Chapter 47 Jaundice and infection
Jaundice is a yellow discoloration of skin and sclera caused by raised levels of bilirubin in the blood (hyperbilirubinaemia). Neonatal jaundice is either physiological or pathological. During the first week of life all neonates have a transient rise in serum bilirubin and about 50% of term babies become jaundiced. This physiological jaundice appears about 48 hrs after birth and usually settles within 10–12 days. Pathological jaundice presents earlier, and is persistent or associated with high bilirubin levels. Causes include increased haemolysis, metabolic and endocrine disorders and infection.
Newborn babies are vulnerable to infection. Defence mechanisms are immature and skin thin and easily damaged. Infections may be acquired before, during or soon after birth and, while some are minor, others can be damaging or life-threatening.
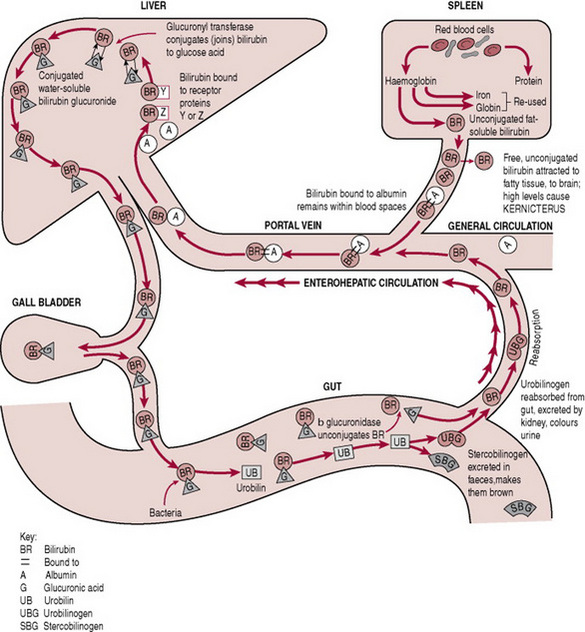
Conjugation of bilirubin
Conjugation changes the end-products of red cell breakdown so they can be excreted in faeces or urine. Understanding this process can increase evidence-based midwifery by increasing knowledge of the importance of such things as early breastfeeding, or early referral for treatment of pathological jaundice.
Ageing, immature or malformed red cells are removed from the circulation and broken down in the reticuloendothelial system (liver, spleen and macrophages). Haemoglobin from these cells is broken down to the by-products of haem, globin and iron.
Two main forms of bilirubin are present in the body:
Three stages are involved in the process of bilirubin conjugation: transport, conjugation and excretion (Fig. 47.1).
Transport of bilirubin
Unconjugated or fat soluble bilirubin is transported to the liver bound to albumin. If not attached to albumin, this unbound or ‘free’ bilirubin can be deposited in extravascular fatty and nerve tissues (skin and brain). Skin deposits of unconjugated or fat soluble bilirubin cause jaundice, while brain deposits can cause bilirubin toxicity or kernicterus (Box 47.1).
Kernicterus (bilirubin toxicity) is an encephalopathy caused by deposits of unconjugated bilirubin in the basal ganglia of the brain. Early signs can be insidious and include lethargy, changes in muscle tone, a high-pitched cry and irritability. These can progress to bilirubin induced neurological dysfunction with muscle hypertonia and possible death. Long-term clinical features can include deafness, blindness, cerebral palsy, developmental delay, learning difficulties and extrapyramidal disturbances such as athetosis, drooling, facial grimace, and chewing and swallowing difficulties (see Shapiro et al 2006).
Kernicterus is usually associated with serum bilirubin levels >340 μmol/L (20 mg/dL), but the critical threshold for long-term morbidity remains unclear. Recent work suggests non-albumin bound or ‘free’ bilirubin correlates better than total bilirubin concentration with bilirubin toxicity. Important risk factors include hypoxia, acidosis, infection, hypothermia and dehydration, all more likely in pre-term and sick-term infants (these can interfere with albumin- binding capacity). Glucose-6-phosphate dehydrogenase (G-6-PD) deficiency is also important as it increases haemoglobin destruction and produces more unconjugated bilirubin.
Kernicterus rarely occurs in healthy, term breastfed babies. However, it does occur. For example, in one group of six infants with kernicterus Maisels & Newman (1995) identified no cause of hyperbilirubinaemia other than breastfeeding. Watchko (2006) found breastmilk feeding almost uniformly present among late pre-term infants with kernicterus. Inadequate establishment of breastfeeding may play a role in hyperbilirubinaemia in some infants with kernicterus. A small subpopulation of breastfed infants with jaundice may be more susceptible if starved (Bertini et al 2001).
If bilirubin neurotoxicity is suspected, a complete neurodevelopmental examination and diagnostic testing are critical. Treatment of kernicterus is usually aggressive and can include phototherapy, intravenous fluids and exchange transfusion. Ongoing follow-up is essential, including complete neurodevelopmental examinations, repeat MRIs, and behavioural hearing evaluations.
(Ahlfors & Wennberg 2004, Kaplan & Hammerman 2004, Karlsson et al 2006, Maisels & Newman 1995, Newman et al 2000, Shapiro et al 2006, Smitherman et al 2006, Watchko 2006).
Conjugation
Once in the liver, unconjugated bilirubin is detached from albumin, combined with glucose and glucuronic acid and conjugation occurs in the presence of oxygen and the enzyme Uridine diphosphoglucuronyl transferase (UDP-GT). The conjugated bilirubin is now water soluble and available for excretion.
Excretion
Conjugated bilirubin is excreted via the biliary system into the small intestine where normal bacteria change the conjugated bilirubin into urobilinogen. This is then oxidized into orange-coloured urobilin. Most is excreted in the faeces, with a small amount excreted in urine (Ahlfors & Wennberg 2004, Kaplan et al 2005).
Jaundice
Jaundice is caused by bilirubin deposits in the skin. In term neonates it appears when serum bilirubin concentrations reach 85–120 μmol/L (5–7 mg/dL), with a head to toe progression as levels increase. Babies of full East Asian parentage have a higher bilirubin level and risk of severe jaundice requiring phototherapy, blood transfusion or rehospitalization than Caucasian infants (Setia et al 2002). Babies of African origin have lower levels. Pre-term babies are more likely to develop jaundice (Tyler & McKiernan 2006). Plate 21 shows a Caucasian baby with physiological jaundice who required 24 hrs of fibreoptic phototherapy, and demonstrates the difference in skin tone between the jaundiced baby and her mother.
Physiological jaundice
Neonatal physiological jaundice occurs when unconjugated (fat soluble) bilirubin is deposited in the skin instead of being taken to the liver for processing into conjugated (water soluble) bilirubin that can be excreted in faeces or urine. It is a normal transitional state affecting up to 50% of term and 80% of premature babies who have a progressive rise in unconjugated bilirubin levels and jaundice on day 3. Physiological jaundice never appears before 24 hrs of life, usually fades by 1 week of age and bilirubin levels never exceed 200–215 μmol/L (12–13 mg/dL).
Causes
In many newborns, a temporary discrepancy exists between red cell breakdown and their ability to transport, conjugate and excrete the resulting bilirubin. Physiological jaundice results from increased red cell breakdown at a time of newborn immaturity.
Increased red cell breakdown
Newborn bilirubin production is more than twice that of normal adults per kilogram of weight. In the hypoxic environment of the uterus the fetus relies on haemoglobin F (fetal haemoglobin), which has a greater affinity for oxygen than does haemoglobin A (adult haemoglobin). When the pulmonary system becomes functional at birth, the large red cell mass must be broken down or haemolised, resulting in increased unconjugated bilirubin.
Decreased albumin-binding capacity
Newborns have lower albumin concentrations and decreased albumin-binding capacity (reducing transport of bilirubin to the liver for conjugation). Some drugs also compete for albumin-binding sites. As binding sites on albumin are used, levels of unbound ‘free’ fat-soluble bilirubin in the blood rise and find tissues with fat affinity (skin and brain).
Enzyme deficiency
Levels of UDP-GT enzyme activity are lower during the first 24 hrs after birth (reducing bilirubin conjugation in the liver). Adult levels are not reached for 6–14 weeks.
Increased enterohepatic reabsorption
This process is increased as the newborn bowel lacks the normal enteric bacteria that break down conjugated bilirubin to urobilinogen (hindering excretion). Newborns also have increased amounts of another enzyme beta-glucuronidase which changes conjugated bilirubin back into the unconjugated state (when it is absorbed back into the system). If feeding is delayed then bowel motility is also decreased, further compromising excretion. Babies of East Asian parentage have enhanced enterohepatic circulation of bilirubin, higher peak bilirubin concentrations and more prolonged jaundice (Ahlfors & Wennberg 2004, Bertini et al 2001, Karlsson et al 2006, Setia et al 2002).
Midwifery care and physiological jaundice
Jaundice in newborns is a challenge for midwives as it is important to distinguish between healthy babies with a normal physiological response who need no active treatment, and those who require serum bilirubin testing (see Management of jaundice). For example, a 1-day-old mildly jaundiced baby requires further investigation. Conversely, a 4-day-old moderately jaundiced baby with good urinary output, who wakes regularly and feeds well does not need invasive blood tests.
One effective use of evidence-based practice in managing hyperbilirubinaemia is helping women feed their babies from birth. Early, frequent feeding assists newborns to cope with increased unconjugated bilirubin by promoting factors that deal with this load. Successful breastfeeding supplies glucose to the liver and increases bowel motility and normal bowel flora. In turn, this helps increase albumin-binding capacity, increases enzyme production for conjugation and decreases enterohepatic reabsorption. As well as reducing jaundice, being with women while they learn to breastfeed extends midwives’ partnership role with women well beyond the birth.
Midwives can further involve families by making them aware of the importance of excessive sleepiness, reluctance to feed and a decrease in wet nappies. The possible time scale of jaundice in some breastfed babies needs to be explained, and parents asked to report any pale stools and dark urine (these may indicate cholestatic liver disease). In formula-fed babies, prolonged jaundice, persistent pale stools and dark urine are rare, and merit immediate attention. If conjugated hyperbilirubinaemia is present, comprehensive diagnostic investigation is required as early diagnosis is critical for the best outcome (Ratnavel & Ives 2005, Tyler & McKiernan 2006).
In recent years, transcutaneous bilirubinometry (TcB) has reduced the number of blood tests in newborns. In home and hospital settings, midwives can use this method to provide a digital assessment of skin pigmentation with an estimate of plasma bilirubin. However, at high serum bilirubin concentrations Grohmann et al (2006) found all three skin test devices, and one of three non-chemical photometric devices underestimated bilirubin levels. TcB may also be less accurate in premature babies (Jangaard et al 2006, Nanjundaswamy et al 2005). For routine newborn care, skin testing can be used first. TcB readings above 200 μmol/L (12 mg/dL), and non-chemical photometric readings above 250 μmol/L (15 mg/dL) require standard laboratory testing (Grohmann et al 2006). With phototherapy, TcB is more accurate using a patched skin area (e.g. under the eyeshield) (Jangaard et al 2006, Nanjundaswamy et al 2005).
Exaggerated or prolonged physiological jaundice
In pre-term babies
Physiological jaundice in pre-term babies is characterized by bilirubin levels of 165 μmol/L (10 mg/dL) or greater by day 3 or 4, with peak concentrations on day 5–7 that return to normal over several weeks. Contributing factors include:
Premature infants are at particular risk of bilirubin production-conjugation imbalance. Good midwifery management of any jaundice in pre-term babies is critical as these babies have a higher risk of kernicterus (see Box 47.1) (Ahlfors & Wennberg 2004, Kaplan et al 2005, Karlsson et al 2006, Newman et al 2000, Tyler & McKiernan 2006, Watchko 2006).
In breastfed babies
The exact mechanism of prolonged jaundice in some breastfeeding babies is still unknown. Some researchers have found a significant relationship between breastfeeding and hyperbilirubinaemia or jaundice. For example, in two samples of 51 387 and 1177 healthy term newborns, one of the predictors of hyperbilirubinaemia or jaundice was exclusive breastfeeding (Newman et al 2000). While breastfed neonates in Bertini et al’s (2001) study of 2174 babies (at 37 weeks) did not have more hyperbilirubinaemia, a small group of jaundiced infants were more susceptible to kernicterus if starved. Although rare, kernicterus can occur in healthy, term, breastfed newborns (Maisels & Newman 1995) and breastmilk feeding is almost always present in pre-term infants with kernicterus (Watchko 2006) (see Box 47.1). Some argue a reliable diagnosis of breastmilk jaundice can only be made by excluding pathological causes (Ratnavel & Ives 2005). Stopping breastfeeding is not necessary. Rather, early midwifery help with establishment of breastfeeding is essential (see management).
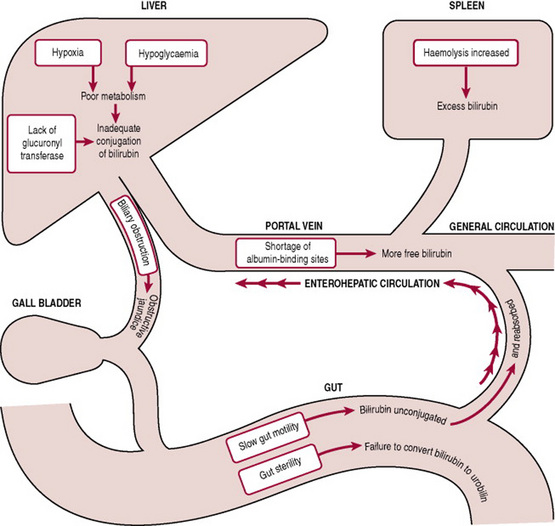
Pathological jaundice
Pathological jaundice in newborns usually appears within 24 hrs of birth, and is characterized by a rapid rise in serum bilirubin. Criteria include:
Causes
The underlying cause of pathological jaundice is any interference in bilirubin production, transport, conjugation or excretion (Fig. 47.2). Any disease or disorder that increases bilirubin production or alters transport or metabolism of bilirubin is superimposed upon normal physiological jaundice.
Production
Increased red cell destruction or haemolysis causes increased bilirubin levels. Causes of this increased haemolysis include:
Conjugation
Immaturity of the neonate’s enzyme system interferes with bilirubin conjugation in the liver. Other factors can include:
Excretion
Conditions interfering with bilirubin excretion can include:
After processing by the liver, most of the bilirubin is conjugated so babies are at less risk of kernicterus. They may, however, require urgent treatment for other serious conditions (see management) (Ahlfors & Wennberg 2004, Joy et al 2005, Kaplan & Hammerman 2004, Kaplan et al 2005, Karlsson et al 2006, Tyler & McKiernan 2006, Ratnavel & Ives 2005, van Dongen et al 2005).
Haemolytic jaundice
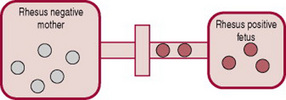
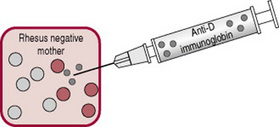
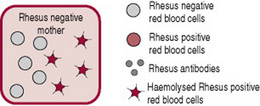
As described above, increased haemoglobin destruction in the fetus or newborn has several causes, for example, Rhesus (RhD) isoimmunization or ABO incompatibility. This increased haemolysis increases bilirubin levels, and causes pathological jaundice. Rhesus (RhD) isoimmunization can occur if blood cells from a Rhesus-positive baby enter a Rhesus-negative mother’s bloodstream. Her blood treats the D antigen on positive blood cells as a foreign substance and produces antibodies. While other causes of increased haemolysis are important, this condition is emphasized because of the midwife’s critical role in the injection of anti-D immunoglobulin (anti-D Ig). Without this anti-D prophylaxis, RhD isoimmunization can cause severe haemolytic disease of the newborn (HDN) with significant mortality and morbidity (National Institute for Health and Clinical Excellence, NICE 2002).
With the effectiveness of anti-D prophylaxis, antibodies against other blood groups are now more common than anti-D (e.g. anti-A, anti-B and anti-Kell). Although few antibodies to blood group antigens other than those in the Rh system cause such severe HDN, some report mortality and morbidity with antibodies other than anti-D. These include anti-E haemolytic disease of the fetus or newborn (Joy et al 2005), and anti-Kell (van Dongen et al 2005). In this chapter, ABO incompatibility is also emphasized, as it is the most frequent cause of mild to moderate haemolysis in neonates.
RhD incompatibility
RhD incompatibility is commonest among Caucasians, about 15% of whom are Rh-negative, compared with 3–5% of African and about 1% of Asian populations (Bianchi et al 2005). Before the introduction of anti-D Ig in 1969, RhD isoimmunization was a major cause of perinatal mortality and morbidity. In England and Wales, about 500 cases of RhD haemolytic disease of the fetus and newborn still occur each year, resulting in 25 to 30 deaths and 15 children with major permanent developmental problems (NICE 2002).
Patterns of Rhesus factor inheritance
RhD incompatibility can occur when a woman with Rh-negative blood type is pregnant with a Rh-positive fetus (Box 47.2).
With a Rhesus-negative (dd) pregnant women the patterns of Rhesus factor inheritance are as follows:
Recent improvements in the extraction and amplification of cell-free fetal DNA in maternal plasma can provide a non-invasive diagnosis of fetal Rhesus D genotype (Bianchi et al 2005). In a meta-analysis of 37 publications (3261 samples) of Rh genotyping from maternal peripheral blood, diagnostic accuracy was 94.8% (Geifman-Holtzman et al 2006). In Bristol UK, the International Blood Group Reference Laboratory provides a fetal blood group genotyping service for RhD isoimmunized pregnant women with heterozygous partners (Finning et al 2004). In the UK, antenatal anti-D Ig prophylaxis is currently given to all Rh-negative pregnant women. Ongoing technique improvements may increase accuracy of testing and enable large-scale, risk-free fetal RhD genotyping using maternal blood (Geifman-Holtzman et al 2006). Such future developments may allow anti-D Ig to be restricted to Rh-positive pregnancies (Finning et al 2004).
Causes of RhD isoimmunization
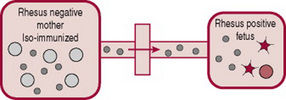
The placenta usually acts as a barrier to fetal blood entering the maternal circulation (Fig. 47.3). However, during pregnancy or birth, fetomaternal haemorrhage (FMH) can occur, when small amounts of fetal Rh-positive blood cross the placenta and enter the Rh-negative mothers blood (Fig. 47.4). The woman’s immune system produces anti-D antibodies (Fig. 47.5). In subsequent pregnancies these maternal antibodies can cross the placenta and destroy the red cells of any Rh-positive fetus (Fig. 47.6).
Figures 47.3–47.8 Rhesus isoimmunization and its prevention.
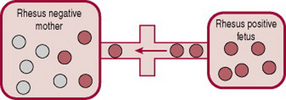
Figure 47.4 Fetal cells enter maternal circulation through ‘break’ in ‘placental barrier’, e.g. at placental separation.
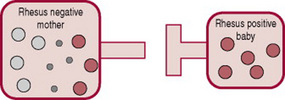
Figure 47.5 Maternal production of Rhesus antibodies following introduction of Rhesus positive blood.
RhD isoimmunization can result from any procedure or incident where positive blood leaks across the placenta, or from any other transfusion of Rh-positive blood (e.g. blood or platelet transfusion or drug use). Haemolytic disease of the fetus and newborn caused by RhD isoimmunization can occur during the first pregnancy. However, in most cases sensitization during the first pregnancy or birth leads to extensive destruction of fetal red blood cells during subsequent pregnancies (Bianchi et al 2005, Finning et al 2004, Geifman-Holtzman et al 2006, NICE 2002).
Prevention of RhD isoimmunization
Most cases of RhD isoimmunization can be prevented by injecting anti-D Ig within 72 hrs of birth or any other sensitizing event (Fig. 47.7). Anti-D Ig is a human plasma-based product that is used to prevent women producing anti-D antibodies. Anti-D Ig is of value to women with non-sensitized Rh-negative blood who have a baby with Rh-positive blood type. It is not used when anti-D antibodies are already present in maternal blood. As well, Anti-D Ig does not protect against the development of other antibodies that cause haemolytic disease of the newborn.
Routine antenatal prophylaxis
In the UK since 2002 (and some other countries), routine antenatal anti-D prophylaxis at 28 and 34 weeks’ gestation is recommended for all non-sensitized Rh-negative women (NICE 2002). With postnatal anti-D Ig prophylaxis, about 1.5% of Rh-negative women still develop anti-D antibodies following a first Rh-positive pregnancy. A meta-analysis (Allaby et al 1999) and Cochrane review (Crowther & Keirse 1999) suggest the antenatal sensitization rate is further reduced by routine antenatal prophylaxis.
Antenatal prophylaxis following sensitizing events
The most recent available evidence suggests anti-D Ig prophylaxis should be given to all non-sensitized Rh-negative women within 72 hrs of the following:
Postnatal prophylaxis
A systematic review of six eligible trials of over 10 000 women found when given within 72 hrs of birth (and other antenatal sensitizing events), anti-D Ig lowered the incidence of Rh isoimmunization 6 months after birth and in a subsequent pregnancy, regardless of the ABO status of the mother and baby (Crowther & Middleton 2001).
Administration of anti-D Ig
As previously outlined, Anti-D Ig destroys any fetal cells in the mother’s blood (Fig. 47.8) before her immune system produces antibodies. Anti-D Ig should not be given to women who are RhD-sensitized, as they have already developed antibodies. The process is as follows:
Dose of anti-D Ig
The dose of anti-D Ig, and the requirement for a test to identify the size of FMH, varies in different countries, as does the need for a follow up screening test (e.g. Kleihauer or flow cytometry) (RCOG 2002). Research evidence on the optimal dose of anti-D Ig is still limited (Crowther & Middleton 2001). The best available evidence suggests an intramuscular dose of 500 IU of anti-D Ig will suppress the immunization that could occur following a FMH of 4–5 mL of RhD positive red cells. Most women have a FMH of less than 4 mL at the birth (cited in RCOG 2002).
In the UK, the following doses of anti-D Ig are recommended:
RhD isoimmunization can also result from not following established protocols, particularly during pregnancy. For example:
Ethical and legal issues
A number of ethical, moral, legal and safety issues surround anti-D Ig, a human plasma-based product (see NICE 2002, RCOG 2002). Women have the right to refuse anti-D Ig treatment. To give informed consent, they need to know the possible consequences of treatment versus non-treatment. In the UK because of the hypothetical risk of transmitting new variant Creutzfeldt–Jakob disease, anti-D Ig use from UK residents has been discontinued. Anti-D Ig is now obtained from paid donors in and outside the EC. Midwives also have rights with respect to anti-D Ig, which they usually administer, in particular, involvement in policy decisions affecting anti-D Ig protocols. To assist midwives in their discussion with families, anti-D Ig information and resources are available, e.g. from NICE 2002. In all countries, midwives need to be aware of the content of consumer information leaflets available nationally and locally.
Management of RhD isoimmunization
Effects of RhD isoimmunization
Destruction of fetal RBCs results in fewer mature and more immature red cells. The fetus becomes anaemic, less oxygen reaches fetal tissue, and oedema and congestive cardiac failure can develop. Increased bilirubin levels also increase the risk of neurological damage from bilirubin deposits in the brain. Lesser degrees of red cell destruction result in haemolytic anaemia, while extensive haemolysis can cause hydrops fetalis and fetal death. Mortality rates are higher for those with hydrops fetalis (van Kamp et al 2005). Early referral to specialist care for women with RhD antibodies is essential. While early specialist care influences fetal outcome (Craparo et al 2005, Ghi et al 2004, van Kamp et al 2005), ongoing midwifery information and support remain important.
Antenatal monitoring and treatment of RhD isoimmunization
Treatment aims to reduce the effects of haemolysis. Intensive fetal monitoring is usually required, and often a high level of intervention throughout the pregnancy. Monitoring and treatment can include:
Postnatal treatment of RhD isoimmunization
Treatment depends upon the baby’s condition. Careful monitoring but less aggressive management may be adequate with mild to moderate haemolytic anaemia and hyperbilirubinaemia. Severely affected babies often require admission to intensive care units. Babies with hydrops fetalis are pale, have oedema and ascites and may be stillborn. In some cases phototherapy can be effective but exchange transfusion is often required, and packed cell transfusion may be needed to increase haemoglobin levels. Babies are at risk of ongoing haemolytic anaemia. Early work with IVIG treatment suggests it can be effective at blocking ongoing haemolysis in babies, who then require shorter duration of phototherapy and less exchange transfusions. In their Cochrane Review Alcock & Liley (2002) recommended further clinical research before routine use of intravenous immunoglobulin for the treatment of isoimmune haemolytic jaundice.
ABO incompatibility
ABO isoimmunization usually occurs when the mother is blood group O and the baby is group A, or less often group B. Type O women are 5.5 times more likely to have sensitization than type A or B as the latter have a protein or antigen not present in type O blood. Individuals with type O blood develop antibodies throughout life from exposure to antigens in food, Gram-negative bacteria or blood transfusion, and by the first pregnancy may already have high serum anti-A and anti-B antibody titres. Some women produce IgG antibodies that can cross the placenta and attach to fetal red cells and destroy them (see effects of RhD isoimmunization). ABO incompatibility is also thought to protect the fetus from Rh incompatibility as the mother’s anti-A and anti-B antibodies destroy any fetal cells that leak into the maternal circulation (David et al 2004). Although first and subsequent babies are at risk, destruction is usually much less severe than with Rh incompatibility. In most cases haemolysis is fairly mild but in subsequent pregnancies can become more severe. ABO erythroblastosis can, rarely, cause severe fetal anaemia and hydrops.
Antibody titres are monitored throughout the pregnancy, but a high level of antenatal intervention is not usually required. Postnatal management depends on the severity of haemolysis and, as with RhD isoimmunization, aims to prevent further haemolysis, reduce bilirubin levels and combat anaemia. After the birth, cord blood can be tested to confirm blood type, check haemoglobin and serum bilirubin levels and identify maternal antibodies on fetal red cells (direct Coombs’ test). If antibodies are present, the baby is monitored for jaundice. As with other causes of haemolysis, if infants require phototherapy it is usually commenced at a lower serum bilirubin level (140–165 μmol/L or 8–10 mg/dL). In rare cases, babies with high serum bilirubin level require exchange transfusion. IVIG administration to newborns with significant hyperbilirubinemia due to ABO haemolytic disease (with a positive direct Coomb’s test) has reduced the need for exchange transfusion (Miqdad et al 2004).
Management of jaundice
For more than 50% of term and 80% of pre-term infants, jaundice is a normal physiological response. However, others are at risk of kernicterus and 1 in 500 newborn babies have liver disease. Good management protocols include careful, individual assessment of each case and a range of therapeutic and diagnostic options.
In countries such as the UK, Australia, New Zealand and many European countries, midwives have an important role in diagnosing and treating jaundice. During the first weeks of the baby’s life, midwives can identify at risk newborns, make follow-up visits when women are discharged from hospital or birth at home, arrange home phototherapy and inform and teach parents. This is particularly so in the UK, with midwives encouraged to extend their public health role and work with women for up to 6 weeks after the birth. This includes responsibility for referrals and communication with other agencies.
Most jaundice beyond 2 weeks of age in healthy term infants is associated with breastfeeding and is benign. However, it can also result from haematological, hepatobiliary, metabolic, endocrine, infectious and genetic disorders, all associated with significant morbidity. If conjugated hyperbilirubinaemia is present, comprehensive diagnostic investigation is required to ensure early diagnosis (Ratnavel & Ives 2005). Prolonged jaundice in premature infants is a frequent clinical problem. Sick, premature infants can develop cholestasis from parenteral nutrition, delayed enteral nutrition, sepsis, hypoxia and umbilical lines. They are at risk of serious liver disease, including extrahepatic biliary atresia (Tyler & McKiernan 2006).
Assessment and diagnosis
Two initial diagnostic questions are:
Individual risk factors
Individual midwifery assessment includes identifying particular risk factors for jaundice. These include any disease or disorder that increases bilirubin production, or alters the transport or excretion of bilirubin (see above and Fig. 47.2). For example:
Treatment strategies
These include phototherapy, exchange transfusion and in some cases drug treatments.
Phototherapy
Indications for phototherapy
Commencement is based on serum bilirubin levels and the individual condition of each baby, particularly when jaundice occurs within the first 12–24 hrs:
Consideration of the above individual factors, and serum bilirubin levels <215 μmol/L (13 mg/dL) are usual before stopping phototherapy. Although bilirubin levels can rise following phototherapy, healthy term babies do not require testing to identify this rebound effect. Rebound to clinically significant levels is more likely with prematurity, a positive direct Coombs test, and in those treated before 72 hrs (Kaplan et al 2006).
Types of phototherapy
Phototherapy reduces levels of unconjugated bilirubin in the blood and decreases the likelihood of neurotoxicity or kernicterus. The skin surface is exposed to high intensity light, which converts fat-soluble unconjugated bilirubin into water-soluble bilirubin that can be excreted in bile and urine. Commercially available phototherapy systems include those delivering light via fluorescent bulbs, halogen quartz lamps, light-emitting diodes and fibreoptic mattresses (Stokowski 2006).
Side-effects of phototherapy
Side-effects of conventional white and blue fluorescent phototherapy can include:
Midwifery care and phototherapy
Midwives and family are usually responsible for infant care either in hospital or at home. Phototherapy may be intermittent or continuous (interrupted only for essential care). In a UK study, fibreoptic home treatment was successful with 22 full term, well babies. No babies required re-admission, and all families preferred home treatment (Walls et al 2004). With both systems, but particularly with conventional phototherapy, babies need to be monitored:
Exchange transfusion
Excess bilirubin is removed from the baby during a blood exchange transfusion. With HDN, sensitized erythrocytes are replaced with blood compatible with both the mother’s and the infant’s serum. In recent years, cord blood screening and advances in phototherapy have reduced exchange transfusion for infants with many haemolytic and enzyme deficiency diseases. Except with very premature babies and Rh incompatibility, exchange transfusion may only be used when phototherapy has failed, or there is a risk of kernicterus.
As with phototherapy, exchange transfusion is considered at a lower serum bilirubin level with haemolysis, in smaller, sick or pre-term babies, and jaundice during the first 12–24 hrs:
In most cases exchange transfusion is carried out in a neonatal intensive care unit (refer to individual hospital protocol). A Cochrane review (Thayyil & Milligan 2006) found insufficient evidence to support a change from double to single volume exchange transfusion for severe jaundice in newborns. Complications can result from the procedure and from blood products. Babies with other medical problems are more likely to have severe complications such as hypocalcaemia, thrombocytopenia and a higher death rate. Necrotizing enterocolitis also increases with exchange transfusions (see Chs 43 and 44).
Drug treatments
Metalloporphyrins are being used experimentally to reduce levels of unconjugated bilirubin in neonates. In a Cochrane Review, Suresh et al (2003) concluded routine treatment of neonatal unconjugated hyperbilirubinaemia with a metalloporphyrin cannot be recommended at present. While treatment may decrease phototherapy and hospitalization, no evidence supported or refuted a decrease in neonatal kernicterus, long-term neurodevelopmental impairment, or increased cutaneous photosensitivity.
Neonatal infection
Modes of acquiring infection
Babies may acquire infections through the placenta (transplacental infection), from amniotic fluid as they traverse the birth canal, or after birth from sources such as carers’ hands, contaminated objects or droplet infection.
Vulnerability to infection
Newborns are more immunodeficient and prone to infection than older children and adults, as full immunocompetence requires innate (natural) and acquired immune responses. Pre-term babies are more vulnerable as placental transfer of IgG mainly occurs after 32 weeks’ gestation. Cytokines in maternal and fetal tissues influence physical immunity of the fetus and neonate. They play a leading role in the perinatal period, with their inter-regulation critical for normal function and maturation of neonatal immunity.
Innate immunity
Babies initially depend on natural or innate immunity that does not require previous exposure to microorganisms and is a first line of defence against infection. This includes intact skin, mucous membranes, gastric acid and digestive enzymes. However, newborn skin is more easily damaged and the bowel is not immediately colonized with normal protective flora (Hale 2007).
Acquired immunity
This antigen specific immunity develops and improves from ongoing exposure to a pathogen or organism. The newborn has some maternal immune protection, but immunoglobulins are deficient. Maternal exposure and transfer of IgG across the placenta provides passive protection during the first months of life, while breastfeeding confers increased immune protection. During the early weeks the baby also has deficiencies in the quantity and the quality of neutrophils.
Of particular importance for midwives is the increasing evidence base supporting vaginal birth and breastfeeding in the functioning and maturation of neonatal host defences. Vaginal birth promotes the production of various cytokines and their receptors, with significantly higher levels when compared to elective caesarean section (Malamitsi-Puchner et al 2005, Nesin & Cunningham-Rundles 2000). Breastfeeding increases the baby’s immune protection through the transmission of secretory IgA in breastmilk (see Ch. 41).
Management of infection
The midwife’s role in the management of fetal and neonatal infection includes prevention, diagnosis and treatment of infection in mother, baby and midwife. Meeting these individual needs may involve high levels of collaboration with other professionals and agencies.
Prevention of infection in the mother
Before, during and after pregnancy, good midwifery practice involves evidence-based prevention that informs each woman of potential sources of infection that may harm her or her child. For example, informing women of such things as the importance of avoiding high risk foods, countries or areas with a high prevalence of some infections and contact with individuals with infectious diseases (see below).
Prevention or treatment of infection in the mother during pregnancy can often prevent or reduce short and long term sequelae in her child (see Ch. 23). In the UK, Group B streptococcus (GBS) is the most frequent cause of serious neonatal bacterial sepsis. In high-risk pregnancies, early-onset neonatal GBS infection can be reduced with antibiotics during birth (Law et al 2005). Similarly, antibiotic use for pre-term rupture of membranes is associated with reduced neonatal morbidity (Kenyon et al 2003).
Prevention of infection in the newborn
A safe environment is of central importance, particularly in hospital where babies are at risk of cross-infection. Careful, frequent hand washing with soap or alcohol remains the most important method of preventing infection. In busy situations cleansing with an alcohol-based hand-rub solution may be the most practical means of improving staff compliance, while wearing gloves further reduces contamination. In their Cochrane Review, Gould et al (2007) recommended research to assess short and longer-term strategies to improve hand hygiene compliance, and determine if this reduced rates of infection. Other midwifery strategies to reduce infection in all environments include:
For asymptomatic term neonates of mothers with risk factors for neonatal infection, insufficient evidence exists to guide clinical practice on prophylactic versus selective antibiotic treatment. Ungerer et al (2004) identified the need for a large randomized controlled trial to compare the effects of prophylactic versus selective antibiotics on infant morbidity, mortality and costs.
Prevention of infection in the midwife
This is an important midwifery practice issue, as all health professionals are at risk of exposure to bloodborne infection. Universal precautions are based on the routine use of techniques that reduce exposure to blood, other body fluids and tissue that may contain bloodborne pathogens, and every client is considered a possible source of infection. In England, the Department of Health (DH) (1998) recommended the following precautions to avoid exposure to body fluids:
Diagnosis of infection
In newborns, early signs of infection may be subtle and difficult to distinguish from other problems. The mother or midwife may simply feel the baby is ‘off colour’ (see Ch. 43). Newborn risk factors include a maternal history of prolonged rupture of membranes, pyrexia during birth, chorioamnionitis, and offensive amniotic fluid.
Treatment of infection
The overall aim is to reduce the risk of septicaemia and life-threatening septic shock in this vulnerable group. Good management includes:
Infections acquired before or during birth
Infections may be acquired through the placenta, from amniotic fluid, or the birth canal. For management of sexually transmissible and reproductive tract infections, see Ch. 23. Other infections are discussed in this chapter, emphasizing those where midwives have a critical preventive role. Viral infections such as rubella and varicella (chickenpox) can be a major cause of fetal morbidity and mortality, as can the parasite toxoplasmosis. Candida albicans, a yeast fungus, as well as causing infant thrush, can also result in systemic candidiasis and death in very pre-term infants.
Rubella
For most immunocompetent children and adults (including pregnant women), the rubella virus causes a mild, insignificant illness spread by droplet infection. Maternal rubella is now rare in many countries with rubella vaccination programmes (Robinson et al 2006). Congenital rubella syndrome (CRS) remains a major cause of developmental anomalies including blindness and deafness (Banatvala & Brown 2004).
Incidence and effects during pregnancy
In most industrialized countries the measles, mumps and rubella (MMR) vaccine has reduced rubella incidence and with it CRS, although in recent years in the UK and some other countries, vaccination rates have declined due to concerns about the vaccine (see prevention). Countries without routine MMR programmes report rates similar to those of industrialized countries before vaccination (Banatvala & Brown 2004). With primary rubella infection during the first 12 weeks of pregnancy maternal-fetal transmission rates are as high as 85%. Intrauterine infection is unlikely when the mother’s rash appears before, or within 11 days after the last menstrual period, and with proven infection later than the 16th week, the risk of severe fetal sequelae is less (Enders et al 1988).
First trimester infection can result in spontaneous abortion and in surviving babies a number of serious and permanent consequences. These include cataracts, sensorineural deafness, congenital heart defects, microcephaly, meningoencephalitis, dermal erythropoiesis, thrombocytopenia and significant developmental delay (Banatvala & Brown 2004, Bedford & Tookey 2006).
Diagnosis and treatment
Diagnosis of congenital rubella may include:
Most women with first trimester infection require a great deal of information and support, and some may request termination of pregnancy. Following the birth, management is symptomatic, emphasizing support for parents, and referrals to ensure best outcomes for babies. Infants with CRS are highly infectious and should be isolated from other infants and pregnant women (but not their own mothers). Long-term follow up is essential, as some problems may not become apparent until babies are older.
Prevention
In the UK, a number of factors may contribute to CRS:
Midwives need to emphasize the importance of avoiding contact with rubella during pregnancy, as reinfection has been reported despite previous vaccination. As part of their extended public health role, midwives can encourage vaccination for seronegative women before and after, (but not during pregnancy), and also discuss the importance of vaccinating their child. Strategies targeting all children, and offering vaccine to susceptible schoolgirls or women before pregnancy offer the best protection against CRS. Those working with pregnant women may also be offered rubella vaccination.
Midwives and other health professionals can also use evidence-based medicine to offer immunization and health education to groups with low rates. For example, in the UK, women in urban areas have the lowest rates of MMR cover (particularly inner city areas). They may also have the highest levels of deprivation (Wright & Polack 2006). Those born outside the UK, e.g. African- and Asian-born women are more susceptible to a rubella outbreak (Bedford & Tookey 2006). Similarly, in Sydney, Australia, country of birth was a strong predictor of immunity, with 65% non-immune Asian-born women compared with 13% Australian-born. Other significant risk factors for non-immunity were maternal age >35 years and nulliparity (Sathanandan et al 2005).
Also essential are evidence-based programmes (e.g. by midwives and health visitors) to address concerns about perceived adverse effects of measles-mumps-rubella vaccine. Often related to higher levels of education, these concerns include associations between MMR vaccine, autism, and Crohn’s disease (Banatvala & Brown 2004, Wright & Polack 2006). In their Cochrane Review, Demicheli et al (2005) found MMR unlikely to be associated with Crohn’s disease, ulcerative colitis, autism or aseptic meningitis. However, overall in the 31 reviewed studies MMR was associated with irritability, febrile convulsions, benign thrombocytopenic purpura, parotitis, joint and limb complaints and aseptic meningitis (mumps). The authors commented on the inadequacy of design and reporting of safety outcomes in MMR vaccine studies, and on the absence of studies on the effectiveness of MMR that fulfilled Cochrane inclusion criteria (see Ch. 51 for further discussion on immunization).
Varicella zoster
Varicella zoster virus (VZV) is a highly contagious DNA virus of the herpes family that causes varicella (chickenpox). Transmitted by respiratory droplets and contact with vesicle fluid, it has an incubation period of 10–20 days and is infectious for 48 hrs before the rash appears until vesicles crust over. After primary infection the virus remains dormant in sensory nerve root ganglia, with any recurrent infection resulting in herpes zoster or shingles (Heininger & Seward 2006). Primary infection during pregnancy can result in serious outcomes (Meyberg-Solomayer et al 2006).
Incidence and effects during pregnancy
Up to 90% of women born in countries such as the UK have had chickenpox before pregnancy, and are seropositive for VZV immunoglobulin G (IgG) antibodies. However, many tropical and subtropical areas have lower rates of chickenpox during childhood, leaving women at increased risk for primary infection during pregnancy (Pinot de Moira et al 2006). In adults chickenpox can also be more severe and may be complicated by pneumonia, hepatitis and encephalitis. A UK confidential enquiry reported 7 maternal deaths associated with varicella infection during pregnancy between 1985 and 1997 (cited in Heuchan & Isaacs 2001).
Fetal effects vary with gestation at the time of maternal infection.
Diagnosis and treatment
Diagnosis of FVS can include a recent history of maternal chickenpox, polymerase chain reaction (PCR) to identify the specific infectious agent, VZV DNA detection in amniotic fluid, and prenatal ultrasound. Continuing improvements in ultrasonography are valuable in confirming the effects of FVS e.g. limb contractures and deformities, cerebral anomalies, borderline ventriculomegaly, intracerebral, intrahepatic and myocardial calcifications, articular effusions, and intrauterine growth retardation (Degani 2006, Meyberg-Solomayer et al 2006).
Most pregnant women with chickenpox will need a great deal of information and support. Women infected during the first 20 weeks may request termination of pregnancy. Although mother and baby should be isolated from others, they should always be kept together. Varicella zoster immune globulin (VZIG) can be offered to seronegative pregnant women who are exposed to chickenpox, within 72 hrs of contact, and always within 10 days. With parental permission, VZIG should also be given to a baby whose mother develops chickenpox between 7 days before and 28 days after the birth, or whose siblings at home have chickenpox (if the mother is seronegative). Informed consent is essential as VZIG is a human blood product (Heuchan & Isaacs 2001, Murguia-de-Sierra et al 2005).
Although no clinical trials could be found to confirm that antiviral chemotherapy prevents CVS, the antiviral drug acyclovir may reduce the mortality and risk of severe disease in some groups, particularly if VZIG is not available. These include pregnant women with severe complications, and newborns if they are unwell or have added risk factors such as prematurity or corticosteroid therapy (Hayakawa et al 2003, Sauerbrei & Wutzler 2000).
Prevention
At the present time, and particularly in the UK, health education remains the most effective midwifery preventive strategy. As with rubella, midwives need to emphasize the importance of avoiding contact with chickenpox during pregnancy. Antenatal screening is only cost effective as part of a screening and vaccination programme or for groups of women who are at increased risk. Varicella childhood immunization programmes are available in some countries, e.g. the USA, Australia, Uruguay, Germany, Taiwan, and Canada (Heininger & Seward 2006).
As part of their extended public health role, and where varicella vaccine is readily available, midwives can encourage vaccination for seronegative women before and after, (but not during pregnancy until safety is proven) and also discuss the importance of vaccinating their child. Where varicella vaccine is readily available, midwives and other health professionals can also offer immunization and health education to groups at increased risk. These include women who have emigrated from tropical countries which have lower childhood chickenpox rates, such as Bangladesh (Pinot de Moira et al 2006).
Using a cohort model, Pinot de Moira et al (2006) assessed costs and benefits of screening and vaccination. For susceptible women, verbal plus serological screening would cost less for both UK- and Bangladesh-born women. Universal screening costs more, but was more effective and could be cost-effective in younger immigrant women.
Toxoplasmosis
Toxoplasmosis is caused by Toxoplasma gondii (T. gondii), a protozoan parasite infecting up to a third of the world’s population. It is found in uncooked meat and cat and dog faeces. Primary infection can be asymptomatic, or characterized by malaise, lymphadenopathy and ocular disease. Primary infection during pregnancy can cause severe damage to the fetus (Montoya & Liesenfeld 2004). Childhood acquired infection also causes half of toxoplasma ocular disease in UK and Irish children (Gilbert et al 2006).
Incidence and effects during pregnancy
A survey of 1897 pregnant women in Kent, UK, identified a seroprevalence rate of 9.1%. A higher seroprevalence was associated with living in a rural location or in Europe as a child (not UK), feeding a dog raw meat and increased age. This 9.1% toxoplasma immune status leaves 90% at risk of primary infection during pregnancy. However, toxoplasma prevalence in the UK has declined since the 1960s (Nash et al 2005). Increasing gestational age at seroconversion increases the risk of mother-to-child transmission (Systematic Review on Congenital Toxoplasmosis, SYROCOT 2007).
Risks for the infected fetus can include intrauterine death, low birthweight, enlarged liver and spleen, jaundice and anaemia, intracranial calcifications, hydrocephalus and retinochoroidal and macular lesions. Infected neonates may be asymptomatic at birth, but can develop retinal and neurological disease. Those with subclinical disease at birth can develop seizures, cognitive and motor problems and reduced cognitive function over time (Gilbert et al 2006, Schmidt et al 2006, SYROCOT 2007). For example, in one group of 38 children with confirmed toxoplasma infection, 58% had congenital infection. Of these, 9% were stillborn, while 32% of the live births had intracranial abnormalities and/or developmental delay, and 45% had retinochoroiditis with no other abnormalities. Of the 42% of children infected after birth, all had retinochoroiditis (Gilbert et al 2006).
Diagnosis and treatment
Diagnosis can be made by direct detection of the parasite, or serological techniques. Antenatally, a T. gondii polymerase chain reaction (PCR) and ultrasound can be used.
Sonographic findings can often enable diagnosis of specific congenital syndromes with serial scanning as conditions change (Degani 2006). Postnatally, T. gondii IgM antibodies may be detected from eluate on the infant’s PKU Guthrie filter paper card (Montoya & Liesenfeld 2004, Schmidt et al 2006).
The effectiveness of antenatal treatment in reducing the congenital transmission of T. gondii is not proven. A meta-analysis of 1438 treated mothers (26 cohorts) also found no evidence that antenatal treatment significantly reduced the risk of clinical symptoms (SYROCOT 2007). Infants with congenital toxoplasmosis are usually treated with pyrimethamine, sulfadiazine and folinic acid for an extended period (Montoya & Liesenfeld 2004, Schmidt et al 2006).
Prevention
Midwives have an essential role in prevention, as health education can result in a 92% reduction in pregnancy seroconversion. Breugelmans et al (2004) found the most effective strategy was a leaflet explaining toxoplasmosis and how to avoid the condition during pregnancy, with this information reinforced in antenatal classes. In the UK, the Toxoplasmosis Trust (1998) educates health professionals and the public (a handbook for midwives is available from the trust). Appropriate information includes advising women about washing kitchen surfaces following contact with uncooked meats, stringent hand washing and avoiding cat and dog faeces.
Of relevance for midwives is an ongoing knowledge of countries with high rates of toxoplasmosis, e.g. France and Brazil, where women may travel. Primary prevention strategies also need to address the toxoplasma ocular disease acquired after birth by UK and Irish children (Gilbert et al 2006).
Maternal serologic screening for toxoplasmosis during pregnancy is offered in some countries. However, controversy exists about primary and secondary prevention (see Montoya & Liesenfeld 2004). Some suggest the current UK policy of not offering prenatal or neonatal screening is supported by the absence of evidence of effective antenatal treatment (Gilbert et al 2006, SYROCOT 2007). The latter reviewers recommended a large randomized clinical trial to identify potential benefits of prenatal treatment.
Candida
Candida is a Gram-positive yeast fungus with a number of strains (see Ch. 23). C. albicans is responsible for most fungal infections, including thrush in infants. Infection can affect the mouth (oral candidiasis), skin (cutaneous candidiasis) and other organs (systemic candidiasis).
Oral candidiasis
Thrush presents as white patches on the baby’s gums, palate and tongue. It can be acquired during birth and from caregivers’ hands or feeding equipment. Raw areas (removed by sucking) on the edge of the infant tongue can assist diagnosis. Risk factors for infant thrush include bottle use during the first 2 weeks, the presence of siblings (Morrill et al 2005), and antibiotic exposure (Dinsmoor et al 2005). Breastfeeding women may also have infected breasts, with flaky or shiny skin of the nipple/areola, sore, red nipples and persistent burning, itching or stabbing pain in the breasts (see Ch. 41). Risk factors for maternal thrush include bottle use in the first 2 weeks after the birth, pregnancy duration of >40 weeks (Morrill et al 2005), and intrapartum antibiotic use (Dinsmoor et al 2005). In a further study of 100 healthy breastfeeding mothers, thrush was best predicted by three or more simultaneous signs or symptoms, or by flaky or shiny skin of the nipple/areola, together or in combination with breast pain (Francis-Morrill et al 2004).
Accurate midwifery diagnosis and treatment of thrush is important for continued breastfeeding. Morrill et al (2005), found 43% of women with thrush 2 weeks after the birth were breastfeeding at 9 weeks, compared with 69% of women with a negative diagnosis. Nystatin is possibly the most effective and least expensive treatment: oral for the baby and topical breast application for the mother (see Wiener 2006 for differential diagnosis and treatment options).
Cutaneous candidiasis
Cutaneous candidiasis presents as a moist papular or vesicular skin rash, usually in the region of the axillae, neck, perineum or umbilicus. Cutaneous fungal infections are found in healthy full-term newborns as well as those who are premature or immunocompromised. Usually benign, recognition and treatment is important in preventing adverse outcomes (Smolinski et al 2005). Management includes keeping the area dry and applying topical nystatin. In pre-term babies the thin cutaneous barrier may contribute to the early onset of systemic Candida infection. Antifungal prophylaxis may be used to prevent systemic Candida colonization, e.g. oral nystatin or fluconazole.
Disseminated candidiasis
Systemic colonization with Candida is associated with such factors as low birth weight, low gestational age, exposure to third-generation cephalosporins, endotracheal intubation, longer stays in the NICU, bacterial sepsis, and colonization of central venous catheter or endotracheal tube (Manzoni et al 2006). Prompt management is essential as the condition can be life threatening, with a high death rate in very low birth weight infants. Complications can include meningitis, endocarditis, pyelonephritis, pneumonia and osteomyelitis. Fluconazole prophylaxis decreases the risk of neonatal candidiasis (Manzoni et al 2006). Treatment may include oral nystatin, fluconazole and amphotericin B. Newer antifungal agents, including voriconazole and caspofungin show promise in treating potentially fatal neonatal fungal infections (Smolinski et al 2005). Clerihew & McGuire (2004), in their Cochrane Review, emphasized the need for a large randomized controlled trial to compare amphotericin B with newer antifungal preparations.
Ophthalmia neonatorum
Ophthalmia neonatorum is a notifiable condition, defined in England as any purulent eye discharge within 21 days of birth, and in Scotland as eye inflammation within 21 days of birth accompanied by a discharge. The condition is usually acquired during vaginal birth. A swab must be taken for culture and sensitivity testing, with immediate medical referral. Differential diagnosis of the organism is essential as chlamydial and gonococcal infections can cause conjunctival scarring, corneal infiltration, blindness and systemic spread (see Ch. 23). Other causes of inflammation must also be excluded. Treatment includes local cleaning and care of the eyes with normal saline, and appropriate drug therapy for the baby and mother if required.
Some infections acquired after birth
After the birth the most common routes for neonatal infection are the umbilicus, broken skin, the respiratory tract and those that result from invasive procedures and devices. Infections acquired after birth are also discussed in Chapters 43 and 44.
Meningitis
Neonatal meningitis is an inflammation of the membranes lining the brain and spinal column caused by such organisms as group B streptococci (GBS), E. coli, Listeria monocytogenes, and less often Candida and herpes. In the UK, neonatal meningitis is most often caused by GBS (Law et al 2005). In Australia and New Zealand, the incidence of GBS early onset neonatal bacterial meningitis decreased significantly between 1993 and 2002, while the incidence of Escherichia coli meningitis remained the same (May et al 2005).
Very early signs may be non-specific, followed by those of meningeal irritation and raised intracranial pressure such as crying, irritability, bulging fontanelle, increasing lethargy, tremors, twitching, severe vomiting, diminished muscle tone and alterations in consciousness. Babies may also present with hemiparesis, horizontal deviation and decreased pupillary reaction of the eye, decreased retinal reflex and an abnormal Moro reflex.
Early diagnosis and treatment are critical to prevent collapse and death. Diagnosis may be confirmed by examination of cerebrospinal fluid (CSF). Very ill babies require intensive care, intravenous fluids and antibiotic therapy. Although acute phase mortality has declined in recent years, long-term neurological complications still occur in many surviving infants. For example, in one group aged 5 years, 23% had a serious disability, with isolation of bacteria from CSF the best single predictor (de Louvois et al 2005). For such infants, long-term comprehensive developmental assessment is essential, including audiometry and vision testing.
Eye infections
Mild eye infections are common in babies and can be treated with routine eye care and antibiotics if required. Other more serious conditions must be excluded, such as ophthalmia neonatorum (see above), trauma, foreign bodies, congenital glaucoma and nasolacrimal duct obstruction.
Skin infections
Newborn skin lesions include septic spots or pustules, either as solitary lesions or clustered in the umbilical and buttock areas. For well babies with limited pustules, regular cleaning with an antiseptic solution is adequate. Antibiotic therapy may be required for more extensive pustules (see Ch. 43). Neonatal fungal skin infections can range from benign conditions such as congenital candidiasis and neonatal cephalic pustulosis to serious infections in very low birthweight or immunocompromised neonates (Smolinski et al 2005).
Respiratory infections
These may be minor (nasopharyngitis and rhinitis) or more severe such as pneumonia (see Chs 43 and 44).
Gastrointestinal tract infections
In newborns these can include gastroenteritis or the more severe NEC. Causative organisms for gastroenteritis include rotavirus, Salmonella, Shigella and a pathogenic strain of E. coli. The secretory IgA in breastmilk offers important protection against these organisms, particularly rotavirus. Treatment depends on the severity of symptoms. With nausea and vomiting, correction of fluid and electrolyte imbalance is urgent to avoid dehydration (see Ch. 43).
Umbilical infection
Signs can include localized inflammation and an offensive discharge. In their Cochrane Review of 10 studies, Zupan et al (2004) found keeping the cord clean was as effective and safe as using antiseptics or antibiotics. Although antiseptics prolonged cord separation time, they also reduced the mother’s concerns. Untreated infection can spread to the liver via the umbilical vein and cause hepatitis and septicaemia. Treatment can include regular cleaning, antibiotic powder and antibiotic therapy (see Ch. 43).
Urinary tract infections
Urinary tract infections can result from bacteria such as E. coli, or less often from a congenital anomaly that obstructs urine flow. The signs are usually those of an early non-specific infection, and diagnosis is usually confirmed through laboratory evaluation of a urine sample (see Ch. 43).
Ahlfors CE, Wennberg RP. Bilirubin-albumin binding and neonatal jaundice. Seminars in Perinatology. 2004;28(5):334-339.
Alcock GS, Liley H. Immunoglobulin infusion for isoimmune haemolytic jaundice in neonates (Cochrane Review). The Cochrane Library. 2002. Online. Available http://www3.interscience.wiley.com/cgi-bin/home, issue 3
Allaby M, Forman K, Touch S, et al. The use of routine anti-D prophylaxis antenatally to Rhesus negative women. Nottingham and Sheffield: Trent Institute for Health Services Research, Universities of Leicester, 1999. ref 99/04
Banatvala JE, Brown DWG. Rubella. Lancet. 2004;363(9415):1127-1137.
Bedford H, Tookey P. Rubella and the MMR vaccine. Nursing Times. 2006;102(5):55-57.
Bertini G, Dani C, Tronchin M, et al. Is breast-feeding really favoring early neonatal jaundice. Pediatrics. 2001;107(3):E41.
Bianchi DW, Avent ND, Costa JM, et al. Noninvasive prenatal diagnosis of fetal rhesus D. Ready for prime(r) time. Obstetrics and Gynecology. 2005;106(4):841-844.
Breugelmans M, Naessens A, Foulon W. Prevention of toxoplasmosis during pregnancy – an epidemiologic survey over 22 consecutive years. Journal of Perinatal Medicine. 2004;32(3):211-214.
Clerihew L, McGuire W. Systemic antifungal drugs for invasive fungal infection in pre-term Infants (Cochrane Review). The Cochrane Library. 2004. Online. Available http://www3.interscience.wiley.com/cgi-bin/home, issue 1
Craparo FJ, Bonati F, Gementi P, et al. The effects of serial intravascular transfusions in ascitic/hydropic RhD-alloimmunized fetuses. Ultrasound in Obstetrics and Gynecology. 2005;25(2):144-148.
Crowther C, Middleton P. Anti-D administration after childbirth for preventing Rhesus alloimmunization. The Cochrane Library. 2001. Online. Available http://www3.interscience.wiley.com/cgi-bin/home, issue 3
Crowther CA, Keirse MJNC. Anti-D administration during pregnancy for preventing Rhesus alloimmunization (Cochrane review). The Cochrane Library. 1999. Online. Available http://www3.interscience.wiley.com/cgi-bin/home, issue 2
David M, Smidt J, Frank CK, et al. Risk factors for fetal-to-maternal transfusion in Rh D-negative women – results of a prospective study on 942 pregnant women. Journal of Perinatal Medicine. 2004;32(3):254-257.
Degani S. Sonographic findings in fetal viral infections: a systematic review. Obstetrical and Gynecological Survey. 2006;61(5):329-336.
de Louvois J, Halket S, Harvey D. Neonatal meningitis in England and Wales: sequelae at 5 years of age – European. Journal of Pediatrics. 2005;164(12):730-734.
Demicheli V, Jefferson T, Rivetti A, et al. Vaccines for measles, mumps and rubella in children (Cochrane Review). The Cochrane Library. 2005. Online. Available http://www3.interscience.wiley.com/cgi-bin/home, issue 4
Dinsmoor MJ, Viloria R, Lief L, et al. Use of intrapartum antibiotics and the incidence of postnatal maternal and neonatal yeast infections. Obstetrics and Gynecology. 2005;106(1):19-22.
Djokomuljanto S, Quah BS, Surini Y, et al. Efficacy of phototherapy for neonatal jaundice is increased by the use of low-cost white reflecting curtains. Archives of Disease in Childhood: Fetal and Neonatal Edition. 2006;91(6):F439-F442.
DH (Department of Health). Guidance for clinical health care workers: protection against infection with blood-borne viruses. Recommendations of the Expert Advisory Group on AIDS and the Advisory Group on Hepatitis. DH, London, 1998.
Ebbesen F, Agati G, Pratesi R. Phototherapy with turquoise versus blue light. Archives of Disease in Childhood: Fetal and Neonatal Edition. 2003;88(5):F430-F431.
Enders G, Pacher UN, Miller E, et al. Outcome of confirmed periconceptional maternal rubella. Lancet. 1988;1(8600):1445-1446.
Finning K, Martin P, Daniels G. A clinical service in the UK to predict fetal Rh (Rhesus) D blood group using free fetal DNA in maternal plasma. Annals of the New York Academy of Sciences. 2004;1022:119-123.
Francis-Morrill J, Heinig MJ, Pappagianis D, et al. Diagnostic value of signs and symptoms of mammary candidosis among lactating women. Journal of Human Lactation. 2004;20(3):288-295.
Geifman-Holtzman O, Grotegut CA, Gaughan JP. Diagnostic accuracy of noninvasive fetal Rh genotyping from maternal blood – a meta-analysis. American Journal of Obstetrics and Gynecology. 2006;195(4):1163-1173.
Ghi T, Brondelli L, Simonazzi G, et al. Sonographic demonstration of brain injury in fetuses with severe red blood cell alloimmunization undergoing intrauterine transfusions. Ultrasound in Obstetrics and Gynecology. 2004;23(5):428-431.
Gilbert R, Tan HK, Cliffe S, et al. Symptomatic toxoplasma infection due to congenital and postnatally acquired infection. Archives of Disease in Childhood. 2006;91(6):495-498.
Gould DJ, Chudleigh JH, Moralejo D, et al. Interventions to improve hand hygiene compliance in patient care (Cochrane Review). The Cochrane Library. 2007. Online. Available http://www3.interscience.wiley.com/cgi-bin/home, issue 2
Grohmann K, Roser M, Rolinski B, et al. Bilirubin measurement for neonates: comparison of 9 frequently used methods. Pediatrics. 2006;117(4):1174-1183.
Hale R. Protecting neonates’ delicate skin. British Journal of Midwifery. 2007;15(4):231-232. 234–235
Hayakawa M, Kimura H, Ohshiro M, et al. Varicella exposure in a neonatal medical centre: successful prophylaxis with oral acyclovir. Journal of Hospital Infection. 2003;54(3):212-215.
Heininger U, Seward JF. Varicella. Lancet. 2006;368(9544):1306-1307.
Heuchan AM, Isaacs D. The management of varicella-zoster virus exposure and infection in pregnancy and the newborn period. Medical Journal of Australia. 2001;174(6):288-292.
Jangaard KA, Curtis H, Goldbloom RB. Estimation of bilirubin using BiliChek, a transcutaneous bilirubin measurement device: effects of gestational age and use of phototherapy. Paediatrics and Child Health. 2006;11(2):79-83.
Joy SD, Rossi KQ, Krugh D, et al. Management of pregnancies complicated by anti-E alloimmunization. Obstetrics and Gynecology. 2005;105(1):24-28.
Kaplan M, Hammerman C. 6 Glucose-6-phosphate dehydrogenase deficiency: a hidden risk for kernicterus – Seminars in Perinatology. 2004;28(5):356-364.
Kaplan M, Kaplan E, Hammerman C, et al. Post-phototherapy neonatal bilirubin rebound: a potential cause of significant hyperbilirubinaemia. Archives of Disease in Childhood. 2006;91(1):31-34.
Kaplan M, Muraca M, Vreman HJ, et al. Neonatal bilirubin production-conjugation imbalance: effect of glucose-6-phosphate dehydrogenase deficiency and borderline prematurity. Archives of Disease in Childhood: Fetal and Neonatal Edition. 2005;90(2):F123-F127.
Karlsson M, Blennow M, Nemeth A, et al. Dynamics of hepatic enzyme activity following birth asphyxia. Acta Paediatrica. 2006;95(11):1405-1411.
Kenyon S, Boulvain M, Neilson J. Antibiotics for pre-term rupture of membranes (Cochrane Review). The Cochrane Library. 2003. Online. Available http://www3.interscience.wiley.com/cgi-bin/home, issue 2
Law MR, Palomaki G, Alfirevic Z, et al. The prevention of neonatal group B streptococcal disease: a report by a working group of the Medical Screening Society. Journal of Medical Screening. 2005;12(2):60-68.
Maayan-Metzger A, Schwartz T, Sulkes J, et al. Maternal anti-D prophylaxis during pregnancy does not cause neonatal haemolysis. Archives of Disease in Childhood: Fetal and Neonatal Edition. 2001;84(1):F60-F62.
Maisels MJ, Newman TB. Kernicterus in otherwise healthy, breast-fed term newborns. Pediatrics. 1995;96(4):730-733.
Malamitsi-Puchner A, Protonotariou E, Boutsikou T, et al. The influence of the mode of delivery on circulating cytokine concentrations in the perinatal period. Early Human Development. 2005;81(4):387-392.
Manzoni P, Farina D, Leonessa ML, et al. Risk factors for progression to invasive fungal infection in pre-term neonates with fungal colonization. Pediatrics. 2006;118(6):2359-2364.
Mari G, Zimmermann R, Moise KJ, et al. Correlation between middle cerebral artery peak systolic velocity and fetal hemoglobin after 2 previous intrauterine transfusions. American Journal of Obstetrics and Gynecology. 2005;193(3):1117-1120.
May M, Daley AJ, Donath S, et al. Early onset neonatal meningitis in Australia and New Zealand, 1992–2002. Archives of Disease in Childhood. 2005;90(4):F324-F327.
Meyberg-Solomayer GC, Fehm T, Muller-Hansen I, et al. Prenatal ultrasound diagnosis, follow-up, and outcome of congenital varicella syndrome. Fetal Diagnosis and Therapy. 2006;21(3):296-301.
Miqdad AM, Abdelbasit OB, Shaheed MM, et al. Intravenous immunoglobulin G (IVIG) therapy for significant hyperbilirubinemia in ABO hemolytic disease of the newborn. Journal of Maternal-Fetal and Neonatal Medicine. 2004;16(3):163-166.
Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet. 2004;363(9425):1962.
Morrill JF, Heinig MJ, Pappagianis D, et al. Risk factors for mammary candidosis among lactating women. Journal of Obstetric, Gynecologic and Neonatal Nursing. 2005;34(1):37-45.
Murguia-de-Sierra T, Villa-Guillen M, Villaneuva-Garcia D, et al. Varicella zoster virus antibody titers after intravenous zoster immune globulin in neonates, and the safety of this preparation. Acta Paediatrica. 2005;94(6):790-793.
Nanjundaswamy S, Petrova A, Mehta R, et al. Transcutaneous bilirubinometry in pre-term infants receiving phototherapy. American Journal of Perinatology. 2005;22(3):127-131.
Nash JQ, Chissel S, Jones J, et al. Risk factors for toxoplasmosis in pregnant women in Kent, United Kingdom. Epidemiology and Infection. 2005;133(3):475-483.
Nesin M, Cunningham-Rundles S. Cytokines and neonates. American Journal of Perinatology. 2000;17(8):393-404.
Newman TB, Xiong B, Gonzales VM, et al. Prediction and prevention of extreme neonatal hyperbilirubinemia in a mature health maintenance organization. Archives of Pediatrics and Adolescent Medicine. 2000;15(11):1140-1147.
NICE (National Institute for Health and Clinical Excellence). Guidance on the use of routine antenatal anti-D prophylaxis for RhD-negative women. Technology appraisal guidance no 41 (TA41), NICE, London, 2002. Online. Available www./nice.org.uk.
Oepkes D, Seaward G, Vandenbussche FPHA, et al. Doppler ultrasonography versus amniocentesis to predict fetal anemia. New England Journal of Medicine. 2006;355(2):156-164.
Pezzati M, Fusi F, Dani C, et al. Changes in skin temperature of hyperbilirubinemic newborns under phototherapy: conventional versus fiberoptic device. American Journal of Perinatology. 2002;19(8):439-443.
Pinot de Moira A, Edmunds WJ, Breuer J. The cost-effectiveness of antenatal varicella screening with post-partum vaccination of susceptibles. Vaccine. 2006;24(9):1298-1307.
Pritchard MA, Beller EM, Norton B. Skin exposure during conventional phototherapy in pre-term infants: a randomized controlled trial. Journal of Paediatrics and Child Health. 2004;40(5/6):270-274.
Ramagnoli C, Zecca E, Papacci P, et al. Which phototherapy system is most effective in lowering serum bilirubin in very pre-term infants? Fetal Diagnosis and Therapy. 2006;21(2):204-220.
Ratnavel N, Ives NK. Investigation of prolonged neonatal jaundice. Current Paediatrics. 2005;15(2):85-91.
RCOG (Royal College of Obstetricians and Gynaecologists). Use of anti-D immunoglobulin for Rh prophylaxis. 2002. Online. Available www.rcog.org.uk.
Robinson JL, Lee BE, Preiksaitis JK, et al. Prevention of congenital rubella syndrome--what makes sense in 2006? Epidemiologic Reviews. 2006;28:81-87.
Sathanandan D, Gupta L, Liu B, et al. Factors associated with low immunity to rubella infection on antenatal screening. Australian and New Zealand Journal of Obstetrics and Gynaecology. 2005;45(5):435-438.
Sauerbrei A, Wutzler P. The congenital varicella syndrome. Journal of Perinatology. 2000;20(8):548-554.
Schmidt DR, Hogh B, Andersen O, et al. The national neonatal screening programme for congenital toxoplasmosis in Denmark: results from the initial four years, 1999–2002. Archives of Disease in Childhood. 2006;91(8):661-665.
Seidman DS, Moise J, Ergaz Z, et al. A prospective randomized controlled study of phototherapy using blue and blue-green light-emitting devices, and conventional halogen-quartz phototherapy. Journal of Perinatology. March 2003;23(2):123-127.
Setia S, Villaveces A, Dhillon P, et al. Neonatal jaundice in Asian, white, and mixed-race infants. Archives of Pediatrics and Adolescent Medicine. 2002;156(3):276-279.
Shapiro SM, Bhutani VK, Johnson L. Hyperbilirubinemia and kernicterus. Clinics in Perinatology. 2006;33(2):387-410.
Smitherman H, Stark AR, Bhutani VK. Early recognition of neonatal hyperbilirubinemia and its emergent management. Seminars in Fetal and Neonatal Medicine. 2006;11(3):214-224.
Smolinski KN, Shah SS, Honig PJ, et al. Neonatal cutaneous fungal infections. Current Opinion in Pediatrics. 2005;17(4):486-493.
Stokowski LA. Fundamentals of phototherapy for neonatal jaundice. Advances in Neonatal Care. 2006;6(6):303-312.
Suresh GK, Martin CL, Soll RF. Metalloporphyrins for treatment of unconjugated hyperbilirubinemia in neonates (Cochrane Review). The Cochrane Library. 2003. Online. Available http://www3.interscience.wiley.com/cgi-bin/home, issue 1
SYROCOT (Systematic Review on Congenital Toxoplasmosis) Study Group. Effectiveness of prenatal treatment for congenital toxoplasmosis: a meta-analysis of individual patients’ data. Lancet. 2007;369(9556):115-122.
Thayyil S, Milligan DWA. Single versus double volume exchange transfusion in jaundiced newborn infants (Cochrane Review). The Cochrane Library. 2006. Online. Available http://www3.interscience.wiley.com/cgi-bin/home, issue 4
Thomas JT, Muller P, Wilkinson C. Antenatal phenobarbital for reducing neonatal jaundice after red cell isoimmunization (Cochrane Review). The Cochrane Library. 2007. Online. Available http://www3.interscience.wiley.com/cgi-bin/home, issue 2
Toxoplasmosis Trust. Toxoplasmosis survey reveals ignorance and misconceptions: daisy chain campaign aims to end an avoidable tragedy. London: Toxoplasmosis Trust, 1998. 18 March
Tyler W, McKiernan PJ. Prolonged jaundice in the pre-term infant – what to do, when and why. Current Paediatrics. 2006;16(1):43-50.
Ungerer RLS, Lincetto O, McGuire W, et al. Prophylactic versus selective antibiotics for term newborn infants of mothers with risk factors for neonatal infection (Cochrane Review). The Cochrane Library. 2004. Online. Available http://www3.interscience.wiley.com/cgi-bin/home, issue 4
van Dongen H, Klumper FJCM, Sikkel E, et al. Non-invasive tests to predict fetal anemia in Kell-alloimmunized pregnancies. Ultrasound in Obstetrics and Gynecology. 2005;25(4):341-345.
van Kamp IL, Klumper FJCM, Oepkes D, et al. Complications of intrauterine intravascular transfusion for fetal anemia due to maternal red-cell alloimmunization. American Journal of Obstetrics and Gynecology. 2005;192(1):165-170.
Walls M, Wright A, Fowlie P, et al. Home phototherapy: a feasible, safe and acceptable practice. Journal of Neonatal Nursing. 2004;10(3):92-94.
Watchko JF. Hyperbilirubinemia and bilirubin toxicity in the late pre-term infant. Clinics in Perinatology. 2006;33(4):839-852.
Wiener S. Diagnosis and management of candida of the nipple and breast. Journal of Midwifery and Women’s Health. 2006;51(2):125-128.
Wright JA, Polack C. Understanding variation in measles-mumps-rubella immunization coverage – a population-based study. European Journal of Public Health. 2006;16(2):137-142.
Zupan J, Garner P, Omari AA. Topical umbilical cord care at birth (Cochrane Review). The Cochrane Library. 2004. Online. Available http://www3.interscience.wiley.com/cgi-bin/home, issue 3