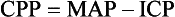The Child with Cerebral Dysfunction
http://evolve.elsevier.com/wong/ncic
Administration of Medication, Ch. 27
Anencephaly, Ch. 11
Brain Tumors, Ch. 36
Controlling Elevated Temperatures, Ch. 27
Cranial Deformities, Ch. 11
Family-Centered Home Care, Ch. 25
High Risk Related to Neurologic Disturbance, Ch. 10
Human Immunodeficiency Virus Infection and Acquired Immunodeficiency Syndrome, Chs. 20 and 35
Hydrocephalus, Ch. 11
Infection Control, Ch. 27
Injuries—The Leading Killer, Ch. 1
Maintaining Healthy Skin, Ch. 27
Neurologic Assessment, Ch. 6
Pain Assessment; Pain Management, Ch. 7
Preparation for Diagnostic and Therapeutic Procedures, Ch. 27
Cerebral Structure and Function
![]() The nervous system is made up of three intimately connected and functioning parts: the central nervous system (CNS), the peripheral nervous system, and the autonomic nervous system. The CNS is composed of two cerebral hemispheres, the brainstem, the cerebellum, and the spinal cord. The peripheral nervous system is composed of the cranial nerves (CNs) that arise from or travel to the brainstem and the spinal nerves that travel to or from the spinal cord and that may be motor (efferent) or sensory (afferent). The autonomic nervous system is composed of the sympathetic and parasympathetic systems, which provide automatic control of vital functions.
The nervous system is made up of three intimately connected and functioning parts: the central nervous system (CNS), the peripheral nervous system, and the autonomic nervous system. The CNS is composed of two cerebral hemispheres, the brainstem, the cerebellum, and the spinal cord. The peripheral nervous system is composed of the cranial nerves (CNs) that arise from or travel to the brainstem and the spinal nerves that travel to or from the spinal cord and that may be motor (efferent) or sensory (afferent). The autonomic nervous system is composed of the sympathetic and parasympathetic systems, which provide automatic control of vital functions.
This chapter is concerned primarily with disturbances of the brain. Chapter 40 discusses the structure and function of the spinal cord and autonomic nervous system in more detail.
Development of the Neurologic System
In contrast to other body tissues, which grow rapidly after birth, the nervous system grows proportionately more rapidly before birth. Two periods of rapid brain cell growth occur during fetal life. At 15 to 20 weeks of gestation there is a dramatic increase in the number of neurons. Another increase in growth rate begins at 30 weeks of gestation and extends to 1 year of age. This rapid growth during infancy continues during early childhood and slows to a more gradual rate during later childhood and adolescence. Brain volume is readily reflected in head circumference, which increases six times as much during the first year as during the second year of life. One half of the postnatal brain growth is achieved by age 1 year, 75% by age 3, and 90% by age 6. Cerebral blood flow (CBF) and oxygen consumption in childhood (up to age 6 years) is almost twice that of adults, which reflects an increased metabolic requirement consistent with growth and development.
The growth and final form of the brain depend on the development and multiplication of neurons. Creation of new cells occurs, in theory, only during the first 100 days of gestation. During the remainder of gestation, cells divide and multiply at the astonishing rate of 250,000 per minute. It is believed that no new nerve cells appear after the sixth month of fetal life. Postnatal growth consists of increasing the amount of cytoplasm around the nuclei of the 10 billion existing cells, increasing the number and intricacy of communications with other cells, and advancing their peripheral axons to keep pace with expanding body dimensions.
The brain constitutes 12% of the body weight at birth. It doubles its weight in the first year, and by age 5 or 6 years its weight at birth has tripled. Thereafter growth slows until in adulthood the brain is only about 2% of the total body weight. The surface configuration of the brain also changes with development. The early embryonic brain surface is smooth, but the sulci deepen with advancing development. This process continues throughout childhood. At birth the cortex is only about one half of its adult thickness, although all the major surface features are present. There is little cortical control over body movements at birth, with movements guided principally by primitive reflexes. (See Chapter 8.) With advancing development and maturation, the brain, through association pathways, exercises increasing control over much of the reflex activity. This allows the growing child to perform progressively complex tasks that require coordinated movements. Persistence of primitive reflexes may suggest defective cortical development.
Cortical control is closely associated with the acquisition of a myelin coating on the nerves. Although nerve fibers are able to conduct impulses without this myelin sheath, the impulses travel at a slower rate and with more likelihood of diffusion. Myelinization of the various nerve tracts in the CNS, which allows progressive neuromotor function, follows the cephalocaudal (head-to-toe) and proximodistal (near-to-far) sequence. It appears first with the fibers of the spinal cord and cranial nerves, then in the brainstem and corticospinal tracts.
Development of the nervous system proceeds on a continuum and generates the most complex structures within the embryo. The brain and spinal cord are among the first of the major organ systems to be recognized in the embryo and one of the last to finish significant development after birth. The rate of myelogenesis accelerates rapidly after birth. In general, the pathways concerned with sensation are myelinated early, before the motor pathways. The acquisition of motor skills depends on the maturation and myelination of the nervous system, and no amount of special training or practice will hasten the process. Most of an infant’s advancing performance is a direct result of brain development indirectly influenced by environmental stimuli.
Central Nervous System
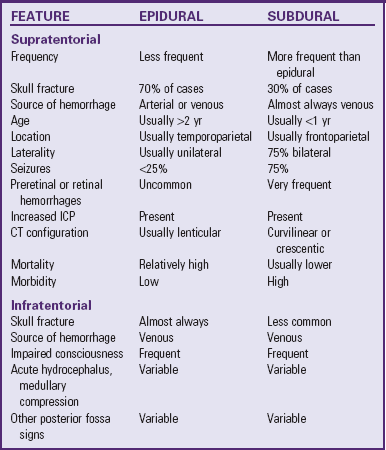
The bony skull forms the strongest covering and provides the primary protection to the brain. It is an expansible structure in the infant and young child due to incomplete ossification of the bones of the skull, but becomes rigid in the older child and adolescent. Blood is supplied to the dura mater by the middle meningeal artery, a branch of the external carotid artery. It enters the skull at a point inferior to the temporal bone, then branches over the surface of the dura, usually encased in a groove in the temporal and parietal bones after 2 years of age. Damage to this artery or to its branches is a common cause of an epidural hematoma.
Brain Coverings
Within the skull, three membranes (the meninges) cover and protect the brain: the dura mater, arachnoid membrane, and pia mater (Fig. 37-1). The tough outer membrane, the dura mater, is a double layer that serves as the outer meningeal layer and the inner periosteum of the cranial bones. These two layers are separated by the epidural space. The dura is closely attached to the skull in infancy, causing slower spread of blood in epidural hemorrhage. Because of this adherence, epidural hemorrhages are uncommon in the first 2 years of life.
PATHOPHYSIOLOGY REVIEW
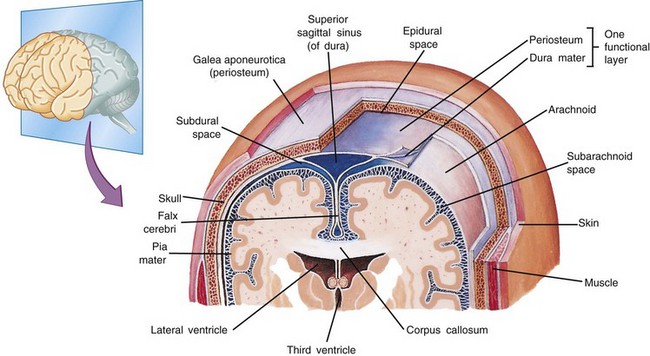
Fig. 37-1 Coronal section of top of head showing meningeal layers. (From Patton KT, Thibodeau GA: Anatomy and physiology, ed 7, St Louis, 2010, Mosby.)
Between these layers of dura inside the skull lie large venous sinuses. Sheets of the dura mater also extend downward and inward to form partitions within the cranium. Projecting downward into the longitudinal fissure is a sheet of dura called the falx cerebri, which separates the cerebral hemispheres, and the falx cerebelli, which separates the cerebellar hemispheres. Another segment is a tentlike structure, the tentorium, which separates the cerebellum from the occipital lobe of the cerebrum. The large gap through which the brainstem passes is the tentorial hiatus, the site of herniation in untreated intracranial pressure (ICP).
The middle meningeal layer, the arachnoid membrane, is a delicate, avascular, weblike structure that loosely surrounds the brain. Between the arachnoid and the dura mater lies the subdural area, a potential space that normally contains only enough fluid to prevent adhesion between the two membranes. During cerebral trauma the fine blood vessels that bridge the subdural space are stretched and ruptured, causing venous blood to escape and spread freely, forming a subdural hemorrhage. The subdural space is small in children; therefore small amounts of blood can increase intracranial hemorrhage significantly.
The innermost covering layer, the pia mater, is a delicate, transparent membrane that, unlike the other coverings, adheres closely to the outer surface of the brain, conforming to the folds (gyri) and furrows (sulci). Within the pial layer lie the arteries and veins of the brain. Between the pia mater and the arachnoid membrane is the subarachnoid space. Cerebrospinal fluid (CSF) fills the entire subarachnoid space surrounding the brain and spinal cord and acts as a protective cushion for the brain tissue. Fibrous filaments known as arachnoid trabeculae provide further protection and help anchor the brain. When the head receives a blow, these attachments allow the arachnoid to slide on the dura, preventing excessive movement.
The Brain
![]() Each section of the brain plays a vital role in regulation and control of body function. Each hemisphere is artificially divided into lobes. Pressure on or damage to these lobes produces observable signs or symptoms directly related to the area of pathology. These signs provide clues to the location of the damage.
Each section of the brain plays a vital role in regulation and control of body function. Each hemisphere is artificially divided into lobes. Pressure on or damage to these lobes produces observable signs or symptoms directly related to the area of pathology. These signs provide clues to the location of the damage.
The two large cerebral hemispheres that occupy the anterior and medial fossae of the skull are separated in the upper part by the longitudinal fissure. This separation is complete anteriorly and posteriorly, but centrally the hemispheres are joined by the block of fibers known as the corpus callosum, the largest fiber bundle in the brain. These fibers interconnect cortical areas of the right and left hemispheres. Destruction of the corpus callosum causes hemispheric independence, or “split brain.”
Situated deeply within each hemisphere and on each side of the midline are the basal ganglia (or cerebral nuclei), which serve as vital sorting areas for messages passing to and from the hemispheres. Connected to the hemispheres by thick bunches of nerve fibers is the brainstem, through which all nerve fibers traverse as they pass from the hemispheres to the cerebellum and spinal cord. The brainstem extends from the base of the hemispheres through the foramen magnum, where it is continuous with the spinal cord. Within the cranium and behind the brainstem is the cerebellum. Any pressure exerted on the intracranial structures can cause compression of the brainstem and prolapse of the cerebellum through the foramen magnum.
Cerebral Blood Flow: The blood supply to the brain tissue is carried by the internal carotid arteries, which branch to supply the various brain segments. The volume of blood to the brain, which constitutes only 17% of the cardiac output, supplies the brain with 20% of the body oxygen. The brain, an “inactive” organ, uses 10 times the oxygen used by the body as a whole. Only the heart uses more oxygen per gram of tissue.
CBF is the result of two opposing forces: cerebral blood pressure (the difference between systemic arterial pressure and cerebral venous pressure) and cerebral vascular resistance. CBF remains constant at a cerebral blood pressure between 50 and 150 mm Hg. Because cerebral venous pressure is usually very low and relatively constant, cerebral blood pressure is determined mainly by systemic arterial pressure.
Autoregulation: ![]() One of the most important factors in the control of CBF is autoregulation, the unique ability of cerebral arterial vessels to change their diameter in response to fluctuating cerebral perfusion pressure (CPP). The CPP is the mean arterial pressure (MAP) minus the ICP:
One of the most important factors in the control of CBF is autoregulation, the unique ability of cerebral arterial vessels to change their diameter in response to fluctuating cerebral perfusion pressure (CPP). The CPP is the mean arterial pressure (MAP) minus the ICP:
As a result, cerebral vessels maintain a constant blood flow during alterations in blood pressure and perfusion caused by body posture, increased ICP, decreased cardiac output, or narrowing or occlusion in the major blood vessels of the neck. Autoregulation fails when the limits of cerebrovascular dilation are reached; at this point CBF decreases, causing clinical symptoms of ischemia (nausea, fainting, dizziness, dim vision). Conversely, increased MAP leads to “breakthrough of autoregulation,” with increased CBF leading to microhemorrhages and cerebral edema. Autoregulation may be impaired locally or globally as a result of trauma or ischemia.
Changes in arterial oxygen pressure (Pao2) or arterial carbon dioxide pressure (Paco2) have a profound effect on autoregulation. Hypercapnia (Paco2 > 40 mm Hg) or increased levels of lactic acid have a pronounced dilating effect on cerebral arterioles, which increases CBF and thus cerebral volume. Hypocapnia (Paco2 of 25 to 30 mm Hg) constricts cerebral arterioles and decreases CBF. Pao2 values between 70 and 100 mm Hg have little effect on the cerebrovascular system. Profound hypoxia (Pao2 < 50 mm Hg) dramatically increases CBF. Consequently maintenance of the airway and effective hyperventilation are of primary importance in the initial management of the neurologically impaired patient. CPP is the most important physiologic determinant because the brain relies on the delivery of oxygen and nutrients to function.
Oxygen: Metabolic requirements for oxygen by the brain are not affected by rest or sleep, but they are reduced by narcosis and coma and are altered by changes in temperature. CBF is not altered when body temperature is between 35° and 40° C (95° and 104° F). Hyperthermia increases oxygen consumption by the brain, and hypothermia decreases oxygen consumption. The brain depends on a constant supply of oxygen-rich blood, and, because the brain’s need for oxygen is great in relation to the volume of blood supplied, it extracts more oxygen from each unit of circulating blood.
Oxygen supply to the brain is compromised when the supply is inadequate as a result of impaired respiration, hypotension, increased ICP or vascular damage, spasm, or compression. Neurons are highly susceptible to elevated Paco2 (a potent vasodilator), and the metabolic damage to brain tissue caused by an inadequate supply of well-oxygenated blood can often exceed the effects of trauma. Respiratory acidosis resulting from increased Paco2 levels can produce symptoms indistinguishable from those of head injury.
Blood-Brain Barrier: The blood-brain barrier (BBB) is an anatomic-physiologic feature of the brain that separates the brain parenchyma from the blood. Unlike capillaries in other parts of the body, cerebral capillaries have no fenestrations or pores. The tight junctions of the vascular endothelium are responsible for the selective nature of the BBB. The mature BBB allows facilitated diffusion of glucose and passive diffusion of water and carbon dioxide but is impermeable to protein and does not permit passage of many active substances. However, the BBB of the fetus and newborn is normally indiscriminately permeable, allowing protein and other large and small molecules to pass freely between the cerebral vessels and the brain. Conditions that cause cerebrovascular dilation (hypertension, hypercapnia, hypoxia, acidosis) disrupt the BBB. Hyperosmotic fluids, which cause shrinkage of vascular endothelium and widen the vascular junctions, also disrupt the BBB.
Increased Intracranial Pressure
![]() The brain, tightly enclosed in the solid bony cranium, is well protected but highly vulnerable to pressure that may accumulate within the enclosure. Its total volume—brain (80%), CSF (10%), and blood (10%)—must remain approximately the same at all times. A change in the proportional volume of one of these components (e.g., increase or decrease in intracranial blood) must be accompanied by a compensatory change in another (e.g., decrease or increase in CSF). In this way the volume and pressure normally remain constant. Examples of compensatory changes are reduction in blood volume, decrease in production of CSF, increase in CSF absorption, or shrinkage of brain mass by displacement of intracellular and extracellular fluid.
The brain, tightly enclosed in the solid bony cranium, is well protected but highly vulnerable to pressure that may accumulate within the enclosure. Its total volume—brain (80%), CSF (10%), and blood (10%)—must remain approximately the same at all times. A change in the proportional volume of one of these components (e.g., increase or decrease in intracranial blood) must be accompanied by a compensatory change in another (e.g., decrease or increase in CSF). In this way the volume and pressure normally remain constant. Examples of compensatory changes are reduction in blood volume, decrease in production of CSF, increase in CSF absorption, or shrinkage of brain mass by displacement of intracellular and extracellular fluid.
Children with open fontanels compensate for increased volume by skull expansion and widened sutures. However, at any age the capacity for spatial compensation is limited. An increase in ICP may be caused by tumors or other space-occupying lesions, accumulation of fluid within the ventricular system, bleeding, or edema of cerebral tissues. Once compensation is exhausted, any further increase in volume results in a rapid rise in ICP.
The early signs and symptoms of increased ICP are often subtle, such as headache, vomiting, personality changes, irritability, and fatigue (Box 37-1). In older children subjective symptoms are headache, especially when lying flat (e.g., on awakening in the morning) or when coughing, sneezing, or bending over, and nausea and vomiting. The child may complain of double vision or blurred vision with movement of the head. Seizures may occur. In children whose cranial sutures have not closed, there is an increase in head circumference and tense or bulging fontanels. Cranial sutures may become diastatic or may split; head circumference can enlarge until the child is 5 years of age if the condition progresses slowly. As pressure increases, the pupils become progressively sluggish in reaction and eventually become fixed and dilated. The level of consciousness progressively deteriorates from drowsiness to eventual coma. Problems related to increased ICP are discussed later in this chapter in relation to head injury. (See Brain Tumors, Chapter 36, and Hydrocephalus, Chapter 11.)
Physiologic and biochemical changes within the cerebral vasculature serve to complicate the primary causes of increased ICP. Especially in cases of trauma, blood flow often initially increases as a result of venous congestion or vasomotor paralysis. If cerebral hypoxia is associated with the cerebral dysfunction, the compensatory vasodilation caused by oxygen deficiency will tend to increase the cerebral flow. However, blood flow is reduced as ICP progressively increases, with diminished blood supply to the brain tissues. The classic responses observed in adults (widening pulse pressure, increased blood pressure) rarely occur in children or are very late signs. Periodic or irregular breathing is an ominous sign of brainstem (especially medullary) dysfunction that often precedes apnea.
Evaluation of Neurologic Status
![]() Earlier chapters discuss methods to evaluate neurologic function in relation to numerous aspects of child care. The neurologic examination is an integral part of the health assessment (see Chapter 6) and newborn assessment (see Chapter 8). Chapter 40 discusses some of the tests used to differentiate neuromuscular disorders. The assessment tools and examinations in this chapter are primarily those used to assess intracranial integrity.
Earlier chapters discuss methods to evaluate neurologic function in relation to numerous aspects of child care. The neurologic examination is an integral part of the health assessment (see Chapter 6) and newborn assessment (see Chapter 8). Chapter 40 discusses some of the tests used to differentiate neuromuscular disorders. The assessment tools and examinations in this chapter are primarily those used to assess intracranial integrity.
![]() Animation—Cervical Nerve Examination
Animation—Cervical Nerve Examination
Assessment: General Aspects
Children younger than 2 years of age require special evaluation because they are unable to respond to directions designed to elicit specific neurologic responses. Early neurologic responses in infants are primarily reflexive; these responses are gradually replaced by meaningful movement in the characteristic cephalocaudal direction of development. This evidence of progressive maturation reflects more extensive myelinization and changes in neurochemical and electrophysiologic properties.
Most information about infants and small children comes from observation of spontaneous and elicited reflex responses. As they develop increasingly complex gross and fine motor skills and communication skills, more sophisticated techniques are used to assess acquisition of developmental milestones. Delay or deviation from expected milestones helps to identify high-risk children. Persistence or reappearance of primitive reflexes indicates a pathologic condition. In evaluating the infant or young child, it is important to obtain the history of the pregnancy, delivery, respiratory status at birth, and neonatal health to determine the possible impact of intrauterine and extrauterine environmental influences known to affect the orderly maturation of the CNS. These influences include maternal infections, chemicals, trauma, medication, illicit drug use, and metabolic insults.
History
A family history can sometimes offer clues regarding possible genetic disorders with neurologic manifestations. A review of family members often identifies conditions that might otherwise be overlooked, especially increased number of miscarriages or siblings or relatives who died at an early age. The nurse asks questions regarding specific neurologic problems, such as intellectual and developmental disabilities, deafness, epilepsy, blindness, unusual movements, weakness, ataxia, stroke, and progressive mental deterioration. History of consanguinity is also important.
A health history provides valuable clues regarding the cause of neurologic dysfunction. Is there a history of injury with loss of consciousness, febrile illness, an encounter with an animal or insect, ingestion of neurotoxic substances, inhalation of chemicals, past illness, or known diabetes mellitus or sickle cell disease? Sudden or progressive alterations in movement or mental abilities may provide clues for investigation. It is also important to ascertain the chronologic course of the illness.
Physical Examination
Physical examination includes observation of the size and shape of the head (particularly in the infant and young child), spontaneous activity and postural reflex activity, and sensory responses. Note whether the patient is lethargic, drowsy, stuporous, alert, active, or irritable. The nurse also observes the overall tone, noting whether there is a normal flexed posture or one of extreme extension, opisthotonos, or hypotonia. Symmetry of movement is also assessed.
Facial features may suggest a specific syndrome. A high-pitched, piercing cry in an infant is often associated with CNS disorders. An abnormal respiratory cycle, such as prolonged apnea, ataxic breathing, paradoxic chest movement, and hyperventilation, may be the result of a neurologic problem.
Older children can be evaluated by the usual methods used in a neurologic examination. In addition, an estimation of the level of development provides essential information about neurologic function. This assessment is discussed throughout the book in relation to evaluation for specific disorders such as intellectual and developmental disabilities, failure to thrive, attention deficit hyperactivity disorder, cerebral palsy, cerebral tumors, and other physical or behavioral problems. Developmental screening tests can assess developmental progress in the young child. (See Appendix A.)
Muscular activity and coordination, including ocular movements and gait, are valuable sources of information. Ocular movements, pupillary response, facial movements, and mouth functions provide clues regarding CNS involvement or impingement. (See Chapter 6 for CNS and reflex testing, p. 176.) Testing reflexes, strength, and coordination and for the presence and location of tremors, twitching, tics, or other unusual movements is also an aspect of the neurologic assessment (Box 37-2). Box 37-3 describes abnormalities of gait that indicate cerebral dysfunction.
Altered States of Consciousness
Consciousness implies awareness—the ability to respond to sensory stimuli and have subjective experiences. Consciousness has two aspects: alertness, an arousal-waking state that includes the ability to respond to stimuli; and cognitive power, which includes the ability to process stimuli and produce verbal and motor responses.
An altered state of consciousness usually refers to varying states of unconsciousness that may be momentary or may last for hours, days, or indefinitely. Unconsciousness is depressed cerebral function—the inability to respond to sensory stimuli and have subjective experiences. Coma is defined as a state of unconsciousness from which the patient cannot be aroused, even with powerful stimuli.
The seat of consciousness, or “alerting area,” of the brain is in the reticular formation—the central core of the brainstem. The reticular formation extends from the midbrain to the medulla. The reticular activating system receives collaterals from and is stimulated by every major somatic and special sensory pathway in the brain. Disturbances of consciousness may occur when any part of the reticular, thalamic, hypothalamic, and cortical circuits is sufficiently impaired. However, the effects may vary according to the areas involved. For example, small lesions of the reticular or hypothalamic regions produce a profound effect, whereas extensive impairment of the cortex is required to produce quantitatively similar results.
Etiology
An altered state of consciousness may be the outcome of several processes that affect the CNS. Impaired neurologic function can result from a direct or indirect cause. Some altered states, such as the diffuse changes observed in encephalitis, are directly related to cerebral insult. Others are the result of dysfunction in other organs or processes. For example, biochemical changes can impair neurologic function without morphologic findings, as in hypoglycemia.
Level of Consciousness
Assessment of level of consciousness (LOC) remains the earliest indicator of improvement or deterioration in neurologic status. LOC is determined by observations of the child’s responses to the environment. Other diagnostic tests, such as motor activity, reflexes, and vital signs, are more variable and do not necessarily directly parallel the depth of the comatose state. The most consistently used terms are described in Box 37-4.
Coma Assessment
Diminished alertness as a result of pathologic conditions occurs on a continuum and is designated as the comatose state, which extends from somnolence at one end to deep coma at the other. To produce coma, one of the following must occur: (1) extensive, diffuse, bilateral cerebral hemispheric destruction (the brainstem may be intact), (2) a lesion in the diencephalon, or (3) destruction of the brainstem down to the level of the lower pons.
Several scales have been devised in an attempt to standardize the description and interpretation of the degree of depressed consciousness. The most popular of these is the Glasgow Coma Scale (GCS), which consists of a three-part assessment: eye opening, verbal response, and motor response. The GCS was created to meet a clinical need to identify criteria for the consciousness level. For clinical purposes, the primary role of observation of the LOC is to detect a life-threatening complication such as cerebral edema. The GCS requires observational skills and is readily reproducible between observers.
A pediatric version of the GCS recognizes that expected verbal and motor responses must be related to the child’s age (Fig. 37-2). The pediatric coma scale does not assess verbal responses as such but records smiling, crying, and interaction. It uses a 6-point motor scale that is inappropriate for children below the age of 6 months. In children under 5 years of age, speech is understood to be any sound at all, even crying. Young children demonstrate orientation by identifying their parents correctly or giving their own names. When assessing LOC in young children, the nurse may find it helpful to have a parent present to help elicit a desired response. An infant or child may not respond in an unfamiliar environment or to unfamiliar voices.
Numeric values are assigned to the levels of response in each category. The sum of these numeric values provides an objective measurement of the patient’s LOC. The lower the score, the deeper the coma. A person with an unaltered LOC would score the highest, 15; a score of 8 or below is generally accepted as a definition of coma; the lowest score, 3, indicates deep coma or death.
The GCS in itself is not sufficient to determine the responses of all children. For example, because a child with quadriplegia cannot respond to commands physically, the child can score very low but be cerebrally intact. Nevertheless, the GCS provides a more objective method for evaluating the state of consciousness in most cases. Severely injured children (GCS ≤ 8) may have a consistent grading of motor response, verbal response, and eye opening.
The GCS at admission is predictive of abnormal neurologic findings at discharge only when profoundly depressed (≤6); otherwise the GCS is not useful as a prognostic tool when used alone (White, Farukhi, Bull, et al, 2001). GCS scores of less than 8 in combination with other abnormal findings (e.g., hypoxia on admission and abnormal computed tomography [CT] results) were associated with poor outcome (Ong, Selladurai, Dhillon, et al, 1996).
Irreversible Coma: There is no precise diagnosis for clinical death. Different tissues undergo permanent damage after varying periods of exposure to an ongoing insult; therefore the brain (especially the cerebrum) has become the tissue of most importance in determining the time of death. The current concept of dying is a process that takes place over a finite interval of time rather than an event that occurs spontaneously. Brain death is the total cessation of brainstem and cortical brain function that results from any condition that causes irreversible widespread brain injury. In children the most common causes are trauma, anoxic encephalopathy, infections, and cerebral neoplasms. The pronouncement of brain death requires two conditions: (1) complete cessation of clinical evidence of brain function (as evidenced by lack of activity on flow study), and (2) irreversibility of the condition. It is essential to establish the absence of a reversible condition, especially a toxic and metabolic disorder, sedative-hypnotic drugs, paralytic agents, hypothermia, hypotension, and surgically remediable conditions (Report of Special Task Force, 1987).
Organ transplantation has created a need to subdivide the process of death to obtain viable tissues at a time when the brain is already dead. The clinical criteria for brain death must be constituted so that there is no error. Although the legal status of the concept of death varies among individual states and communities in the United States, the Task Force for the Determination of Brain Death in Children has established Guidelines for the Determination of Brain Death in Children (see Nursing Care Guidelines box). (See Organ or Tissue Donation and Autopsy, Chapter 23.)
Substantial variability exists in the criteria clinicians use to diagnose brain death (e.g., number of coma examinations, number and duration of apnea tests, Pco2 measurements at the end of the apnea test, ancillary tests used to confirm brain death, organ procurement, and reasons for nonprocurement) (DeVita, 2001; Mathur, Petersen, Stadtler, et al, 2008).
Neurologic Examination
The purpose of the neurologic examination is to establish an accurate, objective baseline of neurologic function. Therefore it is essential that the neurologic examination be documented in a descriptive and detailed fashion, thereby enhancing the ability to detect subtle changes in neurologic status over time. Descriptions of behaviors should be simple, objective, and easily interpreted—for example, “Drowsy but awake and conversationally rational/oriented”; “Sleepy but arousable with vigorous physical stimuli. Pressure to nail base of right hand results in upper extremity flexion/lower extremity extension.”
Vital signs, observation of posture and movement (both spontaneous and elicited), eye examination, CN testing, and reflex testing all provide valuable clues regarding the LOC, the site of involvement, and the probable cause, but they do not necessarily parallel the depth of a comatose state.
Vital Signs
Pulse, respiration, and blood pressure provide information regarding the adequacy of circulation and the possible underlying cause of altered consciousness. Autonomic activity is most intensively disturbed in deep coma and in brainstem lesions. Body temperature is often elevated; sometimes the elevation is extreme. High temperature is most often a sign of an acute infectious process or heatstroke, but may be caused by ingestion of some drugs (especially salicylates, alcohol, and barbiturates) or by intracranial bleeding, especially subarachnoid hemorrhage. Hypothalamic involvement may cause elevated or decreased temperature. Serious infection may produce hypothermia.
The pulse is variable and may be rapid, slow and bounding, or feeble. Blood pressure may be normal, elevated, or very low. The Cushing reflex, or pressor response that causes a slowing of the pulse and an increase in blood pressure, is uncommon in children; when it does occur, it is a very late sign of increased ICP. Medications can also affect vital signs. For assessment purposes, actual changes in pulse and blood pressure are more important than the direction of the change.
Respirations are more often slow, deep, and irregular. Slow and deep breathing often occurs in the heavy sleep caused by sedatives, after seizures, or in cerebral infections. Slow, shallow breathing may result from sedatives or opioids. Hyperventilation (deep and rapid respirations) is usually the result of metabolic acidosis or abnormal stimulation of the respiratory center in the medulla caused by salicylate poisoning, hepatic coma, or Reye syndrome (RS). A pattern of alternating hyperventilation and breath holding during wakefulness is common in Rett syndrome.
Breathing patterns have been described with a number of terms (e.g., apneustic, cluster, ataxic, Cheyne-Stokes). However, it is better to describe what is being observed rather than placing a label on it because the terms are often used and interpreted incorrectly. Periodic or irregular breathing is a sign of brainstem (especially medullary) dysfunction. This is an ominous sign that often precedes complete apnea. The odor of the breath may provide additional clues (e.g., the fruity and acetone odor of ketosis, the foul odor of uremia, the fetid odor of hepatic failure, or the odor of alcohol).
Skin
The skin may offer clues to the cause of unconsciousness. The body surface should be examined for injury, needle marks, petechiae, bites, and ticks. Evidence of toxic substances may be found on the hands, face, mouth, and clothing—especially in small children.
Eyes
Assess pupil size and reactivity (Fig. 37-3). Pupils either react or do not react to light. Pinpoint pupils are commonly observed in poisoning (e.g., opiate or barbiturate poisoning) or in brainstem dysfunction. Widely dilated and reactive pupils are often seen after seizures and may involve only one side. Widely dilated and fixed pupils suggest paralysis of CN III (oculomotor nerve) secondary to pressure from herniation of the brain through the tentorium. A unilateral fixed pupil usually suggests a lesion on the same side. Bilateral fixed pupils usually imply brainstem damage if present for more than 5 minutes. Dilated and nonreactive pupils also occur in hypothermia, anoxia, ischemia, poisoning with atropine-like substances, or prior instillation of mydriatic drugs. Some of the therapies used (e.g., barbiturates) can alter pupil size and reaction.
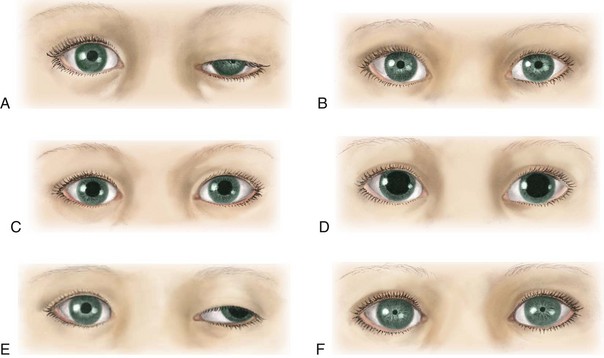
Fig. 37-3 Variations in pupil size with altered states of consciousness. A, Ipsilateral pupillary constriction with slight ptosis. B, Bilateral small pupils. C, Midposition, light fixed to all stimuli. D, Bilateral dilated and fixed pupils. E, Dilated pupils, left eye abducted with ptosis. F, Pinpoint pupils.
The description of eye movements should indicate whether one or both eyes are involved and how the reaction was elicited. Ask the parents if the child has strabismus, which will cause the eyes to appear normal under compromise.
Blinking observed at rest or in response to a sudden loud noise or bright light implies that the pontine reticular formation is intact. The corneal reflex, blinking of the eyelids when the cornea is touched with a wisp of cotton or a camel hair pencil, can test the integrity of the ophthalmic division of CN V (trigeminal nerve). Posttraumatic strabismus indicates CN VI (abducens nerve) damage.
Eye movements are assessed by the doll’s head maneuver, in which the child’s head is rotated quickly to one side and then to the other. When the brainstem centers for eye movement are intact, there is conjugate (paired or working together) movement of the eyes in the direction opposite the head rotation. Absence of this response suggests dysfunction of the brainstem or CN III. Downward or lateral deviation is often observed in association with pupillary dilation in dysfunction of CN III.
The caloric test, or oculovestibular response, is elicited by irrigating the external auditory canal with 10 ml of ice water over a period of approximately 20 seconds (with the head of bed elevated at a 30-degree angle). This test normally causes movement of the eyes toward the side of stimulation. This response is lost when the pontine centers are impaired and thus provides important information in assessment of the comatose patient.
Funduscopic examination reveals additional clues. Because it takes 24 to 48 hours to develop, papilledema (optic disc swelling, indistinct margins, hemorrhages, tortuosity of vessels, absence of venous pulsations), if it develops at all, will not be evident early in the course of unconsciousness. The presence of preretinal hemorrhages in children is usually the result of acute trauma with intracranial bleeding (usually subarachnoid or subdural hemorrhage).
Motor Function
Observation of spontaneous activity, posture, and response to painful stimuli provides clues to the location and extent of cerebral dysfunction. Asymmetric movements of the limbs or the absence of movement suggests paralysis. In hemiplegia the affected limb lies in external rotation and falls uncontrollably when lifted and allowed to drop. Observations should be described rather than labeled.
In the deeper comatose states the child has little or no spontaneous movement, and the musculature tends to be flaccid. There is considerable variability in motor behavior in lesser degrees of coma. For example, the child may be relatively immobile or restless and hyperkinetic; muscle tone may be increased or decreased. Tremors, twitching, and spasms of muscles are common observations. The patient may display purposeless plucking or tossing movements. Combative or negativistic behavior is not uncommon. Hyperactivity is more common in acute febrile and toxic states than in cases of increased ICP. Seizures are common in children and may be present in coma as a result of any cause. Any repetitive or seizure movements are described.
Posturing
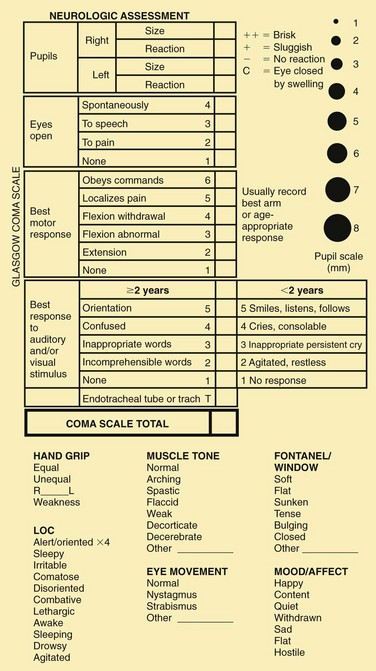
Primitive postural reflexes emerge as cortical control over motor function is lost in brain dysfunction. These reflexes are evident in posturing and motor movements directly related to the area of the brain involved. Posturing reflects a balance between the lower exciting and the higher inhibiting influences, and strong muscles overcome weaker ones. Flexion posturing (Fig. 37-4, A) occurs with severe dysfunction of the cerebral cortex or with lesions to corticospinal tracts above the brainstem. Typical flexion posturing includes rigid flexion, with arms held tightly to the body; flexed elbows, wrists, and fingers; plantar flexed feet; legs extended and internally rotated; and possibly fine tremors or intense stiffness. Extension posturing (Fig. 37-4, B) is a sign of dysfunction at the level of the midbrain or lesions to the brainstem. It is characterized by rigid extension and pronation of the arms and legs, flexed wrists and fingers, clenched jaw, extended neck, and possibly an arched back. Unilateral extension posturing is often caused by tentorial herniation.
Posturing may not be evident when the child is quiet but can usually be elicited by applying painful stimuli, such as a blunt object pressed on the base of the nail. Nurses should avoid applying thumb pressure to the supraorbital region of the frontal bone (risk of orbital damage). Noxious stimuli (e.g., suctioning) will elicit a response, as may turning or touching. When the nurse is describing posturing, the stimulus needed to provoke the response is as important as the reaction.
Reflexes
Testing of certain reflexes, such as those present in an intact spinal cord, may be of limited value. (See Chapter 6.) In general, the corneal, pupillary, muscle-stretch, superficial, and plantar reflexes tend to be absent in deep coma. The state of reflexes is variable in lighter grades of unconsciousness and depends on the underlying pathologic process and the location of the lesion. The doll’s eye reflex maneuver, described previously, reflects paralysis of CN III. The absence of corneal reflexes (CN V) and the presence of a tonic neck reflex are associated with severe brain damage. The Babinski reflex, in which the lateral portion of the foot is stroked, may be of value if it is found to be present consistently in children older than 1 year. A positive Babinski reflex is significant in the assessment of pyramidal tract lesions when it is unilateral and associated with other pyramidal signs. A fluctuating Babinski reflex is often observed after seizures. (See Fig. 8-10, B, p. 250.)
Special Diagnostic Procedures
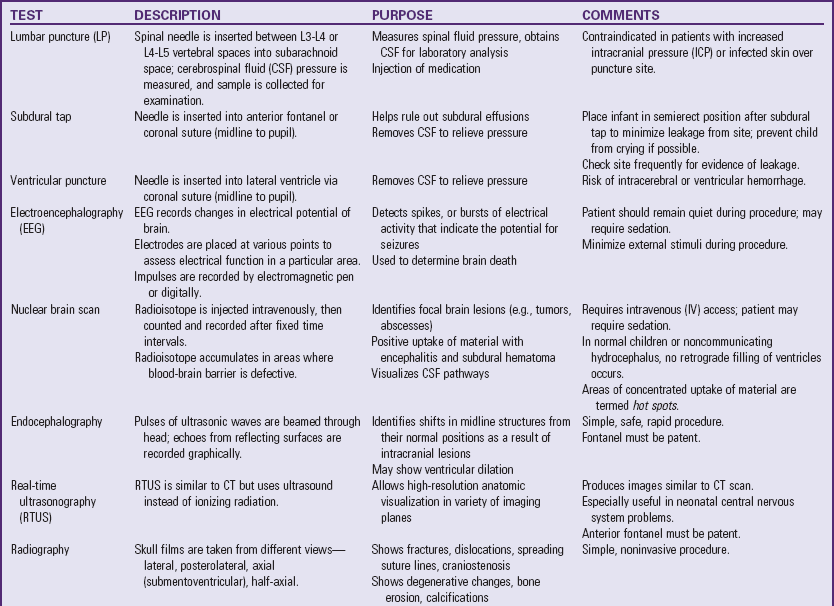
Numerous diagnostic procedures are used for assessment of cerebral function. Laboratory tests that may help determine the cause of unconsciousness include blood glucose, urea nitrogen, and electrolyte (pH, sodium, potassium, chloride, calcium, and bicarbonate) tests; clotting studies, hematocrit, and a complete blood count; liver function tests; blood cultures if there is fever; and sometimes studies to detect lead or other toxic substances, such as drugs.
An electroencephalogram (EEG) may provide important information. For example, generalized random, slow activity suggests suppressed cortical function, and localized slow activity suggests a space-occupying lesion. A flat tracing is one of the criteria used as evidence of brain death. Examination of spinal fluid is carried out when toxic encephalopathy or infection is suspected. Lumbar puncture is ordinarily delayed if intracranial hemorrhage is suspected, and is contraindicated in the presence of ICP because of the potential for brainstem herniation.
Auditory and visual evoked potentials are sometimes used in neurologic evaluation of very young children. Brainstem auditory evoked potentials are useful for evaluating the continuity of brainstem auditory tracts and are particularly useful for detecting demyelinating disease and neoplasms of the brainstem, and for distinguishing between brainstem and cortical lesions. For example, a normal evoked potential in a comatose patient suggests involvement of the cerebral hemispheres.
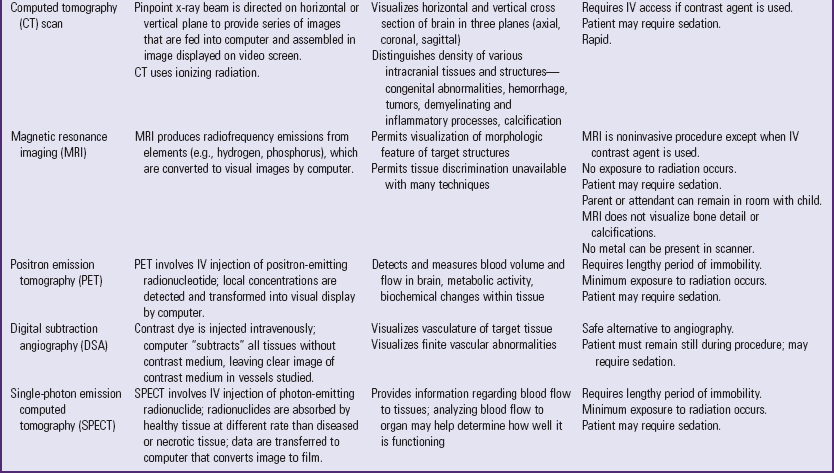
Highly sophisticated tests are carried out with specialized equipment. Two imaging techniques, CT and magnetic resonance imaging (MRI) (Fig. 37-5), assist in diagnosis by scanning both soft tissues and solid matter. Most of these tests are listed in Table 37-1. Because such tests can be threatening to children, the nurse needs to prepare patients for the tests and provide support and reassurance during the tests. (See Preparation for Diagnostic and Therapeutic Procedures, Chapter 27.)
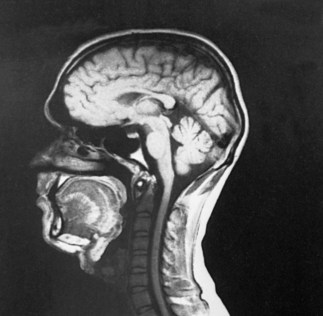
Fig. 37-5 Magnetic resonance imaging. Midsagittal image produces excellent anatomic detail. Note clear delineation of structures such as pituitary gland, brainstem, spinal cord, cerebellum, corpus callosum, and sylvian aqueduct. (Courtesy Philips Medical Systems. From Nolte J: The human brain: an introduction to its functional anatomy, ed 3, St Louis, 1993, Mosby.)
Children who are old enough to understand require careful explanation of the procedure, why it is being done, what they will experience, and how they can help. School-age children usually appreciate a more detailed description of why contrast material is injected. The importance of lying still for tests needs to be stressed. Children unfamiliar with the machines can be shown a picture beforehand. Although radiographic examinations are not painful, the machinery often appears so frightening that the child protests because of anxiety.
This is especially true of CT and MRI, both of which require that the child’s head be placed within a special immobilizing device. Chin and cheek pads are sometimes used to prevent the slightest head movement, and straps are applied to the body to prevent a slight change in body position. The nurse can explain these events to a frightened child by comparing them to an astronaut’s preparation for a space flight. It is important to emphasize to the child that at no time is the procedure painful.
It is helpful for nurses to become acquainted with the equipment and the general environment in which the test will take place so they can better explain the procedure to children at their level of understanding. Written material describing the procedure should be available for parents and may be appropriate to share with children. Equipment is often strange and ominous to children and may be perceived as a frightening monster. They need constant reassurance from a trusted companion. Because children are particularly frightened of needles, they need to be informed of any medication or contrast medium that will be administered intravenously.
The nurse should not expect cooperation from a young child. Sedation may be required. Many different agents are currently used for sedation of children undergoing neurologic diagnostic procedures. Chloral hydrate or benzodiazepines have been used for decades as short-term sedative agents and remain safe methods of pediatric outpatient sedation (Wetzell, 2009). Chloral hydrate is used alone for sedating children for procedures such as MRI. In recent years other sedative agents have been used safely, alone and in combination, for children in the outpatient setting. These include intravenous (IV) sodium pentobarbital (Nembutal), IV fentanyl (Sublimaze), IV midazolam (Versed) (Wetzell, 2009), and intranasal midazolam (Ljungman, Kreuger, Andreasson, et al, 2000; Lloyd, Alredy, and Lloyd, 2000). (See Pain Management, Chapter 7.)
Physical preparation for the diagnostic test may involve administration of a sedative. If so, children should be helped through the preparation and administration and assured that someone will remain with them (if this is possible). Children need continual support and reinforcement during procedures in which they remain conscious. Vital signs and physiologic responses to the procedure are monitored throughout. Many diagnostic procedures performed on an outpatient basis require sedation, and children need recovery time and observation. The nurse should review written instructions with parents if the child is discharged after a procedure. Children who have undergone a procedure with a general anesthetic require postanesthesia care, including positioning to prevent aspiration of secretions and frequent assessment of the vital signs and LOC. In addition, other neurologic functions such as pupillary responses, motor strength, and movement are tested at regular intervals. Any surgical wound resulting from the test is checked for bleeding, CSF leakage, and other complications. Children who undergo repeated subdural taps should have their hematocrit monitored to detect excessive blood loss from the procedure.
Consider children’s emotional reactions to the procedure. They should be allowed and encouraged to express their feelings about the experience through verbal expression and therapeutic play. Parents also seek an explanation of the results of tests and procedures performed on their children. Nurses are in a unique position to provide support and education to parents regarding procedures.
The Child with Cerebral Compromise
Nursing Care of the Unconscious Child
![]() The unconscious child requires nursing attendance with observation, recording, and evaluation of changes in objective signs. These observations provide valuable information regarding the patient’s progress and often serve as a guide to diagnosis and treatment. Therefore careful and detailed observations are essential for the child’s welfare. In addition, vital functions must be maintained and complications prevented through conscientious and meticulous nursing care. The outcome of unconsciousness is variable and ranges from early and complete recovery, to death within a few hours or days, or persistent and permanent unconsciousness, or recovery with varying degrees of residual mental or physical disability. The outcome and recovery of the unconscious child may depend on the level of nursing care and observational skills.
The unconscious child requires nursing attendance with observation, recording, and evaluation of changes in objective signs. These observations provide valuable information regarding the patient’s progress and often serve as a guide to diagnosis and treatment. Therefore careful and detailed observations are essential for the child’s welfare. In addition, vital functions must be maintained and complications prevented through conscientious and meticulous nursing care. The outcome of unconsciousness is variable and ranges from early and complete recovery, to death within a few hours or days, or persistent and permanent unconsciousness, or recovery with varying degrees of residual mental or physical disability. The outcome and recovery of the unconscious child may depend on the level of nursing care and observational skills.
![]() Nursing Care Plan—The Unconscious Child
Nursing Care Plan—The Unconscious Child
Direct emergency measures toward ensuring a patent airway, breathing, and circulation (ABCs); stabilizing the spine when indicated; treating shock; and reducing ICP (if present). Delayed treatment often leads to increased damage. Therapies for specific causes of unconsciousness begin as soon as emergency measures have been implemented; in many cases they occur concurrently. Because nursing care is closely related to the medical management, both are considered here.
Continual observation of the LOC, pupillary reaction, and vital signs is essential to management of CNS disorders. Regular assessment of neurologic status and vital signs is an integral part of the nursing care of unconscious children. The frequency depends on the cause of unconsciousness, the LOC, and the progression of cerebral involvement. Intervals between observations may be as short as every 15 minutes or as long as every 2 hours. Significant alterations are reported immediately.
The temperature is measured every 2 to 4 hours, depending on the child’s condition. An elevated temperature may occur in children with CNS dysfunction; therefore a light covering may be sufficient. Vigorous efforts, such as tepid sponge baths or application of a hypothermia blanket, are needed to prevent brain damage if the rectal temperature exceeds 40° C (104° F).
The LOC is assessed periodically, including pupillary size, equality, and reaction to light. Signs of meningeal irritation, such as nuchal rigidity, need to be assessed. Assessment of LOC also includes response to vocal commands, spontaneous behavior, resistance to care, and response to painful stimuli. Note any abnormal movements, changes in muscle tone or strength, and body position. If a seizure occurs, describe the seizure, including the body areas involved from the beginning to the end of the seizure, and the duration of seizure (see Box 37-11 and Critical Thinking Exercise, p. 1560).
Pain management for the unconscious child requires astute nursing observation and management. Signs of pain include changes in behavior (e.g., increased agitation and rigidity) and alterations in vital signs and perfusion (usually, an increased heart rate, respiratory rate, and blood pressure; and decreased oxygen saturation). Because these findings are not specific for pain, the nurse should be alert for their appearance during times of induced or suspected pain, and for their disappearance after the inciting procedure or the administration of analgesia. A pain assessment record is used to document indications of pain and the effectiveness of interventions. (See Pain Assessment, Chapter 7.) The use of opioids, such as morphine, to relieve pain is controversial because they can mask signs of altered consciousness or depress respirations. However, unrelieved pain activates the stress response, which can elevate ICP. To block the stress response, some authorities advocate the use of analgesics, sedatives, and, in some cases such as head injury, paralyzing agents via continuous IV infusion. A commonly used combination is fentanyl, midazolam, and vecuronium (Norcuron). If there are concerns about assessing the LOC or respiratory depression, naloxone can be used to reverse the opioid effects. Acetaminophen and codeine may also be effective analgesics for mild to moderate pain. Regardless of the drugs used, adequate dosage and regular administration are essential to provide optimum pain relief.
Other measures to relieve discomfort include providing a quiet, dimly lit environment; limiting visitors; preventing any sudden, jarring movement, such as banging into the bed; and preventing an increase in ICP. The latter is most effectively achieved by proper positioning and prevention of straining, such as during coughing, vomiting, or defecating. (See Pain Management, Chapter 7.)
Antiepileptic drugs, such as fosphenytoin (Cerebyx) or phenobarbital, may be ordered for control of seizure activity.
Respiratory Management
Respiratory effectiveness is the primary concern in the care of the unconscious child, and establishment of an adequate airway is always the first priority. Carbon dioxide has a potent vasodilating effect and will increase CBF and ICP. Cerebral hypoxia at normal body temperature that lasts longer than 4 minutes nearly always causes irreversible brain damage.
Children in lighter stages of coma may be able to cough and swallow, but those in deeper states of coma are unable to manage secretions, which tend to pool in the throat and pharynx. Dysfunction of CN IX and X (glossopharyngeal and vagus nerves) places the child at risk of aspiration and cardiac arrest. Therefore position the child with the head and body to the side to prevent aspiration of secretions, and empty the stomach to reduce the likelihood of vomiting. In infants, the blockage of air passages from secretions can happen in seconds. In addition, upper airway obstruction from laryngospasm is a common complication in comatose children.
An oral airway can be used for the child who is suffering a temporary loss of consciousness, such as after a contusion, seizure, or anesthesia. For children who remain unconscious for a longer time, a nasotracheal or orotracheal tube is inserted to maintain the open airway and facilitate removal of secretions. A tracheostomy is performed in cases in which laryngoscopy for introduction of an endotracheal tube would be difficult or dangerous, or for a child who needs long-term ventilatory support. Suctioning is used only as needed to clear the airway, exerting care to prevent increasing ICP. Respiratory status is observed and evaluated regularly. Signs of respiratory distress may indicate a need for ventilatory assistance.
Mechanical ventilation is usually indicated when the respiratory center is involved. (See Chapter 31.) Blood gas analysis is performed regularly, and oxygen is administered when indicated. Moderately severe hypoxia and respiratory acidosis are often present, but are not always evident from clinical manifestations. Hyperventilation often accompanies unconsciousness and may lead to respiratory alkalosis, or it may represent the body’s attempt to compensate for metabolic acidosis. Therefore blood gas and pH determinations are essential guides for electrolyte therapy. Chest physiotherapy is carried out on a regular basis, and the child’s position is changed at least every 2 hours to prevent pulmonary complications.
Intracranial Pressure Monitoring
The selection of the type of ICP monitor should be guided by the clinical presentation and the therapeutic strategy chosen for each child. Indications for inserting an ICP monitor are (1) GCS evaluation of less than 7, (2) GCS evaluation of less than 8 with respiratory assistance, (3) deterioration of condition, and (4) subjective judgment regarding clinical appearance and response.
Four major types of ICP monitors are (1) intraventricular catheter with or without fibroscopic sensors attached to a monitoring system, (2) subarachnoid bolt (Richmond screw), (3) epidural sensor, and (4) anterior fontanel pressure monitor. Transducers for both ventricular and subarachnoid monitoring should be set up without the use of a flush device. Direct ventricular pressure measurement remains the gold standard of ICP monitoring.
The catheter method involves introduction of a catheter into the lateral ventricle on the nondominant side, if known, or placement in the subdural space. The catheter has the advantage of providing a means of extraventricular (or continuous) drainage of CSF to reduce pressure. A drainage bag attached to the system is kept at the level of the ventricles and can be lowered to decrease ICP. This device requires full penetration of the brain, requires skill and experience with placement, and carries the risk of infection.
With the bolt method the end of the bolt is placed into the subarachnoid space. The bolt cannot be adequately secured in a small child’s pliant skull, although special modifications have been developed for children under 6 years of age. The placement of the bolt is not adjusted by anyone except the neurosurgeon who placed the device. The neurosurgeon is notified if a satisfactory wave form is not observed.
An epidural sensor can be placed between the dura and the skull through a burr hole and connected to a stopcock assembly and a transducer, which provides a readout of the pressure. Although less invasive, the epidural sensor may have inconsistent correlation of pressure readings. In infants a fontanel transducer can be used to detect impulses from a pressure sensor and convert them to electrical energy. The electrical energy is then converted to visible waves or numeric readings on an oscilloscope. ICP measurement from the anterior fontanel is noninvasive but may prove to be inaccurate if the equipment is poorly placed or inconsistently recalibrated. Use of the intraparenchymal pressure monitoring device (e.g., Camino) uses fiberoptic technology and performs reliably.
ICP can be increased by direct instillation of solutions; therefore antibiotics are administered systemically if a positive CSF culture is obtained. However, ICP monitoring rarely causes infection. CSF is a body fluid; therefore implement Standard Precautions according to hospital policy. (See Infection Control, Chapter 27.)
Nurses caring for patients with intracranial monitoring devices must be acquainted with the system, assist with insertion, interpret the monitor readings, and be able to distinguish between danger signals and mechanical dysfunction. Because systemic blood pressure, ICP, and therefore CPP are normally lower in children, the child’s age must be taken into account when deciding what constitutes abnormally high ICP or abnormally low CPP.
Several medical measures are available to treat increased ICP resulting from cerebral edema. These include sedation, CSF drainage, and osmotic diuretics. Osmotic diuretics may provide rapid relief of ICP in emergency situations. Although their effect is transient, lasting only about 6 hours, they can be lifesaving in emergencies. These substances are rapidly excreted by the kidneys and carry with them large quantities of sodium and water. Mannitol (or sometimes urea) administered intravenously is the drug most commonly used for rapid reduction of ICP. The infusion is generally given slowly but may be pushed rapidly if there is herniation or impending herniation. Because of the profound diuretic effect of the drug, an indwelling catheter is inserted to ensure bladder emptying. Paco2 should be maintained at 25 to 30 mm Hg to produce vasoconstriction, which reduces CBF, thereby decreasing ICP. Recording and analyzing the child’s volume state, plasma sodium concentration, and serum osmolarity can avert potential fluid and electrolyte problems. Administration of adrenocorticosteroids is not recommended for cerebral edema secondary to head trauma.
Nursing Activities: In cases of high levels of increased ICP, nursing procedures tend to trigger reactive pressure waves in many children. For example, increased intrathoracic or abdominal pressure will be transmitted to the cranium. The goals of monitoring a child who is neurologically compromised include maintaining CPP; controlling ICP, cerebral edema, and factors that increase cerebral metabolism (fever, seizures); and maintaining hemodynamic stability. Take particular care in positioning these patients to avoid neck vein compression that may further increase ICP by interfering with venous return.
Sandbags or other support devices can help maintain correct head position. The child can be propped to one side or the other, and the use of a pressure-relieving or pressure-decreasing mattress decreases the chance of prolonged pressure to vulnerable skin areas. Frequent clinical assessment of the child cannot be replaced by an ICP monitoring device.
It is important to avoid activities that may increase ICP by causing pain or emotional stress. Individualizing nursing activities and minimizing environmental stimuli by decreasing noxious procedures help to control ICP (El Bashir, Laundy, and Booy, 2003). Range-of-motion exercises can be carried out gently, but should not be performed vigorously. Nontherapeutic touch can cause an increase in ICP. Any disturbing procedures to be performed should be scheduled to take advantage of therapies that reduce ICP, such as osmotherapy and sedation. Make efforts to minimize or eliminate environmental noise. Assessment and intervention to relieve pain are important nursing functions to decrease ICP.
Suctioning and percussion are poorly tolerated; therefore these procedures are contraindicated unless the child has concurrent respiratory problems. Hypoxia and the Valsalva maneuver associated with cough acutely elevate ICP. Vibration, which does not increase ICP, accomplishes excellent results and should be tried first if treatment is needed. If suctioning is necessary, it should be used judiciously and preceded by hyperventilation with 100% oxygen, which can be monitored during suctioning with a pulse oxygen sensor reading to determine oxygen saturation.
Nutrition and Hydration
In the unconscious child, fluids and calories are supplied initially by the IV route. (See Chapter 28.) An IV infusion is started early, and the type of fluid administered depends on the patient’s general condition. Fluid therapy requires careful monitoring and adjustment based on neurologic signs and electrolyte determinations. Often, unconscious children cannot tolerate the same amounts of fluid as when they are healthy. Overhydration must be avoided to prevent fatal cerebral edema. When cerebral edema is a threat, fluids may be restricted to reduce the chance of fluid overload. Examine skin and mucous membranes for signs of dehydration. Adjustments to fluid administration are based on urinary output, serum electrolytes and osmolarity, blood pressure, and arterial filling pressure. Observation for signs of altered fluid balance related to abnormal pituitary secretions is a part of nursing care.
Provide long-term nutrition in a balanced formula given by nasogastric or gastrostomy tube. The nasogastric tube is usually taped in place, with care taken to prevent pressure on the nares. Most children have continuous feedings, but if bolus feedings are used, the tube is rinsed with water after each feeding. Tubes are replaced according to institutional policy. Irritation of the nasal mucosa is prevented by alternating nares each time the nasogastric tube is replaced.
Avoid overfeeding to prevent vomiting and the associated risk of aspiration. Stomach contents are aspirated with a syringe and measured before feeding to ascertain the amount remaining in the stomach. The removed contents are refed. If the residual volume is excessive (depending on the child’s size), consult the dietitian and physician regarding the composition and amount of formula and whether changes are required to provide the needed calories and nutrients in a smaller volume.
Altered Pituitary Secretion: An altered ability to handle fluid loads is attributed in part to the syndrome of inappropriate antidiuretic hormone (SIADH) and diabetes insipidus (DI) resulting from hypothalamic dysfunction. (See Chapter 38.) SIADH often accompanies CNS diseases such as head injury, meningitis, encephalitis, brain abscess, brain tumor, and subarachnoid hemorrhage. In the child with SIADH, scant quantities of urine are excreted, electrolyte analysis reveals hyponatremia and hyposmolality, and manifestations of overhydration are evident. It is important to evaluate all parameters because the reduced urinary output might be erroneously interpreted as a sign of dehydration. The treatment of SIADH consists of fluid restriction until serum electrolytes and osmolality return to normal levels. SIADH often occurs in children who have meningitis.
DI may occur after intracranial trauma. In DI there is increased urinary volume and the accompanying danger of dehydration. See Table 37-2 for comparison of fluid changes in SIADH and DI. Adequate replacement of fluids is essential, and observation of electrolyte balance is necessary to detect signs of hypernatremia and hyperosmolality. Exogenous vasopressin may be administered.
Medications
The cause of unconsciousness determines specific drug therapies. Children with infectious processes are given antibiotics appropriate to the disease and the infecting organism. Corticosteroids are prescribed for inflammatory conditions and edema. Cerebral edema is an indication for osmotic diuretics. Antiepileptic medications are prescribed for seizure activity. Sedation in the combative child provides amnesic and anxiolytic properties in conjunction with a paralytic agent. This combination decreases ICP and allows treatment of cerebral edema. Usual drugs include morphine, midazolam, and pancuronium (Pavulon). Midazolam is attractive because of its short half-life.
Deep coma induced by the administration of barbiturates is controversial in the management of ICP. Barbiturates are currently reserved for the reduction of increased ICP when all else has failed. Barbiturates decrease the cerebral metabolic rate for oxygen and protect the brain during times of reduced CPP. Barbiturate coma requires extensive monitoring, including EEG monitoring to assess for seizure activity, cardiovascular and respiratory support, and ICP monitoring to assess response to therapy. Paralyzing agents such as pancuronium also may be needed to aid in performing diagnostic tests, improving effectiveness of therapy, and reducing the risks of secondary complications. Elevation of ICP or heart rate in patients who are being given paralyzing agents or are under sedation may indicate the need for another dose of either or both medications.
Thermoregulation
Hyperthermia often accompanies cerebral dysfunction; if it is present, the nurse implements measures to reduce the temperature to prevent brain damage from hyperthermia and to reduce metabolic demands generated by the increased body temperature. Antipyretics are the method of choice for fever reduction; cooling devices are used for hyperthermia. (See Controlling Elevated Temperatures, Chapter 27.) Laboratory tests and other methods help determine the cause, if any, of the hyperthermia. Treatment with hypothermia and barbiturates increases the risk of iatrogenic complications.
Elimination
A urinary catheter is usually inserted in the acute phase, but diapers may be used and weighed to record urinary output. The child who previously had bowel and bladder control is generally incontinent. If the child remains comatose for a long period, the indwelling catheter may be removed and periodic bladder emptying accomplished by intermittent catheterization. Stool softeners are usually sufficient to maintain bowel function, but suppositories or enemas may be needed occasionally for adequate elimination and to prevent fecal impaction. The passage of liquid stool after a period of no bowel activity is usually a sign of impaction. To avoid this preventable problem, daily recording of bowel activity is essential.
Hygienic Care
Routine measures for cleansing and maintaining skin integrity are an integral part of nursing care of the unconscious child. Skinfolds require special attention to prevent excoriation. The child who is unable to move is prone to develop tissue breakdown and necrosis; therefore the child is placed on a resilient appliance (e.g., alternating-pressure or water-filled mattress) to prevent pressure on prominent areas of the body. The goal is prevention by regular change of position and inspection of vulnerable areas (e.g., the ankle, heels, trochanter, sacrum, and shoulder). Unconscious children undergo numerous invasive procedures, and the skin sites used for these procedures require special assessment and intervention to promote healing and prevent infection. Keep bed linen and any clothing dry and free of wrinkles. Rubbing the back and extremities with lotion stimulates circulation and helps prevent drying of the skin. However, to prevent further tissue damage, do not massage reddened and nonblanching skin. (See Maintaining Healthy Skin, Chapter 27.) If the child requires surgery or radiography, the nurse checks all dressings, bony sites, catheters, and IV access lines before and after the procedure.
Mouth care is performed at least twice daily, since the mouth tends to become dry or coated with mucus. The teeth are carefully brushed with a soft toothbrush or cleaned with gauze saturated with saline. Commercially prepared cleansing devices, such as Toothettes, are convenient for cleansing the mouth and teeth. Lips are coated with ointment to protect them from drying, cracking, or blistering.
The unconscious child is also prone to eye irritation. The corneal reflexes are absent; therefore the eyes are easily irritated or damaged by linen, dust, or other substances that may come in contact with them. Excessive dryness results from incomplete closure of the lids and/or decreased secretions, especially if the child is undergoing osmotherapy to reduce or prevent brain edema.
Keep the child’s hair combed and secure to prevent tangling. Keep the scalp clean with dry or wet shampoos as needed. The child’s head may need to be shaved for tests or surgical procedures. If so, the hair should be saved, if possible, and given to the family.
Positioning and Exercise
The unconscious child is positioned to minimize ICP and to prevent aspiration of saliva, nasogastric secretions, and vomitus. The head of the bed is elevated, and the child is placed in a side-lying or semiprone position. A small, firm pillow is placed under the head, and the uppermost limbs are flexed and supported with pillows. The weight of the body should not rest on the dependent arm. In the semiprone position the child lies with the dependent arm at the side behind the body; the opposite side is supported on pillows, and the uppermost arm and leg are flexed and resting on the pillows. This position prevents undue pressure on the dependent extremities. The dependent position of the face encourages drainage of secretions and prevents the flaccid tongue from obstructing the airway.
Normal range-of-motion exercises help maintain function and prevent contractures of joints. Perform exercises gently to minimize increasing ICP, and with full range of motion. Place a small rolled pad in the palms to help maintain proper positioning of fingers. Footboards or high-top shoes (e.g., running or tennis shoes) can help prevent footdrop, and in some cases splinting is needed to prevent severe contractures of the wrist, knee, or ankle in children.
Stimulation
Sensory stimulation is as important in the care of the unconscious child as it is in the care of the alert child. For the temporarily unconscious or semiconscious child, sensory stimulation helps arouse the child to the conscious state and orient the child in terms of time and place. Auditory and tactile stimulation are especially valuable. Tactile stimulation is not appropriate for a child in whom it may elicit an undesirable response. However, for other children tactile contact often has a relaxing and calming effect. When the child’s condition permits, holding or rocking the child is soothing and provides the body contact needed by young children.
The auditory sense is often intact in a state of coma. Hearing is the last sense to be lost and the first one to be regained; therefore speak to the child as any other child. Conversation around the child should not include thoughtless or derogatory remarks. Soft music is often used to provide auditory stimulation. Singing the child’s favorite songs or reading a favorite story is a strategy used to maintain the child’s contact with a familiar world. Playing songs or favorite stories recorded in the parents’ voices can provide a continuous source of familiar stimulation.
Family Support
Helping the parents of an unconscious child cope with the situation is especially difficult. They may demonstrate all the guilt, fear, hostility, and anxiety of any parent of a seriously ill child. (See Chapter 23.) In addition, these parents face the uncertain outcome of the cerebral dysfunction. The fear of death, cognitive impairment, or permanent physical disability is present. Nursing intervention with parents depends on the nature of the pathologic condition, the parents’ personality, and the parent-child relationship before injury or illness (see Family-Centered Care box).
Awakening from a coma is a gradual process; however, some children regain consciousness within a short time. If there is little or no residual effect, the child is discharged home fairly soon. The parents need the most intensive nursing intervention during the period of crisis and uncertainty. During the recovery phase the nurse gives them information, clarifies it as needed, and encourages them to become involved in the child’s care. Often the child’s hospitalization is brief; however, some children require extended hospitalization for intensive therapy and rehabilitation. The parents of children who die require support and guidance to cope with the reality of the death and to resolve their grief. (See Chapter 23.)
Probably the most difficult situations are those that involve children who never regain consciousness. Unlike losing a child through death, these children lack finality, which often leaves them in a state of suspended grief. Like parents of dying children, parents of comatose children search for any signs of hope. Well-meaning friends and relatives relate instances of miraculous recoveries. The parents seek confirmation and support for such possibilities and assign erroneous meanings to any sign in the child that might be interpreted as evidence of recovery (e.g., reflexive muscle contractions).
At these times nurses need to respond with compassion and honesty. They can acknowledge that miraculous recoveries do occur but are rare. The important message is to maintain open communication with the family.
Like parents who lose a child through death, the parents of a child who is unconscious attempt to construct a representation of the child. They bring items that belong to the child, such as favorite toys or music. This may be interpreted as an attempt to provide stimulation for the child in the hope of eliciting a response, to let the hospital staff know the child as the unique individual he or she was, and to reconstitute an image of the child “lost” to them and for whom they mourn. The nurses’ recognition and understanding of these behaviors and coping mechanisms is important to support the parents in their grief process.
In addition to the process of grieving for the “lost” child, the parents may face difficult decisions. When the child’s brain is so severely damaged that vital functions must be maintained by artificial means, the parents must make the final decision whether to remove the life-support systems. Since this decision is so difficult for parents, the practitioner is frequently placed in a position of making the decision indirectly. After providing the parents with information about what removal from life-support means, the practitioner may suggest that the child be removed from life support to “see if the child can make it without help.” This approach relieves the parents of the decision and can be effective, but it is based on an evaluation of the parents’ intellectual level and emotional state. Sometimes parents may choose to refuse treatment if they believe doing so is best for the child and the family (informed dissent). At other times parents request that “everything possible” be done for the child.
When the child has survived the cerebral insult but is physically and/or mentally limited, either minimally or severely, families must cope with and make decisions about the rehabilitation process and uncertain outcome. The family may need to make decisions whether to place their child in a chronic care facility or to care for their child at home. The drain on financial, emotional, and social resources can be enormous.
For parents who choose to care for their child at home, planning begins early in the recovery process. Family members should become involved with the child’s care as soon as they indicate an interest and ability to do so. They need education and support in learning to care for the child, regular follow-up observation and assessment of the home management, and planning for respite care. Parents need to understand that it is important to plan for periodic relief from the continuous care of the child. (See Discharge Planning and Home Care, Chapter 26, and Family-Centered Home Care, Chapter 25.)
Head Injury
![]() Head injury is a pathologic process involving the scalp, skull, meninges, or brain as a result of mechanical force. According to national statistics and Safe Kids Worldwide,* injuries are the number one health risk for children and the leading cause of death in children older than 1 year of age. Each year, one child in four in the United States suffers an injury serious enough to require medical attention.
Head injury is a pathologic process involving the scalp, skull, meninges, or brain as a result of mechanical force. According to national statistics and Safe Kids Worldwide,* injuries are the number one health risk for children and the leading cause of death in children older than 1 year of age. Each year, one child in four in the United States suffers an injury serious enough to require medical attention.
![]() Critical Thinking Case Study—Head Injury
Critical Thinking Case Study—Head Injury
Etiology
The three major causes of brain damage in childhood, in order of importance, are falls, motor vehicle injuries, and bicycle injuries (Fig. 37-6). Neurologic injury accounts for the highest mortality rate, with boys usually affected twice as often as girls. Falls are the major source of all head injuries in children between the ages 0 to 4 years (Langlois, Rutland-Brown, and Thomas, 2005; Marsh and Whitehead, 2005). In motor vehicle accidents children younger than 2 years of age are almost exclusively injured as passengers, whereas older children may also be injured as pedestrians or cyclists. The majority of deaths from brain trauma caused by bicycle injuries occur between the ages of 5 and 19 years. Bicycle helmet laws have effectively reduced the risk of head injury by 85% and brain injury by 88% (Rivara and Grossman, 2009).

Fig. 37-6 Children possess a sense of adventure and wonder; however, falls remain the leading cause of head injury in children under 5 years of age.
Many of the physical characteristics of children predispose them to craniocerebral trauma. For example, infants are often left unattended on beds, in high chairs, and in other places from which they can fall. Because the head of an infant or toddler is proportionately large and heavy in relation to other body parts, it is the most likely to be injured. Incomplete motor development contributes to falls at young ages, and the natural curiosity and exuberance of children increase their risk for injury.
Pathophysiology
The pathology of brain injury is directly related to the force of impact. Intracranial contents (brain, blood, CSF) are damaged because the force is too great to be absorbed by the skull and musculoligamentous support of the head. Although nervous tissue is delicate, it usually requires a severe blow to cause significant damage.
A child’s response to head injury is different from that of adults. The larger head size in proportion to body size and insufficient musculoskeletal support render the very young child particularly vulnerable to acceleration-deceleration injuries.
Primary head injuries are those that occur at the time of trauma and include skull fractures, contusions, intracranial hematomas, and diffuse injuries. Subsequent complications include hypoxic brain injury, increased ICP, and cerebral edema. The predominant feature of a child’s brain injury is the diffuse amount of swelling that occurs. Hypoxia and hypercapnia threaten the energy requirements of the brain and increase CBF. The added volume across the BBB along with the loss of autoregulation exacerbates cerebral edema. Pressure inside the skull that is greater than arterial pressure results in inadequate perfusion. Because the cranium of very young children has the ability to expand and the thin skull is more compliant, they may tolerate increases in ICP better than older children and adults.
Physical forces act on the head through acceleration, deceleration, or deformation. Acceleration or deceleration is more descriptive of the circumstances responsible for most head injuries. When the stationary head receives a blow, the sudden acceleration causes deformation of the skull and mass movement of the brain. Continued movement of the intracranial contents allows the brain to strike parts of the skull (e.g., the sharp edges of the sphenoid or the irregular surface of the anterior fossa) or the edges of the tentorium.
Although the brain volume remains unchanged, significant distortion and cavitation occur as the brain changes shape in response to the force transmitted from the impact to the skull. This deformation can cause bruising at the point of impact (coup) or at a distance as the brain collides with the unyielding surfaces opposite or far removed from the point of impact (contrecoup) (Fig. 37-7). Thus a blow to the occipital region can cause severe injury to the frontal and temporal areas of the brain.
Fig. 37-7 Mechanical distortion of cranium during closed head injury. A, Preinjury contour of skull. B, Immediate postinjury contour of skull. C, Torn subdural vessels. D, Shearing forces. E, Trauma from contact with floor of cranium. (Redrawn from Grubb RL, Coxe WS: Central nervous system trauma: cranial. In Eliasson SG, Presky AL, Hardin WB, editors: Neurological pathophysiology, New York, 1974, Oxford University Press.)
When a moving head strikes a stationary surface, such as during a fall, sudden deceleration occurs and causes the greatest cerebral injury at the point of impact. Deceleration is responsible for most severe brainstem injuries.
Children with an acceleration-deceleration injury demonstrate diffuse generalized cerebral swelling produced by increased blood volume or by a redistribution of cerebral blood volume (cerebral hyperemia) rather than by the increased water content (edema).
Another effect of brain movement is shearing forces, which are caused by unequal movement or different rates of acceleration at various levels of the brain. A shearing force may tear small arteries that travel from the cerebral surfaces through the meninges to the dural sinuses and cause subdural hemorrhages. Shearing or stretching effects can also be transmitted to nerve fibers. Maximum stress from the shearing force occurs at the interface between structures of different density so that the gray matter (cell body) rapidly accelerates while the white matter (axons) tends to lag behind. Although shearing forces are maximum at the cerebral surface and extend toward the center of rotation within the brain, the most serious effects are often in the area of the brainstem.
Another source of damage occurs when severe compression of the skull causes the brain to be forced through the tentorial opening. This can produce irreparable damage to the brainstem (Fig. 37-8).
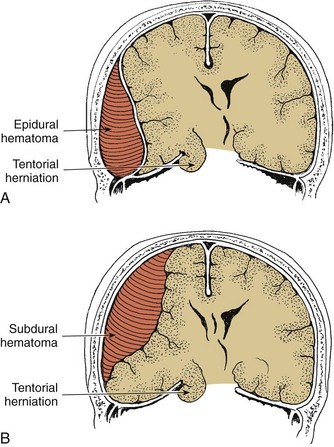
Fig. 37-8 A, Epidural (extradural) hematoma and compression of temporal lobe through tentorial herniation. B, Subdural hematoma.
Patients with mild head injuries have a GCS evaluation of 13 to 15, and those with moderate head injuries have a GCS of 9 to 12; a GCS value of 8 or less indicates severe injury (Marcin and Pollack, 2002).
Concussion: The most common head injury is concussion, an alteration in mental status with or without loss of consciousness, which occurs immediately after a head injury (Lee, 2007). The hallmarks of a concussion are confusion and amnesia. These are often not preceded by loss of consciousness and may occur immediately after the injury or several minutes later. The belief that loss of consciousness is the hallmark of concussion is a common misconception. Blinman, Houseknecht, Snyder, and colleagues (2009) found that fatigue and headache were the most common symptoms after mild traumatic brain injury in a group of 116 children.
The pathogenesis of concussion is still unclear, but it may be a result of shearing forces that cause stretching, compression, and tearing of nerve fibers, particularly in the area of the central brainstem, the seat of the reticular activating system. It has also been suggested that the anatomic alterations of nerve fibers cause the release of large quantities of acetylcholine into the CSF and a reduction in oxygen consumption with increased lactate production.
Contusion and Laceration: The terms contusion and laceration are used to describe actual bruising and tearing of cerebral tissue. Contusions represent petechial hemorrhages or localized bruising along the superficial aspects of the brain at the site of impact (coup injury) or a lesion remote from the site of direct trauma (contrecoup injury). In serious accidents there may be multiple sites of injury.
The major areas of the brain susceptible to contusion or laceration are the occipital, frontal, and temporal lobes. Also, the irregular surfaces of the anterior and middle fossae at the base of the skull are capable of producing bruises or lacerations on forceful impact. Contusions may cause focal disturbances in strength, sensation, or visual awareness. The degree of brain damage in the contused areas varies according to the extent of vascular injury. Signs vary from mild, transient weakness of a limb to prolonged unconsciousness and paralysis. However, the signs and symptoms may be clinically indistinguishable from those of concussion.
Infants who are roughly shaken (shaken baby syndrome) can sustain profound neurologic impairment, seizures, retinal hemorrhages (usually bilateral), and intracranial subarachnoid or subdural hemorrhages (McCabe and Donahue, 2000; Bechtel, Stoessel, Leventahal, et al, 2004). In addition to these classic injuries, other skeletal fractures and injuries may occur (Castiglia, 2001; DiCarlo and Frankel, 2009).
Cerebral lacerations are generally associated with penetrating or depressed skull fractures. However, they may occur without fracture in small children. When brain tissue is actually torn, with bleeding into and around the tear, more severe and prolonged unconsciousness and paralysis usually occur, leaving permanent scarring and some degree of disability.
Fractures: Skull fractures result from a direct blow or injury to the skull and are often associated with intracranial injury. Skull fractures after minor head injury are not uncommon, particularly in children younger than 2 years of age. Infants are at increased risk of skull fractures from minor trauma. Falls are the most common cause of head injury. Many of the falls that resulted in a skull fracture in children younger than 2 years of age involved short distances of less than 3 feet, such as falls from infant carrier car seats (Greenberg, Bolte, and Schunk, 2009).
The types of skull fractures that occur are linear, comminuted, depressed, open, basilar, and growing fractures. As a rule, the faster the blow, the greater the likelihood of a depressed fracture; a low-velocity impact tends to produce a linear fracture.
Linear skull fractures are a single fracture line that starts at the point of maximum impact and spread; however, they do not cross suture lines. Linear skull fractures constitute the majority of childhood skull fractures and typically occur in the parietal bone. Most linear skull fractures are associated with an overlying scalp hematoma, particularly in infants younger than 1 year of age and in the parietal or temporal region (Greenes and Schutzman, 2001). Comminuted fractures consist of multiple associated linear fractures. They usually result from intense impact, often from repeated blows against an object. They may suggest child abuse, particularly if they occur in the occipital bone.
Depressed fractures are those in which the bone is locally broken, usually into several irregular fragments that are pushed inward. The greater the depression, the higher the risk of a tear in the dura or cortical laceration. Depressed skull fractures may be associated with direct underlying parenchymal damage and should be suspected when a child’s head appears misshapen. Surgery may be needed to elevate the depressed bone fragment if there is an associated intracranial hematoma and if the depression is greater than 1 cm (0.4 inch).
Open fractures result in a communication between the skull and the scalp or the mucosa of the upper respiratory tract. The risk of CNS infection is increased with open fractures. Compound fractures consist of a skin laceration overlying the bone fracture. Open fractures that involve the paranasal sinuses or middle ear may lead to leakage of CSF (rhinorrhea or otorrhea). Prophylactic antibiotics are recommended to prevent osteomyelitis.
Basilar fractures involve the bones at the base of the skull in either the posterior or the anterior region. The bones involved are the ethmoid, sphenoid, temporal, or occipital bones and usually result in a dural tear. Because of the proximity of the fracture line to structures surrounding the brainstem, a basal skull fracture is a serious head injury. Approximately 80% of the cases have distinct clinical features. These include leakage of CSF from the nose (CSF rhinorrhea) or from the ear (CSF otorrhea), blood behind the tympanic membrane (hemotympanum), subcutaneous bleeding over the mastoid process that is located posterior to the ear (Battle sign), and subcutaneous bleeding around the orbit (raccoon eyes). CN palsies may occur and primarily involve CN I (olfactory nerve), VIII (vestibulocochlear nerve), VII (facial nerve), and VI (abducens nerve). The diagnosis of basilar fractures is difficult to make from radiographs because of the complex structure of the base of the skull. Therefore a nonenhanced CT is the recommended diagnostic method. Meningitis, although rare, is always a potential risk with CSF leakage. The use of prophylactic antibiotics is controversial, and the trend has been to treat only documented cases of meningitis.
Growing fractures result from a skull fracture with an underlying tear in the dura that fails to heal properly. The enlargement may be caused by a leptomeningeal cyst, dilated ventricles, or herniated brain. The parietal bone is the most common location. The majority of growing skull fractures occur before 3 years of age (Vignes, Jeelani, Jeelani, et al, 2007). Physical examination can reveal the development of a pulsatile mass or enlarging and sunken skull defect. Clinical neurologic symptoms may be delayed for months to years after the initial skull fracture and include headache, seizures, or asymmetric cranial growth.
Complications
The major complications of trauma to the head are hemorrhage, infection, edema, and herniation through the brainstem. Infection is always a hazard in open injuries, and edema is related to tissue trauma. Vascular rupture may occur even in minor head injuries, causing hemorrhage between the skull and cerebral surfaces. Compression of the underlying brain produces effects that can be rapidly fatal or insidiously progressive.
Epidural Hematoma: Epidural (extradural) hematoma is a hemorrhage into the space between the dura and the skull. As the hematoma enlarges, the dura is stripped from the skull; this accumulation of blood results in a mass effect on the brain, forcing the underlying brain contents downward and inward as it expands (see Fig. 37-8, A). Epidural hematomas occur infrequently in infants and children, but may occur after a low-velocity fall (Rocchi, Caroli, Raco, et al, 2005). Child abuse accounts for a significant number of cases of epidural hematomas in infants and children, whereas motor vehicle accidents account for most epidural hematomas in adolescents.
An epidural hemorrhage is usually arterial in origin, most often as a result of a skull fracture that penetrates the groove in the skull occupied by the middle meningeal artery. The low incidence of epidural hematoma in childhood has been attributed to the fact that the middle meningeal artery is not embedded in the bone surface of the skull until approximately 2 years of age. Therefore a fracture of the temporal bone is less likely to lacerate the artery. However, a child’s skull can be indented with sufficient force to tear the middle meningeal artery without causing a fracture. Hemorrhage can also originate from dural veins or the dural sinuses, especially in infants and small children, due to the abundance of dural vasculature in areas of rapid bone growth. In 20% to 40% of children a skull fracture is not detectable.
Because bleeding is generally arterial, brain compression occurs rapidly. Most often the expanding hematoma is located in the parietotemporal region, which forces the medial portion of the temporal lobe under the edge of the tentorium, where it places pressure on nerves and blood vessels. Pressure on the arterial supply and venous return to the reticular formation causes loss of consciousness; pressure on CN III produces dilation and (later) fixation of the ipsilateral pupil. Pressure on the fibers of the pyramidal tract is evidenced by contralateral weakness or paralysis and increased deep tendon reflexes. Extreme pressure may cause brain herniation and death. Expanding epidural hemorrhages may be better tolerated in young children with open sutures that allows for expansion of the skull. In addition, young children have larger subarachnoid and extracellular spaces, which provide space for the expanding hematoma without compression on the brain parenchyma.
The classic clinical picture of an epidural hemorrhage is a lucid interval (momentary unconsciousness) followed by a normal period for several hours, then lethargy or coma due to blood accumulation in the epidural space and compression of the brain (Case, 2008). The child may be seen with varying degrees of impaired consciousness depending on the severity of the traumatic injury. Common symptoms in a child with no neurologic deficit are irritability, headache, and vomiting. In infants less than 1 year of age the most common symptoms are irritability, pallor with anemia, and cephalhematoma. Infants may also have hypotonia, seizures, vomiting, a bulging anterior fontanel, and lethargy.
An epidural hematoma can be detected by an initial CT scan. If the severity of the child’s symptoms is not recognized, herniation and death will result. Cushing triad (systemic hypertension, bradycardia, and respiratory depression) is a late sign of impending brainstem herniation. See Table 37-3 for a comparison of epidural and subdural hematomas.
Subdural Hemorrhage: ![]() A subdural hemorrhage is bleeding between the dura and the arachnoid membrane, which overlies the brain and the subarachnoid space. The hemorrhage may be from two sources: (1) tearing of the veins that bridge the subdural space, and (2) hemorrhage from the cortex of the brain caused by direct brain trauma (see Fig. 37-8, B). Subdural hematomas are much more common than epidural hematomas and occur most often in infancy, with a peak incidence at 6 months (Myhre, Grogaard, Dyb, et al, 2007).
A subdural hemorrhage is bleeding between the dura and the arachnoid membrane, which overlies the brain and the subarachnoid space. The hemorrhage may be from two sources: (1) tearing of the veins that bridge the subdural space, and (2) hemorrhage from the cortex of the brain caused by direct brain trauma (see Fig. 37-8, B). Subdural hematomas are much more common than epidural hematomas and occur most often in infancy, with a peak incidence at 6 months (Myhre, Grogaard, Dyb, et al, 2007).
Unlike epidural hemorrhage, which develops inwardly against the less resistant brain tissue, subdural hemorrhage tends to develop more slowly and spreads thinly and widely, crossing cranial sutures, until it is limited by the dural barriers—the falx and the tentorium. Subdural hematoma is fairly common in infants, often as a result of birth trauma, falls, assaults, or violent shaking. The small subdural space and the dura, which is firmly attached to the skull in this area, are highly vulnerable to increased ICP.
Subdural hemorrhage can cause either acute or chronic subdural hematoma. Acute subdural hematoma may be associated with contusions or lacerations and develops within minutes or hours of injury. Chronic subdural hematoma is more common. The clinical course and manifestations vary, depending on the damage sustained by the brain and the child’s age.
Presenting signs of acute hematoma include irritability, vomiting, increased head circumference, bulging anterior fontanel (in the infant), lethargy, coma, or seizures. In infants with open fontanels, large amounts of intracranial blood may accumulate causing hemorrhagic shock or fever before there are any changes in the neurologic examination (Case, 2008). Retinal hemorrhages and skull and skeletal fractures are suggestive of physical abuse. An infant who has an altered LOC and in whom the CT scan shows subarachnoid hemorrhage or subdural hematoma may have been physically abused. A child with a GCS of 12 or less requires emergency consultation with the neurosurgeon.
Closely observe older children for signs of neurologic deterioration, including altered mental status, vomiting, papillary changes, and signs of increased ICP. Hemiparesis, hemiplegia, and anisocoria (unequal pupils) are signs of brainstem compression and require emergency treatment targeted at decreasing ICP. The surgical management of subdural hematomas depends on the physical examination, size of the hematoma, and presence of other abnormalities on the CT scan. Not all children require surgery or are candidates for surgery. Subdural taps are sometimes the primary treatment in infants or in children with chronic subdural hematomas (Proctor, 2003).
Other Hemorrhagic Lesions: A subarachnoid hemorrhage is bleeding within the subarachnoid space, which is normally filled with CSF. Nontraumatic intracranial hemorrhages are rare in children. The most common risk factors for intracranial hemorrhages are arteriovenous malformations, congenital heart disease, and brain tumors (Lo, Lee, Rusin, et al, 2008). Sudden onset of a severe headache is the hallmark symptom.
Cerebral Edema: Some degree of brain edema is expected after craniocerebral trauma and often accompanies any of the previously mentioned disorders. Cerebral edema peaks at 24 to 72 hours after injury and may account for changes in a child’s neurologic status. Cerebral edema associated with traumatic brain injury may be a result of two different mechanisms: cytotoxic edema or vasogenic edema. Cytotoxic edema is a result of direct cell injury and is caused by intracellular swelling. In many cases the brain cells are irreversibly damaged. Vasogenic edema is due to increased permeability of capillary endothelial cells, resulting in increased intracellular fluid. In vasogenic edema the nerve cells are not primarily injured. Either mechanism can result in increased ICP as a result of increased intracranial volume and changes in CBF as a result of loss of autoregulation and/or hypercapnia or hypoxia. Children at risk for deterioration can be identified by abnormalities seen on admitting noncontrast CT scans (Marcoux, 2005).
Posttraumatic Syndromes: Postconcussion syndrome is a sequela to brain injury with or without loss of consciousness. Controversy exists regarding the definition and pathophysiology of postconcussion syndrome. It typically occurs after a mild head injury but may also occur after moderate to severe head injury. It is a symptom complex that includes headaches, dizziness, fatigue, irritability, anxiety, insomnia, loss of concentration, and memory impairment (Baandrup and Jensen, 2005; Paniak, Reynolds, Phillips, et al, 2002; Yeates, Taylor, Rusin, et al, 2009). Symptoms typically develop within days of the injury and resolve within 3 months. Clinical symptoms of loss of consciousness, posttraumatic amnesia, GCS score of less than 15, disorientation, and other mental status changes are strongly associated with postconcussion syndrome (Yeates, Taylor, Rusin, et al, 2009).
Posttraumatic headaches may occur within 1 week to 3 months after a mild traumatic brain injury. They occur in 25% to 75% of individuals and are typically classified as either tension or migraine headaches (Baandrup and Jensen, 2005).
Posttraumatic seizures occur in a number of children who survive a head injury and are more common in younger children than those over 16 years of age (Baandrup and Jensen, 2005) (see Critical Thinking Exercise). Seizures are more likely to occur in children with severe head injury and usually occur within the first day (Chiaretti, DeBenedictis, Polidori, et al, 2000).
Structural complications may occur as a result of head injuries. Hydrocephalus is seen when there has been subarachnoid hemorrhage or infection. Normal-pressure hydrocephalus is a complication of traumatic brain injury. The clinical signs and symptoms include cognitive deterioration, gait changes, and incontinence. These signs are also seen during posttraumatic amnesia, making early recognition of this syndrome difficult. Focal deficits, including optic atrophy, CN palsies, motor deficits, DI, or aphasia, may be seen. The type of residual effect depends on the location and nature of the trauma.
Diagnostic Evaluation
A detailed health history, both past and present, is essential in evaluating the child with craniocerebral trauma. Certain disorders such as drug allergies, hemophilia, diabetes mellitus, or epilepsy may produce similar symptoms. Even a minor traumatic injury can aggravate a preexisting disease process, thereby producing neurologic signs out of proportion to the injury.
After a minor injury, initial unconsciousness (if present) is brief, and the child ordinarily exhibits a transient period of confusion, somnolence, and listlessness; this period is most often accompanied by irritability, pallor, and one or more episodes of vomiting. A severe head injury requires immediate evaluation and treatment. Because head injuries are often accompanied by injuries in other areas (spine, viscera, extremities), the examination is performed with care to avoid further damage. Box 37-5 lists manifestations of head injury.
Initial Assessment: Priorities in the initial phase in the care of a child with a head injury include assessment of the ABCs (airway, breathing, circulation); neurologic examination focusing on mental status, papillary responses, and motor responses; and assessment for spinal cord injury. The assessment is carried out quickly in relation to vital signs (see Emergency Treatment box).
Ocular signs such as fixed, dilated, and unequal pupils; fixed and constricted pupils; and pupils that are poorly reactive or unreactive to light and accommodation indicate increased ICP or brainstem involvement. It is important to remain with the patient who demonstrates fixed and dilated pupils, since these are ominous signs with the probability of respiratory arrest. Dilated, nonpulsating blood vessels indicate increased ICP before the appearance of papilledema. Retinal hemorrhages often occur with acute head injuries, specifically with shaken baby syndrome.
Funduscopic examination should be performed routinely to detect retinal hemorrhages in a child with CNS trauma. Cortical blindness, defined as a complete bilateral visual loss associated with normal pupillary responses to light, can be a brief or transient consequence of minor head trauma (Hoyt, 2007). Theories of possible causes are vasospasm or localized cerebral edema. Transient blindness after mild head trauma may not be obvious in children unless this diagnosis is considered and evaluated.
Less urgent but important assessments include examination of the scalp for lacerations, widely separated sutures, and the size and tension of fontanels, which indicate intracranial hemorrhage or rapidly developing cerebral edema. Scalp lacerations may require surgical intervention. A significant amount of blood loss can occur from scalp lacerations. An underlying skull fracture should be ruled out by CT scan.
An accurate assessment of clinical signs provides baseline information. Serial evaluations, preferably by a single observer, help detect changes in the neurologic status. Alterations in mental status, evidenced by increased difficulty in rousing the child, mounting agitation, development of focal neurologic signs, or marked changes in vital signs, usually indicate extension or progression of the basic pathologic process.
Evaluation of reflexes provides information about cerebral and pyramidal involvement, although transient abnormalities of the abdominal reflexes and Babinski sign may be present in children with mild head trauma. Conscious, cooperative children are examined for cerebellar signs such as ataxia. Children may display unsteadiness, clumsiness, or tremor with intentional movement after head injury. Temperature may be moderately elevated for 1 or 2 days following an initial mild hypothermia after injury. A persistent fever may indicate subarachnoid hemorrhage or infection.
Special Tests: After a thorough clinical examination, a variety of diagnostic tests are helpful in providing a more definitive diagnosis of the type and extent of the trauma. A hematocrit and urinalysis are typically done. Serum electrolytes and glucose may also be measured in children with severe head injuries; hyperglycemia and disseminated intravascular coagulation are associated with a poor prognosis. The severity of a head injury may not be apparent on clinical examination of the child but is detectable on a CT scan. Whenever the child has a history consistent with a serious head injury (as with an unrestrained occupant in a severe motor vehicle accident or a fall), it is important to perform a scan even if the child initially appears alert and oriented. All children with head injuries who have any alteration of consciousness, headache, vomiting, skull fracture, seizure, or predisposing medical condition should undergo CT scanning.
MRI may be done to further assess cerebral edema or other structural brain abnormalities. A neurobehavioral assessment after early head injury may be useful in documenting cognitive impairment. Skull radiographs are of little benefit in diagnosing skull fractures. Other radiographic tests may be indicated depending on the severity or cause of the trauma. Electroencephalography is not helpful for diagnosis of a head injury but is useful for defining seizures. Lumbar puncture is rarely used for craniocerebral trauma and is contraindicated in the presence of increased ICP because of the possibility of herniation.
Therapeutic Management
The majority of children with mild traumatic brain injury who have not lost consciousness can be cared for and observed at home after careful examination reveals no serious intracranial injury. The nurse should give parents both verbal and written instructions of signs and symptoms that warrant concern and the need for reevaluation. These include persistent or worsening headaches, vomiting, change in mental status or behavior, unsteady gait, or seizure. Parents should bring the child in for examination in 1 or 2 days. The manifestations of epidural hematoma in children do not generally appear until 24 hours or more after injury (see Family-Centered Care box and Emergency Treatment box, p. 1530).
Children with severe injuries, those who have lost consciousness for more than a few minutes, and those with prolonged and continued seizures or other focal or diffuse neurologic signs must be hospitalized until their condition is stable and their neurologic signs have diminished. The child is maintained on NPO status (nothing by mouth) or restricted to clear liquids (if able to take fluids by mouth) until it is determined that vomiting will not occur. IV fluids are indicated in the child who is comatose, displays dulled sensorium, or is persistently vomiting.
The volume of IV fluid is carefully monitored to minimize the possibility of overhydration in case of SIADH and cerebral edema. However, damage to the hypothalamus or pituitary gland may produce DI with its accompanying hypertonicity and dehydration. Fluid balance is closely monitored by daily weight, strict intake and output measurement, and serum osmolality (to detect early signs of water retention).
Sedating drugs are usually withheld in the acute phase. Headache is usually controlled with acetaminophen, although opioids may be needed (see p. 1519). Antiepileptics are used for seizure control. Antibiotics are administered if there are lacerations or penetrating injuries. Prophylactic tetanus toxoid is given as appropriate. (See Chapter 12.) Cerebral edema is managed as described for the unconscious child. Hyperthermia is controlled with a hypothermia blanket.
Surgical Therapy: Scalp lacerations are sutured after careful examination of underlying bone. Depressed fractures require surgical reduction and removal of bone fragments. Torn dura is also sutured (see Atraumatic Care box). A skull fracture depressed more than the thickness of the skull or an intracranial hematoma that causes more than 5 mm (0.2 inch) midline shift is an indication for surgery. Direct pressure should not be applied to a depressed skull fracture. Ping-Pong ball skull fractures in very young infants can correct themselves within a few weeks or may require surgical elevation (Hung, Liao, and Huang, 2005).
Prognosis: The outcome of craniocerebral trauma depends on the extent of injury and complications. However, the outlook is generally more favorable for children than for adults. More than 90% of children with concussions or simple linear fractures recover without symptoms after the initial period. Compared with adults, children have a significantly higher percentage of good outcomes, a lower mortality rate, and a lower incidence of surgical mass lesions after severe head trauma. However, their thinner, softer brain may sustain greater long-term damage than previously suggested.
The concern regarding outcome is increasingly focused on cognitive, emotional, or mental problems. Children may experience a higher frequency of psychologic disturbances after head injury, whereas adults are more prone to complaints of a physical nature. Children may be more vulnerable than adults to long-term cognitive and behavioral dysfunction after diffuse brain injury.
True coma (not obeying commands, eyes closed, and not speaking) usually does not last more than 2 weeks. A child’s eventual outcome can range from brain death to a persistent vegetative state to complete recovery. However, even the best recovery may be associated with personality changes, including mood lability and loss of confidence, impaired short-term memory, headaches, and subtle cognitive impairments. Many children are left with significant disabilities after head injury that appear months later as learning difficulties, behavioral changes, or emotional disturbances (Anderson, Catroppa, Haritou, et al, 2001, 2005; Ewing-Cobbs, Prasad, Kramer, et al, 2006). In general, 90% of the long-term neurologic outcome has been achieved within 6 months to 1 year after the injury.
Nursing Care Management
The hospitalized child requires careful neurologic assessment and evaluation (see p. 1511) repeated as frequently as every 15 minutes to establish a correct diagnosis, identify signs and symptoms of increased ICP, determine clinical management, and prevent many complications. The goals of nursing management of the child with a head injury are to maintain adequate ventilation, oxygenation, and circulation; to monitor and treat increased ICP; to minimize cerebral oxygen requirements; and to support the child and family during the recovery phases.
The child is placed on bed rest, usually with the head of the bed elevated slightly and the head in midline position. ![]() Appropriate safety measures, such as side rails kept up and seizure precautions, are implemented. If the child is extremely restless, hard surfaces may be padded and restraints used to prevent further injury. Individualize care according to the child’s specific needs.
Appropriate safety measures, such as side rails kept up and seizure precautions, are implemented. If the child is extremely restless, hard surfaces may be padded and restraints used to prevent further injury. Individualize care according to the child’s specific needs.
![]() Skill—Implementing Seizure Precautions
Skill—Implementing Seizure Precautions
A key nursing role is to provide sedation and analgesia for the child. The conflict between the need to promote the child’s comfort and relieve anxiety versus the need to assess for neurologic changes presents a dilemma. Both goals can be achieved with close observation of the child’s LOC and response to analgesics (using a pain assessment record) and effective communication with the practitioner. Decreasing restlessness after administration of an analgesic most likely reflects pain control rather than a decreasing LOC. (See Pain Assessment and Pain Management, Chapter 7.)
Children may be restless and irritable, but more often their reaction is to fall asleep when left undisturbed. A quiet environment helps reduce the restlessness and irritability. Bright lights are irritating. This often makes checking the ocular responses more difficult and more aggravating to the child.
Frequent examinations of vital signs, neurologic signs, and LOC are extremely important nursing observations. When possible, they should be performed by a single observer to better detect subtle changes that may indicate worsening of neurologic status. Pupils are checked for size, equality, reaction to light, and accommodation. After the initial elevations usually seen after injury, the vital signs generally return to normal unless there is brainstem involvement.
The most important nursing observation is assessment of the child’s LOC. In the progression of an injury, alterations in consciousness appear earlier than alterations of vital signs or focal neurologic signs (see p. 1512 for evaluation of responsiveness). Frequent examinations of alertness are fatiguing to the child; therefore the child often desires to fall asleep, which may be confused with depressed consciousness. When left alone, the child goes to sleep. It is not uncommon to observe ocular divergence through the partially closed eyelids.
Observations of position and movement provide additional information. Note any abnormal posturing and whether it occurs continuously or intermittently. Questions nurses might ask include:
• Are the child’s hand grips strong and equal in strength?
• Are there any signs of extension or flexion posturing?
• What is the child’s response to stimulation?
• Is movement purposeful, random, or absent?
• Are movement and sensation equal on both sides or restricted to one side only?
The child may complain of headache or other discomfort. The child who is too young to describe a headache may be fussy and resist being handled. The child who suffers from vertigo often vigorously resists being moved from a position of comfort. Forcible movement causes the child to vomit and display spontaneous nystagmus. Seizures are relatively common in children at the time of head injury and may be of any type. Carefully observe any seizure activity and describe it in detail. Children in postictal states are more lethargic, with sluggish pupils.
Document drainage from any orifice. Bleeding from the ear suggests the possibility of a basal skull fracture. Clear nasal drainage is suggestive of an anterior basal skull fracture. Observe the amount and characteristics of the drainage.
Head trauma is often accompanied by other undetected injuries; therefore any bruises, lacerations, or evidence of internal injuries or fractures of the extremities are noted and reported. Associated injuries are evaluated and treated appropriately.
The child with a normal LOC is usually allowed clear liquids unless fluid is restricted. If the child has an IV infusion, it is maintained as prescribed. The diet is advanced to that appropriate for the child’s age as soon as the condition permits. Intake and output are measured and recorded, and any incontinence of bowel or bladder is noted for the child who has been toilet trained.
Observe the child for any unusual behavior, but interpretation of behavior is made in relation to the child’s normal behavior. For example, urinary incontinence during sleep would be of no consequence in a child who routinely wets the bed but would be highly significant for one who is always dry. Parents are invaluable resources in evaluating objective behaviors of their children. Information obtained from parents at, or shortly after, admission is essential in evaluating the child’s behavior (e.g., the ease with which the child is roused normally, the usual sleeping position, how much the child sleeps during the day, the child’s motor activities [rolling over, sitting up, climbing], hearing and visual acuity, appetite, and manner of eating [spoon, bottle, cup]). There would be less concern about a child who falls asleep several times during the day if this is consistent with the child’s usual behavior.
When the child is discharged, advise the parents of probable posttraumatic symptoms that may be expected, such as behavioral changes, sleep disturbances, phobias, and seizures. They should understand observations they need to make and how to contact the practitioner or health facility in case the child develops any unusual signs or symptoms. Emphasize the importance of follow-up evaluation.
Family Support: The emotional and educational support of the family presents a challenge. Witnessing the parents’ grief and helplessness on seeing their child in an intensive care unit connected to monitoring equipment and in an altered state evokes empathy. The nurse can encourage the family to be involved in the child’s care, to bring in familiar belongings, or to make a tape recording of familiar voices and sounds. Parents may need a demonstration on how to touch or cuddle their child and may want to talk about their grief. The nurse can listen attentively, reinforce what is being done to assist the child, and direct parents toward signs and symptoms of recovery to instill hope without promises. Honesty and kindness, along with consistent and competent care, can help families through this difficult time.
Rehabilitation: Rehabilitation and management of the child with permanent brain injury are essential aspects of care. Rehabilitation begins as soon as possible and usually involves the family and a rehabilitation team. The nurse makes a careful assessment of the child’s capabilities, limitations, and probable potential as early as possible, and implements appropriate interventions to maximize the residual capacities. The Brain Injury Association of America* provides information and listings of rehabilitation services and support groups throughout the country.
Pediatric trauma rehabilitation is a national concern. Coordinating care and services for early rehabilitation involves identifying the child’s and family’s response to the traumatic injury and disability, securing available resources, and recognizing the parents’ role in the process.
The child with a disability resulting from head trauma requires assessment on a physical, cognitive, emotional, and social level. The child has experienced separation, pain, sensory deprivation and overload, changes in circadian cycle, and fear of the unknown. Recovery and transition require new coping strategies at the same time that regressive and acting-out behaviors may start. Parents and children need honest communication for decision making. Rehabilitation is recommended when the child is making progress beyond what can be provided in a hospital setting. The Rancho Los Amigos Scale provides a systematic assessment of the progress that a child with a severe head injury may achieve. (See Family-Centered Care box, p. 1523.)
Pediatric rehabilitation focuses on the child’s strengths and needs. The rehabilitation team should include physical medicine; rehabilitation nursing; nutritional counseling; physical, occupational, and speech therapy; special education; and psychologic, neuropsychologic, child life, and social services. Families need to know what to look for when visiting a pediatric rehabilitation center. Before the child’s transfer, the hospital team should provide a detailed care plan of the child’s needs and abilities, especially communication skills, and a description of the child’s usual schedule, nursing care interventions, and the family’s concerns and needs. To augment the care plan, a videotape introducing the child and family and showing any unique aspects of their care can be sent to the rehabilitation center.
Prevention: Preventive strategies are underused in almost all cases of accidental childhood injury. Head injuries occur in the most serious accidents—especially motor vehicle accidents, sports, and falls.
Tremendous strides have been taken in the prevention of cerebral damage after head injury in children. New developments are directed toward the prevention of cellular injury or the primary insult. The roles of calcium, oxyradicals, and prostaglandins are being investigated. However, the greatest benefit lies in prevention of head injuries. Nurses can exert a valuable influence on behalf of children through education. Accidents occur that are preventable because unnecessary risks go unchecked. Inadequate supervision combined with a child’s natural sense of curiosity and exploration can lead to lethal results. Nurses are in the unique position of influencing caregivers in terms of growth and development. Banning the use of infant walkers is an example. This equipment does not help develop motor skills but places infants at risk for head and neck injuries from falls, especially down steps. Public education coupled with legislative support can prevent childhood injuries.
For extensive discussions of childhood injuries, see the information on injury prevention in Chapters 12, 14, 15, 17, and 19.
Submersion Injury
Submersion injury is a major cause of unintentional injury-related death in children ages 1 to 14 years. The term near-drowning is no longer used; instead, the term submersion injury should be used up until the time of drowning-related death (American Heart Association, 2005). Multiple definitions to describe submersion injury or near-drowning exist in the literature (Papa, Hoelle, and Idris, 2005). In 2003 guidelines were established for a uniform definition and reporting for submersion injury incidents (Idris, Berg, Bierens, et al, 2003).
Most cases of submersion injury are accidental, usually involving children who are helpless in water, such as inadequately attended children in or near swimming pools or infants in bathtubs; small children who fall into ponds, streams, and flooded excavations, usually near home; occupants of pleasure boats who fail to wear life preservers; children who have diving accidents; and children who are able to swim but overestimate their endurance. Accidental submersion injury occurs predominantly in males; 40% of children are younger than 5 years, and 90% of cases occur in private swimming pools (Kallas, 2009) (Fig. 37-9).

Fig. 37-9 Water is fascinating for children; however, drowning is the second leading cause of accidental death in unsupervised situations.
Submersion injury can take place in any body of water, including such unlikely places as a pail of water or a toilet bowl. Top-heavy toddlers fall headfirst into a pail of water, their arms become trapped, and they are unable to free themselves. Hot tubs and whirlpool spas have been implicated in childhood submersion injury. The suction created at the outlet is strong enough to trap even larger children underwater. Submersion injury as a form of fatal child abuse also occurs. Homicidal submersion injuries are not witnessed, usually occurring in the home, and the victims are either infants or toddlers.
Pathophysiology
Physiologically most organ systems are affected, especially the pulmonary, cardiovascular, and neurologic systems. The major pulmonary changes that occur in submersion injury are directly related to the length of submersion (regardless of the type and amount of fluid aspirated), the victim’s physiologic response, and the development and degree of immersion hypothermia. Cerebral hypoxia is a major component of morbidity and mortality in these individuals. Therefore early and aggressive resuscitation are imperative.
Physiologic factors that influence the extent of damage from immersion include resistance to asphyxia and anoxia, which shows some individual variation. The temperature of the water plays an important role in developing hypoxemia. Cold water decreases metabolic demands, thereby decreasing the effects of hypoxemia. Cold water also activates the diving reflex. This is a primitive neurologic response, often seen in children, of bradycardia and breath-holding. This neurologic response is triggered by immersion of the face in cold water. Blood is shunted away from the periphery to vital organs (i.e., the brain and heart). Submerged children struggle initially to stay above water, and often breath-hold leads to air hunger. Reflex inspiration eventually occurs, which leads to aspiration or reflex laryngospasm due to water contacting the lower respiratory tract (Salomez and Vincent, 2004). Cardiopulmonary arrest is secondary to hypoxemia and hypothermia.
Pathophysiologic features in submersion injuries are hypoxia, aspiration, and hypothermia. Hypoxia is the primary problem because it results in global cell damage, with different cells tolerating variable lengths of anoxia. Neurons, especially cerebral cells, sustain irreversible damage after 4 to 6 minutes of submersion. The heart and lungs can survive up to 30 minutes. Regardless of the amount of water aspirated, the victim suffers arterial hypoxemia (resulting from atelectasis and shunting of blood through the nonventilated alveoli), combined respiratory acidosis (resulting from retained carbon dioxide), and metabolic acidosis (caused by buildup of acid metabolites because of anaerobic metabolism). Although electrolyte imbalances are contributing factors, they are not the major causes of morbidity and mortality. The pathologic events are directly related to the duration of submersion. The major difficulty is acute ventilatory insufficiency. Approximately 10% of submersion injury victims die without aspirating fluid but succumb from acute asphyxia as a result of prolonged reflex laryngospasm (see Research Focus box).
Aspiration of fluid occurs in the majority of submersion injuries. The aspirated fluid results in pulmonary edema, atelectasis, airway spasm, and pneumonitis, which aggravates hypoxia. It was previously thought that the physiologic response differed between submersion in salt water and fresh water. However, there is no clinically significant difference in human survivors, and the type of water does not alter the therapy or outcome. The duration of submersion and severity of the hypoxia are the main factors that determine outcome (American Heart Association, 2005).
Hypothermia occurs rapidly in infants and children, partly because of their large surface area relative to size and partly as a result of the cold water itself. Profound hypothermia is usually evidence of lengthy submersion.
Clinical Manifestations
Clinical manifestations are directly related to the duration of loss of consciousness and neurologic status after rescue and resuscitation.
Therapeutic Management
With rapid treatment some children can be saved. Resuscitative measures should begin at the scene, and the victim should be transported to the hospital with maximum ventilatory and circulatory support. Many victims need care for some time after aspiration of fluid. In the hospital intensive pulmonary care is implemented and continued according to the patient’s needs.
In general, management of the victim with a submersion injury is based on the degree of cerebral insult. The first priority is to restore oxygen delivery to the cells and prevent further hypoxic damage. A spontaneously breathing child does well in an oxygen-enriched atmosphere; the more severely affected child requires endotracheal intubation and mechanical ventilation. Blood gases and pH are monitored at frequent intervals as a guide to oxygen, fluid, and electrolyte therapies. Rewarming the hypothermic patient is initiated. Seizures may occur due to hypoxia and cerebral edema. Seizures result in increased cerebral oxygen consumption. Therefore it is imperative to aggressively control seizure activity. In addition, blood glucose should be monitored; both hypoglycemia and hyperglycemia are harmful to the brain.
All children who have a submersion injury should be hospitalized for 12 to 48 hours for observation. Although some children do not appear to have sustained adverse effects from the event, respiratory compromise or cerebral edema may occur within 24 hours after the incident. Aspiration pneumonia is a common complication that occurs approximately 48 to 72 hours after the episode. Bronchospasm, alveolar-capillary membrane damage, atelectasis, abscess formation, and acute respiratory distress syndrome are other complications that occur after aspiration of fluid.
Prognosis: The best predictors of a good outcome are length of submersion in nonicy water (5° C [41° F]) of less than 5 minutes and the presence of sinus rhythm, reactive pupils, and neurologic responsiveness at the scene. The worst outcomes—death or severe neurologic impairment—are for children submerged for more than 10 minutes and not responding to advanced life support within 25 minutes. All children without spontaneous purposeful movement and normal brainstem function 24 hours after sustaining a submersion injury suffered severe neurologic deficits or death (Kallas, 2009).
Nursing Care Management
Nursing care depends on the child’s condition. A child who survives may need intensive respiratory nursing care with attention to vital signs, mechanical ventilation or tracheostomy, blood gas determination, chest physiotherapy, and IV infusion. Often the child has sustained a hypoxic insult and requires the same care as an unconscious child.
A difficult aspect in the care of the child who sustained a submersion injury is helping the parents cope with severe guilt reactions. Given the magnitude of the event, parents need repeated assurance that everything possible is being done to treat their child.
If the child dies, the sudden, unexpected nature of the death and the particular circumstances of the accident, especially in terms of guilt for not preventing it, compound the grief. (See Chapter 23.) The parents of the child who is saved from death face the anxiety of not knowing the final outcome—to what extent will their child recover? This situation generates such intense feelings of loneliness and guilt, it is important for families to know they are not alone. They should be reminded frequently that there are people to assist them through this crisis. Additional sources of support that can be recommended include psychiatric and social work consultants, community services, and religious support. Self-help groups are excellent if available in the community.
Nurses often have difficulty relating to the parents if obvious neglect has precipitated the accident and subsequent problems; therefore it is important for those who care for these children and their families to assess their own feelings about the situation, in addition to assessing the family’s coping abilities and resources. Caring for victims of a submersion injury and their families requires the nurse to be sensitive to the needs of the child and the family and to recognize his or her own reactions and emotions.
Prevention: Most submersion injuries are preventable. The most common cause of submersion injury in infants and small children is inadequate adult supervision. Children with known risk factors such as epilepsy and autism require increased surveillance. In general, children are not developmentally ready for formal swimming lessons until after their fourth birthday. All parents and swimming pool owners should be familiar with basic cardiopulmonary resuscitation (CPR). Rapid, basic CPR is one of the keys to improving the outcome (Salomez and Vincent, 2004). Water safety and survival training should be required for all school-age children. Private pools should be fenced on all four sides. Nurses can be active advocates in their communities. Nurses are also in a position to emphasize the importance of adequate adult supervision when children are in or near the water (Kallas, 2009). (See Injury Prevention, Chapters 12, 14, 15, 17, and 19.)
Intracranial Infections
![]() The nervous system is subject to infection by the same organisms that affect other organs of the body. However, the nervous system is limited in the ways in which it responds to injury. Laboratory studies are needed to identify the causative agent. The inflammatory process can affect the meninges (meningitis) or brain (encephalitis).
The nervous system is subject to infection by the same organisms that affect other organs of the body. However, the nervous system is limited in the ways in which it responds to injury. Laboratory studies are needed to identify the causative agent. The inflammatory process can affect the meninges (meningitis) or brain (encephalitis).
Meningitis can be caused by a variety of organisms, but the three main types are (1) bacterial, or pyogenic, caused by pus-forming bacteria, especially meningococci, pneumococci, and group B streptococci; (2) viral, or aseptic, caused by a wide variety of viral agents; and (3) tuberculous, caused by the tuberculin bacillus. The majority of children with acute febrile encephalopathy have either bacterial meningitis or viral meningitis as the underlying cause.
Bacterial Meningitis
![]() Bacterial meningitis is an acute inflammation of the meninges and CSF. The advent of antimicrobial therapy has had a marked effect on the course and prognosis. The introduction of conjugate vaccines against Haemophilus influenzae type b (Hib vaccine) in 1990 and Streptococcus pneumoniae (pneumococcus) in 2000 has led to dramatic changes in the epidemiology of bacterial meningitis (see Evidence-Based Practice box). Today, H. influenzae type b infection has been virtually eradicated among young children in areas in the world where the Hib vaccine is administered routinely (Yogev and Guzman-Cottrill, 2005). Since the introduction of widespread vaccination for S. pneumoniae, the incidence of pneumococcal meningitis in children in the United States has decreased by 55% to 60%. Nonetheless, S. pneumoniae remains an important and frequent cause of bacterial meningitis in children (Kaplan, Mason, Wald, et al, 2004; Nigrovic, Kuppermann, and Malley, 2008).
Bacterial meningitis is an acute inflammation of the meninges and CSF. The advent of antimicrobial therapy has had a marked effect on the course and prognosis. The introduction of conjugate vaccines against Haemophilus influenzae type b (Hib vaccine) in 1990 and Streptococcus pneumoniae (pneumococcus) in 2000 has led to dramatic changes in the epidemiology of bacterial meningitis (see Evidence-Based Practice box). Today, H. influenzae type b infection has been virtually eradicated among young children in areas in the world where the Hib vaccine is administered routinely (Yogev and Guzman-Cottrill, 2005). Since the introduction of widespread vaccination for S. pneumoniae, the incidence of pneumococcal meningitis in children in the United States has decreased by 55% to 60%. Nonetheless, S. pneumoniae remains an important and frequent cause of bacterial meningitis in children (Kaplan, Mason, Wald, et al, 2004; Nigrovic, Kuppermann, and Malley, 2008).
S. pneumoniae remains the most common cause of bacterial meningitis in children between 3 months and 10 years of age despite appropriate treatment. In children older than 1 month and less than 3 months, group B streptococci and gram-negative bacilli were the most frequent pathogens causing bacterial meningitis (Nigrovic, Kuppermann, and Malley, 2008).
Etiology
A variety of bacterial agents can cause bacterial meningitis. Since the introduction of new vaccinations (Hib and PCV7), the pathogens responsible for meningitis have changed. Currently S. pneumoniae and Neisseria meningitidis are the leading causes of bacterial meningitis in children between 3 months and 19 years. S. pneumoniae is the leading cause in children between 3 months and 10 years, and N. meningitidis is the leading cause in children between 10 and 19 years. The distribution of causative pathogens differs in children between 1 and 3 months. The leading causes of neonatal meningitis are group B streptococci (39%) and gram-negative bacilli (32%) (Nigrovic, Kuppermann, and Malley, 2008).
Meningococcal meningitis occurs in epidemic form and is the only type readily transmitted by droplet infection from nasopharyngeal secretions. Although this condition may develop at any age, the risk of meningococcal infection increases with the number of contacts; therefore it occurs predominantly in school-age children and adolescents. There appear to be some seasonal variations. Meningitis caused by pneumococcal and meningococcal infections can occur at any time, but is more common in later winter or early spring.
Maternal factors, such as premature rupture of fetal membranes and maternal infection during the last week of pregnancy, are major causes of neonatal meningitis. Risk factors for children developing meningitis include recent exposure to someone with meningococcal meningitis; recent ear or sinus infection; travel to areas where bacterial meningitis is common such as sub-Saharan Africa; penetrating head trauma; cochlear implant devices; and anatomic defects such as a dermal sinus, urinary tract anomaly, or recent placement of a ventricular shunt (Chavez-Bureno and McCracken, 2005).
Pathophysiology
The most common route of infection is vascular dissemination from a focus of infection elsewhere. For example, organisms from the nasopharynx invade the underlying blood vessels, cross the BBB, and multiply in the CSF. Invasion by direct extension from infections in the paranasal and mastoid sinuses is less common. Organisms also gain entry by direct implantation after penetrating wounds, skull fractures that provide an opening into the skin or sinuses, lumbar puncture or surgical procedures, anatomic abnormalities such as spina bifida, or foreign bodies such as an internal ventricular shunt or an external ventricular device. Once implanted, the organisms spread into the CSF, by which the infection spreads throughout the subarachnoid space.
The infective process is like that seen in any bacterial infection: inflammation, exudation, white blood cell accumulation, and varying degrees of tissue damage. The brain becomes hyperemic and edematous, and the entire surface of the brain is covered by a layer of purulent exudate that varies with the type of organism. For example, meningococcal exudate is most marked over the parietal, occipital, and cerebellar regions; the thick, fibrinous exudate of pneumococcal infection is confined chiefly to the surface of the brain, particularly the anterior lobes; and the exudate of streptococcal infections is similar to that of pneumococcal infections, but thinner.
As infection extends to the ventricles, thick pus, fibrin, or adhesions may occlude the narrow passages and obstruct the flow of CSF.
Clinical Manifestations
The clinical manifestations of acute bacterial meningitis depend to a large extent on the child’s age. The type of organism, the effectiveness of therapy for antecedent illness, and whether it occurs as an isolated entity or as a complication of another illness or injury also influence the clinical manifestation (Box 37-6).
Children and Adolescents: The onset of illness may be abrupt and rapid, or develop progressively over one or several days and may be preceded by a febrile illness (Feigin and Perlman, 2004). Most children with meningitis are seen with fever, chills, headache, vomiting, irritability, and nuchal rigidity that are associated with or quickly followed by alterations in sensorium. Some children are initially seen after having a seizure or have a seizure within the first 48 hours of admission to the hospital (Feigin and Perlman, 2004). The child is extremely irritable and agitated and may develop photophobia, confusion, hallucinations, aggressive behavior, drowsiness, stupor, or coma.
The child resists flexion of the neck (nuchal rigidity). Kernig and Brudzinski signs are positive. Reflex responses are variable, although they show hyperactivity. (See Reflexes, Chapter 6.) The skin may be cold and cyanotic with poor peripheral perfusion.
Other signs and symptoms may appear that are specific to individual organisms. Petechial or purpuric rashes occur in 50% of cases and indicate a meningococcal infection (meningococcemia), especially when the eruption is associated with a septic shock–like state. Joint involvement is seen in meningococcal and H. influenzae infection. A chronically draining ear commonly accompanies pneumococcal meningitis. Escherichia coli infection may be associated with a congenital dermal sinus that communicates with the subarachnoid space.
Infants and Young Children: Between 3 months and 2 years of age the illness is characterized by fever or hypothermia, poor feeding, vomiting, marked irritability, restlessness, seizures, and a bulging or tense fontanel, which are often accompanied by a high-pitched cry.
Neonates: Meningitis in newborn and premature infants is extremely difficult to diagnose. The vague and nonspecific manifestations, which are characteristic of all neonatal sepsis, bear little resemblance to the findings in older children. These infants are usually well at birth but within a few days begin to appear ill. They refuse feedings, have poor sucking ability, and may vomit or have diarrhea. They display poor muscle tone and lack of movement and have a poor cry. Other nonspecific signs that may be present include hypothermia or fever (depending on the infant’s maturity), jaundice, irritability, drowsiness, seizures, respiratory irregularities or apnea, cyanosis, and weight loss. The full, tense, and bulging fontanel may or may not be present until late in the course of the illness, and the neck is usually supple. Untreated, the child’s condition will decline to cardiovascular collapse, seizures, and apnea.
Complications: The incidence of complications from acute bacterial meningitis has been significantly reduced with early diagnosis and vigorous antimicrobial therapy. If infection extends to the ventricles, thick pus, fibrin, or adhesions may occlude the narrow passages, thereby obstructing the flow of CSF and causing obstructive hydrocephalus. Subdural effusions often occur, and thrombosis may occur in meningeal veins or venous sinuses. Destructive changes may take place in the cerebral cortex, and brain abscesses may form by direct extension of the infection or by vascular dissemination. Extension of the infection to the areas of the cranial nerves or compression necrosis from increased pressure may cause deafness, blindness, or weakness or paralysis of facial or other muscles of the head and neck.
One of the most dramatic and serious complications usually associated with meningococcal infections is meningococcal sepsis, or meningococcemia. When the onset is severe, sudden, and rapid, it is known as the Waterhouse-Friderichsen syndrome. The syndrome is characterized by overwhelming septic shock, disseminated intravascular coagulation, massive bilateral adrenal hemorrhage, and purpura (Fig. 37-10). Meningococcemia requires immediate emergency treatment, hospitalization, and intensive care because of the high mortality rate (Feigin and Perlman, 2004; Woods, 2009).
Other acute complications of meningitis include SIADH (see Chapter 38), subdural effusions, seizures, cerebral edema and herniation, and hydrocephalus. Obstruction to the flow of CSF occurs during the acute phase of illness by clumping of purulent material in the drainage channels and during the chronic phase by adhesive arachnoiditis or fibrotic obstruction through any of the ventricular foramina. Postmeningitic complications in neonates include ventriculitis, which results in cystic, walled-off areas of the brain with fluid accumulation and pressure.
Extension of the inflammation to cranial nerves or compression and destruction of the nerves from ICP can produce permanent impairment of vision or hearing and other nerve palsies. CN VIII damage is usually followed by permanent deafness. Other long-term complications include cerebral palsy, cognitive impairments, learning disorder, attention deficit hyperactivity disorder, and seizures.
Hemiparesis and quadriparesis may result from damage caused by arteritis or thrombosis or other mechanisms. Behavioral changes occur in some children. Evidence indicates that psychometric and behavioral defects may be a significant concomitant sign of meningitis in childhood, although it is difficult to determine the degree to which meningitis affects the intelligence of young children. Meningitis in the neonatal period is more likely to cause lifelong impairments, including moderate to severe developmental delay, blindness, deafness, and epilepsy (de Louvois, Halket, and Harvey, 2005).