1. Abedi HR, Torabinejad M, Pitt Ford TR, Bakland LK. The use of mineral trioxide aggregate cement (MTA) as a direct pulp-capping agent. J Endod. 1996;22:199. (abstract)
2. Aeinehchi M, Eslami B, Ghanhariha M, Saffar AS. Mineral trioxide aggregate (MTA) and calcium hydroxide as pulp-capping agents in human teeth: a preliminary report. Int Endod J. 2003;36:225.
3. Agamy HA, Bakry NS, Mounir MMF, Avery DR. Comparison of mineral trioxide aggregate and formocresol as pulp capping agents in pulpotomised primary teeth. Pediatr Dent. 2004;26:302.
4. Akimoto N, Momoi Y, Kohno A, et al. Biocompatibility of Clearfil linear bond 2 and Clearfil AP-X system nonexposed and exposed primate teeth. Quintessence Int. 1998;29:177.
5. Alacam A. Long-term effects of primary teeth pulpotomies with formocresol, glutaraldehyde-calcium hydroxide and glutaraldehyde-zinc oxide-eugenol on succedaneous teeth. J Periodontol. 1989;13:307.
6. Al-Zayer MA, Straffon LH, Feigal RJ, Welch KB. Indirect pulp treatment of primary posterior teeth: a retrospective study. Pediatr Dent. 2003;25:29.
7. American Academy of Pediatric Dentistry. Reference manual: clinical guideline on pulp therapy for primary and young permanent teeth. Pediatr Dent. 2003-2004;25:87.
8. Andreasen F. Transient apical breakdown and its relation to color and sensibility changes. Endod Dent Traumatol. 1986;2:9.
9. Andreasen J, Andreasen F, Andreasen L. Textbook and color atlas of traumatic injuries to the teeth, ed 4. Philadelphia: Wiley-Blackwell; 2008.
10. Andreasen JO, Farik B, Munksgaard EC. Long-term calcium hydroxide as a root canal dressing may increase risk of root fracture. Dent Traumatol. 2002;18:134.
11. Andreasen JO, Ravn JJ. Epidemiology of traumatic dental injuries to primary and permanent teeth in a Danish population sample. Int J Oral Surg. 1972;1:235.
12. Andreasen JO, Ravn JJ. The effect of traumatic injuries to primary teeth on their permanent successors: II–a clinical and radiographic follow-up study of 213 teeth. Scand J Dent Res. 1971;79:284.
13. Andrew P. The treatment of infected pulps in deciduous teeth. Br Dent J. 1955;98:122.
14. Aponte AJ, Hartsook JT, Crowley MC. Indirect pulp capping success verified. J Dent Child. 1966;33:164.
15. Armstrong RL, Patterson SS, Kafrawy AH, Feltman EM. Comparison of Dycal and formocresol pulpotomies in young permanent teeth in monkeys. Oral Surg. 1979;48:160.
16. Attala MN, Noujaim AA. Role of calcium hydroxide in the formation of reparative dentin. J Can Dent Assoc. 1969;35:267.
17. Avram DC, Pulver F. Pulpotomy medicaments for vital primary teeth: surveys to determine use and attitudes in pediatric dental practice and in dental schools throughout the world. J Dent Child. 1989;56:426.
18. Baggett FJ, Mackie IC, Worthington HV. An investigation into the measurement of the working length of immature incisor teeth requiring endodontic treatment in children. Brit Dent J. 1996;181:96.
19. Banchs F, Trope M. Revascularization of immature permanent teeth with apical periodontitis: a new treatment protocol? J Endod. 2004;30:196.
20. Barthel CR, Rosenkranz B, Leuenberg A, Roulet JF. Pulp capping of carious exposures: treatment outcome after 5 and 10 years: a retrospective study. J Endod. 2000;26:525.
21. Bennett CG. Pulpal management of deciduous teeth. Pract Dent Monogr. 1965;May-June:1.
22. Bergenholtz G. Evidence for bacterial causation of adverse pulpal responses in resin-based dental restorations. Crit Rev Oral Biol Med. 2000;11:467.
23. Berk H, Krakow AA. Endodontic treatment in primary teeth. In: Goldman HM, et al, editors. Current therapy in dentistry, vol 5. St Louis: Mosby; 1974.
24. Bevelander G, Benzer D. Morphology and incidence in secondary dentin in human teeth. J Am Dent Assoc. 1943;30:1079.
25. Björndal L. Indirect pulp therapy and stepwise excavation. J Endod. 2008;34(7S):S29.
26. Block RM, Lewis RD, Sheats JB, Fawley J. Cell-mediated immune response to dog pulp tissue altered by formocresol within the root canal. J Endod. 1977;3:424.
27. Bogen G, Kim JS, Bakland LK. Direct pulp capping with mineral trioxide aggregate. An observational study. J Amer Dent Assoc. 2008;139:305.
28. Bonfante G, Kaizer OB, Pegoraro LF, do Valle LA. Fracture strength of teeth with flared root canals restored with glass fibreposts. Int Dent J. 2007;57:153.
29. Borum MK, Andreasen JO. Sequelae of trauma to primary maxillary incisors: I–complications in the primary dentition. Endod Dent Traumatol. 1998;14:31.
30. Burnett S, Walker J. Comparison of ferric sulfate formocresol and a combination of ferric sulfate/formocresol in primary tooth vital pulpotomies: a retrospective radiographic survey. J Dent Child. 2002;69:44.
31. Byers M, Narchi M. The dental injury model: experimental tools for understanding neuro-inflammatory interactions and polymodal nociceptors functions. Crit Rev Oral Biol Med. 1999;10:4.
32. Caldwell RE, Freilich MM, Sandor GKB. Two radicular cysts associated with endodontically treated primary teeth: rationale for long-term follow-up. Ont Dent. 1999;76:29.
33. Caliskan MK. Pulpotomy of carious vital teeth with periapical involvement. Int Endod J. 1995;28:172.
34. Camp JH: Continued apical development of pulpless permanent teeth after endodontic therapy, master’s thesis, Bloomington, 1968, Indiana University School of Dentistry.
35. Carrilho MR, Geraldeli S, Tay F, et al. In vivo preservation of the hybrid layer by chlorhexidine. J Dent Res. 2007;86:529.
36. Carvahlo CAT, Valera MC, Oliveira LD, Camargo CHR. Structural resistance in immature teeth using root reinforcements in vitro. Dent Traumatol. 2005;21:155.
37. Chueh L-H, Huang G T-H. Immature teeth with periradicular pathosis or abscess undergoing apexogenesis: a paradigm shift. J Endod. 2006;32:1205.
38. Cohenca N, Karnis S, Rotstein I. Transient apical breakdown following tooth luxation. Dent Traumatol. 2003;19:289.
39. Coll JA, Sadrian R. Predicting pulpectomy success and its relationship to exfoliation and succedaneous dentition. Pediatr Dent. 1996;18:57.
40. Cotes O, Boj JR, Canalda C, Carreras M. Pulpal tissues reaction to formocresol vs. ferric sulfate in pulpotomized rat teeth. J Clin Ped Dent. 1997;21:247.
41. Coviello J, Brilliant JD. A preliminary clinical study of the use of tricalcium phosphate as an apical barrier. J Endod. 1979;5:6.
42. Cox CF, Suzuki S. Re-evaluating pulpal protection: calcium hydroxide liners vs cohesive hybridization. J Am Dent Assoc. 1994;125:823.
43. Cox CF, Hafez AA, Akimoto N, Otsuki M, Suzuki S, Tarim B. Biocompatibility of primer, adhesive and resin composite systems on non-exposed and exposed pulps of non-human primate teeth. Am J Dent. 1998;11:55. (special issue)
44. Cox CF, Keall CL, Keall HJ, Ostro E, Bergenholtz C. Biocompatibility of surface-sealed dental materials against exposed pulp. J Prosthet Dent. 1987;57:1.
45. Cox CF, Sübay RK, Suzuki S, Suzuki SH, Ostro E. Biocompatibility of various dental materials: pulp healing with a surface seal. Int J Periodont Restorative Dent. 1996;16:241.
46. Cox CF, Bergenholtz G, Fitzgerald M, et al. Capping of the dental pulp mechanically exposed to the oral microflora: a 5-week observation of wound healing in the monkey. J Oral Pathol. 1982;11:327.
47. Cox CF, Bergenholtz G, Heys DR, Syed SA, Fitzgerald M, Heys RJ. Pulp-capping of dental pulp mechanically exposed to oral microflora: a 1- to 2-year observation of wound healing in the monkey. J Oral Pathol. 1985;14:156.
48. Cox CF, Sübay RK, Ostro E, Suzuki S, Suzuki SH. Tunnel defects in dentin bridges: their formation following direct pulp capping. Oper Dent. 1996;21:4.
49. Croll TP, Pascon EA, Langeland K. Traumatically injured primary incisors: a clinical and histological study. J Dent Child. 1987;54:401.
50. Cvek M. A clinical report on partial pulpotomy and capping with calcium hydroxide in permanent incisors with complicated crown fractures. J Endod. 1978;4:232.
51. Cvek M. Prognosis of luxated non-vital maxillary incisors treated with calcium hydroxide and filled with gutta-percha. A retrospective clinical study. Endod Dent Traumatol. 1992;8:45.
52. Cvek M. Treatment of non-vital permanent incisors with calcium hydroxide. Odontol Revy. 1972;23:27.
53. Cvek M, Lundberg M. Histological appearance of pulps after exposure by a crown fracture, partial pulpotomy, and clinical diagnosis of healing. J Endod. 1983;9:8.
54. Cvek M, Granath L, Cleaton-Jones P, Austin J. Hard tissue barrier formation in pulpotomized monkey teeth capped with cyanoacrylate or calcium hydroxide for 10 and 60 minutes. J Dent Res. 1987;66:1166.
55. Cvek M, Cleaton-Jones PE, Austin JC, Andreasen JO. Pulp reactions to exposure after experimental crown fracture or grinding in adult monkey. J Endod. 1982;8:391.
56. Dandashi MB, Nazif MM, Zullo T, Elliott MA, Schneider LG, Czonstkowsky M. An in vitro comparison of three endodontic techniques for primary incisors. Pediatr Dent. 1993;15:254.
57. Davis MJ, Myers R, Switkes MD. Glutaraldehyde: an alternative to formocresol for vital pulp therapy. J Dent Child. 1982;49:176.
58. Dean J, Avery D, McDonald R. McDonald and Avery’s dentistry for the child and adolescent, ed 10. St. Louis: Mosby; 2011.
59. Dominguez MS, Witherspoon DE, Gutmann JL, Opperman LA. Histological and scanning electron microscopy assessment of various vital pulp-therapy materials. J Endod. 2003;29:324.
60. Dummer PMH, Hicks R, Huws D. Clinical signs and symptoms in pulp disease. Int Endod J. 1980;13:27.
61. Dylewski JJ. Apical closure of non-vital teeth. Oral Surg. 1971;32:82.
62. Easlick KA. Operative procedures in management of deciduous molars. Int J Orthod. 1934;20:585.
63. Eidelman E, Holan G, Fuks AB. Mineral trioxide aggregate vs. formocresol in pulpotomized primary molars: a preliminary report. Pediatr Dent. 2001;23:15.
64. Elliott RD, Roberts MW, Burkes J, Phillips C. Evaluation of the carbon dioxide laser on vital human primary pulp tissue. Pediatr Dent. 1999;21:327.
65. El-Meligy OA, Avery DR. Comparison on mineral trioxide aggregate and calcium hydroxide as pulpotomy agents in young permanent teeth (apexogenesis). Pediatr Dent. 2006;28:399.
66. Emmerson C, et al. Pulpal changes following formocresol applications on rat molars and human primary teeth. J South Calif Dent Assoc. 1959;27:309.
67. England MC, Best E. Non induced apical closure in immature roots of dogs’ teeth. J Endod. 1977;3:411.
68. Evans D, Reid J, Strang R, Stirrups D. A comparison of laser Doppler flowmetry with other methods of assessing the vitality of traumatized anterior teeth. Endod Dent Traumatol. 1999;15:284.
69. Fairbourn DR, Charbeneau GT, Loesche WJ. Effect of improved Dycal and I.R.M. on bacteria in deep carious lesions. J Am Dent Assoc. 1980;100:547.
70. Falster CA, Araujo FB, Straffon LH, Nor JE. Indirect pulp treatment: in vivo outcomes of an adhesive resin system vs calcium hydroxide for protection of the dentin-pulp complex. Pediatr Dent. 2002;24:241.
71. Faraco IMJr, Holland R. Response of the pulp of dogs to capping with mineral trioxide aggregate or a calcium hydroxide cement. Dent Traumatol. 2001;17:163.
72. Farooq NS, Coll JA, Kuwabara A. Success rates of formocresol pulpotomy and indirect pulp therapy in the treatment of deep dentinal caries in primary teeth. Pediatr Dent. 2000;22:278.
73. Farsi N, Alamoundi N, Balto K, Al Mushayat A. Clinical assessment of mineral trioxide aggregate (MTA) as direct pulp capping in young permanent teeth. J Clin Pediatr Dent. 2006;31:72.
74. Fayle SA, Welbury RR, Roberts JF. British society of paediatric dentistry: a policy document on management of caries in the primary dentition. Int J Pediatr Dent. 2001;11:153.
75. Fei AL, Udin RD, Johnson R. A clinical study of ferric sulfate as a pulpotomy agent in primary teeth. Pediatr Dent. 1991;13:327.
76. Feigal RJ, Messer HH. A critical look at glutaraldehyde. Pediatr Dent. 1990;12:69.
77. Feltman EM: A comparison of the formocresol pulpotomy techniques and dycal pulpotomy technique in young permanent teeth, master’s thesis, Bloomington, 1972, School of Dentistry, Indiana University.
78. Finn SB, et al, editors. Clinical Pedodontics, ed 3, Philadelphia: WB Saunders, 1967.
79. Fischer DE. Tissue management: a new solution to an old problem. Gen Dent. 1987;35:178-182.
80. Fishman SA, Udin RD, Good DL, Rodef F. Success of electrofulguration pulpotomies covered by zinc oxide and eugenol. Pediatr Dent. 1996;18:385.
81. Frank AL. Therapy for the divergent pulpless tooth by continued apical formation. J Am Dent Assoc. 1966;72:87.
82. Frankel SN. Pulp therapy in pedodontics. Oral Surg. 1972;34:293.
83. Fuchino T. Clinical and histopathological studies of pulpectomy in deciduous teeth. Shikwa Gakuho. 1980;80:971.
84. Fuks AB. Current concepts in vital primary pulp therapy. Eur J Paediatr Dent. 2002;3:115.
85. Fuks AB, Bimstein EC. Clinical evaluation of diluted formocresol pulpotomies in primary teeth of school children. Pediatr Dent. 1981;3:321.
86. Fuks AB, Bimstein E, Bruchimn A. Radiographic and histologic evaluation of the effect of two concentrations of formocresol on pulpotomized primary and young permanent teeth in monkeys. Pediatr Dent. 1983;5:9.
87. Fuks AB, Eidelman E, Pauker N. Root fillings with Endoflas in primary teeth: a retrospective study. J Clin Pediatr Dent. 2002;27:41.
88. Fuks AB, Holan G, Davis JM, Eidelman E. Ferric sulfate versus diluted formocresol in pulpotomized primary molars: long term follow-up. Pediatr Dent. 1997;19:327.
89. Fuks AB, Bimstein E, Guelmann M, Klein H. Assessment of a 2 percent buffered glutaraldehyde solution in pulpotomized primary teeth of school children. J Dent Child. 1990;57:371.
90. Fuks AB, Chosack A, Klein H, Eidelman E. Partial pulpotomy as a treatment alternative for exposed pulps in crown-fractured permanent incisors. Endod Dent Traumatol. 1987;3:100.
91. Fulling HJ, Andreasen JO. Influence of maturation status and tooth type of permanent teeth upon electrometric and thermal pulp testing procedures. Scand J Dent Res. 1976;84:286.
92. Fulling HJ, Andreasen JO. Influence of splints and temporary crowns upon electric and thermal pulp-testing procedures. Scand J Dent Res. 1976;84:291.
93. Fulton R, Ranly DM. An autoradiographic study of formocresol pulpotomies in rat molars using 3H-formaldehyde. J Endod. 1979;5:71.
94. Fuss Z, Trowbridge H, Bender IB, Rickoff B. Assessment of reliability of electrical and thermal pulp testing agents. J Endod. 1986;12:301.
95. Garcia-Godoy F. Evaluation of an iodoform paste in root canal therapy for infected primary teeth. J Dent Child. 1987;54:30.
96. Garcia-Godoy F, Novakovic DP, Carvajal IN. Pulpal response to different application times of formocresol. J Periodontol. 1982;6:176.
97. Garcia-Godoy F, Ranly D. Clinical evaluation of pulpotomies with ZOE as the vehicle for glutaraldehyde. Pediatr Dent. 1987;9:144.
98. Gerlach E. Root canal therapeutics in deciduous teeth. Dent Surv. 1932;8:68.
99. Glendor V. On dental trauma in children and adolescents: incidence, risk, treatment, time and cost. Swed Dent J. 2000;140(Suppl):1.
100. Goldberg F, Kaplan A, Roitman M, Manfre S, Picca M. Reinforcing effect of a resin glass ionomer in the restoration of immature roots in vitro. Dent Traumatol. 2002;18:70.
101. Goldmacher VS, Thilly WG. Formaldehyde is mutagenic for cultured human cells. Mutat Res. 1983;116:417.
102. Granath LE, Hagman G. Experimental pulpotomy in human bicuspids with reference to cutting technique. Acta Odontol Scand. 1971;29:155.
103. Greenberg M. Filling root canals of deciduous teeth by an injection technique. Dent Digest. 1964;67:574.
104. Guelmann M, Brookmyer KL, Villalta P, Garcia-Godoy F. Microleakage of restorative techniques for pulpotomised primary molars. ASDC J Dent Child. 2004;71:209.
105. Guthrie TJ, McDonald RE, Mitchell DF. Dental hemogram. J Dent Res. 1965;44:678.
106. Gwinnett AJ, Tay FR. Early and intermediate time response of the dental pulp to an acid etch technique in vivo. Am J Dent. 1998;11:S35. (special issue)
107. Hadeer AA, Niveen SB, Maha MFM, Avery DR. Comparison of mineral trioxide aggregate and formocresol as pulp-capping agents in pulpotomized primary teeth. Pediatr Dent. 2004;26:302.
108. Ham JW, Patterson SS, Mitchell DF. Induced apical closure of immature pulpless teeth in monkeys. Oral Surg. 1972;33:438.
109. Hamaguchi F, Tsutsui T. Assessment of genotoxicity of dental antiseptics: ability of phenol, guaiacol, p-phenolsulfonic acid, sodium hypochlorite, p-chlorophenol, m-cresol or formaldehyde to induce unscheduled DNA synthesis in cultured Syrian hamster embryo cells. Jpn J Pharmacol. 2000;83:273.
110. Hargreaves K. Seltzer and Bender’s the dental pulp, ed 2. Chicago: Quintessence; 2010.
111. Heasman P, McCracken G. Harty’s dental dictionary, 3rd Edition. London: Churchill Livingstone Elsevier; 2007.
112. Hebling J, Giro EMA, deSouza Costa CA. Biocompatibility of an adhesive system applied to exposed human dental pulp. J Endod. 1999;25:676.
113. Heide S. Pulp reactions to exposure for 4, 24 and 168 hours. J Dent Res. 1980;59:1910.
114. Heide S, Kerekes K. Delayed partial pulpotomy in permanent incisors of monkeys. Int Endod J. 1986;19:78.
115. Hermann BW. Dentinobliteran der Wurzelkanalc nach der Behandlung mit Kalzium. Zahnärztl Rundschau. 1930;39:888.
116. Hernandez R, Bader S, Boston D, Trope M. Resistance to fracture of endodontically treated premolars restored with new generation dentin bonding systems. Int Endod J. 1994;27:281.
117. Hibbard ED, Ireland RL. Morphology of the root canals of the primary molar teeth. J Dent Child. 1957;24:250.
118. Hill S, Berry CW, Seale NS, Kaga M. Comparison of antimicrobial and cytotoxic effects of glutaraldehyde and formocresol. Oral Surg Oral Med Oral Pathol. 1991;71:89.
119. Hobson P. Treatment of deciduous teeth. Part 2. Clinical investigation. Br Dent J. 1970;128:275.
120. Holan G. Development of clinical and radiographic signs associated with dark discolored primary incisors following traumatic injuries: a prospective controlled study. Dent Traumatol. 2004;20:276.
121. Holan G. Tube like mineralizations in the dental pulp of traumatized primary incisors. Endod Dent Traumatol. 1988;14:279.
122. Holan G, Eidelman E, Fuks AB. Long-term evaluation of pulpotomy in primary molars using mineral trioxide aggregate or formocresol as dressing materials. Pediatr Dent. 2005;27:129.
123. Holan G, Fuks AB. A comparison of pulpectomies using ZOE and KRI paste in primary molars: a retrospective study. Pediatr Dent. 1993;15:403.
124. Holan G, Fuks AB. Root canal treatment with ZOE and KRI paste in primary molars: a retrospective study. Pediatr Dent. 1993;15:403.
125. Holan G, Fuks AB. The diagnostic value of coronal dark-gray discoloration in primary teeth following traumatic injuries. Pediatr Dent. 1996;18:224.
126. Holan G, Topf J, Fuks AB. Effect of root canal infection and treatment of traumatized primary incisors on their permanent successors. Endod Dent Traumatol. 1992;8:12.
127. Huang G T-J, Sonoyama W, Liu Y, Liu H, Wang S, Shi S. The hidden treasure in apical papilla: the potential role in pulp/dentin regeneration and bioroot engineering. J Endod. 2008;34:645.
128. Hülsmann M, Pieper K. Use of an electronic apex locator in the treatment of teeth with incomplete root formation. Endod Dent Traumatol. 1989;5:238.
129. Ibricevic H, Al-Jame Q. Ferric sulfate as pulpotomy agent in primary teeth: twenty month clinical follow up. J Clin Pediatr Dent. 2000;24(4):269.
130. Ingle JI, Bakland LK, Baumgartner JC. Ingle’s endodontics, ed 6. New York: BC Decker; 2008.
131. Innes NP, Evans JP, Stirrups DR. The Hall technique; a randomized controlled clinical trial of a novel method of managing carious primary molars in general dental practice: acceptability of the technique and outcomes at 23 months. BMC Oral Health. 2007;7:18.
132. Innes NP, Stirrups DR, Evans DJ, Hall N, Leggate M. A novel technique using preformed metal crowns for managing carious primary molars in general practice–a retrospective analysis. Br Dent J. 2006;200:451.
133. International Agency for Research on Cancer. IARC classifies formaldehyde as carcinogenic to humans. Press release no. 153, June 2004
134. Ireland RL. Secondary dentin formation in deciduous teeth. J Am Dent Assoc. 1941;28:1626.
135. Iwaya S, Ikawa M, Kubota M. Revascularization of an immature permanent tooth with apical periodontitis and sinus tract. Dent Traumatol. 2001;17:185.
136. Jacobsen I. Criteria for diagnosis of pulpal necrosis in traumatized permanent incisors. Scand J Dent Res. 1980;88:306.
137. Jeng HW, Feigal RJ, Messer HH. Comparison of the cytotoxicity of formocresol, formaldehyde, cresol, and glutaraldehyde using human pulp fibroblast cultures. Pediatr Dent. 1987;9:295.
138. Jordon ME. Operative dentistry for children. New York: Dental Items of Interest Publishing Co; 1925.
139. Jung I-L, Lee S-J, Hargreaves KM. Biologically based treatment of immature permanent teeth with pulpal necrosis: a case series. J Endod. 2008;34:876.
140. Junn DJ, McMillan P, Bakland LK, Torabinejad M. Quantitative assessment of dentin bridge formation following pulp-capping with mineral trioxide aggregate (MTA). J Endod. 1998;24:278. (abstract)
141. Kaiser JH: Management of wide-open canals with calcium hydroxide. Paper presented at the meeting of the American Association of Endodontics, Washington, DC, April 17, 1964. Cited by Steiner JC, Dow PR, Cathey GM: Inducing root end closure of nonvital permanent teeth. J Dent Child 35:47, 1968.
142. Kakehashi S, Stanley HR, Fitzgerald RT. The effects of surgical exposures of dental pulps in germ-free and conventional laboratory rats. Oral Surg. 1965;20:340.
143. Karp WB, Korb P, Pashley D. The oxidation of glutaraldehyde by rat tissues. Pediatr Dent. 1987;9:301.
144. Katebzadeh N, Dalton BC, Trope M. Strengthening immature teeth during and after apexification. J Endod. 1998;24:256.
145. Kato S, Fusayama T. Recalcification of artificially decalcified dentin in vivo. J Dent Res. 1970;49:1060.
146. Kennedy DB, El-Kafrawy AH, Mitchell DF, Roche JR. Formocresol pulpotomy in teeth of dogs with induced pulpal and periapical pathosis. J Dent Child. 1973;40:44.
147. King JB, Crawford JJ, Lindahl RL. Indirect pulp capping: a bacteriologic study of deep carious dentine in human teeth. Oral Surg. 1965;20:663.
148. Klein H. Pulp responses to electric pulp stimulator in the developing permanent anterior dentition. J Dent Child. 1978;45:199.
149. Koenigs JF, Heller AL, Brilliant JD, Melfi RC, Driskell TD. Induced apical closure of permanent teeth in adult primates using a resorbable form of tricalcium phosphate ceramic. J Endod. 1975;1:102.
150. Kopel HM, Bernick S, Zacrhrisson E, DeRomero SA. The effects of glutaraldehyde on primary pulp tissue following coronal amputation: an in vivo histologic study. J Dent Child. 1980;47:425.
151. Kubota K, Golden BE, Penugonda B. Root canal filling materials for primary teeth: a review of the literature. J Dent Child. 1992;59:225.
152. Landau MJ, Johnsen DC. Pulpal responses to ferric sulfate in monkeys. J Dent Res. 1988;167:215. Special issue
153. Langeland K. Management of the inflamed pulp associated with deep carious lesion. J Endod. 1981;7:169.
154. Langeland K, Dowden WE, Tronstad L, Langeland LK. Human pulp changes of iatrogenic origin. Oral Surg. 1971;32:943.
155. Laurence RP. A method of root canal therapy for primary teeth, master’s thesis. Atlanta: School of Dentistry, Emory University; 1966.
156. Lawley GR, Schindler WG, Walker WAIII, Kolodrubetl D. Evaluation of ultrasonically placed MTA and fracture resistance intracanal composite resin in a model of apexification. J Endod. 2004;30:167.
157. Laws AJ. Pulpotomy by electro-coagulation. N Z Dent J. 1957;53:68.
158. Lee YL, Lee BS, Lin FH, Yun Lin A, Lan WH, Lin CP. Effects of physiological environments on the hydration behavior of mineral trioxide aggregate. Biomaterials. 2004;25:787.
159. Lekka M, Hume WR, Wolinsky LE. Comparison between formaldehyde and glutaraldehyde diffusion through the root tissues of pulpotomy-treated teeth. J Pedod. 1984;8:185.
160. Lemon RR, Steele PJ, Jeansonne BG. Ferric sulfate hemostasis: effect on osseous wound healing. I. Left in situ for maximum exposure. J Endod. 1993;19:170.
161. Liu J, Chen LR, Chao SY. Laser pulpotomy of primary teeth. J Pediatr Dent. 1999;21:128.
162. Lloyd JM, Scale NS, Wilson CFG. The effects of various concentrations and lengths of application of glutaraldehyde on monkey pulp tissue. Pediatr Dent. 1988;10:115.
163. Loh A, O’Hoy P, Tran X, Charles R, Hughes A, Kubo K, Messer LB. Evidence-based assessment: evaluation of the formocresol versus ferric sulfate primary molar pulpotomy. Pediatr Dent. 2004;26:401.
164. Loos PJ, Han SS. An enzyme histochemical study of the effect of various concentrations of formocresol on connective tissues. Oral Surg. 1971;31:571.
165. Loos PJ, Straffon LH, Han SS. Biological effects of formocresol. J Dent Child. 1973;40:193.
166. Loyola-Rodriguez JP, Garcia-Godoy F, Lindquist R. Growth inhibition of glass ionomer cements on mutans streptococci. Pediatr Dent. 1994;16:346.
167. Machida Y. Root canal therapy in deciduous teeth. Jpn Dent Assoc J. 1983;36:796.
168. Mack ES: Personal communication, 1967.
169. Mack RB, Dean JA. Electrosurgical pulpotomy: a retrospective human study. ASDC J Dent Child. 1993;60:107.
170. Mack RB, Halterman CW. Labial pulpectomy access followed by esthetic composite resin restoration for nonvital maxillary deciduous incisors. J Am Dent Assoc. 1980;100:374.
171. Magloire H, Joffre A, Bleicher F. An in vitro model of human dental pulp repair. J Dent Res. 1996;75:1971.
172. Mansukhani N. Pulpal reactions to formocresol, master’s thesis. Urbana: College of Dentistry, University of Illinois; 1959.
173. Marsh PD. Dental plaque as a microbial biofilm. Caries Res. 2004;38:204.
174. Mass E, Zilberman U. Endodontic treatment of infected primary teeth, using Maisto’s paste. J Dent Child. 1989;56:117.
175. Mass E, Zilberman U. Clinical and radiographic evaluation of partial pulpotomy in carious exposures of permanent molars. Pediatr Dent. 1993;15:257.
176. Mass E, Zilberman U, Fuks AB. Partial pulpotomy: another treatment option for cariously exposed permanent molars. J Dent Child. 1995;62:342.
177. Massler M, Mansukhani H. Effects of formocresol on the dental pulp. J Dent Child. 1959;26:277.
178. Matsumiya S, Susuki A, Takuma S. Atlas of clinical pathology. Tokyo: Tokyo Dental College Press, 1962.
179. Mejàre I, Cvek M. Partial pulpotomy in young permanent teeth with deep carious lesions. Endod Dent Traumatol. 1993;9:238.
180. Messagne J. The transforming growth factor-family. Ann Rev Cell Biol. 1990;6:597.
181. Messer LB, Cline JT, Korf NW. Long-term effects of primary molar pulpotomies on succedaneous bicuspids. J Dent Res. 1980;59:116.
182. Milnes AR. Persuasive evidence that formocresol use in pediatric dentistry is safe. J Can Dent Assoc. 2006;72:247.
183. Milsom KM, Tickle M, Blinkhorn AS. Dental pain and dental treatment of young children attending the general dental service. Br Dent J. 2002;192:280.
184. Mjör IA. Reaction patterns in human teeth. Boca Raton, FL: CRC Press; 1983.
185. Morawa AP, et al. Clinical evaluation of pulpotomies using dilute formocresol. J Dent Child. 1975;42:360.
186. Morawa AP. Clinical studies of human primary teeth following dilute formocresol pulpotomies. J Dent Res. 1974;53:269. (abstract)
187. Mulder GR, van Amerongen WE, Vingerling PA. Consequences of endodontic treatment of primary teeth. II. A clinical investigation into the influence of formocresol pulpotomy on the permanent successor. J Dent Child. 1987;54:35.
188. Murray PE, About I, Lumley PJ, Franquin JC, Remmusat M, Smith AJ. Cavity remaining dentin thickness and pulpal activity. Am J Dent. 2002;15:41.
189. Murray PE, Garcia-Godoy F, Hargreaves KM. Regenerative endodontics: a review of current status and a call for action. J Endod. 2007;33:377.
190. Murray PE, Smith AJ, Garcia-Godoy F, Lumley PJ. Comparison of operative procedure variables on pulp viability in an ex vivo model. Int Endod J. 2008;41:389.
191. Myers DR: Effects of formocresol on pulps of cariously exposed permanent molars, master’s thesis, 1972, College of Dentistry, University of Tennessee, Memphis, TN.
192. Myers DR, Pashley DH, Whitford GM, Sobel RE, McKinney RV. Acute toxicity of high doses of systemically administered formocresol in dogs. Pediatr Dent. 1981;3:37.
193. Myers DR, Shoaf HK, Dirksen TR, Pashley DH, Whitford GM, Reynolds KE. Distribution of 14C-formaldehyde after pulpotomy with formocresol. J Am Dent Assoc. 1978;96:805.
194. Myers DR, Battenhouse MR, Barenie JT, McKinney RV, Singh B. Histopathology of furcation lesions associated with pulp degeneration in primary molars. Pediatr Dent. 1987;9:279.
195. Myers DR, Durham LC, Hanes CM, Barenie JT, McKinney RV. Histopathology of radiolucent furcation lesions associated with pulpotomy-treated primary molars. Pediatr Dent. 1988;10:291.
196. Myers DR, Pashley DH, Lake FT, Burnham D, Kalathoor S, Waters R. Systemic absorption of 14C-glutaraldehyde from glutaraldehyde-treated pulpotomy sites. Pediatr Dent. 1986;8:134.
197. Myers DR, Pashley DH, Whitford GM, McKinney RV. Tissue changes induced by the absorption of formocresol from pulpotomy sites in dogs. Pediatr Dent. 1983;5:6.
198. Myers K, Kaminski E, Lautenschlater E. The effects of mineral trioxide aggregate on the dog pulp. J Endod. 1996;22:198.
199. Nadin G, Goel BR, Yeung CA, Gleny AM: Pulp treatment for extensive decay in primary teeth. Cochrane Database Syst Rev 2003 (1) CD003220.
200. Nair PN, Duncan HF, Pitt Ford TR, Luder HU. Histological, ultrastructural and quantitative investigations on the response of healthy human pulps to experimental capping with mineral trioxide aggregate: a randomized controlled trial. Int Endod J. 2008;41:128.
201. Nakabayashi N. Importance of mini-dumbbell specimen to access tensile strength of restored dentine: historical background and the future perspective in dentistry. J Dent. 2004;32:431.
202. Nakabayashi N, Kojima K, Masuhara E. The promotion of adhesion by the infiltration of monomers into tooth substrates. J Biomed Mater Res. 1982;16:265.
203. Nakashima M, Nagasawa H, Yamada Y, Reddi AH. Regulatory roll of transforming growth factor-β, bone morphogenetic protein-2, and protein-4 on gene expression of extracellular matrix proteins and differentiation on dental pulp cells. Dev Biol. 1994;162:18.
204. Nanci A, editor. Ten Cate’s oral histology, development, structure and function, ed 7, St Louis: Mosby, 2008.
205. Nelson S, Ash M. Wheeler’s dental anatomy: physiology and occlusion, ed 9. St. Louis: Saunders; 2010.
206. Nevins A, Finkelstein F, Laporta R, Borden BG. Induction of hard tissue into pulpless open-apex teeth using collagen-calcium phosphate gel. J Endod. 1978;4:76.
207. Nishino M. Clinico-roentgenographical study of iodoform-calcium hydroxide root canal filling material Vitapex in deciduous teeth. Jpn J Pedod. 1980;18:20.
208. Nocentini S, Moreno G, Coppey J. Survival, DNA synthesis and ribosomal RNA transcription in monkey kidney cells treated by formaldehyde. Mutat Res. 1980;70:231.
209. Nosrat IV, Nosrat CA. Reparative hard tissue formation following calcium hydroxide application after partial pulpotomy in cariously exposed pulps of permanent teeth. Int Endod J. 1998;31:221.
210. Nurko C, Ranly DM, Garcia-Godoy F, Lakshmyya KN. Resorption of a calcium hydroxide/iodoform paste (Vitapex) in root canal therapy for primary teeth: a case report. Pediatr Dent. 2000;22:517.
211. Oen KT, Thompson VP, Vena D, Cauldfield PW, Curro F, Dasanayake A, Ship JA, Limblad A. Attitudes and expectations of treating deep caries: a PEARL Network survey. Gen Dent. 2007;55:197.
212. O’Kane S, Ferguson MWJ. Transforming growth factor βs and wound healing. Int J Biochem Cell Biol. 1997;29:63.
213. Olmez A, Oztas N, Basak F, Sabuncuoglu B. A histopathologic study of direct pulp-capping with adhesive resins. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;86:98.
214. Olsson H, Petersson K, Rohlin M. Formation of a hard tissue barrier after pulp cappings in humans. A systematic review. Int Endod J. 2006;39:429.
215. Orban BJ, editor. Oral histology and embryology, ed 4, St Louis: Mosby, 1957.
216. Oringer MJ. Electrosurgery in dentistry, ed 2. Philadelphia: WB Saunders; 1975.
217. Ørstavik D, Holgslo JK. Mutagenicity of endodontic sealers. Biomats. 1985;6:129.
218. Pameijer CH, Stanley HR. The disastrous effects of the “total etch” technique in vital pulp capping in primates. Am J Dent. 1998;11:S45.
219. Papagiannoulis L. Clinical studies on ferric sulfate as a pulpotomy medicament in primary teeth. Eur J Paediatr Dent. 2002;3:126.
220. Pashley EL, Myers DR, Pashley DH, Whitford GM. Systemic distribution of 14C-formaldehyde from formocresol-treated pulpotomy sites. J Dent Res. 1980;59:603.
221. Patel S, Dawood A, Ford TP, Whaites E. The potential applications of cone beam computed tomography in the management of endodontic problems. Int Endod J. 2007;40:818.
222. Paterson RC, Watts A. Further studies on the exposed germ-free dental pulp. Int Endod J. 1987;20:112.
223. Payne RG, Kenny DJ, Johnston DH, Judd PL. Two-year outcome study of zinc oxide-eugenol root canal treatment for vital primary teeth. J Can Dent Assoc. 1993;59:528.
224. Peron LC, Burkes EJ, Gregory WB. Vital pulpotomy utilizing variable concentrations of paraformaldehyde in rhesus monkeys. J Dent Res. 1976;55:B129. (abstract 269)
225. Physical and Theoretical Chemistry Laboratory. Oxford University http://physchem.ox.ac.uk/MSDS/CR/cresol.html, 2006. (Accessed 15th August 2007.)
226. Pinkham JR, Cassamassimo P, Fields HW, et al, editors. Pediatric dentistry: infancy through adolescence, ed 4, St. Louis: Saunders, 2005.
227. Pisanti S, Sciaky I. Origin of calcium in the repair wall after pulp exposure in the dog. J Dent Res. 1964;43:641.
228. Pitt Ford TR, Torabinejad M, Abedi HR, Bakland LK, Kariyawasam SP. Mineral trioxide aggregate as a pulp-capping material. J Am Dent Assoc. 1996;127:1491.
229. Polydorou O, Pelz K, Hahn P. Antimicrobial effect of an ozone device and its comparison with two dentin-bonding systems. Eur J Oral Sci. 2006;114:349.
230. Pradham DP, Chawla HS, Gauba K, Goyal A. Comparative evaluation of endodontic management of teeth with unformed apices with mineral trioxide aggregate and calcium hydroxide. J Dent Child. 2006;73:79.
231. Primosch RE, Glomb TA, Jerrell RG. Primary tooth pulp therapy as taught in predoctoral pediatric dental programs in the United States. Pediatr Dent. 1997;19:118.
232. Puapichartdumrong P, Ikeda H, Suda H. Influence of the pulpal components in human dentine permeability in vitro. Int Endod J. 2005;38:152.
233. Qudeimat MA, Barrieshi-Nussair KM, Owais AI. Calcium hydroxide vs mineral trioxide aggregates for partial pulpotomy of permanent molars with deep caries. Eur Arch Paediatr Dent. 2007;8:99.
234. Rabinowitch BZ. Pulp management in primary teeth. Oral Surg. 1953;6:542.
235. Ranly DM. Glutaraldehyde purity and stability: implications for preparation, storage, and use as a pulpotomy agent. Pediatr Dent. 1984;6:83.
236. Ranly DM. Pulpotomy therapy in primary teeth: new modalities for old rationales. Pediatr Dent. 1994;16:403.
237. Ranly DM, Amstutz L, Horn D. Subcellular localization of glutaraldehyde. Endod Dent Traumatol. 1990;6:251.
238. Ranly DM, Garcia-Godoy F, Horn D. Time, concentration, and pH parameters for the use of glutaraldehyde as a pulpotomy agent: an in vivo study. Pediatr Dent. 1987;9:199.
239. Ranly DM, Horn D. Distribution, metabolism, and excretin of (14C)glutaraldehyde. J Endod. 1990;16:135.
240. Ranly DM, Horn D, Zislis T. The effect of alternatives to formocresol on antigenicity of protein. J Dent Res. 1985;64:1225.
241. Ricketts DNJ, Kidd EAM, Innes N, Clarkson J: Complete or ultraconservative removal of decayed tissue in unfilled teeth. Cochrane Database Syst Rev 3: CD003808, 2006.
242. Rickman GA, Elbadrawy HE. Effect of premature loss of primary incisors on speech. Pediatr Dent. 1985;7:119.
243. Rifkin A. The root canal treatment of abscessed primary teeth—a three to four year follow-up. J Dent Child. 1982;49:428.
244. Rimondini L, Baroni C. Morphologic criteria for root canal treatment of primary molars undergoing resorption. Endod Dent Traumatol. 1995;11:136.
245. Ritwik P, Cuisia ZV, Dahir P, Musselman RJ: MTA pulpotomies in the primary molars of children: results after 3 years. Presented at the IAPD meeting in New Orleans, October 2003.
246. Roberts SCJr, Brilliant JD. Tricalcium phosphate as an adjunct to apical closure in pulpless permanent teeth. J Endod. 1975;1:263.
247. Robertson A, Lundgren T, Andreasen JO, Dietz W, Hoyer I, Noren JG. Pulp calcifications in traumatized primary incisors: a morphologic and inductive analysis study. Eur J Oral Sci. 1997;105:196.
248. Rodd HD, Boissonade FM. Immunocytochemical investigation of immune cells within human primary and permanent tooth pulp. Int J Paediatr Dent. 2006;16:2.
249. Rodd HD, Boissonade FM. Substance P exposure in human tooth pulp in relation to caries and pain experience. Eur J Oral Sci. 2000;108:476.
250. Rodd HD, Waterhouse PJ, Fuks AB, Fayle SA Moffat M. Pulp therapy for primary molars. UK national clinical guidelines in paediatric dentistry. Int J Paediatr Dent. 2006;16(suppl 1):15.
251. Rolling I, Poulsen S. Formocresol pulpotomy of primary teeth and occurrence of enamel defects on the permanent successors. Acta Odontol Scand. 1978;36:243.
252. Rosenberg B, Murray PE, Namerow K. The effect of calcium hydroxide root filling on dentin fracture strength. Dent Traumatol. 2007;23:26.
253. Ruemping DR, Morton THJr, Anderson MW. Electrosurgical pulpotomy in primates—a comparison with formocresol pulpotomy. Pediatr Dent. 1983;5:14.
254. Sadrian R, Coll JA. A long-term follow-up on the retention of zinc oxide eugenol filler after primary tooth pulpectomy. Pediatr Dent. 1993;15:249.
255. Salako N, Joseph B, Ritwik P, Salonen J, John P, Junaid TA. Comparison of bioactive glass, mineral trioxide aggregate, ferric sulfate, and formocresol as pulpotomy agents in rat molar. Dent Traumatol. 2003;19:314.
256. Sanchez ZMC. Effects of formocresol on pulp-capped and pulpotomized permanent teeth of rhesus monkeys, master’s thesis. Ann Arbor: University of Michigan; 1971.
257. Sarkar NK, Saunderi B, Moiseyevai R, Berzins DW, Kawashima I. Interaction of mineral trioxide aggregate (MTA) with a synthetic tissue fluid. J Dent Res. 2002;81:A-391. (abstract #3155)
258. Sarris S, Tahmassebi JF, Duggal MS, Cross IA. A clinical evaluation of mineral trioxide aggregate for root-end closure of non-vital immature permanent incisors in children–a pilot study. Dent Traumatol. 2007;24:79.
259. Savage NW, Adkins KF, Weir AV, Grundy GE. A histological study of cystic lesions following pulp therapy in deciduous molars. J Oral Pathol. 1986;15:209.
260. Sciaky I, Pisanti S. Localization of calcium placed over amputated pulps in dogs’ teeth. J Dent Res. 1960;39:1128.
261. Schmitt D, Lee J, Bogen G. Multifaceted use of ProRoot MTA root canal repair material. Pediatr Dent. 2001;23:326.
262. Schroder UL. Effect of an extra-pulpal blood clot on healing following experimental pulpotomy and capping with calcium hydroxide. Odontol Revy. 1973;24:257.
263. Schröder V, Wennberg E, Granath LE, Moller H. Traumatized primary incisors: follow-up program based on frequency of periapical osteitis related to tooth color. Swed Dent J. 1997;1:95.
264. Schumacher JW, Rutledge RE. An alternative to apexification. J Endod. 1993;19:529.
265. Seale NS, Glickman GN. Contemporary perspectives on vital pulp therapy: views from the endodontists and pediatric dentists. Pediatr Dent. 2008;30:261.
266. Selliseth N. The significance of traumatized primary incisors on the development and eruption of permanent teeth. Trans Eur Orthod Soc. 1970;46:443.
267. Shabahang S, Torabinejad M, Boyne PP, Adebi H, McMillan P. A comparative study of root-end induction using osteogenic protein-I, calcium hydroxide, and mineral trioxide aggregate in dogs. J Endod. 1999;25:1.
268. Shah N, Logani A, Bhaskar U, Aggarwal V. Efficacy of revascularization to induce apexification/apexogenesis infected, nonvital immature teeth: a pilot clinical study. J Endod. 2008;34:919.
269. Shaw DW, Sheller B, Barrus BD, Morton THJr. Electrosurgical pulpotomy—a 6-month study in primates. J Endod. 1987;13:500.
270. Sheller B, Morton THJr. Electrosurgical pulpotomy: a pilot study in humans. J Endod. 1987;13:69.
271. Shulman ER, Melver FF, Burkes EJJr. Comparison of electrosurgery and formocresol as pulpotomy techniques in monkey primary teeth. Pediatr Dent. 1987;9:189.
272. Sigurdsson A. Pulpal diagnosis. Endod Topics. 2003;5:12.
273. Simon M, van Mullem PJ, Lamers AC. Formocresol: no allergic effect after root canal disinfection in non-presensitized guinea pigs. J Endod. 1982;8:269.
274. Simon S, Rilliard F, Berdal A, Machtou P. The use of mineral trioxide aggregate in one-visit apexification treatment: a prospective study. Int Endod J. 2007;40:186.
275. Smith AJ, Cassidy M, Perry H, Begue-Kirn C, Ruch JV, Lesot H. Reactionary dentinogenesis. Int J Dev Biol. 1995;39:273.
276. Smith NL, Seale NS, Nunn ME. Ferric sulfate pulpotomy in primary molars: a retrospective study. Pediatr Dent. 2000;22:192.
277. Snuggs HM, Cox CF, Powell CF, White KC. Pulp healing and dentinal bridge formation in an acidic environment. Quintessence Int. 1993;24:501.
278. Srinivasan V, Patchett CL, Waterhouse PJ. Is there life after Buckley’s formocresol? Part 1. A narrative review of alternative interventions and materials. Int J Paed Dent. 2006;16:117.
279. Stanley HR, Lundy T. Dycal therapy for pulp exposure. Oral Surg. 1972;34:818.
280. Stanley HR, Pameijer CH. Pulp capping with a new visible-light-curing calcium hydroxide composition (Prisma VLC Dycal). Oper Dent. 1985;10:156.
281. Stanton WG. The non-vital deciduous tooth. Int J Orthod. 1935;21:181.
282. Starkey PE. Management of deep caries of pulpally involved teeth in children. In: Goldman HM, et al, editors. Current therapy in dentistry, vol 3. St Louis: Mosby; 1968.
283. Starkey PE. Treatment of pulpally involved primary molars. In: McDonald RE, et al, editors. Current therapy in dentistry, vol 7. St Louis: Mosby; 1980.
284. Steiner JC, Dow PR, Cathey GM. Inducing root end closure of nonvital permanent teeth. J Dent Child. 1968;35:47.
285. Steiner JC, Van Hassel HJ. Experimental root apexification in primates. Oral Surg. 1971;31:409.
286. Straffon LH, Han SS. Effects of varying concentrations of formocresol on RNA synthesis of connective tissue in sponge implants. Oral Surg. 1970;29:915.
287. Sweet CA. Procedure for the treatment of exposed and pulpless deciduous teeth. J Am Dent Assoc. 1930;17:1150.
288. Swenberg JA, Kerns WD, Mitchell RI, Gralla EJ, Pavkov KL. Induction of squamous cell carcinomas of the rat nasal cavity by inhalational exposure to formaldehyde vapor. Cancer Res. 1980;40:3398.
289. Tagger E, Sarnat H. Root canal therapy of infected primary teeth. Acta Odontol Pediatr. 1984;5:63.
290. Tagger E, Tagger M, Sarnat H. Pulpal reaction for glutaraldehyde and paraformaldehyde pulpotomy dressings in monkey primary teeth. Endod Dent Traumatol. 1986;2:237.
291. Tagger M, Tagger E. Pulp capping in monkeys with Reolite and Life, two calcium hydroxide bases with different pH. J Endod. 1985;11:394.
292. Tatsumi T, Inokoshi S, Yamada T, Hosada H. Remineralization of etched dentin. J Prosthet Dent. 1992;67:617.
293. Tay FR, Pashley DH. Monoblocks in root canals: a hypothetical or tangible goal. J Endod. 2007;33:391.
294. Teixeira FB, Teixeira ECN, Thompson JY, Trope M. Fracture resistance of roots endodontically treated with a new resin filling material. J Am Dent Assoc. 2004;135:646.
295. Teplitsky PE. Formocresol pulpotomies on posterior permanent teeth. J Can Dent Assoc. 1984;50:623.
296. Teuscher GW, Zander HA. A preliminary report on pulpotomy. Northwest Univ Dent Res Grad Q Bull. 1938;39:4.
297. Thibodeau B, Trope M. Pulp revascularization of a necrotic infected immature permanent tooth: case report and review of the literature. Pediatr Dent. 2007;29:47.
298. Thoden van Velzen SK, Feltkamp-Vroom TM. Immunologic consequences of formaldehyde fixation of autologous tissue implants. J Endod. 1977;3:179.
299. Thompson V, Craig RG, Curro FA, Green WS, Ship JA. Treatment of deep carious lesions by complete excavation or partial removal. A critical review. J Am Dent Assoc. 2008;139:705.
300. Tittle KW, Farley J, Linkhardt M, Torabinejad M. Apical closure induction using bone growth factors and mineral trioxide aggregate. J Endod. 1996;22:198. (abstract #41)
301. Trask PA. Formocresol pulpotomy on (young) permanent teeth. J Am Dent Assoc. 1972;85:1316.
302. Tronstad L. Reaction of the exposed pulp to Dycal treatment. Oral Surg. 1974;38:945.
303. Trope M. Regenerative potential of dental pulp. J Endod. 2008;34(7 Suppl):S13.
304. Trope M. Treatment of immature teeth with non-vital pulps and apical periodontitis. Endod Topics. 2006;14:51.
305. Turner C, Courts FJ, Stanley HR. A histological comparison of direct pulp capping agents in primary canines. J Dent Child. 1987;54:423.
306. Tziafas D. The future role of a molecular approach to pulp-dentinal regeneration. Caries Res. 2004;38:314.
307. Tziafas D, Koliniotou-Koumpia E, Tziafa C, Papadimitriou S. Effects of a new antibacterial adhesive on the repair capacity of the pulp-dentine complex in infected teeth. Int Endod J. 2007;40:58.
308. Tziafas D, Molyvdas I. The tissue reaction after capping of dog teeth with calcium hydroxide experimentally crammed into the pulp space. Oral Surg Oral Med Oral Pathol. 1988;65:604.
309. Tziafas D, Smith AJ, Lesot H. Designing new treatment strategies in vital pulp therapy. J Dent. 2000;28:77.
310. Tziafas D, Pantelidou O, Alvanou A, Belibasakis G, Papadimitriou S. The dentinogenic effect of mineral trioxide aggregate (MTA) in short-term capping experiments. Int Endod J. 2002;35:245.
311. Valois CR, Costa EDJr. Influence of the thickness of mineral trioxide aggregate on scaling ability of root-end fillings in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:108.
312. van Amerongen WE, Mulder GR, Vingerling PA. Consequences of endodontic treatment in primary teeth. I. A clinical and radiographic investigation into the influence of the formocresol pulpotomy on the life-span of primary molars. J Dent Child. 1986;53:364.
313. van der Sluis LWM, versluis M, Wu MK, Wesselink PR. Passive ultrasonic irrigation of the root canal: a review of the literature. Int Endod J. 2007;40:415.
314. van Mullen PJ, Simon M, Lamers AC. Formocresol: a root canal disinfectant provoking allergic skin reactions in presensitized guinea pigs. J Endod. 1983;9:25.
315. Venham LL: Pulpal responses to variations in the formocresol pulpotomy technique: a histologic study, master’s thesis, Columbus, 1967, College of Dentistry, Ohio State University.
316. Vij R, Coll JA, Sheldon P, Farooq NS. Caries control and other variables associated with success of primary molar vital pulp therapy. Pediatr Dent. 2004;26:214.
317. WATCH: Working group on action to control chemicals The carcinogenicity of formaldehyde committee paper 2005/6 http://www.hse.gov.uk/aboutus/hsc/iacs/acts/watch/130105/p6.pdf/ Accessed 10th June 2007
318. Waterhouse PJ. ‘New age’ pulp therapy–personal thoughts on a hot debate. Pediatr Dent. 2008;30:247.
319. Waterhouse PJ, Nunn JH, Whitworth JM. Prostaglandin E2 and treatment outcome in pulp therapy of primary molars with carious exposures. Int J Pediatr Dent. 2002;12:116.
320. Webber RT. Apexogenesis versus apexification. Dent Clin North Am. 1984;28:669.
321. Wemes JC, Purdell-Lewis D, Jongebloed W, Vaalburg W. Diffusion of carbon-14-labeled formocresol and glutaraldehyde in tooth structures. Oral Surg. 1982;54:341.
322. Wemes JC, Jansen HW, Purdell-Lewis D, Boering G. Histologic evaluation of the effect of formocresol and glutaraldehyde on the periapical tissues after endodontic treatment. Oral Surg. 1982;54:329.
323. White KC, Cox CF, Kanka J3rd, Dixon DL, Farmer JB, Snuggs HM. Pulpal response to adhesive resin systems applied to acid-etched vital dentin: damp versus dry primer application. Quintessence Int. 1994;25:259.
324. Wilkins FJ, Macleod HD. Formaldehyde induced DNA-protein crosslinks in Escherichia coli. Mutat Res. 1976;36:11.
325. Wilkinson KL, Beeson TJ, Kirkpatrick TC. Fracture resistance of simulated immature teeth filled with Resilon, gutta-percha, or composite. J Endod. 2007;33:480.
326. Witherspoon DE, Small JC, Harris GZ. Mineral trioxide aggregate pulpotomies: a case series outcomes assessment. J Am Dent Assoc. 2006;137:610.
327. Yacobi R, Kenny DJ, Judd PL, Johnston DH. Evolving primary pulp therapy techniques. J Am Dent Assoc. 1991;122:83.
328. Yanpiset K, Vongsavan N, Siggurdsson A, Trope M. Efficacy of laser Doppler flowmetry for the diagnosis of revascularization of reimplanted immature dog teeth. Dent Traumatol. 2001;17:63.
329. Yeung P, Liewehr FR, Moon PC. A quantitative comparison of the fill density of MTA produced by two placement techniques. J Endod. 2006;32:456.
330. Zander HA. Reaction of the pulp to calcium hydroxide. J Dent Res. 1939;18:373.
331. Zurcher E. The Anatomy of the root canals of the teeth of the deciduous dentition and of the first permanent molars. New York: William Wood & Co; 1925.
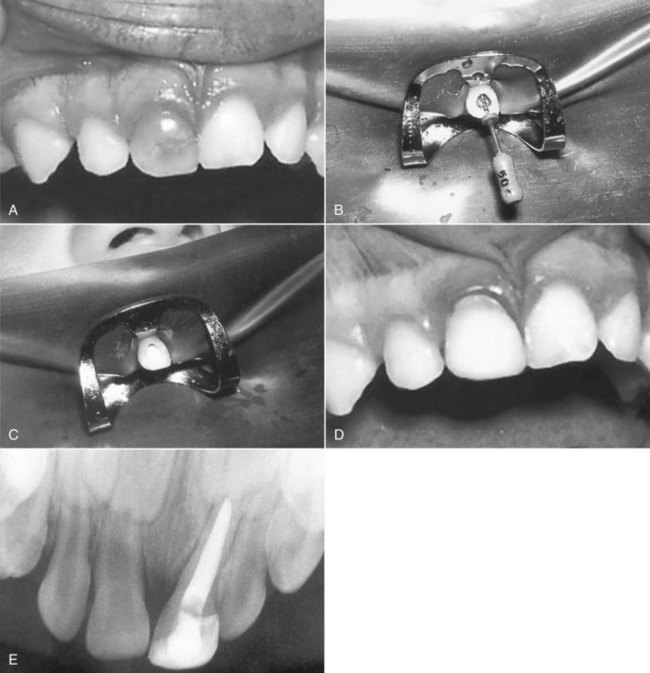
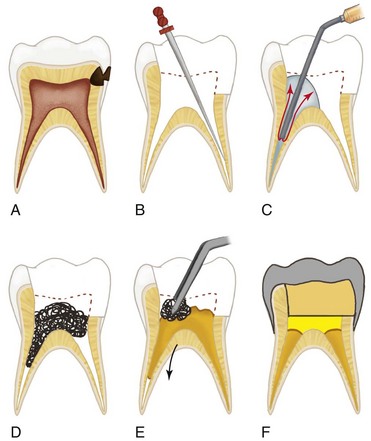
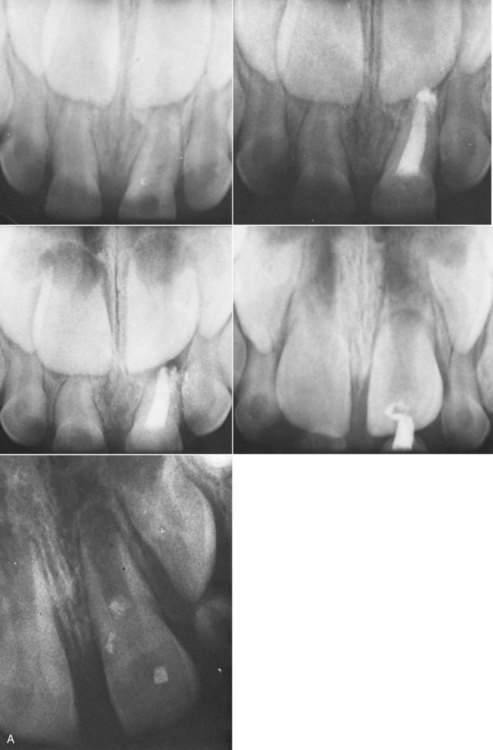
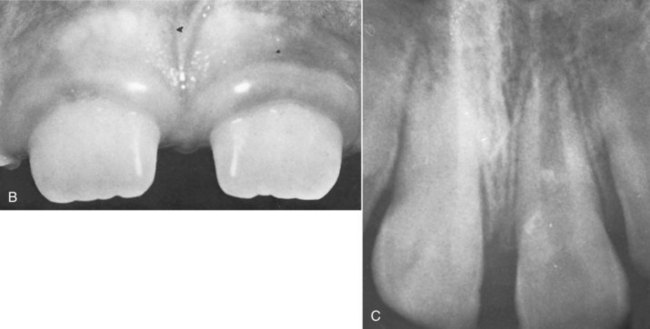
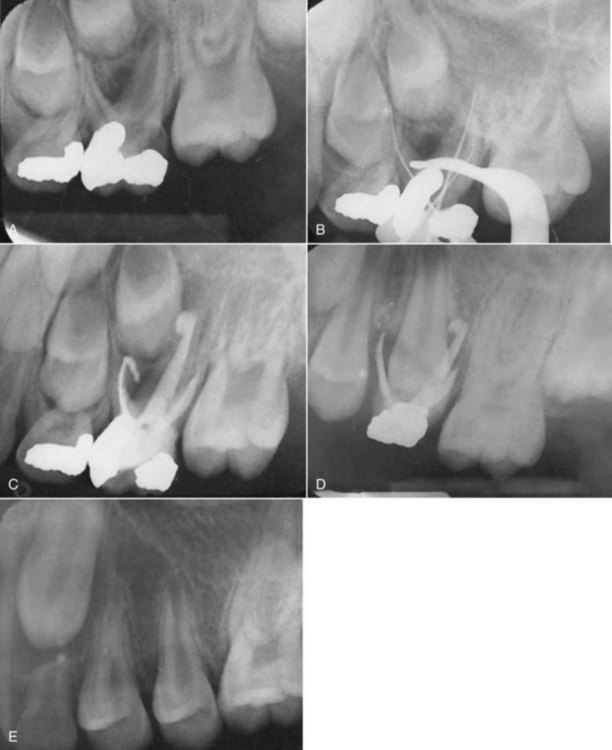
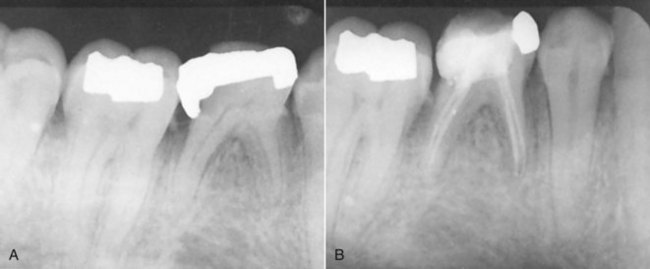
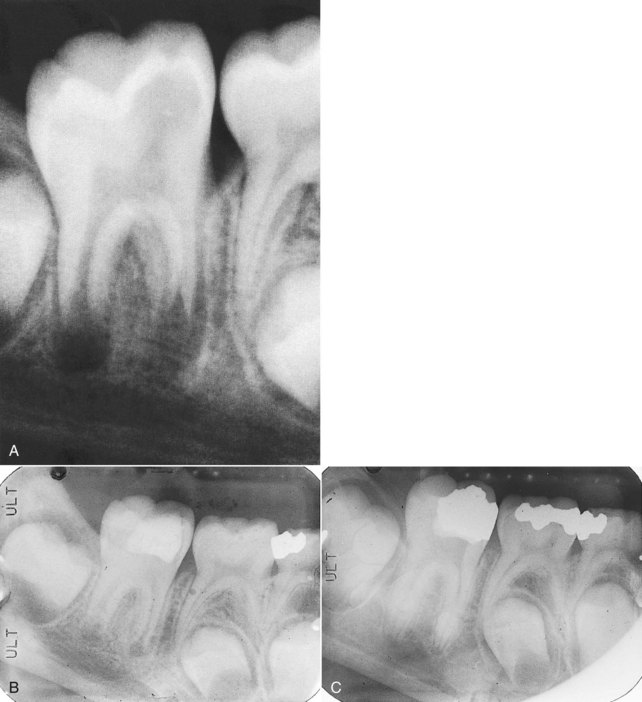
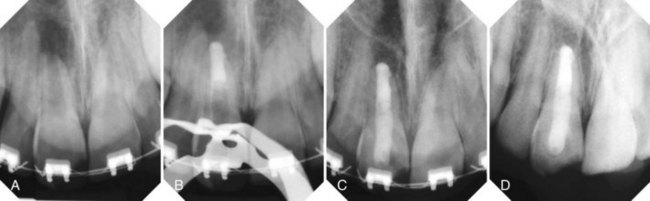
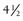 years after root canal treatment, primary tooth is near to exfoliation. E, One year later, premolar is erupted fully and all traces of ZOE have been resorbed.
years after root canal treatment, primary tooth is near to exfoliation. E, One year later, premolar is erupted fully and all traces of ZOE have been resorbed.