Chapter 44 Respiratory problems
Respiratory compromise in the newborn is a common presentation for a variety of diseases, not all of which may be respiratory in origin. This chapter offers a review of the signs and symptoms of respiratory compromise and an examination of some of the common causes of respiratory distress.
The second part of the chapter describes the typical nursing care for a baby who needs intensive care support for a respiratory disease.
Pathophysiology
Anatomical influences
Neonates are susceptible to respiratory compromise, for a number of reasons.
The stages of lung development are, in summary:
Signs of respiratory compromise
Grunting
Grunting is an audible noise heard on expiration. The sound appears when there is partial closure of the glottis as the breath is expired. The baby is attempting to preserve some internal lung pressure and prevent the airways from collapsing completely at the end of the breath.
Retractions
Chest distortions occur due to an increase in the need to create higher inspiratory pressures in a compliant chest. They appear as intercostal, subcostal or sternal recession across the thorax.
Asynchrony
Here the breathing has a ‘see-saw’ pattern as the abdominal movements and the diaphragm work out of unison. This is a result of increased muscle fatigue and the compliant chest wall.
Tachypnoea
This is a compensatory rise in the respiratory rate initiated from the respiratory centre. It is described as a breathing rate above 60, and aims to remove the hypercarbia and prevent hypoxia.
Common respiratory problems
Pneumothorax
Seminal research showed that pneumothoraces are known to occur spontaneously in 1% of the newborn population either during or after birth; however, only one-tenth of this 1% will be symptomatic (Steele et al 1971). A pneumothorax at birth is caused by the large pressures generated by the baby’s first breaths. These may be in the range of up to 40–80cm of water. This can lead to alveoli distension and rupture that allows air to leak to a number of sites most notably the potential space between the lung pleura.
Babies receiving any assisted ventilation have an increased susceptibility to a pneumothorax. This could be due to either maldistribution of the ventilated gas in the lungs, high ventilation settings or baby-ventilator breathing interactions.
Term babies may present with symptoms of respiratory distress on the postnatal ward. Although it is difficult to diagnose a pneumothorax in the absence of a chest X-ray, there may be reduced breath sounds on the affected side, displaced heart sounds and a distorted chest/diaphragm movement with respiration and distension of the chest on the affected side. These signs become harder to detect in the baby with bilateral pneumothoraces.
A baby with a suspected pneumothorax needs an immediate paediatric consultation with the view to an emergency procedure. The procedure will be the placement of a chest drain possibly preceded by a needle aspiration. This will drain the air leak and prevent a further accumulation of the air. The placement of a chest drain is a painful procedure and the baby will need some sedation along with nursing on the neonatal unit. Occasionally a pneumothorax can be managed conservatively with observation and breathing oxygen enriched air to aid the reabsorption of the extra-alveolar gas.
Transient tachypnoea of the newborn
The recorded incidence of transient tachypnoea of the newborn (TTN) varies widely; this is partly a result of the variety of recording methods, differences in radiological interpretation and clear diagnostic features. It is frequently seen as a diagnosis of exclusion of other possible respiratory causes. Nevertheless, the chest X-ray may show a streaky appearance with fluid apparent in the horizontal fissure that confirms the diagnosis.
These infants present with respiratory distress normally restricted to tachypnoea alone with rates up to 120 breaths/min. Occasionally supplemental oxygen is required, but during the 24hrs following birth the condition gradually resolves. The most common predisposing factor for TTN is a caesarean section because the thorax has not been squeezed while the baby descends along the birth canal. This results in lower thoracic pressures after birth. Although these babies tend to require initial care on a neonatal unit, their stay is usually of a short duration with the provision of oxygen and observation.
Infection/pneumonia
A number of infectious disease processes present with signs of respiratory distress in the newborn. All babies presenting with respiratory distress need to be treated for infection until there is proof to the contrary. Wright Lott (2007) notes mortality rates as high as 50% in the developing world and that sepsis rates worldwide range from 0.1% to 0.8%. Pre-term infants have an immature immunological response and hence less resistance to infection.
Pneumonia in the neonate is difficult to diagnose as secretions are difficult to obtain and the radiological appearances can be hard to distinguish. However, pneumonia presenting before 48hrs of age has normally been acquired either at or before birth whereas presentation after 48hrs indicates a late onset infection possibly resulting from hospitalization. All infants with infection require antibiotics but their length of stay on a neonatal unit will vary depending upon the nature of the infection, their signs and their antibiotic course. Babies with a mild infection could be cared for in a transitional care setting.
Meconium aspiration syndrome
Greenough & Roberton (1999) report the incidence for meconium aspiration syndrome in one UK hospital as 0.2/1000 live births; however, this incidence is low and other countries, such as the USA, have higher rates of the disease 2–5/1000 (Greenough & Milner 2005). Fetal asphyxia causes the passage of meconium into the liquor. This meconium is unproblematic unless the baby gasps or breathes in amniotic fluid, potentially inhaling meconium simultaneously. Consequently, it is the babies showing signs of fetal hypoxia who develop signs of meconium aspiration syndrome. A baby can develop meconium aspiration syndrome if stimulated to breathe or gasp, either before or after birth, when there is meconium in the airway that could be inhaled. The passage of meconium into the liquor is rarely seen prior to 34 weeks and Matthews & Warshaw (1979) suggest this is due to the immaturity of the pre-term gastrointestinal tract. A significant number of births have meconium-stained liquor but only a few will cause the severe meconium aspiration syndrome, with its associated mortality that is seen in the NNU. In the majority there is a milder disease process that requires initial supportive treatment but quickly resolves over 24–48hrs.
The initial respiratory distress may be mild, moderate or severe with a gradual deterioration over the first 12–24hrs in the moderate or severe cases. The baby may present with cyanosis, increased work of breathing and a barrel-shaped chest. This chest appearance occurs as a result of gas being trapped, leading to hyperexpansion of the lung fields. The meconium becomes trapped in the airways and causes a ball-valve effect: although air can enter the lung during inhalation, the meconium then blocks the airway during expiration so that air accumulates behind the blockage. This accumulation can then lead to the rupture of the alveoli and cause the baby to develop a pneumothorax. Where the meconium has contact with the lung tissue a pneumonitis occurs and a fertile site for infection is created. Endogenous surfactant is also broken down in the presence of meconium.
These factors in a previously hypoxic baby combine to produce a severe disease process. The external respiration across the alveoli is inhibited, areas of hypoxic lung are bypassed as blood flow shunts away from them and the pattern of fetal circulation across the arterial duct is difficult to break owing to high pulmonary vascular resistance. These infants will need full intensive care and ventilation to prevent further deterioration. Modalities such as ECMO (extracorporeal membrane oxygenation) have been shown to increase survival by 50% (UK Collaborative ECMO Trial Group 1996) while the use of nitric oxide therapy can further assist these severely compromised infants. A number of the most severely affected will be symptomatic for some months with ongoing residual symptoms during early childhood.
Respiratory distress syndrome
The term respiratory distress syndrome (RDS) is used interchangeably with the diagnosis of HMD in the neonatal culture. The diagnosis of HMD is derived from the presence of hyaline membranes in the airways resulting from the damaged epithelium. The disease occurs as a result of the insufficient production of surfactant and is seen most frequently after a premature birth; however, other disorders like maternal diabetes can also inhibit surfactant production. The 50% of babies born before 30 completed weeks’ gestation experience RDS while 1% of all newborn babies may experience RDS (Greenough & Milner 2005).
Surfactant is produced by the type II epithelial cells to reduce the surface tension within the alveoli, preventing their collapse at the end of exhalation. Collapsed alveoli require much greater pressure and exertion to reinflate than do partially collapsed alveoli. The introduction of surfactant therapy into neonatal care has significantly decreased the mortality and morbidity previously seen in RDS. Surfactant consists of several different types of proteins and phospholipids, which also help prevent infection and produce further surfactant.
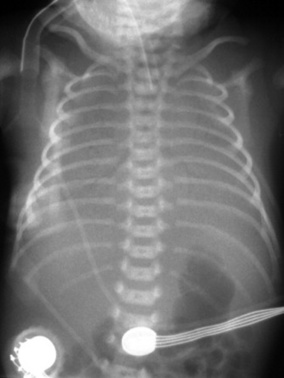
Pre-term babies are born with a small amount of surfactant but as the time from birth increases, the demands outstrip the supply. This gives a clinical picture of an infant with progressive respiratory distress, in which it may be 4hrs before there is a significant presentation. The X-ray has a ground-glass appearance across the lung fields, while severe disease is represented by a ‘white-out’, the greater the density of the ‘white out’ reflecting the severity of the disease, see e.g. Figure 44.1. The infant has an increasing respiratory distress and work of breathing; it may take 48–72hrs to reach the peak of the disease without the administration of surfactant. Resolution of the associated inflammation and the hyaline membrane formation may take up to 7 days in the unsupported baby.
Treatment has been revolutionized since the early 1990s with the administration of surfactant directly into the lungs. Surfactant is also used prophylactically when significant disease is anticipated in the most immature babies and is often administered in the birthing room. Mild disease may need oxygen alone but the more severe will need surfactant and ventilatory support. The length of stay on the NNU is dependent upon the severity of the disease and the gestational age of the baby.
A baby may remain in the NNU for a similar number of weeks to the number of weeks he is born pre-term. This allows the midwife to give the parents a ballpark figure to enable them to adjust accordingly, e.g. ‘your baby is 16 weeks early so you can anticipate a stay on the NNU for up to about 16 weeks’.
Cardiac disease
Cardiac defects affect 1% of births and can be divided into left-sided and right-sided defects. They account for 30% of congenital defects (Jordan & Scott 1989). Although cardiac disease is not a respiratory disease it presents with respiratory symptoms (see also Ch. 46).
Right-sided lesions
The most frequently seen lesions are transposition of the great arteries, tetralogy of Fallot and pulmonary atresia or stenosis (p. 889). These babies typically present as a ‘blue’ baby. On examination, there is little to note other than the presence of cyanosis. Their respiratory distress, if present, is mild and consists of tachypnoea alone. These babies will remain cyanotic in the presence of 100% oxygen. An urgent cardiac assessment will need to be sought.
Left-sided lesions
The most frequently occurring left-sided lesions are hypoplastic left heart syndrome and coarctation of the aorta (see p. 891). These frequently present with neonatal heart failure. Initially the baby may appear irritable, lethargic, sweaty and not interested in feeding. The presence of ‘effortless’ tachypnoea may be seen. This tachypnoea is characterized by the lack of any other sign of respiratory compromise; for example, no grunting is heard and there is no head bobbing and minimal recession. As the heart failure progresses, the infant shows signs of increasing cardiogenic shock and will require full resuscitative measures if left untreated.
When a cardiac condition is suspected a postnatal transfer to a cardiac centre may be necessary for diagnosis and treatment. Parents need clear explanations to enable them to feel supported during this time of anxiety. Prior to departure any resuscitation and stabilization will be managed depending on the advice received from the cardiac centre and the nature of the defect.
Common practice involves the administration of an infusion of prostaglandin to maintain the patency of the arterial duct. The patency of the arterial duct is needed to keep the blood flowing through the heart and around the peripheral circulation. Stabilization may include elective intubation and ventilation of the baby. This assisted mechanical ventilation is commonly provided without oxygen, even if the saturation monitor records saturations in the 80s or 70s, since the additional oxygen may stimulate the arterial duct to close.
Cardiac surgery is complex and some defects require a number of procedures to optimize the baby’s future. It is not possible to treat all the different cardiac defects and for some a poor prognosis remains. However, advances are continuously being made in the management of some of the previously fatal defects, e.g. hypoplastic left heart syndrome (Richens 2007).
What to anticipate in the neonatal unit
The anxious and frightened parents will be confronted with a technological environment with an array of buzzers, bleeps, flashing lights and alarms. It is easy to lose sight of the baby behind the technology, as Plate 18 demonstrates. However, all these machines and alarms will have a role to play in the care of the baby. The second part of the chapter explores the environment of the neonatal unit (NNU) and examines some of the technological and therapeutic support available for neonatal intensive care (NIC).
Respiratory care
Babies showing signs of respiratory compromise require a further assessment by a paediatrician or nurse practitioner. This may necessitate an admission to the NNU for continuous supervision and observation of their respiratory status. They require the skills of practitioners who can monitor and assess their critical changes in status and implement the care that is required. To assist in these observations the use of saturation monitors, transcutaneous monitors, arterial catheter readings and blood gas monitoring are used. When satisfactory oxygenation is not being achieved, the baby may need additional oxygen. This can be delivered via nasal cannula, into the incubator or into a headbox, creating an oxygen-enriched micro-environment.
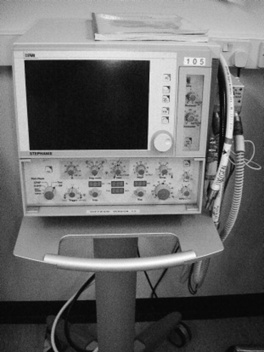
Some babies will need additional ventilatory support to assist the maintenance of an adequate airway or to maximize their respiratory status. Present options for ventilation are mainly pressure controlled although the use of volume controlled strategies is increasing. There is a variety of ventilation styles to choose from. Most frequently these include conventional ventilation (CMV), high frequency oscillation (HFOV) and continuous positive airway pressure (CPAP) and Figure 44.2 shows a neonatal ventilator that offers all three.
CMV
Conventional ventilation techniques are increasingly being delivered in ways similar to the natural breathing patterns. During the critical phase of the illness the ventilator may deliver preset rates and pressures only. However, as the baby stabilizes and improves, ventilators are now able to mimic the babies’ individual rates, pressures or lung volumes thereby minimizing the associated lung trauma.
CPAP
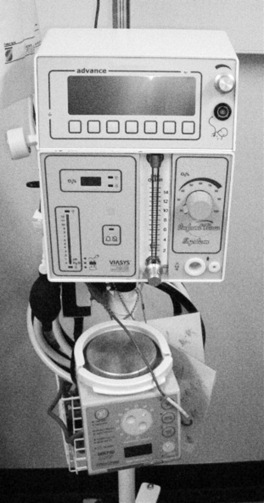
Nasal continuous positive airway pressure (NCPAP) is used either as a therapy for moderate disease or as weaning tool. Pressure is delivered to the nares and oropharynx which, when transmitted through the bronchial tree, distends the alveoli at the end of respiration preventing their collapse. The aim is to avoid the trauma of an endotracheal tube and the bronchiolar damage from ventilation. Figure 44.3 shows a ‘CPAP driver’.
Alternative ventilation options
HFOV
This is commonly used for extreme prematurity, as a rescue therapy or in combination with nitric oxide. When babies are being oscillated they are seen to ‘vibrate’, while some continue to breathe in addition to the ventilation. Pressure is used to reach optimal lung expansion and ‘bounce’ or oscillations are added to help the distribution of the gases through the lung field. Since Johnson et al (2002) published their study research of conventional ventilation versus HFOV, paediatricians have tended to reserve HFOV for a rescue strategy for the critically ill.
ECMO
This involves the oxygenation of the blood supply outside the body. Large cannulae are inserted to remove and reintroduce the blood to the baby. The procedure uses technology similar to cardiac bypass support for the oxygenation of the blood. During this time the lungs are allowed to rest and heal. This type of ventilation is governed by the availability of the treatment and infant suitability. Generally babies need to be at least 34 gestational weeks, weigh >1.8kg and be without a bleeding disorder.
NO
Nitric oxide (NO) is a respiratory gas that is inhaled with each ventilation breath. NO works directly upon the pulmonary vessels with minimal effect to the systemic circulation. It has been licensed for use in Europe since 2001 as a pulmonary vasodilator for pulmonary hypertension in babies at 34 weeks’ gestation. It has been shown that the use of NO reduces the need for ECMO but the role of inhaled NO for pre-term infants remains unclear awaiting further research (Dewhurst et al 2007).
CNEEP
Continuous nasal end expiratory pressure (CNEEP). This is unsuitable for newly delivered premature babies as the tight fixation around the baby’s neck may lead to an increased risk of IVH. However, when this risk has passed, CNEEP works using similar principles to the technology of the iron lung.
These alternative ventilation techniques are available at specialized centres only and may require a postnatal transfer to access. Liquid ventilation is not routinely available in Britain at the time of writing. Box 44.1 shows key points relating to respiratory support.
Cardiovascular support
Cardiac failure in a neonate is rare and the majority of arrests are respiratory in origin. Nevertheless, neonates may require support to maintain an efficient and effective heart beat. Maintenance of an adequate blood pressure (the mean being equivalent to the gestational age) may need pharmaceutical support too. Continuous observation and supervision of the cardiac parameters will be needed, which can be achieved using either an indwelling catheter or non-invasive monitoring. A bradycardia (a heart rate dropping to below 80) may be a sign of various influences such as a blocked endotracheal tube or sepsis. Normally a respiratory cause is considered first before other explanations are explored.
Occasionally, the transition from a fetal to an adult circulation is compromised and the baby develops persistent pulmonary hypertension (PPHN) of the newborn. This condition has previously been called ‘persistent fetal circulation’, which describes the tendency of the blood flow to mimic the circulation within the fetus. The compromise may be due to a primary defect in the pulmonary vasculature or secondary to other factors that raise the pulmonary vascular resistance, for example meconium aspiration syndrome. The use of nitric oxide as the vasodilator of choice has replaced other pharmaceutical strategies for PPHN. Box 44.2 gives a key point to note, relating to cardiovascular support.
Nutrition and hydration
The role and benefits of breastmilk are clearly established (see Ch. 41). These benefits are increased further for the pre-term baby, for whom an immature gut can give rise to feeding difficulties. A pre-term baby has little nutritional reserves and will need supplementation soon after birth to meet the continual demand for glucose from the brain. Although the ideal would be to establish oral breastmilk feeding, for a sick neonate this is not possible as the presence of the endotracheal tube, the absence of a suck reflex or critical illness prevents oral feeding.
The majority of such babies will receive a glucose-based intravenous infusion. Meanwhile the practitioner can give milk feeds via a nasogastric, orogastric or nasojejunal tube and increase the volumes as the baby’s condition allows. Opinions vary concerning the method of administration and the frequency of these early feedings although there is an increasing trend towards regular bolus feeds rather than continuous feeding with a syringe driver (Grant & Denne 1991, Lucas et al 1986, Newell 1998). Nevertheless sick or immature babies need a cautious introduction to milk, whether expressed breastmilk or formula feeds, as these infants are susceptible to necrotizing enterocolitis (NEC).
When a baby is expected to take more than 4–5 days before full feeding is established, then total parenteral nutrition (TPN) is needed to ensure that all nutritional requirements can be met. TPN is normally administered through a central line, either a longline or umbilical catheter. The strength and irritability of the solution necessitate its delivery into a large vein. Weak TPN solutions can be started on the 1st day of life. TPN typically contains amino acids, fats, carbohydrates, minerals, vitamins and trace elements. All help to prevent the depletion of these essential components, assisting growth and development in the pre-term baby. The strength and composition of the TPN can be built up over a few days as the baby demonstrates its ability to tolerate the solution. Although some babies may need TPN for many weeks, it can have some undesirable side-effects upon the liver, giving rise to a conjugated hyperbilirubinaemia or cholestasis. Prolonged TPN is sometimes needed in very immature babies, infants with gastroschisis and those with NEC.
Necrotizing enterocolitis
NEC is a disease seen in the neonatal population and its causes are multifactorial. Prior to birth the gut mucosa is sterile; consequently during the first few days and weeks of life colonization with the normal bacterial flora must occur. This normal process can be altered by delaying feeding when a baby is unwell or pre-term.
The administration of antibiotics can alter the dominant gut flora, as can formula milk. This can lead to a proliferation of anaerobes and bacterial invasion into the gut wall. Bacterial infection can also occur following episodes of hypoxia. Variations in blood volume reducing the arterial blood flow through the mesenteric circulation also predispose to NEC, e.g. intrauterine growth restriction. This causes mucosal ischaemia, which when reperfused leads to oedema, haemorrhage, ulceration and necrosis.
The baby may present with mild symptoms of the disease, a painful distended abdomen, blood in the stool and poor food tolerance. However there may be more acute pathology; symptoms may include air within the gut wall, leading to a perforation, hypovolaemic shock and disseminated intravascular coagulation.
Treatment is initially with antibiotics and medical management. However, surgery is needed if there is a perforation or a failure to respond to the medical therapy. The long term problems for those who do recover can be short gut syndrome and gut stenosis (see also Chs 43 and 47). Box 44.3 is a list of key points relating to nutrition.
A safe environment
Neonatal infection
A neonate has an increased susceptibility to infection owing to the immaturity of the neonatal defence mechanisms. These limitations decrease as gestational age lengthens; nevertheless a term baby still has not achieved the immune responses of an adult. Neonates, and pre-term babies especially, experience reduced immunoglobulin protection. Their responses to infection take longer as the organisms are new and not recognized by the memory cells. Consequently there is a delay in response time, as fresh responses are required for each new organism. The complement cascade, a supplementary defence mechanism of plasma proteins, remains inefficient. The phagocytic cells are restricted in their role and the external defences like the skin are immature. Consequently the neonate has a reduced ability to fight infection, whether contracted prior to birth or after.
The uterus is a sterile environment, which cannot be replicated on the neonatal unit. However, strict infection control measures can be exercised e.g. hands are washed by all caregivers and restricted handling by a few individuals. Nursing a baby in an incubator provides a micro-environment that can assist with barrier nursing. However, neonatal infection, whether prenatal or postnatal, continues to contribute to neonatal mortality hence the use of prophylactic antibiotics is widespread throughout developed countries. Box 44.4 gives a key point relating to infection.
Thermoneutral environment
A baby needs the environment to require minimal metabolic activity to maintain temperature stability. Babies born at the extremes of viability have immature skin owing to reduced keratin in the epidermis, hence heat and water is easily lost. This, along with their immature responses to cold stress and no brown adipose tissue before 28 weeks’ gestation, makes neonates vulnerable to the effects of a cool environment. Babies for whom an inadequate thermoneutral environment is achieved have an increased mortality and morbidity and hence the diligence given to keeping infants warm.
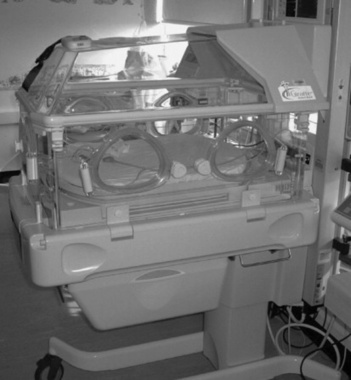
The use of heated boxes has developed into the use of sophisticated incubators as shown in Figure 44.4. These provide an environment where heat can be controlled and a humidified microclimate can be created for the most immature. Continuous attention to thermoregulation can be made with the use of temperature probes placed upon the skin. Warm delivery rooms are routinely used while the practice of putting pre-term babies into plastic bags immediately after birth has become an effective measure for minimizing heat loss (Bjorklund & Hellstrom-Westas 2000).
A stress-free environment
The neonate experiences a number of stressors within the neonatal environment. Once analgesia and sedation have been administered the baby may still show signs of distress and agitation that can lead to long-term patterns of altered behaviours if left unmanaged.
The use of opiates for pain relief and sedation is routine in neonatal care and recently sucking sucrose has become a non-pharmacological option for pain management (Noerr 2001). The recognition of pain in the neonate has prompted a number of pain assessment tools to aid identification as the behavioural responses may not reflect the severity of the pain. The majority involve some subjectivity and the most preferred tools measure contextual, physiological and behavioural responses; CRIES (Krechel & Bildner 1995), the Neonatal Facial Coding System (Grunau et al 1990) and the Premature Infant Pain Profile (Stevens et al 1996) are frequently used examples.
The pre-term baby can also find other ‘routine’ stimuli noxious, e.g. the lighting, the noise levels and handling associated with intensive care. Simple comforting measures can be effective for short term use, especially for the non-ventilated baby. These actions include swaddling, nesting, positioning and non-nutritive sucking from a pacifier. These measures can be learnt by the family to aid the attachment process, as some babies demonstrate disorganized behaviours when presented with the usual parental interactions like cuddling and play. Consideration for the environmental stimuli can also be made e.g. by lowering the lighting and minimizing noise levels. Box 44.5 gives a key point relating to stimuli.
Parents
The importance of attachment for the future relationship between the parents and their baby has been recognized for a number of years now (Klaus & Kennell 1989) and would normally occur as the mother interacts with her baby. Some mothers bond instantaneously, while for others the process occurs more slowly as they meet their baby’s needs. When this normal maternal interaction and role is denied, the process of attachment can be delayed and difficult (Bialoskurski et al 1999). McGrath (2007) identifies factors that can influence a family’s reactions to a child’s hospitalization and lists them as the severity of illness and threat to the baby, previous experiences, familiarity with medical practice and procedures, available support systems, coping strategies, family stresses, cultural or religious beliefs and family communication patterns.
For the majority of parents in the NNU, the question of survival will be paramount. This fear will be intensified with increasing prematurity. The EPICure study data (Costello et al 2000) give the exact data for the outcome of births on the edge of viability. An easily remembered guide suggested by these data is that births at 25 weeks’ gestation have a 50% mortality rate, and the surviving babies have a 50% morbidity rate. The survival of their baby is the initial concern for parents, but once there is less of a danger the need for ongoing daily communication should continue as it helps the parents ‘feel’ for their babies. The mothers rely on this information to help them facilitate an attachment with their baby, since Cox & Bialoskurski (2001) found that, for 99% of mothers, the information regarding their baby’s status was the most important maternal concern during their stay on the NNU.
Some hospital behaviours can become barriers to attachment and nurses and midwives need to be aware of these. Nurses may refer to a baby as ‘their baby’ for a shift duration, while the baby is actually not theirs but belongs to the family. Exclusion from tasks or reports can reinforce that the baby belongs to the hospital, not the family. The baby’s schedule can be controlled by the hospital rather than be family centred, while the family can be treated as guests to their baby rather than partners in care.
The midwife and nursing team can assist the parents to overcome difficulties by improving the quality of their communication, identifying the support structures that are available for parents and by facilitating the parents’ easy and frequent access to their baby. The parents should be enabled to develop the skills needed to care for their own baby and be given the information and empowerment to deliver that care, therefore ensuring that the care is family centred, rather than medical and technology focused. Box 44.6 gives a key point relating to parents.
Bialoskurski M, Cox C, Hayes J. The nature of attachment in the neonatal intensive care unit. Journal of Perinatal and Neonatal Nursing. 1999;13(1):66-77.
Bjorklund LJ, Hellstrom-Westas L. Reducing heat loss at birth in very pre-term infants. Journal of Pediatrics. 2000;137:739-740.
Costello K, Hennessy E, Gibson A, et al. The EPICure study: outcomes to discharge from hospital for babies born at the threshold of viability. Pediatrics. 2000;106:659-671.
Cox CL, Bialoskurski M. Neonatal intensive care: communication and attachment. British Journal of Nursing. 2001;10:668-676.
Dewhurst C, Harigopal S, Subhedar N. Recent advances in inhaled nitric oxide therapy in neonates: A review of the evidence. Infant. 2007;3(2):69-75.
Grant J, Denne SC. Effect of intermittent versus continuous enteral feeding on energy expenditure in premature infants. Journal of Pediatrics. 1991;118:928-932.
Greenough A, Milner AD. Acute respiratory disease. In: Rennie JM, editor. Roberton’s Textbook of Neonatology. 4th edn. Edinburgh: Churchill Livingstone; 2005:468-535.
Greenough A, Roberton NRC. Acute respiratory distress in the newborn. In: Rennie JM, Roberton NRC, editors. Textbook of neonatology. 3rd edn. Edinburgh: Churchill Livingstone; 1999:481-607.
Grunau RVE, Johnston CC, Craig KD. Pain expression in neonates: Facial action and cry responses to invasive and non-invasive procedures. Pain. 1990;42:295-305.
Johnson A, Peacock J, Greenough A, et alUnited Kingdom Oscillation Study Group. High-frequency oscillatory ventilation for the prevention of chronic lung disease of prematurity. New England Journal of Medicine. 2002;347(9):642-663.
Jordan SC, Scott O. Heart disease in paediatrics, 3rd edn. Butterworth-Heinemann, Oxford, 1989;3.
Klaus MH, Kennell JH. Parent–infant bonding, 3rd edn. St Louis: Mosby, 1989.
Krechel S, Bildner J. CRIES: a new neonatal post-operative pain measurement score: initial testing of validity and reliability. Paediatric Anaesthesia. 1995;5(1):53-61.
Lucas A, Bloom S, Aynsley-Green A. Gut hormones and ‘minimal enteral feeding’. Acta Paediatrica Scandinavica. 1986;75:719-723.
Matthews TG, Warshaw JB. Relevance of the gestational age on distribution of meconium passage in utero. Pediatrics. 1979;64:30-31.
McGrath JM. Family: essential partner in care. In: Kenner C, Wright-Lott J, editors. Comprehensive neonatal care: an interdisciplinary approach. 4th edn. Philadelphia: Saunders; 2007:491-509.
Newell S. Enteral nutrition. In: Campbell AGM, McIntosh N, editors. Forfar and Arneil’s textbook of pediatrics. 5th edn. New York: Churchill Livingstone; 1998:152-155.
Noerr B. Sucrose for neonatal procedural pain. Neonatal Network. 2001;20(7):63-67.
Richens T. The preoperative management of severe neonatal left ventricular outflow obstruction. Infant. 2007;3(1):36-40.
Steele RW, Metz JR, Bass JW, et al. Pneumothorax and pneumomediastinum in the newborn. Radiology. 1971;98:629-632.
Stevens B, Johnston C, Petryshen P, et al. Premature infant pain profile: development and initial validation. Clinical Journal of Pain. 1996;12:13-22.
UK Collaborative ECMO Trial Group. UK collaborative randomized trial of neonatal extracorporeal membrane oxygenation. Lancet. 1996;348:75-82.
Wright Lott J. Immune system. In: Kenner C, Wright-Lott J, editors. Comprehensive neonatal care: an interdisciplinary approach. 4th edn. Philadelphia: Saunders; 2007:203.
Kenner C, McGrath J, editors. Developmental care of newborns and infants: a guide for health professionals. St Louis: Mosby, 2004.
A book with a focus on growth, development and behaviours, including the family role and environmental influences. A book to use alongside the more biomedical science books to facilitate a holistic approach to care.
Merenstein G, Gardner S. Handbook of neonatal intensive care, 6th edn. St Louis: Mosby, 2006.
A good authority for a combination of pertinent nursing and medical issues. The book covers topics more relevant for holistic management of babies needing intensive care. Although the work is American the balanced approach to all aspects of neonatology ensures the book retains its recommendation as a resource in Britain.
Rennie, editor. Roberton’s textbook of neonatology, 4th edn., Edinburgh: Churchill Livingstone, 2005.
A comprehensive coverage of all aspects of neonatology. This book provides an invaluable resource for British neonatologists. It covers an extensive range of medical topics and yet retains an easy to read reference approach.
Rennie J, Roberton NRC. A manual of neonatal intensive care, 4th edn. London: Arnold, 2002.
A succinct version of their textbook of neonatology for neonatal intensive care. The book provides essential information for the daily management of infants needing extra care. Ideal as a straightforward reference source.