7 PLEURAL ABNORMALITIES
In this chapter we address several different processes that affect the pleura. The main emphasis is on pleural effusion and pneumothorax.
BASIC ANATOMY
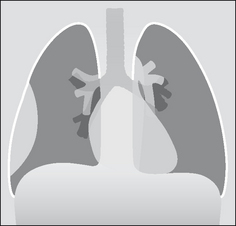
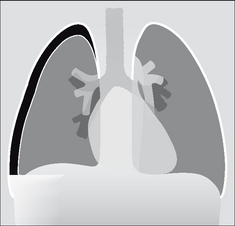
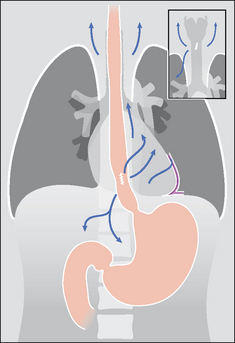
The pleura can be likened to a sac enveloping the lung. This sac has two membranous walls—the inner visceral and the outer parietal.
The pleura is not visible on a normal CXR except where it forms part of a lung fissure or where the two lungs abut each other in the midline (p. 114).
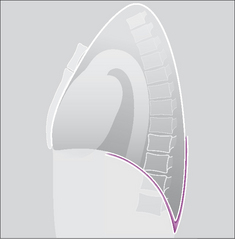
The pleural space is a closed cavity between the layers of the visceral and parietal pleura (Fig. 7.1). A small amount of lubricating fluid lies within the cavity. The lung fissures extend between the lobes of each lung and are lined by two layers of visceral pleura (Figs 7.3-7.5).
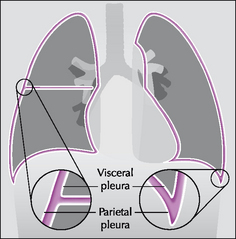
Figure 7.1 The pleural sac, showing the visceral and parietal pleural membranes. The visceral pleura extends into and lines both sides of the horizontal fissure.
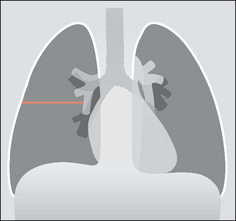
Figure 7.3 Horizontal fissure. As with all the lung fissures it is formed by two layers of visceral pleura. There is a potential space between the two closely opposed layers.
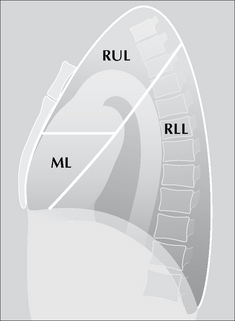
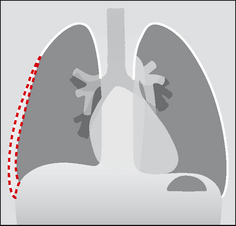
Figure 7.4 The oblique fissure and the horizontal fissure separating the three lobes of the right lung. RUL = right upper lobe; ML = middle lobe; RLL = right lower lobe. Three anatomical points to note: (1) This lung differs from the left lung primarily because the fissures divide it into three lobes. (2) The high position of the apical segment of the lower lobe is often not appreciated. (3) A sliver of the middle lobe touches the anterior aspect of the right dome of the diaphragm.
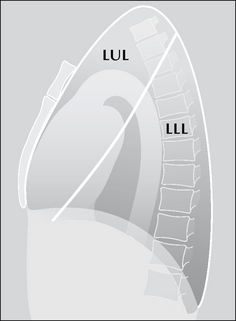
Figure 7.5 The oblique fissure separating the two lobes of the left lung. LUL = left upper lobe; LLL = left lower lobe. Three anatomical points to note: (1) The single fissure divides this lung into two lobes only. (2) The high position of the apical segment of the lower lobe is often not appreciated…particularly when only a frontal CXR is obtained and a lesion is present in this lower lobe segment. (3) A sliver of the upper lobe touches the anterior aspect of the left dome of the diaphragm.
PLEURAL EFFUSION
The pleura is composed of a dynamic membrane of mesothelial cells and a deeper layer of connective tissue containing vessels, nerves and lymphatics. This membrane responds actively to adjacent inflammation and to accumulations of fluid (e.g. from heart failure) within its space.
RECOGNISING THAT A PLEURAL EFFUSION IS PRESENT
Fluid in the pleural space can adopt several different appearances on both erect and supine CXRs1–3.
On the erect frontal CXR
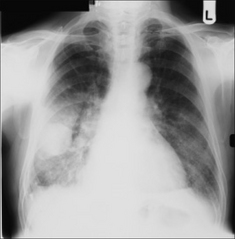
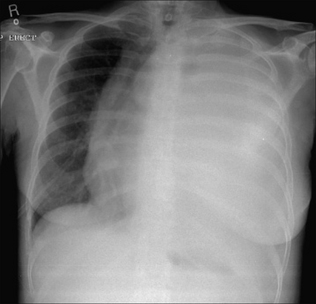
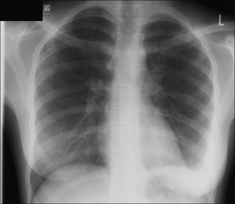
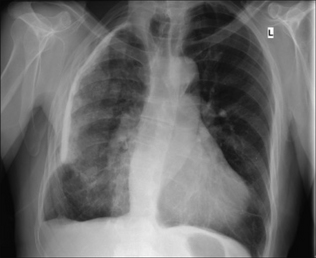
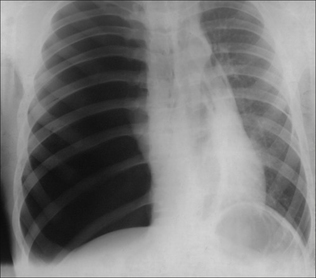
 The commonest appearance is an opaque meniscus at a costophrenic angle. It requires approximately 200–300 ml pleural fluid to efface the normal sharp recess between the diaphragm and the ribs (Fig. 7.6a).
The commonest appearance is an opaque meniscus at a costophrenic angle. It requires approximately 200–300 ml pleural fluid to efface the normal sharp recess between the diaphragm and the ribs (Fig. 7.6a). If the effusion is very large, then the entire hemithorax may be opaque (Chapter 19) and the heart may be pushed towards the normal side(Fig. 7.6c).
If the effusion is very large, then the entire hemithorax may be opaque (Chapter 19) and the heart may be pushed towards the normal side(Fig. 7.6c). There are other patterns:
There are other patterns:
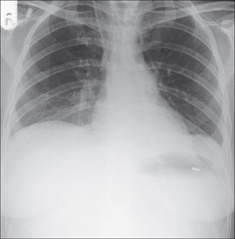
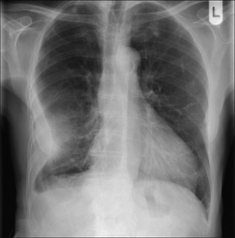
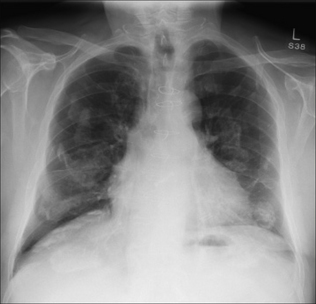
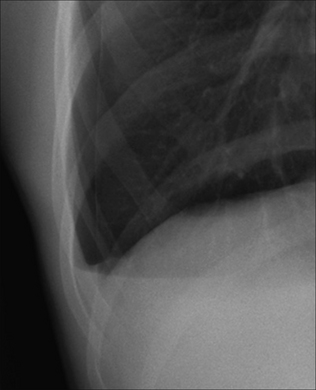
 Subpulmonary. Pooling within the pleural space below the lung. This is a subpulmonary effusion and is a relatively common occurrence. A subpulmonary effusion is usually easier to detect on the left side, where the pool can cause the gastric air bubble to appear widely separate from the (apparent) superior margin of the diaphragm (Fig. 7.11). The normal distance between the dome of the diaphragm and the air in the stomach does not normally exceed 7 mm in 98% of people aged 50 years and over (see Chapter 16, p. 242).
Subpulmonary. Pooling within the pleural space below the lung. This is a subpulmonary effusion and is a relatively common occurrence. A subpulmonary effusion is usually easier to detect on the left side, where the pool can cause the gastric air bubble to appear widely separate from the (apparent) superior margin of the diaphragm (Fig. 7.11). The normal distance between the dome of the diaphragm and the air in the stomach does not normally exceed 7 mm in 98% of people aged 50 years and over (see Chapter 16, p. 242).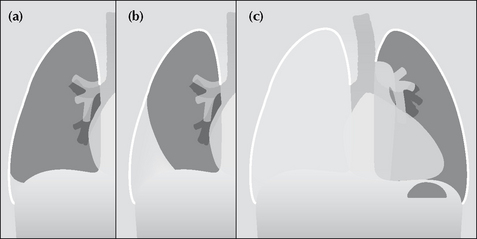
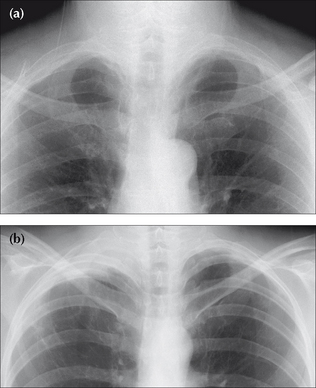
Figure 7.6 From the frontal CXR a rough assessment can be made of thevolume of pleural fluid. (a) Approximately 200–300 ml. (b) Approximately 2 litres.(c) Approximately 5 litres.
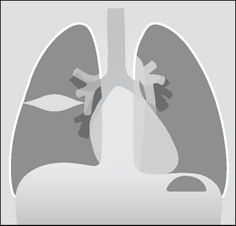
Figure 7.8 An encysted pleural effusion. The fluid has collected between the two layers of the pleura lining the horizontal fissure. The oval or rounded shadow can sometimes be mistaken for a lung tumour.
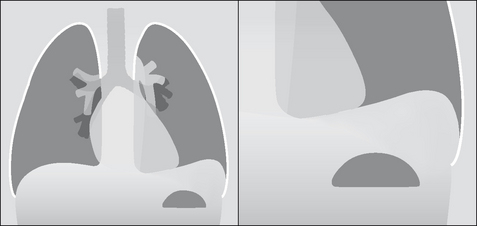
Figure 7.11 Subpulmonary effusion on the left side. The fluid has pooled between the visceral and parietal pleura at the base of the lung. It simulates an elevated dome of the diaphragm. Note the low position of the pushed down gastric air bubble; also, the highest point of the “dome” is situated laterally.
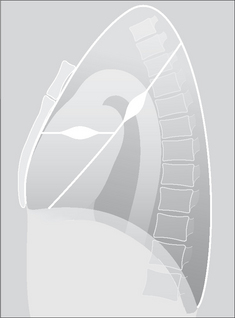
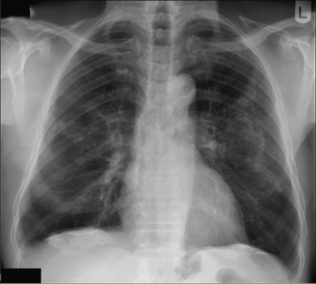
Figure 7.9 Two separate encysted effusions. One is in the horizontal fissure. The other is in the right oblique fissure. Encysted effusions can occur anywhere in the pleural space. They occur most commonly in patients who are in heart failure.
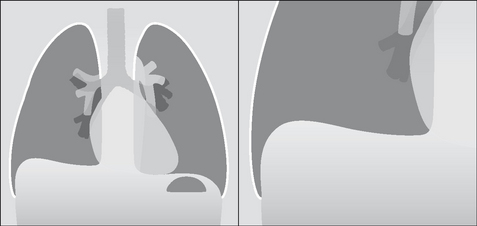
Figure 7.10 Subpulmonary effusion on the right side. This puddle of fluid is often difficult to distinguish from a high dome of the diaphragm. A helpful characteristic: whereas the highest point on a normal dome is invariably central, the highest point on the (apparent) dome is situated laterally when a subpulmonary effusion is present.
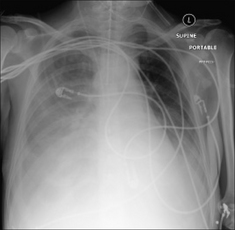
On the supine CXR
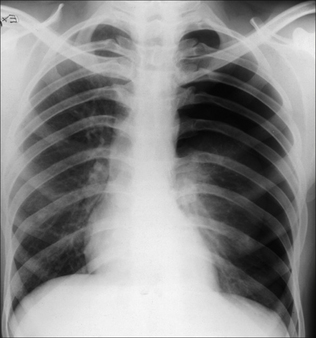
When the patient is supine, pleural fluid layers out in the posterior part of the pleural space. This causes the hemithorax to appear whiter or paler grey than the normal side. In most instances the normal lung vessels will be seen through this shadowing. Approximately 200 ml of fluid needs to be present before an abnormal pale grey appearance is produced2,3.
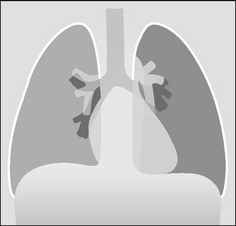
Figure 7.14 The appearance of a (right) pleural effusion on a supine CXR. The pleural space needs to contain approximately 200 ml fluid before the abnormal hemithorax appears paler than the opposite normal side.
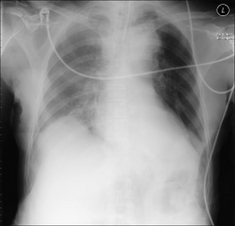
Figure 7.15 Patient in ITU. A right-sided pleural effusion has layered out posteriorly. The right hemithorax is paler than the left hemithorax. Lung vessels are visible on the right side and this supports the diagnosis of pleural fluid rather than lung consolidation… but this distinction can sometimes be difficult.
A frequent puzzle: A patient is very ill, e.g. in the intensive therapy unit (ITU). The CXR shows basal shadowing. Is it mainly fluid or mainly consolidation?
 On a supine CXR it can be very difficult (usually impossible) to determine if the shadowing is predominantly fluid. If thoracocentesis is considered as a therapeutic option then an ultrasound examination will confirm or exclude a significant volume of pleural fluid. Alternatively, a lateral decubitus CXR (p. 231) will usually clarify.
On a supine CXR it can be very difficult (usually impossible) to determine if the shadowing is predominantly fluid. If thoracocentesis is considered as a therapeutic option then an ultrasound examination will confirm or exclude a significant volume of pleural fluid. Alternatively, a lateral decubitus CXR (p. 231) will usually clarify.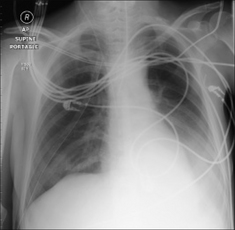
Figure 7.17 Supine CXR. This is the same patient as in Fig. 7.16—on the same day after the large right effusion had been drained. Incidentally, some pleural fluid is also evident on the left side.
A “WHITE OUT” CAUSED BY A MASSIVE PLEURAL EFFUSION
A completely white hemithorax, often referred to as a “white out”, may be caused by a large volume (5–7 litres) of pleural fluid (Fig. 7.18). There are other causes for a CXR white out. These are described in Chapter 19, pp. 264–267.
EMPYEMA
 Pus in the pleural space. Most commonly due to pneumonia. Other causes include trauma, infection outside the thorax, thoracic surgery.
Pus in the pleural space. Most commonly due to pneumonia. Other causes include trauma, infection outside the thorax, thoracic surgery. The appearance on the CXR is essentially the same as that of an uninfected pleural effusion. The diagnosis is usually arrived at by combining evidence of sepsis, pleural fluid on the CXR, and organisms found on needle aspiration.
The appearance on the CXR is essentially the same as that of an uninfected pleural effusion. The diagnosis is usually arrived at by combining evidence of sepsis, pleural fluid on the CXR, and organisms found on needle aspiration. Sometimes an organised empyema will form a walled-off collection, seen as a round or oval shape indenting the lung (Figs 7.19 and 7.20).
Sometimes an organised empyema will form a walled-off collection, seen as a round or oval shape indenting the lung (Figs 7.19 and 7.20).PLEURAL FIBROSIS/THICKENING
ORDINARY PLEURAL THICKENING
This thickening usually occurs at one of two sites:
 At a costophrenic angle as a result of a previous pleural infection. Occasionally it is consequent on a haemorrhagic effusion following trauma. The appearance of fibrotic blunting of a costophrenic angle may be very similar to a small (300 ml) pleural effusion. The overall clinical picture or previous clinical history or a previous CXR will usually help to make the distinction. If there is doubt whether blunting might be fluid, a lateral decubitus radiograph (p. 231) or ultrasound will clarify.
At a costophrenic angle as a result of a previous pleural infection. Occasionally it is consequent on a haemorrhagic effusion following trauma. The appearance of fibrotic blunting of a costophrenic angle may be very similar to a small (300 ml) pleural effusion. The overall clinical picture or previous clinical history or a previous CXR will usually help to make the distinction. If there is doubt whether blunting might be fluid, a lateral decubitus radiograph (p. 231) or ultrasound will clarify. Over the apex of one or both lungs. The cause: either old lung infection(e.g. tuberculosis) or due to compression of lung and pleural tissue by small apical bullae. The concern: if the appearance is unilateral then it can appear identical to a flat apical (Pancoast) carcinoma. See Chapter 10, p. 142. Comparison with a previous CXR often provides reassurance.
Over the apex of one or both lungs. The cause: either old lung infection(e.g. tuberculosis) or due to compression of lung and pleural tissue by small apical bullae. The concern: if the appearance is unilateral then it can appear identical to a flat apical (Pancoast) carcinoma. See Chapter 10, p. 142. Comparison with a previous CXR often provides reassurance.PLEURAL PLAQUES4
Plaques are focal areas of thickening of the parietal pleura due to previous exposure to asbestos. Characteristically they appear as scattered islands of well circumscribed pleural densities (Figs 7.23-7.25). Plaques:
 Are most commonly seen posteriorly and laterally, predominantly affecting the lower third of the thorax.
Are most commonly seen posteriorly and laterally, predominantly affecting the lower third of the thorax.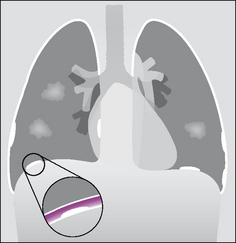
Figure 7.23 To show the various appearances of asbestos related pleural plaques. Seen en face the plaques (two on the right side, one on the left side) appear as islands of low density projected over the lung. In profile, when calcified, they appear as areas of high density against the lateral margins of the thorax, heart, vertebrae and over the domes of the diaphragm. The inset shows that the plaques represent areas of thickening of the parietal pleura.
PLEURAL CALCIFICATION
Other causes of pleural calcification include haemorrhagic effusion, pleural infection +/– empyema (including tuberculosis), and talc inhalation. Usually these causes result in unilateral calcification.
PLEURAL TUMOURS
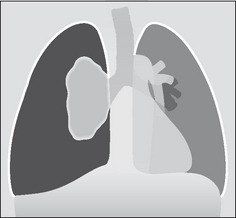
A pleural tumour, whether a primary pleural neoplasm or a secondary deposit from (say) a breast carcinoma, will commonly present with an accompanying effusion (Fig. 7.27).
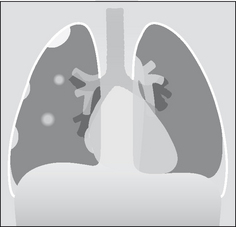
Figure 7.27 Pleural metastases. Nodular metastatic deposits (right hemithorax). Note the small pleural effusion. On the left side no focal lesion is seen other than the pleural effusion. The left pleural appearance is more typical of metastatic disease to the pleura—i.e. the CXR usually shows a pleural effusion only.
Figure 7.28 To illustrate some of the various features that can be seen on a CXR in a patient with a mesothelioma. An extensive pleural mass, sometimes creating an encasement or “peel” surrounding the lung5. A smaller or shrunken ipsilateral hemithorax. A pleural effusion.
An occasional dilemma
A frontal CXR shows a discrete mass hard up against a pleural surface. Is it in the lung, e.g. bronchial carcinoma? Is it arising from the pleura, e.g. breast deposit? Is it a rib lesion indenting the pleura and the lung, e.g. a rib metastasis?Table 7.1 indicates the CXR features that you should look for.
Table 7.1 Pulmonary mass vs Pleural mass vs Extrapleural mass.
| Origin | Characteristic CXR features |
|---|---|
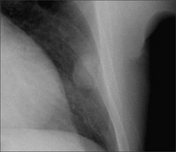
| Intrapulmonary lesion abutting a pleural surface | |
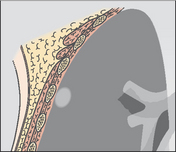 |
 |
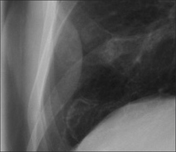
| Intrapleural mass indenting the lung | |
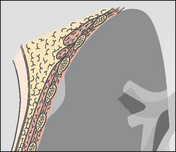 |
 |
| Extrapleural mass but intruding on to or into the lung | |
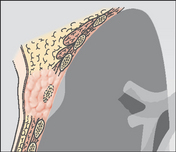 |
 |
PNEUMOTHORAX6-13
Pneumothoraces are common and the erect CXR is usually definitive. Sometimes the CXR appearances are subtle. There are multiple aspects that we need to be familiar with when assessing erect or supine CXRs.
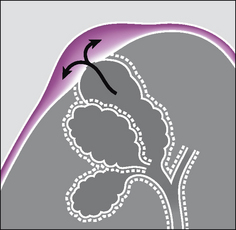
Figure 7.29 Primary spontaneous pneumothorax. An apical pleural bleb thins the visceral pleural membrane. The alveolar wall and the visceral pleura “pop” and air passes from the alveolar bleb into the pleural space.
PNEUMOTHORAX—CAUSES
The groups of individuals who are most at risk of developing a pneumothorax are indicated in Table 7.2.
Table 7.2 Causes of pneumothorax.
| Spontaneous—primary | Spontaneous—secondary | Traumatic/iatrogenic/other |
|---|---|---|
Healthy young adults
 Rupture of a subpleural bleb. A bleb (Fig. 7.29) is an outpouching of the alveolar wall; it bulges and thins the visceral pleural membrane. In general, blebs are present at the lung apices only. Rupture of a subpleural bleb. A bleb (Fig. 7.29) is an outpouching of the alveolar wall; it bulges and thins the visceral pleural membrane. In general, blebs are present at the lung apices only. |
Pre-existing lung disease |
PNEUMOTHORAX—DETECTION
The erect CXR
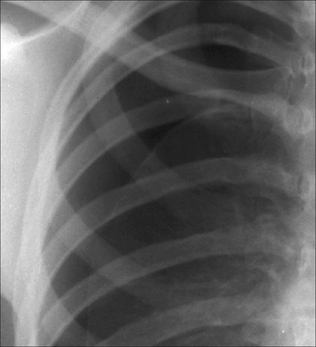
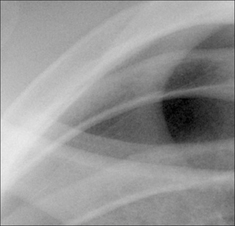
Figure 7.31 Erect CXR. A shallow pneumothorax. Curved visceral pleural membrane at the lung apex and no vessels lateral to the visceral pleural line.
The supine CXR—a pneumothorax can be very subtle8,9
A severely injured patient or a patient in ITU will not be able to sit up. The patient may have extensive lung pathology, e.g. haemorrhage, pneumonia, adult respiratory distress syndrome (ARDS). These factors can affect the position and appearance of air in the pleural space. Remember, an unrecognised pneumothorax may develop into a life-threatening tension pneumothorax in a patient treated with positive pressure ventilation.
Intrapleural air may accumulate in several different positions when a patient is supine. Two areas need to be inspected particularly carefully.
 In the supine position the highest part of the pleural space is at the lung base under the inferior surface of the lung. The lowermost part of the CXR will hold the evidence (Fig. 7.33).
In the supine position the highest part of the pleural space is at the lung base under the inferior surface of the lung. The lowermost part of the CXR will hold the evidence (Fig. 7.33). Look for:
Look for:
 A hyperlucent (i.e. blacker) upper quadrant of the abdomen…blacker because air collecting at the base of the lung overlies the liver (Fig. 7.34).
A hyperlucent (i.e. blacker) upper quadrant of the abdomen…blacker because air collecting at the base of the lung overlies the liver (Fig. 7.34). Air situated in the lateral costophrenic sulcus (the most lateral and inferior aspect of the pleural space)…the so called “deep sulcus sign” (Fig. 7.34).
Air situated in the lateral costophrenic sulcus (the most lateral and inferior aspect of the pleural space)…the so called “deep sulcus sign” (Fig. 7.34). A sharply outlined dome of the diaphragm (Fig. 7.37). This appearance may be particularly prominent if there is adjacent lower lobe pulmonary disease (e.g. pneumonia or ARDS).
A sharply outlined dome of the diaphragm (Fig. 7.37). This appearance may be particularly prominent if there is adjacent lower lobe pulmonary disease (e.g. pneumonia or ARDS). Look for:
Look for:
 A deep and well-defined anterior cardiophrenic sulcus (Figs 7.36 and 7.37). The anterior cardiophrenic sulcus is the most medial part of the pleural space, lying hard up against the inferior margin of the heart. If it appears deep and well-defined, this may be the earliest sign of a small pneumothorax.
A deep and well-defined anterior cardiophrenic sulcus (Figs 7.36 and 7.37). The anterior cardiophrenic sulcus is the most medial part of the pleural space, lying hard up against the inferior margin of the heart. If it appears deep and well-defined, this may be the earliest sign of a small pneumothorax.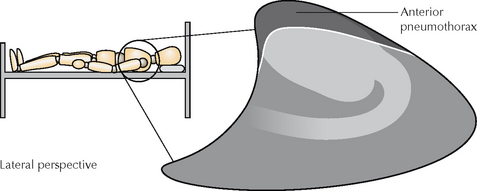
Figure 7.33 Pneumothorax. On a supine CXR the air rises to the highest point in the pleural space—i.e. anteriorly.
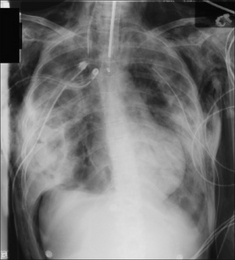
Figure 7.34 Supine CXR. Right-sided pneumothorax showing: (a) a hyperlucent right upper quadrant of the abdomen; and (b) a deep sulcus sign (i.e. intrapleural air collected in the lateral costophrenic sulcus).
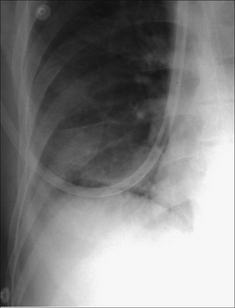
Figure 7.35 Supine CXR. Right-sided pneumothorax revealed by the very sharply defined margin to the dome of the diaphragm. It is sharply defined despite the immediately adjacent lower lobe lung consolidation.
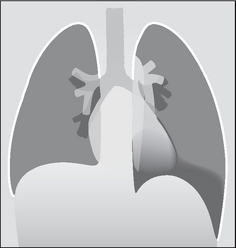
Figure 7.36 Supine radiograph. Left pneumothorax. The left dome of the diaphragm is sharply defined, and the left cardiac border has a fine black margin compared with the right side.
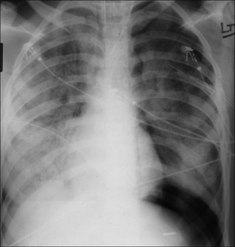
Figure 7.37 ARDS. Supine CXR. A left pneumothorax is revealed by the well-defined dome of the diaphragm and the sharply defined left heart border.
ITU patient has ARDS. CXR—equivocal pneumothorax.
 With a lateral decubitus bedside CXR with the suspect side raised and utilising a horizontal x-ray beam (p. 231).
With a lateral decubitus bedside CXR with the suspect side raised and utilising a horizontal x-ray beam (p. 231).TENSION PNEUMOTHORAX10,11,13
A medical emergency. As air enters the pleural space the normal negative intrapleural pressure (approximately −5 mmHg) becomes atmospheric. Sometimes a valve-like effect occurs and air continues to enter but cannot exit the pleural cavity. The pressure then rises above atmospheric. This build up of tension can cause compression of the inferior vena cava and obstruction to the venous return to the right side of the heart. This is potentially—and rapidly—lethal.
In most instances, the recognition of a tension pneumothorax is primarily a clinical diagnosis. Immediate treatment without recourse to a CXR is mandatory.
Underlying pulmonary or pleural disease (e.g. a patient with a stiff lung) can cause clinical confusion because some of the typical clinical findings—dyspnoea, chest pain, cough, tracheal deviation, diminished breath sounds, cyanosis—may not be present. Therefore it is essential to be familiar with the CXR findings indicating a tension pneumothorax. Two cardinal features to look for:
Recognition on an erect CXR
The visceral pleura is visible, and no vessels are seen beyond the visceral pleural line; the dome of the diaphragm, in most instances, is depressed, flattened or inverted, and the mediastinum is usually displaced towards the opposite side.
ACTION: Urgent placement of a cannula anteriorly. Ideally in the triangle of safety (Fig. 7.40)14,15.
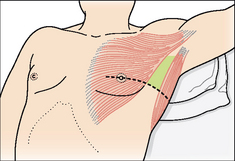
Figure 7.40 Pneumothorax. Safe insertion of an intercostal drain—within the triangle of safety14,15. The margins of the triangle are: the anterior border of the latissimus dorsi, the lateral border of the pectoralis major, a horizontal line at the level of the nipple defining the base of the triangle, and the apex of the triangle just below the axilla. This triangle is the optimum position for insertion of a drain in a non-emergency situation. It is also the preferred site for drain insertion in an emergency when a tension pneumothorax is diagnosed.
Recognition on a supine CXR
Findings as for the erect CXR, but stiff or diseased lung (e.g. extensive pneumonia or ARDS) will sometimes produce a deceptive appearance. Deceptive because the lung may be too solid to collapse or the mediastinum may not be displaced. However, the dome of the diaphragm will usually be flattened/inverted by the raised intrapleural pressure.
The important clinical rule
If the CXR shows evidence of a pneumothorax but the radiographic findings of a tension are equivocal then manage clinically. In other words, if tension is suspected on the clinical findings—then treat as for tension.
An occasional pitfall—mediastinal displacement
A simple pneumothorax—not under tension— can cause mediastinal displacement towards the opposite, normal, side (Fig. 7.41). This can occur when the underlying lung is completely collapsed. The elastic recoil of the lung is no longer counterbalanced by the normal negative pressure in the pleural space. As a consequence the mediastinum moves towards the normal side5.
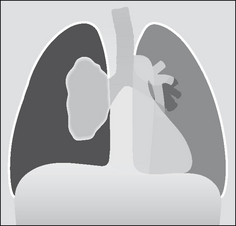
Figure 7.41 Mediastinal displacement—but not a tension pneumothorax. Note that the ipsilateral dome of the diaphragm is neither flattened nor depressed.
A useful guideline: if the ipsilateral dome of the diaphragm is not depressed or flattened do not rush to judgement. A flattened dome is almost always evident when tension is present in the pleural space—though this does not occur in 100% of cases5.
HAEMO- OR HYDRO-PNEUMOTHORAX
Many patients with a spontaneous pneumothorax, whether primary or secondary, will show a little fluid in the pleural space. Usually this is a small amount of blood resulting from a tear of a pleural adhesion.
If a moderate size haemo- or hydro-pneumothorax is present, then an air–fluid level, i.e. a straight line, will be seen at the air–fluid interface (Figs 7.43 and 7.44).
AN OCCASIONAL PUZZLE
Clinically a patient has a pneumothorax, but air is also seen elsewhere in the mediastinum or chest wall. How did it get there? There are two possibilities5:
In some patients (e.g. an asthmatic or treatment with positive pressure ventilation) a pneumothorax does not result from rupture of the visceral pleural membrane. Instead an increase in intra-alveolar pressure causes rupture of an alveolus or a bronchiole, and air dissects through the lung parenchyma along the sheaths of the pulmonary vessels to the hilum and thence into the mediastinum. This mediastinal air can then rupture through the mediastinal parietal pleura and so produce a pneumothorax. Because the mediastinal tissue planes are contiguous with those of the neck and the retroperitoneum the dissecting air from the ruptured alveolus can also cause extensive mediastinal and surgical emphysema (Fig. 7.45).
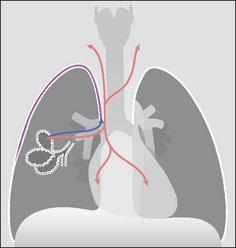
Figure 7.45 To illustrate the mechanism underlying the development of both a pneumothorax and mediastinal emphysema in a patient with asthma. The ruptured alveolus has caused pulmonary interstitial emphysema (PIE) and the air has dissected through the lung to reach the hilum. The air then dissects into the mediastinum and it also crosses the parietal pleura adjacent to the mediastinum to enter the pleural space.
If either of these structures rupture (e.g. external trauma or an oesophageal tear due to vomiting) then air can dissect along the mediastinal tissue planes. The air may then rupture through the mediastinal parietal pleura and enter the pleural space (Fig. 7.46). Occasionally it is the pneumothorax which is the dominant CXR finding, not the surgical emphysema.
PNEUMOTHORAX SIZE—IMPACT ON TREATMENT10–12,15
 Some physicians regard the CXR as a poor tool for measuring the size of a pneumothorax, and they emphasise that it is the clinical status of the patient not the radiographic size of a pneumothorax that should influence the decision as to the appropriate treatment11.
Some physicians regard the CXR as a poor tool for measuring the size of a pneumothorax, and they emphasise that it is the clinical status of the patient not the radiographic size of a pneumothorax that should influence the decision as to the appropriate treatment11. Other physicians utilise the CXR to estimate the size of a pneumothorax in order to help choose between treatment options. Approximate measurements can be made (Fig. 7.47). These descriptive terms are in common usage:
Other physicians utilise the CXR to estimate the size of a pneumothorax in order to help choose between treatment options. Approximate measurements can be made (Fig. 7.47). These descriptive terms are in common usage:
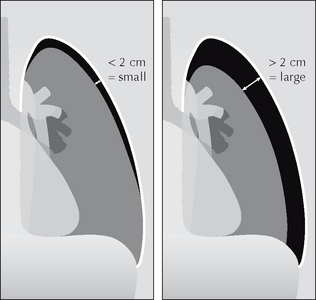
Figure 7.47 Erect CXR. Pneumothorax. Measurement of the rim allows a pneumothorax to be classified as either small or large.
Why are rim measurements regarded as useful? A rim with a diameter of 2 cm equates to a pneumothorax which occupies approximately 50% of the volume of the hemithorax. At this size, and if clinically indicated, the pneumothorax can be treated safely by aspiration12,15.
PNEUMOTHORAX: SOME IMPOSTERS
 Common: a white line on the CXR may be misread as the visceral pleural line. These spurious shadows include clothing artefacts, hair plaits, skin wrinkles (Fig. 7.48), venous lines or tubing, an overlying scapula margin.
Common: a white line on the CXR may be misread as the visceral pleural line. These spurious shadows include clothing artefacts, hair plaits, skin wrinkles (Fig. 7.48), venous lines or tubing, an overlying scapula margin.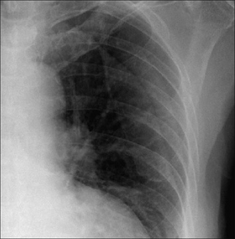
Figure 7.48 Not a pneumothorax. The fine line crossing the left hemithorax is a wrinkle, or fold, of the skin.
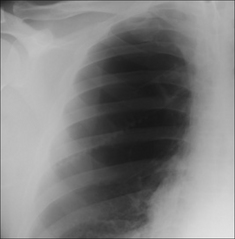
Figure 7.49 Not a pneumothorax. A large bulla. Note that there is no evidence of a visceral pleural line. On an erect CXR a pleural line is almost always evident when a pneumothorax is present.
AN INTERESTING CONDITION—CATAMENIAL PNEUMOTHORAX16
Aetiology/pathology
Recurrent pneumothorax occurring only in relation to menstruation. Pathogenesis remains speculative. One suggestion is that air enters the peritoneal cavity via the unplugged cervix during menstruation. The air then enters the pleural space via a leaky diaphragm. It is of interest that some patients do have pelvic or diaphragmatic endometriosis.
1. Raasch BN, Carsky EW, Lane EJ, et al. Pleural effusion: explanation of some typical appearances. AJR. 1982;139:899-904.
2. Woodring JH. Recognition of pleural effusion on supine radiographs: how much fluid is required? AJR. 1984;142:59-64.
3. Emamian SA, Kaasbol MA, Olsen JF, et al. Accuracy of the diagnosis of pleural effusion on supine chest x-ray. Eur Radiol. 1997;7:57-60.
4. Schwartz DA. New developments in asbestos related pleural disease. Chest. 1991;99:191-198.
5. Fraser RG, Muller NL, Colman NC, Pare PD. Fraser and Pare’s Diagnosis of Diseases of the Chest, 4th ed. Philadelphia, PA: WB Saunders, 1999.
6. O’Connor AR, Morgan WE. Radiological review of pneumothorax. BMJ. 2005;330:1493-1497.
7. Seow A, Kazerooni EA, Pernican PG, et al. Comparison of upright inspiratory and expiratory chest radiographs for detecting pneumothoraces. AJR. 1996;166:313-316.
8. Tocino IM. Pneumothorax in the supine patient: radiographic anatomy. Radiographics. 1985;5:557-586.
9. Cummin ARC, Smith MJ, Wilson AG. Pneumothorax in the supine patient. BMJ. 1987;295:591-592.
10. Baumann MH, Strange C, Heffner JE, et al. Management of spontaneous pneumothorax. An American College of Chest Physicians Delphi consensus statement. Chest. 2001;119:590-602.
11. Baumann MH, Strange C. Treatment of spontaneous pneumothorax: a more aggressive approach? Chest. 1997;112:789-804.
12. Henry M, Arnold T, Harvey J. BTS guidelines for the management of spontaneous pneumothorax. Thorax. 2003;58(suppl ii):39-52.
13. Teplick SK, Clark RE. Various faces of tension pneumothorax. Postgraduate Medicine. 1974;56:87-92.
14. Laws D, Neville E, Duffy J. BTS guidelines for the insertion of a chest drain. Thorax. 2003;58(suppl):55-59.
15. Griffiths JR, Roberts N. Do junior doctors know where to insert chest drains safely? Postgrad Med J. 2005;81:456-458.
16. Carter EJ, Ettensohn DB. Catamenial pneumothorax. Chest. 1990;98:713-716.