minimal ventilatory depression in comparison to opioids. Clonidine has been the most widely used α2 agonist for epidural analgesia; it produces dose-dependent analgesia when given as a bolus. Epidural clonidine has been associated with hypotension and bradycardia because of inhibition of preganglionic sympathetic fibers. This adverse effect is most prevalent at smaller doses, whereas large doses normalize blood pressure because of systemic vasoconstriction, which overrides the central hypotensive effect. Although epidural clonidine has been used as a single agent to provide postoperative analgesia, it has more frequently been used in combination with local anesthetics or opiates to potentiate analgesia and minimize side effects. Optimal ratios for combining α2 agonists with an opioid or local anesthetics are yet to be defined because these drugs exhibit non-linear synergism. For spinal surgery under general anesthesia, patients received epidural clonidine, 25 μg/hr, or placebo infusion postoperatively for 36 hours (Farmery et al 2009). PCA morphine use was less in the clonidine group (35 mg) than in the placebo group (61 mg). Pain scores with movement were less in the clonidine group over the 36-hour period, and the incidence of PONV was reduced. Blood pressure and heart rate were lower in the clonidine group, but the reductions were modest.
Role of Peripheral Nerve Blocks
The increased safety of PNBs that has been made possible in the past 5 years through the use of ultrasound and the decreased quantity of the analgesic solution administered have made this method attractive to practitioners. PNB of the major nerves supplying the lower extremities has emerged as a good alternative (Fowler et al 2008) to the epidural technique for providing postoperative analgesia after surgery on the lower limb, especially with the current anticoagulation guidelines (Horlocker et al 2003). PNB can be achieved by single-shot blockade or by continuous infusion. For lower limb surgery, a femoral nerve block, a sciatic nerve block, an obturator nerve block, or a “3-in-1” block can be performed. Femoral nerve blocks are most commonly used for knee arthroplasty, either alone or in combination with a sciatic nerve block. After completion of the femoral nerve block, the patient is turned laterally for placement of a sciatic perineural catheter via a gluteal approach. Although anatomically an obturator nerve block in combination with a femoral block would provide greater analgesic benefit than a femoral plus sciatic block would, it is technically challenging to achieve an effective obturator block. A “3-in-1” block is intended to block the lateral femoral cutaneous, the femoral, and the obturator nerves.
Several studies have compared the analgesic efficacy and the incidence of side effects with PNBs (femoral alone or femoral plus sciatic) versus epidural analgesia. A systematic review of studies that compared the two techniques concluded that although the analgesic efficacy of both the epidural and PNB techniques was comparable, the incidence of side effects such as hypotension, urinary retention, and nerve injury was much lower with PNBs (Fowler et al 2008). In addition, nerve injuries associated with PNBs present much less adversity than a neuraxial injury. The review also evaluated the potential benefit of adding sciatic blocks to femoral blocks and concluded that there was no additional benefit. Although a lumbar plexus block has greater consistency with regard to blocking the obturator nerve than does an infra-inguinal femoral block (“3-in-1”), it is unclear whether there is any benefit in adding the obturator block. The incidence of quadriceps weakness with PNBs is higher and it can therefore interfere with early mobilization of patients, but there appears to be no difference in rehabilitative outcomes between the two groups at the time of discharge. Data are sparse on whether a continuous femoral nerve block is more effective than a single-shot femoral block. In a randomized trial the continuous mode lowered pain scores and increased opioid consumption significantly more than did the single-shot technique, but the length of stay and functional outcomes did not differ between the two groups (Salinas et al 2006). Although the exact mechanism is not clearly known, the addition of clonidine to a PNB at a dose of 100 μg appears to lead to prolongation of analgesia. Randomized controlled trials have shown that PNBs, especially continuous techniques, provide superior postoperative analgesia and improved outcomes in patients undergoing ACL surgery. For ACL surgery the femoral nerve can be blocked with bupivacaine (0.25–0.5%) or ropivacaine (0.2–0.5%), which can provide analgesia for surgery and the postoperative period.
Patients in whom a perineural femoral nerve catheter is placed typically receive an infusion of 0.1% ropivacaine via a patient-controlled regional anesthesia technique at a basal rate of 5 mL/hr plus a 3–5-mL bolus with a lock-out period of 60 minutes for discharge. The femoral perineural infusion is maintained for up to 24–48 hours postoperatively via disposable ambulatory pumps. Patients deemed appropriate for continuous nerve block analgesia at home need appropriate education before being discharged. The choice of analgesia for the postoperative period depends highly on the surgeon’s choice (Masursky et al 2008) of the timing of physical therapy. The presence of a femoral catheter, especially if the concentration of local anesthetic used is not dilute, can result in significant quadriceps weakness. In fact, there are case reports of patients falling (Williams et al 2007) and further injuring their knee when severe quadriceps weakness is present. Randomized trials comparing femoral nerve catheters with local anesthetics versus intra-articular (IA) injection of bupivacaine and morphine have demonstrated that both postoperative analgesics are equally effective and are associated with low pain scores and reduced narcotic consumption (Woods et al 2006). Preliminary studies suggest that continuous infusion of local anesthetics for peripheral nerve blockade may be very efficient and safe, even on an outpatient basis (Axley and Horn 2010).
A meta-analysis found that continuous peripheral nerve blockade decreased postoperative pain and opioid-related side effects when compared with opioids (Richman et al 2006). Addition of clonidine to local anesthetics, which in animal experiments extends the duration of a nerve block via inhibition of the hyperpolarization-activated cation current (Kroin et al 2004), prolongs the duration of analgesia and motor block in patients by about 2 hours but is associated with risk for orthostatic hypotension and fainting (Pöpping et al 2009).
Wound Instillation of Analgesics
Data obtained from patients undergoing knee replacement surgery support the intraoperative use of a local infiltration technique but not the postoperative use of wound catheter administration; however, there is little evidence to support use of the technique for hip replacement either intraoperatively or with a postoperative wound infusion catheter (Kehlet and Andersen 2011). In laparoscopic gastric procedures, intraperitoneal local anesthetic reduces the intensity of abdominal pain and postoperative opioid consumption (Kahokehr et al 2011). A newly developed liposomal formulation of long-acting bupivacaine is being considered for approval by the FDA. A single injection of the liposomal bupivacaine should last 72 hours and is currently being considered for infiltration of the local surgical site. Regional analgesia with this product has yet to be established.
Intra-articular Injections
IA analgesia has gained popularity for ambulatory surgery because of ease of administration, efficacy in achieving pain relief, and lack of systemic side effects (Rawal 2007). Procedures in which this has been demonstrated include knee and shoulder arthroscopy. However, the results are not conclusive; although several studies demonstrate a positive effect of IA analgesia, many others demonstrate the contrary. In addition, the very recent reports of chondrolysis following IA injection and infusion have led to further debate about its use (Fredrickson et al 2010). A variety of substances, including local anesthetics, opioids, NSAIDs, corticosteroids, α2-adrenergic agonists, and NMDA receptor antagonists, have been used for IA analgesia from as early as 1985 (Hughes 1985). There are many variables in IA analgesia, including varying pharmacokinetic profiles and dosages of individual agents, and this has led to varying efficacy in pain relief and the use of creative combinations of drugs. One such combination that has been investigated is a bupivacaine–morphine combination, which demonstrated positive effects on postoperative pain in multiple studies (Khoury et al 1992, Guler et al 2004, Senthilkumaran et al 2010). After a systematic review it was concluded that IA morphine, 5 mg, was an optimal dose and could provide as much as 27 hours of analgesia postoperatively (Kalso et al 2002). When the dose range effect of IA morphine was examined, it was noted that the analgesic effect was “mild” (Gupta et al 2001).
IA ketorolac has been shown to have analgesic properties when compared with low doses of bupivacaine or morphine (Calmet et al 2004). Some authors have included ketorolac in combination with other drugs in IA injection; when used with bupivacaine and morphine, ketorolac, 60 mg by the IA route, led to a longer duration of analgesia following arthroscopic knee surgery and more patients well enough to return to work on postoperative days 1 and 2 (Ng et al 2006). Addition of a corticosteroid, methylprednisolone, 40 mg, to a morphine–bupivacaine injection also had a similar ability to enhance analgesia and allow patients to return to work earlier (Rasmussen et al 1998). Side effects of steroids, such as delayed wound healing or infection, were not observed with steroid injections (Wang et al 1998). Finally, the use of IA ketamine, 0.5 mg/kg, for outpatient arthroscopic surgery had analgesic effects when compared with placebo, although IA bupivacaine had a greater analgesic effect (Dal et al 2004).
Timing of the IA injection may be important. A single IA injection of morphine followed by another 10 minutes of tourniquet time improved postoperative analgesia (Whitford et al 1997). However, other studies have found no difference with varying tourniquet times (Klinken 1995) or after the tourniquet was deflated completely (Guler et al 2004). The type of procedure has also been linked to varying effects of IA agents. Patients undergoing knee procedures associated with a “high inflammatory response” (e.g., ACL reconstruction, lateral release, patellar shaving, and plica removal) had improved analgesia with morphine in comparison to bupivacaine. In contrast, patients undergoing diagnostic arthroscopy or partial meniscectomy (associated with “low inflammation”) had better pain control with bupivacaine than with morphine (Marchal et al 2003). Use of regional, neuraxial, or systemic analgesia in addition to IA analgesia has been noted as a possible confounding factor in evaluating the efficacy of IA studies. IA analgesia in shoulder surgery has been compared with parenteral analgesics and interscalene brachial plexus blockade (ISB). In patients undergoing arthroscopic acromioplasty, ISB was superior to IA analgesia or suprascapular nerve blockade. When compared with controls, only patients receiving ISB had significant reductions in pain scores and morphine consumption in the postanesthetic care unit (Singelyn et al 2004).
To provide longer-lasting analgesia, continuous IA infusions may be necessary. Following rotator cuff repair, implantation of an IA catheter with infusion of ropivacaine led to improvement in pain control for 12 hours postoperatively but no change in postoperative opioid consumption (Coghlan et al 2009). The authors argued that the use of continuous IA infusions was “not worth the substantial additional costs.” Similar findings were noted after arthroscopic subacromial decompression with a catheter in the subacromial space: improved pain scores but no change in opioid consumption (Harvey et al 2004). Other studies have shown a decrease neither in pain score nor in opioid consumption (Boss et al 2004). These findings have led Fredrickson and colleagues (2010) to question the utility of IA analgesia. Earlier studies were conducted on less painful shoulder procedures (arthroscopic, non–rotator cuff), which may explain the discrepancy with recent findings. Chondrolysis is the disappearance of articular cartilage as a result of dissolution of cartilage matrix and chondrocytes in a short period. This leads to severe osteoarthritis and long-term disability and is considered to be a rare disease of the shoulder (Bailie and Ellenbecker 2009). Animal models have recently indicated the chondrotoxicity of bupivacaine in rabbits (Gomoll et al 2006).
Various substances have been investigated for efficacy in relieving postoperative pain after arthroscopic surgery. The results have been mostly inconclusive. IA injection of opioids and local anesthetics may be safe in the knee. In theory, combinations of agents with different mechanisms of action may allow lower doses of individual drugs while potentiating the analgesic effect. Continuous and patient-controlled IA infusions are also discouraged because prolonged exposure to pharmacologic agents may be the trigger for the development of chondrolysis. Larger-scale studies are necessary to evaluate the benefit of substances other than opioids and local anesthetics. Additionally, determining the timing and optimum dosages and establishing safety profiles are crucial if IA analgesics are to evolve as a component of multimodal analgesic regimens.
Transdermal Fentanyl
The use of patient-controlled delivery has led to the development of other modalities that allow patient control in the delivery of opioid medications. Transdermal delivery systems permit demand dosing of fentanyl at a predetermined interval. The fentanyl HCl iontophoretic transdermal system (ITS) is a patient-controlled approach to analgesic delivery that may avoid some of the problems associated with IV PCA. Fentanyl ITS is a compact, needleless, self-contained system that is preprogrammed to deliver fentanyl, 40 μg, across the skin by means of an imperceptible low-intensity electrical current, a method known as iontophoresis. This system is under further research before being released for human use. Inhaled fentanyl has been tested in pediatric and adult patients. There are investigations of encapsulated liposomal inhaled fentanyl for acute pain—the advantage of this being that it can provide rapid onset and sustained release.
Topical Analgesics
Topical diclofenac exists in several forms, including diclofenac epolamine 1% topical patch, diclofenac sodium 1% topical gel, and diclofenac sodium 1.5% weight-in-weight (w/w) liquid. A recent review showed that topical NSAIDs are not only safe but efficacious as well in the treatment of acute soft tissue injuries and localized regions of pain, acute or chronic (Massey et al 2010). They did find a difference between placebo and topical NSAIDs with regard to local skin irritation, but the systemic side effects were less with the topical form. In fact, most current research points to the fact that topical application of diclofenac could lead to decreased systemic absorption and therefore fewer gastrointestinal and renal adverse events associated with this class of drug.
A review of the diclofenac epolamine topical patch discussed the benefits of the patch as opposed to NSAID gels or creams (McCarberg and Argoff 2010). Such benefits included application of a defined dose of diclofenac, drug delivery over an extended period (typically 12 hours), and ease of application. Application of diclofenac sodium 1% gel versus a placebo vehicle (composition identical to the gel component of the study drug) four times daily for 3 months was investigated for the treatment of osteoarthritis pain (Barthel et al 2009). The results of the study indicated superior analgesia from 1–12 weeks and improved function for the same duration. Diclofenac gel was tolerated as well as placebo. With regard to diclofenac 1.5% w/w liquid, it has been shown to be as efficacious as oral diclofenac in treating arthritis pain (Moen 2009). Gastrointestinal side effects were significantly less common, with local skin reactions being more common. A prospective study established the safety of topical diclofenac 1.5% w/w in a study in which 793 subjects were monitored for an average of 204 days and 144 subjects for 1 year (Shainhouse et al 2010). Application of the study drug, 40 drops four times daily, resulted in local skin reactions (dry skin, contact dermatitis, or dermatitis with vesicles) in 45.1% of the study participants. Twenty-four volunteers indicated a similar overall experience when using diclofenac gel and diclofenac liquid. However, they found the gel to have a less desirable scent and its consistency to be greasier and stickier than the diclofenac liquid (Galer 2010). When side effects have limited oral NSAID use in multimodal analgesia, it may be that intranasal (IN), IV, and topical formulations of NSAIDs could prove to be of benefit in the perioperative period and should be considered as tools that are emerging for multimodal analgesia.
Capsaicin, the active component of chili peppers, is a non-narcotic that acts at the transient receptor potential vanilloid 1 (TRPV1) receptor. It selectively stimulates unmyelinated C-fiber afferent neurons and causes the release of substance P. Following initial depolarization, continued exposure leads to desensitization and terminal die-back of C fibers that lasts several months An ultra-purified capsaicin (ALGRX 4975, 98% pure) has been investigated in a randomized, double-blind, placebo-controlled study of the analgesic efficacy of a single intraoperative wound instillation of 1000 μg of capsaicin after open-mesh groin hernia repair (Aasvang et al 2008). VAS pain scores assessed as area under the curve were significantly lower during the first 3 days postoperatively, but this effect was not observed after 72 hours. Local application of capsaicin during hernia repair does not lead to loss of sensory function in patients (Aasvang et al 2010). Further clinical trials have been carried out in patients undergoing total knee and hip arthroplasty, but the entire data have not been published to date. When capsaicin is used in the perioperative setting, the clinician must administer it well before the end of anesthesia to allow resolution of the acute burning sensation that occurs immediately after its application. The prolonged duration of analgesia produced by capsaicin could be extremely valuable in facilitating earlier rehabilitation after painful orthopedic surgery procedures. In contrast to local anesthetic, capsaicin does not affect motor or autonomic function and therefore will not interfere with postoperative rehabilitation. The capsaicin patch (NGX-4010), though used for various neuropathic chronic pain conditions, may be useful for acute pain in a multimodal fashion. This needs further large-scale randomized controlled trials.
Intranasal Analgesia
IN analgesia has evolved as a new route of administration of analgesics. The NSAID ketorolac given by this route reduced postoperative pain and opioid consumption following major surgery (Moodie et al 2008). When compared with placebo, patients receiving IN ketorolac had improved pain scores for third-molar extraction with bony impaction surgery (Grant and Mehlisch 2010). A randomized controlled trial in patients after abdominal surgery showed 26% less morphine consumed in the first 48 hours postoperatively in the ketorolac group (Singla et al 2010). Both studies remarked on the rapid onset of analgesia, which lasted up to 8 hours, and suggested that IN analgesia can be beneficial in ambulatory or fast-track surgery. The rapid onset and improved analgesia seen with IN ketorolac may be due to its higher penetration via the cribriform plate and into CSF, because it is known that higher CSF levels of NSAIDs are associated with greater analgesia.
Is There a Role for Pre-emptive Analgesia?
In a review of 66 studies (Ong et al 2005), it was concluded that the only pre-emptive treatment that improved all patient outcomes (pain intensity scores, supplemental analgesic consumption, and time to first analgesic consumption) was epidural anesthesia.
A newer concept suggests that the term “pre-emptive analgesia” should be abandoned and replaced with the term “preventive analgesia,” which means that to suppress central sensitization, analgesia should be maintained throughout the perioperative period; recent studies of preventive analgesia for persistent postoperative pain are promising (Pogatzki-Zahn and Zahn 2006).
Surgical Stress Response
Surgical stress and pain elicit a consistent and well-defined metabolic response involving the release of neuroendocrine hormones and cytokines that lead to a myriad of detrimental effects (Blackburn 2011). In addition to the rise in catabolically active hormones such as catecholamines, cortisol, angiotensin II, and antidiuretic hormone, stress causes an increase in adrenocorticotropic hormone, growth hormone, and glucagon (Hagen et al 1980). Epinephrine, cortisol, and glucagon produce hyperglycemia by promoting insulin resistance and gluconeogenesis. They induce protein catabolism and lipolysis to provide substrates for gluconeogenesis. Aldosterone, cortisol, and antidiuretic hormone influence water and electrolyte reabsorption by promoting Na+ and water retention while expending potassium. This contributes to increases in the extravascular fluid compartment both peripherally and within pulmonary parenchymal tissue. Local release of cytokines such as interleukin-2 (IL-2), IL-6, and tumor necrosis factor may contribute to abnormal physiologic responses such as alterations in heart rate, temperature, blood pressure, and ventilation (Michie and Wilmore 1990). In fact, some clinical studies now demonstrate a direct correlation between IL-6 and the extent of surgical trauma and the stress response (Buvanendran et al 2006).
Cardiovascular
The cardiovascular effects of pain are initiated by the release of catecholamines from sympathetic nerve endings and the adrenal medulla, aldosterone and cortisol from the adrenal cortex, and antidiuretic hormone from the hypothalamus and by activation of the renin–angiotensin system. These hormones have direct effects on the myocardium and vasculature, and they augment salt and water retention, which places a greater burden on the cardiovascular system.
The sympathoadrenal release of catecholamines and the effects of angiotensin II may result in hypertension, tachycardia, and dysrhythmias and may lead to myocardial ischemia in susceptible patients as a consequence of the increased oxygen demand. In addition, a significant proportion of perioperative myocardial ischemia is related to reductions in myocardial oxygen supply without hemodynamic aberrations. Activation of the sympathetic nervous system may trigger coronary vasoconstriction, which may result in myocardial ischemia in patients with atherosclerotic coronary artery disease. This may occur through direct activation of cardiac sympathetic nerves, as well as through circulating catecholamines, which may contribute to hypercoagulability, a known mediator of adverse outcomes in patients with ischemic heart disease (Burke et al 2011).
Respiratory
For surgical procedures performed on the thorax and abdomen, pain-induced reflex increases in skeletal muscle tension may lead to decreased total lung compliance, splinting, and hypoventilation. These changes promote atelectasis, contribute to further ventilation–perfusion abnormalities, and result in hypoxemia. In major surgical procedures or in high-risk patients, these respiratory effects of pain may lead to a significant reduction in functional residual capacity ranging from 25–50% of preoperative values (Rawal et al 1984, Tzani et al 2011). Hypoxemia stimulates increases in minute ventilation. Although tachypnea and hypocapnia are common initially, prolonged increases in the work of breathing may result in hypercapnic respiratory failure. Pulmonary consolidation and pneumonitis may occur because of hypoventilation and further aggravate the clinical scenario. These sequelae are especially significant in patients with pre-existing pulmonary disease, upper abdominal and thoracic incisions, advanced age, or obesity.
Gastrointestinal
Pain-induced sympathetic hyperactivity may cause reflex inhibition of gastrointestinal function (Augestad and Delaney 2010). This promotes postoperative ileus, which contributes to postoperative nausea, vomiting, and discomfort and delays resumption of an enteral diet. Failure to resume early enteral feeding may be associated with postoperative morbidity, including septic complications and abnormal wound healing (Moore et al 1992, Dudrick and Palesty 2011).
Immunological
The pain-related stress response suppresses both cellular and humoral immune function (Kurosawa and Kato 2008) and results in lymphopenia, leukocytosis, and depression of the reticuloendothelial system. Many known mediators of the stress response are potent immunosuppressants, and both cortisol and epinephrine infusions decrease neutrophil chemotaxis (Davis et al 1991). These effects can lower resistance to pathogens and may be key factors in the development of perioperative infectious complications (Hopf and Holm 2008). When surgical manipulation of neoplasms causes release of tumor cells, the postoperative stress response may reduce the cytotoxicity of killer T cells. Increases in catecholamines, glucocorticoids, and prostaglandins in response to stress may impair the immunologic responses important for patients with neoplasms (Snyder and Greenberg 2010).
Besides reducing the neuroendocrine stress response, regional anesthesia can decrease myocardial work and oxygen consumption by reducing the heart rate, arterial pressure, and left ventricular contractility. With continuous epidural analgesia with local anesthetics, postoperative pulmonary function is improved (Pöpping et al 2008) and paralytic ileus reduced (Kehlet 2008).
Non-Pharmacological Treatments
Recent trials of transcutaneous electrical nerve stimulation have been positive for moderate postoperative pain and as an adjuvant to opioids (Meissner 2009, Freynet and Falcoz 2010). The efficacy of acupuncture has been more difficult to assess because of the various types of interventions, but a recent review concluded that it reduced opioid consumption and thus was an effective adjuvant for management of postoperative pain (Sun 2008).
Cryotherapy is used to decrease swelling and pain following surgery, but its exact mechanism is not known. Cold therapy has the ability to reduce local inflammation by producing local vasoconstriction and decreasing nerve conduction velocity. The use of cryotherapy has controversial data in terms of the efficacy of analgesia. Multiple factors, including room temperature, the thickness of subcutaneous fat, and the thickness of postoperative dressings, affect the ability to cool the IA space; this may explain the conflicting results reported in the literature on the efficacy of cryotherapy after ACL surgery. A meta-analysis of seven randomized controlled trials demonstrated a significant reduction in pain scores but no improvement in range of motion or postoperative drainage (Raynor et al 2005). Excessive cold can lead to tissue damage from severe hypoxia. Continuous-flow cold therapy has the capacity to produce local vasoconstriction and may reduce bleeding, edema, and local inflammatory mediators.
Risk Factors for Chronic Pain
Persistent post-surgical pain is in general a largely under-recognized clinical problem with an incidence of 10–50%, depending on the surgical procedure (Kehlet et al 2006). Multiple risk factors may contribute to persistent post-surgical pain. In addition, there may be specific mechanisms that result in the acute postoperative pain condition converting to a persistent pain syndrome (Katz and Seltzer 2009).
Presurgical Factors
Preoperative pain intensity is a risk factor for persistent post-surgical pain (Katz and Seltzer 2009). For example, the incidence of chronic pain after total knee arthroplasty is increased in osteoarthritis patients who have more pain before surgery (Brander et al 2003). Recent studies have used experimental pain stimuli (e.g., heat) to quantify each subject’s pain sensitivity (Naert et al 2008, Granot 2009). Although the evidence is conflicting regarding whether a subject’s pain sensitivity predicts acute postoperative pain, there is better evidence that enhanced pain sensitivity contributes to the development of persistent postoperative pain (Granot 2009). Some preoperative psychosocial factors, such as attitude and mood, appear to be more important than others in promoting surgical recovery (Rosenberger et al 2006). In particular, high catastrophizing has been found to be associated with increased postoperative pain severity and an increased incidence of the development of chronic pain (Khan et al 2011). In fact, in examining factors that contribute to poor pain outcomes at 6 months after total knee arthroplasty, preoperative pain catastrophizing was the only psychological measure that predicted poor outcomes (Riddle et al 2010).
Acute Postoperative Factors
Intraoperative nerve injury is believed to be a likely causal mechanism in the development of persistent postoperative pain (Katz and Seltzer 2009). This may explain the high incidence of chronic pain after hernia repair or thoracotomy, two procedures that involve cutting or compression of nerves. Surgical trauma induces neuroplastic changes in pain sensitivity such that mechanical hyperalgesia can be demonstrated both in the area of inflammation (primary hyperalgesia) and in the non-inflamed surrounding tissue (secondary hyperalgesia). Studies have used a technique to measure the area of punctate mechanical hyperalgesia surrounding surgical wounds during the acute postoperative period (Stubhaug et al 1997, Lavond’homme et al 2005), and this may predict which patients will have persistent postoperative pain. Many studies have shown that the intensity of acute postoperative pain is a predictive factor for the development of persistent postoperative pain (Kehlet et al 2006). After breast surgery, clinically meaningful acute postoperative pain independently predicted more intense chronic pain 3 months after surgery (Poleshuck et al 2006).
Postoperative Management Practice
Achievement of optimal results with a continuous epidural analgesia technique requires appropriate perioperative planning and assessment. At the authors’ institution, epidural catheters for postoperative analgesia are commonly placed immediately before induction of operative anesthesia. This practice allows the anesthesiologist to administer a test dose of local anesthetic for evaluation while the patient is still awake. This facilitates the diagnosis of intrathecal, intravascular, or subdural catheter placement and allows confirmation of segmental epidural analgesia when the test dose of local anesthetic is administered. It also allows the continuous epidural infusion to be started during surgery.
This segmental nature of analgesia mandates the need to place an epidural catheter in a location to cover the dermatomes included in the surgical field. A general guideline for catheter locations in various types of surgeries is as follows: thoracic surgery—upper to lower thoracic; upper abdominal and renal surgery—low thoracic to high lumbar; orthopedic procedures of the lower extremities and lower abdominal and gynecologic surgery—lumbar region. Alternatively, catheter placement should be approximately at the dermatomal level that corresponds to a point intersecting the upper one-third and the lower two-thirds of the surgical incision. The differences among the opioids used for epidural analgesia relate to their duration of action and propensity to produce side effects. Patient factors such as advanced age, small body habitus, morbid obesity, history of sleep apnea, and general debilitation should be considered when initiating epidural analgesia because these conditions are associated with a greater propensity for respiratory complications. Reduced concentrations of opioids should be used when initiating epidural analgesia in such patients. Clonidine is used in patients who are taking opioids preoperatively.
Although epidural analgesia is usually effective, patients may occasionally experience inadequate pain relief. A systematic approach is necessary to evaluate and manage inadequate epidural analgesia. The initial step in this process is verification of the integrity of the catheter system, followed by a bolus (5–7 mL) of the epidural solution (typically a combination of dilute local anesthetic with opioid) and analgesic assessment after a short interval (15–30 minutes). If analgesia remains inadequate, a test dose of a local anesthetic solution, such as 2% lidocaine with 1:200,000 epinephrine, can then be given to evaluate the epidural catheter location. The test dose usually yields one of three results. If a bilateral sensory block occurs in a few segmental dermatomes, epidural catheter location is confirmed. In this case the volume of the infusion was probably insufficient for adequate dermatomal coverage, with resultant inadequate analgesia, and increasing the rate of infusion may produce effective analgesia. A unilateral sensory block after administration of a test dose of a local anesthetic is suggestive of the catheter tip residing laterally in or near a neuroforamen. Withdrawal of the catheter 1–2 cm is usually associated with a bilateral sensory block after a subsequent test dose. Once bilateral sensory blockade has been documented, adequate analgesia can be maintained with bolus administration of the epidural solution, followed by adjustment of the continuous epidural infusion or patient-controlled epidural infusion parameters.
Finally, lack of sensory blockade after test dose administration indicates that the epidural catheter does not reside in the epidural space. In this situation, the catheter is removed and the patient is given the option of having another epidural catheter placed or switching to PCA therapy.
Improvements in Monitoring of Postoperative Pain (Numerical Rating Scales and Beyond)
Acute pain is a personal experience, and measurements should rely on recording the patient’s own report because it has been demonstrated that staff underestimates pain and overestimates treatment effects. A patient’s pain and response to treatment should be assessed regularly for several days, depending on the surgical procedure. Although great emphasis has been placed on improving pain in the first 24 hours with various nerve blocks or IV formulations, the major gap may be the transition process from this state to oral analgesics and continuous treatment after discharge from the hospital. Pain assessments should be recorded in a readily available and visible form, together with other vital observations. Monitoring of postoperative pain should reflect not only pain intensity at rest but also activity-associated, dynamic pain and treatment-induced side effects by using standardized methods and protocols. Pain during activity and side effects such as sedation, PONV, and dizziness may prolong rehabilitation and the need for hospital stay.
A number of measurement scales have been used and validated, including categorical scales and the visual analog scale (VAS). Categorical pain intensity scales use words to describe the magnitude of the pain—for example, “no,” “mild,” “moderate,” and “severe” pain. Pain relief scales most often use the descriptors “none,” “slight,” “moderate,” “good,” and “complete.” The VAS uses a 10-cm line with the left end labeled “no pain” and the right end labeled “worst possible pain,” and numerical rating scales-(NRS) of pain intensity asks people to rate their pain from 0 (no pain) to 10 (worst pain). Recent reviews comparing different pain intensity scales have concluded that the VAS and the numerical rating scale are preferred over categorical measures (Dworkin et al 2005, Breivik et al 2008).
Pain relief scales may be perceived as being more convenient than pain intensity scales, particularly in analgesic trials, because patients have the same baseline relief (zero) but could start with different baseline intensity. The approach most commonly used in the immediate postoperative period, at least after major surgical procedures, however, is the use of categorical or visual analogue pain intensity scales. This is due to the fact that most often analgesia is initiated before the patient is awake and thus no baseline relief can be assessed. Sophisticated questionnaires, such as the McGill Pain Questionnaire, may provide a more comprehensive description of the different elements contributing to postoperative pain, but the use of such questionnaires is seldom practicable in daily clinical practice or may not be useful in directing treatment.
The use of neuroimaging for assessment of pain has gained recognition for both acute pain (Iadarola et al 1998) and chronic pain (Apkarian et al 2011). It may also play a vital role in determining the sites of activation for acute postoperative pain (Buvanendran et al 2007).
Advantages of Patient-Controlled Analgesia
IV PCA has been an accepted standard for management of postoperative pain for more than 20 years (Viscusi 2008). The primary advantage of PCA is high patient satisfaction, although there may not be a difference in pain scores. One review found that PCA opioid analgesia produced greater patient satisfaction than did conventional parenteral “as-needed” analgesia, as well as better pain control, but patients using PCA consumed higher amounts of opioids and had a higher incidence of pruritus (Hudcova et al 2006). PCA has several drawbacks. Device safety, in particular, pump programming errors, is always of concern, and newer infusion systems have better software to reduce the incidence of medication errors (Viscusi 2008). In addition, there is still an issue with patient comfort and mobility since the patient is attached to the pump, IV line, and pole.
Procedure-Specific Pain Management
The Procedure-Specific Postoperative Pain Management (PROSPECT) plan provides procedure-specific information on evidence-based pain treatment in various common operations (Kehlet et al 2007).
Major Joint Replacement Surgery
Although general anesthesia is still used in many hospitals for joint replacement, this trend is gradually decreasing as clinical studies (Chu et al 2006) have demonstrated a higher incidence of side effects from general anesthetics and intravenous opioids than from regional anesthesia and analgesia. Total knee arthroplasty is associated with severe postoperative pain, which interferes with early mobility and physical therapy and thereby affects both short- and long-term patient outcomes. Since the advent of regional anesthetic techniques, combined spinal epidural anesthesia followed by continuous epidural analgesia and PCEA has become a common anesthetic–analgesic procedure for joint replacement surgery. Epidural analgesia has been shown to reduce postoperative blood loss, provide superior pain control, and improve postoperative functional outcome in comparison to IV PCA (Choi et al 2003, Campbell et al 2008). Commonly used epidural analgesic solutions include an opioid such as fentanyl, a local anesthetic such as bupivacaine (ropivacaine is currently used by many hospitals because of its preferential sensory blockade properties and cardiovascular safety), or a combination of the two. Common side effects associated with epidural analgesia include hypotension, urinary retention, pruritus, nausea, vomiting, and headache. Significant intraoperative hypotension can lead to PONV and can also be associated with decreased postoperative cognitive function. This may be detrimental to initiation of early physical therapy, which is crucial for improved range of motion of the knee. Serious side effects such as epidural hematoma and the associated nerve damage, respiratory depression, and infection have also been reported, though rarely. For total knee arthroplasty, evidence supports the use of general anesthesia combined with a femoral nerve block for surgery and postoperative analgesia or, alternatively, spinal anesthesia with a local anesthetic plus spinal morphine (Fischer et al 2008).
Genetic Factors
Over the past 10 years a few genetic factors have been identified that are associated with human chronic pain syndromes (Kim et al 2009). However, no strong genetic association was found between monoamine neurotransmitter systems and acute postsurgical pain in patients undergoing oral surgery (Kim et al 2006). In addition, there are no published reports about genes that increase the risk for chronic postoperative pain (Katz and Seltzer 2009).
Conclusions
Several routes of administration of postoperative analgesia have been discussed. The route of administering the postoperative analgesia depends on the type of surgical trauma, as well as the severity of postoperative pain expected. For minor outpatient surgical procedures such as for hernia, local infiltration with a local anesthetic in a multimodal regimen is satisfactory. Conversely, major surgery such as joint replacements or thoracic surgery requires a multimodal regimen in addition to blockade of the neuronal transmission of pain signals.
Acute postoperative pain is a predictable response. Recent research has demonstrated that untreated acute postoperative pain can lead to chronic persistent pain. It is imperative that the health care provider managing acute postoperative pain understand the various options such as multimodal analgesia so that acute pain can be treated and the development of chronic pain from surgery can be prevented.
The references for this chapter can be found at www.expertconsult.com.
References
Aasvang E.K., Hansen J.B., Malmstrøm J., et al. The effect of wound instillation of a novel purified capsaicin formulation on postherniotomy pain: a double-blind, randomized, placebo-controlled study. Anesthesia and Analgesia. 2008;107:282–291.
Aasvang E.K., Hansen J.B., Kehlet H. Late sensory function after intraoperative capsaicin wound instillation. Acta Anaesthesiologica Scandinavica. 2010;54:224–231.
Afilalo M., Stegmann J.U., Upmalis D., et al. Tapentadol immediate release: a new treatment option for acute pain management. Journal of Pain Research. 2010;8:1–89.
American Society of Anesthesiologists. Practice guidelines for acute pain management in the perioperative setting. Anesthesiology. 2004;100:1573–1581.
American Society of Anesthesiologists. Practice guidelines for the perioperative management of patients with obstructive sleep apnea. Anesthesiology. 2006;104:1081–1093.
Apfelbaum J.L., Chen C., Mehta S., et al. Postoperative pain experiences: results from a national survey suggest postoperative pain continues to be undermanaged. Anesthesia and Analgesia. 2003;97:534–540.
Apkarian A.V., Hashmi J.A., Baliki M.N. Pain and the brain: specificity and plasticity of the brain in clinical chronic pain. Pain. 2011;152(Suppl 3):S49–S64.
Aubrun F., Gaillat C., Rosenthal D., et al. Effect of a low-dose ketamine regimen on pain, mood, cognitive function and memory after major gynaecological surgery: a randomized, double-blind, placebo-controlled trial. European Journal of Anaesthesiology. 2008;25:97–105.
Augestad K.M., Delaney C.P. Postoperative ileus: impact of pharmacological treatment, laparoscopic surgery and enhanced recovery pathways. World Journal of Gastroenterology. 2010;16:2067–2074.
Australian and New Zealand College of Anaesthetists, Guidelines on acute pain management, 2007. Available at, http://www.anzca.edu.au/resources/professional-documents/ps41.html. Accessed April 19, 2010
Aveline C., Gautier J.F., Vautier P., et al. Postoperative analgesia and early rehabilitation after total knee replacement: a comparison of continuous low-dose intravenous ketamine versus nefopam. European Journal of Pain. 2009;13:613–619.
Axley M., Horn J.L. Indications and management of continuous infusion of local anesthetics at home. Current Opinion in Anaesthesiology. 2010;23:650–655.
Bailie D.S., Ellenbecker T.S. Severe chondrolysis after shoulder arthroscopy: a case series. Journal of Shoulder and Elbow Surgery. 2009;18:742–747.
Barthel H.R., Haselwood D., Longley S., 3rd., et al. Randomized controlled trial of diclofenac sodium gel in knee osteoarthritis. Seminars in Arthritis and Rheumatism. 2009;39:203–212.
Basbaum A.I., Bautista D.M., Scherrer G., et al. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284.
Beaulieu P. Effects of nabilone, a synthetic cannabinoid, on postoperative pain. Canadian Journal of Anaesthesia. 2006;53:769–775.
Bell R.F., Dahl J.B., Moore R.A., et al. Peri-operative ketamine for acute post-operative pain: a quantitative and qualitative systemic review. Acta Anaesthesiologica Scandinavica. 2005;49:1405–1428.
Benumof J.L. Obstructive sleep apnea in the adult obese patient: implications for airway management. Anesthesiology Clinics of North America. 2002;20:789–811.
Blackburn G.L. Metabolic considerations in management of surgical patients. Surgical Clinics of North America. 2011;91:467–480.
Boss A.P., Maurer T., Seiler S., et al. Continuous subacromial bupivacaine infusion for postoperative analgesia after open acromioplasty and rotator cuff repair: preliminary results. Journal of Shoulder and Elbow Surgery. 2004;13:630–634.
Bourne M.H. Analgesics for orthopedic postoperative pain. American Journal of Orthopedics. 2004;33:128–135.
Brander V.A., Stulberg S.D., Adams A.D., et al. Predicting total knee replacement pain: a prospective, observational study. Clinical Orthopaedics and Related Research. 2003;416:27–36.
Breivik H., Borchgrevink P.C., Allen S.M., et al. Assessment of pain. British Journal of Anaesthesia. 2008;101:17–24.
Brennan T.J. Pathophysiology of postoperative pain. Pain. 2011;152(Suppl):S33–S40.
Brennan T.J., Vandermeulen E.P., Gebhart G.F. Characterization of a rat model of incisional pain. Pain. 1996;64:493–501.
Burke S.L., Lambert E., Head G.A. New approaches to quantifying sympathetic nerve activity. Current Hypertension Reports. 2011;13:249–257.
Buvanendran A., Ali A., Stoub T.R., et al. The use of brain positron emission tomography to identify sites of postoperative pain processing with and without epidural analgesia. Anesthesia and Analgesia. 2007;105:1784–1786.
Buvanendran A., Kroin J.S. Useful adjuvants for postoperative pain management. Best Practice & Research. Clinical Anaesthesiology. 2007;21:31–49.
Buvanendran A., Kroin J.S. Early use of memantine for neuropathic pain. Anesthesia and Analgesia. 2008;107:1093–1094.
Buvanendran A., Kroin J.S. Multimodal analgesia for controlling acute postoperative pain. Current Opinion in Anaesthesiology. 2009;22:588–593.
Buvanendran A., Kroin J.S., Berger R.A., et al. Upregulation of prostaglandin E2 and interleukins in the central nervous system and peripheral tissue during and after surgery in humans. Anesthesiology. 2006;104:403–410.
Buvanendran A., Kroin J.S., Della Valle C.J., et al. Perioperative oral pregabalin reduces chronic pain after total knee arthroplasty: a prospective, randomized, controlled trial. Anesthesia and Analgesia. 2010;110:199–207.
Buvanendran A., Kroin J.S., Kari M., et al. A new knee surgery model in rats to evaluate functional measures of postoperative pain. Anesthesia and Analgesia. 2008;107:300–308.
Buvanendran A., Thillainathan V. Preoperative and postoperative anesthetic and analgesic techniques for minimally invasive surgery of the spine. Spine. 2010;35(Suppl):S274–S280.
Calmet J., Esteve C., Boada S., et al. Analgesic effect of intra-articular ketorolac in knee arthroscopy: comparison of morphine and bupivacaine. Knee Surgery, Sports Traumatology, Arthroscopy. 2004;12:552–555.
Campbell A., McCormick M., McKinlay K., et al. Epidural vs. lumbar plexus infusions following total knee arthroplasty: randomized controlled trial. European Journal of Anaesthesiology. 2008;25:502–507.
Chia Y.Y., Liu K., Wang J.J. Intraoperative high dose fentanyl induces postoperative fentanyl tolerance. Canadian Journal of Anaesthesia. 1999;46:872–877.
Choi P.T., Bhandari M., Scott J., et al. Epidural analgesia for pain relief following hip or knee replacement. Cochrane Database of Systematic Reviews. 2003;3:CD003071.
Chu C.P.W., Yap J.C.C.M., Chen P.P. Postoperative outcome in Chinese patients having primary total knee arthroplasty under general anesthesia/intravenous patient controlled analgesia compared to spinal-epidural anesthesia/analgesia. Hong Kong Medical Journal 2006. 2006;12:442–447.
Chu L.F., Angst M.S., Clark D. Opioid-induced hyperalgesia in humans: molecular mechanisms and clinical considerations. Clinical Journal of Pain. 2008;24:479–496.
Clarke H., Pereira S., Kennedy D., et al. Gabapentin decreases morphine consumption and improves functional recovery following total knee arthroplasty. Pain Research & Management. 2009;14:217–222.
Coghlan J.A., Forbes A., McKenzie D., et al. Efficacy of subacromial ropivacaine infusion for rotator cuff surgery. A randomized trial. Journal of Bone and Joint Surgery. American Volume. 2009;91:1558–1567.
Colombo B., Annovazzi P.O., Comi G. Medications for neuropathic pain: current trends. Neurological Sciences. 2006;27(Suppl 2):S183–S189.
Coursin D.B., Coursin D.B., Maccioli G.A. Dexmedetomidine. Current Opinion in Critical Care. 2007;7:221–226.
Dal D., Tetik O., Altunkaya H., et al. The efficacy of intra-articular ketamine for postoperative analgesia in outpatient arthroscopic surgery. Arthroscopy. 2004;20:300–305.
Dauri M., Faria S., Gatti A., et al. Gabapentin and pregabalin for the acute post-operative pain management. A systematic-narrative review of the recent clinical evidences. Current Drug Targets. 2009;10:716–733.
Davis J.M., Albert J.D., Tracy K.J., et al. Increased neutrophil mobilization and decreased chemotaxis during cortisol and epinephrine infusions. Journal of Trauma. 1991;31:725–731.
Decker M.W., Reuter L.E., Bitner R.S. Nicotinic acetylcholine receptor agonists: a potential new class of analgesics. Current Topics in Medicinal Chemistry. 2004;4:369–384.
De Oliveira G.S., Jr., Almeida M.D., Benzon H.T., et al. Perioperative single dose systemic dexamethasone for postoperative pain: a meta-analysis of randomized controlled trials. Anesthesiology. 2011;115:575–588.
Derry S., Barden J., McQuay H.J., et al. Single dose oral celecoxib for acute postoperative pain in adults. Cochrane Database of Systematic Reviews. 2008;4:CD004233.
Dudrick S.J., Palesty J.A. Historical highlights of the development of enteral nutrition. Surgical Clinics of North America. 2011;91:945–964.
Duedahl T.H., Romsing J., Moniche S., et al. A qualitative systemic review of peri-operative dextromethorphan in post-operative pain. Acta Anaesthesiologica Scandinavica. 2006;50:1–13.
Duflo F., Boselli E., Ryvlin P., et al. Spinal muscarinic and nicotinic subtypes activated by clonidine in postincisional pain. Anesthesiology. 2005;103:1253–1258.
Dworkin R.H., Backonja M., Rowbotham M.C., et al. Advances in neuropathic pain: diagnosis, mechanisms, and treatment recommendations. Archives of Neurology. 2003;60:1524–1534.
Dworkin R.H., O’Connor A.B., Backonja M., et al. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain. 2007;132:237–251.
Dworkin R.H., Turk D.C., Farrar J.T., et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113:9–19.
Eisenach J.C. Muscarinic-mediated analgesia. Life Sciences. 1999;64:549–554.
Eisenach J.C., Tong C. Site of hemodynamic effects of intrathecal α-2 adrenergic agonists. Anesthesiology. 1991;74:766–771.
Engelhardt T., Zaarour C., Naser B., et al. Intraoperative low-dose ketamine does not prevent a remifentanil-induced increase in morphine requirement after pediatric scoliosis surgery. Anesthesia and Analgesia. 2008;107:1170–1175.
Etropolski M., Kelly K., Okamoto A., et al. Comparable efficacy and superior gastrointestinal tolerability (nausea, vomiting, constipation) of tapentadol compared with oxycodone hydrochloride. Advances in Therapy. 2011;28:401–417.
Farmery A.D., Wilson-MacDonald J. The analgesic effect of epidural clonidine after spinal surgery: a randomized placebo-controlled trial. Anesthesia and Analgesia. 2009;108:631–634.
Fishbain D.A., Cole B., Lewis J.E., et al. Do opioids induce hyperalgesia in humans? An evidence-based structured review. Pain Medicine. 2009;10:829–839.
Fischer H.B., Simanski C.J., Sharp C., et al. A procedure-specific systematic review and consensus recommendations for postoperative analgesia following total knee arthroplasty. Anaesthesia. 2008;63:1105–1123.
Fleischli J.W., Adams W.R. Use of postoperative steroids to reduce pain and inflammation. Journal of Foot and Ankle Surgery. 1999;38:232–237.
Fowler S.J., Symons J., Sabato S., et al. Epidural analgesia compared with peripheral nerve blockade after major knee surgery: a systematic review and meta-analysis of randomized trials. British Journal of Anaesthesia. 2008;100:154–164.
Fredrickson M.J., Krishnan S., Chen C.Y. Postoperative analgesia for shoulder surgery: a critical appraisal and review of current techniques. Anaesthesia. 2010;65:608–624.
Freynet A., Falcoz P.E. Is transcutaneous electrical nerve stimulation effective in relieving postoperative pain after thoracotomy? Interactive Cardiovascular and Thoracic Surgery. 2010;10:283–288.
Galer B.S. A comparative subjective assessment study of PENNSAID and Voltaren Gel, two topical formulations of diclofenac sodium. Pain Practice. 2010;20:1–9.
Gilron I. Gabapentin and pregabalin for chronic neuropathic and early postsurgical pain: current evidence and future directions. Current Opinion in Anaesthesiology. 2007;20:456–472.
Gomoll A.H., Kang R.W., Williams J.M., et al. Chondrolysis after continuous intra-articular bupivacaine infusion: an experimental model investigating chondrotoxicity in the rabbit shoulder. Arthroscopy. 2006;22:813–819.
Granot M. Can we predict persistent postoperative pain by testing preoperative experimental pain? Current Opinion in Anaesthesiology. 2009;22:425–430.
Grant G.M., Mehlisch D.R. Intranasal ketorolac for pain secondary to third molar impaction surgery: a randomized, double-blind, placebo-controlled trial. Journal of Oral and Maxillofacial Surgery. 2010;68:1025–1031.
Guler G., Karaoglu S., Akin A., et al. When to inject analgesic agents intra-articularly in anterior cruciate ligament reconstruction: before or after tourniquet releasing. Arthroscopy. 2004;20:918–921.
Gupta A., Bodin L., Holmstrom B., et al. A systematic review of the peripheral analgesic effects of intraarticular morphine. Anesthesia and Analgesia. 2001;93:761–770.
Hagen C., Brandt M.R., Kehlet H. Prolactin, LH, FSH, GH and cortisol response to surgery and the effect of epidural analgesia. Acta Endocrinologica. 1980;94:15–154.
Harrison M.K., Childs A., Carson P.E. Incidence of undiagnosed sleep apnea in patients scheduled for elective total joint arthroplasty. Journal of Arthroplasty. 2003;18:1044–1047.
Harvey G.P., Chelly J.E., Al Samsam T., et al. Patient-controlled ropivacaine analgesia after arthroscopic subacromial decompression. Arthroscopy. 2004;20:451–455.
Hay C.H., de Belleroche J.S. Dexamethasone prevents the induction of COX-2 mRNA and prostaglandins in the lumbar spinal cord following intraplantar FCA in parallel with inhibition of oedema. Neuropharmacology. 1998;37:739–744.
Ho K.M., Ismail H., Lee K.C., et al. Use of intrathecal neostigmine as an adjunct to other spinal medications in perioperative and peripartum analgesia: a meta-analysis. Anaesthesia and Intensive Care. 2005;33:41–53.
Ho K.Y., Gan T.J., Habib A.S. Gabapentin and postoperative pain—a systematic review of randomized controlled trials. Pain. 2006;126:91–101.
Hopf H.W., Holm J. Hyperoxia and infection. Best Practice & Research. Clinical Anaesthesiology. 2008;22:553–569.
Horlocker T.T., Wedel D.J., Benzon H., et al. Regional anesthesia in the anticoagulated patient: defining the risks. Regional Anesthesia and Pain Medicine. 2003;28:172–197.
Huang Y.M., Wang C.M., Wang C.T., et al. Perioperative celecoxib administration for pain management after total knee arthroplasty—a randomized, controlled study. BMC Musculoskeletal Disorders. 2008;9:77.
Hudcova J., McNicol E., Quah C., et al. Patient controlled opioid analgesia versus conventional opioid analgesia for postoperative pain. Cochrane Database of Systematic Reviews. 2006;4:CD003348.
Hughes D.G. Intra-articular bupivacaine for pain relief in arthroscopic surgery (letter). Anesthesia. 1985;40:821.
Hutchison A., Chon E.H., Tucker W.F., et al. Comparison of a fentanyl, morphine, and hydromorphone patient-controlled intravenous delivery for acute postoperative analgesia: a multicenter study of opioid-induced adverse reactions. Hospital Pharmacy. 2006;41:659–663.
Iadarola M.J., Berman K.F., Zeffiro T.A., et al. Neural activation during acute capsaicin-evoked pain and allodynia assessed with PET. Brain. 1998;121:931–947.
Jakobsson J. Preoperative single-dose intravenous dexamethasone during ambulatory surgery: update around the benefit versus risk. Current Opinion in Anaesthesiology. 2010;23:682–686.
Kahn Z.P., Ferguson C.N., Jones R.M. Alpha-2 and imidazoline receptor agonists. Anaesthesia. 1999;54:146–165.
Kahokehr A., Sammour T., Srinivasa S., et al. Systematic review and meta-analysis of intraperitoneal local anaesthetic for pain reduction after laparoscopic gastric procedures. British Journal of Surgery. 2011;98:29–36.
Kalso E., Smith L., McQuay H.J., et al. No pain, no gain: clinical excellence and scientific rigour—lessons learned from IA morphine. Pain. 2002;98:269–275.
Katz J., Seltzer Z. Transition from acute to chronic postsurgical pain: risk factors and protective factors. Expert Review of Neurotherapeutics. 2009;9:723–744.
Kawamata M., Watanabe H., Nishikawa K., et al. Different mechanisms of development and maintenance of experimental incision-induced hyperalgesia in human skin. Anesthesiology. 2002;97:550–559.
Kaya F.N., Sahin S., Owen M.D., et al. Epidural neostigmine produces analgesia but also sedation in women after cesarean delivery. Anesthesiology. 2004;100:381–385.
Kehlet H. Postoperative ileus—an update on preventive techniques. Nature Clinical Practice. Gastroenterology & Hepatology. 2008;5:552–558.
Kehlet H., Andersen L.O. Local infiltration analgesia in joint replacement: the evidence and recommendations for clinical practice. Acta Anaesthesiologica Scandinavica. 2011;55:778–784.
Kehlet H., Jensen T.S., Woolf C.J. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367:1618–1625.
Kehlet H., Wilkinson R.C., Fischer H.B., et al. PROSPECT: evidence-based, procedure-specific postoperative pain management. Best Practice & Research. Clinical Anaesthesiology. 2007;21:149–159.
Kehlet H., Wilmore D.W. Evidence-based surgical care and the evolution of fast-track surgery. Annals of Surgery. 2008;248:189–198.
Khan R.S., Ahmed K., Blakeway E., et al. Catastrophizing: a predictive factor for postoperative pain. American Journal of Surgery. 2011;201:122–131.
Khoury G.F., Chen A.C.N., Garland D.E., et al. Intraarticular morphine, bupivacaine, and morphine/bupivacaine for pain control after knee videoarthroscopy. Anesthesiology. 1992;77:263–266.
Kim H., Clark D., Dionne R.A., et al. Journal of Pain. 2009;10:663–693.
Kim H., Lee H., Rowan J., Brahim J., Dionne R.A., et al. Genetic polymorphisms in monoamine neurotransmitter systems show only weak association with acute post-surgical pain in humans. Molecular Pain. 2006;2:24.
Klinken C. Effects of tourniquet time in knee arthroscopy patients receiving intraarticular morphine combined with bupivacaine. Clinical Forum for Nurse Anesthetists. 1995;6:37–42.
Koppert W., Schmelz M. The impact of opioid-induced hyperalgesia for postoperative pain. Best Practice & Research. Clinical Anaesthesiology. 2007;21:65–83.
Kroin J.S., Buvanendran A., Beck D.R., et al. Clonidine prolongation of lidocaine analgesia after sciatic nerve block in rats is mediated via the hyperpolarization-activated cation current, not by alpha-adrenoreceptors. Anesthesiology. 2004;101:488–494.
Kroin J.S., Buvanendran A., Watts D.E., et al. Upregulation of cerebrospinal fluid and peripheral prostaglandin E2 in a rat postoperative pain model. Anesthesia and Analgesia. 2006;103:334–343.
Kurosawa S., Kato M. Anesthetics, immune cells, and immune responses. Journal of Anesthesia. 2008;22:263–277.
Kwong W.J., Ozer-Stillman I., Miller J.D., et al. Cost-effectiveness analysis of tapentadol immediate release for the treatment of acute pain. Clinical Therapeutics. 2010;32:1768–1781.
Latremoliere A., Woolf C.J. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. Journal of Pain. 2009;10:895–926.
Lavand’homme P., De Kock M., Waterloos H. Intraoperative epidural analgesia combined with ketamine provides effective preventive analgesia in patients undergoing major digestive surgery. Anesthesiology. 2005;103:813–820.
LePage K.T., Ishmael J.E., Low C.M., et al. Differential binding properties of [3H]dextrorphan and [3H]MK-801 in heterologously expressed NMDA receptors. Neuropharmacology. 2005;49:1–16.
Lin T.F., Yeh Y.C., Lin F.S., et al. Effect of combining dexmedetomidine and morphine for intravenous patient-controlled analgesia. British Journal of Anaesthesia. 2009;102:117–122.
Loftus R.W., Yeager M.P., Clark J.A., et al. Intraoperative ketamine reduces perioperative opiate consumption in opiate-dependent patients with chronic back pain undergoing back surgery. Anesthesiology. 2010;113:639–646.
Loubert C., Hinova A., Fernando R. Update on modern neuraxial analgesia in labour: a review of the literature of the last 5 years. Anaesthesia. 2011;66:191–212.
Macario A., Royal M.A. A literature review of randomized clinical trials of intravenous acetaminophen (paracetamol) for acute postoperative pain. Pain Practice. 2011;11:290–296.
Marchal J.M., Delgado-Martinez A.D., Poncela M., et al. Does the type of arthroscopic surgery modify the analgesic effect of intraarticular morphine and bupivacaine? A preliminary study. Clinical Journal of Pain. 2003;29:240–246.
Marret E., Flahault A., Samama C.M., et al. Effects of postoperative, nonsteroidal, antiinflammatory drugs on bleeding risk after tonsillectomy: meta-analysis of randomized, controlled trials. Anesthesiology. 2003;98:1497–1502.
Martin T.J., Buechler N.L., Kahn W., et al. Effects of laparotomy on spontaneous exploratory activity and conditioned operant responding in the rat: a model for postoperative pain. Anesthesiology. 2004;101:191–203.
Masferrer J.L., Reddy S.T., Zweifel B.S., et al. In vivo glucocorticoids regulate cyclooxygenase-2 but not cyclooxygenase-1 in peritoneal macrophages. Journal of Pharmacology and Experimental Therapeutics. 1994;270:1340–1344.
Massey T., Derry S., Moore R.A., et al. Topical NSAIDs for acute pain in adults. Cochrane Database of Systematic Reviews. 2010;6:CD007402.
Masursky D., Dexter F., McCartney C.J., et al. Predicting orthopedic surgeons` preferences for peripheral nerve blocks for their patients. Anesthesia and Analgesia. 2008;106:561–567.
Maund E., McDaid C., Rice S., et al. Paracetamol and selective and non-selective non-steroidal anti-inflammatory drugs for the reduction in morphine-related side-effects after major surgery: a systematic review. British Journal of Anaesthesia. 2011;106:292–297.
McCarberg B.H., Argoff C.E. Topical diclofenac epolamine patch 1.3% for treatment of acute pain caused by soft tissue injury. International Journal of Clinical Practice. 2010;64:1546–1553.
McCarthy G.C., Megalla S.A., Habib A.S. Impact of intravenous lidocaine infusion on postoperative analgesia and recovery from surgery: a systematic review of randomized controlled trials. Drugs. 2010;70:1149–1163.
Meissner W. The role of acupuncture and transcutaneous-electrical nerve stimulation for postoperative pain control. Current Opinion in Anaesthesiology. 2009;22:623–626.
Michie H.R., Wilmore D.W. Sepsis, signals, and surgical sequelae (a hypothesis). Archives of Surgery. 1990;125:531–536.
Moen M.D. Topical diclofenac solution. Drugs. 2009;69:2321–2632.
Moodie J.E., Brown C.R., Bisley E.J., et al. The safety and analgesic efficacy of intranasal ketorolac in patients with postoperative pain. Anesthesia and Analgesia. 2008;107:2025–2031.
Moore F.A., Feliciano D.V., Andrassy R.J., et al. Early enteral feeding, compared with parenteral, reduces postoperative septic complications. The results of a meta-analysis. Annals of Surgery. 1992;216:172–183.
Naert A.L., Kehlet H., Kupers R. Characterization of a novel model of tonic heat pain stimulation in healthy volunteers. Pain. 2008;138:163–171.
Nasir D., Howard J.E., Joshi G.P., et al, A survey of acute pain service structure and function in United States hospitals. Pain Research and Treatment [serial online] 2011. Article ID 934932. Available at, www.hindawi.com/journals/prt/2011/934932. Accessed April 19, 2011
Ng H.P., Nordström U., Axelsson K. Efficacy of intra-articular bupivacaine, ropivacaine or a combination of ropivacaine, morphine, and ketorolac on postoperative pain relief after ambulatory arthroscopic knee surgery: a randomized double-blind study. Regional Anesthesia and Pain Medicine. 2006;31:26–33.
Nishimori M., Ballantyne J.C., Low J.H. Epidural pain relief versus systemic opioid-based pain relief for abdominal aortic surgery. Cochrane Database of Systematic Reviews. 2006;3:CD005059.
Nasir D., Howard J.E., Joshi G.P., et al, A survey of acute pain service structure and function in United States hospitals. Pain Research and Treatment [serial online] 2011. Article ID 934932. Available at, www.hindawi.com/journals/prt/2011/934932. Accessed April 19, 2011
Ong C.K., Lirk P., Seymour R.A., et al. The efficacy of preemptive analgesia for acute postoperative pain management: a meta-analysis. Anesthesia and Analgesia. 2005;100:757–773.
Philips B.D., Liu S.S., Wukovits B., et al. Creation of a novel recuperative pain medicine service to optimize postoperative analgesia and enhance patient satisfaction. Hospital for Special Surgery Journal. 2010;6:61–65.
Pogatzki-Zahn E.M., Zahn P.K. From preemptive to preventive analgesia. Current Opinion in Anaesthesiology. 2006;19:551–555.
Poleshuck E.L., Katz J., Andrus C.H., et al. Risk factors for chronic pain following breast cancer surgery: a prospective study. Journal of Pain. 2006;7:626–634.
Pöpping D.M., Elia N., Marret E., et al. Protective effects of epidural analgesia on pulmonary complications after abdominal and thoracic surgery: a meta-analysis. Archives of Surgery. 2008;143:990–999.
Pöpping D.M., Elia N., Marret E., et al. Clonidine as an adjuvant to local anesthetics for peripheral nerve and plexus blocks: a meta-analysis of randomized trials. Anesthesiology. 2009;111:406–415.
Randinitis E.J., Posvar E.L., Alvey C.W. Pharmacokinetics of pregabalin in subjects with various degrees of renal function. Journal of Clinical Pharmacology. 2003;43:277–283.
Rasmussen S., Larsen A.S., Thomsen S.T., et al. Intra-articular glucocorticoid, bupivacaine and morphine reduces pain, inflammatory response and convalescence after arthroscopic meniscectomy. Pain. 1998;78:131–134.
Rawal N. Postoperative pain treatment for ambulatory surgery. Best Practice & Research. Clinical Anaesthesiology. 2007;21:129–148.
Rawal N., Sjostrand U., Christoffersson E., et al. Comparison of intramuscular and epidural morphine for postoperative analgesia in the grossly obese: influence on postoperative ambulation and pulmonary function. Anesthesia and Analgesia. 1984;63:583–592.
Raynor M.C., Pietrobon R., Guller U. Cryotherapy after ACL reconstruction: a meta-analysis. Journal of Knee Surgery. 2005;18:123–129.
Remérand F., Le Tendre C., Baud A., et al. The early and delayed analgesic effects of ketamine after total hip arthroplasty: a prospective, randomized, controlled, double-blind study. Anesthesia and Analgesia. 2009;109:1963–1971.
Remy C., Marret E., Bonnet F. Effects of acetaminophen on morphine side-effects and consumption after major surgery: meta-analysis of randomized controlled trials. British Journal of Anaesthesia. 2005;94:505–513.
Richman J.M., Liu S.S., Courpas G. Does continuous peripheral nerve block provide superior pain control to opioids? A meta-analysis. Anesthesia and Analgesia. 2006;102:248–257.
Riddle D.L., Wade J.B., Jiranek W.A. Preoperative pain catastrophizing predicts pain outcome after knee arthroplasty. Clinical Orthopaedics and Related Research. 2010;468:798–806.
Rosenberger P.H., Jokl P., Ickovics J. Psychosocial factors and surgical outcomes: an evidence-based literature review. Journal of the American Academy of Orthopaedic Surgeons. 2006;14:397–405.
Rosenquist R.W., Rosenberg J. Postoperative pain guidelines. Regional Anesthesia and Pain Medicine. 2003;28:279–288.
Royal College of Anaesthetists, Guidelines for the provision of anaesthetic services, 2010. Available at, www.rcoa.ac.uk/docs/gpas-acutepain.pdf. Accessed April 19, 2010
Salerno A., Hermann R. Efficacy and safety of steroid use for postoperative pain relief. Journal of Bone and Joint Surgery. American Volume. 2006;88:1361–1372.
Salinas F.V., Liu S.S., Mulroy M.F. The effect of single-injection femoral nerve block versus continuous femoral nerve block after total knee arthroplasty on hospital length of stay and long-term functional recovery within an established clinical pathway. Anesthesia and Analgesia. 2006;102:1234–1239.
Salter M.W. Cellular signalling pathways of spinal pain neuroplasticity as targets for analgesic development. Current Topics in Medicinal Chemistry. 2005;5:557–567.
Sawynok J., Esser M.J., Reid A.R. Antidepressants as analgesics: an overview of central and peripheral mechanisms of action. Journal of Psychiatry & Neuroscience. 2001;26:21–29.
Senthilkumaran S., Tate R., Read J.R.M., et al. Intra-articular morphine and bupivacaine for post-operative analgesia in anterior cruciate ligament reconstruction: a prospective randomized controlled trial. Knee Surgery, Sports Traumatology, Arthroscopy. 2010;18:731–735.
Shainhouse J.Z., Grierson L.M., Naseer Z. A long-term, open-label study to confirm the safety of topical diclofenac solution containing dimethyl sulfoxide in the treatment of the osteoarthritic knee. American Journal of Therapeutics. 2010;17:566–576.
Sindrup S.H., Bach F.W., Madsen C., et al. Venlafaxine versus imipramine in painful polyneuropathy: a randomized, controlled trial. Neurology. 2003;60:1284–1289.
Singelyn F., Lhotel L., Fabre B. Pain relief after arthroscopic shoulder surgery: a comparison of intraarticular analgesia, suprascapular nerve block, and interscalene brachial plexus block. Anesthesia and Analgesia. 2004;99:589–592.
Singla N., Singla S., Minkowitz H.S., et al. Intranasal ketorolac for acute postoperative pain. Current Medical Research and Opinion. 2010;26:1915–1923.
Smirnov G., Terävä M., Tuomilehto H., et al. Etoricoxib for pain management during thyroid surgery—a prospective, placebo-controlled study. Otolaryngology—Head and Neck Surgery. 2008;138:92–97.
Snyder G.L., Greenberg S. Effect of anaesthetic technique and other perioperative factors on cancer recurrence. British Journal of Anaesthesia. 2010;105:106–115.
Stubhaug A., Breivik H., Eide P.K., et al. Mapping of punctate hyperalgesia around a surgical incision demonstrates that ketamine is a powerful suppressor of central sensitization to pain following surgery. Acta Anaesthiologica Scandinavica. 1997;41:1124–1132.
Subramaniam K., Subramaniam B., Steinbrook R.A. Ketamine as adjuvant analgesic to opioids: a quantitative and qualitative systemic review. Anesthesia and Analgesia. 2004;99:482–495.
Sun Y., Gan T.J., Dubose J.W., et al. Acupuncture and related techniques for postoperative pain: a systematic review of randomized controlled trials. British Journal of Anaesthesia. 2008;101:151–160.
Tufanogullari B., White P.F., Peixoto M.P. Dexmedetomidine infusion during laparoscopic bariatric surgery: the effect on recovery outcome variables. Anesthesia and Analgesia. 2008;106:1741–1748.
Tzani P., Chetta A., Olivieri D. Patient assessment and prevention of pulmonary side-effects in surgery. Current Opinion in Anaesthesiology. 2011;24:2–7.
Unnerstall J.R., Kopejtic T.A., Kahr M.J. Distribution of alpha 2 agonist binding sites in the rat and human central nervous system: analysis of some functional, anatomic correlates of the pharmacologic effects of clonidine and related adrenergic agents. Brain Research. 1984;319:69–101.
Viscusi E.R. Patient-controlled drug delivery for acute postoperative pain management: a review of current and emerging technologies. Regional Anesthesia and Pain Medicine. 2008;33:146–158.
Wadhwa A., Clarke D., Goodchild C.S. Large-dose oral dextromethorphan as an adjunct to patient-controlled analgesia with morphine after knee surgery. Anesthesia and Analgesia. 2001;92:448–454.
Wang J.J., Ho S.T., Lee S.C., et al. Intraarticular triamcinolone acetonide for pain control after arthroscopic knee surgery. Anesthesia and Analgesia. 1998;87:1113–1116.
Weinbroum A.A., Bender B., Bickels J., et al. Preoperative and postoperative dextromethorphan provides sustained reduction in postoperative pain and patient-controlled epidural analgesia requirement: a randomized, placebo-controlled, double-blind study in lower-body bone malignancy–operated patients. Cancer. 2003;97:2334–2340.
Wheeler M., Oderda G.M., Ashburn M.A., et al. Adverse events associated with postoperative opioid analgesia: a systematic review. Journal of Pain. 2002;3:159–180.
Whitford A., Healy M., Joshi G.P., et al. The effect of tourniquet release time on the analgesic efficacy of intraarticular morphine after arthroscopic knee surgery. Anesthesia and Analgesia. 1997;84:791–793.
White P.F., Tang J., Wender R.H. The effects of oral ibuprofen and celecoxib in preventing pain, improving recovery outcomes and patient satisfaction after ambulatory surgery. Anesthesia and Analgesia. 2011;112:323–329.
Williams B.A., Kentor M.L., Bottegal M.T. The incidence of falls at home in patients with perineural femoral catheters: a retrospective summary of a randomized clinical trial. Anesthesia and Analgesia. 2007;104:1002.
Wong B.Y., Coulter D.A., Choi D.W., et al. Dextrorphan and dextromethorphan, common antitussives, are antiepileptic and antagonize N-methyl-D-aspartate in brain slices. Neuroscience Letters. 1988;85:261–266.
Woods G.W., O’Connor D.P., Calder C.T. Continuous femoral nerve block versus intra-articular injection for pain control after anterior cruciate ligament reconstruction. American Journal of Sports Medicine. 2006;34:1328–1333.
Woolf C.J. Intrathecal high dose morphine produces hyperalgesia in the rat. Brain Research. 1981;30(209):491–495.
Woolf C.J. Overcoming obstacles to developing new analgesics. Nature Medicine. 2010;16:1241–1247.
Wu C.L., Cohen S.R., Richman J.M., et al. Efficacy of postoperative patient-controlled and continuous infusion epidural analgesia versus intravenous patient-controlled analgesia with opioids: a meta-analysis. Anesthesiology. 2005;103:1079–1088.
Yaksh T.L. Pharmacology of spinal adrenergic systems which modulate spinal nociceptive processing. Pharmacology, Biochemistry, and Behavior. 1985;22:845–858.
Yeh C.C., Wu C.T., Lee M.S., et al. Analgesic effects of preincisional administration of dextromethorphan and tenoxicam following laparoscopic cholecystectomy. Acta Anaesthesiologica Scandinavica. 2004;48:1049–1053.
Zahn P.K., Brennan T.J. Primary and secondary hyperalgesia in a rat model for human postoperative pain. Anesthesiology. 1999;90:863–872.
Zakine J., Samarcq D., Lorne E., et al. Postoperative ketamine administration decreases morphine consumption in major abdominal surgery: a prospective, randomized, double-blind, controlled study. Anesthesia and Analgesia. 2008;106:1856–1861.
Zhang J., Ho K.Y., Wang Y. Efficacy of pregabalin in acute postoperative pain: a meta-analysis. British Journal of Anaesthesia. 2011;106:454–462.
American Society of Anesthesiologists. Practice guidelines for the perioperative management of patients with obstructive sleep apnea. Anesthesiology. 2006;104:1081–1093.
Apkarian A.V., Hashmi J.A., Baliki M.N. Pain and the brain: specificity and plasticity of the brain in clinical chronic pain. Pain. 2011;152(Suppl 3):S49–S64.
Basbaum A.I., Bautista D.M., Scherrer G., et al. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284.
Brennan T.J. Pathophysiology of postoperative pain. Pain. 2011;152(Suppl):S33–S40.
Buvanendran A., Kroin J.S. 2009 Multimodal analgesia for controlling acute postoperative pain. Current Opinion in Anaesthesiology. 2009;22:588–593.
Buvanendran A., Kroin J.S., Berger R.A., et al. Upregulation of prostaglandin E2 and interleukins in the central nervous system and peripheral tissue during and after surgery in humans. Anesthesiology. 2006;104:403–410.
Chu L.F., Angst M.S., Clark D. Opioid-induced hyperalgesia in humans: molecular mechanisms and clinical considerations. Clinical Journal of Pain. 2008;24:479–496.
Katz J., Seltzer Z. Transition from acute to chronic postsurgical pain: risk factors and protective factors. Expert Review of Neurotherapeutics. 2009;9:723–744.
Kehlet H., Jensen T.S., Woolf C.J. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367:1618–1625.
Kehlet H., Wilkinson R.C., Fischer H.B., et al. PROSPECT: evidence-based, procedure-specific postoperative pain management. Best Practice & Research. Clinical Anaesthesiology. 2007;21:149–159.
Latremoliere A., Woolf C.J. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. Journal of Pain. 2009;10:895–926.
Ong C.K., Lirk P., Seymour R.A., et al. The efficacy of preemptive analgesia for acute postoperative pain management: a meta-analysis. Anesthesia and Analgesia. 2005;100:757–773.
Viscusi E.R. Patient-controlled drug delivery for acute postoperative pain management: a review of current and emerging technologies. Regional Anesthesia and Pain Medicine. 2008;33:146–158.
Woolf C.J. Overcoming obstacles to developing new analgesics. Nature Medicine. 2010;16:1241–1247.
Woolf C.J. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152(Suppl):S2–S15.