Diagnostic Evaluation
A lumbar puncture is the definitive diagnostic test. The fluid pressure is measured and samples are obtained for culture, Gram stain, blood cell count, and determination of glucose and protein content. The findings are usually diagnostic. Culture and sensitivity are needed to identify the causative organism. Spinal fluid pressure is usually elevated, but interpretation is often difficult when the child is crying. Sedation with fentanyl and midazolam can alleviate the child’s pain and fear associated with this procedure (see Atraumatic Care box). If there is evidence or suspicion of increased ICP (papilledema, focal neurologic deficits, coma, presence of a CSF shunt, history of hydrocephalus), a CT scan of the head may be warranted before the procedure (Tunkel, Hartman, Kaplan, et al, 2004).
The patient generally has an elevated white blood cell count, often predominantly polymorphonuclear leukocytes. The glucose level is reduced, generally in proportion to the duration and severity of the infection. The relationship between the CSF glucose and serum glucose levels is important in evaluating the glucose content of CSF; therefore a serum glucose sample is drawn approximately one half hour before the lumbar puncture. Protein concentration is usually increased.
A blood culture is advisable for all children suspected of having meningitis and occasionally will be positive when CSF culture is negative. Nose and throat cultures may provide helpful information in some cases.
Therapeutic Management
Acute bacterial meningitis is a medical emergency that requires early recognition and immediate therapy to prevent death and avoid residual disabilities. The initial therapeutic management includes:
The child is isolated from other children, usually in an intensive care unit for close observation. An IV infusion is started to facilitate administration of antimicrobial agents, fluids, antiepileptic drugs, and blood, if needed. The child is placed on a cardiac monitor and in respiratory isolation.
Drugs: Until the causative organism is identified, the choice of antibiotic is based on the known sensitivity of the organism most likely to be the infective agent. After identification of the organism, antimicrobial agents are adjusted accordingly.
Signs of gastrointestinal hemorrhage or secondary infection may complicate steroid administration. Antibiotic treatment with cephalosporins demonstrates superiority for promptly sterilizing the CSF and reducing the incidence of severe hearing impairment.
Nonspecific Measures: Maintaining hydration is a prime concern, and the patient’s condition determines whether IV fluids are needed and the type and amount of fluid. The optimum hydration involves correction of any fluid deficits followed by fluid restriction as ordered to prevent cerebral edema. Cerebral edema and electrolyte disturbances are associated with poor neurologic outcome after bacterial meningitis (Bonthius and Karacay, 2002). Children with bacterial meningitis must be monitored for signs of increased ICP. If needed, measures to decrease ICP are implemented (see p. 1520).
Complications are treated appropriately, such as aspiration of subdural effusion in infants and treatment for disseminated intravascular coagulation syndrome. Shock is managed by restoration of circulating blood volume and maintenance of electrolyte balance. Seizures can occur during the first few days of treatment. These are controlled with the appropriate antiepileptic drug. Hearing loss is not uncommon. The patient should undergo auditory evaluation 6 months after the illness has resolved.
Lumbar puncture is carried out as needed to determine the effectiveness of therapy. The patient is evaluated neurologically during the convalescent period.
Prognosis: Ten percent to 15% of cases of bacterial meningitis are fatal (Centers for Disease Control and Prevention, 2000). The child’s age, duration of illness before antibiotic therapy, rapidity of diagnosis after onset, type of organism, prolonged or complicated seizures, low CSF glucose concentration, and adequacy of therapy are important in the prognosis of bacterial meningitis (see Research Focus box). Bacterial meningitis can result in brain damage, hearing loss, or learning disability (Centers for Disease Control and Prevention, 2000; Prober, 2009).
The sequelae of bacterial meningitis occur most often when the disease occurs in the first 2 months of life and least often in children with meningococcal meningitis. The residual deficits in infants are primarily a result of communicating hydrocephalus and the greater effects of cerebritis on the immature brain. In older children the residual effects are related to the inflammatory process itself or result from vasculitis associated with the disease. Bacterial meningitis continues to cause substantial morbidity in infants and children. The mortality rate and incidence of poor neurologic outcome is highest in patients with pneumococcal meningitis (Prober, 2009).
Hearing impairment is the most common sequela of this disease. Evaluation of CN VIII is needed for at least a 6-month follow-up period to assess for possible hearing loss.
Prevention: Vaccines are available for types A, C, Y, and W-135 meningococci and H. influenzae type b. Routine meningococcal polysaccharide vaccination of children is licensed for use only in children 2 years and older (Pichichero, 2005). Routine vaccinations for H. influenzae type b are recommended for all children beginning at 2 months of age. (See Immunizations, Chapter 12.) Pneumococcal conjugate vaccine is now recommended for all children beginning at 2 months of age (Centers for Disease Control and Prevention, 2009).
Nursing Care Management
![]() Nurses should take the necessary precautions to protect themselves and others from possible infection. Teach parents the proper procedures and supervise them in their application.
Nurses should take the necessary precautions to protect themselves and others from possible infection. Teach parents the proper procedures and supervise them in their application.
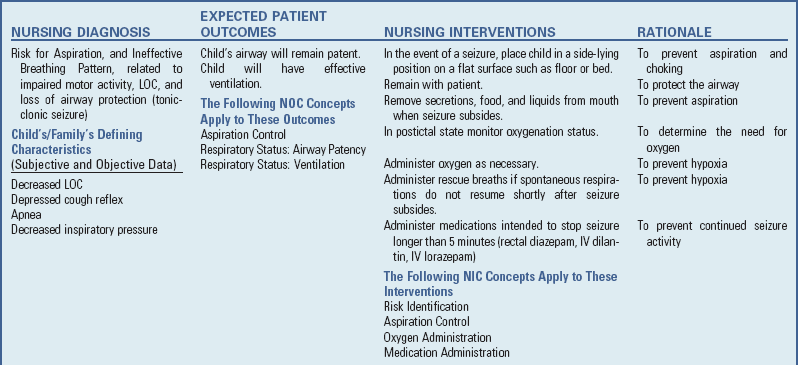
![]() Nursing Care Plan—The Child with Bacterial Meningitis
Nursing Care Plan—The Child with Bacterial Meningitis
Keep the room as quiet as possible, and keep environmental stimuli at a minimum, since most children with meningitis are sensitive to noise, bright lights, and other external stimuli. Most children are more comfortable without a pillow and with the head of the bed slightly elevated. A side-lying position is more often assumed because of nuchal rigidity. The nurse should avoid actions that cause pain or increase discomfort, such as lifting the child’s head. Evaluating the child for pain and implementing appropriate relief measures are important during the initial 24 to 72 hours. Acetaminophen with codeine is often used. Measures are used to ensure safety because the child is often restless and subject to seizures.
The nursing care of the child with meningitis is determined by the child’s symptoms and treatment (see Box 37-6). Observation of vital signs, neurologic signs, LOC, urinary output, and other pertinent data is carried out at frequent intervals. The child who is unconscious is managed as described previously (see pp. 1519-1524), and all children are observed carefully for signs of the complications just described, especially increased ICP, shock, or respiratory distress. Frequent assessment of the open fontanels is needed in the infant because subdural effusions and obstructive hydrocephalus can develop as a complication of meningitis.
Administration of fluids and nourishment is determined by the child’s status. The child with dulled sensorium is usually given nothing by mouth. Other children are allowed clear liquids initially and, if tolerated, progress to a diet suitable for their age. Careful monitoring and recording of intake and output are needed to determine deviations that might indicate impending shock or increasing fluid accumulation, such as cerebral edema or subdural effusion.
One of the most difficult problems in the nursing care of children with meningitis is maintaining IV infusion for the length of time needed to provide adequate antimicrobial therapy (usually 10 days). Because continuous IV fluids are usually not necessary, an intermittent infusion device is used. In some cases children who are recovering uneventfully are sent home with the device, and the parents are taught IV drug administration.
Family Support: The sudden nature of the illness makes emotional support of the child and parents extremely important (see Family-Centered Care box). Parents are upset and concerned about their child’s condition and often feel guilty for not having suspected the seriousness of the illness sooner. They need much reassurance that the natural onset of meningitis is sudden and that they acted responsibly in seeking medical assistance when they did. The nurse encourages the parents to openly discuss their feelings to minimize blame and guilt. The nurse also keeps them informed of the child’s progress and of all procedures, results, and treatments. In the event that the child’s condition worsens, they need the same psychologic care as other parents facing the possible death of their child. (See Chapter 23.)