CHAPTER 6 Defense Mechanisms of the Gingiva
The gingival tissue is constantly subjected to mechanical and bacterial aggressions. The saliva, the epithelial surface, and the initial stages of the inflammatory response provide resistance to these actions. Chapter 2 reviews the role of the epithelium, through its degree of keratinization and turnover rate. This chapter describes the permeability of the junctional and sulcular epithelia and the role of sulcular fluid, leukocytes, and saliva.
Sulcular Fluid
The presence of sulcular fluid, or gingival crevicular fluid (GCF), has been known since the nineteenth century, but its composition and possible role in oral defense mechanisms were elucidated by the pioneering work of Waerhaug122 and Brill and Krasse14 in the 1950s. The latter investigators introduced filter paper into the gingival sulci of dogs previously injected intramuscularly with fluorescein; within 3 minutes the fluorescent material was recovered on the paper strips. This indicated the passage of fluid from the bloodstream through the tissues and exiting via the gingival sulcus.
In subsequent studies, Brill11,12 confirmed the presence of GCF in humans and considered it a “transudate.” However, others73,123 demonstrated that GCF is an inflammatory exudate, not a continuous transudate. In strictly normal gingiva, little or no fluid can be collected.
More recently, interest in the development of tests for the detection or prediction of periodontal disease has resulted in numerous research papers on the components, origin, and function of GCF.
Methods of Collection
The most difficult hurdle to overcome when collecting GCF is the scarcity of material that can be obtained from the sulcus. Many collection methods have been tried.* These methods include the use of absorbing paper strips, twisted threads placed around and into the sulcus, micropipettes, and intracrevicular washings.
The absorbing paper strips are placed within the sulcus (intrasulcular method) or at its entrance (extrasulcular method) (Figure 6-1). The placement of the filter paper strip in relation to the sulcus or pocket is important. The Brill technique inserts it into the pocket until resistance is encountered (Figure 6-1, A). This method produces some degree of irritation of the sulcular epithelium that by itself can trigger the flow of fluid.
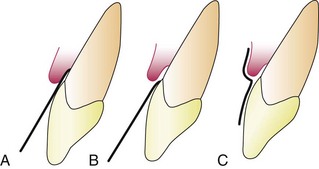
Figure 6-1 Placement of filter strip in gingival sulcus for collection of fluid. A, Intrasulcular method. B and C, Extrasulcular methods.
To minimize this irritation, Löe and Holm-Pedersen73 placed the filter paper strip just at the entrance of the pocket or over the pocket entrance (Figure 6-1, B and C). In this way, fluid seeping out is picked up by the strip, but the sulcular epithelium is not in contact with the paper.
Preweighed twisted threads were used by Weinstein et al.123 The threads were placed in the gingival crevice around the tooth, and the amount of fluid collected was estimated by weighing the sample thread.
The use of micropipettes permits the collection of fluid by capillarity. Capillary tubes of standardized length and diameter are placed in the pocket, and their content is later centrifuged and analyzed.10,12,13
Crevicular washings can be used to study GCF from clinically normal gingiva. One method uses an appliance consisting of a hard acrylic plate covering the maxilla with soft borders and a groove following the gingival margins; it is connected to four collection tubes. The washings are obtained by rinsing the crevicular areas from one side to the other, using a peristaltic pump.21
A modification of the previous method uses two injection needles fitted one within the other so that during sampling, the inside (ejection) needle is at the bottom of the pocket and the outside (collecting) needle is at the gingival margin. The collection needle is drained into a sample tube by continuous suction.101
Permeability of Junctional and Sulcular Epithelia
The initial studies by Brill and Krasse14 with fluorescein were later confirmed with substances such as India ink95 and saccharated iron oxide.21 Substances shown to penetrate the sulcular epithelium include albumin,94 endotoxin,93,98 thymidine,49 histamine,28 phenytoin,114 and horseradish peroxidase.79 These findings indicate permeability to substances with a molecular weight of up to 1000 kD.
Squier and Johnson113 reviewed the mechanisms of penetration through an intact epithelium. Intercellular movement of molecules and ions along intercellular spaces appears to be a possible mechanism. Substances taking this route do not traverse the cell membranes.
Amount
The amount of GCF collected on a paper strip can be evaluated in a variety of ways. The wetted area can be made more visible by staining with ninhydrin; it is then measured planimetrically on an enlarged photograph or with a magnifying glass or a microscope.
An electronic method has been devised for measuring the fluid collected on a “blotter” (Periopaper), employing an electronic transducer (Periotron, Harco Electronics, Winnipeg, Manitoba, Canada) (Figure 6-2). The wetness of the paper strip affects the flow of an electronic current and gives a digital readout. A comparison of the ninhydrin-staining method and the electronic method performed in vitro revealed no significant differences between the two techniques.116
The amount of GCF collected is extremely small. Measurements performed by Cimasoni21 showed that a strip of paper 1.5 mm wide and inserted 1 mm within the gingival sulcus of a slightly inflamed gingiva absorbs about 0.1 mg of GCF in 3 minutes. Challacombe19 used an isotope dilution method to measure the amount of GCF present in a particular space at any given time. His calculations in human volunteers with a mean gingival index of less than 1 showed that the mean GCF volume in proximal spaces from molar teeth ranged from 0.43 to 1.56 µl.
Composition
The components of GCF can be characterized according to individual proteins,73,85,103 specific antibodies, antigens,35,92 and enzymes of several specificities.15 The GCF also contains cellular elements.28,31,125
Many research efforts have attempted to use GCF components to detect or diagnose active disease or to predict patients at risk for periodontal disease.2 So far, more than 40 compounds found in GCF have been analyzed,89 but their origin is not known with certainty. These compounds can be host derived or produced by the bacteria in the gingival crevice, but their source can be difficult to elucidate; examples include β-glucuronidase, a lysosomal enzyme, and lactic acid dehydrogenase, a cytoplasmic enzyme. The source for collagenases may be fibroblasts or polymorphonuclear leukocytes (PMNs, neutrophils),87 or collagenases may be secreted by bacteria.35 Phospholipases are lysosomal and cytoplasmic enzymes but are also produced by microorganisms.15 The majority of GCF elements detected thus far have been enzymes, but there are nonenzymatic substances as well.
Cellular Elements
Cellular elements found in GCF include bacteria, desquamated epithelial cells, and leukocytes (PMNs, lymphocytes, monocytes/macrophages), which migrate through the sulcular epithelium.28,31
Electrolytes
Potassium, sodium, and calcium have been studied in GCF. Most studies have shown a positive correlation of calcium and sodium concentrations and the sodium/potassium ratio with inflammation.56-58 (For further information, see references 12 and 13 and Box 6-2 online).
Organic Compounds
Both carbohydrates and proteins have been investigated. Glucose hexosamine and hexuronic acid are two of the compounds found in GCF.47 Blood glucose levels do not correlate with GCF glucose levels; glucose concentration in GCF is three to four times greater than that in serum.47 This is interpreted not only as a result of metabolic activity of adjacent tissues but also as a function of the local microbial flora.
The total protein content of GCF is much less than that of serum.13,14 No significant correlations have been found between the concentration of proteins in GCF and the severity of gingivitis, pocket depth, or extent of bone loss.7
Metabolic and bacterial products identified in GCF include lactic acid,48 urea,42 hydroxyproline,91 endotoxins,109 cytotoxic substances, hydrogen sulfide,112 and antibacterial factors.27
Many enzymes have also been identified (Boxes 6-1 and 6-2).
BOX 6-2 Compounds and Enzymes (Products) of Possible Bacterial Origin Detected in Gingival Crevicular Fluid
REFERENCES FOR BOXES 6-1 AND 6-2
1 Abiko Y, Hayakawa M, Murai S, et al. Glycylpropyl dipeptidyl amino-peptidase from Bacteroides gingivalis. J Clin Periodontol. 1985;64:106.
2 Adonogianaki E, Mooney J, Kinane DF. The ability of acute gingival crevicular fluid phase proteins to distinguish gingivitis and periodontitis sites. J Clin Periodontol. 1992;19:98.
3 Beighton D, Radford JR, Naylor MN. Glycosidase activity in gingival crevicular fluid in subjects with adult periodontitis or gingivitis. Arch Oral Biol. 1992;37:43.
4 Brandtzaeg P, Mann WA. A comparative study of the lysozyme activity of human gingival pocket fluid, serum and saliva. Acta Odont Scand. 1964;29:441.
5 Buduneli N, Buduneli E, Ciotanar S, et al. Plasminogen activators and plasminogen activator inhibitors in gingival crevicular fluid of cyclosporine A–treated patients. J Clin Periodontol. 2004;31:556.
6 Bulkacz J. Enzymatic activities in gingival fluid with special emphasis on phospholipases. J West Soc Periodontol. 1986;36:145.
7 Bulkacz J, Erbland JF. Exocellular phospholipase activity from a strain of Propionibacterium acnes isolated from a periodontal pocket. J Periodontol. 1997;68:369.
8 Bulkacz J, Erbland JF, MacGregor J. Phospholipase activity in supernatants from cultures of Bacteroides melaninogenicus. Biochem Biophys Acta. 1981;664:148.
9 Bulkacz J, Faull KM. Multiple extracellular phospholipase activities from Prevotella intermedia. Anaerobe. 2009;15:91.
10 Bulkacz J, Garnick J, Barclay JE. Detection of phospholipase activity in crevicular fluid. J Dent Res. 60, 1981. abstract 1187
11 Bulkacz J, Grenett H. Synthesis of prostaglandin-like substances by oral gram negative rods. J Dent Res. 60, 1981. abstract 421
12 Chu L, Bramanti TE, Holt SC, et al. Hemolytic activity in the periodontopathogen Porphyromonas gingivalis: kinetics of enzyme formation and localization. Infect Immun. 1991;59:1932.
13 Chung RM, Grbic JT, Lamster IB. Interleukin-8 and beta-glucuronidase in gingival crevicular fluid. J Clin Periodontol. 1997;24:146.
14 Cimasoni G. Crevicular fluid updated. In: Myers H, editor. Monographs in oral science, vol 12. Basel: S Karger; 1983.
15 Eley BM, Cox SW. Cathepsin B/L-, elastase-, tryptase-, trypsin- and dipeptidyl peptidase IV–like activities in gingival crevicular fluid: a comparison of levels before and after periodontal surgery in chronic periodontitis patients. J Periodontol. 1992;63:412.
16 Fullmer HM, Gibson WA. Collagenolytic activity in gingiva in man. Nature. 1966;209:728.
17 Gibbons RJ, MacDonald JB. Degradation of collagenous substrates by Bacteroides melaninogenicus. J Bacteriol. 1964;81:614.
18 Grbic JT, Singer RE, Jans HH, et al. Immunoglobulin isotypes in gingival crevicular fluid: possible protective of IgA. J Periodontol. 1995;66:55.
19 Gregory RL, Kim DE, Kindel JC, et al. Immunoglobulin-degrading enzymes in localized juvenile periodontitis. J Periodontal Res. 1992;27:176.
20 Huynk C, Roch-Arveiller M, Meyer J, et al. Gingival crevicular fluid of patients with gingivitis or periodontal disease: evaluation of elastase–alpha 1 proteinase inhibitor complexes. J Clin Periodontol. 1992;19:187.
21 Ichimaru E, Imura K, Hara Y, et al. Cystatin activity in gingival crevicular fluid from periodontal disease patients, measured by a new quantitative analysis method. J Periodontal Res. 1992;27:119.
22 Ishikawa I, Cimasoni G, Ahmad-Zadeh C. Possible roles of lysosomal enzymes in the pathogenesis of periodontitis: a study in cathepsin D in human gingival fluid. Arch Oral Biol. 1972;17:111.
23 Jentsch H, Sievert Y, Gocke R. Lactoferrin and other markers from gingival crevicular fluid and saliva before and after periodontal treatment. J Clin Periodontol. 2004;31:511.
24 Jin L, Yu C, Corbet EF. Interleukin-8 and granulocyte elastase in gingival crevicular fluid in relation to periodonytopathogens in untreated adult periodontitis. J Periodontol. 2000;71:929.
25 Kamma JJ, Nakou M, Persson RG. Association of early onset periodontitis microbiota with aspartate aminotransferase activity in gingival crevicular fluid. J Clin Periodontol. 2001;28:1096.
26 Karhuvaara L, Tenovuo J, Sievers G. Crevicular fluid myeloperoxidase: an indicator of acute gingival inflammation. Proc Finish Dent Soc. 1990;86:3.
27 Killian M. Degradation of immunoglobulins A1, A2, and G by suspected principal periodontal pathogens. Infect Immun. 1981;34:757.
28 Kunamitsu K, Ichimaru E, Kato I, et al. Granulocyte medullasin levels in gingival crevicular fluid from chronic adult periodontitis patients and experimental gingivitis subjects. J Periodontal Res. 1990;25:352.
29 Lamster IB, Vogel RI, Hartley LJ, et al. Lactate dehydrogenase, beta-glucuronidase, and arylsulfatase activity in gingival crevicular fluid associated with experimental gingivitis in man. J Periodontol. 1985;56:139.
30 Lamster IB, Kaufman E, Grbic JT, et al. Beta-glucuronidase activity in saliva. J Periodontol. 2003;74:353.
31 Life JS, Johnson NW, Powell JR, et al. Interleukin-1 beta (IL-1b) levels in gingival crevicular fluid fromadults without previous evidence of destructive periodontitis: a cross sectional study. J Clin Periodontol. 1992;19:53.
32 Mancini S, Romanelli R, Laschinger CA, et al. Assessment of a novel screening test for neutrophil collagenase activity in the diagnosis of periodontal disease. J Periodontol. 1999;70:1292.
33 Miyajima K, Ohmo Y, Iwata T, et al. The lactic acid and citric acid content in the gingival fluid of orthodontic patients. Aichi Gakuin Dent Sci. 1991;4:75.
34 Mogi M, Otogoto J. Expression of cathepsin K in gingival crevicular fluid of patients with periodontitis. Arch Oral Biol. 2007;52:894.
35 Mogi M, Otogoto J, Ota N, et al. Interleukin 1 beta, interleukin 6, beta 2-microglobulin, and transforming growth factor-alpha in gingival crevicular fluid from human periodontal disease. Arch Oral Biol. 1999;44:535.
36 Nitzan D, Sperry JF, Wilkins TD. Fibrinolytic activity of oral anaerobic bacteria. Arch Oral Biol. 1978;23:465.
37 Page RC. Host response tests designed for diagnosing periodontal diseases. J Periodontol. 1992;63:356.
38 Perinetti G, Paolantonio M, Ferumunella B. Gingival crevicular fluid alkaline phosphatase activity reflects periodontal healing/recurrent inflammation phases in chronic periodontitis patients. J Periodontol. 2008;79:1200.
39 Perinetti G, Paolantonio M, Serra E, et al. Longitudinal monitoring of subgingival colonization by Actinobacillus actinomycetemcomitans, and crevicular alkaline phosphatase and aspartate aminotransferase activities around orthodontically treated teeth. J Clin Periodontol. 2004;31:60.
40 Puklo M, Guentsch A, Hiemstra PS, et al. Analysis of neutrophil-derived antimicrobial peptides in gingival crevicular fluid suggests importance of cathelicidin LL-37 in the innate immune response against periodontogenic bacteria. Oral Microbiol Immunol. 2008;23:328.
41 Pradeep AR, Roopa Y, Swati PP. Interleukin-4, a T-helper 2 cell cytokine, is associated with the remission of periodontal disease. J Perio Res.. 2008.
42 Rai B, Kharb S, Jain R, Anand S. Biomarkers of periodontitis in oral fluids. J Oral Sci. 2008;50:53.
43 Slots J. Enzymatic characterization of some oral and non-oral gram-negative bacteria with the API ZYM system. J Clin Microbiol. 1981;14:288.
44 Slots J, Dahlen G. Subgingival microorganisms and bacterial virulence factors in periodontitis. Scand J Dent Res. 1985;93:119.
45 Smalley J, Birss AJ, Kay HM, et al. The distribution of trypsin-like enzyme activity in cultures of a virulent and an avirulent strain of Bacteroides gingivalis W50. Oral Microbiol Immunol. 1989;4:178.
46 Sueda T, Cimasoni G, Held AJ. High levels of acid phosphatase in human crevicular fluid. Arch Oral Biol. 1967;12:1205.
47 Talonopoika J. Characterization of fibrin(ogen) fragments in gingival crevicular fluid. Scand J Periodontal Res. 1991;99:40.
48 Tipler LS, Emberg G. Glycosaminoglycan depolimerizing enzymes form oral microorganisms. Arch Oral Biol. 1985;30:391.
49 Totan A, Greabu M, Totan C, Spinu T. Salivary aspartate aminotransferase, alanine aminotransferase and alkaline phosphatase: possible markers in periodontal disease. Clin Chem Lab Med. 2006;44:612.
50 Tsai CC, Ku CH, Ho YP, et al. Changes in gingival crevicular fluid interleukin-4, and interferon-gamma in patients with chronic periodontitis before and after periodontal initial therapy. J Med Scie. 2007;23:1.
51 Tynelius-Brathall G. Hyaluronidase activity in gingival crevicular fluid and in peritoneal exudate leukocytes in dogs. J Periodontal Res. 1972;7:307.
52 Uito VJ. Degradation of basement membrane collagen by proteinases from human gingiva, leukocytes and bacterial plaque. J Periodontol. 1983;54:740.
53 Uito VJ, Grenier D, Chan ECS, et al. Isolation of a chymotrypsin-like enzyme from Treponema denticola. Infect Immun. 1988;56:2717.
54 Yucel O, Berker E, Gariboglee S, et al. Interleukin-11, interleukin-1, interleukin-12 and the pathogenesis of inflammatory periodontal disease. J Clin Periodontol. 2008;35:363.
The methodology used for the analysis of GCF components is as varied as the diversity of those components. Examples include fluorometry for the detection of metalloproteinases28; enzyme-linked immunoabsorbent assay (ELISA) to detect enzyme levels and interleukin-1 beta (IL-1β)71; radioimmunoassays for detecting cyclooxygenase derivatives86 and procollagen III117; high-pressure liquid chromatography (HPLC) to detect timidazole67; and direct and indirect immunodot tests for detection of acute-phase proteins.108
Cellular and Humoral Activity in Gingival Crevicular Fluid
Monitoring periodontal disease is a complicated task because very few noninvasive procedures can follow the initiation and progress of the disease. Analysis of GCF constituents in health and disease may be extremely useful because of GCF’s simplicity and because GCF can be obtained with noninvasive methods.
Analysis of GCF has identified cell and humoral responses in both healthy individuals and those with periodontal disease.64 The cellular immune response includes the appearance of cytokines in GCF (see Box 6-1 online), but there is no clear evidence of a relationship between cytokines and disease. However, interleukin-1 alpha (IL-1α) and IL-1β are known to increase the binding of PMNs and monocytes/macrophages to endothelial cells, stimulate the production of prostaglandin E2 (PGE2) and release of lysosomal enzymes, and stimulate bone resorption.68 Preliminary evidence also indicates the presence of interferon-α in GCF,64 which may have a protective role in periodontal disease because of its ability to inhibit the bone resorption activity of IL-1β.44
Because the amount of fluid recoverable from gingival crevices is small, only the use of very sensitive immunoassays permits the analysis of the specificity of antibodies.27 A study comparing antibodies in different crevices with serum antibodies directed at specific microorganisms did not provide any conclusive evidence about the significance of the antibody presence in GCF in periodontal disease.64
Even though the role of antibodies in the gingival defense mechanisms is difficult to ascertain, the consensus is that in a patient with periodontal disease, (1) a reduction in antibody response is detrimental, and (2) an antibody response plays a protective role.65
Clinical Significance
As mentioned previously, GCF is an inflammatory exudate.73 Its presence in clinically normal sulci can be explained because gingiva that appears clinically normal invariably exhibits inflammation when examined microscopically.
The amount of GCF is greater when inflammation is present34,106 and is sometimes proportional to the severity of inflammation.88 GCF production is not increased by trauma from occlusion78 but is increased by mastication of coarse foods, toothbrushing and gingival massage, ovulation,71 hormonal contraceptives,70 and smoking.80 Other factors that influence the amount of GCF are circadian periodicity and periodontal therapy.
Circadian Periodicity
There is a gradual increase in GCF amount from 6 AM to 10 PM and a decrease afterward.9
Sex Hormones
Female sex hormones increase GCF flow, probably because they enhance vascular permeability.68 Pregnancy, ovulation,67 and hormonal contraceptives69 all increase gingival fluid production.
Mechanical Stimulation
Chewing12 and vigorous gingival brushing stimulate the flow of GCF. Even the minor stimuli represented by intrasulcular placement of paper strips increases the production of fluid.
Periodontal Therapy
There is an increase in GCF production during the healing period after periodontal surgery.3
Drugs in Gingival Crevicular Fluid
Drugs that are excreted through the GCF may be used advantageously in periodontal therapy. Bader and Goldhaber6 demonstrated in dogs that tetracyclines are excreted through the GCF; this finding triggered extensive research that showed a concentration of tetracyclines in GCF compared with serum.43 Metronidazole is another antibiotic that has been detected in human GCF32 (see Chapter 47).
Leukocytes in the Dentogingival Area
Leukocytes have been found in clinically healthy gingival sulci in humans and experimental animals. The leukocytes found are predominantly PMNs. They appear in small numbers extravascularly in the connective tissue adjacent to the bottom of the sulcus; from there, they travel across the epithelium18,45 to the gingival sulcus, where they are expelled (Figures 6-3 and 6-4).
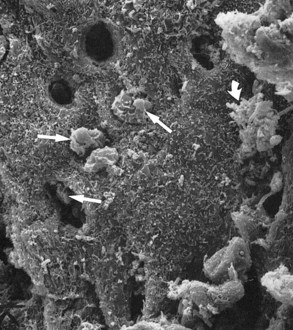
Figure 6-3 Scanning electron microscope view of periodontal pocket wall. Several leukocytes are emerging (straight arrows), some partially covered by bacteria (curved arrow). Empty holes correspond to tunnels through which leukocytes have emerged.
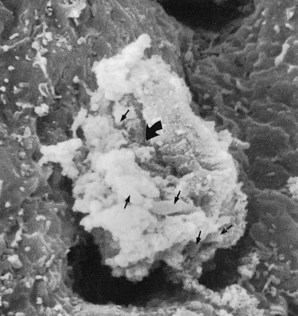
Figure 6-4 Scanning electron microscope view at higher magnification than Figure 6-3. A leukocyte emerging from the pocket wall is covered with bacteria (small arrows). The large curved arrow points to a phagosomal vacuole through which bacteria are being engulfed.
![]() Science Transfer
Science Transfer
Clinicians need to be aware of defense mechanisms of the gingiva because dental treatment can impair gingival defenses. The disruption of the epithelial lining of the gingival sulcus can occur with routine scaling and restorative procedures that involve the subgingival region, resulting in increased risk of inflammation as bacteria and their products have direct contact with the underlying connective tissue. The junctional and oral sulcular epithelium have the capacity to heal and reform the epithelial barrier in 7 to 10 days, and during this time, plaque control must be optimized to limit the risk of initiating periodontal breakdown. The use of gentle minimally traumatic techniques is also important.
Smoking may lessen the defense of the gingiva by impairing polynuclear leukocyte function in the tissues and the gingival sulcus. Further, smokers frequently have less gingival inflammation and bleeding on probing than nonsmokers with the same amount of periodontal attachment loss. Thus patients using tobacco should be carefully monitored for changes in pocket depth and given aggressive periodontal therapy.
Leukocytes are present in sulci even when histologic sections of adjacent tissue are free of inflammatory infiltrate. Differential counts of leukocytes from clinically healthy human gingival sulci have shown 91.2% to 91.5% PMNs and 8.5% to 8.8% mononuclear cells.111,125
Mononuclear cells were identified as 58% B lymphocytes, 24% T lymphocytes, and 18% mononuclear phagocytes. The ratio of T lymphocytes to B lymphocytes was found to be reversed from the normal ratio of about 3 : 1 found in peripheral blood to about 1 : 3 in GCF.125
Leukocytes are attracted by different plaque bacteria54,124 but can also be found in the dentogingival region of germ-free adult animals.74,100 Leukocytes were reported in the gingival sulcus in nonmechanically irritated (resting) healthy gingiva, indicating that their migration may be independent of an increase in vascular permeability.5 The majority of these cells are viable and have phagocytic and killing capacity.62,90,96 Therefore leukocytes constitute a major protective mechanism against the extension of plaque into the gingival sulcus.
Leukocytes are also found in saliva (see following discussion). The main port of entry of leukocytes into the oral cavity is the gingival sulcus.104
Saliva
Salivary secretions are protective in nature because they maintain the oral tissues in a physiologic state (Table 6-1). Saliva exerts a major influence on plaque by mechanically cleansing the exposed oral surfaces, by buffering acids produced by bacteria, and by controlling bacterial activity.
TABLE 6-1 Role of Saliva In Oral Health
| Function | Salivary Components | Probable Mechanism |
|---|---|---|
| Lubrication | Glycoproteins, mucoids | Coating similar to gastric mucin |
| Physical protection | Glycoproteins, mucoids | Coating similar to gastric mucin |
| Cleansing | Physical flow | Clearance of debris and bacteria |
| Buffering | Bicarbonate and phosphate | Antacids |
| Tooth integrity maintenance | ||
| Antibacterial action | Immunoglobulin A | Control of bacterial colonization |
| Lysozyme | Breaks bacterial cell walls | |
| Lactoperoxidase | Oxidation of susceptible bacteria |
Antibacterial Factors
Saliva contains numerous inorganic and organic factors that influence bacteria and their products in the oral environment. Inorganic factors include ions and gases, bicarbonate, sodium, potassium, phosphates, calcium, fluorides, ammonium, and carbon dioxide. Organic factors include lysozyme, lactoferrin, myeloperoxidase, lactoperoxidase, and agglutinins such as glycoproteins, mucins, β2-macroglobulins, fibronectins,117 and antibodies.
Lysozyme is a hydrolytic enzyme that cleaves the linkage between structural components of the glycopeptide muramic acid–containing region of the cell wall of certain bacteria in vitro. Lysozyme works on both gram-negative and gram-positive organisms50; its targets include Veillonella species and Actinobacillus actinomycetemcomitans. It probably repels certain transient bacterial invaders of the mouth.53
The lactoperoxidase-thiocyanate system in saliva has been shown to be bactericidal to some strains of Lactobacillus and Streptococcus82,99 by preventing the accumulation of lysine and glutamic acid, both of which are essential for bacterial growth. Another antibacterial finding is lactoferrin, which is effective against Actinobacillus species.55
Myeloperoxidase, an enzyme similar to salivary peroxidase, is released by leukocytes and is bactericidal for Actinobacillus81 but has the added effect of inhibiting the attachment of Actinomyces strains to hydroxyapatite.16
Salivary Antibodies
As with GCF, saliva contains antibodies that are reactive with indigenous oral bacterial species. Although immunoglobulins G (IgG) and M (IgM) are present, the preponderant immunoglobulin found in saliva is immunoglobulin A (IgA). However, IgG is more prevalent in GCF.115 Major and minor salivary glands contribute all the secretory IgA (sIgA) and lesser amounts of IgG and IgM. GCF contributes most of the IgG, complement, and PMNs that in conjunction with IgG or IgM inactivate or opsonize bacteria.
Salivary antibodies appear to be synthesized locally because they react with strains of bacteria indigenous to the mouth but not with organisms characteristic of the intestinal tract.36,38 Many bacteria found in saliva have been shown to be coated with IgA, and the bacterial deposits on teeth contain both IgA and IgG in quantities greater than 1% of their dry weight.37 It has been shown that IgA antibodies present in parotid saliva can inhibit the attachment of oral Streptococcus species to epithelial cells.33,122 Gibbons et al36-38 suggested that antibodies in secretions may impair the ability of bacteria to attach to mucosal or dental surfaces.
Enzymes
The enzymes normally found in the saliva are derived from the salivary glands, bacteria, leukocytes, oral tissues, and ingested substances; the major enzyme is parotid amylase. Certain salivary enzymes have been reported in increased concentrations in periodontal disease: hyaluronidase and lipase,17 β-glucuronidase and chondroitin sulfatase,41 aspartate aminotransferase and alkaline phosphatase,119 amino acid decarboxylases,41 catalase, peroxidase, and collagenase.60
Proteolytic enzymes in the saliva are generated by both the host and oral bacteria. These enzymes have been recognized as contributors to the initiation and progression of periodontal disease.49,77 To combat these enzymes, saliva contains antiproteases that inhibit cysteine proteases such as cathepsins51 and antileukoproteases that inhibit elastase.87 Another antiprotease, identified as a tissue inhibitor of matrix metalloproteinase (TIMP), has been shown to inhibit the activity of collagen-degrading enzymes.26
High-molecular-weight mucinous glycoproteins in saliva bind specifically to many plaque-forming bacteria. The glycoprotein-bacteria interactions facilitate bacterial accumulation on the exposed tooth surface.33,36-38,124 The specificity of these interactions has been demonstrated. The interbacterial matrix of human plaque appears to contain polymers similar to salivary glycoproteins that may aid in maintaining the integrity of plaque. In addition, these glycoproteins selectively adsorb to the hydroxyapatite to make up part of the acquired pellicle. Other salivary glycoproteins inhibit the sorption of some bacteria to the tooth surface and to epithelial cells of the oral mucosa. This activity appears to be associated with the glycoproteins that possess blood group reactivity.1,33,36,38,122 Another effect of mucin is the deletion of bacterial cells from the oral cavity by aggregation with mucin-rich films.
Glycoproteins and a glycolipid present on mammalian cell surfaces appear to serve as receptors for the attachment of some viruses and bacteria. Thus the close similarity between glycoproteins of salivary secretions and components of the epithelial cell surface suggests that the secretions can competitively inhibit antigen sorption and therefore may limit pathologic alterations.
Salivary Buffers and Coagulation Factors
The maintenance of physiologic hydrogen ion concentration (pH) at the mucosal epithelial cell surface and the tooth surface is an important function of salivary buffers. Their primary effect has been studied in relationship to dental caries. In saliva the most important salivary buffer is the bicarbonate–carbonic acid system.76
Saliva also contains coagulation factors (factors VIII, IX, and X; plasma thromboplastin antecedent [PTA]; Hageman factor) that hasten blood coagulation and protect wounds from bacterial invasion.66 An active fibrinolytic enzyme may also be present.
Leukocytes
In addition to desquamated epithelial cells, the saliva contains all forms of leukocytes, of which the principal cells are PMNs. The number of PMNs varies from person to person at different times of the day and is increased in gingivitis. PMNs reach the oral cavity by migrating through the lining of the gingival sulcus. Living PMNs in saliva are sometimes referred to as orogranulocytes, and their rate of migration into the oral cavity is termed the orogranulocytic migratory rate. Some investigators believe the rate of migration correlates with the severity of gingival inflammation and is therefore a reliable index for assessing gingivitis.111
Role in Periodontal Pathology
Saliva exerts a major influence on plaque initiation, maturation, and metabolism. Salivary flow and composition also influence calculus formation, periodontal disease, and caries. The removal of the salivary glands in experimental animals significantly increases the incidence of dental caries39 and periodontal disease46 and delays wound healing.107
In humans, an increase in inflammatory gingival diseases, dental caries, and rapid tooth destruction associated with cervical or cemental caries is partially a consequence of decreased salivary gland secretion (xerostomia). Xerostomia may result from sialolithiasis, sarcoidosis, Sjögren’s syndrome, Mikulicz’s disease, irradiation, surgical removal of the salivary glands, and other factors (see Chapters 37 and 39).
1 Adinolri M, Mollison PL, Polley MJ, et al. A blood group antibodies. J Exp Med. 1966;123:951.
2 Armitage G. Diagnostic tests for periodontal diseases. Curr Opin Dent. 1992;2:53.
3 Arnold R, Lunstad G, Bissada N, et al. Alterations in crevicular fluid flow during healing following gingival surgery. J Periodontal Res. 1966;1:303.
4 Attstrom R. Presence of leukocytes in the crevices of healthy and clinically inflamed gingiva. J Periodontol. 1970;5:42.
5 Attstrom R, Egelberg J. Emigration of blood neutrophils and monocytes into the gingival crevices. J Periodontal Res. 1970;5:48.
6 Bader HJ, Goldhaber P. The passage of intravenously administered tetracycline in the gingival sulcus of dogs. J Oral Ther. 1966;2:324.
7 Bang J, Cimasoni G. Total protein in human crevicular fluid. J Dent Res. 1971;50:1683.
8 Birkedal-Hansen H, Taylor RE, Zambon JJ, et al. Characterization of collagenolytic activity from strains of Bacteroides gingivalis. J Periodontal Res. 1988;23:258.
9 Bissada NF, Schaffer EM, Haus E. Circadian periodicity of human crevicular fluid. J Periodontol. 1967;38:36.
10 Bjorn HL, Koch G, Lindhe J. Evaluation of gingival fluid measurements. Odont Rev. 1965;16:300.
11 Brill N. The gingival pocket fluid: studies of its occurrence, composition and effect. Acta Odontol Scand. 1969;20(Suppl 32):159.
12 Brill N. Effect of chewing on flow of tissue fluid into gingival pockets. Acta Odontol Scand. 1959;17:277.
13 Brill N, Bronnestam R. Immunoelectrophoretic study of tissue fluid from gingival pockets. Acta Odontol Scand. 1960;18:95.
14 Brill N, Krasse B. The passage of tissue fluid into the clinically healthy gingival pocket. Acta Odontol Scand. 1958;16:223.
15 Bulkacz J. Enzymatic activities in gingival fluid with special emphasis on phospholipases. J West Soc Periodontol. 1986;36:145.
16 Camargo PM de, Miyasaki KT, Wolinsky LE. Host modulation of adherence: the effect of human neutrophil myeloperoxidase on the attachment of Actinomyces viscosus and naeslundii to saliva coated hydroxyapatite. J Periodontal Res. 1988;23:334.
17 Carlsson J, Egelberg J. Local effect of diet on plaque formation and development of gingivitis in dogs. II. Effect of high carbohydrate versus high protein/fat diets. Odont Rev. 1965;16:42.
18 Cattoni M. Lymphocytes in the epithelium of healthy gingiva. J Dent Res. 1951;30:627.
19 Challacombe SJ. Passage of serum immunoglobulin into the oral cavity. In: Lehner T, Cimasoni G, editors. s: Borderland between caries and periodontal disease, vol 2. London: Academic Press; 1980.
20 Cimasoni G. The crevicular fluid. In: Myers H, editor. Monographs in oral science, vol 3. Basel: S Karger; 1974.
21 Cimasoni G. Crevicular fluid updated. In: Myers H, editor. Monographs in oral science, vol 12. Basel: S Karger; 1983.
22 Cobb CM, Brown LR. The effects of exudate from the periodontal pocket on cell culture. Periodontics. 1967;5:5.
23 Dawes C. The chemistry and physiology of saliva. In: Shaw JH, Sweeney EA, Cappuccino CCet al, editors. Textbook of oral biology. Philadelphia: Saunders, 1978.
24 Dawes C, Jenkins GM, Tonge CH. The nomenclature of the integuments of the enamel surface of teeth. Br Dent J. 1963;115:65.
25 Dinarello CA. Interleukin-1 and its biologically related cytokines. In: Cohen S, editor. Lymphokines and the immune response. Boca Raton, Fla: CRC Press, 1990.
26 Drouin L, Overall CM, Sodek J. Identification of matrix metallo-endoproteinase inhibitor (TIMP) in human parotid and submandibular saliva: partial purification and characterization. J Periodontal Res. 1988;23:370.
27 Ebersole JL, Taubman MA, Smith DJ. Gingival crevicular fluid antibody to oral microorganisms. II. Distribution and specificity of local antibody responses. J Periodontal Res. 1985;20:349.
28 Egelberg J. Cellular elements in gingival pocket fluid. Acta Odontol Scand. 1963;21:283.
29 Egelberg J. Gingival exudate measurements for evaluation of inflammatory changes of the gingiva. Odont Rev. 1964;15:381.
30 Egelberg J. Permeability of the dentogingival vessels. II. Clinically healthy gingiva. J Periodontal Res. 1966;1:276.
31 Egelberg J, Attstrom R. Presence of leukocytes within crevices of healthy and inflamed gingiva and their immigration from the blood. J Periodontal Res. 1969;4(Suppl):23.
32 Eisenberg L, Suchow R, Coles RS, et al. The effects of metronidazole administration on clinical and microbiologic parameters of periodontal disease. Clin Prev Dent. 1991;13:28.
33 Ellen RP, Gibbons RJ. Protein associated adherence of Streptococcus pyogenes to epithelial surfaces: prerequisite for virulence. Infect Immun. 1972;5:826.
34 Garnick JJ, Pearson R, Harrell D. The evaluation of the Periotron. J Periodontol. 1979;50:424.
35 Genco RJ, Zambon JJ, Murray PA. Serum and gingival fluid antibodies as an adjunct in the diagnosis of Actinobacillus actinomycetemcomitans–associated periodontal disease. J Periodontol. 1985;56:41.
36 Gibbons RJ, van Houte J. Selective bacterial adherence to oral epithelial surfaces and its role as an ecological determinant. Infect Immun. 1971;3:567.
37 Gibbons RJ, van Houte J. On the formation of dental plaques. J Periodontol. 1973;44:347.
38 Gibbons RJ, van Houte J, Liljemark WF. Some parameters that affect the adherence of S. salivarius to oral epithelial surfaces. J Dent Res. 1972;51:424.
39 Gilda JE, Keyes PH. Increased dental caries activity in the Syrian hamster following desalivation. Proc Soc Exp Biol Med. 1947;66:28.
40 Glas JE, Krasse B. Biophysical studies on dental calculus from germ-free and conventional rats. Acta Odontol Scand. 1962;20:127.
41 Gochman N, Meyer RK, Blackwell RQ, et al. The amino acid decarboxylase of salivary sediment. J Dent Res. 1959;38:998.
42 Golub LM, Borden SM, Kleinberg K. Urea content of gingival crevicular fluid and its relation to periodontal disease in humans. J Periodontal Res. 1971;6:243.
43 Gordon JM, Walker CB, Goodson JM, et al. Sensitive assay for measuring tetracycline levels in gingival crevice fluid. Antimicrob Agents Chemother. 1980;17:193.
44 Gowan M, Mundy GR. Actions of recombinant interleukin 1, interleukin 2 and interferon-gamma on bone resorption in vitro. J Immunol. 1986;136:2478.
45 Grant DA, Orban BJ. Leukocytes in the epithelial attachment. J Periodontol. 1960;31:87.
46 Gupta OH, Blechman H, Stahl SS. The effects of desalivation on periodontal tissues of the Syrian hamster. Oral Surg. 1960;13:470.
47 Hara K, Löe H. Carbohydrate components of the gingival exudate. J Periodontal Res. 1969;4:202.
48 Hasegawa K. Biochemical study of gingival fluid: lactic acid in gingival fluid. Bull Tokyo Med Dent Univ. 1967;14:359.
49 Holt SC, Bramanti TE. Factors in virulence expression and their role on periodontal disease pathogenesis. Crit Rev Oral Biol Med. 1991;2:177.
50 Iacono VC, Bolot PR, Mackay JB, et al. Lytic sensitivity of Actinobacillus actinomycetemcomitans to lysozyme. Infect Immun. 1983;40:773.
51 Isemura S, Ando K, Nakashizoka T, et al. Cystatin S: a cystein-proteinase inhibitor of human saliva. J Biochem. 1984;96:1311.
52 Jensen RL, Folke LEA. The passage of exogenous tritiated thymidine into gingival tissues. J Periodontol. 1974;45:786.
53 Jolles P, Petit JF. Purification and analysis of human saliva lysozyme. Nature. 1963;200:168.
54 Kahnberg KE, Lindhe J, Helden J. Initial gingivitis induced by topical application of plaque extract: a histometric study in dogs with normal gingiva. J Periodontal Res. 1976;11:218.
55 Kalmar JP, Arnold RP. Killing of Actinobacillus actinomycetemcomitans by human lactoferrin. Infect Immun. 1988;56:2552.
56 Kaslick RS, Mandel ID, Chasens AJ, et al. Concentration of inorganic ions in gingival fluid. J Dent Res. 1970;49:887.
57 Kaslick RS, Chasens AI, Mandel ID, et al. Quantitative analysis of sodium, potassium and calcium in gingival fluid from gingiva in varying degrees of inflammation. J Periodontol. 1970;41:93.
58 Kaslick RS, Chasens AI, Mandel ID, et al. Sodium, potassium and calcium in gingival fluid: a study of the relationship of the ions to one another, to circadian rhythms, gingival bleeding, purulence, and to conservative periodontal therapy. J Periodontol. 1970;41:442.
59 Kaslik RS, Chasens AI, Weinstein O, et al. Ultramicromethods for the collection of gingival fluid and quantitative analysis of its sodium content. J Dent Res. 1986;47:1192.
60 King JD. Experimental investigation of periodontal disease in the ferret and in man, with special reference to calculus formation. Dent Pract. 1954;4:157.
61 Kiroshita JJ, Muhlemann HR. Effect of sodium ortho and pyrophosphate on supragingival calculus. Helv Odontol Acta. 1966;10:46.
62 Kowolik MJ, Raeburn JA. Functional integrity of gingival crevicular neutrophil polymorphonuclear leukocytes as demonstrated by nitroblue tetrazolium reduction. J Periodontal Res. 1980;15:483.
63 Krekeler G. Quantitative determination of the gingival sulcus fluid by means of microcapillaries. Dtsch Zahnaertzl Z. 1975;30:544.
64 Lamster IB, Novak MJ. Host mediators in gingival crevicular fluid: implications for the pathogenesis of periodontal disease. Crit Rev Oral Biol Med. 1992;3:31.
65 Lamster IB, Celenti R, Ebersole J. The relationship of serum IgG antibody titers to periodontal pathogens to indicators of the host response in gingival crevicular fluid. J Clin Periodontol. 1990;17:419.
66 Leung SW, Jensen AT. Factors controlling the deposition of calculus. Int Dent J. 1958;8:613.
67 Liew V, Mack G, Tseng P, et al. Single-dose concentrations of timidazole in gingival crevicular fluid, serum and gingival tissue in adults with periodontitis. J Dent Res. 1991;70:910.
68 Life JS, Johnson NW, Powell JR, et al. Interleukin-1 beta (IL-1β) levels in gingival crevicular fluid from adults without previous evidence of destructive periodontitis: a cross sectional study. J Clin Periodontol. 1992;19:53.
69 Lindhe J, Attstrom R. Gingival exudation during the menstrual cycle. J Periodontal Res. 1967;2:194.
70 Lindhe J, Bjorn AL. Influence of hormonal contraceptives on the gingiva of women. J Periodontal Res. 1967;2:1.
71 Lindhe J, Attstrom R, Bjorn AL. Influence of sex hormones on gingival exudate of gingivitis-free female dogs. J Periodontal Res. 1968;3:273.
72 Lisanti VF. Hydrolytic enzymes in periodontal tissues. Ann NY Acad Sci. 1960;85:461.
73 Löe H, Holm-Pedersen P. Absence and presence of fluid from normal and inflamed gingiva. Periodontics. 1965;3:171.
74 Magnusson B. Mucosal changes at erupting molars in germ-free rats. J Periodontal Res. 1969;4:181.
75 Marcus ER, Jooste CP, Driver HS, Hatting J. The quantification of individual proteins in crevicular gingival fluid. J Periodontal Res. 1985;20:444.
76 Mandel I. Relation of saliva and plaque to caries. J Dent Res. 1974;53(Suppl):246.
77 Mandel ID. Markers of periodontal disease susceptibility and activity derived from saliva. In: Johnson NW, editor. Risk markers of oral diseases, vol 3. New York: Cambridge University Press; 1991.
78 Martin LP, Noble WH. Gingival fluid in relation to tooth mobility and occlusal interferences. J Periodontol. 1974;45:444.
79 McDougall WA. Pathways of penetration and effects of horseradish peroxidase in rat molar gingiva. Arch Oral Biol. 1970;15:621.
80 McLaughlin WS, Lovat FM, Macgregor IDM, et al. The immediate effects of smoking on gingival fluid flow. J Clin Periodontol. 1993;20:448.
81 Miyasaki KT, Wilson ME, Genco RJ. Killing of Actinobacillus actinomycetemcomitans by the human peroxide chloride system. Infect Immun. 1986;53:161.
82 Muhlemann HR, Schroeder H. Dynamics of supragingival calculus formation. Adv Oral Biol. 1964;1:175.
83 Nagao M. Influence of prosthetic appliances upon the flow of crevicular tissue fluid. I. Relation between crevicular tissue fluid and prosthetic appliances. Bull Tokyo Med Dent Univ. 1967;14:241.
84 Nakamura M, Slots J. Salivary enzymes: origin and relationship to periodontal disease. J Periodontal Res. 1983;18:559.
85 Novaes ABJr, Ruben MP, Kramer GM. Proteins of the gingival exudate: a review and discussion of the literature. J West Soc Periodontol. 1979;27:12.
86 Offenbacher S, Williams RC, Jeffcoat MK, et al. Effects of NSAIDs on beagle crevicular cyclo-oxygenase metabolites and periodontal bone loss. J Periodontal Res. 1992;27:207.
87 Ohlsson M, Rosengreen M, Tegner H, et al. Quantification of granulocyte elastase inhibitor in human mixed saliva and in pure parotid secretion. Phys Chem. 1983;364:1323.
88 Orban JE, Stallard RE. Gingival crevicular fluid: a reliable predictor of gingival health? J Periodontol. 1969;40:231.
89 Page RC. Host response tests designed for diagnosing periodontal disease. J Periodontol. 1992;63:356.
90 Passo SA, Tsai CC, McArthur WP, et al. Interaction of inflammatory cells and oral microorganisms. IX. The bacterial effect of human PMN leukocytes on isolated plaque microorganisms. J Periodontal Res. 1980;15:470.
91 Paunio K. On the hydroxyproline-containing components in the gingival exudate. J Periodontal Res. 1971;6:115.
92 Pollock JJ, Andors L, Gulumoglu A. Direct measurement of hepatitis B virus antibody and antigen markers in gingival crevicular fluid. Oral Surg Oral Med Oral Pathol. 1984;57:499.
93 Ranney RR, Montgomery EH. Vascular leakage resulting from topical application of endotoxin to the gingiva of the beagle dog. Arch Oral Biol. 1973;18:963.
94 Ranney RR, Zander HA. Allergic periodontal disease in sensitized squirrel monkeys. J Periodontol. 1970;41:12.
95 Ratcliff P. Permeability of healthy gingival epithelium by microscopically observable particles. J Periodontol. 1966;37:291.
96 Renggli HH. Phagocytosis and killing by crevicular neutrophils. In: Lehner T, editor. The borderland between caries and periodontal disease. New York: Grune & Stratton, 1977.
97 Renggli HH, Regolatti B. Intracrevicular sampling of leukocytes using plastic strips. Helv Odont Acta. 1972;16:93.
98 Rizzo AA. Histologic and immunologic evaluation of antigen penetration with oral tissues after topical application. Periodontics. 1970;41:210.
99 Rosebury R, Karshan M. Salivary calculus: dental science and dental art, Philadelphia. Lea & Febiger.. 1938.
100 Rovin S, Costich ER, Gordon HA. The influence of bacteria and irritation in the initiation of periodontal disease in germfree and conventional rats. J Periodontal Res. 1966;1:193.
101 Salonen JI, Paunio KU. An intracrevicular washing method for collection of intracrevicular contents. Scand J Dent Res. 1961;99:406.
102 Sandalli P, Wade AB. Alterations in crevicular fluid flow during healing following gingivectomy and flap procedures. J Periodontal Res. 1969;4:314.
103 Sano K, Nakao M, Shiba A, et al. An ultra-micro assay for proteins in biological fluids other than blood using a combination of agarose gel isoelectric focusing and silver staining. Clin Chim Acta. 1984;137:115.
104 Schiott CR, Löe H. The origin and variation in the number of leukocytes in the human saliva. J Periodontal Res. 1969;4(Suppl):24.
105 Schultz-Haudt S, Bibby BG, Bruce MA. Tissue destructive products of gingival bacteria from nonspecific gingivitis. J Dent Res. 1954;33:624.
106 Shapiro L, Goldman H, Bloom A. Sulcular exudate flow in gingival inflammation. J Periodontol. 1979;50:301.
107 Shen LS, Ghavamzadeh G, Shklar G. Gingival healing in sialadenectomized rats. J Periodontol. 1979;50:533.
108 Sibraa PD, Reinhardt AA, Dyer JK, et al. Acute-phase protein detection and quantification in gingival crevicular fluid by direct and indirect immuno-dot. J Clin Periodontol. 1991;18:101.
109 Simon B, Goldman HM, Ruben MP, et al. The role of endotoxin in periodontal disease. III. Correlation of the amount of endotoxin with the histologic degree of inflammation. J Periodontol. 1971;42:210.
110 Skapski H, Lehner T. A crevicular washing method for investigating immune components of crevicular fluid in man. J Periodontal Res. 1976;11:19.
111 Skougaard MR, Bay I, Kilnkhammer JM. Correlation between gingivitis and orogranulocytic migratory rate. J Dent Res. 1994;48:716.
112 Solis Gaffar MC, Rustogi KN, Gaffar A. Hydrogen sulfide production from gingival crevicular fluid. J Periodontol. 1980;51:603.
113 Squier CA, Johnson NW. Permeability of oral mucosa. Br Med Bull. 1975;31:169.
114 Steinberg AD, Steinberg J, Allen P, et al. The effect of alteration in the sulcular environment upon the movement of 14C-diphenylhydantoin through rabbit sulcular tissues. J Periodontal Res. 1976;11:47.
115 Sueda T, Bang J, Cimasoni G. Collection of gingival fluid for quantitative analysis. J Dent Res. 1969;48:159.
116 Suppipat W, Suppipat N. Evaluation of an electronic device for gingival fluid quantitation. J Periodontol. 1977;48:388.
117 Talonopoika JT, Hamalainen MM. Collagen III aminoterminal propeptide in gingival crevicular fluid before and after periodontal disease. Scand J Dent Res. 1992;100:107.
118 Tomasi TB, Bienenstock J. Secretory immunoglobulins. Adv Immunol. 1968;9:1.
119 Totan A, Greabu M, Totan C, Spinu T. Salivary aspartate aminotransferase, alanine aminotransferase and alkaline pohospharase: possible markers in periodontal disease. Clin Chem Lab Med.. 2006;44:612.
120 Van Winkelhoff AJ, van Steenberger TJM, de Graaff J. The role of black-pigmented Bacteroides in human oral infections. J Clin Periodontol. 1988;15:145.
121 Vogel JJ, Amdur BH. Inorganic pyrophosphate in parotid saliva. Arch Oral Biol. 1967;12:159.
122 Waerhaug J. The gingival pocket: anatomy, pathology deepening and elimination. Odont Tidskaift. 1952;60(Suppl 1):1.
123 Weinstein E, Mandel ID, Salkind A, et al. Studies of gingival fluid. Periodontics. 1967;5:161.
124 Williams RW, Gibbons RG. Inhibition of bacterial adherence by secretory immunoglobulin A: a mechanism of antigen disposal. Science. 1972;177:697.
125 Wilton JMA, Renggli HH, Lehner T. The isolation and identification of mononuclear cells from the gingival crevice in man. J Periodontal Res. 1976;11:243.
