CHAPTER 17 The Role of Endodontics After Dental Traumatic Injuries
A traumatic injury to the tooth results in damage to many dental and periradicular structures, making the management and consequences of these injuries multifactorial. Knowledge of the interrelating healing patterns of these tissues is essential.
This chapter will concentrate on the role of the dentinopulpal complex in the pathogenesis of disease subsequent to dentinal trauma and how treatment of this complex can contribute to favorable healing after an injury.
Unique Aspects of Dental Trauma
Most dental trauma occurs in the 7- to 12-year-old age group and is mainly due to falls and accidents near home or school.23,132 It occurs primarily in the anterior region of the mouth, affecting the maxillary more than the mandibular jaw.28 Serious accidents like automobile crashes can affect any tooth and occur in all age ranges. In many cases, after a traumatic dental injury, endodontic treatment is provided to caries-free, single-rooted, young permanent teeth. If quick and correct treatment for these teeth is provided after injury, the potential for a successful endodontic outcome is very good.
Most Common Types of Dental Trauma
Crown Fractures
Most crown fractures occur in young, caries-free anterior teeth.88,98 This makes maintaining or regaining pulp vitality essential. Luckily, vital pulp therapy supports a good prognosis in these situations if correct treatment and follow-up procedures are carefully followed.
Crown-Root Fractures
Crown-root fractures are first treated periodontally to ensure there is a sufficient and good margin for the restoration to be possible. If the tooth can be maintained from a periodontal point of view, the pulp is treated as with a crown fracture.
Root Fractures
A surprisingly large number of pulps in root-fractured teeth will survive this rather dramatic injury. In almost every case, the apical segment stays vital, and in many cases, the coronal segment stays vital or regains vitality subsequent to the injury. If the coronal segment permanently loses vitality, it should be treated as an immature permanent tooth with nonvital pulp. The apical segment rarely needs treatment.
Luxation Injuries and Avulsion
Luxation injuries and avulsion often result in pulp necrosis and damage to the cemental protective layer of the root. The potential complication of pulp infection in a root that has lost its cemental protective layer makes these injuries potentially catastrophic. Correct emergency and follow-up evaluation, which may include timely endodontic treatment, is critical.
Follow-Up after Dental Trauma
The reader is referred to Chapter 1 for specific descriptions of pulp tests, but a few general statements about pulp tests on traumatized teeth may be helpful in trying to interpret the results.
For decades, controversy has surrounded the validity of thermal and electric tests on traumatized teeth. Only generalized impressions may be gained from these tests following a traumatic injury. They are in reality sensitivity tests for nerve function and do not indicate the presence or absence of blood circulation within the pulpal space. It is assumed that subsequent to traumatic injury, the conduction capability of the nerve endings and/or sensory receptors is sufficiently deranged to inhibit the nerve impulse from an electric or thermal stimulus. This makes the traumatized tooth vulnerable to false-negative readings from such tests.112
Teeth that give a response at the initial examination cannot be assumed to be healthy and continue to give a response over time. Teeth that yield no response cannot be assumed to have necrotic pulps, because they may give a response at later follow-up visits. It has been demonstrated that it may take as long as 9 months for normal blood flow to return to the coronal pulp of a traumatized fully formed tooth. As circulation is restored, the responsiveness to pulp tests returns.66
The transition from no response to a response at a subsequent test may be considered a sign of a healing pulp. The repetitious finding of responses may be taken as a sign of a healthy pulp. The transition from a response to no response may be taken as an indication that the pulp is probably undergoing degeneration, and therefore an intervention in the form of endodontic treatment might be indicated. The persistence of no response would suggest that the pulp has been irreversibly damaged, but even this is not absolute.30
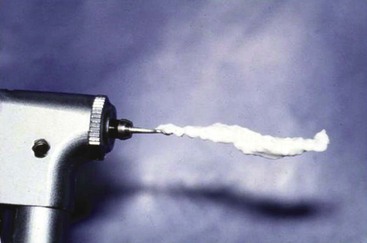
Thermal and electric pulp tests of all anterior teeth (canine to canine) of the maxillary and mandibular jaws should be performed at the time of the initial examination and carefully recorded to establish a baseline for comparison with subsequent repeated tests in later months. These tests should be repeated at 3 weeks, at 3, 6, and 12 months, and at yearly intervals following the trauma. The purpose of the tests is to establish a trend as to the physiologic status of the pulps of these teeth. Particularly in traumatized teeth, carbon dioxide snow (CO2, −78° C) or dichlorodifluoromethane (−40° C) placed on the incisal third of the facial surface gives more accurate responses than a water-ice pencil64,65 (Fig. 17-1). The intense cold seems to penetrate the tooth and covering splints or restorations and reach the deeper areas of the tooth. Neither the dry ice nor the dichlorodifluoromethane spray forms ice water, which could disperse over adjacent teeth or gingiva to give a false-positive response. In trauma evaluation, there is no question that using water-ice pencils should be avoided because of this. Dichlorodifluoromethane spray is a very inexpensive alternative to CO2 snow; how cold it is will give much more reliable responses than water ice. The electric pulp test relies on electric impulses directly stimulating the nerves of the pulp. These tests have limited value in young teeth but are useful in cases where the dentinal tubules are closed and do not allow dentinal fluid to flow in them. This situation is typical of teeth in elderly patients or in traumatized teeth that are undergoing premature sclerosis. In these situations, the thermal tests that rely on fluid flow in the tubules cannot be used, and the electric pulp test becomes important.
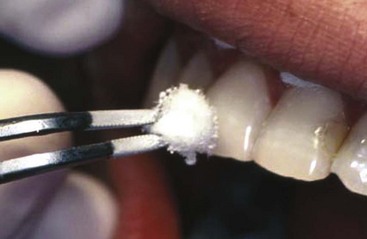
FIG. 17-1 Difluorodichloromethane (−40° F [−40° C]) gas is sprayed on a cotton pellet and then placed on the incisal edge of the maxillary incisor.
Laser Doppler flowmetry (LDF) was introduced in the early 1970s for measurement of blood flow in the retina.81 The technique has also been used to assess blood flow in other tissue systems such as skin and renal cortex. It utilizes a beam of infrared (780 to 820 nm) or near-infrared (632.8 nm) light that is directed into the tissue by optical fibers. As light enters the tissue, it is scattered by moving red blood cells and stationary tissue cells. Photons that interact with moving red blood cells are scattered, and the frequency shifts according to the Doppler principle. Photons that interact with stationary tissue cells are scattered but not Doppler shifted. A portion of the light is returned to a photodetector, and a signal is produced (Fig. 17-2).
Attempts have been made to use LDF technology for pulp vitality diagnosis in traumatized teeth, because this would provide a more accurate reading of the vitality status of the pulp.103,164,165 Studies have shown promising results, indicating that laser Doppler can detect blood flow more consistently and earlier than the standard vitality tests would be expected to render a response. In a study on young dogs, laser Doppler was able to correctly detect blood flow as early as 2 to 3 weeks after avulsion of an immature tooth and at the same time indicate no flow in those which would remain necrotic.165
Presently the cost of a laser Doppler flowmetry machine limits its use in private dental offices; these are used primarily in hospitals and teaching institutions.
Radiographic Examinations
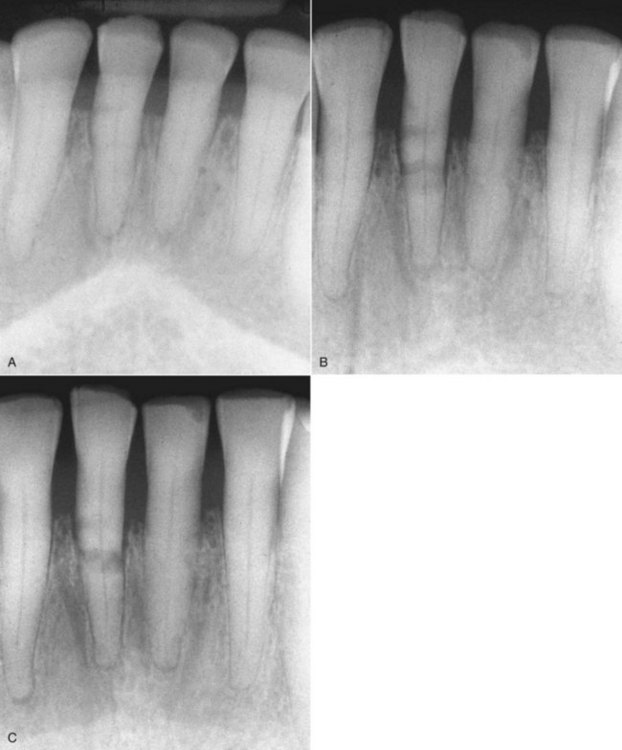
Radiographs are essential tools in the thorough examination of traumatized hard tissues. They may reveal root fractures, subgingival crown fractures, tooth displacements, bone fractures, resorptions of the root, and adjacent bone or foreign objects. One radiograph will be insufficient in most dental trauma cases. In their new guidelines for the management of traumatic dental injuries, the International Association of Dental Traumatology (IADT) has recommended taking at least four different radiographs for almost every injury.58-60 Those four include a direct 90-degree on the axis of the tooth, two with different vertical angulations, and one occlusal film (Fig. 17-3). Multiple radiographs will increase the likelihood of diagnosing root fractures, apical translocations, and other possible injuries.
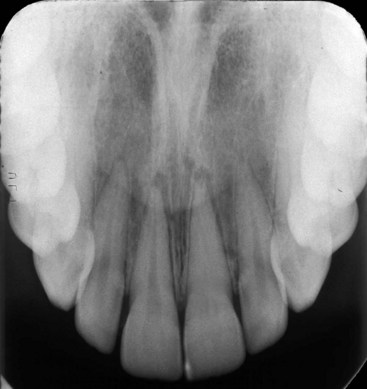
FIG. 17-3 An occlusal film of a luxation injury of central incisors. Both were diagnosed to be laterally luxated with apical translocation. Note that the left central is completely obliterated and has a history of being luxated some years prior.
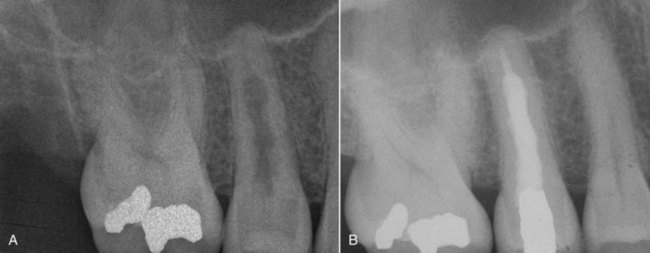
Early detection of root resorption is extremely difficult on dental radiographs, however. The radiodensity of the root requires that a significant amount of root substance is removed to cause enough contrast on the radiograph to allow it to be detected. Thus only resorptive defects on the mesial or distal aspects of the root can be predictably detected after some time, but the facial and palatal or lingual aspects are much harder to see. To overcome these difficulties, it is essential to take as many different horizontal angled radiographs as feasible in cases of suspected root resorption. One should also carefully evaluate the adjacent bone for resorption153 (Fig. 17-4), discussed in more detail later.
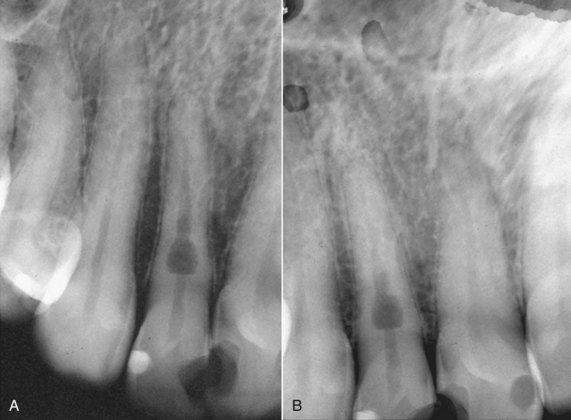
FIG. 17-4 Angled radiographs to show internal resorption. Radiographs from two different horizontal projections depict (A) the lesion within the confines of the root canal on both views and (B) the adjacent bone intact on both views.
New radiographic techniques like tuned-aperture computed tomography (TACT) and cross-sectional CT scans have shown promise in improving the clinician’s ability to diagnose root resorption (see also Chapter 29).105
In instances of soft-tissue laceration, it is advisable to radiograph the injured area prior to suturing to be sure that no foreign objects have been embedded. A soft-tissue radiograph with a normal-sized film briefly exposed at reduced kilovoltage should reveal the presence of many foreign substances, including tooth fragments (Fig. 17-5).
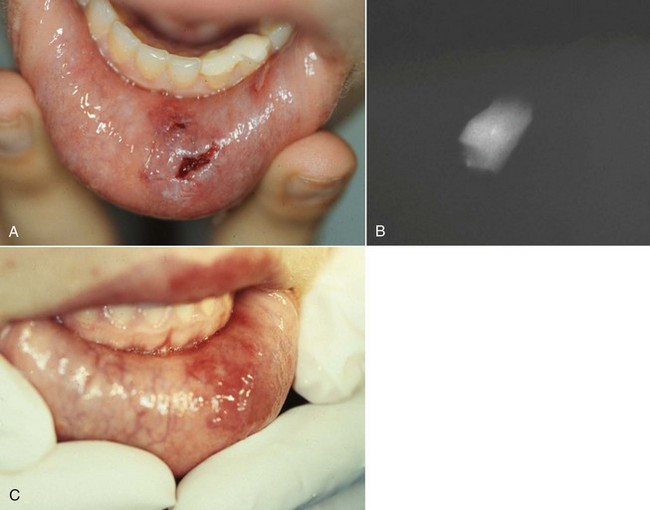
FIG. 17-5 Patient sustained complicated crown fractures on left lower incisors and later on the canine tooth. A, An emergency pulp capping; Band-Aid composite was placed on the teeth. Six days later the patient was referred to the clinic because of a poorly healed laceration on the lip. B, Radiograph of the lip revealed a portion of the lateral crown still in the lip. The patient was anesthetized and the crown removed. C, Healing was quick and uneventful.
In reviewing the films of traumatized teeth, special attention should be directed to the dimension of the root canal space, the degree of apical closure of the root, the proximity of fractures to the pulp, and the relationship of root fractures to the alveolar crest. Whereas conventional periapical films are generally useful, an occlusal or Panorex film can supplement them when the clinician is looking for bone fractures, dislocation of teeth, or the presence of foreign objects.
Crown Fractures
As already mentioned, the primary aim from an endodontic point of view is to maintain pulp vitality after crown fractures.
Crown Infraction
Crown infraction can be defined as an incomplete fracture or crack of enamel, without loss of tooth structure.16
Biologic Consequences
Crown infractions are injuries that carry little danger of resulting in pulp necrosis. Meticulous follow-up over a 5-year period is the most important endodontic preventive measure in these cases. If at any follow-up examination, the reaction to sensitivity tests changes, or if on radiographic assessment, signs of apical or periradicular periodontitis develop or the root appears to have stopped development or is obliterating, endodontic intervention should be considered.
Uncomplicated Crown Fracture
Uncomplicated crown fracture can be defined as fracture of the enamel only or enamel and dentin without pulp exposure.16
Treatment
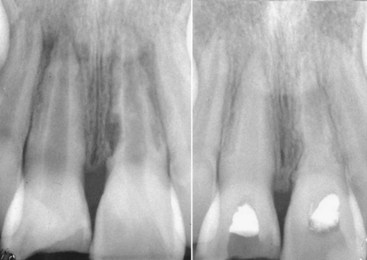
There are two key issues in the treatment of crown fractures. First, all exposed dental tubules need to be closed as soon as reasonably possible. If the broken-off piece is not available, or if it is not possible to reattach it and there is no time to do a full composite restoration at the time of the emergency appointment, a composite Band-Aid or temporary coverage should be placed on all exposed dentin. This is to prevent any ingress of bacteria into the tubules as well as to reduce the patient’s discomfort. The second issue is the remaining dentin thickness. Several studies have confirmed that if the remaining dentin is more than 0.5 mm thick, the tooth can be restored with the restoration of choice, including etching and bonding, and no special attention needs to be given to the pulp.3,41,54,105,140 If, however, the remaining dentin is less than this thickness, a protective layer of hard-setting calcium hydroxide in the deepest part of the dentin exposure does reduce, if not completely prevent, the reactive inflammation of the underling pulp, which is a significantly different reaction compared to the negative reaction to composite bonding systems.3,41,54,105,140
Complicated Crown Fracture
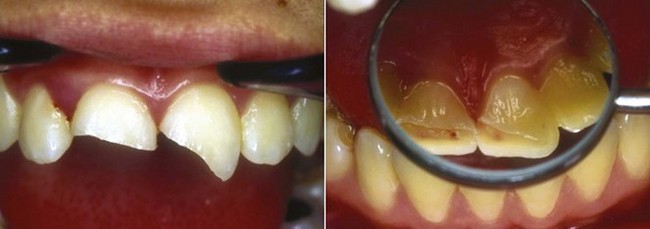
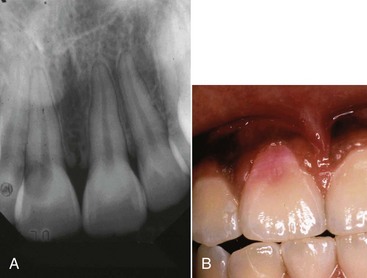
A complicated crown fracture involves enamel, dentin, and pulp1 (Fig. 17-6).
Biologic Consequences
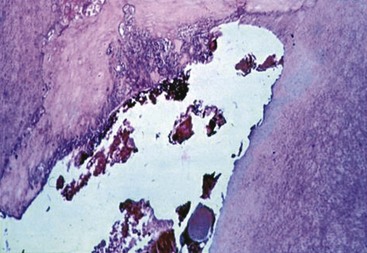
A crown fracture involving the pulp, if left untreated, will always result in pulp necrosis.90 However, the manner and time sequence in which the pulp becomes necrotic allows a great deal of potential for successful intervention to maintain pulp vitality. The first reaction after the injury is hemorrhage and local inflammation (Fig. 17-7).
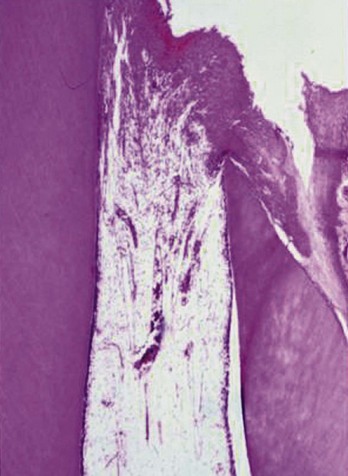
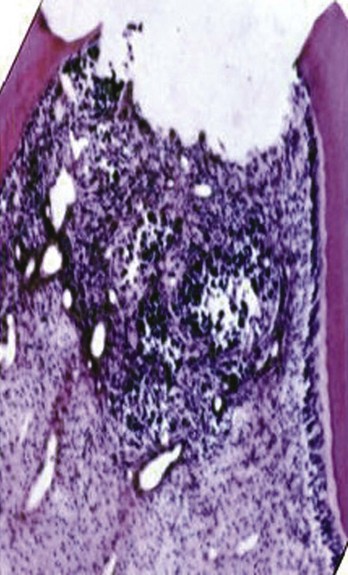
FIG. 17-7 Histologic appearance of the pulp within 24 hours of a traumatic exposure. The pulp proliferated over the exposed dentinal tubules. There is approximately 1.5 mm of inflamed pulp below the surface of the fracture.
Subsequent inflammatory changes are usually proliferative but can be destructive over time. A proliferative reaction is favored in traumatic injuries, since the fractured surface is usually flat, allowing salivary rinsing with little chance of impaction of contaminated debris. Unless impaction of contaminated debris is obvious, it is expected that in the first 24 hours after the injury, a proliferative response with inflammation extending not more than 2 mm into the pulp will be present43,47,75 (see Fig. 17-7). In time, the bacterial challenge will result in local pulpal necrosis and a slow apical progression of the pulpal inflammation (Fig. 17-8).
Treatment
Treatment options for complicated crown fracture are (1) vital pulp therapy, comprising pulp capping, partial pulpotomy, or full pulpotomy; or (2) pulpectomy. Choice of treatment depends on the stage of development of the tooth, the time between trauma and treatment, concomitant periodontal injury, and restorative treatment plan.
Stage of Development of the Tooth
Loss of vitality in an immature tooth can have catastrophic consequences. Root canal treatment on a tooth with a blunderbuss canal is time consuming and difficult. It is probably more important that necrosis of an immature tooth leaves it with thin dentinal walls that are susceptible to fracture both during and after the apexification procedure.91 Every effort must be made to keep the tooth vital, at least until the apex and cervical root have completed their development.
Removal of the pulp in a mature tooth is not as significant as in an immature tooth, since a pulpectomy in a mature tooth has an extremely high success rate.131 However, it has been shown that under optimal conditions, vital pulp therapy (rather than removal) on a mature tooth can be carried out successfully.101,161 Therefore, this form of therapy can be an option under certain circumstances, even though a pulpectomy is the treatment which affords the most predictable success.
In an immature tooth, vital pulp therapy should always be attempted if at all feasible because of the tremendous advantages of maintaining the vital pulp.
Time Between Trauma and Treatment
For 48 hours after a traumatic injury, the initial reaction of the pulp is proliferative, with no more than a 2-mm depth of pulpal inflammation (see Fig. 17-8). After 48 hours, chances of direct bacterial contamination of the pulp increase, with the zone of inflammation progressing apically47; as time passes, the likelihood of successfully maintaining a healthy pulp decreases.
Concomitant Attachment Damage
A periodontal injury will compromise the nutritional supply of the pulp. This fact is particularly important in mature teeth, where the chance of pulp survival is not as good as for immature teeth.14,53
Restorative Treatment Plan
Unlike an immature tooth where the benefits of maintaining vitality of the pulp are so great, in a mature tooth, pulpectomy is a viable treatment option. As pointed out, if performed under optimal conditions, vital pulp therapy after traumatic exposures can be successful. If the restorative treatment plan is simple and a composite resin restoration will suffice as the permanent restoration, this treatment option should be given serious consideration. If a more complex restoration is to be placed (e.g., a crown or bridge abutment), pulpectomy may be the more predictable treatment method.
Vital Pulp Therapy: Requirements for Success
Vital pulp therapy has an extremely high success rate if the following requirements can be adhered to:
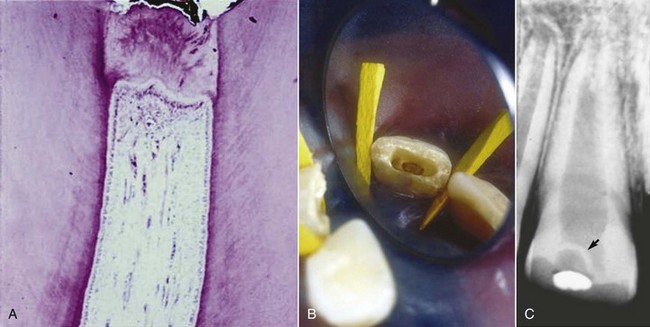
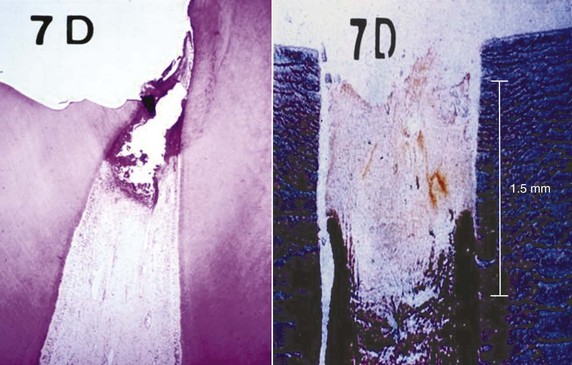
FIG. 17-10 Hard-tissue barrier after calcium hydroxide partial pulpotomy. A, Histologic appearance of replacement odontoblasts and a hard-tissue barrier. B, Clinical appearance of the barrier on removal of the coronal restoration 3 months after placement of the calcium hydroxide. C, Radiographic appearance of the hard-tissue barrier.
A major disadvantage of calcium hydroxide is that it does not seal the fractured surface. Therefore, an additional material must be used to ensure that the pulp is not challenged by bacteria, particularly during the critical healing phase.
Many materials such as zinc oxide eugenol25,151 tricalcium phosphate,77 and composite resin 24 have been proposed as medicaments for vital pulp therapy. None to this date have afforded the predictability of calcium hydroxide used in conjunction with a well-sealed coronal restoration.54,94,128,140 In a dog study, for example, pulps capped directly with various adhesive agents showed moderate to severe inflammatory reactions, with progressive extension of tissue necrosis with time and total absence of continuous hard-tissue bridge formation.94 Application of a calcium hydroxide–based material was characterized by inflammatory cell infiltration, limited tissue necrosis, and partial to complete hard-tissue bridging.94 In a similar study on human teeth in vivo, it was found that Scotchbond Multi-Purpose Plus caused inflammatory changes when applied directly to exposed pulp tissue, but direct capping with Dycal followed by sealing with Scotchbond Multi-Purpose Plus showed favorable results in pulp tissue.140
Mineral trioxide aggregate (MTA) has been shown to be a good pulp capping agent.55,106,109,163 It has a high pH similar to calcium hydroxide when unset145 and after setting will create an excellent bacteria-tight seal.146 It is also hard enough to act as a base for a final restoration.145 Yet MTA does not enjoy the same popularity as calcium hydroxide as a pulp capping agent in treatment of traumatic exposures. There are probably three main reasons. The first is likely to be that because MTA needs a moist environment for at least 6 hours to set properly, the treatment becomes a two-step procedure, compared with a one-step procedure for other medicaments. Using MTA as a pulp capping agent thus necessitates that a wet cotton pellet be placed over it until it is set, and then the permanent restoration can be fabricated at a later time. The second likely reason is that originally, MTA was gray in color and reported to cause discoloration in the tooth crown when used as capping agent in anterior teeth.35 To counter this discoloration problem, a new white version of MTA was marketed a few years ago. Initially there were some concerns that the pulp did not respond to the white version as favorably as it had to the gray one.110 Comparatively few studies have been done comparing the white MTA to the grey one, but most have indicated that the pulp seems to react as well to one as the other.84,106,120 Further studies are warranted to compare the two formulations of MTA to fully elucidate the concerns raised regarding possible differences.4 The third—and probably most common—reason why MTA is not as widely used as it could be is its relatively high cost.
Treatment Methods
Pulp Capping
Pulp capping implies placing the dressing directly onto the pulp exposure without any removal of the soft tissue.24
Indications
None; the success rate of this procedure (80%)62,114 compared to partial pulpotomy (95%)43 indicates that a superficial pulp cap should not be considered after traumatic pulp exposures. The lower success rate is not difficult to understand, since superficial inflammation develops soon after the traumatic exposure. If the treatment is at the superficial level, a number of inflamed (rather than healthy) pulps will be treated, lowering the potential for success. In addition, a bacteria-tight coronal seal is much more difficult to attain in superficial pulp capping, since there is no depth to the cavity to aid in creating the seal, as there is with a partial pulpotomy.
Partial Pulpotomy
Partial pulpotomy implies the removal of coronal pulp tissue to the level of healthy pulp. Knowledge of the reaction of the pulp after a traumatic injury makes it possible to accurately determine this level. This procedure is commonly called the Cvek pulpotomy.
Technique
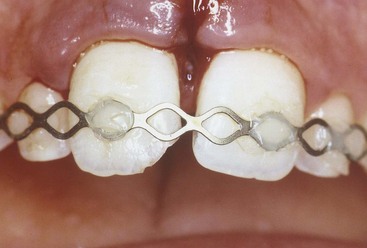
Anesthesia, possibly without a vasoconstrictor, rubber dam placement, and superficial disinfection are performed. A 1- to 2-mm deep cavity is prepared into the pulp, using a high-speed handpiece with a sterile diamond bur of appropriate size with copious water coolant70 (Fig. 17-11). A slow-speed bur or spoon excavator should be avoided. If bleeding is excessive, the pulp is amputated deeper until only moderate hemorrhage is seen. Excess blood is carefully removed by rinsing with sterile saline and the area dried with a sterile cotton pellet. Use of 5% sodium hypochlorite (NaOCl; bleach) has been recommended to rinse the pulpal wound.41 The bleach will cause a chemical amputation of the blood coagulum, remove damaged pulp cells, dentin chips, and other debris, and provides hemorrhage control with minimal damage to the “normal” pulp tissue underneath.
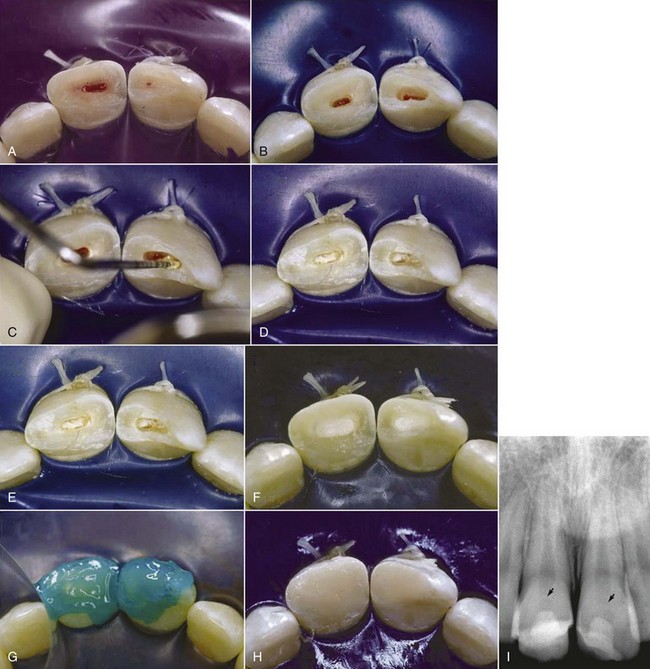
FIG. 17-11 Cvek partial pulpotomy. A, The fractured teeth are cleaned and disinfected; a rubber dam is placed. B, Cavities are prepared at high speed with a round diamond bur 1 to 2 mm into the pulpal tissue. C, Calcium hydroxide on a plugger (D) is placed on the soft tissue of the pulp. E, Care is taken to avoid smearing the walls of the preparation with the calcium hydroxide. F, Cavity preparations are filled with glass ionomer cement. G, Exposed dentin is etched and (H) then covered with composite resin. I, Radiograph exposed 6 months later shows formation of hard-tissue barriers in both teeth.
(Courtesy Dr. Alessandra Ritter.)
Care must be taken not to allow a blood clot to develop; this will compromise the prognosis.43,122 A thin layer of pure calcium hydroxide is mixed with sterile saline or anesthetic solution to a thick mix and carefully placed onto the pulp stump. If the pulp size does not permit additional loss of pulp tissue, a commercial hard-setting calcium hydroxide can be used.139 The prepared cavity is filled with a material with the best chance of a bacteria-tight seal (zinc oxide eugenol or glass ionomer cement) to a level flush with the fractured surface. The material in the pulpal cavity and all exposed dentinal tubules are etched and restored with bonded composite resin. Alternatively, after hemostasis is obtained, the pulp can be capped with MTA, a wet cotton pellet placed on top if it, and the access temporized for the appropriate time. The cotton pellet then needs to be removed as early as possible and the tooth then restored with composite restoration.
Follow-Up
As with pulp capping. Emphasis is placed on evidence of maintenance of responses to sensitivity tests and radiographic evidence of continued root development (Fig. 17-12).
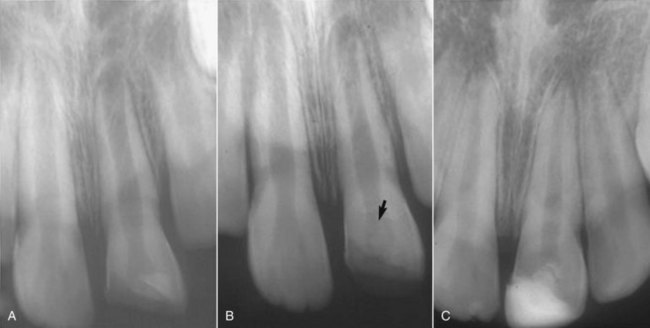
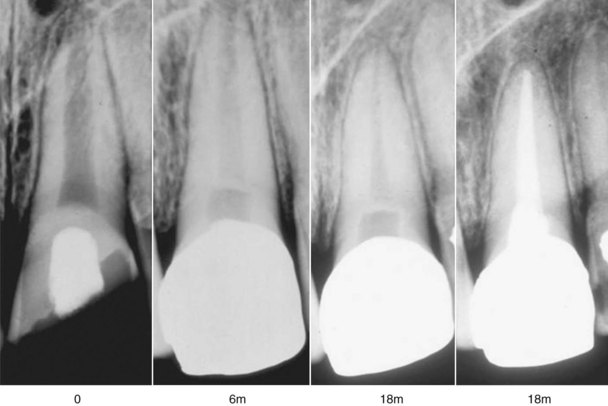
FIG. 17-12 Continued root development after partial pulpotomy. A, Radiograph of immature tooth with a complicated crown fracture. B, At the time of placement of calcium hydroxide after partial pulpotomy. C, Follow-up radiograph confirming that the pulp maintained vitality and the root continued to develop.
Prognosis
This method affords many advantages over pulp capping. Superficial inflamed pulp is removed during preparation of the pulpal cavity. Calcium hydroxide disinfects dentin and pulp and removes additional pulpal inflammation. Most importantly, space is provided for a material that can provide a bacteria-tight seal to allow pulpal healing with hard tissue under optimal conditions. Additionally, coronal pulp remains, which allows sensitivity testing to be carried out at the follow-up visits. Prognosis is extremely good (94% to 96%).43,63
Full Pulpotomy
This procedure involves removal of the entire coronal pulp to a level of the root orifices. This level of pulp amputation is chosen arbitrarily because of its anatomic convenience. Since inflamed pulp sometimes extends past the canal orifices into the root pulp, many mistakes are made that result in treatment of an inflamed rather than noninflamed pulp.
Indications
Full pulpotomy is indicated when it is anticipated that the pulp is inflamed to the deeper levels of the coronal pulp. Traumatic exposures after more than 72 hours or a carious exposure of a young tooth with partially developed apex are two examples of when this treatment may be indicated. Because of the likelihood that the dressing will be placed on an inflamed pulp, full pulpotomy is contraindicated in mature teeth. Benefits only outweigh risks for this treatment form in the immature tooth with incompletely formed apices and thin dentinal walls.
Technique
The procedure begins with anesthesia, rubber dam placement, and superficial disinfection as with pulp capping and partial pulpotomy. The coronal pulp is removed as in partial pulpotomy but to the level of the root orifices. Calcium hydroxide dressing, a bacteria-tight seal, and a coronal restoration are carried out as with partial pulpotomy.
Follow-Up
As with pulp capping and partial pulpotomy. A major disadvantage of this treatment method is that sensitivity testing is not possible owing to the loss of coronal pulp, so radiographic follow-up is extremely important to assess for signs of apical periodontitis and ensure the continuation of root formation.
Prognosis
Since the cervical pulpotomy is performed on pulps, which are expected to have deep inflammation, and the site of pulp amputation is arbitrary, many more mistakes are made that lead to treatment of the inflamed pulp. Consequently the prognosis, which is in the range of 75%, is poorer than partial pulpotomy.67 Because of the inability to evaluate pulp status after full pulpotomy, some authors have recommended pulpectomy routinely after the roots have fully formed (Fig. 17-13). This philosophy is based on the pulpectomy procedure having a success rate in the range of 95%, whereas if apical periodontitis develops, the prognosis of root canal treatment drops significantly to about 80%.125,131
Treatment of the Nonvital Pulp
Immature Tooth: Apexification
Indications
This procedure should be performed in teeth with open apices and thin dentinal walls in which standard instrumentation techniques cannot create an apical stop to facilitate effective root canal filling.
Biologic Consequences
A nonvital immature tooth presents a number of difficulties for adequate endodontic treatment. The canal is often wider apically than coronally, necessitating the use of a filling technique with softened filling material to mold it to the shape of the apical part of the canal. Since the apex is extremely wide, no barrier exists to stop this softened material from moving into and traumatizing the apical periodontal tissues. Also, the lack of apical stop and extrusion of material through the canal might result in a canal that is underfilled and susceptible to leakage. An additional problem in immature teeth with thin dentinal walls is their susceptibility to fracture, both during and after treatment.44
These problems are overcome by stimulating the formation of a hard-tissue barrier to allow for optimal filling of the canal and reinforcing the weakened root against fracture, both during and after apexification.93,143
Technique
Disinfection of the Canal
In the vast majority of cases, nonvital teeth are infected,29,127 so the first phase of treatment is to disinfect the root canal system to ensure periapical healing.37,48 The canal length is estimated with a parallel preoperative radiograph, and after access to the canals is prepared, a file is placed to this estimated length. When the length has been confirmed radiographically, very light filing (because of the thin dentinal walls) is performed with copious irrigation with 0.5% NaOCl.49,136 A lower strength of NaOCl is used because of the increased danger of placing the agent through the apex in immature teeth. The increased volume of irrigant used compensates for this lower concentration of NaOCl. An irrigation needle that can passively reach close to the apical length is useful in disinfecting the canals of these immature teeth. The canal is dried with paper points and a creamy mix of calcium hydroxide (toothpaste thickness) spun into the canal with a Lentulo spiral instrument (Fig. 17-14). Additional disinfecting action of calcium hydroxide is effective after its application for at least 1 week,130 so the continuation of treatment can take place any time after 1 week. Further treatment should not be delayed more than 1 month, since the calcium hydroxide could be washed out by tissue fluids through the open apex, leaving the canal susceptible to reinfection.
Hard-Tissue Apical Barrier
Traditional Method
Formation of the hard-tissue barrier at the apex requires a similar environment to that required for hard-tissue formation in vital pulp therapy: a mild inflammatory stimulus to initiate healing and a bacteria-free environment to ensure that inflammation is not progressive.
As with vital pulp therapy, calcium hydroxide is used for this procedure.44,76,78 Pure calcium hydroxide powder is mixed with sterile saline (or anesthetic solution) to a thick (powdery) consistency (Fig. 17-15). The calcium hydroxide is packed against the apical soft tissue with a plugger or thick point to initiate hard-tissue formation. This step is followed by backfilling with calcium hydroxide to completely fill the canal. The calcium hydroxide is meticulously removed from the access cavity to the level of the root orifices and a well-sealing temporary filling placed in the access cavity. A radiograph is taken, and the canal should appear to have become calcified, indicating that the entire canal has been filled with the calcium hydroxide (Fig. 17-16). Because calcium hydroxide washout is evaluated by its relative radiodensity in the canal, it is prudent to use a calcium hydroxide mixture without the addition of a radiopaquer such as barium sulphate. These additives do not wash out as readily as calcium hydroxide, so if they are present in the canal, evaluation of washout is impossible.
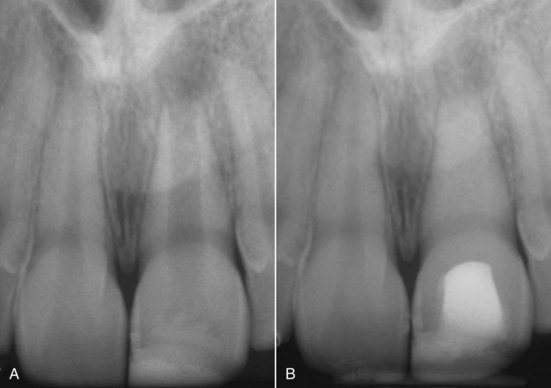
FIG. 17-16 A-B, Root canal that “disappears” after placement of a thick mix of pure calcium hydroxide and then over time washes out again.
(Courtesy Dr. Cecilia Bourguignon.)
At 3-month intervals, a radiograph is exposed to evaluate whether a hard-tissue barrier has formed and if the calcium hydroxide has washed out of the canal. This is assessed to have occurred if the canal can be seen again radiographically. If no washout is evident, it can be left intact for another 3 months. Excessive calcium hydroxide dressing changes should be avoided if at all possible, since the initial toxicity of the material is thought to delay healing.95
When completion of a hard-tissue barrier is suspected, the calcium hydroxide should be washed out of the canal with NaOCl. A file of a size that can easily reach the apex can be used to gently probe for a stop at the apex. When a hard-tissue barrier is indicated radiographically and can be probed with an instrument, the canal is ready for filling.
Mta Barrier
The creation of a physiologic hard-tissue barrier, while quite predictable, takes anywhere from 3 to 18 months with calcium hydroxide. The disadvantages of this long time period are that the patient is required to present for treatment multiple times, and the tooth may fracture during treatment before the thin, weak roots can be strengthened. In addition, one study has indicated that long-term treatment with calcium hydroxide may weaken the roots and make them even more susceptible to fracture.19
MTA has been used to create a hard-tissue barrier quickly after disinfection (see earlier in the section Traditional Method) of the canal (Fig. 17-17). Calcium sulphate is pushed through the apex to provide a resorbable extraradicular barrier against which to pack the MTA. The MTA is mixed and placed into the apical 3 to 4 mm of the canal in a manner similar to the placement of calcium hydroxide. A wet cotton pellet should be placed against the MTA and left for at least 6 hours. After the MTA is fully set, the entire canal is then filled with a root filling material. Some clinicians have suggested that the filling can be placed immediately, since the tissue fluids of the open apex will probably provide enough moisture to ensure that the MTA will set sufficiently. The cervical canal is then reinforced with composite resin to below the marginal bone level as described later (see Fig. 17-17).
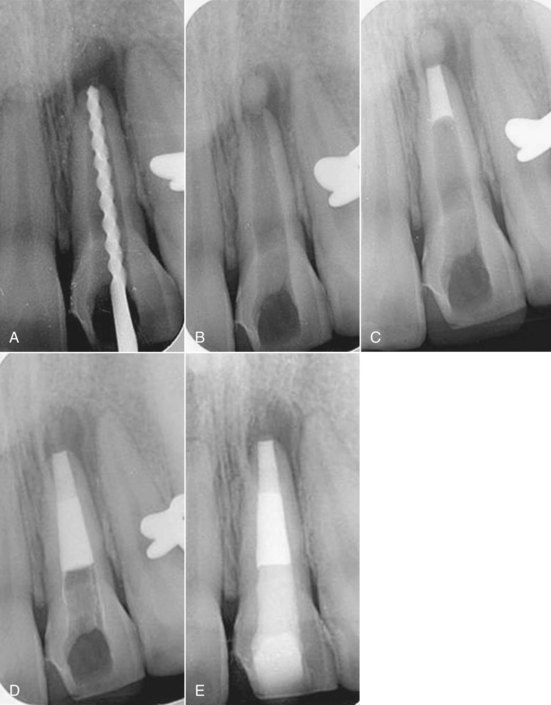
FIG. 17-17 Apexification with mineral trioxide aggregate (MTA). A, The canal is disinfected with light instrumentation, copious irrigation, and a creamy mix of calcium hydroxide for 1 month. B, Calcium sulfate is placed through the apex as a barrier against which the MTA is placed. C, A 4-mm MTA plug is placed at the apex. D, The body of the canal is filled with the Resilon obturation system. E, A bonded resin is placed below the cementoenamel junction (CEJ) to strengthen the root.
(Courtesy Dr. Marga Ree.)
A number of case reports have been published using this MTA apical barrier technique,68,80,100 and it has steadily gained popularity with clinicians. Presently, no prospective long-term outcome study is available comparing its success rate with the traditional calcium hydroxide technique.
Filling the Root Canal
Since the apical diameter is larger than the coronal diameter of most of these canals, a softened filling technique is indicated in these teeth (see Chapter 10). Care must be taken to avoid excessive lateral force during filling, owing to the thin walls of the root. If the hard-tissue barrier was produced by long-term calcium hydroxide therapy, it consists of irregularly arranged layers of coagulated soft tissue, calcified tissue, and cementum-like tissue (Fig. 17-18). Included also are islands of soft connective tissue, giving the barrier a “Swiss cheese” consistency.31,45 Because of the irregular nature of the barrier, it is not unusual for cement or softened filling material to be pushed through it into the apical tissues during filling (Fig. 17-19). Formation of the hard-tissue barrier might be some distance short of the radiographic apex. This is because it forms wherever the calcium hydroxide contacts vital tissue. In teeth with wide, open apices, vital tissue can survive and proliferate from the periodontal ligament a few millimeters into the root canal. Filling should be completed to the level of the hard-tissue barrier and not forced toward the radiographic apex.
Reinforcement of Thin Dentinal Walls
The apexification procedure has become a predictably successful procedure (see Prognosis),61 but the thin dentinal walls still present a clinical problem. Should secondary injuries occur, teeth with thin dentinal walls are more susceptible to fractures that render them unrestorable.51,147 It has been reported that approximately 30% of these teeth will fracture during or after endodontic treatment.93 Consequently, some clinicians have questioned the advisability of the apexification procedure and have opted for more radical treatment procedures, including extraction followed by extensive restorative procedures such as dental implants. Studies have shown that intracoronal bonded restorations can internally strengthen endodontically treated teeth and increase their resistance to fracture.69,91 Thus after root filling, the material should be removed to below the marginal bone level and a bonded resin filling placed (see Fig. 17-17).
Follow-Up
Routine recall evaluation should be performed to determine success in the prevention or treatment of apical periodontitis. Restorative procedures should be assessed to ensure that they in no way promote root fractures.
Prognosis
Periapical healing and the formation of a hard-tissue barrier occurs predictably with long-term calcium hydroxide treatment (79% to 96%).44,93 However, long-term survival is jeopardized by the fracture potential of the thin dentinal walls of these teeth. It is expected that the newer techniques of internally strengthening the teeth described earlier will increase their long-term survivability.
Pulp Revascularization
Regeneration of a necrotic pulp has been considered possible only after avulsion of an immature permanent tooth that was reimplanted within 40 minutes (see later). The advantages of pulp revascularization lie in the possibility of further root development and reinforcement of dentinal walls by deposition of hard tissue, thus strengthening the root against fracture (see also Chapter 16). After reimplantation of an avulsed immature tooth, a unique set of circumstances exists that allows regeneration to take place. The young tooth has an open apex and is short, which allows new tissue to grow into the pulp space relatively quickly. The pulp is necrotic but usually neither degenerated nor infected, so it will act as a matrix into which the new tissue can grow. It has been experimentally shown that the apical part of a pulp may remain vital and after reimplantation proliferate coronally, replacing the necrotized portion of the pulp.26,107,134 Because in most cases, the crown of the tooth is intact and caries-free, bacterial penetration into the pulp space through cracks97 and defects will be a slow process. The race between new tissue growth and infection of the pulp space favors the new tissue.
Regeneration of pulp tissue in a necrotic infected tooth with apical periodontitis has been thought up to now to be impossible.104 However, if it is possible to create an environment similar to that described for the avulsed tooth, regeneration will probably occur. If the canal is effectively disinfected, a matrix into which new tissue can grow is provided, and coronal access is effectively sealed. Regeneration should occur as in an avulsed immature tooth.
Banchs and Trope25 wrote one of the first case reports that suggest it may be possible to replicate the unique circumstances of an avulsed tooth. The case (Fig. 17-20) describes the treatment of an immature second mandibular premolar with radiographic and clinical signs of apical periodontitis along with a sinus tract. The canal was disinfected without mechanical instrumentation but with copious irrigation with 5.25% NaOCl, and the patient was prescribed a mixture of antibiotics consisting of equal amounts of ciprofloxacin, metronidazole, and minocycline for 3 weeks.82
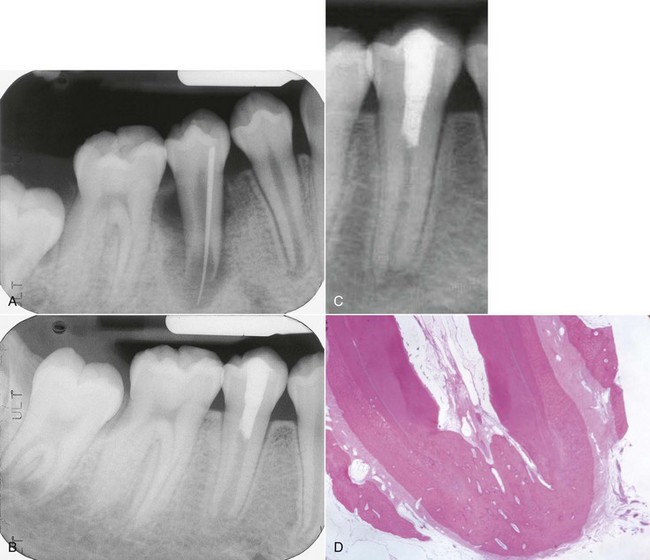
FIG. 17-20 A, Preoperative radiograph showing an immature root with periradicular periodontitis and a traced sinus tract to the apex of the root. B, Radiographic checkup 7 months after treatment that included application of a mixture of ciprofloxacin, metronidazole, and minocycline. C, At 24-month checkup, showing continued root development both in length and width of the root. D, Histologic slide from Thibodeau et al.144 showing thickened root walls, apical development, and healing of apical periodontitis in a dog tooth where a periapical lesion had been created in an immature tooth.
After rinsing out the antibody mixture after three weeks, a blood clot was produced to the level of the cementoenamel junction to provide a matrix for ingrowth of new tissue; this was followed by a deep coronal restoration to provide a bacteria-tight seal. Clinical and radiographic evidence of healing appeared as early as 22 days, the large radiolucency had disappeared within 2 months, and at the 24-month recall it was obvious that the root walls were thick and development of the root below the restoration was similar to the adjacent and contralateral teeth. More recently, published cases and case series reports have further supported that it is possible to have ingrowths of tissue followed by new apposition of dentin and continuation of root formation.89,144 Studies have also confirmed the potent antibacterial properties of the triantibiotic paste used in these cases. Studies are underway to find a synthetic matrix which will act as a more predictable scaffold for new ingrowth of tissue than the blood clot that was used in these examples.144,162 The procedure described here can be attempted in most cases, and if after 3 months there are no signs of regeneration, then the more traditional treatment methods can be initiated.
Crown-Root Fracture
Crown-root fracture is a periodontal rather than an endodontic challenge. The tooth must be treated periodontally to enable a well-sealed coronal restoration. This could be accomplished by simple gingivectomy if the extent of the root component of the fracture is large. Alternatively, the tooth could be orthodontically or surgically extruded such that the exposed surface of the root fracture is treatable. Once the feasibility of the coronal restoration is assured, the particular crown fracture is treated as previously described.
Root Fracture
Root fracture implies fracture of the cementum, dentin, and pulp. These injuries are relatively infrequent, occurring in less than 3% of all dental injuries.166 Immature teeth with vital pulps rarely sustain horizontal root fractures.7 When a root fractures horizontally, the coronal segment is displaced to a varying degree, but generally the apical segment is not displaced. Because the apical pulpal circulation is not disrupted, pulp necrosis in the apical segment is extremely rare. Permanent pulpal necrosis of the coronal segment, requiring endodontic treatment, occurs in about 25% of cases.8,9,86
Diagnosis and Clinical Presentation
Clinical presentation is similar to that of luxation injuries. The extent of displacement of the coronal segment is usually indicative of the location of the fracture and can vary from none, simulating a concussion injury (apical fracture), to severe, simulating an extrusive luxation (cervical fracture).
Radiographic examination for root fractures is extremely important. Since root fractures are usually oblique (facial to palatal) (Fig. 17-21), one periapical radiograph may easily miss its presence. It is imperative to take at least three angled radiographs (45, 90, 110 degrees) so that at least at one angulation, the x-ray beam will pass directly through the fracture line to make it visible on the radiograph (Fig. 17-22).
Treatment
Emergency treatment involves repositioning the segments into close proximity as much as possible. In the case of severe displacement of the coronal segment, its apical extension is frequently lodged in (if not perforating through) the cortical bone facial to the tooth. Forcing the crown facially will not be possible, and the two segments will not be properly aligned. The only way to accomplish reapproximation of the two segments is to release the coronal segment from the bone by gently pulling it slightly downward with finger pressure or extraction forceps, and then once it is loose, rotate it back to its original position (Fig. 17-23). The traditionally recommended113 splinting protocol has been changed from 2 to 4 months with rigid splinting to a semirigid splint to adjacent teeth for 2 to 4 weeks.18 If a long time has elapsed between the injury and treatment, it will likely not be possible to reposition the segments close to their original position, compromising the long-term prognosis for the tooth.
Healing Patterns
Investigators20 have described four types of responses to root fractures:
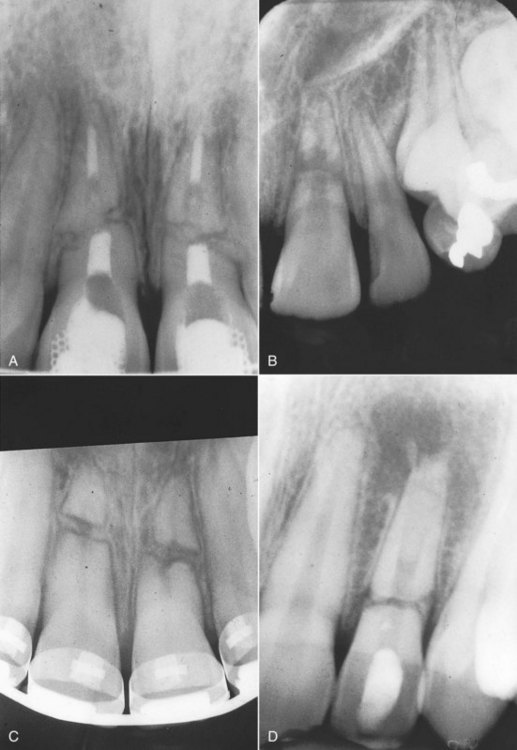
FIG. 17-24 Healing patterns after horizontal root fractures. A, Healing with calcified tissue. B, Healing with interproximal connective tissue. C, Healing with bone and connective tissue. D, Interproximal connective tissue without healing.
The first three types of healing patterns are considered successful. The teeth are usually asymptomatic and respond positively to sensitivity testing. Coronal yellowing is possible because calcific metamorphosis of the coronal segment is not unusual87,166 (see Fig. 17-29).
The fourth type of root fracture response is typical when the coronal segment loses its vitality. The infective products in the coronal pulp cause an inflammatory response and typical radiolucencies at the fracture line20 (see Fig. 17-24, D).
Treatment of Complications
Coronal Root Fractures
Historically it had been thought that fractures in the cervical segment had a poor prognosis, and extraction of the coronal segment was recommended. Research does not support this treatment; in fact, if these coronal segments are adequately splinted, chances of healing do not differ from midroot or apical fractures (Fig. 17-25).166 However, if the fracture occurs at the level of or coronal to the crest of the alveolar bone, the prognosis is extremely poor.
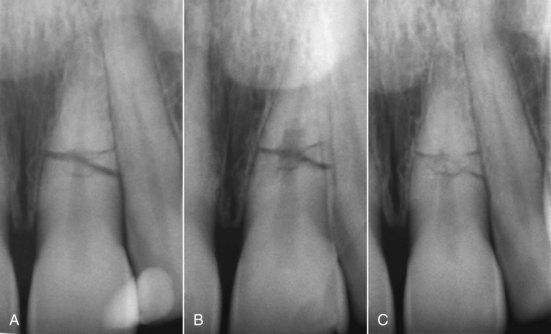
FIG. 17-25 Internal root resorption in the root fracture area; tooth was diagnosed with compound midroot fracture. A, Coronal portion was repositioned and then splinted to the lateral incisor with a composite splint. B, At 6-month recall, the pulp responded normally to cold, but an internal root-resorptive defect was noted on radiograph. No treatment was indicated, because the pulp did respond normally to vitality tests. C, At 42-month recall, the tooth was still asymptomatic, and the pulp still responded to vitality tests. The resorptive defect had healed, and signs of dystrophic calcifications were evident in the apical and coronal segments.
If reapproximation of the fractured segments is not possible, extraction of the coronal segment is indicated. The level of fracture and length of the remaining root are evaluated for restorability. If the apical root segment is long enough, gentle orthodontic eruption of this segment can be carried out to enable a restoration to be fabricated.
Midroot and Apical Root Fractures
Permanent pulpal necrosis occurs in 25% of root fractures.45,86 Initially it is likely that in many cases the pulp in the coronal segment becomes necrotic after the injury, but then because of a very large apical opening in the coronal segment, revascularization is possible if the segments are well reapproximated. In the vast majority of cases, permanent necrosis occurs in the coronal segment, with only the apical segment remaining vital. Therefore, endodontic treatment is indicated in the coronal root segment unless periapical pathology is seen in the apical segment. In most cases, the pulpal lumen is wide at the apical extent of the coronal segment so that long-term calcium hydroxide treatment or an MTA apical plug is indicated (see Apexification). The coronal segment is filled after a hard-tissue barrier has formed apically in the coronal segment and periradicular healing has taken place.
In rare cases when both the coronal and apical pulp are necrotic, treatment is more complicated. Endodontic treatment through the fracture is extremely difficult. Endodontic manipulations, medicaments, and filling materials all have a detrimental effect on healing of the fracture site. If healing of the fracture has been complete and followed by necrosis of the apical segment, the prognosis is much improved.
In more apical root fractures, necrotic apical segments can be surgically removed. This is a viable treatment if the remaining coronal root segment is long enough to provide adequate periodontal support. Removal of the apical segment in midroot fractures leaves the coronal segment with a compromised attachment; endodontic implants have been used to provide additional support to the tooth.
Follow-Up
After the splinting period is completed, follow-up is as with all dental traumatic injuries: at 3, 6, and 12 months and yearly thereafter.
Prognosis
Factors that influence repair include:
Complications are pulp necrosis and root canal obliteration. Pulp necrosis can be treated successfully45,86 by treating the coronal segment with calcium hydroxide to stimulate hard-tissue barrier formation. Root canal obliteration is common if the root segment (coronal or apical) remains vital.
Luxation Injuries
Definitions
Definitions 1 through 5 describe injuries of increasing magnitude in terms of intensity of the injury and subsequent sequelae.
Incidence
Luxation injuries as a group are the most common of all dental injuries, with reported incidences ranging from 30% to 44%.50
Treatment
Teeth that are concussed or subluxated do not need any immediate treatment. Responses to vitality tests should be investigated and noted. Even after mild injury such as subluxation, the pulp might be unresponsive to vitality tests for several weeks if not months.133 When the pulp is unresponsive initially after the trauma, patients should be recalled on a regular basis and monitored for any additional signs of infection of the root canal (see later).
Teeth with lateral and extrusive luxation should be repositioned as soon as possible. In lateral luxation, the apex might be perforating the facial bone plate, and the tooth has to first be slightly and gently pulled down to loosen the hold prior to repositioning it in its original position. Current IADT guidelines call for 2 weeks of physiologic splinting in cases of extrusion luxation and 4 weeks for lateral luxation. Decision on root canal therapy follows the guidelines for avulsion (see later). If the tooth has a fully formed apex and was diagnosed to have moved into (if not through) the cortical plate (apical translocation), there is a good likelihood of the pulp being devitalized, so endodontic treatment should be initiated as early as 2 weeks after the injury. If the apex is still not fully formed, waiting for signs of revascularization is strongly recommended.
Permanent teeth that are intruded are not likely to spontaneously re-erupt, especially if the apex is fully formed.92 Alternative treatment like orthodontic extrusion or immediate surgical reposition should therefore be considered. If orthodontic extrusion is planned for an intruded tooth, it should be initiated as soon as possible, delaying no more than 2 to 3 weeks post trauma. There are only a few studies that have evaluated the true efficacy of this approach. One study157 using a dog model indicated that severely intruded teeth showed signs of ankylosis as early as 11 to 13 days after the trauma, despite initiation of orthodontic movement 5 to 7 days after the injury.
For the surgical approach, one study42 (using a dog model) concluded that “a careful immediate surgical repositioning of a severely intruded permanent tooth with complete root formation has many advantages with few disadvantages.” Another investigation,52 a retrospective study of 58 intruded human teeth, indicated that surgical repositioning resulted in a predictable outcome, with only five of the teeth being lost over the observation period. However, it was also observed that less surgical manipulation positively influenced the healing. If an intruded tooth is immediately reimplanted, it should be splinted for at least 4 weeks, but in most cases the splint needs to be left on the tooth longer.
Biologic Consequences
Luxation injuries result in damage to the attachment apparatus (periodontal ligament and cemental layer), the severity of which is dependent on the type of injury sustained (concussion least, intrusion most). The apical neurovascular supply to the pulp is also affected to varying degrees, resulting in an altered or nonvital tooth.
Healing can be favorable or unfavorable. Favorable healing after a luxation injury occurs if the initial physical damage to the root surface and the resultant inflammatory response to the damaged external root surface is again covered with cementum. An unfavorable response occurs when there is direct attachment of bone to the root, with the root ultimately being replaced by bone.
There are two resorption responses in which the pulp plays an essential role:
External Root Resorption
Caused by an Injury (Alone) to the External Root Surface
If an injury harms the attachment, the byproducts of this mechanical damage stimulate an inflammatory response. The healing response is dependent on the extent of the initial damage.
Localized Injury: Healing With Cementum
When the injury is localized (e.g., after concussion or subluxation injury), mechanical damage to the cementum occurs, resulting in a local inflammatory response and a localized area of root resorption. If no further inflammatory stimulus is present, periodontal healing and root surface repair will occur within 14 days71 (Fig. 17-26). The resorption is localized to the area of mechanical damage, and treatment is not required, since it is free of symptoms and not even visualized radiographically in most cases. In a minority of cases, however, small radiolucencies can be seen on the root surface if the radiograph is taken at a specific angle. It is important to avoid misinterpreting these cases as progressive. The pulp is not involved. If the pulp responds to sensitivity tests, this is a clue that no treatment should be performed. A wait-and-see attitude can allow spontaneous healing to take place. It is important to realize that in the initial stages, radiolucency could be followed by spontaneous repair, and endodontic treatment should not be initiated.
Diffuse Injury: Healing by Osseous Replacement
When the traumatic injury is severe (e.g., intrusive luxation or avulsion with extended dry time), involving diffuse damage on more than 20% of the root surface, an abnormal attachment can occur after healing takes place.96 The initial reaction as always is inflammation, in response to the severe mechanical damage to the root surface. After the initial inflammatory response, a diffuse area of root surface devoid of cementum results. Cells in the vicinity of the denuded root now compete to repopulate it. Often cells which are precursors of bone, rather than the slower-moving periodontal ligament cells, will move across from the socket wall and populate the damaged root. Bone comes into contact with the root without an intermediate attachment apparatus. This phenomenon is termed dentoalveolar ankylosis.96 However, the ankylosis and osseous replacement that follows cannot be reversed and can be considered a physiologic process, since bone resorbs and reforms throughout life. Thus root is resorbed by osteoclasts, but in the reforming stage, bone is laid down instead of dentin, slowly replacing the root with bone. This process is termed osseous replacement152 (Fig. 17-27). The initial inflammation to remove the mechanical debris of the traumatic injury is a pathologic response that in the authors’ opinion may be reversed. In these traumatic cases, however, the resorptive phase includes the root (Fig. 17-28).
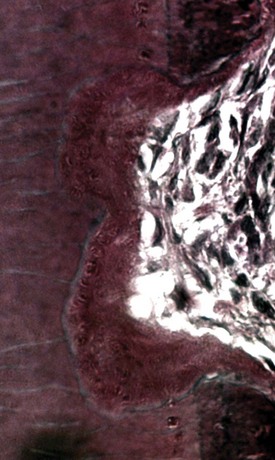
FIG. 17-27 Histologic appearance of osseous replacement. There is direct contact between bone and root structure. Resorptive defects are seen in the bone and root tissue, which is the normal physiologic process of bone turnover. The resorptive defects will be filled by new bone and additional areas resorbed. In this way the entire root will be replaced by bone at a rate dependent on the metabolic rate of the patient.
Treatment
Treatment strategies for limiting the effect of the traumatic injury to the periodontal structures are outside the scope of this chapter, but in general this involves minimizing the initial inflammatory response to the injury. The initial inflammation is destructive in nature and will increase the surface area of root to be covered in the healing phase.21 The smaller the surface area to be covered by new cementum, the higher the chances of favorable repair.
A study has indicated that if Ledermix, a drug combining corticosteroid and tetracycline, is placed in the root canal immediately after a severe trauma where osseous replacement was expected, favorable healing will occur at a very high rate.36 In a more recent study, it was shown that triamcinolone (the corticosteroid portion of the Ledermix paste) was as effective as Ledermix at inhibiting external root resorption.39 Both these studies were done on young dogs and need to be replicated in human studies.
Caused by an Injury to the External Root Surface and Inflammatory Stiumlus in the Root Canal
Recognized inflammatory stimuli that cause root resorption are pressure, pulp space infection, and sulcular infection. This chapter is focused on pulp space infection. Other chapters in this text deal with orthodontic and periodontal issues (see Chapters 18 and 23).
Consequences of Apical Neurovascular Supply Damage
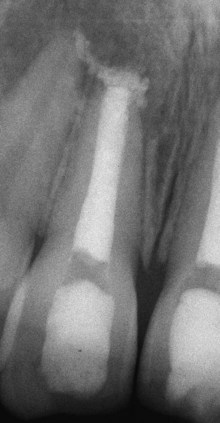
Pulp Canal Obliteration (Calcification)
Pulp canal obliteration is common after luxation injuries (Fig. 17-29). The frequency of pulp canal obliteration appears inversely proportional to pulp necrosis. The exact mechanism of pulp canal obliteration is unknown. It has been theorized that the sympathetic/parasympathetic control of blood flow to the odontoblasts is altered, resulting in uncontrolled reparative dentin.7,15 Another theory is that hemorrhage and blood clot formation in the pulp after injury is a nidus for subsequent calcification if the pulp remains vital.7,15 Pulp canal obliteration can usually be diagnosed within the first year after injury11 and was found to be more frequent in teeth with open apices (>0.7 mm radiographically), in teeth with extrusive and lateral luxation injuries, and in teeth that have been rigidly splinted.11
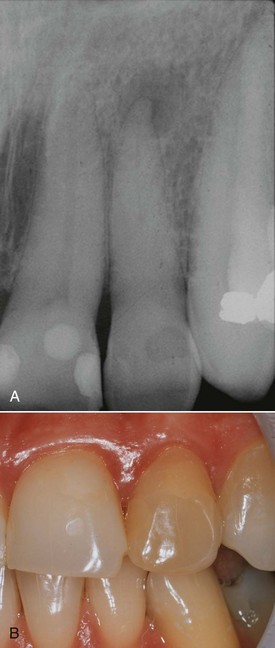
FIG. 17-29 A, Pulp canal calcification after a luxation injury. Radiographic appearance of a calcified maxillary lateral incisor; note that despite calcification, it has now become necrotic and infected, evident by the periapical lesion. B, The typical yellow appearance of the tooth caused by thickened dentin in the pulp chamber.
Pulp Necrosis
The factors most important for the development of pulp necrosis are type of injury (concussion least, intrusion most) and the stage of root development (mature apex > immature apex).10 Pulp necrosis will most likely lead to infection of the root canal system, with problematic consequences.
Pulp Space Infection
Pulp space infection in conjunction with damage to the external root surface results in periradicular root and bone resorption and will continue in its active state as long as the pulpal stimulus (infection) remains. When the root loses its cemental protection, lateral periodontitis with root resorption can result (Fig. 17-30).
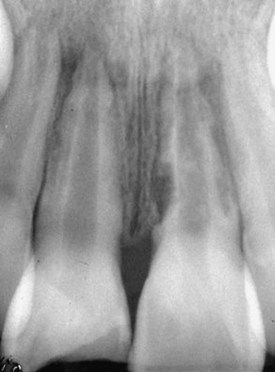
FIG. 17-30 Inflammatory root resorption caused by a pulp space infection. Note the radiolucencies in the root and surrounding bone.
(Courtesy Dr. Fred Barnett.)
To have pulp space infection, the pulp must first become necrotic. Necrosis will occur after a fairly serious injury in which displacement of the tooth results in severing of the apical blood vessels. In mature teeth, pulp regeneration cannot occur and usually by 3 weeks, the necrotic pulp will become infected. For details of the typical bacterial contents of a traumatized necrotic pulp, the reader is referred to Chapter 15 or Bergenholtz.29 Because a serious injury is required for pulp necrosis, it is usual that areas of cemental covering of the root are also affected, resulting in loss of its protective (insulating) quality. Now bacterial toxins can pass through the dentinal tubules and stimulate an inflammatory response in the periodontal ligament. The result is resorption of the root and bone. The periodontal infiltrate consists of granulation tissue with lymphocytes, plasma cells, and polymorphonuclear leukocytes. Multinucleated giant cells resorb the denuded root surface, and this continues until the stimulus (pulp space bacteria) is removed149 (Fig. 17-31). Radiographically the resorption is observed as progressive radiolucent areas of the root and adjacent bone (see Fig. 17-30).
Treatment
Assessing attachment damage due to the traumatic injury and minimizing the subsequent inflammation should be the focus of the emergency visit. The clinician’s attention to pulp space infection should ideally be 7 to 10 days after the injury.155,156 Root canal disinfection removes the stimulus to the periradicular inflammation, thus the resorption will stop.71,155,156 In most cases a new attachment will form, but if a large area of root is affected, osseous replacement can result by the mechanism already described. Again, treatment principles include prevention of pulp space infection or elimination of the bacteria if they are present in the pulp space.
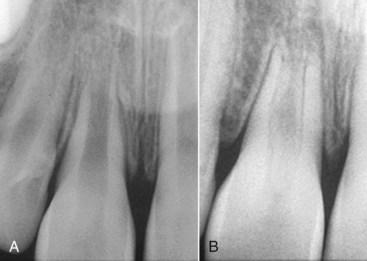
FIG. 17-32 Revascularization of immature root. A tooth with an open apex was replanted soon after the avulsion. The checkup radiograph 12 years later confirms that regrowth has taken place into the pulp chamber. It appears that a bone plug has grown in, and new periodontal ligament has formed, with a lamina dura within the pulp canal.
(Courtesy Dr. Cecilia Bourguignon.)
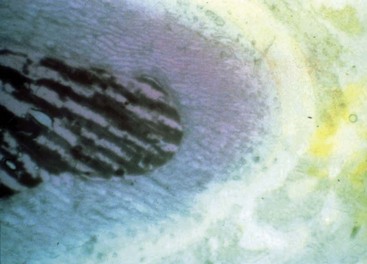
FIG. 17-33 High pH of calcium hydroxide. The root was filled with calcium hydroxide and then cut in cross section. A pH indication shows the high pH in the canal and surrounding root, whereas the surrounding tissue is a neutral pH.
The first visit consists of the microbial control phase, with cleaning and shaping of the canal and the placement of a creamy mix of calcium hydroxide using a Lentulo spiral. The patient is seen in approximately 1 month, at which time the canal is filled with a dense mix of calcium hydroxide. Once filled, the canal should appear radiographically to be calcified, since the radiodensity of calcium hydroxide in the canal is usually similar to the surrounding dentin (see Fig. 17-16). A radiograph is then exposed at 3-month intervals. At each visit the tooth is tested for symptoms of periodontitis. In addition to stopping the resorptive process, calcium hydroxide washout is assessed. Since the root surface is so radiodense as to make assessment of healing difficult, the adjacent bone healing is assessed. If adjacent bone has healed, it is assumed that the resorptive process has stopped in the root as well; then the canal can be obturated with the permanent root filling material (Fig. 17-34).
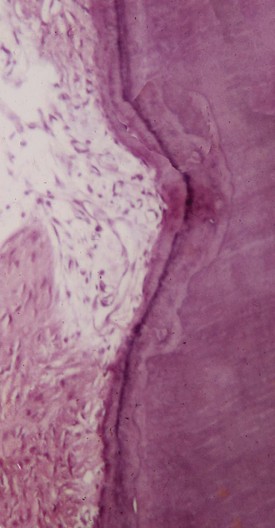
Internal Root Resorption
Internal root resorption is rare in permanent teeth. Internal resorption is characterized by an oval-shaped enlargement of the root canal space.16 External resorption, which is much more common, is often misdiagnosed as internal resorption.
Etiology
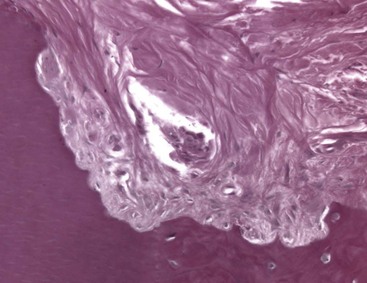
Internal root resorption is characterized by resorption of the internal aspect of the root by multinucleated giant cells adjacent to the granulation tissue in the pulp (Fig. 17-35). Chronic inflammatory tissue is common in the pulp, but only rarely does it result in resorption. There are different theories on the origin of the pulpal granulation tissue involved in internal resorption. The most logical explanation is that it is inflamed pulp tissue due to an infected coronal pulp space. Communication between the coronal necrotic tissue and the vital pulp is through appropriately oriented dentinal tubules159 (see Fig. 17-35). One investigator reports159 that resorption of the dentin is frequently associated with deposition of hard tissue resembling bone or cementum and not dentin. He postulates that the resorbing tissue is not of pulpal origin but is “metaplastic” tissue derived from the pulpal invasion of macrophage-like cells. Others138 concluded that the pulp tissue was replaced by periodontium-like connective tissue when internal resorption was present. In addition to the requirement of the presence of granulation tissue, root resorption takes place only if the odontoblastic layer and predentin are lost or altered.149
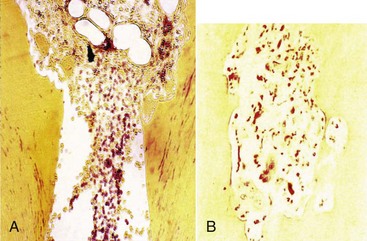
FIG. 17-35 Histologic appearance of internal root resorption. A, Section stained with Brown and Brenn. Bacteria are seen (in the dentinal tubules) communicating between the necrotic coronal segment and the apical granulomatous tissue and resorbing cells. B, An area of active internal root resorption.
(Courtesy Dr. Leif Tronstad.)
Reasons for the loss of predentin adjacent to the granulation tissue are not obvious. Trauma frequently has been suggested as a cause.124,160 Another reason for the loss of predentin might be extreme heat produced when cutting on dentin without an adequate water spray. The heat presumably would destroy the predentin layer, and if later the coronal aspect of the pulp becomes infected, the bacterial products could initiate the typical inflammation in conjunction with resorbing giant cells in the vital pulp adjacent to the denuded root surface. Internal root resorption has been produced experimentally by the application of diathermy.159
Clinical Manifestations
Internal root resorption is usually asymptomatic and is first recognized clinically through routine radiographs. For internal resorption to be active, at least part of the pulp must be vital. The coronal portion of the pulp is often necrotic, whereas the apical pulp that includes the internal resorptive defect can remain vital. Therefore, a negative sensitivity test result does not rule out active internal resorption. It is also possible that the pulp becomes nonvital after a period of active resorption, giving a negative sensitivity test, radiographic signs of internal resorption, and radiographic signs of apical inflammation. Traditionally, the pink tooth has been thought pathognomonic of internal root resorption. The pink color is due to the granulation tissue in the coronal dentin undermining the crown enamel. The pink tooth can also be a feature of subepithelial external inflammatory root resorption, which must be ruled out before a diagnosis of internal root resorption is made.
Radiographic Appearance
The usual radiographic presentation of internal root resorption is a fairly uniform radiolucent enlargement of the pulp canal (Fig. 17-36). Because the resorption is initiated in the root canal, the resorptive defect includes some part of the root canal space, so the original outline of the root canal is distorted.
Histologic Appearance
Like that of other inflammatory resorptive defects, the histologic picture of internal resorption is granulation tissue with multinucleated giant cells (see Fig. 17-35). An area of necrotic pulp is found coronal to the granulation tissue. Dentinal tubules containing microorganisms and communicating between the necrotic zone and the granulation tissue can sometimes be seen129,149,156,159 (see Fig. 17-35). Unlike external root resorption, the adjacent bone is not affected with internal root resorption.
Treatment
Treatment of internal root resorption is conceptually very easy. Since the resorptive defect is the result of the inflamed pulp, and the blood supply to the tissue is through the apical foramina, endodontic treatment that effectively removes the blood supply to the resorbing cells is the treatment approach. After adequate anesthesia is obtained, the canal apical to the internal defect is explored, and a working length short of the radiographic apex is used. The apical canal is thoroughly cleaned and shaped to ensure that the blood supply to the tissue resorbing the root is cut off.
By completion of the root canal instrumentation, it should be possible to obtain a blood-free and dry canal with paper points. Calcium hydroxide is spun into the canal to facilitate the removal of the tissue in the irregular defect at the next visit. At the second visit, the tooth and defect are filled with a warm gutta-percha technique (Fig. 17-37).
Diagnostic Features of External versus Internal Root Resorption
It is often very difficult to distinguish external from internal root resorption, so misdiagnosis and incorrect treatment may result. What follows is a list of typical diagnostic features of each resorptive type.
Radiographic Features
A change of angulation of x-rays should give a fairly good indication of whether a resorptive defect is internal or external. A lesion of internal origin appears close to the canal, whatever the angle of the x-ray (see Fig. 17-36). A defect on the external aspect of the root moves away from the canal as the angulation changes (Fig. 17-38). By using the buccal-object rule, it is usually possible to distinguish whether the external root defect is buccal or lingual-palatal.
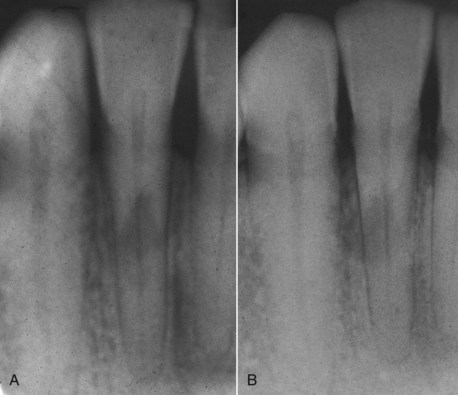
FIG. 17-38 External root resorption. Radiographs from two different horizontal projections depict movement of the lesion to outside the confines of the root canal.
With internal resorption, the outline of the root canal is usually distorted, and the root canal and the radiolucent resorptive defect appear contiguous (see Fig. 17-36). When the defect is external, the root canal outline appears normal and can usually be seen “running through” the radiolucent defect (Fig. 17-39).
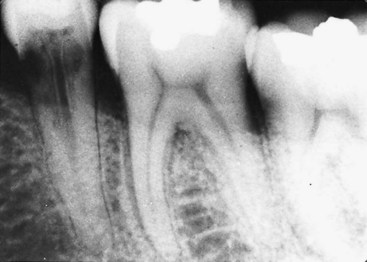
FIG. 17-39 External root resorption on a mandibular premolar 6 years after completing orthodontic treatment. Note the mottled appearance of the resorptive defect and the outline of the root canal within the defect.
External inflammatory root resorption is always accompanied by resorption of the bone in addition to the root (Fig. 17-40); radiolucencies will be apparent in the root and the adjacent bone. Internal root resorption does not involve the bone, and as a rule the radiolucency is confined to the root (see Fig. 17-36). On rare occasions if the internal defect perforates the root, the bone adjacent to it is resorbed and appears radiolucent on the radiograph.
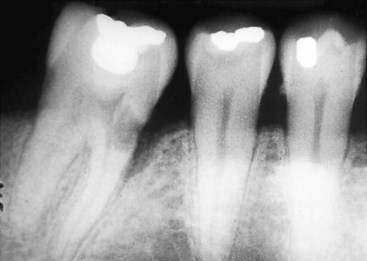
FIG. 17-40 Mandibular molar with subepithelial external inflammatory resorption on its mesial aspect. Note the small opening into the root and the extensive resorption in the dentin; however, the pulp is not exposed. Also note that a resorptive defect is present in the adjacent bone, appearing on the radiograph as similar to an infra-bony pocket.
Vitality Testing
External inflammatory resorption in the apical and lateral aspects of the root involves an infected pulp space, so no response to sensitivity tests supports the diagnosis. However, since subepithelial external root resorption does not involve the pulp (the bacteria are thought to originate in the sulcus of the tooth), a normal response to sensitivity testing is usually associated with this type of resorption. Internal root resorption usually occurs in teeth with vital pulps and responses to sensitivity testing. In teeth that exhibit internal root resorption, it is common to register a no-response to sensitivity testing, since often the coronal pulp has been removed or is necrotic, and the active resorbing cells are more apical in the canal. Also, the pulp might have become necrotic after active resorption took place.
Pink Spot
With apical and lateral external root resorption, the pulp is nonvital, so the granulation tissue that produces the pink spot is not present in these cases. For subepithelial external (Fig. 17-41) and internal root resorption, the pink spot due to the granulation tissue undermining the enamel is a possible sign.
Summary of Possible Diagnostic Features
The majority of misdiagnoses of resorptive defects are made between subepithelial external and internal root resorptions. The diagnosis should always be confirmed while treatment is proceeding. If root canal therapy is the treatment of choice for an apparent internal root resorption, the bleeding within the canal should cease quickly after pulp extirpation, since the blood supply of the granulation tissue is the apical blood vessels. If bleeding continues during treatment—and particularly if it is still present at the second visit—the source of the blood supply is external, and treatment for perforating external resorption should be carried out. On obturation, it should be possible to fill the entire canal from within in internal resorption. Failure to achieve this should make the clinician suspicious of an external lesion that is perforating the root. Finally, if the blood supply of an internal resorption defect is removed on pulp extirpation, any continuation of the resorptive process on recall radiographs should alert the clinician to the possibility that an external resorptive defect was misdiagnosed.
Clinical Management of the Avulsed Tooth
Favorable healing after an avulsion injury requires quick emergency intervention followed by evaluation and possible treatment at decisive times during the healing phase. The urgency of the emergency visit and the multidisciplinary nature of follow-up evaluations require that both the lay public and clinicians from many dental disciplines be knowledgeable about the treatment strategies involved.
Consequences of Tooth Avulsion
Tooth avulsion results in attachment damage and pulp necrosis. The tooth is “separated” from the socket due mainly to tearing of the periodontal ligament that leaves viable periodontal ligament cells on most of the root surface. In addition, small and localized cemental damage occurs from the crushing of the tooth against the socket.
If the periodontal ligament left attached to the root surface does not dry out, the consequences of tooth avulsion are usually minimal.12,135 The hydrated periodontal ligament cells will maintain their viability and repair after replantation, with minimal destructive inflammation as a byproduct. Since the areas of the crushing injury are localized, inflammation stimulated by the damaged tissues will be correspondingly limited, and favorable healing with new replacement cementum is likely to occur after the initial inflammation subsides (see Fig. 17-26).
If excessive drying occurs before replantation, the damaged periodontal ligament cells will elicit a severe inflammatory response over a diffuse area on the root surface. Unlike the situation described earlier where the area to be repaired after the initial inflammatory response is small, here a large area of root surface is affected that must be repaired by new tissue. The slower-moving cementoblasts cannot cover the entire root surface in time, and it is likely that in certain areas, bone will attach directly onto the root surface. In time through physiologic bone recontouring, the entire root will be replaced by bone. As earlier noted, this has been termed osseous replacement or replacement resorption21,153 (see Figs. 17-27, 17-28).
Pulpal necrosis always occurs after an avulsion injury. Although a necrotic pulp itself is not of consequence, the necrotic tissue is extremely susceptible to bacterial contamination. If revascularization does not occur or effective endodontic therapy is not carried out, the pulp space will inevitably become infected. The combination of bacteria in the root canal and cemental damage on the external surface of the root results in an external inflammatory resorption that can be very serious and lead to rapid loss of the tooth149 (see Fig. 17-30).
The consequences after tooth avulsion appear directly related to the severity and surface area of the inflammation on the root surface and resultant damaged root surface that must be repaired. Treatment strategies should always be considered in the context of limiting the extent of the periradicular inflammation, thus tipping the balance toward favorable (cemental) rather than unfavorable (osseous replacement or inflammatory resorption) responses.
Treatment Objectives
Treatment is directed at avoiding or minimizing resultant inflammation due to the two main consequences of the avulsed tooth: attachment damage and pulpal infection.
Attachment damage as a direct result of the avulsion injury cannot be avoided. However, considerable additional damage can occur to the periodontal ligament in the time that the tooth is out of the mouth (primarily because of drying). Treatment is directed at minimizing this damage (and the resultant inflammation) so that the fewest possible complications result. When severe additional damage cannot be avoided, and osseous replacement of the root is considered certain, steps are taken to slow the replacement of the root by bone to maintain the tooth in the mouth for as long as possible.
In the open apex tooth, all efforts are made to promote revascularization of the pulp, thus avoiding pulp space infection. When revascularization fails (in the open apex tooth) or is not possible (in the closed apex tooth), all treatment efforts are made to prevent or eliminate toxins from the root canal space.
Clinical Management
Emergency Treatment at the Accident Site
Replant if possible or place in an appropriate storage medium. As mentioned, damage to the attachment apparatus that occurred during the initial injury is unavoidable but usually minimal. However, all efforts are made to minimize necrosis of the remaining periodontal ligament while the tooth is out of the mouth. Pulpal sequels are not a concern initially and are dealt with at a later stage of treatment.
The single most important factor to assure a favorable outcome after replantation is the speed with which the tooth is replanted.16,22 Of utmost importance is the prevention of drying, which causes loss of normal physiologic metabolism and morphology of the periodontal ligament cells.22,135 Every effort should be made to replant the tooth within the first 15 to 20 minutes.27 This usually requires emergency personnel at the site of the injury with some knowledge of treatment protocol. The clinician should communicate clearly with the person at the site of the accident. Ideally this information should have been given at an earlier time as an educational offering to, for example, school nurses or athletic trainers; failing this, the information can be given over the phone. The aim is to replant a clean tooth with an undamaged root surface as gently as possible, after which the patient should be brought to the office immediately. If doubt exists that the tooth can be replanted adequately, the tooth should quickly be stored in an appropriate medium until the patient can get to the dental office for replantation. Suggested storage media in order of preference are milk, saliva (either in the vestibule of the mouth or in a container into which the patient expectorates), physiologic saline, and water.79 Water is the least desirable storage medium because the hypotonic environment causes rapid cell lysis and increased inflammation on replantation.33,34
Cell culture media in specialized transport containers such as Hank’s Balanced Salt Solution (HBSS) have shown superior ability to maintain the viability of the periodontal ligament fibers for extended periods.154 Presently they are considered impractical, since they need to be present at the accident site before the injury occurs, but if we consider that more than 60% of avulsion injuries occur close to home or school,74 it should be beneficial to have these media available in emergency kits at these sites. It would also be advantageous to have them in ambulances and in the kits of emergency response personnel who are likely to treat the more serious injuries where teeth might otherwise be sacrificed to a more serious life-threatening situation.
Management in the Dental Office
Emergency Visit
Prepare socket, prepare root, replant, construct a functional splint, and administer local and systemic antibiotics.
Recognizing that a dental injury might be secondary to a more serious injury is essential. The attending dental clinician is likely to be the first health care provider the patient sees after a head injury, so ruling out any injuries to the brain (e.g., concussion) and/or central nervous system (CNS) in general is paramount. If on examination a CNS injury is suspected, immediate referral to the appropriate expert is the first priority above and beyond the dental injury. Once a CNS injury has been ruled out, the focus of the emergency visit is the attachment apparatus. The aim is to replant the tooth with a minimum of irreversibly damaged cells (that will cause inflammation) and the maximal number of periodontal ligament cells that have the potential to regenerate and repair the damaged root surface.
Diagnosis and Treatment Planning
If the tooth was replanted at the site of injury, a complete history is taken to assess the likelihood of a favorable outcome. The position of the replanted tooth is assessed and adjusted if necessary. On rare occasions, the tooth may be gently removed to prepare the root to increase the chances of a favorable outcome (see later).
If the patient presents with the tooth out of the mouth, the storage medium should be evaluated and the tooth placed in a more appropriate medium if required. Hank’s Balanced Salt Solution is presently considered the best medium for this purpose. Milk or physiologic saline is also appropriate for storage purposes.
The medical and accident history is taken and a clinical exam carried out, with emphasis on questions about when, how, and where the injury occurred.
The clinical examination should include an examination of the socket to ascertain whether it is intact and suitable for replantation. The socket is gently rinsed with saline, and when cleared of the clot and debris, its walls are examined directly for the presence, absence, or collapse of the socket wall. The socket and surrounding areas including the soft tissues should be radiographed. Three vertical angulations are required for diagnosis of the presence of a horizontal root fracture in adjacent teeth.16 The remaining teeth in both the upper and lower jaws should be examined for injuries such as crown fractures. Any soft-tissue lacerations should be noted and if tooth fragments are missing, explored.
Preparation of the Root
Preparation of the root is dependent on the maturity of the tooth (open versus closed apex) and on the dry time of the tooth before it was placed in a storage medium. A dry time of 60 minutes is considered the point where survival of root periodontal ligament cells is unlikely.
Extraoral Dry Time Less Than 60 Minutes
Closed Apex
The root should be rinsed of debris with water or saline and replanted in as gentle a fashion as possible.
If the tooth has a closed apex, revascularization is not possible,46 but because the tooth was dry for less than 60 minutes (replanted or placed in appropriate medium), the chance for periodontal healing exists. Most importantly, the chance of a severe inflammatory response at the time of replantation is lessened. A dry time of less than 15 to 20 minutes is considered optimal, where periodontal healing would be expected.12,27,135
A continuing challenge is the treatment of the tooth that has been dry for more than 20 minutes (periodontal cell survival is assured) but less than 60 minutes (periodontal cell survival unlikely). In these cases, logic suggests that the root surface consists of some cells with the potential to regenerate and some that will act as inflammatory stimulators.
Open Apex
Gently rinse off debris, soak in doxycycline for 5 minutes or cover with minocycline, replant.
In an open-apex tooth, revascularization of the pulp as well as continued root development is possible (see Fig. 17-32). Investigators46 found in monkeys that soaking the tooth in doxycycline (1 mg in approximately 20 ml of physiologic saline) for 5 minutes before replantation significantly enhanced complete revascularization. This result was confirmed later in dogs by other investigators.116,165 A study found that covering the root with minocycline (Arestin, OraPharma, Inc, Warminster, PA), which attaches to the root for approximately 15 days, further increased the revascularization rate in dogs.116 Although animal studies do not provide us with a prediction of the rate of revascularization in humans, it is reasonable to expect that the same enhancement of revascularization that occurred in two animal species will occur in humans as well. As with the tooth with the closed apex, the open-apex tooth is then gently rinsed and replanted.
Extraoral Dry Time More Than 60 Minutes
Closed Apex
Remove the periodontal ligament by placing in acid for 5 minutes, soak in fluoride, replant.
When the root has been dry for 60 minutes or more, survival of the periodontal ligament cells is not expected.22,135 In such cases, the root should be prepared to be as resistant to resorption as possible (attempting to slow the osseous replacement process). These teeth should be soaked in acid for 5 minutes to remove all remaining periodontal ligament and thus remove the tissue that will initiate the inflammatory response on replantation. The tooth should then be soaked in 2% stannous fluoride for 5 minutes and replanted.32,126 A few years ago, studies were published that indicated that Emdogain (enamel matrix protein, Andover, MA) could be beneficial in teeth with extended extra oral dry times, not only to make the root more resistant to resorption but possibly to stimulate the formation of new periodontal ligament from the socket57,83 (see Fig. 17-8). Unfortunately, more recent studies have shown that the positive effect of Emdogain is only temporary, and most of these teeth start to resorb after a few years.121
If the tooth has been dry for more than 60 minutes and no consideration is given to preserving the periodontal ligament, the endodontics may be performed extraorally. In the case of a tooth with a closed apex, no advantage exists to this additional step at the emergency visit. However, in a tooth with an open apex, endodontic treatment performed after replantation involves a long-term apexification procedure. In these cases, completing the root canal treatment extraorally, where a seal in the blunderbuss apex is easier to achieve, may be advantageous. When endodontic treatment is performed extraorally, it must be performed aseptically with the utmost care to achieve a root canal system that is thoroughly disinfected.
Open Apex
Replant? If yes, treat as with closed-apex tooth. Endodontic treatment may be performed out of the mouth.
Since these teeth are in young patients in whom facial development is usually incomplete, many pediatric clinicians consider the prognosis to be so poor and the potential complications of an ankylosed tooth so severe, that they recommend these teeth not be replanted. Considerable debate exists as to whether it would be beneficial to replant the root even though it will inevitably be lost due to osseous replacement. If the patients are followed carefully and the root submerged by decoronation procedure at the appropriate time,6,56,60 the height, and more importantly the width, of the alveolar bone will be maintained, allowing for easier permanent restoration at the appropriate time when the child’s facial development is complete.
Preparation of the Socket
The socket should be left undisturbed before replantation.16 Emphasis is placed on removal of obstacles within the socket to facilitate replacement of the tooth into the socket.71 It should be lightly aspirated if a blood clot is present. If the alveolar bone has collapsed or may interfere with replantation, a blunt instrument should be inserted carefully into the socket in an attempt to reposition the wall.
Splinting
A splinting technique that allows physiologic movement of the tooth during healing and is in place for a minimal time period results in a decreased incidence of ankylosis.3,13,71 Semirigid (physiologic) fixation for 1 to 2 weeks is recommended.3,5,60 The splint should allow movement of the tooth, should have no memory (so the tooth is not moved during healing), and should not impinge on the gingiva and/or prevent maintenance of oral hygiene in the area. Many splints satisfy the requirements of an acceptable splint. A new titanium trauma splint (TTS) has recently been shown to be particularly effective and easy to use158 (Fig. 17-42). After the splint is in place, a radiograph should be exposed to verify the positioning of the tooth and as a preoperative reference for further treatment and follow-up. When the tooth is in the best possible position, adjusting the bite to ensure that it has not been splinted in a position causing traumatic occlusion is important. One week is sufficient to create periodontal support to maintain the avulsed tooth in position.152 Therefore, the splint should be removed after 1 to 2 weeks. The only exception is with avulsion in conjunction with alveolar fractures, for which 4 to 8 weeks is the suggested time of splinting.152
Management of the Soft Tissues
Soft-tissue lacerations of the socket gingiva should be tightly sutured. Lacerations of the lip are fairly common with these types of injuries. The clinician should approach lip lacerations with some caution; a plastic surgery consult might be prudent. If these lacerations are sutured, care must be taken to clean the wound thoroughly beforehand because dirt or even minute tooth fragments left in the wound affect healing and the esthetic result.
Adjunctive Therapy
Systemic antibiotics given at the time of replantation and prior to endodontic treatment are effective in preventing bacterial invasion of the necrotic pulp and therefore subsequent inflammatory resorption.72 Tetracycline has the additional benefit of decreasing root resorption by affecting the motility of the osteoclasts and reducing the effectiveness of collagenase.117 The administration of systemic antibiotics is recommended, beginning at the emergency visit and continuing until the splint is removed.72 For patients not susceptible to tetracycline staining, the antibiotic of choice is doxycycline twice daily for 7 days at the appropriate dosage for patient age and weight.117,118 Penicillin V 1000 mg as a loading dose, followed by 500 mg 4 times daily for 7 days has also been shown to be beneficial. The bacterial content of the sulcus also should be controlled during the healing phase. In addition to stressing to the patient the need for adequate oral hygiene, the use of chlorhexidine rinses for 7 to 10 days are helpful.
As stated previously, a recent series of studies by our research group found great benefit in removal of the pulp contents at the emergency visit and placing Ledermix or corticosteroid into the root canal.36,39 Apparently the use of the medicament was able to shut down the inflammatory response after replantation to allow for more favorable healing compared to those teeth that did not have the medicament.
The need for analgesics should be assessed on an individual case basis. The use of pain medication stronger than nonprescription nonsteroidal antiinflammatory drugs (NSAIDs) is unusual. The patient should be sent to a physician for consultation regarding a tetanus booster within 48 hours of the initial visit.
Second Visit
This visit should take place 1 to 2 weeks after the trauma. At the emergency visit, emphasis was placed on the preservation and healing of the attachment apparatus. The focus of this visit is the prevention or elimination of potential irritants from the root canal space. These irritants, if present, provide the stimulus for the progression of the inflammatory response, bone and root resorption. Also at this visit, the course of systemic antibiotics is completed; the chlorhexidine rinses can be stopped. At this appointment the splint is removed; the tooth might still have class I or class II mobility after splint removal, but all indications are that it will continue to heal better without the splint.3
Endodontic Treatment
Extraoral Time Less Than 60 Minutes
Closed Apex
Initiate endodontic treatment after 1 to 2 weeks. In cases where endodontic treatment is delayed or signs of resorption are present, treat with long-term calcium hydroxide treatment before obturation.
No chance exists for revascularization of these teeth, therefore endodontic treatment should be initiated at the second visit 7 to 10 days later.14,46 If therapy is initiated at this optimum time, the pulp should be necrotic without (or with minimal) infection.99,155 Endodontic therapy with an effective interappointment antibacterial agent155 over a relatively short period (1 to 2 weeks) is sufficient to ensure effective disinfection of the canal.130 Long-term calcium hydroxide treatment should always be considered when the injury occurred more than 2 weeks before initiation of the endodontic treatment or especially if radiographic evidence of resorption is present.155
The root canal is thoroughly cleaned and shaped, irrigated, and then filled with a thick (powdery) mix of calcium hydroxide and sterile saline (anesthetic solution is also an acceptable vehicle) (see Fig. 17-15). The canal is obturated when a radiographically intact periodontal membrane can be demonstrated around the root (see Fig. 17-34). Calcium hydroxide is an effective antibacterial agent37,130 and favorably influences the local environment at the resorption site, theoretically promoting healing.150 It also changes the environment in the dentin to a more alkaline pH, which may slow the action of the resorptive cells and promote hard-tissue formation.150 However, the changing of the calcium hydroxide should be kept to a minimum (not more than every 3 months) because it has a necrotizing effect on the cells attempting to repopulate the damaged root surface.95
Calcium hydroxide is considered the drug of choice in the prevention and treatment of inflammatory root resorption, but it is not the only medicament recommended in these cases. Some attempts have been made not only to remove the stimulus for the resorbing cells but also to affect them directly. The antibiotic-corticosteroid paste, Ledermix (DENTSPLY [Australia], Melbourne, Australia), is effective in treating inflammatory root resorption by inhibiting the spread of dentinoclasts111 without damaging the periodontal ligament; however, its ability to diffuse through human tooth root has been demonstrated,1 and its release and diffusion is enhanced when used in combination with calcium hydroxide paste.2
Open Apex
Avoid endodontic treatment, and look for signs of revascularization. At the first sign of an infected pulp, initiate apexification procedure.
Teeth with open apices have the potential to revascularize and continue root development; initial treatment is directed toward reestablishing blood supply46,116,164 (Fig. 17-43). The initiation of endodontic treatment is avoided if at all possible, unless definite signs of pulp necrosis (e.g., periradicular inflammation) are present. An accurate diagnosis of pulp vitality is extremely challenging in these cases. After trauma, a diagnosis of necrotic pulp is particularly undesirable, because infection in these teeth is potentially more harmful due to cemental damage accompanying the traumatic injury. External inflammatory root resorption can be extremely rapid in these young teeth, because the tubules are wide and allow irritants to move freely to the external surface of the root.46,164
FIG. 17-43 A, Avulsed tooth soaking in doxycycline. B, Minocycline powder placed on the root surface before replantation.
Patients are recalled every 3 to 4 weeks for pulp vitality testing. Studies indicate that thermal tests with carbon dioxide snow (−78° C) or difluorodichloromethane (−50° C) placed at the incisal edge or pulp horn are the best methods of sensitivity testing, particularly in young permanent teeth.64,65,108 One of these two tests must be included in the pulp vitality testing. Recent reports confirm the superiority of the laser Doppler flowmeter in the diagnosis of revascularization of traumatized immature teeth165; however, the cost of such an instrument precludes its use in the average dental office. Radiographic (apical breakdown and/or signs of lateral root resorption) and clinical (pain to percussion and palpation) signs of pulp pathosis are carefully assessed. At the first sign of pathosis, endodontic treatment should be initiated, and after disinfection of the root canal space an apexification procedure should be carried out.
Extraoral Time More Than 60 Minutes
Closed Apex
As with more than 60 minutes dry time.
These teeth are treated endodontically in the same way as those teeth that had an extraoral time of less than 60 minutes.
Open Apex (If Replanted)
If endodontic treatment was not performed out of the mouth, initiate apexification procedure.
The chance of revascularization in these teeth is extremely poor,153,156 so no attempt is made to revitalize them. An apexification procedure is initiated at the second visit if root canal treatment was not performed at the emergency visit. If endodontic treatment was performed at the emergency visit, the second visit is a recall visit to assess initial healing only.
Temporary Restoration
Effectively sealing the coronal access is essential to prevent infection of the canal between visits. Recommended temporary restorations are reinforced zinc oxide eugenol cement, acid etch composite resin, or glass ionomer cement. The depth of the temporary restoration is critical to its sealability. A depth of at least 4 mm is recommended, so a cotton pellet should not be placed; the temporary restoration is placed directly onto the calcium hydroxide in the access cavity. Calcium hydroxide should first be removed from the walls of the access cavity before placing the temporary restoration, because it is soluble and will wash out when it comes into contact with saliva, leaving a defective temporary restoration.
After initiation of the root canal treatment, the splint is removed. If time does not permit complete removal of the splint at this visit, the resin tacks are smoothed so as not to irritate the soft tissues; the remaining resin is removed at a later appointment.
At this appointment, healing is usually sufficient to perform a detailed clinical examination on the teeth surrounding the avulsed tooth. Pulp vitality tests, reaction to percussion and palpation, and periodontal probing measurements should be carefully recorded for reference at follow-up visits.
Root Filling Visit
At the clinician’s convenience or following long-term calcium hydroxide therapy, when an intact lamina dura is traced.
If the endodontic treatment was initiated 1 to 2 weeks after the avulsion and a thorough examination confirms normality, filling of the root canal at this visit is acceptable. Long-term use of calcium hydroxide is also a proven option for these cases. If endodontic treatment was initiated more than 2 weeks after the avulsion or active resorption is visible, the pulp space must first be disinfected before root filling. Traditionally, the reestablishment of a lamina dura (see Fig. 17-34) is a radiographic sign that the canal bacteria have been controlled. When an intact lamina dura can be traced throughout, root filling can take place.
The canal is cleaned, shaped, and irrigated under strict asepsis (i.e., a rubber dam). After completion of cleaning and shaping, the canal can be filled.
Permanent Restoration
Much evidence exists that coronal leakage caused by defective temporary and permanent restorations results in a clinically relevant amount of bacterial contamination of the root canal after root filling.115 Therefore, the tooth should receive a permanent restoration as soon as possible. The depth of restoration is important for a tight seal, so the deepest restoration possible should be made. A post should be avoided if possible. Because most avulsions occur in the anterior region of the mouth where esthetics is important, composite resins combined with dentin bonding agents are recommended in these cases.
Follow-Up Care
Follow-up evaluations should take place at 3 months, 6 months, and yearly for at least 5 years. If osseous replacement is identified (see Fig. 17-28), a more closely monitored follow-up schedule is indicated. In the case of inflammatory root resorption (see Fig. 17-30), a new attempt at disinfection of the root canal space by standard retreatment might reverse the process. Teeth adjacent to and surrounding the avulsed tooth or teeth may show pathologic changes long after the initial accident, so these teeth too should be tested at follow-up visits and the results compared to those collected soon after the accident.
1. Abbott PV, Heithersay GS, Hume WR. Release and diffusion through human tooth roots in vitro of corticosteroid and tetracycline trace molecules from Ledermix paste. Endod Dent Traumatol. 1988;4:55.
2. Abbott PV, Hume WR, Heithersay GS. Effects of combining Ledermix and calcium hydroxide pastes on the diffusion of corticosteroid and tetracycline through human tooth roots in vitro. Endod Dent Traumatol. 1989;5:188.
3. About I, Murray PE, Franquin JC, Remusat M, Smith AJ. The effect of cavity restoration variables on odontoblast cell numbers and dental repair. J Dent. 2001;29:109.
4. Accorinte Mde L, Holland R, Reis A, et al. Evaluation of mineral trioxide aggregate and calcium hydroxide cement as pulp-capping agents in human teeth. J Endod. 2008;34:1.
5. Andersson L, Friskopp J, Blomlof L. Fiber-glass splinting of traumatized teeth. ASDC J Dent Child. 1983;50:21.
6. Andersson L, Malmgren B. The problem of dentoalveolar ankylosis and subsequent replacement resorption in the growing patient. Aust Endod J. 1999;25:57.
7. Andreasen FM. Pulpal healing after luxation injuries and root fracture in the permanent dentition. Endod Dent Traumatol. 1989;5:111.
8. Andreasen FM, Andreasen JO. Resorption and mineralization processes following root fracture of permanent incisors. Endod Dent Traumatol. 1988;4:202.
9. Andreasen FM, Andreasen JO, Bayer T. Prognosis of root-fractured permanent incisors: prediction of healing modalities. Endod Dent Traumatol. 1989;5:11.
10. Andreasen FM, Pedersen BV. Prognosis of luxated permanent teeth–the development of pulp necrosis. Endod Dent Traumatol. 1985;1:207.
11. Andreasen FM, Zhijie Y, Thomsen BL, Andersen PK. Occurrence of pulp canal obliteration after luxation injuries in the permanent dentition. Endod Dent Traumatol. 1987;3:103.
12. Andreasen JO. Effect of extra-alveolar period and storage media upon periodontal and pulpal healing after replantation of mature permanent incisors in monkeys. Int J Oral Surg. 1981;10:43.
13. Andreasen JO. Etiology and pathogenesis of traumatic dental injuries. A clinical study of 1,298 cases. Scand J Dent Res. 1970;78:329.
14. Andreasen JO. Luxation of permanent teeth due to trauma. A clinical and radiographic follow-up study of 189 injured teeth. Scand J Dent Res. 1970;78:273.
15. Andreasen JO. Review of root resorption systems and models. Etiology of root resorption and the homeostatic mechanisms of the periodontal ligament. In: Davidovitch Z, editor. The biological mechanisms of tooth eruption and resorption. Birmingham, Ala: EB-SCOP Media, 1989.
16. Andreasen JO, Andreasen FM, Andersson L. Textbook and color atlas of traumatic injuries to the teeth, ed 4. Hoboken, NJ: Wiley-Blackwell; 2007.
17. Andreasen JO, Andreasen FM, Mejare I, Cvek M. Healing of 400 intra-alveolar root fractures. 1. Effect of pre-injury and injury factors such as sex, age, stage of root development, fracture type, location of fracture and severity of dislocation. Dent Traumatol. 2004;20:192.
18. Andreasen JO, Andreasen FM, Mejare I, Cvek M. Healing of 400 intra-alveolar root fractures. 2. Effect of treatment factors such as treatment delay, repositioning, splinting type and period and antibiotics. Dent Traumatol. 2004;20:203.
19. Andreasen JO, Farik B, Munksgaard EC. Long-term calcium hydroxide as a root canal dressing may increase risk of root fracture. Dent Traumatol. 2002;18:134.
20. Andreasen JO, Hjorting-Hansen E. Intraalveolar root fractures: radiographic and histologic study of 50 cases. J Oral Surg. 1967;25:414.
21. Andreasen JO, Hjorting-Hansen E. Replantation of teeth. I. Radiographic and clinical study of 110 human teeth replanted after accidental loss. Acta Odontol Scand. 1966;24:263.
22. Andreasen JO, Kristerson L. The effect of limited drying or removal of the periodontal ligament. Periodontal healing after replantation of mature permanent incisors in monkeys. Acta Odontol Scand. 1981;39:1.
23. Andreasen JO, Ravn JJ. Epidemiology of traumatic dental injuries to primary and permanent teeth in a Danish population sample. Int J Oral Surg. 1972;1:235.
24. Arakawa M, Kitasako Y, Otsuki M, Tagami J. Direct pulp capping with an auto-cured sealant resin and a self-etching primer. Am J Dent. 2003;16:61.
25. Banchs F, Trope M. Revascularization of immature permanent teeth with apical periodontitis: new treatment protocol? J Endod. 2004;30:196.
26. Barrett AP, Reade PC. Revascularization of mouse tooth isografts and allografts using autoradiography and carbon-perfusion. Arch Oral Biol. 1981;26:541.
27. Barrett EJ, Kenny DJ. Avulsed permanent teeth: a review of the literature and treatment guidelines. Endod Dent Traumatol. 1997;13:153.
28. Bastone EB, Freer TJ, McNamara JR. Epidemiology of dental trauma: a review of the literature. Aust Dent J. 2000;45:2.
29. Bergenholtz G. Micro-organisms from necrotic pulp of traumatized teeth. Odontol Revy. 1974;25:347.
30. Bhaskar SN, Rappaport HM. Dental vitality tests and pulp status. J Am Dent Assoc. 1973;86:409.
31. Binnie WH, Rowe AH. A histological study of the periapical tissues of incompletely formed pulpless teeth filled with calcium hydroxide. J Dent Res. 1973;52:1110.
32. Bjorvatn K, Selvig KA, Klinge B. Effect of tetracycline and SnF2 on root resorption in replanted incisors in dogs. Scand J Dent Res. 1989;97:477.
33. Blomlof L. Milk and saliva as possible storage media for traumatically exarticulated teeth prior to replantation. Swed Dent J Suppl. 1981;8:1.
34. Blomlof L, Lindskog S, Andersson L, Hedstrom KG, Hammarstrom L. Storage of experimentally avulsed teeth in milk prior to replantation. J Dent Res. 1983;62:912.
35. Bortoluzzi EA, Broon NJ, Bramante CM, et al. Mineral trioxide aggregate with or without calcium chloride in pulpotomy. J Endod. 2008;34:172.
36. Bryson EC, Levin L, Banchs F, Abbott PV, Trope M. Effect of immediate intracanal placement of Ledermix Paste(R) on healing of replanted dog teeth after extended dry times. Dent Traumatol. 2002;18:316.
37. Bystrom A, Claesson R, Sundqvist G. The antibacterial effect of camphorated paramonochlorophenol, camphorated phenol and calcium hydroxide in the treatment of infected root canals. Endod Dent Traumatol. 1985;1:170.
38. Canakci V, Akgul HM, Akgul N, Canakci CF. Prevalence and handedness correlates of traumatic injuries to the permanent incisors in 13 17-year-old adolescents in Erzurum, Turkey. Dent Traumatol. 2003;19:248.
39. Chen H, Teixeira FB, Ritter AL, Levin L, Trope M. The effect of intracanal anti-inflammatory medicaments on external root resorption of replanted dog teeth after extended extra-oral dry time. Dental Traumatol. 2008;24:74.
40. Cox CF, Keall CL, Keall HJ, Ostro E, Bergenholtz G. Biocompatibility of surface-sealed dental materials against exposed pulps. J Prosthet Dent. 1987;57:1.
41. Cox CF, Tarim B, Kopel H, et al. Technique sensitivity: biological factors contributing to clinical success with various restorative materials. Adv Dent Res. 2001;15:85.
42. Cunha RF, Pavarini A, Percinoto C, et al. Influence of surgical repositioning of mature permanent dog teeth following experimental intrusion: a histologic assessment. Dent Traumatol. 2002;18:304.
43. Cvek M. A clinical report on partial pulpotomy and capping with calcium hydroxide in permanent incisors with complicated crown fracture. J Endod. 1978;4:232.
44. Cvek M. Prognosis of luxated non-vital maxillary incisors treated with calcium hydroxide and filled with gutta-percha. A retrospective clinical study. Endod Dent Traumatol. 1992;8:45.
45. Cvek M. Treatment of non-vital permanent incisors with calcium hydroxide. IV. Periodontal healing and closure of the root canal in the coronal fragment of teeth with intra-alveolar fracture and vital apical fragment. A follow-up. Odontol Revy. 1974;25:239.
46. Cvek M, Cleaton-Jones P, Austin J, Lownie J, Kling M, Fatti P. Effect of topical application of doxycycline on pulp revascularization and periodontal healing in reimplanted monkey incisors. Endod Dent Traumatol. 1990;6:170.
47. Cvek M, Cleaton-Jones PE, Austin JC, Andreasen JO. Pulp reactions to exposure after experimental crown fractures or grinding in adult monkeys. J Endod. 1982;8:391.
48. Cvek M, Hollender L, Nord CE. Treatment of non-vital permanent incisors with calcium hydroxide. VI. A clinical, microbiological and radiological evaluation of treatment in one sitting of teeth with mature or immature root. Odontol Revy. 1976;27:93.
49. Cvek M, Nord CE, Hollender L. Antimicrobial effect of root canal debridement in teeth with immature root. A clinical and microbiologic study. Odontol Revy. 1976;27:1.
50. Da Silva AC, Passeri LA, Mazzonetto R, et al. Incidence of dental trauma associated with facial trauma in Brazil: a 1-year evaluation. Dent Traumatol. 2004;20:6.
51. Deutsch AS, Musikant BL, Cavallari J, et al. Root fracture during insertion of prefabricated posts related to root size. J Prosthet Dent. 1985;53:786.
52. Ebeleseder KA, Santler G, Glockner K, et al. An analysis of 58 traumatically intruded and surgically extruded permanent teeth. Endod Dent Traumatol. 2000;16:34.
53. Eklund G, Stalhane I, Hedegard B. A study of traumatized permanent teeth in children aged 7-15 years. Part III. A multivariate analysis of post-traumatic complications of subluxated and luxated teeth. Sven Tandlak Tidskr. 1976;69:179.
54. Ersin NK, Eronat N. The comparison of a dentin adhesive with calcium hydroxide as a pulp-capping agent on the exposed pulps of human and sheep teeth. Quintessence Int. 2005;36:271.
55. Faraco IMJr, Holland R. Response of the pulp of dogs to capping with mineral trioxide aggregate or a calcium hydroxide cement. Dent Traumatol. 2001;17:163.
56. Filippi A, Pohl Y, von Arx T. Decoronation of an ankylosed tooth for preservation of alveolar bone prior to implant placement. Dent Traumatol. 2001;17:93.
57. Filippi A, Pohl Y, von Arx T. Treatment of replacement resorption with Emdogain preliminary results after 10 months. Dent Traumatol. 2001;17:134.
58. Flores MT, Malmgren B, Andersson L, et al. Guidelines for the management of traumatic dental injuries. I. Fractures and luxations of permanent teeth. Dent Traumatol. 2007;23:66.
59. Flores MT, Malmgren B, Andersson L, et al. Guidelines for the management of traumatic dental injuries. II. Avulsion of permanent teeth. Dent Traumatol. 2007;23:130.
60. Flores MT, Malmgren B, Andersson L, et al. Guidelines for the management of traumatic dental injuries. III. Primary teeth. Dent Traumatol. 2007;23:196.
61. Frank AL. Therapy for the divergent pulpless tooth by continued apical formation. J Am Dent Assoc. 1966;72:87.
62. Fuks AB, Bielak S, Chosak A. Clinical and radiographic assessment of direct pulp capping and pulpotomy in young permanent teeth. Pediatr Dent. 1982;4:240.
63. Fuks AB, Cosack A, Klein H, Eidelman E. Partial pulpotomy as a treatment alternative for exposed pulps in crown-fractured permanent incisors. Endod Dent Traumatol. 1987;3:100.
64. Fulling HJ, Andreasen JO. Influence of maturation status and tooth type of permanent teeth upon electrometric and thermal pulp testing. Scand J Dent Res. 1976;84:286.
65. Fuss Z, Trowbridge H, Bender IB, Rickoff B, Sorin S. Assessment of reliability of electrical and thermal pulp testing agents. J Endod. 1986;12:301.
66. Gazelius B, Olgart L, Edwall B. Restored vitality in luxated teeth assessed by laser Doppler flowmeter. Endod Dent Traumatol. 1988;4:265.
67. Gelbier MJ, Winter GB. Traumatised incisors treated by vital pulpotomy: a retrospective study. Br Dent J. 1988;164:319.
68. Giuliani V, Baccetti T, Pace R, Pagavino G. The use of MTA in teeth with necrotic pulps and open apices. Dent Traumatol. 2002;18:217.
69. Goldberg F, Kaplan A, Roitman M, Manfre S, Picca M. Reinforcing effect of a resin glass ionomer in the restoration of immature roots in vitro. Dent Traumatol. 2002;18:70.
70. Granath LE, Hagman G. Experimental pulpotomy in human bicuspids with reference cutting technique. Acta Odontol Scand. 1971;29:155.
71. Hammarstrom L, Lindskog S. General morphological aspects of resorption of teeth and alveolar bone. Int Endod J. 1985;18:93.
72. Hammarstrom L, Pierce A, Blomlof L, Feiglin B, Lindskog S. Tooth avulsion and replantation–a review. Endod Dent Traumatol. 1986;2:1.
73. Hebling J, Giro EM, Costa CA. Biocompatibility of an adhesive system applied to exposed human dental pulp. J Endodon. 1999;25:676.
74. Hedegard B, Stalhane I. A study of traumatized permanent teeth in children 7-15 years. I. Sven Tandlak Tidskr. 1973;66:431.
75. Heide S, Mjor IA. Pulp reactions to experimental exposures in young permanent monkey teeth. Int Endod J. 1983;16:11.
76. Heithersay GS. Calcium hydroxide in the treatment of pulpless teeth with associated pathology. J Br Endod Soc. 1962;8:74.
77. Heller AL, Koenigs JF, Brilliant JD, Melfi RC, Driskell TD. Direct pulp capping of permanent teeth in primates using a resorbable form of tricalcium phosphate ceramic. J Endod. 1975;1:95.
78. Herforth A, Strassburg M. [Therapy of chronic apical periodontitis in traumatically injuring front teeth with ongoing root growth.]. Dtsch Zahnarztl Z. 1977;32:453.
79. Hiltz J, Trope M. Vitality of human lip fibroblasts in milk, Hanks balanced salt solution and Viaspan storage media. Endod Dent Traumatol. 1991;7:69.
80. Holland R, Bisco Ferreira L, de Souza V, Otoboni Filho JA, Murata SS, Dezan E. Reaction of the lateral periodontium of dogs’ teeth to contaminated and noncontaminated perforations filled with mineral trioxide aggregate. J Endod. 2007;33:1192.
81. Holloway GAJr, Watkins DW. Laser Doppler measurement of cutaneous blood flow. J Invest Dermatol. 1977;69:306.
82. Hoshino E, Kurihara-Ando N, Sato I, et al. In-vitro antibacterial susceptibility of bacteria taken from infected root dentine to a mixture of ciprofloxacin, metronidazole and minocycline. Int Endod J. 1996;29:125.
83. Iqbal MK, Bamaas N. Effect of enamel matrix derivative (Emdogain) upon periodontal healing after replantation of permanent incisors in beagle dogs. Dent Traumatol. 2001;17:36.
84. Iwamoto CE, Erika A, Pameijer CH, Barnes D, Romberg EE, Jefferies S. Clinical and histological evaluation of white ProRoot MTA in direct pulp capping. Am J Dent. 2006;19:85.
85. Jacobsen I. Root fractures in permanent anterior teeth with incomplete root formation. Scand J Dent Res. 1976;84:210.
86. Jacobsen I, Kerekes K. Diagnosis and treatment of pulp necrosis in permanent anterior teeth with root fracture. Scand J Dent Res. 1980;88:370.
87. Jacobsen I, Zachrisson BU. Repair characteristics of root fractures in permanent anterior teeth. Scand J Dent Res. 1975;83:355.
88. Jarvinen S. Fractured and avulsed permanent incisors in Finnish children. A retrospective study. Acta Odontol Scand. 1979;37:47.
89. Jung IY, Lee SJ, Hargreaves KM. Biologically based treatment of immature permanent teeth with pulpal necrosis: a case series. J Endod. 2008;34:876.
90. Kakehashi S, Stanley HR, Fitzgerald RJ. The effect of surgical exposures on dental pulps in germ-free and conventional laboratory rats. Oral Surg. 1965;20:340.
91. Katebzadeh N, Dalton BC, Trope M. Strengthening immature teeth during and after apexification. J Endod. 1998;24:256.
92. Kenny DJ, Barrett EJ, Casas MJ. Avulsions and intrusions: the controversial displacement injuries. J Can Dent Assoc. 2003;69:308.
93. Kerekes K, Heide S, Jacobsen I. Follow-up examination of endodontic treatment in traumatized juvenile incisors. J Endod. 1980;6:744.
94. Koliniotou-Koumpia E, Tziafas D. Pulpal responses following direct pulp capping of healthy dog teeth with dentine adhesive systems. J Dent. 2005;33:639.
95. Lengheden A, Blomlof L, Lindskog S. Effect of delayed calcium hydroxide treatment on periodontal healing in contaminated replanted teeth. Scand J Dent Res. 1991;99:147.
96. Lindskog S, Pierce AM, Blomlof L, Hammarstrom L. The role of the necrotic periodontal membrane in cementum resorption and ankylosis. Endod Dent Traumatol. 1985;1:96.
97. Love RM. Bacterial penetration of the root canal of intact incisor teeth after a simulated traumatic injury. Endod Dent Traumatol. 1996;12:289.
98. Love RM, Ponnambalam Y. Dental and maxillofacial skeletal injuries seen at the University of Otago School of Clinicianry, New Zealand 2000-2004. Dent Traumatol. 2008;24:170.
99. Lundin SA, Noren JG, Warfvinge J. Marginal bacterial leakage and pulp reactions in Class II composite resin restorations in vivo. Swed Dent J. 1990;14:185.
100. Maroto M, Barberia E, Planells P, Vera V. Treatment of a non-vital immature incisor with mineral trioxide aggregate (MTA). Dent Traumatol. 2003;19:165.
101. Masterton JB. The healing of wounds of the dental pulp. An investigation of the nature of the scar tissue and of the phenomena leading to its formation. Dent Pract Dent Rec. 1966;16:325.
102. Mejare I, Hasselgren G, Hammarstrom LE. Effect of formaldehyde-containing drugs on human dental pulp evaluated by enzyme histochemical technique. Scand J Dent Res. 1976;84:29.
103. Mesaros S, Trope M, Maixner W, Burkes EJ. Comparison of two laser Doppler systems on the measurement of blood flow of premolar teeth under different pulpal conditions. Int Endod J. 1997;30:167.
104. Myers WC, Fountain SB. Dental pulp regeneration aided by blood and blood substitutes after experimentally induced periapical infection. Oral Surg Oral Med Oral Pathol. 1974;37:441.
105. Murray PE, Smith AJ, Windsor LJ, Mjor IA. Remaining dentine thickness and human pulp responses. Int Endod J. 2003;33:36.
106. Nair PN, Duncan HF, Pitt Ford TR, Luder HU. Histological, ultrastructural and quantitative investigations on the response of healthy human pulps to experimental capping with mineral trioxide aggregate: a randomized controlled trial. Int Endod J. 2008;41:128.
107. Ohman A. Healing and sensitivity to pain in young replanted human teeth. An experimental, clinical and histological study. Odontol Tidskr. 1965;73:166.
108. Ohman A. Healing and sensitivity to pain in young replanted human teeth: an experimental and histologic study. Odontol Tidskr. 1965;73:166.
109. Parirokh M, Kakoei S. Vital pulp therapy of mandibular incisors: a case report with 11-year follow up. Aust Endod J. 2006;32:75.
110. Perez AL, Spears R, Gutmann JL, Opperman LA. Osteoblasts and MG-63 osteosarcoma cells behave differently when in contact with ProRoot MTA and White MTA. Int Endod J. 2003;36:564.
111. Pierce A, Lindskog S. The effect of an antibiotic/corticosteroid paste on inflammatory root resorption in vivo. Oral Surg Oral Med Oral Pathol. 1987;64:216.
112. Pileggi R, Dumsha TC, Myslinksi NR. The reliability of electric pulp test after concussion injury. Endod Dent Traumatol. 1996;12:16.
113. Rabie G, Barnett F, Tronstad L. Long-term splinting of maxillary incisor with intra alveolar root fracture. Endod Dent Traumatol. 1988;4:99.
114. Ravn JJ. Follow-up study of permanent incisors with complicated crown fractures after acute trauma. Scand J Dent Res. 1982;90:363.
115. Ray HA, Trope M. Periapical status of endodontically treated teeth in relation to the technical quality of the root filling and the coronal restoration. Int Endod J. 1995;28:12.
116. Ritter AL, Ritter AV, Murrah V, Sigurdsson A, Trope M. Pulp revascularization of replanted immature dog teeth after treatment with minocycline and doxycycline assessed by laser Doppler flowmetry, radiography, and histology. Dent Traumatol. 2004;20:75.
117. Sae-Lim V, Wang CY, Choi GW, Trope M. The effect of systemic tetracycline on resorption of dried replanted dogs’ teeth. Endod Dent Traumatol. 1998;14:127.
118. Sae-Lim V, Wang CY, Trope M. Effect of systemic tetracycline and amoxicillin on inflammatory root resorption of replanted dogs’ teeth. Endod Dent Traumatol. 1998;14:216.
119. Saroglu I, Sonmez H. The prevalence of traumatic injuries treated in the pedodontic clinic of Ankara University, Turkey, during 18 months. Dent Traumatol. 2002;18:299.
120. Sawicki L, Pameijer CH, Emerich K, Adamowicz-Klepalska B. Histological evaluation of mineral trioxide aggregate and calcium hydroxide in direct pulp capping of human immature permanent teeth. Am J Dent. 2008;21:262.
121. Schjott M, Andreasen JO. Emdogain does not prevent progressive root resorption after replantation of avulsed teeth: a clinical study. Dental Traumatol. 2005;21:46.
122. Schroder U. Reaction of human dental pulp to experimental pulpotomy and capping with calcium hydroxide (Thesis). Odont Revy. 1973;24(Suppl 25):97.
123. Schroder U, Granath LE. Early reaction of intact human teeth to calcium hydroxide following experimental pulpotomy and its significance to the development of hard tissue barrier. Odontol Revy. 1971;22:379.
124. Seltzer S. Endodontology. Philadelphia: Lea & Febiger; 1988.
125. Seltzer S, Bender IB, Turkenkopf S. Factors affecting successful repair after root canal therapy. J Am Dent Assoc. 1963;52:651.
126. Selvig KA, Zander HA. Chemical analysis and microradiography of cementum and dentin from periodontically diseased human teeth. J Periodont. 1962;33:103.
127. Shuping GB, Orstavik D, Sigurdsson A, Trope M. Reduction of intracanal bacteria using nickel-titanium rotary instrumentation and various medications. J Endod. 2000;26:751.
128. Silva GA, Lanza LD, Lopes-Junior N, Moreira A, Alves JB. Direct pulp capping with a dentin bonding system in human teeth: a clinical and histological evaluation. Oper Dent. 2006;31:297.
129. Silverman S. The dental structures in primary hyperparathyroidism. Oral Surg. 1962;15:426.
130. Sjogren U, Figdor D, Spangberg L, Sundqvist G. The antimicrobial effect of calcium hydroxide as a short-term intracanal dressing. Int Endod J. 1991;24:119.
131. Sjogren U, Hagglund B, Sundqvist G, Wing K. Factors affecting the long-term results of endodontic treatment. J Endod. 1990;16:498.
132. Skaare AB, Jacobsen I. Dental injuries in Norwegians aged 7-18 years. Dent Traumatol. 2003;19:67.
133. Skieller V. The prognosis for young teeth loosened after mechanical injuries. Acta Odontol Scand. 1960;18:171.
134. Skoglund A, Tronstad L. Pulpal changes in replanted and autotransplanted immature teeth of dogs. J Endod. 1981;7:309.
135. Soder PO, Otteskog P, Andreasen JO, Modeer T. Effect of drying on viability of periodontal membrane. Scand J Dent Res. 1977;85:164.
136. Spangberg L, Rutberg M, Rydinge E. Biologic effects of endodontic antimicrobial agents. J Endod. 1979;5:166.
137. Stalhane I, Hedegard B. Traumatized permanent teeth in children aged 7-15 years. Sven Tandlak Tidskr. 1975;68:157.
138. Stanley HR. Diseases of the dental pulp. In: Tieck RW, editor. Oral pathology. New York: McGraw-Hill Company, 1965.
139. Stanley HR, Lundy T. Dycal therapy for pulp exposures. Oral Surg Oral Med Oral Pathol. 1972;34:818.
140. Subay RK, Demirci M. Pulp tissue reactions to a dentin bonding agent as a direct capping agent. J Endod. 2005;31:201.
141. Swift EJJr, Trope M. Treatment options for the exposed vital pulp. Pract Periodontics Aesthet Dent. 1999;11:735.
142. Tapias MA, Jimenez-Garcia R, Lamas F, Gil AA. Prevalence of traumatic crown fractures to permanent incisors in a childhood population: Mostoles, Spain. Dent Traumatol. 2003;19:119.
143. Teixeira FB, Teixeira EC, Thompson JY, Trope M. Fracture resistance of roots endodontically treated with a new resin filling material. J Am Dent Assoc. 2004;135:646.
144. Thibodeau B, Teixeira F, Yamauchi M, Caplan DJ, Trope M. Pulp revascularization of immature dog teeth with apical periodontitis. J Endod. 2007;33:680.
145. Torabinejad M, Hong CU, McDonald F, Pitt Ford TR. Physical and chemical properties of a new root-end filling material. J Endod. 1995;21:349.
146. Torabinejad M, Rastegar AF, Kettering JD, Pitt Ford TR. Bacterial leakage of mineral trioxide aggregate as a root-end filling material. J Endod. 1995;21:109.
147. Trabert KC, Caput AA, Abou-Rass M. Tooth fracture–a comparison of endodontic and restorative treatments. J Endod. 1978;4:341.
148. Tronstad L. Reaction of the exposed pulp to Dycal treatment. Oral Surg Oral Med Oral Pathol. 1974;38:945.
149. Tronstad L. Root resorption–etiology, terminology and clinical manifestations. Endod Dent Traumatol. 1988;4:241.
150. Tronstad L, Andreasen JO, Hasselgren G, Kristerson L, Riis I. pH changes in dental tissues after root canal filling with calcium hydroxide. J Endod. 1980;7:17.
151. Tronstad L, Mjor IA. Capping of the inflamed pulp. Oral Surg Oral Med Oral Pathol. 1972;34:477.
152. Trope M. Clinical management of the avulsed tooth: present strategies and future directions. Dent Traumatol. 2002;18:1.
153. Trope M. Root resorption of dental and traumatic origin: classification based on etiology. Pract Periodontics Aesthet Dent. 1998;10:515.
154. Trope M, Friedman S. Periodontal healing of replanted dog teeth stored in Viaspan, milk and Hank’s balanced salt solution. Endod Dent Traumatol. 1992;8:183.
155. Trope M, Moshonov J, Nissan R, Buxt P, Yesilsoy C. Short vs. long-term calcium hydroxide treatment of established inflammatory root resorption in replanted dog teeth. Endod Dent Traumatol. 1995;11:124.
156. Trope M, Yesilsoy C, Koren L, Moshonov J, Friedman S. Effect of different endodontic treatment protocols on periodontal repair and root resorption of replanted dog teeth. J Endod. 1992;18:492.
157. Turley PK, Joiner MW, Hellstrom S. The effect of orthodontic extrusion on traumatically intruded teeth. Am J Orthod. 1984;85:47.
158. von Arx T, Filippi A, Buser D. Splinting of traumatized teeth with a new device: TTS (Titanium Trauma Splint). Dent Traumatol. 2001;17:180.
159. Wedenberg C, Lindskog S. Experimental internal resorption in monkey teeth. Endod Dent Traumatol. 1985;1:221.
160. Wedenberg C, Zetterqvist L. Internal resorption in human teeth–a histological, scanning electron microscopic, and enzyme histochemical study. J Endod. 1987;13:255.
161. Weiss M. Pulp capping in older patients. N Y State Dent J. 1966;32:451.
162. Windley W, Teixeira F, Levin L, Sigurdsson A, Trope M. Disinfection of immature teeth with a triple antibiotic paste. J Endod. 2005;31:439.
163. Witherspoon DE, Small JC, Harris GZ. Mineral trioxide aggregate pulpotomies: a case series outcomes assessment. J Am Dent Assoc. 2006;137:610.
164. Yanpiset K, Trope M. Pulp revascularization of replanted immature dog teeth after different treatment methods. Endod Dent Traumatol. 2000;16:211.
165. Yanpiset K, Vongsavan N, Sigurdsson A, Trope M. Efficacy of laser Doppler flowmetry for the diagnosis of revascularization of reimplanted immature dog teeth. Dent Traumatol. 2001;17:63.
166. Zachrisson BU, Jacobsen I. Long-term prognosis of 66 permanent anterior teeth with root fracture. Scand J Dent Res. 1975;83:345.