CHAPTER 9 Gingival Enlargement
Increase in size of the gingiva is a common feature of gingival disease. Accepted current terminology for this condition is gingival enlargement or gingival overgrowth. These are strictly clinical descriptive terms and avoid the erroneous pathologic connotations of terms used in the past, such as “hypertrophic gingivitis” or “gingival hyperplasia.”
The many types of gingival enlargement can be classified according to etiologic factors and pathologic changes as follows:
Using the criteria of location and distribution, gingival enlargement is designated as follows:
The degree of gingival enlargement can be scored as follows16:
Inflammatory Enlargement
Gingival enlargement may result from chronic or acute inflammatory changes; chronic changes are much more common. In addition, inflammatory enlargements usually are a secondary complication to any of the other types of enlargement, creating a combined gingival enlargement. In these cases, it is important to understand the double etiology and treat them adequately.
Chronic Inflammatory Enlargement
Clinical Features
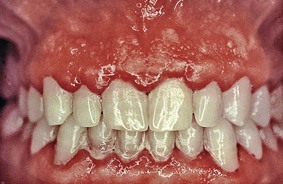
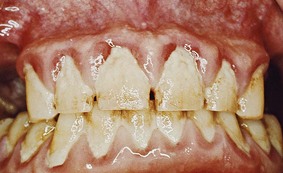
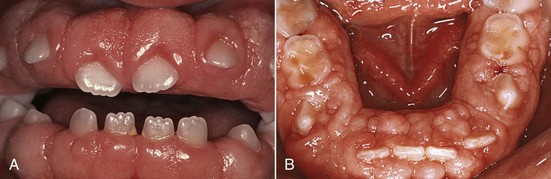
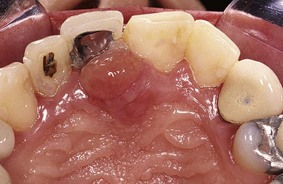
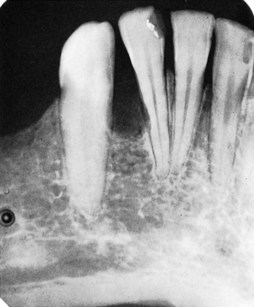
Chronic inflammatory gingival enlargement originates as a slight ballooning of the interdental papilla and marginal gingiva. In the early stages, it produces a life preserver–shaped bulge around the involved teeth. This bulge can increase in size until it covers part of the crowns. The enlargement may be localized or generalized and progresses slowly and painlessly, unless it is complicated by acute infection or trauma (Figures 9-1 and 9-2).
Occasionally, chronic inflammatory gingival enlargement occurs as a discrete sessile or pedunculated mass resembling a tumor. It may be interproximal or on the marginal or attached gingiva. The lesions are slow-growing masses and usually painless. They may undergo spontaneous reduction in size, followed by exacerbation and continued enlargement. Painful ulceration sometimes occurs in the fold between the mass and the adjacent gingiva.
Histopathology
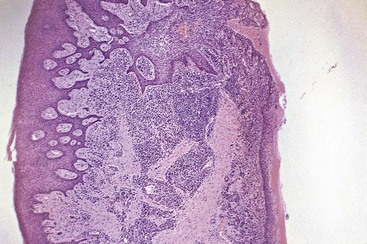
Chronic inflammatory gingival enlargements show the exudative and proliferative features of chronic inflammation (Figure 9-3). Lesions that are clinically deep red or bluish red are soft and friable with a smooth, shiny surface, and they bleed easily. They also have a preponderance of inflammatory cells and fluid, with vascular engorgement, new capillary formation, and associated degenerative changes. Lesions that are relatively firm, resilient, and pink have a greater fibrotic component with an abundance of fibroblasts and collagen fibers.
Etiology
Chronic inflammatory gingival enlargement is caused by prolonged exposure to dental plaque. Factors that favor plaque accumulation and retention48 include poor oral hygiene, irritation by anatomic abnormalities, and improper restorative and orthodontic appliances.
Gingival Changes Associated with Mouth Breathing
Gingivitis and gingival enlargement are often seen in mouth breathers. The gingiva appears red and edematous, with a diffuse surface shininess of the exposed area. The maxillary anterior region is the common site of such involvement. In many cases the altered gingiva is clearly demarcated from the adjacent unexposed normal gingiva (Figure 9-4). The exact manner in which mouth breathing affects gingival changes has not been demonstrated. Its harmful effect is generally attributed to irritation from surface dehydration. However, comparable changes could not be produced by air drying the gingiva of experimental animals.67
Acute Inflammatory Enlargement
Gingival Abscess
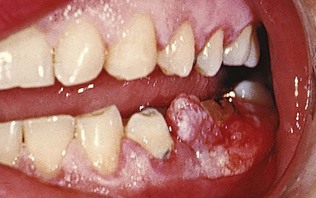
A gingival abscess is a localized, painful, rapidly expanding lesion that usually has a sudden onset (Figure 9-5). It is generally limited to the marginal gingiva or interdental papilla. In its early stages, it appears as a red swelling with a smooth, shiny surface. Within 24 to 48 hours, the lesion usually becomes fluctuant and pointed with a surface orifice from which a purulent exudate may be expressed. The adjacent teeth are often sensitive to percussion. If permitted to progress, the lesion generally ruptures spontaneously.
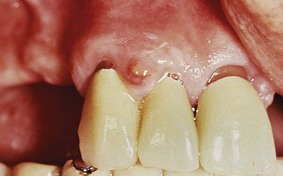
Figure 9-5 Gingival abscess on facial gingival surface, in space between cuspid and lateral incisor, unrelated to the gingival sulcus area.
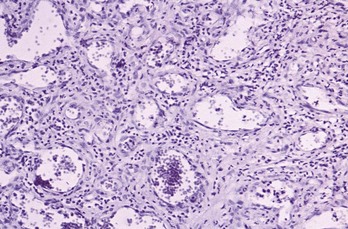
Histopathology
The gingival abscess consists of a purulent focus in the connective tissue, surrounded by a diffuse infiltration of polymorphonuclear leukocytes (PMNs), edematous tissue, and vascular engorgement. The surface epithelium has varying degrees of intracellular and extracellular edema, invasion by leukocytes, and sometimes ulceration.
Etiology
Acute inflammatory gingival enlargement results from bacteria carried deep into the tissues when a foreign substance (e.g., toothbrush bristle, piece of apple core, or lobster shell fragment) is forcefully embedded into the gingiva. The lesion is confined to the gingiva and should not be confused with periodontal or lateral abscesses.
Periodontal (Lateral) Abscess
Periodontal abscesses involve the supporting periodontal tissues and generally produce enlargement of the gingiva. For a detailed description of periodontal abscesses, see Chapter 13.
![]() Science Transfer
Science Transfer
Gingival enlargement is seen in many different forms and has many different etiologies. Often there is a history of medication that has induced gingival hyperplasia where the response to plaque bacteria has an accentuation of tissue proliferation, e.g., hydantoin, calcium channel blockers, and immunosuppressants. Other causes such as pregnancy, puberty, mouth breathing, leukemia, sarcoidosis, and Wegener’s granulomatosis are also to be considered. A localized gingival enlargement that has no obvious etiological basis should be biopsied so that malignant neoplastic disease is identified and treated and also to provide the basis for treatment of other less dangerous entities such as fibromas, papillomas, peripheral giant cell granulomas, and gingival cysts.
Drug-Induced Gingival Enlargement
Gingival enlargement is a well-known consequence of the administration of some anticonvulsants, immunosuppressants, and calcium channel blockers and may create speech, mastication, tooth eruption, and aesthetic problems.
Clinical and microscopic features of the enlargements caused by the different drugs are similar.19,87 These are presented first, followed by a description of the particular features of each drug.
General Information
Clinical Features
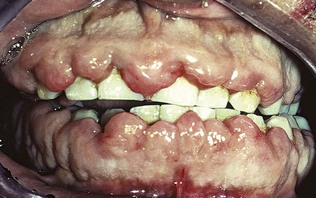
The growth starts as a painless, beadlike enlargement of the interdental papilla and extends to the facial and lingual gingival margins (Figure 9-6). As the condition progresses, the marginal and papillary enlargements unite and may develop into a massive tissue fold covering a considerable portion of the crowns; they may interfere with occlusion (Figure 9-7).
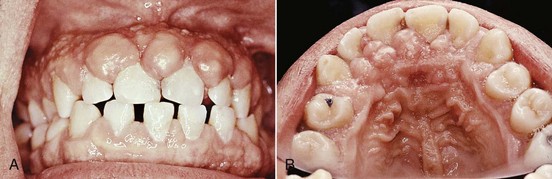
Figure 9-6 Gingival enlargement associated with phenytoin therapy. A, Facial view; note the prominent papillary lesions and the firm, nodular surface. B, Occlusal view of upper jaw.
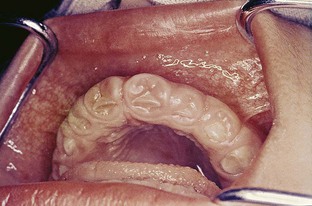
Figure 9-7 Phenytoin gingival enlargement in a 5-year-old child covering most of the clinical crowns of teeth.
When uncomplicated by inflammation, the lesion is mulberry shaped, firm, pale pink, and resilient, with a minutely lobulated surface and no tendency to bleed. The enlargement characteristically appears to project from beneath the gingival margin, from which it is separated by a linear groove. However, the presence of the enlargement makes plaque control difficult, often resulting in a secondary inflammatory process that complicates the gingival overgrowth caused by the drug.
The resultant enlargement then becomes a combination of the increase in size caused by the drug and the complicating inflammation caused by bacteria. Secondary inflammatory changes not only add to the size of the lesion caused by the drug but also produce a red or bluish red discoloration, obliterate the lobulated surface demarcations, and increase bleeding tendency (Figure 9-8).
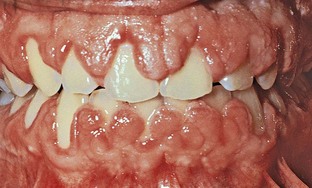
Figure 9-8 Combined gingival enlargement resulting from the inflammatory involvement of a phenytoin-induced overgrowth.
The enlargement is usually generalized throughout the mouth but is more severe in the maxillary and mandibular anterior regions. It occurs in areas in which teeth are present, not in edentulous spaces, and the enlargement disappears in areas from which teeth are extracted. Hyperplasia of the mucosa in edentulous mouths has been reported but is rare.26,27
Drug-induced enlargement may occur in mouths with little or no plaque and may be absent in mouths with abundant deposits. Some investigators, however, believe that inflammation is a prerequisite for development of the enlargement, which therefore could be prevented by plaque removal and fastidious oral hygiene.22,37,79,102 Oral hygiene by means of toothbrushing or use of a chlorhexidine toothpaste91 reduces the inflammation but does not lessen or prevent the overgrowth. Hassell et al42,44 have hypothesized that in noninflamed gingiva, fibroblasts are less active or even quiescent and do not respond to circulating phenytoin, whereas fibroblasts within inflamed tissue are in an active state as a result of the inflammatory mediators and the endogenous growth factors present.
A genetic predisposition is a suspected factor45,84 in determining whether a person treated with phenytoin will develop gingival enlargement or not.
The enlargement is chronic and slowly increases in size. When surgically removed, it recurs. Spontaneous disappearance occurs within a few months after discontinuation of the drug. See Chapter 58 for treatment of gingival enlargements, including the substitution of drugs that do not induce gingival overgrowth.
Histopathology
Drug-induced gingival enlargement consists of a pronounced hyperplasia of the connective tissue and epithelium (Figure 9-9). There is acanthosis of the epithelium, and elongated rete pegs extend deep into the connective tissue, which exhibits densely arranged collagen bundles with an increase in the number of fibroblasts and new blood vessels.90 An abundance of amorphous ground substance has also been reported.68 Structural changes in the outer epithelial cell surface have been reported in cyclosporine-induced enlargements.3
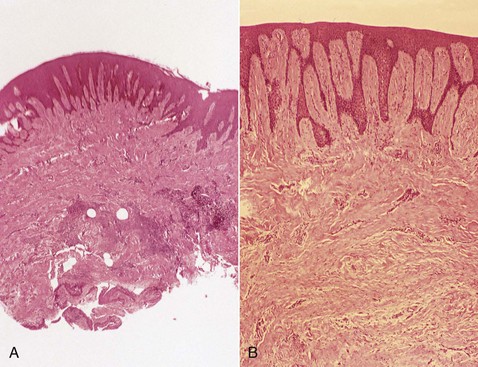
Figure 9-9 Microscopic view of gingival enlargement associated with phenytoin therapy. A, Hyperplasia and acanthosis of the epithelium and densely collagenous connective tissue, with evidence of inflammation in the area adjacent to the gingival sulcus (pocket). B, High-power view showing extension of deep rete pegs into the connective tissue.
The enlargement begins as a hyperplasia of the connective tissue core of the marginal gingiva and increases by its proliferation and expansion beyond the crest of the gingival margin. An inflammatory infiltrate may be found at the bottom of the sulcus, or pocket. Cyclosporine enlargements usually have a more highly vascularized connective tissue with foci of chronic inflammatory cells, particularly plasma cells.75
The “mature” phenytoin enlargement has a fibroblast/collagen ratio equal to that of normal gingiva from normal individuals, suggesting that at some point in the development of the lesion, fibroblastic proliferation must have been abnormally high.43 Recurring phenytoin enlargements appear as granulation tissue composed of numerous young capillaries and fibroblasts and irregularly arranged collagen fibrils with occasional lymphocytes.
Anticonvulsants
The first drug-induced gingival enlargements reported were those produced by phenytoin (Dilantin). Dilantin is a hydantoin, introduced by Merritt and Putnam69 in 1938 for the treatment of all forms of epilepsy, except petit mal. Shortly thereafter, its relationship with gingival enlargement was reported.35,57
Other hydantoins known to induce gingival enlargement are ethotoin (Peganone) and mephenytoin (Mesantoin). Other anticonvulsants that have the same side effect are the succinimides (ethosuximide [Zarontin], methsuximide [Celontin]), and valproic acid [Depakene]).38
Gingival enlargement occurs in about 50% of patients receiving the drug,110 although different authors have reported incidences from 3% to 84.5%.2,35,80 It occurs more often in younger patients.5 Its occurrence and severity are not necessarily related to the dosage after a threshold level has been exceeded.97 Phenytoin appears in the saliva. There is no consensus, however, on whether the severity of the overgrowth is related to the levels of phenytoin in plasma or saliva.2,5,6,117 Some reports indicate a relation between the drug dosage and the degree of gingival overgrowth.54,58
Tissue culture experiments indicate that phenytoin stimulates proliferation of fibroblast-like cells98 and epithelium.99 Two analogs of phenytoin (1-allyl-5-phenylhydantoinate and 5-methyl 5-phenylhydantoinate) have a similar effect on fibroblast-like cells.99 Fibroblasts from a phenytoin-induced gingival overgrowth show increased synthesis of sulfated glycosaminoglycans in vitro.53 Phenytoin may induce a decrease in collagen degradation as a result of the production of an inactive fibroblastic collagenase.42
Experimental attempts to induce gingival enlargement with phenytoin administration in laboratory animals have been successful only in the cat,50 the ferret, and the Macaca speciosa monkey.104 In experimental animals, phenytoin causes gingival enlargement that is independent of local inflammation.
In cats, one of the metabolic products of phenytoin is 5-(parahydroxyphenyl)-5-phenylhydantoin; administration of this metabolite to cats also induces gingival enlargement in some cases. This led Hassell et al43 to hypothesize that gingival enlargement may result from the genetically determined ability or inability of the host to deal effectively with prolonged administration of phenytoin.
Systemic administration of phenytoin accelerates the healing of gingival wounds in nonepileptic humans101 and increases the tensile strength of healing abdominal wounds in rats.25,100 The administration of phenytoin may precipitate a megaloblastic anemia66 and folic acid deficiency.105
In conclusion, the pathogenesis of gingival enlargement induced by phenytoin is not known, but some evidence links it to a direct effect on specific, genetically predetermined subpopulations of fibroblasts, inactivation of collagenase, and plaque-induced inflammation.
Immunosuppressants
Cyclosporine is a potent immunosuppressive agent used to prevent organ transplant rejection and to treat several diseases of autoimmune origin.20 Its exact mechanism of action is not well known, but it appears to selectively and reversibly inhibit helper T cells, which play a role in cellular and humoral immune responses. Cyclosporin A (Sandimmune, Neoral) is administered intravenously or by mouth, and dosages greater than 500 mg/day have been reported to induce gingival overgrowth.24
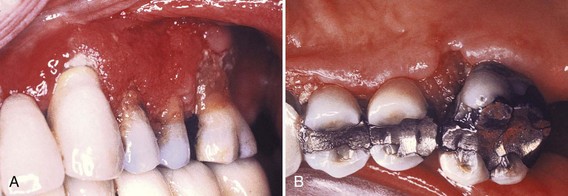
Cyclosporine-induced gingival enlargement is more vascularized than phenytoin enlargement (Figures 9-10 and 9-11)86,92,119. Its occurrence varies according to different studies from 25% to 70%,89 it affects children more frequently, and its magnitude appears to be related more to the plasma concentration than to the patient’s periodontal status.96 Gingival enlargement is greater in patients who are medicated with both cyclosporine and calcium channel blockers.102,112,113
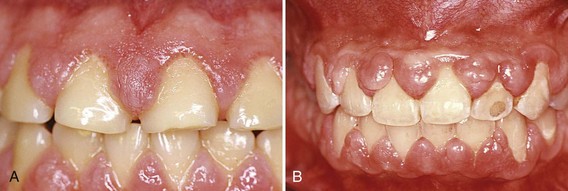
Figure 9-10 Cyclosporine-associated gingival enlargement. A, Mild involvement located particularly on papillae between teeth #9 and 10 and teeth #10 and 11. B, Advanced generalized enlargement.
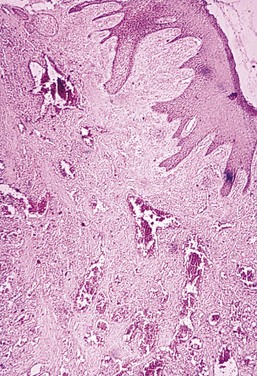
Figure 9-11 Microscopic view of cyclosporine-associated gingival enlargement. Note the epithelial hyperplasia and fibrous stroma with abundant vascularization.
The microscopic finding of many plasma cells plus the presence of an abundant amorphous extracellular substance has suggested that the enlargement is a hypersensitivity response to the cyclosporine.68
In experimental animals (rats), oral administration of cyclosporine was reported also to induce abundant formation of new cementum.4
In addition to gingival enlargement, cyclosporine induces other major side effects such as nephrotoxicity, hypertension, and hypertrichosis. Another immunosuppressive drug, tacrolimus, has been used effectively and is also nephrotoxic, but it results in much less severe hypertension, hypertrichosis, and gingival overgrowth.7,71,103
Calcium Channel Blockers
Calcium channel blockers are drugs developed for the treatment of cardiovascular conditions such as hypertension, angina pectoris, coronary artery spasms, and cardiac arrhythmias. They inhibit calcium ion influx across the cell membrane of heart and smooth muscle cells, blocking intracellular mobilization of calcium. This induces direct dilation of the coronary arteries and arterioles, improving oxygen supply to the heart muscle; it also reduces hypertension by dilating the peripheral vasculature.
These drugs are the dihydropyridine derivatives (amlodipine [Lotrel, Norvasc], felodipine [Plendil], nicardipine [Cardene], nifedipine [Adalat, Procardia]), the benzothiazine derivatives (diltiazem [Cardizem, Dilacor XR, Tiazac]), and the phenylalkylamine derivatives (verapamil [Calan, Isoptin, Verelan, Covera HS]).38
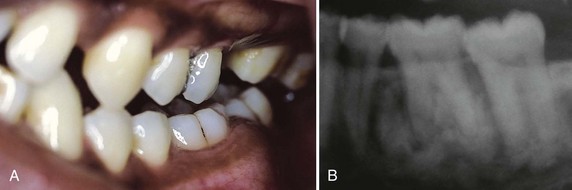
Some of these drugs can induce gingival enlargement. Nifedipine, one of the most often used,39,63,65,77 induces gingival enlargement in 20% of patients.8 Diltiazem, felodipine, nitrendipine, and verapamil also induce gingival enlargement.14,46 The dihydropyridine derivative, isradipine, can replace nifedipine in some cases and does not induce gingival overgrowth.116
Nifedipine is also used with cyclosporine in kidney transplant recipients, and the combined use of both drugs induces larger overgrowths.13 Nifedipine gingival enlargement has been induced experimentally in rats, where it appears to be dose dependent32; in humans, however, this dose dependency is not clear. One report indicates that nifedipine increases the risk of periodontal destruction in patients with diabetes mellitus type 2.64
Idiopathic Gingival Enlargement
Idiopathic gingival enlargement is a rare condition of undetermined cause. It has been designated by such terms as gingivomatosis, elephantiasis, idiopathic fibromatosis, hereditary gingival hyperplasia, and congenital familial fibromatosis.
Clinical Features
The enlargement affects the attached gingiva, as well as the gingival margin and interdental papillae, in contrast to phenytoin-induced overgrowth, which is often limited to the gingival margin and interdental papillae (see Figure 9-6). The facial and lingual surfaces of the mandible and maxilla are generally affected, but the involvement may be limited to either jaw. The enlarged gingiva is pink, firm, and almost leathery in consistency and has a characteristic minutely pebbled surface (Figure 9-12). In severe cases the teeth are almost completely covered, and the enlargement projects into the oral vestibule. The jaws appear distorted because of the bulbous enlargement of the gingiva. Secondary inflammatory changes are common at the gingival margin.
Etiology
The cause is unknown, and thus the condition is designated as “idiopathic.” Some cases have a hereditary basis,28,120,121 but the genetic mechanisms involved are not well understood. A study of several families found the mode of inheritance to be autosomal recessive in some cases and autosomal dominant in others.52,84 In some families the gingival enlargement may be linked to impairment of physical development.56 The enlargement usually begins with the eruption of the primary or secondary dentition and may regress after extraction, suggesting that the teeth (or the plaque attached to them) may be initiating factors. The presence of bacterial plaque is a complicating factor.
Gingival enlargement has been described in tuberous sclerosis, which is an inherited condition characterized by a triad of epilepsy, mental deficiency, and cutaneous angiofibromas.107,111
Enlargements Associated with Systemic Diseases
Many systemic diseases can develop oral manifestations that may include gingival enlargement. These diseases and conditions can affect the periodontium by two different mechanisms, as follows:
Conditioned Enlargements
Conditioned enlargements occur when the systemic condition of the patient exaggerates or distorts the usual gingival response to dental plaque. The specific manner in which the clinical picture of conditioned gingival enlargement differs from that of chronic gingivitis depends on the nature of the modifying systemic influence. Bacterial plaque is necessary for the initiation of this type of enlargement. However, plaque is not the sole determinant of the nature of the clinical features.
The three types of conditioned gingival enlargement are hormonal (pregnancy, puberty), nutritional (associated with vitamin C deficiency), and allergic. Nonspecific conditioned enlargement is also seen.
Enlargement in Pregnancy
Pregnancy gingival enlargement may be marginal and generalized or may occur as single or multiple tumor-like masses (see Chapters 27 and 38).
During pregnancy, there is an increase in levels of both progesterone and estrogen, which by the end of the third trimester reach levels 10 and 30 times the levels during the menstrual cycle, respectively.1 These hormonal changes induce changes in vascular permeability, leading to gingival edema and an increased inflammatory response to dental plaque. The subgingival microbiota may also undergo changes, including an increase in Prevotella intermedia.61,83
Marginal Enlargement
Marginal gingival enlargement during pregnancy results from the aggravation of previous inflammation, and its incidence has been reported as 10%18 and 70%.122
The clinical picture varies considerably. The enlargement is usually generalized and tends to be more prominent interproximally than on the facial and lingual surfaces. The enlarged gingiva is bright red or magenta, soft, and friable and has a smooth, shiny surface. Bleeding occurs spontaneously or on slight provocation.
Tumorlike Gingival Enlargement
The so-called pregnancy tumor is not a neoplasm; it is an inflammatory response to bacterial plaque and is modified by the patient’s condition. It usually appears after the third month of pregnancy but may occur earlier. The reported incidence is 1.8% to 5%.67
The lesion appears as a discrete, mushroomlike, flattened spherical mass that protrudes from the gingival margin or more often from the interproximal space and is attached by a sessile or pedunculated base (Figure 9-13). It tends to expand laterally, and pressure from the tongue and the cheek perpetuates its flattened appearance. Generally dusky red or magenta, it has a smooth, glistening surface that often exhibits numerous deep-red, pinpoint markings. It is a superficial lesion and usually does not invade the underlying bone. The consistency varies; the mass is usually semifirm, but it may have various degrees of softness and friability. It is usually painless unless its size and shape foster accumulation of debris under its margin or interfere with occlusion, in which case, painful ulceration may occur.
Histopathology
Gingival enlargement in pregnancy is called angiogranuloma. Both marginal and tumorlike enlargements consist of a central mass of connective tissue, with numerous diffusely arranged, newly formed, and engorged capillaries lined by cuboid endothelial cells (Figure 9-14), as well as a moderately fibrous stroma with varying degrees of edema and chronic inflammatory infiltrate. The stratified squamous epithelium is thickened, with prominent rete pegs and some degree of intracellular and extracellular edema, prominent intercellular bridges, and leukocytic infiltration.
Although the microscopic findings are characteristic of gingival enlargement in pregnancy, they are not pathognomonic because they cannot be used to differentiate pregnant and nonpregnant patients.67
Most gingival disease during pregnancy can be prevented by the removal of plaque and calculus, as well as the institution of fastidious oral hygiene at the outset. In pregnancy, treatment of the gingiva that is limited to the removal of tissue without complete elimination of local irritants is followed by recurrence of gingival enlargement. Although spontaneous reduction in the size of gingival enlargement typically follows the termination of pregnancy, complete elimination of the residual inflammatory lesion requires the removal of all plaque deposits and factors that favor its accumulation. For additional information on gingival disease in pregnancy, see Chapter 58.
Enlargement in Puberty
Enlargement of the gingiva is sometimes seen during puberty (see Chapter 27). It occurs in both male and female adolescents and appears in areas of plaque accumulation.
The size of the gingival enlargement greatly exceeds that usually seen in association with comparable local factors. It is marginal and interdental and is characterized by prominent bulbous interproximal papillae (Figure 9-15). Often, only the facial gingivae are enlarged, and the lingual surfaces are relatively unaltered; the mechanical action of the tongue and the excursion of food prevent a heavy accumulation of local irritants on the lingual surface.
Gingival enlargement during puberty has all the clinical features generally associated with chronic inflammatory gingival disease. It is the degree of enlargement and its tendency to recur in the presence of relatively scant plaque deposits that distinguish pubertal gingival enlargement from uncomplicated chronic inflammatory gingival enlargement. After puberty the enlargement undergoes spontaneous reduction but does not disappear completely until plaque and calculus are removed.
A longitudinal study of 127 children 11 to 17 years of age showed a high initial prevalence of gingival enlargement that tended to decline with age.108 When the mean number of inflamed gingival sites per child was determined and correlated with the time at which the maximum number of inflamed sites was observed and with the oral hygiene index at that time, a pubertal peak in gingival inflammation unrelated to oral hygiene factors clearly occurred. A longitudinal study of subgingival microbiota of children between ages 11 and 14 and their association with clinical parameters has implicated Capnocytophaga species in the initiation of pubertal gingivitis.72 Other studies have reported that hormonal changes coincide with an increase in the proportion of Prevotella intermedia and Prevotella nigrescens.74,118
Enlargement in Vitamin C Deficiency
Enlargement of the gingiva is generally included in classic descriptions of scurvy. Acute vitamin C deficiency itself does not cause gingival inflammation, but it does cause hemorrhage, collagen degeneration, and edema of the gingival connective tissue. These changes modify the response of the gingiva to plaque to the extent that the normal defensive delimiting reaction is inhibited, and the extent of the inflammation is exaggerated,33,34 resulting in the massive gingival enlargement seen in scurvy (Figure 9-16) (see Chapter 27).
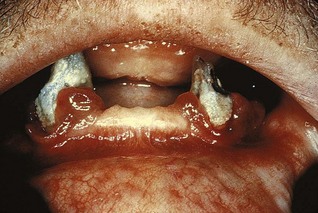
Figure 9-16 Gingival enlargement in patient with vitamin C deficiency. Note the prominent hemorrhagic areas.
(Courtesy Dr. Gerald Shklar, Boston.)
Gingival enlargement in vitamin C deficiency is marginal; the gingiva is bluish red, soft, and friable and has a smooth, shiny surface. Hemorrhage, occurring either spontaneously or on slight provocation, and surface necrosis with pseudomembrane formation are common features.
Histopathology
In vitamin C deficiency the gingiva has a chronic inflammatory cellular infiltration with a superficial acute response. There are scattered areas of hemorrhage, with engorged capillaries. Marked diffuse edema, collagen degeneration, and scarcity of collagen fibrils or fibroblasts are striking findings.
Plasma Cell Gingivitis
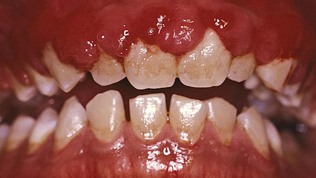
Plasma cell gingivitis consists of a mild marginal gingival enlargement that extends to the attached gingiva. The gingiva appears red, friable, and sometimes granular and bleeds easily; usually it does not induce a loss of attachment (Figure 9-17). This lesion is located in the oral aspect of the attached gingiva and therefore differs from plaque-induced gingivitis.
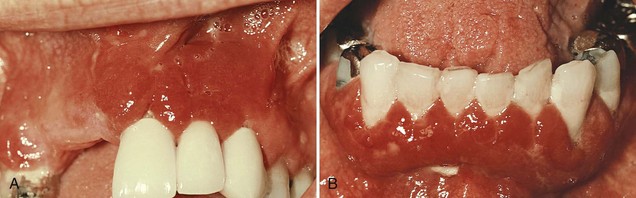
Figure 9-17 Plasma cell gingivitis. A, Diffuse lesions on facial surface of the anterior maxilla. B, Mandibular lesions.
(Courtesy Dr. Kim D. Zussman, Thousand Oaks, CA.)
Histopathology
In plasma cell gingivitis the oral epithelium shows spongiosis and infiltration with inflammatory cells; ultrastructurally, there are signs of damage in the lower spinous layers and the basal layers. The underlying connective tissue contains a dense infiltrate of plasma cells that also extends to the oral epithelium, inducing a dissecting type of injury.76
An associated cheilitis and glossitis have been reported.55,94 Plasma cell gingivitis is thought to be allergic in origin, possibly related to components of chewing gum, dentifrices, or various diet components. Cessation of exposure to the allergen brings resolution of the lesion.
In rare instances, marked inflammatory gingival enlargements with a predominance of plasma cells can appear; these are associated with rapidly progressive periodontitis.78
A solitary plasma cell tumor or plasmocytoma has been described in the nasopharynx and rarely in the oral mucosa.12,92 It is a slow-growing pedunculated tumor with a pink and smooth surface and is composed of normal plasma cells. It is usually benign, but in rare cases, it can be an oral manifestation of multiple myeloma, a malignant tumor of the bone marrow.
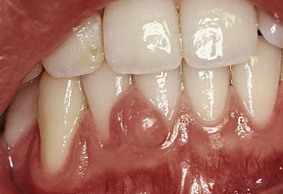
Nonspecific Conditioned Enlargement (Pyogenic Granuloma)
Pyogenic granuloma is a tumorlike gingival enlargement that is considered an exaggerated conditioned response to minor trauma (Figure 9-18). The exact nature of the systemic conditioning factor has not been identified.11 Pyogenic granuloma is similar in clinical and microscopic appearance to the conditioned gingival enlargement seen in pregnancy. Differential diagnosis is based on the patient’s history.
Treatment consists of removal of the lesions plus the elimination of irritating local factors. The recurrence rate is about 15%.
Systemic Diseases That Cause Gingival Enlargement
Several systemic diseases may result in gingival enlargement by different mechanisms. These are uncommon cases and are only briefly discussed.
Leukemia
Leukemic gingival enlargement may be diffuse or marginal and localized or generalized (see Chapter 27). It may appear as a diffuse enlargement of the gingival mucosa, an oversized extension of the marginal gingiva (Figure 9-19), or a discrete tumorlike interproximal mass.
Clinical Features
In leukemic enlargement the gingiva is generally bluish red and has a shiny surface. The consistency is moderately firm, but there is a tendency toward friability and hemorrhage, occurring either spontaneously or on slight irritation. Acute painful necrotizing ulcerative inflammatory involvement may occur in the crevice formed at the junction of the enlarged gingiva and the contiguous tooth surfaces.
Patients with leukemia may also have a simple chronic inflammation without the involvement of leukemic cells and may present with the same clinical and microscopic features seen in patients without the systemic disease. Most cases, however, reveal features of both simple chronic inflammation and leukemic infiltrate.
True leukemic enlargement often occurs in acute leukemia but may also be seen in subacute leukemia. It seldom occurs in chronic leukemia.
Histopathology
Gingival enlargements in leukemic patients show various degrees of chronic inflammation. Mature leukocytes and areas of connective tissue are infiltrated with a dense mass of immature and proliferating leukocytes, the specific nature of which varies with the type of leukemia. Engorged capillaries, edematous and degenerated connective tissue, and epithelium with various degrees of leukocytic infiltration and edema are found. Isolated surface areas of acute necrotizing inflammation with a pseudomembranous meshwork of fibrin, necrotic epithelial cells, PMNs, and bacteria are often seen.
Granulomatous Diseases
Wegener’s Granulomatosis
Wegener’s granulomatosis is a rare disease characterized by acute granulomatous necrotizing lesions of the respiratory tract, including nasal and oral defects. Renal lesions develop, and acute necrotizing vasculitis affects the blood vessels. The initial manifestations of Wegener’s granulomatosis may involve the orofacial region and include oral mucosal ulceration, gingival enlargement,47 abnormal tooth mobility, exfoliation of teeth, and delayed healing response.17
The granulomatous papillary enlargement is reddish purple and bleeds easily on stimulation (see Figure 12-30).
Histopathology
Chronic inflammation occurs, with scattered giant cells and foci of acute inflammation and microabscesses covered by a thin, acanthotic epithelium. Vascular changes have not been described with gingival enlargement in Wegener’s granulomatosis, probably because of the small size of the gingival blood vessels.51
The cause of Wegener’s granulomatosis is unknown, but the condition is considered an immunologically mediated tissue injury.23 At one time the usual outcome was death from kidney failure within a few months, but more recently the use of immunosuppressive drugs has produced prolonged remissions in more than 90% of patients.60
Sarcoidosis
Sarcoidosis is a granulomatous disease of unknown etiology. It starts in individuals in their twenties or thirties, predominantly affects blacks, and can involve almost any organ, including the gingiva, in which a red, smooth, painless enlargement may appear.
Histopathology
Sarcoid granulomas consist of discrete, noncaseating whorls of epithelioid cells and multinucleated, foreign body–type giant cells with peripheral mononuclear cells.88
Neoplastic Enlargement (Gingival Tumors)
This section provides only a brief description of some of the more common neoplastic and pseudoneoplastic lesions of the gingiva. The reader is referred to texts on oral pathology for more comprehensive coverage.88,92
Benign Tumors of the Gingiva
Epulis is a generic term used clinically to designate all discrete tumors and tumorlike masses of the gingiva. It serves to locate the tumor but not to describe it. Most lesions referred to as “epulis” are inflammatory rather than neoplastic.
Neoplasms account for a comparatively small proportion of gingival enlargements and make up a small percentage of the total number of oral neoplasms. In a survey of 257 oral tumors, approximately 8% occurred on the gingiva.70 In another study of 868 growths of the gingiva and palate, of which 57% were neoplastic and the remainder inflammatory, the following incidence of tumors was noted: carcinoma, 11.0%; fibroma, 9.3%; giant cell tumor, 8.4%; papilloma, 7.3%; leukoplakia, 4.9%; mixed tumor (salivary gland type), 2.5%; angioma, 1.5%; osteofibroma, 1.3%; sarcoma, 0.5%; melanoma, 0.5%; myxoma, 0.45%; fibropapilloma, 0.4%; adenoma, 0.4%; and lipoma, 0.3%.9
Fibroma
Fibromas of the gingiva arise from the gingival connective tissue or from the periodontal ligament. They are slow-growing, spherical tumors that tend to be firm and nodular but may be soft and vascular. Fibromas are usually pedunculated. Hard fibromas of the gingiva are rare; most of the lesions diagnosed clinically as “fibromas” are inflammatory enlargements.93
Histopathology
Fibromas are composed of bundles of well-formed collagen fibers with a scattering of fibrocytes and a variable vascularity.
The so-called giant cell fibroma contains multinucleated fibroblasts. In another variant, mineralized tissue (bone, cementum-like material, and dystrophic calcifications) may be found; this type of fibroma is called peripheral ossifying fibroma.
Papilloma
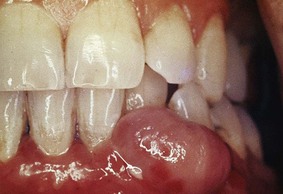
Papillomas are benign proliferations of surface epithelium that are in many but not all cases associated with the human papillomavirus (HPV). Gingival papillomas appear as solitary wartlike or cauliflower-like protuberances (Figure 9-20). They may be small and discrete or broad, hard elevations with minutely irregular surfaces.
Peripheral Giant Cell Granuloma
Giant cell lesions of the gingiva arise interdentally or from the gingival margin, occur most frequently on the labial surface, and may be sessile or pedunculated. They vary in appearance from smooth, regularly outlined masses to irregularly shaped, multilobulated protuberances with surface indentations (Figure 9-21). Ulceration of the margin is occasionally seen. The lesions are painless, vary in size, and may cover several teeth. They may be firm or spongy, and the color varies from pink to deep red or purplish blue. There are no pathognomonic clinical features whereby these lesions can be differentiated from other forms of gingival enlargement. Microscopic examination is required for definitive diagnosis.
The prefix “peripheral” is needed to differentiate them from comparable lesions that originate within the jawbone (central giant cell granulomas).
In some cases the giant cell granuloma of the gingiva is locally invasive and causes destruction of the underlying bone (Figure 9-22). Complete removal leads to uneventful recovery.
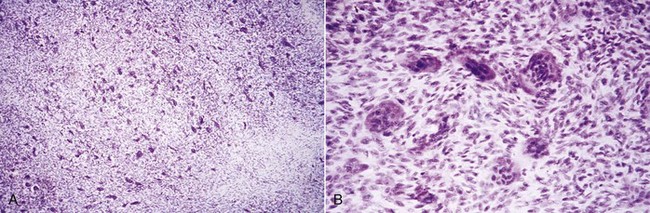
Figure 9-22 A, Microscopic survey of peripheral giant cell granuloma. B, High-power study of the lesion demonstrating the giant cells and intervening stroma that make up the major portion of the mass.
Histopathology
The giant cell granuloma has numerous foci of multinuclear giant cells and hemosiderin particles in a connective tissue stroma (see Figure 9-22). Areas of chronic inflammation are scattered throughout the lesion, with acute involvement occurring at the surface. The overlying epithelium is usually hyperplastic, with ulceration at the base. Bone formation occasionally occurs within the lesion (Figure 9-23).
Central Giant Cell Granuloma
Giant cell lesions arise within the jaws and produce central cavitation. They occasionally create a deformity of the jaw that makes the gingiva appear enlarged.
Mixed tumors, salivary gland type of tumors, and plasmacytomas of the gingiva have also been described but are not often seen.
Leukoplakia
Leukoplakia is a strictly clinical term defined by the World Health Organization as a white patch or plaque that does not rub off and cannot be diagnosed as any other disease. The cause of leukoplakia remains obscure, although it is associated with the use of tobacco (smoke or smokeless). Other probable factors are Candida albicans, HPV-16 and HPV-18, and trauma. Leukoplakia of the gingiva varies in appearance from a grayish white, flattened, scaly lesion (Figure 9-24) to a thick, irregularly shaped, keratinous plaque.
Most leukoplakias (80%) are benign; the remaining 20% are malignant or premalignant, and only 3% of these are invasive carcinomas.31 Biopsy of all leukoplakias is necessary, selecting the most suspicious area, to arrive at a correct diagnosis and institute proper therapy.31,92
Histopathology
Leukoplakia exhibits hyperkeratosis and acanthosis. Premalignant and malignant cases have a variable degree of atypical epithelial changes that may be mild, moderate, or severe, depending on the extent of involvement of the epithelial layers. When dysplastic changes involve all layers, it is diagnosed as a carcinoma in situ, and this may become invasive carcinoma when the basement membrane is breached.31 Inflammatory involvement of the underlying connective tissue is a common associated finding.
Gingival Cyst
Gingival cysts of microscopic proportions are common, but they seldom reach a clinically significant size.73 When they do, they appear as localized enlargements that may involve the marginal and attached gingiva. The cysts occur in the mandibular canine and premolar areas, most often on the lingual surface. They are painless, but with expansion, they may cause erosion of the surface of the alveolar bone. The gingival cyst should be differentiated from the lateral periodontal cyst (see Chapter 13), which arises within the alveolar bone, adjacent to the root, and is developmental in origin. Gingival cysts develop from odontogenic epithelium or from surface or sulcular epithelium traumatically implanted in the area. Removal is followed by uneventful recovery.
Histopathology
A gingival cyst cavity is lined by a thin, flattened epithelium with or without localized areas of thickening. Less frequently, the following types of epithelium can be found: unkeratinized stratified squamous epithelium, keratinized stratified squamous epithelium, and parakeratinized epithelium with palisading basal cells.16
Malignant Tumors of the Gingiva
Carcinoma
Oral cancer accounts for less than 3% of all malignant tumors in the body but is the sixth most common cancer in males and the twelfth in females.75 The gingiva is not a frequent site of oral malignancy (6% of oral cancers62).
Squamous cell carcinoma is the most common malignant tumor of the gingiva. It may be exophytic, presenting as an irregular outgrowth, or ulcerative, appearing as flat, erosive lesions. It is often symptom free, going unnoticed until complicated by inflammatory changes that may mask the neoplasm but cause pain; sometimes it becomes evident after tooth extraction. These masses are locally invasive, involving the underlying bone and periodontal ligament of adjoining teeth and the adjacent mucosa (Figure 9-25). Metastasis is usually confined to the region above the clavicle; however, more extensive involvement may include the lung, liver, or bone.
Malignant Melanoma
Malignant melanoma is a rare oral tumor that tends to occur in the hard palate and maxillary gingiva of older persons.75,85 It is usually darkly pigmented and is often preceded by localized pigmentation.21 It may be flat or nodular and is characterized by rapid growth and early metastasis. It arises from melanoblasts in the gingiva, cheek, or palate. Infiltration into the underlying bone and metastasis to cervical and axillary lymph nodes are common.
Sarcoma
Fibrosarcoma, lymphosarcoma, and reticulum cell sarcoma of the gingiva are rare; only isolated cases have been described in the literature.40,110 Kaposi’s sarcoma often occurs in the oral cavity of patients with acquired immunodeficiency syndrome (AIDS), particularly in the palate and the gingiva (see Chapter 19).
Metastasis
Tumor metastasis to the gingiva occurs infrequently. Such metastasis has been reported with various tumors, including adenocarcinoma of the colon,49 lung carcinoma, melanoma,30 renal cell carcinoma,15 hypernephroma,81 chondrosarcoma,110 and testicular tumor.29
The low incidence of oral malignancy should not mislead the clinician. Ulcerations that do not respond to therapy in the usual manner, as well as all gingival tumors and tumorlike lesions, must be biopsied and submitted for microscopic diagnosis (see Chapter 35).
False Enlargement
False enlargements are not true enlargements of the gingival tissues but may appear as such as a result of increases in size of the underlying osseous or dental tissues. The gingiva usually presents with no abnormal clinical features except the massive increase in size of the area.
Underlying Osseous Lesions
Enlargement of the bone subjacent to the gingival area occurs most often in tori and exostoses, but it can also occur in Paget’s disease, fibrous dysplasia, cherubism, central giant cell granuloma, ameloblastoma, osteoma, and osteosarcoma. Figure 9-26 shows fibrous dysplasia (florid type) in a 38-year-old black female that induced an osseous enlargement in the mandibular molar area appearing as a gingival enlargement. The gingival tissue can appear normal or may have unrelated inflammatory changes.
Underlying Dental Tissues
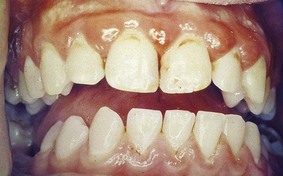
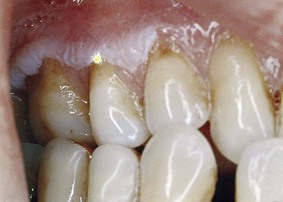
During the various stages of eruption, particularly of the primary dentition, the labial gingiva may show a bulbous marginal distortion caused by superimposition of the bulk of the gingiva on the normal prominence of the enamel in the gingival half of the crown. This enlargement has been termed developmental enlargement and often persists until the junctional epithelium has migrated from the enamel to the cementoenamel junction.
In a strict sense, developmental gingival enlargements are physiologic and usually present no problems. However, when such enlargement is complicated by marginal inflammation, the composite picture gives the impression of extensive gingival enlargement (Figure 9-27). Treatment to alleviate the marginal inflammation, rather than resection of the enlargement, is sufficient in these patients.
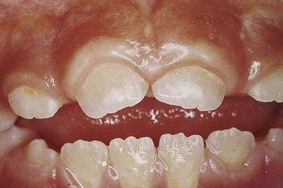
Figure 9-27 Developmental gingival enlargement. The normal bulbous contour of the gingiva around the incompletely erupted anterior teeth is accentuated by chronic inflammation.
NOTE: Chapter 85 in the online version of this book presents numerous examples of gingival enlargements.
1 Amar S, Chung KM. Influence of hormonal variation on the periodontium in women. Periodontol 2000. 1994;6:79.
2 Angelopoulos AP, Goaz PW. Incidence of diphenylhydantoin gingival hyperplasia. Oral Surg. 1972;34:898.
3 Ashrafi SH, Slaski K, Thu K, et al. Scanning electron microscopy of cyclosporine-induced gingival overgrowth. Scan Microsc. 1996;10:219.
4 Ayanoglou CM, Lesty C. New cementum formation induced by cyclosporin A: a histological, ultrastructural and histomorphometric study in the rat. J Periodontol. 1997;32:543.
5 Babcock JR. Incidence of gingival hyperplasia associated with Dilantin therapy in a hospital population. J Am Dent Assoc. 1965;71:1447.
6 Babcock JR, Nelson GH. Gingival hyperplasia and Dilantin content of saliva. J Am Dent Assoc. 1964;68:195.
7 Bader G, Lejeune S, Messner M. Reduction of cyclosporine-induced gingival overgrowth following a change to tacrolimus: a case history involving a liver transplant patient. J Periodontol. 1988;69:729.
8 Barclay S, Thomason JM, Idle JR, et al. The incidence and severity of nifedipine-induced gingival overgrowth. J Clin Periodontol. 1992;19:311.
9 Bernick S. Growth of the gingiva and palate. II. Connective tissue tumors. Oral Surg. 1948;1:1098.
10 Bernier JL, Tiecke RW. Nevus of the gingiva. J Oral Surg. 1950;8:165.
11 Bhaskar SN, Jacoway JR. Pyogenic granuloma: clinical features, incidence, histology and result of treatment. J Oral Surg. 1966;24:391.
12 Bhaskar SN, Levin MP, Frisch J. Plasma cell granuloma of periodontal tissues: report of 45 cases. Periodontics. 1988;6:272.
13 Bökenkamp A, Bohnhorst B, Beier C, et al. Nifedipine aggravates cyclosporine A–induced hyperplasia. Pediatr Nephrol. 1994;8:181.
14 Brown RS, Sein P, Corio R, et al. Nitrendipine-induced gingival hyperplasia. Oral Surg. 1990;70:593.
15 Buchner A, Begleiter A. Metastatic renal cell carcinoma in the gingiva mimicking a hyperplastic lesion. J Periodontol. 1980;51:413.
16 Buchner A, Hansen AS. The histomorphologic spectrum of the gingival cyst in the adult. Oral Surg. 1979;48:532.
17 Buckley DJ, Barrett AP, Bilous AM, et al. Wegener’s granulomatosis—are gingival lesions pathognomonic? J Oral Med. 1987;42:169.
18 Burket LW. Oral medicine. Philadelphia: Lippincott; 1946.
19 Butler RT, Kalkwarf KL, Kaldhal WB. Drug-induced gingival hyperplasia: phenytoin, cyclosporine and nifedipine. J Am Dent Assoc. 1987;114:56.
20 Calne R, Rolles K, White DJ, et al. Cyclosporin-A initially as the only immunosuppressant in 34 recipients of cadaveric organs: 32 kidneys, 2 pancreas and 2 livers. Lancet. 1979;2:1033.
21 Chaudry AP, Hampel A, Gorlin RJ. Primary malignant melanoma of the oral cavity: a review of 105 cases. Cancer. 1958;11:923.
22 Ciancio SG, Yaffe SJ, Catz CC. Gingival hyperplasia and diphenylhydantoin. J Periodontol. 1972;43:411.
23 Cotran RS, Kumar V, Robbins SL. Robbins’ pathologic basis of disease, ed 4. Philadelphia: Saunders; 1989.
24 Daley TD, Wysocki GP, Day C. Clinical and pharmacologic correlations in cyclosporine-induced gingival hyperplasia. Oral Surg. 1986;62:417.
25 DaCosta ML, Regan MC, al Sader M, et al. Diphenyl hydantoin sodium promotes early and marked angiogenesis and results in increased collagen deposition and tensile strength in healing wounds. Surgery. 1988;123:287.
26 Dallas BM. Hyperplasia of the oral mucosa in an edentulous epileptic. NZ Dent J. 1963;59:54.
27 Dreyer WP, Thomas CJ. DPH-induced hyperplasia of the masticatory mucosa in an edentulous epileptic patient. Oral Surg. 1978;45:701.
28 Emerson TG. Hereditary gingival hyperplasia: a family pedigree of four generations. Oral Surg. 1965;19:1.
29 Fantasia JE, Chen A. A testicular tumor with gingival metastasis. Oral Surg. 1979;48:64.
30 Ferreti Bonan PR, Laranjeira AL, Martelli HJr, et al. Synchronous metastatic melanoma presenting as gingival and facial swelling: a case report and review of the literature. J Periodontol. 2008;79:2371.
31 Fowler CB. Benign and malignant neoplasms of the periodontium. Periodontol 2000. 1999;21:33.
32 Fu E, Nieh S, Hiao CT, et al. Nifedipine-induced gingival overgrowth in rats: brief review and experimental study. J Periodontol. 1988;69:765.
33 Glickman I. The periodontal tissues of the guinea pig in vitamin C deficiency. J Dent Res. 1948;27:9.
34 Glickman I. The effect of acute vitamin C deficiency upon the response of the periodontal tissues of the guinea pig to artificially induced inflammation. J Dent Res. 1948;27:201.
35 Glickman I, Lewitus M. Hyperplasia of the gingiva associated with Dilantin (sodium diphenyl hydantoinate) therapy. J Am Dent Assoc. 1941;28:1991.
36 Hagen JD, Soule EH, Gores RJ. Granular cell myoblastoma of the oral cavity. Oral Surg. 1961;14:454.
37 Hall WB. Dilantin hyperplasia: a preventable lesion. J Periodontal Res. 1969;4(suppl):36.
38 Hallmon WW, Rossmann JA. The role of drugs in the pathogenesis of gingival overgrowth. Periodontol 2000. 1999;21:176.
39 Hancock RH, Swan RH. Nifedipine-induced gingival overgrowth. J Clin Periodontol. 1992;19:12.
40 Hardman FG. Secondary sarcoma presenting clinical appearance of fibrous epulis. Br Dent J. 1949;86:109.
41 Hassell TM. Epilepsy and the oral manifestations of phenytoin therapy. In: Monographs in oral science. New York: S Karger; 1981.
42 Hassell TM. Evidence for production of an inactive collagenase by fibroblasts from phenytoin-enlarged human gingiva. J Oral Pathol. 1982;11:310.
43 Hassell TM, Burtner AP, McNeal D, et al. Oral problems and genetic aspects of individuals with epilepsy. Periodontol 2000. 1994;6:68.
44 Hassell TM, Page RC. The major metabolite of phenytoin (Dilantin) induces gingival overgrowth in cats. J Periodontal Res. 1978;13:280.
45 Hassell TM, Page RC, Lindhe J. Histologic evidence of impaired growth control in diphenylhydantoin gingival overgrowth in man. Arch Oral Biol. 1978;23:381.
46 Heijl L, Sundin Y. Nitrendipine-induced gingival overgrowth in dogs. J Periodontol. 1989;60:104.
47 Hernandez G, Serrano C, Porras L, et al. Strawberry-like gingival tumor as the first clinical sign of Wegener’s granulomatosis. J Periodontol. 2008;79:1297.
48 Hirschfeld I. Hypertrophic gingivitis: its clinical aspect. J Am Dent Assoc. 1932;19:799.
49 Humphrey AA, Amos NH. Metastatic gingival adenocarcinoma from primary lesion of colon. Am J Cancer. 1936;28:128.
50 Ishikawa J, Glickman I. Gingival response to the systemic administration of sodium diphenyl hydantoinate (Dilantin) in cats. J Periodontol. 1961;32:149.
51 Israelson H, Binnie WH, Hurt WC. The hyperplastic gingivitis of Wegener’s granulomatosis. J Periodontol. 1981;52:81.
52 Jorgenson RJ, Cocker ME. Variation in the inheritance and expression of gingival fibromatosis. J Periodontol. 1974;45:472.
53 Kantor ML, Hassell TM. Increased accumulation of sulfated glycosaminoglycans in cultures of human fibroblasts from phenytoin-induced gingival overgrowth. J Dent Res. 1983;62:383.
54 Kapur RN, Grigis S, Little TM, et al. Diphenylhydantoin-induced gingival hyperplasia: its relation to dose and serum level. Dev Med Child Neurol. 1973;15:483.
55 Kerr DA, McClatchey KD, Regezi JA. Allergic gingivostomatitis (due to gum chewing). J Periodontol. 1971;42:709.
56 Kilpinen E, Raeste AM, Collan Y. Hereditary gingival hyperplasia and physical maturation. Scand J Dent Res. 1978;86:118.
57 Kimball O. The treatment of epilepsy with sodium diphenylhydantoinate. JAMA. 1939;112:1244.
58 Klar LA. Gingival hyperplasia during Dilantin therapy: a survey of 312 patients. J Public Health Dent. 1973;33:180.
59 Klingsberg J, Cancellaro LA, Butcher EO. Effects of air drying in rodent oral mucous membrane: a histologic study of simulated mouth breathing. J Periodontol. 1961;32:38.
60 Kornblut AD, Wolff SM, de Fries HE, et al. Wegener’s granulomatosis. Laryngoscope. 1980;90:1453.
61 Kornman KS, Loesche WJ. The subgingival microbial flora during pregnancy. J Periodontal Res. 1980;15:111.
62 Krolls SO, Hoffman S. Squamous cell carcinomas of the oral soft tissues: a statistical analysis of 14,253 cases by age, sex and race of patients. J Am Dent Assoc. 1976;92:571.
63 Lederman D, Lummerman H, Reuben S, et al. Gingival hyperplasia associated with nifedipine therapy. Oral Surg. 1984;57:620.
64 Li X, Luan Q, Wang X, et al. Nifedipine intake increases the risk of periodontal destruction in subjects with type 2 diabetes mellitus. J Periodontol. 2008;79:2054.
65 Lucas RM, Howell LP, Wall RA. Nifedipine-induced gingival hyperplasia: a histochemical and ultrastructural study. J Periodontol. 1985;56:211.
66 Lustberg A, Goldman D, Dreskin OH. Megaloblastic anemia due to Dilantin therapy. Ann Intern Med. 1961;54:153.
67 Maier AW, Orban B. Gingivitis in pregnancy. Oral Surg. 1949;2:334.
68 Mariani G, Calastrini C, Carinci F, et al. Ultrastructural features of cyclosporine A–induced gingival hyperplasia. J Periodontol. 1993;64:1092.
69 Merritt H, Putnam T. Sodium diphenylhydantoinate in the treatment of convulsive disorders. JAMA. 1938;111:1068.
70 McCarthy FP. A clinical and pathological study of oral disease. JAMA. 1941;116:16.
71 Mihatsch MJ, Kyo M, Morozumi K, et al. The side-effects of cyclosporine-A and tacrolimus. Clin Nephrol. 1988;49:356.
72 Mombelli A, Lang NP, Burgin WB, et al. Microbial changes associated with the development of puberty gingivitis. J Periodontal Res. 1990;25:331.
73 Moskow BS. The pathogenesis of the gingival cyst. Periodontics. 1966;4:23.
74 Nakagawa S, Fujii H, Machida Y, et al. A longitudinal study from prepuberty to puberty of gingivitis: correlation between the occurrence of Prevotella intermedia and sex hormones. J Periodontol. 1994;21:658.
75 Neville BW, Damm DD, Allen CM, et al. Oral and maxillofacial pathology. Philadelphia: Saunders; 1995.
76 Newcomb GM, Seymour GJ, Adkins KF. An unusual form of chronic gingivitis: an ultrastructural, histochemical and immunologic investigation. Oral Surg. 1982;53:488.
77 Nishikawa S, Tada H, Hamasaki A, et al. Nifedipine-induced gingival hyperplasia: a clinical and in vitro study. J Periodontol. 1991;62:30.
78 Nitta H, Kameyama Y, Ishikawa I. Unusual gingival enlargement with rapidly progressive periodontitis: report of a case. J Periodontol. 1993;64:1008.
79 Nuki K, Cooper SH. The role of inflammation in the pathogenesis of gingival enlargement during the administration of diphenylhydantoin sodium in cats. J Periodontal Res. 1972;7:91.
80 Panuska HJ, Gorlin RJ, Bearman JE, et al. The effect of anticonvulsant drugs upon the gingiva: a series of 1048 patients. J Periodontol. 1961;32:15.
81 Persson PA, Wallenino K. Metastatic renal carcinoma (hypernephroma) in the gingiva of the lower jaw. Acta Odontol Scand. 1961;19:289.
82 Pollack RP. Neurofibroma of the palatal mucosa: a case report. J Periodontol. 1990;61:456.
83 Raber-Durlacher JE, van Steenbergen TJM, van der Velde U, et al. Experimental gingivitis during pregnancy and postpartum: clinical, endocrinological and microbiological aspects. J Clin Periodontol. 1994;21:549.
84 Raeste AM, Collan Y, Kilpinen E. Hereditary fibrous hyperplasia of the gingiva with varying penetrance and expressivity. Scand J Dent Res. 1978;86:357.
85 Rapini RP, Golitz LE, Greer ROJr. Primary malignant melanoma of the oral mucosa. Cancer. 1985;55:1543.
86 Rateitschak-Pluss EM, Hefti A, Lortscher R, et al. Initial observation that cyclosporin A induces gingival enlargement in man. J Clin Periodontol. 1983;10:237.
87 Rees TD. Drugs and oral disorders. Periodontology 2000. 1998;18:21.
88 Rees TD, Disorders affecting the periodontium, editor, Periodontol 2000, 21, 1999, 145
89 Romito GA, Pustiglioni FE, Saraiva L, et al. Relationship of subgingival and salivary microbiota to gingival overgrowth in heart transplant patients following cyclosporine A therapy. J Periodontol. 2004;75:918.
90 Rostock MH, Fry HR, Turner JE. Severe gingival overgrowth associated with cyclosporine therapy. J Periodontol. 1986;57:294.
91 Russell BJ, Bay LM. Oral use of chlorhexidine gluconate toothpaste in epileptic children. Scand J Dent Res. 1978;86:52.
92 Sapp JP, Eversole LR, Wysocki GP. Contemporary oral and maxillofacial pathology. St Louis: Elsevier; 2004.
93 Schneider LC, Weisinger E. The true gingival fibroma: an analysis of 129 fibrous gingival lesions. J Periodontol. 1978;49:423.
94 Serio FG, Siegel MA, Slade BE. Plasma cell gingivitis of unusual origin: a case report. J Periodontol. 1978;49:423.
95 Seymour RA, Jacobs DJ. Cyclosporine and the gingival tissues. J Clin Periodontol. 1992;19:1.
96 Seymour RA, Smith DG, Rogers SR. The comparative effects of azathioprine and cyclosporine on some gingival health parameters of renal transplant patients. J Clin Periodontol. 1987;14:610.
97 Seymour RA, Thomason JM, Ellis JS. The pathogenesis of drug-induced gingival overgrowth. J Clin Periodontol. 1996;23:165.
98 Shafer WG. Effect of Dilantin sodium analogues on cell proliferation in tissue culture. Proc Soc Exp Biol Med. 1960;106:205.
99 Shafer WG. Effect of Dilantin sodium on various cell lines in tissue culture. Proc Soc Exp Biol Med. 1961;108:694.
100 Shafer WG, Beatty RE, Davis WB. Effect of Dilantin sodium on tensile strength of healing wounds. Proc Soc Exp Biol Med. 1958;98:348.
101 Shapiro M. Acceleration of gingival wound healing in nonepileptic patients receiving diphenylhydantoin sodium. Exp Med Surg. 1958;16:41.
102 Slavin J, Taylor J. Cyclosporine, nifedipine and gingival hyperplasia. Lancet. 1987;ii:739.
103 Spencer CM, Goa KL, Gillis JC. Tacrolimus: an update of its pharmacology and drug efficacy in the management of organ transplantation. Drugs. 1997;54:925.
104 Staple PH, Reed MJ, Mashimo PA. Diphenylhydantoin gingival hyperplasia in Macaca arctoides: a new human model. J Periodontol. 1977;48:325.
105 Stein GM, Lewis H. Oral changes in a folic acid deficient patient precipitated by anticonvulsant drug therapy. J Periodontol. 1973;44:645.
106 Stevenson ARL, Austin BW. A case of ameloblastoma presenting as an exophytic gingival lesion. J Periodontol. 1990;61:378.
107 Stirrups D, Inglis J. Tuberous sclerosis with nonhydantoin gingival hyperplasia: report of a case. Oral Surg. 1980;49:211.
108 Sutcliffe P. A longitudinal study of gingivitis and puberty. J Periodontal Res. 1972;7:52.
109 Sznajder N, Dominguez FV, Carraro JJ, Lis G. Hemorrhagic hemangioma of the gingiva: report of a case. J Periodontol. 1973;44:579.
110 Taicher S, Mazar A, Hirschberg A, et al. Metastatic chondrosarcoma of the gingiva mimicking a reactive exophytic lesion: a case report. J Periodontol. 1991;623:223.
111 Thomas D, Rapley J, Strathman R, et al. Tuberous sclerosis with gingival overgrowth. J Periodontol. 1992;63:713.
112 Thomason JM, Seymour RA, Rice N. The prevalence and severity of cyclosporine and nifedipine-induced gingival overgrowth. J Clin Periodontol. 1993;20:37.
113 Thomason JM, Seymour RA, Ellis JS, et al. Determinants of gingival overgrowth severity in organ transplant patients. J Clin Periodontol. 1996;23:628.
114 Traeger KA. Cyst of the gingiva (mucocele): report of a case. Oral Surg. 1961;14:243.
115 Wedgwood D, Rusen D, Balk S. Gingival metastases from primary hepatocellular carcinoma. Oral Surg. 1979;47:263.
116 Westbrook P, Bednarczyk EM, Carlson M, et al. Regression of nifedipine-induced gingival hyperplasia following switch to a same class calcium channel blocker, isradipine. J Periodontol. 1997;68:645.
117 Westphal P. Salivary secretion and gingival hyperplasia in diphenylhydantoin-treated guinea pigs. Sven Tandlak Tidskr. 1969;62:505.
118 Wojcicki CJ, Harper DS, Robinson PJ. Differences in periodontal-disease associated microorganisms in prepubertal, pubertal and postpubertal children. J Periodontol. 1987;58:219.
119 Wysocki G, Gretsinger HA, Laupacis A, et al. Fibrous hyperplasia of the gingiva: a side effect of cyclosporin A therapy. Oral Surg. 1983;55:274.
120 Zackin SJ, Weisberger D. Hereditary gingival fibromatosis. Oral Surg. 1961;14:828.
121 Ziskin DE, Zegarelli E. Idiopathic fibromatosis of the gingivae. Ann Dent. 1943;2:50.
122 Ziskin DE, Blackberg SM, Stout AP. The gingivae during pregnancy. Surg Gynecol Obstet. 1933;57:719.