Chapter 16 Monitoring Fluid Therapy and Complications of Fluid Therapy
Intravenous administration of fluids to veterinary patients is very common, and placement of an intravenous catheter is one of the most common invasive procedures performed in veterinary practice. Monitoring the patient’s response to fluid therapy and considering the potential for complications arising from these products and the presence of a vascular access catheter are fundamental features of treatment.
Intravenous fluids are “drugs,” and fluid therapy is a “prescription,” and should be considered as such to avoid potential complications resulting from inappropriate selection, underdosing, and overdosing.59 Selection of fluid type and volume is a major component of the therapeutic plan and should include careful assessment of tissue and intravascular losses, acid-base and electrolyte status, age and species of the animal, nature of illness or injury, acute or chronic history, hematocrit and serum albumin concentration, coagulation status, cardiorespiratory function, and cost. The animal’s illness or injury is a dynamic event, and selection of fluid type and volume may change according to the patient’s response to fluid therapy and with improvement or deterioration of the underlying problem. Therefore constant monitoring to achieve desired endpoints is required. This chapter will introduce the various monitoring techniques frequently used in veterinary practice, the potential for misinterpretation, and complications associated with fluid therapy and catheters.
The patient’s history must be considered when formulating a fluid therapy plan. Rapid loss of intravascular fluid such as occurs in sepsis associated with third-space sequestration requires judicious fluid selection and rapid replacement, whereas chronic loss in a patient with adequate perfusion can be afforded a less aggressive approach to prevent excessive diuresis and iatrogenic electrolyte disturbances. The history must include the patient’s age and previously diagnosed organ dysfunction. Fluid administration to geriatric patients or those with heart disease must be more cautious than administration to young otherwise healthy individuals. Physical examination should identify the compartment most affected by the fluid deficit: intravascular volume depletion with perfusion deficit such as occurs in acute hemorrhage, tissue water loss (dehydration) with normal perfusion, or depletion of both compartments (perfusion deficits and dehydration) indicating a large deficit of total body water.
Intravascular volume (perfusion) deficits are managed and monitored differently than are tissue water deficits. Unless severe total body water loss is present, the dehydrated animal may still have adequate tissue perfusion as indicated by a heart rate within normal range, a normal digital pulse and capillary refill time, normal acid-base status, normal blood lactate concentration, adequate urine production and appropriate concentrating ability, and normal renal and hepatic function (unless primary problems are known to exist with these organs). However, hypoxia resulting from anemia may contribute to end-organ dysfunction or injury despite adequate perfusion.
A thorough physical examination must accompany monitoring using the various technical devices available. Although monitoring central venous pressure (CVP), systemic arterial blood pressure (SABP), pulmonary capillary wedge pressure (PCWP), and cardiac output (CO) provides very useful (and sometimes essential) information, monitoring the patient by physical examination and biochemical evaluation of organ function also are very important, with improvement of these being the ultimate endpoints for achieving success with fluid therapy. As with the various monitoring devices, standard guidelines for assessing fluid deficits and overload by physical examination exist. However, there are many caveats, and interpretation is not necessarily clear-cut nor can it be assumed that absolute numbers or specific findings are related to fluid volume alone (Tables 16-1 through 16-3). Each patient must be assessed individually based on history, physical findings, and laboratory data.
Table 16-1 Physical Signs Associated with Dehydration
| Percent Dehydration | Physical Signs |
|---|---|
| <5 | Not detectable |
| 5-6 | Mild loss of skin elasticity |
| 6-8 | Definite loss of skin elasticity |
| May have dry mucous membranes | |
| May have depressed globes within orbits | |
| 8-10 | Persistent skin tent with slow return because of loss of skin elasticity |
| 10-12 | Persistent skin tent because of loss of skin Elasticity |
| Depressed globes within orbits | |
| Dry mucous membranes | |
| Signs of perfusion deficits (CRT >2 sec, tachycardia) | |
| 12-15 | Signs of shock |
| Death |
Note: The association between % dehydration and circulatory compromise must also be considered with rate of fluid loss. Chronic fluid loss may result in severe dehydration, but perfusion may be adequate; however, fluid loss occurring acutely will result in circulatory collapse at an estimated lower level of hydration. Therefore perfusion status cannot consistently be used to assess hydration status.
CRT, capillary refill time.
Table 16-2 Confounding Factors of Physical Findings Associated with Dehydration
| Assessment | Confounding Factors |
|---|---|
| Skin turgor (“tent”) | Young animals with subcutaneous fat |
| Obese animals with subcutaneous fat | |
| Cachectic animals | |
| Geriatric animals with loss of tissue elasticity | |
| Mucous membranes | |
| Dry | Panting, tachypnea, dyspnea |
| Moist | Nauseated, vomiting, drinking |
| Position of the globe | Cachexia |
| Perfusion status | Affected by rate of fluid loss; chronic loss may not affect perfusion parameters until a large volume is lost |
Table 16-3 Physical Findings Associated with Inadequate Tissue Perfusion
| Assessment | Confounding Factors |
|---|---|
| Mucous Membranes | |
| Pale pink | Vasoconstriction caused by pain or anxiety Anemia |
| Pale | Volume loss overestimated because of vasoconstriction caused by pain or anxiety |
| Dark pink or red | Vasodilatation and may be interpreted as normal volume |
| Hemoconcentration may be interpreted as normal volume | |
| Capillary Refill Time | |
| <1 sec may be considered adequate perfusion | |
| Difficult to interpret if peripherally vasoconstricted because of pain or anxiety |
Monitoring
Assessment of the volume of fluid required to correct fluid deficits in all compartments cannot be accurately derived. Therefore our therapy always is empirical and based on history, physical examination, and laboratory findings. We derive our therapeutic plan using mathematical formulas based on an estimated percentage of intravascular or tissue loss. Our assessment may not be accurate, and therefore the volume of fluid given should be titrated to the patient’s needs and the physiologic responses to the fluid administered. The extent and invasiveness of monitoring used to assess these responses are dependent on the severity of illness and stability of the patient, other therapies administered, the interrelationship among variables affecting the hemodynamic profile (e.g., anemia and perfusion, CVP and mechanical ventilation), the availability of various monitoring devices, and the level of expertise of the clinician and support staff. This latter point is very important because interpretation of an isolated result, especially when monitoring devices are not frequently used, can complicate treatment regimens.
Endpoints of fluid resuscitation
During administration of any fluid, basic monitoring techniques should be performed, including heart rate (HR), respiratory rate (RR), pulse pressure, capillary refill time (CRT), mucous membrane (MM) color, mentation, and temperature and color of the distal limbs and digits. Although abnormalities in these parameters may not be sensitive indicators of hypovolemia,60 a general goal for endpoints of resuscitation should be values within the normal range for the size and species of animal with recovery to normal mentation and warm, pink digits. Monitoring urine production with a goal of 0.5 to 1.0 mL/kg/hr also is a useful assessment of adequate volume, and 1.0 to 2.0 mL/kg is considered optimal with normal renal function, providing that the urine specific gravity is within a normal range of concentration (1.020 to 1.030). Lower urine specific gravity values with small volumes of urine may suggest a lack of concentrating ability, resulting in the urine produced rather than adequate volume and glomerular filtration rate. A higher urine specific gravity indicates the requirement for continuing fluid therapy in a patient with normal concentrating ability. When administering fluids to critically ill animals, measuring preload, stroke volume, or CO (targeted to specific values) is superior to measuring systemic blood pressure. Central venous and PCWP are surrogate markers of preload, but use of the pulmonary artery catheter (PAC) to measure the PCWP has been shown to be inconsistent, of questionable value, and associated with increased morbidity in human medicine and is being used less frequently.39 This technique is also difficult to perform in general practice and therefore not recommended. The CVP and SABP measurements frequently are used in veterinary medicine to guide fluid resuscitation. Measurement of CVP and SABP are associated with major pitfalls (see Arterial Blood Pressure and Central Venous Pressure section discussed later), but are still of value when used in conjunction with the physical examination. Suggested measurements for conditions requiring optimal resuscitation include 5 to 8 mm Hg (6.5 to 10.5 cm H2O) CVP, 80 to 100 mm Hg mean arterial pressure (MAP), and 100 to 120 mm Hg systolic blood pressure (SBP), and for patients in which the goal is adequate resuscitation (i.e., those with ongoing noncompressible hemorrhage), a MAP of 65 mm Hg and a SBP of 90 to 95 mm Hg are acceptable, physical findings are normal, until hemorrhage is controlled either spontaneously or surgically. Pulmonary contusions and other pulmonary conditions predisposing to capillary leak with increased hydrostatic pressure are additional indications for cautious adequate resuscitation. The patient’s base deficit or blood lactate concentration also may be used to assess perfusion. The goal should be to achieve an adjusted base excess of 0 to +4 mEq/L and a lactate concentration less than 1.4 mmol/L in cats and 2.0 mmol/L in dogs. Where a central line (jugular catheter) is in place, a recent study has identified venous saturation (ScvO2) of 68% in dogs to reflect a minimal adequacy of perfusion, which is similar to that reported in humans.31 However, as mortality significantly decreased above this value, it is recommended that fluid resuscitation with a goal of ScvO2 >70% be achieved.
Intravascular Volume
Blood is composed of plasma and red cells and is separated from the interstitial and intracellular compartments by the vascular walls. Measurements of pressure within this system, such as CVP, MAP, or SBP, are used as indirect assessments of blood volume. However, various physiologic or pathophysiologic conditions may lead to an increase or decrease in pressure with or without loss or gain of fluid.
Central venous pressure
The CVP is a measure of the hydrostatic pressure within the intrathoracic vena cavae.1,30 The CVP is slightly higher than the right atrial pressure (RAP), and RAP is quantitatively similar to right ventricular pressure at end diastole30 or preload.12 However, CVP does not reliably predict right ventricular end-diastolic volume.64 Accurate placement of the catheter and consistency in positioning of the animal are extremely important in interpretation of results and determination of trends.30 CVP measurements may be obtained from the caudal vena cava in cats.48 Keeping in mind potential pitfalls, measurement of the CVP during a fluid challenge, such as would be administered in hypovolemia or acute renal failure, can be valuable in assessing the effect of therapy. In the hypovolemic patient, for example, if no appreciable increase in CVP30 is observed after a fluid bolus, additional fluid or colloid should be administered (refer to Chapter 15 for an in-depth discussion of CVP ). It has been my experience that a rapid infusion of 20 mL/kg of a crystalloid may not be “tolerated” in some patients regardless of cardiovascular status (Table 16-4). Nausea, vomiting, shivering, and restlessness are frequently noted in these individuals (Box 16-1). The reason for the observed signs may be associated with a vagally mediated baroreceptor reflex secondary to atrial stretch. Should CVP increase above an acceptable range after such a challenge in an animal with acute renal failure, fluid administration should be curtailed or stopped (Table 16-4). These are general recommendations, and CVP does not reliably predict whether administration of a fluid bolus will or will not significantly increase CO under all conditions.66,67 Factors other than intravascular volume that influence CVP measurements include cardiac function (e.g., systolic or diastolic dysfunction), pulmonary hypertension (e.g., pulmonary thromboembolic disease), venous compliance (e.g., increased systemic vascular resistance), and intrathoracic pressure (e.g., pleural effusion, pneumothorax, pericardial effusion, mechanical ventilation). Although mechanical ventilation affects CVP, threshold values of CVP in ventilated patients still may be of value to predict hemodynamic instability when assessed in response to increasing airway pressure induced by positive end-expiratory pressure (PEEP).38 In this study of patients with acute lung injury, subjects with CVP less than 10 mm Hg usually had decreased CO when challenged with increasing PEEP, whereas those with CVP greater than 10 mm Hg had increased, decreased, or unchanged CO.
Table 16-4 Interpretation of CVP Values in Response to a Rapid Infusion of 20 mL/kg Crystalloid or 5 mL/kg Colloid20[30]
| Interpretation of Response | Response to Infusion |
|---|---|
| Euvolemia and normal cardiac function | 2-4 cm H2O increase from baseline returning to baseline in 15 min |
| Increased venous blood volume, reduced cardiac compliance, or both | An increase in CVP maintained >4 cm H2O above baseline |
| Normal blood volume | A slow (15 min) return to baseline |
| Increased blood volume relative to cardiac performance | A prolonged (>30 min) return to baseline |
| Markedly reduced intravascular volume; requires further resuscitation | Minimal to no increase in CVP |
| Reduced intravascular volume and accommodation of fluid within the intravascular space and subsequent reduction in vascular tone; requires further resuscitation | An increase in CVP with rapid (<5 min) return to baseline |
| Further resuscitation | Raise CVP by 2-4 cm H2O within first few minutes of bolus therapy. If falls rapidly to baseline, repeat bolus therapy until CVP 5-10 cm H2O (3-7 mm Hg) requiring 10-15 min to fall; at this point, blood volume and venous return are optimal relative to cardiac performance. |
| CVP ~7-9 cm H2O (10-12 mm Hg) with normal intrapleural and intraabdominal pressures | Higher volume may predispose to pulmonary edema; continued fluid resuscitation probably will not improve cardiac output |
Arterial blood pressure
Although systemic blood pressure is not an absolute measure of volume, it is frequently monitored during periods of bolus fluid administration when managing shock. When extensive monitoring is required, direct arterial pressure measurements should be obtained. However, on presentation, it may not be possible to successfully perform arterial catheterization, and pressures may be obtained with oscillometric or Doppler monitors. However, when the limbs are poorly perfused or the patient is cold, the oscillometric and Doppler methods are insensitive, and it is difficult to obtain accurate measurements, especially in small animals. In my experience, the coccygeal artery, with the cuff positioned as far proximal as possible, tends to be more reliable in this instance. The MAP is dependent on CO and systemic vascular resistance (SVR), according to the equation MAP = CO × SVR. Therefore adequate MAP does not necessarily indicate adequate CO if SVR is increased as may occur in a compensatory sympathetic response. During acute blood loss, especially in otherwise young healthy animals, the compensatory response can be quite dramatic and result in nearly normal or normal MAP. If resuscitation is based on normal MAP or SBP alone, inadequate resuscitation with continued poor perfusion likely will occur until the patient decompensates. However, if normal MAP or SBP is accompanied by a physical examination (see Physical Findings section) that indicates the presence of a sympathetic response, the clinician will be aware of the requirement for additional resuscitation or analgesics (Table 16-3). In this setting, it is difficult to know how much blood has been lost and the contribution of pain and anxiety. Pain, anxiety, and hypothermia also contribute to the sympathetic response, and the findings observed may be more a result of these factors than of fluid and blood loss. In this setting, intravascular volume loss may be overestimated, resulting in excessive fluid administration. Therefore fluid requirements and monitoring progress should be assessed based on several factors in addition to pressure measurements. These considerations include a relatively pain-free patient and an improvement in physical findings (see Physical Findings section).
Cardiac output measurements
Several techniques are available to measure CO, but most are technically challenging. The lithium dilution cardiac output (LiDCO) and PulseCO (both from LiDCO, London) have been investigated for use in humans,39 and in large45 and small animals.55 Briefly, isotonic lithium chloride is injected as a bolus via a central or peripheral vein, and a concentration-time curve is generated by an arterial ion-selective electrode attached to an arterial manometer system. The CO is calculated from the lithium dose and the area under the concentration time curve before recirculation. The PulseCO hemodynamic monitor was developed for use in conjunction with the LiDCO to give a beat-by-beat estimate of CO that is derived from analysis of the arterial trace. Although these systems have limitations, their use in veterinary research indicates potential value in clinical practice in anesthetized animals or nonmoving critically ill animals. Movement, flexion, and extension of the catheterized limb contribute to erroneous results (personal observations). A great advantage of this system is that a central catheter is not required, and continuous CO can be measured. Measuring CO during fluid resuscitation has definite advantages over determination of SABP because the former is a more accurate measure of volume. Predetermined goals for CO, stroke volume, and oxygen delivery can be set and monitored with this system. For critically ill patients being mechanically ventilated, intermittent cardiac output measurements can be obtained by the use of a partial CO2 rebreathing noninvasive system (NICO, Novametrix Medical Systems Inc, Wallingford, Conn.).15 This system requires body weight, Spo2, Fio2, Pao2, and Paco2, all of which are available in ventilated patients, and the CO2 sensor measurement obtained from expired CO2 at the endotracheal tube and ventilation circuit.21
Physical findings
As previously mentioned, SABP may be normal in patients with hypovolemia caused by blood loss, and therefore the physical examination must be considered in conjunction with SABP when assessing adequate resuscitation. Cool limbs, rectal temperature below normal, increased HR and RR, paler than normal MM color, prolonged CRT, and depressed mentation all indicate poor perfusion, regardless of blood pressure readings. If SABP is normal and the patient is free of pain but HR and RR are high and MMs still pale, a compensated stage of shock may exist, and further resuscitation is required. When assessing response to fluid therapy in animals with pain, an opioid analgesic (preferably hydromorphone or fentanyl) should be administered to control pain. The sympathetic response associated with pain and anxiety will be reduced, allowing the clinician to assess cardiovascular dynamics solely associated with the blood loss. Administration of these opioids commencing with a low-dose and careful titration to effect does not compromise the cardiovascular system47 and will allow better assessment of the patient because the effect of the sympathetic response to pain will be eliminated from consideration. It is advised, however, that depressed animals receive fluid therapy for a few minutes before opioid administration until mentation is improved. Depression (not associated with head trauma) indicates poor cerebral perfusion due to fluid or blood loss usually greater than 30% of the intravascular volume, and a potential slight reduction in SABP due to the opioid may compromise cerebral perfusion pressure further. Hypothermia may be a result of poor perfusion caused by low circulating volume or by a primary cause that may interfere with achieving resuscitation goals; clinical impression suggests this may be especially so in cats. Marked hypothermia results in bradycardia and decreased CO62; therefore, warming during resuscitation is necessary. Again, opioid analgesic administration should be administered once the patient’s mentation improves. Increased RR also may be associated with pulmonary injury, disease, or fluid overload. An improvement in attitude (i.e., improved cerebral perfusion) should be noted with adequate fluid resuscitation. The CRT and MM color, pulse pressure, and urine production also should improve. Palpation of the bladder and monitoring urine produced by assessing bladder size can be useful when urinary bladder catheterization cannot be performed.
Packed cell volume and total solids (or protein)
It is essential to obtain baseline packed cell volume (PCV) and total solids (TS) or total protein concentration on admission. During a traumatic event, sympathetic stimulation results in splenic contraction, especially in the dog, increasing the PCV and potentially giving the impression that hemorrhage has not occurred. A normal PCV may be observed after trauma even with clinically relevant blood loss. The TS in this setting will be lower than normal (6.0 to 8.0 g/dL), confirming blood loss. Monitoring these tests as frequently as every 15 minutes during resuscitation may be necessary to evaluate ongoing blood loss and the requirement for administration of red blood cells (PRBCs), whole blood, or hemoglobin-based oxygen-carrying solutions (HBOCs). A definitive recommendation regarding transfusion of blood products has not been established in veterinary patients, but the use of PRBCs, whole blood, or HBOCs is suggested when the PCV decreases to less than 25% in the dog or less than 20% in the cat, especially when ongoing resuscitation is required. The use of HBOCs may be an effective adjunct to limited resuscitation from hemorrhagic shock51 while reducing complications of aggressive fluid therapy, However, complications associated with these solutions have been reported in cats.20
Administration of a colloid has been recommended when the TS is less than 4.0 g/dL (less than 40 g/L) to avoid a clinically relevant decrease in colloid osmotic pressure (COP),50 which may predispose to tissue and pulmonary edema, especially when additional crystalloid fluids are to be administered. The refractometer reading for hetastarch is 4.5 g/dL (45 g/L) and that for pentastarch is 7.5 g/dL (75 g/L). After these colloids are administered, the TS measurement is difficult to interpret and cannot be extrapolated to a COP measurement. Response to administration of colloids must be assessed by direct COP measurement, CO determination, or improvement in clinical signs.
Urine production
During hypovolemia and dehydration, renal blood flow is decreased. When blood volume is decreased by hemorrhage, the decreased pressures result in activation of the sympathetic nervous system, including renal sympathetic nerves. Sodium and water are conserved by constriction of the glomerular arterioles, decreased glomerular filtration rate (GFR), increased tubular reabsorption of salt and water, and activation of the renin angiotensin aldosterone system. Decreased arterial pressure also results in secretion of antidiuretic hormone (ADH).28 Together, these actions serve to replenish the intravascular space and return blood volume toward normal. As a consequence of these effects, a very small volume of hypertonic urine is produced. In addition to renal blood flow and GFR, urine volume also is dependent on the concentrating ability of the kidneys. If underlying renal tubular dysfunction is present, increased urine volume may not reflect adequate renal perfusion and GFR. When renal function is otherwise normal, however, urine production and specific gravity are useful parameters to monitor when assessing intravascular volume. Urine output has been referred to as the “poor man’s cardiac output.” Intravenous fluid therapy also will expand the intravascular space and consequently increase urine volume.
Assessment of Urine Output
Careful monitoring is necessary to ensure that urine production is maintained by adequate fluid replacement (Chapter 22). Normal urine production is between 0.5 and 2 mL/ kg/hr but varies with the concentrating ability of the kidneys. The goal is to maintain urine output of 1 to 2 mL/kg/hr with a urine specific gravity of approximately 1.026 (dog) and 1.035 (cat). However, if there is loss of concentrating ability (e.g., renal tubular injury, Escherichia coli pyelonephritis), urine output can be extremely high (25 to 40 mL/kg/hr), and specific gravity may be in the hyposthenuric or isosthenuric range, hence the importance of measuring of urine output and specific gravity. If urine output is decreased, the patient should be assessed for possible third-space loss, capillary leak, increased temperature, vomiting, diarrhea, salivation, or inadequate resuscitation as causes. In addition, alterations in vasopressin (antidiuretic hormone or ADH) activity and intravascular sodium, may result in marked hyposthenuric polyuria or concentrated oliguria. [Chapter Hypernatremia, Hyponatremia]. Calculating appropriate fluid requirement is important because administering an excessive volume of fluids will result in diuresis, medullary washout, and electrolyte disturbances (especially potassium). Ongoing diuresis may require a prolonged hospital stay for correction of resulting fluid and electrolyte imbalances. Measurement of urine volume can be accomplished by:
1. Collection of urine when the animal voids
3. Intermittent or continuous urinary bladder catheterization
4. Placing preweighed towels or pads under the animal and weighing them after voiding. Any increase in towel or pad weight over baseline, unless otherwise soiled, is assumed to be the result of urine. The volume of urine voided can be estimated by assuming 1000 mL equals 1000 g (1 kg or 2.2 lb). This technique underestimates urine produced because some urine may remain in the cage.
Weighing the animal several times a day will assist in estimating fluid loss or gain. If the animal’s weight declines despite fluid therapy, it is assumed that ongoing losses such as high urine output, vomiting, diarrhea, salivation, or evaporative losses caused by fever or hyperthermia are in excess of fluids administered. A weight loss of 0.1 to 0.3 kg body weight per 1000 kcal energy requirement (approximate caloric requirement for a caged 20-kg dog) per day is anticipated in an anorexic animal. Third-space losses must be assessed by other means because weight loss will not be evident. After urine flow has been established, regardless of the underlying problem, ongoing fluid requirements are calculated as follows:
1. Divide the day into six 4-hour intervals, four 6-hour intervals, or three 8-hour intervals.
2. Determine urine produced during each time interval, and add the estimated insensible and ongoing losses for that period.
3. Determine ongoing losses in vomitus, diarrhea, and saliva for the period selected.
4. Determine insensible loss at 20 mL/kg/day. In addition, for each degree Celsius above 38.5° C, add 10% of the normal daily maintenance fluid requirement (i.e., if the normal daily requirement is 1 L and temperature is 40.5° C, then 200 mL should be added). Divide this total amount by 6, 4, or 3 depending on the interval selected above.
5. This volume of fluid, in addition to the amount determined by urine produced and ongoing losses, is to be delivered during the next period. Daily weight is advised in all hospitalized patients because unnoticed polyuria or inadequate intravenous or oral fluids for an individual patient may result in weight loss, and excessive intravenous fluid administration may result in unnecessary weight gain.
Perfusion
Lactate
The blood lactate concentration may be used as an indicator of perfusion to monitor resuscitation and is discussed further in Chapter 10. Strict adherence to collection (i.e., blood should be collected in heparin) and processing (i.e., immediate) of blood samples is required. Lactate measurements can be performed on arterial or venous samples.34,43 Normal blood lactate concentrations are less than 2.0 mmol/L in dogs, with 3 to 5 mmol/L representing a mild increase, 5 to 8 mmol/L a moderate increase, and more than 8 mmol/L a severe increase. Normal blood lactate concentrations are less than 1.46 mmol/L in cats. When inadequate oxygen delivery to tissues occurs, cells revert to anaerobic metabolism, and lactate production increases. Obtaining an initial lactate measurement in all severely ill patients can serve as a useful method of evaluating severity of illness or injury. This was illustrated in a recent study of blunt trauma in dogs.74 Monitoring lactate concentrations as a method of measuring total body oxygen metabolism will provide information about the state of oxygen delivery and adequacy of resuscitation. With restoration of adequate perfusion and oxygen delivery, aerobic metabolism is resumed with a reduction in lactate production. A recent study in human septic patients demonstrated that lactate clearance derived from calculating the change in lactate concentration from two blood samples drawn at two time points (before and after treatment [e.g., fluid bolus]) was not inferior to ScvO2 measurements as a marker of adequacy of oxygen delivery. A lactate clearance of more than 10% ([lactate initial—lactate resuscitation/lactate initial]/100%) may be a useful monitoring technique in assessing improved perfusion status, especially in situations where appropriate equipment is not available to measure saturation of oxygen in central venous or jugular vein samples.40
The most common cause of hyperlactatemia is hypoperfusion and tissue hypoxia (type A), but increased lactate concentrations also may be caused by increased production secondary to alkalosis, hypoglycemia, various drugs, and systemic illness (type B).9 As an example, in those with acute liver failure and sepsis, hyperlactatemia is not necessarily associated with hypoxia. In this situation, the liver becomes a net producer of both lactate and pyruvate.8 Similarly, in those with sepsis, hyperlactatemia is a consequence of enhanced glycolysis and increased release of lactate from the intestine and the periphery. Therefore hypermetabolism must be considered as a cause for hyperlactatemia when no other indications of inadequate perfusion or tissue hypoxia are present.8 Type A hyperlactatemia also includes relative hypoxia, in which energy requirements exceed demand such as may occur in strenuous exercise and in extreme muscle activity (e.g., seizures, trembling, struggling).35 This effect resolves rapidly after cessation of the activity.
Acid-Base Status
Many illnesses and trauma are associated with acid-base disturbances, often, metabolic acidosis. Hypoperfusion and tissue hypoxia result in metabolic acidemia unless a comorbid condition results in metabolic alkalosis, generating a mixed disturbance in which blood pH may be within normal limits. In one study, 95% of animals referred to a tertiary referral center were diagnosed with metabolic acidosis.41 Knowing the metabolic status of a patient is an extremely important part of the overall assessment of the animal and provides information about the potential origin of the abnormality and the appropriate fluid to select. Eliminating the underlying problem ultimately will correct the abnormal metabolic status, but until it can be resolved, providing optimal therapy to improve outcome is essential. When blood gas analysis is not available, acidemic patients with 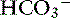 loss usually can be identified as having increased serum chloride concentration, decreased total CO2, and normal anion gap, information that can be obtained from a serum biochemistry profile. If acidemia is caused by the addition of an unmeasured anion (e.g., lactate, glycolate), the serum chloride concentration usually is normal, but the anion gap is increased. Where inappropriate administration of 0.9% sodium chloride is administered, a hyperchloremic metabolic acidosis frequently occurs. Alkalemic patients, however, often are hypochloremic. Monitoring acid-base status provides additional information about improved perfusion and resolution of the illness, as well as the potential need for a change in fluid therapy as the disease process changes. For example, a dog with vomiting caused by pyloric obstruction commonly will exhibit a hypochloremic metabolic alkalosis and hyponatremia; 0.9% sodium chloride is the fluid of choice. Once the underlying problem is resolved and alkalosis has been corrected, continuing with 0.9% sodium chloride may result in hyperchloremic acidosis; therefore a change to a balanced electrolyte solution typically is recommended.
loss usually can be identified as having increased serum chloride concentration, decreased total CO2, and normal anion gap, information that can be obtained from a serum biochemistry profile. If acidemia is caused by the addition of an unmeasured anion (e.g., lactate, glycolate), the serum chloride concentration usually is normal, but the anion gap is increased. Where inappropriate administration of 0.9% sodium chloride is administered, a hyperchloremic metabolic acidosis frequently occurs. Alkalemic patients, however, often are hypochloremic. Monitoring acid-base status provides additional information about improved perfusion and resolution of the illness, as well as the potential need for a change in fluid therapy as the disease process changes. For example, a dog with vomiting caused by pyloric obstruction commonly will exhibit a hypochloremic metabolic alkalosis and hyponatremia; 0.9% sodium chloride is the fluid of choice. Once the underlying problem is resolved and alkalosis has been corrected, continuing with 0.9% sodium chloride may result in hyperchloremic acidosis; therefore a change to a balanced electrolyte solution typically is recommended.
Hydration
Physical findings used to assess hydration are skin turgor, position of the globes within the orbits, and moistness of mucous membranes (see Table 16-1).10 Assessment of these findings should be noted on admission; however, there are confounding factors of the assessment which must be considered (see Table 16-2) when calculating volume requirements to avoid overestimates and underestimates. However, frequent monitoring of these findings is useful when monitoring response to therapy and fluid requirements for ongoing management. Weight gain has been recommended as a monitor of hydration after fluid therapy, and in my experience, weighing the animal has proved to be a useful adjunct of fluid management. However, weight gain cannot always be predicted.29a
Fluid and electrolyte disturbances
Serum electrolyte concentrations
Serum electrolyte concentrations require frequent monitoring in a patient with a dynamic illness. In the patient with hypochloremic alkalosis and hyponatremia described previously, caution is required when increasing the sodium content of the fluids administered. In patients with serum sodium less than 125 (dogs) to 130 (cats)mEq/L for greater than 24 hours, an increase of more than 0.5 mEq/L/hr of sodium may result in a development of central nervous system lesions. Similar concerns exist when attempting to decrease serum sodium concentration in patients with hypernatremia for longer than 24 hours. Serum potassium concentration is influenced by acidosis, alkalosis, and the underlying illness, and hyperkalemia or hypokalemia may contribute substantially to morbidity. Potassium supplementation often is required in many illnesses, but the need for potassium supplementation should be carefully evaluated and not assumed. Although abnormalities in serum calcium concentration are not as frequent, hypocalcemia and hypercalcemia also have diagnostic and therapeutic implications. Hyperchloremia and hypochloremia can affect the acid-base status of the patient and warrant vigilance and correction as necessary. Hypomagnesemia is a common finding in critical care patients with many underlying illnesses. The electrocardiogram (ECG) may be a useful monitoring tool when electrolyte disturbances exist (e.g., hyperkalemia, hypocalcemia) and result in dysrhythmias; however, one cannot rely on the ECG to diagnose electrolyte abnormalities. For a detailed discussion on the individual electrolyte disturbances, the reader is referred to the specific chapters in this book.
Colloid osmometry
Under normal conditions, blood volume and extracellular fluid volume are controlled in parallel to each other. However, there are situations in which the distribution of extracellular fluid between the interstitial space and blood can vary. The principal factors that can cause accumulation of fluid in the interstitial space include (1) increased capillary hydrostatic pressure, (2) decreased plasma COP (oncotic pressure), (3) increased permeability of the capillaries, and (4) obstruction of the lymphatic vessels.28 With the exception of lymphatic obstruction, these conditions frequently are preexistent in critically ill small animal patients or may develop as a consequence of fluid administration. The endothelium represents a semipermeable membrane that normally prevents loss of proteins.
The COP of whole blood obtained from normal dogs is 19.95 ± 2.1 (range, 15.3 to 26.3) mm Hg and for plasma is 17.5 ± 3.0 mm Hg. In whole blood obtained from normal cats, COP is 24.7 ± 3.7 (range, 17.6 to 33.1) mm Hg and in plasma 19.8 ± 2.4 mm Hg.
Complications
Potential complications associated with selection of fluids
Crystalloid Solutions
As previously mentioned, crystalloid fluids should be considered drugs because their various compositions will influence many ionic interactions and shifts in plasma. The type of fluid selected will influence resolution of alkalosis or acidosis. Alkalemia and acidemia will affect the pathologic condition experienced by the animal. If an inappropriate selection is made, such as 0.9% sodium chloride in a patient with hyperchloremia and decreased strong ion difference or in another condition resulting in acidosis, acidosis will worsen, and acidosis has been shown to increase morbidity.71 Likewise, in a patient with alkalosis, administration of an alkalinizing solution potentially will contribute to morbidity as electrolyte composition of plasma is altered (e.g., hypokalemia, ionized hypocalcemia, hyperammonemia), and a shift of the oxyhemoglobin dissociation curve to the left, with associated problems, occurs. Administration of dextrose also can alter the electrolyte composition of plasma. Intracellular shifts of phosphorus and potassium may occur during dextrose infusions, and careful monitoring and supplementation of these ions are required in patients with hypophosphatemia and hypokalemia. The addition of dextrose makes the solution more acidic because of the oxidation of the sugar. The physiologic acid-base effect of an infusion of 5% dextrose also will be a trend toward acidosis because of the effective free water infusion and the effects of glucose metabolism under different patient conditions.80 Another potential concern with the administration of 5% dextrose in water is the generation of free water when additional water is not needed. Dextrose-containing solutions may be indicated for specific situations (i.e., pure water loss).
Lactated Ringer’s solution contains lactate as a bicarbonate precursor. Lactate is metabolized in the liver, and it has been suggested that administration of this fluid may increase lactate concentration in animals with severe liver disease. However, the clinical importance of this effect must be determined on an individual basis. Mild hyperlactatemia has been noted in dogs with lymphosarcoma receiving lactated Ringer’s solution.
Because of the calcium content of lactated Ringer’s solution, blood transfusions should not be given through the same fluid administration set. Lactated Ringer’s solution will result in microscopic clot formation in blood products.69
Acetated polyionic solutions (Plasma-Lyte 148, Plasma-Lyte A, Baxter Corp, Mississauga, Ontario, Canada) (Normosol-R, Abbott Laboratories Ltd., Montreal, Quebec, Canada) contain acetate as the alkalinizing component. Acetate is metabolized in muscle cells, and therefore specific organ dysfunction (e.g., kidney disease, liver disease) is not a contraindication for its use. It has been suggested that these solutions not be administered to animals with diabetic ketoacidosis (DKA) because acetate is a ketone precursor and may promote ketone production. This concern appears to be theoretical because concurrent treatment for DKA with insulin prevents further ketone production. Many patients with DKA are acidemic, and crystalloid solutions containing acetate have been my choice for fluid therapy for several years. Rapid administration of polyionic acetate solutions may precipitate vasodilatation and hypotension in animals that already are hypovolemic.65 Although this is likely a rare event, with no prospective studies investigating this in veterinary medicine, monitoring blood pressure during administration of acetated crystalloid solutions is recommended. Hypertonic saline solutions have been recommended for various conditions and have several positive attributes. However, rate of infusion is important, and rapid infusions may result in bronchoconstriction and shallow breathing.72
Colloid Solutions
Synthetic colloid solutions are recommended for many clinical situations, and the commonly used synthetic colloid solutions are formulated in 0.9% sodium chloride. The primary acid-base effect of the colloid solutions on plasma is acidification. The electrolyte preparations that accompany these macromolecules (e.g., 0.9% saline) also have an important effect on acid-base equilibrium after infusion.46
Considering the frequency of use of these products in veterinary practice, very little has been published with regard to complications after their administration. A very recent review on the use of colloids in humans states that “crystalloids in general, and albumin in many conditions except in patients with closed head injury (SAFE), have been shown to be safer than older starches in critically ill patients. They should therefore be considered the gold standard for future safety trials”. The review summarizes that synthetic colloids are without superior effect in critically ill adults and children but must be considered harmful depending on the cumulative dose administered. This review also comments on the lack of evidence for the superiority of 6% HES/0.4 (the most recent colloid marketed) compared with crystalloids.3 Some of the concerns for the use of colloids are coagulopathy, renal injury and edema.
Coagulopathy
A clinical study evaluating the safety and efficacy of hetastarch after a total dosage of between 9 and 59 mL/kg in dogs with varying problems and associated hypoalbuminemia reported minor hetastarch-associated changes in coagulation tests that were of little clinical relevance.77 However, none of these dogs had preexisting coagulopathies. A potential complication of colloid administration is hemorrhage, if a preexisting condition exists in a patient with a moderate coagulopathy. Hemorrhage associated with administration of synthetic colloids has been reported in some human patients.4 A recent veterinary study identified a prolonged platelet closure time in healthy dogs administered 20 mL/kg of hetastarch (670/0.75). The conclusions of this study were that the potential platelet dysfunction, in addition to the effects of hemodilution, may increase the risk of bleeding.76
Renal Injury
Synthetic colloids are eliminated primarily by renal excretion, and caution must be used when administering these products as rapid volume expanders to patients with oliguria unless oliguria is determined to be caused by hypovolemia or hypotension. These products should not be administered to patients with anuric renal failure or congestive heart failure because of concern about volume overload. Interference with renal function has been reported in human patients receiving synthetic colloids, and most commonly this observation has been associated with use of dextran 40.16 A reduction in the GFR also has been noted in human surgical and trauma patients receiving synthetic colloids.6 A recent Cochrane review, however, concluded that in surgical and trauma patients there was no difference with respect to risk of kidney failure or need for dialysis between patients treated with HES and other fluids, however, it was noted that there were too few studies to absolutely conclude association of risk. On the other hand, administration of HES to septic patients revealed a 55% increased risk of developing kidney failure and a 59% increased risk of requiring dialysis. Products with lower molecular weight and degree of substitution are reported to have better safety profiles; however, insufficient evidence exists in the literature at this time to support this.11
Where oncotic pressure is normal, caution is warranted with colloid administration because a high oncotic pressure may result in renal dysfunction. Based on the observation that renal dysfunction was shown to parallel an increase in plasma oncotic pressure, which was sufficient to oppose hydraulic filtration pressure within Bowman’s capsule, the concept of hyperoncotic renal failure has been proposed in human medicine.68 This syndrome has been reported to occur with dextrans, hydroxyethylstarch, gelatin, and hyperoncotic albumin; therefore, no specific colloid solution of itself is directly nephrotoxic. As this problem has been identified predominantly in renal transplant patients and those with sepsis, it is advised that careful consideration be given to the volume of colloid administered in conjunction with an adequate volume of crystalloid to meet the minimum daily water requirement.68 The frequency of renal dysfunction associated with administration of synthetic colloids in veterinary patients is not known; however, it is wise to consider the human reports when administering the various colloids to veterinary patients.
Pulmonary Edema
Another potential complication that may occur is leakage of the small molecules (<50,000 Da)—contained in the currently used synthetic colloid solutions (e.g., hetastarch and pentastarch) —into the pulmonary interstitium when administered to animals with capillary leak syndrome.32,61 A review of fluid therapy in sepsis with capillary leakage concluded that additional studies, including patients with specific diseases, considering various aspects of the colloids administered and with specific endpoints for fluid resuscitation, should be conducted before definitive recommendations can be made for colloid administration.54 In the meanwhile, appreciating potential adverse effects, selecting appropriate patients, and monitoring during treatment should reduce the morbidity associated with administration of these solutions.
As with other colloidal solutions, caution with administration of the HBOCs is warranted to avoid complications, especially associated respiratory problems. A study of 72 cats receiving a HBOC solution, developed pulmonary edema (n = 8) and/or pleural effusion (n = 21).20 It was concluded that this complication could potentially be associated with a rapid infusion rate and/or large volume of infusion of approximately 14 mL/kg.20
Immune-Mediated Reactions
Human serum albumin [HSA]
Many critically ill veterinary patients are hypoalbuminemic, and this frequently contributes to morbidity and mortality as a result of interstitial, pulmonary, and visceral edema, and the loss of the many other physiologic functions of albumin. Administration of 25% HSA to these critically ill patients to reverse the adverse effects of hypoalbuminemia has been reported.56 While administration of 25% HSA to seriously ill animals has shown benefit, complications associated with immune-mediated adverse effects occur when administered to healthy animals.19 Therefore, careful consideration must be given before the administration of 25% HSA, which should be restricted to severe hypoalbuminic and seriously ill patients. In this setting the benefits will potentially outweigh the risks. Patient follow-up for up to 4 weeks is warranted to ensure early signs of immune-mediated reactions are detected and treated with corticosteroids.58 Canine lyophilized albumin (Animal Blood Resources International) is now available and may also be beneficial in hypoalbuminemic patients.
Species-specific blood products
Transfusions of blood products may result in both acute or delayed immune-mediated reactions. While these are relatively rare events, it is essential to be familiar with prevention and treatment (Chapter 24).
Potential complications associated with the volume of fluids administered
Diuresis and Electrolyte Losses
The volume of fluids administered tends to be empirically derived, and response to therapy must serve to guide ongoing requirements. Excessive administration serves no therapeutic benefit and frequently results in patient morbidity. A mechanism conserved across species through evolution is the renal-body fluid system for arterial pressure control. Urine output can double when intravascular volume and pressure increase even a few millimeters of mercury above normal, a response termed pressure diuresis. In addition to water loss, a concomitant sodium loss occurs.23 If this diuresis goes undetected, an excess of all electrolytes is excreted in the urine because of decreased reabsorption in the proximal and distal renal tubules.24 In some instances, resultant hypokalemia can be quite profound, especially in cats.
The ability to concentrate urine relies on the high osmolarity of the renal medullary interstitial fluid, which provides the osmotic gradient necessary for water reabsorption. Transport of sodium, potassium, chloride, and other ions into the medullary interstitium, along with urea, maintains this osmotic gradient. Increased medullary blood flow, which can occur with excessive fluid administration, will wash out the hyperosmotic interstitium, thereby reducing renal concentrating ability.26 A vicious cycle then is established in which fluid loss occurs because of a lack of concentrating ability. When this occurs, gradual fluid reduction is required to reestablish the hyperosmotic gradient and maintain hydration.
Edema
Administration of fluids can lead to interstitial and pulmonary edema when the patient’s illness is associated with inflammation, anuric or oliguric renal failure, cardiac insufficiency, hypoalbuminemia, or with administration of a large overdose of fluids resulting in hypervolemia and a subsequent increase in interstitial hydrostatic pressure.
Interstitial Edema
Under normal conditions there is a slight negative pressure (−3 mm Hg) in most loose subcutaneous tissues of the body. This negative pressure holds the tissues together and offers some resistance to fluid flux caused by the low compliance of the tissue. With small increases in interstitial fluid volume, a large increase in hydrostatic pressure occurs, resisting additional filtration across the capillary. However, with large increases in intravascular hydrostatic pressure and further capillary filtration, the interstitial volume will increase. When the colloid osmotic gradient fails and the lymphatic system is overloaded, the tissue safety factors are overcome with little resistance to further fluid loss. When the interstitial pressure reaches 0 mm Hg, the compliance of the tissue is increased, facilitating larger volumes of fluid to accumulate in the tissues with little change in interstitial hydrostatic pressure. This effect is called stress relaxation. The proteoglycan meshwork within the interstitium is disrupted as the increased volume of fluid pushes the brush pile of proteoglycans apart, allowing the fluid to flow freely through the tissues.27 When this occurs, pitting edema is detected by pressing on an area of skin and noting pitting for several seconds until the fluid flows back into the area. Edema also may occur in patients with moderate to severe capillary leak after administration of moderate volumes of crystalloid solutions. Assessing interstitial tissue edema is an essential component of monitoring during fluid administration. Three body regions that are useful to evaluate are the hock because nonedematous animals, regardless of the amount of body fat, have well-defined lateral saphenous veins, Achilles tendons, and bony prominences; the mandibles and intermandibular space because these areas also are well defined in most animals; and the movement of the skin and subcutaneous tissues over the torso. With the development of interstitial edema, these anatomic regions become less defined, and a “jelly-like” appearance of the skin develops. If these findings are generalized, overhydration resulting from excessive fluid administration or capillary leak can be assumed. These regions should always be examined for baseline assessment before fluid administration. Chemosis also may occur with overhydration, but this finding tends to occur later than those previously mentioned. Bandages placed around the neck to secure a catheter into the jugular vein may cause edema of the head and chemosis unassociated with overhydration. Likewise, edema of a distal limb may occur if it is the dependent limb or a bandage is placed above the hock or carpus. Although body weight did not change in the majority of animals treated for dehydration during a 24- to 48-hour period,41 body weight should be monitored because an increase above that calculated to treat dehydration may indicate fluid overload if confirmed by other physical findings. Fluid losses into third spaces will increase body weight without improvement in overall fluid repletion.
When edema is noted in subcutaneous tissues, it is likely that a similar degree of edema also exists in body organs. It has been my observation at necropsy that edema of the brain, gastrointestinal tract, heart, liver, and kidney coexists with subcutaneous edema. This finding may account for some of the clinical signs observed, including depression, vomiting, cardiac arrhythmias, coagulopathy, and oliguria (see later discussion).
Pulmonary Edema
Pulmonary interstitial fluid dynamics differ from those of other tissues. Pulmonary capillary pressure is lower (approximately 7 mm Hg); interstitial fluid pressure in the lung is more negative (−8 to −5 mm Hg) than that of peripheral subcutaneous tissue; and the pulmonary capillaries are relatively permeable to protein molecules, rendering the COP of the pulmonary interstitial fluid approximately 14 mm Hg. These differences favor fluid movement from the alveoli into the interstitium and lymphatics.25 Pulmonary edema occurs in the same manner as does edema elsewhere in the body. Therefore the conditions discussed above can result in pulmonary edema after an excessive volume of crystalloid, and potentially colloid, is administered. As pulmonary interstitial pressure increases into the positive pressure range and the lymphatics are unable to remove this fluid, it leaks into the alveolar space. In the absence of capillary leak disorders, when the pulmonary capillary pressure exceeds 25 mm Hg (approximately 18 mm Hg above normal) in normal dogs, fluid accumulates in the lungs. Experiments performed on dogs showed that pulmonary capillary pressure must increase to a value at least equal to the COP of the plasma inside the capillaries before clinically relevant pulmonary edema occurs.25 The COP of normal dogs is 19.95 ± 2.1 (range, 15.3 to 26.3) mm Hg, and measuring COP in addition to evaluating physical findings helps guide fluid management. This information applies to normal dogs and not dogs with capillary leak conditions or those that are hypoproteinemic. When COP is decreased, as in patients with hypoproteinemia, edema formation may occur even at lower hydrostatic pressures. In experimental models, edema begins to form at 11 mm Hg when COP is decreased.29
Monitoring physical signs (see Table 16-2) can be effective in assessing potential fluid overload. The author has observed shivering, restlessness, nausea (as indicated by swallowing and licking the lips), and rarely vomiting in some animals as a response to an excessive rate of infusion of a balanced electrolyte solution. These signs stopped within 1 or 2 minutes after discontinuing or reducing the fluid rate for a short period, and the observed behavior was reproduced when a high rate of fluid administration was reestablished. These animals did not have identifiable cardiac disease. While monitoring CVP in one animal with oliguric renal failure that demonstrated this behavior, CVP rapidly increased from 1 cm H2O to 11 cm H2O. The fluids were discontinued, but when restarted, the same behavior occurred when CVP reached 10 to 11 cm H2O again. The fluid rate in this instance was reestablished at a more reasonable level for this dog. The reason for the observed signs may be associated with a vagally mediated baroreceptor reflex secondary to atrial stretch. Monitoring respiratory rate and effort is simple, and a slight but consistent increase can be an early clue to fluid overload and development of pulmonary edema. A slight but consistent reduction in oxygen saturation (Spo2) over a few minutes as detected by pulse oximetry may be another indication of pulmonary edema. Confirmation by a reduction in Pao2 on arterial blood gases may be warranted to confirm the Spo2 readings. As an aside, titration of oxygen via nasal cannula can be performed using pulse oximetry. As the Spo2 reading consistently hovers around 92% to 95%, this would be the oxygen flow rate to administer to avoid potential oxygen toxicity while providing adequate oxygenation. Radiographic assessment of the pulmonary vasculature can be used to monitor fluid administration. In animals, the width of the pulmonary vein should be less than 1.5 times the width of the pulmonary artery, and fluid overload should be considered if the measured difference exceeds this value. In human patients, changes in vascular pedicle width has proven to be a valuable method for monitoring fluid balance in the intensive care unit.52 The radiographic appearance of pulmonary edema, increased lung sounds such as crackles, and cyanosis indicate a late stage of edema with severe patient compromise. Capillary permeability is increased during systemic inflammatory conditions, endothelial injury, pneumonia, and pancreatitis, and capillary leak would be expected to occur with administration of smaller fluid volumes. Monitoring in affected animals must be diligent with even slight changes being a potential warning sign of pulmonary edema. Pancreatitis is a relatively common problem in cats and dogs requiring fluid therapy. In humans, approximately 33% of pancreatitis patients will develop acute lung injury and acute respiratory distress syndrome.36 This complication is caused by changes in the pulmonary endothelium associated with the systemic inflammatory process, liberation of pancreatic digestive enzymes (especially elastase), and damage by neutrophils that results in enhanced capillary leak.13,37 These changes also occur in small animals.33
In a prospective observational study of human septic patients, a positive cumulative fluid balance had a significant negative influence on survival.83 A retrospective study of human patients with septic shock and acute lung injury confirmed the association between fluid accumulation and mortality after onset of septic shock.63 While there are no veterinary studies evaluating cumulative fluid overload, clinical observation in septic or capillary leak conditions in veterinary patients indicates similar findings to those in human patients.
Fluid administration is a lifesaving procedure in many situations. The rate and volume of administration must be carefully considered in the individual patient based on their unique clinical and physical situation. Fluid accumulation and fluid overload are frequent findings in human and veterinary critically ill patients and in those suffering from severe acute kidney injury. A recent review on this topic focuses on the consequences associated with fluid overload in critically ill patients, with or without associated acute kidney injury, and discusses the potential mechanisms by which acute kidney injury can contribute to fluid overload and whether fluid overload can also contribute to kidney dysfunction.17
Effusions
When edema occurs in tissues, effusion may occur in potential spaces (e.g., pleural cavity, pericardial cavity, peritoneal cavity, joint cavities). The extent to which effusion occurs will depend on the severity of the fluid overload and capillary leak. Fluid pressure in these potential spaces in the normal state is negative and similar to that of subcutaneous tissue. The interstitial hydrostatic pressure normally is −7 to −8 mm Hg in the pleural cavity, −3 to −5 mm Hg in the joint spaces, and −5 to −6 mm Hg in the pericardial cavity.22 The abdominal cavity is prone to effusion (i.e., ascites). Pleural and peritoneal effusions may be present because of the disease process even before fluids are administered, and both often are present in patients with moderate to severe pancreatitis. Small volumes of fluid in the peritoneal and pleural cavity are difficult to detect on physical examination. Baseline assessment of the chest should be made by auscultation and thoracic radiography, and gentle ballottement and radiography or ultrasonography can be used to assess the abdomen. Further monitoring may be required during fluid therapy, and a change in the type of fluid administered may be required. With increasing effusion and decreased COP, a colloid should be considered and cautiously administered.
As previously mentioned, fluid overload in critical illness is an area of increasing attention, especially in the pathogenesis of abdominal compartment syndrome with volume overload having an association with adverse clinical outcomes in critically ill patients.18,82
Fluid accumulation can contribute to organ dysfunction by various different mechanisms. The resulting tissue edema of abdominal organs may directly impair function such as gut absorption or kidney excretion as examples. The abdominal compartment syndrome has been increasingly recognized in medical and surgical patients following intense volume resuscitation with consequent acute formation of ascites and edema.49
Blood Loss
Potentially deleterious effects of aggressive fluid therapy to treat patients after trauma before full assessment can increase morbidity and mortality. After trauma, the term “shock” frequently is used to describe a patient that is tachycardic, tachypneic, and has weaker than normal pulses, paler than normal MMs, and CRT of more than 2 seconds. However, mentation also is an important part of the assessment. If the patient has depressed mentation of varying degree but without known brain injury, this finding suggests clinically important blood loss and shock. However, if the patient still is alert, the physical findings may be the result of a sympathetic response caused by pain and fright and not necessarily associated with blood loss. If the initial clinical response, in the latter case, is to immediately institute aggressive fluid therapy to treat presumed shock, one must consider the potential role of the neuroendocrine response in producing these clinical signs with or without blood loss. As an example relating blood loss to clinical signs, a 30-kg blood donor normally can donate 450 mL blood (i.e., 20% of blood volume) without obvious clinical signs. However, it is prudent to consider that a traumatized patient or one with a coagulopathy is bleeding and consider all potential sites of ongoing hemorrhage. If hemorrhage is present, one must determine its severity based on clinical signs, physical examination findings, and serial monitoring of physiologic and laboratory test results. Crystalloids, colloids, or blood products should be administered at calculated rates based on clinical signs, physical examination findings, and laboratory data. Immediate compression of an area of hemorrhage should be performed. When compression is not feasible (i.e., within the abdomen or thorax), a careful and skilled approach to volume resuscitation must be conducted. Trauma patients also may have pulmonary contusions. Aggressive fluid therapy in these patients may cause pulmonary edema, and pulmonary status must be evaluated and monitored. If pulmonary contusions are noted, judicious fluid administration to adequate endpoints should be carried out. It should not be assumed that these patients are necessarily hypovolemic. On many occasions, intravenous fluid therapy is not required, and the need for fluid therapy must be assessed on an individual basis.
Noncompressible Hemorrhage
Laboratory studies performed in the 1950s and 1960s set guidelines for the standard approach to resuscitation of patients with hypotensive hemorrhagic shock84 and focused on early, aggressive administration of crystalloid solutions and blood products. The goal was to restore intravascular volume and vital signs toward normal as quickly as possible regardless of the site of hemorrhage. However, these guidelines have come into question. Early laboratory studies used controlled hemorrhage models, whereas hemorrhage occurring in the clinical setting as a result of blunt or penetrating trauma is uncontrolled until definitive therapy controls bleeding. More recent hemorrhage models have demonstrated that aggressive fluid resuscitation may be harmful and may result in increased hemorrhage and mortality. Using splenic injury models, aggressive fluid therapy significantly increased hemorrhage and mortality.42,78 Availability of blood products in veterinary practice is limited, and ongoing hemorrhage will be fatal. Other studies demonstrated that achieving a MAP of 40 to 60 mm Hg improved survival compared with a MAP of 80 mm Hg.79 By achieving a MAP of 40 mm Hg and allowing hemostasis and clot formation with a gradual increase in MAP over several hours, survival was significantly increased when compared with immediate resuscitation to a MAP of 80 mm Hg.7 (The consequences of a MAP of <60 mm Hg for a few hours have not been reported in cats and dogs in the clinical setting). Although a clot is formed immediately, it tends to be soft and “jelly-like” and inadequate to maintain vascular integrity at high pressures. Allowing a little time for the clot to become a more rigid hemostatic plug facilitates hemostasis.75 Clinical trials in human patients are underway to further investigate this strategy. A recent review found no evidence to suggest that prehospital intravenous fluid resuscitation was beneficial and found some evidence that it may be harmful.14 However, this evidence was not conclusive. A UK Consensus Statement suggests a more cautious approach to fluid management than previously advocated and concludes that further research is required on hypotensive (i.e., cautious) resuscitation versus delayed or no fluid replacement, especially in those with blunt trauma.14 In my experience, a more cautious approach to resuscitation in blunt abdominal trauma patients with blood pressure correction to a MAP of 65 mm Hg, systolic pressure of approximately 95-100 mm Hg, with resulting physiologic parameters of decreased HR, decreased RR, and improvement in MM color and CRT, and urine produced at ~0.5 mL/kg/hour has proved successful. However, when physiologic parameters do not improve, surgical exploration is warranted. Unnecessary aggressive fluid therapy in a slightly hypotensive or normotensive animal before adequate clot formation could increase blood pressure and disrupt a clot on a lacerated vein or on a splenic or hepatic fracture. Minimum resuscitation to that required to afford adequate perfusion until such time as the hemorrhage is controlled makes more sense clinically. Volume resuscitation should be aggressive when the patient’s condition is life threatening. These patients are easily identified, and rapid resuscitation to an adequate hemodynamic state (MAP, approximately 65 mm Hg; systolic, approximately 90 to 95 mm Hg) is warranted until hemorrhage is controlled surgically or spontaneously stops. With blood volume loss of 30% or more, transfusion of whole blood, packed cells, and plasma or colloids is required in addition to crystalloids. Many abdominal trauma patients can be managed this way if blood products are readily available. However, surgical intervention may be necessary when resuscitative efforts are not successful. The clinician must remember that abdominocentesis will be negative if hemorrhage into the retroperitoneal space has occurred. Conversely, blood loss into the pericardial space resulting in tamponade requires aggressive fluid therapy while preparing the patient for pericardiocentesis. In the acute setting, only very small volumes of blood within the pericardial space are required to cause tamponade.
Coagulopathy
In addition to the above concerns for clot strength with aggressive fluid administration, trauma patients with blood loss receiving a large volume of crystalloid and colloid fluids to raise blood pressure are at great risk for dilutional and dysfunctional coagulopathy.44,81 In addition, the shock state plus room temperature fluids result in hypothermia. Acidosis often is present in such patients, and is made markedly worse by the use of normal saline. This combination enhances the loss and function of coagulation factors with the inevitable exacerbation of bleeding. As noted in the colloid section, hydroxyethyl starch may augment problems with hemostasis. Hetastarch (670/0.75) has been shown to alter platelet function in healthy dogs, which may increase the risk of bleeding.77 To avoid, or reduce, the coagulopathic complications associated with fluid administration, appropriate blood products, such as whole blood, warm balanced electrolyte fluids, and a calculated volume of synthetic colloid (see Chapter 27) should be administered. As a guide for requirement for fresh frozen plasma or fresh blood, ongoing monitoring and assessment of hemostatic function is essential. Periodic measurement of the activated clotting time using a tissue activator (i.e., tubes containing celite or kaolin [The Actalyke Story. The Science. Helena Laboratories, Beaumont, Tex. 1997].) is a very cheap test, and was prolonged in hemorrhage, hypothermia, and combined hypothermia and hemorrhage in a pig shock model.53 This test was more sensitive than PT (did not prolong at all) and aPTT (prolonged only in combined hemorrhage and hypothermia) in detecting clotting changes since platelets and other blood clotting components are included in the test. Thromboelastography is also useful as this test was also altered in all three scenarios, and detects the mechanisms associated with coagulation abnormalities;53 however, this is a very expensive test precluding it as a practical trending test in the resuscitative setting where frequent measurements are required. A study monitoring the ACT in human trauma patients during resuscitation in the operating room, concluded that a low ACT reflects the initial hypercoagulability associated with major trauma and an elevated ACT is an objective indicator that the coagulation system reserve is near exhaustion.2 As the ACT is prolonged in brain trauma (brain tissue is a trigger for DIC), this test cannot be reliably used as an assessment of dilutional coagulopathy where head injury is present.
As a guide for the normal range for ACT, obtained using various techniques, before establishment of the normal range for ACT within each individual institution, refer to Table 16-5.
Compressible Hemorrhage
When hemorrhage is compressible (i.e., that occurring from a limb), pressure is easily applied and hemorrhage stopped. Fluid resuscitation then can occur to optimal requirements without concern of further blood loss.
Autotransfusion of Blood
Autotransfusion of blood, in the peracute setting, from the thorax or abdomen can be lifesaving in an exsanguinating animal if no other source of hemoglobin is available. However, one should anticipate serious complications that will arise if blood is autotransfused from abdominal hemorrhage of several hours duration or associated with intestinal or colonic injury and leakage of contents, or that which has occurred as a result of neoplasia. Frequently, hemorrhage associated with neoplasia does not represent a single episode of bleeding but rather one of several with some episodes of hemorrhage occurring days to weeks previously. Various metabolic products in this accumulated blood are triggers for disseminated intravascular coagulation even when filtered. The potential for facilitating metastatic spread of the tumor also is frequently debated.
Approach to Fluid Selection and Volume to Avoid Complications
The volume of fluid to be administered is dependent on the situation at hand. The cause and severity of the hypovolemic state are important factors in fluid (e.g., crystalloid, colloid, blood products) selection and volume of resuscitation. It is important to ascertain whether fluids are required. Traumatized patients do not always have blood loss. When an intravascular volume deficit is confirmed, it is useful to construct a mental algorithm when managing patients with hypovolemia. It is best to start with the question, “Is there hemorrhage or not?” If “yes,” “is the hemorrhage compressible or noncompressible?” If it is compressible, the patient should be managed to an optimal goal of resuscitation. If it is noncompressible, the patient should be resuscitated to an adequate hemodynamic state as described in the section on Endpoints of Fluid Resuscitation. Other questions include “Is there hemorrhage associated with pulmonary contusions?” If so, the patient should be resuscitated to adequate perfusion (see Endpoints of Fluid Resuscitation section). “Is surgical intervention required?” If so, the patient should be resuscitated to an adequate hemodynamic state and then to optimal perfusion when the hemorrhage is controlled (see Endpoints of Fluid Resuscitation section). “If hemorrhage is not present, is capillary leak present or not? If capillary leak is not present, is COP normal or not?” If COP is normal, the patient should be resuscitated to optimal perfusion (see Endpoints of Fluid Resuscitation section). Crystalloids frequently are adequate. If not, a synthetic or natural colloid may be required. If capillary leak is present, fluid selection is crucial, and different types of fluids frequently are necessary. Resuscitation to optimal perfusion should be attempted, but resuscitation to adequate perfusion frequently is all that can be attained until the underlying illness is resolved.
Recognizing the underlying cause of hypovolemia and the appropriate type of fluid to administer is important. Being aware of associated problems that will necessitate cautious fluid selection, volume, and rate of administration is key to success. The difference between optimal and adequate perfusion endpoints must be understood. Continued monitoring appropriate for the condition being managed will reduce the frequency of complications that can be associated with administration of fluids. There is no single method to monitor the adequacy of fluid resuscitation. Using a combination of methods and appreciating the limitations of each under the various conditions that are being used will assist the clinician in making the best possible assessment.
Complications associated with routes of fluid administration
Fluids can be delivered by several routes: enteral, subcutaneous, intraosseous, intraperitoneal, and most commonly, intravenous. Complications associated with intravenous catheters are discussed in detail in Chapter 15.
Enteral Route
The enteral route can be used effectively to rehydrate otherwise stable veterinary patients, assuming enteral routes of fluid loss (e.g., vomiting, diarrhea) are not present. A nasoesophageal tube is easily placed, and a maintenance electrolyte solution (Normosol-M®, Plasma-Lyte 56®) can be delivered via a fluid administration set.59 Potential complications with the enteral route include positioning of the tube within the airway, vomiting if the administration rate is too high, and hyperkalemia if potassium supplementation is excessive.
Subcutaneous Route
This route of administration is very commonly used in veterinary practice. However, it is not recommended for animals with moderate to severe dehydration or for those with circulatory compromise. Circulation to the skin is reduced in volume-depleted animals, resulting in slow absorption. If absorption is poor or an excessive volume of fluid is administered, pooling of the fluid may occur in the subcutaneous tissues, and then fluid may gravitate ventrally. Pooling of fluid results in discomfort and lowers body temperature. Lactated Ringer’s solution and 0.9% saline are fluids of choice for the subcutaneous route. Acetated polyionic solutions (Plasma-Lyte 148, Plasma-Lyte A, and Normosol-R) appear to cause severe discomfort when administered subcutaneously. The author has observed excessive vocalization and other painful behavior after subcutaneous administration of Plasmalyte 148. These effects may be associated with the acetate itself because the p. 6 of this solution is comparable with that of lactated Ringer’s solution and is greater than that of 0.9% sodium chloride. Dextrose-containing solutions should not be administered via this route. Abscess formation and cellulitis also may be complications of this route if aseptic technique is not followed carefully.
Intraosseous
The bone marrow of the femur and humerus occasionally is more easily accessed than small collapsed veins in neonatal and pediatric small animal patients. Strict aseptic technique is required to avoid infection resulting in abscess formation and sepsis. This procedure is painful, and lidocaine should be infiltrated through the skin, subcutaneous tissue, and to the periosteum before attempting catheter placement. Iatrogenic injury to the regional nerves also may occur. Although this route is frequently advocated for our very young and small patients, this is rarely performed at the Ontario Veterinary College because placement of a 2-inch peripheral intravenous catheter into the jugular vein is preferred. However, where intravascular access cannot be obtained and intraosseous access is required, the EZ-IO Intraosseous Infusion system (Vidacare—distributed by MILA International, Erlanger, Ky.) is suggested as this technology allows clinicians to access the intraosseous space quickly and relatively painlessly. This technique is also useful for fluid delivery in severely hypovolemic animals with collapsed veins.
Intraperitoneal
This route is rarely used, but relatively rapid absorption of crystalloid solutions occurs from this site.70 Concerns using this route include pathologic conditions of the abdomen and the risk of peritonitis should contamination occur. Solutions containing acetate (e.g., Plasma-Lyte, Normosol products) should be avoided because they appear to be very painful when introduced into the abdomen. Lactated Ringer’s solution and 0.9% saline are advised for this route.
Intravenous
The most common route for fluid administration is the intravenous route. Catheters are placed in peripheral veins (e.g., saphenous, cephalic) or a jugular vein. Strict aseptic technique is required (see Chapter 15). Complications may occur if surgical preparation of the venipuncture site is not performed. Inflammation at the venipuncture site may indicate infection or phlebitis caused by movement of the catheter or the rigid material of the catheter (Teflon). In a study in which Violon catheters (Insyte, Becton Dickinson, Franklin Lakes, N.J.) were placed after strict aseptic skin preparation, catheter dwell time could be extended up to 10 days with peripheral catheters and longer with jugular catheters.57 These catheters become very flexible and soft when warmed to body temperature, and catheter replacement every 72 hours is not necessary when using these methods. Reduced inflammation associated with Violon catheters appears to result in minimal discomfort for the animal, and rarely do dogs and cats attempt to remove the catheter. At the Ontario Veterinary College, catheters only are removed when inflammation or fever of unknown origin occurs, or if the catheter is grossly contaminated. Such catheter tips are submitted for culture, and rarely (approximately one annually) is bacterial contamination identified. Inflammation of the catheter insertion site tends to occur at 72 hours in patients with vasculitis (e.g., immune-mediated hemolytic anemia).57 Vasculitis may also predispose to thrombosis (e.g., immune-mediated hemolytic anemia, pancreatitis). Jugular vein catheterization should be avoided if possible in such animals, but many require frequent blood sampling or parenteral nutrition or both, often necessitating a central catheter. Heparin administration may reduce the potential for thrombosis.
Extravasation of fluids can be a serious complication if the fluid is hyperosmolar (e.g., amino acids, >5% dextrose in electrolyte solutions), contains vasoconstrictive drugs (e.g., epinephrine, norepinephrine), or contains certain chemotherapeutic agents. To reduce or prevent phlebitis associated with hyperosmolar solutions, a central vein should be used to deliver these solutions. If a peripheral vein must be used, a 24- to 22-gauge catheter rather than 20- to 18-gauge catheter should be selected. A minimum catheter length of 2 inches should be selected where possible, unless the patient is very small. Unlike the standard approach, where a subcutaneous tunnel before venipuncture is recommended, it is important that the short (<2 inches) catheter be placed directly through the skin and into the vein at a minimal angle to avoid excessive tunneling before the catheter enters the vein. This will ensure that the whole length of the catheter enters the vein, reducing the chance of the catheter backing out of the vein, resulting in leakage of the hyperosmolar solution (these should be considered for short-term use only). The catheter must be secured well to prevent movement of the skin and dislodgement of the catheter. Where loose skin is a problem, a 2-inch, 22- or 20-gauge catheter is essential. Hyperosmolar solutions should be administered slowly through small bore catheters to avoid displacement from the vein. The area of catheter placement routinely should be assessed each time the veterinarian or technician examines the patient. The bandage does not have to be removed (exceptions are hyperosmolar and vasoactive solutions), but the limb should be palpated for heat, swelling, and discomfort. If infusion pumps are used and the alarm signals occlusion, the catheter and entry site should be examined carefully.
Destruction of the catheter or administration set will occur if the animal bites it, and hemorrhage may be a complication if this event is not witnessed. Serious hemorrhage also may occur if the connector on an arterial catheter becomes dislodged and may require blood transfusion if a large amount of blood loss occurs.
Finally. catheters should not be disconnected when the patient is moved. The intravenous line should be clamped as close to the catheter site as possible, the catheter flushed with a 1 unit heparin/mL saline solution, and the bags carried with the patient. Disconnecting the lines promotes microbial contamination and the potential for sepsis.
1 Aldrich J., Haskins S. Monitoring the critically ill patient. In: Bonagura J.D., Kirk R.W., editors. Current veterinary therapy XII. Philadelphia: WB Saunders; 1995:98-105.
2 Aucar J.A., Norman P., Whitten E. Intraoperative detection of traumatic coagulopathy using the activated coagulation time. Shock. 2003;19(5):404-407.
3 Christiane S., Hartog M.D., Michael Bauer M.D., Konrad Reinhart M.D. The Efficacy and Safety of Colloid Resuscitation in the Critically Ill. Anesth Analg. 2011;112:156-164.
4 Baldassarre S., Vincent J.L. Coagulopathy induced by hydroxyethylstarch. Anesth Analg. 1997;84:451-453.
5 Bateman S.W., Mathews K.A. Comparison of axillary and heating block methods of activated clotting time (ACT) in dogs. J Vet Emerg Crit Care. 1999;9:79-82.
6 Boldt J. Hydroxyethylstarch as a risk factor for acute renal failure: is a change of clinical practice indicated? Drug Saf. 2002;25:837-846.
7 Burris D., Rhee P., Kaufmann C., et al. Controlled resuscitation for uncontrolled hemorrhagic shock. J Trauma. 1999;46:216-223.
8 Clemmesen O., Ott P., Larsen F.S. Splanchnic metabolism in acute liver failure and sepsis. Curr Opin Crit Care. 2004;10:152-155.
9 Cohen R.D., Woods R.F. Clinical and biochemical aspects of lactic acidosis. Boston: Blackwell Scientific. 1976:42.
10 Cornelius L.M. Fluid therapy in small animal practice. J Am Vet Med Assoc. 1980;176:110-114.
11 Dart A.B., Mutter T.C., Ruth C.A. Hydroxyethyl starch (HES) versus other fluid therapies: effects on kidney function. Cochrane Database Syst Rev. (1):2010. Art.No: CD007594. DOI: 10.1002/14651858.CD007594.pub2
12 de Laforcade A.M., Rozanski A. Central venous pressure and arterial blood pressure measurements. Vet Clin North Am Small Anim Pract. 2001;31:1163-1174.
13 Downey G.P., Dong O., Kruger J. Regulation of neutrophil activation in acute lung injury. Chest. 1999;116(Suppl. 1):46S-54S.
14 Dretzke J., Sandercock J., Bayliss S., et al. Clinical effectiveness and cost-effectiveness of prehospital intravenous fluids in trauma patients. Health Technol Assess. 2004;8:iii. 1–103
15 Ethier M.R., Mathews K.A., Valverde, et al. Evaluation of the efficacy and safety of two sedative-analgesic protocols in assisted ventilation in healthy dogs. Am J Vet Res. 2008;69:1351-1359.
16 Ferraboli R., Malheiro P.S., Abdulkader R.C., et al. Anuric acute renal failure caused by dextran-40 administration. Ren Fail. 1997;19:303-306.
17 Boucharda J., Mehta R.L. Fluid accumulation and acute kidney injury: consequence or cause. Curr Opin Crit Care. 2009;15:509-513.
18 Foland J.A., Fortenberry J.D., Warshaw B.L., et al. Fluid overload before continuous hemofiltration and survival in critically ill children: a retrospective analysis. Crit Care Med. 2004;32:1771-1776.
19 Francis A., Martin L., Haldorson G.J., et al. Adverse reactions suggestive of type III hypersensitivity in six healthy dogs given human serum albumin. J Am Vet Med Assoc. 2007;230(6):873-879.
20 Gibson G.R., Callan M.B., Hoffman V. Use of a hemoglobin-based oxygen-carrying solution in cats: 72 cases (1998-2000). J Am Vet Med Assoc. 2002;221:96.
21 Gunkel C., Valverde A., Morey T., Hernandez J., Robertson S.A. Comparison of non-invasive cardiac output measurement by partial carbon dioxide rebreathing with the lithium dilution method in anesthetized dogs. J Vet Emerg Crit Care. 2004;14:187-195.
22 Guyton A.C., Hall J.E. Body fluid compartments. In: Guyton A.C., Hall J.E., editors. Textbook of medical physiology. 10th ed. Philadelphia: WB Saunders; 2000:264.
23 Guyton A.C., Hall J.E. Dominant role of the kidney in long-term regulation of arterial pressure and in hypertension: the integrated system for pressure control. In: Guyton A.C., Hall J.E., editors. Textbook of medical physiology. 10th ed. Philadelphia: WB Saunders; 2000:329.
24 Guyton A.C., Hall J.E. Integration of renal mechanisms for control of blood volume and extracellular fluid volume; and renal regulation of potassium, calcium, phosphate and magnesium. In: Guyton A.C., Hall J.E., editors. Textbook of medical physiology. 10th ed. Philadelphia: WB Saunders; 2000:329.
25 Guyton A.C., Hall J.E. Pulmonary circulation, pulmonary edema, pleural fluid. In: Guyton A.C., Hall J.E., editors. Textbook of medical physiology. 10th ed. Philadelphia: WB Saunders; 2000:444-451.
26 Guyton A.C., Hall J.E. Regulation of extracellular fluid osmolarity and sodium concentration. In: Guyton A.C., Hall J.E., editors. Textbook of medical physiology. 10th ed. Philadelphia: WB Saunders; 2000:313.
27 Guyton A.C., Hall J.E. The body fluid compartments: extracellular and intracellular fluids; interstitial fluid and edema. In: Guyton A.C., Hall J.E., editors. Textbook of medical physiology. 10th ed. Philadelphia: WB Saunders; 2000:264.
28 Guyton A.C., Hall J.E. The kidneys and body fluids. In: Guyton A.C., Hall J.E., editors. Textbook of medical physiology. 10th ed. Philadelphia: WB Saunders; 2000:332-335.
29 Guyton A.C., Lindsey A.W. Effect of elevated left atrial pressure and decreased plasma protein concentration on the development of pulmonary edema. Circ Res. 1959;7:649-657.
29a Hansen B., DeFrancesco T. Relationship between hydration estimate and body weight change after fluid therapy in critically ill dogs and cats. J Vet Emerg Crit Care. 2002;12:235-243.
30 Hansen B. Technical aspects of fluid therapy: catheters and monitoring of fluid therapy. In: DiBartola S., editor. Fluid therapy in small animal practice. 3rd ed. Philadelphia: WB Saunders; 2000:344-376.
31 Hayes G., Mathews K.A., Boston S., Dewey C. Central Venous Oxygen Saturation and Associations with Outcome in Canine ICU patients. Abstract Int Vet Emerg Crit Care Symposium. 2009. Chicago
32 Holbeck S., Grande P. Effects on capillary fluid permeability and fluid exchange of albumin, dextran, gelatin and hydroxyethylstarch in cat skeletal muscle. Crit Care Med. 2000;28:1089-1095.
33 Holm J.L., Chan D.L., Rozanski E.A. Acute pancreatitis in dogs. J Vet Emerg Crit Care. 2003;13:201-213.
34 Hughes D., Drobatz K.J. Comparison of plasma lactate concentration from cephalic, jugular, and femoral arterial blood samples in normal dogs. J Vet Emerg Crit Care. 1996;6:115.
35 Hughes D. Lactate measurement: diagnostic, therapeutic, and prognostic implications. In: Bonagura J.D., editor. Kirk’s current veterinary therapy XIII. Philadelphia: WB Saunders; 2000:112-116.
36 Jacobs M.L., Daggett W.M., Civette J.M. Acute pancreatitis: analysis of factors influencing survival. Ann Surg. 1977;185:43-51.
37 Jaffray C., Yang J., Carter G. Pancreatic elastase activates pulmonary nuclear factor kappa B and inhibitory kappa B, mimicking pancreatitis-associated adult respiratory distress syndrome. Surgery. 2000;128:225-231.
38 Jellinek H., Krafft P., Fitzgerald R.D., et al. Right atrial pressure predicts hemodynamic response to apneic positive airway pressure. Crit Care Med. 2000;28:672-678.
39 Jonas M.M., Tanser S.J. Lithium dilution measurement of cardiac output and arterial pulse waveform analysis: an indicator dilution calibrated beat-by-beat system for continuous estimation of cardiac output. Curr Opin Crit Care. 2002;8:257-261.
40 Jones A.E., Shapiro N.I., Trzeciak S., et al. Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy: A randomized clinical trial
41 Kilborn S., Pook H., Bonnett B. Comparison of three different methods of total carbon dioxide measurement. Vet Clin Pathol. 1995;24:22-27.
42 Krausz M.M., Bashenko Y., Hirsh M. Crystalloid or colloid resuscitation of uncontrolled hemorrhagic shock after moderate splenic injury. Shock. 2000;13:230-236.
43 Lagutchik M.S., Ogilvie G.K., Wingfield W.E. Lactate levels in critically ill and injured dogs. J Vet Emerg Crit Care. 1996;6:119.
44 Lier H., Krep H., Schroeder S., et al. Preconditions of hemostasis in trauma: A review. The influence of acidosis, hypocalcemia, anemia and hypothermia on functional hemostasis in trauma. J Trauma. 2008;65:951-960.
45 Linton R.A., Young L.E., Marlin D.J., et al. Cardiac output measured by lithium dilution, thermodilution and transesophageal Doppler echocardiography in anesthetized horses. Am J Vet Res. 2000;61:731-737.
46 Liskaser F., Story D.A. The acid-base physiology of colloid solutions. Curr Opin Crit Care. 1999;5:440-442.
47 Machado C.G., Dyson D.H., Mathews K.A. Evaluation of induction by use of a combination of oxymorphone and diazepam or hydromorphone and diazepam and maintenance of anesthesia by use of isoflurane in dogs with experimentally induced hypovolemia. Am J Vet Res. 2005;66(7):1227-1237.
48 Machon R.G., Raffe M.R., Robinson E.P. Central venous pressure measurements in the caudal vena cava of cats. J Vet Emerg Crit Care. 1995;5:121-129.
49 Maerz L., Kaplan L.J. Abdominal compartment syndrome. Crit Care Med. 2008;36(4 Suppl.):S212-S215.
50 Mandell D.C., King L.G. Fluid therapy in shock. Vet Clin North Am Small Anim Pract. 1988;28:623-644.
51 Manning J.E., Katz L.M., Brownstein M.R., et al. Bovine hemoglobin-based oxygen carrier (HBOC-210) for resuscitation of uncontrolled, exsanguinating liver injury in swine. Shock. 2000;13:152-159.
52 Martin G.S., Ely W., Carroll F.E., et al. Findings on the portable chest radiograph correlates with fluid balance in critically ill patients. Chest. 2002;122:2087-2095.
53 Martini W.Z., Cortez D.S., Dubick M.T.T., et al. Thromboelastography is better than PT, aPT and activated clotting time in detecting clinically relevant clotting abnormalities after hypothermia, hemorrhagic shock and resuscitation in pigs. J Trauma. 2008;65:535-543.
54 Marx G. Fluid therapy in sepsis with capillary leakage. Eur J Anaesthesiol. 2003;20:429-442.
55 Mason D.J., O’Grady M., Woods J.P., et al. Comparison of a central and a peripheral (cephalic v) injection site for the measurement of cardiac output using the lithium-dilution cardiac output technique in anesthetized dogs. Can J Vet Res. 2002;66:207-210.
56 Mathews K.A., Barry M. The use of 25% human serum albumin: outcome and efficacy in raising serum albumin and systemic blood pressure in critically ill dogs and cats. J Vet Emerg Crit Care. 2005;15:110-180.
57 Mathews K.A., Brooks M., Valliant A.E. A prospective study of intravenous catheter contamination. J Vet Emerg Crit Care. 1996;6:33-37.
58 Mathews K.A. The therapeutic use of 25% human serum albumin in critically ill dogs and cats. Vet Clin Small Anim. 2008;38:595-605.
59 Mathews K.A. The various types of parenteral fluids and their indications. Vet Clin North Am Small Anim. 1998;28:483-513.
60 McGee A., Abernethy W.B.III, Simel D.L. The rational clinical examination. Is this patient hypovolemic? JAMA. 1999;281:1022-1029.
61 Mishler J.M. Synthetic plasma volume expanders: their pharmacology, safety and clinical efficacy. Clin Haematol. 1984;13:75-92.
62 Moon P.F., Ilkiw J.E. Surface-induced hypothermia in dogs: 19 cases (1987-1989). J Am Vet Med Assoc. 1993;202:437-444.
63 Murphy C.V., Schramm G.E., Doherty J.A., et al. The importance of fluid management in acute lung injury secondary to septic shock. Chest. 2009;136:102-109.
64 Nelson L. The new pulmonary artery catheter: continuous venous oximetry, right ventricular ejection fraction and continuous cardiac output. New Horiz. 1997;5:251-258.
65 Pascoe P.J. Perioperative management of fluid therapy. In: DiBartola S., editor. Fluid therapy in small animal practice. 2nd ed. Philadelphia: WB Saunders; 2000:307.
66 Pinsky M.R. Functional hemodynamic monitoring. Intensive Care Med. 2002;28:386-388.
67 Reuter D.A., Felbinger T.W., Schmidt C., et al. Stroke volume variations for assessment of cardiac responsiveness to volume loading in mechanically ventilated patients after cardiac surgery. Intensive Care Med. 2002;28:392-398.
68 Roche A.M., James M.F.M. Colloids and Crystalloids: does it matter to the kidney? Curr Opin Crit Care. 2009;15:520-524.
69 Ryden S.E., Oberman H.A. Compatibility of common intravenous solutions with CPD blood. Transfusion. 1975;15:250-255.
70 Schaer M. General principles of fluid therapy in small animal medicine. Vet Clin North Am Small Anim Pract. 1989;19:203-210.
71 Scheingraber S., Rehm M., Sehmisch C., et al. Rapid saline infusion produces hyperchloremic acidosis in patients undergoing gynecologic surgery. Anesthesiology. 1999;90:1265-1270.
72 Schertel E.R., Tobias T.A. Hypertonic fluid therapy. In: DiBartola S.P., editor. Fluid therapy in small animal practice. Philadelphia: WB Saunders; 1992:471.
73 See A.M., Swindells K.L., Sharman M.J., et al. Activated coagulation times in normal cats and dogs using MAX-ACT tubes. Aust Vet J. 2009;87:292-295.
74 Simpson S.A., Syring R., Otto C.M. Severe blunt trauma in dogs: 235 cases (1997-2003). J Vet Emerg Crit Care. 2009;1996:588-602.
75 Sixma J.J., Wester J. The hemostatic plug. Semin Hematol. 1977;14:265-299.
76 Smart L., Jandrey K.E., Kass P.H., et al. The effect of hetastarch (670/0.75) in vivo on platelet closure time in the dog. J Vet Emerg Crit Care. 2009;19(5):444-449.
77 Smiley L.E., Garvey M.S. The use of hetastarch as adjunct therapy in 36 dogs with hypoalbuminemia: a phase two clinical trial. J Vet Intern Med. 1994;8:195-200.
78 Solomonov E., Hirsh M., Yahiya A. The effect of vigorous fluid resuscitation in uncontrolled hemorrhagic shock after massive splenic injury. Crit Care Med. 2000;28:749-754.
79 Stern S.A., Kowalenko T., Younger J. Comparison of the effects of bolus vs slow infusion of 7.5% NaCl/6% Dextran-70 in a model of near-lethal uncontrolled hemorrhage. Shock. 2000;14:616-622.
80 Story D.A., Bellomo R. The acid-base physiology of crystalloid solutions. Curr Opin Crit Care. 1999;5:436-439.
81 Tieu B.H., Holcomb J.B., Schreiber M.A. Coagulopathy: Its pathophysiology and treatment in the injured patient. World J Surg. 2007;31:1055-1064.
82 Van Biesen W., Yegenaga I., Vanholder R., et al. Relationship between fluid status and its management on acute renal failure (ARF) in intensive care unit (ICU) patients with sepsis: a prospective analysis. J Nephrol. 2005;18:54-60.
83 Vincent J.L., Sakr Y., Sprung C.L., et al. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006;34:344-353.
84 Wiggers C.J. Physiology of shock. New York: Commonwealth Fund. 1950:121-143.