Chapter 42 The healthy low birthweight baby
It is now generally accepted that healthy babies between 32 and 37 weeks’ gestation, with a birthweight of 1.7–2.5kg, do not need automatic admission to a neonatal intensive care unit (NICU) but instead can be cared for on a postnatal ward, with their mother. There is no doubt that low birthweight (LBW) babies are more vulnerable to illness compared with appropriately grown term babies, and extra monitoring may be a necessary precaution, particularly during the period of adaptation to extrauterine life and the early neonatal period. Caring for healthy LBW babies on a postnatal ward is thought to be advantageous because it removes them from the greater hazard of infection that occurs in neonatal units, prevents separation of mother and baby and finally, eliminates the need for parents to visit the neonatal unit, which is for many, an alien and intimidating environment (Fowlie & McHaffie 2005). There is growing evidence that the majority of these babies remain well, will have minimal or no illness in the neonatal period (Roberton 1999) and can be cared for by midwives as the lead professional. It therefore follows that neonatal nurses will be more able to focus their care on the critically ill, and those babies that are healthy but <1.70kg, thus utilizing neonatal nurse skills and cot resources to their best effect.
Introduction
Between 6% and 7% of all babies born in the UK weigh <2500g at birth. In 1977, the World Health Organization (WHO 1977a) recommended that babies who weigh <2500g should be called low birthweight (LBW). According to Tucker & McGuire (2005) pre-term babies make up two-thirds of LBW babies with the other one-third being small for their gestational age (SGA). Spencer (2003) asserts that 70% of these LBW babies will weigh between 2000 and 2500g. The concepts and categories that surround LBW are complex. The following classification describes the different types of LBW babies seen in practice.
Classification of babies by weight and gestation
Definitions of low birthweight are based upon weight alone and do not consider the gestational age of the baby. Likewise, definitions of gestational age disregard any considerations of birthweight.
Weight
As neonatal technology and care have become more effective, new birthweight categories have been devised to further define babies by birthweight. The low birthweight categories are:
Gestational age
A pre-term baby is one born before completion of the 37th gestational week. Gestational weeks are calculated from the first day of the last menstrual period (LMP) and have no relevance to the baby’s weight, length, head circumference, or indeed any other measurement of fetal or neonatal size. As ultrasound scanning has become more accurate, the expected date of delivery (EDD) is sometimes re-calculated at 11–12 weeks, based on the estimation of the fetal crown–rump length. When this data is available, the LMP calculation is disregarded.
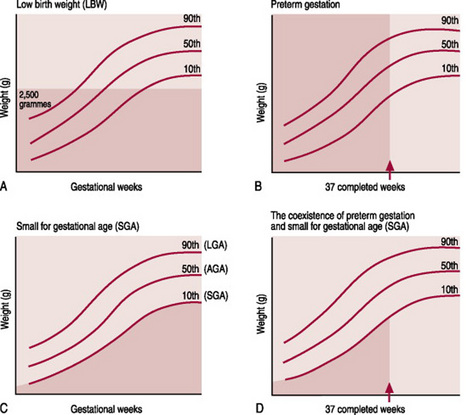
Thus, it is the relationship between these two separate considerations of weight (for assessment of growth) and gestational age (for assessment of maturity) that is of great importance. This relationship can be plotted on centile charts (Fig. 42.1); these charts visually demonstrate that growth is appropriate, excessive or diminished for gestational age and that the baby is either pre-term, term or post-term. They are based on measurements of fetal growth that have been collected over the last 20 years, from multiple ultrasound measurements, but have limitations. Farrer (2005) believes that to act as a more accurate tool, growth charts should be derived from studies of local populations, because genetically derived growth differences exist between countries, cultures and lifestyles. Tucker & McGuire (2005) also assert that in most of the UK, data on birthweight is collected routinely, but often there is uncertainty and incomplete recording of estimates of gestation.
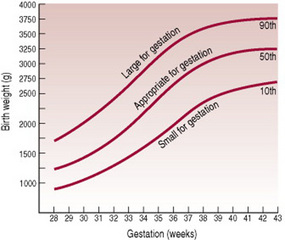
Figure 42.1 A centile chart, showing weight and gestation.
(From Simpson 1997, with permission from Baillière Tindall.)
Various types of LBW babies can be described:
Babies are considered large for their gestational age (LGA) at any weight when they fall above the 90th centile. Therefore, it follows that both term and pre-term babies can be SGA, AGA or LGA (Fig. 42.2).
The small for gestational age (SGA) baby
Expert care of newborn babies requires an understanding of intrauterine growth patterns as they relate to gestational age. A baby’s clinical course following birth is largely determined by these factors.
Intrauterine growth restriction rate (IUGR)
Tappero & Honeyfield (2003) define IUGR as a rate of fetal growth that is less than normal for the population and for the growth potential of a specific baby.
The relationship between IUGR and SGA
In the past, the terms IUGR and SGA were used interchangeably. Although related, they are not synonymous. IUGR is a failure of normal fetal growth caused by multiple adverse effects on the fetus, whereas SGA describes a baby whose weight is lower than population norms. SGA babies are defined as having a birth weight below the 10th centile for gestational age, or <2 standard deviations below the mean (the 50th centile) for the gestational age. Thus, all IUGR babies may not be SGA and all SGA babies may not be small as a result of growth restriction (Gomella et al 1999). Indeed, Farrer (2005) contends that at least half of the SGA babies in Britain have no known aetiology. They are proportionately small; their weight, length and head circumference are all on the 3rd centile or below. It is generally accepted that their small parents or grandparents genetically determine their smallness. They are well, healthy babies who need to be treated accordingly and do not need overzealous labeling, which may lead to unwarranted wasteful and potentially harmful interventions (Farrer 2005).
Causes of IUGR
Fetal growth is regulated by maternal, placental and fetal factors and represents a mix of genetic mechanisms and environmental influences through which growth potential is expressed. The mechanisms that appear to limit fetal growth are multifactorial and are presented in Box 42.1.
Box 42.1 Causes of intrauterine growth restriction
Sources: Blackburn (2003), Mupanemunda & Watkinson (2005), Spencer (2003), Tappero & Honeyfield (2003).
Classification of IUGR
Factors that influence fetal growth can be intrinsic or extrinsic.
Intrinsic factors
These are factors that operate from within the fetus, that arise from chromosomal or genetic abnormalities, or alternatively from infective agents that are transplacental in origin and act by altering the normal process of cell division.
Extrinsic factors
These are factors that influence the fetus through its intrauterine environment.
The clinical appearance and behaviour of the baby at birth can provide relevant information as to the type of growth disruption that may have been sustained and help the midwife to anticipate any future potential problems. There are two types of IUGR clinically recognized.
Asymmetric growth (sometimes called acute)
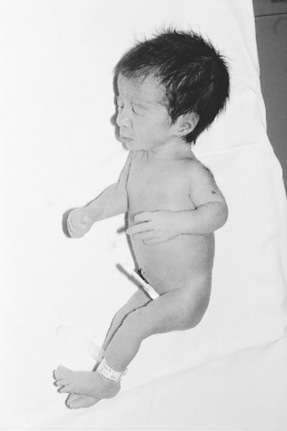
Here the fetal weight is reduced out of proportion to the length and head circumference; this is thought to be caused by extrinsic factors such as pregnancy-induced hypertension (Mupanemunda & Watkinson 2005) that adversely affect fetal nutrition during the latter stages of gestation. The head looks disproportionately large compared with the body, but the head circumference is usually within normal parameters and brain growth is usually spared (McGrath et al 2004). The bones are within gestational norms for length and density but the anterior fontanelle may be larger than expected, owing to diminished membranous bone formation. The abdomen looks scaphoid, or sunken owing to shrinkage of the liver and spleen, which surrender their stores of glycogen and red blood cell mass, respectively as the fetus adapts to the adverse conditions of the uterus. Since the ratio of brain mass to liver mass is large, hypoglycaemia is more likely to be seen in such babies. There is decreased subcutaneous fat deposition and the skin is loose, which can give the baby a wizened, old appearance. Vernix caseosa is frequently reduced or absent as a result of diminished skin perfusion and, in the absence of this protective covering, the skin is continuously exposed to amniotic fluid, and its cells will begin to desquamate (shed); thus the skin appears pale, dry and coarse and, if the baby is of a mature gestation, may be stained with meconium. Unless severely affected, these babies appear hyperactive and hungry with a lusty cry (Fig. 42.3).
Symmetric growth (chronic)
Symmetric IUGR is due either to decreased growth potential of the fetus, as a result of congenital infection or chromosomal/genetic defects (intrinsic), or to extrinsic factors that are active early in gestational life (e.g. the effects of maternal smoking, or poor dietary intake), or a combination of intrinsic and extrinsic factors (Mupanemunda & Watkinson 2005). The head circumference, length and weight are all proportionately reduced for gestational age (Gomella et al 1999). These babies are diminutive in size, do not appear wasted, have subcutaneous fat appropriate for their size and their skin is taut. They are generally vigorous and less likely to be hypoglycaemic or polycythaemic, but, because the insult began early with possible interruptions to cell division, they may suffer major congenital abnormalities and can be a source of infection to carers, as a result of transplacental infection.
The pre-term baby
This describes a baby born before the end of the 37th gestational week, regardless of birthweight. Most of these babies are appropriately grown and, whereas some are SGA, a small number are LGA (these tend to be infants of diabetic mothers). The factors that play a role in the initiation of pre-term labour are largely unknown, are described as multifactorial and in large part overlay with factors that impair fetal growth. They are divided into those labours that start spontaneously and those where a decision is made to terminate a viable pregnancy before term (referred to as elective causes) (Box 42.2).
Box 42.2 Causes of pre-term labour
Sources: Blackburn (2003), Harlow & Spencer (1999), Tucker & McGuire (2005).
Characteristics of the pre-term baby
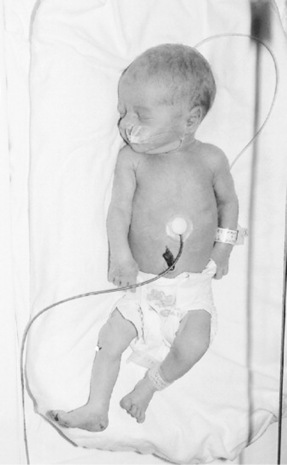
The appearance at birth of the pre-term baby will depend upon the gestational age. The following description will focus upon the baby born during the last trimester of pregnancy. Pre-term babies rarely become large enough in utero to develop muscular flexion and fully adopt the fetal position (Young 1996) and as a result their posture appears flattened with hips abducted, knees and ankles flexed. Lissauer et al (2006) describe a generally hypotonic baby with a weak and feeble cry. The head is in proportion to the body; the skull bones are soft with large fontanelles and wide sutures. The chest is small and narrow and appears underdeveloped owing to minimal lung expansion during fetal life. The abdomen is prominent because the liver and spleen are large and abdominal muscle tone is poor (Fig. 42.4). The liver is large because it receives a good supply of oxygenated blood via the fetal circulation and is active in the production of red blood cells as a result of erythropoiesis. The umbilicus appears low in the abdomen because linear growth is cephalocaudal (it is more apparent nearer to the head than the feet), by virtue of the fetal circulation oxygenation. Subcutaneous fat is laid down from 28 weeks’ gestation, therefore its presence and abundance will affect the redness and transparency of the skin. Vernix caseosa is abundant in the last trimester and tends to accumulate at sites of dense lanugo growth (i.e. the face, ears, shoulders and sacral region) and protects the skin from amniotic fluid maceration. The ear pinna is flat with little curve, the eyes bulge and the orbital ridges are prominent. The nipple areola is poorly developed and barely visible. The cord is white, fleshy and glistening. The plantar creases are absent before 36 weeks but soon start to appear as fluid loss occurs through the skin. In girls the labia majora fail to cover the labia minora and in boys the testes descend into the scrotal sac at about the 37th gestational week (Gardner & Johnson 2006).
Management at birth of the healthy LBW baby
Given the unpredictability of the LBW baby and the birth process, the role of the midwife in the birthing room is to prepare adequately the environment, staff and parents for certain eventualities. This takes the form of asking the multiprofessional team (second midwife, paediatrician and neonatal nurse), to be on standby for the birth. The incidence of perinatal asphyxia and congenital abnormality is greater in SGA babies and the baby with a scaphoid abdomen could be physically normal, albeit thin, but alternatively could deteriorate quickly if presenting with a diaphragmatic hernia. Current cot availability in the NICU, transitional care unit (as applicable) and postnatal ward should be known. The labour room ambient temperature should ideally be between 23 and 25 °C; the neonatal resuscitaire should be checked and ready for use.
The Apgar score is traditionally scored at 1 and 5min and acts as a guide in helping staff communicate the resuscitative needs of neonates immediately after birth. Some consider it has limited usefulness in LBW babies because it was originally devised by Apgar in 1953 for term babies and does not take into account how gestational age, congenital abnormalities or sedation affect the score (Juretschke 2000). Early labeling of the LBW baby is particularly important because separation of mother and baby could happen at any time if the baby’s condition becomes unstable. The midwife should cut the cord to leave an extra length, in case access to the umbilical vessels should be necessary at a later time. A detailed but expedient examination of the baby should be conducted by the midwife in the presence of the parents and recorded accordingly. It is particularly important in SGA babies to check that the anal sphincter is patent, which could provide information on the internal integrity of the gastrointestinal system, particularly the oesophagus. This is a good time to allay any parental anxieties about their baby’s general appearance, which may be quite different to what they were expecting (Redshaw 1997). Once it is established that the baby appears healthy, the midwife may attempt to normalize the care by emphasizing to the parents the importance of preventing cold stress and suggest skin-to-skin contact for a period of up to 50min (Philipp & Radford 2006). Lee (2006a) reports that skin-to-skin contact was originally introduced by Klaus & Kennell in the 1970s and later was developed as kangaroo care as an alternative to cot care for LBW babies because of their special physical needs (Klaus & Kennell 1982). The re-introduction of skin-to-skin contact has now been generalized to newborns by the WHO/UNICEF UK Baby Friendly Initiative (1989). The midwife should ensure that the baby is thoroughly dried before skin-to-skin contact is attempted, to prevent evaporative heat losses. This will, according to Sedin (2006) secure the baby’s conductive heat transfer gains, and help him to become physically stabilized. Whether the baby will find the breast is open to speculation according to Carfoot et al (2003). Their systematic review based on term infants failed to establish whether skin-to-skin contact had any effect on timing of the first breastfeed or duration of breastfeeding. If the mother chooses not to engage in skin-to-skin contact, the father may wish to do so (Lee 2006a) but if not, the baby can be dressed, wrapped and held by his parents. The baby’s axilla temperature should be maintained between 36.4 °C and 37 °C (Thureen et al 2005).
Assessment of gestational age
It is generally considered essential to obtain an accurate assessment of gestational age, first to anticipate the problems a baby is more likely to develop and secondly to provide valid comparisons with respect to morbidity and mortality statistics (Tappero & Honeyfield 2003). In 1970, Dubowitz et al developed a scoring system for the assessment of gestational age in babies <5 days old, based upon neurological and physical (external) characteristics. The purpose of the assessment, which is still used today, is to confirm the stated gestation, reveal any discrepancies and provide a reliable estimate when uncertainty exists. Despite its usefulness, the accuracy of the assessment is less reliable in babies who have suffered a neurological insult such as birth asphyxia or chronic exposure to drugs. Such babies will obtain a lower score on neurological criteria. Other affected babies are those who have suffered asymmetric IUGR. Their sole creases have extra wrinkles as a result of exposure to amniotic fluid and this feature may confer upon them an enhanced maturity, whereas breast tissue formation, female external genitalia and ear cartilage will give the appearance of less maturity because of diminished amounts of adipose tissue and so they may be underscored on physical assessment, if they are near to term. Hence, gestational age assessments based on physical criteria can be misleading. Mupanemunda & Watkinson (2005) do not totally endorse the Dubowitz scoring system, believing that its margins of error are too wide. They further contend that the handling of LBW babies for the assessment is problematic because it may de-stabilize an otherwise well baby. With the developments of more accurate dating by antenatal ultrasound techniques, it is argued that there is less justification for a full assessment of gestational age in well LBW babies. The exception is applied when the mother deliberately conceals her pregnancy, is unable to communicate or unwilling to divulge requisite information, or is unreliable (e.g. a drug-abusing mother with unfavourable social circumstances). Therefore any assessments that are carried out should be carefully conducted, with the view that no harm should be caused as a result of the process.
Care of the healthy LBW baby
Many of the care issues relevant to the LBW baby apply to both the pre-term and the SGA infant. Where differences do exist, these will receive further consideration. The following areas of focus, although presented separately, do by their nature influence each other and should not be regarded as stand-alone entities.
Principles of thermoregulation
Thermoregulation is the balance between heat production and heat loss and the ideal body temperature should be between 36.5 ° and 37.3 °C. The prevention of cold stress, which may lead to hypothermia which is a body temperature below 36 °C, is critical for the intact survival of the LBW baby. Newborn babies are unable to shiver, move very much, or ask for an extra blanket and therefore rely upon physical adaptations that generate heat by raising their basal metabolic rate and utilizing brown fat deposits. Thus, exposure to cool environments can result in multisystem physiological changes, which significantly challenge the baby’s health status. As body temperature falls, tissue oxygen consumption rises as the baby attempts to raise its metabolic rate by burning glucose to generate energy and heat. Care measures should aim to provide an environment that supports thermoneutrality. This is otherwise known as the ‘neutral thermal environment’, a range of ambient temperatures within which the metabolic rate is minimal, the baby is neither gaining or losing heat, oxygen consumption is minimal and the core- to-skin temperature gradient is small (Blackburn 2003).
Thermoregulation and the healthy mature SGA baby
In the neonate, the head accounts for at least one-fifth of the total body surface area and brain heat production is thought to be 55% of total metabolic heat production. Rapid heat loss due to the large head-to-body ratio and large surface area is exaggerated, particularly in the asymmetrically grown SGA baby. Wide sutures and large fontanelles add to the heat-losing tendency. On the plus side, they have increased skin maturity but often depleted stores of subcutaneous fat, which are used for insulation. Their raised basal metabolic rate helps them to produce heat but their high energy demands in the presence of poor glycogen stores and minimal fat deposition can soon lead to hypoglycaemia and then hypothermia. Once the baby is thoroughly dried, a pre-warmed hat will minimize heat loss from the head.
Thermoregulation and the healthy pre-term baby
All pre-term babies are prone to heat loss because their ability to produce heat is compromised by their immaturity, so factors such as their large ratio of surface area to weight, their varying amounts of subcutaneous fat and their ability to mobilize brown fat stores (which have been laid down from 28 weeks) will be affected by their gestational age (Blackburn 2003). During cooling, immaturity of the heat-regulating centres in the hypothalamus and medulla oblongata fail, in different degrees, to recognize and marshall adequately coordinated homeostatic controls. In addition, pre-term babies are often unable to increase their oxygen consumption effectively through normal respiratory function and their calorific intake is often inadequate to meet increasing metabolic requirements (Anderson et al 2006). Furthermore, their open resting postures increase their surface area and along with insensible water losses, these factors render the pre-term baby more susceptible to evaporative heat losses (Sedin 2006). Gestational age and the weight of the baby will influence the type of care initiated. Gardner & Johnson (2006) and Fransson et al (2005) report that skin-to-skin contact is a more effective measure for re-warming cool babies than incubator care. When the baby is not receiving skin-to-skin contact with either parent and the baby is under 2kg, the warm conditions in an incubator can be achieved either by heating the air to 30–32 °C (air mode) or by servo-controlling the baby’s body temperature at a desired set point (36 °C). In servo mode, a thermocouple is taped to the upper abdomen and the incubator heater maintains the skin at that site at a preset constant (see Fig. 42.4). Within the incubator they are clothed with bedding, in a room temperature of 26 °C. Most pre-term babies between 2.0 and 2.5kg will be cared for in a cot, in a room temperature of 24 °C.
Hypoglycaemia
Hypoglycaemia and the healthy LBW baby
The term hypoglycaemia refers to a low blood glucose concentration and in itself is not a medical condition, but a feature of illness or a failure to adapt from the fetal state of continuous transplacental glucose consumption to the extrauterine pattern of intermittent nutrient supply (WHO 1997b). It is more likely to occur in conditions where babies become cold or where the initiation of early feeding (within the first hour) is delayed. Counterregulatory mechanisms (for a more detailed account on hypoglycaemia, see Ch. 48) maintain the blood glucose at safe limits to protect tissue viability; however, it is generally questioned whether LBW babies are able to counter-regulate as effectively as appropriately grown term babies and some caution is recommended (WHO 1997b). The aim of management is to maintain the true blood sugar above the level considered to be the lowest level of normal, which is 2.6mmol/dL (WHO 1997b). However, this does not mean that every LBW baby should be routinely screened. Well LBW babies who show no clinical signs of hypoglycaemia, are demanding and taking nutritive feeds on a regular basis and maintaining their body temperature, do not need screening for hypoglycaemia. The emphasis of care is placed upon the concept of adequate feeding and the cornerstone of success is the midwife’s ability to assess skillfully whether the baby is feeding sufficiently well to meet energy requirements. Midwives should be guided by their local policies regarding use of reagent strips, but if a baby, despite being fed, presents with clinical signs of hypoglycaemia then a venous sample should be taken by the paediatrician to assess the true blood sugar level, and this should be dispatched to the laboratory for verification purposes. A blood glucose that remains <2.6mmol/dL, despite the baby’s further attempts to feed by breast or colostrum by cup, may warrant transfer to the NICU, because glucose by intravenous bolus may be necessary to correct the metabolic disturbance. In addition, the midwife should consider that there may be some underlying medical condition that may call for more thorough investigation.
Hypoglycaemia and the healthy mature SGA baby
If the SGA baby does not suffer any effects of perinatal asphyxia or develop cold stress, the remaining potential problem is hypoglycaemia. Glycogen storage begins at the beginning of the third trimester and, due to altered placental transport of nutrients during this time, asymmetrically grown babies have reduced glycogen stores in liver and skeletal muscles (Ogata 1999). Their greater brain-to-body mass and a tendency towards polycythaemia increase their energy demands and, since both the brain and the red blood cells are obligatory glucose users, these factors can increase glucose requirements. Mature SGA babies with an asymmetric growth pattern will usually feed within the first 30min of birth and will demand feeds every 2–3hrs thereafter, to make up for lost time. Feeding is thought to mature the blunted counterregulatory response, so their susceptibility to hypoglycaemia is relatively short-lived and limited to the first 48hrs following birth. If the baby is taking formula milk, feeds are usually calculated at 90mL/kg on the first day, with 30mL increments/day. Anderson et al (2006) warn however that overfeeding a growth restricted baby has been considered a cause of adult obesity and diabetes.
Hypoglycaemia and the pre-term baby
The pre-term baby may be sleepier, and attempts to take the first feed may reflect gestational age. Total feed requirements (60mL/kg on the first day, with 30mL/kg increments/day) may not be taken directly from the breast and supplementary feeds can be given by cup.
Feeding the LBW baby
Both pre-term and SGA babies benefit from human milk because it contains long chain polyunsaturated omega-3 fatty acids, which are thought to be essential for the myelination of neural membranes and for retinal development. Pre-term breastmilk has a higher concentration of lipids, protein, sodium, calcium and immunoglobulins, a low osmolarity, and lipases and enzymes that improve digestion and absorption (Jones & Spencer 1999). The uniqueness of the mother’s milk for her own baby cannot be overstated. For any mother to commit to the challenge of breastfeeding her LBW baby, and in particular the pre-term baby, she should be thoroughly prepared both cognitively and emotionally by the midwife, so that her expectations are realistic as she anticipates the likely sequence of events that her baby may take her through (Lang 2002). First, she needs to understand what her baby may be able to achieve related to his development, which is based upon the combined influences of his gestational age at birth and his postnatal age. For a baby to feed for nutritive purposes, the coordination of breathing with suck and swallow reflexes reflect neurobehavioral maturation and organization and is thought to occur between 32 and 36 weeks’ gestation (Jones & Spencer 2005, Pinelli & Symington 2001). Pre-term babies are limited in their ability to suck because of their weak musculature and flexor control, which is important for firm lip and jaw closure (Blackburn 2003, Hurst & Meier 2005). Before 32 weeks, they will need to be tube fed on a regular basis, usually on a 3-hourly regimen with preset amounts of breastmilk, hind milk or formula milk based on postnatal age and present weight, to provide the necessary calories for growth, but not at the expense of energy expenditure (King 2005).
It is now common practice for parents to tube feed their own baby. Als & Butler (2006) believe that parents should provide physical support for head, trunk and shoulders as sucking is part of the flexor pattern of development and may be enhanced by giving the baby something to grasp. Jones & Spencer (2005) note that the pre-term head is very heavy for the weak musculature of the neck and would, if not supported, result in considerable head lag, so correct positioning and attachment to the breast can be made much more difficult to achieve (Hurst & Meier 2005). Poor head alignment can result in airway collapse which may lead to apnoea and bradycardia therefore support from the midwife is essential when initiating breastfeeding. Tube feeding has the advantage that the tube can be left in situ during a cup or breastfeed and has been shown to eliminate the need to introduce bottles into a breastfeeding regimen (Philipp & Radford 2006). McGrath (2004) recommends that some of the softer silastic tubes can be left in place for a month or more and this removes the necessity for regular replacement however several problems have been identified with tube feeding. Babies are preferential nose breathers and the presence of a nasogastric tube will inevitably take up part of their available airway. Their prolonged use has been associated with delay in the development of sucking and swallowing reflexes simply because the mouth is bypassed. For these reasons, cup-feeding has been used in addition to tube feeding in order to provide the baby with a positive oral experience, to stimulate saliva and lingual lipases to aid digestion (Lang 2002), and to accelerate the transition from naso/oral gastric feeding to breastfeeding. Oral gastric tubes have been associated with vagal stimulation and have resulted in bradycardia and apnoea. Preference for nasal or oral gastric tubes remains open to debate (McGrath 2004).
Certain behaviours, such as licking and lapping are well established before sucking and swallowing (Ritchie 1998) and when babies are given the opportunity, it is not unusual to see them as early as 28 and 29 weeks, licking milk that has been expressed onto the nipple by their mother. Thus, babies between 30 and 32 weeks’ gestation can be given expressed breastmilk (EBM) by cup. Lang (2002) makes the point that tongue movement is vital in the efficient stripping of the milk ducts, so cup-feeding can be seen as developmental preparation for breastfeeding. Between 32 and 34 weeks’ gestation, cup-feeding can act as the main method of feeding, with the baby taking occasional complete breastfeeds. Cup-feeds tend to last about the same duration it takes to give a feed by bottle. More importantly, however, they utilize little energy, which is a crucial factor in their favour for LBW babies.
A pre-term baby of <35 weeks’ gestation can be gently wrapped /swaddled prior to a feed and this is thought to provide reassurance and comfort, not unlike the unique close-fitting tactile stimulation of the uterus. McGrath (2004) argues that this approach supports development of flexion as well as decreasing disorganized behaviours that could detract from feeding success. A pre-term baby may easily tire and the mother can be taught to start the flow of milk by hand expressing, before attaching him to the breast. Long pauses between sucks are to be expected. This burst–pause pattern is a signal of normal development and seems to occur earlier with breastfeeding. The baby may appear to be asleep and a change in position may remind him of the task in hand, but it is thought to be a mistake to force a reluctant baby to feed (Sparshott 1997). If it is obvious that the baby is more interested in sleeping, the mother can complete the feed by tube.
From 35 weeks onwards, cup-feeding can be gradually replaced by complete breastfeeding (Lang 2002). Progress in the feeding method is often dependent upon the pre-term baby, who cues his mother that he is ready to take milk from the breast. In the interim, she must express her milk so that she can maintain her milk supply and provide the milk for cup-feeding as necessary. An unrushed feed can take up to an hour to complete. Any advances on this timeframe should be reviewed in terms of the quality of the baby–nipple attachment (see Ch. 41), maternal milk flow (which may be affected by anxiety or other factors) and the general condition of the baby (e.g. the development of physiological jaundice). Feeding frequency can vary between 6 and 10 feeds/day. The baby should be left to establish his own volume requirements and feeding pattern. The mother should be encouraged to rely upon her own instincts and common sense so that the rhythm of total care she adopts in hospital will thoroughly prepare her for when she goes home (Lang 2002). Often the difference between early and late transfer home is more dependent upon the mother’s positive attitude and skill development than the baby’s maturity and inherent abilities.
The care environment: promoting health and development of the healthy LBW baby
The importance of providing an appropriate environment for the healthy LBW baby cannot be overstressed and according to Sparshott (1997), the ideal environment should resemble home, which provides a cycle of day and night, regular nourishment, rest, stimulation and loving attention. The midwife’s role is to create such an environment, primarily for the physical development of the baby, but at the same time to provide psychological support for the mother and her family. The mother’s desire to be involved is seen as an essential element in the success of caring for LBW babies in hospital.
The normal sensory requirements of the developing brain depend upon subtle influences, first from the uterus and then from the breast (Reed & Freer 2000). Any disruption to this natural arrangement renders the LBW baby vulnerable to influences in the care environment that can result in poor coordination as a result of delays in the development of different subsystems (autonomic, motor, sensory, etc.). Reid (2000) believes maternal role development depends upon the mother’s self-esteem and her perception of mothering. By attempting to adapt the care environment to be more like the intrauterine environment, the midwife can help parents to become aware of their baby’s behavioural and autonomic cues and utilize them in organizing care according to their baby’s individual tolerance. The ethos of care is for them to listen and learn from their baby, to come to know and see him as an individual, competent for his stage of development and not merely ‘a baby born too early’, or a dysfunctional term infant. They will be encouraged (but not cajoled) into taking a major role in their baby’s emerging developmental agenda, come to understand the situation in which they find themselves, become more able to reset their expectations and thus offer more baby-led support (Lee 2006b, Reed & Freer 2000).
According to McGrath et al (2004) the emerging task of the term newborn baby is increasing alertness, with growing responsiveness to the outside world. By comparison, a pre-term baby is at a stage of development that is more concerned with their internal world. Term babies have stable function of the autonomic and motor systems. Pre-term babies will be at different stages of this development, depending on their gestational age and health status. They will spend more time in rapid eye movement (REM) sleep or drowsy states and have difficulty in achieving deep sleep. They are unable to shut out stimulation that prevents them from sleeping and resting, and sudden noise hazards provoke stress reactions, which can adversely affect respiratory, cardiovascular and digestive stability. The term baby is able to shut out such stimuli for rest and sleep purposes. The degree to which SGA term babies have been affected by their unique intrauterine experience is difficult to assess in the short term, but hyperactivity is seen as a feature of an adaptive stress reaction. These babies, like their pre-term counterparts, need an environment that supports their level of robustness. Environmental disturbances, excessive or prolonged handling and even activities like feeding may add an extra physiological burden to an already compromised state. Social contact is considered a vital element for the development of parent–child interaction, yet stereotypical notions of social contact that revolve around practical care giving and feeding may not be suitable for some babies and when these activities are clustered together, may draw too heavily on the baby’s physical resources. When the baby is over-stimulated and wishes to terminate the interaction, certain cues according to Vergara & Bigsby (2004) are known as coping signals and are recognized as fist clenching, furrowing of the brow, gaze aversion, splayed fingers and yawning. Should the baby wish to initiate or continue an interaction, he tends to demonstrate approach signals such as raised eyebrows, head raising and engagement in different degrees of eye contact with his social partners. The midwife can reassure parents, that by paying attention to their baby’s behavioural cues, they can work with his capabilities, which is crucial for maintaining his healthy status (Als & Butler 2006).
Handling and touch
Kangaroo care (KC) is used to promote closeness between a baby and mother and involves placing the nappy-clad baby upright between the maternal breasts for skin-to-skin contact (see Plate 20). The LBW baby remains beneath the mother’s clothing for varying periods of time that suit the mother. Some mothers may have repeated contacts throughout the day; others may prefer specific periods around which they plan their day’s activities. There are no rules or time limitations applied, but contact should be reviewed if there are any clinical signs of neonatal distress. For the benefits of kangaroo care, see Box 42.3.
Box 42.3 Benefits of kangaroo care
Sources: Jones & Hartmann (2005), Padden & Glen (1997), Sparshott (1997), WHO (1997b).
Noise and light hazards
The time spent in a postnatal ward should be a time of rest and recuperation for both the mother and her LBW baby. Noise should be kept to a minimum. Harrison et al (2004) assert that all extraneous noises should be eliminated from clinical areas such as musical toys and mobiles, harsh clattering footwear, telephones, radios, intercom systems and raised voices. Clinicians should be aware of noise hazards such as the closing of incubator portholes, use of peddle bins, ward doors and general equipment. Ward areas can be carpeted and quiet signs can be posted to remind visitors not to disrupt the peace (Young 1996). In dimmed lighting conditions, pre-term babies are more able to improve their quality of sleep and alert status. Reduced light levels at night will help to promote the development of circadian rhythms and diurnal cycles. Light levels can be adjusted during the day with curtains or blinds to shade windows and protect the room from direct sunlight. Screens to shield adjacent babies from phototherapy lights are essential.
Sleeping position
Hunter (2004) reports that pre-term babies have reduced muscle power and bulk, with flaccid muscle tone, therefore their movements are erratic, weak or flailing. They exert energy to maintain their body position against the pull of gravity. Without support they may, to differing degrees, develop head, shoulder and hip flattening, which in turn can lead to poor mobility. Nesting the more immature pre-term babies into soft bedding, in addition to the use of close flexible boundaries, helps to keep their limbs in midline flexion; however, it is vital that they are nursed in a supine position to prevent asphyxia. Lying the baby in the supine position is thought to be effective in promoting engagement in self-regulatory behaviours such as exploration of the face and mouth, hand and foot clasping, boundary searching, and flexion and extension of the limbs. Pressure on the occiput should over time ensure a more rounded head.
Sudden infant death syndrome (SIDS)
Placing healthy LBW babies to sleep in the prone position has been theoretically eradicated from neonatal practice and Fleming et al (2004) reiterate that all babies should be placed in the supine position and it is incumbent upon health care professionals to accustom the baby and educate the parents in adopting this approach. The midwife needs to explain how the issues apply to individual families and take into consideration the time of year, gestational age and postnatal age when care is transferred to the community midwife. Parental training on ‘what to do if my baby stops breathing’ is becoming part of a routine preparation for transfer home, although this degree of preparedness can empower some parents but frighten others. The decision to receive training should be the parent’s choice (Resuscitation Council 2006).
The prevention of infection
LBW babies, particularly pre-term babies, are especially vulnerable to infections because of the immaturity of their host defence systems (Mupanemunda & Watkinson 1999). (See Chs 43 and 47 for further details.)
The provision of neonatal care: the question of venue and facilities
The decision to transfer a healthy LBW baby to a postnatal ward, a transitional unit or a NICU will depend upon the baby’s gestational age and weight. Perhaps more influential will be the availability of facilities and level of staffing that exists at the time. Traditionally, the advantage of a separate transitional care unit has been to divert medium risk babies away from the NICU, and this has been of great value (Bromley 2000), however, some would argue that part of its attraction has been to take healthy LBW babies that otherwise would have been roomed-in with their mothers on postnatal wards. Some offer rooming-in facilities but some are used as an intermediate unit where, in addition to healthy LBW babies, there are convalescing NICU graduates (Farrer 2005), term babies with congenital abnormalities, babies with feeding problems (particularly where tube-feeding is required) and those babies who need antibiotic therapy. Alternatively, transitional care provision can be found on a postnatal ward in a protected area, or transitional care cots can be interspersed randomly throughout the ward. Postnatal ward care is seen as optimal as separation from their babies and lack of contact are reported by mothers as the worst features of having a baby that needs specialist care (Redshaw 1997) but postnatal wards are often busy, noisy places. For women who need hospital postnatal care, midwives need to make the postnatal environment more conducive for rest, relaxation and recuperation (Wray & Davies 2007). Spitzer (2005) believes that mothers are cued by the environment in which they find themselves. If they are less stressed, able to care for their own baby and enjoy being with other postnatal women and being cared for by midwives, these factors may help them to perceive their hospital postnatal experience as a normative life event and better prepare them for when they go home (see Box 42.4 for the benefits and limitations of caring for LBW babies on postnatal wards). Postnatal visiting by the community midwife should continue for as long as necessary to help the family adapt to their new responsibilities (Nursing and Midwifery Council 2004).
Box 42.4 Benefits and limitations of caring for healthy LBW babies on postnatal wards
Sources: Bromley (2000), Lee (2006b), Redshaw (1997).
Benefits to the mother
Limitations that affect the mother
Als H, Butler S. Neurobehavioural development of the pre-term infant. Martin RJ, Fanaroff AA, Walsh MC, editors. Fanaroff and Martin’s neonatal-perinatal medicine. Diseases of the fetus and infant, Vol. 2. London: Elsevier Mosby, 2006.
Anderson MS, Wood LL, Keller J, et al. Enteral nutrition. In: Merenstein GB, Gardner SL, editors. Handbook of neonatal intensive care. St Louis: Elsevier Mosby, 2006.
Blackburn ST. Maternal, fetal and neonatal physiology. A clinical perspective. Saunders, St Louis, 2003.
Bromley P. Transitional care: let’s think again. Journal of Neonatal Nursing. 2000;6:60-64.
Carfoot S, Williamson PR, Dickson R. A systematic review of randomized controlled trials evaluating the effect of mother/baby skin to skin care on successful breast-feeding. Midwifery. 2003;19:148-155.
Dubowitz LMS, Dubowitz V, Golberg C. Clinical assessment of gestational age in the newborn infant. Journal of Paediatrics. 1970;77:1-10.
Farrer K. Fetal growth, intrauterine growth retardation and small for gestational age babies. In: Rennie J, editor. Roberton’s Textbook of neonatology. 4th edn. London: Churchill Livingstone; 2005:389-398.
Fleming PJ, Blair PS, Sidebotham PD, et al. Investigating sudden unexpected deaths in infancy and childhood and caring for bereaved families: an integrated multi-agency approach. British Medical Journal. 2004;328:331-334.
Fowlie PW, McHaffie H. Supporting parents in the neonatal unit. In: McGuire W, Fowlie PW, editors. ABC of pre-term birth. Oxford: Blackwell Publishing, 2005.
Fransson AL, Karlsson H, Nilsson K. Temperature variation in newborn babies: importance of physical contact with the mother. Archives of Disease in Children. Fetal Neonatal Edition. 2005;90:F500-F504.
Gardner SL, Johnson JL. Initial nursery care. In: Merenstein GB, Gardner SL, editors. Handbook of neonatal intensive care. St Louis: Elsevier Mosby, 2006.
Gomella TL, Cunningham MD, Eyal FG, et al. Neonatology. Stamford: Appleton & Lange, 1999.
Harrison LL, Lotas MJ, Jorgensen KM. Environmental issues. In: Kenner C, McGrath JM, editors. Developmental care of newborns and infants. A guide for health professionals. St Louis: Mosby Elsevier, 2004.
Harlow FD, Spencer AD. Obstetrics for the neonatologist. In: Rennie JM, Roberton NRC, editors. Textbook of neonatology. Edinburgh: Churchill Livingstone; 1999:157-173.
Hunter J. Positioning. In: Kenner C, McGrath. Developmental care of newborns and infants. A guide for health professionals. St Louis: Mosby, 2004.
Hurst N, Meier P. Breast-feeding the pre-term infant. In: Riordan J, editor. Breast-feeding and human lactation. London: Jones and Bartlett, 2005.
King C. Nutritional requirements. In: Jones E, King C, editors. Feeding and nutrition in the pre-term infant. London: Elsevier Churchill Livingstone, 2005.
Klaus MH, Kennell JH. Parent-infant bonding. London: Mosby, 1982.
Jones E, Hartmann PE. Milk Expression. In: Jones E, King C, editors. Feeding and nutrition in the pre-term infant. London: Churchill Livingstone, 2005.
Jones L, Spencer A. Successful pre-term breast-feeding. Practising Midwife. 1999;2:54-57.
Jones E, Spencer SA. How to achieve successful pre-term breast-feeding. Infant. 2005;1(4):14-17.
Juretschke LJ. Apgar scoring: its use and meaning for today’s newborn. Neonatal Network. 2000;9:17-19.
Lang S. Breast-feeding special care babies. London: Baillière Tindall, 2002.
Lee B. Pre-term birth: a medical miracle with an emotional cost? RSM Forum. Midwives. 2006;9:60-62.
Lee B. Pre-term birth: a medical miracle with an emotional cost? RSM Forum. Midwives. 2006;9:92-94.
Lissauer T, Fanaroff AA, Rodriguez RJ, et al. Neonatology at a glance. London: Blackwell, 2006.
McGrath JM. Feeding. In: Kenner C, McGrath JM, editors. Developmental care of newborns and infants. A guide for health care professionals. St Louis: Mosby, 2004.
McGrath JM, Kenner C, Amspacher KA. Factors that can influence fetal development. In: Kenner C, McGrath JM, editors. Developmental care of newborns and infants. A guide for health professionals. St Louis: Mosby Elsevier, 2004.
Mupanemunda RH, Watkinson M. Key topics in neonatology. Oxford: Bios Scientific, 1999.
Mupanemunda R, Watkinson M. Key topics in neonatology. London: Taylor and Francis, 2005.
Nursing and Midwifery Council. Midwives rules and standards. London: NMC, 2004.
Ogata ES. Carbohydrate homeostasis. In: Avery GB, Fletcher MA, McDonald MG, editors. Neonatology, physiopathology and management of the newborn. London: Lippincott, Williams & Wilkins; 1999:699-712.
Padden T, Glen S. Maternal experiences of pre-term birth and neonatal care. Journal of Reproductive and Infant Psychology. 1997;15:121-139.
Philipp BL, Radford A. Baby-friendly: snappy slogan or standard of care? Archives of Disease in Children. 2006;91:145-149.
Pinelli K, Symington A. Non-nutritive sucking for promoting physiologic stability and nutrition in pre-term infants (Cochrane review). The Cochrane Library. (issue 3):2001. Update Software, Oxford
Redshaw ME. Mothers of babies requiring special care: attitudes and experiences. Journal of Reproductive and Infant Psychology. 1997;15:109-120.
Reed T, Freer Y. Developmental nursing care. In: Boxwell G, editor. Neonatal intensive care. London: Routledge, 2000.
Reid TL. Maternal identity in pre-term birth. Journal of Child Health Care. 2000;4:23-29.
Resuscitation Council UK. Newborn life support resuscitation at birth, 2nd edn. Kent: TT Litho Printers, 2006.
Ritchie JF. Immature sucking response in premature babies: cup-feeding as a tool in increasing maintenance of breast-feeding. Journal of Neonatal Nursing. 1998;14:13-17.
Roberton NRC. Fetal growth, intrauterine growth retardation and small for gestational age babies. In: Rennie JM, Roberton NRC, editors. Textbook of neonatology. London: Churchill Livingstone; 1999:389-398.
Sedin G. The thermal environment of the newborn infant. Martin RJ, Fanaroff AA, Walsh MC, editors. Fanaroff and Martin’s neonatal-perinatal medicine. Diseases of the fetus and infant, Vol. 1. London: Elsevier Mosby, 2006.
Sparshott M. Pain, distress and the newborn baby. Oxford: Blackwell Science, 1997.
Spencer N. Weighing the evidence – how is birth weight determined? Oxon: Radcliffe Medical Press, 2003.
Spitzer AR. Care of the family in the neonatal intensive care unit. In: Spitzer AR, editor. Intensive care of the fetus and neonate. Philadelphia: Elsevier Mosby, 2005.
Tappero EP, Honeyfield ME. Physical assessment of the newborn. A comprehensive approach to the art of physical examination. NICU.INK, Santa Rosa, 2003.
Thureen PJ, Deacon J, Hernandez JA, et al. Assessment and care of the well newborn. St Louis: Elsevier, 2005.
Tucker J, McGuire W. Epidemiology of pre-term birth. In: McGuire W, Fowlie PW, editors. ABC of pre-term birth. Oxford: BMJ Blackwell Publishing, 2005.
Vergara ER, Bigsby R. Developmental and therapeutic interventions in the NICU. London: Paul Brookes Publishing, 2004.
WHO/UNICEF. Joint statement – protecting, promoting and supporting breast-feeding. Geneva: World Health Organization, 1989.
WHO. Manual of international statistical classification of diseases, injuries and causes of death. Geneva: World Health Organization, 1977. Vol 1
WHO. Hypoglycaemia of the newborn. Review of the literature. World Health Organization, Geneva, 1997.
Wray J, Davies L. What women want from postnatal care. Midwives. 2007;10:131.
Young J. Development care of the premature baby. London: Baillière Tindall, 1996.
Lissauer T, Fanaroff AA, Rodriguez RJ, Weindling M. Neonatology at a glance. London: Blackwell, 2006.
This book provides a concise overview of neonatology with topics confined to one or two pages with colour illustrations and photographs, ideal for the reader who is relatively new to the subject area and needs to have the important points of discussion made clear and free from unnecessary detail.