CHAPTER 13 The Periodontal Pocket
The periodontal pocket, defined as a pathologically deepened gingival sulcus, is one of the most important clinical features of periodontal disease. All different types of periodontitis, as outlined in Chapter 4, share histopathologic features such as tissue changes in the periodontal pocket, mechanisms of tissue destruction, and healing mechanisms. They differ, however, in their etiology, natural history, progression, and response to therapy.32
Classification
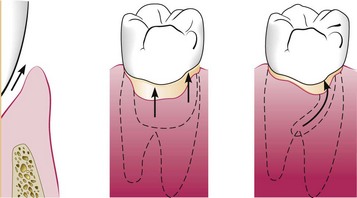
Deepening of the gingival sulcus may occur by coronal movement of the gingival margin, apical displacement of the gingival attachment, or a combination of the two processes (Figure 13-1). Pockets can be classified as follows:
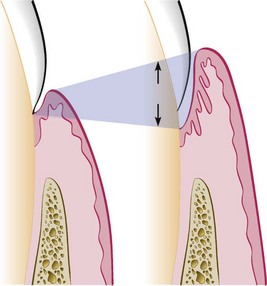
Figure 13-1 Illustration of pocket formation indicating expansion in two directions (arrows) from the normal gingival sulcus (left) to the periodontal pocket (right).
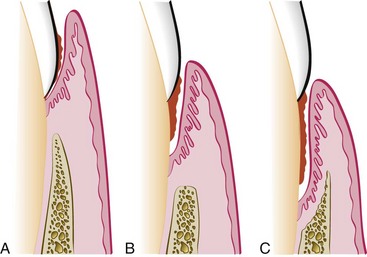
Figure 13-2 Different types of periodontal pockets. A, Gingival pocket. There is no destruction of the supporting periodontal tissues. B, Suprabony pocket. The base of the pocket is coronal to the level of the underlying bone. Bone loss is horizontal. C, Intrabony pocket. The base of the pocket is apical to the level of the adjacent bone. Bone loss is vertical.
Pockets can involve one, two, or more tooth surfaces and can be of different depths and types on different surfaces of the same tooth and on approximating surfaces of the same interdental space.30,38 Pockets can also be spiral (i.e., originating on one tooth surface and twisting around the tooth to involve one or more additional surfaces) (Figure 13-3). These types of pockets are most common in furcation areas.
Clinical Features
Clinical signs that suggest the presence of periodontal pockets include a bluish red, thickened marginal gingiva; a bluish red, vertical zone from the gingival margin to the alveolar mucosa; gingival bleeding and suppuration; tooth mobility; diastema formation; and symptoms such as localized pain or pain “deep in the bone.” The only reliable method of locating periodontal pockets and determining their extent is careful probing of the gingival margin along each tooth surface (Figure 13-4 and Table 13-1). On the basis of depth alone, however, it is sometimes difficult to differentiate between a deep normal sulcus and a shallow periodontal pocket. In such borderline cases, pathologic changes in the gingiva distinguish the two conditions.
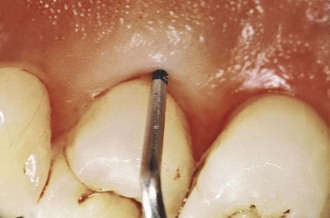
Figure 13-4 Probing of a deep periodontal pocket. Entire length of the periodontal probe has been inserted to the base of the pocket in palatal surface of first premolar.
TABLE 13-1 Correlation of Clinical and Histopathologic Features of the Periodontal Pocket
| Clinical Features | Histopathologic Features |
|---|---|
| 1. Gingival wall of pocket presents various degrees of bluish red discoloration; flaccidity; a smooth, shiny surface; and pitting on pressure. | 1. The discoloration is caused by circulatory stagnation; the flaccidity, by destruction of gingival fibers and surrounding tissues; the smooth, shiny surface, by atrophy of epithelium and edema; and the pitting on pressure, by edema and degeneration. |
| 2. Less frequently, gingival wall may be pink and firm. | 2. In such cases, fibrotic changes predominate over exudation and degeneration, particularly in relation to outer surface of pocket wall. However, despite external appearance of health, inner wall of pocket invariably presents some degeneration and is often ulcerated (see Figure 13-15). |
| 3. Bleeding is elicited by gently probing soft tissue wall of pocket. | 3. Ease of bleeding results from increased vascularity, thinning and degeneration of epithelium, and proximity of engorged vessels to inner surface. |
| 4. When explored with a probe, inner aspect of pocket is generally painful. | 4. Pain on tactile stimulation is caused by ulceration of inner aspect of pocket wall. |
| 5. In many cases, pus may be expressed by applying digital pressure. | 5. Pus occurs in pockets with suppurative inflammation of inner wall. |
For a more detailed discussion of the clinical aspects of periodontal pockets, see Chapter 30.
Pathogenesis
The initial lesion in the development of periodontitis is the inflammation of the gingiva in response to a bacterial challenge. Changes involved in the transition from the normal gingival sulcus to the pathologic periodontal pocket are associated with different proportions of bacterial cells in dental plaque. Healthy gingiva is associated with few microorganisms, mostly coccoid cells and straight rods. Diseased gingiva is associated with increased numbers of spirochetes and motile rods.40,41,43 However, the microbiota of diseased sites cannot be used as a predictor of future attachment or bone loss because their presence alone is not sufficient for disease to start or progress.35
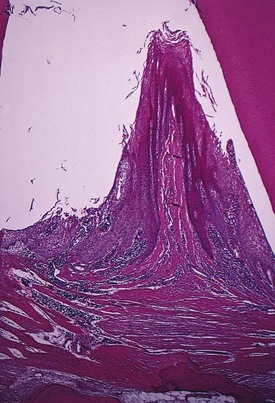
Pocket formation starts as an inflammatory change in the connective tissue wall of the gingival sulcus. The cellular and fluid inflammatory exudate causes degeneration of the surrounding connective tissue, including the gingival fibers. Just apical to the junctional epithelium, collagen fibers are destroyed20,61 and the area is occupied by inflammatory cells and edema (Figure 13-5).
Early concepts assumed that after the initial bacterial attack, periodontal tissue destruction continued to be linked to bacterial action. More recently, it was established that the host’s immunoinflammatory response to the initial and persistent bacterial attack unleashes mechanisms that lead to collagen and bone destruction. These mechanisms are related to various cytokines, some produced normally by cells in noninflamed tissue and others by cells involved in the inflammatory process such as polymorphonuclear leukocytes (PMNs), monocytes, and other cells, leading to collagen and bone destruction. This chapter describes the histologic aspects of gingival inflammation and tissue destruction. For further information on the molecular biology aspects of these mechanisms of tissue destruction the reader is referred to Chapter 25 and online.
The two mechanisms associated with collagen loss are (1) collagenases and other enzymes secreted by various cells in healthy and inflamed tissue, such as fibroblasts,74 PMNs,73 and macrophages,53 become extracellular and destroy collagen (these enzymes that degrade collagen and other matrix macromolecules into small peptides are called matrix metalloproteinases75) and (2) fibroblasts phagocytize collagen fibers by extending cytoplasmic processes to the ligament-cementum interface and degrade the inserted collagen fibrils and the fibrils of the cementum matrix.20,21
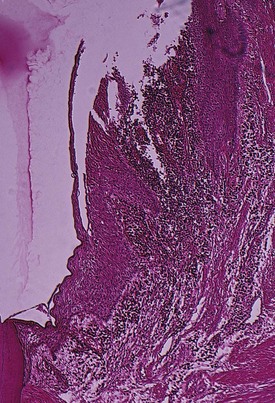
As a consequence of the loss of collagen, the apical cells of the junctional epithelium proliferate along the root, extending fingerlike projections two or three cells in thickness (Figure 13-6).
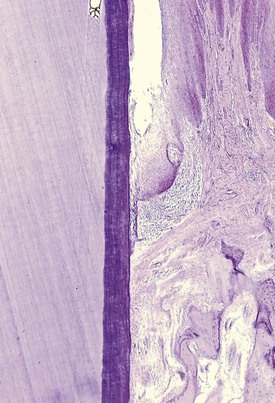
Figure 13-6 Low-power view of base of periodontal pocket and apical area. Note the dense inflammatory infiltrate on area of destroyed collagen fibers and the thin, fingerlike extension of epithelium covering the cementum denuded of fibers.
As a result of inflammation, PMNs invade the coronal end of the junctional epithelium in increasing numbers (Figure 13-7). The PMNs are not joined to one another or to the epithelial cells by desmosomes. When the relative volume of PMNs reaches approximately 60% or more of the junctional epithelium, the tissue loses cohesiveness and detaches from the tooth surface. Thus the coronal portion of the junctional epithelium detaches from the root as the apical portion migrates, resulting in its apical shift, and the oral sulcular epithelium gradually occupies an increasing portion of the sulcus (then a pocket) lining.62
Extension of the junctional epithelium along the root requires the presence of healthy epithelial cells. Marked degeneration or necrosis of the junctional epithelium impairs rather than accelerates pocket formation. (This occurs in necrotizing ulcerative gingivitis resulting in an ulcer and not in pocket formation.)
Degenerative changes seen in the junctional epithelium at the base of periodontal pockets are usually less severe than those in the epithelium of the lateral pocket wall (see Figure 13-7). Because migration of the junctional epithelium requires healthy, viable cells, it is reasonable to assume that the degenerative changes seen in this area occur after the junctional epithelium reaches its position on the cementum.
The degree of leukocyte infiltration of the junctional epithelium is independent of the volume of inflamed connective tissue, thus this process may occur in gingiva with only slight signs of clinical inflammation.61
With continued inflammation, the gingiva increases in bulk and the crest of the gingival margin extends coronally. The apical cells of the junctional epithelium continue to migrate along the root and its coronal cells continue to separate from it. The epithelium of the lateral wall of the pocket proliferates to form bulbous, cordlike extensions into the inflamed connective tissue. Leukocytes and edema from the inflamed connective tissue infiltrate the epithelium lining the pocket, resulting in various degrees of degeneration and necrosis.
The transformation of a gingival sulcus into a periodontal pocket creates an area where plaque removal becomes impossible, and a feedback mechanism is established. The rationale for pocket reduction is based on the need to eliminate areas of plaque accumulation.
Histopathology
Changes occurring in the initial stages of gingival inflammation are presented in Chapter 7. Once the pocket is formed, several microscopic features are present, as discussed in this section.
Soft Tissue Wall
The connective tissue is edematous and densely infiltrated with plasma cells (approximately 80%), lymphocytes, and a scattering of PMNs. The blood vessels are increased in number, dilated, and engorged, particularly in the subepithelial connective tissue layer.10 The connective tissue exhibits varying degrees of degeneration. Single or multiple necrotic foci are occasionally present.52 In addition to exudative and degenerative changes, the connective tissue shows proliferation of the endothelial cells, with newly formed capillaries, fibroblasts, and collagen fibers (see Figure 13-5).
The junctional epithelium at the base of the pocket is usually much shorter than that of a normal sulcus. Although marked variations are found as to length, width, and condition of the epithelial cells,63 usually the coronoapical length of the junctional epithelium is reduced to only 50 to 100 µm.14 The cells may be well formed and in good condition or may exhibit slight-to-marked degeneration (see Figures 13-6 and 13-9).
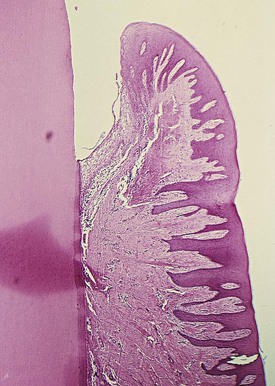
The most severe degenerative changes in the periodontal pocket occur along the lateral wall (see Figure 13-7). The epithelium of the lateral wall of the pocket presents striking proliferative and degenerative changes. Epithelial buds or interlacing cords of epithelial cells project from the lateral wall into the adjacent inflamed connective tissue and may extend farther apically than the junctional epithelium (Figures 13-8, A, and 13-9). These epithelial projections, as well as the remainder of the lateral epithelium, are densely infiltrated by leukocytes and edema from the inflamed connective tissue. The cells undergo vacuolar degeneration and rupture to form vesicles. Progressive degeneration and necrosis of the epithelium lead to ulceration of the lateral wall, exposure of the underlying inflamed connective tissue, and suppuration. In some cases, acute inflammation is superimposed on the underlying chronic changes.
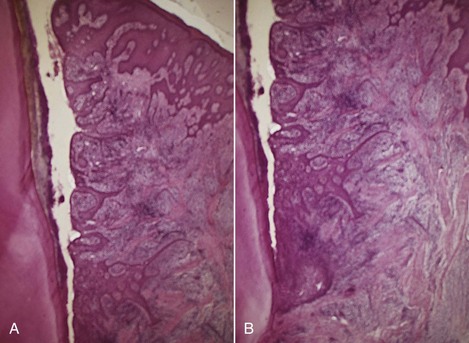
Figure 13-8 A, Lateral wall of periodontal pocket showing epithelial proliferative and atrophic changes and marked inflammatory infiltrate and destruction of collagen fibers. B, Slightly apical view of the same case showing the shortened junctional epithelium.
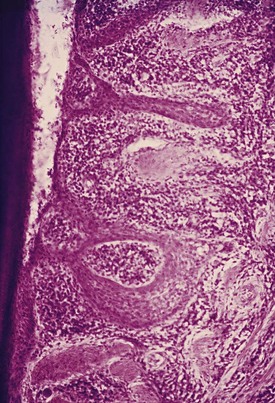
Figure 13-9 Base of periodontal pocket showing extensive proliferation of lateral epithelium next to atrophic areas, dense inflammatory infiltrate, remnants of destroyed collagen fibers, and the junctional epithelium, apparently in a less altered state than lateral pocket epithelium.
A comparative study of gingival changes in aggressive and chronic periodontitis revealed more pronounced degenerative changes in the epithelium of aggressive cases with more open intercellular spaces, with microclefts and necrotic areas.35
The severity of the degenerative changes is not necessarily related to pocket depth. Ulceration of the lateral wall may occur in shallow pockets, and deep pockets are occasionally observed in which the lateral epithelium is relatively intact or shows only slight degeneration.
The epithelium at the gingival crest of a periodontal pocket is generally intact and thickened, with prominent rete pegs.
For a detailed electron microscopic study of the pocket epithelium in experimentally induced pockets in dogs, see Müller-Glauser and Schröder.49
Bacterial Invasion
Bacterial invasion of the apical and lateral areas of the pocket wall has been described in human chronic periodontitis. Filaments, rods, and coccoid organisms with predominant gram-negative cell walls have been found in intercellular spaces of the epithelium.25,26 Hillmann et al35 have reported the presence of Porphyromonas gingivalis and Prevotella intermedia in the gingiva of aggressive periodontitis cases. Actinobacillus actinomycetemcomitans has also been found in the tissues.16,47,58
Bacteria may invade the intercellular space under exfoliating epithelial cells, but they are also found between deeper epithelial cells and accumulating on the basement lamina. Some bacteria traverse the basement lamina and invade the subepithelial connective tissue60 (Figures 13-10 and 13-11).
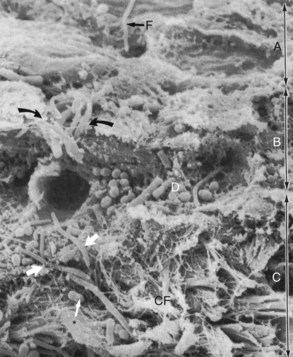
Figure 13-10 Scanning electron micrograph of section of pocket wall in advanced periodontitis in human specimen showing bacterial penetration into the epithelium and connective tissue. Scanning electron microscope view of surface of pocket wall (A), sectioned epithelium (B), and sectioned connective tissue (C). Curved arrows point to areas of bacterial penetration into the epithelium. Thick white arrows point to bacterial penetration into the connective tissue through a break in the continuity of the basal lamina. CF, Connective tissue fibers; D, accumulation of bacteria (rods, cocci, filaments) on basal lamina; F, filamentous organism on surface of epithelium. Asterisk points to coccobacillus in connective tissue.
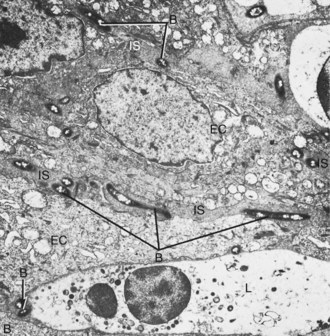
Figure 13-11 Transmission electron micrograph of the epithelium in the periodontal pocket wall showing bacteria in the intercellular spaces. B, Bacteria; EC, epithelial cell; IS, intercellular space; L, leukocyte about to engulf bacteria. (×8000.)
The presence of bacteria in the gingival tissues has been interpreted by different investigators as bacterial invasion or as “passive translocation” of plaque bacteria.42 This important point has significant clinicopathologic implications and has not yet been clarified.17,39,43
Mechanisms of Tissue Destruction
The inflammatory response triggered by bacterial plaque unleashes a complex cascade of events aimed at destroying and removing bacteria, necrotic cells, and deleterious agents. However, this process is nonspecific and in an attempt to restore health, the host’s cells, such as neutrophiles, macrophages, fibroblasts, epithelial cells and others, produce proteinases, cytokines, and prostaglandins that can damage or destroy the tissues.
Chapter 25 describes in detail these aspects of inflammation and the mechanisms of tissue destruction at the molecular level.
Microtopography of Gingival Wall
Scanning electron microscopy has permitted the description of several areas in the soft tissue (gingival) wall of the periodontal pocket in which different types of activity take place.59 These areas are irregularly oval or elongated and adjacent to one another and measure about 50 to 200 µm. These findings suggest that the pocket wall is constantly changing as a result of the interaction between the host and the bacteria. The following areas have been noted:
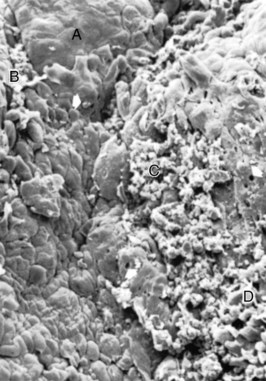
Figure 13-12 Scanning electron frontal micrograph of the periodontal pocket wall. Different areas can be seen in the pocket wall surface. A, Area of quiescence; B, bacterial accumulation; C, bacterial-leukocyte interaction; D, intense cellular desquamation. Arrows point to emerging leukocytes and holes left by leukocytes in the pocket wall. (×800.)
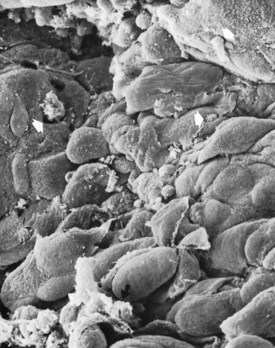
Figure 13-13 Scanning electron micrograph of the periodontal pocket wall, frontal view, in a patient with advanced periodontitis. Note the desquamating epithelial cells and leukocytes (white arrows) emerging onto the pocket space. Scattered bacteria can also be seen (black arrow). (×1500.)
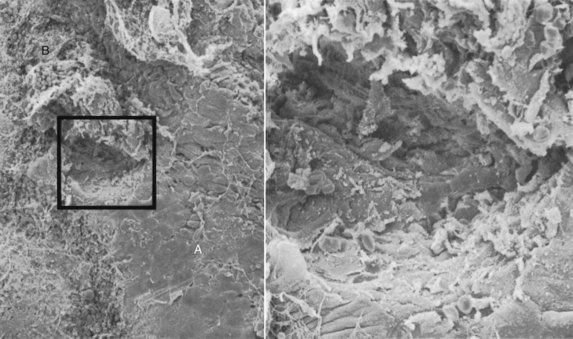
Figure 13-14 Left, Area of ulceration in the lateral wall of a deep periodontal pocket in a human specimen. A, Surface of pocket epithelium in a quiescent state; B, area of hemorrhage. (×800.) Right, Magnification of the square on left. Connective tissue fibers and cells can be seen in the bottom of the ulcer. (Scanning electron microscopy, ×3000.)
![]() Science Transfer
Science Transfer
Clinicians should use their knowledge of the histopathology of periodontal disease as a basis for clinical decision making. As inflammation spreads apically to involve the connective tissue apical to the junctional epithelium, gingivitis then becomes periodontitis. Periodontal probing with gentle pressure generally allows the probe to extend to the apical end of the junctional epithelium in patients with gingival health; however, when inflammatory changes result in less dense collagen fibers in early and advanced periodontitis, special care must be taken not to push the probe too far apically, which results in inaccurate pocket depth recordings and pain for the patient.
One of the earliest and common changes in gingivitis is ulceration of the gingival sulcular epithelium that results in bleeding on probing, which will persist as the disease progresses to periodontitis. This is a sign of continuing bacterial destruction of periodontal tissues. In more severe disease, purulence may be seen in the gingival fluid, which indicates more dramatic and aggressive tissue breakdown.
The transition from one area to another could result from bacteria accumulating in previously quiescent areas and triggering the emergence of leukocytes and the leukocyte-bacteria interaction. This would lead to intense epithelial desquamation and finally to ulceration and hemorrhage.
Periodontal Pockets as Healing Lesions
Periodontal pockets are chronic inflammatory lesions and thus are constantly undergoing repair. Complete healing does not occur because of the persistence of the bacterial attack, which continues to stimulate an inflammatory response, causing degeneration of the new tissue elements formed in the continuous effort at repair.
The condition of the soft tissue wall of the periodontal pocket results from the interplay of the destructive and constructive tissue changes. Their balance determines clinical features such as color, consistency, and surface texture of the pocket wall. If the inflammatory fluid and cellular exudate predominate, the pocket wall is bluish red, soft, spongy, and friable, with a smooth, shiny surface; at the clinical level, this is generally referred to as an edematous pocket wall. If there is a relative predominance of newly formed connective tissue cells and fibers, the pocket wall is more firm and pink, clinically referred to as a fibrotic pocket wall (see Table 13-1 and Chapter 30).
Edematous and fibrotic pockets represent opposite extremes of the same pathologic process not different disease entities. They are subject to constant modification, depending on the relative predominance of exudative and constructive changes.
Fibrotic pocket walls may be misleading because they do not necessarily reflect what is taking place throughout the pocket wall. The most severe degenerative changes in periodontal tissues occur adjacent to the tooth surface and subgingival plaque. In some cases, inflammation and ulceration on the inside of the pocket are walled off by fibrous tissue on the outer aspect (Figure 13-15). Externally the pocket appears pink and fibrotic, despite the inflammatory changes occurring internally.
Pocket Contents
Periodontal pockets contain debris consisting principally of microorganisms and their products (enzymes, endotoxins, and other metabolic products), gingival fluid, food remnants, salivary mucin, desquamated epithelial cells, and leukocytes. Plaque-covered calculus usually projects from the tooth surface (Figure 13-16). Purulent exudate, if present, consists of living, degenerated, and necrotic leukocytes; living and dead bacteria; serum; and a scant amount of fibrin.46 The contents of periodontal pockets filtered free of organisms and debris have been demonstrated to be toxic when injected subcutaneously into experimental animals.31
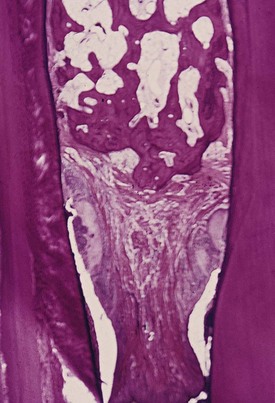
Figure 13-16 Interdental papilla with ulcerated suprabony periodontal pockets on its mesial and distal aspects. Calculus is present on the approximal tooth surfaces and within the gingiva.
Pus is a common feature of periodontal disease, but it is only a secondary sign. The presence of pus or the ease with which it can be expressed from the pocket merely reflects the nature of the inflammatory changes in the pocket wall. It is not an indication of the depth of the pocket or the severity of the destruction of the supporting tissues. Extensive pus formation may occur in shallow pockets, whereas deep pockets may exhibit little or no pus. Localized accumulation of pus constitutes an abscess, which is discussed later in this chapter.
Root Surface Walls
The root surface wall of periodontal pockets often undergoes changes that are significant because they may perpetuate the periodontal infection, cause pain, and complicate periodontal treatment.11
As the pocket deepens, collagen fibers embedded in the cementum are destroyed and cementum becomes exposed to the oral environment. Collagenous remnants of Sharpey’s fibers in the cementum undergo degeneration, creating an environment favorable to the penetration of bacteria. Viable bacteria have been found in the roots of 87% of periodontally diseased noncarious teeth.2 Bacterial penetration into the cementum can be found as deep as the cementodentinal junction1,19 and may also enter the dentinal tubules.29,31 Penetration and growth of bacteria leads to fragmentation and breakdown of the cementum surface and results in areas of necrotic cementum, separated from the tooth by masses of bacteria.
Pathologic granules9 have been observed with light and electron microscopy6,7 and may represent areas of collagen degeneration or areas in which collagen fibrils have not been fully mineralized initially.
In addition, bacterial products, such as endotoxins,3,5 have also been detected in the cementum wall of periodontal pockets. When root fragments from teeth with periodontal disease are placed in tissue culture, they induce irreversible morphologic changes in the cells of the culture. Such changes are not produced by normal roots.33 Diseased root fragments also prevent the in vitro attachment of human gingival fibroblasts, whereas normal root surfaces allow the cells to attach freely.4 When placed in the oral mucosa of the patient, diseased root fragments induce an inflammatory response even if they are autoclaved.44
These changes manifest clinically as softening of the cementum surface, which is usually asymptomatic, but painful when a probe or explorer penetrates the area. They also constitute a possible reservoir for reinfection of the area after treatment. In the course of treatment, these necrotic areas are removed by root planing until a hard, smooth surface is reached. Cementum is very thin in the cervical areas, and scaling and root planing often remove it entirely, exposing the underlying dentin. Sensitivity to cold may result until secondary dentin is formed by the pulp tissue.
Decalcification and Remineralization of Cementum
Areas of increased mineralization65 are probably a result of an exchange, on exposure to the oral cavity, of minerals and organic components at the cementum-saliva interface. The mineral content of exposed cementum increases,64 and the minerals that are increased in diseased root surfaces include calcium,67 magnesium,50,67 phosphorus,50 and fluoride.50 Microhardness, however, remains unchanged.55,81 The development of a highly mineralized superficial layer may increase the tooth resistance to decay.4
The hypermineralized zones are detectable by electron microscopy and are associated with increased perfection of the crystal structure and organic changes suggestive of a subsurface cuticle.64,65 These zones have also been seen in microradiographic studies66 as a layer 10 to 20 mm thick, with areas as thick as 50 mm. No decrease in mineralization was found in deeper areas, thereby indicating that increased mineralization does not come from adjacent areas. A loss of or reduction in the crossbanding of collagen near the cementum surface27,28 and a subsurface condensation of organic material of exogenous origin64 have also been reported.
Areas of demineralization are often related to root caries. Exposure to oral fluid and bacterial plaque results in proteolysis of the embedded remnants of Sharpey’s fibers; the cementum may be softened and may undergo fragmentation and cavitation.34 Unlike enamel caries, root surface caries tend to progress around rather than into the tooth.48 Active root caries lesions appear as well-defined yellowish or light-brown areas, are frequently covered by plaque, and have a softened or leathery consistency on probing. Inactive lesions are well-defined darker lesions with a smooth surface and a harder consistency on probing.24
The dominant microorganism in root surface caries is Actinomyces viscosus,72 although its specific role in the development of the lesion has not been established.24 Other bacteria, such as Actinomyces naeslundii, Streptococcus mutans, Streptococcus salivarius, Streptococcus sanguis, and Bacillus cereus, have been found to produce root caries in animal models. Quirynen et al54 reported that when plaque levels and pocket depths decrease after periodontal therapy (both nonsurgical and surgical), a shift in oral bacteria occurs, leading to a reduction in periodontal pathogens and an increase in S. mutans and the development of root caries.
A prevalence rate study of root caries in 20- to 64-year-old individuals revealed that 42% had one or more root caries lesions and that these lesions tended to increase with age.37
The tooth may not be painful, but exploration of the root surface reveals the presence of a defect, and penetration of the involved area with a probe causes pain. Caries of the root may lead to pulpitis, sensitivity to sweets and thermal changes, or severe pain. Pathologic exposure of the pulp occurs in severe cases. Root caries may be the cause of toothache in patients with periodontal disease and no evidence of coronal decay.
Caries of the cementum requires special attention when the pocket is treated. The necrotic cementum must be removed by scaling and root planing until firm tooth surface is reached, even if this entails extension into the dentin.
Areas of cellular resorption of cementum and dentin are common in roots unexposed by periodontal disease.68 These areas are of no particular significance because they are symptom free, and as long as the root is covered by the periodontal ligament, they are likely to undergo repair. However, if the root is exposed by progressive pocket formation before repair occurs, these areas appear as isolated cavitations that penetrate into the dentin. These areas can be differentiated from caries of the cementum by their clear-cut outline and hard surface. They may be sources of considerable pain, requiring placement of a restoration.
Surface Morphology of Tooth Wall.78
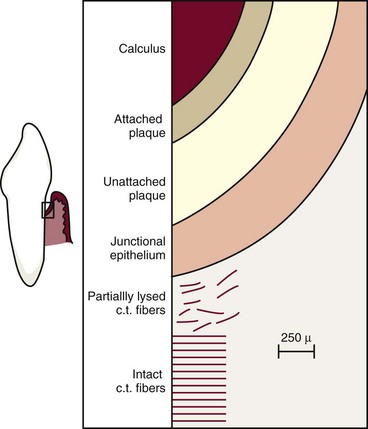
The following zones can be found in the bottom of a periodontal pocket (Figure 13-17):
Zones 3, 4, and 5 make up the “plaque-free zone” seen in extracted teeth.8,12,36,57,78 The total width of the plaque-free zone varies according to the type of tooth (wider in molars than in incisors) and the depth of the pocket (narrower in deeper pockets).56 It is important to remember that the term plaque-free zone refers only to attached plaque because unattached plaque contains a variety of gram-positive and gram-negative morphotypes, including cocci, rods, filaments, fusiforms, and spirochetes. The most apical zone contains predominantly gram-negative rods and cocci.76
Periodontal Disease Activity
For many years the loss of attachment produced by periodontal disease was thought to be a slow but continuously progressive phenomenon. More recently, as a result of studies on the specificity of plaque bacteria, the concept of periodontal disease activity has evolved.
According to this concept, periodontal pockets go through periods of exacerbation and quiescence, resulting from episodic bursts of activity followed by periods of remission. Periods of quiescence are characterized by a reduced inflammatory response and little or no loss of bone and connective tissue attachment. A buildup of unattached plaque, with its gram-negative, motile, and anaerobic bacteria (see Chapter 23), starts a period of exacerbation in which bone and connective tissue attachment are lost and the pocket deepens. This period may last for days, weeks, or months and is eventually followed by a period of remission or quiescence in which gram-positive bacteria proliferate and a more stable condition is established. Based on a study of radioiodine125I absorptiometry, McHenry et al45 confirmed that bone loss in untreated periodontal disease occurs in an episodic manner.
These periods of quiescence and exacerbation are also known as periods of inactivity and periods of activity. Clinically, active periods show bleeding, either spontaneously or with probing, and greater amounts of gingival exudate. Histologically, the pocket epithelium appears thin and ulcerated, and an infiltrate composed predominantly of plasma cells,19 PMNs,53 or both are seen. Bacterial samples from the pocket lumen analyzed with dark-field microscopy show high proportions of motile organisms and spirochetes.43
Methods to detect periods of activity or inactivity are currently being investigated (see online).
Site Specificity
Periodontal destruction does not occur in all parts of the mouth at the same time but rather on a few teeth at a time or even only some aspects of some teeth at any given time. This is referred to as the site specificity of periodontal disease. Sites of periodontal destruction are often found next to sites with little or no destruction. Therefore the severity of periodontitis increases with the development of new disease sites and with the increased breakdown of existing sites.
Pulp Changes Associated with Periodontal Pockets
The spread of infection from periodontal pockets may cause pathologic changes in the pulp. Such changes may give rise to painful symptoms or may adversely affect the response of the pulp to restorative procedures. Involvement of the pulp in periodontal disease occurs through either the apical foramen or the lateral pulp canals after pocket infection reaches then. Atrophic and inflammatory pulpal changes occur in such cases (see Chapters 52 and 63).
Relationship of Attachment Loss and Bone Loss to Pocket Depth
The severity of the attachment loss in pocket formation is generally but not always correlated with the depth of the pocket. This is because the degree of attachment loss depends on the location of the base of the pocket on the root surface, whereas pocket depth is the distance between the base of the pocket and the crest of the gingival margin. Pockets of the same depth may be associated with different degrees of attachment loss (Figure 13-18), and pockets of different depths may be associated with the same amount of attachment loss (Figure 13-19).
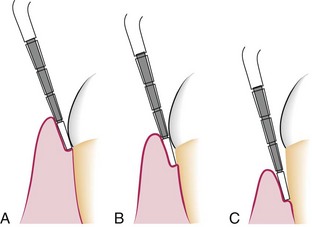
Figure 13-18 Same pocket depth with different amounts of recession. A, Gingival pocket with no recession. B, Periodontal pocket of similar depth as in A but with some degree of recession. C, Pocket depth same as in A and B but with still more recession.
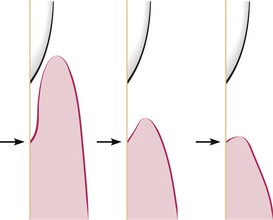
Figure 13-19 Different pocket depths with the same amount of attachment loss. Arrows point to bottom of the pocket. The distance between the arrow and the cementoenamel junctions remains the same despite different pocket depths.
Severity of bone loss is generally, but not always, correlated with pocket depth. Extensive attachment and bone loss may be associated with shallow pockets if the attachment loss is accompanied by recession of the gingival margin, and slight bone loss can occur with deep pockets.
Area Between Base of Pocket and Alveolar Bone
Normally, the distance between the apical end of the junctional epithelium and the alveolar bone is relatively constant. The distance between the apical extent of calculus and the alveolar crest in human periodontal pockets is most constant, having a mean length of 1.97 mm (±33.16%).71,77
The distance from attached plaque to bone is never less than 0.5 mm and never more than 2.7 mm.78-80 These findings suggest that the bone-resorbing activity induced by the bacteria is exerted within these distances. However, the finding of isolated bacteria or clumps of bacteria in the connective tissue60 and on the bone surface26 may modify these considerations.
Relationship of Pocket to Bone
In infrabony pockets, the base of the pocket is apical to the crest of the alveolar bone, and the pocket wall lies between the tooth and the bone. The bone loss is in most cases vertical. In suprabony pockets on the other hand, the base is coronal to the crest of the alveolar bone and the pocket wall lies coronal to the bone. The type of bone loss is always horizontal.
This creates some microscopic differences that have some therapeutic importance. They are the relationship of the soft tissue wall of the pocket to the alveolar bone, the pattern of bone destruction, and the direction of the transseptal fibers of the periodontal ligament15 (Figures 13-20 to 13-22).
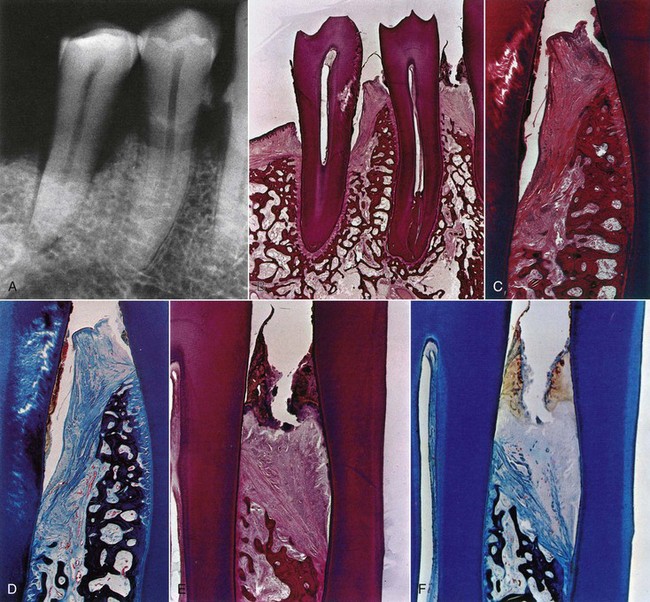
Figure 13-20 Radiographic and microscopic features of intrabony pockets. A, Radiograph of mandibular canine and premolar area showing angular bone loss mesial to second premolar. Type of bone loss between first premolar and canine is not radiographically apparent. B, Mesiodistal histologic section of teeth seen in A showing intrabony pocket mesial to second premolar, as well as suprabony pockets distal to second premolar and mesial and distal to first premolar. The mesial suprabony pocket of first premolar appears coronal to a vertical bone loss. C, Higher-power view of area between the premolars. Note the angular bone loss and transseptal fibers covering the bone. D, Higher-power view of area between the premolars stained with Mallory connective tissue stain, clearly showing the direction of transseptal fibers. E, Higher-power view of area between first premolar and canine. Note the abundant calculus, dense leukocytic infiltration of the gingiva, and angulation of transseptal fibers and bone. The pocket is still suprabony. F, Mallory stain of similar area as in E. Note destruction of the gingival fibers caused by inflammation and the angular fibers formed over angular bone loss and less affected by the inflammation. Transseptal fibers extend from distal surface of premolar over crest of alveolar bone into the intrabony pocket. Note the leukocytic infiltration of the transseptal fibers.
(From Glickman I, Smulow J: Periodontal disease: clinical, radiographic and histopathologic features, Philadelphia, 1974, Saunders.)
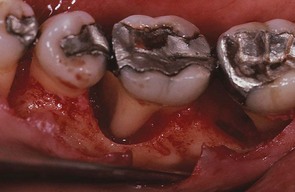
Figure 13-21 After raising a flap for the treatment of the infrabony pockets, the vertical bone loss around the mesial roots of the mandibular first and second molars can be seen.
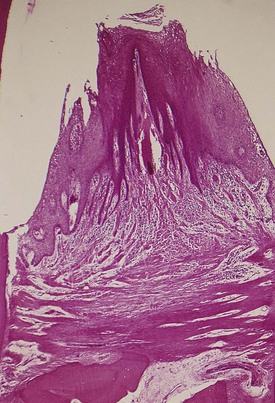
Figure 13-22 Two suprabony pockets in an interdental space. Note the normal horizontal arrangement of the transseptal fibers.
In suprabony pockets, the alveolar crest gradually attains a more apical position in relation to the tooth but retains its general morphology and architecture. The interdental fibers that run over the bone from one tooth to the other maintain their usual horizontal direction. In infrabony pockets, the morphology of the alveolar crest changes completely, with the formation of an angular bony defect. The interdental fibers in this case run over the bone in an oblique direction between the two teeth of the interdental space.15,80 This may affect the function of the area and also necessitate a modification in treatment techniques (see Chapters 60 and 62).13,15 Table 13-2 summarizes the distinguishing features of suprabony and infrabony pockets. The classification of infrabony pockets is discussed in Chapter 14.
TABLE 13-2 Distinguishing Features of Suprabony and Intrabony Periodontal Pockets
| Suprabony Pocket | Intrabony Pocket |
|---|---|
| 1. Base of pocket is coronal to level of alveolar bone. | 1. Base of pocket is apical to crest of alveolar bone so that the bone is adjacent to soft tissue wall (see Figure 13-2). |
| 2. Pattern of destruction of underlying bone is horizontal. | 2. Pattern of bone destruction is vertical (angular) (see Figures 13-20 and 13-21). |
| 3. Interproximally, transseptal fibers that are restored during progressive periodontal disease are arranged horizontally in the space between base of pocket and alveolar bone (see Figure 13-22). | 3. Interproximally, transseptal fibers are oblique rather than horizontal. They extend from cementum beneath base of pocket along alveolar bone and over crest to cementum of adjacent tooth (see Figure 13-20). |
| 4. On facial and lingual surfaces, periodontal ligament fibers beneath pocket follow their normal horizontal-oblique course between the tooth and bone. | 4. On facial and lingual surfaces, periodontal ligament fibers follow angular pattern of adjacent bone. They extend from cementum beneath base of pocket along alveolar bone and over crest to join with outer periosteum. |
Periodontal Abscess
A periodontal abscess is a localized purulent inflammation in the periodontal tissues (Figure 13-23). It is also known as a lateral abscess or parietal abscess. Abscesses localized in the gingiva, caused by injury to the outer surface of the gingiva, and not involving the supporting structures are called gingival abscesses. Gingival abscesses may occur in the presence or absence of a periodontal pocket (see Chapter 9).
Periodontal abscess formation may occur in the following ways:
Periodontal abscesses are classified according to location as follows:
Microscopically, an abscess is a localized accumulation of viable and nonviable PMNs within the periodontal pocket wall. The PMNs liberate enzymes that digest the cells and other tissue structures, forming the liquid product known as pus, which constitutes the center of the abscess. An acute inflammatory reaction surrounds the purulent area, and the overlying epithelium exhibits intracellular and extracellular edema and invasion of leukocytes (Figure 13-24).
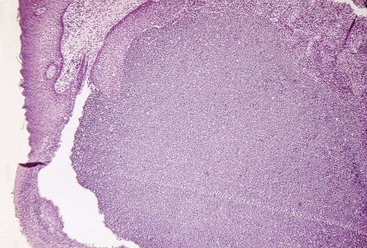
Figure 13-24 Microscopic view of a periodontal abscess showing dense accumulation of polymorphonuclear leukocytes (PMNs) covered by squamous epithelium.
The localized acute abscess becomes a chronic abscess when its purulent content drains through a fistula into the outer gingival surface or into the periodontal pocket and the infection causing the abscess is not resolved.
Bacterial invasion of tissues has been reported in abscesses; the invading organisms were identified as gram-negative cocci, diplococci, fusiforms, and spirochetes. Invasive fungi were also found and were interpreted as being “opportunistic invaders.”22 Microorganisms that colonize the periodontal abscess have been reported to be primarily gram-negative anaerobic rods.51
Lateral Periodontal Cyst
The periodontal cyst, also called lateral periodontal cyst, is an uncommon lesion that produces localized destruction of the periodontal tissues along a lateral root surface, most often in the mandibular canine-premolar area.23,70 It is considered to be derived from rests of Malassez or other proliferating odontogenic rests.69
A periodontal cyst is usually asymptomatic, without grossly detectable changes, but it may present as a localized, tender swelling. Radiographically, an interproximal periodontal cyst appears on the side of the root as a radiolucent area bordered by a radiopaque line. Its radiographic appearance cannot be differentiated from that of a periodontal abscess.
Microscopically, the cystic lining may be (1) a loosely arranged, thin, nonkeratinized, epithelium, sometimes with thicker proliferating areas or (2) an odontogenic keratocyst.23
1 Adriaens PA, DeBoever JA. Ultrastructural study of bacterial invasion in roots of periodontally diseased, caries-free human teeth. J Dent Res. 1986;65:770.
2 Adriaens PA, DeBoever JA, Loesche WJ. Bacterial invasion in root cementum and radicular dentin of periodontally diseased teeth in humans: a reservoir of periodontopathic bacteria. J Periodontol. 1988;59:222.
3 Aleo JJ, Vandersall DC. Cementum: recent concepts related to periodontal disease therapy. Dent Clin North Am. 1980;24:627.
4 Aleo JJ, DeRenzis FA, Farber PA. In vitro attachment of human gingival fibroblasts to root surfaces. J Periodontol. 1975;46:639.
5 Aleo JJ, DeRenzis FA, Farber PA, et al. The presence and biologic activity of cementum-bound endotoxin. J Periodontol. 1974;45:672.
6 Armitage GC, Christie TM. Structural changes in exposed cementum. I. Light microscopic observations. J Periodontal Res. 1973;8:343.
7 Armitage GC, Christie TM. Structural changes in exposed cementum. II. Electronmicroscopic observations. J Periodontal Res. 1973;8:356.
8 Bass CC. A demonstrable line on extracted teeth indicating the location of the outer border of the epithelial attachment. J Dent Res. 1946;25:401.
9 Bass CC. A previously undescribed demonstrable pathologic condition in exposed cementum and the underlying dentine. Oral Surg. 1951;4:641.
10 Bonakdar MPS, Barber PM, Newman HN. The vasculature in chronic adult periodontitis: a qualitative and quantitative study. J Periodontol. 1997;68:50.
11 Bosshardt DD, Selvig KA. Dental cementum: the dynamic tissue covering the root. Periodontol 2000. 1997;13:41.
12 Brady JM. A plaque-free zone on human teeth: scanning and transmission electron microscopy. J Periodontol. 1973;44:416.
13 Carranza FA, Carranza FAJr. The management of alveolar bone in the treatment of the periodontal pocket. J Periodontol. 1956;27:29.
14 Carranza FAJr. Histometric evaluation of periodontal pathology: a review of recent studies. J Periodontol. 1967;38:741.
15 Carranza FAJr, Glickman I. Some observations on the microscopic features of the infrabony pockets. J Periodontol. 1957;28:33.
16 Christersson LA, Albini B, Zambon JJ, et al. Tissue localization of Actinobacillus actinomycetemcomitans in human periodontitis. I. Light, immunofluorescence and electronmicroscopic studies. J Periodontol. 1987;58:529.
17 Coons DB, Charbeneau TD, Rivera-Hidalgo F. Quantification of bacterial penetration in spontaneous periodontal disease in beagle dogs. J Periodontol. 1989;60:23.
18 Daly CG, Seymour GJ, Kieser JB, et al. Histological assessment of periodontally involved cementum. J Clin Periodontol. 1982;9:266.
19 Davenport RHJr, Simpson DM, Hassell TM. Histometric comparison of active and inactive lesions of advanced periodontitis. J Periodontol. 1982;53:285.
20 Deporter DA, Brown DJ. Fine structural observations on the mechanisms of loss of attachment during experimental periodontal disease in the rat. J Periodontal Res. 1980;15:304.
21 Deporter DA, Ten Cate AR. Collagen resorption by periodontal ligament fibroblasts at the hard tissue–ligament interfaces of the mouse molar periodontium. J Periodontol. 1980;51:429.
22 DeWitt GV, Cobb CM, Killoy WJ. The acute periodontal abscess: microbial penetration of the soft tissue wall. Int J Periodont Restor Dent. 1985;5:39.
23 Fantasia JE. Lateral periodontal cyst: an analysis of 46 cases. Oral Surg Oral Med Oral Pathol. 1979;48:237.
24 Fejerskov O, Nyvad B. Pathology and treatment of dental caries in the aging individual. In: Holm-Pedersen P, Löe H, editors. Geriatric dentistry. Copenhagen: Munksgaard, 1986.
25 Frank RM. Bacterial penetration in the apical wall of advanced human periodontitis. J Periodontal Res. 1980;15:563.
26 Frank RM, Voegel RC. Bacterial bone resorption in advanced cases of human periodontitis. J Periodontal Res. 1978;13:251.
27 Furseth R. Further observations on the fine structure of orally exposed carious human dental cementum. Arch Oral Biol. 1971;16:71.
28 Furseth R, Johanson E. The mineral phase of sound and carious human dental cementum studies by electron microscopy. Acta Odontol Scand. 1970;28:305.
29 Giuliana G, Ammatuna P, Pizzo G, et al. Occurrence of invading bacteria in radicular dentin of periodontally diseased teeth: microbiological findings. J Clin Periodontol. 1997;24:478.
30 Glickman I, Smulow JB. Periodontal disease: clinical, radiographic, and histopathologic features. Philadelphia: Saunders; 1974.
31 Graham JW. Toxicity of sterile filtrate from parodontal pockets. Proc R Soc Med. 1937;30:1165.
32 Greenstein G, Lamster I. Changing periodontal paradigms: therapeutic implications. Int J Periodont Restor Dent. 2000;20:337.
33 Hatfield CG, Baumhammers A. Cytotoxic effects of periodontally involved surfaces of human teeth. Arch Oral Biol. 1971;16:465.
34 Herting HC. Electron microscope studies of the cementum surface structures of periodontally healthy and diseased teeth. J Dent Res. 1967;46(suppl):127.
35 Hillmann G, Dogan S, Gewrtsen W. Histopathological investigation of gingival tissue from patients with rapidly progressive periodontitis. J Periodontol. 1998;69:195.
36 Hoffman ID, Gold W. Distances between plaque and remnants of attached periodontal tissues on extracted teeth. J Periodontol. 1971;42:29.
37 Katz RV, Hazen SP, Chilton NW, et al. Prevalence and intraoral distribution of root caries in an adult population. Caries Res. 1982;16:265.
38 Krayer JW, Rees TD. Histologic observations on the topography of a human periodontal pocket viewed in transverse step-serial sections. J Periodontol. 1993;64:585.
39 Liakoni H, Barber P, Newman HN. Bacterial penetration of pocket soft tissues in chronic adult and juvenile periodontitis cases: an ultrastructural study. J Clin Periodontol. 1987;14:22.
40 Lindhe J, Liljenberg B, Listgarten MA. Some microbiological and histopathological features of periodontal disease in man. J Periodontol. 1980;52:264.
41 Listgarten MA. Structure of the microbial flora associated with periodontal health and disease in man. J Periodontol. 1976;47:1.
42 Listgarten MA. Pathogenesis of periodontitis. J Clin Periodontol. 1986;13:418.
43 Listgarten MA, Hellden L. Relative distributions of bacteria at clinically healthy and periodontally diseased sites in humans. J Clin Periodontol. 1978;5:665.
44 Lopez NJ, Belvederessi M, de la Sotta R. Inflammatory effects of periodontally diseased cementum studied by autogenous dental root implants in humans. J Periodontol. 1980;51:582.
45 McHenry KR, Hausman E, Genco RJ, et al. 125I absorptiometry: alveolar bone mass measurements in untreated periodontal disease. J Dent Res. 1981;60(suppl A):387. (abstract)
46 McMillan L, Burrill DY, Fosdick LS. An electron microscope study of particulates in periodontal exudate. J Dent Res. 1958;37:51. (abstract)
47 Meyer DH, Screenivasan PK, Fives-Taylor PM. Evidence for the invasion of a human oral cell line by Actinobacillus actinomycetemcomitans. Infect Immun. 1991;59:2719.
48 Mount GJ. Root surface caries: a recurrent dilemma. Aust Dent J. 1986;31:288.
49 Müller-Glauser W, Schröder HE. The pocket epithelium: a light and electron microscopic study. J Periodontol. 1982;53:133.
50 Nakata T, Stepnick R, Zipkin I. Chemistry of human dental cementum: the effect of age and exposure on the concentration of F, Ca, P and Mg. J Periodontol. 1972;43:115.
51 Newman MG, Sims TN. The predominant cultivable microbiota of the periodontal abscess. J Periodontol. 1979;50:350.
52 Orban B, Ray HG. Deep necrotic foci in the gingiva. J Periodontol. 1948;19:91.
53 Page RC, Schroeder HH. Structure and pathogenesis. In: Schluger S, Youdelis R, Page R, editors. Periodontal disease. Philadelphia: Lea & Febiger, 1977.
54 Quirynen M, Gizani S, Mongardini C, et al. The effect of periodontal therapy on the number of cariogenic bacteria in different intraoral niches. J Clin Periodontol. 1999;26:322.
55 Rautiola CA, Craig RG. The micro hardness of cementum and underlying dentin of normal teeth and teeth exposed to periodontal disease. J Periodontol. 1961;32:113.
56 Saglie FR, Johansen JR, Flotra L. The zone of completely and partially destroyed periodontal fibers in pathologic pockets. J Clin Periodontol. 1975;2:198.
57 Saglie FR, Johansen JR, Tollefsen T. Plaque-free zones on human teeth in periodontitis. J Clin Periodontol. 1975;2:190.
58 Saglie FR, Marfany A, Camargo P. Intragingival occurrence of Actinobacillus actinomycetemcomitans and Bacteroides gingivalis in active destructive periodontal lesions. J Periodontol. 1988;59:259.
59 Saglie FR, Carranza FAJr, Newman MG, et al. Scanning electron microscopy of the gingival wall of deep periodontal pockets in humans. J Periodontal Res. 1982;17:284.
60 Saglie FR, Newman MG, Carranza FAJr, et al. Bacterial invasion of gingiva in advanced periodontitis in humans. J Periodontol. 1982;53:217.
61 Schroeder HE. Quantitative parameters of early human gingival inflammation. Arch Oral Biol. 1970;15:383.
62 Schroeder HE, Attstrom R, The borderland between caries and periodontal disease, vol 2, 1980, Grune & Stratton, London
63 Schroeder HE, The periodontium, 1986, Springer Verlag, Berlin
64 Selvig KA. Ultrastructural changes in cementum and adjacent connective tissue in periodontal disease. Acta Odontol Scand. 1966;24:459.
65 Selvig KA. Biological changes at the tooth-saliva interface in periodontal disease. J Dent Res. 1969;48(suppl):846.
66 Selvig KA, Hals E. Periodontally diseased cementum studied by correlated microradiography, electron probe analysis and electron microscopy. J Periodontal Res. 1977;12:419.
67 Selvig KA, Zander HA. Chemical analysis and microradiography of cementum and dentin from periodontally diseased human teeth. J Periodontol. 1962;33:303.
68 Sottosanti JS. A possible relationship between occlusion, root resorption, and the progression of periodontal disease. J West Soc Periodontol. 1977;25:69.
69 Spouge JD. A new look at the rests of Malassez: a review of their embryological origin, anatomy, and possible role in periodontal health and disease. J Periodontol. 1980;51:437.
70 Standish SN, Shafer WG. The lateral periodontal cyst. J Periodontol. 1958;29:27.
71 Stanley HR. The cyclic phenomenon of periodontitis. Oral Surg Oral Med Oral Pathol. 1955;8:598.
72 Syed SA, Loesche WJ, Pape HL, et al. Predominant cultivable flora isolated from human root surface caries plaque. Infect Immun. 1975;11:727.
73 Taichman N. Potential mechanisms of tissue destruction in periodontal disease. J Dent Res. 1968;47:928.
74 Takada T, Donath K. The mechanism of pocket formation: a light microscopic study of undecalcified human material. J Periodontol. 1988;59:215.
75 Ten Cate AR. Fibroblasts and their products. In Ten Cate JR, editor: Oral histology: development, structure, and function, ed 4, St Louis: Mosby, 1994.
76 Vrahopoulos TP, Barber PM, Newman HN. The apical border plaque in severe periodontitis: an ultrastructural study. J Periodontol. 1995;66:113.
77 Wade AB: The relation between the pocket base, the epithelial attachment and the alveolar process. In Les Parodontopathies, 16th ARPA Congress, Vienna, 1960.
78 Waerhaug J. The gingival pocket. Odont Tidsk. 60(suppl 1), 1952.
79 Waerhaug J. The angular bone defect and its relationship to trauma from occlusion and downgrowth of subgingival plaque. J Clin Periodontol. 1979;6:61.
80 Waerhaug J. The infrabony pocket and its relationship to trauma from occlusion and subgingival plaque. J Periodontol. 1979;50:355.
81 Warren EB, Hanse NM, Swartz ML, et al. Effects of periodontal disease and of calculus solvents on microhardness of cementum. J Periodontol. 1964;35:505.
82 Wittwer JW, Dickler EH, Toto PD. Comparative frequencies of plasma cells and lymphocytes in gingivitis. J Periodontol. 1969;40:274.
83 Zander HA. The attachment of calculus to root surfaces. J Periodontol. 1953;24:16.