Pharmacologic Management
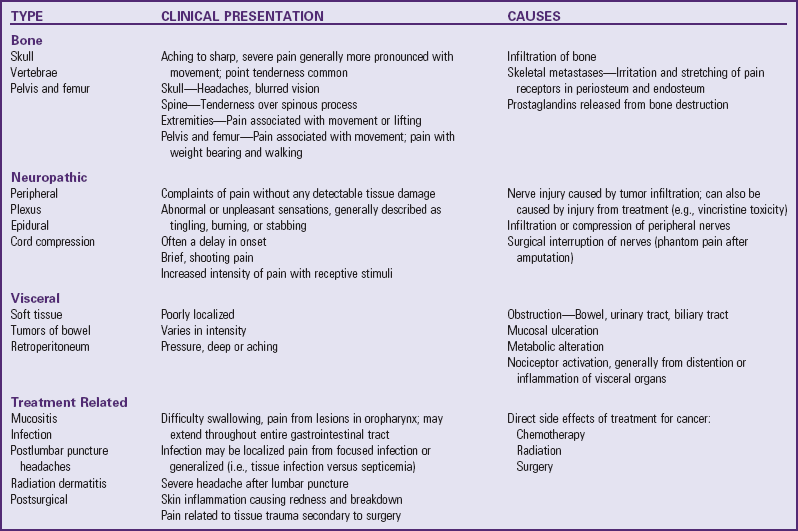
![]() Nonopioids, including acetaminophen (Tylenol, Paracetamol) and nonsteroidal antiinflammatory drugs (NSAIDs), are suitable for mild to moderate pain (Table 7-4). Opioids are needed for moderate to severe pain (Table 7-5). A combination of the two analgesics acts on the pain system on two levels: nonopioids primarily act at the peripheral nervous system and opioids primarily act at the central nervous system. This approach provides increased analgesia without increased side effects. Several combinations, such as acetaminophen with codeine, may have increasing doses of the opioid but a constant dose of the nonopioid (Table 7-6). Before increasing the opioid, it may be preferable to increase the nonopioid component, for example, adding one regular-strength acetaminophen tablet (325 mg) to acetaminophen 300 mg with codeine 15 mg (Tylenol No. 2) before advancing to acetaminophen 300 mg with codeine 30 mg (Tylenol No. 3) or codeine 60 mg (Tylenol No. 4). However, if this approach is not successful, pain management will require a stronger opioid (see Table 7-5).
Nonopioids, including acetaminophen (Tylenol, Paracetamol) and nonsteroidal antiinflammatory drugs (NSAIDs), are suitable for mild to moderate pain (Table 7-4). Opioids are needed for moderate to severe pain (Table 7-5). A combination of the two analgesics acts on the pain system on two levels: nonopioids primarily act at the peripheral nervous system and opioids primarily act at the central nervous system. This approach provides increased analgesia without increased side effects. Several combinations, such as acetaminophen with codeine, may have increasing doses of the opioid but a constant dose of the nonopioid (Table 7-6). Before increasing the opioid, it may be preferable to increase the nonopioid component, for example, adding one regular-strength acetaminophen tablet (325 mg) to acetaminophen 300 mg with codeine 15 mg (Tylenol No. 2) before advancing to acetaminophen 300 mg with codeine 30 mg (Tylenol No. 3) or codeine 60 mg (Tylenol No. 4). However, if this approach is not successful, pain management will require a stronger opioid (see Table 7-5).
TABLE 7-4
NONSTEROIDAL ANTIINFLAMMATORY DRUGS (NSAIDs) APPROVED FOR CHILDREN*
note: Newer formulations of NSAIDs selectively inhibit one of the enzymes of cyclooxygenase (COX-2, which is responsible for pain transmission) but do not inhibit the other (COX-1). Inhibition of COX-1 decreases prostaglandin production, which is necessary for normal organ function. For example, prostaglandins help maintain gastric mucosal blood flow and barrier protection, regulate blood flow to the liver and kidneys, and facilitate platelet aggregation and clot formation. Theoretically, the COX-2 NSAIDs provide similar analgesic and antiinflammatory benefits with fewer gastric and platelet side effects than the nonselective agents. COX-2 NSAIDs are approved for use in patients >18 years of age.
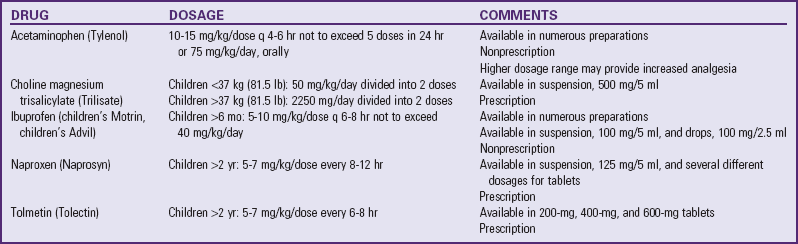
*All NSAIDs in this table (except acetaminophen) have significant antiinflammatory, antipyretic, and analgesic actions. Acetaminophen has a weak antiinflammatory action, and its classification as an NSAID is controversial. Patients respond differently to various NSAIDs; therefore changing from one drug to another may be necessary for maximum benefit. Acetylsalicylic acid (aspirin) is also an NSAID but is not recommended for children because of its possible association with Reye syndrome. The NSAIDs in this table have no known association with Reye syndrome. However, caution should be exercised in prescribing any salicylate-containing drug (e.g., choline magnesium trisalicylate) for children with known or suspected viral infection. Side effects of ibuprofen, naproxen, and tolmetin include nausea, vomiting, diarrhea, constipation, gastric ulceration, bleeding nephritis, and fluid retention. Acetaminophen and choline magnesium trisalicylate are well tolerated in the gastrointestinal tract and do not interfere with platelet function. NSAIDs (except acetaminophen) should not be given to patients with allergic reactions to salicylates. All the NSAIDs should be used cautiously in patients with renal impairment.
Data from Lacy CF: Lexi-Comp’s drug information handbook 2009-2010, ed 18, Hudson, Ohio, 2009, Lexi-Comp, Inc.
TABLE 7-5
DOSAGE OF SELECTED OPIOIDS FOR CHILDREN
IM, Intramuscular; IV, intravenous; PCA, patient-controlled analgesia.
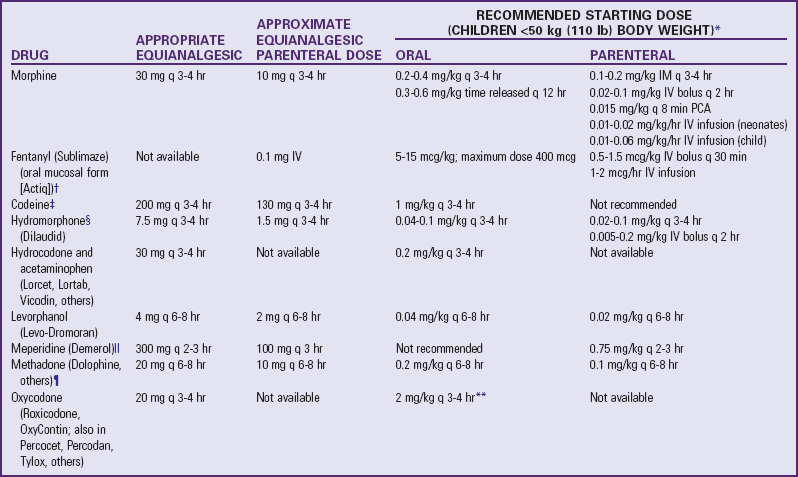
note: Published tables vary in suggested doses that are equianalgesic to morphine. Clinical response is criterion that must be applied for each patient; titration to clinical response is necessary. Because there is not complete cross-tolerance among these drugs, it is usually necessary to use a lower than equianalgesic dose when changing drugs and to retitrate to response.
caution: Recommended doses do not apply to patients with renal or hepatic insufficiency or other conditions affecting drug metabolism and kinetics.
*caution: Doses listed for patients with body weight <50 kg (110 lb) cannot be used as initial starting doses in infants <6 months of age. For nonventilated infants <6 months, the initial opioid dose should be about  to
to 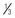 of the dose recommended for older infants and children. For example, morphine could be used at a dose of 0.03 mg/kg instead of the traditional 0.1 mg/kg.
of the dose recommended for older infants and children. For example, morphine could be used at a dose of 0.03 mg/kg instead of the traditional 0.1 mg/kg.
†Actiq is indicated only for management of breakthrough cancer pain in patients with malignancies who are already receiving and are tolerant to opioid therapy, but it can be used for preoperative or preprocedural sedation and analgesia.
‡caution: Codeine doses above 65 mg often are not appropriate because of diminishing incremental analgesia with increasing doses but continually increasing constipation and other side effects. Dosages are from McCaffery M, Pasero C: Pain: a clinical manual, ed. 2, St. Louis, 1999, Mosby.
§For morphine, hydromorphone, and oxymorphone, rectal administration is an alternate route for patients unable to take oral medications, but equianalgesic doses may differ from oral and parenteral doses because of pharmacokinetic differences.
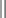 Meperidine is not recommended for continuous pain control (e.g., postoperatively) because of risk of normeperidine toxicity.
Meperidine is not recommended for continuous pain control (e.g., postoperatively) because of risk of normeperidine toxicity.
¶Initial dose is 10%-25% of equianalgesic morphine dose. Parenteral Dolophine is no longer available in the United States.
**caution: Doses of aspirin and acetaminophen in combination with opioid or nonsteroidal antiinflammatory drug preparations must also be adjusted to patient’s body weight. Daily dose of acetaminophen should not exceed 75 mg/kg, or 4000 mg.
Data from Acute Pain Management Guideline Panel: Acute pain management: operative or medical procedures and trauma: clinical practice guideline, AHCPR Pub. No. 92-0032, Rockville, Md, 1992, Agency for Health Care Policy and Research, Public Health Service, US Department of Health and Human Services; Berde C, Ablin A, Glazer J, et al: American Academy of Pediatrics Report of the Subcommittee on Disease-Related Pain in Childhood Cancer, Pediatrics 86(5 pt 2):820, 1990.
TABLE 7-6
bid, Twice a day; hs, at bedtime; IV, intravenous; NSAIDs, nonsteroidal antiinflammatory drugs; PO, by mouth; prn, as needed; q, every; tid, three times a day.
![]() Skill—Calculating Safe Dosages for Children
Skill—Calculating Safe Dosages for Children
![]() Pediatric Drug Dosage Calculations
Pediatric Drug Dosage Calculations
Oxycodone is available without a nonopioid in an immediate release and a controlled release preparation (OxyContin). The oxycodone dose can be safely increased without the risk of toxicity from excessive acetaminophen use. Actions of various opioids differ. Morphine is considered the gold standard for the management of severe pain. When morphine is not a suitable opioid, drugs such as hydromorphone (Dilaudid) and fentanyl (Sublimaze) are effective substitutes. Although fentanyl is used as an anesthetic in the operating room, it is classified as an analgesic. It can be safely administered by nurses by the intravenous (IV), intramuscular (IM), transmucosal, and transdermal routes (Algren, Gursoy, Johnson, et al, 1998; Golianu, Krane, Galloway, et al, 2000).
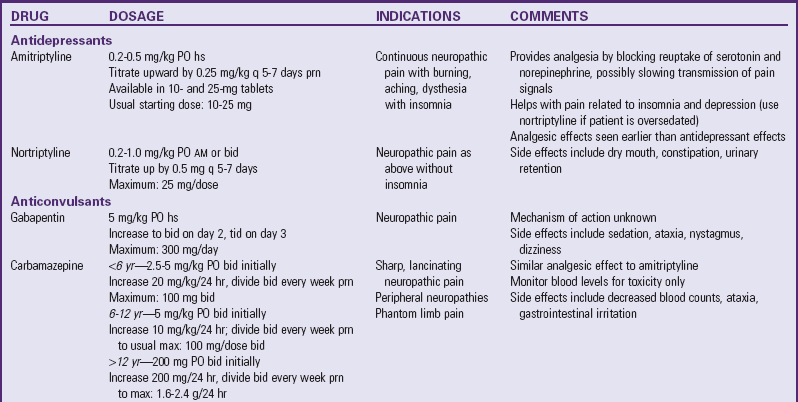
Several drugs, known as coanalgesics or adjuvant analgesics, may be used alone or with opioids to control pain symptoms and opioid side effects. Drugs frequently used to relieve anxiety, cause sedation, and provide amnesia are diazepam (Valium) and midazolam (Versed); however, these drugs are not analgesics and should be used to enhance the effects of analgesics, not as a substitute for analgesics. Other adjuvants include tricyclic antidepressants (e.g., amitriptyline, imipramine) and antiepileptics (e.g., gabapentin, carbamazepine, clonazepam) for neuropathic pain (see Table 7-6), stool softeners and laxatives for constipation, antiemetics for nausea and vomiting, diphenhydramine for itching, steroids for inflammation and bone pain, and dextroamphetamine and caffeine for possible increased pain and sedation (Table 7-7) (McCaffery and Pasero, 1999).
TABLE 7-7
MANAGEMENT OF OPIOID SIDE EFFECTS



hs, At bedtime; IV, intravenous; PO, by mouth; PR, by rectum; prn, as needed; q, every; tid, three times a day.
The use of placebos to determine whether the patient is having pain is unjustified and unethical; a positive response to a placebo, such as a saline injection, is common in patients who have a documented organic basis for pain. Therefore the deceptive use of placebos does not provide useful information about the presence or severity of pain. The use of placebos can cause side effects similar to those of opioids, can destroy the patient’s trust in the health care staff, and raises serious ethical and legal questions. The American Society of Pain Management Nursing has issued a position statement against the use of placebos to treat pain (McCaffery and Pasero, 1999).
Children (except infants younger than about 3 to 6 months) metabolize drugs more rapidly than adults. Younger children may require higher doses of opioids to achieve the same analgesic effect. Therefore the therapeutic effect and duration of analgesia vary. Children’s dosages are usually calculated according to body weight, except in children with a weight greater than 50 kg (110 lb), where the weight formula may exceed the average adult dose. In this case the adult dose is used.
A reasonable starting dose of opioid for infants under 6 months who are not mechanically ventilated is one fourth to one third of the recommended starting dose for older children. The infant is monitored closely for signs of pain relief and respiratory depression. The dose is titrated to effect. Because tolerance can develop rapidly, large doses may be needed for continued severe pain (McCaffery and Pasero, 1999). If pain relief is inadequate, the initial dose is increased (usually by 25% to 50% if pain is moderate, or by 50% to 100% if pain is severe) to provide greater analgesic effectiveness. Decreasing the interval between doses may also provide more continuous pain relief. A major difference between opioids and nonopioids is that nonopioids have a ceiling effect, which means that doses higher than the recommended dose will not produce greater pain relief. Opioids do not have a ceiling effect other than that imposed by side effects; therefore larger dosages can be safely given for increasing severity of pain.
Parenteral and oral dosages of opioids are not the same. Because of the first-pass effect, an oral opioid is rapidly absorbed from the gastrointestinal tract and is partially metabolized in the liver before reaching the central circulation. Therefore oral dosages must be larger to compensate for the partial loss of analgesic potency to achieve equianalgesia (equal analgesic effect). Conversion factors (Table 7-8) for selected opioids must be used when a change is made from IV (preferred) or IM to oral. Immediate conversion from IM or IV to the suggested equianalgesic oral dose may result in a substantial error. For example, the dose may be significantly more or less than what the child requires. Small changes ensure small errors. Several routes of analgesic administration can be used (Box 7-3), and the most effective and least traumatic route of administration should be selected.
TABLE 7-8
EQUIANALGESIA OF SELECTED ANALGESICS
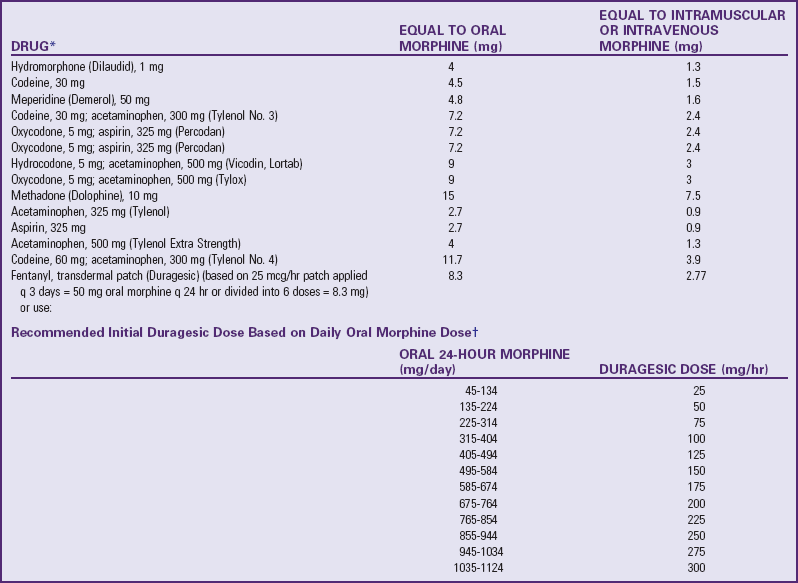
note: When converting to oral oxycodone from oral morphine, an appropriate conservative estimate is 15-20 mg of oxycodone per 30 mg of morphine; however, when converting to oral morphine from oral oxycodone, an appropriate conservative estimate is 30 mg of morphine per 30 mg of oxycodone (McCaffery M, Pasero C: Pain: a clinical manual, ed. 2, St. Louis, 1999, Mosby).
*Oral medication with exception of fentanyl.
†Data from Duragesic package insert, Janssen Pharmaceutical Products, Titusville, NJ, 2001.
Courtesy Betty R. Ferrell, PhD FAAN, 1999. Used with permission.
Preventive pain control is best provided through continuous IV infusion rather than intermittent boluses. If intermittent boluses are given, make certain the intervals between doses do not exceed the drug’s expected duration of effectiveness. For extended pain control with fewer administration times, drugs that provide longer duration of action (e.g., some NSAIDs, time-released morphine or oxycodone, methadone, levorphanol) can be used.
Continuous analgesia is not always appropriate, since not all pain is continuous. Frequently, temporary pain control or conscious sedation is needed to provide analgesia before a scheduled procedure. When pain can be predicted, the drug’s peak effect should be timed to coincide with the painful event. For example, with opioids the peak effect is approximately a half hour for the IV route; with nonopioids the peak effect occurs about 2 hours after oral administration. For rapid onset and peak of action, opioids that quickly penetrate the blood-brain barrier (e.g., IV fentanyl) provide excellent pain control.
Patient-Controlled Analgesia
A significant advance in the administration of IV, epidural, or subcutaneous analgesics is the use of patient-controlled analgesia (PCA). As the name implies, the patient controls the amount and frequency of the analgesic, which is typically delivered through a special infusion device. Children who are physically able to “push a button” (i.e., 5 to 6 years of age) and who can understand the concept of pushing a button to obtain pain relief can use PCA (Maxwell and Yaster, 2000). Although it is controversial, parents and nurses have used the IV PCA system for the child. Nurses can efficiently use the infusion device on a child of any age to administer analgesics to avoid signing for and preparing opioid injections every time one is needed (Fig. 7-7). When PCA is used as “nurse- or parent-controlled” analgesia, the concept of patient control is negated, and the inherent safety of PCA needs to be monitored. Research has reported safe and effective analgesia in children when the patient, parent, or nurse controlled the PCA (Algren, Gursoy, Johnson, et al, 1998; Maxwell and Yaster, 2000).
PCA infusion devices typically allow for three methods or modes of drug administration to be used alone or in combination:
1. Patient-administered boluses that can be infused only according to the preset amount and lockout interval (time between doses). More frequent attempts at self-administration may mean the patient needs the dose and time adjusted for better pain control.
2. Nurse-administered boluses that are typically used to give an initial loading dose to increase blood levels rapidly and to relieve breakthrough pain (pain not relieved with the usual programmed dose).
3. Continuous basal rate infusion that delivers a constant amount of analgesic and prevents pain from returning during those times, such as sleep, when the patient cannot control the infusion.
As with any type of analgesic management plan, continued assessment of the child’s pain relief is essential for the greatest benefit from PCA. Typical uses of PCA are for controlling pain from surgery, sickle cell crisis, trauma, and cancer. Morphine is the drug of choice for PCA and usually comes in a concentration of 1 mg/ml (Table 7-9). Other options are hydromorphone (0.2 mg/ml) and fentanyl (0.01 mg/ml).
TABLE 7-9
SUGGESTED INTRAVENOUS PATIENT-CONTROLLED ANALGESIA OPIOID INFUSION ORDERS

From Yaster M, Krance EJ, Kaplan RF, et al: Pediatric pain management and sedation handbook, St. Louis, 1997, Mosby.
Hydromorphone is often used when patients are not able to tolerate side effects such as pruritus and nausea from the morphine PCA (Algren, Gursoy, Johnson, et al, 1998; Maxwell and Yaster, 2000). Some physicians may still prescribe meperidine. However, meperidine is the least potent and shortest-acting of the synthetic opioids and the least effective in providing analgesia for severe pain. More important, it may increase the risk of seizures when administered chronically because of the excitatory effects on the nervous system of its metabolite, normeperidine. Some authors (Nadvi, Sarnaik, and Ravindranath, 1999) have argued that the incidence of meperidine-associated seizures is extremely small (0.4% of patients; 0.06% of admissions) and the risk of seizures should not dissuade clinicians from using this drug. However, the American Pain Society recommends that meperidine be reserved for brief treatment courses for patients who have reported and demonstrated its effectiveness, or who have allergies or uncorrectable intolerances to other opioids. Meperidine should not be used for longer than 48 hours or in dosages greater than 600 mg/24 hr (Max, Payne, Edwards, et al, 1999).
Epidural Analgesia
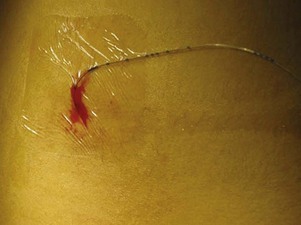
Epidural analgesia is used to manage pain in selected cases. Although an epidural catheter can be inserted at any vertebral level, it is usually placed into the epidural space of the spinal column at the lumbar or caudal level (Fig. 7-8). The thoracic level is usually reserved for older children or adolescents who have had an upper abdominal or thoracic procedure, such as a lung transplant. An opioid (usually fentanyl, hydromorphone, or preservative-free morphine, which is often combined with a long-acting local anesthetic such as bupivacaine or ropivacaine) is instilled via single or intermittent bolus, continuous infusion, or patient-controlled epidural analgesia. Analgesia results from the drug’s effect on opiate receptors in the dorsal horn of the spinal cord, rather than the brain. As a result, respiratory depression is rare, but if it occurs, it develops slowly, typically 6 to 8 hours after administration (Golianu, Krane, Galloway, et al, 2000). Properly securing the epidural catheter with an occlusive dressing decreases the possibility of soiling or inadvertently displacing the catheter (Fig. 7-9). Careful monitoring of sedation level and respiratory status is critical to prevent opioid-induced respiratory depression. Assessment of pain and the skin condition around the catheter site are important aspects of nursing care (Golianu, Krane, Galloway, et al, 2000).
Transmucosal and Transdermal Analgesia
Oral transmucosal fentanyl (Oralet) provides nontraumatic preoperative and preprocedural analgesia and sedation (Golianu, Krane, Galloway, et al, 2000). Fentanyl is also available as a transdermal patch (Duragesic). Although contraindicated for acute pain management, it may be used for older children and adolescents who have cancer pain or sickle cell pain or for patients who are opioid tolerant.
One of the most significant improvements in the ability to provide atraumatic care to children is the anesthetic cream LMX4 (a 4% liposomal lidocaine cream) or EMLA (a eutectic mixture of local anesthetics) (Abdelkefi, Abdennebi, Mellouli, et al, 2004; Choi, Irwin, Hui, et al, 2003; Egekvist and Bjerring, 2000; Gad, Olsen, Lysgaard, et al, 2005; Rogers and Ostrow, 2004; Santiago, Abad, Fernandez, et al, 2000; Uziel, Berkovitch, Gazarian, et al, 2003). The eutectic mixture (lidocaine 2.5% and prilocaine 2.5%), whose melting point is lower than that of the two anesthetics alone, permits effective concentrations of the drug to penetrate intact skin (see Evidence-Based Practice box, p. 207, and Fig. 7-10). Transdermal patches such as Synera are effective methods to administer topical analgesia before painful procedures. A recent review of the evidence comparing these patches to EMLA is found in the Evidence-Based Practice box (p. 208).
In some situations there is not enough time for topical preparations like LMX or EMLA to take effect, and refrigerant sprays such as ethyl chloride and fluorimethane can be used (Reis and Holubkov, 1997). When sprayed on the skin, these sprays vaporize, rapidly cool the area, and provide superficial anesthesia. Hospital formularies may have other products with lidocaine, prilocaine, or amethocaine topical preparations that require less time for application.
The intradermal route is sometimes used to inject a local anesthetic, typically lidocaine, into the skin to reduce the pain from a lumbar puncture, bone marrow aspiration, or venous or arterial access. One problem with the use of lidocaine is the stinging and burning that initially occur. However, the use of buffered lidocaine with sodium bicarbonate (see Evidence-Based Practice box, p. 210) reduces the stinging sensation (Wong and Pasero, 1997a, 1997b). Warming the lidocaine to 37° C (98.6° F) may accomplish the same effect (McCaffery and Pasero, 1999). A needle-free injection system also be can used to provide intradermal anesthesia (see Evidence-Based Practice box, p. 209).
Timing of Analgesia
The right timing for administering analgesics depends on the type of pain. For continuous pain control, such as for postoperative or cancer pain, a preventive schedule of medication around the clock (ATC) is effective. The ATC schedule avoids the low plasma concentrations that permit breakthrough pain. If analgesics are administered only when pain returns (a typical use of the prn, or “as needed,” order), pain relief may take several hours. This may require higher doses, leading to a cycle of undermedication of pain alternating with periods of overmedication and drug toxicity. This cycle of erratic pain control also promotes “clock watching,” which may be erroneously equated with addiction. Nurses can effectively use prn orders by giving the drug at regular intervals, since “as needed” should be interpreted as “as needed to prevent pain,” not “as little as possible.”
Monitoring Side Effects
Both NSAIDs and opioids have side effects, although the major concern is with those from opioids (Box 7-4). Respiratory depression is the most serious complication and is most likely to occur in sedated patients. The respiratory rate may decrease gradually or respirations may cease abruptly; lower limits of normal are not established for children, but any significant change from a previous rate calls for increased vigilance. A slower respiratory rate does not necessarily reflect decreased arterial oxygenation; an increased depth of ventilation may compensate for the altered rate. If respiratory depression or arrest occurs, be prepared to intervene quickly (see Nursing Care Guidelines box).
Although respiratory depression is the most dangerous side effect, constipation is a common, and sometimes serious, side effect of opioids, which decrease peristalsis and increase anal sphincter tone. Prevention with stool softeners and laxatives is more effective than treatment once constipation occurs. Dietary treatment, such as increased fiber, is usually not sufficient to promote regular bowel evacuation. However, dietary measures, such as increased fluid and fruit intake, and physical activity are encouraged. Pruritus from epidural or IV infusion is treated with low doses of IV naloxone, nalbuphine, or diphenhydramine. Nausea, vomiting, and sedation usually subside after 2 days of opioid administration, although oral or rectal antiemetics are sometimes necessary.
Both tolerance and physical dependence can occur with prolonged use of opioids (see Community Focus box). Physical dependence is a normal, natural, physiologic state of “neuroadaptation.” When opioids are abruptly discontinued without weaning, withdrawal symptoms occur 24 hours later and reach a peak within 72 hours. Symptoms of withdrawal include signs of neurologic excitability (irritability, tremors, seizures, increased motor tone, insomnia), gastrointestinal dysfunction (nausea, vomiting, diarrhea, abdominal cramps), and autonomic dysfunction (sweating, fever, chills, tachypnea, nasal congestion, rhinitis). Withdrawal symptoms can be anticipated and prevented by weaning patients from opioids that were administered for more than 5 to 10 days. Adherence to a weaning protocol to prevent or minimize withdrawal symptoms from opioids is required. A weaning flowsheet (Fig. 7-11, A) may be used to assess the efficacy of opioid weaning in neonates (Franck and Vilardi, 1995; Franck, Vilardi, Durand, et al, 1998). In older infants and young children (7 months to 10 years) the Withdrawal Assessment Tool–1 (Fig. 7-11, B) may be use to assess and monitor withdrawal symptoms in pediatric critically ill children who are exposed to opioids and benzodiazepines for prolonged periods (Franck, Harris, Soetenga, et al, 2008).
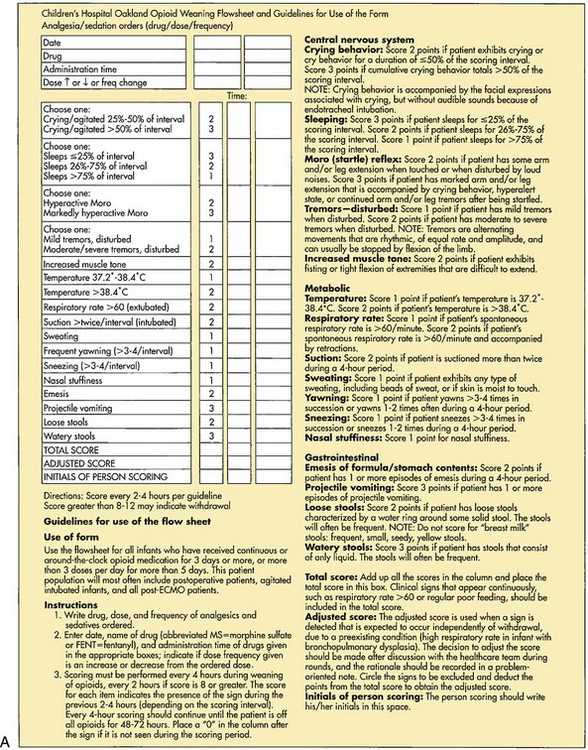

Fig. 7-11 A, Weaning flowsheet to monitor opioid weaning in neonates. B, Withdrawal assessment tool for infants and children. SBS, State behavioral scale. (A, Modified from Franck L, Vilardi J: Assessment and management of opioid withdrawal in ill neonates, Neonatal Netw 14[2]:39-48, 1995; B, © 2007 LS Franck and MAQ Curley. All rights reserved. Reprinted in Franck LS, Harris SK, Soetenga DJ, et al: The Withdrawal Assessment Tool–1 (WAT–1): an assessment instrument for monitoring opioid and benzodiazepine withdrawal symptoms in pediatric patients, Pediatr Crit Care Med 9[6]:577, 2008. *From Curley MQ, Harris SK, Fraser KA, et al: State behavioral scale: a sedation assessment instrument for infants and young children supported on mechanical ventilation, Pediatr Crit Care Med 7(2):107-114, 2008.)
Tolerance occurs when the dose of an opioid needs to be increased to achieve the same analgesic effects that was previously achieved at a lower dose (see Community Focus box). Tolerance may develop after 10 to 21 days of morphine administration. Treatment of tolerance involves increasing the dose or decreasing the duration between doses. Treatment of physical dependence involves gradually reducing the dose over several days to prevent withdrawal symptoms, as follows (Max, Payne, Edwards, et al, 1999):
• Gradually reduce dose (similar to tapering of steroids).
• Give half of previous daily dose every 6 hours for first 2 days.
• Then reduce dose by 25% every 2 days. Continue this schedule until the patient reaches a total daily dose of 0.6 mg/kg of morphine (or equivalent). After 2 days on this dose, discontinue opioid.
• You may also switch to oral methadone, using one fourth of equianalgesic dose as initial weaning dose and proceeding as described above.
Parents and older children may fear addiction when opioids are prescribed. The nurse should address these concerns with assurance that any such risk is extremely low. It may be helpful to ask the question, “If you did not have this pain, would you want to take this medicine?” The answer is invariably no, which reinforces the solely therapeutic nature of the drug. It is also important to avoid making statements to the family such as “We don’t want you to get used to this medicine,” or “By now you shouldn’t need this medicine,” which may reinforce the fear of becoming addicted. Whereas both physical dependence and tolerance are physiologic states, addiction or psychologic dependence is a psychologic state and implies a “cause-effect” mode of thinking, such as “I need the drug because it makes me feel better.” Infants and children do not have the cognitive ability to make the cause-effect association and therefore cannot become addicted. The use of opioid analgesics early in life has not been demonstrated to increase the risk for addiction later in life. Nurses need to explain to parents the differences among physical dependence, tolerance, and addiction and allow them to express concerns about the use and duration of use of opioids. Infants and children, when treated appropriately with opioids, may be at risk for physical tolerance and physical dependence, but not psychologic dependence or addiction (McCaffery and Pasero, 1999).
Evaluation of Effectiveness of Pain Regimen
The response to therapy should be evaluated 15 to 30 minutes after each dose, and titration should continue to the highest achievable amount of relief (Max, Payne, Edwards, et al, 1999). In a retrospective study that examined the pain experience of children with sickle cell disease, evidence of pain relief from medications was documented for less than half (44.8%) of the patients in the emergency department (ED) (Jacob and Mueller, 2008). Even though The Joint Commission required documentation of pain assessments with vital signs, evidence of pain relief was not documented in 41.4% of the episodes. Titration methods in the ED or during the course of hospitalization, if used, were not reflected in the amount of medications received by the children (Jacob and Mueller, 2008; Jacob, Miaskowski, Savedra, et al, 2003a, 2003b).
In a study of nursing practice related to pain assessment and management in different pediatric specialty units, nurses noted complaints of pain, but seldom documented specific pain scores or responses to analgesics after administration (Jacob and Puntillo, 2000). Pain scores were not available before and after analgesics, and it was therefore not possible to conclude whether analgesics were effective. Nurses therefore need to evaluate and monitor pain in a timely fashion after administration of analgesic and titrate dosage to effect. Nurses also need to make recommendations for an alternate analgesic; for addition of another analgesic; or for a combination of analgesics, adjuvants, and nonpharmacologic strategies.
Consequences of Untreated Pain in Infants
Despite current research on the neonate’s experience of pain, infant pain often remains inadequately managed. The mismanagement of infant pain is partially the result of misconceptions regarding the effects of pain on the neonate and the lack of knowledge of immediate and long-term consequences of untreated pain. Infants respond to noxious stimuli through physiologic indicators (increased heart rate and blood pressure, variability in heart rate and intracranial pressure, and decreases in Sao2 and skin blood flow) and behavioral indicators (muscle rigidity, facial expression, crying, withdrawal, and sleeplessness) (Anand, Grunau, and Oberlander, 1997; Bildner and Krechel, 1996). The physiologic and behavioral changes, as well as a variety of neurophysiologic responses to noxious stimulation, are responsible for acute and long-term consequences of pain.
Several harmful effects occur with unrelieved pain, particularly when pain is prolonged. Pain triggers a number of physiologic stress responses in the body, and they lead to negative consequences that involve multiple systems. Unrelieved pain may prolong the stress response and adversely affect an infant’s or child’s recovery, whether it is from trauma, surgery, or disease. (See Research Focus box.)
Poorly controlled acute pain can predispose patients to chronic pain syndromes. See Box 7-5 for a list of numerous complications of untreated pain in infants. A guiding principle in pain management is that prevention of pain is always better than treatment (Benjamin, Swinson, and Nagel, 2000). Pain that is established and severe is often more difficult to control. When pain is unrelieved, sensory input from injured tissues reaches spinal cord neurons and may enhance subsequent responses. Long-lasting changes in cells within spinal cord pain pathways may occur after a brief painful stimulus and may lead to the development of chronic pain conditions. Basbaum (1999a, 1999b) reported a series of studies that emphasize a distinct neurochemistry of acute and persistent pain and concluded that persistent pain is not merely a prolonged acute pain symptom of some other disease. Underlying physiologic mechanisms lead to the persistence of pain (Marx, 2004; Woolf and Salter, 2000).
Anand and Hickey (1987) described additional responses of infants to painful stimuli from their own unpublished and other scientific studies. Chemical and hormonal responses were observed following noxious stimuli without the use of an anesthetic or analgesic. Such responses included increases in β-endorphin secretion (an endogenous opioid), plasma renin activity, plasma epinephrine and norepinephrine, catecholamines, growth hormone, glucagon, aldosterone, and other corticosteroids. The result of these chemical and hormonal increases includes the breakdown of fat and carbohydrate stores; prolonged hyperglycemia; and increased serum lactate, pyruvate, total ketone bodies, and nonesterified fatty acids. Such consequences can lead to a greater morbidity for neonates in the NICU. Several experimental studies revealed a significant decrease in these responses when adequate analgesia was used before the painful procedure. One study showed that the standardization of postoperative pain management strategies for infants in the NICU led to the following improvements: (1) decreased length of time to extubation, (2) decreased length of stay, (3) better fluid management, and (4) reduced side effects of opioids. The authors also noted improved pain management documentation, decreased cost, and decreased nursing time (Furdon, Eastman, Benjamin, et al, 1998) (see Atraumatic Care box).
An experience known as the windup phenomenon has been attributed to a decreased pain threshold and chronic pain. Central and peripheral mechanisms that occur in response to noxious tissue injury have been studied in an attempt to explain a prolonged neonatal response to pain characteristic of the windup phenomenon. After exposure to noxious stimuli, multiple levels of the spinal cord experience an altered excitability. This altered excitability may cause nonnoxious stimuli, such as routine nursing care and handling, to be perceived as noxious stimuli. The nonnoxious stimuli produce the same physiologic response to stress that noxious stimuli would produce, leading to chronic pain. Long-term exposure to chronic pain may be responsible for more biologic and clinical consequences in critically ill premature infants than acute pain (Anand, Grunau, and Oberlander, 1997).
Researchers have found that nerve damage resulting from tissue injury stimulates collateral nerve growth by surrounding undamaged nerves. This collateral growth is responsible for inappropriate innervation in the spinal cord, which processes information from the surrounding undamaged nerves (Anand, Grunau, and Oberlander, 1997). Based on evidence from a study of human infants and adult rats exposed to neonatal pain, additional long-term consequences of neonatal pain include potential emotional temperament changes in infancy or childhood, accentuated hormonal stress responses in adult life, a preference for alcohol, and decreased exploratory behaviors (Anand, Grunau, and Oberlander, 1997).
Consequences of a history of extremely low birth weight and early pain exposure that may be attributed to pain and environmental stress include increased prevalence of neurologic deficits, psychosocial problems, and neurobehavioral disorders. Additional sequelae include cognitive deficits, learning disorders, poor motor performance, behavioral problems, attention deficits, poor adaptive behavior, inability to cope with novel situations, problems with impulsivity and social control, and learning deficits (Anand, Grunau, and Oberlander, 1997).
The limited available knowledge with respect to the consequences of infant pain suggests serious potential deleterious effects of untreated pain (see Box 7-5). Prevention of acute pain and treatment of chronic pain have been documented as beneficial in reducing the morbidity and mortality associated with frequent exposure to pain in premature infants (Anand and Carr, 1989; Anand and Hickey, 1987, 1992). Nurses who care for infants and children should consider the potential acute and long-term effects of pain on their young patients and be advocates in treating and preventing pain.