The Child with Cancer
http://evolve.elsevier.com/wong/ncic
Administration of Medication, Ch. 27
Anaphylaxis, Ch. 29
Anemia, Ch. 35
Biologic Development (Adolescence), Ch. 19
Bone Marrow Aspiration or Biopsy, Ch. 27
The Child with Cerebral Dysfunction, Ch. 37
Dental Health, Ch. 14
Drug Reactions, Ch. 18
Epistaxis (Nosebleeding), Ch. 35
Family-Centered Care of the Child with Chronic Illness or Disability, Ch. 22
Family-Centered Care of the Child During Illness and Hospitalization, Ch. 26
Family-Centered End-of-Life Care, Ch. 23
Immunizations, Ch. 12
Infection Control, Ch. 27
Lumbar Puncture, Ch. 27
Pain Assessment; Pain Management, Ch. 7
Physical Examination, Ch. 6
Preparation for Diagnostic and Therapeutic Procedures, Ch. 27
Stomatitis, Ch. 16
Sunburn, Ch. 18
Surgical Procedures, Ch. 27
Venous Access Devices, Ch. 28
Cancer in Children
Few situations in nursing exceed the challenges of caring for a child with cancer. Despite the dramatic improvements in survival rates for these children, the family’s needs are tremendous as they cope with a serious physical illness and the fear that the child will not be cured. Nurses should base support of patients and their families on the premise that communication promotes understanding and clarity. With understanding, fear diminishes and hope emerges, and in the presence of hope, anything is possible.
This chapter summarizes the clinical presentation and nursing care issues for the most common types of pediatric cancer. Chapter 22 discusses the general psychologic needs of these children and their families in terms of chronic illness. Chapter 23 discusses situations when the disease is life threatening and death is a possibility.
Epidemiology
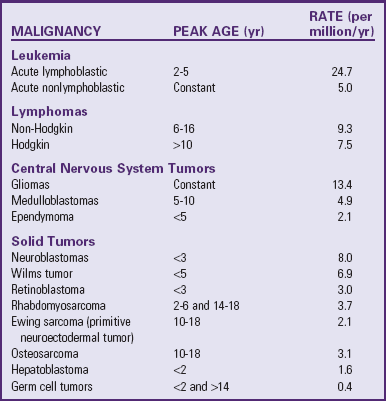
Childhood cancer is rare; approximately 12,400 cases of cancer are diagnosed in children younger than 20 years of age in the United States each year. Despite the relatively low incidence, approximately 1600 children under the age of 15 years die from their disease each year, making cancer the leading cause of death from disease in this age-group. The incidence of cancer in children and adolescents is approximately 15 per 100,000 children (Ries, Smith, Gurney, et al, 1999).
The incidence of specific subtypes of childhood cancer can vary according to age, sex and race. For example, males have a higher overall incidence of cancer compared with females, with a ratio of 1.2 : 1. This is due to the higher incidence of acute lymphoblastic leukemia (ALL), lymphoma, and medulloblastoma—the most common types of childhood cancer—in young boys. Unlike adults, Caucasian children have an overall higher incidence of cancer compared to African-American children. This is accounted for by the higher incidence in ALL and central nervous system (CNS) tumors in Caucasian children. The incidence of childhood cancer is more pronounced during the first year of life with a second peak from ages 2 to 3 years, followed by declining rates until age 9. Although each specific type of cancer has its own age distribution, overall incidence steadily rises from age 9 through adolescence (Scheurer, Bondy, and Gurney, 2011) (see Table 36-1 and Research Focus box).
Etiology
Often one of the first questions parents of newly diagnosed children with cancer ask is, “How did my child get this and did I do something to cause it?” Parents are also understandably concerned with the question of the likelihood that their other children will get cancer. The cause of cancer is not known. Although there are numerous hypotheses concerning its origin, the most enduring theory is that some genetic alteration results in the unregulated proliferation of cells. Significant advances have been made in our understanding of cell proliferation, programmed cell death (apoptosis), genes that activate tumor growth (oncogenes), and genes that keep tumor growth in check (tumor suppressor genes). Cancer is the result of multiple genetic events but is not necessarily hereditary. Overall, the incidence of cancers caused by direct inheritance is low.
In the early 1970s Alfred Knudson described the “two-hit hypothesis.” This explanation of cancer inheritance is best described in retinoblastoma. Like most genes, the retinoblastoma gene (Rb) is present in two copies on each cell. It is a tumor suppressor gene, responsible for controlling cell growth. When just one of these copies is lost—the “first hit”—the cell remains normal. However, when the second copy is lost—“second hit”—abnormal cell proliferation occurs and retinoblastoma develops (Knudson, Hethcote, and Brown, 1975). A child can inherit one altered copy of the retinoblastoma gene from a mother or father. Therefore it takes only one more hit for retinoblastoma to develop. Retinoblastoma can be either inherited or sporadic. Familial retinoblastoma manifests earlier and is more commonly bilateral. Population studies of siblings and offspring of children with cancer suggest that there is not, in general, a strong constitutional genetic component for childhood cancers other than retinoblastoma (MacDonald and Lessick, 2000).
Perhaps the most well-known inherited cancer predisposition syndrome is Li-Fraumeni syndrome, which is mainly due to constitutional (in all cells) mutation in the tumor suppressor gene, p53. This syndrome is characterized by early incidence of sarcomas, brain tumors, premenopausal breast cancer, and multiple primary tumors. However, other tumors have also been described within the spectrum of this syndrome.
Chromosome abnormalities have been identified in many childhood malignancies and are important in the development of various types of cancer. Chromosome abnormalities can be confined to the tumor or can be present in all cells; the latter are called germ-line mutations. Chromosome abnormalities can be due to translocations (a rearrangement of information between two chromosomes) or abnormal numbers of chromosomes. For example, many well-established chromosome translocations have been identified in childhood leukemia and solid tumors (see Research Focus box).
Other genetic syndromes that can affect genes or chromosomes and are associated with a predisposition to cancer include Fanconi anemia, Bloom syndrome, Beckwith-Weidemann syndrome, neurofibromatosis type 1, ataxia-telangiectasia, and Klinefelter syndrome.
Children with immunodeficiencies, such as Wiskott-Aldrich syndrome or acquired immunodeficiency syndrome, or children whose immune system has been suppressed, such as following transplant procedures, are at a greater risk for developing various cancers. Of major concern is the increased risk of secondary cancers in some children successfully treated for their primary malignancy.
Risk Factors
Lifestyle-related risks are the main factors that increase the risk of cancer in adults, but have little to no effect on childhood cancer. There is relatively little information to support a strong environmental role in the development of childhood cancer. However, some risk factors are well established. Known risk factors include exposure to ionizing radiation, carcinogenic drugs, immunosuppressive therapy, infections such as Epstein Barr virus, race, and genetic conditions (as previously described).
Although drugs, particularly those containing radioisotopes and immunosuppressive agents, can increase the risk of developing childhood cancer, the one drug most recognized for its carcinogenic effect is diethylstilbestrol. This drug has not been given in the United States since 1971. In the past, large doses of this hormone given to pregnant women to prevent abortion caused adenocarcinoma of the vagina in a significant proportion of the female offspring when they reached adolescence and early adulthood (Hatch, Herbst, Hoover, et al, 2000).
Prevention
Knowledge of the risk factors that increase the likelihood of cancer holds the promise of prevention. Unfortunately, in children the known carcinogens are limited. Therefore at present there is really no known prevention.
Health professionals do have two roles, however. One is aimed at preventing adult type of cancers by educating parents and children about the hazards of known carcinogens, particularly the effects of cigarette smoking and excessive exposure to sunlight. Lung cancer is the leading cause of death from cancer in adults, and malignant melanoma is the leading cause of death from diseases of the skin. Children at higher risk for skin cancer are those with light-colored eyes, complexion, and hair; those who sunburn easily; those who live near the equator; and those with freckles associated with sunburn (Cercato, Nagore, Ramazzotti, et al, 2008). Not only these children but all children should be protected from overexposure to the sun. (See Chapter 18.) In addition, to provide early detection of other types of cancer, males should learn testicular self-examination, and female adolescents should learn breast self-examination and seek periodic health examinations, including a Papanicolaou smear.
Second, health care professionals need to be aware of the cardinal symptoms of childhood cancer (Box 36-1). Unfortunately, fever and pain are manifestations of common childhood disorders and, without a high index of suspicion, may be attributed to minor ailments. The other signs are subtle and easily missed. If parents suspect an abnormality, their concerns must be taken seriously. The greatest weapons against all forms of cancer are early detection and treatment.
Diagnostic Evaluation
The evaluation of a child suspected of having cancer may take several days to complete. Specific signs and symptoms depend on the type of cancer and its location. The essential components of a comprehensive evaluation for childhood cancer include complete history and review of symptoms, physical examination, laboratory tests, diagnostic imaging, diagnostic procedures (lumbar puncture [LP], bone marrow aspirate, and biopsy), and surgical pathology.
Complete History
History of present illness—Onset of symptoms, severity and duration, alleviating or potentiating factors
History of previous illnesses—Communicable diseases, infections, medication history, previous hospitalizations or surgeries, exposure to blood products, immunization status
Family history—Family members with prior cases of cancer: type, age at diagnosis, treatment, and outcome
Present health status of family members—History of illness or disease in other family members
Developmental factors—Milestones obtained, recent regression in any milestones
Review of Symptoms
Skin—History of bruising or bleeding, lesions, lumps, open sores
Head, eyes, ears, nose, and throat (HEENT)—History of trauma, vision disturbances, proptosis, pupil discoloration, unequal pupils, eye muscle weakness, infection, difficulty swallowing
Heart—History of murmur or thrill
Lungs—History of infection, asthma or reactive airway disease, cough, wheezing, shortness of breath, dyspnea
Abdomen—History of abdominal swelling, pain, mass, change in bowel or bladder patterns
Musculoskeletal—History of weakness in extremities, limited range of motion, tenderness or swelling, joint pain
Neurologic—Loss of developmental milestones, altered consciousness, decreased sensations, abnormal reflexes, abnormal cerebellar functions, headaches, seizures
Lymphatic—History of enlarged lymph nodes, frequent infections
Hematologic—History of bruising, nosebleeds or gum bleeding, paleness, fatigue, bloody or tarry-colored stools
Physical Examination
See Physical Examination, Chapter 6.
General—Orientation, general state of health
Skin—Petechiae or ecchymosis, lesions or sores, presence of blood from gum or nose, color of skin
HEENT—Macrocephaly, bulging fontanel, evidence of infection, proptosis, pupil discoloration, anisocoria, extraocular movements not intact, limited peripheral vision, nystagmus, leukocoria
Heart—Murmur or thrill, peripheral pulses
Lungs—Evidence of infection, rales or rhonchi, decreased breath sounds, dyspnea, tachypnea
Abdomen—Hepatosplenomegaly, mass, decreased bowel sounds, striae
Neurologic—Altered consciousness, altered sensation, abnormal reflexes, abnormal cerebellar functions, unstable gait, dysarthria, cranial nerve deficits
Laboratory Tests
Several laboratory tests must be performed to accurately diagnose and treat children with cancer. The majority of patients have a complete blood count, serum chemistries, liver function tests, coagulation studies, and urinalysis done on initial presentation. For example, patients with leukemia often have a low hemoglobin; low platelet count; and low, normal, or high white blood counts. In addition, these patients may have elevated lactate dehydrogenase, creatinine, and uric acid, which require close monitoring when therapy is initiated. Frequent complete blood counts are necessary to monitor effects of therapy and in some hematologic malignancies, response to therapy.
Blood chemistry yields important information with regards to kidney, liver, bone function, and electrolyte balance. These tests are important to help detect the extent of disease and also to monitor for side effects during therapy. For example, a patient with bone metastasis may have elevated alkaline phosphatase. Elevations in blood urea nitrogen and creatinine may reflect kidney damage from chemotherapy agents. Consequently, regular blood chemistries and urinalysis are standard procedures through the course of the disease.
Diagnostic Procedures
![]() An LP is a routine test employed in leukemia, brain tumors, and other cancers that may metastasize to the CNS. An LP is also used to administer intrathecal drugs in patients with various malignancies such as leukemia.
An LP is a routine test employed in leukemia, brain tumors, and other cancers that may metastasize to the CNS. An LP is also used to administer intrathecal drugs in patients with various malignancies such as leukemia.
![]() Critical Thinking Exercise—Bone Marrow Test
Critical Thinking Exercise—Bone Marrow Test
A bone marrow test is performed by aspirating marrow with a large or fine bore needle. A bone marrow biopsy is performed by obtaining a piece of bone through a special type of needle. These tests are performed to determine the presence or absence of tumor or response to therapy in this specific location. For example, the specific type of leukemia can be identified by examination of the patient’s bone marrow and core biopsy. Also, patients with other solid tumors, like neuroblastoma, may have spread of disease to the bone marrow, which can be determined by these procedures.
Diagnostic Imaging
Modern day diagnostic imaging has greatly improved our ability to accurately diagnose childhood cancers. The most commonly employed modes of imaging include chest x rays, computed tomography (CT), magnetic resonance imaging (MRI). More recently, positron emission tomography (PET) is being used increasingly in a variety of pediatric malignancies such as Hodgkin disease and sarcomas. Interventional radiology is playing an increasing role in the diagnosis and management of pediatric malignancies.
Pathologic Evaluation
A biopsy is necessary to establish the diagnosis of a malignancy. Beyond, telling us what type of cancer the patient has, this tissue sample can also be sent for various biologic studies that define the patient’s prognosis and allow health care providers to tailor therapy according to the risk group. For example, a bone marrow biopsy determines whether the patient has acute lymphocytic leukemia or acute myelocytic leukemia, but also tells what specific subtype of leukemia the patient has and how aggressively it should be treated. Similarly, patients with neuroblastoma undergo a biopsy of the tumor to establish the diagnosis and to evaluate the tumor for N-myc amplification, which determines the type of treatment they receive.
Treatment Modalities
The use of multimodal therapy consisting of surgery, chemotherapy, and radiotherapy; enrollment of large numbers of children in cooperative group clinical trials or protocols; and improvements in supportive care have greatly increased the survival of children with cancer. Eighty percent of these patients are now expected to be cured of their disease.
Current efforts are aimed at increasing the survival of patients with high-risk tumors, decreasing the acute and long-term side effects of treatment, and studying the biology of the diseases to better identify patients who are at different risk levels for disease recurrence and can therefore benefit from risk-adapted therapies.
Surgery
The main goal of surgery, besides obtaining biopsies, is to remove all traces of tumor and restore normal body functioning. Surgery is most successful when the tumor is encapsulated and localized (confined to the site of origin). It may only be palliative when the cancer is regional (metastasized to an area adjacent to the original site) or advanced (widespread throughout the body). Obviously the best prognosis is directly related to early detection of the tumor.
Because the majority of pediatric cancers respond well to chemotherapy, more conservative surgical excision is increasingly used in a variety of tumors in an attempt to preserve function and cosmesis. For example, in some types of bone cancer, such as osteosarcoma, patients are successfully treated with resection of the diseased portion of the bone rather than amputation. There is an increasing emphasis on the use of combination drug therapy and radiotherapy after limited surgical intervention.
Chemotherapy
Chemotherapy may be the primary form of treatment, or it may be an adjunct to surgery or radiotherapy. The majority of chemotherapy agents work by interfering with the function or production of nucleic acids, deoxyribonucleic acid (DNA), or ribonucleic acid (RNA). Although several drugs with antineoplastic capabilities have been effective in treating different forms of cancer, the remarkable survival rates have been the result of improved combination drug regimens. Combining drugs allows for optimum cell cycle destruction with minimum toxic effects and decreased resistance by the cancer cells to the agent. For example, VAC (vincristine [Oncovin], doxorubicin [Adriamycin], and cyclophosphamide [Cytoxan]) combines complementary cytotoxic effects with nonsimilar side effects. Doxorubicin and cyclophosphamide are myelosuppressive, whereas vincristine is neurotoxic.
In addition to more effective combinations of drugs, several advances in the administration of chemotherapy have permitted continuous or intermittent IV administration without multiple venipunctures. The use of venous access devices (catheters and implantable infusion ports) has greatly facilitated safe and effective drug administration with minimum discomfort for the child. (See Chapter 28.) Continuous infusions over an extended period using syringe pumps have made possible the administration of certain drugs, such as cytosine arabinoside, in higher doses with less toxicity than when the drug is administered intermittently.
Chemotherapeutic agents can be classified according to their primary mechanism of action. Alkylating agents replace a hydrogen atom of a molecule by an alkyl group. The irreversible combination of alkyl groups with nucleotide chains, particularly DNA, causes unbalanced growth of unaffected cell constituents so that the cell eventually dies. These agents have a steep dose-response curve and, for this reason, can be used in high-dose therapy regimens. Examples of alkylating agents include cyclophosphamide, ifosfamide, cisplatin (Platinol), and dacarbazine. Antimetabolites resemble essential metabolic elements needed for cell growth but are sufficiently altered in molecular structure to inhibit further synthesis of DNA or RNA; their maximum effect occurs in cells that are actively producing DNA. Examples of antimetabolites include methotrexate and mercaptopurine. Plant alkaloids arrest cells in metaphase (a phase of mitosis) by binding to microtubular protein needed for spindle formation. Examples include vincristine and vinblastine. Antitumor antibiotics are natural products that interfere with cell division by reacting with DNA in such a way as to prevent further replication of DNA and transcription of RNA. Examples include doxorubicin and daunomycin.
Both adrenal and gonadal hormones have antineoplastic properties. The precise mechanism of action is still unclear. Adrenocorticosteroids, in theory, bind with DNA and alter the transcription process. Although there are a number of cortisone preparations, prednisone and dexamethasone are most frequently used.
A number of agents are not categorized according to the preceding classifications. For example, l-asparaginase is an enzyme isolated from extracts of bacterial cultures of Escherichia coli or Erwinia carotovora. It hydrolyzes l-asparagine, an amino acid, to l-aspartic acid, which prevents the cell from synthesizing protein needed for DNA and RNA synthesis. Because l-asparagine is synthesized by normal cells but must be exogenously supplied to certain leukemic and lymphoma cells, administration of the enzyme destroys the essential exogenous supply while sparing normal cells of untoward effects.
An understanding of the actions and side effects of these drugs is essential to nursing care of children with cancer. Unfortunately, almost all drugs are not selectively cytotoxic for malignant cells, and other cells with a high rate of proliferation, such as the bone marrow elements and hair, skin, and epithelial cells of the gastrointestinal tract, are also affected. Frequently the problems related to the destruction of these normal cells require more nursing care than the disease itself.
More recently, a number of targeted agents called tyrosine kinase inhibitors have been developed and are being used in a variety of pediatric and adult malignancies. Examples of some of these agents include imatinib, sunitinib, and sorafenib.
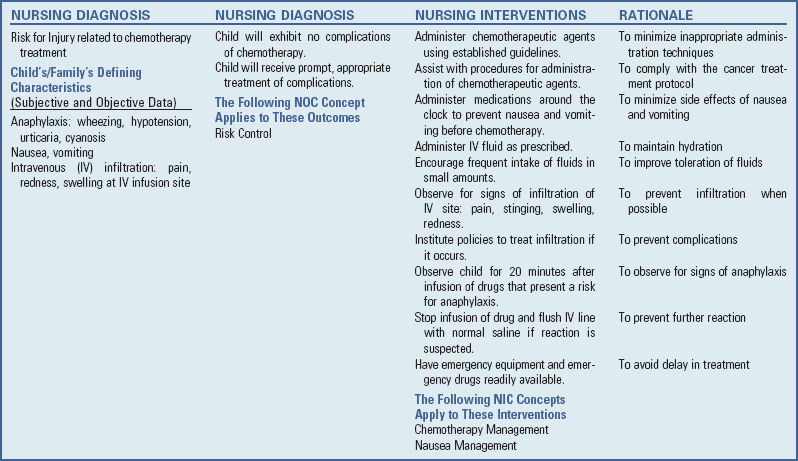
Precautions in Administering and Handling Chemotherapeutic Agents: Many chemotherapeutic agents are vesicants (sclerosing agents) that can cause severe cellular damage if even minute amounts of the drug infiltrate surrounding tissue. Only nurses experienced with chemotherapeutic agents should administer vesicants (Fig. 36-1). Guidelines are available* and must be followed meticulously to prevent tissue damage to patients. Interventions for extravasation vary, but each nurse should be aware of the institution’s policies before giving any vesicant and implement them at once if indicated.
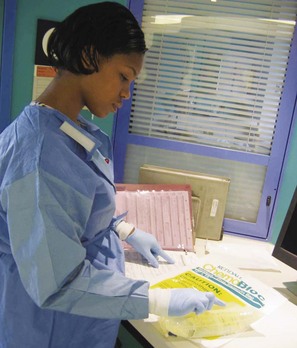
Fig. 36-1 Nurses caring for children with cancer require expertise in the safe administration of chemotherapy.
In addition to extravasation, a potentially fatal complication is anaphylaxis, especially from l-asparaginase, bleomycin, cisplatin, and etoposide (VP-16). (See Chapter 29.) Hypersensitivity reactions to these chemotherapeutic agents are characterized by urticaria, angioedema, flushing, rashes, difficulty breathing, hypotension, and nausea or vomiting. Nursing responsibilities include prevention of, recognition of, and preparation for serious reactions. Prevention begins with a careful history of known allergies and education of the patient and family regarding signs and symptoms to report. (See Chapter 6.)
If a reaction is suspected, the nurse discontinues the drug, flushes the IV line and maintains with saline, and monitors the child’s vital signs and subsequent responses.
In addition to the nurse’s many responsibilities nurses in regard to the child and family, nurses must also use safeguards to protect themselves. Handling chemotherapeutic agents may present risks to handlers and to their offspring, although the exact degree of risk is not known. The Oncology Nursing Society has published comprehensive guidelines for safe practice issues related to administration of chemotherapy.† They have also established safe management procedures for chemotherapy administered in the home (Oncology Nursing Society, 2004). Basic nursing guidelines are in the Nursing Care Guidelines box.
Radiotherapy
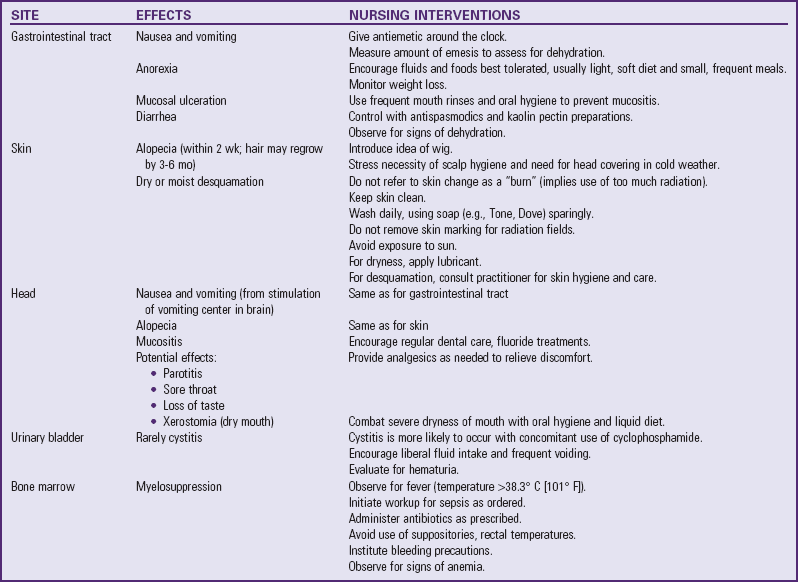
Radiotherapy is frequently used in the treatment of childhood cancer, usually in conjunction with chemotherapy or surgery. It can be used for curative purposes and for palliation to relieve symptoms by shrinking the size of the tumor. Recent advances in radiotherapy have optimized its beneficial effects and minimized many of the undesirable side effects, although high-dose irradiation is associated with many serious late effects.
Ionizing radiation is cytotoxic in at least three different ways: (1) damaging the pyrimidine bases cytosine, thymine, and uracil, needed for the synthesis of nucleic acids; (2) causing single-strand breaks in the DNA or RNA molecule; or (3) causing double helical–strand breaks in these molecules. The effect of disturbing cellular metabolic and reproductive functions is either sublethal or lethal damage. Lethal damage refers to the death of the cell. Sublethal damage refers to injured cells that may subsequently be repaired. Many of the acute side effects are the result of lethal damage to radiosensitive tissue, particularly proliferating cells such as those of the bone marrow, gastrointestinal tract, and hair follicles. Late effects are usually the result of cell death.
The acute untoward reactions from radiotherapy depend primarily on the area to be irradiated. Total-body irradiation is associated with the most severe reactions and is employed to prepare the immune system for bone marrow transplantation (BMT). Table 36-2 summarizes the acute effects of radiotherapy and nursing interventions that may be helpful in mitigating or preventing them. In limited areas of the country, proton beam radiation is available. Protons are positively charged subatomic particles. Protons deposit energy differently than x-ray beams. There is no “exit dose” beyond the tumor involved in proton radiotherapy. Therefore the potential benefit is in long-term effects to organs surrounding the target area. For example, some brain tumor patients receive radiation to the spine. With traditional forms of radiotherapy, long-term effect to nearby vital organs like the heart and lungs are possible. Although research on the potential beneficial effects of proton therapy is still in the early stages, theoretically these organs would not be affected with the use of proton therapy (Lee, Bilton, Famigletti, et al, 2005).
Biologic Response Modifiers
Biologic response modifiers (BRMs) modify the relationship between tumor and host by therapeutically changing the host’s biologic response to tumor cells. These agents or interventions may affect the host’s immunologic mechanisms (immunotherapy); have direct antitumor activity; or stimulate cell growth, reducing the hematologic toxicity associated with chemotherapy (Smith, 2011). Much of the current work in biotherapy is directed toward the use of monoclonal antibodies in the diagnosis and treatment of cancers. Through a complex process, special cells are fused to form a hybrid clone, or hybridoma, that produces antibodies that recognize a single specific antigen—hence the term monoclonal antibody (mono meaning one and clone meaning exact duplicate). These clones are then frozen, maintained in culture, or grown as tumors in mice to produce large quantities of the antibody in ascites fluid (Trahan, Green, and Murray, 2001). Although monoclonal antibodies have many prospective uses, their current role has been in diagnosing subclasses of leukemia cells to enhance understanding of which types of leukemia respond to different treatments and to determine whether the subclass is related to the prognosis. Researchers have also used monoclonal antibodies to deplete allogeneic bone marrow of T cells to reduce graft-versus-host disease (GVHD) and to selectively eliminate malignant cells from autologous marrow for transplanting back into the patient (Smith, 2011). Results from these studies have been encouraging, but further work is needed to define the role monoclonal antibodies and other BRMs will have in cancer care.
Bone Marrow Transplantation
Another approach to the treatment of childhood cancer is BMT. Candidates for transplantation are children who have malignancies that are unlikely to be cured by other means (see Family-Centered Care box). BMT allows for administration of lethal doses of chemotherapy, often combined with radiotherapy, to rid the body of all cancer cells (Locatelli, Giorgiani, Di-Cesare-Merlone, 2008). Once the body is free of malignant cells and the immune system is suppressed to prevent rejection of the transplanted marrow, the donor marrow or stem cells or the cells previously stored from the patient are given to the patient by IV transfusion. The newly transfused marrow or stem cells begin to produce functioning nonmalignant blood cells. In essence, the recipient accepts a new blood-forming organ.
The selection process for a suitable donor and the potential complications in transplantation are related to the human leukocyte antigen (HLA) system complex. Some of the major HLA antigens are A, B, C, D, and DR. There is a wide diversity for each of these HLA loci. For example, more than 20 different HLA-A antigens and more than 40 different HLA-B antigens can be inherited. The genes are inherited as a single unit, or haplotype. A child inherits one unit from each parent; thus a child and each parent have one identical and one nonidentical haplotype. Because the possible haplotype combinations among siblings follow the laws of mendelian genetics, there is a 1 in 4 chance that two siblings have two identical haplotypes and are perfectly matched at the HLA loci.
The importance of HLA matching is to prevent the serious complication of GVHD. Because the child’s immune system is essentially rendered nonfunctional, the recipient is unlikely to reject the bone marrow. However, the donor’s marrow may contain antigens not matched to the recipient’s antigens, which begin attacking body cells. The more closely the HLA systems match, the less likely GVHD is to develop. However, it can occur even with a perfect HLA match because of unidentified and thus unmatched histocompatibility antigens (Bollard, Krance, and Heslop, 2011).
Different types of BMT are now performed in children with cancer. Allogeneic BMT involves matching a histocompatible donor with the recipient. However, allogeneic BMT is limited by the presence of a suitable marrow donor. Because of the limited numbers of patients having HLA-identical siblings, other types of allogeneic transplants have been developed. Umbilical cord blood stem cell transplantation is a new source of hematopoietic stem cells for use in children with cancer (Rocha, Wagner, Sobocinski, et al, 2000). Because stem cells can be found with high frequency in the circulation of newborns, cord blood transplantation has become an alternative for some children (Rocha, Wagner, Sobocinski, et al, 2000). The benefit of using umbilical cord blood is the blood’s relative immunodeficiency at birth, allowing for partially matched, unrelated cord blood transplants to be successful, with a lower risk of GVHD-related problems (Frey, Guess, Allison, et al, 2009).
Autologous BMTs use the patient’s own marrow that was collected from disease-free tissue, frozen, and sometimes treated to remove malignant cells. Children with solid tumors such as neuroblastoma, Hodgkin disease, non-Hodgkin lymphoma (NHL), rhabdomyosarcoma, Ewing sarcoma, and Wilms tumor have been treated with autologous BMTs.
Peripheral stem cell transplants (PSCTs) are also used in children with cancer. PSCT, a type of autologous transplant, differs in the way stem cells are collected from the patient. Colony-stimulating factor (CSF) is first given to stimulate the production of many stem cells (Lanzkowsky, 2005; Matsubara, Makimoto, Takayama, et al, 2001). Once the white blood cell count is high enough, the stem cells are collected by an apheresis machine. This machine filters out peripheral stem cells from whole blood and returns the remainder of the blood cells and plasma to the child. Stem cells have been collected without problems in very small children weighing 20 kg (44 lb) or less (Sevilla, Gonzalez-Vicent, Madero, et al, 2002). The peripheral stem cells are then frozen until the patient is ready for the PSCT.
Complications of Therapy
Although tremendous advances have been achieved through current modes of cancer therapy, the successes are not without consequences. Numerous side effects are expected with chemotherapy and radiotherapy (see Nursing Care Plan, pp. 1470-1472). Other complications that are less common but generally more serious are described here.
Pediatric Oncologic Emergencies
Tumor Lysis Syndrome: Life-threatening conditions may develop in children with cancer as a result of the malignancy and or aggressive treatment modalities. Acute tumor lysis syndrome has hallmark metabolic abnormalities that are the direct result of rapid release of intracellular contents during the lysis of malignant cells. This typically occurs in patients with ALL or Burkitt lymphoma during the initial treatment period but may occur spontaneously before onset of therapy. Tumor lysis syndrome may also occur in other malignancies that have a large tumor burden, are very sensitive to chemotherapy, or have a rapid proliferative rate. The hallmark metabolic abnormalities of tumor lysis syndrome include hyperuricemia, hypocalcemia, hyperphosphatemia, hyperkalemia, and uremia. The crystallization of uric acid in the renal tubules can also lead to acute renal failure and death (Coiffier, Altman, Pui, et al, 2008).
Risk factors for development of tumor lysis syndrome include high white blood cell count at diagnosis, large tumor burden, sensitivity to chemotherapy, and high proliferative rate. In addition to the described metabolic abnormalities, children may develop a spectrum of clinical symptoms, including flank pain, lethargy, nausea and vomiting, oliguria, pruritus, tetany, and altered level of consciousness.
Management of tumor lysis syndrome consists of early identification of patients at risk, prophylactic measures, and early interventions. Patients at risk for tumor lysis syndrome should have serum chemistries and urine pH monitored frequently, strict record of intake and output, and aggressive IV fluids. Medications to reduce uric acid formation and promote excretion of by-products of purine metabolism, such as allopurinol, are often used. If tumor lysis syndrome occurs, IV hydration continues and the specific metabolic abnormalities are treated. Hyperuricemia is now effectively treated with recombinant urate oxidase, or rasburicase. This medication converts uric acid to allantoin, which is more soluble in urine. Exchange transfusions are sometimes necessary to reduce the metabolic consequences of massive tumor lysis, especially in children with a high tumor burden.
Hyperleukocytosis: Hyperleukocytosis, defined as a peripheral white blood cell count greater than 100,000/mm3, can lead to capillary obstruction, microinfarction, and organ dysfunction. Children experience respiratory distress and cyanosis. They also experience neurologic changes, including altered level of consciousness, visual disturbances, agitation, confusion, ataxia, and delirium. Management consists of rapid cytoreduction by chemotherapy, hydration, urinary alkalinization, and allopurinol. Leukophoresis or exchange transfusion may be necessary.
Superior Vena Cava Syndrome: Obstruction may create an oncologic emergency for a child with cancer. Space-occupying lesions located in the chest, especially from Hodgkin disease and NHL, may cause superior vena cava syndrome (SVCS) (compression of mediastinal structures), leading to airway compromise and potentially to respiratory failure. SVCS has also occurred with central venous catheters from the formation of a thrombus or a fibrotic reaction (McCloskey, 2002).
Children are initially seen with cyanosis of the face, neck, and upper chest; facial and upper extremity edema; and distended neck veins. They may have dyspnea from airway obstruction. Management consists of airway protection and alleviation of respiratory distress. Rapid treatment is initiated, and symptoms typically improve with as the disease is effectively treated.
Spinal Cord Compression: Different malignancies can invade or impinge on the spinal cord, causing acute symptoms of cord compression. Symptoms can include pain, sensation change, extremity weakness, loss of bowel and bladder function, and respiratory insufficiency. Children with primary CNS tumors can have tumors that originate or spread to the spinal cord. Other solid tumors, like neuroblastoma or rhabdomyosarcoma, can metastasize to the spinal cord and cause compression. Careful physical examination is essential in early detection of symptoms. Treatment may include corticosteroids to reduce associated edema and alleviate symptoms and rapid initiation of treatment such as emergent radiation or laminectomy if indicated.
Disseminated Intravascular Coagulation: Overwhelming infections in the immunocompromised child constitute an emergency situation. Gram-negative sepsis can result in numerous complications, including disseminated intravascular coagulation (DIC), created by bacteria or fungus causing damage to the endothelial system. Life-threatening hemorrhage can occur from DIC in combination with thrombocytopenia (platelet count of 20,000/mm3) and leukocytosis (leukocyte count of 100,000/mm3). Leukocytosis can cause intracranial bleeding from increased viscosity of the blood. The resulting leukocytosis leads to vascular damage and subsequent hemorrhage.
Nursing Care Management
This section presents an overview of general nursing concepts that apply to most childhood cancers. Specific nursing care for children with a particular type of cancer is discussed under each disease section later in this chapter. This discussion focuses on the physical aspects of care (see Nursing Care Plan). Chapter 22 (chronic illness) and in Chapter 23 (terminal illness) present the emotional aspects.
Signs and Symptoms of Cancer in Children
![]() Early detection is critical to early treatment and eventual cure. Cancers in children are often difficult to recognize. Therefore being alert to the persistence of unusual symptoms is essential (see Box 36-1). This chapter discusses some of the more significant clues to pediatric cancer.
Early detection is critical to early treatment and eventual cure. Cancers in children are often difficult to recognize. Therefore being alert to the persistence of unusual symptoms is essential (see Box 36-1). This chapter discusses some of the more significant clues to pediatric cancer.
![]() Nursing Care Plan—The Child with Cancer
Nursing Care Plan—The Child with Cancer
Pain may be an early or late initial sign of cancer and requires a careful history of its onset, characteristics, location, intensity, and alleviating factors. Pain may be generalized or present at a specific location. For example, bone pain occurs in approximately 20% of children with leukemia. Pain, swelling, and tenderness at the tumor site may be the initial sign in bone tumors.
Fever is a frequent occurrence during childhood and is caused by numerous illnesses, including cancer. The major cause of fever in cancer patients is infection, especially related to neutropenia. The malignant process itself can also cause fever. This is often referred to as tumor-associated fever. The exact mechanism as to how the malignancy causes a fever is not known. There are multiple theories, including the release of pyrogens or toxins by the tumor or the hypersensitivity reaction response to tumors that activates other body cells to release pyrogens.
A careful skin assessment will reveal signs and symptoms of a low platelet count. Ecchymosis and petechiae are most commonly found on the child’s extremities, and gum or nose bleeding may occur when the platelet count falls below 20,000/mm3.
The child with malignant invasion of the bone marrow often appears pale, with symptoms of lethargy, weight loss, and generalized malaise. These symptoms may be attributed to anemia caused by the replacement of normal cells with malignant cells in the bone marrow. The nurse should assess for signs and symptoms of anemia. (See Chapter 35.)
An abdominal mass is a typical finding in children with Wilms tumor and neuroblastoma. An abdominal mass in a child must be evaluated for a malignancy.
Swollen lymph glands are another common finding in children. However, enlarged, firm, lymph nodes in a child with fever for more than 1 week, a recent history of weight loss, or an abnormal chest x-ray film may indicate a serious disease and should be evaluated further.
The presence of a white reflection as opposed to the normal red pupillary reflex in the pupil of a child’s eye is the classic sign of retinoblastoma. Squinting, strabismus, or swelling can indicate other solid tumors of the eye.
The child with a brain tumor develops signs and symptoms according to the exact area of the brain involved. The nurse must perform a thorough assessment to identify the specific area of tumor involvement (see Table 36-4).
Managing Side Effects of Treatment
Cancer care encompasses more than treatments aimed at eliminating the malignant cells. Because of the delicate balance between killing malignant cells and preserving functional cells, supportive therapy is frequently needed during those times that serious damage occurs to normal body tissues.
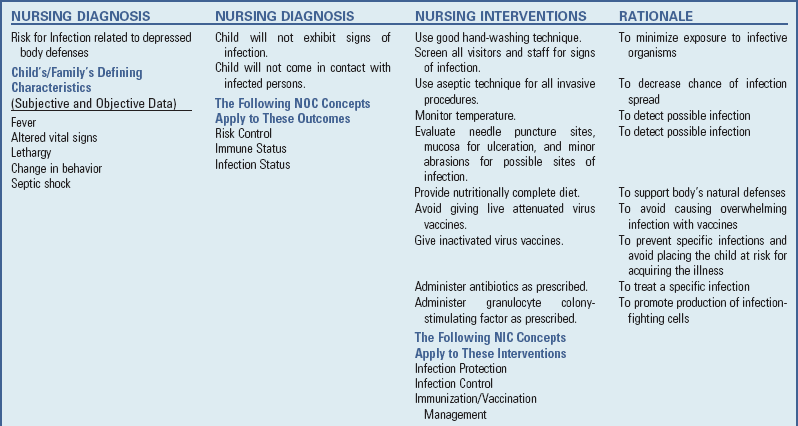
Infection
A major concern for the child receiving treatment for cancer is the risk for the development of complications secondary to the treatment. Major complications include fever, bleeding, and anemia.
The nurse caring for the child with fever must be aware of the signs and symptoms of septic shock, as discussed in Chapter 29. The child with fever who has an absolute neutrophil count (ANC) lower than 500/mm3 is at risk for (see Nursing Care Guidelines box):
The child with fever is evaluated for potential sites of infection, such as from a needle puncture, mucosal ulceration, minor abrasion, or skin tears (e.g., a hangnail). Although the body may not be able to produce an adequate inflammatory response to the infection and the usual clinical signs of infection may be partially expressed or absent, fever will occur. Therefore monitor the temperature closely. To identify the source of infection, the health care team takes blood, stool, urine, and nasopharyngeal cultures and chest x-ray films.
Once infection is suspected, broad-spectrum IV antibiotic therapy is begun before the organism is identified and may be continued for 7 to 10 days. If the child does not have a venous access device, a heparin lock should be inserted to prevent the inconvenience of multiple venipunctures in maintaining a patent IV line and to avoid limitations in activity caused by the IV line.
The organisms most lethal to these children are (1) viruses, particularly varicella (chickenpox), herpes zoster, herpes simplex, measles, rubella, mumps, and poliomyelitis; (2) Pneumocystis carinii (a protozoan); (3) fungi, especially Candida albicans; (4) gram-negative bacteria, such as Pseudomonas aeruginosa, E. coli, and Proteus and Klebsiella organisms; and (5) gram-positive bacteria, especially Staphylococcus aureus, Staphylococcus epidermidis, and group A β-hemolytic streptococci (Quadri and Brown, 2000). As prophylaxis against these various organisms, broad-spectrum antibiotics are usually prescribed. Ensuring compliance with this long-term regimen is an important nursing responsibility.
Prophylaxis against P. carinii is routinely given to most children during treatment for cancer (American Academy of Pediatrics, 2006). Trimethoprim-sulfamethoxazole (Bactrim, Septra) is usually given three times a week during treatment.
CSFs, a family of glycoprotein hormones that regulate the reproduction, maturation, and function of blood cells, are now routinely used as supportive measures to prevent the side effects caused by low blood counts. CSFs promote stem cell proliferation and stimulate a more rapid maturation of the cells, allowing them to enter the bloodstream earlier. G-CSF (filgrastim [Neupogen], pegfilgrastim [Neulasta]) directs granulocyte development and can decrease the duration of neutropenia following immunosuppressive therapy. This reduces the incidence and duration of infection in children receiving treatment for cancer. G-CSF is also being used to decrease the bone marrow recovery time after BMT (Matsubara, Makimoto, Takayama, et al, 2001). G-CSF is usually administered intravenously or subcutaneously 24 hours after chemotherapy is discontinued and is given for 10 to 14 days. G-CSF is discontinued when the ANC surpasses 10,000/mm3. The pegylated or long-acting form of G-CSF, pegfilgrastim, is given only once after completion of therapy and typically has its peak efficacy (highest white blood cell count) about 8 to 10 days after administration. During G-CSF therapy, children may experience bone pain, fever, rash, malaise, and headaches.
Prevention of infection continues as a priority after discharge from the hospital. Some institutions allow the child to return to school when the ANC is above 500/mm3. Other institutions place no restrictions on the child, regardless of the blood count. If the level falls below this value, cautious isolation from crowded areas, such as shopping centers or subways, is advisable. At all times, encourage family members to practice good hand washing to avoid introducing pathogens into the home (see Critical Thinking Exercise).
Hemorrhage
Before the use of transfused platelets, hemorrhage was a leading cause of death in children with some types of cancer. Now most bleeding episodes can be prevented or controlled with judicious administration of platelet concentrates or platelet-rich plasma. Severe spontaneous internal hemorrhage varies, but usually does not occur until the platelet count is 10,000/mm3 or less (Hockenberry and Kline, 2011; Rossetto and McMahon, 2000).
Because infection increases the tendency toward hemorrhage, and because bleeding sites become more easily infected, take special care to avoid performing skin punctures whenever possible. When performing finger sticks, venipunctures, intramuscular injections, and bone marrow tests, employ aseptic technique with continued observation for bleeding. Meticulous mouth care is essential, since gingival bleeding with resultant mucositis is a frequent problem. Because the rectal area is prone to ulceration from various drugs, hygiene is essential. To prevent additional trauma, avoid rectal temperatures and suppositories. Frequent turning and the use of a pressure-reducing mattress under bony prominences prevent development of pressure sores and decubital ulcers.
Platelet transfusions are generally reserved for active bleeding episodes that do not respond to local treatment and that may occur during induction or relapse therapy. Epistaxis and gingival bleeding are the most common. The nurse teaches parents and other children measures to control nosebleeding. Applying pressure at the site without disturbing clot formation is the general rule. Two of the problems with multiple platelet transfusions are the risk of febrile reactions and decreased life span of the platelets. Platelet concentrates normally do not have to be cross-matched for blood group or type. However, because platelets contain specific antigen components similar to blood group factors, children who receive multiple transfusions may become sensitized to a platelet group other than their own. Therefore platelets are cross-matched with the donor’s blood components whenever possible.
Transfused platelets generally survive in the body for 1 to 3 days. The peak effect is reached in about 2 hours and decreased by half in 24 hours. Therefore, after a transfusion, the nurse observes and records the approximate time when hemostasis of bleeding sites occurs. Delayed hemostasis is evidence of platelet destruction. For long-term patients, multiple transfusion therapy becomes progressively less effective.
During bleeding episodes the parents and child need much emotional support (see Critical Thinking Exercise). The sight of oozing blood is upsetting. Often parents request a platelet transfusion, unaware of the necessity of trying local measures first. The nurse can help calm their anxiety by explaining the reason for delaying a platelet transfusion until absolutely necessary. Because compatible donors decrease the risk of antigen formation in the recipient, the nurse should encourage parents to locate suitable donors for eventual blood use.
Children at home who have low platelet counts (usually <100,000/mm3) should avoid activities that might cause injury or bleeding, such as riding bicycles or skateboards, roller skating or in-line skating, climbing trees or playground equipment, and contact sports such as football or soccer. Once the platelet count rises, these restrictions are not necessary. In addition, aspirin and aspirin-containing products are not used; for mild pain or significantly elevated temperature, acetaminophen is substituted.
Anemia
Initially anemia may be profound from complete replacement of the bone marrow by cancer cells. During induction therapy, blood transfusions with packed red blood cells may be necessary to raise the hemoglobin to levels approaching 10 g/dl. The usual precautions in caring for the child are instituted. (See Chapter 35.)
Anemia is also a consequence of drug-induced myelosuppression. Although not as severely affected as the white blood cells, erythrocyte production may be delayed. Because children have an amazing capacity to withstand low hemoglobin levels, the best approach is to allow the child to regulate activity with reasonable adult supervision. It may be necessary for the parents to alert the schoolteacher to the child’s physical limitations, particularly in terms of strenuous activity.
Nausea and Vomiting
The nausea and vomiting that occur shortly after administration of several of the drugs and as a result of cranial or abdominal irradiation can be profound. 5-Hydroxytryptamine-3 receptor antagonists are the antiemetics of choice to manage nausea and vomiting caused by chemotherapy and radiotherapy (Culy, Bhana, and Plosker, 2001). The advantage of these agents over conventional drugs is that they produce no extrapyramidal side effects, such as difficulty speaking or swallowing, shuffle walk, slow movements, trembling, stiffness of the arms and legs, or loss of balance. Multiple studies have shown ondansetron (Zofran) to be effective for patients receiving cisplatin, cyclophosphamide, ifosfamide, and anthracyclines (Anastasia, 2000). Ondansetron in combination with dexamethasone has been more effective than ondansetron alone (Culy, Bhana, and Plosker, 2001) and has been superior to metoclopramide (Reglan) for cisplatin-induced emesis (Culy, Bhana, and Plosker, 2001; American Society of Health-System Pharmacists, 1999).
For mild to moderate vomiting, phenothiazine-type drugs remain the mainstay of therapy. Promethazine (Phenergan), chlorpromazine (Thorazine), prochlorperazine (Compazine), or trimethobenzamide (Tigan) may be effective agents. Metoclopramide is a more effective antiemetic for severe vomiting. Unfortunately, the drug causes a number of side effects in children, particularly extrapyramidal reactions, such as muscle tremors or twitching, agitation, grimacing, dysarthria, and oculogyric crisis (fixation of eyes in one position for minutes or hours). Metoclopramide should be administered with dexamethasone or diphenhydramine (Benadryl) (Krane, Casillas, and Zeltzer, 2011).
Synthetic cannabinoids are now being used in children undergoing chemotherapy. A drug that has yielded promising results is tetrahydrocannabinol, or dronabinol. Dronabinol helps control nausea and vomiting and also is an effective appetite stimulant (Lohr, 2008).
The most beneficial regimen for antiemetic control has been the administration of the antiemetic before the chemotherapy begins (30 minutes to 1 hour before) and regular (not as-needed) administration every 2, 4, or 6 hours for at least 24 hours after chemotherapy. The goal is to prevent the child from ever experiencing nausea or vomiting, since this can prevent the development of anticipatory symptoms (the conditioned response of developing nausea and vomiting before receiving the drug). Other nonpharmacologic interventions (similar to those discussed for pain management in Chapter 7) can be useful in controlling posttherapy and anticipatory nausea and vomiting. Giving the antineoplastic drug with a mild sedative at bedtime is also helpful for some children, and there is evidence that nighttime administration of drugs such as methotrexate and 6-mercaptopurine may be more effective cytotoxically than morning administration.
Altered Nutrition
Altered nutrition is a common side effect of treatment. Continued assessment of the child’s nutritional status must occur throughout treatment. Record regular evaluation of the child’s intake. The child’s height, weight, and head circumference (for children <3 years of age) must be measured routinely during visits to the hospital or clinic. When appropriate, monitor energy reserves, evaluated by skinfold measurements. Biochemical assays may be helpful in some children and include serum prealbumin, transferrin, and albumin (Han-Markey, 2000; Nitenberg and Raynard, 2000). Box 36-2 lists criteria for nutrition intervention in children with cancer.
Nutritional status is important to maintain because it compromised nutritional status can contribute to reduced tolerance to treatment, altered metabolism of chemotherapy drugs, prolonged episodes of neutropenia, and increased risk for infection.
Supportive nutrition measures include oral supplements with high-protein and high-calorie foods. Ways to increase calories include substituting cream for milk, adding tofu (high in protein) to most meals, and serving full-fat yogurt and ice cream instead on nonfat or low-fat items. Cooking with butter; putting sugar on cereal; and making high-calorie snacks such as trail mix, peanut butter, or dried fruit readily available for the child are other ways to increase calories. Enteral feeding may be necessary when children are unable to maintain the necessary calories to prevent weight loss. The use of parenteral hyperalimentation is used most frequently for children who have digestive problems, after surgery, or with BMT. Chapter 27 discusses these interventions in more detail.
Despite such approaches, some children still do not eat. Theories to explain persistent anorexia include that it is (1) a physical effect related to the cancer that is nonspecific; (2) a conditioned aversion to food from nausea and vomiting during treatment; (3) a response to stress in the environment, related to eating or to the child’s condition; (4) a result of depression; (5) a control mechanism when so much else has been imposed on the child; and (6) an opportunity to express anger at parents and punish them for “allowing” the child to become sick. When loss of appetite and weight persists, the nurse should investigate the family situation to determine whether any of these variables are contributing to the problem.
Mucosal Ulceration
One of the most distressing side effects of several drugs is gastrointestinal mucosal cell damage, which results in ulcers anywhere along the alimentary tract. Oral ulcers (stomatitis) are red, eroded, painful areas in the mouth or pharynx. (See Stomatitis, Chapter 16.) Similar lesions may extend along the esophagus and occur in the rectal area. They greatly compound anorexia because eating is extremely uncomfortable.
When oral ulcers develop, the following interventions are helpful: (1) a bland, moist, soft diet; (2) use of a soft sponge toothbrush (Toothette) or cotton-tipped applicator; (3) frequent mouth rinses with normal saline (using a solution of 1 tsp of table salt and 1 pint of water) or sodium bicarbonate and salt mouth rinses (using a solution of 1 tsp of baking soda and 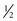 tsp of table salt in 1 quart of water); and (4) local anesthetics without alcohol, such as a solution of diphenhydramine and Maalox (aluminum and magnesium hydroxide) or UlcerEase (Velez, Tamara, and Mintz, 2004; Scully, Epstein, and Sonis, 2004). Although local anesthetics are effective in temporarily relieving the pain, many children dislike the taste and numb feeling they produce.
tsp of table salt in 1 quart of water); and (4) local anesthetics without alcohol, such as a solution of diphenhydramine and Maalox (aluminum and magnesium hydroxide) or UlcerEase (Velez, Tamara, and Mintz, 2004; Scully, Epstein, and Sonis, 2004). Although local anesthetics are effective in temporarily relieving the pain, many children dislike the taste and numb feeling they produce.
Oral preparations used to prevent or treat mucositis include UlcerEase, which is used to soothe mucositis and gum irritations; chlorhexidine gluconate (Peridex) is effective against candidal and bacterial infections (Velez, Tamara, and Mintz, 2004; Scully, Epstein, and Sonis, 2004). Antifungal troches (lozenges) or mouth rinse is typically used prophylactically in patients with myelosuppression, especially for children who have undergone BMT.
Administering mouth care is particularly difficult in infants and toddlers. A satisfactory method of cleaning the gums is to wrap a piece of gauze around a finger; soak it in saline or plain water; and swab the gums, palate, and inner cheek surfaces with the finger. Mouth rinses are best accomplished with plain water or saline because the child cannot gargle or spit out excess fluid. Children should perform mouth care routinely before and after any feeding and as often as every 2 to 4 hours to rid mucosal surfaces of debris, which becomes an excellent medium for bacterial and fungal growth.
Dental hygiene can become a serious problem if the child wears an orthodontic appliance. The accumulated debris on braces is difficult to remove without vigorous brushing, and the appliance itself traumatizes the gums. Sometimes braces are removed during chemotherapy.
Difficulty eating is a major problem with stomatitis and may warrant hospitalization if the child refuses fluids. The child usually chooses the foods that are best tolerated. Surprisingly, some children prefer salty foods to bland ones. Drinking can usually be encouraged if a straw is used to bypass the ulcerated oral mucosa. The nurse should encourage parents to relax any eating pressures because the anorexia accompanying stomatitis is well justified. In addition, because it is a temporary condition, once the ulcers heal, the child can resume good food habits. Ordinarily, severe mucosal ulceration indicates a need for decreased chemotherapy until complete healing takes place, usually within a week. Analgesics, including opioids, may be needed when treatment cannot be altered, such as during BMT.
If rectal ulcers develop, meticulous toilet hygiene, warm sitz baths after each bowel movement, and an occlusive ointment applied to the ulcerated area promote healing; the use of stool softeners is necessary to prevent further discomfort. Parents should record bowel movements because the child may voluntarily avoid defecation to prevent discomfort. Rectal temperatures and suppositories are avoided because they may further traumatize the affected area.
Neurologic Problems
Vincristine, and to a lesser extent vinblastine, can cause various neurotoxic effects, one of the more common of which is severe constipation from decreased bowel innervation. Opioids further aggravate constipation. The nurse advises parents to record bowel movements and to notify the practitioner of a change in stool habits. Physical activity and stool softeners are helpful in preventing the problem, but laxatives, such as MiraLax, or enemas are often necessary to stimulate evacuation. Dietary changes such as increased fiber may not be effective, since the increased bulk tends to increase fecal distention and discomfort without producing the necessary mechanical stimulation.
Footdrop and weakness and numbness of the extremities may cause difficulty in walking or in fine hand movement. The nurse should look for these problems and warn parents of these side effects, which are reversible once the drug is stopped. If the child is on bed rest, a footboard is used to preserve proper alignment. If weakness occurs while the child is attending school, a temporary alteration of activity may be necessary. Parents should inform the teacher of the situation to avoid unrealistic expectations of the child’s abilities.
Another side effect that can be severe is jaw pain. Analgesics may help relieve the discomfort. Children may avoid movement by not talking or chewing, although continuous chewing, such as with gum, may actually reduce the pain. Because the pain is temporary, usually lasting for a day or two, the child can be given fluids through a straw.
A neurologic syndrome, postirradiation somnolence, may develop 5 to 8 weeks after CNS irradiation and last for 4 to 15 days. It is characterized by somnolence with or without fever, anorexia, and nausea and vomiting. Parents should be warned of the possibility of such symptoms and encouraged to seek medical evaluation, since somnolence may be an early indicator of long-term neurologic sequelae after cranial irradiation.
Hemorrhagic Cystitis
Sterile hemorrhagic cystitis is a side effect of chemical irritation to the bladder from chemotherapy or radiotherapy. It can be prevented by (1) a liberal oral or parenteral fluid intake (at least one and a half times the recommended daily fluid requirement [2 L/m2/day]); (2) frequent voiding immediately after feeling the urge, including immediately before bed and after arising (may include one nighttime void); (3) administration of the drug early in the day to allow for sufficient fluids and frequent voiding; and (4) administration of mesna, a drug that inhibits the urotoxicity of cyclophosphamide and ifosfamide (Lanzkowsky, 2005).
In most cases IV fluids are given before, during, and after the drug to ensure adequate hydration, thereby eliminating the need for the child’s drinking large amounts of fluid. If oral home administration is prescribed, the family needs specific instructions on exactly how much fluid the child must have.
Alopecia
Hair loss is a side effect of several chemotherapeutic drugs and cranial irradiation. Not all children lose their hair during drug therapy. However, retaining hair is the exception rather than the rule. It is better to warn children and parents of this side effect than to allow them to think it is only a remote possibility.
The family should know that the hair falls out in clumps, causing patchy baldness. To lessen the trauma of seeing large amounts of hair on bed linen or clothing, the child can wear a disposable surgical cap to collect the shed hair during the period of greatest hair loss, or the hair can be cut short. Families should also be aware that wigs are tax deductible and that hair regrows in 3 to 6 months. The hair frequently is darker, thicker, and curlier than before.
If the child chooses not to wear a wig, attention to some type of head covering is important, especially in cold or sunny climates. Scalp hygiene is also important. The scalp should be washed regularly as with any other body part.
Many children demonstrate increased tolerance to hair loss on reinduction therapy. Rather than complete baldness, the child may experience thinning of the hair. If the hair is cut short, kept clean, and blow-dried with an electric hair drier, it usually can look full enough to make a wig unnecessary. This can be a tremendous psychologic boost to the child who is already depressed about learning of a relapse and the need for additional chemotherapy.
Steroid Effects
Short-term steroid therapy produces no acute toxicities and often results in two beneficial reactions: increased appetite and a sense of well-being. However, it does produce physical changes and alterations in body image, which, although not clinically significant, can be extremely distressing to older children. One of these is cushingoid appearance. The child’s face becomes rounded and puffy. (See Fig. 38-5.) Unlike hair loss, little can be done to camouflage this obvious change, although careful avoidance of salt and salt-containing foods can help reduce fluid accumulation. It is not unusual for other children to make fun of the child. It is helpful to reassure the child that, after cessation of the drug, the facial contours will return to normal. If the child resumes activity early in the course of treatment, the change may be less noticeable to peers than after a long absence. Also, the use of loose-fitting clothes, such as warm-up outfits, can help camouflage the change in weight.
In contrast, parents may appreciate the full, rounded appearance because it simulates the look of a well-nourished, healthy child. Because of their own needs, they may be less able to understand the child’s misery over altered body image. The nurse can foster a better understanding between the parents and child by encouraging both parties to openly discuss their feelings.
Children receiving steroid therapy do look healthy. The moon face, red cheeks, supraclavicular fat pads, protuberant abdomen, and fluid retention indicate weight gain. However, the actual weight gain resulting from increased muscle mass and subcutaneous tissue may be small. Therefore the nurse should evaluate weight gain by observing the extremities and measuring skinfold thickness and arm circumference during steroid therapy to determine whether the weight gain is a result of increased dietary intake.
Shortly after beginning steroid therapy, children may experience a number of mood changes, which range from feelings of well-being and euphoria to depression and irritability. If parents are unaware of these drug-induced changes, they may become unduly concerned. Therefore the nurse should warn them of the reactions and encourage them to discuss the behavioral changes with each other and the child.
Nursing Care During Bone Marrow Transplantation
Many of the side effects previously discussed occur in the child undergoing BMT. However, because of the aggressive preconditioning programs used to remove the marrow and the use of growth factors to promote engraftment of transplanted stem cells, these children are usually hospitalized for several weeks after BMT. Because of the risk of infection, the unit may employ such measures as strict hand washing, screening visitors, laminar airflow rooms, and institutional isolation policies.
BMT patients must have numerous procedures performed, such as the insertion of a venous access device, administration of intensive chemotherapy and irradiation, and continued meticulous personal hygiene. During the period after transplantation and before the new marrow begins adequately replacing granulocytes, the child is extremely susceptible to infection. Interstitial or nonbacterial pneumonia is another serious complication with a high mortality rate.
However, the most common complication in allogeneic transplants is GVHD, which can affect the skin, gastrointestinal tract, liver, heart, lungs, lymphoid tissue, and marrow. GVHD is characterized by a hardening of the tissues and drying of the mucous membranes. The severity of the manifestations varies, but once vital organs are affected, death can ensue. Treatment involves the use of steroids or azathioprine (Imuran). However, these immunosuppressive drugs further increase the risk of infection. All blood products should be irradiated to minimize the introduction of additional antigens.
Another unfortunate posttransplant possibility is recurrence of the malignancy after engraftment. Emphasis is now placed on the prevention of GVHD, using various agents such as cyclosporine, methotrexate, and steroids (Bollard, Krance, and Heslop, 2011).
Skin breakdown and delayed wound healing frequently occur in the patient undergoing BMT. Preventive interventions to minimize pressure on dependent areas of the skin include the use of pressure-relieving or pressure-reducing beds or mattresses and frequent turning. Measures to promote healing when breakdown occurs include frequent sitz baths for the perianal area; transparent dressings, such as Tegaderm, over bony prominences; and protective skin barriers, such as hydrocolloid dressings or occlusive ointments. Throughout this long ordeal the family is concerned about successful engraftment and fears fatal complications. Consequently nurses need to provide sensitive care and maintain a supportive attitude during the many crises that may arise. If the procedure is not successful, the care needed by these families is consistent with that required by the family of any child with a life-threatening disorder. (See Chapter 23.)
Preparation for Procedures
Children in particular need psychologic preparation for the various treatment modalities, which often involve surgery, IV injections, bone marrow aspiration, and LP. The diagnostic procedures initially employed to confirm the diagnosis and those that are repeated to monitor treatment are often a source of discomfort and stress to the child and family. Even noninvasive procedures such as imaging and radiologic tests are frightening to a young child. Many of these tests require the child to lie absolutely motionless for a prolonged time in a confined space with little or no communication with a supportive adult. Consequently infants and young children are usually sedated, and older children need an explanation of what to expect and reminders during the test of how much longer they must remain still. The same principles for preparing children for procedures that are discussed in Chapter 27 apply here, including the option of having parents stay with the child whenever possible. (See Nursing Care Guidelines box, p. 1001.) Children who undergo repeated tests need additional preparation and emotional support to decrease their stress.
Two procedures, bone marrow studies and LPs, are so commonly performed in many types of childhood cancer that they deserve special consideration in preparing children (Fig. 36-2). Both tests can be frightening to children because they are done behind the child’s field of vision. Professionals caring for children with cancer recommend the use of sedation for the initial procedures and subsequent developmentally appropriate support using both pharmacologic and nonpharmacologic approaches. (See Chapter 27).
Lidocaine 4% preparations adequately penetrate intact skin and are used as a local anesthetic before intrusive procedures, including venipunctures, implanted port access, LPs, and subcutaneous or intramuscular injections of growth factors or other drugs (Hockenberry and Kline, 2011). (See Fig. 27-7.) Local intradermal anesthesia is frequently used for LP and bone marrow examination. To reduce the stinging sensation from lidocaine, sodium bicarbonate should be added. (See Pain Management, Chapter 7.) Deeper infiltration of the muscle and periosteum of the bone with buffered lidocaine further reduces the pain from the large-bore aspiration or biopsy needle entering the bone.
For bone marrow studies, LPs, and other procedures, children of preschool age and beyond should be prepared beforehand. If this is not possible, the nurse should explain each step of the procedure as it occurs, stressing what will be done and what it will feel like. If each step is explained beforehand, having the child recall the next step during the procedure can be a distraction mechanism.
Physical care after the procedures is minimum. A small pressure bandage is applied to the bone marrow puncture site, and an adhesive bandage is applied to the LP site. No activity restriction is necessary after the bone marrow test, although the site is usually sore and the child may prefer to remain quiet. Recommendations after LP vary. If medication was instilled, the child may be placed in a slight Trendelenburg position to facilitate circulation of the medicated spinal fluid.
Pain Management
Nurses must be knowledgeable about the basic pathophysiology of cancer pain and treatment-related side effects. The World Health Organization’s three-step analgesic pain ladder should be incorporated into the approach to pain management for every child with cancer (McMain, 2008; Hellsten, 2000). Nurses must acquire extensive knowledge of nonopioid and opioid analgesics used in pediatric pain management. (See Chapter 7.) Interdisciplinary pain management teams are used in many pediatric cancer centers. These teams serve as consultants and provide expertise in the assessment and management of pain. The nurse often serves as the coordinator of care, playing a key role in cancer pain management.
Chapter 7 discusses pharmacologic management of disease-related pain, which involves a variety of methods. It may take more than a trial of one type of medication to find the appropriate agent to manage a patient’s pain. The route of administration must be considered as well. Providing “pain relief” by administering painful intramuscular injections, as an alternative to the IV route, is not appropriate therapy because many oral preparations are now available with comparable efficacy. Nonsteroidal antiinflammatory drugs (NSAIDs), acetaminophen with codeine, oxycodone, and morphine are commonly used agents in the management of disease-related pain (Kumar, Rajagopal, and Naseema, 2000). All are available in the oral form, and morphine and the NSAID ketorolac (Toradol) are available as IV preparations. Appropriate dosing is imperative. Doses are titrated to increase the amount of analgesia and minimize side effects.
Health Promotion
Children with cancer require the same basic health supervision as any child. Sometimes the overwhelming needs and demands placed on the family, coupled with the singular concern focused on the cancer by both family and practitioners, result in a lack of attention to normal health care needs. Nurses should monitor the type of primary care the child receives, using as a guideline recommendations for health supervision. Areas of particular concern are growth, physical and cognitive development, and neurologic status. Two other areas are also important: (1) dental care because of potential side effects from treatment, and (2) immunizations because of concern with live virus vaccines and immunosuppression.
Dental Care
Irradiation to the head and neck can cause a number of late complications (Armenian, Meadows, and Bhatia, 2011). Some are irreversible, such as facial asymmetry, but those affecting the teeth and gums (caries, periodontal disease) benefit from excellent oral hygiene, including regular use of systemic and topical fluoride. (See Dental Health, Chapter 14.) There is also evidence of delayed or absent development of the permanent teeth (Armenian, Meadows, and Bhatia, 2011; Oeffinger, Eshelman, Tomlinson, et al, 2000). Depending on the child’s age, this can be a source of acute psychologic distress, especially during early school-age years, when “losing a tooth” is a status symbol. Children need to be aware of this possibility and need help to explain the delay to peers.
Daily toothbrushing and flossing are encouraged in children with granulocyte counts in excess of 500/mm3 and platelet counts above 40,000/mm3. Fluoride rinses are used as discussed in Chapter 14. Oral hygiene for children whose counts are below these parameters is limited to wiping the teeth with moistened gauze sponges or Toothettes.
Immunizations
Viral replication after the administration of live vaccine for polio, measles, rubella, and mumps can cause serious disease in immunocompromised children. The child receiving chemotherapy for cancer should not receive live, attenuated vaccines. Inactivated vaccines can be given to immunosuppressed children, but the immune response is likely to be suboptimum, so delaying vaccinations is usually recommended (American Academy of Pediatrics, 2006). Children who are immunosuppressed should not receive the varicella vaccine (American Academy of Pediatrics, 2006). Siblings and other family members can receive the live measles, mumps, and rubella vaccine and the varicella vaccine without risk to the child who is immunosuppressed.
An important indication for isolation is an outbreak of childhood disease, especially chickenpox. Ideally the school nurse should work with the treating practitioner to decide the optimum time for school reattendance. If the child has been exposed to the varicella virus, varicella-zoster immune globulin given within 72 hours may favorably alter the course of the disease, or antiviral agents, such as acyclovir, may be given if the child develops varicella. These antiviral agents are effective in preventing serious disease if given during the first 3 days of the appearance of symptoms (American Academy of Pediatrics, 2006). Without treatment, death from disseminated varicella (about 7%) is usually caused by pneumonia; other serious although nonfatal complications include hepatitis, pancreatitis, meningitis, and bacterial skin infections. (See also Immunizations, Chapter 12.)
Family Education
Nurses working with children who have cancer have a significant supportive role in helping the family understand the various therapies, preventing or managing expected side effects or toxicities, and observing for late effects of treatment. Education is a constant feature of the nursing role, especially in terms of new treatments, clinical trials, and home care. Because of the anxiety generated by the diagnosis of cancer, some families may resort to unproven methods of treatment. These unorthodox approaches may produce unnecessary harm by themselves or, if benign, render injury because other proven modes of therapy are avoided. In many instances this causes financial burden and emotional strife among family members.
Nurses are instrumental in helping families avoid the trap of seeking unproven and potentially unsafe “remedies” by encouraging the families to discuss concerns and questions openly with their health care provider. Some families feel pressure from well meaning friends and relatives to “leave no stone unturned” or “try everything.” Communicating openly and effectively with families about the diagnosis and forms of therapy and discussing alternative therapies is essential to building a trusting relationship with a patient and his or her family. This will help ensure that the family discusses any possible treatments they are considering, and their physician can guide them regarding the efficacy and safety. Nurses must be fortified with knowledge to substantiate present treatment protocols and to discredit unauthorized methods. The American Cancer Society and local and state medical societies are reliable sources of information concerning research on investigational versus quack methods of cancer therapy. The Association of Pediatric Hematology/Oncology Nurses* has developed numerous educational materials for family and child teaching. The American Childhood Cancer Organization† is an international organization providing support, education, and advocacy programs for children with cancer and their families.
Instruction regarding home care frequently involves teaching about medication schedules, observing for side effects or toxicities that require further evaluation, taking measures to prevent or manage these problems, and caring for special devices such as central venous catheters.‡ Compliance is an important issue, since poor adherence to drug regimens can result in a relapse. Every effort must be made to ensure that the family understands the importance of adhering to the prescribed treatment schedule and measures to improve compliance. (See Chapter 27.)
Cessation of Therapy
Care does not end when the child completes therapy. With the increasing awareness of late effects, nurses play an important role in the assessment of the child for problems such as delayed growth, secondary malignancies, and disturbances in any body system. The family needs to be aware of the importance of continued medical supervision. Other health care professionals caring for the child, such as school nurses, family physicians, and dentists, should be informed of the child’s cancer diagnosis. As children reach adulthood, they may benefit from genetic counseling regarding cancers that are likely to be inherited. If the possibility of sterility exists, pretreatment sperm banking may be offered to adolescent boys, which allows additional options regarding family planning in adulthood (see Nursing Care Plan, pp. 1470-1472). The Children’s Oncology Group in collaboration with the American Academy of Pediatrics (2009) has developed guidelines for long term follow-up care for pediatric cancer survivors. Nurses involved with these children should be familiar with these guidelines and use all opportunities to teach patients and families regarding needed continued care.
Cancers of the Blood and Lymph Systems
Acute Leukemias
Leukemia is a broad term given to a group of malignant diseases of the bone marrow and lymphatic system. It is a complex disease of varying heterogeneity. Consequently classification has become increasingly complex, sophisticated, and essential because identification of the subtype of leukemia has therapeutic and prognostic implications. The following is an overview of the major classification systems currently used.
Morphology: In children, two forms are generally recognized: acute lymphoblastic leukemia (ALL) and acute myelogenous leukemia (AML). Synonyms for ALL include lymphatic, lymphocytic, lymphoid, and lymphoblastoid leukemia.
ALL is the most common form of childhood cancer. The annual incidence is 3 or 4 cases per 100,000 Caucasian children (Margolin, Rabin, Steuber, et al, 2011). It occurs more frequently in boys than in girls and in Caucasians than in African-Americans. The peak onset is between 2 and 5 years of age. It is one of the forms of pediatric cancer that has demonstrated dramatic improvements in survival rates. Before the use of antileukemic agents in 1948, a child with ALL lived 2 to 3 months. Current long-term disease-free survival rates for children with ALL approach 80% in major research centers.
AML accounts for about 20% of all cases of childhood leukemia and has an annual incidence of 5 to 7 cases per million. The incidence is similar for males and females, and higher rates are seen during the first 2 years of life. Approximately 40% to 50% of children with AML can be cured of their disease.
Classification: ![]() Because of the confusion and inconsistency in classifying the leukemias, ALL and AML are further subdivided according to another system known as the French-American-British (FAB) system. In the FAB system the subtypes are determined after a thorough study of the morphology (structure) and cytochemical reactivity of the leukemic cells. Accordingly, ALL is morphologically classified into three subtypes: L1, L2, and L3. L1 is the most common subtype (Landier, 2001; Margolin, Rabin, Steuber, et al, 2011). AML is classified into eight subtypes.
Because of the confusion and inconsistency in classifying the leukemias, ALL and AML are further subdivided according to another system known as the French-American-British (FAB) system. In the FAB system the subtypes are determined after a thorough study of the morphology (structure) and cytochemical reactivity of the leukemic cells. Accordingly, ALL is morphologically classified into three subtypes: L1, L2, and L3. L1 is the most common subtype (Landier, 2001; Margolin, Rabin, Steuber, et al, 2011). AML is classified into eight subtypes.
Cytochemical Markers: Leukemic cells demonstrate different reactions when they are exposed to certain chemicals. For example, terminal deoxynucleotidyl transferase is able to provide excellent differentiation between ALL and AML. Several other chemicals are available to further differentiate various cell types.
Chromosome Studies: Chromosome analysis of leukemic cells has become an important tool in the diagnosis and management of patients with ALL and AML. For example, children with trisomy 21 have 20 times the risk of other children for developing ALL (Plon and Malkin, 2011). Children with more than 50 chromosomes (DNA index >1.16) have a better prognosis. Similarly, patients with ALL and trisomies of chromosomes 4 and 10 have a good prognosis with a low risk of treatment failure (Margolin, Rabin, Steuber, et al, 2011).
In addition, several structural changes (translocations) in the leukemic cells have been identified and are associated with clinical outcome. For example, the presence of a t(12;21) (p13;q22) translocation is associated with a good clinical outcome in ALL. The t(9;22)(q34;p11) translocation, known as the Philadelphia chromosome, is found in approximately 5% of children with ALL and is associated with a poor prognosis. For patients with AML, cytogenetic classification plays an important role in the prognosis and therapy. For example, patients with a t(8;21)(q22;q22) or t(15;17)(q22;q12) have a favorable prognosis.
Cell-Surface Immunologic Markers: Most childhood leukemias are of B-cell lineage. Early pre–B-cell (common) ALL, characterized by the presence of cytoplasmic immunoglobulins, is the most frequent type of cancer found in children. Most (>80%) of these children have the common acute lymphocytic leukemia antigen (CALLA) on their cell surface (Margolin, Rabin, Steuber, et al, 2011). Children with ALL who are CALLA positive have better survival rates.
Other subtypes of ALL include B-cell ALL that secretes immunoglobulin on the cell surface and T-cell ALL (revealed by the presence of T-cell surface antigens and heat-stable rosette formation in the presence of sheep red blood cells). Some groups of patients have a characteristic combination of surface markers that usually correlates with a specific subtype of leukemia.
Pathologic and Related Clinical Manifestations
Leukemia is an unrestricted proliferation of immature white blood cells in the blood-forming tissues of the body. Although not a “tumor” as such, the leukemic cells demonstrate the neoplastic properties of solid cancers. Thus the resultant pathologic and clinical manifestations of the disease are caused by infiltration and replacement of any tissue of the body with nonfunctional leukemic cells. Highly vascular organs, such as the spleen and liver, are most severely affected.
To understand the pathophysiology of the leukemic process, it is important to clarify two common misconceptions. First, although leukemia is an overproduction of white blood cells, most often in the acute form the leukocyte count is low. Instead, the peripheral blood smear and, more definitively, the bone marrow examination reveal greatly elevated counts of immature cells, or blasts. Second, these immature cells do not deliberately attack and destroy the normal blood cells or vascular tissues. Cellular destruction occurs through the process of infiltration and subsequent competition for metabolic elements. The following discussion elaborates on the pathologic process and related clinical manifestations in the most susceptible organs of the body (Fig. 36-3).
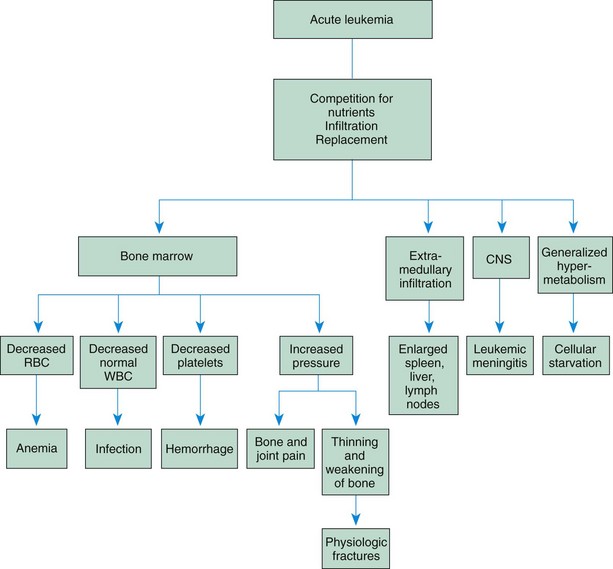
Fig. 36-3 Principal sites of tissue involvement in leukemia. CNS, Central nervous system; RBC, red blood cells; WBC, white blood cells.
Bone Marrow Dysfunction: In all types of leukemia the proliferating cells depress bone marrow production of the formed elements of the blood by competing for and depriving the normal cells of the essential nutrients for metabolism. The three main consequences are (1) anemia from decreased erythrocytes, (2) infection from neutropenia, and (3) bleeding from decreased platelet production.
The invasion of the bone marrow with leukemic cells gradually causes a weakening of the bone and a tendency toward fractures. As leukemic cells invade the periosteum, increasing pressure causes severe pain. The most frequent presenting signs and symptoms of leukemia are a result of infiltration of the bone marrow. These include fever, pallor, fatigue, anorexia, hemorrhage (usually petechiae), and bone and joint pain. In the presence of neutropenia the body’s normal bacterial flora can become aggressive pathogens. Any break in the skin is a potential site of infection. Frequently, vague abdominal pain is caused by areas of inflammation from normal flora within the intestinal tract.
Disturbance of Involved Organs: The spleen, liver, and lymph glands demonstrate marked infiltration, enlargement, and eventually fibrosis. Hepatosplenomegaly is typically more common than lymphadenopathy.
The next most important site of involvement is the CNS. Less than 5% of patients with ALL and about 20% of patients with AML have CNS involvement. The use of prophylactic CNS intrathecal therapy has dramatically decreased the incidence of CNS relapse in these patients.
The usual effect of leukemic infiltration of the meninges is increased intracranial pressure (ICP). The pathogenesis is presumably attributable to invasion of the arachnoid by proliferating cells, which then interfere with the flow of cerebrospinal fluid in the subarachnoid space and at the base of the brain. The increased fluid pressure causes dilation of all four ventricles and consequently the signs and symptoms normally associated with this condition, such as severe headache, vomiting, papilledema, irritability, lethargy, and eventually coma. Irritation of the meninges also causes pain and stiffness in the neck and back.
Additional sites of involvement may be the cranial nerves (most often cranial nerve VII, or the facial nerve) and spinal nerves, particularly of the lumbosacral plexus, hypothalamus, and cerebellum. Clinical manifestations for these sites are directly related to the area involved. For example, with lumbosacral invasion, the patient has weakness in the lower extremities, pain radiating down the legs to the feet, and difficulty in voiding. Although such signs may suggest a brain tumor, the absence of localized signs often leads to the discovery of CNS involvement in leukemia.
Other sites that may become invaded with leukemic cells include the kidneys, testes, prostate, ovaries, gastrointestinal tract, and lungs. With long-term survivors becoming increasingly common, such extramedullary sites of leukemic invasion, especially the testes, are becoming more important clinically.