Chapter 12 The fetus
The midwife’s role in embryological and fetal development is focused on health education for maternal and fetal well-being. This involves providing parents with information about the effects of maternal lifestyle, such as diet, smoking, alcohol, drugs and exercise, on fetal growth and development (see Ch. 13). Additionally, an understanding of fetal development is of value when a baby is born before term.
Time scale of development
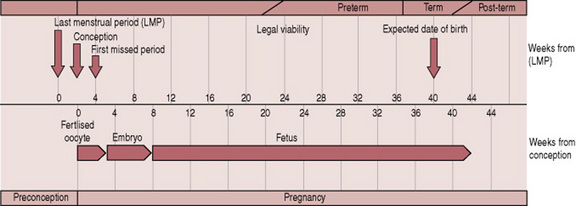
Embryological development is complex and occurs from weeks 2–8; and includes the development of the zygote in the first 2–3 weeks after fertilization. Fetal development occurs from week 8 until birth. The interval from the beginning of the last menstrual period (LMP) until fertilization is not part of pregnancy. However, this period is important for the calculation of the expected date of birth. Figure 12.1 illustrates the comparative lengths of these prenatal events.
A summary of embryological and fetal development categorized into 4-week periods is provided in Box. 12.1. This should be used to complement the text below.
Fetal growth and maturation
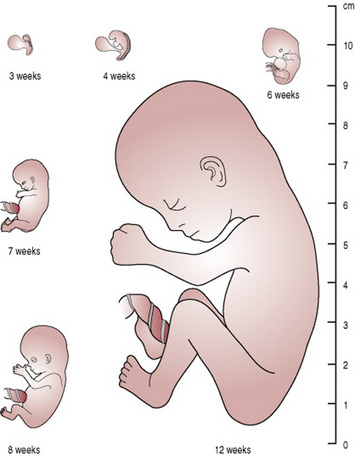
From the 9th week, fetal growth is rapid. Tissues grow by cell proliferation, cell enlargement and accretion of extracellular material. An adequate supply of nutrients and oxygen from the placenta to the fetus is crucial for growth. In developed countries the average birth weight is around 3400 g and half of this is reached by 30 weeks’ gestation. The fetus gains approximately 25 g/day between weeks 32 and 40. A visual representation of growth in terms of height is provided in Figure 12.2.
As fetal growth is an indicator of fetal health and well-being, monitoring of growth is crucial. This is done by visual observation of the uterus for size, fundal height measurements and ultrasonography.
The cardiovascular system
The early development of the cardiovascular system in the 3rd week coincides with the lack of yolk sac, and the urgent need to supply the growing embryo with oxygen and nutrients from the maternal blood through the placenta.
The cardiovascular system is the first to function in the embryo. The heart and vascular system commences development in the 3rd week, and by the 4th week a primitive heart is visible and is beginning to function, beating at around 22 days. Blood is pumped around the vessels from the 4th week. The first signs of the heart are the appearance of paired endothelial strands in the cardiogenic mesoderm, which canalize to become heart tubes and then fuse to become a tubular heart.
By the 4th week, three paired veins drain into the heart. The vitelline veins return poorly oxygenated blood from the yolk sac. The hepatic veins and the portal vein develop from the vitelline veins and their networks. The umbilical vein carries oxygenated blood from the placenta to the embryo. A structure called the ductus venosus develops to connect the umbilical vein to the inferior vena cava. The common cardinal veins return poorly oxygenated blood from the embryo. These veins constitute the main venous drainage system of the embryo, draining the cranial and caudal parts of the embryo, respectively.
There are three phases of red blood cell formation: the yolk sac period from weeks 3–13; the hepatic/liver period from weeks 5–36; and the bone marrow period from weeks 12 throughout life. Red blood cells known as erythrocytes produced by the yolk sac and liver contain fetal haemoglobin. Fetal haemoglobin (HbF) has a much greater affinity for oxygen and is found in greater concentrations (18–20 g/dL at term) than adult haemoglobin (HbA), thus enhancing the transfer of oxygen across the placental site. Fetal erythrocytes have a life span of 90 days, shorter than adult erythrocytes, which is around 120 days. The short life span of fetal erythrocytes contributes to neonatal physiological jaundice (see Ch. 47). Genes passed from both parents determine the fetal blood group and Rhesus factor.
The respiratory system
The development of the respiratory system begins in the 4th week. The lower respiratory tract and lungs develop simultaneously. The lungs originate from a bud growing out of the pharynx, which sub-divides again and again to form the branching structure of the bronchial tree. Lung development occurs on several levels and continues after birth until about 8 years of age when the full number of bronchioles and alveoli will have developed. The development of type II alveolar cells commences around 20 weeks. These cells are necessary for the production of surfactant, a lipoprotein that reduces the surface tension in the alveoli and assists gaseous exchange. The amount of surfactant increases until the lungs are mature at 30–34 weeks.
There is some movement of the thorax from the 3rd month of fetal life and more definite diaphragmatic movements from the 6th month. This does not constitute breathing as gaseous exchange is via the placenta.
At term, the lungs contain about 100 mL of lung fluid. About one-third of this is expelled during birth and the rest is absorbed and carried away by the lymphatics and blood vessels as air takes its place.
Babies born before 24 weeks’ gestation have a reduced chance of survival owing to the immaturity of the capillary system in the lungs and the lack of surfactant (see Ch. 44).
The urogenital system
The urogenital system is divided functionally into the urinary/renal system and the genital/reproductive system. Both systems develop from the intermediate mesoderm.
The kidneys develop from the 4th week and produce small amounts of urine from the 9th week. They become more functional around the 15th week when more urine is produced. The urine does not constitute a route for excretion as elimination of waste products is via the placenta. The urine forms much of the amniotic fluid and production increases with maturity.
The superior vesical arteries arise from the first few centimetres of the hypogastric arteries, which lead to the umbilical arteries. A single umbilical artery at birth is suggestive of abnormalities of the renal tract (see Ch. 46).
The sex of the embryo is determined at fertilization. The gonads develop in the 5th week from intermediate mesoderm. In the two sexes, genital development is similar and is referred to as the indifferent state of sexual development. Differentiation occurs from the 7th week, but female gonad development occurs slowly and the ovaries may not be identifiable until the 10th week. External genitalia in both sexes develops in the 9th week, but males and females are not distinguishable until about the 12th week.
The endocrine system
The adrenal glands develop from mesoderm and neural crest cells from the 6th week, and grow to 10–20 times larger than the adult adrenals. Their size regresses during the first year of life. They produce the precursors for placental formation of oestriols. They influence maturation of the lungs, liver and epithelium of the digestive tract. Also, it is thought that they play a part in the initiation of labour; the exact mechanism is not fully understood (Johnson & Everitt 2000).
The digestive system
The primitive gut develops from the endodermal layer of the yolk sac in the 4th week. It starts as a straight tube, and proceeds on several levels: foregut; midgut and hindgut. By the 5th week, the foregut (oesophagus, stomach and duodenum) is visible. The liver, gallbladder and pancreas bud form the gut tube around the 4th–5th week. The liver grows rapidly and from the 5th–10th week fills much of the abdominal cavity. It is responsible for about 10% of fetal weight by the 9th week. Towards the end of pregnancy, iron stores are laid down in the liver. The liver cells produce bile from the 12th week. The midgut (small intestine, caecum and vermiform appendix, ascending colon and transverse colon) undertakes much of its development in the 6th week, while the hindgut (rectum and anal canal) completes its development in the 7th week.
Around 12 weeks, the digestive tract is well formed and the lumen is patent. Most digestive juices are present before birth and act on the swallowed substances to form meconium. Bile enters the duodenum from the bile duct during the 13th week, giving the intestinal contents a dark green colour. Meconium is normally retained in the gut until after birth when it is passed as the first stool of the newborn.
Insulin is secreted from 10 weeks and glucagon from 15 weeks; both increase steadily with increasing fetal age.
The nervous system
The brain begins to develop from around day 19 and three structures are visible: forebrain, midbrain and hindbrain. By the 5th week the major regions, the thalamus and the hypothalamus are differentiated.
The neural tube is derived from the ectoderm. The ectoderm folds inwards by a complicated process to form the neural tube, which is then covered over by skin. Closure of the neural tube is essential and takes place by 26 days. This process is occasionally incomplete, leading to open neural tube defects (see Ch. 46).
The development of the sense organs, including the transmission of sensory input to the brain and output from the brain occurs under complex processes. The eyes and ears are associated with the development of the head and neck, which begin early and continues until the cessation of growth in the late teens. Although the eyes develop from around 22 days, for normal vision to occur many complex structures within the eye must properly relate to neighbouring structures. The eye is complete by 20 weeks but the eyelids are fused until around the 6th month. The developing eyes are sensitive to light.
The inner ear, which contains the structures for hearing and balance, commences early but is not complete until around 25 weeks.
Motor output controlled by the basal ganglia in the form of movement begins around 8 weeks. These movements are not usually felt by the mother until around 16 weeks, and are referred to as quickening. As the nervous system matures fetal behaviour becomes more complex and more defined. The fetus develops behavioral patterns: sleep with no eye or body movements; sleep with periodic eye and body movements known as REM sleep; wakefulness with subtle eye and limb movements; active phase with vigorous eye and limb movements.
Integumentary, skeletal and muscular systems
The epidermis develops from a single layer of ectoderm to which other layers are added. By the end of the 1st month, a thin outer layer of flattened cells covers the embryo. Further development continues until the 6th month. From 18 weeks, the fetus is covered with a white, creamy substance called vernix caseosa. This protects the skin from the amniotic fluid and from any friction against itself. Hair begins to develop during the 9th–12th week. At 20 weeks, the fetus is covered with a fine downy hair called lanugo; at the same time the head, hair and eyebrows begin to form. Lanugo is shed from 36 weeks and by term, there is little left. Fingernails develop from about 10 weeks but the toenails do not form until about 18 weeks. By term, the nails usually extend beyond the fingertips.
All skeletal tissue arises from the mesodermal and neural crest cells but skeletal tissue in different parts of the body are diverse in morphology and tissue architecture. The skeleton first appears as cartilage and at specific periods the cartilage is replaced by true bone through a process of ossification. However, the skull and facial bones develop from direct ossification with no intermediate cartilage stage. Skeletal, cardiac and smooth muscle is formed and continues into childhood (Carlson 2004, Moore & Persaud 2003).
The fetal circulation
The placenta is the source of oxygenation, nutrition and elimination of waste for the fetus. There are several temporary structures in addition to the placenta and the umbilical cord that enable the fetal circulation (Fig. 12.3) to occur. These are:
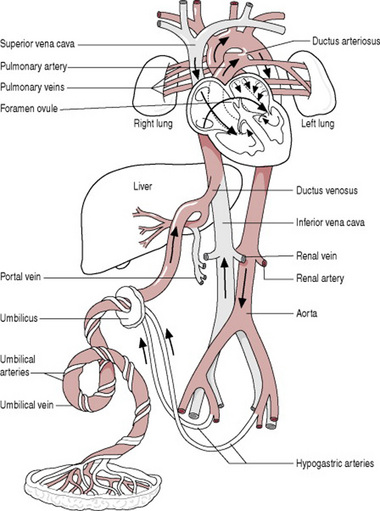
Figure 12.3 A diagram of the fetal circulation. The arrows show the course taken by the blood. The temporary structures are labelled in colour.
The fetal circulation takes the following course:
Oxygenated blood from the placenta travels to the fetus in the umbilical vein. The umbilical veins divide into two branches; one that supplies the portal vein in the liver, the other the ductus venosus joining the inferior vena cava. Most of the oxygenated blood that enters the right atrium passes across the foramen ovale to the left atrium and from here into the left ventricle, and then the aorta. The head and upper extremities receive approximately 50% of this blood via the coronary and carotid arteries, and the subclavian arteries respectively. The rest of the blood travels down the descending aorta, mixing with deoxygenated blood from the right ventricle.
Deoxygenated blood collected from the upper parts of the body returns to the right atrium in the superior vena cava. Blood that has entered the right atrium from the superior vena cava and inferior vena cava passes into the right ventricle. A little blood travels to the lungs in the pulmonary artery, for their development. Most blood passes through the ductus arteriosus into the descending aorta. This blood, although low in oxygen and nutrients is sufficient to supply the lower body. It is also by this means that deoxygenated blood travels back to the placenta via the internal iliac arteries, which lead into the hypogastric arteries, which lead into the umbilical arteries.
Adaptation to extrauterine life
At birth, there is a dramatic alteration to the fetal circulation and an almost immediate change occurs. The cessation of umbilical blood flow causes a cessation of flow in the ductus venosus, a fall in pressure in the right atrium and closure of the foramen ovale. As the baby takes the first breath, the lungs inflate, and there is a rapid fall in pulmonary vascular resistance. The ductus arteriosus constricts due to bradykinin released from the lungs on initial inflation. The effect of bradykinin is dependant on the increase in arterial oxygen. In the term baby, the ductus arteriosus closes within the first few days of birth.
These structural changes become permanent and become as follows:
Adaptation to extrauterine life also involves (Stables & Rankin 2004):
The fetal skull
The fetal head is large in relation to the fetal body compared with the adult (Fig. 12.4). Additionally, it is large in comparison with the maternal pelvis and is the largest part of the fetal body to be born. Adaptation between the skull and the pelvis is necessary to allow the head to pass through the pelvis without complications. The bones of the vault are thin and pliable and if subjected to great pressure damage to the underlying delicate brain may occur. Important intracranial membranes, venous sinuses and structures can be seen in Figures 12.5 and 12.6.
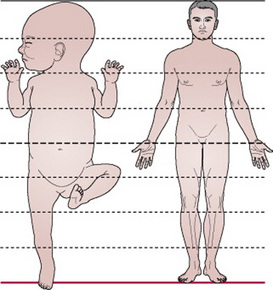
Figure 12.4 Comparison of a baby’s proportions to those of an adult. The baby’s head is wider than the shoulders and one-quarter of the total length.
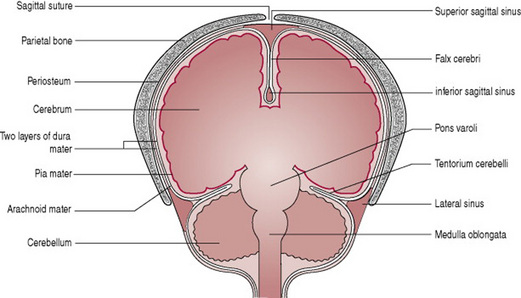
Figure 12.5 Coronal section through the fetal head to show intracranial membranes and venous sinuses.
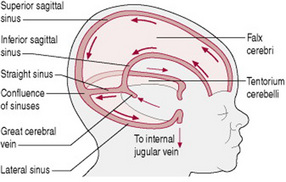
Figure 12.6 Diagram showing intracranial membranes and venous sinuses. Arrows show direction of blood flow.
Divisions of the fetal skull
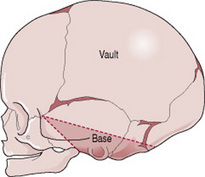
The skull is divided into the vault, the base and the face (Fig. 12.7). The base comprises bones that are firmly united to protect the vital centres in the medulla. The face is composed of 14 small bones which are also firmly united and non-compressible. The vault is the large, dome-shaped part above an imaginary line drawn between the orbital ridges and the nape of the neck.
The bones of the vault
The bones of the vault (Fig. 12.8) are laid down in membrane. They ossify from the centre outwards in a process known as ossification. Ossification is incomplete at birth, leaving small gaps between the bones, known as the sutures and fontanelles. The ossification centre on each bone appears as a protuberance. Ossification of the skull is not complete until early adulthood.
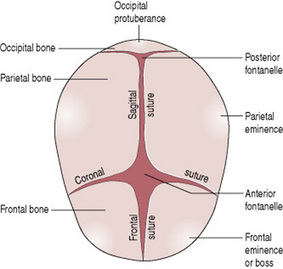
Figure 12.8 View of fetal head from above (head partly flexed), showing bones, sutures and fontanelles.
Sutures and fontanelles
The sutures are the cranial joints formed where two bones meet. Where two or more sutures meet, a fontanelle is formed (Fig. 12.8). The sutures and fontanelles described below permit a degree of overlapping of the skull bones during labour.
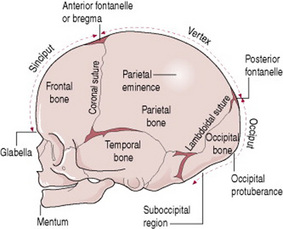
Regions and landmarks of the fetal skull
The skull is further separated into regions and within these, there are important landmarks (Fig. 12.9). The midwife feels for the landmarks on vaginal examination as they help ascertain the position of the fetal head
Diameters of the fetal skull
Knowledge of the diameters of the skull alongside the diameters of the pelvis allows the midwife to determine the relationship between the fetal head and the mother’s pelvis.
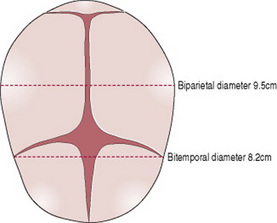
There are six longitudinal diameters (Fig. 12.10) and two transverse diameters (Fig. 12.11).
Figure 12.10 Diagram showing the longitudinal diameters of the fetal skull.
| Diameter | Length (cm) |
|---|---|
| SOB, sub-occipitobregmatic | 9.5 |
| SOF, sub-occipitofrontal | 10.0 |
| OF, occipitofrontal | 11.5 |
| MV, mentovertical | 13.5 |
| SMV, sub-mentovertical | 11.5 |
| SMB, sub-mentobregmatic | 9.5 |
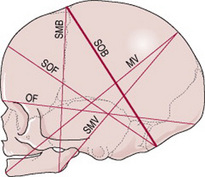
The longitudinal diameters are:
Knowledge of the diameters of the trunk is also important for the delivery of the shoulders and breech (Box 12.2).
Box 12.2 Diameters of the fetal trunk
Bisacromial diameter 12 cm
This is the distance between the acromion processes on the two shoulder blades and is the dimension that needs to pass through the pelvis for the shoulders to be born. The articulation of the clavicles on the sternum allows forward movement of the shoulders, which may reduce the diameter slightly.
Presenting diameters
Some presenting diameters are more favourable than others for easy passage through the pelvis and this will depend on the attitude of the head. This term attitude is used to describe the degree of flexion or extension of the head on the neck. The attitude of the head determines which diameters will present in labour and therefore influences the outcome.
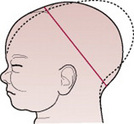
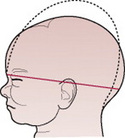
The presenting diameters of the head are those that are at right angles to the curve of Carus. There are always two, a longitudinal diameter and a transverse diameter. The presenting diameters determine the presentation of the fetal head, for which there are three:
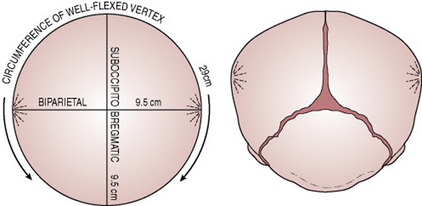
Figure 12.12 Diagram showing the dimensions presenting when the fetal head is well flexed in a vertex presentation.
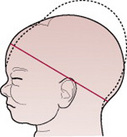
When the head is deflexed, the presenting diameters are the occipitofrontal (11.5 cm) and the biparietal (9.5 cm). This situation often arises when the occiput is in a posterior position. If it remains so, the diameter distending the vaginal orifice will be the occipitofrontal (11.5 cm).
Moulding
The term moulding is used to describe the change in shape of the fetal head that takes place during its passage through the birth canal. Alteration in shape is possible because the bones of the vault allow a slight degree of bending and the skull bones are able to override at the sutures. This overriding allows a considerable reduction in the size of the presenting diameters, while the diameter at right angles to them is able to lengthen owing to the give of the skull bones (Fig. 12.13). The shortening of the fetal head diameters may be by as much as 1.25 cm. The dotted lines in Figures 12.14-12.19 illustrate moulding in the various presentations.
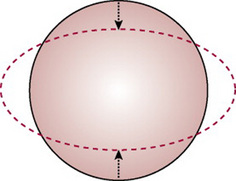
Figure 12.13 Demonstration of the principle of moulding. The diameter compressed is diminished; the diameter at right angles to it is elongated.
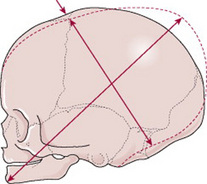
Figure 12.14 Moulding in a normal vertex presentation with the head well flexed. The sub-occipitobregmatic diameter is reduced and the mentovertical elongated.
Figures 12.15–12.19 Series of diagrams showing moulding when the head presents. Moulding is shown by the dotted line.
Additionally, moulding is a protective mechanism and prevents the fetal brain from being compressed as long as it is not excessive, too rapid or in an unfavourable direction. The skull of the pre-term infant is softer and has wider sutures than that of the term baby, and hence may mould excessively.
Venous sinuses are closely associated with the intracranial membranes (Fig. 12.6) and if membranes are torn due to excessive moulding, there is danger of bleeding. A tear of the tentorium cerebelli may result in bleeding from the great cerebral vein.
Carlson BM. Human embryology and developmental biology, 3rd edn. Philadelphia: Mosby, 2004.
Johnson MH, Everitt BJ. Essential reproduction, 5th edn. Oxford: Blackwell Science, 2000.
Moore KL, Persaud TVN. Before we are born, essentials of embryology and birth defects, 6th edn. London: Saunders, 2003.
Stables D, Rankin J. Physiology in childbearing, 2nd edn. London: Elsevier, 2004.
Coad J, Dunstall M. Anatomy and physiology for midwives. Edinburgh: Mosby, 2001.
A detailed discussion of embryonic and fetal development appears in Ch. 9. The fetal circulation and adaptation to neonatal life are addressed in Ch. 15 along with other adaptations at birth.
England MA. A colour atlas of life before birth. Netherlands: Wolfe Medical Publications, 1983.
This text serves to illustrate embryological and fetal development in photographic form. For the student who requires a detailed understanding of prenatal events and in particular the hormonal influences, this book is unsurpassed. It is important to be aware that it addresses reproduction in various species and discusses how much our knowledge of the physiology of sheep or goats can inform us of events in the human. Useful statements at the head of each small section give the principles that are relevant.
Wolpert L. The triumph of the embryo. Oxford: Oxford University Press, 1991.
Originating in a series of Christmas lectures at the Royal Institution, this text explores the unifying principles that may account for the way embryos develop. Written for the non-specialist, it invites the reader to think broadly and aims to inspire as well as instruct.