Chapter 10 Glaucoma
Introduction
Aqueous secretion
Aqueous humour is produced in two steps:
Aqueous outflow
Anatomy
Physiology
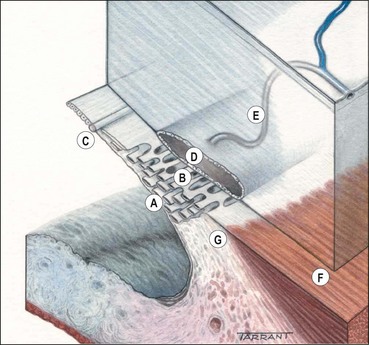
Aqueous flows from the posterior chamber via the pupil into the anterior chamber, from where it exits the eye by two different routes (Fig. 10.3):
Intraocular pressure
The IOP is determined by the balance between the rate of aqueous secretion and aqueous outflow. The latter is in turn related to the resistance encountered in the outflow channels and to the level of episcleral venous pressure. The rate of aqueous outflow is proportional to the difference between the intraocular and episcleral venous pressure.
Concept of normal intraocular pressure
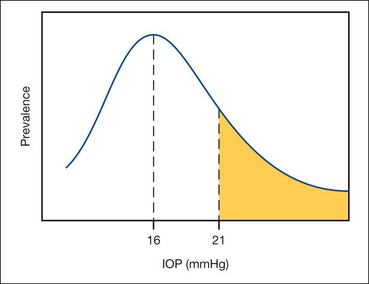
The distribution of IOP within the general population has a range of 11–21 mmHg. Although there is no absolute pathological point, 21 mmHg is considered the upper limit of normal and levels above this are viewed with suspicion. However, in some patients glaucomatous damage occurs with IOPs less than 21 mmHg (normal-tension or normal-pressure glaucoma) whilst others remain unscathed with IOPs up to 30 mmHg (ocular hypertension). Although the actual level of IOP is important in the development of glaucomatous damage, other factors are also significant.
Fluctuation
Normal IOP varies with the time of day, heartbeat, blood pressure level and respiration. The diurnal pattern varies, with a tendency to be higher in the morning and lower in the afternoon and evening. Normal eyes manifest a mean diurnal pressure variation of 5 mmHg; ocular hypertensive or glaucomatous eyes, however, exhibit a wider fluctuation. A single normal reading, particularly if taken during late afternoon, may therefore be misleading and it may be necessary to take several readings at different times of day (‘phasing’). In clinical practice phasing during the morning hours may be sufficient because 80% of patients peak between 8.00 a.m. and noon.
Overview of glaucoma
Definition
It is difficult to define glaucoma precisely, as it encompasses a diverse group of disorders. All forms of the disease have in common a potentially progressive and characteristic optic neuropathy which is associated with visual field loss as damage progresses, and in which intraocular pressure is usually a key modifying factor. On a molecular level, glaucoma of diverse aetiology is linked by the presence of endothelial leucocyte adhesion molecule-1 (ELAM-1), which indicates activation of a stress response in trabecular meshwork cells.
Epidemiology
Glaucoma affects up to 2% of those over the age of 40 years globally, and up to 10% over the age of 80; 50% may be undiagnosed. In a population of European or African ethnic origin, primary open-angle glaucoma (POAG) is the most common form. On a worldwide basis, primary angle-closure constitutes up to half of cases, with particularly high prevalence in individuals of Far Eastern descent.
Classification
Glaucoma may be congenital (developmental) or acquired. Sub-classification into open-angle and angle-closure types is based on the mechanism by which aqueous outflow is impaired with respect to the anterior chamber angle configuration. Distinction is also made between primary and secondary glaucoma; in the latter a recognizable ocular or non-ocular disorder contributes to elevation of IOP.
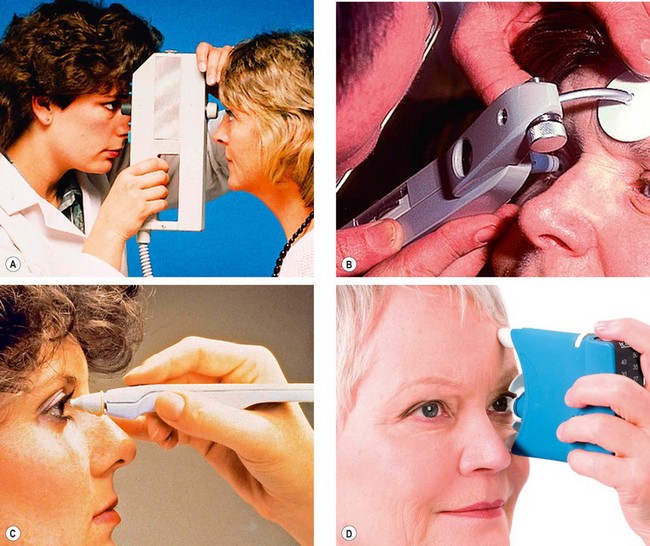
Tonometry
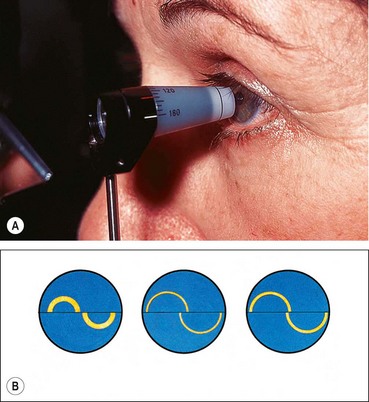
Goldmann tonometry
Principles
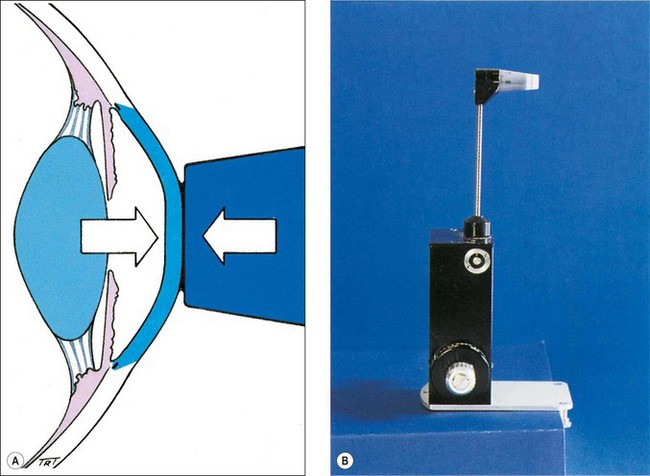
Goldmann applanation tonometry (GAT) is based on the Imbert–Fick principle, which states that for an ideal, dry, thin-walled sphere, the pressure (P) inside the sphere equals the force (F) necessary to flatten its surface divided by the area (A) of flattening (i.e. P = F/A). Theoretically, average corneal rigidity and the capillary attraction of the tear meniscus cancel each other out when the flattened area has the 3.06 mm diameter contact surface of the Goldmann prism (Fig. 10.4A), which is applied to the cornea with a variable amount of measurable force from which the IOP is deduced. The Goldmann tonometer is shown in Figure 10.4B. Disposable tonometer prisms and tonometer caps have been introduced to counter fears of infection from reusable prisms.
Technique
Sources of error
Other types of tonometry
Gonioscopy
Introduction
Overview
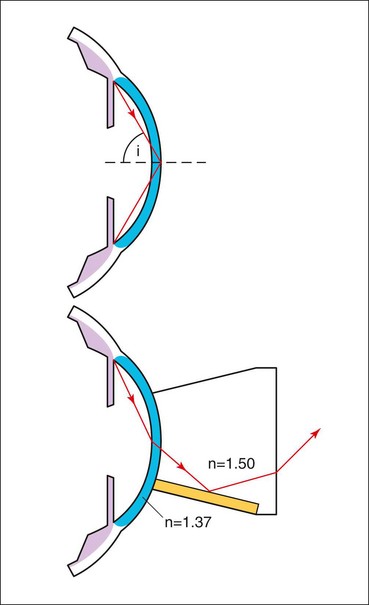
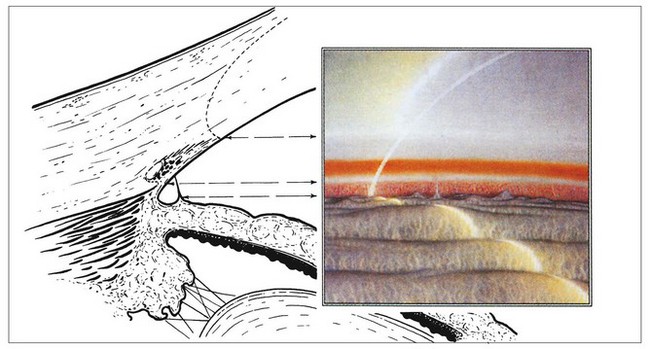
Optical principles
The angle of the anterior chamber cannot be visualized directly through the intact cornea because light from angle structures undergoes ‘total internal reflection’ at the anterior surface of the precorneal tear film (Fig. 10.8). Because the refractive index of a goniolens is similar to that of the cornea, it eliminates total internal reflection by replacing the tear film-air interface with a new tear film-goniolens interface. Light rays can then be viewed as they exit the contact lens. The two main types of goniolenses are indirect and direct (see below).
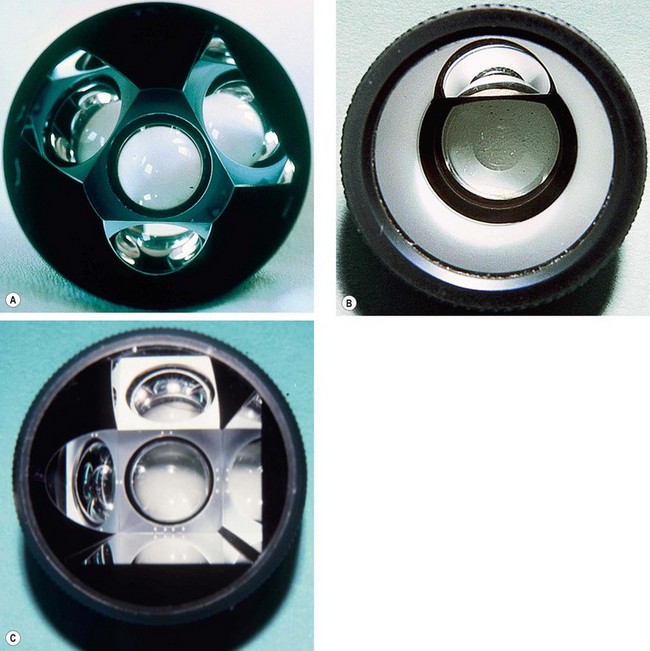
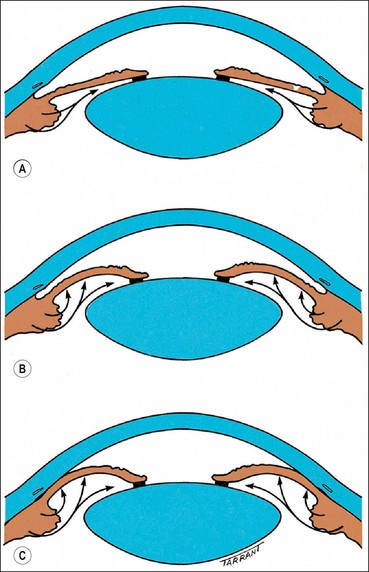
Indirect gonioscopy
Indirect goniolenses use a mirror to reflect rays from the angle such that they exit the lens at much less than the critical angle. They provide a mirror image of the opposite angle and can be used only in conjunction with a slit lamp.
Non-indentation gonioscopy
Indentation gonioscopy
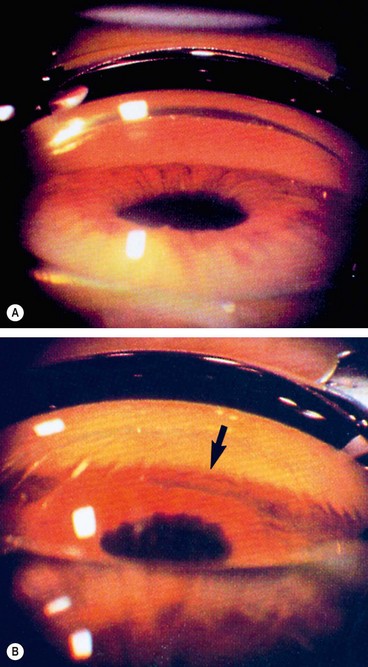
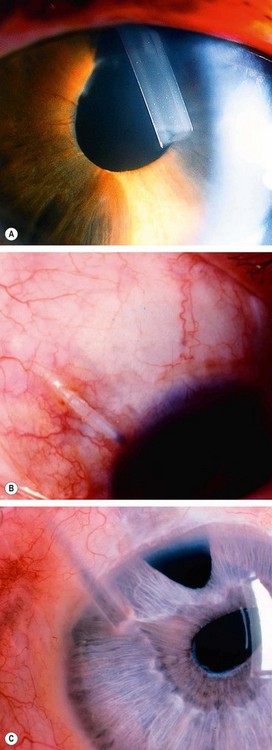
Fig. 10.11 Indentation gonioscopy in appositional angle closure. (A) Total angle closure prior to indentation; (B) during indentation the entire angle becomes visible (arrow) and the cornea develops folds
(Courtesy of W Alward, from Color Atlas of Gonioscopy, Wolfe 1994)
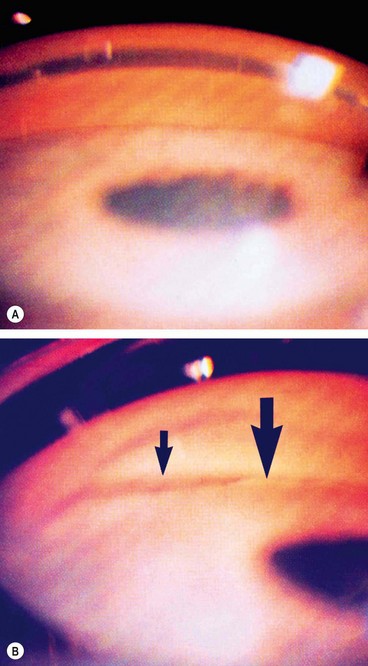
Fig. 10.12 Indentation gonioscopy in partial synechial angle closure. (A) Total angle closure prior to indentation; (B) during indentation part of the angle becomes open (small arrow) and the remainder remains closed (large arrow) due to PAS
(Courtesy of W Alward, from Color Atlas of Gonioscopy, Wolfe 1994)
Direct gonioscopy
Direct goniolenses work by constructing the viewing surface of the lens in a domed or slanted configuration such that exiting light rays strike the contact lens/air interface at a steeper than critical angle so that they will pass through to the observer. This approach is called ‘direct’ because light rays from the angle are viewed directly, without reflection inside the lens. They do not require a slit-lamp and are used with the patient in the supine position, typically under general anaesthesia in the evaluation and surgical treatment of infantile glaucoma.
Identification of angle structures
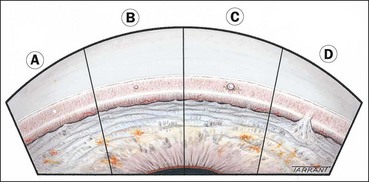
Figure 10.15 shows the anatomy of angle structures.
Grading of angle width
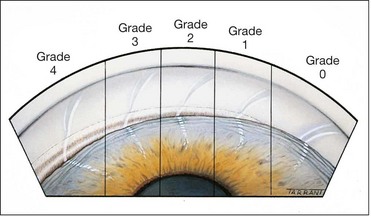
Shaffer system
The Shaffer system records the angle in degrees between two imaginary lines tangential to the inner surface of the trabeculum and the anterior surface of the iris about one-third of the distance from its periphery. In practice, the angle is graded by many according to the visibility of various structures. The system assigns a numerical grade to each quadrant of the angle as below (Fig. 10.17); it should be borne in mind that most angles are narrowest superiorly.
Other systems
Evaluation of the optic nerve head
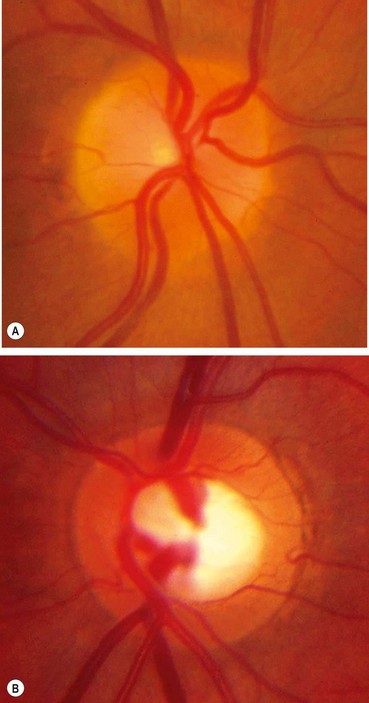
Normal optic nerve head
Neuroretinal rim
The neuroretinal rim (NRR) is the tissue between the outer edge of the cup and the optic disc margin. The normal rim has an orange or pink colour and a characteristic configuration in most healthy eyes: the inferior rim is the broadest followed by the superior, nasal and temporal (the ‘ISNT’ rule).
Optic disc size
Optic disc size is important in deciding if a cup-disc (C/D) ratio is normal. Normal median vertical diameter for non-glaucomatous discs is 1.50 mm in a Caucasian population. It can be assessed clinically as follows:
Table 10.2 Correction factors for estimating optic disc diameter
| Lens | Correction factor |
|---|---|
| Volk 60 D | × 0.88–1.0 |
| Nikon 60 D | Around 1.0 |
| Volk 90 D | ×1.3 |
| Volk 78 D | ×1.1 |
| Goldmann 3-mirror | ×1.27 |
Cup–disc ratio
The C/D ratio indicates the diameter of the cup expressed as a fraction of the diameter of the disc; the vertical rather than the horizontal ratio is generally used in clinical practice. The NRR occupies a relatively similar cross-sectional area in different eyes.
Changes in glaucoma
In many cases, it is not possible to decide with certainty whether an individual optic disc is glaucomatous. The clinical findings and results of investigation should be considered together to guide management. Glaucomatous damage results in characteristic signs involving (a) the optic nerve head, (b) the peripapillary area and (c) the retinal nerve fibre layer.
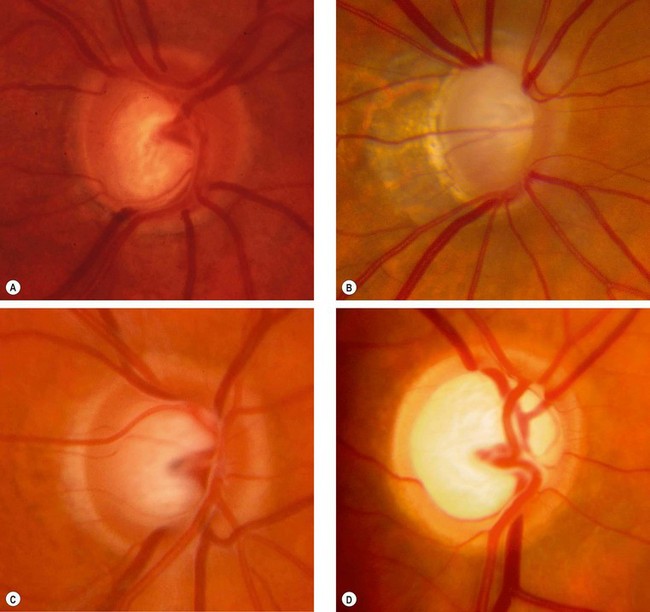
Optic nerve head
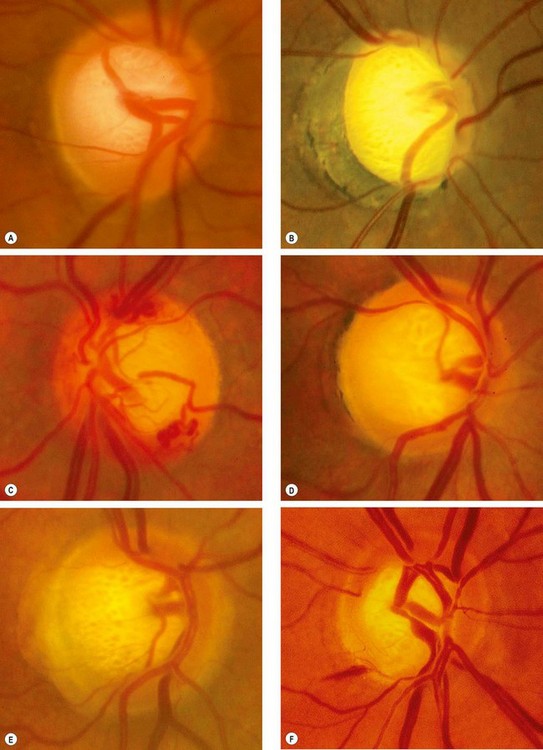
The spectrum of disc damage in glaucoma ranges from highly localized tissue loss with notching of the NRR to diffuse concentric enlargement of the cup, as well as changes in vasculature. Pathological cupping is caused by an irreversible decrease in the number of nerve fibres, glial cells and blood vessels. A documented increase in cup size is always significant. If an eye with a small optic disc and correspondingly small cup develops glaucoma, the cup will increase in size, but even in the presence of substantial damage may still be smaller than that of a large physiological cup, so overall disc diameter must be taken into account as discussed above. Assessment of the thickness, symmetry and colour of the NRR is of substantial importance (see ‘ISNT’ rule above).
Subtypes of glaucomatous damage
The appearance and pattern of disc damage may correlate with subtypes of glaucoma and provide clues as to the pathogenic mechanisms involved. Four ‘pure’ glaucomatous disc appearances have been described, although the majority of discs are unclassifiable.
Non-specific signs of glaucomatous damage
Other disc signs of glaucomatous damage, though of variable specificity, include:
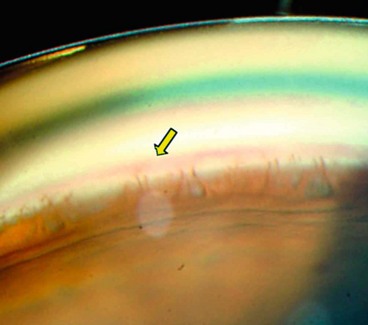
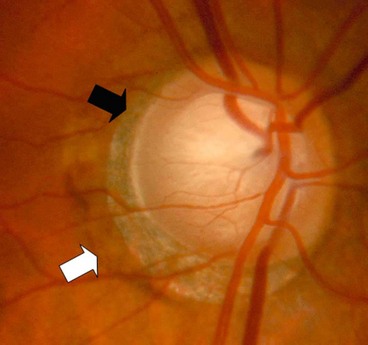
Peripapillary changes
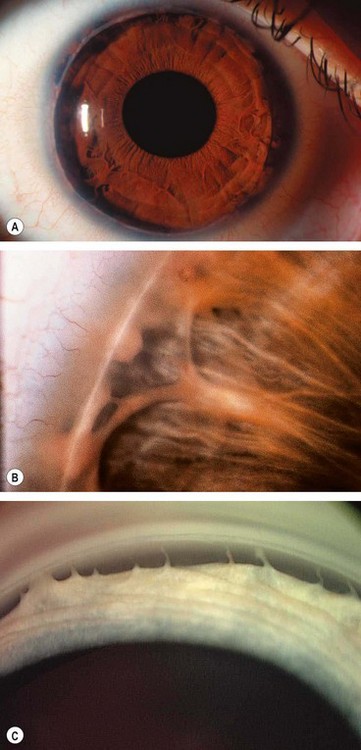
Peripapillary atrophy surrounding the optic nerve head may be of significance in glaucoma (Fig. 10.21) and may be a sign of early damage in patients with ocular hypertension.
It is important to note the distinction from the scleral lip or rim, the white band of exposed sclera central to the beta zone
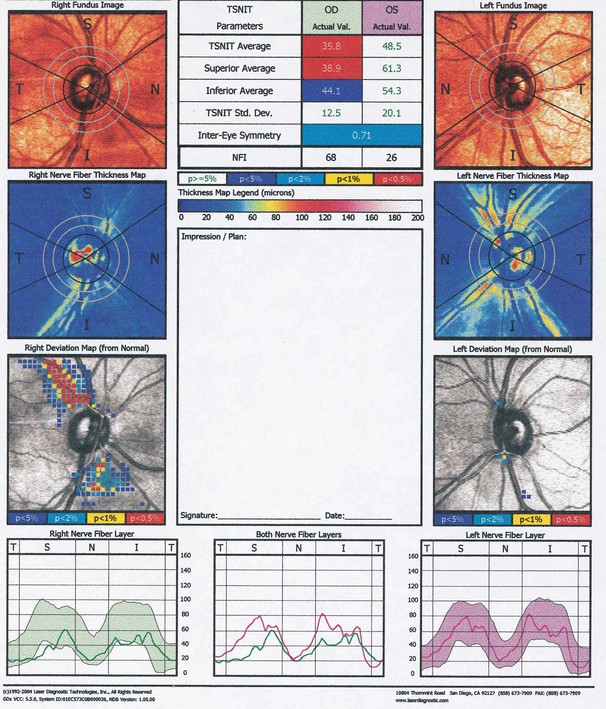
Retinal nerve fibre layer
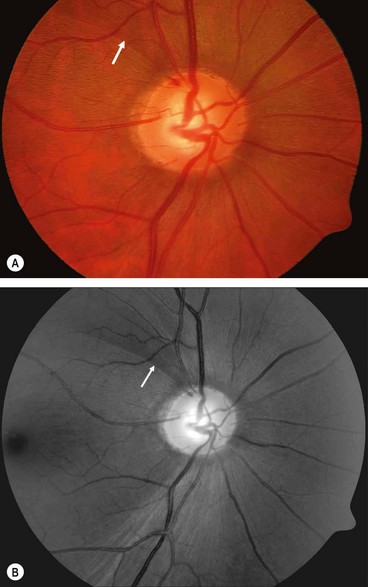
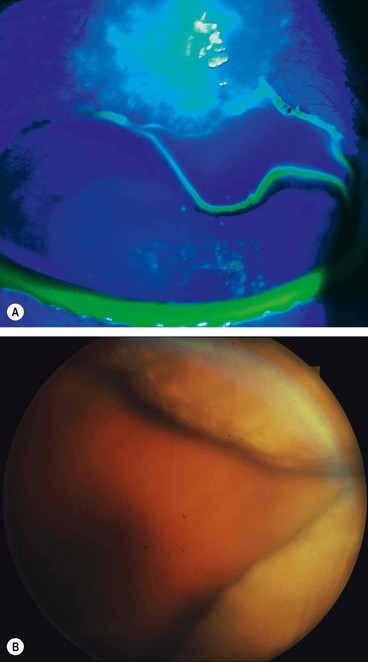
In glaucoma subtle retinal nerve fibre layer (RNFL) defects precede the development of detectable optic disc and visual field changes; their onset often follows disk haemorrhages. Two patterns occur: (a) localized wedge-shaped defects (Fig. 10.22A) and (b) diffuse defects that are larger and have indistinct borders. Red-free (green) light increases the contrast between normal retina and defects on slit-lamp biomicroscopy and typically makes identification easier (Fig. 10.22B). Defects may be easier to detect on (black-and-white) photographs than during clinical examination. Optical coherence tomography (OCT) and scanning laser polarimetry are highly effective means of quantifying the RNFL. It should be noted that RNFL defects are not specific to glaucoma, and can be seen in a range of neurological disease, as well as apparently normal individuals.
Imaging in glaucoma
Stereo disc photography
Stereo photography has historically been regarded as the reference standard in optic disc imaging, and remains a valuable option. The images are taken by repositioning the camera slightly between shots, either manually or using a stereo separator built into the camera.
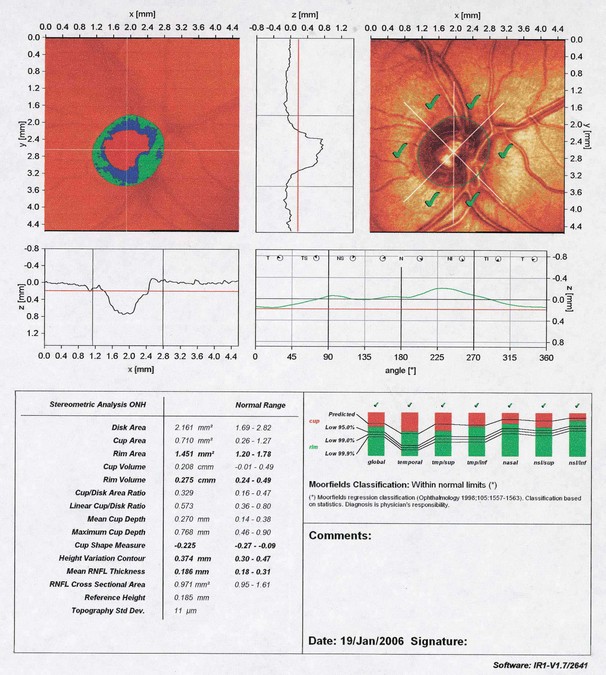
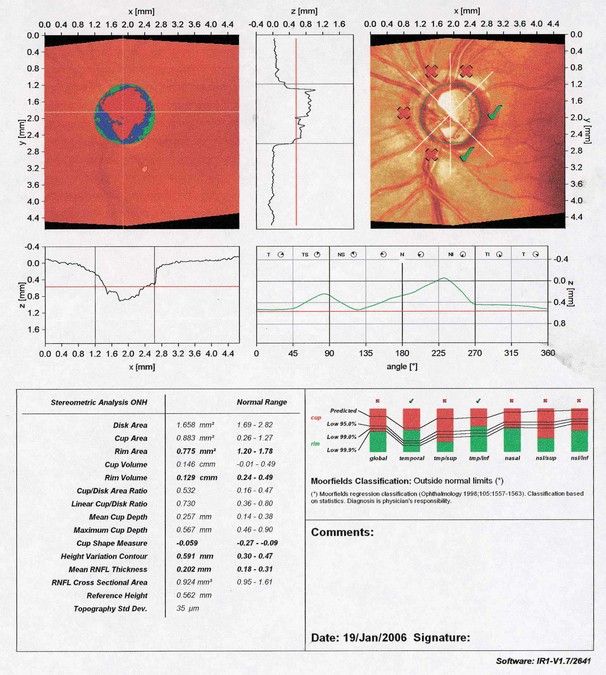
Confocal scanning laser tomography
Scanning laser polarimetry
Optical coherence tomography
OCT has become a routine part of the management of macular and other retinal disease, but is also widely used for the assessment of glaucoma. The principles are discussed in detail in Chapter 14. The following imaging strategies are applicable to glaucoma:
Anterior chamber depth measurement
Objective measurement of the depth of the anterior chamber is sometimes clinically useful in glaucoma management. Indications include monitoring of progression in conditions where the anterior chamber is shallowed such as post-trabeculectomy hypotony and cilio-lenticular block, and as a diagnostic tool, including the comparison of the two eyes. Older methods involved using a slit-lamp with or without a special attachment, but an accurate and repeatable measurement can be obtained using ultrasonographic or optical interferometric methods (e.g. ACD function on Zeiss IOLMaster). Utility and accuracy is limited in pseudophakic eyes.
Perimetry
Definitions
Types of perimetry
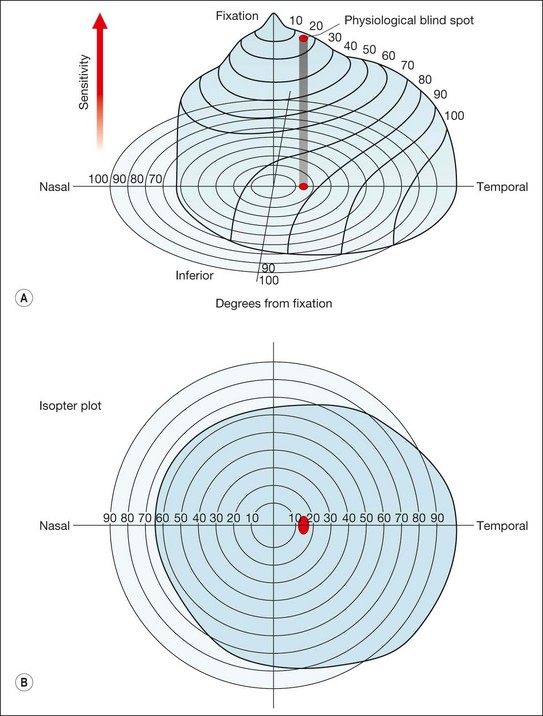
Kinetic
Kinetic perimetry is a two-dimensional assessment of the boundary of the hill of vision. It involves the presentation of a moving stimulus of known luminance or intensity from a non-seeing area to a seeing area until it is perceived (Fig. 10.27A). The stimulus is moved at a steady speed along various meridia (clock hours) and the point of perception is recorded on a chart. By joining these points along different meridians an isopter is plotted for that stimulus intensity. Using stimuli of different intensities a contour map of the visual field with several different isopters can be plotted. Kinetic perimetry can be performed by simple confrontation or by means of a perimeter such as the Goldmann.
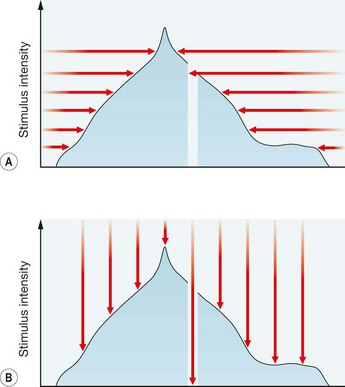
Static
A method of assessing fields in which the location of a stimulus remains fixed at a certain location within the field, with the intensity increased until it is seen by the subject (or decreased until it is no longer seen). In other words, the target intensity is increased (or decreased) until threshold is reached (Fig. 10.27B). The most frequently used automated perimeter is the Humphrey Field Analyzer (HFA); others include the Henson, Dicon and Octopus. Automated static perimetry now constitutes the method used for the great majority of visual fields monitoring in the care of patients with glaucoma.
Suprathreshold
Suprathreshold perimetry involves testing with stimuli of luminance above the expected normal threshold levels for an age-matched population to assess whether these are detected; in other words, testing to check that a subject can see stimuli that would be seen by a normal person of the same age. It enables testing to be carried out rapidly to indicate whether function is grossly normal or not. However, it is not highly quantitative, and so is usually reserved for screening.
Threshold
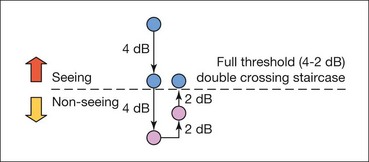
Threshold perimetry is used for detailed assessment of the hill of vision by plotting the threshold luminance value in various locations in the visual field and comparing the results with age-matched ‘normal’ values. In Humphrey perimetry (see below), a stimulus of higher than expected intensity is presented; if seen, the intensity is decreased in 4 dB steps until it is no longer seen (‘staircasing’). The stimulus is then increased again in 2 dB steps until seen once more (Fig. 10.28). If the stimulus is not seen initially, its intensity is increased in 4 dB steps until seen, then decreased in 2 dB steps until no longer seen. Essentially, the threshold is crossed in one direction with large increments, then crossed again to ‘fine-tune’ the result with smaller increments. Threshold testing is quantitatively detailed and is therefore used for monitoring glaucomatous fields.
Sources of error
The skill of the perimetrist in setting up the test, explaining the procedure to the patient, reassuring the patient and monitoring performance is fundamental to obtaining an accurate field. However, errors may still occur as a result of one or more of the following factors:
Humphrey Field Analyzer
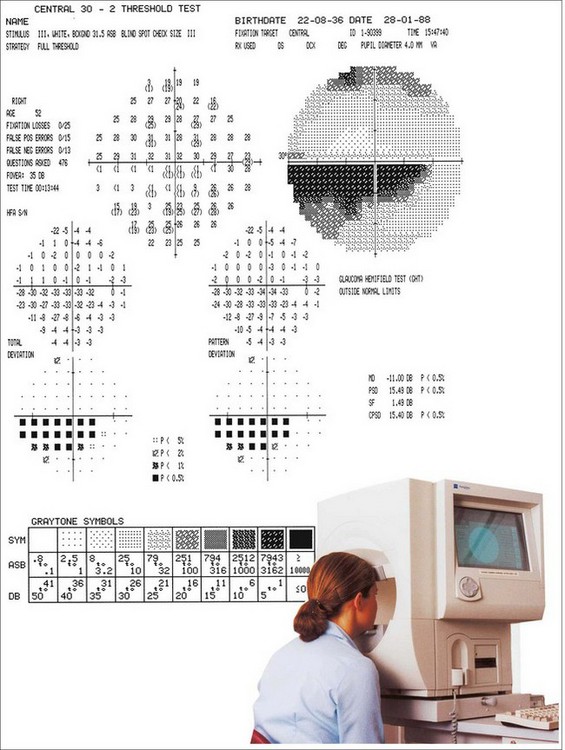
The Humphrey Field Analyzer (HFA) consists of a hemispherical bowl onto which a target can be projected at any location in the visual field (Fig. 10.29).
Testing patterns
Most important defects in glaucoma occur within the central 30° radius of field, so this is the area most commonly tested.
Testing strategies
Displays
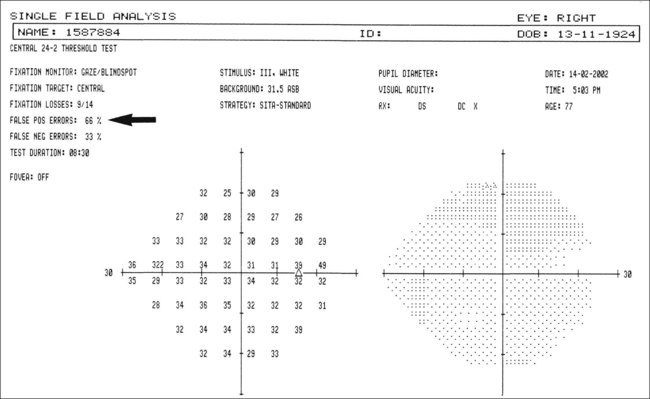
Reliability indices
Reliability indices reflect the extent to which the patient’s results are reliable and should be analyzed first. If grossly unreliable, further analysis of a visual field printout is of little value. In patients who consistently fail to achieve good reliability indices it may be useful to switch to a suprathreshold strategy or kinetic perimetry.
Glaucoma hemifield test
The glaucoma hemifield test (GHT) is a means, available in the 24–2 and 30–2 HFA testing patterns, of assessing the visual field for damage in a pattern commonly seen in glaucoma. The GHT compares five corresponding areas on the superior and inferior fields (as glaucomatous change is typically vertically asymmetrical). It also assesses overall sensitivity.
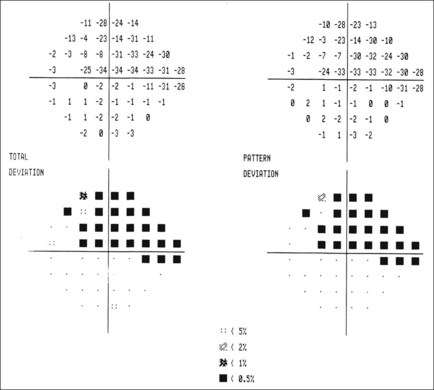
Global indices
Essentially, the global indices represent a statistical summary of the field in a single number; they are used principally used to monitor progression of glaucomatous damage rather than for initial diagnosis.
Computer analysis of serial fields
Adoption into routine clinical practice of computer software for analysis of serial visual fields has, to date, been slow due to several factors. A large number of reliable fields need to be carried out (usually over an extended period) before analysis is effective. Clinical impression frequently differs from software-based interpretation, and different software packages have tended to show poor correlation in assessing the same data. Resource constraints may also have played a part. The quality of available software has been improving steadily, and there are signs that gradual integration may take place. Newer analysis programs include ‘Progressor’ and ‘PeriData’. In the former, each test location is subjected to linear regression analysis, and an indication of stability and of the speed of any deterioration is given using coloured graphical representation.
Short-wave automated perimetry
Short-wave automated perimetry (SWAP) uses a blue stimulus on a yellow background. Sensitivity to blue light (mediated by blue cone photoreceptors) is adversely affected relatively early in glaucoma. SWAP is more sensitive to early glaucomatous defects but has not been widely adopted because cataract decreases sensitivity to blue light (the brunescing lens acts as a yellow filter) and patients frequently dislike the lengthy test. It is available on newer HFA models.
Frequency-doubling contrast test
Ocular hypertension
Definition
In the general population the mean IOP is 16 mmHg; two standard deviations on either side of this gives a ‘normal’ IOP range 11–21 mmHg. The distribution is Gaussian with the curve skewed to the right (Fig. 10.34).
Risk factors for developing glaucoma
The Ocular Hypertension Treatment Study (OHTS) was a multicentre longitudinal trial. In addition to looking at the effect of treatment in ocular hypertensives (IOP <32 mmHg), invaluable landmark information was gained about the effect of a range of putative risks for conversion from OHT to glaucoma; the percentage of OHT patients likely to develop glaucoma taking key factors into account is set out in Tables 10.3 and 10.4 (median follow-up was 72 months). Additional considerations are discussed below. Limitations included the possibility that early glaucomatous damage was already present in some of the patients classified as having OHT. The fact that some percentages appear anomalous may be due to the relatively low numbers in different subcategories.
Table 10.3 Risk of developing glaucoma according to IOP (intraocular pressure) and CCT (central corneal thickness)

The following factors were significant on multivariate analysis:
The following factors were significant on univariate analysis only; they were not significant in isolation but were over-ridden when the factors considered above were taken into account.
Factors examined in the OHTS but not found to be significant are listed below.
Pre-perimetric glaucoma
This concept refers to glaucomatous damage, usually manifested by a suspicious optic disc and/or the presence of retinal nerve fibre layer defects, in which no visual field abnormality has developed. The field testing modality for this purpose is usually taken as standard achromatic automated perimetry.
Management
In the OHTS, untreated patients with ocular hypertension had a 9.5% cumulative risk of developing POAG after 5 years; treatment (which aimed to reduce IOP by 20% or more and to reach 24 mmHg or less) reduced this to 4.4%. Hence, when deciding on whether to start treatment it is important to take into account that it will be necessary to treat a large number of patients in order to prevent the development of glaucoma in a single individual.
Primary open-angle glaucoma
Introduction
Definition
Primary open-angle glaucoma (POAG), also referred to as chronic simple glaucoma, is a generally bilateral disease of adult onset characterized by:
POAG is the most prevalent type of glaucoma in individuals of European and African ethnic origin. It affects both sexes equally.
Risk factors
Genetics
Mutations at 15 loci in the human genome have so far been identified as associated with POAG and are designated primary open angle glaucoma-1A (GLC1A) to GLC1O. Four susceptible genes have been identified: the MYOC gene (chromosome 1q21-q31), coding for the glycoprotein myocilin that is found in the trabecular meshwork and other ocular tissues, the OPTN gene on chromosome 10p, which codes for optineurin, the WDR36 gene on chromosome 5q22, and the NTF4 gene on chromosome 19q13.3. Among them MYOC is the most frequently mutated gene in POAG: a study of unrelated POAG patients found myocilin mutations in at least 4% of the adults. A number of different mutations have been described in the MYOC gene, though the normal function of myocilin and its role in causing glaucoma is as yet undetermined. If a single family member develops glaucoma prior to age 35 years, the chances that the genetic defect is a mutation is the myocilin gene may be as high as 33%.
Steroid responsiveness
A proportion of the population develops an elevation in IOP in response to a course of topical steroid; more potent steroids have a greater propensity to elevate IOP, as does greater frequency of instillation. This tendency is more marked in patients with POAG and their close relatives. Intra- and periocular steroid administration, including periocular application of steroid skin cream and nasal administration, are also prone to elevate IOP. Systemic steroids are much less prone to cause elevation of IOP, but substantial, probably dose-dependent, rises can occur and some authorities have advocated screening for all patients on systemic steroids, perhaps those on dexamethasone in particular. The precise mechanism of the ‘steroid response’ is uncertain, but it may be mediated by an increase in trabecular meshwork cell myocilin production.
Pathogenesis of glaucomatous optic neuropathy
Retinal ganglion cell death in glaucoma occurs predominantly through apoptosis (programmed cell death) rather than necrosis. The preterminal event is calcium ion influx into the cell body and an increase in intracellular nitric oxide; glutamine metabolism is intrinsically involved. After initial injury, a cascade of events results in astrocyte and glial cell proliferation, and alterations in the extracellular matrix of the lamina cribrosa, with subsequent optic nerve head remodelling. Multiple factors are likely to be involved, but the mechanisms remain relatively speculative: the process of glaucomatous damage and the relationship with IOP and other potential influences is still poorly understood. One or both of the following mechanisms may be involved:
Insults via both mechanisms might lead to reduction in axoplasmic flow, interference with the delivery of nutrients or removal of metabolic products, deprivation of neuronal growth factors, oxidative injury and the initiation of immune-mediated damage.
Screening
Universal population screening for glaucoma has not been demonstrated to be cost-effective, and current practice restricts screening to high-risk groups, such as older individuals, those with a history of POAG in a close family member over the age of 40, and people of black ethnicity. In these groups, screening tends to be performed sporadically via routes such as commercial sight tests, which may lead to the relative exclusion of underprivileged economic groups. Population screening with tonometry alone is unsatisfactory, since it will label as normal a significant number of cases with other features of POAG such as cupping and visual field loss. Even with the added criterion of a vertical cup–disc ratio of >0.4, only a proportion of potential POAG patients will be identified. It is therefore prudent for routine screening eye examinations to include visual field examination as well as tonometry and ophthalmoscopy.
Diagnosis
History
Examination
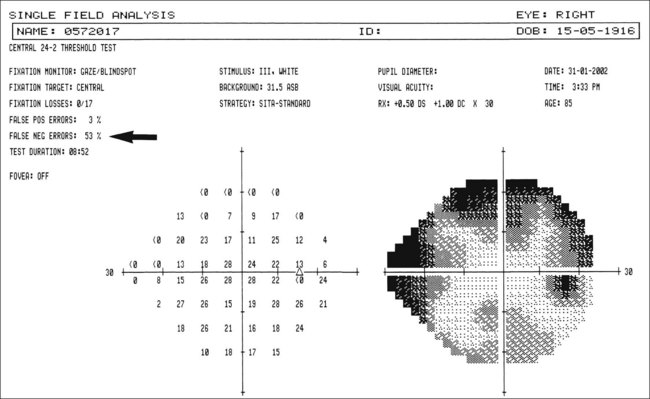
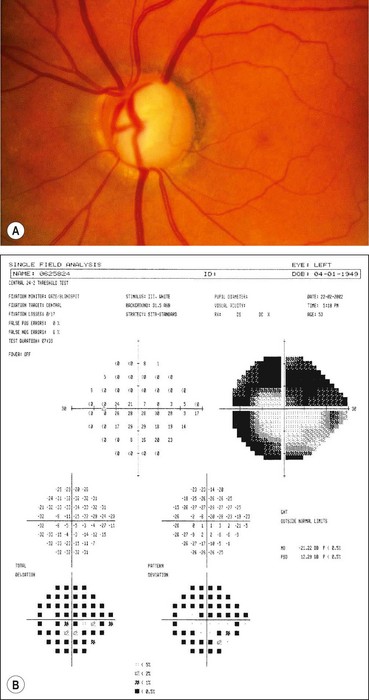
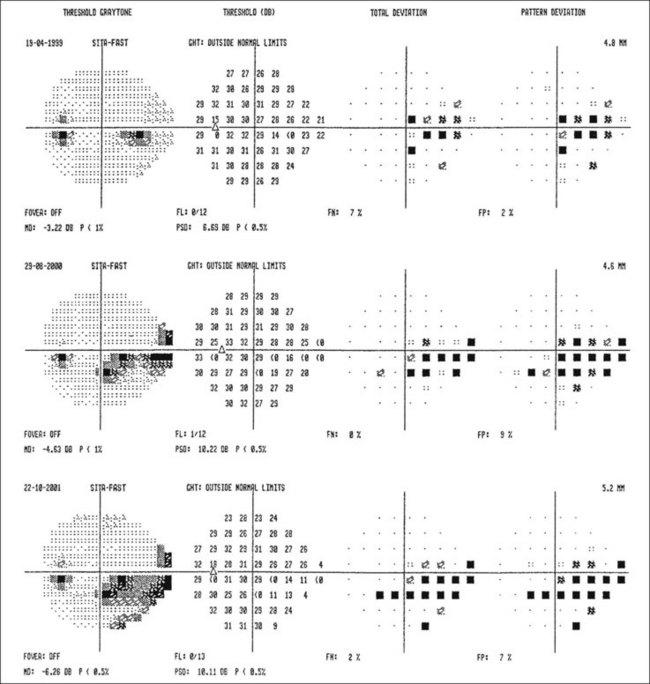
Visual field defects
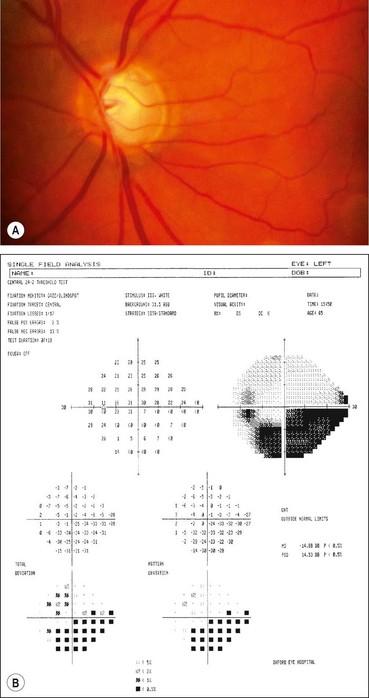
Fig. 10.37 Severe damage. (A) Marked cupping; (B) dense arcuate scotoma/nasal step connecting with the blind spot
Global indices should always be taken into account (Fig. 10.39); on average annual deterioration in mean total deviation of just over 1.0 dB can be expected in treated patients.
Management
The primary aim of treatment is to prevent functional impairment of vision within the patient’s lifetime by slowing the rate of ganglion cell loss closer to that of the normal population (approximately 5000/year). Currently the only proven method of achieving this is the lowering of IOP.
Patient instruction
An explanation should be offered concerning the nature of the disease, and an explanatory booklet provided. The timing of medication use should be specified, and the patient educated in the technique of eye drop instillation. At follow-up visits the patient’s proficiency at instilling drops should be checked. In order to maximize drug contact time with the anterior segment and to minimize systemic absorption the patient should be instructed either to perform lacrimal sac occlusion (by applying fingertip pressure at the medial canthus) or to close the eyes for about 3 minutes after instillation. Common or severe potential adverse effects should be explained at the commencement of treatment and their occurrence enquired about at follow-up visits.
Treatment goals
Medical therapy
Laser trabeculoplasty
In argon laser trabeculoplasty (ALT) or selective laser trabeculoplasty (SLT) argon or Nd:YAG laser is applied to the trabeculum to enhance aqueous outflow and lower IOP. The therapeutic effect is highly variable, and when effective tends to be transient, although typically lasting for months to years. The following are the main indications:
Surgery
Trabeculectomy is the surgical procedure most commonly performed for glaucoma, although non-penetrating surgery is gaining in popularity. If significant lens opacity is present, phacoemulsification alone may be associated with a fall in IOP; alternatively it can be combined with a filtration procedure (phacotrabeculectomy). Progressive damage is thought to be less likely after surgery than with medical therapy, probably because the resultant IOP is often significantly lower and less likely to fluctuate, and because compliance is no longer a factor. The following are the main indications:
Prognosis
The great majority of patients diagnosed with POAG will not become blind in their lifetime, but the rate of glaucoma progression varies considerably.
Normal-pressure glaucoma
Definition
Normal-pressure glaucoma (NPG), also referred to as normal- or low-tension glaucoma, is a variant of POAG. It is characterized by:
The distinction between NPG and POAG is based on an epidemiologically-derived range of normal IOP. It is essentially an arbitrary division and may not have significant clinical value, though there is the possibility that a spectrum exists in which, towards the NPG end, IOP-independent factors are of increasing relative importance.
Pathogenesis
Any aetiological factors distinct from those in POAG have not been conclusively determined although various mechanisms have been postulated including anomalies of local and systemic vascular function, structural optic nerve anomalies and autoimmune disease. With the introduction of widespread central corneal thickness (CCT) assessment, NPG in some patients has been explained by very low CCT, and overall CCT in patients with NPG is lower than in POAG. A small proportion of NPG patients have been found to have marked nocturnal IOP spikes, sometimes only detected on testing in the supine position.
Risk factors
Differential diagnosis
Diagnosis
History and examination are essentially the same as for POAG but specific points warrant attention:
Treatment
Further lowering of IOP is effective in reducing progression in some patients. However, as many untreated patients will not deteriorate, in most cases progression should be demonstrated before commencing treatment. Exceptions to this general principle include advanced damage, particularly if threatening central vision, and young age. Regular assessment including perimetry should be performed at 4–6 monthly intervals. With treatment that reduces IOP by 30% from baseline, 80% of patients are stable and 20% show progression.
Primary angle-closure glaucoma
Introduction
Overview
The term ‘angle-closure’ refers to occlusion of the trabecular meshwork (TM) by the peripheral iris (iridotrabecular contact – ITC), obstructing aqueous outflow. Angle-closure can be primary, when it occurs in an anatomically predisposed eye, or secondary to another ocular condition. Primary angle-closure glaucoma may be responsible for up to half of all cases of glaucoma globally, with a particularly high prevalence in individuals of Far Eastern descent. It is typically associated with greater speed of progression and visual morbidity than POAG.
Classification
Recently classification has moved away from a symptom-based approach (acute, subacute and chronic), to reflect the stages in the natural history of the disease. This takes into account that the majority of patients are asymptomatic.
Mechanism
The mechanisms involved in angle-closure can be categorized according to the anatomical level (anterior to posterior) at which causative forces act. In many patients more than one level is contributory.
Diagnosis
Symptoms
Signs
Provocation testing
Although investigations are usually unnecessary provocation testing may aid decision-making in some circumstances. For example, in patients with only partially opened angles following laser iridotomy, to assess the propensity to develop a steep increase in IOP and so determine whether further intervention (e.g. iridoplasty) might be appropriate. In the dark room/prone provocation test the patient sits face down in a dark room for one hour. The IOP is checked and a rise of 8 mmHg or more is taken as being of significance but may also sometimes occur in normal eyes. A positive response is virtually always abolished following lens extraction.
Treatment
Primary angle-closure suspect (PACS)
Chronic presentation of PAC and PACG
Acute and subacute presentation of PAC and PACG
Treatment intensity should be individualized dependent on severity. Hospital admission is usually required in an acute presentation, though not necessarily when subacute.
Differential diagnosis of an acute elevation of IOP
A key indicator that PAC/PACG may not be responsible is an open angle in a fellow eye.
Classification of secondary glaucoma
Open-angle
Secondary open-angle glaucoma can be subdivided on the basis of the site of aqueous outflow obstruction as follows:
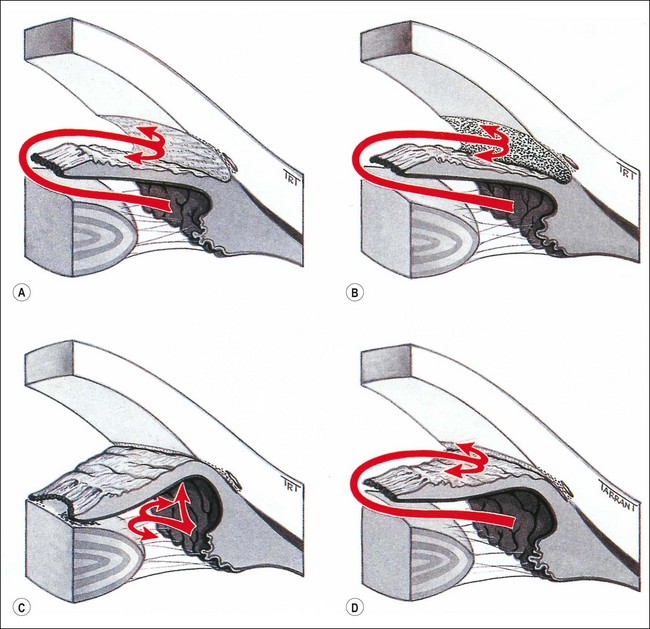
Fig. 10.48 Pathogenesis of secondary glaucoma. (A) Pre-trabecular obstruction; (B) trabecular obstruction; (C) angle-closure with pupillary block; (D) angle-closure without pupillary block
Trabecular glaucomas may also be caused by alteration of the trabecular fibres themselves by:
Angle-closure
Secondary angle-closure is caused by impairment of aqueous outflow secondary to apposition between the peripheral iris and the trabeculum. Classification is based according to the presence or absence of pupillary block:
Pseudoexfoliation
Pseudoexfoliation syndrome
Introduction
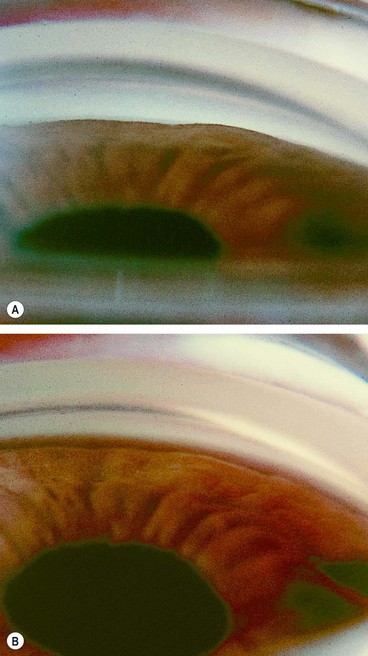
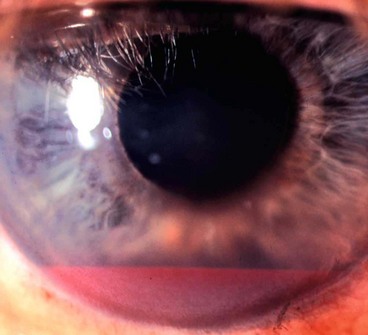
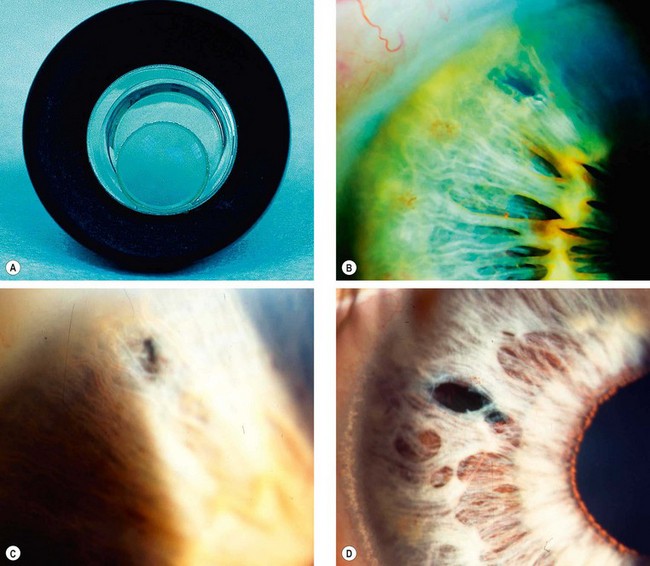
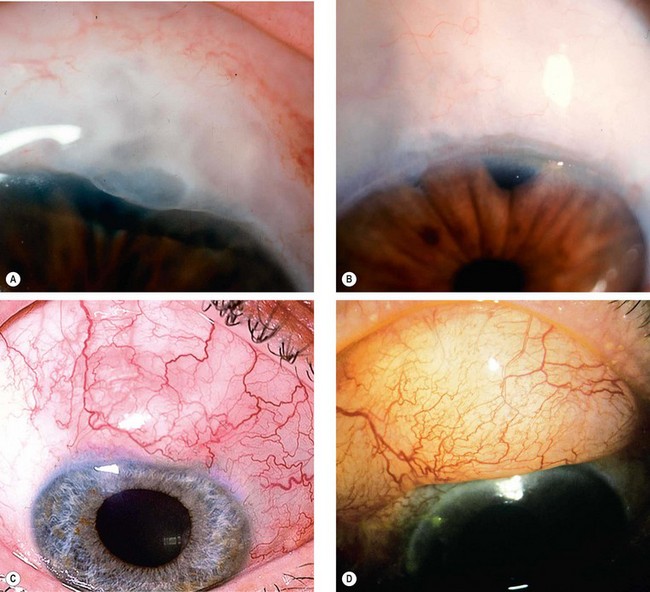
The pseudoexfoliation syndrome (PXF), sometimes known as exfoliation syndrome, is a relatively common cause of chronic open-angle glaucoma, though subtle signs are easily overlooked. When an eye with PXF develops secondary open-angle glaucoma the condition is referred to as pseudoexfoliation glaucoma (PXG). PXF is more common in females but males appear to be at higher risk of developing glaucoma. The condition is particularly common in Scandinavia. A high risk of developing PXF and PXG is conferred by mutations in the LOXL1 gene at locus 15q22, coding for elastic fibre components of the extracellular matrix. The cumulative risk of glaucoma in eyes with PXF is 5% at 5 years and 15% at 10 years.
Pathogenesis
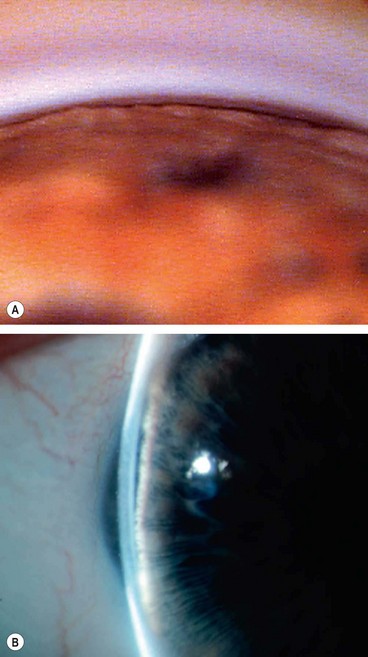
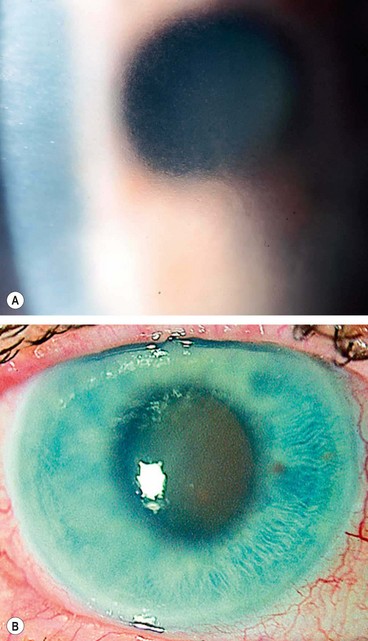
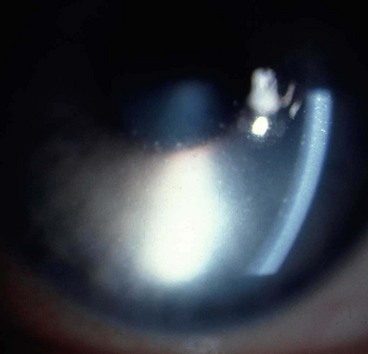
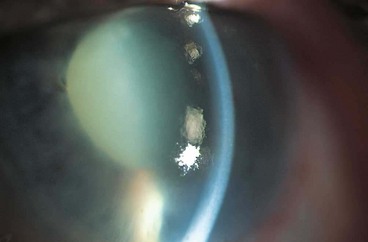
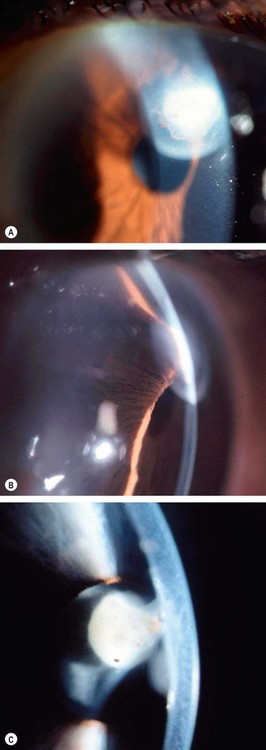
A grey-white fibrillary extracellular material composed of a protein core surrounded by glycosaminoglycans is produced by abnormal basement membranes of ageing epithelial cells in the trabeculum, equatorial lens capsule, iris and ciliary body. The material is then deposited on the anterior lens capsule (Fig. 10.49A), zonules, ciliary body, iris, trabeculum, anterior vitreous face and conjunctiva. In addition to its occurrence within the eye, exfoliative fibrillopathy has been reported in skin and visceral organs, suggesting that PXF may be an ocular manifestation of a systemic disorder; PXFS is associated with an increasing number of vascular disorders, hearing loss and Alzheimer disease.
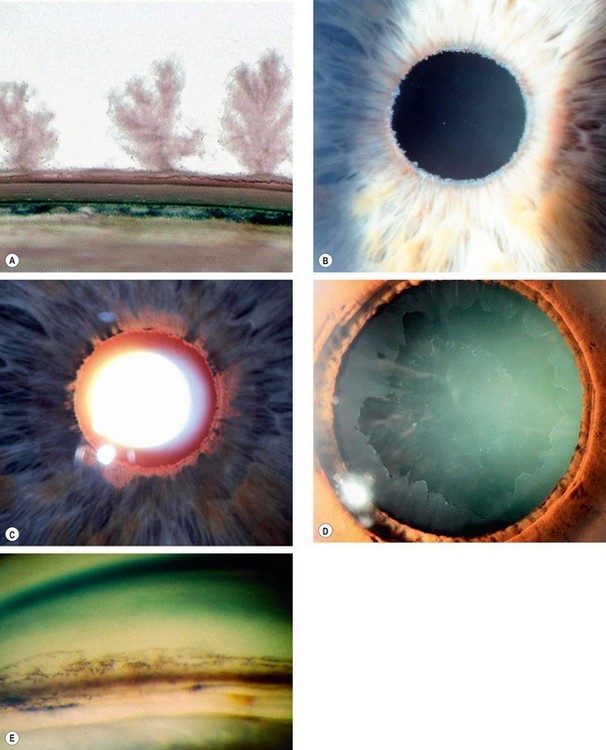
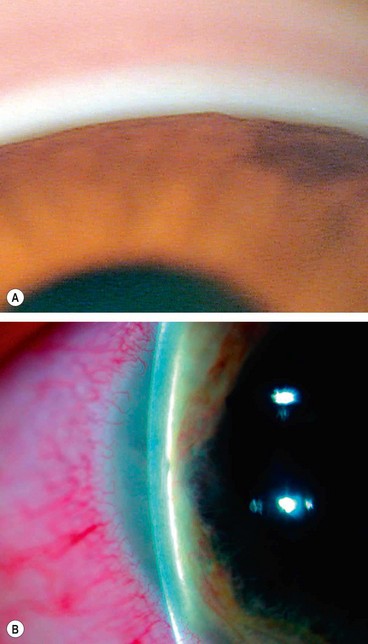
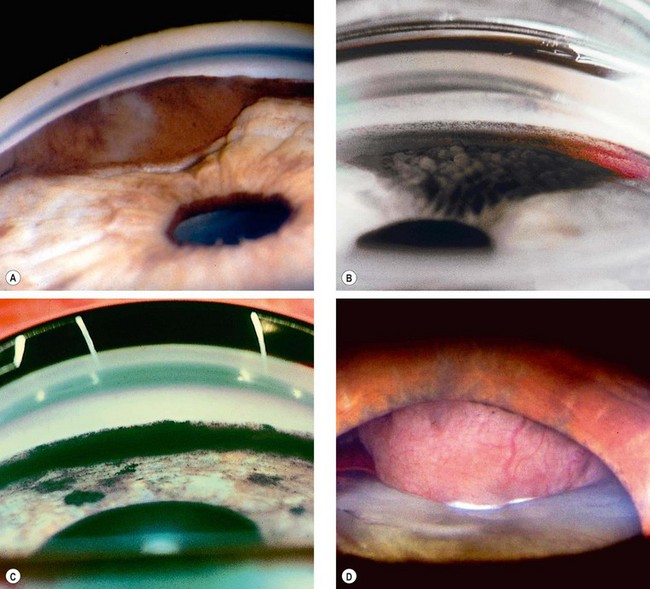
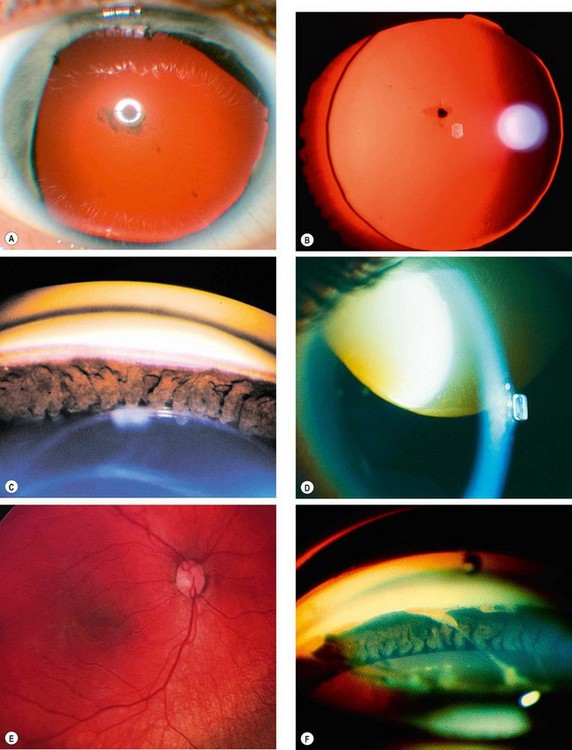
Fig. 10.49 Pseudoexfoliation syndrome. (A) Christmas-tree like deposits of pseudoexfoliative material (PXF) on the lens capsule; (B) PXF on the pupillary margin; (C) transillumination defect corresponding to sphincter iris atrophy; (D) PXF on the lens; (E) gonioscopy shows patchy trabecular hyperpigmentation and Sampaolesi line
(Courtesy of J Harry and G Misson, from Clinical Ophthalmic Pathology, Butterworth-Heinemann 2001– fig. A; M Jager – fig. D; J Schuman, V Christopoulos, D Dhaliwal, M Kahook and R Noecker, from Lens and Glaucoma, in Rapid Diagnosis in Ophthalmology, Mosby 2008 – fig. F)
Diagnosis
Pseudoexfoliation glaucoma
Pathogenesis
Probable causes of elevation of IOP include trabecular blockage due to a combination of ‘clogging up’ of the trabeculum by PXF material and/or pigment released from the iris.
Diagnosis
Treatment
Prognosis
The prognosis is worse than in POAG; the IOP is often significantly elevated and may also exhibit great fluctuation. Severe damage may develop rapidly. It is therefore important to monitor patients closely, and some practitioners feel that review should take place at intervals of no more than 6 months for patients with PXF.
Pigment dispersion
Pigment dispersion syndrome
Introduction
Pigment dispersion syndrome (PDS) is a usually bilateral condition characterized by the liberation of pigment granules from the iris pigment epithelium and their deposition throughout the anterior segment. PDS primarily affects whites and may be inherited as AD with variable penetrance. There is a significant linkage between the disease phenotype and genetic markers located on 7q35-36. Myopia predisposes to the phenotypical manifestations and the development of a secondary open-angle ‘pigmentary’ glaucoma. However, some manifestations of PDS may be extremely subtle and go undetected.
Pathogenesis
Pigment shedding is caused by the mechanical rubbing of the posterior pigment layer of the iris against packets of lens zonules as a result of excessive posterior bowing of the mid-peripheral portion of the iris. It is postulated that an increase in anterior chamber pressure (relative to the posterior chamber) occurs due to ‘reverse pupil block’, with resultant posterior bowing of the iris and iridozonular touch (Fig. 10.50A). This is supported by the observation that neutralization of reverse pupil block with peripheral iridotomy flattens the iris and decreases iridozonular contact (Fig. 10.50B). The pigment epithelium itself may be abnormally susceptible to shedding. In some patients strenuous exercise may precipitate episodes of pigment dispersion associated with a rise in IOP. Pigment granules are released into the aqueous humour, dispersed by aqueous currents and deposited on all anterior chamber structures, including the zonular fibres and ciliary body.
Diagnosis
Pigmentary glaucoma
Pathogenesis
Elevation of IOP appears to be caused by pigmentary obstruction of the intertrabecular spaces and damage to the trabeculum secondary to denudation, collapse and sclerosis.
Risk factors
About a third of patients with PDS develop ocular hypertension or chronic open-angle glaucoma after 15 years. Men are affected twice as frequently as women. The optic disc may be more susceptible to the effects of elevated IOP because of the underlying myopia. It is therefore important to regularly follow patients with the condition, particularly myopic males with Krukenberg spindles. However, initial IOP, cup–disc ratio and degree of trabecular hyperpigmentation are not helpful in identifying those who will eventually develop glaucoma. Patients with pigmentary glaucoma have an increased incidence of steroid responsiveness.
Diagnosis
Treatment
Prognosis
Over time the control of IOP becomes easier and occasionally the IOP may spontaneously revert to normal; this may or may not be associated with a decrease in trabecular pigmentation. Patients with undetected previous pigmentary glaucoma may later be erroneously diagnosed as having NPG.
Differential diagnosis
Neovascular glaucoma
Introduction
Pathogenesis
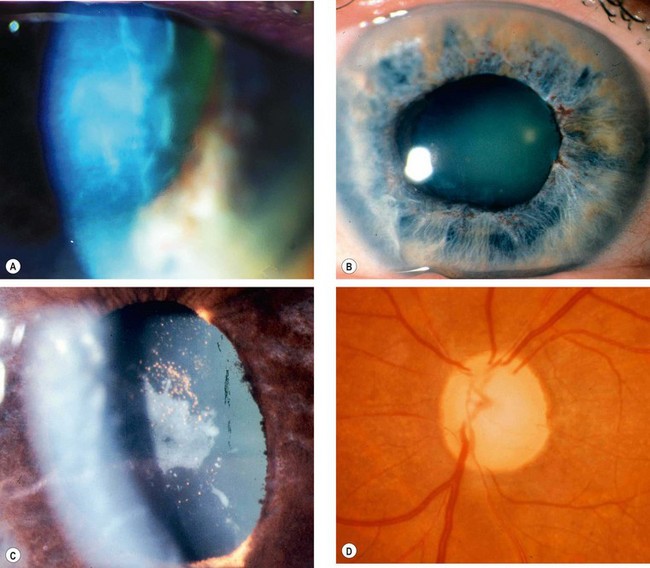
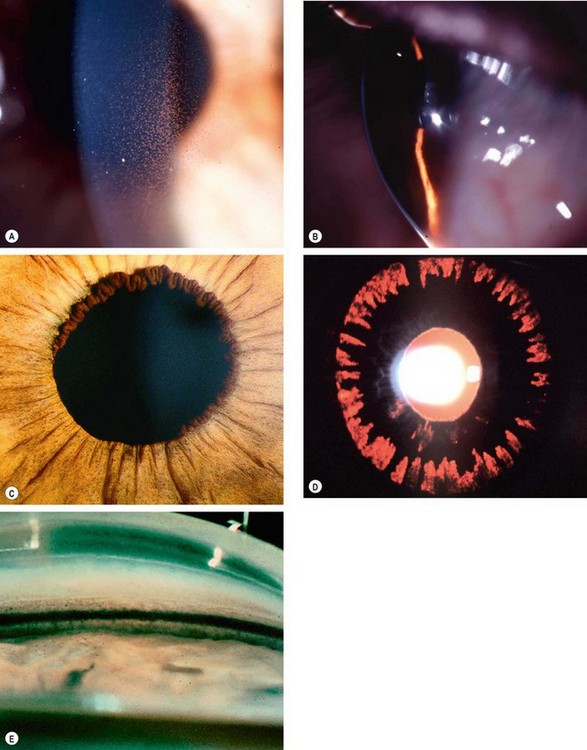
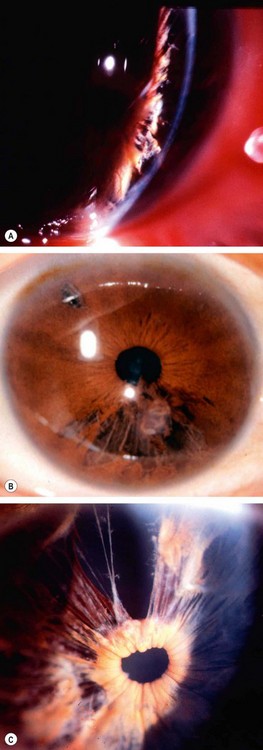
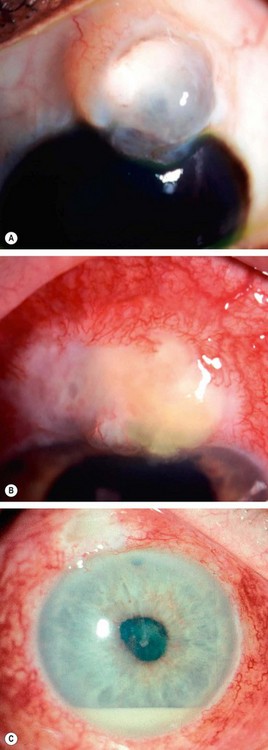
Neovascular glaucoma (NVG) is an aggressive condition which occurs as a result of iris neovascularization (rubeosis iridis). The common aetiological factor is severe, diffuse and chronic retinal ischaemia. It is postulated that hypoxic retinal tissue produces growth factors in an attempt to revascularize hypoxic areas; the most important of these is vascular endothelial growth factor (VEGF). Apart from inducing retinal neovascularization (proliferative retinopathy) such factors also diffuse into the anterior segment and initiate rubeosis iridis and neovascularization in the angle of the anterior chamber. The latter initially impairs aqueous outflow in the presence of an open angle and later contracts to produce a secondary angle-closure glaucoma which is usually severe and relentless (Fig. 10.52A).
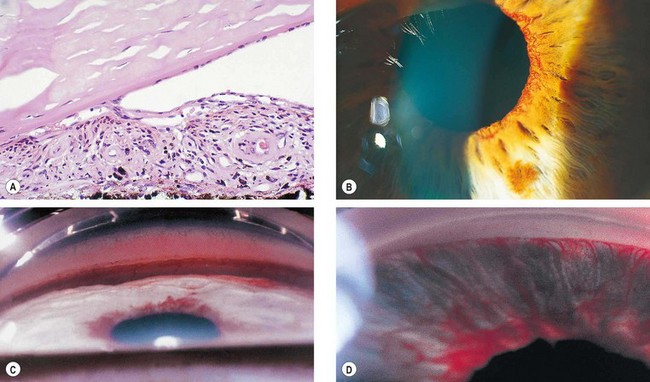
Fig. 10.52 Neovascular glaucoma. (A) Rubeosis iridis and angle-closure by PAS; (B) tiny capillary tufts on the pupil margin; (C) invasion of angle structures by new vessels; (D) progressive synechial angle-closure
(Courtesy of J Harry and G Misson, from Clinical Ophthalmic Pathology, Butterworth-Heinemann 2001 – fig. A)
Causes of rubeosis iridis
Rubeosis iridis
Diagnosis
In chronological order rubeosis develops as follows:
Treatment
Secondary open-angle glaucoma
Diagnosis
Neovascular tissue proliferates across the face of the angle (Fig. 10.52C). Here the new blood vessels arbourize and form a fibrovascular membrane which blocks the trabeculum, giving rise to secondary open-angle glaucoma.
Treatment
Secondary angle-closure glaucoma
Diagnosis
If rubeosis continues to progress the angle becomes progressively closed by contraction of fibrovascular tissue with pulling of the peripheral iris over the trabeculum (Fig. 10.52D and see Fig. 10.52A). The angle thus closes circumferentially in a zipper-like fashion resulting in very high IOP, severe visual impairment, congestion of the globe and pain. The prognosis for visual function is generally poor by this stage, though aggressive management can achieve comfort and retain useful sight in some cases.
Treatment
Inflammatory glaucoma
Introduction
Overview
Elevation of intraocular pressure (IOP) secondary to intraocular inflammation frequently presents a diagnostic and therapeutic challenge. The elevation of IOP may be transient and innocuous, or persistent and severely damaging. The prevalence of secondary glaucoma increases with chronicity and severity of disease. Secondary glaucoma is particularly common in Fuchs uveitis syndrome and chronic anterior uveitis associated with juvenile idiopathic arthritis. Posterior uveitis is less likely to affect the aqueous outflow pathway and consequently less likely to lead to IOP elevation.
Diagnostic dilemmas
Angle-closure glaucoma with pupillary block
Pathogenesis
Secondary angle-closure is caused by posterior synechiae extending for 360° (seclusio pupillae) which obstruct aqueous flow from the posterior to the anterior chamber (Fig. 10.53A). The resultant increased pressure in the posterior chamber produces anterior bowing of the peripheral iris (iris bombé – Fig. 10.53B) resulting in shallowing of the anterior chamber and apposition of the iris to the trabeculum and peripheral cornea (Fig. 10.53C). Such an inflamed iris easily sticks to the trabeculum and the iridocorneal contact may become permanent with the development of PAS.
Angle-closure glaucoma without pupillary block
Open-angle glaucoma
In acute anterior uveitis
In acute anterior uveitis the IOP is usually normal or subnormal due to concomitant ciliary shutdown. Occasionally, however, secondary open-angle glaucoma develops due to obstruction of aqueous outflow, most commonly as the acute inflammation is subsiding and ciliary body function is returning. This effect, which is often transient and innocuous, may be steroid-induced or caused by a combination of the following mechanisms:
In chronic anterior uveitis
In chronic anterior uveitis the main mechanism for reduced outflow facility is thought to be trabecular scarring and/or sclerosis secondary to chronic trabeculitis. The exact incidence and importance of this mechanism is, however, difficult to determine as most eyes also have some degree of synechial angle-closure. Because of the variable appearance of the angle on gonioscopy, definitive diagnosis of trabecular damage is difficult. Theoretically, the angle should be open and, in some eyes, a gelatinous exudate resembling ‘mashed potatoes’ is seen on the trabeculum. Treatment is as for secondary synechial angle-closure glaucoma.
Treatment
Medical
Laser iridotomy
Surgery
Posner–Schlossman syndrome
Posner–Schlossman syndrome (glaucomatocyclitic crisis) is characterized by recurrent attacks of unilateral, acute secondary open-angle glaucoma associated with mild anterior uveitis. The cause of the raised IOP is presumed to be acute trabeculitis. There is some evidence that the herpes simplex virus may play a pathogenic role. Posner–Schlossman syndrome is a rare condition typically affecting young adults, 40% of whom are positive for HLA-Bw54. Males are affected more frequently than females. The IOP is elevated for between a few hours and several days. The attacks are unilateral, although 50% of patients have bilateral involvement at different times. The intervals between attacks vary and, with time, usually become longer. Patients should be followed even after the attacks have completely subsided, because a significant percentage will develop chronic open-angle glaucoma.
Diagnosis
Lens-related glaucoma
Phacolytic glaucoma
Pathogenesis
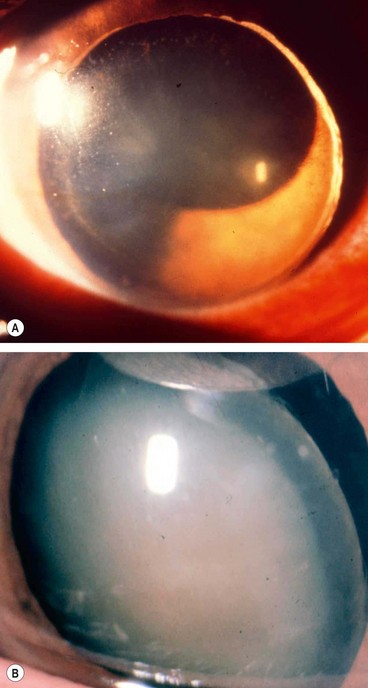
Phacolytic glaucoma (lens protein glaucoma) is open-angle glaucoma occurring in association with a hypermature cataract. Trabecular obstruction is caused by high molecular weight lens proteins which have leaked through the intact capsule into the aqueous humour. Macrophages containing lens proteins may also contribute to trabecular blockage (Fig. 10.57A and B). Phacolytic glaucoma should not be confused with phacoanaphylactic (phacoantigenic) uveitis which is an autoimmune granulomatous reaction to lens proteins occurring in an eye with a ruptured capsule.
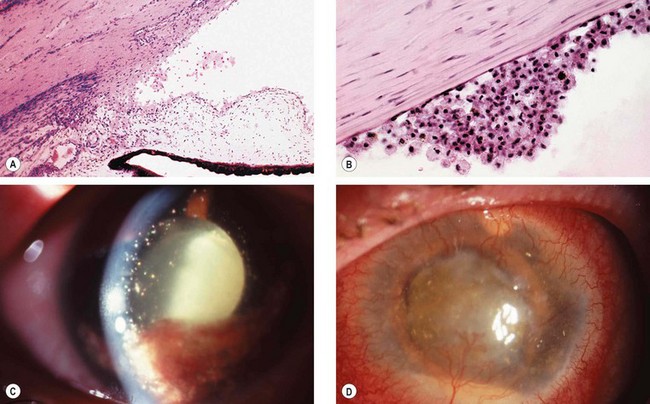
Fig. 10.57 Phacolytic glaucoma. (A) Lens protein-containing macrophages in the angle; (B) lens protein-containing macrophages on the corneal endothelium similar to keratic precipitates; (C) hypermature cataract and lens protein-containing macrophages floating in the aqueous; (D) neglected end-stage glaucoma with corneal vascularization and a small pseudohypopyon
(Courtesy of J Harry – figs A and B)
Diagnosis
Phacomorphic glaucoma
Pathogenesis
Phacomorphic glaucoma is an acute secondary angle-closure glaucoma precipitated by an intumescent cataractous lens. Equatorial age-related growth of the lens slackens the suspensory ligament and allows the lens to move anteriorly. Associated anteroposterior growth leads to increased iridolenticular contact and potentiates pupillary block and iris bombé.
Diagnosis
Treatment
Treatment is initially similar to acute PACG, but miotics are omitted as they tend to increase iris-lens apposition and shift the lens anteriorly. Systemic hyperosmotic agents may be required more commonly than in PACG. Laser iridotomy may be worthwhile but is often not possible (due to corneal oedema or lens-cornea proximity) or ineffective. Definitive treatment consists of early cataract extraction, ideally when the IOP is normal and the eye quiet.
Lens dislocation into the anterior chamber
Causes
Diagnosis
The dislocated lens causes acute pupillary block and a sudden and severe elevation of IOP with associated visual impairment. This constitutes an acute emergency because lenticulocorneal contact may cause permanent endothelial damage.
Treatment
The IOP is initially reduced with osmotic agents. Subsequent management is dependent on the absence or presence of some remaining zonular attachments and the hardness of the lens as follows:
Incarcerated lens in the pupil
Traumatic glaucoma
Hyphaema
Pathogenesis
A traumatic hyphaema may be associated with IOP elevation due to trabecular blockage by red blood cells. Pupillary occlusion by a blood clot may be superimposed on an angle-closure component. Secondary haemorrhage, often more severe than the primary bleed, may develop within 3–5 days of the initial injury. Patients with sickle-cell haemoglobinopathies are at increased risk of developing complications associated with traumatic hyphaema.
Risk of glaucoma
Although most traumatic hyphaemas are relatively innocuous and transient, severe and prolonged elevation of IOP may damage the optic nerve and cause blood staining of the cornea; the latter can progress very rapidly. The size of a hyphaema is a useful indicator of visual prognosis and risk of complications:
Treatment
Angle recession glaucoma
Pathogenesis
Angle recession involves rupture of the face of the ciliary body, the portion that lies between the iris root and the scleral spur, due to blunt trauma. Although a large percentage of eyes with traumatic hyphaema exhibit some degree of angle recession, only 6–9% develop glaucoma after 10 years. The rise in IOP is secondary to associated trabecular damage rather than from angle recession itself; however, the risk of glaucoma is directly related to the extent of angle recession. Since glaucoma may not develop until months or even years after the initial injury, angle recession mandates periodic review.
Diagnosis
Iridocorneal endothelial syndrome
Classification
The iridocorneal endothelial (ICE) syndrome typically affects one eye of a middle-aged woman. It consists of the following three very rare and frequently overlapping disorders: (a) progressive iris atrophy, (b) iris naevus (Cogan–Reese) syndrome and (c) Chandler syndrome.
Pathogenesis
The common link between the three variants of ICE syndrome is an abnormal corneal endothelial cell layer which has the capacity to proliferate and migrate across the angle and onto the surface of the iris. The term ‘proliferative endotheliopathy’ has therefore been suggested to describe this disorder. The ICE syndrome may progress to glaucoma, corneal decompensation or both. Glaucoma is due to synechial angle closure secondary to contraction of this abnormal tissue. Polymerase chain reaction shows the presence of herpes simplex virus DNA in a substantial percentage of ICE syndrome corneal specimens, suggesting that the condition may be of viral origin.
General features
Specific features
In their purest form, the three conditions are easily distinguished from each other. However, there is frequently considerable overlap and clear differentiation may be difficult. Occasionally, during follow-up one condition can be observed changing into another. The differentiation depends primarily on the iris changes.
Glaucoma in intraocular tumours
Approximately 5% of eyes with intraocular tumours develop a secondary elevation of IOP. Depending on the location of the tumour one or more of the following mechanisms may be responsible:
Trabecular block
Trabecular block may be the result of one of the following:
Secondary angle closure
Secondary angle closure may be the result of one of the following:
Glaucoma in epithelial ingrowth
Pathogenesis
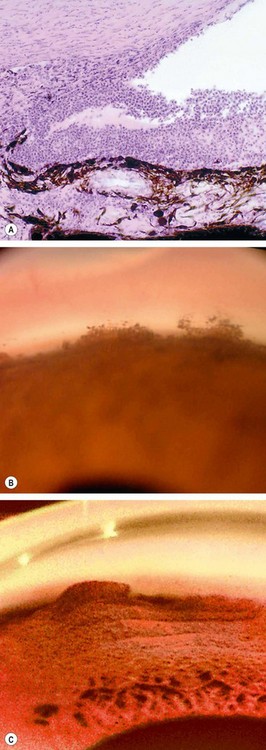
Epithelial ingrowth is a rare and potentially blinding complication of anterior segment surgery or trauma. Conjunctival or corneal epithelial cells migrate through the wound and proliferate in the anterior segment, in a cystic or diffuse manner. The latter is characterized by the proliferation of sheets of epithelial cells over the posterior cornea, trabeculum, iris and ciliary body (Fig. 10.65A) and is more commonly associated with secondary glaucoma than the cystic variety. Elevation of IOP is caused by a combination of often pre-existing PAS, obstruction of the trabeculum by the epithelial membrane, and desquamated epithelial and inflammatory cells.
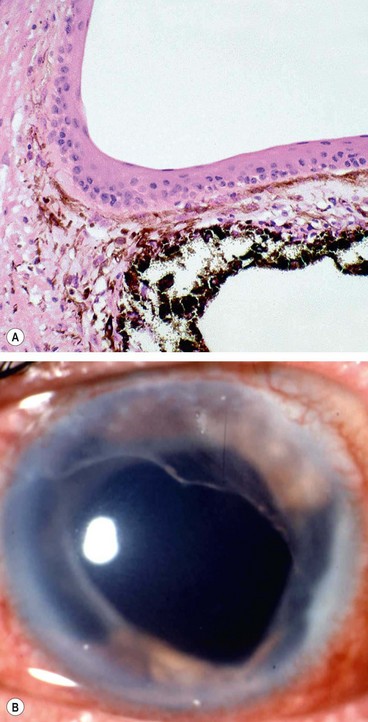
Fig. 10.65 Diffuse epithelial ingrowth. (A) Stratified squamous epithelium lining the anterior iris surface and filtration angle; (B) translucent membrane with a scalloped border involving the posterior corneal surface
(Courtesy of J Harry and G Misson, from Clinical Ophthalmic Pathology, Butterworth-Heinemann 2001 – fig. A)
Diagnosis
Treatment
The aim of treatment is to eradicate the invading epithelium to avoid recurrence or the conversion of epithelial cysts into diffuse epithelialization with consequent intractable glaucoma.
Glaucoma in iridoschisis
Iridoschisis is a rare condition which typically affects the elderly and is often bilateral. It is associated with underlying angle-closure glaucoma in at least 90% of cases. It is thought that acute or intermittent angle-closure results in iris atrophy as a result of high IOP.
Primary congenital glaucoma
Introduction
Genetics
Most cases of primary congenital glaucoma (PCG) are sporadic. In approximately 10% inheritance is AR with incomplete penetrance. To date, PCG has been linked to three loci: 2p21 (GLC3A), for which the responsible gene is CYP1B1, 1p36 (GLC3B) and 14q24 (GLC3C), for which the genes are not yet identified.
Pathogenesis
Impaired aqueous outflow in PCG is caused by maldevelopment of the angle of the anterior chamber, unassociated with any other major ocular anomalies (isolated trabeculodysgenesis). Clinically, trabeculodysgenesis is characterized by absence of the ciliary body band due to translucent amorphous material that obscures the trabeculum (Fig. 10.67B).
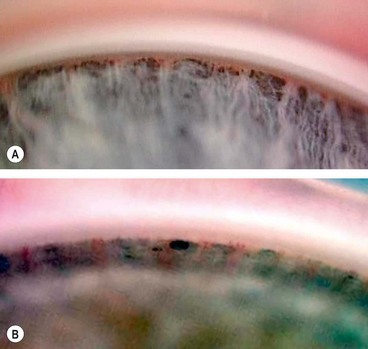
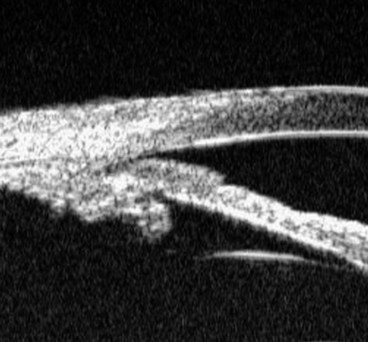
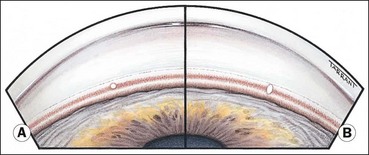
Fig. 10.67 (A) Normal infant angle shows the iris root, prominent ciliary body band but no discernible scleral spur and trabeculum; (B) angle in congenital glaucoma shows the iris root but not the ciliary body band due to translucent amorphous tissue that obscures the trabeculum
(Courtesy of K Nischal)
Classification
Diagnosis
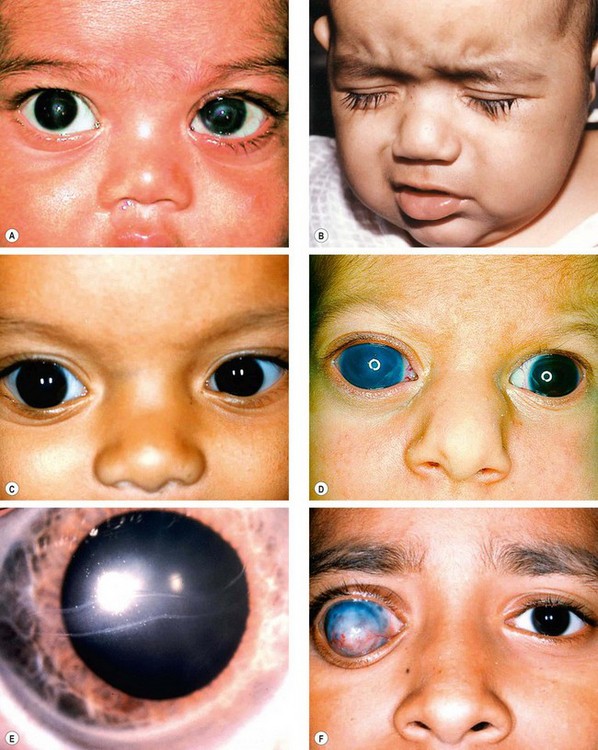
Although PCG is the most common of the congenital glaucomas, it is still a very rare condition, affecting 1 : 10 000 births; 65% of patients are boys. The clinical features depend on the age of onset and the level of IOP. Both eyes are affected in 75% of cases although involvement is frequently asymmetrical.
Management
Initial evaluation
The initial evaluation should be performed under general anaesthesia with intravenous ketamine, since this lowers IOP less than other agents. Examination of the optic discs should be undertaken first, followed by measurement of IOP and corneal diameters, and finally gonioscopy.
Surgery
Follow-up
The patients should be reviewed 1 month after initial surgery. The IOP and corneal diameters should be monitored at regular intervals because progressive enlargement of the corneal diameter is as important a sign of uncontrolled congenital glaucoma analagous to progressive visual field loss in adult glaucoma. Cycloplegic refraction should be performed at 6-monthly intervals. About 50% of patients suffer visual loss from optic nerve damage, anisometropic amblyopia, corneal scarring, cataract and lens subluxation. A buphthalmic eye is also susceptible to traumatic damage.
Differential diagnosis
Iridocorneal dysgenesis
Posterior embryotoxon
Posterior embryotoxon is an isolated innocuous finding in 10% of the general population.
Axenfeld–Rieger syndrome
Pathogenesis and genetics
Axenfeld–Rieger syndrome is a spectrum of disorders designated in current nomenclature by the following eponyms: (a) Axenfeld anomaly, (b) Rieger anomaly and (c) Rieger syndrome. Gene loci have been mapped to 4q25 (PITX2 gene), 6p25 (FKHL7) and 13q14 (RIEG2). All patients with Axenfeld–Rieger syndrome, irrespective of ocular manifestations, share the following features:
Axenfeld anomaly
This is characterized by posterior embryotoxon with attachment of strands of peripheral iris tissue (Fig. 10.72C).
Rieger anomaly
Rieger syndrome
Rieger syndrome is linked to the region of the epidermal growth factor gene on chromosome 4. It is characterized by Rieger anomaly in association with the following extraocular malformations:
Peters anomaly
Peters anomaly is an extremely rare but serious condition which is bilateral in 80% of cases. It is the result of defective neural crest cell migration in the 6th to 8th weeks of fetal development, during which time the anterior segment of the eye is formed. It is not a homogeneous condition and may vary from mild to severe.
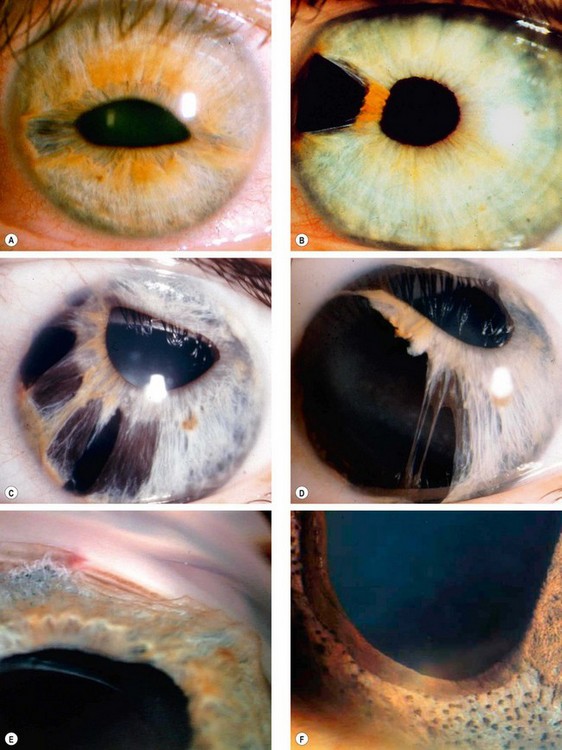
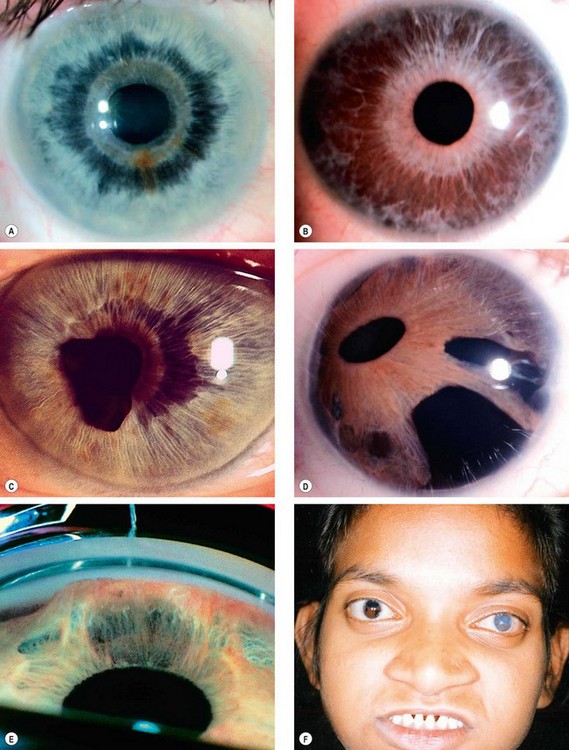
Aniridia
Genetics
Aniridia (AN) is a rare bilateral condition that may have life-threatening associations. It occurs as a result of abnormal neuroectodermal development secondary to a mutation in the PAX6 gene linked to 11p13. PAX6 is adjacent to gene WT1, mutation of which predisposes to Wilms tumour.
Classification
All patients with sporadic aniridia should have abdominal ultrasonography (to detect Wilms tumour) every 3 months until 5 years of age, every 6 months until 10 years of age and annually until 16 years of age or until molecular genetic analysis confirms an intragenic mutation without extragenic involvement.
Diagnosis
Glaucoma
Glaucoma occurs in approximately 75% of patients and usually presents in late childhood or adolescence. It is caused by synechial angle-closure secondary to the pulling forward of rudimentary iris tissue by contraction of pre-existing fibres that bridge the angle (Fig. 10.75F). Treatment is difficult and the prognosis guarded.
Glaucoma in phacomatoses
Sturge–Weber syndrome
Sturge–Weber syndrome (encephalotrigeminal angiomatosis) is a congenital, sporadic phacomatosis (see Ch. 1).
Pathogenesis of glaucoma
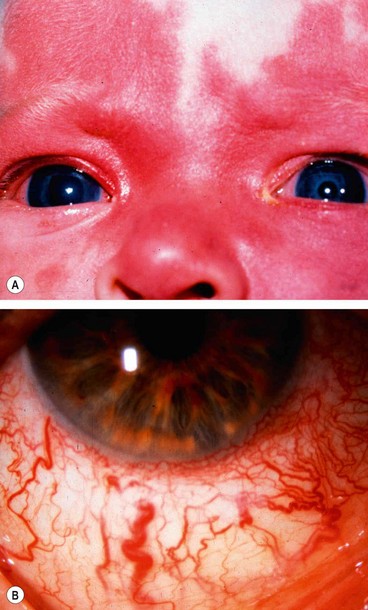
Glaucoma develops in about 30% of patients ipsilateral to the facial haemangioma, especially if the lesion affects the upper eyelid. Elevation of IOP occurs within the first 2 years of life in 60% of glaucoma patients and may result in buphthalmos (Fig. 10.76A). In the remainder, glaucoma may develop at any time from infancy to adulthood. The pathogenesis is controversial and often obscure.
Treatment
Neurofibromatosis type 1
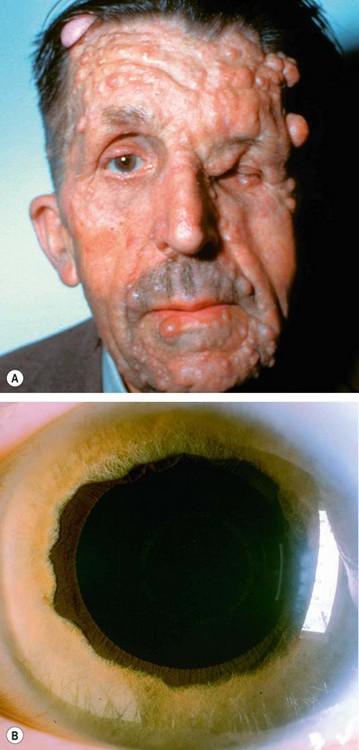
Neurofibromatosis is a disorder that primarily affects cell growth of neural tissues. Inheritance is AD with irregular penetrance and variable expressivity (see Ch. 19). Glaucoma is relatively rare and, when present, is usually unilateral and congenital. About 50% of patients with glaucoma have an ipsilateral plexiform neurofibroma of the upper eyelid or exhibit facial hemiatrophy (Fig. 10.77A). One or more of the following may be the causative mechanism:
Glaucoma medications
Most glaucoma medications are administered topically. As a general rule, treatment is indicated whenever glaucomatous damage is deemed likely to occur. The decision on which medication to prescribe depends not only on the type of glaucoma, but also on the patient’s medical history (e.g. presence of asthma or bradycardia). This requires a detailed knowledge of the potential side-effects. To improve compliance, patients should be fully informed not only about their disease but also the medications used, how to administer the drug, and what side-effects to expect. The efficacy of therapy should be regularly evaluated and the regimen altered to improve efficacy, if appropriate, or to reduce adverse effects.
Beta-blockers
Pharmacology
Adrenergic neurones secrete noradrenaline at sympathetic postganglionic nerve endings. Adrenergic receptors are of the following four main types:
Beta-blockers are drugs that antagonize the effects of catecholamines at beta receptors. Non-selective beta-blockers are equipotent at beta-1 and beta-2 receptors, while cardioselective are more potent at beta-1 receptors. The advantage of the latter, at least in theory, is that the bronchoconstrictive effect of beta-2 blockade is minimized. Betaxolol is the only cardioselective agent currently available for the treatment of glaucoma.
Mode of action
Beta-blockers reduce IOP by decreasing aqueous secretion and are therefore useful in all types of glaucoma, irrespective of the state of the angle. The exact pharmacological basis for this is unclear. However, in approximately 10% of cases the pressure response decreases with time: tachyphylaxis. This may occur within a few days of starting treatment (‘short-term escape’) or within a few months (‘long-term drift’). As a general rule, little additional effect is obtained if a topical beta-blocker is used in a patient who is already on a systemic beta-blocker. During sleep, aqueous flow is normally less than half the daytime flow and beta-blockers have limited effect. When a beta-blocker is used in combination with brimonidine or a topical carbonic anhydrase inhibitor, an additional 15% reduction in IOP may be achieved. When combined with a prostaglandin analogue, the reduction is even greater (20%).
Side-effects
Preparations
Alpha-2 agonists
Alpha-2 agonists decrease IOP by both decreasing aqueous secretion and enhancing uveoscleral outflow. Because the drugs cross the blood-brain barrier they should not be used in children.
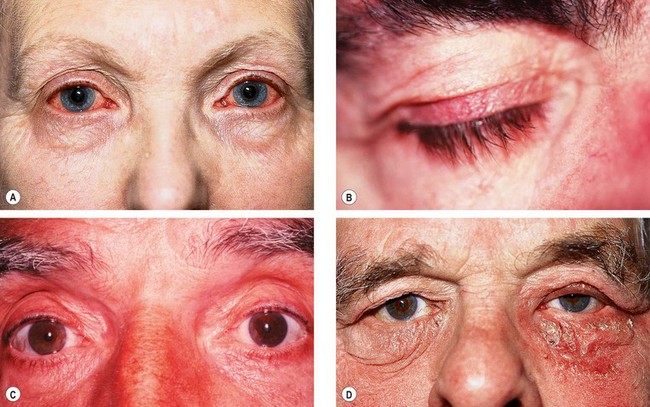
Fig. 10.78 Side-effects of topical medication. (A) Allergic conjunctivitis due to brimonidine; (B) lengthening and hyperpigmentation of lashes due to prostaglandin analogues; (C) darkening of irides due to prostaglandin analogues; (D) blepharoconjunctivitis due to topical carbonic anhydrase inhibitors
(Courtesy of J Salmon – fig. A; P Watson – fig. C)
Prostaglandin analogues
This group of agents have a sustained IOP-lowering effect which probably extends for several days in most patients.
Pharmacology
Prostanoid receptors are located on many ocular tissues, with involvement in functions such as regulation of intraocular pressure and blood flow.
Preparations
Side-effects
Topical carbonic anhydrase inhibitors
The carbonic anhydrase inhibitors (CAIs) are chemically related to the sulphonamides. They lower IOP by inhibiting aqueous secretion.
Miotics
Pharmacology
Miotics are parasympathomimetic drugs that act by stimulating muscarinic receptors in the sphincter pupillae and ciliary body.
Ocular side-effects include miosis, brow ache, myopic shift and exacerbation of symptoms of cataract. Visual field defects appear denser and larger.
Preparations
Combined preparations
Combined preparations with similar ocular hypotensive effects to the sum of the individual components improve convenience and patient compliance. They are also more cost effective. Examples include:
Systemic carbonic acid inhibitors
Preparations
Systemic side-effects
Systemically administered CAIs may be useful as short-term treatment, particularly in patients with acute glaucoma. Because of their systemic side-effects long-term use is reserved for patients at high risk of visual loss. The patient should always be warned of potential side-effects as this decreases anxiety and improves compliance.
Osmotic agents
Physiological principles
Osmotic pressure is dependent on the number rather than on the size of solute particles in a solution. Lower molecular weight solutes therefore exert a greater osmotic effect per gram. Osmotic agents remain intravascular, thus increasing blood osmolality. They lower IOP by creating an osmotic gradient between blood and vitreous so that water is ‘drawn out’ from the vitreous. The higher the gradient, the greater the reduction in IOP. To be effective in the eye, an osmotic agent must therefore be unable to penetrate the blood-aqueous barrier. If penetration occurs, an osmotic equilibrium is set up and any further effect is lost. Osmotic agents are therefore of limited value in the treatment of inflammatory glaucomas in which the integrity of the blood-aqueous barrier is compromised.
Clinical uses
When a temporary drop in IOP is required that cannot be achieved by other means.
Side-effects
Preparations
Laser therapy
Argon laser trabeculoplasty
Overview
Argon laser trabeculoplasty (ALT) involves the application of discrete laser burns to the trabecular meshwork. This enhances aqueous outflow and lowers IOP. ALT is performed in open-angle glaucomas, usually as an adjunct to medical therapy. It is believed that the procedure causes increased outflow facility by the following mechanisms: (a) mechanical tightening of the trabecular meshwork to open the adjacent, untreated trabecular spaces, and/or (b) inducing cell division and migration of macrophages to clear the trabecular meshwork of debris. ALT is ineffective in paediatric glaucoma and in most secondary glaucomas, with the exception of pigmentary and pseudoexfoliation.
Technique
Follow-up
Four to 6 weeks should be allowed for the treatment to take effect. If the IOP is reduced significantly by 6 weeks, gradual withdrawal of medication may be attempted, although total withdrawal is seldom possible. The main aim of ALT is to obtain a safe IOP; the reduction of medication is usually a secondary consideration. If IOP remains high and only 180° has been treated, the remaining 180° is treated. Following 360° ALT, re-treatment is less likely to be beneficial and filtration surgery merits consideration.
Complications
Results
Selective laser trabeculoplasty
Selective laser trabeculoplasty (SLT) is a relatively new procedure which uses a 532 nm frequency-doubled, Q-switched Nd:YAG laser, which selectively targets melanin pigment in the trabecular meshwork cells, leaving non-pigmented structures unscathed. Targeting is easier than with ALT, which may lead to more consistent results being achieved. It may be safer than ALT as there is no thermal tissue damage and it is thought that treatment can therefore be repeated. Initial results show that it is probably as effective as ALT.
Nd:YAG laser iridotomy
Technique
Technical problems
Complications
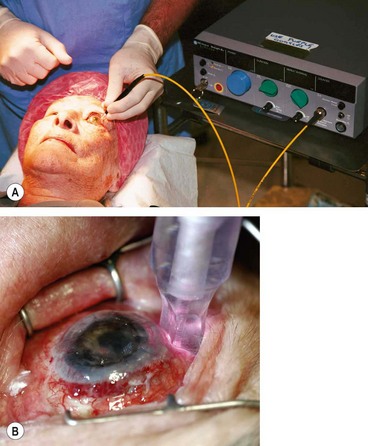
Diode laser cycloablation
Diode laser ablation (cyclodiode) lowers IOP by destroying part of the secretory ciliary epithelium, thereby reducing aqueous secretion. In the past it was used mainly in uncontrolled end-stage secondary glaucoma with minimal visual potential, mainly to control pain. However, it is now apparent that it can safely be used in eyes with good vision which may be retained provided control of IOP is adequate. More than one treatment session is commonly required for adequate pressure control.
Laser iridoplasty
Laser iridoplasty is performed to widen the anterior chamber angle by causing contraction of the peripheral iris away from the angle recess. It can be used to attempt to break an episode of acute angle-closure, but is more commonly applied on an elective basis (see ‘Primary angle-closure).
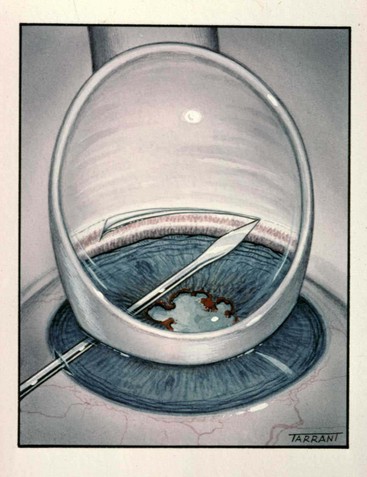
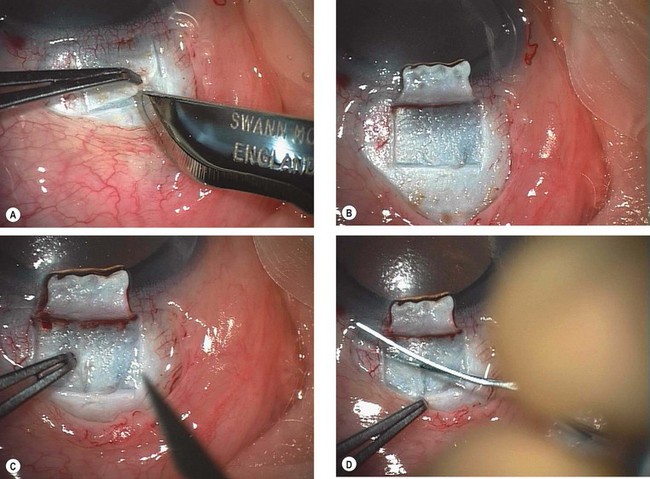
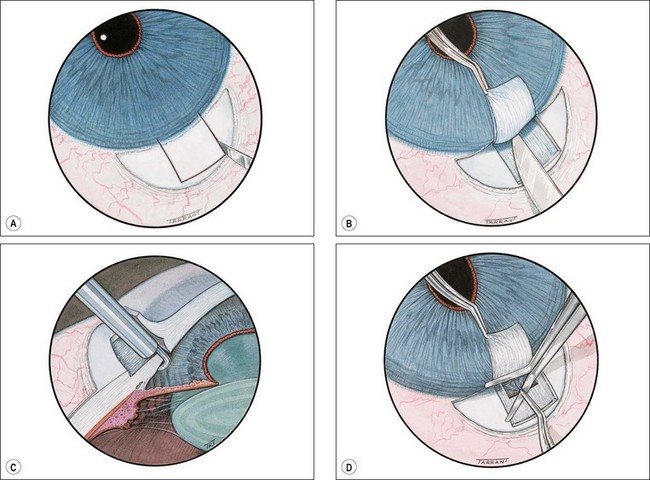
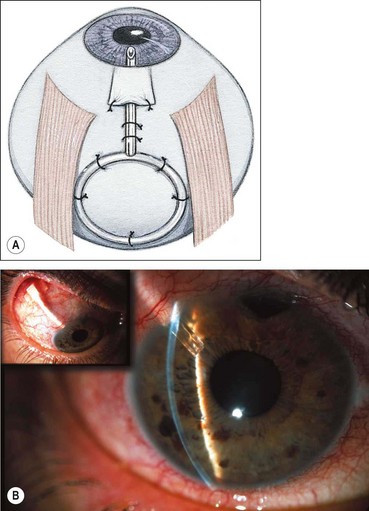
Trabeculectomy
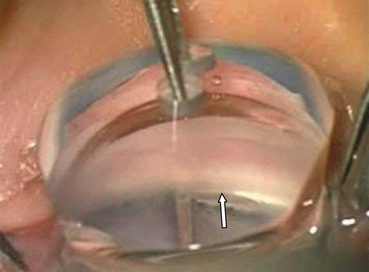
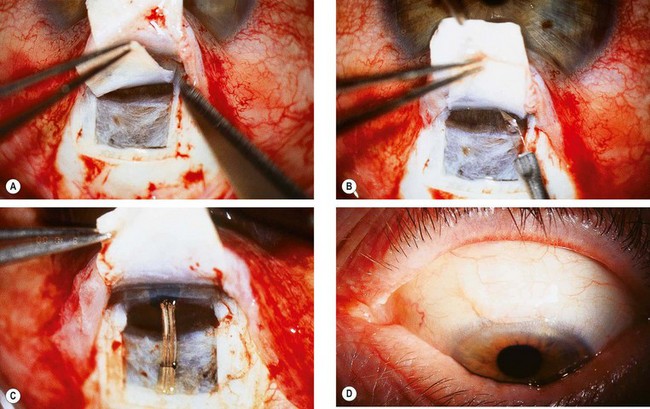
Trabeculectomy lowers IOP by creating a fistula, to allow aqueous outflow from the anterior chamber to the sub-Tenon space. The fistula is protected or ‘guarded’ by a superficial scleral flap (Fig. 10.83). The procedure is usually performed when medical therapy has failed to achieve adequate control of IOP.
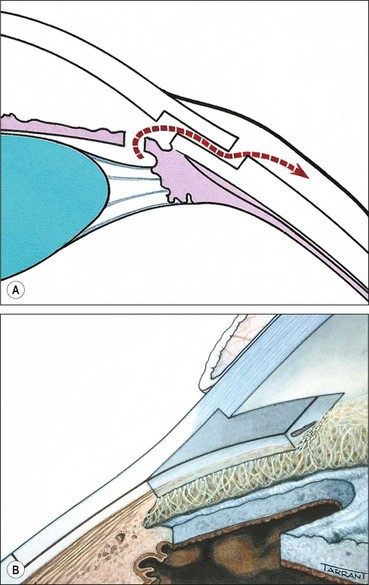
Fig. 10.83 Trabeculectomy principles. (A) Pathway of aqueous egress following trabeculectomy; (B) appearance from inside the eye following completion
Technique
Shallow anterior chamber
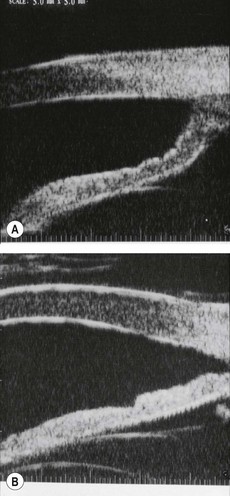
A shallow anterior chamber may be due to (a) pupillary block, (b) overfiltration or (c) malignant glaucoma (aqueous misdirection). Severe and sustained shallowing is uncommon (Fig. 10.85A and B), the chamber re-forming spontaneously in most cases. However, those that do not may develop severe complications such as peripheral anterior synechiae, corneal endothelial damage (Fig. 10.85C) and cataract (Fig. 10.85D).
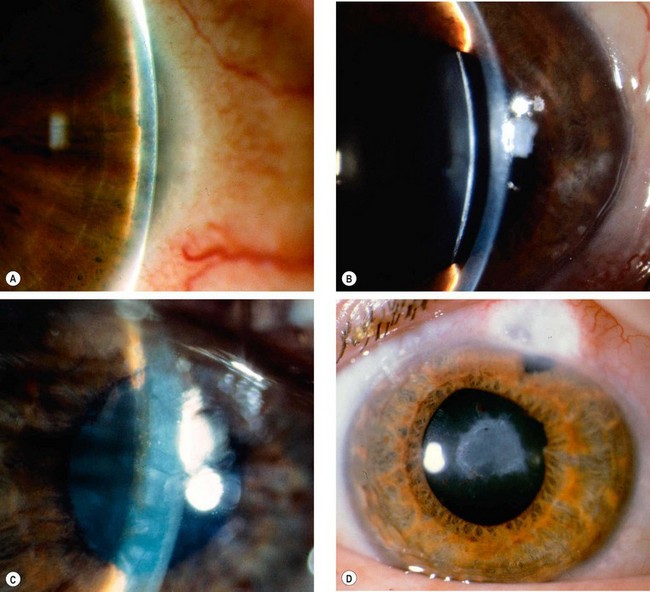
Fig. 10.85 Shallow anterior chamber. (A) Peripheral iris-corneal apposition; (B) pupillary border-corneal apposition; (C) lenticulo-corneal apposition resulting in corneal oedema; (D) cataract following inappropriate management
(Courtesy of J Schuman, V Christopoulos, D Dhaliwal, M Kahook and R Noecker, from Lens and Glaucoma, in Rapid Diagnosis in Ophthalmology, Mosby 2008 – fig. A)
Overfiltration
Overfiltration may be caused by scleral flap leakage due to insufficient resistance to outflow by the lamellar scleral flap, but bleb leakage through an inadvertent buttonhole or due to inadequate closure of the conjunctiva and Tenon capsule is perhaps the most common cause.
Malignant glaucoma
Malignant glaucoma is rare but serious. It is caused by anterior rotation of the ciliary processes and iris root, often with aqueous misdirection (ciliolenticular block); blockage of aqueous flow occurs in the vicinity of the pars plicata of the ciliary body, so that aqueous is forced backwards into the vitreous.
Failure of filtration
Diagnosis
A normally functioning bleb should be slightly elevated, avascular and show superficial microcysts (Fig. 10.87A). Poor filtration is indicated by increasing IOP and a bleb with one of the following appearances:
Causes
Causes of failure can be classified according to the site of blockage:
Management
Management of filtration failure depends on the cause and may involve one or more of the following:
Late bleb leakage
Bleb-associated bacterial infection and endophthalmitis
Glaucoma filtration-associated infection is classified as limited to the bleb (blebitis) or endophthalmitis, although there is some overlap. The incidence of blebitis following trabeculectomy with mitomycin has been estimated to be up to 5% per year but many studies show a for lower rate.
Pathogenesis
Adjunctive antifibrotic agents (mitomycin C, 5-fluorouracil) are frequently used to increase the success of glaucoma filtration surgery. The use of these agents can lead to a very thin-walled drainage bleb (Fig. 10.88A) that significantly increases the risk of late-onset infection. The infection presumably gains access directly through the thin and avascular wall of the drainage bleb. All patients with such blebs should be warned of the possibility of late infection and strongly advised to report immediately should they develop a red and sticky eye, or blurred vision (RSVP – red, sticky, visual loss, pain).
Blebitis
Endophthalmitis
Non-penetrating surgery
Overview
In non-penetrating filtration surgery the anterior chamber is not entered and the internal trabecular meshwork is preserved, thus reducing the incidence of postoperative overfiltration and hypotony and its potential sequelae. Two lamellar scleral flaps are fashioned and the deep flap excised leaving behind a thin membrane consisting of trabeculum/Descemet membrane through which aqueous diffuses from the anterior chamber to the subconjunctival space. The surgery is technically challenging and requires meticulous dissection of a deep scleral flap without entering the delicate anterior trabecular meshwork.
Indications
The main indication for non-penetrating surgery is POAG, although other open-angle glaucomas may also be amenable. In general the IOP reduction is less than that achieved by trabeculectomy, so that topical medication often needs to be recommenced. Conventional filtration is therefore still the procedure of choice when the target IOP is in the low teens though it is probably associated with a lower risk of ‘snuffing out’ central vision when advanced damage is present.
Technique
Trabectome
The Trabectome is a novel microelectrosurgical device which approaches the angle ab interno under direct vision using a gonioscopy lens, to remove a strip of trabecular meshwork and inner wall of Schlemm canal (‘trabeculotomy’). Whilst it does not seem to lower the intraocular pressure as effectively as trabeculectomy, the safety profile is better.
Antimetabolites in filtration surgery
Indications
Adjunctive antimetabolites inhibit the natural healing response that may preclude successful filtration surgery. They should, however, be used with caution because of the serious nature of potential complications, and usually considered in the presence of known risk factors for failure of trabeculectomy. In uncomplicated glaucoma the use of low-dose antimetabolites may improve long-term control of IOP but this benefit should be weighed against possible complications such as corneal epithelial defects, chronic hypotony and late-onset bleb leakage.
5-fluorouracil
5-fluorouracil (5-FU) inhibits DNA synthesis and is active on the ‘S’ phase (synthesis phase) of the cell cycle. Fibroblastic proliferation is inhibited, but fibroblastic attachment and migration are unaffected. It is the antimetabolite of choice in elderly patients who have risk factors for failure. The drug can be used in one or both of the following ways:
Mitomycin C
Mitomycin C (MMC) is an alkylating agent rather than an antimetabolite, and selectively inhibits DNA replication, mitosis and protein synthesis. The drug inhibits proliferation of fibroblasts, suppresses vascular ingrowth and is much more potent than 5-FU. Optimum concentration and exposure time is not known and vary between 0.2–0.5 mg/mL and 1–5 minutes. In general, low or intermediate risk indicates use of a low concentration (0.2 mg/mL), whilst high risk implies the need for a higher concentration (0.4–0.5 mg/mL). Higher concentrations and extended exposure times are associated with an increased risk of complications. The technique of intraoperative application is the same as for 5-FU and great care should be taken to prevent contamination of the anterior chamber. MMC can also be applied externally to the bleb with a sponge in the postoperative period.
Drainage shunts
Shunts using episcleral explants
Types
These create a communication between the anterior chamber and sub-Tenon space. All such shunts consist of a tube attached to a posterior episcleral explant. Some contain pressure-sensitive valves for regulation of aqueous flow. Reduction of IOP is due to passive, pressure-dependent flow of aqueous across the capsular wall.
Indications
Complications
Results
The results depend on the type of glaucoma. In general, an IOP in the mid-teens is achieved, though as with trabeculectomy, topical medication is typically required. The long-term success rate in neovascular glaucoma is often disappointing because of progressive retinal disease with loss of vision and late development of phthisis bulbi. Adjunctive mitomycin C may enhance the success rate of drainage shunt surgery but is associated with a higher complication rate.