1. Aars H, et al. Effects of autonomic reflexes on tooth pulp blood flow in man. Acta Physiol Scand. 1992;146:423-429.
2. Aars H, Brodin P, Anderson E. A study of cholinergic and b-adrenergic components in the regulation of blood flow in the tooth pulp and gingiva of man. Acta Physiol Scand. 1993;148:441.
3. Accorinte ML, et al. Response of human dental pulp capped with MTA and calcium hydroxide powder. Oper Dent. 2008;33:488-495.
4. Ahlberg K, Brännström M, Edwall L. The diameter and number of dentinal tubules in rat, cat, dog and monkey: a comparative scanning electronic microscopic study. Acta Odontol Scand. 1975;33:243.
5. Alitalo K, Tammela T, Petrova TV. Lymphangiogenesis in development and human disease. Nature. 2005;438:946-953.
6. Amess TR, Matthews B The effect of topical application of lidocaine to dentin in the cat on the response of intra-dental nerves to mechanical stimuli: proceedings of the International Conference on Dentin/Pulp Complex Shimono M Maeda T Suda H Takahashi K 1996 Quintessence Publishing Co Tokyo
7. Andelin WE, et al. Identification of hard tissue after experimental pulp capping using dentin sialoprotein (DSP) as a marker. J Endod. 2003;29:646-650.
8. Anderson LC, Vakoula A, Veinote R. Inflammatory hypersensitivity in a rat model of trigeminal neuropathic pain. Arch Oral Biol. 2003;48:161.
9. Andreasen JO. Luxation of permanent teeth due to trauma. A clinical and radiographic follow-up study of 189 injured teeth. Scand J Dent Res. 1970;78:273.
10. Anneroth G, Norberg KA. Adrenergic vasoconstrictor innervation in the human dental pulp. Acta Odontol Scand. 1968;26:89-93. May
11. Arola D, Rouland JA, Zhang D. Fatigue and fracture of bovine dentin. Exp Mech. 2002;42:380.
12. Artese L, et al. Vascular endothelial growth factor (VEGF) expression in healthy and inflamed human dental pulps. J Endod. 2002;28:20-23.
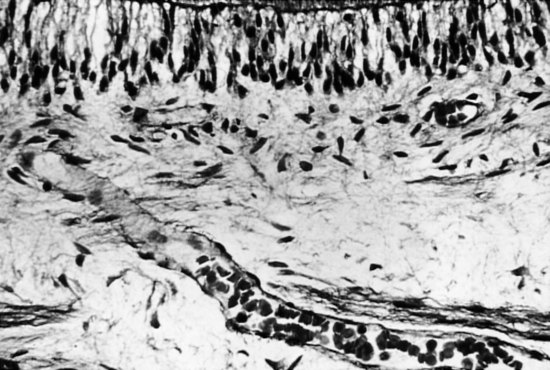
13. Avery JK. Structural elements of the young normal human pulp. Oral Surg Oral Med Oral Pathol. 1971;32:113-125.
14. Awawden L, Lundy FT, Shaw C, Kennedy JG, Lamey PJ. Quantitative analysis of substance P, neurokinin A, and calcitonin gene-related peptide in pulp tissue from painful and healthy human teeth. Int Endod J. 2002;36:30.
15. Bajaj D, Sundaram N, Nazari A, Arola D. Dehydration and fatigue crack growth in dentin. Biomaterials. 2006;27:2507-2517.
16. Banchs F, Trope M. Revascularization of immature permanent teeth with apical periodontitis: new treatment protocol? J Endod. 2004;30:196-200.
17. Baume LJ. The biology of pulp and dentine. In: Myers HM, editor. Monographs in oral science, vol 8. Basel: S Karger AG; 1980.
18. Bender IB, Landau MA, Fonsecca S, Trowbridge HO. The optimum placement-site of the electrode in electric pulp testing of the 12 anterior teeth. J Am Dent Assoc. 1989;118:305.
19. Bergenholtz G. Evidence for bacterial causation of adverse pulpal responses in resin-based dental restorations. Crit Rev Oral Biol Med. 2000;11:467.
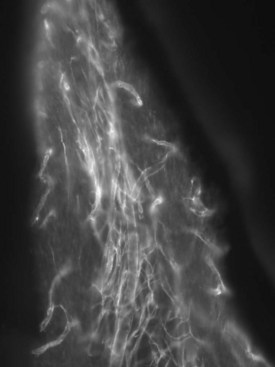
20. Berggreen E, Haug SR, Mkony LE, Bletsa A. Characterization of the dental lymphatic system and identification of cells immunopositive to specific lymphatic markers. Eur J Oral Sci. 2009;117:34-42.
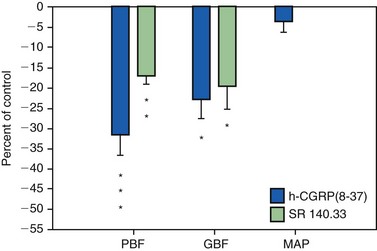
21. Berggreen E, Heyeraas KJ. Effect of the sensory neuropeptide antagonists h-CGRP(8–37) and SR 140.33 on pulpal and gingival blood flow in ferrets. Arch Oral Biol. 2000;45:537-542.
22. Berggreen E, Heyeraas KJ. Role of K+ATP channels, endothelin A receptors, and effect of angiotensin II on blood flow in oral tissues. J Dent Res. 2003;82:33-37.
23. Berggreen E, Heyeraas KJ. The role of sensory neuropeptides and nitric oxide on pulpal blood flow and tissue pressure in the ferret. J Dent Res. 1999;78:1535-1543.
24. Bernick S, Nedelman C. Effect of aging on the human pulp. J Endod. 1975;1:88.
25. Bevilacqua MP, et al. Interleukin-1 activation of vascular endothelium. Effects on procoagulant activity and leukocyte adhesion. Am J Pathol. 1985;121:394-403.
26. Bhussary BR. Modification of the dental pulp organ during development and aging. In: Finn SB, editor. Biology of the Dental Pulp Organ: a Symposium. Birmingham: University of Alabama Press, 1968.
27. Biesterfeld RC, Taintor JF, Marsh CL. The significance of alterations of pulpal respiration: a review of the literature. J Oral Pathol. 1979;8:129.
28. Bishop MA, Malhotra MP. An investigation of lymphatic vessels in the feline dental pulp. Am J Anat. 1990;187:247.
29. Bishop MA, Malhotra M, Yoshida S. Interodontoblastic collagen (von Korff fibers) and circumpulpal dentin formation: an ultrathin serial section study in the cat. Am J Anat. 1991;191:67.
30. Bishop MA, Yoshida S. A permeability barrier to lanthanum and the presence of collagen between odontoblasts in pig molars. J Anat. 1992;181:29.
31. Bletsa A, et al. Cytokine signalling in rat pulp interstitial fluid and transcapillary fluid exchange during lipopolysaccharide-induced acute inflammation. J Physiol. 2006;573(Pt 1):225-236.
32. Bongenhielm U, Haegerstrand A, Theodorsson E, Fried K. Effects of neuropeptides on growth of cultivated rat molar pulp fibroblasts. Regul Pept. 1995;60:2391-2398.
33. Bonucci E. Matrix vesicles: their role in calcification. In: Linde A, editor. Dentin and dentinogenesisis, Vol I. Boca Raton: CRC Press; 1984:135-154.
34. Borda E, et al. Nitric oxide synthase and PGE2 reciprocal interactions in rat dental pulp: cholinoceptor modulation. J Endod. 2007;33:142-147.
35. Botero TM, et al. TLR4 mediates LPS-induced VEGF expression in odontoblasts. J Endod. 2006;32:951-955.
36. Bowles WR, et al. beta 2-Adrenoceptor regulation of CGRP release from capsaicin-sensitive neurons. J Dent Res. 2003;82:308-311.
37. Brännström M. Communication between the oral cavity and the dental pulp associated with restorative treatment. Oper Dent. 1984;9:57.
38. Brännström M. The transmission and control of dentinal pain. In: Grossman LJ, editor. Mechanisms and control of pain. New York: Masson Publishing USA, 1979.
39. Brännström M, Aström A. A study of the mechanism of pain elicited from the dentin. J Dent Res. 1964;43:619.
40. Breschi L, Lopes M, Gobbi P, Mazzotti G, Falconi M, Perdigao J. Dentin proteoglycans: an immunocytochemical FEISEM study. J Biomed Mater Res. 2002;61:40.
41. Brown AC, Yankowitz D. Tooth pulp tissue pressure and hydraulic permeability. Circ Res. 1964;15:42-50.
42. Butler WT, D’Sousa RN, Bronckers AL, Happonen RP, Somerman MJ. Recent investigations on dentin specific proteins. Proc Finn Dent Soc. 1992;88(suppl 1):369.
43. Butler WT, Ritchie H. The nature and functional significance of dentin extracellular matrix proteins. Int J Dev Biol. 1995;39:213-222.
44. Byers MR. Dynamic plasticity of dental sensory nerve structure and cytochemistry. Arch Oral Biol. 1994;39(suppl):13S.
45. Byers MR. Neuropeptide immunoreactivity in dental sensory nerves: variations related to primary odontoblast function and survival. In: Shimono M, Takahashi K, editors. Dentin/Pulp Complex. Tokyo: Quintessence Publishing Co, 1996.
46. Byers MR, Chudler EH, Iadarola MJ. Chronic tooth pulp inflammation causes transient and persistent expression of Fos in dynorphin-rich regions of rat brainstem. Brain Res. 2000;861:191-207.
47. Byers MR, Närhi MVO. Dental injury models: experimental tools for understanding neuroinflammatory interactions and polymodal nociceptor functions. Crit Rev Oral Biol Med. 1999;10:4.
48. Byers MR, Närhi MVO. Nerve supply of the pulpodentin complex and response to injury. In: Hargreaves K, Goodis H, editors. Seltzer and Bender’s dental pulp. Chicago: Quintessence Publishing Co, 2002.
49. Byers MR, Narhi MV, Mecifi KB. Acute and chronic reactions of dental sensory nerve fibers to cavities and desiccation in rat molars. Anat Rec. 1988;221:872-883.
50. Byers MR, Schatteman GC, Bothwell MA. Multiple functions for NGF-receptor in developing, aging and injured rat teeth are suggested by epithelial, mesenchymal and neural immunoreactivity. Development. 1990;109:461.
51. Byers MR, Sugaya A. Odontoblast process in dentin revealed by fluorescent Di-I. J Histochem Cytochem. 1995;43:159.
52. Byers MR, Suzuki H, Maeda T. Dental neuroplasticity, neuro-pulpal interactions and nerve regeneration. Microsc Res Tech. 2003;60:503.
53. Byers MR, Taylor PE. Effect of sensory denervation on the response of rat molar pulp to exposure injury. J Dent Res. 1993;72:613.
54. Byers MR, Wheeler EF, Bothwell M. Altered expression of NGF and P75 NGF-receptor by fibroblasts of injured teeth precedes sensory nerve sprouting. Growth Factors. 1992;6:41-52.
55. Camps J, Pashley DH. In vivo sensitivity to air blasts and scratching of human root dentin. J Periodontol. 2003;74:1589.
56. Camps J, Salomon JP, Van Meerbeek B, Tay F, Pashley D. Dentin deformation after scratching with clinically-relevant forces. Arch Oral Biol. 2003;48:527.
57. Carter JM, Sorensen SE, Johnson RR, Teitelbaum RL, Levine MS. Punch shear testing of extracted vital and endodontically treated teeth. J Biomech. 1983;16:841.
58. Casasco A, et al. Immunohistochemical localization of endothelin-like immunoreactivity in human tooth germ and mature dental pulp. Anat Embryol (Berl). 1991;183:515-520.
59. Chaudhary P, Martenson ME, Baumann TK. Vanilloid receptor expression and capsaicin excitation of rat dental primary afferent neurons. J Dent Res. 2001;80:1518.
60. Chu SC, et al. Induction of vascular endothelial growth factor gene expression by proinflammatory cytokines in human pulp and gingival fibroblasts. J Endod. 2004;30:704-707.
61. Coffey CT, Ingram MJ, Bjöandal AM. Analysis of dentinal fluid. Oral Surg. 1970;30:835.
62. Costos CAS, Hebling J, Hanks CT. Current status of pulp capping with dentin adhesive systems: a review. Dent Mater. 2000;16:188.
63. Coure E. Ultrastructural changes during the life cycle of human odontoblasts. Arch Oral Biol. 1986;31:643.
64. Cox CF, Bogen G, Kopel HM, Ruby JP. Repair of pulpal injury by dental materials. Chap. 14. In: Hargreaves K, Goodis H, editors. Seltzer and Bender’s dental pulp. Chicago: Quintessence Publishing Co, 2002.
65. Cox CF, White KC, Ramus DL, Farmer JB, Snuggs HM. Reparative dentin: factors affecting its deposition. Quintessence Int. 1992;23:257.
66. Csillag M, Berggreen E, Fristad I, Haug SR, Bletsa A, Heyeraas KJ. Effect of electrical tooth stimulation on blood flow and immunocompetent cells in rat dental pulp after sympathectomy. Acta Odontol Scand. 2004;62:305-312.
67. Cuicchi B, Bouillaguet S, Holz J, Pashley D. Dentinal fluid dynamics in human teeth, in vivo. J Endod. 1995;21:191.
68. Cvek M, Granath L, Lundberg M. Failures and healing in endodontically treated non-vital anterior teeth with posttraumatically reduced pulpal lumen. Acta Odontol Scand. 1982;40:223-228.
69. Dahl E, Major IA. The fine structure of the vessels in the human dental pulp. Acta Odontol Scand. 1973;31:223-230.
70. Dahl T, Sabsay B, Veis A. Type I collagen-phosphophoryn interactions: specificity of the monomer-monomer binding. J Struct Biol. 1998;123:162.
71. Dia XF, ten Cate AR, Limeback H. The extent and distribution of intratubular collagen fibrils in human dentine. Arch Oral Biol. 1991;36:775.
72. Diamond J. The effect of injecting acetylcholine into normal and regenerating nerves. J Physiol (Lond). 1959;145:611.
73. Diamond RD, Stanley HR, Swerdlow H. Reparative dentin formation resulting from cavity preparation. J Prosthet Dent. 1966;16:1127.
74. Diogenes A, Akopian AN, Hargreaves KM. NGF up-regulates TRPA1: implications for orofacial pain. J Dent Res. 2007;86:550-555.
75. D’Souza RN, Bachman T, Baumgardner KR, Butler WT, Litz M. Characterization of cellular responses involved in reparative dentinogenesis in rat molars. J Dent Res. 1995;74:702.
76. Ebihara A, Tokita Y, Izawa T, Suda H. Pulpal blood flow assessed by laser Doppler flowmetry in a tooth with a horizontal root fracture. Oral Surg Oral Med Oral Path. 1996;81:229.
77. Eda S, Saito T. Electron microscopy of cells displaced into the dentinal tubules due to dry cavity preparation. J Oral Pathol. 1978;7:326.
78. Edwall L, Kindlová M. The effect of sympathetic nerve stimulation on the rate of disappearance of tracers from various oral tissues. Acta Odontol Scand. 1971;29:387.
79. Edwall B, et al. Neuropeptide Y (NPY) and sympathetic control of blood flow in oral mucosa and dental pulp in the cat. Acta Physiol Scand. 1985;125:253-264.
80. Eissmann HF, Radke RA. Postendodontic restoration. In Cohen S, Burns RC, editors: Pathways of the pulp, ed 4, St Louis: Mosby, 1987.
81. El-Backly RM, et al. Regeneration of dentine/pulp-like tissue using a dental pulp stem cell/poly(lactic-co-glycolic) acid scaffold construct in New Zealand white rabbits. Aust Endod J. 2008;34:52-67.
82. Embery G. Glycosaminoglycans of human dental pulp. J Biol Buccale. 1976;4:229-236.
83. Embery G, Hall R, Waddington R, Septier D, Goldberg M. Proteoglycans in dentinogenesis. Crit Rev Oral Biol Med. 2001;12:331.
84. England MC, Pellis EG, Michanowicz AE. Histopathologic study of the effect of pulpal disease upon nerve fibers of the human dental pulp. Oral Surg Oral Med Oral Pathol. 1974;38:783.
85. Engström C, Linde A, Persliden B. Acid hydrolases in the odontoblast-predentin region of dentinogenically active teeth. Scand J Dent Res. 1976;84:76.
86. Evans D, Reid T, Strang R, Stirrups D. A comparison of laser Doppler flowmetry with other methods of assessing vitality in traumatized anterior teeth. Endod Dent Traumatol. 1999;15:284.
87. Fearnhead RW. Innervation of dental tissues. In: Miles AEW, editor. Structural and chemical organization of the teeth, vol 1. New York: Academic Press; 1967.
88. Ferrara N. Vascular endothelial growth factor. Eur J Cancer. 1996;32A:2413-2422.
89. Ferrari M, Mason PN, Goracci C, Pashley DH, Tay FR. Collagen degradation in endodontically-treated teeth after clinical function. J Dent Res. 2004;88:414.
90. Finkelman RD, Mohan S, Jennings JC, Taylor AK, Jepsen S, Baylink DJ. Quantitation of growth factors IGF-1, SGF/IGF-11 and TGF-b in human dentin. J Bone Miner Res. 1990;5:717.
91. Firestone AR, Wheatley AM, Thüer UW. Measurement of blood perfusion in the dental pulp with laser Doppler flowmetry. Int J Microcirc Clin Exp. 1997;17:298.
92. Fish WE. An experimental investigation of enamel, dentine and the dental pulp. London: John Bale, Sons and Danielson; 1932.
93. Fisher AK. Respiratory variations within the normal dental pulp. J Dent Res. 1967;46:424.
94. Fisher AK, Schumacher ER, Robinson NR, Sharbondy GP. Effects of dental drugs and materials on the rate of oxygen consumption in bovine dental pulp. J Dent Res. 1957;36:447.
95. Fisher AK, Walters VE. Anaerobic glycolysis in bovine dental pulp. J Dent Res. 1968;47:717.
96. Fitzgerald M, Chiego DJ, Heys DR. Autoradiographic analysis of odontoblast replacement following pulp exposure in primate teeth. Arch Oral Biol. 1990;35:707.
97. Fogel HM, Marshall FJ, Pashley DH. Effects of distance of the pulp and thickness on the hydraulic conductance of human radicular dentin. J Dent Res. 1988;67:1381.
98. Fraser JR, et al. Uptake and degradation of hyaluronan in lymphatic tissue. Biochem J. 1988;256:153-158.
99. Fried K. Changes in pulp nerves with aging. Proc Finn Dent Soc. 1992;88(suppl 1):517.
100. Fried K, et al. Target finding of pain nerve fibers: neural growth mechanisms in the tooth pulp. Physiol Behav. 2007;92:40-45.
101. Fristad I, Heyeraas KJ, Kvinnsland I. Nerve fibres and cells immunoreactive to neurochemical markers in developing rat molars and supporting tissues. Arch Oral Biol. 1994;39:633-646.
102. Fristad I, Jacobsen EB, Kvinnsland IH. Coexpression of vasoactive intestinal polypeptide and substance P in reinnervating pulpal nerves and in trigeminal ganglion neurones after axotomy of the inferior alveolar nerve in the rat. Arch Oral Biol. 1998;43:183-189.
103. Fristad I, Kvinnsland IH, Jonsson R, Heyeraas KJ. Effect of intermittent long-lasting electrical tooth stimulation on pulpal blood flow and immunocompetent cells: a hemodynamic and immunohistochemical study in young rat molars. Exp Neurol. 1997;146:230-239.
104. Fuss Z, Trowbridge H, Bender IB, Rickoff B, Sorin S. Assessment of reliability of electrical and thermal pulp testing agents. J Endod. 1986;12:301.
105. Gani O, Visvisian C. Apical canal diameter in the first upper molar at various ages. J Endod. 1999;10:689.
106. Garant PR. The organization of microtubules within rat odontoblast processes revealed by perfusion fixation with glutaraldehyde. Arch Oral Biol. 1972;17:1047.
107. Garberoglio R, Brännström M. Scanning electron microscopic investigation of human dentinal tubules. Arch Oral Biol. 1976;21:355.
108. Gaucher C, Boukpessi T, Septier D, et al. Dentin noncollagenous matrix proteins in familiar hypophosphatemic rickets. Cells, Tissues, Organs. 2009;189:219-223.
109. George A, Bannon L, Sabsay B, et al. The carboxyl-terminal domain of phosphophoryn contains unique extended triplet amino acid repeat sequences forming ordered carboxyl-phosphate interaction ridges that may be essential in the biomineralization process. J Biol Chem. 1996;271:32869-32873.
110. George CH, Kendall JM, Evans WH. Intracellular trafficking pathways on assembly of connexins into tight junctions. J Biol Chem. 1999;274:8678.
111. Gibbs JL, Hargreaves KM. Neuropeptide Y Y1 receptor effects on pulpal nociceptors. J Dent Res. 2008;87:948-952.
112. Gilbert TM, Pashley DH, Anderson RW. Response of pulpal blood flow to intra-arterial infusion of endothelin. J Endod. 1992;18:228-231.
113. Gloe T, Pohl U. Laminin binding conveys mechanosensing in endothelial cells. News Physiol Sci. 2002;17:166.
114. Gold M. Tetrodotoxin-resistant Na currents and inflammatory hyperalgesia. Proc Natl Acad Sci U S A. 1999;96:7645.
115. Goldberg M, Lasfargues J-J. Dentin-pulpal complex revisited. J Dent. 1995;23:15.
116. Goldberg M, Six N, Decup F, Lasfargues JJ, Salih E, Tompkins K, et al. Bioactive molecules and the future of pulp therapy. Am J Dent. 2003;16:66.
117. Goldberg M, Takagi M. Dentine proteoglycans: composition, ultrastructure and functions. Histochem J. 1993;25:781.
118. Goldberg M, et al. Inflammatory and immunological aspects of dental pulp repair. Pharmacol Res. 2008;58:137-147.
119. Gotjamanos T. Cellular organization in the subodontoblastic zone of the dental pulp. II. Period and mode of development of the cell-rich layer in rat molar pulps. Arch Oral Biol. 1969;14:1011.
120. Gregg JM, Dixon AD. Somatotopic organization of the trigeminal ganglion. Arch Oral Biol. 1973;18:487.
121. Grossman ES, Austin JC. Scanning electron microscope observations on the tubule content of freeze-fractured peripheral vervet monkey dentine (Cercopithecus pygerythrus),. Arch Oral Biol. 1983;28:279.
122. Gunji T. Morphological research on the sensitivity of dentin. Arch Histol Jpn. 1982;45:45.
123. Guzy CE, Nicholls JI. In vitro comparison of intact endodontically-treated teeth with and without endo-post reinforcement. J Prosthet Dent. 1979;42:39.
124. Hahn C-L, Falkler WAJr, Siegel MA. A study of T cells and B cells in pulpal pathosis. J Endod. 1989;15:20.
125. Hahn C-L, Overton B. The effects of immunoglobulins on the convective permeability of human dentin in vivo. Arch Oral Biol. 1997;42:835.
126. Hals E, Tonder KJ. Elastic pseudoelastic tissue in arterioles of the human and dog dental pulp. Scand J Dent Res. 1981;89:218-227.
127. Hamersky PA, Weimer AD, Taintor JF. The effect of orthodontic force application on the pulpal tissue respiration rate in the human premolar. Am J Orthod. 1980;77:368.
128. Han SS. The fine structure of cells and intercellular substances of the dental pulp. In: Finn SB, editor. Biology of the dental pulp organ. Birmingham: University of Alabama Press; 1968:103.
129. Hargreaves KM. Pain mechanisms of the pulpodentin complex. In: Hargreaves KM, Goodis HE, editors. Seltzer and Bender’s dentin pulp. Chicago: Quintessence Publishing Co, 2002.
130. Hargreaves KM, Bowles WR, Jackson DL. Intrinsic regulation of CGRP release by dental pulp sympathetic fibers. J Dent Res. 2003;82:398-401.
131. Harris R, Griffin CJ. Fine structure of nerve endings in the human dental pulp. Arch Oral Biol. 1968;13:773.
132. Hartmann A, Azerad J, Boucher Y. Environmental effects on laser Doppler pulpal blood-flow measurements in man. Arch Oral Biol. 1996;41:333.
133. Hashioka K, et al. Relationship between clinical symptoms and enzyme-producing bacteria isolated from infected root canals. J Endod. 1994;20:75-77.
134. Hattler AB, Listgarten MA. Pulpal response to root planing in a rat model. J Endod. 1984;10:471.
135. Haug SR, Heyeraas KJ. Effects of sympathectomy on experimentally induced pulpal inflammation and periapical lesions in rats. Neuroscience. 2003;120:827-836.
136. Haug SR, Heyeraas KJ. Modulation of dental inflammation by the sympathetic nervous system. J Dent Res. 2006;85:488-495.
137. Helfer AR, Melwick S, Schilder H. Determination of the moisture content of vital and pulpless teeth. Oral Surg Oral Med Oral Pathol. 1972;34:661.
138. Hermanstyne TO, Markowitz K, Fan L, Gold MS. Mechanotransducers in rat pulpal afferents. J Dent Res. 2008;87:834-838.
139. Herr P, Holz J, Baume LJ. Mantle dentine in man: a quantitative study microradiographic study. J Biol Buccale. 1986;14:139.
140. Heyeraas KJ. Pulpal hemodynamics and interstitial fluid pressure: balance of transmicrovascular fluid transport. J Endod. 1989;15:468-472.
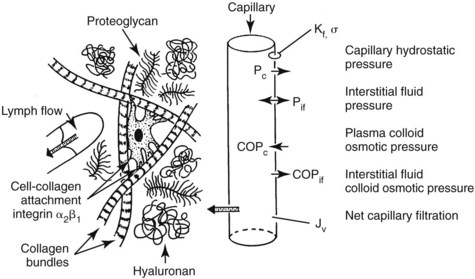
141. Heyeraas KJ, Berggreen E. Interstitial fluid pressure in normal and inflamed pulp. Crit Rev Oral Biol Med. 1999;10:328.
142. Heyeraas KJ, Jacobsen EB, Fristad I Vascular and immunoreactive nerve fiber reactions in the pulp after stimulation and denervation: proceedings of the International Conference on Dentin/Pulp Complex Shimono M Maeda T Suda H Takahashi K 1996 Quintessence Publishing Co Tokyo 162
143. Heyeraas KJ, Kvinnsland I. Tissue pressure and blood flow in pulpal inflammation. Proc Finn Dent Soc. 1992;88(Suppl 1):393-401.
144. Heyeraas KJ, et al. Effect of electrical tooth stimulation on blood flow, interstitial fluid pressure and substance P and CGRP-immunoreactive nerve fibers in the low compliant cat dental pulp. Microvasc Res. 1994;47:329-343.
145. Hikiji A, Yamamoto H, Sunakawa M, Suda H. Increased blood flow and nerve firing in the cat canine tooth in response to stimulation of the second premolar pulp. Arch Oral Biol. 2000;45:53.
146. Hildebrand C, et al. Teeth and tooth nerves. Prog Neurobiol. 1995;45:165-222.
147. Hirakawa S, Detmar M. New insights into the biology and pathology of the cutaneous lymphatic system. J Dermatol Sci. 2004;35:1-8.
148. Hirvonen T, Närhi M, Hakumäki M. The excitability of dog pulp nerves in relation to the condition of dentine surface. J Endod. 1984;10:294.
149. Holland GR. Morphological features of dentine and pulp related to dentine sensitivity. Arch Oral Biol. 1994;39(suppl):3S.
150. Holland GR. The extent of the odontoblast process in the cat. Am J Anat. 1976;121:133.
151. Holland GR. The odontoblast process: form and function. J Dent Res. 1985;64(special issue):499.
152. Howe CA, McKendry DJ. Effect of endodontic access preparation on resistance to crown-root fracture. J Am Dent Assoc. 1990;121:712.
153. Ibricevic H, et al. Identification of alpha 2 adrenoceptors in the blood vessels of the dental pulp. Int Endod J. 1991;24:279-289.
154. Ikeda H, Tokita Y, Suda H. Capsaicin-sensitive A fibers in cat tooth pulp. J Dent Res. 1997;76:1341.
155. Inoue H, Kurosaka Y, Abe K. Autonomic nerve endings in the odontoblast/predentin border and predentin of the canine teeth of dogs. J Endodon. 1992;18:149.
156. Isidor F, Odman P, Brondum K. Intermittent loading of teeth restored using prefabricated carbon fiber posts. Int J Prosthodont. 1996;9:131.
157. Itthagarum A, Tay FR. Self-contamination of deep dentin by dentinal fluid. Am J Dent. 2000;13:195.
158. Jacobsen EB, Heyeraas KJ. Pulp interstitial fluid pressure and blood flow after denervation and electrical tooth stimulation in the ferret. Arch Oral Biol. 1997;42:407-415.
159. Jernvall J, et al. Evidence for the role of the enamel knot as a control center in mammalian tooth cusp formation: non-dividing cells express growth stimulating Fgf-4 gene. Int J Dev Biol. 1994;38:463-469.
160. Johnsen DC, Harshbarger J, Rymer HD. Quantitative assessment of neural development in human premolars. Anat Rec. 1983;205:421.
161. Johnsen D, Johns S. Quantitation of nerve fibers in the primary and permanent canine and incisor teeth in man. Arch Oral Biol. 1978;23:825.
162. Johnson G, Brännström M. The sensitivity of dentin: changes in relation to conditions at exposed tubule apertures. Acta Odontol Scand. 1974;32:29.
163. Jones PA, Taintor JF, Adams AB. Comparative dental material cytotoxicity measured by depression of rat incisor pulp respiration. J Endod. 1979;5:48.
164. Jontell M, Okiji T, Dahlgren U, Bergenholtz G. Immune defense mechanisms of the dental pulp. Crit Rev Oral Biol Med. 1998;9:179.
165. Katoh Y, Yamaguchi R, Shinkai K, et al. Clinicopathological study on pulp-irritation of adhesive resinous materials (report 3). Direct capping effects on exposed pulp of Macaca fascicularis. Jpn J Conserv Dent. 1997;40:163.
166. Kayaoglu G, Orstavik D. Virulence factors of Enterococcus faecalis: relationship to endodontic disease. Crit Rev Oral Biol Med. 2004;15:308-320.
167. Kaye H, Herold RC. Structure of human dentine. I. Phase contrast, polarization, interference, and bright field microscopic observations on the lateral branch system. Arch Oral Biol. 1966;11:355.
168. Kelley KW, Bergenholtz G, Cox CF. The extent of the odontoblast process in rhesus monkeys (Macaca mulatta) as observed by scanning electron microscopy. Arch Oral Biol. 1981;26:893.
169. Kerezoudis NP, Olgart L, Edwall L. CGRP(8–37) reduces the duration but not the maximal increase of antidromic vasodilation in dental pulp and lip of the rat. Acta Physiol Scand. 1994;151:73-81.
170. Kerezoudis NP, Olgart L, Edwall L. Involvement of substance P but not nitric oxide or calcitonin gene-related peptide in neurogenic plasma extravasation in rat incisor pulp and lip. Arch Oral Biol. 1994;39:769-774.
171. Kerezoudis NP, et al. Activation of sympathetic nerves exerts an inhibitory influence on afferent nerve-induced vasodilation unrelated to vasoconstriction in rat dental pulp. Acta Physiol Scand. 1993;147:27-35.
172. Kettunen P, Thesleff I. Expression and function of FGFs-4, -8, and -9 suggest functional redundancy and repetitive use as epithelial signals during tooth morphogenesis. Dev Dyn. 1998;211:256-268.
173. Kettunen P, et al. Coordination of trigeminal axon navigation and patterning with tooth organ formation: epithelial-mesenchymal interactions, and epithelial Wnt4 and Tgfbeta1 regulate semaphorin 3a expression in the dental mesenchyme. Development. 2005;132:323-334.
174. Khullar SM, Fristad I, Brodin P, Kvinnsland IH. Upregulation of growth associated protein 43 expression and neuronal co-expression with neuropeptide Y following inferior alveolar nerve axotomy in the rat. J Peripher Nerv Syst. 1998;3:79-90.
175. Kim S, Edwall L, Trowbridge H, Chien S. Effects of local anesthetics on pulpal blood flow in dogs. J Dent Res. 1984;63:650.
176. Kim S, Schuessler G, Chien S. Measurement of blood flow in the dental pulp of dogs with the 133xenon washout method. Arch Oral Biol. 1983;28:501.
177. Kim S, Trowbridge HO, Dorscher-Kim JE. The influence of 5-hydroxytryptamine (serotonin) on blood flow in the dog pulp. J Dent Res. 1986;65:682-685.
178. Kim SK, et al. Antagonistic effect of D-myo-inositol-1,2,6-trisphosphate (PP56) on neuropeptide Y-induced vasoconstriction in the feline dental pulp. Arch Oral Biol. 1996;41:791-798.
179. Kim S, et al. Effects of selected inflammatory mediators on blood flow and vascular permeability in the dental pulp. Proc Finn Dent Soc. 1992;88(suppl 1):387-392.
180. Kim S, et al. Functional alterations in pulpal microcirculation in response to various dental procedures and materials. Proc Finn Dent Soc. 1992;88(suppl 1):65-71.
181. Kim S, Dorscher-Kim JE, Liu M. Microcirculation of the dental pulp and its autonomic control. Proc Finn Dent Soc. 1989;85:279-287.
182. Kimberly CL, Byers BR. Inflammation of rat molar pulp and periodontium causes increased calcitonin-gene-related peptide and axonal sprouting. Anat Rec. 1988;222:289.
183. Kinney JH, Balooch M, Marshall SJ, Marshall GWJr, Weihs TP. Hardness and Young’s modulus of human peritubular and intertubular dentin. Arch Oral Biol. 1996;41:9-13.
184. Kinney JH, Marshall SJ, Marshall GW. The mechanical properties of human dentin: a critical review and reevaluation of the dental literature. Crit Rev Oral Biol Med. 2003;14:13.
185. Kinney JH, Pople JA, Driessen CH, Breunig TM, Marshall GW, Marshall SJ. Intrafibrillar mineral may be absent in dentinogenesis imperfecta type II (D1–11). J Dent Res. 2001;80:1555.
186. Kollar EJ, Lumsden AG. Tooth morphogenesis: the role of the innervation during induction and pattern formation. J Biol Buccale. 1979;7:49-60.
187. Kontturi-Nähri V, Närhi M. Testing sensitive dentin in man. Int Endod J. 1993;26:4.
188. Kramer IRH. The distribution of blood vessels in the human dental pulp. In: Finn SB, editor. Biology of the dental pulp Organ. Birmingham: University of Alabama Press; 1968:361.
189. Kroeger DC, Gonzales F, Krivoy W. Transmembrane potentials of cultured mouse dental pulp cells. Proc Soc Exp Biol Med. 1961;108:134.
190. Kvinnsland IH, Luukko K, Fristad I, Kettunen P, Jackson DL, Fjeld K, et al. Glial cell line-derived neurotrophic factor (GDNF) from adult rat tooth serves a distinct population of large-sized trigeminal neurons. Eur J Neurosci. 2004;19:2089-2098.
191. Langeland K, Langeland LK. Histologic study of 155 impacted teeth. Odontol Tidskr. 1965;73:527.
192. Langeland K, Langeland LK. Pulp reactions to cavity and crown preparations. Aust Dent J. 1970;15:261.
193. Lantelme RL, Handleman SL, Herbison RJ. Dentin formation in periodontally diseased teeth. J Dent Res. 1976;55:48.
194. Laurent TC, et al. The catabolic fate of hyaluronic acid. Connect Tissue Res. 1986;15:33-41.
195. Lechner JH, Kalnitsky G. The presence of large amounts of type III collagen in bovine dental pulp and its significance with regard to the mechanism of dentinogenesis. Arch Oral Biol. 1981;26:265-273.
196. Lesot H, Osman M, Ruch JV. Immunofluorescent localization of collagens, fibronectin and laminin during terminal differentiation of odontoblasts. Dev Biol. 1981;82:371.
197. Lesot H, et al. Epigenetic signals during odontoblast differentiation. Adv Dent Res. 2001;15:8-13.
198. Levi-Montalcini R. The nerve growth factor: its mode of action on sensory and sympathetic nerve cells. Harvey Lect. 1966;60:217-259.
199. Lewinstein I, Grajower R. Root dentin hardness of endodontically treated teeth. J Endod. 1981;7:421.
200. Lilja J. Innervation of different parts of the predentin and dentin in a young human premolar. Acta Odontol Scand. 1979;37:339.
201. Lilja J, Noredenvall K-J, Brännström M. Dentin sensitivity, odontoblasts and nerves under desiccated or infected experimental cavities. Swed Dent J. 1982;6:93.
202. Linde A. The extracellular matrix of the dental pulp and dentin. J Dent Res. 1985;64(special issue):523.
203. Linde A. A study of the dental pulp glycosamino-glycans from permanent human teeth and rat and rabbit incisors. Arch Oral Biol. 1973;18:49-59.
204. Linde A, Goldberg M. Dentinogenesis. Crit Rev Oral Biol Med. 1993;4:679-728.
205. Linde A, Lundgren T. From serum to the mineral phase. The role of the odontoblast in calcium transport and mineral formation. Int J Dev Biol. 1995;39:213-222.
206. Liu L, Simon SA. Capsaicin, acid and heat-evoked currents in rat trigeminal ganglion neurons: relationship to functional VR1 receptors. Physiol Behav. 2000;69:363.
207. Lohinai Z, Szekely AD, Benedek P, Csillag A. Nitric oxide synthetase containing nerves in the cat and dog dental pulps and gingiva. Neurosci Lett. 1997;227:91.
208. Lohinai Z, et al. Evidence for the role of nitric oxide in the circulation of the dental pulp. J Dent Res. 1995;74:1501-1506.
209. Lumsden AG. The developing innervation of the lower jaw and its relation to the formation of tooth germs in mouse. In: TEETH; Form, function and evolution. New York: Columbia University Press; 1982:32-43.
210. Lundberg JM, Änggård A, Fahrenkrug J, Hökfelt T, Mutt V. Vasoactive intestinal polypeptide in cholinergic neurons of exocrine glands: functional significance of coexisting transmitters for vasodilation and secretion. Proc Natl Acad Sci U S A. 1980;77:1651-1655.
211. Lundberg JM, Fried G, Fahrenkrug J, Holmstedt B, Hökfelt T, Lagercrantz H, et al. Subcellular fractionation of cat submandibular gland: comparative studies on the distribution of acetylcholine and vasoactive intestinal polypeptide (VIP). Neuroscience. 1981;6:1001-1010.
212. Lundgren T, Nannmark U, Linde A. Calcium ion activity and pH in the odontoblast-predentin region: ion-selective microelectrode measurements. Calcif Tissue Int. 1992;50:134.
213. Luthman J, Luthman D, Hökfelt T. Occurrence and distribution of different neurochemical markers in the human dental pulp. Arch Oral Biol. 1992;37:193.
214. Luukko K, Kvinnsland IH, Kettunen P. Tissue interactions in the regulation of axon pathfinding during tooth morphogenesis. Dev Dyn. 2005;234:482-488.
215. Luukko K, et al. Identification of a novel putative signaling center, the tertiary enamel knot in the postnatal mouse molar tooth. Mech Dev. 2003;120:270-276.
216. Luukko K, et al. Secondary induction and the development of tooth nerve supply. Ann Anat. 2008;190:178-187.
217. Madison S, Whitsel E, Suarez-Roca H, Maixner W. Sensitizing effects of leukotriene B4 on intradentinal primary afferents. Pain. 1992;49:99.
218. Maeda T, Honma S, Takano Y. Dense innervation of radicular human dental pulp as revealed by immunocytochemistry for protein gene-product 9.5. Arch Oral Biol. 1994;39:563.
219. Maita E, Simpson MD, Tao L, Pashley DH. Fluid and protein flux across the pulpodentin complex of the dog, in vivo. Arch Oral Biol. 1991;36:103.
220. Maltos KL, et al. Vascular and cellular responses to pro-inflammatory stimuli in rat dental pulp. Arch Oral Biol. 2004;49:443-450.
221. Mangkornkarn C, Steiner JC. In vivo and in vitro glycosaminoglycans from human dental pulp. J Endod. 1992;18:327-331.
222. Marbach JJ, Raphael KG. Phantom tooth pain: a new look at an old dilemma. Pain Med. 2000;1:68-77.
223. Marfurt CF, Zaleski EM, Adams CE, Welther CL. Sympathetic nerve fibers in rat orofacial and cerebral tissues as revealed by the HRP-WGA tracing technique: a light and electron microscopic study. Brain Res. 1986;366:373-378.
224. Marion D, Jean A, Hamel H, Kerebel LM, Kerebel B. Scanning electron microscopic study of odontoblasts and circumferential dentin in a human tooth. Oral Surg Oral Med Oral Pathol. 1991;72:473.
225. Martin-de las Heras S, Valenzuela A, Overall CM. The matrix metalloproteinases gelatinase A in human dentine. Arch Oral Biol. 2000;45:757.
226. Martinez-Insua A, de Silva L, Rilo B, Santana U. Comparison of the fracture resistance of pulpless teeth restored with a cast post and core or carbon-fiber post with a composite core. J Prosthet Dent. 1998;80:527.
227. Matsuo S, Ichikawa H, Henderson TA, Silos-Santiago I, Barbacid M, Arends JJ, et al. trkA modulation of developing somatosensory neurons in oro-facial tissues: tooth pulp fibers are absent in trkA knockout mice. Neuroscience. 2001;105:747-760.
228. Matthews B, Andrew D. Microvascular architecture and exchange in teeth. Microcirculation. 1995;2:305-313.
229. Matthews B, Andrew D, Amess TR: The functional properties of intradental nerves: proceedings of the International Conference on Dentin/Pulp Complex, ed. by Shimono M.
230. Matthews B, Vongsavan N. Interactions between neural and hydrodynamic mechanisms in dentine and pulp. Arch Oral Biol. 1994;39(suppl 1):87S.
231. McGrath PA, Gracely RH, Dubner R, Heft MW. Non-pain and pain sensations evoked by tooth pulp stimulation. Pain. 1983;15:377-388.
232. Meyer MW, Path MG. Blood flow in the dental pulp of dogs determined by hydrogen polarography and radioactive microsphere methods. Arch Oral Biol. 1979;24:601.
233. Michelich V, Pashley DH, Whitford GM. Dentin permeability: a comparison of functional versus anatomical tubular radii. J Dent Res. 1978;57:1019.
234. Michelich VJ, Schuster GS, Pashley DH. Bacterial penetration of human dentin in vitro. J Dent Res. 1980;59:1398.
235. Mitsiadis TA, De Bari C, About I. Apoptosis in developmental and repair-related human tooth remodeling: a view from the inside. Exp Cell Res. 2008;314:869-877.
236. Mjör IA, Nordahl I. The density and branching of dentinal tubules in human teeth. Arch Oral Biol. 1996;41:401.
237. Moe K, et al. Development of the pioneer sympathetic innervation into the dental pulp of the mouse mandibular first molar. Arch Oral Biol. 2008;53:865-873.
238. Mohamed SS, Atkinson ME. A histological study of the innervation of developing mouse teeth. J Anat. 1983;136(Pt 4):735-749.
239. Mullaney TP, Howell RM, Petrich JD. Resistance of nerve fibers to pulpal necrosis. Oral Surg. 1970;30:690.
240. Murray PE, About I, Lumley PJ, Franquin JC, Remusat M, Smith AJ. Human odontoblast cell numbers after dental injury. J Dent. 2000;28:277.
241. Murray PE, Hafez AA, Windsor LJ, Smith AJ, Cox CF. Comparison of pulp responses following restoration of exposed and non-exposed cavities. J Dent. 2002;30:213.
242. Murray PE, Lumley PJ, Ross HF, Smith AJ. Tooth slice organ culture for cytotoxicity assessment of dental materials. Biomaterials. 2000;21:1711.
243. Naftel JP, et al. Course and composition of the nerves that supply the mandibular teeth of the rat. Anat Rec. 1999;256:433-447.
244. Nagaoka S, Miyazaki Y, Liu HJ, Iwamoto Y, Kitano M, Kawagoe M. Bacterial invasion into dentinal tubules of human vital and nonvital teeth. J Endod. 1995;21:70.
245. Nair PN, et al. Histological, ultrastructural and quantitative investigations on the response of healthy human pulps to experimental capping with mineral trioxide aggregate: a randomized controlled trial. Int Endod J. 2008;41:128-150.
246. Nakamura O, Gohda E, Ozawa M, et al. Immunohistochemical studies with a monoclonal antibody on the distribution of phosphophoryn in predentin and dentin. Calcif Tissue Int. 1985;37:491-500.
247. Nalla RK, Imberi V, Kinney JH, Staininec M, Marshall SJ, Richie RO. In vitro fatigue behavior of human dentin with implications for life predictions. J Biomed Mater Res. 2003;64A:10.
248. Nalla RK, Kinney JH, Marshall SJ, Richie RO. On the in vitro fatigue behavior of human dentin: effect of mean stress. J Dent Res. 2004;83:211.
249. Närhi M. Activation of dental pulp nerves of the cat and the dog with hydrostatic pressure. Proc Finn Dent Soc. 1978;74(suppl 5):1.
250. Närhi M, Jyväsjärvi E, Hirronen T. Activation of heat-sensitive nerve fibers in the dental pulp of the cat. Pain. 1982;14:317.
251. Närhi M, Jyväsjärvi E, Virtanen A, Huopaniemi T, Ngassapa D, Hirvonen T. Role of intradentinal A- and C-type nerve fibers in dental pain mechanisms. Proc Finn Dent Soc. 1992;88(suppl 1):507.
252. Närhi M, Virtanen A, Kuhta J, Huopaniemi T. Electrical stimulation of teeth with a pulp tester in the cat. Scand J Dent Res. 1979;87:32.
253. Närhi M, Yamamoto H, Ngassapa D. Function of intradental nociceptors in normal and inflamed teeth. In: Shimono M, Maeda T, Suda H, Takahashi K, editors. Dentin/pulp complex. Tokyo: Quintessence Publishing Co; 1996:136.
254. Närhi M, Yamamoto H, Ngassapa D, Hirvonen T. The neurophysiological basis and the role of inflammatory reactions in dentine hypersensitivity. Arch Oral Biol. 1994;39(suppl):23S.
255. Nawroth PP, Stern DM. Modulation of endothelial cell hemostatic properties by tumor necrosis factor. J Exp Med. 1986;163:740-745.
256. Ngassapa D, Närhi M, Hirvonen T. The effect of serotonin (5-HT) and calcitonin gene-related peptide (CGRP) on the function of intradental nerves in the dog. Proc Finn Dent Soc. 1992;88(suppl 1):143.
257. Nicol GD, Vasko MR. Unraveling the story of NGF-mediated sensitization of nociceptive sensory neurons: ON or OFF the Trks? Molecular interventions. Feb 2007;7:26-41.
258. Nishioka M, Shiiya T, Ueno K, Suda H. Tooth replantation in germ-free and conventional rats. Endod Dent Traumatol. 1998;14:163.
259. Nitzan DW, Michaeli Y, Weinreb M, Azaz B. The effect of aging on tooth morphology: a study on impacted teeth. Oral Surg Oral Med Oral Pathol. 1986;61:54.
260. O’Neil RG, Brown RC. The vanilloid receptor family of calcium-permeable channels: molecular integrators of microenvironmental stimuli. News Physiol Sci. 2003;18:226.
261. Ochoa JL, Torebjork E, Marchettini P, Sivak M. Mechanism of neuropathic pain: cumulative observations, new experiments, and further speculation. In: Fields HL, Dubner R, Cervero F, editors. Advances in pain research and therapy. New York: Raven Press; 1985:431.
262. Oehmke MJ, Knolle E, Oehmke H-J. Lymph drainage in the human dental pulp. Microsc Res Tech. 2003;62:187.
263. Ogawa K, Yamashita Y, Ichijo T, Fusayama T. The ultrastructure and hardness of the transparent layer of human carious dentin. J Dent Res. 1983;62:7.
264. Okiji T, Kawashima N, Kosaka T, Matsumoto A, Kobayashi C, Suda H. An immunohistochemical study of the distribution of immunocompetent cells, especially macrophages and Ia antigen-presenting cells of heterogeneous populations, in normal rat molar pulp. J Dent Res. 1992;71:1196.
265. Okiji T, et al. Involvement of arachidonic acid metabolites in increases in vascular permeability in experimental dental pulpal inflammation in the rat. Arch Oral Biol. 1989;34:523-528.
266. Olgart LM, Edwall L, Gazelius B. Involvement of afferent nerves in pulpal blood-flow reactions in response to clinical and experimental procedures in the cat. Arch Oral Biol. 1991;36:575-581.
267. Olgart LM, Edwall L, Gazelius B. Neurogenic mediators in control of pulpal blood flow. J Endod. 1989;15:409-412.
268. Olgart LM, Gazelius B, Brodin E, Nilsson G. Release of substance P-like immunoreactivity from the dental pulp. Acta Physiol Scand. 1977;101:510.
269. Olgart L, Kerezoudis NP. Nerve-pulp interactions. Arch Oral Biol. 1994;39(suppl):47S.
270. Orchardson R, Cadden SW. An update on the physiology of the dentine-pulp complex. Dent Update. 2001;28:200-206. 208–209, 2001
271. Orchardson R, Gillam DG. Managing dentin hypersensitivity. J Am Dent Assoc. 2006;137:990-998. quiz 1028–1029
272. Oxlund H, Manschot J, Viidik A. The role of elastin in the mechanical properties of skin. J Biomech. 1988;21:213-218.
273. Parsons RJ, McMaster PD. The effect of the pulse upon the formation and flow of lymph. J Exp Med. 1938;68:353-376.
274. Pashley DH. Dentin conditions and disease. In: Lazzari G, editor. CRC handbook of experimental dentistry. Boca Raton, FL: CRC Press; 1983:97.
275. Pashley DH. Dentin permeability and dentin sensitivity. Proc Finn Dent Soc. 1992;88(suppl 1):31.
276. Pashley DH. Dentin permeability: theory and practice. In: Spangberg L, editor. Experimental endodontics. Boca Raton, FL: CRC Press; 1990:19.
277. Pashley DH. Dynamics of the pulpodentin complex. Crit Rev Oral Biol Med. 1996;7:104.
278. Pashley DH. Potential treatment modalities for dentin hypersensitivity—in office products. In Addy M, Orchardson R, editors: Tooth wear and sensitivity. London: Martin-Dunitz Publishers; 2000.
279. Pashley DH, Matthews WG. The effects of outward forced convective flow on inward diffusion in human dentin in vitro. Arch Oral Biol. 1993;38:577.
280. Pashley DH, Pashley EL, Carvalho RM, Tay FR. Effects of dentin permeability on restorative dentistry. Dent Clin North Am. 2002;46:211.
281. Pashley DH, Tay FR, Yiu C, Hashimoto M, Breschi B, Carvalho RM, et al. Collagen degradation by host-derived enzymes during aging. J Dent Res. 2004;83:216.
282. Pashley DH, Zhang Y, Agee KA, Rouse CJ, Carvalho RM, Russell CM. Permeability of demineralized dentin to HEMA. Dent Mater. 2000;16:7.
283. Pimenta FJ, Sa AR, Gomez RS. Lymphangiogenesis in human dental pulp. Int Endod J. 2003;36:853-856.
284. Pissiotis E, Spängberg L. Dentin permeability to bacterial proteins in vitro. J Endod. 1994;20:118.
285. Poggi P, et al. Ultrastructural localization of elastin-like immunoreactivity in the extracellular matrix around human small lymphatic vessels. Lymphology. 1995;28:189-195.
286. Pohto P, Antila R. Innervation of blood vessels in the dental pulp. Int Dent J. 1972;22:228-239.
287. Prati C, Cervellati F, Sanasi V, Montebugnoli L. Treatment of cervical dentin hypersensitivity with resin adhesives: 4 week evaluation. Am J Dent. 2001;14:378.
288. Prescott RS, et al. In vivo generation of dental pulp-like tissue by using dental pulp stem cells, a collagen scaffold, and dentin matrix protein 1 after subcutaneous transplantation in mice. J Endod. 2008;34:421-426.
289. Qian XB, Naftel JP. Effects of neonatal exposure to anti-nerve growth factor on the number and size distribution of trigeminal neurones projecting to the molar dental pulp in rats. Arch Oral Biol. 1996;41:359-367.
290. Qin C, Baba O, Butler WT. Post-translational modifications of SIBLING proteins and their roles in osteogenesis and dentinogenesis. Crit Rev Oral Biol Med. 2004;15:126-136.
291. Rapp R, el-Labban NG, Kramer IR, Wood D. Ultrastructure of fenestrated capillaries in human dental pulps. Arch Oral Biol. 1977;22:317.
292. Reader A, Foreman DW. An ultrastructural qualitative investigation of human intradental innervation. J Endod. 1981;7:493.
293. Renton T, Yiangou Y, Plumpton C, Tate S, Bountra C, Anand P. Sodium channel Nav1.8 immunoreactivity in painful human dental pulp. BMC Oral Health. 2005;5:5.
294. Roberts-Clark D, Smith AJ. Angiogenic growth factors in human dentine matrix. Arch Oral Biol. 2000;42:1013.
295. Rodd HD, Boissonade FM. Comparative immunohistochemical analysis of the peptidergic innervation of human primary and permanent tooth pulp. Arch Oral Biol. 2002;47:375.
296. Rodd HD, Boissonade FM. Innervation of human tooth pulp in relation to caries and dentition type. J Dent Res. Jan 2001;80:389-393.
297. Rodd HD, Boissonade FM, Day PF. Pulpal status of hypomineralized permanent molars. Pediatr Dent. 2007;29:514-520.
298. Rutherford B. BMP-7 gene transfer into inflamed ferret-dental pulps. Eur J Oral Sci. 2001;109:422.
299. Rutherford B, Fitzgerald M. A new biological approach to vital pulp therapy. Crit Rev Oral Biol Med. 1995;6:218.
300. Rutherford RB, Spanberg L, Tucker M, Rueger D, Charette M. The time-course of the induction of reparative dentine formation in moneys by recombinant human osteogenic protein-1. Arch Oral Biol. 1994;39:833.
301. Ryan TJ, Mortimer PS, Jones RL. Lymphatics of the skin. Neglected but important. Int J Dermatol. 1986;25:411-419.
302. Sakamoto N, et al. Identification of hyaluronidase activity in rabbit dental pulp. J Dent Res. 1981;60:850-854.
303. Sakurai K, Okiji T, Suda H. Co-increase of nerve fibers and HLA-DR- and/or factor XIIIa-expressing dendritic cells in dentinal caries-affected regions of the human dental pulp: an immunohistochemical study. J Dent Res. 1999;78:1596.
304. Sasaki S. Studies on the respiration of the dog tooth germ. J Biochem (Tokyo). 1959;46:269.
305. Sasano T, Kuriwada S, Sanjo D. Arterial blood pressure regulation of pulpal blood flow as determined by laser Doppler. J Dent Res. 1989;68:791.
306. Sasano T, Kuriwada S, Shoji N, Sanjo D, Izumi H, Karita K. Axon reflex vasodilatation in cat dental pulp elicited by noxious stimulation of the gingiva. J Dent Res. 1994;73:1797.
307. Sasano T, Shoji N, Kuriwada S, Sanjo D, Izumi H, Karita K. Absence of parasympathetic vasodilatation in cat dental pulp. J Dent Res. 1995;74:1665-1670.
308. Schmid-Schonbein GW. Microlymphatics and lymph flow. Physiol Rev. 1990;70:987-1028.
309. Schüpbach P, Lutz F, Finger WT. Closing of dentin tubules by Gluma desensitizer. Eur J Oral Sci. 1997;105:414.
310. Scott JN, Weber DF. Microscopy of the junctional region between human coronal primary and secondary dentin. J Morphol. 1977;154:133.
311. Seltzer S, Bender IB, Ziontz M. The interrelationship of pulp and periodontal disease. Oral Surg. 1963;16:1474.
312. Senger DR, et al. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983-985.
313. Sessle BJ. Recent developments in pain research: central mechanisms of orofacial pain and its control. J Endod. 1986;12:435-444.
314. Sessle BJ. The neurobiology of facial and dental pain: present knowledge, future directions. J Dent Res. 1987;66:962.
315. Shortland PJ, Jacquin MF, De Maro JA, Kwan CL, Hu JW, Sessle BJ. Central projections of identified trigeminal primary afferents after molar pulp differentiation in adult rats. Somatosens Mot Res. 1995;12:227.
316. Shulman K, et al. Expression of vascular permeability factor (VPF/VEGF) is altered in many glomerular diseases. J Am Soc Nephrol. 1996;7:661-666.
317. Shuttleworth CA, Ward JL, Hirschmann PN. The presence of type III collagen in the developing tooth. Biochim Biophys Acta. 1978;535:348-355.
318. Sigal MJ, Pitaru S, Aubin JE, Ten Cate AR. A combined scanning electron microscopy and immunofluorescence study demonstrating that the odontoblast process extends to the dentinoenamel junction in human teeth. Anat Rec. 1984;210:453.
319. Smith AJ, Garde C, Cassidy N, Ruch JV, Lesot H. Solubilization of dentin extracellular matrix by calcium hydroxide. J Dent Res. 1995;74:829. (abstract)
320. Smith AJ, Sloan AJ, Matthews JB, Murray PE, Lumley P. Reparative processes in dentine and pulp. In: Addy M, Embery G, Edger WM, Orchardson R, editors. Tooth wear and sensitivity: clinical advances in restorative dentistry. Martin Dunitz Publishers, 2000.
321. Smith AJ, Tobias RS, Cassidy N, Plant CG, Browne RM, Begue-Kirn C, et al. Odontoblast stimulation in ferrets by dentine matrix components. Arch Oral Biol. 1994;39:13.
322. Souza PP, et al. Regulation of angiotensin II receptors levels during rat induced pulpitis. Regul Pept. 2007;140:27-31.
323. Stanley HR, White CL, McCray L. The rate of tertiary (reparative) dentin formation in the human tooth. Oral Surg. 1966;21:180.
324. Stenvik A, Iverson J, Mjör IA. Tissue pressure and histology of normal and inflamed tooth pulps in Macaque monkeys. Arch Oral Biol. 1972;17:1501.
325. Stern D, et al. An endothelial cell-dependent pathway of coagulation. Proc Natl Acad Sci U S A. 1985;82:2523-2527.
326. Sunakawa M, Tokita Y, Suda H. Pulsed Nd:YAG laser irradiation of the tooth pulp in the cat. II. Effect of scanning lasing. Lasers Surg Med. 2000;26:477-488.
327. Swift ML, Byers MR. Effects of aging responses of nerve fibers to pulpal inflammation in rat molars analyzed by quantitative immunohistochemistry. Arch Oral Biol. 1992;37:901.
328. Tagami J, Hosoda H, Burrow MF, Nakajima M. Effect of aging and caries on dentin permeability. Proc Finn Dent Soc. 1992;88(suppl 1):149.
329. Takahashi K, Kishi Y, Kim S. A scanning electron microscope study of the blood vessels of dog pulp using corrosion resin casts. J Endodon. 1982;8:131.
330. Tanaka T. The origin and localization of dentinal fluid in developing rat molar teeth studied with lanthanum as a tracer. Arch Oral Biol. 1980;25:153-162.
331. Telles PD, et al. Lipoteichoic acid up-regulates VEGF expression in macrophages and pulp cells. J Dent Res. 2003;82:466-470.
332. Thesleff I. The genetic basis of tooth development and dental defects. Am J Med Genet A. 2006;140:2530-2535.
333. Thomas HF. The extent of the odontoblast process in human dentin. J Dent Res. 1979;58(D):2207.
334. Thomas HF, Payne RC. The ultrastructure of dentinal tubules from erupted human premolar teeth. J Dent Res. 1983;62:532.
335. Thomas JJ, Stanley HR, Gilmore HW. Effects of gold foil condensation on human dental pulp. J Am Dent Assoc. 1969;78:788.
336. Tokita Y, Sunakawa M, Suda H. Pulsed ND: YAG laser irradiation of the tooth pulp in the cat. I. Effect of spot lasing. Lasers Surg Med. 2000;26:477.
337. Tönder KJ. Blood flow and vascular pressure in the dental pulp. Summary. Acta Odontol Scand. 1980;38:135-144.
338. Tönder KJ. Effect of vasodilating drugs on external carotid and pulpal blood flow in dogs: “stealing” of dental perfusion pressure. Acta Physiol Scand. 1976;97:75-87.
339. Tönder KJH, Kvininsland I. Micropuncture measurements of interstitial fluid pressure in normal and inflamed dental pulp in cats. J Endod. 1983;9:105.
340. Tönder KH, Naess G. Nervous control of blood flow in the dental pulp in dogs. Acta Physiol Scand. 1978;104:13-23.
341. Torebjörk HE, Hanin RG. Perceptual changes accompanying controlled preferential blocking of A and C fiber responses in intact human skin nerves. Exp Brain Res. 1973;16:321.
342. Torneck CD. Dentin-pulp complex. In: Ten Cate AR, editor. Oral histology: development, structure, and function. ed 5. St Louis: Mosby; 1998:150.
343. Torneck CD, Kwan CL, Hu JW. Inflammatory lesions of the tooth pulp induce changes in brainstem neurons of the rat trigeminal subnucleus oralis. J Dent Res. 1996;75:553.
344. Trantor IR, Messer HH, Birner R. The effects of neuropeptides (calcitonin-gene-related peptide and substance P) on cultured human pulpal cells. J Dent Res. 1995;74:1066.
345. Trowbridge HO. Pathogenesis of pulpitis resulting from dental caries. J Endod. 1981;7:52.
346. Trowbridge HO, Franks M, Korostoff E, Emling R. Sensory response to thermal stimulation in human teeth. J Endod. 1980;6:405.
347. Trowbridge HO, Shibata F. Mitotic activity in epithelial rests of Malassez. Periodontics. 1967;5:109.
348. Trowbridge HO, Silver DR. Review of current approaches to in-office management of tooth hypersensitivity. Dent Clin North Am. 1990;16:561.
349. Trowbridge HO, Stewart JCB, Shapiro IM Assessment of indurated, diffusely calcified human dental pulps. In Proceedings of the International Conference on Dentin/Pulp Complex 1996 Quintessence Publishing Co Tokyo 297
350. Turner DF. Immediate physiological response of odontoblasts. Proc Finn Dent Soc. 1992;88(suppl 1):55.
351. Turner D, Marfurt C, Sattelburg C. Demonstration of physiological barrier between pulpal odontoblasts and its perturbation following routine restorative procedures: a horseradish peroxidase tracing study in the rat. J Dent Res. 1989;68:1262.
352. Uddman R, et al. Occurrence of VIP nerves in mammalian dental pulps. Acta Odontol Scand. 1980;38:325-328.
353. Vaahtokari A, et al. The enamel knot as a signaling center in the developing mouse tooth. Mech Dev. 1996;54:39-43.
354. Vainio S, et al. Identification of BMP-4 as a signal mediating secondary induction between epithelial and mesenchymal tissues during early tooth development. Cell. 1993;75:45-58.
355. van Amerongen JP, Lemmens IG, Tonino GJ. The concentration, extractability and characterization of collagen in human dental pulp. Arch Oral Biol. 1983;28:339-345.
356. Van Hassel HJ. Physiology of the human dental pulp. Oral Surg Oral Med Oral Path. 1971;32:126.
357. Van Hassel HJ, Brown AC. Effect of temperature changes on intrapulpal pressure and hydraulic permeability in dogs. Arch Oral Biol. 1969;14:301-315.
358. Vashishth D, Tanner KE, Bonfield W. Experimental validation of a microcracking-based toughening mechanism for cortical bone. J Biomech. 2003;36:121.
359. Veis A. Mineral-matrix interactions in bone and dentin. J Bone Miner Res. 1993;Suppl 2:S493-S497.
360. Vickers ER, Cousins MJ. Neuropathic orofacial pain part 1–prevalence and pathophysiology. Aust Endod J. 2000;26:19-26.
361. Vongsavan N, Matthews B. Fluid flow through cat dentine in vivo. Arch Oral Biol. 1992;37:175-185.
362. Vongsavan N, Matthews B. The permeability of cat dentine in vivo and in vitro. Arch Oral Biol. 1991;36:641.
363. Vongsavan N, Matthews B. The relation between fluid flow through dentine and the discharge of intradental nerves. Arch Oral Biol. 1994;39(suppl):140S.
364. Vongsavan N, Matthews B. The vascularity of dental pulp in cats. J Dent Res. 1992;71:1913-1915.
365. Wakisaka S. Neuropeptides in the dental pulp: their distribution, origins and correlation. J Endod. 1990;16:67.
366. Wakisaka S, Ichikawa H, Akai M. Distribution and origins of peptide- and catecholamine-containing nerve fibres in the feline dental pulp and effects of cavity preparation on these nerve fibres. J Osaka Univ Dent Sch. 1986;26:17-28.
367. Wakisaka S, Sasaki Y, Ichikawa H, Matsuo S. Increase in c-fos-like immunoreactivity in the trigeminal nucleus complex after dental treatment. Proc Finn Dent Soc. 1992;88(suppl 1):551-555.
368. Wall PD. Alterations in the central nervous system after deafferentation: connectivity control. In: Bonica JJ, Lindblom U, Iggo A, editors. Advances in pain research and therapy, vol 5. New York: Raven Press; 1983:677.
369. Wang RZ, Weiner S. Strain-structure relations in human teeth using Moire fringes. J Biomech. 1998;31:135.
370. Warfvinge J, Dahlen G, Bergenholtz G. Dental pulp response to bacterial cell wall material. J Dent Res. 1985;64:1046.
371. Weber DF. Human dentine sclerosis: a microradiographic study. Arch Oral Biol. 1974;19:163.
372. Weber DK, Zaki AL. Scanning and transmission electron microscopy of tubular structure presumed to be human odontoblast processes. J Dent Res. 1986;65:982.
373. Weiger R, Axmann-Kremar D, Lost C. Prognosis of conventional root canal treatment reconsidered. Endodon Dent Traumatol. 1998;14:1.
374. Weiner S, Vies A, Beniash E, Arad T, Dillon JW, Sabsay B, et al. Peritubular dentin formation: crystal organization and the macromolecular constituents in human teeth. J Struct Biol. 1999;126:27.
375. Weinstock M, Leblond CP. Synthesis, migration and release of precursor collagen by odontoblasts as visualized by radioautography after 3H-proline administration. J Cell Biol. 1974;60:92.
376. Weinstock A, Weinstock M, Leblond CP. Autoradiographic detection of 3H-fucose incorporation into glycoprotein by odontoblasts and its deposition at the site of the calcification front in dentin. Calcif Tissue Res. 1972;8:181.
377. Wiig H, Aukland K, Tenstad O. Isolation of interstitial fluid from rat mammary tumors by a centrifugation method. Am J Physiol Heart Circ Physiol. 2003;284:H416-H424.
378. Wiig H, et al. The role of the extracellular matrix in tissue distribution of macromolecules in normal and pathological tissues: potential therapeutic consequences. Microcirculation. 2008;15:283-296.
379. Winter HF, Bishop JG, Dorman HL. Transmembrane potentials of odontoblasts. J Dent Res. 1963;42:594.
380. Woodnutt DA, Wager-Miller J, O’Neill PC, Bothwell M, Byers MR. Neurotrophin receptors and nerve growth factor are differentially expressed in adjacent nonneuronal cells of normal and injured tooth pulp. Cell Tissue Res. 2000;299:225-236.
381. Yamada T, Nakamura K, Iwaku M, Fusayama T. The extent of the odontoblast process in normal and carious human dentin. J Dent Res. 1983;62:798.
382. Yamaguchi M, Kojima T, Kanekawa M, Aihara N, Nogimura A, Kasai K. Neuropeptides stimulate production of interleukin-1 beta, interleukin-6, and tumor necrosis factor-alpha in human dental pulp cells. Inflamm Res. 2004;53:199-204.
383. Yamamura T. Differentiation of pulpal wound healing. J Dent Res. 1985;64(special issue):530.
384. Yang BH, Piao ZG, Kim Y-B. Activation of vanilloid receptor 1 (VR1) by eugenol. J Dent Res. 2003;82:781.
385. Yu CY, Boyd NM, Cringle SJ. An in vivo and in vitro comparison of the effects of vasoactive mediators on pulpal blood vessels in rat incisors. Arch Oral Biol. 2002;47:723-732.
386. Yu CY, Boyd NM, Cringle SJ, Alder VA, Yu DY. Oxygen distribution and consumption in rat lower incisor pulp. Arch Oral Biol. 2002;47:529.
387. Zerlotti E. Histochemical study of the connective tissue of the dental pulp. Arch Oral Biol. 1964;9:149.
388. Zhang J, Kawashima N, Suda H, Nakano Y, Takano Y, Azuma M. The existence of CD11c+ sentinel and F4/80+ interstitial dendritic cells in dental pulp and their dynamics and functional properties. Int Immunol. 2006;18:1375-1384.