CHAPTER 37 Periodontal Treatment of Medically Compromised Patients
Many patients seeking dental care have significant medical conditions that may alter both the course of their oral disease and the therapy provided. Older periodontal patients will have a greater likelihood of having underlying disease. Therefore the therapeutic responsibility of the clinician includes identification of the patient’s medical problems to formulate proper treatment plans. Thorough medical histories are paramount.82 If significant findings are unveiled, consultation with or referral of the patient to an appropriate physician may be indicated. This ensures correct patient management and provides medicolegal coverage to the clinician.
This chapter covers common medical conditions and associated periodontal management. Review of each topic area is general, and the reader is encouraged to consult other references for more detailed coverage of specific disorders. Understanding these conditions will enable the clinician to treat the total patient, not merely the periodontal reflection of underlying disease.
Cardiovascular Diseases
Cardiovascular diseases are the most prevalent category of systemic disease in the United States (US) and many other countries, and they are more common with increasing age.109 Health histories should be closely scrutinized for cardiovascular problems. These conditions include hypertension, angina pectoris, myocardial infarction (MI), previous cardiac bypass surgery, previous cerebrovascular accident (CVA), congestive heart failure (CHF), presence of cardiac pacemakers or automatic cardioverter-defibrillators, and infective endocarditis (IE).
In most cases the patient’s physician should be consulted, especially if stressful or prolonged treatment is anticipated. Short appointments and a calm, relaxing environment help minimize stress and maintain hemodynamic stability.
![]() Science Transfer
Science Transfer
In managing periodontal therapy in medically compromised patients, the clinician should always obtain a consultation from the patient’s physician, who will determine the necessary precautions that should accompany periodontal treatment. Changes in recommendations for medically compromised patients are continually occurring. Recently, changes have been suggested for protecting patients with artificial joints from the bacteremias associated with periodontal and other invasive dental procedures. Currently, the American Association of Orthopedic Surgeons (AAOS) recommends systemic antibiotic coverage to accompany dental procedures associated with bacteremias for all patients with artificial joints. Dentists should follow the recommendations from the patient’s orthopedic surgeon and utilize the appropriate protocol.
Dentists have the responsibility to understand the role of periodontal inflammation in accentuating certain systemic diseases (e.g., arteriosclerosis, diabetes, and preterm low-birth-weight infants. Thus all clinicians need to be cognizant of the systemic implications of periodontal diseases and their treatment and should stay up to date to provide accurate and detailed information during consultation with the patient’s physician.
Hypertension
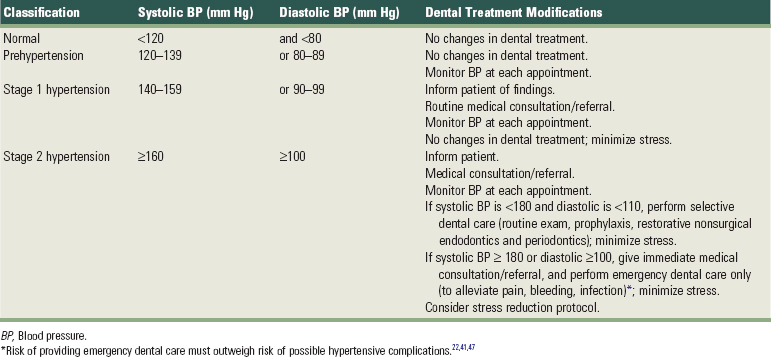
Hypertension, the most common cardiovascular disease, affects more than 50 million American adults, many of whom are undiagnosed.40 In 2003, the National Heart, Lung and Blood Institute issued revised guidelines for evaluation and management of hypertension.22,41,47 The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC-7) guidelines22 simplified the classification of blood pressure (Table 37-1).
Compared with previous classification3,6 schemes that focused on diastolic blood pressure (BP), the JNC-7 guidelines22 emphasize the importance of systolic BP greater than 140 mm Hg. Systolic blood pressure greater than 140 mm Hg is considered a greater risk factor for cardiovascular disease than elevated diastolic pressure. JNC-7 also introduced a category known as prehypertension to replace the more innocuous terms “high normal” and “borderline” hypertension. People with systolic BP between 120 and 139 mm Hg or diastolic BP between 80 and 89 mm Hg are classified as “prehypertensive.” Hypertension is now classified into only two categories versus three under past classification schemes for simplicity and because treatment for categories 2 and 3 was essentially the same. Stage 1 hypertension is defined by systolic pressure of 140 to 159 mm Hg or diastolic pressure of 90 to 99 mm Hg. Stage 2 hypertension is defined by a systolic pressure greater than 160 mm Hg or diastolic pressure greater than 100 mm Hg.
Hypertension is not diagnosed on a single elevated BP recording. Rather, classification is usually based on the average value of two or more BP readings taken at two or more appointments. The higher value of either the systolic or diastolic pressure determines the patient’s classification. Patients with hypertension enter the dental practice every day and are particularly common among the older population seen in most periodontal practices. Evidence from the Framingham Heart Study revealed that people with normal BP at age 55 still have a 90% risk of becoming hypertensive later in life.110
Hypertension is divided into primary and secondary types. Primary (essential) hypertension occurs when no underlying pathologic abnormality can be found to explain the disease. Approximately 95% of all hypertensive patients have primary hypertension. The remaining 5% have secondary hypertension, in which an underlying etiology can be found and often treated. Examples of the conditions responsible for secondary hypertension are renal disease, endocrinologic changes, and neurogenic disorders.
In early hypertension, the patient may be asymptomatic. If not identified and diagnosed, hypertension may persist and increase in severity, leading eventually to coronary artery disease, angina, MI, CHF, CVA, or kidney failure.54 The dental office can play a vital role in the detection of hypertension and maintenance care of the patient with hypertensive disease. The first dental office visit should include two BP readings spaced at least 10 minutes apart, which are averaged and used as a baseline. Before the clinician refers a patient to a physician because of elevated BP, readings should be taken at a minimum of two appointments, unless the measurements are extremely high (i.e., systolic pressure >180 mm Hg or diastolic pressure >100 mm Hg). The periodontal recall system is an ideal method for hypertension detection and monitoring. Almost three of every four adult patients with hypertension in the US do not control their BP well enough to attain the goal of systolic pressure less than 140 mm Hg and diastolic pressure less than 90 mm Hg.19 Lack of compliance with antihypertensive therapy is the primary reason for this failure. Dentists can help achieve greater success in managing hypertension by taking BP readings at each periodontal recall visit.
Periodontal procedures should not be performed until accurate BP measurements and histories have been taken to identify those patients with significant hypertensive disease. The time of day also should be recorded since BP varies significantly throughout the day.74 Table 37-1 outlines appropriate medical referral or consultation and dental treatment modifications, depending on the patient’s stage of hypertension.
Dental treatment for hypertensive patients is generally safe as long as stress is minimized.54,60 If a patient is currently receiving antihypertensive therapy, consultation with the physician may be warranted regarding the current medical status, medications, periodontal treatment plan, and patient management. Many physicians are not knowledgeable about the nature of specific periodontal procedures. The dentist must inform the physician regarding the estimated degree of stress, length of the procedures, and complexity of the individualized treatment plan. Morning dental appointments were once suggested for hypertensive patients. However, recent evidence indicates that BP generally increases around awakening and peaks at midmorning.15,74,102 Lower BP levels occur in the afternoon; therefore afternoon dental appointments may be preferred.
No routine periodontal treatment should be given to a patient who is hypertensive and not under medical management. For patients with systolic BP greater than 180 mm Hg or diastolic BP greater than 110 mm Hg, treatment should be limited to emergency care until hypertension is controlled. Analgesics are prescribed for pain and antibiotics for infection. Acute infections may require surgical incision and drainage, although the surgical field should be limited because excessive bleeding may be seen with elevated BP.
When treating hypertensive patients, the clinician should not use a local anesthetic containing an epinephrine concentration greater than 1 : 100,000 nor should a vasopressor be used to control local bleeding. Local anesthesia without epinephrine may be used for short procedures (<30 minutes). In a patient with hypertensive disease, however, it is important to minimize pain by providing profound local anesthesia to avoid an increase in endogenous epinephrine secretion.54,60
The benefits of the small doses of epinephrine used in dentistry far outweigh the potential for hemodynamic compromise. The smallest possible dose of epinephrine should be used, and aspiration before injection of local anesthetics is critical. Intraligamentary injection is generally contraindicated because hemodynamic changes are similar to intravascular injection.101 If the hypertensive patient exhibits anxiety, use of conscious sedation in conjunction with periodontal procedures may be warranted107 (see Chapter 55).
Beta-adrenergic receptor antagonists, or β-blockers, are typically used to treat hypertension (Table 37-2). β-blockers are either cardioselective, blocking only β-1 cardiac receptors (β1 receptors), or nonselective, blocking both β-1 cardiac receptors and β-2 peripheral receptors (β2 receptors). Epinephrine, an α-adrenergic and β-adrenergic agonist, produces an increase in heart rate through direct stimulation of cardiac β-1 receptors. Epinephrine also stimulates α-adrenergic receptors, producing vasoconstriction of arteries, as well as β-2 receptors, causing vasodilation of skeletal muscle arterioles. Administration of local anesthetics containing epinephrine to patients taking nonselective β-blockers (e.g., propranolol, nadolol) may cause elevated BP.119 Epinephrine-induced α-adrenergic stimulation results in vasoconstriction and increased BP. Because the patient’s nonselective medication has blocked the β-2 receptors, epinephrine will not stimulate the normal compensatory β-2 receptor–induced vasodilation. This may result in dramatically increased BP, followed by reflex bradycardia mediated by the vagus nerve and carotid baroreceptors. The end result is a patient with severe hypertension and bradycardia, resulting in a dangerous decrease in vascular perfusion and possible death. Because of this potential complication, epinephrine-containing local anesthetics should be used cautiously and only in very small amounts in patients taking nonselective β-blockers, with careful monitoring of vital signs.54,119
TABLE 37-2 Nonselective and Selective β-Adrenergic Receptor Antagonists (β-Blockers)
| Generic Name | Trade Name |
|---|---|
| Nonselective β-Blockers | |
| Carvedilol | Coreg |
| Carteolol hydrochloride | Cartrol |
| Nadolol | Corgard |
| Penbutolol sulfate | Levatol |
| Pindolol | Visken |
| Propranolol hydrochloride | Inderal; Inderal LA |
| Timolol maleate | Blocadren |
| Selective β-Blockers | |
| Acebutolol hydrochloride | Sectral |
| Atenolol | Tenormin |
| Betaxolol hydrochloride | Kerlone |
| Bisoprolol fumarate | Zebeta |
| Metoprolol tartrate | Lopressor |
| Metoprolol succinate | Toprol-XL |
The clinician should be aware of the many side effects of various antihypertensive medications. Postural hypotension is common and can be minimized by slow positional changes in the dental chair.54 Depression is a side effect of which many patients are unaware. Nausea, sedation, oral dryness, lichenoid drug reactions, and gingival overgrowth are associated with certain classes of antihypertensive agents.60
Ischemic Heart Diseases
Ischemic heart disease (Figure 37-1) includes disorders such as angina pectoris and myocardial infarction. Angina pectoris occurs when myocardial oxygen demand exceeds supply, resulting in temporary myocardial ischemia.41 Patients with a history of unstable angina pectoris (angina that occurs irregularly or on multiple occasions without predisposing factors) should be treated only for emergencies and then in consultation with their physician. Patients with stable angina (angina that occurs infrequently, is associated with exertion or stress, and is easily controlled with medication and rest) can undergo elective dental procedures. Because stress often induces an acute anginal attack, stress reduction is important. Profound local anesthesia is vital, and conscious sedation may be indicated for anxious patients107 (see Chapter 55). Supplemental oxygen delivered by nasal cannula may also help prevent intraoperative anginal attacks.
Patients who treat acute anginal attacks with nitroglycerin should be instructed to bring their medication to dental appointments. Nitroglycerin should also be kept in the office emergency medical kit. For particularly stressful procedures, the patient may take a nitroglycerin tablet preoperatively to prevent angina, although this generally is not necessary. The patient’s nitroglycerin should be readily accessible on the dental tray in case it is needed during treatment. Because the shelf-life of nitroglycerin is relatively short, the expiration date of the patient’s nitroglycerin should be noted, as should the expiration date of the nitroglycerin in the office’s emergency medical kit. Also, patients with angina may be taking longer-acting forms of nitroglycerin (tablet, patch), β-blockers, or calcium channel blockers (also used in treatment of hypertension) for prevention of angina. Restrictions on use of local anesthetics containing epinephrine are similar to those for the patient with hypertension. In addition, intraosseous injection with epinephrine-containing local anesthetics using special systems (e.g., Stabident, Fairfax Dental) should be done cautiously in patients with ischemic heart disease, because it results in transient increases in heart rate and myocardial oxygen demand.79
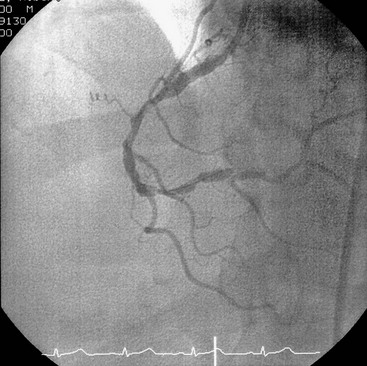
Figure 37-1 Coronary angiogram. Atherosclerosis can result in narrowing of the coronary arteries and onset of signs and symptoms of ischemic heart disease.
If the patient becomes fatigued or uncomfortable or has a sudden change in heart rhythm or rate during a periodontal procedure, the procedure should be discontinued as soon as possible. A patient who has an anginal episode in the dental chair should receive the following emergency medical treatment:
In recent years, nitroglycerin lingual spray formulations have been popular in hospital pharmacies because of the increased shelf life as compared to nitroglycerin tablets.73 The lingual spray has been reported to provide a greater and more rapid vasodilation with a longer duration of action.28,86 The convenience and advantages of a nitroglycerin lingual spray are appealing, but the accuracy of dose delivery has been questioned and warrants additional studies before it can be recommended to replace the known tablet regimen.73
Myocardial infarction (MI) is the other category of ischemic heart disease encountered in dental practice. Dental treatment is generally deferred for at least 6 months after MI because peak mortality occurs during this time.33 After 6 months, MI patients can usually be treated using techniques similar to those for the stable angina patient.
Cardiac (aortocoronary) bypass, femoral artery bypass, angioplasty, and endarterectomy have become common surgical procedures in patients with ischemic heart disease. If one of these procedures was performed recently, the physician should be consulted before elective dental therapy to determine the degree of heart damage or arterial occlusive disease, the stability of the patient’s condition, and the potential for infective endocarditis or graft rejection. Prophylactic antibiotics are not usually necessary for cardiac bypass patients unless recommended by the cardiologist.
Congestive Heart Failure
Congestive heart failure (CHF) is a condition in which the pump function of the heart is unable to supply sufficient amounts of oxygenated blood to meet the body’s needs.33 CHF usually begins with left ventricular failure caused by a disproportion between the hemodynamic load and the capacity to handle that load. CHF may be caused by a chronic increase in workload (as in hypertension or aortic, mitral, pulmonary, or tricuspid valvular disease), direct damage to the myocardium (as in MI or rheumatic fever), or an increase in the body’s oxygen requirements (as in anemia, thyrotoxicosis, or pregnancy).
Patients with poorly controlled or untreated CHF are not candidates for elective dental procedures. These individuals are at risk for sudden death, usually from ventricular arrhythmias.32 For patients with treated CHF, the clinician should consult with the physician regarding the severity of CHF, underlying etiology, and current medical management. Medical management of CHF may include use of calcium channel blockers, direct vasodilators, diuretics, angiotensin-converting enzyme (ACE) inhibitors, α-receptor blockers, and cardiotonic agents such as digoxin.30,46 Each of these medications has potential side effects that may have an impact on periodontal therapy. Because of the presence of orthopnea (inability to breathe unless in an upright position) in some CHF patients, the dental chair should be adjusted to a comfortable level for the patient rather than being placed in a supine position. Short appointments, stress reduction with profound local anesthesia and possibly conscious sedation, and use of supplemental oxygen should be considered.33,54
Cardiac Pacemakers and Implantable Cardioverter-Defibrillators
Cardiac arrhythmias are most often treated with medications; however, some are also treated with implantable pacemakers or automatic cardioverter-defibrillators.32,54,81 Pacemakers are usually implanted in the chest wall and enter the heart transvenously. Automatic cardioverter-defibrillators are more often implanted subcutaneously near the umbilicus and have electrodes passing into the heart transvenously or directly attached to the epicardium. Consultation with the patient’s physician allows determination of the underlying cardiac status, the type of pacemaker or automatic cardioverter-defibrillator, and any precautionary measures to be taken. Older pacemakers were unipolar and could be disrupted by dental equipment that generated electromagnetic fields, such as ultrasonic and electrocautery units. Newer units are bipolar and are generally not affected by dental equipment. Automatic cardioverter-defibrillators activate without warning when certain arrhythmias occur. This may endanger the patient during dental treatment because such activation often causes sudden patient movement. Stabilization of the operating field during periodontal treatment with bite blocks or other devices can prevent unexpected trauma.
Infective Endocarditis
Infective endocarditis (IE) is a disease in which microorganisms colonize the damaged endocardium or heart valves.35 Although the incidence of IE is low, it is a serious disease with a poor prognosis, despite modern therapy. The term infective endocarditis is preferred to the previous term bacterial endocarditis because the disease can also be caused by fungi and viruses. The organisms most often encountered in IE are α-hemolytic streptococci (e.g., Streptococcus viridans). However, nonstreptococcal organisms often found in the periodontal pocket have been increasingly implicated, including Eikenella corrodens, Aggregatibacter actinomycetemcomitans, Capnocytophaga, and Lactobacillus species.14
IE has been divided into acute and subacute forms. The acute form involves virulent organisms, generally nonhemolytic streptococci and strains of staphylococci, which invade normal cardiac tissue, produce septic emboli, and cause infections that run a rapid, generally fatal course. The subacute form, on the other hand, results from colony formation on damaged endocardium or heart valves by low-grade pathogenic organisms; the classic example is rheumatic carditis consequent to rheumatic fever.
Since the last American Heart Association (AHA) publication on prevention of IE in 1997,25 many have questioned the efficacy of antimicrobial prophylaxis to prevent IE in patients who undergo dental or other procedures and have suggested that the AHA guidelines should be revised.29,103 Members of the Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the AHA Council on Cardiovascular Disease in the Young, and a national and international group of experts on IE extensively reviewed data published on the prevention of IE. The committee concluded that only an extremely small number of IE cases might be prevented by antibiotic prophylaxis for dental procedures (even if such therapy was 100% effective). Consequently, the guidelines were changed and published in a 2008 report.117 The new guidelines advise that IE prophylaxis should only be recommended for cardiac conditions with the highest risk of adverse outcome from IE (Box 37-1). For these patients, antibiotic prophylaxis is recommended for all dental procedures that involve manipulation of the gingival tissues, periapical tissues, or perforation of the oral mucosa. Antibiotic prophylaxis is not indicated for individuals on the basis of an increased lifetime risk of contracting IE.
BOX 37-1 Cardiac Conditions Associated with the Highest Risk of Adverse Outcome from Endocarditis for which Prophylaxis with Dental Procedures Is Recommended*
The practice of periodontics is intimately concerned with the prevention of IE. However, bacteremia may occur even in the absence of dental procedures, especially in individuals with poor oral hygiene and significant periodontal inflammation. In fact, IE is much more likely to result from frequent exposure to random bacteremias associated with daily activities than caused by a dental procedure.117 Thus the prevention of periodontal inflammation is paramount. The AHA states that patients who are at risk for IE should “establish and maintain the best possible oral health to reduce potential sources of bacterial seeding.” To provide adequate preventive measures for IE, the clinician’s major concern should be to reduce the microbial population in the oral cavity so as to minimize soft tissue inflammation and bacteremia.
Preventive measures to reduce the risk of IE should consist of the following:
TABLE 37-3 Recommended Antibiotic Prophylaxis Regimens for Periodontal Procedures in Adults At Risk for Infective Endocarditis
| Regimen | Antibiotic | Dosage* |
|---|---|---|
| Standard oral regimen | Amoxicillin | 2.0 g 30–60 minutes before procedure |
| Alternate regimen for patients allergic to amoxicillin/penicillin | Clindamycin | 600 mg 30–60 minutes before procedure |
| or | ||
| Azithromycin or clarithromycin | 500 mg 30–60 minutes before procedure | |
| or | ||
| Cephalexin or cefadroxil† | 2.0 g 30–60 minutes before procedure | |
| Patients unable to take oral medications | Ampicillin | 2.0 g intramuscularly or intravenously within 30 minutes before procedure |
| Patients unable to take oral medications and allergic to penicillin | Clindamycin | 600 mg intravenously within 30 minutes before procedure (must be diluted and injected slowly) |
| or | ||
| Cefazolin* | 1.0 g intramuscularly or intravenously within 30 minutes before procedure | |
* Adult dosages listed. Children’s dosages are lower.
† Cephalosporins should not be used in patients with immediate-type hypersensitivity reactions to penicillins (e.g., urticaria, angioedema, anaphylaxis).
The following guidelines should aid in the development of periodontal treatment plans for patients susceptible to IE:
Cerebrovascular Accident
A cerebrovascular accident (CVA), or stroke, results from ischemic changes (e.g., cerebral thrombosis caused by an embolus) or hemorrhagic phenomena. Hypertension and atherosclerosis are predisposing factors for CVA and should alert the clinician to evaluate the patient’s medical history carefully for the possibility of early cerebrovascular insufficiency and to be aware of symptoms of the disease. A physician’s referral should precede periodontal therapy if the signs and symptoms of early cerebrovascular insufficiency are evident.
To prevent a repeat stroke, active infections should be treated aggressively because even minor infection may alter blood coagulation and trigger thrombus formation and ensuing cerebral infarction. The clinician should counsel the patient about the importance of thorough oral hygiene.83 Poststroke weakness of the facial area or paralysis of extremities may make oral hygiene procedures extremely difficult.65 The clinician may need to modify oral hygiene instruments for ease of use, perhaps in consultation with an occupational therapist. Long-term chlorhexidine rinses may greatly aid in plaque control.
Dental clinicians should treat post-CVA patients with the following guidelines in mind:
Endocrine Disorders
Diabetes
The diabetic patient requires special precautions before periodontal therapy. The two major types of diabetes are type 1 (formerly known as “insulin-dependent diabetes”) and type 2 (formerly called “non–insulin-dependent diabetes”).56 Over the past decade, the medical management of diabetes has changed significantly in an effort to minimize the debilitating complications associated with this disease.2,7 Patients are more tightly managing their blood glucose levels (glycemia) through diet, oral agents, and insulin therapy.55
If the clinician detects intraoral signs of undiagnosed or poorly controlled diabetes, a thorough history is indicated.78 The classic signs of diabetes include polydipsia (excessive thirst), polyuria (excessive urination), and polyphagia (excessive hunger, often with unexplained concurrent weight loss). If the patient has any of these signs or symptoms, or if the clinician’s index of suspicion is high, further investigation with laboratory studies and physician consultation is indicated. Periodontal therapy has limited success in the presence of undiagnosed or poorly controlled diabetes.
If a patient is suspected of having undiagnosed diabetes, the following procedures should be performed:
BOX 37-2 Data from American Diabetes Association: Diabetes Care 26(suppl 1):5, 2003.
Diagnostic Criteria for Diabetes Mellitus
Diabetes mellitus may be diagnosed by any one of three different laboratory methods. Whichever method is used, it must be confirmed on a subsequent day by using any one of the following three methods.
* The third method is not recommended for routine clinical use.
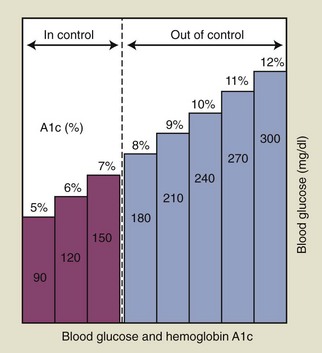
If a patient is known to have diabetes, it is critical that the level of glycemic control be established before initiating periodontal treatment. The fasting glucose and casual glucose tests provide “snapshots” of the blood glucose concentration at the time the blood was drawn; these tests reveal nothing about long-term glycemic control. The primary test used to assess glycemic control in a known diabetic individual is the glycosylated (or glycated) hemoglobin (Hb) assay (Box 37-3). Two different tests are available, the HbA1 and the HbA1c assay; the HbA1c is used more often.55 This assay has been shown by a large international study to provide an accurate measure of the average blood glucose concentrations over the preceding 2 to 3 months.63 Table 37-4 lists the average blood glucose concentrations for HbA1c values from that study, and Figure 37-2 is a simplified graphic representation of the data. The therapeutic goal for many patients is to achieve and maintain an HbA1c below 8%. Patients with relatively well-controlled diabetes (HbA1c < 8%) usually respond to therapy in a manner similar to nondiabetic individuals.23,105,116 Poorly controlled patients (HbA1c > 10%) often have a poor response to treatment, with more postoperative complications and less favorable long-term results56,105 (see Figure 27-3). Improvements in HBA1c values after periodontal therapy may provide an indication of the potential response.
BOX 37-3 Laboratory Evaluation of Diabetes Control: Glycosylated Hemoglobin Assay (HbA1c)*
| 4%–6% | Normal |
| <7% | Good diabetes control |
| 7%–8% | Moderate diabetes control |
| >8% | Action suggested to improve diabetes control |
* American Diabetes Association guidelines.
TABLE 37-4 Comparison of Hba1c Values to Estimated Average Glucose Measurements
| HbA1c (%) | Estimated Average Glucose (mg/dl) |
|---|---|
| 5.0 | 97 |
| 5.5 | 111 |
| 6.0 | 126 |
| 6.5 | 140 |
| 7.0 | 154 |
| 7.5 | 169 |
| 8.0 | 183 |
| 8.5 | 197 |
| 9.0 | 212 |
| 9.5 | 226 |
| 10.0 | 240 |
| 10.5 | 255 |
| 11.0 | 269 |
| 11.5 | 283 |
| 12.0 | 298 |
As discussed in Chapter 27, periodontal infection may worsen glycemic control and should be managed aggressively. Diabetic patients with periodontitis should receive oral hygiene instructions, mechanical debridement to remove local factors, and regular maintenance. When possible, an HbA1c of less than 10% should be established before surgical treatment is performed. Systemic antibiotics are not needed routinely, although recent evidence indicates that tetracycline antibiotics in combination with scaling and root planing may positively influence glycemic control. If the patient has poor glycemic control and surgery is absolutely needed, prophylactic antibiotics may be given; penicillins are most often used for this purpose. Frequent reevaluation after active therapy is needed to assess treatment response and prevent recurrence of periodontitis.
Almost all diabetic patients use glucometers for immediate blood glucose self-monitoring. These devices use capillary blood from a simple fingerstick to provide blood glucose readings in seconds. Diabetic patients should be asked whether they have glucometers and how often they use them. Because these devices provide instantaneous assessment of blood glucose, they are highly beneficial in the dental office environment. The following guidelines should be observed:
The most common dental office complication seen in diabetic patients taking insulin is symptomatic low blood glucose, or hypoglycemia (Box 37-4). Hypoglycemia is also associated with the use of numerous oral agents (Table 37-5). In patients receiving conscious sedation, the warning signs of an impending hypoglycemic episode may be masked, making the patient’s glucometer one of the best diagnostic aids. Hypoglycemia does not usually occur until blood glucose levels fall below 60 mg/dl. However, in patients with poor glycemic control who have prolonged hyperglycemia (high blood glucose levels), a rapid drop in glucose can precipitate signs and symptoms of hypoglycemia at levels well above 60 mg/dl.
TABLE 37-5 Oral Agents Used in Management of Diabetes
| Agent | Action | Risk of Hypoglycemia |
|---|---|---|
| Stimulate pancreatic insulin secretion | ++ | |
| Stimulate pancreatic insulin secretion | +++ | |
| Stimulate pancreatic insulin secretion | + | |
| Stimulate rapid pancreatic insulin secretion (different mechanism than sulfonylureas) | + | |
| Block production of glucose by liver; improve tissue sensitivity to insulin | − | |
| Improve tissue sensitivity to insulin | − | |
| Slow absorption of some carbohydrates from gut, decreasing postprandial peaks in glycemia | − | |
| Inhibit the enzyme DPP-4; enable the pancreas to produce more insulin but only after food ingestion | − | |
| Combination agents | Combine two different oral agents into single drug | Risk depends on which drugs are combined |
As medical management of diabetes has intensified over the last decade, the incidence of severe hypoglycemia has risen.5 The clinician should question diabetic patients about past episodes of hypoglycemia. Hypoglycemia is more common in patients with better glycemic control. When planning dental treatment, it is best to schedule appointments before or after periods of peak insulin activity. This requires knowledge of the pharmacodynamics of the drugs being taken by the diabetic patient. Patients taking insulin are at greatest risk, followed by those taking sulfonylurea agents. Metformin and thiazolidinediones generally do not cause hypoglycemia (see Table 37-5).
Insulins are classified as rapid-acting, short-acting, intermediate-acting, or long-acting agents (Table 37-6). The categories vary in their onset, peak, and duration of activity. It is important that the clinician establish exactly which insulins the diabetic patient takes, the amount, the number of times per day, and the time of the last dose. Periodontal treatment often can be timed to avoid peak insulin activity. Many diabetic patients take multiple injections each day, in which case it is difficult, if not impossible, to avoid peak insulin activity. Checking the pretreatment glucose with the patient’s glucometer, checking again during long procedures, and checking again at the end of the procedure provides a better understanding of the patient’s insulin pharmacodynamics and help prevent hypoglycemia.
If hypoglycemia occurs during dental treatment, therapy should be immediately terminated. If a glucometer is available, the blood glucose level should be checked. Treatment guidelines include the following55:
Emergencies resulting from hyperglycemia are rare in the dental office. They generally take days to weeks to develop. However, the glucometer may be used to rule out hyperglycemic emergencies such as diabetic ketoacidosis, a life-threatening event.
Because periodontal therapy may render the patient unable to eat for some time, adjustment in insulin or oral agent dosages may be required. It is absolutely critical that patients eat their normal meal before dental treatment. Taking insulin without eating is the primary cause of hypoglycemia. If the patient is restricted from eating before treatment (e.g., for conscious sedation), normal insulin doses will need to be reduced. As a general guideline, well-controlled diabetic patients having routine periodontal treatment may take their normal insulin doses as long as they also eat their normal meal. If the procedures are going to be particularly long, the insulin dose before treatment may need to be reduced. Likewise, if the patient will have dietary restrictions after treatment, insulin or sulfonylurea dosages may need to be reduced.
Consultation with the patient’s physician is prudent and allows both practitioners to review the proposed treatment plan and determine any modifications needed. When periodontal surgery is indicated, it is usually best to limit the size of the surgical fields so that the patient will be comfortable enough to resume a normal diet immediately.
Thyroid and Parathyroid Disorders
Periodontal therapy requires minimal alterations in the patient with adequately managed thyroid disease.70,97 Patients with thyrotoxicosis and those with inadequate medical management should not receive periodontal therapy until their conditions are stabilized. Patients with a history of hyperthyroidism should be carefully evaluated to determine the level of medical management, and they should be treated in a way that limits stress and infection. Hyperthyroidism may cause tachycardia and other arrhythmias, increased cardiac output, and myocardial ischemia. Medications such as epinephrine and other vasopressor amines should be given with caution in patients with treated hyperthyroidism, although the small amounts used in dental anesthetics rarely cause problems.97,119 These drugs should not be given to patients with thyrotoxicosis or poorly controlled thyroid disorders. Patients with hypothyroidism require careful administration of sedatives and narcotics because of the potential for excessive sedation.
Routine periodontal therapy may be provided to patients with parathyroid disease once that disorder has been identified and the proper medical treatment given. However, patients who have not received medical care may have significant renal disease, uremia, and hypertension. Also, if hypercalcemia or hypocalcemia is present, the patient may be more prone to cardiac arrhythmias.
Adrenal Insufficiency
Acute adrenal insufficiency is associated with significant morbidity and mortality as a result of peripheral vascular collapse and cardiac arrest. Therefore, the periodontist should be aware of the clinical manifestations (Box 37-5 online) and ways of preventing acute adrenal insufficiency in patients with histories of primary adrenal insufficiency (Addison’s disease) or secondary adrenal insufficiency (most often caused by use of exogenous glucocorticosteroids).
The use of systemic corticosteroids is common in patients with allergic, endocrine, respiratory, joint, intestinal, neurologic, renal, liver, skin, and connective tissue disorders. Significant complications associated with corticosteroid use include alterations in glucose metabolism (steroid-induced diabetes), increased risk of infection, altered wound healing, osteoporosis, skin disorders, cataracts, glaucoma, and suppression of the hypothalamic-pituitary-adrenal (HPA) axis.37,54 In the normal healthy patient, stress activates the HPA axis, stimulating increased endogenous cortisol production by the adrenal glands. Exogenous steroids may suppress the HPA axis and impair the patient’s ability to respond to stress with increased endogenous cortisol production, leading to the potential for acute adrenal crisis (see Box 37-5). The degree of adrenal suppression depends on the drugs used, dose, duration of administration, time elapsed since steroid therapy was terminated, and route of administration.
It has been common practice in the past to administer prophylactic systemic steroids before dental treatment for patients who are taking or who recently have taken exogenous steroids. Such steroid supplementation may not be required for many periodontal procedures.37 In fact, adrenal crisis is rare in dentistry, especially when associated with secondary adrenal suppression caused by steroid use.59 Shapiro et al96 found that patients taking 5 to 20 mg/day of prednisone maintained at least some adrenal reserve after immediate termination of steroid therapy. Higher doses may suppress the adrenal glands to a greater degree. Although exogenous steroids may suppress normal adrenal cortisol secretion for an extended period, the ability of the adrenal gland to respond to stress may return quickly after termination of steroid therapy.
Despite its rarity, the severe consequences of adrenal crisis suggest caution in patient management. Before providing extensive dental treatment to a patient with a history of recent or current steroid use, physician consultation is indicated to determine whether the patient’s treatment needs warrant supplemental steroids. Use of a stress reduction protocol and profound local anesthesia will help minimize the physical and psychologic stress associated with therapy and reduce the risk of acute adrenal crisis.
Current evidence indicates that a vast majority of individuals with adrenal insufficiency can receive routine dental treatment without the need for supplemental glucocorticosteroids.18,48 Patients currently taking corticosteroids generally have enough exogenous and endogenous cortisol to handle routine dental procedures if their usual dose is taken within two hours of the planned procedure. Thus, for most patients, supplemental corticosteroid administration is not required when uncomplicated minor surgical procedures, including periodontal surgery, are performed with local anesthesia with or without sedation.48
Individuals who may be at risk for adrenal crisis requiring supplementation include those who are undergoing lengthy, major surgical procedures, those expected to have significant blood loss, and those who have extremely low adrenal function. For these individuals, consultation with their physician and steroid supplementation is indicated. Low adrenal function can be identified with an adrenocorticotropic hormone (ACTH) stimulation test. A rapid assay is also available to determine the degree of adrenal reserve by measurement of serum cortisol levels 30 and 60 minutes after IV administration of synthetic corticotropin.96
For the patient who is identified as being at risk, the need for corticosteroid prophylaxis depends on the drug used because of the variance in equivalent therapeutic doses (Table 37-7 online).
TABLE 37-7 Equivalent Doses of Corticosteroids
| Corticosteroid | Equivalent Dose (mg) |
|---|---|
| Cortisone | 25 |
| Hydrocortisone | 20 |
| Prednisone | 5 |
| Prednisolone | 5 |
| Methyl prednisone | 5 |
| Methylprednisolone | 4 |
| Triamcinolone | 4 |
| Dexamethasone | 0.75 |
| Betamethasone | 0.6 |
Glucocorticosteroid coverage regimens vary, but most provide a twofold to fourfold increase in coverage, depending on the stress produced by the procedure. In an emergency situation, when testing is not possible, increasing the steroid dose before the procedure can decrease the chances of acute adrenal crisis.
Management of the patient in an acute adrenal insufficiency crisis is as follows:
Hemorrhagic Disorders
Patients with a history of bleeding problems caused by disease or drugs should be managed to minimize risks of hemorrhage. Identification of these patients through the health history, clinical examination, and clinical laboratory tests is paramount. Health questioning should cover (1) history of bleeding after previous surgery or trauma, (2) past and present drug history, (3) history of bleeding problems among relatives, and (4) illnesses associated with potential bleeding problems.
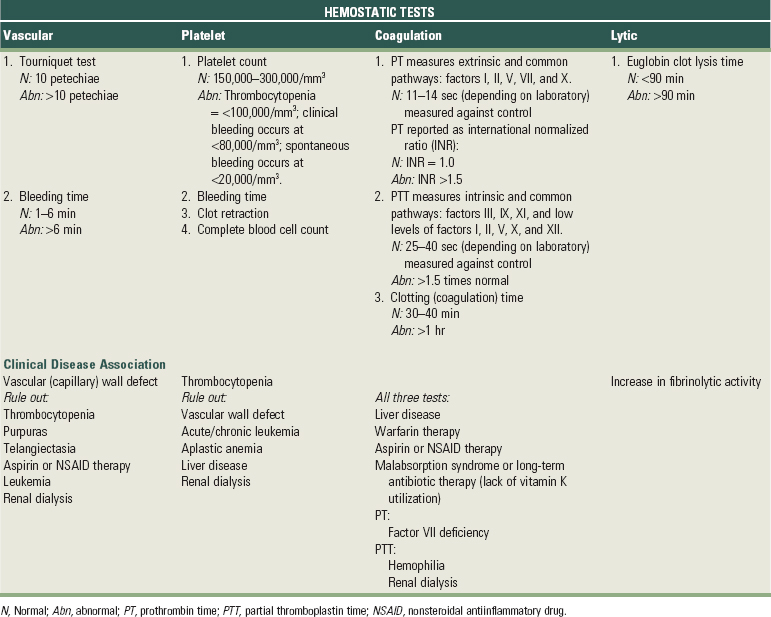
Clinical examination should detect the presence of jaundice, ecchymosis, spider telangiectasia, hemarthrosis, petechiae, hemorrhagic vesicles, spontaneous gingival bleeding, and gingival hyperplasia. Laboratory tests should include methods to measure the hemostatic, coagulation, or lytic phases of the clotting mechanism, depending on clues regarding which phase is involved (Table 37-8). These tests include bleeding time, tourniquet test, complete blood cell count, prothrombin time (PT), partial thromboplastin time (PTT), and coagulation time.
Bleeding disorders may be classified as coagulation disorders, thrombocytopenic purpuras, or nonthrombocytopenic purpuras.
Coagulation Disorders
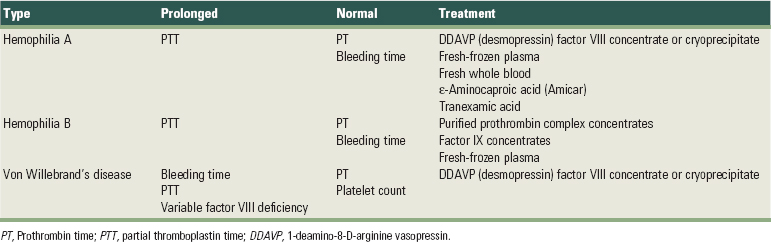
The main inherited coagulation disorders include hemophilia A, hemophilia B and von Willebrand’s disease78,88 (Table 37-9 online). Hemophilia A results in a deficiency of coagulation factor VIII, and the clinical severity of the disorder depends on the level of factor VIII remaining.67 Patients with severe hemophilia who have less than 1% of normal factor VIII levels may have severe bleeding on the slightest provocation, whereas those with more moderate hemophilia (1% to 5% factor VIII) have less frequent spontaneous hemorrhage but still bleed with minimal trauma.54 Patients with mild hemophilia (6% to 30% factor VIII) rarely bleed spontaneously but may still have hemorrhage after severe trauma or during surgical procedures. The clinician should consult the patient’s physician before dental treatment to determine the risk for bleeding and treatment modifications required. To prevent surgical hemorrhage, factor VIII levels of at least 30% are needed.54,67 Parenteral 1-deamino-8-D-arginine vasopressin (DDAVP; desmopressin) can be used to raise factor VIII levels twofold to threefold in patients with mild or moderate hemophilia. DDAVP has the significant advantage of avoiding the risk of viral disease transmission from factor VIII infusion and is considered the drug of choice in responsive patients. Most patients with moderate and severe hemophilia require infusion of factor VIII concentrate before surgical procedures. Before 1985, the risk of viral disease transmission from these infusions was high. In recent years, virally safe, highly purified monoclonal antibody or recombinant DNA factor VIII products have come into widespread use.
Hemophilia B, or Christmas disease, results in a deficiency of factor IX. The severity of the disorder depends on the relative amount of existing factor IX. Surgical therapy requires a factor IX level of 30% to 50% and is usually achieved by administration of purified prothrombin complex concentrates or factor IX concentrates.67
Von Willebrand’s disease results from a deficiency of von Willebrand factor, which mediates adhesion of platelets to the injured vessel wall and is required for primary hemostasis. Von Willebrand factor also carries the coagulant portion of factor VIII in the plasma. The disorder has three major subtypes with a wide range of clinical severity. In fact, many cases of von Willebrand’s disease go undiagnosed, and bleeding during dental treatment may be the first sign of the underlying disease. More severe forms require preoperative factor VIII concentrate or cryoprecipitate infusion. Patients with milder forms respond favorably to administration of DDAVP before periodontal surgery or tooth extraction.67,68
Periodontal treatment may be performed in patients with these coagulation disorders, provided that sufficient precautions are taken. Probing, scaling, and prophylaxis can usually be done without medical modification. More invasive treatment, such as local block anesthesia, root planing, or surgery, dictate prior physician consultation.
During treatment, local measures to ensure clot formation and stability are of major importance. Complete wound closure and application of pressure will reduce hemorrhage. Antihemostatic agents, such as oxidized cellulose or purified bovine collagen, may be placed over surgical sites or into extraction sockets. The antifibrinolytic agent ε-aminocaproic acid (Amicar), given orally or via IV, is a potent inhibitor of initial clot dissolution.44 Tranexamic acid is a more potent antifibrinolytic agent than Amicar and has been shown to prevent excessive oral hemorrhage after periodontal surgery and tooth extraction.77 It is available as an oral rinse and may be used either alone or in combination with systemic tranexamic acid for several days after surgery.99
Not all coagulation disorders are hereditary. Liver disease may affect all phases of blood clotting because most coagulation factors are synthesized and removed by the liver. Long-term alcohol abusers or chronic hepatitis patients often demonstrate inadequate coagulation. Coagulation may be impaired by vitamin K deficiency, often caused by malabsorption syndromes, or by prolonged antibiotic administration, which alters the intestinal microflora that produces vitamin K. Dental treatment planning for patients with liver disease should include the following:
Anticoagulant Medications
The most common cause of abnormal coagulation may be drug therapy. Patients with prosthetic heart valves or histories of MI, CVA, or thromboembolism are frequently placed on anticoagulant therapy using coumarin derivatives such as dicumarol and warfarin.42,51 These drugs are vitamin K antagonists that decrease production of vitamin K–dependent coagulation factors II, VII, IX, and X. The effectiveness of anticoagulation therapy is monitored by PT laboratory test. The recommended level of therapeutic anticoagulation for most patients is an INR of 2.0 to 3.0, with prosthetic heart valve patients generally in the 2.5 to 3.5 range.42 Traditional recommendations for periodontal treatment are as follows:
NOTE: Discontinuing anticoagulant therapy before dental surgery (as previously stated) was common in the past. However, many clinicians may no longer recommend discontinuing anticoagulation for many procedures because this has significant potential risks to patient health.43,90 Recent evidence related to the risks of altering anticoagulant therapy along with the lack of evidence for bleeding complications suggest that treating patients without reducing or discontinuing medications may be more prudent. See later section on anticoagulant/antiplatelet therapy under section for a detailed explanation of these more recent considerations.
Heparin is generally used for short-term anticoagulation and is given IV (usually in a hospital environment). It is a powerful anticoagulant with a duration of action of 4 to 8 hours. Periodontal treatment is rarely required while a patient is taking heparin.
Antiplatelet Medications
Aspirin interferes with normal platelet aggregation and can result in prolonged bleeding. Because it binds irreversibly to platelets, the effects of aspirin last at least 4 to 7 days. Aspirin is generally used in small doses of 325 mg or less per day, which usually does not alter bleeding time. In general, patients taking low doses of aspirin daily do not need to discontinue aspirin therapy before periodontal procedures.90 However, higher doses may increase bleeding time and predispose the patient to postoperative bleeding.54 For patients taking more than 325 mg of aspirin per day, aspirin may need to be discontinued 7 to 10 days before surgical therapy that might result in significant bleeding, in consultation with the physician. Nonsteroidal antiinflammatory drugs (NSAIDs), such as ibuprofen, also inhibit platelet function. Because NSAIDs bind reversibly, the effect is transitory, lasting only a short time after the last drug dose. The bleeding time is used when questions arise about the potential effect of aspirin or NSAIDs. Aspirin should not be prescribed for patients who are receiving anticoagulation therapy or who have illnesses related to bleeding tendencies.
Thrombocytopenic Purpuras
Thrombocytopenia is defined as a platelet count of less than 100,000/mm3. Bleeding caused by thrombocytopenia may be seen with idiopathic thrombocytopenic purpuras, radiation therapy, myelosuppressive drug therapy (e.g., chemotherapy), leukemia, or infections. Purpuras are hemorrhagic diseases characterized by extravasation of blood into the tissues under the skin or mucosa, producing spontaneous petechiae (small red patches) or ecchymoses (bruises).
Periodontal therapy for patients with thrombocytopenia should be directed toward reducing inflammation by removing local irritants to avoid the need for more aggressive therapy.54,67 Oral hygiene instructions and frequent maintenance visits are paramount. Physician referral is indicated for a definitive diagnosis and to determine any alterations in planned therapy. Scaling and root planing are generally safe unless platelet counts are less than 60,000/mm3. No surgical procedures should be performed unless the platelet count is greater than 80,000/mm3. Platelet transfusion may be required before surgery. Surgical technique should be as atraumatic as possible, and local hemostatic measures should be applied.
Nonthrombocytopenic Purpuras
Nonthrombocytopenic purpuras result from either vascular wall fragility or thrombasthenia (impaired platelet aggregation). Vascular wall fragility may result from hypersensitivity reactions, scurvy, infections, chemicals (phenacetin, aspirin), dysproteinemia, and other causes. Thrombasthenia occurs in uremia, Glanzmann’s disease, aspirin ingestion, and von Willebrand’s disease.67 Both types of nonthrombocytopenic purpura may result in immediate bleeding after gingival injury. Treatment consists primarily of direct pressure applied for at least 15 minutes. This initial pressure should control the bleeding unless coagulation times are abnormal or reinjury occurs. Surgical therapy should be avoided until the qualitative and quantitative platelet problems are resolved.
Blood Dyscrasias
Numerous disorders of red and white blood cells may affect the course of periodontal therapy. Alterations in wound healing, bleeding, tissue appearance, and susceptibility to infection may occur. Clinicians should be aware of the clinical signs and symptoms of blood dyscrasias, availability of screening laboratory tests, and need for physician referral.
Leukemia
Altered periodontal treatment for patients with leukemia is based on their enhanced susceptibility to infections, bleeding tendency, and the effects of chemotherapy.54 The treatment plan for leukemia patients is as follows:
Agranulocytosis
Patients with agranulocytosis (cyclic neutropenia and granulocytopenia) have an increased susceptibility to infection. The total white blood cell count is reduced, and granular leukocytes (neutrophils, eosinophils, basophils) are reduced or disappear. These disorders are often marked by early, severe periodontal destruction.115 When possible, periodontal treatment should be done during periods of disease remission. At such times, treatment should be as conservative as possible while reducing potential sources of systemic infection. After physician consultation, severely affected teeth should be extracted. Oral hygiene instruction should include use of chlorhexidine rinses twice daily. Scaling and root planing should be performed carefully under antibiotic protection.
Renal Diseases
The most common causes of renal failure are glomerulonephritis, pyelonephritis, kidney cystic disease, renovascular disease, drug nephropathy, obstructive uropathy, and hypertension.39,45 Renal failure may result in severe electrolyte imbalances, cardiac arrhythmias, pulmonary congestion, CHF, and prolonged bleeding.54 Because the dental management of patients with renal disease may need to be drastically altered, physician consultation is necessary to determine the stage of renal disease, regimen for medical management, and alterations in periodontal therapy. The patient in chronic renal failure has a progressive disease that ultimately may require kidney transplantation or dialysis. It is preferable to treat the patient before, rather than after, transplant or dialysis.
The following treatment modifications should be used:
The patient who is receiving dialysis requires modifications in treatment planning.39,45 The three modes of dialysis are intermittent peritoneal dialysis (IPD), chronic ambulatory peritoneal dialysis (CAPD), and hemodialysis. Only hemodialysis patients require special precautions. These patients have a high incidence of viral hepatitis, anemia, and prolonged hemorrhage. The risk for hemorrhage is related to anticoagulation during dialysis, platelet trauma from dialysis, and the uremia that develops with renal failure.54 Hemodialysis patients have either an internal arteriovenous fistula or an external arteriovenous shunt. This shunt is often located in the arm and must be protected from trauma. Thus, in addition to guidelines for patients with chronic renal disease, the following recommendation are made for those receiving hemodialysis:
The renal transplant patient’s greatest foe is infection. Transplant patients take immunosuppressive drugs that greatly reduce resistance to infection.80 Excessive bleeding may occur during or after periodontal treatment because of drug-induced thrombocytopenia, anticoagulation, or both. A periodontal abscess is a potentially life-threatening situation; therefore a dental team approach should be used before transplantation to determine which teeth can be easily maintained. Many organ transplant centers now include dental examination in their standard pretransplant protocol. Teeth with severe bone and attachment loss, furcation invasion, periodontal abscesses, or extensive surgical requirements should be extracted, leaving an easily maintainable dentition. In addition to the recommendations for patients with chronic renal failure, the following should be considered for the renal transplant patient:
Liver Diseases
Liver diseases may range from mild conditions to complete liver failure. Major causes of liver disease include drug toxicity, cirrhosis, viral infections (e.g., hepatitis B and C), neoplasms, and biliary tract disorders.121 Because the liver is the site of production for most of the clotting factors, excessive bleeding during or after periodontal treatment may occur in patients with severe liver disease. Many drugs are metabolized in the liver; thus liver disease alters normal drug metabolism. Treatment recommendations for patients with liver disease include the following:
For the liver transplant patient, as with kidney and other organ transplantation, infection is a major concern. Transplant patients take immunosuppressive drugs that greatly reduce resistance to infection.80 Excessive bleeding may occur during or after periodontal treatment because of drug-induced thrombocytopenia, anticoagulation, or both. Dental or periodontal infections are potentially life-threatening. A pretransplantation evaluation is recommended to determine which teeth can be maintained without risk of infection. Teeth with severe bone and attachment loss, furcation invasion, periodontal abscesses, or extensive surgical requirements should be extracted.
Pulmonary Diseases
The periodontal treatment of a patient with pulmonary disease may require alteration, depending on the nature and the severity of the respiratory problem. Pulmonary diseases range from obstructive lung diseases (e.g., asthma, emphysema, bronchitis, or acute obstruction) to restrictive ventilatory disorders caused by muscle weakness, scarring, obesity, or any condition that could interfere with effective lung ventilation.69,87 Combined restrictive-obstructive lung disease may also develop.
The clinician should be aware of the signs and symptoms of pulmonary disease, such as increased respiratory rate, cyanosis, clubbing of the fingers, chronic cough, chest pain, hemoptysis, dyspnea or orthopnea, and wheezing. Patients with these problems should be referred for medical evaluation and treatment. Most patients with chronic lung disease may undergo routine periodontal therapy if they are receiving adequate medical management. Caution should be practiced in relation to any treatment that may depress respiratory function.
Acute respiratory distress may be caused by slight airway obstruction or depression of respiratory function. Because of their limited vital lung capacity, these patients also have decreased cough effectiveness.87 They must continually deal with the mental anxiety caused by air hunger and alter their position in attempts to improve their ventilatory efficiency.
The following guidelines should be used during periodontal therapy:
Medications and Cancer Therapies
Some medications prescribed to cure, manage, or prevent diseases have effects on periodontal tissues, wound healing, or the host immune response that require an understanding, appreciation, and in some cases, modification of treatment. Bisphosphonates, anticoagulation medications, antiplatelet medications, steroids, chemotherapy, and radiation therapy are briefly addressed here. Readers are encouraged to seek additional information and advice from other sources.
Bisphosphonates
Bisphosphonate medications are primarily used to treat cancer (IV administration) and osteoporosis (oral administration). They act by inhibiting osteoclastic activity, which leads to less bone resorption, less bone remodeling, and less bone turnover.83a The use of bisphosphonates in cancer treatment is aimed at preventing the often lethal imbalance of osteoclastic activity. In the treatment of osteoporosis, the goal is simply to harness osteoclastic activity to minimize or prevent bone loss. The major difference in the use of bisphosphonates for cancer versus osteoporosis is the potency and route of administration. Potency is influenced by the chemical properties and pharmacokinetics of these agents with bone. See Chapter 27 for a description of the chemical structure, activity, and role of bisphosphonates in the development of bisphosphonate-related osteonecrosis of the jaw (BRONJ).
Clinically, BRONJ presents as exposed alveolar bone occurring spontaneously or after a dental procedure (see Figure 27-29 Figure 27-30 ). Individuals treated with high potency, nitrogen-containing bisphosphonates, especially those administered via IV for cancer treatment (e.g., zoledronate), appear to be at greater risk for BRONJ than individuals taking oral bisphosphonates for prevention and treatment of osteoporosis. The incidence in patients treated for cancer has been reported to range from 2.5% to 5.4%.114a Estimating the incidence in patients taking oral bisphosphonates for osteoporosis is more difficult but appears to range from 0.007% to 0.04%.112a Even if this is an underestimation of the actual risk for individuals taking oral bisphosphonates, the incidence appears to be low. The risk for individuals treated with oral bisphosphonates for a period of less than 3 years appears to be minimal or zero.52 Regular use of oral bisphosphonates for a period greater than 3 years suggests a risk profile that increases with time and length of use.52
As with many multifactorial diseases and conditions, it is likely that factors in addition to bisphosphonate therapy contribute to the individual risk of BRONJ. Potential risk factors thought to contribute to the development of BRONJ include systemic corticosteroid therapy, smoking, alcohol, poor oral hygiene, chemotherapy, radiotherapy, diabetes, and hematologic disease.28 Reported factors or conditions leading to BRONJ include extractions, root canal treatment, periodontal infections, periodontal surgery, and dental implant surgery.52a Clearly, both periodontal disease and treatment (especially surgery) pose a risk for patients treated with bisphosphonates. The bacterial-induced inflammatory process of periodontitis that causes bone resorption can lead to bone necrosis. Likewise, periodontal treatment, especially surgery, may cause bone necrosis in the presence of bisphosphonates. Caution is warranted for any patient who has been or will be treated with bisphosphonates.
Health care providers need to evaluate patients carefully, communicate with medical health care providers, inform patients, and consider treatment options and risks carefully. A careful intraoral examination is prudent for all patients treated with bisphosphonate therapy (IV or oral) to determine whether bone exposures exist and to assess any local conditions that might predispose them to the development of BRONJ. A thorough medical history should be reviewed, evaluated, and recorded with details about any bisphosphonate treatment, including medication type, dose, route of administration, and duration. Comorbidities, such as previous and current medications, treatments, and existing disease or pathology, should be considered. Radiographs should be carefully evaluated for signs of bisphosphonate toxicity. Finally, Marx has suggested that a laboratory blood test for the serum C-terminal telopeptide fragment of type I collagen (CTX) can be used as a means of assessing an individual’s risk of developing BRONJ.52 Marx reports that lower CTX values are associated with greater risk (Table 37-10). It is important to recognize that these values are based on retrospective evaluation of patients with osteonecrosis of the jaws and that prospective studies to validate these findings have not been done. The CTX laboratory test is a measure of the specific C-terminal fragment of type I collagen cleaved by osteoclasts and serves as a good indicator of bone resorption activity. However, its use as a measure of risk for BRONJ is controversial and not confirmed by prospective studies.
TABLE 37-10 C-Terminal Telopeptide Laboratory Risk Assessment for Bisphosphonate Therapy
| C-Terminal Telopeptide (CTX) Value | Risk for BRONJ |
|---|---|
| 300–600 pg/ml (normal) | None |
| 150–299 pg/ml | None or minimal |
| 101–149 pg/ml | Moderate |
| ≤100 pg/ml | High |
Data from Marx RE: Oral and intravenous bisphosphonate-induced osteonecrosis of the jaws, Hanover Park, MD, 2007, Quintessence Publishing Co, Inc..
Optimal periodontal/oral health should be achieved and maintained for all patients. For individuals treated with IV bisphosphonates, invasive treatment, such as extractions, periodontal surgery, implant surgery, and bone augmentation procedures, should be avoided. Caution and careful consideration of risks must be considered before any treatment for individuals with a history of taking oral bisphosphonates for periods longer than 3 years. This is an area of current research that will undoubtedly continue to evolve as the pathophysiology is better understood. Providers are encouraged to consult other sources for updates on this important topic.
Anticoagulant/Antiplatelet Therapy
Many patients with a variety of conditions are placed on anticoagulant or antiplatelet medications to prevent thrombosis (blood clotting) or thromboembolism. Examples of patients at risk who may be on anticoagulant or antiplatelet therapy include those with heart value replacements, heart rhythm disorders, and congenital heart defects, as well as individuals with a history or risk of myocardial infarction, stroke, or deep vein thrombosis. These medications, although effective in reducing the risk of thrombosis, may increase the risk for bleeding complications, especially in patients undergoing surgical procedures.
As stated earlier, traditional management of patients on anticoagulant or antiplatelet therapy was to discontinue therapy about 3 to 5 (antiplatelet) or 7 to 10 (anticoagulant) days before planned surgical procedures. Recent evidence and new thoughts regarding the management of patients on anticoagulant or antiplatelet therapy suggest that treating them (e.g., periodontal surgery, extractions, etc) without altering their anticoagulant/antiplatelet mediations is safe and does not lead to intraoperative or postoperative bleeding complications. Furthermore, the increased risk of morbidity/mortality in those individuals discontinuing the anticoagulant or antiplatelet therapy may be significant.
Controlled clinical studies have demonstrated that intraoperative bleeding is not likely to be a problem with simple extractions or periodontal surgery if antiplatelet therapy (e.g., aspirin) is continued.12,50 In these studies, there were no episodes of uncontrolled bleeding, all bleeding was controlled with local measures, and there were no cases of postoperative bleeding problems. Conversely, the risk of stopping antiplatelet therapy may be serious. In a retrospective evaluation of 52 patients undergoing cataract surgery, 1 in 10 whose antiplatelet therapy was stopped or reduced suffered a stroke.85
Similarly, clinical studies of patients on anticoagulant therapy undergoing extractions and other oral surgical procedures have demonstrated minimal bleeding problems when therapy is continued.17,20,113,114,120 In a review of the literature, Wahl et al114 reported that only 12 patients (<1.3%, 950 total) receiving continuous anticoagulant therapy required more than local measures to control hemorrhage after minor oral surgical procedures (2400 total). Most of the 950 patients had anticoagulation levels that were well above currently recommended therapeutic levels. Only 3 patients (<0.31%) had anticoagulation levels within or below currently recommended therapeutic levels. In contrast, 5 of 526 (0.95%) patients who experienced 575 interruptions of continuous anticoagulant therapy, suffered serious embolic complications; four of these patients died. In a prospective study of 131 patients undergoing 511 extractions, patients whose oral anticoagulant therapy was reduced 72 hours before surgery to achieve an INR of 1.5 to 2.0 (target 1.8) were found to have postoperative bleeding warranting subsequent local intervention in 10 cases (15.1%).84 There were only six cases (9.2%) in the group that continued their oral anticoagulant therapy (mean INR = 2.9).
These studies suggest that the risks of serious morbidity associated with discontinuing anticoagulant/antiplatelet therapy should be avoided and that the risk of bleeding while maintaining this therapy is minimal.
Corticosteroids
Approximately 5% of the adults in the US habitually take corticosteroids for the treatment of various conditions, potentially putting them at risk for secondary adrenal insufficiency.48 Patients who habitually use corticosteroids have an increased likelihood of developing hypertension, osteoporosis, and peptic ulcer disease. Care should be taken to minimize the risk of adverse outcomes in these patients. BP should be monitored, and medications that may exacerbate peptic ulceration (e.g., acetylsalicylic acid [ASA], NSAIDs) should be avoided.
Stressful events, such as trauma, illness, surgery, emotional upset, or athletic events, normally increase circulating endogenous cortisol levels through stimulation of the hypothalamic-pituitary-adrenal (HPA) axis. Pain appears to increase the requirement for cortisol release.76 There is concern that the normal release of cortisol in response to stressful events, such as a dental procedure, may be impaired in patients exposed to habitual corticosteroid use. Hence the concern for whether patients who are habitually taking corticosteroids require perioperative supplementation for dental procedures. Historically, recommendations were based on the medication type, amount, and duration of corticosteroid use. However, current thinking about the need for perioperative corticosteroid supplementation has been adjusted.
Studies investigating the stress response to minor general and oral surgical procedures conclude that significant increases in cortisol are generally not seen until 1 to 5 hours after surgery and appear to be associated more with postoperative pain and the loss of local anesthesia than with the preoperative and intraoperative stress of the procedure.13,94,95 In fact, the administration of adequate analgesics in the postoperative period can diminish the release (requirement) of cortisol.13
Evidence indicates that a vast majority of individuals with adrenal insufficiency can receive routine dental treatment without the need for supplemental glucocorticosteroids.18,48 Patients currently taking corticosteroids generally have enough exogenous and endogenous cortisol to handle routine dental procedures if their usual dose is taken within 2 hours of the planned procedure. Thus, for most patients, supplemental corticosteroid administration is not required when uncomplicated minor surgical procedures, including periodontal surgery, are performed with local anesthesia with or without sedation.48 Topical corticosteroids generally have minimal HPA effect, and steroid supplementation is not required for these patients.
Individuals who may be at risk for adrenal crisis requiring supplementation include those who are undergoing lengthy, major surgical procedures, those expected to have significant blood loss and those who have extremely low adrenal function. Low adrenal function can be identified with an ACTH stimulation test. For these individuals, consultation with the physician and steroid supplementation is indicated.
Immunosuppression and Chemotherapy
Immunosuppressed patients have impaired host defenses as a result of an underlying immunodeficiency or drug administration (primarily related to organ transplantation or cancer chemotherapy).57,91 Because chemotherapy is often cytotoxic to bone marrow, destruction of platelets and red and white blood cells results in thrombocytopenia, anemia, and leukopenia. Immunosuppressed individuals are at greatly increased risk for infection, and even minor periodontal infections can become life threatening if immunosuppression is severe.75,78 Intraorally, bacterial, viral, and fungal infections may manifest. Patients receiving bone marrow transplantation require special attention because these patients receive extremely high-dose chemotherapy and are particularly susceptible to dissemination of oral infections.
Treatment in these patients should be directed toward the prevention of oral complications that could be life threatening. The greatest potential for infection occurs during periods of extreme immunosuppression; therefore treatment should be conservative and palliative. It is always preferable to evaluate the patient before initiation of chemotherapy.57,91 Teeth with a poor prognosis should be extracted, with thorough debridement of remaining teeth to minimize the microbial load. The clinician must teach and emphasize the importance of good oral hygiene. Antimicrobial rinses, such as chlorhexidine, are recommended, especially for patients with chemotherapy-induced mucositis, to prevent secondary infection.
Chemotherapy is usually performed in cycles, with each cycle lasting several days, followed by intervening periods of myelosuppression and recovery. If periodontal therapy is needed during chemotherapy, it is best done the day before chemotherapy is given, when white blood cell counts are relatively high. Coordination with the oncologist is critical. Dental treatment should be done when white cell counts are above 2000/mm3, with an absolute granulocyte count of 1000 to 1500/mm3.54
Radiation Therapy
The use of radiotherapy, alone or in conjunction with surgical resection, is common in the treatment of head and neck tumors. The side effects of ionizing radiation include dramatic perioral changes of significant concern to dental health personnel.41,58,92 The extent and severity of mucositis, dermatitis, xerostomia, dysphagia, gustatory alteration, radiation caries (Figure 37-3), vascular changes, trismus, temporomandibular joint degeneration, and periodontal change depend on the type of radiation used, fields of irradiation, number of ports, types of tissues in the fields, and dosage.
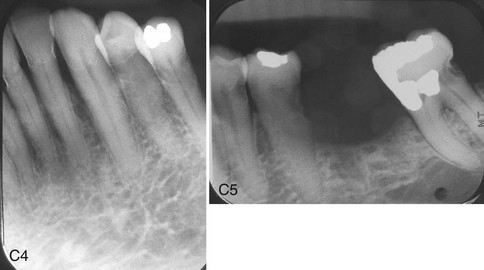
Figure 37-3 A, Clinical view of patient with radiation caries. Notice how the caries primarily affects the smooth tooth surfaces and the cusp tips. B, Radiographs of anterior teeth of 52-year-old male with postradiation caries. Patient received 6000-cGy radiation treatment to the posterior mandible and base of tongue for squamous cell carcinoma. Radiation caries developed within 1 year after radiation treatment, affecting the cervical areas and incisal edges of the anterior teeth. C, Radiographs of the mandibular teeth of 60-year-old male with postradiation caries. Patient received chemotherapy and 65-Gy intensity modulated radiation therapy (IMRT) to the lower head and neck for pharyngeal carcinoma. Radiation caries affecting the cervical areas of most remaining teeth. Notice the radiopaque layer on the outer surface of carious areas attributed to remineralization treatment with fluoride.
(A and C courtesy Dr. Eric Sung, Hospital Dentistry, University of California Los Angeles.)
Patients scheduled to receive head and neck radiation therapy require dental consultation at the earliest possible time to reduce the morbidity of the known perioral side effects.89 Preirradiation treatment depends on the patient’s prognosis, compliance, and residual dentition in addition to the fields, ports, dose, and immediacy of radiotherapy. The initial visit should include panoramic and intraoral radiographs, a clinical dental examination, a periodontal evaluation, and a physician consultation. The physician should be asked about the amount of radiation to be administered, extent and location of the lesion, nature of any surgical procedures already performed or to be performed, number of radiation ports, exact fields to be irradiated, mode of radiation therapy, and patient’s prognosis (i.e., likelihood of metastasis). Preirradiation treatment should commence immediately after the physician consultation. The first decision should involve possible extractions because radiation can cause side effects that interfere with healing.
For head and neck squamous cell carcinomas, the radiation dose is usually 5000 to 7000 cGy (centigray; 1 cGy = 1 rad) delivered in a fractionated method (150-200 cGy/day over a 6- to 7-week course).16,58 This is considered “full-course” radiation treatment, and the degree of perioral side effects depends on which tissues are irradiated, that is, the radiation fields. If this dose is administered to the salivary gland tissues, xerostomia will ensue. The parotid is the most radiosensitive of the salivary glands; saliva may become extremely viscous or nonexistent, depending on the dose delivered to the particular gland. Xerostomia causes a decrease in the normal salivary cleansing mechanisms, buffering capacity of saliva, and pH of oral fluids.58 Oral bacterial populations shift to a preponderance of cariogenic forms (e.g., Streptococcus mutans, Actinomyces spp., Lactobacillus spp.). Radiation-induced caries may progress rapidly and primarily affects smooth tooth surfaces (see Figure 37-3).
High-dose radiation therapy results in hypovascularity of irradiated tissues with a reduction in wound-healing capacity.65,111,112 Most severe among the resulting oral complications is osteoradionecrosis (ORN). Decreased vascularity renders the bone less capable of resolving trauma or infection. Such events may cause severe destruction of bone. The risk of ORN continues for the remainder of the patient’s life and does not decrease with time.53
Periodontal disease can be a precipitating factor in ORN.21,34 Tooth extraction after radiation treatment involves a high risk for developing ORN, and surgical flap procedures are generally discouraged after radiation. For these reasons, it is important that the clinician address the patient’s periodontal disease before radiation begins, whenever possible. Teeth that are nonrestorable or severely periodontally diseased should be extracted, ideally at least 2 weeks before radiation.58 Extractions should be performed in a manner that allows primary closure. Mucoperiosteal flaps should be gently elevated; teeth should be extracted in segments; alveolectomy should be performed, allowing no rough bony spicules to remain; and primary closure should be provided without tension. It is unnecessary to extract teeth that can be retained with conservative restorative, endodontic, or periodontal therapy. However, prudence dictates extraction of questionable teeth because periodontal treatment after irradiation may be limited to nonsurgical forms of therapy. Flap surgery or extraction of teeth after radiation may lead to ORN. Management of ORN is often difficult and costly, involving progressively more aggressive treatment if bone does not respond to conservative therapy. Costly hyperbaric oxygen therapy is frequently required for complete resolution.
During radiation therapy, patients should receive weekly prophylaxis, oral hygiene instruction, and professionally applied fluoride treatments, unless mucositis prevents such treatment. Patients should be instructed to brush daily with a 0.4% stannous or 1.0% sodium fluoride gel. Custom gel trays allow optimum fluoride application.111 All remaining teeth should receive thorough debridement (scaling and root planing).
Postirradiation follow-up consists of palliative treatment given as indicated. Viscous lidocaine may be prescribed for painful mucositis, and salivary substitutes may be given for xerostomia. Daily topical fluoride application and oral hygiene are the best means of preventing radiation caries over time. A long-term, 3-month recall interval is ideal.
Prosthetic Joint Replacement
The main treatment consideration for patients with prosthetic joint replacements relates to the potential need for antibiotic prophylaxis before periodontal therapy. Currently, no scientific evidence indicates that prophylactic antibiotics prevent late prosthetic joint infections, which might occur from transient bacteremia induced by dental treatment.26,54,93 Furthermore, although dental-induced bacteremia could theoretically cause prosthetic joint infection, scant reports demonstrate dental treatment as a source of joint infection, and none of these actually documents a cause-and-effect relationship.23,82,108 Thus, in previous reports, the American Dental Association (ADA), American Academy of Orthopedic Surgeons (AAOS), American Academy of Oral Medicine (AAOM), and British Society for Antimicrobial Chemotherapy (BSAC) all agreed that routine antibiotic prophylaxis before dental treatment was not indicated for most patients with prosthetic joint replacements.1,4,8,31,106 However, antibiotic prophylaxis was indicated for almost all patients within the first 2 years after joint replacement and for so-called high-risk patients, including those with previous prosthetic joint infections, immunosuppression, rheumatoid arthritis, systemic lupus erythematosus, type 1 diabetes, hemophilia, and malnourishment.4 Importantly, many authors consider patients with severe periodontal disease or other potential dental infections to be “high risk,” and antibiotic prophylaxis may be indicated for these patients before dental treatment.64,106
In February 2009, the AAOS released an “information statement” regarding antibiotic prophylaxis for bacteremia in patients with joint replacements.10 Their earlier information statement, developed jointly with the ADA, was published in 19974 and revised in 2003.8 It intended to reduce the risk to patients from unnecessary use of antibiotics. The 2009 information statement, which was not developed collaboratively with the ADA, will greatly increase the number of patients exposed to antibiotics because it states that all patients with prosthetic joint replacements should be given antibiotic prophylaxis prior to invasive procedures. Commentaries that challenge the statement have been published in the Journal of the American Dental Association (JADA)38 and the Journal of the Canadian Dental Association (JCDA).62
Although the evidence demonstrates no need for antibiotic prophylaxis in most patients, many orthopedic surgeons still prefer such treatment,98 most likely because of the high morbidity and potential mortality associated with prosthetic joint infections. Consultation with the patient’s orthopedic surgeon before periodontal treatment is in the patient’s best interest and may help assess the risk for joint infection relative to current dental status and type of periodontal treatment planned. Because individuals with significant periodontal disease are considered “high risk,” antibiotic prophylaxis before treatment is common in the periodontal practice (Table 37-11).4
TABLE 37-11 Antibiotic Regimens for Prevention of Prosthetic Joint Infections*
| Patient Characteristics | Drug Regimen |
|---|---|
| Patients not allergic to penicillins | Cephalexin, cephradine, or amoxicillin: 2 g orally 1 hour before dental procedure |
| Patients allergic to penicillins | Clindamycin: 600 mg orally 1 hour before dental procedure |
| Patients not allergic to penicillins, but unable to take oral medication | Cefazolin 1 g or ampicillin 2 g intramuscularly or intravenously 1 hour before dental procedure |
| Patients allergic to penicillins, and unable to take | Clindamycin 600 mg intravenously 1 hour before dental oral medications procedure (must be diluted and injected slowly) |
* No second doses were recommended.
Data from American Dental Association and American Academy of Orthopedic Surgeons: Am Dent Assoc J 128:1004-1008, 1997.
Providers are encouraged to monitor the status of these important statements and implied guidelines from other resources.
Pregnancy
The aim of periodontal therapy for the pregnant patient is to minimize the potential exaggerated inflammatory response related to pregnancy-associated hormonal alterations. Meticulous plaque control, scaling, root planing, and polishing should be the only nonemergency periodontal procedures performed.
The second trimester is the safest time to perform treatment. However, long, stressful appointments, as well as periodontal surgical procedures, should be delayed until the postpartum period. As the uterus increases in size during the second and third trimesters, obstruction of the vena cava and aorta may occur if the patient is placed in a supine position. The reduction in return cardiac blood supply may cause supine hypotensive syndrome, with decreased placental perfusion.104 Decreasing BP, syncope, and loss of consciousness may occur. This can be prevented by placing the patient on her left side or simply by elevating the right hip 5 to 6 inches during treatment. Appointments should be short, and the patient should be allowed to change positions frequently. A fully reclined position should be avoided if possible.
Other precautions during pregnancy relate to the potential toxic or teratogenic effects of therapy on the fetus. Ideally, no medications should be prescribed. However, analgesics, antibiotics, local anesthetics, and other drugs may be required during pregnancy, depending on the patient’s needs. Before being prescribed, all drugs should be reviewed for potential adverse effects on the fetus.54,104
As for all patients, use of dental radiographs during pregnancy should be kept to a minimum. The small amount of radiation exposure during diagnostic dental radiography poses little, if any, risk to the fetus as long as the mother is properly shielded.11,104 The ADA has stated that “normal radiographic guidelines do not need to be altered because of pregnancy.”11 Use of a properly positioned lead apron is an absolute requirement. Chapter 38 includes a detailed discussion of the management of the female patient, including pregnancy.
Infectious Diseases
Because many infectious diseases are occult in nature and because medical histories are often inaccurate or incomplete, all periodontal patients should be treated as though they have an infectious disease.27 Protection of patients, clinicians, and office staff requires use of universal (standard) precautions for all patients, maximizing prevention of infection and cross-contamination. This section provides a brief discussion of hepatitis, human immunodeficiency virus (HIV) and acquired immunodeficiency syndrome (AIDS), and tuberculosis in relation to the precautions required in periodontal therapy.
Hepatitis
To date, six distinct viruses causing viral hepatitis have been identified: hepatitis A, B, C, D, E, and G viruses.24,36,49 In addition, a single-stranded deoxyribonucleic acid (DNA) virus known as transfusion-transmitted virus has recently been identified in cases of acute and chronic hepatitis49,118; its role as an etiologic agent is still undetermined. These forms of viral hepatitis differ in their virology, epidemiology, and prophylaxis (Table 37-12 online). Because the majority of hepatitis infections are undiagnosed, the clinician must be aware of high-risk groups, such as renal dialysis patients, health care workers, immunosuppressed patients, patients who have received multiple blood transfusions, homosexuals, drug users, and institutionalized patients.
Hepatitis A virus (HAV) and hepatitis E virus (HEV) are both self-limiting infections with no associated chronic liver disease. These viruses are primarily transmitted via the fecal-oral route. HAV transmission in the US usually results from close personal household, sexual, or day care center contact. Conversely, HEV is transmitted mainly through fecally contaminated drinking water and is thus relatively uncommon in the US. Currently, a vaccination is available to protect against HAV infection but not HEV infection.
Hepatitis B virus (HBV) infection may result in chronic liver disease and a chronic carrier state. Chronic HBV infection develops in about 5% to 10% of infected individuals, with much higher rates among infants and children. Because it is transmitted primarily through a hematogenous route, HBV is a major concern for health care workers; the highest rates of HBV infection are found among dentists and oral surgeons.36 Percutaneous or permucosal injury with contaminated instruments or needles is the most common route of infection in the dental office. The HBV vaccine is recommended for all health care workers.
Hepatitis D virus (HDV) is a defective virus that requires the presence of HBV for its survival, replication, and infectivity. The HDV genetic material is packaged within the HBV surface-antigen coating. Thus prevention of HDV infection is similar to prevention of HBV and relies strongly on HBV vaccination. Once antibody titers to HBV are elevated to a protective level, the patient is also protected against HDV infection.
Hepatitis C virus (HCV) is probably the most serious of all viral hepatitis infections because of its high chronic infection rate. Only 15% of patients infected with HCV recover completely; 85% develop chronic HCV infection, which dramatically increases the risk for cirrhosis, hepatocellular carcinoma, and liver failure. In fact, HCV infection is the leading cause of liver transplantation in the US. Unfortunately, no vaccine is available for HCV. Because HCV is transmitted primarily through a percutaneous or permucosal route, health care workers are at risk from injury with contaminated instruments.
Hepatitis G virus (HGV) is a ribonucleic acid (RNA) virus, and its epidemiology and virology are not fully understood. HGV rarely occurs as a solitary infection, usually appearing as a co-infection with hepatitis A, B, or C. HGV is known to be transmitted through the blood and has frequently been associated with transfusions.
Transfusion-transmitted virus (TTV) was first identified in 1997 and is common in the general population.49,61 The virus is often present in patients with hepatitis and chronic liver disease. Similar to HGV, TTV may not actually cause a specific form of hepatitis. Patients infected with TTV may develop a chronic carrier state without having clinically evident disease, or they may develop frank disease. Evidence indicates that TTV can be transmitted not only by the blood (e.g., transfusions) but also via a fecal-oral route and from mother to child. Also, as with HGV, TTV is often associated with HCV.
The following guidelines are offered for treating hepatitis patients:
If a percutaneous or permucosal injury occurs during dental treatment of a HBV carrier, current Centers for Disease Control and Prevention (CDC) guidelines recommend administration of hepatitis B immune globulin (HBIG).71 The HBV vaccine should also be administered if the injured individual has not previously received it. Unfortunately, postexposure prophylaxis with immune globulin or antiviral agents is generally ineffective if a percutaneous injury occurs during treatment of a hepatitis C carrier.24,72
HIV and AIDS
Since the beginning of the AIDS epidemic, a wide range of oral lesions has been associated with HIV infection; these lesions are discussed in Chapter 19.
As with hepatitis, not all HIV-infected patients know that they are infected when they report for dental treatment. Furthermore, individuals with known HIV infection may not admit their status on the medical history. Therefore every patient receiving dental treatment should be managed as a potentially infected person, using universal precautions for all therapy.
Extensive periodontal treatment plans must be considered in regard to the patient’s systemic health, prognosis, and survival time.54 Large variations in progression of HIV disease exist among individuals, and selection of an appropriate treatment plan depends on the state of the patient’s overall health. Although there appear to be few contraindications to routine dental treatment for many HIV-infected patients, the periodontal treatment plan is influenced by the patient’s overall systemic health and coincident oral infections or diseases. An awareness of oral disorders associated with HIV infection may allow the clinician to recognize previously undiagnosed disease or to modify treatment protocols appropriately.
Tuberculosis
The patient with tuberculosis should receive only emergency care, following the guidelines listed in the section on hepatitis. If the patient has completed chemotherapy, the patient’s physician should be consulted regarding infectivity and the results of sputum cultures for Mycobacterium tuberculosis. When medical clearance has been given and the sputum culture results are negative, these patients may be treated normally. Any patient who gives a history of poor medical follow-up (e.g., lack of yearly chest radiographs) or shows signs or symptoms indicative of tuberculosis should be referred for evaluation. Adequate treatment of tuberculosis requires a minimum of 18 months, and thorough posttreatment follow-up should include chest radiographs, sputum cultures, and a review of the patient’s symptoms by the physician at least every 12 months.
1 Management of dental patients with prosthetic joints. Council on Dental Therapeutics. J Am Dent Assoc. 1990;121:537-538.
2 The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977-986.
3 The fifth report of the Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure (JNC V). Arch Intern Med. 1993;153:154-183.
4 American Dental Association and American Academy of Orthopaedic Surgeons. Advisory statement. Antibiotic prophylaxis for dental patients with total joint replacements. J Am Dent Assoc. 1997;128:1004-1008.
5 Hypoglycemia in the Diabetes Control and Complications Trial. The Diabetes Control and Complications Trial Research Group. Diabetes. 1997;46:271-286.
6 The sixth report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure (JNC VI). Arch Intern Med. 1997;157:2413-2446.
7 Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837-853.
8 American Dental Association and American Academy of Orthopaedic Surgeons. Antibiotic prophylaxis for dental patients with total joint replacements. J Am Dent Assoc. 2003;134:895-899.
9 American Diabetes Association. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2003;5(suppl 1):5.
10 American Association of Orthopaedic Surgeons. Information Statement: antibiotic prophylaxis for bacteremia in patients with joint replacements. Available www.aaos.org/about/papers/advistmt/1033.asp, 2009.
11 American Dental Association Council on Dental Materials, IaE. Recommendations in radiographic practices: an update. J Am Dent Assoc. 1989;118:115.
12 Ardekian L, Gaspar R, Peled M, Brener B, Laufer D. Does low-dose aspirin therapy complicate oral surgical procedures? J Am Dent Assoc. 2000;131:331-335.
13 Banks P. The adreno-cortical response to oral surgery. Br J Oral Surg. 1970;8:32-44.
14 Barco CT. Prevention of infective endocarditis: a review of the medical and dental literature. J Periodontol. 1991;62:510-523.
15 Black HR. The coronary artery disease paradox. Am J Hypertens. 1996;9:2S-10S.
16 Blaha PJ, Reeve CM. Periodontal treatment for patients with cancer. Curr Opin Periodontol. 1994:64-70.
17 Blinder D, Manor Y, Martinowitz U, Taicher S. Dental extractions in patients maintained on oral anticoagulant therapy: comparison of INR value with occurrence of postoperative bleeding. Int J Oral Maxillofac Surg. 2001;30:518-521.
18 Bromberg JS, Baliga P, Cofer JB, et al. Stress steroids are not required for patients receiving a renal allograft and undergoing operation. J Am Coll Surg. 1995;180:532-536.
19 Burt VL, Whelton P, Roccella EJ, et al. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988-1991. Hypertension. 1995;25:305-313.
20 Campbell JH, Alvarado F, Murray RA. Anticoagulation and minor oral surgery: should the anticoagulation regimen be altered? J Oral Maxillofac Surg. 2000;58:131-135. discussion 135-136
21 Carl W. Local radiation and systemic chemotherapy: preventing and managing the oral complications. J Am Dent Assoc. 1993;124:119-123.
22 Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560-2572.
23 Christgau M, Palitzsch KD, Schmalz G, et al. Healing response to non-surgical periodontal therapy in patients with diabetes mellitus: clinical, microbiological, and immunologic results. J Clin Periodontol. 1998;25:112-124.
24 Cleveland JL, Gooch BF, Shearer BG, Lyerla RL. Risk and prevention of hepatitis C virus infection. Implications for dentistry. J Am Dent Assoc. 1999;130:641-647.
25 Dajani AS, Taubert KA, Wilson W, et al. Prevention of bacterial endocarditis: recommendations by the American Heart Association. J Am Dent Assoc. 1997;128:1142-1151.
26 Deacon JM, Pagliaro AJ, Zelicof SB, Horowitz HW. Prophylactic use of antibiotics for procedures after total joint replacement. J Bone Joint Surg Am. 1996;78:1755-1770.
27 DePaola LG. Managing the care of patients infected with bloodborne diseases. J Am Dent Assoc. 2003;134:350-358.
28 Ducharme A, Dupuis J, McNicoll S, et al. Comparison of nitroglycerin lingual spray and sublingual tablet on time of onset and duration of brachial artery vasodilation in normal subjects. Am J Cardiol. 1999;84:952-954. A958
29 Durack DT. Antibiotics for prevention of endocarditis during dentistry: time to scale back? Ann Intern Med. 1998;129:829-831.
30 Eisenberg MJ, Brox A, Bestawros AN. Calcium channel blockers: an update. Am J Med. 2004;116:35-43.
31 Eskinazi D, Rathbun W. Is systematic antimicrobial prophylaxis justified in dental patients with prosthetic joints? Oral Surg Oral Med Oral Pathol. 1988;66:430-431.
32 Estes NA3rd, Weinstock J, Wang PJ, et al. Use of antiarrhythmics and implantable cardioverter-defibrillators in congestive heart failure. Am J Cardiol. 2003;91:45D-52D.
33 Findler M, Garfunkel AA, Galili D. Review of very high-risk cardiac patients in the dental setting. Compendium. 1994;15(58):60-64.
34 Galler C, Epstein JB, Guze KA, et al. The development of osteoradionecrosis from sites of periodontal disease activity: report of 3 cases. J Periodontol. 1992;63:310-316.
35 Genco RJ, Offenbacher S, Beck J, Rees T. Cardiovascular Diseases and Oral Infections. In: Rose LF, Genco RJ, Cohen DW, Mealey BL, editors. Periodontal medicine. Toronto: BC Decker, 1999.
36 Gillchrist J. Hepatitis viruses A, B, C, D, E, and G: implications for dental personnel. J Am Dent Assoc. 1999;130:509.
37 Glick M. Glucocorticosteroid replacement therapy: a literature review and suggested replacement therapy. Oral Surg Oral Med Oral Pathol. 1989;67:614-620.
38 Glick M. Clinical guidelines: to follow or folly? You decide. J Am Dent Assoc. 2009;140:824-826.
39 Gudapati A, Ahmed P, Rada R. Dental management of patients with renal failure. Gen Dent. 2002;50:508-510.
40 Hajjar I, Kotchen TA. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988-2000. JAMA. 2003;290:199-206.
41 Herman WW, Konzelman JLJr, Prisant LM. New national guidelines on hypertension: a summary for dentistry. J Am Dent Assoc. 2004;135:576-584. quiz 653-574
42 Herman WW, Konzelman JLJr, Sutley SH. Current perspectives on dental patients receiving coumarin anticoagulant therapy. J Am Dent Assoc. 1997;128:327-335.
43 Jeske AH, Suchko GD. Lack of a scientific basis for routine discontinuation of oral anticoagulation therapy before dental treatment. J Am Dent Assoc. 2003;134:1492-1497.
44 Johnson WT, Leary JM. Management of dental patients with bleeding disorders: review and update. Oral Surg Oral Med Oral Pathol. 1988;66:297-303.
45 Kerr AR. Update on renal disease for the dental practitioner. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92:9-16.
46 Konstam MA. Improving clinical outcomes with drug treatment in heart failure: what have trials taught? Am J Cardiol. 2003;91:9D-14D.
47 Lenfant C, Chobanian AV, Jones DW, Roccella EJ. Seventh report of the Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7): resetting the hypertension sails. Hypertension. 2003;41:1178-1179.
48 Little JW, Falace DA, Miller CS, Rhodus NL. Dental management of the medically compromised patient. St. Louis: Mosby; 2002.
49 Lodi G, Bez C, Porter SR, Scully C, Epstein JB. Infectious hepatitis C, hepatitis G, and TT virus: review and implications for dentists. Spec Care Dentist. 2002;22:53-58.
50 Madan GA, Madan SG, Madan G, Madan AD. Minor oral surgery without stopping daily low-dose aspirin therapy: a study of 51 patients. J Oral Maxillofac Surg. 2005;63:1262-1265.
51 Martinowitz U, Mazar AL, Taicher S, et al. Dental extraction for patients on oral anticoagulant therapy. Oral Surg Oral Med Oral Pathol. 1990;70:274-277.
52 Marx RE. Oral and intravenous bisphosphonate-induced osteonecrosis of the jaws. Hanover Park: Quintessence Publishing Co, Inc; 2007.
52a Marx RE, Sawatari Y, Fortin M, et al. Bisphosphonate-induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: risk factors, recognition, prevention, and treatment. J Oral Maxillofac Surg. 2005;63:1567-1575.
53 Marx RE, Johnson RP. Studies in the radiobiology of osteoradionecrosis and their clinical significance. Oral Surg Oral Med Oral Pathol. 1987;64:379-390.
54 Mealey BL. Periodontal implications: medically compromised patients. Ann Periodontol. 1996;1:256-321.
55 Mealey BL. Impact of advances in diabetes care on dental treatment of the diabetic patient. Compend Contin Educ Dent. 1998;19:41-44. 46-48, 50 passim; quiz 60
56 Mealey BL. Diabetes Mellitus. In: Rose LF, Genco RJ, Cohen DW, Mealey BL, editors. Periodontal medicine. Hamilton: BC Decker, 2000.
57 Mealey BL, Semba SE, Hallmon WW. Dentistry and the cancer patient: Part 1–Oral manifestations and complications of chemotherapy. Compendium. 1994;15:1252. 1254, 1256 passim; quiz 1262
58 Mealey BL, Semba SE, Hallmon WW. The head and neck radiotherapy patient: Part 2–Management of oral complications. Compendium. 1994;15:442. 444, 446-452 passim; quiz 458
59 Miller CS, Little JW, Falace DA. Supplemental corticosteroids for dental patients with adrenal insufficiency: reconsideration of the problem. J Am Dent Assoc. 2001;132:1570-1579. quiz 1596-1577
60 Muzyka BC, Glick M. The hypertensive dental patient. J Am Dent Assoc. 1997;128:1109-1120.
61 Naoumov NV. TT virus–highly prevalent, but still in search of a disease. J Hepatol. 2000;33:157-159.
62 Napenas JJ, Lockhart PB, Epstein JB. Comment on the 2009 American Academy of Orthopaedic Surgeons’ information statement on antibiotic prophylaxis for bacteremia in patients with joint replacements. J Can Dent Assoc. 2009;75:447-449.
63 Nathan DM, Kuenen J, Borg R, et al. Translating the A1C assay into estimated average glucose values. Diabetes Care. 2008;31:1473-1478.
64 Norden CW. Antibiotic prophylaxis in orthopedic surgery. Rev Infect Dis. 1991;13(Suppl 10):S842-S846.
65 Ostuni E. Stroke and the dental patient. J Am Dent Assoc. 1994;125:721-727.
66 Pallasch TJ, Slots J. Antibiotic prophylaxis and the medically comprised patient. Periodontal 2000. 1996;10:107.
67 Patton LL, Ship JA. Treatment of patients with bleeding disorders. Dent Clin North Am. 1994;38:465-482.
68 Petrover M, ZCohen CI. The use of desmopressin in the management of two patients with von Willebrand’s disease undergoing periodontal surgery: 2 case reports. J Periodontal. 1990;61:239.
69 Phillips YY, Hnatiuk OW. Diagnosing and monitoring the clinical course of chronic obstructive pulmonary disease. Respir Care Clin N Am. 1998;4:371-389.
70 Pinto A, Glick M. Management of patients with thyroid disease: oral health considerations. J Am Dent Assoc. 2002;133:849-858.
71 Prevention CfDCa. Immunization of health-care workers: recommendation of the Advisory committee on Immunization practices (ACIP) and the Hospital Infection Control Practices Advisory Committee (HICPAC). MMWR. 1997;46(RR-18):1.
72 Prevention CfDCa. Recommendations for follow-up of health-care workers after occupational exposure to hepatitus C virus. MMWR. 1997;46:603.
73 Price JW, Price JR. Accuracy and precision of metered doses of nitroglycerin lingual spray. Am J Health Syst Pharm. 2008;65:1556-1559.
74 Raab FJ, Schaffer EM, Guillaume-Cornelissen G, Halberg F. Interpreting vital sign profiles for maximizing patient safety during dental visits. J Am Dent Assoc. 1998;129:461-469.
75 Raber-Durlacher JE, Epstein JB, Raber J, et al. Periodontal infection in cancer patients treated with high-dose chemotherapy. Support Care Cancer. 2002;10:466-473.
76 Raff H, Norton AJ, Flemma RJ, Findling JW. Inhibition of the adrenocorticotropin response to surgery in humans: interaction between dexamethasone and fentanyl. J Clin Endocrinol Metab. 1987;65:295-298.
77 Ramstrom G, Sindet-Pedersen S, Hall G, et al. Prevention of postsurgical bleeding in oral surgery using tranexamic acid without dose modification of oral anticoagulants. J Oral Maxillofac Surg. 1993;51:1211-1216.
78 Rees TD, Mealey BL. Periodontal treatment of the medically compromised patient. In: Rose LF, Mealey BL, Genco RJ, Cohen DW, editors. Periodontics: medicine, surgery, and implants. St. Louis: Mosby Elsevier, 2004.
79 Replogle K, Reader A, Nist R, et al. Cardiovascular effects of intraosseous injections of 2 percent lidocaine with 1:100,000 epinephrine and 3 percent mepivacaine. J Am Dent Assoc. 1999;130:649-657.
80 Rhodus NL, Little JW. Dental management of the renal transplant patient. Compendium. 1993;14:518-524. 526, 528 passim; Quiz 532
81 Rhodus NL, Little JW. Dental management of the patient with cardiac arrhythmias: an update. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;96:659-668.
82 Rose L, Steinberg B. Clinical history and laboratory tests. In: Rose L, Genco R, Cohen D, Mealey B, editors. Periodontal medicine. Hamilton: BC Decker, 2000.
83 Rose LF, Mealey B, Minsk L, Cohen DW. Oral care for patients with cardiovascular disease and stroke. J Am Dent Assoc. 2002;133(Suppl):37S-44S.
83a Russell RG. Bisphosphonates: mode of action and pharmacology. Pediatrics. 2007;119(Suppl 2)):S150-S162.
84 Sacco R, Sacco M, Carpenedo M, Moia M. Oral surgery in patients on oral anticoagulant therapy: a randomized comparison of different INR targets. J Thromb Haemost. 2006;4:688-689.
85 Saitoh AK, Saitoh A, Taniguchi H, Amemiya T. Anticoagulation therapy and ocular surgery. Ophthalmic Surg Lasers. 1998;29:909-915.
86 Sato H, Koretsune Y, Taniguchi T, et al. Studies on the response of nitroglycerin oral spray compared with sublingual tablets for angina pectoris patients with dry mouth. A multicenter trial. Arzneimittelforschung. 1997;47:128-131.
87 Scannapieco FA. Respiratory diseases. In: Rose LF, Genco RJ, Mealey BL, editors. Periodontal medicine. Toronto: BC Decker, 1999.
88 Schardt-Sacco D. Update on coagulopathies. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:559-563.
89 Schiodt M, Hermund NU. Management of oral disease prior to radiation therapy. Support Care Cancer. 2002;10:40-43.
90 Scully C, Wolff A. Oral surgery in patients on anticoagulant therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:57-64.
91 Semba SE, Mealey BL, Hallmon WW. Dentistry and the cancer patient: Part 2–Oral health management of the chemotherapy patient. Compendium. 1994;15:1378. 1380-1377; quiz 1388
92 Semba SE, Mealey BL, Hallmon WW. The head and neck radiotherapy patient: Part 1–Oral manifestations of radiation therapy. Compendium. 1994;15:250. 252-260; quiz 261
93 Seymour RA, Whitworth JM. Antibiotic prophylaxis for endocarditis, prosthetic joints, and surgery. Dent Clin North Am. 2002;46:635-651.
94 Shannon IL, Isbel GM, Hester WR. Stress in dental patients. IV. Effect of local anesthetic administration on serum free 17-hydroxycorticosteroid patterns. J Oral Surg Anesth Hosp Dent Serv. 1963;21:50-54.
95 Shannon IL, Isbell GM, Prigmore JR, Hester WR. Stress in dental patients. II. The serum free 17-hydroxycorticosteroid response in routinely appointed patients undergoing simple exodontia. Oral Surg Oral Med Oral Pathol. 1962;15:1142-1146.
96 Shapiro R, Carroll PB, Tzakis AG, et al. Adrenal reserve in renal transplant recipients with cyclosporine, azathioprine, and prednisone immunosuppression. Transplantation. 1990;49:1011-1013.
97 Sherman RG, Lasseter DH. Pharmacologic management of patients with diseases of the endocrine system. Dent Clin North Am. 1996;40:727-752.
98 Shrout MK, Scarbrough F, Powell BJ. Dental care and the prosthetic joint patient: a survey of orthopedic surgeons and general dentists. J Am Dent Assoc. 1994;125:429-436.
99 Sindet-Pedersen S, Stenbjerg S, Ingerslev J. Control of gingival hemorrhage in hemophilic patients by inhibition of fibrinolysis with tranexamic acid. J Periodontal Res. 1988;23:72-74.
100 Slots J, Rosling BG, Genco RJ. Suppression of penicillin-resistant oral Actinobacillus actinomycetemcomitans with tetracycline. Considerations in endocarditis prophylaxis. J Periodontol. 1983;54:193-196.
101 Smith G. Peridontal ligament injection: evaluation of systematic effects. Oral Surg Oral Med Oral Pathol. 1983;56:571.
102 Smolensky MH. Chronobiology and chronotherapeutics. Applications to cardiovascular medicine. Am J Hypertens. 1996;9:11S-21S.
103 Strom BL, Abrutyn E, Berlin JA, et al. Dental and cardiac risk factors for infective endocarditis. A population-based, case-control study. Ann Intern Med. 1998;129:761-769.
104 Tarsitano BF, Rollings RE. The pregnant dental patient: evaluation and management. Gen Dent. 1993;41:226-234. quiz 233-224
105 Tervonen T, Karjalainen K. Periodontal disease related to diabetic status. A pilot study of the response to periodontal therapy in type 1 diabetes. J Clin Periodontol. 1997;24:505-510.
106 Thyne GM, Ferguson JW. Antibiotic prophylaxis during dental treatment in patients with prosthetic joints. J Bone Joint Surg Br. 1991;73:191-194.
107 Tibbetts LS. Conscious Sedation. In: Rose LF, Genco RJ, Mealey BL, Cohen DW, editors. Periodontics: medicine, surgery, and implants. St. Louis: Mosby, Elsevier, 2004.
108 Tong DC, Rothwell BR. Antibiotic prophylaxis in dentistry: a review and practice recommendations. J Am Dent Assoc. 2000;131:366-374.
109 Umino M, Nagao M. Systemic diseases in elderly dental patients. Int Dent J. 1993;43:213.
110 Vasan RS, Beiser A, Seshadri S, et al. Residual lifetime risk for developing hypertension in middle-aged women and men: The Framingham Heart Study. JAMA. 2002;287:1003-1010.
111 Vissink A, Burlage FR, Spijkervet FK, et al. Prevention and treatment of the consequences of head and neck radiotherapy. Crit Rev Oral Biol Med. 2003;14:213-225.
112 Vissink A, Jansma J, Spijkervet FK, et al. Oral sequelae of head and neck radiotherapy. Crit Rev Oral Biol Med. 2003;14:199-212.
112a Wade ML, Suzuki JB. Issues related to diagnosis and treatment of bisphosphonate-induced osteonecrosis of the jaws. Grand Rounds Oral Systemic Med. 2007;2:46-53.
113 Wahl MJ. Dental surgery in anticoagulated patients. Arch Intern Med. 1998;158:1610-1616.
114 Wahl MJ. Myths of dental surgery in patients receiving anticoagulant therapy. J Am Dent Assoc. 2000;131:77-81.
114a Wang EP, Kaban LB, Strewler GJ, et al. Incidence of osteonecrosis of the jaw in patients with multiple myeloma and breast or prostate cancer on intravenous bisphosphonate therapy. J Oral Maxillofac Surg. 2007;65:1328-1331.
115 Watanabe K. Prepubertal periodontitis: a review of diagnostic criteria, pathogenesis, and differential diagnosis. J Periodontal Res. 1990;25:31-48.
116 Westfelt E, Rylander H, Blohme G, et al. The effect of periodontal therapy in diabetics. Results after 5 years. J Clin Periodontol. 1996;23:92-100.
117 Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis and Kawasaki Disease Committee Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. J Am Dent Assoc. 2008;139(Suppl):3S-24S.
118 Winsom C, Siegel MA. Advances on the diagnosis and management of human viral hepatitis. Dent Clin North Am. 2003;47:431.
119 Yagiela JA. Adverse drug interactions in dental practice: interactions associated with vasoconstrictors. Part V of a series. J Am Dent Assoc. 1999;130:701-709.
120 Zanon E, Martinelli F, Bacci C, et al. Safety of dental extraction among consecutive patients on oral anticoagulant treatment managed using a specific dental management protocol. Blood Coagul Fibrinolysis. 2003;14:27-30.
121 Ziccardi VB, Abubaker AO, Sotereanos GC, Patterson GT. Maxillofacial considerations in orthotopic liver transplantation. Oral Surg Oral Med Oral Pathol. 1991;71:21-26.