Chapter 21 Trauma
Eyelid trauma
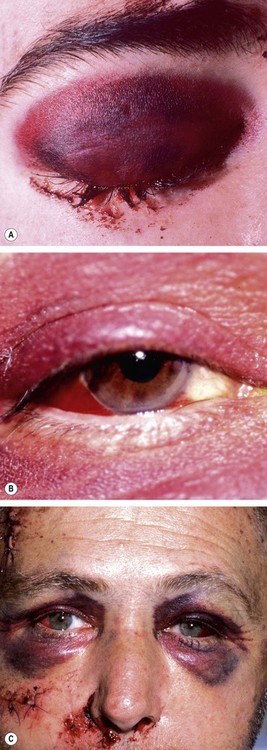
Periocular haematoma
A ‘black eye’, consisting of a haematoma (focal collection of blood) and/or periocular ecchymosis (diffuse bruising) and oedema is the most common blunt injury to the eyelid or forehead and is generally innocuous. It is, however, very important to exclude the following more serious conditions:
Laceration
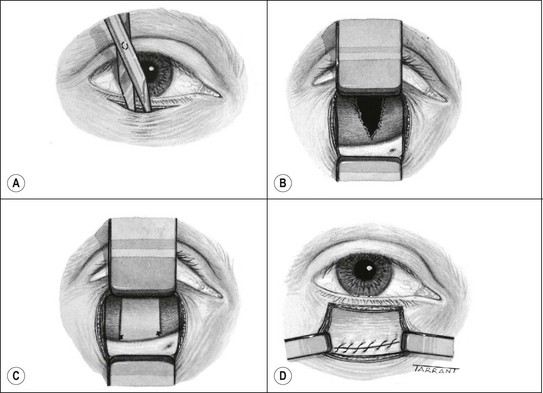
The presence of a lid laceration, however insignificant, mandates careful exploration of the wound and examination of the globe. Any lid defect should be repaired by direct closure whenever possible, even under tension, since this affords the best functional and cosmetic results.
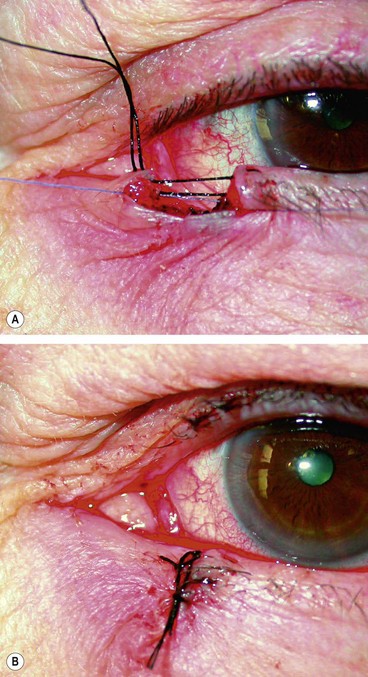
Fig. 21.2 Repair of lid laceration. (A) Initial approximation of the tarsal plate with an absorbable suture and lid margin with a silk suture; (B) completed repair
(Courtesy of J Nerad, K Carter and M Alford, from Oculoplastic and Reconstructive Surgery, in Rapid Diagnosis in Ophthalmology, Mosby 2008)
It is very important to ensure that the patient’s tetanus immunization status is satisfactory after any injury. Without any prior immunization (unlikely) give 250 units of human tetanus immunoglobulin intramuscularly (IM); if previously immunized but a booster has not been administered within the last 10 years, give IM or subcutaneous tetanus toxoid.
Orbital fractures
Blow-out orbital floor fracture
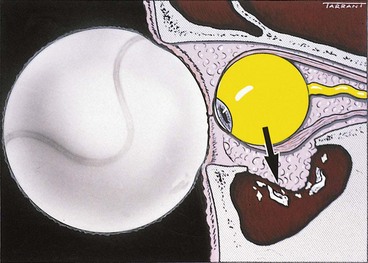
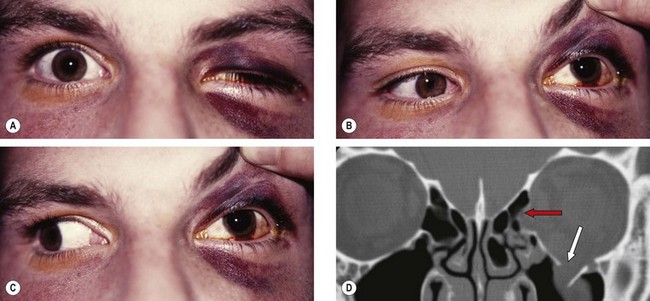
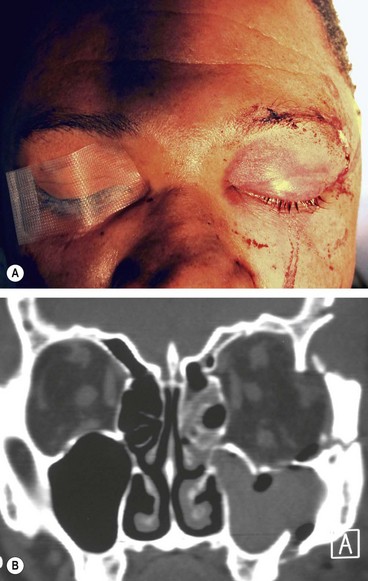
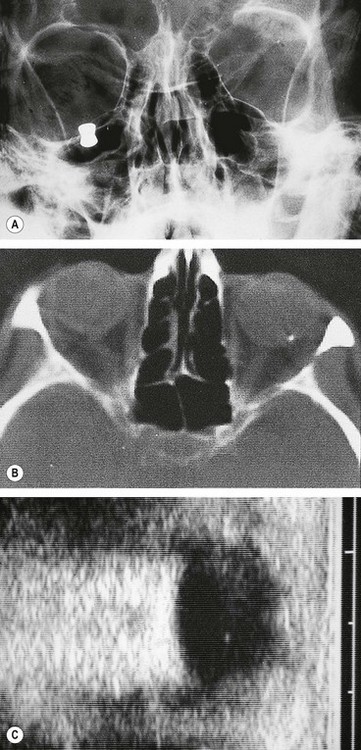
A blow-out fracture of the orbital floor is typically caused by a sudden increase in the orbital pressure by an impacting object which is greater in diameter than the orbital aperture (about 5 cm), such as a fist or tennis ball (Fig. 21.3), so that the eyeball itself is displaced and transmits rather than absorbs the impact. Since the bones of the lateral wall and the roof are usually able to withstand such trauma, the fracture most frequently involves the floor of the orbit along the thin bone covering the infraorbital canal. Occasionally, the medial orbital wall may also be fractured; a ‘pure’ blow-out fracture does not involve the orbital rim whereas an ‘impure’ fracture involves the rim and/or adjacent facial bones. Clinical features vary with the severity of trauma and the time interval between injury and examination.
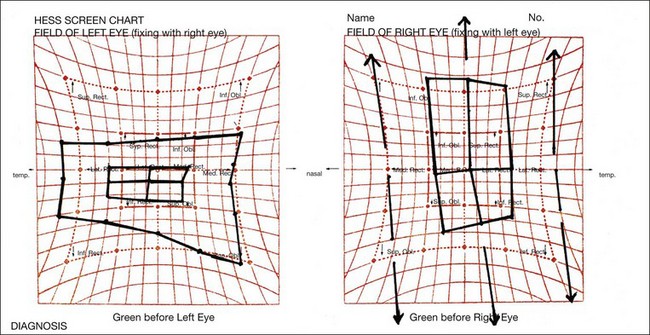
Diagnosis
Treatment
Blow-out medial wall fracture
Medial wall orbital fractures are usually associated with floor fractures; isolated fractures are less common.
Roof fracture
Roof fractures are rarely encountered by ophthalmologists. Isolated fractures, caused by falling on a sharp object (Fig. 21.8) or a blow to the brow or forehead, are most common in children. Complicated fractures, caused by major trauma with associated displacement of the orbital rim or significant disturbance of other craniofacial bones, typically affect adults.
Lateral wall fracture
Acute lateral wall fractures are rarely encountered by ophthalmologists. Because the lateral wall of the orbit is more solid than the other walls, a fracture is usually associated with extensive facial damage (Fig. 21.9).
Trauma to the globe
Introduction
Definitions
Principles of evaluation
Blunt trauma
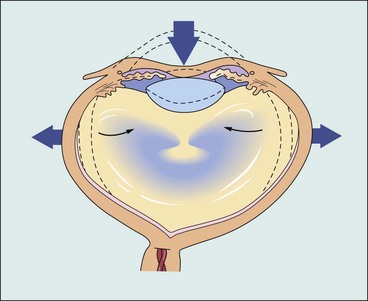
The most common causes of blunt trauma are squash balls, elastic luggage straps and champagne corks. Severe blunt trauma to the globe results in anteroposterior compression with simultaneous expansion in the equatorial plane (Fig. 21.11) associated with a transient but severe increase in intraocular pressure. Although the impact is primarily absorbed by the lens-iris diaphragm and the vitreous base, damage can also occur at a distant site such as the posterior pole. The extent of ocular damage depends on the severity of trauma and tends largely to be concentrated to either anterior or posterior segment. Apart from obvious ocular damage, blunt trauma commonly results in long-term effects; the prognosis is therefore necessarily guarded.
Corneal
Hyphaema
Anterior uvea
The anterior uvea may sustain structural and/or functional damage.
Intraocular pressure
It is important for IOP to be monitored carefully, particularly in the early period following trauma. Elevation can occur for a variety of reasons including hyphaema (above) and inflammation (see Ch. 10). In contrast, the ciliary body may react to severe blunt trauma by temporary cessation of aqueous secretion (‘ciliary shock’) resulting in hypotony; it is important for an occult open injury to be excluded as the cause of the hypotony. Tears extending into the face of the ciliary body (angle recession) are associated with a risk of later glaucoma.
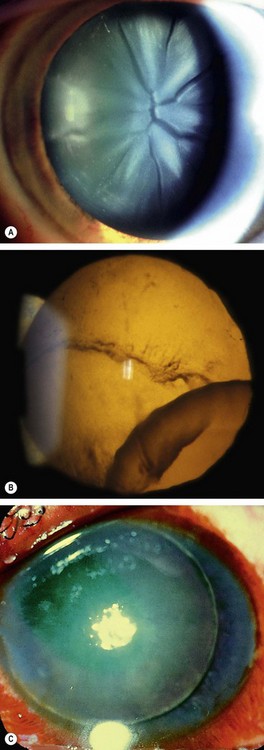
Lenticular
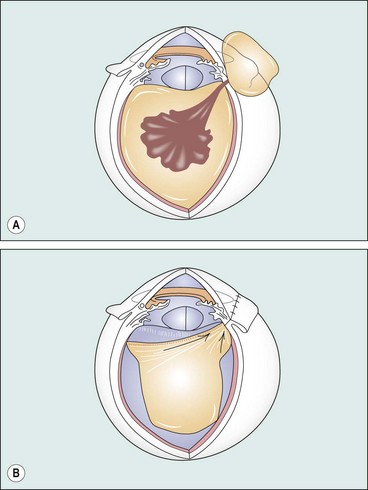
Globe rupture
Rupture of the globe may result from severe blunt trauma. The rupture is usually anterior, in the vicinity of the Schlemm canal, with prolapse of structures such as the lens, iris, ciliary body and vitreous (Fig. 21.16); an anterior rupture may be masked by extensive subconjunctival haemorrhage. An occult posterior rupture can be associated with little visible damage to the anterior segment, but should be suspected if there is asymmetry of anterior chamber depth – the anterior chamber of an affected eye is classically deep, with posterior rotation of the iris–lens diaphragm – and intraocular pressure in the affected eye is low. Gentle B-scan ultrasonography may demonstrate a posterior rupture, but CT or MR may be necessary; MR is not performed if there is a risk of ferrous intraocular foreign body. The principles of scleral rupture repair are described later.
Vitreous haemorrhage
Vitreous haemorrhage may occur, often in association with posterior vitreous detachment. Pigment cells (’tobacco dust’) may be seen floating in the anterior vitreous, and though not necessarily associated with a retinal break, should always prompt a careful retinal assessment.
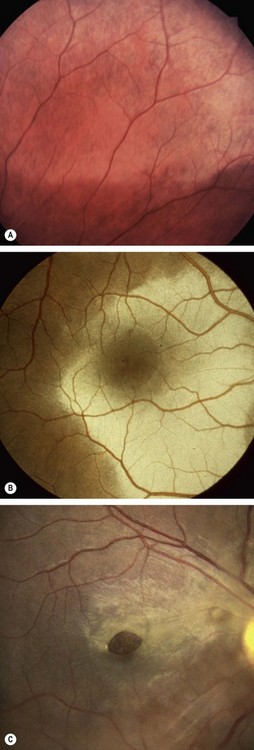
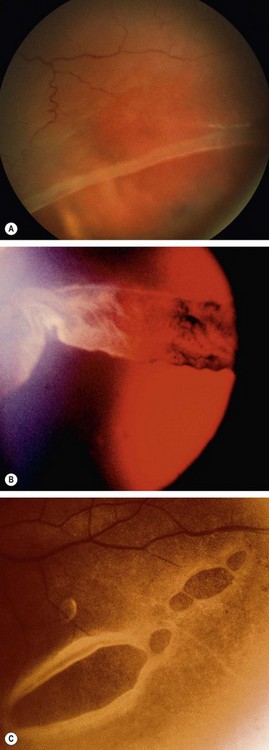
Commotio retinae
Commotio retinae is caused by concussion of the sensory retina resulting in cloudy swelling which gives the involved area a grey appearance. Commotio most frequently affects the temporal fundus (Fig. 21.17A). If the macula is involved, a ‘cherry-red spot’ may be seen at the fovea (Fig. 21.17B). Severe involvement may be associated with intraretinal haemorrhage, sometimes affecting the macula. The prognosis in mild cases is good with spontaneous resolution within 6 weeks. Sequelae to more severe commotio may include progressive pigmentary degeneration and macular hole formation (Fig. 21.17C).
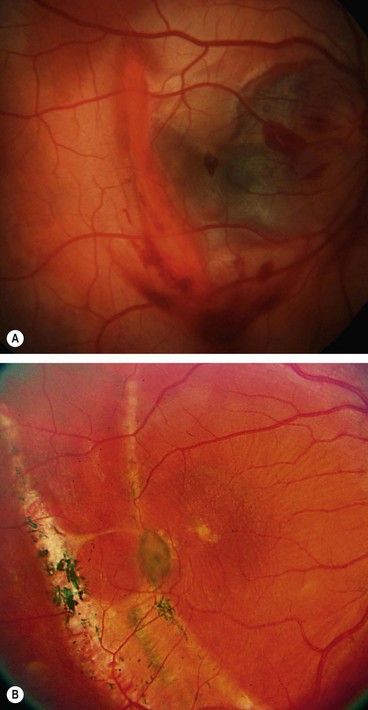
Choroidal rupture
Choroidal rupture involves the choroid, Bruch membrane and retinal pigment epithelium (RPE); it may be direct or indirect. Direct ruptures are located anteriorly at the site of impact and run parallel with the ora serrata. Indirect ruptures occur opposite the site of impact. A fresh rupture may be partially obscured by subretinal haemorrhage (Fig. 21.18A), which may break through the internal limiting membrane with resultant subhyaloid or vitreous haemorrhage. Weeks to months later, on absorption of the blood, a white crescentic vertical streak of exposed underlying sclera concentric with the optic disc becomes visible. The visual prognosis is poor if the fovea is involved. An uncommon late complication is choroidal neovascularization (Fig. 21.18B) which may result in haemorrhage, scarring and further visual deterioration.
Retinal breaks and detachment
Trauma is responsible for about 10% of all cases of retinal detachment (RD) and is the most common cause in children, particularly boys. A great variety of breaks may develop in traumatized eyes either at the time of impact or subsequently.
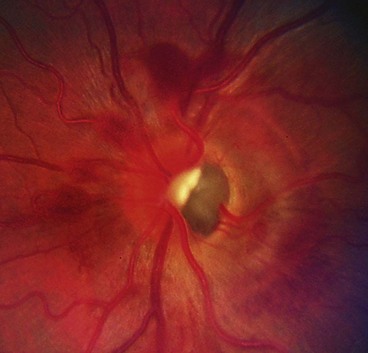
Optic nerve
Shaken baby syndrome
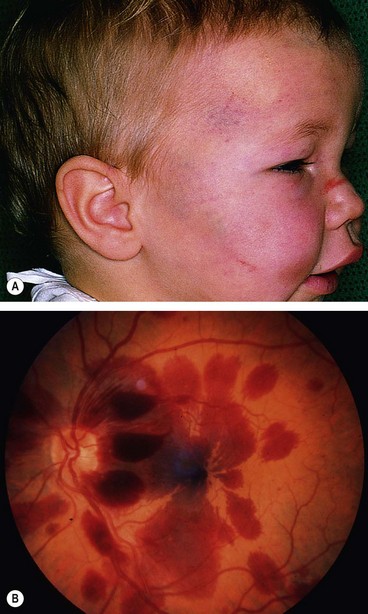
Shaken baby syndrome (non-accidental head injury, abusive head trauma) is a form of physical abuse occurring typically in children under the age of 2 years. Mortality is more than 25%, and it is responsible for up to 50% of deaths from child abuse. It is caused principally by violent shaking, often in association with impact injury to the head, and should be considered in conjunction with a specialist paediatrician whenever characteristic ophthalmic features are identified. The pattern of injury results from rotational acceleration and deceleration of the head, in contrast to the linear forces generated by falls. It is thought that direct trauma is not the main mechanism of brain damage; brainstem traction injury causes apnoea, consequent hypoxia leading to raised intracranial pressure and ischaemia.
Penetrating trauma
Causes
Penetrating injuries are three times more common in males than females, and typically occur in a younger age group (50% aged 15–34). The most frequent causes are assault, domestic and occupational accidents, and sport. The extent of the injury is determined by the size of the object, its speed at the time of impact and its composition. Sharp objects such as knives cause well-defined lacerations of the globe. However, the extent of damage caused by flying foreign bodies is determined by their kinetic energy. For example, an air gun pellet is large and although relatively slow-moving has a high kinetic energy and can thus cause considerable ocular damage. In contrast, a fast-moving fragment of shrapnel has a low mass and therefore will cause a well-defined laceration with relatively less intraocular damage than an air gun pellet. Of paramount immediate importance is the risk of infection with any penetrating injury. Endophthalmitis or panophthalmitis, often more severe than the initial injury, may ensue with loss of the eye. Risk factors include delay in primary repair, ruptured lens capsule and a dirty wound. Any eye with an open injury should be covered by a protective eye shield upon diagnosis.
Corneal
The technique of primary repair depends on the extent of the wound and associated complications such as iris incarceration, flat anterior chamber and damage to intraocular contents.
Scleral
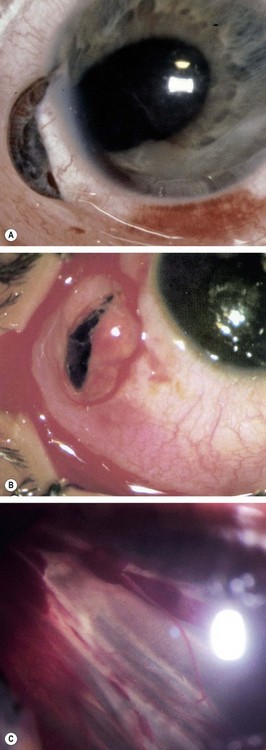
Fig. 21.23 Penetrating scleral wounds. (A) Anterior circumferential scleral laceration with iridociliary prolapse; (B) radial anterior scleral laceration with ciliary and vitreous prolapse; (C) fibrous proliferation
(Courtesy of Wilmer Institute – fig. A; EM Eagling and MJ Roper-Hall, from Eye Injuries, Butterworths 1986 – fig. B)
Retinal detachment
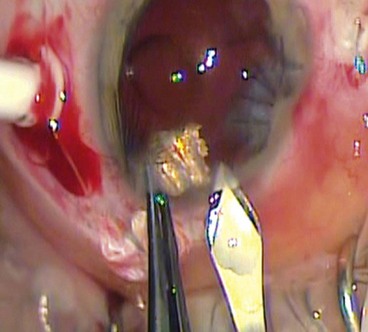
Traumatic tractional retinal detachment may result from vitreous incarceration in the wound and the presence of blood within the vitreous gel which acts as a stimulus to fibroblastic proliferation along the planes of incarcerated vitreous (Fig. 21.24A). The contraction of such anterior epiretinal membranes leads to a shortening and a rolling effect on the peripheral retina in the region of the vitreous base and eventually to an anterior tractional retinal detachment (Fig. 21.24B). A retinal break may develop several weeks later leading to a sudden extension of subretinal fluid and consequent visual loss.
Superficial foreign bodies
Subtarsal
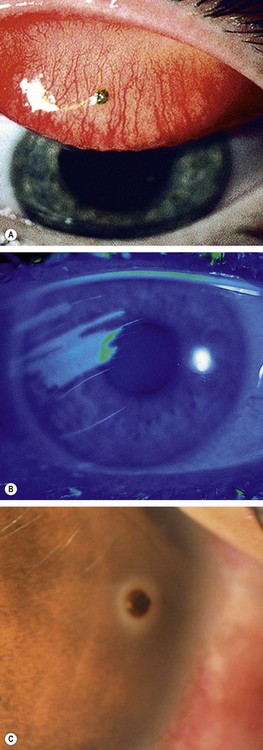
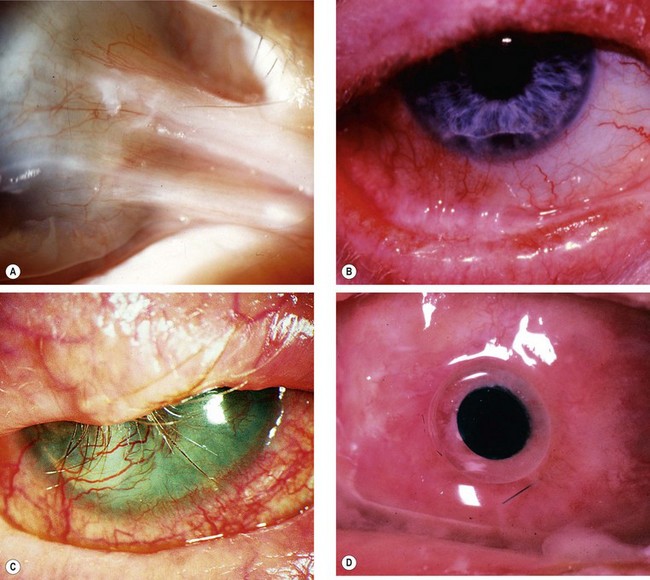
Small foreign bodies such as particles of steel, coal or sand often impact on the corneal or conjunctival surface. They may be washed along the tear film into the lacrimal drainage system or adhere to the superior tarsal conjunctiva (Fig. 21.25A) in the subtarsal sulcus and abrade the cornea with every blink, when a pathognomonic pattern of linear corneal abrasions may be seen (Fig. 21.25B).
Corneal
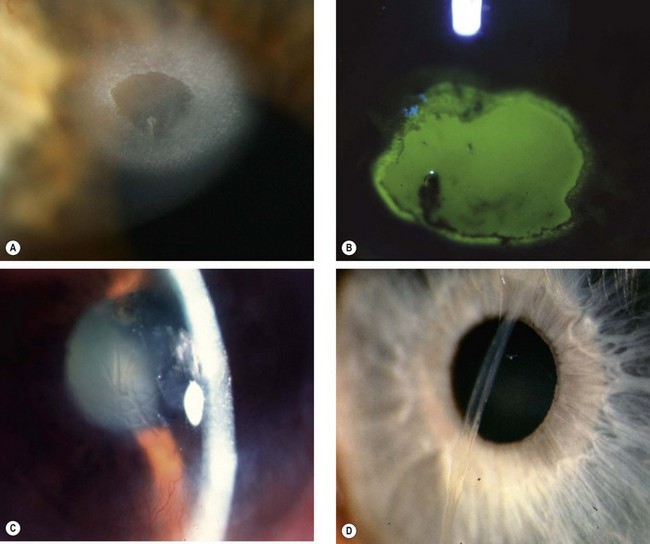
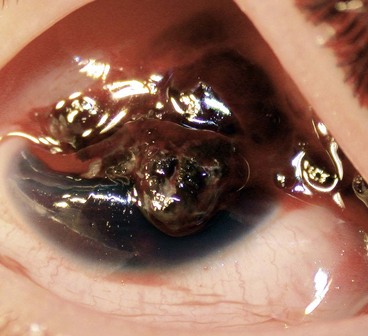
Intraocular foreign bodies
An intraocular foreign body (IOFB) may traumatize the eye mechanically, introduce infection or exert other toxic effects on the intraocular structures. Once in the eye, the foreign body may lodge in any of the structures it encounters; thus it may be located anywhere from the anterior chamber to the retina and choroid (Fig. 21.26). Notable mechanical effects include cataract formation secondary to capsular injury, vitreous liquefaction, and retinal haemorrhages and tears. Stone and organic foreign bodies are associated with a higher rate of infection, and this is particularly high with soil-contaminated or vegetable matter, when prophylaxis with intravitreal antibiotics is required. Many substances including glass, many plastics, gold and silver are inert. However, iron and copper may undergo dissociation and result in siderosis and chalcosis respectively.
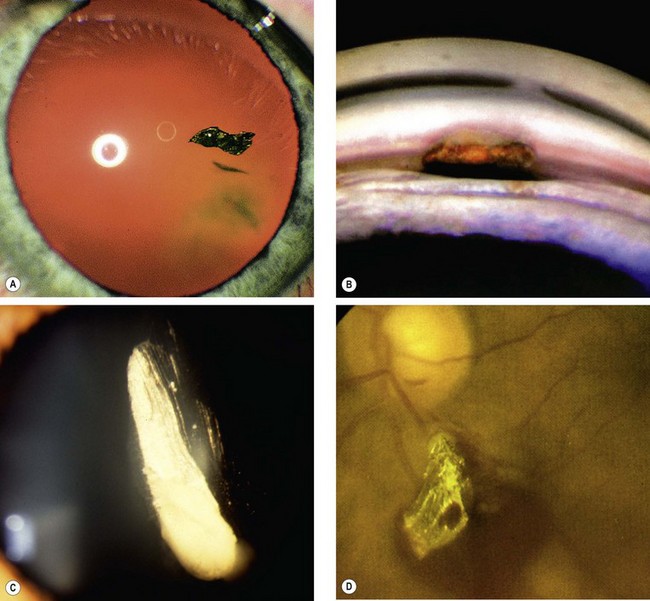
Fig. 21.26 Intraocular foreign bodies. (A) In the lens; (B) in the angle; (C) in the anterior vitreous; (D) on the retina with associated preretinal haemorrhage
(Courtesy of R Curtis – fig. B: EM Eagling and MJ Roper-Hall, from Eye Injuries, Butterworths 1986 – fig. D)
Initial management
Technique of removal
Siderosis
Steel is the most common foreign body constituent, typically projected into the eye by hammering or power tool use. A ferrous IOFB undergoes dissociation resulting in the deposition of iron in the intraocular epithelial structures, notably the lens epithelium, iris and ciliary body epithelium and the sensory retina, where it exerts a toxic effect on cellular enzyme systems, with resultant cell death.
Chalcosis
The ocular reaction to an intraocular foreign body with a high copper content involves a violent endophthalmitis-like picture, often with progression to phthisis bulbi. On the other hand, an alloy such as brass or bronze, with a relatively low copper content, results in chalcosis. Electrolytically-dissociated copper becomes deposited intraocularly, resulting in a picture similar to that seen in Wilson disease. Thus a Kayser–Fleischer ring develops, as does an anterior ‘sunflower’ cataract. Retinal deposition results in golden plaques visible ophthalmoscopically. Since copper is less retinotoxic than iron, degenerative retinopathy does not develop and visual function may be preserved.
Enucleation
Primary enucleation should be performed only for very severe injuries, with no prospect of retention of vision when it is impossible to repair the sclera (see Fig. 21.16). Secondary enucleation may be considered following primary repair if the eye is severely and irreversibly damaged, particularly if it is also unsightly and uncomfortable. The time delay also allows the patient valuable time to mentally and emotionally adapt to the prospect of losing an eye. Based on anecdotal evidence, it has been recommended that enucleation should be performed within 10 days of the original injury in order to prevent the very remote possibility of sympathetic ophthalmitis (see Ch. 11). However, objective evidence for this is lacking.
Bacterial endophthalmitis
Endophthalmitis develops in about 8% of cases of penetrating trauma with retained foreign body.
Chemical injuries
Causes
Chemical injuries range in severity from the trivial to the potentially blinding. The majority are accidental, and a few due to assault. Two-thirds of accidental burns occur at work and the remainder at home. Alkali burns are twice as common as acid burns since alkalis are more widely used both at home and in industry. The severity of a chemical injury is related to the properties of the chemical, the area of affected ocular surface, duration of exposure (including retention of particulate chemical on the surface of the globe or under the upper lid) and related effects such as thermal damage. Alkalis tend to penetrate more deeply than acids, as the latter coagulate surface proteins, forming a protective barrier. The most common involved alkalis are ammonia, sodium hydroxide and lime. The commonest acids implicated are sulphuric, sulphurous, hydrofluoric, acetic, chromic and hydrochloric. Ammonia and sodium hydroxide may produce severe damage because of rapid penetration. Hydrofluoric acid used in glass etching and cleaning also tends to rapidly penetrate the eye, whilst sulphuric acid may be complicated by thermal effects and high velocity impact after car battery explosions.
Pathophysiology
Management
Emergency treatment
A chemical burn is the only eye injury that requires emergency treatment without first taking a history and performing a careful examination. Immediate treatment is as follows:
Grading of severity
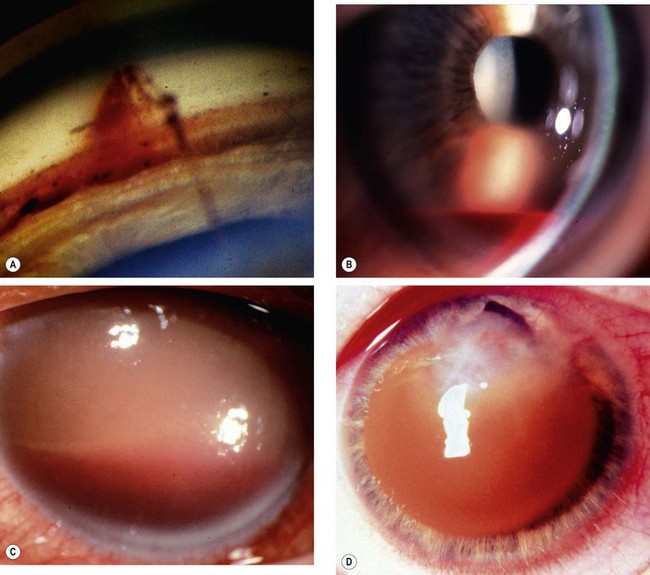
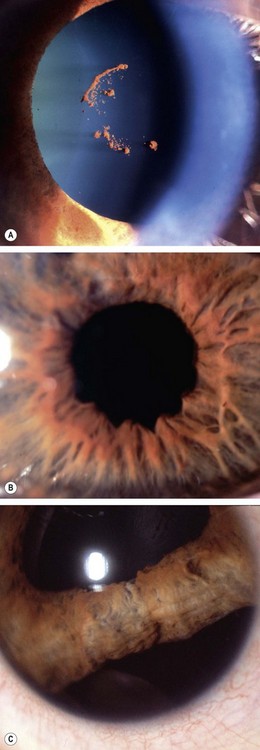
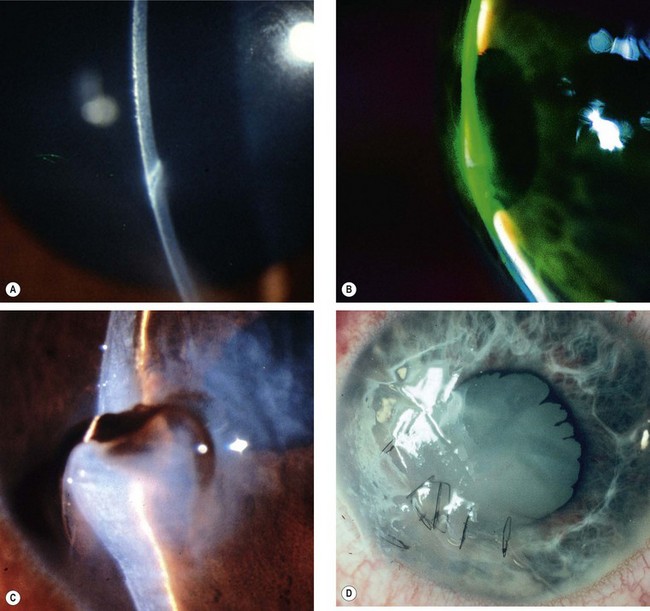
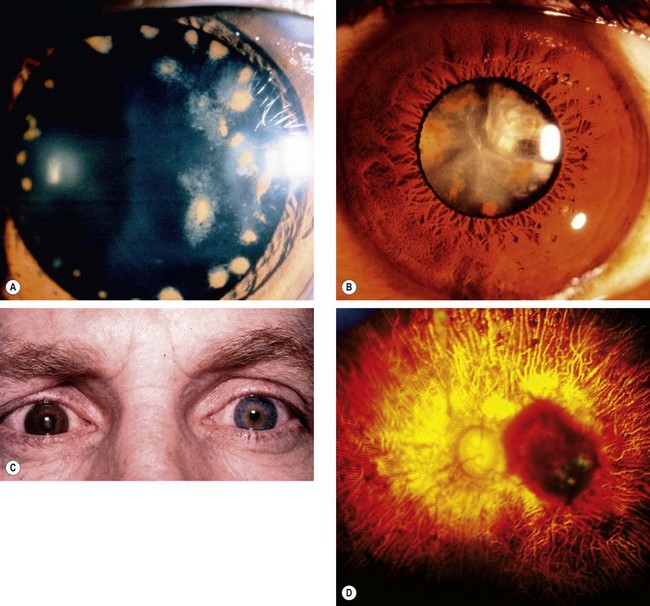
Acute chemical injuries are graded to plan appropriate subsequent treatment and afford an indication of likely ultimate prognosis. Grading is performed on the basis of corneal clarity and severity of limbal ischaemia (Roper-Hall system); the latter is assessed by observing the patency of the deep and superficial vessels at the limbus (Fig. 21.29A).
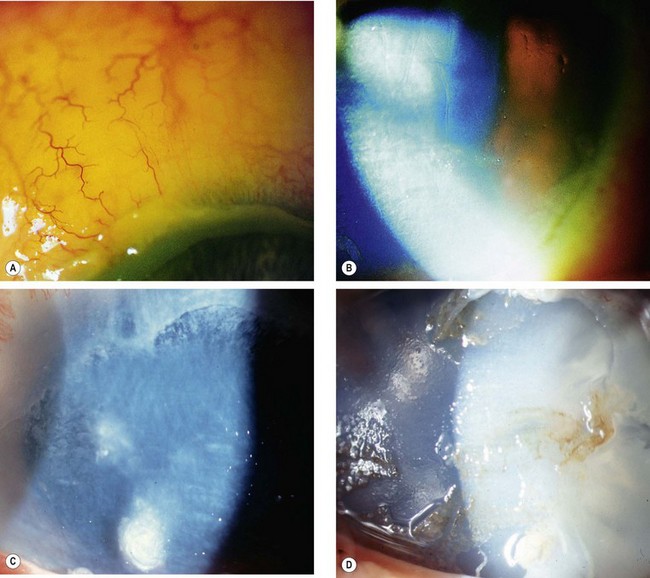
Fig. 21.29 Chemical burns. (A) Limbal ischaemia; (B) grade 2 – corneal haze but visible iris details; (C) grade 3 – corneal haze obscuring iris details; (D) grade 4 – total corneal opacification
Other features to note at initial assessment are the extent of corneal and conjunctival epithelial loss, iris changes, status of the lens and intraocular pressure.
Medical treatment
Most mild (grade 1 and 2) injuries are treated with topical antibiotic ointment for about a week, with topical steroids and cycloplegics if necessary. The main aims of treatment of more severe burns are to reduce inflammation, promote epithelial regeneration and prevent corneal ulceration. For moderate-severe injuries, preservative-free drops should be used.