Chapter 9 Lens
Acquired cataract
Age-related cataract
Subcapsular cataract
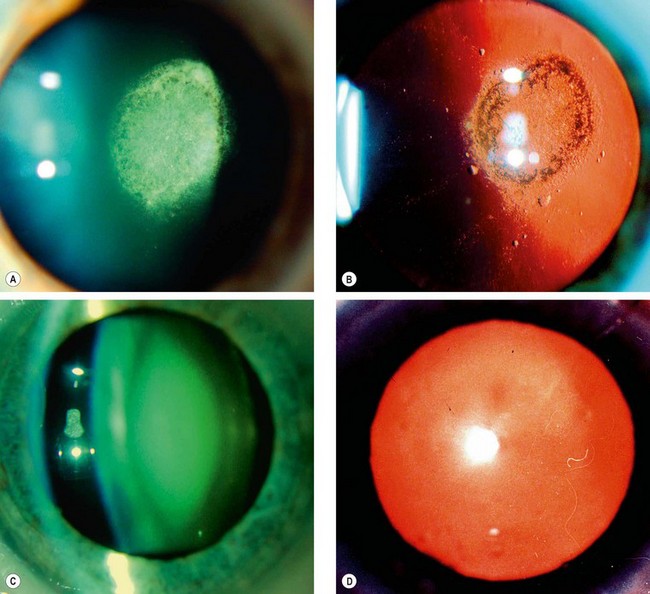
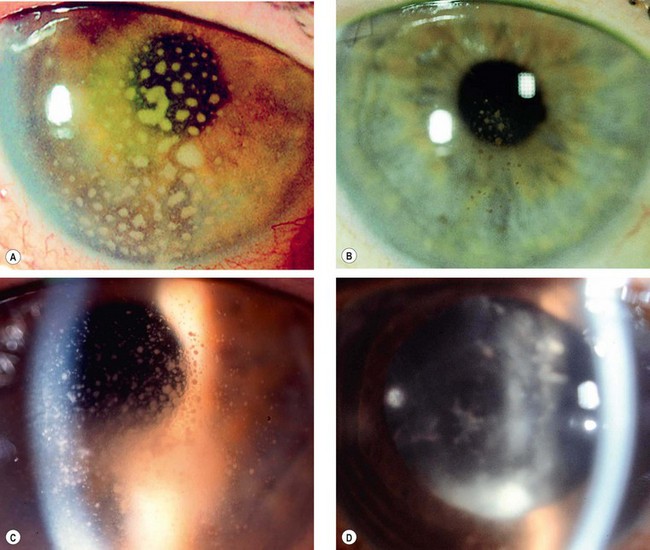
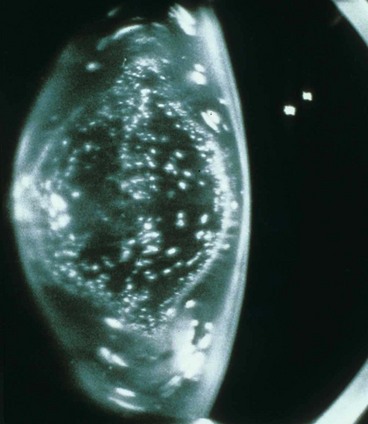
Anterior subcapsular cataract lies directly under the lens capsule and is associated with fibrous metaplasia of the lens epithelium. Posterior subcapsular opacity lies just in front of the posterior capsule and has a vacuolated, granular, or plaque-like appearance on oblique slit-lamp biomicroscopy (Fig. 9.1A) and appears black on retroillumination (Fig. 9.1B). Due to its location at the nodal point of the eye, a posterior subcapsular opacity has a more profound effect on vision than a comparable nuclear or cortical cataract. Near vision is frequently impaired more than distance vision. Patients are particularly troubled under conditions of miosis, such as produced by headlights of oncoming cars and bright sunlight.
Nuclear cataract
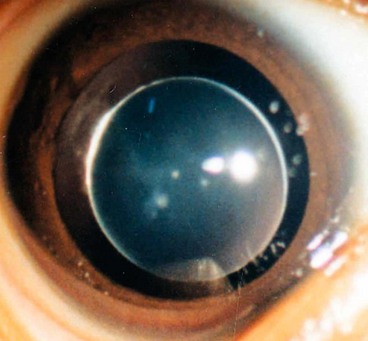
Nuclear cataract starts as an exaggeration of the normal ageing changes involving the lens nucleus. It is often associated with myopia due to an increase in the refractive index of the nucleus, and also with increased spherical aberration. Some elderly patients may consequently be able to read without spectacles again (’second sight of the aged’). Nuclear sclerosis is characterized in its early stages by a yellowish hue due to the deposition of urochrome pigment. This type of cataract is best assessed with oblique slit-lamp biomicroscopy (Fig. 9.1C) and not by retroillumination (Fig. 9.1D). When advanced the nucleus appears brown.
Cortical cataract
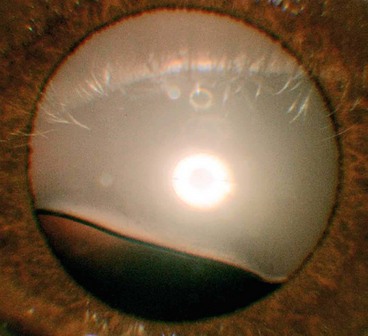
Cortical cataract may involve the anterior, posterior or equatorial cortex. The opacities start as clefts and vacuoles between lens fibres due to hydration of the cortex. Subsequent opacification results in typical cuneiform (wedge-shaped) or radial spoke-like opacities, often initially in the inferonasal quadrant (Fig. 9.2A and B). Patients with cortical opacities frequently complain of glare due to light scattering.
Christmas tree cataract
Christmas tree cataract, which is uncommon, is characterized by striking polychromatic needle-like deposits in the deep cortex and nucleus (Fig. 9.2C and D); they may be solitary or associated with other opacities.
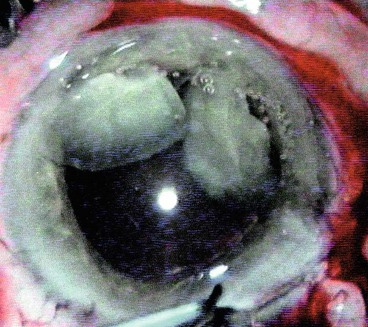
Cataract maturity
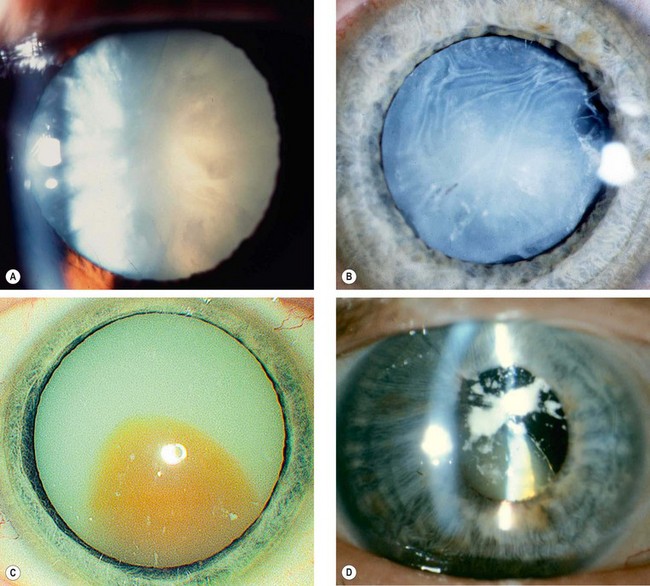
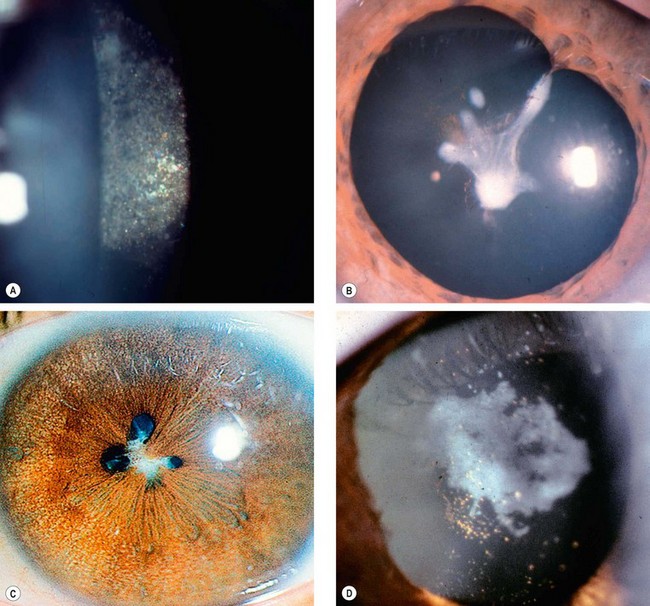
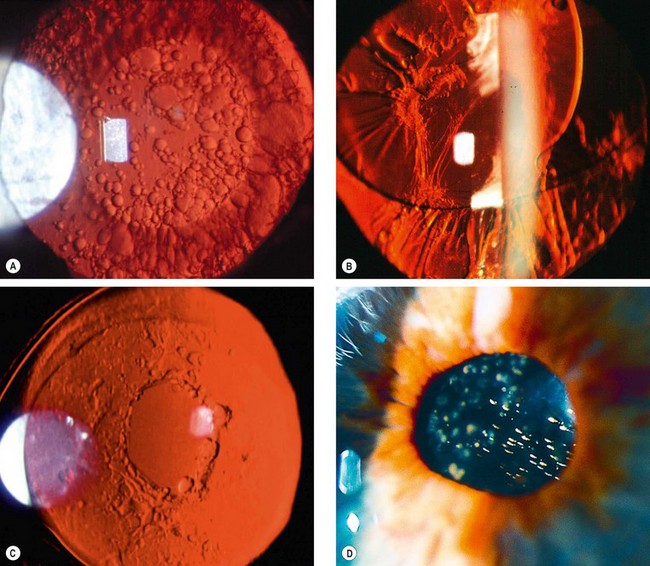
Fig. 9.3 Cataract maturity. (A) Mature cataract; (B) hypermature cataract with wrinkling of the anterior capsule; (C) Morgagnian cataract with liquefaction of the cortex and inferior sinking of the nucleus; (D) total liquefaction and absorption of the cortex with inferior sinking of the lens
(Courtesy of P Gili – fig. D)
Cataract in systemic diseases
Diabetes mellitus
Hyperglycaemia is reflected in a high level of glucose in the aqueous humour, which diffuses into the lens. Here glucose is metabolized by aldose reductase into sorbitol, which then accumulates within the lens, resulting in secondary osmotic overhydration of the lens substance. In mild degree, this may affect the refractive index of the lens with consequent fluctuation of refraction pari passu with the plasma glucose level (hyperglycaemia resulting in myopia and vice versa). Cortical fluid vacuoles develop and later evolve into frank opacities.
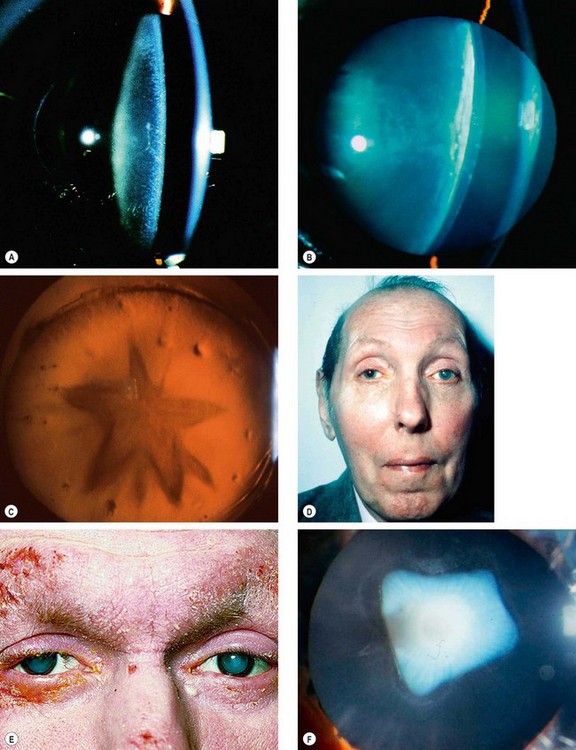
Fig. 9.4 Cataract in systemic disease. (A) Diabetic snowflake cataract; (B) advanced diabetic cataract; (C) stellate posterior subcapsular cataract in myotonic dystrophy; (D) advanced left cataract in a patient with myotonic dystrophy; (E) bilateral advanced cataracts in atopic dermatitis; (F) shield-like anterior subcapsular cataract in atopic dermatitis
(Courtesy of A Fielder – fig. A; J Schuman, V Christopoulos, D Dhaliwal, M Kahook and R Noecker, from Lens and Glaucoma, in Rapid Diagnosis in Ophthalmology, Mosby 2008 – fig. B; L Merin – fig. D)
Myotonic dystrophy
Myotonic dystrophy is an AD condition characterized by delayed muscular relaxation after cessation of voluntary effort (myotonia – see Ch. 19). About 90% of patients develop visually innocuous, fine cortical iridescent opacities in the 3rd decade which evolve into visually disabling stellate posterior subcapsular opacities (Fig. 9.4C) by the 5th decade that may progress to maturity (Fig. 9.4D); occasionally cataract may predate myotonia.
Atopic dermatitis
About 10% of patients with severe atopic dermatitis develop cataracts in the 2nd–4th decades; these are often bilateral and may mature quickly (Fig. 9.4E). Shield-like dense anterior subcapsular plaque which wrinkles the anterior capsule is characteristic (Fig. 9.4F). Posterior subcapsular opacities resembling a complicated cataract may also occur.
Neurofibromatosis type 2
NF2 (see Ch. 19) is associated with cataract in about 60% of patients. It tends to develop prior to the age of 30 years. They may be posterior subcapsular or capsular, cortical or mixed.
Secondary cataract
A secondary (complicated) cataract develops as a result of some other primary ocular disease.
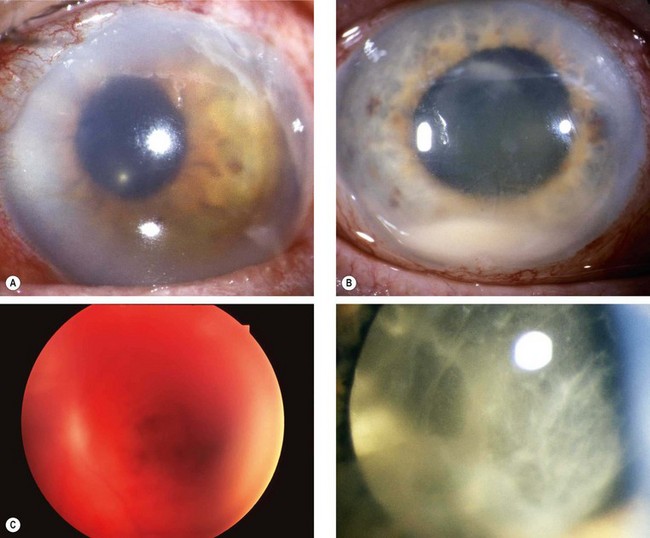
Chronic anterior uveitis
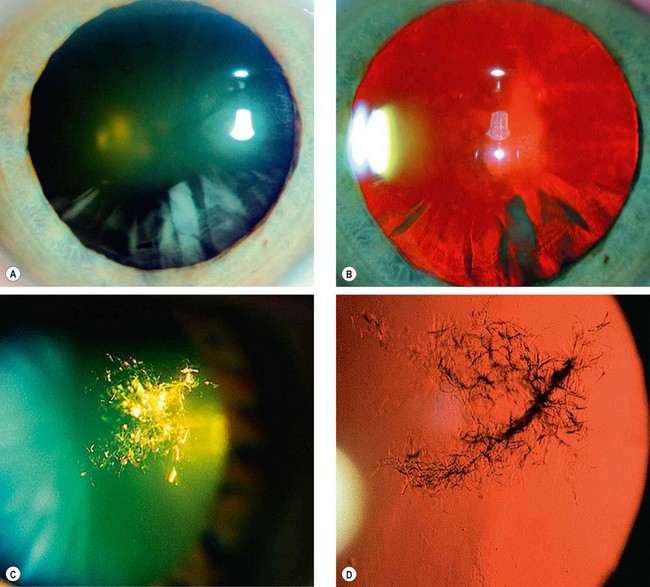
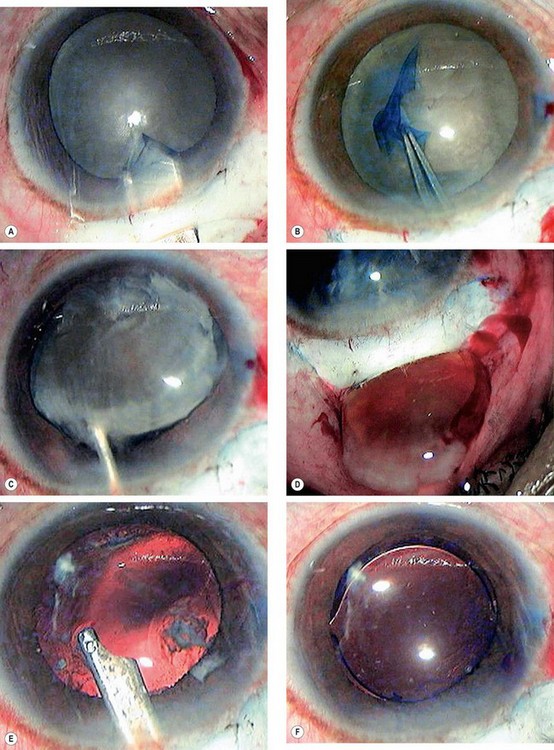
Chronic anterior uveitis is the most common cause. The incidence is related to the duration and activity of intraocular inflammation that results in prolonged breakdown of the blood–aqueous and/or blood–vitreous barrier. The use of steroids, topically and systemically, is also important. The earliest finding is a polychromatic lustre at the posterior pole of the lens which may not progress if the uveitis is arrested. If the inflammation persists, posterior (Fig. 9.5A) and anterior opacities (Fig. 9.5B and C) develop that may progress to maturity. The opacities appear to progress more rapidly in the presence of posterior synechiae.
Acute congestive angle-closure
Acute congestive angle-closure may cause small, grey-white, anterior, subcapsular or capsular opacities within the pupillary area (glaukomflecken – Fig. 9.5D). They represent focal infarcts of the lens epithelium and are almost pathognomonic of past acute angle-closure glaucoma.
High myopia
High (pathological) myopia is associated with posterior subcapsular lens opacities and early-onset nuclear sclerosis, which may ironically increase the myopic refractive error. Simple myopia, however, is not associated with such cataract formation.
Hereditary fundus dystrophies
Hereditary fundus dystrophies, such as retinitis pigmentosa, Leber congenital amaurosis, gyrate atrophy and Stickler syndrome, may be associated with posterior subcapsular lens opacities (see Ch. 15). Cataract surgery may occasionally improve visual acuity even in the presence of severe retinal changes.
Traumatic cataract
Trauma is the most common cause of unilateral cataract in young individuals and may include the following.
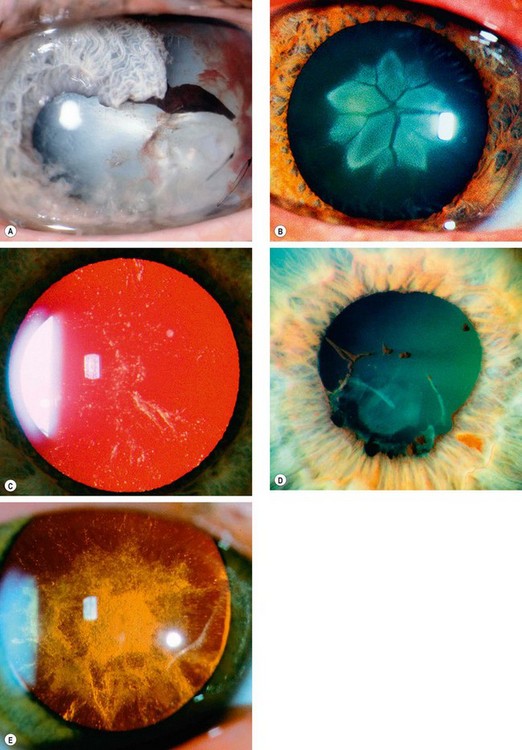
Fig. 9.6 Causes of traumatic cataract. (A) Penetrating trauma; (B) blunt trauma; (C) electric shock and lightning strike; (D) infrared radiation (glassblower’s cataract); (E) ionizing radiation
(Courtesy of J Schuman, V Christopoulos, D Dhaliwal, M Kahook and R Noecker, from Lens and Glaucoma, in Rapid Diagnosis in Ophthalmology, Mosby 2008 – figs C-E)
Management of age-related cataract
Preoperative considerations
Indications for surgery
Systemic preoperative assessment
For elective surgery, a general medical history is taken and any problems managed accordingly. Table 9.1 sets out suggested further enquiry and action in relation to a range of systemic diseases. Routine preoperative general medical examination, blood tests and ECG are not usually required for local anaesthesia.
Table 9.1 Management of general medical conditions prior to elective surgery
| Condition | Further questions/examination | Action |
|---|---|---|
| Diabetes mellitus | Well-controlled? Will need blood test (finger-prick may be sufficient, consider additional tests if necessary) | If control poor, may need to defer surgery and contact patient’s physician Medication and food and drink intake as usual on the day of surgery for local anaesthesia |
| Systemic hypertension | If systolic >170 or diastolic >100 may need physician opinion | Consider contacting physician for optimization; defer surgery if necessary as risk of suprachoroidal haemorrhage may be elevated |
| Actual or suspected myocardial infarction (MI) in the past | Date of MI? | Defer surgery for at least 6 months from date of MI. Contact physician/anaesthetist if concerns about current cardiovascular status |
| Angina | Stable/well-controlled? | Bring glyceryl trinitrate (GTN) spray on day of surgery. If unstable, contact physician or anaesthetist |
| Respiratory disease | Is chest function currently optimal? Can the patient lie flat? |
If the patient cannot lie flat, may need to discuss with operating surgeon. Trial of lying flat (at least half an hour) Remind patient to bring any inhalers to hospital |
| Rheumatic fever, transplanted or prosthetic heart valve, previous endocarditis | Does the patient usually require prophylactic antibiotic cover for operations? | Antibiotic prophylaxis only exceptionally required for ophthalmic surgery e.g. removal of an infected eye |
| Stroke in the past | Date of stroke? Particular residual difficulties? |
Defer surgery for at least 6 months from date of stroke. Many have positional/other practical consequences |
| Rheumatoid arthritis | Does the patient have any problems lying flat or with neck position? | If in doubt about patient’s ability to position appropriately, may need to discuss with operating surgeon |
| Jaundice in the past | What was the underlying diagnosis? | If viral hepatitis suspected, note prominently as special precautions to avoid needlestick injury may be necessary |
| HIV infection | If there are any high-risk factors, has the patient undergone an HIV test in the past? | Special precautions to avoid needlestick injury may be necessary |
| Sickle status | For patients of southern Asian and Afro-Caribbean ethnic origin, enquire about sickle status | Blood test if unknown and general anaesthesia planned |
| Parkinson disease or other cause of substantial tremor | Is the patient able to maintain head stability sufficiently to cooperate with local anaesthesia and surgery? | If not, may require general anaesthesia |
| Epilepsy | Is the condition well-controlled? | General anaesthesia may be preferred |
| Myotonic dystrophy | Has the patient undergone surgery and anaesthesia in the past? | If general anaesthesia is planned, an anaesthetic opinion should be obtained well in advance of surgery |
Ophthalmic preoperative assessment
A detailed and pertinent ophthalmic evaluation is required. Following a past ophthalmic history, the following should be considered:
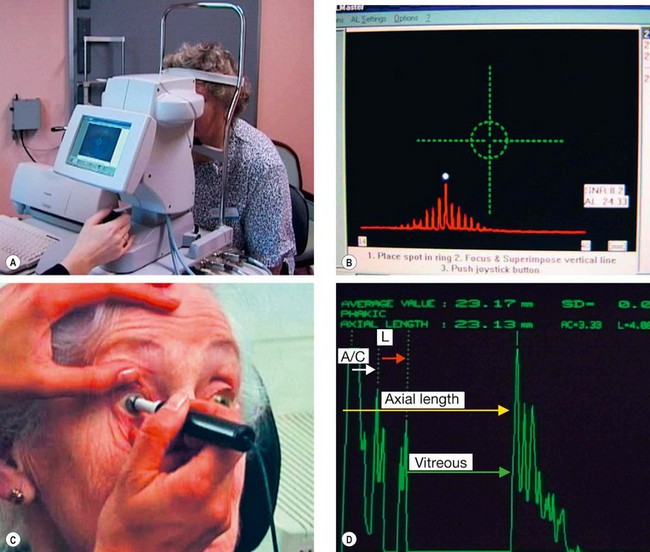
Biometry
Biometry facilitates calculation of the lens power likely to result in the desired postoperative refractive outcome; in its basic form this involves the measurement of two ocular parameters, keratometry and axial (anteroposterior) length.
Postoperative refraction
Intraocular lenses
Positioning
An IOL consists of an optic and the haptics. The optic is the central refracting element, and the haptics the arms or loops which sit in contact with the ocular structures (capsular bag, ciliary sulcus or anterior chamber angle) for stable optimal positioning (centration) of the optic. Modern cataract surgery, with preservation of the capsular ‘bag’, affords positioning of the IOL in the ideal location – ‘in the bag’. Complicated surgery, with rupture of the posterior capsule, may necessitate alternative positioning in the posterior chamber with the haptics in the ciliary sulcus (a 3-piece IOL only, not 1-piece including those with plate haptics, as these may not be stable), or in the anterior chamber (AC) with the haptics supported in the angle – AC positioning requires a specific lens type, an ‘AC-IOL’.
Design
Anaesthesia
The vast majority of cataract surgery is performed under local anaesthesia (LA) although general anaesthesia is required in some circumstances such as children and many young adults, very anxious patients, some patients with learning difficulties, epilepsy, dementia and those with a head tremor.
Phacoemulsification
Introduction
Phacoemulsification (‘phaco’) has become the preferred method of cataract extraction over the last 15 years. The smaller incision of phacoemulsification is associated with little induced postoperative astigmatism and early stabilization of refraction (usually 3 weeks for 3.0 mm incisions but less for sub-2.5 mm incisions). Postoperative wound-related problems such as iris prolapse have been almost eliminated. One disadvantage of phaco is that it requires complex machinery to break up the lens nucleus and remove it through a small incision. Considerable training and practice is required to learn the techniques adequately.
Phacodynamics
The surgeon must understand the machine dynamics and the interaction of fluidics in treating different forms of cataract. The various machines behave differently but the basic mechanism is similar. Choosing appropriate settings makes surgery safer and easier.
Pumps
Handpiece
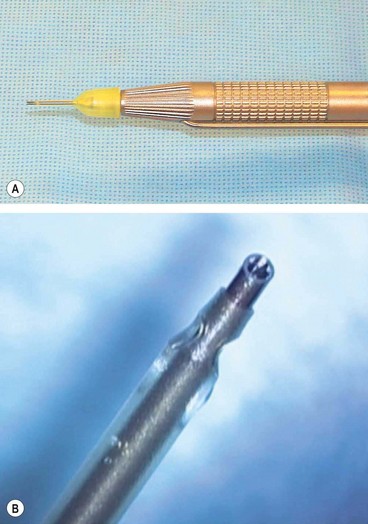
The phaco handpiece (Fig. 9.10A) contains a series of piezoelectric crystals which act as rapid switching devices, causing the tip to vibrate at ultrasonic frequencies. The tip itself consists of a hollow titanium needle 0.7–1.1 mm in diameter with an enclosing sleeve (Fig. 9.10B) to protect the cornea from thermal and mechanical damage. Differently-shaped phaco needles have various characteristics in terms of cutting and holding nuclear material. Emulsification of the lens is the result of the following phenomena:
Viscoelastics
Viscoelastics are biopolymers whose main constituents are glycosaminoglycans and hydroxypropylmethylcellulose. All have the propensity to cause raised intraocular pressure unless carefully removed at the end of surgery.
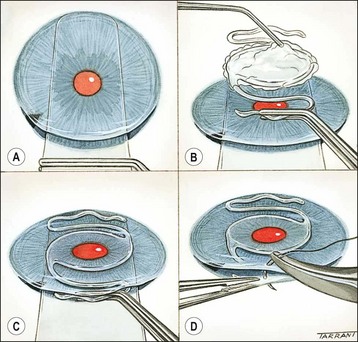
Technique
It is beyond the scope of this book to describe the technique in detail; the following are the basic steps:
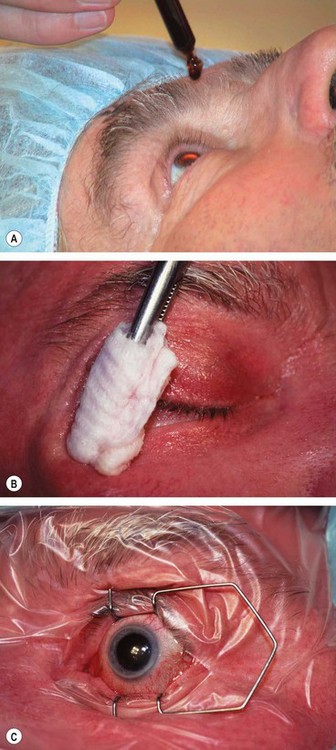
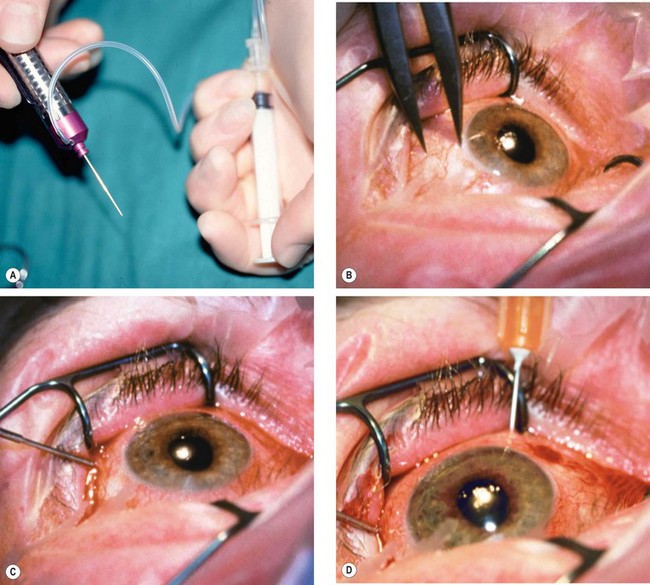
Fig. 9.11 Preparation. (A) Povidone-iodine 5% is instilled; (B) skin is painted; (C) drapes isolate the eyelids from the operating field with insertion of a speculum.
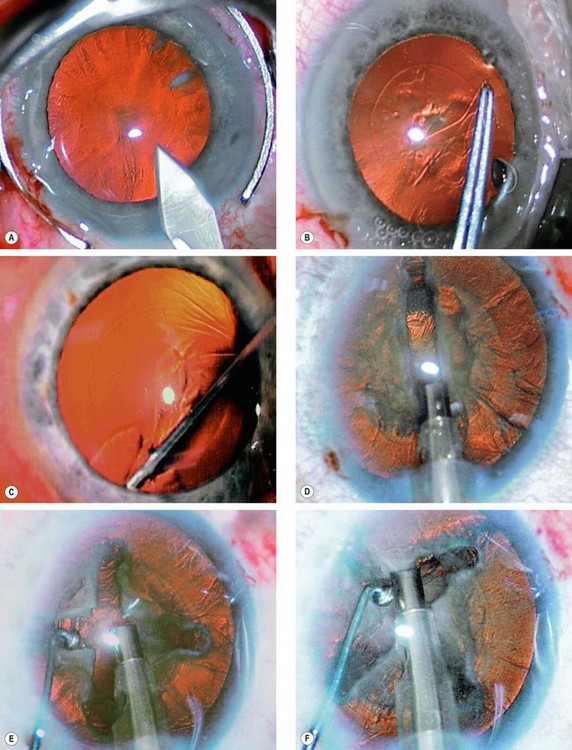
Fig. 9.12 Four quadrant (’divide and conquer’) phacoemulsification. (A) Corneal incision; (B) capsulorhexis; (C) hydrodissection; (D) nucleus is grooved; (E) nucleus is cracked; (F) each nuclear quadrant is emulsified and aspirated
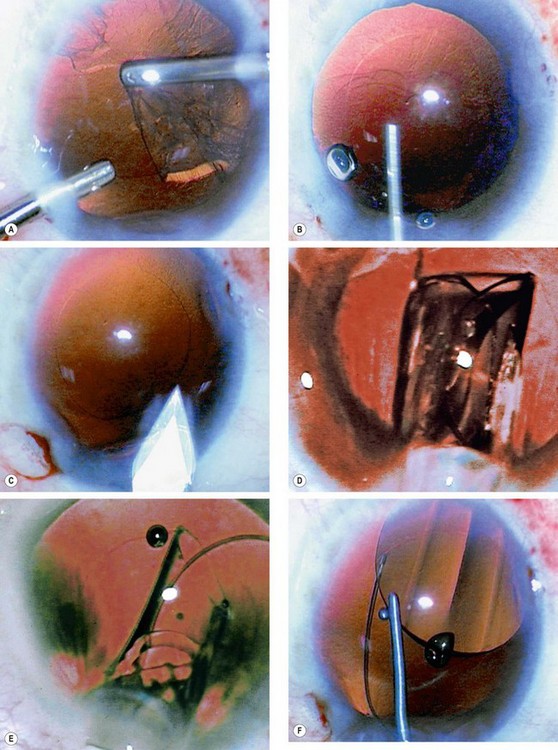
Fig. 9.13 Completion of phacoemulsification. (A) Cortical lens matter is pulled centrally and aspirated: (B) injection of viscoelastic into the capsular bag; (C) incision is enlarged; (D) cartridge nozzle with IOL is introduced through the incision; (E) IOL is slowly injected into the eye; (F) IOL is dialled into position if necessary
Small incision manual cataract surgery
Small incision manual cataract surgery is an effective alternative to phacoemulsification in countries where very high volume surgery with inexpensive instrumentation is required. The procedure is fast and has a low rate of complications, and can be performed on a dense cataract. The technique is as follows:
Operative complications
Rupture of the posterior lens capsule
Capsular rupture may be accompanied by vitreous loss, posterior migration of lens material, and rarely expulsive haemorrhage. Sequelae to vitreous loss, particularly if inappropriately managed, include chronic cystoid macular oedema, retinal detachment, endophthalmitis, updrawn pupil, uveitis, vitreous touch, vitreous wick syndrome, glaucoma and posterior dislocation of the IOL.
Posterior loss of lens fragments
Dislocation of fragments of lens material into the vitreous cavity after zonular dehiscence or posterior capsule rupture is rare but potentially serious as it may result in glaucoma, chronic uveitis, retinal detachment and chronic cystoid macular oedema. Initially, any uveitis or raised intraocular pressure must be treated. It may be reasonable to adopt a conservative approach for small fragments, but larger fragments, certainly a quadrant or more, will virtually always require removal by pars plana vitrectomy.
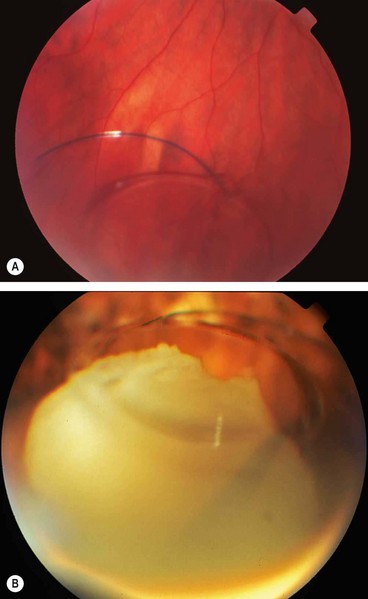
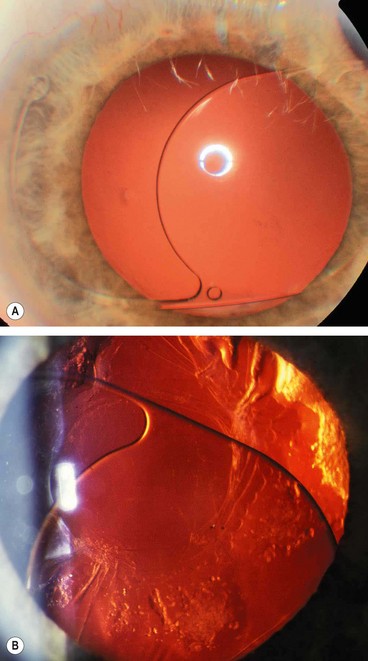
Posterior dislocation of IOL
Dislocation of an IOL into the vitreous cavity (Fig. 9.17A) is a rare but serious complication particularly if accompanied by loss of lens material (Fig. 9.17B). If the IOL is left in the posterior segment it may lead to vitreous haemorrhage, retinal detachment, uveitis and chronic cystoid macular oedema. Treatment involves pars plana vitrectomy with removal, repositioning or exchange of the IOL depending on the extent of capsular support.
Suprachoroidal haemorrhage
A suprachoroidal haemorrhage involves a bleed into the suprachoroidal space from a ruptured long or short posterior ciliary artery. If sufficiently severe it may result in extrusion of intraocular contents (‘expulsive’ haemorrhage). It is a dreaded complication, but extremely rare (0.04%) with phacoemulsification. Contributing factors include advanced age, glaucoma, increased axial length, systemic cardiovascular disease, vitreous loss, and conversion from phacoemulsification to ECCE. A high intraoperative index of suspicion is critical, and if there is any suggestion of a suprachoroidal haemorrhage the operation should be terminated and the incision sutured immediately.
Acute postoperative endophthalmitis
Pathogenesis
The estimated incidence of acute endophthalmitis following cataract surgery is approximately 0.3%. Toxins produced by the infecting bacteria and the host inflammatory responses cause rapid and irreversible photoreceptor damage, and these effects can continue long after the ocular contents have been rendered sterile.
Prophylaxis
Because of the low rate of endophthalmitis it is very difficult to prove that any method of prophylaxis is effective or superior to any other. The following are likely to be beneficial:
Differential diagnosis
If there is any doubt about the diagnosis, treatment should be that of infectious endophthalmitis. Early recognition leads to a better outcome.
Identification of pathogens
Samples for culture should be obtained from aqueous and vitreous to confirm the diagnosis. However, negative culture does not necessarily rule out infection and treatment should be continued. An operating theatre with experienced staff is the best setting, but samples can be taken in a minor procedures operating room if necessary to avoid delay, making sure that all equipment is available prior to starting.
Treatment
Table 9.2 Preparation of antibiotics for intravitreal injection
| Ceftazidime (broad spectrum, including Pseudomonas) |
| Vancomycin (action primarily against Gram-positive organisms) |
| Only saline, not WFI, should be used with vancomycin |
| As A–E above, again preferably starting with a 500 mg ampoule. |
| Amikacin (alternative to ceftazidime; as carries a higher risk of retinal infarction, use only if well-defined penicillin or cephalosporin allergy); lower intravitreal dose than ceftazidime and vancomycin |
| Note different dilution procedure to ceftazidime and vancomycin |
Subsequent management
Subsequent management proceeds according to culture results and clinical findings. Ultrasonography may be useful in assessing response to treatment.
Delayed-onset postoperative endophthalmitis
Pathogenesis
Delayed-onset endophthalmitis following cataract surgery develops when an organism of low virulence becomes trapped within the capsular bag (’saccular endophthalmitis’). Organisms can become sequestered within macrophages, protected from eradication but with continued expression of bacterial antigen.
It has an onset ranging from 4 weeks to years (mean of 9 months) postoperatively and typically follows uneventful cataract extraction with a posterior chamber intraocular lens. It may rarely be precipitated by Nd:YAG laser capsulotomy, which releases the organism into the vitreous. The infection is caused most frequently by P. acnes and occasionally S. epidermidis, Corynebacterium spp. or Candida parapsilosis.
Diagnosis
Posterior capsular opacification
Visually significant posterior capsular opacification (PCO) is the most common late complication of uncomplicated cataract surgery. Apart from reducing visual acuity, PCO may impair contrast sensitivity, cause difficulties with glare or give rise to monocular diplopia. The incidence of PCO is reduced when the capsulorhexis opening is in complete contact with the anterior surface of the IOL. PMMA (and probably to a lesser extent hydrogel) IOLs are particularly prone to PCO, but otherwise implant design is more important than material; notably, a square edge to the optic appears to inhibit PCO.
Signs
Treatment
Treatment involves the creation of an opening in the posterior capsule with the Nd:YAG laser.
Anterior capsular fibrosis and contraction
Since the advent of continuous curvilinear capsulorhexis, contraction of the anterior capsular opening (capsulophimosis) has become a relatively common postoperative complication. It can occur as early as several weeks after surgery and is accompanied by prominent subcapsular fibrosis (Fig. 9.22). The contraction typically progresses for up to three months, and if severe, may require Nd: YAG laser anterior capsulotomy. The severity of contraction is related to the optic material; the highest rate is with plate-haptic silicone IOLs and the lowest with 3-piece acrylic optic-PMMA haptic IOLs. A small capsulorhexis may also predispose to contraction.
Miscellaneous postoperative complications
Malposition of IOL
Although uncommon, malposition may be associated with both optical and structural problems. Annoying visual aberrations include glare, haloes, and monocular diplopia if the edge of the IOL becomes displaced into the pupil.
Cystoid macular oedema
Symptomatic CMO is relatively uncommon following uncomplicated phacoemulsification and in most cases it is mild and transient. It occurs more often after complicated surgery and has a peak incidence at 6–10 weeks, although the interval may be much longer.
Retinal detachment
Rhegmatogenous retinal detachment, although uncommon following uneventful ECCE or phaco, may be associated with the following risk factors:
Congenital cataract
Aetiology
Congenital cataracts occur in about 3 in 10 000 live births. Two-thirds of cases are bilateral and the cause can be identified in about half of those affected. The most common cause is genetic mutation, usually autosomal dominant (AD); other causes include chromosomal abnormalities, metabolic disorders and intrauterine infections. The underlying aetiological factors in unilateral cases remain less clear and the cause can be identified only in approximately 10%. Unilateral cataracts are usually sporadic, without a family history or systemic disease, and affected infants are usually full-term and healthy.
Inheritance
Isolated hereditary cataracts account for about 25% of cases. The mode is most frequently AD but may be autosomal recessive (AR) or X-linked (X-L). The morphology of the opacities and frequently the need for surgery are usually similar in parent and offspring. Isolated inherited congenital cataracts carry a better visual prognosis than those with coexisting ocular and systemic abnormalities.
Morphology
The morphology of congenital cataract is important because it may indicate a likely aetiology, mode of inheritance and effects on vision.
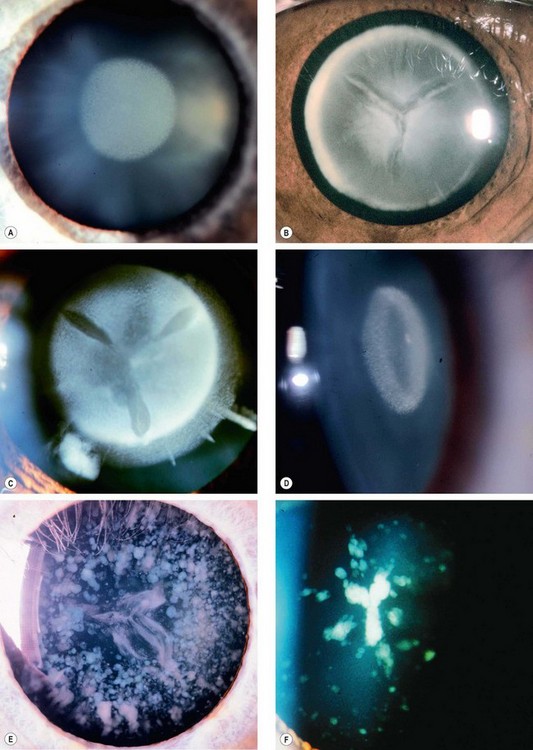
Fig. 9.25 Congenital cataracts. (A) Nuclear; (B) lamellar; (C) dense lamellar with ‘riders’; (D) coronary; (E) dense blue dot; (F) sutural and fine blue dot
(Courtesy of R Bates – fig. E)
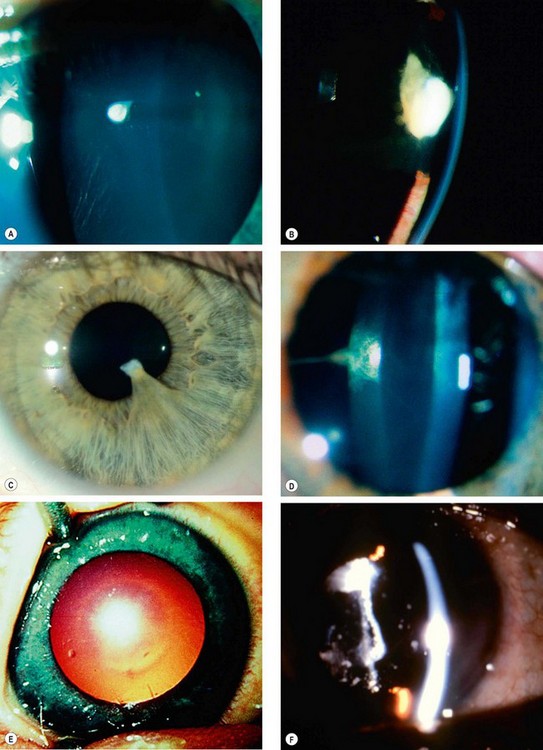
Fig. 9.26 Congenital cataracts. (A) Flat anterior polar; (B) pyramidal anterior polar; (C) anterior polar with persistent pupillary membrane; (D) posterior polar associated with Mittendorf dot; (E) ‘oil droplet’; (F) membranous
(Courtesy of J Schuman, V Christopoulos, D Dhaliwal, M Kahook and R Noecker, from Lens and Glaucoma, in Rapid Diagnosis in Ophthalmology, Mosby 2008 – fig. D; K Nischal – fig. E)
Systemic metabolic associations
Many systemic paediatric conditions may be associated with congenital cataract. The vast majority are extremely rare and of interest only to paediatric ophthalmologists. The general ophthalmologist should, however, be aware of the following conditions:
Galactoasemia
Lowe syndrome
Fabry’ disease
Mannosidosis
Associated intrauterine infections
Congenital rubella
Toxoplasmosis
Associated chromosomal abnormalities
Down syndrome (trisomy 21)
Associated skeletal syndromes
Hallermann–Streiff–François syndrome
Nance–Horan syndrome
Management
Ocular examination
Since a formal estimate of visual acuity cannot be obtained in the neonate, reliance is required on the density and morphology of the opacity, associated ocular findings and the visual behaviour of the child in order to assess the visual significance of the opacity. It is also important to examine parents and siblings.
Systemic investigations
Unless there is an established hereditary basis for the cataracts, the investigation of the infant with bilateral cataracts should include the following:
Treatment
Timing is crucial and the main considerations are as follows:
Postoperative complications
Cataract surgery in children carries a higher incidence of complications than in adults.
Visual rehabilitation
Although the technical difficulties of performing cataract surgery in infants and young children have mostly been resolved, visual results are hampered by amblyopia. With regard to optical correction for the aphakic child, the two main considerations are age and laterality of aphakia.
Ectopia lentis
Ectopia lentis refers to a displacement of the lens from its normal position. The lens may be completely dislocated, rendering the pupil aphakic (luxated), or partially displaced, still remaining in the pupillary area (subluxated). Ectopia lentis may be hereditary or acquired. Acquired causes include trauma, a large eye (e.g. high myopia, buphthalmos), anterior uveal tumours and hypermature cataract. Only hereditary causes are discussed below.
Without systemic associations
With systemic associations
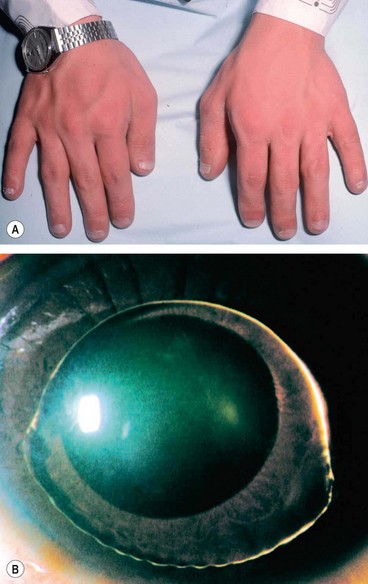
Marfan syndrome
Weill–Marchesani syndrome
Weill–Marchesani syndrome is a rare systemic connective tissue disease, conceptually the converse of Marfan syndrome.
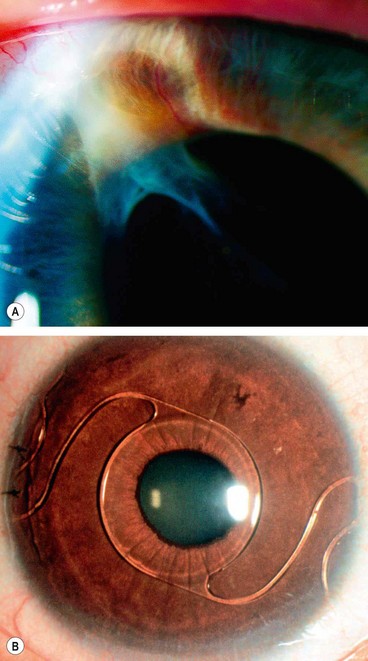
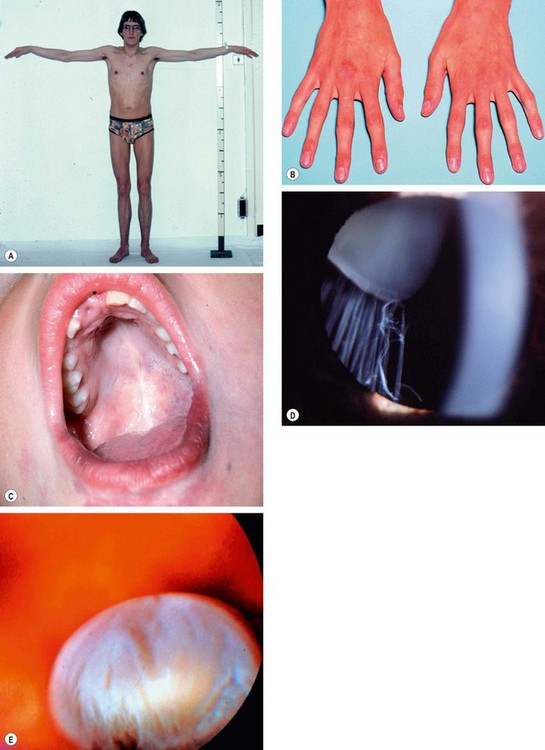
Homocystinuria
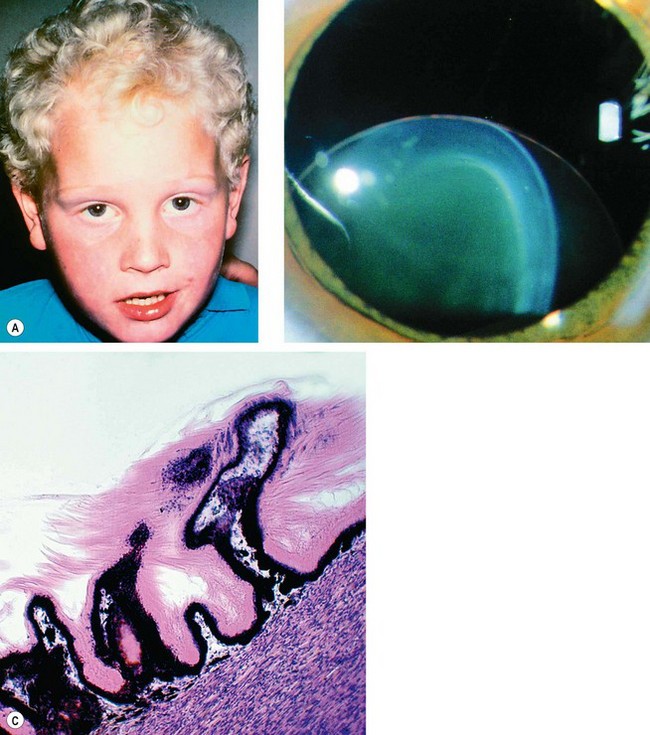
Fig. 9.31 Homocystinuria. (A) Coarse blond hair; (B) inferior subluxation with zonular disintegration; (C) histology shows matted zonular fibres lying over the ciliary epithelium
(Courtesy of J Schuman, V Christopoulos, D Dhaliwal, M Kahook and R Noecker, from Lens and Glaucoma, in Rapid Diagnosis in Ophthalmology, Mosby 2008 – fig. B; J Harry and G Misson, from Clinical Ophthalmic Pathology, Butterworth-Heinemann 2001 – fig. C)
Other systemic associations
Management
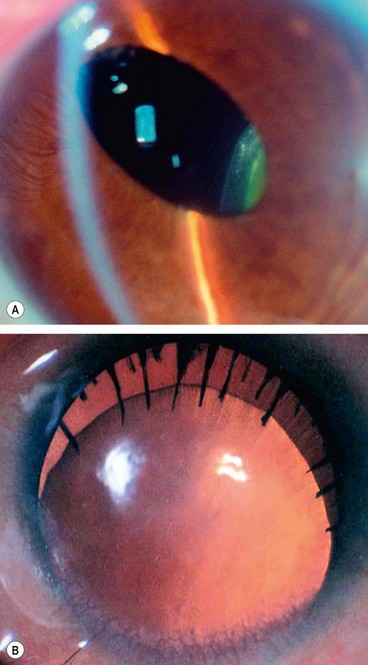
The main complications of ectopia lentis are (a) refractive error (lenticular myopia), (b) optical distortion due to astigmatism and/or lens edge effect, (c) glaucoma (see Ch. 10) and, rarely, (d) lens-induced uveitis.
Abnormalities of shape
Anterior lenticonus
Posterior lenticonus
Lentiglobus
Lentiglobus is a very rare, usually unilateral, generalized hemispherical deformity of the lens which may be associated with posterior polar lens opacity.
Microspherophakia
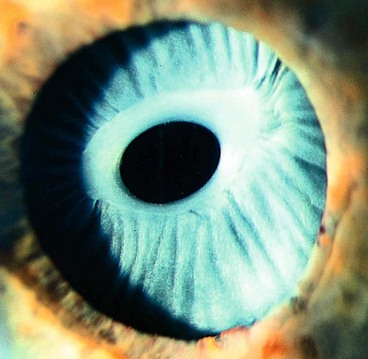
Coloboma
A coloboma is characterized by notching (segmental agenesis) at the inferior equator (Fig. 9.36) with corresponding absence of zonular fibres. It is not a true coloboma as there is no focal absence of a tissue layer due to failure of closure of the optic fissure. Occasionally a lens coloboma may be associated with a coloboma of the iris or fundus.