chapter 36 Surgery of the Penis and Urethra
Improvements in microsurgery, tissue transfer techniques, and tissue handling have expanded the repertoire of the urologic surgeon and the genitourinary reconstructive surgeon in particular. Urologists are now able to reconstruct congenital, and acquired genitourinary abnormalities with greater facility. Microvascular and microneurosurgical techniques have made it possible to construct a phallus that allows a patient to void while standing and to enjoy erotic sensibility. Because the phallus has both erotic sensibility and protective sensation, the patient can eventually have a prosthetic implantation that allows an acceptable sexual life. This chapter discusses the general principles of male genital reconstructive surgery; specifics include male urethral surgery, surgery for congenital and traumatic penile lesions, and complex fistula and obliterative issues associated with the posterior urethra.
Principles of Reconstructive Surgery
Many techniques in reconstructive surgery require the transfer of tissue. Skin is one of those tissues, and its properties vary from individual to individual and from place to place on the same individual. Variable characteristics such as color, texture, thickness, extensibility, innate skin tension, and blood supply can be useful in various situations.
The term tissue transfer implies the movement of tissue for purposes of reconstruction. Unlike extirpative surgery, the transfer of tissue for reconstruction requires an intimate knowledge of the anatomy of both the donor and the recipient sites, as well as of the principles that will allow that tissue to survive once it is transferred.
The skin can be used as a model. The superficial layer of the skin is termed the epidermis (thickness, 0.8 to 1 mm). The deep layer of the skin is termed the dermis. The dermis has two layers: a superficial layer, the adventitial dermis (also called the papillary or periadnexal dermis, depending on the anatomy), and a deep layer, the reticular dermis. For genitourinary reconstruction, skin without adnexal structures is often used; thus, the papillary dermis is synonymous with the adventitial dermis. Other tissues commonly transferred for genitourinary reconstruction include bladder and oral mucosa. The bladder epithelium is the superficial layer of the bladder; the deep layer of the bladder is termed the lamina propria, with superficial and deep layers. The oral mucosa is the superficial layer of much of the oral cavity, which also has a deeper layer termed the lamina propria, again with superficial and deep layers.
All tissue has physical characteristics: extensibility, inherent tension, and the viscoelastic properties of stress relaxation and creep. The physical characteristics of a transferred unit are primarily a function of the helical arrangement of collagen along with the elastin cross-linkages. The collagen-elastin structure is suspended in a mucopolysaccharide matrix that influences the viscoelastic properties.
Tissue can be transferred as a graft (Fig. 36–1A and B). The term graft implies that tissue has been excised and transferred to a graft host bed, where a new blood supply develops by a process termed take. Take requires approximately 96 hours and occurs in two phases. The initial phase, imbibition, requires about 48 hours. During that phase, the graft survives by “drinking” nutrients from the adjacent graft host bed, and the temperature of the graft is less than the core body temperature. The second phase, inosculation, also requires about 48 hours and is the phase in which true microcirculation is reestablished in the graft. During that phase, the temperature of the graft rises to core body temperature. The process of take is influenced by both the nature of the grafted tissue and the conditions of the graft host bed. Processes that interfere with the vascularity of the graft host bed thus interfere with graft take.
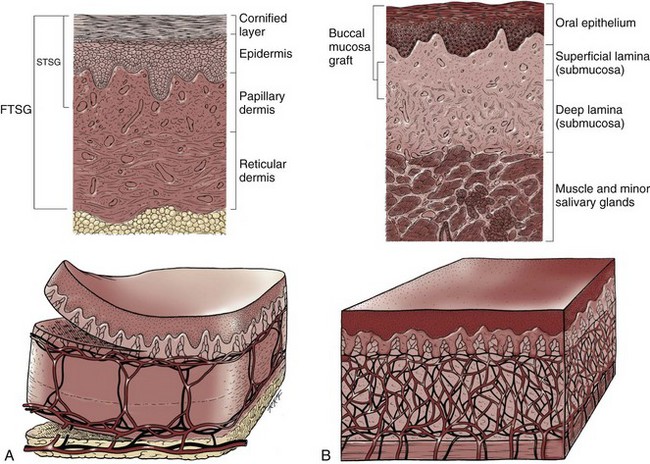
Figure 36–1 Cross-sectional diagrams (histologic appearance above, microvasculature below) of the skin. A, Cross-sectional diagrams of skin. B, Cross-sectional diagrams of oral mucosa.
(From Jordan GH, Schlossberg SM. Using tissue transfer for urethral reconstruction. Contemp Urol 1993;13:23.)
The epidermal, or epithelial layer, is a covering, the barrier to the “outside,” and is adjacent to the superficial dermis, or superficial lamina. At approximately that interface is the superficial plexus. In the case of skin, the plexus is the intradermal plexus. There are some lymphatics in the superficial dermal or tunica layer. On the undersurface of the deep dermal layer or deep lamina is the deep plexus. In the case of skin, this is the subdermal plexus. The deep dermis contains most of the lymphatics and greater collagen content than found in the superficial dermal layer. The deep or reticular dermis is generally thought to account for the physical characteristics of the tissue.
Thus if a graft is a split-thickness unit, that graft carries the epidermis or the covering. That graft also exposes the superficial dermal (intradermal or intralaminar) plexus. In most grafts, that superficial plexus is composed of small but numerous vessels. This thus conveys favorable vascular characteristics to a split-thickness unit. The unit has few lymphatics, and the physical characteristics are not carried, which accounts for the tendency of split-thickness units to be brittle and less durable. The reticular dermis is not carried with the split-thickness unit (Jordan, 1993).
The mesh graft is usually an application of the split-thickness graft. After the harvest of a sheet graft, the sheet is placed on a carrier that cuts systematically placed slits in the graft. These slits can expand the graft by various ratios (i.e., 1.5 : 1, 2 : 1, 3 : 1). For most genital reconstructive surgery, the slits are not for expansion but rather to allow subgraft collections to escape; in some cases, the slits allow the graft to conform better to irregular graft host beds (e.g., the testes in split-thickness skin graft scrotal construction). It has also been proposed that mesh grafts take readily because of increased levels of growth factors, possibly as a function of the slits. In general, full-thickness skin grafts are not meshed (Schreiter and Koncz, 1983; Jordan, 1993).
If a graft is a full-thickness unit, it carries the covering and the superficial dermis or lamina with all the characteristics attributable to that layer. It also, however, carries the deep dermis or deep lamina. In skin, the subdermal plexus is exposed. In most cases, that plexus is composed of larger vessels that are more sparsely distributed. The graft is thus fastidious in its vascular characteristics. A full-thickness unit carries most of the lymphatics, and the physical characteristics are likewise carried with the transferred tissue (Devine et al, 1976; Jordan, 1993; Wessels and McAninch, 1996). An off-the-shelf substance called Integra is currently available. Integra (Integra LifesSciences Corporation, Plainsboro, NJ 08536, 1-877-444-1122) is used to “prepare” graft recipient sites. After a period, the Integra material becomes infiltrated with granulation tissue and after about 10 days to 2 weeks can be grafted with split-thickness skin. The resultant graft is said to have both a dermal layer as well as the graft covering. We have limited experience with the material at this time.
When the grafts that are most commonly used in genitourinary reconstructive surgery are examined, the split-thickness skin graft has favorable vascular characteristics but tends to contract and be brittle when mature. The full-thickness skin graft tends to have more fastidious vascular characteristics, but it does not contract as much and is more durable when mature (see Fig. 36–1A). There is a difference between genital full-thickness skin (penile and preputial skin grafts) and extragenital full-thickness skin. This is probably a reflection of the increased mass of the graft in extragenital skin grafts. This increased mass makes the graft more fastidious, and the poor results reported with urethral reconstruction with extragenital full-thickness skin grafts are probably due to poor or ischemic take (Webster et al, 1984; Webster, 1987; Jordan, 1993). The posterior auricular graft (Wolfe graft) is an exception to the rule concerning extragenital skin. The postauricular skin is thin and overlies the temporalis fascia and is thought to be carried on numerous perforators. The subdermal plexus of this graft thus mimics the characteristics of the intradermal plexus, and the total mass of the graft is more like that of the split-thickness unit. In the bladder epithelial graft, there is a superficial and a deep plexus; however, the plexuses are connected by many more perforators. Thus bladder epithelial grafts tend to have more favorable vascular characteristics. In the case of the oral mucosal grafts, there is a panlaminar plexus. Thus the oral mucosal graft can be thinned somewhat, provided a sufficient amount of deep lamina is carried to preserve the physical characteristics (see Fig. 36–1B). The oral mucosal grafts are thought to have optimal vascular characteristics (Humby, 1941; Memmelaar, 1947). The thinned graft diminishes the total graft mass while preserving the physical characteristics and not adversely affecting the vascular characteristics. The enthusiasm for the buccal mucosal graft thus seems well founded. The fact that the graft has a “wet epithelial” surface is likewise thought to be a favorable characteristic for many cases of urethral reconstructive surgery. The lingual, labial, and buccal grafts all vary in thickness and somewhat in substance. Because the labial mucosal grafts are thin, many surgeons prefer that donor site for reconstruction of the fossa navicularis (Jordan, 1993).
A series reporting the use of “buccal mucosal” onlay grafts with mid- and long-term results seems to suggest durability for these grafts. In that series (Fichtner et al, 2004), 67 patients were described, all with follow-up exceeding 5 years and some with 10 years of follow-up. All failures occurred within 12 months of the original procedure.
The dermal graft has been used for years to augment the tunica albuginea of the corpora cavernosa. When it is harvested, the graft exposes both the intradermal plexus and the deep dermal plexus. The dermal graft thus takes readily (is not fastidious) and has the physical characteristics normal to skin. When it is properly prepared, the tunica vaginalis graft is essentially peritoneum. The tendency of peritoneum to take readily is well documented both in the literature that examines adhesion formation and in the urology literature concerning the application of peritoneal grafts for reconstruction of the urinary tract. The literature fails to accurately define what the surgeon can expect regarding physical characteristics (Jordan, 1993). Tunica vaginalis grafts have proved useful for small defects of the tunica albuginea of the corpora cavernosa, but there is a tendency to aneurysmal dilation when they are used for larger defects. Tunica vaginalis grafts have been tried for urethral reconstruction with uniformly poor results.
As described in the urologic literature, vein grafts are perhaps not true grafts according to the terminology used in this chapter. Vein patches are widely used in vascular surgery. The premise is that the vein survives by endothelial direct perfusion and reestablishment of vein wall blood flow by perfusion of the vasa vasorum. The vascular literature is at odds with this concept. The intima is the endothelial layer; it is thin and easily injured during the process of vein harvest and preparation, with areas of endothelial sloughing noted. Inflammatory cells and fibrin adhere to the exposed basement membrane. However, the endothelium does regenerate in the first 6 weeks. The media is a combination of smooth muscle and interlaced collagen. After graft harvest, smooth muscle injury is prominently noted and is thought to be related to warm ischemia. In more mature grafts, much of the smooth muscle is replaced by a process of fibrous transformation with collagen deposition. The adventitia is a loose collagenous network interspersed with vasa vasorum. Mature vein grafts show evidence of take to the vasa vasorum. However, the adventitia becomes incorporated by periadventitial connective tissues. Thrombosis in the vasa vasorum, early in the process of take, is not an unusual phenomenon. When vein grafts are exposed to arterial pressure and shear stress forces, the process colloquially described as “arterialization” occurs and is associated with changes of the vessel wall elastic properties, and the graft becomes rigid with low compliance. Once these changes are noted, at least when veins are used for vessel replacement, the graft remains noncompliant throughout the remaining life of the graft (Szilagyi et al, 1973; Fuchs et al, 1978; Tolhurst and Haeseker, 1982). Vein “grafts” are currently being widely used for replacement of defects of the tunica albuginea of the corpora cavernosa. The pertinent points with regard to the transfer of vein patches to the corpora cavernosa and their long-term behavior, however, have been inferred from the current vascular literature. Dermal grafts have been tried for urethral reconstruction, also with poor results in general. Rectal mucosal grafts have also been proposed for urethral reconstruction but little is known about their graft take. In general, the vascularity of the bowel mucosa is based on the vascularity of the underlying muscle, with the mucosa carried on perforators. Little is found in the literature regarding the process of take of these grafts.
Tissue can be transferred as a flap. The term flap implies that the tissue is excised and transferred with the blood supply either preserved or surgically reestablished at the recipient site. Flaps can be classified by a number of criteria. We can classify flaps on the basis of their vascularity and thus characterize flaps as either random flaps (Fig. 36–2) or axial flaps (Fig. 36–3). A random flap is a flap without a defined cuticular vascular territory. The flap is carried on the dermal or laminar plexuses; the dimensions of random flaps can vary widely from individual to individual and from body site to body site. The term axial flap means that there is a defined vessel in the base of the flap. There are three types of axial flaps. The direct cuticular axial flap is a flap based on a vessel superficial to the superficial layer of the deep body wall fascia (see Fig. 36–3A). The classic example of a direct cuticular flap is the groin flap. A musculocutaneous flap (Fig. 36–4A), on the other hand, is based on the vascularity to the muscle. The overlying skin paddle is carried on perforators. If the muscle alone is carried as a flap, the overlying skin survives as a random unit. The fasciocutaneous system of vascularity (Fig. 36–4B) is similar to the musculocutaneous system. However, the deep blood supply is carried on the fascia (both deep and superficial layers), and the overlying skin paddle is based again on perforators. Thus one can transfer a fascial flap based on the deep blood supply associated with the flap; the overlying skin, if it is not carried with the flap, remains as a random unit (Ponten, 1981; Tolhurst and Haeseker, 1982; Cormack and Lamberty, 1984). It has been argued that fascia is relatively avascular and hence cannot serve as “the blood supply” to the fasciocutaneous unit. Indeed, the fascial layer, in reality, acts as a trellis—thus the vessels are carried much like the limbs of a vine (Jordan, 1993).
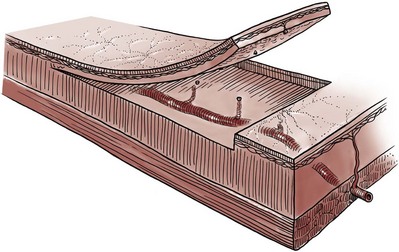
Figure 36–2 Random flap. The arterial perforators have been interrupted, and flap survival depends on the intradermal and subdermal plexuses.
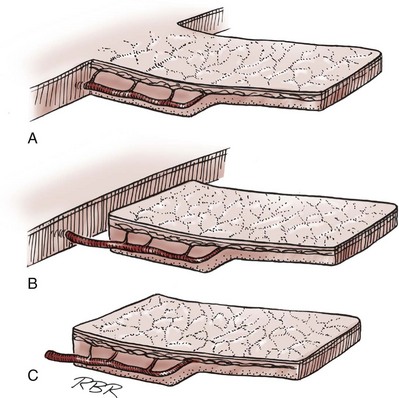
Figure 36–3 Axial flaps. Large vessels enter the base of the flaps. Survival depends on these vessels and on the random distal vascularity. A, Peninsula flap. The vascular continuity and the cutaneous continuity in the flap base are intact. B, Island flap. The vascular pedicle is intact; the cuticular continuity has been divided. These axial vessels are unsupported (dangling). C, Microvascular free-transfer flap. The free-flap cuticular and vascular connections are interrupted at the base of the flap. Vascular continuity is reconstituted in the recipient area by a microsurgical anastomosis.
(A to C, From Jordan GH, McCraw JB. Tissue transfer techniques for genitourinary reconstructive surgery. AUA Update Series 1988;7:lesson 10.)
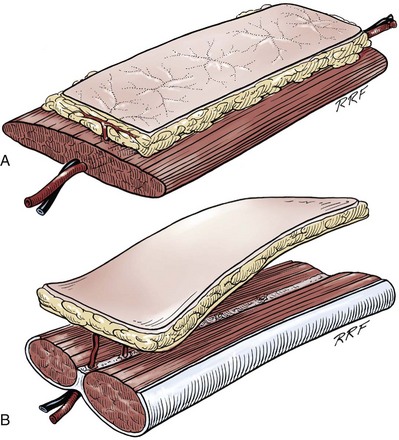
Figure 36–4 A, Musculocutaneous flap. Musculocutaneous perforators from the artery to a muscle vascularize the skin and overlying subcutaneous fat. They may be transferred as free flaps but are usually transferred locally, left attached to the vascular pedicle. B, Fasciocutaneous flap. Perforating blood vessels from rich plexuses on the superficial and deep aspects of the fascia connect to perforator vessels that communicate with the microvasculature of the overlying paddle. In genital reconstruction, these flaps are based on the dartos fascia of the penis or are free flaps from the forearm.
(A and B, From Jordan GH, McCraw JB. Tissue transfer techniques for genitourinary reconstructive surgery. AUA Update Series 1988;7:lesson 10.)
A flap can also be classified by the elevation technique. A peninsular flap is a flap in which the vascular continuity and the cutaneous continuity of the flap base are left intact (see Figs. 36-2 and 36-3A). An island flap (see Fig. 36–3B) is a flap in which the vascular continuity is maintained; however, the cuticular continuity is divided. Thus a true island flap is elevated on dangling vessels. The microvascular free transfer flap (free flap) (see Fig. 36–3C) has both the vascular continuity and the cuticular continuity interrupted. The vascular continuity is then reestablished at the recipient site.
Again, there is confusion in terminology. In genitourinary reconstructive surgical procedures, we tend to use the term island flap. As already mentioned, a true island flap is elevated on dangling vessels. The usual case, however, is that a skin island or paddle is elevated either on the muscle, as in the gracilis musculocutaneous flap, or on the fascia, as in local genital skin flaps. The term island flap is not synonymous with the terms skin island and skin paddle. The usefulness of these flaps and grafts is illustrated in the discussion of the surgical techniques in this chapter. There is continued interest in the use of tissue-cultured grafts or “manufactured” grafts. The likelihood of someday, soon, being able to successfully use off-the-shelf grafts or sheets of cultured material is right around the corner (Chen et al, 1999; Atala, 2002; Rotariu et al, 2002; El-Kassaby et al, 2003; Bhargava et al, 2004).
Key Points: Principles of Reconstructive Surgery
Anatomy of the Penis and Male Perineum
See Figs. 36-5 to 36-13 on the Expert Consult website![]() .
.
The anatomic relationships of the male genitourinary structures in the penis and male perineum must precede the discussion of specific reconstructive surgical techniques; for a complete anatomic description please see Chapter 2.
The penile shaft (Fig. 36–5) comprises three erectile bodies: the two corpora cavernosa and the corpus spongiosum containing the urethra, along with their enveloping fascial layers, nerves, and vessels, all covered by skin. All these structures continue into the perineum. The corpora cavernosa contain erectile tissue within a dense elastic sheath of connective tissue called the tunica albuginea. The corpora cavernosa are not separate structures but constitute a single space with free communication through an incompetent midline septum that becomes more complete toward the base of the penis. This erectile tissue contains arteries, nerves, muscle fibers, and venous sinuses lined with flat endothelial cells, and these features fill the corpora cavernosa, making its cut surface look like that of a sponge. This tissue is separated from the tunica albuginea by a thin layer of areolar connective tissue.
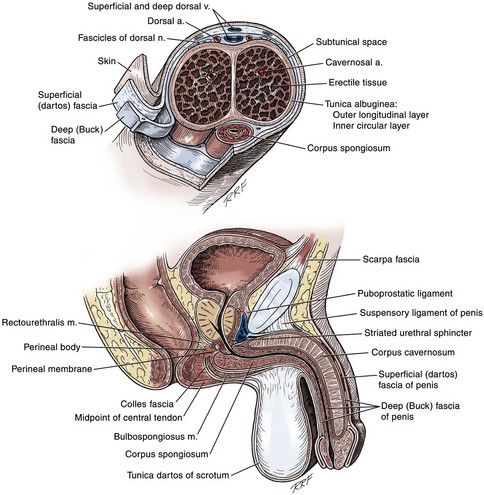
Figure 36–5 Top, Cross section of the penis at the junction of its middle and distal thirds. The septum is correctly illustrated as strands that interweave with the tunica albuginea both ventrally and dorsally. Bottom, Diagram of a sagittal section of the penis and perineum illustrating the fascial layers.
The third erectile body, the corpus spongiosum, lies in the ventral groove between the two corpora cavernosa. The tunica albuginea (adventitia) of the corpus spongiosum is thinner than the tunica albuginea of the corpora cavernosa, and the corpus spongiosum contains less erectile tissue than the corpora cavernosa. The urethra traverses the length of the penis within the corpus spongiosum. At its distal end, the corpus spongiosum expands to form the glans penis. The urethral meatus is slitlike, lying slightly on the ventral aspect of the tip of the glans, with its long axis oriented vertically. At its base, the penis is supported by two ligaments, composed primarily of elastic fibers that are continuous with the fascia of the penis. Posterior to this attachment, the right and left corpora cavernosa diverge, and the corpus spongiosum broadens between the two crura to form the bulbospongiosus (bulb) (Fig. 36–6).
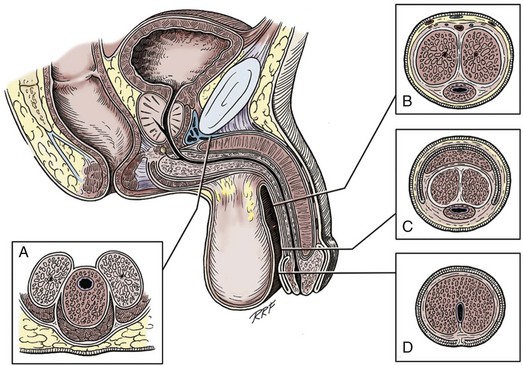
Figure 36–6 Diagrammatic cross sections of the anterior urethra. A, The bulbous urethra. The urethra is eccentrically placed in the corpus spongiosum. Proximally, the corpora cavernosa have split into individual crura, with the urethra lying against the triangular ligament. B, In the shaft of the penis, the urethra is more centrally placed with relation to the corpus spongiosum, and the corpora cavernosa are intimately fused, separated only by septal fibers. C, At the coronal margin, the urethra remains relatively centrally placed, and the corpora cavernosa are fused, again separated by septal fibers. The spongy tissue of the corpus spongiosum has become incorporated as the deep tissues of the glans. D, The fossa navicularis widens somewhat in caliber and is totally surrounded by the spongy erectile tissue of the glans penis. The urethra here is relatively ventrally placed in relation to the body of the corpus spongiosum.
(A to D, From Jordan GH. Complications of interventional techniques of urethral stricture disease: direct visual internal urethrotomy, stents and laser. In: Carson C, editor. Topics in clinical urology: complications of interventional techniques. New York: Igaku-Shoin; 1996. p. 86–94.)
Figure 36–5 also illustrates the relationship of the erectile bodies and the urethra to the structures in the perineum. For the discussion of trauma and reconstruction, it is the consensus opinion of a World Health Organization conference convened in Stockholm in 2002 that the common use of the terms anterior urethra and posterior urethra be put aside and that the urethra be subdivided into six separate areas. These portions of the urethra are illustrated in Figure 36–7.
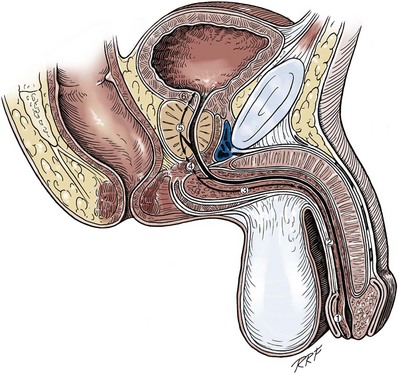
Figure 36–7 Sagittal section of the pelvis. The urethra is subdivided into the following sections: 1, fossa navicularis; 2, pendulous or penile urethra; 3, bulbous urethra; 4, membranous urethra; 5, prostatic urethra; 6, bladder neck. By common usage, the divisions of the fossa navicularis, pendulous urethra, and bulbous urethra compose the anterior urethra; and the divisions of the membranous urethra, prostatic urethra, and bladder neck compose the posterior urethra.
(Modified from Devine CJ Jr, Angermeier KW. Anatomy of the penis and male perineum. AUA Update Series 1994;8:11.)
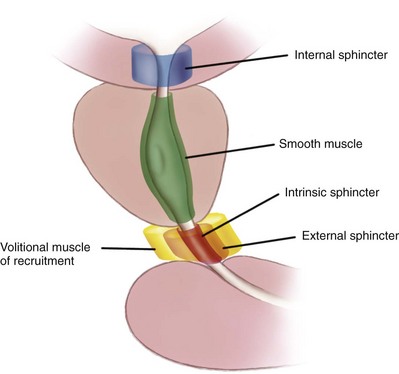
A submucosal layer is noted throughout the length of the urethra. Five “sphincters” are recognized (Fig. 36–8).
In the penis, the erectile bodies are surrounded by Buck fascia, dartos fascia, and skin. Buck fascia is the tough, elastic layer immediately adjacent to the tunica albuginea (see Fig. 36–5). On the superior aspect of the corpora cavernosa, the deep dorsal vein, paired dorsal arteries, and multiple branches of the dorsal nerves are contained within the envelope of Buck fascia. In the midline groove on the underside of the corpora cavernosa, Buck fascia splits to surround the corpus spongiosum. Consolidation of the fascial layers (Fig. 36–9A to C), lateral to the corpus spongiosum, attaches it to the tunica albuginea of the corpora cavernosa. Attached distally to the undersurface of the glans penis at the corona, Buck fascia extends into the perineum, enclosing each crus of the corpora cavernosa and the bulb of the corpus spongiosum, and firmly fixing these structures to the pubis, ischium, and inferior fascia of the perineal membrane (urogenital diaphragm).
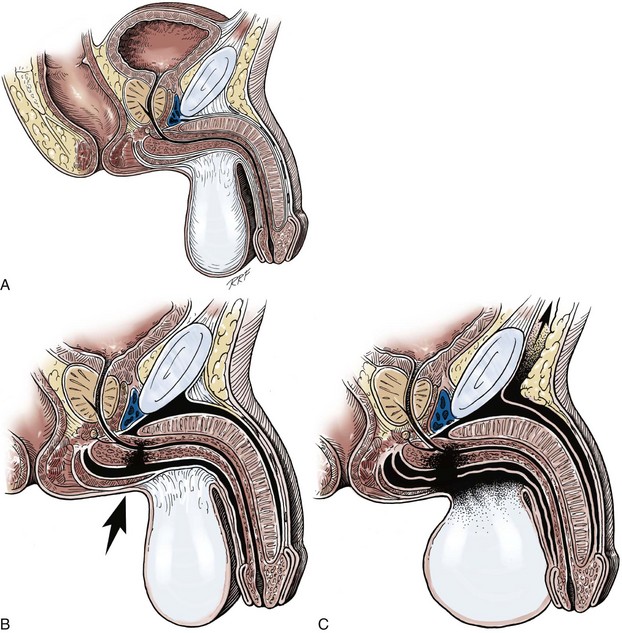
Figure 36–9 Cross sections of the pelvis. A, The normal attachment of the fasciae enveloping the penile structures. The dartos fascia is contiguous with the Scarpa fascia onto the abdomen, with the tunica dartos of the scrotum, with the Colles fascia on the perineum, and over the thigh—eventually to insert at the fascia lata. B, With trauma to the pelvis or perineum, the corpus spongiosum is injured; the hematoma, however, is confined by the attachment of the Buck fascia. C, With trauma to the perineum or pelvis, the corpus spongiosum is injured and the Buck fascia is violated; the hematoma thus can spread throughout the confines of the extended dartos fascia–tunica dartos system.
Distally, the skin of the penis is confluent with the glabrous skin covering the glans. At the corona, it is folded on itself to form the foreskin (prepuce) that overlies the glans. The dartos fascia, a layer of areolar tissue remarkable for its lack of fat, separates these two layers of skin and continues into the perineum, where it fuses with the layers of the superficial perineal (Colles) fascia. In the penis, the dartos fascia is loosely attached to the skin and the deeper layer of Buck fascia, and contains the superficial arteries, veins, and nerves of the penis.
Blood is supplied to the skin of the penis by the left and right superficial external pudendal vessels (Fig. 36–10A), which arise from the first portion of the femoral artery, cross the upper medial portion of the femoral triangle, and divide into two main branches, running dorsolaterally and ventrolaterally in the shaft of the penis, with collateralization across the midline. At intervals, fine branches split off to the skin, forming a rich subdermal vascular plexus that can sustain the skin after its underlying dartos fascia has been mobilized. The arteries are accompanied by venous tributaries that are more prominent and easily seen than the arteries. Because of its remarkable thinness and mobility, and the character of its vascular supply, the skin covering the penis is an ideal substitute—in some cases, for urethral reconstruction. The blood supply to the scrotal wall and ventral penile skin is based on the posterior scrotal artery, a superficial vessel from the deep internal pudendal artery (Fig. 36–10B). As with the superficial external pudendal tributaries, the posterior scrotal system provides a series of tributaries carried within the tunica dartos.
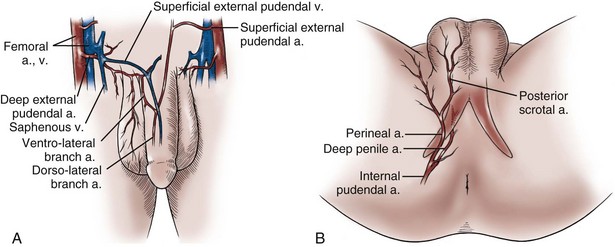
Figure 36–10 Illustration of the vasculature to the genital skin. A, The superficial external pudendal vessels arborize to become the fascial blood supply contained in the dartos fascia of the penis. B, The scrotal artery is a terminal branch of the deep internal pudendal artery. This artery is thought to arborize in the tunica dartos of the scrotum and Colles fascia of the perineum. The perineal artery continues lateral to the groin crease onto the thigh and extends toward the groin.
Venous Drainage
The penis is drained by three venous systems: superficial, intermediate, and deep (Fig. 36–11) (Aboseif et al, 1989). The superficial veins contained in the dartos fascia on the dorsolateral aspects of the penis unite at its base to form a single superficial dorsal vein. The superficial dorsal vein usually drains into the left saphenous vein (rarely into the right), and occasionally forms two trunks that drain into both. Veins from more superficial tissue may drain into the external superficial pudendal veins.
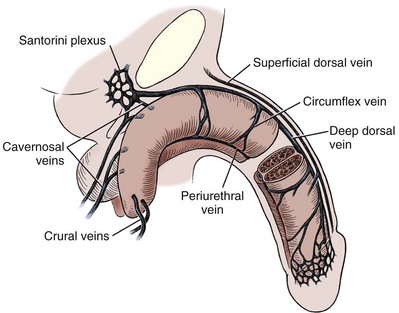
Figure 36–11 Diagram illustrates the venous drainage of the deep structures of the penis.
(From Horton CE, Stecker JF, Jordan GH. Management of erectile dysfunction, genital reconstruction following trauma and transsexualism. In: McCarthy JG, editor. Plastic surgery, vol 6. Philadelphia: WB Saunders; 1990. p. 4213–45.)
The intermediate system contains the deep dorsal and circumflex veins, lying within and beneath Buck fascia. Emissary veins begin within the erectile space of the corpora cavernosa and, following a perpendicular or oblique course through the tunica albuginea, emerge from the lateral and dorsal surfaces of the corpora cavernosa to empty into the circumflex veins or the deep dorsal vein. The circumflex veins are channels, usually more prominently present in the distal two thirds of the penile shaft. They arise from the corpus spongiosum, on the ventrum of the penis, and often receive the emissary veins as they travel around the lateral aspect of the corpora cavernosa, passing beneath the dorsal arteries and nerves to empty into the deep dorsal vein. The circumflex veins can also become confluent ventrally, forming periurethral veins on each side. These may become important in the treatment of impotence caused by veno-occlusive incompetence.
The deep dorsal vein is formed by five to eight small veins emerging from the glans penis to form the retrocoronal plexus, which drains into the deep dorsal vein that may consist of more than one vein lying in the midline groove between the corporal bodies. In a number of patients, there is a connection between the superficial and deep dorsal veins. The vein gathers blood from the emissary and circumflex veins, and passing beneath the pubis at the level of the suspensory ligament, it leaves the shaft of the penis at the crus and drains into the periprostatic plexus.
The deep drainage system consists of the crural and cavernosal veins. The crural veins arise in the midline, in the space between the crura. Normally, they are small and almost indiscernible, joining the deep dorsal vein or the periprostatic plexus. If the deep dorsal vein has been ligated or obliterated after trauma, striking development of these veins can be noted as the intracrural space is entered during the perineal dissection for urethral repair. Emissary veins in the proximal third of the crura, near their attachment to the ischial tuberosities, join to form several thin-walled trunks on the dorsomedial surface of each corpus cavernosum. Some pass medially, joining the dorsal or crural veins, or, extending proximally, enter the periprostatic plexus. Most consolidate into one or two cavernosal veins on each side. Running in the penile hilum, deep and medial to the cavernosal arteries and nerves, they join to form a large venous channel that drains into the internal pudendal vein. Three or four small cavernosal veins emerge from the dorsolateral surface of each crus and course laterally between the bulbospongiosus and the crus of the penis for 2 to 3 cm before draining into the internal pudendal veins. These usually insignificant vessels become larger, and can be noted more readily in patients with veno-occlusive erectile dysfunction. The internal pudendal veins (usually two) run together with the internal pudendal artery and nerve in the Alcock canal to empty into the internal iliac vein.
Arterial System
The blood supply to the deep structures of the penis is derived from the common penile artery, which is a continuation of the internal pudendal artery after it gives off the perineal branch (Fig. 36–12). From that point, the artery is termed the common penile artery and travels along the medial margin of the inferior pubic ramus. As it nears the urethral bulb, the artery divides into its three terminal branches, as follows:
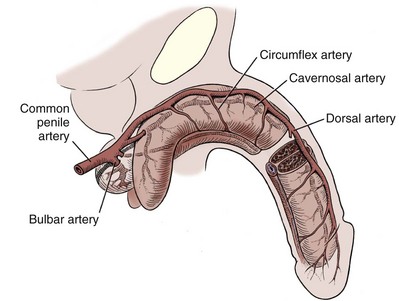
Figure 36–12 Diagram illustrates the arterial supply to the deep structures of the penis.
(From Horton CE, Stecker JF, Jordan GH. Management of erectile dysfunction, genital reconstruction following trauma and transsexualism. In: McCarthy JG, editor. Plastic surgery, vol 6. Philadelphia: WB Saunders; 1990. p. 4213–45.)
The bulbourethral artery is a short artery or arteries of relatively large caliber that pierce the Buck fascia to enter the bulbospongiosus. These arteries are oriented almost parallel to the path of the membranous urethra.
The dorsal artery generally travels along the dorsum of the penis between the deep dorsal vein medially and the dorsal nerves laterally, with a coiled rather than a straight configuration. The artery uncoils as the penis elongates with erection, allowing flow to be maintained. Along its course, it gives off 3 to 10 circumflex branches (the circumflex cavernosal arteries) that accompany the circumflex veins around the lateral surface of the corpora cavernosa, and that provide vascularity to the corpus spongiosum. Its terminal branches arborize in the glans penis. In many patients, branches penetrate the tunica and connect to the cavernosal arteries. The functional significance of these perforators varies from individual to individual.
The cavernosal artery, usually a single artery, arises on each side as the terminal branch of the penile artery. It enters the corpus cavernosum at the hilum and runs the length of the penile shaft, splitting off the many helicine arteries that constitute the arterial portion of the erectile apparatus. The arteries frequently branch before entering the corporeal body. Sometimes a branch enters the opposite corpus cavernosum, and occasionally a single artery branches in the penile shaft to supply both sides.
Lymphatics
Lymph drainage from the glans penis collects in large trunks in the area of the frenulum. The lymph vessels circle to the dorsal aspect of the corona, where they unite with those from the other side. The vessels traverse the penis beneath Buck fascia, terminating mostly in the deep inguinal lymph nodes of the femoral triangle. Some drainage is to the presymphyseal lymph nodes, and by way of these to the lateral lymph nodes of the external iliac group.
Nerve Supply
The nerves of the penis are derived from the pudendal and cavernosal nerves. The pudendal nerves supply somatic motor and sensory innervation to the penis. The cavernosal nerves are a combination of the parasympathetic and visceral afferent fibers and constitute the autonomic nerves of the penis. These provide the nerve supply to the erectile apparatus.
The pudendal nerves enter the perineum with the internal pudendal vessels through the lesser sciatic notch at the posterior border of the ischiorectal fossa. They run in the fibrofascial pudendal Alcock canal to the edge of the urogenital diaphragm. Each dorsal nerve of the penis arises in the Alcock canal respectively as the first branch of the pudendal nerve. Traveling ventral to the main pudendal trunk above the internal obturator and under the levator ani, the dorsal nerves perforate the transverse perinei muscles to arrive on the dorsum of the penis and continue distally along the respective dorsolateral penile surface lateral to the dorsal artery. On the shaft, their fascicles fan out to supply proprioceptive and sensory nerve terminals in the tunica of the corpora cavernosa and sensory terminals in the skin. These nerves terminate in the glans penis.
Perineum
The perineum is the diamond-shaped outlet bounded anteriorly by the pubic arch and the arcuate ligaments of the pubis, posteriorly by the tip of the coccyx, and laterally by the inferior rami of the pubis and ischium. A transverse line between the ischial tuberosities divides the perineum into an anterior triangle containing the external urogenital organs and a posterior anal triangle (see Fig. 36–12A and B).
Colles Fascia
In the anterior triangle, Colles fascia (Fig. 36–13A) attaches at its posterior margin to the perineal body at the posteroinferior margin of the urogenital diaphragm. The fascia curves below the superficial transverse perinei muscles and projects forward as two layers attached laterally to the ischium and the inferior ramus of the pelvis. The loose superficial layer is fatty and is continuous with the more substantial dartos fascia (tuncia dartos) of the scrotum. In the scrotum, the dartos fascial layer contains muscle fibers that cause the rugous appearance of the scrotum. The fascia also projects (but without muscle fibers) into the midline, to form the septum between the halves of the scrotum. The median raphe in the skin delineates the separation of the halves of the scrotum and is continued anteriorly as a darkly colored streak in the ventral midline of the penis and posteriorly as the median raphe of the perineum terminating at the anus.
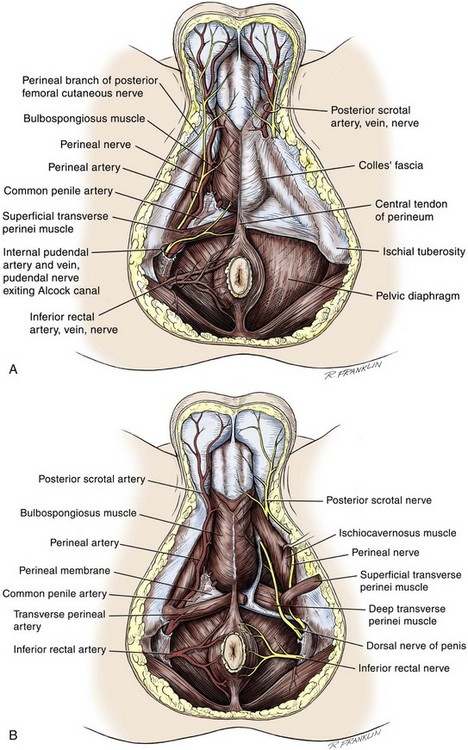
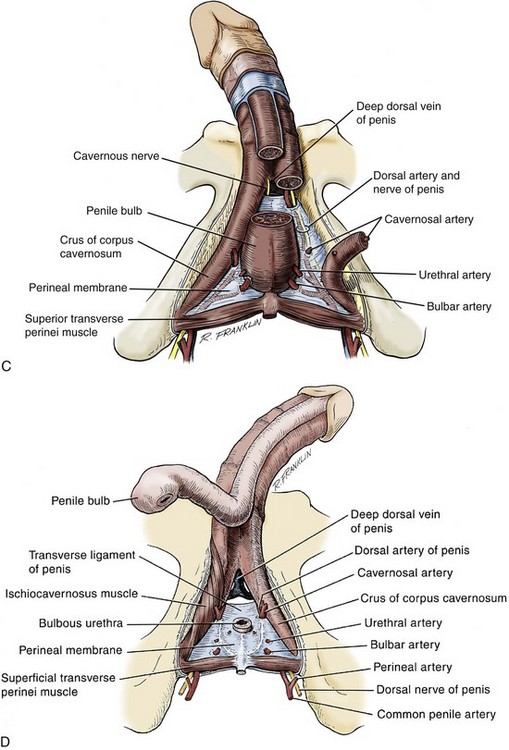
Figure 36–13 “Peel-away” diagrams of the anatomy of the perineum. A, The skin and subcuticular tissues have been removed. B, In the anterior perineal triangle, Colles fascia has been removed. In the posterior anal triangle, the pelvic diaphragm has been removed. Note the division of the superficial transverse perinei muscle, exposing the deep transverse perinei muscle. C, The anterior perineal triangle has been dissected to expose the erectile bodies. D, The corpus spongiosum has been divided at the departure of the urethra from the penile bulb. The intracrural space is exposed.
(A to D, From Devine Jr CJ, Angermeier KW. Anatomy of the pelvis and male perineum. AUA Update Series 1994;13:1015.)
The deep membranous layer of Colles fascia is a more substantial layer that forms a roof over the scrotal cavity, separating it from the superficial perineal pouch. At the anterior aspect of the scrotum, Colles fascia joins with the dartos fascia (tunica dartos) of the scrotum, and a fold of this fascia projects backward beneath the fibers of the midline fusion of the ischiocavernosus muscle (bulbospongiosus muscle). At the base of the penis, it is continuous with the dartos fascia of the penis. Thickenings of the fascia, at this level, form the two suspensory ligaments of the penis. First, the outer fundiform ligament, which is continuous with the lower end of the linea alba, splits into laminae that surround the body of the penis and unite beneath it. Second, the inner triangle-shaped suspensory ligament is attached to the anterior aspect of the symphysis pubis and blends with the dartos fascia of the penis below it.
Anteriorly, Colles fascia fuses and becomes continuous with the membranous layer of the subcutaneous connective tissue of the anterior abdominal wall (Scarpa fascia). Laterally, Colles fascia fuses to the pubic arch and with the fascia lata. Posteriorly, Colles fascia sweeps beneath the transverse perinei muscles, fusing with the posterior aspect of the perineal membrane. The space beneath the continuous plane formed by these fascial attachments is the superficial perineal pouch, in which infections or extravasation of urine and collections of blood (after trauma to the urethra) may be confined (see Fig. 36–9A to C).
Superficial Perineal Space
In males, the superficial perineal space contains the continuation of the corpora cavernosa, the proximal part of the corpus spongiosum and urethra, the muscles associated with them, and the branches of the internal pudendal vessels and pudendal nerves (Fig. 36–13B).
The ischiocavernous muscles cover the crura of the corpora cavernosa. They attach to the inner surfaces of the ischium and ischial tuberosities on each side and insert at the midline into Buck fascia, surrounding the crura at their junction below the arcuate ligament of the penis. The midline fusion of the ischiocavernosus muscles–bulbospongiosus muscles is in the midline of the perineum. They are attached to the perineal body posteriorly and to each other in the midline, as they encompass the bulbospongiosus and crura of the corpora cavernosa at the base of the penis. These muscles are confluent with the ischiocavernous muscles laterally and at their insertion into Buck fascia, covering the dorsal vessels and nerves at the base of the penis.
Central Perineal Tendon (Perineal Body)
Lying just anterior to the anus, as a part of the plane separating the anterior and posterior perineal triangles, the perineal body is formed by the interconnection of eight muscles of the perineum (see Fig. 36–13A and B). The perineal body receives fibers from the anterior portion of the anal sphincter and is the central point of insertion of the superficial transverse perinei muscles that arise at the ischial tuberosities. The bulbospongiosus muscle (midline fusion of the ischiocavernosus muscle) is fixed to the perineal body by its most posterior fibers. The deep transverse perinei muscles and fibers from the anterior portions of the levator ani muscles attach to the deep aspect of the perineal body.
Deep Perineal Space
The urogenital diaphragm constitutes the deep perineal space (see Fig. 36–13C and D). It is contained within two layers of fascia and incompletely covers the outlet of the pelvis anterior to the deep layer of the perineal body. The deep layer of fascia is an indistinct structure—the continuation of the endopelvic obturator fascia. The superficial fascia attaches laterally to the ischial rami and the inferior ramus of the pubis. This fascia blends with the deep layer behind the perineal body and anteriorly, where it terminates with a thickened edge, the transverse perineal ligament. A space between this ligament and the arcuate ligament of the pubis accommodates the deep dorsal vein of the penis.
The deep perineal pouch (see Fig. 36–13D) contains the deep transverse perinei muscles, the external sphincter of the urethra, the bulbourethral (Cowper) glands, and the blood vessels and nerves associated with the structures within it. The sphincter urethral muscle fibers arise from the medial surface of the inferior pubic rami and pass medially toward the urethra, where they meet the fibers from the opposite side. In males, the muscle encircles the membranous urethra to function as the somatic sphincter of the urethra (Haertsch, 1981).
Key Points: Anatomy of the Penis and Male Perineum
Generalities of Reconstructive Surgical Techniques
With any surgical procedure, there are basic rules and surgeons’ biases as to the best way to perform a certain operation. Certainly, this is true for reconstructive procedures of the external genitalia. In this section, the differences are highlighted.
Reconstructive surgery is performed with all efforts aimed at minimizing tissue injury and promoting healing. Adequate visualization is essential. Surgical loupes are used by almost all surgeons performing both adult and pediatric reconstructive genital surgery. A headlight or suction with attached light often adds to visualization, especially in deep perineal surgery. In penile cases such as reconstruction of the fossa navicularis or correction of penile curvature, bipolar cautery is used exclusively. With cautery, the electric charge is grounded either to a pad (monopolar) or to the opposite tong of the forceps (bipolar). It is easy to see that in most instances, the field effects of the electricity are more confined with bipolar cautery. Because electricity is dissipated by conductors (in the case of human tissue, vessels and nerves), there is a possibility of damage to these delicate structures. In other cases, monopolar cautery can be used in the superficial structures, but bipolar cautery is better during dissection around the corpus spongiosum, elevation of penile and scrotal flaps, division of the perineal intracorporeal space, and dissection of the dorsal neurovascular structures.
Appropriate instruments for genitourinary reconstructive surgery can commonly be found in a plastic surgery tray or on the peripheral vascular tray in the typical operating room. Some examples are fine tenotomy scissors, fine forceps, a variety of skin hooks, and delicate needle holders. Sharp scissors that cut with minimal collateral trauma are essential. These instruments minimize tissue injury from manipulation and permit more precise dissection. For urethral surgery, a set of bougie à boule sizers is essential to check the caliber of the urethral lumen. McCrea urethral sounds are a nice addition to the typical van Buren sounds available in the usual operating room. For calibration, sounds do not replace the need for bougie à boule calibrators. For posterior urethral reconstruction, a sound to pass through the cystostomy tract and prostate to find the proximal end for the reconstruction is often helpful. We find that a Haygrove staff serves this role nicely. Some centers use the cystoscope for this purpose, and often it suffices well, whereas at other times it is not as effective as the Haygrove-style sound. The choice of suture material clearly evolves on the basis of the surgeon’s experience and bias. However, there are some common principles with which most surgeons would agree. First, in urethral surgery, absorbable suture is the rule. Typical choices for most surgeons are braided absorbable sutures or the family of monofilament absorbable sutures. Chromic suture is rarely used now because the choices of other absorbable sutures seem superior in virtually all cases. Thus, in the case of tension-free closures, very small sutures can be used. In some cases, tying the suture can be awkward, and thus a larger suture may be warranted, even though the anastomosis is tension free. The caliber of suture should be the smallest possible to line up the tissue, which is typically not under tension. There is no reason to use suture that is stronger than the tissues that are being sutured. Fine suture such as 5-0 and 6-0 chromic or polyglactin can be used to suture the epithelium to the adventitia of the corpus spongiosum to control bleeding. For a flap or graft repair, 4-0 to 6-0 suture is usually adequate. For primary anastomosis of the corpus spongiosum or for a posterior urethral reconstruction, 3-0 suture may be appropriate because of tying concerns. The needle should be tapered if possible except when, as in urethroplasty, for example, severe spongiofibrosis or scarring is present. Some of the typical choices are taper needles, such as RB-1, TF, and SH-1, and cutting needles, such as P-3 and PC-3. The UR-6 half-circle taper needle that is often used in radical prostatectomy can be helpful for deep perineal anastomosis of the urethra.
Surgical position and retraction are critical to attaining good results. If possible, procedures are done with the patient supine or prone. Many procedures that previously were done with the patient in the lithotomy position can be done with the patient in the frog-leg or split-leg position. For penile surgery, a Scott retractor with stay hooks,* the Jordan-Bookwalter perineal retractor set,† or the Omni-Tract perineal retractor‡ are helpful. Lithotomy or exaggerated lithotomy positions are used only for the minimal time necessary. With appropriate padding for the foot and positioning without pressure on the back of the leg, the complications in the low lithotomy position are minimal. When the patient is in the supine, split-leg, and low lithotomy positions, venous compression stockings can be used. The controversy in positioning revolves around the use of the exaggerated lithotomy position. It is our preference to use this position for all bulbar and posterior urethral reconstructions. Other surgeons use a lower lithotomy position. We find the more exaggerated position to be safe and believe that it provides unequaled access to the deep perineal structures (Angermeier and Jordan, 1994). Details of positioning, as we do it, are covered later. To minimize the patient’s time in the exaggerated position, all graft harvesting or flap elevation is done with the patient in the flat supine position.
In addition to proper diagnosis and planning, the surgical technique is important for the overall success of reconstructive surgery. Unlike the results of extirpative surgery, the results of reconstructive surgery depend on methods that minimize tissue damage and maximize wound healing. The key ingredients are adequate visualization, appropriate choice of suture, delicate tissue handling, appropriate positioning, and adequate retraction.
Key Points: Reconstructive Surgical Techniques
Selected Processes
Urethral Hemangioma
Although urethral hemangioma is a rare condition, it is usually persistent and offers a challenge to the surgeon when excision is deemed necessary. Patients typically present with hematuria or a bloody urethral discharge and, occasionally, with obstructive symptoms. The lesions may be either single or multiple, and the urethral meatus is a common location. Although diagnosis is often made at cystoscopy, which readily visualizes the dilated blood vessels, the lesion often extends beyond the point at which it is seen with cystoscopy.
Because all reported cases of urethral hemangioma have been benign, management depends on the size and location of the lesion. Asymptomatic lesions do not require treatment and should be observed, because hemangiomas can regress spontaneously. Symptomatic lesions that require treatment must be completely excised to prevent recurrence.
Although electrofulguration has been reported as a possible treatment of urethral hemangioma, it should be used only to control an acute episode. For smaller lesions, laser treatment has been successful and produces less scarring. Lasers that are used for this purpose include argon, potassium titanyl phosphate (KTP) (532 nm), and neodymium:yttrium-aluminum-garnet (Nd:YAG). The preferred treatment of larger lesions is open excision and urethral reconstruction. This, in some cases, means circumferential reconstruction. It is clear that tubed graft reconstruction should be avoided; tubed flap reconstruction or tubed construction with mixed tissue transfer could be considered, although staged reconstruction is probably preferable. In addition, good initial success has been reported with polidocanol as a sclerosing agent for extensive urethral hemangiomas.
Reiter Syndrome
Reiter syndrome is characterized by a classic triad of arthritis, conjunctivitis, and urethritis. In addition, some patients have had an episode of diarrhea that preceded the development of arthritis. In most cases, however, the classic triad is not present, and patients present with only arthritis affecting the knees, ankles, and feet in an asymmetrical distribution. The history of urethritis is obtained on detailed questioning.
Urethral involvement is usually mild, self-limited, and a minor portion of the disease. In approximately 10% to 20% of patients, a glanular lesion is present. Referred to as circinate balanitis, this lesion is diagnostic of Reiter syndrome and typically appears as a shallow, painless ulcer with gray borders. On occasion, the lesion appears as small, red macules, 1 to 2 mm in diameter. When the urethritis is mild and self-limited, no treatment is necessary.
In rare cases, urethritis causes severe inflammation with necrosis of the mucosa, producing uncompromising stricture disease. We have not been successful in excision and replacement of the urethra in these cases. Alternatively, we perform a perineal urethrostomy and excise the entire distal urethra. This approach may decrease the rheumatic manifestations associated with Reiter syndrome.
Lichen Sclerosus (Balanitis Xerotica Obliterans)
Lichen sclerosus (LS) is the preferred term for what was previously known as balanitis xerotica obliterans (BXO). Lichen sclerosus is a chronic inflammatory disorder of the skin of uknown origin. No specific mechanism of disease has been elucidated. There are several acquired scarring disorders of the skin associated with pathology of the basement membrane, such as mucous membrane pemphigoid, that may be shown as related (Bernard et al, 1992; Akporiaye et al, 1997). The reported incidence of LS in the western population is 1 in 300; however, the worldwide prevalence may be substantially different (Wallace, 1971; Dogliotti et al, 1974; Jacyk and Isaac, 1979; Datta et al, 1993). The peak ages of recognition in women are bimodal, with many cases noted before puberty but with another peak presenting in postmenopausal women (Tasker and Wojnarowska, 2003). In men, LS seems to peak between the ages of 30 to 50; however, incidences of LS have been described in people of all ages, from infants to the elderly (Tasker and Wojnarowska, 2003). LS is commonly found at the time of circumcision when performed beyond the neonatal period (McKay et al, 1975; Rickwood et al, 1980; Garat et al, 1986; Ledwig and Weigland, 1989; Meuli et al, 1994). The most common cause of meatal stenosis, LS appears as a whitish plaque that may involve the prepuce, glans penis, urethral meatus, and fossa navicularis. If only the foreskin is involved, circumcision may be curative (Akporiaye et al, 1997). In the authors’ experience, LS usually begins as a meatal or perimeatal process in the circumcised patient, but it may involve other areas of the preputial space in the uncircumcised patient. In uncircumcised men, the prepuce becomes edematous and thickened, and often may be adherent to the glans (Bainbridge et al, 1971). Diagnosis is made through biopsy. Several reports have suggested the association with chronic infection by a spirochete, Borrelia burgdorferi (Tuffanelli, 1987; Dillon and Ghassan, 1995; Shelley et al, 1999).
The term BXO was first applied by Stühmer in 1928. Freeman and Laymon showed that BXO and LS were probably the same process (Freeman and Laymon, 1941; Laymon and Freeman, 1944). The first report of what was probably LS was published by Weir in 1875. He described a case of vulvar and oral “ichthyosis” (Weir, 1875). In 1976, the International Society for the Study of Vulvar Disease unified the nomenclature devising a new classification system and proposed the term lichen sclerosus (Friedrich, 1976). As mentioned previously, the cause of LS has not been defined. A number of mechanisms have been proposed. Koebner phenomenon relates the development of LS to trauma to an affected area (Lee and Phillips, 1994). The etiology has also been suggested to be an autoimmune disease (Goolamali et al, 1974; Marren et al, 1995). Sander hypothesized that reactive oxidative stress contributed to the sclerotic, immunologic, and carcinogenic process in LS (Sander et al, 2004). As mentioned, the infectious cause has also been implicated (Tuffanelli, 1987; Ross et al, 1990). That has been called into question because the organism has not been uniformly demonstrated in the lesions. It has also been proposed that LS has a genetic origin, based on the observation of a familial distribution of cases (Marren et al, 1995). There have been reports of concomitant existence of the disease in identical twins (Fallic et al, 1997; Thomas and Kennedy, 1986) and nonidentical twins (Cox et al, 1986), with coexistence of dermatosis. The disease has also been seen in mothers and daughters (Shirer and Ray, 1987). Studies on the human leukocyte antigen (HLA) have suggested a genetic component in patients afflicted with LS (Marren et al, 1995). The combination of topical steroids and antibiotics may help stabilize the inflammatory process. Conservative therapy may be warranted in patients whose meatus can easily be maintained at 14 to 16 French (Staff, 1970). In these cases, intermittent catheterization with lubrication of the catheter and meatal dilator with 0.05% clobetasol (brand name Temovate) may be adequate treatment. Long-term antibiotic therapy may also be helpful to improve the inflammation, because secondary infection of the inflamed tissue may occur. We have typically used tetracycline, but a trial of long-term penicillin or advanced-generation erythromycin therapy may be warranted (Shelley et al, 1999). This nonsurgical approach to treatment is used in patients who are not good surgical candidates for other medical reasons or in older patients, and in younger patients who demonstrate stable disease. Secrest has proposed a link between hypogonadism and LS in male patients. He has consistently shown diminished testosterone levels in patients afflicted with LS and has a study underway analyzing whether replacement androgen therapy may be helpful (Secrest et al, 2008).
In young patients with severe meatal stenosis, surgery is indicated. Because patients with long-standing meatal stenosis often have severe proximal urethral stricture disease, retrograde urethrography should be performed before the initiation of therapy. The etiology of stricture disease associated with LS is unclear. Possible causes include iatrogenic stricture resulting from repeated instrumentation and pressure voiding associated with meatal stenosis causing secondary intravasation of urine into the glans Littre (Fig. 36–14). In cases of early LS with only meatal involvement resulting in stenosis of the fossa navicularis, prompt reconstruction seems to be successful in the long term and seems to avoid the sequelae of panurethral stricture disease. Most surgeons now believe that because LS is a disease of genital skin, better tissue for reconstruction is the oral mucosa, and techniques are discussed later (Mundy, 1994; Bracka, 1999). Long-standing cases with a long length of urethral stricture are amenable to techniques of reconstruction but are very challenging. It is becoming clear that except in the case of urethral stricture disease confined only to the meatus and fossa navicularis, staged oral graft reconstruction, at least in the short to mid term, seems to provide superior durable results. This may also be the case in cases confined to the meatus and fossa navicularis, because a recent analysis of patients reconstructed with the ventral transverse skin island technique showed a 50% recurrence rate even in those patients. The weakness of that analysis, is that the data did not include biopsy proof that all patients had LS (Virasoro et al, 2007). We also are seeing more and more patients who present with a buried penis. This phenomenon occurs when the skin of the penile shaft has been lost because of severe inflammation, and the penis is trapped in the penopubic and scrotal area. These patients are often profoundly overweight, and many are diabetic; they have often had prior surgical procedures. Their management is complex and ultimately determined by their desire and need for functional reconstruction. In some patients with severe urethral stricture disease, we have completely reconstructed the urethra; in others, we have simply performed a perineal urethrostomy. Perineal urethrostomy is usually technically straightforward, because the rule in most patients with lichen sclerosus is to spare the proximal anterior urethra. We have proposed that, in many cases, the sparing of the proximal anterior urethra demonstrates the distribution of the glands of Littre for a given patient. Younger patients have requested mobilization and release of the penis with placement of a split-thickness skin graft. However, because the inflammation involves the glans penis (which is not removed), the secondary inflammation may also involve the skin graft. Therefore lifelong monitoring of these patients for the secondary effects of inflammation is necessary.
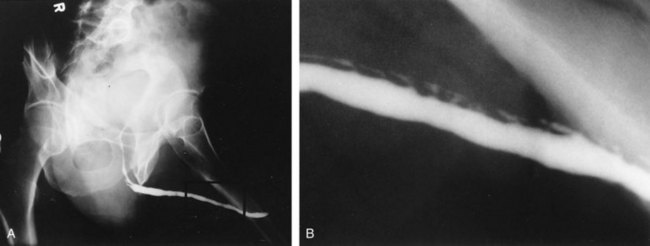
Figure 36–14 A and B, Urethrogram in a patient with urethral stricture disease associated with lichen sclerosus–balanitis xerotica obliterans. It illustrates the intravasation of contrast material into the dilated glands of Littre during voiding.
(From Jordan GH. Management of membranous urethral strictures via the perineal approach. In: McAninch J, Carroll P, Jordan GH, editors. Traumatic and reconstructive urology. Philadelphia: WB Saunders; 1996.)
Several reports have suggested the development of squamous cell carcinoma in patients with a long history of lichen sclerosus (Doré et al, 1990; Pride et al, 1993).
Amyloidosis
Although a rare disease, amyloidosis of the urethra should be considered in the evaluation of any patient with a urethral mass. Patients may present with hematuria, dysuria, or urethral obstruction. Because the differential diagnosis includes urethral neoplasm, cystoscopy with transurethral biopsy is indicated. Once the diagnosis is made, treatment should be based only on symptoms. Most patients can be observed expectantly and not require aggressive treatment. Some will require treatment for urethral stricture. Progression and recurrence are rare (Walzer et al, 1983; Dounis et al, 1985; Crook et al, 2002).
Urethrocutaneous Fistula
A urethrocutaneous fistula is a tract lined with epithelium that leads from the urethra to the skin. The size of a fistula can vary from pinpoint to large. Urethral fistulas may be a complication of urethral surgery or develop secondary to periurethral infection associated with inflammatory strictures or treatment of a urethral growth (condyloma or papillary tumor). Treatment of a urethral fistula must be directed not only to the defect but also to the underlying process that led to its development. Treatment therefore varies according to the cause of the fistula. In cases of urethral reconstruction, especially reconstruction for hypospadias, fistula often occurs or recurs because of distal obstruction and high-pressure voiding. Additionally, in some cases where multiple attempts at fistula closure have been attempted and failed, the condition of the tissue adjacent to the fistula are so scarred that staged reconstruction is needed to import “better tissue.”
After urethral surgery, fistulas can develop immediately or as delayed complications. An early fistula is the result of poor local healing, possibly secondary to hematoma, infection, or tension with closure. In addition, a mere breakdown of the urethral or overlying skin closure, or both, could occur. Very occasionally, with aggressive local care and continued urinary diversion, the fistula closes spontaneously.
Several techniques are used for fistula closure. Endoscopic and radiographic evaluation of the urethra must be performed before the repair in all cases. If the fistula is small and closure of the hole will not decrease the lumen of the urethra, a button of skin is removed from around the fistula, and its edges are cut flush with the urethral wall. The urethra is closed with small (6-0 or 7-0) absorbable suture, inverting the epithelial edge, and the repair is tested to ensure that it is watertight. The authors prefer either polyglycolic acid (Vicryl) or polydioxanone suture. Subsequent layers are designed and closed to avoid superimposed suture lines. Without question, the safest diversion is a suprapubic catheter. However, in many cases, a silicone stent that reduces pressure during voiding for 7 to 14 days suffices. The operating microscope can be useful for the closure of small fistulas, allowing the use of 8-0 polyglycolic acid suture and limiting the size of the associated skin incision.
If the fistula is so large that simple closure will compromise the lumen of the urethra, then often local flaps will be required. However, if the adjacent tissues are thin and poorly visualized, then closure of the fistula may become a staged urethral reconstruction as mentioned above. For larger fistulas, a suprapubic tube for diversion is probably prudent. The mobilization of flaps, such as the tunica dartos flap, may be necessary to secure adequate tissue interposition and avoidance of superimposed suture lines.
Fistulas associated with inflammatory strictures occur as periurethral tracts and develop secondary to high-pressure voiding of infected urine. As multiple tracts develop, this problem becomes what is known as a “watering pot perineum.” Repair requires suprapubic drainage, and treatment of the infection requires incision and drainage of any abscesses present. We widely excise the fistula tracts and associated inflammatory tissue and wait 4 to 6 months before repairing the underlying stricture. Flap reconstruction, if donor tissues are available, may be used. However, a staged graft procedure (discussed later) is also an excellent choice. One must be cautious in the patient with urethral fistulas but without a chronic history of obstructive voiding symptoms. In many cases, fistula or periurethral abscess may be the hallmark symptom of urethral carcinoma.
Urethral Diverticulum
A congenital diverticulum is a transitional cell epithelium-lined pouch that is the result of either a distention of a segment of the urethra or the attachment of a structure to the urethra by a narrow neck (i.e., a müllerian remnant). In males, a congenital anterior urethral diverticulum may result from incomplete development of the urethra, with a defect in only the ventral wall and subsequent distention of this segment by the hydraulic force of the voiding stream (Valdivia et al, 1986; Bedos and Cibert, 1989; Ozgok et al, 1994). Furthermore, the downstream lip of the defect may serve as a valvular obstruction, increasing the pressure in the lumen and subsequently the diverticulum enlarges. Another possible etiology is injury of the urethra that may cause an intraspongiosal hematoma. This could create a paraurethral space and subsequent diverticulum or defects (fistula or diverticulum). These can also be associated with urethral strictures (Bryden and Gough, 1999). It has also been suggested that congenital diverticula may represent giant cystic dilation of Cowper ducts (Gil-Vernet, 1977; Jiminez Cruz and Rioja Sanz, 1993). We do not favor this proposed suggested etiology because the diverticula seem to be slightly more distal than the expected location of Cowper ducts, and in our experience with reconstruction of a considerable number of these diverticula, no proximal limb of the ducts seems to exist in them. In many cases, endoscopic unroofing of the diverticulum remedies the voiding symptoms; although, after unroofing it is not uncommon for the patient to note postvoid dribbling. Open repair essentially excises the redundancy of the urethra associated with the diverticulum. If the lumen is comprised, then dorsal onlay by either graft or flap can be useful.
A congenital diverticulum in the prostatic urethra may be a large remnant of the müllerian duct associated with defects of diminished virilization. However, it often occurs in proximal hypospadias and represents an enlarged utricle (Devine et al, 1980). These diverticula may not be demonstrated with voiding urethrography but are demonstrated with cystoscopy or retrograde urethrography. The tip of a urethral catheter tends to catch in this opening, necessitating the use of something to direct the catheter tip toward the true lumen. Other than necessitating caution during evaluation, they do not usually cause problems or require treatment unless they are very large.
Large utricles can accumulate urine with voiding and then decompress after voiding. If they are large enough, the stasis of urine can be associated with recurrent urinary tract infection or difficult-to-manage “incontinence.” A surgical approach to small lesions can be through a suprapubic incision, possibly opening the bladder to go through the center of the trigone. However, large diverticula can be approached trans-sacrally (Peña and Devries, 1982). Although this is a complex procedure, it seems to be associated with much less morbidity than an abdominal or a perineal approach and provides superior exposure. We excise the diverticulum after exposing and dissecting its communication with the urethra. After ensuring that there is no distal obstruction to interfere with healing, we close the urethra.
Diverticula of the female urethra are covered in Chapter 78.
Paraphimosis, Balanitis, and Phimosis
Paraphimosis, or painful swelling of the foreskin distal to a phimotic ring, occurs if the foreskin remains retracted for a prolonged time. Swelling is sufficient to make reduction of the foreskin over the glans difficult. In the very young child, paraphimosis is often seen after the foreskin has been traumatically reduced during an examination, or sometimes by overzealous parental attempts at hygiene. It serves to say that traumatic, sudden reduction of a tight foreskin should be avoided in all ages and circumstances. To reduce a paraphimosis, gentle steady pressure must be applied to the foreskin to decrease the swelling. Especially with a child, this is best accomplished in a quiet room by a parent squeezing it in the hand. Elastic wrap may be helpful in some cases. Putting an ice pack on the area for a short time before gentle compression helps, not with the swelling but as an analgesic. When the swelling has been reduced, the surgeon can push against the glans with the thumbs, pulling on the foreskin with the fingers. Because paraphimosis tends to recur, a dorsal slit at a minimum or a circumcision should be carried out as an elective procedure at a later date. An occasional patient presents with acute paraphimosis that has been present for many hours to days. This is typically seen in an adolescent who is reluctant to reveal the problem to his parents. In these cases, reduction may be impossible and should be dealt with by emergency dorsal slit or circumcision. Considerable postoperative edema is the rule in these cases.
Balanitis, or inflammation of the glans, can occur as a result of poor hygiene, from failure to retract and clean under the foreskin. The subsequent swelling makes cleaning more difficult, but the inflammation usually responds to local care and antibiotic ointment. Oral antibiotic therapy may occasionally be necessary. Balanoposthitis is a severe balanitis and occurs when the phimotic band is tight enough to retain inflammatory secretions, creating what amounts to a preputial cavity abscess. On occasion, an emergent dorsal slit is required.
Phimosis, or the inability to retract the foreskin, can result from repeated episodes of balanitis. In older patients, balanitis may be a presenting sign of diabetes. In these cases, circumcision may be warranted.
Urethral Meatal Stenosis
A small urethral meatus in the newborn will probably not be called to a urologist’s attention unless the stenosis is associated with other congenital deformities (e.g., hypospadias) or causes voiding difficulties or urinary tract infection (Allen and Summers, 1974). If the urethral meatus of a boy appears exceptionally narrow and there are associated symptoms, a meatotomy should be considered. For this decision to be made, voiding should be observed to note that the meatus opens as a full, forceful stream is passed. If the stream is narrow and excessively forceful, stenosis is probably present. The occluding skin is generally a thin layer that can sometimes be seen to pouch out, with the meatus opening at the dorsal lip as the child voids. Meatal stenosis in a boy appears to be a consequence of circumcision that then allows subsequent ammoniacal meatitis. If the child is seen with ammoniacal meatitis, we usually start meatal dilation with 0.05% clobetasol cream. Within a week, the process seems to settle down. Anecdotally, the fusion of the ventral-meatal skin that causes meatal stenosis can be avoided. Parents must be counseled about the cause, that is, a wet diaper pressing for prolonged periods against the tip of the glans.
A ventral urethral meatotomy can at times be accomplished with the use of local anesthesia. In the young child, general anesthesia is the preferred approach, avoiding trauma to the child, the parents, and the urologist. It is important to insert the anesthetic needle into the skin fold from the underside, so that the tip of the needle can be observed and controlled. If insertion is done from the outside, the needle will pass through both layers of the fold, and a wheal cannot be raised because of leakage of the anesthetic solution. After the meatotomy, the edges of the cut will seal together unless they are kept open. The tip of a meatal dilator is the best instrument for this purpose. The child’s parents are instructed to gently separate the edges with the tip of the dilator three times a day for 7 to 10 days. The surgeon should observe the parents carry out this procedure. Pediatric meatal dilators (see the later product reference) are available; however, the tip of an ophthalmic antibiotic tube also works well, and the antibiotic ointment can be used as the lubricant.
Meatal stenosis occurs in adults after inflammation, specific or nonspecific urethral infection, and trauma (especially in association with indwelling catheters, urethral instrumentation, or radical prostatectomy in some cases). It may also be the result of the failure of a previous hypospadias repair. To perform a ventral meatotomy in a normally developed penis in adolescents and adults, it is often necessary to place sutures to approximate the urethral mucosal edge to control bleeding. This step usually requires three sutures: one at the apex and one on either side. We have found a dilator made by Cook Urological* to be helpful in keeping the meatus open. In some cases, it may be necessary to perform a dorsal rather than a ventral meatotomy. This can be accomplished as a Y-V-plasty after the excision of any scarred ridge of neourethra. Dorsal meatotomy, although effective in opening the meatus, often creates a cosmetically less-than-optimal shape of the meatus. In the adult, it is unusual for the meatal stenosis to be an isolated finding. The stricture process usually involves the fossa navicularis to some extent as well.
Circumcision
Controversy continues about whether neonatal circumcision should or should not be performed (Poland, 1990; Schoen, 1990). Much attention has been focused on this issue, but despite this, many little boys in the United States are circumcised. Ritual circumcision will continue; however, in ritual circumcision, it is not necessary to remove the skin but only to draw blood. It is important not to circumcise any boy with a penile abnormality (e.g., hypospadias, chordee) that may require the foreskin during repair. An indication for circumcision in the young boy presents when the child has had recurrent urinary tract infections thought to be associated with the redundant preputial skin.
Most circumcisions performed just after birth are done with the Gomco clamp or one of the plastic disposable devices made for this purpose. Care should be taken to free the foreskin from the glans completely and to apply appropriate tension when the foreskin is pulled into the clamp. To prevent either a too generous or an inadequate circumcision, we find it useful to carefully mark the foreskin so that the correct level is ascertained. At this center, we do neonatal circumcision with a penile block for anesthesia.
The most common complication is bleeding due to inadequate control with vascular compression. Application of an epinephrine-soaked sponge may help in controlling a minimal ooze. Infection can also occur and responds to local care. Any resulting skin separation should be repaired after the inflammation resolves. Sometimes, too much skin is removed, or the urethra is included in the clamp, resulting in a fistula. In many if not most cases in which excess skin is removed, closure can still be accomplished with aggressive frenuloplasty along with remaining skin closure by transposition of the remaining skin. If the entire penis is “scalped,” it may be best managed with a split-thickness skin graft or with reapplication of the excised foreskin, after it is prepared properly as a graft. In complicated cases, burying the penis in the scrotum and repairing it at a later date may be prudent. Monopolar electrocautery should be avoided in a neonatal circumcision, because penile loss from the field distribution of the current can occur. The use of monopolar cautery with a Gomco or similar clamping device must be avoided, because devastating loss of tissue can occur.
At present, whether a newborn who has lost his penis because of a circumcision mishap should be gender reassigned is under review by the North American Task Force on Intersexuality (Oesterling et al, 1987; Gearhart and Rock, 1989). Our experience with phallic construction now includes a number of children and youths who had been converted to a female after a circumcision accident. As they passed through puberty, they realized that this sexual assignment was wrong. We believe that with the present knowledge of reconstructive techniques, the matter is not clear, one way or the other. However, most of these boys could undergo reconstruction in such a manner as to preserve reproductive function.
In adults, circumcision can be done with local anesthesia, by blocking the dorsal nerves at the base of the penis and circumferentially infiltrating the superficial layers of the penile base. In men and older boys, we favor a sleeve circumcision. With the foreskin in its retracted position, a marking pen outlines an incision, leaving a small preputial cuff. This mark should go straight across the base of the frenulum. This incision is made and carried through the dartos fascia to the superficial lamina of the Buck fascia. The foreskin is reduced, and a second incision is marked, following the outlines of the coronal margin and the V of the frenulum on the ventral side. The frenulum usually retracts into a V. In some cases, the frenulum can be lengthened by closing the edges of the V in a longitudinal orientation for a short length (frenuloplasty). If frenuloplasty is done, the proximal incision does not need to follow the V of the retracted frenulum because the ventral skin is straight. We make the skin incision and fulgurate bleeding vessels with bipolar cautery as the incision is deepened and the skin edge mobilized. In older boys and adults, the vessels are more substantial and not easily sealed by compression, no matter how vigorous. Thus circumcision clamps can be ineffective and are not recommended even though larger sizes are available. After the sleeve of preputial skin has been removed, hemostasis is obtained and the skin edges are reapproximated.
In smaller boys, some may consider this sleeve procedure to be tedious and difficult. If this is the case, after the skin is marked, a dorsal slit is made through both layers of the prepuce back to the level of the corona. Following the marks, the two layers of the preputial skin are incised. Bleeders are controlled, and the skin edges are reapproximated.
Complications should be uncommon. Most patients develop some hyperesthesia of the glans, which resolves. A hematoma is probably the most common immediate complication. Some patients notice minor cosmetic imperfections that are functionally insignificant. One of the most distressing problems we see, however, is a patient who complains that the surgeon has removed too much skin. To avoid this unfortunate occurrence, a circumcision should be done precisely, and, whatever the procedure to be carried out, the incisions should first be marked with the skin lying undistorted on the shaft. Adults requesting circumcision must be carefully evaluated from a psychosexual standpoint because many of these patients, who are the most persistent in requesting circumcision, become the most dissatisfied after the surgery.
Circumcision has been shown now in numerous studies to provide protection for men in areas where HIV is very prevalent (Tobian et al, 2009).
Failed Hypospadias Repair
In treating a patient in whom hypospadias repair has failed, it is important to obtain all available records to help determine what may have contributed to his complications. A hypospadias repair may fail because of an inadequate correction of chordee or an inadequate urethra, with a stricture, fistula, or diverticulum (Winslow et al, 1986). Many times, it is readily apparent from the records that not all aspects of the hypospadias deformity (i.e., ventrally displaced meatus, ventral chordee, and some expression of inadequacy of ventral tissue fusion) were addressed in the previous repairs. Adults with urethral strictures are often seen who have had hypospadias surgery as children. Depending on the age of the patient and the preference of the treating urologist, a variety of different techniques may have been used to repair the original hypospadias. Many of these patients have persistent chordee and a subcoronal meatus. Adults have also been seen who have had long-standing evidence of urethral fistula. In addition, some patients may have clinical findings not related to hypospadias that should have been recognized previously, especially when hypospadias is part of an overlying intersex problem. In years past, problems associated with previous failures have been caused by errors in design, technique, or postoperative care (Devine et al, 1978). With more modern techniques available and with most hypospadias treated by surgeons with considerable experience, failures seem to be associated with perioperative infections or other factors that adversely affect wound healing. Complex hypospadias repair failures are currently encountered with much less frequency, and most that are encountered are in patients who had previous procedures more than 15 to 20 years ago. Truly, their complications resulted not from poorly designed surgery at the time but rather from the “state of the art” at the time.
Evaluation of a failed hypospadias repair includes retrograde urethrography, voiding cystourethrography, and cystoscopy. In an older patient, a reliable preoperative assessment of residual chordee can be made on the basis of the history and photographs taken at home. In younger patients, complete evaluation of more complex situations with use of anesthesia may be necessary.
In the adult patient, a detailed discussion must occur regarding the positive and negative aspects of the various approaches. Patients who were initially operated on before the late 1970s probably underwent either a graft or some form of repair using almost exclusively ventral tissue. Some of these patients still have the remnants of a dorsal hood or enough dorsal skin for a dorsal transverse penile skin island type of reconstruction to be performed.
We believe that surgical correction of complex cases requires an aggressive approach by the surgeon (Secrest et al, 1993). However, with the advent and very common usage of the tubed incised plate repair, initially described by Snodgrass (1999), the nature of failures is different and the approaches also remarkably different. It is the authors’ observation that the number of failed surgeries is less, the nature of graft salvage techniques is remarkably different, and the method of addressing residual curvature is also different. It is possible to reincise the “urethral plate” and tabularize it if the plate is not scarred, possible to graft the plate dorsally if it is, and, if the tissues are badly scarred, then the tendency to revert to staged reconstruction has now been adopted by many surgeons (Snodgrass et al, 2009). Certainly, the use of flaps has a place in corrective procedures, and the excision of scarred tissues causing residual curvature, likewise its place. However, the procedure of plication or corporoplasty techniques for correction of residual curvature has, for the most part, become the standard of care. Certainly graft techniques for correction of curvature are used, but with far less frequency than in years past.
Residual Genital Abnormality in Patients with Closed or Diverted Exstrophy
General
Residual genital defects in men who have had exstrophy repaired as children can cause functional, aesthetic, and psychologic problems. The effects of these problems are compounded in men who have undergone urinary diversion and who must wear stomal appliances, although with the improvement of continent diversions, this is less of a factor. Except in the most severe forms of bladder exstrophy or cloacal exstrophy—when the penis or the halves of the bifid penis are truly inadequate—successful reconstruction is possible. Even then, if normal testes are present, the success of newer techniques of phallic construction (see subsequent discussion) should lend support to considerating the option of raising such a child as a boy, possibly preserving his reproductive potential through puberty. In these very difficult cases, we think that the parents must be presented with both options, gender reassignment versus eventual phalloplasty. Remarkable progress has been made in the treatment of difficult cases (Johnston, 1975; Hendren, 1979; Jeffs, 1979; Snyder, 1990; Perovic et al, 1992; Gearhart et al, 1994; Mitchell and Bagli, 1996), as well as in techniques of primary closure. However, many patients need further genital surgery, because they experience the hypertrophic growth spurt of the penis associated with puberty.
The goals of reconstructive surgery in male patients with exstrophy or epispadias are to produce a dangling penis with erectile bodies of satisfactory length and shape to allow sexual function and to construct a urethra that serves as a conduit for the passage of urine and ejaculate. However, experience has shown that in the diverted exstrophy patient with only a bladder remnant, construction of a urethra that is essentially defunctionalized is difficult. These urethras all eventually seem to fibrose and stenose. The bladder neck remnant thus becomes a cyst that is often colonized. Bouts of virulent epididymitis or the formation of what is really a bladder neck remnant abscess begin to occur. We have now seen two patients who developed carcinoma of the prostate in a bladder neck remnant. The diagnosis in these patients was difficult and the resultant surgery even more difficult. Neither patient did well from the standpoint of treatment of the carcinoma. Both were seen before the aggressive use and better understanding of prostate-specific antigen.
Many patients who have undergone surgery as children do not present for correction of inadequacies of the external genitalia until after they have completed puberty and realize that their situation has not improved and is not likely to improve. Some have been in sexual situations and have encountered problems. We employ a systematic approach to accomplishing the reconstruction necessary to correct the anatomic defects in these patients (Devine et al, 1980; Winslow et al, 1988). Surgery is undertaken in a sequential fashion beginning with the simplest procedure that will achieve the desired functional result.
Lower abdominal wall scarring can be corrected or defects can be closed by fashioning peripenile flaps that are shaped like a W (see Fig. 36–19). In many patients, there may be wide diastasis recti that is really a ventral hernia. Anchoring of meshes or Gore-Tex can be difficult, and in several cases we have resorted to a fibular bone microvascular free transfer to reconstruct the continuity of the pubis, allowing effective closure of the abdominal hernia.
Surgical Technique
The adult reconstructive surgeon’s place, now, with the more effective primary closures, is in the correction of hernia as mentioned above or in the patient who has an inadequate penis either due to the deformity, scarring from multiple surgeries, or has had prior gender reassignment yet recognizes and chooses their male gender.
Key Points: Selected Processes
Trauma to the Genitalia
This topic is primarily covered in Chapter 88, but an additional description is also included in the electronic version of this chapter. Please see the Expert Consult website![]() for this section, as well as Fig. 36–15.
for this section, as well as Fig. 36–15.
Figure 36–15 Technique of microscopic replantation after amputation of the penis. A, The typical appearance of a penile amputation injury. B, The urethra, corpora cavernosa, and dorsal neurovascular structures are exposed and minimally debrided. C, A two-layer spatulated urethral anastomosis is completed. Microvascular coaptation of the dorsal vein, dorsal artery, and dorsal nerves is accomplished. D, Coverage is accomplished with the native skin. If the patient is circumcised, the sleeve of skin between the amputation injury and the old circumcision scar should not be discarded. Should chronic edema develop, revision can be accomplished at a later date. Note: Diversion is by way of a suprapubic cystostomy tube. The reconstructed urethra is stented.
(A to D, From Jordan GH, Gilbert DA. Management of amputation injuries of the male genitalia. Urol Clin North Am 1989;16:359–67.)
Penetrating Trauma to the Penis
Penetrating injuries of the penis can involve the urethra, the corporeal bodies, or both. Our choice in managing the acute injury is exploration and attempted immediate anatomic repair, perhaps placing a suprapubic tube should extensive urethral reconstruction be required. With bullet trauma, the velocity and the construction of the projectile are important factors. Small, “slow”-bullet injuries of the urethra can be successfully reconstructed primarily; however, larger high-velocity wounds may require diversion and delayed reconstruction. Some projectiles are designed to fragment and, even if “low velocity,” can cause considerable adjacent tissue damage. Later reconstruction is directed at urethral stricture (if it occurs after the initial injury), or there is curvature of the penis secondary to damage of the corporeal bodies, or both are present. Fistulas that result from penetrating trauma to the penis are usually treatable by primary closure with interposition of superficial tissue layers between the urethra and the skin. Large fistulas may require more complex tissue transfer and, in many ways, become more of a problem of urethral reconstruction as for stricture. The principles are discussed elsewhere in the chapter. Recent military actions, in which injury is due to blasts with shrapnel and high-velocity nonfragmenting projectiles, have redefined the thinking on mechanisms surrounding penetrating trauma. For example, it has been learned that high-velocity projectiles can truly penetrate peripheral structures with little cavitation effect, not the effect previously noted with wounds to the abdomen and chest.
Amputation of the Penis
Amputation is the ultimate penetrating penile injury. If the patient presents acutely with the amputated distal part of his penis, microvascular replantation is the favored approach (Fig. 36–15). If there is no microvascular surgeon at the center where the patient presents, he should be transferred. The amputated portion of the penis should be cleaned, wrapped in a sponge soaked in sterile saline, and placed in a sterile zipper-sealed plastic bag. This is kept in ice slush, and replantation can be accomplished as long as 18 to 24 hours after amputation. Often, the amputation is self-inflicted, usually during an acute psychotic break. This should not preclude replantation unless the patient adamantly refuses such treatment. Even then, with a court order or the agreement of two or more surgeons, replantation may be undertaken. Current legal opinion regarding a patient’s right of refusal is remarkably unhelpful in clarifying circumstances in which a patient refuses treatment but may not really be capable of true informed consent. Applying for a court order may be the safest method for obtaining consent. The patient’s condition or other circumstances may prevent his transfer for microvascular replantation. If so, replantation by the technique described by McRoberts and colleagues (1968) should be carried out. This and other series show that a high degree of success can be expected after replantation without microvascular reanastomosis (Chapple et al, 2004; Morey et al, 2004).
If the patient presents with the distal part having been disposed, or otherwise unavailable, the wound should be closed. The penis has often been stretched out during the amputation, and an excess of skin has been removed, leaving a length of intact but denuded shaft structures proximal to the amputation wound. We close the corporeal bodies with 4-0 or 5-0 polydioxanone suture, widely spatulate the urethral meatus, and immediately cover the penile shaft with a split-thickness skin graft. Others bury the shaft beneath the skin of the scrotum. In some of these patients, primary grafting of the stump allows a functional penis. However, many require phallic construction or penile reconstruction later.
A number of sophisticated techniques for reconstruction of the traumatized penis are available. The forearm flaps have become the mainstay of penile reconstructive procedures (see subsequent discussion). The initial stage of reconstruction of the amputated penis consists of mobilization of the penile and urethral stumps.
Degloving Injuries of the Penis
Degloving injuries occur when the skin of the penis or scrotum is trapped and stripped from the deeper structures, exposing the uninjured corpora cavernosa and the testes. The tear is deep to the elastic dartos fascia. Bleeding is usually not a problem, because there are not many large vessels in this space. The appearance of the “bare” testes and penile shaft, however, is impressive.
When the patient presents, the wounds should be dressed in sterile saline-soaked bandages. A delay of approximately 24 hours is sufficient to define the extent of the damage. Most degloving injuries can then be managed acutely with immediate reconstruction by the application of split-thickness skin grafts. The shaft is covered with a sheet graft of split-thickness skin. The testes are sutured together in the midline, fixed in their anatomically correct position, and covered with a meshed split-thickness skin graft. In the acute trauma situation, we have not used Integra to prepare the graft site. We have had good success with acute grafting. The parietal tunica vaginalis is opened, and the graft is placed directly on the testes. After take of this graft, the meshing gives the appearance of rugae. With time, the effect of gravity on the testes causes the reconstructed scrotum to become pendulous and sometimes even redundant. In this repair, split-thickness skin grafts are more successful than full-thickness skin grafts because the host bed is less than optimal after a degloving injury. Although split-thickness grafts cannot be employed for single-stage urethroplasty because of contraction, contraction has not been a problem with such grafts applied to the penile shaft or testes. Adequate shaft sensation is achieved by means of the deep structures beneath the graft. Should the testis be avulsed as part of the injury, replantation is usually not an option because the process of stretching of the vessels before breaking leads to an unpredictable intimal injury.
Some surgeons bury the shaft of the penis in a subcutaneous tunnel on the abdomen and bury the testes in subcutaneous thigh pouches. McDougal (1983) has described a technique to mobilize the buried testes with the overlying thigh skin, combining scrotal reconstruction with testicular replacement. In patients so managed, this is a good way of transposing the testes and overlying tissues to an anatomically correct location. When we have managed patients who have been previously treated acutely with the placement of the testes and penis in subcutaneous tunnels, we have mobilized the testes and the penile shaft from their tunnels and immediately applied grafts of split-thickness skin, as already discussed (Morey et al, 2004).
Genital Burns
The ability to reconstruct the damage caused by genital burns often depends on how well the normal structures have been maintained after the acute injury. Careful debridement is the rule in acute management of genital burns. Corporeal tissue cannot be replaced with transferred tissue. The physiologic functions of genital tissues cannot be accurately duplicated. The unique vascularity of genital tissue allows less aggressive rather than more aggressive debridement.
Devastating urethral injuries occur with many burns. Reconstruction of the urethra is dependent on the nature of the injuries. When the urethra has been nearly obliterated, there usually is not sufficient uninvolved, nonhirsute local genital tissue that can be transferred for urethral reconstruction. Vascularized tissue must be imported to support reconstruction of the urethra with graft techniques. In many patients, the penis has become incarcerated in contracted scar tissue after the acute injury is healed. Successful transposition of a gracilis musculocutaneous flap introduces compliant vascular tissue and skin into the area, allowing release of the penile shaft. Subsequently, the penile shaft can be covered with a split-thickness skin graft. In some patients, the genital scarring is so severe that microvascular transfer of a free flap is necessary to replace the penile shaft.
For many patients, reconstruction requires a number of stages. In several of our patients, the urethra has been obliterated literally from the entry of the membranous urethra into the bulbospongiosus to the tip of the penis. A perineal urethrostomy has been required while transfer of vascular tissues to the area of the perineum and penis was accomplished. When these tissues are in place, subsequent reconstruction of the urethra can be undertaken with meshed split-thickness skin grafts or buccal mucosal grafts. For coverage of large perineal or groin defects, the posterior thigh flap offers excellent bulky, sensate tissue.
Radiation Trauma
Radiation trauma to the penis occurs in two subsets of patients: patients in whom radiation has been used therapeutically for a lesion on the penis and those in whom radiation to the pelvis has caused chronic lymphedema. Therapeutic radiation can produce chronic suppurative gangrene. In most cases, these lesions are not amenable to reconstruction and are best managed by partial penectomy and later reconstruction, when the patient is proved to be cancer free. Also, we have treated several patients who developed tissue atrophy and further fibrosis after radiation therapy for Peyronie disease. Delivered at near-tumoricidal doses, this radiation made dermal graft repair much more difficult.
In patients who have had pelvic irradiation, the genitalia usually have one or more of the following: lymphedema, cellulitis, weeping of fluid, or lymphangiectasia. If cellulitis is part of the problem, prolonged treatment (i.e., for months) should be considered before reconstruction. We have, in fact, had several patients with recurrent cellulitis who had prolonged antibiotic therapy (we use ciprofloxacin); not only did their cellulitis become quiescent, but the lymphedema resolved significantly.
The genitalia of patients with lymphedema can be readily reconstructed. Lymphedema of the penis involves the tissues of the dartos fascia and the dermal layer of the skin. In the penis, the lymphedematous tissue can be excised by removing the dartos fascia and skin, dissecting in the layer immediately superficial to Buck fascia. In the scrotum, Colles fascia–tunica dartos and skin of the scrotum must be removed. When the lymphedematous tissue has been excised, the testes are free, and, as in a degloving injury, they must be fixed in the midline in an anatomically correct position. The scrotal skin peripheral to the edema is often normal and can be advanced to cover the testes. The shaft of the penis should be covered with a split-thickness skin graft. If the scrotum cannot be closed, a meshed split-thickness skin graft is used to cover the testes, as described previously. Grafts provide optimal reconstruction in these patients. Not uncommonly, these patients have hydroceles; the parietal tunica vaginalis must be excised, and grafting can be done directly onto the visceral tunica vaginalis of the testes. If there are hydroceles, the process often is “systemic” and not local. In these cases, reconstruction using the lateral scrotal skin is seldom effective. The use of Integra, previously mentioned, may have a place in these circumstances.
Unlike the full-thickness skin graft, split-thickness skin carries little of the reticular dermis and hence few of the lymphatic channels. Reaccumulation of lymphedema will occur within a full-thickness skin graft and can recur in a thick split-thickness graft. Local skin flaps should be avoided as previously mentioned. They often reaccumulate lymphedema once they have been transposed to the area of the genitalia. As previously noted, grafts do not develop sensation. However, good sensation usually develops, derived from the deep structures. The glans almost never accumulates disabling edema, and the sensation of the glans remains intact because the lymphedematous tissue has been excised in the plane superficial to Buck fascia, sparing the dorsal nerves of the penis. In many cases of genital lymphedema, the posterior scrotum and the lateral scrotal wall are spared from the edematous process; in those cases, the bulk of the scrotum is excised, and closure is accomplished with use of the posterior and lateral scrotum. If the edematous process involves the lower extremities also, it is best to reconstruct the scrotum with a graft as opposed to the local tissues.
Direct radiation to the penis can cause urethral injury. However, it is unusual for the urethra to be injured without damage to adjacent structures. Often, because of the vascularity of the corpus spongiosum, minimal debridement can be accomplished, leaving the patient with a fistula that can be reconstructed at a later date. The success of such reconstruction depends on the damage that the radiation has done to the adjacent structures.
Key Points: Trauma to the Genitalia
Urethral Stricture Disease
The term urethral stricture refers to anterior urethral disease, or a scarring process involving the spongy erectile tissue of the corpus spongiosum (spongiofibrosis) (Fig. 36–16). The spongy erectile tissue of the corpus spongiosum underlies the urethral epithelium, and, in some cases, the scarring process extends through the tissues of the corpus spongiosum and into adjacent tissues. Contraction of this scar reduces the urethral lumen. For example, if a normal urethra measures 30 French, its diameter is 10 mm and the area of the lumen is approximately 78 mm2. If scarring has resulted in a urethra that measures 15 French, the lumen is only 55 mm2, or 29% reduced. It is therefore evident that scar contraction caused by anterior urethral stricture disease can, for a while, be asymptomatic, but because the lumen is further reduced can be associated with marked voiding symptoms.
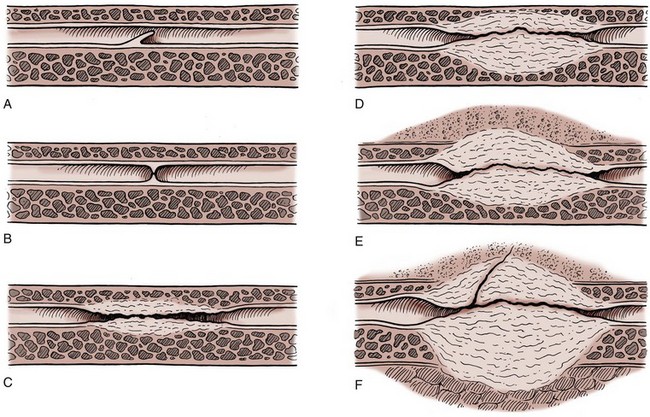
Figure 36–16 The anatomy of anterior urethral strictures includes, in most cases, underlying spongiofibrosis. A, Mucosal fold. B, Iris constriction. C, Full-thickness involvement with minimal fibrosis in the spongy tissue. D, Full-thickness spongiofibrosis. E, Inflammation and fibrosis involving tissues outside the corpus spongiosum. F, Complex stricture complicated by a fistula. This can proceed to the formation of an abscess, or the fistula may open to the skin or the rectum.
(A to F, From Jordan GH. Management of anterior urethral stricture disease. Probl Urol 1987;1:199–225.)
In contrast, posterior urethral “strictures” are not included in the common definition of urethral stricture. Posterior urethral stricture is an obliterative process in the posterior urethra that has resulted in fibrosis and is generally the effect of distraction in that area caused by either trauma or radical prostatectomy. Although the distraction defect can be lengthy in some cases, the actual process involving the tissues of the urethra is usually confined. By consensus of the World Health Organization conference, the term stricture is limited to the anterior urethra. Distraction defects are those processes of the membranous urethra associated with pelvic fracture. Other narrowings of the posterior urethra are termed urethral contractures or stenoses (Bhargava et al, 2004).
Urethral Anatomy
Although urethral anatomy is described in the earlier section on anatomy, it is useful to re-emphasize key anatomic points. The bulbous urethra is eccentrically placed in relation to the corpus spongiosum and is much closer to the dorsum of the penile structures (see Fig. 36–6). As one moves distally, the pendulous or penile urethra becomes more centrally placed within the corpus spongiosum.
The genital skin has a dual (proximal and distal) and bilateral blood supply, forming a fasciocutaneous system (see Fig. 36–10). The corpus spongiosum receives blood from the common penile artery, the terminal branch of the internal pudendal artery (see Fig. 36–12). The corpus spongiosum also has a dual blood supply—a proximal blood supply and a retrograde blood supply through the dorsal arteries as they arborize in the glans penis.
Etiology
Any process that injures the urethral epithelium or the underlying corpus spongiosum to the point that healing results in a scar can cause an anterior urethral stricture. Today, most urethral strictures are the result of trauma (usually straddle trauma). This trauma to the urethra often goes unrecognized until the patient presents with voiding symptoms resulting from the obstruction of the stricture or scar. In most straddle trauma, the injury to the bulbous urethra is readily reconstructable (Park and McAninch, 2004). Unfortunately, iatrogenic trauma to the urethra still exists, but with the development of small endoscopes and the limitation of indications for cystoscopy in boys, we see fewer iatrogenic strictures today than in the past. The place of idiopathic urethrorrhagia with regard to strictures in children is not clear; some question whether it may be a cause of strictures in young boys irrespective of the child’s having undergone an endoscopic procedure (Rourke et al, 2003). To date, no specific inciting factor has been identified as causing idiopathic urethrorrhagia. We appear to have histologic results from a patient with resolving urethrorrhagia that shows portions of tissues covered in part by squamous epithelium; other parts are covered by transitional epithelium; there are several areas of denuded epithelium with acute hemorrhage and neutrophilic infiltration; a few foci of microcalcification were shown; several mucus glands were found within the submucosal connective tissue and a few collections of amorphous material, likely mucin. These areas stain negatively, however, with a special stain for amyloid. There is no evidence of viral cytopathic effect or malignancy. Thus, what is not seen is evidence of bacterial infection, viral inclusions, and so on. We are, however, seeing an increase in strictures associated with lichen sclerosus, and those strictures clearly behave much more like inflammatory strictures than traumatically induced isolated scars. Finally, posterior urethral injuries, traumatic by definition, result in obliterative or near-obliterative defects that are associated with extensive fibrosis interposed between the distracted ends of the urethra.
On the other hand, inflammatory strictures associated with gonorrhea were the most commonly seen in the past and are less common now. With the advent of prompt and effective antibiotic treatment, gonococcal urethritis now progresses less often to gonococcal urethral strictures. The place of Chlamydia and Ureaplasma urealyticum (i.e., nonspecific urethritis) in the development of anterior urethral strictures is not clear. To date, no clear association between nonspecific urethritis and the development of anterior urethral stricture has been established.
There is, as mentioned earlier, a definite association between the development of an inflammatory stricture and lichen sclerosus–balanitis xerotica obliterans (LS-BXO). LS-BXO usually begins with inflammation of the glans and inevitably causes meatal stenosis, if not a true stricture of the fossa navicularis. The cause of this distal penile skin and urethral inflammation is not known. Literature suggests the possibility that bacterial infection is the cause of the resultant skin changes. There is some evidence to suggest that the progression of the stricture eventually to involve the anterior urethra extensively may be due to high-pressure voiding that causes intravasation of urine into the glands of Littre, inflammation of these glands, and, perhaps, microabscesses and deep spongiofibrosis. Whether the urethral changes and eventual fibrosis are also related to bacterial injury has not, to our knowledge, been well defined. Although the use of antibiotics seems, in these patients, to limit obstructive voiding symptoms, to our knowledge the literature does not show resolution of the stricture process with the use of antibiotics.
What is known as a congenital stricture is an entity that is difficult to understand. If, in embryologic development, a stricture is found at a natural place where a fusion of structures occurs (i.e., the posterior and anterior urethra), a congenital stricture might be a reasonable assumption. However, the term congenital stricture is used by some to define a stricture for which there is no identifiable cause. We propose, however, that it is reasonable to define a stricture as congenital only if it is not an inflammatory stricture, it is a short-length stricture, and it is not associated with a history of or potential for urethral trauma. This then limits the term congenital stricture to strictures of the anterior urethra found in infants before they attempt erect ambulation. So defined, congenital strictures will clearly be the rarest encountered.
Diagnosis and Evaluation
Patients who have urethral strictures most often present with obstructive voiding symptoms or urinary tract infections such as prostatitis and epididymitis. Some patients also present with urinary retention. However, on close inquiry, most of these patients are found to have tolerated notable voiding obstructive symptoms for a long time before progressing to complete obstruction.
Not uncommonly, when a patient cannot void, an attempt is made to pass a urethral catheter. If the catheter does not pass, the nature of the obstruction is determined by dynamic retrograde urethrography. Thus, most cases are managed with acute dilation, and clearly there are many instances in which this is not the best course for the patient. When there is doubt, we determine the nature of the stricture when possible, and selectively place a suprapubic cystostomy catheter to treat the acute situation and allow time for a more appropriate treatment plan to be devised. The practice of blind passage of filiforms and blind dilation without knowledge of the anatomy of the urethral stricture is condemned. Although detailed imaging is not always available, flexible endoscopy is almost universally available in the United States. At the least, the stricture can be visualized and attempted guide wire placement under direct vision is attempted.
For an appropriate treatment plan to be devised, it is important to determine the location, length, depth, and density of the stricture (spongiofibrosis). The length and location of the stricture can be determined with radiography, urethroscopy, and ultrasonography. The depth and density of the scar in the spongy tissue can be deduced from the physical examination, the appearance of the urethra in contrast-enhanced studies, and the amount of elasticity noted on urethroscopy. The depth and density of fibrosis are difficult to determine objectively. The absolute length of spongiofibrosis may not be evident on ultrasound evaluation. Ultrasound examination clearly can augment contrast-enhanced studies and is accurate in determining the length of narrow-caliber annularity (Morey and McAninch, 1996a). Contrast studies of the urethra are best carried out by or under the direct supervision of the surgeon responsible for treatment of the patient.
In 1979, McCallum and Colapinto described the use of dynamic radiographic studies and emphasized the need for these studies to be dynamic as opposed to static (Fig. 36–17) (McCallum and Colapinto, 1979a, 1979b). At our center, imaging includes dynamic studies that are accomplished during retrograde injection of contrast material and while the patient is voiding. Even with gentle technique, extravasation during retrograde urethrography is possible in patients in whom the urethra is markedly inflamed. For this reason, contrast studies should be carried out with contrast material that is suitable for intravenous injection and used either directly from the bottle or diluted according to the manufacturers’ guidelines. Contrast materials that have been thickened with lubricating jelly or anesthetic gels can be a source of problems, and offer little with regard to enhancement of radiographic studies, nor do they make the studies more comfortable. Real-time ultrasound evaluation of the urethra after it has been filled with a lubricating jelly or saline has been described by Morey and McAninch (1996a, 1996b). It is a misconception, however, that ultrasonography always directly visualizes the spongiofibrosis. However, Morey and McAninch (1996a, 1996b) believe that ultrasonography of the bulbous urethra possibly more accurately determines the length of the stricture, which could be important in considering an anastomotic repair. If the patient is not in steep lateral oblique position for retrograde urethrography, the length of the stricture will be underestimated. Finally, it should be understood that during contrast-enhanced urethrography, more than one projection may be necessary to visualize the stricture. Magnetic resonance is also being explored as an adjunct to the evaluation of urethral stricture, as well as pelvic fracture urethral injuries. In our experience, the use of magnetic resonance for routine strictures or pelvic fracture urethral distraction defects is not routinely beneficial. In the case of urethral tumors, we have found magnetic resonance to be invaluable. The experience of others is commensurate with ours (Pavlica et al, 2003). In the occasional pelvic fracture urethral distraction defect, the alignment of the two urethral ends can be nicely defined.
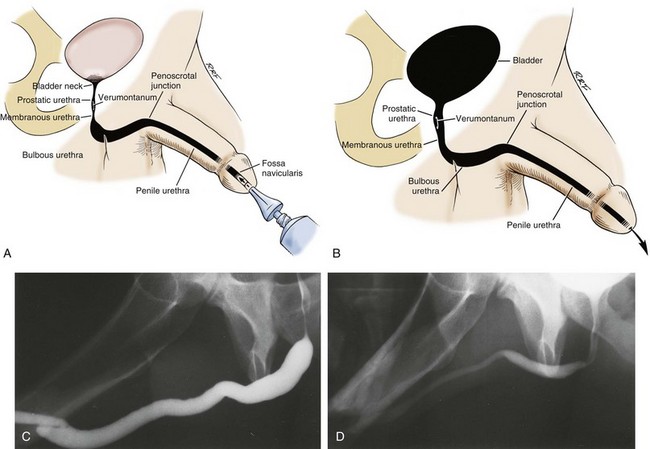
Figure 36–17 A, Representation of a dynamic retrograde urethrogram with the criteria of McCallum illustrated. B, Representation of a dynamic voiding urethrogram with the criteria of McCallum illustrated. C, A normal retrograde urethrogram. D, A normal voiding urethrogram.
(A and B, Modified from McCallum RW. The adult male urethra. Radiol Clin North Am 1979;17:227–44.)
Endoscopic examination may be necessary after the contrast studies. The flexible cystoscope has simplified this evaluation, and when local anesthesia is used, there is little discomfort associated with it. The scope can be passed to the stricture, and many times it is not necessary to pass it beyond that level. In addition, it is not always necessary, and usually not beneficial, to dilate the stricture at the time of the initial endoscopic evaluation. Pediatric endoscopic equipment has proved to be extremely valuable for examination of the urethra proximal to a narrow-caliber area without the need to dilate the narrowest area. In the patient who cannot void and has a suprapubic tube, combined contrast studies with endoscopy are helpful in defining the stricture anatomy (Fig. 36–18).
Figure 36–18 A series of radiographs demonstrating the usefulness of the combination of contrast enhancement with endoscopy. A, A retrograde urethrogram shows a totally obliterative process involving the proximal bulbous urethra. B, The patient was successful in relaxing to void; however, there is suggestion of a wide-caliber annular area proximal to the obliterative process of the bulbous urethra. C, Endoscopy through the suprapubic cystostomy tube clarifies the anatomy of the proximal urethra and demonstrates the length of the obliterative process.
It is imperative, however, to completely evaluate the urethra proximal and distal to the stricture with endoscopy and bougienage during surgery to ensure that all the involved urethra is included in the reconstruction. Whereas hydraulic pressure generated by voiding may keep segments proximal to the stricture patent, unless these segments are included in the repair, they are at risk for contraction after obstruction of the narrow-caliber segment is relieved with reconstruction. For this reason, any abnormal areas of the urethra that are proximal to a narrow-caliber segment of the stricture must be treated with suspicion. If the lumen does not appear to demonstrate evidence of diminished compliance, we presume that area to be uninvolved in active stricture disease. However, coning down of the urethra suggests its involvement in the scar.
In some patients, the urethra proximal to a narrow area may remain confusing with regard to its potential for continued constriction after reconstruction. In select patients, we have found it useful to place a suprapubic tube to defunctionalize the urethra. After 6 to 8 weeks, if there is to be constriction of an area that was hydrodilated with voiding, the tendency for that constriction to occur should become apparent.
Key Points: Urethral Stricture
Treatment
Although the treatment of urethral stricture disease dates to the foundations of our specialty, significant progress made during the last 50 years allows many of the most complex strictures to be reliably reconstructed in one stage. In the past, a concept known as the reconstructive ladder was used as a treatment guideline for urethral strictures. That concept was based on the principle that the simplest procedure should always be attempted first, and sometimes repeated after failure, before moving on to more complex approaches. This approach is considered archaic in modern urethral reconstruction.
Both the patient and the physician must have a good understanding of the goal of treatment before the treatment choice is made. To this end, treatment options should be discussed with the patient, with care taken to emphasize the anticipated outcome with regard to potential cure. Some patients may prefer stricture management and choose to have periodic dilations in the office, at home, or in the hospital rather than undergo technically detailed open surgery. Others may have cure as a goal and choose surgical management. Many surgical procedures today have short- and midterm results approaching long-term success rates of more than 90% to 95% for many strictures.
Dilation
Urethral dilation is the oldest and simplest treatment of urethral stricture disease, and for the patient with an epithelial stricture without spongiofibrosis, it may be curative. The goal of this treatment, a concept that is frequently forgotten, is to stretch the scar without producing more scarring. If bleeding occurs during dilation, the stricture has been torn rather than stretched, possibly further injuring the involved area.
The least traumatic method to stretch the urethra is to use soft techniques over multiple treatment sessions. We believe that the safest method of urethral dilation currently available involves the use of urethral balloon-dilating catheters. These catheters may be attached to a filiform tip or passed over a guide wire or may come with an integral coudé tip. For initial dilation, we favor the use of balloons placed over wires that have been passed through the stricture under endoscopic control.
Dilation can be curative, and, in the literature, in correctly selected patients, has short and midterm efficacy rates equal to internal urethrotomy. Selection criteria will be discussed in the following section that discusses internal urethrotomy. The literature does not compare internal urethrotomy and dilation in randomized selection, thus we do not have a true comparison but rather comparison by retrospective analysis (Steenkamp et al, 1997).
Internal Urethrotomy
Internal urethrotomy refers to any procedure that opens the stricture by incising it transurethrally. The urethrotomy procedure involves incision through the scar to healthy tissue to allow the scar to expand (release of scar contracture) and the lumen to heal enlarged. The goal is for the resultant larger luminal caliber to be maintained after healing.
With epithelial apposition, wound healing occurs by primary intention. Internal urethrotomy does not provide an epithelial approximation but, rather, aims to separate the scarred epithelium so that the healing occurs by secondary intention. In healing by secondary intention, epithelialization progresses from the wound edges. As it progresses from the wound edge, epithelialization slows. In an effort to aid epithelialization, nature invokes the forces of wound contraction, not to be confused with scar contraction. Wound contraction closes the wound defect and limits the size of the area that requires epithelialization, hastening the healing of the surface defect. In the case of internal urethrotomy, however, wound contraction merely tries to reapproximate the edges of the scar, putting a race into effect. If epithelialization progresses completely before wound contraction significantly narrows the lumen, the internal urethrotomy may be a success. If wound contraction significantly narrows the lumen before completion of epithelialization, the stricture has recurred. The study by Dubey and colleagues (2005) shows the extent of luminal narrowing to be a predictor of success with internal urethrotomy: the narrower the percent of narrowing, the worse the outcome, with a cutoff of 74% narrowing.
Many surgeons have learned to perform internal urethrotomy by making a single incision at the 12-o’clock position. This location might be questioned, however, on the basis of the location of the urethra within the corpus spongiosum (see Fig. 36–25). On examination of a cross section of the corpus spongiosum, it can be seen that the thinnest portion of the anterior aspect is from 10-o’clock to 2-o’clock. The distance between the anterior wall of the urethra and the corpora cavernosa is likewise short in the bulbous urethra, and a single 12-o’clock cut could rapidly penetrate the corpus spongiosum and extend into the triangular ligament; although it might not enter the corpora cavernosa, a deep cut could enter the intracrural space. Distally, although the anterior aspect of the corpus spongiosum is thicker, on the other hand, a deep incision in the more distal aspects of the anterior urethra will certainly enter the corpora cavernosa, and these incisions have been associated with erectile dysfunction thought to be due to local cavernosal veno-occlusive dysfunction. Vigorous incisions at 10-o’clock and 2-o’clock in the bulbous urethra risk the same problem. If deep spongiofibrosis is present, stricture cure is impossible by internal urethrotomy, and these deep incisions are therefore unnecessary.
The most common complication of internal urethrotomy is recurrence of stricture. Less commonly noted complications of internal urethrotomy include bleeding (almost always associated with erections immediately after the procedure) and extravasation of irrigation fluid into the perispongiosal tissues. These complications are rare today, however, because of the less frequent use of aggressive internal urethrotomy as a treatment modality for urethral strictures. Normal saline should be used as the irrigant when direct visual internal urethrotomy is performed. Additionally with the use of deep urethrotomy incisions, another complication can be creation of a fistula between the corpus spongiosum and the corpora cavernosa, and thus cavernosal veno-occlusive dysfunction.
A major problem with assessing the success rates of internal urethrotomy is that the nature of the strictures that have been treated with internal urethrotomy has been poorly reported. In addition, the literature is not clear regarding the goal of internal urethrotomy. For many, an internal urethrotomy is successful if it offers temporary relief. Therefore, in many cases, internal urethrotomy has been reported as successful despite the fact that it has been associated with eventual stricture recurrence. A report published by Santucci and McAninch (2001) (Rosen et al, 1994) using actuarial techniques shows the curative success rate of internal urethrotomy to be approximately 20%. Evaluations by Pansadoro and Emiliozzi (1996) and others show the curative success rate of direct visual internal urethrotomy to be approximately 30% to 35%. Their analysis also shows that there is virtually no increase in success rate with a second internal urethrotomy. The data show that strictures at the bulbous urethra that are less than 1.5 cm in length and not associated with dense, deep spongiofibrosis (i.e., straddle injuries) can be managed with internal urethrotomy, with a 74% moderately long-term success rate. Pansadoro’s study did not have any long-term successes for treated strictures outside the bulbous urethra. The variables associated with success of internal urethrotomy have been verified by other studies (Heyns et al, 1998). A number of studies now show that the success of reconstruction is diminished by multiple prior urethral dilations and internal urethrotomy (Stone et al, 1983; Albers et al, 1996; Heyns et al, 1998; Boccon-Gibod, 2005). Success rates with internal urethrotomy are not equal to those of open urethral reconstruction (Mandhani et al, 2005). A number of analyses have sought to compare the cost effectiveness of the practice of internal urethrotomy initially prior to consideration of open reconstruction. The analyses all differ in method, and thus not surprisingly differ in findings (Rourke and Jordan, 2005; Wright et al, 2006; Wessells, 2009).
Several techniques have been employed to oppose the process of wound contraction and to prevent stricture recurrence. One method is to leave an indwelling Foley catheter for as long as 6 weeks after urethrotomy, in the hope that the urethra will mold around the catheter as it heals. Studies have shown, however, that the failure rate of long-term catheterization after internal urethrotomy is similar to that seen with 3 to 7 days of catheterization, and even 6 weeks is not sufficient time to oppose the forces of wound contraction.
Another technique used to oppose the forces of wound contraction after internal urethrotomy is home self-catheterization or home urethral obturation. After internal urethrotomy, patients generally have an indwelling catheter placed for a period of 3 to 5 days. When the catheter is removed, the patient is started on a urethral obturation regimen. Most regimens require more frequent catheterizations early in the recovery period, with a tapering schedule during the next 3 to 6 months. Anecdotally, many have reported an improved cure rate with self-catheterization in combination with internal urethrotomy. It has been our experience, however, that the stricture inevitably recurs when the patient stops self-obturation, regardless of how long it has been used. That being understood, this can effectively manage the problems when it is combined with a urethral dilating regimen in the properly motivated patient. Colchicine, because it binds tubulin, has been used along with internal urethrotomy (Carney et al, 2007). Initial findings in a nonrandomized study also suggest that, perhaps by pharmacologically blocking tubulin and thus possibly wound contracture, the results of internal urethrotomy may be better.
Urethral stents (removable or permanently implantable) are another modality used in opposing the forces of wound contraction after internal urethrotomy or dilation. Removable urethral stents are designed to prevent the process of epithelialization from incorporating the stent into the urethral wall and are left in place for as long as 6 months to 1 year before they are removed. The greatest experience with these removable stents comes from Israel (Yachia and Beyar, 1991), and centers there report good success in small series. The Memokath stent is currently being used in studies in the United States. It is a removable stent made of nitinol.
The majority of experience with permanently implantable stents comes from Europe and the United Kingdom. Milroy (1993) has reported a success rate of 84% at 4.5 years with use of the permanently implantable UroLume (Rousseau et al, 1987; Sigwart et al, 1987; Milroy et al, 1988, 1989; Sarramon et al, 1990; Ashken et al, 1991; Krah et al, 1992; Sneller and Bosch, 1992; Verhamme et al, 1993; Badlani et al, 1995; Milroy and Allen, 1996; Jordan, 1997; Tillem et al, 1997; Brandes and McAninch, 1998; Shah et al, 2003). The UroLume, made of an alloy, is designed to be incorporated into the wall of the urethra and corpus spongiosum. Available data show that the stent is best employed for relatively short strictures of the bulbous urethra associated with minimal spongiofibrosis. However, these are the strictures that are most successfully reconstructed with open techniques that offer better long-term success rates. The North American Study Group 11-year data have been published (Shah et al, 2003). Of 179 patients originally enrolled in the North American Study, 24 patients completed 11 years of follow-up. The overall success rate for all patients enrolled at 11 years is less than 30% (Shah et al, 2003). A 10-year follow-up study from the Netherlands (De Vocht et al, 2003) reported results thought to “weaken the optimistic early results”; of 15 patients implanted, only 2 were satisfied with their stent at 10 years.
Permanently implantable stents are associated with some unique complications. The stents must be placed only in the bulbous urethra, and when they are placed beyond the area of the scrotal urethra, placement has been associated with pain on sitting and intercourse. Some patients (particularly young patients) complain of perineal pain, often with vigorous activity, even after implantation of the stent in the deep bulbous urethra. In addition, longer, bulbous strictures require two stents that are overlapped. These stents can migrate away from each other, leaving a gap between them where recurrence of stricture is inevitable. When this occurs, the stricture recurrence is excised and a third stent is placed to span the gap.
There are also specific contraindications to the use of the UroLume. Patients who have undergone prior substitution urethral reconstruction, particularly where skin has been incorporated into the urethra, have been shown to be poor candidates for implantation with the UroLume stent because contact of the stent with the skin is associated with a virulent hypertrophic reaction. These patients experience postvoid dribbling, and the hypertrophic reaction can be so severe in some cases that functional recurrence of the stricture results. Another subset of patients shown to be poor candidates for the UroLume is those with strictures associated with deep spongiofibrosis. Patients who fall into this category are those who have had urethral distraction injuries and straddle injuries associated with deep fibrosis. As of this writing, the UroLume is approved by the U.S. Food and Drug Administration. The product insert clearly contraindicates use of the UroLume in patients with pelvic fracture urethral distraction defects. Many greeted the arrival of the UroLume as a panacea. Experience is accumulating not only with use of the UroLume but also with misuse of the device, and its use should therefore be approached with the utmost caution. Some centers in Europe are now advocating use of the UroLume only in patients who are older than 50 years, or who have other significant medical problems that make the option of lengthy open urethral reconstruction less appealing. At our center, we have seen teenagers implanted, many patients implanted for pelvic fracture urethral distraction, patients with stents palpable on the shaft of the penis, and patients with both prostatic stents and urethral stents—all absolute contraindications. We have seen patients with as many as six stents in place, and although the maximal number of stents is not absolutely defined, if the first stent does not work, it is probably wise to refer the patient for reconstruction, which is possible with acceptable results (86% with mean follow-up of 25 months) (Barbagli et al, 1999). Placement of more stents, except when two stents were originally placed and then migrated from each other, is probably futile. However, in carefully selected elderly patients, the UroLume can be life changing for the patients, with minimal morbidity associated with the implantation procedure, in a situation where early and midterm success is adequate. The place of permanently implanted stents seems to be challenged as newer temporary/removable urethral stents are introduced.
Lasers
Types of lasers that have been used for the treatment of urethral stricture disease include carbon dioxide, argon, KTP, Nd : YAG, holmium : YAG, and excimer lasers. The ideal laser for use in the treatment of urethral stricture disease is one that totally vaporizes tissue, exhibits negligible peripheral tissue destruction, is not absorbed by water, and is easily propagated along a fiber. Although the carbon dioxide laser appears to be ideally suited, it must be used with a gas cystoscope, which carries the potential threat of a carbon dioxide embolus.
For both the argon and the Nd : YAG lasers, the predominant mode of action is thermal necrosis, which leads to a significant potential for peripheral tissue injury, rather than vaporization. The Nd : YAG laser has also been used with a bare fiber in the contact mode. A bare fiber carries with it a risk of forward scatter. When it is used in the contact mode, the YAG energy is transferred to a sapphire tip. Advocates of the use of a contact laser suggest that it obliterates the scar by vaporization; however, results with use of these fibers are no better than those with direct cold-knife visual internal urethrotomy.
A KTP laser is essentially an Nd : YAG laser that has passed through a KTP crystal, resulting in a reduced depth of penetration. A KTP laser urethrotomy is accomplished by passing the fiber over the scar tissue to make urethrotomy cuts. The holmium : YAG laser has properties similar to the KTP laser, and like the KTP laser, it provides both direct contact cutting and vaporization with minimal forward scatter. Experience with the holmium : YAG laser is accumulating; anecdotally, it may have a place in the management of some strictures, in particular strictures that are relatively isolated and short.
The excimer laser is a true vaporizing laser that has little forward scatter or peripheral tissue necrosis associated with it. Little experience with this laser has been reported, but future investigation is clearly warranted.
To date, the results of laser urethrotomy are mixed. With the advent of new lasers and experience with them, however, future data may show better results.
Open Reconstruction
Excision and Reanastomosis
It has now been demonstrated with certainty that the most dependable technique of anterior urethral reconstruction is the complete excision of the area of fibrosis, with a primary reanastomosis of the normal ends of the anterior urethra (Fig. 36–19) (Russell, 1914). The best results are achieved when the following technical points are observed: the area of fibrosis is totally excised; the urethral anastomosis is widely spatulated, creating a large ovoid anastomosis; and the anastomosis is tension free.
Figure 36–19 Techniques for excision and primary reanastomosis of anterior urethral stricture. A, The bulbospongiosus is released from its attachment to the perineal body. The arteries to the bulb are not divided. This technique allows the urethra to be mobilized distally. This technique combined with development of the intracrural space can shorten the path of the urethra by approximately 1 to 1.5 cm. B, Technique of a primary spatulated anastomosis after excision of an anterior urethral stricture.
(A and B, From Jordan GH. Principles of plastic surgery. In: Droller MJ, editor. Surgical management of urologic disease: an anatomic approach. Philadelphia: Mosby–Year Book; 1992. p. 1218–37.)
The success of this procedure relies on vigorous mobilization of the corpus spongiosum. With vigorous mobilization, dissection of Buck fascia to improve compliance, development of the intracrural space, and detachment of the bulbospongiosus from the perineal body, significant lengths of stricture can be excised and reanastomosed. Strictures of 1 to 2 cm are generally easily excised with reanastomosis. In some cases, strictures as long as 3 to 5 cm can be totally excised and a primary reanastomosis of the anterior urethra performed. For very proximal short-length bulbous strictures, tension-free anastomosis can be facilitated by the dissection of the membranous urethra (Fig. 36–20). As a rule, the closer the stricture is to the membranous urethra, the longer it can be and still be reconstructed with anastomotic techniques. For many proximal strictures, a single-layer anastomosis is preferable. When the length of stricture precludes total excision of fibrosis with primary anastomosis, tissue transfer is required. Morey published a series of patients who had stricture excision with anastomosis for strictures up to 5 cm. He makes the point that the younger patient has more compliant tissue, thus allowing the limits to be stretched (Morey and Kizer, 2006).
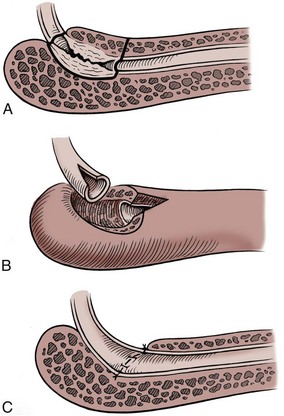
Figure 36–20 Technique of excision of very proximal bulbous urethral stricture with reanastomosis. This technique is facilitated by dissection of the membranous urethra. A, The area of the stricture is defined for excision. B, The stricture is excised and both ends of the urethra are spatulated on the dorsal aspect. C, The anastomosis is complete.
Morey also reported an interesting variant of excision with anastomosis for anterior stricture. In that case report, a patient had two independent areas of stricture apparently separated by totally normal urethra and corpus spongiosum. The authors excised both areas of stricture independently with respective anastomosis of each site. Although this case was successful, we think that the authors’ considerable experience allowed them to achieve a successful result, and a safer reconstruction with use of onlay or augmented onlay might have been better (DeCastro et al, 2002).
Jordan reported the use of a vessel-sparing excision and reanastomosis of the bulbar urethra. The dissection is similar to the standard excision and reanastomosis (Fig. 36–21), except once the triangular ligament is divided and the intracrural space developed, the space between the membranous urethra and the proximal vasculature is developed and these vessels are preserved (Fig 36–22). The urethra is then divided with the stenotic segment excised, the ends are spatulated, and then the reanastomosis is done. Advantages of preserving the proximal blood supply to the bulbar urethra include those patients whose distal blood supply is compromised by trauma, previous surgery, or hypospadias. Another theoretical advantage would be a decrease in the risk of erectile dysfunction. Further studies need to be done to confirm our initial excellent results and the above advantages (Jordan, et al 2007; Gur and Jordan, 2008).
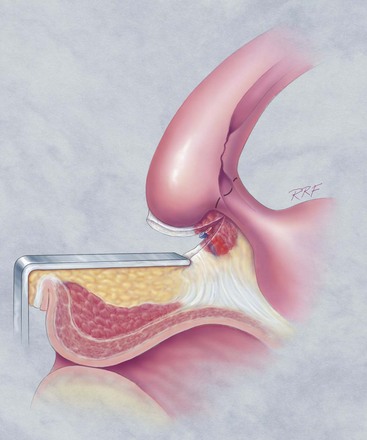
Figure 36–21 Diagrammatic representation of the dissection of the proximal corpus spongiosum, bulbospongiosum, and the membranous urethra. Illustrated is the customary technique for dividing the urethra through the juncture of the membranous urethra with the proximal bulbous urethra—to perform an excision of stricture with a primary anastomosis. In this illustration, the proximal vasculature has been ligated and divided. The urethra can then be divided at the distal most limits of the membranous urethra.
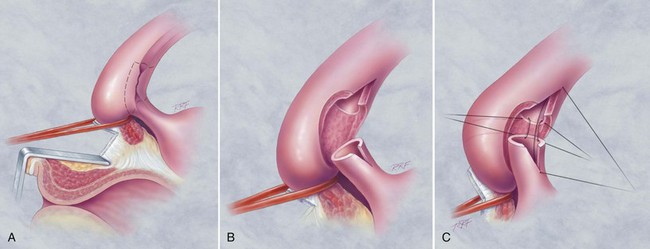
Figure 36–22 Illustrated is the technique of vessel-sparing excision with primary anastomosis. The proximal corpus spongiosum, bulbospongiosum, and area of the proximal vessels and membranous urethra have been dissected. A, Illustrated is the dissection of the space between the proximal vasculature and the membranous urethra. In this technique, the arteries to the bulb can be preserved, and the membranous urethra can be divided at its juncture with the bulbous urethra. The area of proximal stricture can be excised. B, The illustration of the stricture having been excised prior to placement of the anastomotic sutures. C, Anastomotic sutures are placed to effect the spatulated anastomosis. At this center, customarily we alternate polydioxanone sutures with Monocryl; however, any acceptable absorbable suture can be used. Note the membranous urethra is spatulated on the dorsum as is the proximal bulbous urethra.
Four grafts that have been successfully used for primary urethral reconstruction are the full-thickness skin graft, the bladder epithelial graft, the oral mucosal graft and the rectal mucosal graft. Oral mucosal grafts as mentioned can be taken from the cheek (buccal), the lip (labial), and the undersurface of the tongue (lingual). Split-thickness skin grafts have been used for staged anterior urethral reconstruction (Humby, 1941; Memmelaar, 1947; Pressman and Greenfield, 1953; Devine et al, 1976; Hendren and Crooks, 1980; Schreiter and Koncz, 1983; Webster et al, 1984; Hendren and Reda, 1986; Ransley et al, 1987; Burger et al, 1992; Jordan, 1993; El-Kassaby et al, 1996; Wessels and McAninch, 1996). The characteristics and microvascularity of some of those grafts are discussed earlier in this chapter in the section Principles of Reconstructive Surgery.
In years past, graft reconstruction of the urethra was almost abandoned in favor of flap reconstruction techniques. However, since the late 1990s, there has been a resurgence of interest in the use of grafts (Wessells and McAninch, 1996) and, specifically, the use of buccal mucosal grafts (Hellstrom et al, 1996; Weinberg et al, 2002; Barbagli et al, 2003; Elliott et al, 2003; Bhargava and Chapple, 2004; Kellner et al, 2004; Xu et al, 2004; Dubey et al, 2005). Grafts have been most successfully employed in the area of the bulbous urethra, where the urethra is invested by the bulk of the ischiocavernosus muscles. However, the use of grafts, other than in the area of the bulbous urethra, and, in some cases, the use of tubed reconstruction is reported in increasing numbers. The grafts can be applied to the ventrum of the urethra; however, a ventral urethrotomy seems to be advantageous only if the use of the spongioplasty maneuver is contemplated (Fig. 36–23). The spongioplasty procedure requires that the corpus spongiosum adjacent to the area of the stricture be relatively normal and free of fibrosis. There are data to support the superiority of results with the dorsal onlay technique and other reports showing no difference in success. In the past, we preferred to use lateral graft onlay (see Fig. 36–23) or dorsal graft onlay (see Fig. 36–23). Placement of the urethrostomy laterally allows exposure of the urethra while cutting through the corpus spongiosum, where it is relatively thinner, thus limiting bleeding and maximizing exposure. In addition, in the bulbous urethra, the graft can be sutured to the underlying muscle bed in the hope of improving graft host bed immobilization and approximation.
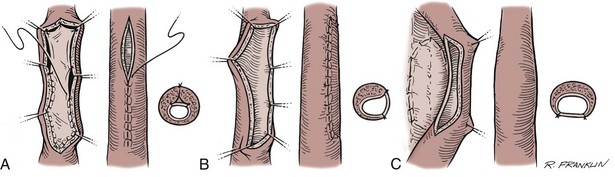
Figure 36–23 Diagram of various techniques of graft onlay. A, Ventral onlay with spongioplasty. B, Lateral onlay with quilting to the ischiocavernosus muscle. C, Dorsal onlay with spread fixation of the graft.
The Monseur (1980) urethral reconstruction was applied in only a few select centers. In this technique, the urethrostomy was made through the stricture on the dorsal wall. The edges of the stricture were then sutured open to the underlying triangular ligament or corpora cavernosa, or both. Barbagli and associates (1995) have modified the Monseur technique (Fig. 36–24). In that modification, the urethrostomy is performed through the stricture on the dorsal wall. In the area of the urethrostomy, a graft is then applied and spread fixed to the triangular ligament or corpora cavernosa, or to both. In turn, the edges of the stricturotomy are then sutured to the edges of the graft as well as to the adjacent structures. The results of this technique are excellent. The ventral and dorsal graft onlay techniques can both be used with stricture excision and strip anastomosis (augmented anastomotic procedure) (Fig. 36–25). For proximal strictures, the vessel-sparing technique of augmented anastomosis depends on the surgeon’s ability to excise the scarred epithelium and underlying corpus spongiosum tissue without the need to completely divide the corpus spongiosum.
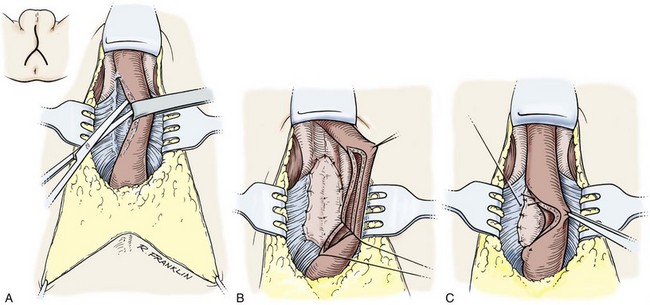
Figure 36–24 Technique of dorsal graft onlay popularized by Barbagli. A, The corpus spongiosum is detached from the triangular ligament and corpora cavernosa. B, A dorsal urethrostomy is performed. The graft is spread fixed to the corpora cavernosa. Note the pie-crusting incision. C, The edges of the stricturotomy are then sutured to the graft as well as to the corpora cavernosa.
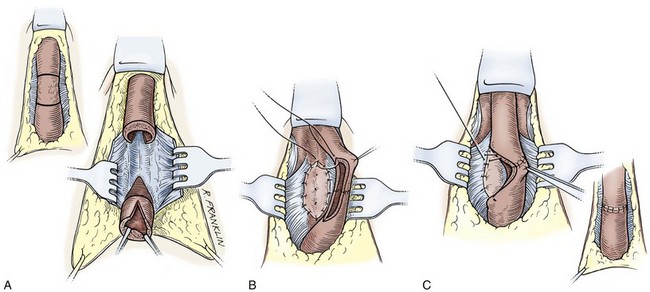
Figure 36–25 Technique of augmented anastomosis with graft onlay. A, The corpus spongiosum is detached from the triangular ligament and the corpora cavernosa. The area of spongiofibrosis is identified and marked, and the area of narrowest caliber stricture is excised. The urethral ends are then spatulated on the dorsum. B, A two-layer floor strip anastomosis is performed and the graft is spread fixed to the corpora cavernosa. Note the pie-crusting incisions and the mattress sutures. C, The edges of the stricturotomy are then sutured to the graft as well as to the corpora cavernosa.
Another option is the two-staged application of a mesh split-thickness skin graft, buccal mucosal graft, or posterior auricular full-thickness skin graft. In the first stage of the staged graft procedure, a medium-thickness split-thickness skin graft, a buccal mucosal graft, or a Wolfe graft is placed over the dartos fascia. If the graft is placed immediately onto the tunica albuginea or corpora cavernosa, the inability to mobilize the graft makes second-stage tubularization difficult. However, there is an advantage to having at least a midline strip of the graft adherent to the corpora cavernosa. At a later date, second-stage surgery is performed to tubularize the graft. Whereas Schreiter and Noll (1989), who first described the procedure of mesh split-thickness skin graft, often proceeded to the second stage within 3 to 4 months, we wait 12 months between the first- and second-stage surgeries if a split-thickness skin graft is used. This procedure has been found useful for select cases in both the United States and Europe. In the United States, its use has mostly been confined to the most difficult cases, however, with single-stage reconstruction still applied to the majority. As already mentioned, staged graft techniques have been effectively used in the complicated hypospadias patient. Staged buccal graft operations have been successfully employed in the LS-BXO patients with midterm follow-up. In addition, in the complicated hypospadias patient, staged buccal grafts and posterior auricular skin grafts have been successfully employed (Fig. 36–26).
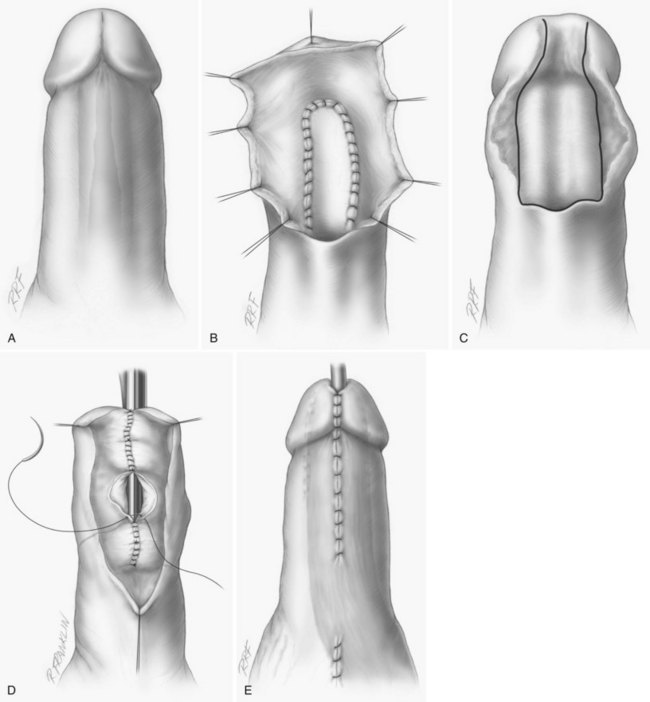
Figure 36–26 Staged reconstruction of a distal anterior urethral stricture. A, The appearance of the penis with the urethra (shaded area shows the location of a tight stenosis of the fossa navicularis that extends into the distal pendulous urethra). B, The distal narrow stricture of the fossa navicularis has been excised and stricturotomy performed into the normal urethra proximal to the excised tissue. A buccal graft has been applied to the defect, but the bolster dressing has not yet been applied. C, After 6 months, the graft is mature. The illustration shows a Tiersch tube ready for closure. D, The Tiersch tube is closed with a watertight suture line. The distal urethra is usually calibrated to create a urethral lumen of approximately 28 Fr. E, Glans reconstruction and closure of the distal shaft has been performed (the shaded area shows the tunica dartos flap that carries a parietal tunica vaginalis island). The flap is mobilized in this case from left hemiscrotum, and transposed to cover the entire area of the urethral reconstruction.
A number of applications of genital skin islands, mobilized on either the dartos fascia of the penis or the tunica dartos of the scrotum, have been proposed for the repair of urethral stricture disease. In the past, these “flap operations” were considered separate procedures. We suggest that all these procedures are really different applications of a single concept, as proposed by the microinjection studies of Quartey (1983). Skin islands can be viewed as passengers on fascial flaps, and the design of flaps for urethral reconstruction can be paralleled to the design of flaps for reconstruction in general.
There are three important considerations for the use of flaps in urethral reconstruction: the nature of the flap tissue, the vasculature of the flap, and the mechanics of flap transfer. The skin must be nonhirsute for urethral reconstruction. In addition, for donor site consideration, it is most convenient to use the areas of redundant nonhirsute genital skin.
If the redundancy is dorsal, the skin island can be oriented transversely and mobilized on the dorsal dartos fascia after the techniques described by Duckett and Standoli in 1984 (Fig. 36–27) (Duckett, 1986, 1992; El-Kassaby et al, 1986; Duckett et al, 1993). If there is redundancy of the ventral skin, the skin island can be mobilized as a ventral longitudinal island. These islands can be either vigorously mobilized on a ventrolaterally oriented dartos fascial flap for transposition to the perineum or less vigorously mobilized and transposed and inverted into a pendulous urethral stricture defect (Fig. 36–28) (Orandi, 1972). Ventral islands can be oriented transversely (Fig. 36–29) as well as longitudinally. Longer skin islands can be mobilized by orienting the island both ventrally and transversely at the distal extent. This “hockey stick” orientation allows islands as long as 7 to 9 cm (Fig. 36–30). For distal strictures of the anterior urethra, including the fossa and meatus as well as the pendulous urethra, the islands can be advanced to reconstruct to the level of the meatus by either developing glans wings or elevating the ventral glans.
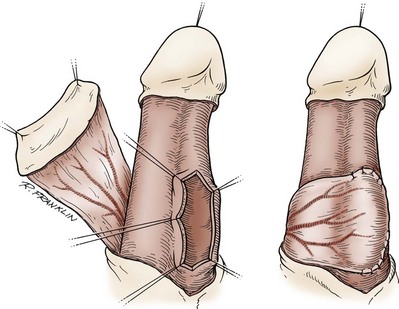
Figure 36–27 A dorsal transverse island of penile skin applied to a stricture of the urethra. The flap has been elevated on the dartos fascia, and a lateral incision has been made into the urethra. The flap is secured in place (right).
(From Jordan GH. Management of anterior urethral stricture disease. In: Webster GD, editor. Problems in urology. Philadelphia: JB Lippincott; 1987. p. 217.)
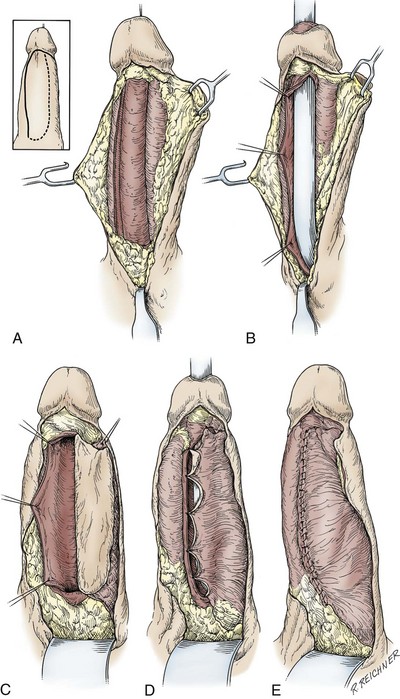
Figure 36–28 Penile longitudinal skin island. The incisions to be made to mobilize the flap are demonstrated in the inset. The heavy line is the primary incision made full thickness through the dartos fascia and superficial Buck fascia lateral to the corpus spongiosum. A, Dissection elevates the dartos fascial flap well past the corpus spongiosum in the midline. B, A lateral urethrostomy placed to face the flap has opened the entire length of the stricture. C, The skin paddle of the flap has been developed by making the incision outlined by the dotted line (inset) and undermining the skin lateral to it. The medial edge of the flap has been fixed to the edge of the stricturotomy. D, The flap is inverted into the defect. E, A watertight subepithelial suture line has been completed with a running absorbable monofilament suture. The skin will be closed with subcutaneous sutures and interrupted cutaneous sutures.
(A to E, From Jordan GH. Management of anterior urethral stricture disease. In: Webster GD, editor. Problems in urology. Philadelphia: JB Lippincott; 1987. p. 214.)
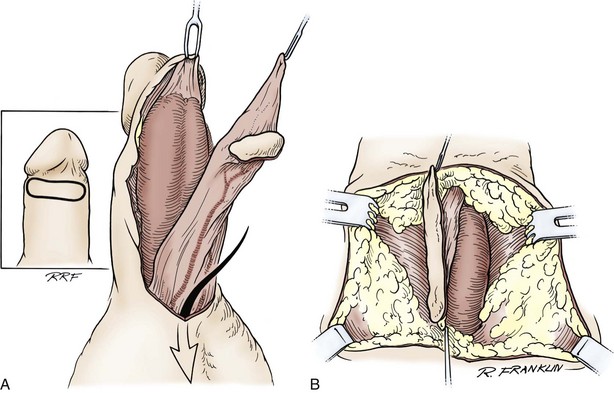
Figure 36–29 A ventral transverse skin island is elevated on the penile dartos fascia, inverted to the area of the perineum where flap onlay is accomplished. A, The skin island is elevated on the dartos fascia. B, The appearance of the flap transposed to the area of the perineum for onlay in a proximal bulbous urethral stricture.
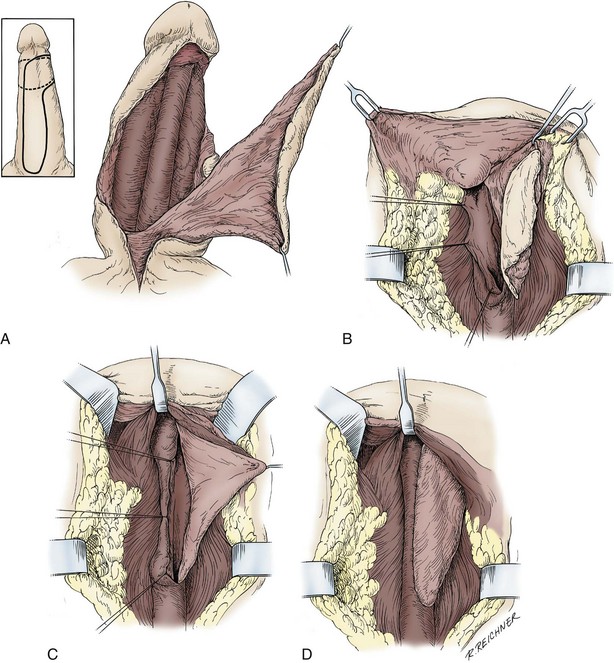
Figure 36–30 Ventral skin island for long bulbous stricture. The skin paddle of the flap is developed on the ventral midline of the penis and can be extended around the penile shaft at its distal end. A, The paddle of the flap has been incised and its pedicle elevated. This pedicle includes Buck fascia and dartos fascia, denuding the tunica of the corpus spongiosum and the corpora cavernosa. The pedicle (the dartos fascia bilaterally) is based on the superficial external pudendal vessels and the internal pudendal vessels in the scrotum. Development of this pedicle allows the flap to be moved to any area of the urethra. B, The flap has been passed through a tunnel beneath the scrotum developed by dissection along the corpus spongiosum. A laterally placed urethrostomy has opened the urethral stricture. C, The deep edge of the flap is secured by the suture techniques previously described. D, Anastomosis of the flap has been completed. The pedicle can be seen extending beneath the scrotum.
(A to D, From Jordan GH, McCraw JB. Tissue transfer techniques for genitourinary surgery, part III. AUA Update Series 1988;7:lesson 11.)
Where there is general redundancy to the penile skin, the islands can be oriented circumferentially. These “circular skin islands” are mobilized on the entire penile dartos fascia, and the mechanics of transposition suggest that they are most efficient when they are ventrally based, with the pedicle split dorsally. In some cases, circular skin islands as long as 15 cm can be obtained (El-Kassaby et al, 1986; McAninch, 1993; Miller and McAninch, 1993). The so-called Q flap circular island design can provide even longer islands, sometimes necessary for complex long-length anterior urethral reconstruction (Morey et al, 2000).
Many times, it is beneficial to combine the excision of the stricture with a skin island onlay (Fig. 36–31) or a graft onlay (see Fig. 36–25, augmented anastomosis). We have found that segments of very narrow caliber (nearly or totally obliterating) are difficult to deal with. These segments can often be completely excised; a roof or floor strip anastomosis of the urethra is performed, and the remaining urethrotomy defect is filled with either a graft or a skin island onlay. In some patients, there are relatively large nonhirsute areas of the scrotal skin that can be elevated on the tunica dartos of the scrotum. This flap has, in the past, been maligned in the literature. However, we and others have extensive experience with these flaps and, in select cases, have had very good results. The fascial flap must be based laterally, and so oriented, these flaps have been shown to be extremely reliable. Because the tunica dartos has a significant muscle component, the skin island must be carefully tailored. If these skin islands are correctly tailored at the outset, they are not attended with diverticular development as some have believed they were in the past. We now have used these flaps in more than 80 patients with excellent results and good long-term follow-up (>12 years at this writing). Scrotal skin islands are not our first choice; however, for difficult cases, they remain a reasonable option.
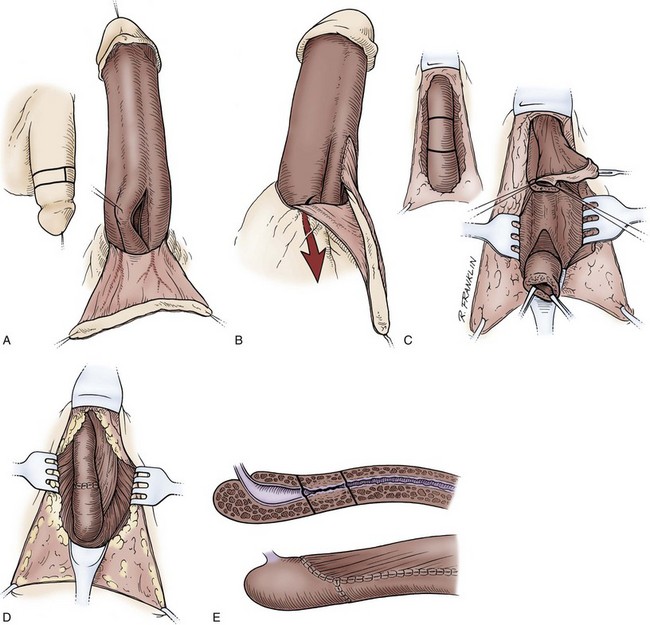
Figure 36–31 Illustration of reconstruction in a patient with a long anterior urethral stricture with a relatively short narrow-caliber section (technique of augmented anastomosis with circular skin island). A, A circular skin island is elevated on the dartos fascia. The patient is positioned flat on the table. B, The skin island onlay is begun, the rest of the flap is placed into the perineal dissection, and the penis is closed; the patient is then repositioned in the lithotomy position. C, The flap is retrieved through the perineal dissection. The narrow-caliber section is excised, and the urethra is spatulated on the dorsum. D, The onlay is completed, and the floor strip anastomosis is closed. E, Schematic of the surgery.
(A to E, From Stack RS, Schlossberg SM, Jordan GH. Reconstruction of anterior urethral strictures by the technique of excision and primary anastomosis. Atlas Urol Clin North Am 1997;5:11–21.)
These procedures using skin islands oriented on the penile dartos fascia have also been useful for reconstruction of the fossa navicularis (Cohney, 1963; Blandy and Tresidder, 1967; Brannen, 1976; De Sy, 1984; Jordan, 1987; Armenakas et al, 1998). In the past, meatal strictures and strictures of the fossa navicularis were managed with repeated dilations or sequential meatotomies. Because these meatotomies were seldom successful in the long term, techniques were developed that allowed the spatulation of random penile skin flaps into the meatotomy defects. These procedures functionally improved the results; however, the cosmetic appearance of the penis was less than optimal. With the use of skin islands elevated on the dartos fascia, excellent functional as well as cosmetic results became the norm. Again, the design of these islands must take into consideration the location of hair on the shaft of the penis, as well as the mechanics of flap transfer (i.e., transposition versus advancement) (Figs. 36-32 and 36-33). In addition, full-thickness skin has been used to reconstruct the fossa navicularis, but when they can be avoided, skin grafts are not thought appropriate for reconstruction in cases of LS-BXO. As already mentioned, there is question about the use of skin islands in general in patients with LS-BXO.
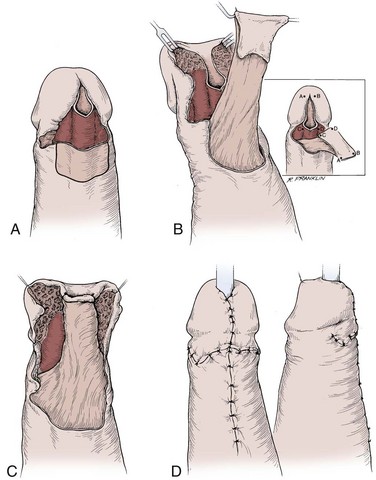
Figure 36–32 Technique of reconstruction of the fossa navicularis after Jordan. A, The ventral corpus spongiosum is exposed and the urethra opened ventrally through the area of stenosis. A transverse ventral skin island is outlined on the distal penile skin. B, The skin island is elevated on the ventral dartos fascia. C, The skin island is transposed and inverted into the meatotomy defect (inset). D, The appearance of the penis closed after the procedure.
(A to C, From Jordan GH. Reconstruction of the fossa navicularis. J Urol 1987;138:1210; D, from Jordan GH. Reconstruction of the meatus–fossa navicularis using flap techniques. In: Schreiter F, editor. Plastic-reconstructive surgery in urology. Stuttgart: Georg Thieme; 1999. p. 338–44.)
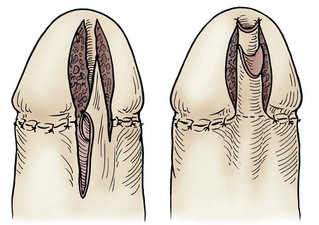
Figure 36–33 Technique after De Sy, in which a ventral longitudinal skin island is advanced into the meatotomy defect. The skin island is developed by deepithelialization of a portion of the longitudinal flap.
(From Jordan GH. Management of anterior urethral stricture disease. Probl Urol 1987;1:199–225.)
The literature is clear that onlay procedures (graft or flap) are attended with a higher success rate than tubularized grafts or tubularized skin islands (Hendren and Crooks, 1980). Tubularized grafts and skin islands should therefore be avoided, if possible. When tubularized segments cannot be avoided, the length of these segments can be limited by combining aggressive mobilization and excision. Without question, tubularized flaps provide better results than tubularized grafts. Where extremely long segments of the anterior urethra require reconstruction, a flap can be used distally and augmented by graft onlay proximally (Wessells et al, 1997). Where tubed reconstruction is required, the combination of a graft spread fixed to reestablish the “urethral plate” with flap onlay, in a small series with only short follow-up, seems perhaps to be better than tubed flap reconstruction, even when it is employed in the onlay-tube-onlay configuration (Morey, 2001).
A flap procedure that can be used as an alternative to the split-thickness skin grafts when nonhirsute skin is unavailable is the epilated midline genital skin island. Like the split-thickness skin graft, this procedure must be viewed as a staged procedure, with the epilations being the initial stage or stages. Epilation can be accomplished with either a narrow-gauge needle and monopolar cautery or epilation needles and machines. The interval between the epilations must be 6 to 8 weeks, and urethral reconstruction cannot be accomplished until 10 to 12 weeks after the last epilation. The actual stricture repair involves elevation of the midline skin island, based on both the dartos fascia of the penis and the tunica dartos of the scrotum. As with nonhirsute scrotal skin islands in general, the importance of meticulous tailoring of the scrotal portion of the island cannot be overemphasized.
Mundy (1994) analyzed a large series of urethral reconstructions. His data show that when follow-up is limited to 1 year, the success rate with tissue transfer clusters is about 95%. With longer follow-up, however, there is deterioration over time. With excision and primary anastomosis, the success seen at 1 year seems to be more durable, however, and does not appear to deteriorate at the same rate with time. We have reported our long-term data for excision and primary anastomosis with anterior urethral stenosis in 220 patients with a mean follow-up of 44 months; three recurrences were noted, two within the first 6 months and a third at 4 years. The rate of postoperative erectile dysfunction is 2%, with the severe straddle population being at increased risk. Wessells and McAninch (1998), in a meta-analysis of graft onlay procedures compared with flap procedures, showed equivalent results for graft operations and flap procedures, and graft onlay procedures are technically far easier to perform. There clearly are some cases where flap reconstruction would be expected to provide superior results (i.e., radiation strictures, patients with multiple operations, pendulous strictures). However, with the increased knowledge gained by the enthusiastic application of graft reconstruction, there is currently a redefined paradigm for anterior reconstruction. Whereas grafts have been used successfully for all segments of the anterior urethra, many think that flaps are best suited for distal reconstruction and grafts for proximal reconstruction, all other variables equivalent (Greenwell et al, 1999).
The issue of postoperative erectile dysfunction must be discussed. Our rates for anterior urethral anastomotic reconstruction are quoted earlier. In an analysis by Coursey, 200 urethroplasty patients were studied. Overall, the rate of erectile dysfunction after urethroplasty was approximately equal to that with circumcision. However, longer-segment reconstructions did have a higher risk of postoperative erectile dysfunction, although in many, over time, the patient’s erectile function improved (Coursey et al, 2001).
Special mention must be made regarding reconstruction for strictures associated with LS-BXO. With the advent of flap techniques, many centers embraced these techniques for these strictures. However, analysis of results from these patients treated at several large centers has shown a very high recurrence rate. Consequently, these centers adjusted the techniques by applying staged graft techniques (see Fig. 36–26). Interestingly, staged graft techniques using skin grafts again had a very high recurrence rate in a number of analyses. Theoretically, because LS-BXO is a skin condition, the use of skin as a flap, single-stage graft, or staged graft does not preclude involvement of the skin with the inflammatory process (Lee and Phillips, 1994; Akporiaye et al, 1997). Thus surgeons at a number of centers believe that staged oral graft techniques should be employed for reconstruction of strictures associated with LS-BXO. Short follow-up suggests better success with this approach. Long-term follow-up is not available. In a review of our experience in patients with a fossa navicularis stricture and LS-BXO, we noted a 50% recurrence in the stricture with a ventral transverse skin island (Virasoro et al, 2007).
Key Points: Treatment of Urethral Stricture
Pelvic fracture urethral Injuries
Pelvic fracture urethral injuries are the result of blunt pelvic trauma and accompany about 10% of pelvic fracture injuries. Although it is possible to totally disrupt the urethra with a straddle injury, these injuries most commonly involve only the bulbous urethra. However, the ensuing spongiofibrosis can be associated with complete obliteration of the urethra. Distraction injuries are for all intents unique to the membranous urethra. Pelvic fracture distraction injuries of the membranous urethra have been compared with plucking an apple (prostate) off its stem (the membranous urethra). This analogy implies that the injury most frequently occurs at the apex of the prostate. Experience shows that this is not the case, however, and the most frequent point of distraction is at the departure of the bulbous urethra from the membranous urethra (Andrich and Mundy, 2001; Mouraviev and Santucci, 2005). The distraction can, however, involve all or any portion of the membranous urethra between the departure of the bulbous urethra and the apex of the prostate. In the postpubescent male, the injury seldom involves the prostatic urethra. In the prepubescent male, in whom the prostatic urethra is more fragile, the injury can extend into that area.
Many injuries appear not to totally distract the entire circumference of the urethra. Instead, a strip of epithelium is left intact. In these patients, the placement of an aligning catheter may allow the urethra to heal virtually unscarred or with an easily managed stenosis. Because of flexible endoscopy equipment, the placement of an aligning catheter is relatively straightforward. If distraction is complete, the catheter then serves to align the obliterated urethral ends, and reconstruction is facilitated. Because of the ready availability of flexible cystoscopes, some centers are now acutely evaluating these injuries only with endoscopy. Those enthusiastic for this approach believe that not only can the injury be completely evaluated but the entire process, including the placement of an aligning catheter, is expedited (Kielb et al, 2001). Aligning catheters are just what the name implies—a guide, not a mechanism for placing traction on the bladder and prostate. Aligning catheters also seem to act as a drain as the pelvic hematoma liquefies, and perhaps the presence of the catheter may allow more rapid and complete resolution of the process (Cohen et al, 1991; Herschorn et al, 1992; Rehman et al, 1998; Mouraviev et al, 2005).
Evaluation
As with the repair of any stricture or stenosis, it is important to define the precise anatomy of the pelvic fracture injury before treatment is undertaken (McCallum and Colapinto, 1979a, 1979b); this includes the depth, density, length, and location. In pelvic fracture urethral distraction defects, the depth and density of fibrosis are predictable. Although the location of the distraction injury has been demonstrated to be an important factor in continence after reconstruction, this information should be a factor only in counseling of patients before the reconstruction and not in the treatment approach. The length of the defect is an important consideration and must be determined as precisely as possible.
Contrast studies are a first-line tool for the evaluation of pelvic fracture urethral injury. A cystogram outlines the bladder and provides information about rostral displacement of the proximal urethra. A lack of contrast material in the posterior urethra gives some information, albeit inconclusive, about the integrity of the bladder neck.
When the patient is successful in relaxing to void and the cystogram outlines the posterior urethra, a simultaneous retrograde urethrogram nicely outlines the length of the injury defect. However, this situation is the exception rather than the rule, and retrograde urethrography is most useful for determining whether the anterior urethra is normal. If the anterior urethra is normal, it has been our experience, as well as that of others, that a successful anastomotic repair is ensured. In fact, a primary anastomosis has been shown to be possible even with some involvement of the anterior urethra. Even in cases of prior failed posterior urethral reconstruction, primary anastomotic repair is often feasible, although the failure rate is slightly higher in these cases (Chapple and Pang, 1999; Flynn et al, 2003; Koraitim, 2003; Shenfeld et al, 2004). Thus primary anastomosis is unquestionably the goal in all these patients until it is proved impossible to perform.
When the proximal urethra is not visualized on a simultaneous cystogram with urethrogram, endoscopy through the suprapubic tract in combination with retrograde urethrography can be used to outline the defect. After the endoscopic appearance of the bladder neck is assessed, the flexible endoscope can be advanced through the bladder neck and into the posterior urethra to the level of the obstruction. The appearance of the bladder neck on contrast studies or on antegrade endoscopy does not accurately predict the ultimate function of the bladder neck after urethral reconstruction (Iselin and Webster, 1999). A simultaneous retrograde urethrogram will then outline the anterior urethra, with the space not visualized representing the injury defect.
Some have also advocated magnetic resonance imaging for the evaluation of patients with pelvic fracture urethral injuries. We have had little experience with this modality for that purpose; however, we have found the information obtained on the few studies that we have done to be useful. In these cases, there was the question of bone interposition into the injury defect, and magnetic resonance imaging nicely outlined that. We evaluated a case in which the prostatic urethra appeared obliterated. On magnetic resonance imaging, one could easily see that the prostate was not only distracted from the membranous urethra but also distracted from the bladder. This information was essential to planning of subsequent reconstruction in this case. It would seem intuitively obvious that knowing the length of distraction would be helpful in determining the precise approach and steps necessary for reconstruction. However, the literature is not clear on this matter (Andrich et al, 2003; Koraitim, 2004), and it is our experience that the surgeon must be prepared to exercise all options of reconstruction in virtually all such cases (McCallum and Colapinto, 1979a, 1979b).
Repair
The timetable for the reconstruction of pelvic fracture urethral injuries is determined by the type and extent of associated injuries. If possible, it is desirable to proceed within 4 to 6 months after trauma. However, orthopedic injuries of the lower extremities often necessitate a delay in proceeding with urethral reconstruction (Mundy, 1991; Follis et al, 1992; Brandes and Borrelli, 2001). Most pelvic fracture patients have inferior venal caval filters placed, because of the risk of deep venous thrombosis (DVT) and pulmonary embolism (PE) at the time of trauma. It is our practice to start all of these patients on low-dose anticoagulation and to continue it for about 10 days to 2 weeks after surgery.
In the majority of cases, pelvic fracture urethral injuries are not long, and the resultant obliteration is amenable to a technically straightforward mobilization of the corpus spongiosum with a primary anastomotic technique. The classic reconstruction consists of a spatulated anastomosis of the proximal anterior urethra to the apical prostatic urethra. Experience has demonstrated, however, that anastomosis of the proximal anterior urethra to any segment of the posterior urethra (apical, prostatic, or below) can be successfully accomplished by a widely spatulated anastomosis in which optimal epithelial apposition is achieved. About 10% of pelvic fracture urethral injuries are associated with more complex injuries and can be associated with fistulas (most commonly urethral rectal fistulas). Reconstruction of these injuries is technically more demanding.
Several series support the concept that the bulk of pelvic fracture urethral injuries, even the most difficult cases, can be managed by the perineal approach (Webster et al, 1983, 1990; Webster and Sihelnik, 1985; Koraitim, 1985, 1997; Morey et al, 1996; Flynn et al, 2003). In fact, a transpubic or an abdominal-perineal approach, as pioneered by Waterhouse and colleagues (1973), in our experience, is not necessary for the reconstruction of distraction injuries. In addition, pubectomy can be associated with long-term sequelae that include shortening of the penis, destabilization of erection, and destabilization of the pelvis, resulting in a chronic pain syndrome with exercise. However, there are surgeons who continue to rely heavily on the transpubic approach (Koraitim, 1997; Das et al, 2004).
Alternatively, the above-and-below approach does have merit when concomitant surgery is planned in the region of the bladder neck. We have found, and Iselin and Webster (1999) have reported that the competence of the bladder neck is difficult to assess accurately before the reestablishment of urethral continuity. In the past, great reliance was placed on whether the bladder neck was closed or open on cystography. We now know, however, that contrast material can opacify the prostatic urethra when the bladder neck is more than adequately competent for continence. Similarly, confidence has been placed in the appearance of the bladder neck on endoscopic examination through the suprapubic tube. Again, even when an obvious scar is noted to involve the bladder neck, follow-up of these patients after the urethral reconstruction establishes continuity of the urethra and finds many patients with more than adequate continence. Still other patients are believed to have incontinence due to scar incarceration of the bladder neck, caused by the extensive fibrosis left behind by resolution of the hematoma. In our experience, however, this is an infrequent occurrence, and the appearance of the bladder neck by any modality available is not predictive of continence. Therefore, it is currently our practice to reestablish the continuity of the urethra and, when there are concerns about continence, forewarn the patient before the urethral reconstruction. If these patients find that they experience less-than-adequate continence postoperatively, the problem is addressed in a subsequent procedure (Bhargava et al, 2004).
At the time of reconstruction, before the patient is placed in the lithotomy position, endoscopy is performed through the meatus and again through the suprapubic tube sinus. Endoscopy on the table is designed to ensure that there is no concomitant vesicolithiasis. This endoscopy is performed with a rigid endoscope, which is manipulated through the suprapubic tube sinus and the bladder neck and positioned against the area of total obliteration. On gentle manipulation of the endoscope, if the impulse of the endoscope tip is felt on the patient’s perineum, the impulse will be palpable when the perineum is opened and an instrument is manipulated through the bladder neck during reconstruction. If the impulse is not palpable perineally at this time, it may not be palpable during dissection, and in those cases, we create a temporary vesicostomy. This allows us to position an instrument reliably through the bladder neck, because the vesicostomy allows the surgeon to palpably identify the bladder neck before instrumentation of the posterior urethra. This maneuver has eliminated the occurrence of false passages with use of a sound such as the Haygrove staff through the suprapubic site, and also has eliminated the occurrence of misanastomosis of the anterior urethra to sites other than the apical proximal urethra.
We prefer the use of the exaggerated lithotomy position for the perineal approach (Fig. 36–34). This position is safe and provides optimal exposure to the area of the membranous and apical prostatic urethra (Angermeier and Jordan, 1994; Miller et al, submitted). A custom Skytron table, modified to allow the exaggerated lithotomy position, and a Stille-Scandia table, designed to place patients in the lithotomy position, are our preferences. The legs are carefully positioned in the Allen- or Guardian-style stirrups. Care is taken to avoid pressure on the lateral aspects of the lower extremities and calf muscles. The patient’s hips are elevated into position by raising the buttocks portion of the operating table. The boots are positioned to avoid stretch injuries of the common peroneal nerves (see Fig. 36–34).
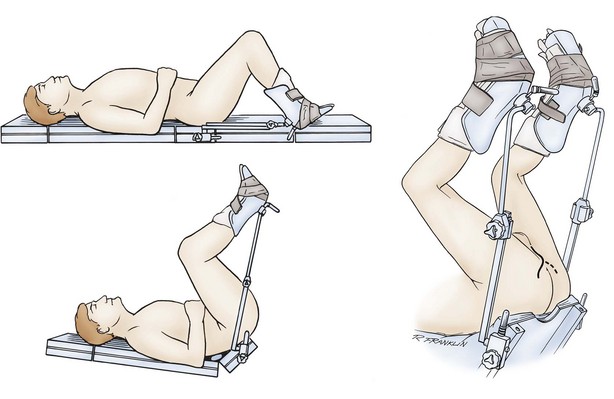
Figure 36–34 Patient placed in an exaggerated lithotomy position. The hips have been rotated into position by elevation of the buttocks portion of a specially modified table. The legs are suspended from boot-style stirrups with as little flexion of the hips and knees as allowed by the design of the stirrups.
(From Angermeier KW, Jordan GH. Complications of the exaggerated lithotomy position: a review of 177 cases. J Urol 1994;151:866–8.)
After the patient is correctly positioned, the perineal approach to reconstruction begins with an incision and dissection anterior to the transverse perinei musculature (anterior perineal triangle). This is in contrast to the approach posterior to the transverse perinei musculature (posterior anal triangle), which is useful for perineal prostatectomy. We use a λ-shaped incision (Fig. 36–35) that is carried sharply down to the midline fusion of the ischiocavernosus musculature (see Fig. 36–35A), then beneath the scrotum, to expose the uninvested portion of the corpus spongiosum. We then place a self-retaining ring retractor.
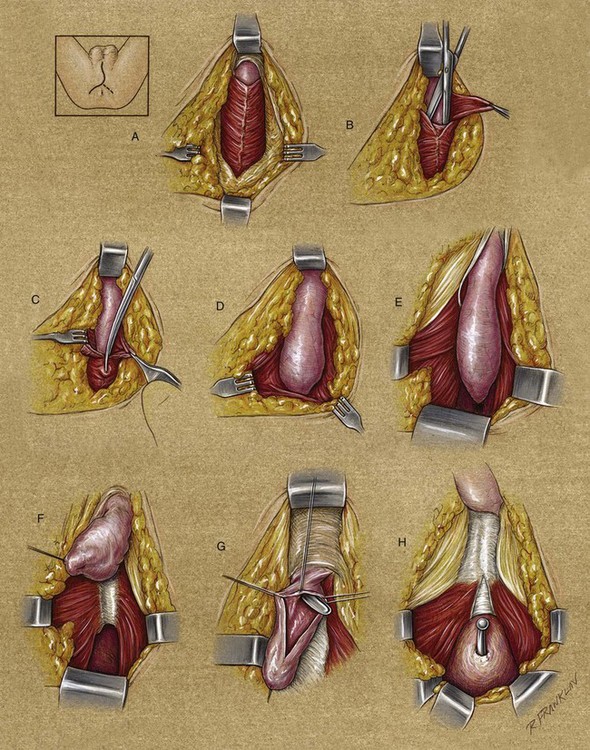
Figure 36–35 Diagram of a perineal repair of a membranous urethral stricture. A λ incision extends from the midline of the scrotum to the ischial tuberosities. A, Colles fascia has been opened to expose the midline fusion of the ischiocavernosus muscles and the tunica of the corpus spongiosum distal to the edge of the muscles. B, The scissors are introduced to develop the space between the muscle and the bulb of the urethra. C, An incision is made in the midline with the scissors, exposing the length of the bulb. D, The ischiocavernosus muscle is retracted to expose the full length of the bulb. E, The self-retaining retractor is placed to expose the inferior fascia of the genitourinary diaphragm. The bulb of the corpus spongiosum (bulbospongiosum) can now be mobilized to gain access to the fibrosed area of the urethra. F, The fibrosed urethra is incised, freeing the bulb. G, The anterior urethra is opened to make an adequate lumen. H, The Haygrove staff has been passed through the suprapubic cystostomy. Resection of the fibrotic distraction defect has allowed it to pass into the perineum.
The fusion of the ischiocavernosus musculature is divided, and the musculature is cleanly dissected from the corpus spongiosum and bulbospongiosum (see Fig. 36–35B to D). The corpus spongiosum is detached from the triangular ligament and corpora cavernosa (see Fig. 36–35E), the bulbospongiosum is detached from the perineal body, and the dissection is carried farther down to the infrapubic space. Posterior detachment of the bulbospongiosum is carried anteriorly, and the dissection is eventually carried through the area of fibrosis (see Fig. 36–35F).
In some cases, the proximal blood supply is encountered and must be controlled. We have found that these arteries are easily controlled with a sharp tip hemostat and monopolar cautery. Suture ligature should be avoided in the case of the arteries to the bulbospongiosum because of their proximity to the nerves as they are coursing into the corpora cavernosa.
We then divide the triangular ligament and vigorously develop the intracrural space down to the pubis (Fig. 36–36). If the dorsal vein is encountered, it is ligated and divided. It is important to make sure that the arteries were not rolled into the intracrural space if the tissues were dislocated during trauma. It is not uncommon to see the penetration of the cavernosal arteries or the dorsal arteries, or both, into this space. If there is doubt about the nature of the vessels encountered, Doppler sonography should be used. When the pubis is exposed, the periosteal elevator can be gently introduced onto the retropubic surface, releasing and allowing the descent of the tissues from beneath the pubis.
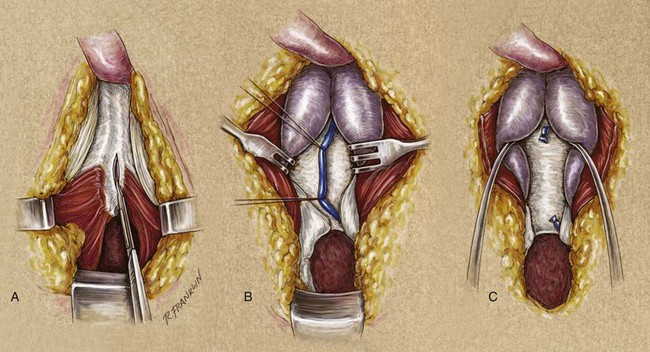
Figure 36–36 Division of the triangular ligament and development of the intracrural space. A, When the prostatic urethra is displaced, and the arc that the urethra must traverse needs to be shortened, that length can be shortened by incision of the triangular ligament. B, Incision and mobilization of the perichondrium and periosteum of the symphysis pubis to allow placement of retractors without trauma to the erectile bodies. Lateral displacement of the crura will expose the dorsal vein of the penis; after careful identification, the vein can be ligated and divided. C, Completion of the dissection affords additional exposure for resection of the fibrosis that surrounds the apex of the prostate and the proximal end of the disrupted urethra.
(A to C, From Jordan GH. Reconstruction of the meatus–fossa navicularis using flap techniques. In: Schreiter F, editor. Plastic-reconstructive surgery in urology. Stuttgart: Georg Thieme; 1999. p. 338–44.)
We then introduce a Haygrove staff into the suprapubic sinus and through the bladder neck to the distal limits of the posterior urethra (see Fig. 36–35G and H). The impulse is palpated, and the fibrosis is resected until normal tissue planes are encountered. The tissue is submitted for histologic examination. The tip of the Haygrove staff is eventually concealed only by the normal urethral epithelium, at which point we open the epithelium and control it with either a skin hook or a stitch. We then perform endoscopy to ensure that the urethrotomy is at the distal limits of the posterior urethra. If a tension-free anastomosis is not thought possible, we mobilize the corpus spongiosum beneath the scrotum from its attachment to the corpora cavernosa. Aggressive mobilization of the corpus spongiosum is the last maneuver undertaken, because it is thought to have possible ill effects on the retrograde blood supply, which in the pelvic fracture patient may be tenuous. Meticulous detachment of the investment of Buck fascia from the corpus spongiosum increases the compliance of the corpus and limits the need for aggressive mobilization.
It is important to try to avoid the creation of chordee during the repair of a distraction injury. To prevent chordee, the attachment cannot be carried beyond the area of the penoscrotal attachment. It is warranted in some cases, however, to preoperatively counsel patients that they may have some chordee after aggressive mobilization that results in a primary anastomotic repair. Primary anastomotic repairs carry success rates in the high 90% range. If a technique of tissue transfer is needed, the long-term cure rates may eventually only be in the mid 80% range. Most of these patients are young. Successful, durable reconstruction is of paramount importance. If chordee results, it is most often mild and not disabling sexually; in our and other surgeons’ minds, it is probably a fair trade for optimizing the urethral reconstruction. Development of the intracrural space—mobilization of the corpus spongiosum, infrapubectomy, and, if needed, rerouting of the corpus spongiosum—shortens the course that the corpus spongiosum must traverse and allows reconstruction without attendant chordee.
The proximal urethrotomy is spatulated so that it accepts at least a 32 French bougie à boule, and 10 to 12 anastomotic sutures are placed and tagged to allow identification of their position in the proximal anastomosis. We have used a combination of 3-0 Monocryl and 3-0 polydioxanone sutures for this purpose. No special needles are required for the placement of these sutures. However, a Heaney needle driver and a Ravitch needle driver can be useful in difficult cases. A lighted sucker is also beneficial at this point in the procedure. We recently have used a posterior retractor blade with integral light bundle and have found it very helpful with visualization.
After spatulation of the proximal urethrotomy and placement of the sutures, we spatulate the proximal portion of the anterior urethra. The spatulation is continued until the urethrotomy accepts a 30 to 32 French bougie à boule, and the anastomotic sutures are placed in their respective locations. Before seating the anastomosis, we introduce a soft silicone (Silastic) ribbed urethral stenting catheter through the anastomosis under direct vision. The wound is then copiously irrigated to reduce the clot around the area of the anastomosis, and the anastomosis is seated.
Next, we reattach the corpus spongiosum to the corpora cavernosa and the bulbospongiosum to the perineal body. We then place a small suction drain deep to the closure of the ischiocavernosus musculature and Colles fascia, and a second one superficial to that closure and beneath the subcutaneous closure.
In those cases in which the proximal urethra is significantly distracted in a rostral direction, the surgeon must be prepared to perform infrapubectomy (Fig. 36–37) or corporeal rerouting, or both (Fig. 36–38). The performance of the infrapubectomy, along with the development of the intercrural space, allows exposure of the apical prostatic urethra. When the prostatic urethra remains rostrally displaced, the impulse of the sound or instrument placed through the cystostomy tract into the bladder neck is often not readily apparent. In these situations, it is comforting to be able to palpate the bladder neck and the properly placed sound before embarking on a dissection beneath the pubis. In addition, if the rostral distraction is significant, the path of the anterior urethra over the hilum of the penis into the infrapubectomy often does not allow a tension-free anastomosis, and the infrapubectomy can be continued beneath one side of the corpora cavernosa, allowing rerouting of the corpus spongiosum (see Fig. 36–38).
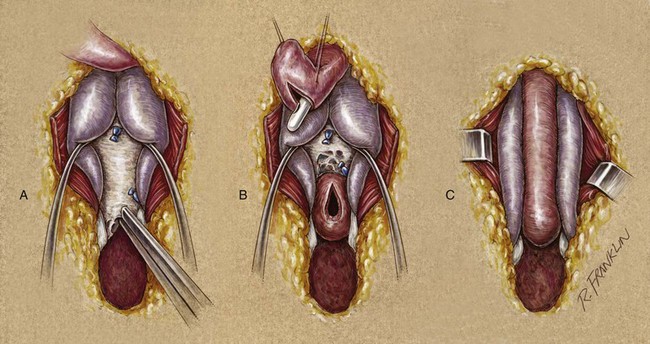
Figure 36–37 Infrapubectomy. If the prostate is elevated behind the symphysis pubis (A), the inferior aspect of the symphysis is resected with a Kerrison rongeur. As much of the bone can be removed as necessary (B), to afford a simple approximation of the ends of the urethra (C).
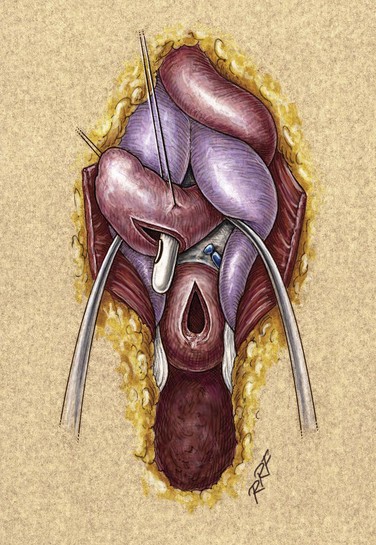
Figure 36–38 Resection of the pubis and rerouting of the urethra around the crus. When the prostate is markedly displaced, it may be necessary to expand the infrapubectomy. Sometimes, despite separation of the crura to the full extent possible, the two ends of the urethra do not meet when they are brought directly through the crus. It is necessary to bring the urethra lateral to one of the crura to make up this length.
Postoperative Management
We use a small soft silicone (Silastic) stenting catheter. Urine is diverted by way of the suprapubic cystostomy, and the urethral catheter is plugged and serves as a stent only. After the reconstruction, patients are initially kept at bed rest for 24 to 48 hours and then ambulated and discharged with the suprapubic catheter and stenting urethral catheter in place. They are also discharged on a regimen of oxybutynin and a suppressive antibiotic. The drains are removed as drainage allows.
A voiding trial with contrast material is performed between 21 and 28 days postoperatively. Patients are directed to stop taking oxybutynin 24 hours before the voiding trial. In anastomoses that are technically straightforward, the trial is performed at 21 days, and in those cases with more rostral distraction of the proximal urethra, the trial is delayed for 3 to 5 days longer. The trial involves removing the urethral catheter, filling the patient’s bladder with contrast material, and instructing him to void. We do not use pericatheter retrograde urethrography for the evaluation of patients who have undergone urethral reconstruction. The voiding film is examined to ensure that there is not extravasation and that the reanastomosis appears widely patent. A urine culture specimen is also obtained, and the suprapubic catheter is plugged. The patient is allowed to void through the urethra for 5 to 7 days, and the suprapubic catheter is then removed. Patients remain on the suppressive antibiotic regimen until they are tube free, and a culture and a sensitivity test are performed. At that time, they are prescribed a short regimen of culture-specific antibiotic.
Approximately 6 months postoperatively, and again 1 year later, the patients are evaluated with flexible endoscopy. At that time, we consider the reconstruction to be mature, and it should be widely patent. Absent the reappearance of symptoms, we refer further follow-ups to the referring urologist.
We have almost completely replaced postoperative retrograde studies with flexible endoscopy. We have not found flow studies to be valuable in observing these patients and, in many cases (anterior urethral reconstruction), have found that retrograde urethrography is more confusing than helpful.
With the use of the techniques discussed or similar techniques, curative rates for reconstruction of posterior pelvic fracture urethral injuries are in the high 90% range. In large centers, failures are not due to technical problems (i.e., anastomotic restenosis). In general, failures are indicative of ischemia of the proximal corpus spongiosum with ensuing stenosis of the mobilized corpus spongiosum. This occurs because, with mobilization, the corpus spongiosum, in essence, becomes a flap with the vascular pedicle being the retrograde vascularity from the arborization of the dorsal arteries through the glans (Fig. 36–39).
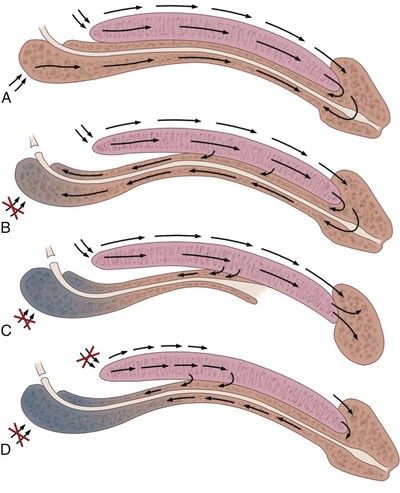
Figure 36–39 Diagrammatic representation of the deep vasculature of the penis. A, In the normal situation, through the common penile artery, flow is directed to the tip of the penis with arborization into the spongy erectile tissue of the glans penis. This provides retrograde flow into the corpus spongiosum. If the arteries of the bulb are intact, there is also antegrade arterial flow to the corpus spongiosum. B, With interruption of the arteries to the bulb and mobilization of the corpus spongiosum, all flow to the corpus spongiosum is retrograde through the common penile arterial system. C, In the case of hypospadias, in which the distal corpus spongiosum may have been interrupted, with proximal mobilization of the corpus spongiosum and therefore division of the arteries to the bulb. Even if the common penile circulation is intact to the tip of the penis, it may not adequately provide retrograde vascularity to the corpus spongiosum; hence, ischemic stenosis can ensue. D, In the case of injury to the common penile artery, with elevation of the proximal corpus spongiosum and division of the arteries to the bulb, blood flow to the proximal corpus spongiosum may not be adequate, leading to ischemic necrosis or ischemic stenosis.
We have studied this phenomenon in our trauma patients and have now arrived at conclusions that we believe allow us to predict the patients at risk for this ischemic atrophy phenomenon. Initially, we used pudendal angiography to study all our trauma patients who seemed to be at risk for bilateral deep internal pudendal artery injury at the time of trauma. These were patients who had evidence of injury to the dorsal penile nerves, patients in whom reconstruction had failed at other centers, patients with lateral impact pelvic fractures, and patients whose pelvic fractures were of the “windswept” variety (Brandes and Borrelli, 2001). We found that many patients had evidence of either unilateral or bilateral pudendal artery lesions but that most had evidence of vascular reconstitution. Patients with an intact pudendal artery on one side often were potent and were reliably cured with reconstruction. Patients with only reconstituted vessels, either unilateral or bilateral, never were potent but were reliably reconstructed. We found that these patients were optimal candidates for penile arterial revascularization to improve potency. Because we noted this relationship to potency, we began looking at our patients with duplex ultrasonography. We found that patients with normal pudendal arteries, either unilateral or bilateral, demonstrated normal arterial parameters on duplex evaluation. Those with only reconstituted arteries, either bilateral or unilateral, never had normal arterial parameters on duplex ultrasonography.
This now allows us to proceed to pudendal angiography only in those patients with abnormal arterial parameters on duplex ultrasonography; the patients with normal findings on ultrasonography predictably do well with reconstruction. Our data also show that patients do well with reconstruction if they have at least one side that is reconstituted, and those patients at risk for ischemic stenosis are only those with bilateral complete obstruction of the internal pudendal vessels. In such a patient, we now perform penile arterial revascularization to augment the vascularity and, with that accomplished, then proceed to urethral reconstruction (Jordan, 2005; Davies et al, 2009). We are currently evaluating new technology to see if we can get the same information regarding the deep vasculature without need for either duplex ultrasonography or angiography in many patients. The SPY Imaging System (Novadaq, Bonita Springs, FL) is a novel noninvasive technique of evaluating vasculature. It uses indocinen green (ICG) dye, which is a fluorescent compound injected intravenously. It binds to plasma proteins and consequently remains intravascular, making it ideal for imaging the arterial supply. A laser is used to excite the ICG dye and make it fluoresce. The filtered fluorescent images are captured by a charge-coupled device video camera demonstrating the vasculature. In many cases of pelvic fracture urethral distraction defects, erectile dysfunction is a consequence of the injury, although clearly in some patients, erectile dysfunction results from the reconstructive surgery. We think that the incidence of injury to the pudendal arteries is drastically underreported and underrecognized. Thus, we, along with others, believe that in many of these cases, the cause of erectile dysfunction is vascular (Brandes and Borrelli, 2001). However, others think that in most, the cause is neurologic (Shenfeld et al, 2003).
Summary
Using the maneuvers outlined, we have found that virtually all distraction injuries can be reconstructed through a perineal approach with an anastomotic technique. Although the above-and-below approach is used when concomitant bladder neck surgery is performed, the inability to accurately identify those patients has led us to perform bladder neck surgery at a second setting. We have therefore abandoned a transpubic approach as applied to posterior urethral distraction injuries.
Although we favor primary reconstruction of posterior urethral distraction injuries, others choose to manage these injuries endoscopically (Barry, 1989). We have found that the endoscopic management of pelvic fracture urethral injuries is not a simple procedure and must be undertaken only by a skilled and experienced surgeon. Many of these procedures can be categorized as a “cut-for-light” procedure. Although there are surgeons who report success, the majority of cut-for-light procedures are not done with sufficient precision to allow adequate realignment of the urethra. We have seen many disasters that have resulted from these procedures and in most cases condemn the use of these modalities. In addition, no cut-for-light series compares favorably, with regard to long-term success rates, with series from large centers that use primary anastomotic techniques (Levine and Wessells, 2001).
In 1989, Marshall described his method of using stereotactic techniques for endoscopic alignment of the ends of the urethra. He emphasized the length of time it takes to obtain precise alignment before undertaking the endoscopic portion of the procedure. In his procedure, he passes a wire through the aligned ends of the urethra, minimally dilating the channel, and widening it with transurethral resection. The scar is stabilized by a period of self-catheterization. A communication with Marshall has determined that he has limited the applications of this procedure and does not advocate it as a primary modality. Patients whose medical condition, age, or concomitant orthopedic injury prevents them from being placed in the exaggerated lithotomy position, or reconstructed using a transpubic approach, may be managed with this technique.
In children, the goals of surgery are the same as in adults. In our experience, most children can be reconstructed by the same perineal exposure as in adults. Exposure is clearly more difficult, but nonetheless perineal anastomosis can be done (Hafez et al, 2005). However, the posterior, sagittal trans-sphincteric approach has been proposed as a better approach in children (Mathews et al, 1998; Peña and Hong, 2004). We agree that the posterior approach is an elegant method of exposure; however, our observation is that with this approach, surgeons tend to resort to techniques of substitution reconstruction where primary anastomosis could be done and, in our opinion, is superior. With our experience accumulating using the vessel-sparing approach to anterior urethral reconstruction—both primary anastomosis and augmented anastomosis—we have extended the technique to select patients with pelvic fracture urethral reconstruction and have found the approach feasible with good results in a small number of patients. The advantage however has not been proven.
Key Points: Distraction Injuries of the Urethra