Chapter 11 The History and Physical Examination
The ubiquitous nature of arteries, veins, and lymphatic vessels allows for any region of the body to develop vascular disease. This chapter describes the vascular medical history and physical examination—the core components of evaluating patients with vascular diseases. Application of these methods and tailored use of special examination maneuvers facilitate the diagnosis of vascular disease, especially when used in conjunction with vascular tests described elsewhere in this section. This chapter will review the cardinal complaints of patients with vascular disease, and then the physical findings associated with common arterial, venous, and lymphatic diseases. More specific features of the vascular history and examination are discussed in the relevant chapters of each vascular disease.
Vascular History
The medical history is the foundation of the physician-patient interaction, guiding the physical examination, testing, and treatment decisions. A comprehensive medical history can identify the diagnosis the vast majority of the time, but an inadequate one can result in excess testing and inappropriate therapy.
Arterial Disease
Symptoms of arterial disease typically arise as a result of either arterial stenoses or occlusions, though aneurysms also may cause symptoms. The important historical features of arterial disease in selected regional circulations are reviewed first.
Peripheral artery disease
In addition to carotid and coronary artery disease (CAD), peripheral artery disease (PAD) is one of the most common clinical manifestations of atherosclerosis. Approximately 50% of patients with PAD have symptoms, described in the following discussion as typical or atypical, and the remainder are asymptomatic. The importance of making the diagnosis of PAD, even in the absence of symptoms, derives from the prognostic information implicit with its diagnosis (see Chapter 16). Notably, patients with PAD often have coexisting coronary and cerebrovascular atherosclerosis, and are two- to fourfold more likely than patients without PAD to die of cardiovascular disease.1
Therefore, the history of patients with PAD should seek to determine whether the patient has known risk factors for atherosclerosis, and whether or not there are concomitant clinical manifestations of atherosclerosis. The historian should elicit information regarding dyslipidemia, diabetes mellitus, hypertension, family history of premature atherosclerosis, and cigarette smoking. Historical evidence of CAD, including prior myocardial infarction (MI), symptoms of angina, or prior coronary revascularization procedures and history of stroke or symptoms of cerebrovascular ischemia, including hemiparesis, hemiparesthesia, aphasia, or amaurosis fugax, should be sought and documented.
Intermittent Claudication
A cardinal symptom of PAD is intermittent claudication (see Chapter 18). Claudication occurs when limb skeletal muscle ischemia is produced with effort because increased muscle energy requirements are not served by sufficient augmentation in blood supply. Symptoms develop intermittently with activity; the blood flow limitation imposed by peripheral artery stenosis typically does not compromise muscular function at rest. Claudication is variably described as aching, heaviness, burning, fatigue, cramping, and/or tightness in the affected limb. Symptoms occur with reproducible amounts of exercise: one block of walking, one flight of stairs, or 5 minutes on a bicycle, for example. Discomfort may develop in any muscular portion of the leg—buttocks, hip, thigh, calf, or foot. Areas of the limb to develop discomfort are related to the arterial segments with stenoses. Iliac artery disease typically produces hip or buttock claudication, whereas femoral artery disease causes thigh or calf claudication. Arm claudication is unusual, but may occur in patients with innominate, subclavian, axillary, or brachial artery stenosis. Cessation of activity relieves the exercising muscle’s demand-supply mismatch and enables restoration of oxidative metabolism. Therefore, patients typically report that discontinuation of activity relieves the discomfort after several minutes.
Atypical symptoms also occur and may include reduction of leg discomfort despite continued effort, gait disturbance, and slower walking speed.2 Atypical claudication may be more common than traditional symptoms because of the high frequency of other conditions present in this older age group: spinal stenosis, venous insufficiency, and degenerative join disease. Patients with intermittent claudication often slow their walking speed by a third to regulate muscle use and prolong walking distance. Thus, when a physician solicits the history of walking impairment, patients may report no change in distance walked before symptoms occur, despite a progressive decline in functional ability.3
Several questionnaires for PAD have been devised and validated. These provide a standard to accompany the interview when querying patients about symptoms of PAD. The Rose questionnaire was the initial PAD-related questionnaire, but limited diagnostic sensitivity minimized its utility.4,5 The San Diego questionnaire is a modified version of the Rose Questionnaire and a more reliable instrument to assess intermittent claudication (see Chapter 16).6 The disease-specific Walking Impairment Questionnaire has been validated and can be used to assess walking difficulty in patients with PAD. It has four subscales: severity of pain with walking, distance, speed, and stair climbing.7
Critical Limb Ischemia
Critical limb ischemia (CLI) occurs when limb blood flow is inadequate to meet the metabolic demands of the tissues at rest.8 This may result in persistent pain, especially in the acral portions of the leg (toes, ball of the foot, heel). Additional foot symptoms include sensitivity to cold, joint stiffness, and hypesthesia. As a consequence of the effects of gravity on perfusion pressure, patients may report worsening of pain with leg elevation, or even when lying in bed, and reduction in pain with limb dependency (e.g., when the feet hang over the bed onto the floor). Critical limb ischemia may cause tissue breakdown (ulceration) or gangrene.
Acute Limb Ischemia
Acute limb ischemia is most often due to embolism or in situ thrombosis (see Chapter 46).9 Other causes include arterial dissection or trauma. The presentation of acute arterial occlusion ranges from asymptomatic loss of a pulse, to worsened claudication, to sudden onset of severe pain at rest. Symptoms may develop suddenly over several hours, or over several days. Acute ischemic symptoms are more likely to occur when no or few collateral vessels are present, rather than when there is a well-developed collateral network. Acute arterial occlusion may cause symptoms in any portion of the leg distal to the obstruction. The five Ps—pain, pallor, poikilothermia, paresthesias, and paralysis—characterize the historical features and findings of patients with acute limb ischemia. Severity of symptoms does not discriminate among etiologies.
Atheroembolism
Atheroembolism is embolization of atherosclerotic debris that compromises distal arteries (TAO) (see Chapter 47). Atheroemboli vary in composition, from larger fibroplatelet particles that occlude small arteries to cholesterol emboli, nanometers in size, that occlude arterioles. Causes of atheroemboli include catheterization and cardiovascular surgery, but approximately half of such events occur without a known precipitant.10
Symptoms reflect occlusion of the small distal vessels in the limb, and patients will commonly present with calf, foot, or toe pain and areas of violaceous discoloration or cyanosis in the toes (blue toe syndrome). Symptoms develop hours to days after the event; ulcerations may develop and are slow to resolve. Symptoms may be unilateral or bilateral, depending upon the origin of emboli proximal to or beyond the aortic bifurcation. If atheroemboli arise proximal to the renal arteries, renal insufficiency is a potential sequela.
Other Peripheral Artery Diseases
Uncommon diseases of the peripheral arteries should be considered in patients with claudication or evidence of ischemia, but whose age falls below that typically affected by atherosclerosis or in those with atypical symptoms. These diseases include thromboangiitis obliterans (TAO) (see Chapter 44), Takayasu arteritis (see Chapter 42), and giant-cell arteritis (see Chapter 43), and vascular compression syndromes, such as those affecting the thoracic outlet, iliac artery, and popliteal artery (see Chapter 62).
Takayasu arteritis is a large-vessel vasculitis that generally occurs between the ages of 20 and 40 years. Women are more likely to develop the disease than men. Constitutional and vascular symptoms occur (e.g., fevers, weight loss, fatigue, arthralgias, myalgias) and may be present months to years without overt evidence of vascular disease. About 50% of patients complain of muscle or joint pains, and headache has been reported in up to 40%. More than 50% of patients will have a diminished pulse or claudication of an upper extremity. Approximately 30% of patients will report neck pain and have a tender carotid artery (i.e., carotidynia). Lightheadedness is also common and may be secondary to vertebral artery involvement.
Patients with giant-cell arteritis (GCA) are typically older than 50 years of age. Giant-cell arteritis predominantly affects the branches of the thoracic aorta and the intracranial arteries. Some 50% of patients have constitutional symptoms related to inflammation, and 50% have coexisting polymyalgia rheumatica. The most common complaint is headache that typically affects the occipital or temporal region; it occurs in over 60% of patients with GCA. In patients with headache, scalp tenderness may be present. Partial or complete vision loss develops in 20% of patients, and approximately 50% of these individuals report amaurosis fugax (i.e., transient episodes that involve one eye and last 10 minutes or less [see Chapter 43]). Patients may present with upper limb claudication, and 40% report jaw claudication. Tongue claudication and swallowing difficulties are less common.
Thromboangiitis obliterans (Buerger’s disease) is a small- to medium-vessel vasculitis that affects the distal vessels of the arms or legs, and usually occurs before 40 years of age in cigarette smokers.11 It affects more men than women. The classic triad of TAO is claudication, Raynaud phenomenon, and superficial thrombophlebitis. Claudication of the hands or feet may progress to ulceration of the fingers or toes.11
Neurovascular Compression Syndromes
Claudication in the upper extremities raises the possibility of thoracic outlet syndrome (see Chapter 62).12 Compression of the axillary or subclavian artery by a cervical rib, abnormal insertion of the scalene anticus muscle, or apposition of the clavicle and first rib may result in arterial compression during head turning, arm use above or behind the head, or arm extension. Weakness, burning, aching, or fatigue in the arms can result. Examples of triggers include wall painting, hair washing, and housecleaning.
Popliteal artery entrapment should be considered in a young person with leg claudication but preserved pulses at rest.13 Anatomical variants in the course of the popliteal artery may result in its compression by the gastrocnemius muscle during exercise and can cause symptoms of claudication.
Vasospastic and related diseases
Raynaud phenomenon is the most common vasospastic disorder encountered in clinical practice14 (see Chapter 48). Patients typically report that the digits become pale or cyanotic during cold exposure. Fingers are most commonly affected, but the toes develop symptoms in 40% of affected individuals. Less commonly involved areas include the tongue, nose, and ear lobes. Patients may experience paresthesias or pain in the digits if ischemia persists. With rewarming and release of vasospasm, digital rubor due to reactive hyperemia may develop. A pulsating or flushed feeling may accompany the hyperemic phase. All color phases are not required for diagnosis. Indeed, with an appropriate history, the diagnosis can be made with only one color change.
There are two categories of Raynaud phenomenon: primary and secondary. Differentiating between the two is important because of the information it provides about cause and prognosis. Primary Raynaud’s disease is benign, typically affects fingers (and toes) symmetrically, and recovery is predictable with rewarming. Some 70% to 80% of patients with primary Raynaud’s disease are women. In patients with secondary Raynaud phenomenon, pallor may occur in only one or several digits. In severe cases, cyanosis is unremitting and tissue loss may occur. Raynaud phenomenon that has its onset after age 45 years should prompt an investigation for an underlying cause. The history should include questions to elicit evidence of disease or conditions that cause secondary Raynaud phenomenon, including connective tissue disorders, arterial occlusive disease, trauma (vibration, hypothenar hand injury), neurovascular compression syndromes, blood dyscrasias, and drug use.
Acrocyanosis is a vascular disorder characterized by bluish discoloration of the hands and feet exacerbated by cold exposure (see Chapter 49). Unlike Raynaud phenomenon, the discoloration is not confined to the digits, and pallor does not occur. Warming, however, can ameliorate cyanosis and restore normal skin color. Acrocyanosis typically occurs in persons aged 20 to 45 years, and women are affected more often than men.
Pernio is a vascular inflammatory disorder in which skin lesions and swelling occur in fingers and toes, particularly in cold moist climates (see Chapter 51). Other exposed portions of the body may be affected. The typical lesions described by the patient are pruritic and painful blisters or superficial ulcers.
The complex regional pain syndromes, reflex sympathetic dystrophy (RSD), and causalgia are associated with limb symptoms, often following a relatively minor injury. Hand or foot pain is a frequent complaint. This may be associated with hyperpathia, hyperesthesias, coolness, cyanosis, hyperhidrosis, and swelling. Symptoms are typically out of proportion to severity of the initial injury. Patients may observe brittle nails that develop ridges, and report muscle, skin, and subcutaneous tissue wasting and limited joint mobility in the affected limb.
Renal artery disease
No symptoms specific to renal artery stenosis are elicited by history. Unlike other end organs, symptoms of chronic renal ischemia are not localized to the kidney, but reflect systemic pathophysiological alterations that result from activation of the renin angiotensin system and disturbances of salt and water balance. Historical clues that raise suspicion of renal artery stenosis include onset of hypertension before age 30 or after age 55, malignant hypertension, hypertension refractory to three concurrently prescribed antihypertensive medications, azotemia subsequent to administration of an angiotensin-converting enzyme (ACE) inhibitor or angiotensin receptor blocker, unexplained azotemia, recurrent congestive heart failure, and episodic pulmonary edema (see Chapter 23). Renal artery stenosis should be considered in patients with these clinical clues, particularly if they have evidence of atherosclerosis in other regional circulations (e.g., CAD, PAD, aortic disease).
Mesenteric artery disease
Most patients with atherosclerosis of the celiac, superior mesenteric, or inferior mesenteric arteries are asymptomatic unless two or all three of these arteries are occluded. Symptoms of chronic mesenteric ischemia include postprandial epigastric or midabdominal pain that may radiate to the back (see Chapter 27). Onset of abdominal discomfort is 15 to 30 minutes after eating, and symptoms may persist for several hours. Patients tend to avoid food to prevent these symptoms, and weight loss ensues.
Carotid artery disease
The majority of patients with significant stenoses of the common or internal carotid arteries are asymptomatic (see Chapter 30). When they do occur, symptoms may be temporary (minutes to hours) or fixed, indicating a transient ischemic attack (TIA) or stroke, respectively. Symptoms of carotid artery disease reflect compromise of the neural territory subtended by its principal intracranial branch, the middle cerebral artery, and include contralateral hemiparesis, hemiparesthesia, and aphasia. Ipsilateral amaurosis fugax or blindness may also occur because the ophthalmic artery is supplied by the internal carotid artery.
The prevalence of carotid artery disease is increased in patients with CAD or PAD, both of which increase the risk of stroke by two- to fourfold.
Venous and Lymphatic Systems
A history soliciting evidence of venous and lymphatic diseases is required when patients complain of leg pain or swelling, or express concerns regarding leg ulcers, varicose veins, or localized inflammation on a limb. In patients presenting with leg edema, the history should seek to determine whether the swelling is secondary to venous or lymphatic diseases, trauma, arthritis, or whether it is associated with a systemic condition such as congestive heart failure, cirrhosis, nephrotic syndrome, renal insufficiency, or endocrinopathy (e.g., hypothyroidism, Cushing’s syndrome).
Deep vein thrombosis
Patients with thrombosis of a deep vein of a limb may present with swelling or discomfort, or no symptoms at all (see Chapter 52). Symptoms are usually but not always unilateral. Historical queries should seek potential causes of deep vein thrombosis (DVT) when it is suspected. Information regarding recent trauma, surgery, hospitalization, prolonged period of immobility, cancer, thrombophilic disorder, or family history of venous thrombosis should be acquired. An uncommon cause of left leg DVT is May-Thurner syndrome, in which the left iliac vein is compressed by the right iliac artery. In patients with arm symptoms, questions should seek evidence of indwelling catheters or cancer, since these are the most common causes of upper extremity DVT. In addition, a history of repetitive arm motion should be sought when considering the possibility of Paget-Schroetter syndrome, in which compression of the axillosubclavian vein by muscular, tendinous, or bony components of the thoracic outlet may cause thrombosis. Thrombosis or extrinsic compression of the superior vena cava may cause symptoms of superior vena cava syndrome, which include headache, face and neck fullness and flushing, and bilateral arm swelling.
Superficial thrombophlebitis
Thrombosis of a superficial vein is a local phenomenon that presents with pain and tenderness over the affected vein. Predisposing factors sought by history include intravenous catheters, varicose veins, injury, and malignancy. It is important to consider the possibility of malignancy, especially pancreatic, lung, and ovarian cancers in patients with recurrent or migratory superficial thrombophlebitis (i.e., Trousseau’s syndrome). Uncommon disorders associated with superficial thrombophlebitis include TAO and Behçet’s syndrome.
Chronic venous insufficiency
Venous insufficiency should be considered in patients who present with chronic unilateral or bilateral leg swelling. Causes of venous insufficiency include deep venous obstruction and deep venous valvular incompetence (also see Chapter 55). Approximately 30% of patients with DVT will ultimately develop chronic venous insufficiency.15 Valvular incompetence may be a consequence of recanalized venous thrombus or a primary valvular abnormality. Queries should address the duration of leg swelling, knowledge of prior DVT, presence of focal hyperpigmentation, pain, pruritus, or ulcers. Symptoms may include a heavy, dull, or “bursting” sensation of the edematous leg. Patients may report that discomfort in the affected leg increases with dependency and improves with leg elevation. Some individuals with severe leg swelling note that calf discomfort worsens with walking, a symptom termed venous claudication.
Varicose veins
Most patients with varicose veins do not have specific symptoms, but present to a physician’s office with cosmetic concerns. Symptoms of varicose veins include leg discomfort or aching, particularly with prolonged standing. These symptoms are most likely to occur along long segments of the greater and lesser saphenous veins and their tributaries. Burning or pruritus may develop, particularly if complicated by accompanying skin ulceration.
Lymphedema
Lymphedema should be considered in patients who present with limb swelling (see Chapter 58). This condition may affect the arms or legs and is usually unilateral, although it can be bilateral. Lymphedema should be suspected if limb swelling occurs early in life, particularly during childhood or adolescence. Congenital lymphedema typically appears at birth or shortly thereafter. Lymphedema praecox often presents around puberty but can occur anytime before age 35. Lymphedema tarda generally occurs after age 35. Lymphedema is also associated with genetic disorders such as Turner’s and Noonan’s syndromes. It is important to elicit history of conditions that may predispose a patient to lymphedema, including recurrent skin infection, lymphangitis, filariasis, trauma, malignancy of the lymphatic system, and radiation or surgical resection of lymph nodes and lymphatic vessels as adjunctive therapy for cancer.
Lymphangitis
Patients with lymphangitis may report an erythematous patch or linear streak that affects the limb and tends to propagate proximally over time. The erythematous area may be painful and tender. These patients usually present with systemic signs of infection, including fever and shaking chills. History might determine whether lesions induced by trauma or infection may have served as a portal of entry.
Vascular Examination
As in any comprehensive physical examination, vital signs (blood pressure, heart rate, respiratory rate) should be assessed and recorded. Blood pressure should be measured in both arms and preferably in supine, seated, and upright positions. Overall appearance of the patient should be noted.
The vascular examination includes inspection, palpation, and auscultation of vascular structures in many areas of the body. A systematic approach ensures a complete evaluation, so the examination described in this chapter will cover principal anatomical regions that are particularly relevant to the peripheral vasculature. The heart, lungs, and neurological and musculoskeletal systems should be examined, but details of these examinations are beyond the scope of this chapter.
Limbs
The limbs should be inspected carefully, assessing their appearance, symmetry, color, and evidence of edema or muscle wasting.
Pulse examination
The pulse examination of the arms and legs is a critical part of the vascular examination. Asymmetry, decreased intensity, or absence of pulses provide clinical evidence of PAD and indicate the location of stenotic lesions. Some examiners describe pulses as absent, diminished, or normal, or use a numerical scale (e.g., from 0 [absent] to 2 + [normal]). Bounding pulses may be evidence of aortic valve insufficiency, and dilated expansive pulses a sign of ectasia or aneurysm.
Pulses of the arms—brachial, radial, and ulnar pulses—should be palpated using two or three fingertips. The brachial pulse is superficial and in the medial third of the antecubital fossa. The radial pulse, also superficial, can be found over the stylus of the radius near the base of the thumb (Fig. 11-1). The ulnar pulse is palpated on the volar aspect of the wrist, over the head of the ulnar bone. Wrist support by the examiner improves pulse detection by decreasing overlying muscle tension.
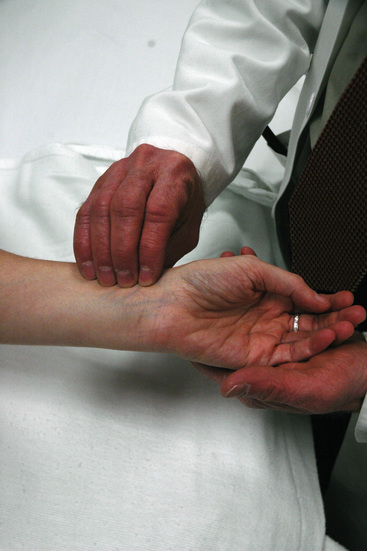
Figure 11-1 Palpating radial pulse.
Examiner, using three or four fingers, lightly palpates the superficial radial pulse over stylus of radius near base of thumb.
Pulse examination of the leg (femoral, popliteal, posterior tibial, and dorsalis pedis pulses) should be undertaken with the patient supine. The femoral pulse is located deep, below the inguinal ligament, about midway between the symphysis pubis and iliac spine. Obesity may obscure local landmarks. Lateral rotation of the leg, pannus retraction, and two hands may be required for adequate palpation. On occasion, the increase in flow velocity caused by a stenosis may create a thrill in the common or superficial femoral artery that is appreciated by palpation of the femoral pulse, or a bruit that can be heard with auscultation.
Palpation of the popliteal pulse can be difficult. The leg should be straight yet relaxed to decrease overlying muscle stiffness. The popliteal pulse should be palpated with three fingers from each hand while the thumbs are applying moderate opposing force to the top of the knee (Fig. 11-2). The popliteal pulse typically can be found at the junction of the medial and lateral thirds of the fossa. In contrast to superficial pulses like the radial or dorsalis pedis pulse, the popliteal pulse is diffuse and deep. Widened popliteal pulses may be indicative of popliteal artery aneurysm.
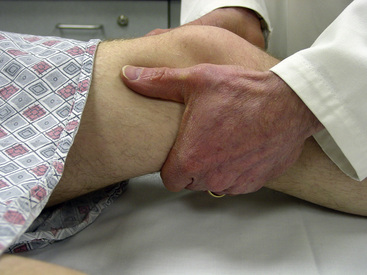
Figure 11-2 Palpating popliteal pulse.
Popliteal pulse requires moderate pressure for its appreciation. Examiner uses both thumbs for moderate opposing force while placing digits two, three, and four in lateral third of popliteal fossa. Patient’s leg should be relaxed while examiner induces mild flexion. A widened popliteal artery pulse may indicate presence of aneurysm.
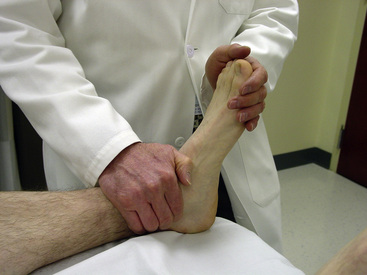
The posterior tibial pulse can be found slightly below and behind the medial malleolus. Counterpressure with the thumb and passive dorsiflexion of the foot may increase the likelihood of palpation (Fig. 11-3). The posterior tibial pulse should be present. Its absence is diagnostic for PAD. In contrast, the dorsalis pedis pulse, which can be appreciated just lateral to the extensor tendon on the dorsum of the foot, normally may be absent in 2% to 12% of persons.
The allen test
The radial and ulnar arteries supply blood flow to the hand. Within the hand, these arteries form the superficial and deep palmar arches, enabling blood supply to the digits from either vessel; 5% to 10% of the population has a congenitally incomplete arch. Disease states associated with interruption of the palmar arch include connective tissue diseases like the CREST variant (calcinosis, Raynaud phenomenon, esophageal dysmotility, sclerodactyly, telangiectasia) of scleroderma, vasculitides like TAO, and thromboemboli. The Allen test can differentiate between a complete and incomplete palmar arch. The examiner occludes both the radial and ulnar pulses (Fig. 11-4), and the patient then opens and closes the fist several times, creating palmar pallor. Upon release of one pulse, normal skin color should return within seconds. The other artery is then tested and observed similarly. Persistent pallor is indicative of an incomplete palmar arch or occluded artery distal to the remaining pulse occluded by the examiner.
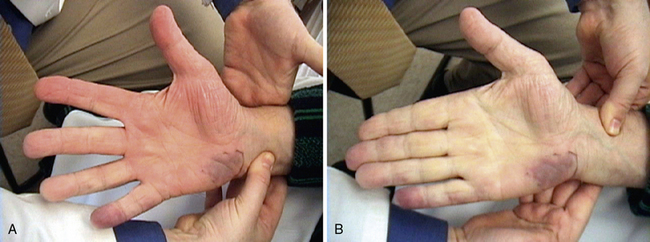
The Allen test determines presence or absence of a complete palmar arch. Both radial and ulnar pulses are occluded while patient opens and closes hand to create palmar pallor. Once pallor is evident, examiner releases one pulse. In this example, patient presented with persistent fifth digit and hypothenar cyanosis. A, Release of radial artery pulse results in expected hyperemia and palmar erythema. B, In contrast, release of ulnar artery pulse does not result in palmar erythema, indicating proximal occlusion in ulnar segment of palmar arch. This test is considered a positive Allen test.
Nearly three quarters of all patients with TAO, will have a positive Allen test, and 50% will report Raynaud phenomenon. Digital ischemia in these patients is more likely to progress and cause persistent cyanosis and lead to digital ulcers. Patients with TAO also may develop migratory superficial thrombophlebitis, which appears as painful, tender, red nodules.
Thoracic outlet maneuvers
Thoracic outlet syndrome results from compression of the neurovascular bundle as it leaves the thoracic cavity. Each component of the bundle may be affected, including the brachial plexus, subclavian/axillary artery, and subclavian/axillary vein.
Thoracic outlet maneuvers seek to elicit positional interruption of arterial flow. During the examination, the physician holds the radial pulse in one hand and maneuvers the arm with the other. The subclavian artery is auscultated in the supraclavicular fossa. An abnormal thoracic outlet maneuver is characterized by development of a subclavian bruit followed by loss of the radial pulse. Several thoracic outlet maneuvers have been described, and each may be relevant to compression at different sites in the thoracic outlet. Each side is examined in sequence. The Adson maneuver assesses the segment of the subclavian artery in the scalene triangle. The patient rotates his/her head toward the symptomatic side, extends the neck (i.e., looking up and over the shoulder), and simultaneously performs an exaggerated inspiration. The costoclavicular maneuver assesses the segment of the subclavian artery coursing between the clavicle and first rib. The patient thrusts the shoulders back and inferiorly. The hyperabduction maneuver evaluates the subclavian artery as it courses near the insertion of the pectoralis major muscle. The patient is seated and the head is looking forward. The arm is abducted 180 degrees to a position along the side of the head. Abduction of the arm to 90 degrees may be combined with external rotation in evaluating symptoms suggestive of thoracic outlet syndrome (Fig. 11-5). This maneuver is often used to assess subclavian venous or arterial compression during ultrasonography or angiography.
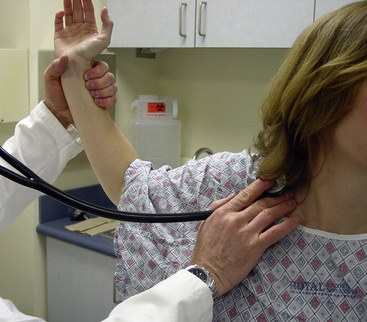
Figure 11-5 Elevated Arm Stress Test (EAST) may be used to evaluate subclavian artery as it courses near insertion of pectoralis major muscle.
Patient initially sits looking forward while arms are abducted 90 degrees, elbows are abducted 90 degrees, and patient repeatedly makes a clenched fist. During maneuver, radial pulse should be palpated while subclavian artery is auscultated. Loss of pulse or development of subclavian artery bruit is a positive study.
For patients in whom clinical suspicion for thoracic outlet syndrome is present, sensitivity and specificity for these provocative tests are 72% and 53%, respectively.16 Routine application of these maneuvers is not warranted, however, since up to 50% of the population may have a positive finding. Indeed, in one study of 64 randomly selected subjects, application of these maneuvers in a nonspecific manner overdiagnosed the syndrome more than threefold.17 Patients with carpal tunnel syndrome present a particularly difficult group for these maneuvers and generate a false-positive rate of nearly 50%.18
Limb ischemia
Skin color and temperature can provide information about severity of limb arterial perfusion. The feet, hands, fingers and toes should be examined for temperature and skin color, and the nails for evidence of fragility and pitting. Limb temperature can best be appreciated using the back of the examiner’s hand. Temperature changes of adjacent segments on the ipsilateral limb and comparisons with the contralateral limb can be made. Presence of foot pallor while the leg is horizontal is indicative of poor perfusion and may be a sign of ischemia. Foot pallor may be precipitated in patients with PAD (who do not have CLI) by elevating the patient’s leg to 60 degrees for 1 minute. Repetitive dorsiflexion and plantar flexion of the foot may also precipitate pallor on the sole of the foot when PAD is present. To qualitatively assess collateral blood flow, the leg is then lowered as the patient moves to the seated position. This is done to elicit rubor, indicative of reactive hyperemia, and determine pedal vein refill time. The time to development of dependent rubor is indicative of the severity of PAD. Severe PAD and poor collateral blood flow may prolong reactive hyperemia by more than 30 seconds. Normally, pedal venous refill occurs in less than 15 seconds. Moderate PAD subserved by collateral vessels is suspected if venous refill is 30 to 45 seconds; severe disease with poor collateral development is likely when venous filling time is longer than 1 minute.
Ulcers
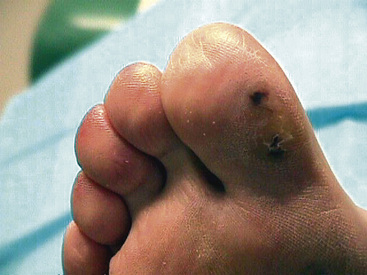
Ischemia arising from arterial occlusive disease or emboli may cause formation of ischemic ulcers (see Chapter 60). They tend to be small, annular, pale, and desiccated (Fig. 11-6) and are usually located in distal areas of the limbs (e.g., toes, heels, fingertips). Ischemic ulcers vary in size but may be as small as 3 mm in diameter. Arterial ischemic ulcers are tender. Neurotrophic ulcers that develop in patients with diabetes typically occur at sites of trauma, such as areas of callus formation, bony prominence, or parts of the foot exposed to mild chronic trauma caused by ill-fitting shoes. Ischemic ulcers also develop in diabetic patients with PAD and may have features of neuropathic ulcers. Without proper treatment, ulceration may progress to tissue necrosis and gangrene. Gangrene can be characterized as an area of dead tissue that blackens, mummifies, and sloughs.
Digital vasospasm
It is unusual for patients with Raynaud phenomenon to present to the physician’s office during an attack in which the fingers are blanched. Moreover, it is difficult to precipitate digital ischemia in these patients, even with local cold exposure, such as placing the hands in ice water. Digital ischemia may be apparent in patients with fixed obstructive lesions of the digital arteries. Persistent digital ischemia may occur in patients with connective tissue disorders such as scleroderma or systemic lupus erythematosus, atheroemboli, TAO, or atherosclerosis. The fingers and toes are cool and appear cyanotic or pale. Fissures, pits, ulcerations, or necrosis or gangrene may be evident on the ischemic digits (see Fig. 11-6).
Livedo reticularis
Livedo reticularis can be described as a lacelike or netlike pattern in the skin (Fig. 11-7). The “laces” may vary in color from red to blue and surround a central area of clearing. Cold exposure exacerbates the changes in hue. Both primary and secondary forms may occur and may be complicated by ulceration. The primary benign form is more common in women. The secondary forms are usually associated with vasculitis, atheroemboli, hyperviscosity syndromes, endocrine abnormalities, and infections. In the secondary forms of livedo reticularis, lesions may be more diffuse and ominous. Purpuric lesions and cutaneous nodules that progress to ulceration in response to cold may develop.
Edema
The limbs should be evaluated for edema. The most common location is in the legs, adjacent to the malleoli and over the tibia. With deep digital palpation, development of a divot or finger impression is indicative of pitting edema. Edema can be graded in each leg or arm as absent, mild, moderate, or severe or on a numerical scale of 4, with 0 being the absence of edema. Unilateral edema may be evidence of DVT, chronic venous insufficiency, or lymphedema.
The most common physical findings of DVT include unilateral leg swelling, warmth, and erythema. The affected vein may be tender. A common femoral vein cord is detected by palpating along its course just below the inguinal ligament vein, and a femoral vein cord would be appreciated along the anteromedial aspect of the thigh. In the absence of obvious edema, a subtle clue may be unilateral absence of contours of the thigh, calf, or ankle. Muscular groups subtended by the thrombosed vein may be edematous due to poor venous drainage, conferring a boggy feeling to the affected calf or thigh muscles. Inflammation associated with a thrombosis may make the leg feel warm.
Homans sign is nonspecific and misses the diagnosis as commonly as it makes it. In John Homans’s essay on lower extremity venous thrombosis, he states, “The clinical signs of a deep thrombosis of the muscles of the calf are entirely lacking when the individual lies or even reclines in bed. It is possible there may be a little discomfort upon forced dorsiflexion of the foot (tightening of the posterior muscles) but it is not yet clear whether or not this is a sign upon which to depend.”19
Presence of thrombus just below the skin makes the diagnosis of superficial thrombophlebitis relatively easy. The patient may present with local venous engorgement, a palpable cord, warmth, erythema, or tenderness.
Chronic venous insufficiency
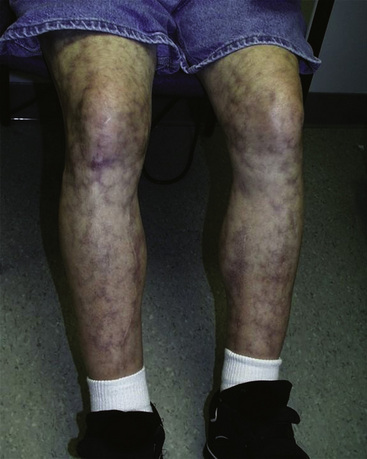
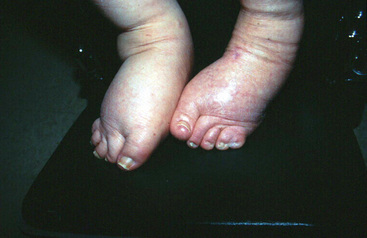
With chronic venous insufficiency, the physical examination may demonstrate fibrosis, tenderness, excoriation, and skin induration from hyperkeratosis, cellulitis, and ulceration (Fig. 11-8). Chronic venous edema may impart hemosiderin deposition in the skin and confer a brawny appearance, typically in the pretibial calf. The severity of chronic venous disease may be classified using the CEAP (clinical signs, etiology, anatomy, pathophysiology) classification20 (Table 11-1).
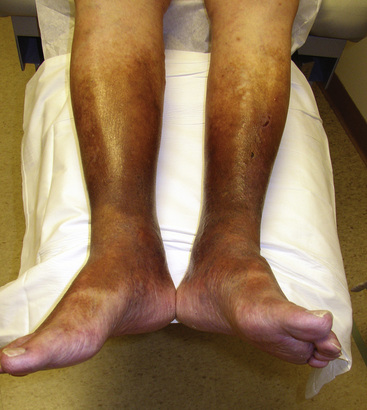
Figure 11-8 Skin changes of chronic venous insufficiency.
Chronic venous insufficiency and edema result in deposition of hemosiderin, causing darkening and toughening of skin and giving calf a brawny appearance. Note small superficial venous ulcers mid-calf above shin.
Table 11-1 CEAP Clinical Classification
| Class | Clinical signs |
|---|---|
| 0 | No visible or palpable signs of venous disease |
| 1 | Telangiectasis or reticular veins |
| 2 | Varicose veins |
| 3 | Edema |
| 4 | Skin changes ascribed to venous disease (e.g., pigmentation, venous eczema, lipodermatosclerosis) |
| 5 | Skin changes as defined above, with healed ulceration |
| 6 | Skin changes as defined above, with active ulceration |
CEAP, clinical signs, etiology, anatomy, pathophysiology.
In contrast to arterial ulcers, which are circumscribed and pallid, venous ulcers are large with irregular borders, erythematous, and moist, giving the skin a shiny appearance. They are usually located near the medial or lateral malleolus. Venous ulcers may be painless, but many are associated with pain.21,22
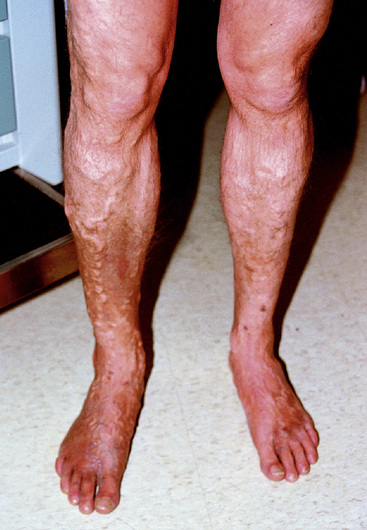
Varicose veins
Varicose veins are dilated, serpentine, superficial veins. If they cluster, they may feel and appear like a bunch of grapes (Fig. 11-9). Varicose veins should be inspected and palpated. Areas of erythema, tenderness, or induration may identify superficial thrombophlebitis. Varicose veins are most prominent with leg dependence (e.g., with standing). Once filled, the veins may be balloted, and a fluid wave may be detected. Venous telangiectasias, also known as spider veins, are commonly confused with varicose veins. Spider veins are typically small, cutaneous veins in a caput medusa pattern.
Superficial venous varicosities may be primary or result from deep venous thrombosis or insufficiency. An examiner can distinguish between superficial venous insufficiency and deep venous insufficiency at the bedside using the Brodie-Trendelenburg test. With the patient lying supine, the leg is elevated to 45 degrees and a tourniquet applied after the veins have drained. The patient then stands. The veins below the tourniquet should fill slowly. If venous refill distal to the site of tourniquet application occurs in less than 30 seconds, this is evidence of an incompetent deep and perforator system. Slower refills suggest a competent deep and perforator system. The varicose veins are examined upon tourniquet release. Superficial venous insufficiency will be confirmed with rapid retrograde superficial venous filling.
The Perthes test can differentiate between deep venous insufficiency and a deep venous obstruction as the cause of varicose veins. The patient is asked to stand, and once the superficial veins are engorged, a tourniquet is applied around the mid-thigh. The patient then walks for 5 minutes. If the varicose veins collapse below the level of the tourniquet, the perforator veins are presumed competent and the deep veins patent. If the superficial veins remain engorged, either the superficial and/or communicating veins are incompetent. If the varicose veins increase in prominence, and walking causes leg pain, the deep veins are occluded.
Lymphedema
During the initial stages of lymphedema, leg swelling will be similar to venous insufficiency: soft and pitting. Extension of edema into the foot to the origin of the toes may help differentiate lymphedema from venous edema (Fig. 11-10). In addition, the inability to pinch skin on the toes, the Stemmer sign, also may differentiate early lymphedema from venous edema. Subsequently, the limb becomes wooden as progressive deposition of protein-rich fluid causes induration and fibrosis of affected tissues. Lymphedema increases production of subcutaneous and adipose tissue, thickening the skin. Advanced disease may be identified when the leg feels wooden, edema is no longer pitting, and the limb is enlarged; the skin may appear verrucous at the toes. Palpation for lymphadenopathy should be performed when considering secondary causes of lymphedema.
Lymphangitis
Lymphangitis can usually be visualized as a red streak that extends proximally from an inciting lesion. If left untreated, the entire limb may become edematous, erythematous, and warm, without evidence of venous congestion or impairment of arterial flow. Commonly, the regional lymph nodes are indurated.
Neck Examination
The neck is inspected for any areas of swelling or asymmetry. Jugular venous pressure is assessed to investigate the possibility of a volume overloaded state or congestive heart failure. Patients typically are placed at 45 degrees and the height of jugular venous pressure estimated. If necessary, the angle of head elevation should be adjusted to see the top of the jugular venous column.
The carotid arteries are palpated between the trachea and the sternocleidomastoid muscles. In older patients especially, the carotid body may be sensitive, and carotid pulses may induce bradycardia and hypotension. Pulses should be symmetrical with a rapid upstroke. Pulse asymmetry may indicate a proximal carotid or brachiocephalic stenosis. Parvus and tardus pulses (decreased amplitude and a delayed slow upstroke) may indicate aortic valve stenosis or proximal occlusive disease. Stenosis of the carotid bifurcation or internal carotid artery usually does not affect carotid pulse contour or amplitude. Occasionally, severe stenosis will create a thrill that can be appreciated by palpation.
The carotid pulses are auscultated to elicit evidence of bruits. Bruits are caused by blood flow turbulence as a result of arterial stenosis, extrinsic compression, aneurysmal dilation, or arteriovenous connection. The bell of the stethoscope is recommended to appreciate low-frequency bruits and eliminate any adventitious sounds heard through the diaphragm. The entire cervical portion of each carotid artery should be auscultated, including the segment near the angle of the jaw where the carotid bifurcation is often located (Fig. 11-11). Auscultation of the subclavian arteries for bruits is performed in the supraclavicular fossa and between the lateral aspect of the clavicle and pectoralis muscle. Although the proximal location of a bruit defines the area of turbulent flow, a bruit may be appreciated for an additional several centimeters. The sensitivity and specificity of a carotid bruit for the presence of stenosis ranges from 50% to 79% and 61% to 91%.23 The pitch of bruits increases with worsening severity. Continuation of the bruit into diastole is another marker of severity and implies advanced stenosis. Paradoxically, severe stenosis causing subtotal arterial occlusion may not evoke an audible bruit.
Abdominal Vascular Examination
Vascular examination of the abdomen is performed as the patient lies supine on the examining table, with legs outstretched. From this position, the abdominal wall should be relaxed and not rigid. Prior to palpation, the abdomen should be inspected. Engorged superficial veins in the abdomen indicate the possibility of inferior vena cava obstruction. After inspection, all four quadrants are auscultated with the stethoscope. The presence of bruits is indicative of aortic or branch vessel occlusive disease. Bruits may arise as a result of mesenteric, renal, or aortic disease. Following auscultation, the abdomen is palpated for masses and to detect an aortic aneurysm. Deepest palpation can generally be obtained by gradually increasing pressure in the midline using both hands (Fig. 11-12). In asthenic patients, the aorta can be palpated. In subjects with a waist size greater than 40 inches, the likelihood of palpating an aneurysm is quite limited.
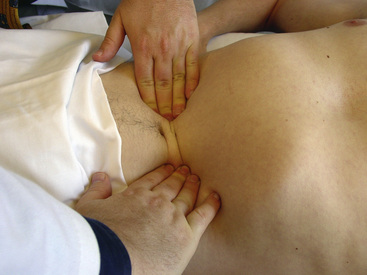
Figure 11-12 Abdominal palpation for aneurysm.
Examiner, using progressively increasing force, palpates until aorta can be defined between both sets of fingers. Examiner should appreciate lateral pulsation with every heart beat. Aneurysm sizing is performed by estimating distance between closest fingers of each hand.
Presence of an aneurysm can be determined when there is a distinct and expansive pulsatile configuration to the aorta. An aneurysm should be sized by determining the lateral borders with both hands, and the space estimated with a measuring tape. Tenderness during the abdominal vascular examination is unusual and may suggest aneurysmal expansion, an inflammatory aneurysm, or a contained rupture. Nonaortic pathology, including appendicitis, cholecystitis, diverticulitis, and peritonitis, are more common causes of tenderness.
1 Fowkes F.G., Murray G.D., Butcher I., et al. Ankle brachial index combined with Framingham risk score to predict cardiovascular events and mortality: a meta-analysis. JAMA. 2008;300:197–208.
2 McDermott M.M., Greenland P., Liu K., et al. Leg symptoms in peripheral arterial disease: associated clinical characteristics and functional impairment. JAMA. 2001;286:1599–1606.
3 McDermott M.M., Liu K., Greenland P., et al. Functional decline in peripheral arterial disease: associations with the ankle brachial index and leg symptoms. JAMA. 2004;292:453–461.
4 Rose G.A. The diagnosis of ischaemic heart pain and intermittent claudication infield surveys. Bull World Health Org. 1962;27:645–658.
5 Coyne K.S., Margolis M.K., Gilchrist K.A., et al. Evaluating effects of method of administration on walking impairment questionnaire. J Vasc Surg. 2003;38:296–304.
6 Criqui M.H., Denenberg J.O., Bird C.E., et al. The correlation between symptoms and non-invasive test results in patients referred for peripheral arterial disease testing. Vasc Med. 1996;1:65–71.
7 Regensteiner J.G., Gardner A., Hiatt W.R. Exercise testing and exercise rehabilitation for patients with peripheral arterial disease: status in 1997. Vasc Med. 1997;2:147–155.
8 Rajagopalan S., Grossman P.M. Management of chronic critical limb ischemia. Cardiol Clin. 2002;20:535–545.
9 Jaffery Z., Thornton S.N., White C.J. Acute limb ischemia. Am J Med Sci. 2011;342:226–234.
10 Fukumoto Y., Tsutsui H., Tsuchihashi M., et al. The incidence and risk factors of cholesterol embolization syndrome, a complication of cardiac catheterization: a prospective study. J Am Coll Cardiol. 2003;42:211–216.
11 Olin J.W. Thromboangiitis obliterans (Buerger’s disease). N Engl J Med. 2000;343:864–869.
12 Mackinnon S.E., Novak C.B. Thoracic outlet syndrome. Curr Probl Surg. 2002;39:1070–1145.
13 Levien L.J. Popliteal artery entrapment syndrome. Semin Vasc Surg. 2003;16:223–231.
14 Wigley F.M. Clinical practice. Raynaud’s phenomenon. N Engl J Med. 2002;347:1001–1008.
15 Bergan J.J., Schmid-Schonbein G.W., Smith P.D., et al. Chronic venous disease. N Engl J Med. 2006;355:488–498.
16 Gillard J., Perez-Cousin M., Hachulla E., et al. Diagnosing thoracic outlet syndrome: contribution of provocative tests, ultrasonography, electrophysiology, and helical computed tomography in 48 patients. Joint Bone Spine. 2001;68:416–424.
17 Warrens A.N., Heaton J.M. Thoracic outlet compression syndrome: the lack of reliability of its clinical assessment. Ann R Coll Surg Engl. 1987;69:203–204.
18 Nord K.M., Kapoor P., Fisher J., et al. False positive rate of thoracic outlet syndrome diagnostic maneuvers. Electromyogr Clin Neurophysiol. 2008;48:67–74.
19 Homans J. Venous thrombosis in the lower limbs: its relation to pulmonary embolism. Am J Surg. 1937;38:316–326.
20 Beebe H.G., Bergan J.J., Bergqvist D., et al. Classification and grading of chronic venous disease in the lower limbs. A consensus statement, Int Angiol. 1995;14:197–201.
21 de Araujo T., Valencia I., Federman D.G., et al. Managing the patient with venous ulcers. Ann Intern Med. 2003;138:326–334.
22 Nemeth K.A., Harrison M.B., Graham I.D., et al. Pain in pure and mixed aetiology venous leg ulcers: a three-phase point prevalence study. J Wound Care. 2003;12:336–340.
23 Magyar M.T., Nam E.M., Csiba L., et al. Carotid artery auscultation–anachronism or useful screening procedure? Neurol Res. 2002;24:705–708.