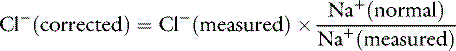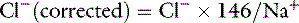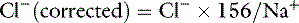Chapter 4 Disorders of Chloride
Hyperchloremia and Hypochloremia
“Whereas for a long time it was assumed that chloride ions were reabsorbed entirely passively with sodium — the “mendicant” role of chloride — more recent studies suggest that several distinctive reabsorptive transport mechanisms operate in parallel.”86
Chloride constitutes approximately two thirds of the anions in plasma and the remainder of extracellular fluid (ECF). It also is the major anion filtered by the glomeruli and reabsorbed in the renal tubules. Chloride is important not only for maintaining osmolality but also actively participates in acid-base regulation.
Chloride is present in plasma at a mean concentration of approximately 110 mEq/L in dogs and 120 mEq/L in cats.17 Chloride concentration in venous samples is 3 to 4 mEq/L lower than those in arterial samples when cells are separated from plasma anaerobically.92 The intracellular concentration of chloride is much lower than its plasma concentration and is dependent on the resting membrane potential of the cell. Muscle cells, for example, have a resting membrane potential of approximately −68 mV and an average chloride concentration ([Cl−]) of 2 to 4 mEq/L, whereas red blood cells have a resting membrane potential of approximately −15 mV and an average [Cl−] of 60 mEq/L.55 This higher intracellular concentration of chloride ions in erythrocytes allows chloride to move in and out of the red blood cells very effectively, as dictated by electrical charges on either side of the cell membrane. This is an important difference from other cells and is the basis of the so-called “chloride-shift” in the red cell membrane.55 The chloride ion distribution in various body fluids is summarized in Box 4-1.
Box 4-1 Chloride Ion in Various Body Fluids
From de Morais HSA: Chloride ion in small animal practice: the forgotten ion, J Vet Emerg Crit Care 2:11–24, 1992.
Chloride metabolism
Gastrointestinal tract
Under normal conditions, humans produce 1 to 2 L of gastric juice daily. The sodium concentration ([Na+]) and [Cl−] of gastric juice is quite variable, ranging from 20 to 100 mEq/L and 120 to 160 mEq/L, respectively.76 In the jejunum, sodium is absorbed actively against small electrochemical gradients and also through relatively large mucosal “pores” in the proximal bowel. Chloride absorption in the jejunum generally follows sodium to maintain electroneutrality. It is believed that chloride reabsorption in the jejunum occurs via the paracellular route in response to the transepithelial potential generated by active sodium transport.24 The ileum is less permeable to ions than the jejunum. Absorption of chloride and secretion of bicarbonate in the ileum are coupled by processes that may involve active transport of one or both ions. Highly efficient absorption of sodium and chloride occurs in the colon, where 90% of the sodium and chloride entering is reabsorbed. There appears to be no direct or indirect coupling between sodium and chloride or bicarbonate reabsorption in the distal colon. Active chloride reabsorption and bicarbonate secretion occur in the distal colon. Chloride also can be secreted in the jejunum, ileum, and colon.24,76 Pancreatic juice usually is not rich in chloride ions. However, there is a reciprocal relationship between chloride and bicarbonate concentration in pancreatic fluid that is dependent on flow rate, with chloride being the major anion at lower rates of secretion.24
Kidneys
The kidneys play an important role in the regulation of plasma chloride concentration. After sodium, chloride is the most prevalent ion in the glomerular ultrafiltrate. Most of the chloride filtered is reabsorbed in the renal tubules. The traditional view of epithelial transport in the kidneys represents the chloride ion as an obedient passive partner that follows the actively transported sodium ion. This view does not apply to many epithelia, including specific nephron segments. Chloride transport is intimately related to sodium and fluid transport and to cellular acid-base metabolism.86
Chloride reabsorption in the proximal tubule is actively and passively linked to active sodium reabsorption. A formate-chloride exchange mechanism exists in the luminal membrane of proximal tubular cells and is responsible for active chloride reabsorption.81 Reabsorbed chloride returns to the systemic circulation at the basolateral membrane primarily by a potassium chloride (K+- Cl−) cotransporter. Of filtered chloride, approximately 50% to 60% is reabsorbed by the proximal convoluted and straight tubules. Chloride reabsorption occurs transcellularly in the thick ascending limb of Henle’s loop, leading to the generation of a lumen-positive transepithelial voltage. Sodium is reabsorbed transcellularly or paracellularly, and the transepithelial voltage drives the latter process. Chloride ion delivery is the rate-limiting step in this process, and net sodium chloride (NaCl) transport increases directly with fluid [Cl−] concentration. Loop diuretics such as furosemide and bumetanide act in the loop of Henle by competing for the chloride site on the Na+-K+-2 Cl−carrier.24,46,81,86
A comprehensive model explaining sodium chloride transport in the distal tubule is not yet available. This is because of, in part, the cellular heterogeneity of this nephron segment and differences among species and because a portion of this nephron segment is not accessible to micropuncture techniques in rats, the most extensively studied species. Thiazide diuretics act by inhibiting the Na+- Cl− carrier in the early distal tubule, apparently at the chloride site.46 Conversely, loop diuretics do not block NaCl reabsorption at this site. Chloride ion transport in the collecting tubule is closely related to bicarbonate transport.81 Little is known about chloride transport in the medullary collecting tubules. In the cortical collecting tubules, however, the paracellular pathway, which is highly conductive for chloride ions, is an important route for reabsorption of chloride by diffusion down an electrochemical gradient. An increase in the lumen-positive transepithelial potential difference (TPD) decreases net chloride reabsorption, whereas a decrease in TPD increases chloride reabsorption. Therefore, hormones that change TPD in the cortical collecting tubule can affect chloride reabsorption. Experimentally, administration of deoxycorticosterone acetate (DOCA) twice daily resulted in a mild increase in [Na+] and no change in [Cl−].54 The resulting increase in strong ion difference (SID; the difference between all strong cations and all strong anions in plasma; see Chapter 13) was associated with a mild increase in bicarbonate ion concentration ([HCO3−]). Administration of DOCA in sodium-supplemented dogs caused a significant increase in plasma [Na+] and [HCO3−] with no change in plasma [Cl−].64 When NaHCO3, instead of NaCl, was added to the diet, [Na+] and [HCO3−] increased significantly, whereas [Cl−] decreased. Increased urinary loss of chloride is believed to be associated with hyperadrenocorticism. In a study of 117 dogs with hyperadrenocorticism, only 12 had [Cl−] below 105 mEq/L.61 However, 25 of these dogs had hypernatremia, and the [Cl−] could have been low relative to the [Na+]. The mean [Na+] was 149.9 mEq/L, and the mean [Cl−] was 108 mEq/L (mean [Cl−] after correcting for changes in free water was 105 mEq/L). The cortical collecting duct is the main site of action for mineralocorticoids and glucocorticoids.19 Administration of DOCA increases TPD in rats and rabbits, increasing sodium reabsorption in the cortical collecting tubules. Such an effect could explain the observed changes in chloride and sodium concentrations in dogs with hyperadrenocorticism.
Chloride and acid-base balance
Metabolic acidosis
Metabolic acidoses are traditionally divided into hyperchloremic (normal anion gap [AG]) and normochloremic (high AG) based on the AG and [Cl−]. The AG is the difference between measured cations (sodium and potassium) and measured anions (chloride and bicarbonate) (see Chapters 9 and 10). Physiologically, there is no AG because electroneutrality must be maintained and the AG is the difference between the unmeasured anions (UA) and unmeasured cations (UC+). The AG is a simplification that is helpful clinically. Metabolic acidosis usually results from an increase in the concentration of a strong anion. Strong anions are anions that are completely dissociated at the pH of body fluids (e.g., Cl−, lactate, ketoanions). If the strong anion added is chloride, the sum of the measured anions ([Cl−] + [HCO3−]) will remain the same, and the AG will not change (so-called hyperchloremic or normal AG acidosis). If the strong anion added is an unmeasured anion (e.g., lactate), [Cl−] will remain normal, whereas [HCO3−] will decrease. The sum of the measured anions will decrease, thus increasing the AG (so-called normochloremic or high AG acidosis).
The acid-base status of plasma is regulated by changing Pco2 in the lungs and SID in the kidneys, the latter being accomplished mainly by differential reabsorption of sodium and chloride ions in the renal tubules. Chloride is the most prevalent strong anion in the ECF. At a constant [Na+], a decrease in [Cl−] increases SID causing hypochloremic alkalosis, whereas an increase in [Cl−] decreases SID causing hyperchloremic acidosis. The effects of increasing [Cl−] without changing [Na+] can be understood when considering a fluid with an SID = 0 (e.g., 0.9% NaCl where [Na+] = [Cl−] and thus SID = [Na+] − [Cl−] = 0).14 It is known that 0.9% NaCl administration leads to metabolic acidosis. The classic explanation is that infusion of a fluid without bicarbonate dilutes [HCO3−] in plasma and leads to acidosis. However, the degree of acidosis after normal saline infusion correlates best with the amount of chloride given and with the increase in serum [Cl−].98 There was a weaker correlation with the volume administered and no increase in plasma volume, calling into question the traditional concept of dilutional acidosis.
Chloride in metabolic alkalosis
Chloride participates in the genesis, maintenance, and correction of metabolic alkalosis because decreases in [Cl−] increase SID, causing metabolic alkalosis. The role of chloride is supported by the inverse relationship between chloride and bicarbonate in metabolic alkalosis,6 the fact that chloride depletion is accompanied by increased plasma [HCO3−],74 and the fact that chronic metabolic alkalosis cannot be produced experimentally if chloride is available in the diet.59 In addition, during recovery from chronic hypercapnia, the compensatory increase in [HCO3−] will not normalize if dietary chloride is restricted.88
Chloride was first linked to metabolic alkalosis in dogs when MacCallum and colleagues63 observed hypochloremia and an increase in “alkali reserve” in dogs with loss of gastric fluid caused by pyloric obstruction. The classic hypothesis associates the genesis and maintenance of metabolic alkalosis primarily with volume contraction. According to this hypothesis, volume depletion accompanying alkalosis augments fluid reabsorption in the proximal tubules. Alkalosis is maintained because bicarbonate ions are preferentially reabsorbed in this segment.40 Volume expansion suppresses fluid and bicarbonate reabsorption, and more bicarbonate and chloride ions are delivered to distal nephron segments, which possess greater capacity to reabsorb chloride than bicarbonate. Chloride then is retained, bicarbonate is excreted, and alkalosis is corrected.40 In addition to volume expansion, provision of chloride was also a feature of studies used to substantiate this hypothesis. The classic hypothesis can be viewed from a different perspective in which changes in chloride are the cause of the alkalosis.39 In rats, chloride ion depletion alone plays a role in the genesis and maintenance of metabolic alkalosis.35-37,62 In rats with chronic hypochloremic alkalosis, chloride repletion (and correction of alkalosis) can be achieved without administration of sodium, without volume expansion, and without an increase in the glomerular filtration rate (GFR).96 The correction phase is associated with a decrease in plasma renin activity but with no change in plasma aldosterone concentration. It also has been shown that maintenance and correction of hypochloremic alkalosis primarily are dependent on total body chloride and its influence on renal function, and not on the demands of sodium and fluid homeostasis.38 Ultimately, the correction of alkalosis is dependent on the kidneys.40 The principal mechanisms by which the kidneys correct metabolic alkalosis probably operate in the collecting ducts, especially in the cortical segment, where HCO3- can either be secreted or reabsorbed.39
Expanding the ECF without providing chloride does not correct hypochloremic alkalosis. Furosemide-induced hypochloremic alkalosis in humans eating an NaCl-free diet supplemented with 60 mEq potassium per day can be corrected with orally administered KCl without changes in weight or ECF volume.84 In this study, five NaCl-depleted control subjects were given furosemide and a combination of KCl and NaCl intravenously to maintain their sodium deficit while correcting their chloride deficit. Subjects who were selectively sodium depleted did not become alkalotic.84 It also has been shown that a 25% increase in ECF volume (created by intravenous infusion of 6% bovine albumin in 5% dextrose) has no effect on hypochloremic alkalosis in a rat model of hypochloremic alkalosis.38
These studies demonstrate that ECF volume, GFR, effective circulating volume, and sodium balance are not independent variables in the generation and maintenance of metabolic alkalosis.72 However, it still could be concluded that chloride induces potassium conservation that in turn inhibits bicarbonate reabsorption, because potassium balance was corrected even in studies during which choline-Cl instead of KCl was used to correct the alkalosis. When NaCl is supplied without potassium, however, alkalosis is corrected despite a persistent potassium deficit,4,71 and administration of potassium without chloride does not correct alkalosis.56 It has been speculated that hypokalemia in rats may cause hypochloremia by impairing recycling of potassium at the luminal membrane in the thick ascending limb of Henle’s loop. This, in turn, impairs the effectiveness of the Na+-K+-2 Cl− carrier, decreasing net chloride reabsorption.40 It still is controversial whether hypokalemia induces metabolic alkalosis in humans.34 Contrary to what occurs in rats, isolated potassium deficiency in dogs causes mild metabolic acidosis.8,9 Chronic potassium depletion also is associated with metabolic acidosis in cats.25
Studies in rats with experimentally induced normovolemic and hypovolemic hypochloremic alkalosis showed no difference in the renal handling of chloride and bicarbonate between alkalotic and normal animals in the proximal convoluted tubule, loop of Henle, or distal convoluted tubule.40 Key adjustments in anion excretion during the maintenance and correction of hypochloremic alkalosis were suspected to occur in the collecting tubule, especially in the cortical segment.39 Sodium-independent chloride and bicarbonate transport, and secretion or reabsorption of HCO3− occur at this site.39,40 Alterations in the delivery of HCO3− and Cl− to the collecting tubules also may be important.39
The chloride depletion hypothesis for the genesis and maintenance of metabolic alkalosis was proposed as an extension of the classic hypothesis.39,40 It states that chloride alone is essential for correction of the hypochloremic alkalosis and that it does so by a renal mechanism. Volume depletion is a common but not essential feature of the maintenance phase of alkalosis, and its persistence does not preclude correction of alkalosis. If adequate chloride is provided, restoration of depleted volume, however, may hasten correction of alkalosis by increasing the GFR and decreasing proximal tubular reabsorption of fluid and bicarbonate.39,40 The manner by which exogenous Cl− repletion is detected and the kidneys are signaled to excrete HCO3−, and the cellular mechanisms by which these events occur in the various nephron segments, remain to be determined.39
Role of chloride in adaptation to acid-base disturbances
Chloride excretion is an important mechanism in the kidneys’ adaptation to metabolic acidosis and chronic respiratory acid-base disturbances. In metabolic acidosis, the kidneys increase net acid excretion (primarily by enhanced NH4Cl excretion) beginning on day 1 and reaching a maximum after 5 to 6 days.81 The increase in chloride ion excretion without an associated increase in sodium ion excretion increases plasma SID and returns [HCO3−] and pH toward normal.
The increase in Pco2 in chronic respiratory acidosis causes intracellular [H+] to increase in the renal tubular cells, resulting in stimulation of net acid excretion (primarily as NH4Cl).81 Chloruresis, negative chloride balance, enhanced fractional and absolute bicarbonate reabsorption, and enhanced net acid excretion typically are associated with the renal response to chronic respiratory acidosis.40 The loss of chloride ions in the urine decreases urinary SID, because Cl− is accompanied by NH4+ (a weak cation) rather than Na+. Thus, plasma SID and consequently [HCO3−] are increased. Hypochloremia is a common finding in dogs with chronic hypercapnia.65,78,87,94 Conversely, renal H+ ion excretion is decreased in chronic respiratory alkalosis. This effect probably is mediated by a decrease in intracellular [H+]. In this setting, there is a decrease in NH4Cl excretion in urine and an increase in renal reabsorption of Cl−. The increase in Cl− reabsorption decreases plasma SID and consequently [HCO3−] is responsible for the hyperchloremia observed in dogs with chronic hypocapnia.42
Clinical approach to chloride disorders
Corrected chloride
Changes in chloride concentration can result from changes in water balance or can be caused by a gain or loss of chloride. When changes in [Cl−] are caused by water balance alterations (i.e., increase or decrease in free water), [Na+] also changes. Changes in [Cl−] and [Na+] are proportional in this setting (e.g., a 10% gain in free water decreases [Cl−] and [Na+] by 10%). To account for changes in water balance, [Cl−] must be evaluated in conjunction with evaluation of changes in [Na+].17 Therefore, patient [Cl−] is “corrected” for changes in [Na+] concentration:17
where Cl− (measured) and Na+ (measured) are the patient’s chloride and sodium concentrations, respectively, and Na+ (normal) is the mean normal sodium concentration.
Assuming mean values for [Na+] of 146 mEq/L in dogs and 156 mEq/L in cats, and for [Cl−] of 110 mEq/L in dogs and 120 mEq/L in cats,20 the Cl− (corrected) can be estimated17 in dogs as:
and in cats as:
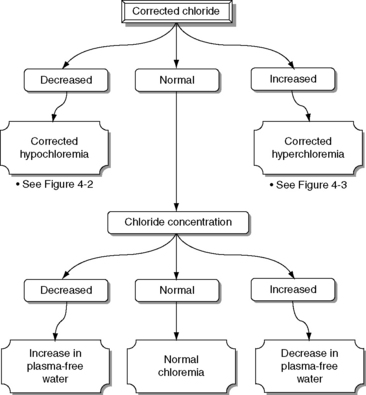
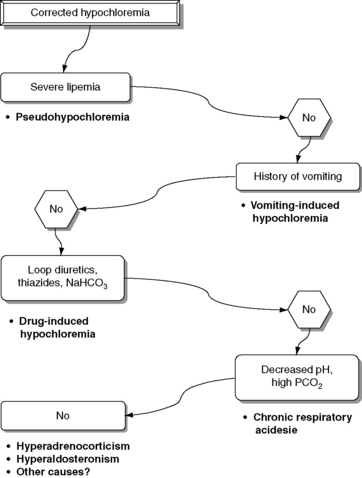
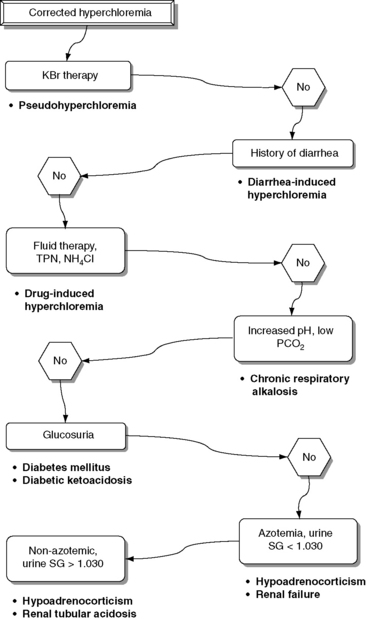
Normal Cl− (corrected) is approximately 107 to 113 mEq/L in dogs and approximately 117 to 123 mEq/L in cats.17 These values may vary between different laboratories and different analyzers. Newer analyzers report higher chloride values unless the chloride calibration is deliberately changed.102 Using the Cl− (corrected) permits the division of chloride disorders into artifactual and corrected chloride changes (Table 4-1). In artifactual chloride changes, changes in free water are solely responsible for the chloride changes, whereas in corrected chloride changes, chloride itself is primarily changed. Algorithms for the evaluation of chloride abnormalities are presented in Figures 4-1 through 4-3.
Chloride measurements
Reference intervals for [Cl−] and [Na+] vary depending on the analytical method and performing laboratory, and these factors should be considered when interpreting and comparing clinical results. Breed-related changes for chloride concentration were not identified in dogs or cats.80,90 Chloride ions can be measured in plasma, serum, or blood; serum is preferred because serum chloride is stable for months. Chloride concentrations most commonly are measured by potentiometry, which is based on ion electrical potential. When the ion electrode is immersed in a solution containing chloride ions, an electrode potential proportional to the logarithm of the chloride ion activity is generated. Chloride ions then are measured by the chloride ion electrode based on this principle in combination with a reference electrode. Because of the much greater solubility of AgCl compared with AgI, the chloride electrode will be irreversibly damaged if immersed in solutions containing iodide ions, resulting in a falsely increased chloride concentration. A high interference also is observed when bromide and cyanide ions are measured, and the chloride electrode will only give reliable results if these ions are absent or in minimal amounts when compared with chloride ions. Care must be taken when using different analyzers to measure chloride. Direct potentiometry is commonly used in blood gas analyzers and point-of-care electrolyte analyzers, whereas indirect potentiometry is commonly used in the large chemistry analyzers located in the central laboratory. Direct potentiometry reveals the true sodium concentration (activity), whereas in indirect potentiometry, the concentration of the ion is diluted to an activity near unity.47 Because the concentration will take into account the original volume and dilution factor, any excluded volume (lipids, proteins) introduces an error (usually very small). However, significant differences between point-of-care analysis and the central laboratory have been found clinically69 for sodium and chloride concentrations, and these differences may be worse in hypoalbuminemic human patients.91 Some laboratories measure potassium by enzymatic spectrophotometry. This method is prone to interference by hemolysis and hyperproteinemia.5
Clinical disturbances
Disorders associated with normal CL (corrected)
Artifactual Hypochloremia and Artifactual Hyperchloremia
A change in the water content of plasma without an imbalance in the content of electrolytes dilutes or concentrates anions and cations. Consequently, [Cl−] and [Na+] will change in parallel. These changes usually are recognized by changes in sodium concentration (hypernatremia or hyponatremia), and this ion (and changes in osmolality) should receive primary attention (see Chapter 3).
High chloride concentration with normal Cl− (corrected) (artifactual hyperchloremia) usually is associated with pure water loss (e.g., diabetes insipidus, essential hypernatremia) or hypotonic losses (e.g., osmotic diuresis). Patients with hypernatremia caused by sodium gain (e.g., hypertonic saline or NaHCO3 administration, hyperadrenocorticism) tend to have abnormal Cl− (corrected). Low chloride concentration with normal Cl− (corrected) (artifactual hypochloremia) has been associated with congestive heart failure, hypoadrenocorticism, and third-space loss of sodium and chloride. It also is associated with gastrointestinal loss, although in this setting one ion often is lost in excess of the other (e.g., chloride in patients with vomiting of stomach contents, sodium in patients with diarrhea), and the Cl− (corrected) may be abnormal. Patients with hypoadrenocorticism may present with corrected hyperchloremia as a result of mineralocorticoid deficiency.
Patients with artifactual hypochloremia tend to have decreased SID and therefore a tendency toward acidosis (so-called dilutional acidosis), whereas patients with artifactual hyperchloremia tend to have increased SID and a tendency toward alkalosis (so-called concentration alkalosis).18 These are the only situations in which [HCO3−] changes in the same direction as [Cl−], and the change in [Cl−] is more pronounced.16
Disorders associated with abnormal CL− (corrected)
Corrected Hypochloremia
Decreased Cl− (corrected) is associated with a tendency toward alkalosis (hypochloremic alkalosis) caused by the associated increase in SID.18 Pseudohypochloremia may occur whenever chloride ion concentration is measured with a technique that is not ion-selective in lipemic or hyperproteinemic samples.21,45 Chloride concentration in lipemic samples (triglyceride concentration >600 mg/dL) is underestimated by titrimetric methods but overestimated when colorimetric methods are used.45 Clinical signs associated with pure hypochloremia in dogs and cats have not been reported but probably are related to the metabolic alkalosis that accompanies hypochloremia.17 However, it has been shown that in euvolemic chloride depletion, GFR decreases acutely by as much as 15% to 20%, probably as a result of changes in tubuloglomerular feedback and internal shifts of fluid out of the ECF.40,41 The clinical importance of these experimental observations is unknown, but hypochloremia itself may potentiate the decrease in GFR associated with hypovolemia in the most common causes of corrected hypochloremia (e.g., vomiting of stomach contents, therapy with loop diuretics). Chloride ion depletion also stimulates renin secretion in rats despite concurrent volume expansion and potassium infusion.1 Renin release caused by hypochloremia probably is mediated by the macula densa. Any resultant increase in aldosterone secretion would increase potassium excretion in the urine and contribute to hypokalemia.
Corrected hypochloremia may be caused by excessive loss of chloride relative to sodium or by administration of substances containing proportionately more sodium than chloride as compared with the normal ECF composition. The former can occur with administration of diuretics that cause chloride ion wasting (e.g., loop diuretics, thiazides) or when the fluid lost has a high [Cl−], as in the case of vomiting of stomach contents or gastric conduit urinary diversions.18,30,67 Loss of plasma during exercise in greyhounds also leads to corrected hypochloremia as a result of a greater loss of Cl− than Na+.93 Interestingly, anticipation of exercise leads to a corrected hyperchloremia in sled dogs.3 Chloride concentration normalizes after exercise in those dogs. The administration of substances containing proportionately more sodium than chloride (e.g., NaHCO3) increases [Na+] without increasing [Cl−], therefore causing a decrease in Cl− (corrected). Corrected hypochloremia in dogs with hyperadrenocorticism has been discussed previously. Corrected hypochloremia has been observed in dogs 1 week after daily administration of prednisone at a dose of 0.55 mg/kg every 12 hours15 and 3 to 6 days after administration of methylprednisolone acetate (5 mg/kg) to cats.77 Unlike dogs with hypoadrenocorticism that have corrected hyperchloremia caused by the lack of mineralocorticoids, dogs with gastrointestinal diseases that mimic hypoadrenocorticism (i.e., presence of hyperkalemia and hyponatremia)22 tend to develop corrected hypochloremia. In cats, acute tumor lysis syndrome,10 primary hypoadrenocorticism,75 anemia, hemorrhagic pleural effusion,100 and diabetic ketoacidosis13 have been associated with corrected hypochloremia. Vomiting may have been a contributory factor in the corrected hypochloremia observed in some of these cats.
An increase in renal chloride ion excretion and a decrease in plasma [Cl−] have been observed in dogs with experimentally induced chronic respiratory acidosis.65,78,87,94 Consequently, patients with chronic hypercapnia may be presented with corrected hypochloremia.50 Potential causes of corrected hypochloremia are listed in Box 4-2, and an algorithm for the differential diagnosis of corrected hypochloremia is presented in Figure 4-2.
Box 4-2 Causes of Corrected Hypochloremia
Excessive loss of chloride relative to sodium
Vomiting of stomach contents⁎
Selected gastrointestinal diseases associated with hyperkalemia and hyponatremia in dogs without hypoadrenocorticism (eg, trichuriasis, salmonellosis, perforated duodenal ulcer)
Therapy with thiazides or loop diuretics⁎
⁎ Most important causes in small animal practice.
Treatment of patients with corrected hypochloremia should be directed at correcting the SID. Special attention also should be paid to the sodium concentration. Renal Cl− conservation is enhanced in hypochloremic states, and renal chloride ion reabsorption does not return to normal until plasma [Cl−] is restored to normal or near normal.40 Therefore, patients with normal renal function should be expected to respond to therapy if the underlying disease process is corrected and chloride is provided. In cases in which expansion of extracellular volume is desired, intravenous infusion of 0.9% NaCl is the treatment of choice.30 If hypokalemia also is present, KCl should be added to the fluid administered. In the rare situation in which volume expansion is not necessary, chloride can be administered using salts without sodium (e.g., KCl, NH4Cl). Use of NaCl or KCl requires normal renal function to correct hypochloremia, whereas NH4Cl requires intact hepatic and renal function.57
Corrected Hyperchloremia
Increased Cl− (corrected) is associated with a tendency toward acidosis (hyperchloremic acidosis) because of a decrease in SID. Pseudohyperchloremia may occur in patients receiving potassium bromide because bromide and other halides (e.g., iodides) are measured as chloride.21,29 Bromide interferes with every chloride assay to some extent, but ion-selective electrodes are the most vulnerable to bromide interference.27-29 If colorimetric methods are used to measure chloride concentration, other pigments such as hemoglobin and bilirubin may cause pseudohyperchloremia.21 Lipemia also can cause pseudohyperchloremia when colorimetric methods are used.45 Emulsified lipids in the photoelectric cell induce scattering of light, resulting in overestimation of the true chloride content. This effect overcomes the decrease in chloride caused by an increase in the plasma water fraction.45
Specific clinical signs associated with pure hyperchloremia in dogs and cats have not been reported but probably are related to the metabolic acidosis that accompanies hyperchloremia.17 Potential causes of corrected hyperchloremia are listed in Box 4-3, and an algorithm for the differential diagnosis of corrected hyperchloremia is presented in Figure 4-3.
Box 4-3 Causes of Corrected Hyperchloremia
Excessive gain of chloride relative to sodium
Therapy with chloride salts (NH4Cl, KCl)
Fluid therapy (e.g., 0.9% NaCl, hypertonic saline, KCl-supplemented fluids)⁎⁎
Corrected hyperchloremia can be caused by chloride retention in renal failure97,101 or by administration of NH4Cl in cats12,31,60,89 and dogs.48,49 Type I renal tubular acidosis also is associated with hyperchloremic acidosis in dogs23,79 and cats.7,26,99 The exact mechanism by which hyperchloremic acidosis occurs in distal renal tubular acidosis is not completely understood. However, there is a decrease in ammonium excretion,81 and chloride replaces bicarbonate in the plasma, causing hyperchloremia.57 Patients with diarrhea develop corrected hyperchloremia because of loss of fluid with high sodium and lower chloride ion concentrations than those of plasma.
Patients with diabetes mellitus may have ketoacidosis with normal AG (hyperchloremia). The ketoacids are excreted in the urine at low serum concentrations; thus, a patient with normal or near normal extracellular volume, renal perfusion, and GFR may excrete the ketoacids as fast as they are generated. The kidneys retain chloride in place of ketones in this situation, increasing chloride concentration while the AG remains unchanged.33 Patients with diabetes also can develop corrected hyperchloremia during the resolving phase of the ketoacidotic crisis.43,73 The hyperchloremia of the recovery phase has at least three causes. First, the administration of large volumes of isotonic saline can increase chloride concentration more than sodium concentration; second, KCl often is infused in large doses; and third, the ketones are excreted in urine in exchange for NaCl.2,70 In cats, however, ketoacidosis was associated with corrected hypochloremia in at least one report.13 No information was provided about whether the cats in this report had vomited. In at least one ketoacidotic dog, corrected hypochloremia also was found.100 Patients with hypoadrenocorticism and hypoaldosteronism have chloride retention and hyperchloremic metabolic acidosis because of the lack of mineralocorticoids. These patients typically have decreased serum sodium and chloride concentrations caused by lack of aldosterone. The hyponatremia is more pronounced than the hypochloremia, and Cl− (corrected) is increased.17 Well-hydrated dogs with mineralocorticoid deficiency that are able to maintain serum sodium concentration usually have a mildly increased serum chloride concentration.
Drugs that cause chloride retention also can cause hyperchloremia. Potassium-sparing diuretics such as spironolactone act by decreasing the number of open aldosterone-sensitive sodium channels in the principal cells of the cortical collecting tubules.81 Inhibition of sodium reabsorption at this site leads to hyperkalemia and hyperchloremic acidosis. Acetazolamide inhibits carbonic anhydrase in the proximal tubule, resulting in bicarbonaturia, urinary alkalinization, and in rats, but not in dogs, reduction in renal ammoniagenesis.32,44 Chloride reabsorption proceeds normally in the ascending loop of Henle, resulting in chloride retention,58 and use of acetazolamide is associated with hyperchloremia and metabolic acidosis.51,81,83 Parenteral nutrition can cause hyperchloremia, because some solutions have high concentrations of cationic amino acids (e.g., lysine-HCl, arginine-HCl) that release chloride and generate hydrogen ions.52
Fluid therapy is another important cause of hyperchloremia in hospitalized patients. Administration of isotonic saline, lactated Ringer’s solution, or isotonic saline with 5% dextrose has been associated with corrected hyperchloremia in dogs.11,82 Hyperchloremia can be exacerbated by intravenous infusion of 0.9% sodium chloride.57 Isotonic sodium chloride solution supplemented with 20 mEq/L of KCl has a final sodium concentration of 154 mEq/L and a chloride concentration of 174 mEq/L. This solution has a much higher chloride concentration than plasma and is a common cause of corrected hyperchloremia in hospitalized patients.17 Corrected hyperchloremia also has been associated with salt poisoning in dogs20,53 and with administration of hypertonic saline in dogs and pigs.17,68 Experimentally, chronic respiratory alkalosis causes renal chloride retention in dogs.42 The observed hyperchloremia is part of the normal renal adaptation to chronic respiratory acid-base disorders. Therefore, patients with chronic hypocapnia can be expected to have corrected hyperchloremia.
Contrary to what is observed in Greyhounds while racing, exercise in other breeds is not associated with corrected hypochloremia. Corrected hyperchloremia was identified in sled dogs anticipation of exercise,3 and during prolonged endurance exercise.66 However, corrected chloride concentration did not change in sled dogs during high intensity sprint exercise of extended (>10 miles) duration.95 Corrected hyperchloremia also was observed in dogs during and after agility competition.85
Treatment of corrected hyperchloremia should be directed at correction of the underlying disease process. The effects of fluid therapy on chloride concentration should be anticipated, especially in patients with diabetes mellitus or abnormal renal function. Special attention should be given to plasma pH because patients with corrected hyperchloremia tend to be acidotic. Bicarbonate therapy can be instituted whenever plasma pH is less than 7.2 or bicarbonate concentration is less than 12 mEq/L in patients with hyperchloremic metabolic acidosis.
Conclusion
Although it is the major anion in ECF, chloride has not received much attention in the clinical setting. It should be remembered that the chloride ion also is important in the metabolic regulation of acid-base balance. The kidneys regulate acid-base balance by changing the amount of chloride that is reabsorbed with sodium. Chloride is important in determining the patient’s SID, and therefore changes in chloride concentration will reflect the patient’s acid-base status. Corrected hypochloremia is associated with increased SID and metabolic alkalosis. Chloride is the only anion in ECF that can contribute to a substantial increase in SID. Administration of chloride is necessary for correction of hypochloremic metabolic alkalosis. Corrected hyperchloremia is associated with decreased SID and metabolic acidosis. Treatment with sodium bicarbonate should be carried out in hyperchloremic patients with a pH of less than 7.2.
1 Abboud H.E., Luke R.G., Galla J.H., et al. Stimulation of renin by acute selective chloride depletion in the rat. Circ Res. 1979;44:815-821.
2 Adrogué H.J., Eknoyan G., Suki W.K. Diabetic ketoacidosis: role of the kidneys in the acid-base homeostasis re-evaluated. Kidney Int. 1984;25:591-598.
3 Angle C.T., Wakshlag J.J., Gillete R.L., et al. Hematologic, serum biochemical, and cortisol changes associated with anticipation of exercise and short duration high-intensity exercise in sled dogs. Vet Clin Pathol. 2009;38:370-374.
4 Atkins E.L., Schwartz W.B. Factors governing correction of the alkalosis associated with potassium deficiency: the critical role of chloride in the recovery process. J Clin Invest. 1962;41:218-229.
5 Bernardini D., Gerardi G., Contiero B., et al. Interference of haemolysis and hyperproteinemia on sodium, potassium, and chloride measurements in canine serum samples. Vet Res Commun. 2009;33(Suppl. 1):S173-26.
6 Bia M., Thier S.O. Mixed acid-base disturbances: a clinical approach. Med Clin North Am. 1981;65:347-361.
7 Brown S.A., Spyridakis L.K., Crowell W.A. Distal renal tubular acidosis and hepatic lipidosis in a cat. J Am Vet Med Assoc. 1986;189:1350-1352.
8 Burnell J.M., Dawbron J.K. Acid base parameters in potassium depletion in the dog. Am J Physiol. 1970;218:1583-1589.
9 Burnell J.M., Teubner E.J., Simpson D.P. Metabolic acidosis accompanying potassium deprivation. Am J Physiol. 1974;227:329-333.
10 Calia C.M., Hohenhaus A.E., Fox P.R., et al. Acute tumor lysis syndrome in a cat with lymphoma. J Vet Intern Med. 1996;10:409-411.
11 Chew D.J., Leonard M., Muir W.W.III. Effect of sodium bicarbonate infusion on serum osmolality, electrolyte concentration, and blood gas tensions in cats. Am J Vet Res. 1991;52:12-17.
12 Ching S.V., Fettman M.J., Hamar D.W., et al. The effect of chronic dietary acidification using ammonium chloride on acid-base and mineral metabolism in the adult cat. J Nutr. 1989;111:902-915.
13 Christopher M.M., Broussard J.D., Peterson M.E. Heinz body formation associated with ketoacidosis in cats. J Vet Intern Med. 1995;9:24-31.
14 Constable P.D. Hyperchloremic acidosis: the classic example of strong ion acidosis. Anesth Analg. 2003;96:919-922.
15 Corrigan A.M., Behrend E.N., Martin L.G., et al. Effect of glucocorticoid administration on serum aldosterone concentration in clinically normal dogs. Am J Vet Res. 2010;71:649-654.
16 de Morais H.S.A. A nontraditional approach to acid-base disorders. In: DiBartola S.P., editor. Fluid therapy in small animal practice. Philadelphia: WB Saunders; 1992:297-320.
17 de Morais H.S.A. Chloride ion in small animal practice: the forgotten ion. J Vet Emerg Critic Care. 1992;2:11-24.
18 de Morais H.S.A., Muir III W.W. Strong ions and acid-base disorders. In: Bonagura J.D., Kirk R.W., editors. Current veterinary therapy XII. 12th ed. Philadelphia: WB Saunders; 1995:121-127.
19 de Rouffignac C., Elalouf J.M. Hormonal regulation of chloride transport in the proximal and distal nephron. Annu Rev Physiol. 1988;50:123-140.
20 DiBartola S.P., de Morais H.S.A. Case examples. In: DiBartola S.P., editor. Fluid therapy in small animal practice. Philadelphia: WB Saunders; 1992:599-688.
21 DiBartola S.P., Green R.A., de Morais H.S.A. Electrolyte and acid-base abnormalities. In: Willard M.D., Tvedten H., Turnwald G.H., editors. Small animal clinical diagnosis by laboratory methods. 2nd ed. Philadelphia: WB Saunders; 1994:97-114.
22 DiBartola S.P., Johnson S.E., Davenport D.J., et al. Clinicopathologic findings resembling hypoadrenocorticism in dogs with primary gastrointestinal disease. J Am Vet Med Assoc. 1985;187:60.
23 DiBartola S.P., Leonard P.O. Renal tubular acidosis in a dog. J Am Vet Med Assoc. 1982;180:70-73.
24 Dobbins J. Gastrointestinal disorders. In: Arieff A.I., DeFronzo R.A., editors. Fluid, electrolyte, and acid-base disorders. New York: Churchill Livingstone; 1985:827-849.
25 Dow S.W., Fettman M.J., Smith K.R., et al. Effects of dietary acidification and potassium depletion on acid-base balance, mineral metabolism and renal function in adult cats. J Nutr. 1990;120:569-578.
26 Drazner F.H. Distal renal tubular acidosis associated with chronic pyelonephritis in a cat. Calif Vet. 1980;34:15-21.
27 Driscoll J.L., Martin H.F. Detection of bromism by an automated chloride method. Clin Chem. 1966;12:314-318.
28 Elin R.J., Robertson E.A. Bromide interference with determination of chloride by each of four methods (letter). Clin Chem. 1981;27:778-779.
29 Emancipator K., Kroll M.H. Bromide interference: is less really better? Clin Chem. 1990;8:1470-1473.
30 Fencl V., Rossing T.H. Acid-base disorders in critical care medicine. Annu Rev Med. 1989;40:17-29.
31 Finco D.R., Barsanti J.A., Brown S.A. Ammonium chloride as an urinary acidifier in cats: efficacy, safety and rationale for its use. Mod Vet Pract. 1986;67:537-541.
32 Fine A. Effects of carbonic anhydrase inhibition on renal ammoniagenesis in the dog. Pharmacology. 1986;33:217-220.
33 Gabow P.A. Disorders associated with altered anion gap. Kidney Int. 1985;27:472-483.
34 Galla J.H. Metabolic alkalosis. J Am Soc Nephrol. 2000;11:369-373.
35 Galla J.H., Bonduris D.N., Luke R.G. Correction of chloride depletion metabolic alkalosis (CDA) without volume expansion (abstract). Clin Res. 1982;30:540A.
36 Galla J.H., Bonduris D.N., Luke R.G. Effect of hypochloremia in glomerular filtration rate (GFR) on euvolemic rats (abstract). Clin Res. 1982;30:785A.
37 Galla J.H., Bonduris D.N., Luke R.G. The correction of acute chloride-depletion alkalosis in the rat without volume expansion. Am J Physiol. 1983;244:F217-F221.
38 Galla J.H., Bonduris D.N., Luke R.G., et al. Effects of chloride and extracellular fluid volume on bicarbonate reabsorption along the nephron in metabolic alkalosis in the rat: reassessment of the classical hypothesis of the pathogenesis of metabolic alkalosis. J Clin Invest. 1987;80:41-50.
39 Galla J.H., Gifford J.D., Luke R.G., et al. Adaptations to chloride-depletion alkalosis. Am J Physiol. 1991;261:R771-R781.
40 Galla J.H., Luke R.G. Chloride transport and disorders of acid-base balance. Annu Rev Physiol. 1988;50:141-158.
41 Garella S., Cohen J.J., Northrup T.E. Chloride-depletion metabolic alkalosis induces ECF volume depletion via internal fluid shifts in nephrectomized dogs. Eur J Clin Invest. 1991;21:273-279.
42 Gennari F.J., Goldstein M.B., Schwartz W. The nature of the renal adaptation to chronic hypocapnia. J Clin Invest. 1972;51:1722-1730.
43 Goodkin D.A., Krishna G.G., Narins R.G. The role of the anion gap in detecting and managing mixed metabolic acid-base disorders. Clin Endocrinol Metab. 1984;13:333-349.
44 Gougoux A., Vinay P., Zizian L., et al. Effect of acetazolamide on renal metabolism and ammoniagenesis in the dog. Kidney Int. 1987;31:1279-1290.
45 Graber M.L., Quigg R.J., Slempsey W.E., et al. Spurious hyperchloremia and decreased anion gap in hyperlipidemia. Ann Intern Med. 1983;98:607-609.
46 Greger R. Chloride transport in thick ascending loop, distal convolution, and collecting duct. Annu Rev Physiol. 1988;50:111-122.
47 Gunnerson K.J. Clinical review: the meaning of acid-base abnormalities in the intensive care unit part I — epidemiology. Crit Care. 2005;9(5):508-516.
48 Halperin M.L., Bun-Chen C. Influence of acute hyponatremia on renal ammoniagenesis in dogs with chronic metabolic acidosis. Am J Physiol. 1990;258:F328-F332.
49 Halperin M.L., Vinay P., Gougoux A., et al. Regulation of the maximum rate of renal ammoniagenesis in the acidotic dog. Am J Physiol. 1985;248:F607-F615.
50 Hara Y., Nezu Y., Harada Y., et al. Secondary chronic respiratory acidosis in a dog following the cervical cord compression by an intradural glioma. J Vet Med Sci. 2002;64:863-866.
51 Haskins S.C., Munger R.J., Helphrey M.G., et al. Effects of acetazolamide on blood acid-base and electrolyte values in dogs. J Am Vet Med Assoc. 1981;179:792-796.
52 Heird W.C., Dell B., Driscoll J.M., et al. Metabolic acidosis resulting from intravenous alimentation with synthetic amino acids. N Engl J Med. 1972;287:943-945.
53 Hughes D.E., Sokolowski J. Sodium chloride poisoning in the dog. Canine Pract. 1978;5:28-31.
54 Hulter H.N., Licht J.H., Sebastian A. K+ deprivation potentiates the renal acid excretory effect of mineralocorticoid: obliteration by amiloride. Am J Physiol. 1979;236:F48-F57.
55 Jones N.L. Blood gases and acid base physiology, 2nd ed. New York: Thieme Medical Publishers; 1987.
56 Kassirer J.P., Berkman P.M., Lawrenz D.R., et al. The critical role of chloride in the correction of hypokalemic alkalosis in man. Am J Med. 1965;38:172-189.
57 Koch S.M., Taylor R.W. Chloride ion in intensive care medicine. Crit Care Med. 1992;20:227-240.
58 Kreisberg R.A., Wood B.C. Drugs and chemical-induced metabolic acidosis. Clin Endocrinol Metab. 1983;12:391-411.
59 Lemieux G., Gervais M. Acute chloride depletion alkalosis: effect of anions on its maintenance and correction. Am J Physiol. 1964;207:1279-1286.
60 Lemieux G., Lemieux C., Duplessis S., et al. Metabolic characteristics of cat kidney: failure to adapt to metabolic acidosis. Am J Physiol. 1990;259:R277-R281.
61 Ling G., Stabenfeldt G.H., Comer K.M., et al. Canine hyperadrenocorticism: pretreatment clinical and laboratory evaluation of 117 cases. J Am Vet Med Assoc. 1979;174:1211-1215.
62 Luke R.G., Galla J.H. Chloride-depletion alkalosis with a normal extracellular fluid volume. Am J Physiol. 1983;254:F419-F424.
63 MacCallum W.G., Lintz J., Vermilye H.N., et al. The effect of pyloric obstruction relation to gastric tetany. Bull John Hopkins Hosp. 1920;31:1-7.
64 Madias N.E., Bossed W.H., Adrogué H.J. Ventilatory response to chronic metabolic acidosis and alkalosis in the dog. J Appl Physiol. 1984;56:1640-1646.
65 Madias N.E., Wolf C.J., Cohen J.J. Regulation of acid-base equilibrium in chronic hypercapnia. Kidney Int. 1985;27:538-543.
66 McKenzie E.C., Jose-Cullineras E., Hinchcliff K.W., et al. Serum chemistry alterations in Alaskan sled dogs during five successive days of prolonged endurance exercise. J Am Vet Med Assoc. 2007;230:1486-1492.
67 McLoughlin M.A., Walshaw R., Thomas M.W., et al. Gastric conduit urinary diversion in normal dogs. Part II. Hypochloremic metabolic alkalosis. Vet Surg. 1992;21:33-39.
68 Moon P.F., Kramer G.C. Hypertonic saline-dextran resuscitation from hemorrhagic shock induces transient mixed acidosis. Crit Care Med. 1995;23:323-331.
69 Morimatsu H., Rocktaschel J., Bellomo R., et al. Comparison of point-of-care versus central laboratory measurement of electrolyte concentrations on calculations of the anion gap and the strong ion difference. Anesthesiology. 2003;98(5):1077-1084.
70 Narins R.G., Emmett M. Simple and mixed acid-base disorders: a practical approach. Medicine. 1980;59:161-187.
71 Needle M.A., Kaloyanides G.J., Schwartz W.B. The effects of selective depletion of hydrochloric acid on acid-base and electrolyte equilibrium. J Clin Invest. 1964;43:1836-1846.
72 Norris S.H., Kurtzman N.A. Does chloride play an independent role in the pathogenesis of metabolic alkalosis? Semin Nephrol. 1988;7:101-108.
73 Oh M.S., Carrol H.J., Goldstein D.A., et al. Hyperchloremic acidosis during the recovery phase of diabetic ketosis. Ann Intern Med. 1978;89:925-927.
74 Penman R.W., Luke R.F., Jarboe T.M. Respiratory effects of hypochloremic alkalosis and potassium depletion in the dog. J Appl Physiol. 1972;33:170-174.
75 Peterson M.E., Greco D.S., Orth D.N. Primary hypoadrenocorticism in ten cats. J Vet Intern Med. 1989;3:55-58.
76 Phillips S.F. Small and large intestinal disorders: associated fluid and electrolyte complications. In: Maxwell M.H., Kleeman C.R., Narins R.G., editors. Clinical disorders of fluid and electrolyte metabolism. New York: McGraw-Hill; 1987:865-877.
77 Ployngam T., Tobias A.H., Smith S.A., et al. Hemodynamic effects of methylprednisolone acetate administration in cats. Am J Vet Res. 2006;67:583-587.
78 Polak A., Haynie G.D., Hays R.M., et al. Effects of chronic hypercapnia on electrolyte and acid-base equilibrium. I. Adaptation. J Clin Invest. 1961;40:1223-1237.
79 Polzin D.J., Stevens J.B., Osborne C.A. Clinical application of the anion gap in evaluation of acid-base disorders in dogs. Comp Cont Educ Pract Vet. 1982;4:1021-1033.
80 Reynolds B.S., Concordet D., Germain C.A., et al. Breed dependency of reference intervals for plasma biochemical values in cats. J Vet Intern Med. 2010;24:809-818.
81 Rose B.D. Clinical physiology of acid-base and electrolyte disorders, 3rd ed. New York: McGraw-Hill; 1989.
82 Rose R.J. Some physiological and biochemical effects of the intravenous administration of five different electrolyte solutions in the dog. J Vet Pharmacol Ther. 1979;2:279-289.
83 Rose R.J., Caner J. Some physiological and biochemical effects of acetazolamide in the dog. J Vet Pharmacol Ther. 1979;2:215-221.
84 Rosen R.A., Bruce J.A., Dubovsky E.V., et al. On the mechanism by which chloride corrects metabolic alkalosis in man. Am J Med. 1988;84:449-458.
85 Rovira S., Muñoz A., Benito M. Fluid and electrolyte shifts during and after agility competitions in dogs. J Vet Med Sci. 2007;69:31-35.
86 Schild L., Giebisch G., Green R. Chloride transport in the proximal renal tubule. Annu Rev Physiol. 1988;50:97-110.
87 Schwartz W.B., Brackelt N.C., Cohen J.J. The response of extracellular hydrogen ion concentration to graded degrees of chronic hypercapnia: the physiologic limits of defense of pH. J Clin Invest. 1965;44:291-301.
88 Schwartz W.B., Hays R.M., Polak A., et al. Effects of chronic hypercapnia on electrolyte and acid-base equilibrium. II. Recovery, with special reference to the influence of chloride intake. J Clin Invest. 1961;40:1238-1249.
89 Senior D.F., Sundstrom D.A., Wolfson B.B. Testing the effects of ammonium chloride and d-methionine on the urinary pH of cats. Vet Med. 1986;81:88-93.
90 Sharkey L., Gjevre K., Hegstad-Davies R., et al. Breed-associated variability in serum biochemical analytes in four large-breed dogs. Vet Clin Pathol. 2009;38:375-380.
91 Story D.A., Morimatsu H., Egi M., et al. The effect of albumin concentration on plasma sodium and chloride measurements in critically ill patients. Anesth Analg. 2007;104(4):893-897.
92 Tietz N.W., Pruden E.L., Sigaard-Andersen O. Electrolytes, blood gases, and acid-base balance. Section one. Electrolytes. In: Tietz N.W., editor. Textbook of clinical chemistry. Philadelphia: WB Saunders; 1986:1172-1191.
93 Toll P.W., Gaehtgens P., Neuhaus D., et al. Fluid, electrolyte, and packed cell volume shifts in racing greyhounds. Am J Vet Res. 1995;56:227-232.
94 van Ypersele de Strihou C., Gulyassy P.F., Schwartz W.B. Effects of chronic hypercapnia on electrolyte and acid-base equilibrium. III. Characteristics of the adaptive and recovery process as evaluated by provision of alkali. J Clin Invest. 1962;41:2246-2253.
95 Wakshlag J., Snedden K., Reynolds A. Biochemical and metabolic changes due to exercise in sprint-racing sled dogs: implications for postexercise carbohydrate supplements and hydration management. Vet Ther. 2004;5:52-59.
96 Wall B.M., Byrum G.V., Galla J.H., et al. Importance of chloride for the correction of chronic metabolic alkalosis in the rat. Am J Physiol. 1987;253:F1031-F1039.
97 Warnock D.G. Uremic acidosis. Kidney Int. 1988;34:278-287.
98 Waters J.H., Miller L.R., Clack S., et al. Causes of metabolic acidosis in prolonged surgery. Crit Care Med. 1999;27:2142-2146.
99 Watson A.D., Culvenor J.A., Middleton D.J., et al. Distal renal tubular acidosis in a cat with pyelonephritis. Vet Rec. 1986;119:65-68.
100 Whitehair K.J., Haskins S.C., Whitehair J.G., et al. Clinical applications of quantitative acid-base chemistry. J Vet Intern Med. 1995;9:1-12.
101 Widmer B., Gerhardt R.E., Harrington J.T., et al. Serum electrolyte and acid base composition: the influence of graded degrees of chronic renal failure. Arch Intern Med. 1979;139:1099-1102.
102 Winter S.D., Pearson J.R., Gabow P.A., et al. The fall of serum anion gap. Arch Intern Med. 1990;150:311-313.