Infections
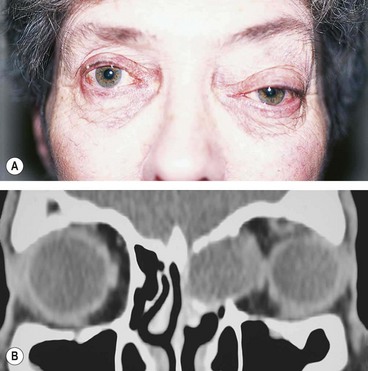
Preseptal cellulitis
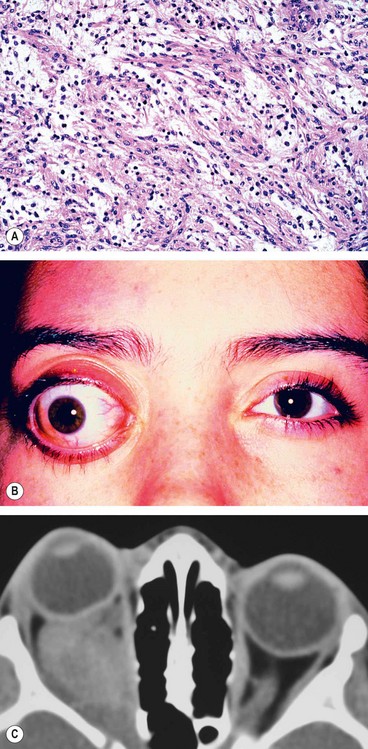
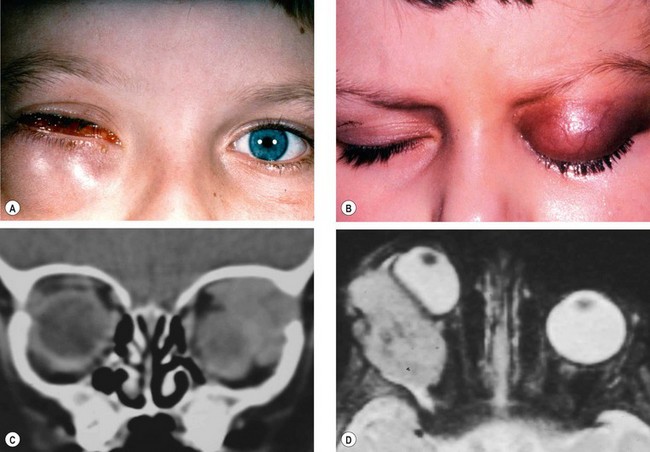
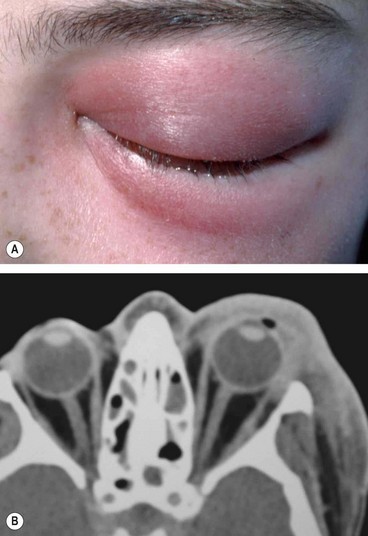
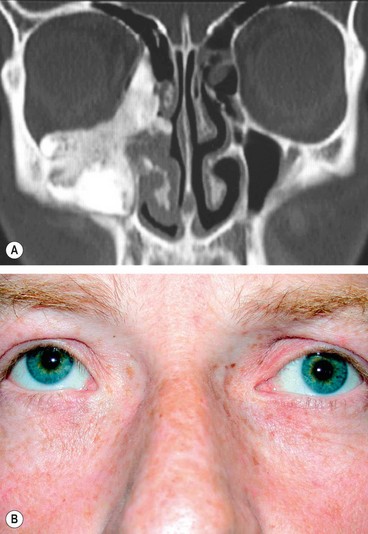
Preseptal cellulitis is an infection of the subcutaneous tissues anterior to the orbital septum. Although not strictly an orbital disease, it is included here because it must be differentiated from the much less common but potentially more serious orbital cellulitis. Occasionally rapid progression to orbital cellulitis may occur.
1
Causes
a
Skin trauma such as laceration or insect bites. The offending organism is usually
S. aureus or
S. pyogenes.
b
Spread of local infection, such as from an acute hordeolum, dacryocystitis or sinusitis.
c
From remote infection of the upper respiratory tract or middle ear by haematogenous spread.
2
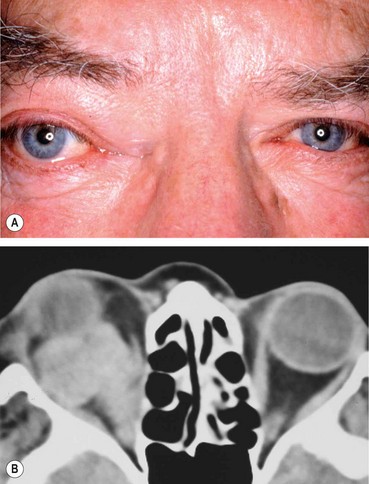
Signs
•
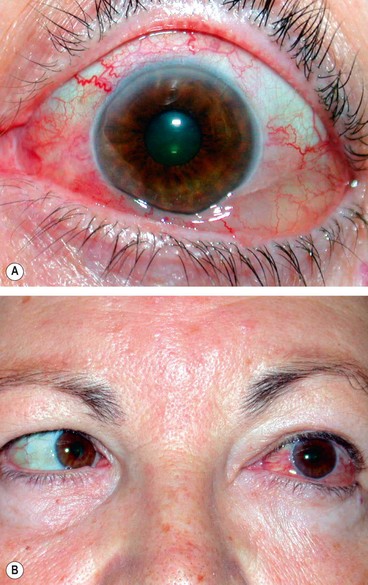
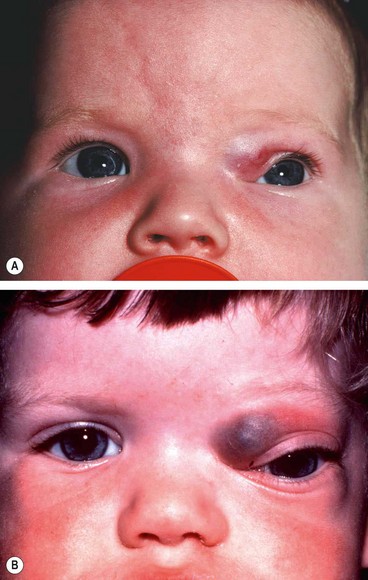
Unilateral tender and red lid with periorbital oedema (
Fig. 3.13A).
•
In contrast to orbital cellulitis proptosis and chemosis are absent; visual acuity, pupillary reactions and ocular motility are unimpaired.
3
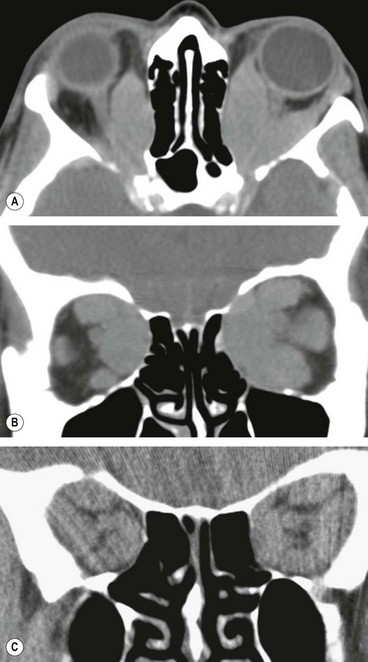
CT shows opacification anterior to the orbital septum (
Fig. 3.13B).
4
Treatment is with oral co-amoxiclav 500/125 mg every 8 hours. Severe infection may require intravenous antibiotics.
Bacterial orbital cellulitis
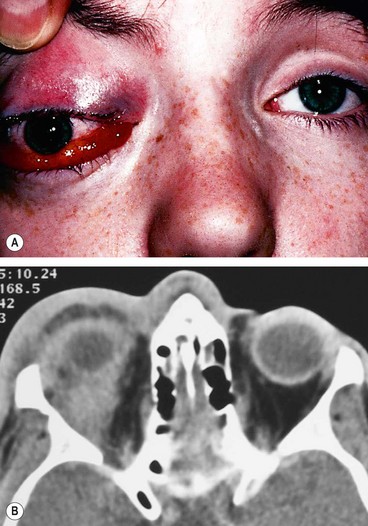
Bacterial orbital cellulitis is a life-threatening infection of the soft tissues behind the orbital septum. It can occur at any age but is more common in children. The most common causative organisms are S. pneumoniae, S. aureus, S. pyogenes and H. influenzae.
Pathogenesis
1
Sinus-related, most commonly ethmoidal, typically affects children and young adults.
2
Extension of preseptal cellulitis through the orbital septum.
3
Local spread from adjacent dacryocystitis, mid-facial or dental infection. The last condition may cause orbital cellulitis via an intermediary maxillary sinusitis.
5
Post-traumatic develops within 72 hours of an injury that penetrates the orbital septum. The typical clinical features may be masked by associated laceration or haematoma.
6
Post-surgical may complicate retinal, lacrimal or orbital surgery.
Diagnosis
1
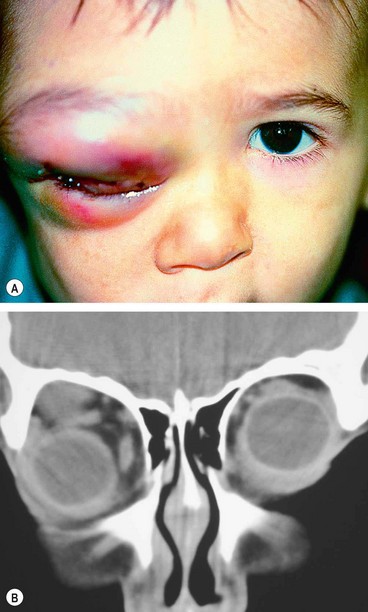
Presentation is with the rapid onset of severe malaise, fever, pain and visual impairment.
2
Signs
•
Unilateral tender warm and red periorbital and lid oedema.
•
Proptosis, often obscured by lid swelling, is most frequently lateral and downwards.
•
Optic nerve dysfunction.
3
CT shows opacification posterior to the orbital septum (
Fig. 3.14B).
Complications
1
Ocular complications include exposure keratopathy, raised intraocular pressure, occlusion of the central retinal artery or vein, endophthalmitis and optic neuropathy.
2
Intracranial complications, which are rare but extremely serious, include meningitis, brain abscess and cavernous sinus thrombosis. The last is an extremely serious complication which should be suspected when there is evidence of bilateral involvement, rapidly progressive proptosis and congestion of the facial, conjunctival and retinal veins. Additional features include abrupt progression of all clinical signs associated with prostration, severe headache, nausea and vomiting.
3
Subperiosteal abscess is most frequently located along the medial orbital wall.
Treatment
1
Hospital admission with otolaryngological assessment and frequent ophthalmic review is mandatory.
2
Antibiotic therapy involves intravenous ceftazidime, with oral metronidazole to cover anaerobes. Vancomycin
is a useful alternative in the context of penicillin allergy. Antibiotic therapy should be continued until the patient has been apyrexial for 4 days.
3
Monitoring of optic nerve function every 4 hours by testing pupillary reactions, visual acuity, colour vision and light brightness appreciation.
4
Investigations, where appropriate, include the following:
•
CT of the orbit, sinuses and brain. Orbital CT is particularly useful to exclude a subperiosteal abscess.
•
Lumbar puncture if meningeal or cerebral signs develop.
5
Surgical intervention in which the infected sinuses and orbital collections are drained should be considered in the following circumstances:
•
Lack of response to antibiotics.
•
Subperiosteal or intracranial abscess.
•
Atypical picture, which may merit a biopsy.
Rhino-orbital mucormycosis
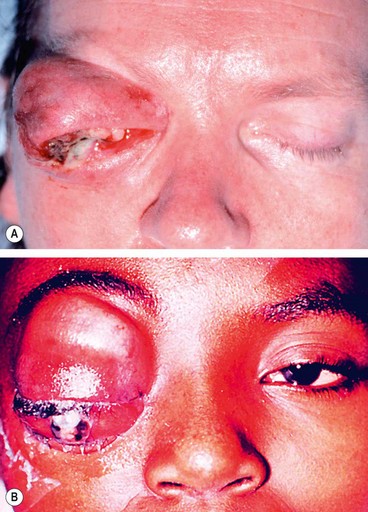
Mucormycosis is a very rare opportunistic infection caused by fungi of the family Mucoraceae, which typically affects patients with diabetic ketoacidosis or immunosuppression. This aggressive and often fatal infection is acquired by the inhalation of spores, which give rise to an upper respiratory infection. The infection then spreads to the contiguous sinuses and subsequently to the orbit and brain. Invasion of blood vessels by the hyphae results in occlusive vasculitis with ischaemic infarction of orbital tissues.
1
Presentation is with gradual onset facial and periorbital swelling, diplopia and visual loss.
2
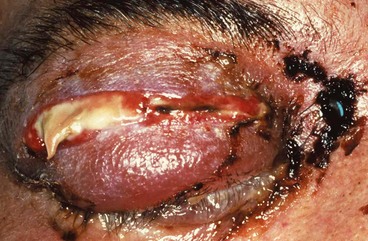
Signs
•
Ischaemic infarction superimposed on septic necrosis is responsible for the black eschar which may develop on the palate, turbinates, nasal septum, skin and eyelids (
Fig. 3.15).
•
Progression is slower than in bacterial orbital cellulitis.
3
Complications include retinal vascular occlusion, multiple cranial nerve palsies and cerebrovascular occlusion.
4
Treatment
•
Intravenous antifungal agents such as amphotericin.
•
Daily packing and irrigation of the involved areas with amphotericin.
•
Wide excision of devitalized and necrotic tissues.
•
Adjunctive hyperbaric oxygen may be helpful.
•
Correction of the underlying metabolic defect, if possible.
•
Exenteration may be required in unresponsive cases.
Non-Infective Inflammatory disease
Idiopathic orbital inflammatory disease
Idiopathic orbital inflammatory disease (IOID), previously referred to as orbital pseudotumour, is an uncommon disorder characterized by non-neoplastic, non-infective, space-occupying orbital lesions. The inflammatory process may involve any or all of the orbital soft tissues, resulting in, for example, myositis, dacryoadenitis, optic perineuritis or scleritis. Histopathological analysis reveals pleomorphic inflammatory cellular infiltration followed by reactive fibrosis, but has thus far shown no correlation between clinicopathological features and the subsequent course of the disease. Unilateral disease is the rule in adults, although in children bilateral involvement may occur. Simultaneous orbital and sinus involvement is a rare distinct entity.
Diagnosis
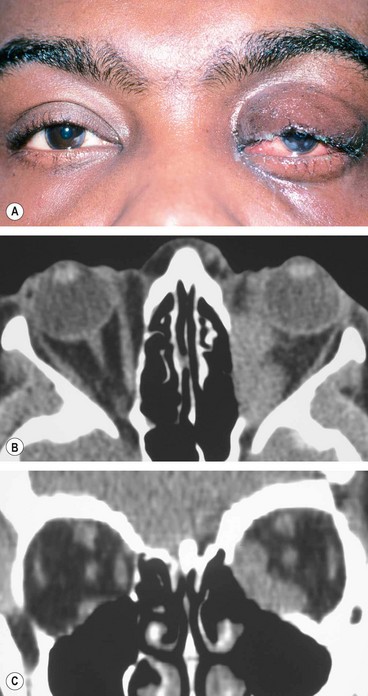
1
Presentation is with acute periorbital redness, swelling and pain (
Fig. 3.16A).
2
Signs
•
Congestive proptosis and ophthalmoplegia may occur.
•
Optic nerve dysfunction, particularly if the inflammation involves the posterior orbit.
3
CT shows ill-defined orbital opacification and loss of definition of contents (
Fig. 3.16B and C).
4
Course. This follows one of the following patterns:
•
Spontaneous remission after a few weeks without sequelae.
•
Intermittent episodes of activity with eventual remission.
•
Severe prolonged inflammation eventually leading to progressive fibrosis of orbital tissues, resulting in a ‘frozen orbit’ characterized by ophthalmoplegia, which may be associated with ptosis and visual impairment caused by optic nerve involvement.
Treatment
1
Observation, for relatively mild disease, in anticipation of spontaneous remission.
2
Biopsy is generally required in persistent cases to confirm the diagnosis and rule out neoplasia.
3
NSAIDs are often effective and may precede steroid therapy.
4
Systemic steroids should be administered only after the diagnosis has been confirmed, as they may mask other pathology such as infection and Wegener granulomatosis. Oral prednisolone, initially 60–80 mg/day, is later tapered and discontinued, depending on clinical response, although it may need to be reintroduced in the event of recurrence.
5
Radiotherapy may be considered if there has been no improvement after 2 weeks of adequate steroid therapy. Even a low dose treatment (i.e. 10 Gy) may produce remission.
6
Antimetabolites such as methotrexate or mycophenolate mofetil may be necessary in the context of resistance to both steroids and radiotherapy.
7
Systemic infliximab, a tumour necrosis factor inhibitor, may be effective in recurrent or recalcitrant cases that have failed to respond to conventional therapy.
Differential diagnosis
1
Bacterial orbital cellulitis should be considered when onset is acute and the anterior orbital tissues are markedly inflamed. A trial of systemic antibiotics may be necessary before the correct diagnosis becomes apparent.
2
Severe acute TED shares many features with IOID, but is commonly bilateral while IOID is usually unilateral.
3
Systemic disorders such as Wegener granulomatosis, polyarteritis nodosa and Waldenström macroglobulinaemia
may manifest orbital involvement similar to IOID.
4
Malignant orbital tumours, particularly metastatic.
5
Ruptured dermoid cyst may evoke a secondary painful granulomatous inflammatory reaction.
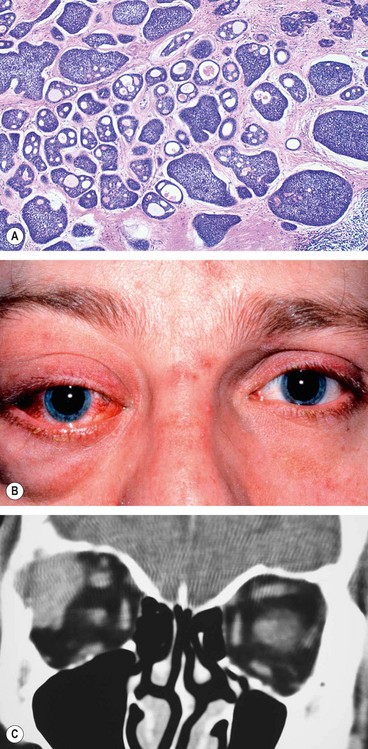
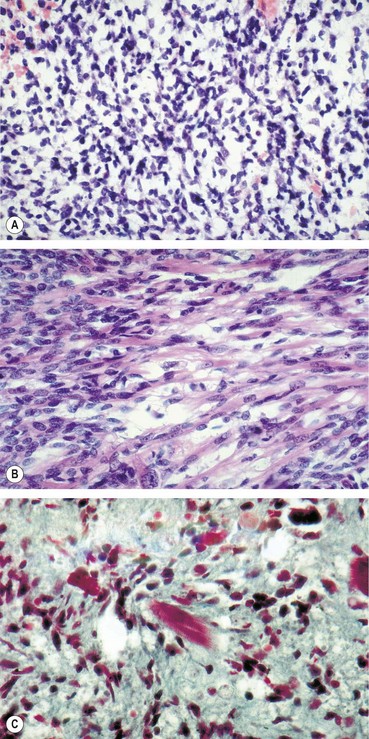
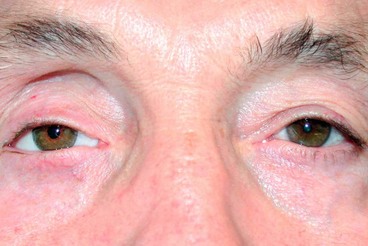
Orbital myositis
Orbital myositis is an idiopathic, non-specific inflammation of one or more extraocular muscles and is considered a subtype of IOID.
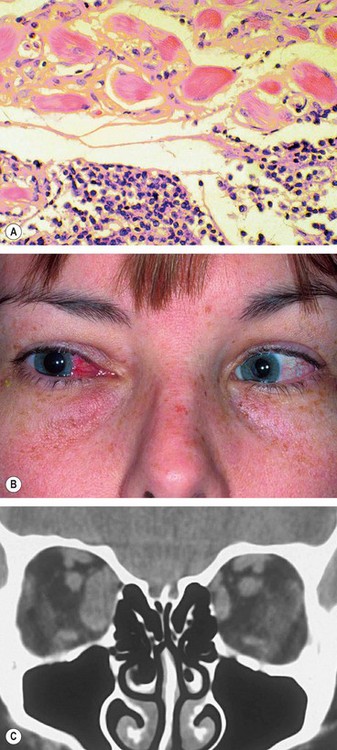
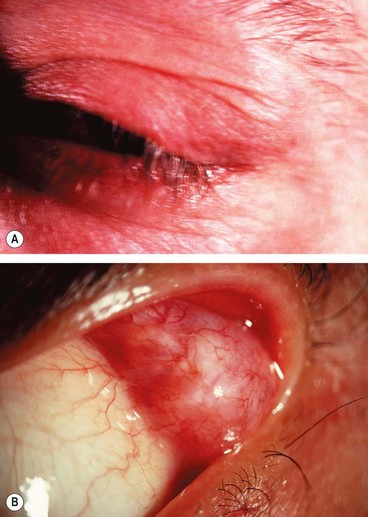
1
Histology shows a chronic inflammatory cellular infiltrate in relation to muscle fibres (
Fig. 3.17A).
2
Presentation is usually in early adult life with acute pain, exacerbated by eye movement, and diplopia.
3
Signs
•
Lid oedema, ptosis and chemosis.
•
Pain and diplopia associated with eye movements.
•
Vascular injection over the involved muscle (
Fig. 3.17B).
•
In chronic cases the affected muscle may become fibrosed, with permanent restrictive myopathy.
4
CT shows enlargement of the affected muscles (
Fig. 3.17C), with or without involvement of the tendons of insertion.
5
Differential diagnosis includes orbital cellulitis, TED and Tolosa–Hunt syndrome (see below).
6
Course
•
Acute non-recurrent involvement which resolves spontaneously within 6 weeks.
•
Chronic disease characterized by either a single episode persisting for longer than 2 months (often for years) or recurrent attacks.
7
Treatment is aimed at relieving discomfort and dysfunction, shortening the course and preventing recurrences.
a
NSAIDs may be adequate in mild disease.
b
Systemic steroids are generally required and usually produce dramatic improvement, although recurrences occur in 50% of cases.
c
Radiotherapy is also effective, particularly in limiting recurrence.
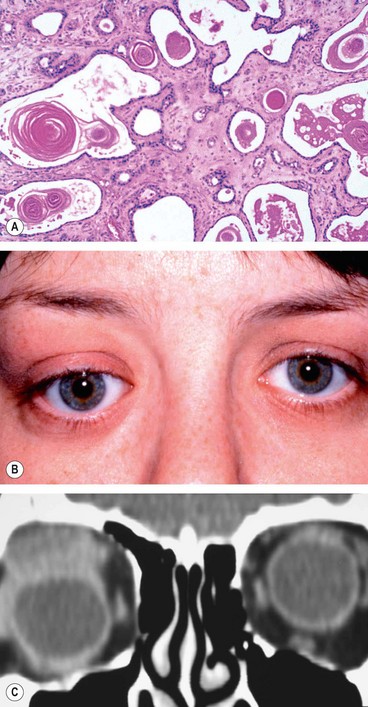
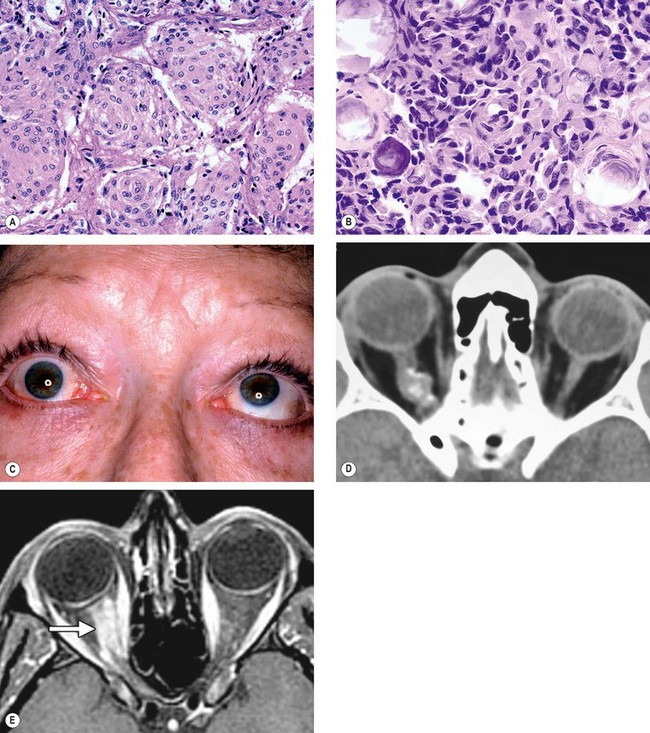
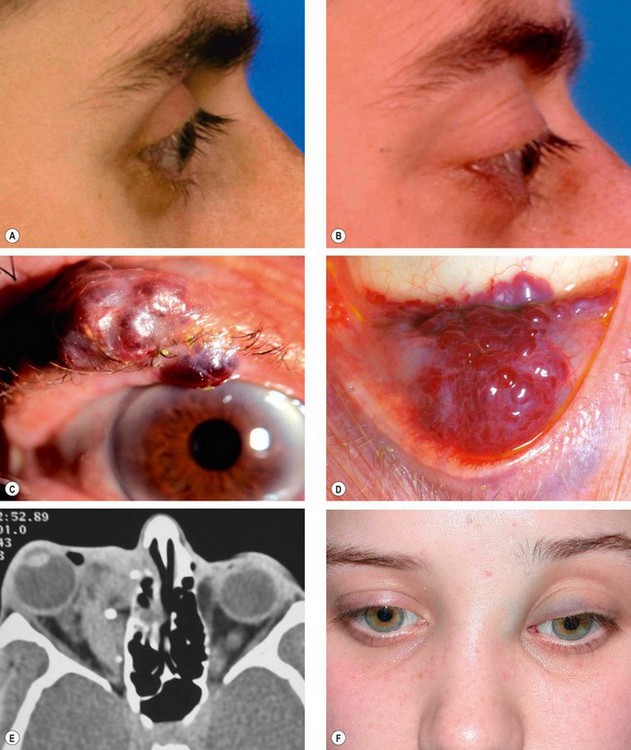
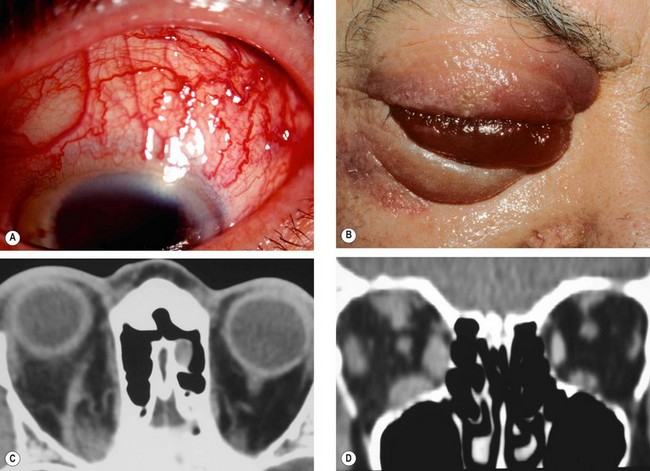
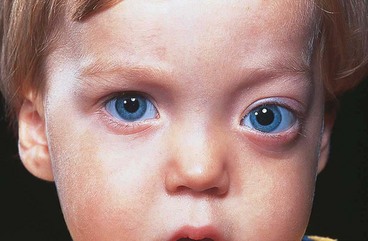
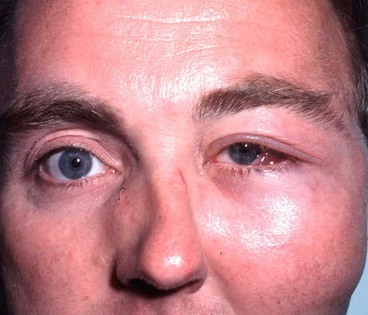
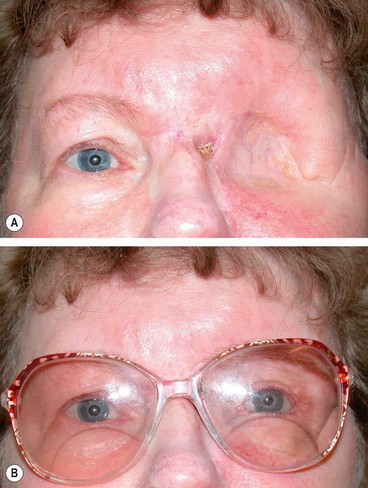
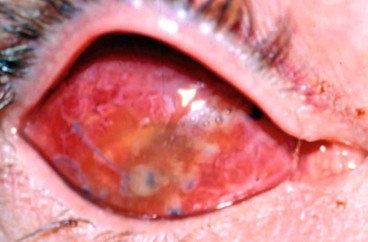
Acute dacryoadenitis
Lacrimal gland involvement occurs in about 25% of patients with IOID. More commonly, dacryoadenitis occurs in isolation, resolves spontaneously and does not require treatment. Occasionally it may be caused by mumps, mononucleosis, and rarely bacteria.
1
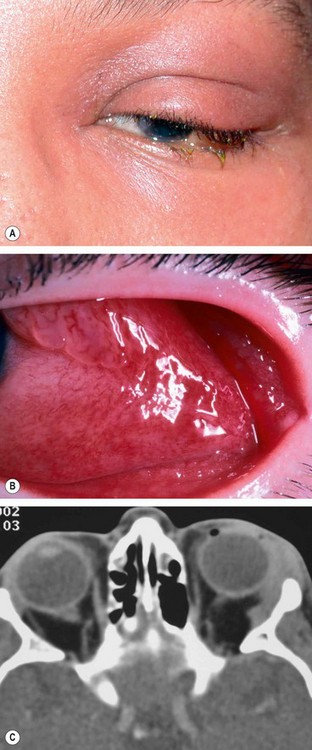
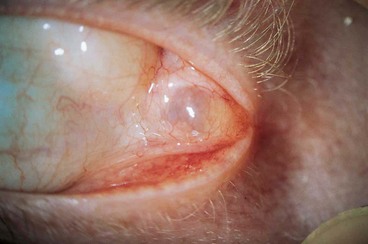
Presentation is with acute discomfort in the region of the lacrimal gland.
2
Signs
•
Swelling of the lateral aspect of the eyelid giving rise to a characteristic S-shaped ptosis and slight downward and inward dystopia (
Fig. 3.18A).
•
Tenderness over the lacrimal gland fossa.
•
Injection of the palpebral portion of the lacrimal gland and adjacent conjunctiva (
Fig. 3.18B).
•
Lacrimal secretion may be reduced.
3
CT shows enlargement of the gland and involvement of adjacent tissues (
Fig. 3.18C).
4
Differential diagnosis
a
Ruptured dermoid cyst may cause localized inflammation in the region of the lacrimal gland.
b
Malignant lacrimal gland tumours may cause pain but the onset is not usually acute.
Tolosa–Hunt syndrome
Tolosa–Hunt syndrome is a diagnosis of exclusion. It is a rare idiopathic condition caused by non-specific granulomatous inflammation of the cavernous sinus, superior orbital fissure and/or orbital apex. The clinical course is characterized by remissions and recurrences.
1
Presentation is with diplopia associated with ipsilateral periorbital or hemicranial pain.
2
Signs
•
Proptosis, if present, is usually mild.
•
Ocular motor nerve palsies often with involvement of the pupil.
•
Sensory loss along the distribution of the first and second divisions of the trigeminal nerve.
3
Treatment is with systemic steroids.
Wegener granulomatosis
Wegener granulomatosis may involve the orbit, often bilaterally, usually by contiguous spread from the paranasal sinuses or nasopharynx. Primary orbital involvement is less common. The possibility of Wegener granulomatosis should be considered in any patient with bilateral orbital inflammation, particularly if associated with sinus pathology. The antineutrophilic cytoplasmic antibody (cANCA) is a useful serological test.
1
Signs
•
Proptosis, orbital congestion and ophthalmoplegia.
•
Dacryoadenitis and nasolacrimal duct obstruction.
•
Coexistent manifestations include scleritis, peripheral ulcerative keratitis, intraocular inflammation and retinal vascular occlusions.
2
Treatment
a
Systemic cyclophosphamide and steroids are very effective. In resistant cases ciclosporin, azathioprine, antithymocyte globulin or plasmapheresis may be useful.
b
Surgical orbital decompression may be required for severe orbital involvement.
Vascular malformations
Varices
Varices consist of weakened segments of the orbital venous system of variable length and complexity. Intrinsic to the circulation, they enlarge with increased venous pressure, their distensability varying with the residual thickness and strength of their walls. Most cases are unilateral and the most frequent site is upper nasal. Phleboliths are present in about 20% of cases.
1
Presentation ranges from early childhood to late middle age.
2
Signs
•
Intermittent non-pulsatile proptosis not associated with a bruit.
•
As the orbital veins are devoid of valves, rapidly reversible proptosis may be precipitated or accentuated by increasing venous pressure through coughing, straining, Valsalva manoeuvre (
Fig. 13.19A and B), assuming the dependent position or external compression of the jugular veins.
3
Associations include varices of the eyelids (
Fig. 13.19C) and conjunctiva (
Fig. 13.19D) which may also be accentuated by performing the Valsalva manoeuvre.
5
Complications include acute haemorrhage and thrombosis. Patients with long-standing lesions may develop atrophy of surrounding fat and enophthalmos associated with a deepened superior sulcus in the resting position (
Fig. 13.19F), reversible with an increase in venous pressure.
6
Treatment by surgical excision is technically difficult and often incomplete because the lesions are friable and bleed easily. Indications include recurrent thrombosis, pain, severe proptosis and optic nerve compression.
Lymphangioma
Lymphangiomas are not neoplasms but abortive, non-functional, benign, vascular malformations. Although haemodynamically isolated from the circulation, bleeding into the lumen may occur with resultant blood-filled ‘chocolate’ cysts. Lymphangiomas may be confused with orbital venous anomalies and haemangiomas.
1
Presentation is usually in early childhood.
2
Signs
•
Anterior lesions typically manifest several soft bluish masses in the upper nasal quadrant (
Fig. 3.20).
•
Posterior lesions may cause slowly progressive proptosis, or initially may lie dormant and later present with the sudden onset of painful proptosis (
Fig. 3.21A and B) secondary to spontaneous haemorrhage, which may be associated with optic nerve compression.
•
The blood subsequently becomes encysted with the formation of chocolate cysts which may regress spontaneously with time.
•
Involvement of the oropharynx may be present (
Fig. 3.21C).
3
Treatment involving surgical excision is difficult because lymphangiomas are friable, not encapsulated, bleed easily and may infiltrate normal orbital tissues. Persistent sight-threatening chocolate cysts can be drained or removed sub-totally by controlled vaporization using a carbon dioxide laser.
Carotid-cavernous fistula
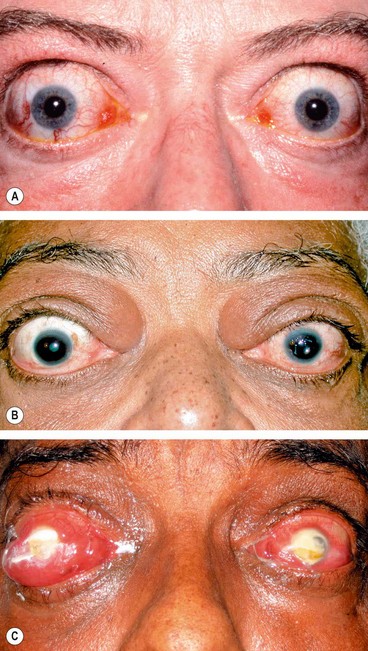
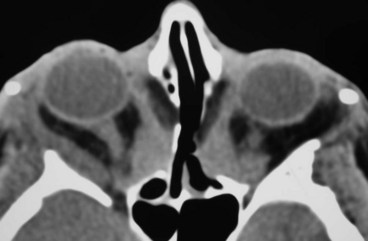
An arteriovenous fistula is an acquired abnormal communication between an artery and a vein. The blood within the affected vein becomes ‘arterialized’, the venous pressure rises, and venous drainage may be altered in both rate and direction. The arterial pressure and perfusion are also reduced. A carotid-cavernous fistula is one such communication, between the carotid artery and the cavernous sinus. When arterial blood flows anteriorly into the ophthalmic veins, ocular manifestations occur because of venous and arterial stasis around the eye and orbit, increased episcleral venous pressure and a decrease in arterial blood flow to the cranial nerves within the cavernous sinus. Carotid-cavernous fistulae can be classified on the basis of: (a) aetiology (spontaneous and traumatic), (b) haemodynamics (high and low flow) and (c) anatomy (direct and indirect).
Direct carotid-cavernous fistula
Pathogenesis
Representing 50% of all cases, direct fistulae are high-flow shunts in which carotid artery blood passes directly into the cavernous sinus through a defect in the wall of the intracavernous portion of the internal carotid artery as a result of the following:
1
Trauma is responsible for 75% of cases. A basal skull fracture may cause a tear in the intracavernous internal carotid artery with sudden and dramatic onset of symptoms and signs.
2
Spontaneous rupture of an intracavernous carotid aneurysm or an atherosclerotic artery accounts for the remainder. Middle-aged hypertensive women are at particular risk. Spontaneous fistulae usually have lower flow rates and less severe symptoms than traumatic.
Diagnosis
1
Presentation may be days or weeks after head injury with the classic triad of pulsatile proptosis, conjunctival chemosis and a whooshing noise in the head.
2
Signs are usually ipsilateral to the fistula but may be bilateral or even contralateral because of the vascular connections across the midline between the two cavernous sinuses.
•
Ptosis (due to 3rd nerve involvement) and haemorrhagic chemosis (
Fig. 3.22B). The presence of ptosis may be helpful in differentiating this condition from acute TED.
•
Pulsatile proptosis associated with a bruit and a thrill, both of which can be abolished by ipsilateral carotid compression in the neck. A cephalic bruit may also be present.
•
Increased intraocular pressure from elevated episcleral venous pressure and orbital congestion.
•
Anterior segment ischaemia, characterized by corneal epithelial oedema, aqueous cells and flare, and in severe cases iris atrophy, cataract and rubeosis iridis.
•
Ophthalmoplegia occurs in 60–70% of cases due to the ocular motor nerve damage by initial trauma or an intracavernous aneurysm or by the fistula itself. The 6th nerve is most frequently affected because of its free-floating location within the cavernous sinus. The 3rd and 4th nerves, situated in the lateral wall of the sinus, are less frequently involved. Engorgement and swelling of extraocular muscles may also contribute to defective ocular motility.
•
Fundus examination may show optic disc swelling, venous dilatation and intraretinal haemorrhages from venous stasis and impaired retinal blood flow. Preretinal or vitreous haemorrhages are rare.
2
Vision. Immediate visual loss may be due to ocular or optic nerve damage at the time of head trauma. Delayed visual loss may occur as a result of exposure keratopathy, secondary glaucoma, central retinal vein occlusion, anterior segment ischaemia or ischaemic optic neuropathy.
3
Special investigations. CT and MR may demonstrate prominence of the superior ophthalmic vein (
Fig. 3.22C) and diffuse enlargement of extraocular muscles (
Fig. 3.22D). Definitive diagnosis involves CT and MR angiography.
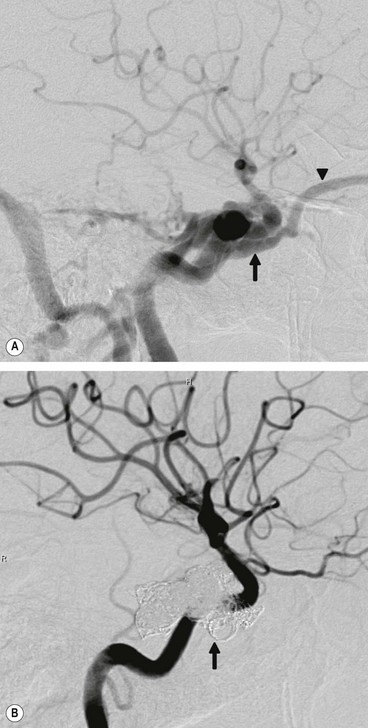
Treatment
Most carotid-cavernous fistulae are not life-threatening; the organ at major risk is the eye. Surgery is indicated if spontaneous closure does not occur. A post-traumatic fistula is much less likely to close on its own than a spontaneous fistula because of higher blood flow. The current treatment of choice involves endovascular embolization with coils (Fig. 3.23) or balloons which may be transvenous or transarterial.
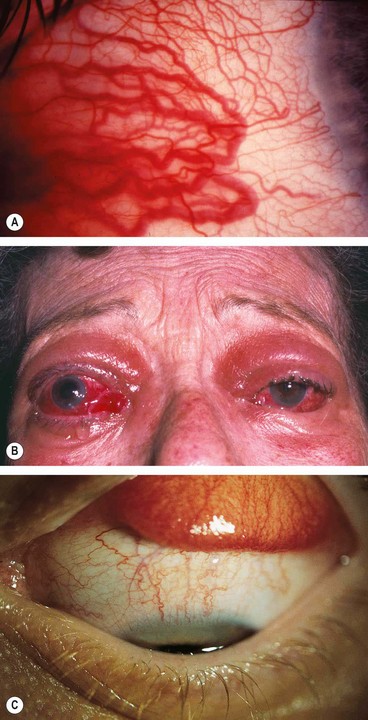
Indirect carotid-cavernous fistula
In an indirect carotid-cavernous fistula (‘dural shunt’), the intracavernous portion of the internal carotid artery remains intact. Arterial blood flows through the meningeal branches of the external or internal carotid arteries indirectly into the cavernous sinus. Due to slow blood flow, the clinical features are more subtle than in a direct fistula. The condition may therefore be misdiagnosed or missed altogether.
1
Types
•
Between meningeal branches of the internal carotid artery and the cavernous sinus.
•
Between meningeal branches of the external carotid artery and the cavernous sinus.
•
Between meningeal branches of both the external and internal carotid arteries and the cavernous sinus.
2
Causes
a
Congenital malformations, in which the onset of symptoms is precipitated by intracranial vascular thrombosis.
b
Spontaneous rupture, especially in hypertensive patients, which may be precipitated by minor trauma or straining.
3
Presentation is with gradual onset of redness of one or both eyes caused by conjunctival vascular engorgement.
4
Signs are variable.
•
Mild epibulbar injection with or without chemosis (
Fig. 3.24A).
•
Exaggerated ocular pulsation best detected on applanation tonometry.
•
‘Corkscrew-like’ epibulbar vessels is a common later sign.
•
Raised intraocular pressure.
•
Mild proptosis occasionally associated with a soft bruit.
•
Ophthalmoplegia caused by 6th nerve palsy (
Fig. 3.24B) or swelling of extraocular muscles in marked cases.
•
Fundus may be normal or manifest moderate venous dilatation.
5
Differential diagnosis includes chronic conjunctivitis, thyroid eye disease, glaucoma caused by other pathology and orbital arteriovenous malformations which may mimic dural shunts.
6
Treatment, if required, usually involves endovascular embolization, although not via the transarterial route.
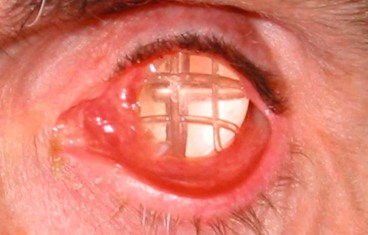
Cystic lesions
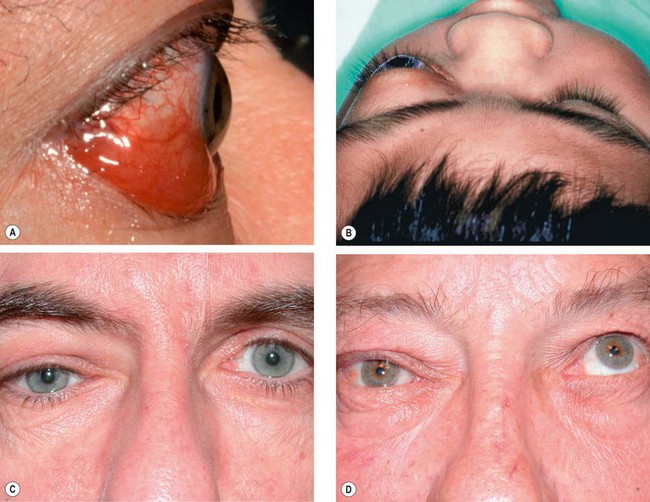
Dacryops
A dacryops is a ductal cyst of the lacrimal gland. It is a relatively uncommon orbital cystic lesion that is frequently bilateral.
1
Signs. A round, cystic lesion, originating from the palpebral portion of the lacrimal gland, which protrudes into the superior fornix (
Fig. 3.25).
2
Treatment involves excision or marsupialization.
Dermoid cyst
An orbital dermoid cyst is a choristoma derived from displacement of ectoderm to a subcutaneous location along embryonic lines of closure. Dermoids are lined by keratinized stratified squamous epithelium (like skin), have a fibrous wall and contain dermal appendages such as sweat glands, sebaceous glands and hair follicles. Epidermoid cysts do not contain such adnexal structures. Dermoids may be (a) superficial or (b) deep, located anterior or posterior to the orbital septum respectively.
Superficial dermoid cyst
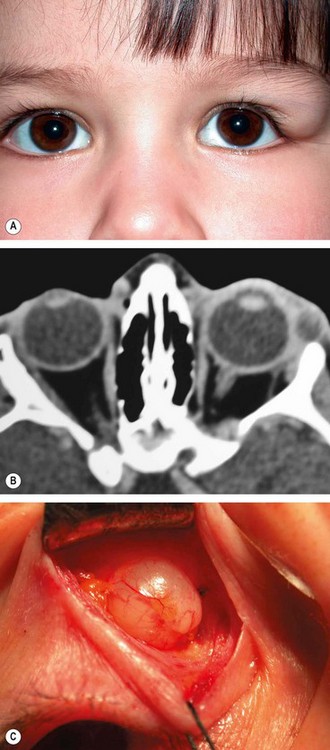
1
Presentation is in infancy with a painless nodule most commonly located in the superotemporal and occasionally the superonasal part of the orbit.
2
Signs
•
A firm round smooth non-tender mass 1–2 cm in diameter (
Fig. 3.26A), mobile under the skin but usually tethered to the adjacent periosteum.
•
The posterior margins are easily palpable, denoting lack of deeper origin or extension.
3
CT shows a heterogeneous well-circumscribed lesion (
Fig. 3.26B).
4
Treatment is by excision
in toto (
Fig. 3.26C), taking care not to rupture the lesion, since leaking of keratin into the surrounding tissue results in severe granulomatous inflammation.
Deep dermoid cyst
1
Presentation is in adolescence or adult life.
2
Signs
•
Proptosis, dystopia or a mass lesion with indistinct posterior margins (
Fig. 3.27A).
•
Rupture may incite an inflammatory reaction.
•
Some deep dermoids, associated with bony defects, may extend into the inferotemporal fossa or intracranially.
3
CT shows a well-circumscribed cystic lesion (
Fig. 3.27B).
4
Treatment by excision
in toto is advisable because deep dermoids enlarge and may leak their contents
into adjacent tissues inducing a painful granulomatous inflammation, often followed by fibrosis. If incompletely excised, dermoids may recur and cause persistent low-grade inflammation.
Sinus mucocele
A mucocele develops when the drainage of normal para-nasal sinus secretions is obstructed due to infection, allergy, trauma, tumour or congenital narrowing. A slowly expanding cystic accumulation of mucoid secretions and epithelial debris develops and gradually erodes the bony walls of the sinuses, causing symptoms by encroaching upon surrounding tissues. Orbital invasion occurs usually from frontal or ethmoidal mucoceles, and rarely from those arising in the maxillary sinus.
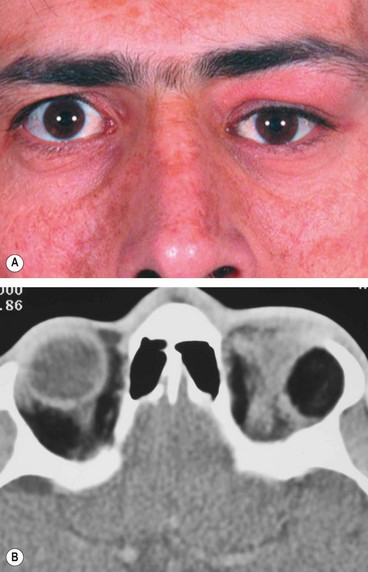
1
Presentation is in adult life with proptosis or dystopia (
Fig. 3.28A), diplopia or epiphora. Pain is uncommon unless secondary infection develops (mucopyocele).
2
CT shows a soft tissue mass with thinning or erosion of the bony walls of the sinus (
Fig. 3.28B).
3
Treatment involves complete removal of the mucocele.
Encephalocele
An encephalocele is formed by herniation of intracranial contents through a congenital defect of the base of the skull. A meningocele contains only dura whilst a meningoencephalocele also contains brain tissue. Orbital encephalocele may be (a) anterior (fronto-ethmoidal) or (b) posterior, which is associated with dysplasia of the sphenoid bone.
1
Presentation is usually during infancy.
2
Signs
•
Anterior encephaloceles involve the superomedial part of the orbit and displace the globe forwards and laterally (
Fig. 3.29A).
•
Posterior encephaloceles displace the globe forwards and downwards (
Fig. 3.29B).
•
The displacement increases on straining or crying and may be reduced by manual pressure.
•
Pulsating proptosis may occur due to communication with the subarachnoid space but, because the communication is not vascular, there is neither a thrill nor a bruit.
3
CT shows the bony defect responsible for the herniation (
Fig. 3.29C).
4
Differential diagnosis
a
Of anterior encephaloceles includes other causes of medial canthal swellings such as dermoid cysts and amniontoceles of the lacrimal sac.
b
Of posterior encephaloceles includes other orbital lesions that present during early life such as capillary haemangioma, juvenile xanthogranuloma, teratoma and microphthalmos with cyst.
5
Associations
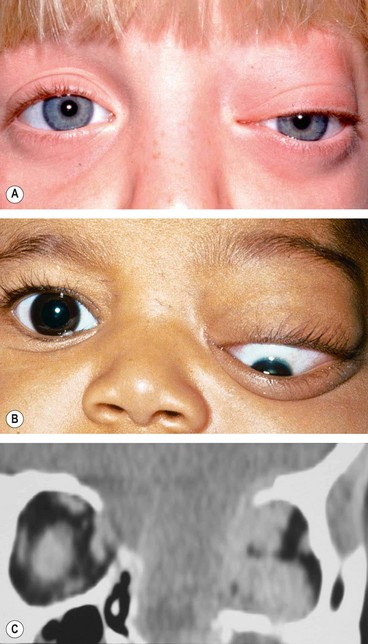
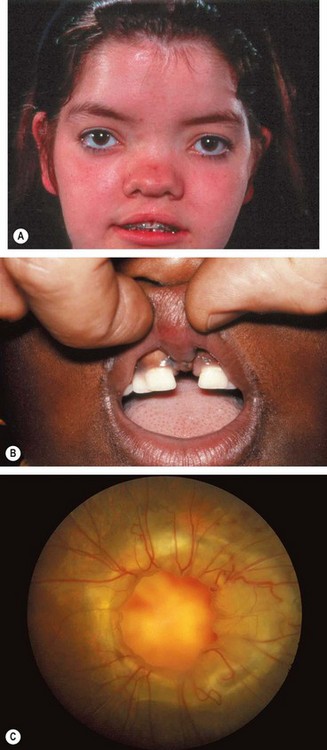
a
Other bony abnormalities such as hypertelorism (
Fig. 3.30A), broad nasal bridge and cleft palate (
Fig. 3.30B).
b
Ocular associations include microphthalmos, orbital varices, colobomas and the morning glory syndrome (
Fig. 3.30C).
c
Neurofibromatosis type 1 (NF1) is frequently associated with posterior encephalocele.
Tumours
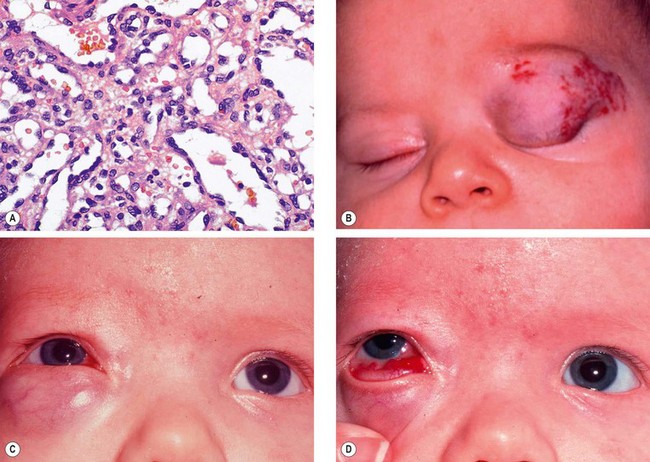
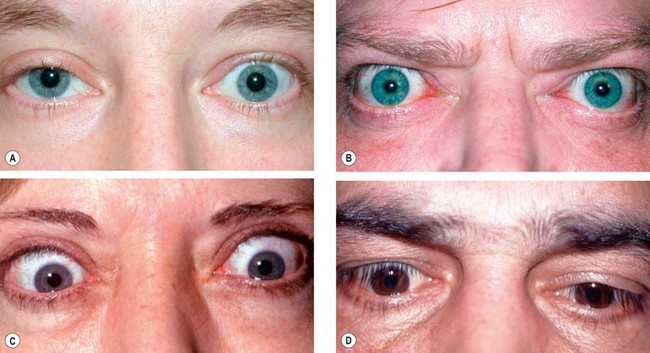
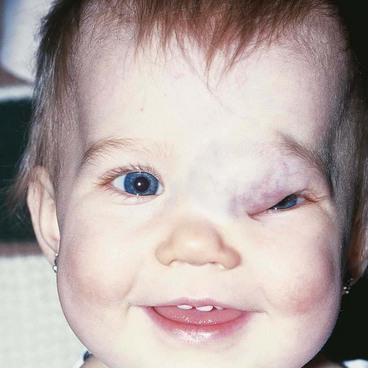
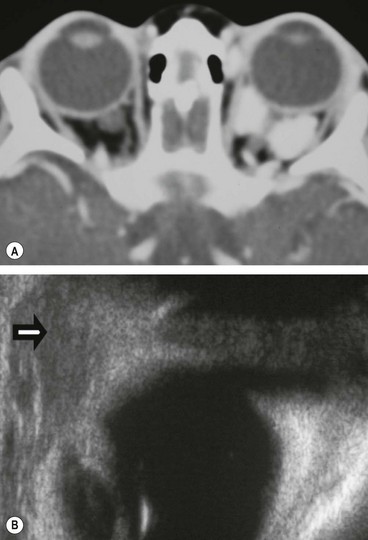
Capillary haemangioma
Capillary haemangioma is the most common tumour of the orbit and periorbital areas in childhood. Girls are affected more commonly than boys. It may present as a small isolated lesion of minimal clinical significance, or as a large disfiguring mass that can cause visual impairment and systemic complications. The tumour is composed of anastomosing small vascular channels without true encapsulation (Fig. 3.31A).
Diagnosis
The tumour is classified according to its location with respect to the skin and orbital septum as follows: (a) cutaneous, (b) purely preseptal, (c) preseptal with an extraconal element and (d) combination of preseptal, extraconal and intraconal.
1
Presentation is usually in the first few weeks of life (approximately 30% are present at birth).
2
Signs
•
Superficial cutaneous lesions are bright red.
•
Preseptal tumours appear dark blue or purple through the overlying skin (
Fig. 3.31B and C) and are most frequently located superiorly.
•
A large tumour may enlarge and change in colour to a deep blue during crying or straining, but both pulsation and a bruit are absent.
•
Deep orbital tumours give rise to unilateral proptosis without skin discoloration.
•
Haemangiomatous involvement of the palpebral or forniceal conjunctiva is common and may be an important diagnostic clue (
Fig. 3.31D).
•
Additional haemangiomas on the eyelids or elsewhere are common.
3
CT may be required for deep involvement when the diagnosis is not apparent on inspection. The lesion appears as a homogeneous enhancing soft tissue mass in the anterior orbit or as an extraconal mass with ‘finger-like’ posterior expansions (
Fig. 3.32A). The orbital cavity may show enlargement but there is no bony erosion.
4
Ultrasound is used to demonstrate the anatomical relations and extent of the tumour (
Fig. 3.32B).
5
The course is characterized by rapid growth 3–6 months after diagnosis (
Fig. 3.33), followed by a slower phase of natural resolution in which 30% of lesions resolve by the age of 3 years and 70% by the age of 7 years.
Treatment
1
Indications
•
Amblyopia secondary to induced astigmatism, anisometropia, and occlusion.
•
Optic nerve compression.
•
A severe cosmetic blemish, necrosis or infection.
2
Laser treatment may be used to close blood vessels in superficial skin lesions less than 2 mm in thickness.
3
Steroid injection of triamcinolone acetonide 40 mg/mL or betamethasone 4 mg/mL into the lesion, if cutaneous or preseptal is usually effective during the early active stage. A maximum of 1–2 mL should be injected using multiple entry sites. The tumour usually begins to regress within 2 weeks but, if necessary, second and third injections can be given after about 2 months. It is advisable not to inject deep into the orbit for fear of causing occlusion of the central retinal artery due to retrograde introduction of the solution into the central retinal artery. Other complications include skin depigmentation and necrosis, bleeding and fat atrophy. Adrenal suppression and failure to thrive have also been reported.
4
Systemic steroids administered daily over several weeks may be used, particularly if there is a large orbital component.
5
Systemic beta-blockers constitute a new and very promising treatment modality.
6
Local resection with cutting cautery may reduce the bulk of an anterior circumscribed tumour, but is usually reserved for the late inactive stage.
Systemic associations
Children with large capillary haemangiomas may have the following conditions:
1
High-output heart failure may occur in a small minority of patients with very large fast-growing visceral haemangiomas.
2
Kasabach–Merritt syndrome, characterized by thrombocytopenia and low levels of coagulant factors associated with rapidly expanding haemangiomas of the trunk, extremities and abdominal viscera.
3
Maffucci syndrome which is characterized by multiple skin or visceral haemangiomas associated with enchondromatosis.
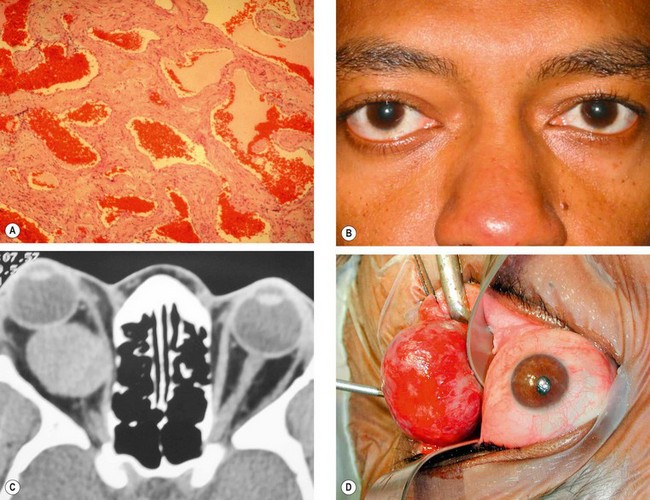
Cavernous haemangioma
Cavernous haemangioma is a vascular malformation that occurs in adults, with a female preponderance of 70%. Although it may develop anywhere in the orbit, it most frequently occurs within the lateral part of the muscle cone just behind the globe, and behaves like a low-flow arteriovenous malformation.
1
Histology shows endothelial-lined vascular channels of varying size separated by fibrous septae (
Fig. 3.34A).
2
Presentation is in the 4th–5th decades with slowly progressive unilateral proptosis. Growth may be accelerated by pregnancy.
3
Signs
•
Axial proptosis (
Fig. 3.34B) which may be associated with optic disc oedema and choroidal folds.
•
A lesion at the orbital apex may compress the optic nerve without causing significant proptosis.
•
Gaze-evoked transient blurring of vision may occur.
4
CT shows a well-circumscribed oval lesion with slow contrast enhancement (
Fig. 3.34C).
5
Treatment. Many cavernous haemangiomas are detected by chance on scans performed for unrelated reasons and observation alone is often appropriate. Symptomatic lesions require surgical excision in most cases because they gradually enlarge. The cavernous
haemangioma, unlike its capillary counterpart, is usually well encapsulated and relatively easy to remove (
Fig. 3.34D).
Pleomorphic lacrimal gland adenoma
Pleomorphic adenoma (benign mixed-cell tumour) is the most common epithelial tumour of the lacrimal gland and is derived from the ducts and secretory elements including myoepithelial cells.
1
Histology. The inner layer of cells forms glandular tissue that may be associated with squamous differentiation and keratin production (
Fig. 3.35A); the outer cells undergo metaplastic change leading to the formation of myxoid tissue.
2
Presentation is in the 2nd–5th decades with a painless, slowly progressive proptosis or swelling in the superolateral part of the orbit, usually of more than a year’s duration. Old photographs may reveal an abnormality many years prior to presentation.
3
Orbital lobe tumour
•
Smooth, firm, non-tender mass in the lacrimal gland fossa with inferonasal dystopia (
Fig. 3.35B).
•
Posterior extension may cause proptosis, ophthalmoplegia and choroidal folds.
•
CT shows a round or oval mass, with a smooth outline and indentation but not destruction of the lacrimal gland fossa (
Fig. 3.35C). The lesion may also indent the globe. Calcification may be shown.
4
Palpebral lobe tumour
•
This is less common and tends to grow anteriorly causing upper lid swelling without dystopia (
Fig. 3.36A).
•
The lesion may be visible to inspection (
Fig. 3.36B).
5
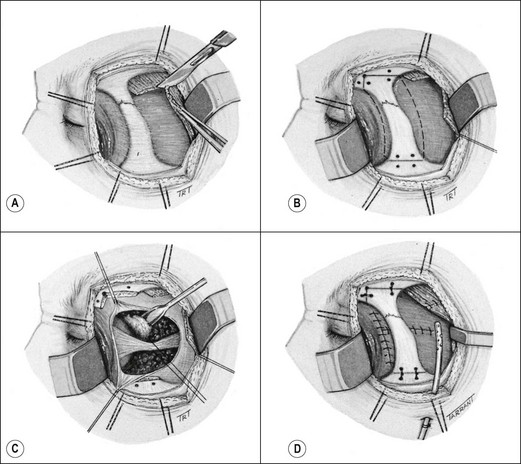
Treatment involves surgical excision. If the diagnosis is strongly suspected, it is wise to avoid prior biopsy, to avoid tumour seeding into adjacent orbital tissue, although this may not always be possible in the context of diagnostic uncertainty. Tumours of the palpebral lobe are usually resected, along with a margin of normal tissue, through an anterior (trans-septal) orbitotomy. Those of the orbital portion are excised through a lateral orbitotomy as follows:
b
The underlying bone is drilled for subsequent wiring (
Fig. 3.37B).
c
The lateral orbital wall is removed and the tumour excised including a margin of adjacent tissue and periorbita (
Fig. 3.37C).
d
The lateral orbital wall and temporalis are repaired (
Fig. 3.37D).
6
Prognosis is excellent provided excision is complete and without disruption of the capsule. Incomplete excision or preliminary incisional biopsy may result in seeding of the tumour into adjacent tissues, with recurrences and occasionally malignant change.
Lacrimal gland carcinoma
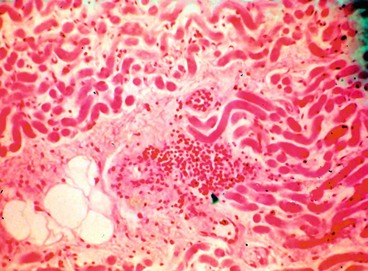
Lacrimal gland carcinoma is a rare tumour which carries a high morbidity and mortality. In order of frequency the main histological types are: (a) adenoid cystic, (b) pleomorphic adenocarcinoma, (c) mucoepidermoid and (d) squamous cell.
1
Histology of an adenoid cystic carcinoma shows nests of basaloid cells with numerous mitoses (
Fig. 3.38A).
2
Presentation is in the 4th–5th decades with a history shorter than that of a benign tumour. Pain is a frequent feature of malignancy but may also occur
with inflammatory lesions. A malignant mixed-cell tumour may present in three main clinical settings:
•
After incomplete or piecemeal excision of a benign pleomorphic adenoma, followed by one or more recurrences over a period of several years with eventual malignant transformation.
•
As a long-standing proptosis or swollen upper lid which suddenly starts to increase.
•
Without a previous history of a pleomorphic adenoma as a rapidly growing lacrimal gland mass, usually of several months’ duration.
3
Signs
•
A mass in the lacrimal area causing inferonasal dystopia.
•
Posterior extension, with involvement of the superior orbital fissure, may give rise to epibulbar congestion, proptosis, periorbital oedema and ophthalmoplegia (
Fig. 3.38B).
•
Hypoaesthesia in the region supplied by the lacrimal nerve.
•
Optic disc swelling and choroidal folds.
4
Investigations
a
CT shows a globular lesion with irregular serrated edges, often with contiguous erosion or invasion of bone (
Fig. 3.38C). Calcification in the tumour is commonly seen.
b
Biopsy is necessary to establish the histological diagnosis. Subsequent management depends on the extent of tumour invasion of adjacent structures as seen on imaging studies.
c
Neurological assessment is mandatory because adenoid-cystic carcinoma exhibits perineural spread and may extend into the cavernous sinus.
5
Treatment involves excision of the tumour and adjacent tissues. Extensive tumours may require orbital exenteration (see
Fig. 3.52) or mid-facial resection, but the prognosis for life is frequently poor. Radiotherapy combined with local resection may prolong life and reduce pain.
Optic nerve glioma
Optic nerve glioma is a slow-growing, pilocytic astrocytoma which typically affects children. Approximately 30% of patients have associated NF1 (see Ch. 19) and in these patients the prognosis is better. Malignant gliomas are rare and almost always occur in adults. They have a very poor prognosis with almost certain death within 1 year of diagnosis.
1
Histology shows spindle-shaped pilocytic astrocytes and glial filaments (
Fig. 3.39A).
2
Presentation is most frequently in the 1st decade (median age 6.5 years) with slowly progressive visual loss, followed later by proptosis, although this sequence may occasionally be reversed. Acute loss of vision as a result of haemorrhage into the tumour is uncommon.
3
Signs
•
Proptosis often non-axial, with temporal or inferior dystopia (
Fig. 3.39B).
•
The optic nerve head, initially swollen subsequently becomes atrophic.
•
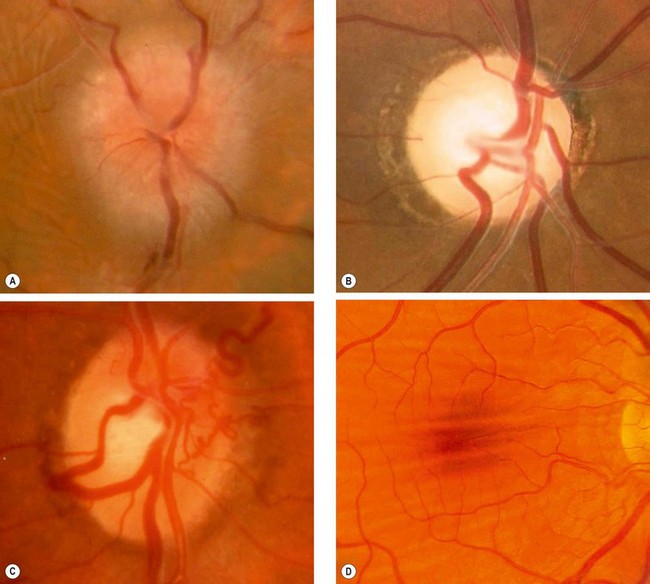
Opticociliary collaterals (see
Fig. 3.4C) and central retinal vein occlusion are occasionally seen.
•
Intracranial spread to the chiasm and hypothalamus may develop.
4
CT in patients with associated NF1 shows a fusiform enlargement of the optic nerve with a clear cut margin produced by the intact dural sheath (
Fig. 3.39C). In patients without NF1 the nerve is more irregular and shows low-density areas.
5
MR may be useful in showing intracranial extension (see
Fig. 19.101A).
6
Treatment may not be required in patients with no evidence of growth, good vision and no cosmetic deformity. Surgical excision with preservation of the globe is required in those with large or growing tumours that are confined to the orbit, particularly if vision is poor and proptosis significant. Radiotherapy may be combined with chemotherapy for tumours with intracranial extension that precludes surgical excision.
7
Prognosis for life in childhood tumours is variable. Some have an indolent course with little growth, while others may extend intracranially and threaten life.
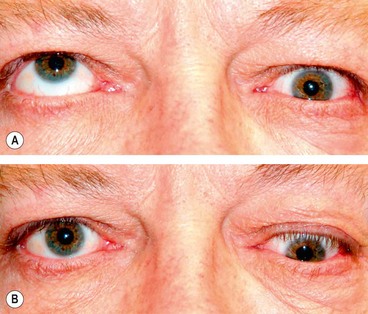
Optic nerve sheath meningioma
Optic nerve sheath meningiomas arise from meningothelial cells of the arachnoid villi surrounding the intraorbital or, less commonly, the intracanalicular portion of the optic nerve. In some cases the tumour merely encircles the optic nerve whilst in others it invades the nerve by growing along the fibrovascular pial septa. Primary optic nerve sheath meningiomas are less common than optic nerve gliomas and, as with other meningiomas, typically affect middle-aged women.
1
Histology shows two different types:
a
Meningothelial are characterized by varying-sized irregular lobules of meningothelial cells separated by fibrovascular strands (
Fig. 3.40A).
b
Psammomatous show psammoma bodies among proliferating meningothelial cells (
Fig. 3.40B).
2
Presentation is with gradual unilateral visual impairment. Transient obscurations of vision may be the presenting symptom.
3
Signs. The classical triad is (a)
visual loss, (b)
optic atrophy and (c)
opticociliary shunt vessels. However, the simultaneous occurrence of all three signs in one individual is uncommon. The sequence of involvement is as follows and is opposite of that seen in tumours outside the dural sheath.
•
Optic nerve dysfunction and chronic disc swelling followed by atrophy.
•
Opticociliary collaterals (see
Fig. 3.4C), found in about 30% of cases, regress as optic atrophy supervenes.
•
Restrictive motility defects, particularly in upgaze (
Fig. 3.40C), occur because the tumour may ‘splint’ the optic nerve.
•
Proptosis caused by intraconal spread usually develops after the onset of visual loss.
4
CT shows thickening and calcification of the optic nerve (
Fig. 3.40D).
5
MR more clearly delineates smaller tumours and those around the optic canal (
Fig. 3.40E).
6
Treatment may not be required in middle-aged patients with slow-growing tumours because the prognosis is good. Surgical excision is required in young patients with aggressive tumours, particularly if the eye is blind. Fractionated stereotactic radiotherapy may be appropriate in selected cases or as adjunctive treatment following surgery.
7
Prognosis for life is good in adults, although the tumour may be more aggressive in children.
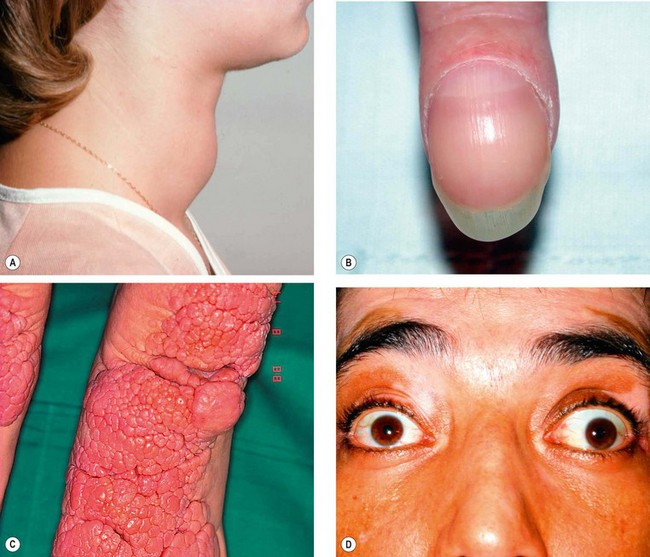
Plexiform neurofibroma
Plexiform neurofibroma is the most common peripheral neural tumour of the orbit and occurs almost exclusively in association with NF1.
1
Presentation is in early childhood with periorbital swelling.
2
Signs
•
Diffuse involvement of the orbit with disfiguring hypertrophy of periocular tissues (
Fig. 3.41A).
•
Involvement of the eyelids causes mechanical ptosis with a characteristic S-shaped deformity (see
Fig. 1.19B).
•
On palpation the involved tissues are said to resemble a ‘bag of worms’.
3
CT shows the extent of orbital involvement (
Fig. 3.41B).
4
Treatment is often unsatisfactory and complete surgical removal is extremely difficult. Orbital surgery should be avoided if at all possible because of the intricate relationship between the tumour and important orbital structures.
Isolated neurofibroma
Isolated (localized) neurofibroma is less common and is associated with NF1 in about 10% of cases.
1
Presentation is in the 3rd–4th decades with insidious mildly painful proptosis unassociated with visual impairment or ocular motility dysfunction.
2
Treatment by excision is usually straightforward because the tumour is well-circumscribed and relatively avascular.
Lymphoma
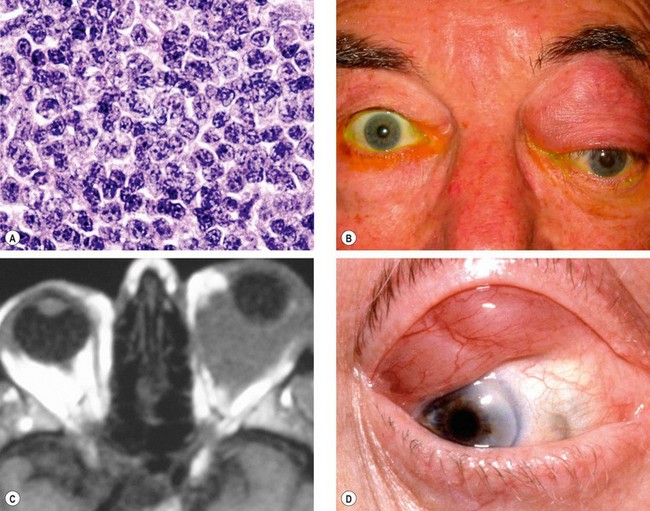
Lymphomas of the ocular adnexa constitute approximately 8% of all extranodal lymphomas. They represent one end of the spectrum of lymphoproliferative lesions, at the other end of which lies benign reactive lymphoid hyperplasia. Accurate diagnosis of the type of lymphoma has improved considerably with the introduction of immunological staining methods. The vast majority of orbital lymphomas are of B-cell origin and most are composed of ‘small’ B cells (Fig. 3.42A).
1
Presentation is insidious and usually in old age.
2
Signs
•
Any part of the orbit may be affected (
Fig. 3.42B and C) and occasionally involvement is bilateral.
•
Anterior lesions may be palpated and have a rubbery consistency (
Fig. 3.42D).
•
Occasionally the lymphoma may be confined to the conjunctiva or lacrimal glands, sparing the orbit.
3
Systemic investigations in patients with hypercellular lymphoid lesions of the orbit include chest radiographs, serum immunoprotein electrophoresis, thoraco-abdominal CT to detect possible retroperitoneal involvement and, if necessary, bone marrow aspiration.
4
Course. This is variable and relatively unpredictable. In some patients histological features raise suspicion of malignancy and yet the lesion resolves spontaneously or with the help of steroids. Conversely, what appears to be a benign lymphoid hyperplasia may be followed several years later by the development of lymphoma. Small lymphoproliferations and those involving only the conjunctiva have the best prognosis.
5
Treatment involves radiotherapy for localized lesions and by chemotherapy for disseminated disease.
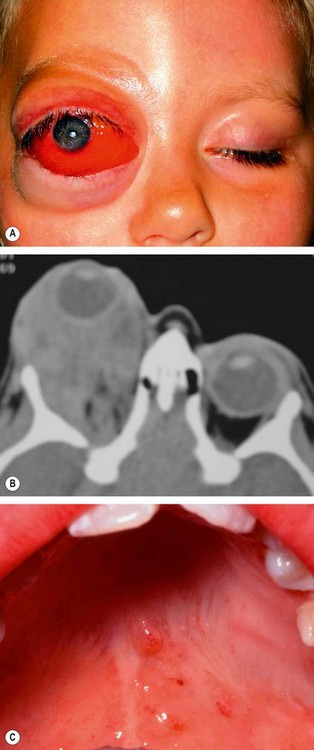
Embryonal sarcoma
Embryonal sarcoma (traditionally designated ‘rhabdomyosarcoma’) is the most common primary orbital malignancy of childhood. The tumour is derived from undifferentiated mesenchymal cell rests, which have the potential to differentiate into striated muscle. They do not arise from striated muscle, and the term rhabdomyosarcoma is appropriate only if there is evidence of differentiation into muscle. The main role of the ophthalmologist is diagnosis by incisional biopsy, followed by prompt referral to a paediatric oncologist.
1
Histology
a
Undifferentiated tumours show a mass of loosely arranged mesenchymal cells (
Fig. 3.43A).
b
Differentiated tumours show elongated and strap-like with a ‘tadpole’ or ‘tennis-racket’ configuration (rhabdomyoblasts –
Fig. 3.43B) with or without cross-striations (
Fig. 3.43C).
2
Presentation is in the 1st decade (average 7 years) with rapidly progressive unilateral proptosis which may initially mimic an inflammatory process.
3
Signs
•
The tumour is most commonly superonasal or retrobulbar followed by superior and inferior (
Fig. 3.44A).
•
Swelling and injection of overlying skin develop later but the skin is not warm (
Fig. 3.44B).
•
In advanced cases tumour spread to the sinuses may be seen.
4
CT shows a poorly defined mass of homogeneous density, often with adjacent bony destruction (
Fig. 3.44C).
5
MR shows a poorly defined mass of homogeneous density, often with adjacent bony destruction (
Fig. 3.44D)
6
Systemic investigations for evidence of metastatic spread include chest X-ray, liver function tests, bone marrow biopsy, lumbar puncture and skeletal survey. The most common sites for metastases are lung and bone.
7
Treatment involves radiotherapy and chemotherapy with vincristine, actinomycin and cyclophosphamide. Surgical excision is reserved for the rare recurrent or radio-resistant tumour.
8
Prognosis depends on the stage and location of disease at the time of diagnosis. Patients with tumours localized to the orbit have a 95% cure rate.
9
Differential diagnosis includes orbital cellulitis which also presents in children with similar acute signs although in embryonal sarcoma the skin is not warm. Other tumours such as metastatic neuroblastoma and myeloid sarcoma may also present with a rapidly growing orbital mass (see below).
Adult metastatic tumours
Orbital metastases are an infrequent cause of proptosis and are much less common than metastases to the choroid. If the orbit is the initial manifestation of the tumour, the ophthalmologist may be the first person to see the patient. In order of frequency the most common primary sites are breast, bronchus, prostate, skin (melanoma), gastrointestinal tract and kidney.
1
Presentation
•
A mass causing dystopia and proptosis is the most common (
Fig. 3.45A).
•
Infiltration of orbital tissues characterized by ptosis, diplopia, brawny indurated periorbital skin and a firm orbit, with resistance to manual retropulsion of the globe.
•
Enophthalmos with scirrhous tumours.
•
Chronic orbital inflammation.
•
Primarily with involvement of the cranial nerves (II, III, IV, V, VI) and only mild proptosis with lesions at the orbital apex.
2
CT (
Fig. 3.45B) and
MR show a non-encapsulated mass.
3
Fine needle biopsy is useful for histological confirmation. If this fails, open biopsy may be required.
4
Treatment is aimed at preserving vision and relieving pain because most patients die within 1 year. Radiotherapy is the mainstay of treatment. Orbital exenteration rarely may be required if other modalities fail to control intolerable symptoms.
Childhood metastatic tumours
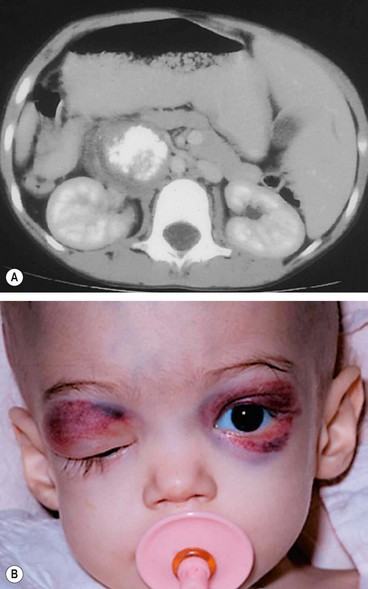
Neuroblastoma
1
Systemic features. Neuroblastoma is one of the most common childhood malignancies. It arises from primitive neuroblasts of the sympathetic chain, most commonly in the abdomen (
Fig. 3.46A), followed by the thorax and pelvis. Presentation is usually in early childhood; in almost half of all cases the tumour is disseminated at diagnosis with an appalling prognosis.
2
Orbital metastases may be bilateral and typically present with an abrupt onset of proptosis accompanied by a superior orbital mass and lid ecchymosis (
Fig. 3.46B).
Myeloid sarcoma
1
Systemic features. Myeloid sarcoma (granulocytic sarcoma) is a localized tumour composed of malignant cells of myeloid origin. Because the tumour may exhibit a characteristic green colour it was formerly referred to as chloroma. Myeloid sarcoma may occur as a manifestation of established myeloid leukaemia or it may precede the disease.
2
Orbital involvement usually presents at about age 7 years with rapid onset of proptosis, sometimes bilateral, which may be associated with ecchymosis and lid oedema. When orbital involvement precedes systemic leukaemia, the diagnosis may be difficult.
Langerhans cell histiocytosis
1
Systemic features. Langerhans cell histiocytosis is a rare group of disorders due to clonal proliferations of histiocytes. Presentation ranges from localized disease usually causing bone destruction (eosinophilic granuloma), through multifocal bone involvement to a fulminant systemic disease. Soft tissues are less commonly involved, but cutaneous and visceral involvement may occur.
2
Orbital involvement consists of unilateral or bilateral osteolytic lesions and soft tissue involvement, typically in the superotemporal quadrant (
Fig. 3.47). Patients with solitary lesions tend to have a more benign course and respond well to treatment with local curettage and intralesional steroid injection or
radiotherapy. Systemic disease responds poorly and has a high mortality.
Orbital invasion from adjacent structures
Sinus tumours
Malignant tumours of the paranasal sinuses, although rare, may invade the orbit and carry a poor prognosis unless diagnosed early. It is therefore important to be aware of both their otorhinolaryngological and ophthalmic features.
1
Maxillary carcinoma is by far the most common sinus tumour to invade the orbit (
Fig. 3.48).
•
Otorhinolaryngological manifestations include facial pain and swelling, epistaxis and nasal discharge.
•
Ophthalmic features include upward dystopia, diplopia and epiphora.
2
Ethmoidal carcinoma may cause lateral dystopia.
3
Nasopharyngeal carcinoma may spread to the orbit through the inferior orbital fissure; proptosis is a late finding.
Bony invasion
1
Intracranial meningioma arising from the sphenoidal ridge may invade the orbit by direct spread and cause proptosis (see
Fig. 19.56). Occasionally tumours arising from the tuberculum sellae or olfactory groove may invade the orbital through the superior orbital fissure or optic canal.
2
Fibrous dysplasia is a benign developmental disorder leading to slowly-developing irregular expansion of bone with a mass effect on adjacent structures (
Fig. 3.49A). Within the orbital region this may cause facial asymmetry, proptosis, dystopia (
Fig. 3.49B) and visual loss. Most orbital disease is due to the monostotic form whereas polyostotic disease is associated with endocrine disorders and cutaneous pigmentation (McCune-Albright syndrome).
Orbital invasion from eyelid, conjunctival and intraocular tumours
Orbital invasion may occur from eyelid malignancies such as basal cell carcinoma, squamous cell carcinoma or sebaceous gland carcinoma, from conjunctival tumours, in particular melanoma (Fig. 3.50A), and from intraocular tumours such as choroidal melanoma or retinoblastoma (Fig. 3.50B).
Craniosynostoses
The craniosynostoses are a rare group of congenital conditions in which an abnormally-shaped skull results from premature closure of skull sutures.
Crouzon syndrome
Crouzon syndrome is caused primarily by premature fusion of the coronal and sagittal sutures.
1
Inheritance is usually AD, but 25% of cases represent a fresh mutation. The gene (FGFR2) has been isolated to chromosome 10.
2
Systemic features
•
Short anteroposterior head distance and wide cranium due to premature fusion.
•
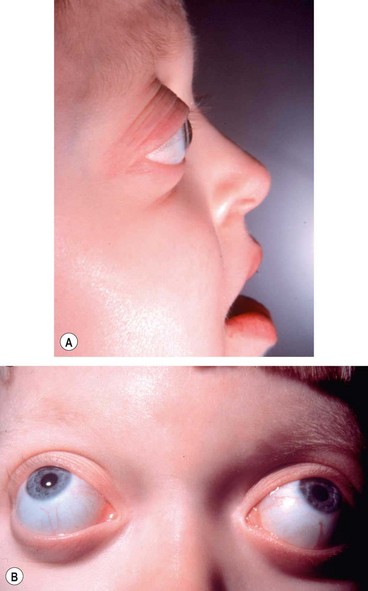
Midfacial hypoplasia and curved ‘parrot-beak’ nose which gives rise to a ‘frog-like’ facies and mandibular prognathism (
Fig. 3.58A).
•
Inverted V-shaped palate.
•
Acanthosis nigricans is seen in one subtype.
3
Ocular features
•
Proptosis due to shallow orbits is the most conspicuous feature.
•
Hypertelorism (wide separation of the orbits).
•
Ametropia and amblyopia.
•
Vision-threatening complications include exposure keratopathy and optic atrophy, due to chronic papilloedema and cerebral hypoperfusion secondary to sleep apnoea.
4
Ocular associations include blue sclera, cataract, ectopia lentis, glaucoma, coloboma, megalocornea and optic nerve hypoplasia.
Apert syndrome
Apert syndrome (acrocephalosyndactyly) is the most severe of the craniosynostoses and may involve all the cranial sutures.
1
Inheritance is AD, but in the majority of cases it is sporadic and associated with older parental age. It is frequently the result of mutations in FGFR2.
2
Systemic features
•
Oxycephaly with flattened occiput and steep forehead.
•
Horizontal groove above the supraorbital ridge.
•
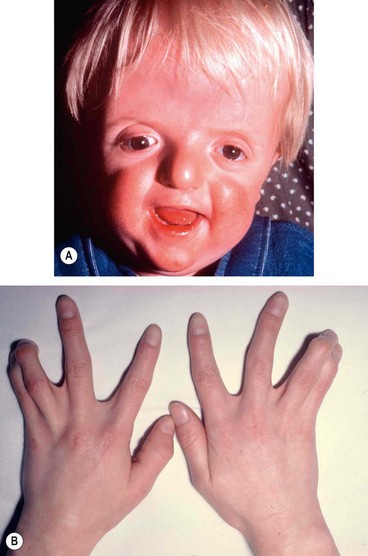
Midfacial hypoplasia with a ‘parrot-beak’ nose and low-set ears (
Fig. 3.59A).
•
High-arched palate, cleft palate and bifid uvula.
•
Anomalies of the heart, lungs and kidneys.
•
Acneiform skin eruptions on the trunk and extremities.
•
Developmental delay in 30% of cases.
3
Ocular features
•
Shallow orbits, proptosis and hypertelorism are generally less pronounced than in Crouzon syndrome.
•
Extorted slant of the palpebral apertures.
•
Vision-threatening complications as in Crouzon syndrome.
4
Ocular associations include keratoconus, ectopia lentis and congenital glaucoma.
Pfeiffer syndrome
1
Inheritance is AD with genetic heterogeneity.
2
Systemic features
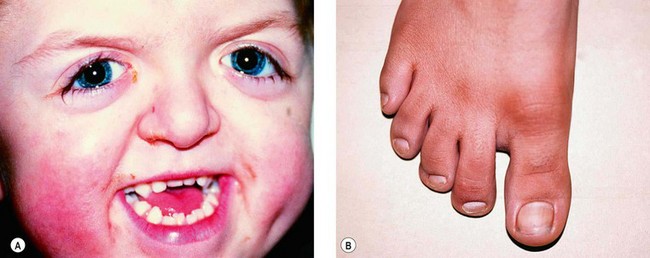
•
Midfacial hypoplasia and down-slanting palpebral fissures (
Fig. 3.60A).
•
Broad thumbs and great toes (
Fig. 3.60B), and soft tissue syndactyly.
•
Occasionally clover-leaf skull.
•
Mental handicap is uncommon.
•
Shallow orbits and hypertelorism similar to Apert syndrome.
•
Vision-threatening complications as in Crouzon syndrome.